Abstract
Background
Long‐term physical conditions affect 10% to 12% of children and adolescents worldwide. These individuals are at greater risk of developing psychological problems, particularly anxiety and depression, sometimes directly related to their illness or medical care (e.g. health‐related anxiety). There is limited evidence regarding the effectiveness of psychological therapies for treating anxiety and depression in this population. Therapies designed for children and adolescents without medical issues may or may not be appropriate for use with those who have long‐term physical conditions.
Objectives
This review was undertaken to assess the effectiveness and acceptability of psychological therapies in comparison with controls (treatment‐as‐usual, waiting list, attention placebo, psychological placebo, or non‐psychological treatment) for treating anxiety and depression in children and adolescents with long‐term physical conditions.
Search methods
We searched Ovid MEDLINE (1950‐ ), Embase (1974‐ ), PsycINFO (1967‐ ) and the Cochrane Central Register of Controlled Trials (CENTRAL) to 27 September 2018. An earlier search of these databases was conducted via the Cochrane Common Mental Disorders Controlled Trial Register (CCMD‐CTR) (all years to May 2016). In addition, we searched the Web of Science (Core Collection) (12 October 2018) and conducted a cited reference search for reports of all included trials. We handsearched relevant conference proceedings, reference lists of included articles, and grey literature.
Selection criteria
Randomised controlled trials (RCTs), cluster‐randomised trials and cross‐over trials of psychological therapies for treating anxiety or depression in children with long‐term physical conditions were included.
Data collection and analysis
Abstracts and complete articles were independently reviewed by two authors. Discrepancies were addressed by a third author. Odds ratio (OR) was used for comparing dichotomous data and standardised mean differences (SMD) for comparing continuous data. Meta‐analysis was undertaken when treatments, participants, and the underlying clinical question were similar. Otherwise, narrative analysis of data was undertaken.
Main results
Twenty‐eight RCTs and one cross‐over trial with 1349 participants were included in the review. Most participants were recruited from community settings and hospital clinics in high‐income countries. For the primary outcome of treatment efficacy, short‐term depression (versus any control), there was low‐quality evidence from 16 trials involving 1121 participants suggesting that psychological therapies may be more effective than control therapies (SMD ‐0.31, 95% CI ‐0.59 to ‐0.03; I2 = 79%). For the primary outcome of treatment efficacy, short‐term anxiety (versus any control), there was inadequate evidence of moderate‐quality from 13 studies involving 578 participants to determine whether psychological therapies were more effective than control conditions (SMD ‐0.26, CI ‐0.59 to 0.07, I2 = 72%). Planned sensitivity analyses could not be undertaken for risk of bias due to the small number of trials that rated high for each domain. Additional sensitivity analysis demonstrated that psychological interventions specifically designed to reduce anxiety or depression were more effective than psychological therapies designed to improve other symptoms or general coping. There was some suggestion from subgroup analyses that they type of intervention (Chi² = 14.75, df = 5 (P = 0.01), I² = 66.1%), the severity of depression (Chi² = 23.29, df = 4 (P = 0.0001), I² = 82.8%) and the type of long‐term physical condition (Chi² = 10.55, df = 4 (P = 0.03), I² = 62.1%) may have an impact on the overall treatment effect.There was qualitative (reported), but not quantitative evidence confirming the acceptability of selected psychological therapies for anxiety and depression. There was low‐quality evidence that psychological therapies were more effective than control conditions in improving quality of life (SMD 1.13, CI 0.44 to 1.82, I2 = 89%) and symptoms of long‐term physical conditions (SMD ‐0.34, CI ‐0.6 to ‐0.06, I2 = 70%), but only in the short term. There was inadequate low‐quality evidence to determine whether psychological therapies were more effective than control conditions at improving functioning in either the short term or long term. No trials of therapies for addressing health‐related anxiety were identified and only two trials reported adverse effects; these were unrelated to psychological therapies. Overall, the evidence was of low to moderate quality, results were heterogeneous, and only one trial had an available protocol.
Authors' conclusions
A limited number of trials of variable quality have been undertaken to assess whether psychological therapies are effective for treating anxiety and depression in children and adolescents with long‐term physical conditions. According to the available evidence, therapies specifically designed to treat anxiety or depression (especially those based on principles of cognitive behaviour therapy (CBT)) may be more likely to work in children and adolescents who have mild to moderate levels of symptoms of these disorders, at least in the short term. There is a dearth of therapies specifically designed to treat health‐related anxiety in this age group.
Plain language summary
Psychological therapies for anxiety and depression in children and adolescents with long‐term physical conditions
Why is this review important?
More than one in ten children and adolescents have long‐term physical conditions such as asthma, diabetes, and cancer. They are more likely to develop psychological problems like anxiety or depression. Treating these problems early can prevent difficulties with family life, school, and future mental health problems. It is currently unclear whether psychological therapies (talking therapies) designed for children and adolescents without medical issues are appropriate for use with this population.
Who will be interested in this review?
This review will be of interest to mental and medical healthcare providers, service users, and service commissioners.
What questions does this review aim to answer?
This review aims to answer the following questions: 1) Are psychological therapies better than a range of other therapies in reducing symptoms of anxiety and depression in children and adolescents with long‐term physical conditions? and 2) Are psychological therapies acceptable to this audience?
Which studies were included in the review?
We searched a number of databases to find all high‐quality trials of psychological therapies for anxiety or depression in children and adolescents aged 18 years or less with long‐term physical conditions and symptoms of anxiety or depression, published from 1970 to September 2018. We included twenty‐nine studies with a total of 1349 people in the review and rated the overall quality of the studies as 'low to moderate'.
What does the evidence from the review tell us?
A handful of psychological therapies have been researched in children and adolescents with long‐term physical conditions. Most of these were developed for use with children and adolescents who do not have long‐term physical conditions. Some of these, particularly those based on principles of cognitive behaviour therapy (CBT) and therapies specifically designed to treat depression or anxiety, are effective at reducing mild symptoms of these conditions in the short term. There is limited evidence that such therapies are acceptable to young people and that they can improve quality of life and symptoms of long‐term physical conditions. There is currently a lack of therapies for addressing health‐related anxiety in this population.
What should happen next?
Further research should be undertaken to develop more effective psychological therapies to treat anxiety and depression in children and adolescents with long‐term physical conditions.
Summary of findings
Summary of findings for the main comparison. Psychological therapy compared to any comparator for anxiety and depression in children and adolescents with long‐term physical conditions.
| Psychological therapy compared to any comparator for anxiety and depression in children and adolescents with long‐term physical conditions | ||||||
| Patient or population: anxiety and depression in children and adolescents with long‐term physical conditions Setting: Intervention: psychological therapy Comparison: any comparator | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | comments | |
| Risk with any comparator | Risk with Psychological therapy | |||||
| Treatment efficacy: depression short‐term | The mean depression short‐term was 0 | SMD 0.31 lower (0.59 lower to 0.03 lower) | ‐ | 1121 (16 RCTs) | ⊕⊕⊝⊝ LOW 1 2 3 | A SMD of 0.31 is a small effect size |
| Treatment efficacy: anxiety short‐term | The mean anxiety short‐term was 0 | SMD 0.26 lower (0.59 lower to 0.07 higher) | ‐ | 578 (13 RCTs) | ⊕⊕⊕⊝ MODERATE 1 2 | The confidence interval crosses the line of no effect |
| Quality of life short‐term | The mean quality of life short‐term was 0 | SMD 1.13 higher (0.44 higher to 1.82 higher) | ‐ | 464 (7 RCTs) | ⊕⊕⊝⊝ LOW 1 2 3 | A SMD of 1.13 is a large effect size |
| Functioning short‐term | The mean functioning short‐term was 0 | SMD 0.49 higher (0.3 lower to 1.29 higher) | ‐ | 483 (7 RCTs) | ⊕⊕⊝⊝ LOW 1 2 3 | The confidence interval crosses the line of no effect |
| Status of long‐term physical condition short‐term | The mean long‐term physical condition short‐term was 0 | SMD 0.34 lower (0.61 lower to 0.06 lower) | ‐ | 823 (14 RCTs) | ⊕⊕⊝⊝ LOW 1 2 3 | A SMD of 0.34 is a small effect size |
| Dropouts due to adverse events | No data available | ‐ | ‐ | ‐ | ‐ | ‐ |
| Suicide‐related behaviour | No data available | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 High degree of inconsistency between results
2 Substantial heterogeneity
3 Upper/lower CI crosses the effect size (SMD) of 0.5 in either direction
Background
Description of the condition
The terms 'long‐term conditions' and 'chronic illnesses of childhood' are variably defined in the literature, but usually include physical, psychological, or cognitive problems lasting more than three months and that impair functioning (Van der Lee 2007). It is estimated that 10% to 12% of children internationally are affected by long‐term physical conditions (Van Cleave 2010). Asthma is the most common long‐term physical condition of childhood, followed by diabetes, and then epilepsy (Burkart 2002). Less common long‐term physical conditions include respiratory conditions such as cystic fibrosis and bronchiectasis, cardiovascular conditions such as congenital heart disease, gastrointestinal conditions such as Crohn’s disease, renal conditions such as chronic kidney disease, neurological conditions such as muscular dystrophy, chronic pain, cancer, and others. Due to improvements in hygiene, immunisation, and access to medical care reducing the impact of acute illness, in some developed countries, the prevalence of long‐term physical conditions is now greater than that of many acute illnesses (Halfon 2010). Epidemiological studies show that the risk of psychological difficulties, especially anxiety and depression, is substantially increased in children and adolescents with such long‐term physical conditions (Cadman 1987; Gortmaker 1990; Newacheck 1991; Opolski 2005; Pinquart 2011a; Pinquart 2011b; Pless 1971; Weiland 1992).
Anxiety disorders are common, occurring in 2.6% to 5.2% of children under 12 years and 5% to 19% of all children and adolescents (Costello 2004). The presentation of anxiety disorders varies with age, from separation anxiety, undifferentiated worries, and somatic complaints in younger children to specific phobias, panic disorder, and social anxiety in older children and adolescents. Childhood anxiety disorders often persist into adolescence and early adulthood (Secenti 2017), and yet they often remain untreated or diagnosed late (Pao 2011). Anxiety disorders are associated with poor academic performance, and personal and social dysfunction (Pine 2009). They may also co‐occur with depression (Kovacs 1989), substance abuse (Kushner 1990), attention‐deficit/hyperactivity disorder (ADHD), and conduct disorder (Bittner 2007), and are associated with suicidal behaviours and death by suicide (Hill 2011). Anxiety has been identified in children and young people with long‐term physical conditions as an area of clinical significance (Benton 2007; Pao 2011). It may arise from a number of different mechanisms, including confrontation by dangerous stimuli such as threatening symptoms of illness or distressing procedures and unpredictable events, increased fear of death in life‐threatening diseases, having a reduced sense of control over one’s circumstances, experiencing peer rejection or parental overprotection and experiencing illness‐specific symptoms such as shortness of breath in asthma (Pinquart 2011a). Risk factors for developing anxiety in people with long‐term conditions include younger age, female gender, and type of illness (Hermanns 2005).
Depression is another common, yet under‐recognised, problem experienced by children with an overall prevalence of 0.4% to 2.5% in primary school children, and from 0.4% to 8.3% in adolescents (Birmaher 1996a). A 30‐year study of American children indicated a depression rate of 2.8% in children under the age of 13 years and 5.6% in young people aged 13 to 18 years (Costello 2004). Rates rise rapidly during adolescence (Fergusson 2001). By adulthood, around 25% of young people have suffered from a depressive disorder (Lewinsohn 1993; Lewinsohn 1998). Depression is associated with poor academic performance, social dysfunction, substance abuse, and attempted and death by suicide (Birmaher 1996; Birmaher 1996a; Brent 1986; Brent 2002; Fleming 1993; Rao 1995; Rhode 1994). Even subthreshold depression (defined as symptoms of depression that do not reach the cut‐off for formal diagnosis) is associated with a increased risk of depression (Gonzales‐Tejera 2005), substance abuse (Judd 2002), suicidal behaviours (Fergusson 2006), and mortality (Cuijpers 2002). Depression may be comorbid with anxiety in 15.9% to 61.9% of children identified as either anxious or depressed, and measures of anxiety and depression are highly correlated (Brady 1992). Depression has also been identified as occurring more commonly in children and adolescents with long‐term physical conditions (Dantzer 2003; Pinquart 2011b). Depressive symptoms have been reported in as many as 40% of children with a long‐term physical condition and socialisation problems (Denny 2014). Risk factors for depression in chronic illness are thought to include low self‐esteem and negative attributional style (Burke 1999).
The likelihood of psychosocial problems such as anxiety or depression is governed by numerous broader factors including the adaptive capacities of parents, the sociocultural context of hospitalisation, and the nature of particular hospital experiences, including the degree and duration of discomfort and pain (Lewis 2003). The child’s internal abilities to cope with stress and adapt to illness also vary in relation to the child’s developmental stage and temperament (Lewis 2003). Disordered parenting, abuse, divorce, and poverty are also serious risk factors (Lewis 2003). Costs for families include increased burden of care and health problems for family members, especially mothers and siblings (Eiser 1997). To date, models of psychological problem development have included deficit‐centred approaches in which it is assumed that emotional and behavioural problems are the inevitable consequence of long‐term physical conditions (Drotar 1978) and multidimensional approaches in which the balance between resistance and resilience factors determines the development of psychological problems in people with long‐term physical conditions (Wallander 2003).
The importance of treating anxiety and depression in people with long‐term physical conditions goes beyond the clinical outcomes for each of these conditions. Even mild depression is known to impair motivation to access medical care and adherence to medical treatment plans (Turner 2000). Depression can limit pain management (Breitbart 1995), worsen other physical outcomes and related disability (Glassman 1998; de Groot 2001; Saravay 1996), negatively influence family relationships (Breitbart 1995), increase medical costs by up to 50% (Simon 2005), and lead to suicide in people with long‐term physical conditions (Harris 1997). There is some evidence that early identification and treatment of anxiety and depression might improve mental and physical health‐related outcome in adults with long‐term physical conditions (Lustman 2000; Pollock 2000; Sharpe 2001), Although such evidence is currently more limited in children and adolescents, it still stands to reason that this might also be true in this age group.
Description of the intervention
Psychological therapies have been used to treat anxiety or depression in children with long‐term conditions. Studies of anxiety and depression have been combined within this review due to the high rates of comorbidity of these conditions and the fact that these disorders are often treated simultaneously in clinical settings. Psychological therapies are defined as any psychotherapeutic treatment (talking therapy) scientifically designed to change cognition or behaviour (or both) with the intention of improving outcomes (Eccleston 2012). Evidence for the efficacy of therapies for psychological problems in children with long‐term physical conditions has been comprehensively evaluated. The majority of interventions specifically designed for children and adolescents with long‐term physical conditions focus on compliance with medical treatment, education about their medical condition, and improving aspects of medical care. Psychological issues, especially anxiety and depression, are usually addressed using standard psychological therapies which may or may not have been tested in this population. Access to such therapies may be limited, depending upon the availability of community child and adolescent mental health services, paediatric consultation liaison services, and other appropriate community‐based health services.
How the intervention might work
The cause of both anxiety and depression are complex and include biological, psychological, and social factors (Cicchetti 1998; Davidson 2002; Goodyer 2000; Lewinsohn 1994; ; McCauley 2001). We expect that the majority of interventions designed to address these conditions will include an element of education about the psychological problem being addressed, and be based upon the principles of cognitive behavioural therapy (CBT), interpersonal therapy (IPT), or family therapy. However, potential mechanisms for the main categories of psychological therapies are listed below.
Behaviour therapies aim to change patients' behaviour towards their symptoms using operant conditioning (use of rewards and punishment). Common components used to treat anxiety and depression include psychoeducation (Guerney 1971), relaxation training (Lowe 2002), and behavioural activation (BA) (Jacobsen 1996; Martell 2001). Biofeedback techniques (using instruments to help individuals become aware of physical processes and sensations) may also be used (Schwartz 2003).
Cognitive behaviour therapy (CBT) helps to link thoughts, feelings, and behaviour, and targets the situations or triggers that generate emotional responses. Cognitive appraisal of triggers and altering cognitions in order to change mood and behaviour are encouraged. CBT for depression is based on the cognitive model of depression (Beck 1976) which proposes that individuals prone to depression have cognitive distortions which result in a negative view of themselves, the world, and the future. People with "pessimistic attribution styles" (Abramson 1978) have a bias toward viewing negative events as stable and self‐induced, versus positive events as transient and out of their control. This leads to a state of 'learned helplessness' (Petersen 1993; Seligman 1979) and hopelessness, as well as passivity in the face of challenges (McCauley 2001). CBT for depression in children and adolescents involves helping the child to: (1) recognise and evaluate their thoughts and identify different levels of mood in themselves, (2) recognise thoughts and behaviours that have contributed to this mood, (3) develop coping strategies to address them via effective problem‐solving, and (4) evaluate outcomes. CBT has been shown to improve depression in children and adolescents (Harrington 1998; Reinecke 1998; Weisz 2006) and prevent relapse (Paykel 1999), although long‐term results in studies have contradictory findings (Fonagy 2005). CBT for anxiety is based on Beck’s cognitive model of anxiety which proposes that fear and anxiety are learnt responses that can be 'unlearnt'. CBT for anxiety in children and adolescents involves helping the child to: (1) recognise anxious feelings and bodily reactions, (2) clarify thoughts or cognitions in anxiety‐provoking situations, (3) develop effective coping skills via modified self‐talk, modelling, reality, or in vivo exposure (Silverman 1996), role playing, and relaxation training, and (4) evaluate outcomes. An element of treatment known as systematic desensitisation involves pairing anxiety stimuli, in vivo or by imagination, in a gradually‐increasing hierarchy with competing relaxing stimuli such as pleasant images and muscle relaxation (James 2013). Recent advances have identified optimal methods of delivering exposure work such as affect labelling, using retrieval cues and undertaking exposure in multiple contexts (Craske 2014).
Third wave CBTs include acceptance and commitment therapy (ACT) (Hayes 1999; Hayes 2004), compassionate mind training (CMT), also known as compassion‐focused therapy (Gilbert 2005; Gilbert 2009), functional analytic psychotherapy (FAP) (Kohlenberg 1991), metacognitive therapy for depression (Wells 2008; Wells 2009) and dialectical behaviour therapy (DBT) (Koons 2001; Linehan 1993). These approaches use a combination of cognitive, behavioural, and mindfulness techniques to assist people to manage situations without thought suppression or experiential avoidance (Hoffman 2008).
Psychodynamic therapies aim to resolve internal conflicts stemming from difficulties in past relationships and experiences (for example, sexual abuse). Such conflicts are thought to cause anxiety or psychic pain and are 'repressed' into the unconscious through the use of defence mechanisms (Bateman 2000). Although some defence mechanisms are adaptive, some are developmentally immature and can cause harm. Psychoanalytic (sometimes called psychodynamic psychotherapy) attempts to explore, through talking, play (with younger children) and the formation of a therapeutic relationship, how earlier experiences influence and perhaps seriously distort current thoughts, feelings, behaviours (actions), and relationships (McQueen 2008).
Humanistic therapies include grief therapy, supportive therapy and transactional analysis. These therapies are based on the premise that people are ‘self‐actualising’, that is, they have an inherent tendency to develop their potential (Maslow 1970; Rogers 1951) and that they are self‐aware, free to choose how they live, and are responsible for the choices they make. Individualised, rather than manualised or prescribed methods, are undertaken to help them address their situation (Cain 2002).
Intergrative therapies include interpersonal therapy (IPT) which addresses interpersonal conflict, difficulty with role transitions, and experiences of loss, all of which are well known as risk factors in the development of depressive disorder in young people (Birmaher 1996; Lewinsohn 1994; McCauley 2001). IPT has been proposed to work by activating several interpersonal change mechanisms including: (1) enhancing social support, (2) decreasing interpersonal stress, (3) facilitating emotional processing, and (4) improving interpersonal skills (Lipsitz 2013). It has been proven to be effective in the treatment of teenage depression (Bolton 2007; Mufson 1996; Mufson 2004).
Systemic therapies include family therapy which is based on the premise that family members can influence one another's well‐being and have a significant effect on both the development of symptoms and the outcomes of interventions (Carr 2006). There are a number of forms of family therapy including structural family therapy (Liebman 1974; Minuchin 1978) which centres on individual physiological vulnerability, dysfunctional transactional styles, and the role the sick child plays in facilitating conflict avoidance. Systems therapy, including Milan and post‐Milan family therapy, attempts to elicit changes in the family dynamic by presenting information that encourages family members to reflect on their own behaviour within the family dynamic (Selvini 1978). Strategic family therapy acknowledges the effect of the illness on all family members and focuses on inducing change in symptoms by highlighting paradoxical intentions of family members (Madanes 1981). Attachment‐based family therapy (ABFT), which focuses on the development of secure attachment relationships within the family, has been shown to be better than waiting‐list control for treating depression, to lead to faster resolution of depressive symptoms, and less suicidal ideation (Diamond 2002). ABFT has also been shown to lead to greater client and family satisfaction and retention when combined with CBT than when CBT is used alone for treating anxiety in young people (Siqueland 2005).
Why it is important to do this review
A few existing Cochrane reviews have already investigated the value of psychological therapies for anxiety and depression in children and adolescents. One review has addressed the prevention of depression in children and adolescents without addressing those with long‐term conditions (Hetrick 2016). Two reviews have addressed the treatment of depression (Cox 2014) and anxiety (James 2013) in children and adolescents, but again not in those with long‐term conditions. Two reviews have addressed psychological interventions for depression in adolescents with a single condition such as congenital heart disease or pain (Fisher 2018; Lane 2013) and one review focusses on interventions for parents rather than children (Eccleston 2012). One non‐Cochrane review (Bennett 2015) has examined the effectiveness of psychological therapies for anxiety and depression in children and adolescents with long‐term physical conditions, but only to a limited extent without any meta‐analysis of data.
This review aims to fill a gap in the literature by evaluating whether currently available psychological therapies are effective for the treatment of anxiety and depression in children and adolescents with long‐term physical conditions. Establishing this evidence will provide comment on current best practice and serve to guide the development of new forms and modalities of treatment for this growing population. Due to the unique qualities of e‐health interventions and the rapidly growing nature of this new field of health, e‐health interventions for addressing anxiety and depression in children and adolescents with long‐term physical conditions are being considered separately from non‐e‐health interventions by the same authors in a related review (Thabrew 2018).
Objectives
To assess the effectiveness and acceptability of psychological therapies in comparison with controls (treatment‐as‐usual, waiting list, attention placebo, psychological placebo, or non‐psychological treatment) for treating anxiety and depression in children and adolescents with long‐term physical conditions.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) and cluster‐randomised trials. Cross‐over trials were also included, though we only used data from the first phase in order to avoid carry‐over effects. We excluded observational studies, quasi‐randomised trials, and non‐randomised trials. We did not exclude any study on the basis of language or publication status.
Types of participants
Participant characteristics
We included trials performed on children and adolescents aged up to 18 years (or at least 80% of the sample within this age range). In the case that a trial presented data separately for child/adolescents and adults, we would have incorporated the relevant data; however, this situation did not arise.
Diagnosis
We included studies performed on participants with any single or mixed long‐term physical condition(s) of more than three months duration, who also had depression/subthreshold depression or anxiety, or both. Depressive and anxiety disorders were reliably diagnosed through structured clinical interviews, and symptom severity was assessed by either patient‐ or clinician‐administered validated rating scales (Sadock 2005) based on DSM‐III, ‐IV or ‐V (American Psychological Association 2013) or ICD‐9 or ‐10 (World Health Organization 1992) criteria.
Comorbidities
Those with any mixed long‐term conditions and with both anxiety and/or depression were included; we included studies of those who may also have any other type of comorbid physical (e.g. asthma, diabetes, epilepsy) or mental health condition (e.g. attention deficit and hyperactivity disorder, obsessive compulsive disorder, schizophrenia).
Setting
We included studies conducted in hospital and community settings.
Types of interventions
Experimental intervention
Experimental interventions included any individual or group‐based psychological or psychologically‐oriented therapy excluding e‐health therapies (which are addressed in a companion review, Thabrew 2017) designed with the primary aim of treating clinical or subthreshold levels of anxiety or depression and tested in children and adolescents with long‐term conditions. These may have included parent participation, but not interventions that were designed only for parents. The interventions we considered were guided by the HIRED approach to psychological therapies (Hunot 2013; Shinohara 2013) and included:
behaviour therapies (e.g. relaxation training, Lowe 2002);
cognitive behaviour therapies (e.g. CBT, Beck 1976);
third wave CBTs (e.g. ACT, Hayes 1999);
psychodynamic therapies (e.g. psychoanalytic therapy, McQueen 2008);
humanistic therapies (e.g. person‐centred psychotherapy, Rogers 1951);
integrative therapies (e.g. Birmaher 1996);
systemic therapies (e.g. structural family therapy, Minuchin 1978); and
other psychologically‐oriented therapies (e.g. bibliotherapy, Russell 1958; art therapy, Uttley 2015).
Comparator intervention
Comparator interventions included any of the following.
Attention placebo (AP): a control condition that was regarded as inactive by both researchers and by participants in a trial.
Psychological placebo (PP): a control condition that was regarded as inactive in a trial by researchers but is regarded as active by the participants.
Non‐psychological therapies (NPT): e.g. pharmacotherapy for depression or anxiety.
Treatment‐as‐usual (TAU): participants could receive any appropriate medical care during the course of the study on a naturalistic basis, including standard psychological or pharmacotherapeutic care, usual care, or no treatment.
Waiting list (WL): as in TAU, patients in the WL condition could receive any appropriate medical care during the course of the study on a naturalistic basis; however, unlike in the TAU comparator intervention, those in the WL will receive the intervention after the period of waitlist.
Types of outcome measures
Outcome measures were focused on the individual child rather than the wider family. We evaluated the difference between the treatment group and the control group separately for anxiety and depression using the following outcomes.
Primary outcomes
1. Treatment efficacy: changes in severity of anxiety and depression symptoms measured separately using validated scales for each of these conditions (e.g. Children's Depression Inventory (CDI) for childhood depression (Kovacs 1989), State‐Trait Anxiety Inventory (STAI) for anxiety (Spielberger 1983)). Clinician‐rated scales were analysed separately from those rated by children, young people, parents, and others (e.g. teachers). Statistically‐significant results were interpreted with regard to the clinical significance of each scale (possibly using T‐scores, if these were available for all scales).
2. Treatment acceptability: measured via validated scales (e.g. Treatment Satisfaction Questionnaire (Regnault 2011)) or qualitatively determined by participant report. In addition, we examined the number of participants who dropped out for any reason and adverse events.
Secondary outcomes
3. Changes in caseness (remission/response), as defined by study authors or measured using cut‐offs on similar validated scales for each of these conditions.
4. Suicide‐related behaviour, i.e. number of: a) deaths by suicide, b) suicide attempts and c) episodes of self harm, either reported or measured using validated scales (e.g. Suicide Behaviour Questionnaire‐Revised (SBQ‐R), Osman 2001).
5. Improvement in quality of life measured using validated scales (e.g. Pediatric Quality of Life Scale (PedsQL), Varni 2004).
6. Functioning as a proxy for psychological well‐being measured using validated scales (e.g. Children's Global Assessment Scale (CGAS), Shaffer 1983).
7. Status of long‐term physical condition using validated scales (e.g. Abdominal Pain Index (API), Walker 1997).
8. Adherence to treatment of long‐term physical condition.
9. School/college attendance (e.g. reduction in number of days missed).
10. Economic benefits (e.g. reduction of costs of treatment, number of appointments with general practitioners, use of additional treatments, ability to study or work).
Timing of outcome assessment
Clustering and comparison of outcome measures at similar time periods was undertaken. The primary time point was short‐term (at the end of treatment or up to three months, whichever was first measured, to evaluate immediate therapeutic effect). Long‐term (three to six months beyond the end of treatment) outcome measures were assessed separately. If multiple long‐term measures were provided, we used the one furthest from the intervention as this was most relevant to understanding the enduring nature of its therapeutic effect.
Hierarchy of outcome measures
For trials presenting a range of symptom measures (i.e. more than one depression scale or more than one anxiety scale), we used the scale ranked highest according to the following five criteria: appropriateness to children and adolescents; reliability; construct validity; agreement with clinical interview; track record in psychopharmacological research. These are the same criteria that are used in our accompanying published review examining e‐health interventions (Thabrew 2018), and based on work originally implemented in a Cochrane review by Hazell and colleagues (Hazell 2002; Hazell 2013).
For depression, the ranking from highest to lowest scales was as follows: Schedule for Affective Disorders and Schizophrenia for School‐Age Children (Kiddie‐SADS (Kaufman 1997)), Children's Depression Rating Scale (CDRS (Poznanski 1985)), Bellevue Index of Depression (BID (Petti 1978)), Children's Depression Inventory (CDI (Kovacs 1985), Hamilton Depression Rating Scale (HAM‐D (Hamilton 1967)), Depressive Adjective Checklist (DACL (Lubin 1965)), then others (Hazell 2002).
For anxiety, the ranking of scales was based on appropriateness to children and adolescents, reliability, construct validity, agreement with clinical interview, and track record in psychotherapeutic research. From highest to lowest, this would be as follows: Anxiety Disorder Interview Schedule (ADIS (Silverman 1988)), Multi‐dimensional Anxiety Scale for Children (MASC (March 1997)), Paediatric Anxiety Rating Scale (PARS (PARS 2002)), Social Phobia and Anxiety Inventory for Children (SPAI‐C (Beidel 2000)), Social Anxiety Scale for Children‐Revised (SASC‐R (La Greca 1988)), Fear Survey Schedule for Children‐Revised (FSSC (Olendick 1983)), Revised Children’s Manifest Anxiety Scale (RCMAS (Reynolds 1978)), State‐Trait Anxiety Inventory for Children (STAI‐C (Spielberger 1983)), Screen for Child Anxiety Related Emotional Disorders (SCARED (Birmaher 1999)), Hamilton Anxiety Rating Scale (HARS (Maier 1988)), then others (based on Myers 2002).
Search methods for identification of studies
Cochrane Common Mental Disorders Controlled Trials Register (CCMD‐CTR)
The Cochrane Common Mental Disorders (CCMD) Group maintains a specialised register of randomised controlled trials, the CCMD‐CTR. This register contains over 40,000 reference records (reports of RCTs) for anxiety disorders, depression, bipolar disorder, eating disorders, self‐harm, and other mental disorders within the scope of this Group. The CCMD‐CTR is a partially studies‐based register with more than 50% of reference records tagged to approximately 12,500 individually PICO‐coded study records. Reports of trials for inclusion in the register are collated from (weekly) generic searches of MEDLINE (1950‐), Embase (1974‐) and PsycINFO (1967‐), quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), and review‐specific searches of additional databases. Reports of trials are also sourced from international trial registries, drug companies, the handsearching of key journals, conference proceedings, and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCMD's core search strategies (used to identify RCTs) can be found on the Group's website, with an example of the core MEDLINE search displayed in Appendix 1.
The CCMD‐CTR is current to June 2016 only, with the move of the editorial base from the University of Bristol to York.
The search of the CCMD‐CTR was superseded with a cross‐search of Ovid MEDLINE, Embase and PsycINFO (2016 to 27 September 2018).
Electronic searches
The Cochrane Group's Information Specialist initially searched the CCMD‐CTR (all years to 6 May 2016), using the following terms.
CCMD‐CTR‐Studies Register:
Condition = (anxiety or depressi* or mood or mutism or neuroses or neurotic or “obsessive compulsive” or panic or *phobi* or psychoneuroses or “stress disorder*” or “psychological stress” or “school refusal”) and Comorbidity = not empty and Age Group = (child or adolescent)
CCMD‐CTR‐References Register:
This search included a more sensitive set of terms to find additional untagged/uncoded reports of RCTs (Appendix 2).
The CCMD's information specialist conducted complementary searches on the following bibliographic databases, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource.
The Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO); searched 9 June 2016, 18 August 2017 and 27 September 2018 (Appendix 3).
Other Cochrane Library databases (CDSR, DARE, HTA); searched 9 June 2016 and 18 August 2017
A cross‐search of Ovid MEDLINE, Embase and PsycINFO; searched 18 August 2017 and 27 September 2018 (Appendix 4).
We searched the following resources (all years to 16 August 2016, 18 August 2017 and 12 October 2018):
Web of Scence Core Collection (Science, Social Science and Conference Proceeding indices (SCI, SSCI, CPCI‐S, CPCI‐SSH; searched 18 August 2016 and 31 August 2017) (employing the same search strategy as displayed in Appendix 2, but amending NEXT to NEAR/x and adding an RCT filter (random* OR "cross over" OR crossover or trial OR trials).
International trial registries via the World Health Organization's trials portal (ICTRP) and ClinicalTrials.gov all years to 12 October 2018, to identify unpublished or ongoing trials.
We did not restrict our search by date, language, or publication status.
Searching other resources
Handsearching
We handsearched conference proceedings (those titles not already indexed in Embase or PsycINFO, or already handsearched within Cochrane) of the Annual Meeting of the American Academy of Child and Adolescent Psychiatry (AACAP) (2000 onwards).
Reference lists
We checked the reference lists of all included studies and relevant systematic reviews to identify additional studies missed from the original electronic searches (for example, unpublished or in‐press citations). We also conducted a cited reference search on the Web of Science for reports of all included studies.
Grey literature
We searched sources of grey literature via the following websites: Open Grey http://www.opengrey.eu/ and the National Guidlines Clearing House www.guideline.gov/.
Correspondence
We contacted trialists and subject experts for information on unpublished or ongoing studies or to request additional trial data.
Data collection and analysis
Selection of studies
Two authors (HT and JH) independently screened the titles and abstracts of studies identified by the above search. Studies that obviously did not fulfil inclusion criteria at this stage of the screening process were discarded. Eligible or potentially‐eligible articles were retrieved for full‐text inspection by two authors (HT and JH) independently. We resolved any discrepancies by discussion or by involving a third author (KS), as necessary. We listed the reasons for exclusions in the table Characteristics of excluded studies. The selection process was described in enough detail to complete a PRISMA flow diagram.
Data extraction and management
Two authors (HT and KS) independently extracted data on trial characteristics, the methodology, participant characteristics, intervention characteristics, outcome measures, and outcome data using Covidence software. We attempted to contact authors at least twice to obtain additional information, when required. After agreement, data for analysis was transferred to RevMan 5.3 into the format required to include the maximal numbers of studies (we were able to extract in every case: events, mean and standard deviations (SDs), and total number of participants for each group. Any disagreements were resolved by discussion or with the help of the third author (SH).
Main planned comparisons
Psychological therapies for anxiety or depression versus attention placebo (AP).
Psychological therapies for anxiety or depression versus psychological placebo (PP).
Psychological therapies for anxiety or depression versus non‐psychological therapies (NPT).
Psychological therapies for anxiety or depression versus treatment‐as‐usual (TAU).
Psychological therapies for anxiety or depression versus waiting list (WL).
For definitions of interventions and comparators, see Types of interventions. We combined all types of psychological therapy in the main analyses, and conducted subgroup analyses to investigate any differences between them (where data allowed).
Assessment of risk of bias in included studies
Risk of bias was assessed for each included study using the Cochrane 'Risk of bias' tool (Higgins 2011). The following domains were considered.
Sequence generation: was the allocation sequence adequately generated?
Allocation concealment: was allocation adequately concealed?
Blinding of participants and care providers for each main outcome or class of outcomes: was knowledge of the allocated treatment adequately prevented during the study?
Blinding of outcome assessors for each main outcome or class of outcomes: was knowledge of the allocated treatment adequately prevented during the study?
Incomplete outcome data for each main outcome or class of outcomes: were incomplete outcome data adequately addressed?
Selective outcome reporting: were reports of the study free of any suggestion of selective outcome reporting?
Other sources of bias: was the study apparently free of other problems that could put it at high risk of bias? Additional items to be included here were therapist qualifications, treatment fidelity, and researcher allegiance/conflict of interest.
A description of what was reported to have happened in each study was reported independently by two authors (HT and KS) and a judgement on the risk of bias was made for each domain within and across studies, based on the following three categories.
Low risk of bias.
Unclear risk of bias.
High risk of bias.
Disagreements were resolved by discussion or with the help of the third author (SH).
For cluster‐randomised trials, the risk of bias was assessed by considering recruitment bias, baseline imbalance, loss of cluster, incorrect analysis, and comparability with individual randomised trials.
The level of risk of bias was noted in both the body of the review and the 'Risk of bias' summary figures.
Measures of treatment effect
Odds ratio (OR) was used for comparing dichotomous data and standardised mean differences (SMD) to analyse continuous data when different scales were used across studies to measure an outcome, and mean difference when the same scale was used across studies or when there was only one study included in a meta‐analysis. . A SMD effect size of 0.2 was considered small, 0.5 was considered medium and ≥ 0.8 was considered large (Pace 2011). When an effect was discovered, a number needed to treat for an additional beneficial outcome (NNTB) for the primary outcome was calculated from the odds ratio (www.nntonline.net/visualrx/), as this value was less likely to be affected by the side (benefit or harm) in which the data were entered (Cates 2002; Deeks 2000).
Meta‐analyses were only undertaken where this was meaningful, i.e. if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense. We narratively described skewed data reported as medians and interquartile ranges.
Unit of analysis issues
Cluster‐randomised trials
We planned to include and analyse any identified cluster‐randomised trials as long as proper adjustment for the intra‐cluster correlation was undertaken as described in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Cross‐over trials
Due to the risk of carry‐over effects in cross‐over trials, only data from the first phase of such studies were used.
Studies with multiple treatment groups
Where studies had additional arms that were not psychological therapies, we only included data relating to the therapy and one control arm in the review. If a study had more than two arms that met the inclusion criteria, for example, two psychological therapies and a control arm, the number of people in the control arm was split equally to produce two (or more) pairwise comparisons.
Dealing with missing data
We contacted the authors for apparently missing data. We only used imputed data if this was on the basis of appropriate multiple imputation or modelling using maximum likelihood estimation (including last observation carried forward). Where trials did not report the SDs of continuous measure scores and the original authors were unable to provide SDs, we calculated the SD from the standard error (SE) or P values (Altman 1996), or from CI, t‐values, or P values as described in section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If this was not possible, we used the baseline SD. If means were based on imputed data and these were all that was available, we used the number of observed cases.
Assessment of heterogeneity
Before pooling results and carrying out any meta‐analysis, we considered clinical heterogeneity and the role of subgroup analyses to address it. We quantified statistical heterogeneity using the I2 statistic with data entered in the way (benefit or harm) that yielded the lowest amount.
The amount, depending on the value obtained for the I2 statistic (Higgins 2003), was qualified as:
might not be important (0% to 40%);
may represent moderate heterogeneity (30% to 60%);
may represent substantial heterogeneity (50% to 90%);
may represent considerable heterogeneity (75% to 100%).
Assessment of reporting biases
If more than 10 studies were identified and selected, we planned to enter data from them into a funnel plot (trial effect versus trial size) to evaluate overt publication bias. We accepted that a symmetrical funnel plot was likely to indicate low publication bias and an asymmetric funnel plot was likely to indicate possible publication bias. The number of studies required to reduce the P value of a statistically significant finding to 0.05 (not statistically significant) was also to be used to evaluate the robustness of the findings. A high classical fail‐safe number indicated that the conclusions were unlikely to be reversed by new studies, while a low classical fail‐safe number indicated that they may be more likely to be reversed in the future. Finally, we used Duval and Tweedie’s trim and fill analysis (Duval 2000) to estimate what the effect size (OR, risk ratio, etc.) would be if there was no publication bias.
Data synthesis
When available and sufficiently clinically and statistically homogenous, we combined final score data from included trials in meta‐analyses. As we were anticipating heterogeneity of data, we planned on analysing the data using RevMan 5.3 using a random‐effects model for analysis. We presented the characteristics of included and excluded studies in tables. We presented the 'Risk of bias' assessment in a 'Risk of bias' graph. We presented results for each comparison as forest plots, when appropriate. We provided narrative summaries for comparisons with fewer than two available studies and those with a moderate or high level of statistical heterogeneity following exploration of heterogeneity.
Subgroup analysis and investigation of heterogeneity
For each condition (anxiety or depression), in order to better understand the factors which contributed to effective intervention, subgroup analyses were planned for the primary outcome, as follows.
Type of experimental therapy (e.g. CBT, other therapy). This was undertaken because different types of therapies are known to have varied underlying theoretical bases and often result in different effect sizes (e.g. Watanabe 2007).
Type of control therapy (e.g. active comparators (such as attention placebo, psychological placebo, and other non‐psychological therapies) and non‐active comparators (such as treatment‐as‐usual and waiting list), as defined by previous researchers (Weisz 2006). Control intervention type has been shown to impact upon effect sizes (Furakawa 2014).
Modality of delivery (e.g. individual, group). Different modalities of therapy have been shown to result in different effect sizes during the treatment of a range of conditions (Wierzbicki 1987).
Dose of treatment (number of completed sessions divided into less than six sessions weeks compared with six or more sessions or weeks). Although different therapies will have different total duration, it is of interest to identify therapies that most efficiently result in symptomatic improvement.
Form of measurement (e.g. self‐rated, parent‐rated, clinician‐rated). Different types of rating scale have been shown to contribute differently to the prediction of outcomes (Uher 2012).
Type of long‐term physical condition (e.g. asthma, diabetes). This was undertaken to identify whether these therapies are more or less effective for children (0 to eight years, nine to 12 years old) and young people (13 to 15 years and 16 to 18 years old) with different types of physical illness and in order to make recommendations regarding the targeted use of these therapies.
Category of depressive symptoms (divided into subthreshold, mild, moderate, severe and uncertain). There is a possibility that subthreshold and 'clinical' depressive symptoms may respond differently to therapies (Costello 1992).
Target of intervention. Interventions targeted at children or adolescents may be differently effective to those targeted at families (Aydin 2014).
Participant factors (e.g. sex, age). Younger and older people have been shown to have different effect sizes following similar therapies (Bennett 2013) so results were analysed according to four clinically relevant subgroups of age (0 to eight years, nine to 12 years, 13 to 15 years, and 16 to 18 years old).
Sensitivity analysis
In order to test the robustness of decisions made in the review process, three planned sensitivity analyses were carried out for the primary outcomes based on allocation concealment, blinding of outcome assessors, and dropout rates (studies in which more than 20% of participants did not complete post‐intervention assessments were removed). Allocation concealment and blinding of outcome assessors have been shown to have a significant impact on treatment effect (Schulz 1995). One post hoc sensitivity analysis was conducted for the primary outcomes based on whether or not therapies were specifically designed to address anxiety or depression. This was undertaken because we realised that our original inclusion criteria were so broad that many of the included trials were actually designed to treat other symptoms or functioning and our other analyses did not allow us to identify the effectiveness of therapies directed toward reducing anxiety or depression in the target population.
'Summary of findings' table
We constructed a 'Summary of findings' table for each comparison between psychological and other interventions, with regard to the following outcomes:
Treatment efficacy: short‐term depression (measuring the change in severity of depressive symptoms by the end of treatment);
Treatment efficacy: short‐term anxiety (measuring the change in severity of anxiety symptoms by the end of treatment);
Short‐term quality of life;
Short‐term functioning;
Short‐term status of long‐term physical condition;
Dropouts due to adverse effects (in the short term);
Suicide‐related behaviour (including the number of a) deaths by suicide, b) suicide attempts, and c) episodes of deliberate self harm, either reported or measured using validated scales in the short term (Osman 2001)).
In the 'Summary of findings' tables we used the principles of the GRADE approach (Guyatt 1998) to assess the extent to which there could be confidence that the obtained effect estimate reflected the true underlying effect. The quality of a body of evidence was judged on the basis of the included studies’ risks of bias, the directness of the evidence, unexplained heterogeneity, imprecision, and the risk of publication bias. We used the average rate in all the arms of included trials as the ’assumed risk’ for each outcome. As we were not aiming to target any particularly high or low risk populations, all the tables were for medium‐risk populations.
Results
Description of studies
Results of the search
We found 10,814 citations from searches run by CCMD (to 27 September 2018) and our own searches (to 12 Oct 2018), from which 67 abstracts were identified as potentially relevant. Following review of the full texts reports we excluded 32 trials, included 31 papers describing 28 trials and placed four papers in awaiting classification. All 28 trials contributed data to the narrative analysis and 22 trials contributed data to the meta‐analysis. See Figure 1 for further details.
1.
Primsa Flow Diagram
Included studies
Twenty eight trials were included in this review (see Table 2 for an overview). Characteristics of individual trials are presented in the Characteristics of included studies table.
1. Summary of included trials.
| Trial Author | Year | Intervention | Number of participants | Age range of participants in years (mean) | Long‐term physical condition | Psychological condition (Anxiety/ Depression |
| Comparator: Attention Placebo (AP) | ||||||
| Freedenberg | 2017 | Mindfulness | 46 | 12‐18 (14.8) | Cardiac disease | Depression |
| Hickman | 2015 | CBT | 32 | 13‐17 (NR) | Headache | Depression Anxiety |
| Kanstrup | 2016 | ACT | 30 | 14‐18 (16) | Chronic pain | Depression |
| Kashikar Zuck | 2012 | CBT | 114 | 11‐18 (15.5) | Fibromyalgia | Depression |
| Levy | 2010 | CBT | 200 | 7‐17 (NR) | Abdominal pain | Depression |
| Wei | 2017 | Counselling | 85 | 11‐16 (NR) | Diabetes | Depression Anxiety |
| Comparator: Psychological Placebo | ||||||
| Kashikar Zuck | 2005 | Coping skills training | 30 | 13‐17 (15.83) | Fibromyalgia | Depression |
| Martinovich | 2006 | CBT | 104 | 13‐19 (NR) | Epilepsy | Depression |
| Szigethy | 2014 | CBT | 217 | 9‐17 (14.3) | Inflammatory bowel disease | Depression |
| Comparator: Non‐Psychological Therapy (NPT) | ||||||
| Grey | 1998 | Coping skills training | 82 | 13‐20 (NR) | Diabetes | Depression |
| Comparator: Treatment‐As‐Usual (TAU) | ||||||
| Bignall | 2015 | Breathing retraining and asthma education | 33 | 12‐17 (15.47) | Asthma | Anxiety |
| Chiang | 2009 | Self‐management and relaxation | 48 | 6‐14 (NR) | Asthma | Anxiety |
| Li | 2016 | Family therapy | 104 | 13‐20 (NR) | Epilepsy | Depression Anxiety |
| Rostami | 2016 | 74 | 11‐21 (NR) | Diabetes | Depression Anxiety |
|
| Sharma | 2017 | CBT | 63 | 10‐19 (13.91) | Headache | Anxiety |
| Szigethy | 2007 | CBT | 41 | 11‐17 (14.29) | Inflammatory bowel disease | Depression |
| Thompson | 2012 | CBT | 30 | 11‐17 (14.29) | Inflammatory bowel disease | Depression |
| Varni | 1993 | Social skills training | 64 | 5‐13 (8.3) | Cancer | Depression Anxiety |
| Comparator: Waiting list | ||||||
| Detling Miller | 2008 | Breathing and progressive muscle relaxation | 26 | 12‐18 (NR) | Diabetes | Anxiety |
| Griffiths | 1996 | CBT | 51 | 10‐12 (NR) | Headache | Depression Anxiety |
| Hains | 2000 | Stress management training | 15 | 12‐15 (NR) | Diabetes | Anxiety |
| Moghanloo | 2015 | ACT | 34 | 7‐15 (NR) | Diabetes | Depression |
| Not included in meta‐analysis | ||||||
| Beebe | 2010 | Art therapy | 22 | 7‐14 (NR) | Asthma | Depression Anxiety |
| Bhana | 2014 | VUKA (culturally tailored cartoon) | 65 | 10‐13 (NR) | HIV | Depression |
| Bussone | 1998 | Biofeedback relaxation | 35 | 11‐15 (NR) | Headache | Anxiety |
| Chadi | 2016 | Mindfulness | 19 | 13‐18 (15.8) | Chronic pain | Depression Anxiety |
| Grey | 2009 | Coping skills training | 82 | 8‐20 (NR) | Diabetes | Depression |
| van der Veek | 2013 | CBT | 104 | 7‐18 (NR) | Abdominal pain | Depression |
| van Dijk Lokkart | 2016 | Psychosocial training and physical activity | 68 | 8‐18 (13) | Cancer | Anxiety |
ACT = acceptance and commitment therapy AP = attention placebo CBT = cognitive behaviour therapy HIV =human immunodeficiency virus NPT = non‐psychological therapy NR = not recorded PP = psychological placebo TAU = treatment‐as‐usual VUKA: 'Let's wake up' in Zulu WL = waiting‐list
Design
All of the included trials were randomised controlled trials and were published between 1993 (Varni 1993) to 2017 (Freedenberg 2017). One cross‐over trial was included (Kashikar‐Zuck 2005), but only data from the first phase was used, as we had planned. One trial (Szigethy 2007) had long‐term follow‐up published in a secondary paper described long‐term follow‐up from a previous trial. No relevant cluster‐randomised trials were identified.
