Abstract
Oxygen three-isotope analysis by secondary ion mass spectrometry of chondrule olivine and pyroxene in combination with electron microprobe analysis were carried out to investigate 24 FeO-poor (type I) and 2 FeO-rich (type II) chondrules from the Kaba (CV) chondrite. The Mg#’s of olivine and pyroxene in individual chondrules are uniform, which confirms that Kaba is one of the least thermally metamorphosed CV3 chondrites. The majority of chondrules in Kaba contain olivine and pyroxene that show indistinguishable Δ17O values (= δ17O − 0.52 × δ18O) within analytical uncertainties, as revealed by multiple spot analyses of individual chondrules. One third of chondrules contain olivine relict grains that are either 16O-rich or 16O-poor relative to other indistinguishable olivine and/or pyroxene analyses in the same chondrules. Excluding those isotopically recognized relicts, the mean oxygen isotope ratios (δ18O, δ17O, and Δ17O) of individual chondrules are calculated, which are interpreted to represent those of the final chondrule melt. Most of these isotope ratios plot on or slightly below the primitive chondrule mineral (PCM) line on the oxygen three-isotope diagram, except for the pyroxene-rich type II chondrule that plots above the PCM and on the terrestrial fractionation line. The Δ17O values of type I chondrules range from ~ −8‰ to ~ −4‰; the pyroxene-rich type II chondrule yields ~0‰, the olivine-rich type II chondrule ~ −2‰. In contrast to the ungrouped carbonaceous chondrite Acfer 094, the Yamato 81020 CO3, and the Allende CV3 chondrite, type I chondrules in Kaba only possess Δ17O values below −3‰ and a pronounced bimodal distribution of Δ17O values, as evident for those other chondrites, was not observed for Kaba.
Investigation of the Mg#-Δ17O relationship revealed that Δ17O values tend to increase with decreasing Mg#’s, similar to those observed for CR chondrites though data from Kaba cluster at the high Mg# (>98) and the low Δ17O end (−6‰ and −4‰). A mass balance model involving 16O-rich anhydrous dust (Δ17O = −8‰) and 16O-poor water ice (Δ17O = +2‰) in the chondrule precursors suggests that type I chondrules in Kaba would have formed in a moderately high dust enriched protoplanetary disk at relatively dry conditions (~50–100× dust enrichment compared to Solar abundance gas and less than 0.6× ice enhancement relative to CI chondritic dust). The olivine-rich type II chondrule probably formed in a disk with higher dust enrichment (~2000× Solar).
1. INTRODUCTION
Chondrules - a main constituent of most chondritic meteorites - are spherical, melted objects that formed by transient high-temperature events in the proto-planetary disk (e.g., Hewins, 1996; Rubin, 2000; Connolly and Desch, 2004; Ciesla, 2005; Morris et al., 2012). Since the recognition of isotopic anomalies in the Allende CV chondrite in the early 1970s (Clayton et al., 1973, 1977), numerous oxygen isotope studies on primitive carbonaceous chondrites have targeted the variabilities among bulk meteorites, as well as those of CAIs and chondrules (e.g, Clayton, 1993; Clayton and Mayeda, 1999; Jones et al., 2004; Krot et al., 2006; Yurimoto et al., 2008, and references therein). In general, oxygen isotope ratios of chondrules in carbonaceous chondrites plot in the oxygen three-isotope diagram below the terrestrial fractionation (TF) line and along a slope ~1 line, for instance parallel to the CCAM (carbonaceous chondrite anhydrous minerals) line, while those in ordinary chondrites cluster slightly above the TF line (e.g., Clayton 1993).
By using secondary ion mass spectrometers (SIMS), high precision (sub-‰) mineral-scale isotope data of chondrule minerals have become increasingly available in recent years (e.g., Chaussidon et al., 2008; Kita et al., 2010; Connolly and Huss, 2010; Rudraswami et al., 2011; Ushikubo et al., 2012; Schrader et al., 2013, 2014, 2017; Tenner et al., 2013, 2015; Nagashima et al., 2015). In contrast to bulk analyses, in-situ measurements of primary chondrule minerals, such as olivine, low-Ca pyroxene and mesostasis phases allow for an evaluation of the isotopic homogeneity of single chondrules. A growing number of studies have established that most chondrules are internally homogeneous in terms of their Δ17O (= δ17O − 0.52 × δ18O) values except for minor occurrence of relict olivine grains (e.g., Kita et al., 2010; Rudraswami et al., 2011; Tenner et al., 2013, 2015, 2017; Schrader et al., 2017). In particular, according to the analyses of ungrouped carbonaceous chondrite Acfer 094, in which both thermal metamorphism and aqueous alteration in the parent body was minimal, Ushikubo et al. (2012) clearly demonstrated that oxygen isotope ratios of olivine and pyroxene phenocrysts are indistinguishable from those of glassy mesostasis. Presuming that this is the general case, i.e., chondrule phenocrysts and mesostasis have indistinguishable Δ17O values at the time of chondrule formation, it is now possible to deduce the composition of the isotopically homogenized melt by measuring chondrule phenocrysts in other chondrites even if oxygen isotope ratios of glassy mesostasis were altered due to parent body processes (e.g., in Semarkona LL3 by Kita et al., 2010).
It has been suggested that chondrules formed in an open system with respect to major oxides, such as MgO and SiO2, which evaporated and re-condensed during melting (e.g., Tissandier et al., 2002; Alexander, 2004; Nagahara et al., 2008). Under a dust-enriched environment (e.g., Ebel and Grossman, 2000), the ambient gas present during chondrule formation would have oxygen isotope ratios similar to those of average solid precursors, since oxygen in the ambient gas would predominately originate from these precursors (e.g., Ushikubo et al., 2012). Further, Ushikubo et al. (2012) suggested that the internally homogeneous oxygen isotope ratios within a single chondrule were the result of isotope exchange between ambient gas and chondrule melt that occurred rapidly due to evaporation and re-condensation of major oxides from the chondrule melt.
The Mg#’s of chondrules, defined as the molar MgO/(MgO+FeO) % of mafic chondrule minerals, mainly depend on the redox conditions during chondrule-formation (Ebel and Grossman, 2000). Oxygen fugacities required to form FeO-poor (type I) or FeO-rich (type II) chondrules in carbonaceous chondrites (log fO2: up to iron-wüstite buffer for type II; IW – 6 to − 2 for type I chondrules; e.g., Ebel and Grossmann, 2000; Tenner et al., 2015) are higher than estimates for the Solar nebula (log fO2: ~ IW − 6 at 1600K; e.g., Krot et al., 2000). The more oxidizing redox conditions were likely imposed by enhancement (relative to Solar abundances) of dust particles and H2O ice (e.g., Ebel and Grossman, 2000; Fedkin and Grossman, 2006, 2016; Schrader et al., 2013; Tenner et al., 2015). In carbonaceous chondrites, many studies have found type I chondrules to be generally 16O-rich compared to type II chondrules (e.g., Kunihiro et al., 2004, 2005; Connolly and Huss, 2010; Schrader et al., 2013). Further, Ushikubo et al. (2012) and Tenner et al. (2013) observed a bimodal distribution of Δ17O values that is related to Mg#’s of chondrules in Acfer 094 and Y-81020 (CO3), respectively. The majority of chondrules possess Δ17O values of ~ −5‰ and Mg#>97; other chondrules show Δ17O values of ~ −2‰ and a wide range of Mg# (100–40). In CB and CH chondrites, the majority of chondrules are type I with Δ17O values of ~ −2‰ (Krot et al., 2010), while type II chondrules have Δ17O values of ~ +1.5‰ (Nakashima et al., 2010). In the case of CR chondrites, the majority of chondrules are type I showing a larger range of Δ17O values from −6‰ to −1‰, while minor type II chondrules span from −2‰ to 0‰ (e.g., Connolly and Huss, 2010; Schrader et al., 2013, 2014, 2017; Tenner et al., 2015).
The overall tendency of higher chondrule Δ17O values in combination with lower Mg#’s across carbonaceous chondrites suggests the existence of 16O-poor water ice among chondrule precursors in carbonaceous chondrite forming regions (e.g., Connolly and Huss, 2010; Ushikubo et al., 2012; Schrader et al., 2013). By detailed examination of Mg#’s and corresponding Δ17O values among type I chondrules in CR chondrites, Tenner et al. (2015) observed a monotonic increase in Δ17O values with decreasing Mg#, which was never observed in other chondrite groups before. Tenner et al. (2015) uses an oxygen isotope mass balance model in combination with expressions that link dust enrichment and abundance of water ice to the fO2 of the chondrule melt in order to explain Δ17O values and Mg#’s of chondrules. By applying Δ17O values of −6‰ and +5‰ for anhydrous dust and water ice, respectively, the model estimated that type I chondrules in CR formed under 100–200× dust-enrichments relative to solar composition gas with variable amounts of water ice from 0 to 0.8× the nominal water ice content of CI chondritic dust. Tenner et al. (2015) stated that CR chondrites, Acfer 094, CO, and perhaps CV chondrites all contain chondrules with high Mg#’s (>98) and Δ17O values ranging from −6‰ to −4‰. This range of compositions was also found to be the dominant chondrule type in the Y-82094 ungrouped carbonaceous chondrite (Tenner et al., 2017).
The literature data about the Mg#-Δ17O relationship for chondrules from CV chondrites, as summarized in Tenner et al. (2015), requires re-examination because of the following reasons. Earlier SIMS studies are mostly not at sub-‰ precision level and mainly include olivine analyses and only a limited amount of low-Ca pyroxene analyses (e.g., Choi et al., 2000; Jones et al., 2004; Libourel and Chaussidon, 2011), hence, rendering it difficult to evaluate the mineral-scale isotopic homogeneity of these chondrules. Chaussidon et al. (2008) measured oxygen isotope ratios of several chondrule constituents in Vigarano CV3 and, for a somewhat limited number of chondrules, in the Mokoia and Efremovka CV3 chondrites; however, corresponding information about the mineral chemistry, especially the Mg#’s for each individual chondrule, were not provided. Conversely, a complete data set of oxygen isotope ratios of chondrule phases, including olivine, low-Ca pyroxene, and, where applicable, spinel and plagioclase was published by Rudraswami et al. (2011) for Allende, but the evaluation of the Mg#-Δ17O relationship is made difficult by significant thermal metamorphism experienced by this chondrite (e.g., Krot et al., 1995; Bonal et al., 2006). Since diffusion of Mg and Fe in olivine and pyroxene is considerably faster than diffusion of oxygen (Dohmen and Chakraborty, 2007; Farver, 2010), chondrules more likely preserve primary oxygen isotopic compositions than Mg#’s when subjected to thermal metamorphism. For example, even though many olivine grains show higher FeO contents (lower Mg#’s) than coexisting low-Ca pyroxenes in chondrules of the Allende CV3, most chondrules are still homogeneous in oxygen isotope ratios at analytical precisions (Rudraswami et al., 2011).
