Significance
The pneumococcus, a bacterium frequently carried in the nasopharynx, is responsible for a wide spectrum of infections in humans, including severe invasive pneumococcal diseases (IPDs). In nontropical climates, IPDs typically display a marked winter seasonality, a striking but still enigmatic aspect of pneumococcal epidemiology. Here we used dynamic models of carriage transmission and disease, confronted with detailed IPD incidence data, to elucidate the components and the mechanisms underlying the seasonality of IPDs. We find that temporal variations in climate, influenza-like illnesses, and interindividual contacts explain most of the seasonal variability in IPDs. We quantify the potential impact of seasonally timed interventions, a type of control measures that exploit pneumococcal seasonality to help reduce IPDs.
Keywords: pneumococcus, influenza, climate, infectious disease seasonality, epidemiology
Abstract
Infections caused by Streptococcus pneumoniae—including invasive pneumococcal diseases (IPDs)—remain a significant public health concern worldwide. The marked winter seasonality of IPDs is a striking, but still enigmatic aspect of pneumococcal epidemiology in nontropical climates. Here we confronted age-structured dynamic models of carriage transmission and disease with detailed IPD incidence data to test a range of hypotheses about the components and the mechanisms of pneumococcal seasonality. We find that seasonal variations in climate, influenza-like illnesses, and interindividual contacts jointly explain IPD seasonality. We show that both the carriage acquisition rate and the invasion rate vary seasonally, acting in concert to generate the marked seasonality typical of IPDs. We also find evidence that influenza-like illnesses increase the invasion rate in an age-specific manner, with a more pronounced effect in the elderly than in other demographics. Finally, we quantify the potential impact of seasonally timed interventions, a type of control measures that exploit pneumococcal seasonality to help reduce IPDs. Our findings shed light on the epidemiology of pneumococcus and may have notable implications for the control of pneumococcal infections.
The pneumococcus (Streptococcus pneumoniae) is a bacterium that frequently colonizes the human nasopharynx, particularly that of young children (1). Upon reaching other body sites, the pneumococcus can cause a variety of conditions, ranging from mild infections of the upper respiratory tract to severe invasive pneumococcal diseases (IPDs) (1). Although widespread immunization with conjugate vaccines has had a marked impact in many populations (2), IPDs remain a substantial cause of morbidity and mortality, especially in low-income countries (3). Seasonality is a striking, but still enigmatic aspect of IPD epidemiology (4). In nontropical climates, IPDs typically display regular, marked seasonal variations, with a zenith of cases during winter and a nadir during summer (5–9). Previous work has examined candidate seasonal factors that could contribute to this pattern, such as climate (6, 7, 10), cocirculating pathogens [e.g., influenza viruses (11, 12)], or variations of interindividual contact rates (13). Nevertheless, our understanding of pneumococcal seasonality remains fragmentary (4, 14). Elucidating the drivers of pneumococcal seasonality could provide critical insight into the mechanisms of transmission and carriage, which subsequently lead to disease.
To bridge this gap, here, we analyzed detailed IPD surveillance data in France, collected between 2000 and 2010, alongside parallel data on climate, cocirculating influenza-like illnesses (ILIs), and timing of school holidays. Using likelihood-based inference and semimechanistic models of transmission, carriage, and disease, we systematically explored a range of hypotheses about the mechanisms and the components of pneumococcal seasonality. We found that IPD seasonality was best explained by an amalgam of all of the seasonal factors considered. Furthermore, we showed that both the invasion rate and the carriage acquisition rate varied seasonally and acted in concert to produce the strong seasonality typical of IPDs. Finally, we assessed the potential impact of seasonally timed interventions, which exploit pneumococcal seasonality to help control IPDs.
Methods
Choice of Spatial Scale.
Mainland France covers an area of about 550,000 km2, with a latitude span of ∼9° and a longitude span of ∼13°. An oceanic climate (Köppen–Geiger group Cfb) predominates, although Mediterranean climates (Köppen–Geiger groups Csa and Csb) are present in the southeast regions. In a previous study based on IPD data in five regions spanning mainland France (5), we did not find evidence of marked geographical differences of IPD seasonality. Here, we therefore chose to work with country-level data. In a sensitivity analysis, we nevertheless verified the robustness of our results by carrying out model simulations at higher spatial resolution in France (SI Appendix, Table S4) and in tropical climates (SI Appendix, Fig. S6).
IPD Data.