Sample sizes
Sample sizes ranged from 15 (Hains 2000) to 217 (Szigethy 2014). Sample sizes for each trial were as follows: 15 participants (Hains 2000); 19 participants (Chadi 2016); 22 participants (Beebe 2010); 26 participants (Detling Miller 2008); 30 participants (Kanstrup 2016; Kashikar‐Zuck 2005); 32 participants (Hickman 2015); 33 participants (Bignall 2015); 34 participants (Moghanloo 2015); 35 participants (Bussone 1998); 46 participants (Freedenberg 2017); 41 participants (Szigethy 2007); 48 participants (Chiang 2009); 51 participants (Griffiths 1996); 63 participants (Sharma 2017); 64 participants (Varni 1993); 65 participants (Bhana 2014; Grey 1998); 68 participants (Van Dijk Lokkart 2016); 74 participants (Rostami 2016); 82 participants (Grey 2009); 85 participants (Wei 2017); 104 participants (Li 2016;Martinović 2006; Van der Veek 2013); 114 participants (Kashikar‐Zuck 2012); 200 participants (Levy 2010) and 217 participants (Szigethy 2014).
Setting
Sixteen trials were completed in the United States of America (Beebe 2010; Bignall 2015; Bussone 1998; Detling Miller 2008; Freedenberg 2017; Grey 1998; Grey 2009; Hains 2000; Hickman 2015; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Levy 2010; Szigethy 2007; Szigethy 2014; Varni 1993), two were completed in the Netherlands (Van der Veek 2013; Van Dijk Lokkart 2016), China (Chiang 2009; Li 2016, and Iran (Moghanloo 2015; Rostami 2016), and one in Canada (Chadi 2016), Australia (Griffiths 1996), England (Wei 2017), Sweden (Kanstrup 2016), Serbia and Montenegro (Martinović 2006), and South Africa (Bhana 2014). All trials recruited from a hospital or outpatient population with the exception of two trials (Beebe 2010; Bignall 2015) which recruited from a school‐based population, and one trial (Griffiths 1996) which recruited community participants via advertisement.
Participants
Participants ranged between four years and 21 years of age. Age ranges for individual trials were as follows; seven to 14 years, mean not reported (Beebe 2010); 10 to 13 years, mean not reported (Bhana 2014); 12 to 17 years, mean 15.47 years (Bignall 2015); 11 to 15 years, mean not reported (Bussone 1998); 13 to 18 years, mean 15.8 years (Chadi 2016); six to 14 years, mean not reported (Chiang 2009); 12 to 18 years, mean not reported (Detling Miller 2008); 12 to 18 years, mean 14.8 years (Freedenberg 2017); 13 to 20 years, mean not reported (Grey 1998); eight to 12 years, mean not reported (Grey 2009); 10 to 12 years, mean not reported (Griffiths 1996); 12 to 15 years, mean not reported (Hains 2000); 13 to 17 years, mean not reported (Hickman 2015); 14 to 18 years, mean 16 years (Kanstrup 2016); 13 to 17 years, mean 15.83 years (Kashikar‐Zuck 2005); 11 to 18 years, mean 15.5 years (Kashikar‐Zuck 2012); seven to 17 years, mean not reported (Levy 2010); 13 to 20 years, mean not reported (Li 2016); 13 to 19 years, mean not reported (Martinović 2006); seven to 15 years, mean not reported (Moghanloo 2015); 11 to 21 years, mean not reported (Rostami 2016); 10 to 19 years, mean 13.91 (Sharma 2017); 11 to 17, mean 14.29 (Szigethy 2007); nine to 17 years, mean 14.3 years (Szigethy 2014); seven to 18 years, mean not reported (Van der Veek 2013); eight to 18 years, mean 13.0 years (Van Dijk Lokkart 2016); five to 13 years, mean 8.3 years (Varni 1993); and 11 to 16 years, mean not reported (Wei 2017).
The ethnicity of participants varied across trials. Individual breakdowns were as follows (ethnicity terms provided are those used in individual trial reports): 100% black South Africans of Zulu descent (Bhana 2014); 100% African‐American (Bignall 2015); 64% white, 32% Hispanic, 4% black (Chadi 2016); 100% Chinese (Chiang 2009); 87% white, 11% black/Hispanic (Grey 1998); 93% white, 7% black/Hispanic (Grey 2009); 87% white, 7% African‐American, 7% Asian‐American (Hains 2000); 31% white, 16% black, 6% American‐Indian/Alaskan native, 3% Asian, 44% Hispanic (Hickman 2015); 93% Caucasian, 7% African‐American (Kashikar‐Zuck 2005); 90% white, 6% black/black African, 2% American‐Indian/Alaskan, 1% Asian, 1% other (Kashikar‐Zuck 2012); 96% Caucasian, 4% other (Levy 2010); 49% Arab, 34% Lor, 17% Persian (Rostami 2016); 78% white, 15% African‐American, 2% Latina, 5% other (Szigethy 2007); 89% white, 11% other (Szigethy 2014); 48% white, 36% Hispanic, 9% Asian, 5% black, 2% American‐Indian (Varni 1993). Thirteen trials did not specify the ethnicities of their participants (Beebe 2010; Bussone 1998; Detling Miller 2008; Freedenberg 2017; Griffiths 1996; Kanstrup 2016; Li 2016; Martinović 2006; Moghanloo 2015; Sharma 2017; Van der Veek 2013; Van Dijk Lokkart 2016; Wei 2017).
Trial participants had a range of long‐term physical conditions, including chronic pain (Chadi 2016; Kanstrup 2016), abdominal pain (Levy 2010; Van der Veek 2013), headaches (Bussone 1998; Griffiths 1996; Hickman 2015; Sharma 2017), fibromyalgia (Kashikar‐Zuck 2005; Kashikar‐Zuck 2012), diabetes mellitus (Detling Miller 2008;Grey 1998; Grey 2009; Hains 2000; Moghanloo 2015; Rostami 2016; Wei 2017), inflammatory bowel disease (Szigethy 2007; Szigethy 2014), asthma (Beebe 2010; Bignall 2015; Chiang 2009), cancer (Van Dijk Lokkart 2016; Varni 1993), cardiac disease (Freedenberg 2017), epilepsy (Li 2016; Martinović 2006), and human immunodeficiency virus (HIV) infection (Bhana 2014). All trials were undertaken with outpatient or community samples. No trials used an inpatient population.
Depression was measured in twenty‐one trials (Beebe 2010; Bhana 2014; Chadi 2016; Freedenberg 2017; Grey 1998; Grey 2009; Griffiths 1996; Hickman 2015; Kanstrup 2016; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Levy 2010; Li 2016; Martinović 2006; Moghanloo 2015; Rostami 2016; Szigethy 2007; Szigethy 2014; Van der Veek 2013; Varni 1993; Wei 2017) and anxiety was measured in fifteen trials (Beebe 2010; Bignall 2015; Bussone 1998; Chadi 2016; Chiang 2009; Detling Miller 2008; Griffiths 1996; Hains 2000; Hickman 2015; Li 2016; Rostami 2016; Sharma 2017; Van Dijk Lokkart 2016; Varni 1993; Wei 2017).
Participants had severe baseline levels of depression in two trials (Hickman 2015; Li 2016), a moderate level of depression in one trial (Szigethy 2007), mild levels of depression in five trials (Kanstrup 2016; Martinović 2006; Moghanloo 2015; Rostami 2016; Szigethy 2014) and subthreshold levels of depression in eight trials (Bhana 2014; Freedenberg 2017; Grey 1998; Grey 2009; Kashikar‐Zuck 2005; Levy 2010; Van der Veek 2013; Varni 1993). Baseline levels of depression were not reported in three trials (Beebe 2010; Chadi 2016; Van Dijk Lokkart 2016) and were unclear due to the type of scales used in three trials (Griffiths 1996; Kashikar‐Zuck 2012; Wei 2017). Participants had severe baseline levels of anxiety in two trials (Bignall 2015; Hickman 2015), a moderate to severe level of anxiety in one trial (Sharma 2017), a mild to moderate level of anxiety in one trial (Varni 1993), mild levels of anxiety in four trials (Bussone 1998; Hains 2000; Li 2016; Rostami 2016), and subthreshold levels of anxiety in two trials (Freedenberg 2017; Van der Veek 2013). Baseline levels of anxiety were not reported in three trials (Beebe 2010; Chadi 2016; Van Dijk Lokkart 2016) and were unclear due to the type of scales used in four trials (Chiang 2009; Detling Miller 2008; Griffiths 1996; Wei 2017).
Severity of the long‐term physical condition was measured in a variety of ways and likely reflected the diverse range of clinical problems and study methodologies. For asthma, severity was rated as “persistent and requiring daily treatment” (Beebe 2010); by Asthma Control Test (ACT) score of 20 or less (Bignall 2015); and by medication use, asthma signs and symptoms, Peak Expiratory Flow Rate (PEFR), and by being rated as moderate to severe on the Asthma APGAR Score (AAS) (Chiang 2009). For pain, a severity rating was frequently used (Chadi 2016;Kanstrup 2016; Kashikar‐Zuck 2005; Levy 2010); other trials measured symptom frequency (Bussone 1998; Griffiths 1996; Levy 2010); pain‐related or symptom‐related disability (Kanstrup 2016; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Levy 2010), mean Children’s Somatization Inventory rating (Levy 2010); medication use (Griffiths 1996), mean Headache Impact Test (HIT) rating (Sharma 2017), and headache‐related disability and pain interference (Hickman 2015;). In diabetes, HbA1c levels were used (Detling Miller 2008; Grey 1998; Grey 2009; Hains 2000; Rostami 2016; Wei 2017) and fasting blood sugar (FBS) (Rostami 2016). For neurological conditions, both Li 2016 and Martinović 2006 measured seizure frequency. In the gastrointestinal (GI) group, disease severity was measured by the Paediatric Chron’s Disease Activity Index (PCDAI) (Szigethy 2007; Szigethy 2014) and the Clinical Score of Kozarek (Szigethy 2007; Szigethy 2014); the percentage of participants taking medication (Szigethy 2007); or the mean Abdominal Pain Index (API) (Van der Veek 2013). Six trials did not report a severity measure (Bhana 2014; Detling Miller 2008; Freedenberg 2017; Moghanloo 2015; Van Dijk Lokkart 2016; Varni 1993).
The inclusion criteria varied across the trials due to the diverse nature of the trials included. The most commonly cited inclusion criteria included participants receiving stable treatment (Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Li 2016; Moghanloo 2015; Sharma 2017); the ability to communicate in the language of the study (Bignall 2015; Van der Veek 2013); and being free from 'other' health problems (Grey 1998; Grey 2009). Two trials did not specify any inclusion criteria (Detling Miller 2008; Griffiths 1996). See Characteristics of included studies for details of individual trials.
There was more consistency in exclusion criteria. A number of trials excluded participants with previously diagnosed or moderate to severe mental health problems such as depression, panic disorder, psychosis, or substance abuse (Hickman 2015; Kanstrup 2016; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Li 2016; Martinović 2006; Moghanloo 2015; Szigethy 2007; Szigethy 2014; Wei 2017). Some also excluded participants with comorbid medical conditions or underlying pathology that could influence the presenting problem (Chadi 2016; Hickman 2015; Kanstrup 2016; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Levy 2010; Li 2016; Martinović 2006; Moghanloo 2015; Wei 2017). Seven trials excluded participants with cognitive or communication difficulties including not being able to speak or communicate in the language of the trial (Chadi 2016; Freedenberg 2017; Kanstrup 2016; Levy 2010; Rostami 2016; Szigethy 2007; Van Dijk Lokkart 2016). Other trials excluded participants using medication (Kashikar‐Zuck 2012; Li 2016; Moghanloo 2015; Szigethy 2007; Szigethy 2014), receiving or requiring ongoing or changing treatment relative to the clinical target (Bussone 1998; Kanstrup 2016; Moghanloo 2015; Rostami 2016; Szigethy 2007; Szigethy 2014; Van Dijk Lokkart 2016), living too far from the treatment provider (Chadi 2016), experiencing suicidal ideation or behaviours (Chadi 2016; Kanstrup 2016; Szigethy 2007), participants with intellectual disability or developmental delay (Chadi 2016; Kanstrup 2016; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Levy 2010; Li 2016; Martinović 2006; Van Dijk Lokkart 2016), who had previously participated in a clinical trial (Chiang 2009), had lack of access to technology relevant to the trial (Freedenberg 2017), known child protection issues (Wei 2017) or mobility issues (Van Dijk Lokkart 2016). Nine trials did not specify any exclusion criteria (Beebe 2010; Bhana 2014; Detling Miller 2008; Grey 1998; Grey 2009; Griffiths 1996; Sharma 2017; Van der Veek 2013; Varni 1993).
Baseline differences between groups
Around two‐third of trials (n = 14) reported that there were no differences between the groups at baseline (Bignall 2015; Chiang 2009; Grey 1998; Hickman 2015; Kanstrup 2016; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Li 2016;Martinović 2006; Moghanloo 2015; Rostami 2016; Szigethy 2007; Varni 1993; Wei 2017). One trial implied that there were differences at baseline between the groups but did not specify this further (Van Dijk Lokkart 2016). Bhana 2014 reported that the intervention group had a greater proportion of participants that received child support grants, while Freedenberg 2017 and Van der Veek 2013 found that the intervention group had higher baseline levels of anxiety. Grey 2009 found that the intervention group was more likely to be of white ethnicity and mothers had higher levels of education. Similarly, Hains 2000 found differences in ethnicity between the groups. Levy 2010 found that participants in the intervention group had greater levels of parent‐rated pain while those in the control group had greater levels of pain minimisation coping skills. Sharma 2017 found that participants in the treatment group were less likely to be using medication at baseline than the control group. Three baseline group differences were found by Szigethy 2014; ethnicity (CBT 94.6%, Supported Nondirective Therapy; SNDT 84.1%); surgical resection rate (CBT 5.6%, SNDT 14.2%); and raw mean baseline Childrens Depression Rating Scale‐Revised (CDRS‐R) scores (CBT 45.1, SNDT 48.9). Four trials did not report if there were any group differences at baseline (Beebe 2010; Bussone 1998; Detling Miller 2008; Griffiths 1996).
Interventions
Nine trials (Griffiths 1996; Hickman 2015; Kashikar‐Zuck 2012; Levy 2010; Martinović 2006; Sharma 2017; Szigethy 2007; Szigethy 2014; Van der Veek 2013) evaluated cognitive behaviour therapy (CBT), three trials (Grey 1998; Grey 2009; Kashikar‐Zuck 2005) evaluated coping skills training, one trial (Beebe 2010) evaluated art therapy, one trial (Bhana 2014) evaluated 'VUKA' a culturally tailored cartoon storyline and curriculum, one trial (Bignall 2015) evaluated a breathing retraining and standard asthma education programme, one trial (Bussone 1998) evaluated a biofeedback training relaxation group, one trial (Chadi 2016) evaluated a mindfulness‐based intervention, one trial (Chiang 2009) evaluated self‐management and relaxation‐breathing training, one trial (Detling Miller 2008) evaluated anxiety‐reduction with deep breathing and progressive muscle relaxation exercises, one trial (Freedenberg 2017) evaluated Mindfulness‐Based Stress Reduction, one trial (Hains 2000) evaluated a stress‐management training intervention, one trial (Kanstrup 2016) evaluated a group‐based acceptance and commitment therapy, one trial (Li 2016) evaluated systemic family therapy with antiepileptic drugs, one trial (Moghanloo 2015) evaluated acceptance and commitment therapy, one trial (Rostami 2016) evaluated education and emotional support, one trial (Van Dijk Lokkart 2016) evaluated psychosocial training and a physical activity programme, one trial (Varni 1993) evaluated social skills training, and one trial (Wei 2017) evaluated nondirective behavioural counselling.
Components of CBT‐based therapies included psychoeducation, progressive muscle relaxation and breathing exercises, cognitive monitoring and thought challenging, autogenic relaxation, mental imagery, cued relaxation, relapse prevention, and homework to practise learnt skills. Some therapies added working with parents and children to modify family responses to illness and wellness behaviours (Levy 2010) and specific components to educate children to improve cognitions and behaviours related to medical conditions such as inflammatory bowel disease (IBD) (Szigethy 2007; Szigethy 2014). Components of coping skills training included role‐play of social situations, social problem‐solving, recognition of associations between thoughts, feelings, and behaviour and guided self‐dialogue, stress management, conflict resolution around illness‐specific stressors, and sometimes homework to practise learnt techniques (Grey 1998; Grey 2009; Kashikar‐Zuck 2005). Components of other interventions were more variable; art therapy Beebe 2010) consisted of an opening activity, discussion of a weekly topic and art intervention, art making, an opportunity for the participants to share their feelings related to the art they created, and a closing activity. An HIV education programme (Bhana 2014) consisted of ten steps covering AIDS‐related loss and bereavement, HIV transmission and treatment knowledge, disclosure of HIV status to others, youth identity, acceptance and coping with HIV, adherence to medical treatment, stigma and discrimination, caregiver‐child communication, particularly on sensitive topics such as puberty and HIV, puberty, identifying and developing strategies to keep children safe in high‐risk situations where sexual behaviour and drug use were possible, and social support. A relaxation therapy protocol for children with asthma (Bignall 2015) consisted of diaphragmatic breathing, asthma‐specific guided imagery and progressive muscle relaxation. A biofeedback‐assisted relaxation therapy (Bussone 1998) consisted of relaxation exercises for eight muscle groups (lower arms, upper arms, legs, abdomen, chest, shoulders, eyes, and forehead) associated with auditory feedback and a period of self‐control (the feedback signal was turned off, while subject was instructed to continue attempting to relax). Two mindfulness‐based therapies included an eight‐week mindfulness programme adapted for adolescent concerns and preferences (Chadi 2016) and combination of mindfulness techniques to alleviate symptoms, and cognitive restructuring to look at stressors in a different way (Freedenberg 2017). A self‐management and relaxation training programme (Chiang 2009) consisted of a mixture of deep breathing, progressive muscle relaxation and biofeedback. An anxiety management programme for adolescents with diabetes (Detling Miller 2008) included deep breathing and progressive muscle relaxation. A stress‐management training intervention (Hains 2000) involved education about stress, cognitive restructuring and problem‐solving skills as well as application of these skills. Two trials of acceptance and commitment therapy involved group‐based pain education and discussion about pain and symptoms in relation to values‐oriented behavioural activation (Kanstrup 2016) and a combination of values clarification, building a commitment, seeing control as the problem, seeking alternatives to control (willing to see other possibilities, acceptance), cognitive diffusion, viewing self as context, acceptance and commitment, internal dialogue, and relapse prevention (Moghanloo 2015). A systemic family therapy intervention (Li 2016) included investigating differences and interactions of family behaviours, positively assigning meaning, orientating resources, investigating the dynamics of changes, investigating the targets of changes, and promoting the changes. An education and emotional support therapy (Rostami 2016) consisted of information about diabetes and its complications; causes; methods of care and self‐care; blood sugar control; emotional support; and general lifestyle advice. A psychosocial training and physical activity programme (Van Dijk Lokkart 2016) consisted of psychoeducation and cognitive behavioural strategies to improve emotional and social coping, as well as a combination of cardiorespiratory and strength exercises. A social skills training intervention (Varni 1993) consisted of social cognitive problem‐solving, assertiveness training, handling teasing and name calling. Finally, a nondirective behavioural counselling programme (Wei 2017) consisted of a client‐centred approach in which adolescents were provided time to express any issues or concerns.
Eleven therapies included modules for both children and parents, while sixteen included modules only for children. The duration of interventions varied between the studies as follows: seven modules of one hour per week (Beebe 2010), six modules over three months (Bhana 2014), two modules of 30 minutes each (Bignall 2015), ten modules of 20 minutes each plus four follow‐up sessions (Bussone 1998), eight sessions (Chadi 2016), a single session with 30 minutes of relaxation training followed by twelve weeks of practice at least three times per week for 30 minutes (Chiang 2009), a single session and weekly telephone follow‐up over six weeks (Detling Miller 2008), six modules (Freedenberg 2017), eight group sessions, daily self‐management sessions, monthly outpatient sessions (Grey 1998), six modules (Grey 2009), eight modules (Griffiths 1996), six modules (Hains 2000), three office sessions of 30 minutes and four telephone sessions of 20 minutes over seven weeks (Hickman 2015), eighteen sessions of two hours each, including a minimum of ten adolescent sessions and four joint (child and parent) sessions (Kanstrup 2016), six sessions of one hour and two telephone follow‐ups toward the end of eight weeks (Kashikar‐Zuck 2005), eight sessions of 45 minutes each over eight weeks (Kashikar‐Zuck 2012), three sessions over two weeks (Levy 2010), six sessions (Li 2016), eight sessions over eight weeks and four further sessions over four weeks (Martinović 2006), ten sessions (Moghanloo 2015), eight sessions of two hours each over eight weeks (Rostami 2016), twelve modules (Sharma 2017), nine to eleven sessions of 60 minutes each (Szigethy 2007), up to 12 sessions over three months (nine key sessions with three sessions of flexible content) plus three parent sessions (Szigethy 2014), six sessions of 45 minutes each (Van der Veek 2013), two 45‐minute sessions of physical training per week and six 60‐minute sessions of child psychotherapy, two sessions of 60 minutes each of parent psychotherapy (Van Dijk Lokkart 2016), three sessions and two boosters at three and six weeks following the return to school (Varni 1993), and six weekly sessions with follow‐up at six and twelve months (Wei 2017). All therapies included some form of homework, usually behavioural assignments, relaxation training and practice, telephone calls, physical therapy, and psychotherapy. Most therapies were delivered in a predetermined format, with a set number of sessions over a specific time period. One trial of CBT (Levy 2010) allowed flexibility for sessions to be delivered by telephone (which was chosen by participants almost a third of the time). Two trials of primary and secondary control enhancement therapy ‐ physical illness (PASCET‐PI) (Szigethy 2007; Szigethy 2014) allowed for sessions to be flexibility delivered in accordance with visits for medical treatment and by telephone.
Nine trials used treatment‐as‐usual (TAU) as the control therapy. In the first (Bignall 2015), TAU included education focused on the pathophysiology of asthma, standard symptom management techniques (e.g. rescue medication use and avoiding triggers), and basic principles of the mind‐body connection as it relates to asthma (e.g. how thoughts and emotions influence medication use). Participants were reminded to stay on their medication regimen based on their providers’ instructions. Educational handouts reviewing the information discussed were provided. In the second (Chiang 2009), TAU included an education program with five units: (a) reforming asthma cognition, (b) correct usage of asthma drugs, (c) establishing a safe home environment, (d) monitoring with a peak flow meter, and (e) keeping an asthma diary. An educational booklet that included personal care plans, pages to allow recording of peak flowmeter levels (with one meter given to each family), and a diary to record asthma signs/symptoms was provided. Any kind of psychological therapy was allowed over the same duration as the primary intervention. In the third (Li 2016), TAU included only medication with no additional clinic visits. In the fourth (Rostami 2016), TAU included an undefined education group. In the fifth (Sharma 2017), TAU consisted of pharmacotherapy without any active intervention from the researcher. In the sixth and seventh (Szigethy 2007), TAU included routine care and the provision of an information sheet for parents about depression. In the eighth (Van Dijk Lokkart 2016), TAU varied between hospitals according to local guidelines, but did not include any routine exercise or psychosocial training. In the ninth (Varni 1993), TAU included a minimum of two hours individual attention as part of a school reintegration programme and equal time with a research assistant via five sessions of play interaction. Six trials (Beebe 2010; Bhana 2014; Chadi 2016; Detling Miller 2008; Hains 2000; Moghanloo 2015) used a waiting‐list control. Five trials used active control therapies as follows: placebo relaxation group in order to control for attention, expectations for improvement, and effects from sitting quietly for an extended period (Bussone 1998), online video support group (Freedenberg 2017), headache education about surrounding lifestyle triggers of headaches. e.g. environmental headache triggers, medication triggers of headaches, hormonal headache triggers, dietary triggers of headaches, headache management tips, and the importance of hydration (Hickman 2015) ; psychoeducation focused on education about gastrointestinal system anatomy and function, nutrition guidelines and reading of food product labels (Levy 2010) and intensive medical care (Van der Veek 2013). Four trials used psychological placebo as control therapies as follows: self‐monitoring with weekly diaries for eight weeks in which the participants merely recorded their average pain level for each day (on a 0 to 10 VAS), sleep quality for each night on a 1 to 3 scale (good, not so good, bad), and their pain medication (Kashikar‐Zuck 2005), education about fibromyalgia, pain medications, general lifestyle issues such as diet, sleep, and exercise, and the impact of juvenile fibromyalgia syndrome on the participant's lifestyle (Kashikar‐Zuck 2012), supportive psychotherapy (Martinović 2006) and supported nondirective therapy during which children were encouraged to talk about whatever was bothering them (Szigethy 2014). One trial used a non‐psychological placebo as control therapy (Grey 1998). This involved intensive diabetes management with administration of three or more daily insulin injections or an external insulin pump, self‐monitoring of blood glucose at least four times daily, monthly outpatient visits, and interim telephone contacts.
All trials allowed participants to use adjunctive pharmacologic and nonpharmacologic therapies to manage long‐term physical conditions. Only one trial (Chadi 2016) included participants taking serotonin‐specific reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs) that could have impacted on symptoms of anxiety and depression.
Outcomes
Primary outcomes
Treatment efficacy was evaluated using validated scales that measured changes in the severity of symptoms of either depression or anxiety.
Changes in the severity of depression symptoms were measured using the Beck Youth Depression Inventory (BYDI) scale in three trials (Beebe 2010; Chadi 2016; Hickman 2015), Children's Depression Inventory (CDI) scale in ten trials (Bhana 2014; Grey 1998; Grey 2009; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Levy 2010; Szigethy 2007; Szigethy 2014; Van Dijk Lokkart 2016; Varni 1993), Beck Depression Inventory (BDI) scale in two trials (Martinović 2006; Rostami 2016), Children's Depression Scale (CDS) in one trial (Griffiths 1996), Hospital Anxiety And Depression Scale (HADS) in one trial (Freedenberg 2017), CESD‐C scale in one trial (Kanstrup 2016), Hamilton Depression Scale (HAMD) in one trial (Li 2016), Reynolds' Child Depression Rating Scale (RCDS) in one trial (Moghanloo 2015), Revised Child Anxiety and Depression Scale in one trial (Van der Veek 2013) and the depression subscale of the Wellbeing Questionnaire in one trial (Wei 2017).
Changes in the severity of anxiety symptoms were measured using the Beck Youth Anxiety Inventory (BYAI) scale in three trials (Beebe 2010; Chadi 2016; Hickman 2015), State Trait Anxiety Inventory (STAI) scale in six trials (Bignall 2015; Bussone 1998; Detling Miller 2008; Hains 2000; Sharma 2017; Varni 1993), Chinese Children’s Anxiety scale (CCAS) in one trial (Chiang 2009), HADS in one trial (Freedenberg 2017), Children's Manifest Anxiety Scale in one trial (Griffiths 1996), Hamilton Anxiety Scale (HAMA) scale in one trial (Li 2016), Beck Anxiety Inventory scale in one trial (Rostami 2016), Revised Child Anxiety and Depression scale in one trial (Van der Veek 2013), and the anxiety subscale of the Wellbeing Questionnaire in one trial (Wei 2017).
Treatment acceptability was not measured by any trials using validated scales. Therefore, it was assessed according to the other criteria stipulated in the methods section.
Secondary outcomes Changes in 'caseness' (remission/response) of anxiety or depression were not reported by any of the included trials. Neither was suicide‐related behaviour, defined as a) the number of deaths by suicide, b) suicide attempts, and c) episodes of self‐harm, either reported or measured using validated scales (e.g. SBQ‐R, Osman 2001).
Improvement in quality of life following intervention was measured in nine trials using the PedsQL Asthma Module (Beebe 2010), PedsQL (Bignall 2015), PedsQL 4.0 (Chadi 2016, van Dijk Lokkart 2016), Diabetes Quality of Life: Youth (DQOLY) (Grey 1998; Grey 2009), PedsQL Emotional functioning subscale (Kashikar‐Zuck 2012), Quality of Life in Epilepsy Inventory (QOLIE‐31) (Martinović 2006) and KIDSCREEN‐27 (van der Veek 2013).
Functioning, as a proxy for psychological well‐being, was measured using the Functional Disability Inventory (FDI) in four trials (Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Levy 2010; Van der Veek 2013), Functional Disability Index in one trial (Kanstrup 2016) and Children's Global Assessment Scale (CGAS) in two trials (Sharma 2017, Szigethy 2007).
The status of the long‐term physical condition was measured using HbA1c (haemoglobin A1c or glycated haemoglobin test) in four trials (Grey 1998; Grey 2009, Hains 2000; Rostami 2016), total Pain Index in one trial (Bussone 1998), peak expiratory flow rate (PEFR) in one trial (Chiang 2009), Pain Interference Index in one trial (Kanstrup 2016), Visual Analogue Scale in one trial (Kashikar‐Zuck 2005), pain severity (measured using Visual Analogue Scale) in one trial (Kashikar‐Zuck 2012), GI Symptom subscale, Children's Somatization Inventory in one trial (Levy 2010), seizure frequency in one trial (Li 2016), Headache Impact Test in one trial (Sharma 2017), PCDAI (Pediatric Crohn's Disease Activity Index Calculator) or PUCAI (Paediatric Ulcerative Colitis Activity Index) in one trial (Szigethy 2014) and Abdominal Pain Index (API) in one trial (Van der Veek 2013).
Adherence to treatment of the long‐term physical condition was not assessed by any of the trials. Neither were school or college attendance (e.g. reduction in number of days missed) or economic benefits (e.g. reduced cost of treatment, number of appointments with general practitioner, use of additional treatments, ability to trial or work).
Excluded studies
Thirty two trials were excluded from the review. Ten trials were excluded because there was no control group (Belsky 1994; Chalder 2002; Garcia Perez 2010; Gauntlett Gilbert 2013; Gulewitsch 2012; Hesse 2015; Long 2011; Malboeuf Hurtubise 2016; OsterhausSo 1993; Riley 2015). Eight trials were excluded due to the study population being adult (Barsevick 2002; BrownLk 2016; Grover 2002; Naar King 2010; Pless 1994; Ribeiro 2008; Stapersma 2018; Yorke 2017) and one as the patient population was hospitalised (Stubbe 2008). Four trials were excluded because the intervention did not met the criteria for the review (Chernoff 2002; Lemanek 2009; Lyon 2014; Shoshani 2016), and nine were excluded because changes in anxiety and depression were not measured (AmbrosinoJm 2008; Bauman 1994; Chalder 2004. Gebert 1998; Groß 2013; Jeppesen 2012; Saedi 2012; Scholten 2013; Westrupp 2015). For further details of these trials, please refer to the table of Characteristics of excluded studies.
Ongoing studies
There were no ongoing studies included in this review.
Studies awaiting classification
A total of four trials are awaiting classification. Insufficient data was available for Chadi 2016a; Coupey 1991; Hood 2014; Yang H 2004 and unfortunately no further information was obtained despite contacting the authors. See Characteristics of studies awaiting classification.
Risk of bias in included studies
For details of the 'Risk of bias' judgements for each trial using GRADE criteria, see Characteristics of included studies. A graphical representation of the overall risk of bias in included trials is presented in Figure 2 and Figure 3.
2.
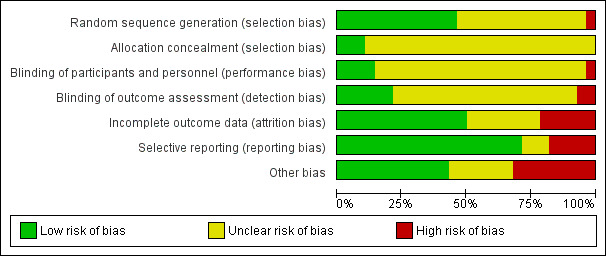
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
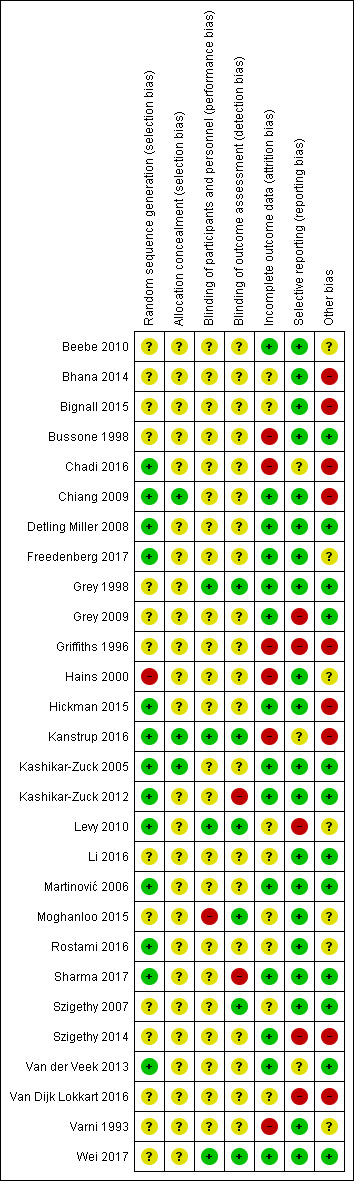
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The risk of bias due to random sequence generation was rated as unclear for 15 trials, low for 13 trials and high for one trial (Hains 2000). In this last trial, the method of randomisation was not described and one participant from the intervention group reportedly swapped with a participant from the control group due to parental scheduling. The risk of bias due to allocation concealment was considered unclear in 26 trials, and low in three trials (Chiang 2009; Kanstrup 2016; Kashikar‐Zuck 2005) with none rated as high risk of bias.
Blinding
The risk of bias due to blinding of participants and research assistants was rated unclear for 23 trials, low for four trials (Grey 1998; Kanstrup 2016; Levy 2010; Wei 2017) and high for one trial (Moghanloo 2015). In this last trial, participants were not blinded to treatment group and it would have been obvious if they were or were not receiving the intervention. It was also unclear whether personnel were blinded. The risk of bias due to blinding of outcome assessors was rated as unclear in 21 trials, low in 5 trials (Grey 1998; Kanstrup 2016; Levy 2010; Moghanloo 2015; Wei 2017), and high in two trials (Kashikar‐Zuck 2012; Sharma 2017).
Incomplete outcome data
The risk of bias due to incomplete outcome data was rated as low in 14 trials and unclear in nine trials. In six trials the risk of bias was rated as high (Bussone 1998; Chadi 2016; Griffiths 1996; Hains 2000; Kanstrup 2016; Varni 1993).
There were no dropouts recorded during the following trials (Beebe 2010; Chadi 2016; Grey 1998; Li 2016; Martinović 2006; Rostami 2016; Sharma 2017; Van der Veek 2013). Rates of dropouts between randomisation and completion of other trials were as follows: both groups 6/65 (individual losses not specified) (Bhana 2014), intervention group 2/18 versus control group 1/15 (Bignall 2015), intervention group 0/20 versus control group 5/15 (Bussone 1998), intervention group 7/29 versus control group 7/33 (Chiang 2009), both groups 10/36 (individual losses not specified) (Detling Miller 2008), intervention group 4/30 versus control group 2/22 (Freedenberg 2017), intervention group 12/65 versus control group 17/46 (Grey 2009), intervention group 2/17 versus control group 5/17 (Griffiths 1996), intervention group 0/8 versus control group 1/7 (Hains 2000), intervention group 2/18 versus control group 2/18 (Hickman 2015), intervention group 4/24 versus control group 12/24 (Kanstrup 2016), intervention group 1/15 versus control group 2/15 (Kashikar‐Zuck 2005) intervention group 7/57 versus control group 7/57 (Kashikar‐Zuck 2012), intervention group 13/100 versus control group 11/100 (Levy 2010), intervention group 3/20 versus control group 3/20 (Moghanloo 2015), intervention group 1/22 versus control group 0/19 (Szigethy 2007), intervention group 20/110 versus control group 19/107 (Szigethy 2014), intervention group 8/30 versus control group 7/38 (Van Dijk Lokkart 2016), both groups (individual losses not specified) 4/64 (Varni 1993), intervention group 3/33 versus control group 2/33 (Wei 2017). Please note that these rates differed from the rates of dropouts between baseline and post‐intervention measurement used in our sensitivity analysis.
Methods of dealing with missing data were: intention to treat analysis in six trials (Chadi 2016; Grey 2009; Kashikar‐Zuck 2005; Szigethy 2007; Szigethy 2014; Van der Veek 2013), multiple imputation in seven trials (Griffiths 1996; Hains 2000; Hickman 2015; Kanstrup 2016; Kashikar‐Zuck 2012; Levy 2010; Wei 2017) and unclear methods in fifteen trials (Beebe 2010; Bhana 2014; Bignall 2015; Bussone 1998; Chiang 2009; Detling Miller 2008; Freedenberg 2017; Grey 1998; Li 2016; Martinović 2006; Rostami 2016; Rostami 2016; Sharma 2017; Van Dijk Lokkart 2016; Varni 1993).
Selective reporting
Five trials were rated at high risk for selective reporting. In one trial (Griffiths 1996), only some data were presented in numerical form while the remainder were presented graphically, making it hard to accurately interpret. In three trials (Grey 2009; Levy 2010; Szigethy 2014), only some of the data were reported. Only one trial (Van Dijk Lokkart 2016) had an available trial protocol with clear deviations from this in the report of the trial results.
Other potential sources of bias
Nine trials (Bhana 2014; Bignall 2015; Chadi 2016; Chiang 2009; Griffiths 1996; Hickman 2015, Kanstrup 2016; Szigethy 2014; Van Dijk Lokkart 2016) were rated at high risk of bias as they were conducted by developers of psychological therapies.
Effects of interventions
See: Table 1
See: Table 1 for the main comparison, psychological interventions compared to any comparator for anxiety and depression in children and adolescents with long‐term physical conditions.
As adequate datasets were only available for some of the primary and secondary outcomes we had planned to assess, meta‐analysis was undertaken for these outcomes (see comparisons 1 to 6 below). Eligible trials reported pre‐intervention and post‐intervention scores using different scales for almost all outcomes, therefore Standardised Mean Differences (SMDs) were used to pool results in accordance with the recommendation of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Of the six trials not included in the meta‐analysis, two trials (Beebe 2010; Chadi 2016) provided change scores rather than absolute values for pre‐intervention and post‐intervention outcomes; two trials (Bhana 2014; Van der Veek 2013) provided pre‐ and post‐intervention scores without standard deviations that were not made available despite contacting the authors; and two trials (Grey 2009; Van Dijk Lokkart 2016) only measured symptoms of depression at baseline. Results from these trials indicated that a family‐based psychosocial intervention to promote mental health among adolescents with HIV infection was better than waiting‐list control at improving adherence to treatment of the long‐term physical condition but not any better than waiting‐list control at improving symptoms of depression (Bhana 2014); art therapy was no better than waiting‐list control at improving symptoms of depression, symptoms of anxiety, or quality of life (Beebe 2010); a mindfulness‐based intervention for adolescents with chronic pain was no better than waiting‐list control at improving symptoms of depression, symptoms of anxiety, quality of life, or symptoms of long‐term physical condition (Chadi 2016); coping skills training in school‐age children with type 1 diabetes was no better than group education at improving symptoms of depression, quality of life, or symptoms of long‐term physical condition (Grey 2009); CBT was no better than intensive medical treatment for improving symptoms of anxiety, symptoms of depression, functioning, or quality of life (Van der Veek 2013); and a combined physical and psychosocial intervention programme for childhood cancer patients was no better than care‐as‐usual at improving quality of life and symptoms of depression (Van Dijk Lokkart 2016).
There was qualitative, but not quantitative evidence of the acceptability of some psychological therapies. Four trials (Bignall 2015; Chadi 2016; Chiang 2009; Freedenberg 2017) described psychological therapies as being acceptable to children, one trial (Bhana 2014) described psychological therapies as being acceptable to parents and two trials described psychological therapies as being acceptable to both children and parents (Hickman 2015; Van der Veek 2013). Only one trial (Chiang 2009) had a dropout rate of greater than 20% between baseline and immediately post‐intervention (the cut‐off used for our sensitivity analysis). Three trials (Grey 1998; Kashikar‐Zuck 2012; Van der Veek 2013) reported on adverse effects that may have contributed to reduced acceptability of psychological therapies. In the first trial (Van der Veek 2013), no adverse effects were identified by either participants who received CBT or those who received intensive medical care. In the second trial (Grey 1998), rates of severe hypoglycaemia were no greater with coping skills training than with intensive therapy. In the third trial (Kashikar‐Zuck 2012), adverse event rates (asthma flare‐ups, sinus infections, strep throat, gastrointestinal infections, and abdominal pain) were similar between participants who received CBT and fibromyalgia education and none of the adverse events were attributable to psychological therapies.
Comparison 1: Psychological therapies for anxiety or depression versus any comparator
Twenty two trials including 1349 participants (Bignall 2015; Bussone 1998; Chiang 2009; Detling Miller 2008; Freedenberg 2017; Grey 1998; Griffiths 1996; Hains 2000; Hickman 2015; Kanstrup 2016; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Levy 2010; Li 2016; Martinović 2006; Moghanloo 2015; Rostami 2016; Sharma 2017; Szigethy 2007; Szigethy 2014; Varni 1993; Wei 2017) contributed meta‐analysable data to this comparison. See also: Table 1.
Primary outcomes
1.1 Treatment efficacy: depression and anxiety, short‐term and long‐term
There was low‐quality evidence from sixteen trials involving 904 participants (Freedenberg 2017; Grey 1998; Griffiths 1996; Hickman 2015; Kanstrup 2016; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Levy 2010; Li 2016; Martinović 2006; Moghanloo 2015; Rostami 2016; Szigethy 2007; Szigethy 2014; Varni 1993; Wei 2017) that psychological therapies were more effective than any comparator in reducing symptoms of depression immediately post‐intervention (SMD ‐0.31, CI ‐0.59 to ‐0.03, I2 = 79%) (Analysis 1.1, Figure 4). This difference diminished in the long term, with evidence from five trials involving 258 participants (Kashikar‐Zuck 2012; Martinović 2006; Szigethy 2007; Varni 1993; Wei 2017) providing inadequate evidence to demonstrate that psychological therapies were more effective than any comparator in reducing symptoms of depression at three to six‐month follow‐up (SMD ‐0.24, CI ‐0.68 to 0.19, I2 = 62%) (Analysis 1.2). There was inadequate evidence of moderate quality from thirteen trials involving 578 participants (Bignall 2015; Bussone 1998; Chiang 2009; Detling Miller 2008; Freedenberg 2017; Griffiths 1996; Hains 2000; Hickman 2015; Li 2016; Rostami 2016; Sharma 2017; Varni 1993; Wei 2017) to demonstrate that psychological therapies were more effective than any comparator in reducing symptoms of anxiety immediately post‐intervention (SMD ‐0.26, CI ‐0.59 to 0.07, I2 = 72%) (Analysis 1.3, Figure 5). Similarly, it could not be determined from four trials involving 131 participants (Bussone 1998; Hains 2000; Varni 1993; Wei 2017) that psychological therapies were no more effective than any comparator in reducing symptoms of anxiety at three to six‐month follow‐up (SMD ‐0.23, CI ‐0.66 to 0.20, I2 = 24%) (Analysis 1.4). Due to the high degree of heterogeneity of most of these results, they should be interpreted with caution.
1.1. Analysis.
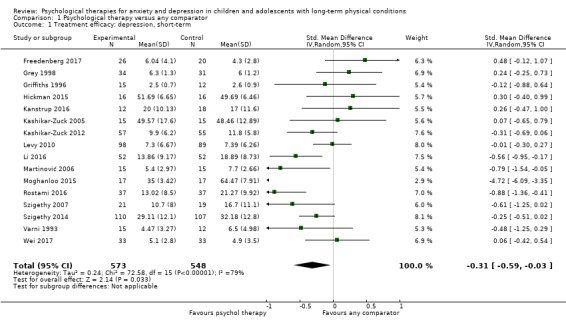
Comparison 1 Psychological therapy versus any comparator, Outcome 1 Treatment efficacy: depression, short‐term.
4.
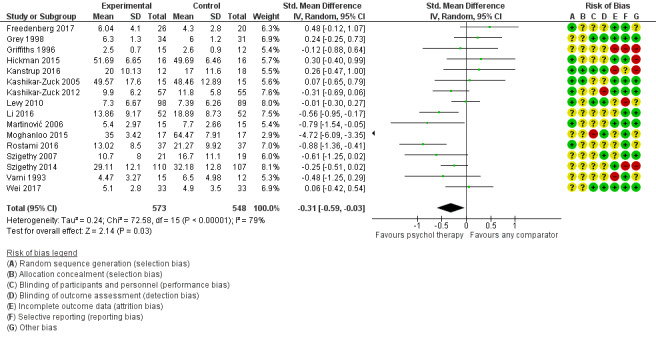
Forest plot of comparison: Psychological therapy versus any comparator, outcome: 1.1 Treatment efficacy: depression short‐term
1.2. Analysis.
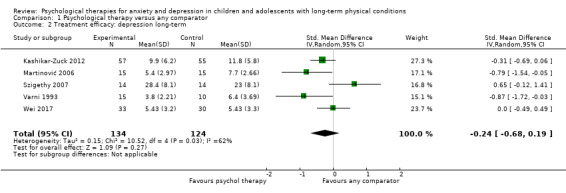
Comparison 1 Psychological therapy versus any comparator, Outcome 2 Treatment efficacy: depression long‐term.
