Abstract
Background
At least one‐third of community‐dwelling people over 65 years of age fall each year. Exercises that target balance, gait and muscle strength have been found to prevent falls in these people. An up‐to‐date synthesis of the evidence is important given the major long‐term consequences associated with falls and fall‐related injuries
Objectives
To assess the effects (benefits and harms) of exercise interventions for preventing falls in older people living in the community.
Search methods
We searched CENTRAL, MEDLINE, Embase, three other databases and two trial registers up to 2 May 2018, together with reference checking and contact with study authors to identify additional studies.
Selection criteria
We included randomised controlled trials (RCTs) evaluating the effects of any form of exercise as a single intervention on falls in people aged 60+ years living in the community. We excluded trials focused on particular conditions, such as stroke.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Our primary outcome was rate of falls.
Main results
We included 108 RCTs with 23,407 participants living in the community in 25 countries. There were nine cluster‐RCTs. On average, participants were 76 years old and 77% were women. Most trials had unclear or high risk of bias for one or more items. Results from four trials focusing on people who had been recently discharged from hospital and from comparisons of different exercises are not described here.
Exercise (all types) versus control
Eighty‐one trials (19,684 participants) compared exercise (all types) with control intervention (one not thought to reduce falls). Exercise reduces the rate of falls by 23% (rate ratio (RaR) 0.77, 95% confidence interval (CI) 0.71 to 0.83; 12,981 participants, 59 studies; high‐certainty evidence). Based on an illustrative risk of 850 falls in 1000 people followed over one year (data based on control group risk data from the 59 studies), this equates to 195 (95% CI 144 to 246) fewer falls in the exercise group. Exercise also reduces the number of people experiencing one or more falls by 15% (risk ratio (RR) 0.85, 95% CI 0.81 to 0.89; 13,518 participants, 63 studies; high‐certainty evidence). Based on an illustrative risk of 480 fallers in 1000 people followed over one year (data based on control group risk data from the 63 studies), this equates to 72 (95% CI 52 to 91) fewer fallers in the exercise group. Subgroup analyses showed no evidence of a difference in effect on both falls outcomes according to whether trials selected participants at increased risk of falling or not.
The findings for other outcomes are less certain, reflecting in part the relatively low number of studies and participants. Exercise may reduce the number of people experiencing one or more fall‐related fractures (RR 0.73, 95% CI 0.56 to 0.95; 4047 participants, 10 studies; low‐certainty evidence) and the number of people experiencing one or more falls requiring medical attention (RR 0.61, 95% CI 0.47 to 0.79; 1019 participants, 5 studies; low‐certainty evidence). The effect of exercise on the number of people who experience one or more falls requiring hospital admission is unclear (RR 0.78, 95% CI 0.51 to 1.18; 1705 participants, 2 studies, very low‐certainty evidence). Exercise may make little important difference to health‐related quality of life: conversion of the pooled result (standardised mean difference (SMD) ‐0.03, 95% CI ‐0.10 to 0.04; 3172 participants, 15 studies; low‐certainty evidence) to the EQ‐5D and SF‐36 scores showed the respective 95% CIs were much smaller than minimally important differences for both scales.
Adverse events were reported to some degree in 27 trials (6019 participants) but were monitored closely in both exercise and control groups in only one trial. Fourteen trials reported no adverse events. Aside from two serious adverse events (one pelvic stress fracture and one inguinal hernia surgery) reported in one trial, the remainder were non‐serious adverse events, primarily of a musculoskeletal nature. There was a median of three events (range 1 to 26) in the exercise groups.
Different exercise types versus control
Different forms of exercise had different impacts on falls (test for subgroup differences, rate of falls: P = 0.004, I² = 71%). Compared with control, balance and functional exercises reduce the rate of falls by 24% (RaR 0.76, 95% CI 0.70 to 0.81; 7920 participants, 39 studies; high‐certainty evidence) and the number of people experiencing one or more falls by 13% (RR 0.87, 95% CI 0.82 to 0.91; 8288 participants, 37 studies; high‐certainty evidence). Multiple types of exercise (most commonly balance and functional exercises plus resistance exercises) probably reduce the rate of falls by 34% (RaR 0.66, 95% CI 0.50 to 0.88; 1374 participants, 11 studies; moderate‐certainty evidence) and the number of people experiencing one or more falls by 22% (RR 0.78, 95% CI 0.64 to 0.96; 1623 participants, 17 studies; moderate‐certainty evidence). Tai Chi may reduce the rate of falls by 19% (RaR 0.81, 95% CI 0.67 to 0.99; 2655 participants, 7 studies; low‐certainty evidence) as well as reducing the number of people who experience falls by 20% (RR 0.80, 95% CI 0.70 to 0.91; 2677 participants, 8 studies; high‐certainty evidence). We are uncertain of the effects of programmes that are primarily resistance training, or dance or walking programmes on the rate of falls and the number of people who experience falls. No trials compared flexibility or endurance exercise versus control.
Authors' conclusions
Exercise programmes reduce the rate of falls and the number of people experiencing falls in older people living in the community (high‐certainty evidence). The effects of such exercise programmes are uncertain for other non‐falls outcomes. Where reported, adverse events were predominantly non‐serious.
Exercise programmes that reduce falls primarily involve balance and functional exercises, while programmes that probably reduce falls include multiple exercise categories (typically balance and functional exercises plus resistance exercises). Tai Chi may also prevent falls but we are uncertain of the effect of resistance exercise (without balance and functional exercises), dance, or walking on the rate of falls.
Plain language summary
Exercise for preventing falls in older people living in the community
Background
At least one‐third of community‐dwelling people over 65 years of age fall each year. Exercises that target balance, gait and muscle strength have previously been found to prevent falls in these people.
Review aim
To assess the effects (benefits and harms) of exercise interventions for preventing falls in older people living in the community.
Search date
We searched the healthcare literature for reports of randomised controlled trials relevant to this review up to 2 May 2018. In such studies, people are allocated at random to receive one of two or more interventions being compared in the study. Leaving group allocation to chance helps ensure the participant populations are similar in the intervention groups.
Study characteristics
This review includes 108 randomised controlled trials with 23,407 participants. These were carried out in 25 countries. On average, participants were 76 years old and 77% were women.
Certainty of the evidence
The majority of trials had unclear or high risk of bias, mainly reflecting lack of blinding of trial participants and personnel to the interventions. This could have influenced how the trial was conducted and outcome assessment. The certainty of the evidence for the overall effect of exercise on falls was high. Risk of fracture, hospitalisation, medical attention and adverse events were not well reported and, where reported, the evidence was low‐ to very low‐certainty. This leads to uncertainty regarding drawing conclusions from the evidence for these outcomes.
Key results
Eighty‐one trials compared exercise (all types) versus a control intervention that is not thought to reduce falls in people living in the community (who also had not recently been discharged from hospital). Exercise reduces the number of falls over time by around one‐quarter (23% reduction). By way of an example, these data indicate that if there were 850 falls in 1000 people followed over one year, exercise would result in 195 fewer falls. Exercise also reduces the number of people experiencing one or more falls (number of fallers) by around one‐sixth (15%) compared with control. For example, if there were 480 fallers who fell in 1000 people followed over one year, exercise would result in 72 fewer fallers. The effects on falls were similar whether the trials selected people who were at an increased risk of falling or not.
We found exercise that mainly involved balance and functional training reduced falls compared with an inactive control group. Programmes involving multiple types of exercise (most commonly balance and functional exercises plus resistance exercises) probably reduced falls, and Tai Chi may also reduce falls. We did not find enough evidence to determine the effects of exercise programmes classified as being mainly resistance exercises, dance, or walking programmes. We found no evidence to determine the effects of programmes that were mainly flexibility or endurance exercise.
There was considerably less evidence for non‐fall outcomes. Exercise may reduce the number of people experiencing fractures by over one‐quarter (27%) compared with control. However, more studies are needed to confirm this. Exercise may also reduce the risk of a fall requiring medical attention. We did not find enough evidence to determine the effects of exercise on the risk of a fall requiring hospital admission. Exercise may make very little difference to health‐related quality of life. The evidence for adverse events related to exercise was also limited. Where reported, adverse events were usually non‐serious events of a musculoskeletal nature; exceptionally one trial reported a pelvic stress fracture and a hernia.
Summary of findings
Background
Description of the condition
At least one‐third of community‐dwelling people over 65 years of age fall each year (Campbell 1990; Tinetti 1988), and the rate of fall‐related injuries increases with age (Peel 2002). Falls can have serious consequences, such as fractures and head injuries (Peel 2002). Around 10% of falls result in a fracture (Campbell 1990; Tinetti 1988); fall‐associated fractures in older people are a significant source of morbidity and mortality (Burns 2016). Although most fall‐related injuries, such as bruising, lacerations and sprains, are less serious, they can still lead to pain, reduced function and substantial healthcare costs (Burns 2016).
Falls are associated with reduced quality of life (Stenhagen 2014), and can have psychological consequences: fear of falling and loss of confidence that can result in self‐restricted activity levels leading to a reduction in physical function and social interactions (Yardley 2002). Paradoxically, this restriction of activities may increase the risk of further falls by contributing to deterioration in physical abilities. Both injurious and non‐injurious falls can have these psychological and subsequent physical impacts.
Despite early attempts to achieve a consensus definition of a 'fall' (Anonymous 1987), many definitions still exist in the literature. It is particularly important for studies to use a clear, simple definition of a fall. An international researchers' consensus statement defines a fall as "an unexpected event in which the participant comes to rest on the ground, floor, or lower level" (Lamb 2005). The wording recommended when asking study participants is: "In the past month, have you had any fall including a slip or trip in which you lost your balance and landed on the floor or ground or lower level?" (Lamb 2005). 'Lower level' refers to a surface lower than the person's starting position so, for example, falling from a standing position to unintentionally sitting on a bed would be considered a fall.
In addition to the physical and psychological consequences for individuals and their families, falls can have important financial impacts on individuals, families and health and community care systems (Burns 2016). For example, falling is an independent predictor of admission to residential aged care facilities (Tinetti 1997).
Description of the intervention
Exercise is a physical activity that is planned, structured and repetitive and aims to improve or maintain physical fitness (Caspersen 1985). There is a wide range of possible types of exercise, such as strengthening exercise, balance and co‐ordination exercise and aerobic exercise. Exercise programmes often include one or more types of exercise. The Prevention of Falls Network Europe (ProFaNE) developed a taxonomy that classifies exercise type as: i) gait, balance, and functional (task) training; ii) strength/resistance (including power); iii) flexibility; iv) three‐dimensional (3D) exercise (e.g. Tai Chi, Qigong, dance); v) general physical activity; vi) endurance; and vii) other kinds of exercises (Lamb 2011). The taxonomy allows for more than one type of exercise to be delivered within a programme.
Formal exercise programmes are delivered by a wide range of individuals ranging from health professionals (such as physiotherapists, also known as physical therapists) and exercise professionals (such as trained fitness leaders) to trained volunteers. Exercise programmes may be supervised, unsupervised or involve a mixture of both.
This review considers all types of exercise and all delivery methods.
Exercise can also be delivered as part of a multiple component intervention, where people also receive one or more other fall or fracture prevention interventions, such as home‐hazard modification and vitamin D supplementation. The effects of multiple component interventions that include exercise are assessed in Hopewell 2018.
How the intervention might work
Many aspects of physical functioning deteriorate with increased age and inactivity. Impairments in muscle strength, balance control and gait are particularly strong risk factors for falls (Tinetti 1988). For example, those with poor leg extensor strength were found to be 43% more likely to fall at home than their stronger counterparts (Menant 2017). Systematic reviews have found that those with gait problems have twice the odds of falling than those without (Deandrea 2010), and that measures of balance and mobility such as the Berg Balance Scale, Timed Up and Go Test, and Five Times Sit‐to‐Stand Test can identify individuals at greater risk of future falls (Lusardi 2017).
Exercises that address these impairments are therefore likely to reduce the risk of falling. As Cochrane Reviews have now found that exercise improves both strength (Liu 2009), and balance (Howe 2011) in older people, exercise is likely to have a fall prevention effect through its impact on these key fall risk factors. A Cochrane Review found that exercise reduces the fear of falling (Kendrick 2014), which is also a strong predictor of falls.
A previous Cochrane Review found exercise as a single intervention, prevents falls (Gillespie 2012), and to be the most commonly tested single fall prevention intervention. Economic evaluations accompanying randomised trials have found exercise to be a cost‐effective fall‐prevention strategy (Davis 2010).
Exercise interventions have been found to be effective when delivered in a group‐based setting or on an individual basis. The optimal features of successful fall prevention exercise programmes are not yet clear, but programmes that are multicomponent (e.g. target both strength and balance; Gillespie 2012), and programmes that include balance training, appear to be particularly effective (Sherrington 2017).
Different approaches to exercise will have advantages and disadvantages in terms of cost, 'enjoyability', accessibility and impacts on various body systems and outcomes. These advantages and disadvantages are likely to vary between individuals and in different settings.
Exercise has the potential to lead to adverse events such as cardiovascular episodes and musculoskeletal injuries if not carefully prescribed and undertaken (Thompson 2013). Exercise may also increase the risk of falls, particularly in higher risk individuals. For example, exercise interventions aiming to improve balance and ultimately lessen the risk of falling, often involve a 'challenge' to balance that simultaneously puts the person at greater risk of falling (Sherrington 2017). The risk may be increased if an exercise participant becomes fatigued (due to deconditioning or as a result of comorbidities or medications) or are not encouraged to use support when needed (Skelton 2001). Trials and reviews should therefore record and report adverse events.
As the majority of fractures in older people involve falls, exercise has the potential to prevent fractures. Systematic reviews have suggested that exercise may prevent fractures (Gillespie 2012), and fall‐related injuries (Robertson 2002).
Why it is important to do this review
An update of the effects of exercise interventions on falls is warranted given the number of new trials published, the increasing number of older people living in the community and the major long‐term consequences associated with falls and fall‐related injuries to both the individual and to society.
It is also important to understand to what extent interventions designed to prevent falls will also prevent fall‐associated fractures, the need for medical attention and improve quality of life. Different exercise programmes may have different effects on falls and so careful analysis of the impact of different programmes is crucial to optimise the prescription of exercise interventions and inform public health promotion initiatives for healthy ageing. Additionally, looking for adverse events associated with the different exercise programmes, such as exercise‐related falls and muscle strains, is also important.
Objectives
To assess the effects (benefits and harms) of exercise interventions for preventing falls in older people living in the community.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), either individual or cluster randomised, evaluating the effects of exercise interventions on falls or fall‐related fractures in older people living in the community. We excluded trials that explicitly used methods of quasi‐randomisation (e.g. allocation to groups by alternation or date of birth).
Types of participants
We included trials if they specified an inclusion criterion of 60 years of age or over. Trials that included younger participants were included if the mean age minus one standard deviation was more than 60 years. We included trials where the majority of participants were living in the community, either at home or in places of residence that, on the whole, do not provide residential health‐related care or rehabilitative services; for example, retirement villages, or sheltered housing. Trials with mixed populations (community and higher dependency places of residence) were eligible for inclusion if data were provided for subgroups based on setting or the numbers in higher dependency residences were very few and balanced in the comparison groups.
We excluded studies that only included participants affected by particular clinical conditions that increase the risk of falls, such as stroke, Parkinson's disease, multiple sclerosis, dementia, hip fracture and severe visual impairment. Several of these topic areas are covered by other Cochrane Reviews (Canning 2015; Verheyden 2013). We acknowledge that some individuals with these (and other) health conditions may be included in studies of the general community; these we included.
As in our protocol, we also included trials recruiting participants in hospital if the majority were discharged to the community, where the majority of the intervention was delivered and falls recorded. As we considered such trials, whose participants were recently discharged from hospital, to be a distinct category we reported them separately.
Types of interventions
This review included all exercise interventions tested in trials that measured falls in older people. The intention was to include trials where exercise was a single intervention as opposed to a component of a broader intervention. We included trials where an additional low‐contact intervention (e.g. information on fall prevention) was given to one or both groups if we judged that the main purpose of the study was to investigate the role of exercise.
We classified exercise programmes on the basis of the primary exercise category and noted the presence of additional, secondary, exercise categories. Based on the Prevention of Falls Network Europe (ProFaNE) taxonomy (Lamb 2011), as shown in Appendix 1, we classified exercise programmes in the included trials as primarily involving the following exercise categories: i) gait, balance, co‐ordination and functional task training (referred to as 'balance and functional exercises' for simplicity); ii) strength/resistance training (including power training, using resistance so referred to as 'resistance exercises'); iii) flexibility; iv) three‐dimensional (3D) exercise (with separate Tai Chi and dance subcategories); iv) general physical activity (walking programmes); v) endurance; and vi) other kinds of exercises. We also formed another category for exercise programmes that included more than one of the above categories as the primary exercise category, e.g. a programme with 15 minutes of gait, balance, co‐ordination and functional task training followed by 15 minutes of strength/resistance training. We examined the descriptions of interventions used in individual trials and categorised the intervention accordingly. For example, some forms of yoga may have been categorised as flexibility exercise and others as 3D exercise.
We compared each of these types of exercise with control, comprising either 'usual care' (i.e. no change in usual activities) or a control intervention (i.e. an intervention that is not thought to reduce falls, such as general health education, social visits, very gentle exercise, or 'sham' exercise not expected to impact on falls).
We first undertook an 'umbrella' comparison of 'exercise (all types) versus control', explored the impact of the use of an increased risk of falls as a trial inclusion criterion and the impact of participant age on the overall impact of exercise on falls, then set out the following comparisons.
Balance and functional exercises versus control.
Resistance exercises versus control.
Flexibility training versus control.
3D (including Tai Chi, Qigong) exercise versus control.
3D (dance) exercise versus control.
Walking programme versus control.
Endurance training versus control.
Other kinds of exercise versus control.
Multiple categories of exercise versus control (i.e. exercise programmes including more than one of the above categories versus control).
We also planned to undertake the following secondary comparisons of different exercise programmes.
Different types of exercise, based on the above categories.
Different modes of delivery (e.g. group versus individual) of the same type of exercise.
Different doses (e.g. higher intensity versus lower intensity) of the same type of exercise.
Types of outcome measures
Primary outcomes
Rate of falls (falls per person‐year)
Secondary outcomes
Number of people who experienced one or more falls (risk of falling)
Number of people who experienced one or more fall‐related fractures
Number of people who experienced one or more falls that resulted in hospital admission (newly listed outcome April 2018)
Number of people who experienced one or more falls that required medical attention
Health‐related quality of life, measured using validated scale, e.g. EQ‐5D or similar (newly listed outcome April 2018)
Number of people who experienced one or more adverse events (see below)
We chose 'rate of falls' as the single primary outcome for ease of interpretation of the results of the review. Furthermore, the rate of falls is likely to be more sensitive to change than the proportion of fallers, especially in samples with high fall rates. As falls are count data, dichotomisation to falling versus not falling represents a loss of information. Therefore, many trials use the rate of falls as their primary outcome and use negative binomial regression to compare the rates between intervention and control groups, as recommended in Robertson 2005.
Adverse events needed to be monitored closely in all groups using the same methods over the entire study period to be included in the analysis.
Other outcomes
We recorded and reported mortality data, distinguishing where possible, between those who were lost to the trials because they had died and those whose death was explicitly linked to trial participation.
We recorded and reported data regarding intervention adherence, cost and cost‐effectiveness, where available.
Timing of outcome measurement
The primary outcome included one time point from each study. For studies with outcomes measured at multiple time points, we used the closest to 18 months in the primary analysis. We included a separate longer‐term outcome for studies with follow‐up at more than 18 months after randomisation. To maximise the use of available information, we also included studies with just one time point that was longer than 18 months in the primary analysis.
Search methods for identification of studies
Electronic searches
Our search extended the searches performed up to February 2012 in Gillespie 2012. We searched: the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (February 2012 to 2 May 2018); the Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Register of Studies Online) (2012 Issue 2 to 2018 Issue 5); MEDLINE (including Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and MEDLINE Daily) (January 2012 to 30 April 2018); Embase (March 2012 to 2018 Week 18); the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (February 2012 to 2 May 2018); and the Physiotherapy Evidence Database (PEDro) (2012 to 2 May 2018), using tailored search strategies. We did not apply any language restrictions.
In MEDLINE, we combined subject‐specific search terms with the sensitivity‐ and precision‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011). The search strategies for CENTRAL, MEDLINE, Embase, CINAHL and PEDro are shown in Appendix 2.
We also searched the World Health Organisation International Clinical Trials Registry Platform (WHO ICTRP) and ClinicalTrials.gov for ongoing and recently completed trials (May 2018) (see Appendix 2).
Searching other resources
We checked reference lists of other systematic reviews as well as contacting researchers in the field to assist in the identification of ongoing and recently completed trials.
Data collection and analysis
The intended methodology for data collection and analysis was described in our published protocol (Sherrington 2016), which was based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Pairs of review authors (CS, AT, NJF, ZAM) screened the title, abstract and descriptors of identified studies for possible inclusion. From the full text, two review authors (CS, AT, NJF, ZAM) independently assessed potentially eligible trials for inclusion and resolved any disagreement through discussion. We contacted authors for additional information as necessary.
Data extraction and management
Pairs of review authors (CS, AT, NJF, ZAM, GW) independently extracted data using a pretested data extraction form (based on the one used in Gillespie 2012). We extracted data from both newly included trials and those included in Gillespie 2012. For the latter trials, however, we primarily extracted information and data for additional outcomes that were not collected previously for Gillespie 2012. Disagreement was resolved by consensus or third party adjudication. Review authors were not blinded to authors and sources. Review authors did not assess their own trials.
We used the standardised data extraction form to record the following items.
General information: review author's name; date of data extraction; study ID; first author of study; author's contact address (if available); citation of paper; and trial objectives.
Trial details: trial design; location; setting; sample size; inclusion and exclusion criteria (with particular note of whether there was exclusion for cognitive impairment); comparability of groups; length of follow‐up; stratification; stopping rules; and funding source.
'Risk of bias' assessment and justification for this judgement: sequence generation; allocation concealment; blinding (participants, personnel, outcome assessors); incomplete outcome data; selective outcome reporting; and other bias (recall bias).
Characteristics of participants: age; gender; ethnicity; the number randomised, analysed and lost to follow‐up; and dropouts in each arm (with reasons).
Interventions: experimental and control interventions; details of exercise programme (duration, frequency, intensity and individual‐ or group‐based delivery, level of supervision); timing of intervention; uptake of intervention (acceptance of exercise intervention), whether studies assessed adherence (compliance) with interventions and associated data (e.g. number of sessions attended); and additional co‐interventions (such as motivational strategies, additional information or support given to participants).
Outcomes measured: rate of falls; number of people experiencing one or more falls; number of people who experienced one or more fall‐related fractures; number of people who experienced one or more falls requiring medical attention; and number of people who experienced adverse events.
Other details: cost and cost‐effectiveness information related to fall outcomes.
We retrieved data from both full‐text and abstract reports of studies. Where these sources did not provide sufficient information, we contacted study authors for additional details. We also used data sourced from personal communication reported by Gillespie 2012.
In response to feedback on an earlier draft of this review we extended our data extraction to extract data on the number of people who experienced one or more falls resulting in hospital admission, mortality and health‐related quality of life (Differences between protocol and review).
We recorded and reported data on fracture, hospitalisation, medical attention, and health‐related quality of life only where separate data were available by intervention group.
Assessment of risk of bias in included studies
Pairs of two review authors (CS, AT, NJF, ZAM, GW) independently assessed risk of bias using Cochrane's 'Risk of bias' tool as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Review authors were not blinded to authors and sources. Review authors did not assess their own trials. Disagreement was resolved by consensus or third party adjudication (CS).
As outlined in Appendix 3 we assessed the following domains: random sequence generation (selection bias); allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); and selective outcome reporting bias. We also assessed bias in the recall of falls due to less reliable methods of ascertainment (Hannan 2010). We rated risk of bias as either low, high or unclear for each domain.
Specifically for trials using cluster‐randomisation, we considered the risk of additional bias relating to recruitment, baseline imbalance, loss of clusters, incorrect analysis and comparability with individually‐randomised trials, as described in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
We reported the treatment effects for rate of falls as rate ratios (RaRs) with 95% confidence intervals (CIs). For the number of fallers, number of participants experiencing fall‐related fractures, fall‐related hospital admission, falls that required medical attention and adverse events, we reported risk ratios (RRs) and 95% CIs.
The rate of falls is the total number of falls per unit of person‐time that falls were monitored (e.g. falls per person‐year). The RaR compares the rate of falls in any two groups during each trial. We used a RaR (for example, incidence RaR or hazard ratio (HR) for all falls) with 95% CI if these were reported in the paper. If both adjusted and unadjusted RaRs were reported, we used the unadjusted estimate unless the adjustment was for clustering. If a RaR was not reported, but appropriate raw data were available, we used Excel to calculate a RaR and 95% CI. We used the reported rate of falls (falls per person‐year) in each group and the total number of falls for participants contributing data, or we calculated the rate of falls in each group from the total number of falls and the actual total length of time falls were monitored (person‐years) for participants contributing data. In cases where data were only available for people who had completed the study, or where the trial authors had stated there were no losses to follow‐up, we assumed that these participants had been followed up for the maximum possible period.
The risk ratio (RR) compares the number of people who fell once or more (fallers) between groups. We used a reported estimate of the RR, HR for first fall, or odds ratio (OR)) and 95% CI if available. If both adjusted and unadjusted estimates were reported we used the unadjusted estimate, unless the adjustment was for clustering. If an OR was reported, or an effect estimate and 95% CI was not, and appropriate data were available, we calculated a RR and 95% CI using the 'csi' command in Stata. For the calculations, we used the number of participants contributing data in each group, if this was known; if not reported, we used the number randomised to each group. The same approach was used for the number of people experiencing fractures, falls requiring medical attention and adverse events. Data regarding the number of people in each group experiencing the additional variables of falls resulting in hospitalisation and death were entered into Review Manager 5 directly (Review Manager 2014).
For continuous outcomes (health‐related quality of life), we presented the mean difference (MD) with 95% CIs where the same outcome measure was used, or standardised mean difference (SMD) with 95% CIs for outcomes measured using different scales. Final values, which were used in preference to change scores, were always available where these outcomes were reported.
Unit of analysis issues
For trials which were cluster randomised, for example by medical practice, we performed adjustments for clustering, as described in Higgins 2011, if this was not done in the published report. We used an intraclass correlation coefficient (ICC) of 0.01 as reported in Smeeth 2002. We ignored the possibility of a clustering effect in trials that randomised by household. We anticipated that trials would be unlikely to report details of clustering by household and that the clustering effect by household would be very small (if any).
The pooled exercise versus control comparisons necessitated the inclusion of more than one pair‐wise comparison (intervention versus control) from the same trial in the same meta‐analysis. Where multiple comparisons from the same trial were included in the same meta‐analysis the standard errors were inflated by 25% and the number of control participants shown in the analyses was 'shared' between different comparisons by dividing by the number of intervention groups in the same analysis. For example, if a trial had 100 participants in a control group, 100 participants in a resistance training group, and 100 participants in a balance training group, the standard errors in the resistance versus control and balance versus control comparisons would be inflated by 25% and the number of control participants would be shown as 50 in both the resistance versus control and balance versus control comparisons.
We did not include outcomes collected at different time points in the same trial in the same analysis.
Dealing with missing data
Some missing data are inevitable in studies of older people, given the increased risk of ill health and death, and the length of delivery of the intervention in fall prevention trials. We attempted to contact study investigators for any key missing or unclear data or information in their trial; clarification on outcome data was only sought for number of falls and number of people who experienced falls. We undertook sensitivity analyses excluding trials with more than 20% loss to follow‐up or where the loss to follow‐up was unclear.
Assessment of heterogeneity
Where we considered study interventions to be sufficiently similar to be combined in meta‐analyses, we assessed heterogeneity of treatment effects by visual inspection of forest plots and by using the Chi² test (with a significance level at P < 0.10) and the I² statistic. We based our interpretation of the I² results on that suggested by Higgins 2011: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% may represent very substantial (‘considerable’) heterogeneity.
Assessment of reporting biases
We constructed and visually inspected funnel plots for outcomes that included more than 10 data points.
Data synthesis
For our primary comparison, we pooled data from all relevant trials without stratification. We originally planned to present the umbrella comparison of exercise versus control subgrouped by the main exercise categories (Sherrington 2016). This change was made in response to editorial input and the request for additional subgroup and sensitivity analyses in a commissioning brief relating to the National Institute for Health and Care Excellence (NICE) guideline CG161 (NICE 2013).
We presented separate analyses for studies that recruited people in hospitals and delivered interventions after discharge as we considered these were a distinct population compared with general community‐dwelling older adults.
We grouped similar exercise interventions using the fall prevention classification system (taxonomy) developed by the Prevention of Falls Network Europe (ProFaNE) (Lamb 2011). Full details are available in Appendix 1 and the ProFaNE Taxonomy Manual.
When considered appropriate, we pooled results of comparable studies using random‐effects models. We used 95% CIs throughout. We planned not to pool data where there was considerable heterogeneity (I² ≥ 75%) that could not be explained by the diversity of methodological or clinical features among trials.
When considered appropriate, we pooled data using the generic inverse variance method in Review Manager 5 (Review Manager 2014). This method enables pooling of the adjusted and unadjusted treatment effect estimates (rate ratios or risk ratios) reported in the individual studies or which can be calculated from data presented in the published article (see Measures of treatment effect). The generic inverse variance option in Review Manager 5 requires entering the natural logarithm of the rate ratio or risk ratio and its standard error for each trial; we calculated these in Excel. For continuous outcomes (health‐related quality of life), we presented MDs with 95% CIs where the same outcome measure was used, or SMDs with 95% CIs for outcomes measured using different scales.
Where it was inappropriate to pool data, we present trial‐level data in the analyses and tables for illustrative purposes.
The statistician was not blind to study or group.
Subgroup analysis and investigation of heterogeneity
We undertook subgroup analyses for the fall and fracture outcomes for the pooled (all‐exercise types) versus control analyses to compare the effect of exercise on falls and fractures in trials that did and did not use an increased risk of falls as an inclusion criterion. In response to a request (Differences between protocol and review) to explore the potential effects of stratification by age (based on a threshold of 75 years), we undertook subgroup analyses for the falls and fracture outcomes for the pooled (all‐exercise types) versus control analyses. We compared the effects on falls outcomes in trials with predominantly older populations (defined by inclusion criteria 75 years or above, lower range limit more than 75 years, or mean age minus one standard deviation more than 75 years) and those with predominantly younger populations.
Prompted by feedback at editorial review, we extended the following subgroup analyses (originally established for different exercise categories) to the all‐exercise types versus control for fall outcomes: a) individual versus group‐based exercise; and b) exercise delivered by people with different qualifications (e.g. health professionals versus trained fitness leaders).
We presented separate analyses stratified by the different ProFaNE exercise intervention categories outlined above, and performed subgroup analyses for the fall and fracture outcomes. We then used subgroup analyses to explore effects within the different ProFaNE exercise intervention categories. When there were at least 10 trials in a comparison, we carried out subgroup analyses to compare effects in trials of: a) higher versus lower falls risk at enrolment (i.e. trials with participants selected for inclusion based on history of falling or other specific risk factors for falling versus trials with unselected participants); b) individual versus group‐based exercise; and c) exercise delivered by people with different qualifications.
We used the test for subgroup differences available in Review Manager 2014 to determine whether there was evidence for a difference in treatment effect between subgroups.
Sensitivity analysis
We carried out 10 sensitivity analyses to explore the stability of the results.
Sensitivity analysis 1 (participant age)
In response to a specific request (Differences between protocol and review) to explore the potential effects of changing the age threshold from 60 to 65 years for inclusion into the review, we set out a series of sensitivity analyses to explore the effects of removing trials that would have been excluded from the review if a 65 year or older inclusion threshold had been applied.
Sensitivity analyses 2‐5 (risk of bias in included trials)
To assist with the GRADE rating we undertook sensitivity analyses for all outcomes in the 'Summary of findings' table by removing trials with a high risk of bias in any item.
To explore the possible impact of risk of bias on the primary pooled estimates of treatment effect, we examined the effects of the following.
Inclusion of trials at high or unclear risk of selection bias from inadequate concealment of allocation.
Inclusion of trials at high or unclear risk of detection bias from inadequate blinding of outcome assessors.
Inclusion of trials at high or unclear risk of attrition bias from incomplete outcome data.
Sensitivity analyses 6‐7 (meta‐analysis decisions)
We also examined the impact on the results of the removal of the cluster‐randomised trials and the use of fixed‐effect rather than random‐effects models for data pooling.
Sensitivity analysis 8 (multiple exercise category components)
In order to assist in the interpretation of the results of the type of exercise subgroup 'multiple categories of exercise' comparisons, we undertook a sensitivity analyses for both falls outcomes which only included trials that were coded as having the two primary components balance/functional exercises and resistance exercises.
Sensitivity analyses 9a and 9b (different exercise type coding)
To explore the possible impact of how we classified exercise interventions, we examined the effects of the following for both falls outcomes.
Classification of interventions based on the Otago Exercise Program as multiple categories of exercise.
Classification of any intervention that included balance and functional exercises plus strength exercises as multiple categories of exercise.
Assessing the certainty of evidence and 'Summary of findings' tables
We used the GRADE approach to assess the quality of evidence related to all outcomes listed in the Types of outcome measures (Schünemann 2017). Using GRADEpro GDT (GRADEPro GDT 2015), we assessed the certainty of the evidence as ‘high’, ‘moderate’, ‘low’ or ‘very low’ depending on the presence and extent of five factors: risk of bias; inconsistency of effect; indirectness; imprecision; and publication bias. We prepared 'Summary of finding' tables featuring the seven listed outcomes for the umbrella comparison (exercise (all types) versus control) and for the rate of falls, risk of falling, fall‐related fractures and adverse events for the primary exercise categories versus control comparisons, where data were available (Types of interventions). We used standardised qualitative statements to describe the different combinations of effect size and the certainty of evidence (Cochrane Norway 2017).
Results
Description of studies
Results of the search
A total of 8007 records were downloaded from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (7), CENTRAL (1650), MEDLINE (1601), Embase (2998), CINAHL (1104), PEDro (139), the WHO ICTRP (317), and ClinicalTrials.gov (191). We identified 359 studies from a prior Cochrane Review (Gillespie 2012), and other systematic reviews. We also found one study after the search process in September 2018 (Li 2018b)
Removal of duplicates and spurious records resulted in 4006 references. Upon screening of these, we excluded 3541 records and we obtained copies of 465 papers for consideration. A screening of these led to the removal of a further 230 records. The final round of study selection based on 235 reports resulted in the inclusion of 108 studies (194 reports), the exclusion of 21 studies (23 reports) (see Characteristics of excluded studies) and identification of 16 ongoing studies (Ongoing studies). Two further studies await classification (Jagdhane 2016; Li 2018b).
We contacted authors of two studies to request additional details to assess eligibility, and received responses from both studies; we included Hamrick 2017 and excluded Hinrichs 2016.
A flow diagram summarising the study selection process is shown in Figure 1.
1.
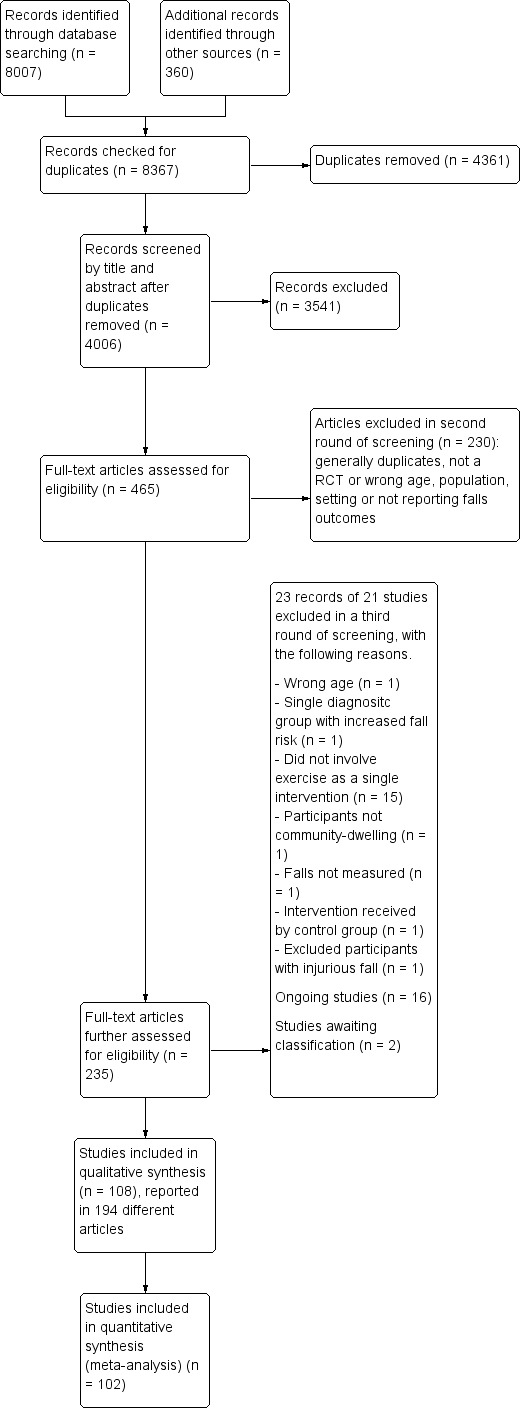
Study flow diagram.
Included studies
This review includes 108 trials with 23,407 participants. Details are provided in the Characteristics of included studies and are briefly summarised below. Due to the size of the review, not all links to references have been inserted in the following text but can be viewed in Appendix 4. Characteristics of the included studies are summarised in Table 8 and Table 9.
1. Study design, length of follow‐up, setting and trial size.
| Study IDa | Study design | No. arms (clusters) | Length of follow‐up (months) | Setting | No. randomised | No. analysedb | % lost to follow‐up |
| Gait, balance, and functional training | |||||||
| Almeida 2013 | Parallel | 3 | 4 | Brazil | 119 | 76 | 36% |
| Arantes 2015 | Parallel | 2 | 3 | Brazil | 30 | 28 | 7% |
| Arkkukangas 2015 | Parallel | 2 | 3 | Sweden | 45 | 40 | 11% |
| Barnett 2003 | Parallel | 2 | 12 | Australia | 163 | 150 | 8% |
| Boongrid 2017 | Parallel | 2 | 12 | Thailand | 439 | 437 | 0% |
| Campbell 1997 | Parallel | 2 | 24 | New Zealand | 233 | 233 | 0% |
| Clegg 2014 | Parallel | 2 | 3 | United Kingdom | 84 | 70 | 17% |
| Clemson 2010 | Parallel | 2 | 6 | Australia | 34 | 34 | 0% |
| Clemson 2012 (Life Program) | Parallel | 3 | 12 | Australia | 317 | 317 | 0% |
| Cornillon 2002 | Parallel | 2 | 12 | France | 303 | 303 | 0% |
| Dadgari 2016 | Cluster | 2 (25) | 6 | Iran | 551 | 317 | 42% |
| Dangour 2011 | Cluster | 2 (28) | 24 | Chile | 984 | 619 | 37% |
| Day 2002 | Parallel | 2 | 18 | Australia | 272 | 272 | 0% |
| Duque 2013 | Parallel | 2 | 9 | Australia | 60 | 60 | 0% |
| El‐Khoury 2015 | Parallel | 2 | 24 | France | 706 | 706 | 0% |
| Gschwind 2015 | Parallel | 2 | 6 | Germany, Spain, Australia | 153 | 136 | 11% |
| Halvarsson 2013 | Parallel | 2 | 15 | Sweden | 59 | 48 | 19% |
| Halvarsson 2016 (balance group) | Parallel | 3 | 3 | Sweden | 96 | 69 | 28% |
| Hamrick 2017 | Parallel | 2 | 6 | USA | 43 | 38 | 12% |
| Hirase 2015 | Parallel | 3 | 4 | Japan | 93 | 86 | 8% |
| Iliffe 2015 (FaME and OEP groups) | Cluster | 3 (42) | 18 | United Kingdom | 1254 | 709 | 43% |
| Iwamoto 2009 | Parallel | 2 | 5 | Japan | 68 | 67 | 1% |
| Karinkanta 2007 (balance group) | Parallel | 4 | 12 | Finland | 149 | 144 | 3% |
| Kerse 2010 | Parallel | 2 | 12 | New Zealand | 193 | 193 | 0% |
| Korpelainen 2006 | Parallel | 2 | 30 | Finland | 160 | 160 | 0% |
| Kovacs 2013 | Parallel | 2 | 12 | Hungary | 76 | 72 | 5% |
| Lin 2007 | Parallel | 2 | 6 | Taiwan | 100 | 100 | 0% |
| Liu‐Ambrose 2008 | Parallel | 2 | 12 | Canada | 74 | 59 | 30% |
| Liu‐Ambrose 2004 (agility group) | Parallel | 3 | 6 | Canada | 104 | 98 | 6% |
| Lord 1995 | Parallel | 2 | 12 | Australia | 197 | 169 | 14% |
| Lord 2003 | Cluster | 2 (20) | 12 | Australia | 551 | 508 | 8% |
| Luukinen 2007 | Parallel | 2 | 16 | Finland | 486 | 437 | 10% |
| Madureira 2007 | Parallel | 2 | 12 | Brazil | 66 | 60 | 9% |
| McMurdo 1997 | Parallel | 2 | 24 | Scotland | 118 | 92 | 22% |
| Miko 2017 | Parallel | 2 | 12 | Hungary | 100 | 97 | 3% |
| Morgan 2004 | Parallel | 2 | 12 | USA | 294 | 229 | 22% |
| Nitz 2004 | Parallel | 2 | 6 | Australia | 73 | 45 | 38% |
| Reinsch 1992 | Cluster | 2 (16) | 12 | USA | 230 | 230 | 0% |
| Robertson 2001a | Parallel | 2 | 12 | New Zealand | 240 | 240 | 0% |
| Sakamoto 2013 | Parallel | 2 | 6 | Japan | 1365 | 865 | 37% |
| Sales 2017 | Parallel | 2 | 12 | Australia | 66 | 48 | 27% |
| Siegrist 2016 | Cluster | 2 (40) | 12 | Germany | 378 | 378 | 0% |
| Skelton 2005 | Parallel | 2 | 9 | United Kingdom | 81 | 81 | 0% |
| Smulders 2010 | Parallel | 2 | 12 | Netherlands | 96 | 92 | 4% |
| Trombetti 2011 | Parallel | 2 | 6 | Switzerland | 134 | 134 | 0% |
| Weerdesteyn 2006 | Parallel | 2 | 7 | Netherlands | 58 | 58 | 0% |
| Wolf 1996 (balance group) | Parallel | 3 | 8 | USA | 200 | 200 | 0% |
| Yang 2012 | Parallel | 2 | 6 | Australia | 165 | 121 | 27% |
| Strength/resistance (including power) | |||||||
| Ansai 2015 (resistance group) | Parallel | 3 | 4 | Brazil | 69 | 68 | 1% |
| Carter 2002 | Parallel | 2 | 5 | Canada | 93 | 80 | 14% |
| Fiatarone 1997 | Parallel | 2 | 4 | USA | 34 | 0 | N/A |
| Grahn Kronhed 2009 | Parallel | 2 | 12 | Sweden | 65 | 65 | 0% |
| Karinkanta 2007 (resistance group) | Parallel | 4 | 12 | Finland | 149 | 144 | 3% |
| Latham 2003c | Parallel | 2 | 6 | New Zealand and Australia | 243 | 222 | 9% |
| Liu‐Ambrose 2004 (resistance group) | Parallel | 3 | 6 | Canada | 104 | 98 | 6% |
| Vogler 2009 (seated group)c | Parallel | 3 | 12 | Australia | 180 | 171 | 5% |
| Woo 2007 (resistance group) | Parallel | 3 | 12 | China | 180 | 176 | 33% |
| 3D | |||||||
| Day 2015 | Parallel | 2 | 12 | Australia | 503 | 409 | 19% |
| Huang 2010 | Cluster | 2 (4) | 5 | Taiwan | 115 | 78 | 32% |
| Li 2005 | Parallel | 2 | 6 | USA | 256 | 188 | 27% |
| Logghe 2009 | Parallel | 2 | 12 | Netherlands | 269 | 269 | 0% |
| Merom 2016 | Cluster | 2 (23) | 12 | Australia | 530 | 522 | 2% |
| Taylor 2012 | Parallel | 2 | 17 | New Zealand | 684 | 684 | 0% |
| Voukelatos 2007 | Parallel | 2 | 6 | Australia | 702 | 684 | 3% |
| Wolf 2003 | Cluster | 2 (20) | 11 | USA | 311 | 286 | 8% |
| Wolf 1996 (Tai Chi group) | Parallel | 3 | 8 | USA | 200 | 200 | 0% |
| Woo 2007 (Tai Chi group) | Parallel | 3 | 12 | China | 180 | 176 | 3% |
| Wu 2010 (com‐ex group) | Parallel | 3 | 4 | USA | 64 | 64 | 0% |
| Wu 2010 (home‐ex group) | Parallel | 3 | 4 | USA | 64 | 64 | 0% |
| Wu 2010 (tel‐ex group) | Parallel | 3 | 4 | USA | 64 | 64 | 0% |
| General physical activity | |||||||
| Ebrahim 1997 | Parallel | 2 | 24 | United Kingdom | 165 | 102 | 38% |
| Resnick 2002 | Parallel | 2 | 6 | USA | 20 | 17 | 15% |
| Voukelatos 2015 | Parallel | 2 | 12 | Australia | 386 | 339 | 12% |
| Multiple primary exercise categories | |||||||
| Ansai 2015 (multicomponent group)d | Parallel | 3 | 4 | Brazil | 69 | 68 | 1% |
| Beyer 2007d | Parallel | 2 | 12 | Denmark | 65 | 53 | 18% |
| Brown 2002d | Parallel | 2 | 14 | Australia | 99 | 71 | 28% |
| Buchner 1997 | Parallel | 2 | 25 | USA | 105 | 100 | 5% |
| Bunout 2005d | Parallel | 2 | 12 | Chile | 298 | 241 | 19% |
| Cerny 1998d | Parallel | 2 | 6 | USA | 28 | 28 | 0% |
| Clemson 2012 (structured group)d | Parallel | 3 | 12 | Australia | 317 | 317 | 0% |
| Gill 2016d | Parallel | 2 | 42 | USA | 1635 | 1635 | 0% |
| Haines 2009c,d | Parallel | 2 | 6 | Australia | 53 | 53 | 0% |
| Halvarsson 2016 (balance and physical activity group) | Parallel | 3 | 3 | Sweden | 96 | 69 | 28% |
| Hauer 2001d | Parallel | 2 | 6 | Germany | 57 | 56 | 2% |
| Irez 2011d | Parallel | 2 | 3 | Turkey | 60 | 60 | 0% |
| Kamide 2009d | Parallel | 2 | 6 | Japan | 57 | 43 | 25% |
| Karinkanta 2007 (resistance and balance groups)d | Parallel | 4 | 12 | Finland | 149 | 144 | 3% |
| Kim 2014d | Parallel | 2 | 12 | Japan | 105 | 103 | 2% |
| Lehtola 2000 | Parallel | 2 | 10 | Finland | 131 | 131 | 0% |
| Means 2005d | Parallel | 2 | 6 | USA | 338 | 238 | 30% |
| Ng 2015d | Parallel | 2 | 12 | Singapore | 98 | 92 | 6% |
| Park 2008 | Parallel | 2 | 11 | Korea | 50 | 45 | 10% |
| Rubenstein 2000 | Parallel | 2 | 3 | USA | 59 | 59 | 0% |
| Sherrington 2014c,d | Parallel | 2 | 12 | Australia | 340 | 340 | 0% |
| Suzuki 2004d | Parallel | 2 | 20 | Japan | 52 | 44 | 15% |
| Uusi‐Rasi 2015d | Parallel | 2 | 24 | Finland | 205 | 186 | 9% |
| Vogler 2009 (weightbearing group)c | Parallel | 3 | 12 | Australia | 180 | 171 | 5% |
| Exercise versus exercise | |||||||
| Ballard 2004 | Parallel | 2 | 16 | USA | 40 | 39 | 3% |
| Barker 2016 | Parallel | 2 | 6 | Australia | 53 | 44 | 17% |
| Clemson 2012 | Parallel | 3 | 12 | Australia | 317 | 286 | 10% |
| Davis 2011 | Parallel | 3 | 9 | Canada | 155 | 155 | 0% |
| Freiberger 2007 | Parallel | 2 | 24 | Germany | 134 | 127 | 5% |
| Helbostad 2004 | Parallel | 2 | 12 | Norway | 77 | 68 | 12% |
| Hwang 2016 | Parallel | 2 | 18 | Taiwan | 456 | 334 | 27% |
| Iliffe 2015 | Cluster | 3 (42) | 18 | United Kingdom | 1254 | 709 | 43% |
| Karinkanta 2007 | Parallel | 4 | 12 | Finland | 149 | 144 | 3% |
| Kemmler 2010 | Parallel | 2 | 18 | Germany | 246 | 227 | 8% |
| Kwok 2016 | Parallel | 2 | 12 | Singapore | 80 | 80 | 0% |
| Kyrdalen 2014 | Parallel | 2 | 3 | Norway | 125 | 94 | 25% |
| LaStayo 2017 | Parallel | 2 | 12 | USA | 134 | 112 | 16% |
| Liston 2014 | Parallel | 2 | 6 | United Kingdom | 21 | 15 | 29% |
| Liu‐Ambrose 2004 | Parallel | 3 | 6 | Canada | 104 | 98 | 6% |
| Lurie 2013 | Parallel | 2 | 3 | USA | 64 | 59 | 8% |
| Mirelman 2016 | Parallel | 2 | N/A | Belgium, Israel, Italy, Netherlands, and United Kingdom | 152 | 0 | N/A |
| Morone 2016 | Parallel | 2 | 3 | Italy | 38 | 0 | N/A |
| Morrison 2018 | Parallel | 2 | 3 | USA | 65 | 46 | 29% |
| Okubo 2016 | Parallel | 2 | 16 | Japan | 105 | 90 | 14% |
| Shigematsu 2008 | Parallel | 2 | 8 | Japan | 68 | 68 | 0% |
| Steadman 2003 | Parallel | 2 | 1 | United Kingdom | 199 | 133 | 33% |
| Taylor 2012 | Parallel | 2 | 17 | New Zealand | 684 | 684 | 0% |
| Verrusio 2017 | Parallel | 2 | 12 | Italy | 150 | 147 | 2% |
| Wolf 1996 | Parallel | 3 | 8 | USA | 200 | 200 | 0% |
| Yamada 2010 | Parallel | 2 | 12 | Japan | 60 | 58 | 3% |
| Yamada 2012 | Parallel | 2 | 12 | Japan | 157 | 145 | 8% |
| Yamada 2013 | Parallel | 2 | 12 | Japan | 264 | 230 | 13% |
a Categorised by primary exercise category. b Number analysed for fall data. c Post‐hospital discharge study. d Indicates the primary interventions include gait, balance, and functional training and strength/resistance training.
2. Key characteristics of participants and intervention approach.
| Study IDa | Age (mean) | % Women | High risk of falls | Duration of intervention (weeks) | Intervention delivered by health professional | Group exercise | Intervention progressed |
| Gait, balance, and functional training | |||||||
| Almeida 2013 | 79 | 83% | Yes | 16 | Yes | Yes | NR |
| Arantes 2015 | 73 | 100% | Yes | 12 | Yes | Yes | Yes |
| Arkkukangas 2015 | 83 | 71% | No | 12 | Yes | No | Yes |
| Barnett 2003 | 75 | 67% | Yes | 52 | No | Yes | Yes |
| Boongrid 2017 | 74 | 83% | Yes | 52 | Yes | No | Yes |
| Campbell 1997 | 84 | 100% | Yes | 52 | Yes | No | Yes |
| Clegg 2014 | 79 | 71% | Yes | 12 | Yes | No | Yes |
| Clemson 2010 | 81 | 47% | Yes | 26 | Yes | No | Yes |
| Clemson 2012 (Life Program) | 83 | 55% | Yes | 52 | Yes | No | Yes |
| Cornillon 2002 | 71 | 83% | No | 52 | No | No | No |
| Dadgari 2016 | 70 | 49% | Yes | 24 | No | No | Yes |
| Dangour 2011 | 66 | 68% | No | 104 | No | Yes | Yes |
| Day 2002 | 76 | 59% | No | 18 | No | Yes | No |
| Duque 2013 | 77 | 62% | Yes | 6 | Yes | No | Yes |
| El‐Khoury 2015 | 79 | 100% | Yes | 104 | No | Yes | Yes |
| Gschwind 2015 | 75 | 61% | No | 16 | No | No | Yes |
| Halvarsson 2013 | 77 | 71% | Yes | 12 | Yes | Yes | Yes |
| Halvarsson 2016 (balance group) | 76 | 98% | Yes | 12 | Yes | Yes | Yes |
| Hamrick 2017 | 70 | 79% | No | 8 | No | Yes | Yes |
| Hirase 2015 | 82 | 70% | Yes | 16 | Yes | No | No |
| Iliffe 2015 | 73 | 62% | No | 24 | No | OEP: no; FaME: Yes | Yes |
| Iwamoto 2009 | 76 | 90% | No | 20 | No | Yes | No |
| Karinkanta 2007 (balance group) | 73 | 100% | No | 52 | No | Yes | No |
| Kerse 2010 | 81 | 58% | No | 26 | No | No | Yes |
| Korpelainen 2006 | 73 | 100% | No | 130 | Yes | Yes | Yes |
| Kovacs 2013 | 69 | 100% | No | 25 | Yes | Yes | Yes |
| Lin 2007 | 77 | 51% | Yes | 16 | Yes | No | Yes |
| Liu‐Ambrose 2004 (agility group) | 79 | 100% | No | 25 | No | Yes | No |
| Liu‐Ambrose 2008 | 83 | 71% | Yes | 26 | Yes | No | Yes |
| Lord 1995 | 71 | 100% | No | 52 | No | Yes | No |
| Lord 2003 | 80 | 86% | No | 52 | No | Yes | No |
| Luukinen 2007 | 88 | 79% | Yes | 70 | Yes | No | Yes |
| Madureira 2007 | 74 | 100% | No | 40 | Yes | Yes | No |
| McMurdo 1997 | 65 | 100% | No | 60 | No | Yes | No |
| Miko 2017 | 69 | 100% | No | 52 | Yes | Yes | Yes |
| Morgan 2004 | 81 | 71% | Yes | 8 | Yes | Yes | Yes |
| Nitz 2004 | 76 | 92% | Yes | 10 | Yes | Yes | No |
| Reinsch 1992 | 74 | 80% | No | 52 | No | Yes | No |
| Robertson 2001a | 84 | 68% | No | 52 | Yes | No | Yes |
| Sakamoto 2013 | 80 | 82% | Yes | 26 | No | No | Yes |
| Sales 2017 | 73 | 69% | Yes | 18 | Both | Yes | Yes |
| Siegrist 2016 | 78 | 75% | Yes | 16 | Yes | Yes | Yes |
| Skelton 2005 | 72 | 100% | Yes | 36 | No | Yes | Yes |
| Smulders 2010 | 71 | 94% | Yes | 5.5 | Yes | Yes | Yes |
| Trombetti 2011 | 76 | 96% | Yes | 26 | No | Yes | Yes |
| Weerdesteyn 2006 | 74 | 77% | Yes | 5 | No | Yes | Yes |
| Wolf 1996 (balance group) | 76 | 81% | No | 15 | No | No | Yes |
| Yang 2012 | 81 | 44% | Yes | 26 | Yes | No | No |
| Strength/resistance (including power) | |||||||
| Ansai 2015 (resistance group) | 82 | 68% | Yes | 16 | No | Yes | Yes |
| Carter 2002 | 69 | 100% | No | 20 | No | Yes | No |
| Fiatarone 1997 | 82 | 94% | Yes | 16 | No | No | No |
| Grahn Kronhed 2009 | 71 | 100% | No | 16 | Yes | Yes | Yes |
| Karinkanta 2007 (resistance group) | 73 | 100% | No | 52 | No | Yes | Yes |
| Latham 2003b | 80 | 53% | Yes | 10 | Yes | No | Yes |
| Liu‐Ambrose 2004 (resistance group) | 79 | 100% | No | 25 | No | Yes | Yes |
| Vogler 2009 (seated group) | 80 | 79% | Yes | 12 | Yes | No | Yes |
| Woo 2007 (resistance group) | 69 | 50% | No | 52 | No | Yes | No |
| 3D | |||||||
| Day 2015 | 77 | 70% | Yes | 48 | No | Yes | Yes |
| Huang 2010 | 71 | 30% | No | 22 | No | Yes | No |
| Li 2005 | 77 | 70% | No | 26 | No | Yes | No |
| Logghe 2009 | 77 | 71% | Yes | 13 | No | Yes | No |
| Merom 2016 | 85% | No | 52 | No | Yes | Yes | |
| Taylor 2012 | 75 | 73% | Yes | 20 | No | Yes | No |
| Voukelatos 2007 | 69 | 84% | No | 16 | No | Yes | No |
| Wolf 1996 (Tai Chi group) | 76 | 81% | No | 15 | No | Yes | Yes |
| Wolf 2003 | 81 | 94% | Yes | 48 | No | Yes | Yes |
| Woo 2007 (Tai Chi group) | 69 | 50% | No | 52 | No | Yes | No |
| Wu 2010 (com‐ex group) | 75 | 84% | Yes | 15 | No | Yes | No |
| Wu 2010 (home‐ex group) | 75 | 84% | Yes | 15 | No | No | No |
| Wu 2010 (tel‐ex group) | 75 | 84% | Yes | 15 | No | No | No |
| General physical activity | |||||||
| Ebrahim 1997 | 67 | 100% | No | 104 | Yes | No | Yes |
| Resnick 2002 | 88 | 100% | No | 26 | No | Yes | Yes |
| Voukelatos 2015 | 73 | 74% | No | 48 | No | No | No |
| Multiple primary exercise categories | |||||||
| Ansai 2015 (multicomponent group)c | 82 | 68% | Yes | 16 | No | Yes | Yes |
| Beyer 2007c | 78 | 100% | Yes | 26 | Yes | Yes | Yes |
| Brown 2002c | 79% | No | 16 | Yes | Yes | Yes | |
| Buchner 1997 | 75 | 51% | Yes | 25 | No | Yes | Yes |
| Bunout 2005c | 75 | 70% | No | 52 | No | Yes | Yes |
| Cerny 1998c | 71 | No | 24 | No | Yes | NR | |
| Clemson 2012 (structured group)c | 83 | 55% | Yes | 52 | Yes | No | Yes |
| Gill 2016c | 79 | 67% | Yes | 96 | No | Yes | Yes |
| Haines 2009b,c | 81 | 60% | Yes | 8 | Yes | No | Yes |
| Halvarsson 2016 (balance and physical activity group) | 76 | 98% | Yes | 12 | Yes | Yes | Yes |
| Hauer 2001c | 82 | 100% | Yes | 12 | Yes | Yes | Yes |
| Irez 2011c | 75 | 100% | No | 12 | No | Yes | Yes |
| Kamide 2009c | 71 | 100% | No | 26 | Yes | No | No |
| Karinkanta 2007 (resistance and balance groups)c | 73 | 100% | No | 52 | No | Yes | Yes |
| Kim 2014c | 78 | 100% | Yes | 52 | No | Yes | Yes |
| Lehtola 2000 | 74 | 80% | No | 26 | No | Yes | Yes |
| Means 2005c | 74 | 57% | No | 6 | Yes | Yes | Yes |
| Ng 2015c | 70 | 61% | Yes | 12 | No | Yes | Yes |
| Park 2008 | 68 | 100% | No | 48 | NR | Yes | No |
| Rubenstein 2000 | 75 | 0% | Yes | 12 | No | Yes | Yes |
| Sherrington 2014b,c | 81 | 74% | Yes | 52 | Yes | No | Yes |
| Suzuki 2004c | 78 | 100% | No | 26 | No | Yes | No |
| Uusi‐Rasi 2015c | 74 | 100% | Yes | 104 | Yes | Yes | Yes |
| Vogler 2009b (weightbearing group) | 80 | 79% | Yes | 12 | Yes | No | Yes |
| Exercise versus exercise | |||||||
| Ballard 2004 | 73 | 100% | Yes | 15 (Low intensity = 2) | No | Yes | NR |
| Barker 2016 | 69 | 88% | Yes | 12 | Yes | Pilates group: Yes; HEP group: No | Yes |
| Clemson 2012 | 83 | 55% | Yes | 52 | Yes | No | Yes |
| Davis 2011 | 78 | 100% | No | 52 | No | Yes | No |
| Freiberger 2007 | 76 | 44% | Yes | 16 | No | No | Yes |
| Helbostad 2004 | 81 | 81% | Yes | Yes | Yes | Combined training:No; Home training: Yes. | |
| Hirase 2015 | 82 | 70% | Yes | 16 | Yes | No | No |
| Hwang 2016 | 72 | 67% | Yes | 24 | Tai Chi: No; other group: Yes | No | Yes |
| Karinkanta 2007 | 73 | 100% | No | 52 | No | Yes | Yes |
| Kemmler 2010 | 69 | 100% | No | 78 | No | Yes | High intensity: Yes; low intensity: No |
| Kwok 2016 | 70 | 85% | Yes | 12 | Yes | Yes | Yes |
| Kyrdalen 2014 | 83 | 73% | Yes | 12 | Yes | Group: Yes; Home: No | Yes |
| LaStayo 2017 | 76 | 65% | Yes | 12 | Yes | Yes | Yes |
| Liston 2014 | 77 | 85% | Yes | 8 | Yes | Yes | OEP: Yes; Stretching: No. |
| Liu‐Ambrose 2004 | 79 | 100% | No | 25 | No | Yes | Yes |
| Lurie 2013 | 80 | 59% | Yes | Variable | Yes | No | Yes |
| Mirelman 2016 | 83 | 35% | Yes | 6 | No | No | Yes |
| Morone 2016 | 69 | 100% | Yes | 8 | Yes | Yes | No |
| Morrison 2018 | 67 | 48% | No | 12 | No | Balance: Yes; Wii: No | Balance: No; Wii: Yes |
| Okubo 2016 | 71 | 63% | No | 64 | No | Yes | Yes |
| Shigematsu 2008 | 69 | 63% | No | 12 | No | Yes | No |
| Steadman 2003 | 83 | 82% | Yes | 6 | Yes | Yes | Yes |
| Taylor 2012 | 75 | 73% | Yes | 20 | No | Yes | No |
| Verrusio 2017 | 65 | 53% | Yes | 52 | Yes | No | NR |
| Wolf 1996 | 76 | 81% | No | 15 | No | Yes | Yes |
| Yamada 2010 | 80 | Unknown | No | Yes | Yes | Yes | |
| Yamada 2012 | 86 | 81% | No | 24 | Yes | Yes | Yes |
| Yamada 2013 | 77 | 57% | No | 24 | No | No | Yes |
a Categorised by primary exercise category. b Post‐hospital discharge study. c Indicates the primary interventions include gait, balance, and functional training and strength/resistance training.
We contacted authors of 49 included studies to request additional details regarding study design and outcome data and received responses for 26 trials; this resulted in additional information that is used in the review for 10 studies (Arkkukangas 2015; Clegg 2014; Dadgari 2016; Hamrick 2017; Kerse 2010; Kovacs 2013; Lord 2003; Morrison 2018; Sales 2017; Siegrist 2016). Trialists of the other 16 studies either reported they had no data to supply or they supplied data that could not be used in the review (Ansai 2015; Beyer 2007; Cerny 1998; Dangour 2011; Davis 2011; Duque 2013; Gschwind 2015; Huang 2010; Kyrdalen 2014; LaStayo 2017; Lurie 2013; Morgan 2004; Morone 2016; Okubo 2016; Park 2008; Resnick 2002). This account does not include the studies for which further information or data were sought or supplied regarding trials included in Gillespie 2012.
Trial design
All included studies were randomised controlled trials (RCTs). The majority of trials were individually randomised and nine were cluster randomised; either by unit of residence (Huang 2010; Lord 2003; Merom 2016; Wolf 2003), health centre (Dadgari 2016; Dangour 2011; Iliffe 2015; Siegrist 2016), or senior centre (Reinsch 1992). The included trials had 230 groups. Most trials (n = 95) had two groups included in this review (usually intervention and control), 10 studies had three groups (two intervention and one control: Almeida 2013; Ansai 2015; Clemson 2012; Halvarsson 2016; Hirase 2015; Iliffe 2015; Liu‐Ambrose 2004; Vogler 2009; Wolf 1996; Woo 2007; all intervention: Davis 2011; Wu 2010), and one study had four groups (3 intervention, 1 control) (Karinkanta 2007).
Trial size
The median number of participants randomised per trial was 134 (interquartile range (IQR) 65 to 262). The trials ranged in sample size from 20 participants in Resnick 2002 to 1635 participants in Gill 2016.
Trial setting
The included trials were carried out in 25 countries, the most common being Australia (19 trials), USA (18 trials), Japan (11 trials), the UK (7 trials), Finland (5 trials), Brazil (4 trials), Canada (4 trials), Germany (4 trials), New Zealand (4 trials), Sweden (4 trials), the Netherlands (3 trials), and Taiwan (3 trials). The remaining trials were conducted in Chile (2 trials), France (2 trials), Hungary (2 trials), Italy (2 trials), Norway (2 trials), Singapore (2 trials), China (1 trial), Denmark (1 trial), Iran (1 trial), Korea (1 trial), Switzerland (1 trial), Thailand (1 trial) and Turkey (1 trial). Of the three multinational trials, Gschwind 2015 included participants in Germany, Spain and Australia; Mirelman 2016 recruited from Belgium, Israel, Italy, Netherlands and the UK and Latham 2003 from Australia and New Zealand. See Appendix 4.
Participants
There were 23,407 participants randomised and 20,007 with fall data at follow‐up. Overall, 77% of included participants were women. All participants were women in 28 trials (see Appendix 4), and men in one trial (Rubenstein 2000). The average participant age in the included trials was 76 years. The inclusion/exclusion criteria and other participant details are listed for each study in the Characteristics of included studies.
Sixteen trials (15%) would have been excluded if the review inclusion criteria had been set at 65+ years of age (see Appendix 4).
Sixty included studies (56%) specified a history of falling or evidence of one or more risk factors for falling in their inclusion criteria (see Appendix 4).
Seventy‐two trials (67%) excluded participants with cognitive impairment, either defined as an exclusion criterion or implied by the stated requirement to be able to give informed consent and/or to follow instructions (see Appendix 4).
Four trials (4%) only included people who had recently been discharged from hospital (Haines 2009; Latham 2003; Sherrington 2014; Vogler 2009). It is possible other trials also included some participants who had been recently discharged from hospital or the emergency department, however this was not quantified.
Interventions
Exercise was compared with a control intervention (one that is not thought to reduce falls, such as general health education, social visits, very gentle exercise, or 'sham' exercise) in 81 trials (19,684 participants) in people not recently discharged from hospital, and four trials (816 participants) in people who were recently discharged from hospital (Haines 2009; Latham 2003; Sherrington 2014; Vogler 2009). Twenty‐three trials, with 3527 participants, compared the effect of different types of exercise in people not recently discharged from hospital, and one trial (180 participants) compared the effect of different types of exercise in the post‐hospital population (Vogler 2009). Four trials (1021 participants) compared group versus individual exercise (Barker 2016; Helbostad 2004; Iliffe 2015; Kyrdalen 2014), and three trials (879 participants) compared high‐ versus low‐dose exercise (Ballard 2004; Davis 2011; Taylor 2012); see Appendix 4).
When interventions are grouped by the type of intervention (descriptors), as described in Data synthesis, there were 230 groups; 146 intervention arms and 84 control arms. There were 13 multiarm studies included in the review; 12 trials had three arms (Almeida 2013; Ansai 2015; Clemson 2012; Davis 2011; Halvarsson 2016; Hirase 2015; Iliffe 2015; Liu‐Ambrose 2004; Vogler 2009; Wolf 1996; Woo 2007; Wu 2010), and one trial had four arms (Karinkanta 2007). Buchner 1997 had four arms; however, because fall data were not available for individual intervention groups we made an a priori decision to report fall outcomes for all three exercise groups combined compared with control group. In 76 (52%) intervention arms, the exercise intervention was delivered in a group setting; in 43 (29%) intervention arms, it was delivered individually; and 27 (18%) intervention arms involved a combination of group‐based and individual exercise (see Appendix 4). In 67 (46%) intervention arms, the intervention was delivered by a health professional; in the 77 (53%) intervention arms where the intervention was not delivered by a trained health professional, personnel included trained physical educators, trained exercise leaders and Tai Chi instructors; in one intervention arm, the intervention was delivered by both types of personnel (Sales 2017); and in one trial the personnel were not specified (Park 2008).
The intervention arms were grouped by their primary exercise modality into six categories (Appendix 5) using the ProFaNE taxonomy (Appendix 1).
Most intervention arms (n = 78; 53%) included balance and functional exercises as the primary intervention (ProFaNE taxonomy code gait/balance/co‐ordination/functional task training).
Strength/resistance training was the primary component of 9 (6%) intervention arms.
Flexibility training was the primary component of one (1%) intervention arms.
3D training (constant repetitive movement through all three spatial planes) was the primary component of 15 (10%) intervention arms.
General physical activity (walking groups) was the primary component of 6 (4%) intervention arms.
Endurance training alone was the primary component of one (1%) intervention arm.
Multiple categories of ProFaNE taxonomy were the primary intervention in 37 (25%) intervention arms. The majority (n = 19, 51%) of these intervention arms included balance and functional exercise as well as resistance training.
The number of studies, and how many of these are cluster‐RCTs, for the main exercise versus control comparison for each primary exercise category is summarised below, with further details including numbers of participants presented in Table 10, and associated study IDs in Appendix 6 (all trials) and Appendix 7 (trials contributing data to the rate of falls analysis). Note that these do not include the four post‐hospital discharge RCTs.
3. Numbers of studies and participants included in the exercise versus control comparison for each primary exercise category.
| Comparisona | Number of trials (cluster) b | Number of participants randomised | Number of participants analysed for any one outcome | Number of trials (cluster) with participants analysed for rate of falls outcome c,d | Number of participants analysed for rate of falls outcome d |
| Exercise (all types) versus control | 81 (9) | 19684 | 13518 | 59 (6) | 12,981 |
| Balance and functional exercises versus control | 48 (6) | 11860 | 8288 | 39 (4) | 7920 |
| Resistance exercises versus control | 7 | 694 | 327 | 5 | 327 |
| Flexibility versus control | 0 | 0 | 0 | 0 | 0 |
| 3D exercise (Tai Chi) versus control | 10 (2) | 3284 | 2677 | 7 (1) | 2655 |
| 3D exercise (dance) versus control | 1 (1) | 530 | 522 | 1 (1) | 522 |
| General physical activity (walking programme) versus control | 3 | 571 | 441 | 2 | 441 |
| Endurance training versus control | 0 | 0 | 0 | 0 | 0 |
| Other kinds of exercise versus control | 0 | 0 | 0 | 0 | 0 |
| Multiple categories of exercise versus control | 21 | 4073 | 1623 | 11 | 1374 |
aExercise (all types) combines all categories of exercise. Multiple categories of exercise include studies containing two or more primary categories of exercise, as categorised using the ProFaNE taxonomy. The remaining analyses include only one primary category of exercise, as categorised using the ProFaNE taxonomy. bStudy IDs are shown in Appendix 6. cStudy IDs are shown in Appendix 7. dThese data apply to the follow‐up (at the time point included in main analysis) for the primary outcome (rate of falls) for the individual trials.
Exercise (all types) versus control: 81 RCTs (9 cluster‐RCTs).
Balance and functional exercises versus control: 48 RCTs (6 cluster‐RCTs).
Resistance exercises versus control: 7 RCTs.
Flexibility versus control: 0 RCTs.
3D exercise (Tai Chi) versus control: 10 RCTs (2 cluster‐RCTs).
3D exercise (dance) versus control: 1 RCTs (1 cluster‐RCT).
General physical activity (walking programme) versus control: 3 RCTs.
Endurance training versus control: 0 RCTs.
Other kinds of exercise versus control: 0 RCTs.
Multiple categories of exercise versus control: 21 RCTs.
The duration of the exercise intervention in these 81 trials ranged from 5 to 130 weeks; it was one year or more in 24 trials (30%) and two years or more in five trials (6%) (Table 9).
Additional details of the number of studies and number of participants included in the primary analysis (exercise versus control on rate of falls) for each primary category of exercise are shown in Appendix 8.
Outcomes
The source of data used for calculating outcomes for each trial for generic inverse variance analysis is shown in Appendix 9. Rate of falls was reported in 34 trials, and could be calculated from a further 43 trials. Data on risk of falling (number of fallers) were available in 17 trials and could be calculated for a further 61. Raw data for rate of falls and number of fallers, when available, are shown in Appendix 10. Six trials met our inclusion criteria but did not include data that could be included in these analyses (Almeida 2013; Fiatarone 1997; Mirelman 2016; Morone 2016; Morrison 2018; Resnick 2002). Two of these trials contained inadequate data to include in an analysis (Fiatarone 1997; Resnick 2002), but reported no significant between‐group difference in number of falls, and two trials reported zero falls in each group (Almeida 2013; Morrison 2018). Morone 2016 did not present fall data, but found balance training using Wii‐fit may have a greater effect on balance outcomes compared with conventional balance training. Mirelman 2016 found treadmill plus virtual reality training may be more effective in preventing falls than treadmill alone, six months after the end of a six‐week training period. The raw data for non‐fall outcomes for these studies are shown in Appendix 11.
Eleven trials reported a fracture outcome, two trials reported number of falls requiring hospitalisation, and five trials reported the number of people experiencing a fall requiring medical attention. Death was recorded in 40 trials and was listed as a reason for loss to follow‐up in all of these trials except Wolf 2003, which also assessed death as an adverse event. Deaths were not reported by group in two trials (Day 2002; Lord 1995; Appendix 12). None of the deaths were explicitly linked to the trial participation.
Adverse events
Two trials, including one in the post‐hospital population, measured the number of people experiencing adverse events in both groups throughout the trial period (Iliffe 2015; Latham 2003). No other studies reported adverse events that were monitored closely in all groups over the entire study period. Adverse events reported to any degree are described in Appendix 13. Adverse events were reported to a degree in the intervention and control groups in 16 trials, in the intervention group only in 13 trials, in two intervention groups in seven trials, and in two intervention plus control group in five trials.
Adherence
Adherence was measured in 78 studies and adherence data were reported in 77 studies (Appendix 14). The measures used to quantify adherence varied: the majority of studies summarised proportion of classes attended (n = 53) or proportion of scheduled sessions completed (n = 20), three studies quantified the amount of exercise performed (Boongrid 2017; Okubo 2016; Sherrington 2014), and two studies described the proportion of participants who started the programme (El‐Khoury 2015; Skelton 2005).
Excluded studies
We eliminated 253 reports on full‐text review. We retained 21 studies (23 reports) as excluded studies as they initially appeared to meet the inclusion criteria but were subsequently excluded (see Excluded studies for links to references, and the Characteristics of excluded studies and Appendix 15 for details). Of the identified trials:
one trial did not meet the review's inclusion criterion for age (Pereira 1998);
one trial included participants with a particular clinical condition that increases the risk of falls (Hsu 2017);
one trial included participants who were not community‐dwelling (DeSure 2013);
15 trials did not involve exercise as a single intervention;
one trial included an ineligible comparator (Ohtake 2013);
one trial did not measure falls (Hinrichs 2016);
one trial withdrew three of the six fallers from the study because the falls resulted in injuries (Morris 2008).
Studies awaiting classification
Two studies are awaiting classification. Li 2018b is a large study (n = 670) comparing the effect of Tai Ji Quan, multimodal exercise and stretching in older people at high risk of falls. The other is a small (n = 6) study (Jagdhane 2016).
Ongoing studies
We identified 16 ongoing trials (see the Characteristics of ongoing studies). Seven trials are currently open to recruitment CTRI/2018/01/011214; NCT02617303; NCT02926105; NCT03211429; NCT03320668; NCT03417531; NCT03462654), and nine are ongoing but no longer recruiting (ACTRN 12613001161718; ACTRN 12615000138583; ACTRN 12615000865516; ISRCTN71002650; NCT01029171; NCT02126488; NCT02287740; NCT03404830; NCT03455179).
The median target sample size is 402 (IQR 280‐670) and two of the ongoing trials are cluster randomised (ACTRN 12613001161718; ISRCTN71002650). Half of the trials (8/16, 50%) specify increased fall‐risk as an inclusion criterion. Eight studies are investigating the effect of a programme of multiple categories of exercise (ACTRN 12615000865516; CTRI/2018/01/011214; ISRCTN71002650; NCT01029171; NCT02287740; NCT02617303; NCT02926105; NCT03455179), including four using the Otago Exercise Program (ACTRN 12615000865516; NCT01029171; NCT02617303; NCT02926105). There are three trials on resistance training (ACTRN 12613001161718; NCT03404830; NCT03455179), one on Tai Chi (NCT03211429), one on balance training (ACTRN 12615000138583), and a study evaluating slip training on the treadmill (NCT02126488). Two studies compare group versus individual delivery, using the LiFE Program (NCT03462654) and Otago Exercise Program (NCT03320668). There are no studies investigating the effect of flexibility training, general physical activity or endurance training alone.
Risk of bias in included studies
Details of the 'Risk of bias' assessment across all included trials and for each individual item in the included trials are shown in Characteristics of included studies, Figure 2 and Figure 3.
2.
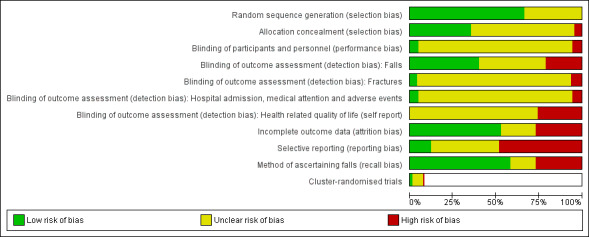
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
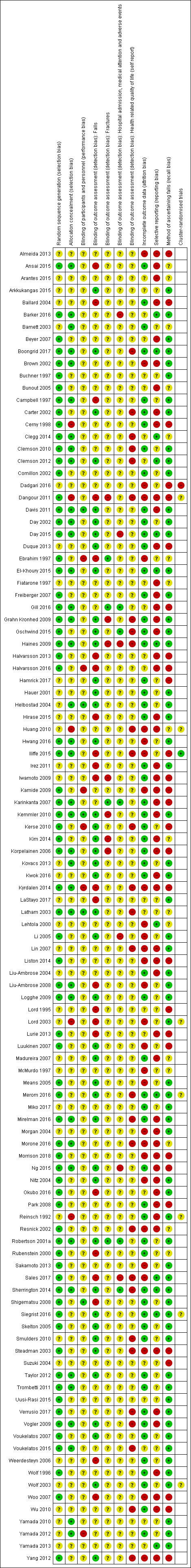
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged the risk of bias in generation of the allocation sequence as low in 67% (n = 72/108) of trials, unclear in 33% (n = 36/108) and high in zero trials. We assessed the methods of concealment of the allocation prior to group assignment as low risk of bias in 35% (n = 38/108), unclear in 60% (n = 65/108) and high in the remaining 5% (5/108) of trials (Cerny 1998; Dangour 2011; Huang 2010; Lord 2003; Reinsch 1992).
Blinding
Blinding of participants and personnel
In the majority of studies (90%, n = 97/108) it was not possible to blind the personnel and participants to group allocation. As the likelihood of awareness of group allocation introducing performance bias was not clear, we assessed the risk of bias for non‐blinding as unclear for these trials. We judged the impact of performance bias as low in 5% (n = 5/108) of trials, unclear in 89% (97/108) of trials and high in 6% (6/108) of trials.
Blinding of outcome assessment
We assessed the risk of bias for blinding of outcome assessment separately for the following outcomes.
-
Rate of falls and risk of falling
We judged the risk of detection bias in relation to the methods of ascertainment of the rate and/or risk of falls to be low in 40% (n = 43/108), high in 21% (n = 23/108) and unclear in 39% (n = 42/108) of the included trials.
-
Risk of fractures
In trials reporting on the risk of fracture, we assessed the risk of bias for blinding of outcome assessment for the rate of fractures. We judged the risk of detection bias in relation to the methods of ascertainment of fractures to be low in 20% (n = 4/20), high in 35% (n = 7/20) and unclear in 45% (n = 9/20) of the included trials that measured fractures.
-
Requiring hospital admission/medical attention, adverse events
In trials reporting on the risk of hospital admission and/or requiring medical attention and/or adverse events, we judged the risk of detection bias in relation to the method of ascertainment of these outcomes to be low in 15% (5/33) of trials, unclear in 67% (22/33) and high in 18% (6/33) of trials.
-
Health‐related quality of life
In trials reporting on health‐related quality of life we judged the risk of detection bias in relation to the method of ascertainment of health‐related quality of life to be high in all studies (23/23), due to participants in these studies being unblinded to their allocated group and health‐related quality of life being a self‐reported outcome.
Incomplete outcome data
We judged the risk of bias due to incomplete outcome data to be low in 53% (n = 57/108), unclear in 20% (n = 22/108) and high in the remaining 27% of trials (n = 29/108).
Selective reporting
We assessed the risk of bias due to selective reporting of falls outcomes as low in 12% (n = 13/108) of studies, unclear in 40% (n = 43/108) and high in 48% (52/108).
Other potential sources of bias
Bias in the recall of falls due to less reliable methods of ascertainment
We assessed 58% of included studies (n = 63/108) as being at low risk of bias in the recall of falls (i.e. falls were recorded concurrently using recommended methods of monthly diaries or postcards). We judged the risk of bias to be high in 27% of trials (n = 29/108), in that ascertainment of falling episodes was by participant recall, at intervals during the study or at its conclusion. In 15% of trials (n = 16/108) the risk of bias was unclear, as retrospective recall was for a short period only, or details of ascertainment were not described.
Bias due to cluster‐randomisation
We assessed the nine cluster‐randomised trials for risk of bias associated with recruitment methods, baseline imbalance, loss of clusters, incorrect analysis and comparability with individually‐randomised trials. We judged the risk of bias due to factors associated with cluster‐randomised trials to be low in one (11%) trial, unclear in seven trials (78%) and high in the remaining trial (11%, Dadgari 2016).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Summary of findings for the main comparison. Summary of findings: exercise (all types) versus control (e.g. usual activities).
| Exercise (all types) versus control (e.g. usual activities) for preventing falls in older people living in the community | ||||||
|
Patient or population: Older people living in the community (trials focusing on people recently discharged from hospital were not included) Settings: Community, either at home or in places of residence that, on the whole, do not provide residential health‐related care Intervention: Exercise of all typesa Comparison: Usual care (no change in usual activities) or a control (non‐active) interventionb | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Exercise (all types) | |||||
| Rate of falls (falls per person‐years) Follow‐up: range 3 to 30 months |
All studies population |
Rate ratio 0.77 (0.71 to 0.83)d |
12,981 (59 RCTs) | ⊕⊕⊕⊕ highe | Overall, there is a reduction of 23% (95% CI 17% to 29%) in the number of falls Guide to the data: If 1000 people were followed over 1 year, the number of falls in the overall population would be 655 (95% CI 604 to 706) compared with 850 in the group receiving usual care or attention control. In the unselected population, the corresponding data are 466 (95% CI 430 to 503) compared with 605 in the group receiving usual care or attention control. In the selected higher‐risk population, the corresponding data are 924 (95% CI 852 to 996) compared with 1200 in the control group |
|
| 850 per 1000c | 655 per 1000 (604 to 706) | |||||
| Not selected for high risk population | ||||||
| 605 per 1000c | 466 per 1000 (430 to 503) | |||||
| Selected for high risk population | ||||||
| 1200 per 1000c | 924 per 1000 (852 to 996) | |||||
| Number of people who experienced one or more falls Follow‐up: range 3 to 25 months |
All studies population | RR 0.85 (0.81 to 0.89)g | 13,518 (63 RCTs) | ⊕⊕⊕⊕ highe | Overall, there is a reduction of 15% (95% CI 11% to 19%) in the number of people who experienced one or more falls Guide to the data: If 1000 people were followed over 1 year, the number of people who experienced one or more falls in the unselected population would be 408 (95% CI 389 to 428) compared with 480 in the group receiving usual care or attention control. In the unselected population, the corresponding data are 323 (95% CI 308 to 339) compared with 380 in the group receiving usual care or attention control. In the selected higher‐risk population, the corresponding data are 425 (95% CI 405 to 445) compared with 500 in the control group. |
|
| 480 per 1000f | 408 per 1000 (389 to 428) | |||||
| Not selected for high risk population | ||||||
| 380 per 1000f | 323 per 1000 (308 to 339) | |||||
| Selected for high risk population | ||||||
| 500 per 1000f | 425 per 1000 (405 to 445) | |||||
| Number of people who experienced one or more fall‐related fractures Follow‐up: range 4 to 42 months |
All studies populationh | RR 0.73 (0.56 to 0.95) | 4047 (10 RCTs) | ⊕⊕⊝⊝ lowi | Overall, there may be a reduction of 27% (95% CI 5% to 44%) in the number of people who experienced one or more fall‐related fractures Guide to the data: If 1000 people were followed over 1 year, the number of people who experienced one or more fall‐related fractures may be 47 (95% CI 36 to 61) compared with 64 in the control group |
|
| 64 per 1000 | 47 per 1000 (36 to 61) | |||||
| Number of people who experienced one of more falls that resulted in hospital admission Follow‐up: range 3 to 42 months |
All studies populationh | RR 0.78 (0.51 to 1.18) | 1705 (2 RCTs) |
⊕⊝⊝⊝ very lowj | The evidence is very low certainty, hence we are uncertain of the findings of a reduction of 22% (95% CI 49% reduction to 18% increase) in the number of people who experienced one or more falls that required hospital admission. Of note is that the 95% CI includes the possibility of both reduced and increased hospitalisation. Guide to the data: If 1000 people were followed over 1 year, the number of people who experience one or more falls that required hospital admission in the general risk population may be 45 (95% CI 30 to 68) compared with 57 in the group receiving usual care or attention control |
|
| 57 per 1000 | 45 per 1000 (29 to 68) | |||||
| Number of people who experienced one or more falls that required medical attention. Follow‐up: range 6 to 24 months |
All studies populationh | RR 0.61 (0.47 to 0.79) | 1019 (5 RCTs) |
⊕⊕⊝⊝ lowk | Overall, there may be a reduction of 39% (95% CI 21% to 53%) in the number of people who experienced one or more falls that required medical attention Guide to the data: If 1000 people were followed over 1 year, the number of people who experienced one or more falls that required medical attention may be 129 (95% CI 100 to 167) compared with 211 in the group receiving usual care or attention control |
|
| 211 per 1000 | 129 per 1000 (100 to 167) | |||||
| Health‐related quality of life Follow‐up: range 3 to 24 months (A higher score indicates better quality of life) |
‐ | The mean health‐related quality of life score in the intervention groups was 0.03 standard deviations lower (0.10 lower to 0.04 higher) | ‐ | 3172 (15 RCTs) |
⊕⊕⊝⊝ lowl | SMD was calculated from 4 trials with EQ‐5D, 5 trials with SF‐36, 3 trials with SF12, 1 trial with QUALEFFO‐41, 1 trial with WHOQOL‐BREF, and 1 with Assessment of QOL EQ‐5D: Mean difference = −0.0026 (95% CI −0.0086 to 0.0034). SMD was converted back to MD using EQ‐5D scale (0 to 1), based on data for 4 trials (6 comparisons) reporting endpoint scores.m MID for the EQ‐5D is typically 0.074 (Walters 2005) SF36: Mean difference = −0.36 (95% CI −1.20 to 0.48). SMD was converted back to MD using SF‐36 scale, based on data for 5 trials.m MID for the SF‐36 is typically 3 to 5 ( Walters 2003) |
| Adverse events | See comment | Not estimable | 6019 (27 RCTs) |
⊕⊝⊝⊝n very low | Adverse events were reported to various degrees, but predominantly in the intervention groups, in the 27 RCTs, 14 of which reported no adverse events. Aside from 2 serious adverse events (1 pelvic stress fracture and 1 inguinal hernia surgery) reported in 1 trial, the rest were non‐serious adverse events, primarily of a musculoskeletal nature. There was a median of 3 events (range 1 to 26) in the exercise groups | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MID: minimally important difference; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aExercise is a physical activity that is planned, structured and repetitive and aims to improve or maintain physical fitness. There is a wide range of possible types of exercise, and exercise programmes often include one or more types of exercise. We categorised exercise based on the Prevention of Falls Network Europe (ProFaNE) taxonomy that classifies exercise type as: i) gait, balance, and functional [task] training; ii) strength/resistance (including power); iii) flexibility; iv) three‐dimensional (3D) exercise (e.g. Tai Chi, Qigong, dance); v) general physical activity; vi) endurance; and vii) other kind of exercises. The taxonomy allows for more than one type of exercise to be delivered within a programme. bA control intervention is one that is not thought to reduce falls, such as general health education, social visits, very gentle exercise, or 'sham' exercise not expected to impact on falls. cThe all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 59 RCTs. We calculated the risk in the control group using the median falls per person‐year for the subgroups of trials for which a) an increased risk of falls was not an inclusion criterion (29 RCTs, 6123 participants), or b) increased risk of falls was an inclusion criterion (30 RCTs, 6858 participants). dSubgroup analysis found no difference based on whether risk of falls was an inclusion criterion or not (test for subgroup differences: Chi2 = 0.90, df = 1, P = 0.34, I2 = 0%). eThere was no downgrading, including for risk of bias, as results were essentially unchanged with removal of the trials with a high risk of bias on one or more items. fThe all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 63 RCTs. We calculated the risk in the control group using the median proportion of fallers for the subgroups of trials for which a) an increased risk of falls was not an inclusion criterion (28 RCTs, 6347 participants), or b) increased risk of falls was an inclusion criterion (35 RCTs, 7171 participants). gSubgroup analysis found no difference based on whether risk of falls was an inclusion criterion or not (test for subgroup differences: Chi2 = 0.94, df = 1, P = 0.33, I2 = 0%). hWe calculated the risk in the control group based on the number of events and the number of participants in the control group for this outcome. i Downgraded by two levels due to imprecision (few events and wide CI due to small sample size), and risk of publication bias (likelihood of reporting fractures only if there was a treatment effect; with some indication on viewing the funnel plot). jDowngraded by two levels due to imprecision (low event rate and wide confidence intervals) and because most of the 81 studies included in the review for this comparison do not contribute to the outcome. We further downgraded the evidence by one level for risk of bias because the evidence was dominated by one trial that was at high risk of bias in one or more items. kDowngraded by two levels due to imprecision and the high probability of publication bias (only 5 of 89 RCTs included in the review reported the outcome). We did not downgrade for risk of bias as results were essentially unchanged with removal of the trials at a high risk of bias in one or more items. lDowngraded by two levels due to inconsistency (there was considerable heterogeneity (I² = 76%)) and risk of bias (removing studies with high risk of bias in one or more items had a marked impact on results). mIn order to express the MD in the unit‐specific measurement instruments (ED‐5D and SF‐36), we multiplied the SMD by a typical among‐person standard deviation for that scale, using the pooled standard deviation of baseline scores in the largest study in the analysis. For EQ‐5D, Iliffe 2015 has a combined SD of 0.086; for SF36, Dangour 2011 has combined SD of 12.04. nDowngraded by three levels due to limitations in design of studies, suggesting a very serious risk of bias and incomplete data. Only one trial measured the number of people experiencing adverse events in both groups throughout the trial period (Iliffe 2015).