Here we present SIMS oxygen three-isotope analysis of chondrules from Kaba in order to understand the chondrule formation environment and oxygen isotope reservoirs for the CV chondrite-forming region. Kaba is one of the least thermally metamorphosed CV3 chondrites (Kimura and Ikeda, 1998; Krot et al., 1998; Grossman and Brearley, 2005; Bonal et al., 2006, Busemann et al., 2007) and a member of the Bali-like oxidized subgroup (McSween, 1977). In particular, representative dust-enrichment and ice-enhancement factors of the local disk region are evaluated using the oxygen isotope mass balance model of Tenner et al. (2015) by applying model parameters suitable for the CV chondrule-forming region. Unlike Allende, chondrules in Kaba likely preserve primary Mg#’s in addition to Δ17O values that are representative for conditions during chondrule formation, an assumption tested by comparing Mg# of olivine and pyroxene within each chondrule.
2. ANALYTICAL TECHNIQUES
2.1. Electron microscopy
One thin section of Kaba (USNM 1052–1) was imaged (BSE, SE) and the petrography of chondrules investigated with a Hitachi S-3400N scanning electron microscope (SEM). The chemical composition of olivine and pyroxene grains were determined by a Cameca SX-51 electron microprobe (20nA, 15keV, fully focused beam); plagioclase and high-Ca pyroxene of the mesostasis were analyzed by a Cameca SXFive (10nA, 15keV, 10μm beam). The SEM and both electron microprobes are hosted at the Department of Geoscience at UW-Madison. On both electron microprobes, concentrations of the oxides SiO2, TiO2, Al2O3, Cr2O3, MgO, FeO, MnO, CaO, K2O, and Na2O were acquired and counting times on the peak and background were set to 10s and 5 s, respectively. The 3σ detection limits (wt%) for these oxides (in the order mentioned above) were at most: 0.05, 0.06, 0.04, 0.07, 0.05, 0.07, 0.07, 0.04, 0.03, and 0.06, respectively. Data reduction, including ZAF/φ(ρz) corrections, was done with the “Probe for EPMA” software suite (Donovan, 2015). The following standards were used for olivine and pyroxene analyses (on Cameca SX-51): Ti: rutile, Al: jadeite, Cr: synthetic Cr2O3, Fe: synthetic hematite, Mn: manganese olivine, Ca: wollastonite, K: microcline, Na: jadeite, and depending on mineral type and composition: Si: synthetic enstatite, synthetic forsterite, Fo83, manganese olivine; Mg: synthetic enstatite, synthetic forsterite, Fo83, amphibole. Analyses of plagioclase and high-Ca pyroxene in the chondrule mesostasis were performed on a Cameca SXFive, using the same suite of standards except for the following elements: Si: synthetic enstatite, An78, An67; Al: jadeite, An95; Fe: fayalite; Ca: An78, An95; Na: jadeite, albite, An78.
2.2. SIMS oxygen isotope analyses
The in-situ oxygen three-isotope analysis of olivine and pyroxene was carried out during two separate sessions with the Cameca IMS 1280 SIMS at the UW-Madison using multi-collector Faraday cups and following analytical protocols similar to those of Kita et al. (2010) and Tenner et al. (2013). In both sessions, Cs+ primary ion intensity was set to ~3 nA in order to generate a secondary 16O− intensity of ~3×109 cps (counts per second). During the first session, the primary beam was tuned to form an ellipsoid-shaped spot of 14×10 μm, similar to the condition used in previous studies (e.g, Tenner et al., 2015, 2017). In the second session, the primary beam aperture was enlarged (>200 μm, normally 100–150 μm) and resulted in a diffused beam shape (Gaussian beam in both sessions). Consequently, we reduced the beam size to ~10 μm but rastered 5 × 5 μm over the sample surface to produce a roundish spot of 12 μm diameter. The intensity of 16O1H− was monitored automatically at the end of each analysis by using a X-deflector between sector magnet and detectors in order to correct the contribution from tailing of the 16O1H− peak to the 17O− signal. The correction was always insignificant (≤0.1‰).
Typically, 12–18 analyses of unknowns were bracketed by 8 analyses (4 before and 4 after unknowns) of the San Carlos olivine standard (δ18O = 5.32‰ VSMOW, Kita et al., 2010) to monitor the drift of instrumental bias and the external (spot-to-spot) reproducibility. External reproducibility, which represents the uncertainty of individual analyses of unknowns (Kita et al., 2009), was typically ~0.4, ~0.3, and ~0.4‰ (2SD) for δ17O, δ18O, and Δ17O, respectively. Olivine and pyroxene form solid solutions series that show systematic difference in instrumental biases in δ18O (e.g., Tenner et al., 2013), which are calibrated using three olivine (Fo100, 89, 60) and three low-Ca pyroxenes (En97, 85, 70) and one diopside (En50Wo50) standard (Kita et al., 2010; Nakashima et al., 2013) that cover the range of chemical compositions observed in olivine and pyroxene in Kaba chondrules.
2.3. Selection of chondrules and positions of SIMS analysis
To minimize the sampling bias that is potentially introduced by preferential selection of chondrules by e.g., shape, texture, or degree of alteration, every chondrule larger or equal ~750 μm in diameter (or longest direction) was selected from a single polished thin section of Kaba (USNM 1052–1) for oxygen isotope analysis based on the evaluation of high-resolution BSE and SE images of the entire thin section. Due to scarcity of type II chondrules in Kaba, one small (~150 μm) chondrule comprising FeO-rich olivine was included for SIMS analysis as well as one smaller chondrule (~600 μm) comprising FeO-rich pyroxene. All selected chondrules are labeled (K1 to K26) in the BSE mosaic image of the Kaba USNM 1052–1 thin section shown in Appendix EA1. At this point, exploratory SEM-EDS measurements were carried out to examine chemical zoning of minerals and to identify opaque phases. Per chondrule, at least 8 locations that are larger than 15 μm and free of cracks and inclusions were selected for SIMS analyses, preferably 4 for olivine and 4 for pyroxene, to be able to evaluate the isotopic homogeneity of individual chondrules. High-Ca pyroxene or plagioclase in the mesostasis of chondrules were not analyzed for oxygen three-isotopes, because they are usually smaller than 15 μm. The chemical compositions of minerals at each position were determined by EPMA and utilized later for instrumental bias correction of SIMS analyses. Finally, after SIMS analysis, each individual SIMS pit was imaged by SEM (see Appendix EA2) to evaluate whether cracks or inclusions were hit and whether the desired position was accurately sampled by the ion beam. Individual SIMS analyses were rejected from results if the primary beam overlapped two different mineral grains or an elevated OH signal in combination with visible cracks or cavities within pits indicate a compromised analysis.
2.4. Data reduction for host Δ17O values of individual chondrules
In order to estimate representative oxygen isotope ratios of the last chondrule melt, Ushikubo et al. (2012) and Tenner et al. (2013) established a data reduction scheme to calculate the host Δ17O value of a single chondrule as the mean value of multiple olivine and pyroxene analyses that are within a critical value from the mean. The same data reduction scheme was applied for chondrules in Kaba by using 0.6‰ (Δ17O) as the critical value, which is the mean 3SD of the bracketing San Carlos olivine standard during the 2 SIMS sessions. In a first step, the mean Δ17O value from multiple analyses of an individual chondrule was calculated. If all data are within ±0.6‰ from the mean, this mean Δ17O value is considered to represents that of the host chondrule. The host Δ17O value should be determined by at least 2 data points. In a second step, for chondrules that contain data exceeding ±0.6‰ from the mean, a subset of data is selected, which preferably includes all pyroxene analyses and a new mean is calculated, only including this new subset. Subsequently, the subset is tested again for the variability criterion. The excluded olivine analyses, deviating more than 0.6‰ from the mean, are potential relict grains that may not reflect Δ17O values of the final chondrule melt.
The host δ17O and δ18O values are calculated with the same subset of analyses that were used for calculating the host Δ17O values. Uncertainties (at 95% confidence level) reported for host δ17O and δ18O values comprise the propagation of three types of uncertainties related to the analysis of unknowns, the instrumental bias correction based on the bracket standard, and the ultimate uncertainty of SIMS oxygen isotope analyses: Uncertainties (unc.) are calculated as the sum of those three components that are (i) twice the standard error of those analyses that constitute the mean, where SD is standard deviation of the unknown analyses or that of the standard bracket whichever larger, (ii) twice the standard error of the analyses of the corresponding San Carlos olivine bracket, and (iii) a fixed value to account for possible mass-dependent fractionation in mean isotope ratios because of sample geometry and topography, as well as the reproducibility of calibration standards (±0.3‰ for δ18O, ±0.15‰ for δ17O, Kita et al., 2009, 2010). Uncertainties of host Δ17O values only consider components (i) and (ii).
3. RESULTS
3.1. Petrography of chondrules
In the Kaba thin section, 7 type IA (<10% modal abundance of low-Ca pyroxenes), 3 type IB (<10% olivine), 14 type IAB, 1 type IIA, and 1 type IIB were analyzed for oxygen isotope compositions. The two individual chondrules K8 and K9 are part of one compound object and discussed together as chondrule K8+9 (Fig. 1a). Petrographic descriptions of each chondrule analyzed in this study is presented in the Appendix EA3. Chondrule sizes range from 0.6 – 3mm (diameter or longest direction); the type IIA chondrule is considerably smaller (0.15mm). Throughout this paper no distinction is made between complete chondrules and chondrule fragments. General textures of most investigated chondrules are best described as porphyritic.
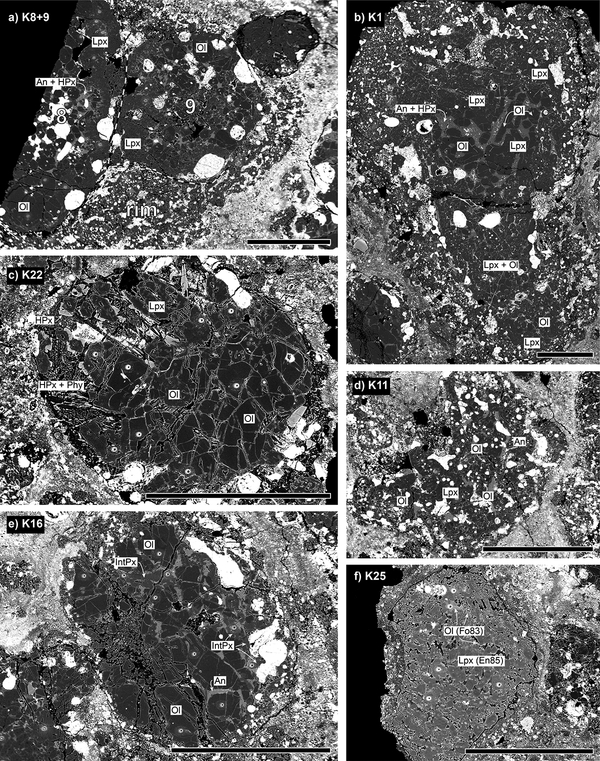
Figure 1:
Representative BSE images of chondrules from the Kaba USNM 1052–1 thin section showing SIMS pits for oxygen three-isotope analyses (except for K1). (a) compound chondrule K8+9 (IAB) enclosed in igneous rim comprising pyroxene, olivine and opaque phases. (b) K1 (IAB) composed of coarse-grained olivine and pyroxene phenocrysts, as well as anorthite and high-Ca pyroxene in mesostasis at the chondrule center; enclosed in relatively fine-grained and opaque phases-rich rim. (c) K22 (IAB) contains coarse-grained olivine and low-Ca pyroxene. Altered mesostasis comprises phyllosilicates and high-Ca pyroxenes, latter forming euhedral overgrowths on low-Ca pyroxenes. (d) low-Ca pyroxene poikilically encloses olivine in K11 (IB). (e) K16 (IA) comprises small amount of mesostasis and intermediate pyroxene alongside olivine. (f) FeO-rich low-Ca pyroxenes (En85) in K25 (IIB) possess overgrowth of FeO-rich olivine (Fo83). Ol: olivine, Lpx: low-Ca pyroxene, HPx: high-Ca pyroxene, IntPx: intermediate pyroxene, An: anorthite, Phy: phyllosilicates; scale in all images: 500μm.