1.3. Analysis.
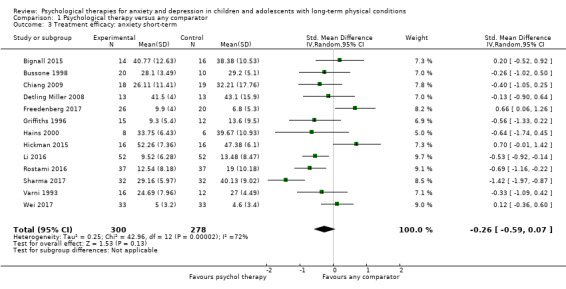
Comparison 1 Psychological therapy versus any comparator, Outcome 3 Treatment efficacy: anxiety short‐term.
5.
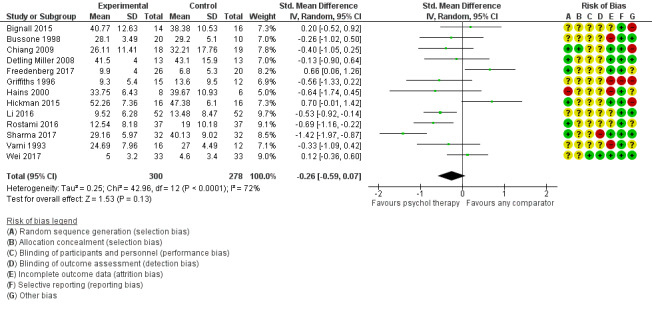
Forest plot of comparison: Psychological therapy versus any comparator, outcome: 1.3 Treatment efficacy: anxiety short‐term
1.4. Analysis.
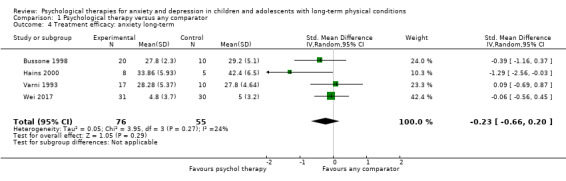
Comparison 1 Psychological therapy versus any comparator, Outcome 4 Treatment efficacy: anxiety long‐term.
1.2 Treatment acceptability
No data were available for this outcome.
Secondary outcomes
1.3 Change in caseness (remission/response)
No data were available for this outcome.
1.4 Suicide‐related behaviour
No data were available for this outcome.
1.5 Quality of life: short‐term and long‐term
There was low‐quality evidence from seven trials involving 464 participants (Beebe 2010; Bignall 2015; Grey 1998; Martinović 2006; Moghanloo 2015; Szigethy 2014; Wei 2017) that psychological therapies were more effective than any comparator in improving quality of life immediately post‐intervention (SMD 1.13, CI 0.44 to1.82, I2 = 89%) (Analysis 1.5, Figure 6). At three to six‐month follow‐up, three of these trials, involving 59 participants, that measured long‐term changes in quality of life (Beebe 2010; Martinović 2006; Wei 2017) did not demonstrate a sustained difference between psychological interventions and any comparator in this domain (SMD 0.71, CI ‐0.52 to 1.93, I2 = 88%]) (Analysis 1.6).
1.5. Analysis.
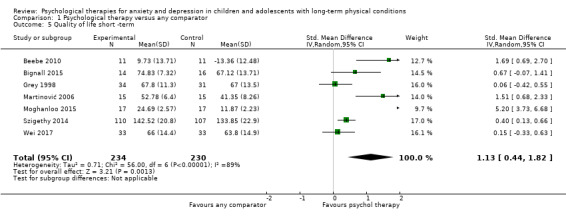
Comparison 1 Psychological therapy versus any comparator, Outcome 5 Quality of life short ‐term.
6.
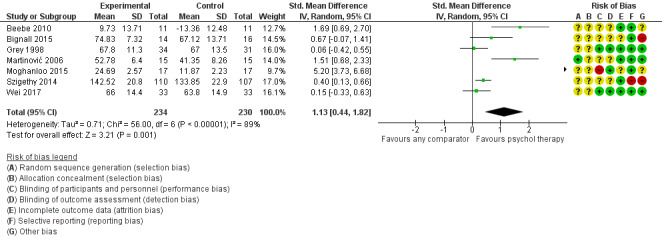
Forest plot of comparison: Psychological therapy versus any comparator, outcome: 1.5 Quality of life short‐term
1.6. Analysis.
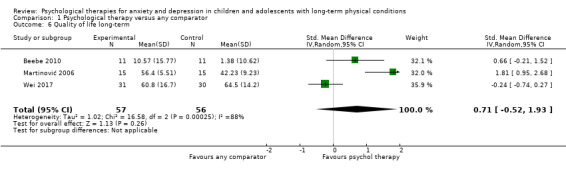
Comparison 1 Psychological therapy versus any comparator, Outcome 6 Quality of life long‐term.
1.6 Functioning: short‐term and long‐term
There was inadequate low quality‐evidence from seven trials involving 483 participants (Hickman 2015; Kanstrup 2016; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Levy 2010; Sharma 2017; Szigethy 2014) to demonstrate that psychological therapies were more effective than any comparator in improving functioning immediately post‐intervention (SMD 0.49, CI ‐0.30 to 1.29, I2 = 93%) (Analysis 1.7, Figure 7). Similarly, two of these trials involving 142 participants (Kashikar‐Zuck 2012; Szigethy 2007) were unable to demonstrate that psychological therapies were more effective than any comparator in improving functioning at three to six‐month follow‐up (SMD ‐0.04, CI ‐0.80 to 0.71, I2 = 71%) (Analysis 1.8).
1.7. Analysis.

Comparison 1 Psychological therapy versus any comparator, Outcome 7 Functioning short‐term.
7.
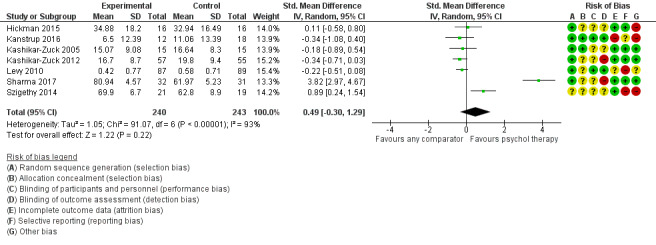
Forest plot of comparison: Psychological therapy versus any comparator, outcome: 1.7 Functioning short‐term
1.8. Analysis.
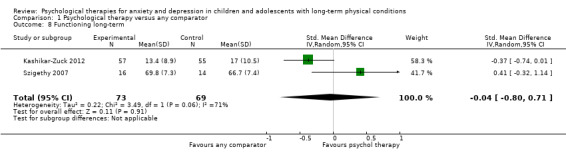
Comparison 1 Psychological therapy versus any comparator, Outcome 8 Functioning long‐term.
1.7 Status of long‐term physical condition: short‐term and long‐term
There was low‐quality evidence from fourteen trials involving 823 participants (Bignall 2015; Bussone 1998; Chiang 2009; Detling Miller 2008; Grey 1998; Hains 2000; Hickman 2015; Kanstrup 2016; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Levy 2010; Li 2016; Rostami 2016; Sharma 2017) that psychological therapies were more effective than any comparator in reducing symptoms of long‐term physical conditions immediately post‐intervention (SMD ‐0.34, CI ‐0.61 to ‐0.06, I2 = 70) (Analysis 1.9, Figure 8). This difference was not sustained according to evidence from two trials involving 142 participants (Bussone 1998; Kashikar‐Zuck 2012) at three to six‐month follow‐up (SMD ‐0.53, CI ‐1.36 to 0.29, I2 = 72%) (Analysis 1.10).
1.9. Analysis.

Comparison 1 Psychological therapy versus any comparator, Outcome 9 Status of long‐term physical condition short‐term.
8.
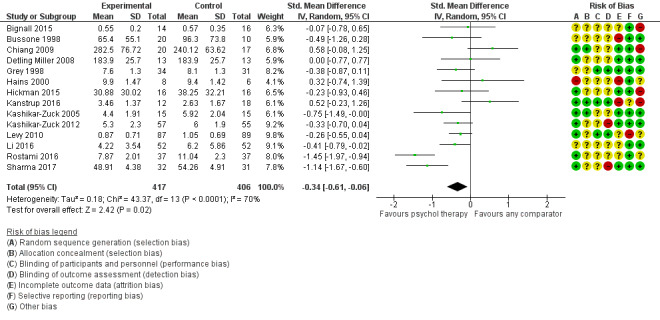
Forest plot of comparison: Psychological therapy versus any comparator, outcome: 1.9 Status of long‐term physical condition short‐term
1.10. Analysis.
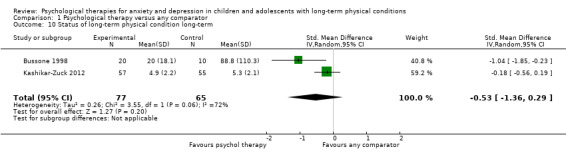
Comparison 1 Psychological therapy versus any comparator, Outcome 10 Status of long‐term physical condition long‐term.
1.8 Adherence to treatment of long‐term physical condition
No data were available for this outcome.
1.9 School/college attendance
No data were available for this outcome.
1.10 Economic benefits
No data were available for this outcome.
Comparison 2: Psychological therapies for anxiety or depression versus attention placebo (AP)
Seven trials with 535 participants (Bussone 1998; Freedenberg 2017; Hickman 2015; Kanstrup 2016; Kashikar‐Zuck 2012; Levy 2010; Wei 2017) contributed data to this comparison.
Primary outcomes
2.1 Treatment efficacy: depression and anxiety, short‐term and long‐term
It could not be determined from six trials involving 473 participants (Freedenberg 2017; Hickman 2015; Kanstrup 2016; Kashikar‐Zuck 2012; Levy 2010; Wei 2017) whether psychological therapies were more effective than attention placebo in reducing symptoms of depression immediately post‐intervention (SMD 0.03, 95% CI ‐0.19 to 0.25; I2 = 23%) (Analysis 2.1). A similar result was obtained from one trial involving 66 participants (Wei 2017) at three to six‐month follow‐up (SMD 0.00, 95% CI ‐0.49 to 0.49; I2 = 0%) (Analysis 2.2). It could not be determined from four trials involving 154 participants (Bussone 1998; Freedenberg 2017; Hickman 2015; Wei 2017) whether psychological therapies were more effective than attention placebo in reducing symptoms of anxiety immediately post‐intervention (SMD 0.31, 95% CI ‐0.10 to 0.73, I2 = 43%) (Analysis 2.3). A similar result was obtained from two trials involving 91 participants (Bussone 1998; Wei 2017) at three to six‐month follow‐up (SMD ‐0.16, ‐0.58 to 0.26, I2 = 0%) (Analysis 2.4)
2.1. Analysis.
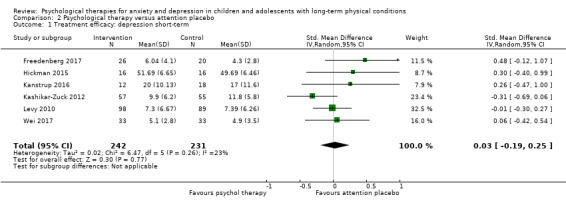
Comparison 2 Psychological therapy versus attention placebo, Outcome 1 Treatment efficacy: depression short‐term.
2.2. Analysis.
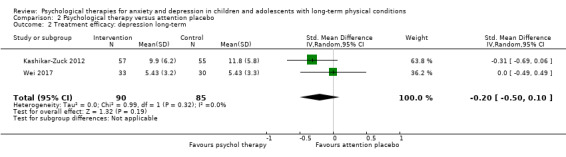
Comparison 2 Psychological therapy versus attention placebo, Outcome 2 Treatment efficacy: depression long‐term.
2.3. Analysis.
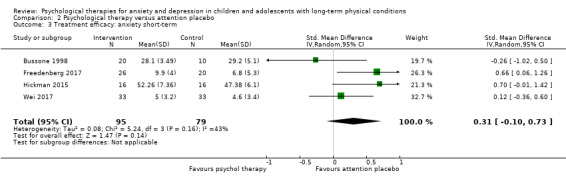
Comparison 2 Psychological therapy versus attention placebo, Outcome 3 Treatment efficacy: anxiety short‐term.
2.4. Analysis.
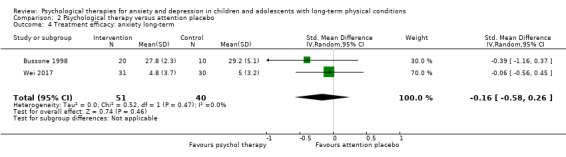
Comparison 2 Psychological therapy versus attention placebo, Outcome 4 Treatment efficacy: anxiety long‐term.
2.2 Treatment acceptability
No data were available for this outcome.
Secondary outcomes
2.3 Change in caseness (remission/response)
No data were available for this outcome.
2.4 Suicide‐related behaviour
No data were available for this outcome.
2.5 Quality of life: short‐term and long‐term
It could not be determined from one trial involving 66 participants (Wei 2017) whether psychological therapies were more effective than attention placebo in improving quality of life immediately post‐intervention (SMD 0.15, 95% CI ‐0.33 to 0.63; I2 = 0%) (Analysis 2.5), nor at three to six‐month follow‐up (SMD ‐0.24, 95% CI ‐0.74 to 0.27; I2 = 0%) (Analysis 2.6).
2.5. Analysis.
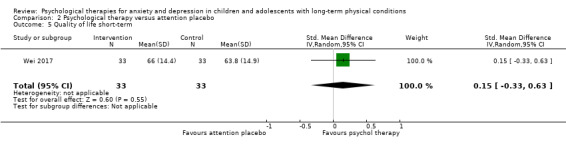
Comparison 2 Psychological therapy versus attention placebo, Outcome 5 Quality of life short‐term.
2.6. Analysis.
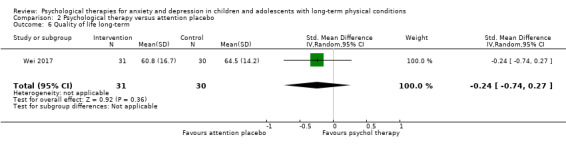
Comparison 2 Psychological therapy versus attention placebo, Outcome 6 Quality of life long‐term.
2.6 Functioning: short‐term and long‐term
It could not be determined from four trials involving 350 participants (Hickman 2015; Kanstrup 2016; Kashikar‐Zuck 2012; Levy 2010) whether psychological therapies were more effective than attention placebo in improving functioning immediately post‐intervention (SMD ‐0.24, 95% CI ‐0.45 to ‐0.02; I2 = 0%) (Analysis 2.7). Nor could it be determined from one trial (Kashikar‐Zuck 2012) whether psychological therapies were more effective than attention placebo in improving functioning at three to six‐month follow‐up (SMD ‐0.37, 95% CI ‐0.74 to 0.01; I2 = 0%) (Analysis 2.8).
2.7. Analysis.
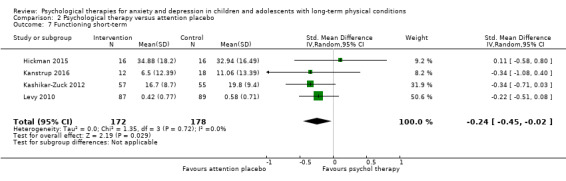
Comparison 2 Psychological therapy versus attention placebo, Outcome 7 Functioning short‐term.
2.8. Analysis.
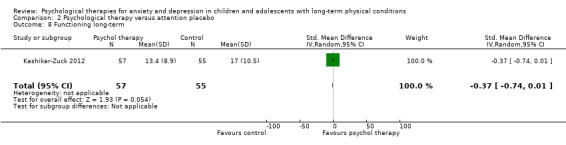
Comparison 2 Psychological therapy versus attention placebo, Outcome 8 Functioning long‐term.
2.7 Status of long‐term physical condition: short‐term and long‐term
It could not be determined from five trials involving 380 participants (Bussone 1998; Hickman 2015; Kanstrup 2016; Kashikar‐Zuck 2012; Levy 2010) whether psychological therapies were more effective than attention placebo in reducing symptoms of long‐term physical conditions immediately post‐intervention (SMD ‐0.23, 95% CI ‐0.46 to 0.01; I2 = 13%) (Analysis 2.9). Similarly, it could not be determined from two trials involving 142 participants (Bussone 1998; Kashikar‐Zuck 2012) that psychological therapies were more effective than attention placebo in reducing symptoms of long‐term physical conditions at three to six‐month follow‐up (SMD ‐0.53, 95% CI ‐1.36 to 0.29; I2 = 72%) (Analysis 2.10).
2.9. Analysis.
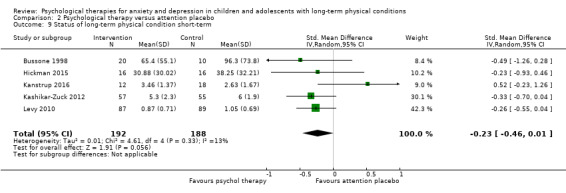
Comparison 2 Psychological therapy versus attention placebo, Outcome 9 Status of long‐term physical condition short‐term.
2.10. Analysis.
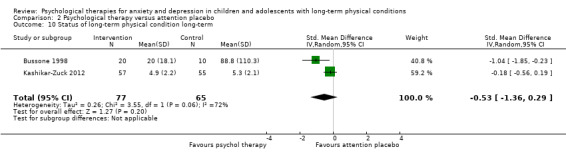
Comparison 2 Psychological therapy versus attention placebo, Outcome 10 Status of long‐term physical condition long‐term.
2.8 Adherence to treatment of long‐term physical condition
No data were available for this outcome.
2.9 School/college attendance
No data were available for this outcome.
2.10 Economic benefits
No data were available for this outcome.
Comparison 3: Psychological therapies for anxiety or depression versus psychological placebo (PP)
Three trials with 277 participants (Kashikar‐Zuck 2005; Martinović 2006; Szigethy 2014) contributed data to this comparison.
Primary outcomes
3.1 Treatment efficacy: depression and anxiety, short‐term and long‐term
It could not be determined from three trials involving 277 participants (Kashikar‐Zuck 2005; Martinović 2006; Szigethy 2014) whether psychological therapies were more effective than psychological placebo in reducing symptoms of depression immediately post‐intervention (SMD ‐0.28, 95% CI ‐0.63 to 0.07; I2 = 28%) (Analysis 3.1). However, at three to six‐month follow‐up, one of these trials involving 30 participants (Martinović 2006) demonstrated an improvement in this domain for those receiving psychological therapy compared with psychological placebo i (SMD ‐0.79, 95% CI ‐1.54 to ‐0.05; I2 = 0%) (Analysis 3.2). None of the trials in this group measured changes in anxiety either immediately post‐intervention or at three to six‐month follow‐up.
3.1. Analysis.
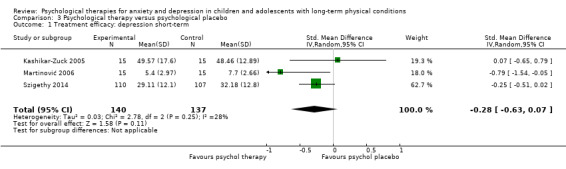
Comparison 3 Psychological therapy versus psychological placebo, Outcome 1 Treatment efficacy: depression short‐term.
3.2. Analysis.
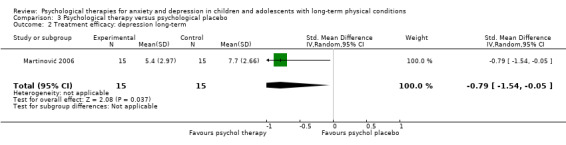
Comparison 3 Psychological therapy versus psychological placebo, Outcome 2 Treatment efficacy: depression long‐term.
3.2 Treatment acceptability
No data were available for this outcome.
Secondary outcomes
3.3 Change in caseness (remission/response)
No data were available for this outcome.
3.4 Suicide‐related behaviour
No data were available for this outcome.
3.5 Quality of life: short‐term and long‐term
It could not be determined from two trials involving 227 participants (Martinović 2006; Szigethy 2014) whether psychological therapies were more effective than psychological placebo in improving quality of life immediately post‐intervention (SMD 0.88 CI ‐0.20 to 1.96, I2 = 84%) (Analysis 3.3). However, at three to six‐month follow‐up, one of these trials involving 30 participants (Martinović 2006) demonstrated an improvement in this domain (SMD 1.81, 95% CI 0.95 to 2.68; I2 = 0%) (Analysis 3.4).
3.3. Analysis.
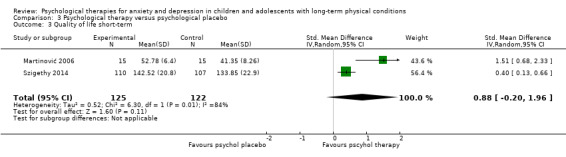
Comparison 3 Psychological therapy versus psychological placebo, Outcome 3 Quality of life short‐term.
3.4. Analysis.
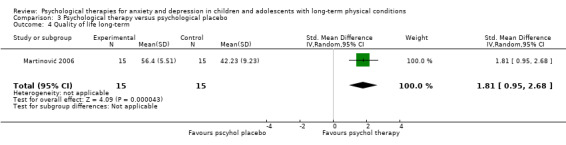
Comparison 3 Psychological therapy versus psychological placebo, Outcome 4 Quality of life long‐term.
3.6 Functioning: short‐term and long‐term
It could not be determined from one trial involving 30 participants (Kashikar‐Zuck 2005) whether psychological therapies were more effective than psychological placebo in improving functioning immediately post‐intervention (SMD ‐0.18, 95% CI ‐0.89 to 0.54; I2 = 0%) (Analysis 3.5). No trials in this group measured functioning at three to six‐month follow‐up.
3.5. Analysis.

Comparison 3 Psychological therapy versus psychological placebo, Outcome 5 Functioning short‐term.
3.7 Status of long‐term physical condition: short‐term and long‐term
One trial involving 30 participants (Kashikar‐Zuck 2005) demonstrated a short‐term improvement in the status of long‐term physical condition for those receiving psychological therapy compared with psychological placebo (SMD ‐0.75, 95% CI ‐1.49 to ‐0.00; I2 = 0%) (Analysis 3.6). No trials in this group measured the status of long‐term physical conditions at three to six‐month follow‐up.
3.6. Analysis.
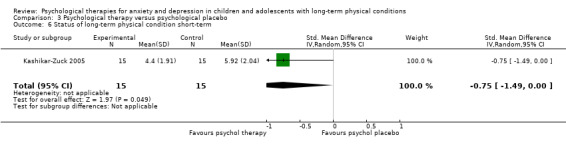
Comparison 3 Psychological therapy versus psychological placebo, Outcome 6 Status of long‐term physical condition short‐term.
3.8 Adherence to treatment of long‐term physical condition
No data were available for this outcome.
3.9 School/college attendance
No data were available for this outcome.
3.10 Economic benefits
No data were available for this outcome.
Comparison 4: Psychological therapies for anxiety or depression versus non‐psychological therapies (NPT)
One trial including 65 participants (Grey 1998) contributed data to this comparison.
Primary outcomes
4.1 Treatment efficacy: depression and anxiety, short‐term and long‐term
It could not be determined from one trial with 65 participants (Grey 1998) whether psychological therapies (in this case, CBT) were more effective than non‐psychological therapies in reducing symptoms of depression immediately post‐intervention (MD 0.30, 95% CI ‐0.31 to 0.91; I2 = 0%) (Analysis 4.1). This trial did not measure symptoms of depression at three to six‐month follow‐up. Nor did it measure symptoms of anxiety either immediately post‐intervention or at three to six‐month follow‐up.
4.1. Analysis.
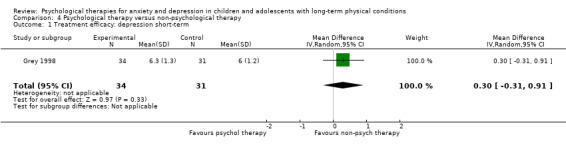
Comparison 4 Psychological therapy versus non‐psychological therapy, Outcome 1 Treatment efficacy: depression short‐term.
4.2 Treatment acceptability
Secondary outcomes
4.3 Change in caseness (remission/response)
No data were available for this outcome.
4.4 Suicide‐related behaviour
No data were available for this outcome.
4.5 Quality of life: short‐term and long‐term
It could not be determined from one trial involving 65 participants (Grey 1998) whether psychological therapies were more effective than non‐psychological therapies in improving quality of life immediately post‐intervention (MD 0.80, 95% CI ‐5.28 to 6.88; I2 = 0%) (Analysis 4.2). This trial did not measure quality of life at three to six‐month follow‐up.
4.2. Analysis.
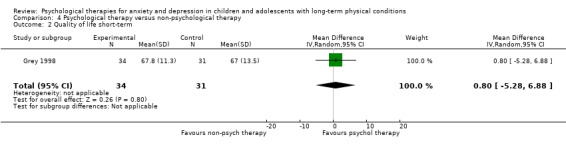
Comparison 4 Psychological therapy versus non‐psychological therapy, Outcome 2 Quality of life short‐term.
4.6 Functioning: short‐term and long‐term
No data were available for this outcome.
4.7 Status of long‐term physical condition: short‐term and long‐term
It could not be determined from one trial involving 65 participants (Grey 1998) whether psychological therapies were more effective than non‐psychological therapies in improving symptoms of long‐term physical conditions immediately post‐intervention (MD ‐0.50, 95% CI ‐1.13 to 0.13; I2 = 0%) (Analysis 4.3). This trial did not measure symptoms of long‐term physical conditions at three to six‐month follow‐up.
4.3. Analysis.
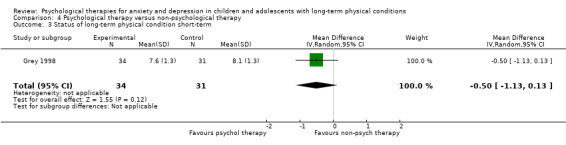
Comparison 4 Psychological therapy versus non‐psychological therapy, Outcome 3 Status of long‐term physical condition short‐term.
4.8 Adherence to treatment of long‐term physical condition
No data were available for this outcome.
4.9 School/college attendance
No data were available for this outcome.
4.10 Economic benefits
No data were available for this outcome.
Comparison 5: Psychological therapies for anxiety or depression versus treatment‐as‐usual (TAU)
Seven trials with 582 participants (Bignall 2015; Chiang 2009; Li 2016; Rostami 2016; Sharma 2017; Szigethy 2007; Varni 1993) contributed data to this comparison.
Primary outcomes
5.1 Treatment efficacy: depression and anxiety, short‐term and long‐term
Four trials involving 245 participants (Li 2016; Rostami 2016; Szigethy 2007; Varni 1993) demonstrated that psychological therapies were more effective than treatment‐as‐usual in reducing symptoms of depression immediately post‐intervention (SMD ‐0.65, CI ‐0.91 to ‐0.39, I2= 0%) (Analysis 5.1). Conversely, it could not be determined from two trials involving 53 participants (Szigethy 2007; Varni 1993) whether psychological therapies were more effective than treatment‐as‐usual in reducing symptoms of depression at three to six‐month follow‐up (SMD ‐0.10, CI ‐1.59 to 1.39, I2 = 85%) (Analysis 5.2). Six trials involving 337 participants (Bignall 2015; Chiang 2009; Li 2016; Rostami 2016; Sharma 2017; Varni 1993) demonstrated that psychological therapies were more effective than treatment‐as‐usual in reducing symptoms of anxiety immediately post‐intervention (SMD ‐0.57, 95% CI ‐0.96 to ‐0.17; I2 = 65%) (Analysis 5.3). However, it could not be determined from one trial involving 27 participants (Varni 1993) whether psychological therapies were more effective than treatment‐as‐usual in reducing symptoms of anxiety at three to six‐month follow‐up (SMD 0.09, 95% CI ‐0.69 to 0.87; I2 = 0%) (Analysis 5.4).
5.1. Analysis.
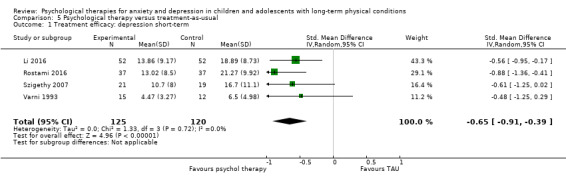
Comparison 5 Psychological therapy versus treatment‐as‐usual, Outcome 1 Treatment efficacy: depression short‐term.
5.2. Analysis.
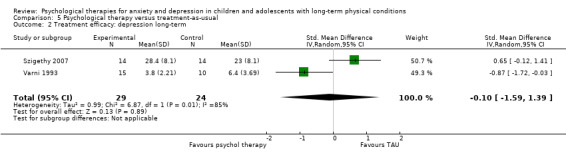
Comparison 5 Psychological therapy versus treatment‐as‐usual, Outcome 2 Treatment efficacy: depression long‐term.
5.3. Analysis.
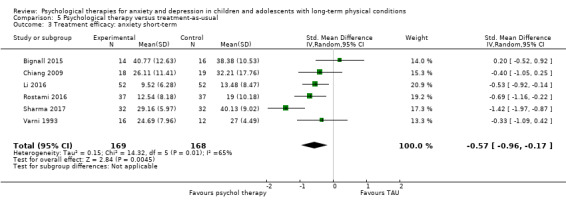
Comparison 5 Psychological therapy versus treatment‐as‐usual, Outcome 3 Treatment efficacy: anxiety short‐term.
5.4. Analysis.
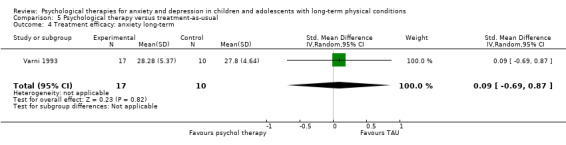
Comparison 5 Psychological therapy versus treatment‐as‐usual, Outcome 4 Treatment efficacy: anxiety long‐term.
5.2 Treatment acceptability
No data were available for this outcome.
Secondary outcomes
5.3 Change in caseness (remission/response)
No data were available for this outcome.
5.4 Suicide‐related behaviour
No data were available for this outcome.
5.5 Quality of life: short‐term and long‐term
It could not be determined from one trial involving 30 participants (Bignall 2015) whether psychological therapies were more effective than treatment‐as‐usual in improving quality of life immediately post‐intervention (SMD 0.67, 95% CI ‐0.07 to 1.41; I2 = 0%) (Analysis 5.5). No trials in this group measured functioning at three to six‐month follow‐up.
5.5. Analysis.

Comparison 5 Psychological therapy versus treatment‐as‐usual, Outcome 5 Quality of life short‐term.
5.6 Functioning: short‐term and long‐term
It could not be determined from two trials involving 103 participants (Sharma 2017; Szigethy 2007) whether psychological therapies were more effective than treatment‐as‐usual in improving functioning immediately post‐intervention (SMD 2.34, CI ‐0.53 to 5.21, I2 = 97%) (Analysis 5.6). Nor could it be determined from one follow‐up trial involving 30 participants (Szigethy 2007) whether psychological therapies were more effective than treatment‐as‐usual in improving functioning at three to six‐month follow‐up (SMD 0.41, 95% CI ‐0.32 to 1.14; I2 = 0%) (Analysis 5.7).
5.6. Analysis.

Comparison 5 Psychological therapy versus treatment‐as‐usual, Outcome 6 Functioning short‐term.
5.7. Analysis.
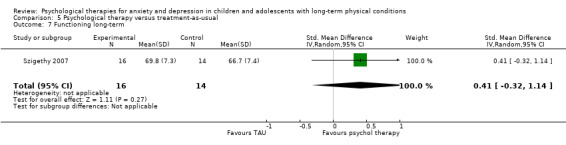
Comparison 5 Psychological therapy versus treatment‐as‐usual, Outcome 7 Functioning long‐term.
5.7 Status of long‐term physical condition: short‐term and long‐term
It could not be determined from five trials involving 308 participants (Bignall 2015; Chiang 2009; Li 2016; Rostami 2016; Sharma 2017) whether psychological therapies were more effective than attention placebo in reducing symptoms of long‐term physical conditions immediately post‐intervention (SMD ‐0.52, 95% CI ‐1.18 to 0.14; I2 = 87%) (Analysis 5.8). None of the trials in this group measured changes in symptoms of long‐term physical conditions at three to six‐month follow‐up.
5.8. Analysis.
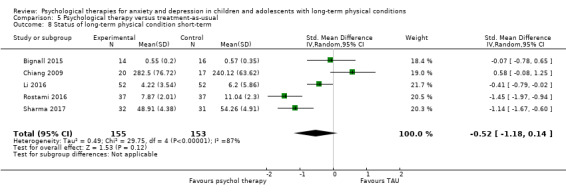
Comparison 5 Psychological therapy versus treatment‐as‐usual, Outcome 8 Status of long‐term physical condition short‐term.
5.8 Adherence to treatment of long‐term physical condition
No data were available for this outcome.
5.9 School/college attendance
No data were available for this outcome.
5.10 Economic benefits
No data were available for this outcome.
Comparison 6: Psychological therapies for anxiety or depression versus waiting list (WL)
Four trials including 100 participants (Detling Miller 2008; Griffiths 1996; Hains 2000; Moghanloo 2015) contributed data to this comparison.
Primary outcomes
6.1 Treatment efficacy: depression and anxiety, short‐term and long‐term
It could not be determined from two trials involving 61 participants (Griffiths 1996; Moghanloo 2015) whether psychological therapies were more effective than waiting list in reducing symptoms of depression immediately post‐intervention (SMD ‐2.39, CI ‐6.89 to 2.12, I2 = 97%) (Analysis 6.1). No trials in this group measured symptoms of depression at three to six‐month follow‐up. It could not be determined from three trials involving 67 participants (Detling Miller 2008; Griffiths 1996; Hains 2000) whether psychological therapies were more effective than waiting list in reducing symptoms of anxiety immediately post‐intervention (SMD ‐0.40, 95% CI ‐0.89 to 0.09; I2 = 0%) (Analysis 6.2). However, one of these trials (Hains 2000) demonstrated that psychological therapies were more effective than waiting list in reducing symptoms of anxiety at three to six‐month follow‐up (SMD ‐1.29, 95% CI ‐2.56 to ‐0.03; I2 = 0%) (Analysis 6.3).
6.1. Analysis.
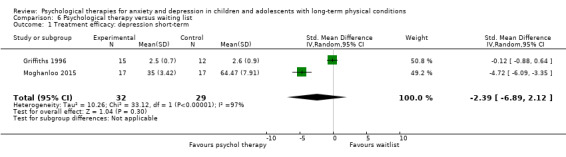
Comparison 6 Psychological therapy versus waiting list, Outcome 1 Treatment efficacy: depression short‐term.
6.2. Analysis.
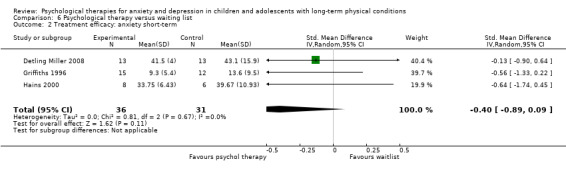
Comparison 6 Psychological therapy versus waiting list, Outcome 2 Treatment efficacy: anxiety short‐term.
6.3. Analysis.
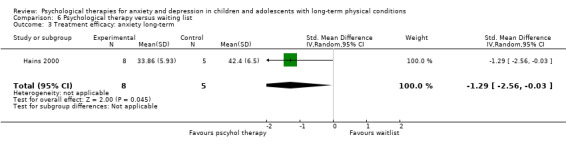
Comparison 6 Psychological therapy versus waiting list, Outcome 3 Treatment efficacy: anxiety long‐term.
6.2 Treatment acceptability
No data were available for this outcome.
Secondary outcomes
6.3 Change in caseness (remission/response)
No data were available for this outcome.
6.4 Suicide‐related behaviour
No data were available for this outcome.
6.5 Quality of life: short‐term and long‐term
One trial involving 34 participants (Moghanloo 2015) demonstrated that psychological therapies were significantly more effective than waiting list in improving quality of life immediately post‐intervention (SMD 5.20, 95% CI 3.73 to 6.68; I2 = 0%) (Analysis 6.4). This trial did not measure quality of life at three to six‐month follow‐up.
6.4. Analysis.

Comparison 6 Psychological therapy versus waiting list, Outcome 4 Quality of life short‐term.
6.6 Functioning: short‐term and long‐term
No data were available for this outcome.
6.7 Status of long‐term physical condition: short‐term and long‐term
It could not be demonstrated from two trials involving 40 participants (Detling Miller 2008; Hains 2000) whether psychological therapies were more effective than waiting list in reducing symptoms of long‐term physical conditions immediately post‐intervention (SMD 0.11, 95% CI ‐0.51 to 0.73; I2 = 0%) (Analysis 6.5). This trial did not measure changes in symptoms of long‐term physical conditions at three to six‐month follow‐up.
6.5. Analysis.
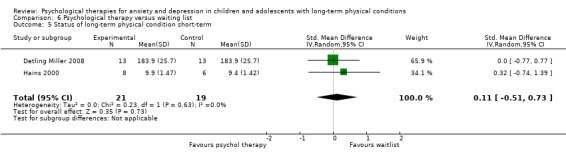
Comparison 6 Psychological therapy versus waiting list, Outcome 5 Status of long‐term physical condition short‐term.
6.8 Adherence to treatment of long‐term physical condition
No data were available for this outcome.
6.9 School/college attendance
No data were available for this outcome.
6.10 Economic benefits
No data were available for this outcome.
Subgroup analyses
Subgroup analyses were undertaken for the outcomes of treatment efficacy (short‐term depression and treatment efficacy and short‐term anxiety) for which sufficient data of different types were available. The factors investigated in subgroup analysis included: type of experimental therapy, modality of delivery, dose of therapy, type of long‐term physical condition, category of depressive symptoms, category of anxiety symptoms, and target of therapy. All included trials used self‐report outcome measures and all but two trials (Griffiths 1996; Wei 2017) included mixed age groups of participants, rendering subgroup analysis based on these criteria irrelevant.
7.1 Type of experimental therapy
Two trials involving 64 participants who had undertaken ACT, eleven trials involving 592 participants who had undertaken CBT, one trial involving 46 participants who had undertaken mindfulness therapy, one trial involving 74 participants who had undertaken education therapy, one trial involving 66 participants who had undertaken supportive therapy, and three trials involving 196 participants who had undertaken other therapies were included in the subgroup analysis of the type of experimental therapy on the outcome treatment efficacy of short‐term depression . Results indicated that the type of experimental therapy made a difference to this outcome (Chi² = 14.75, df = 5 (P = 0.01), I² = 66.1%) with CBT and education therapies being more effective than other types of therapy (Analysis 7.1).
7.1. Analysis.
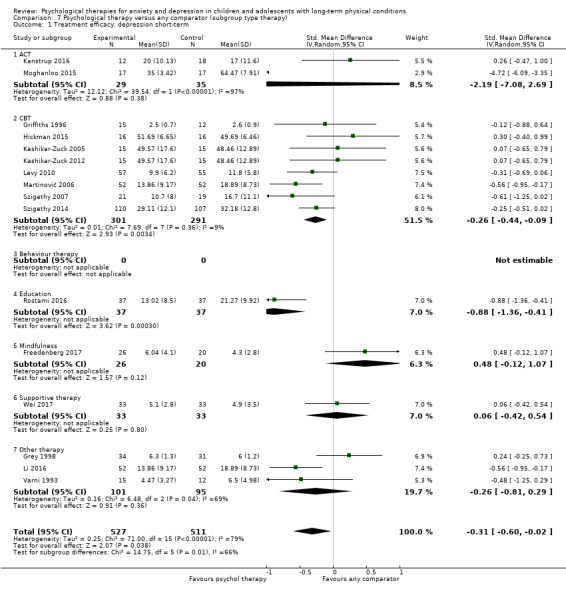
Comparison 7 Psychological therapy versus any comparator (subgroup type therapy), Outcome 1 Treatment efficacy: depression short‐term.
Four trials involving 137 participants who had undertaken CBT, education therapy, one trial involving 46 participants who had undertaken mindfulness therapy, three trials involving 193 participants who had undertaken behaviour therapy, one trial involving 66 participants who had undertaken supportive therapy, and two trials involving 58 participants who had undertaken other therapies were included in the subgroup analysis of the effect of the type of experimental therapy on changes in the outcome treatment efficacy of short‐term anxiety. Results indicated that the type of experimental therapy made a difference to this outcome (Chi² = 16.52, df = 5 (P = 0.005), I² = 69.7%) with a single trial (Rostami 2016) demonstrating that education therapy was more effective than other types of therapy. Interestingly, a single trial of mindfulness therapy (Freedenberg 2017) demonstrated a significant effect for the comparator (an online support group) (Analysis 7.2).
7.2. Analysis.
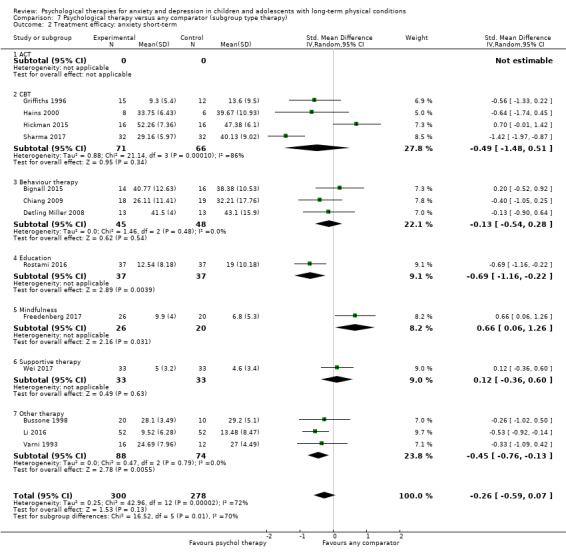
Comparison 7 Psychological therapy versus any comparator (subgroup type therapy), Outcome 2 Treatment efficacy: anxiety short‐term.
7.2 Mode of delivery
Eeven trials involving 573 participants who had undertaken individual therapy and five trials involving 548 participants who had undertaken group therapy were Included in the subgroup analysis of the effect of mode of delivery on the outcome treatment efficacy of short‐term depression. Results indicated that the mode of delivery made no difference to this outcome (Chi² = 0.89, df = 1 (P = 0.35), I² = 0%) (Analysis 8.1).
8.1. Analysis.
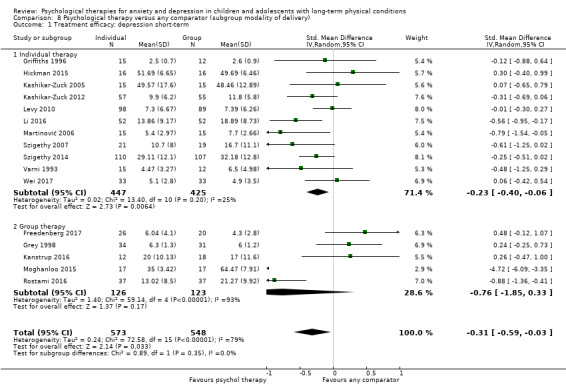
Comparison 8 Psychological therapy versus any comparator (subgroup modality of delivery), Outcome 1 Treatment efficacy: depression short‐term.
Nine trials involving 300 participants who had undertaken individual therapy and four trials involving 278 participants who had undertaken group therapy were included in the subgroup analysis of the effect of mode of delivery on the outcome treatment efficacy of short‐term anxiety. Results indicated that the mode of delivery made no difference to this outcome (Chi² = 0.59, df = 1 (P = 0.44), I² = 0%) (Analysis 8.2).
8.2. Analysis.
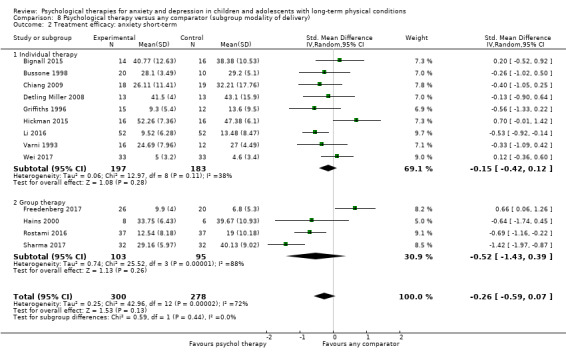
Comparison 8 Psychological therapy versus any comparator (subgroup modality of delivery), Outcome 2 Treatment efficacy: anxiety short‐term.
7.3 Dose of therapy
Four trials involving 369 participants who had undertaken fewer than six sessions or six weeks of therapy (considered 'brief') (Pitceathly 2009) and twelve trials involving 757 participants who had undertaken more than six sessions or six weeks of therapy were included in the subgroup analysis of the effect of dose of therapy on the outcome treatment efficacy of short‐term depression. Results indicated that the dose of therapy made no difference to this outcome (Chi² = 0.76, df = 1 (P = 0.38), I² = 0%) (Analysis 9.1).
9.1. Analysis.
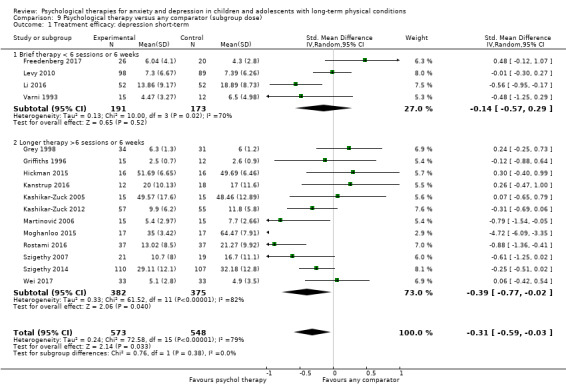
Comparison 9 Psychological therapy versus any comparator (subgroup dose), Outcome 1 Treatment efficacy: depression short‐term.
Seven trials involving 285 participants who had undertaken fewer than six sessions or six weeks of therapy (considered 'brief') (Pitceathly 2009) and six trials involving 293 participants who had undertaken more than six sessions or six weeks of therapy were included in the subgroup analysis of the effect of dose of therapy on the outcome treatment efficacy of short‐term anxiety. Results indicated that the dose of therapy made no difference to this outcome (Chi² = 0.37, df = 1 (P = 0.54), I² = 0%) (Analysis 9.2).
9.2. Analysis.

Comparison 9 Psychological therapy versus any comparator (subgroup dose), Outcome 2 Treatment efficacy: anxiety short‐term.