Summary of findings 2. Summary of findings: balance and functional exercises versus control (e.g. usual activities).
| Balance, and functional exercises versus control (e.g. usual activities) for preventing falls in older people in the community | ||||||
|
Patient or population: Older people living in the community (trials focusing on people recently discharged from hospital were not included) Settings: Community, either at home or in places of residence that, on the whole, do not provide residential health‐related care Intervention: Exercise, type = gait, balance, and functional (task) traininga Comparison: Usual care (no change in usual activities) or a control (non‐active) interventionb | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Exercise (gait, balance, and functional [task] training) | |||||
| Rate of falls (falls per person‐years) Follow‐up: range 3 to 30 months | All studies population | Rate ratio 0.76 (0.70 to 0.81) | 7920 (39 RCTs) |
⊕⊕⊕⊕d high | Overall, there is a reduction of 24% (95% CI 19% to 30%) in the number of falls Guide to the data based on the all‐studies estimate. If 1000 people were followed over 1 year, the number of falls would be 646 (95% CI 595 to 689) compared with 850 in the group receiving usual care or attention control |
|
| 850 per 1000c | 646 per 1000 (595 to 689) | |||||
| Specific exercise population | ||||||
| 930 per 1000c | 707 per 1000 (651 to 754) | |||||
| Number of people who experienced one of more falls Follow‐up: range 3 to 24 months |
All studies population | RR 0.87 (0.82 to 0.91) | 8288 (37 RCTs) |
⊕⊕⊕⊕d high | Overall, there is a reduction of 13% (95% CI 9% to 18%) in the number of people who experienced one or more falls. Guide to the data based on the all‐studies estimate. If 1000 people were followed over 1 year, the number of people who experienced one or more falls would be 418 (95% CI 394 to 437) compared with 480 in the group receiving usual care or attention control |
|
| 480 per 1000e |
418 per 1000 (394 to 437) |
|||||
| Specific exercise population | ||||||
| 549 per 1000e | 478 per 1000 (451 to 500) | |||||
| Number of people who experienced one or more fall‐related fractures. Follow‐up: range 6 to 30 months |
All studies population | RR 0.44 (0.25 to 0.76) | 2139 (7 RCTs) |
⊕⊕⊝⊝g low | Overall, there may be a reduction of 56% (95% CI 24% to 75%) in the number of people who experienced one or more fall‐related fractures Guide to the data. If 1000 people were followed over 1 year, the number of people who experienced one or more fall‐related fractures may be 29 (95% CI 16 to 49) compared with 64 in the group receiving usual care or attention control |
|
| 64 per 1000f | 29 per 1000 (16 to 49) | |||||
| Adverse events | See comment | Not estimable | 4167 (15 RCTs) |
⊕⊝⊝⊝h very low | Adverse events were reported on in 15 of the 48 trials with gait, balance, and functional (task) training as the primary intervention in exercise versus control analyses in trials. Adverse events were reported for both intervention and control groups (11 trials) or just the intervention group (4 trials). 200 adverse events were reported; most were non‐serious adverse events of a musculoskeletal nature; 173 were in a single study including 2 intervention groups. Other adverse events included shortness of breath in 4 participants; and 1 participant with palpitations. One study reported a pelvic stress fracture in an intervention group | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aUsing Prevention of Falls Network Europe (ProFaNE) taxonomy, gait, balance, and functional [task] training is: gait training = specific correction of walking technique, and changes of pace, level and direction; balance training = transferring bodyweight from one part of the body to another or challenging specific aspects of the balance systems; functional training = functional activities, based on the concept of task specificity. Training is assessment‐based, tailored and progressed. Exercise programs included in this analysis contained a single primary exercise category (gait, balance, and functional [task] training); these exercise programs may also include secondary categories of exercise. bA control intervention is one that is not thought to reduce falls, such as general health education, social visits, very gentle exercise, or 'sham' exercise not expected to impact on falls. c The all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 59 all‐exercise types RCTs. The specific exercise population risk was based on the number of events and the number of participants in the control group for this outcome over the 39 RCTs. dWe did not downgrade for risk of bias, as results were essentially unchanged with the removal of the trials with a high risk of bias in one or more items. eThe all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 63 all‐exercise types RCTs. The specific exercise population risk was based on the number of events and the number of participants in the control group for this outcome over the 37 RCTs.
fThe all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 10 all‐exercise types RCTs. Based on the number of events and the number of participants in the control group for this outcome over the seven RCTs, the assumed risk in the control group was 43 per 1000. gDowngraded by two levels due to risk of bias (removing studies with high risk of bias on one or more items had a marked impact on results), and imprecision (few events and wide CI due to small sample size). hDowngraded by three levels due to limitations in design of studies, suggesting a high likelihood of bias (no trials in this analysis measured the number of participants experiencing adverse events in both groups throughout the trial period).
Summary of findings 3. Summary of findings: resistance exercises versus control (e.g. usual activities).
| Resistance exercises versus control (e.g. usual activities) for preventing falls in older people in the community | ||||||
|
Patient or population: Older people living in the community (trials focusing on people recently discharged from hospital were not included) Settings: Community, either at home or in places of residence that, on the whole, do not provide residential health‐related care Intervention: Exercise, type = resistance traininga Comparison: Usual care (no change in usual activities) or a control (non‐active) interventionb | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Exercise (resistance training) | |||||
| Rate of falls (falls per person‐years) Follow‐up: range 4 to 12 months |
All studies population | Rate ratio 1.14 (0.67 to 1.97) | 327 (5 RCTs) | ⊕⊝⊝⊝d very low | The evidence is of very low certainty, hence we are uncertain of the findings of an increase of 14% (95% CI 33% reduction to 97% increase) in the number of falls. Guide to the data based on the all‐studies estimate. If 1000 people were followed over 1 year, the number of falls would be 969 (95% CI 570 to 1675) compared with 850 in the group receiving usual care or attention control |
|
| 850 per 1000c | 969 per 1000 (570 to 1675) | |||||
| Specific exercise population | ||||||
| 630 per 1000c |
719 per 1000 (423 to 1242) |
|||||
| Number of people who experienced 1 or more falls Follow‐up: range 4 to 12 months |
All studies population | RR 0.81 (0.57 to 1.15) | 163 (2 RCTs) |
⊕⊝⊝⊝f very low | The evidence is of very low certainty, hence we are uncertain of the findings of a decrease of 19% (95% CI 43% reduction to 15% increase) in the number of people who experienced one or more falls Guide to the data based on the all‐studies estimate. If 1000 people were followed over 1 year, the number of people who experienced one or more falls would be 389 (95% CI 274 to 552) compared with 480 in the group receiving usual care or attention control |
|
| 480 per 1000e | 389 per 1000 (274 to 552) | |||||
| Specific exercise population | ||||||
|
864 per 1000e |
700 per 1000 (493 to 994) | |||||
| Number of people who experienced 1 or more fall‐related fractures | All studies population | RR 0.97 (0.14 to 6.49) | 73 (1 RCT) | ⊕⊝⊝⊝h very low | The evidence is of very low certainty, hence we are uncertain of the findings of a decrease of 3% (95% CI 86% reduction to 549% increase) The very small number of events (3 fractures in all) means that these data are not informative |
|
| 64 per 1000g | 63 per 1000 (9 to 416) | |||||
| Adverse events | See comment | Not estimable | 64 (1 RCT) | ⊕⊝⊝⊝i very low | Adverse events were reported on in one of the five trials with resistance training as the primary intervention in exercise versus control analyses. The study reported 10 musculoskeletal complaints in the intervention group and one musculoskeletal complaint in the control group. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aUsing Prevention of Falls Network Europe (ProFaNE) taxonomy, resistance training is any type of weight training (contraction of muscles against resistance to induce a training effect in the muscular system). Resistance is applied by body weight or external resistance. Training is assessment‐based, tailored and progressed. Exercise programmes included in this analysis had resistance training as the single primary exercise category; these exercise programmes may also include secondary categories of exercise. bA control intervention is one that is not thought to reduce falls, such as general health education, social visits, very gentle exercise, or 'sham' exercise not expected to impact on falls. cThe all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 59 all‐exercise types RCTs. The specific exercise population risk was based on the number of events and the number of participants in the control group for this outcome over the 5 RCTs. dDowngraded by three levels due to risk of inconsistency (there was substantial heterogeneity (I² = 67%)), imprecision (wide CI due to small sample size), and risk of bias (removing studies with high risk of bias in one or more items had a marked impact on results). eThe all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 63 all‐exercise types RCTs. The specific exercise population risk was based on the number of events and the number of participants in the control group for this outcome over the 2 RCTs. fDowngraded by one level due to risk of bias (removing studies with high risk of bias on one or more items had a marked impact on results), and downgraded by two levels due to imprecision (small number of trials and participants, wide CI). gThe all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 10 all‐exercise types RCTs. Based on the number of events and the number of participants in the control group for this outcome in the sole RCT, the assumed risk in the control group was 28 per 1000. hDowngraded by three levels for imprecision (wide CI, single study, very few events). iDowngraded by three levels due to only one study reporting adverse events and limitations in design of studies, suggesting a high likelihood of bias (number of participants experiencing adverse events was not reported in the same manner in both groups throughout the trial period).
Summary of findings 4. Summary of findings: 3D (Tai Chi) exercise versus control (e.g. usual activities).
| 3D (Tai Chi) exercise versus control (e.g. usual activities) for preventing falls in older people in the community | ||||||
|
Patient or population: Older people living in the community (trials focusing on people recently discharged from hospital were not included) Settings: Community, either at home or in places of residence that, on the whole, do not provide residential health‐related care Intervention: Exercise, type = 3D (Tai Chi) traininga Comparison: Usual care (no change in usual activities) or a control (non‐active) interventionb | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Exercise (3D (Tai Chi)) | |||||
| Rate of falls (falls per person‐year) Follow‐up: range 6 to 17 months |
All studies population | Rate ratio 0.81 (0.67 to 0.99) | 2655 (7 RCTs) | ⊕⊕⊝⊝d low | Overall, there may be a reduction of 19% (95% CI 1% to 33%) in the number of falls. Guide to the data based on the all‐studies estimate. If 1000 people were followed over 1 year, the number of falls may be 689 (95% CI 570 to 842) compared with 850 in the group receiving usual care or attention control |
|
| 850 per 1000c | 689 per 1000 (570 to 842) | |||||
| Specific exercise population | ||||||
| 1020 per 1000c | 827 per 1000 (684 to 1010) | |||||
| Number of people who experienced one or more falls Follow‐up: range 5 to 17 months |
All studies population | RR 0.80 (0.70 to 0.91) | 2677 (8 RCTs) |
⊕⊕⊕⊕f high | Overall, there is a reduction of 20% (95% CI 9% to 30%) in the number of people who experienced one or more falls Guide to the data based on the all‐studies estimate. If 1000 people were followed over 1 year, the number of people who experienced one or more falls would be 384 (95% CI 336 to 437) compared with 480 in the group receiving usual care or attention control |
|
| 480 per 1000e | 384 per 1000 (336 to 437) | |||||
| Specific exercise population | ||||||
| 437 per 1000e | 350 per 1000 (306 to 398) | |||||
| Number of people who experienced one or more fall‐related fractures | See comment | Not estimable | See comment | ‐ | This outcomes was not reported | |
| Adverse events | See comment | Not estimable | 474 (2 RCTs) |
⊕⊝⊝⊝g very low | Adverse events were reported in two of 10 trials (474 participants) with 3D (Tai Chi) as the primary intervention. There were no occurrences of adverse events | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aUsing Prevention of Falls Network Europe (ProFaNE) taxonomy, 3D (Tai Chi) training uses upright posture, specific weight transferences and movements of the head and gaze, during constant movement in a fluid, repetitive, controlled manner through three spatial planes. Exercise programmes included in this analysis had 3D (Tai Chi) training as the single primary exercise category; these exercise programmes may also include secondary categories of exercise. bA control intervention is one that is not thought to reduce falls, such as general health education, social visits, very gentle exercise, or 'sham' exercise not expected to impact on falls. cThe all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 59 all‐exercise types RCTs. The specific exercise population risk was based on the number of events and the number of participants in the control group for this outcome over the seven RCTs. dDowngraded by two levels due to inconsistency (there was substantial heterogeneity (I² = 74%)), and risk of bias (removing studies with high risk of bias in one or more items had a marked impact on results). eThe all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 63 all‐exercise types RCTs. The specific exercise population risk was based on the number of events and the number of participants in the control group for this outcome over the eight RCTs. fWe did not downgrade for risk of bias, as results were essentially unchanged with removal of the trials with a high risk of bias in one or more items. gDowngraded by three levels due to only 30% of trials reporting adverse events to any degree, and limitations in the design of studies suggesting a high likelihood of bias (no trials in this analysis measured the number of participants experiencing adverse events in both groups throughout the trial period).
Summary of findings 5. Summary of findings: 3D (dance) exercise versus control (e.g. usual activities).
| 3D (dance) exercise versus control (e.g. usual activities) for preventing falls in older people in the community | ||||||
|
Patient or population: Older people living in the community (trials focusing on people recently discharged from hospital were not included) Settings: Community, either at home or in places of residence that, on the whole, do not provide residential health‐related care Intervention: Exercise, type = 3D (dance) traininga Comparison: Usual care (no change in usual activities) or a control (non‐active) interventionb | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Exercise (3D [dance]) | |||||
| Rate of falls (falls per person‐years) Follow‐up: 12 months |
All studies population | Rate ratio 1.34 (0.98 to 1.83) | 522 (1 RCT) |
⊕⊝⊝⊝d very low | The evidence is of very low certainty, hence we are uncertain of the findings of an increase of 34% (95% CI 2% reduction to 83% increase) in the number of falls Guide to the data based on the all‐studies estimate If 1000 people were followed over 1 year, the number of falls may be 1139 (95% CI 833 to 1556) compared with 850 in the group receiving usual care or attention control |
|
| 850 per 1000c | 1139 per 1000 (833 to 1556) | |||||
| Specific exercise population | ||||||
| 800 per 1000c | 1072 per 1000 (784 to 1464) | |||||
| Number of people who experienced one or more falls Follow‐up: 12 months |
All studies population | RR 1.35 (0.83 to 2.20) | 522 (1 RCT) |
⊕⊝⊝⊝d very low | The evidence is of very low certainty, hence we are uncertain of the findings of an increase of 35% (95% CI 17% reduction to 120% increase) in the number of people who experienced one or more falls Guide to the data based on the all‐studies estimate If 1000 people were followed over 1 year, the number of people who experienced one or more falls may be 648 (95% CI 399 to 1056) compared with 480 in the group receiving usual care or attention control |
|
| 480 per 1000e | 648 per 1000 (399 to 1056) | |||||
| Specific exercise population | ||||||
| 583 per 1000e | 787 per 1000 (484 to 1283) | |||||
| Number of people who experienced one or more fall‐related fractures | Not estimable | Not estimable | See comment | ‐ | This outcome was not reported | |
| Adverse events | See comment | Not estimable | 522 (1 RCT) |
⊕⊝⊝⊝f very low | Adverse events were reported for the intervention group only (275 participants) in the one trial in this analysis. There were no occurrences of adverse events | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aUsing Prevention of Falls Network Europe (ProFaNE) taxonomy, 3D (dance) training uses dynamic movement qualities, patterns and speeds whilst engaged in constant movement in a fluid, repetitive, controlled manner through three spatial planes. Exercise programmes included in this analysis had 3D (dance) training as the single primary exercise category; these exercise programmes may also include secondary categories of exercise. bA control intervention is one that is not thought to reduce falls, such as general health education, social visits, very gentle exercise, or 'sham' exercise not expected to impact on falls. cThe all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 59 all‐exercise types RCTs. The specific exercise population risk was based on the number of events and the number of participants in the control group for this outcome in the sole RCT. dGraded very low due to serious imprecision (only one cluster‐RCT, with a wide CI due to small sample size). eThe all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 63 all‐exercise types RCTs. The specific exercise population risk was based on the number of events and the number of participants in the control group for this outcome in the sole RCT. fDowngraded by three levels due to limitations in the design of studies, suggesting a high likelihood of bias (the trial measured the number of participants experiencing adverse events in the exercise group).
Summary of findings 6. Summary of findings: walking programme (general physical activity) versus control (e.g. usual activities).
| General physical activity (including walking) training versus control (e.g. usual activities) for preventing falls in older people in the community | ||||||
|
Patient or population: Older people living in the community (trials focusing on people recently discharged from hospital were not included) Settings: Community, either at home or in places of residence that, on the whole, do not provide residential health‐related care Intervention: Exercise, type = general physical activity (including walking) traininga Comparison: Usual care (no change in usual activities) or a control (non‐active) interventionb | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Exercise (general physical activity [including walking]) | |||||
| Rate of falls (falls per person‐years) Follow‐up: range 12 to 24 months |
All studies population | Rate ratio 1.14 (0.66 to 1.97) | 441 (2 RCTs) |
⊕⊝⊝⊝d very low | The evidence is of very low certainty, hence we are uncertain of the findings of an increase of 14% (95% CI 34% reduction to 97% increase) in the number of falls Guide to the data based on the all‐studies estimate If 1000 people were followed over 1 year, the number of falls may be 969 (95% CI 561 to 1675) compared with 850 in the group receiving usual care or attention control |
|
| 850 per 1000c | 969 per 1000 (561 to 1675) | |||||
| Specific exercise population | ||||||
| 670 per 1000c | 764 per 1000 (443 to 1320) | |||||
| Number of people who experienced one or more falls Follow‐up: range 12 to 24 months |
All studies population | RR 1.05 (0.71 to 1.54) | 441 (2 RCTs) |
⊕⊝⊝⊝f very low | The evidence is of very low certainty, hence we are uncertain of the findings of an increase of 5% (95% CI 29% reduction to 54% increase) in the number of people who experienced one or more falls Guide to the data based on the all‐studies estimate If 1000 people were followed over 1 year, the number of people who experienced one or more falls may be 504 (95% CI 341 to 740) compared with 480 in the group receiving usual care or attention control |
|
| 480 per 1000e | 504 per 1000 (341 to 740) | |||||
| Specific exercise population | ||||||
| 374 per 1000e | 393 per 1000 (266 to 576) | |||||
| Number of people who experienced one or more fall‐related fractures | All studies population | RR 0.66 (0.11 to 3.76) | 97 (1 RCT) | ⊕⊝⊝⊝h very low | The evidence is of very low certainty, hence we are uncertain of the findings of a reduction of 34% (95% CI 89% reduction to 276% increase) in the number of people who experienced one or more fall‐related fractures Guide to the data If 1000 people were followed over 1 year, the number of people who experienced one or more fall‐related fractures may be 43 (95% CI 7 to 241) compared with 64 in the group receiving usual care or attention control |
|
| 64 per 1000g | 43 per 1000 (7 to 241) | |||||
| Adverse events | See comment | Not estimable | See comment | ‐ | This outcome was not reported | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aUsing Prevention of Falls Network Europe (ProFaNE) taxonomy, physical activity is any movement of the body, produced by skeletal muscle, that causes energy expenditure to be substantially increased. Recommendations regarding intensity, frequency and duration are required in order to increase performance. Exercise programmes included in this analysis had general physical activity (including walking) training as the single primary exercise category; these exercise programmes may also include secondary categories of exercise. bA control intervention is one that is not thought to reduce falls, such as general health education, social visits, very gentle exercise, or 'sham' exercise not expected to impact on falls. cThe all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 59 all‐exercise types RCTs. The specific exercise population risk was based on the number of events and the number of participants in the control group for this outcome in the two RCTs. dDowngraded by three levels due to inconsistency (there was substantial heterogeneity (I² = 67%)), imprecision (wide CI), and risk of bias (removing studies with high risk of bias on one or more items had a marked impact on results). eThe all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 63 all‐exercise types RCTs. The specific exercise population risk was based on the number of events and the number of participants in the control group for this outcome in the two RCTs. fDowngraded by three levels due to inconsistency (there was moderate heterogeneity (I² = 50%), imprecision (wide CI), and risk of bias (removing studies with high risk of bias on one or more items had a marked impact on results). gThe all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 10 all‐exercise types RCTs. Based on the number of events and the number of participants in the control group for this outcome in the only RCT, the assumed risk in the control group was 84 per 1000.
hDowngraded three levels due to risk of bias and imprecision (single study, wide CI).
Summary of findings 7. Summary of findings: multiple categories of exercise versus control (e.g. usual activities).
| Multiple categories of exercise (often including, as primary interventions: gait, balance, and functional (task) training plus resistance training) versus control (e.g. usual activities) for preventing falls in older people in the community | ||||||
|
Patient or population: Older people living in the community (trials focusing on people recently discharged from hospital were not included) Settings: Community, either at home or in places of residence that, on the whole, do not provide residential health‐related care Intervention: Exercise, type = Multiple types of exercisea Comparison: Usual care (no change in usual activities) or a control (non‐active) interventionb | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Exercise (multiple types (including, as primary interventions: gait, balance, and functional (task) training, plus resistance training)) | |||||
| Rate of falls (falls per person‐years) Follow‐up: range 3 to 25 months |
All studies population | Rate ratio 0.66 (0.50 to 0.88)d | 1374 (11 RCTs) |
⊕⊕⊕⊝e moderate | Overall, there is probably a reduction of 34% (95% CI 12% to 50%) in the number of falls Guide to the data based on the all‐studies estimate If 1000 people were followed over 1 year, the number of falls would probably be 561 (95% CI 425 to 748) compared with 850 in the group receiving usual care or attention control |
|
| 850 per 1000c | 561 per 1000 (425 to 748) | |||||
| Specific exercise population | ||||||
| 1180 per 1000c | 779 per 1000 (590 to 1039) | |||||
| Number of people who experienced one or more falls Follow‐up: range 3 to 25 months |
All studies population | RR 0.78 (0.64 to 0.96) | 1623 (17 RCTs) |
⊕⊕⊕⊝g moderate | Overall, there is probably a reduction of 22% (95% CI 4% to 36%) in the number of people who experienced one or more falls Guide to the data based on the all studies estimate. If 1000 people were followed over 1 year, the number of people who experienced one or more falls would probably be 375 (95% CI 308 to 461) compared with 480 in the group receiving usual care or attention control. |
|
| 480 per 1000f | 375 per 1000 (308 to 461) | |||||
| Specific exercise population | ||||||
| 374 per 1000f | 296 per 1000 (243 to 364) | |||||
| Number of people who experienced one or more fall‐related fractures | 64 per 1000h | 55 per 1000 (40 to 75) | RR 0.85 (0.62 to 1.16) | 1810 (3 RCTs) |
⊕⊕⊝⊝i low | Overall, there may be a reduction of 15% (95% CI 38% reduction to 16% increase) in the number of people who experienced one or more fall‐related fractures Guide to the data If 1000 people were followed over 1 year, the number of people who experienced one or more fall‐related fractures would probably be 55 (95% CI 40 to 75) compared with 64 in the group receiving usual care or attention control |
| Adverse events | See comment | Not estimable | 1177 (10 RCTs) |
⊕⊝⊝⊝j very low | Adverse events were reported in 10 of the 21 trials with multiple primary intervention categories, in the exercise versus control analyses in these trials. Adverse events were reported for both intervention and control groups (5 trials), or the intervention group only (5 trials). There were a total of 43 adverse events reported. Most were non‐serious of a musculoskeletal nature. There was reported exacerbation of pre‐existing osteoarthritis conditions in one trial and inguinal hernia surgery was reported in one intervention arm of another trial | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aExercise programmes included in this analysis had more than one primary exercise category. We categorised exercise based on the Prevention of Falls Network Europe (ProFaNE) taxonomy that classifies exercise type as: i) gait, balance, and functional (task) training; ii) strength/resistance (including power); iii) flexibility; iv) three‐dimensional (3D) exercise (e.g. Tai Chi, Qigong, dance); v) general physical activity; vi) endurance; and vii) other kind of exercises. The programmes often included, as the primary intervention, gait, balance, and functional (task) training plus resistance training. The exercise programmes may also include secondary categories of exercise. bA control intervention is one that is not thought to reduce falls, such as general health education, social visits, very gentle exercise, or 'sham' exercise not expected to impact on falls. cThe all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 59 all‐exercise types RCTs. The specific exercise population risk was based on the number of events and the number of participants in the control group for this outcome over the 11 RCTs. dSensitivity analyses revealed little difference in the results when only trials that include the most common two components (balance and functional exercises plus resistance exercises) were pooled (RaR 0.69, 95% CI 0.48 to 0.97; 1084 participants; 8 studies; I² = 72%). eDowngraded by one level due to inconsistency (there was substantial heterogeneity (I² = 65%)). We did not downgrade for risk of bias, as results were essentially unchanged with removal of the trials at a high risk of bias in one or more items. fThe all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 63 all‐exercise types RCTs. The specific exercise population risk was based on the number of events and the number of participants in the control group for this outcome over the 17 RCTs. gDowngraded by one level due to risk of bias (removing studies with high risk of bias in one or more items had a marked impact on results). hThe all‐studies population risk was based on the number of events and the number of participants in the control group for this outcome over the 10 all‐exercise types RCTs. Based on the number of events and the number of participants in the control group for this outcome over three RCTs, the assumed risk in the control group was 87 per 1000. iDowngraded by one level due to risk of bias and by one level due to imprecision. jDowngraded by three levels for limitations in the design of studies, suggesting a high likelihood of bias (no trials in this analysis measured the number of participants experiencing adverse events in both groups throughout the trial period).
Exercise (all types) versus control
Overview of results reporting format
For each outcome described below we report the overall pooled effects of all exercise interventions (including the subgroup analyses for age, baseline risk of falling, personnel, and group delivery, for the falls outcomes) then the effects in studies testing interventions within each exercise category of the ProFaNE taxonomy (Appendix 1; Appendix 5), as well as the results of studies of exercise interventions that included multiple categories. For analyses with more than 10 included comparisons (both rate of falls and number of people experiencing one or more falls comparisons for balance and functional exercises, and multiple categories of exercise) we also report the results of the three prespecified subgroup analyses (increased fall risk as a study entry criterion, exercise delivery by a health professional, group versus individual delivery).
The findings are summarised and the absolute impact of interventions illustrated in 'Summary of findings' tables for the overall 'exercise versus control' comparison and for separate primary exercise categories for which there are data. No trials compared primarily flexibility exercise, endurance exercise or other exercise type versus control.
The results for the four trials comparing exercise (all types) versus control in people who had been recently discharged from hospital are presented separately, after this main comparison.
Rate of falls (falls per person‐year)
Exercise (all types) reduces the rate of falls by 23% compared with control (rate ratio (RaR) 0.77, 95% confidence interval (CI) 0.71 to 0.83; 12,981 participants, 59 studies, I² = 55%; high‐certainty evidence; Analysis 1.1).
1.1. Analysis.
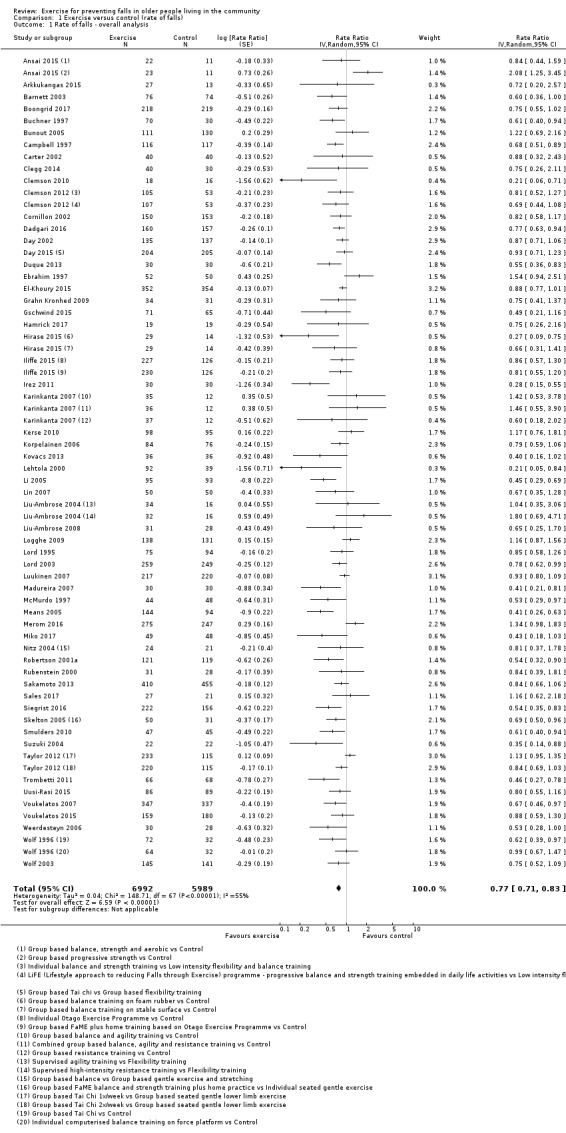
Comparison 1 Exercise versus control (rate of falls), Outcome 1 Rate of falls ‐ overall analysis.
Subgroup analysis by falls risk at baseline, found there was probably little or no difference in the effect of exercise (all types) on the rate of falls in trials where all participants were at an increased risk of falling (RaR 0.80, 95% CI 0.72 to 0.88; 6858 participants, 30 studies, I² = 56%) compared with trials that did not use increased risk of falling as an entry criterion (RaR 0.74, 95% CI 0.65 to 0.84; 6123 participants, 29 studies, I² = 53%); test for subgroup differences: Chi² = 0.90, df = 1, P = 0.34, I² = 0% (Analysis 1.2).
1.2. Analysis.
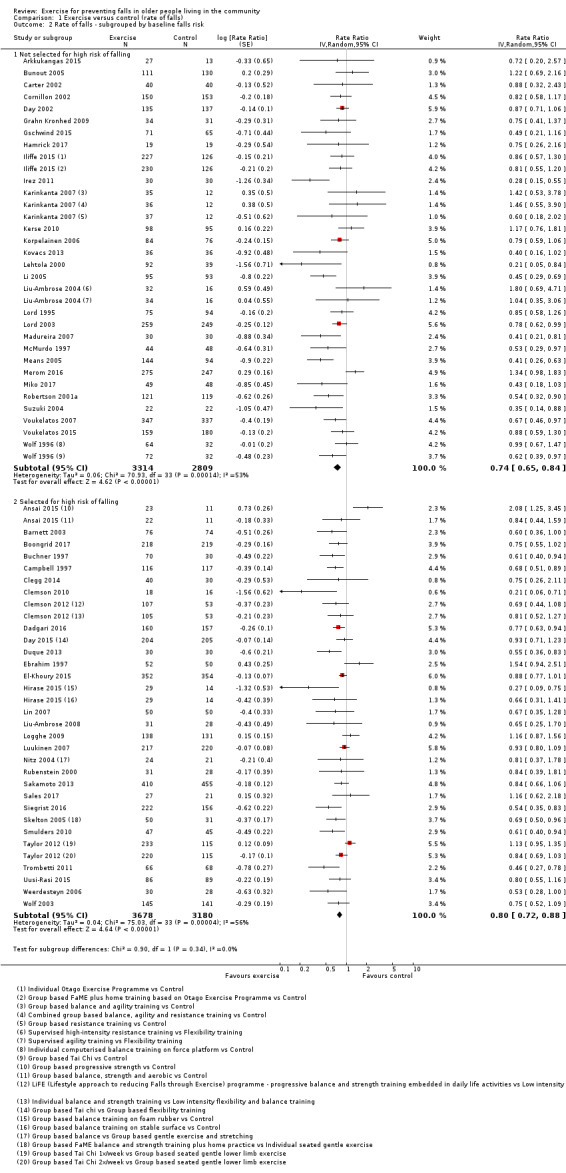
Comparison 1 Exercise versus control (rate of falls), Outcome 2 Rate of falls ‐ subgrouped by baseline falls risk.
Subgroup analysis by participant age found there was probably little or no difference in the effect of exercise (all types) on the rate of falls in trials where participants were aged 75 years or older (RaR 0.83, 95% CI 0.72 to 0.97; 3376 participants, 13 studies, I² = 54%) compared with trials where participants were aged less than 75 years (RaR 0.75, 95% CI 0.69 to 0.82; 9605 participants, 46 studies, I² = 55%); test for subgroup differences: Chi² = 1.36, df = 1, P = 0.24, I² = 27% (Analysis 1.3).
1.3. Analysis.
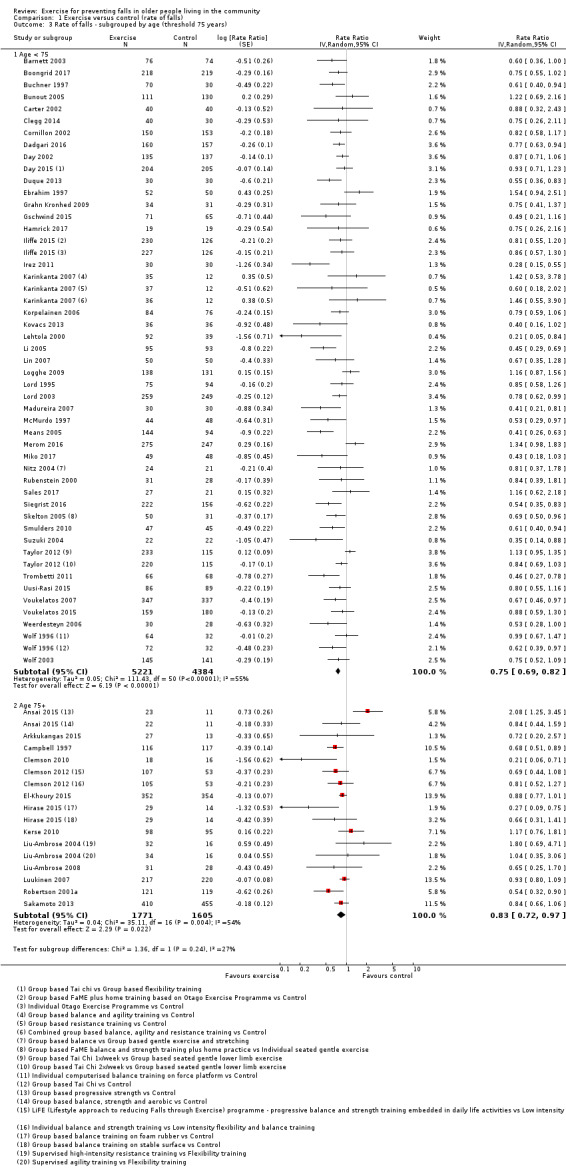
Comparison 1 Exercise versus control (rate of falls), Outcome 3 Rate of falls ‐ subgrouped by age (threshold 75 years).
Subgroup analyses found a larger effect of exercise (all types) in trials where interventions were delivered by a health professional (RaR 0.69, 95% CI 0.61 to 0.79; 4511 participants, 25 studies, I² = 47%) than in trials where the interventions were delivered by trained instructors who were not health professionals (RaR 0.82, 95% CI 0.75 to 0.90; 8470 participants, 34 studies, I² = 57%); test for subgroup differences: Chi² = 4.44, df = 1, P = 0.04, I² = 78% (Analysis 1.4). Notably, both approaches resulted in reductions in the rate of falls.
1.4. Analysis.
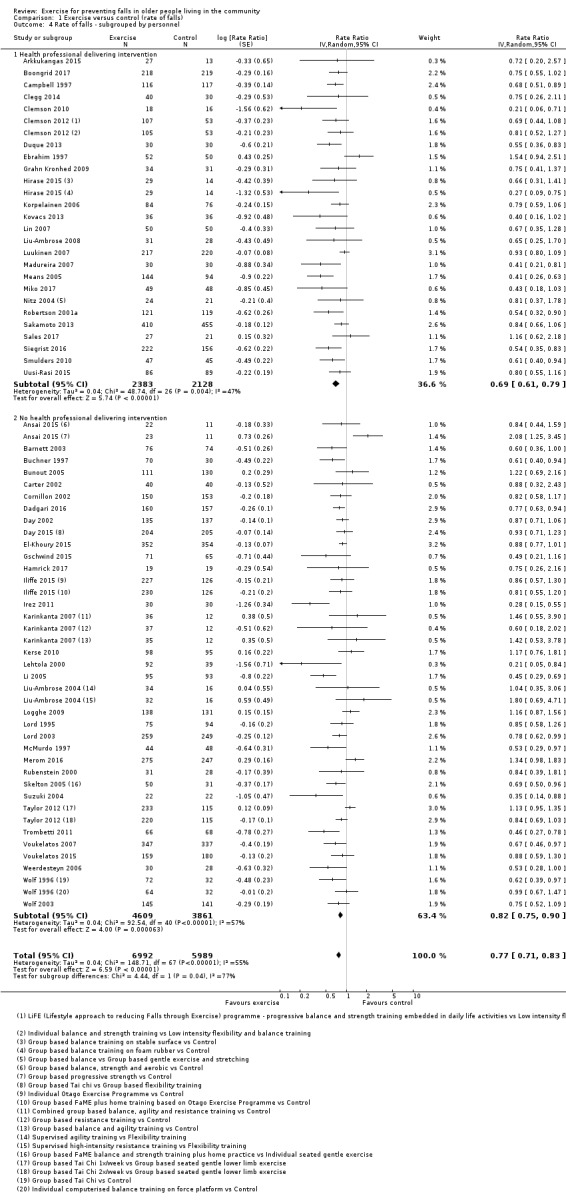
Comparison 1 Exercise versus control (rate of falls), Outcome 4 Rate of falls ‐ subgrouped by personnel.
Subgroup analyses found there may be no difference in the effect of exercise (all types) on the rate of falls where interventions were delivered in a group setting (RaR 0.76, 95% CI 0.69 to 0.85; 8163 participants, 40 studies, I² = 62%) compared with trials where interventions were delivered individually (RaR 0.79, 95% CI 0.71 to 0.88; 4818 participants, 21 studies, I² = 35%); test for subgroup differences: Chi² = 0.21, df = 1, P = 0.65, I² = 0% (Analysis 1.5). Two three‐group studies, appear in both subgroups (Iliffe 2015; Wolf 1996).
1.5. Analysis.
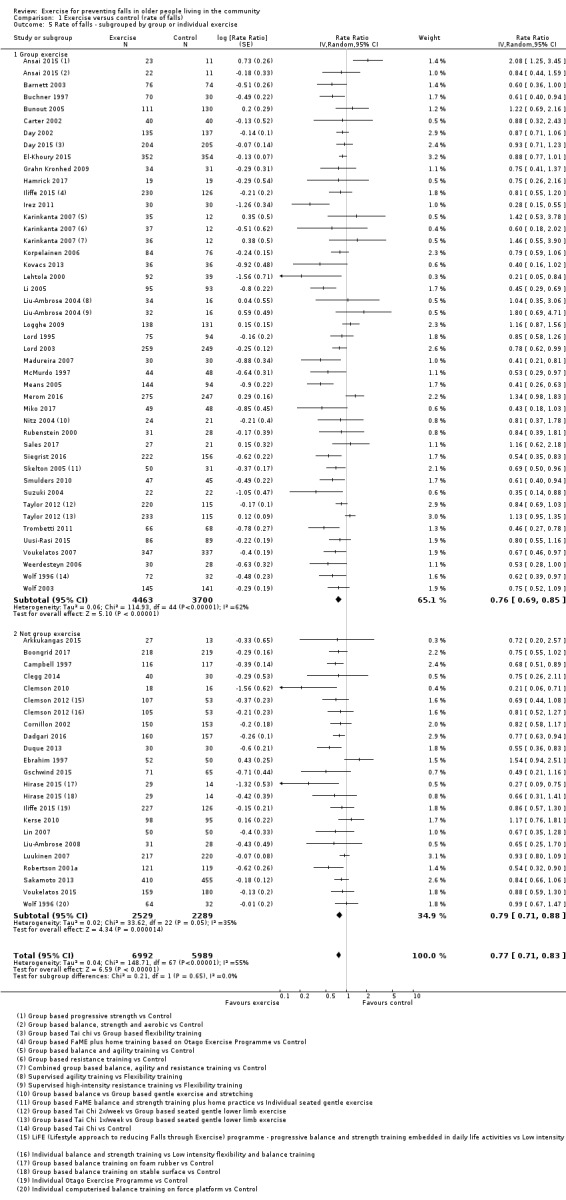
Comparison 1 Exercise versus control (rate of falls), Outcome 5 Rate of falls ‐ subgrouped by group or individual exercise.
Subgroup analysis by exercise type showed a variation in the effects of the different types of exercise on rate of falls, the visual impression being confirmed by the statistically significant test for subgroup differences: Chi² = 17.18, df = 5, P = 0.004, I² = 70.9% (Analysis 1.6).
1.6. Analysis.
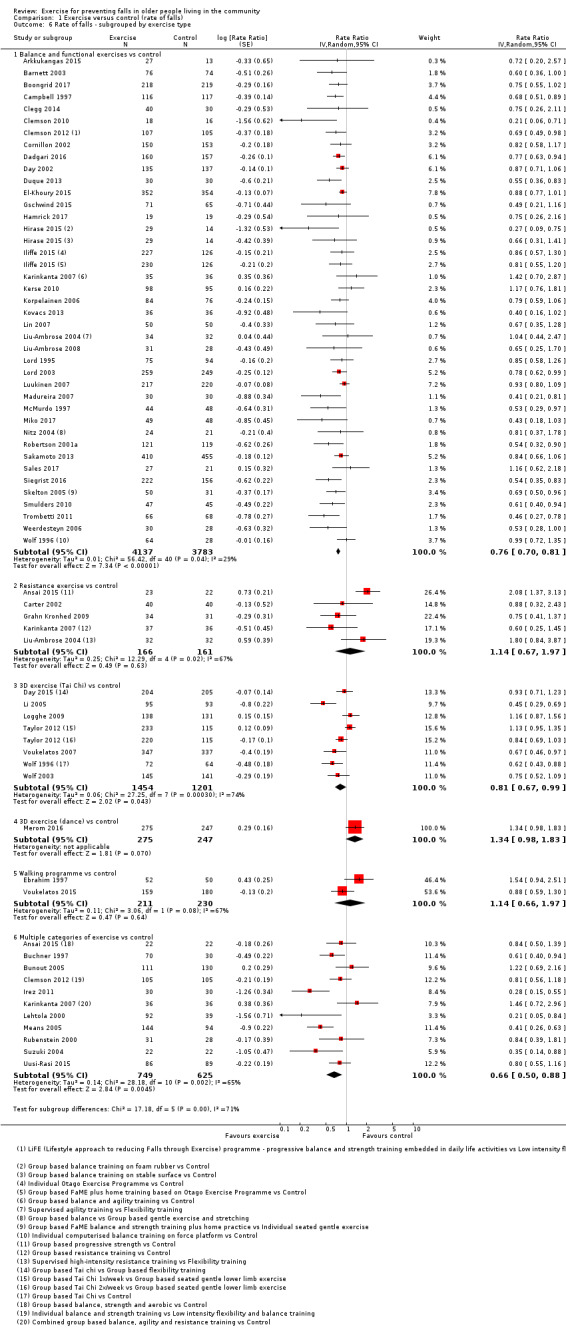
Comparison 1 Exercise versus control (rate of falls), Outcome 6 Rate of falls ‐ subgrouped by exercise type.
Different categories of primary exercise versus control
Balance and functional exercises versus control
Exercise interventions that were classified as being primarily gait, balance, co‐ordination or functional task training using the ProFaNE taxonomy, reduce the rate of falls by 24% compared with control (RaR 0.76, 95% CI 0.70 to 0.81; 7920 participants, 39 studies, I² = 29%, high‐certainty evidence; Analysis 1.6).
Subgroup analyses found little or no difference in the effect of balance and functional exercises on the rate of falls in trials where all participants were at an increased risk of falling (RaR 0.72, 95% CI 0.65 to 0.80; 4602 participants, 21 studies, I² = 38%) compared with trials that did not use increased risk of falling as an entry criterion (RaR 0.80, 95% CI 0.72 to 0.90; 3355 participants, 18 studies, I² = 17%); test for subgroup differences: Chi² = 1.99, df = 1, P = 0.16, I² = 50% (Analysis 8.1).
8.1. Analysis.
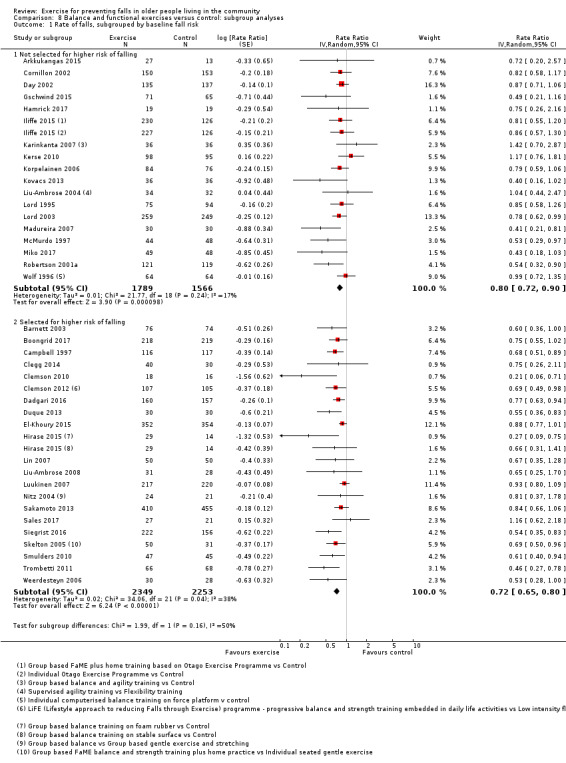
Comparison 8 Balance and functional exercises versus control: subgroup analyses, Outcome 1 Rate of falls, subgrouped by baseline fall risk.
Subgroup analyses found a larger effect of balance and functional exercises in trials where interventions were delivered by a health professional (RaR 0.67, 95% CI 0.58 to 0.65; 2960 participants, 20 studies, I² = 37%) than in trials where the interventions were delivered by trained instructors who were not health professionals (RaR 0.82, 95% CI 0.76 to 0.88; 4997 participants, 19 studies, I² = 9%); test for subgroup differences: Chi² = 6.72, df = 1, P = 0.01, I² = 85% (Analysis 8.3). Notably, both approaches resulted in statistically significant reductions in the rate of falls.
8.3. Analysis.
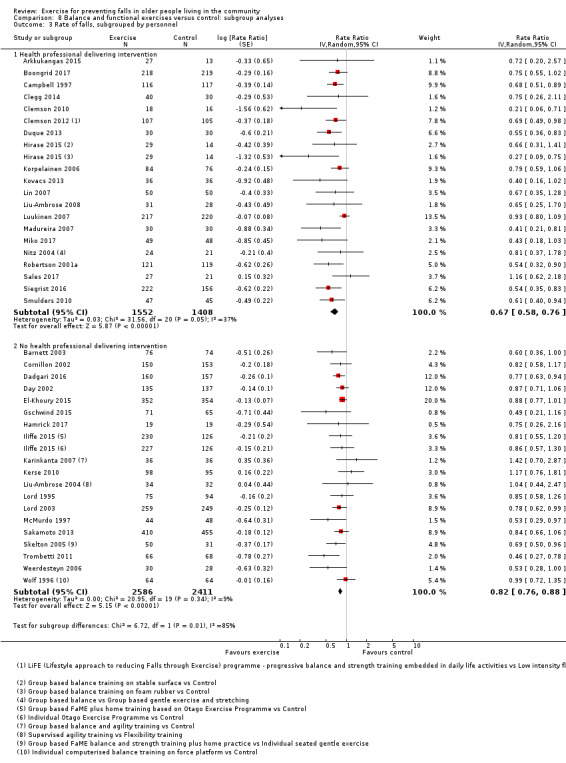
Comparison 8 Balance and functional exercises versus control: subgroup analyses, Outcome 3 Rate of falls, subgrouped by personnel.
Subgroup analyses found little or no difference in the effect of balance and functional exercises on the rate of falls in trials where interventions were delivered in a group setting (RaR 0.73, 95% CI 0.65 to 0.82; 3620 participants, 20 studies, I² = 34%) compared with trials where interventions were delivered individually (RaR 0.77, 95% CI 0.70 to 0.85; 4589 participants, 20 studies, I² = 28%); test for subgroup differences: Chi² = 0.47, df = 1, P = 0.50, I² = 0% (Analysis 8.5).
8.5. Analysis.
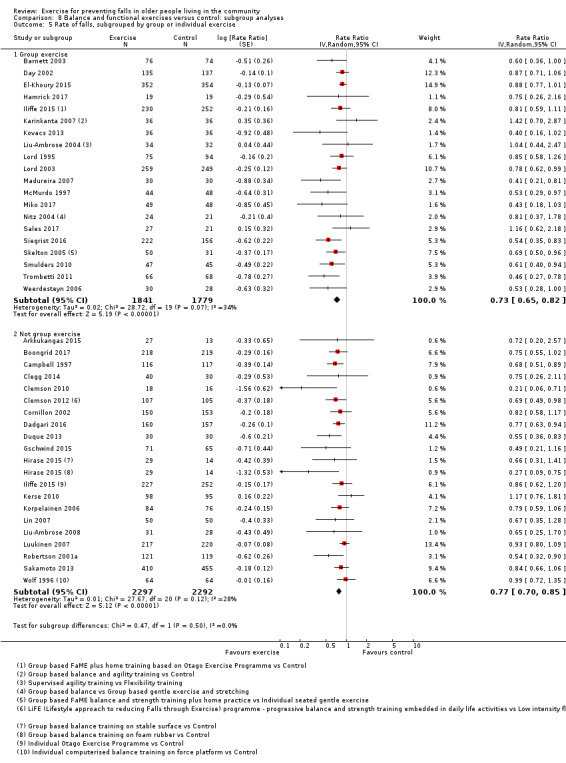
Comparison 8 Balance and functional exercises versus control: subgroup analyses, Outcome 5 Rate of falls, subgrouped by group or individual exercise.
Resistance exercises versus control
We are uncertain whether exercises, classified as being primarily resistance or strength exercises using the ProFaNE taxonomy, reduce the rate of falls compared with control (RaR 1.14, 95% CI 0.67 to 1.97; 327 participants, 5 studies, I² = 67%; very low‐certainty evidence; Analysis 1.6).
3D (Tai Chi) exercise versus control
Exercise interventions that were classified as 3D (Tai Chi or similar) may reduce the rate of falls by 19% compared with control (RaR 0.81, 95% CI 0.67 to 0.99; 2655 participants, 7 studies, I² = 74%; low‐certainty evidence; Analysis 1.6).
3D (dance) exercise versus control
We are uncertain whether exercises, classified as being primarily 3D (dance) using the ProFaNE taxonomy, reduce the rate of falls compared with control (RaR 1.34, 95% CI 0.98 to 1.83; 522 participants, 1 study; very low‐certainty evidence; Analysis 1.6).
Walking programme versus control
We are uncertain whether exercises, classified as being primarily walking programmes using the ProFaNE taxonomy, reduce the rate of falls compared with control (RaR 1.14, 95% CI 0.66 to 1.97; 441 participants, 2 studies; I² = 67%; very low‐certainty evidence; Analysis 1.6).
Multiple categories of exercise versus control
Exercise interventions that include multiple categories of the ProFaNE taxonomy (most commonly balance and functional exercises plus resistance exercises) probably reduce the rate of falls by 34% compared with controls (RaR 0.66, 95% CI 0.50 to 0.88; 1374 participants, 11 studies; I² = 65%; moderate‐certainty evidence; Analysis 1.6).
Sensitivity analyses revealed little difference in the results when we pooled only trials that include the most common two components (balance and functional exercises plus resistance exercises) (RaR 0.69, 95% CI 0.48 to 0.97; 1084 participants, 8 studies; I² = 72%; Analysis 19.1).
19.1. Analysis.
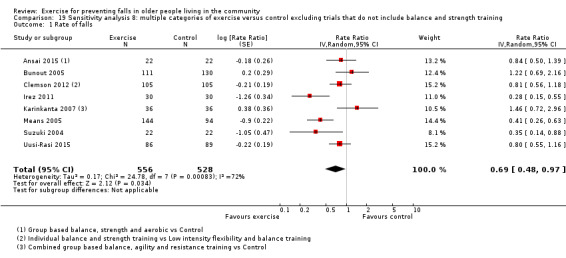
Comparison 19 Sensitivity analysis 8: multiple categories of exercise versus control excluding trials that do not include balance and strength training, Outcome 1 Rate of falls.
Subgroup analyses found there is probably little or no difference in the effect of exercise interventions that included multiple categories on the rate of falls in trials where all participants were at an increased risk of falling (RaR 0.77, 95% CI 0.63 to 0.94; 618 participants, 5 studies, I² = 0%) compared with trials that did not use increased risk of falling as an entry criterion (RaR 0.54, 95% CI 0.29 to 0.99; 763 participants, 6 studies, I² = 79%); test for subgroup differences: Chi² = 1.19, df = 1, P = 0.27, I² = 16.2% (Analysis 9.1).
9.1. Analysis.
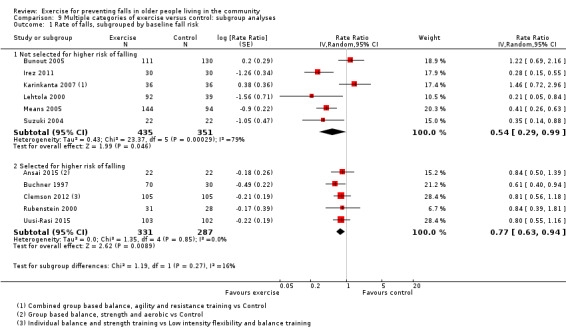
Comparison 9 Multiple categories of exercise versus control: subgroup analyses, Outcome 1 Rate of falls, subgrouped by baseline fall risk.
Subgroup analyses found there is probably little or no difference in the effect of exercise interventions that included multiple categories on rate of falls in trials where interventions were delivered by health professionals (RaR 0.65, 95% CI 0.43 to 0.99; 653 participants, 3 studies, I² = 72%) compared with trials where interventions were delivered by trained instructors who were not health professionals (RaR 0.66, 95% CI 0.44 to 0.99; 751 participants; 8 studies, I² = 67%); test for subgroup differences: Chi² = 0, df = 1, P = 0.96, I² = 0% (Analysis 9.3).
9.3. Analysis.
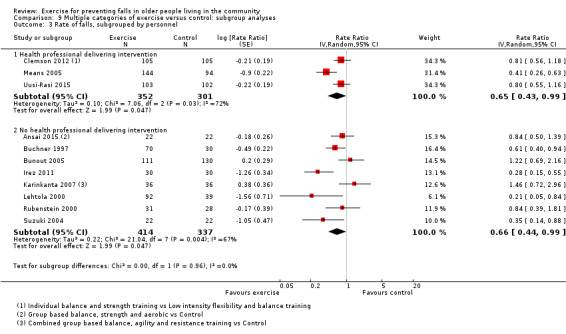
Comparison 9 Multiple categories of exercise versus control: subgroup analyses, Outcome 3 Rate of falls, subgrouped by personnel.
Subgroup analyses found there is probably little or no difference in the effect of exercise interventions that included multiple categories on the rate of falls in trials where interventions were delivered in a group setting (RaR 0.64, 95% CI 0.46 to 0.89; 1194 participants, 10 studies, I² = 67%) compared with trials where interventions were delivered individually (RaR 0.81, 95% CI 0.56 to 1.18; 210 participants, 1 study); test for subgroup differences: Chi² = 0.86, df = 1, P = 0.35, I² = 0% (Analysis 9.5).
9.5. Analysis.
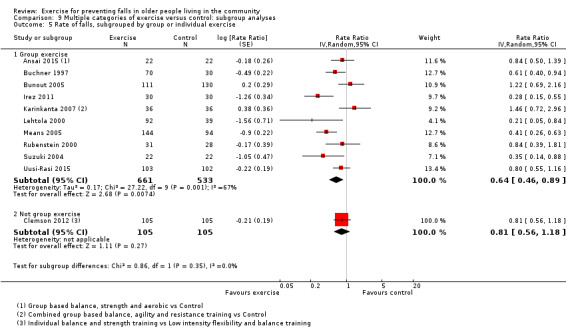
Comparison 9 Multiple categories of exercise versus control: subgroup analyses, Outcome 5 Rate of falls, subgrouped by group or individual exercise.
Long‐term follow‐up rate of falls (secondary outcome)
Five studies reported the rate of falls at more than 18 months after randomisation. Data from four studies, pooled by exercise category, are presented in Analysis 1.7. Balance and functional exercises may reduce the rate of falls in the long term (RaR 0.82, 95% CI 0.66 to 1.01; 858 participants, 2 studies; I2 = 41%; low‐certainty evidence). The long‐term effects of a walking programme tested in Ebrahim 1997 (97 participants) and a multiple exercise programme, including balance and strength training tested in Uusi‐Rasi 2015 (175 participants) are unclear (Analysis 1.7). Data from Iliffe 2015 were not included in Analysis 1.7 because the follow‐up period, which differed from the other four studies, was a one‐year period started six months after programme completion. There was no evidence of a difference in rate of falls for either exercise programme (FaME programme: RaR 0.94, 95% CI 0.62 to 1.41; 202 participants; Otago Exercise Program: RaR 1.04, 95% CI 0.69 to 1.55; 201 participants).
1.7. Analysis.
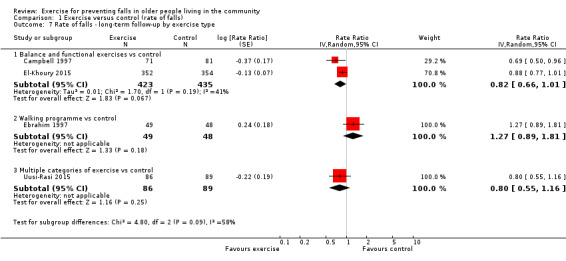
Comparison 1 Exercise versus control (rate of falls), Outcome 7 Rate of falls ‐ long‐term follow‐up by exercise type.
Number of people who experienced one or more falls (risk of falling)
Exercise (all types) reduces the number of people experiencing one or more falls by 15% compared with control (risk ratio (RR) 0.85, 95% CI 0.81 to 0.89; 13,518 participants, 63 studies, I² = 26%; high‐certainty evidence; Analysis 2.1).
2.1. Analysis.
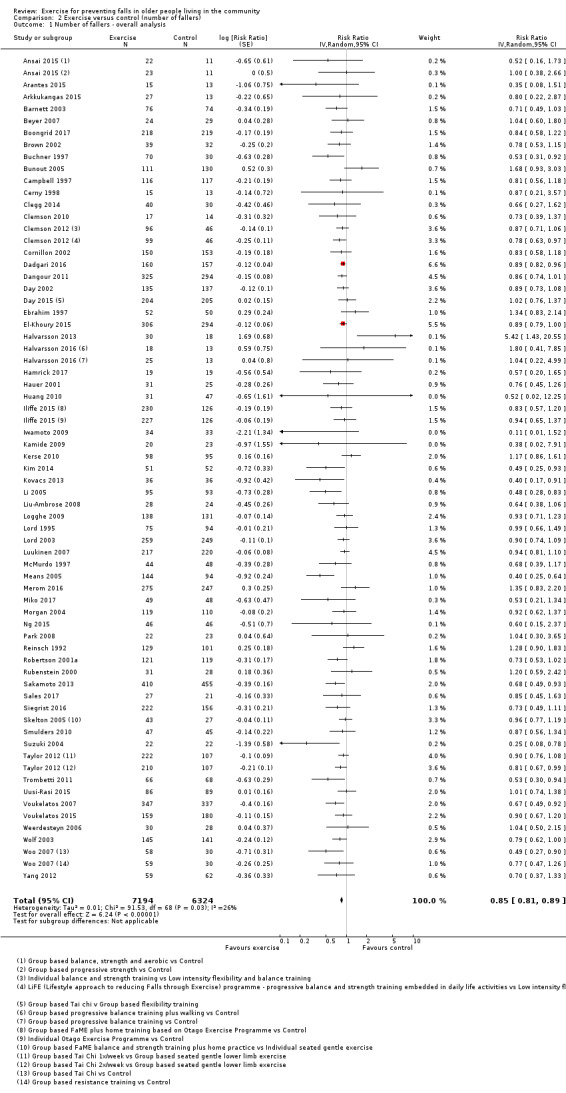
Comparison 2 Exercise versus control (number of fallers), Outcome 1 Number of fallers ‐ overall analysis.
Subgroup analysis by falls risk at baseline found there was little or no difference in the effect of exercise (all types) on the number of people experiencing one or more falls in trials where all participants were at an increased risk of falling (RR 0.87, 95% CI 0.83 to 0.91; 7171 participants, 35 studies, I² = 1%) compared with trials that did not use increased risk of falling as an entry criterion (RR 0.82, 95% CI 0.73 to 0.92; 6347 participants, 28 studies, I² = 45%); test for subgroup differences: Chi² = 0.94, df = 1, P = 0.33, I² = 0% (Analysis 2.2).
2.2. Analysis.
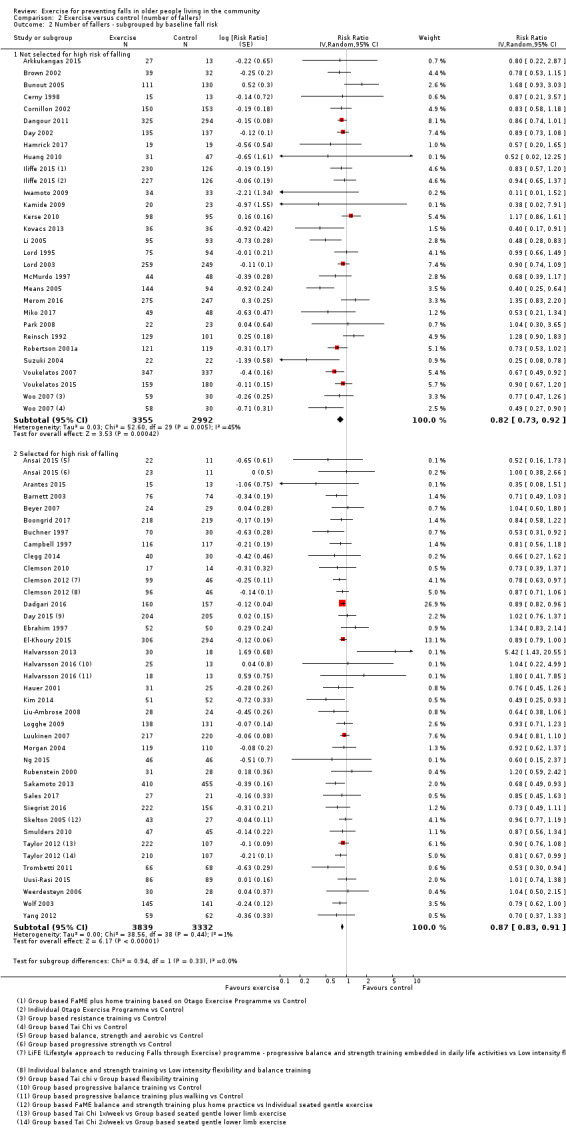
Comparison 2 Exercise versus control (number of fallers), Outcome 2 Number of fallers ‐ subgrouped by baseline fall risk.
Subgroup analysis by participant age found there was little or no difference in the effect of exercise (all types) on the number of people experiencing one or more falls in trials where participants were aged 75 years or older (RR 0.86, 95% CI 0.80 to 0.92; 3172 participants, 13 studies, I² = 0%) compared with trials where participants were aged less than 75 years (RR 0.85, 95% CI 0.79 to 0.91; 10,346 participants, 50 studies, I² = 33%); test for subgroup differences: Chi² = 0.07, df = 1, P = 0.79, I² = 0% (Analysis 2.3).
2.3. Analysis.
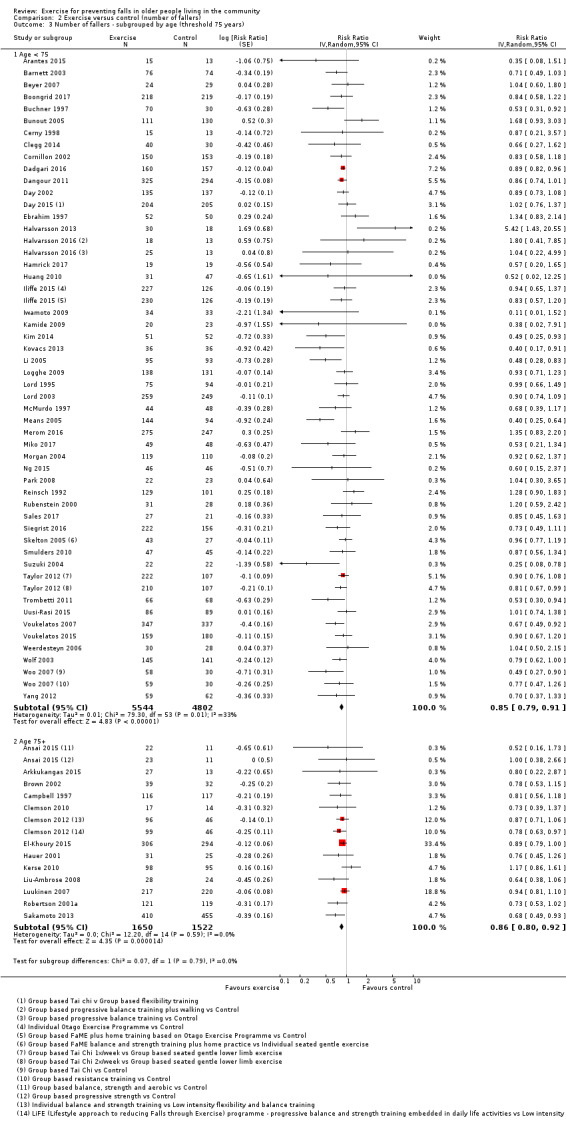
Comparison 2 Exercise versus control (number of fallers), Outcome 3 Number of fallers ‐ subgrouped by age (threshold 75 years).
Subgroup analyses by personnel delivering exercise found there was little or no difference in the effect of exercise (all types) on the number of people experiencing one or more falls in trials where interventions were delivered by a health professional (RR 0.82, 95% CI 0.74 to 0.91; 3747 participants, 26 studies, I² = 25%) than in trials where the interventions were delivered by trained instructors who were not health professionals (RR 0.86, 95% CI 0.81 to 0.92; 9726 participants, 36 studies, I² = 29%); test for subgroup differences: Chi² = 0.63, df = 1 (P = 0.43), I² = 0% (Analysis 2.4). The personnel providing the exercise programme was not identified in Park 2008.
2.4. Analysis.
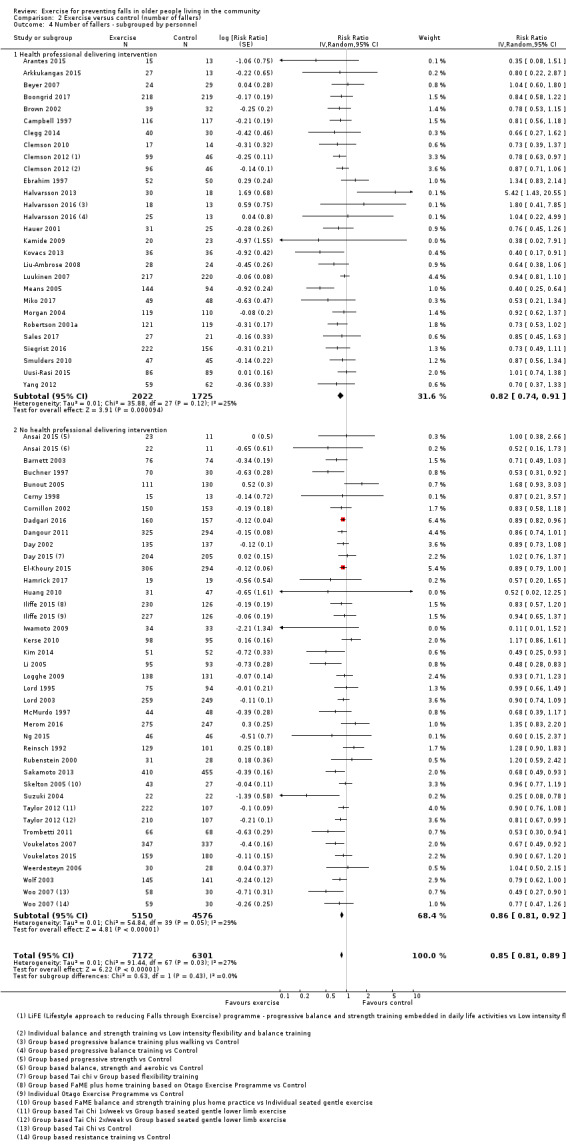
Comparison 2 Exercise versus control (number of fallers), Outcome 4 Number of fallers ‐ subgrouped by personnel.
Subgroup analyses found there may be no difference in the effect of exercise (all types) on the number of people experiencing one or more falls in trials where interventions were delivered in a group setting (RR 0.83, 95% CI 0.78 to 0.90; 9219 participants, 48 studies, I² = 33%) compared with trials where interventions were delivered individually (RR 0.88, 95% CI 0.83 to 0.93; 4299 participants, 16 studies; I² = 0%); test for subgroup differences: Chi² = 1.14, df = 1, P = 0.29, I² = 12% (Analysis 2.5). One three‐group study appears in both subgroups (Iliffe 2015).
2.5. Analysis.
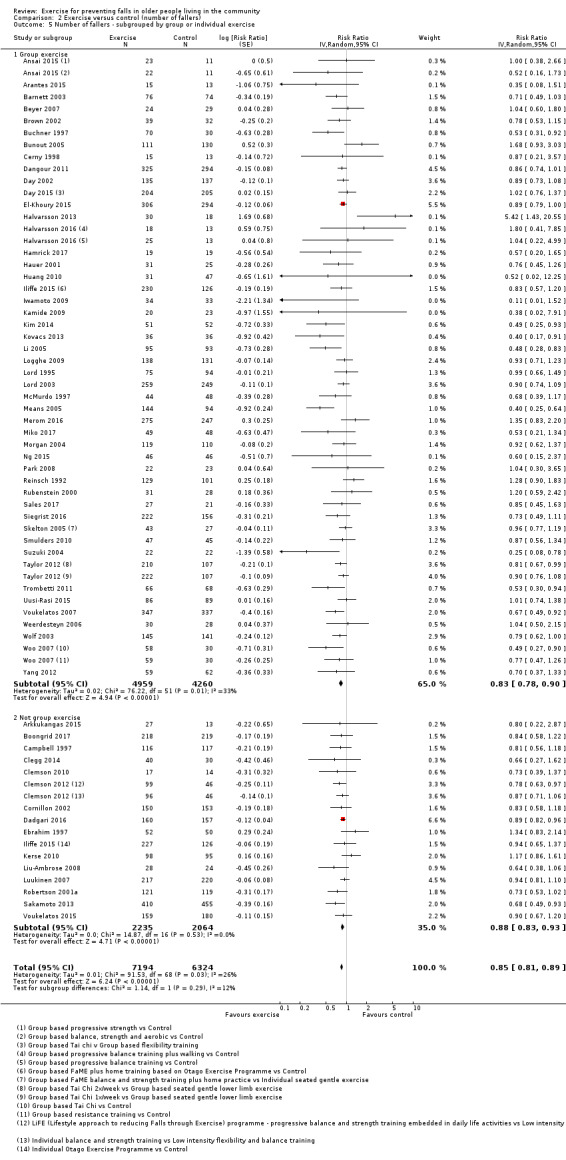
Comparison 2 Exercise versus control (number of fallers), Outcome 5 Number of fallers ‐ subgrouped by group or individual exercise.
The subgroup analysis by exercise type provided a visual impression of potential subgroup differences of effect of different exercises on the numbers of fallers, but the test for subgroup differences did not show a statistically significant result: test for subgroup differences: Chi² = 6.45, df = 5, P = 0.26, I² = 22.5% (Analysis 2.6).
2.6. Analysis.
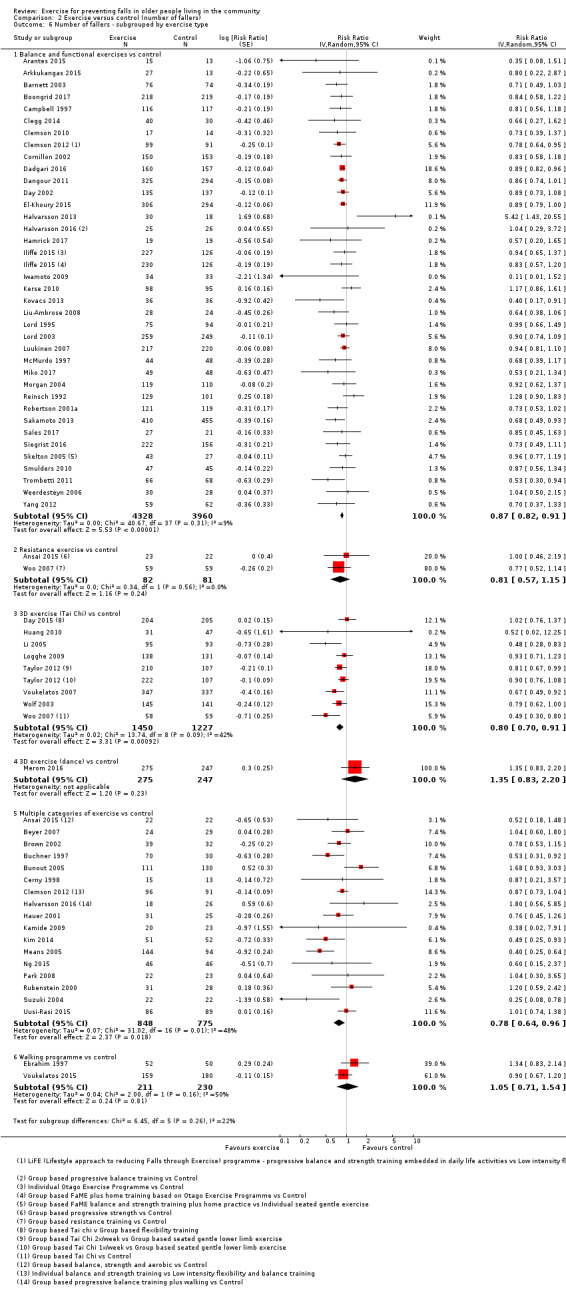
Comparison 2 Exercise versus control (number of fallers), Outcome 6 Number of fallers ‐ subgrouped by exercise type.
Different categories of primary exercise versus control
Balance and functional exercises versus control
Exercise interventions that were classified as being primarily gait, balance, co‐ordination or functional task training using the ProFaNE taxonomy, reduce the number of people experiencing one or more falls by 13% compared with control (RR 0.87, 95% CI 0.82 to 0.91; 8288 participants, 37 studies, I² = 9%; high‐certainty evidence; Analysis 2.6).
Subgroup analyses found little or no difference in the effect of balance and functional exercises on the number of people experiencing one or more falls in trials where all participants were at an increased risk of falling (RR 0.86, 95% CI 0.81 to 0.91; 4639 participants, 22 studies, I² = 6%) compared with trials that did not use increased risk of falling as an entry criterion (RR 0.88, 95% CI 0.80 to 97; 3649 participants, 15 studies, I² = 18%); test for subgroup differences: Chi² = 0.21, df = 1, P = 0.65, I² = 0% (Analysis 8.2).
8.2. Analysis.
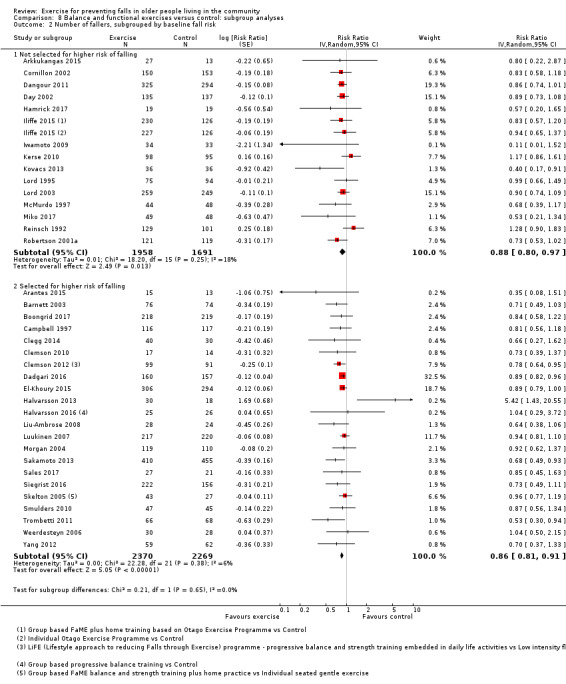
Comparison 8 Balance and functional exercises versus control: subgroup analyses, Outcome 2 Number of fallers, subgrouped by baseline fall risk.
Subgroup analyses found little or no difference in the effect of balance and functional exercises on the number of people experiencing one or more falls in trials where interventions were delivered by health professionals (RR 0.82, 95% CI 0.75 to 0.90; 2894 participants, 19 studies, I² = 5%) compared with trials where interventions were delivered by trained instructors who were not health professionals (RR 0.89, 95% CI 0.84 to 0.94; 5394 participants, 18 studies, I² = 11%); test for subgroup differences: Chi² = 1.71, df = 1, P = 0.19, I² = 41% (Analysis 8.4).
8.4. Analysis.
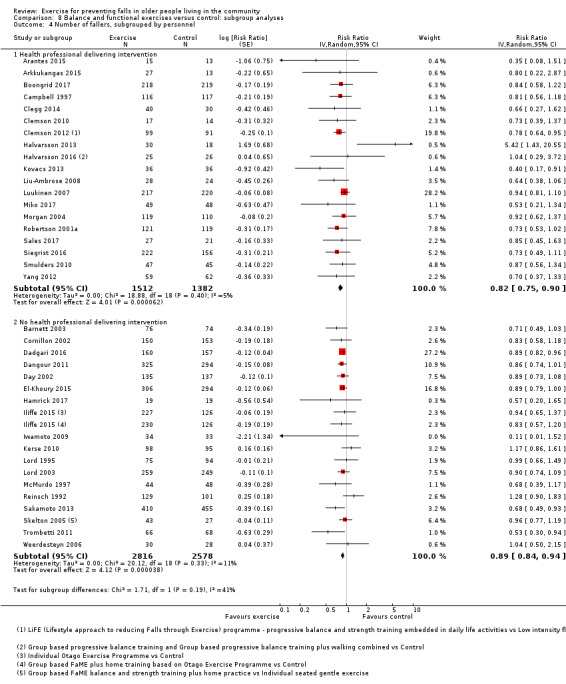
Comparison 8 Balance and functional exercises versus control: subgroup analyses, Outcome 4 Number of fallers, subgrouped by personnel.
Subgroup analyses also found little or no difference in the effect of balance and functional exercises on the number of people experiencing one or more falls in trials where interventions were delivered in a group setting (RR 0.87, 95% CI 0.80 to 0.95; 4465 participants, 22 studies, I² = 19%) compared with trials where interventions were delivered individually (RR 0.87, 95% CI 0.82 to 0.92; 4075 participants, 16 studies, I² = 0%); test for subgroup differences: Chi² = 0.01, df = 1 (P = 0.92), I² = 0% (Analysis 8.6).
8.6. Analysis.
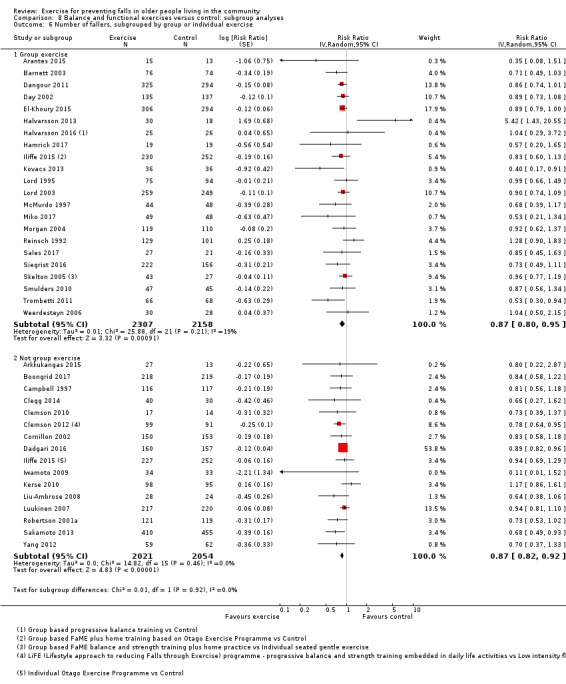
Comparison 8 Balance and functional exercises versus control: subgroup analyses, Outcome 6 Number of fallers, subgrouped by group or individual exercise.
Resistance exercises versus control
We are uncertain whether exercise, classified as being primarily resistance or strength exercises, reduces the number of people experiencing one or more falls compared with control (RR 0.81, 95% CI 0.57 to 1.15; 163 participants, 2 studies, I² = 0%; very low‐certainty evidence; Analysis 2.6).
3D (Tai Chi) exercise versus control
Exercise interventions that were classified as 3D (Tai Chi or similar) reduce the number of people experiencing one or more falls by 20% compared with control (RR 0.80, 95% CI 0.70 to 0.91; 2677 participants, 8 studies, I² = 42%; high‐certainty evidence; Analysis 2.6).
3D (dance) exercise versus control
We are uncertain whether exercise, classified as being primarily 3D (dance), reduces the number of people experiencing one or more falls compared with control (RR 1.35, 95% CI 0.83 to 2.20; 522 participants, 1 study; very low‐certainty evidence; Analysis 2.6). We assessed the certainty of the evidence as very low due to there being wide CIs in the single trial.
Walking programme versus control
We are uncertain whether exercise, classified as being primarily walking programmes, reduces the number of people experiencing one or more falls compared with control (RR 1.05, 95% CI 0.71 to 1.54; 441 participants, 2 studies, I² = 50%; Analysis 2.6), as we assessed the certainty of the evidence as very low.
Multiple categories of exercise versus control
Exercise interventions that included multiple categories of the ProFaNE taxonomy probably reduce the number of people experiencing one or more falls by 22% compared with control (RR 0.78, 95% CI 0.64 to 0.96; 1623 participants, 17 studies, I² = 48%; moderate‐certainty evidence; Analysis 2.6).
Sensitivity analyses revealed little difference in the results when we pooled only trials that included the two most common components (balance and functional exercises plus resistance exercises) (RR 0.76, 95% CI 0.61 to 0.95; 1375 participants, 13 studies; I² = 53%; Analysis 19.2).
19.2. Analysis.
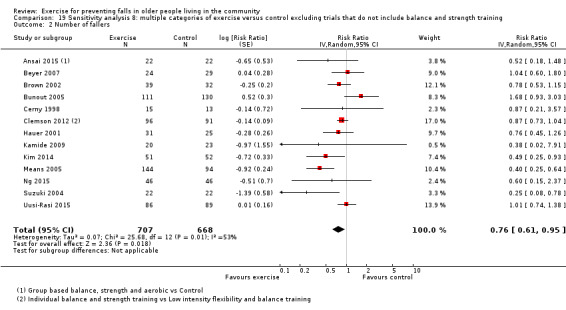
Comparison 19 Sensitivity analysis 8: multiple categories of exercise versus control excluding trials that do not include balance and strength training, Outcome 2 Number of fallers.
Subgroup analyses found there may be little or no difference in the effect of exercise interventions that included multiple categories on the number of people experiencing one or more falls in trials where all participants were at an increased risk of falling (RR 0.84, 95% CI 0.71 to 1.00; 913 participants, 10 studies, I² = 19%) compared with trials that did not use increased risk of falling as an entry criterion (RR 0.70, 95% CI 0.41 to 1.19; 710 participants, 7 studies, I² = 67%); test for subgroup differences: Chi² = 0.42, df = 1, P = 0.52, I² = 0% (Analysis 9.2).
9.2. Analysis.
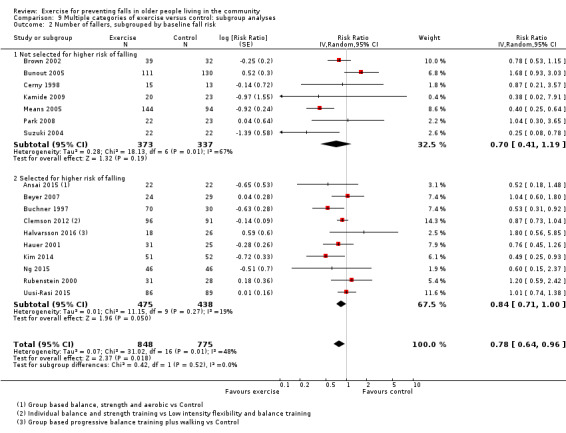
Comparison 9 Multiple categories of exercise versus control: subgroup analyses, Outcome 2 Number of fallers, subgrouped by baseline fall risk.
Subgroup analyses found there may be little or no difference in the effect of exercise interventions that included multiple categories on the number of people experiencing one or more falls in trials where interventions were delivered by health professionals (RR 0.81, 95% CI 0.65 to 1.02; 867 participants, 8 studies, I² = 50%) compared with trials where interventions were delivered by trained instructors who were not health professionals (RR 0.70, 95% CI 0.45 to 1.10; 711 participants, 8 studies, I² = 57%); test for subgroup differences: Chi² = 0.34, df = 1, P = 0.56, I² = 0% (Analysis 9.4).
9.4. Analysis.
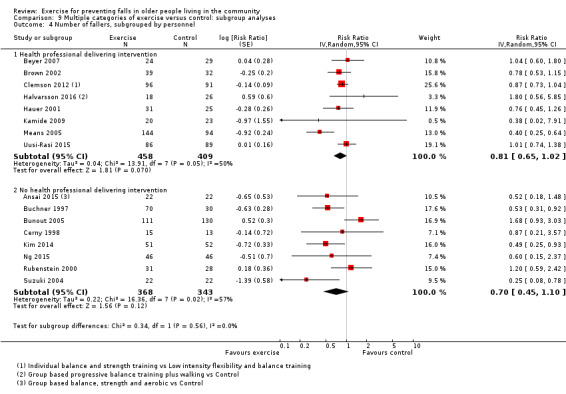
Comparison 9 Multiple categories of exercise versus control: subgroup analyses, Outcome 4 Number of fallers, subgrouped by personnel.
Subgroup analyses found there may be little or no difference in the effect of exercise interventions that included multiple categories on the number of people experiencing one or more falls in trials where interventions were delivered in a group setting (RR 0.77, 95% CI 0.60 to 1.00; 1301 participants, 14 studies, I² = 57%) compared with trials where interventions were delivered individually (RR 0.86, 95% CI 0.72 to 1.03; 322 participants, 3 studies, I² = 0%); test for subgroup differences: Chi² = 0.45, df = 1 (P = 0.50), I² = 0% (Analysis 9.6).
9.6. Analysis.
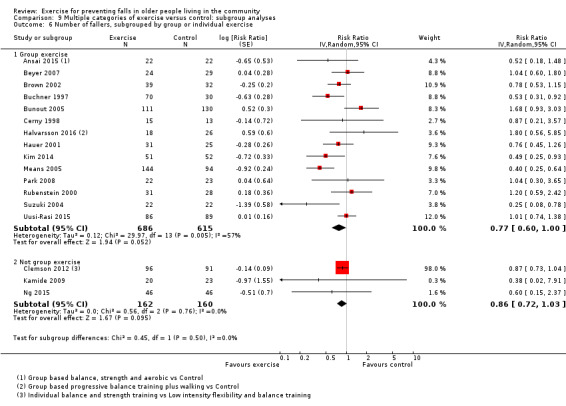
Comparison 9 Multiple categories of exercise versus control: subgroup analyses, Outcome 6 Number of fallers, subgrouped by group or individual exercise.
Long‐term follow‐up
Data from the three studies reporting on the number of people experiencing one or more falls at more than 18 months after randomisation are shown in Analysis 2.7. Balance and functional exercises may reduce the number of fallers in the long term (RR 0.86, 95% CI 0.78 to 0.94; 1325 participants, 2 studies; I² = 0%; low‐certainty evidence) but there is no evidence of difference for a multiple exercise programme (including balance and strength training) tested in Uusi‐Rasi 2015 (RR 1.01, 95% CI 0.74 to 1.38; 175 participants; low‐certainty evidence).
2.7. Analysis.
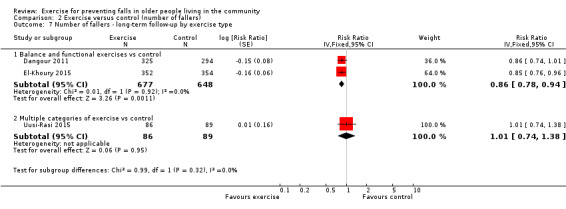
Comparison 2 Exercise versus control (number of fallers), Outcome 7 Number of fallers ‐ long‐term follow‐up by exercise type.
Number of people who experienced one or more fall‐related fractures
Exercise (all types) may reduce the number of people experiencing one or more fall‐related fractures by 27% compared with control (RR 0.73, 95% CI 0.56 to 0.95; 4047 participants, 10 studies, I² = 0%; low‐certainty evidence; Analysis 3.1).
3.1. Analysis.
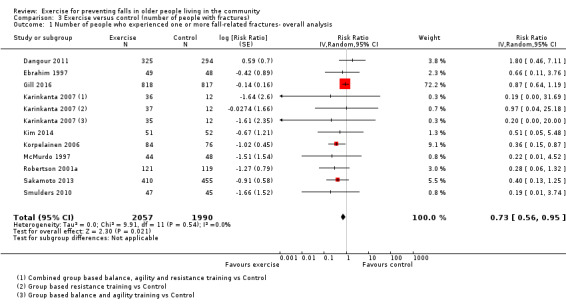
Comparison 3 Exercise versus control (number of people with fractures), Outcome 1 Number of people who experienced one or more fall‐related fractures‐ overall analysis.
Subgroup analysis by falls risk at baseline found there may be little or no difference in the effect of exercise (all types) on the number of people experiencing one or more fall‐related fractures in trials where all participants were at an increased risk of falling (RR 0.80, 95% CI 0.60 to 1.07; 2792 participants, 5 studies, I² = 0) compared with trials that did not use increased risk of falling as an entry criterion (RR 0.48, 95% CI 0.26 to 0.91; 1255 participants, 5 studies, I² = 0%); test for subgroup differences: Chi² = 2.05, df = 1, P = 0.15, I² = 50.6% (Analysis 3.2).
3.2. Analysis.
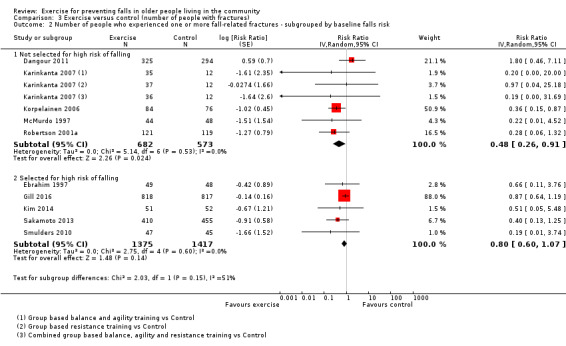
Comparison 3 Exercise versus control (number of people with fractures), Outcome 2 Number of people who experienced one or more fall‐related fractures ‐ subgrouped by baseline falls risk.
Subgroup analyses found there may be little or no difference in the effect of exercise (all types) on the number of people experiencing one or more fall‐related fractures in trials where participants were aged 75 years or older (RR 0.61, 95% CI 0.31 to 1.20; 2740 participants, 3 studies, I² = 42%) compared with trials where participants were aged less than 75 years (RR 0.53, 95% CI 0.29 to 0.96; 1308 participants, 7 studies, I² = 0%); test for subgroup differences: Chi² = 0.1, df = 1, P = 0.75, I² = 0% (Analysis 3.3).
3.3. Analysis.
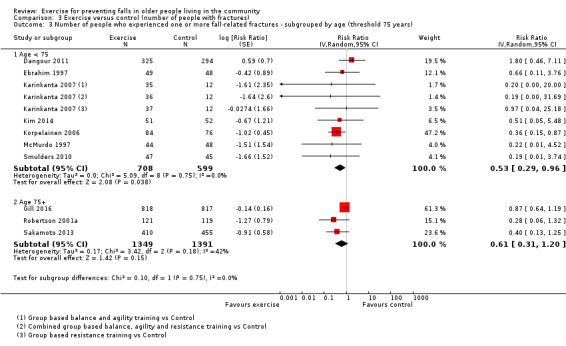
Comparison 3 Exercise versus control (number of people with fractures), Outcome 3 Number of people who experienced one or more fall‐related fractures ‐ subgrouped by age (threshold 75 years).
The subgroup analysis by exercise type did not show subgroup differences on the effects on fall‐related fractures: test for subgroup differences: Chi² = 4.22, df = 3, P = 0.24, I² = 28.9% (Analysis 3.4).
3.4. Analysis.
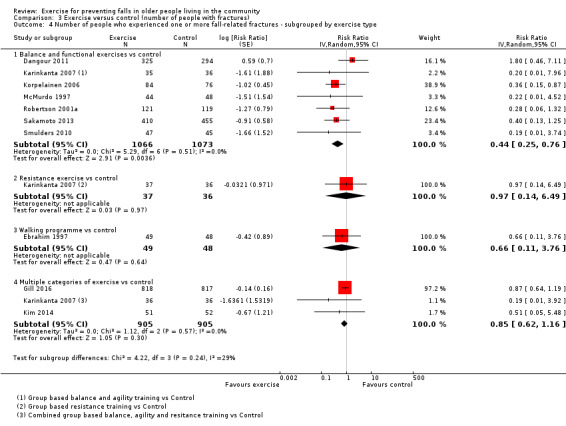
Comparison 3 Exercise versus control (number of people with fractures), Outcome 4 Number of people who experienced one or more fall‐related fractures ‐ subgrouped by exercise type.
Different categories of primary exercise versus control
Balance and functional exercises versus control
Exercise interventions that were classified as being primarily gait, balance, co‐ordination or functional task training using the ProFaNE taxonomy, may reduce the number of people experiencing one or more fall‐related fractures by 56% compared with control (RR 0.44, 95% CI 0.25 to 0.76; 2139 participants, 7 studies, I² = 0%; low‐certainty evidence; Analysis 3.4).
Resistance exercises versus control
We are uncertain whether exercises, classified as being primarily resistance or strength exercises using the ProFaNE taxonomy, reduce the number of people experiencing one or more fall‐related fractures compared with control (RR 0.97, 95% CI 0.14 to 6.49; 73 participants; 1 study; very low‐certainty of evidence due to single study with very wide CI; Analysis 3.4).
3D exercise versus control
We did not find any studies that looked at the impact of 3D exercises (Tai Chi or dance) on the number of people experiencing one or more fall‐related fractures compared with control.
Walking programme versus control
We are uncertain whether exercises, classified as being primarily walking programmes using the ProFaNE taxonomy, reduce the number of people experiencing one or more fall‐related fractures compared with control (RR 0.66, 95% CI 0.11 to 3.76; 97 participants, 1 study; very low‐certainty evidence due to a single study with very wide CI; Analysis 3.4).
Multiple categories of exercise versus control
Exercise interventions that include multiple categories of the ProFaNE taxonomy, may slightly reduce the number of people experiencing one or more fall‐related fractures compared with control; however, the 95% CI includes the possibility of both reduced and increased numbers of people experiencing fall‐related fractures (RR 0.85, 95% CI 0.62 to 1.16; 1810 participants, 3 studies, I² = 0%; low‐certainty evidence; Analysis 3.4).