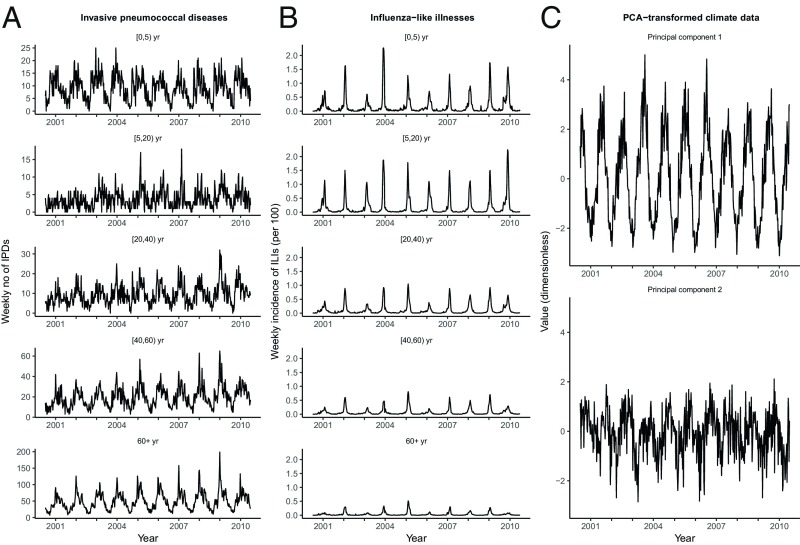
The IPD incidence data were available from the Epibac network, a nationwide, hospital voluntary-based sentinel surveillance system described previously (5, 15, 16). An IPD case was defined by the isolation of S. pneumoniae or the detection of pneumococcal DNA by PCR in cerebrospinal fluid (meningitis) or only in blood (nonmeningitis bacteremia). As shown in Fig. 1A, the data consisted of country-level, weekly time series of IPD cases from wk 27/2000 to wk 26/2010 (522 wk overall), stratified into five epidemiologically relevant age groups: [0, 5), [5, 20), [20, 40), [40, 60), and 60+ y. We corrected for underreporting by applying an observation model to model outputs (SI Appendix, Supplementary Methods).
Fig. 1.
Time series of IPDs, ILIs, and climate in mainland France, 2000/wk 27 to 2010/wk 26. (A) Weekly number of IPDs in different age groups. For visual clarity, the y axis values differ between panels. (B) Weekly incidence (per 100 population) of ILIs in different age groups. (C) Weekly values of climate. The meteorological variables considered were the average temperature, vapor pressure (a measure of absolute humidity), relative humidity, and duration of insolation. We here represent the values of the first two components derived from a principal component analysis (PCA); these two components captured about 97% of the variability and were used instead of the individual variables in all of the analyses (see SI Appendix, Supplementary Methods for further details on the PCA).
ILI Data.
ILI incidence data were available from the French Sentinelles network, a nationwide surveillance system based on a sample of general practitioners across France (17). An ILI case was defined clinically as sudden onset of fever (≥39 °C), associated with myalgia and respiratory symptoms (e.g., cough and sore throat). According to previous evidence, this specific case definition makes ILI a good proxy for influenza infection (5). As for the IPD data, the ILI data consisted of weekly, age-stratified time series of cases during wk 27/2000 to wk 26/2010 (Fig. 1B). Correction factors were applied to account for the age-specific pattern of health-seeking behavior observed in France, in particular the high probability of consulting a physician for an ILI infection in young children (SI Appendix, Table S1).
Meteorological Data.
Daily meteorological records from nine weather stations located near the most populated cities across France were provided by Météo-France, the French national meteorological service. The following variables were considered, based on previous associational evidence (6, 7, 10): daily average temperature (measured in degrees Celsius), hours of sunshine, average relative humidity (in %), and average vapor pressure (a measure of absolute humidity, measured in hPa). Before analysis, the data were preprocessed in two steps. First, the data were averaged temporally and spatially to create weekly time series representing the average climatic conditions in mainland France. Second, because these variables were markedly correlated, we conducted a principal component analysis to summarize them (SI Appendix, Figs. S2 and S3). The first two components, displayed in Fig. 1C, captured about 97% of the variability and were used instead of the individual meteorological variables in all of the analyses.
Interindividual Contact Data.
Data on age-specific contact rates were available from an empirical study of self-reported contacts in France (ref. 18 and SI Appendix, Fig. S1), comparable to the POLYMOD study in other European countries (19). The POLYMOD contact matrix from Great Britain was also tested in a sensitivity analysis (SI Appendix, Fig. S7 and Table S5).
Demographic Data.
Annual birth rates and age-specific annual population estimates in France were available from the French National Institute of Statistics and Economic Studies. The smoothed estimates were used to calculate age-specific migration rates, so that the simulated population sizes approximately equaled the observed population sizes (SI Appendix, Supplementary Data).
Model and Hypotheses Formulation.
To identify the mechanisms underlying the seasonal variations of IPDs, we formulated an age-structured, semimechanistic model of pneumococcal transmission, carriage, and subsequent invasive disease (20, 21). A feature of this model was the inclusion of two stages of carriage (early and late) to test the hypothesis that disease risk was not uniform over the duration of carriage (22). To analyze pneumococcal seasonality, the model further incorporated seasonal variations of ILIs (Fig. 1B), climate (Fig. 1C), and interindividual contact rates. The impact of ILIs was modeled mechanistically, by means of a pneumococcus–ILI coinfection model that integrated different mechanisms of interaction (21, 23). Specifically, we examined three different hypotheses of interaction: an individual carrying pneumococcus and infected with ILI was assumed (i) to contribute more to pneumococcal transmission or (ii) to be at higher risk of contracting an IPD; an individual not carrying pneumococcus but infected with ILI was assumed (iii) to have a higher risk of acquiring pneumococcal carriage. In contrast to ILIs, and in the absence of information to inform a fully mechanistic model, the impact of climate was modeled semimechanistically. Specifically, we considered four meteorological variables identified in previous ecological studies (6, 7, 10), summarized—via a principal component analysis (PCA)—by two principal components to bypass collinearity issues (Fig. 1C). We then constructed a background seasonal function that incorporated these two components, in addition to annual and semiannual harmonic terms representing a potentially unexplained seasonality. The model also incorporated seasonal variations of contacts between schoolchildren, timed according to the calendar of summer and Christmas holidays in France. Finally, we also considered a potential increase of contacts between children and the elderly during Christmas school holidays, a hypothesis previously put forth to explain the early-winter peaks of IPDs (24). In sum, our model incorporated most of the seasonal factors thought to contribute to pneumococcal epidemiology.