7.4 Type of long‐term physical condition
Three trials including 240 participants with diabetes, two trials including 134 participants with epilepsy, two trials including 257 participants with inflammatory bowel disease, six trials including 418 participants with pain disorders, and two trials including 73 participants with other disorders were included in the subgroup analysis of the outcome treatment efficacy of short‐term depression. Results indicated that the type of long‐term physical condition made a difference to the outcome treatment efficacy of short‐term depression (Chi² = 10.55, df = 4 (P = 0.03), I² = 62.1%) (Analysis 10.1). Participants with epilepsy and inflammatory bowel disease responded better to psychological interventions than those with other types of long‐term physical condition.
10.1. Analysis.
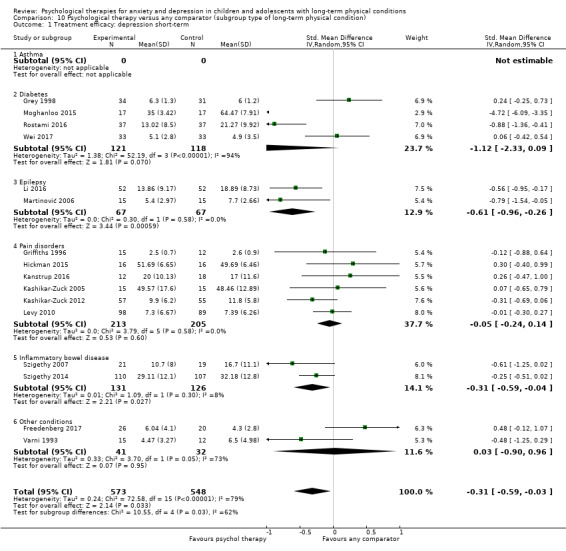
Comparison 10 Psychological therapy versus any comparator (subgroup type of long‐term physical condition), Outcome 1 Treatment efficacy: depression short‐term.
Two trials including 67 participants with asthma, four trials including 180 participants with diabetes, one trial including 104 participants with epilepsy, four trials including 153 participants with pain disorders, and two trials including 74 participants with other disorders were included in the subgroup analysis of the outcome treatment efficacy of short‐term anxiety short‐term. Results indicated that the type of long‐term physical condition made a difference to the outcome treatment efficacy of short‐term anxiety (Chi² = 2.61, df = 4 (P = 0.63), I² = 0%) (Analysis 10.2). Participants with epilepsy appeared to respond better to psychological interventions than those with other types of long‐term physical condition.
10.2. Analysis.
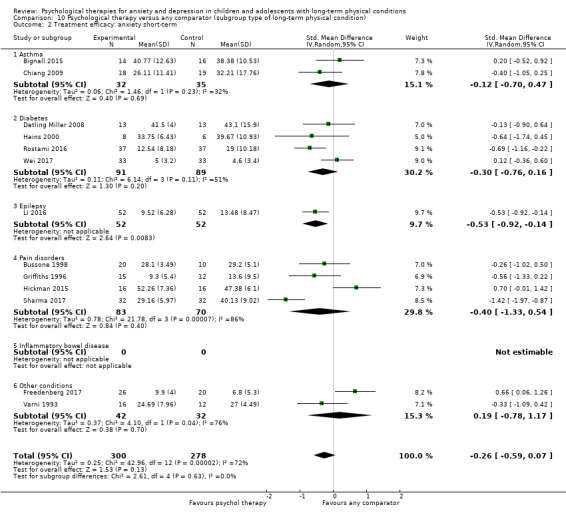
Comparison 10 Psychological therapy versus any comparator (subgroup type of long‐term physical condition), Outcome 2 Treatment efficacy: anxiety short‐term.
7.5 Category of depressive or anxiety symptoms
Five trials including 355 participants with subthreshold symptoms of depression, five trials including 385 participants with mild symptoms of depression, one trial including 40 participants with moderate symptoms of depression, two trials including 136 participants with severe symptoms of depression, and three trials including 205 participants with uncertain symptoms of depression were included in the subgroup analysis of the outcome treatment efficacy of short‐term depression. Results indicated that the category of depressive symptoms made a difference to the outcome treatment efficacy of short‐term depression (Chi² = 9.38, df = 4 (P = 0.05), I² = 57.4%) (Analysis 11.1). Participants with mild symptoms of depression appeared to benefit the most from psychological interventions, compared with those who had subthreshold, moderate, severe, or uncertain symptoms.
11.1. Analysis.
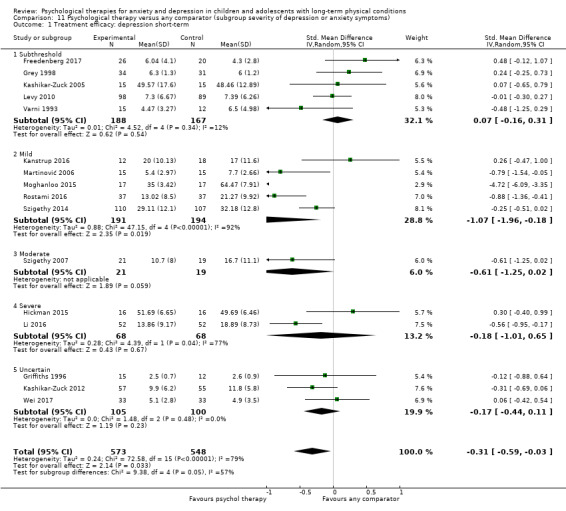
Comparison 11 Psychological therapy versus any comparator (subgroup severity of depression or anxiety symptoms), Outcome 1 Treatment efficacy: depression short‐term.
One trial including 46 participants with subthreshold symptoms of anxiety, five trials including 249 participants with mild symptoms of anxiety, two trials including 92 participants with moderate symptoms of anxiety, two trials including 62 participants with severe symptoms of anxiety, and three trials including 26 participants with uncertain symptoms of anxiety were included in the subgroup analysis of the outcome treatment efficacy of short‐term anxiety. Results indicated that the category of anxiety symptoms made a difference to the outcome treatment efficacy of short‐term anxiety (Chi² = 23.91, df = 4 (P < 0.0001), I2 = 83.3%) (Analysis 11.2). Participants with mild symptoms of anxiety appeared to benefit the most from psychological interventions, compared with those who had subthreshold, moderate, severe, or uncertain symptoms.
11.2. Analysis.
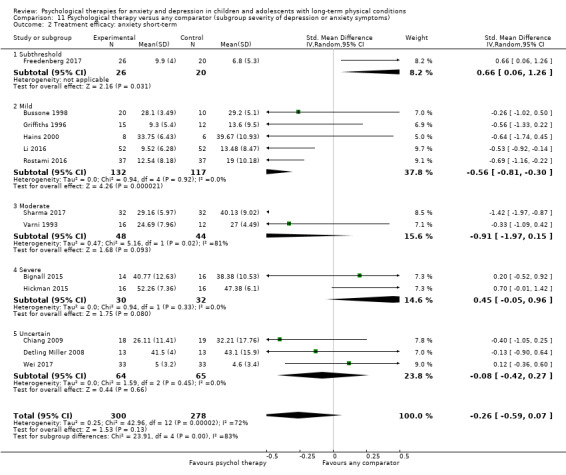
Comparison 11 Psychological therapy versus any comparator (subgroup severity of depression or anxiety symptoms), Outcome 2 Treatment efficacy: anxiety short‐term.
7.6 Target of therapy
Eight trials including 374 participants targeted at children alone and eight trials including 747 participants targeted at both children and families were included in the subgroup analysis of the outcome treatment efficacy of short‐term depression. The type of target of therapy made no difference to the outcome (Chi² = 0.62, df = 1 (P = 0.43), I² = 0%) (Analysis 12.1).
12.1. Analysis.
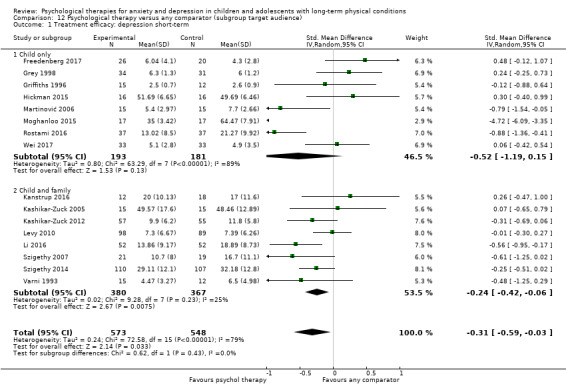
Comparison 12 Psychological therapy versus any comparator (subgroup target audience), Outcome 1 Treatment efficacy: depression short‐term.
Eight trials including 374 participants targeted at children alone and eight trials including 747 participants targeted at both children and families were included in the subgroup analysis of the outcome treatment efficacy of short‐term anxiety. Similar to the results above, the type of target of therapy made no difference to the outcome (Chi² = 0.94, df = 1 (P = 0.33), I² = 0%) (Analysis 12.2).
12.2. Analysis.
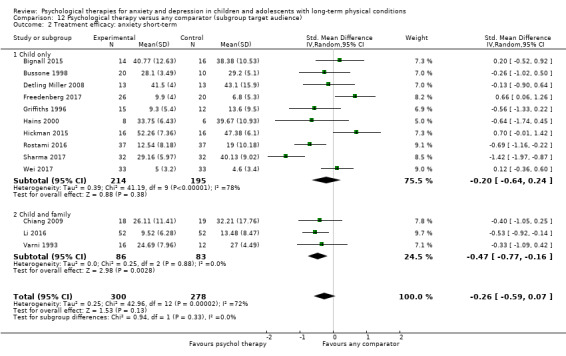
Comparison 12 Psychological therapy versus any comparator (subgroup target audience), Outcome 2 Treatment efficacy: anxiety short‐term.
Sensitivity analyses
Sensitivity analyses were conducted for the primary outcomes of change in symptoms of depression immediately post‐intervention and change in symptoms of anxiety immediately post‐intervention based on three preplanned criteria: allocation concealment, blinding of outcome assessors, and dropout rates (with more than 20% between pre‐intervention and follow‐up being significant) and one previously unplanned criterion, trials of psychological therapies designed to treat anxiety or depression. None of the trials that contributed to either primary outcome were rated at high risk for allocation concealment, so it was not possible to undertake a sensitivity analysis using this criterion. Only one trial (Sharma 2017) that contributed to the meta‐analysis of change in symptoms of anxiety immediately post‐intervention was rated at high risk for blinding of outcome assessors and exclusion of this trial did not significantly alter the overall outcome. Similarly, only one trial (Chiang 2009) that contributed to the meta‐analysis of change in symptoms of anxiety immediately post‐intervention was rated at high risk for dropouts and exclusion of this trial did not significantly alter the overall outcome. When trials of psychological therapies that were not specifically designed to treat anxiety or depression were excluded, there was a significant difference in effect sizes. The SMD for change in symptoms of depression immediately post‐intervention (including only the following trials: Li 2016; Martinović 2006; Moghanloo 2015; Rostami 2016; Szigethy 2007; Szigethy 2014) increased from ‐0.31 (‐0.59, ‐0.03), a small effect size, to ‐1.03 (‐1.64, ‐0.41), a large effect size (Analysis 1.11, Figure 9). Similarly, the SMD for change in symptoms of anxiety immediately post‐intervention (including the following trials: Chiang 2009; Detling Miller 2008; Li 2016; Rostami 2016; Sharma 2017) increased from ‐0.26 (‐0.59, 0.07), a nonsignificant effect size, to ‐0.66 (‐1.05, ‐0.28) (Analysis 1.12, Figure 10), a moderate effect size.
1.11. Analysis.
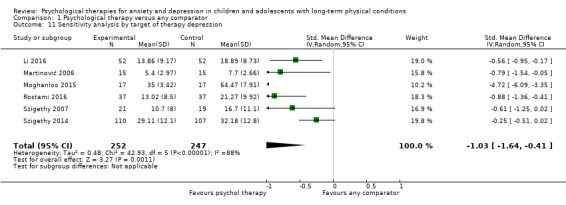
Comparison 1 Psychological therapy versus any comparator, Outcome 11 Sensitivity analysis by target of therapy depression.
9.
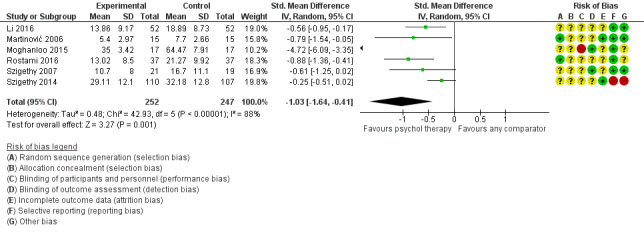
Forest plot of comparison: 1 Psychological therapy versus any comparator, outcome: 1.11 Sensitivity analysis by target of therapy: treatment efficacy: depression short‐term
1.12. Analysis.
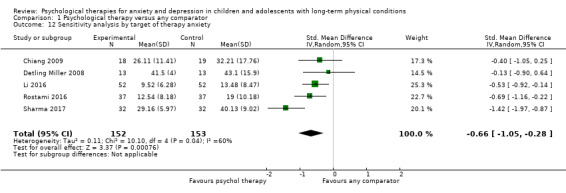
Comparison 1 Psychological therapy versus any comparator, Outcome 12 Sensitivity analysis by target of therapy anxiety.
10.
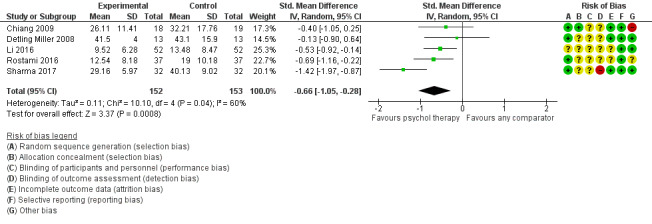
Forest plot of comparison: 1 Psychological therapy versus any comparator, outcome: 1.12 Sensitivity analysis by target of therapy: treatment efficacy: anxiety short‐term
Reporting Bias
The presence and impact of publication bias was minimised through the use of an exhaustive systematic review procedure. Nevertheless, we inspected funnel plots for the primary outcome measures of changes in symptoms of anxiety and depression to assess the likely presence of publication bias. There was no evidence of major funnel plot asymmetry for either primary outcome measure (see Figure 11 and Figure 12). It should be noted that SMDs are naturally correlated with their respective standard errors and therefore this can result in spurious funnel plot asymmetry (see Higgins 2008b, section 10.4.3)
11.
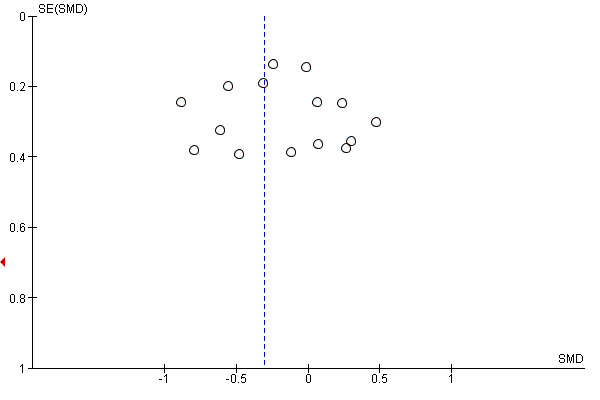
Funnel plot of comparison: Psychological therapy versus any comparator, outcome: 1.1 Treatment efficacy: depression short‐term
12.
Funnel plot of comparison: Psychological therapy versus any comparator, outcome: 1.3 Treatment efficacy: anxiety short‐term
Discussion
Summary of main results
The primary objectives of this review were to evaluate the treatment efficacy and acceptability of psychological therapies for anxiety and depression in children and adolescents with long‐term physical conditions. As per our findings, there was low‐quality evidence that psychological therapies were more effective than control therapies in reducing symptoms of depression in the short term, but not the long term. There was inadequate low‐quality evidence to determine whether psychological therapies were more effective than control therapies in reducing symptoms of anxiety either in the short term or the long term. There was qualitative (reported), but not quantitative evidence confirming the acceptability of selected psychological therapies for anxiety and depression.
Secondary objectives of this review included identifying changes in 'caseness' of anxiety or depression, quality of life, status of long‐term physical conditions, adherence to treatment of long‐term physical conditions, functioning, quality of life, school/college attendance, and economic benefits associated with the use of psychological therapies. Meta‐analysable data were only available for some of these outcomes. There was low‐quality evidence that psychological therapies were more effective than control therapies in improving quality of life and symptoms of long‐term physical conditions, but primarily in the short term. There was inadequate low‐quality evidence to determine whether psychological therapies were more effective than control therapies at improving functioning in either the short term or long term.
Subgroup analysis revealed that CBT and education therapies were more effective than other types of therapy at reducing symptoms of depression and education therapy was more effective than other types of therapy at reducing symptoms of anxiety. Therapies delivered to participants with mild levels of depression and anxiety were more effective than those delivered to participants with subthreshold, moderate, or severe symptoms. Children and adolescents with some types of long‐term physical conditions, such as epilepsy and inflammatory bowel disease, appeared to respond better to psychological therapies. However, this may be an artefact related to differences in trial quality. The mode of delivery (individual versus group), dose of therapy (brief versus longer than six sessions or six weeks) and target of therapy (child versus parent and child) did not make any difference to overall outcomes. Psychological therapies were more effective when tested against psychological placebo and treatment‐as‐usual than with attention placebo, non‐psychological therapy and waiting‐list controls. As most trials included mixed age groups, it was not possible to subgroup participants by age. The limited number of trials in some subgroup analyses mean that these results should be interpreted with care. Planned sensitivity analyses for risk of bias could not be undertaken due to the small number of trials rated at high risk of bias for each domain. Additional sensitivity analysis revealed that psychological interventions specifically designed to reduce anxiety or depression were more effective at doing so than psychological therapies designed to reduce pain or to improve coping and social skills. Our main findings are summarised in the Summary of main results for the main comparison.
Overall completeness and applicability of evidence
Although we identified a limited number of studies from which to draw conclusions, given the comprehensive nature of the search and its update during the review process, we are confident that our findings are representative of the current level of evidence in this area. Included in our analysis were trials that had been undertaken over a twenty‐five year period and, unsurprisingly, given changes in publication standards, the quality of reporting was better for newer trials than for older ones. Most trials were conducted in high‐income countries, making it difficult to ascertain the likely effectiveness of these therapies for children and adolescents living in lower‐income countries. Many trials were designed to test the effectiveness of psychological therapies on dimensions other than anxiety or depression. Fortunately, subgroup and sensitivity analysis allowed us to better understand the features of psychological therapies and the type of audience associated with the greatest clinical effectiveness. Included trials were undertaken with children and adolescents with a range of long‐term physical conditions. However, as most trials included mixed age groups, it was not possible to distinguish between psychological therapies that may be more useful for children and those that may be more useful for adolescents. As each psychological therapy was only investigated associated with a single long‐term physical condition, its effectiveness for individuals with other long‐term physical conditions is uncertain. As most trials were undertaken with community samples, the value of psychological therapies to hospitalised individuals also remains unclear. Inconsistent description of baseline anxiety and depression limits certainty regarding the ideal audience for currently available psychological therapies. Only six trials were tested against the most stringent type of control, namely attention placebo. Most trials focused on short‐term clinical effectiveness of psychological therapies, making long‐term outcomes less clear. Acceptability was poorly described in trial reports and there were clear gaps in evidence regarding adverse effects, change in 'caseness' of anxiety and depression, adherence to treatment of long‐term physical condition, suicidality, school attendance, and economic benefits of psychological therapies.
Quality of the evidence
Overall, the evidence was of low‐ to moderate‐quality according to the GRADE framework and results were heterogeneous. Of the 28 trials included in this review, only 16 had meta‐analysable data for the primary outcome of change in depressive symptoms immediately post‐intervention and 13 had meta‐analysable data for the primary outcome of change in anxiety symptoms immediately post‐intervention. Only one trial (Van Dijk Lokkart 2016) had an available trial protocol, making the others at unclear risk of selective reporting. The main reasons that trials were downgraded were inconsistency, heterogeneity, and wide confidence intervals for meta‐analysed data. Although there was no evidence of reporting bias according to funnel plot evaluation, previous reviewers (Sansom‐Daly 2014) have drawn attention to the ‘file drawer’ effect of smaller studies with negative short‐term results. Despite conducting as thorough a search as we could of key databases, trial registries, and other sources, we may have missed some studies of existing psychological therapies.
Potential biases in the review process
None of the review authors were involved in any of the included trials. It is likely that the results of this review reflect the limited number of trials of psychological therapies designed to treat anxiety and depression in children and adolescents with long‐term physical conditions.
Agreements and disagreements with other studies or reviews
This review follows Cochrane reviews of the prevention of depression (Hetrick 2016) and treatment of depression (Cox 2014) and anxiety (James 2013) in children and adolescents without long‐term physical conditions, reviews of psychological therapies for depression in children and adolescents with specific conditions such as congenital heart disease (Lane 2013) or pain disorders (Fisher 2018), and a review of psychological therapies for parents of children with long‐term physical conditions (Eccleston 2012). It also follows a non‐Cochrane review of psychological interventions for children and adolescents with long‐term physical conditions (Bennett 2015) which was much smaller than ours (comprising 10 trials, two RCTs and no meta‐analysis). Similar to the reviews by Bennett and Eccleston, we identified CBT‐based therapies as the most effective types of therapies for treating anxiety and depression in children and adolescents with long‐term physical conditions. Similar to a review of depression treatment in adults with long‐term physical conditions (Rizzo 2011), we observed that therapies designed for treating depression were more effective than those designed to treat other symptoms or to improve coping in a more general sense. When restricted to these parameters, the therapies included in our review demonstrated better effect sizes than those delivered to children in the general population (Weisz 2017). Reasons for this could possibly include children with long‐term physical conditions experiencing more 'reactive' forms of depression that respond better to therapy; the receipt of simultaneous care from other health professionals increasing their overall level of attention; and pre‐existing coping strategies for managing medical issues contributing toward improved mental health recovery. Like the reviews by Bennett and Eccleston, we did not find adequate measurement and reporting of adverse outcomes for any psychological therapies, making it hard to be certain of the disadvantages of such therapies. Similar to a review of adolescents and young adults with cancer (Sansom‐Daly 2014), we found that despite the perceived benefits of peer support, individually‐delivered therapies were more effective than group‐based therapies. This may be related to the nature of the problems being addressed and the need for a more individually‐tailored focus during the delivery of such therapies. As previously mentioned, included trials were more focused on the needs of adolescents than younger children. A non‐Cochrane review of supportive care interventions for young children with long‐term physical conditions (Robb 2014) does highlight some value for these therapies in reducing symptoms of anxiety. However, as most of the trials in this review were not RCTs, they did not meet criteria for inclusion in our review. We did not find any significant discrepancies between our findings and those of related reviews or studies. Differences in some of our results are likely to be related to differences in review methodology.
Authors' conclusions
Implications for practice.
Although 28 trials were included in the overall analysis and 22 trials were included in the meta‐analysis, only 16 trials of variable quality evaluated depression and 13 trials of variable quality evaluated anxiety, limiting the certainty with which we can make conclusions about the use of psychological therapies for treating these conditions in children and adolescents with long‐term physical conditions. According to the available evidence, therapies specifically designed to treat depression (especially those based on principles of cognitive behaviour therapy (CBT)) are more likely to work in children and adolescents who have mild symptoms of this disorder. The evidence with regard to the treatment of anxiety is less clear. There is a dearth of therapies specifically designed to treat health‐related anxiety in this age group.
Implications for research.
We offer the following recommendations for future research in this area. All trials should use validated measures of anxiety or depression and be reported according to CONSORT guidelines (Schulz 2010) in order to ensure the availability of comparable datasets. Where possible, attention control groups should be used to better distinguish the specific effects of psychological therapies from the generic benefit of receiving therapeutic attention. Larger and better designed trials of existing and new psychological therapies should be undertaken by researchers independent from the design of such therapies to be more certain of their effectiveness. More trials focused on anxiety and depression as primary outcomes are needed. Data on long‐term clinical effectiveness, acceptability, functional change, and economic gains should be collected together with data on adverse effects to evaluate potential benefits and harms of psychological therapies. As there are many existing psychological therapies for treating anxiety and depression in children, but few trials have been undertaken with children who have long‐term physical conditions, more research is needed with this subgroup. Larger trials should stratify participants by age group and examine developmentally‐related differences in the effectiveness of psychological therapies. Given the known relationship between medical illness or treatment and anxiety, further research is needed into potentially shared coping strategies and therapies that specifically address health‐related anxiety. Increasing the number of trials undertaken in lower‐ and middle‐income countries and different cultures would help to distinguish locally useful therapies from more globally dispersible therapies. As co‐design of therapies with end‐users is likely to improve their acceptability (Hetrick 2018), more research involving co‐design methodology should also be undertaken. Further research is needed to understand the effectiveness of existing psychological therapies on children with different long‐term physical conditions, as well as the essential ingredients and minimum viable dose of psychological therapies. Finally, future updates of this review should report on adherence to these recommendations.
What's new
| Date | Event | Description |
|---|---|---|
| 2 January 2019 | Amended | Correction made to search date in Plain Language Summary |
Acknowledgements
The authors acknowledge the valuable contributions of the Cochrane Common Mental Disorders (CCMD) group, including Sarah Dawson (Information Specialist), Lindsay Robertson (Systematic Reviewer), Jess Hendon (Managing Editor), Jessica Sharp (Former Managing Editor) and Rachel Churchill (Co‐ordinating Editor).
This review was supported by funding from the Oakley Foundation and Starship Foundation in New Zealand.
Cochrane Group funding acknowledgement
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Common Mental Disorders Group.
Disclaimer
The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health and Social Care.
Appendices
Appendix 1. MEDLINE search for Specialised Register
The search strategy listed below is the weekly OVID Medline search used to inform the Group’s specialised register. It is based on a list of terms for all conditions within the scope of the Cochrane Common Mental Disorders Group plus a sensitive RCT filter.
OVID MEDLINE search strategy, used to inform the Cochrane Common Mental Disorders Group's Specialised Register A weekly search alert based on condition + RCT filter only 1. [MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/ 2. [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf. 3. [RCT filter]: (controlled clinical trial.pt. or randomized controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomized controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.) 4. (1 and 2 and 3)
Records are screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs are tagged to the appropriate study record. Similar weekly search alerts are also conducted on OVID Embase and PsycINFO, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource.
Appendix 2. Review search: CCMD‐CTR‐References Register
CCMD‐CTR‐References Register was searched using a sensitive set of terms for age group, condition and comorbidity:
[Age Group] #1. (child* or boy* or girl* or infant* or juvenil* or minors or paediatric* or pediatric* or school* or preschool* or pre‐school* or kindergarten or nursery or adolesc* or preadolesc* or pre‐adolesc* or pubert* or pubescen* or prepube* or pre‐pube* or high‐school or teen* or (young next (adult* or people or patient* or men* or women* or mother* or male or female or survivor* or offender* or minorit*)) or youth* or student* or undergrad* or college or campus or classroom):ti,ab [Condition: anxiety/depression] #2. ((emotion* or psycholog* or mental) next (health or stress* or problem* or disturb* or aspect* or state* or ill*)):ti,ab,kw,ky,emt,mh,mc #3. (depress* or mood or anxiety or *phobi* or PTSD or post‐trauma* or posttrauma or “post trauma*” or panic* or OCD or obsess* or compulsi* or GAD or "stress disorder*" or “stress reaction*” or "acute stress" or “psychological stress” or “school refusal” or mutism or neurosis or neuroses or neurotic or psychoneuro*):ti,ab,kw,ky,emt,mh,mc [Comorbidity: chronic physical illness] #4. (“physical* ill*” or “medical* ill*” or “chronic disease” or (chronic* NEXT (ill* or condition*1 or disease* or disorder* or health)) or (long term NEXT (condition*1 or sick*)) or “medical* morbid*” or (medical* NEXT (comorbid* or co morbid*)) or multimorbid* or (multi* NEXT (morbid* or “co morbid*” or comorbid* or physical))):ti,ab,kw,ky,emt,mh,mc #5. (AIDS or allerg* or angina or aneurysm or “ankylosing spondylitis” or arthropath* or arthriti* or arthrosis or arthroses or asthma* or “atrial fibrillation” or “autoimmune disease*” or “back pain” or blindness or “brain atroph*” or (bone NEXT (disease* or disorder*)) or ((bronchi* or bowel) NEXT (disease* or disorder*)) or bypass or (cancer or neoplasm* or neoplastic or malignan*) or (cardiac NEXT (arrest or arrhythmia* or surg*)) or cardiomyopath* or ((cardiovascular or coronary) NEAR2 (disease* or disorder* or event*)) or “cerebral palsy” or (cerebrovascular NEAR2 (disease* or disorder* or event*)) or “chronic obstructive” or COPD or pain or cirrhosis or colitis or “congenital abnormalit*” or (congential NEAR3 (disease or disorder*)) or coxarthrosis or Crohn* or Cushing* or “cystic fibrosis” or cystitis) #6. (deaf* or deformit* or disabled or (physical NEXT (deform* or disab* or impair*)) or dermatitis or dermato* or dorsopath* or diabet* or “digestive system*” or duoden* or dystonia or eczema or (endocrine NEXT (disease* or disorder*)) or enuresis or epilep* or “eye disease*” or (“fatigue syndrome” or “chronic fatigue”) or fibromyalgia or fibrosis or “food hypersensitivity” or (gastr* NEXT (disease* or disorder*)) or gastritis or “genetic disorder*” or gout or (glomerul* NEXT (disease* or disorder*)) or headache* or ((h?emic or lymph*) NEXT (disease* or disorder*)) or h?ematuria or h?emophili* or h?emorrhag* or ((hearing or visual or vision) NEAR2 (aid* or impair* or loss)) or hemiplegi* or hepatitis or h?emodialysis or ((renal or kidney) NEXT (disease* or disorder* or failure)) or (heart NEXT (disease* or disorder* or failure or surg*)) or HIV or “human immunodeficiency virus” or hypertensi* or hypotensi*) #7. (“inflammatory disease*” or incontinen* or “irritable bowel” or isch?emi* or (joint NEXT (disease* or disorder*)) or kyphosis or leuk?emia or ((liver or hepatic) NEXT (disease* or disorder* or failure)) or lordosis or “lung disease*” or “lupus erythemat*” or lymphoma or “macular degeneration” or migraine* or “movement disorder*” or musculoskeletal or necrotizing or nephrotic* or neuromuscular or “multiple sclerosis” or myeloma) #8. (“nephrotic syndrome” or ((nutritional or metabolic) NEXT (disease* or disorder or syndrome*)) or (organ* NEAR2 (transplant* or recipient*)) or (neurological NEXT (disease* or disorder*)) or occlusion* or obesity or obese or orthop?edic* or osteo* or “otitis media” or otorhinolaryngology* or otosclerosis or pancrea* or papulosquamous or paraplegi* or parkinson* or “peripheral vascular” or “pick disease*” or pneumoconiosis or polio* or polyarthropath* or polyarteritis or polyarthrosis or polyneuropath* or psoriasis or parapsoriasis or (pulmonary NEAR2 (disease* or disorder*))) #9. ((respiratory NEXT (disease* or disorder*)) or retinopathy or rheumat* or sclerosis or scoliosis or “sickle cell an?emia” or ((skin or “connective tissue”) NEXT (disease* or disorder*)) or (“sleep disorder*” or “sleep apn?ea” or insomnia* or dyssomnia* or hypersomnia*) or “spina bifida” or “spinal muscular atropy” or spondylo* or stenosis* or stoma* or (stroke or strokes or “cerebral infarct*”) or tetraplegi* or ((thyroid NEAR (disease* or disorder* or dysfunction*)) or hyperthyroidism or hypothyroidism) or tuberculosis or (systemic NEAR (disorder* or disease*)) or ulcer* or (urogenital NEXT (disease* or disorder*)) or vasculopath* or (vascular NEAR (disease* or disorder*)) or vestibular or ((virus or viral) NEXT disease)) #10. (#1 and (#2 or #3) and (#4 or #5 or #6 or #7 or #8 or #9))
Key to field codes: ti: title; ab: abstract; kw: CCMD keywords; ky: additional keywords; emt: EMTREE subject headings; mh:MeSH subject headings; mc: MeSH check words
Appendix 3. Review search: CENTRAL via CRSO
The Cochrane Central Register of Controlled Trials (CENTRAL) was searched (via the Cochrane Register of Studies Online (CRSO)), using a sensitive set of terms for age group, condition, comorbidity and intervention:
[Age Group] #1 (child* or boy* or girl* or infant* or juvenil* or minors or paediatric* or pediatric* or school* or preschool* or pre‐school* or kindergarten or nursery or adolesc* or preadolesc* or pre‐adolesc* or pubert* or pubescen* or prepube* or pre‐pube* or high‐school or teen* or (young next (adult* or people or patient* or men* or women* or mother* or male or female or survivor* or offender* or minorit*)) or youth* or student* or undergrad* or college or campus or classroom):ti,ab [Condition: anxiety/depression] #2 ((emotion* or psycholog* or mental) next (health or stress* or problem* or disturb* or aspect* or state* or ill*)) #3 (depress* or mood or anxiety or *phobi* or PTSD or post‐trauma* or posttrauma or “post trauma*” or panic* or OCD or obsess* or compulsi* or GAD or "stress disorder*" or “stress reaction*” or "acute stress" or “psychological stress” or “school refusal” or mutism or neurosis or neuroses or neurotic or psychoneuro*) [Comorbidity: chronic physical illness] #4 (“physical* ill*” or “medical* ill*” or “chronic disease” or (chronic* NEXT (ill* or condition*1 or disease* or disorder* or health)) or (long term NEXT (condition*1 or sick*)) or “medical* morbid*” or (medical* NEXT (comorbid* or co morbid*)) or multimorbid* or (multi* NEXT (morbid* or “co morbid*” or comorbid* or physical))) #5 (allerg* or angina or aneurysm or “ankylosing spondylitis” or arthropath* or arthriti* or arthrosis or arthroses or asthma* or “atrial fibrillation” or “autoimmune disease*” or “back pain” or blindness or “brain atroph*” or (bone NEXT (disease* or disorder*)) or ((bronchi* or bowel) NEXT (disease* or disorder*)) or bypass or (cancer or neoplasm* or neoplastic or malignan*) or (cardiac NEXT (arrest or arrhythmia* or surg*)) or cardiomyopath* or ((cardiovascular or coronary) NEAR2 (disease* or disorder* or event*)) or “cerebral palsy” or (cerebrovascular NEAR2 (disease* or disorder* or event*)) or “chronic obstructive” or COPD or pain or cirrhosis or colitis or “congenital abnormalit*” or (congential NEAR3 (disease or disorder*)) or coxarthrosis or Crohn* or Cushing* or “cystic fibrosis” or cystitis) #6 (deaf* or deformit* or disabled or (physical NEXT (deform* or disab* or impair*)) or dermatitis or dermato* or dorsopath* or diabet* or “digestive system*” or duoden* or dystonia or eczema or (endocrine NEXT (disease* or disorder*)) or enuresis or epilep* or “eye disease*” or (“fatigue syndrome” or “chronic fatigue”) or fibromyalgia or fibrosis or “food hypersensitivity” or (gastr* NEXT (disease* or disorder*)) or gastritis or “genetic disorder*” or gout or (glomerul* NEXT (disease* or disorder*)) or headache* or ((h?emic or lymph*) NEXT (disease* or disorder*)) or h?ematuria or h?emophili* or h?emorrhag* or ((hearing or visual or vision) NEAR2 (aid* or impair* or loss)) or hemiplegi* or hepatitis or h?emodialysis or ((renal or kidney) NEXT (disease* or disorder* or failure)) or (heart NEXT (disease* or disorder* or failure or surg*)) or HIV or “human immunodeficiency virus” or hypertensi* or hypotensi*) #7 (“inflammatory disease*” or incontinen* or “irritable bowel” or isch?emi* or (joint NEXT (disease* or disorder*)) or kyphosis or leuk?emia or ((liver or hepatic) NEXT (disease* or disorder* or failure)) or lordosis or “lung disease*” or “lupus erythemat*” or lymphoma or “macular degeneration” or migraine* or “movement disorder*” or musculoskeletal or necrotizing or nephrotic* or neuromuscular or “multiple sclerosis” or myeloma) #8 (“nephrotic syndrome” or ((nutritional or metabolic) NEXT (disease* or disorder or syndrome*)) or (organ* NEAR2 (transplant* or recipient*)) or (neurological NEXT (disease* or disorder*)) or occlusion* or obesity or obese or orthop?edic* or osteo* or “otitis media” or otorhinolaryngology* or otosclerosis or pancrea* or papulosquamous or paraplegi* or parkinson* or “peripheral vascular” or “pick disease*” or pneumoconiosis or polio* or polyarthropath* or polyarteritis or polyarthrosis or polyneuropath* or psoriasis or parapsoriasis or (pulmonary NEAR2 (disease* or disorder*))) #9 ((respiratory NEXT (disease* or disorder*)) or retinopathy or rheumat* or sclerosis or scoliosis or “sickle cell an?emia” or ((skin or “connective tissue”) NEXT (disease* or disorder*)) or (“sleep disorder*” or “sleep apn?ea” or insomnia* or dyssomnia* or hypersomnia*) or “spina bifida” or “spinal muscular atropy” or spondylo* or stenosis* or stoma* or (stroke or strokes or “cerebral infarct*”) or tetraplegi* or ((thyroid NEAR (disease* or disorder* or dysfunction*)) or hyperthyroidism or hypothyroidism) or tuberculosis or (systemic NEAR (disorder* or disease*)) or ulcer* or (urogenital NEXT (disease* or disorder*)) or vasculopath* or (vascular NEAR (disease* or disorder*)) or vestibular or ((virus or viral) NEXT disease)) #10 ((#1 and (#2 or #3) and (#4 or #5 or #6 or #7 or #8 or #9)) [Intervention: psychological therapies] #11 MESH DESCRIPTOR Psychotherapy EXPLODE ALL TREES #12 ((psychologic* or behavio?r or cognitive) adj3 (intervent* or therap* or treat* or manag*)):ti,ab #13 (abreaction or “acting out” or (acceptance NEAR2 commitment) or “activity scheduling” or adlerian or “analytical therap*” or “anger control” or “anger management” or “art therap*” or “assertive* training” or “attention bias modification” or “autogenic training” or autosuggestion or “aversion therap*” or “balint group” or “behavio* activation” or “behavio* contracting” or “behavio* modification” or “behavio* therap*” or bibliotherap* or “body therap*” or “brief therapy” or catharsis or “client cent* therapy” or “cognitive behavio*”or “cognitive therap*” or CBT or cCBT or iCBT or “cognitive rehabilitation” or “cognitive restructur*” or “colour therap*” or “color therap*” or “compassion focus*” or “compassionate therap*” or “conjoint therap*” or “contingency management” or “conversion therap*” or “conversational therap*” or countertransference or “coping skill*” or counsel* or “covert sensitization” or “crisis intervention” or “crisis management”) #14 ((dialectic* NEAR2 therap*) or "diffusion therap*" or "distraction therap*" or (dream* NEAR3 analys*) or "eclectic therap*" or "emotion* focus* therap*" or "emotional freedom technique" or "encounter group therap*" or existential or experiential or "exposure therap*" or "expressive therap*" or "eye movement desensiti#ation" or "family therap*" or "focus oriented" or "free association" or freudian or "functional analysis" or gestalt or griefwork or "group therap*" or "guided image*" or "holistic therap*" or humanistic or hypnosis or hypnotherapy or hypnoti#zability or "implosive therap*" or "insight therap*" or "integrative therap*" or "interpersonal therap*" or Jungian or kleinian) #15 (logotherap* or "logo therap*" or meditation or "mental healing" or metacognitive or meta‐cognitive or milieu or "mind train*" or mindfulness or morita or "multimodal therap*" or music or "narrative therap*" or "nondirective therap*" or non‐directive therap* or "nondirective therap*" or "non‐specific therap*" or "nonspecific therap*" or "object relations" or "personal construct therap*" or "person cent* therap*" or "persuasion therap*" or "pet therap*" or "animal therap*" or "play therap*" or ((pleasant or pleasing) NEAR2 event*) or "present cent* therap*" or "primal therap*" or "problem focus* therap*" or "problem sol*" or "process experiential" or psychoanaly* or psychodrama or psychodynamic or psychoeducat* or psychotherap*) #16 ("rational emotive" or "reality therap*" or "reciprocal inhibition" or "relationship therap*" or "relaxation stress management" or "relaxation technique*" or "relaxation therap*" or "relaxation training" or "reminiscence therap*" or rogerian or "role play*" or schema or "self analys*" or "self esteem building" or "sensitivity training" or "sleep phase chronotherap*" or "socioenvironment* therap*" or "social skill*" or sociotherap* or "solution focused therap*" or "stress management" or "support group*" or (support NEAR3 psycho*) or "supportive therap*" or "systematic desensiti*" or "systemic *therap*" or "therapeutic communit*" or "therapeutic technique" or "third wave" or "time limited therap*" or "transference therap*" or "transactional analysis" or transtheoretical or "validation therap*") #17 (#11 OR #12 OR #13 OR #14 OR #15 OR #16) #18 (#10 AND #17)
Appendix 4. Review search update 2017/2018
In August 2017 the CCMD group's information specialist updated the search of CENTRAL and conducted a new cross‐search on the Ovid databases (2016 onwards) (MEDLINE, Embase and PsycINFO). This was because the CCMD‐CTR was out‐of‐date at the time.
Date of search: 18 August 2017 CENTRAL retrieved 209 records, Ovid XSearch 790. These were de‐duplicated against each other and records retrieved in May 2016, leaving 787 new records to screen.
In September 2018 the searches were updated once more. Date of search: 27 September 2018, (2017 onwards). CENTRAL retrieved 270 records, Ovid XSearch 731. The records were de‐duplicated against each other and all previous search results, leaving 539 new records to screen.
1. The Cochrane Central Register of Controlled Trials (CENTRAL) CENTRAL was searched (via the Cochrane Register of Studies Online (CRSO)) Issue 8, 2017 and Issue 9 2018. The search terms are listed in Appendix 3.
2. Ovid XSearch (Embase, MEDLINE, PsycINFO)
Ovid databases searched: PsycINFO 1806 to August Week 2 2017, Embase 1974 to 2017 Week 33, Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to 18 Aug 2017.
Date limited: 1 Jan 2016 to 18‐Aug‐2017 (and 1‐Jan‐2017 to 27‐Sept‐2018).