Some type IAB chondrules show a predominately concentric layering. In particular, concentric layering within individual chondrules is well developed in the compound chondrule K8+9 (Fig. 1a), as well as in chondrules K1 (Fig. 1b) and K20 (Appendix EA1, Page 20). In these examples, inner portions of chondrules comprise olivine, mesostasis, and in the case of the individual chondrule K8 also abundant opaque phases. Inner portions are mantled by pyroxenes, often containing olivine inclusions, and are enclosed with fine-grained rims dominated by pyroxene and dispersed opaque phases. The texture and mineralogy of the irregular-shaped chondrule K12 (see Appendix EA1, Page 12) resembles those of these fine-grained rims. Textures of the type IAB chondrules K3 and K19 (see Appendix EA1) indicate a similar concentric layering but fine-grained rims are absent.
In general, olivine occurs in type I chondrules (i) as anhedral grains (e.g., K3, K5, K8+9) or, rarely, as euhedral phenocrysts (K1) and small euhedral crystals in mesostasis (K4), (ii) in the form of irregular-shaped olivine masses (e.g., K19; K22, Fig. 1c), and (iii) as small euhedral grains poikilically enclosed in low-Ca pyroxene (e.g., K1, K24; K11, Fig. 1d). In type IA chondrules, olivine grains can be coarse-grained and embedded in varying amounts of interstitial mesostasis (K7, K10; K16, Fig. 1e). Chondrule K6 (IA) comprises olivine that contains metal grains, typical for “dusty olivine” relicts. Except for its occurrence in fine-grained rims, low-Ca pyroxene is usually subhedral (or even euhedral) and often exhibits a poikilitic texture caused by small individual inclusions (e.g., K11, K15, K24) or clusters of olivine grains (K13). The type IIB chondrule K25 comprises subhedral pyroxene that exhibits thin (<20μm) overgrows of olivine (K25, Fig. 1f).
Chondrules contain varying amounts of opaque phases (e.g., high abundance: K11, K12, K14, K23; low: K3, K7, K16, K22) that are evenly distributed within chondrules (e.g., K11, K12) or localized at their margins (e.g., K8+9, Fig 1a; K16, Fig. 1e). In chondrule K8+9, larger blobs of opaque phases are abundant at the interface of chondrule interior and rim (Fig. 1a). As confirmed by SEM-EDS analyses, opaque phases are mainly magnetite, Fe-Ni sulfides, and, rarely, Fe-Ni alloys (only in K1). The mesostasis is altered in most of the chondrules and comprises abundant phyllosilicates that probably replaced glassy mesostasis and plagioclase (Fig. 1c). High-Ca pyroxene often forms euhedral overgrows on low-Ca pyroxenes (Fig. 1c), sometimes also worm-like intergrow textures with plagioclase (see Appendix EA1, Page 14).
3.2. Mineral chemistry of olivine, pyroxene, and mesostasis phases
Mg#’s of olivine in type I chondrules are restricted to a narrow range between 98.5 and 99.8 (99.3 ±0.58, 2SD) and zoning caused by thermal metamorphism is insignificant (complete set of EPMA analyses in Appendix EA4). Thin FeO-rich halos around opaques or small holes in olivine, once filled by opaque minerals, are evidence for small-scale diffusion and excluded from analyses. Some olivine in chondrule K1 shows higher than typical Cr2O3 (up to 0.69wt%) and MnO (up to 0.49wt%) values compared to the mean of all olivine analyses (Cr2O3: 0.36 ±0.27wt%; MnO: 0.18 ±0.25wt%; 2SD). Chondrules K3, K7, and K22 contain olivine grains that yield CaO contents slightly higher than 0.5wt% (up to 0.59wt%). Olivine in the type IIA chondrule (K26) is zoned in Fe content and yields Mg#’s of 66.2 and 57.7 in the center and close to margins, respectively. The thin olivine overgrowths on pyroxene in the type IIB chondrule (K25) possess Mg#’s of about 83.
Low-Ca pyroxene (Wo<3) is Fe-poor (mean Mg# type I: 99.1 ±0.51, 2SD) and shows the characteristic twinning of clinoenstatite when observed under crossed polarizers. In addition to low-Ca pyroxene, some type I chondrules (K5, K6, K8+9, K10, K13, K16) contain “intermediate pyroxene” (IntPx) often associated with mesostasis and distinguished from low-Ca pyroxene by higher Wo contents (Wo3–5); lamellar twins are often absent in intermediate pyroxene. The fine-grained rim enclosing chondrules K8 and K9 comprises low-Ca and intermediate pyroxene. In chondrule K10, intermediate and high-Ca pyroxenes in the mesostasis are the only pyroxenes; in chondrule K16, low-Ca pyroxene is only present in insignificant amounts at chondrule margins whereas intermediate pyroxene fills interstitials of coarse olivine grains (Fig. 1e). Pyroxene in the type IIB chondrule (K25, Fig. 1f) is En85Fs14Wo1 (Mg#: 85.7). In general, high-Ca pyroxenes (En55–65Fs<2Wo34–44) that coexist with plagioclase (An91–98) in the mesostasis of several chondrules can be considered as aluminian augites (Al > 0.1 apfu, atoms per formula unit). In these aluminian augites, the Ca(R3+)AlSiO6 component, where R3+ is predominately Al, can exceed 15 mol% in a few cases. Ti contents of high-Ca pyroxenes are low (<0.05 apfu).
3.3. Oxygen three-isotope ratios
A total of 235 SIMS oxygen isotope analyses of olivine (133) and pyroxene (102) were performed. Thereof 16 had to be rejected from final results, mostly because (i) the spot area covered two different phases (e.g., K1: 98.Ol, 104.Lpx; see Appendix EA2, Page 1), (ii) abundant cracks (e.g., K4: 234.Lpx), (iii) the beam hit cavities in grains (e.g., K23: 309.Ol) or (iv) due to other surface imperfections. SIMS analyses are referred to in this paper by citing the chondrule identifier, followed by the analysis number and an abbreviation of the mineral analyzed. The complete oxygen three-isotope dataset of this study, including rejected analyses, is provided in Appendix EA5. Individual oxygen three-isotope diagrams of all analyzed chondrules are given in Appendix EA6.
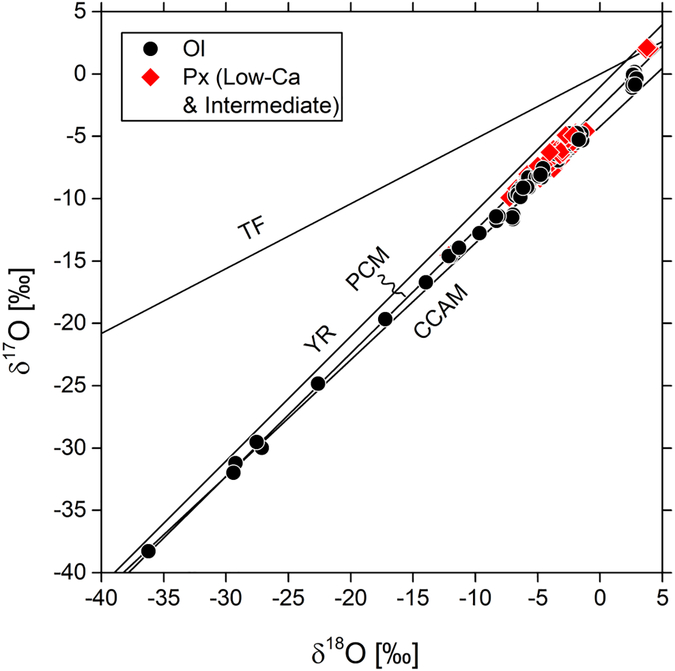
Most oxygen isotope ratios of olivine and pyroxene plot either on the PCM or in between the PCM and CCAM lines (Fig. 2). The δ18O and δ17O values of all individual analyses range from ≈ −36‰ to +4‰ and −38‰ to +2‰, respectively (Δ17O ≈ −19‰ - 0‰). Some of the most 16O-rich analyses (e.g., Δ17O ≈ −15‰, K4: 235.O1, 236.O1) show internal errors (derived from cycle-by-cycle variations within a single spot analysis) that are larger than typical (< 0.5‰), indicating heterogeneous oxygen three isotope ratios within an analyzed volume. Typical olivine and pyroxene in type I chondrules yield δ18O and δ17O values in between −12‰ and −1‰ and from −15‰ to −5‰, respectively (Δ17O ≈ −8‰ - −3‰.). Pyroxene in the type IIB (δ18O: +3.7‰ - +4.0‰, δ17O: +2.0‰ - +2.1‰, Δ17O: 0.0‰ - +0.2‰) and olivine in the FeO-rich type IIA chondrule (δ18O: +2.6‰ - +2.9‰, δ17O: −1.1 - −0.4‰, Δ17O: −2.5‰ - −1.9‰) are 16O-poor compared to those in type I chondrules. Analyses of olivine overgrowths on pyroxene of the type IIB chondrule (K25: 327.O1, 328.O1) have been rejected because of irregular SIMS pits, yet their Δ17O values (Δ17O: 0.09 ±0.01‰, n=2, 2SD) are indistinguishable within analytical uncertainty from those of the pyroxene (0.06 ±0.19‰, n=3, 2SD) in the same chondrule.