Because pneumococcal carriage precedes disease, the seasonal variations of IPDs can be ascribed to seasonality in the carriage acquisition rate or in the rate of progression from carriage to disease, i.e., the invasion rate. To identify the source of IPD seasonality, we formulated five hypotheses about the seasonalities of the acquisition and the invasion rates. The first and second hypotheses proposed that either the acquisition rate or the invasion rate varied seasonally. The third hypothesis proposed that both rates varied seasonally with a similar timing, but a possibly lower amplitude [e.g., as a result of bacterial population bottlenecks during transmission (25)] for the acquisition rate. The fourth hypothesis proposed that both the invasion and the acquisition rates varied seasonally, but the latter rate as a result of differences of contacts between school terms and school holidays in children. Finally, the fifth hypothesis combined the third and the fourth hypotheses.
Model Implementation and Estimation.
The model was represented as a set of deterministic differential equations, completed by a negative-binomial stochastic observation model to correct for underreporting of IPD cases. To test our different hypotheses about pneumococcal seasonality, we conducted maximum likelihood estimation via trajectory matching using the R pomp package (26). For every hypothesis, the estimation was completed in several steps, starting from a broad search of parameter space, followed by a refined search to pinpoint the maximum likelihood estimate. The convergence was checked by inspecting the sliced log-likelihood around every estimated parameter. Finally, a parametric bootstrap was used to calculate approximate confidence intervals at the 95% level. The parsimony of competing hypotheses was quantified using the Akaike Information Criterion (AIC). Complete details are provided in SI Appendix, Supplementary Methods.
Results
Components of Pneumococcal Seasonality.
The results regarding the components of pneumococcal seasonality were unambiguous in our study (SI Appendix, Table S2). Irrespective of the assumptions about the seasonalities of the acquisition and the invasion rate, models that incorporated climate at lag 1 wk and ILIs at lag 0 wk received more support from the data (as judged by the AIC). Irrespective of the assumptions about climate and ILIs, we also found evidence that both the invasion rate and the acquisition rate varied seasonally. Hence, the seasonality of IPDs was best explained by a model that incorporated all of the seasonal drivers and mechanisms considered. The nature of the interaction with ILIs was also unequivocal in this model (SI Appendix, Table S3), with strong evidence that ILIs increased the invasion risk, but not the transmission risk (relative risk: 1.0 [1.0, 4.3]) nor the acquisition risk (relative risk: 1.0 [1.0, 2.1]). Of note, the estimated relative risk of invasion was higher in the elderly (relative risk: 146 [89, 188]) than in individuals aged <5 y (relative risk: 49 [30, 68]) or [5, 60) y (relative risk: 59 [40, 72]). Finally, this model also estimated a large (7 [6–9]-fold) increase of contacts between children and the elderly during Christmas and a disease risk concentrated during early carriage (100 [73–100]% of disease occurring during the first third of carriage duration).
To assess the ability of our best (i.e., with lowest AIC) model to explain pneumococcal seasonality, we compared the data to model simulations (SI Appendix, Fig. S5). This revealed an excellent model–data agreement (generalized R2 of 0.79), although the model did not capture well IPD peaks around October in children <5 y. To further assess the robustness of our results and the predictive power of our models, we conducted three additional analyses. First, we simulated all of the models to compare their predictions in five geographical regions spanning continental France (5). The results confirmed our main conclusions: the models incorporating climate and ILIs, with seasonality in both the invasion and the acquisition rates, provided a better fit while retaining correct predictive power in every region (R2 ranging from 0.39 to 0.56, SI Appendix, Table S4). Second, we refitted all of the models using a contact matrix derived from the POLYMOD study in Great Britain (SI Appendix, Fig. S7). We found our estimates to be almost unchanged in this case (SI Appendix, Table S5). Third, we ran out-of-fit model predictions using climatic data in Mae Sot, Thailand and in Sibanor, The Gambia, both tropical locations (Köppen–Geiger group Aw) with two marked—dry and rainy—seasons. We found our model predictions to be qualitatively consistent with longitudinal carriage data in both locations (refs. 27 and 28 and SI Appendix, Fig. S6). The robustness of our results to variations in fixed model parameters, data resolution, and climatic conditions strengthens the evidence for our conclusions regarding the components and the mechanisms of pneumococcal seasonality.
Dissecting Pneumococcal Seasonality.