Long‐term follow‐up
Three studies, each testing a different exercise category, reported the number of people who experienced fractures more than 18 months after randomisation (Dangour 2011; Ebrahim 1997; Gill 2016). The effect of exercise on fractures at long‐term follow‐up is unclear (RR 0.93, 95% CI 0.69 to 1.25; 2351 participants, 3 studies; very low‐certainty; Analysis 3.5). Only the data (6 versus 4 fractures at 24 months compared with 2 versus 3 at 12 months) for Ebrahim 1997 differed from that presented in the main analysis (Analysis 3.1).
3.5. Analysis.
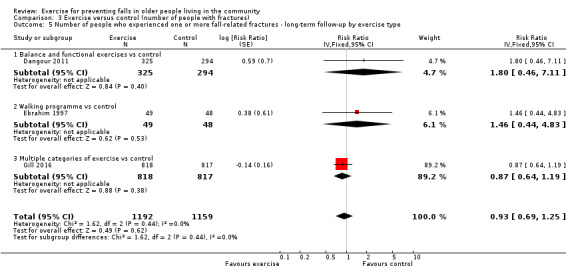
Comparison 3 Exercise versus control (number of people with fractures), Outcome 5 Number of people who experienced one or more fall‐related fractures ‐ long‐term follow‐up by exercise type.
Number of people who experienced one or more falls that resulted in hospital admission
Only two studies reported this outcome (Clegg 2014; Gill 2016). We are uncertain of the finding that exercise (all types) makes little or no difference to the number of people who experience one or more falls requiring hospital admission compared with control (RR 0.78, 95% CI 0.51 to 1.18; 1705 participants, 2 studies, I² = 0%; very low‐certainty evidence, downgraded three levels due to high risk of bias, imprecision (wide CI) and because a large number of studies included in the review do not contribute data to the outcome; Analysis 4.1).
4.1. Analysis.
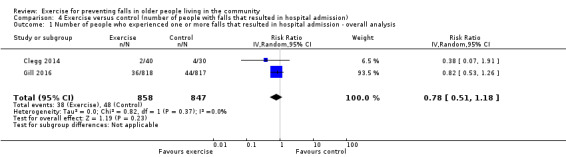
Comparison 4 Exercise versus control (number of people with falls that resulted in hospital admission), Outcome 1 Number of people who experienced one or more falls that resulted in hospital admission ‐ overall analysis.
Number of people who experienced one or more falls that required medical attention
Exercise (all types) may reduce the number of people who experience one or more falls requiring medical attention by 39% compared with control (RR 0.61, 95% CI 0.47 to 0.79; 1019 participants, 5 studies (7 comparisons), I² = 3%; low‐certainty evidence downgraded due to imprecision and risk of publication bias; Analysis 5.1).
5.1. Analysis.
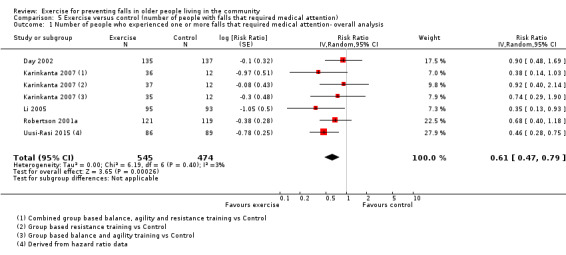
Comparison 5 Exercise versus control (number of people with falls that required medical attention), Outcome 1 Number of people who experienced one or more falls that required medical attention‐ overall analysis.
Different categories of primary exercise versus control
Balance and functional exercises versus control
Exercise interventions that were classified as being primarily gait, balance, co‐ordination or functional task training using the ProFaNE taxonomy, may make little or no difference to the number of people who experienced one or more falls requiring medical attention compared with control (RR 0.76, 95% CI 0.54 to 1.09; 583 participants, 3 studies, I² = 0%; low‐certainty evidence; Analysis 5.2).
5.2. Analysis.
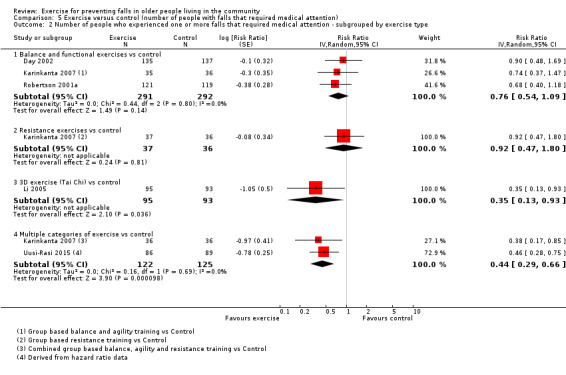
Comparison 5 Exercise versus control (number of people with falls that required medical attention), Outcome 2 Number of people who experienced one or more falls that required medical attention ‐ subgrouped by exercise type.
Resistance exercises versus control
Exercises classified as being primarily resistance or strength exercises using the ProFaNE taxonomy, may make little or no difference to the number of falls requiring medical attention compared with control (RR 0.92, 95% CI 0.47 to 1.80; 73 participants, 1 study; very low‐certainty evidence; Analysis 5.2).
3D (Tai Chi) exercise versus control
Exercise interventions that were classified as 3D (Tai Chi or similar) may reduce the number of falls requiring medical attention by 65% compared with control (RR 0.35, 95% CI 0.13 to 0.93; 188 participants, 1 study; low‐certainty evidence; Analysis 5.2).
Walking programme versus control
This outcome was not reported.
Multiple categories of exercise versus control
Exercise interventions that include multiple categories of the ProFaNE taxonomy, may reduce the rate of falls requiring medical attention (RR 0.44, 95% CI 0.29 to 0.66; 247 participants, 2 studies, I² = 0%; low‐certainty evidence; Analysis 5.2).
Long‐term follow‐up
Two studies reported on this outcome at more than 18 months after randomisation (Karinkanta 2007; Uusi‐Rasi 2015). Pooled data from these two studies showed exercise (all types) may reduce the number of people who experience one or more falls requiring medical attention in the long term (RR 0.54, 95% CI 0.37 to 0.78; 319 participants, 2 studies; low‐certainty evidence; Analysis 5.3). The same data from both studies were used in Analysis 5.1 and Analysis 5.3.
5.3. Analysis.
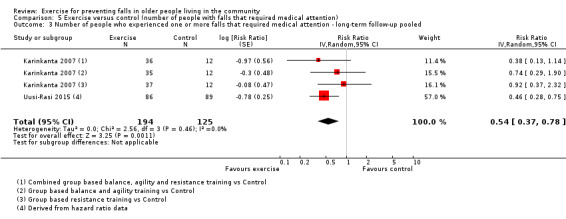
Comparison 5 Exercise versus control (number of people with falls that required medical attention), Outcome 3 Number of people who experienced one or more falls that required medical attention ‐ long‐term follow‐up pooled.
Health‐related quality of life
We were able to pool data from 15 of the 23 trials that assessed health‐related quality of life in people not recently discharged from hospital. Based on pooled standardised mean difference (SMD) results from the 15 trials (17 comparisons) that reported final scores, exercise interventions may make little or no difference to people's reported health‐related quality of life compared with those who received usual care or an attention control; however, the 95% CI includes the possibility of both increased and reduced quality of life (SMD ‐0.03, 95% CI ‐0.10 to 0.04; 3172 participants, 15 studies; I2 = 76%; low‐quality evidence downgraded two levels due to inconsistency (there was considerable heterogeneity, 76%), and risk of bias (removing studies with high risk of bias on two or more items had a marked impact on results; Analysis 6.1).
6.1. Analysis.
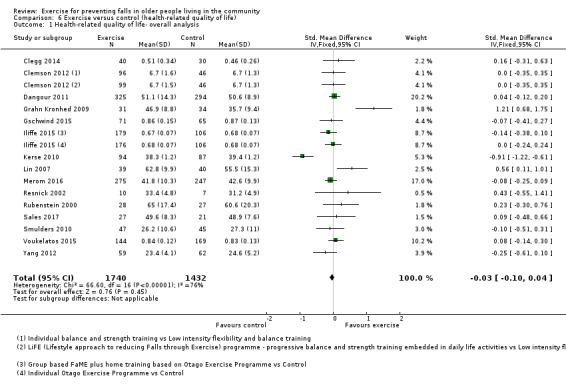
Comparison 6 Exercise versus control (health‐related quality of life), Outcome 1 Health‐related quality of life‐ overall analysis.
Four trials (6 comparisons) reported end point scores using the EQ‐5D; the SMD converted back to mean difference (MD) ‐0.0026 points (95% CI ‐0.0086 to 0.0034) on the 0 to 1 EQ‐5D scale, which is less than the minimally important difference of 0.074 (Walters 2005). For the five trials that measured health‐related quality of life using SF‐36, converting these data to the SF‐36 scale (0 worst to 100 best) indicates that the estimated MD of 0.36 (95% CI ‐1.20 to 0.47) is not clinically important, as the minimally important difference is usually 3 to 5 (Walters 2003).
Appendix 16 provides summary information for all 23 trials including three post‐hospital studies and those which we could not include in the meta‐analysis (e.g. because they used unique outcome measures or reported median, IQR or P value), the results of which are similar to the above.
Number of people who experienced one or more adverse events
Twenty‐seven trials reported on adverse event to some degree (Appendix 13). Fourteen of the trials reporting on adverse events stated there were no adverse events.
Iliffe 2015 measured the number of people experiencing adverse events in both groups throughout the trial period and reported 59 events classified as 'adverse reactions' or 'possible adverse reactions' in the group receiving FaME intervention, 60 in the OEP group and 45 in the control group; the majority were reports of musculoskeletal pain and none were serious. No other studies reported adverse events that were monitored closely in all groups over the entire study period. A serious adverse effect was a pelvic stress fracture reported in Clemson 2012. The remaining trials reported non‐serious adverse events of a musculoskeletal nature, with a median of three events (range 1 to 26) in the intervention group. The majority of reported adverse events were of a musculoskeletal nature and not serious. Of the studies that reported adverse events, a greater proportion of the strength‐only exercises were associated with adverse events than in the gait, balance and functional training or multiple exercise categories.
Different categories of primary exercise
Balance and functional exercises versus control
Adverse events were reported in 15 of the 48 trials, including exercise interventions that were classified as being primarily gait, balance, co‐ordination or functional task training using the ProFaNE taxonomy. Two hundred adverse events were reported; most were non‐serious adverse events of a musculoskeletal nature, one trial (two intervention arms) reported 128 of these adverse events (Iliffe 2015), one intervention arm reported shortness of breath in four participants (Liu‐Ambrose 2004), another trial reported palpitations in a participant (Sakamoto 2013), and one trial reported a pelvic stress fracture (Clemson 2012). See Appendix 13.
Resistance exercises versus control
Adverse events were reported in one trial, including exercises classified as being primarily resistance or strength exercises using the ProFaNE taxonomy (Liu‐Ambrose 2004). The study reported 10 musculoskeletal complaints in the intervention group and one musculoskeletal complaint in the control group.
3D (Tai Chi) exercise versus control
Adverse events were reported in two of 10 trials with 3D (Tai Chi) as the primary intervention. There were zero occurrences of adverse events.
3D (dance) exercise versus control
Adverse events were reported in the one trial in this analysis, in the intervention group only. There were zero occurrences of adverse events.
Walking programme versus control
This outcome was not reported.
Multiple categories of exercise versus control
Adverse events were reported in 10 of the 21 trials of exercise interventions that include multiple categories of the ProFaNE taxonomy. Adverse events were reported for both intervention and control groups (5 trials), or the intervention group only (5 trials). There was a total of 43 adverse events reported. The majority were non‐serious and of a musculoskeletal nature. There was reported exacerbation of pre‐existing osteoarthritis conditions (Uusi‐Rasi 2015), and inguinal hernia surgery was reported in one intervention arm (Clemson 2012).
Number of people who died
Death was primarily reported as a reason for loss to follow‐up in all 30 trials with separate group data. Exercise (all types) may reduce the number of people who died compared with control; however, the 95% CI includes the possibility of both reduced death and increased death with exercise (RR 0.86, 95% CI 0.66 to 1.12; 10,037 participants, 30 studies, I² = 0%; low‐certainty evidence (downgraded one level due to risk of bias, as results changed, becoming statistically significant, with removal of the 14 trials with a high risk of bias on one or more items; and one level for indirectness, as the outcome was assessed indirectly as a reason for loss to follow‐up; Analysis 7.1). The risk of death did not differ between the trials including people selected or not‐selected for risk of falling: test for subgroup differences: Chi² = 0.19, df = 1, P = 0.67, I² = 0% (Analysis 7.2). None of the deaths were explicitly linked to trial participation.
7.1. Analysis.
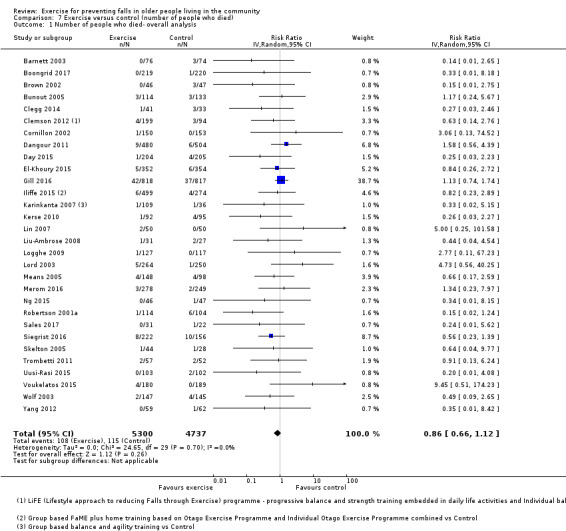
Comparison 7 Exercise versus control (number of people who died), Outcome 1 Number of people who died‐ overall analysis.
7.2. Analysis.
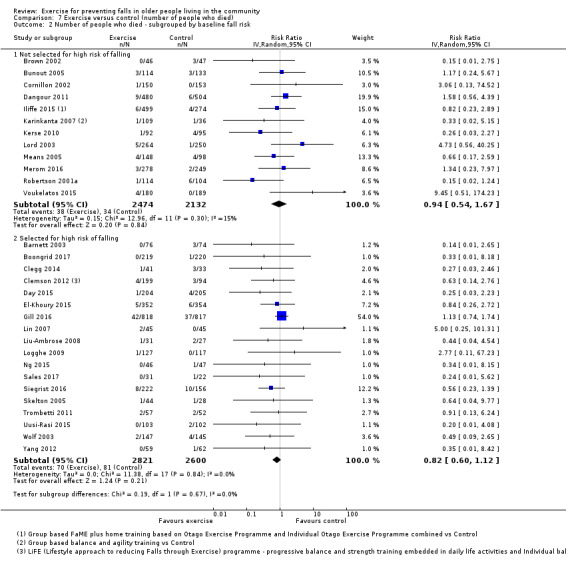
Comparison 7 Exercise versus control (number of people who died), Outcome 2 Number of people who died ‐ subgrouped by baseline fall risk.
Exercise (all types) versus control tested in people who had recently been discharged from hospital
Four studies investigated outcomes in people who had recently been discharged from hospital (Haines 2009; Latham 2003; Sherrington 2014; Vogler 2009). Results of individual studies for rate of falls (3 trials) are shown in Analysis 10.1; number of falls (4 trials) in Analysis 10.2; health‐related quality of life (3 trials) in Analysis 10.3; and mortality (4 trials) in Analysis 10.4. Given the diversity of interventions, we did not pool data. It is noted that overall, the effects of exercise on falls appear smaller (or in the opposite direction in the case of Sherrington 2014) in these studies compared with studies in the general older population (very low‐certainty evidence).
10.1. Analysis.
Comparison 10 Exercise versus control (by exercise type, in people after hospital stays), Outcome 1 Rate of falls.
10.2. Analysis.
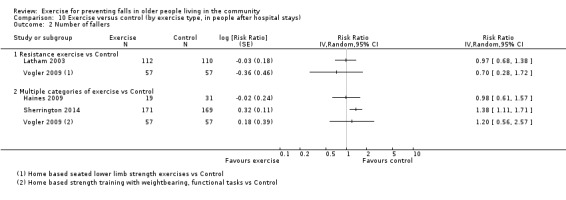
Comparison 10 Exercise versus control (by exercise type, in people after hospital stays), Outcome 2 Number of fallers.
10.3. Analysis.
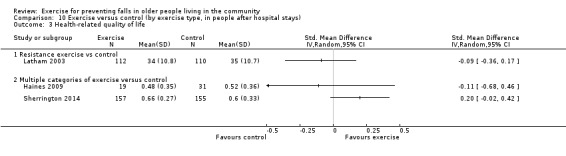
Comparison 10 Exercise versus control (by exercise type, in people after hospital stays), Outcome 3 Health‐related quality of life.
10.4. Analysis.
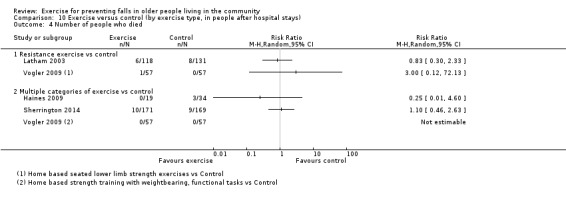
Comparison 10 Exercise versus control (by exercise type, in people after hospital stays), Outcome 4 Number of people who died.
All four studies reported on adverse events to some degree (Appendix 13). Latham 2003 measured the number of people experiencing adverse events in both groups throughout the trial period and reported that 18 participants had back and knee pain directly attributable to the exercise programme; there were no details of the five participants with adverse events in the control group. The remaining trials reported non‐serious adverse events of a musculoskeletal nature.
Exercise versus exercise
Comparisons of different types of exercise
The results of individual trials directly comparing different types of exercise are shown for rate of falls in Analysis 11.1, with long‐term rate of falls data in Analysis 11.2; number of fallers in Analysis 11.3; number with fall‐related fractures in Analysis 11.4; number requiring medical attention in Analysis 11.5; quality of life in Analysis 11.6; and mortality in Analysis 11.7. Given the variability between programmes, we did not undertake any meta‐analyses for these comparisons for any of the outcomes. Overall there is very low‐certainty evidence for each comparison.
11.1. Analysis.
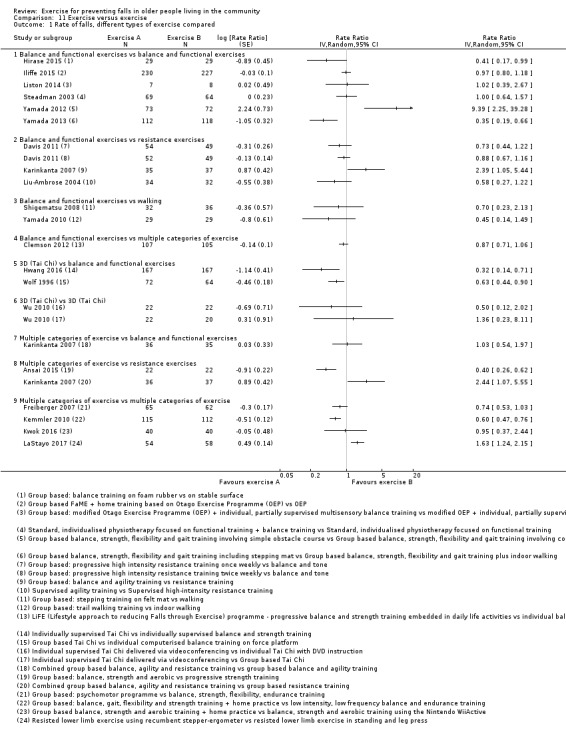
Comparison 11 Exercise versus exercise, Outcome 1 Rate of falls, different types of exercise compared.
11.2. Analysis.

Comparison 11 Exercise versus exercise, Outcome 2 Rate of falls >18 months, different types of exercise compared.
11.3. Analysis.
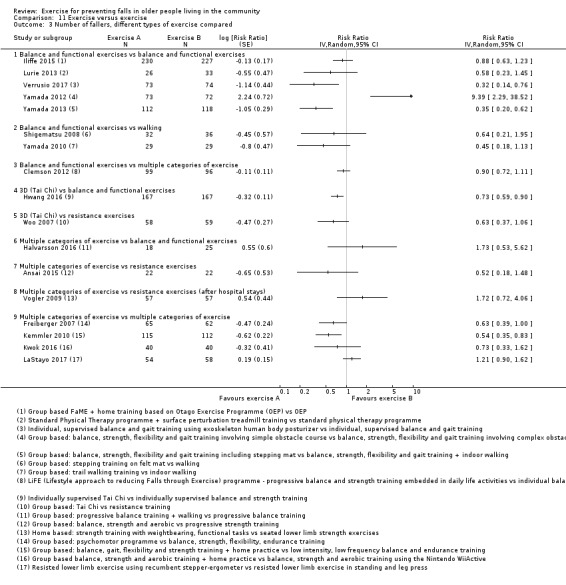
Comparison 11 Exercise versus exercise, Outcome 3 Number of fallers, different types of exercise compared.
11.4. Analysis.
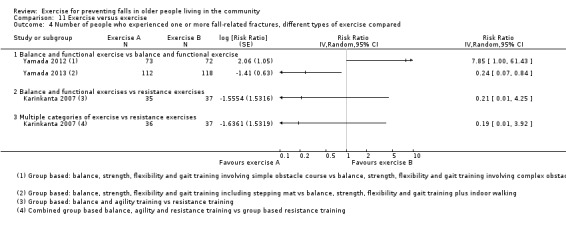
Comparison 11 Exercise versus exercise, Outcome 4 Number of people who experienced one or more fall‐related fractures, different types of exercise compared.
11.5. Analysis.
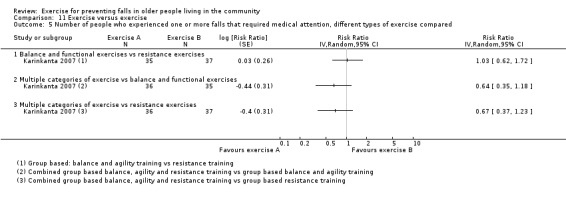
Comparison 11 Exercise versus exercise, Outcome 5 Number of people who experienced one or more falls that required medical attention, different types of exercise compared.
11.6. Analysis.
Comparison 11 Exercise versus exercise, Outcome 6 Quality of life, different types of exercise compared.
11.7. Analysis.
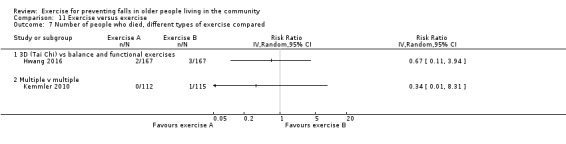
Comparison 11 Exercise versus exercise, Outcome 7 Number of people who died, different types of exercise compared.
Most of the trials in these analyses did not find significant differences in the fall prevention effects of different programmes, but most were not likely to be adequately powered to detect differences between different exercise programmes.
A few studies did find greater effects of particular programmes. For example, Kemmler 2010 found greater effects on the rate of falls of a more intensive programme delivered twice a week compared with a low intensity programme delivered once a week. Studies by Yamada et al found greater fall prevention effects of complex obstacle negotiation training compared with simple training (Yamada 2012), and greater effects of multidimensional stepping compared with walking (Yamada 2013). Both these interventions were delivered in addition to group exercise primarily targeting balance. Hwang 2016 found greater effects of Tai Chi than supervised balance and strength training on the rate of falls and the number of people falling. All these findings require confirmation in different and larger studies.
Different modes of delivery (e.g. group versus individual) of the same type of exercise
The results of individual trials that provided direct comparisons between the same programmes being delivered in group‐based settings and individually are shown for rate of falls in Analysis 11.8; number of fallers in Analysis 11.9; number requiring hospital admission in Analysis 11.10; quality of life in Analysis 11.11; and mortality in Analysis 11.12. All results were inconclusive; the five trials were too small to draw conclusions (Barker 2016; Helbostad 2004; Iliffe 2015; Kyrdalen 2014; Wu 2010).
11.8. Analysis.
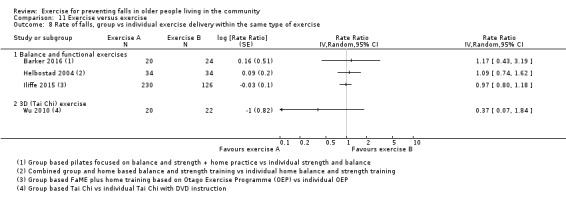
Comparison 11 Exercise versus exercise, Outcome 8 Rate of falls, group vs individual exercise delivery within the same type of exercise.
11.9. Analysis.
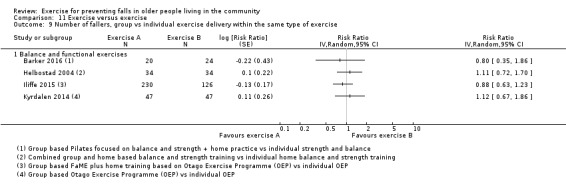
Comparison 11 Exercise versus exercise, Outcome 9 Number of fallers, group vs individual exercise delivery within the same type of exercise.
11.10. Analysis.
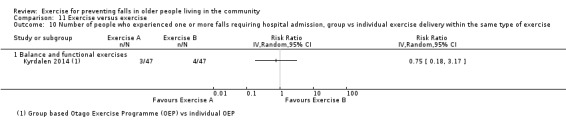
Comparison 11 Exercise versus exercise, Outcome 10 Number of people who experienced one or more falls requiring hospital admission, group vs individual exercise delivery within the same type of exercise.
11.11. Analysis.
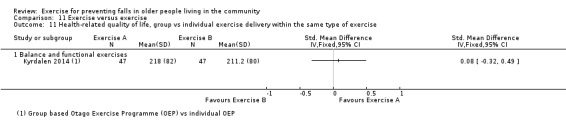
Comparison 11 Exercise versus exercise, Outcome 11 Health‐related quality of life, group vs individual exercise delivery within the same type of exercise.
11.12. Analysis.
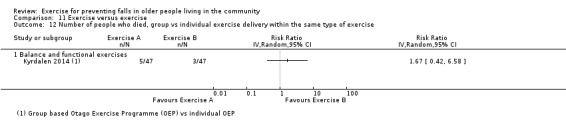
Comparison 11 Exercise versus exercise, Outcome 12 Number of people who died, group vs individual exercise delivery within the same type of exercise.
Different doses (e.g. higher intensity versus lower intensity) of the same type of exercise
The results of the individual trials that directly compared higher with lower doses of the same type of exercise are shown for rate of falls in Analysis 11.13, number of fallers in Analysis 11.14, and mortality in Analysis 11.15. Taylor 2012 found a greater impact on the rate of falls when Tai Chi classes were delivered twice rather than once per week. The other two trials were too small to draw conclusions (Ballard 2004; Davis 2011).
11.13. Analysis.
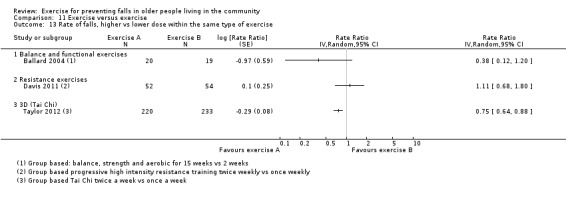
Comparison 11 Exercise versus exercise, Outcome 13 Rate of falls, higher vs lower dose within the same type of exercise.
11.14. Analysis.
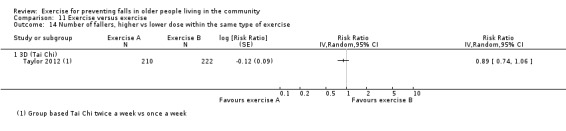
Comparison 11 Exercise versus exercise, Outcome 14 Number of fallers, higher vs lower dose within the same type of exercise.
11.15. Analysis.
Comparison 11 Exercise versus exercise, Outcome 15 Number of people who died, higher vs lower dose within the same type of exercise.
Number of people who experienced one or more adverse events
No studies reported adverse events that were monitored closely in all groups over the entire study period. Adverse events reported to any degree are described in Appendix 13. Three of the 10 trials reporting on adverse events stated there were no adverse events. The remaining trials reported non‐serious adverse events of a musculoskeletal nature.
Economic data
We identified 12 out of the 108 studies that reported economic data. These included reports of costs of intervention or health service use and/or the results of trial‐based cost‐effectiveness or cost‐utility analyses (Appendix 17).
As in Gillespie 2012, the perspectives taken, the cost items measured and valued, and the type of healthcare resources included in the calculation of incremental cost‐effectiveness ratios (ICERs) all varied, so that comparison of ICERs for the interventions remains difficult even for evaluations carried out within similar health systems. Nonetheless, the results from several studies demonstrate the potential cost‐effectiveness of fall prevention interventions. One trial of the Otago Exercise Program showed cost savings in those aged 80 years and over resulting from fewer hospital admissions (Robertson 2001a). Davis 2011 reported that both once and twice weekly resistance training dominated control (balance and tone) classes in terms of both falls and quality‐adjusted life years (i.e. were less costly and more effective).
Other studies provide information on the cost per fall prevented from the delivery of exercise interventions. For example, Voukelatos 2007 reported AUD 1683 per fall prevented from group‐based Tai Chi and Davis 2009 reports a cost of CAD 247 per fall prevented from a group‐based exercise programme compared with guideline‐based care.
Sensitivity analyses
For each of these, the impact on the pooled exercise versus control fall rate outcome is summarised in Appendix 18. The results of the sensitivity analyses can be seen in Analyses 12 to 20.
Sensitivity analysis 1, removing trials that included participants aged < 65 years: Analysis 12.1 (rate of falls: pooled data); Analysis 12.2 (rate of falls: grouped by exercise); Analysis 12.3 (number of fallers: pooled data); Analysis 12.4 (number of fallers: grouped by exercise); Analysis 12.5 (fracture: pooled data); Analysis 12.6 (fracture: grouped by exercise type); Analysis 12.7 (medical attention: pooled data); Analysis 12.8 (medical attention: subgrouped by exercise).
Sensitivity analysis 2, removing trials with high risk of bias on any item: Analysis 13.1 (rate of falls: pooled data); Analysis 13.2 (rate of falls: subgrouped by exercise); Analysis 13.3 (number of fallers: pooled data); Analysis 13.4 (number of fallers: subgrouped by exercise type); Analysis 13.5 (fracture: pooled data).
Sensitivity analysis 3, removing trials with unclear or high risk of bias on allocation concealment: Analysis 14.1 (rate of falls: pooled data).
Sensitivity analysis 4, removing trials with unclear or high risk of bias on assessor blinding: Analysis 15.1 (rate of falls: pooled data).
Sensitivity analysis 5, removing trials with unclear or high risk of bias on incomplete outcome data: Analysis 16.1 (rate of falls: pooled data).
Sensitivity analysis 6, removing cluster‐randomised trials: Analysis 17.1 (rate of falls: pooled data).
Sensitivity analysis 7, all trials, fixed‐effect meta‐analysis: Analysis 18.1 (rate of falls: pooled data).
Sensitivity analysis 8, multiple categories of exercise versus control, removing trials that do not include balance and strength training: Analysis 19.1 (rate of falls: pooled data); Analysis 19.2 (number of fallers: pooled data).
Sensitivity analysis 9a, classification of interventions based on the Otago Exercise Program as multiple categories of exercise: Analysis 20.1 (rate of falls: pooled data); Analysis 20.2 (number of fallers: pooled data).
Sensitivity analysis 9b, classification of interventions that included balance and functional exercises plus strength exercises as multiple categories of exercise: Analysis 20.3 (rate of falls: pooled data); Analysis 20.4 (number of fallers: pooled data).
12.1. Analysis.
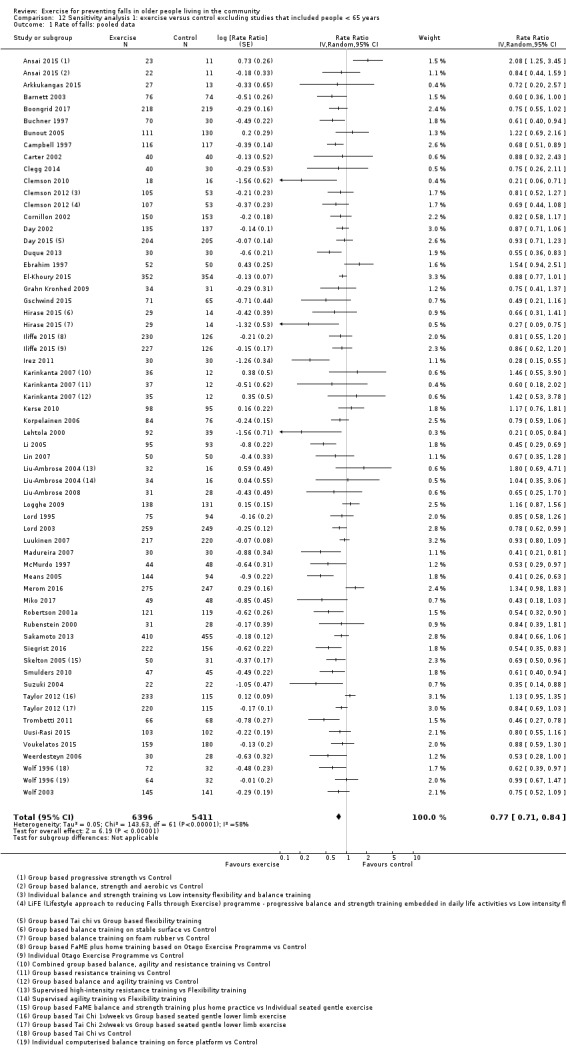
Comparison 12 Sensitivity analysis 1: exercise versus control excluding studies that included people < 65 years, Outcome 1 Rate of falls: pooled data.
12.2. Analysis.
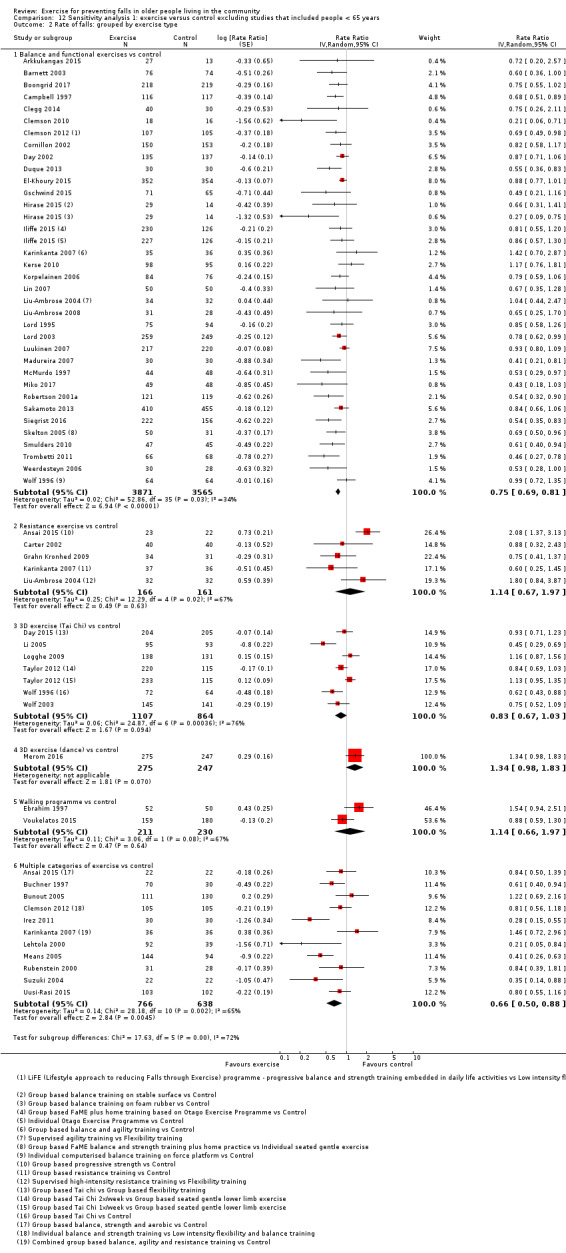
Comparison 12 Sensitivity analysis 1: exercise versus control excluding studies that included people < 65 years, Outcome 2 Rate of falls: grouped by exercise type.
12.3. Analysis.
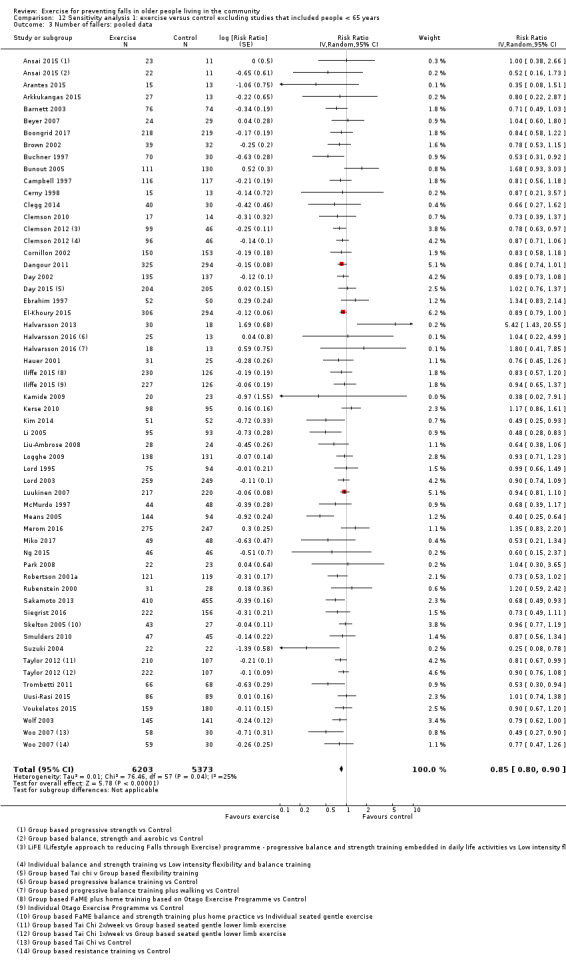
Comparison 12 Sensitivity analysis 1: exercise versus control excluding studies that included people < 65 years, Outcome 3 Number of fallers: pooled data.
12.4. Analysis.
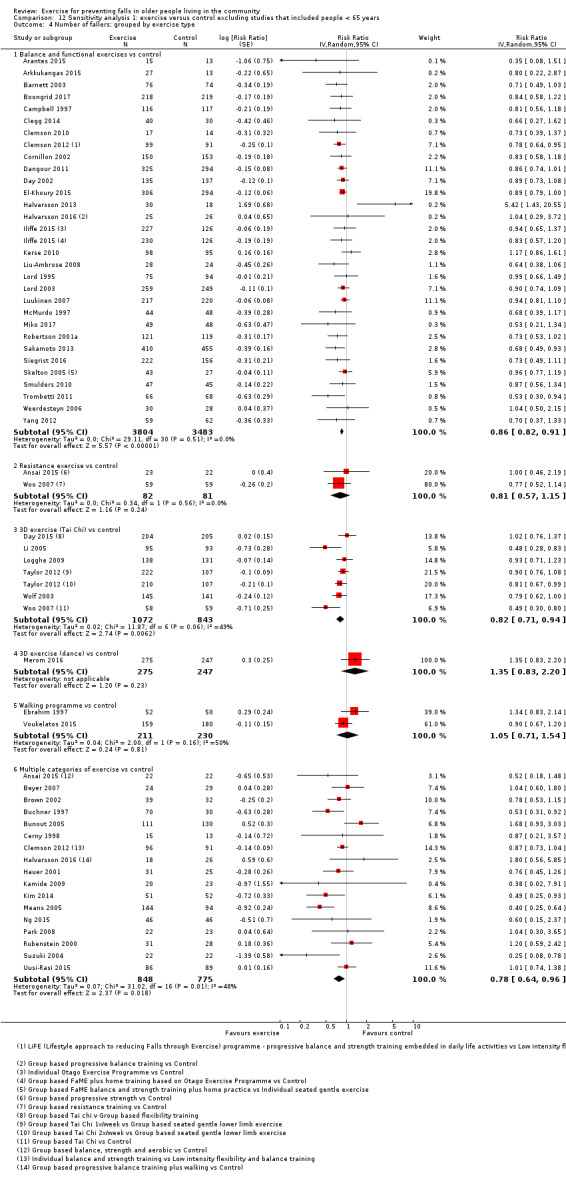
Comparison 12 Sensitivity analysis 1: exercise versus control excluding studies that included people < 65 years, Outcome 4 Number of fallers: grouped by exercise type.
12.5. Analysis.
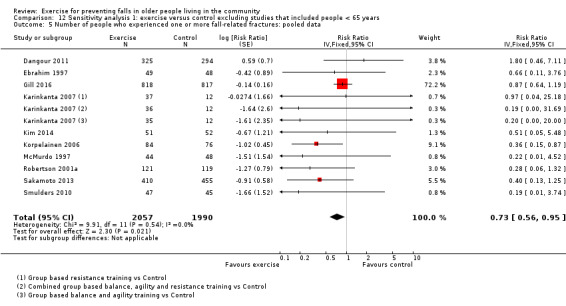
Comparison 12 Sensitivity analysis 1: exercise versus control excluding studies that included people < 65 years, Outcome 5 Number of people who experienced one or more fall‐related fractures: pooled data.
12.6. Analysis.
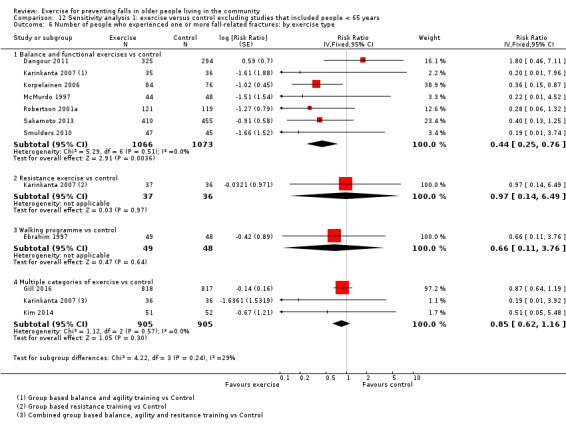
Comparison 12 Sensitivity analysis 1: exercise versus control excluding studies that included people < 65 years, Outcome 6 Number of people who experienced one or more fall‐related fractures: by exercise type.
12.7. Analysis.
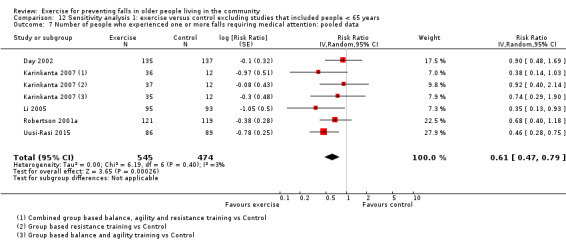
Comparison 12 Sensitivity analysis 1: exercise versus control excluding studies that included people < 65 years, Outcome 7 Number of people who experienced one or more falls requiring medical attention: pooled data.
12.8. Analysis.
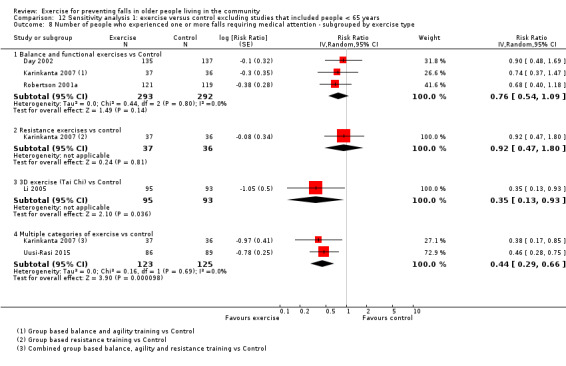
Comparison 12 Sensitivity analysis 1: exercise versus control excluding studies that included people < 65 years, Outcome 8 Number of people who experienced one or more falls requiring medical attention ‐ subgrouped by exercise type.
13.1. Analysis.
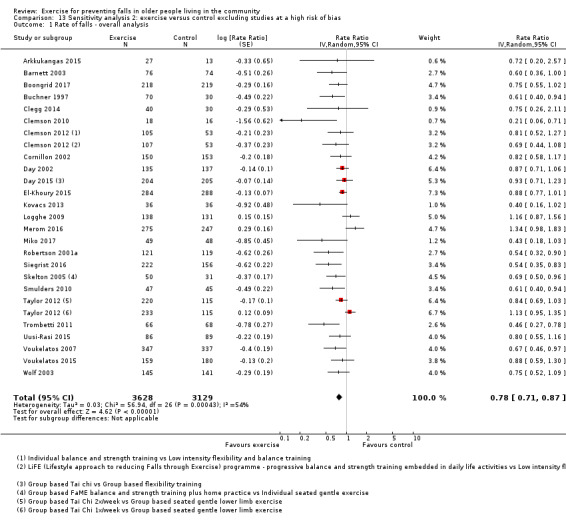
Comparison 13 Sensitivity analysis 2: exercise versus control excluding studies at a high risk of bias, Outcome 1 Rate of falls ‐ overall analysis.
13.2. Analysis.
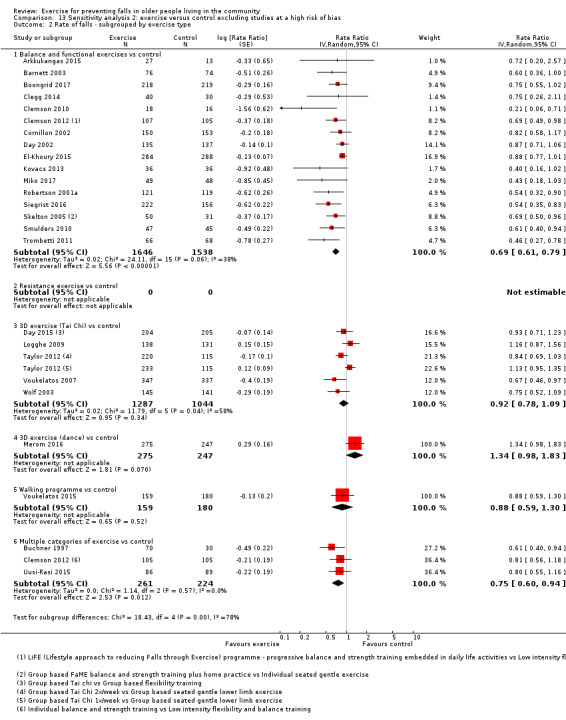
Comparison 13 Sensitivity analysis 2: exercise versus control excluding studies at a high risk of bias, Outcome 2 Rate of falls ‐ subgrouped by exercise type.
13.3. Analysis.
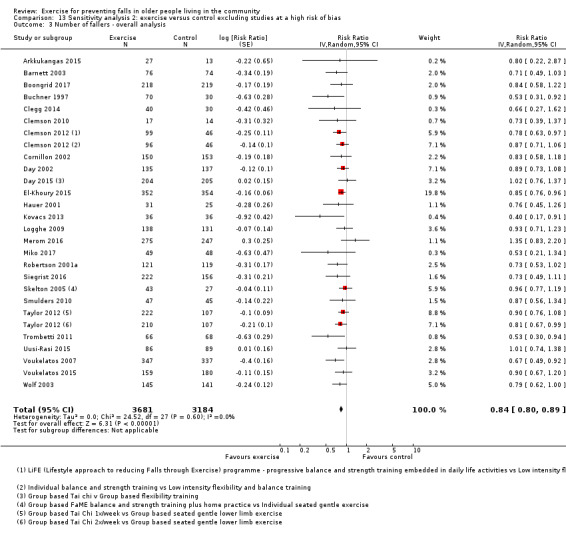
Comparison 13 Sensitivity analysis 2: exercise versus control excluding studies at a high risk of bias, Outcome 3 Number of fallers ‐ overall analysis.
13.4. Analysis.
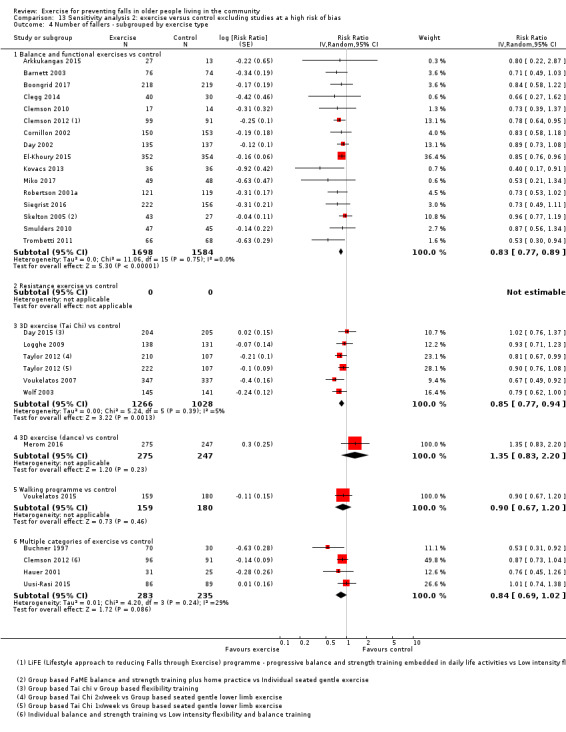
Comparison 13 Sensitivity analysis 2: exercise versus control excluding studies at a high risk of bias, Outcome 4 Number of fallers ‐ subgrouped by exercise type.
13.5. Analysis.
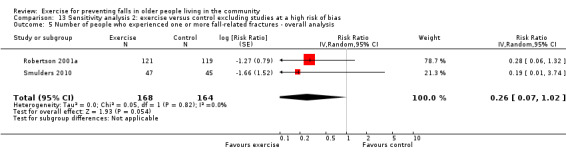
Comparison 13 Sensitivity analysis 2: exercise versus control excluding studies at a high risk of bias, Outcome 5 Number of people who experienced one or more fall‐related fractures ‐ overall analysis.
14.1. Analysis.
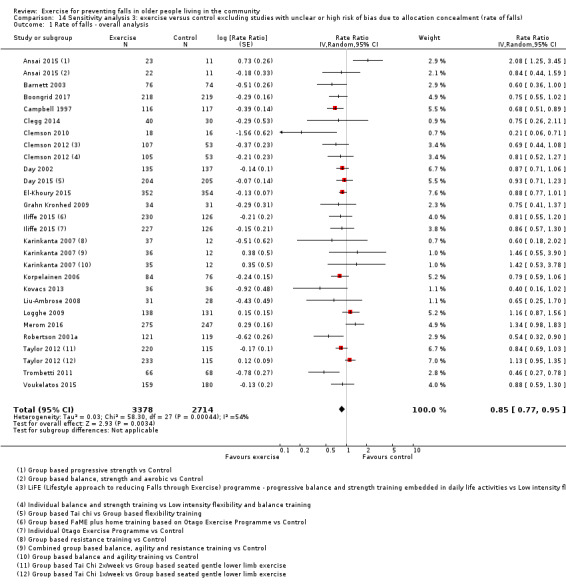
Comparison 14 Sensitivity analysis 3: exercise versus control excluding studies with unclear or high risk of bias due to allocation concealment (rate of falls), Outcome 1 Rate of falls ‐ overall analysis.
15.1. Analysis.
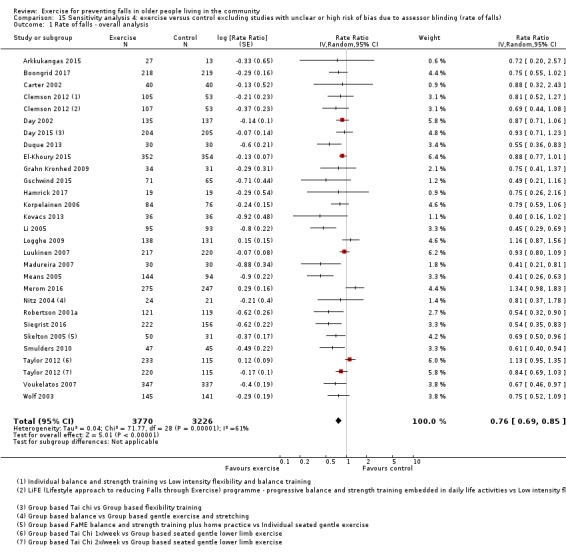
Comparison 15 Sensitivity analysis 4: exercise versus control excluding studies with unclear or high risk of bias due to assessor blinding (rate of falls), Outcome 1 Rate of falls ‐ overall analysis.
16.1. Analysis.
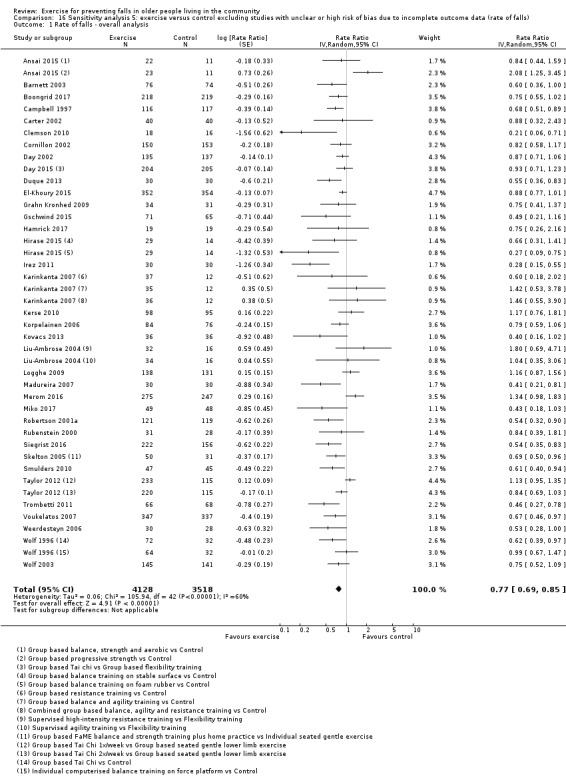
Comparison 16 Sensitivity analysis 5: exercise versus control excluding studies with unclear or high risk of bias due to incomplete outcome data (rate of falls), Outcome 1 Rate of falls ‐ overall analysis.
17.1. Analysis.
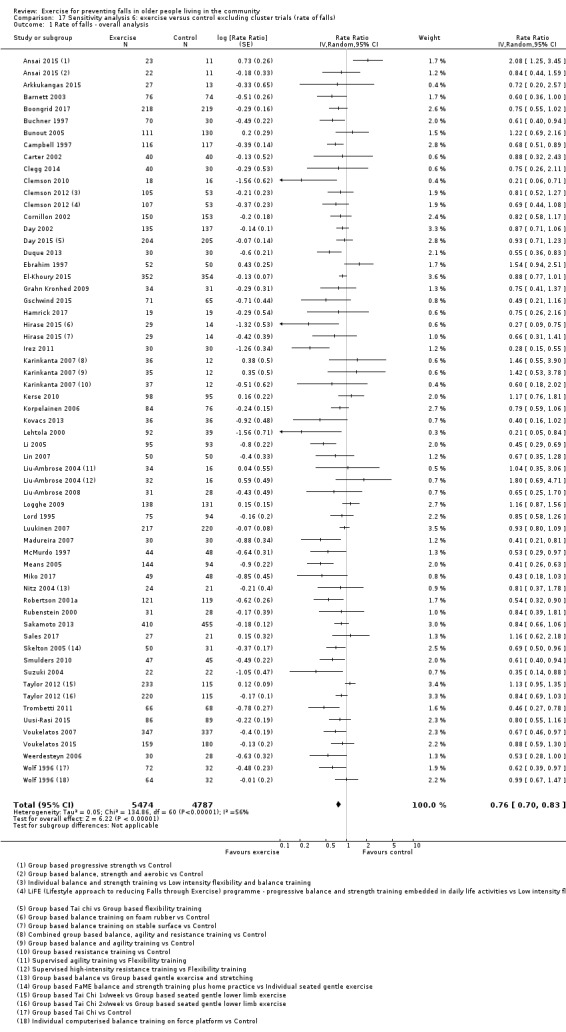
Comparison 17 Sensitivity analysis 6: exercise versus control excluding cluster trials (rate of falls), Outcome 1 Rate of falls ‐ overall analysis.
18.1. Analysis.
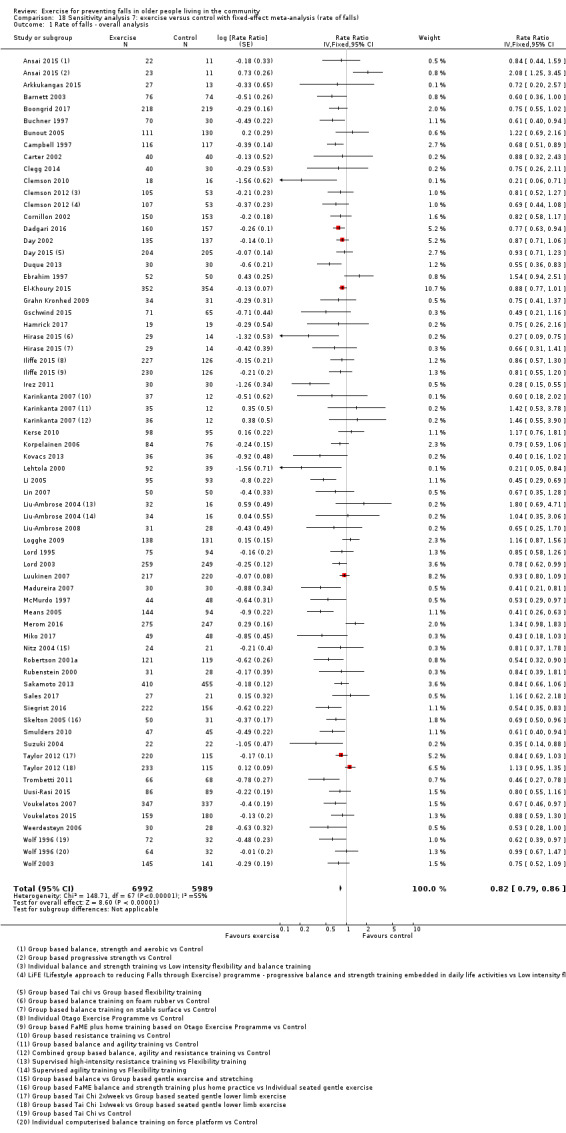
Comparison 18 Sensitivity analysis 7: exercise versus control with fixed‐effect meta‐analysis (rate of falls), Outcome 1 Rate of falls ‐ overall analysis.
20.1. Analysis.
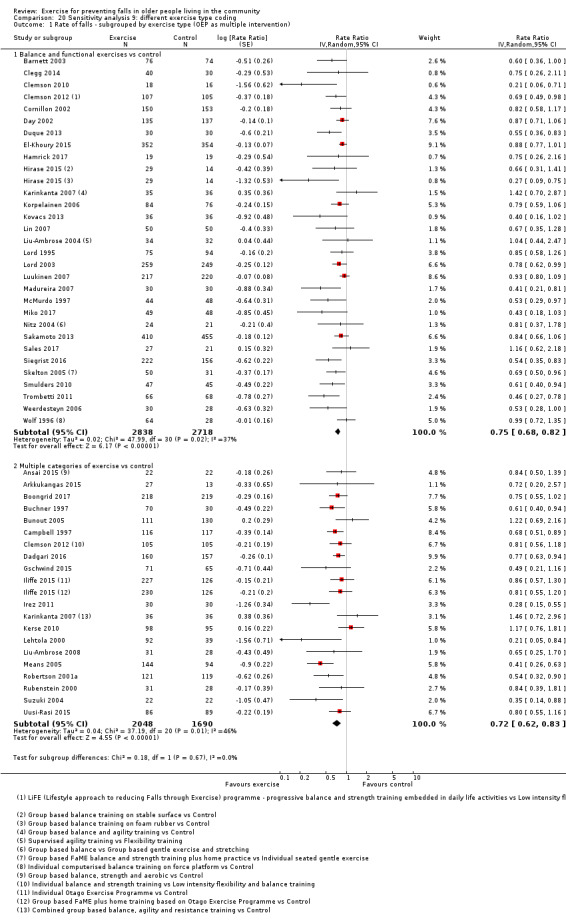
Comparison 20 Sensitivity analysis 9: different exercise type coding, Outcome 1 Rate of falls ‐ subgrouped by exercise type (OEP as multiple intervention).
20.2. Analysis.
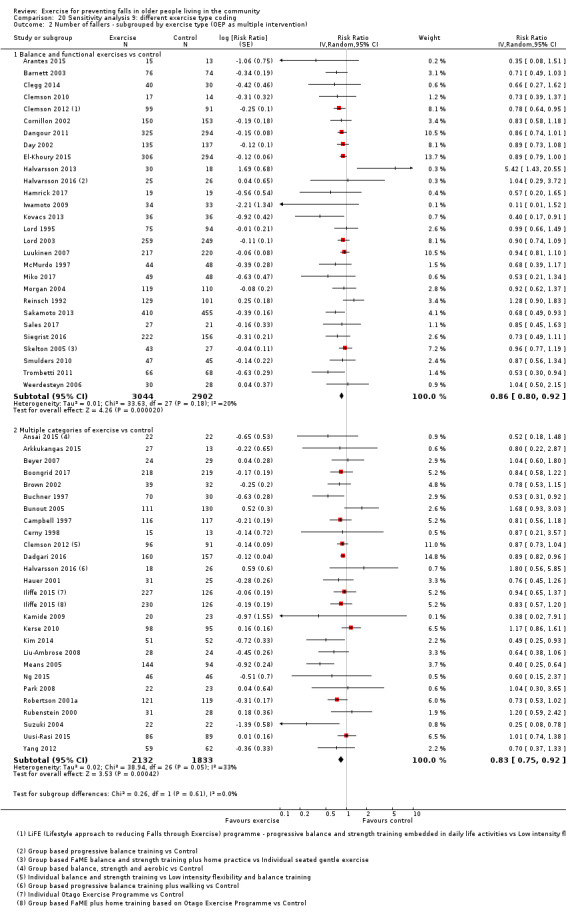
Comparison 20 Sensitivity analysis 9: different exercise type coding, Outcome 2 Number of fallers ‐ subgrouped by exercise type (OEP as multiple intervention).
20.3. Analysis.
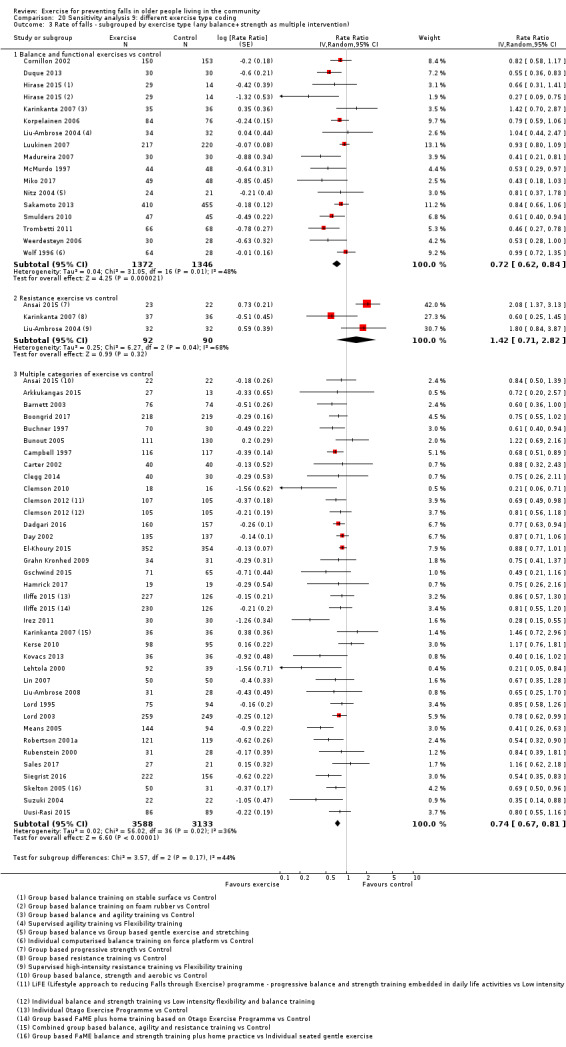
Comparison 20 Sensitivity analysis 9: different exercise type coding, Outcome 3 Rate of falls ‐ subgrouped by exercise type (any balance+strength as multiple intervention).
20.4. Analysis.
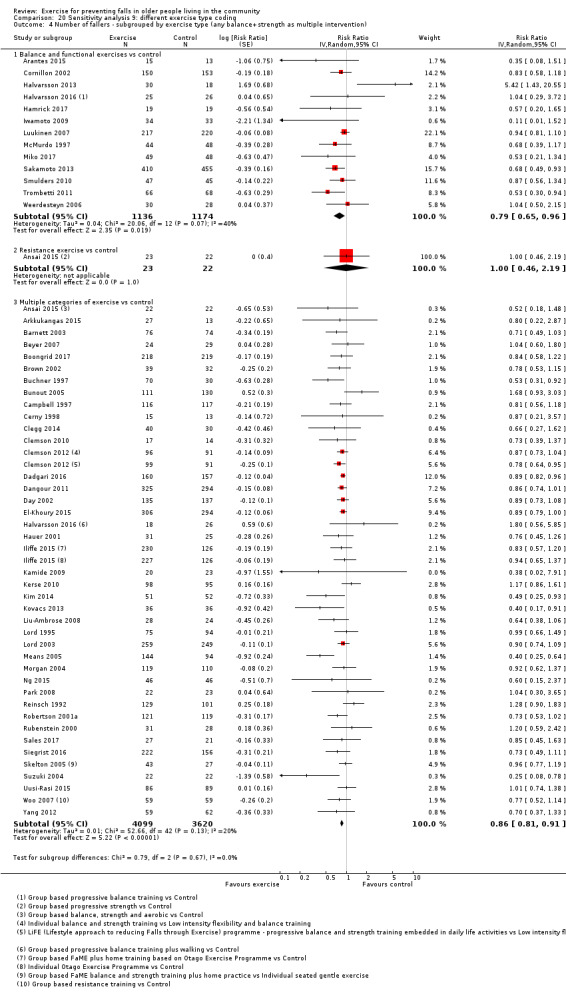
Comparison 20 Sensitivity analysis 9: different exercise type coding, Outcome 4 Number of fallers ‐ subgrouped by exercise type (any balance+strength as multiple intervention).
As shown in Appendix 18; the nine sensitivity analyses (based on age of included participants, risk of bias, cluster trials, fixed‐effect analyses, and categorisation of interventions) made little difference to the results of the primary pooled analysis. This indicates the robustness of the review's primary findings and methods.
In undertaking the GRADE assessment we downgraded the certainty of evidence based on sensitivity analysis (removal of trials with one or more items at high risk of bias) for the following comparisons.
Fall outcome: resistance exercises versus control, Tai Chi versus control, walking programme versus control.
Faller outcome: resistance exercises versus control, walking programme versus control, multiple categories of exercise versus control.
Fracture outcome: exercise (all types) versus control, balance and functional exercises versus control, multiple versus control.
Health‐related quality of life outcome: exercise (all types) versus control.
Heterogeneity
This review's primary analyses display minimal to substantial heterogeneity with P < 0.05 for the Chi² test and I² values up to 74%. This variability was not explained by our subgroup analyses. We consider this likely to represent between‐study differences in the exact nature of programmes (e.g. dose, intensity, adherence) and target populations, which requires ongoing investigation. Given the overall positive impact of the programmes and the stability of results, we do not consider this to preclude the meta‐analyses we have undertaken.
Funnel plots
The funnel plots in Figure 4; Figure 5; Figure 6; Figure 7; Figure 8 and Figure 9 do show some asymmetry, particularly for the fracture outcomes. We used this information in the GRADE assessment to downgrade the strength of the evidence for the fracture outcomes but did not consider the asymmetry sufficient to downgrade the level of evidence for the other outcomes.
4.
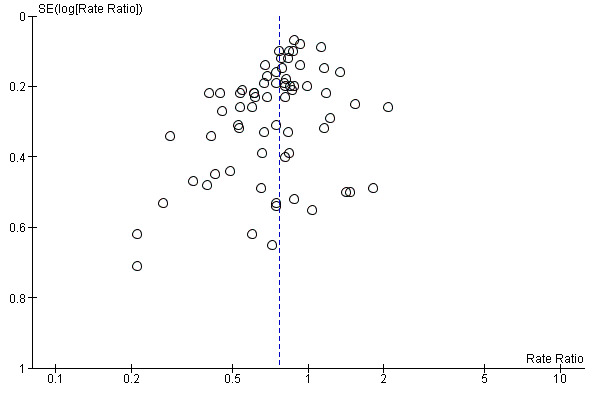
Funnel plot of comparison: 1 Exercise versus control (rate of falls), outcome: 1.1 Rate of falls ‐ overall analysis.
5.
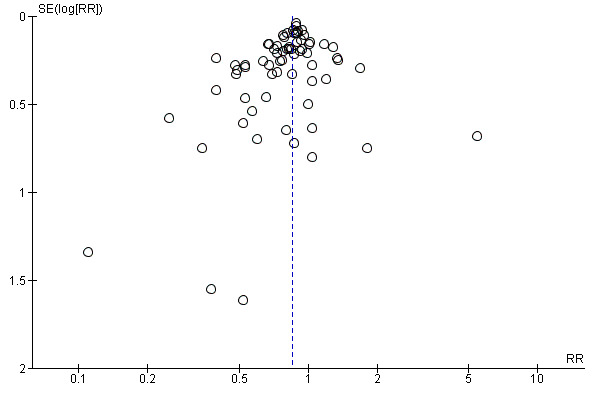
Funnel plot of comparison: 2 Exercise versus control (number of fallers), outcome: 2.1 Number of fallers ‐ overall analysis.
6.
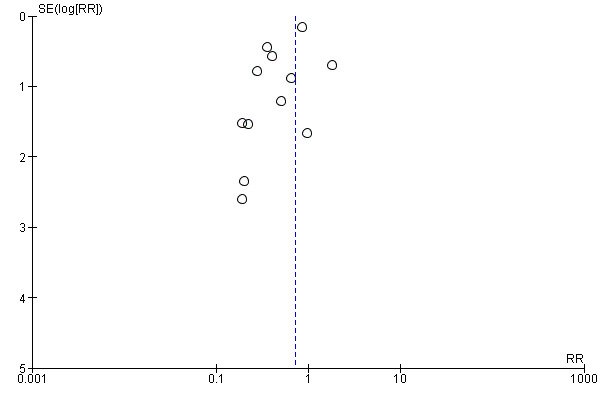
Funnel plot of comparison: 3 Exercise versus control (number of people with fractures), outcome: 3.1 Number of people who experienced one or more fall‐related fractures‐ overall analysis.
7.
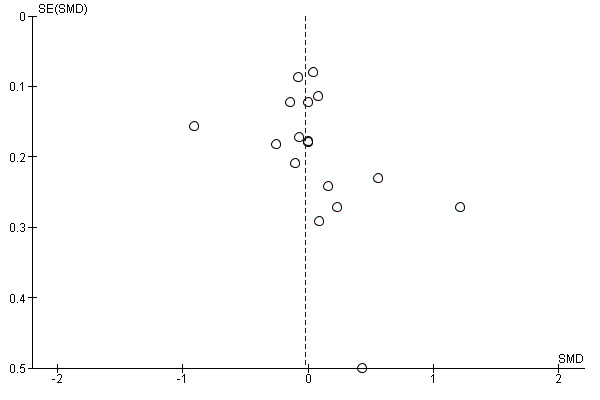
Funnel plot of comparison: 6 Exercise versus control (health‐related quality of life), outcome: 6.1 Health‐related quality of life‐ overall analysis.
8.
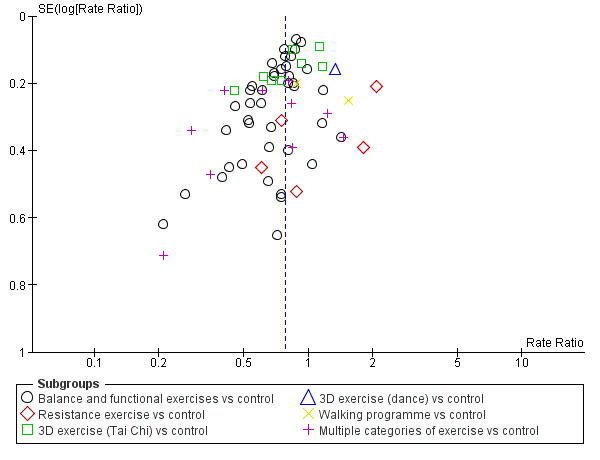
Funnel plot of comparison: 1 Exercise versus control (rate of falls), outcome: 1.6 Rate of falls ‐ subgrouped by exercise type.
9.
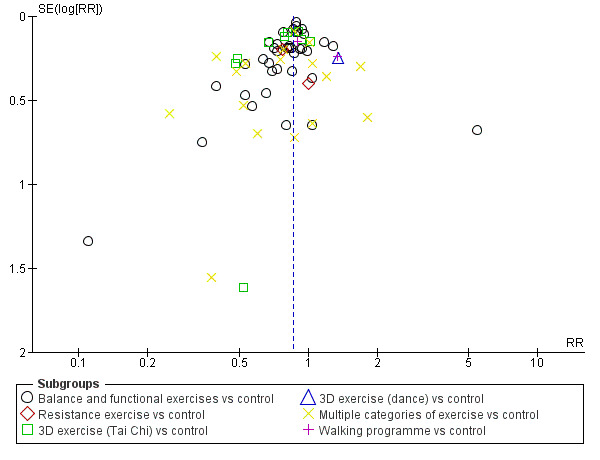
Funnel plot of comparison: 2 Exercise versus control (number of fallers), outcome: 2.6 Number of fallers ‐ subgrouped by exercise type.
Discussion
Summary of main results
This review includes 108 trials with 23,407 participants, who were older people living in the community. Of these, 81 trials (19,684 participants) contributed the evidence for the main 'exercise versus control' intervention (one that is not thought to reduce falls) comparison; these did not include the four trials that included only people who had been recently discharged from hospital. After summarising the results for this comparison, we summarised the evidence for the primary exercise categories versus control comparisons, where data were available. Our illustrative risks for dichotomous outcomes presented in Table 1, are based on counts (number of events divided by the number of participants) for those trials included in the analysis for that outcome. In Table 1, we also based our illustrative risks for falls outcomes on the median values obtained from the subgroups of trials for which: a) an increased risk of falls was not an inclusion criterion (not selected population); or b) increased risk of falls was an inclusion criterion. In the other 'Summary of findings' tables, we used the 'all‐exercise versus control' studies risks to illustrate the absolute risks for falls and fracture outcomes; we supplemented the falls outcomes by illustrative risks based on count data for the specific exercise category summarised.
Exercise (all types) versus control
There is high‐certainty evidence that falls can be prevented by exercise programmes, as summarised in Table 1. Exercise reduces both the rate of falls (reported in 59 randomised controlled trials (RCTs)) and the number of people experiencing falls (reported in 63 RCTs). Subgroup analyses did not reveal differences in effect on both falls outcomes according to whether trials were selected for high risk of falling or not. Hence, the overall rate of falls and number of fallers results were applied when estimating absolute risks in the following lower and higher risk of falls categories. As shown below, the absolute numbers of falls or numbers of fallers prevented are greater in the higher risk populations.
For the overall risk category, based on an illustrative risk of 850 falls per 1000 person‐years in the control group, there were 195 (23%) fewer falls per 1000 person‐years in the exercise group (95% confidence interval (CI) 144 (17%) to 246 (29%) fewer). Based on an illustrative risk of 480 fallers per 1000 older people in the control group, there were 72 (15%) fewer fallers per 1000 older people in the exercise group (95% CI 52 (11%) to 91 (19%) fewer).
For the non‐selected lower risk category, based on an illustrative risk of 605 falls per 1000 person‐years in the control group, there were 139 (23%) fewer falls per 1000 person‐years in the exercise group (95% CI 102 (17%) to 175 (29%) fewer). Based on an illustrative risk of 380 fallers per 1000 older people in the control group, there were 57 (15%) fewer fallers per 1000 older people in the exercise group (95% CI 41 (11%) to 72 (19%) fewer).
For the selected higher risk category, based on an illustrative risk of 1200 falls per 1000 person‐years in the control group, there were 276 (23%) fewer falls per 1000 person‐years in the exercise group (95% CI 204 (17%) to 348 (29%) fewer). Based on an illustrative risk of 500 fallers per 1000 older people in the control group, there were 75 (15%) fewer fallers per 1000 older people in the exercise group (95% CI 55 (11%) to 95 (19%) fewer).
Subgroup analyses did not reveal differences in effect on both falls outcomes according to whether trials included younger and older populations based on a 75 year cut‐off. There was, however, a greater reduction on the rate of falls from exercises (all types) in trials where interventions were delivered by a health professional than in trials where trained instructors who were not health professionals delivered the interventions; however, both approaches reduced the rate of falls. This finding did not apply to the subgroup analysis for number of fallers. Subgroup analyses did not reveal differences in effect on both falls outcomes according to whether interventions were delivered in a group setting or delivered individually.
The test for subgroup differences for when subgrouped by exercise type revealed significant subgroup differences for rate of falls, a finding that endorsed our prespecified intention to report separate analyses by primary exercise type (see below).
Far fewer studies reported on number of people who experienced fall‐related fractures (10 RCTs), fall‐related hospital admission (2 RCTs) and medical attention (5 RCTs). Exercise may reduce the number of people with fall‐related fractures: 27% reduction, 95% CI 5% to 44% reduction. Based on an illustrative risk, derived from the study data, of 64 people with fall‐related fractures per 1000 older people in the control group, there were 17 fewer people with fall‐related fractures per 1000 older people in the exercise group (95% CI 3 to 28 fewer). Exercise may make little or no difference to the number of people who experience one or more falls requiring hospital admission; reduction 22%, 95% CI 49% reduction to 18% increase. Based on an illustrative risk of 57 people with fall‐related hospital admission per 1000 older people in the control group, there were 12 fewer people with fall‐related hospital admissions per 1000 older people in the exercise group (95% CI 28 fewer to 11 more). Exercise may reduce the number of people who experience one or more falls requiring medical attention: 39% reduction, 95% CI 21% to 53% reduction. Based on an illustrative risk of 211 people with falls that required medical attention per 1000 older people in the control group, there were 82 fewer people with fall‐related medical attention per 1000 older people in the exercise group (95% CI 44 to 111 fewer).
Exercise may make little important difference to people‐reported health‐related quality of life compared with control: conversion of the pooled result (standardised mean difference (SMD) ‐0.03, 95% CI ‐0.10 to 0.04; 15 RCTs) to the EQ‐5D and SF‐36 scores showed the respective 95% CIs were much smaller than minimally important differences for both scales.
We are uncertain of the evidence for adverse events, which were incompletely reported and mainly for the exercise groups only in 27 RCTs (6019 participants). Fourteen trials reported no adverse events. Aside from two serious adverse events (1 pelvic stress fracture and 1 inguinal hernia surgery) reported in one trial, the remainder were non‐serious adverse events, primarily of a musculoskeletal nature.
Different exercise types versus control
'Summary of findings' tables, summarising the evidence for the rate of falls, risk of falling, fall‐related fractures and adverse events, are presented for the primary exercise categories for which data are available. There are no data available for flexibility exercise or endurance exercise versus control. The following should be viewed in terms of the data available for each exercise type. The few direct comparisons of different exercise types were clinically heterogeneous and we did not undertake any meta‐analyses for these comparisons for any of the outcomes.
Balance and functional exercises
This was compared with control in 48 trials. As summarised in Table 2, there is high‐certainty evidence that balance and functional exercises reduce the rate of falls and the number of people who experience falls. There is low‐certainty evidence that this type of exercise programme may help prevent fall‐related fractures. Adverse events, which were incompletely reported, were mainly non‐serious adverse events of a musculoskeletal nature.
Resistance (strength) exercises
This was compared with control in seven trials. As summarised in Table 3, we are uncertain of the effects of resistance training on the rate of falls and number of fallers. We are uncertain of the effects on fall‐related fractures; only three participants had fractures in the single trial reporting this outcome. Adverse events, which were incompletely reported, were non‐serious adverse events of a musculoskeletal nature.
3D exercise: Tai Chi
This was compared with control in 10 trials. As summarised in Table 4, there is low‐certainty evidence that Tai Chi may reduce the rate of falls and high‐certainty evidence that Tai Chi reduces the number of people who experience falls. Fall‐related fractures were not reported. The two trials reporting on adverse events, reported none.
3D exercise: dance
This was compared with control in one trial. As summarised in Table 5, we uncertain of findings of little effect of dance training on rate of falls or numbers of fallers. Fall‐related fractures were not reported. The trials reported there had been no adverse events in the dance group.
General physical activity: walking programme
This was compared with control in three trials. As summarised in Table 6, we are uncertain of the effects of walking programmes on rate of falls and number of people who experience falls. We are uncertain of the effects on fall‐related fractures; only 10 participants had fractures in the single trial reporting this outcome. All three trials reported there had been no adverse events.
Multiple categories of exercise
Multiple categories of exercise (most commonly balance and functional exercises plus resistance exercises) were compared with control in 21 trials. As summarised in Table 7, there is moderate‐certainty evidence that these interventions probably reduce rate of falls and number of fallers. Sensitivity analyses revealed little difference in the results when only the trials that included the most commonly two components (balance and functional exercises plus resistance exercises) as primary outcomes were pooled. Sensitivity analyses also revealed little difference in the results when any intervention that included balance and functional exercises plus strength exercises, as primary or secondary interventions, was classified as multiple types of exercise (Appendix 18). There is low‐certainty evidence that these interventions may have little effect on fall‐related fractures. Adverse events, which were incompletely reported, were mainly non‐serious adverse events of a musculoskeletal nature.
Subgroup analyses
Our prespecified subgroup analyses were performed on falls outcomes for balance and functional exercises and multiple categories of exercise. As for the overall exercise versus control comparison, subgroup analysis did not suggest a difference in effects on falls outcomes between trials that used increased risk of falls as an inclusion criterion to those in trials that did not. Also consistent with the overall exercise versus control comparison, there was greater reduction on the rate of falls from balance and functional exercises in trials where interventions were delivered by a health professional than in trials where the interventions were delivered by trained instructors who were not health professionals; although both approaches resulted in reductions in the rate of falls. There was no difference in the reduction on rate of falls from multiple primary types of exercise in trials where interventions were delivered by a health professional than in trials where the intervention was not delivered by a health professional. Other subgroup analyses did not detect differences in effects of exercises in trials where interventions were delivered in a group setting compared with trials where interventions were delivered individually. We did not explore the interaction between subgroups. For example, higher risk people are likely to require health professional input for safe exercise prescription.
Adverse events
Forty‐one of the 108 included trials reported on adverse events to some degree (31 exercise versus control trials, of which four trials included people recently discharged from hospital, and 10 exercise versus exercise trials). Seventeen trials reported an absence of adverse events, one trial reported a pelvic fracture and an inguinal hernia surgery (Clemson 2012), and the remaining trials primarily reported non‐serious musculoskeletal events. Only two trials, one of which included post‐discharge from hospital participants, reported adverse events in both exercise and control groups over the whole trial period, perhaps reflecting the cost and complexity of such monitoring.
Exercise (all types) versus control in people who had recently been discharged from hospital
Four heterogeneous studies investigated outcomes in people who had recently been discharged from hospital. We did not pool the data available for rate of falls, number of fallers and health‐related quality of life given the small numbers of trials and diversity of the interventions. Overall, the very low‐certainty evidence, downgraded for risk of bias, inconsistency and imprecision evidence is insufficient to draw any conclusions.
Comparisons of different types, modes of delivery and doses of exercise
Given the variability between programmes, we did not undertake any meta‐analyses of comparisons between different types of exercise. Most of the trials in these analyses did not find significant differences in the fall prevention effects of different programmes, but most were not likely to be adequately powered to detect differences between different exercise programmes. When comparing different exercise types delivered within the same studies we found some indication that higher doses of exercise were associated with a greater impact on the rate of falls and the number of people falling.
Economic data
Of the 12 studies included in this review that reported economic evaluation, some give an indication of value for money for the interventions tested. Variations in the methods used, however, made comparisons across studies difficult. There was some, although limited, evidence that fall prevention strategies can be cost‐saving during the trial period, and may also be cost‐effective over the participants’ remaining lifetime; however, it should be noted that these analyses usually fail to include the cost of identifying the target population, which can be substantial and can impact on cost‐effectiveness measures (Eldridge 2005). Additional studies have modelled the impact and cost‐effectiveness of a public health falls prevention programme in Australia (Farag 2015), undertaken secondary analyses to estimate cost‐effectiveness of implementing the Otago Exercise Program in Norway (Hektoen 2009), performed cost–benefit analysis of fall prevention interventions (Campbell 1999; Carande‐Kulis 2015; Clemson 2004a; Li 2005), and undertaken a literature review and developed a tool to estimate the cost‐effectiveness of fall prevention interventions in the community (Public Health England 2018).
Overall completeness and applicability of evidence
Trial design and participants
The 108 trials included in this review included 23,407 community‐dwelling older people, who were predominantly women (77%). A wide range of ages were included as few trials set upper age limits. Participant characteristics varied greatly due to the recruitment methods used, and the inclusion and exclusion criteria applied. Participants in most trials were healthy volunteers; however, some trials recruited people who were attending outpatient clinics. Sixty trials (56%) recruited participants with a history of falls or one or more risk factors for falling.
We excluded trials that tested exercise interventions for preventing falls in people affected by particular conditions, such as stroke, Parkinson’s disease, multiple sclerosis, hip fracture and dementia from this review as we considered that the results of interventions for these conditions were not necessarily applicable to older people as a whole. Fall prevention trials in these populations also often include a wider age range which would result in some being excluded from this review; Cochrane Reviews for each of these specific groups (including all age groups) would be preferable for summarising the evidence. The majority of trials (67%) excluded older people who were cognitively impaired, therefore the results of this review may not be applicable to this high risk group.
Most trials were relatively small (median = 134 participants), with a mean age of 76 (ranging from a mean age of 65 to a maximum mean age of 88 years). Thirty‐seven trials reported 12‐month follow‐up, with 49 reporting less than 12 months and 22 reporting more than 12 months follow‐up. Trials were undertaken over 25 years from 1992 to 2017.
Setting
Exercise‐based fall prevention interventions tested in a further 58 RCTs were included in this review compared with Gillespie 2012. The included trials were conducted in 25 countries using a variety of healthcare models. These different healthcare systems and structures may have impacted upon the effectiveness of some interventions. There remains a paucity of studies undertaken in low‐income economies.
Interventions
We classified the exercise interventions using the ProFaNE guidelines. This classification system is clearly described(Lamb 2011; Appendix 1); however, we acknowledge there is a degree of subjectivity in the classification of exercise interventions based on brief descriptions in trial reports. We conducted post‐hoc sensitivity analyses to explore the effects of recategorising trials with a secondary component of strength training as having multiple primarily exercise categories and found this made little difference to the results (Appendix 18). The duration of exercise intervention in the 81 exercise versus control trials ranged from 5 to 130 weeks; it being one year or more in 30% of these.
Outcomes
We sought data for rate of falls, number of people falling, number of people sustaining a fall‐related fracture, number of people who experienced falls leading to medical attention, number of people who had a fall‐related hospital admission, health‐related quality of life and number of people who experienced adverse events. However, few studies provided fracture, medical attention, hospital admission, health‐related quality of life and full adverse events data. As the analyses and Appendix 10 demonstrate, some studies provided data for both falls and fallers, as recommended in Lamb 2005, and others provided data for one or other falls outcomes.
The outcome of interest, falling, was not always clearly defined, which is a source of concern. Comparability of future research findings would be enhanced by the adoption of the consensus definition of a fall developed for trials in community‐dwelling populations by the Prevention of Falls Network Europe (ProFaNE) (Lamb 2005). The included studies also varied in the methods used for falls ascertainment, recording, analysing and reporting. Studies should use accepted protocols for recording of falls data, including daily recording of falls with monthly or more frequent follow‐up by the researchers who are blind to group allocation (Lamb 2005). At least 26% of included trials did not do this despite evidence of a 25% underreporting of falls when data were collected retrospectively by telephone at the end of a three‐month period, compared with data collected daily and returned monthly over the same period (Hannan 2010). There are difficulties in using fall diaries over long time periods however, with trial dropouts due to over‐burden of paperwork reported by Iliffe 2015.
The lack of consistent measurement of adverse events in trials requires attention by trialists. We found just two studies that measured adverse events in both groups throughout the trial period. Although it is worth noting that the burden on trial resources and participants of full documentation of adverse events is probably a key reason this has not been done to date. Trials of exercise interventions do not tend to be as well‐resourced as trials of pharmacological interventions in which adverse event monitoring is routine.
This review only included data for the risk of fractures and injurious falls, rather than for the rates of fractures and injurious falls; however, it is important to note that several trials have identified an impact of exercise on rates of fall‐related fracture (Karinkanta 2007; Korpelainen 2006; Kemmler 2010), as well as rates of injurious falls (Uusi‐Rasi 2015). There is also evidence of an impact of exercise on the rate of falls requiring medical care, over and above the impact from other types of interventions (Fitzharris 2010).
Other considerations relating to applicability
We decided not to pool studies undertaken in people who had recently been discharged from hospital with studies undertaken among general older populations. It is well documented that people who have recently been discharged form hospital are at a particularly high risk of falls (Mahoney 1994), and as such may require different intervention approaches. There is increasing awareness that many older people deteriorate physically during a hospital admission (Oliver 2017). We note that a number of recent studies of interventions have been undertaken in this population and among emergency department attendees (Harper 2017; Matchar 2017; Oliver 2017); however, there is still uncertainly of the best treatment for this population and a separate review may be needed in future.
For the control groups of the trials that did not have increased risk of falls as an inclusion criterion, the median rate of falls (if 1000 people were followed over 1 year, there would be 605 falls) and the median proportion of fallers (if 1000 people were followed over 1 year, 380 would experience one or more falls) are similar to estimates of fall risk and rate in the general community derived from large population studies (AIHW 2018; Lord 2011; NICE 2018). This indicates that participants in trials that do not recruit based on fall risk, are representative of the general community, rather than being at low risk of falls.
Subgroup analyses comparing the effects on falls outcomes in trials with predominantly older populations and those with predominantly younger populations should be interpreted with some caution, as implementation of one of the categorisation criteria (mean age minus 1 SD > 75) may result in some younger people in the older group and vice versa.
Ongoing studies
The 16 identified ongoing studies may contribute to research priorities. Six ongoing studies, two of which have a larger sample size (exceeding 400 participants), will evaluate the relative impact of different exercise programmes (NCT02126488; NCT03211429; NCT03404830; NCT03455179; n > 400 (NCT02287740; NCT02926105). Two studies will investigate individual versus group delivery of the LiFE programme (NCT03462654), and Otago Exercise Program (NCT03320668). Also, one large trial awaiting classification studied the difference between three types of exercise, including flexibility exercise (Li 2018b). Fall‐related fractures are listed as outcomes in only two trials (ISRCTN71002650; NCT02617303). Two trials, in India (CTRI/2018/01/011214), and Columbia (NCT03211429), will contribute to the understanding of the effect of exercise on falls in emerging economies. In addition, research is underway to investigate strategies for optimal translation of effective exercise programmes from the research setting to clinical and community settings (Carpenter 2018; Hawley‐Hague 2017).
Certainty of the evidence
This review, containing 108 trials (23,407 participants) provides moderate‐ to high‐certainty evidence of the effectiveness of exercise‐based interventions for preventing falls among community‐dwelling people aged 60 years and over.
We have summarised the GRADE certainty of evidence in seven 'Summary of findings' tables: Table 1 (Exercise (all types) versus control); Table 2 (Balance and functional exercises versus control); Table 3 (Resistance exercises versus control); Table 4 (3D (Tai Chi) exercise versus control)); Table 5 (3D (dance) exercise versus control)); Table 6 (Walking programme versus control); Table 7 (Multiple categories of exercise versus control).
The certainty of the evidence ranged from high to very low. We downgraded outcomes by one level for risk of bias if the results changed with removal of the trials with a high risk of bias on one or more items. We downgraded one level for inconsistency where heterogeneity was greater than 60%. In addition, we downgraded the level of evidence for imprecision by one or two levels due to the wide confidence intervals, often reflecting the small number of participants and trials. We downgraded where the risk of small sample bias was evident on funnel plot and downgraded one level for fall‐related hospital admission and fall‐related medical attention because a large number of studies included in the review do not contribute to the outcome.
Sensitivity analyses revealed the results for the falls outcomes to be stable (see Appendix 18) suggesting that the results are robust to key risks of bias and essentially unchanged by methodological choices in the conduct of the review. In undertaking the GRADE assessment we downgraded the certainty of evidence based on sensitivity analysis (removal of trials with one or more items at high risk of bias) for one or both falls outcomes for several types of exercise (resistance, Tai Chi, walking, multiple) and for the overall fracture and quality of life outcomes. It is noteworthy that many of the sensitivity analyses undertaken regarding risk of bias revealed a stability of the results of this review.
Rates of fractures and injurious falls were not prespecified outcomes in this review. More trials reported the outcome in this way than anticipated. We would be in favour of reporting these outcomes in future versions of this review.
Potential biases in the review process
We conducted a comprehensive search of the published literature using multiple databases and also searched clinical trial registries for completed trials for which full reports had not been identified. Two review authors who were blinded to each other's results performed screening and data extraction in duplicate to minimise bias. Despite this thorough search strategy, we acknowledge the possibility that some relevant trials may have been missed, especially if they were published in languages other than English.
Two review authors independently classified the exercise interventions using the ProFaNE guidelines (Lamb 2011), including assigning intervention categories to primary or secondary status. We recognise there is some subjectivity in this classification system, particularly for those interventions containing more than one category of exercise. Sensitivity analyses that tested the effects of recategorising primary balance and functional exercise trials with a secondary component of strength training indicated that this did not importantly affect the results.