Search Terms: 1. (child* or boy* or girl* or infant* or juvenil* or minors or paediatric* or pediatric* or school* or preschool* or pre‐school* or kindergarten or nursery or adolesc* or preadolesc* or pre‐adolesc* or pubert* or pubescen* or prepube* or pre‐pube* or high‐school or teen* or (young adj3 (adult* or people or patient* or men* or women* or mother* or male or female or survivor* or offender* or minorit*)) or youth* or student* or undergrad* or college or campus or classroom).ti,ab,kf,kw,id,hw. 2. ((emotion* or psycholog* or mental) adj3 (health or stress* or problem* or disturb* or aspect* or state* or ill*)).ti,ab,kf,kw,id,hw. 3. (depress* or mood or anxiety or agoraphobi* or phobi* or PTSD or post‐trauma* or posttrauma or post trauma* or panic* or OCD or obsess* or compulsi* or GAD or stress disorder* or stress reaction* or acute stress or psychological stress or school refusal or mutism or neurosis or neuroses or neurotic or psychoneuro*).ti,ab,kf,kw,id,hw. 4. or/2‐3 5. (physical* ill* or medical* ill* or chronic disease or (chronic* adj3 (ill* or condition*1 or disease* or disorder* or health)) or (long term adj3 (condition*1 or sick*)) or medical* morbid* or (medical* adj3 (comorbid* or co morbid*)) or multimorbid* or (multi* adj (morbid* or co morbid* or comorbid* or physical))).ti,ab,kf,kw,id,hw. 6. (allerg* or angina or aneurysm or ankylosing spondylitis or arthropath* or arthriti* or arthrosis or arthroses or asthma* or atrial fibrillation or autoimmune disease* or back pain or blindness or brain atroph* or (bone adj (disease* or disorder*)) or ((bronchi* or bowel) adj (disease* or disorder*)) or bypass or (cancer or neoplasm* or neoplastic or malignan*) or (cardiac adj (arrest or arrhythmia* or surg*)) or cardiomyopath* or ((cardiovascular or coronary) adj2 (disease* or disorder* or event*)) or cerebral palsy or (cerebrovascular adj2 (disease* or disorder* or event*)) or chronic obstructive or COPD or pain or cirrhosis or colitis or congenital abnormalit* or (congential adj3 (disease or disorder*)) or coxarthrosis or Crohn* or Cushing* or cystic fibrosis or cystitis).ti,ab,kf,kw,id,hw. 7. (deaf* or deformit* or disabled or (physical adj (deform* or disab* or impair*)) or dermatitis or dermato* or dorsopath* or diabet* or digestive system* or duoden* or dystonia or eczema or (endocrine adj (disease* or disorder*)) or enuresis or epilep* or eye disease* or (fatigue syndrome or chronic fatigue) or fibromyalgia or fibrosis or food hypersensitivity or (gastr* adj (disease* or disorder*)) or gastritis or genetic disorder* or gout or (glomerul* adj (disease* or disorder*)) or headache* or ((h?emic or lymph*) adj (disease* or disorder*)) or h?ematuria or h?emophili* or h?emorrhag* or ((hearing or visual or vision) adj2 (aid* or impair* or loss)) or hemiplegi* or hepatitis or h?emodialysis or ((renal or kidney) adj (disease* or disorder* or failure)) or (heart adj (disease* or disorder* or failure or surg*)) or HIV or human immunodeficiency virus or hypertensi* or hypotensi*).ti,ab,kf,kw,id,hw. 8. (inflammatory disease* or incontinen* or irritable bowel or isch?emi* or (joint adj (disease* or disorder*)) or kyphosis or leuk?emia or ((liver or hepatic) adj (disease* or disorder* or failure)) or lordosis or lung disease* or lupus or lymphoma or macular degeneration or migraine* or movement disorder* or musculoskeletal or necrotizing or nephrotic* or neuromuscular or multiple sclerosis or myeloma).ti,ab,kf,kw,id,hw. 9. (nephrotic syndrome or ((nutritional or metabolic) adj (disease* or disorder or syndrome*)) or ((organ* or kidney or stem cell) adj2 (transplant* or recipient*)) or (neurological adj (disease* or disorder*)) or occlusion* or obesity or obese or orthop?edic* or osteo* or otitis media or otorhinolaryngolog* or otosclerosis or pancrea* or papulosquamous or paraplegi* or parkinson* or (peripheral adj (arterial or vascular)) or pick disease* or pneumoconiosis or polio* or polyarthropath* or polyarteritis or polyarthrosis or polyneuropath* or psoriasis or parapsoriasis or (pulmonary adj2 (disease* or disorder*))).ti,ab,kf,kw,id,hw. 10. ((respiratory adj (disease* or disorder*)) or retinopathy or rheumat* or sclerosis or scoliosis or sickle cell an?emia or ((skin or connective tissue) adj (disease* or disorder*)) or (sleep disorder* or sleep apn?ea or insomnia* or dyssomnia* or hypersomnia*) or spina bifida or spinal muscular atropy or spondylo* or stenosis* or stoma* or (stroke or strokes or cerebral infarct*) or tetraplegi* or ((thyroid adj (disease* or disorder* or dysfunction*)) or hyperthyroidism or hypothyroidism) or tuberculosis or (systemic adj5 (disorder* or disease*)) or ulcer* or (urogenital adj (disease* or disorder*)) or vasculopath* or (vascular adj5 (disease* or disorder*)) or vestibular or ((virus or viral) adj disease)).ti,ab,kf,kw,id,hw. 11. or/5‐10 12. exp Psychotherapy/ or exp Psychotherapeutic Techniques/ 13. exp Child Psychotherapy/ or exp Adolescent Psychotherapy/ 14. ((psychologic* or behavio?r or cognitive) adj3 (intervent* or therap* or treat* or manag*)).ti,ab,id,kf,kw. 15. (abreaction or acting out or (acceptance adj2 commitment) or activity scheduling or adlerian or analytical therap* or anger control or anger management or art therap* or assertive* training or attention bias modification or autogenic training or autosuggestion or aversion therap* or balint or behavio* activation or behavio* contracting or behavio* modification or behavio* therap* or bibliotherap* or body therap* or brief therapy or catharsis or client cent* therapy or cognitive behavio* or cognitive therap* or CBT or cCBT or iCBT or cognitive rehabilitation or cognitive restructur* or colour therap* or color therap* or compassion focus* or compassionate therap* or conjoint therap* or contingency management or conversion therap* or conversational therap* or countertransference or coping skill* or counsel* or covert sensitization or crisis intervention or crisis management).ti,ab,kf,kw,id,hw. 16. ((dialectic* adj2 therap*) or diffusion therap* or distraction therap* or (dream* adj3 analys*) or eclectic therap* or emotion* focus* therap* or emotional freedom technique or encounter group therap* or existential or experiential or exposure therap* or expressive therap* or eye movement desensiti#ation or family therap* or focus oriented or free association or freudian or functional analysis or gestalt or griefwork or group therap* or guided image* or holistic therap* or humanistic or hypnosis or hypnotherapy or hypnoti#zability or implosive therap* or insight therap* or integrative therap* or interpersonal therap* or Jungian or kleinian).ti,ab,id,kf,kw,hw. 17. (logotherap* or logo therap* or meditation or mental healing or metacognitive or meta‐cognitive or milieu or mind train* or mindfulness or morita or multimodal therap* or music or narrative therap* or nondirective therap* or non‐directive therap* or nondirective therap* or non‐specific therap* or nonspecific therap* or object relations or personal construct therap* or person cent* therap* or persuasion therap* or pet therap* or animal therap* or play therap* or ((pleasant or pleasing) adj2 event*) or present cent* therap* or primal therap* or problem focus* therap* or problem sol* or process experiential or psychoanaly* or psychodrama or psychodynamic or psychoeducat* or psychotherap*).ti,ab,kf,kw,id,hw. 18. (rational emotive or reality therap* or reciprocal inhibition or relationship therap* or relaxation stress management or relaxation technique* or relaxation therap* or relaxation training or reminiscence therap* or rogerian or role play* or schema or self analys* or self esteem building or sensitivity training or sleep phase chronotherap* or socioenvironment* therap* or social skill* or sociotherap* or solution focused therap* or stress management or support group* or (support adj3 psycho*) or supportive therap* or systematic desensiti* or systemic *therap* or therapeutic communit* or therapeutic technique or third wave or time limited therap* or transference therap* or transactional analysis or transtheoretical or validation therap*).ti,ab,kf,kw,id,hw. 19. or/12‐18 20. trial.ti. 21. (randomi#ed or randomi#ation or randomi#ing).ti,ab,kf,kw,id. 22. (RCT or at random or (random* adj3 (assign* or allocat* or control* or crossover or cross‐over or design* or divide* or division or number))).ti,ab,kf,kw,id. 23. placebo.hw,ti,ab,kf,kw,id. 24. ((control* adj2 (trial or study or group)) and (placebo or waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,kf,kw,id,hw. 25. Randomized Controlled Trial.sh,pt. 26. Double Blind Procedure/ 27. Double Blind Method/ 28. (clinical trial or empirical study).md. 29. ((single or double or triple) adj2 (blind* or mask* or dummy)).ti,ab,kf,kw,id. 30. or/20‐29 31. ((animal or nonhuman) not (human and (animal or nonhuman))).hw. 32. (30 not 31) 33. (1 and 4 and 11 and 19 and 32) 34. (2016* or 2017* or 2018*).yr,em,dd,dc,ed. 35. (33 and 34) 36. (case adj (control* or report?)).ti,kf,kw,id,hw. 37. (review or letter or comment*).ti,hw,pt. 38. (dental or dentist* or an?esthes*).ti,hw,jw. 39. or/36‐38 40. (35 not 39)
Appendix 5. Characteristics of excluded studies
| Study | Reason for exclusion |
| AmbrosinoJm 2008 | Outcome: did not measure anxiety or depression |
| Barsevick 2002 | Participants: adult not children |
| Bauman 1994 | Outcome: did not measure anxiety or depression |
| Belsky 1994 | Design: pilot study. Not a randomised controlled trial |
| Brierley 2013 | Design: systematic review. Not a randomised controlled trial |
| BrownLk 2016 | Participants: adult not children |
| Chalder 2002 | Design: uncontrolled study. Not a randomised controlled trial |
| Chalder 2004 | Outcome: did not measure anxiety or depression |
| Chernoff 2002 | Intervention: targets families not children |
| Eccleston 2014 | Design: systematic review. Not a randomised controlled trial |
| Fisher 2014 | Design: systematic review. Not a randomised controlled trial |
| Garcia Perez 2010 | Design: prospective cohort study. Not a randomised controlled trial |
| Gauntlett Gilbert 2013 | Design: uncontrolled trial. Not a randomised controlled trial |
| Gebert 1998 | Outcome: did not measure anxiety or depression |
| Glasscoe 2008 | Design: systematic review. Not a randomised controlled trial |
| Goldbeck 2014 | Design: systematic review. Not a randomised controlled trial |
| Graham 2016 | Design: systematic review. Not a randomised controlled trial |
| Groß 2013 | Outcome: did not measure anxiety or depression |
| Grover 2002 | Participants: adult not children |
| Gulewitsch 2011 | Design: systematic review. Not a randomised controlled trial |
| Gulewitsch 2012 | Design: pilot study. Not a randomised controlled trial |
| Hampson 2001 | Design: systematic review. Not a randomised controlled trial |
| Hesse 2015 | Design: pilot study. Not a randomised controlled trial |
| Jeppesen 2012 | Outcome: did not measure anxiety or depression |
| Lemanek 2009 | Intervention: wrong type of intervention |
| Long 2011 | Design: pilot study. Not a randomised controlled trial |
| Lyon 2014 | Intervention: wrong type of intervention |
| Malboeuf Hurtubise 2014 | Duplicate article |
| Malboeuf Hurtubise 2016 | Design: pilot study. Not a randomised controlled trial |
| May 2005 | Design: systematic review. Not a randomised controlled trial |
| Muglia Wechsler 2014 | Design: systematic review. Not a randomised controlled trial |
| Naar King 2010 | Participants: adult not children |
| NICE 2014 | Design: systematic review. Not a randomised controlled trial |
| OsterhausSo 1993 | Design: not a randomised controlled trial |
| Pai 2006 | Design: systematic review. Not a randomised controlled trial |
| Pless 1994 | Participants; adults not children |
| Prasko 2010 | Design: systematic review. Not a randomised controlled trial |
| Rastogi 2012 | Design: systematic review. Not a randomised controlled trial |
| Ribeiro 2008 | Participants: adults not children |
| Riley 2015 | Design: wrong study design |
| Robb 2014 | Design: systematic review. Not a randomised controlled trial |
| Saedi 2012 | Design: wrong study design |
| Scholten 2013 | Outcome: did not measure anxiety or depression |
| Seitz 2009 | Design: systematic review. Not a randomised controlled trial |
| Shoshani 2016 | Design: wrong study design |
| Stapersma 2018 | Participants: adult population (<80% of participants were 18 years) |
| Stubbe 2008 | Participants: not limited to children with chronic illness |
| Timmer 2011 | Design: systematic review. Not a randomised controlled trial |
| Yorke 2007 | Design: systematic review. Not a randomised controlled trial |
| Westrupp 2015 | Outcome: wrong outcomes |
| Yorke 2017 | Partcipants: adult population (<80% of participants were 18 years) |
Footnotes
Data and analyses
Comparison 1. Psychological therapy versus any comparator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment efficacy: depression, short‐term | 16 | 1121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.59, ‐0.03] |
| 2 Treatment efficacy: depression long‐term | 5 | 258 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.68, 0.19] |
| 3 Treatment efficacy: anxiety short‐term | 13 | 578 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.59, 0.07] |
| 4 Treatment efficacy: anxiety long‐term | 4 | 131 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.66, 0.20] |
| 5 Quality of life short ‐term | 7 | 464 | Std. Mean Difference (IV, Random, 95% CI) | 1.13 [0.44, 1.82] |
| 6 Quality of life long‐term | 3 | 113 | Std. Mean Difference (IV, Random, 95% CI) | 0.71 [‐0.52, 1.93] |
| 7 Functioning short‐term | 7 | 483 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [‐0.30, 1.29] |
| 8 Functioning long‐term | 2 | 142 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.80, 0.71] |
| 9 Status of long‐term physical condition short‐term | 14 | 823 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.61, ‐0.06] |
| 10 Status of long‐term physical condition long‐term | 2 | 142 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.36, 0.29] |
| 11 Sensitivity analysis by target of therapy depression | 6 | 499 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.64, ‐0.41] |
| 12 Sensitivity analysis by target of therapy anxiety | 5 | 305 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.05, ‐0.28] |
Comparison 2. Psychological therapy versus attention placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment efficacy: depression short‐term | 6 | 473 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.19, 0.25] |
| 2 Treatment efficacy: depression long‐term | 2 | 175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.50, 0.10] |
| 3 Treatment efficacy: anxiety short‐term | 4 | 174 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.10, 0.73] |
| 4 Treatment efficacy: anxiety long‐term | 2 | 91 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.58, 0.26] |
| 5 Quality of life short‐term | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.33, 0.63] |
| 6 Quality of life long‐term | 1 | 61 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.74, 0.27] |
| 7 Functioning short‐term | 4 | 350 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.45, ‐0.02] |
| 8 Functioning long‐term | 1 | 112 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.74, 0.01] |
| 9 Status of long‐term physical condition short‐term | 5 | 380 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.46, 0.01] |
| 10 Status of long‐term physical condition long‐term | 2 | 142 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.36, 0.29] |
Comparison 3. Psychological therapy versus psychological placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment efficacy: depression short‐term | 3 | 277 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.63, 0.07] |
| 2 Treatment efficacy: depression long‐term | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.54, ‐0.05] |
| 3 Quality of life short‐term | 2 | 247 | Std. Mean Difference (IV, Random, 95% CI) | 0.88 [‐0.20, 1.96] |
| 4 Quality of life long‐term | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 1.81 [0.95, 2.68] |
| 5 Functioning short‐term | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.89, 0.54] |
| 6 Status of long‐term physical condition short‐term | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.49, ‐0.00] |
Comparison 4. Psychological therapy versus non‐psychological therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment efficacy: depression short‐term | 1 | 65 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.31, 0.91] |
| 2 Quality of life short‐term | 1 | 65 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐5.28, 6.88] |
| 3 Status of long‐term physical condition short‐term | 1 | 65 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.13, 0.13] |
Comparison 5. Psychological therapy versus treatment‐as‐usual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment efficacy: depression short‐term | 4 | 245 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.91, ‐0.39] |
| 2 Treatment efficacy: depression long‐term | 2 | 53 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.59, 1.39] |
| 3 Treatment efficacy: anxiety short‐term | 6 | 337 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.96, ‐0.17] |
| 4 Treatment efficacy: anxiety long‐term | 1 | 27 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.69, 0.87] |
| 5 Quality of life short‐term | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [‐0.07, 1.41] |
| 6 Functioning short‐term | 2 | 103 | Std. Mean Difference (IV, Random, 95% CI) | 2.34 [‐0.53, 5.21] |
| 7 Functioning long‐term | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.32, 1.14] |
| 8 Status of long‐term physical condition short‐term | 5 | 308 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.18, 0.14] |
Comparison 6. Psychological therapy versus waiting list.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment efficacy: depression short‐term | 2 | 61 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.39 [‐6.89, 2.12] |
| 2 Treatment efficacy: anxiety short‐term | 3 | 67 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.89, 0.09] |
| 3 Treatment efficacy: anxiety long‐term | 1 | 13 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐2.56, ‐0.03] |
| 4 Quality of life short‐term | 1 | 34 | Std. Mean Difference (IV, Fixed, 95% CI) | 5.20 [3.73, 6.68] |
| 5 Status of long‐term physical condition short‐term | 2 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.51, 0.73] |
Comparison 7. Psychological therapy versus any comparator (subgroup type therapy).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment efficacy: depression short‐term | 16 | 1038 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.60, ‐0.02] |
| 1.1 ACT | 2 | 64 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.19 [‐7.08, 2.69] |
| 1.2 CBT | 8 | 592 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.44, ‐0.09] |
| 1.3 Behaviour therapy | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Education | 1 | 74 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.36, ‐0.41] |
| 1.5 Mindfulness | 1 | 46 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.12, 1.07] |
| 1.6 Supportive therapy | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.42, 0.54] |
| 1.7 Other therapy | 3 | 196 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.81, 0.29] |
| 2 Treatment efficacy: anxiety short‐term | 13 | 578 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.59, 0.07] |
| 2.1 ACT | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 CBT | 4 | 137 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.48, 0.51] |
| 2.3 Behaviour therapy | 3 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.54, 0.28] |
| 2.4 Education | 1 | 74 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.16, ‐0.22] |
| 2.5 Mindfulness | 1 | 46 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.06, 1.26] |
| 2.6 Supportive therapy | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.36, 0.60] |
| 2.7 Other therapy | 3 | 162 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.76, ‐0.13] |
Comparison 8. Psychological therapy versus any comparator (subgroup modality of delivery).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment efficacy: depression short‐term | 16 | 1121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.59, ‐0.03] |
| 1.1 Individual therapy | 11 | 872 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.40, ‐0.06] |
| 1.2 Group therapy | 5 | 249 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.85, 0.33] |
| 2 Treatment efficacy: anxiety short‐term | 13 | 578 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.59, 0.07] |
| 2.1 Individual therapy | 9 | 380 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.42, 0.12] |
| 2.2 Group therapy | 4 | 198 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.43, 0.39] |
Comparison 9. Psychological therapy versus any comparator (subgroup dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment efficacy: depression short‐term | 16 | 1121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.59, ‐0.03] |
| 1.1 Brief therapy < 6 sessions or 6 weeks | 4 | 364 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.57, 0.29] |
| 1.2 Longer therapy >6 sessions or 6 weeks | 12 | 757 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.77, ‐0.02] |
| 2 Treatment efficacy: anxiety short‐term | 13 | 578 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.59, 0.07] |
| 2.1 Brief therapy < 6 sessions or 6 weeks | 7 | 285 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.52, 0.22] |
| 2.2 Longer therapy > 6 sessions or 6 weeks | 6 | 293 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.95, 0.22] |
Comparison 10. Psychological therapy versus any comparator (subgroup type of long‐term physical condition).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment efficacy: depression short‐term | 16 | 1121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.59, ‐0.03] |
| 1.1 Asthma | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Diabetes | 4 | 239 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐2.33, 0.09] |
| 1.3 Epilepsy | 2 | 134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.96, ‐0.26] |
| 1.4 Pain disorders | 6 | 418 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.24, 0.14] |
| 1.5 Inflammatory bowel disease | 2 | 257 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.59, ‐0.04] |
| 1.6 Other conditions | 2 | 73 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.90, 0.96] |
| 2 Treatment efficacy: anxiety short‐term | 13 | 578 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.59, 0.07] |
| 2.1 Asthma | 2 | 67 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.70, 0.47] |
| 2.2 Diabetes | 4 | 180 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.76, 0.16] |
| 2.3 Epilepsy | 1 | 104 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.92, ‐0.14] |
| 2.4 Pain disorders | 4 | 153 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.33, 0.54] |
| 2.5 Inflammatory bowel disease | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Other conditions | 2 | 74 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.78, 1.17] |
Comparison 11. Psychological therapy versus any comparator (subgroup severity of depression or anxiety symptoms).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment efficacy: depression short‐term | 16 | 1121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.59, ‐0.03] |
| 1.1 Subthreshold | 5 | 355 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.16, 0.31] |
| 1.2 Mild | 5 | 385 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.96, ‐0.18] |
| 1.3 Moderate | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.25, 0.02] |
| 1.4 Severe | 2 | 136 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐1.01, 0.65] |
| 1.5 Uncertain | 3 | 205 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.44, 0.11] |
| 2 Treatment efficacy: anxiety short‐term | 13 | 578 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.59, 0.07] |
| 2.1 Subthreshold | 1 | 46 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.06, 1.26] |
| 2.2 Mild | 5 | 249 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.81, ‐0.30] |
| 2.3 Moderate | 2 | 92 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.97, 0.15] |
| 2.4 Severe | 2 | 62 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.05, 0.96] |
| 2.5 Uncertain | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.42, 0.27] |
Comparison 12. Psychological therapy versus any comparator (subgroup target audience).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment efficacy: depression short‐term | 16 | 1121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.59, ‐0.03] |
| 1.1 Child only | 8 | 374 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.19, 0.15] |
| 1.2 Child and family | 8 | 747 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.42, ‐0.06] |
| 2 Treatment efficacy: anxiety short‐term | 13 | 578 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.59, 0.07] |
| 2.1 Child only | 10 | 409 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.64, 0.24] |
| 2.2 Child and family | 3 | 169 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.77, ‐0.16] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beebe 2010.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: community (school for children located on the campus of National Jewish Health, Denver, Colorado) Outcome measures used: Beck Youth Inventories; Paediatric Quality of Life Inventory (PEDSQL) Asthma Module completed at baseline, post‐intervention (7 weeks) and 6 months after the completion of the intervention |
|
| Participants |
Type of chronic illness: asthma (persistent) Inclusion criteria: 7‐14 years old; diagnosed with asthma Exclusion criteria: not described Baseline characteristics Overall Number: 22 Sex (males (%)): not reported Age in years (SD): not reported Ethnicities: not reported Depressive symptoms ‐ rating: not reported Anxiety symptoms ‐ rating: not reported Art therapy Number: not reported Sex (males (%)): not reported Age in years (SD): not reported Ethnicities: not reported Depressive symptoms ‐ rating: not reported Anxiety symptoms ‐ rating: not reported Waiting‐list control Number: not reported Sex (males (%)): not reported Age in years (SD): not reported Ethnicities: not reported Depressive symptoms ‐ rating: not reported Anxiety symptoms ‐ rating: not reported Baseline differences: not reported |
|
| Interventions |
Intervention characteristics Art therapy Description of intervention: one hour per week over seven weeks following a manualised structure Modality: group Dose: 7 weekly modules of 60 minutes duration Parent or caregiver involvement: completion of questionnaires only Therapist involvement: art therapist involved in all sessions Waiting‐list control Duration: 7 weeks Parent or caregiver involvement: completion of questionnaires only |
|
| Outcomes |
Specific depression measures: Beck Youth Inventory‐ no data included in meta‐analysis Specific anxiety measures: Beck Youth Inventory ‐ no data included in meta‐analysis Quality of life: PedsQL asthma module |
|
| Identification |
Sponsorship source: not stated Country: USA Comments: n/a Authors name: Anya Beeb Institution: National Jewish Health Email: beebea@njhealth.com Address: National Jewish Health, 1400 Jackson Street, Denver, Colorado, United States |
|
| Notes | Authors contacted for additional data, but no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: randomisation not clearly described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: blinding of participants not clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: blinding of outcome assessors not clearly described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: only one dropout, controls were the same as intervention group participants |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all outcome measures appeared to be reported |
| Other bias | Unclear risk | Judgement comment: no other sources of bias identified |
Bhana 2014.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatient clinics at two hospitals in Durban Outcome measures used: Children’s Depression Inventory (CDI) completed at baseline and at post‐intervention (3 months) |
|
| Participants |
Type of chronic illness: HIV Inclusion criteria: aged 10‐14 years old; aware of HIV status; both the caregiver and child provided written consent and assent Exclusion criteria: not stated Baseline Characteristics Overall Number: 65 Age in years (SD): not stated Sex (males (%)): 32 (52%) Ethnicities: all participants were Black South Africans of Zulu ethnicity with most speaking both English and Zulu Depressive symptoms ‐ rating: not reported VUKA Number: 33 Sex (males (%)): not reported Age: not reported Depressive symptoms ‐ rating: CDI 3.31 (SD not reported) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold Waiting‐list control Number: 32 Sex (males (%)): not reported Age in years (SD): not reported Depressive symptoms ‐ rating: CDI 3.19 (SD not reported) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold Baseline differences: no significant differences except the proportion of families receiving child support grants (100% in site 2 compared to less than 75% for site 1). |
|
| Interventions |
Intervention characteristics VUKA Audience: child and parent Description of intervention: a culturally‐tailored cartoon storyline and curriculum. Vuka tells the story of a 12‐year‐old orphaned boy with AIDS dealing with the challenges of diagnosis and treatment. It provides step‐by‐step guidance for counsellors to facilitate discussions and problem‐solving within and between families in multi‐family groups. Modality: group Dose: 6 sessions over three months Manualised or non‐manualised: manualised Parent or caregiver involvement: parents involved in groups and feedback questionnaires Therapist involvement: delivered to the participant by counsellors Waiting‐list control Duration: three months Parent or caregiver involvement: completion of assessment (1 session) |
|
| Outcomes | Specific depression measures: Children’s Depression Inventory (CDI)‐ no data included in meta‐analysis | |
| Identification |
Sponsorship source: not stated Country: South Africa Comments: contacted author to clarify data details (table 2 and table 3 data are confusing, no outcomes have been entered), author response needs action (email forwarded to Hiran and listed in contacted authors table) Authors name: Arvin Bhana Institution: Human Sciences Research Council, Human Social Development Email: arvin.bhana@mrc.ac.za Address: Human Sciences Research Council, Human Social Development, Durban, South Africa |
|
| Notes | Numbers for groups amended following email from author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: randomisation not clearly described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: blinding of participants not clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: blinding of outcome assessors not clearly described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: there was insufficient information about how missing data was handled |
| Selective reporting (reporting bias) | Low risk | Judgement comment: poor quality of reporting ‐ different numbers in different tables between the two sites, hard to interpret |
| Other bias | High risk | Judgement comment: the study was conducted by the developers of the intervention |
Bignall 2015.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: high school Outcome measures used: State Trait Anxiety Inventory – Trait version (STAI‐T); Asthma Control Test (ACT); Pediatric Quality of Life Inventory (PEDSQL); Forced expiratory volume (FEV) completed at baseline and at post‐intervention (1 month) |
|
| Participants |
Type of chronic illness: asthma Inclusion criteria: self‐report of African‐American or black ethnicity, English‐speaking, diagnosis of asthma via the school‐based health centre (SBHC) referrals and a self‐reported history of a provider‐diagnosed asthma and a raw score of 20 or less on the Asthma Control Test (ACT) Exclusion criteria: raw score greater than 20 on the Asthma Control Test, individuals who opted out of the study by calling the research team Baseline characteristics Overall Number: 30 Sex (males (%)): 8 (34%) Age in years (SD): 15.38 (2.97) Ethnicities: African‐American Anxiety symptoms ‐ rating: not reported Relaxation/breathing retraining intervention Number: 14 Sex (males (%)): 4 (33.3%) Age in years (SD): 15.52 years (1.5) Anxiety symptoms ‐ rating (SD): STAI‐T 41.62 (3.51) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): severe Educational intervention Number: 16 Sex (males (%)): 4 (33.3%) Age in years (SD): 15.29 (1.04) Anxiety symptoms ‐ rating (SD): STAI‐T 39.98 (3.16) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): severe Baseline differences: there were no significant differences at baseline |
|
| Interventions |
Intervention characteristics Breathing retraining and standard asthma education programme Audience: child Description of intervention: asthma education with a set of relaxation/breathing retraining skills for improving asthma control and anxiety. Modality: individual Dose: 2 sessions of 30 minutes over one month with a follow‐up phone call Manualised or non‐manualised: manualised Parent or caregiver involvement: none Therapist involvement: session delivery and follow‐up phone call Educational intervention (TAU) Audience: child Description of intervention: standard treatment‐as‐usual asthma education focused on the pathophysiology of asthma, standard symptom management techniques, and basic principles of the mind‐body connection as it relates to asthma Modality: individual Dose: 2 sessions of 30 minutes over one month with follow‐up phone call Manualised or non‐manualised: non‐manualised Therapist involvement: session delivery and follow‐up phone call |
|
| Outcomes |
Specific anxiety measures: the State Trait Anxiety Inventory (STAI) Improvement in quality of life: Pediatric Quality of Life Inventory (PEDSQL) Status of long‐term physical condition: FEV1 (forced expiratory volume); Asthma Control Test (ACT) |
|
| Identification |
Sponsorship source: Community Academic Partnership institutional grant Country: USA Comments: n/a Authors name: Whitney J. Bignall (correspondence: Sian Cotton) Institution: University of Cincinnati Email: sian.cotton@uc.edu Address: Division of Integrative Medicine,Department of Family and Community Medicine, University of Cincinnati, P.O. Box 670566, Cincinnati, OH 45267‐0566 USA |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: randomisation process not clearly described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: blinding of participants not clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: blinding of outcome assessors not clearly described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: 3 participants dropped out and it was unclear how their data was handled |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all outcomes were reported |
| Other bias | High risk | Judgement comment: study carried out by developers of the intervention |
Bussone 1998.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatient clinic Outcome measures: State Trait Anxiety Inventory – State (STAI‐S); Pain Total Index completed at baseline, 3, 6, and 12 months |
|
| Participants |
Type of chronic illness: pain (headache) Inclusion criteria: aged between 11‐15 years; satisfied the International Headache Society criteria for episodic tension‐type headache; experienced a minimum of one headache per week; neurological examination and routine laboratory tests were negative Exclusion criteria: currently or previously receiving preventative prescribed pharmacological treatment for headache Baseline characteristics Overall Number: 30 Sex (males (%)): 15 (50%) Age in years (SD): not reported Severity of chronic illness: at least 1 headache per week (moderate severity), mean headache Duration in years (SD): 2.7 (2.0) Ethnicities: not reported Anxiety symptoms ‐ rating (SD): not reported Social Skills Intervention Number: 20 Sex (males (%)): 10 (50%) Age in years (SD): 11.1 (2.6) Severity of chronic illness: at least 1 headache per week of moderate severity Duration in years (SD): 2.6 (2.0) years Anxiety symptoms ‐ rating (SD): mean state anxiety 30.1 (3.4) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Placebo control Number: 10 Sex (males (%)): 5 (15%) Age in years (SD): 13.5 (+/‐ 1.5) Severity of chronic illness: at least 1 headache per week (moderate severity) Duration in years (SD): 2.7 (2.0) years Anxiety symptoms ‐ rating (SD): mean state anxiety 30.1 (5.1) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Baseline differences: not described |
|
| Interventions |
Intervention characteristics Biofeedback training relaxation group Audience: child Description of intervention: four sessions of progressive muscle relaxation training, lasting approximately 20 minutes per session. Six sessions of biofeedback training lasting 21 min and subjects were instructed not to practice relaxation at home in order to provide a pure test of in‐clinic treatment alone Modality: not reported Dose: 10 sessions (20 minutes each) + 4 follow‐up sessions with at least two days between any two sessions Manualised or non‐manualised: manualised Parent or caregiver involvement: not reported Therapist involvement: delivery of sessions Placebo relaxation group Description of intervention: 10 session program (2 visits per week for 5 weeks total) where participants were instructed to remain calm and attempt to relax. EMG monitoring took place throughout as per the intervention group but no feedback was given Modality: not reported Dose: 10 sessions (20 minutes each) + 4 follow‐up sessions with at least two days between any two sessions Manualised or non‐manualised: manualised Parent or caregiver involvement: not reported Therapist involvement: delivery of sessions |
|
| Outcomes |
Specific anxiety measures: State Trait Anxiety Inventory – state (STAI‐S) Status of Long Term Physical Condition: Pain Total Index |
|
| Identification |
Sponsorship source: Research grant from the National Institute of Neurological Disorders and Stroke, NS‐29855 Country: USA Comments: n/a Authors name: G Bussone Institution: University of West Florida Email: fandrasi@uwf.edu Address: Frank Andrasik, Behavioral Medicine Laboratory, University of West Florida, 11000 University Parkway, Pensacola,FL 32514, USA. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: method of randomisation not clearly described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: blinding of participants not clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: blinding of outcome assessors not clearly described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: 5 participants dropped out and were omitted from the analysis |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all collected data appears to be reported. |
| Other bias | Low risk | Judgement comment: no other sources of bias identified |
Chadi 2016.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatient clinic Questionnaires used: Beck Youth Depression Inventory (BYDI); Beck Youth Anxiety Inventory (BYAI); Pediatric Quality of Live Inventory (PEDSQL) completed at baseline, 1 week, post‐intervention (8 weeks), 11 and 18 weeks |
|
| Participants |
Type of chronic illness: chronic pain Inclusion criteria: 13‐18 years old; seeing a psychiatrist for follow‐up; has a condition that results in chronic pain for at least three months Exclusion criteria: lives more than one hour from treatment centre; unable to speak French; untreated psychosis or depression; active suicidal ideation unknown to the referral physician; intellectual disability Baseline characteristics Overall Number: 19 Sex (males (%)): 0 (0%) Age in years (SD): 15.8 (13.9‐17.8) Ethnicities: 16 (84%) white, 2 (11%) Hispanic, 1 (5%) black Depressive symptoms ‐ rating: not reported Anxiety symptoms ‐ rating (SD): not reported Mindfulness‐based Intervention Number: 10 Sex (males (%)): 0 (0%) Age in years (SD): 16.1 (13.8‐17.8) Severity of chronic illness: 5.9/10 (3.5‐7.8) Ethnicities: 9 (90%) white, 1 (10%) Hispanic, 0 (0%) black Depressive symptoms ‐ rating: not reported Anxiety symptoms ‐ rating (SD): not reported Waiting‐list control Number: 9 Sex (males (%)): 0 (0%) Age in years (SD): 15.6 (13.9‐16.8) Severity of chronic illness: 6.0/10 (4.8‐7.4) Ethnicities: 7 (78%) white, 1 (11%) Hispanic, 1 (11%) black Depressive symptoms ‐ rating: not reported Anxiety symptoms ‐ rating (SD): not reporte Baseline differences: none reported |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention Description of intervention: 8 weekly 90‐minute sessions led by psychiatry residents and adapted to adolescent concerns and preferences Modality: group Dose: 8 weekly sessions of 90 minutes Manualised or non‐manualised: manualised Parent or caregiver involvement: none Therapist involvement: therapist(s) ran the group sessions Waiting‐list control Duration: 8 weeks |
|
| Outcomes |
Specific depression measures: Beck Youth Depression Inventory ‐ no data included in meta‐analysis Specific anxiety measures: Beck Youth Anxiety Scale‐ no data included in meta‐analysis Improvement in quality of life: Pediatric Quality of Life Inventory (PEDSQL)‐ no data included in meta‐analysis |
|
| Identification |
Sponsorship source: American Academy of Pediatrics and Bell Canada Mental Health Fund Country: Canada Comments: n/a Authors name: Nicholas Chadi Institution: Centre Hospitalier Universitaire Sainte Justine Email: nicholas.chadi@umontreal.ca Address: Departments of Pediatrics, Child and Adolescent Psychiatry, Clinical Biochemistry, Centre Hospitalier Universitaire Sainte‐Justine, Montreal, Quebec, Canada |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: randomisation was done using a computer‐generated randomisation list and permuted block design, with block sizes of two or four. Participants were assigned to the experimental or the waiting‐list control group using a 1/1 ratio. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: participants were not blinded but the researcher was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: self‐report measures used. Unclear how feasibility measures were rated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: less than 30% loss to follow‐up and less than 20% difference between groups. However, absolute numbers lost per group and reasons not described. Outcome data for some metrics not reported (e.g. MDD and other Beck Youth Inventories). |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: not all Beck Inventories reported and some summarised data for outcomes |
| Other bias | High risk | Judgement comment: some of the authors were the inventors of the intervention |
Chiang 2009.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatient clinic Measures used: Chinese children’s anxiety scale; Forced Expiration Flow (FEV) completed at baseline and post‐intervention (12 weeks) |
|
| Participants |
Type of chronic illness: asthma Inclusion criteria: diagnosed with moderate‐to‐severe asthma at least 6 months before the study, experienced more than 5 asthmatic episodes per year, an AAS (asthma severity) score of 2, regularly treated with asthma medication in a paediatric clinic Exclusion criteria: participated in any other clinical trials Baseline characteristics Overall Number: 37 Sex (males (%)): 30 (62.5%) Age in years (SD): not specified Severity of chronic illness: moderate to severe Ethnicities: Chinese Anxiety symptoms ‐ rating (SD): not reported Self‐management and relaxation‐breathing training Number: 18 Sex (males (%)): 15 (68.2%) Age in years (SD): not specified Anxiety symptoms ‐ rating (SD): 31.73 (12.27) Self‐management program Number: 19 Sex (males (%)): 15 (57.7) Age in years (SD): not specified Depressive symptoms ‐ rating: not collected Anxiety symptoms ‐ rating (SD): mean 29.96 (11.96) Baseline differences: none. |
|
| Interventions |
Intervention characteristics Self‐management and relaxation‐breathing training Audience: child and parent Description of intervention: children were taught progressive muscle relaxation with the assistance of biofeedback. A relaxation CD, one‐page instruction sheet, and training was provided to parents to support their children. All participants also received the education programme described for the control group. Modality: child and family Dose: 1 x 30 min session of relaxation training followed by 12 weeks of practice at least 3 times/week for 30 minutes Manualised or non‐manualised: manualised Parent or caregiver involvement: parents involved in session and assisted children (especially younger ones) to practice relaxation strategies Therapist involvement: implement the self‐management education program each session, coach children in 30 minutes of relaxation‐breathing training each session, perform reminder phone calls weekly Asthma education program Type of control: treatment‐as‐usual Audience: child and parent Description of intervention: education program with five units: (a) reforming asthma cognition, (b) correct usage of asthma drugs, (c) establishing a safe home environment, (d) monitoring with a peak flow meter, and (e) keeping an asthma diary. Educational booklet on personal care plans, peak flowmeter records (with one meter given to each family), and using a diary to record asthma signs/symptoms was provided. Modality: child and family Dose: unclear Time required and duration, including homework: unclear Manualised or non‐manualised: manualised Parent or caregiver involvement: parents involved in session Therapist involvement: implemented the self‐management education program |
|
| Outcomes |
Specific anxiety measures: Chinese Children’s Anxiety Scale (CCAS) Status of long‐term physical condition: Peak expiratory flow rate (PEFR) |
|
| Identification |
Sponsorship source: this research was supported by grants from ChinaMedical University (No. CMU‐92‐NS02), National ScienceCouncil (NSC‐92‐2314‐B039‐019 and NSC‐93‐2314‐B039‐005), Taiwan, Republic of China Country: Republic of China Comments: n/a Authors name: Li‐Chi Chiang Institution: China Medical University and China Medical Teaching Hospital Email: lichi514@seed.net.tw Address: School of Nursing, China Medical University and China Medical University Hospital, Taichung 40402, Taiwan, ROC |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: children were randomly assigned to the experimental or comparison groups using a 223 randomised block design. This randomisation procedure was confidentially conducted by an administrator in the clinic. Coin toss method used to allocate to group. |
| Allocation concealment (selection bias) | Low risk | Judgement comment: a list of treatment assignments linked with case number was generated and kept by the first author and the study statistician. The codes and treatment assignments were not released to any subjects, staff, and paediatric physicians, other than those mentioned until the completion of data analysis. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: likely single‐blind design. Not clearly stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: blinding of outcome assessors not clearly described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: appropriate statistical models were used for handling missing data |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all outcomes appeared to be reported |
| Other bias | High risk | Judgement comment: lead author was a developer of the intervention |
Detling Miller 2008.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatient clinic Outcome measures: Youth State Trait Inventory (STAI‐Y); Competitive State Anxiety Inventory‐2; blood glucose completed at baseline and post‐intervention (6 weeks) |
|
| Participants |
Type of chronic illness: type 1 diabetes Inclusion criteria: diagnosis of type 1 diabetes at least 1 year prior, 13 to 19 years old, no diabetic complications requiring medical treatment, no comorbid conditions, adequate blood glucose control by an HbAlc level at or below 8.5%, currently competing in an organised sport and competing for at least one year, experienced self‐assessed performance anxiety that was detrimental to blood glucose control (self‐report), no current participation or history of participation in any other anxiety‐reduction program or psychotherapy related to anxiety Exclusion criteria: not described Baseline characteristics Overall Number: 26 Sex (males (%)): 14 (53.8) Age in years (SD): not specified Severity of chronic illness: not reported Ethnicities: not reported Anxiety symptoms ‐ rating (SD): not reported Anxiety‐reduction group Number: 13 Sex (males (%)): 6 (46.2) Age in years (SD): 14.4 (1.2) Severity of chronic illness: mild Ethnicities: not reported Anxiety symptoms ‐ rating (SD): 36.7 (1.8) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): moderate Waiting‐list control Number: 13 Sex (males (%)): 8 (62%) Age in years (SD): 15.2 (1.9) Ethnicities: not stated Anxiety symptoms ‐ rating (SD): 34.8 (2.4) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): moderate Baseline differences: no significant differences |
|
| Interventions |
Intervention characteristics Relaxation training Audience: child Description of intervention: individuals were taught deep breathing and PMR exercises and provided with a compact disc recording of the anxiety‐reduction techniques to practice at home once a day for 6 weeks. Modality: individual Dose: single session and weekly telephone follow‐up over 6 weeks. Participants were encouraged to practice their relaxation daily. Manualised or non‐manualised: manualised Parent or caregiver involvement: none Therapist involvement: delivered intervention and weekly telephone follow‐up Pamphlet on diabetes and physical activity while on waiting list Description of intervention: educational information on diabetes and with recommendations to be physically active while on waiting list Dose: 6 weeks Parent or caregiver involvement (quantify/describe): none |
|
| Outcomes |
Specific anxiety measures: State Trait Anxiety Inventory – Youth Competitive State Anxiety Inventory‐2: no data included in meta‐analysis Status of long‐term physical condition: blood glucose (mg/dL) |
|
| Identification |
Sponsorship source: this project was fully funded by The C. Charles Jackson Foundation. Abbott Diabetes Care provided the Freestyle Flash® meters and testing strips at no charge. Country: USA Comments: Dissertation project ‐ baseline was used only to determine eligibility. Pre‐intervention (pre‐competition) measures served as a baseline therefore. Authors name: Nicole J. Detling Miller Institution: University of Utah Email: not provided Address: ProQuest Information and Learning Compay, 300 North Zeeb Road, P.O. Box 1346, Ann Arbour, MI 48106‐1346 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: randomised using a random number table |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: blinding of participants not clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: self‐report measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: all planned outcome measures reported |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all planned outcome measures reported |
| Other bias | Low risk | Judgement comment: no other sources of bias identified |
Freedenberg 2017.