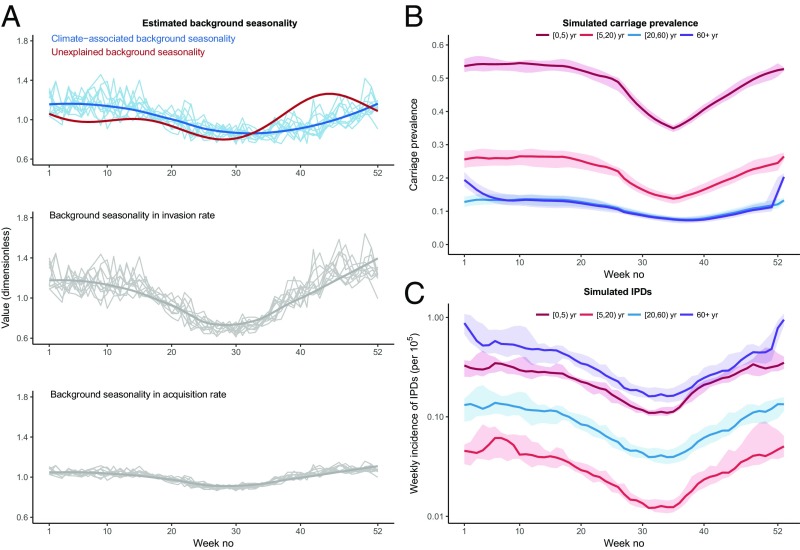
According to the best model’s estimates, the background seasonal function included a climate-associated component that gradually increased from summer to winter and an unexplained component that displayed a shallow trough during early summer and a peak in early November (Fig. 2A, Top). As a result, the estimated invasion rate displayed large-amplitude oscillations over the year (approximately ±40% around the seasonal mean, Fig. 2A, Middle). Despite a similar seasonal shape, the background seasonality in the acquisition rate displayed smaller-amplitude variations over the year (approximately ±10% around the seasonal mean, Fig. 2A, Bottom). In addition to this background seasonality, the acquisition rate incorporated seasonal variations of contact rates, resulting from reduced contacts between schoolchildren during school holidays and increased contacts between children and the elderly during Christmas holidays (SI Appendix, Table S3). The overall effect of the seasonal ingredients composing the acquisition rate is apparent in model simulations of age-specific carriage prevalence (Fig. 2B). In schoolchildren, the predicted carriage prevalence was stable during winter and spring; it gradually decreased during summer holidays and reincreased from school resumption until the end of the year. In contrast, carriage prevalence was more uniform in adults, except for a marked, but transient increase in the elderly during Christmas and the start of the new year. This pattern of seasonal carriage prevalence, combined with the seasonally varying invasion rate, resulted in large-amplitude variations of IPDs (Fig. 2C). Hence, our results demonstrated that both the acquisition rate and the invasion rate varied seasonally and acted in concert to produce the pronounced seasonality typical of IPDs.
Fig. 2.
Dissecting pneumococcal seasonality. (A) (Top) The shape of the estimated background seasonality, which comprised an unexplained seasonality (modeled using a Fourier series with two harmonics, red line) and a climate-associated seasonality (blue lines). For the climate-related seasonality, each light blue line represents a distinct epidemiological year; the dark blue line is the average seasonal shape. (Middle) The estimated seasonality in the invasion rate (that is, the rate at which pneumococcal carriers contract an IPD), which was the product of the unexplained seasonality and the climate-associated seasonality. (Bottom) The estimated seasonality in the carriage acquisition rate, which had a similar seasonal shape but damped oscillations. In the Middle and Bottom, each light gray line represents a distinct epidemiological year; the dark gray line is the average seasonal shape. (B) Median (range) simulated carriage prevalence in different age groups. (C) Median (range) simulated IPD incidence (per 100,000 population) in different age groups. All of the estimates and simulations presented in this figure are from the best model.
Estimated Impact of ILIs and Climate.
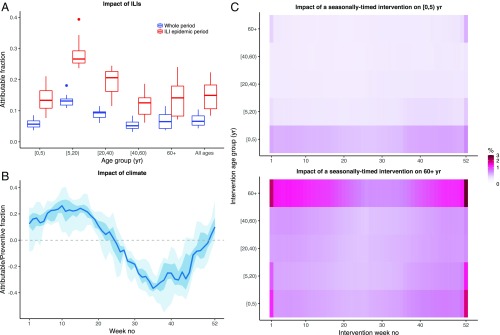
To further interpret our estimates, we computed the attributable fraction, a population-level measure of impact (Fig. 3A). The large individual-level effect of ILIs on the invasion risk translated into a pronounced population-level effect during periods of peak ILI activity (median [IQR] attributable fraction: 0.15 [0.11, 0.18]), but a more modest effect overall (median [IQR]: 0.07 [0.05, 0.08]). Notably, the impact of ILIs on IPDs was estimated to vary with age, with a peak in children aged [5, 20) y (median [IQR]: 0.13 [0.12, 0.14]) and a smaller effect in young children (median [IQR]: 0.06 [0.05, 0.07]) and the elderly (median [IQR]: 0.06 [0.04, 0.09]). In the absence of evidence that ILIs affected either pneumococcal acquisition or transmission, this age-specific effect was explained by the changes of ILIs over age (Fig. 1B) and their age-specific individual effect on invasion risk. We note, however, that an intervention targeting ILIs in a given age group can reduce ILI transmission in the population at large and may, therefore, still induce indirect effects on IPDs in other age groups. Compared with ILIs, climate had a more pronounced, but also more variable impact (Fig. 3B). This impact changed gradually over the season, resulting in more cases of IPDs during winter (median [IQR] attributable fraction: 0.18 [0.11, 0.23]) and spring (attributable fraction 0.16 [0.08, 0.23]), but fewer cases during summer (preventive fraction −0.23 [−0.32, −0.14]) and fall (preventive fraction −0.17 [−0.29, −0.08]).
Fig. 3.