We recorded and reported data on fracture, hospitalisation, medical attention and health‐related quality of life only where it was reported by intervention group. To check whether this could be a source of potential bias, we conducted an audit of fracture reporting in the 48 trials with balance, function and gait exercise interventions. Of the 10 trials reporting fracture outcomes, we included seven reporting fracture outcomes by intervention group in the analysis. We did not include the three other studies in the analysis because they either did not report fractures by group (Skelton 2005), they reported fractures during the intervention period but not during follow‐up (Iliffe 2014), or they just reported a fracture (1 pelvic stress fracture) as an adverse event (Clemson 2012). This provided some reassurance that our approach for these secondary and generally under‐reported outcomes did not have an important impact on the results.
Agreements and disagreements with other studies or reviews
Our review adds to the existing body of evidence and supports the findings of Gillespie 2012, whereby multiple component group‐based exercise was found to reduce the rate of falls (rate ratio (RaR) 0.71, 95% confidence interval (CI) 0.63 to 0.82; 16 trials, 3622 participants) and the risk of falling (risk ratio (RR) 0.85, 95% CI 0.76 to 0.96; 22 trials, 5333 participants). Similar results were found for individually‐delivered multiple component exercise that reduced the rate of falls (RaR 0.68, 95% CI 0.58 to 0.80; 951 participants, 7 trials) and the number of people falling (RR 0.78, 95% CI 0.64 to 0.94; 714 participants, 6 trials). The review by Gillespie 2012, also found that Tai Chi reduced the rate of falls (RaR 0.72, 95% CI 0.52 to 1.00; 1563 participants, 5 trials) and the number of people falling (RR 0.71, 95% CI 0.57 to 0.87; 1625 participants, 6 trials). Group‐based balance or functional exercises also demonstrated a statistically significant reduction in the rate of falls (RaR 0.72, 95% CI 0.55 to 0.94; 519 participants, 4 trials) but not in the number of people falling (RR 0.81, 0.62 to 1.07; 453 participants, 3 trials). This influential review has informed, and been the basis of, many guidelines and policy documents internationally.
We extended the findings of Gillespie 2012 by recoding intervention programmes (Appendix 1), in an attempt to identify a primary exercise component for each included study and reserving the 'multiple component' category for trials in which the intervention programme had an equal focus on each of the multiple components. As a result, more studies in our review are classified as balance and functional exercises and fewer as multiple component programmes. We hope that this change will be of assistance to those seeking to design exercise intervention programmes.
The present review also adds to our previous non‐Cochrane review (Sherrington 2017), that used different methodology (multivariable metaregression) yet reached similar conclusions about the importance of the inclusion of exercises that safely challenge balance in fall prevention exercise programmes. Other recent analyses have reached similar findings, including a large network meta‐analysis (Tricco 2017).
The importance of exercise in fall prevention suggests that greater attention be given to the widespread implementation of a life course approach to healthy ageing, i.e. lifelong exercise to maximise physical functioning in older age, as suggested by the World Health Organization (WHO 2015).
Authors' conclusions
Implications for practice.
Well‐designed exercise programmes reduce the rate of falls and the number of people experiencing falls amongst older people living in the community (high‐certainty evidence).
The effects of exercise programmes are uncertain for other non‐falls outcomes, mainly reflecting the considerable under‐reporting of these outcomes in the included trials. Exercise may reduce the number of people experiencing one or more fall‐related fractures and the number of people experiencing one or more falls requiring medical attention (low‐certainty evidence). We are uncertain about the effect of exercise programmes on the number of people who experience one or more falls requiring hospital admission. Exercise may make little important difference to health‐related quality of life (low‐certainty evidence). The reporting of adverse events was poor; where reported these were usually non‐serious and predominantly musculoskeletal.
Effective exercise programmes that reduce both falls outcomes primarily involve balance and functional exercises (high‐certainty evidence) or include multiple exercise categories, most commonly balance and functional exercises plus resistance exercises (moderate‐certainty evidence). Tai Chi reduces the number of people experiencing falls (high‐certainty evidence) and may reduce the rate of falls (low‐certainty evidence). We are uncertain about the effect of programmes involving primarily resistance exercises, dance or walking, as there is insufficient evidence on these. There are no data available for flexibility exercise or endurance exercise versus control.
Exercise programmes were effective regardless of whether they were delivered individually or in groups, by health professionals or trained non‐health professionals, to younger or older populations (based on a 75 year age threshold) or to those identified at a higher risk of falls or not selected for risk of falls. There is likely to be a greater absolute impact in people identified at increased risk of falling, but there is benefit also for those who are at more general risk in the community. Although trial follow‐up ranged from 3 to 18 months in the main comparison, there may also be longer‐term benefits of introducing fall prevention exercise habits in people in the general community. Notably too, the duration of most of the exercise programmes was 12 weeks or over and nearly one‐third lasted a year or more. These findings highlight the importance of primary prevention.
There is currently insufficient evidence to determine the effects of exercise programmes for people recently discharged from hospital. There is also insufficient information from direct comparisons to determine whether there are differences in the effectiveness of different types, modes of delivery and doses of exercise.
Implications for research.
Further work is needed to understand the relative impact of different exercise programmes. Such studies will need to be very large to be adequately powered to detect effects between interventions.
Large studies are also needed to establish the impact of fall prevention interventions on fall‐related fractures and falls requiring medical attention, as such falls are particularly costly to health systems and impactful for individuals.
During the development of priority topics for future research, the current evidence base should be considered in conjunction with the areas studied in the ongoing trials.
Individual participant data meta‐analysis could contribute further to the investigation of differential effects of exercise in people of different ages and baseline fall risks, as these are individual‐level rather than trial‐level characteristics. We recommend researchers follow the Prevention of Falls Network Europe (ProFaNE) guidelines for the conduct of falls trials (Lamb 2005).
Further research is required to establish the effectiveness of fall prevention programmes in emerging economies, where the burden of falls is increasing more rapidly than in high‐income countries due to rapidly ageing populations (WHO 2015).
There is an urgent need to investigate strategies to enhance implementation of effective exercise‐based fall prevention interventions into routine care of older people by healthcare professionals and community organisations.
As it is possible that interventions designed to increase physical activity could increase falls due to increased exposure to risk, we suggest that those undertaking trials of physical activity interventions in older people consider monitoring falls.
Future studies should use the consensus definition of a fall developed for trials in community‐dwelling populations by ProFaNE (Lamb 2005), consistent methods of falls ascertainment, and consistent measurement of adverse events in both groups throughout the trial period. Future research should use the ProFaNE descriptors to categorise interventions (Lamb 2011), but should be clear how this was operationalised. Appendix 1 outlines how this guide was operationalised in the present review and may provide a useful resource.
Notes
This review provides updated evidence for one of the intervention categories (exercise) covered in the Cochrane Review 'Interventions for preventing falls in older people living in the community' (Gillespie 2012). Some of the wording in several sections of the protocol, such as Background/Description of the condition, was taken from Gillespie 2012. This reflects shared authorship of the two publications, but also attempts to maintain a continuity with the Gillespie 2012 review, as well as links between our review and other proposed reviews that will cover other intervention categories, such as multifactorial and multiple component interventions (Hopewell 2018).
Editorial management and appraisal for this review were conducted by the Cochrane Fast‐Track Service (Managing Editor: Helen Wakeford; Associate Editor: Liz Bickerdike; Information Specialist Advisor: Ruth Foxlee) with additional oversight and appraisal by the Cochrane Bone, Joint and Muscle Trauma Group (Managing Editor: Joanne Elliott; Co‐ordinating Editor: Helen Handoll). Approval for publication given by Helen Handoll. This review was copy‐edited by Kate Cahill and Clare Dooley.
Support to the authors for implementing the requirement by NICE for additional analyses to inform the update of their guideline on Falls in older people was provided by Helen Handoll and Liz Bickerdike, with facilitation by Joanne Elliott and Helen Wakeford. This aspect was under the aegis of Michael Brown, Senior Editor of the Cochrane Acute and Emergency Care Network.
Acknowledgements
We are very grateful for helpful feedback from editors Liz Bickerdike, Helen Handoll and Helen Wakeford; and external referees Elizabeth Burns, Helen Hawley, Dawn Skelton and Edgar Ramos Vieir on drafts of the review. We also thank Joanne Elliott and Helen Wakeford for editorial support on the review and Kate Cahill and Clare Dooley for copy‐editing. We thank Joanne Elliott for assistance with developing the search strategy. We would like to acknowledge the helpful feedback on the review from consumer peer reviewers: Federica Davolio, Auxiliadora Fraiz and Marina Sartini.
We are also grateful to the authors of Gillespie 2012, particularly Lesley Gillespie and Clare Robertson, for the development of methods and procedures and assistance with this review.
We are grateful to Courtney West, Connie Jensen and Christoper Ng for assistance with searching and data extraction.
This project was partly funded by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group. Additional funding for the review was via the NIHR (UK): NIHR Cochrane Reviews of National Institute for Care and Excellence (NICE) Priority scheme, project reference: NIHR127512. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Categories of exercise (ProFaNE): definitions and application
| Exercise category | ProFaNE description | How the category criteria were applied in this reviewa |
| Gait, balance, and functional training | Gait training involves specific correction of walking technique (e.g. posture, stride length and cadence) and changes of pace, level and direction. Balance training involves the efficient transfer of bodyweight from one part of the body to another or challenges specific aspects of the balance systems (e.g. vestibular systems). Balance retraining activities range from the re‐education of basic functional movement patterns to a wide variety of dynamic activities that target more sophisticated aspects of balance. Functional training uses functional activities as the training stimulus, and is based on the theoretical concept of task specificity. All gait, balance and functional training should be based on an assessment of the participant’s abilities prior to starting the programme; tailoring of the intervention to the individuals abilities; and progression of the exercise programme as ability improves | Selected as exercise category if the intervention met the baseline assessment, tailoring and progression criteria. Selected as primary category for interventions where most exercises were conducted standing and where the intervention focus and most time spent was on exercise in this category |
| Strength/resistance (including power) | The term 'resistance training' covers all types of weight training i.e. contracting the muscles against a resistance to ‘overload’ and bring about a training effect in the muscular system. The resistance is an external force, which can be one’s own body placed in an unusual relationship to gravity (e.g. prone back extension) or an external resistance (e.g. free weight). All strength/resistance training should be based on an assessment of the participant’s abilities prior to starting the programme; tailoring the intervention to the individual's abilities; and progression of the exercise programme as ability improves | Selected as exercise category if the intervention met the baseline assessment, tailoring and progression criteria. Selected as primary category for interventions where additional resistance was used or where it was clear that overload was sufficient without external resistance and where the intervention focus and most time spent was on exercise in this category |
| Flexibility | Flexibility training is the planned process by which stretching exercises are practised and progressed to restore or maintain the optimal range of movement (ROM) available to a joint or joints. The ranges of motion used by flexibility programmes may vary from restoration/maintenance of the entire physiological range of motion, or alternatively, maintenance of range that is essential to mobility or other functions | Selected as exercise category if the intervention met the progression of stretching criterion. Selected as primary category for interventions where flexibility training was a stated aim of the intervention and where the intervention focus and most time spent was on exercise in this category |
| 3D | 3D training involves constant movement in a controlled, fluid, repetitive way through all three spatial planes or dimensions (forward and back, side to side, and up and down). Tai Chi and Qi Gong incorporate specific weight transferences and require upright posture and subtle changes of head position and gaze direction. Dance involves a wide range of dynamic movement qualities, speeds and patterns | Selected as exercise category if the intervention involved Tai Chi or dance. Selected as primary category for interventions where the intervention focus and most time spent was on exercise in this category |
| General physical activity | Physical activity is any bodily movement produced by skeletal muscle contraction resulting in a substantial increase in energy expenditure. Physical activity has both occupational, transportation and recreational components and includes pursuits like golf, tennis, and swimming. It also includes other active pastimes like gardening, cutting wood, and carpentry. Physical activity can provide progressive health benefits and is a catalyst for improving health attitudes, health habits, and lifestyle. Increasing habitual physical activity should be with specific recommendations as to duration, frequency and intensity if a physical or mental health improvement is indicated | Selected as exercise category if the intervention included unstructured physical activity. We classed programmes that included unstructured walking as this category. Selected as primary category for interventions where the intervention focus and most time spent was on exercise in this category |
| Endurance | Endurance training is aimed at cardiovascular conditioning and is aerobic in nature and simultaneously increases the heart rate and the return of blood to the heart | Selected as exercise category if the intervention focused on structured aerobic training. We classed programmes that included treadmill walking as this category. Selected as primary category for interventions where the intervention focus and most time spent was on exercise in this category |
| Other | Other kinds of exercises not described | Selected as exercise category if the intervention did not meet the other categories listed and where the intervention focus and most time spent was on exercise in this category |
| aInterventions were allocated a secondary category if some but not all criteria were met by the intervention or where the category was not the primary focus of the intervention, or both | ||
Appendix 2. Search strategies (February 2012 to 2 May 2018)
CENTRAL (CRS Online)
#1 MESH DESCRIPTOR Accidental Falls EXPLODE ALL TREES #2 (falls or faller*):TI,AB,KY #3 #1 or #2 #4 MESH DESCRIPTOR Aged EXPLODE ALL TREES #5 (senior* or elder* or old* or aged or ag?ing or postmenopausal or community dwelling):TI,AB,KY #6 #4 or #5 #7 #3 and #6
MEDLINE (Ovid Interface)
1 Accidental Falls/ 2 (falls or faller*1).tw. 3 or/1‐2 4 exp Aged/ 5 (senior*1 or elder* or old* or aged or ag?ing or postmenopausal or community dwelling).tw. 6 or/4‐5 7 3 and 6 8 Randomized controlled trial.pt. 9 Controlled clinical trial.pt. 10 randomized.ab. 11 placebo.ab. 12 Clinical trials as topic/ 13 randomly.ab. 14 trial.ti. 15 8 or 9 or 10 or 11 or 12 or 13 or 14 16 exp Animals/ not Humans/ 17 15 not 16 18 7 and 17
Embase (Ovid Interface)
1 Falling/ 2 (falls or fallers).tw. 3 or/1‐2 4 exp Aged/ 5 (senior*1 or elder* or old* or aged or ag?ing or postmenopausal or community dwelling).tw. 6 or/4‐5 7 3 and 6 8 exp Randomized Controlled Trial/ or exp Single Blind Procedure/ or exp Double Blind Procedure/ or Crossover Procedure/ 9 (random* or RCT or placebo or allocat* or crossover* or 'cross over' or trial or (doubl* adj1 blind*) or (singl* adj1 blind*)).ti,ab. 10 8 or 9 11 (exp Animal/ or animal.hw. or Nonhuman/) not (exp Human/ or Human cell/ or (human or humans).ti.) 12 10 not 11 13 7 and 12
CINAHL (Ebsco)
S1 (MH "Accidental Falls") S2 TI ( falls or faller* ) OR AB ( falls or faller* ) S3 S1 OR S2 S4 (MH "Aged+") S5 TI ( senior* or elder* or old* or aged or ag?ing or postmenopausal or community dwelling ) OR AB ( senior* or elder* or old* or aged or ag?ing or postmenopausal or community dwelling ) S6 S4 OR S5 S7 S3 AND S6 S8 PT Clinical Trial S9 (MH "Clinical Trials+") S10 TI clinical trial* OR AB clinical trial* S11 TI ( (single blind* or double blind*) ) OR AB ( (single blind* or double blind*) ) S12 TI random* OR AB random* S13 S8 OR S9 OR S10 OR S11 OR S12 S14 S7 AND S13
PEDro
Advanced search option selected
Abstract and Title: fall* Method: clinical trial Sub discipline: gerontology
New record added since: (date of last review entered here)
ClinicalTrials.gov
(prevent OR reduce OR reduction OR risk) AND (fall OR fallers) AND (exercise OR training)
WHO ICTRP
prevent* AND fall* AND exercise* OR reduc* AND fall* AND exercise* OR risk* AND fall* AND exercise* OR prevent* AND fall* AND train* OR reduc* AND fall* AND train* OR risk* AND fall* AND exercise*
Appendix 3. 'Risk of bias' assessment tool
| Domain | Criteria for judging risk of bias |
| Random sequence generation relating to selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
|
| Allocation concealment relating to selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
|
| Blinding of participants and personnel relating to performance bias due to knowledge of the allocated interventions by participants and personnel carrying out the interventions |
|
| Blinding of outcome assessment relating to detection bias due to knowledge of the allocated interventions by outcome assessors |
|
| Incomplete outcome data relating to attrition bias due to amount, nature or handling of incomplete outcome data |
|
| Selective outcome reporting relating to bias due to the selective reporting or non‐reporting of findings |
|
| Method of ascertaining falls relating to bias in the recall of falls due to unreliable methods of ascertainment |
|
| Cluster‐randomised trials relating to bias due to factors particular to cluster‐randomised trials |
|
We adapted this from Table 8.5.a 'The Cochrane Collaboration's tool for assessing risk of bias' and Table 8.5.d 'Criteria for judging risk of bias in the 'Risk of bias' assessment tool' (Higgins 2011).
Appendix 4. Description of included studies: reference links
Appendix 5. Categories of exercise (ProFaNE) in interventions in the included trials
| Study ID | Gait/balance/functional training | Strength/resistance training | Flexibility | 3D (Tai Chi, dance etc) | General physical activity | Endurance | Other |
|
Almeida 2013 Fully supervised group‐based balance and strength training |
Primary | Secondary | Secondary | ‐ | ‐ | ‐ | ‐ |
|
Almeida 2013 Minimally supervised group‐based balance and strength training |
Primary | Secondary | Secondary | ‐ | ‐ | ‐ | ‐ |
|
Ansai 2015 Group‐based balance, strength and aerobic training |
Primary | Primary | ‐ | ‐ | ‐ | Primary | ‐ |
|
Ansai 2015 Group‐based progressive strength training |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | |
|
Arantes 2015 Group‐based balance training |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Arkkukangas 2015 Individual Otago Exercise Program |
Primary | Secondary | ‐ | ‐ | Secondary | ‐ | ‐ |
|
Ballard 2004 Group‐based balance, strength and aerobic training for 15 weeks |
Primary | Secondary | ‐ | ‐ | ‐ | Secondary | ‐ |
|
Ballard 2004 Group‐based balance, strength and aerobic training for 2 weeks |
Primary | Secondary | ‐ | ‐ | ‐ | Secondary | ‐ |
|
Barker 2016 Group‐based Pilates focused on balance and strength plus home practice |
Primary | Secondary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Barker 2016 Individual strength and balance |
Primary | Secondary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Barnett 2003 Group‐based balance, strength and aerobic training |
Primary | Secondary | ‐ | ‐ | ‐ | Secondary | ‐ |
|
Beyer 2007 Group‐based balance, strength and flexibility training |
Primary | Primary | Primary | ‐ | ‐ | ‐ | ‐ |
|
Boongrid 2017 Individual Otago Exercise Program |
Primary | Secondary | ‐ | ‐ | Secondary | ‐‐ | |
|
Brown 2002 Group‐based balance, strength and aerobic training |
Primary | Primary | ‐ | ‐ | ‐ | Secondary | Secondary ‐ co‐ordination activities |
|
Buchner 1997 Group‐based strength training (combined with endurance and combined groups in analysis)* |
Primary | ||||||
|
Buchner 1997 Group‐based stationary cycling (combined with resistance and combined groups in analysis)* |
‐ | ‐ | ‐ | ‐ | ‐ | Primary | ‐ |
|
Buchner 1997 Group‐based stationary cycling + strength training (combined with endurance and resistance groups in analysis)* |
‐ | Primary | ‐ | ‐ | ‐ | Primary | ‐ |
|
Bunout 2005 Group‐based balance, strength and walking |
Primary | Primary | ‐ | ‐ | ‐ | Primary | ‐ |
|
Campbell 1997 Individual Otago Exercise Program |
Primary | Secondary | ‐ | ‐ | Secondary | ‐ | ‐ |
|
Carter 2002 Group‐based Osteofit strength training |
Secondary | Primary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Cerny 1998 Group‐based balance, strength, flexibility, aerobic training and brisk walking |
Primary | Primary | Primary | ‐ | ‐ | Primary | ‐ |
|
Clegg 2014 Individual balance and strength training |
Primary | Secondary | |||||
|
Clemson 2010 LiFE (Lifestyle approach to reducing Falls through Exercise) programme‐ balance and strength training embedded in daily life activities |
Primary | Secondary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Clemson 2012 LiFE (Lifestyle approach to reducing Falls through Exercise) programme‐ balance and strength training embedded in daily life activities |
Primary | Secondary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Clemson 2012 Individual balance and strength training |
Primary | Primary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Cornillon 2002 Group‐based balance and gait training |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Dadgari 2016 Individual Otago Exercise Program |
Primary | Secondary | ‐ | ‐ | Secondary | ‐ | ‐ |
|
Dangour 2011 Group‐based balance and strength |
Primary | Secondary | ‐ | ‐ | Secondary | ‐ | ‐ |
|
Davis 2011 Group‐based progressive high intensity resistance training once weekly |
‐ | Primary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Davis 2011 Group‐based progressive high intensity resistance training twice weekly |
‐ | Primary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Davis 2011 Group‐based balance and tone |
Primary | Secondary | |||||
|
Day 2002 Group‐based balance and strength |
Primary | Secondary | Secondary | ‐ | ‐ | ‐ | ‐ |
|
Day 2015 Group‐based Tai Chi |
‐ | ‐ | ‐ | Primary | ‐ | ‐ | ‐ |
|
Duque 2013 Virtual reality balance training |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | Secondary‐ visual‐vestibular rehabilitation |
|
Ebrahim 1997 Brisk walking |
‐ | ‐ | ‐ | ‐ | Primary | ‐ | ‐ |
|
El‐Khoury 2015 Group‐based balance and strength plus home practice |
Primary | Secondary | Secondary | ‐ | ‐ | ‐ | ‐ |
|
Fiatarone 1997 Individual high‐intensity progressive resistance training |
‐ | Primary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Freiberger 2007 Group‐based psychomotor programme |
Primary | Primary | ‐ | ‐ | ‐ | ‐ | Primary‐ perceptual training |
|
Freiberger 2007 Group‐based balance, strength, flexibility, endurance |
Primary | Primary | Primary | Primary | |||
|
Gill 2016 Group and home‐based balance, strength, flexibility and walking training |
Primary | Primary | Secondary | Primary | ‐ | ‐ | |
|
Grahn Kronhed 2009 Group‐based strength and balance training |
Secondary | Primary | Secondary | ‐ | ‐ | Secondary | ‐ |
|
Gschwind 2015 Individual balance and strength training using exergames |
Primary | Secondary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Haines 2009 Home strength and balance program with DVD/workbook |
Primary | Primary | ‐ | Primary | ‐ | ‐ | ‐ |
|
Halvarsson 2013 Group‐based progressive balance training |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Halvarsson 2016 Group‐based progressive balance training |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Halvarsson 2016 Group‐based progressive balance training plus walking |
Primary | ‐ | ‐ | ‐ | Primary | ‐ | ‐ |
|
Hamrick 2017 Home exercise group |
Primary | ‐ | Secondary | ‐ | ‐ | ‐ | ‐ |
|
Hauer 2001 Group‐based progressive strength and balance training |
Primary | Primary | ‐ | ‐ | Primary | ‐ | ‐ |
|
Helbostad 2004 Combined group and home‐based balance and strength training |
Primary | Secondary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Helbostad 2004 Individual home balance and strength training |
Primary | Secondary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Hirase 2015 Group‐based balance training on foam rubber |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Hirase 2015 Group‐based balance training on stable surface |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Huang 2010 Group‐based Tai Chi |
‐ | ‐ | ‐ | Primary | ‐ | ‐ | ‐ |
|
Hwang 2016 Individually supervised balance and strength training |
Primary | Secondary | Secondary | ‐ | ‐ | ‐ | ‐ |
|
Hwang 2016 Individually supervised Tai Chi |
‐ | ‐ | ‐ | Primary | ‐ | ‐ | ‐ |
|
Iliffe 2015 Individual Otago Exercise Program |
Primary | Secondary | ‐ | ‐ | Secondary | ‐ | ‐ |
|
Iliffe 2015 Group‐based FaME plus home training based on Otago Exercise Program |
Primary | Secondary | ‐ | ‐ | Secondary | ‐ | ‐ |
|
Irez 2011 Group‐based pilates |
Primary | Primary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Iwamoto 2009 Group‐based balance and gait training |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Kamide 2009 Individual balance and strength training |
Primary | Primary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Karinkanta 2007 Group‐based balance and agility training |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Karinkanta 2007 Group‐based resistance training |
‐ | Primary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Karinkanta 2007 Combined group‐based balance, agility and resistance training |
Primary | Primary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Kemmler 2010 Group‐based balance, gait flexibility and strength training plus home practice |
Primary | Primary | Primary | ‐ | ‐ | Secondary | ‐ |
|
Kemmler 2010 Group‐based low intensity, low frequency balance and endurance training |
Primary | ‐ | Primary | ‐ | ‐ | Secondary | ‐ |
|
Kerse 2010 Individual Otago Exercise Program |
Primary | Secondary | ‐ | ‐ | Secondary | ‐ | ‐ |
|
Kim 2014 Group‐based balance and strength training |
Primary | Primary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Korpelainen 2006 Group‐based balance and strength training plus home practice |
Primary | Secondary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Kovacs 2013 Group‐based balance and strength based on Otago Exercise Program |
Primary | Secondary | ‐ | ‐ | Secondary | ‐ | ‐ |
|
Kwok 2016 Group‐based balance, strength and aerobic training plus home practice |
Primary | Primary | ‐ | ‐ | ‐ | Primary | ‐ |
|
Kwok 2016 Balance, strength and aerobic training using the Nintendo WiiActive |
Primary | Primary | ‐ | ‐ | ‐ | Primary | ‐ |
|
Kyrdalen 2014 Group‐based Otago Exercise Program |
Primary | Secondary | ‐ | ‐ | Secondary | ‐ | ‐ |
|
Kyrdalen 2014 Individual Otago Exercise Program |
Primary | Secondary | ‐ | ‐ | Secondary | ‐ | ‐ |
|
LaStayo 2017 Resisted lower limb exercise in standing and leg press |
Primary | Primary | Secondary | ‐ | Secondary | ‐ | ‐ |
|
LaStayo 2017 Resisted lower limb exercise using recumbent stepper‐ergometer |
Primary | Primary | Secondary | ‐ | Secondary | ‐ | ‐ |
|
Latham 2003 Resistance exercise |
‐ | Primary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Lehtola 2000 Group‐based balance and flexibility training plus walking and home practice |
Primary | ‐ | Primary | ‐ | Primary | ‐ | ‐ |
|
Li 2005 Group‐based Tai Chi |
‐ | ‐ | ‐ | Primary | ‐ | ‐ | ‐ |
|
Lin 2007 Individual balance, strength and flexibility training |
Primary | Secondary | Secondary | ‐ | ‐ | ‐ | ‐ |
|
Liston 2014 Group‐based modified Otago Exercise Program plus individual, partially supervised multisensory balance training |
Primary | Secondary | ‐ ‐ |
‐ | Secondary | ‐ | ‐ |
|
Liston 2014 Group‐based modified Otago Exercise Program plus individual, partially supervised flexibility training |
Primary | Secondary | Secondary | ‐ | Secondary | ‐ | ‐ |
|
Liu‐Ambrose 2004 Supervised, high‐intensity resistance training |
‐ | Primary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Liu‐Ambrose 2004 Supervised agility training |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Liu‐Ambrose 2008 Individual Otago Exercise Program |
Primary | Secondary | ‐ | ‐ | Secondary | ‐ | ‐ |
|
Logghe 2009 Group‐based Tai Chi |
‐ | ‐ | ‐ | Primary | ‐ | ‐ | ‐ |
|
Lord 1995 Group‐based balance, strength, gait training |
Primary | Secondary | Secondary | ‐ | ‐ | ‐ | ‐ |
|
Lord 2003 Group‐based balance, strength, gait training |
Primary | Secondary | Secondary | ‐ | ‐ | ‐ | ‐ |
|
Lurie 2013 Standard Physical Therapy programme + surface perturbation treadmill training |
Primary | Secondary | Secondary | ‐ | ‐ | ‐ | Secondary‐ slip and trip training |
|
Lurie 2013 Standard Physical Therapy programme |
Primary | Secondary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Luukinen 2007 Individual balance and gait training |
Primary | ‐ | ‐ | ‐ | Secondary | ‐ | ‐ |
|
Madureira 2007 Group‐based balance training and walking plus home practice |
Primary | ‐ | ‐ | ‐ | Secondary | ‐ | ‐ |
|
McMurdo 1997 Group‐based balance training |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Means 2005 Group‐based balance, strength, flexibility, gait training and walking |
Primary | Primary | Primary | ‐ | Secondary | ‐ | ‐ |
|
Merom 2016 Group‐based social dancing |
‐ | ‐ | ‐ | Primary | ‐ | Secondary | ‐ |
|
Miko 2017 Individual, partially supervised balance training |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Mirelman 2016 Individual, supervised treadmill training |
Primary | ‐ | ‐ | ‐ | ‐ | Primary | ‐ |
|
Mirelman 2016 Individual, supervised treadmill training plus virtual reality |
Primary | ‐ | ‐ | ‐ | ‐ | Secondary | ‐ |
|
Morgan 2004 Group‐based strength, balance and gait training |
Primary | Secondary | Secondary | ‐ | ‐ | ‐ | ‐ |
|
Morone 2016 Group‐based balance training using Wii‐Fit |
Primary | ‐ | ‐ | ‐ | Secondary | ‐ | ‐ |
|
Morone 2016 Group‐based balance training |
Secondary | ‐ | Primary | ‐ | ‐ | ‐ | ‐ |
|
Morrison 2018 Group‐based balance training |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Morrison 2018 Home‐based strength, balance and aerobic Wii Fit programme |
Primary | ‐ | ‐ | ‐ | ‐ | Secondary | ‐ |
|
Ng 2015 Group‐based strength and balance training plus home practice |
Primary | Primary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Nitz 2004 Group‐based balance |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Okubo 2016 Group‐based Tai Chi and Otago Exercise Program plus home practice |
Secondary | Secondary | ‐ | Primary | Secondary | ‐ | ‐ |
|
Okubo 2016 Group‐based brisk walking |
‐ | ‐ | ‐ | ‐ | Primary | ‐ | ‐ |
|
Park 2008 Strength and balance and endurance training |
Primary | Secondary | Secondary | ‐ | ‐ | Primary | ‐ |
|
Reinsch 1992 Group‐based balance and strength training |
Primary | Secondary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Resnick 2002 Individual or group‐based walking |
‐ | ‐ | ‐ | ‐ | Primary | ‐ | ‐ |
|
Robertson 2001a Individual Otago Exercise Program |
Primary | Secondary | ‐ | ‐ | Secondary | ‐ | ‐ |
|
Rubenstein 2000 Group‐based balance, strength and endurance |
Secondary | Primary | ‐ | ‐ | ‐ | Primary | ‐ |
|
Sakamoto 2013 One leg stand balance training |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | |
|
Sales 2017 Group‐based strength, balance, co‐ordination, mobility and flexibility |
Primary | Secondary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Sherrington 2014 home‐based strength and balance programme |
Primary | Primary | ‐ | ‐ | ‐ | ||
|
Shigematsu 2008 Group‐based stepping training on felt mat |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Shigematsu 2008 Group‐based walking |
Primary | ‐ | ‐ | ‐ | Primary | ‐ | |
|
Siegrist 2016 Group‐based balance, strength, power and gait training plus home practice |
Primary | Secondary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Skelton 2005 Group‐based FaME balance and strength training plus home practice |
Primary | Secondary | ‐ | ‐ | Secondary | ‐ | ‐ |
|
Smulders 2010 Group‐based balance and gait training using an obstacle avoidance course |
Primary | ‐ | ‐ | ‐ | Secondary | ‐ | Secondary‐ training in fall techniques, lifting techniques |
|
Steadman 2003 Standard, individualised physiotherapy focused on functional training plus balance training |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Steadman 2003 Standard, individualised physiotherapy focused on functional training |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Suzuki 2004 Group‐based strength, balance and gait training plus home practice |
Primary | Primary | Primary | Primary | ‐ | ‐ | ‐ |
|
Taylor 2012 Group‐based Tai Chi, 2x/ week |
‐ | ‐ | ‐ | Primary | ‐ | ‐ | ‐ |
|
Taylor 2012 Group‐based Tai Chi, 1x/ week |
‐ | ‐ | ‐ | Primary | ‐ | ‐ | ‐ |
|
Trombetti 2011 Group‐based balance and gait training |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Uusi‐Rasi 2015 Group‐based balance and strength training plus home practice |
Primary | Primary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Verrusio 2017 Individual, supervised balance and gait training using exoskeleton human body posturizer |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Verrusio 2017 Individual, supervised balance and gait training |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Vogler 2009 home‐based seated lower limb strength exercises |
‐ | Primary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Vogler 2009 home‐based strength training with weightbearing, functional tasks |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Voukelatos 2007 Group‐based Tai Chi |
‐ | ‐ | ‐ | Primary | ‐ | ‐ | ‐ |
|
Voukelatos 2015 Individual walking programme |
‐ | ‐ | ‐ | ‐ | Primary | ‐ | ‐ |
|
Weerdesteyn 2006 Group‐based balance and gait training using an obstacle avoidance course |
Primary | ‐ | ‐ | ‐ | Secondary | ‐ | ‐ |
|
Wolf 1996 Group‐based Tai Chi |
‐ | ‐ | ‐ | Primary | ‐ | ‐ | ‐ |
|
Wolf 1996 Individual, computerised balance training on force platform. |
Primary | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Wolf 2003 Group‐based Tai Chi |
‐ | ‐ | ‐ | Primary | ‐ | ‐ | ‐ |
|
Woo 2007 Group‐based Tai Chi |
‐ | ‐ | ‐ | Primary | ‐ | ‐ | ‐ |
|
Woo 2007 Group‐based resistance training |
Secondary | Primary | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Wu 2010 Individual, supervised Tai Chi delivered via videoconferencing |
‐ | ‐ | ‐ | Primary | ‐ | ‐ | ‐ |
|
Wu 2010 Group‐based Tai Chi |
‐ | ‐ | ‐ | Primary | ‐ | ‐ | ‐ |
|
Wu 2010 Individual Tai Chi with DVD instruction |
‐ | ‐ | ‐ | Primary | ‐ | ‐ | ‐ |
|
Yamada 2010 Group‐based trail walking |
Primary | Secondary | Secondary | ‐ | Secondary | ‐ | ‐ |
|
Yamada 2010 Group‐based indoor walking |
Secondary | Secondary | Secondary | ‐ | Primary | ‐ | ‐ |
|
Yamada 2012 Group‐based balance, strength, flexibility and gait training involving complex obstacle course |
Primary | Secondary | Secondary | ‐ | Secondary | ‐ | ‐ |
|
Yamada 2012 Group‐based balance, strength, flexibility and gait training involving simple obstacle course |
Primary | Secondary | Secondary | ‐ | Secondary | ‐ | ‐ |
|
Yamada 2013 Group‐based balance, strength, flexibility and gait training including stepping mat |
Primary | Secondary | Secondary | ‐ | Secondary | ‐ | ‐ |
|
Yamada 2013 Group‐based balance, strength, flexibility and gait training plus indoor walking |
Primary | Secondary | Secondary | ‐ | Secondary | ‐ | ‐ |
|
Yang 2012 Individual Otago Exercise Program |
Primary | Secondary | ‐ | ‐ | Secondary | ‐ | ‐ |
* Intervention groups combined due to fall data not being available for individual intervention groups.
Appendix 6. Study IDs for the 81 studies included in the exercise (all types) versus control comparison
* = multigroup trial appearing in more than one category.
Appendix 7. Study IDs for the 59 exercise versus control studies included in rate of falls analysis
* = multigroup trial appearing in more than one category
Appendix 8. Number of studies and participants in primary analysis (exercise versus control on rate of falls), by primary category of exercise
| Comparisona | Number of trials (cluster)b | Number of trials with no secondary exercise categoriesc | Number of participants randomised | Number of participants analysedd |
| Exercise (all types) versus control | 59 (6) | 15 | 16363 | 12981 |
| Balance and functional exercises versus control | 39 (4) | 7 | 9815 | 7920 |
| Resistance exercises versus control | 5 | 3 | 480 | 327 |
| Flexibility versus control | 0 | 0 | 0 | 0 |
| 3D exercise (Tai Chi) versus control | 7 (1) | 6 | 2794 | 2655 |
| 3D exercise (dance) versus control | 1 (1) | 0 | 530 | 522 |
| General physical activity (walking program) versus control | 2 | 2 | 551 | 441 |
| Endurance training versus control | 0 | 0 | 0 | 0 |
| Other kinds of exercise versus control | 0 | 0 | 0 | 0 |
| Multiple categories of exercise versus control | 11 | N/A | 1783 | 1374 |
aExercise (all types) combines all categories of exercise. Multiple categories of exercise include studies containing two or more primary categories of exercise, as categorised using the ProFaNE taxonomy. The remaining analyses include only one primary category of exercise, as categorised using the ProFaNE taxonomy. bStudy IDs are shown in Appendix 7. cThe number of trials where the intervention programme did not include a secondary exercise from another exercise category using the ProFaNE taxonomy. dThese data apply to the follow‐up (at the time point included in main analysis) for the primary outcome (rate of falls) for the individual trials
Appendix 9. Source of data for generic inverse variance analysis (see footnotes for explanation of codes)
| Study ID | Source for rate ratio: falls | Source for risk ratio: fallers | Source for risk ratio: number with fractures | Source for risk ratio: number with falls requiring medical attention | Source for risk ratio: number with adverse events | Source for risk ratio: hospitalisation | Source for risk ratio: death |
| Almeida 2013 | ND | ND | NA | NA | ND | NA | NA |
| Ansai 2015 | NA | 7 | NA | NA | NA | NA | NA |
| Arantes 2015 | NA | 7 | NA | NA | NA | NA | NA |
| Arkkukangas 2015 | 3 | 7 | NA | NA | ND | NA | NA |
| Ballard 2004 | 3 | NA | NA | NA | ND | NA | NA |
| Barker 2016 | 1 | 7 | NA | ND | ND | NA | NA |
| Barnett 2003 | 1 | 5 | NA | NA | NA | NA | 7 |
| Beyer 2007 | NA | 7 | NA | NA | ND | NA | NA |
| Boongrid 2017 | 1 | 4 | NA | NA | ND | NA | 7 |
| Brown 2002 | NA | 7 | NA | NA | NA | NA | 7 |
| Buchner 1997 | 1 | 4 | NA | NA | NA | NA | NA |
| Bunout 2005 | 3 | 7 | NA | NA | NA | NA | 7 |
| Campbell 1997 | 2 | 4 | NA | NA | NA | NA | NA |
| Carter 2002 | 3 | NA | NA | NA | ND | NA | NA |
| Cerny 1998 | NA | 7 | NA | NA | NA | NA | NA |
| Clegg 2014 | 3 | 5 | NA | NA | NA | 7 | 7 |
| Clemson 2010 | 1 | 7 | NA | NA | NA | NA | NA |
| Clemson 2012 | 1 (ex v control), 3 (ex v ex) | 7 | NA | NA | ND | NA | 7 |
| Cornillon 2002 | 3 | 7 | NA | NA | NA | NA | 7 |
| Dadgari 2016 | 3c | 7c | NA | NA | NA | NA | 7 |
| Dangour 2011 | NA | 7c | 7 | NA | NA | NA | NA |
| Davis 2011 | 1, 3 | NA | NA | NA | ND | NA | NA |
| Day 2002 | 1, 3 | 4 | NA | 7 | NA | NA | 7 |
| Day 2015 | 3 | 7 | NA | ND | NA | NA | 7 |
| Duque 2013 | 3 | NA | NA | NA | NA | NA | NA |
| Ebrahim 1997 | 3 | 7 | 7 | NA | NA | NA | NA |
| El‐Khoury 2015 | 2b | 7 | ND | ND | ND | NA | 7 |
| Fiatarone 1997 | NA | ND | NA | NA | NA | NA | NA |
| Freiberger 2007 | 1 | 7 | NA | NA | NA | NA | NA |
| Gill 2016 | NA | NA | 7 | NA | NA | 7 | 7 |
| Grahn Kronhed 2009 | 3 | NA | NA | NA | NA | NA | NA |
| Gschwind 2015 | 3 | NA | NA | NA | ND | NA | NA |
| Haines 2009 | 1 | 7 | 7 | ND | ND | NA | NA |
| Halvarsson 2013 | NA | 7 | NA | NA | NA | NA | NA |
| Halvarsson 2016 | NA | 7 | ND | NA | NA | NA | NA |
| Hamrick 2017 | 3 | 7 | NA | NA | NA | NA | NA |
| Hauer 2001 | NA | 5 | NA | NA | ND | NA | NA |
| Helbostad 2004 | 3 | 7 | NA | NA | NA | NA | NA |
| Hirase 2015 | 3 | NA | NA | NA | NA | NA | NA |
| Huang 2010 | NA | 7c | NA | NA | NA | NA | NA |
| Hwang 2016 | 1 | 7 | NA | NA | NA | NA | 7 |
| Iliffe 2015 | 3 | 7 | NA | NA | NA | NA | 7 |
| Irez 2011 | 3 | NA | NA | NA | NA | NA | NA |
| Iwamoto 2009 | ND | 7 | ND | ND | ND | NA | NA |
| Kamide 2009 | ND | 7 | NA | NA | NA | NA | NA |
| Karinkanta 2007 | 3 | NA | 7 | 7 | NA | NA | 7 |
| Kemmler 2010 | 3 | 5 | ND | NA | ND | NA | 7 |
| Kerse 2010 | 3 | 7 | NA | NA | NA | NA | 7 |
| Kim 2014 | NA | 7 | 7 | NA | NA | NA | NA |
| Korpelainen 2006 | 3 | NA | 7 | NA | ND | NA | NA |
| Kovacs 2013 | 3 | 7 | ND | NA | NA | NA | NA |
| Kwok 2016 | 1a | 7 | NA | NA | ND | NA | NA |
| Kyrdalen 2014 | NA | 7 | NA | NA | NA | ND | 7 |
| LaStayo 2017 | 3 | 7 | NA | NA | NA | NA | NA |
| Latham 2003 | 3 | 4 | NA | NA | 7 | NA | 7 |
| Lehtola 2000 | 1 | NA | NA | NA | NA | NA | NA |
| Li 2005 | 2a | 4 | NA | 7 | ND | NA | NA |
| Lin 2007 | 3 | NA | NA | NA | NA | NA | 7 |
| Liston 2014 | 3 | ND | NA | NA | NA | NA | NA |
| Liu‐Ambrose 2004 | 3 | ND | NA | NA | ND | NA | NA |
| Liu‐Ambrose 2008 | 1 | 7 | NA | NA | NA | NA | 7 |
| Logghe 2009 | 2 | 7 | NA | NA | NA | NA | 7 |
| Lord 1995 | 3 | 5 | NA | NA | NA | NA | 7 |
| Lord 2003 | 1a | 7 | NA | NA | NA | NA | 7 |
| Lurie 2013 | NA | 7 | NA | NA | NA | NA | NA |
| Luukinen 2007 | 2 | 7 | NA | NA | NA | NA | NA |
| Madureira 2007 | 3 | NA | NA | NA | NA | NA | NA |
| McMurdo 1997 | 3 | 7 | 7 | NA | NA | NA | NA |
| Means 2005 | 3 | 7 | NA | NA | ND | NA | 7 |
| Merom 2016 | 1b | 5b | NA | NA | ND | NA | 7 |
| Miko 2017 | 3 | 7 | NA | NA | NA | NA | NA |
| Mirelman 2016 | ND | NA | NA | NA | ND | NA | NA |
| Morgan 2004 | NA | 7 | NA | NA | NA | NA | NA |
| Morone 2016 | ND | ND | NA | NA | NA | NA | NA |
| Morrison 2018 | NA | ND | NA | NA | NA | NA | NA |
| Ng 2015 | NA | 7 | NA | NA | ND | NA | 7 |
| Nitz 2004 | 3 | NA | NA | NA | ND | NA | NA |
| Okubo 2016 | NA | NA | NA | NA | NA | NA | NA |
| Reinsch 1992 | NA | 7c | NA | ND | ND | NA | NA |
| Resnick 2002 | ND | NA | NA | NA | NA | NA | NA |
| Robertson 2001a | 1 | 7 | 7 | 7 | NA | NA | 7 |
| Rubenstein 2000 | 3 | 7 | NA | NA | ND | NA | NA |
| Sakamoto 2013 | 3 | 7 | 7 | NA | ND | NA | NA |
| Sherrington 2014 | 1 | 5 | ND | ND | ND | NA | 7 |
| Sales 2017 | 3 | 7 | NA | NA | ND | NA | 7 |
| Shigematsu 2008 | 3 | 7 | NA | NA | ND | NA | NA |
| Siegrist 2016 | 1b | 7b | ND | NA | ND | NA | 7 |
| Skelton 2005 | 1 | 7 | ND | NA | ND | NA | 7 |
| Smulders 2010 | 1 | 7 | 7 | NA | NA | NA | NA |
| Steadman 2003 | 3 | NA | NA | NA | NA | NA | NA |
| Suzuki 2004 | 3 | 7 | NA | NA | NA | NA | NA |
| Taylor 2012 | 3 | 7 | NA | NA | NA | NA | 7 |
| Trombetti 2011 | 1 | 4 | NA | NA | ND | NA | 7 |
| Uusi‐Rasi 2015 | 1 | 4 | NA | 4 | ND | NA | 7 |
| Verrusio 2017 | NA | 7 | ND | NA | NA | NA | NA |
| Vogler 2009 | NA | 7 | NA | NA | ND | NA | 7 |
| Voukelatos 2007 | 1 | 4 | NA | NA | NA | NA | NA |
| Voukelatos 2015 | 1 | 5 | NA | NA | NA | NA | 7 |
| Weerdesteyn 2006 | 3 | 7 | NA | NA | NA | NA | NA |
| Wolf 1996 | 3 | NA | NA | NA | NA | NA | NA |
| Wolf 2003 | 2b | 7c | NA | NA | ND | NA | 7 |
| Woo 2007 | NA | 7 | NA | NA | NA | NA | NA |
| Wu 2010 | 3 | NA | NA | NA | NA | NA | NA |
| Yamada 2010 | 1 | 7 | NA | NA | ND | NA | NA |
| Yamada 2012 | 1 | 7 | ND | NA | ND | NA | NA |
| Yamada 2013 | 1 | 7 | ND | NA | ND | NA | NA |
| Yang 2012 | NA | 7 | NA | NA | NA | NA | 7 |
Abbreviations:
Codes for source of rate ratio 1: incidence rate ratio reported by trial authors 2: hazard ratio/relative hazard (multiple events) reported by trial authors 3: incidence rate ratio calculated by review authors a: adjusted for confounders by trial authors b: adjusted for clustering by trial authors c: adjusted for clustering by review authors Codes for source of risk ratio: 4: hazard ratio/relative hazard (first fall only) reported by trial authors 5: relative risk reported by trial authors 6: odds ratio reported by trial authors 7: relative risk calculated by review authors a: adjusted for confounders by trial authors b: adjusted for clustering by trial authors c: adjusted for clustering by review authors NA: not applicable. Falls (for rate ratio) or fallers (for risk ratio) or number of people sustaining a fracture (for risk ratio) or number with falls requiring medical attention (for risk ratio) or number with adverse events (for risk ratio) or number of people with falls requiring hospital admission (for risk ratio) or death (for risk ratio) not reported as an outcome in the trial ND: outcomes relating to falls or fallers or fractures or falls requiring medical attention or adverse events or hospital admission or death were reported, but there were no useable data
Appendix 10. Raw data for rate of falls and number of fallers when available
| Study ID | Intervention group: falls per person‐year | Control group: falls per person‐year | Intervention group: number of fallers | Intervention group: number in analysis | Control group: number of fallers | Control group: number in analysis | Follow‐up |
| Almeida 2013 | 0 | 0 | 0 | 28 | 0 | 26 | 4 mo |
| Ansai 2015 multiple/resistance vs control | 4.06/10.14 | 4.88 | 4/8 | 22/23 | 8 | 22 | 4 mo |
| Arantes 2015 | ‐ | ‐ | 2 | 15 | 5 | 13 | 3 mo |
| Arkkukangas 2015 | 0.89 | 1.23 | 5 | 27 | 3 | 13 | 3 mo |
| Ballard 2004 | 0.16 | 0.41 | ‐ | 20 | ‐ | 19 | 16 mo |
| Barker 2016 | 1.17 | 1.16 | 6 | 20 | 9 | 24 | 6 mo |
| Barnett 2003 | 0.61 | 0.95 | 27 | 76 | 37 | 74 | 12 mo |
| Beyer 2007 | ‐ | ‐ | 12 | 24 | 14 | 29 | 12 mo |
| Boongrid 2017 | 0.30 | 0.40 | 51 | 218 | 61 | 219 | 12 mo |
| Brown 2002 | ‐ | ‐ | 20 | 39 | 21 | 32 | 14 mo |
| Buchner 1997 | 0.49 | 0.81 | 29 | 70 | 18 | 30 | 25 mo |
| Bunout 2005 | 0.23 | 0.18 | 23 | 111 | 16 | 130 | 12 mo |
| Campbell 1997 12 mo/24 mo vs control | 0.87/0.83 | 1.34/1.19 | 53 | 116/71 | 62 | 117/81 | 24 mo |
| Carter 2002 | 0.46 | 0.52 | ‐ | 40 | ‐ | 40 | 5 mo |
| Cerny 1998 | ‐ | ‐ | 3 | 15 | 3 | 13 | 6 mo |
| Clegg 2014 | 0.70 | 0.93 | 7 | 40 | 8 | 30 | 3 mo |
| Clemson 2010 | ‐ | ‐ | 8 | 18 | 9 | 16 | 6 mo |
| Clemson 2012 LiFE/ structured vs control | 1.66/1.90 | 2.28 | 60/65 | 105/107 | 71 | 106 | 12 mo |
| Cornillon 2002 | 0.39 | 0.47 | 39 | 150 | 48 | 153 | 12 mo |
| Dadgari 2016 | 2.52 | 3.28 | 138 | 160 | 154 | 157 | 6 mo |
| Dangour 2011 | ‐ | ‐ | 189 | 325 | 198 | 294 | 24 mo |
|
Davis 2011 x1/x2 wkly resistance vs balance/tone |
0.74/0.82 | 1.06 | ‐ | 52/54 | ‐ | 49 | 9 mo |
| Day 2002 | 1.05 | 1.20 | 76 | 135 | 87 | 137 | 18 mo |
| Day 2015 | 0.62 | 0.58 | 65 | 204 | 64 | 205 | 12 mo |
| Duque 2013 | 2.2 | 4 | ‐ | 30 | — | 40 | 9 mo |
|
Ebrahim 1997 12 mo/24 mo vs control |
0.80/0.70 | 0.52/0.55 | 25/‐ | 52/49 | 18/‐ | 50/48 | 24 mo |
| El‐Khoury 2015 | 0.79 | 0.92 | 189 | 352 | 222 | 354 | 24 mo |
|
Freiberger 2007 12 mo/24 mo Fitness vs strength & balance |
0.90/— | 1.22/‐ | 19/‐ | 65/48 | 29/— | 62/49 | 24 mo |
| Gill 2016 | ‐ | ‐ | ‐ | 818 | ‐ | 817 | 42 mo |
| Grahn Kronhed 2009 | 0.6 | 0.8 | ‐ | 31 | ‐ | 34 | 12 mo |
| Gschwind 2015 | 0.25 | 0.50 | ‐ | 71 | ‐ | 65 | 6 mo |
| Haines 2009 | ‐ | ‐ | 11 | 19 | 20 | 34 | 6 mo |
| Halvarsson 2013 | ‐ | ‐ | 18 | 30 | 2 | 18 | 15 mo |
|
Halvarsson 2016 balance/ balance+walking vs control |
‐ | ‐ | 4/5 | 25/18 | 4 | 26 | 3 mo |
| Hamrick 2017 | 0.63 | 0.84 | 4 | 19 | 7 | 19 | 6 mo |
| Hauer 2001 | ‐ | ‐ | 14 | 31 | 15 | 25 | 6 mo |
| Helbostad 2004 | 1.45 | 1.33 | 20 | 34 | 18 | 34 | 12 mo |
|
Hirase 2015 foam/ stable surface vs control |
0.72/1.77 | 2.7 | ‐ | 29/29 | ‐ | 28 (14 in analysis) | 4 mo |
| Huang 2010 | ‐ | ‐ | 0 | 31 | 2 | 47 | 5 mo |
|
Hwang 2016 Tai Chi vs lower extremity |
0.08 | 0.16 | 72 | 167 | 99 | 167 | 18 mo |
|
Iliffe 2015 FAME/ OEP vs control, (18 mo/30mo) |
0.64/0.66 | 0.76 | ‐ | 230/227 | ‐ | 252 | 30 mo |
| Irez 2011 | 1.48 | 5.2 | ‐ | 30 | ‐ | 30 | 3 mo |
| Iwamoto 2009 | 0.00 | 0.29 | 0 | 34 | 4 | 33 | 5 mo |
| Kamide 2009 | ‐ | ‐ | 0 | 20 | 1 | 23 | 6 mo |
|
Karinkanta 2007 balance/resistance/bal+resistance vs control |
0.51/0.21/0.53 | 0.36 | ‐ | 35/37/36 | ‐ | 36 | 12 mo |
| Kemmler 2010 | 0.17 | 0.28 | ‐ | 112 | — | 115 | 18 mo |
| Kerse 2010 | 0.48 | 0.41 | 47 | 98 | 39 | 95 | 12 mo |
| Kim 2014 | ‐ | ‐ | 10 | 51 | 21 | 52 | 12 mo |
| Korpelainen 2006 | 0.42 | 0.53 | ‐ | 84 | ‐ | 76 | 30 mo |
| Kovacs 2013 | 0.42 | 0.17 | 6 | 36 | 15 | 36 | 12 mo |
| Kwok 2016 | ‐ | ‐ | 8 | 40 | 11 | 40 | 12 mo |
| Kyrdalen 2014 | ‐ | ‐ | 19 | 47 | 17 | 47 | 3 mo |
| Latham 2003 | 1.02 | 1.07 | 60 | 112 | 64 | 110 | 6 mo |
| LaStayo 2017 stepper‐ergometer resistance vs traditional resistance | 2.78 | 1.40 | 36 | 54 | 32 | 58 | 12 mo |
| Lehtola 2000 | 0.15 | 0.24 | ‐ | 92 | ‐ | 39 | 10 mo |
| Li 2005 | 0.80 | 1.57 | 27 | 95 | 43 | 93 | 6 mo |
| Lin 2007 | 0.58 | 0.88 | ‐ | 50 | ‐ | 50 | 6 mo |
| Liston 2014 | 2.29 | 2.25 | ‐ | 7 | ‐ | 8 | 6 mo |
| Liu‐Ambrose 2004 resistance/agility vs stretching | 1.13/0.65 | 0.63 | ‐ | 32/34 | ‐ | 32 | 6 mo |
| Liu‐Ambrose 2008 | ‐ | ‐ | 12 | 31 | 16 | 28 | 12 mo |
| Logghe 2009 | ‐ | ‐ | 58 | 138 | 59 | 131 | 12 mo |
| Lord 1995 | 0.53 | 0.63 | 26 | 75 | 33 | 94 | 12 mo |
| Lord 2003 | 0.67 | 0.85 | 109 | 259 | 117 | 249 | 12 mo |
| Lurie 2013 | ‐ | ‐ | 5 | 26 | 11 | 33 | 3 mo |
| Luukinen 2007 | 1.23 | 1.15 | 126 | 217 | 136 | 220 | 16 mo |
| Madureira 2007 | 0.96 | 0.40 | ‐ | 30 | ‐ | 30 | 12 mo |
| McMurdo 1997 | 0.17 | 0.32 | 13 | 44 | 21 | 48 | 24 mo |
| Means 2005 | 0.48 | 1.18 | 22 | 144 | 36 | 94 | 6 mo |
| Merom 2016 | 1.03 | 0.80 | 133 | 275 | 144 | 247 | 12 mo |
| Miko 2017 | 0.14 | 0.33 | 6 | 49 | 11 | 48 | 12 mo |
| Morgan 2004 | ‐ | ‐ | 34 | 119 | 34 | 110 | 12 mo |
| Morrison 2018 Wii vs balance | 0 | 0 | 0 | 14 | 0 | 32 | 3 mo |
| Ng 2015 | ‐ | ‐ | 3 | 46 | 5 | 46 | 12 mo |
| Nitz 2004 | 1.00 | 1.24 | ‐ | 24 | ‐ | 21 | 6 mo |
|
Okubo 2016 walking vs balance |
‐ | ‐ | ‐ | 50 | ‐ | 40 | 16 mo |
| Park 2008 | ‐ | ‐ | 4 | 22 | 4 | 23 | 11 mo |
| Reinsch 1992 | ‐ | ‐ | 55 | 129 | 34 | 101 | 12 mo |
| Resnick 2002 | ‐ | ‐ | ‐ | 10 | ‐ | 7 | 6 mo |
| Robertson 2001a | 0.69 | 1.01 | 38 | 121 | 51 | 119 | 12 mo |
| Rubenstein 2000 | 1.68 | 2.00 | 12 | 28 | 9 | 31 | 3 mo |
| Sales 2017 | 0.89 | 0.76 | 11 | 27 | 10 | 21 | 12 mo |
| Sakamoto 2013 | 0.28 | 0.33 | ‐ | 410 | ‐ | 455 | 6 mo |
| Sherrington 2014 | ‐ | ‐ | 11 | 169 | 15 | 171 | 4 mo |
| Shigematsu 2008 | 0.23 | 0.33 | 4 | 32 | 7 | 36 | 8 mo |
| Siegrist 2016 | 1.3 | 2.4 | 93 | 222 | 77 | 156 | 12 mo |
| Skelton 2005 | ‐ | ‐ | 35 | 50 | 23 | 31 | 9 mo |
| Smulders 2010 | 0.72 | 1.18 | 21 | 47 | 23 | 45 | 12 mo |
| Steadman 2003 | 7.13 | 7.13 | ‐ | 69 | ‐ | 64 | 1 mo |
| Suzuki 2004 | 0.16 | 0.46 | 3 | 22 | 12 | 22 | 20 mo |
|
Taylor 2012 Tai Chi x1 week/ x2 week v low level ex. |
1.55/1.16 | 1.38 | 132/111 | 233/220 | 140 (70 for analysis) | 231 (115 for analysis) | 17 mo |
| Trombetti 2011 | 0.7 | 1.6 | 19 | 66 | 32 | 68 | 6 mo |
| Uusi‐Rasi 2015 | 1.21 | 1.18 | ‐ | 91 | ‐ | 95 | 24 mo |
| Verrusio 2017 | — | — | 6 | 73 | 19 | 74 | 12 mo |
| Vogler 2009 | |||||||
| Voukelatos 2007 | 0.50 | 0.75 | 71 | 347 | 81 | 337 | 6 mo |
| Voukelatos 2015 | ‐ | ‐ | 54 | 159 | 68 | 180 | 12 mo |
| Weerdesteyn 2006 | 0.89 | 1.68 | 10 | 30 | 9 | 28 | 7 mo |
| Wolf 1996 Tai Chi/ balance training vs education | 0.86/1.53 | 1.29 | ‐ | 72/64 | ‐ | 64 | 8 mo |
| Wolf 2003 | ‐ | ‐ | 69 | 145 | 85 | 141 | 11 mo |
| Woo 2007 Tai Chi/ resistance vs control | ‐ | ‐ | 15/24 | 58/59 | 31/31 | 59 | 12 mo |
| Wu 2010 Telecommunication‐based Tai Chi/ home‐based Tai Chi vs group Tai Chi | 0.47/0.94 | 0.35 | ‐ | 22/22 | ‐ | 20 | 4 mo |
| Yamada 2010 | ‐ | ‐ | 5 | 29 | 11 | 29 | 12 mo |
| Yamada 2012 | ‐ | ‐ | 19 | 73 | 2 | 72 | 12 mo |
| Yamada 2013 | ‐ | ‐ | 13 | 112 | 39 | 118 | 12 mo |
| Yang 2012 | ‐ | ‐ | 12 | 59 | 18 | 62 | 6 mo |
mo: months
Appendix 11. Raw data for fall‐related fracture, falls requiring medical attention, falls requiring hospital admission and death, when available
| Study ID | Intervention group: fall‐ related fracture | Control group: fall‐related fracture | Intervention group: falls requiring medical attention | Control group: falls requiring medical attention | Intervention group: falls requiring hospital admission | Control group: falls requiring hospital admission | Intervention group: number in analysis | Control group: number in analysis | Follow‐up |
| Almeida 2013 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Ansai 2015 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Arantes 2015 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Arkkukangas 2015 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Ballard 2004 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Barker 2016 | ‐ | ‐ | 3 | 8 | ‐ | ‐ | 20 | 24 | 6 mo |
| Barnett 2003 | ‐ | ‐ | 28 | 38 | ‐ | ‐ | 76 | 74 | 12 mo |
| Beyer 2007 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Boongrid 2017 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Brown 2002 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Buchner 1997 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Bunout 2005 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Campbell 1997 12 mo/24 mo | ‐ | ‐ | 27/103 | 43/110 | ‐ | ‐ | 116/71 | 117/81 | 24 mo |
| Carter 2002 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Cerny 1998 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | 15 | 13 | 6 mo |
| Clegg 2014 | ‐ | ‐ | ‐ | ‐ | 2 | 4 | 41 | 33 | 3 mo |
| Clemson 2010 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Clemson 2012 LiFE/ structured v control | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Cornillon 2002 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Dadgari 2016 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Dangour 2011 | 10 | 5 | ‐ | ‐ | ‐ | ‐ | 412 | 406 | 24 mo |
|
Davis 2011 x1/x2 wkly resistance v balance/tone |
‐ | ‐ | 0/0 | 0 | ‐ | ‐ | 52/54 | 49 | 9 mo |
| Day 2002 | ‐ | ‐ | 16 | 18 | ‐ | ‐ | 135 | 137 | 18 mo |
| Day 2015 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Duque 2013 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Ebrahim 1997 | 6 | 4 | ‐ | ‐ | ‐ | ‐ | 49 | 48 | 24 mo |
| El‐Khoury 2015 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Freiberger 2007 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Gill 2016 | 66 | 76 | ‐ | ‐ | 46 | 44 | 818 | 817 | 42 mo |
| Grahn Kronhed 2009 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | 31 | 34 | 12 mo |
| Gschwind 2015 | 0 | 0 | 0 | 0 | ‐ | ‐ | 71 | 65 | 6 mo |
| Haines 2009 | 1 | 2 | 5 | 26 | ‐ | ‐ | 19 | 34 | 6 mo |
| Halvarsson 2013 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Halvarsson 2016 balance/ balance + walking v control |
0 | 0 | 0 | 0 | ‐ | ‐ | 25/18 | 26 | 3 mo |
| Hamrick 2017 | 0 | 0 | 0 | 0 | ‐ | ‐ | 19 | 19 | 6 mo |
| Hauer 2001 | 0 | 0 | 0 | 0 | ‐ | ‐ | 31 | 25 | 6 mo |
| Helbostad 2004 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Hirase 2015 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Huang 2010 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Hwang 2016 Tai Chi v lower extremity |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Iliffe 2015 FAME/ OEP v control |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Irez 2011 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Iwamoto 2009 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Kamide 2009 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Karinkanta 2007 balance/resistance/bal+resistance |
0/2/1 | 1 | 17/16/11 | 17 | ‐ | ‐ | 36/37/36 | 36 | 12 mo |
|
Kemmler 2010 high intensity / low intensity |
‐ | ‐ | 0 | 0 | ‐ | ‐ | 115 | 113 | 18 mo |
| Kerse 2010 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Kim 2014 | 1 | 2 | ‐ | ‐ | ‐ | ‐ | 51 | 52 | 12 mo |
| Korpelainen 2006 | 6 | 16 | ‐ | ‐ | ‐ | ‐ | 84 | 76 | 30 mo |
| Kovacs 2013 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Kwok 2016 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Kyrdalen 2014 OEP group / OEP home |
‐ | ‐ | ‐ | ‐ | 3 | 4 | 62 | 63 | 3 mo |
| Latham 2003 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| LaStayo 2017 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Lehtola 2000 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Li 2005 | ‐ | ‐ | 5 | 14 | ‐ | ‐ | 95 | 93 | 6 mo |
| Lin 2007 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Liston 2014 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Liu‐Ambrose 2004 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Liu‐Ambrose 2008 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Logghe 2009 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Lord 1995 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Lord 2003 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Lurie 2013 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Luukinen 2007 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Madureira 2007 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| McMurdo 1997 | 0 | 2 | ‐ | ‐ | ‐ | ‐ | 44 | 48 | 24 mo |
| Means 2005 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Merom 2016 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Miko 2017 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Morgan 2004 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Morrison 2018 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Ng 2015 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Nitz 2004 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Okubo 2016 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Park 2008 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Reinsch 1992 | ‐ | ‐ | 4 | 1 | ‐ | ‐ | 129 | 101 | 12 mo |
| Resnick 2002 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Robertson 2001a | 2 | 7 | 18 | 26 | ‐ | ‐ | 114 | 104 | 12 mo |
| Rubenstein 2000 | 0 | 0 | 0 | 0 | ‐ | ‐ | 28 | 31 | 3 mo |
| Sales 2017 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sakamoto 2013 | 4 | 11 | ‐ | ‐ | ‐ | ‐ | 410 | 455 | 6 mo |
| Sherrington 2014 | 14 | 15 | 61 | 53 | ‐ | ‐ | 171 | 169 | 4 mo |
| Shigematsu 2008 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Siegrist 2016 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Skelton 2005 | NDa | NDa | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 9 mo |
| Smulders 2010 | 1 | 3 | 0 | 2 | ‐ | ‐ | 47 | 45 | 12 mo |
| Steadman 2003 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Suzuki 2004 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | 22 | 22 | 20 mo |
|
Taylor 2012 Tai Chi x1 week/ x2 week v low level ex. |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Trombetti 2011 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Uusi‐Rasi 2015 | ‐ | ‐ | HR | HR | ‐ | ‐ | 91 | 97 | 24 mo |
| Verrusio 2017 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Vogler 2009 seated v weightbearing training |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Voukelatos 2007 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Voukelatos 2015 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Weerdesteyn 2006 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Wolf 1996 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Wolf 2003 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Woo 2007 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Wu 2010 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Yamada 2010 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Yamada 2012 | 1 | 8 | ‐ | ‐ | ‐ | ‐ | 73 | 72 | 12 mo |
| Yamada 2013 | 3 | 13 | ‐ | ‐ | ‐ | ‐ | 112 | 118 | 12 mo |
| Yang 2012 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
mo: months; HR: hazard ratio data only; NDa: no data presented by group
Appendix 12. Raw data for death, when available
| Study ID | Intervention group: death | Control group: death | Intervention group: number in analysis | Control group: number in analysis | Follow‐up |
| Barnett 2003 | 0 | 3 | 76 | 74 | 12 mo |
| Boongrid 2017 | 0 | 1 | 219 | 220 | 12 mo |
| Brown 2002 | 0 | 3 | 46 | 47 | 14 mo |
| Bunout 2005 | 3 | 3 | 111 | 133 | 12 mo |
| Clegg 2014 | 1 | 3 | 41 | 33 | 3 mo |
| Clemson 2012 LiFE/structured vs control | 1/3 | 3 | 100/99 | 94 | 12 mo |
| Cornillon 2002 | 1 | 0 | 150 | 153 | 12 mo |
| Dangour 2011 | 9 | 6 | 412 | 406 | 24 mo |
| Day 2002 | NRa | NRa | 135 | 137 | 18 mo |
| Day 2015 | 1 | 4 | 204 | 205 | 12 mo |
| El‐Khoury 2015 | 5 | 6 | 352 | 354 | 24 mo |
| Gill 2016 | 42 | 37 | 818 | 817 | 42 mo |
| Haines 2009c | 0 | 3 | 19 | 34 | 6 mo |
|
Hwang 2016 Tai Chi vs lower extremity |
2 | 3 | 169 | 170 | 18 mo |
|
Iliffe 2015 FAME/OEP vs control |
3/3 | 4 | 243/256 | 274 | 18 mo |
|
Karinkanta 2007 balance/resistance/bal+resistance |
1/0/0 | 1 | 36/37/36 | 36 | 12 mo |
|
Kemmler 2010 high intensity/low intensity |
0 | 1 | 115 | 113 | 18 mo |
| Kerse 2010 | 1 | 4 | 92 | 95 | 12 mo |
|
Kyrdalen 2014 OEP group/OEP home |
6 | 3 | 62 | 63 | 3 mo |
| Latham 2003c | 6 | 8 | 118 | 118 | 6 mo |
| Lin 2007 | 2 | 0 | 45 | 45 | 6 mo |
| Liu‐Ambrose 2008 | 1 | 2 | 31 | 27 | 12 mo |
| Logghe 2009 | 1 | 0 | 127 | 117 | 12 mo |
| Lord 1995 | NRb | NRb | 75 | 94 | 12 mo |
| Lord 2003 | 5 | 1 | 264 | 250 | 12 mo |
| Means 2005 | 4 | 4 | 148 | 98 | 6 mo |
| Merom 2016 | 3 | 2 | 278 | 249 | 12 mo |
| Ng 2015 | 0 | 1 | 46 | 47 | 12 mo |
| Robertson 2001a | 1 | 6 | 114 | 104 | 12 mo |
| Sales 2017 | 0 | 1 | 31 | 22 | 12 mo |
| Sherrington 2014c | 10 | 9 | 171 | 169 | 4 mo |
| Siegrist 2016 | 8 | 10 | 222 | 156 | 12 mo |
| Skelton 2005 | 1 | 1 | 44 | 28 | 9 mo |
|
Taylor 2012 Tai Chi x 1 week/ x 2 week vs low‐level exercise |
2/0 | 7 | 182/174 | 181 | 17 mo |
| Trombetti 2011 | 2 | 2 | 57 | 52 | 6 mo |
| Uusi‐Rasi 2015 | 0 | 2 | 91 | 97 | 24 mo |
|
Vogler 2009c seated vs weight‐bearing training |
1 | 1 | 58 | 58 | 3 mo |
| Voukelatos 2015 | 4 | 0 | 180 | 189 | 12 mo |
| Wolf 2003 | 2 | 4 | 147 | 141 | 11 mo |
| Yang 2012 | 0 | 1 | 59 | 62 | 6 mo |
mo: months
NR: not reported. adata not presented by group; total deaths = 15. bdata not presented by group; total deaths = 3. cPost‐hospital discharge trials.
Appendix 13. Adverse events. Studies that reported on adverse events
| Study IDa | Group in which adverse events were reported | Adverse events reported in intervention group(s)b | Adverse events reported in control groupb | ||
| Adverse events reported | Number in intervention group(s) | Adverse events reported | Number in control group | ||
| Gait, balance, and functional training | |||||
| Almeida 2013 | Two intervention groups and control | 0, 0 | 28 | 0 | 26 |
| Boongrid 2017 | Intervention and control | Knee pain (n = 2) | 218 | Knee pain (n = 2) | 219 |
| Clemson 2012 LiFEc | Intervention only | Pelvic stress fracture (n = 1) | 105 | ‐ | 106 |
| El‐Khoury 2015 | Intervention only | Painful wrist (n = 1), twisted ankle (n = 1), bruises (n = 5), lumbago (n = 1) | 352 | ‐ | 354 |
| Gschwind 2015 | Intervention and control | 0 | 71 | 0 | 65 |
| Iliffe 2015 FaME/OEP groupsd | Two intervention groups and control | FaME: 59 (including 'pulled muscles', exacerbation of back/knee pain, muscle/joint soreness) OEP: 69 (including 'pulled muscles', venous problems, exacerbation of back/knee/hip pain and sciatica) |
230/227 | 45 (including exacerbation of back pain) | 252 |
| Iwamoto 2009 | Intervention and control | 0 | 34 | 0 | 33 |
|
Liu‐Ambrose 2004 agility groupc |
Two intervention groups and control | Agility intervention group: Musculoskeletal complaints (n = 3), shortness of breath (n = 4) | 34 | Musculoskeletal complaint (n = 1) | 32 |
| Nitz 2004 | Intervention and control | 0 | 24 | 0 | 21 |
| Reinsch 1992 | Intervention and control | Pain, bruise, minor injury | 129 | Pain, bruise, minor injury | 101 |
| Sakamoto 2013 | Intervention only | Knee pain (n = 2), lower limb pain (n = 1), palpitations (n = 1) | 410 | ‐ | 455 |
| Sales 2017 | Intervention only | Falls during exercise session, no injury (n = 2) | 27 | ‐ | 21 |
| Siegrist 2016 | Intervention and control | 0 | 222 | 0 | 156 |
| Skelton 2005 | Intervention and control | 0 | 50 | 0 | 31 |
| Trombetti 2011 | Intervention and control | 0 | 66 | 0 | 68 |
| Strength/resistance (including power) | |||||
| Latham 2003f | Intervention and control | Back and knee pain directly attributable to the exercise programme (n = 18) | 112 | n = 5 (no further details) | 110 |
| Liu‐Ambrose 2004 Resistance groupc | Two intervention groups and control | Resistance intervention group: Musculoskeletal complaints (n = 10) | 32 | Musculoskeltal complaint (n = 1)4 | 32 |
|
Vogler 2009 Seated groupf |
Two intervention groups and control | Musculoskeletal symptoms in all groups: lower back, hip, knee pain | All groups n = 171 | ||
| 3D (Tai Chi) | |||||
| Li 2005 | Intervention and control | 0 | 95 | 0 | 93 |
| Wolf 2003 | Intervention and control | 0 | 145 | 0 | 141 |
| 3D (Dance) | |||||
| Merom 2016 | Intervention only | 0 | 275 | ‐ | 247 |
| Multiple primary exercise categories | |||||
| Arkkukangas 2015 | Intervention only | 0 | 27 | ‐ | 13 |
| Beyer 2007e | Intervention only | Mild and transient pain symptoms: knee (n = 3), hip (n = 1), thigh/gluteal/groin/hamstrings (n = 3), back (n = 2), ankle (n = 1) | 24 | ‐ | 29 |
| Carter 2002 | Intervention and control | 0 | 40 | Grade 1 quadriceps strain (n = 1) |
40 |
| Clemson 2012 structuredc,e | Intervention only | Groin strain and inguinal hernia surgery (n = 1) | 107 | ‐ | 106 |
| Haines 2009e,f | Intervention only | Muscle soreness (n = 1) | 19 | ‐ | 34 |
| Hauer 2001e | Intervention and control | 0 | 31 | 0 | 25 |
| Korpelainen 2006 | Intervention only | Musculoskeletal problems (n = 3) | 84 | ‐ | 76 |
| Means 2005e | Intervention only | 0 | 144 | ‐ | 94 |
| Ng 2015e | Intervention and control | Joint pain, hip and knee (n = 2); relieved after adjusting training regimen | 46 | 0 | 46 |
| Rubenstein 2000 | Intervention and control | 0 | 28 | 0 | 31 |
| Sherrington 2014e,f | Intervention only | Finger pain following grip strength assessment; thigh pain after assessment, low back pain, calf pain, knee pain, exacerbation of hernia symptoms, pre‐existing conditions (mainly musculoskeletal) limited progression of exercises (n = 12) | 169 | ‐ | 171 |
| Uusi‐Rasi 2015 | Intervention and control | Mild musculoskeletal overuse symptoms, pre‐existing osteoarthritic symptoms (n = 25) | 91 | Mild musculoskeletal overuse symptoms (n = 1) | 95 |
| Exercise versus exercise only | Group in which adverse events were reported | Adverse events reported in intervention group | Adverse events reported in intervention group | ||
| Adverse events reported | Number in intervention group | Adverse events reported | Number in intervention group | ||
| Ballard 2004 | One intervention group | 15 weeks ex group: Hip pain (n = 1) | 20 | 19 | |
| Barker 2016 | One intervention group | Pilates group: Hip pain (n = 1) | 20 | 24 | |
| Davis 2011 | Two intervention groups and control | 1x/week group: Musculoskeletal complaints (n = 14) | 52; | 2 a week group: Musculoskeletal complaints (n = 5) Balance and tone group: Musculoskeletal complaints (n = 4) |
2 a week group = 54; Balance and tone group = 49 |
| Kemmler 2010 | Two intervention groups | 0 | 112 | 0 | 115 |
| Kwok 2016 | Two intervention groups | 0 | 40 | 0 | 40 |
| Mirelman 2016 | Two intervention groups | Treadmill group: Death from natural causes (n = 1), myocardial infarctions (n = 2), exacerbated orthopaedic‐related pain or arthritis (n = 3), rhabdomyolysis (n = 4). (All deemed not to be caused by the interventions) | 136 | Virtual reality group: Stroke (n = 1), exacerbated orthopaedic‐related pain or arthritis (n = 4), herpes zoster (n = 1) (All deemed not to be caused by the interventions) | 146 |
| Shigematsu 2008 | Two intervention groups | 0 | 32 | 0 | 36 |
| Yamada 2010 | Two intervention groups | Muscle ache and fatigue | 29 | Muscle ache and fatigue | 29 |
| Yamada 2012 | Two intervention groups | Muscle ache and fatigue | 73 | Muscle ache and fatigue | 72 |
| Yamada 2013 | Two intervention groups | Muscle ache and fatigue | 112 | Muscle ache and fatigue | 118 |
aCategorised by primary exercise category. bAt time point used in falls analysis (if available). cStudy with two intervention groups plus a control group; intervention groups reported across multiple rows. dIncluded events classified as adverse reactions and possible adverse reactions. eIndicates the primary interventions include gait, balance, and functional training plus strength/resistance training. fParticipants recently discharged from hospital.
Appendix 14. Adherence
| Study IDa | Adherence was measured | Adherence data were reported | Measurement of adherence | Reported adherence resultsb |
| Gait, balance, and functional training | ||||
| Almeida 2013 | No | No | ‐ | ‐ |
| Arantes 2015 | Yes | Yes | Adherence to exercise programme | Mean (range) number of sessions attended: exercise group = 22.1 (20 to 24), control group = 10.8 (10 to 12) |
| Barnett 2003 | Yes | Yes | Attendance | 33.7% of participants attended > 30/37 classes |
| Boongrid 2017 | Yes | Yes | Repetitions, sets, duration | 56.8% exercised ≥ 120 minutes a week at 12 months |
| Campbell 1997 | Yes | No | ‐ | ‐ |
| Clegg 2014 | Yes | Yes | Attendance | Mean attendance = 46% |
| Clemson 2010 | No | No | ‐ | ‐ |
| Clemson 2012 LiFE | Yes | Yes | Adherence to exercise programme | 76% still exercising at 6 months |
| Cornillon 2002 | No | No | ‐ | ‐ |
| Dadgari 2016 | No | No | ‐ | ‐ |
| Dangour 2011 | Yes | Yes | Attendance | Adherence: 38% |
| Day 2002 | Yes | Yes | Attendance | Mean (SD) number of sessions attended = 10 (3.8) of 15 sessions |
| Duque 2013 | Yes | Yes | Adherence to exercise programme | Adherence: 97% |
| El‐Khoury 2015 | Yes | Yes | Started exercise programme | Started the programme = 84%. Attended > 1 month = 73% |
| Gschwind 2015 | Yes | Yes | Adherence to exercise programme | Median (IQR): number of times iStoppFalls system used = 42 (57); duration = 11.7 hours (22); number of times balance exergames were performed = 24 (30); duration = 4.0 hours (6.9); number of strength exercises performed = 20 (31); duration = 7.9 hours (13.4) |
| Halvarsson 2013 | Yes | Yes | Attendance | Mean (range) adherence to the training sessions, intervention group: 87% (71% to 100%) |
| Halvarsson 2016 balance | Yes | Yes | Attendance | Mean (range) attendance, intervention group: 89% (66% to 100%) |
| Hamrick 2017 | Yes | Yes | Attendance | Mean attendance at yoga classes: 92% |
| Hirase 2015 | Yes | Yes | Attendance | Proportion of classes attended, foam rubber: 95.5%; stable surface: 93.3%; control: 91.2% |
| Iliffe 2015 | Yes | Yes | Attendance | Proportion attended ≥ 75% classes, group ex + OEP group: 40%. Attained ≥ 75% home exercise prescription of 90 minutes/week, OEP: 37% |
| Iwamoto 2009 | Yes | Yes | Attendance | Attendance at 3‐week programme: 100% |
| Karinkanta 2007 balance | Yes | Yes | Attendance | Mean attendance: 59% |
| Kerse 2010 | Yes | Yes | Adherence to exercise programme | Intervention group: exercised ≥ 2 a week = 55% of participants; walked ≥ 2 a week = 59%; exercised ≥ 3 a week = 25%; walked ≥ 3 a week = 37%; programme almost daily = 20%. Control group: completed all visits = 86% of participants |
| Kovacs 2013 | Yes | Yes | Adherence to exercise programme | Mean (range) attendance (/50 sessions): 80.6% (56% to 100%) |
| Lin 2007 | No | No | ‐ | ‐ |
| Liu‐Ambrose 2008 | Yes | Yes | Adherence to exercise programme | Intervention group. Completed programme ≥ 1 a week = 68% of participants; ≥ 2 a week = 57% of participants; ≥ 3 a week = 25% of participants |
| Liu‐Ambrose 2004 agility group | Yes | Yes | Attendance | Attendance, agility training group: 87.3%; stretching group: 78.8% |
| Lord 1995 | Yes | Yes | Attendance | Attendance at ≥ 60% classes: 75%. For these attendees, mean (range) number of classes attended: 60 (26 ‐ 82) |
| Lord 2003 | Yes | Yes | Attendance | Mean proportion of sessions attended: 42.3% |
| Luukinen 2007 | No | No | ‐ | ‐ |
| Madureira 2007 | Yes | Yes | Adherence to exercise programme | Proportion of participants who attended 100% of sessions: 60%. Proportion of participants who did home exercise ≥ 1 a week: 76.7%; ≥ 1 to 4 a week: 36.7%; every day: 40% |
| McMurdo 1997 | Yes | Yes | Attendance | Mean (range) proportion of sessions attended: 76% (46% to 100%) |
| Miko 2017 | No | No | ‐ | ‐ |
| Morgan 2004 | Yes | Yes | Attendance | Mean proportion of the 24 scheduled sessions attended: 70%. Participants who dropped out of the study completed an average of 31.7% of the exercise sessions compared with 82.9% completed session by those who did not drop out |
| Nitz 2004 | No | No | ‐ | ‐ |
| Reinsch 1992 | No | No | ‐ | ‐ |
| Robertson 2001a | Yes | Yes | Adherence to exercise programme | Performed exercises ≥ 2x/week = 72% of participants; ≥ 3x/week = 43% of participants. Walked ≥ 2x/week = 71% of participants |
| Sakamoto 2013 | Yes | No | Adherence to exercise programme | No data |
| Sales 2017 | Yes | Yes | Attendance | Mean adherence: 79.6% |
| Siegrist 2016 | Yes | Yes | Attendance | Proportion of participants who attended > 10 sessions: 82%. Proportion of participants who performed home exercise programme ≥ 10x: 46% |
| Smulders 2010 | Yes | Yes | Attendance | Proportion of sessions completed: 92.8%. Proportion of participants who completed 100% of sessions: 53.2% |
| Trombetti 2011 | Yes | Yes | Attendance | Mean attendance at exercise programme: 78% |
| Weerdesteyn 2006 | Yes | Yes | Attendance | Mean attendance at exercise sessions: 87% |
| Wolf 1996 balance | No | No | ‐ | ‐ |
| Yang 2012 | Yes | Yes | Adherence to exercise programme | Proportion of intervention participants with full adherence: 44.1%; exercised < 2x/week: 13.6% |
| Strength/resistance (including power) | ||||
| Ansai 2015 resistance | Yes | Yes | Adherence to exercise programme | 56.5% performed ≥ 24 sessions for 16 weeks (50% intervention) |
| Fiatarone 1997 | No | No | ‐ | ‐ |
| Grahn Kronhed 2009 | Yes | Yes | Attendance | Mean attendance at scheduled sessions, exercise group: 24/30 sessions (median (range) 25 (13 ‐ 30) |
| Karinkanta 2007 resistance | Yes | Yes | Attendance | Mean attendance: 74% |
| Latham 2003 | Yes | Yes | Attendance, exercise intensity | Mean adherence: 82% of prescribed sessions. Mean (SD) exercise intensity at the end of training: 51% (13%) of 1 RM; 25% of participants reached the high intensity desired by the intervention |
| Liu‐Ambrose 2004 resistance | Yes | Yes | Attendance | Attendance, agility training group: 87.3%; stretching group: 78.8% |
| Vogler 2009 seated group | Yes | Yes | Attendance | Proportion of sessions completed: 70% |
| Woo 2007 resistance | No | No | ‐ | ‐ |
| 3D | ||||
| Day 2015 | Yes | Yes | Attendance, hours | Mean (SD) class attendance (/96 classes offered): intervention 34.4 (SD 26.9); control 41.3 (26.1). Mean intervention dose = 25.8 hours |
| Huang 2010 | No | No | ‐ | ‐ |
| Li 2005 | Yes | Yes | Attendance | ‐ |
| Logghe 2009 | Yes | Yes | Attendance | Attendance at ≥ 80% of lessons: 47% |
| Merom 2016 | Yes | Yes | Attendance | Median (IQR) attendance to sessions was 56% (IQR 26% to 77%) (approximately 45 sessions) |
| Voukelatos 2007 | No | No | ‐ | ‐ |
| Wolf 2003 | Yes | Yes | Attendance | Mean (SD) attendance in the Tai Chi group: 76% (19); control group 81% (17) |
| Wolf 1996Tai Chi | No | No | ‐ | ‐ |
| Woo 2007 Tai Chi | No | No | ‐ | ‐ |
| Wu 2010 Com‐ex | No | No | ‐ | ‐ |
| Wu 2010 Home‐ex | No | No | ‐ | ‐ |
| Wu 2010 Tel‐ex | Yes | Yes | Attendance | Mean (SD) attendance in Tel‐ex group: 69% (27); Comm‐ex: 71% (27); Home‐ex: 38% (46). Mean (SD) total exercise time (hours): Tel‐ex: 30 (12); Comm‐ex: 31 (12); Home‐ex 17 (21) |
| General physical activity | ||||
| Ebrahim 1997 | No | No | ‐ | ‐ |
| Resnick 2002 | Yes | Yes | Adherence to exercise programme | 7/10 intervention participants adhered to the recommended walking programme (20 minutes 3 a week). 2/10 engaged in a regular walking programme but did not meet the recommended dose. 1 did not engage in any exercise. None of the 7 control group participants started an exercise programme during the course of the study |
| Voukelatos 2015 | No | No | ‐ | ‐ |
| Exercise versus exercise | ||||
| Ballard 2004 | No | No | ‐ | ‐ |
| Barker 2016 | Yes | Yes | Adherence to exercise programme | Proportion attended over 75% of the classes: 95% |
| Davis 2011 | No | No | ‐ | ‐ |
| Helbostad 2004 | No | No | ‐ | ‐ |
| Hwang 2016 | Yes | Yes | Attendance | Proportion attended >20 sessions: Tai Chi group 78%; lower limb group 72% |
| Kemmler 2010 | No | No | ‐ | ‐ |
| Kwok 2016 | No | ‐ | ‐ | ‐ |
| Kyrdalen 2014 | Yes | Yes | Attendance | Mean(SD) attendance, OEP group: 21.9 (SD 2.7) out of the possible 24 exercise sessions; OEP home: 32.8 (2.8) out of the recommended 36 exercise sessions |
| LaStayo 2017 | Yes | Yes | Attendance | In both groups, all participants completed ≥ requisite minimum 18/36 exercise classes and > 90% of participants who > 28/36 exercise classes |
| Liston 2014 | No | No | ‐ | ‐ |
| Lurie 2013 | No | No | ‐ | ‐ |
| Mirelman 2016 | No | Yes | ‐ | ‐ |
| Morone 2016 | No | No | ‐ | ‐ |
| Morrison 2018 | Yes | Yes | Adherence to exercise programme | Proportion who completed the training or all sessions in Wii group: < 50% |
| Okubo 2016 | Yes | Yes | Repetitions, sets, duration | Mean (SD) exercise, balance group: 1.4 (0.5) sets/day, for 4.6 (2.0) days/week; walking group: 45.2 (24.5) min/day of walking for 4.3 (1.7) days week |
| Shigematsu 2008 | No | No | ‐ | ‐ |
| Skelton 2005 | Yes | Yes | Started exercise programme | Proportion of intervention participants who completed > 1 intervention session: 73% |
| Steadman 2003 | No | No | ‐ | ‐ |
| Taylor 2012 | Yes | Yes | Attendance | Median (IQR) attendance at exercise programme: 79% (49% to 90%) |
| Verrusio 2017 | No | No | ‐ | ‐ |
| Yamada 2010 | Yes | Yes | Attendance | Median (IQR) adherence: 100% (74% to 100%) for each group |
| Yamada 2012 | Yes | Yes | Attendance | Median (IQR) adherence in complex course group: 96% (88% to 100%); simple course group: 96% (88% to 100%) |
| Yamada 2013 | Yes | Yes | Attendance | Median (IQR) adherence, multitarget stepping programme: 93% (83% to 96%); walking programme: 92% (83% to 96%) |
| Multiple primary exercise categories | ||||
| Ansai 2015 multicomponent* | Yes | Yes | Adherence to exercise programme | 34.7% performed ≥ 24 sessions for 16 weeks (50% intervention) |
| Arkkukangas 2015 | Yes | Yes | Adherence to exercise programme | Adherent = 73, not adherent = 27. Definition of adherence unclear |
| Beyer 2007c | Yes | Yes | Attendance | Training compliance was on average 79% (42 ‐ 100%) |
| Brown 2002c | Yes | Yes | Attendance | Mean attendance 84.6% (22/26 sessions), range 62% to 100% |
| Buchner 1997 | No | No | ‐ | ‐ |
| Bunout 2005c | Yes | Yes | Attendance | 58% attended > 50% of sessions |
| Carter 2002 | Yes | Yes | Attendance | Attendance: 89% |
| Cerny 1998c | No | No | ‐ | ‐ |
| Clemson 2012 structuredc | Yes | Yes | Adherence to exercise programme | 71% still exercising at 6 months |
| Freiberger 2007c | Yes | Yes | Attendance | Proportion of intervention participants participating in > 75% of sessions: 77% |
| Gill 2016c | Yes | Yes | Attendance | Mean attendance at scheduled sessions, physical activity group: 68%; median (IQR) 71% (50% to 83%) |
| Haines 2009c | Yes | Yes | Adherence to exercise programme | Number of intervention participants who adhered to exercise in week 8: ≥ 1 a week = 8/19; ≥ 2 a week = 4/19 |
| Hauer 2001c | Yes | Yes | Adherence to exercise programme | Mean adherence, intervention group: 85.4%; control group: 84.2% |
| Irez 2011c | Yes | Yes | Attendance | Proportion of sessions completed: 92% |
| Kamide 2009* | Yes | Yes | Adherence to exercise programme | Intervention participants. Completed intervention > 3 a week, 19/23 (82.6%) participants; completed intervention > 2 a wk, 21/23 (91.3%) participants |
| Karinkanta 2007 resistance and balance groupsc | Yes | Yes | Attendance | Mean attendance: 67% |
| Kim 2014c | Yes | Yes | Attendance; exercise sessions at home | Intervention group. Mean (range) attendance at sessions: 75.3% (64% ‐ 86%); mean frequency of home exercise programme: 3.4 a week; mean exercise time: 24.9 minutes |
| Korpelainen 2006 | Yes | Yes | Attendance | Intervention group. Mean attendance at sessions: 75%; mean frequency of home exercise programme: 3 a week |
| Lehtola 2000 | Yes | Yes | Adherence to exercise programme | "Active participants": 52 participants; "Passive participants": 20 |
| Means 2005c | No | No | ‐ | ‐ |
| Ng 2015c | Yes | Yes | Attendance | Mean attendance: physical training 85%, control 94% |
| Park 2008 | No | No | ‐ | ‐ |
| Rubenstein 2000 | Yes | Yes | Attendance | Exercise participants attended 84% of the exercise sessions |
| Sherrington 2014c | Yes | Yes | Reps, sets, duration | Proportion of prescribed repetitions completed in 12th month: 47% |
| Suzuki 2004c | Yes | Yes | Attendance | Mean attendance at exercise classes: 75.3% |
| Uusi‐Rasi 2015c | Yes | Yes | Attendance | Mean (range) attendance at group training: 72.8% (0% to 97.4%); home training sessions: 66.1% (0% to 100%) |
| Vogler 2009 Weight‐bearing group | Yes | Yes | Attendance | Proportion of sessions completed: 62% |
aCategorised by primary exercise category. bAt time point used in falls analysis (if available). cIndicates the primary interventions include gait, balance, plus functional training and strength/resistance training.