Figure 2:
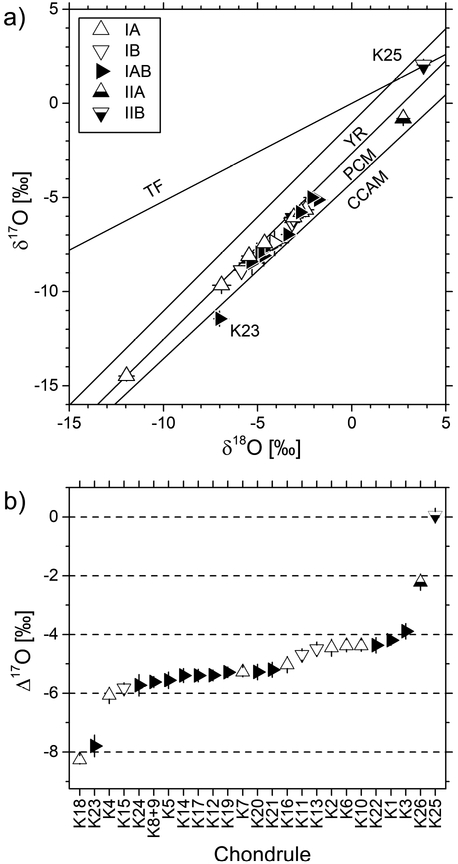
Oxygen three-isotope diagram showing olivine (n=124) and pyroxene (n=95) analyses from chondrules in Kaba. Most analyses plot on the primitive chondrule mineral (PCM; Ushikubo et al., 2012) line or in between the PCM and CCAM (Clayton et al., 1977) lines; analyses of chondrule K25 plot above PCM and on terrestrial fractionation (TF) line. YR: Young & Russel line (Young and Russell, 1998).
3.4. The presence of isotopic relict grains and host chondrule Δ17O values
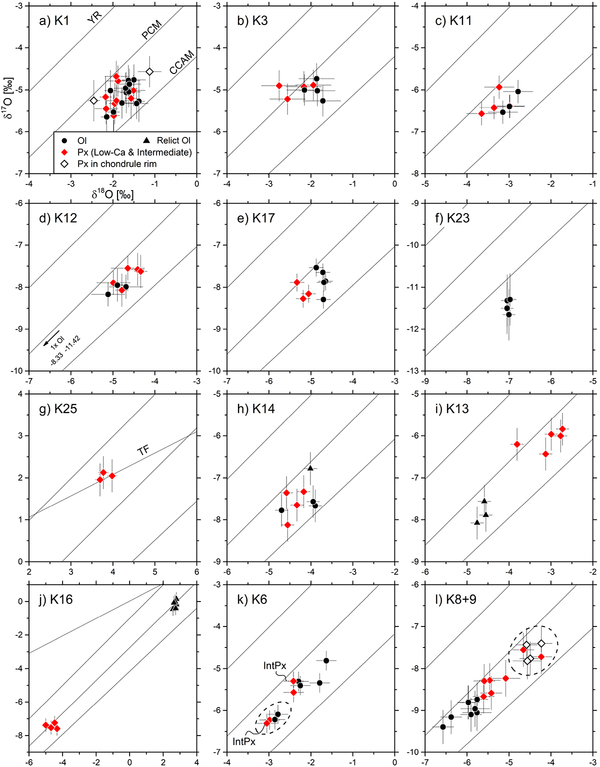
Figure 3 provides oxygen three-isotope diagrams for selected chondrules and Fig. 4 shows Δ17O values of olivine and pyroxene as well as mean chondrule Δ17O values. For all chondrules examined, at least 3, but usually more than 7 analyses are indistinguishable within analytical uncertainties (Fig 3a–g). 11 out of 25 chondrules comprise olivine analyses that are variable in Δ17O values beyond the threshold, and some of them are considered to represent analyses of relict grains. Most relict olivine grains are 16O-rich relative to their host, but in some chondrules (see Fig. 4; K18, K16) relict olivine grains are 16O-poor relative to the corresponding host. Olivine analyses in chondrules K5, K13, and K16 form clusters with mean Δ17O values that are distinguishable from those of the corresponding pyroxene clusters. Therefore, in these cases, the host Δ17O values are determined by using only pyroxene analyses although a part of the olivine analyses in chondrules K5 and K13 are within the variability threshold. In chondrules K6, K8+9, and K24, the internal variability of Δ17O is marginal compared to the threshold of 0.6‰.
Figure 3:
Oxygen three-isotope diagrams of individual olivine and pyroxene analyses of representative chondrules in Kaba. Error bars show external reproducibility (2SD) obtained by analyses of the bracketing olivine standard. (a-g) Individual analyses are indistinguishable in terms of their Δ17O values within the variability threshold of ±0.6‰. (h) One olivine analysis in chondrule K14 slightly exceeds variability threshold. (i-j) Olivine and pyroxene analyses form distinct clusters, while (relict) olivine are either 16O-rich (K13) or 16O-poor (K16) relative to pyroxene. (k-l) analyses of K6 and K8+9 show clearly resolvable variability in δ18O and δ17O values (2SD: 1.0‰ – 1.4‰) but Δ17O values are within variability threshold (±0.6‰). Both chondrules are marginally heterogeneous. In K6 (k), analyses located in right hand part of chondrule are relatively more 16O-rich (circled data points). In K8+9 (l) pyroxene analyses in the rim and a few locations of the chondrule interior (circled data points) are 16O-poor relative to the rest of the analyses.
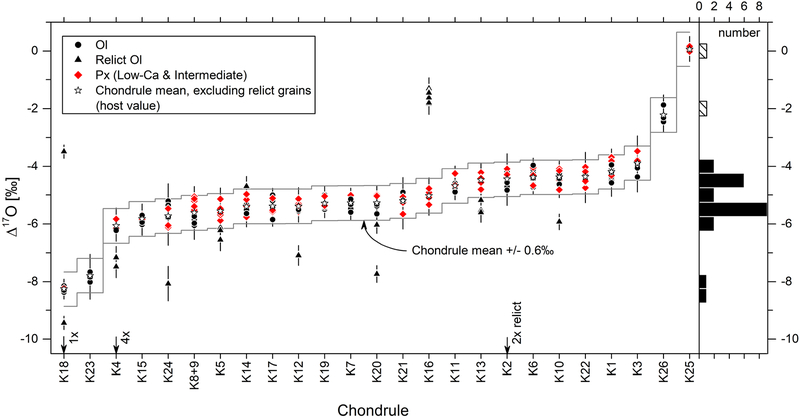
Figure 4:
Δ17O values of olivine and pyroxene analyses per individual chondrule. Error bars show external reproducibility (2SD) obtained by analyses of the bracketing standard. Chondrules sorted according to increasing host chondrule Δ17O values (stars) which are obtained by averaging Δ17O values of isotopically homogeneous olivine and/or pyroxene analyses after excluding isotopic relicts (triangles). Δ17O values of relict olivine exceed the variability threshold (grey line; ±0.6‰, mean external precision on bracketing standard, 3SD). Most type I chondrules show host chondrule Δ17O values in between −6‰ and −4‰ as illustrated by the histogram on the right-hand side of the graph.
Most chondrules in the Kaba 1052–1 thin section (21 out of 25) yield almost continuous host Δ17O values in between −6.1 and −3.9‰ (δ18O: −6.9 - −2.1‰, δ17O: −9.7 - −5.0‰, see Fig. 4 and Table 1). Chondrules K18 (IA) and K23 (IAB) possess the lowest host Δ17O values of - 8.3‰ (δ18O: −12.0‰, δ17O: −14.5‰) and −7.8‰ (δ18O: −7.0‰, δ17O: −11.4‰), respectively. The type IIB chondrule (K25) and the type IIA chondrule (K26) show the most 16O-poor compositions with host Δ17O values of 0.1‰ (δ18O: 3.8‰, δ17O: 2.0‰) and −2.2‰ (δ18O: 2.7‰, δ17O: −0.8‰), respectively.
Table 1 :
Average mineral chemistry and host oxygen isotope ratios (‰, VSMOW) of individual chondrules in the polished thin section of Kaba CV3 (USNM 1052–1).
| Chondrule | Type | OI-ra | OI-ha | Pxa | Mg#b | Woc | δ18 | Od | δ17 | Od | Δ17 | Od |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K1 (core) | IAB | - | 12 | 9 | 99.0 | 1 | −1.79 | ±0.33 | −5.15 | ±0.24 | −4.22 | ±0.17 |
| K1 (rim) | - | - | - | 2 | 98.4 | 1 | −1.79 | ±1.36 | −4.91 | ±0.72 | −3.98 | ±0.29 |
| K1 (all) | IAB | - | 12 | 11 | 98.8 | 1 | −1.79 | ±0.34 | −5.13 | ±0.24 | −4.20 | ±0.17 |
| K2 | IA | 2 | 6 | 2 | 99.2 | 2 (1–2) | −3.07 | ±0.50 | −6.05 | ±0.32 | −4.45 | ±0.29 |
| K3 | IAB | - | 4 | 4 | 99.3 | 1 | −2.12 | ±0.42 | −5.00 | ±0.24 | −3.89 | ±0.26 |
| K4 | IA | 6 | 1 | 2 | 99.5 | 1 | −6.91 | ±0.49 | −9.66 | ±0.44 | −6.07 | ±0.28 |
| K5 | IAB | 3 | - | 5 | 99.3 | 3 (1–5) | −4.46 | ±0.47 | −7.87 | ±0.45 | −5.56 | ±0.28 |
| K6 | IA | - | 6 | 4 | 99.3 | 3 (1–3) | −2.45 | ±0.44 | −5.66 | ±0.37 | −4.39 | ±0.21 |
| K7 | IA | - | 5 | 3 | 99.4 | 1 | −5.45 | ±0.40 | −8.11 | ±0.22 | −5.28 | ±0.16 |
| K8 | IAB | - | 6 | 4 | 99.3 | 1 | −5.75 | ±0.40 | −8.78 | ±0.31 | −5.79 | ±0.19 |
| K9 | IAB | - | 2 | 3 | 99.4 | 2 (1–5) | −5.34 | ±0.86 | −8.30 | ±0.65 | −5.53 | ±0.27 |
| K8+9 (rim) | - | - | - | 4 | 99.1 | 4 (1–5) | −4.47 | ±0.36 | −7.61 | ±0.30 | −5.28 | ±0.23 |
| K8+9 (all) | IAB | - | 8 | 11 | 99.3 | 2 (1–5) | −5.37 | ±0.46 | −8.41 | ±0.36 | −5.62 | ±0.19 |
| K10 | IA | 1 | 4 | 5 | 99.0 | 4 (4–5) | −2.67 | ±0.37 | −5.77 | ±0.29 | −4.38 | ±0.19 |
| K11 | IB | - | 3 | 4 | 99.2 | 1 (1–3) | −3.16 | ±0.39 | −6.33 | ±0.26 | −4.68 | ±0.19 |
| K12 | IAB | 1 | 3 | 5 | 99.1 | 1 (1–2) | −4.73 | ±0.37 | −7.85 | ±0.26 | −5.39 | ±0.18 |
| K13 | IB | 3 | - | 5 | 99.0 | 2 (1–4) | −3.08 | ±0.49 | −6.09 | ±0.29 | −4.48 | ±0.23 |
| K14 | IAB | 1 | 3 | 4 | 99.1 | 1 | −4.31 | ±0.39 | −7.64 | ±0.29 | −5.40 | ±0.24 |
| K15 | IB | - | 4 | 4 | 99.4 | 1 | −5.85 | ±0.41 | −8.87 | ±0.25 | −5.83 | ±0.18 |
| K16 | IA | 6 | - | 4 | 99.2 | 5 | −4.62 | ±0.42 | −7.43 | ±0.29 | −5.03 | ±0.27 |
| K17 | IAB | - | 5 | 3 | 99.4 | 1 | −4.90 | ±0.36 | −7.94 | ±0.26 | −5.40 | ±0.21 |
| K18 | IA | 3 | 3 | 1 | 99.6 | 1 | −11.96 | ±0.41 | −14.48 | ±0.28 | −8.26 | ±0.14 |
| K19 | IAB | - | 5 | 2 | 99.3 | 1 | −4.37 | ±0.65 | −7.56 | ±0.42 | −5.29 | ±0.18 |
| K20 | IAB | 2 | 3 | 2 | 99.2 | 1 | −4.10 | ±0.75 | −7.41 | ±0.61 | −5.28 | ±0.26 |
| K21 | IAB | - | 5 | 3 | 99.2 | 1 | −3.41 | ±0.34 | −6.97 | ±0.30 | −5.20 | ±0.26 |
| K22 | IAB | - | 4 | 4 | 98.8 | 1 | −2.74 | ±0.53 | −5.79 | ±0.38 | −4.37 | ±0.26 |
| K23 | IAB | - | 4 | - | 99.5 | - | −7.02 | ±0.31 | −11.44 | ±0.40 | −7.79 | ±0.37 |
| K24 | IAB | 1 | 3 | 4 | 99.4 | 1 | −5.29 | ±0.69 | −8.48 | ±0.63 | −5.73 | ±0.36 |
| K25 | MB | - | - | 3 | 85.7 | 1 | 3.82 | ±0.35 | 2.04 | ±0.30 | 0.06 | ±0.24 |
| K26 | IIA | - | 4 | - | 62.9e | - | 2.74 | ±0.34 | −0.80 | ±0.38 | −2.22 | ±0.28 |
Ol-r, Ol-h, and Px represent the number of SIMS analyses from relict olivine, host olivine, and low-Ca pyroxene, respectively.