Predicted impact of different interventions. (A) Fraction of IPDs attributable to ILIs according to age (x axis), calculated during the whole study period (blue boxplots) or the ILI epidemic periods (defined as ±6 wk around the ILI peak week every year, red boxplots). For every epidemiological year, ILIs were set to 0 in a given age group; the resulting number of IPDs in that age group during that year was calculated and compared with that of the base model (with ILIs). The attributable fraction represents the relative decrease (compared with the base model) in the number of IPDs. Each boxplot shows the year-to-year variation in the attributable fraction. (B) Relative excess of IPDs due to climate, according to week number (x axis). For every epidemiological year, the climate covariates of the best model were set to 0; the resulting overall number of IPDs during that year was calculated and compared with that of the base model. The fraction represents the relative decrease (if positive) or increase (if negative) in the number of IPDs, compared with the base model. For every week number, year-to-year variability is summarized by the median (blue line), the interquartile range (dark blue ribbon), and the range (blue ribbon). (C) Predicted impact of a seasonally timed intervention. We simulated the impact of reducing the contacts of a target age group (y axis) during a target week number (x axis) throughout the study period. The heatmap shows the predicted relative decrease (in %) of IPDs in [0, 5) y (Top) and 60+ y (Bottom), the two age groups most at risk for IPDs. For visual clarity, the color scale is square-root transformed. See SI Appendix, Supplementary Results for complete details of the simulation protocol and SI Appendix, Fig. S9, showing the predicted impact in all of the age groups.
Predicted Impact of a Seasonally Timed Intervention.
In addition to the hypothetical interventions targeted at ILIs or climate described above, the seasonality in the carriage acquisition rate suggests that reducing interindividual contacts at specific times of the year might be an effective way to control IPDs. The success of such an intervention, however, will hinge on the pattern of age-specific contacts and the identification of core transmitter age groups. To examine this, we performed numerical experiments in which contacts from a target age group were reduced (e.g., by enhancing barrier precautions, such as improving hand hygiene or using protective masks) during a specific week of the year, throughout the study period. The predicted effect of such a seasonally timed intervention on young children and the elderly—the two demographics most at risk for IPDs—is shown in Fig. 3C. To control IPDs in young children (Fig. 3C, Top), the predicted best strategy was to reduce the frequency of same-age contacts during fall and winter. By contrast, targeting other age groups was predicted to have much lower impact, except for a transient effect of reducing contacts from the elderly during Christmas holidays. A different picture emerged regarding the control of IPDs in the elderly (Fig. 3C, Bottom). Although the best strategy remained to reduce same-age contacts during winter and fall, the predicted impact of other age groups was more pronounced. In particular, young children were the second most impactful age group, with a seasonally varying effect of reducing their contacts highest during fall and winter, particularly during Christmas holidays. Similar results were obtained in other age groups (SI Appendix, Fig. S9), with indication of a seasonal impact of the timed intervention, highest when targeted, first, at same-age contacts and, second, at contacts involving young children. We found these results to be robust with an alternative contact matrix, derived from the POLYMOD study in Great Britain (SI Appendix, Fig. S10). These results emphasize the assortative nature of pneumococcal transmission, in addition to the key role of young children as core transmitters to other age groups. Furthermore, they provide a proof of concept of the potential usefulness of interventions that exploit seasonality to control IPDs.
Discussion
The main goal of this study was to elucidate the mechanisms of pneumococcal seasonality, by leveraging detailed IPD incidence data in France. To do so, we developed semimechanistic models of pneumococcal transmission, carriage, and disease that incorporated seasonal variations of ILIs, climate, and interindividual contact rates—all seasonal factors previously proposed to contribute to pneumococcal epidemiology. Using likelihood-based statistical inference methods, we systematically evaluated the support of multiple hypotheses about the components and the mechanisms of pneumococcal seasonality. We found that pneumococcal seasonality was best explained by a conjunction of all of the seasonal factors considered. In addition, we found evidence that both the invasion rate and the carriage acquisition rate varied seasonally and acted in concert to generate the marked seasonality typical of IPDs in France. Finally, we explored the impact of seasonally timed interventions, which aim at exploiting pneumococcal seasonality to control IPDs.
Our results indicated that a substantial part of IPD variability was explained by seasonal variations of climate. These results are broadly consistent with previous ecological studies that estimated an association between IPDs and climatic drivers, such as temperature (7, 10), humidity (6), UV radiation (6, 10), or sunshine duration (7). Also in keeping with our findings in France, a recent modeling study found evidence of higher pneumococcal transmission during dry and cool seasons (28). Although the underlying biological mechanisms are unknown, experimental evidence from other respiratory pathogens—such as influenza (29)—suggests that climatic conditions could also affect the survival or the transmission of pneumococcus. Alternatively, it has been proposed that climate modulates the host susceptibility to pneumococcal diseases (14). In support of this hypothesis, a recent study demonstrated marked seasonal variations in many markers of human immunity, in particular a more proinflammatory response—known to facilitate the presence of pneumococcus in the nasopharynx (30)—during European winter (31). Irrespective of the mechanisms involved, a simple and testable prediction of our model is that pneumococcal seasonality varies with climate and, therefore, with latitude. The observation of a latitudinal gradient in the timing of bacterial meningitis (32) and the reverse seasonality of IPDs in the Southern hemisphere (9) provide preliminary evidence that supports this prediction. Our preliminary analyses in two tropical climates are also consistent with this prediction, but applying our models to analyze data from other climates would be useful to further elucidate pneumococcal seasonality.