Appendix 15. Description of excluded studies: reference links
| Reason for exclusion | Links to references |
| Types of participants | |
| Not meeting age criteria | N = 1: Pereira 1998 |
| In a single diagnostic group with increased risk of falls | N = 1: Hsu 2017 |
| Not predominantly community‐dwelling | N = 1: DeSure 2013 |
| Types of intervention | |
| Not exercise as a single intervention | N = 15: Alkan 2011; Beling 2009; Clemson 2004b; Fahlström 2017; Gianoudis 2014; Iwamoto 2012; Lee 2013; Leung 2014; Li 2018a; Olsen 2014; Pai 2014; Rossi‐Izquierdo 2017; Steinberg 2000; Swanenburg 2007; Ueda 2017 |
| Type of control | |
| Control did not meet inclusion criteria | N = 1: Ohtake 2013 |
| Type of outcome | |
| Falls not measured | N = 1: Hinrichs 2016 |
| Participants with injurious falls excluded | N = 1: Morris 2008 |
Appendix 16. Raw data for quality of life outcome where available
| Study ID | Outcome measure | Outcome format | Intervention group quality of life | Intervention group number in analysis | Control group quality of life | Control group number in analysis | Data included in analysis |
| Boongrid 2017 | Thai EQ‐5D | Mean (SD) Baseline 6 month |
7.37 (?) 7.7 (?) |
219 | 7.35 (?) 7.4 (?) |
220 | None |
| Carter 2002 | Osteoporosis‐specific health‐related quality of life | Mean (95% CI) change 5 month‐ baseline (adjusted) | ‐0.31 (−2.98 to 2.37) | 40 | −0.48 (−3.00 to 2.37) | 40 | None |
| Clegg 2014 | EQ‐5D | Mean (SD) Baseline 3 month |
0.53 (0.30) 0.51 (0.34) |
40 | 0.52 (0.25) 0.46 (0.26) |
30 | EQ‐5D |
| Clemson 2010 | SF‐36 ‐ physical SF‐36 ‐ mental |
Median (IQR) change 0 to 6 months | 0.6 (−5.0 to 10.1) −1.1 (−8.4 to 0) |
17 | 2.3 (−5/3 to 6.3) −2.9 (−10.9 to 5.7) |
14 | None |
| Clemson 2012 (LIFE) | EQ‐5D | Mean (SD) Baseline 6 month 12 month |
7.1 (1.4) 6.6 (1.3) 6.7 (1.5) |
99 | 7.0 (1.4) 7.2 (1.6) 6.7 (1.3) |
91 | EQ‐5D 12 months |
| Clemson 2012 (Structured) | EQ‐5D | Mean (SD) Baseline 6 month 12 month |
6.9 (1.5) 6.9 (1.5) 6.7 (1.6) |
96 | 7.0 (1.4) 7.2 (1.6) 6.7 (1.3) |
91 | EQ‐5D 12 months |
| Dangour 2011 | SF‐36 ‐ physical SF‐36 ‐ mental |
Mean (SD) Baseline 24 month Baseline 24 month |
51.2 (6.7) 51.1 (14.3) 49.3 (9.1) 49.2 (6.3) |
325 | 49.8 (6.3) 50.6 (8.9) 49.4 (7.9) 48.3 (6.3) |
294 | SF‐36 physical 24 months |
| Grahn Kronhed 2009 | SF‐36 ‐ physical SF‐36 ‐ mental QUALEFFO‐41 |
Mean (SD) Baseline 12 month Baseline 12 month mean (SD) change |
44.8 (9.3) 46.9 (8.8) 49.2 (9.7) 53.0 (8.0) ‐0.7 (5.0) |
31 | 36.7 (10.8) 35.7 (9.4) 48.9 (10.3) 47.6 (11.0) −0.2 (5.5) |
34 | SF‐36 physical 12 months |
| Gschwind 2015 | EQ‐ 5D utility score EQ‐5D VAS |
Mean (SD) Baseline 6 month Baseline 6 month |
0.86 (0.11) 0.86 (0.15) 79.2 (14.7) 80.9 (13.7) |
71 | 0.86 (0.13) 0.87 (0.13) 81.7 (12.7) 79.9 (14.6) |
65 | EQ‐ 5D utility score 6 months |
| Haines 2009 | EQ‐5D utility score EQ‐5D VAS |
Mean (SD) Baseline 6 month Baseline 6 month |
0.58 (0.32) 0.48 (0.35) 66.7 (14.3) 58.9 (21.4) |
19 | 0.65 (0.25) 0.52 (0.36) 67.5 (18.9) 58.1 (25.0) |
31 | EQ‐5D utility 6 months |
| Iliffe 2015 FAME | EQ‐5D SF‐12 physical SF‐12 mental OPQOL |
Mean (SD) Baseline 12 month Baseline 12 month Baseline 12 month Baseline 12 month |
0.67 (0.09) 0.67 (0.07) 38.7 (5.6) 38.9 (4.9) 49.6 (6.0) 48.7 (5.8) 129.4 (13.5) 132.3 (16.0) |
179 | 0.68 (0.08) 0.68 (0.07) 38.7 (5.5) 39.1 (5.0) 49.9 (6.1) 49.2 (5.6) 130.8 (13.5) 134.8 (14.8) |
212 | EQ‐5D 12 months |
| Iliffe 2015 OEP | EQ‐5D SF‐12 physical SF‐12 mental OPQOL |
Mean (SD) Baseline 12 month Baseline 12 month Baseline 12 month Baseline 12 month |
0.68 (0.09) 0.68 (0.07) 38.8 (5.6) 39.3(4.7) 50.2 (5.9) 49.05 (5.1) 129.4 (12.7) 133.7 (15.0) |
176 | 0.68 (0.08) 0.68 (0.07) 38.7 (5.5) 39.1 (5.0) 49.9 (6.1) 49.2 (5.6) 130.8 (13.5) 134.8 (14.8) |
212 | EQ‐5D 12 months |
| Kerse 2010 | SF‐36 physical SF‐36 mental |
Mean (SD) Baseline 6 month 12 month Baseline 6 month 12 month |
39.0 (1.2) 39.5 (1.2) 38.3 (1.2) 51.2 (0.9) 54.7 (0.7) 55.4 (0.7) |
94 | 39.3 (1.1) 37.9 (1.3) 39.4 (1.2) 48.7 (1.0) 53.7 (0.9) 52.7 (0.0) |
87 | SF‐36 physical 12 months |
|
Kyrdalen 2014 (group versus home OEP) |
SF‐36 physical SF‐36 mental |
Mean (95%CI) Baseline 3 month 6 month Baseline 3 month 6 month |
178.2 (158.6 to 197.7) 232.9 (211.0 to 254.8) 218.0 (194.5 to 241.1) 237.3 (217.2 to 257.3) 286.4 (263.6 to 309.2) 269.1 (244.4 to 293.9) |
47 | 192.3 (172.4 to 212.2) 202.1 (179.6 to 224.6) 212.2 (188.4 to 234.1) 254.3 (233.9 to 274.7) 276.0 (252.4 to 299.5) 289.2 (265.2 to 313.2) |
47 | SF‐36 physical 6 months |
| Latham 2003 | SF‐36 physical | Mean (95%CI) 3 month 6 month |
34 (32 to 36) 35 (33 to 37) |
112 | 35 (33 to 37) 37 (35 to 39) |
110 | SF‐36 physical 6 months |
| Lin 2007 | WHOQOL‐BREF Physical Psychological Social Environmental |
Mean (SD) Baseline 6 month 8 month Baseline 6 month 8 month Baseline 6 month 8 month Baseline 6 month 8 month |
51.0 (17.9) 59.0 (12.5) 62.8 (9.9) 55.2 (13.6) 62.9 (13.2) 64.4 (12.6) 69.9 (11.4) 71.9 (10.0) 75.4 (9.4) 64.1 (12.5) 70.2 (9.4) 74.9 (6.8) |
39 | 48.9 (17.3) 52.6 (13.8) 55.5 (15.3) 55.7 (16.0) 53.8 (17.0) 56.3 (17.6) 68.8 (10.6) 63.8 (14.8) 66.3 (13.3) 62.5 (9.8) 62.1 (14.4) 65.1 (14.3) |
40 | WHOQOL‐BREF physical 8 months |
| Merom 2016 | SF‐12 Physical Mental |
Mean (SD) Baseline 12 month Baseline 12 month |
43.2 (8.6) 41.8 (10.3) 53.0 (8.1) 52.7 (8.7) |
274 | 44.6 (8.7) 42.6 (9.9) 51.9 (7.4) 51.8 (8.2) |
247 | SF‐12 Physical 12 months |
| Resnick 2002 | SF‐12 Physical Mental |
Mean (SD) Baseline 2 month 6 month Baseline 2 month 6 month |
31.1 (5.8) 33.7 (4.7) 33.4 (4.8) 48.3 (3.0) 48.4 (2.6) 47.0 (5.2) |
10 | 32.7 (6.7) 32.2 (7.3) 31.2 (4.9) 46.9 (3.0) 47.1 (3.4) 46.8 (3.2) |
7 | SF‐12 Physical 6 months |
| Rubenstein 2000 | SF‐36 Physical functioning Physcial role limits Health perceptions Health question |
Mean (SD) Baseline 3 month Baseline 3 month Baseline 3 month Baseline 3 month |
59.6 (24.8) 65.0 (17.4) 66.9 (36.7) 75.0 (34.0) 60.0 (19.1) 64.3 (18.2) 51.8 (26.3) 67.9 (21.4) |
28 | 62.2 (21.0) 60.6 (20.3) 53.7 (38.4) 57.4 (35.2) 58.9 (19.5) 61.1 (19.9) 50.9 (20.2) 46.3 (22.7) |
27 | SF‐36 Physical functioning 3 months |
| Sales 2017 | SF‐12 Physical Mental |
Mean (SD) Baseline 12 month Baseline 12 month |
46.9 (7.6) 49.6 (8.3) 53.1 (9.8) 54.5 (7.0) |
27 | 49.1 (7.9) 48.9 (7.6) 51.4 (6.1) 51.6 (7.9) |
21 | SF‐12 Physical, 12 months |
| Sherrington 2014 | EQ‐5D utility SF‐12 Physical Mental |
Mean (SD) Baseline 12 month Baseline 12 month Baseline 12 month |
0.63 (0.23) 0.66 (0.27) 37.44 (8.9) 40.37 (8.29) 54.71 (6.5) 55.87 (5.02) |
157 | 0.62 (0.23) 0.60 (0.33) 38.17 (8.36) 39.27 (9.26) 54.70 (6.79) 55.19 (7.09) |
155 | EQ‐5D utility 12 months |
| Smulders 2010 | QUALEFFO‐41 | Mean (SD) Baseline 6 weeks 12 month |
25.2 (10.0) 25.4 (10.9) 26.2 (10.6) |
47 | 28.7 (10.9) 26.3 (10.6) 27.3 (11.0) |
45 | QUALEFFO‐41 12 months |
|
Steadman 2003 (balance vs physio) |
Euroqol VAS | Mean (SD) Baseline 6 weeks 3 month 6 month |
57.8 (19.7) 65.1 (19.6) 65.1 (17.7) 64.4 (19.9) |
69 | 59.4 (17.2) 64.9 (17.3) 65.7 (16.9) 64.5 (17.4) |
64 | Euroqol VAS 6 months |
|
Verrusio 2017 (HBP v physio) |
SF‐36 Physical Mental |
Mean (SD) Baseline 3 month Baseline 3 month |
52.1 (6.0) 52.2 (5.4) |
73 | 52.7 (7.1) 53.1 (5.3) |
74 | None (too hard to read follow‐up data from figure) |
| Voukelatos 2015 | Australian QoVL | Mean (95% CI) Baseline 12 month |
0.81 (0.79 to 0.83) 0.84 (0.82 to 0.86) |
144 | 0.81 (0.79 to 0.83) 0.83 (0.81 to 0.85) |
169 | AQoL 12 months |
|
Wu 2010 Telecommunication‐based Tai Chi vs group Tai Chi |
SF‐36 Physical Mental |
Mean change (SD) | 7.3 (16,3) 2.9 (18.1) |
22 | 9.0 (15.8) 6.2 (11.9) |
20 | None |
|
Wu 2010 home‐based Tai Chi vs group Tai Chi |
SF‐36 Physical Mental |
Mean change (SD) | 6.7 (14.7) ‐0.2 (8.0) |
22 | 9.0 (15.8) 6.2 (11.9) |
20 | None |
| Yang 2012 | Assessment of quality of life | Mean (SD) Baseline 6 months |
24.8 (4.8) 23.4 (4.1) |
59 | 25.0 (4.5) 24.6 (5.2) |
62 | QoL, 6 months |
Appendix 17. Studies reporting cost‐effectiveness, cost‐utility, or costs (intervention and/or healthcare resource use) related to fall outcomes
| Study ID (source if not primary reference), sample, efficacy analyses, type of evaluation | Intervention(s) and comparator (N in analysis) | Perspective(s), type of currency, price year, time horizon | Cost items measured | Mean (SD) intervention cost per person | Healthcare service costs | Incremental cost per fall prevented/per QALY gained |
| •Buchner 1997 •Patients from a HMO, mild deficits in strength and balance, mean age 75 years •Analysis •Cost analysis |
•Centre‐based endurance training or strength training, or both, supervised for 24 to 26 weeks then self‐supervised (N = 75) vs no intervention (N = 30) |
•HMO •US dollar •Not specified (presumed 1992) •Period 7 to 18 months after randomisation |
•Hospital costs, ancillary outpatient costs (from HMO computerised records) |
‐ | •Hospitalised control participants more likely to have hospital costs > USD 5000 (P < 0.05) •Ancillary outpatient costs 7 ‐ 18 months after randomisation: Exercise: USD 270 Control: USD 285 (no significant difference) |
‐ |
| •Campbell 1997 and Campbell 1999 (Robertson 2001b) •Women aged ≥ 80 years from 17 general practices, mean age (SD) 84.1 (3.3) years •Analysis •Cost‐effectiveness analysis |
•Specific set of muscle strengthening and balance retraining exercises individually prescribed at home (OEP) by physiotherapist, 4 home visits and monthly phone calls in year 1, phone contact only in year 2 (N = 116) vs social visits and usual care (N = 117) |
•Societal •New Zealand dollar •1995 •During participation in trial (up to 2 years) |
•Intervention costs (recruitment, programme delivery, overheads) •Healthcare costs resulting from falls (actual costs of hospital admissions and outpatient services, estimates of GP visits and other costs) •Total healthcare resource use (actual costs of hospital admissions and outpatient services) |
In research setting: •NZD 173 (0) in year 1 •NZD 22 (0) in year 2 |
•No difference between the 2 groups for healthcare costs resulting from falls or for total healthcare costs •27% of hospital admission costs during trial resulted from falls |
At 1 year: •NZD 314 per fall prevented (programme implementation costs only) At 2 years: •NZD 265 per fall prevented (programme implementation costs only) |
| •Dangour 2011 (Walker 2009) •People aged 65 to 67.9 years living in low‐middle socioeconomic status municipalities in Santiago, Chile •Analysis •Cost analysis |
•Multicomponent exercise classes, 2 x 1‐hour supervised classes a week for 24 months (10 health centres, N = 854) vs remainder (10 health centres, N = 811) |
•Societal and health system •Chilean peso converted to US dollar •2007 •During 2‐year trial |
From 93 exit interviews: •Physical activity intervention |
•USD 164 for physical activity intervention |
‐ | •Not calculated (neither intervention reduced risk of falling; cost‐effectiveness of physical activity intervention reported as USD 4.84 per extra metre walked) |
| •Davis 2011 (Liu‐Ambrose 2010) •Community‐living women aged 65 to 75 years •Analysis •Cost‐effectiveness analysis, cost‐utility analysis |
•Once weekly resistance training (N = 54) vs twice‐weekly balance and tone classes (N = 49) •Twice‐weekly resistance training (N = 51) vs twice‐weekly balance and tone classes (N = 49) |
•Health service •Canadian dollar •2008 •9 months |
•Costs of delivering the interventions (staff time, room use, equipment, building overhead costs); visits to health professionals; all visits, admissions, and procedures in hospital; laboratory and diagnostic tests |
•CAD 353 once‐weekly resistance training •CAD 706 twice‐weekly resistance training •CAD 706 twice‐weekly balance and tone classes |
•Mean healthcare costs resulting from falls, mean total healthcare costs respectively: CAD 547, CAD 1379 once‐weekly resistance training •CAD 184, CAD 1684 twice‐weekly resistance training •CAD 162, CAD 1772 twice‐weekly balance and tone classes |
•Both once‐ and twice‐weekly resistance training dominated balance and tone classes in terms of both falls and QALYs (i.e. less costly, more effective) |
| •Day 2002 (McLean 2015) •Community‐dwelling people identified from the electoral roll, mean age 76.1 years •Analysis •Cost‐effectiveness analysis Cost‐utility analysis |
Exercise group, 1‐hour class a week, 15 weeks, plus daily home exercises designed by physiotherapist (N = 135) vs no intervention (N = 137) | •Healthcare •Australian dollar (costs converted from Australian Ddllar to GBP using 2010 purchasing‐power parity) •2010 •18 months |
•Intervention cost (labour, equipment, venue hire, music and consumables) •Healthcare costs: (General Practitioner, ambulance services, emergency department visits, hospital admissions) |
•AUD 52 | •AUD 33. for exercise group; AUD 39. for control group |
ICER per: •Fall prevented 652 •Injurious fall prevented 1176 •Fracture prevented 26,236 •QALY 51,483 |
| •Iliffe 2014 and Iliffe 2015 •Community‐dwelling people with mean age 73 years •Analysis •Cost‐effectiveness analysis Cost‐utility analysis |
1. home‐based Otago exercise programme (OEP) (N = 410) 30 minutes, 3 a week, 24 weeks vs Control group: no intervention (N = 457) 2. Community centre‐based Falls Management Exercise (FaME) group (N = 387) 1 hour, weekly + home exercises based on OEP 30 minutes, 2 a week for 24 weeks vs Control group: no intervention (N = 457) 3. OEP vs FaME |
•Healthcare •GBP •2011 •52 weeks |
•Cost of delivering the intervention (venue hire, procurement of exercise equipment, instructors, training and reimbursement of instructors and mentors). •Cost of primary care service use (GP, practice nurse, out‐of‐hours, other). |
OEP London = GBP 88, Nottingham = GBP 117 FaME: London = GBP 269; Nottingham = GBP 218 |
OEP GBP 404; FaME GBP 412.; usual care GBP 367 |
Cost‐effectiveness analysis not conducted due to no between‐group difference in QALY |
| •Kemmler 2010 •Women aged ≥ 65 living independently •Analysis 4.1, 4.2 •Cost analysis |
•Multicomponent exercise, 2 60‐minute classes and 2 20‐minute home training sessions weekly for 18 months (N = 115) vs control (low‐intensity exercise classes 60 minutes once‐weekly for 10 weeks followed by 10 weeks of rest) (N = 112) •All participants received calcium (1500 m/d) and cholecalciferol (500 IU/d) supplements |
•Health system •Euro (Germany) •Not specified •During participation in 18‐month trial |
•Total healthcare costs (no details provided) |
‐ | •EUR 2255 (2596) exercise group and EUR 2780 (3318) control group for mean total healthcare costs (P = 0.20) |
‐ |
| • Liu‐Ambrose 2008 (Davis 2009) •Women and men aged ≥ 70 years recruited from 2 referral‐based falls clinics •Analysis •Cost‐effectiveness analysis |
•Specific set of muscle strengthening and balance retraining exercises individually prescribed at home (OEP) by trained physiotherapist for 1 year (N = 36) vs guideline care (N = 38) •All participants received falls risk assessment, comprehensive geriatric assessment and treatment |
•Health system •Canadian dollar •Not specified •12 months |
•Cost of delivering the intervention •Cost of the falls clinic |
•CAD 14,285 | ‐ | •CAD 247 per fall prevented (comparable to incremental cost‐effectiveness ratios in New Zealand studies of the Otago Exercise Program) |
| •Robertson 2001a •Men and women aged ≥ 75 years from 17 general practices, mean (SD) age 80.9 (4.2) years •Analysis •Cost‐effectiveness analysis |
•Specific set of muscle‐strengthening and balance‐retraining exercises individually prescribed at home (OEP) by trained district nurse, supervised by physiotherapist, 5 home visits and monthly phone calls for 1 year (N = 121) vs usual care (N = 119) | •Health system •New Zealand dollar •1998 •During participation in 1‐year trial |
•Intervention costs (training, recruitment, programme delivery, supervision of exercise instructor, overheads) •Hospital admission costs resulting from fall injuries during trial (actual costs of hospital admissions) |
In community health service setting: •NZD 432 (0) for 1 year |
•5 hospital admissions due to fall injuries in control group, none in exercise group (cost savings of NZD 47,818) | •NZD 1803 per fall prevented (programme implementation costs only) ‐ NZD 7471 per injurious fall prevented (programme implementation costs only) •NZD 155 per fall prevented (programme implementation costs and hospital admission cost savings) ‐ NZD 640 per injurious fall prevented (programme implementation costs and hospital admission cost savings) |
| •Sherrington 2014 (Farag 2015a) •Community‐dwelling people aged 60 years and over, discharged from hospital •Analysis •Cost‐effectiveness analysis Cost‐utility analysis |
•Weight‐bearing Exercise for Better Balance (WEBB) programme, 15 – 20 minutes up to 6 times weekly for 12 months (N = 171) vs usual care (N = 169) | •Health and community care funder perspective (Australia) •Australian Dollar •2012 •1 year |
•Costs of delivering the interventions (travel, staff, equipment, phone calls) •Cost of health service use (respite care, residential aged care, hospital admission, emergency department presentation, general practitioner, specialist and nursing services, allied health, social support services) |
AUD 751 for WEBB AUD 0 for usual care |
AUD 12,029 for WEBB AUD 10,327 for usual care |
AUD 77,403 per QALY gained |
| •Uusi‐Rasi 2015 (Patil 2016) •Community‐dwelling women with mean age •74 years • Analysis •Cost‐effectiveness analysis |
•No exercise + placebo •No exercise + vitamin D 800 IU/day •Exercise + placebo: supervised group training classes 2 a week for first year, and 1 a week for second year (N = 91) vs No exercise + placebo (control) (N = 95) •Exercise + vitamin D 800 IU/day |
•Societal •Euros (Finland) •2011 •2 years |
•Intervention costs (salaries, administration costs) •Healthcare costs (fall‐related health care costs for all injurious falls reported during the intervention period) |
Total costs (intervention and healthcare): EUR 30.9 (95) for no exercise + placebo; EUR 206.9 (786) for no exercise + vitamin D 800IU/day; EUR 73.4 (104) for exercise + placebo; EUR 188.0 (454) for exercise + vitamin D 800IU/day |
‐ | ICER all intervention (excluding outliers): EUR 220.7 (220.7) for no exercise + placebo EUR 17,600 (exc) for no exercise + vitamin D 800 IU/day EUR 2670 (708.3) for exercise + placebo EUR 3820 (3820) for exercise + vitamin D 800IU/day |
| •Voukelatos 2007 (Haas 2006) •Healthy community‐living people aged ≥ 60 years, mean (SD) age 69 (6.5) years •Analysis •Cost‐effectiveness analysis |
•Tai Chi classes 1 hour weekly for 16 weeks (N = 347) vs no intervention (N = 337) |
•Public health system (NSW Health) •Australian dollar •Not specified (presumed 2001) •During 24‐week trial period |
•Intervention costs (cost of venues, advertising, instructors) •Health service use related to falls from healthcare use diary and hospital records, valued at standard costs (GP, specialist, tests, hospitalisations, medications) |
•AUD 245 (0) intervention group plus charge AUD 44 per participant |
•Mean total healthcare costs higher for Tai Chi group (AUD 55) than control group (AUD 17) (P < 0.001) |
•AUD 1683 per fall prevented (includes cost offset by charging AUD 44 per instruction course) |
See also Davis 2010 GP: general practitioner; HMO: health maintenance organisation; OEP: Otago Exercise Program; QALY: quality‐adjusted life‐year
Appendix 18. Sensitivity analyses: exploring impact on results (rate of falls outcome)
| Sensitivity analysis | Pooled impact of exercise on fall rate, Rate ratio, 95% CI |
| Primary analysis, all trials, random‐effects meta‐analysis | 0.77, 0.71 to 0.83; participants = 12,981; studies = 59 |
| Sensitivity analysis 1, removing trials that included participants aged < 65 years | 0.77, 0.71 to 0.84; participants = 11,807; studies = 53 |
| Sensitivity analysis 2, removing trials with high risk of bias on any itema | 0.78, 0.71 to 0.87; participants = 6757; studies = 25 |
| Sensitivity analysis 3, removing trials with unclear or high risk of bias on allocation concealment | 0.85, 0.77 to 0.95; participants = 6092; studies = 22 |
| Sensitivity analysis 4, removing trials with unclear or high risk of bias on assessor blinding (falls outcome) | 0.76, 0.69 to 0.85; participants = 6996; studies = 27 |
| Sensitivity analysis 5, removing trials with unclear or high risk of bias on incomplete outcome data | 0.77, 0.69 to 0.85; participants = 7646; studies = 36 |
| Sensitivity analysis 6, removing cluster‐randomised trials | 0.76, CI 0.70 to 0.83; participants = 10,261; studies = 53 |
| Sensitivity analysis 7, all trials, fixed‐effect meta‐analysis | 0.82, 0.79 to 0.86; participants = 12,981; studies = 59 |
| Sensitivity analysis 8, multiple categories of exercise versus control, excluding trials that do not include balance and strength training | 0.69, 0.48 to 0.97; participants = 1084; studies = 8 |
| Primary analysis, subgrouped by exercise type Balance and functional exercises versus control Multiple categories of exercise versus control |
0.76, CI 0.70 to 0.81; participants = 7920; studies = 39 0.66, CI 0.50 to 0.88; participants = 1374; studies = 11 |
| Sensitivity analysis 9a, classification of interventions based on the Otago Exercise Program as multiple categories of exercise Balance and functional exercises versus control Multiple categories of exercise versus control |
0.75, 0.68 to 0.82; participants = 5556; studies = 30 0.72, 0.62 to 0.83; participants = 3738; studies = 20 |
| Sensitivity analysis 9b, classification of interventions that included balance and functional exercises plus strength exercises as multiple categories of exercise Balance and functional exercises versus control Multiple categories of exercise versus control |
0.72, 0.62 to 0.84; participants = 2718; studies = 16 0.74, 0.67 to 0.81; participants = 6721; studies = 35 |
aAfter removing trials assessed as high risk of bias in one or more key domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessors (detection bias), and incomplete outcome data (attrition bias).
Data and analyses
Comparison 1. Exercise versus control (rate of falls).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls ‐ overall analysis | 59 | 12981 | Rate Ratio (Random, 95% CI) | 0.77 [0.71, 0.83] |
| 2 Rate of falls ‐ subgrouped by baseline falls risk | 59 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Not selected for high risk of falling | 29 | 6123 | Rate Ratio (Random, 95% CI) | 0.74 [0.65, 0.84] |
| 2.2 Selected for high risk of falling | 30 | 6858 | Rate Ratio (Random, 95% CI) | 0.80 [0.72, 0.88] |
| 3 Rate of falls ‐ subgrouped by age (threshold 75 years) | 59 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Age < 75 | 46 | 9605 | Rate Ratio (Random, 95% CI) | 0.75 [0.69, 0.82] |
| 3.2 Age 75+ | 13 | 3376 | Rate Ratio (Random, 95% CI) | 0.83 [0.72, 0.97] |
| 4 Rate of falls ‐ subgrouped by personnel | 59 | 12981 | Rate Ratio (Random, 95% CI) | 0.77 [0.71, 0.83] |
| 4.1 Health professional delivering intervention | 25 | 4511 | Rate Ratio (Random, 95% CI) | 0.69 [0.61, 0.79] |
| 4.2 No health professional delivering intervention | 34 | 8470 | Rate Ratio (Random, 95% CI) | 0.82 [0.75, 0.90] |
| 5 Rate of falls ‐ subgrouped by group or individual exercise | 59 | 12981 | Rate Ratio (Random, 95% CI) | 0.77 [0.71, 0.83] |
| 5.1 Group exercise | 40 | 8163 | Rate Ratio (Random, 95% CI) | 0.76 [0.69, 0.85] |
| 5.2 Not group exercise | 21 | 4818 | Rate Ratio (Random, 95% CI) | 0.79 [0.71, 0.88] |
| 6 Rate of falls ‐ subgrouped by exercise type | 59 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 6.1 Balance and functional exercises vs control | 39 | 7920 | Rate Ratio (Random, 95% CI) | 0.76 [0.70, 0.81] |
| 6.2 Resistance exercise vs control | 5 | 327 | Rate Ratio (Random, 95% CI) | 1.14 [0.67, 1.97] |
| 6.3 3D exercise (Tai Chi) vs control | 7 | 2655 | Rate Ratio (Random, 95% CI) | 0.81 [0.67, 0.99] |
| 6.4 3D exercise (dance) vs control | 1 | 522 | Rate Ratio (Random, 95% CI) | 1.34 [0.98, 1.83] |
| 6.5 Walking programme vs control | 2 | 441 | Rate Ratio (Random, 95% CI) | 1.14 [0.66, 1.97] |
| 6.6 Multiple categories of exercise vs control | 11 | 1374 | Rate Ratio (Random, 95% CI) | 0.66 [0.50, 0.88] |
| 7 Rate of falls ‐ long‐term follow‐up by exercise type | 4 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 7.1 Balance and functional exercises vs control | 2 | 858 | Rate Ratio (Random, 95% CI) | 0.82 [0.66, 1.01] |
| 7.2 Walking programme vs control | 1 | 97 | Rate Ratio (Random, 95% CI) | 1.27 [0.89, 1.81] |
| 7.3 Multiple categories of exercise vs control | 1 | 175 | Rate Ratio (Random, 95% CI) | 0.80 [0.55, 1.16] |
Comparison 2. Exercise versus control (number of fallers).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of fallers ‐ overall analysis | 63 | 13518 | Risk Ratio (Random, 95% CI) | 0.85 [0.81, 0.89] |
| 2 Number of fallers ‐ subgrouped by baseline fall risk | 63 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Not selected for high risk of falling | 28 | 6347 | Risk Ratio (Random, 95% CI) | 0.82 [0.73, 0.92] |
| 2.2 Selected for high risk of falling | 35 | 7171 | Risk Ratio (Random, 95% CI) | 0.87 [0.83, 0.91] |
| 3 Number of fallers ‐ subgrouped by age (threshold 75 years) | 63 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Age < 75 | 50 | 10346 | Risk Ratio (Random, 95% CI) | 0.85 [0.79, 0.91] |
| 3.2 Age 75+ | 13 | 3172 | Risk Ratio (Random, 95% CI) | 0.86 [0.80, 0.92] |
| 4 Number of fallers ‐ subgrouped by personnel | 62 | 13473 | Risk Ratio (Random, 95% CI) | 0.85 [0.81, 0.89] |
| 4.1 Health professional delivering intervention | 26 | 3747 | Risk Ratio (Random, 95% CI) | 0.82 [0.74, 0.91] |
| 4.2 No health professional delivering intervention | 36 | 9726 | Risk Ratio (Random, 95% CI) | 0.86 [0.81, 0.92] |
| 5 Number of fallers ‐ subgrouped by group or individual exercise | 63 | 13518 | Risk Ratio (Random, 95% CI) | 0.85 [0.81, 0.89] |
| 5.1 Group exercise | 48 | 9219 | Risk Ratio (Random, 95% CI) | 0.83 [0.78, 0.90] |
| 5.2 Not group exercise | 16 | 4299 | Risk Ratio (Random, 95% CI) | 0.88 [0.83, 0.93] |
| 6 Number of fallers ‐ subgrouped by exercise type | 63 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 6.1 Balance and functional exercises vs control | 37 | 8288 | Risk Ratio (Random, 95% CI) | 0.87 [0.82, 0.91] |
| 6.2 Resistance exercise vs control | 2 | 163 | Risk Ratio (Random, 95% CI) | 0.81 [0.57, 1.15] |
| 6.3 3D exercise (Tai Chi) vs control | 8 | 2677 | Risk Ratio (Random, 95% CI) | 0.80 [0.70, 0.91] |
| 6.4 3D exercise (dance) vs control | 1 | 522 | Risk Ratio (Random, 95% CI) | 1.35 [0.83, 2.20] |
| 6.5 Multiple categories of exercise vs control | 17 | 1623 | Risk Ratio (Random, 95% CI) | 0.78 [0.64, 0.96] |
| 6.6 Walking programme vs control | 2 | 441 | Risk Ratio (Random, 95% CI) | 1.05 [0.71, 1.54] |
| 7 Number of fallers ‐ long‐term follow‐up by exercise type | 3 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 7.1 Balance and functional exercises vs control | 2 | 1325 | Risk Ratio (Fixed, 95% CI) | 0.86 [0.78, 0.94] |
| 7.2 Multiple categories of exercise vs control | 1 | 175 | Risk Ratio (Fixed, 95% CI) | 1.01 [0.74, 1.38] |
Comparison 3. Exercise versus control (number of people with fractures).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of people who experienced one or more fall‐related fractures‐ overall analysis | 10 | 4047 | Risk Ratio (Random, 95% CI) | 0.73 [0.56, 0.95] |
| 2 Number of people who experienced one or more fall‐related fractures ‐ subgrouped by baseline falls risk | 10 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Not selected for high risk of falling | 5 | 1255 | Risk Ratio (Random, 95% CI) | 0.48 [0.26, 0.91] |
| 2.2 Selected for high risk of falling | 5 | 2792 | Risk Ratio (Random, 95% CI) | 0.80 [0.60, 1.07] |
| 3 Number of people who experienced one or more fall‐related fractures ‐ subgrouped by age (threshold 75 years) | 10 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Age < 75 | 7 | 1307 | Risk Ratio (Random, 95% CI) | 0.53 [0.29, 0.96] |
| 3.2 Age 75+ | 3 | 2740 | Risk Ratio (Random, 95% CI) | 0.61 [0.31, 1.20] |
| 4 Number of people who experienced one or more fall‐related fractures ‐ subgrouped by exercise type | 10 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 Balance and functional exercises vs control | 7 | 2139 | Risk Ratio (Random, 95% CI) | 0.44 [0.25, 0.76] |
| 4.2 Resistance exercise vs control | 1 | 73 | Risk Ratio (Random, 95% CI) | 0.97 [0.14, 6.49] |
| 4.3 Walking programme vs control | 1 | 97 | Risk Ratio (Random, 95% CI) | 0.66 [0.11, 3.76] |
| 4.4 Multiple categories of exercise vs control | 3 | 1810 | Risk Ratio (Random, 95% CI) | 0.85 [0.62, 1.16] |
| 5 Number of people who experienced one or more fall‐related fractures ‐ long‐term follow‐up by exercise type | 3 | 2351 | Risk Ratio (Fixed, 95% CI) | 0.93 [0.69, 1.25] |
| 5.1 Balance and functional exercises vs control | 1 | 619 | Risk Ratio (Fixed, 95% CI) | 1.80 [0.46, 7.11] |
| 5.2 Walking programme vs control | 1 | 97 | Risk Ratio (Fixed, 95% CI) | 1.46 [0.44, 4.83] |
| 5.3 Multiple categories of exercise vs control | 1 | 1635 | Risk Ratio (Fixed, 95% CI) | 0.87 [0.64, 1.19] |
Comparison 4. Exercise versus control (number of people with falls that resulted in hospital admission).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of people who experienced one or more falls that resulted in hospital admission ‐ overall analysis | 2 | 1705 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.51, 1.18] |
Comparison 5. Exercise versus control (number of people with falls that required medical attention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of people who experienced one or more falls that required medical attention‐ overall analysis | 5 | 1019 | Risk Ratio (Random, 95% CI) | 0.61 [0.47, 0.79] |
| 2 Number of people who experienced one or more falls that required medical attention ‐ subgrouped by exercise type | 5 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Balance and functional exercises vs control | 3 | 583 | Risk Ratio (Random, 95% CI) | 0.76 [0.54, 1.09] |
| 2.2 Resistance exercises vs control | 1 | 73 | Risk Ratio (Random, 95% CI) | 0.92 [0.47, 1.80] |
| 2.3 3D exercise (Tai Chi) vs control | 1 | 188 | Risk Ratio (Random, 95% CI) | 0.35 [0.13, 0.93] |
| 2.4 Multiple categories of exercise vs control | 2 | 247 | Risk Ratio (Random, 95% CI) | 0.44 [0.29, 0.66] |
| 3 Number of people who experienced one or more falls that required medical attention ‐ long‐term follow‐up pooled | 2 | 319 | Risk Ratio (Random, 95% CI) | 0.54 [0.37, 0.78] |
Comparison 6. Exercise versus control (health‐related quality of life).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Health‐related quality of life‐ overall analysis | 15 | 3172 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.10, 0.04] |
| 2 Health‐related quality of life ‐ subgrouped by baseline fall risk | 15 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Not selected for high risk of falling | 8 | 2420 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.24, 0.23] |
| 2.2 Selected for high risk of falling | 7 | 752 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.12, 0.22] |
6.2. Analysis.
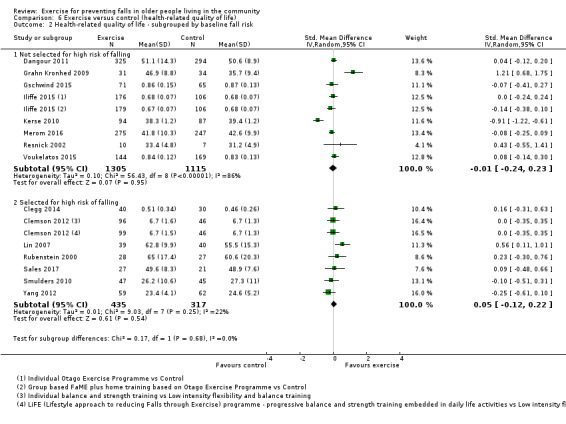
Comparison 6 Exercise versus control (health‐related quality of life), Outcome 2 Health‐related quality of life ‐ subgrouped by baseline fall risk.
Comparison 7. Exercise versus control (number of people who died).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of people who died‐ overall analysis | 30 | 10037 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.66, 1.12] |
| 2 Number of people who died ‐ subgrouped by baseline fall risk | 30 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Not selected for high risk of falling | 12 | 4606 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.54, 1.67] |
| 2.2 Selected for high risk of falling | 18 | 5421 | Risk Ratio (IV, Random, 95% CI) | 0.82 [0.60, 1.12] |
Comparison 8. Balance and functional exercises versus control: subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls, subgrouped by baseline fall risk | 39 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Not selected for higher risk of falling | 18 | 3355 | Rate Ratio (Random, 95% CI) | 0.80 [0.72, 0.90] |
| 1.2 Selected for higher risk of falling | 21 | 4602 | Rate Ratio (Random, 95% CI) | 0.72 [0.65, 0.80] |
| 2 Number of fallers, subgrouped by baseline fall risk | 37 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Not selected for higher risk of falling | 15 | 3649 | Risk Ratio (Random, 95% CI) | 0.88 [0.80, 0.97] |
| 2.2 Selected for higher risk of falling | 22 | 4639 | Risk Ratio (Random, 95% CI) | 0.86 [0.81, 0.91] |
| 3 Rate of falls, subgrouped by personnel | 39 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Health professional delivering intervention | 20 | 2960 | Rate Ratio (Random, 95% CI) | 0.67 [0.58, 0.76] |
| 3.2 No health professional delivering intervention | 19 | 4997 | Rate Ratio (Random, 95% CI) | 0.82 [0.76, 0.88] |
| 4 Number of fallers, subgrouped by personnel | 37 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 Health professional delivering intervention | 19 | 2894 | Risk Ratio (Random, 95% CI) | 0.82 [0.75, 0.90] |
| 4.2 No health professional delivering intervention | 18 | 5394 | Risk Ratio (Random, 95% CI) | 0.89 [0.84, 0.94] |
| 5 Rate of falls, subgrouped by group or individual exercise | 39 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 5.1 Group exercise | 20 | 3620 | Rate Ratio (Random, 95% CI) | 0.73 [0.65, 0.82] |
| 5.2 Not group exercise | 20 | 4589 | Rate Ratio (Random, 95% CI) | 0.77 [0.70, 0.85] |
| 6 Number of fallers, subgrouped by group or individual exercise | 37 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 6.1 Group exercise | 22 | 4465 | Risk Ratio (Random, 95% CI) | 0.87 [0.80, 0.95] |
| 6.2 Not group exercise | 16 | 4075 | Risk Ratio (Random, 95% CI) | 0.87 [0.82, 0.92] |
Comparison 9. Multiple categories of exercise versus control: subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls, subgrouped by baseline fall risk | 11 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Not selected for higher risk of falling | 6 | 786 | Rate Ratio (Random, 95% CI) | 0.54 [0.29, 0.99] |
| 1.2 Selected for higher risk of falling | 5 | 618 | Rate Ratio (Random, 95% CI) | 0.77 [0.63, 0.94] |
| 2 Number of fallers, subgrouped by baseline fall risk | 17 | 1623 | Risk Ratio (Random, 95% CI) | 0.78 [0.64, 0.96] |
| 2.1 Not selected for higher risk of falling | 7 | 710 | Risk Ratio (Random, 95% CI) | 0.70 [0.41, 1.19] |
| 2.2 Selected for higher risk of falling | 10 | 913 | Risk Ratio (Random, 95% CI) | 0.84 [0.71, 1.00] |
| 3 Rate of falls, subgrouped by personnel | 11 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Health professional delivering intervention | 3 | 653 | Rate Ratio (Random, 95% CI) | 0.65 [0.43, 0.99] |
| 3.2 No health professional delivering intervention | 8 | 751 | Rate Ratio (Random, 95% CI) | 0.66 [0.44, 0.99] |
| 4 Number of fallers, subgrouped by personnel | 16 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 Health professional delivering intervention | 8 | 867 | Risk Ratio (Random, 95% CI) | 0.81 [0.65, 1.02] |
| 4.2 No health professional delivering intervention | 8 | 711 | Risk Ratio (Random, 95% CI) | 0.70 [0.45, 1.10] |
| 5 Rate of falls, subgrouped by group or individual exercise | 11 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 5.1 Group exercise | 10 | 1194 | Rate Ratio (Random, 95% CI) | 0.64 [0.46, 0.89] |
| 5.2 Not group exercise | 1 | 210 | Rate Ratio (Random, 95% CI) | 0.81 [0.56, 1.18] |
| 6 Number of fallers, subgrouped by group or individual exercise | 17 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 6.1 Group exercise | 14 | 1301 | Risk Ratio (Random, 95% CI) | 0.77 [0.60, 1.00] |
| 6.2 Not group exercise | 3 | 322 | Risk Ratio (Random, 95% CI) | 0.86 [0.72, 1.03] |
Comparison 10. Exercise versus control (by exercise type, in people after hospital stays).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 3 | Rate Ratio (Random, 95% CI) | Totals not selected | |
| 1.1 Resistance exercise vs control | 1 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Multiple categories of exercise vs control | 2 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of fallers | 4 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| 2.1 Resistance exercise vs Control | 2 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Multiple categories of exercise vs Control | 3 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Health‐related quality of life | 3 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Resistance exercise vs control | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Multiple categories of exercise versus control | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of people who died | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Resistance exercise vs control | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Multiple categories of exercise vs control | 3 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 11. Exercise versus exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls, different types of exercise compared | 20 | Rate Ratio (Random, 95% CI) | Totals not selected | |
| 1.1 Balance and functional exercises vs balance and functional exercises | 6 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Balance and functional exercises vs resistance exercises | 3 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Balance and functional exercises vs walking | 2 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Balance and functional exercises vs multiple categories of exercise | 1 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 3D (Tai Chi) vs balance and functional exercises | 2 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 3D (Tai Chi) vs 3D (Tai Chi) | 1 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Multiple categories of exercise vs balance and functional exercises | 1 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Multiple categories of exercise vs resistance exercises | 2 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Multiple categories of exercise vs multiple categories of exercise | 4 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Rate of falls >18 months, different types of exercise compared | 1 | Rate Ratio (Random, 95% CI) | Totals not selected | |
| 2.1 Multiple categories of exercise vs multiple categories of exercise | 1 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of fallers, different types of exercise compared | 17 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| 3.1 Balance and functional exercises vs balance and functional exercises | 5 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Balance and functional exercises vs walking | 2 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Balance and functional exercises vs multiple categories of exercise | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 3D (Tai Chi) vs balance and functional exercises | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 3D (Tai Chi) vs resistance exercises | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Multiple categories of exercise vs balance and functional exercises | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Multiple categories of exercise vs resistance exercises | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Multiple categories of exercise vs resistance exercises (after hospital stays) | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Multiple categories of exercise vs multiple categories of exercise | 4 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of people who experienced one or more fall‐related fractures, different types of exercise compared | 3 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| 4.1 Balance and functional exercise vs balance and functional exercise | 2 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Balance and functional exercises vs resistance exercises | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Multiple categories of exercise vs resistance exercises | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of people who experienced one or more falls that required medical attention, different types of exercise compared | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| 5.1 Balance and functional exercises vs resistance exercises | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Multiple categories of exercise vs balance and functional exercises | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Multiple categories of exercise vs resistance exercises | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Quality of life, different types of exercise compared | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Balance and functional exercises versus balance and functional exercises | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Number of people who died, different types of exercise compared | 2 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 7.1 3D (Tai Chi) vs balance and functional exercises | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Multiple v multiple | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Rate of falls, group vs individual exercise delivery within the same type of exercise | 4 | Rate Ratio (Random, 95% CI) | Totals not selected | |
| 8.1 Balance and functional exercises | 3 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 3D (Tai Chi) exercise | 1 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Number of fallers, group vs individual exercise delivery within the same type of exercise | 4 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| 9.1 Balance and functional exercises | 4 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Number of people who experienced one or more falls requiring hospital admission, group vs individual exercise delivery within the same type of exercise | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 10.1 Balance and functional exercises | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Health‐related quality of life, group vs individual exercise delivery within the same type of exercise | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Balance and functional exercises | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Number of people who died, group vs individual exercise delivery within the same type of exercise | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 12.1 Balance and functional exercises | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Rate of falls, higher vs lower dose within the same type of exercise | 3 | Rate Ratio (Random, 95% CI) | Totals not selected | |
| 13.1 Balance and functional exercises | 1 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Resistance exercises | 1 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 3D (Tai Chi) | 1 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Number of fallers, higher vs lower dose within the same type of exercise | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| 14.1 3D (Tai Chi) | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Number of people who died, higher vs lower dose within the same type of exercise | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 12. Sensitivity analysis 1: exercise versus control excluding studies that included people < 65 years.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls: pooled data | 53 | 11807 | Rate Ratio (Random, 95% CI) | 0.77 [0.71, 0.84] |
| 2 Rate of falls: grouped by exercise type | 53 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Balance and functional exercises vs control | 34 | 7436 | Rate Ratio (Random, 95% CI) | 0.75 [0.69, 0.81] |
| 2.2 Resistance exercise vs control | 5 | 327 | Rate Ratio (Random, 95% CI) | 1.14 [0.67, 1.97] |
| 2.3 3D exercise (Tai Chi) vs control | 6 | 1971 | Rate Ratio (Random, 95% CI) | 0.83 [0.67, 1.03] |
| 2.4 3D exercise (dance) vs control | 1 | 522 | Rate Ratio (Random, 95% CI) | 1.34 [0.98, 1.83] |
| 2.5 Walking programme vs control | 2 | 441 | Rate Ratio (Random, 95% CI) | 1.14 [0.66, 1.97] |
| 2.6 Multiple categories of exercise vs control | 11 | 1404 | Rate Ratio (Random, 95% CI) | 0.66 [0.50, 0.88] |
| 3 Number of fallers: pooled data | 52 | 11576 | Risk Ratio (Random, 95% CI) | 0.85 [0.80, 0.90] |
| 4 Number of fallers: grouped by exercise type | 54 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 Balance and functional exercises vs control | 30 | 7287 | Risk Ratio (Random, 95% CI) | 0.86 [0.82, 0.91] |
| 4.2 Resistance exercise vs control | 2 | 163 | Risk Ratio (Random, 95% CI) | 0.81 [0.57, 1.15] |
| 4.3 3D exercise (Tai Chi) vs control | 6 | 1915 | Risk Ratio (Random, 95% CI) | 0.82 [0.71, 0.94] |
| 4.4 3D exercise (dance) vs control | 1 | 522 | Risk Ratio (Random, 95% CI) | 1.35 [0.83, 2.20] |
| 4.5 Walking programme vs control | 2 | 441 | Risk Ratio (Random, 95% CI) | 1.05 [0.71, 1.54] |
| 4.6 Multiple categories of exercise vs control | 17 | 1623 | Risk Ratio (Random, 95% CI) | 0.78 [0.64, 0.96] |
| 5 Number of people who experienced one or more fall‐related fractures: pooled data | 10 | 4047 | Risk Ratio (Fixed, 95% CI) | 0.73 [0.56, 0.95] |
| 6 Number of people who experienced one or more fall‐related fractures: by exercise type | 10 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 6.1 Balance and functional exercises vs control | 7 | 2139 | Risk Ratio (Fixed, 95% CI) | 0.44 [0.25, 0.76] |
| 6.2 Resistance exercise vs control | 1 | 73 | Risk Ratio (Fixed, 95% CI) | 0.97 [0.14, 6.49] |
| 6.3 Walking programme vs control | 1 | 97 | Risk Ratio (Fixed, 95% CI) | 0.66 [0.11, 3.76] |
| 6.4 Multiple categories of exercise vs control | 3 | 1810 | Risk Ratio (Fixed, 95% CI) | 0.85 [0.62, 1.16] |
| 7 Number of people who experienced one or more falls requiring medical attention: pooled data | 5 | 1019 | Risk Ratio (Random, 95% CI) | 0.61 [0.47, 0.79] |
| 8 Number of people who experienced one or more falls requiring medical attention ‐ subgrouped by exercise type | 5 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 8.1 Balance and functional exercises vs Control | 3 | 585 | Risk Ratio (Random, 95% CI) | 0.76 [0.54, 1.09] |
| 8.2 Resistance exercises vs control | 1 | 73 | Risk Ratio (Random, 95% CI) | 0.92 [0.47, 1.80] |
| 8.3 3D exercise (Tai Chi) vs Control | 1 | 188 | Risk Ratio (Random, 95% CI) | 0.35 [0.13, 0.93] |
| 8.4 Multiple categories of exercise vs control | 2 | 248 | Risk Ratio (Random, 95% CI) | 0.44 [0.29, 0.66] |
Comparison 13. Sensitivity analysis 2: exercise versus control excluding studies at a high risk of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls ‐ overall analysis | 25 | 6757 | Rate Ratio (Random, 95% CI) | 0.78 [0.71, 0.87] |
| 2 Rate of falls ‐ subgrouped by exercise type | 25 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Balance and functional exercises vs control | 16 | 3184 | Rate Ratio (Random, 95% CI) | 0.69 [0.61, 0.79] |
| 2.2 Resistance exercise vs control | 0 | 0 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 3D exercise (Tai Chi) vs control | 5 | 2331 | Rate Ratio (Random, 95% CI) | 0.92 [0.78, 1.09] |
| 2.4 3D exercise (dance) vs control | 1 | 522 | Rate Ratio (Random, 95% CI) | 1.34 [0.98, 1.83] |
| 2.5 Walking programme vs control | 1 | 339 | Rate Ratio (Random, 95% CI) | 0.88 [0.59, 1.30] |
| 2.6 Multiple categories of exercise vs control | 3 | 485 | Rate Ratio (Random, 95% CI) | 0.75 [0.60, 0.94] |
| 3 Number of fallers ‐ overall analysis | 26 | 6865 | Risk Ratio (Random, 95% CI) | 0.84 [0.80, 0.89] |
| 4 Number of fallers ‐ subgrouped by exercise type | 26 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 Balance and functional exercises vs control | 16 | 3282 | Risk Ratio (Random, 95% CI) | 0.83 [0.77, 0.89] |
| 4.2 Resistance exercise vs control | 0 | 0 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 3D exercise (Tai Chi) vs control | 5 | 2294 | Risk Ratio (Random, 95% CI) | 0.85 [0.77, 0.94] |
| 4.4 3D exercise (dance) vs control | 1 | 522 | Risk Ratio (Random, 95% CI) | 1.35 [0.83, 2.20] |
| 4.5 Walking programme vs control | 1 | 339 | Risk Ratio (Random, 95% CI) | 0.90 [0.67, 1.20] |
| 4.6 Multiple categories of exercise vs control | 4 | 518 | Risk Ratio (Random, 95% CI) | 0.84 [0.69, 1.02] |
| 5 Number of people who experienced one or more fall‐related fractures ‐ overall analysis | 2 | 332 | Risk Ratio (Random, 95% CI) | 0.26 [0.07, 1.02] |
Comparison 14. Sensitivity analysis 3: exercise versus control excluding studies with unclear or high risk of bias due to allocation concealment (rate of falls).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls ‐ overall analysis | 22 | 6092 | Rate Ratio (Random, 95% CI) | 0.85 [0.77, 0.95] |
Comparison 15. Sensitivity analysis 4: exercise versus control excluding studies with unclear or high risk of bias due to assessor blinding (rate of falls).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls ‐ overall analysis | 27 | 6996 | Rate Ratio (Random, 95% CI) | 0.76 [0.69, 0.85] |
Comparison 16. Sensitivity analysis 5: exercise versus control excluding studies with unclear or high risk of bias due to incomplete outcome data (rate of falls).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls ‐ overall analysis | 36 | 7646 | Rate Ratio (Random, 95% CI) | 0.77 [0.69, 0.85] |
Comparison 17. Sensitivity analysis 6: exercise versus control excluding cluster trials (rate of falls).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls ‐ overall analysis | 53 | 10261 | Rate Ratio (Random, 95% CI) | 0.76 [0.70, 0.83] |
Comparison 18. Sensitivity analysis 7: exercise versus control with fixed‐effect meta‐analysis (rate of falls).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls ‐ overall analysis | 59 | 12981 | Rate Ratio (Fixed, 95% CI) | 0.82 [0.79, 0.86] |
Comparison 19. Sensitivity analysis 8: multiple categories of exercise versus control excluding trials that do not include balance and strength training.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 8 | 1084 | Rate Ratio (Random, 95% CI) | 0.69 [0.48, 0.97] |
| 2 Number of fallers | 13 | 1375 | Risk Ratio (Random, 95% CI) | 0.76 [0.61, 0.95] |
Comparison 20. Sensitivity analysis 9: different exercise type coding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls ‐ subgrouped by exercise type (OEP as multiple intervention) | 48 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Balance and functional exercises vs control | 30 | 5556 | Rate Ratio (Random, 95% CI) | 0.75 [0.68, 0.82] |
| 1.2 Multiple categories of exercise vs control | 20 | 3738 | Rate Ratio (Random, 95% CI) | 0.72 [0.62, 0.83] |
| 2 Number of fallers ‐ subgrouped by exercise type (OEP as multiple intervention) | 52 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Balance and functional exercises vs control | 28 | 5946 | Risk Ratio (Random, 95% CI) | 0.86 [0.80, 0.92] |
| 2.2 Multiple categories of exercise vs control | 26 | 3965 | Risk Ratio (Random, 95% CI) | 0.83 [0.75, 0.92] |
| 3 Rate of falls ‐ subgrouped by exercise type (any balance+strength as multiple intervention) | 50 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Balance and functional exercises vs control | 16 | 2718 | Rate Ratio (Random, 95% CI) | 0.72 [0.62, 0.84] |
| 3.2 Resistance exercise vs control | 3 | 182 | Rate Ratio (Random, 95% CI) | 1.42 [0.71, 2.82] |
| 3.3 Multiple categories of exercise vs control | 35 | 6721 | Rate Ratio (Random, 95% CI) | 0.74 [0.67, 0.81] |
| 4 Number of fallers ‐ subgrouped by exercise type (any balance+strength as multiple intervention) | 53 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 Balance and functional exercises vs control | 13 | 2310 | Risk Ratio (Random, 95% CI) | 0.79 [0.65, 0.96] |
| 4.2 Resistance exercise vs control | 1 | 45 | Risk Ratio (Random, 95% CI) | 1.0 [0.46, 2.19] |
| 4.3 Multiple categories of exercise vs control | 41 | 7719 | Risk Ratio (Random, 95% CI) | 0.86 [0.81, 0.91] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Almeida 2013.
| Methods | Study design: RCT Number of study arms: 3 Length of follow‐up: 4 months |
|
| Participants | Setting: Sao Paulo, Brazil Number of participants: 119 Number analysed: 76 Number lost to follow‐up: 43 Sample: community‐dwelling Age (years): mean 79.1 (SD 4.6) Sex: 83% female Inclusion criteria: non‐institutionalised, able to walk independently, had at least 1 fall in the previous year, not enrolled in a regular exercise programme Exclusion criteria: any self‐reported conditions that would preclude exercise prescription and physical activity for older people, systolic or diastolic BP > 170 and 130 mm Hg, respectively, inability to follow written instructions and unable to obtain constant support for that task |
|
| Interventions | 1. Fully‐supervised group‐based balance and strength training: own body weight used for strength training, received home hazard reduction information and monthly phone calls; 50‐minute sessions, 3 a week for 4 months 2. Minimally‐supervised group‐based balance and strength training: own body weight used for strength training, received home hazard reduction information and monthly phone calls; 1 x 50‐minute session, alternate weeks for 4 months. Brochure provided with same exercises to be performed at home 3 x a week for 4 months 3. Control: no exercise intervention, participants asked not to engage in any other exercise programme during the study |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) |
|
| Duration of the study | 16 weeks | |
| Adherence | None reported | |
| Notes | Source of funding: São Paulo State Funding Agency Economic information: not reported Data could not be analysed due to zero events for falls (and thus fallers) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After baseline assessments, participants were randomly assigned to one of the 3 groups". Insufficient information about sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Information about falls collected at 4‐month assessment in both groups. Blinding of assessors was not stated. Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% missing outcome data, unbalanced losses across groups and reasons for missing data across groups not specified |
| Selective reporting (reporting bias) | High risk | Falls were measured but number of falls not reported. Fall outcomes and adverse events were not prespecified in the Methods section. There was no protocol or trial registration |
| Method of ascertaining falls (recall bias) | High risk | Information about falls collected at 4‐month assessment |
Ansai 2015.
| Methods | Study design: RCT Number of study arms: 3 Length of follow‐up: 4 months | |
| Participants | Setting: São Paulo, Brazil Number of participants: 69 Number analysed: 68 Number lost to follow‐up: 1 Sample: community‐dwelling Age (years): mean 82.4 (SD 2.4) Sex: 68% female Inclusion criteria: aged > 80, community‐dwelling, sedentary, able to walk alone, available to attend training site 3 a week Exclusion criteria: presence of any injury listed in the absolute contraindications of the Physical Activity Readiness Medical Examination, relative cognition, neurological or musculoskeletal contraindications making participation in protocols impossible, MMSE score below the cut‐off designated by educational level minus 1 SD |
|
| Interventions | 1. Group‐based balance, strength and aerobic training: cycle ergometer used for aerobic training, strength exercises (upper limbs, abdominals, squats, ankles) progressed using Borg scale and increments of 1 kg, balance activities with increasing difficulty; 1 hour, 3 a week for 16 weeks 2. Group‐based progressive strength training: leg press, chest press, calf raise, back extension, abdominal and rowing, 3 sets of 10 ‐ 12 RM using gym equipment; 1 hour, 3 a week, 16 weeks 3. Control: no intervention |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) |
|
| Duration of the study | 23 weeks | |
| Adherence | 1. Group‐based balance, strength and aerobic training group: 35% performed ≥ 24 sessions for 16 weeks (50% intervention) 2. Group‐based progressive strength training group: 56% performed ≥ 24 sessions for 16 weeks (50% intervention) |
|
| Notes | Source of funding: Federal University of São Carlos
Economic information: not reported 16‐week data used due to proportion of fallers not being clear for longer follow‐up periods Email communication regarding fall data, response received, data not included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐number generator |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | High risk | Blinding of assessor not specified; as falls were reported by telephone or during training, assume assessor not blinded to group allocation |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of outcome data are missing (6%) and losses are balanced across groups |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of falls and adverse events were not reported |
| Method of ascertaining falls (recall bias) | Unclear risk | Provided with fall calendar, falls reported by retrospective recall once a month, by telephone or during training |
Arantes 2015.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 3 months | |
| Participants | Setting: Belo Horizonte, Brazil Number of participants: 30 Number analysed: 28 Number lost to follow‐up: 2 Sample: community‐dwelling Age (years): Intervention mean = 73.9 (SD 7.7); Control mean = 72.2 (SD 5.7) Sex: 100% female Inclusion criteria: age 65 years +, history of 1 or more falls in the previous year, at risk for falling (at least 2 risk factors assessed by the QuickScreen Falls Risk Assessment), classified as prefrail (phenotype proposed by Fried 2001), able to walk 6 m independently Exclusion criteria: cognitive impairment (evaluated by MMSE), presence of neurological disease, acute vestibular dysfunction in past month, initiation of any other intervention during study period |
|
| Interventions | 1. Group‐based balance training: exercises increased in difficulty; 1 hour, 2 a week, 12 weeks 2. Control group: neck and upper limb stretches and movements; 1 hour, 1 a week, 12 weeks |
|
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 12 weeks | |
| Adherence | Adherence measured by session attendance 1. Group‐based balance training group: average number of sessions attended: 22.1 (range; 20 ‐ 24) 2. Control group: average number of sessions attended: 10.8 (range 10 ‐ 12) |
|
| Notes | Source of funding: CNPq and FAPEMIG
Economic information: not reported Paper states "falls were registered for 1 year after randomisation" but these results not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The allocation was made through a computer program" |
| Allocation concealment (selection bias) | Unclear risk | Concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blind to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Quote: "The assessments were performed before and immediately after the end of intervention, always by the same evaluators, and they were blinded in all the moments of the study". Unclear whether these same assessors made monthly telephone calls to collect fall data. |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of outcome data are missing (7%), with losses only from control group, due to starting another intervention (n = 1) and family problems (n = 1) |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of falls and adverse events were not reported |
| Method of ascertaining falls (recall bias) | Unclear risk | Quote: "The subjects were contacted monthly by telephone and asked about the occurrence of falls in that period" |
Arkkukangas 2015.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 3 months | |
| Participants | Setting: 3 different municipalities, Sweden Number of participants: 45 Number analysed: 40 Number lost to follow‐up: 5 Sample: community‐dwelling Age (years): mean 83 (range 75 ‐ 103) Sex: 71% female Inclusion criteria: ≥ 75 yrs, walk independently in home, understand written and oral information in Swedish language Exclusion criteria: < 25 MMSE, ongoing regular physical therapy due to injury ± illness, terminal care |
|
| Interventions | Randomised into 3 groups: 2 intervention groups (1 Individual Otago Exercise Programme, 1 Otago Exercise Programme + Motivational Interview group) and 1 control group. The Individual Otago Exercise Programme and Otago Exercise Programme + Motivational Interviewing groups were combined in this review 1. Individual Otago Exercise Programme: home‐based programme 3 a week, walking programme 4 a week, for 12 weeks, received written recommendations for falls prevention 2. Control group: no intervention, received written recommendations for falls prevention |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) |
|
| Duration of the study | 12 weeks | |
| Adherence | Not reported | |
| Notes | Source of funding: Mälardalen University
Economic information: not reported Email communication to obtain fall data, response received, data included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Predetermined randomisation list made by an independent statistician". Blocks of 3, 6, 9, or 12 participants. Method of generating the randomisation list not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment of predetermined list not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blind to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls collected by fall calendar in both groups Quote: "fall calendar, which was followed up by the physiotherapist every month". "Four physiotherapists performed the measurements single blindly." Assume fall calendar was followed up by 1 of the blinded physiotherapists |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of outcome data are missing (11%). Unbalanced losses in intervention (n = 4) and control (n = 0) groups, but reason for missing data not specified |
| Selective reporting (reporting bias) | Unclear risk | Adverse events were not a prespecified outcome and were not reported for all groups. No trial protocol or prospective trial registration |
| Method of ascertaining falls (recall bias) | Low risk | Fall calendar, followed up monthly by physiotherapist. Quote: "Four PTs performed the measurements single blindly" |
Ballard 2004.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 16 months | |
| Participants | Setting: USA Number of participants: 40 Number analysed: 39 Number lost to follow‐up: 1 Sample: community‐dwelling Age (years): mean 72.9 (SD 6) Sex: 100% female Inclusion criteria: aged ≥ 65; ambulatory; community‐dwelling; history of falling in previous year or fear of future fall; able to undertake moderate exercise Exclusion criteria: cardiovascular disease or extreme vertigo that might prohibit moderate exercise; requiring walker for support |
|
| Interventions | 1. Group‐based balance, strength and aerobic training for 15 weeks: elastic bands used for strength training, 6 home‐safety education classes; 1 hour, 3 a week, for 15 weeks 2. Group‐based balance, strength and aerobic training for 2 weeks: elastic bands used for strength training, 6 home‐safety education classes; 1 hour, 3 a week, for 2 weeks |
|
| Outcomes | 1. Rate of falls | |
| Duration of the study | 64 weeks | |
| Adherence | Adherence measured by session attendance, exercising at 1 year, frequency of exercise at 1 year Participants attended 83% (± 9%) of the exercise sessions At 1‐year follow‐up: 1. Group‐based balance, strength and aerobic training for 15 weeks plus home practice group: Continued exercise format as in intervention group: No = 7, Yes = 13 Exercise format performed 2 a week=5; performed ≥ 3 a week = 8. 2. Group‐based balance, strength and aerobic training for 2 weeks plus home practice with videotape group: Started exercise format as in intervention group: No = 17, Yes = 2 Exercise format performed 2 a week = 1; performed 3 x ar week = 1 |
|
| Notes | Source of funding: not reported
Economic information: not reported Data not used for number of people falling as not clear on total proportion of fallers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "assigned to exercise and control groups using stratified randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blind to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | High risk | Falls data were collected in both groups at the 6 home‐safety education sessions, assume assessors not blinded. Fall data also collected by telephone at 1 year; blinding of telephone assessors not reported |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of outcome data are missing (3%). Missing data are from 1 exercise group participant and unlikely to be related to outcome |
| Selective reporting (reporting bias) | High risk | Number of fallers was reported in only 1 group. Adverse events were not prespecified or reported |
| Method of ascertaining falls (recall bias) | High risk | Falls identified retrospectively during intervention at each home‐safety class (every 2 months), and by telephone follow‐up 1 year after end of intervention |
Barker 2016.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months | |
| Participants | Setting: Melbourne, Australia Number of participants: 53 Number analysed: 44 Number lost to follow‐up: 9 Sample: community‐dwelling Age (years): mean 69 Sex: 88% female Inclusion criteria: ≥ 60 years, at risk of sustaining a fall injury based on a telephone screen developed by the research team, able to negotiate a set of 10 stairs independently without a gait aid Exclusion criteria: cognitive impairment (telephone MMSE < 17), acute medical condition that impaired safe performance of exercise (e.g. unstable BP, chronic back pain, acute MI), cancer diagnosis within the past 5 years or receiving active treatment for cancer, uncontrolled chronic conditions (e.g. diabetes, hypertension), already participating in Pilates or other formal exercise (≥ 60 minutes a week for ≥ 4 weeks during the 12 weeks prior to screening for eligibility) |
|
| Interventions | 1. Group‐based Pilates focused on balance and strength plus home practice: group performed predominantly in standing with minimal‐to‐no upper limb support, used Pilates equipment; 1 hour, 2 a week, 12 weeks, and tailored home exercises performed 20 minutes daily; participants paid AUD 36.50 per class 2. Individual strength and balance: tailored home exercise performed 20 minutes daily for 12 weeks Both groups received a fall and fracture prevention information and exercise brochure |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) |
|
| Duration of the study | 24 weeks | |
| Adherence | Adherence measured by class attendance, time recorded exercising 1. Group‐based Pilates focused on balance and strength plus home practice group: 95% attended over 75% of the classes; mean hours of exercise recorded at 24 weeks = 59.5 2. Individual strength and balance group: mean hours of exercise recorded at 24 weeks = 40.8 |
|
| Notes | Source of funding: Monash University Faculty of Medicine, Nursing and Health Sciences Strategic Grant Scheme Economic information: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, permuted, block randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blind to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Falls assessed by monthly calendar and telephone calls in all groups. Blinding of assessors of fall calendars / phone calls was not stated. Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | High risk | Adverse events were "monitored by therapists delivering pilates classes or spontaneously reported by participants to the research staff", therefore assessors not blinded |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of outcome data are missing (17%). Unbalanced losses in intervention (n = 4) and control (n = 9) groups, with reasons for missing data inconsistent across groups. Missing data have been imputed using appropriate methods (last observation carried forward) |
| Selective reporting (reporting bias) | Low risk | Prespecified falls outcomes reported in prospective trial protocol |
| Method of ascertaining falls (recall bias) | Low risk | Monthly calendar and telephone calls |
Barnett 2003.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months | |
| Participants | Setting: Sydney, Australia Number of participants: 163 Number analysed: 150 Number lost to follow‐up: 13 Sample: older people identified as at risk of falling by general practitioner or hospital physiotherapist using assessment tool Age (years): mean 74.9 (SD 10.9) Sex: 67% female Inclusion criteria: age > 65 years; identified as 'at risk' of falling (1 or more of the following risk factors: lower limb weakness, poor balance, slow reaction time) Exclusion criteria: cognitive impairment; degenerative conditions, e.g. Parkinson's disease or medical condition involving neuromuscular, skeletal, or cardiovascular system that precluded taking part in exercise programme |
|
| Interventions | 1. Group‐based balance, strength and aerobic training: exercises increased in difficulty, strength training using own body weight; 1 hour a week for 4 terms for 1 year (37 classes) plus home exercise based on class content + diaries to record participation
2. Control: no exercise intervention Both groups received information on strategies for avoiding falls, e.g. hand and foot placement if loss of balance occurred |
|
| Outcomes | 1. Rate of falls
2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who died |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by class attendance, frequency of home programme 1. Group‐based balance, strength and aerobic training group: Median number of classes attended: 23 (range 0 – 36) Number attended 30 or more classes: 28 (34%) Attending exercise classes at end of trial and performing home programme ≥ 1 a week: 91%, with 13% performing exercises daily |
|
| Notes | Source of funding: Bankstown‐Lidcombe hospital Economic information: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised in matched blocks" (N = 6) |
| Allocation concealment (selection bias) | Low risk | Consecutively‐numbered, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Both groups received information on strategies for avoiding falls and intervention group also received structured weekly exercise sessions. Blinding not reported, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Falls reported by participants who were aware of their group allocation, by postal surveys monthly in both groups. Telephone interview if not returned by 2 weeks. Unclear whether those conducting telephone check were unblinded |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of outcome data are missing (8%). Balanced losses in intervention (n = 7) and control (n = 6) groups, with reasons for missing fall data unclear |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Unclear risk | Interval recall. Falls identified by postal survey at the end of each calendar month. Phoned if not returned within 2 weeks |
Beyer 2007.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months | |
| Participants | Setting: Copenhagen, Denmark Number of participants: 65 Number analysed: 53 Number lost to follow‐up: 12 Sample: women with a history of a fall identified from hospital records Age (years): range 70 ‐ 90 Sex: 100% female Inclusion criteria: community‐dwelling; at a relatively high risk of falls, defined as either ≥ 80 years old or ≥ 65 years with history of a fall in the previous 12 months or a timed 'up and go' test score of at least 15 seconds; home‐dwelling; aged 70 ‐ 90 years; history of a fall requiring treatment in ED but not hospitalisation; able to come to training facility Exclusion criteria: lower limb fracture in last 6 months; neurological diseases, unable to understand Danish; cognitively impaired (MMSE < 24) |
|
| Interventions | 1. Group‐based balance, strength and flexibility training: gym equipment used for strengthening, 1 hour, 2 a week, for 6 months 2. Control: no intervention; offered intervention after 1 year | |
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by training compliance 1. Group‐based balance, strength and flexibility training group: mean training compliance 79% (42 ‐ 100%) |
|
| Notes | Source of funding: Danish Medical Association Research Fund, Danish Medical Research Council
Economic information: not reported Email communication regarding fall data, response received, data not included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…using the minimization method with the aid of a computer program for randomization" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Falls were recorded in both allocated groups using the same method (a monthly falls calendar), but no mention of blinding of personnel confirming falls or carrying out data entry. Insufficient information to make a judgement |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of outcome data are missing (18%). Unbalanced losses in intervention (n = 10) and control (n = 4) groups, with reasons for missing fall data differing between the 2 groups (intervention group: n = 3 did not start training, 4 = ill, 1 = fracture, 2 = lost to follow‐up; control group: n = 1 dropped out as unhappy with group allocation, 1 = ill, 1 = fracture, 1 = spouse ill) |
| Selective reporting (reporting bias) | High risk | The study prespecified falls "were monitored in all participants during the study period", but number of falls was not reported |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "A falls calendar was sent to every participant on the first day of each month" for 1 year |
Boongrid 2017.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months | |
| Participants | Setting: Bangkok, Thailand Number of participants: 439 Number analysed: 437 Number lost to follow‐up: 2 Sample: community‐dwelling Age (years): mean 73.8 (SD 6.7) Sex: 83% female Inclusion criteria: ≥ 65 years, mild‐to‐moderate balance dysfunction, able to provide written informed consent. Exclusion criteria: moderate‐to severe cognitive problems, a neurological condition that severely influenced their gait and mobility (e.g. Parkinson’s disease, stroke with hemiparesis), acute arthritis, any unstable or terminal illnesses that would preclude the planned exercises and were unlikely to resolve, unable to communicate well in Thai, already participating in regular strengthening exercise (e.g. yoga, Tai Chi) |
|
| Interventions | 1. Individual Otago Exercise Programme and walking plan; video disk, manuals and weekly calendars provided, telephone calls every 2 weeks, and home visit in 3, 6, 9, 12 months 2. Control group: no intervention Both groups received fall prevention education and home safety information through video disk recorder media and books |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who died |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by proportion exercising ≥120 minutes a week at 3 months 1. Individual Otago Exercise Programme and walking plan group: 30% exercised ≥ 120 minutes a week at 3 months; 32% exercised ≥ 120 minutes a week at 6 months; 57% exercised ≥ 120 minutes a week at 3 months |
|
| Notes | Source of funding: Development potentials of Thai People Project, Mahidol University Economic information: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A block randomization was applied to generate random sequence lists by an investigator who was not involved in data collection or administering interventions" |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes and sequence kept confidential |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blinded to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls were recorded on daily calendar in all groups. Research assistants who conducted interviews were blinded to group allocation |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Method of ascertaining adverse events is unclear |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants were not blind to allocated group |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of outcome data are missing (< 1%). Balanced losses in intervention and control groups |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified in study protocol were reported. Adverse events not specified in protocol but were reported in results |
| Method of ascertaining falls (recall bias) | Low risk | Falls were self‐recorded on a daily calendar, plus interviews by blinded research assistants at 3, 6, 9 and 12 months |
Brown 2002.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 14 months | |
| Participants | Setting: Perth, Western Australia Number of participants: 99 Number analysed: 71 Number lost to follow‐up: 28 Sample: men and women recruited by press releases in 11 newspapers and information brochures distributed to organisations, GPs, etc; 6 pairs of people with the same residential address randomised to the same group Age (years): N = 101 aged 75 to 84, N = 48 aged 85 to 94 Sex: 79% female Inclusion criteria: age ≥ 75; community‐living; independent in basic ADL; able to walk 20 m without personal assistance Exclusion criteria: cognitive impairment (MMSE ≤ 24); various conditions, e.g. angina, claudication, cerebrovascular disease, low or high blood pressure, major systemic disease, mental illness |
|
| Interventions | Randomised into 3 groups: 2 intervention groups (1 group‐based balance, strength and aerobic training, and 1 social intervention group) and 1 control group. Only group‐based balance, strength and aerobic training and control group included in this review 1. Group‐based balance, strength and aerobic training: individualised and progressed, elastic tubing and free weights used for strength training, home practice of a functional task; 1 hour, 2 a week, 16 weeks 2. Control group: no intervention |
|
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) 2. Number of people who died |
|
| Duration of the study | 56 weeks | |
| Adherence | Adherence measured by session attendance 1. Group‐based balance, strength and aerobic training group: mean attendance; 85% (22 ‐ 26 sessions), range of 62 ‐ 100% (16 sessions) |
|
| Notes | Source of funding: not reported
Economic information: not reported Only group‐based balance, strength and aerobic training and control group included in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised into one of three groups using a table of random numbers" |
| Allocation concealment (selection bias) | Low risk | Randomised into one of 3 groups "by a physiotherapist uninvolved in the study." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blind to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Fall data collected in same manner in each group. Study reports outcome assessors were blinded, but it is unclear whether blinded assessors conducted the telephone follow‐ups for falls |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of outcome data are missing (28%). Unbalanced losses in intervention and control groups |
| Selective reporting (reporting bias) | High risk | Fall data were collected but number of falls not reported |
| Method of ascertaining falls (recall bias) | Low risk | Participants provided details of falls in monthly report sheet returned in reply‐paid addressed envelopes. No mention of telephone calls |
Buchner 1997.
| Methods | Study design: RCT Number of study arms: 2 for analysis Length of follow‐up: 25 months | |
| Participants | Setting: Seattle, USA Number of participants: 105 Number analysed: 100 Number lost to follow‐up: 5 Sample: random sample of HMO members (FICSIT intervention groups only) Age (years): mean 75 Sex: 51% female Inclusion criteria: aged 68 ‐ 85; unable to do 8‐step tandem gait test without errors; below 50th percentile in knee extensor strength for height and weight Exclusion criteria: active cardiovascular, pulmonary, vestibular, and bone disease; positive cardiac stress test; body weight > 180% ideal; major psychiatric illness; active metabolic disease; chronic anaemia; amputation; chronic neurological or muscle disease; inability to walk; dependency in eating, dressing, transfer or bathing; terminal illness; inability to speak English or complete written forms |
|
| Interventions | Randomised into 7 groups: 6 intervention groups (3 FICSIT trial ‐ group‐based stationary cycling, group‐based strength training, group‐based combined endurance and strength training; and 3 MoveIT trial), and 1 control group. This paper reports on the 3 FICSIT groups and the control group
1. Group‐based stationary cycling: stationary cycles used for arms and legs, supervised classes; 1 hour (30 ‐ 35 minutes endurance exercise), 3 a week for 6 months followed by unsupervised exercise
2. Group‐based strength training: weight machines used for upper and lower body (2 sets of 10 reps per set, 50 ‐ 60% 1 RM for set 1 and 75% of 1 RM for set 2), supervised classes; 1 hour, 3 a week for 6 months followed by unsupervised exercise 3. Group‐based combined endurance and strength training: 20 minutes of endurance training and 1 set of strength training exercises (75% 1 RM) 4. Control: usual activity levels but "allowed to exercise after 6 months" |
|
| Outcomes | 1. Rate of falls
2. Number of people who experienced 1 or more falls (risk of falling) "A priori decision" to report fall outcomes for "any exercise" (all 3 exercise groups combined) compared with control group |
|
| Duration of the study | Up to 100 weeks, median 72 weeks | |
| Adherence | Exercise groups: 14 dropouts (19%), participants who remained in the study attended 95% sessions Control group; 1 dropout (3%) |
|
| Notes | Source of funding: National Institute on Aging, Centers for Disease Control and Prevention, Department of Veterans Affairs Economic information: Healthcare service costs: hospitalised control participants more likely to have hospital costs > USD 5000 (P < 0.05); no significant difference in ancillary outpatient costs between groups at 7 ‐ 18 months Seattle FICSIT trial. Only 1.3% of original sample randomised. Falls not primary outcome. Other outcomes assessed at end of intervention (6 months) then "control group allowed to exercise after 6 months" (7/30 participants did). Cost analysis reported in primary reference | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, quote: "using a variation of randomly permuted blocks" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Falls reported by participants who were aware of their group allocation. Quote: "Most study outcomes were measured by blinded examiners..." but unclear whether this applies to personnel carrying out telephone follow‐up of falls |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of outcome data are missing (5%). Unbalanced losses between intervention groups (n = 2 in each of the 3 groups) and control (n = 0) group. Reason for missing data unclear |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Low risk | Falls reported immediately by mail, also monthly postcard return; telephone follow‐up if no postcard received |
Bunout 2005.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months | |
| Participants | Setting: Santiago, Chile Number of participants: 298 Number analysed: 241 Number lost to follow‐up: 57 Sample: men and women Age (years): mean 75 (SD 5) Sex: 70% female Inclusion criteria: "elderly subjects" consenting to participate; able to reach community centre Exclusion criteria: severe disabling condition; cognitive impairment (MMSE < 20) |
|
| Interventions | 1. Group‐based balance, strength and walking: moderate intensity strength training using functional weight‐bearing exercises, progressive resistance TheraBands; 1 hour, 2 a week, 1 year 2. Control: no intervention | |
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who died |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by attendance at > 50% sessions 1. Group‐based balance, strength and walking group: 42% non‐compliant (attended < 50% sessions) |
|
| Notes | Source of funding: University of Chile
Economic information: not reported Journal website for supplementary data www.ageing.oupjournals.org. Additional data obtained from author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using computer‐generated random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Falls reported at follow‐up clinics by participants who were aware of their group allocation. Blinding of researchers at follow‐up not reported |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of outcome data are missing (19%). Number lost from each group is unclear |
| Selective reporting (reporting bias) | High risk | Falls data were collected but number of fallers was not reported; adverse events were not reported |
| Method of ascertaining falls (recall bias) | Unclear risk | Interval recall. Falls ascertained at monthly outpatient clinic or by telephone |
Campbell 1997.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 24 months | |
| Participants | Setting: Dunedin, New Zealand Number of participants: 233 Number analysed: 233 Number lost to follow‐up: 0 Sample: women identified from general practice registers Age (years): mean 84.1 (SD 3.1) Sex: 100% female Inclusion criteria: at least 80 years old; community‐living Exclusion criteria: cognitive impairment; not ambulatory in own residence; already receiving physiotherapy |
|
| Interventions | 1. Individual Otago Exercise Programme: home‐based programme prescribed in 4 x 1‐hour visits in first 2 months, 30‐minute exercise, 3 a week plus walk outside home 3 a week. Regular phone contact after first 2 months 2. Control: social visit by research nurse x 4 in first 2 months. Regular phone contact | |
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 52 weeks. 2‐year data reported in Campbell 1999 | |
| Adherence | Not reported | |
| Notes | Source of funding: Accident Rehabilitation and Compensation Insurance Corporation of New Zealand, Department of Veterns Affairs, USA
Economic information: Mean cost per person (intervention): NZD 173 in year 1, NZD 22 in year 2. Healthcare service costs: no difference between the 2 groups resulting from falls or for total healthcare costs, 27% hospital admission costs resulted from fall. Incremental cost per fall prevented/per QALY gained: at 1 year = NZD 314 (programme implementation costs only); at 2 years = NZD 265 (programme implementation costs only) Otago Exercise Programme manual can be obtained from www.cdc.gov/HomeandRecreationalSafety/Falls/compendium/1.2_otago.html. Cost‐effectiveness analysis reported (Robertson 2001ac). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation schedule developed using computer‐generated numbers |
| Allocation concealment (selection bias) | Low risk | Assignment by independent person off‐site |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | High risk | Falls reported by participants who were aware of group allocation. Blinding of adjudicator reported, but researcher making telephone contact was aware of group allocation as she also did social visits (personal communication reported by Gillespie 2012) |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing outcome data for falls |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Low risk | Falls recorded daily on postcard calendars, mail registration monthly by postcard, telephone follow‐up |
Carter 2002.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 5 months | |
| Participants | Setting: Vancouver, Canada Number of participants: 93 Number analysed: 80 Number lost to follow‐up: 13 Sample: community‐dwelling osteoporotic women Age (years): mean 69 (SD 3) Sex: 100% female Inclusion criteria: aged 65 ‐ 75 years; residents of greater Vancouver; osteoporotic (based on BMD) Exclusion criteria: < 5 years post‐menopause; weighed > 130% ideal body weight; other contraindications to exercising; already doing > 8 hours/week moderate‐to‐hard exercise; planning to be out of city > 4 week during 20‐week programme |
|
| Interventions | 1. Group‐based Osteofit strength and gait training: strengthening and stretching exercises using progressive resistance Theraband elastic bands and small free weights, 40 minutes, 2 a week, for 20 weeks, bimonthly social seminar 2. Control: usual activities, bimonthly social seminar separate from intervention group | |
| Outcomes | 1. Rate of falls | |
| Duration of the study | 20 weeks | |
| Adherence | Adherence measured by class attendance 1. Group‐based Osteofit strength and gait training class: 89% |
|
| Notes | Source of funding: BC Medical Services Foundation of the Vancouver Foundation, British Columbia Sports Medicine Foundation, RBC Foundation Economic information: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer‐generated programme |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls recorded in falls calendars in both groups. Quote: "All data were collected by trained researchers blinded to group assignment" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants not blind to allocated group |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of outcome data are missing (5%). Minor imbalance in withdrawals in intervention (n = 5) and control (n = 8) groups, with balanced reasons for withdrawal between the groups |
| Selective reporting (reporting bias) | High risk | Fall data were collected but number of fallers was not reported; adverse events were not reported |
| Method of ascertaining falls (recall bias) | Low risk | Falls recorded in falls calendars returned monthly |
Cerny 1998.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months | |
| Participants | Setting: California, USA Number of participants: 28 Number analysed: 28 Number lost to follow‐up: 0 Sample: community‐dwelling "well elderly" (proportion of women not stated); some pairs of people randomised to the same group where they were (e.g. dependent on the other for transport) Age (years): mean 71 (SD 4) Inclusion criteria: none described Exclusion criteria: none described |
|
| Interventions | 1. Group‐based balance, strength, flexibility, aerobic training and brisk walking: 1½ hours, 3 a week, 6 months 2. Control: no intervention | |
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 24 weeks | |
| Adherence | Not reported | |
| Notes | Source of funding: not reported
Economic information: not reported Contact with lead author but no full paper or report prepared Email communication about fall data, response received, data not included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by coin toss. Individually randomised but some clusters, e.g. couples or 2 women where 1 was dependent on the other for transport (personal communication reported in Gillespie 2012) |
| Allocation concealment (selection bias) | High risk | Coin toss on site |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Unclear if assessors were blinded, insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing outcome data for falls |
| Selective reporting (reporting bias) | High risk | Fall data were collected but number of falls was not reported; adverse events were not reported |
| Method of ascertaining falls (recall bias) | High risk | Assume retrospective recall and 3‐ and 6‐month assessment |
Clegg 2014.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 3 months | |
| Participants | Setting: Bradford, United Kingdom Number of participants: 84 Number analysed: 70 Number lost to follow‐up: 14 Sample: community‐dwelling Age (years): mean 79 (SD 9.2) Sex: 71% female Inclusion criteria: living at home in assisted‐living sites, housebound, recently discharged from elderly medicine outpatient clinic, had a case manager, attending a day centre or respite care Exclusion criteria: unable to stand and walk independently, currently participating in exercise programme, registered blind, poorly‐controlled angina, another household member in the trial, severe dementia, palliative care |
|
| Interventions | 1. Individual balance and strength training: no special equipment required and manual provided, leg strengthening for basic mobility tasks, 5 face‐to‐face home visits, 7 telephone calls, < 15 minutes exercise sessions, 3 a day, 5 a week, 12 weeks 2. Control group: usual care |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who experienced 1 or more falls requiring hospital admission 4. Health‐related quality of life 5. Number of people who died |
|
| Duration of the study | 12 weeks | |
| Adherence | Adherence measured by completion of programme, diary completion 1. Individual balance and strength training group: 70% completed the 12‐week programme (n = 28); 27/28 (96%) diaries returned mean diary completion = 64% mean recorded total adherence = 46% mean recorded partial or total intervention adherence = 67% |
|
| Notes | Source of funding: Dunhill Medical Trust, Royal College of Physicians Joint Research Fellowship
Economic information: not reported Email communication to obtain fall data, response received, data included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generation of randomsation sequence by independent research unit |
| Allocation concealment (selection bias) | Low risk | Storage of randomsation sequence by independent research unit |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Unclear whether falls were confirmed using the same method in both groups and unclear who assessed falls. Assessors of performance/questionnaire outcomes intended to be blinded but Quote: "were frequently unblinded". Impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Assessors of performance/questionnaire outcomes intended to be blinded, but Quote: "were frequently unblinded". Impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants not blind to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of outcome data are missing (17%). Minor unbalance in withdrawals in intervention (n = 5) and control (n = 9) groups, with some unbalance in reasons for withdrawal between the groups (intervention: 3 = withdrew, 1 = lost to follow‐up, 1 = died; control: 4 = withdrew, 2 = lost to follow‐up, 3 = died) |
| Selective reporting (reporting bias) | Low risk | Falls outcomes were prospectively specified in trial registery. Adverse events reported |
| Method of ascertaining falls (recall bias) | Unclear risk | Method of fall recording not stated |
Clemson 2010.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months | |
| Participants | Setting: Sydney, Australia Number of participants: 34 Number analysed: 34 Number lost to follow‐up: 0 Sample: volunteer community‐dwelling men and women recruited by various strategies Age (years): mean 82 (SD 5.9) Sex: 47% female Inclusion criteria: aged > 70 years; ≥ 2 falls or an injurious fall in previous year Exclusion criteria: cognitive impairment; no conversational English; unable to walk independently; resident in nursing home or hostel; unstable or terminal illness that would preclude planned exercises; neurological conditions, e.g. Parkinson's disease |
|
| Interventions | 1. LiFE (Lifestyle approach to reducing Falls through Exercise) programme ‐ progressive balance and strength training embedded in daily life activities: taught in 5 home visits + 2 booster visits over 3 months + 2 phone calls; 6‐month programme 2. Control group: no intervention | |
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 24 weeks | |
| Adherence | Not reported | |
| Notes | Source of funding: University of Sydney Bridging Grant Economic information: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was conducted … using a random numbers table" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was conducted by an investigator not involved in assessment or intervention …" "Once baseline assessments were completed by the research assistant (RA), participants were then allocated in order of completion from the generated lists by the blinded investigator" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Quote: "An RA who was not involved in the intervention and masked to the group allocation conducted all assessments. Falls surveillance was by daily calendar, which participants mailed monthly, using pre‐addressed envelopes to the RA. An investigator telephoned any participant who failed to return the calendar or who reported a fall." Unclear whether the investigator carrying out the telephone calls was blind to group allocation |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants unblinded to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall outcome data are missing (9%). Balance in withdrawals in intervention (n = 1) and control (n = 2) groups, with balanced reasons for withdrawal between the groups |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "Falls surveillance was by daily calendar, which participants mailed monthly, using pre‐addressed envelopes …" |
Clemson 2012.
| Methods | Study design: RCT Number of study arms: 3 Length of follow‐up: 12 months | |
| Participants | Setting: Sydney, Australia Number of participants: 317 Number analysed: 317 Number lost to follow‐up: 0 Sample: community‐dwelling Age (years): mean 83.4 Sex: 55% female Inclusion criteria: men and women ≥ 70 yrs, ≥ 2 falls or 1 injurious fall in past 12 months determined by self‐report Exclusion criteria: moderate to severe cognitive problems, no conversational English, inability to walk independently, neurological condition severely influencing gait and mobility, resident in a nursing home or hostel, unstable or terminal medical illness precluding the planned exercises and unlikely to resolve |
|
| Interventions | 1. LiFE (Lifestyle approach to reducing Falls through Exercise) programme ‐ progressive balance and strength training embedded in daily life activities: performed throughout the day, taught in 5 home visits + 2 booster visits over 3 months + 2 phone calls. Manual provided for increasing intensity and challenge. 6‐month programme. 2. Individual balance and strength training: progressive exercises performed 3 a week, taught in 5 home visits + 2 booster visits over 3 months + 2 phone calls. 6‐month programme. 3. Control: Low‐intensity flexibility and balance training; gentle and flexibility exercises in sitting, lying down, or standing while holding on, not progressed, 2 sessions + 1 booster session + 6 follow‐up phone calls. 6 months |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Health‐related quality of life 4. Number of people who died |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by sessions performed. Mean adherence to programme over first 6 months for each group/still exercising at 6 months reported: 1. LiFE (Lifestyle approach to reducing Falls through Exercise) programme group: 47% (SD 33)/81 (76%) 2. Individual balance and strength training group: 35% (SD 29)/63 (60%) 3. Control group: 47% (SD 34)/74 (71%) |
|
| Notes | Source of funding: Australian National Health and Medical Research Council Economic information: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was ... concealed by using an automated secure website that was operated by an off‐site independent service" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Fall data collected using same method in each group. Fall event surveillance was conducted by a research assistant blinded to group allocation |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants unblinded to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of fall outcome data are missing (10%). Minor imbalance in withdrawals in LiFE (n = 8), structured programme (n = 9) and control (n = 14) groups, with reasons for loss of fall data unclear |
| Selective reporting (reporting bias) | Low risk | Falls outcomes were prospectively specified in trial registry. Adverse events reported |
| Method of ascertaining falls (recall bias) | Low risk | Daily calendar mailed monthly, follow‐up phone call for missing calendars or fall reported by blinded researcher |
Cornillon 2002.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months | |
| Participants | Setting: St Étienne, France Number of participants: 303 Number analysed: 303 Number lost to follow‐up: 0 Sample: community‐dwelling and independent in ADL Age (years): mean 71 Sex: 83% female Inclusion criteria: aged > 65; living at home; ADL‐independent; consented Exclusion criteria: cognitively impaired (MMSE < 20); obvious disorder of walking or balance |
|
| Interventions | 1. Group‐based balance and gait training, information on fall risk, and balance and sensory training, 1 a week, 8 weeks 2. Control: normal activities | |
| Outcomes | 1. Rate of falls
2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who died |
|
| Duration of the study | 52 weeks | |
| Adherence | 102 people (68%) participated in at least 6 sessions, 14 (9%) participated in 1 ‐ 5 sessions and 34 (23%) did not participate in any sessions (due to refusal, health, or dissatisfaction with the proposed programme) | |
| Notes | Source of funding: not reported Economic information: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by random‐number tables |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blind to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Falls recorded on 6‐monthly falls calendars in both groups. No telephone contact described. Blinding of study personnel recording data from the calendars not described |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Method of ascertaining adverse events unclear. Blinding of study personnel not described |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | Unclear risk | Prespecified falls outcomes reported, adverse events reported. No trial protocol or prospective trial registration |
| Method of ascertaining falls (recall bias) | Low risk | Prospective. Falls recorded on monthly falls calendars |
Dadgari 2016.