Mean Mg# (MgO/(MgO+FeO) mol%) of olivine and pyroxene excluding relict olivine.
Mean Wo component of low-Ca pyroxenes in mol%; ranges shown in parenthesis, if significantly variable.
Quoted uncertainties are at 95% confidence level. Analyses of relict olivine are not included in the mean values.
Olivine zoned in Mg# (core ~58, rim ~66).
4. DISCUSSION
4.1. Evaluation of host chondrule oxygen isotope ratios
The primary aim of this SIMS oxygen isotope study of olivine and low-Ca pyroxene phenocrysts is to determine the oxygen isotope ratios of the last chondrule-forming melt that would record the average oxygen isotope ratios of the local dust-enriched disk. For clarity, the term “last chondrule-forming melt” is used here to refer to the product of the last major melting event that is recorded in most chondrules of one chondrite. These melts interacted with the ambient gas before or during crystallization of chondrule minerals (e.g., Tissandier et al., 2002; Hewins and Zanda, 2012; Nagahara and Ozawa, 2012; Di Rocco and Pack, 2015; Marrocchi and Chaussidon, 2015) and there is evidence that chondrule phenocrysts and evolved melts were not in chemical equilibrium (e.g., Libourel et al., 2006). This disequilibrium, in turn, raises some doubts whether Δ17O values of olivine and low-Ca are actual representative for those of the last chondrule-forming melt. However, Ushikubo et al. (2011) showed for Acfer 094 (ungr. CC), the most pristine carbonaceous chondrite known to date, that chondrule phenocrysts (excluding relicts) and mesostasis phases such as high-Ca pyroxenes, plagioclase, and glass are indistinguishable in respect to Δ17O values. Also, high-Ca pyroxenes, plagioclase, and olivine show indistinguishable Δ17O values in chondrules from two CR chondrites (Tenner et al., 2015) and Yamato-82094 (ungr. CC, Tenner et al., 2017). Moreover, it is likely that systematically higher Δ17O values of chondrule glass in Semarkona (LL, Kita et al., 2010) or plagioclase in 375 Kaba (CV, Krot and Nagashima, 2016) are due to parent body processes and not caused by gasmelt exchange during plagioclase crystallization or glass formation.
Porphyritic chondrules can contain mineral grains that didn’t completely melt during the chondrule-forming event and are identified as relict grains either by chemical compositions and/or isotope ratios (e.g., Jones et al., 2004; Kunihiro et al., 2004; Krot et al., 2006; Berlin et al., 2011; Rudraswami et al., 2011; Ushikubo et al., 2012; Schrader et al., 2013; Tenner et al., 2013). Relict olivine grains predate “host” minerals that crystallized from the final chondrule melt (Nagahara, 1981; Jones, 1996; Wasson and Rubin, 2003). Classical examples of those relict olivine are 16O-rich forsteritic cores in type II chondrules of carbonaceous chondrites (e.g., Yurimoto and Wasson, 2002; Kunihiro et al., 2004, 2005; Rudraswami et al., 2011; Ushikubo et al., 2012; Schrader et al., 2013; Tenner et al., 2013). However, in most of the cases, isotopically distinct olivine relict grains are chemically and petrographically indistinguishable from other olivine of the same chondrule and only identified by SIMS analyses at sub-‰ precisions (e.g., Rudraswami et al., 2011; Ushikubo et al., 2012; Tenner et al., 2013, 2015, 2017). In order to deduce representative oxygen isotope ratios of the chondrule-forming environment, it has proven useful to define mean “host” oxygen isotope ratios. These host values have analyses of those isotopic relict grains excluded from chondrule means (e.g., Kunihiro et al., 2004; Ushikubo et al., 2012; Tenner et al., 2017).
In 11 of 22 chondrules, analyzed olivine grains are indistinguishable from pyroxene in the same chondrule, i.e., chondrules are relict grain-free and contain both minerals. The total number of chondrules cited here excludes chondrules K23 and K25 where no valid olivine or pyroxene analyses, respectively, are available as well as K26 that doesn’t contain pyroxene. Moreover, 19 out of 22 chondrules, shown in Fig. 5a, are either relict grain-free or contain at least one olivine with Δ17O values indistinguishable from pyroxene of the same chondrule; the remainder comprises three chondrules, K5, K13, and K16, where all olivine are considered to represent relict grains. Since olivine is the liquidus phase succeeded by low-Ca pyroxene during crystallization of type I chondrule melts, there is a possibility that a changing melt Δ17O value during crystallization would be reflected in differing Δ17O values of both minerals, e.g., higher Δ17O values of pyroxenes relative to those of olivine (e.g., Chaussidon et al., 2008); such systematic relationship was clearly not observed in this study. It is therefore suggested that olivine and pyroxene crystallized from a homogenized melt in respect to Δ17O and that the mean chondrule Δ17O values of chondrule olivine and pyroxene can be used as a proxy for those of the last chondrule-forming melt.
Figure 5:
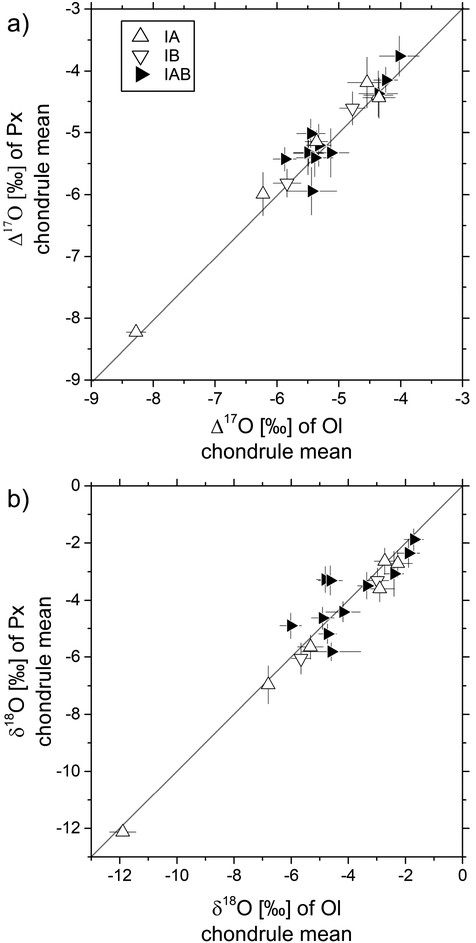
Mean olivine and pyroxene (a) Δ17O and (b) δ18O values of individual chondrules. (a) Chondrule means (Δ17O) of olivine and pyroxene (excluding relict olivine) are shown for chondrules that have indistinguishable olivine and pyroxene analyses. Error bars show the propagated uncertainties of the mean values (see Section 2.4. and Tab. 1). (b) Mean olivine δ18O values are slightly higher than those of pyroxene but variations are within propagated uncertainties.
There could be, however, a tendency of olivine to show slightly higher δ18O values than pyroxene of the same chondrule, but caution is advised because variations are within analytical uncertainties (see Fig. 5b). Similar observations were made earlier for different chondrites (Tenner et al., 2013, 2015) and attributed to evaporative loss of light isotopes before olivine crystallization followed by re-condensation of light isotopes before or during pyroxene crystallization (Tenner et al, 2015). Since such processes result only in mass-dependent fractionation, chondrule Δ17O values and conclusions based thereof are not affected by this type of gas-melt interaction.
Mean chondrule Δ17O values determined for Kaba are independent of the modal abundance of olivine and pyroxene as shown in Fig. 6 which is in line with previous SIMS oxygen isotope studies on unequilibrated carbonaceous chondrites (Rudraswami et al., 2011; Ushikubo et al., 2012; Tenner et al., 2013, 2015). Apart from the Mg#’s, this study found no connection between oxygen isotope ratios and chemical compositions of olivine or pyroxene. For example, there exists no systematic difference of pyroxene δ17O, δ18O, or Δ17O depending on the Wo content (i.e., low-Ca pyroxene vs. Intermediate pyroxene). In agreement with previous SIMS oxygen isotope studies on chondrule in carbonaceous chondrites (e.g., Rudraswami et al., 2011; Ushikubo et al., 2012; Tenner et al., 2015), most host chondrule Δ17O values plot on the PCM line or between the PCM and CCAM lines (see Fig. 6); notable exceptions are chondrules K25 (type IIB) and K23 (IAB). In the latter case (K23), isotopic compositions of olivine are potentially fractionated.
Figure 6:
Host chondrule isotope ratios (δ18O, δ17O, and Δ17O) and textures of chondrules. Error bars represent propagated uncertainty of the mean values. (a) Oxygen 3-isotope diagram showing chondrule means. Reference lines are the same as those in Fig. 2. Host values of chondrules plot on the PCM line or slightly below. Exceptions are chondrules K23 (IAB) and K25 (IIB). (b) Host Δ17O values are independent of chondrule texture among type I chondrules. Type II chondrules are 16O-poor compared to type I chondrules.