| Methods |
Study design: Randomised controlled trial Study grouping: parallel group Setting: outpatient clinic Measures used: Hospital Anxiety and Depression Scale (HADS) completed at baseline and post‐intervention (6 weeks) |
|
| Participants |
Type of chronic illness: Congenitial Heart Disease (CHD) Inclusion criteria: aged 12–18 years, a diagnosis of CHD and/or arrhythmias, and/or a cardiac device, or postural orthostatic tachycardia syndrome. Exclusion criteria: lack of English fluency in the participant or their caregiver; inability to complete the study measures; lack of access to Skype technology for the video online group. Baseline characteristics Overall Number: 46 Sex (males (%)): 17 (37.0%) Age in years (SD): not reported Ethnicities: not reported Depressive symptoms – rating (SD): not reported Anxiety symptoms ‐ rating (SD): not reported Mindfulness‐Based Stress Reduction (MBSR) Number: 26 Sex (males (%)): 8 (31%) Age in years (SD): 15.1 (1.8) Type of chronic illness: 10 (46%) congenital heart disease, 12 (54%) POTS Ethnicities: not reported Depressive symptoms – rating (SD): mean HADS score 6.3 (4.5) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold (8) Anxiety symptoms ‐ rating (SD): mean score 10.5 (4.0) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Video online intervention Number: 20 Sex (males (%)): 9 (45%) Age in years (SD): 14.5 (1.6) Type of chronic illness: 10 (50%) congenital heart disease, 10 (50%) POTS Ethnicities: not stated Depressive symptoms – rating (SD): mean HADS score 4.6 (3.0) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold Anxiety symptoms ‐ rating (SD): mean score 7.1 (6.2) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure: subthreshold Baseline differences: MBSR group had significantly higher baseline anxiety |
|
| Interventions |
Intervention characteristics Mindfulness‐based stress reduction (MBSR) Audience: child Description of intervention: deep breathing exercises, meditation, and yoga. Group discussions focusing on stressors related to their cardiac‐associated issues and noticing the physical sensations that accompany these thoughts. Group discussions about fears related to their illness, device, and physical sensations, and their behavioural responses to these fears. Use of mindfulness techniques to alleviate symptoms and cognitive restructuring. Modality: group Dose: 6 sessions of 60‐minute duration over six weeks plus 15 minutes of practice per day Manualised or non‐manualised: manualised Parent or caregiver involvement: not reported Therapist involvement: delivery of sessions Online video support group Audience: child Description of intervention: six sessions of 60‐minute duration via Skype. The first half of the weekly discussion included questions or topics about health and/or cardiac issues based on requests sent in by the participants earlier in the week. The second half of the discussion consisted of whatever the group chose to talk about with the group leader observing Modality: group Dose: 6 x 60‐minute sessions over 6 weeks Manualised or non‐manualised: manualised Parent or caregiver involvement: not reported Therapist involvement: facilitated all sessions |
|
| Outcomes |
Specific depression measures: Hospital Anxiety and Depression Inventory (HADS) – depression subscale (HADS) Specific anxiety measures: Hospital Anxiety and Depression Inventory (HADS) – anxiety subscale (HADS) |
|
| Identification |
Sponsorship source: William and Joanne Conway Research Chair at Children’s National Health System in Washington, DC. Country: United States of America Comments: n/a Authors name: Vicki A. Freedenberg Institution: The George Washington University Email: vfreeden@cnmc.org Address: Department of Pediatrics, The George Washington University, Washington, DC, USA |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: simple randomisation was used but no information about what this entailed |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: blinding of participants not clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: self‐report outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: missing data accounted for |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all planned outcomes reported |
| Other bias | Unclear risk | Judgement comment: authors may have developed the intervention as per the publication: Freedenberg VA, Thomas SA, Friedmann E. A pilot study of a mindfulness based stress reduction program in adolescents with implantable cardioverter defibrillators or pacemakers. Pediatric Cardiology 2015, 36:786–795. |
Grey 1998.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatient clinic Measures used: Children’s Depression Inventory (CDI); Diabetes Quality of Life Scale: Youth; HbA1c completed at baseline and post‐intervention (3 months) |
|
| Participants |
Type of chronic illness: type 1 diabetes Inclusion criteria: aged between 12‐20 years; no comorbid health problem except for treated hypothyroidism; treated with insulin for at least 1 year; recent HbAlc between 7% and 14%; no severe hypoglycaemic events within the past 6 months; in school grade appropriate to age within 1 year Exclusion criteria: not described Baseline characteristics Overall Number: 65 Sex (males (%)): 28 (43.1) Age in years (SD): not reported Ethnicities: 60 (77%) white, 5 (23%) black/Hispanic Depressive symptoms – rating (SD): not reported Coping skills training Number: 34 Sex (males (%)): 15(44%) Age in years (SD): 15.8 (2.1) Severity of chronic illness: HbA1C = 8.9 (1.8) ‐ moderate Ethnicities: 31 (91%) white, 3 (9%) black/Hispanic Depressive symptoms – rating (SD): mean CDI 7.9 (1.3) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold Intensive management Number: 31 Sex (males (%)): 13 (42%) Age in years (SD): 15.0 (2.3) Severity of chronic illness: HbA1C = 9.0 (1.6) ‐ moderate Ethnicities: 29 (94%) white, 2 (6%) black/Hispanic Depressive symptoms ‐ rating: mean CDI 6.6 (1.8) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold Baseline differences: no differences at baseline |
|
| Interventions |
Intervention characteristics Coping skills training plus intensive diabetes management Audience ‐ child/child and parent: child Description of intervention: intensive management with the addition of role‐playing various social situations so that appropriate coping behaviour could be modelled. Emphasis on social problem‐solving, social skills training, cognitive behaviour modification, and conflict resolution. Modality: group Dose: 8 weekly group sessions of 60‐90 minutes, daily self‐management sessions, monthly outpatient sessions for a period of three months Manualised or non‐manualised: manualised Parent or caregiver involvement: none Therapist involvement: facilitation of role‐play group Intensive diabetes management Type of control: non‐psychological placebo Audience: child Description of intervention: administration of three or more daily insulin injections or an external insulin pump, self‐monitoring of blood glucose at least four times daily, monthly outpatient visits, and interim telephone contacts Modality: individual Dose: daily self‐management sessions, monthly outpatient sessions for a duration of three months Manualised or non‐manualised: non‐manualised Parent or caregiver involvement: not described Therapist involvement: none. |
|
| Outcomes |
Specific depression measures: the Children's Depression Inventory (CDI) Improvement in quality of life: Diabetes Quality of Life: Youth (DQOLY) Status of long‐term physical condition: HbA1c (haemoglobin A1c or glycated haemoglobin test) |
|
| Identification |
Sponsorship source: National Institute of Nursing Research, Culpeper Foundation, Yale Children's Clinical Research Center Country: USA Comments: n/a Authors name: Margaret Grey Institution: Yale University School of Nursing Email: margaret.grey@yale.edu Address: 100 Church St. SouthPO. Box 9740,New Haven, CT 06536‐0740 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: randomisation stated, but method not described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: study personnel were blinded. Study used self‐report data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: there were no dropouts from the study |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all planned outcomes reported |
| Other bias | Low risk | Judgement comment: no other sources of bias identified |
Grey 2009.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatient clinic Measures used: the Children’s Depression Inventory (CDI); HbA1c completed at baseline, and 1, 3, 6, and 12 months |
|
| Participants |
Type of chronic illness: type 1 diabetes Inclusion criteria: aged 8‐12 years; diagnosed with type 1 diabetes and treated with insulin for at least 6 months; free of other significant health problems; in school grade appropriate to within 1 year of child's age Exclusion criteria: not described Baseline characteristics Overall Number: 82 Sex (males (%)): 32 (39%) Age in years (SD): not reported Severity of chronic illness: not reported Ethnicities: not reported Depressive symptoms – rating (SD): not reported Coping skills training Number: 53 Sex (males (%)): 23 (43%) Age in years (SD): 9.9 (1.5) Severity of chronic illness: mean HbA1C 6.98 (1.33) Ethnicities: 44 (83%) white, 9 (17%) other Depressive symptoms – rating (SD): CDI 7.0 (6.1) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold Group education Number: 29 Sex (males (%)): 9 (31%) Age in years (SD): 9.9 (1.4) Severity of chronic illness: mean HbA1c 7.11 (1.21) Ethnicities: 26 (90%) white, 3 (10%) other Depressive symptoms – rating (SD): CDI 5.5 (4.5) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold Baseline differences: increased likelihood for white children and children whose mothers had higher education to receive the intervention |
|
| Interventions |
Intervention characteristics Coping skills training Audience: child and parent Description of intervention: sessions addressed specific coping skills including communication, social problem‐solving, recognition of associations between thoughts, feelings, and behaviour and guided self‐dialogue, stress management, and conflict resolution around diabetes‐specific stressors. Dose: 6 weekly sessions of 90 minutes Manualised or non‐manualised: manualised Parent or caregiver involvement: parents involved in all sessions Therapist involvement: delivery of sessions Education Audience: child and parent Description of intervention: education about diabetes management Dose: 4 weekly sessions of 90 minutes duration Manualised or non‐manualised: manualised Parent or caregiver involvement: parents involved in all sessions Therapist involvement: delivery of sessions |
|
| Outcomes |
Specific depression measures: Children's Depression Inventory (CDI) Improvement in quality of life: Diabetes Quality of Life Scale ‐ Satisfaction scale Status of long‐term physical condition: HbA1c (haemoglobin A1c or glycated haemoglobin test) |
|
| Identification |
Sponsorship source: supported by a grant from the National Institute for Nursing Research (National Institute of Health R01NR004009;PI: Margaret Grey, DrPH, RN, FAAN) Country: USA Authors name: Margaret Grey (correspondence: Robin Whittemore) Institution: Yale School of Nursing Email: robin.whittemore@yale.edu Address: c/o Robin Whittemore, Yale School of Nursing, 100 Church Street South, New Haven, CT 06536‐0740 |
|
| Notes | Comments: Square root of CDI scores (depressive outcome measure) were taken to satisfy normal distribution. These numbers were used for depression outcome measures rather than raw CDI scores. Author contacted for post‐intervention data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: participants randomised via sealed envelope technique, but details of this process not clear |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: blinding of participants not clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: self‐report meaures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: data for all participants was included in the final analysis. Despite aiming for a total of 100 participants, the authors were only able to recruit 82 participants. |
| Selective reporting (reporting bias) | High risk | Judgement comment: actual figures for some post‐intervention measurements were not provided |
| Other bias | Low risk | Judgement comment: no other sources of bias were identified |
Griffiths 1996.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: community sample recruited via newspaper advertisements Measures used: Children’s Depression Scale (CDS); Children’s Manifest Anxiety Scale (CMAS) completed at baseline and post‐intervention (8 weeks) |
|
| Participants |
Type of chronic illness: headaches/migraines Inclusion criteria: aged 10‐12 years; at least one headache per week for 6 months; could attend sessions with a parent; diagnosis of migraine or tension headache or combined migraine/tension headache Exclusion criteria: currently receiving psychological treatment for headaches Baseline characteristics Overall Sex (males (%)): 21 (50%) Age in years (SD): not reported Type of chronic illness: 8 (19%) migraine; 14 (33%) tension; 20 (48%) combined Ethnicities: not reported Depressive symptoms – rating (SD): not reported Anxiety symptoms ‐ rating (SD): not reported Clinic‐based cognitive behaviour therapy Number: 15 Sex (males (%)): 7 (46.7%) Age in years (SD): 11.4 (0.58) Type of chronic illness: 2 (13%) migraine; 9 (60%) tension; 4 (27%) combined Ethnicities: not reported Depressive symptoms – rating (SD): CDS 3.0 (0.9) Anxiety symptoms ‐ rating (SD): CMAS 14.5 (6.3) Home‐based cognitive behaviour therapy Number: 12 Sex (males (%)): 7 (46.7%) Age in years (SD): 11.5 (0.58) Type of chronic illness: 3 (20%) migraine; 4 (27%) tension; 8 (53%) combined Ethnicities: not reported Depressive symptoms – rating (SD): CDS 2.7 (0.7) Anxiety symptoms ‐ rating (SD): CMAS 11.7 (4.9) Waiting‐list control Number: 27 Sex (males (%)): 7 (58.3%) Age in years (SD): 11.1 (0.58) Type of chronic illness: 3 (25%) migraine; 1 (8%) tension; 8 (67%) combined Severity of chronic illness: chronicity in years: 1 (1); 1‐2 (1); 2‐5 (7); 5 (3) Ethnicities: not reported Depressive symptoms – rating (SD): CDS 2.8 (0.9) Anxiety symptoms ‐ rating (SD): CMAS 15.6 (9.0) Baseline differences: Group 1 (clinic‐based sample) had more males than females and more participants with tension headache than migraine or combined headache). Group 2 (home‐based sample) had more females than males and more participants with combined headache than migraine or tension headache alone. Group 3 (control sample) had more males than females and more combined headache than migraine or tension headache alone. |
|
| Interventions |
Intervention characteristics Clinic‐based cognitive behaviour therapy Audience: child Description of intervention: 8 sessions cognitive behaviour therapy program Modality: group Dose: 8 weekly sessions of 90 minutes duration Manualised or non‐manualised: manualised Parent or caregiver involvement: parents attended an assessment session and were telephoned weekly by therapists between weeks 2‐6 Therapist involvement: delivered sessions and completed follow‐up phone calls Home‐based cognitive behaviour therapy Audience: child Description of intervention: 8 sessions cognitive behaviour therapy program. Treatment was delivered by a work at home manual, except treatment sessions 1, 4, and 8 which were completed at the clinic. Dose: individual Dose: 8 weekly sessions of 90 minutes duration Manualised or non‐manualised: manualised Parent or caregiver involvement: completion of assessment session; participation in two brief monitoring phone calls lasting between 5‐12 minutes Therapist involvement: sessions 1, 4, and 8 delivery plus brief phone calls to parents at weeks 2 and 6 Waiting‐list control Audience: child Description of intervention: self‐monitoring of headaches and medication intake. Time required and duration, including homework: 8 weeks Parent or caregiver involvement: completion of assessment session; participation in two brief monitoring phone calls lasting between 5‐12 minutes |
|
| Outcomes |
Specific depression measures: Children's Depression Scale (CDS) Change anxiety measures: Children's Manifest Anxiety Scale (CMAS) |
|
| Identification |
Sponsorship source: not stated Country: Australia Authors name: Jennifer D. Griffiths Institution: University of Western Australia Email: not stated Address: Department of Pychology, The University of Western Australia, Nedlands, Perth, Australia 6907 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: randomisation not clearly described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: blinding of participants not clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: self‐report measures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: only full datasets were analysed. Nine participants dropped out during the study. Reasons for doing so were not adequately described. |
| Selective reporting (reporting bias) | High risk | Judgement comment: only some data (e.g. anxiety and depression scores pre/post) presented in numerical form. Other data (e.g. headache scores) presented graphically, making it impossible to use in this review. Author could not be contacted for clarification as no email address provided and not on ResearchGate. Data collected more than 10 years ago, so unlikely to be available via institution. |
| Other bias | High risk | Judgement comment: the intervention was devised by the study authors |
Hains 2000.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatient clinic Measures used: State Trait Anxiety Inventory (STAI); HbA1c completed at baseline, post‐intervention (6 weeks), and approximately 10 weeks (1 month post‐intervention) |
|
| Participants |
Type of chronic illness: type 1 diabetes Recruitment: diabetes outpatient clinic Inclusion criteria: in seventh or eighth grade, receiving outpatient treatment for type 1 diabetes, HbA1c values greater than 9.0% during previous clinic visit Exclusion criteria: parent and child did not give written consent Baseline characteristics Overall Number: 14 Sex (males (%)): 6/14 (42.9%) Age in years (SD): not reported Severity of chronic illness: moderate (HbA1C > 9) Ethnicities: 13 (86%) white, 1 (7%) African‐American, 1 (7%) Asian‐American Anxiety symptoms ‐ rating (SD): not reported Stress‐management training intervention Number: 8 Sex (males (%)): 3/8(38%) Severity of chronic illness: HbA1c greater than 9.0% Age in years (SD): not reported Ethnicities: 8 (100%) white Anxiety symptoms ‐ rating (SD): mean state anxiety 39 (SD 7.45) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Waiting‐list control Number: 7 Sex (males (%)): 3 (50%) Age in years (SD): not reported Ethnicities: 5 (72%) white, 1 (14%) African‐American, 1 (14%) Asian‐American Anxiety symptoms ‐ rating (SD): mean state anxiety 38.5 (8.74) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Baseline differences: All of the youths were white with the exception of 1 African‐American girl and 1 Asian‐American boy in the control group. No other group differences stated. |
|
| Interventions |
Intervention characteristics Stress‐management training interventions Audience: child Description of intervention: stress management curriculum based on CBT and problem‐solving therapy Modality: group Dose: 6 weekly sessions of 60 minutes duration Manualised or non‐manualised: manualised Parent or caregiver involvement: not stated Therapist involvement: delivery of sessions Waiting‐list control Audience: child Dose: nil sessions. Only completion of questionnaires before, post‐test and 1 month follow‐up |
|
| Outcomes |
Specific anxiety measures: State Trait Anxiety Inventory (STAI) Status of long‐term physical condition: HbA1c (haemoglobin A1c or glycated haemoglobin test) |
|
| Identification |
Sponsorship source: this study was supported in part by LifeScan Company Country: USA Authors name: Anthony Hains Institution: University of Wisconsin Email: aahains@uwm.edu Address: Department of Educational Psychology, PO Box 413m University of Wisconsin‐Milwaukee, WI53201 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement comment: method of randomisation not described. In addition, one participant from intervention group swopped with a participant from the control group due to parental scheduling. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: blinding of participants not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: 1 participant dropped out of control group, but timing not clear and the results were not included in the final dataset. No information about how the data from the participant that dropped out was handled. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all planned outcomes reported |
| Other bias | Unclear risk | Judgement comment: the intervention may have been developed by the authors, but this was not explicitly described |
Hickman 2015.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatient clinic Outcome measures: Beck Youth Inventories (BYI); Pediatric Migraine Disability Assessment (PEDMIDAS); Parent Perception of Pain Interference (PPPI) completed at baseline and post‐intervention (variable time‐frame) |
|
| Participants |
Type of chronic illness: chronic daily headaches Inclusion criteria: diagnosis of chronic daily headaches, aged 13 to 17 years, parent or guardian able to accompany teen to clinic visit, ability to speak English, enrolment in high school, presence of mild to moderate depression symptoms (score of 55 to 69 on the Beck Youth Depression Inventory) Exclusion criteria: presence of a prior diagnosed mental health condition or of clinical pathology as the underlying cause of headaches Baseline characteristics COPE‐HEP Number: 16 Sex (males (%)): 3(18.8%) Age in years (SD): 15.38 (0.96) Severity of chronic illness: moderate Ethnicities: 5 (31%) white, 4 (25%) black, 1 (6.3% (American‐Indian/Alaskan native), 0 (0%) Asian, 6 (37.5%) Hispanic Depressive symptoms – rating (SD): Beck Youth Inventory 60.0 (4.51) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): severe Anxiety symptoms ‐ rating (SD): Beck Youth Inventory 59.25 (9.57) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): severe Headache education Number: 16 Sex (males (%)): 6 (38%) Age in years (SD): 14.8 (1.17) Severity of chronic illness: moderate Ethnicities: 5(31%) white, 1 (6.3%) black, 1 (6.3%) American‐Indian/Alaskan native, 1 (6.3%) Asian, 8 (50% Hispanic) Depressive symptoms – rating (SD): Beck Youth Inventory 57.56 (3.35) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): severe Anxiety symptoms ‐ rating (SD): Beck Youth Inventory 50.94 (8.31) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): severe Baseline differences: no statistically significant differences on demographic variables at baseline |
|
| Interventions |
Intervention characteristics Cognitive Behavioural skills building (COPE‐HEP) Audience: unclear Description of intervention: CBT intervention delivered in office and over telephone sessions Modality: individual Dose: 3 x 30‐min office sessions and 4 x 20‐min telephone sessions over 7 weeks Manualised or non‐manualised: manualised Parent or caregiver involvement: unclear Therapist involvement: delivery of intervention sessions Headache education Audience: child Description of intervention: office sessions with topics surrounding lifestyle triggers of headaches, environmental headache triggers, medication triggers of headaches, hormonal headache triggers, dietary triggers of headaches, headache management tips, and the importance of hydration Modality: individual Dose: 7 weekly sessions and completion of homework tasks Manualised or non‐manualised: manualised Parent or caregiver involvement: unclear Therapist involvement: delivery of session |
|
| Outcomes |
Specific depression measures: Beck Youth Inventory (Depression Scale) Specific anxiety measures: Beck Youth Inventory (Anxiety Scale) Status of long term physical conditions: Parent Perception of Pain Interference (PPPI) Functional disability: Pediatric Migraine Disability Assessment (PediMIDAS) |
|
| Identification |
Sponsorhip source: National Institute of Nursing Research/National Institutes of Health Country: US Name: Carolyn Hickman Institution: Phoenix Children’s Hospital Email: chickman@phoenixchildrens.com Address: Phoenix Children’s Hospital 1919 East Thomas Road Phoenix, Arizona 85016 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: online randomisation was used |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no description of visibility of randomisation process to participants/researchers/outcome assessors |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: blinding of participants not clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: blinding of outcome assessors not clearly described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: missing data accounted for and ITT used |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no evidence of selective outcome reporting |
| Other bias | High risk | Judgement comment: the primary author is the developer of the researched intervention |
Kanstrup 2016.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatient clinic Outcome measures: Center for Epidemiological Studies Depression (CES‐DC); Functional Disability Index (FDI); Pain Interference Scale (PIS) completed at baseline, mid‐intervention (7 weeks) and post‐intervention (14 weeks) |
|
| Participants |
Type of chronic illness: chronic pain Inclusion criteria: attending tertiary care pain clinic; aged 14‐18 years old; pain duration of more than 6 months; previously ineffective pain treatments; reported substantial pain‐related disability Exclusion criteria: improvement was expected without treatment; psychiatric comorbidity was considered the main reason for disability, required immediate intervention or was assumed to interfere with the planned intervention; substantial risk for suicide; substantial cognitive dysfunction or reduced proficiency in Swedish; ongoing or planned treatments; pain was recurrent rather than continuous; pain was fully explained by a pathophysiological process, e.g., cancer Baseline characteristics Overall Number: 30 Sex (males (%)): 6 (20.0%) Age in years (SD): 16.0 (1.6) Severity of chronic illness: Current pain intensity on a 0‐6 scale 3.31 (1.4) Ethnicities: not reported Depressive symptoms – rating (SD): mean CES‐DC = 28 Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Group‐based ACT therapy Number: 12 Sex (males (%)): 1 (8.3%) Age in years (SD): 16.3 (1.5) Type of chronic illness: 10 (83%) headache, 4 (32%) abdominal pain, 7 (58%) back pain, 4 (33%) joint pain, 9 (75%) other pain Severity of chronic illness: moderate (mean pain intensity 3.76 (0.96) on a 0‐6 scale Ethnicities: not reported Depressive symptoms – rating (SD): mean CES‐DC 26.0 (SD not reported) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Individual ACT therapy Number: 18 Sex (males (%)): 5 (27.8%) Age in years (SD): mean age 15.8 (1.6) Type of chronic illness: 17 (94%) headache, 8 (44%) abdominal pain, 6 (33%) back pain, 1 (6%) joint pain, 9 (50%) other pain, 1 (6%) CRPS Severity of chronic illness: moderate (mean pain intensity 3.0 (1.66) on a 0‐6 scale Ethnicities: not reported Depressive symptoms – rating (SD): CES‐DC 28.5 (SD not reported) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Anxiety symptoms ‐ rating (SD): not assessed Baseline differences: no differences reported. |
|
| Interventions |
Intervention characteristics Group based acceptance and commitment therapy Audience: child and parent Description of intervention: four phases treatment based on acceptance and committed therapy and focusing on preparing for behaviour change; shifting perspective; acceptance of pain symptoms and cognitive defusion and values‐oriented behaviour activation. The parent support program was embedded in the treatment and comprised of four sessions (Sessions 3, 6, 11, and 12). Session 12 was a joint session, with both adolescents and parents participating. Modality: group Dose: 18 sessions of 120 minutes each, including a minimum of 10 adolescent sessions and 3 separate parent and 1 joint (child + parent) sessions Manualised or non‐manualised: manualised Parent or caregiver involvement: participation in 4 joint sessions with child Therapist involvement: delivery of treatment Individual acceptance and commitment therapy Audience: child and parent Description of intervention: four phases treatment based on acceptance and committed therapy and focusing on preparing for behaviour change; shifting perspective; acceptance of pain symptoms and cognitive defusion and values‐oriented behaviour activation. The parent support program was embedded in the treatment and comprised of four sessions (Sessions 3, 6, 11, and 12). Session 12 was a joint session, with both adolescents and parents participating. Modality: individual Dose: 18 sessions x 45 mins each with 3 separate parent and 1 joint (child + parent) sessions Manualised or non‐manualised: manualised Parent or caregiver involvement: participation in 4 joint sessions with child Therapist involvement: delivery of treatment |
|
| Outcomes |
Specific depression measures: Center for Epidemiological Studies Depression – children’s scale (CES‐DC) Improvement in function: Functional Disability Index Status of long‐term physical condition: Pain Interference Index |
|
| Identification |
Sponsorship source: funding for Kanstrup was provided from the Doctoral School in Health Care Sciences at Karolinska Institutet and from the Functional Area Medical Psychology at Karolinska University Hospital. Funding for Kemani was provided from the Functional Area Medical Psychology at Karolinska University Hospital. Funding for Wiwe Lipsker was provided from the KID‐funding at Karolinska Institutet and from the Functional Area Medical Psychology at Karolinska University Hospital. Lekander was funded by Stockholm University (Stress Research Institute) and Karolinska Institutet (Osher Center for Integrative Medicine). Financial support for Holmström and Wicksell was provided through the regional agreement on medical training and clinical research (ALF) between Stockholm City Council and Karolinska Institutet. Country: Sweden Authors name: Marie Kanstrup Institution: Karolinska University Hospital Email: Marie.Kanstrup@ki.se Address: Functional Area Medical Psychology, Functional Unit Behavioral Medicine, Karolinska University Hospital Solna, P8:01, 171 76 Stockholm, Sweden |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: the randomisation sequences were generated via an online randomisation service accessible at https://www.random.org/ |
| Allocation concealment (selection bias) | Low risk | Judgement comment: an administrator who was not involved in treatment delivery randomised the participants, placed the information in coded sealed envelopes and informed the participants about which condition they had been assigned to by opening the envelopes in their presence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: participants blinded until the point of allocation (post‐baseline assessment) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: self‐report measures were used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: only those with complete data were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: some outcomes such as SD not included |
| Other bias | High risk | Judgement comment: intervention developed by authors |
Kashikar‐Zuck 2005.
| Methods |
Study design: randomised controlled trial Study grouping: cross‐over Setting: outpatient clinic Outcome measures: Children’s Depression Inventory (CDI); Pain severity visual analogue scale (VAS) completed at baseline, post‐intervention (8 weeks) and 16 weeks |
|
| Participants |
Type of chronic illness: juvenile primary fibromyalgia syndrome Inclusion criteria: Aged 13‐17 years, diagnosed with juvenile primary fibromyalgia; stabilised on medication for at least 4 weeks prior to enrolment; average pain level of at least 3 (mild pain) over the preceding 2 weeks on a 10 cm visual analogue scale; functional disability score greater than 7 (mild disability) on the Functional Disability Inventory Exclusion criteria: existing comorbid rheumatic disease; documented developmental delay, or impairment; major depressive disorder Baseline characteristics Overall Number: 30 Sex (males (%)): 0 (0%) Age in years (SD): mean 15.38 (1.26) Severity of chronic illness: average pain rating (VAS) of 5.30 or more (moderate) Ethnicities: 28 (93%) Caucasian, 2 (7%) African‐American Depressive symptoms – rating (SD): not reported Coping Skills Training Number: 15 Sex (males (%)): 0 (0%) Age in years (SD): not reported Severity of chronic illness: average pain rating (VAS) of 5.71/10 Ethnicities: not reported Depressive symptoms – rating (SD): mean CDI T score = 56.07 (12.42) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold Self‐monitoring Number: 15 Sex (males (%)): 0 (0%) Age in years (SD): not reported Severity of chronic illness: average pain rating (VAS) of 5.30/10 Ethnicities: not reported Depressive symptoms – rating (SD): mean CDI T score = 48.46 (12.89) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold Anxiety symptoms ‐ rating (SD): not reported Baseline differences: no significant differences |
|
| Interventions |
Intervention characteristics Coping skills training Audience: child and parent Description of intervention: psychoeducation, relaxation strategies; distraction and activity pacing techniques; cognitive techniques to deal with negative thoughts and mood difficulties; problem‐solving to anticipate and plan for difficult or stressful situations and improve sleep hygiene Modality: child, with parent involved in 3 sessions Dose: 6 weekly 60 minutes sessions and 2 telephone follow‐ups toward the end of 8 weeks Manualised or non‐manualised: manualised Parent or caregiver involvement: parents involved in 3 sessions Therapist involvement: delivered sessions Self‐monitoring Audience: child Description of intervention: self‐monitoring using weekly diaries for 8 weeks Modality: individual Dose: no contact, diaries provided, and outcome measures collected at 4 and 8 weeks Manualised or non‐manualised: non‐manualised Parent or caregiver involvement: no involvement Therapist involvement: no involvement |
|
| Outcomes |
Specific depression measures: Children’s Depression Inventory (CDI) Improvement in function: Functional Disability Inventory (FDI) Status of long‐term physical condition: Pain rating Visual Analogue Scale (VAS) |
|
| Identification |
Sponsorship source: Cincinnati Children’s Hospital Research Foundation, National Institutes of Health Country: USA Authors name: Susmita Kashikar‐Zuck Institution: Cincinnati Children’s Hospital Medical Center Email: Susmita.Kashikar‐Zuck@cchmc.org Address: Psychology Division, MLC 3015Cincinnati Children’s Hospital Medical CenterCincinnati, OH 45229. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: random allocation using a computer generated pseudo random number table and block allocation |
| Allocation concealment (selection bias) | Low risk | Judgement comment: sealed envelope opened by blinded researcher |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: unclear about participants. Research assistants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: rheumatologists and occupational therapists conducting 'tender point' examinations blinded to group. All other outcome measures self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: few dropouts from either group, management of missing data using ITT with last available value carried forward for missing data |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all planned outcomes reported |
| Other bias | Low risk | Judgement comment: no other sources of bias evident |
Kashikar‐Zuck 2012.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatient clinic Outcome measures: Children’s Depression Inventory (CDI); Functional Disability Inventory (FDI); Pediatric Quality of Life Scale (PEDSQL emotional functioning subscale); pain severity visual analogue scale (VAS) completed at baseline, post‐intervention (week 8) and week 26 |
|
| Participants |
Type of chronic illness: juvenile fibromyalgia syndrome Inclusion criteria: children aged 11‐18 years; diagnosed with juvenile FMS; were receiving stable medications for 8 weeks; reported average pain severity 4 on a 0–10‐cm visual analogue scale (VAS); obtained a score 7 on the Functional Disability Inventory (FDI); experienced a mild disruption in daily activities due to juvenile FMS symptoms Exclusion criteria: other rheumatic disease; documented developmental delay; current panic disorder, major depression, or lifetime bipolar disorder or psychosis; use of opioids Baseline characteristics Overall Number: 112 Sex (males (%)): 9 (7.9) Age in years (SD): 15.0 (1.8) Severity of chronic illness: average pain severity of 5.7/10 (VAS) Ethnicities: white, African‐American, Asian, American‐Indian/native Alaskan, other (numbers not reported) Depressive symptoms – rating (SD): CDI 13.2 (1.4) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Cognitive behavioural therapy Number: 57 Sex (males (%)): 3 (5.3) Age in years (SD): 15.2 (1.8) Severity of chronic illness: average pain severity of 5.7/10 (VAS) Ethnicities: white, African‐American, American‐Indian/native Alaskan, other (no numbers provided) Depressive symptoms – rating (SD): CDI 13.3 (6.4) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Fibromyalgia education Number: 55 Sex (males (%)): 6 (10.5) Age in years (SD): 14.9 (1.7) Severity of chronic illness: average pain severity of 5.8/10 (VAS) Ethnicities: white, African‐American, Asian (numbers not reported) Depressive symptoms – rating (SD): CDI 12.9 ( 5.6) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Baseline differences: no significant differences |
|
| Interventions |
Intervention characteristics Cognitive behaviour therapy Audience: child and parent Description of intervention: psychoeducation about behavioural pain management; muscle relaxation; distraction strategies; activity pacing; problem‐solving; relapse prevention strategies. Homework and parent training included to encourage constant use of skills. Modality: individual Dose: 8 x 45 min sessions over 8 weeks plus homework activities Manualised or non‐manualised: manualised Parent or caregiver involvement: attend three sessions to receive training in behavioural management techniques and support skills their children were learning Therapist involvement: delivery of treatment Education Audience: child and parent Description of intervention: general education about fibromyalgia and lifestyle Modality: individual Dose: 8 x 45 min sessions over 8 weeks Manualised or non‐manualised: manualised Parent or caregiver involvement: attend three educational sessions Therapist involvement: delivery of sessions |
|
| Outcomes |
Specific depression measures: Children’s Depression Inventory (CDI) Improvement in quality of life: Pediatric Quality of Life Inventory (PEDSQL) emotional functioning subscale ‐ no data included in meta‐analysis Improvement in function: Functional Disability Inventory (FDI) Status of long‐term physical condition: pain severity (measured using Visual Analogue Scale with anchors of 0‐10) |
|
| Identification |
Sponsorship source: Supported by the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01‐AR‐050028 to Dr.Kashikar‐Zuck) Country: USA Authors name: Susmita Kashikar‐Zuck Institution: Cincinnati Children's Hospital Medical Center Email: Susmita.Kashikar‐Zuck@cchmc.org Address: Division of Behavioral Medicine and Clinical Psychology, MLC 3015,Cincinnati Children’s Hospital Medical Center, 3333 Burnet Avenue,Cincinnati, OH 45229 |
|
| Notes | Outcome measures: post‐intervention = week 9, long‐term follow up = 6 months following end of treatment (all time points not available for all outcomes) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no information available in the manuscript |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: single‐blind design used. PI, study physicians, study coordinator and assessment staff blinded to treatment condition throughout trial. Participants asked not to divulge what treatment they were receiving to their study physician |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: self‐report outcomes completed by participants who were not blinded to treatment condition |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: ITT analysis used to deal with missing data |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all planned outcomes reported |
| Other bias | Low risk | Judgement comment: no other sources of bias identified |
Levy 2010.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: hospital outpatient clinic and community sample obtained via advertisement Outcome measures: Children’s Depression Inventory (CDI); Fucntional Disability Index (FDI) completed at baseline, 1 week, post‐intervention (week 3) and 6 months |
|
| Participants |
Type of chronic illness: chronic abdominal pain Inclusion criteria: aged 7–17 years, three or more episodes of recurrent abdominal pain during a 3‐month period; child and parent cohabited for the past 5 years or, in cases of divided custody, for at least half of the child's lifetime. Exclusion criteria: positive physical or laboratory findings which would explain the abdominal pain; any chronic gastrointestinal disease; lactose intolerance as diagnosed by the attending physician; major surgery within the past year; developmental disabilities requiring full‐time special education or impairing ability to communicate; non‐English speaking Baseline characteristics Overall Number: 187 Sex (males (%)): 55 (27.5%) Age in years (SD): not reported Severity of chronic illness: not reported Ethnicities: not reported Depressive symptoms – rating (SD): not reported Social learning/cognitive behaviour therapy Number: 98 Sex (males (%)): 29 (29%) Age in years (SD): 11.12 (2.6) Severity of chronic illness (SD): mean CSI 1.25 (0.71) Ethnicities: 85 (93.4%) Caucasian Depressive symptoms ‐ rating: mean CDI 9.06 (6.67) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold Education support Number: 89 Sex (males (%)): 26 (26%) Age in years (SD): 11.3 (2.5) Severity of chronic illness (SD): mean CSI 1.1 (0.69) Ethnicities: 87 (97.8%) Caucasian Depressive symptoms ‐ rating: mean CDI 7.75 (6.26) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold Baseline differences: participants in the intervention group were reported to have greater levels of parent‐rated pain and those in the control group were reported to have greater levels of pain minimisation coping skills |
|
| Interventions |
Intervention characteristics Social learning/cognitive behaviour therapy Audience: child and parent Description of intervention: three main components ‐ relaxation training; working with parents and children to modify family responses to illness and wellness behaviours; cognitive restructuring to address and alter dysfunctional cognitions Modality: child and family Dose: 3 sessions of 75 minutes duration over two weeks and homework exercises between sessions Manualised or non‐manualised: manualised Parent or caregiver involvement: parents were involved in all sessions Therapist involvement: delivered sessions Education support Audience: child and parent Description of intervention: three sessions focused on education about GI system anatomy and function, nutrition guidelines, and reading of food product labels Modality: child and parent Dose: 3 sessions of 75 minutes duration delivered over two weeks and homework between sessions Manualised or non‐manualised: manualised Parent or caregiver involvement: parents involved in all sessions Therapist involvement: delivered sessions |
|
| Outcomes |
Specific depression measures: Children’s Depression Inventory (CDI) Improvement in function: Functional Disability Inventory (FDI) Status of long‐term physical condition: GI Symptom subscale, Children's Somatization Inventory (CSI) |
|
| Identification |
Sponsorship source: this study was supported by grant number 5R01HD036069 from the National Institutes of Health—National Institute of Child Health and Human Development Country: USA Authors name: Rona Levy Institution: University of Washington Email: rlevy@uw.edu Address: School of Social Work, University of Washington, Mailstop354900, Seattle, Washington 98105, USA |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: participants were blind to their group assignment until commencement of the first treatment session |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: self‐report measures with blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: management of missing data not fully explained |
| Selective reporting (reporting bias) | High risk | Judgement comment: some data not fully reported (e.g. actual post‐intervention data and SDs) |
| Other bias | Unclear risk | Judgement comment: the intervention may have been designed by the study authors |
Li 2016.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: paediatric outpatient clinic Outcome measures: Hamilton Depression Rating Scale (HAM‐D); Hamilton Anxiety Rating Scale (HAM‐A); seizure frequency completed at baseline and post‐intervention (3 months) |
|
| Participants |
Type of chronic illness: epilepsy Inclusion criteria: aged 13–20 years old; receiving stable anti‐epileptic drug treatment; engaged in education; read and expresses self well; Wechsler Intelligence Scale (WISC‐R) score greater or equal to 90 points Exclusion criteria: epilepsy associated with other diseases; long‐term administration of antidepressants and anti‐psychotics drugs that would affect the nervous system besides antiepileptic drugs; Wechsler intelligence scale score less than 90 points; hearing loss or vision disorders; consciousness and status epilepticus; mental illness; history of alcohol, drug abuse, and psychoactive substances Baseline characteristics Overall Number: 104 Sex (males (%)): 51 (49%) Age in years (SD): not reported Severity of chronic illness: not reported Ethnicities: not reported Depressive symptoms – rating (SD): not reported Anxiety symptoms ‐ rating (SD): not reported Systemic family therapy Number: 52 Sex (males (%)): 26 (50%) Age in years (SD): 17.14 (1.82) Type of chronic illness: 33 (63%) tonic‐clonic/complex partial seizures, 19 (37%) other types of seizures Severity of chronic illness (SD): seizure frequency 6.5 (6.77) Ethnicities: not reported Depressive symptoms – rating (SD): mean HAM‐D = 22.55 (9.76) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): severe Anxiety symptoms ‐ rating (SD): mean HAM‐A = 13.41 (7.83) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Medication control Number: 52 Sex (males (%)): 25 (48.08%) Age in years (SD): 16.98 (2.06) Type of chronic illness: 34 (65%) tonic clonic/complex‐partial seizures, 18 (35%) other types of seizures Severity of chronic illness (SD): seizure frequency 7.0 (6.85) Ethnicities: not reported Depressive symptoms – rating (SD): mean HAM‐D = 20.35 (9.55) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): Severe Anxiety symptoms ‐ rating (SD): mean HAM‐A = 13.76 (SD8.76) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Baseline differences: no significant differences |
|
| Interventions |
Intervention characteristics Systemic family therapy with antiepileptic drugs Audience: child and parent Description of intervention: topics included investigating the differences and interactions of family behaviours, positively assigning their meaning, orientating the resources, investigating the dynamics of changes, investigating the targets of changes, and promoting the changes. Modality: child and family Dose: 80 minutes per session for six sessions, with four homework topics for 3 months Manualised or non‐manualised: manualised Parent or caregiver involvement: parents attended all sessions Therapist involvement: delivered sessions Medication control Audience: child Description of intervention: medication only, no additional clinic visits Modality: individual Duration of 3 months |
|
| Outcomes |
Specific depression measures: Hamilton Depression Scale (HAM‐D) Specific anxiety measures: Hamilton Anxiety Scale (HAM‐A) Status of long‐term physical condition: seizure frequency |
|
| Identification |
Sponsorship source: this work was supported by Science and Technology research project of Chongqing Municipal Educational Commission (No. KJ080322), the project of Chongqing Municipal Health Bureau (No. 2010‐2‐015), the project of Chongqing Education and Science topics (No. 2012‐GX‐123), and the project of Medical research topics of Chongqing Municipal Health Bureau (No. 2013‐2‐033) Country: China Authors name: Jing Li Institution: the First Affiliated Hospital of Chongqing Medical University Email: jlxfcn@126.com Address: Department of Neurology, the First Affiliated Hospital of Chongqing Medical University, No. 1 Friendship Road, Chongqing 400016, China |
|
| Notes | Therapy designed to address anxiety/depression | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: no dropouts reported and no missing data mentioned (nor any process for managing this) |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all planned outcomes reported |
| Other bias | Low risk | Judgement comment: no other biases identified |
Martinović 2006.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Comments: outpatient clinic Outcome measures: Beck Depression Inventory (BDI); Quality of Life in Epilepsy Inventory (QOLEI‐31) completed at baseline, 6, and 9 months |
|
| Participants |
Type if illness: epilepsy Inclusion criteria: newly diagnosed epilepsy; subthreshold depression; normal intelligence Exclusion criteria: epilepsy caused by progressive cerebral lesion; mental retardation; diagnosis of depression, psychotic symptoms, schizophrenia, bipolar disorder, social phobia, agoraphobia, or panic disorder Baseline characteristics Overall Number: 30 Sex (males (%)): 12 (40%) Age in years (SD): not reported Ethnicities: not reported Depressive symptoms – rating (SD): not reported Cognitive behavioural intervention Number: 15 Sex (males (%)): 6 (67%) Age in years (SD): 17.2 (2.5) Type of chronic illness: 6 (40%) generalised epilepsy, 9 (60%) partial epilepsy Severity of chronic illness: 10 (66%) seizure‐free, 5 (33%) uncontrolled Ethnicities: not reported Depressive symptoms – rating (SD): BDI = 8.2 (0.94) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Supportive psychotherapy Number: 15 Sex (males (%)): 6 (67%) Age in years (SD): 17.6 (2.2) Type of chronic illness: 5 (33%) generalised epilepsy, 10 (66%) partial epilepsy Severity of chronic illness: 8 (53%) seizure‐free, 7 (47%) uncontrolled Ethnicities: not reported Depressive symptoms – rating (SD): BDI = 8.1 (0.96) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Baseline differences: no significant differences |
|
| Interventions |
Intervention characteristics Cognitive behavioural intervention Audience: child Description of intervention: delivered as part of an individualised treatment plan and designed to teach participants to recognise and correct all main types of cognitive errors. Sessions included activity plans, relaxation, identification and correction of thought distortions — cognitive restructuring, role playing, development of social skills, and problem‐solving Modality: individual Dose: 8 sessions over 8 weeks and 4 further sessions over 4 months (total of 12 sessions over 6 months) Manualised or non‐manualised: manualised Parent or caregiver involvement: unclear Therapist involvement: delivery of sessions Supportive psychotherapy Audience: child Description of intervention: described by the authors as "TAU". Sessions consisting of therapeutic counselling where participants were instructed to note, in a treatment diary, the occurrence of negative thoughts and countermeasures taken (positive thoughts). Negative and positive thoughts were rated on a 4‐point scale, and the results at baseline and the 6‐ and 9‐month follow‐ups were compared. Modality: individual Dose: 8 sessions over 8 weeks and 4 further sessions over 4 months (total of 12 sessions over 6 months) Manualised or non‐manualised: manualised Parent or caregiver involvement: unclear Therapist involvement: unclear |
|
| Outcomes |
Specific depression measures: the Beck Depression Inventory (BDI) Improvement in quality of life: Quality of Life in Epilepsy Inventory (QOLIE‐31) |
|
| Identification |
Sponsorship source: not stated Country: Serbia and Montenegro Authors name: Zarko Martinovic Institution: Department of Epilepsy and Clinical Neurophysiology, Institute of Mental Health Email: yscn@sezampro.yu Address: Department of Epilepsy and Clinical Neurophysiology, Institute of Mental Health11000 Belgrade, Palmoticeva 37, Serbia and Montenegro |
|
| Notes | Three different depression measures used (BDI, CES‐D, and HAMD‐reported BDI scores) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: randomised number sequence |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: blinding of participants not clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: blinding of outcome assessors not clearly described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: outcomes measured on all 30/32 participants who remained in study (1 left from each group within 1‐2 months and did not have any available post‐‐intervention outcomes) |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no obvious reporting bias |
| Other bias | Low risk | Judgement comment: no other sources of bias identified |
Moghanloo 2015.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatient clinic Outcome measures: Reynold’s Child Depression Inventory (RCDI); Satisfaction with Life Scale (SLS) completed at baseline and post‐intervention (10 weeks) |
|
| Participants |
Type of chronic illness: type 1 diabetes Inclusion criteria: less than 15 years old; diabetes diagnosis for at least one year without having a major psychiatric disorder; continuing medical treatment process normally Exclusion criteria: determined by treating physician, included: needing significant change in the dose of insulin administered during the research, acute or chronic medical illness that makes problems in venesection or intolerance in long sessions, cinching up severe medical complications of diabetes, receiving psychiatric treatment, or use of psychotropic drugs and drug abuse during the study period Baseline characteristics Overall Number: 34 Sex (males (%)): 17 (50%) Age in years (SD): not reported Ethnicities: not reported Depressive symptoms – rating (SD): not reported Acceptance and commitment therapy Number: 17 Sex (males (%)): 8 (47.1%) Age in years (SD): 10.35 (2.91) Severity of chronic illness: not reported Ethnicities: not reported Depressive symptoms – rating (SD): Reynolds' Child Depression Scale 63.81 (7.64) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Control Number: 17 Sex (males (%)): 9 (53.%) Age in years (SD): 10.59 (3.16) Severity of chronic illness: not reported Ethnicities: not reported Depressive symptoms – rating (SD): Reynolds' Child Depression Scale 63.4 (7.86) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Baseline differences: no significant differences |
|
| Interventions |
Intervention characteristics Acceptance and commitment therapy Audience: child Description of intervention: sessions included exercises and metaphors to teach topics including building a therapeutic contract and functional analysis, creative helplessness, values clarification and building a commitment, control as the problem, alternatives to control, cognitive diffusion, self as context, acceptance and commitment, internal dialogue, and relapse prevention Modality: group Dose: 10 sessions of 90 minutes over 10 weeks Manualised or non‐manualised: manualised Parent or caregiver involvement: not described Therapist involvement: delivered the group treatment Waiting list Audience: child Description of intervention: no sessions. Pre and post‐period data collection only |
|
| Outcomes |
Specific depression measures: Reynolds' Child Depression Rating Scale (RCDS) Improvement in quality of life: Satisfaction with Life Scale (SLS) |
|
| Identification |
Sponsorship source: not reported Country: Iran Authors name: Vahid Moghanloo Institution: Islamic Azad University Email: roghayehataie@gmail.com Address: Islamic Azad University, Parsabad, IR Iran |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: participants were not blind to group and it would have been obvious if they were or were not receiving the intervention. Unclear if personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: all assessments of participants were conducted by a psychologist who was not the therapist and who was blind to subject’s treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: unclear how missing data was handled and no comparison made between those that dropped out and those that did not |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all planned outcomes reported |