Regarding influenza viruses, a number of experimental studies have examined their interaction with pneumococcus (12). The evidence garnered from these studies consistently demonstrated that influenza viruses have a facilitatory effect on pneumococcus, by increasing acquisition, bacterial load, transmission, or disease severity. Understanding how these individual-level mechanisms in animal models translate into population-level patterns in humans, however, is not straightforward. Indeed, population-based studies have estimated an at most modest contribution of influenza to IPDs (Refs. 5, 7, 8, and 33, reviewed in ref. 11.). As proposed by Shrestha et al. (23), this discrepancy may be explained by the fact that a large individual-level interaction results in a much lower population-level effect, whose identification depends on the interannual variability of influenza peaks. Our results, which also point to a disconnect between the individual- and population-level scales, entirely support this view. Unlike Shrestha et al., however, we found little evidence that ILIs affected pneumococcal acquisition, but two differences are worth noting. First, we explicitly modeled pneumococcal carriage, in addition to disease, so that our estimates may not be directly comparable. Second, our study focused on two disease outcomes (pneumococcal meningitis and bacteremia) whose epidemiology may differ from that of pneumococcal pneumonia. Indeed, a US study documented differences of seasonality between pneumonia and nonpneumonia IPDs and suggested the existence of different mechanisms leading to the two disease outcomes (33). To further test this hypothesis, a natural follow-up would be to apply our models to pneumococcal pneumonia data. Regarding the specific outcomes considered here, our results are consistent with those of Opatowski et al. (21), although a more pronounced effect on pneumococcal carriage transmission was found in that study. In addition, our estimate of the relative risk of carriage acquisition in ILI-infected individuals (1.0 [1.0, 2.1], SI Appendix, Table S3) is not incompatible with that of a case-control study in young children (2.19 [1.02, 4.69], ref. 34).
Our results have public health implications. First, we found that the population-level effect of ILIs on pneumococcal circulation—via either increased transmission or acquisition—was minor. Importantly, therefore, interventions targeted at ILIs are not expected to produce marked indirect effects on pneumococcal carriage. Second, in keeping with previous studies (7, 8), we estimated that the overall fraction of IPDs due to ILIs was modest. Considering the severity and the high incidence of IPDs in certain regions, however, interventions that aim at reducing ILIs could still prevent a large number of IPDs, in particular in the context of influenza pandemics (35). Our results suggest that such interventions should be directed to individuals aged [5, 20), in whom the burden of ILIs and its subsequent direct impact on IPDs are highest. Third, our results confirmed the key role of young children as core transmitters of pneumococcus, who should therefore be the prime target of control efforts that aim to reduce pneumococcal carriage. Finally, our findings support the concept of a senescence of the immune system in the elderly (36), which may help explain the high burden of IPDs in that age group. Hence, interventions targeted at the elderly are another important component of control efforts, although we predict they will have limited indirect effects.
To interpret our results more generally, we point out that both ILIs and climate were estimated to have a much higher effect on invasion than on either acquisition or transmission. Seconding a previous study (33), we propose that seasonal variations in pneumococcal carriage density may explain these results. Indeed, we expect such variations to have a much higher effect on invasion than on transmission, because bottlenecks (i.e., reductions in bacterial population size) are presumably tighter during between-host transmission than during within-host invasion. Because we did not explicitly model carriage density, this interpretation is speculative. However, previous experimental (25) and epidemiological (37) studies have suggested that carriage density in an important factor in transmission and invasion.
Several limits of our study are worth noting. First, in the absence of longitudinal carriage data, we made pragmatic assumptions based on available evidence. Specifically, we calibrated our models to reproduce a decrease of carriage prevalence with age, a robust signature of pneumococcal epidemiology (38). Regarding the seasonality of carriage prevalence in high-income countries, most [but not all (13)] longitudinal studies reported small-amplitude variations of carriage over the year (39–41). Our model-based hindcasts (Fig. 2B) of carriage prevalence are broadly consistent with those observations. However, our result regarding the increase of contact rates and of carriage prevalence in the elderly during Christmas holidays should be confronted with new empirical data. Importantly, our estimate of the risk of disease during early carriage was sensitive to this transient effect, as evidenced by the fact that the information about that parameter was lost after removing data during Christmas holidays (95% confidence interval [0, 1]). Acknowledging these limitations, our results nevertheless demonstrate that IPD incidence data contain dynamic information about the transmission of pneumococcal carriage. Second, different types and subtypes of influenza can vary in transmissibility and virulence and may have a different impact on pneumococcus (11). Future work could therefore extend the models proposed here to incorporate more detailed information on influenza viruses, if available. Third, other cocirculating pathogens, not considered here, have been proposed to interact with pneumococcus (42). Incorporating additional candidate pathogens may help further understand pneumococcal seasonality, in particular the part that remained unexplained by our models (Fig. 2A). In SI Appendix, we present preliminary evidence suggesting that the respiratory syncytial virus [quantified as the number of visits to emergency departments for bronchiolitis in children <5 y (SI Appendix, Fig. S8)] may also interact with pneumococcus. Finally, our model ignores a number of complexities associated with pneumococcal epidemiology, foremost the differences of fitness between the different serotypes (43). Previous studies, however, indicated that IPD seasonality changed little after the introduction of conjugate vaccines, despite substantial serotype replacement (5, 8), suggesting that the seasonal drivers act comparably on the different serotypes.