| Methods | Study design: Cluster‐RCT
Number of study arms: 2 Number of clusters: 25 Length of follow‐up: 6 months |
|
| Participants | Setting: Shahroud, Iran Number of participants: 551 Number analysed: 317 Number lost to follow‐up: 234 Sample: community‐dwelling Age (years): mean 70.6 (SD 5.1) Sex: 49% female Inclusion criteria: ≥ 60 years, able to walk ≥ 10 m, permanent residency in an urban area in past 12 months, previous falls, had a female family member (to maintain homogeneity) as a caregiver (aged 18 ‐ 50) with health literacy (able to read instructional booklet and explaining the content to the researchers) Exclusion criteria: acute or chronic disease restricting exercise, unable to walk independently for 10 m, hip replacement surgery or lower extremity fracture/s in past 12 months, orthopaedic surgeon recommending not to participate due to severe articular involvement limiting physical activity or any other reason, elderly people with high level of activity in past 12 months |
|
| Interventions | 1. Individual Otago Exercise Programme: home programme with monthly visits in the presence of family caregiver/s, 45‐minute sessions, 3 x ar week, 6 months 2. Control group: given a booklet on general health for elderly people published by the 'Iranian Ministry of Health, Treatment and Medical Education' |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) |
|
| Duration of the study | 24 weeks | |
| Adherence | Not reported | |
| Notes | Source of funding: Shahroud University of Medical Sciences
Economic information: not reported Email communication to obtain fall data, response received, data included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only mentions block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Cluster‐RCT. Individual participant recruitment was undertaken after group allocation. The method of concealment is not described and it is unclear whether recruitment was undertaken by a person who was unblinded and may have had knowledge of participant characteristics |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Method of ascertaining falls was not clear in either group. Blinding of assessors not described |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall outcome data are missing (42%). Balanced withdrawals in intervention (n = 119) and control (n = 115) groups; reasons for loss of fall data unclear |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | High risk | Falls outcome: Quote: "was examined before and after the exercise training program" (6 months). Method of ascertaining falls at 6 months was not clear |
| Cluster‐randomised trials | High risk | Individuals were recruited to the trial after the clusters were randomised and personnel recruiting participants were not blinded to cluster; baseline comparability of clusters was not reported; missing outcomes for clusters or within clusters were not reported; no accounting for clustering in analysis; results comparable with individually randomised trials |
Dangour 2011.
| Methods | RCT (cluster‐randomised by health centre, 2 x 2 factorial design) Study design: Cluster‐RCT Number of study arms: 2 Number of clusters: 28 (20 clusters only for fallers and fractures) Length of follow‐up: 24 months |
|
| Participants | Setting: Santiago, Chile Number of participants: 984 Number analysed: 619 Number lost to follow‐up: 365 Sample: randomly sampled households in health centre catchment areas and health centre registries Age (years): range 65 ‐ 68 Sex: 68% female Inclusion criteria (clusters): health centres with > 400 residents aged 65 ‐ 67.9 years in low‐middle economic status municipalities Exclusion criteria (individuals): unable to walk unaided; seeking medical advice for unplanned 3 kg weight loss over 3 months; planning to move house within 3 months; already enrolled in national Programme of Complementary Feeding for the Older Population (PACAM) or consuming PACAM programme supplements; scoring ≥ 6 on Pfeffer screen (poor cognitive function) |
|
| Interventions | Randomised into 3 groups: 2 intervention groups (1 group‐based balance and strength, and 1 nutritional supplements group) and 1 control group. Only group‐based balance and strength and control group included in this review
1. Group‐based balance and strength: supervised sessions for functional weight‐bearing exercises; 1 hour, 2 a week, 24 months 2. Control group: no intervention |
|
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) 2. Number of people who experienced 1 or more fall‐related fractures 3. Health‐related quality of life 4. Number of people who died |
|
| Duration of the study | 108 weeks | |
| Adherence | Adherence measured as attendance at sessions offered 1. Group‐based balance and strength group: 38% |
|
| Notes | Source of funding: London School of Hygiene and Tropical Medicine, London, UK
Economic information: Mean cost per person (intervention) USD 164 for physical activity intervention. Incremental cost per fall prevented/per QALY gained: cost effectiveness of physical activity intervention reported as USD 4.84 per extra metre walked Cost analysis reported in primary reference Number of clusters allocated to intervention: 5; number of clusters allocated to control: 5; number of clusters analysed (intervention): 5; number of clusters analysed (control): 5 Email communication about fall data, response received, data not included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing of lots Quote: "The center names (clusters) were put into a hat. The four treatment arms (nutritional supplementation, nutritional supplementation+physical activity, physical activity, control) were randomly numbered 1–4. As each name was drawn out of the hat by a member of the study team, it was assigned to the next treatment number until each arm contained five clusters" |
| Allocation concealment (selection bias) | High risk | Cluster RCT. Individual participant recruitment was undertaken after group allocation. The method of concealment is not described and it is unclear whether recruitment was undertaken by a person who was unblinded and may have had knowledge of participant characteristics |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | High risk | Falls assessed via participant recall in both groups. Although assessors of the primary outcomes (pneumonia, physical function) were blind to group allocation, this was not mentioned, therefore assumed not to apply, for secondary outcomes (included fallers) |
| Blinding of outcome assessment (detection bias) Fractures | High risk | Fractures were self‐reported, not confirmed by the results of radiological examination or from primary care case record |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants were not blinded to allocated group |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall outcome data are missing (37%). Unbalanced withdrawals in intervention (n = 155) and control (n = 209) groups; reasons for loss of fall data unclear |
| Selective reporting (reporting bias) | High risk | Fall data were collected but number of falls was not reported; adverse events were not reported |
| Method of ascertaining falls (recall bias) | High risk | Participant recall for falls was at 12 and 24 months. For secondary outcomes including Quote: "self‐reported incidence of falls" ... "Participants in the original 20 clusters were re‐interviewed after 12 and 24 mo for outcome data" |
| Cluster‐randomised trials | Unclear risk | Individuals were recruited to the trial after the clusters were randomised and personnel recruiting participants were not blind to cluster; baseline characteristics of clusters and participants were similar between trial arms; missing outcomes for clusters or within clusters were not reported; accounted for the clustered design in the analysis; results comparable with individually randomised trials |
Davis 2011.
| Methods | Study design: RCT Number of study arms: 3 Length of follow‐up: 9 months | |
| Participants | Setting: Vancouver, Canada Number of participants: 155 Number analysed: 155 Number lost to follow‐up: 0 Sample: community‐dwelling women Age (years): mean 70 (range 65 ‐ 75) Sex: 100% female Inclusion criteria: aged 65 ‐ 75; cognitively intact; visual acuity 20/40 or better Exclusion criteria: resistance training in the last 6 months; medical condition for which exercise is contraindicated; neurogenerative disease; taking cholinesterase inhibitors; depression; on hormone replacement therapy during previous 12 months |
|
| Interventions | 1. Group‐based progressive high‐intensity resistance training classes: gym equipment and free weights used with a "progressive, high intensity protocol", 1 a week, 1 year 2. Group‐based progressive high‐intensity resistance training classes: gym equipment and free weights used with a "progressive, high intensity protocol", 2 a week, 1 year 3. Group‐based balance and tone: stretching, range of motion, pelvic floor, balance, relaxation exercises using body weight alone, 2 a week, 1 year | |
| Outcomes | 1. Rate of falls | |
| Duration of the study | 52 weeks | |
| Adherence | Not reported | |
| Notes | Source of funding: The Vancouver Foundation, Natural Sciences and Engineering Research Council of Canada, Michael Smith Foundation for Health Research, the Canada Foundation for Innovation Economic information: Mean cost per person (intervention): CAD 353 once‐weekly resistance training, CAD 706 twice‐weekly resistance training, CAD 706 twice‐weekly balance and tone classes. Mean healthcare costs resulting from falls, mean total healthcare costs respectively: CAD 547, CAD 1379 once‐weekly resistance training; CAD 184, CAD 1684 twice‐weekly resistance training; CAD 162, CAD 1772 twice‐weekly balance and tone classes. Incremental cost per fall prevented/per QALY gained: both once‐ and twice‐weekly resistance training less costly and more effective than balance and tone classes Cost‐effectiveness analysis and cost utility analysis reported in primary reference Email communication about fall data, response received, data not included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization sequence was generated by www.randomization.com." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization sequence … was concealed until interventions were assigned. This sequence was held independently and remotely by the research coordinator" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants or personnel but both groups received an exercise intervention so unlikely to introduce bias |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Fall calendars used to assess falls in all groups. Quote: "The assessors were blinded to the participants' assignments" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Method of ascertaining adverse events unclear |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | High risk | Fall data were collected but number of fallers was not reported |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "We used monthly fall diary calendars to track all falls for each participant during the 12‐month study period." |
Day 2002.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 18 months | |
| Participants | Setting: Melbourne, Australia Number of participants: 272 Number analysed: 272 Number lost to follow‐up: 0 Sample: community‐dwelling men and women identified from electoral roll Age (years): mean 76.1 (SD 5.0) Sex: 60% female Inclusion criteria: aged ≥ 70; community‐dwelling and able to make modifications; expected to remain in area for 2 years (except for short absences); have approval of family physician Exclusion criteria: undertaken regular to moderate exercise with a balance component in previous 2 months; unable to walk 10 to 20 m without rest or help or having angina; severe respiratory or cardiac disease; psychiatric illness prohibiting participation; dysphasia; recent major home modifications; education and language adjusted score > 4 on the short portable mental status questionnaire |
|
| Interventions | Randomised into 8 groups: only 1 intervention group (group‐based balance and strength) and 1 control group included in this review 1. Group‐based balance and strength, plus daily home exercises tailored by physiotherapist: 1‐hour class a week, 15 weeks 2. Control group: no intervention. Received brochure on eye care for over‐40‐year olds |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who experienced 1 or more falls requiring medical attention |
|
| Duration of the study | 18 months | |
| Adherence | Adherence measured by class attendance, frequency of home programme 1. Group‐based balance and strength group: 401/541 participants started a class; mean number of sessions attended, 10 (SD 3.8); 328/401 attended > 50% of their sessions; mean number of additional home exercise sessions, 9 a month |
|
| Notes | Source of funding: Australian National Health and Medical Research Council, Victorian Department of Human Services (Aged Care), City of Whitehorse, Victorian Health Promotioin Foundation, Rotary, National Safety Council Economic information: Mean cost per person (intervention) AUD 52, AUD 33 for exercise group, AUD 39 for control group. Incremental cost per fall prevented/per QALY gained: ICER per fall prevented AUD 652, injurious fall prevented AUD 1176, fracture prevented AUD 26,236, QALY AUD 51,483 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by "adaptive biased coin" technique, to ensure balanced group numbers |
| Allocation concealment (selection bias) | Low risk | Computer‐generated by an independent third party contacted by telephone |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | All participants used monthly falls diary, with telephone contact from a researcher blinded to group allocation if not returned in 5 days |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Low risk | Falls reported using monthly postcard to record daily falls. Telephone follow‐up if calendar not returned within 5 working days of the end of each month, or reporting a fall |
Day 2015.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months | |
| Participants | Setting: Melbourne, Australia Number of participants: 503 Number analysed: 409 Number lost to follow‐up: 94 Sample: community‐dwelling men and women Age (years): mean 70 Sex: 70% female Inclusion criteria: ≥ 70 years and older, community residents, and preclinically disabled as defined by Fried 2001. Exclusion criteria: already participating in Tai Chi or a vigorous exercise programme (other physical activity was allowed), adjusted score > 4 on the Short Portable Mental Status Questionnaire, major unstable cardiopulmonary disease, life‐threatening illness, major psychiatric illness unless stable on treatment, or did not have approval to participate from their local doctor |
|
| Interventions | 1. Group based Tai Chi (Modified Sun style Tai‐Chi): 1‐hour session, 2 a week, up to 48 weeks. Participants paid AUD 3 a class 2. Control: Group‐based flexibility training conducted primarily in the seated position with some leg exercises performed in standing, holding on to the back of a chair, 1‐hour session, 2 a week, up to 48 weeks. Participants paid AUD 3 a class |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1e or more falls (risk of falling) 3. Number of people who experienced 1 or more falls requiring hospital admission 4. Number of people who died |
|
| Duration of the study | 48 weeks | |
| Adherence | Adherence measured by class attendance 1. Group‐based Tai Chi group: mean number of classes attended during the first 24‐week period, 25.8 (SD 15.9), median 30; mean number of classes attended during the full 48 weeks, 34.4 (SD 26.9), median 33.5 2. Group‐based flexibility training group: mean number of classes attended during the first 24‐week period, 27.4 (SD 13.4), median 30; mean number of classes attended during the full 48 weeks, 41.3 (SD 26.1), median 39.0 |
|
| Notes | Source of funding: Australian National Health and Medical Research Council Economic information: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomized by the study statistician (D.J.) by using a computerized random number generator and a minimization algorithm" |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation list was e‐mailed directly to the exercise program administrator who managed exercise class delivery, independent of the research staff involved in the data collection" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to group allocation Quote: "Although class leaders and participants were not blinded to group assignment, they were told that we were comparing the 2 exercise programs" |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Participants reported falls for up to 48 weeks using a monthly post‐card calendar system, supplemented with telephone follow up for missing calendars Quote: "The interviewer was blind to group assignment" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | High risk | A blinded interviewer ascertained injury from participant self‐report |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall outcome data are missing (18%). Loss of fall data was balanced in intervention (n = 46) and control (n = 48) groups; reason for loss of fall data was 'refused calendars' in all in both groups |
| Selective reporting (reporting bias) | Low risk | Prespecified falls outcomes reported. Prospective trial registration |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "Participants reported falls for up to 48 weeks using a monthly post‐card calendar system, supplemented with telephone follow up for missing calendars. Reported falls were followed up with a telephone interview to record the circumstances of the fall and any resulting injuries and subsequent treatment. Interviews were completed for 96.3% of reported falls." |
Duque 2013.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 9 months | |
| Participants | Setting: Penrith, Australia Number of participants: 60 Number analysed: 60 Number lost to follow‐up: 0 Sample: community‐dwelling people attending the Falls and Fractures Clinic, Nepean Hospital Age (years): Intervention mean = 79.33 (SD 10), control mean = 75 (SD 8) Sex: 62% female Inclusion criteria: fallen within 6 months of assessment, poor performance in balance assessed using posturography component of the Balance Rehabilitaion Unit (BRU) virtual reality system Exclusion criteria: severe visual impairment, inability to walk independently with a cane or walker, inability to stand unaided for 60 secs, score of < 22/30 in MMSE, PD or any neuromuscular conditions, Geriatric Depression Scale (GDS) > 8/15, inability to understand or answer the study questionnaires |
|
| Interventions | 1. Virtual reality balance training: performed in standing, 30‐minute session, 2 a week, 6 weeks 2. Control group: usual care, general recommendations and care plan on falls prevention |
|
| Outcomes | 1. Rate of falls | |
| Duration of the study | 36 weeks | |
| Adherence | Adherence not defined. Proportion that progressed through levels reported: 1. Virtual reality balance training group: 97%; most of the participants (91%) reached ≥ 10/15 possible levels in every group of virtual exercises |
|
| Notes | Source of funding: Nepean Medical Research Foundation, Department of Geriatric Medicine at Nepean Hospital
Economic information: not reported Email communication regarding fall data, response received, data not included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Fall outcomes were recorded using the same method in both groups Quote: "to prevent any assessment bias, different physiotherapists with no access to the subjects’ data were specifically assigned to perform either assessment or training" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | High risk | Fall data were collected but number of fallers was not reported. Adverse events not reported |
| Method of ascertaining falls (recall bias) | High risk | The occurrence of falls was retrospectively assessed by asking the participant (1) whether they have suffered a fall, and (2) the number of falls during the 6 months prior to the assessment |
Ebrahim 1997.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 24 months (also 12 months) | |
| Participants | Setting: London, UK Number of participants: 165 Number analysed: 102 Number lost to follow‐up: 63 Sample: community‐dwelling women Age (years): Intervention mean = 66.4 (SD 7.8), Control mean = 68.1 (SD 7.8) Sex: 100% female Inclusion criteria: postmenopausal women who had sustained a fracture in the upper arm in the past 2 years recruited from 2 East London Hospitals Exclusion criteria: women being treated with bisphosphonates, if expected survival was < 1 year, cognitive impairment, too frail to withstand brisk walking or travelling for measurements |
|
| Interventions | 1. Individual Brisk Walking: intensity progressed, monthly telephone contact, advice from nurse about general health and balanced diet, walked 40 minutes, 3 a week, 2 years 2. Control group: simple upper limb exercises, monthly telephone contact, advice from nurse about general health and balanced diet |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who experienced 1 or more fall‐related fractures |
|
| Duration of the study | 2 years | |
| Adherence | Adherence not defined. Participation in programme reported: 1. Individual Brisk Walking group: adherence not defined, 49/81 (60.5%) continued programme, with all remaining participants exercising ≥ 40 min, 3 a week 2. Control group: adherence not defined, 48/84 (57.14%) continued programme |
|
| Notes | Source of funding: The Wolfson Family Trust Economic information: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned" using "computer generated" allocation |
| Allocation concealment (selection bias) | Unclear risk | Series of prepared envelopes but did not mention "opaque" or "sealed" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blind to allocated group. Research personnel were not blind to group, yet delivered the intervention to both groups and assessed fall outcome, which increases the risk of bias |
| Blinding of outcome assessment (detection bias) Falls | High risk | Falls ascertained by the same method in both groups. The research nurse delivering intervention to groups also conducted the monthly telephone calls to monitor the occurrence of falls, therefore was not blinded |
| Blinding of outcome assessment (detection bias) Fractures | Low risk | Fracutres were assessed in all groups using radiological examination, by personnel blinded to group allocation |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall outcome data are missing (38%). Loss of fall data was unbalanced in intervention (n = 17) and control (n = 12) groups; reason for loss of fall data was unclear |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Unclear risk | Monthly telephone calls |
El‐Khoury 2015.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 24 months | |
| Participants | Setting: France Number of participants: 706 Number analysed: 706 Number lost to follow‐up: 0 Sample: community‐dwelling women Age (years): Intervention mean = 79.8 (SD 2.8), Control mean = 79.6 (SD 2.8) Sex: 100% female Inclusion criteria: Women aged 75 ‐ 85 living in the community, diminished balance or gait capacities (assessed by 6 m walking time and tandem walk test) Exclusion criteria: > 12.5 seconds to walk 6 m, unable to stand for 10 sec with feet together, medical conditions precluding exercise, expected to move away in next 6 months, difficulty attending exercise classes regularly, already attending exercise classes |
|
| Interventions | 1. Group‐based balance and strength, 1 hour a week for 2 years, plus tailored home practice performed weekly 2. Control group: no intervention, offered 4 exercise sessions at end of trial Both groups offered fall prevention brochures and newsletters |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who died |
|
| Duration of the study | 104 weeks | |
| Adherence | Adherence measured by programme attendance 1. Group‐based balance and strength group: 58/352 (16%) never started the programme; 38/352 (11%) attended a few classes in the first month only |
|
| Notes | Source of funding: “Assistance Publique‐Hôpitaux de Paris” (AP‐HP), French Ministry of Health, French National Research Agency, National Institute of Health Prevention and Education, Council of the Ile‐de‐France region Economic information: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation lists were computer generated, based on randomly permuted blocks of varying size (2, 4 or 6, randomly sampled with equal probability)…stratified for study centre and body weight" |
| Allocation concealment (selection bias) | Low risk | Baseline assessment and randomisation lists installed on assessors laptop, where Quote: "at the end of the baseline examination, the programme automatically determined the eligibility of each woman, based on her examination results; if she was eligible and agreed to participate, it randomly assigned her into the experimental intervention or the control group" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls ascertained by the same method in both groups Quote: "Investigator blinded to group assignment" phoned those who reported falls |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | In both groups Quote: "if a fracture of admission to hospital was reported, a copy of the radiologist's report or medical record was requested to confirm the severity of the injuries". Blinding of assessor unclear |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | Low risk | Fall outcomes prespecified in prospective trial registratio were reported, adverse events reported |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "Participants were asked to mark the exact date of any fall on pre‐addressed, prepaid monthly calendar postcards, and to return the cards at the end of the corresponding month". A blinded assessor telephoned those who reported falls. |
Fiatarone 1997.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 4 months | |
| Participants | Setting: USA Number of participants: 34 Number analysed: no fall data Sample: frail older people Age (years): mean 82 (SD 1) Sex: 94% female Inclusion criteria: community‐dwelling older people; moderate to severe functional impairment Exclusion criteria: none given |
|
| Interventions | 1. Individual high‐intensity progressive resistance training, 11 different upper and lower limb exercises with arm and leg weights, 2 weeks instruction and then weekly phone calls, performed 3 a week, 16 weeks 2. Control: wait‐list control. Weekly phone calls | |
| Outcomes | Reported number of people sustaining 1 or more adverse effects of intervention | |
| Duration of the study | 16 weeks | |
| Adherence | Not reported | |
| Notes | Source of funding: not reported
Economic information: not reported Abstract only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Insufficient information to permit judgement. |
| Selective reporting (reporting bias) | High risk | Falls not mentioned in Methods, fall outcome mentioned in results, adverse events not reported |
| Method of ascertaining falls (recall bias) | Unclear risk | Interval recall. Falls identified weekly by phone call |
Freiberger 2007.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 24 months | |
| Participants | Setting: Erlangen, Germany Number of participants: 134 Number analysed: 127 Number lost to follow‐up: 7 Sample: community‐dwelling Age (years): mean 76.1 (SD 4.1) Sex: 44% female Inclusion criteria: ≥ 70 years, fallen in past 6 months, fear of falling, signed informed consent, completing baseline assessment Exclusion criteria: unable to walk independently, cognitive impairment (< 25 on the Digit Symbol Substitution Test) |
|
| Interventions | Randomised into 3 groups: 2 intervention groups (group‐based psychomotor programme and group‐based balance, strength, flexibility, endurance) and 1 control group. Only the 2 intervention groups were included in this review 1. Group‐based psychomotor programme: strength training using dumbbells, free weights and body weight, increasing difficulty of balance exercises, motor co‐ordination, competence training, perceptual training, and home exercises; sessions 1 hour, 2 a week for 16 weeks 2. Group‐based balance, strength, flexibility, endurance: strength training using dumbbells, free weights and body weight, plus home exercises; sessions 1 hour, 2 a week for 16 weeks |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by session attendance 1. Group‐based psychomotor programme: 82% attended at least 24/32 sessions 2. Group‐based balance, strength, flexibility, endurance group: 84% attended at least 24/32 sessions |
|
| Notes | Source of funding: The Robert Bosch Foundation, Siemens Health Insurance Economic information: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐number generator |
| Allocation concealment (selection bias) | Unclear risk | Quote: "All randomizations were concealed". No other information given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Falls ascertained by the same method in both groups. Blinding of assessors performing the telephone interview was not specified |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall outcome data are missing (5%). Loss of fall data was balanced in the balance programme (n = 4) and psychomotor programme (n = 3) groups; reason for loss of fall data was unclear |
| Selective reporting (reporting bias) | High risk | Fall data were collected but number of falls was not reported. Adverse events not reported |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "falls were collected prospectively using a monthly fall calendar between months 12 and 24; fall sheets were mailed in at the end of the month. Up to five follow‐up telephone calls were made in the event of no response after each month. If falls were reported, details were collected during a structured telephone interview" |
Gill 2016.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 42 months | |
| Participants | Setting: USA Number of participants: 1635 Number analysed: 1635 Number lost to follow‐up: 0 Sample: community‐dwelling Age (years): Intervention mean = 78.7 (SD 5.2), control mean = 79.1 (SD 5.2) Sex: 67% female Inclusion criteria: aged 70 ‐ 89 years, < 20 minutes/week structured exercise in past month and < 125 minutes/week of moderate physical activity, short physical performance battery score ≤ 9 out of 12, could walk 400 m in 15 minutes or less without assistance or aid, no major cognitive impairment, safely participate in the intervention as determined by medical history, physical exam, and electrocardiography Exclusion criteria: not reported |
|
| Interventions | 1. Group‐ and home‐based balance, strength, flexibility and walking training: individualised and progressed, used ankle weights for strength training; 1‐hour sessions, 2 a week, home exercises 3 ‐ 4 a week for 24 ‐ 42 months depending on time of enrolment 2. Control group: attended weekly health education group for 26 weeks and monthly sessions thereafter, plus 5 ‐ 10 minutes stretching exercises |
|
| Outcomes | 1. Number of people who experienced 1 or more fall‐related fractures 2. Number of people who experienced 1 of more falls requiring hospital admission 3. Number of people who died |
|
| Duration of the study | Up to 168 weeks | |
| Adherence | Adherence measured by attendance at sessions 1. Group‐ and home‐based balance, strength, flexibility and walking training group: attended mean of 63% of scheduled sessions, median 71% (interquartile range 50 ‐ 83%) 2. Control: attended mean of 73% of the scheduled sessions, median 82% (63 ‐ 90%) |
|
| Notes | Source of funding: National Institute of Health, National Institute of Aging, National Heart, Lung and Blood Institute Economic information: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomised..through a secure web based data management system using a permuted block algorithm (with random block lengths) stratified by field center and sex" |
| Allocation concealment (selection bias) | Low risk | Quote: "Secure web based data management system" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Fractures | Low risk | Question by blinded assessor: Quote: “did a doctor tell you that you fractured or broke a bone?” If yes, Quote: "Two experts blinded to group randomization subsequently reviewed and adjudicated independently relevant medical records, including those from all hospital admissions.” A fall‐related fracture required the fulfilment of 4 prespecified criteria |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Low risk | Quote: "Two experts blinded to group randomization subsequently reviewed and adjudicated independently relevant medical records, including those from all hospital admissions.” |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | No fall data |
| Selective reporting (reporting bias) | High risk | The question "have you fallen?" was asked but was not prespecified |
| Method of ascertaining falls (recall bias) | High risk | Questioned by blinded assessors every 6 months: Since (last visit date), did a doctor tell you that you fractured or broke a bone? (If yes) Did you break a bone as a result of a fall? and Other than the conditions we just asked you about, were you admitted to a hospital overnight for any other reasons since (last visit date)? Since (last visit date), have you fallen? Did this fall result in an inability to leave home for at least one week? |
Grahn Kronhed 2009.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months | |
| Participants | Setting: Linköping, Sweden Number of participants: 65 Number analysed: 65 Number lost to follow‐up: 0 Sample: women with osteoporosis identified from Linköping Hospital, Osteoporosis Unit files Age (years): mean 71.4, range 60 to 81 Sex: 100% female Inclusion criteria: BMD measured within previous 9 months and T‐score ≤ −2.5 SD Exclusion criteria: enrolled in a pharmacological RCT; requiring indoor walking aids; cognitively impaired (MMSE < 20); severe heart disease, malignancy, recent arthroplasty, unhealed fractures; unable to understand Swedish |
|
| Interventions | 1. Group‐based strength and balance training: supervised and progressed using body weight, pulleys, leg press, exercises on balance boards and weight shifting on trampoline; 1 hour, 2 a week for 4 months 2. Control: no intervention. Instructed not to change exercise routines for 1 year | |
| Outcomes | 1. Rate of falls 2. Health‐related quality of life |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by completion of sessions 1. Group‐based strength training group: completed mean of 24/30 sessions (median = 25, range 13 ‐ 30) |
|
| Notes | Source of funding: Östergötland County Council and the Faculty of Health Sciences, Linköping University, Region Västra Götaland, the Stohne’s foundation, and Sanofi‐AventisÖstergötland County Council and the Faculty of Health Sciences, Linköping University, Region Västra Götaland, the Stohne’s foundation, and Sanofi‐Aventis
Economic information: not reported No participants sustained a fracture during follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method not described but assume it was truly random, given that Quote: "an independent statistical unit randomised the participants" |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent statistical unit randomized the participants" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls ascertained by the same method in both groups Quote: "… participants were followed‐up concerning … falls … for 1 year by the independent statistical unit." Probably blind to allocated group or at least unlikely to introduce bias. |
| Blinding of outcome assessment (detection bias) Fractures | High risk | Participant‐reported fractures with no description of confirmation |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants not blind to allocated group |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | High risk | Fall data obtined but number of fallers not reported. Adverse events not reported |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "... participants reported number of falls each week for the 1‐year study period" |
Gschwind 2015.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months | |
| Participants | Setting: Cologne, Germany; Valencia, Spain; Sydney, Australia Number of participants: 153 Number analysed: 136 Number lost to follow‐up: 17 Sample: community‐dwelling Age (years): mean 74.7 (SD 6.3) Sex: 61% female Inclusion criteria: ≥ 65 years, living in the community, able to walk 20 m without a walking aid, able to watch television ± glasses from 3 m distance, have enough space for system use (3.5 m2) Exclusion criteria: insufficient language skills to understand the study procedures, cognitive impairment, medical conditions precluding participation in a regular exercise programme (i.e. uncontrolled hypertension, severe neurological disorder, acute cancer, psychiatric disorder, acute infection) |
|
| Interventions | 1. Individual balance and strength training using exergames: home programme of balance exercises (Weight‐bearing Exercise for Better Balance (WEBB) programme (www.webb.org.au) + technology exergames and feedback, 40‐minute sessions, 3 a week, and progressive strengthening exercises based on the Otago Exercise Programme, 15 ‐ 20 minute sessions, 3 a week for 16 weeks 2. Control group: no intervention |
|
| Outcomes | 1. Rate of falls 2. Health‐related quality of life |
|
| Duration of the study | 24 weeks | |
| Adherence | Adherence was monitored automatically by iStopFalls system 1. Individual balance and strength training using exergames groups: used the iStopFalls system 42 times (median, IQR = 3.9) for a total duration of 11.7 hours (median, IQR = 22.0) |
|
| Notes | Source of funding: European Union's Seventh Framework Program, NHMRC
Economic information: not reported Email communication regarding fall data, response received, data not included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Were randomised by permuted block‐ randomisation (ratio1:1) using a unique computer‐generated random number for identification. Participants who lived in the same household were treated as one unit and randomised into the same block" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls ascertained by the same method in both groups Quote: "Falls frequency .. monitored with monthly diaries for 6 months. Participants were contacted by phone when the diaries were not returned." "Staff performing the assessments was.. blinded to group allocation" It is likely, although not certain, that staff conducting follow‐up calls were blinded to group |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Low risk | In both groups Quote: "falls frequency and adverse events were monitored with monthly diaries for 6 months". "Staff performing the assessments was.. blinded to group allocation" It is likely, although not certain, that staff conducting follow‐up calls were blinded to group |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants were unblinded to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall outcome data are missing (11%). Loss of fall data was balanced in the intervention (n = 7) and control (n = 10) groups; reason for missing data was unclear |
| Selective reporting (reporting bias) | High risk | Fall data obtined but number of fallers not reported |
| Method of ascertaining falls (recall bias) | Low risk | Falls frequency and adverse events were monitored with monthly diaries for 6 months Participants were contacted by phone when the diaries were not returned |
Haines 2009.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months | |
| Participants | Setting: Brisbane, Australia Number of participants: 53 Number analysed: 53 Number lost to follow‐up: 0 Sample: patients in geriatric rehabilitation, medical, or surgical units in Princess Alexandra Hospital Age (years): mean 80.7 (SD 7.7) Sex: 60% female Inclusion criteria: aged > 65 years; gait instability or walking with a mobility aid; discharged from hospital to community‐dwelling Exclusion criteria: unstable severe cardiac disease; cognitive impairment; aggressive behaviour; restricted weight‐bearing status; referred for post‐discharge community rehabilitation services |
|
| Interventions | 1. Home‐based strength and balance programme with DVD/workbook: lower limb strength and balance exercises with 6 levels of difficulty, 3 ‐ 7 a week. DVD player provided if required. At least 1 home visit from project PT, then telephone contact weekly for 8 weeks, then 18 weeks without active encouragement 2. Control: did not receive programme materials, visits or telephone calls | |
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Health‐related quality of life 4. Number of people who died |
|
| Duration of the study | 26 weeks | |
| Adherence | Exercise group: exercise adherence monitored by weekly phone calls by the physio for 8 weeks
Week 1: N = 15 exercised ≥ 1, N = 12 exercised ≥ 2/week
Week 2: N = 15 exercised ≥ 1, N = 11 exercised ≥ 2/week
Week 3: N = 13 exercised ≥ 1, N = 8 exercised ≥ 2/week
Week 4: N = 12 exercised ≥ 1, N = 9 exercised ≥ 2/week Week 5; N = 11 exercised ≥ 1, N = 8 exercised ≥ 2/week Week 6: N = 9 exercised ≥ 1, N = 4 exercised ≥ 2/week |
|
| Notes | Source of funding: Queensland Health, Allied Health Advisory, Community Rehabilitation Workforce Project Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random allocation sequence was generated by an investigator (TH) using a computerized random number generator” |
| Allocation concealment (selection bias) | Low risk | Quote: “This sequence was entered into sealed, consecutively numbered, opaque envelopes. Each envelope corresponding to the participants study number (allocated in the order in which participants consented to participate in the study) was opened following completion of the baseline assessment. The envelopes containing the allocation sequence were secured within a locked office.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to intervention, effect of not blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Quote: “All participants received monthly follow‐up phone calls from the blinded outcome assessor” |
| Blinding of outcome assessment (detection bias) Fractures | High risk | The only evidence for fractures was from self‐reports from participants |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | High risk | Number of falls resulting in medical review (GP or hospital medical officer or emergency department) were self‐reports |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants not blindde to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall outcome data are missing (6%). Loss of data was due to 3 deaths in the control group. Unlikely this was linked to outcome |
| Selective reporting (reporting bias) | Low risk | Prespecified fall and adverse event outcomes reported. Trial prospectively registered |
| Method of ascertaining falls (recall bias) | Low risk | Quote: “Participants in both groups were provided with a log for recording falls and details surrounding them.” “All participants received monthly follow‐up phone calls from the blinded outcome assessor.” |
Halvarsson 2013.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 15 months | |
| Participants | Setting: Stockholm, Sweden Number of participants: 59 Number analysed: 48 Number lost to follow‐up: 11 Sample: community‐dwelling Age (years): mean 77 (range 67 ‐ 93) Sex: 71% female Inclusion criteria: ≥ 65 years, fear of falling or an experience of a fall during the previous 12 months, or both, ability to walk unaided indoors and a MMSE score ≥ 24 Exclusion criteria: severely impaired vision or hearing, severe cancer, severe pain, neurological disease or damage with symptoms, dizziness requiring medical care, or heart and respiratory problems that might affect participation |
|
| Interventions | 1. Group‐based progressive balance training: 45 minute sessions, 3 a week for 12 weeks 2. Control group: usual activities and offered intervention following the study period |
|
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 65 weeks | |
| Adherence | Adherence measured by attendance at sessions 1. Group‐based progressive balance training group: 71 – 100% (n = 24 ‐ 36), mean 87% (n = 31) |
|
| Notes | Source of funding: Stockholm County Council and Karolinska Institute, the Torsten and Ragnar Söderberg Foundation, and Johanniterorden, Sister Kenny Foundation in Minneapolis Economic information: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization to group allocation was done in blocks, with a 2:1 ratio in favor of the intervention group, by the subjects themselves drawing a allocation slip" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | High risk | Quote: "were told not to reveal group allocation to the assessors. However, most of the participants did reveal which group they belonged to at the time of the first follow‐up, resulting in non‐masked assessors at long‐term follow‐up" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of fall outcome data are missing (19%). Loss of fall data was unbalanced in intervention (n = 8) and control (n = 3) groups; reason for loss of fall data was unclear |
| Selective reporting (reporting bias) | High risk | Falls measured but number of falls not reported. Adverse events not reported |
| Method of ascertaining falls (recall bias) | High risk | Quote: "Fall frequency was assessed at baseline and during the time between the follow‐ups by asking the participants to recall if they had fallen during the last year" |
Halvarsson 2016.
| Methods | Study design: RCT Number of study arms: 3 Length of follow‐up: 3 months | |
| Participants | Setting: Stockholm, Sweden Number of participants: 96 Number analysed: 69 Number lost to follow‐up: 27 Sample: community‐dwelling Age (years): Intervention mean 76 (range 67 ‐ 86), Control mean 75 (range 66 ‐ 84) Sex: 98% female Inclusion criteria: age ≥ 65 years afraid of falling or having experienced at least one fall in the last 12 month s, or both, and independence in ambulation Exclusion criteria: fractures during the last year, MMSE score < 24, severely decreased vision, or other diseases or constraints that might interfere with participation in the exercise programme |
|
| Interventions | 1. Group‐based progressive balance training: supervised and tailored exercises, 45 minute sessions, 3 a week for 12 weeks 2. Group‐based progressive balance training plus walking: supervised and tailored exercises, 45‐minute sessions, 3 a week for 12 weeks, plus walking (preferably with poles) for ≥ 30 minutes, 3 a week for 12 weeks 3. Control group: no intervention, offered the same balance training at the end of the study |
|
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 60 weeks | |
| Adherence | Adherence measured in sessions attended Participants attending ≥ 66% sessions included in follow‐up. Adherence rate to the training sessions was 89% (range 66 ‐ 100%) 2. Group‐based progressive balance training plus walking: all except 1 participant fulfilled the added physical activity intervention |
|
| Notes | Source of funding: Stockholm County Council, Karolinska Institutet (ALF), Swedish Research Council, Health Care Sciences Postgraduate School at Karolinska Institutet
Economic information: not reported 3‐month data used due to proportion of fallers not being clear for longer follow‐up period |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomised...using web‐based software" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blind to allocated group. Research personnel were not blind to group, yet delivered the intervention to both groups and assessed fall outcome, which increases the risk of bias |
| Blinding of outcome assessment (detection bias) Falls | High risk | Quote: "The test leaders were blinded to group allocation at baseline; however, it was no longer possible after baseline testing, because some of the test leaders were also involved in the balance training" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of fall outcome data are missing (3%). Loss of fall data was unbalanced in balance (n = 9) balance + walking (n = 13) and control (n = 5) groups; reason for loss of fall data was unbalanced |
| Selective reporting (reporting bias) | High risk | Falls measured but number of falls not reported. |
| Method of ascertaining falls (recall bias) | High risk | Quote: "Participants reported .. at each follow‐up whether they had fallen during the time since the previous follow‐up session". Follow‐up was at 3, 9 and 15 months |
Hamrick 2017.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months | |
| Participants | Setting: Wisconsin, USA Number of participants: 43 Number analysed: 38 Number lost to follow‐up: 5 Sample: community‐dwelling Age (years): mean 69.9 (range 60 ‐ 88) Sex: 79% female Inclusion: 60 years and older; able to walk 150 feet without assistive devices; cognitively intact as evidenced by correct answers to the Memory Impairment Screen; able to provide informed consent Exclusion criteria: pelvic or lower extremity injury in the previous 6 months that required temporary use of an assistive device, including crutches, for > 7 days; inability to provide informed consent; neurologic condition that impairs strength or balance including herniated lumbar disc with nerve root compression, previous stroke with residual lower extremity weakness, Parkinson’s Disease, multiple sclerosis, muscular dystrophy and other neuromuscular diseases; cardiac or other medical condition with previous physician instructions to avoid low‐intensity exercise; terminal condition with rapid progression of disease and not expected to live > 6 months; pelvic or lower extremity orthopaedic surgery in the previous 12 months.; practised yoga at home or in a classroom setting in the past 6 months |
|
| Interventions | 1. Home‐exercise group: instructed to practice 3 yoga home poses for 10 minutes + 5 minutes of relaxation (breathing techniques) daily for 8 weeks 2. Relaxation group: instructed to practice 5 minutes of relaxation daily for 8 weeks Both groups attended 60‐minute yoga classes, 2 a week for 8 weeks |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) |
|
| Duration of the study | 26 weeks | |
| Adherence | Attendance in the 16 yoga sessions was 92% | |
| Notes | Source of funding: Wisconsin Partnership Program Economic information: not reported Email communication to obtain fall data, response received, data included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned 1:1 by concealed allocation at enrollment". Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Participants were randomly assigned 1:1 by concealed allocation at enrollment". Method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and yoga instructors were not blinded to group allocation, but the impact of non‐blinding is unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Quote: "participants underwent assessment at baseline and within 1 week of completing the classes by one of the authors who was blinded to participant home exercise assignment. We conducted a telephone survey about falls ... 2 months and 4 months after completing the class" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall outcome data are missing (11%). Loss of fall data was balanced in the treatment groups |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | High risk | Logs were given to inspire tracking of falls but logs were not collected. Telephone survey about falls 2 months and 4 months after completion of the intervention |
Hauer 2001.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months | |
| Participants | Setting: Germany Number of participants: 57 Number analysed: 56 Number lost to follow‐up: 1 Sample: women recruited at the end of ward rehabilitation in a geriatric hospital Age (years): mean 82 (SD 4.8), range 75 ‐ 90 Sex: 100% female Inclusion criteria: ≥ 75 years; fall(s) as reason for admission to hospital or recent history of injurious fall leading to medical treatment; residing within study community Exclusion criteria: acute neurological impairment; severe cardiovascular disease; unstable chronic or terminal illness; major depression; severe cognitive impairment; musculoskeletal impairment preventing participation in training regimen; falls known to be due to a single, identifiable disease, e.g. stroke or hypoglycaemia |
|
| Interventions | 1. Group‐based progressive strength and balance training: gym equipment, pulleys and body weight used for 'high‐intensity' progressive strength training; 45‐minute sessions, 3 a week, for 12 weeks
2. Control group: flexibility, calisthenics, ball games, and memory tasks while seated, 60‐minute sessions, 3 a week, for 12 weeks Both groups also received identical physiotherapy with balance and strength training components excluded (25 mins, 2 a week) |
|
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 26 weeks | |
| Adherence | Adherence was measured in training lists 1. Group‐based strength and balance training group: 23/31 completed study, 85.4% adherence 2. Control group: 22/26 completed study, 84.2% adherence |
|
| Notes | Source of funding: Ministerium für Wissenschaft, Forschung und Kunst Baden‐Wuerttemberg, University of Heidelberg Economic information: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear whether participants were blinded, but control group received placebo activities and both groups received identical physiotherapy sessions |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls ascertained by the same method in both groups. Staff documenting falls were blinded to group assignment |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Method of determining adverse events was not described |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall outcome data are missing (2%). 1 control participant had no fall data due to moving residence |
| Selective reporting (reporting bias) | Unclear risk | Prespecified falls outcomes reported. Adverse events reported but not prespecified. No trial protocol or prospective trial registration |
| Method of ascertaining falls (recall bias) | Low risk | Prospective. Daily diaries collected every 2 weeks |
Helbostad 2004.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months | |
| Participants | Setting: 6 local districts in Trondheim, Norway
Number of participants: 77
Number analysed: 68
Number lost to follow‐up: 9
Sample: volunteers recruited through newspapers and invitations from health workers
Age (years): mean 81 (SD 4.5)
Sex: 81% female Inclusion criteria: aged ≥ 75; fallen in last year and / or using walking aid indoors or outdoors Exclusion criteria: exercising 1 or more times weekly; terminal illness; cognitive impairment (MMSE < 22); recent stroke; unable to tolerate exercise |
|
| Interventions | 1. Combined group and home‐based balance and strength training: individually‐tailored progressive resistance exercises, functional balance training, 1 hour sessions, 2 x ar week, for 12 weeks + home exercises as below (2) 2. Individual home‐balance and strength training: 4 non‐progressive functional balance and strength exercises using own body weight, 2 a day, for 12 weeks, plus 3 education group meetings |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1or more falls (risk of falling) | |
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured as sessions participated, frequency of home sessions 1. Group‐ and home‐based balance and strength training: mean training sessions participated 21/24 (range 14 ‐ 24); mean home training sessions completed a day 1.35 (SD = 0.51) 2. Individual balance and strength training: mean group meetings participated 2.5/3 (range 0 ‐ 3); mean home training sessions completed a day 1.29 (SD = 0.54) |
|
| Notes | Source of funding: Norwegian Foundation for Research in Physiotherapy, Norwegian Research Council, University of Bergen Economic information: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised into one of two exercise programs" |
| Allocation concealment (selection bias) | Low risk | Randomised by independent research office using sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Cluster‐randomised trial comparing 2 types of exercise intervention. Low risk of performance bias |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls ascertained by the same method in both groups. Assessors blind to participants' assignment |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall outcome data are missing (12%). Loss of fall data was balanced in the home training (n = 4) and combined training (n = 5) groups. Reasons for data loss were balanced in the 2 groups |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Low risk | Monthly falls diary (prepaid postcard), telephone call if no response or fall reported |
Hirase 2015.
| Methods | Study design: RCT Number of study arms: 3 Length of follow‐up: 4 months | |
| Participants | Setting: Nagasaki and Unzen, Japan Number of participants: 93 Number analysed: 86 Number lost to follow‐up: 7 Sample: community‐dwelling Age (years): Foam rubber intervention mean = 82.1 (SD 5.5),sStable surface intervention mean = 82.0 (SD 5.7), Control group: 82.2 (SD 6.3) Sex: 70% female Inclusion criteria: > 65 years, living at home, able to walk with or without a cane, assessed to be at high falls risk (≥ 4 risk factors using falls assessment questionnaire) Exclusion criteria: participated in exercise ≥ 4 a month before the intervention, musculoskeletal, neurological, or cardiovascular disorders that may be aggravated by exercise, unable to respond to interview questions because of cognitive impairment |
|
| Interventions | 1. Group‐based balance training on foam rubber pad: 10 exercises performed in a standing position, 60‐minute sessions, weekly for 4 months; plus 3 home‐based exercises performed daily 2. Group‐based balance training on stable flat surface: same balance training programme as foam rubber mat group but performed on a stable flat surface; 60‐minute sessions, weekly for 4 months; plus 3 home‐based exercises performed daily 3. Control group: weekly social programmes at a day centre for 4 months |
|
| Outcomes | 1. Rate of falls | |
| Duration of the study | 16 weeks | |
| Adherence | Adherence measured as class attendance, frequency of home programme 1. Group‐based balance training on foam rubber pad: 96% attendance of all possible classes. Performed the home‐based exercise programme 3.5 (SD: 2.0) days a week 2. Group‐based balance training on stable flat surface: 93% attendance of all possible classes. Performed the home‐based exercise programme 3.4 (SD: 2.3) days a week 3. Control group: 91% attendance of all possible programmes |
|
| Notes | Source of funding: NR, Department of Locomotive Rehabilitation Science, Unit of Rehabilitation Sciences, Graduate School of Biomedical Sciences, Nagasaki University Economic information: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Process not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "using the sealed envelope method" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | High risk | Quote: "The number of additional falls was recorded every week by a physical therapist working in each day center"" "Physical therapists working in the day centers assessed the participants and implemented the intervention program." Assume assessors not blinded |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall outcome data are missing (7%). Loss of fall data was balanced in the groups (n = 3 in foam rubber group, n = 2 in stable and control groups), with all withdrawals due to hospital admission |
| Selective reporting (reporting bias) | High risk | Falls measured, but number of fallers not reported. Adverse events not reported |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "a diary with a monthly sheet to record the number of additional falls during the follow‐up period. The number of additional falls was recorded every week by a physical therapist working in each day center" |
Huang 2010.
| Methods | Study design: Cluster RCT Number of study arms: 2 Number of clusters: 4 (2 clusters included in this review) Length of follow‐up: 5 months | |
| Participants | Setting: Taipei, Taiwan Number of participants: 115 Number analysed: 78 Number lost to follow‐up: 37 Sample: people registered as living in 4 randomly‐selected villages Age (years): mean 71.5 (SD 0.6) in people not lost to follow‐up Sex: 30% female Inclusion criteria: aged > 65 years; living in a non‐organised community of Taiwan Exclusion criteria: immobile; living outside registered living area |
|
| Interventions | Randomised into 4 groups: 3 intervention groups (1 group‐based Tai Chi, 1 education group, 1 Tai Chi plus education group) and 1 control group. Only group‐based Tai Chi and control groups included in this review 1. Group‐based Tai Chi: 13 simple movements, 40‐minute sessions, 3 a week for 20 weeks 2. Control group: usual care |
|
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) 2. Health‐related quality of life |
|
| Duration of the study | 20‐72 weeks | |
| Adherence | Not reported | |
| Notes | Source of funding: The National Science Council, Taiwan
Economic information: not reported Reported results not adjusted for clustering. Raw data at 5 months used in the review and adjusted for clustering. No raw data for 18 months so not possible to adjust for clustering. Number of clusters allocated to intervention: 1; number of clusters allocated to control: 1; number of clusters analysed (intervention): 1; number of clusters analysed (control): 1 Email communication regarding fall data, response received, data not included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The three intervention groups and one control group were then assigned randomly to one each of the four selected villages." |
| Allocation concealment (selection bias) | High risk | Individual participant recruitment was undertaken after group allocation of the 4 villages. There was no mention of active blinding of research team members recruiting participants |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Insufficient information to determine how falls were monitored in each group or whether assessors were blinded |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants were not blinded to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall outcome data are missing (32%). Loss of fall data was unbalanced in the Tai Chi (n = 34) and control (n = 3) groups, with the reasons for withdrawal not clear |
| Selective reporting (reporting bias) | High risk | Falls measured, but number of falls not reported. Adverse events not reported |
| Method of ascertaining falls (recall bias) | Unclear risk | No mention of how falls were monitored Quote: "The fall or non‐fall situation was checked at preintervention, postintervention and at one and half year later with the aim of examining the effectiveness of the interventions" |
| Cluster‐randomised trials | Unclear risk | Individuals were recruited to the trial after the clusters were randomised and personnel recruiting participants were not blind to cluster; clusters were not comparable at baseline for gender or education level; missing outcomes for clusters or within clusters were not reported; did not account for clustering in analysis; results comparable with individually randomised trials |
Hwang 2016.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 18 months |
|
| Participants | Setting: Taipei, Taiwan Number of participants: 456 Number analysed: 334 Number lost to follow‐up: 122 Sample: community‐dwelling Age (years): mean 72 Sex: 67% female Inclusion criteria: aged ≥ 60 who received fall‐related medical attention ‐ an older person was presumed to have recovered from a fall injury within 6 months and who could walk independently were invited by telephone to enrol in the study and participate in the baseline assessment Exclusion criteria: major unstable cardiopulmonary disease (ischaemic chest pain or shortness of breath on mild exertion), cognitive impairment (MMSE score < 24), and contraindications to physical exercise (e.g. severe arthritis that limits exercise capability) |
|
| Interventions | 1. Individually‐supervised Tai Chi: taught individually each week for 24 consecutive weeks, 60‐minute sessions, 1 a week for 6 months 2. Individually‐supervised balance and strength training: exercises at increasing difficulty levels using own body weight; 60‐minute sessions, 1 a week for 6 months |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who died |
|
| Duration of the study | 72 weeks | |
| Adherence | Adherence measured as participation in sessions 1. Individually‐supervised Tai Chi group: 145 (78%) people participated in 20 or more sessions 2. Supervised balance and strength training group: 132 (72%) people participated in 20 or more sessions |
|
| Notes | Source of funding: National Health Research Institute, Ministry of Science Technology Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Statisticans using computer‐generated sequence; block‐randomised in groups of 8 |
| Allocation concealment (selection bias) | Low risk | Using an automated secure website operated by an off‐site independent service |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Research assistants who conducted fall‐related phone calls were blinded to allocation |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall outcome data are missing (27%) |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "Falls were prospectively monitored and recorded daily using a diary, and these records were mailed monthly to the study coordinator." "When a participant failed to return the diary or provided incomplete data, two research assistants blinded to the group assignment provided telephone reminders, making a maximum of five calls. Monthly follow‐up of fall records was continued in participants who were unavailable for certain periods". |
Iliffe 2015.
| Methods | Study design: Cluster‐RCT Number of study arms: 3 Number of clusters: 42 Length of follow‐up: 18 months |
|
| Participants | Setting: London and Nottingham, UK Number of participants: 1254 Number analysed: 709 Number lost to follow‐up: 545 Sample: community‐dwelling Age (years): mean 73 (range 65 ‐ 94) Sex: 62% female Inclusion criteria: ≥ 65 years, registered with participating general practices, living independently (not in residential or nursing homes), physically able to attend group exercise Exclusion criteria: ≥ 3 falls in the past year, ≥ 150 minutes of moderate‐vigorous physical activity a week, uncontrolled medical conditions and significant cognitive impairment |
|
| Interventions | 1. Individual Otago Exercise Programme: leg strengthening, balance exercises and walking plan, 30 minute, 3 a week for 24 weeks 2. Group‐based FaME plus home training based on Otago Exercise Programme: leg and trunk strengthening, balance, flexibility, functional floor skills, walking plan, 1‐hour group session a week for 24 weeks + 30‐minute home exercises sessions, 2 a week for 24 weeks 3. Control group: no intervention |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Health‐related quality of life 4. Number of people who died |
|
| Duration of the study | 96 weeks | |
| Adherence | Adherence measured as home sessions completed, or class attendance 1. Individual Otago Exercise Programme: 149 (37%) participants reported they achieved ≥ 75% of the home exercise prescription (90 minutes a week) 2. Group‐based FaME plus home training based on Otago Exercise Programme: 150 participants (40%) attended 75% (or more) of classes |
|
| Notes | Source of funding: Health Technology Assessment programme of the National Institute for Health Research Economic information: Mean cost per person (intervention) OEP London GBP 88, Nottingham GBP 117; FaME: London GBP 269, Nottingham GBP 218. Health service cost OEP GBP 404, FaME GBP 412, usual care GBP 367. Incremental cost per fall prevented/per QALY gained: no between‐group difference in QALY. Number of clusters allocated to OEP: 14; Number of clusters allocated to FaME: 14; number of clusters allocated to control: 14; number of clusters analysed (OEP): 14; number of clusters analysed (FaME): 14; number of clusters analysed (control): 14 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatments will be assigned…using computer generated random number tables, embedded in a computer programme for minimisation" |
| Allocation concealment (selection bias) | Low risk | Quote: "Practices were allocated to intervention or usual care, only after all participants had been recruited. The practices, their patients and the researchers undertaking baseline assessments were all blinded to allocation until this point" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | High risk | Falls were measured using the same method in all groups. The researchers assessing outcomes were not blinded |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants not blinded to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall outcome data are missing (44%) at 18‐month follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | High risk | Self‐completed fall diaries (completed monthly during the 6‐month intervention period and every 3 months from 6 to 24 months follow‐up). Telephone contact with non‐responders and fallers |
| Cluster‐randomised trials | Low risk | After all participants from a practice had been recruited, the practice was individually allocated to a study arm by the London co‐ordinating centre; baseline comparability of clusters was not reported; missing outcomes for clusters or within clusters were not reported; accounted for the clustered design in the analysis; results comparable with individually randomised trials |
Irez 2011.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 3 months |
|
| Participants | Setting: Turkey Number of participants: 60 Number analysed: 60 Number lost to follow‐up: 0 Sample: community‐dwelling women Age (years): Intervention mean 72.8 (SD 6.7), Control mean 78.0 (SD 5.7) Sex: 100% female Inclusion criteria: Healthy, > 65 years of age, relatively sedentary (undertaking no leisure time physical activity or < 30 minutes of physical activity a day) for at least a year Exclusion criteria: Any significant health problem or orthopaedic problem that would keep them from fully participating in the intervention protocol or the inability to attend at least 80% of the training sessions, or both |
|
| Interventions | 1. Group‐based Pilates: mat exercises, used TheraBand elastic resistance bands, Pilates or exercise balls; 60 minutes, 3 a week for 12 weeks 2. Control group: usual activity |
|
| Outcomes | 1. Rate of falls | |
| Duration of the study | 12 weeks | |
| Adherence | Adherence measured as sessions completed 1. Group‐based Pilates group: completed 32/36 sessions (92% participation rate) |
|
| Notes | Source of funding: Mugla University, School of Physical Education and Sports Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | High risk | Fall calendars were returned to the treating physiotherapist, who also conducted follow‐up phone‐calls |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | High risk | Falls measured, but number of fallers not reported. Adverse events not reported. |
| Method of ascertaining falls (recall bias) | Low risk | Falls calendars, completed daily. Calendars were returned to the treating physiotherapist at the end of each month. Physiotherapists followed up non‐returns |
Iwamoto 2009.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 5 months |
|
| Participants | Setting: Tokyo, Japan Number of participants: 68 Number analysed: 67 Number lost to follow‐up: 1 Sample: volunteer patients from Department of Orthopaedic Surgery (2 hospitals) and Orthopaedic Clinics (3) Age (years): mean 76.4 (SD 5.6), range 66 ‐ 88 Sex: 90% female Inclusion criteria: aged > 50 years; fully ambulatory; able to complete physical assessments Exclusion criteria: using walking aids; severe kyphosis due to osteoporotic vertebral fractures; acute illness; severe cardiovascular disease |
|
| Interventions | 1. Group‐based balance and gait training: supervised exercise programme (calisthenics, balance, muscle power, walking ability training); 30 minutes, 3 a week for 20 weeks 2. Control group: no exercise |
|
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 20 weeks | |
| Adherence | Adherence not defined. Completion rate: 1. Group‐based balance and gait training group: all participants completed the 5‐month trial; adherence not defined 2. Control group: 33/34 participants completed trial |
|
| Notes | Source of funding: Keio University School of Medicine Economic information: not reported Place of residence not specified, i.e. not specifically community‐dwelling, but not preventing falls in hospital or specifically in an institution |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects were randomly divided into two groups ..." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The subjects were randomly divided into two groups ..." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | High risk | Assessor blinding is unclear, but assume obtaining "information regarding falls and fractures .... every week by directly asking the participants" occured for exercise participants during class and control participants were assessed at 2½ and 5 months |
| Blinding of outcome assessment (detection bias) Fractures | High risk | Fractures appear to be self‐reported with no confirmation from medical records |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Method of ascertaining adverse events unclear |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall outcome data are missing (2%). Only missing data are from 1 control participant due to noncompliance |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of fallers not reported |
| Method of ascertaining falls (recall bias) | High risk | Quote: "The incidence of fall and fracture … was assessed 2.5 and 5 months after the start of the trial. In particular, information regarding falls and fractures was obtained every week by directly asking the participants." No mention of diaries or calendars. Retrospective recall. Possibly only the intervention group were asked every week (at class) and remainder at 2½ and 5 months. |
Kamide 2009.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months |
|
| Participants | Setting: Kanagawa, Japan Number of participants: 57 Number analysed: 43 Number lost to follow‐up: 14 Sample: women registered at an employment agency for older people (see Notes) Age (years): mean 71 (SD 3.6) Sex: 100% female Inclusion criteria: aged ≥ 65 years; community‐dwelling; independently mobile; no restriction on physical activities Exclusion criteria: cerebrovascular, cardiopulmonary, neuromuscular, liver, or kidney disease; hyperparathyroidism; unstable diabetes mellitus or hypertension; fracture of spine or lower limbs; taking prednisolone; exercising regularly |
|
| Interventions | 1. Individual balance and strength training: home‐based exercises, Theraband used for moderate‐intensity lower‐limb strength training, no home visits but monthly telephone or mail contact; performed ≥ 3 days a week for 24 weeks 2. Control: usual activities, telephone or mail contact from PT every 3 months |
|
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured as frequency of sessions completed 1. Individual balance and strength training group: 19 of 23 (83%) intervention participants completed > 3 a week, 21 of 23 (91%) intervention participants completed > 2 a week |
|
| Notes | Source of funding: Univers Foundation, Tokyo Economic information: not reported Employment agency providing light work or volunteer activities for older people and encouraging social activities |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random assignment procedure was performed using random numbers generated by a computer program ..." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The subjects were randomly assigned to either the home‐based exercise group or the control group". Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and therapists aware of group allocation. Intervention group: Quote: "the therapist contacted each subject by telephone or mail every month to maintain their motivation." Control group: Quote: "The subjects who were assigned to the control group were instructed to continue with their usual daily activities, with no restrictions on their exercise activities. A therapist contacted them every 3 months by telephone or mail." |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Quote: "Functional capacity, physical function, and bone mineral density were assessed in all subjects in both groups before and after the 6‐month intervention. The staff performing the assessments were blinded to each subject's group assignment. Falls were also assessed before and after the 12‐month followup." Unclear if assessors were blinded. Assume method of fall asessment was the same in both groups |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall outcome data are missing (25%). |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of falls not reported |
| Method of ascertaining falls (recall bias) | High risk | Quote: "Falls were also assessed before and after the 12‐month followup." No concurrent recording described. No mention of frequent telephone monitoring |
Karinkanta 2007.
| Methods | Study design: RCT Number of study arms: 4 Length of follow‐up: 12 months |
|
| Participants | Setting: Tampere, Finland Number of participants: 149 Number analysed: 144 Number lost to follow‐up: 5 Sample: community‐dwelling women Age (years): Balance group mean 72.9 (SD 2.3), Combined group mean 72.9 (SD 2.2), Resistance group mean 72.7 (SD 2.5), Control group mean 72.0 (SD 2.1) Sex: 100% female Inclusion criteria: Willingness to participate, aged 70 ‐ 79 years, female, full understanding of the study procedures, no history of any illness that would contraindicate exercise or limiting participation in exercise, no history of any illness that affects the bones or balance, No uncorrected vision problems, not taking medications known to affect balance or bone metabolism (for 12 months prior to recruitment) Exclusion criteria: Already involved in intense exercise > twice a week BMD score T score < −2.5 in femoral neck |
|
| Interventions | 1. Group‐based balance and agility training: static and dynamic balance, agility training, jumps and other impacts, and changes of direction exercises, 50‐minute sessions, 3 a week for 12 months 2. Group‐based balance and strength training: strength and balance training as described in (1) and (3) on alternate weeks, 50‐minute sessions, 3 a week for 12 months 3. Group‐based resistance training: tailored resistance exercises for large muscle groups using machines tailored up to 70 ‐ 80% of 1RM, 50‐minute sessions, 3 a week for 12 months 4. Control group: asked to maintain same level of activity |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or fall‐related fractures 3. Number of people who experienced a fall requiring medical attention |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured as attendance rate 1. Group‐based balance and agility training: mean attendance rate 59% 2. Group‐based balance and strength training: mean attendance rate 67% 3. Group‐based resistance training: mean attendance rate 74% |
|
| Notes | Source of funding: Academy of Finland, the Finnish Ministry of Education, and the Medical Research Fund of the Tampere University Hospital Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomization list" |
| Allocation concealment (selection bias) | Low risk | Blinded statistician allocated participants |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Assume falls assessed using same method for all participants. Unclear whether researcher assessing files was blinded |
| Blinding of outcome assessment (detection bias) Fractures | Low risk | Medical files examined for fractures by researcher blinded to group allocation |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Low risk | Medical files examined for injurious falls by researcher blinded to group allocation |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall outcome data are missing (3%). Missing data were balanced between balance group (n = 2), combination group (n = 2) and control (n = 1), with 2 participants dying (1 balance, 1 control) and the remaining 3 losing interest |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of fallers not reported. Adverse events not reported |
| Method of ascertaining falls (recall bias) | High risk | Medical files examined for injurious falls |
Kemmler 2010.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 18 months |
|
| Participants | Setting: Erlangen‐Nuremberg area, Germany Number of participants: 246 Number analysed: 227 Number lost to follow‐up: 19 Sample: female members of Siemens Health Insurance living in Erlangen‐Nuremberg area Age (years): mean 69 (SD 4) Sex: 100% female Inclusion criteria: aged ≥ 65; community‐dwelling; consenting Exclusion criteria: diseases affecting bone metabolism or fall risk; medication affecting bone metabolism or fall risk; history of profound coronary heart diseases (stroke, cardiac events), acute or chronic inflammatory diseases, or secondary osteoporosis; participation in exercise studies during previous 2 years; very low physical capacity (< 50 W during ergometry) |
|
| Interventions | 1. Group‐based balance, gait, flexibility and strength training plus home practice: progressive high‐intensity exercise programme (aerobic dance, static and dynamic balance training, functional gymnastics, isometric strength training, and stretching for trunk, hip, and thigh, and upper body exercises using elastic belts), 60‐minute, 2 a week; plus progressive strength and flexibility home exercises, 20‐minute, 2 a week for 18 months 2. Group‐based low‐intensity, low‐frequency balance and endurance training: low‐ to moderate‐intensity "Wellness programme" (relaxation, games/interaction, general co‐ordination, endurance, balance, dances, body sensitivity, muscle strength, breathing, and flexibility); 1 hour, 1 a week for 10 weeks then 10 week rest |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who died |
|
| Duration of the study | 72 weeks | |
| Adherence | Adherence measured as session attendance, frequency of home training 1. Group‐based balance, gait, flexibility and strength training plus home practice: mean attendance rate, 76% (SD 8%) group training, 42% (SD 5%) for home training 2. Control: mean attendance rate, 72% (SD 9%) |
|
| Notes | Source of funding: Siemens Betriebs Krankenkasse, Behinderten‐ und Rehabilitations‐ Sportverband Bayern, Netzwerk Knochengesundheit e.V., Opfermann Arzneimittel GmbH, Thera‐Band, Institute of Sport Science, Institute of Medical Physics Economic information: Mean total healthcare service costs: Exercise group EUR 2255, Control group EUR 2780 Cost analysis in primary reference |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated block randomization" |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence and group assignment were performed by the Institute of Biometry and Epidemiology. Participants were enrolled by the Institute of Medical Physics" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was blinded for the outcome assessors and participants ..." "To blind the participants, the control group performed a program that focused on well‐being and was designed not to cause physical adaptations" "The effectiveness of the blinding in the control group was proven in structured interviews conducted by the primary investigators at the end of the 18 months". Assume no blinding of personnel; impact is unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls assessed using same method for all participants. Outcome assessors were blind to allocation |
| Blinding of outcome assessment (detection bias) Fractures | High risk | Quote: "Injurious falls and overall fractures were monitored daily with the use of fall calendars compiled by the participants. Outcome assessors contacted subjects who fell and nonresponders monthly by telephone". No report of radiological confirmation of fractures |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall outcome data are missing (8%). Missing data were balanced between high‐intensity (n = 8) and low‐intensity (n = 11) groups, with balanced reasons for loss of data in the 2 groups |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of falls was not reported. Adverse events were not reported |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "Injurious falls and overall fractures were monitored daily with the use of fall calendars compiled by the participants. Outcome assessors contacted subjects who fell and nonresponders monthly by telephone." |
Kerse 2010.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: Auckland, New Zealand Number of participants: 193 Number analysed: 193 Number lost to follow‐up: 0 Sample: community‐dwelling Age (years): mean 81.1 (SD 4.4) Sex: 58% female Inclusion criteria: aged 75 years or older, were community‐dwelling, were able to communicate in English to complete assessments, positive depression screen (answered yes to 2 of the 3 depression screen questions) and that they had no severe dementia or unstable medical conditions precluding participation in a physical activity programme Exclusion criteria: see inclusion criteria |
|
| Interventions | 1. Individual Otago Exercise Programme: home‐based programme which comprised moderate‐intensity balance retraining, 'progressive resistance' lower limb‐strengthening exercises, upper limb strengthening, walking, goal setting, and social enrichment; leg and arm weights used (1, 2, 3 kg); ≥ 30 minutes, 3 a week for 6 months; total of 8 x 1‐hour visits to discuss, adjust the programme and motivate 2. Control group: 8 social visits with standardised conversation for a similar amount of time to the intervention participants |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Health‐related quality of life 4. Number of people who died |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured as number of visits received, frequency of exercises 1. Individual Otago Exercise Programme: 81/97 participants (84%) received all the intervention visits, 6/97 had < 6 visits; During the first 6 months: 29% exercised ≥ 3 a week and 37% walked ≥ 3 a week 65% exercised ≥ 2 a week and 63% walked ≥ 2 a week At 12 months: 25% exercised ≥ 3 a week and 37% walked ≥ 3 a week 55% exercised ≥ 2 a week and 59% walked ≥ 2 a week 7 participants performed the programme almost daily 2. Control group: 86% completed all visits |
|
| Notes | Source of funding: New Zealand Health Research Council, University of Auckland Research Committee Economic information: not reported Email communication to obtain fall data, response received, data included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Assessment of falls was the same in both groups Quote: "The research nurses conducting follow‐up assessments were blinded to the participants’ group allocation. To maintain this blinding, immediately before the follow‐up visits, participants were reminded by a telephone call from a researcher not to talk to the assessment nurses about the physical activity program or who had been visiting them." |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants not blinded to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing falls data |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | High risk | Interval recall. Falls were ascertained by self‐report at 6 months and 12 months |
Kim 2014.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: Tokyo, Japan Number of participants: 105 Number analysed: 103 Number lost to follow‐up: 2 Sample: community‐dwelling women Age (years): Intervention mean 77.83 (SD 4.21), Control mean 77.83 (SD 4.15) Sex: 100% female Inclusion criteria: age ≥ 70 years; experienced at least 1 fall incident in the previous year; and no missing fall‐related baseline data Exclusion criteria: severe knee or back pain; severe walking disability; and unstable cardiac conditions |
|
| Interventions | 1. Group‐based balance and strength: increased difficulty of exercises, used resistance bands or ankle weights for strength training; 60‐minute, 2 a week for 3 months; plus 1‐hour exercise classes 1 a month during 1‐year follow‐up; home programme encouraged ≥ 3 a week during 1‐year follow‐up 2. Control group: Health education. 60‐minute class once a month for 3 months, a total of 3 times |
|
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) 2. Number of people who experienced 1 or more fall‐related fractures |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured as session attendance, frequency of home exercises, mean exercise time 1. Group‐based balance and strength group: mean attendance rate during intervention, 75% (range 64 – 86%); mean frequency home exercises 3.4 a week; mean exercise time 24.9 minutes |
|
| Notes | Source of funding: Ministry of Health and Welfare of Japan, Japan Society for the Promotion of Science Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The allocation process was blinded". Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Fall diaries were collected at 1‐year follow‐up Quote: "The investigators evaluating the effects of the exercise treatment were blind to intervention allocations" |
| Blinding of outcome assessment (detection bias) Fractures | High risk | Participants were asked about fractures by face‐to‐face interview at baseline, 3 month and 1 year. No radiological confirmation |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall outcome data are missing (2%). Missing data were balanced between the exercise (n = 2) and control (n = 1) groups, with reasonable reasons for loss of data in the 2 groups (exercise: reduced motivation = 1, hospitalisation = 1; control: moved house = 1) |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of falls was not reported. Adverse events were not reported |
| Method of ascertaining falls (recall bias) | Unclear risk | Falls diary, distributed at 3‐month assessment and collected at 1‐year follow‐up |
Korpelainen 2006.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 30 months |
|
| Participants | Setting: Oulu, Finland Number of participants: 160 Number analysed: 160 Number lost to follow‐up: 0 Sample: birth cohort of women Age (years): mean 73 (SD 1.2) Sex: 100% female Inclusion criteria: hip BMD > 2 less than the reference value Exclusion criteria: "medical reasons"; use of a walking aid other than a stick; bilateral total hip joint replacement; unstable chronic illness; malignancy; medication known to affect bone density; severe cognitive impairment; involvement in other interventions |
|
| Interventions | 1. Group‐based balance and strength training plus home practice: exercises increased in difficulty and used no special equipment; 1‐hour session, weekly, plus 20 minutes daily at home for 6 months each year; plus twice‐yearly seminars on nutrition, health, medical treatment and fall prevention 2. Control: twice‐yearly seminars on nutrition, health, medical treatment, and fall prevention | |
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more fall‐related fractures | |
| Duration of the study | 130 weeks | |
| Adherence | Adherence measured as session attendance and frequency of home programme 1. Group‐based balance and strength training plus home‐practice group: mean attendance at sessions; 77% during the first supervised 6‐month period, 75% during the second supervised period and 74% during the last supervised 6 months; mean frequency of performing home programme was 3 a week |
|
| Notes | Source of funding: Finnish Ministry of Education, the Finnish Cultural Foundation, University of Oulu, Deaconess Institute of Oulu, Juho Vainio Foundation, Miina Sillanpää Foundation, Research Foundation of Orion Corporation Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each participant received sequentially, according to the original identification numbers, the next random assignment in the computer list". |
| Allocation concealment (selection bias) | Low risk | The randomisation was "provided by a technical assistant not involved in the conduction of the trial" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blind to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls measured using the same method in each group Quote: "The assessors in direct contact with participants during the study did not know to which group they had been allocated" |
| Blinding of outcome assessment (detection bias) Fractures | High risk | No radiological evidence for fractures |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing falls data |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of fallers was not reported |
| Method of ascertaining falls (recall bias) | High risk | 3‐monthly retrospective recall |
Kovacs 2013.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: Budapest, Hungary Number of participants: 76 Number analysed: 72 Number lost to follow‐up: 4 Sample: community‐dwelling women Age (years): Intervention mean 68.5 (SD 5.3), Control mean 68.3 (SD 6.4) Sex: 100% female Inclusion criteria: Women aged 60 years of age or over, lived in community setting Exclusion criteria: GP did not recommend their participation because of having progressive neurological or unstable cardiovascular diseases that would limit participation in the exercise programme, having severe pain in lower limb in weight‐bearing positions or participation in regular physical exercise programme (sport or physiotherapy) in the past 6 months |
|
| Interventions | 1. Group‐based balance and strength training plus home‐practice: exercises and competition games with no special equipment, 60‐minute sessions, 2 a week for 25 weeks 2. Control group: asked not to start any type of regular exercise programme and maintain their usual activities, offered participation in the next programme |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured as the percentage of the number of sessions completed out of the total 50 sessions 1. Group‐based balance and strength training plus home‐practice group: 81% (range 56 ‐ 100%) |
|
| Notes | Source of funding: Quality‐Metric Incorporated Economic information: not reported Email communication to obtain fall data, response received, data included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Blocked randomisation was performed (with a block size of 4 and 6)". Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Consecutively numbered opaque identical sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Fall calendars were distributed and collected by a physiotherapist who was not involved in the exercise programme and who was not informed about the participants’ group allocation. Blinding assumed |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall outcome data are missing (5%). Missing data were balanced between the exercise (n = 2) and control (n = 2) groups |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported, (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Low risk | Fall calendar, collected monthly |
Kwok 2016.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: Singapore Number of participants: 80 Number analysed: 80 Number lost to follow‐up: 0 Sample: community‐dwelling Age (years): mean 80 Sex: 85% female Inclusion criteria: not participating in any routine exercise programme, participants with MFES scores ≤ 9 and could comprehend English, Mandarin or a local dialect Exclusion criteria: people with neurological disorders |
|
| Interventions | 1. Group‐based balance, strength and aerobic training plus home practice: gym equipment used for cardiovascular training, strength training prescribed at 10 or 15 repetitive maximum; 1‐hour sessions, weekly for 12 weeks, 20 minutes of home balance and strength exercises from week 13 on non‐intervention days 2. Balance, strength and aerobic training using the Nintendo WiiActive: supervision provided for gaming exercises with the Wii balance board, calisthenics and resistance band and calisthenics used for cadiovascular training, resistance band used for strengthening; 20 minutes, weekly for 12 weeks, 20 minutes of home exercises from week 13 |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured as session attendance and home exercise compliance 1. Group‐based balance, strength and aerobic training plus home‐practice group: mean exercise session attendance 9.4 (SD 3.2); mean home exercise compliance 2.1 days a week (SD 1.2) 2. Balance, strength and aerobic training using the Nintendo WiiActive group: mean exercise session attendance 9.5 (SD 2.5); mean home exercise compliance 2.4 days per week (SD 1.4) |
|
| Notes | Source of funding: The SingHealth Foundation, Singapore Physiotherapy Association Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Generated the random allocation sequence". Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Consecutively‐numbered, sealed envelope. Opaque not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Quote: "Baseline and follow‐up measurements were performed by trained and blinded research assistants". Assume this includes monthly telephone follow‐up of fall‐tracking |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of falls was not reported |
| Method of ascertaining falls (recall bias) | Low risk | Participants tracked monthly fall incidence on a recording sheet and were contacted monthly through telephone or mobile phone short messages to minimise recall bias |
Kyrdalen 2014.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 3 months |
|
| Participants | Setting: 11 communities in southeast Norway Number of participants: 125 Number analysed: 94 Number lost to follow‐up: 31 Sample: community‐dwelling Age (years): mean 82.5 (SD 5.7) Sex: 73% female Inclusion criteria: home‐dwelling, at increased fall risk (defined as answering yes on either criterion 1 or 2 below, and in addition yes on 2 or more of criteria 3 ‐ 9: 1) had fallen at least once during the previous 12 months; 2) had self‐reported balance or gait problems; 3) had Parkinson’s disease or had suffered a stroke; 4) had 4+ concomitant diseases; 5) needed a handrail or support while rising from a chair; 6) used 4+ prescribed medications; 7) had reduced cognitive function as assessed by a geriatrician; 8) had BMI < 20, and 9) had reduced vision for their age Exclusion criteria: a score of 23/30 or less on the MMSE or not able to walk without support from another person |
|
| Interventions | 1. Group‐based Otago Exercise Programme: 45 minutes 2 a week for 12 weeks plus outdoor walking for 30 minutes, ≥ 3 a week for 12 weeks 2. Individual Otago Exercise Programme: 30 minutes, 3 a week for 12 weeks, plus outdoor walking for 30 minutes, ≥ 3 a week for 12 weeks Both groups received 4 home visits to check programme plus 4 telephone calls |
|
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) 2. Number of people who experienced 1 of more falls requiring hospital admission 3. Health‐related quality of life 4. Number of people who died |
|
| Duration of the study | 12 weeks | |
| Adherence | Adherence measured as session attendance 1. Group‐based Otago Exercise Programme: attended mean of 21.9 out of 24 sessions (SD 2.7) 2. Individual Otago Exercise Programme: attended mean 32.8 out of 36 recommended sessions (SD 2.8) |
|
| Notes | Source of funding: Norwegian Fund for Post‐Graduate Physiotherapy Training Economic information: not reported Email communication regarding fall data, response received, data not included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A Web‐based block randomization procedure with varying group size, developed by the Applied Clinical Research Unit at the Norwegian University of Science and Technology, was used" |
| Allocation concealment (selection bias) | Low risk | Centralised "web‐based" randomisation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) Falls | High risk | Baseline to 3 months: fall calendars collected by unblinded exercise instructors at intervention sessions. 3 ‐ 6 months: falls collected retrospectively at 6‐month interview with blinded assessor |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Method of ascertaining hospital admission is unclear |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants not blinded to group allocaiton |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall outcome data are missing (25%) |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of falls was not reported |
| Method of ascertaining falls (recall bias) | High risk | Baseline to 3 months: falls were recorded on fall calendars which were collected by unblinded exercise instructors during twice‐weekly group sessions (intervention group) or at home visits in weeks 1, 2, 4 and 8 (control group). Non‐returns or incomplete calendars were followed up with the participant or next of kin; the person collecting this information unclear. 3 ‐ 6 months: falls collected retrospectively at 6‐month interview with blinded assessor |
LaStayo 2017.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: Utah, USA Number of participants: 134 Number analysed: 112 Number lost to follow‐up: 22 Sample: community‐dwelling Age (years): mean 76.1 (SD 7.18) Sex: 65% female Inclusion criteria: at least 65 years of age or older; had experienced at least 1 fall in the previous 12 months; community‐dwelling; ambulatory with a gait speed ranging from of 0.42 to 1.3 m/s; able to recall all 3 items (or 1 to 2 items with a normal clock drawing test) on the Mini‐CogTM instrument for dementia screening; managing 2 or more co‐morbid conditions, though cleared by their physician to participate in a 60‐minute (with rests) multicomponent exercise fall reduction programme (MCEFRP) Exclusion criteria: progressive diagnosed neurologic disease (e.g. Parkinson’s, multiple sclerosis, Guillain‐Barre, Alzheimers); any dystrophies or rheumatologic conditions that primarily affects muscle (e.g. muscular dystrophy, polymyalgia rheumatica); already participated in a MCEFRP or if they were currently performing (or had performed) regular (3 times a week) aerobic (defined as hiking, fast‐walking, jogging, running swimming or cycling) or resistance (defined as weight training with bands, cable, free‐weights or weight‐machines) exercise over the past 12 months; any of the absolute contraindications for a MRI scan |
|
| Interventions | Participants trained for 60 minutes per session, 3 times a week for 3 months as part of the multicomponent exercise fall reduction program that included aerobic training (recumbent trainer, cycle erg or treadmill), flexibility exercise, 15 ‐ 20‐minute individualised balance exercises, upper‐limb resistance training and lower‐limb resistance training The 2 lower‐limb resistance training programmes were: 1) Traditional (TRAD) resistance exercise: 3 sets of 15 repetitions of a seated bilateral leg‐press exercise at 70% 1 RM. Also, standing multidirectional straight‐leg exercises with a weighted cuff placed just proximal to the ankle. The training loads for this exercise were increased as tolerated every 2 weeks, provided the participants could complete 3 sets of 15 repetitions with appropriate form 2) Resistance exercise by negative, eccentrically‐induced, work (RENEW): progressive resistive eccentric exercise of the knee and hip extensor muscles using a recumbent stepper‐ergometer. The duration of each resistance training session was progressively increased to a maximum 15‐minute duration during weeks 5 – 12 |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) |
|
| Duration of the study | 52 weeks | |
| Adherence | All participants completed the prespecified requisite minimum 18 MCEFRP sessions and ≥ 90% adhered to at least 29 of the 36 exercise sessions | |
| Notes | Source of funding: National Institute of Aging of the National Institutes of Health Economic information: not reported Email communication regarding fall data, response received, data not included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomisation process with blocks of ten insured equivalency in the number of subjects adn the same proportion of men and women were assigned into each of the groups" |
| Allocation concealment (selection bias) | Unclear risk | Allocation not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not specified. Assume participants and presonnel not blinded. Impact of non‐blinding is unknown |
| Blinding of outcome assessment (detection bias) Falls | High risk | Assessors were not blinded to group |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of fall outcome data are missing (16%). Missing data were not balanced between the RENEW (n = 14) and traditional (n = 8) groups, with more participants dropping out in the first 3 months in the RENEW group (9 dropouts compared with 4 dropouts). The reasons for the dropouts are not clear |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Low risk | From 0 ‐ 3 months intervention personnel asked about falls at weekly intervention sessions. 4 ‐ 12 months falls were recorded by monthly stamped postcards, with telephone contact if a fall was reported or postcards were not returned |
Latham 2003.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months |
|
| Participants | Setting: 5 hospitals in Auckland, New Zealand and Sydney, Australia Number of participants: 243 Number analysed: 222 Number lost to follow‐up: 21 Sample: frail older people recently discharged from hospital Age (years): mean 79 Sex: 53% female Inclusion criteria: aged ≥ 65, considered frail (1 or more health problems, e.g. dependency in an ADL, prolonged bed rest, impaired mobility, or a recent fall); no clear indication or contraindication to either of the study treatments Exclusion criteria: poor prognosis and unlikely to survive 6 months; severe cognitive impairment; physical limitations that would limit adherence to exercise programme; unstable cardiac status; large ulcers around ankles that would preclude use of ankle weights; living outside hospitals’ geographical zone; not fluent in English |
|
| Interventions | 1. Exercise: quadriceps exercises using adjustable ankle cuff weights 3 a week for 10 weeks. First 2 sessions in hospital, remainder at home. Monitored weekly by physiotherapist: alternating home visit with telephone calls
2. “Attention” control: frequency‐matched telephone calls and home visits from research physical therapist including general enquiry about recovery, general advice on problems, support
3. Vitamin D: single oral dose of 6 x 1.25 mg calciferol (300,000 IU) 4. Vitamin D control: placebo tablets |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Health‐related quality of life 5. Number of people who died |
|
| Duration of the study | 26 weeks | |
| Adherence | Adherence was monitored through a participant diary 1. Exercise: adhered to 82% of prescribed sessions (mean 24.6 of 30 sessions). Mean exercise intensity at the end of training was 51% ± 13% of 1 RM, only 25% of participants were able to reach the high intensity desired by the intervention |
|
| Notes | Source of funding: Health Research Council of New Zealand, Auckland University of Technology Research Fund, Lenore Wilson Estate Economic information: not reported Detailed description of exercise regimen given in paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study biostatistician‐generated random sequence. Block randomisation technique |
| Allocation concealment (selection bias) | Low risk | Computerised centralised randomisation scheme |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial with 4 arms with varying risks of bias (factorial design). 2 arms double‐blind, placebo‐controlled (low risk) and 2 arms exercise and attention control with matched frequency of visits where impact of non‐blinding likely to be low or unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Placebo‐controlled arms: falls reported by participants who were blinded to group allocation (and assessor blinded to group allocation). Exercise and exercise control arms: falls reported by participants who were aware of their group allocation but assessor blinded to group allocation |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Quote: "The field research staff recorded all adverse events, and a blinded assessor coded them". Assume field research staff were not blinded. Assume adverse events were recorded using same methods in both groups (as visits were frequency‐matched) |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Trial participants in exercise and placebo‐controlled groups were not blinded to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of fall outcome data are missing (9%). There was a minor imbalance in missing data between the resistance (n = 8) and control (n = 13) groups, with the resistance group missing data due to death (n = 6) and refusal (n = 2), and the control group missing data due to death (n = 8) and refusal (n = 5) |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes reported. No protocol or prospective trial registration |
| Method of ascertaining falls (recall bias) | Unclear risk | Prospective. Falls recorded in fall diary with weekly reminders for first 10 weeks. Nurses examined fall diaries and sought further details about each fall at 3‐ and 6‐month visits. Reminder phone call between visits |
Lehtola 2000.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 10 months |
|
| Participants | Setting: Finland Number of participants: 131 Number analysed: 131 Number lost to follow‐up: 0 Sample: community‐dwelling Age (years): Intervention mean 72.3 (SD 1.6), Control mean 72.4 (SD 1.6) Sex: 80% female Inclusion criteria: community‐dwelling adults aged 70 ‐ 75 Exclusion criteria: people in institutional care, people who on testing required a mobility aid, or had physical or cognitive impairments e.g. dementia, RA, OA, cardiac or respiratory conditions |
|
| Interventions | 1. Group‐based balance and flexibility training plus walking and home practice: 60‐minute class, 1 a week for 20 weeks; walking with sticks 20 minutes, > 3 a week for 24 weeks; home exercises 20 minutes, > 3 a week for 24 weeks 2. Control group: usual care |
|
| Outcomes | 1. Rate of falls | |
| Duration of the study | 40 weeks | |
| Adherence | Participants completed diary collected monthly 1. Group‐based balance and flexibility training plus walking and home practice group: 'Active' participants: 52 participants; 'Passive': 20 participants |
|
| Notes | Source of funding: not reported Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unable to assess due to language |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess due to language |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to assess due to language |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Unable to assess due to language |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | Risk of falls and adverse events not reported |
| Selective reporting (reporting bias) | Low risk | No missing fall data |
| Method of ascertaining falls (recall bias) | Unclear risk | Unable to assess due to language |
Li 2005.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months |
|
| Participants | Setting: Legacy Health System, Portland, Oregon, USA Number of participants: 256 Number analysed: 188 Number lost to follow‐up: 68 Sample: people enrolled in HMO Age (years): mean 77.5 (SD 5), range 70 to 92 Sex: 70% female Inclusion criteria: age ≥ 70; physician clearance to participate; inactive (no moderate to strenuous activity in last 3 months); walks independently Exclusion criteria: chronic medical problems that would limit participation; cognitive impairment |
|
| Interventions | 1. Group‐based Tai Chi: 1 hour, 3 a week for 26 weeks 2. Control group: low‐level stretching 1 hour, 3 a week for 26 weeks | |
| Outcomes | 1. Rate of falls
2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who experienced 1 or more falls requiring medical attention |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured as class attendance 1. Group‐based Tai Chi group: median compliance; 61 sessions (range 30 ‐ 77). 92 (80%) attended 50+ sessions 2. Control group: median compliance; 61 sessions (range 35 ‐ 78). 87 (81%) attended 50+ sessions |
|
| Notes | Source of funding: National Institutes of Health, National Institute on Aging Economic information: not reported 6‐month fall data used as total over 12‐month period not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blind to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls reported by participants who were aware of their group allocation, using the same method on both groups. Fall diaries coded by blinded research assistant |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | High risk | The only evidence for requiring medical attention was from self‐reports from participants |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall outcome data are missing (27%) |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes was not reported (adverse events were not reported) |
| Method of ascertaining falls (recall bias) | Low risk | Prospective. Falls recorded on daily fall calendars, collected on a monthly basis |
Lin 2007.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months |
|
| Participants | Setting: Taiwan Number of participants: 100 Number analysed: 100 Number lost to follow‐up: 0 Sample: residents of rural agricultural area Age (years): mean 76.5 Sex: 51% female Inclusion criteria: medical attention for a fall in previous 4 weeks, ≥ 65 years Exclusion criteria: none described |
|
| Interventions | Randomised into 3 groups: 2 intervention groups (1 individual balance, strength and flexibility training group, 1 home safety assessment and modification group) and 1 control group. Only Individual balance, strength and flexibility training group and control group included in this review 1. Individual balance, strength and flexibility training: Home‐based exercises with physiotherapist, used 1 kg ankle weights for strengthening if able, 40 ‐ 60‐minute sessions, 3 x or more a week for 4 months 2. Control: 1 social visit by a public health worker 30 to 40‐minute every 2 weeks for 4 months with fall prevention pamphlets provided |
|
| Outcomes | 1. Rate of falls 2. Health‐related quality of life 3. Number of people who died |
|
| Duration of the study | 16 weeks | |
| Adherence | Not reported | |
| Notes | Source of funding: Bureau of Health Promotion, Department of Health, National Science Council Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomised. Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Quote: "Participants were asked to report their falls by telephone or postcard; they were also contacted by telephone every 2 weeks to ascertain the occurrence of falling". The method of ascertaining falls was the same in all groups. Blinding of assessors not reported |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants were not blinded to allocated group |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall outcome data are missing (21%) |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of fallers was not reported. Adverse events were not reported |
| Method of ascertaining falls (recall bias) | Low risk | Prospective. Reported falls by telephone or postcard when they occurred. Phoned every 2 weeks to ascertain occurrence of falls |
Liston 2014.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months |
|
| Participants | Setting: London, UK Number of participants: 21 Number analysed: 15 Number lost to follow‐up: 6 Sample: Secondary care‐based falls clinic Age (mean): Otago Exercise Programme + multisensory mean 77.8 years; Otago Exercise Programme + stretching mean 76.7 years Sex: 85% female Inclusion criteria: ≥ 65 years, ≥ 2 non‐syncopal falls during the previous 12 months, no previous diagnosis of vestibular dysfunction, referred after multifactorial assessment for the locally‐provided ‘routine’ modified Otago Exercise Programme classes Exclusion criteria: where falls were considered by the attending physician as due to acute illness without significant underlying instability, medication side effects, or musculoskeletal or neurologic disease significantly affecting postural stability |
|
| Interventions | Randomised into 3 groups: 2 intervention groups (1 group‐based modified Otago Exercise Programme plus individual, partiall‐supervised multisensory balance training, and 1 group‐based modified Otago Exercise Programme plus individual, partially‐supervised flexibility training) and 1 control group. Only the 2 intervention groups were included in this review 1. Group‐based modified Otago Exercise Programme plus individual, partially‐supervised multisensory balance training: 1‐hour class, 2 a week, + 45‐minute supervised home sessions providing additional customised multisensory balance exercises for 8 weeks 2. Group‐based modified Otago Exercise Programme plus individual, partially‐supervised flexibility training: 1‐hour class, 2 a week, + 45‐minute supervised home stretching programme for 8 weeks |
|
| Outcomes | 1. Rate of falls | |
| Duration of the study | 24 weeks | |
| Adherence | Not reported | |
| Notes | Source of funding: King’s College London PhD studentship Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐number generator |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Quote: "Outcome measures were assessed at baseline, four and eight weeks (end of treatment), and were performed by a rater blinded to intervention group….. Six‐months postintervention, a telephone follow‐up recorded retrospective falls history". Unclear if falls were collected by an assessor blinded to treatment group |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall outcome data are missing (29%) |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of fallers was not reported. Adverse events were not reported |
| Method of ascertaining falls (recall bias) | High risk | Quote: "Six‐months postintervention, a telephone follow‐up recorded retrospective falls history...for the previous six‐months" |
Liu‐Ambrose 2004.