| Other bias | Unclear risk | Judgement comment: no other sources of bias identified although some participants reported data verbally so was not confidential and this may have impacted their reporting |
Rostami 2016.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatient clinic Outcome measures: Beck Depression Inventory (BDI); Beck Anxiety Iventory (BAI); HbA1c at baseline and 3 months |
|
| Participants |
Type of chronic illness: type 1 diabetes Inclusion criteria: living in the city of Ahvaz; confirmation of the diagnosis of diabetes by a physician; no previous group‐support training; no history of uncontrolled underlying disease; lack of long‐term complications of diabetes; no history of severe anxiety and depression and other mental illnesses before suffering diabetes Exclusion criteria: psychiatric treatment or drug abuse during the study; hospitalisation during the execution of the study Baseline characteristics Overall Number: 74 Sex (males (%)): 30 (40.5%) Age in years (SD): not reported Severity of chronic illness: not reported Ethnicities: 36 (48%) Arab; 25 (34%) Lor; 13 (18%) Persian Depressive symptoms – rating (SD): not reported Anxiety symptoms ‐ rating (SD): not reported Group training on depression and anxiety Number: 37 Sex (males (%)): 13 (35.2%) Age in years (SD): not reported Severity of chronic illness (SD): mean HbA1c 10.68 (2.15) Ethnicities: 13 (35%) Lor, 19 (51%) Arab, 5 (14%) Persian Depressive symptoms – rating (SD): BDI 16.1 (10.76) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Anxiety symptoms – rating (SD): 16.75 (10.54) Anxiety symptoms ‐ category(none/subthreshold/clinical range/unsure): mild Treatment‐as‐usual Number: 37 Sex (males (%)): 17 (46%) Age in years (SD): not reported severity of chronic illness: mean HbA1c 10.17 (2.09) Ethnicities: 12 (35%) Lor, 17 (46%) Arab, 8 (21%) Persian Depressive symptoms – rating (SD): BDI 20.0 (10.73) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Anxiety symptoms ‐ rating (SD): 18.32 (10.39) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Baseline differences: no significant differences |
|
| Interventions |
Intervention characteristics Group training on depression and anxiety Audience: child Description of intervention: 8 modules covering information about diabetes and its complications; causes; methods of care and self‐care; blood sugar control; emotional support; and general lifestyle advice. Modality: group Dose: 8 weekly sessions of 120 minutes Manualised or non‐manualised: manualised Parent or caregiver involvement: not described Therapist involvement: delivery of group Treatment‐as‐usual Audience: child Dose: 8 weeks Manualised or non‐manualised: non‐manualised |
|
| Outcomes |
Specific depression measures: Beck Depression Inventory (BDI) Specific anxiety measures: Beck Anxiety Inventory (BAI) Status of long‐term physical condition: HbA1c (haemoglobin A1c or glycated haemoglobin test) |
|
| Identification |
Sponsorship source: the study was part of the MSc dissertation of the first author and financially supported by the Ahvaz University of Medical Sciences Country: Iran Authors name: Shahnaz Rostami Institution: Ahvaz Jundishapur University of Medical Sciences Email: marjannaseri33@gmail.com Address: Marjan Naseri, Nursing Care Research Center in Chronic Disease, Nursing and Midwifery School, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran |
|
| Notes | Therapy designed to address anxiety/depression | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: simple random assignment using a computer generated table of 1s and 2s |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: blinding of personnel not clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: self‐report measures used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: method of managing missing data not described. It is not clear if all participants completed the trial. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all planned outcomes appear to be reported |
| Other bias | Unclear risk | Judgement comment: intervention may have been developed by the study authors |
Sharma 2017.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatients clinic Outcome measures: State Trait Anxiety Inventory (STAI); Headache Impact Test (HIT); Children’s Global Assessment Scale (CGAS) completed at baseline and post‐intervention (12 weeks) |
|
| Participants |
Type of chronic illness: headaches/migraines Inclusion criteria: aged 10‐19 years; current ICD‐10 diagnosis of anxiety disorder; current diagnosis of primary headache; basic reading and writing ability; no significant change in medication regimen in previous 4 weeks; no history suggestive of organic disorder Exclusion criteria: not reported Baseline characteristics Overall Number: 64 Sex (males (%)): 33 (52.4%) Age in years (SD): 13.9 (2.43) Type of chronic illness: 49 (78%) tension headache; 14 (22%) migraine Severity of chronic illness: not reported Ethnicities: not reported Anxiety symptoms ‐ rating (SD): not reported Transdiagnostic cognitive behaviour therapy Number: 32 Sex (males (%)): 17 (53.1%) Age in years (SD): 13.9 (2.6) Type of chronic illness: 24 (75%) tension headache, 8 (25%) migraine Severity of chronic illness (SD): mean HIT 59.72 (6.39) Ethnicities: not reported Anxiety symptoms ‐ rating (SD): mean STAI‐S 41 (9.39) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): moderate‐severe Treatment‐as‐usual Number: 32 Sex (males (%)): 16 (51.6%) Age in years (SD): 13.87 (2.2) Type of chronic illness: 25 (81%) tension headache, 6 (19%) migraine Severity of chronic illness (SD): mean HIT 58.23 (5.73) Ethnicities: not reported Anxiety symptoms ‐ rating (SD): mean STAI‐S 42.42 (8.71) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): moderate‐severe Baseline differences: TCBT participants less likely to be using headache medication than TAU participants. |
|
| Interventions |
Intervention characteristics Transdiagnostic cognitive behaviour therapy (TCBT) Audience: child Description of intervention: psychoeducation about anxiety disorders and headache; challenging myths; treatment components; strategies to manage and challenge anxiety; relaxation; exposure to anxiety provoking scenarios; problem‐solving skills; assertiveness training; cognitive restructuring; use of headache diaries to monitor Modality: group Dose: 12 weekly sessions of 120 minutes duration Manualised or non‐manualised: manualised Parent or caregiver involvement: not reported Therapist involvement: sessions delivered by a therapist Treatment‐as‐usual Audience: child Description of intervention: TAU consisted of pharmacotherapy without any active intervention from the researcher Duration: 12 weeks |
|
| Outcomes |
Specific anxiety measures: State Trait Anxiety Inventory (STAI‐State) Improvement in function: Children's Global Assessment Scale (CGAS) Status of long‐term physical condition: Headache Impact Test (HIT) |
|
| Identification |
Sponsorship source: not described Country: India Authors name: Pragya Sharma Institution: All India Institute of Medical Sciences Email: pragya.cp@gmail.com Address: not provided |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: computer‐generated block randomisation was undertaken |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: not clearly described. Implies that assessors were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: clinician‐rated outcome measures were not undertaken by an independent clinician |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no participants lost to follow‐up. All participant data was included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all planned outcomes were reported |
| Other bias | Low risk | Judgement comment: authors developed the treatment manual |
Szigethy 2007.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatient clinic Outcome measures: Children’s Depression Inventory (CDI); Children’s Global Assessment Scale (CGAS) completed at baseline and post‐intervention (12‐14 weeks) |
|
| Participants |
Type of chronic illness: inflammatory bowel disease Inclusion criteria: CDI score of 9 or greater and/or CDI‐P score of 9 at step 2; aged 11‐17 years; biopsy‐confirmed inflammatory bowel disorder Exclusion criteria: unable to speak English; current mood disorder or psychotic disorder; antidepressant medication within two weeks of assessment; substance use/dependence within one month of study enrolment; suicide attempt within one month of study enrolment; previous depression requiring hospitalisation; failure of previous manualised CBT of at least 8 sessions Baseline characteristics Overall Number: 40 Sex (males (%)): 20 (48.8%) Age in years (SD): 14.99 (2.01) Severity of chronic illness: not reported Ethnicities: 31 (78.1%) white; 6 (14.6%) African‐American; 1 (2.4%) Latina; 2 (4.9%) other Depressive symptoms – rating (SD): not reported Cognitive behavioural therapy (PASCET‐PI) Number: 21 Sex (males (%)): 12 (54%) Age in years (SD): 14.95 (2.33) Type of chronic illness: inflammatory bowel disease Severity of chronic illness: 50.0% on steroids Ethnicities: 19 (90.9%) white, 2 (9.1%) African‐American Depressive symptoms – rating (SD): CDI = 25.7 (10.8) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): moderate Treatment‐as‐usual + information sheet Number: 19 Sex (males (%)): 10 (52.5%) Age in years (SD): 15.02 (1.83) Type of chronic illness: inflammatory bowel disease Severity of chronic illness: 42.9% on steroids Ethnicities: 15 (79.9%) white, 4 (29.4%) African‐American Depressive symptoms – rating (SD): CDI = 21.8 (8.1) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): moderate Baseline differences: no significant differences |
|
| Interventions |
Intervention characteristics Cognitive behaviour therapy (PASCET‐PI) Audience: child and parent Description of intervention: targets depression, and skill development to improve cognitions and behaviours related to IBD. Uses the physical illness narrative to address cognitive distortions; relaxation and guided imagery for pain; and behavioural motivation Modality: individual Dose: 9‐11 60 min sessions (up to 3 delivered via telephone) Manualised or non‐manualised: manualised Parent or caregiver involvement: three independent parent sessions designed to educate parents about IBD and MDD, as well as teaching them to become CBT coaches Therapist involvement: delivery of sessions Treatment‐as‐usual + information sheet Audience: child Description of intervention: treatment‐as‐usual (not described) plus the provision of an information sheet for parents about depression Modality: individual Manualised or non‐manualised: non‐manualised |
|
| Outcomes | Specific depression measures: Children`s Depression Inventory (CDI) | |
| Identification |
Sponsorship source: National Institute Mental Health, Wolpow Family Fund Country: USA Authors name: Eva Szigethy Institution: University of Pittsburgh Medical Center Email: szigethye@upmc.edu. Address: Children’s Hospital of Pittsburgh3705 Fifth AvenueRoom 2423, DeSoto WingPittsburgh, PA 15213 |
|
| Notes | Therapy designed to address depression | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: randomisation not clearly described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: blinding of participants not clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: outcome assessors were blinded to the treatment group of participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: three participants discontinued the intervention. Management of missing data not clearly described. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all planned outcomes reported |
| Other bias | Low risk | Judgement comment: no other biases identified |
Szigethy 2014.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatient clinic at two children's hospitals Outcome measures: Children’s Depression Rating Scale (CDRS‐R) Pediatric’s Chrons Disease Activity Index (PCDAI); Pediatric Ulcerative Colitis Activity Index (PUCAI) completed at baseline and post‐intervention (3 months) |
|
| Participants |
Type of chronic illness: gastrointestinal problems Inclusion criteria: 9‐17 years; identified as having IBS by the Porto Criteria; diagnosis of major or minor depression as defined by the DSM‐IV‐TR or the KSADS‐PL Exclusion criteria: lifetime episode of psychotic or bipolar disorder or eating disorder requiring hospitalisation; suicide attempt within one month of the assessment; hospitalisation for MDD within three months of assessment; use of antidepressants within one month of assessment; substance use by history; iatrogenic opiate use within one month of assessment; current psychotherapy engagement Baseline characteristics Overall Number: 217 Sex (males (%)): 102 (47.2%) Age in years (SD): 14.3 Type of chronic illness: 161 (74%) Crohn's disease, 56 (26%) ulcerative colitis Severity of chronic illness: not reported Ethnicities: mixed Depressive symptoms – rating (SD): not reported Cognitive behaviour therapy Number: 110 Sex (males (%)): 54 (49.1%) Age in years (SD): 14.3 (2.5) Type of chronic illness: 79 (72%) Crohn's, 27 (28%) ulcerative colitis Severity of chronic illness: PCDAI (Crohn's) 21.0 (SD:16.2), PUCAI (UC) 23.3 (SD: 24.9) Ethnicities: 104 (94.6%) white, 6 (5.4%) other Depressive symptoms – rating (SD): CDRS‐R 45.1 (12.1) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Supported nondirective therapy Number: 107 Sex (males (%)): 48 (44.9%) Age in years (SD): 14.3 (2.3) Type of chronic illness: 74 (69%) Crohn's, 26 (31%) ulcerative colitis Severity of chronic illness: PDCAI (Crohn's) 22.4 (SD: 16.9), PUCAI 25.8 (SD: 23.8) Ethnicities: 90 (84.1%) white, 17 (15.9%) other Depressive symptoms – rating (SD): CDRS‐R 48.9 (12.8) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): mild Baseline differences: Ethnicity (CBT 94.6%, SNDT 84.1%), surgical resection rate (CBT 5.6%, SNDT 14.2%), and raw mean baseline CDRSR scores (CBT 45.1, SNDT 48.9). |
|
| Interventions |
Intervention characteristics Cognitive behaviour therapy Audience ‐ child / child and parent: child and parent Description of intervention: CBT modules delivered either face‐to‐face or over the telephone. Topics included behavioural activation, relaxation strategies, challenging negative thinking, and relapse prevention. In addition to this, there were 3 parent sessions. Modality: child and family Dose: up to 12 sessions of 45 minutes over three months (9 key sessions with 3 sessions of flexible content) + 3 parent sessions Manualised or non‐manualised: manualised Parent or caregiver involvement: 3 individual sessions Therapist involvement: delivery of sessions Supported nondirective therapy Audience: child and parent Description of intervention: supportive psychotherapy where children were encouraged to talk about whatever was bothering them. In addition to this, there were 3 parent sessions. Modality: individual (child and family) Dose: 12 sessions of 45‐minute duration over three months + 3 parent sessions Manualised or non‐manualised: non‐manualised Parent or caregiver involvement: 3 individual sessions Therapist involvement: delivery of sessions |
|
| Outcomes |
Specific depression measures: Children’s Depression Inventory (CDI) Quality of life: IMPACT‐III Questionnaire Status of long‐term physical condition: Pediatric Chrons Diease Activity Index (PCDAI) or Pediatric Ulcerative Colitis Activity Index (PUCAI) ‐ no data included in meta‐analysis |
|
| Identification |
Sponsorship source: the National Institute of Mental Health (NIMH) through grants R01 MH077770 and NCT00534911. Country: United States of America Authors name: Eva Szigethy Institution: Children's Hospital of Pittsburgh Email: szigethye@upmc.edu Address: Children’s Hospital of Pittsburgh, 4401 Penn Avenue, 3rd Floor Plaza Building, Pittsburgh, PA 15224 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: block randomisation used, but mechanism (computerised/manual) not described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: not clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: self‐report by participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: included all participants in the analysis including those that dropped out |
| Selective reporting (reporting bias) | High risk | Judgement comment: only some data presented. Post‐intervention data regarding depression and symptoms of IBD (and SDs) not reported. |
| Other bias | High risk | Judgement comment: some of the study authors were the developers of the intervention |
Van der Veek 2013.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatient clinic Outcome measures: Reyonds Children’s Anxiety and Depression Scale (RCADS); Abdominal Pain Index (API); Functional Disability Inventory (FDI); KIDSCREEN‐27 completed at baseline, post‐intervention (6 weeks), 6, and 12 months |
|
| Participants |
Types of chronic illness: abdominal pain (AP) Inclusion criteria: aged 7‐18 years of age; fulfilled the Rome III criteria for paediatric AP‐related functional gastrointestinal disorders; AP is main complaint; AP present at least once per week for at least 2 months before diagnosis; no evidence of an inflammatory, anatomic, metabolic, or neoplastic process that explains the patient’s symptoms; absence of a psychiatric disorder that required treatment before treatment of FAP; Dutch‐speaking; AP present during the 2 weeks before inclusion Exclusion criteria: not described Baseline characteristics Overall Number: 104 Sex (males (%)): not reported Age in years (SD): not reported Severity of chronic illness: not reported Ethnicities: not reported Depressive symptoms – rating (SD): not reported Anxiety symptoms ‐ rating (SD): not reported Cognitive behaviour therapy Number: 52 Sex (males (%)): 15 (28.8%) Age in years (SD): 11.94 (2.61) Type of chronic illness: 34 (65%) FAP syndrome, 11 (21%) IBS, 7 (14%) other/combination Severity of chronic illness: mean API 32.18 (SD not reported) Ethnicities: not reported Depressive symptoms – rating (SD): RCADS (dep) 3.92 (SD not reported) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold Anxiety symptoms ‐ rating (SD): mean RCADS (anx) 10.61 (SD not reported) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold Intensive medical care Number: 56 Sex (males (%)): 14 (26.9%) Age in years (SD): 11.87 (2.93) Type of chronic illness): 30 (54%) FAP syndrome, 18 (32%) IBS, 8 (14%) other/combination Severity of chronic illness (SD): API 33.56 (SD not reported) Ethnicities: not described Depressive symptoms – rating (SD): RCADS (dep) 3.72 (SD not reported) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold Anxiety symptoms ‐ rating (SD): RCADS (anx) 8.54 (SD not reported) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold Baseline differences: greater prevalence of anxiety disorders in the IMC group at baseline. |
|
| Interventions |
Intervention characteristics Cognitive behaviour therapy Audience ‐ child / child and parent: child and parent Description of intervention: 1 standard (relaxation training) and 3 optional (cognitive therapy, behaviour therapy directed at child, behaviour therapy directed at parent) modules Modality: individual Dose: 6 weekly sessions of 45 minutes duration Manualised or non‐manualised: manualised Parent or caregiver involvement: parents present during all sessions for participants under 12 years of age and for first, middle, and last session for participants over 12 years of age Therapist involvement: delivery of sessions Intensive medical care Audience: child and parent Description of intervention: medical education delivered by paediatrician to address the complaints raised by the parents Modality: child and family Dose: 6 weekly sessions of 20‐30 minutes Manualised or non‐manualised: non‐manualised Parent or caregiver involvement: parents generally involved in every session. In children 13 years and older, children were able to be seen on their own for a maximum of half of the sessions. Therapist involvement : paediatric gastroenterologists delivered the sessions |
|
| Outcomes |
Specific depression measures: Revised Child Anxiety and Depression Scale — short ‐ no data included in meta‐analysis Specific anxiety measures: Revised Child Anxiety and Depression Scale — short Improvement in quality of life: KIDSCREEN‐27 ‐ no data included in meta‐analysis Improvement in function: Functional Disability Inventory (FDI) ‐ no data included in meta‐analysis Status of long‐term physical condition: Abdominal Pain Index (API) ‐ no data included in meta‐analysis |
|
| Identification |
Sponsorship source: the Dutch Digestive Foundation, grant SWO 05‐09 Country: Netherlands Authors name: Shelley van der Veek Institution: Leiden University Email: sveek@fsw.leidenuniv.nl Address: Department of Child and Family Studies, Leiden university, PO Box 9555, 2300 RB Leiden, Netherlands |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: computerised randomisation undertaken |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: not clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: child and parent self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: full sample analysed using intention to treat principle |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: some data not provided (e.g. standard deviations for key results) |
| Other bias | Low risk | Judgement comment: no other biases identified |
Van Dijk Lokkart 2016.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatients from four paediatric oncology centres Outcome measures: Children’s Depression Inventory (CDI); Pediatric Quality of Life Inventory (PEDSQL) completed at baseline, post‐intervention (12‐weeks), and 12 months |
|
| Participants |
Type of chronic illness: cancer Inclusion criteria: 8‐18 years of age; diagnosed with a childhood malignancy; had completed treatment less than 12 months prior; had previously been treated with chemotherapy and/or radiotherapy Exclusion criteria: requiring stem cell treatment and/or growth hormone therapy; wheelchair dependent; difficulty riding a bike; unable to read or write; unable to self‐reflect; difficulty following instructions; learning disabilities Baseline characteristics Overall Number: 68 Sex (males (%)): 46 (52.9%) Age in years (SD): not reported Severity of chronic illness: not reported Ethnicities: not reported Depressive symptoms – rating (SD): not reported Anxiety symptoms ‐ rating (SD): not reported Social skills intervention Number: 30 Sex (males (%)): 16 (53.3%) Age in years (SD): 13.0 (3.0) Type of chronic illness: cancer – 20 (66%) leukaemia/lymphoma, 2 (7%) brain tumours, 8 (27%) other solid tumours Severity of chronic illness: not reported Ethnicities: not reported Depressive symptoms – rating (SD): not reported Anxiety symptoms ‐ rating (SD): not reported Treatment‐as‐usual Number: 38 Sex (males (%)): 20 (52.6%) Age in years (SD): 12.6 (3.1) Type of chronic illness: 26 (69%) leukaemia/lymphoma, 5 (13%) brain tumours, 7 (18%) other solid tumours Severity of chronic illness: not reported Ethnicities: not reported Depressive symptoms – rating (SD): not reported Anxiety symptoms ‐ rating (SD): not reported Baseline differences: implied that there were differences at baseline but did not report on these |
|
| Interventions |
Intervention characteristics Psychosocial training and physical activity program Audience: child and parent Description of intervention: psychoeducation and cognitive behavioural strategies to improve emotional and social coping. Intensive exercise program consisting of a combination of cardiorespiratory and strength exercises Modality: child and family Dose: 2 x 45 min sessions of physical training per week; 6 x 60 min sessions of child psychotherapy; 2 x 60 min sessions of parent psychotherapy delivered over 12 weeks Manualised or non‐manualised: manualised Parent or caregiver involvement): parents involved in 2 sessions and outcome measurement Therapist involvement: delivery of sessions Treatment‐as‐usual Audience: child and parent Description of intervention: varied between hospitals according to local guidelines, but did not include any routine exercise or psychosocial training Dose: not reported Manualised or non‐manualised: not manualised Parent or caregiver involvement: not described Therapist involvement: not reported |
|
| Outcomes |
Specific depression measures: Children’s Depression Iventory (CDI) ‐ no data included in meta‐analysis Improvement in quality of life: Pediatric Quality of Life Inventory (PEDSQL) Generic Core Scale ‐ no data included in meta‐analysis |
|
| Identification |
Sponsorship source: the Alpe d’HuZes/KWF Fund. The research grant is bestowed upon the Dutch Cancer Society (grant number: ALPE 2009‐4305), the RopaRun and the VUmc Childhood Cancer Research (VONK). Country: Netherlands Authors name: Elisabeth van Dijk Lokkart Institution: VU University Medical Center Email: vandijk@vumc.nl Address: VU University Medical Center, Department of Medical Psychology, P.O. Box 7057,1007 MB Amsterdam, The Netherlands |
|
| Notes | While CDI (depression) was assessed, the authors only described the results in a narrative form ‐ no data were provided and available for extraction | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: block randomisation undertaken by an independent data manager, but exact method not described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: not clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: self‐report used but method of blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: method of managing missing data not clearly described |
| Selective reporting (reporting bias) | High risk | Judgement comment: only some outcomes reported (authors contacted for additional details) |
| Other bias | High risk | Judgement comment: authors developed the intervention and this may have biased their analysis |
Varni 1993.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatients from two cancer centres Outcome measures: Children’s Depression Inventory (CDI); State Trait Anxiety Inventory – children’s version (STAI‐C) completed at baseline, 6, and 9 months |
|
| Participants |
Type of chronic illness: cancer Inclusion criteria: newly diagnosed with cancer; in Grade K to 8; English‐speaking; receiving medical treatment at one of the medical centres participating in the study Exclusion criteria: not described Baseline characteristics Overall Number: 64 Sex (males (%)): 48 (51.3%) Age in years (SD): not reported Severity of chronic illness: not reported Ethnicities: 31 (48%) white; 23 (36%) Hispanic; 6 (9%) Asian; 3 (5%) black; 1 (2%) American‐Indian Depressive symptoms – rating (SD): not reported Anxiety symptoms ‐ rating (SD): not reported Social skills intervention Number: 33 Sex (males (%)): 23 (70%) Age in years (SD): 8.3 (2.4) Type of chronic illness: 19 (58%) acute lymphoblastic leukaemia, 5 (15%) Hodgkin's lymphoma, 9 (27%) other types of cancer Severity of chronic illness: not described Ethnicities: 17 (52%) white, 11 (33%) Hispanic, 4 (12%) Asian, 1 (3%) American‐Indian Depressive symptoms – rating (SD): CDI 4.87 (4.03) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold Anxiety symptoms ‐ rating (SD): mean STAIC 29.75 (6.37) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): mild to moderate Waiting‐list control Number: 31 Sex (males (%)): 15 (48.4%) Age in years (SD): 8.0 (2.4) Type of chronic illness: 17 (55%) acute lymphoblastic leukaemia, 2 (6%) Hodgkin's lymphoma, 12 (39%) other types of cancer Severity of chronic illness: not described Ethnicities: 14 (45%) white, 12 (39%) Hispanic, 2 (6%) Asian, 3 (10%) black Depressive symptoms – rating (SD): CDI 6.83 (SD 4.15) Depressive symptoms ‐ category (none/subthreshold/clinical range/unsure): subthreshold Anxiety symptoms ‐ rating (SD): STAIC 24.69 (SD 7.69) Anxiety symptoms ‐ category (none/subthreshold/clinical range/unsure): mild to moderate Baseline differences: no significant differences at baseline. |
|
| Interventions |
Intervention characteristics Social skills intervention Audience: child. Parent participated in one training session at the beginning of the intervention Description of intervention: Structured social skills training focusing on social cognitive problem‐solving; assertiveness training; handling teasing and name calling Modality: individual Dose: three weekly sessions of 60 minutes duration and 2 boosters at 3 and 6 weeks following the return to school Manualised or non‐manualised: manualised Parent or caregiver involvement: one session at the beginning of the training Therapist involvement: delivery of sessions Standard treatment ‐ routine school reintegration services Audience: child Description of intervention: minimum of 2 hrs individual attention as part of school reintegration programme and equal time with a research assistant via 5 sessions of play interaction Modality: individual Dose: 120‐minute initial session with 5 follow‐up sessions of unspecified duration Manualised or non‐manualised: non‐manualised Parent or caregiver involvement: not described Therapist involvement: not specified |
|
| Outcomes |
Specific depression measures: Children's Depression Inventory (CDI) Specific anxiety measures: State Trait Anxiety Inventory for Children (STAIC) |
|
| Identification |
Sponsorship source: not stated Country: United States of America Authors name: James W Varni Institution: University of California Email: not stated Address: Psychosocial Behavioral Sciences Program, Division of Hematology‐Oncology, Children's Hospital and Health Center, 3020 Children's Way, San Diego, California 92123 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: stratified randomisation used, but process not clearly described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: no information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: self‐report and parent‐report |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: some discrepancies between numbers in tables ‐ missing data and management not clearly described |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all planned outcome measures reported |
| Other bias | Unclear risk | Judgement comment: the intervention may have been developed by the study authors |
Wei 2017.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Setting: outpatient clinic Outcome measures: Wellbeing Questionnaire (WBQ) completed at baseline, 3, and 24 months |
|
| Participants |
Type of chronic illness: type 1 diabetes Inclusion criteria: 11‐16 years; diagnosed with T1DM at least 12 months prior; attending study clinics; Exclusion criteria: other serious chronic illness; significant psychiatric illness; child protection issues Baseline characteristics Overall Number: 66 Sex (males (%)): not reported Age in years (SD): not reported Severity of chronic illness: not reported Ethnicities: not reported Depressive symptoms – rating (SD): not reported Anxiety symptoms ‐ rating (SD): not reported CBT experimental group Number: 33 Sex (males (%)): 19 (44%) Age in years (SD): 13.2 (11.4) Severity of chronic illness: mean HbA1C (SD) = 8.7 (6.4) Ethnicities: not stated Depressive symptoms – rating (SD): WBQ (dep) 5.5 (0.57) Anxiety symptoms ‐ rating (SD): WBQ (anx) 4.7 (0.55) Nondirective behavioural counselling Number: 33 Sex (males (%)): 19 (45%) Age in years (SD): 14.1 (11.7) Severity of chronic illness: mean HbA1C (SD) = 8.4 (6.4) Ethnicities: not stated Depressive symptoms – rating (SD): 6.3 (0.48) Anxiety symptoms ‐ rating (SD): 5.9 (0.52) Baseline differences: no differences at baseline |
|
| Interventions |
Intervention characteristics CBT experimental group Audience: child Description of intervention: the programme addresses developing and maintaining a therapeutic relationship; cognitive restructuring; identifying negative automatic thoughts, recognising associations between thoughts, feelings, and behaviour, and replacing with more balanced thoughts; problem‐solving, assertiveness training, and relaxation. Modality: individual Dose: 6 weekly sessions with follow‐up sessions at 6 and 12 months Manualised or non‐manualised: manualised Parent or caregiver involvement: not described Therapist involvement: delivery of sessions Nondirective behavioural counselling Audience: child Description of intervention: client‐centred, nondirective counselling that provided time for the young person to express any issues/concerns Modality: individual Dose: 6 weekly sessions with follow‐up sessions at 6 and 12 months Manualised or non‐manualised: non‐manualised Parent or caregiver involvement: not described Therapist involvement: delivery of session |
|
| Outcomes |
Improvement in quality of life: Wellbeing Questionnaire (WBQ); Diabetes Quality of Life for Youths Improvement in function: Diabetes Self‐Efficacy Scale ‐ no data included in meta‐analysis Status of long‐term physical condition: HbA1c (haemoglobin A1c or glycated haemoglobin test) ‐ no data included in meta‐analysis |
|
| Identification |
Sponsorship source: Diabetes UK, Grant/Award number: RD01/0002114; National Institute of Health Research Country: England Authors name: Christina Wei Institution: Bristol Royal Hospital for Children Email: Liz.Crowne@UHBristol.nhs.uk Address: Department Paediatric Endocrinology Diabetes, Bristol Royal Hospital for Children; University Hospitals Bristol, NHS Foundation Trust, Bristol, UK |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: randomisation process not clearly described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: blinding process described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: self‐report and HbA1C |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: analyses used (Fisher's Exact Tests) all for the inclusion of subjects with missing data |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all planned outcomes reported |
| Other bias | Low risk | Judgement comment: no other sources of bias identified |
ACT: Acceptance and Committment Therapy
anx: Anxiety AP: Attention Placebo API: Abdominal Pain Index BAI: Beck Anxiety Inventory BDI: Beck Depression Inventory BYAI: Beck Youth Anxiety Inventory BYDI: Beck Youth Depression Inventory BYI: Beck Youth Inventory CCAS: Chinese Children's Anxiety Scale CDI: Children's Depression Inventory CDI‐P: Children's Depression Inventory ‐ Parent CDRS‐R: Childrens Depression Rating Scale ‐ Revised CDS: Childrens Depression CES‐DC: Center for Epidemiologic Studies Scale for Children CGAS: Children's Global Assessment Scale CMAS: Children's Manifest Anxiety Scale COPE: Coping Oriaentiations to Problems Experienced Inventory CPI: California Pyschological Inventory CRPS: Complex Regional Pain Syndrome CSI: Children's Somatization Inventory dep: Depression DQOLY: Diabetes Quality of Life for Youth DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders ‐ version 4, text revision EMG: Electromyelography FAP: Functional Analytic Psychotherapy FDI: Functional Disability Inventory FEV: Forced Expiratory Volume FMS: Fibromyalgia Syndrome GI: Gastrointestinal HADS: Hospital Anxiety and Depression Scale HAM‐A: Hamilton Anxiety Scale HAM‐D: Hamilton Depression Scale HbA1c: Haemoglobin A1C HIT: Headache Impact Test HIV: Human Immunodeficiency Virus IBD: Inflammatory Bowel Disease IBS: Irritable Bowel Syndrome ICD‐10: International Classification of Disease ‐ version 10 IMPACT‐III: Measure of health‐related quality of life in children with inflammatory bowel disease ITT: Intention to treat KIDSCREEN‐27: Measure of children's quality of life KSADS‐PL: Schedule for Affective Disorders and Schizphrenia for School‐aged children ‐ Present and Lifetime version MBSR: Mindfulness Based Stress Reduction MDD: Major Depressive Disorder PASCET‐PI: Primary and Secondary Control Enhancement Training ‐ Physical Illness PCDAI: Paediatric Chron's Disease Activity Index PEDMIDAS: Paediatric Migrain Disability Assessment PEDSQL: Pediatric Quality of Life Scale PEFR: Peak Expiratory Flow Rate PI: Primary investigator PIS:Patient information sheet PMR: Progressive Muscle Relaxation PPPI: Parent Perception of Pain Interference POTS: Postural Orthostatic Tachycardia Syndrome PUCAI: Paediatric Ulcerative Colitis Activity Index QOLEI‐31: Quality of Life in Epilepsy Inventory RCADS: Revised Children's Anxiety and Depression Scale RCDI: Reynold's Child Depression Inventory RCDS: Reynold's Child Depression Scale SBHC: School‐Based Health Centre SD: Standard Deviation SLS: Satisfaction with Life Scale SNDT: Supported Non‐Directive Therapy STAIC: State‐Trait Anxiety Inventory for Children STAI‐S: State‐Trait Anxiety Inventory State version STAI‐T: State‐Trait Anxiety Inventory Trait version STAI‐Y: State‐Trait Anxiety Inventory for Youth T1DM: Type 1 Diabetes Mellitus TAU: Treatment As Usual TCBT: Transdiagnostic Cognitive Behaviour Therapy VAS: Visual Analogue Scale VUKA: ' 'Let's wake up' in Zulu WBQ: Wellbeing Questionnaire WISC‐R: Weschler Intelligence Scale ‐ Revised version
Changes in the severity of depression symptoms were measured using the Beck Youth Depression Inventory (BYDI) scale in three trials (Beebe 2010; Chadi 2016; Hickman 2015), Children's Depression Inventory (CDI) scale in ten trials (Bhana 2014; Grey 1998; Grey 2009; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Levy 2010; Szigethy 2007; Szigethy 2014; Van Dijk Lokkart 2016; Varni 1993), Beck Depression Inventory (BDI) scale in two trials (Martinović 2006; Rostami 2016), Children's Depression Scale (CDS) in one trial (Griffiths 1996), Hospital Anxiety And Depression Scale (HADS) in one trial (Freedenberg 2017), CESD‐C scale in one trial (Kanstrup 2016), Hamilton Depression Scale (HAMD) in one trial (Li 2016), Reynolds' Child Depression Rating Scale (RCDS) in one trial (Moghanloo 2015), Revised Child Anxiety and Depression Scale in one trial (Van der Veek 2013) and the depression subscale of the Wellbeing Questionnaire in one trial (Wei 2017).
Changes in the severity of anxiety symptoms were measured using the Beck Youth Anxiety Inventory (BYAI) scale in three trials (Beebe 2010; Chadi 2016; Hickman 2015), State Trait Anxiety Inventory (STAI) scale in six trials (Bignall 2015; Bussone 1998; Detling Miller 2008; Hains 2000; Sharma 2017; Varni 1993), Chinese Children’s Anxiety scale (CCAS) in one trial (Chiang 2009), HADS in one trial (Freedenberg 2017), Children's Manifest Anxiety Scale in one trial (Griffiths 1996), Hamilton Anxiety Scale (HAMA) scale in one trial (Li 2016), Beck Anxiety Inventory scale in one trial (Rostami 2016), Revised Child Anxiety and Depression scale in one trial (Van der Veek 2013), and the anxiety subscale of the Wellbeing Questionnaire in one trial (Wei 2017).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AmbrosinoJm 2008 | Wrong outcomes |
| Barsevick 2002 | Adult population |
| Bauman 1994 | Wrong outcomes |
| Belsky 1994 | Wrong study design |
| BrownLk 2016 | Adult population |
| Chalder 2002 | Wrong study design |
| Chalder 2004 | Wrong outcomes |
| Chernoff 2002 | Wrong intervention |
| Garcia Perez 2010 | Wrong study design |
| Gauntlett Gilbert 2013 | Wrong study design |
| Gebert 1998 | Wrong outcomes |
| Grover 2002 | Adult population |
| Groß 2013 | Wrong outcomes |
| Gulewitsch 2012 | Wrong study design |
| Hesse 2015 | Wrong study design |
| Jeppesen 2012 | Wrong outcomes |
| Lemanek 2009 | Wrong intervention |
| Long 2011 | Wrong study design |
| Lyon 2014 | Wrong intervention |
| Malboeuf Hurtubise 2016 | Wrong study design |
| Naar King 2010 | Adult population |
| OsterhausSo 1993 | Wrong study design |
| Pless 1994 | Adult population |
| Ribeiro 2008 | Adult population |
| Riley 2015 | Wrong study design |
| Saedi 2012 | Wrong outcomes |
| Scholten 2013 | Wrong outcomes |
| Shoshani 2016 | Wrong intervention |
| Stapersma 2018 | Adult population (<80% of participants were 18 years) |
| Stubbe 2008 | Wrong patient population |
| Westrupp 2015 | Wrong outcomes |
| Yorke 2017 | Adult population (<80% of participants were 18 years) |
Characteristics of studies awaiting assessment [ordered by study ID]
Chadi 2016a.
| Methods | Single‐centre, single‐blinded, prospective, experimental, longitudinal trial using an active intervention and a waiting‐list control |
| Participants | Adolescents with chronic pain of three or more months duration |
| Interventions | 8‐week mindfulness curriculum |
| Outcomes | Self‐reported quality of life, pain perception, anxiety, depression, psychological distress, and cortisol levels |
| Notes | Abstract only available. Author contacted to provide further information but no response received |
Coupey 1991.
| Methods | Single‐site randomised controlled trial using an active intervention and a no‐treatment control |
| Participants | Adolescents with chronic illness |
| Interventions | Two months social skills training program and a four‐month job placement versus no‐treatment control |
| Outcomes | Self‐esteem and psychiatric symptoms |
| Notes | Abstract only available. Author contacted to provide further information but no response received |
Hood 2014.
| Methods | Multisite randomised controlled trial using active intervention and waiting‐list control |
| Participants | Adolescents with type 1 diabetes mellitus |
| Interventions | Nine group sessions of depression prevention program comprising cognitive‐behaviour and problem‐solving therapies versus educational control group |
| Outcomes | Depression, resilience, and diabetes outcome (BGM and A1c) |
| Notes | Abstract only available. Author contacted to provide further information but no response received |
Yang H 2004.
| Methods | Single‐site randomised controlled trial using active intervention and treatment‐as‐usual |
| Participants | Children with asthma |
| Interventions | Four weeks of nightly relaxation therapy before bedtime versus treatment‐as‐usual |
| Outcomes | Anxiety, depression, and asthma symptoms |
| Notes | Abstract only available. Author contacted to provide further information but no response received |
A1C: blood test that reflects your average blood glucose levels
BGM: Blood glucose metre
CBT: Cognitive Behavioural Therapy
RCT: Randomized controlled trial
TAU: Treatment as usual
Differences between protocol and review
Due to recent advances in technology and linkage with RevMan software, we used Covidence software to collate and analyse data instead of the table we presented in our protocol. We also amended our methodology for evaluating treatment acceptability. Validated scales and participant report were prioritised over dropout rates and adverse events, however, all of these dimensions were considered during the analysis of this outcome.
Contributions of authors
| Task | Who has agreed to undertake the task? |
| Drafting the protocol | Hiran Thabrew, Karolina Stasiak, Sarah Hetrick, Sally Merry |
| Developing a search strategy (in conjunction with CCMD’s Information Specialist) | Hiran Thabrew, Karolina Stasiak, Stephen Wong, Sarah Hetrick |
| Selecting which trials to include (2 people + 1 arbiter in the event of dispute) | Hiran Thabrew, Stephen Wong, April Highlander |
| Extracting data from trials (3 people + 1 arbiter in the event of dispute) | Hiran Thabrew, Jessica Huss, April Highlander, Karolina Stasiak |
| Undertaking 'Risk of bias' assessments (2 people + 1 arbiter in the event of dispute) | Hiran Thabrew, Sarah Hetrick, Karolina Stasiak |
| Entering data into RevMan (Cochrane software) | Hiran Thabrew, Karolina Stasiak, Sarah Hetrick |
| Carrying out the analysis | Hiran Thabrew, Sarah Hetrick, Karolina Stasiak |
| Interpreting the analysis | Hiran Thabrew, Sarah Hetrick, Karolina Stasiak, Sally Merry |
| Drafting the final review | Hiran Thabrew, Karolina Stasiak, Liesje Donkin, Sarah Hetrick, Sally Merry |
| Producing the 'Summary of findings' tables | Hiran Thabrew, Sarah Hetrick, Karolina Stasiak |
| Checking final review meets all mandatory MECIR standards before submission | Hiran Thabrew, Sarah Hetrick |
| Keeping the review up to date | Hiran Thabrew, Karolina Stasiak, Sarah Hetrick, Sally Merry |
Sources of support
Internal sources
-
University of Auckland, New Zealand.
Salaries of authors
External sources
-
Oakley Foundation, New Zealand.
Equipment and research assistance
-
Starship Foundation, New Zealand.
Equipment and research assistance
-
National Institute for Health Research (NIHR), UK.
Single largest funder of Cochrane Common Mental Disorders
Declarations of interest
Sally Merry and Karolina Stasiak have been involved in designing and trialing SPARX, an online‐ and CD‐ROM‐based interactive health game for adolescents with depression. Hiran Thabrew, Sarah Hetrick, Liesje Donkin, Jessica Huss, April Highlander, and Stephen Wong, do not have any known conflicts of interest to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Beebe 2010 {published data only}
- Beebe A, Gelfand EW, Bender B. A randomized trial to test the effectiveness of art therapy for children with asthma. Journal of Allergy and Clinical Immunology 2010;126:263‐6, 266.e1. [DOI] [PubMed] [Google Scholar]
Bhana 2014 {published data only}
- Bhana A, Mellins CA, Petersen I, Alicea S, Myeza N, Holst H, et al. The VUKA family program: piloting a family‐based psychosocial intervention to promote health and mental health among HIV infected early adolescents in South Africa. AIDS Care 2014;26:1‐11. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bignall 2015 {published data only}
- Bignall WJR, Luberto CM, Cornette AF, Haj‐Hamed M, Cotton S. Breathing retraining for African‐American adolescents with asthma: a pilot study of a school‐based randomized controlled trial. Journal of Asthma 2015;52:889‐96. [DOI] [PubMed] [Google Scholar]
Bussone 1998 {published data only}
- Bussone G, Grazzi L, D'Amico D, Leone M, Andrasik F. Biofeedback‐assisted relaxation training for young adolescents with tension‐type headache: a controlled study. Cephalalgia 1998;18(7):463‐7. [DOI: 10.1111/j.1468-2982.1998.1807463.x] [DOI] [PubMed] [Google Scholar]
Chadi 2016 {published data only}
- Chadi N, McMahon A, Vadnais M, Malboeuf‐Hurtubise C, Djemli A, Dobkin PL, et al. Mindfulness‐based intervention for female adolescents with chronic pain: A pilot randomized trial. Journal of the Canadian Academy of Child and Adolescent Psychiatry 2016;25(3):159‐68. [PMC free article] [PubMed] [Google Scholar]
Chiang 2009 {published data only}
- Chiang LC, Ma WF, Huang JI, Tseng LF, Hsueh KC. Effect of relaxation‐breathing training on anxiety and asthma signs/symptoms of children with moderate‐to‐severe asthma: a randomized controlled trial. International Journal of Nursing Studies 2009;46:1061‐70. [DOI] [PubMed] [Google Scholar]
Detling Miller 2008 {published data only}
- Detling MNJ. The effects of anxiety reduction techniques on anxiety and blood glucose control in adolescent athletes with type 1 diabetes. Dissertation Abstracts International Section A: Humanities and Social Sciences 2008;68:4646. [Google Scholar]
Freedenberg 2017 {published data only}
- Freedenberg VA, Hinds PS, Friedmann E. Mindfulness‐based stress reduction and group support decrease stress in adolescents with cardiac diagnoses: A randomized two‐group study. Pediatric Cardiology 2017;38(7):1‐11. [DOI: ] [DOI] [PubMed] [Google Scholar]
Grey 1998 {published data only}
- Grey M, Boland EA, Davidson M, Yu C, Sullivan‐Bolyai S, Tamborlane WV. Short‐term effects of coping skills training as adjunct to intensive therapy in adolescents. Diabetes Care 1998;21:902‐8. [DOI] [PubMed] [Google Scholar]
Grey 2009 {published data only}
- Grey M, Whittemore R, Jaser S, Ambrosino J, Lindemann E, Liberti L, et al. Effects of coping skills training in school‐age children with type 1 diabetes. Research in Nursing & Health 2009;32:405‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffiths 1996 {published data only}
- Griffiths JD, Martin PR. Clinical‐versus home‐based treatment formats for children with chronic headache. British Journal of Health Psychology 1996;1(2):151‐66. [DOI: 10.1111/j.2044-8287.1996.tb00499.x] [DOI] [Google Scholar]
Hains 2000 {published data only}
- Hains AA, Davies WH, Parton E, Totka J, Amoroso‐Camarata J. A stress management intervention for adolescents with type 1 diabetes. Diabetes Educator 2000;26:417‐24. [DOI] [PubMed] [Google Scholar]
Hickman 2015 {published data only}
- Hickman C, Jacobson D, Melnyk BM. Randomized controlled trial of the acceptability, feasibility, and preliminary effects of a cognitive behavioral skills building intervention in adolescents with chronic daily headaches: A pilot study. Journal of Pediatric Health Care 2015;29:5‐16. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanstrup 2016 {published data only}
- Kanstrup M, Wicksell RK, Kemani M, Lipsker CW, Lekander M, Holmstrom L. A clinical pilot study of individual and group treatment for adolescents with chronic pain and their parents: Effects of acceptance and commitment therapy on functioning. Children 2016;3(4):30. [DOI: 10.3390/children3040030] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kashikar‐Zuck 2005 {published data only}
- Kashikar‐Zuck S, Swain NF, Jones BA, Graham TB. Efficacy of cognitive‐behavioral intervention for juvenile primary fibromyalgia syndrome. Journal of Rheumatology 2005;32:1594‐602. [PubMed] [Google Scholar]
Kashikar‐Zuck 2012 {published data only}
- Kashikar ZS, Ting TV, Arnold LM, Bean J, Powers SW, Graham TB, et al. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia: a multisite, single‐blind, randomized, controlled clinical trial. Arthritis and Rheumatism 2012;64:297‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levy 2010 {published data only}
- Levy RL, Langer SL, Walker LS, Romano JM, Christie DL, Youssef N, et al. Cognitive‐behavioral therapy for children with functional abdominal pain and their parents decreases pain and other symptoms. American Journal of Gastroenterology 2010;105(4):946‐56. [DOI: 10.1038/ajg.2010.106] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li J, Wang XF, Meng, HQ, Zeng, KB, Quan, FY, Liu, F. Systemic family therapy of comorbidity of anxiety and depression with epilepsy in adolescents. Psychiatry Investigation 2016;13(3):305‐10. [DOI: 10.4306/pi.2016.13.3.305] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinović 2006 {published data only}
- Martinović Z, Simonović P, Djokić R. Preventing depression in adolescents with epilepsy. Epilepsy & Behavior 2006;9:619‐24. [DOI: 10.1016/j.yebeh.2006.08.017] [DOI] [PubMed] [Google Scholar]
Moghanloo 2015 {published data only}
- Moghanloo VA, Moghanloo RA, Moazezi M. Effectiveness of acceptance and commitment therapy for depression, psychological well‐being and feeling of guilt in 7‐15 years old diabetic children. Iranian Journal of Pediatrics 2015;25(4):e2436. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rostami 2016 {published data only}
- Rostami S, Naseri M, Dashtbozorgi B, Zarea K, Qhahfarrokhi KR, Haghighizadeh MH. Effects of Group Training on Depression and Anxiety among Patients with Type I Diabetes: a Randomized Clinical Trial. International Journal of Pediatrics 2016;4(5):1777‐86. [Google Scholar]
Sharma 2017 {published data only}
- Sharma P, Mehta M, Sagar R. Efficacy of transdiagnostic cognitive‐behavioral group therapy for anxiety disorders and headache in adolescents. Journal of Anxiety Disorders 2017;46:78‐84. [DOI: 10.1016/j.janxdis.2016.11.001] [DOI] [PubMed] [Google Scholar]
Szigethy 2007 {published data only}
- Szigethy E, Kenney E, Carpenter J, Hardy DM, Fairclough D, Bousvaros A, et al. Cognitive‐behavioral therapy for adolescents with inflammatory bowel disease and subsyndromal depression. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46:1290‐8. [DOI: 10.1097/chi.0b013e3180f6341f] [DOI] [PubMed] [Google Scholar]
- Thompson RD, Craig A, Crawford EA, Fairclough D, Gonzalez‐Heydrich J, Bousvaros A. Longitudinal results of cognitive behavioral treatment for youths with inflammatory bowel disease and depressive symptoms. Journal of Clinical Psychology in Medical Settings 2012;19:329‐37. [DOI: 10.1007/s10880-012-9301-8] [DOI] [PubMed] [Google Scholar]
Szigethy 2014 {published data only}
- Keerthy D, Youk A, Srinath AI, Malas N, Bujoreanu S, Bousvaros A, et al. Effect of psychotherapy on healthcare utilization in children with inflammatory bowel disease and depression. Journal of Pediatric Gastroenterology and Nutrition 2017;63(3):658‐64. [ISSN:0277‐2116 (Print)1536‐4801 (Electronic)0277‐2116 (Linking)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szigethy E, Bujoreanu SI, Youk AO, Weisz J, Benhayon D, Fairclough D, et al. Randomized efficacy trial of two psychotherapies for depression in youth with inflammatory bowel disease. Journal of the American Academy of Child & Adolescent Psychiatry 2014;53:726‐35. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Veek 2013 {published data only}
- Veek S, Derkx B, Haan E, Benninga MA, Boer F. Cognitive behavior therapy for children with functional abdominal pain: Preliminary results of a randomized controlled trial conference abstract. Gastroenterology [abstracts from Digestive Disease Week]. Chicago, IL United States: AGA Journals, May 7‐10] 2011; Vol. 140:S94.
- Veek SM, Derkx BH, Benninga MA, Boer F, Haan E. Cognitive behavior therapy for pediatric functional abdominal pain: a randomized controlled trial. Pediatrics 2013;132:e1163‐e1172. [DOI: ] [DOI] [PubMed] [Google Scholar]
Van Dijk Lokkart 2016 {published data only}
- Dijk‐Lokkart EM, Braam KI, Dulmen‐den Broeder E, Kaspers GJL, Takken T, Grootenhuis, MA, et al. Effects of a combined physical and psychosocial intervention program for childhood cancer patients on quality of life and psychosocial functioning: results of the QLIM randomized clinical trial. Psycho‐Oncology 2016;25(7):815‐22. [DOI: ] [DOI] [PubMed] [Google Scholar]
Varni 1993 {published data only}
- Varni JW, Katz ER, Colegrove Jr R, Dolgin M. The Impact of Social Skills Training on the Adjustment of Children with Newly Diagnosed Cancer1. Journal of Pediatric Psychology 1993;18(6):751‐767. [DOI: 10.1093/jpepsy/18.6.751] [DOI] [PubMed] [Google Scholar]
Wei 2017 {published data only}
- Wei C, Allen RJ, Tallis PM, Ryan FJ, Hunt LP, Shield JP, et al. Cognitive behavioural therapy stabilises glycaemic control in adolescents with type 1 diabetes‐Outcomes from a randomised control trial. Pediatric Diabetes 2017;19(1):106‐113. [DOI: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
AmbrosinoJm 2008 {published data only}
- Ambrosino JM, Fennie K, Whittemore R, Jaser S, Dowd MF, Grey M. Short‐term effects of coping skills training in school‐age children with type 1 diabetes. Pediatric Diabetes 2008;9:74‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barsevick 2002 {published data only}
- Barsevick AM, Sweeney C, Haney E, Chung E. A systematic qualitative analysis of psychoeducational interventions for depression in patients with cancer (Structured abstract). Oncology Nursing Forum 2002;29(1):73‐84. [DOI] [PubMed] [Google Scholar]
Bauman 1994 {published data only}
- Bauman LJ, Coupey SM, Lauby JI, Koeber C, Silver EJ, Stein R. Long‐term mental‐health effects of TEEN, a social skills program for inner‐city adolescents with chronic health conditions. Pediatric Research 1994;35:A3. [Google Scholar]
Belsky 1994 {published data only}
- Belsky J, Khanna P. The effects of self‐hypnosis for children with cystic fibrosis: a pilot study. American Journal of Clinical Hypnosis 1994;36:282‐92. [DOI] [PubMed] [Google Scholar]
BrownLk 2016 {published data only}
- Brown LK, Kennard BD, Emslie GJ, Mayes Tl, Whiteley LB, Bethel J, et al. Effective treatment of depressive disorders in medical clinics for adolescents and young adults living with HIV: a controlled trial. Journal of Acquired Immune Deficiency Syndromes 2016;71:38‐46. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chalder 2002 {published data only}
- Chalde T, Tong J, Deary V. Family cognitive behaviour therapy for chronic fatigue syndrome: an uncontrolled study. Archives of Disease in Childhood 2002;86(2):95‐7. [DOI: 10.1136/adc.86.2.95] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chalder 2004 {published data only}
- Chalder T, Deary V, Husain K. Family focused cognitive behaviour therapy versus psycho‐education for chronic fatigue syndrome in 11‐18 year olds: a randomised controlled trial. 32nd Congress of the British Association for Behavioural and Cognitive Psychotherapies (jointly with the European Association of Behavioural and Cognitive Therapies); 2004 September 7 ‐ 11; Manchester. 2004; Vol. 40, issue 8:205.