4.2. Consistent Mg#’s of chondrule olivine and pyroxene
Pristine oxygen isotope ratios and Mg#’s are a precondition for reliable conclusions about the chondrule-forming environment and consistent Mg#’s of olivine and pyroxenes are an important indicator whether thermal metamorphism could have disturbed primary values. According to the equilibrium condensation model of Ebel and Grossmann (2000), olivine and orthopyroxene initially show similar Mg#’s of about 98 at the time the last melt disappears (Ebel and Grossmann, 2000, therein Fig. 8, dust enrichment factor: 100). Due to diffusive exchange with Fe-rich minerals, olivine and pyroxene could become more FeO-rich during parent body metamorphism. Importantly, the interdiffusion coefficient of Fe and Mg in olivine is about 2 log units larger than in orthopyroxene (e.g., Dohmen and Chakraborty, 2007; Dohmen et al., 2016, and references therein), i.e., diffusion rates are higher in olivine than in orthopyroxene. Therefore, low degrees of thermal metamorphism manifests itself first in elevated olivine Mg#’s whereas pyroxene Mg#’s remain unchanged. Further, because diffusion of Fe and Mg is fast relative to that of oxygen (Dohmen and Chakraborty, 2007), matching Mg# indicate that both minerals probably record primary oxygen isotope ratios. However, it should be noted that fluids could have enhanced oxygen diffusion in those silicates (Farver, 2010, and references therein). In this study we found consistent Mg#’s of most olivine pyroxenes within individual chondrules from Kaba, providing evidence for unaltered Mg-Fe ratios and Δ17O values. Those values can now be used to infer possible dust enrichment and ice enhancement factors of the CV chondrule-forming region.
Figure 8:
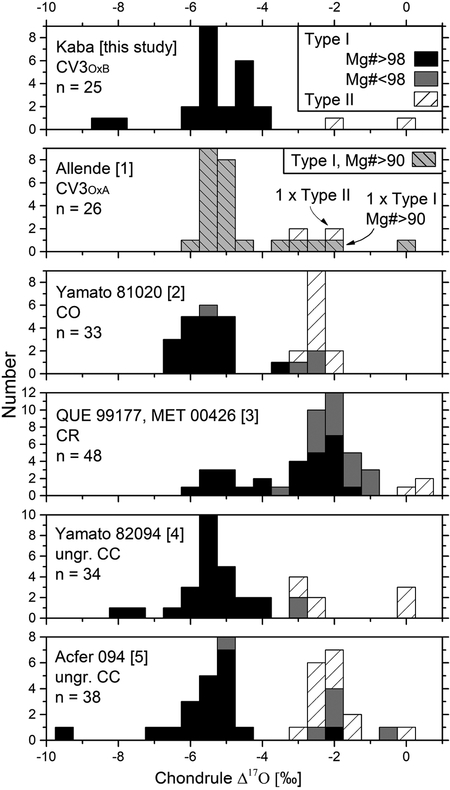
Histograms of host Δ17O values from chondrules in Kaba and further carbonaceous chondrites. Like Kaba, all listed carbonaceous chondrites contain abundant type I chondrules with Δ17O values in the range of ~ −6‰ and −4‰, though no type I chondrules in Kaba show Δ17O values > −3‰ that are common in the other carbonaceous chondrites except Yamato 82094. Because Allende experienced significant thermal metamorphism, Mg#’s of chondrules are disturbed and no distinction is being made between type I chondrules with Mg#<98 or >98. Literature data are from [1] Rudraswami et al. (2011), [2] Tenner et al. (2013), [3] Tenner et al. (2015), [4] Tenner et al. (2017), [5] Ushikubo et al. (2012).
4.3. Relating oxygen isotope compositions of chondrules to levels of dust enrichment in the chondrule forming region
Tenner et al. (2015) proposed a simple yet powerful model to relate the Δ O values and Mg#’s of host chondrules to the extent of dust enrichment and the contribution of distinct precursor oxygen reservoirs. The oxygen isotope characteristics of host chondrules are described by an oxygen isotope mass balance that involves Solar gas (Δ17O = −28.4‰), anhydrous silicate dust (−5.9‰) as well as organics (+11.8‰) and H2O ice (+5.1‰) in the dust as precursor oxygen reservoirs. At a given amount of water ice, chondrule Δ17O values strongly increase with rising dust enrichment but continue to increase only marginally at dust enrichment factors higher than 100 because at these conditions, mass balance is dominated by nearly constant amounts of oxygen from the anhydrous silicate precursor dust and the water ice.
For instance, if a chondrule formed in a region with 100 times (CI) dust enrichment (H/O ~37) relative to Solar nebula values, 43% and 53% of the oxygen in the chondrule would come from precursor anhydrous silicates and water ice in the dust, respectively; only a minor fraction of 4% would be inherited from the Solar gas and organics in the dust (Tenner et al., 2015, see their Table 3). Hence, when applying the model originally calibrated for CR chondrules to chondrules in CV chondrites, new Δ17O values for the precursor silicates and water ice need to be defined whereas a difference in Δ17O values for the organics in both regions can be ignored. Following Tenner et al. 2015, oxygen isotope ratios for both reservoirs are defined based on the assumption that the lowest measured chondrule Δ17O values (mean of K18 and K23: −8.0‰) are representative for the effective Δ17O value of the anhydrous precursor silicates and that chondrules with higher Δ17O values are formed by addition of 16O-poor water ice. Since type II chondrules likely formed at highly dust-enriched conditions, the Δ17O value of the water ice is calculated using the measured Δ17O value of chondrule K26 (−2.2‰, type II) in the following way: at a dust enrichment factor of 1000, the mass balance involves oxygen from the sources water ice, anhydrous silicate dust, and organics in the following proportions 54:44:2 (Tenner et al., 2015); solving the mass balance for the Δ17O value of water ice, results in a value of +2‰.
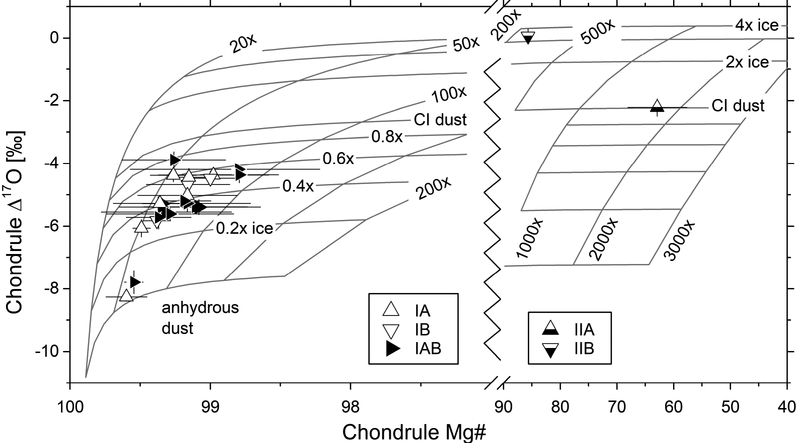
Host chondrule Δ17O and Mg#’s from Kaba are shown in Fig. 7 in combination with modeled dust and ice enrichment factors. Error bars for Mg#’s denote maximum-minimum ranges including olivine and pyroxene analyses in individual chondrules of Kaba. Data suggests that higher chondrule Mg#’s correlate with lower Δ17O values which is in line with results for chondrules from CR chondrites (Schrader et al., 2013, 2014, 2017; Tenner et al., 2015). According to the model of Tenner et al. (2015), values of type I host chondrules in Kaba correspond to low dust enrichment factors between 50× and 100× and low amounts of water ice (anhydrous CI dust to 0.6× the nominal amount of ice in dust of CI composition) in the dust. The type IIB chondrule could have formed at 300× dust enrichment and ~3× water ice. However, formation of this chondrule might be unrelated to that of other chondrules because δ18O values are fractionated in such a way that analyses plot off the PCM and on the TF line. This distinguishes them from analyses of all other chondrules and suggests that at least one different precursor reservoir contributed to their formation (e.g., Clayton et al., 1983; Tenner et al., 2015). The type II chondrule K26 possibly formed at dust enrichments of 2000×. Results from this study support the idea that Mg#’s and Δ17O values may be correlated even for very Mg-rich chondrules with Mg#’s above 99. For these reducing conditions (logfO2: IW – 3.5), increasing Δ17O values in combination with decreasing Mg#’s may be due to a small increase in the dust enrichment factor or water ice contents or a combination of both parameters (see Fig. 7), as noted by, e.g., Tenner et al. (2015).
Figure 7:
Plot showing host Δ17O values and Mg#’s of chondrules superimposed by oxygen isotope mixing curves of constant dust enrichment and ice enhancement from the model of Tenner et al. (2015). The model adopts the following Δ17O values for the various oxygen reservoirs: anhydrous silicate dust, −8.0‰; Solar gas, −28.4‰; water ice, +2.0‰; organics in the dust, +11.3‰. Inferred anhydrous dust enrichment and ice enhancement factors for type I chondrules are 50–100× relative to Solar abundance and from anhydrous to ~0.6× the water ice content of dust of CI composition, respectively. Mg#’s and Δ17O values of the type II chondrules suggest higher : (IIB: ~300×, IIA: ~2000×) dust enrichment factors and water ice contents (1–4× the amount of water ice of dust of CI composition). The error bars for Mg#’s represent the range (min-max); for host (mean) Δ17O values, error bars are propagated uncertainties (see Tab. 1).
Ice enhancement and, to a lower degree, dust enrichment factors inferred from chondrule Δ17O values vary with the assumed isotope ratios of the contributing reservoirs. For example, overestimating the Δ17O value of anhydrous silicate dust in the precursor by 5‰ (Δ17O = −12‰ instead of −8‰) has only a limited effect on inferred maximum dust enrichments (slight decrease) but increases the maximum estimated ice enhancement factor to 1× the nominal amount of ice in CI dust (type I chondrules), when keeping other parameters constant. As discussed by Tenner et al. (2015), alternative Δ17O values of water ice (e.g., +80‰) can significantly decrease the inferred ice enhancement factor while only moderately affecting dust enrichment factors, e.g., shifting maximum enrichment factors obtained for Kaba type I chondrules from 100× to 200× CI dust relative to Solar abundance. In conclusions, dust enrichment factors inferred from chondrules in Kaba are likely reasonable estimates for the chondrule forming environment, while ice enhancement factors are less accurately known and sensitive to the applied Δ17O values of reservoirs.
In the scope of the model by Marrocchi and Chaussidon (2015), relict olivine grains represent the direct silicate precursor material for the individual chondrule they are included in. Differences between isotope ratios of individual chondrules are, consequently, due to isotopically distinct precursor olivine rather than due to influence of 16O-poor water ice. Further, Marrocchi and Chaussidon (2015) suggest that, if present, isotopic disequilibrium between chondrule olivine and pyroxene (Δ18Opx-ol) represents a possible proxy for dust enrichment in the chondrule-forming region. Accordingly, isotopically homogeneous chondrules would indicate high (>100 times) dust enrichments. A Δ18Opx-ol of approximately +1.5 like observed in chondrules K5 and K13 would amount to dust enrichments smaller than 5×, considering precursor olivine of −5% (Marrocchi and Chaussidon, 2015, see their Table S1). Within the framework of that model, 16O-poor relict olivine as a source for 16O-rich pyroxene as found in chondrule K16 is only conceivable with 16O-rich gas and extremely low dust enrichment factors (< 0.001). The model predictions such as the low dust enrichment factors for the three chondrules are based on the explicit assumption that most olivine are relict grains. Although it can’t be ruled out that some olivine grains with Δ17O values similar to pyroxenes are actually relict grains, there is recent chemical and isotopic evidence from chondrules in CR chondrites (Schrader et al., 2014) that argue against such a scenario where most olivine in type I chondrules are relict (e.g., Libourel and Krot, 2007; Libourel and Chaussidon, 2011).