| Methods | Study design: RCT Number of study arms: 3 Length of follow‐up: 6 months |
|
| Participants | Setting: British Colombia (BC), Canada Number of participants: 104 Number analysed: 98 Number lost to follow‐up: 6 Sample: women with osteoporosis or osteopenia diagnosed at BC Women's Hospital and Health Centre; individuals with low BMD identified through Osteoporosis Society of Canada; advertising Age (years): mean 79 (SD 3), range 75 ‐ 85 Sex: 100% female Inclusion criteria: women aged 75 ‐ 85; osteoporosis or osteopenia (BMD total hip or spine T score at least 1 SD below young normal sex‐matched area BMD of the Lunar reference database); resident in greater Vancouver Exclusion criteria: living in care facility; non‐white race; regularly exercising twice a week or more; history of illness or a condition affecting balance (stroke, Parkinson's disease); unable to safely participate in exercise programme; MMSE 23 or less |
|
| Interventions | 1. Supervised, high‐intensity resistance training: progressive strengthening using gym equipment and free weights; 50 minutes, 2 a week for 25 weeks 2. Supervised agility training: training to challenge hand‐eye and foot‐eye co‐ordination, and dynamic, standing and leaning balance, and reaction time (ball games, relay races, dance movements, obstacle courses wearing hip protectors); 50 minutes, 2 a week for 25 weeks. 3. Control group: sham exercises (stretching, deep breathing, relaxation, posture education); 50 minutes, 2 a week for 25 weeks | |
| Outcomes | 1. Rate of falls | |
| Duration of the study | 25 weeks | |
| Adherence | Adherence measured by class attendance. 1. Supervised, high‐intensity resistance training group: 85% compliance 2. Supervised agility training group: 87% compliance 3. Control group: 79% compliance |
|
| Notes | Source of funding: Vancouver Foundation (BCMSF), Canadian Institutes of Health Research, Michael Smith Foundation for Health Research, Peter Wall Institute for Advanced Studies at the University of British Columbia, Canada Foundation for Innovation Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described but stratified by baseline performance in postural sway |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | All participants asked to keep falls diary. Study described as "single blind" which indicates that assessors were blinded, but unclear whether personnel recording falls outcomes were blinded |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Assessors of adverse events were not blinded to group allocation. Participants were questioned about the presence of adverse events after each exercise session, therefore assume the 3 groups were assessed using the same method and with the same frequency |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall outcome data are missing (6%). The missing data were balanced between groups (2 missing from each group at final assessment) |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of fallers was not reported. Adverse events were not reported |
| Method of ascertaining falls (recall bias) | Low risk | Prospective. Quote: "Falls documented using monthly falls calendars" |
Liu‐Ambrose 2008.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: Vancouver, Canada Number of participants: 74 Number analysed: 59 Number lost to follow‐up: 15 Sample: people attending a falls clinic after presenting at ED or to GP with a fall or fall‐related injury (41/59 completing baseline assessment) Age (years): mean 82.2 (SD 6.3) (in 59 participants completing baseline assessment) Sex: 71% female Inclusion criteria: aged ≥ 70; community‐dwelling; attending 1 of 2 falls clinics (criteria for attending clinic: history of a fall and considered at risk for further falls); able to walk at least 3 m; 1 additional non‐syncopal fall in previous year (if index fall was suspected to be due to carotid sinus syndrome); at risk of further falls (TUG test > 15 seconds or PPA z‐score of ≥ 1) Exclusion criteria: progressive neurological condition (e.g. Parkinson's disease); life expectancy < 12 months; cognitively impaired (MMSE score < 24) |
|
| Interventions | 1. Individual Otago Exercise Programme: 30 minutes, 3 a week for 6 months plus walking for ≥ 2 a week 2. Control: no exercise intervention; semi‐structured interview about presenting fall and experience seeking care for the fall at ED Both groups received falls risk factor assessment and comprehensive geriatric assessment followed by 'Guideline Care' through falls clinic |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who died |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by programme completion 1. Individual Otago Exercise Programme: 7/28 (25%) completed programme ≥ 3 a week. 16/28 (57%) completed programme ≥ 2 a week. 19/28 (68%) completed programme at ≥ 1 a week |
|
| Notes | Source of funding: Canadian Institutes of Health Research Economic information: Mean cost per person (intervention) CAD 14,285. Incremental cost per fall prevented/per QALY gained: CAD 247 per fall prevented Cost‐effectiveness analysis reported in Davis 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization sequence was computer generated (www.randomization.com)" |
| Allocation concealment (selection bias) | Low risk | Quote: "The Family Practice Research Coordinator at the University of British Columbia held this sequence independently and remotely" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blind to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | High risk | Falls self‐reported and Quote: "A research assistant who was not blinded to treatment group" phoned participants at the end of each month |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall outcome data are missing (30%) |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "Ascertainment of falls ... documented on monthly calendars that were returned in prepaid preaddressed envelopes at the end of each month." "A research assistant who was not blinded to treatment group but was unaware of the study hypotheses made three attempts by telephone to contact participants at the end of each month. The purpose of each phone call was to inquire about falls (both groups) ... for all participants regardless of whether the calendar was returned." |
Logghe 2009.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: 2 industrial towns in the western Netherlands Number of participants: 269 Number analysed: 269 Number lost to follow‐up: 0 Sample: registered with participating 23 general practices Age (years): mean 77 (SD 4.6) Sex: 71% female Inclusion criteria: aged ≥ 70; community‐dwelling; high falls risk (1 or more falls in previous year or 2 or more risk factors for falling (disturbed balance, mobility problems, dizziness, using benzodiazepines or diuretics)) Exclusion criteria: none described |
|
| Interventions | 1. Group‐based Tai Chi: 1 hour, 2 a week for 13 weeks + fall‐prevention brochure 2. Control: fall‐prevention brochure | |
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who died |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by lesson attendance 1. Group‐based Tai Chi: 47% attended 80% of lessons |
|
| Notes | Source of funding: Netherlands Organization for Health Research and Development (ZonMw) Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent research assistant performed a prestratified block randomization using a computer‐generated randomization list" |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent research assistant performed ... randomization" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls self‐reported but Quote: "The blinded research assistant contacted the participant when forms were missing or incomplete, and they then completed the forms together over the telephone". Falls were recorded and confrimed using the same method in both groups |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "At baseline, the participants received a falls calendar and the instruction to fill it out on a daily basis for 1 year ... The fall calendars were collected monthly by mail. The blinded research assistant contacted the participant when forms were missing or incomplete, and they then completed the forms together over the telephone" |
Lord 1995.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: Australia Number of participants: 197 Number analysed: 169 Number lost to follow‐up: 28 Sample: women recruited from a schedule from a previous epidemiologic study. Fitness level not defined Age (years): mean 71.6 (SD 5.4), range 60 ‐ 85 Sex: 100% female Inclusion criteria: living independently in the community Exclusion criteria: unable to speak English |
|
| Interventions | 1. Group‐based balance, strength, gait training: exercise class not requiring any special equipment; 1 hour, 2 a week for 4 x 10 ‐ 12‐week terms, with 2‐week inter‐term breaks and 5‐week Christmas/summer break 2. Control: no intervention | |
| Outcomes | 1. Rate of falls
2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who died (not reported by group) |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by class attendance 1. Group‐based balance, strength, gait training: 75/100 attended 26+ classes; of those 75, mean of 60 classes (73%), range 26 ‐ 82 classes (max classes = 82) |
|
| Notes | Source of funding: National Health and Medical Research Council of Australia Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | High risk | Falls reported by participants who were aware of their group allocation. Assessors not blinded to treatment status. |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of fall outcome data are missing (14%). There was an imbalance in missing data between the intervention (n = 25) and control (n = 3) groups. It is unclear whether the reason for missing outcome data is related to true outcome, but the missing intervention‐group data included 13 dropouts, 3 deaths, 1 stroke, 2 injurious falls and 4 medical conditions that precluded participation. Reason for missing control group data is unclear |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | High risk | Interval recall. Fall ascertainment questionnaires sent out every 2 months. Telephone call if questionnaire not returned |
Lord 2003.
| Methods | RCT. Cluster‐randomised by village. Stratified by accommodation (self‐care or intermediate care) and by cluster size (< 75 or at least 75 residents) Study design: Cluster‐RCT Number of study arms: 2 Number of clusters: 20 Length of follow‐up: 12 months |
|
| Participants | Setting: retirement villages, Sydney, Australia Number of participants: 551 Number analysed: 508 Number lost to follow‐up: 43 Sample: recruited from self‐care apartment villages (78%) and intermediate‐care hostels (22%) Age (years): mean 79.5 (SD 6.4), range 62 ‐ 95 Sex: 86% female Inclusion criteria: resident in one of 20 retirement villages Exclusion criteria: MMSE < 20; already attending exercise classes of equivalent intensity; medical conditions that precluded participation as determined by nurse or physician (neuromuscular, skeletal, cardiovascular); in hospital or away at recruitment time |
|
| Interventions | Randomised into 3 groups: 1 intervention group (group‐based balance, strength, gait training) and 2 control groups (1 seated flexibility and relaxation activities, 1 no group activity). Only the intervention group and control group with no activity included in this review 1. Group‐based balance, strength, gait training: within village site, instructor‐led class not requiring any special equipment; 1 hour, 2 a week for 52 weeks 2. Control: no group activity |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who died |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by class attendance, range for both groups 0‐100%. 1. Group‐based balance, strength, gait training: mean number of classes attended 42%; IQR: 10 ‐ 62 classes 2. Control group: mean number of classes attended 45%; IQR: 6 ‐ 50 classes |
|
| Notes | Source of funding: National Health and Medical Research Council of Australia, New South Wales Health, MBF (Australia) Economic information: not reported Number of clusters allocated to intervention: 10; number of clusters allocated to control: 10; number of clusters analysed (intervention): 10; number of clusters analysed (control): 10 Email communication to obtain fall data, response received, data included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | High risk | Cluster‐RCT. Individual participant recruitment was undertaken after group allocation. The method of concealment is not described and it is likely that recruitment was undertaken by a person who was unblinded and may have known participant characteristics |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | High risk | Falls reported by completion of questionnaire monthly by all participants; if not returned telephone calls were made. No mention of blinding of personnel carrying out phone calls, but in intermediate‐care sites, falls record book was kept by nursing staff (unblinded) |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall data were missing (43%) |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Low risk | Retrospective. Falls ascertained by questionnaires given to residents every month, with follow‐up phone calls or home visit for non‐responders. In addition nurses recorded falls in falls record book in intermediate‐care hostels |
| Cluster‐randomised trials | Unclear risk | Individuals were recruited to the trial after the clusters were randomised. Personnel recruiting participants were not blind to cluster; baseline comparison of the intervention arms is reported, but not baseline comparability of clusters; missing outcomes for clusters or within clusters were not reported; accounted for the clustered design in the analysis; results comparable with individually‐randomised trials |
Lurie 2013.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 3 months |
|
| Participants | Setting: USA Number of participants: 64 Number analysed: 59 Number lost to follow‐up: 5 Sample: outpatients Age (mean): 80 Sex: 59% female Inclusion criteria: physically able to use a treadmill, willing to be randomised, willing to participate in a phone interview 3 months after discharge from PT, considered at risk of falls by primary care provider Exclusion criteria: inability to use a treadmill (e.g. severe spinal issues such kyphosis, osteoporosis, or compression fractures that inhibit their ability to stand for more than a few minutes at a time), not a candidate for gait and balance training (e.g. balance issues were purely vestibular) as determined by their physical therapist |
|
| Interventions | 1. Standard Physical Therapy programme + surface perturbation treadmill training: programme as (2) plus treadmill simulating a trip and slip. Number and frequency of sessions was clinically determined by each therapist. 12 weeks 2. Standard Physical Therapy programme: individualised exercise (strengthening, flexibility or balance, or both) and mobility training supervised in‐clinic and home programme not requiring any special equipment. Number and frequency of sessions was clinically determined by each therapist. 12 weeks |
|
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 12 weeks | |
| Adherence | Not reported | |
| Notes | Source of funding: The Dartmouth Center for Clinical and Translational Science Economic information: not reported Email communication regarding fall data, response received, data not included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were assigned using permuted block randomization stratified by site and gender" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Allocation concealment was ensured until after participants enrolled and completed the baseline fall risk assessment". Method of allocation concealment not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear risk, participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | High risk | Assessors of falls were not blinded to group allocation Quote: "Another limitation of this study was the inability to blind testers to treatment group allocation" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of fall outcome data are missing (5%). Missing data were not balanced between groups; all missing data were from the surface perturbation treadmill training programme (1 because they did not meet the inclusion criteria, 4 did not return for treatment) |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of falls was not reported. Adverse events were not reported |
| Method of ascertaining falls (recall bias) | High risk | Asked by telephone at 3 months: " In the past 3 months have you fallen?” |
Luukinen 2007.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 16 months |
|
| Participants | Setting: Oulu, Finland Number of participants: 486 Number analysed: 437 Number lost to follow‐up: 49 Sample: identified from population and geriatric registers of Oulu Age (years): mean 88 (SD 3) Sex: 79% female Inclusion criteria: age ≥ 85; home‐dwelling; ≥ 1 risk factor for falling (≥ 2 falls in previous year, loneliness, poor self‐rated health, poor visual acuity/hearing, depression, poor cognition, impaired balance, chair rise, slow walking speed, difficulty with at least 1 ADL, able to walk outdoors, up or down stairs) Exclusion criteria: none described |
|
| Interventions | 1. Individual balance and gait training: Individual plan for home exercise (3 a day) or group exercise, walking exercises, self‐care exercises (duration and frequency not described). Interventions carried out by OT or physiotherapist or both 2. Control: asked to visit GP without written intervention form | |
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 16 months median falls follow‐up | |
| Adherence | Not reported | |
| Notes | Source of funding: Ministry of Health and Social Affairs of Finland Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done by the study statistician using a random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Ascertinment of falls was the same in each group and performed by blinded assessor Quote: "Fall recording was based on regular phone calls to all participants made every second month by a research nurse ... unaware of the randomization and the interventions." |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall data were missing (49%) |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | High risk | Interval recall Quote: "Fall recording was based on regular telephone interviews once in 2 months, but did not include diary reporting" |
Madureira 2007.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: São Paulo, Brazil Number of participants: 66 Number analysed: 60 Number lost to follow‐up: 6 Sample: women attending osteometabolic disease outpatient clinic Age (years): mean 74 (SD 4.7) Sex: 100% female Inclusion criteria: aged > 65; with osteoporosis Exclusion criteria: secondary osteoporosis, visual deficiency, hearing deficiency, vestibular alteration, unable to walk more than 10 m independently, contraindications for exercise training; planning to be out of town for > 4 weeks during study |
|
| Interventions | 1. Group‐based balance training and walking plus home practice: 1 hour a week for 40 weeks. Encouraged to continue same exercises at home, 30 minutes 3 a week 2. Control: osteoporosis treatment, "instructions to prevent falls", and 3‐monthly clinic visits | |
| Outcomes | 1. Rate of falls | |
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by class participation and frequency of home exercises 1. Group‐based balance training and walking plus home practice: 60% attended all exercise sessions at the club; 77% performed home exercises ≥ 1 a week, 40% exercised every day and 37% performed the exercises 1 ‐ 4 a week |
|
| Notes | Source of funding: not reported Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomized consecutively into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blind to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | In both groups, falls were self‐reported but recorded in medical record every 3 months by "the Osteometabolic Outpatient Clinic physician blinded to the group assignment" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall data were missing (6%). Missing data were balanced between the interention (n = 3) and control (n = 3) groups |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of fallers was not reported. Adverse events were not reported |
| Method of ascertaining falls (recall bias) | Unclear risk | Quote: "During the study, patients in both groups received a calendar and were instructed to write down falls, which were included in the same electronic medical record every 3 months by the Osteometabolic Outpatient Clinic physician blinded to the group assignment." No mention of more frequent telephone follow‐up |
McMurdo 1997.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 24 months |
|
| Participants | Setting: Dundee, Scotland UK Number of participants: 118 Number analysed: 92 Number lost to follow‐up: 26 Sample: women recruited by advertisement Age (years): mean 64.5, range 60 ‐ 73 Sex" 100% female Inclusion criteria: community‐dwelling; post‐menopausal Exclusion criteria: conditions or drug treatment likely to affect bone |
|
| Interventions | 1. Group‐based balance training: programme of weight‐bearing exercise to music, 45 minutes, 3 a week, 30 weeks a year, over 2 years, plus 1000 mg calcium carbonate daily 2. Control: 1000 mg calcium carbonate daily | |
| Outcomes | 1. Rate of falls
2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who experienced 1 or more fall‐related fractures |
|
| Duration of the study | 104 weeks | |
| Adherence | Adherence measured by class attendance. Mean tablet complicance was 97% in both groups 1. Group‐based balance training group: Mean class attendance, 76%; range 46 ‐ 100% |
|
| Notes | Source of funding: Scottish Home and Health Department; Renacare supplied calcium carbonate tablets Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blind to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Falls reported by participants who were aware of their group allocation. Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Method of recording fractures is unclear |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall data were missing (26%) |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Unclear risk | No description about ascertainment of falls |
Means 2005.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months |
|
| Participants | Setting: Arkansas, USA Number of participants: 338 Number analysed: 238 Number lost to follow‐up: 100 Sample: volunteers from 17 senior citizens' centres Age (years): mean 73.5 Sex: 57% female Inclusion criteria: aged ≥ 65 years; able to walk at least 30 feet without assistance from others; able to follow instructions and give consent Exclusion criteria: resident in a nursing home; acute medical problems; cognitive impairment |
|
| Interventions | 1. Group‐based balance, strength, flexibility, gait training and walking: self‐perceived moderate intensity, 90‐minute sessions, 3 a week for 6 weeks 2. Control: group seminars on non‐health‐related topics of interest to senior citizens. Same time and frequency as intervention group | |
| Outcomes | 1. Rate of falls
2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who died |
|
| Duration of the study | 26 weeks | |
| Adherence | Adherence measured by retention/attrition rate Attrition data: 1. Group‐based balance, strength, flexibility, gait training and walking: n = 12 never attended exercise sessions after 6 weeks 2. Control: n = 23 never attended seminars after 6 weeks |
|
| Notes | Source of funding: National Institute on Aging, Rehabilitation Research and Development Service, Department of Veterans Affairs Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by coin flip |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and treatment personnel not mentioned in report, but unlikely. Insufficient information to make judgement on impact of lack of blinding. |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls reported using the same method in each group, by participants who were aware of their group allocation. Assessor blinded to group allocation |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Adverse events were obtained in the same manner in each group. Quote: "Research staff .. involved in collection of evaluation data did not know the participants' group assignemnt at the time of their evaluation". Adverse events were self‐reported and were not clarified using medical records |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall data were missing (30%) |
| Selective reporting (reporting bias) | Unclear risk | Prespecified falls outcomes reported. No prospective trial registration or protocol |
| Method of ascertaining falls (recall bias) | Low risk | Prospective. Recorded on pre‐printed postcards weekly with telephone calls to non‐correspondents to optimise compliance |
Merom 2016.
| Methods | Study design: Cluster‐RCT Number of study arms: 2 Number of clusters: 23 Length of follow‐up: 12 months |
|
| Participants | Setting: Sydney, Australia Number of participants: 530 Number analysed: 522 Number lost to follow‐up: 8 Sample: living in retirement village Age (years): Age > 80 years: 39% Sex: 85% female Inclusion criteria: Eligible participants had to be a resident of the village; be able to walk at least 50 m; agree to undergo physical and cognitive testing; plan to stay in the village for the next 12 months; and obtain medical clearance to participate in the study Exclusion criteria: Participants were excluded if they planned to leave the village for 3 months or more during the trial period, or if they scored < 24 on the MMSE in the baseline assessment indicating cognitive impairment |
|
| Interventions | 1. Group‐based social dancing: folk dancing or ballroom dancing classes with gradual increase in cognitive complexity and cardiovascular effort; 1 hour, 2 a week, for 12 months 2. Control group: usual activities, and asked not to join a dance class during the trial period, placed on a wait list for the dance classes at the end of trial. |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Health‐related quality of life 4. Number of people who died |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by session attendance 1. Group‐based social dancing group: median session attendance was 56%, (IQR 26 – 77%) or approximately 45 sessions. The median attendance was lower for folk (55%) than ballroom dancing (60%) |
|
| Notes | Source of funding: NHMRC Economic information: not reported Number of clusters allocated to intervention: 12; number of clusters allocated to control: 11; number of clusters analysed (intervention): 12; number of clusters analysed (control): 11 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation method, constrained using minimisation |
| Allocation concealment (selection bias) | Unclear risk | The relative timing of the randomisation of clusters and recruitment of participants is unclear. It is unclear whether personnel recruiting participants were blinded to intervention group to which the cluster was randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls were recorded using the same method in each group Quote: "The recording of falls from participant diaries was performed by research staff blind to allocation" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants were not blinded to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall data were missing (1%). There were missing fall data from an equal number of participants in the intervention group (n = 4) and the control group (n = 4). The reason for missing fall data was not clear |
| Selective reporting (reporting bias) | Low risk | Prespecified falls and adverse event outcomes reported. Prospective trial registration available and specifies the same fall outcomes as those in the trial report |
| Method of ascertaining falls (recall bias) | Low risk | Participants were asked to record “F” (fall) or “N” (no fall) each day using monthly calendars (diaries), which were returned by mail at the end of each month. Participants who reported a fall were interviewed by telephone to obtain details about where the fall(s) occurred; whether the fall resulted in injuries; and whether any treatment was sought. Participants who did not return their calendars within 2 weeks were telephoned by study researchers and verbal responses were recorded. At the end of the call, they were also requested to return their calendar by mail to maintain completeness |
| Cluster‐randomised trials | Unclear risk | The relative timing of the randomisation of clusters and recruitment of participants is unclear. There was attempt at concealment, Quote: "Retirement villages were randomised by the trial statistician... The trial statistician advised the study coordinator of the village’s allocation, and the study coordinator arranged the delivery of the intervention. Allocation was thus concealed from the research team that were recruiting villages and participants and performing the baseline assessments" Baseline comparison of the intervention arms is reported, but not baseline comparability of clusters; Quote: "Retention to the 12‐month assessment varied markedly by village ranging from 60% to 92%"; accounted for the clustered design in the analysis; results comparable with individually‐randomised trials |
Miko 2017.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: Budapest, Hungary Number of participants: 100 Number analysed: 97 Number lost to follow‐up: 3 Sample: community‐dwelling women Age (years): Intervention group mean 69.3 (SD 4.6), Control group mean 69.1 (SD 5.3) Sex: 100% female Inclusion criteria: women with osteoporosis, classified using the World Health Organization diagnostic criteria for established osteoporosis in postmenopausal women were eligible: bone mineral density T‐score lower than −2.5 in the lumbar spine, femoral neck or total femur region, and a history of at least 1 osteoporotic fracture Exclusion criteria: visual deficiency, severe auditiory or vestibular deficiency, advanced locomotor diseases, women who used assistive walking devices or who were unable to walk independently more than 10 metres, progressive neurological or unstable cardiovascular diseases and participation in a regular physical exercise programme in the past 6 months |
|
| Interventions | 1. Individual, partially‐supervised balance training: supervised by physiotherapist in back, torso and lower‐extremity muscle‐strengthening exercises and balance training. Progressed through 3 levels; 30‐minute sessions, 2 a week, for 1 year, plus home programme 1 hour a day 2. Control group: Received osteoporosis treatment only |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) |
|
| Duration of the study | 52 weeks | |
| Adherence | Not reported | |
| Notes | Source of funding: no funding received Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Numbered series of prefilled envelopes. Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A numbered series of prefilled envelopes specifying the group". No report of the location and whether envelopes opaque or sealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Falls reported using same method in each group. Unclear if personnel recording/confirming fall outcomes were blind to group allocation |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall data were missing (3%). There were missing fall data from 1 intervention participant (due to loss of interest) and 2 control participants (1 due to loss of interest, 1 without explanation). |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Low risk | Fall diary kept to record any fall and the circumstances. No follow‐up phone calls noted |
Mirelman 2016.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months |
|
| Participants | Setting: Belgium, Israel, Italy, the Netherlands, and the UK Number of participants: 152 Number analysed: no fall data Sample: community‐dwelling Age (years): mean 82.6 Sex: 35% female Inclusion criteria: aged 60 − 90 years, able to walk ≥ 5 minutes unassisted, stable medication for the past month, self‐reported ≥ 2 falls within 6 months before screening; individuals with mild cognitive impairment were included if they had a score of 0·5 on the Clinical Dementia Rating scale Exclusion criteria: psychiatric comorbidity (e.g. major depressive disorder in accordance with DSM IV criteria); history of stroke, traumatic brain injury, or other neurological disorders (other than Parkinson’s disease and mild cognitive impairment, for those groups); acute lower back or lower extremity pain; peripheral neuropathy; rheumatic and orthopaedic diseases; or a clinical diagnosis of dementia or severe cognitive impairment (MMSE score < 21) |
|
| Interventions | 1. Individual, supervised treadmill training: progressed with treadmill duration and speed; 45‐minute session, 3 a week for 6 weeks 2. Individual, supervised treadmill training plus virtual reality: as (1) plus received projected images of the virtual environment (e.g. obstacles, distractors) that necessitated continual adjustment of steps; 45‐minute session, 3 a week for 6 weeks |
|
| Outcomes | 1. Health‐related quality of life | |
| Duration of the study | 26 weeks | |
| Adherence | Adherence measured by number of completed sessions of the 18 sessions: 1. Individual, supervised treadmill training: 16·82 (SD 1·81) 2. Individual, supervised treadmill training plus virtual reality: 16·62 (SD 1·78) |
|
| Notes | At baseline 130 participants had Parkinson's disease, 43 mild cognitive impairment, 109 idiopathic falls. Falls data unavailable only for non‐Parkinson's disease participants Source of funding: European Commission Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "By use of computer‐based allocation, participants were randomly assigned" |
| Allocation concealment (selection bias) | Low risk | Group allocation performed by a third party not involved in the day‐to‐day running of the study; treating therapist notified by e‐mail to ensure concealed allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls recorded using same method in each group Quote: "Falls were recorded without knowledge of training group" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | The method of recording adverse events was unclear |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants unblinded to intervention group |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% had missing data (7%) for the study. Missing data were balanced between the treadmill training group (n = 12) and treadmill plus virtual reality group (n = 8), with reasons for missing data similar between groups (e.g. 2 adverse events in treadmill group, 3 adverse events in virtual reality group) |
| Selective reporting (reporting bias) | High risk | Falls measured, but number of fallers is not presented |
| Method of ascertaining falls (recall bias) | Low risk | Participants received a falls calendar, which they were provided as a paper version, web‐based calender, or a smartphone application. Research staff contacted all participants every month to maximise compliance |
Morgan 2004.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: community and assisted‐living facilities Florida, USA Number of participants: 294 Number analysed: 229 Number lost to follow‐up: 65 Sample: recruited from Miami Department of Veterans Affairs Medical Centre, 9 assisted‐living facilities, private physical therapy clinic Age (years): mean 80.5 (SD 7.5) Sex: 71% female Inclusion criteria: aged ≥ 60; hospital admission or bedrest for ≥ 2 days in previous month Exclusion criteria: medical conditions precluding exercise programme (angina, severe osteoporosis, etc.); MMSE < 23 (unable to follow instructions); using oxygen therapy at home; planned inpatient treatment or evaluation in 2 months following recruitment; requiring human assistance, wheelchair or artificial limbs to walk |
|
| Interventions | 1. Group‐based strength, balance and gait training: seated and standing exercises with no special equipment used, supervised by a physical therapist assisted by a physical therapy assistant; 45 minutes, 3 a week for 8 weeks 2. Control: usual activities | |
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by completion of scheduled exercise sessions 1. Group‐based strength, balance and gait training: completed an average of 70% of the 24 scheduled exercise sessions |
|
| Notes | Source of funding: not reported Economic information: not reported SAFE‐GRIP (Study to Assess Falls among Elderly Geriatric Rehabilitation Intensive Program) Email communication about fall data, response received, data not included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stratified by sex, age (< 75 and ≥ 75), falls history in previous month (fall/no fall). Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blinded to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Blinding not described. Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall data are missing (22%) |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of falls was not reported. Adverse events were not reported |
| Method of ascertaining falls (recall bias) | Low risk | Prospective. Pre‐dated postcard diaries returned every 2 weeks |
Morone 2016.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 3 months |
|
| Participants | Setting: Italy Number of participants: 38 Number analysed: 38 Number lost to follow‐up: 0 Sample: community‐dwelling Age (years): mean 68.93 (SD 4.18) Sex: 100% female Inclusion criteria: women; no or irregular physical or educational programmes for balance (or not performed for the last 2 years); age > 65 years; presence of a reduction in balance measured by the Berg Balance Scale (< 45); presence of bone loss (T score > 1.5 and < 2.5) as measured by central DEXA scan Exclusion criteria: presence of any orthopaedic, cardiovascular or oncologic pathology that could affect the balance ability; fracture/s in past year |
|
| Interventions | 1. Group‐based balance training using Wii‐Fit: Wii Fit programme (balance, yoga, standing leg strengthening) supervised by a physiotherapist, 1‐hour session, 2 a week for 8 weeks 2. Group‐based balance training: conventional balance exercises (flexibility, lying muscle strengthening, balance on unstable balance platform, postural exercises in supine) supervised by a physiotherapist, 1‐hour session, 2 a week for 8 weeks |
|
| Outcomes | No outcomes included in review | |
| Duration of the study | 12 weeks | |
| Adherence | Not reported | |
| Notes | Source of funding: not reported Economic information: not reported No fall data in paper. Email communication about fall data, no response received. No fall data included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated list" |
| Allocation concealment (selection bias) | Low risk | Quote: "allocation was concealed by covering each number of the list with an opaque adhesive label" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to group allocation. Effect of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Falls were recorded using the same method in both groups. It is unclear whether assessors were blinded when collecting fall data |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants not blind to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | No fall data available |
| Selective reporting (reporting bias) | High risk | Fall outcome prespecified but fall data not presented |
| Method of ascertaining falls (recall bias) | Unclear risk | Quote: "participants enrolled in both groups recorded in a specific diary the falls or events related to falls during the 3‐month follow‐up" |
Morrison 2018.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 3 months |
|
| Participants | Setting: Virginia, USA Number of participants: 65 Number analysed: 46 Number lost to follow‐up: 19 Sample: community‐dwelling Age (years): mean 66.99 (SD 5.42) Sex: 48% female Inclusion criteria: Type 2 diabetes Exclusion criteria: significant cardiovascular disease, unstable proliferative retinopathy, end‐stage renal disease, or uncontrolled hypertension; no balance or resistance training during the previous year |
|
| Interventions | 1. Group‐based balance training: balance and postural control exercises closely mimicking the type of training performed during unsupervised (Wii Fit) training. a) warm‐up (lower‐limb stretching); b) mostly balance exercises including heel‐toe walking. calf raises, forward leans, single‐leg balance, and basic yoga stretches (the yoga stretches selected were the same as those offered within the Wii program); 40‐minute sessions, 3 a week for 12 weeks 2. Home‐based strength, balance and aerobic Wii Fit programme: aerobics, yoga, strength training, and balance using the Wii Fit Balance System and software programme. 1‐hour interactive tutorial on using the equipment, exercised unsupervised at home, 40‐minute sessions, 3 a week for 12 weeks |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls |
|
| Duration of the study | 12 weeks | |
| Adherence | Not reported | |
| Notes | Source of funding: American Diabetes Association Economic information: not reported Email communication to obtain fall data, response received, data included in review (there were no falls) Data could not be analysed due to zero events for falls (and thus fallers) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Concealment not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not specified |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Falls was measured using the same measures in all groups. Blinding not specified |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | Large loss (> 20%) to follow‐up |
| Selective reporting (reporting bias) | High risk | Fall outcome prespecified but fall data not presented |
| Method of ascertaining falls (recall bias) | High risk | Quote: "Individuals were instructed to record the number of falls they had during the 12‐week exercise intervention" |
Ng 2015.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: Singapore Number of participants: 98 Number analysed: 92 Number lost to follow‐up: 6 Sample: community‐dwelling Age (years): mean 70.0 (SD 4.7) Sex: 61% female Inclusion criteria: Prefrail and frail older adults were identified based on 5 CHS criteria defining physical frailty: unintentional weight loss, slowness, weakness, exhaustion, and low activity,which were scored 1 if present and 0 if absent. The total summed scores ranging from 0 to 5 were used to classify a participant as robust (score = 0), prefrail (score = 1 to 2), or frail (score = 3 to 5). Prefrail or frail older adults were eligible for the trial if they were aged 65 years and above, able to walk without personal assistance, and living at home Exclusion criteria: People were excluded if they had significant cognitive impairment (MMSE score 23 or less); major depression; severe audiovisual impairment; any progressive,degenerative neurologic disease; terminal illness with life expectancy < 12 months; were participating in other interventional studies; or were unavailable to participate for the full duration of the study |
|
| Interventions | Randomised into 5 groups: 4 intervention groups (1 physical exercise group, 1 nutritional intervention group, 1 cognitive training group, 1 combination intervention group) and 1 control group. Only the physical exercise group and control group were included in this review 1. Group‐based strength and balance training plus home practice: resistance and functional exercises of moderate and tailored to progress in intensity; using free weights, different floor surfaces, treadmill; 90 minutes, 2 a week for 12 weeks, and 12‐week home programme 2. Control group: access to 1 standard care from health and aged care services that were normally available to older people, and given artificially sweetened liquid, 2 capsules and 1 tablet (ingredients: cornstarch, lactose, magnesium stearate) |
|
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) 2. Number of people who died |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by training sessions completed 1. Group‐based strength and balance training plus home practice: 85% compliance 2. Control group: 94% compliance |
|
| Notes | Source of funding: NHMRC Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Central computerized randomization procedure" |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment was allocated by a project manager not involved in the enrollment, intervention,or assessment." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Quote: "Outcome assessments were performed at baseline, 3 months, 6 months, and 12 months by assessors who were blinded to the participants’ group allocation". Falls were self‐reported at these time points. Falls were measured using the same method in all groups |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | High risk | Adverse events were recorded by the interventional nurses who also administered treatment and were therefore not blinded to group |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall data are missing (6%). Missing data were blanced in the 2 groups (physical training: 1 withdrew, 1 unable to contact; control: 3 withdrew, 1 died) |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of fallers was not reported. Adverse events were not reported |
| Method of ascertaining falls (recall bias) | High risk | Falls were self‐reported at 3‐month, 6‐month, and 12‐month assessments |
Nitz 2004.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months |
|
| Participants | Setting: Brisbane, Australia Number of participants: 73 Number analysed: 45 Number lost to follow‐up: 28 Sample: volunteers recruited through advertising and fliers Age (years): mean 75.8 (SD 7.8) Sex: 92% female Inclusion criteria: aged > 60; living independently in the community; at least 1 fall in previous year Exclusion criteria: unstable cardiac condition, living too far from exercise class site, unable to guarantee regular attendance |
|
| Interventions | 1. Group‐based balance: using workstation (circuit training) format, 1 hour a week for 10 weeks 2. Control: Group‐based gentle exercise and stretching, 1 hour a week for 10 weeks | |
| Outcomes | 1. Rate of falls | |
| Duration of the study | 24 weeks | |
| Adherence | Adherence measured as participants who completed the study 1. Group‐based balance group: 24 2. Group‐based gentle exercise and stretching group: 21 |
|
| Notes | Source of funding: not reported Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blinded to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Quote: "Partipants used a calendar on which each day was marked for a fall ... or incident free day" Quote: "The physiotherapists who undertook all assessments of the participants were blinded to the intervention group allocation" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall data are missing (38%) |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of fallers was not reported. Adverse events were not reported |
| Method of ascertaining falls (recall bias) | Low risk | Falls ascertained by marked calendar returned monthly |
Okubo 2016.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 16 months |
|
| Participants | Setting: Japan Number of participants: 105 Number analysed: 90 Number lost to follow‐up: 15 Sample: community‐dwelling Age (years): mean 70.1 (3.8) Sex: 63% female Inclusion criteria: aged 65 ‐ 79 years, not care‐dependent or support‐dependent, on a Japanese long‐term care insurance system, not restricted from exercising by a doctor, without regular exercise habits Exclusion criteria: high risk of falling (≥ 2 of the following: using a walking aid, knee pain, using 4 or more medications, history of recurrent falls/fractures during the previous year), were unable to participate in either of the 2 groups or had participated in another clinical trial during the previous year |
|
| Interventions | 1. Group‐based Tai Chi and Otago plus home practice: health lectures (20 minutes), warm‐up (10 ‐ 15 minutes), recreational activity (0 ‐ 10 minutes), balance training and muscle strengthening of the legs, based on OEP (15 ‐ 20 minutes) and Tai Chi (30 ‐ 40 minutes), and a cool‐down (10 ‐ 15 minutes); 2‐hour sessions, 1 a week for 12 weeks. Home balance and muscle strengthening exercises, 3 ‐ 5 days a week during 3‐month supervised and 13‐month unsupervised periods 2. Group‐based brisk walking: health lectures (20 minutes), a warm‐up (10 ‐ 15 minutes), recreational activity (0 ‐ 10 minutes), brisk walking on a pedestrian road (30 ‐ 50 minutes) and a cool‐down (10 ‐ 15 minutes). 2‐hour sessions, 1 a week. Home exercise of walking for 30 ‐ 50 minutes, 3 ‐ 5 days a week was also recommended during the 3‐month supervised and 13‐month unsupervised follow‐up periods | |
| Outcomes | No outcomes included in the review | |
| Duration of the study | 56 weeks | |
| Adherence | 1. Group exercise: an average of 1.4 ± 0.5 sets/day were carried out for 4.6 ± 2.0 days/week 2. Group exercise: an average of 45.2 ± 24.5 min/day of walking for 4.3 ± 1.7 days/week | |
| Notes | Source of funding: Japan Society for the Promotion of Science Economic information: not reported Email communication about fall data, response received, data not included in review. Falls outcomes reported in trial were 'falls per physically active person‐day' and 'falls per person‐step' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “computer‐generated random numbers” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blinded to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | High risk | No blinding was applied |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of fall data were missing (14%). There were missing data from 10 walking‐group participants (knee pain n = 3, time issue n = 6, misfortune n = 1) and 5 balance‐group participants (knee pain n = 1, time issue n = 3, transfer issue n = 1) |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of fallers was not reported. Adverse events were not reported |
| Method of ascertaining falls (recall bias) | Low risk | Participants were asked to record the number of falls and trips daily in their fall calendars, and turn them in every month until the end of the 16th month. Falls from bicycles were excluded |
Park 2008.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 11 months |
|
| Participants | Setting: Korea Number of participants: 50 Number analysed: 45 Number lost to follow‐up: 5 Sample: Community‐dwelling participants in a community learning centre for seniors and senior members of local clubs Age (years): mean 68.35 (SD 3.47) Sex: 100% female Inclusion criteria: community‐dwelling (e.g. in a private dwelling, apartment, residential facility); ambulatory (with or without an aid); competent to give consent; residents of Busan, Korea; aged 65 years Exclusion criteria: < 5 years after menopause; history of chronic disease that might influence BMD, physical activity and balance ability; history of ovariectomy or diseases known to affect bone metabolism (e.g. cancer, renal disease, rheumatoid arthritis); current medication with bisphosphonate, oestrogens, or other hormonal preparations; weigh > 130% ideal body weight; other contraindications to participating in a regular exercise programme; already doing moderate or hard exercise for more than 7 hours a week |
|
| Interventions | 1. Exercise group: Stretching for 9 minutes, strength training for 10 minutes followed by 23 minutes of weight‐bearing exercise at an intensity above 65 – 75% of the maximal heart rate, and 18 minutes of balance and posture correction training. The programme was conducted 3 times a week for 48 weeks 2. Control group: retained their sedentary lifestyle participation in physical exercise |
|
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 48 weeks | |
| Adherence | Not reported | |
| Notes | Source of funding: Korea Science and Engineering Foundation Economic information: not reported Email communication regarding fall data, response received, data not included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomly assigned (by a computer generated program)" |
| Allocation concealment (selection bias) | Unclear risk | Concealment not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blinded to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Blinding not specified |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall data were missing (10%). Missing data were balanced in intervention (n = 3) and control (n = 2) groups. The reason for missing data was unclear |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of falls was not reported. Adverse events were not reported |
| Method of ascertaining falls (recall bias) | High risk | Retrospective. Participants were asked "Did you have any falls during the past one year? What was the reason for the fall?" |
Reinsch 1992.
| Methods | RCT (cluster‐randomised by senior centre. 2 x 2 factorial design) Study design: Cluster‐RCT Number of study arms: 2 Number of clusters: 16 Length of follow‐up: 12 months |
|
| Participants | Setting: Los Angeles County and Orange County, California, USA Number of participants: 230 Number analysed: 230 Number lost to follow‐up: 0 Sample: recruited from 16 senior centres Age (years): mean 74.2 (SD 6.0) Sex: 80% female Inclusion criteria: aged > 60 Exclusion criteria: none listed |
|
| Interventions | Randomised into 4 groups: 3 intervention groups (1 group‐based balance and strength training, 1 cognitive‐behavioural training, 1 exercise and cognitive training) and 1 control group (discussion group). Only the group‐based balance and strength training and control group were included in this review 1. Group‐based balance and strength training: no special equipment used; 1 hour, 3 a week for 52 weeks 2. Control group: health and interest discussion group, 1 hour, 1 a week for 52 weeks |
|
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 52 weeks | |
| Adherence | Dropout/noncompliance defined as missing ⅓ or more of the classes taught at their centre 1. Group‐based balance and strength training: 13/57 noncompliance (44/57 compliance) 2. Control group: 8/50 noncompliance (42/50 compliance) |
|
| Notes | Source of funding: NIH, AARP Andrus Foundation, Roosevelt Warm Springs Foundation Economic information: not reported MacRae paper includes a subset of results for only 2 arms of the study, in Los Angeles county only Number of clusters allocated to intervention: 4; number of clusters allocated to control: 4; number of clusters analysed (intervention): 4; number of clusters analysed (control): 4 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned to treatments" |
| Allocation concealment (selection bias) | High risk | Quote:" A biostatistician not involved in the study randomized general practices into the intervention or control group by using computer‐generated random numbers. After the randomization, the general practitioners enrolled patients for the study according to the inclusion and exclusion criteria". The method of concealment is not described and assume the recruiting general practitioners were unblinded and may have had knowledge of participant characteristics |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blinded to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Falls reported by participants who were aware of their group allocation. Blinding of research assistant not described |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Method for recording medical attention and adverse events was unclear. Appears to be self‐report |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of falls was not reported |
| Method of ascertaining falls (recall bias) | Low risk | Prospective. Monthly diaries plus weekly phone calls or visits |
| Cluster‐randomised trials | Unclear risk | Individual participant recruitment was undertaken after group allocation. The method of concealment is not described and it is likely that recruitment was undertaken by a person who was unblinded and may have had knowledge of participant characteristics; baseline characteristics of clusters were not reported; missing outcomes for clusters or within clusters were not reported; did not account for the clustered design in the analysis; results comparable with individually‐randomised trials |
Resnick 2002.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months |
|
| Participants | Setting: Baltimore, USA Number of participants: 20 Number analysed: 17 Number lost to follow‐up: 3 Sample: women in a continuing‐care retirement community Age (years): mean 88 (SD 3.7) Sex: 100% female Inclusion criteria: able to walk 50 feet with or without assistive device; sedentary lifestyle Exclusion criteria: cognitive impairment (MMSE > 20); terminal illness; medical condition precluding participation in aerobic exercise |
|
| Interventions | 1. Individual or group‐based walking: with visits from nurse practitioner to support and set goals, exercise for 20 minutes, 3 a week, for 6 months 2. Control: no intervention | |
| Outcomes | 1. Health‐related quality of life | |
| Duration of the study | 26 weeks | |
| Adherence | Adherence measured by meeting the recommended 20 minutes, 3 a week walking programme 1. Individual or group‐based walking group: 7 participants adhered to the recommended walking programme. 2 engaged in a regular walking programme but did not meet the recommended 20 minutes 3 a week. 1 did not engage in any exercise 2. Control group: 0 participants started an exercise programme during the course of the study |
|
| Notes | Source of funding: not reported Economic information: not reported Participants lived independently in apartments, and could walk independently. (Personal correspondence). Pilot study with no usable data. Email communication about fall data, response received, data not included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by coin flip (personal communication as reported by Gillespie 2012) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blind to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Falls reported by participants who were aware of their group allocation. Blinding of research assistant not described. |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants not blinded to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | No fall data available |
| Selective reporting (reporting bias) | High risk | Fall outcome prespecified but fall data not presented |
| Method of ascertaining falls (recall bias) | Unclear risk | Quote: "based on self‐report". No additional information |
Robertson 2001a.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: West Auckland, New Zealand Number of participants: 240 Number analysed: 240 Number lost to follow‐up: 0 Sample: identified from computerised registers at 17 general practices Age (years): mean 80.9 (SD 4.2), range 75 ‐ 95 Sex: 68% female Inclusion criteria: aged ≥ 75; living at home Exclusion criteria: unable to walk around own residence; already receiving physiotherapy; unable to understand trial requirements |
|
| Interventions | 1. Individual Otago Exercise Programme: home exercises plus walking plan prescribed by nurse at 1 week (1 hour) and at 2, 4, 8 weeks, and 6 months (half‐hour) plus monthly telephone call to maintain motivation; exercised 3 a week and walked 2 a week for 1 year 2. Control: usual care | |
| Outcomes | 1. Rate of falls
2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who experienced 1 or more fall‐related fractures 4. Number of people who experienced 1 or more falls requiring medical attention 5. Number of people who died |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by completion of the trial, frequency of exercise programme 1. Individual Otago Exercise Programme: 113 participants completed the trial. 43% (n = 49) carried out their exercise programme ≥ 3 times a week. 72% (n = 81) carried out their exercise programme ≥ 2 times a week and 71% (n = 80) walked at least ≥ 2 times a week during the 1‐year follow‐up 2. Control: 98 participants completed the trial |
|
| Notes | Source of funding: Health Funding Authority Northern Division, Accident Rehabilitation and Compensation Insurance Corporation of New Zealand, Trustbank Otago Community Trust medical research fellowship Economic information: Mean cost per person (intervention) in community health service setting NZD 432 for 1 year. Healthcare service costs: 5 hospital admissions due to fall injuries in control group, none in exercise group (cost savings of NZD 47,818). Incremental cost per fall prevented/per QALY gained: NZD 1803 per fall prevented (programme implementation costs only), NZD 7471 per injurious fall prevented (programme implementation costs only), NZD 155 per fall prevented (programme implementation costs and hospital admission cost savings), NZD 640 per injurious fall prevented (programme implementation costs and hospital admission cost savings). District nurse had no previous experience in exercise prescription. Received 1 week's training from research group's physiotherapist, Mean who also made site visits and phone calls to monitor quality. Otago Exercise Programme manual can be obtained from www.cdc.gov/HomeandRecreationalSafety/Falls/compendium/1.2_otago.html. Cost‐effectiveness analysis reported in primary reference |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using allocation schedule developed using computer‐generated numbers |
| Allocation concealment (selection bias) | Low risk | Assignment by independent person off‐site |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blinded to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls reported by participants who were aware of their group allocation. Phoned by independent assessor blinded to allocation. Person classifying fall events also blinded to allocation |
| Blinding of outcome assessment (detection bias) Fractures | Low risk | A blinded assessor telephoned participants who fell to record injuries as a result of the fall. Quote: “The circumstances of “serious” injuries were confirmed from hospital and general practice records. The investigator classifying fall events remained blind to group allocation” |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Low risk | A blinded assessor telephoned participants who fell to record injuries as a result of the fall. Quote: “The circumstances of “serious” injuries were confirmed from hospital and general practice records. The investigator classifying fall events remained blind to group allocation” |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Low risk | Active fall registration with daily postcard calendars returned monthly, plus telephone calls |
Rubenstein 2000.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 3 months |
|
| Participants | Setting: California, USA Number of participants: 59 Number analysed: 59 Number lost to follow‐up: 0 Sample: men recruited from Veterans Administration ambulatory care centre (volunteers) Age (years): mean 74 Sex: 0% female Inclusion criteria: aged ≥ 70; ambulatory; ≥ 1 fall risk factor: lower limb weakness, impaired gait, impaired balance, > 1 fall in previous 6 months Exclusion criteria: exercised regularly; severe cardiac or pulmonary disease; terminal illness; severe joint pain; dementia; medically unresponsive depression; progressive neurological disease |
|
| Interventions | 1. Group‐based balance, strength and endurance: using free weights, elastic bands, bicycle, treadmill; 90 minutes, 3 a week for 12 weeks 2. Control: usual activities | |
| Outcomes | 1. Rate of falls
2. Number of people who experienced 1 or more falls (risk of falling) 3. Health‐related quality of life |
|
| Duration of the study | 12 weeks | |
| Adherence | Adherence measured by session attendance 1. Group‐based balance, strength and endurance group: attended 84% of the exercise sessions |
|
| Notes | Source of funding: Department of Veterans Affairs, Health Services Research and Development Service, Disabled American Veterans Charities of Greater Los Angeles Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in blocks of 16 to 20 at 3‐ to 6‐month intervals, using randomly‐generated sequence cards in sealed envelopes |
| Allocation concealment (selection bias) | Unclear risk | Cards in sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blind to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | High risk | Falls reported by participants who were aware of their group allocation. Fall data were gathered in different settings for the intervention and control goups. The person ascertaining falls was aware of group allocation |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Method of recording fractures is unclear |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Method of recording adverse events is unclear |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | Unclear risk | Falls were prespecified in Methods section and reported in Results. Adverse events not prespecified. No protocol paper or prospective trial registration |
| Method of ascertaining falls (recall bias) | Unclear risk | No active fall registration. Fall ascertainment for intervention group at weekly classes. Controls phoned every 2 weeks |
Sakamoto 2013.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months |
|
| Participants | Setting: Japan Number of participants: 1365 Number analysed: 865 Number lost to follow‐up: 500 Sample: community‐dwelling Age (years): Intervention: male: mean 80.5 (SD 4.1); female: mean 80.1 (SD 4) Control: male: mean 80.7 (SD 4); female: mean 80.5 (SD 4.1) Sex: 82% female Inclusion criteria: > 75 years of age, lived at home and visited an orthopaedic clinic or hospital for an orthopaedic handicap and could stand on 1 leg (both right and left, with the eyes open for ≤ 15 seconds (the Ministry of Health, Labour, and Welfare of Japan designates men and women 75+ years of age who can stand on 1 leg with eyes open for ≤ 15 s as having musculoskeletal ambulation disability symptom complex), ability to communicate and those who could continue training Exclusion criteria: People with Parkinson’s disease or other conditions that made them susceptible to falls, people with artificial joints, and people with cognitive disorders |
|
| Interventions | 1. 1‐leg stand balance training: trained each leg with eyes open for 1 minute, 3 a day for 6 months 2. Control group: no intervention |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who experienced 1 or more fall‐related fractures |
|
| Duration of the study | 26 weeks | |
| Adherence | Not reported | |
| Notes | Source of funding: Ministry of Health, Labour, and Welfare of Japan Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 10 x 5 (= 50) random number tables with 5 x 5 (25) numbers were prepared and 2 ten‐faced dice (one green, one yellow) were thrown to decide which table to use. Two six‐faced dice were then thrown to select the number within the chosen random number table to decide whether the institution would be designated an exercise or non‐exercise institution" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not specified but assume participants and personnel were unblinded. Impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | The record of falls/exercise was checked at an outpatient orthopaedic clinic monthly. Blinding not specified |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Self‐report on calendar, then fracture was confirmed and recorded by a doctor. Unclear if doctor was blinded to group |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Participants surveyed at 6 months for adverse events. Blinding not specified |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall data missing (37%) |
| Selective reporting (reporting bias) | Unclear risk | Falls and adverse events were prespecified in Methods section and reported in Results. No protocol paper or prospective trial registration |
| Method of ascertaining falls (recall bias) | Low risk | Instructed to record exercise/falls/fracture every day. The record was checked at the time of examination at outpatient orthopaedic clinic once a month |
Sales 2017.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: Australia Number of participants: 66 Number analysed: 48 Number lost to follow‐up: 18 Sample: community‐dwelling Age (years): mean 73.0 (SD 8.3) Sex: 69% female Inclusion: living in the community; aged between 60 and 90 years; 1 or more falls in the previous 12 months or concerned about having a fall; generally active and independent in the community; no more than a single point stick used for regular outdoors walking (at least 3 times a week) Exclusion: any uncontrolled non‐musculoskeletal conditions that would make testing difficult and uncomfortable, such as chronic obstructive airways disease and congestive heart failure; pre‐existing neurological or orthopaedic condition that affects lower‐limb strength; partial foot amputation or ulceration or foot fractures; any uncontrolled musculoskeletal conditions that may affect ambulation (rheumatoid arthritis, gout, etc.); medical condition or physical impairment judged by the medical practitioner to contraindicate inclusion |
|
| Interventions | 1. Group‐based strength, balance, co‐ordination, mobility and flexibility: circuit‐based class, 1‐hour sessions, 2 a week for 18 weeks 2. Control: continue with their usual daily activities. Social activities with research team (9 meetings of 2 hours duration over 18 weeks of intervention) |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Health‐related quality of life 4. Number of people who died |
|
| Duration of the study | 52 weeks | |
| Adherence | Attendance at classes was measured. An average of 35 sessions were run for each group of participants | |
| Notes | Source of funding: Gandel Philanthropy Economic information: not reported Detailed description of exercise intervention given in protocol paper Email communication to obtain fall data, response received, data included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomization stratification by gender... blocks of 12 participants will be recruited at a time" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "opaque not concealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blinded to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | High risk | Quote: "Assessors and participants will not be blinded to their respective group allocation". Assume assessor collating calendars was not blinded |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | High risk | Adverse events were self‐reported after undertaking exercise sessions. Assessors not blinded |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants were not blinded to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall data were missing (27%) |
| Selective reporting (reporting bias) | Low risk | Falls, risk of falls and adverse events are reported and the prospective trial registration prespecifies the same fall outcomes as those in the trial report |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "Participants will be requested to record any falls and physical activity or exercise experienced using a monthly calendar for 12 months from the baseline assessment. At the end of each month the calendar will be returned to the researchers in a reply paid envelope. If the calendar is not returned within two weeks of the end of a month, the participant will be followed up with a phone call". |
Sherrington 2014.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: Sydney, Australia Number of participants: 340 Number analysed: 340 Number lost to follow‐up: 0 Sample: community‐dwelling Age (years): mean 81.2 (SD 8.0) Sex: 74% female Inclusion criteria: aged 60 years and over and had been admitted to and subsequently discharged from 9 aged care, rehabilitation and orthopaedic wards at 4 public hospitals in Sydney, Australia Exclusion criteria: resided in a high‐care residential facility (nursing home); had cognitive impairment (a MMSE score < 24); had insufficient English language to understand procedures; were unable to walk more than 1 m even with an assistive device or the help of 1 person; or had a medical condition precluding a 12‐month home exercise program (e.g. unstable cardiac disease or progressive neurological disease) |
|
| Interventions | 1. Home‐based strength and balance programme: Weight‐bearing Exercise for Better Balance exercise programme + 32‐page education booklet about fall prevention, home programme of lower limb balance and strengthening exercises for 20 ‐ 30‐minute sessions, up to 6 a week for 12 months; home visits: 10 over 12 months 2. Control group: Usual care from health and community services + 32‐page education booklet about fall prevention | |
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Health‐related quality of life 4. Number of people who died |
|
| Duration of the study | 52 weeks | |
| Adherence | Participants who actually exercised 1. Weight‐bearing Exercise group: 1 month: 90%, 3 months: 81%, 8 months: 66%, 12 months: 60% | |
| Notes | Source of funding: Australian National Health and Medical Research Council, Australian National Health and Medical Research Council Research Fellowships Economic information: Mean cost per person (intervention): WEBB AUD 751. Healthcare service costs: WEBB AUD 12,029, usual care AUD 10,327. Incremental costs per fall prevented/per QALY gained: AUD 77,403 per QALY gained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number schedule with randomly‐ordered blocks of 2, 4, and 6 |
| Allocation concealment (selection bias) | Low risk | Quote: “Ensure concealed randomisation to groups, the randomisation schedule was generated in advance by and only accessible to the first author who was not involved in participant recruitment, interviews or assessments” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Same method used to ascertain falls in both groups. Blinded research assistants recorded and confirmed falls |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Method of ascertaining fractures not specified |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Low risk | Adverse events were monitored using the exercise diaries and recorded by blinded assessors |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants were not blinded to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | Low risk | Falls, risk of falls and adverse events are reported and the trial protocol paper prespecifies the same fall outcomes as those in the trial report |
| Method of ascertaining falls (recall bias) | Low risk | Monthly falls calendar. Participants who did not return calendars or who reported a fall were telphoned by blinded research assistants |
Shigematsu 2008.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 8 months |
|
| Participants | Setting: Kawage, Mie, Japan Number of participants: 68 Number analysed: 68 Number lost to follow‐up: 0 Sample: randomly‐selected people meeting inclusion criteria Age (years): mean 69 (SD 3) Sex: 63% female Inclusion criteria: 65 ‐ 74 years old; community‐dwelling Exclusion criteria: severe neurological or cardiovascular disease; mobility‐limiting orthopaedic conditions |
|
| Interventions | 1. Group‐based stepping training on felt mat: step direction and performance progressed on felt mat at own pace, 70‐minute sessions, 2 a week for 12 weeks; group "divided" at 12 weeks and continued sessions for a further 12 weeks 2. Group‐based walking: instructed to increase number of daily steps in supervised outdoor walking, 40‐minute sessions, 1 a week for 12 weeks; as above, group divided and half continued walking for a further 12 weeks | |
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 52 weeks with 32 weeks follow‐up after the intervention | |
| Adherence | Adherence measured by session attendance 1. Group‐based stepping training on felt mat: participants attended 21.8 ± 2.9 of 24 sessions (90.9% ± 12.1%) Dropouts: 0. The participants conscientiously exercised for 40 minutes throughout the regimen 2. Group‐based walking: participants attended 9.3 ± 2.6 of 11 sessions (84.2% ± 23.7%). Dropouts: 5 |
|
| Notes | Source of funding: not reported Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomly allocated.. by a public health nurse who used a computerized random number generation program in which the numbers 0 and 1 corresponded to the two groups, respectively" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study described as "single‐blind", presumably meaning that participants were blind to whether they were in the intervention or control groups as both groups received an exercise intervention. Treatment personnel presumably unblinded but judged that lack of blinding unlikely to introduce bias |
| Blinding of outcome assessment (detection bias) Falls | High risk | Study described as "single‐blind" because both groups received an exercise intervention. Assessors presumably unblinded |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | Unclear risk | Prespecified falls outcomes reported. Adverse events were reported but not prespecified. No protocol paper or trial registration |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "All the persons received a pre‐paid postcard at the beginning of each month, which they returned at the beginning of the next month". Instructed to record falls on a daily basis. Phoned or face‐to‐face interview if falls reported |
Siegrist 2016.
| Methods | Study design: Cluster‐RCT Number of study arms: 2 Number of clusters: 40 Length of follow‐up: 12 months |
|
| Participants | Setting: Munich, Germany Number of participants: 378 Number analysed: 378 Number lost to follow‐up: 0 Sample: community‐dwelling Age (years): mean 78.1 (SD 5.9) Sex: 75% female Inclusion criteria: community‐dwelling senior citizens aged ≥ 65 years with increased physical fall risk included in the trial. Increased fall risk was defined as 1 or more falls in the past 12 months, low physical function (Timed‐up‐and‐Go‐Test or Chair‐Stand‐Test > 10 seconds) or subjective or objective balance deficits or fear of falling. At least 1 criterion was necessary for inclusion into the study. Exclusion criteria: Those individuals who did not live independently or suffered from physical or mental restrictions that interfered with the assessment of physical fall risk or participation in an exercise program were excluded. |
|
| Interventions | 1. Group‐based balance, strength, power and gait training plus home practice: no additional equipment required, increasing levels of difficulty, behavioural aspects, a self‐management programme and perceptual and functional training conducted by a fall prevention instructor (physiotherapist or sports scientist); 1 hour a week for 16 weeks 2. Control group: no guidelines for preventing falls apart from individual GP's experience |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who died |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by session attendance, frequency of home programme 1. Group‐based balance, strength, power and gait training plus home practice group: 82% participated in more than 10 training sessions. 46% of the participants performed the home‐exercise programme 10 times or more (average 6.7 times) |
|
| Notes | Source of funding: Bavarian State Ministry of the Environment and Public Health Economic information: not reported Number of clusters allocated to intervention: 20; number of clusters allocated to control: 20; number of clusters analysed (intervention): 17 (3 general practices dropped out after randomisation and before recruiting participants); number of clusters analysed (control): 16 (4 general practices dropped out after randomisation and before recruiting participants) Email communication to obtain fall data, response received, data included in review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...using computer‐generated random numbers..". |
| Allocation concealment (selection bias) | Unclear risk | Cluster RCT. Individuals were recruited to the trial after the clusters were randomised. It is very likely personnel recruiting participants were not blind to cluster |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blind to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls reported using the same method in both groups and followed‐up by blinded assessor |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | Low risk | Falls and adverse event outcomes were reported as prespecified in protocol paper |
| Method of ascertaining falls (recall bias) | Low risk | Daily fall calendar, posted monthly Quote: "When a fall was reported, detailed information was obtained through structured telephone interviews by trained assistants" |
| Cluster‐randomised trials | Unclear risk | Individuals were recruited to the trial after the clusters were randomised. It is likely personnel recruiting participants were not blind to cluster; baseline comparability of clusters not reported; missing outcomes for clusters or within clusters were not reported (and 7 general practices dropped out after randomisation but before recruiting participants); accounted for the clustered design in the analysis; results comparable with individually‐randomised trials |
Skelton 2005.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 9 months |
|
| Participants | Setting: United Kingdom Number of participants: 81 Number analysed: 81 Number lost to follow‐up: 0 Sample: women recruited using posters, newspapers and radio stations Age (years): mean 72.8 (SD 5.9) Sex: 100% female Inclusion criteria: aged ≥ 65; living independently in own home; ≥ 3 falls in previous year Exclusion criteria: acute rheumatoid arthritis; uncontrolled heart failure or hypertension; significant cognitive impairment; significant neurological disease or impairment; previously diagnosed osteoporosis |
|
| Interventions | 1. Group‐based Falls Management Exercise (FaME) balance and strength training plus home practice: the exercise classes were balance‐specific, individually‐tailored and targeted training for dynamic balance, strength, bone, endurance, flexibility, gait and functional skills, training to improve ‘righting’ or ‘correcting’ skills to avoid a fall, backward‐chaining and functional floor exercises: 1‐hour sessions, 1 a week for 26 weeks; plus home exercises, 30 minutes, 2 a week for 36 weeks 2. Control: no exercise class. Home‐based seated exercises 2 a week for 36 weeks | |
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who experienced fall‐related fractures (outcome not reported by group) 3. Number of people who died |
|
| Duration of the study | Total of 132 weeks on average 46.5 weeks (on average) of pre‐intervention falls monitoring 36 weeks of intervention 49.7 weeks (on average) of follow‐up |
|
| Adherence | Adherence measured by retention/attrition rate 1. Falls Management Exercise group: 17% refused to participate, with another 10% dropping out of the exercise sessions after initial entry |
|
| Notes | Source of funding: Research Into Ageing, Dunhill Medical Trust, Barnwood House Trust, Save and Prosper Educational Trust Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by random‐number tables by an observer unconnected to the trial |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blinded to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls reported by participants who were aware of their group allocation Quote: "The information from the diaries was recorded by an observer blinded to the subject’s group who also contacted subjects if diaries had not been returned for two weeks or more" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Method of ascertaining adverse events is unclear |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall data were missing (14%). Data were missing from 7 participants in the intervention group (ill helath n = 4, nursing home n = 2, death n = 1), and 4 control participants (ill helath n = 2, nursing home n = 1, death n = 1) |
| Selective reporting (reporting bias) | Unclear risk | Fall outcomes were prespecified in protocol paper and reported. Adverse events were not prespecified but were reported |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "Both groups completed daily falls diaries... Diaries were returned every 2 weeks by post to the investigator..." Telephone contact if dairies not returned for 2 weeks or more |
Smulders 2010.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: Nijmegan, Netherlands Number of participants: 96 Number analysed: 92 Number lost to follow‐up: 4 Sample: identified from databases of DXA scans, mail out to members of Dutch Osteoporosis Patient Council; advertising Age (years): mean 71.0 (SD 4.7) Sex: 94% female Inclusion criteria: community‐dwelling; aged > 65; osteoporosis (DXA; femoral neck or lower‐back T score ≤ −2.5); ≥ 1 falls in previous year; able to walk 15 minutes without walking device Exclusion criteria: severe cardiac, pulmonary, or musculoskeletal disorders or disorders associated with higher fall risk (e.g. neurologic disorders) |
|
| Interventions | 1. Group‐based balance and gait training using an obstacle avoidance course: 11 sessions between 1 ‐ 2½ hours including education, balance, gait training using obstacle course, for 5½ weeks 2. Control: usual care |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who experienced 1 or more fall‐related fractures 4. Health‐related quality of life |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by session attendance 1. Group‐based balance and gait training using an obstacle avoidance course group: 93% attendance at total number of sessions. More than half (53%) of the participants did not miss a session |
|
| Notes | Source of funding: Center for Organization of Healthcare Research Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After a baseline assessment M1, the researcher performed block randomization using non–see‐through envelopes. The probability of allocation to the exercise group was independent of recruitment method" |
| Allocation concealment (selection bias) | Unclear risk | Non‐see‐through envelopes but not sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Quote: "Fall calendars were scored by an independent researcher who was blinded to group allocation" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Method of reporting fractures is unclear |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants not blind to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall data were missing (4%) with missing data balanced between groups and balanced reasons for missing data |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "After the intervention had ended, participants registered their falls for 1 year on fall calendars that had to be returned every month… When no fall calendar was received within 2 weeks after the start of the month, the participant was reminded by telephone" |
Steadman 2003.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 1 month |
|
| Participants | Setting: London, UK Number of participants: 199 Number analysed: 133 Number lost to follow‐up: 66 Sample: attendees at a hospital multidisciplinary falls clinic Age (years): mean 82.7 (SD 5.6) Sex: 82% female Inclusion criteria: ≥ 60 years; Berg Balance Scale < 45 after "adequate management of potential risk factors" Exclusion criteria: amputation; unable to walk 10 metres; recent stroke; progressive neurological disorder; unstable medical condition; severe cognitive impairment |
|
| Interventions | 1. Standard, individualised physiotherapy focused on functional training plus balance training: performance of functional activities, plus repetition and progression of balance and walking exercises, 45‐minute sessions, 2 sessions a week for 6 weeks 2. Standard, individualised physiotherapy focused on functional training: performance of functional activities but no defined repetition or progression, 45‐minute sessions, 2 sessions a week for 4 weeks plus telephone follow‐up in final 2 weeks | |
| Outcomes | 1. Rate of falls | |
| Duration of the study | 24 weeks | |
| Adherence | Structured observation schedules were used randomly to monitor adherence to treatment protocols in both groups. 1. Standard, individualised physiotherapy focused on functional training: the protocol of therapy was being adhered to in all 48 participants observed receiving enhanced balance training 2. Control: the protocol of therapy was being adhered to in all 55 participants observed receiving conventional physiotherapy alone |
|
| Notes | Source of funding: not reported Economic information: not reported Falls reported in past month at 6 weeks used in analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blind to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Fall data collected using same method in both groups Quote: "A therapist who was not involved with randomization or delivering the interventions completed baseline and outcome assessments" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants not blinded to intervention group |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall data are missing (33%) |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of fallers are not reported. Adverse events are not reported |
| Method of ascertaining falls (recall bias) | High risk | Interval recall. Falls data collected for previous month at 6 weeks, 12 weeks and 24 weeks. |
Suzuki 2004.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 20 months |
|
| Participants | Setting: Tokyo, Japan Number of participants: 52 Number analysed: 44 Number lost to follow‐up: 8 Age (years): mean 78 (SD 3.9), range 73 to 90 Sex: 100% female Sample and inclusion criteria: women in the Tokyo Metropolitan Institute of Gerontology Longitudinal Interdisciplinary Study on Aging attending a comprehensive geriatric health examination; living at home Exclusion criteria: unable to measure muscle strength, poor mobility due to hemiplegia, poorly‐controlled blood pressure, communication difficulties due to impaired hearing |
|
| Interventions | 1. Group‐based strength, balance and gait training plus home practice: 0.5 ‐ 1.5 kg weights and light‐medium rubber bands used for strengthening, 1‐hour class, fortnightly for 6 months plus individual home‐based exercises 30 minutes daily, 3 a week 2. Control: pamphlet and advice on falls prevention | |
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 87 weeks | |
| Adherence | Adherence measured by session attendance 1. Group‐based strength, balance and gait training plus home practice: attendance ranged from 64 ‐ 86%, with a mean of 75%. 15 participants (54%) attended all 10 sessions. 6 who attended 0 ‐ 3 times were regarded as failing to master the exercise programme. Among the 22 participants who completed the intervention, 21 (96%) participated in > 7 sessions |
|
| Notes | Source of funding: Tokyo Metropolitan Government Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomized" but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Falls reported by participants who were aware of their group allocation, using same method in each group. Does not state whether outcome assessors were blind to allocation |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | < 20% of fall data are missing (15%). Mild imbalance in missing data from intervention group (n = 6) and control group (n = 2). Reason for missing data in the control group is unclear |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | High risk | Retrospective recall. Falls and fractures recorded retrospectively at interview at 8 months and 20 months after intervention |
Taylor 2012.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 17 months |
|
| Participants | Setting: Auckland, Christchurch and Dunedin, New Zealand Number of participants: 684 Number analysed: 684 Number lost to follow‐up: 0 Sample: community‐dwelling Age (years): mean 74.5 (SD 6.5) Sex: 73% female Inclusion criteria: ≥ 65 years old (55 years if Ma ¯ori or Pacific Islander to account for ethnic disparities in health), had experienced at least 1 fall in the previous 12 months or were considered to be at risk of falling using the Falls Risk Assessment Tool (FRAT > 1). Exclusion criteria: unable to walk independently (with or without walking aid), chronic medical condition that would limit participation in low‐ to moderate‐intensity exercise, severe cognitive limitations (score < 23 on the Telephone MMSE), participated in Tai Chi within the last year, or currently participating in an organized exercise programme aimed at improving strength and balance |
|
| Interventions | 1. Group‐based Tai Chi, 2 a week: 1‐hour class, 2 a week for 20 weeks 2. Group‐based Tai Chi, 1 a week: 1‐hour class, 1 a week for 20 weeks 3. Control: Group‐based seated gentle lower‐limb exercise, stretching, low‐level strength, and low‐level cardiovascular exercise; 1‐hour class, 1 a week for 20 weeks |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) |
|
| Duration of the study | 68 weeks | |
| Adherence | Adherence measured by percentage of sessions attended. 1. Group‐based Tai Chi, 2 a week: median attendance rate 72% (IQR 44 – 88%) 2. Group‐based Tai Chi, 1 a week: median attendance rate 79% (IQR 49 – 90%) 3. Group‐based seated gentle lower‐limb exercise: median attendance rate 67% (IQR 10 – 85%) |
|
| Notes | Source of funding: Accident Compensation Corporation Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Web‐based, computer‐generated blocked random number system (generated by the study biostatistician)" |
| Allocation concealment (selection bias) | Low risk | Quote: "At the end of the baseline assessment, each participant was given a sealed opaque envelope containing group allocation details and was instructed to open the envelope after leaving the assessment venue and not to discuss the assignment with any of the assessors" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blinded to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Quote: "Participants who did not return their monthly calendars had reminder telephone calls within 2 weeks, and assessors blinded to group allocation collected data related to any falls over the telephone" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | Unclear risk | Prespecified falls outcomes reported. Trial registration was retrospective and does not note adverse events |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "Participants recorded fall incidents as they occurred on provided calendars that they returned monthly by mail" |
Trombetti 2011.
| Methods | Study design: RCT (cross‐over at 6 months) Number of study arms: 2 Length of follow‐up: 6 months |
|
| Participants | Setting: Geneva, Switzerland Number of participants: 134 Number analysed: 134 Number lost to follow‐up: 0 Sample: volunteers recruited by advertising etc. Age (years): 75.5 (SD 6.9) Sex: 96% female Inclusion criteria: aged ≥ 65; community‐dwelling; no previous experience of Jaques‐Dalcroze eurhythmics (except during childhood); high risk of falling (≥ 1 fall after the age of 65, impaired balance, or physically frail) Exclusion criteria: neurological or orthopaedic disease seriously affecting gait and balance; progressive or unstable medical conditions limiting participation; dependent on walking aids, e.g. canes and walkers |
|
| Interventions | 1. Group‐based balance and gait training: music‐based multitask exercise programme gradually increasing in difficulty to challenge balance, 1 hour, 1 a week for 6 months 2. Control: received intervention after 6 months |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who died |
|
| Duration of the study | 26 weeks | |
| Adherence | Adherence measured by percentage completed study, class attendance 1. Group‐based balance and gait training: mean attendance rate; 78%. 83% completed the intervention, of whom 77% attended at least 20 classes (i.e. 80% of the classes) |
|
| Notes | Source of funding: Loterie Romande Geneva, Carigest SA, Gertrude Hirzel Foundation, Leenaards Foundation, Oltramare Foundation, Eagle Foundation, Foundation for Geneva (Georges Junod Fund), Delta réseau de soins Geneva, Helsana Economic information: not reported Falls data from 6 months (before cross‐over) used for analysis in the review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomized … according to a computer‐generated list … using a permuted block randomization design" |
| Allocation concealment (selection bias) | Low risk | Quote: "subjects were randomized … according to a computer‐generated list prepared by an independent statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded to allocated group but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Participants self‐reported falls Quote: "Participants who failed to return the diary or provided incomplete data were contacted by telephone." Not clear whether this assessor was blind to group allocation |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Method of ascertaining adverse events, and presence of blinding, unclear |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | Unclear risk | Fall outcomes were prespecifed in the prospective trial registration. Adverse events (part of the minimum set of expected outcomes) were noted only in the results |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "Falls were prospectively monitored for 12 months and recorded daily using a diary mailed monthly to the study coordinator. Participants who failed to return the diary or provided incomplete data were contacted by telephone" |
Uusi‐Rasi 2015.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 24 months |
|
| Participants | Setting: Tampere, Finland Number of participants: 205 Number analysed: 186 Number lost to follow‐up: 19 Sample: community‐dwelling women Age (years): mean 74 (SD 3.0) Sex: 100% female Inclusion criteria: 70 ‐ 80 years, living at home independently; had fallen at least once during the previous year; no contraindication to exercise; understands the procedures of the study, voluntarily agrees to undergo all measurements and signs informed consent Exclusion criteria: moderate to vigorous exercise > 2 hours a week; regular use of vitamin D or calcium + vitamin D supplements; a recent fracture (during preceding 12 months); contraindication or inability to participate in the exercise programme; a marked decline in the basic ADL; cognitive impairments (MMSE, MMSE‐test); primary hyperthyroidism; and degenerative conditions, such as Parkinson's disease. |
|
| Interventions | Randomised into 4 groups: 3 intervention groups (1 vitamin D and exercise, 1 placebo and exercise, 1 vitamin D without exercise) and 1 control group (placebo without exercise). Only the placebo and exercise and the control groups were included in this review 1. Group‐based balance and strength training plus home practice: balance, weight‐bearing, agility and functional exercises; weight machines, pulleys and free weights used for strength training; 2 a week for the first year, and 1 a week for the second year, plus home training 5 ‐ 15 minutes performed on all rest days 2. Control group: usual pre‐study level of physical activity |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who experienced 1 or more falls that required medical attention 4. Number of people who died |
|
| Duration of the study | 104 weeks | |
| Adherence | Adherence measured by session attendance, home training completion 1. Group‐based balance and strength training plus home practice: attendance at all offered group training; 73% (range, 0 ‐ 97.4%). Attendance at all home training sessions; 66.1% (range 0 ‐ 100%) |
|
| Notes | Source of funding: Academy of Finland, Ministry of Education and Culture, Competitive Research Fund of Pirkanmaa Hospital District, Juho Vainio Foundation Hazard ratios but not numbers reported for "medically attended fallers" Economic information: Total costs (intervention and healthcare): EUR 30.9 for no exercise + placebo; EUR 206.9 for no exercise + vitamin D 800 IU/day; EUR 73.4 for exercise + placebo; EUR 188.0 for exercise + vitamin D 00 IU/day. Incremental costs per fall/per QALY gained: EUR 220.7 for no exercise + placebo, EUR 17,600 for no exercise + vitaminD 800 IU/day, EUR 2670 for exercise + placebo, EUR 3820 for exercise + vitamin D 800 IU/day |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study statistician (K.T.) generated the participant list using validated randomization software. He was blinded to the study participants and their characteristics and randomly allocated them into 4 groups (simple randomization)" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Falls ascertained by self report. Unclear whether staff conducting follow‐up telephone calls were blinded |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Method of ascertaining adverse events and injurious falls was not clear |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of fall data are missing (9%). Missing fall data had mild imbalance, with intervention group (n = 12; lost interest n = 3, health reasons n = 9) and control group (n = 7; lost interest n = 2, health reasons n = 3, died n = 2) |
| Selective reporting (reporting bias) | Unclear risk | Fall outcomes were prespecifed in the prospective trial registration. Adverse events (part of the minimum set of expected outcomes) were noted only in the Results |
| Method of ascertaining falls (recall bias) | Low risk | Prospective fall diaries returned monthly by mail, and details of each registered fall were ascertained by a telephone call |
Verrusio 2017.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: Rome, Italy Number of participants: 150 Number analysed: 147 Number lost to follow‐up: 3 Sample: outpatients Age (years): mean 64.9 (SD 4.6) Sex: 53% female Inclusion criteria: young old people (60 – 69 years), normal or corrected vision, Tinetti score 19 ‐ 24 Exclusion criteria: medical condition that prevented safe participation in an exercise programme, peripheral artery occlusive disease, diabetic neuropathy, history of stroke, history of inflammatory arthritis, history of vertebral fragility fractures or hip or leg fractures or both in the previous 24 months, systolic blood pressure 200 mmHg or diastolic blood pressure 110 mmHg, or both |
|
| Interventions | 1. Individual, supervised balance and gait training using exoskeleton human body posturiser: moderate intensity, 1 hour, 3 a week for 12 months 2. Individual supervised walking, balance and posture training: moderate intensity, 1 hour, 3 a week, for 12 months |
|
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 52 weeks | |
| Adherence | Not reported | |
| Notes | Source of funding: not reported Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomly assigned into two groups following simple randomization procedures (computerized random numbers)" |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | It is unclear whether the assessors recording falls were blinded to group allocation |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants were not blinded to group alloction |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall data are missing (2%). The missing data were balanced between the groups with 2 lost to follow‐up in the intervention group and 1 in the control group |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of falls was not reported |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "The number of falls will be monitored with daily fall diaries. Diaries will be collected monthly through the mail. Details of each registered fall will be ascertained by the investigator" |
Vogler 2009.
| Methods | Study design: RCT Number of study arms: 3 Length of follow‐up: 12 months |
|
| Participants | Setting: Sydney, Australia Number of participants: 180 Number analysed: 171 Number lost to follow‐up: 9 Sample: community‐dwelling Age (years): mean 80 (SD 7) Sex: 83% female Inclusion criteria: 65+ years hospital inpatients Exclusion criteria: medical contraindications to exercise. MMSE score < 25 out of 30, discharge to high‐care residential facility |
|
| Interventions | 1. Home‐based seated lower‐limb strength exercises: seated exercises targeting hip flexion, extension, abduction, knee flexion and extension, and ankle plantar‐ and dorsiflexion; resistance via cuff weights and exercise bands with aim of 10 ‐ 12 RM, 3 a week for 12 weeks; approximately 12 a month; checked and progressed 8 times over 12 weeks 2. Home‐based strength training with weight‐bearing, functional tasks: weight‐bearing (WB) exercise in standing, targeting lower‐limb strength, e.g. heel raises, partial squats, sit‐to‐stand, and stepping forward and sideways up onto blocks. Resistance by weight‐loaded waist belts, with aim of 10 ‐ 12 RM. Also exercise targeting WB task performance, e.g. reaching, tandem stand, 3 times a week for 12 weeks; approximately 12 times a month; checked and progressed 8 times over 12 weeks 3. Control group: social visits, frequency‐matched, each 1 hour duration | |
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) 2. Number of people who died |
|
| Duration of the study | 12 weeks | |
| Adherence | 1. Seated exercise group: completed 70% of 36 recommended exercise sessions 2. WB group: completed 62% of 36 recommended exercise sessions |
|
| Notes | Source of funding: NHMRC, Good Age Trust Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "performed in blocks of 15 subjects by computer‐generated random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: “Group allocations for each subject were concealed in opaque envelopes” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls were measured using the same method in each group Quote: “The outcome assessor remained unaware of group allocation" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants were not blinded to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall data were missing (5%). Missing data were balanced, with 3 participants missing from each group |
| Selective reporting (reporting bias) | High risk | Falls were measured but number of falls were not reported |
| Method of ascertaining falls (recall bias) | Low risk | Weekly fall incidence questionnaire |
Voukelatos 2007.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months |
|
| Participants | Setting: Sydney, Australia Number of participants: 702 Number analysed: 684 Number lost to follow‐up: 18 Sample: community‐dwelling Age (years): mean 69 (SD 6.5), range 69 ‐ 70 Sex: 84% female Inclusion criteria: aged > 60; community‐dwelling Exclusion criteria: degenerative neurological disease; severely debilitating stroke; metastatic cancer; severe arthritis; unable to walk across a room independently; unable to use English |
|
| Interventions | 1. Group‐based Tai Chi: style of Tai Chi differed between classes depending on Tai Chi instructor; 1‐hour class, 1 a week for 16 weeks. Cost AUD 44 2. Control: instructed not to take part in a Tai Chi programme and placed on 24‐week waiting list, then offered Tai Chi programme | |
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 24 weeks | |
| Adherence | Adherence measured by retention/attrition rate 1. Group‐based Tai Chi: dropout: 6. 76 participants provided falls data but did not complete the 16‐week balance assessment 2. Control: dropout: 12. 81 participants provided falls data but did not complete the 16‐week balance assessment |
|
| Notes | Source of funding: New South Wales Health Department Economic information: Mean cost per person (intervention): AUD 245 plus charged AUD 44 per participant. Healthcare service costs: Tai Chi group AUD 55, control group AUD 17. Incremental cost per fall prevented/per QALY gained: AUD 1683 per fall prevented (includes cost offset by charging AUD 44 per instruction course).Cost‐effectiveness analysis reported in Haas 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization list ... was prepared for each venue using randomly permuted blocks of four or six" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and instructors conducting classes in intervention group were not blinded. Control participants were asked not to take classes during the study period, but may have accessed other fall‐prevention interventions. Insufficient evidence to make judgement on impact of lack of blinding. |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls were recorded using the same method in both groups. Outcome assessors were blinded to group assignment |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall data were missing (3%). Missing data were balanced across groups, with 6/347 participants missing from the intervention group and 12/249 missing from the control group. The reasons for missing data were balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "Participants were given falls calendars and were instructed to record on the calendar each day for 24 weeks whether they had had a fall." Pre‐paid postage calendars returned at the end of each month, with telephone call if not returned within 2 weeks. |
Voukelatos 2015.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: Sydney, Australia Number of participants: 386 Number analysed: 339 Number lost to follow‐up: 47 Sample: community‐dwelling Age (years): mean 73.2 (range 65 ‐ 90) Sex: 74% female Inclusion criteria: 65 years and over community‐dwelling inactive (i.e. < 120 minutes of exercise a week) mobile (i.e. able to walk at least 50 m with minimal aid); able to communicate in English Exclusion criteria: medical condition precluding participation in the study, participating in another study |
|
| Interventions | 1. Individual walking programme: 48‐week self‐paced walking programme by manual; focused on walking duration (12 weeks), walking intensity (12 weeks), maintaining the level of walking achieved in the previous stages (24 weeks); 6 telephone calls to help modify and support adherence 2. Control group: Mailed information about health issues, 6 telephone calls to discuss health information |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Health‐related quality of life 4. Number of people who died |
|
| Duration of the study | 48 weeks | |
| Adherence | Not reported | |
| Notes | Source of funding: NSW Ministry of Health Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation scheme used randomised permuted blocks of 6 and 4 prepared by the chief investigator |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Blinding not described. Insufficient information to permit judgement. |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants not blinded to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of fall data were missing (12%). Missing data were unbalanced across groups, with 33/192 participants missing from the intervention group and 14/194 missing from the control group. The reasons for missing fall data at 24 months were not clear |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Low risk | Monitored for 48 weeks through monthly calendars. When participants reported a fall, they were contacted by telephone to confirm the fall and document any fall‐related injuries |
Weerdesteyn 2006.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 7 months |
|
| Participants | Setting: Nijmegan, The Netherlands Number of participants: 58 Number analysed: 58 Number lost to follow‐up: 0 Sample: recruited using newspaper advertisements Age (years): mean 74 (SD 6) Sex: 77% female Inclusion criteria: ≥ 65 years; community‐dwelling; ≥ 1 fall in previous year; able to walk 15 minutes without a walking aid Exclusion criteria: severe cardiac, pulmonary, or musculoskeletal disorders; pathologies associated with increased falls risk, e.g. Parkinson's disease; osteoporosis; using psychotropic drugs |
|
| Interventions | 3 arms described, but 1 not randomised. Only randomised groups were included in this review 1. Group‐based balance and gait training using an obstacle avoidance course: daily tasks and walking progressed with cognitive tasks and visual constraints, 1½ hours, 2 a week for 5 weeks 2. Control: no training | |
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) |
|
| Duration of the study | 28 weeks | |
| Adherence | Adherence measured by session attendance 1. Group‐based balance and gait training using an obstacle avoidance course: mean attendance rate to the exercise sessions; 87% for both low‐intensity exercise group and walking exercise group. 51% of exercise participants attended the maximum number of 10 sessions |
|
| Notes | Source of funding: Organization for Healthcare Research, Eurokinesis Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Block randomization (3 blocks of 20) with gender stratification with equal probability for either exercise or control group assignment" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The group allocation sequence was concealed (to both researchers and participants) until assignment of interventions". "We had participants draw a sealed envelope with group allocation ticket from a box containing all remaining envelopes in the block" (personal communication reported in Gillespie 2012) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blinded to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | High risk | Falls reported by participants who were aware of their group allocation. Outcome assessors were not blinded to assignment (personal communication from Dr Weeredesteyn, as reported in Gillespie 2012) |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing falls data |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes not reported (adverse events not reported) |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "Falls were monitored monthly using pre‐addressed, reply‐paid fall registration cards." Asked whether a fall had occurred in the past month. Sent a reminder if no registration card received |
Wolf 1996.
| Methods | Study design: RCT Number of study arms: 3 Length of follow‐up: 8 months |
|
| Participants | Setting: Atlanta, USA Number of participants: 200 Number analysed: 200 Number lost to follow‐up: 0 Sample: residing in an independent living facility, recruited by advertising and direct contact Age (years): mean 76.2 (SD 4.7) Sex: 81% female Inclusion criteria: aged > 70; ambulatory; living in unsupervised environment; agreeing to participate weekly for 15 weeks with 4‐month follow‐up Exclusion criteria: debilitating conditions, e.g. cognitive impairment, metastatic cancer, crippling arthritis, Parkinson's disease, major stroke, profound visual defects |
|
| Interventions | 1. Group‐based Tai Chi: progression to reduce base of support and towards single stance, 2 sessions a week for 15 weeks, individual contact with instructor approximately 45 minutes a week 2. Individual, computerised balance training on force platform: increasing sway with no foot movement using visual feedback from monitor with eyes open and closed, 1 a week for 15 weeks, individual contact with instructor approximately 45 minutes a week 3. Control: group discussions of topics of interest to older people with gerontological nurse, 1 hour a week for 15 weeks | |
| Outcomes | 1. Rate of falls | |
| Duration of the study | 87 weeks | |
| Adherence | Adherence measured by attendance at sessions. Inability to make up 2 missed consecutive sessions defined as dropout 1. Group‐based Tai Chi: 6/72 dropped out, 92% retention 2. Individual, computerised balance training on force platform: 4/64 dropped out, 94% retention 3. Control: 3/64 dropout, 95% retention |
|
| Notes | Source of funding: NIH Cooperative Grant Economic information: not reported Atlanta FICSIT trial (Province 1995). 1997 paper included under this Study ID reports on a subgroup of the trial, reporting on outcomes other than falls |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using computer‐generated fixed randomisation procedure |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blind to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Falls measured using same method in each group. Does not state whether outcome assessors were blind to allocation |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | High risk | Falls measured, but number of fallers not reported. Adverse events not reported |
| Method of ascertaining falls (recall bias) | Low risk | Falls ascertained by monthly calendar, or by monthly phone call from project staff |
Wolf 2003.
| Methods | Study design: Cluster‐RCT Number of study arms: 2 Number of clusters: 20 Length of follow‐up: 11 months |
|
| Participants | Setting: Atlanta, USA Number of participants: 311 Number analysed: 286 Number lost to follow‐up: 25 Sample: congregate living facilities (independent living facilities) recruited in pairs by whether Housing and Urban Development (N = 14) or private (N = 6). At least 15 participants recruited per site Age (years): mean 80.9 (SD 6.2), range 70 to 97 Sex: 94% female Inclusion criteria: aged ≥ 70; ≥ 1 fall in previous year; transitioning to frailty Exclusion criteria: frail or vigorous elderly; major cardiopulmonary disease; cognitive impairment (MMSE < 24); contraindications for exercise, e.g. major orthopaedic conditions; mobility restricted to wheelchair; terminal cancer; evidence of other progressive or unstable neurological or medical conditions |
|
| Interventions | 1. Group‐based Tai Chi: progressed from using upright support to 2 minutes of Tai Chi without support; 1‐hour class progressing to 90 minutes, 2 a week for 48 weeks 2. Control group: wellness education programme (Instruction on fall prevention, exercise and balance, diet and nutrition, pharmacological management, legal issues, changes in body function, mental health issues. Interactive material provided but no formal instruction in exercise); 1 hour a week for 48 weeks | |
| Outcomes | 1. Rate of falls
2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who died |
|
| Duration of the study | 48 weeks | |
| Adherence | Adherence measured by group attendance 1. Group‐based Tai Chi group: mean attendance rate; 76 ± 19% (range 6 ‐ 100%) 2. Control group: mean attendance rate; 81 ± 17% (range 10 ‐ 100%) |
|
| Notes | Source of funding: NIH Grant Economic information: not reported "Transitioning to frailty" if not vigorous or frail; based on age, gait/balance, walking activity for exercise, other physical activity for exercise, depression, use of sedatives, vision, muscle strength, lower extremity disability (Speechley 1991) Number of clusters allocated to intervention: 10; number of clusters allocated to control: 10; number of clusters analysed (intervention): 10; number of clusters analysed (control): 10 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Facilities stratified by socioeconomic status and randomised in pairs Quote: "First site in the pair was randomized to an intervention. The second site received the other intervention" |
| Allocation concealment (selection bias) | Unclear risk | Cluster‐RCT. Insufficient information to permit judgement, although allocation of second site in the pair could be predicted after the first site was randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blinded to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Falls reported using the same method in each group. Outcome assessors blinded to assignment |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | The method of ascertaining adverse events was unclear |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall data were missing (8%). Missing data were balanced across groups (13/158 missing from the intervention group and 12/153 missing from the control group) and the reasons for missing data were balanced across groups |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes reported. No published study protocol or prospective trial registration |
| Method of ascertaining falls (recall bias) | Low risk | Prospective. Falls recorded on forms and submitted to instructor weekly + phone call |
| Cluster‐randomised trials | Unclear risk | The relative timing of the randomisation of clusters and recruitment of participants is unclear; baseline characteristics of clusters not reported; missing outcomes for clusters or within clusters were not reported; accounted for the clustered design in the analysis; results comparable with individually‐randomised trials |
Woo 2007.
| Methods | Study design: RCT Number of study arms: 3 Length of follow‐up: 12 months |
|
| Participants | Setting: Hong Kong, China Number of participants: 180 Number analysed: 176 Number lost to follow‐up: 4 Sample: recruited by notices posted in 4 community centres in Shatin township Age (years): mean 69 (SD 2.6), range 65 ‐ 74 Sex: 50% female Inclusion criteria: able to walk > 8 m without assistance Exclusion criteria: neurological disease which impaired mobility; shortness of breath or angina on walking up 1 flight of stairs; dementia; already performing Tai Chi or resistance training exercise |
|
| Interventions | 1. Group‐based Tai Chi: Yang style Tai Chi, 3 a week for 52 weeks 2. Group‐based resistance training: used a medium‐strength Theraband for arm and leg exercises, 3 a week for 52 weeks 3. Control: no exercise prescribed | |
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) | |
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by attendance rate 1. Group‐based Tai Chi group: mean attendance rate 81% with no attrition between 6 and 12 months 2. Group‐based resistance group: mean attendance rate 76% with no attrition between 6 and 12 months |
|
| Notes | Source of funding: Council of Hong Kong Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated blocked randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel implementing the intervention not blind to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | High risk | Assessors not blinded to group allocation Quote: "Falls were ascertained by diary and reported to the staff running the interventions" (personal communication reported in Gillespie 2012). |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall data were missing (33%) |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of falls not reported. Adverse events not reported |
| Method of ascertaining falls (recall bias) | High risk | Quote: "Falls were ascertained by diary and reported to the staff running the interventions." (personal communication) but this could not apply to the control group (personal communication reported in Gillespie 2012) |
Wu 2010.