In conclusion, we systematically dissected the seasonality of pneumococcus, building on detailed IPD incidence data in France. Our results bring together a number of previous lines of evidence and add significant knowledge of the mechanisms that govern transmission, carriage, and disease. We anticipate that dynamic models, such as those presented here, will prove to be valuable tools to further elucidate the seasonality of pneumococcus and, it is likely, of other bacterial respiratory pathogens.
Supplementary Material
Acknowledgments
We thank Pejman Rohani for helpful comments on the manuscript and Guillaume Béraud for providing the contact data in France. This work was supported directly by internal resources of the French National Institute for Health and Medical Research, the Institut Pasteur, and the University of Versailles–Saint-Quentin-en-Yvelines. This study received funding from the Île-de-France region (Domaine d’intérêt majeur Malinf) and from the French Government’s “Investissement d’Avenir” program, Laboratoire d’Excellence “Integrative Biology of Emerging Infectious Diseases” (Grant ANR-10-LABX-62- IBEID).
Footnotes
Conflict of interest statement: L.O. and M.D.d.C. have received research funding from Pfizer (through their research unit) on a project related to meningococcal epidemiology. L.O. has received consulting fees from WHO for work on antimicrobial resistance.
This article is a PNAS Direct Submission.
Data deposition: Data and R codes are available from the Dryad Digital Repository, datadryad.org (doi:10.5061/dryad.2j3c073).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1812388116/-/DCSupplemental.
References
- 1.Simell B, et al. Pneumococcal Carriage Group The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines. 2012;11:841–855. doi: 10.1586/erv.12.53. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378:1962–1973. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wahl B, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000-15. Lancet Glob Health. 2018;6:e744–e757. doi: 10.1016/S2214-109X(18)30247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Hoek AJ, Miller E. Editorial commentary: Seasonal changes in pneumococcal disease—Still much of an enigma. Clin Infect Dis. 2014;58:195–196. doi: 10.1093/cid/cit726. [DOI] [PubMed] [Google Scholar]
- 5.Domenech de Cellès M, et al. Characterizing and comparing the seasonality of influenza-like illnesses and invasive pneumococcal diseases using seasonal waveforms. Am J Epidemiol. 2018;187:1029–1039. doi: 10.1093/aje/kwx336. [DOI] [PubMed] [Google Scholar]
- 6.Kuster SP, Tuite AR, Kwong JC, McGeer A, Fisman DN. Toronto Invasive Bacterial Diseases Network Investigators Evaluation of coseasonality of influenza and invasive pneumococcal disease: Results from prospective surveillance. PLoS Med. 2011;8:e1001042. doi: 10.1371/journal.pmed.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicoli EJ, et al. Influenza and RSV make a modest contribution to invasive pneumococcal disease incidence in the UK. J Infect. 2013;66:512–520. doi: 10.1016/j.jinf.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter ND, et al. Active Bacterial Core Surveillance Team Influenza circulation and the burden of invasive pneumococcal pneumonia during a non-pandemic period in the United States. Clin Infect Dis. 2010;50:175–183. doi: 10.1086/649208. [DOI] [PubMed] [Google Scholar]
- 9.Watson M, Gilmour R, Menzies R, Ferson M, McIntyre P. New South Wales Pneumococcal Network The association of respiratory viruses, temperature, and other climatic parameters with the incidence of invasive pneumococcal disease in Sydney, Australia. Clin Infect Dis. 2006;42:211–215. doi: 10.1086/498897. [DOI] [PubMed] [Google Scholar]
- 10.White ANJ, et al. Let the sun shine in: Effects of ultraviolet radiation on invasive pneumococcal disease risk in Philadelphia, Pennsylvania. BMC Infect Dis. 2009;9:196. doi: 10.1186/1471-2334-9-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Peterson ME, Campbell H, Nair H. Association of seasonal viral acute respiratory infection with pneumococcal disease: A systematic review of population-based studies. BMJ Open. 2018;8:e019743. doi: 10.1136/bmjopen-2017-019743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahl J, et al. Risk factors for pneumococcal carriage in day care centers: A retrospective study during a 10-year period. Pediatr Infect Dis J. 2014;33:536–538. doi: 10.1097/INF.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 14.Dowell SF, Whitney CG, Wright C, Rose CE, Jr, Schuchat A. Seasonal patterns of invasive pneumococcal disease. Emerg Infect Dis. 2003;9:573–579. doi: 10.3201/eid0905.020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lepoutre A, et al. Microbiologists of Epibac; ORP Networks Impact of the pneumococcal conjugate vaccines on invasive pneumococcal disease in France, 2001-2012. Vaccine. 2015;33:359–366. doi: 10.1016/j.vaccine.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Domenech de Cellès M, et al. 2019. Data from “Unraveling the seasonal epidemiology of pneumococcus.” Dryad Digital Repository, 10.5061/dryad.2j3c073. Deposited December 21, 2018.