4.4. Comparison to data from the Allende CV3 and other carbonaceous chondrites
It can be seen from the previous discussion that host chondrule Δ17O values in combination with mean Mg#’s provide important information about the contribution of different oxygen reservoirs to chondrule formation. With histograms presented in Fig. 8, host chondrule Δ17O values of Kaba are compared to those of various groups of carbonaceous chondrites in order to identify common chondrule populations. Literature data in Fig. 8 are selected based on the following criterions: (1) The precision of individual chondrule means are small compared to bin size (<0.5‰), (2) the chondrule means were obtained from multiple analyses (n>4) of single chondrule at sub‰ precision in order to exclude obvious relict olivine analysis, and (3) the total number of chondrules in the dataset are statistically meaningful (n≥20) and generally cover a representative set of chondrule types. It is important to note that frequencies of host Δ17O values can be prone to misinterpretations because available chondrule data may not be completely representative for the meteorite. In many studies, chondrules are primarily selected based on the size of mineral grains due to spatial resolution of the SIMS method. For example, grain size limitations might have biased Allende data towards type IA chondrules (Rudraswami et al., 2011). Also, minor chondrule types, such as type II chondrules in carbonaceous chondrites and Al-rich chondrules are often over-selected compared to their actual representative abundances (e.g., Connolly and Huss, 2010; Ushikubo et al., 2012; Schrader et al., 2013; Tenner et al., 2017).
Histograms of host chondrule Δ17O values of Kaba, Allende, Yamato 81020, two CRs, Yamato 82094, and Acfer 094 (for references see Fig. 8) show at least one common mode of Δ17O values in between −6‰ and −4‰. Yamato 81020, the CR chondrites, and Acfer 094 also contain a significant fraction of chondrules with Δ17O values of ~ −2‰, which are observed in Kaba, Allende, and Yamato 82094 as minor components. Except for Yamato 81020, all carbonaceous chondrites mentioned in Fig. 8 contain chondrules with Δ17O values of about 0‰. Only the Kaba, Acfer 094, and Yamato 82094 chondrites contain chondrules with Δ17O values lower than −7‰. In general, type I chondrules are 16O-rich in comparison to type II chondrules and show a negative correlation between chondrule Mg# and Δ17O values, as indicated by different grey tones in Fig. 8. More precisely, excluding the two CR chondrites, most type I chondrules possess host Δ17O values in the range between −6‰ and −4‰ with a minor mode at about −2‰ that mainly comprises chondrules with Mg#’s below 98. In contrast to the strong bimodal host Δ17O values from the Acfer 094 and Yamato 81020 chondrites, data on type I chondrules from the CR chondrites suggest a continuum of Δ17O values ranging from −6‰ to −1‰. At large, it appears that type II chondrules only possess Δ17O values higher than −4‰ whereas type I chondrules can show host Δ17O values from −10‰ up to 0‰.
Analyzed chondrules from the Kaba CV3 chondrite differ from those in the aforementioned chondrites, especially Allende, in two major aspects: First, host chondrule Mg#’s are consistently above 98 and, second, there exist no type I chondrules in Kaba that show Δ17O values higher than ~ −3‰. In Allende chondrules, the difference in Mg#’s is due to secondary processes, so that high Mg#’s among many Kaba chondrules represent the primary characters of chondrules in CV. However, the lack of type I chondrules with Δ17O > −3‰ among chondrules in Kaba studied here could be due to sampling bias because only chondrules from a single thin section of Kaba were analyzed. Precursors of type I chondrules with 16O-poor signature (Δ17O > −3‰) are indicated from the analysis of chondrule K16 (IA, Fig. 1e), in which relatively 16O-rich pyroxene analyses define the host value (Δ17O = −5.03 ±0.27‰, unc.) by excluding 16O-poor (Δ17O = −1.57 ±0.22‰, unc.) relict olivine analysis. Such 16O-poor relict olivine was also found in Allende chondrules (Rudraswami et al., 2011).
5. CONCLUSIONS
Olivine and pyroxene in chondrules from the Kaba USNM 1052–1 thin section were analyzed for oxygen three-isotopes (SIMS) and mineral chemistry (EPMA) in order to evaluate the degree of isotopic homogeneity in chondrules and the relationship of chondrule Δ17O values and Mg#’s. More than one third of the analyzed chondrules in the Kaba CV3 contain isotopic olivine relict grains that are generally 16O-rich relative to chondrule means; however, three chondrule also comprise 16O-poor relict grains. Excluding those isotopic relicts, mean chondrule Δ17O values were calculated and it was demonstrated for most of the chondrules that olivine and pyroxene are isotopically indistinguishable and, consequently, that both minerals likely crystallized from the last chondrule-forming melt.
Except for two type II chondrules, the analyzed thin section only contains Mg-rich chondrules with high and uniform Mg#’s (host: 99.6 − 98.8). Consistent Mg#’s of olivine and pyroxene in individual chondrules support the interpretation that Kaba was only marginally affected by thermal metamorphism. The majority of type I chondrules possesses host Δ17O values in the range between −6‰ and −4‰ and only two type I chondrules show more 16O-rich compositions (~ −8‰). The type IIB and IIA chondrules are relatively 16O-poor and yield Δ17O values of 0‰ and −2‰, respectively. The Δ17O values of type I chondrules in Kaba tend to increase continuously with decreasing Mg#’s.
In conclusion, type I chondrules in the Kaba CV3 chondrite are witnesses of an oxygen reservoir with an effective Δ17O value of approximately −5‰ which is also commonly found in other groups of carbonaceous chondrites (e.g., Jones et al., 2004; Krot et al., 2006; Libourel and Chaussidon, 2011; Rudraswami et al., 2011; Ushikubo et al., 2013; Tenner et al., 2013, 2015; Schrader et al., 2014). Interestingly, host Δ17O values of type I chondrules in Kaba don’t show a pronounced bimodal distribution as it is evident for other carbonaceous chondrites such as Acfer 094 and Yamato 81020. At this point it is not clear whether the predominance of Δ17O values in between −6‰ and −4‰ is an exclusive property of the investigated thin section or representative for Kaba in general.
If the analyzed chondrules in Kaba are representative of CV chondrites, the correlation of Δ17O and Mg#’s points to similar principals that governed formation of type I chondrules in CV and CR chondrites. This includes a possible involvement of water ice as an oxidizing 16O-poor agent. Inferred dust enrichment factors for the local disk environment are only moderately high (50–100×) and dust was almost anhydrous (anhydrous to 0.6× the nominal ice in dust of CI composition), based on investigations of type I chondrules in the Kaba CV3.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Glenn MacPherson (National Museum of Natural History, Smithsonian Institution) for the allocation of Kaba thin section for this study. We are grateful to John Fournelle for help with EPMA measurements, Jim Kern for SIMS support, and Nöel Chaumard for discussion. Reviews of Devin Schrader and two anonymous reviewers significantly improved the manuscript. This work is supported by NASA Cosmochemistry Program (NNX14AG29G). WiscSIMS is partly supported by NSF (EAR13–55590).
REFERENCES
- Alexander CMO’D (2004) Chemical equilibrium and kinetic constraints for chondrule and CAI formation conditions. Geochim. Cosmochim. Acta 68, 3943–3969. [Google Scholar]
- Berlin J, Jones RH and Brearley AJ (2011) Fe-Mn systematics of type IIA chondrules in unequilibrated CO, CR, and ordinary chondrites. Meteorit. Planet. Sci 46, 513–533. [Google Scholar]
- Bonal L, Quirico E, Bourot-Denise M and Montagnac G (2006) Determination of the petrologic type of CV3 chondrites by Raman spectroscopy of included organic matter. Geochim. Cosmochim. Acta 70, 1849–1863. [Google Scholar]
- Busemann H, Alexander CMO’D and Nittler LR (2007) Characterization of insoluble organic matter in primitive meteorites by microRaman spectroscopy. Meteorit. Planet. Sci 42, 1387–1416. [Google Scholar]
- Chaussidon M, Libourel G and Krot AN (2008) Oxygen isotopic constraints on the origin of magnesian chondrules and on the gaseous reservoirs in the early Solar System. Geochim. Cosmochim. Acta 72, 1924–1938. [Google Scholar]
- Choi B-G, Krot AN and Wasson JT (2000) Oxygen-isotopes in magnetite and fayalite in CV chondrites Kaba and Mokoia. Meteorit. Planet. Sci 35, 1239–1248. [Google Scholar]
- Ciesla FJ (2005) Chondrule-forming Processes – An Overview In Chondrites and the protoplanetary disk (eds. Krot AN, Scott ERD and Reipurth B). Astronomical Society of the Pacific, San Francisco, CA: pp. 811–820. [Google Scholar]
- Clayton RN (1993) Oxygen Isotopes in Meteorites. Annu. Rev. Earth Planet. Sci 21, 115–149. [Google Scholar]
- Clayton RN and Mayeda TK (1999) Oxygen isotope studies of carbonaceous chondrites. Geochim. Cosmochim. Acta 63, 2089–2104. [Google Scholar]
- Clayton RN, Grossman L and Mayeda TK (1973) A Component of Primitive Nuclear Composition in Carbonaceous Meteorites. Science 182, 485–488. [DOI] [PubMed] [Google Scholar]
- Clayton RN, Onuma N, Grossman L and Mayeda TK (1977) Distribution of the pre-Solar component in Allende and other carbonaceous chondrites. Earth Planet. Sc. Lett 34, 209–224. [Google Scholar]
- Clayton RN, Onuma N, Ikeda Y, Mayeda TK, Hutcheon ID, Olsen EJ and Molini-Velsko C (1983) Oxygen isotopic compositions of chondrules in Allende and ordinary chondrites In Chondrules and their origins (ed. King EA). Lunar and Planetary Institute, Huston, TX: pp. 37–43. [Google Scholar]