Chernoff 2002 {published data only}
- Chernoff RG, Ireys HT, DeVet KA, Kim YJ. A randomized, controlled trial of a community‐based support program for families of children with chronic illness: pediatric outcomes. Archives of Pediatrics & Adolescent Medicine 2002;156:533‐9. [DOI] [PubMed] [Google Scholar]
Garcia Perez 2010 {published data only}
- Garcia‐Perez L, Perestelo‐Perez L, Serrano‐Aguilar P, Trujillo‐Martin MD. Effectiveness of a psychoeducative intervention in a summer camp for children with type 1 diabetes mellitus. Diabetes Educator 2010;36(2):310‐7. [DOI: 10.1177/0145721710361784] [DOI] [PubMed] [Google Scholar]
Gauntlett Gilbert 2013 {published data only}
- Gauntlett‐Gilbert J, Connell H, Clinch J, McCracken LM. Acceptance and values‐based treatment of adolescents with chronic pain: outcomes and their relationship to acceptance. Journal of Pediatric Psychology 2013;38(1):72‐81. [DOI: 10.1093/jpepsy/jss098] [DOI] [PubMed] [Google Scholar]
Gebert 1998 {published data only}
- Gebert N, Hummelink R, Konning J, Staab D, Schmidt S, Szczepanski R, et al. Efficacy of a self‐management program for childhood asthma ‐ A prospective controlled study. Patient Education and Counseling 1998;35(3):213‐20. [DOI: 10.1016/s0738-3991(98)00061-5] [DOI] [PubMed] [Google Scholar]
Groß 2013 {published data only}
- Groß M, Warschburger P. Evaluation of a cognitive‐behavioral pain management program for children with chronic abdominal pain: a randomized controlled study. International Journal of Behavioral Medicine 2013;20:434‐43. [DOI] [PubMed] [Google Scholar]
Grover 2002 {published data only}
- Grover N, Kumaraiah V, Prasadrao PS, D'Souza G. Cognitive behavioural intervention in bronchial asthma. Journal of the Association of Physicians of India 2002;50:896‐900. [PubMed] [Google Scholar]
Gulewitsch 2012 {published data only}
- Gulewitsch MD, Schauer JS, Hautzinger M, Schlarb AA. Therapy of functional abdominal pain in childhood. Concept, acceptance and preliminary results of a short hypnotherapeutic‐behavioural intervention. Schmerz 2012;26(2):160‐7. [DOI: 10.1007/s00482-011-1139-8] [DOI] [PubMed] [Google Scholar]
Hesse 2015 {published data only}
- Hesse T, Holmes LG, Kennedy‐Overfelt V, Kerr LM, Giles LL. Mindfulness‐based intervention for adolescents with recurrent headaches: a pilot feasibility study. Evidence‐Based Complementary and Alternative Medicine 2015;Article ID 508958:1‐9. [DOI: 10.1155/2015/508958] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeppesen 2012 {published data only}
- Jeppesen JH, Herlin T, Christensen AE, Leegaard A, Thastum M. A waitlist controlled trial of the efficacy of a psychological treatment program for children with juvenile idiopathic arthritis and their parents abstract. Annals of the Rheumatic Disease 2012;71:739. [DOI: ] [Google Scholar]
Lemanek 2009 {published data only}
- Lemanek KL, Ranalli M, Lukens C. A randomized controlled trial of massage therapy in children with sickle cell disease. Journal of Pediatric Psychology 2009;34(10):1091‐6. [DOI: 10.1093/jpepsy/jsp015] [DOI] [PubMed] [Google Scholar]
Long 2011 {published data only}
- Long KA, Ewing LJ, Cohen S, Skoner D, Gentile D, Koehrsen J, et al. Preliminary evidence for the feasibility of a stress management intervention for 7‐ to 12‐year‐olds with asthma. Journal of Asthma 2011;48(2):162‐70. [DOI: 10.3109/02770903.2011.554941] [DOI] [PubMed] [Google Scholar]
Lyon 2014 {published data only}
- Lyon ME, Jacobs S, Briggs L, Cheng YI, Wang J. A longitudinal, randomized, controlled trial of advance care planning for teens with cancer: Anxiety, depression, quality of life, advance directives, spirituality. Journal of Adolescent Health 2014;54(6):710‐7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Malboeuf Hurtubise 2016 {published data only}
- Malboeuf‐Hurtubise C, Achille M, Muise L, Beauregard‐Lacroix R, Vadnais M, Lacourse E. A mindfulness‐based meditation pilot study: lessons learned on acceptability and feasibility in adolescents with cancer. Journal of Child and Family Studies 2016;25(4):1168‐77. [DOI: 10.1007/s10826-015-0299-z] [DOI] [Google Scholar]
Naar King 2010 {published data only}
- Naar‐King S, Parsons JT, Murphy D, Kolmodin K, Harris DR. A multisite randomized trial of a motivational intervention targeting multiple risks in youth living with HIV: initial effects on motivation, self‐efficacy, and depression. Journal of Adolescent Health 2010;46:422‐8. [S1054‐139X(09)00607‐7 [pii] 10.1016/j.jadohealth.2009.11.198 [doi]; 10.1016/j.jadohealth.2009.11.198 [doi]] [DOI] [PMC free article] [PubMed] [Google Scholar]
OsterhausSo 1993 {published data only}
- Osterhaus SO, Passchier J, Helm‐Hylkema H, Jong KT, Orlebeke JF, Grauw AJ, et al. Effects of behavioral psychophysiological treatment on schoolchildren with migraine in a nonclinical setting: predictors and process variables. Journal of Pediatric Psychology 1993;18:697‐715. [DOI] [PubMed] [Google Scholar]
Pless 1994 {published data only}
- Pless IB, Feeley N, Gottlieb L, Rowat K, Dougherty G, Willard B. A randomized trial of a nursing intervention to promote the adjustment of children with chronic physical disorders. Pediatrics 1994;94:70‐5. [PubMed] [Google Scholar]
Ribeiro 2008 {published data only}
- Ribeiro LH, Jennings F, Jones A, Furtado R, Natour J. Effectiveness of a back school program in low back pain. Clinical and Experimental Rheumatology 2008;26(1):81‐8. [PubMed] [Google Scholar]
Riley 2015 {published data only}
- Riley AR, Duke DC, Freeman KA, Hood KK, Harris MA. Depressive symptoms in a trial behavioral family systems therapy for diabetes: a post hoc analysis of change. Diabetes Care 2015;38:1435‐40. [DOI: ] [DOI] [PubMed] [Google Scholar]
Saedi 2012 {published data only}
- Saedi S, Najafabadi NM, Roustaei A. The effect of cognitive‐religious group therapy on psychological profile and hopefulness in adolescents with cancer in ahvaz. Iranian Journal of Psychiatry 2012;Conference: 5th International Congress of Iranian Association of Child and Adolescents Psychiatry Tehran Iran, Islamic Republic of. Conference Start: 20121008 Conference End: 20121010. Conference Publication::128. [Google Scholar]
Scholten 2013 {published data only}
- Scholten L, Willemen AM, Last BF, Maurice‐Stam H, Dijk EM, Ensink E, et al. Efficacy of psychosocial group intervention for children with chronic illness and their parents. Pediatrics 2013;131(4):E1196‐E1203. [DOI: 10.1542/peds.2012-2222] [DOI] [PubMed] [Google Scholar]
Shoshani 2016 {published data only}
- Shoshani A, Mifano K, Czamanski‐Cohen J. The effects of the Make a Wish intervention on psychiatric symptoms and health‐related quality of life of children with cancer: a randomised controlled trial. Quality of Life Research 2016;25(5):1209‐18. [DOI: 10.1007/s11136-015-1148-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stapersma 2018 {published data only}
- Stapersma L, Brink G, Ende J, Szigethy EM, Beukers R, Korpershoek TA, et al. Effectiveness of disease‐specific cognitive behavioral therapy on anxiety, depression, and quality of life in youth with inflammatory bowel disease: a randomised controlled trial. Journal of Pediatric Psychology 2018;43(9):967‐80. [DOI: 10.1093/jpepsy/jsy029] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stubbe 2008 {published data only}
- Stubbe D. A focus on reducing anxiety in children hospitalized for cancer and diverse pediatric medical diseases through a self‐engaging art intervention. Dissertation Abstracts International: Section B: The Sciences and Engineering 2008;69:3881. [Google Scholar]
Westrupp 2015 {published data only}
- Westrupp EM, Northam E, Lee KJ, Scratch SE, Cameron F. Reducing and preventing internalizing and externalizing behavior problems in children with type 1 diabetes: a randomized controlled trial of the Triple P‐Positive Parenting Program. Pediatric Diabetes 2015;16(7):554‐63. [DOI: 10.1111/pedi.12205] [DOI] [PubMed] [Google Scholar]
Yorke 2017 {published data only}
- Yorke J, Adair P, Doyle AM, Dubrow‐Marshall L, Fleming S, Holmes L, et al. A randomised controlled feasibility trial of group cognitive behavioural therapy for people with severe asthma. Journal of Asthma 2018;54(5):543‐54. [DOI: 10.1080/02770903.2016.1229335] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Chadi 2016a {published data only}
- Chadi N, McMahon A, Luu TM, Haley N. A randomized pilot study of an adapted mindfulness‐based intervention for adolescents with chronic pain. Journal of Adolescent Health. Conference: 2016 Annual Meeting of the Society for Adolescent Health and Medicine, SAHM 2016. Washington, DC United States. Conference Start: 20160309. Conference End: 20160312. Conference Publication: (var.pagings) 2016;58(2 SUPPL. 1):S66‐S67. [Google Scholar]
Coupey 1991 {published data only}
- Coupey S, Bauman L, Lauby J, Koeber C, Stein R. Mental‐health effects of a social skills intervention for adolescents with chronic illness. Pediatric research 1991;29:A3. [DOI] [Google Scholar]
Hood 2014 {published data only}
- Hood KK, Weissberg‐Benchell J. Supporting teen problem solving (STEPS): Depression prevention for teens with T1D. Diabetes 2014;74th Scientific Sessions of the American Diabetes Association San Francisco, CA United States. Conference Start: 20140613 Conference End: 20140617:A206. [DOI: ] [Google Scholar]
Yang H 2004 {published data only}
- Yang HB, Tang YQ, Yi ZW. Effect of relaxation training on psychosomatic symptoms of children with asthma. Zhongguo Linchuang Kangfu 2004;8:8392‐3. [DOI] [Google Scholar]
Additional references
Abramson 1978
- Abramson LY, Seligman MEP, Teasdale I. Learned helplessness in humans: critique and reformulation. Journal of Abnormal Psychology 1978;87(1):49‐59. [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
American Psychological Association 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. USA: American Psychological Association, 2013. [Google Scholar]
Aydin 2014
- Aydin A. Parental involvement in cognitive‐behavioral therapy for children with anxiety disorders. Turk Psikiyatri Dergisi [Turkish Journal of Psychiatry] 2014;25(3):181‐9. [PubMed] [Google Scholar]
Bateman 2000
- Bateman A, Brown D, Pedder J. Introduction to psychotherapy: An outline of psychodynamic principles and practice. 3rd Edition. London (UK): Routledge, 2000. [Google Scholar]
Beck 1976
- Beck AT. Cognitive Therapy and the Emotional Disorders. New York (NY): International Universities Press, 1976. [Google Scholar]
Beidel 2000
- Beidel DC, Turner SM, Hamlin K, Morris TL. The Social Phobia and Anxiety Inventory for Children (SPAI‐C): external and discriminative validity. Behavior Therapy 2000;31(1):75‐87. [Google Scholar]
Bennett 2013
- Bennett K, Manassis K, Walter SD, Cheung A, Wilansky‐Traynor P, Diaz‐Granados N, et al. Cognitive behavioral therapy age effects in child and adolescent anxiety: an individual patient data meta‐analysis. Depression and Anxiety 2013;30(9):829‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 2015
- Bennett S, Shafran R, Coughtrey A, Walker S, Heyman I. Psychological therapies for mental health disorders in children with chronic physical illness: a systematic review. Archives of Diseases in Childhood 2015;100:308‐16. [DOI: 10.1136/archdischild-2014-307866] [DOI] [PubMed] [Google Scholar]
Benton 2007
- Benton TD, Ifeagwu JA, Smith–Whitley K. Anxiety and depression in children and adolescents with sickle cell disease. Current Psychiatry Reports 2007;9(2):114‐21. [DOI] [PubMed] [Google Scholar]
Birmaher 1996
- Birmaher B, Ryan N, Williamson D, Brent D, Kaufman J, Dahl R, et al. Childhood and adolescent depression: A review of the past 10 years. Part I. Journal of the American Academy of Child and Adolescent Psychiatry 1996;35(11):1427‐39. [DOI] [PubMed] [Google Scholar]
Birmaher 1996a
- Birmaher B, Ryan N, Williamson D, Brent D. Childhood and adolescent depression: a review of the past 10 years, Part II. Journal of the American Academy of Child and Adolescent Psychiatry 1996;35(12):1575‐83. [DOI] [PubMed] [Google Scholar]
Birmaher 1999
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the screen for child anxiety related emotional disorders scale (SCARED): a replication study. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38:1230‐6. [DOI] [PubMed] [Google Scholar]
Bittner 2007
- Bittner A, Egger HL, Erkanli A, Costello JE, Foley DL, Angold A. What do childhood anxiety disorders predict?. Journal of Child Psychology and Psychiatry 2007;48(12):1174‐83. [DOI] [PubMed] [Google Scholar]
Bolton 2007
- Bolton P, Bass J, Betancourt T, Speelman L, Onyango G, Clougherty KF, et al. Interventions for depression symptoms among adolescent survivors of war and displacement in northern Uganda: a randomized controlled trial. JAMA 2007;298(5):519‐27. [DOI] [PubMed] [Google Scholar]
Brady 1992
- Brady EU, Kendall PC. Comorbidity of anxiety and depression in children and adolescents. Psychological Bulletin 1992;111(2):244‐55. [DOI] [PubMed] [Google Scholar]
Breitbart 1995
- Breitbart W. Identifying patients at risk for and treatment of major psychiatric complications of cancer. Supportive Care in Cancer 1995;3:45‐60. [DOI] [PubMed] [Google Scholar]
Brent 1986
- Brent DA, Kalas R, Edelbrock C, Costello AJ, Dulcan MK, Conover N. Psychopathology and its relationship to suicidal ideation in childhood and adolescence. Journal of the American Academy of Child and Adolescent Psychiatry 1986;25(5):666‐73. [DOI] [PubMed] [Google Scholar]
Brent 2002
- Brent DA, Birmaher B. Adolescent depression. New England Journal of Medicine 2002;347(9):667‐71. [DOI] [PubMed] [Google Scholar]
Burkart 2002
- Burkart P. Children’s adherence to recommended asthma self‐management. Pediatric Nursing 2002;28(2):409‐15. [PubMed] [Google Scholar]
Burke 1999
- Burke P, Elliott M. Depression in pediatric chronic illness. Psychosomatics 1999;40(1):5‐17. [DOI] [PubMed] [Google Scholar]
Cadman 1987
- Cadman D, Boyle M, Szatmari P. Chronic illness, disability and mental and social well‐being: findings of the Ontario Child Health Study. Pediatrics 1987;79:805‐13. [PubMed] [Google Scholar]
Cain 2002
- Cain DJ, Seeman J. Humanistic Psychotherapies: Handbook of Research and Practice. Washington DC: American Psycholoigcal Association, 2002. [Google Scholar]
Carr 2006
- Carr A. The Handbook of Child and Adolescent Clinical Psychology. 2nd Edition. London (UK): Routledge, 2006. [Google Scholar]
Cates 2002
- Cates CJ. Simpson's paradox and calculation of number needed to treat from meta‐analysis. BMC Medical Research Methodology 2002;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cicchetti 1998
- Cicchetti D, Toth SL. The development of depression in children and adolescents. American Psychologist 1998;53(2):221‐41. [DOI] [PubMed] [Google Scholar]
Costello 1992
- Costello EJ, Shugart MA. Above and below the threshold: severity of psychiatric symptoms and functional impairment in a pediatric sample. Pediatrics 1992;90(3):359‐68. [PubMed] [Google Scholar]
Costello 2004
- Costello EJ, Egger HL, Angold A. Developmental epidemiology of anxiety disorders. In: Ollendick TH, March JS editor(s). Phobic and Anxiety Disorders in Children and Adolescents: A Clinician's Guide to Effective Psychosocial and Pharmacological Interventions. New York: Oxford University Press, 2004:61‐91. [Google Scholar]
Cox 2014
- Cox GR, Callahan P, Churchill R, Hunot V, Merry SN, Parker AG, et al. Psychological therapies versus antidepressant medication, alone and in combination for depression in children and adolescents. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD008324.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Craske 2014
- Craske MG, Treanor M, Conway CC, Zbozinek T, Vervliet B. Maximizing exposure therapy: an inhibitory learning approach. Behaviour Research and Therapy 2014;58:10‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuijpers 2002
- Cuijpers P, Smit F. Excess mortality in depression: a meta‐analysis of community studies. Journal of Affective Disorders 2002;72(3):227‐36. [DOI] [PubMed] [Google Scholar]
Dantzer 2003
- Dantzer C, Swendsen J, Maurice‐Tison S, Salamon R. Anxiety and depression in juvenile diabetes: a critical review. Clinical Psychology Review 2003;23:787‐800. [DOI] [PubMed] [Google Scholar]
Davidson 2002
- Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, Cohen JD. Neural and behavioral substrates of mood and mood regulation. Biological Psychiatry 2002;52:478‐502. [DOI] [PubMed] [Google Scholar]
de Groot 2001
- Groot M, Anderson R, Freedland K, Clouse R, Lustman P. Association of depression and diabetes complications: a meta‐analysis. Psychosomatic Medicine 2001;63:619‐30. [DOI] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. 8th International Cochrane Colloquium; 2000 Oct 25‐28; Cape Town (SA). The Cochrane Collaboration, 2000.
Denny 2014
- Denny S, Silva M, Fleming T, Clark T, Merry S, Ameratunga S, et al. The prevalence of chronic health conditions impacting on daily functioning and the association with emotional well‐being among a national sample of high school students. Journal of Adolescent Health 2014;54(4):410‐15. [DOI] [PubMed] [Google Scholar]
Diamond 2002
- Diamond GS, Reis BF, Diamond GM, Siqueland L, Isaacs L. Attachment‐based family therapy for depressed adolescents: a treatment development study. Journal of the American Academy of Child and Adolescent Psychiatry 2000;41(10):1190‐96. [DOI] [PubMed] [Google Scholar]
Drotar 1978
- Drotar D. Psychological research in pediatric settings: lessons from the field. Journal of Pediatric Psychology 1978;19:63‐79. [DOI] [PubMed] [Google Scholar]
Duval 2000
- Duval S, Tweedie R. Trim and fill: A simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56(2):453‐63. [DOI] [PubMed] [Google Scholar]
Eccleston 2012
- Eccleston C, Palermo TM, Fisher E, Law E. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD009660.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eiser 1997
- Eiser C. Effects of chronic illness on children and their families. Advances in Psychiatric Treatment 1997;3:204‐10. [Google Scholar]
Fergusson 2001
- Fergusson DM, Horwood LJ. The Christchurch health and development study: review of findings on child and adolescent mental health. Australian and New Zealand Journal of Psychiatry 2001;35:287‐96. [DOI] [PubMed] [Google Scholar]
Fergusson 2006
- Fergusson DM, Horwood LJ, Ridder EM, Beautrais AL. Subthreshold depression in adolescence and mental health outcomes in adulthood. Archives of General Psychiatry 2005;62(1):66‐72. [DOI] [PubMed] [Google Scholar]
Fisher 2018
- Fisher E, Law E, Dudeney J, Palermo TM, Stewart G, Eccleston C. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database of Systematic Reviews 2018, Issue 10. [DOI: 10.1002/14651858.CD003968.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleming 1993
- Fleming JE, Boyle MH, Offord DR. The outcome of adolescent depression in the Ontario child health study follow‐up. Journal of the American Academy of Child and Adolescent Psychiatry 1993;32(1):28‐33. [DOI] [PubMed] [Google Scholar]
Fonagy 2005
- Fonagy P, Target M, Cottrell D, Phillips J, Kurtz Z. What works for whom: a critical review of treatments for children and adolescents. 1st Edition. New York (NY): The Guilford Press, 2005. [Google Scholar]
Furakawa 2014
- Furukawa TA, Noma H, Caldwell DM, Honyashiki M, Shinohara K, Imai H, et al. Waiting list may be a nocebo condition in psychotherapy trials: a contribution from network meta‐analysis. Acta Psychiatrica Scandinavica 2014;130(3):181‐92. [DOI] [PubMed] [Google Scholar]
Gilbert 2005
- Gilbert PJ. Compassion: Conceptualisations, Research and Use in Psychotherapy. New York (NY): Brunner‐Routledge, 2005. [Google Scholar]
Gilbert 2009
- Gilbert PJ. The Compassionate Mind. London (UK): Constable & Robinson, 2009. [Google Scholar]
Glassman 1998
- Glassman AH, Shapiro PA. Depression and the course of coronary artery disease. American Journal of Psychiatry 1998;55:4‐11. [DOI] [PubMed] [Google Scholar]
Gonzales‐Tejera 2005
- González‐Tejera G, Canino G, Ramírez R, Chávez L, Shrout P, Bird H, et al. Examining minor and major depression in adolescents. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2005;46(8):888‐99. [DOI] [PubMed] [Google Scholar]
Goodyer 2000
- Goodyer IM, Tamplin A, Herbert J, Altham PME. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high‐risk adolescents. British Journal of Psychiatry 2000;177(6):499‐504. [DOI] [PubMed] [Google Scholar]
Gortmaker 1990
- Gortmaker SL, Walker DK, Weitzman M. Chronic conditions, socioeconomic risks and behavioural problems in children and adolescents. Pediatrics 1990;85:267‐76. [PubMed] [Google Scholar]
Guerney 1971
- Guerney B Jr, Stollak G, Guerney L. The practicing psychologist as educator ‐ An alternative to the medical practitioner model. Professional Psychology 1971;2:276‐82. [Google Scholar]
Guyatt 1998
- Guyatt GH, Juniper EF, Walter SD, Griffith LE, Goldstein RS. Interpreting treatment effects in randomised trials. BMJ 1998;316(7132):690‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halfon 2010
- Halfon N, Newacheck W. Evolving notions of childhood chronic illness. JAMA 2010;303(7):665‐6. [DOI] [PubMed] [Google Scholar]
Hamilton 1967
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology 1967;6:278‐96. [DOI] [PubMed] [Google Scholar]
Harrington 1998
- Harrington R, Clark A. Prevention and early intervention for depression in adolescence and early adult life. European Archives of Psychiatry and Clinical Neuroscience 1998;248:32‐45. [DOI] [PubMed] [Google Scholar]
Harris 1997
- Harris EC, Barraclough BM. Suicide as an outcome for mental disorders: a meta‐analysis. British Journal of Psychiatry 1997;170:205‐23. [DOI] [PubMed] [Google Scholar]
Hayes 1999
- Hayes SC, Strosahl K, Wilson KG. Acceptance and Commitment Therapy: An Experiential Approach to Behavior Change. New York (NY): Guilford Press, 1999. [Google Scholar]
Hayes 2004
- Hayes SC, Masuda A, Bassett R, Luoma J, Guerrero LF. DBT, FAP, and ACT: how empirically oriented are the new behavior therapy technologies?. Behavior Therapy 2004;35:35‐54. [Google Scholar]
Hazell 2002
- Hazell P, O'Connell D, Heathcote D, Henry D. Tricyclic drugs for depression in children and adolescents. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD002317.pub2] [DOI] [PubMed] [Google Scholar]
Hazell 2013
- Hazell P, Mirzaie M. Tricyclic drugs for depression in children and adolescents. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD002317.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hermanns 2005
- Hermanns N, Kulzer B, Krichbaum M, Kubiak T, Haak T. Affective and anxiety disorders in a German sample of diabetic patients: prevalence, comorbidity and risk factors. Diabetic Medicine 2005;22:293‐300. [DOI] [PubMed] [Google Scholar]
Hetrick 2016
- Hetrick SE, Cox GR, Witt KN, Bir JJ, Merry SN. Cognitive behavioural therapy (CBT), third‐wave CBT and interpersonal therapy (IPT) based interventions for preventing depression in children and adolescents. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD003380.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hetrick 2018
- Hetrick SE, Robinson SE, Robinson J, Burge E, Blandon R, Mobilio B, Rice SM, Simmons MB, Alvarez‐Jimenez A, Davey CG. Youth Co‐design Of A Mobile Phone app to facilitate self‐monitoring and management of mood symptoms in young people with major depression, suicidal ideation and self‐harm. JMIR Mental Health In Press (accepted 24 Nov 2017). [DOI] [PMC free article] [PubMed]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Available from www.cochrane‐handbook.org.
Hill 2011
- Hill RM, Castellanos D, Pettit JW. Suicide‐related behaviors and anxiety in children and adolescents: a review. Clinical Psychology Review 2011;31(7):1133‐44. [DOI] [PubMed] [Google Scholar]
Hoffman 2008
- Hofmann SG, Asmundson GJG. Acceptance and mindfulness‐based therapy: New wave or old hat?. Clinical Psychology Review 2008;28(1):1‐16. [DOI] [PubMed] [Google Scholar]
Hunot 2013
- Hunot V, Moore TH, Caldwell DM, Furukawa TA, Davies P, Jones H, et al. 'Third wave' cognitive and behavioural therapies versus other psychological therapies for depression. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD008704.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobsen 1996
- Jacobson NS, Dobson KS, Truax PA, Addis ME, Koerner K, Gollan JK, et al. A component analysis of cognitive‐behavioral treatment for depression. Consulting and Clinical Psychology 1996;64(2):295‐304. [DOI] [PubMed] [Google Scholar]
James 2013
- James A, James G, Cowdrey F, Soler A, Choke A. Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD004690.pub3] [DOI] [PubMed] [Google Scholar]
Judd 2002
- Judd LL, Schettler PJ, Akiskal HS. The prevalence, clinical relevance, and public health significance of subthreshold depressions. Psychiatric Clinics of North America 2002;25(4):685‐98. [DOI] [PubMed] [Google Scholar]
Kaufman 1997
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School‐Age Children ‐ Present and Lifetime Version (KSADS‐PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36:980‐8. [DOI] [PubMed] [Google Scholar]
Kohlenberg 1991
- Kohlenberg RJ, Tsai M. Functional Analytic Psychotherapy: Creating Intense and Curative Therapeutic Relationships. London (UK): Plenum Press, 1991. [Google Scholar]
Koons 2001
- Koons CR, Robins CJ, Tweed JL, Lynch TR, Gonzalez AM, Morse JQ, et al. Efficacy of dialectical behaviour therapy in women veterans with borderline personality disorder. Behavior Therapy 2001;32:371‐90. [Google Scholar]
Kovacs 1985
- Kovacs M. The Children's Depression Inventory (CDI). Psychopharmacology Bulletin 1985;21:995‐8. [PubMed] [Google Scholar]
Kovacs 1989
- Kovacs M, Gatsonis C, Paulauskas S, Richards C. Depressive disorders in childhood. IV. A longitudinal study of comorbidity with and the risk for anxiety disorders. Archives of General Psychiatry 1989;46:776‐82. [DOI] [PubMed] [Google Scholar]
Kushner 1990
- Kushner M, Sher K, Beitman B. The relation between alcohol problems and the anxiety disorders. American Journal of Psychiatry 1990;147:685‐95. [DOI] [PubMed] [Google Scholar]
La Greca 1988
- Greca AM, Dandes SK, Wick P, Shaw K, Stone WL. Development of the Social Anxiety Scale for Children: reliability and concurrent validity. Journal of Clinical Child Psychology 1988;17:84‐91. [Google Scholar]
Lane 2013
- Lane DA, Millane TA, Lip GYH. Psychological interventions for depression in adolescent and adult congenital heart disease. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD004372.pub2] [DOI] [PubMed] [Google Scholar]
Lewinsohn 1993
- Lewinsohn PM, Hops H, Poberts RE, Seeley JR, Andrews JA. Prevalence and incidence of depression and other DSM‐IIIR disorders in high school students. Journal of Abnormal Psychology 1993;102:133‐4. [DOI] [PubMed] [Google Scholar]
Lewinsohn 1994
- Lewinsohn PM, Roberts RE, Seeley JR, Rohde P, Gotlib IH, Hops H. Adolescent psychopathology, II: Psychosocial risk factors for depression. Journal of Abnormal Psychology 1994;103(2):302‐15. [DOI] [PubMed] [Google Scholar]
Lewinsohn 1998
- Lewinsohn PM, Rohde P, Seely JR. Major depressive disorder in older adolescents: prevalence, risk factors, and clinical implications. Clinical Psychology Review 1998;18(7):765‐94. [DOI] [PubMed] [Google Scholar]
Lewis 2003
- Lewis M, Vitulano L. Biopsychosocial issues and risk factors in the family when the child has a chronic illness. Child and Adolescent Psychiatric Clinics of North America 2003;12:389‐99. [DOI] [PubMed] [Google Scholar]
Liebman 1974
- Liebman R, Minuchin S, Baker L. An integrated treatment program for anorexia nervosa. American Journal of Psychiatry 1974;131(4):432‐6. [PubMed] [Google Scholar]
Linehan 1993
- Linehan M. Cognitive‐behavioral Treatment of Borderline Personality Disorder. New York (NY): Guilford Press, 1993. [Google Scholar]
Lipsitz 2013
- Lipsitz JD, Markowitz JC. Mechanisms of change in interpersonal therapy (IPT). Clinical Psychology Review 2013;33(8):1134‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowe 2002
- Lowe B, Breining K, Wilke S, Wellmann R, Zipfel S, Eich W. Quantitative and qualitative effects of Feldenkrais, progressive muscle relaxation, and standard medical treatment in patients after acute myocardial infarction. Psychotherapy Research 2002;12(2):179‐91. [Google Scholar]
Lubin 1965
- Lubin B. Adjective Checklists for Measurement of Depression. JAMA 1965;12(1):57‐62. [DOI] [PubMed] [Google Scholar]
Lustman 2000
- Lustman P, Freedland K, Griffith L, Clouse R. Fluoxetine for depression in diabetes: a randomized double‐blind placebo‐controlled trial. Diabetes Care 2000;23:618‐23. [DOI] [PubMed] [Google Scholar]
Madanes 1981
- Madanes C. Strategic Family Therapy. San Francisco (CA): Jossey Bass, 1981. [Google Scholar]
Maier 1988
- Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. Journal of Affective Disorders 1988;14(1):61‐8. [DOI] [PubMed] [Google Scholar]
March 1997
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36:554‐65. [DOI] [PubMed] [Google Scholar]
Martell 2001
- Martell CR, Addis ME, Jacobson NS. Depression in Context: Strategies for Guided Action. New York (NY): W.W. Norton, 2001. [Google Scholar]
Maslow 1970
- Maslow AH. Motivation and Personality. New York (NY): Harper and Row, 1970. [Google Scholar]
McCauley 2001
- McCauley E, Pavlidis K, Kendall K. Developmental precursors of depression: the child and the social environment. In: Goodyer IM editor(s). The Depressed Child and Adolescent. 2nd Edition. Cambridge University Press, 2001:46‐78. [Google Scholar]
McQueen 2008
- McQueen D, Kennedy R, Sinason V, Maxted F. Psychoanalytic Psychotherapy After Child Abuse: The Treatment of Adults and Children who have Experienced Sexual Abuse, Violence and Neglect in Childhood. London (UK): Karmac, 2008. [Google Scholar]
Minuchin 1978
- Minuchin S, Rosman BL, Baker L. Psychosomatic Families: Anorexia Nervosa in Context. Oxford, UK: Harvard University Press, 1978. [Google Scholar]
Mufson 1996
- Mufson L, Moreau D, Weissman M. Focus on relationships: interpersonal psychotherapy for adolescent depression. In: Hibbs ED, Jensen PS editor(s). Psychosocial Treatments for Child and Adolescent Disorders: Empirically Based Strategies for Clinical Practice. Washington (DC): APA, 1996:137‐56. [Google Scholar]
Mufson 2004
- Mufson L, Dorta KP, Wickramaratne P, Nomura Y. A randomized effectiveness trial of interpersonal psychotherapy for depressed adolescents. Archives of General Psychiatry 2004;61(6):577‐84. [DOI] [PubMed] [Google Scholar]
Myers 2002
- Myers K, Winters NC. Ten year review of rating scales. II: Scales for internalizing disorders. Journal of the American Academy of Child and Adolescent Psychiatry 2002;41(6):634‐59. [DOI] [PubMed] [Google Scholar]
Newacheck 1991
- Newacheck PW, McManus MA, Fox HB. Prevalence and impact of chronic Illness among adolescents. American Journal of Diseases of Childhood 1991;145:1367‐73. [DOI] [PubMed] [Google Scholar]
Olendick 1983
- Olendick TH. Reliability and validity of the Revised Fear Survey Schedule for Children (FSSC‐R). Behaviour Research and Therapy 1983;21(6):652‐92. [DOI] [PubMed] [Google Scholar]
Opolski 2005
- Opolski M, Wilson I. Asthma and depression: a pragmatic review of the literature and recommendations for research. Clinical Practice and Epidemiology in Mental Health 2005;1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osman 2001
- Osman A, Bagge CL, Guitierrez PM, Konick LC, Cooper BA, Barrios FX. The Suicide Behaviour Questionnaire ‐ Revised (SBQ‐R). Validation with clinical and non‐clinical samples. Assessment 2001;5:443‐54. [DOI] [PubMed] [Google Scholar]
Pace 2011
- Pace NL. Research methods for meta‐analyses. Best Practice & Research. Clinical Anaesthesiology 2011;25(4):523‐33. [DOI] [PubMed] [Google Scholar]
Pao 2011
- Pao M, Bosk A. Anxiety in medically ill children and adolescents. Depression Anxiety 2011;28:29‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
PARS 2002
- The Research Units on Pediatric Psychopharmacology Anxiety Study Group. The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. Journal of the American Academy of Child and Adolescent Psychiatry 2002;41(9):1061‐9. [DOI] [PubMed] [Google Scholar]
Paykel 1999
- Paykel ES, Scott J, Teasdale JD, Johnson AL, Garland A, Moore R, et al. Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Archives of General Psychiatry 1999;56(9):829‐35. [DOI] [PubMed] [Google Scholar]
Petersen 1993
- Petersen AC, Compas BE, Brooks‐Gunn J, Stemmler M, Ey S, Grant KE. Depression in adolescence. American Psychologist 1993;48(2):155‐68. [DOI] [PubMed] [Google Scholar]
Petti 1978
- Petti TA. Depression in hospitalized child psychiatry patients: approaches to measuring depression. Journal of the American Academy of Child Psychiatry 1978;17:49‐59. [DOI] [PubMed] [Google Scholar]
Pine 2009
- Pine DS, Helfinstein SM, Bar‐Haim Y, Nelson E, Fox NA. Challenges in developing novel treatments for childhood disorders: lessons from research on anxiety. Neuropsychopharmacology 2009;34(1):213‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinquart 2011a
- Pinquart M, Shen Y. Anxiety in children andadolescents with chronic physical illnesses: A meta‐analysis. Acta Paediatrica 2011;100:1069‐76. [DOI] [PubMed] [Google Scholar]
Pinquart 2011b
- Pinquart M, Shen Y. Depressive symptoms in children and adolescents with chronic physical illness: an updated meta‐analysis. Journal of Pediatric Psychology 2011;36(4):375‐84. [DOI] [PubMed] [Google Scholar]
Pitceathly 2009
- Pitceathly C, Maguire P, Fletcher I, Parle M, Tomenson B, Creed F. Can a brief psychological intervention prevent anxiety or depressive disorders in cancer patients? A randomised controlled trial. Annals of Oncology 2009;20(5):928‐34. [DOI: ] [DOI] [PubMed] [Google Scholar]
Pless 1971
- Pless IB, Roghmann KJ. Chronic illness and its consequences: observations based on three epidemiologic surveys. Journal of Pediatrics 1971;79:351‐59. [DOI] [PubMed] [Google Scholar]
Pollock 2000
- Pollock B, Laghrissi‐Thode F, Wagner W. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. Journal of Clinical Psychopharmacology 2000;20:137‐40. [DOI] [PubMed] [Google Scholar]
Poznanski 1985
- Poznanski EO, Freeman LN, Mokros HB. Children's Depression Rating Scale ‐ Revised. Psychopharmacology Bulletin 1985;21(4):979‐89. [Google Scholar]
Rao 1995
- Rao U, Ryan ND, Birmaher B, Dahl RE, Williamson DE, Kaufman J, et al. Unipolar depression in adolescence: clinical outcome in adulthood. Journal of the American Academy of Child and Adolescent Psychiatry 1995;43(5):566‐78. [DOI] [PubMed] [Google Scholar]
Regnault 2011
- Regnault A, Balp MM, Kulich K, Estève L, Viala‐Danten M. Validation of the treatment satisfaction questionnaire for medication (TSQM) in cystic fibrosis (CF). Cystic Fibrosis 2011;10:S85. [DOI] [PubMed] [Google Scholar]
Reinecke 1998
- Reinecke MA, Ryan NE, DuBios DL. Cognitive‐behavioral therapy of depression and depressive symptoms during adolescence: a review and meta‐analysis. Journal of the American Academy of Child and Adolescent Psychiatry 1998;37(1):26‐34. [DOI] [PubMed] [Google Scholar]
Reynolds 1978
- Reynolds CR, Richmond BO. What I Think and Feel: a revised measure of children's manifest anxiety. Abnormal Child Psychology 1978;6:271‐80. [DOI] [PubMed] [Google Scholar]
Rhode 1994
- Rhode P, Lewinsohn PM, Seeley JR. Are adolescents changed by an episode of major depression?. Journal of the American Academy of Child and Adolescent Psychiatry 1994;33(9):1289‐98. [DOI] [PubMed] [Google Scholar]
Rizzo 2011
- Rizzo M, Creed F, Goldberg D, Meader N, Pilling S. A systematic review of non‐pharmacological treatments for depression in people with chronic physical health problems. Journal of Psychosomatic Research 2011;71(1):18‐27. [DOI] [PubMed] [Google Scholar]
Robb 2014
- Robb SL, Hanson‐Abromeit D. A review of supportive care interventions to manage distress in young children with cancer and parents. Cancer nursing 2014;1(37(4)):E1‐26. [DOI] [PubMed] [Google Scholar]
Rogers 1951
- Rogers C. Client‐centered Therapy: Its Current Practice, Implications and Theory. London (UK): Constable, 1951. [Google Scholar]
Russell 1958
- Russell D. Some research on the impact of reading. English Journal 1958;47(2):398‐411. [Google Scholar]
Sadock 2005
- Sadock BJ, Sadock VA. Kaplan & Sadock's Comprehensive Textbook of Psychiatry. Philadelphia: Lippincott, Williams and Wilkins, 2005. [Google Scholar]
Sansom‐Daly 2014
- Sansom‐Daly U, Wakefield C, Bryant R, Ellis S, Doolan E, Cohn RJ. Adapting evidence‐based psychological therapy to the computer screen for adolescent and young adult cancer survivors: Preliminary results from the ’recapture life’ randomised controlled trial. Asia‐Pacific journal of Clinical Oncology 2014;10:36. [Google Scholar]
Saravay 1996
- Saravay SM. Psychiatric interventions in the medically ill: outcomes and effectiveness research. Psychiatric Clinics of North America 1996;19:467‐80. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine 2010;8(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwartz 2003
- Schwartz M, Andrasik F. Biofeedback: A Practitioner's Guide. 3rd Edition. New York (NY): Guilford Press, 2003. [Google Scholar]
Secenti 2017
- Secinti E, Thompson EJ, Richards M, Gaysina D. Research Review: Childhood chronic physical illness and adult emotional health ‐ a systematic review and meta‐analysis. Journal of Child Psychology and Psychiatry 2017;58(7):753‐69. [DOI: 10.1111/jcpp.12727] [DOI] [PubMed] [Google Scholar]
Seligman 1979
- Seligman MEP, Abramson LY, Semmel A, Baeyer C. Depressive attributional style. Journal of Abnormal Psychology 1979;88:242‐7. [DOI] [PubMed] [Google Scholar]
Selvini 1978
- Selvini Palazzoli M, Boscolo L, Cecchin G, Prata G. Paradox and Counterparadox. New York (NY): Aronson, 1978. [Google Scholar]
Shaffer 1983
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, et al. A Children's Global Assessment Scale (CGAS). Archives of General Psychiatry 1983;40(11):1228‐31. [DOI] [PubMed] [Google Scholar]
Sharpe 2001
- Sharpe L, Sensky T, Timberlake N, Ryan B, Brewin C, Allard S. A blind, randomized, controlled trial of cognitive‐behavioural intervention for patients with recent onset rheumatoid arthritis: preventing psychological and physical morbidity. Pain 2001;89:275‐83. [DOI] [PubMed] [Google Scholar]
Shinohara 2013
- Shinohara K, Honyashiki M, Imai H, Hunot V, Caldwell DM, Davies P, et al. Behavioural therapies versus other psychological therapies for depression. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD008696.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silverman 1988
- Silverman WK, Nelles WB. The Anxiety Disorders Interview Schedule for Children. Journal of the American Academy of Child and Adolescent Psychiatry 1988;27(6):772‐8. [DOI] [PubMed] [Google Scholar]
Silverman 1996
- Silverman WK, Kurtines WM. Anxiety and Phobic Disorders: A Pragmatic Approach. New York (NY): Plenum Press, 1996. [Google Scholar]
Simon 2005
- Simon GE, Katon WJ, Lin EH, Ludman E, VonKorff M, Ciechanowski P, et al. Diabetes complications and depression as predictors of health care costs. General Hospital Psychiatry 2005;27(5):344‐51. [DOI] [PubMed] [Google Scholar]
Siqueland 2005
- Siqueland L, Rynn M, Diamond GS. Cognitive behavioral and attachment based family therapy for anxious adolescents: phase I and II studies. Journal of Anxiety Disorders 2005;19(4):361‐81. [DOI] [PubMed] [Google Scholar]
Spielberger 1983
- Spielberger CD, Gorssuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State‐Trait Anxiety Inventory. Florida: Consulting Psychologists Press, Inc, 1983. [Google Scholar]
Thabrew 2017
- Thabrew H, Stasiak K, Hetrick SE, Wong S, Merry SN. eHealth interventions for anxiety and depression in children and adolescents with long‐term physical conditions. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD012489] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thabrew 2018
- Thabrew H, Stasiak K, Hetrick SE, Wong S, Huss JH, Merry SN. E‐Health interventions for anxiety and depression in children and adolescents with long‐term physical conditions. Cochrane Database of Systematic Reviews 2018, Issue 8. [DOI: 10.1002/14651858.CD012489] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2000
- Turner J, Brian K. Emotional dimensions of chronic disease. Western Journal of Medicine 2000;172(2):124‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uher 2012
- Uher R, Perlis RH, Placentino A, Dernovšek MZ, Henigsberg N, Mors O, et al. Self‐report and clinician‐rated measures of depression severity: can one replace the other?. Depression and Anxiety 2012;29:1043‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uttley 2015
- Uttley L, Scope A, Stevenson M, Rawdin A, Taylor Buck E, Sutton A, et al. Systematic review and economic modelling of the clinical effectiveness and cost‐effectiveness of art therapy among people with non‐psychotic mental health disorders. Health Technology Assessment 2015; Vol. 18:1‐120. [DOI] [PMC free article] [PubMed]
Van Cleave 2010
- Cleave J, Gortmaker SL, Perrin JM. Dynamics of Obesity and Chronic Health Conditions Among Children and Youth. JAMA 2010;303(7):623‐30. [DOI] [PubMed] [Google Scholar]
Van der Lee 2007
- Lee JH, Mokkink LB, Grootenhuis MA, Heymans HS, Offringa M. Definitions and measurement of chronic health conditions in childhood: a systematic review. JAMA 2007;297(24):2741‐51. [DOI] [PubMed] [Google Scholar]
Varni 2004
- Varni JW, Burwinkle TM, Katz ER. The PedsQL in pediatric cancer pain. A prospective longitudinal analysis of pain and emotional distress. Journal of Developmental and Behavioral Pediatrics 2004;25:239‐46. [DOI] [PubMed] [Google Scholar]
Walker 1997
- Walker LS, Smith CA, Garber J, Slyke DA. Development and validation of the pain response inventory for children. Psychological Assessment 1997;9:392‐405. [Google Scholar]
Wallander 2003
- Wallander JL, Thompson RJ, Alriksson‐Schmidt AR. Psychosocial adjustment of children with chronic physical conditions. In: Roberts MC editor(s). Handbook of Pediatric Psychology. 3rd Edition. New York (NY): Guilford Press, 2003:141‐58. [Google Scholar]
Watanabe 2007
- Watanabe N, Hunot V, Omori IM, Churchill R, Furukawa TA. Psychotherapy for depression among children and adolescents: a systematic review. Acta Psychiatrica Scandinavica 2007;116(2):84‐95. [DOI] [PubMed] [Google Scholar]
Weiland 1992
- Weiland SK, Pless IB, Roghmann KJ. Chronic illness and mental health problems in pediatric practice: results from a survey of primary care providers. Pediatrics 1992;89:445‐9. [PubMed] [Google Scholar]
Weisz 2006
- Weisz JR, McCarty CA. Effects of Psychotherapy for depression in children and adolescents: what we can (and can't) learn from meta‐analysis and component profiling. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(7):879‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weisz 2017
- Weisz JR, Kuppens SN, Sofie N, Mei Y, Eckshtain D, Ugueto A, et al. What five decades of research tells us about the effects of youth psychological therapy: A multilevel meta‐analysis and implications for science and practice. American Psychologist 2017;72(2):79‐117. [DOI] [PubMed] [Google Scholar]
Wells 2008
- Wells A, Fisher PL. Metacognitive therapy in recurrent and persistent depression: a multiple‐baseline study of a new treatment. Cognitive Therapy and Research 2008;33(3):291. [Google Scholar]
Wells 2009
- Wells A. Metacognitive Therapy for Anxiety and Depression. New York (NY): Guildford, 2009. [Google Scholar]
Wierzbicki 1987
- Wierzbicki M, Bartlett T. The efficacy of group and individual cognitive therapy for mild depression. Cognitive Therapy and Research 1987;11(3):337‐42. [Google Scholar]
World Health Organization 1992
- World Health Organization. The ICD‐10 Classification of Mental and Behavioral Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization, 1992. [Google Scholar]