- 17.Valleron AJ, et al. A computer network for the surveillance of communicable diseases: The French experiment. Am J Public Health. 1986;76:1289–1292. doi: 10.2105/ajph.76.11.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Béraud G, et al. The French connection: The first large population-based contact survey in France relevant for the spread of infectious diseases. PLoS One. 2015;10:e0133203. doi: 10.1371/journal.pone.0133203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mossong J, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domenech de Cellès M, et al. Interaction of vaccination and reduction of antibiotic use drives unexpected increase of pneumococcal meningitis. Sci Rep. 2015;5:11293. doi: 10.1038/srep11293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Opatowski L, et al. Assessing pneumococcal meningitis association with viral respiratory infections and antibiotics: Insights from statistical and mathematical models. Proc Biol Sci. 2013;280:20130519. doi: 10.1098/rspb.2013.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray BM, Converse GM, 3rd, Dillon HC., Jr Epidemiologic studies of Streptococcus pneumoniae in infants: Acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980;142:923–933. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 23.Shrestha S, et al. Identifying the interaction between influenza and pneumococcal pneumonia using incidence data. Sci Transl Med. 2013;5:191ra84. doi: 10.1126/scitranslmed.3005982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walter ND, Taylor TH, Jr, Dowell SF, Mathis S, Moore MR. Active Bacterial Core Surveillance System Team Holiday spikes in pneumococcal disease among older adults. N Engl J Med. 2009;361:2584–2585. doi: 10.1056/NEJMc0904844. [DOI] [PubMed] [Google Scholar]
- 25.Zafar MA, Kono M, Wang Y, Zangari T, Weiser JN. Infant mouse model for the study of shedding and transmission during Streptococcus pneumoniae monoinfection. Infect Immun. 2016;84:2714–2722. doi: 10.1128/IAI.00416-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King AA, Nguyen D, Ionides EL. Statistical inference for partially observed Markov processes via the R package pomp. J Stat Software. 2016;69:1–43. [Google Scholar]
- 27.Bojang A, et al. Seasonality of pneumococcal nasopharyngeal carriage in rural Gambia determined within the context of a cluster randomized pneumococcal vaccine trial. PLoS One. 2015;10:e0129649. doi: 10.1371/journal.pone.0129649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Numminen E, et al. Climate induces seasonality in pneumococcal transmission. Sci Rep. 2015;5:11344. doi: 10.1038/srep11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaman J, Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci USA. 2009;106:3243–3248. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat Rev Microbiol. 2018;16:355–367. doi: 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dopico XC, et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat Commun. 2015;6:7000. doi: 10.1038/ncomms8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paireau J, Chen A, Broutin H, Grenfell B, Basta NE. Seasonal dynamics of bacterial meningitis: A time-series analysis. Lancet Glob Health. 2016;4:e370–e377. doi: 10.1016/S2214-109X(16)30064-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberger DM, et al. Seasonal drivers of pneumococcal disease incidence: Impact of bacterial carriage and viral activity. Clin Infect Dis. 2014;58:188–194. doi: 10.1093/cid/cit721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grijalva CG, et al. The role of influenza and parainfluenza infections in nasopharyngeal pneumococcal acquisition among young children. Clin Infect Dis. 2014;58:1369–1376. doi: 10.1093/cid/ciu148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinberger DM, et al. Impact of the 2009 influenza pandemic on pneumococcal pneumonia hospitalizations in the United States. J Infect Dis. 2012;205:458–465. doi: 10.1093/infdis/jir749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krone CL, van de Groep K, Trzciński K, Sanders EA, Bogaert D. Immunosenescence and pneumococcal disease: An imbalance in host-pathogen interactions. Lancet Respir Med. 2014;2:141–153. doi: 10.1016/S2213-2600(13)70165-6. [DOI] [PubMed] [Google Scholar]
- 37.Brotons P, et al. Nasopharyngeal bacterial load as a marker for rapid and easy diagnosis of invasive pneumococcal disease in children from Mozambique. PLoS One. 2017;12:e0184762. doi: 10.1371/journal.pone.0184762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Polain de Waroux O, Flasche S, Prieto-Merino D, Edmunds WJ. Age-dependent prevalence of nasopharyngeal carriage of Streptococcus pneumoniae before conjugate vaccine introduction: A prediction model based on a meta-analysis. PLoS One. 2014;9:e86136. doi: 10.1371/journal.pone.0086136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray BM, Turner ME, Dillon HC., Jr Epidemiologic studies of Streptococcus pneumoniae in infants. The effects of season and age on pneumococcal acquisition and carriage in the first 24 months of life. Am J Epidemiol. 1982;116:692–703. doi: 10.1093/oxfordjournals.aje.a113452. [DOI] [PubMed] [Google Scholar]
- 40.Hussain M, et al. A longitudinal household study of Streptococcus pneumoniae nasopharyngeal carriage in a UK setting. Epidemiol Infect. 2005;133:891–898. doi: 10.1017/S0950268805004012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Syrjänen RK, Kilpi TM, Kaijalainen TH, Herva EE, Takala AK. Nasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years old. J Infect Dis. 2001;184:451–459. doi: 10.1086/322048. [DOI] [PubMed] [Google Scholar]
- 42.Weinberger DM, Klugman KP, Steiner CA, Simonsen L, Viboud C. Association between respiratory syncytial virus activity and pneumococcal disease in infants: A time series analysis of US hospitalization data. PLoS Med. 2015;12:e1001776. doi: 10.1371/journal.pmed.1001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lipsitch M, et al. Estimating rates of carriage acquisition and clearance and competitive ability for pneumococcal serotypes in Kenya with a Markov transition model. Epidemiology. 2012;23:510–519. doi: 10.1097/EDE.0b013e31824f2f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.