- Connolly HC Jr. and Desch SJ (2004) On the origin of the “kleine Kügelchen” called Chondrules. Chem. Erde - Geochem 64, 95–125. [Google Scholar]
- Connolly HC Jr. and Huss GR (2010) Compositional evolution of the protoplanetary disk. Geochim. Cosmochim. Acta 74, 2473–2483. [Google Scholar]
- Di Rocco T and Pack A (2015) Triple oxygen isotope exchange between chondrule melt and water vapor. Geochim. Cosmochim. Acta 164, 17–34. [Google Scholar]
- Dohmen R and Chakraborty S (2007) Fe–Mg diffusion in olivine II. Phys. Chem. Minerals 34, 409–430. [Google Scholar]
- Dohmen R, Ter Heege JH, Becker H-W and Chakraborty S (2016) Fe-Mg interdiffusion in orthopyroxene. Am. Mineral 101, 2210–2221. [Google Scholar]
- Donovan JJ (2015) Probe for EPMA v. 11.1.5 Probe Software, Inc, Eugene, OR. [Google Scholar]
- Ebel DS and Grossman L (2000) Condensation in dust-enriched systems. Geochim. Cosmochim. Acta 64, 339–366. [Google Scholar]
- Farver JR (2010) Oxygen and Hydrogen Diffusion in Minerals. Rev. Mineral. Geochem 72, 447–507. [Google Scholar]
- Fedkin AV and Grossman L (2006) The Fayalite Content of Chondritic Olivine: Obstacle to Understanding the Condensation of Rocky Material In Meteorites and the Early Solar System II (eds. Lauretta DS and McSween H). University of Arizona Press, Tucson: pp. 279–294. [Google Scholar]
- Fedkin AV and Grossman L (2016) Effects of dust enrichment on oxygen fugacity of cosmic gases. Meteorit. Planet. Sci 51, 843–850. [Google Scholar]
- Grossman JN and Brearley AJ (2005) The onset of metamorphism in ordinary and carbonaceous chondrites. Meteorit. Planet. Sci 40, 87–122. [Google Scholar]
- Hewins RH (1996) Chondrules and the protoplanetary disk: an overview In Chondrules and the protoplanetary disk (eds. Hewins RH, Jones RH and Scott ERD). Cambridge Univ. Press, Cambridge; pp. 3–9. [Google Scholar]
- Hewins RH and Zanda B (2012) Chondrules. Meteorit. Planet. Sci 47, 1120–1138. [Google Scholar]
- Hua X, Huss GR, Tachibana S and Sharp TG (2005) Oxygen, silicon, and Mn-Cr isotopes of fayalite in the Kaba oxidized CV3 chondrite. Geochim. Cosmochim. Acta 69, 1333–1348. [Google Scholar]
- Jones RH (1996) FeO-rich, porphyritic pyroxene chondrules in unequilibrated ordinary chondrites. Geochim. Cosmochim. Acta 60, 3115–3138. [Google Scholar]
- Jones RH, Leshin LA, Guan Y, Sharp ZD, Durakiewicz T and Schilk AJ (2004) Oxygen isotope heterogeneity in chondrules from the Mokoia CV3 carbonaceous chondrite. Geochim. Cosmochim. Acta 68, 3423–3438. [Google Scholar]
- Kimura M and Ikeda Y (1998) Hydrous and anhydrous alterations of chondrules in Kaba and Mokoia CV chondrites. Meteorit. Planet. Sci 33, 1139–1146. [Google Scholar]
- Kita NT, Ushikubo T, Fu B and Valley JW (2009) High precision SIMS oxygen isotope analysis and the effect of sample topography. Chem. Geol 264, 43–57. [Google Scholar]
- Kita NT, Nagahara H, Tachibana S, Tomomura S, Spicuzza MJ, Fournelle JH and Valley JW (2010) High precision SIMS oxygen three isotope study of chondrules in LL3 chondrites. Geochim. Cosmochim. Acta 74, 6610–6635. [Google Scholar]
- Krot AN and Nagashima K (2016) Evidence for Oxygen-Isotope Exchange in Chondrules and Refractory Inclusions During Fluid-Rock Interaction on the CV Chondrite Parent Body. Meteorit. Planet. Sci. Suppl 51, A249. [Google Scholar]
- Krot AN, Scott ERD and Zolensky ME (1995) Mineralogical and chemical modification of components in CV3 chondrites. Meteorit. Planet. Sci 30, 748–775. [Google Scholar]
- Krot AN, Petaev MI, Scott ERD, Choi B-G, Zolensky ME and Keil K (1998) Progressive alteration in CV3 chondrites. Meteorit. Planet. Sci 33, 1065–1085. [Google Scholar]
- Krot AN, Fegley B, Lodders K and Palme H (2000) Meteoritical and astrophysical constraints on the oxidation state of the Solar nebula In Protostars and Planets IV (eds. Mannings V, Boss AP and Russell SS). University of Arizona Press, Tucson: pp. 1019–1054. [Google Scholar]
- Krot AN, Yurimoto H, McKeegan KD, Leshin LA, Chaussidon M, Libourel G, Yoshitake M, Huss GR, Guan Y and Zanda B (2006) Oxygen isotopic compositions of chondrules. Chem. Erde - Geochem 66, 249–276. [Google Scholar]
- Krot AN, Nagashima K, Ciesla FJ, Meyer BS, Hutcheon ID, Davis AM, Huss GR and Scott ERD (2010) Oxygen Isotopic Composition of the Sun and Mean Oxygen Isotopic Composition of the Protosolar Silicate Dust. Astrophys. J 713, 1159–1166. [Google Scholar]
- Kunihiro T, Rubin AE, McKeegan KD and Wasson JT (2004) Oxygen-isotopic compositions of relict and host grains in chondrules in the Yamato 81020 CO3.0 chondrite. Geochim. Cosmochim. Acta 68, 3599–3606. [Google Scholar]
- Kunihiro T, Rubin AE and Wasson JT (2005) Oxygen-isotopic compositions of low-FeO relicts in high-FeO host chondrules in Acfer 094, a type 3.0 carbonaceous chondrite closely related to CM. Geochim. Cosmochim. Acta 69, 3831–3840. [Google Scholar]
- Libourel G and Krot AN (2007) Evidence for the presence of planetesimal material among the precursors of magnesian chondrules of nebular origin. Earth Planet. Sc. Lett 254, 1–8. [Google Scholar]
- Libourel G and Chaussidon M (2011) Oxygen isotopic constraints on the origin of Mg-rich olivines from chondritic meteorites. Earth Planet. Sc. Lett 301, 9–21. [Google Scholar]
- Libourel G, Krot AN and Tissandier L (2006) Role of gas-melt interaction during chondrule formation. Earth Planet. Sc. Lett 251, 232–240. [Google Scholar]
- Marrocchi Y and Chaussidon M (2015) A systematic for oxygen isotopic variation in meteoritic chondrules. Earth Planet. Sc. Lett 430, 308–315. [Google Scholar]
- Morris MA, Boley AC, Desch SJ and Athanassiadou T (2012) Chondrule formation in bow shocks around eccentric planetary embryos. Astrophys. J 752, 27. [Google Scholar]
- Nagahara H (1981) Evidence for secondary origin of chondrules. Nature 292, 135–136. [Google Scholar]
- Nagahara H and Ozawa K (2012) The role of exchange reactions in oxygen isotope fractionation during CAI and chondrule formation. Meteorit. Planet. Sci 47, 1209–1228. [Google Scholar]
- Nagahara H, Kita NT, Ozawa K and Morishita Y (2008) Condensation of major elements during chondrule formation and its implication to the origin of chondrules. Geochim. Cosmochim. Acta 72, 1442–1465. [Google Scholar]
- Nagashima K, Krot AN and Huss GR (2015) Oxygen-isotope compositions of chondrule phenocrysts and matrix grains in Kakangari K-grouplet chondrite. Geochim. Cosmochim. Acta 151, 49–67. [Google Scholar]
- Nakashima D, Kimura M, Yamada K, Noguchi T, Ushikubo T and Kita NT (2010) Study of chondrules in CH chondrites - I: oxygen isotope ratios of chondrules. Meteorit. Planet. Sci. Suppl 45, A148. [Google Scholar]
- Nakashima D, Kita NT, Ushikubo T, Noguchi T, Nakamura T and Valley JW (2013) Oxygen three-isotope ratios of silicate particles returned from asteroid Itokawa by the Hayabusa spacecraft. Earth Planet. Sc. Lett 379, 127–136. [Google Scholar]
- Rubin AE (2000) Petrologic, geochemical and experimental constraints on models of chondrule formation. Earth Sci. Rev 50, 3–27. [Google Scholar]
- Rudraswami NG, Ushikubo T, Nakashima D and Kita NT (2011) Oxygen isotope systematics of chondrules in the Allende CV3 chondrite. Geochim. Cosmochim. Acta 75, 7596–7611. [Google Scholar]
- Schrader DL, Connolly HC Jr., Lauretta DS, Nagashima K, Huss GR, Davidson J and Domanik KJ (2013) The formation and alteration of the Renazzo-like carbonaceous chondrites II. Geochim. Cosmochim. Acta 101, 302–327. [Google Scholar]
- Schrader DL, Nagashima K, Krot AN, Ogliore RC and Hellebrand E (2014) Variations in the O-isotope composition of gas during the formation of chondrules from the CR chondrites. Geochim. Cosmochim. Acta 132, 50–74. [Google Scholar]
- Schrader DL, Nagashima K, Krot AN, Ogliore RC, Yin Q-Z, Amelin Y, Stirling CH and Kaltenbach A (2017) Distribution of 26Al in the CR chondrite chondrule-forming region of the protoplanetary disk. Geochim. Cosmochim. Acta 201, 275–302. [Google Scholar]
- Tenner TJ, Ushikubo T, Kurahashi E, Kita NT and Nagahara H (2013) Oxygen isotope systematics of chondrule phenocrysts from the CO3.0 chondrite Yamato 81020. Geochim. Cosmochim. Acta 102, 226–245. [Google Scholar]
- Tenner TJ, Nakashima D, Ushikubo T, Kita NT and Weisberg MK (2015) Oxygen isotope ratios of FeO-poor chondrules in CR3 chondrites. Geochim. Cosmochim. Acta 148, 228–250. [Google Scholar]
- Tenner TJ, Kimura M and Kita NT (2017) Oxygen isotope characteristics of chondrules from the Yamato-82094 ungrouped carbonaceous chondrite: Further evidence for common O-isotope environments sampled among carbonaceous chondrites. Meteorit. Planet. Sci 52, 268–294. [Google Scholar]
- Tissandier L, Libourel G and Robert F (2002) Gas-melt interactions and their bearing on chondrule formation. Meteorit. Planet. Sci 37, 1377–1389. [Google Scholar]
- Ushikubo T, Nakashima D, Kimura M, Tenner TJ and Kita NT (2013) Contemporaneous formation of chondrules in distinct oxygen isotope reservoirs. Geochim. Cosmochim. Acta 109, 280–295. [Google Scholar]
- Wasson JT and Rubin AE (2003) Ubiquitous low-FeO relict grains in type II chondrules and limited overgrowths on phenocrysts following the final melting event. Geochim. Cosmochim. Acta 67, 2239–2250. [Google Scholar]
- Young ED and Russell SS (1998) Oxygen Reservoirs in the Early Solar Nebula Inferred from an Allende CAI. Science 282, 452–455. [PubMed] [Google Scholar]
- Yurimoto H and Wasson JT (2002) Extremely rapid cooling of a carbonaceous-chondrite chondrule containing very 16O-rich olivine and a 26Mg-excess. Geochim. Cosmochim. Acta 66, 4355–4363. [Google Scholar]
- Yurimoto H, Krot AN, Choi B-G, Aleon J, Kunihiro T and Brearley AJ (2008) Oxygen Isotopes of Chondritic Components. Rev. Mineral. Geochem 68, 141–186. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.