| Methods | Study design: RCT Number of study arms: 3 Length of follow‐up: 4 months |
|
| Participants | Setting: Burlington, Vermont, USA Number of participants: 64 Number analysed: 64 Number lost to follow‐up: 0 Sample: volunteers recruited by advertising, referrals, flyers etc. Age (years): mean 75.4 (SD 7) Sex: 84% female Inclusion criteria: age ≥ 65; community‐dwelling; at risk of falling (≥ 1 fall in past year or ≤ 50% on ABC Scale); able to walk and do weight‐bearing exercises with or without assistive devices; no plans to be away > 2 weeks during study period; sufficient cognition and attention to follow directions; have a television (TV) and Internet access; sufficient visual acuity to mimic instructor's movements on TV screen; consenting; with primary care physician approval to participate Exclusion criteria: unable to walk/exercise independently; unable to travel to community centre; having certain exercise‐limiting conditions including musculoskeletal, cardiac, neurological, pulmonary etc |
|
| Interventions | Delivered by 3 methods with same content and same instructor: 1. Individual, supervised Tai Chi delivered by videoconferencing: "Tel‐ex" yang style Tai Chi home‐based interactive by TV screen, live and supervised in real‐time, 1 hour a day, 3 days a week for 15 weeks 2. Group‐based Tai Chi: "Comm‐ex" yang style Tai Chi class held in community facility, live and supervised in real‐time, 1 hour a day, 3 days a week for 15 weeks 3. Individual Tai Chi with DVD instruction: "Home‐ex" yang style Tai Chi exercise from home but not connected to instructor during the 15 weeks, received written instructions for DVD programme, DVD with 45 x 1‐hour sessions, identical exercises to live class instruction groups; 1 hour a day, 3 days a week for 15 weeks |
|
| Outcomes | 1. Rate of falls | |
| Duration of the study | 15 weeks | |
| Adherence | Adherence measured by total exercise time 1. Individual, supervised Tai Chi delivered by videoconferencing: total exercise time 30 ± 12 hours (69 ± 27%) 2. Group‐based Tai Chi: total exercise time 31 ± 12 hours (71 ± 27%) 3. Individual Tai Chi with DVD instruction: total exercise time 17 ± 21 hours (38 ± 46%) |
|
| Notes | Source of funding: not reported Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Those who consented were enrolled in the study and were randomly assigned into the Tele‐ex, Commex, and Home‐ex groups. To ensure balance among the 3 groups on important potential confounders, randomization was stratified by sex, age (65–74y vs 75y), and time expected to be away during the study period (1 wk vs 1–2 wk). Blocked randomization was used within strata." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | All 3 groups received a fall‐prevention intervention (Tai Chi). Unclear whether there is potential for performance bias |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Falls were measured using the same method in each group. Unclear whether assessor was blinded |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants not blinded to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | No missing fall data |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of fallers not reported. Adverse events not reported |
| Method of ascertaining falls (recall bias) | High risk | Quote: "Fall incidents were assessed by a Fall History Form that recorded the number of falls in the ... past 15 weeks" |
Yamada 2010.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: Kyoto, Japan Number of participants: 60 Number analysed: 58 Number lost to follow‐up: 2 Sample: people recruited using advertising in local press (proportion of women not stated) Age (years): not stated Inclusion criteria: aged ≥ 65; community‐dwelling; visited primary care physician in previous 3 years; MMSE ≥ 24; able to walk independently (with or without a cane): willing to participate in group exercise classes lasting ≥ 6 months; access to transportation; minimal hearing and visual impairments; no regular exercise in previous 12 months Exclusion criteria: severe cardiac pulmonary, or musculoskeletal disorders; neurological conditions associated with falling (stroke, Parkinson's disease); osteoporosis; use of psychotropic drugs |
|
| Interventions | 1. Group‐based trail walking training: 90‐minute class (moderate intensity aerobic exercise, progressive strengthening with rubber band, flexibility and balance exercises) including trail walking between flags as quickly as possible, 1 a week for 16 weeks 2. Group‐based indoor walking: 90‐minute class (moderate‐intensity aerobic exercise, progressive strengthening with rubber band, flexibility and balance exercises) including supervised indoor walking session at a comfortable pace (up to 30 minute on 300‐foot loop); 1 a week for 16 weeks |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by completion of 16 scheduled sessions 1. Group‐based trail walking training: median relative adherence; 100% (25th – 75th percentile, 94 – 100%) 2. Group‐based indoor walking: median relative adherence; 100% (25th – 75th percentile, 94 – 100%) |
|
| Notes | Source of funding: not reported Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were block randomized in blocks of four" |
| Allocation concealment (selection bias) | Low risk | Quote: "Using this sequence, opaque envelopes bearing group names were numbered and the 60 participants were then randomly as signed to the TWE (n = 30) or walking (W) group (n = 30)" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Both groups received an exercise intervention. Unclear whether there was any risk of performance bias |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Unclear whether person ascertaining falls was blinded to allocated group |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of fall data were missing (3%). The missing data were balanced between groups, with 1 withdrawal from each group |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes reported. No published study protocol or prospective trial registration |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "The participants were asked to record any falls in fall diaries that were mailed to the research assistants every month." |
Yamada 2012.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: Japan Number of participants: 157 Number analysed: 145 Number lost to follow‐up: 12 Sample: community‐dwelling Age (years): mean 86 Sex: 81% female Inclusion criteria: ≥ 75 years old, community‐dwelling, had visited a primary care physician within the past 3 years, no severe cognitive impairment, walk independently (or with a cane), willingness to participate in group exercise classes for at least 6 months, had access to transportation, no significant hearing and vision impairments, no regular exercise in the past 12 months Exclusion criteria: severe cardiac, pulmonary or musculoskeletal disorders, co‐morbidities associated with greater risk of falls, such as Parkinson disease and stroke, and use of psychotropic drugs |
|
| Interventions | 1. Group‐based balance, strength, flexibility and gait training involving complex obstacle course: 45‐minute exercise session ('moderate‐intensity' aerobic‐dance exercise, progressive strength training using elastic band, progressive balance exercises); plus walking as quickly as possible in a progressively difficult field of obstacles 2 times a session. 1 session a week for 24 weeks 2. Group‐based balance, strength, flexibility and gait training involving simple obstacle course: 45‐minute exercise session ('moderate‐intensity' aerobic‐dance exercise, progressive strength training using elastic band, progressive balance exercises); plus walking at a self‐selected speed along a simple level walkway of 15 m with obstacles 6 times a session. 1 session a week for 24 weeks |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who experienced 1 or more fall‐related fractures |
|
| Duration of the study | 52 weeks | |
| Adherence | Adherence measured by completion of programme 1. Group‐based balance, strength, flexibility and gait training involving complex obstacle course group: median relative adherence; 96% (25th ‐ 75th percentile, 88 – 100%) 2. Group‐based balance, strength, flexibility and gait training involving simple obstacle course group: median relative adherence; 96% (25th ‐ 75th percentile, 88 – 100%) |
|
| Notes | Source of funding: not reported Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods not described |
| Allocation concealment (selection bias) | Low risk | Quote: "Opaque envelopes bearing group names were numbered" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel implementing the intervention not blinded to allocated group, but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Not specifically reported if the research assistants collecting fall outcomes were blinded Quote: "research assistants collected fall outcomes… a physiotherapist blinded to group allocation collected secondary outcome measures" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Quote: “The diagnosis of fractures was based on radiological evidence of fracture”. Unclear if assessors were blinded to group allocation |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Method of measuring adverse events was unclear |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Low risk | Less than 20% of fall data were missing (8%). The missing data were balanced between groups, with 6 withdrawals from each group. The reasons for withdrawals were unclear |
| Selective reporting (reporting bias) | Unclear risk | Minimum set of expected outcomes reported. No published study protocol or prospective trial registration |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "The participants were asked to record any falls in fall diaries mailed every month by research assistants. If participants failed to send the fall diaries, research assistants collected data on falls over the telephone" |
Yamada 2013.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 12 months |
|
| Participants | Setting: Japan Number of participants: 264 Number analysed: 230 Number lost to follow‐up: 34 Sample: community‐dwelling Age (years): Training group mean 76.2 (SD 8.5); Control group mean 77.2 (SD 7.6) Sex: 57% female Inclusion criteria: 65 years old, community‐dwelling, frail, certified for long‐term care insurance service requirement, no severe cognitive impairment, ability to walk independently (or with cane), willing to participate in group exercise classes for at least 6 months, access to transportation, no significant hearing or vision impairment, and had not exercised regularly in the previous 12 months Exclusion criteria: serious visual impairment (cataract, glaucoma, or colour blindness), severe cardiac, pulmonary, or musculoskeletal disorders, comorbidities associated with greater risk of falls, such as Parkinson's disease and stroke, and use of psychotropic drugs |
|
| Interventions | 1. Group‐based balance, strength, flexibility and gait training including stepping mat: 30‐minute exercise sessions (moderate aerobic‐dance warm‐up, mild progressive resistance with elastic band, progressive balance exercises); plus walking on multitarget stepping mat test repeated 4 times, 2 times a week for 24 weeks 2. Group‐based balance, strength, flexibility and gait training plus indoor walking: 30‐minute exercise sessions (moderate aerobic‐dance warm‐up, mild progressive resistance with elastic band, progressive balance exercises); plus indoor 50 m walking programme, 2 times a week for 24 weeks |
|
| Outcomes | 1. Rate of falls 2. Number of people who experienced 1 or more falls (risk of falling) 3. Number of people who experienced 1 or more fall‐related fractures |
|
| Duration of the study | 52 weeks | |
| Adherence | 1. Group‐based balance, strength, flexibility and gait training including stepping mat group: median relative adherence; 93% (IQR 83 – 96%) 2. Group‐based balance, strength, flexibility and gait training plus indoor walking group: median relative adherence, 92% (IQR 83 – 96%) |
|
| Notes | Source of funding: Health Labor Sciences, Ministry of Health, Labor and Welfare Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods not described |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded. Effect of non‐blinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Unclear risk | Not specifically reported if the research assistants collecting fall outcomes were blinded Quote: "research assistants collected fall outcomes… a physiotherapist blinded to group allocation collected secondary outcome measures" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Quote: “All participants who had fallen were contacted by telephone and interviewed using a structured questionnaire about the fall and its consequences. Fractures were diagnosed based on radiological evidence of fracture”. Unclear if assessors were blinded to group allocation |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Method of measuring adverse events was unclear |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Falls and fallers | Unclear risk | Less than 20% of fall data were missing (13%). The missing data were unbalanced between groups, with 20 withdrawals from the intervention group and 14 from the control group. The reasons for withdrawals were unclear |
| Selective reporting (reporting bias) | Low risk | Minimum set of expected outcomes reported. No published study protocol or prospective trial registration |
| Method of ascertaining falls (recall bias) | Low risk | Quote: "The participants were asked to record any falls in fall diaries mailed every month by research assistants. If participants failed to send the fall diaries, research assistants collected data on falls over the telephone" |
Yang 2012.
| Methods | Study design: RCT Number of study arms: 2 Length of follow‐up: 6 months |
|
| Participants | Setting: Melbourne, Australia Number of participants: 165 Number analysed: 121 Number lost to follow‐up: 44 Sample: community‐dwelling Age (years): Intervention mean 81 (5.9); Control mean 80.1 (6.4) Sex: 44% female Inclusion criteria: aged 65 years or over, living in the community, being community ambulant, requiring no walking aid or using a single‐point stick only, experiencing no more than 1 fall in the previous 12 months, having concerns about balance, and had mild balance dysfunction (i.e. Functional Reach Test score < 26 cm, Step Test score < 13 steps/15 seconds, Five‐Time Sit‐to‐Stand Test time > 17.9 seconds, had > 3 abnormal scores on the NeuroCom Balance Master) Exclusion criteria: balance performance within normal limits |
|
| Interventions | 1. Individual Otago Exercise Programme: Tailored home programme with no upper‐limb support. Ankle weights and exercise manual provided. 20‐minute sessions, 5 times a week, for 24 weeks, plus ≥ 30 minutes daily walking 2. Control group: provided with a fall‐prevention information booklet and continued with usual activities |
|
| Outcomes | 1. Number of people who experienced 1 or more falls (risk of falling) 2. Health‐related quality of life 3. Number of people who died |
|
| Duration of the study | 24 weeks | |
| Adherence | Adherence measured by sessions performed 1. Individual Otago Exercise Programme: 26 (44%) full adherence, 8 participants (14%) reported exercising less than twice a week on average |
|
| Notes | Source of funding: Australian Government Department of Veterans’ Affair Economic information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described in sufficient detail to allow a definite judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unblinded but impact of unblinding unknown |
| Blinding of outcome assessment (detection bias) Falls | Low risk | Quote: "Assessors were blinded to group assignment" |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Hospital admission, medical attention and adverse events | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Health related quality of life (self report) | High risk | Participants not blinded to group allocation |
| Incomplete outcome data (attrition bias) Falls and fallers | High risk | More than 20% of fall data were missing (27%) |
| Selective reporting (reporting bias) | High risk | Falls were measured, but number of falls not reported. Adverse events not reported |
| Method of ascertaining falls (recall bias) | High risk | Relied on recall over 1 month. Preliminary information on falls was collected based on participants’ self‐report (retrospective recall) at the 6‐month reassessment |
ABC Scale: Activities‐specific Balance Confidence Scale ADL: activities of daily living BMD: bone mineral density DXA: dual‐energy X‐ray absorptiometry (a way of measuring bone density) ED: emergency department FaME: Falls Management Exercise FICSIT: frailty and injuries: co‐operative studies of intervention techniques GP: general practitioner HMO: health maintenance organisation m: metres MMSE: Mini Mental State Examination OT: occupational therapist PT: physical therapist/physiotherapist RCT: randomised controlled trial SD: standard deviation TUG: Timed Up and Go test wk: week x: times <: less than >: more than
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alkan 2011 | RCT. Community‐dwelling women > 65 years old. Excluded as intervention was not exercise |
| Beling 2009 | RCT. Community‐dwelling. Age mean 80 years. Excluded as intervention was not exercise |
| Clemson 2004b | RCT. Community‐dwelling. Age mean 78 years. Excluded as intervention was not exercise |
| DeSure 2013 | RCT. Excluded as sample was from an assisted‐living community unit |
| Fahlström 2017 | RCT. Excluded due to multiple interventions delivered |
| Gianoudis 2014 | RCT. Community‐dwelling. Age mean 67 years. Excluded as intervention was not just exercise |
| Hinrichs 2016 | RCT. Community‐dwelling. Age mean 80 years. Excluded as falls not measured |
| Hsu 2017 | RCT. Excluded as an inclusion criterion was subcortical ischaemic vascular cognitive impairment, a particular clinical condition that increases the risk of falls |
| Iwamoto 2012 | RCT. Community‐dwelling. Age mean 74 years. Excluded as intervention whole‐body vibration without exercise |
| Lee 2013 | RCT. Community‐dwelling older adults. Excluded as intervention was multifactorial. |
| Leung 2014 | RCT. Community‐dwelling. Age > 60 years. Excluded as intervention was whole‐body vibration without exercise |
| Li 2018a | RCT. Age > 60 years. Excluded as intervention incorporated functional electrical stimulation |
| Morris 2008 | RCT. 3/26 participants were withdrawn from the study due to injuries resulting from a fall. This equated to 50% of the participants who fell during the trial being excluded from the results |
| Ohtake 2013 | RCT. Community‐dwelling. Aged > 65 years. Excluded due to the control group |
| Olsen 2014 | RCT. Community‐dwelling older women. Excluded due to multiple interventions, not just exercise |
| Pai 2014 | RCT. Community‐dwelling older adults. Excluded as intervention was not exercise |
| Pereira 1998 | RCT. Community‐dwelling. Excluded as mean age 57 (SD 4) |
| Rossi‐Izquierdo 2017 | RCT. Aged > 60. Excluded as intervention involved vestibular rehabilitation |
| Steinberg 2000 | RCT. Older community‐dwellers. Excluded due to multiple interventions |
| Swanenburg 2007 | RCT. Community‐dwelling. Age mean 71 years. Excluded due to multiple interventions |
| Ueda 2017 | RCT. Community‐dwelling. Excluded as the difference in intervention between groups was hazard reduction using floor plans |
Characteristics of studies awaiting assessment [ordered by study ID]
Jagdhane 2016.
| Methods | RCT |
| Participants | 6 older adults, mean (SD) age 73.3 (5) years |
| Interventions | Intervention group: 4 weeks of anticipatory postural adjustment training |
| Outcomes | Timed‐Up and Go, single‐limb stance, and Activities‐specific Balance Confidence scale |
| Notes | Awaiting full‐text paper to determine if falls were measured |
Li 2018b.
| Methods | RCT |
| Participants | Community‐dwelling, 70 years or older, fell in past year or impaired mobility, mean (SD) age 77.7 (5.6) years |
| Interventions | 3 intervention groups, each with 2 60‐minute classes a week for 24 weeks: i) Tai Ji Quan; ii) multimodal exercise programme; iii) stretching |
| Outcomes | Incidence of falls at 6 months |
| Notes | Published 7 days before Cochrane Review submitted. Results stated that at 6 months, the incidence rate ratio (IRR) was significantly lower in the Tai Ji Quan group (IRR 0.43, 95% CI 0.31 to 0.56, P = 0.01), and multimodal exercise (IRR 0.60, 95% CI 0.45 to 0.80, P = 0.001), compared with the stretching group |
Characteristics of ongoing studies [ordered by study ID]
ACTRN 12613001161718.
| Trial name or title | Effectiveness of dual‐task functional power training for preventing falls in older people: Study protocol for a cluster‐randomised controlled trial |
| Methods | Cluster RCT |
| Participants | Target sample size: 280 Inclusion criteria: aged 65 years and over, at an increased risk of falling, currently resident in retirement villages, able to speak English, walk unaided or with minimal assistance (walking stick or walker) or at least 50 metres and be cognitively intact; clearance from local doctor prior to exercising if has any contraindicated medical conditions to exercise Exclusion criteria: current or prior participation in a structured progressive resistance training programme and/or organised balance training > 1 a week in the past 3 months, acute or terminal illness likely to compromise exercise participation, unstable or ongoing cardiovascular/respiratory disorders, musculoskeletal or neurological diseases disrupting voluntary movement or that might limit training, upper‐ or lower‐extremity fracture in the past 3 months, visual impairment not corrected with glasses |
| Interventions | 1. Exercise programme involving dual‐task functional power training (DT‐FPT), 2 twice a week supervised for 6 months, 'step‐down' maintenance for 6 months, follow‐up after 6 months 2. Usual care control group |
| Outcomes | 1. Number of falls over the 6‐, 12‐, 18‐month period; details of the fall location, cause, injury, treatment and the healthcare utilisation 2. Changes in lower‐limb functional muscle strength and power, isometric knee extensor, dorsi‐flexor and hand‐grip strength, dynamic balance and reaction time, gait, Instrumental Activities of Daily Living (IADL), quality of life, cognitive function and fall‐related self‐efficacy |
| Starting date | 23 October 2015 |
| Contact information | Centre for Physical Activity and Nutrition Research, School of Exercise and Nutrition Sciences, Deakin University, Burwood, Victoria, Australia Email: rmdaly@deakin.edu.au |
| Notes |
ACTRN 12615000138583.
| Trial name or title | Standing Tall ‐ a home‐based exercise programme using mobile technology for preventing falls in older people |
| Methods | RCT |
| Participants | Target sample size: 500 Inclusion criteria: ≥ 70 years old, community‐dwelling, English‐speaking, independent in ADL, able to walk household distances without the use of a walking aid, willingness to give informed consent and comply with the study protocol Exclusion criteria: unstable or acute medical condition that precludes exercise participation, progressive neurological condition (such as Parkinson’s disease, multiple sclerosis, Meniere's disease), cognitively‐impaired, defined as a Pfeiffer Short Portable Mental Status Questionnaire (SPMSQ) score < 8, currently participating in a fall‐prevention programme |
| Interventions | 1. Balance training delivered through a tablet computer in people’s homes, unsupervised for > 2 hours a week for 2 years 2. Control group: usual care + health promotion education programme relevant to older adults delivered through the tablet computer with weekly fact sheets |
| Outcomes | 1. Number of people falling over 12 months 2. Rate of falls over 12 months 3. Questionnaire measure of concern about falling using the iconographical Falls Efficacy Scale 4. Clinical measures of balance, gait, choice reaction stepping time, Timed Up and Go Test, Sit‐to‐Stand Test 5. Concern about falling, quality of life, depressive symptoms, acceptability and enjoyment of intervention, exercise self‐efficacy, healthcare use, physical activity levels, adverse events |
| Starting date | 17 February 2015 ‐ 15 December 2017 |
| Contact information | Dr Kim Delbaere Address NeuRA Barker St Randwick 2031 NSW Australia Phone +61 2 9399 1066 Email k.delbaere@neura.edu.au |
| Notes |
ACTRN 12615000865516.
| Trial name or title | Balance Exercise and Strength Training (BEST) programme for older people living at home |
| Methods | RCT |
| Participants | Target sample size: 576 Inclusion criteria: aged 65 years and over, and living at home or independently in the community (e.g. self‐care unit in residential aged care facility) in the Illawarra Shoalhaven Local Health District Exclusion criteria: Residing in nursing home; cognitive impairment; inability to walk 10 metres despite assistance from walking aid; insufficient English language skills; a progressive neurological disease e.g. Parkinson’s disease; recent fracture/joint replacement; a medical condition precluding exercise, e.g. unstable cardiac disease, uncontrolled hypertension, uncontrolled metabolic diseases; unable to obtain a medical clearance; currently participating in an exercise programme similar to either study programme 2 or more times a week |
| Interventions | 1. Lower‐limb group will receive a home‐based exercise programme for the lower limb based on the Otago Exercise Program 2. Upper‐limb group will receive an exercise programme designed to improve upper limb function |
| Outcomes | 1. Rate of falls 2. Upper limb function 3. Strength and balance, physical activity, falls efficacy, quality of life, health service usage, attitudes to exercise |
| Starting date | 26 October 2015 |
| Contact information | Professor Cathie Sherrington The University of Sydney Musculoskeletal Health Sydney, School of Public Health PO Box M179 Missenden Road NSW 2050 Australia Email cathie.sherrington@sydney.edu.au |
| Notes |
CTRI/2018/01/011214.
| Trial name or title | Falls and fractures: A physiotherapy approach to prediction and prevention in healthcare |
| Methods | RCT |
| Participants | Men and women, aged 60 ‐ 80 years. Moderate risk in fracture risk assessment tool and Berg balance scale |
| Interventions | 1. Exercise protocol as in the standard guidelines. Physiotherapy interventions including flexibility, mobility, strengthening and balancing exercises 4 times a week for up to 6 weeks with follow‐up every week. 2. Lifestyle modifications and ergonomical advice |
| Outcomes | Berg balance scale Lower extremity functional scale |
| Starting date | 21 March 2017 |
| Contact information | Dr. Bhoomika Brahmbhatt Sainath Hospital, Physiotherapy department, Exercise therapy division, Room no 301, Bopal‐Ghuma Road, Ahmedabad‐380058 Ahmadabad GUJARAT, India Ph. 9099015220 Email: bhumika2207@gmail.com |
| Notes |
ISRCTN71002650.
| Trial name or title | The design and development of a complex multifactorial falls assessment intervention for falls prevention: The Prevention of Falls Injury Trial (PreFIT) |
| Methods | 3‐arm cluster‐RCT and economic evaluation |
| Participants | N = 9821 Inclusion criteria: ≥ 70 years old, living in the community or in sheltered accommodation Exclusion criteria: terminally ill, residential and nursing homes |
| Interventions | 3 arms: 1. Written advice 2. Written advice plus structured exercise 3. Written advice plus multifactorial fall prevention (MFFP) The total duration of follow‐up for all trial arms is 18 months (updated 13 August 2015: was previously 12 months) The total duration of treatment varies across trial arms as follows: 1. Advice: 30 minutes 2. Exercise: 12 weeks (2 x 1‐hour sessions a week) 3. MFFP: 8 weeks (depending on individual risk factors, but typically 6 x 30‐minute sessions over 8 weeks) |
| Outcomes | 1. Number of people sustaining peripheral fractures 2. Time to first fracture 3. Rate of falls, quality of life, emotional and physical function, mortality 4. Resource use, out‐of‐pocket expenses |
| Starting date | September 2010 |
| Contact information | Prof Sarah Lamb Warwick Clinical Trials Unit The University of Warwick Gibbet Hill Campus Coventry CV4 7AL United Kingdom +44 (0)24 7615 0404 Email: s.lamb@warwick.ac.uk |
| Notes |
NCT01029171.
| Trial name or title | Action Seniors!: A 12‐month randomized controlled trial of a home‐based strength and balance retraining programme in reducing falls |
| Methods | RCT |
| Participants | Target sample size: 344 Inclusion criteria: adults ≥ 70 years old attending a Falls Prevention Clinic Service; understands, speaks, and reads English proficiently; MMSE 8 score > 24/30; had 1 documented non‐syncopal fall in the last 12 months and 1 of the following: 1) A Physiological Profile Assessment (PPA) score of at least 1 SD above normal; OR 2) Timed Up and Go Test (TUG) performance of > 15 seconds; OR 3) 1 additional documented non‐syncopal fall in the previous 12 months; expected to live > 12 months; community‐dwelling (i.e. not residing in a nursing home, extended care unit, or assisted‐care facility); able to walk 3 metres with or without an assistive device; and able to provide written informed consent Exclusion criteria: diagnosed with a neurodegenerative disease (e.g. Parkinson's disease); diagnosed with dementia (of any type); stroke; clinically significant peripheral neuropathy or severe musculoskeletal or joint disease; or history indicative of carotid sinus sensitivity (i.e. syncopal falls) |
| Interventions | 1. Intervention: Otago Exercise Program; home‐based balance and strength retraining programme 2. Control: Usual care as prescribed by geriatrician |
| Outcomes | 1. Falls over a 12‐month period 2. Physiological falls risk; mobility; cognitive function; and economic evaluation |
| Starting date | November 2009 |
| Contact information | Teresa Liu‐Ambrose Aging, Mobility, and Cognitive Neuroscience Laboratory, Vancouver Falls Prevention Clinic, University of British Columbia CANADA |
| Notes |
NCT02126488.
| Trial name or title | Effect of adaptive training for balance recovery |
| Methods | RCT |
| Participants | N = 308 Inclusion criteria: ≥ 65 years old, healthy, no known history of musculoskeletal, neurological, cardiovascular, or pulmonary impairment that may affect their ability to perform the testing procedures Exclusion criteria: Ultrasound calcaneus bone mineral density T score < −2.5 (osteoporotic), MMSE score < 25 (cognitive impairment) |
| Interventions | 1. Treadmill slip perturbation: perturbation training on a treadmill with precisely‐controlled slip‐like displacements and then encounter an unannounced novel slip during over‐ground walking 2. Treadmill training placebo: placebo training (on the same treadmill for the same duration but without perturbation) but encounter an identical novel slip during their over‐ground walking 3. Observation training: watching a training video and slides, so when exposed to an identical novel slip in over‐ground walking, they will know where and how the slip is going to occur and how to resist a fall |
| Outcomes | 1. Fall incidence, 1 year 2. Dynamic stability, 6 months |
| Starting date | June 2014 |
| Contact information | Yi‐Chung (Clive) Pai, University of Illinois at Chicago |
| Notes |
NCT02287740.
| Trial name or title | Prevention of falls among older adults in community settings |
| Methods | RCT |
| Participants | Target sample size: 670 Inclusion criteria: ≥ 70 years, living independently in the community; ≥ 1 fall in the preceding 12 months referral from a healthcare provider indicating the participant is at risk of falls; no participation in daily and/or structured vigorous physical activity or walking for exercise ≥ 15 minutes or muscle‐strengthening activities on 2 or more days a week in previous 3 months; walking independently, with or without the use of an assistive device; no severe cognitive impairment; able to exercise safely as determined by healthcare provider; willingness to be randomly assigned to an intervention condition and complete the 6‐month intervention and 6‐month follow‐up Exclusion Criteria: any medical or physical condition deemed unacceptable by their physician or healthcare provider; planned to leave the study area within the next 12 months |
| Interventions | All training sessions are 2 a week, 6 months. 1. Tai Ji Quan, moving for better balance: core 8‐form routine training with built‐in variations and a subroutine of integrated therapeutic movements 2. Multimodal exercise: aerobic, strength, balance, and flexibility exercises 3. Stretching: primarily seated exercises accompanied by breathing, stretching, and relaxation |
| Outcomes | 1. Number of falls in 6 months 2. Cost per fall prevented determined by calculating total intervention cost estimates divided by number of falls observed during the 6‐month intervention |
| Starting date | 1 November 2014 |
| Contact information | Fuzhong Li, Ph.D Oregon Research Institute |
| Notes |
NCT02617303.
| Trial name or title | Prevention of falls and its consequences in elderly people |
| Methods | RCT |
| Participants | Target sample size: 402 Inclusion criteria: ≥ 75 to 89 years, assigned to primary care team; living in the community; using assisted mobility devices are included; ranking the Folstein MMSE test; expectation of permanence in the area for at least 18 months; agree to participate in the study by informed consent; in the case of a caregiver of person with dementia who assumes the realisation of the exercise programme and the following of tips, may be included; Exclusion Criteria: current participation in another trial or institutional programme of guided physical activity; hip or knee operation or major injury or both, or any other intervention in the last 6 months; unable to follow an aerobic physical activity programme; in Home Care Programmes or Nursing Homes at baseline or during the training phase; terminal or severe cancer cases; disabled prior to or during the study period; have not been visited in reference's Health Center in the last 2 years (displacement/transfer); very advanced dementia that precludes following the instructions in the exercise programme and nurse's instructions. In case of a caregiver who assumes the realisation of exercise programme and the following of tips, patients of the Health Center who will be displaced, or temporarily shifted (> 2months/year) may be included |
| Interventions | 1. Otago Exercise Program exercises, consisting of a set of aerobic exercises affecting gait, balance, stability and are adapted for older people to support them both in groups and individually; 3 months followed by a loyalty phase (1 year) to consolidate the exercise programme. Falls and fractures monitored quarterly for 15 months 2. Usual practice: normal medical treatment will be provided by family physicians and nurses |
| Outcomes | 1. Reduction in falls measured with a questionnaire at baseline and quarterly over 15 months 2. Reduction of fracture, fear of falling, measured with questionnaire 3. Physical measures of strength, balance, motion, endurance 4. Number of appointments at the practice 5. Nursing Home admission measured through questionnaire at 15 months 6. Drug reduction |
| Starting date | September 2015 |
| Contact information | Rafael Azagra, PhD Insitut Català de la Salut Universitat Autònoma de Barcelona, SPAIN Email: rafael.azagra@uab.cat |
| Notes |
NCT02926105.
| Trial name or title | Comparison of home‐based exercise programmes for falls prevention and quality of life in older adults |
| Methods | RCT |
| Participants | Target sample size: 405 Inclusion criteria: ≥ 65 years old, living in their own home, having a history of falls in the previous 12 months or perceiving fear of falling (≥ 20 points on FES‐I: Falls Efficacy Scale ‐ international version), able to walk without auxiliary tools in their home, signed informed consent Exclusion criteria: having severe vision impairment that does not permit the reading of the exercise‐programme booklet and that does not permit the completion of the monthly diaries, receiving physiotherapeutic treatment with balance learning, having cognitive impairment (< 25 points on the Folstein MMSE |
| Interventions | 1. 'Test and Exercise home‐based tailored balance and functional strength tests and exercises, 3 a week, 12 months + 8 physiotherapist home visits 2. Otago home‐based programme: tailored balance, strength, walking exercises, 3 a week over 12 months + 8 physiotherapist home visits 3. Active‐Control: receive the 'Helsana' booklet with recommendations and 10 exercises, 3 a week, 12 months |
| Outcomes | 1. Number of falls, 1 year 2. Fear of falling 3. Severity of falls 4. Risk of fall 5. Quality of life 6. Exercise adherence |
| Starting date | October 2016 |
| Contact information | Anne‐Gabrielle Mittaz Hager, MS HES‐SO Valais‐Wallis Leukerbad, Valais, Switzerland, 3954 Telephone: +41 79 609 90 63 Email: gaby.mittaz@hevs.ch |
| Notes |
NCT03211429.
| Trial name or title | Effectiveness of three interventions to reduce fear of falling and improve functionality in the elderly |
| Methods | RCT |
| Participants | N = 110 Inclusion criteria: ≥ 60 years old, healthy, community‐dwelling, reported fear of falling, 'Leganés Cognitive Test' ≥ 23, SPPB (short physical performance battery) ≤ 9 Exclusion criteria: some cognitive impairment or medical condition or both that may affect the intervention, permanent use of wheelchair, people who have received prior protocolised management for fear of falling |
| Interventions | 1. Cognitive behavioural therapy: teach participants how to deal with their concerns about falls and related avoidance of activity 2. Tai Chi: training in the Yang style of 24 movement 3. Postural control exercise: individually‐adjusted progressive, specific and functional postural control training |
| Outcomes | 1. Fear of falling 2. Functional mobility 3. Falls 4. Depression 5. Handgrip 6. Daily life activities 7. Self‐rated health 8. Postural control |
| Starting date | June 2016 |
| Contact information | Carmen L Curcio, PhD Universidad de Caldas Manizales, Caldas, Colombia, 170004 573184665019 Email: carmen.curcio@ucaldas.edu.co |
| Notes |
NCT03320668.
| Trial name or title | Efficacy of the Otago Exercise Program delivered as group training versus individually‐tailored training |
| Methods | RCT |
| Participants | Target sample size: 728 Inclusion criteria: 65 ‐ 80 years, people who belong (ascribed) to primary healthcare centres of the same health area, non‐institutionalised, independence for walking, provide informed consent for participation Exclusion Criteria: residential period in the Health Basic Area of the primary health centre < 9 months, or < 9 months life expectancy in the health area of the primary healthcare centre; mild and moderate cognitive impairment; sight impairment or hearing impairment which prevents following the intervention (according to the diagnosis from medical history); absolute contraindication to perform physical exercise (according to the diagnosis from medical history) |
| Interventions | 1. Individual Otago Exercise Program (OEP): individual education in 5 sessions + telephone call to follow‐up 2. Group OEP: OEP education to 10 people groups in 5 sessions + telephone calls to follow‐up |
| Outcomes | 1. Percentage of falls, 12 months 2. Adverse events 3. Adherence 4. Participant satisfaction |
| Starting date | 10 January 2017 |
| Contact information | Laura Albornos‐Muñoz Instituto de Salud Carlos III, SPAIN Telephone: 34 918222517 Email: lalbornos@isiii.es |
| Notes |
NCT03404830.
| Trial name or title | Effects of a program of high intensity exercise by intervals on the risk of falls for the physical condition and the state of health in people over 60 years |
| Methods | RCT |
| Participants | Target sample size: 45 Inclusion criteria: Men and women, aged 60 ‐ 80 years Exclusion criteria: diseases that may alter balance and functional activity (such as auditory or vestibular alterations), central or peripheral neurological disorders, other rheumatological diseases, or serious psychiatric or somatic diseases |
| Interventions | 1 and 2: training twice a week for 12 weeks 1. High‐intensity interval training (HIIT) group: Squat training with the Suspension Training System (TRX). The session will be divided into 4 x 4‐minute intervals at an intensity of 90 ‐ 95% of the maximum heart rate, followed by 3‐minute active rest intervals of 50 ‐ 70%. Followed by 10 minutes of exercises of joint range 2. Moderate‐intensity continuous training (MICT) group: Squat training with the Suspension Training System (TRX) with an intensity close to 70% of their maximum heart rate maintained for 40 minutes. The session will conclude with a return to calm of 10 minutes of joint width and stretching. 3. No intervention group |
| Outcomes | 1. Gait and balance parameters 2. Mobility 3. Balance 4. Strength 5. Balance confidence 6. Falls self‐efficacy 7. Body composition 8. Health‐related quality of life |
| Starting date | September 2017 |
| Contact information | Agustín Aibar Almazán University of Jaén, SPAIN |
| Notes |
NCT03417531.
| Trial name or title | Sarcopenia prevention with a targeted exercise and protein supplementation program |
| Methods | RCT, 2x2 factorial design, triple‐blinded |
| Participants | Target sample size: 800 participants Inclusion: age 80+; at least 1 of 5 Cardiovascular Health Study frailty criteria ( i) weight loss of > 4.5 kg in the last 12 months; ii) reduced grip strength in Martin Vigorimeter test: men ≤ 64 kPa, women ≤ 42 kPa; iii) standardised question on exhaustion as published by Fried et al. (Fried 2001); iv) gait speed < 1 m/s; v) 6‐minute walk test < 300 metres; Injurious (any injury) low trauma fall in the last 12 months prior to enrolment; At risk of malnutrition or established malnutrition based on the Mini Nutritional Assessment (MNA) screening tool (score ≤ 11); Community‐dwelling or assisted living Exclusion: MMSE < 24; inability to come to the trial centres; inability to walk at least 3 meters with or without walking aid; severe kidney impairment; inability to follow exercise instruction or inability to take protein powder mixed in drink or food; severe gait impairment or diseases with a risk of recurrent falling; major visual or hearing impairment or other serious illness that would preclude participation (e.g. alcohol abuse, alcoholic disease); inability to read/speak/write in German; living in a nursing home; contraindication to treatment (e.g. allergy); contraindication to the vitamin D standard of care therapy |
| Interventions | 1. Protein supplement plus active exercise: Participants will ingest twice daily 23.7 g of L‐leucine‐enriched whey protein isolate powder (equivalent to 20 g of protein) and perform a simple home exercise strength programme (3 x 30 minutes a week) 2. Active comparator: Protein‐free supplement plus active exercise: Participants will ingest twice daily 23.7 g of a protein‐free, isocaloric powder blend and perform a simple home exercise strength programme (3 x 30 minutes a week) 3. Active comparator: Protein supplement plus control exercise: Participants will ingest twice daily 23.7 g of L‐leucine‐enriched whey protein isolate powder (equivalent to 20 g of protein) and perform a joint flexibility home exercise programme (3 x 30 minutes a week) 4. Sham comparator: Protein‐free supplement plus control exercise: Participants will ingest twice daily 23.7 g of a protein‐free, isocaloric powder blend and perform a joint flexibility home exercise programme (3 x 30 minutes a week) |
| Outcomes | 1. Rate of falling 2. Mobility 3. Fallers, number of people with injurious falls 4. Frailty 5. Sarcopenia 6. Institutionalisation 7. Health care utilisation |
| Starting date | May 2018 |
| Contact information | Heike A. Bischoff‐Ferrari University of Zurich, SWITZERLAND Ph: +41 44 255 27 57 Email: heike.bischoff@usz.ch |
| Notes |
NCT03455179.
| Trial name or title | Effects of slow‐speed traditional resistance training, high‐speed resistance training and multicomponent training with variable resistances on molecular, body composition, neuromuscular, physical function and quality of life variables in older adults |
| Methods | RCT |
| Participants | Target sample size: 192 participants Inclusion criteria: Age > 60 years; physically independent (able to walk 100 meters without a walking aid and climb 10 steps without rest); medical certificate of suitability or fitness to practice resistance training activities; no plans to leave the area during the intervention; cognitive ability to understand, follow the instructions and sign the informed consent form; free of any antioxidant supplements for at least 6 weeks before the start of this study. Exclusion criteria: Presence of cardiovascular, musculoskeletal, renal, liver or neuromuscular disorders that would prevent the participant from performing the exercises; body weight changes > 10% in the previous year; intake of prescription medications that were expected to alter the results of the study; history of malignant neoplasms; engagement in regular strength training during the previous 6 months; participating in another research project involving dietary, exercise and/or pharmaceutical intervention; MMSE < 24/30; Severe visual or hearing impairment |
| Interventions | 1. Slow‐speed traditional resistance training. Resistance training with variable resistances (elastic band) at high intensity and slow‐speed (2 seconds of concentric contraction and 2 seconds of eccentric contraction) twice a week over 20 weeks 2. High‐speed resistance training. Resistance training with variable resistances (elastic band) at low intensity and high‐speed ('as fast as possible' for the concentric contraction, pause for 1 second and 2 ‐ 3 seconds for the eccentric contraction) twice a week over 20 weeks 3. Multicomponent training. Training sessions with balance, resistance, aerobic, flexibility and co‐ordination components twice a week over 20 weeks 4. Control. Maintain usual physical activity habits and diet |
| Outcomes | 1. Muscle biochemistry 2. Muscle strength 3. Function 4. Mobility 5. Body composition 6. Falls |
| Starting date | March 2018 |
| Contact information | Prof. Juan Carlos Colado Sánchez Department of Physical Education and Sports University of Valencia, SPAIN Spain, 46010 |
| Notes |
NCT03462654.
| Trial name or title | Comparison of a group‐delivered and individually‐delivered lifestyle‐integrated functional exercise (LiFE) programme in older persons |
| Methods | RCT |
| Participants | Target sample size: 300 participants Inclusion criteria: Aged 70 years or older; speaks German; able to read newspaper; able to walk 200 meters with or without walking aid; home‐dwelling; 2 or more falls in the past 12 months OR 1 injurious fall in the past 12 months OR subjective decline in balance and strength in the past 12 months together with Timed Up and Go Test time > 13.5 seconds; available for intervention participation for 11 weeks Exclusion criteria: Cognitive impairment (MoCA < 23); current participation in an organised exercise class > 1 a week in the past 3 months; moderate‐ to vigorous‐intensity physical activity ≥ 150 minutes a week in the past 3 months; a list of 8 medical conditions |
| Interventions | 1. Individual LiFE (iLiFE). In iLiFE, LiFE activities to increase strength, improve balance, and promote physical activity as well as habitualisation strategies are introduced and taught in 7 highly individualised, one‐to‐one home visits 2. Group LiFE (gLiFE). In gLiFE, the same LiFE activities as performed in iLiFE are introduced and taught in 7 group sessions with 8 ‐ 12 participants. Implementation and habitualisation strategies will be addressed within the group setting, making use of group dynamics and processes |
| Outcomes | 1. Fall incidence expressed as number of falls per amount of physical activity 2. Cost‐effectiveness of iLiFE and gLiFE (incremental cost‐effectiveness ratios (ICERs) of delivering iLiFE and gLiFE) |
| Starting date | April 2018 |
| Contact information | Carl‐Philipp Jansen Heidelberg University, Network Aging Research, GERMANY ph. +49 6221 548144 Email: jansen@nar.uni‐heidelberg.de |
| Notes |
ADL: activities of daily living m: metres MMSE: Mini Mental State Examination RCT: randomised controlled trial SD: standard deviation TUG: Timed Up and Go test wk: week x: times <: less than >: more than ≥: greater than or equal to
Differences between protocol and review
Changes and clarifications to protocol
Types of participants
We clarified that we considered studies that focused on people who had been recently discharged from hospital ‐ typically, trial participants would be recruited in hospital prior to discharge ‐ were a distinct category.
Types of interventions
We clarified that our umbrella comparison was exercise (all types) versus control. We clarified that comparisons of different types, modes of delivery or doses of exercise were secondary comparisons. We redefined comparisons of different intensities of exercise as different doses of exercise to reflect the way dose was reported in the included trials.
We recoded intervention programmes in the included studies rather than using codes from Gillespie 2012, as we considered it more relevant for practice to divide studies on the basis of the primary intervention component rather than the presence of certain components. We examined the descriptions of interventions used in individual trials and categorised the intervention based on the ProFaNE taxonomy (Lamb 2011). We classified exercise programmes on the basis of the primary exercise category and noted the presence of additional, secondary, exercise categories. The exercise categories follow: i) gait, balance, co‐ordination and functional task training (referred to as 'balance and functional exercises' for simplicity); ii) strength/resistance training (including power training, using resistance so referred to as 'resistance exercises'); iii) flexibility; iv) three‐dimensional (3D) exercise (with Tai Chi or dance subcategories); iv) general physical activity (walking programmes); v) endurance; vi) other kind of exercises. We formed an additional category for exercise programmes that included more than one of the above categories as the primary exercise category. As indicated in our protocol, some forms of yoga were categorised as flexibility exercise and others as 3D exercise, depending on the content of the intervention in the individual trial.
Types of outcomes
We added two outcomes for consistency with a related review on multifactorial and multiple component interventions (Hopewell 2018): number of people who experienced one or more falls that resulted in hospital admission, and health‐related quality of life.
While we collected all reports of adverse events, we stipulated that these needed to be monitored closely in all groups using the same methods over the entire study period to be included in the data analysis.
We clarified that outcomes collected within 18 months of randomisation would be included in the primary analyses. Outcomes collected more than 18 months after randomisation were considered long‐term outcomes that would be pooled and reported separately. The 18 months threshold was a pragmatic choice that allowed for some slippage in the 12‐month follow‐up; these data could actually be collected later on, such as between 13 and 15 months.
Data extraction and management
In particular, we evaluated whether trials excluded participants with cognitive impairment. This was to aid assessment of the generalisability of the review's results.
We clarified that we recorded and reported data on fracture, hospitalisation, medical attention and health‐related quality of life only where it was reported by group. Additionally, we returned to trial authors where data were missing for falls outcomes only.
Risk of bias assessment
We applied 'risk of bias' assessments for the primary outcome (rate of falls). In addition, we reported blinding of outcome assessment (detection bias) separately for four groups of outcomes (falls; fractures; medical attention, hospital admission and adverse events; and health‐related quality of life).
We have added an assessment of risk of bias specifically for trials using cluster‐randomised trials. We assessed the risk of additional bias relating to recruitment, baseline imbalance, loss of clusters, incorrect analysis and comparability with individually‐randomised trials, as described in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
In light of more recent recommendations in the interpretation of funnel plots (Sterne 2011), we did not refer to the examination of funnel plots as purely assessing publication and reporting bias.
Data synthesis ‐ decisions for pooling data
We decided not to pool the results of studies that recruited people in hospitals and delivered interventions after discharge with the other trials of people living in the community. This was because, on reflection, we considered post‐hospital patients to be distinct from general community‐dwelling older adults. Thus, while the post‐hospital studies are included, we analysed them separately rather than pooling together with the general community‐dwelling older adults.
We followed the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and primarily used random‐effects meta‐analyses (where meta‐analysis was considered appropriate) as we considered it more likely that there was a range of true effects rather than a single effect of exercise on falls. We then undertook sensitivity analyses to assess the impact on the conclusions of the fixed‐effect analyses.
Subgroup analysis
Given the need for caution in conducting subgroup analyses, we set out a criterion that we would only perform a subgroup analysis where there were at least 10 trials in a comparison.
Sensitivity analysis
In order to aid interpretation of the sensitivity analyses, we decided not to group three risk of bias domains together. Instead, we conducted separate sensitivity analyses for each risk of bias domain to examine the effects of including trials at high or unclear risk of selection, detection and attrition bias.
In order to assist in the interpretation of the results of the type of exercise subgroup 'multiple categories of exercise' comparisons, we undertook additional sensitivity analyses for both falls outcomes which only included trials that were coded as having the two primary components balance/functional exercises and resistance exercises.
GRADE assessment
We used the updated GRADE assessment criteria, which expressed our judgement of the quality of the evidence in terms of 'certainty' rather than 'quality'.
'Summary of findings' tables
We clarified our intention to produce a 'Summary of findings' table for our umbrella comparison (exercise (all types) versus control); the outcomes shown included the two new secondary outcomes (hospital admission and health‐related quality of life); see next section. We also limited the number of outcomes in the 'Summary of findings' tables for the different primary exercise category versus control comparisons to four outcomes: rate of falls, risk of falling, fall‐related fractures and adverse events. This reflected the sparse data for other outcomes and that these are subgroup comparisons.
Changes to protocol in response to a commissioning brief relating to NICE guideline CG161
To enhance the direct usefulness of the review to decisions relating to the NICE clinical guideline (CG161; NICE 2013), we made the following changes to the protocol in response to a commissioning brief (April 2018).
We set the umbrella comparison as 'exercise (all types) versus control'.
We added two new secondary outcomes to Types of outcome measures: number of people who experienced one of more falls that resulted in hospital admission, and health‐related quality of life. In addition, we recorded and reported mortality data. We reported collecting these data in Data extraction and management.
We added in the details of the measures of treatment effect we would use for continuous outcomes in Measures of treatment effect.
In view of the different cut‐offs used to define the populations of older people of 60 years in this review (Types of participants), and 65 years in CG161, we examined how many trials would have been excluded if the age limit was raised to 65 years and set out a sensitivity analysis to explore the effects of excluding these from the exercise (all types) versus control comparison.
We set out a subgroup analysis to compare the effects on falls outcomes in trials with predominantly older populations (based on the proposed threshold of 75 years) and those with predominantly younger populations (Subgroup analysis and investigation of heterogeneity).
We set out a subgroup analysis for the fall and fracture outcomes for the pooled (all‐exercise types) versus control analyses to compare the effect of exercise on falls and fractures in trials that did and did not use an increased risk of falls as an inclusion criterion (Subgroup analysis and investigation of heterogeneity).
Peer referee feedback
In response to peer referee feedback, we extended two subgroup analyses (qualifications of personnel delivering the exercise programmes; group versus individual exercise programmes) to the all types of exercise analyses versus control comparisons for the falls outcomes.
To explore the possible impact of how we classified exercise interventions, we conducted additional sensitivity analyses to examine the effects on both falls outcomes of the:
classification of interventions based on the Otago Exercise Program as multiple categories of exercise; and
classification of any intervention that included balance and functional exercises plus strength exercises as multiple categories of exercise.
Contributions of authors
All authors have contributed to the production of this review.
CS was involved in screening, data extraction, data analysis, co‐led the writing of the review and acted as guarantor of the review. NF was involved in screening, data extraction, data analysis, and co‐led the writing of the review. AT was involved in screening, data extraction, data analysis, and contributed to writing the review. GW and ZM were involved in screening, data extraction, data analysis, and contributed to writing the review. KH was involved in data extraction, data analysis, contributed to writing the review and commented on drafts of the review. LC, SH and SL contributed to writing the review and commented on drafts of the review.
Sources of support
Internal sources
School of Public Health, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia.
Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences (NDORMS), University of Oxford, Oxford, UK.
Faculty of Health Sciences, The University of Sydney, Lidcombe, Australia.
External sources
National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group, UK.
Australian National Health and Medical Research Council fellowships contribute to the salaries of CS and AT, Australia.
NIHR Cochrane Reviews of NICE Priority scheme, project reference NIHR127512, UK.
Declarations of interest
Several authors (CS, AT, SH, KH and SL) are currently running trials of fall prevention interventions; including the following ongoing trials in this review (ACTRN 12615000138583; ACTRN 12615000865516; ISRCTN71002650). These trials are all funded by national grant agencies.
No review author was involved in study selection or processing of any trials in which they were or are involved.
CS is an author of several trials considered in this review, including four included trials (Merom 2016; Sherrington 2014; Vogler 2009; Voukelatos 2015). NF has no known conflicts of interest. GW has no known conflicts of interest. AT has no known conflicts of interest. ZM has no known conflicts of interest. KH is an author of several trials considered in this review, including one included trial (Sherrington 2014). LC is an author of several trials considered in this review, including two included trials (Clemson 2010; Clemson 2012). SH has no known conflicts of interest. SL is lead author of the ProFaNE consensus for falls guidance and is an author of one of the trials considered in this review.
New
References
References to studies included in this review
Almeida 2013 {published data only}
- Almeida TL, Alexander NB, Nyquist LV, Montagnini ML, Santos AC, Rodrigues GH, et al. Minimally supervised multimodal exercise to reduce falls risk in economically and educationally disadvantaged older adults. Journal of Aging and Physical Activity 2013;21(3):241‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ansai 2015 {published data only (unpublished sought but not used)}
- Ansai JH, Rebelatto JR. Effect of two physical exercise protocols on cognition and depressive symptoms in oldest‐old people: A randomized controlled trial. Geriatrics and Gerontology International 2015;15(9):1127‐34. [DOI] [PubMed] [Google Scholar]
Arantes 2015 {published data only (unpublished sought but not used)}
- Arantes PM, Dias JM, Fonseca FF, Oliveira AM, Oliveira MC, Pereira LS, et al. Effect of a program based on balance exercises on gait, functional mobility, fear of falling, and falls in prefrail older women: a randomized clinical trial. Topics in Geriatric Rehabilitation 2015;31(2):113‐20. [DOI: 10.1097/TGR.0000000000000056] [DOI] [Google Scholar]
Arkkukangas 2015 {published and unpublished data}
- Arkkukangas M. Fall data [personal communication]. Email to: Z. Michaleff. 18 August 2015.
- Arkkukangas M, Johnson ST, Hellström K, Söderlund A, Eriksson S, Johansson AC. A feasibility study of a randomised controlled trial comparing fall prevention using exercise with or without the support of motivational interviewing. Preventive Medicine Reports 2015;2:134‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballard 2004 {published data only (unpublished sought but not used)}
- Ballard JE, McFarland C, Wallace LS, Holiday DB, Roberson G. The effect of 15 weeks of exercise on balance, leg strength, and reduction in falls in 40 women aged 65 to 89 years. Journal of the American Medical Women's Association 2004;59(4):255‐61. [MEDLINE: ] [PubMed] [Google Scholar]
Barker 2016 {published data only}
- Barker AL, Talevski J, Bohensky MA, Brand CA, Cameron PA, Morello RT. Feasibility of Pilates exercise to decrease falls risk: a pilot randomized controlled trial in community‐dwelling older people. Clinical Rehabilitation 2016;30(10):984‐96. [DOI] [PubMed] [Google Scholar]
Barnett 2003 {published data only}
- Barnett A, Smith B, Lord SR, Williams M, Baumand A. Community‐based group exercise improves balance and reduces falls in at‐risk older people: a randomised controlled trial. Age and Ageing 2003;32(4):407‐14. [DOI] [PubMed] [Google Scholar]
Beyer 2007 {published data only (unpublished sought but not used)}
- Beyer N, Simonsen L, Bulow J, Lorenzen T, Jensen D V, Larsen L, et al. Old women with a recent fall history show improved muscle strength and function sustained for six months after finishing training. Aging Clinical and Experimental Research 2007;19(4):300‐9. [DOI] [PubMed] [Google Scholar]
Boongrid 2017 {published data only}
- Boongrid C, Keesukphan P, Phiphadthakusolkul S, Rattanasiri S, Thakkinstian A. Effects of a simple home‐based exercise program on fall prevention in older adults: A 12‐month primary care setting, randomized controlled trial. Geriatrics and Gerontology International 2017;17(11):2157‐63. [DOI: 10.1111/ggi.13052] [DOI] [PubMed] [Google Scholar]
Brown 2002 {published data only}
- Brown A P. Reducing falls in elderly people: A review of exercise interventions. Physiotherapy Theory and Practice 1999;15(2):59‐68. [Google Scholar]
- Brown AI. Functional Adaptation to Exercise in Elderly Subjects [thesis]. Perth (Australia): Curtin University of Technology, 2002 (espace.curtin.edu.au/handle/20.500.11937/401). [Google Scholar]
- Piotrowski A, Cole J, Allison G. The influence of functional ability and physical and social intervention on falls in elderly subjects. XVIth Congress of the International Association of Gerontology; 1997 August 19‐23; Adelaide (Australia). 2002.
Buchner 1997 {published data only}
- Buchner DM, Cress ME, Lateur BJ, Esselman PC, Margherita AJ, Price R, et al. The effect of strength and endurance training on gait, balance, fall risk, and health services use in community‐living older adults. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 1997;52(4):M218‐24. [DOI] [PubMed] [Google Scholar]
- Buchner DM, Cress ME, Wagner EH, Lateur BJ. The role of exercise in fall prevention: Developing targeting criteria for exercise programs. In: Vellas B, Toupet M, Rubenstein L, Albarede JL, Christen Y editor(s). Falls, Balance and Gait Disorders in the Elderly. Amsterdam: Elsevier, 1992:55‐68. [Google Scholar]
- Buchner DM, Cress ME, Wagner EH, Lateur BJ, Price R, Abrass IB. The Seattle FICSIT/MoveIt study: the effect of exercise on gait and balance in older adults. Journal of the American Geriatrics Society 1993;41(3):321‐5. [DOI] [PubMed] [Google Scholar]
Bunout 2005 {published and unpublished data}
- Bunout D. [Personal communication]. Personal communication reported by Gillespie 2012.
- Bunout D, Barrera G, Avendano M, Maza P, Gattas V, Leiva L, et al. Results of a community‐based weight‐bearing resistance training programme for healthy Chilean elderly subjects. Age and Ageing 2005;34(1):80‐3. [DOI] [PubMed] [Google Scholar]
Campbell 1997 {published data only}
- Campbell AJ, Robertson MC, Gardner MM, Norton RN, Buchner DM. Falls prevention over 2 years: a randomized controlled trial in women 80 years and older. Age and Ageing 1999;28(6):513‐8. [DOI] [PubMed] [Google Scholar]
- Campbell AJ, Robertson MC, Gardner MM, Norton RN, Buchner DM. Psychotropic medication withdrawal and a home‐based exercise program to prevent falls: a randomized, controlled trial. Journal of the American Geriatrics Society 1999;47(7):850‐3. [DOI] [PubMed] [Google Scholar]
- Campbell AJ, Robertson MC, Gardner MM, Norton RN, Tilyard MW, Buchner DM. Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. BMJ 1997;315(7115):1065‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. Home‐based exercises to prevent falls in elderly women. New Zealand Journal of Physiotherapy 1998;26(3):6. [Google Scholar]
- Gardner MM, Buchner DM, Robertson MC, Campbel AJ. Practical implementation of an exercise‐based falls prevention programme. Age and Ageing 2001;30(1):77‐83. [DOI] [PubMed] [Google Scholar]
- Robertson MC. Development of a falls prevention programme for elderly people: evaluation of efficacy, effectiveness, and efficiency [thesis]. Dunedin (NZ): University of Otago, 2001. [Google Scholar]
- Robertson MC, Campbell AJ, Gardner MM, Devlin N. Preventing injuries in older people by preventing falls: a meta‐analysis of individual‐level data. Journal of the American Geriatrics Society 2002;50(5):905‐11. [DOI] [PubMed] [Google Scholar]
- Robertson MC, Devlin N, Scuffham P, Gardner MM, Buchner DM, Campbell AJ. Economic evaluation of a community based exercise programme to prevent falls. Journal of Epidemiology and Community Health 2001;55(8):600‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carter 2002 {published data only (unpublished sought but not used)}
- Carter ND, Khan KM, McKay HA, Petit MA, Waterman C, Heinonen A, et al. Community‐based exercise program reduces risk factors for falls in 65‐ to 75‐year‐old women with osteoporosis: randomized controlled trial. Canadian Medical Association Journal 2002;167(9):997‐1004. [PMC free article] [PubMed] [Google Scholar]
- Carter ND, Khan KM, Petit MA, Heinonen A, Waterman C, Donaldson MG, et al. Results of a 10 week community based strength and balance training programme to reduce fall risk factors: a randomised controlled trial in 65‐75 year old women with osteoporosis. British Journal of Sports Medicine 2001;35(5):348‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cerny 1998 {published and unpublished data}
- Cerny K. [Personal communication]. Personal communication reported by Gillespie 2012.
- Cerny K, Blanks R, Mohamed O, Schwab D, Robinson B, Russo A, et al. The effect of a multidimensional exercise program on strength, range of motion, balance and gait in the well elderly. Gait and Posture 1998;7(2):185‐6. [Google Scholar]
Clegg 2014 {published and unpublished data}
- Clegg A. Faller outcome data [personal communication]. Email to: C. West. 4 June 2018.
- Clegg A, Barber S, Young J, Iliffe S, Forster A. The Home‐based Older People's Exercise (HOPE) trial: a pilot randomised controlled trial of a home‐based exercise intervention for older people with frailty. Age and Ageing 2014;43(5):687‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clemson 2010 {published data only}
- Clemson L, Singh MF, Bundy A, Cumming RG, Weissel E, Munro J, et al. LiFE Pilot Study: a randomised trial of balance and strength training embedded in daily life activity to reduce falls in older adults. Australian Occupational Therapy Journal 2010;57(1):42‐50. [DOI] [PubMed] [Google Scholar]
Clemson 2012 {published data only}
- Clemson L, Fiatarone Singh MA, Bundy A, Cumming RG, Manollaras K, O'Loughlin P, et al. Integration of balance and strength training into daily life activity to reduce rate of falls in older people (the LiFE study): randomised parallel trial. BMJ 2012;345:e4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cornillon 2002 {published data only}
- Cornillon E, Blanchon MA, Ramboatsisetraina P, Braize C, Beauchet O, Dubost V. Effectiveness of falls prevention strategies for elderly subjects who live in the community with performance assessment of physical activities (before‐after) [Impact d'un programme de prevention multidisciplinaire de la chute chez le sujet age autonome vivant a domicile, avec analyse avant‐apres des performances physiques]. Annales de Readaptation et de Medecine Physique 2002;45(9):493‐504. [DOI] [PubMed] [Google Scholar]
Dadgari 2016 {published and unpublished data}
- Dadgari A. Fall outcomes [personal communication]. Email to: N Fairhall. 29 August 2017.
- Dadgari A. Number of males / females [personal communication]. Email to: C Ng. 4 September 2017.
- Dadgari A. Number of study participants per health centre [personal communication]. Email to: C Sherrington. 1 January 2018.
- Dadgari A, Aizan Hamid T, Hakim MN, Chaman R, Mousavi SA, Poh Hin L, et al. Randomized control trials on Otago Exercise Program (OEP) to reduce falls among elderly community dwellers in Shahroud, Iran. Iran Red Crescent Medical Journal 2016;18(5):e26340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dangour 2011 {published data only (unpublished sought but not used)}
- Dangour AD, Albala C, Aedo C, Elbourne D, Grundy E, Walker D, et al. A factorial‐design cluster randomised controlled trial investigating the cost‐effectiveness of a nutrition supplement and an exercise programme on pneumonia incidence, walking capacity and body mass index in older people living in Santiago, Chile: the CENEX study protocol. Nutrition Journal 2007;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangour AD, Albala C, Allen E, Grundy E, Walker DG, Aedo C, et al. Effect of a nutrition supplement and physical activity program on pneumonia and walking capacity in Chilean older people: a factorial cluster randomized trial. PLoS Medicine 2011;8(4):e1001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN48153354. Cost‐effectiveness of nutritional supplementation and exercise programme among older people in Santiago, Chile. controlled‐trials.com/ISRCTN48153354 (first received 22 July 2005).
- Walker DG, Aedo C, Albala C, Allen E, Dangour AD, Elbourne D, et al. Methods for economic evaluation of a factorial‐design cluster randomised controlled trial of a nutrition supplement and an exercise programme among healthy older people living in Santiago, Chile: the CENEX study. BMC Health Services Research 2009;9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2011 {published data only (unpublished sought but not used)}
- Davis JC, Marra CA, Beattie BL, Robertson MC, Najafzadeh M, Graf P, et al. Sustained cognitive and economic benefits of resistance training among community‐dwelling senior women: a 1‐year follow‐up study of the Brain Power study. Archives of Internal Medicine 2010;170(22):2036‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JC, Marra CA, Liu‐Ambrose TY. Falls‐related self‐efficacy is independently associated with quality‐adjusted life years in older women. Age and Ageing 2011;40(3):340‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JC, Marra CA, Robertson MC, Khan KM, Najafzadeh M, Ashe MC, et al. Economic evaluation of dose‐response resistance training in older women: a cost‐effectiveness and cost‐utility analysis. Osteoporosis International 2011;22(5):1355‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JC, Marra CA, Robertson MC, Najafzadeh M, Liu‐Ambrose T. Sustained economic benefits of resistance training in community‐dwelling senior women. Journal of American Geriatrics Society 2011;59(7):1232‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu‐Ambrose T, Davis JC, Nagamatsu LS, Hsu CL, Katarynych LA, Khan KM. Changes in executive functions and self‐efficacy are independently associated with improved usual gait speed in older women. BMC Geriatrics 2010;10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu‐Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12‐month randomized controlled trial. Archives of Internal Medicine 2010;170(2):170‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Day 2002 {published data only}
- Day L, Fildes B, Gordon I, Fitzharris M, Flamer H, Lord S. Randomised factorial trial of falls prevention among older people living in their own homes. BMJ 2002;325(7356):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzharris MP, Day L, Lord SR, Gordon I, Fildes B. The Whitehorse NoFalls trial: effects on fall rates and injurious fall rates. Age and Ageing 2010;39(6):728‐33. [DOI] [PubMed] [Google Scholar]
- McLean K, Day L, Dalton A. Economic evaluation of a group‐based exercise program for falls prevention among the older community‐dwelling population. BMC Geriatrics 2015;15(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Day 2015 {published data only}
- Day L, Hill KD, Stathakis VZ, Flicker L, Segal L, Cicuttini F, et al. Impact of Tai‐Chi on falls among preclinically disabled older people: a randomized controlled trial. Journal of the American Medical Directors Association 2015;16(5):420‐6. [DOI] [PubMed] [Google Scholar]
Duque 2013 {published data only (unpublished sought but not used)}
- Duque G, Boersma D, Loza‐Diaz G, Hassan S, Suarez H, Geisinger D, et al. Effects of balance training using a virtual‐reality system in older fallers. Clinical Interventions in Aging 2013;8:257‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebrahim 1997 {published data only}
- Ebrahim S, Thompson PW, Baskaran V, Evans K. Randomized placebo‐controlled trial of brisk walking in the prevention of postmenopausal osteoporosis. Age and Ageing 1997;26(4):253‐60. [DOI] [PubMed] [Google Scholar]
El‐Khoury 2015 {published data only}
- El‐Khoury F, Cassou B, Latouche A, Aegerter P, Charles MA, Dargent‐Molina P. Effectiveness of two year balance training programme on prevention of fall induced injuries in at risk women aged 75‐85 living in community: Ossebo randomised controlled trial. BMJ 2015;351:h3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiatarone 1997 {published data only}
- Fiatarone MA, O'Neill EF, Doyle Ryan N, Clements K. Efficacy of home‐based resistance training in frail elders. World Congress of Gerontology, 16th Congress of the International Association of Gerontology; 1997 August 19‐23; Adelaide, South Australia. 1997:323 (abstract no. 985). [CN‐00847936]
Freiberger 2007 {published data only}
- Freiberger E, Haberle L, Spirduso WW, Zijlstra GA. Long‐term effects of three multicomponent exercise interventions on physical performance and fall‐related psychological outcomes in community‐dwelling older adults: a randomized controlled trial. Journal of the American Geriatrics Society 2012;60(3):437‐46. [DOI] [PubMed] [Google Scholar]
- Freiberger E, Menz H B, Abu‐Omar K, Rutten A. Preventing falls in physically active community‐dwelling older people: a comparison of two intervention techniques. Gerontology 2007;53(5):298‐305. [DOI] [PubMed] [Google Scholar]
Gill 2016 {published data only}
- Gill TM, Pahor M, Guralnik JM, McDermott MM, King AC, Buford TW, et al. Effect of structured physical activity on prevention of serious fall injuries in adults aged 70‐89: randomized clinical trial (LIFE Study). BMJ 2016;352:i245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grahn Kronhed 2009 {published data only (unpublished sought but not used)}
- Grahn Kronhed AC, Hallberg I, Odkvist L, Moller M. Effect of training on health‐related quality of life, pain and falls in osteoporotic women. Advances in Physiotherapy 2009;11(3):154‐65. [Google Scholar]
Gschwind 2015 {published data only (unpublished sought but not used)}
- Gschwind YJ, Eichberg S, Ejupi A, Rosario H, Kroll M, Marston HR, et al. ICT‐based system to predict and prevent falls (iStoppFalls): results from an international multicenter randomized controlled trial. European Review of Aging and Physical Activity 2015;12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haines 2009 {published data only}
- Haines TP, Russell T, Brauer SG, Erwin S, Lane P, Urry S, et al. Effectiveness of a video‐based exercise programme to reduce falls and improve health‐related quality of life among older adults discharged from hospital: a pilot randomized controlled trial. Clinical Rehabilitation 2009;23(11):973‐85. [DOI] [PubMed] [Google Scholar]
Halvarsson 2013 {published data only (unpublished sought but not used)}
- Halvarsson A, Franzén E, Farén E, Olsson E, Oddsson L, Ståhle A. Long‐term effects of new progressive group balance training for elderly people with increased risk of falling ‐ a randomized controlled trial. Clinical Rehabilitation 2013;27(5):450‐8. [DOI] [PubMed] [Google Scholar]
- Halvarsson A, Oddsson L, Olsson E, Farén E, Pettersson A, Ståhle A. Effects of new, individually adjusted, progressive balance group training for elderly people with fear of falling and tend to fall: a randomized controlled trial.[Erratum in Clinical Rehabilitation 2012 Nov;26(11):1055]. Clinical Rehabilitation 2011;25(11):1021‐31. [DOI] [PubMed] [Google Scholar]
Halvarsson 2016 {published data only (unpublished sought but not used)}
- Halvarsson A, Franzén E, Ståhle A. Balance training with multi‐task exercises improves fall‐related self‐efficacy, gait, balance performance and physical function in older adults with osteoporosis: a randomized controlled trial. Clinical Rehabilitation 2015;29(4):365‐75. [DOI] [PubMed] [Google Scholar]
- Halvarsson A, Oddsson L, Franzén E, Ståhle A. Long‐term effects of a progressive and specific balance‐training programme with multi‐task exercises for older adults with osteoporosis: a randomized controlled study. Clinical Rehabilitation 2016;30(11):1049‐59. [DOI] [PubMed] [Google Scholar]
Hamrick 2017 {published and unpublished data}
- Hamrick I. Sample size, instructor:participant ratio and faller outcome data [personal communication]. Email to: N Fairhall. 14 December 2017, 19 May 2018.
- Hamrick I, Mross P, Christopher N, Smith PD. Yoga's effect on falls in rural, older adults. Complementary Therapies in Medicine 2017;35:57‐63. [DOI] [PubMed] [Google Scholar]
Hauer 2001 {published and unpublished data}
- Hauer K. [Personal communication]. Personal communication reported by Gillespie 2012.
- Hauer K, Pfisterer M, Schuler M, Bartsch P, Oster P. Two years later: a prospective long‐term follow‐up of a training intervention in geriatric patients with a history of severe falls. Archives of Physical Medicine and Rehabilitation 2003;84(10):1426‐32. [DOI] [PubMed] [Google Scholar]
- Hauer K, Rost B, Rutschle K, Opitz H, Specht N, Bartsch P, et al. Exercise training for rehabilitation and secondary prevention of falls in geriatric patients with a history of injurious falls. Journal of the American Geriatrics Society 2001;49(1):10‐20. [DOI] [PubMed] [Google Scholar]
- Hauer K, Specht N, Schuler M, Bartsch P, Oster P. Intensive physical training in geriatric patients after severe falls and hip surgery. Age and Ageing 2002;31(1):49‐57. [DOI] [PubMed] [Google Scholar]
- Oster P, Hauer K, Specht N, Rost B, Baertsch P, Schlierf G. Strength and coordination training for prevention of falls in the elderly [Kraft‐ und koordinationstraining zur sturzpravention im alter]. Zeitschrift fur Gerontologie und Geriatrie 1997;30(4):289‐92. [PubMed] [Google Scholar]
Helbostad 2004 {published data only}
- Helbostad JL, Moe‐Nilssen R, Sletvold O. Comparison of two types of exercise regimes on selected functional abilities for community‐dwelling elderly at risk of falling. XVI Conference of the International Society for Postural Gait Research; 2003 March 23‐27; Sydney (Australia). 2004.
- Helbostad JL, Sletvold O, Moe‐Nilssen R. Effects of home exercises and group training on functional abilities in home‐dwelling older persons with mobility and balance problems. A randomized study. Aging Clinical and Experimental Research 2004;16(2):113‐21. [DOI] [PubMed] [Google Scholar]
- Helbostad JL, Sletvold O, Moe‐Nilssen R. Home training with and without additional group training in physically frail old people living at home: effect on health‐related quality of life and ambulation. Clinical Rehabilitation 2004;18(5):498‐508. [DOI] [PubMed] [Google Scholar]
Hirase 2015 {published data only (unpublished sought but not used)}
- Hirase T, Inokuchi S, Matsusaka N, Okita M. Effects of a balance training program using a foam rubber pad in community‐based older adults: a randomized controlled trial. Journal of Geriatric Physical Therapy 2015;38(2):62‐70. [DOI] [PubMed] [Google Scholar]
Huang 2010 {published data only (unpublished sought but not used)}
- Huang HC, Liu CY, Huang YT, Kernohan WG. Community‐based interventions to reduce falls among older adults in Taiwan ‐ long time follow‐up randomised controlled study. Journal of Clinical Nursing 2010;19(7‐8):959‐68. [DOI] [PubMed] [Google Scholar]
Hwang 2016 {published data only}
- Hwang HF, Chen SJ, Lee‐Hsieh J, Chien DK, Chen CY, Lin MR. Effects of home‐based tai chi and lower extremity training and self‐practice on falls and functional outcomes in older fallers from the emergency department ‐ a randomized controlled trial. Journal of the American Geriatrics Society 2016;64(3):518‐25. [DOI] [PubMed] [Google Scholar]
Iliffe 2015 {published data only}
- Gawler S, Skelton DA, Dinan‐Young S, Masud T, Morris RW, Griffin M, et al. Reducing falls among older people in general practice: The ProAct65+ exercise intervention trial. Archives of Gerontology and Geriatrics 2016;67:46‐54. [DOI] [PubMed] [Google Scholar]
- Iliffe S, Kendrick D, Morris R, Griffin M, Haworth D, Carpenter H, et al. Promoting physical activity in older people in general practice: ProAct65+ cluster randomised controlled trial. British Journal of General Practice 2015;65(640):e731‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliffe S, Kendrick D, Morris R, Masud T, Gage H, Skelton D, et al. Multicentre cluster randomised trial comparing a community group exercise programme and home‐based exercise with usual care for people aged 65 years and over in primary care. Health Technology Assessment 2014;18(49):vii‐xxvii, 1‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Irez 2011 {published data only}
- Irez GB, Ozdemir RA, Evin R, Irez SG, Korkusuz F. Integrating pilates exercise into an exercise program for 65+ year‐old women to reduce falls. Journal of Sports Science and Medicine 2011;10(1):105‐11. [PMC free article] [PubMed] [Google Scholar]
Iwamoto 2009 {published data only}
- Iwamoto J, Suzuki H, Tanaka K, Kumakubo T, Hirabayashi H, Miyazaki Y, et al. Preventative effect of exercise against falls in the elderly: A randomized controlled trial. Osteoporosis International 2009;20(7):1233‐40. [DOI] [PubMed] [Google Scholar]
Kamide 2009 {published data only}
- Kamide N, Shiba Y, Shibata H. Effects on balance, falls, and bone mineral density of a home‐based exercise program without home visits in community‐dwelling elderly women: a randomized controlled trial. Journal of Physiological Anthropology 2009;28(3):115‐22. [DOI] [PubMed] [Google Scholar]
Karinkanta 2007 {published data only (unpublished sought but not used)}
- Karinkanta S, Heinonen A, Sievanen H, Uusi‐Rasi K, Pasanen M, Ojala K, et al. A multi‐component exercise regimen to prevent functional decline and bone fragility in home‐dwelling elderly women: randomized, controlled trial. Osteoporosis International 2007;18(4):453‐62. [DOI] [PubMed] [Google Scholar]
- Karinkanta S, Kannus P, Uusi‐Rasi K, Heinonen A, Sievanen H. Combined resistance and balance‐jumping exercise reduces older women's injurious falls and fractures: 5‐year follow‐up study. Age and Ageing 2015;44(5):784‐9. [DOI] [PubMed] [Google Scholar]
- Karinkanta S, Nupponen R, Heinonen A, Pasanen M, Sievanen H, Uusi‐Rasi K, et al. Effects of exercise on health‐related quality of life and fear of falling in home‐dwelling older women. Journal of Aging and Physical Activity 2012;20(2):198‐214. [DOI] [PubMed] [Google Scholar]
Kemmler 2010 {published data only}
- Kemmler W, Stengel S, Engelke K, Haberle L, Kalender WA. Exercise effects on bone mineral density, falls, coronary risk factors, and health care costs in older women: the randomized controlled senior fitness and prevention (SEFIP) study. Archives of Internal Medicine 2010;170(2):179‐85. [DOI] [PubMed] [Google Scholar]
- Kemmler W, Stengel S, Engelke K, Haberle L, Mayhew JL, Kalender WA. Exercise, body composition, and functional ability: a randomized controlled trial. American Journal of Preventive Medicine 2010;38(3):279‐87. [DOI] [PubMed] [Google Scholar]
- Kemmler W, Stengel S, Engelke K, Kalender WA. Exercise decreases the risk of metabolic syndrome in elderly females. Medicine and Science in Sports and Exercise 2009;41(2):297‐305. [DOI] [PubMed] [Google Scholar]
- NCT00267839. Effect of exercise on risk‐factors of elderly women. clinicaltrials.gov/show/NCT00267839 (first received 21 December 2005).
Kerse 2010 {published and unpublished data}
- ACTRN12605000475640. Late life intervention to improve function in elderly patients with depression. www.anzctr.org.au/trial_view.aspx?ID=736 (first received 23 September 2005).
- Kerse N. Fall outcome data [personal communication]. Email to: C West Vol. 18 June 2018.
- Kerse N, Falloon K, Moyes SA, Hayman KJ, Dowell T, Kolt GS, et al. DeLLITE depression in late life: an intervention trial of exercise. Design and recruitment of a randomised controlled trial. BMC Geriatrics 2008;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerse N, Hayman KJ, Moyes SA, Peri K, Robinson E, Dowell A, et al. Home‐based activity program for older people with depressive symptoms: DeLLITE‐‐a randomized controlled trial. Annals of Family Medicine 2010;8(3):214‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2014 {published data only (unpublished sought but not used)}
- Kim H, Yoshida H, Suzuki T. Falls and fractures in participants and excluded non‐participants of a fall prevention exercise program for elderly women with a history of falls: 1‐year follow‐up study. Geriatrics and Gerontology International 2014;14(2):285‐92. [DOI] [PubMed] [Google Scholar]
Korpelainen 2006 {published data only (unpublished sought but not used)}
- Korpelainen R, Keinanen‐Kiukaanniemi S, Heikkinen J, Vaananen K, Korpelainen J. Effect of exercise on extraskeletal risk factors for hip fractures in elderly women with low BMD: a population‐based randomized controlled trial. Journal of Bone and Mineral Research 2006;21(5):772‐9. [DOI] [PubMed] [Google Scholar]
- Korpelainen R, Keinanen‐Kiukaanniemi S, Heikkinen J, Vaananen K, Korpelainen J. Effect of impact exercise on bone mineral density in elderly women with low BMD: a population‐based randomized controlled 30‐month intervention. Osteoporosis International 2006;17(1):109‐18. [DOI] [PubMed] [Google Scholar]
- Korpelainen R, Keinanen‐Kiukaanniemi S, Nieminen P, Heikkinen J, Vaananen K, Korpelainen J. Long‐term outcomes of exercise: follow‐up of a randomized trial in older women with osteopenia. Archives of Internal Medicine 2010;170(17):1548‐56. [DOI] [PubMed] [Google Scholar]
- NCT00655577. Exercise and prevention of hip fractures. clinicaltrials.gov/show/NCT00655577 (first received 10 April 2008).
Kovacs 2013 {published and unpublished data}
- Kovacs E. Fall outcome data [personal communication]. Email to: C West 18 June 2018.
- Kovacs E, Prokai L, Meszaros L, Gondos T. Adapted physical activity is beneficial on balance, functional mobility, quality of life and fall risk in community‐dwelling older women: a randomized single‐blinded controlled trial. European Journal of Physical and Rehabilitation Medicine 2013;49(3):301‐10. [PubMed] [Google Scholar]
Kwok 2016 {published data only}
- Kwok BC, Pua YH. Effects of WiiActive exercises on fear of falling and functional outcomes in community‐dwelling older adults: a randomised control trial. Age and Ageing 2016;45(5):621‐7. [DOI] [PubMed] [Google Scholar]
Kyrdalen 2014 {published data only (unpublished sought but not used)}
- Kyrdalen IL, Moen K, Rõysland AS, Helbostad JL. The Otago Exercise Program performed as group training versus home training in fall‐prone older people: a randomized controlled trial. Physiotherapy Research International 2014;19(2):108‐16. [DOI] [PubMed] [Google Scholar]
LaStayo 2017 {published data only (unpublished sought but not used)}
- LaStayo P, Marcus R, Dibble L, Wong B, Pepper G. Eccentric versus traditional resistance exercise for older adult fallers in the community: a randomized trial within a multi‐component fall reduction program. BMC Geriatrics 2017;17:149. [DOI: 10.1186/s12877-017-0539-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Latham 2003 {published data only}
- Latham NK, Anderson CS, Lee A, Bennett DA, Moseley A, Cameron ID. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: The Frailty Interventions Trial in Elderly Subjects (FITNESS). Journal of the American Geriatrics Society 2003;51(3):291‐9. [DOI] [PubMed] [Google Scholar]
Lehtola 2000 {published data only (unpublished sought but not used)}
- Lehtola S, Hanninen L, Paatalo M. The incidence of falls during a six‐month exercise trial and four‐month followup among home dwelling persons aged 70‐75 years [Kaatumistapaturmien ilmaantuvuus 70‐75‐vuotiailla oululaisilla liikuntaintervention ja sen jälkeisen seurannan aikana]. Liikuntatiede 2000;6:41‐6. [Google Scholar]
Li 2005 {published data only}
- Li F, Harmer P, Fisher K J, McAuley E, Chaumeton N, Eckstrom E, et al. Tai Chi and fall reductions in older adults: a randomized controlled trial. Journals of Gerontology, Series A: Biological Sciences and Medical Sciences 2005;60(2):187‐94. [DOI] [PubMed] [Google Scholar]
- Li F, Harmer P, Fisher KJ, McAuley E. Tai Chi: improving functional balance and predicting subsequent falls in older persons. Medicine and Science in Sports and Exercise 2004;36(12):2046‐52. [DOI] [PubMed] [Google Scholar]
Lin 2007 {published data only (unpublished sought but not used)}
- Lin MR, Wolf SL, Hwang HF, Gong SY, Chen CY. A randomized, controlled trial of fall prevention programs and quality of life in older fallers. Journal of the American Geriatrics Society 2007;55(4):499‐506. [DOI] [PubMed] [Google Scholar]
Liston 2014 {published data only}
- Liston MB, Alushi L, Bamiou DE, Martin FC, Hopper A, Pavlou M. Feasibility and effect of supplementing a modified OTAGO intervention with multisensory balance exercises in older people who fall: a pilot randomized controlled trial. Clinical Rehabilitation 2014;28(8):784‐93. [DOI] [PubMed] [Google Scholar]
Liu‐Ambrose 2004 {published data only}
- Liu‐Ambrose T, Khan KM, Eng JJ, Janssen PA, Lord SR, McKay HA. Resistance and agility training reduce fall risk in women aged 75 to 85 with low bone mass: a 6‐month randomized, controlled trial. Journal of the American Geriatrics Society 2004;52(5):657‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu‐Ambrose T, Khan KM, Eng JJ, Lord SR, McKay HA. Balance confidence improves with resistance or agility training. Increase is not correlated with objective changes in fall risk and physical abilities. Gerontology 2004;50(6):373‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu‐Ambrose T, Khan KM, Eng JJ, Lord SR, McKay HA. Strength or agility training significantly reduces fall risk compared to posture training in 75 to 85 year old women with low bone density: a six month RCT. XVIth conference of the International Society for Postural and Gait Research; 2003 March 23‐27;Sydney (Australia). 2004.
- Liu‐Ambrose TY, Khan KM, Eng JJ, Gillies GL, Lord SR, McKay HA. The beneficial effects of group‐based exercises on fall risk profile and physical activity persist 1 year postintervention in older women with low bone mass: follow‐up after withdrawal of exercise. Journal of the American Geriatrics Society 2005;53(10):1767‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu‐Ambrose 2008 {published data only}
- Davis J, Guy P, Liu‐Ambrose T, Donaldson M, Robertson M, Khan K, et al. Cost‐effectiveness analysis of the Otago home‐based strength and balance retraining in senior fallers. Journal of Nutrition, Health and Aging 2009;13(Suppl 1):S436. [DOI: 10.1007/s12603-009-0095-9] [DOI] [Google Scholar]
- Donaldson MG. Falls risk in frail seniors: clinical and methodological studies [thesis]. Vancouver (CA): University of British Columbia, 2007. [Google Scholar]
- Liu‐Ambrose T, Donaldson MG, Ahamed Y, Graf P, Cook WL, Close J, et al. Otago home‐based strength and balance retraining improves executive functioning in older fallers: a randomized controlled trial. Journal of the American Geriatrics Society 2008;56(10):1821‐30. [DOI] [PubMed] [Google Scholar]
- NCT00323596. Trial of a home based strength and balance retraining program in reducing falls risk factors. clinicaltrials.gov/show/NCT00323596 (first received 9 May 2006).
Logghe 2009 {published data only}
- Logghe IH, Verhagen AP, Rademaker AC, Zeeuwe PE, Bierma‐Zeinstra SM, Rossum E, et al. Explaining the ineffectiveness of a Tai Chi fall prevention training for community‐living older people: A process evaluation alongside a randomized clinical trial (RCT). Archives of Gerontology and Geriatrics 2011;52(3):357‐62. [DOI] [PubMed] [Google Scholar]
- Logghe IH, Zeeuwe PE, Verhagen AP, Wijnen‐Sponselee RM, Willemsen SP, Bierma‐Zeinstra SM, et al. Lack of effect of Tai Chi Chuan in preventing falls in elderly people living at home: a randomized clinical trial. Journal of the American Geriatrics Society 2009;57(1):70‐5. [DOI] [PubMed] [Google Scholar]
- Zeeuwe PE, Verhagen AP, Bierma‐Zeinstra SM, Rossum E, Faber MJ, Koes BW. The effect of Tai Chi Chuan in reducing falls among elderly people: design of a randomized clinical trial in the Netherlands. BMC Geriatrics 2006;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lord 1995 {published data only}
- Lord SR, Ward JA, Williams P, Strudwick M. The effect of a 12‐month exercise trial on balance, strength, and falls in older women: a randomized controlled trial. Journal of the American Geriatrics Society 1995;43(11):1198‐206. [DOI] [PubMed] [Google Scholar]
- Lord SR, Ward JA, Williams P, Zivanovic E. The effects of a community exercise program on fracture risk factors in older women. Osteoporosis International 1996;6(5):361‐7. [DOI] [PubMed] [Google Scholar]
Lord 2003 {published and unpublished data}
- Lord S. Number of fallers outcome data [personal communication]. Email to: C West Vol. 18 June 2018.
- Lord SR, Castell S, Corcoran J, Dayhew J, Matters B, Shan A, et al. The effect of group exercise on physical functioning and falls in frail older people living in retirement villages: a randomized, controlled trial. Journal of the American Geriatrics Society 2003;51(12):1685‐92. [DOI] [PubMed] [Google Scholar]
Lurie 2013 {published data only (unpublished sought but not used)}
- Lurie JD, Zagaria AB, Pidgeon DM, Forman JL, Spratt KF. Pilot comparative effectiveness study of surface perturbation treadmill training to prevent falls in older adults. BMC Geriatrics 2013;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luukinen 2007 {published data only}
- Luukinen H, Lehtola S, Jokelainen J, Vaananen‐Sainio R, Lotvonen S, Koistinen P. Pragmatic exercise‐oriented prevention of falls among the elderly: a population‐based, randomized, controlled trial. Preventive Medicine 2007;44(3):265‐71. [DOI] [PubMed] [Google Scholar]
- Luukinen H, Lehtola S, Jokelainen J, Vaananen‐Sainio R, Lotvonen S, Koistinen P. Prevention of disability by exercise among the elderly: a population‐based, randomized, controlled trial. Scandanavian Journal of Primary Health Care 2006;24(4):199‐205. [DOI] [PubMed] [Google Scholar]
Madureira 2007 {published data only (unpublished sought but not used)}
- Madureira MM, Bonfá E, Takayama L, Pereira RM. A 12‐month randomized controlled trial of balance training in elderly women with osteoporosis: improvement of quality of life. Maturitas 2010;66(2):206‐11. [DOI] [PubMed] [Google Scholar]
- Madureira MM, Pereira RM. A 12‐month randomized controlled trial of balance training in elderly women with osteoporosis: Improvement of quality of life and reduction of falls. Arthritis and Rheumatism 2009;10:1496. [DOI] [PubMed] [Google Scholar]
- Madureira MM, Takayama L, Gallinaro AL, Caparbo VF, Costa RA, Pereira RM. Balance training program is highly effective in improving functional status and reducing the risk of falls in elderly women with osteoporosis: a randomized controlled trial. Osteoporosis International 2007;18(4):419‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
McMurdo 1997 {published data only}
- McMurdo ME, Mole PA, Paterson CR. Controlled trial of weight bearing exercise in older women in relation to bone density and falls. BMJ 1997;314(7080):569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Means 2005 {published data only}
- Means KM, Rodell DE, O'Sullivan PS. Balance, mobility, and falls among community‐dwelling elderly persons: effects of a rehabilitation exercise program. American Journal of Physical Medicine and Rehabilitation 2005;84(4):238‐50. [DOI] [PubMed] [Google Scholar]
Merom 2016 {published data only}
- Merom D, Mathieu E, Cerin E, Morton RL, Simpson JM, Rissel C, et al. Social dancing and incidence of falls in older adults: a cluster randomised controlled trial. PLoS Medicine 2016;13(8):e1002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miko 2017 {published data only}
- Miko I, Szerb I, Szerb A, Poor G. Effectiveness of balance training programme in reducing the frequency of falling in established osteoporotic women: a randomized controlled trial. Clinical Rehabilitation 2017;31(2):217‐24. [DOI] [PubMed] [Google Scholar]
Mirelman 2016 {published data only (unpublished sought but not used)}
- Mirelman A, Rochester L, Maidan I, Del D, Alcock L, Nieuwhof F, et al. Addition of a non‐immersive virtual reality component to treadmill training to reduce fall risk in older adults (V‐TIME): a randomised controlled trial. Lancet 2016;388(10050):1170‐82. [DOI] [PubMed] [Google Scholar]
Morgan 2004 {published data only (unpublished sought but not used)}
- DeVito CA, Morgan RO, Duque M, Abdel‐Moty E, Virnig BA. Physical performance effects of low‐intensity exercise among clinically defined high‐risk elders. Gerontology 2003;49(3):146‐54. [DOI] [PubMed] [Google Scholar]
- Morgan RO, Virnig BA, Duque M, Abdel‐Moty E, Devito CA. Low‐intensity exercise and reduction of the risk for falls among at‐risk elders. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2004;59(10):1062‐7. [DOI] [PubMed] [Google Scholar]
- NCT00013078. Safe‐Grip fall/injuries intervention: a randomized controlled trial. clinicaltrials.gov/show/NCT00013078 (first received 16 March 2001).
Morone 2016 {published data only (unpublished sought but not used)}
- Morone G, Paolucci T, Luziatelli S, Iosa M, Piermattei C, Zangrando F, et al. Wii Fit is effective in women with bone loss condition associated with balance disorders: a randomized controlled trial. Aging Clinical and Experimental Research 2016;28(6):1187‐93. [DOI] [PubMed] [Google Scholar]
Morrison 2018 {published and unpublished data}
- Morrison S. Fall outcome data, randomisation and intervention details [personal communication]. Email to: N Fairhall. 15 June 2018.
- Morrison S, Simmons R, Colberg SR, Parson HK, Vinik I. Supervised balance training and Wii Fit‐based exercises lower falls risk in older adults with type 2 diabetes. Journal of the American Medical Directors Association 2018;19(2):185.e7‐185.e13. [DOI] [PubMed] [Google Scholar]
Ng 2015 {published data only (unpublished sought but not used)}
- Ng TP, Feng L, Nyunt MS, Feng L, Niti M, Tan BY, et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: a randomized controlled trial. American Journal of Medicine 2015;128(11):1225‐36 e1. [DOI] [PubMed] [Google Scholar]
Nitz 2004 {published and unpublished data}
- Nitz JC. [Personal communication]. Personal communication reported by Gillespie 2012.
- Nitz JC, Choy NL. The efficacy of a specific balance‐strategy training programme for preventing falls among older people: A pilot randomised controlled trial. Age and Aging 2004;33(1):52‐8. [DOI] [PubMed] [Google Scholar]
Okubo 2016 {published data only (unpublished sought but not used)}
- Okubo Y, Osuka Y, Jung S, Rafael F, Tsujimoto T, Aiba T, et al. Walking can be more effective than balance training in fall prevention among community‐dwelling older adults. Geriatrics and Gerontology International 2016;16(1):118‐25. [DOI] [PubMed] [Google Scholar]
Park 2008 {published data only (unpublished sought but not used)}
Reinsch 1992 {published data only (unpublished sought but not used)}
- El‐Faizy M, Reinsch S. Home safety intervention for the prevention of falls. Physical and Occupational Therapy in Geriatrics 1994;12(3):33‐49. [Google Scholar]
- MacRae PG, Feltner ME, Reinsch S. A 1‐year exercise program for older women: effects on falls, injuries, and physical performance. Journal of Aging and Physical Activity 1994;2:127‐42. [Google Scholar]
- Reinsch S, MacRae P, Lachenbruch PA, Tobis JS. Attempts to prevent falls and injury: a prospective community study. Gerontologist 1992;32(4):450‐6. [DOI] [PubMed] [Google Scholar]
- Tobis J, Reinsch S, McRae P, Lachenbruch T. Experimental intervention at senior centres for the prevention of falls. Journal of the American Geriatrics Society 1990;38(8):A28. [Google Scholar]
Resnick 2002 {published data only (unpublished sought but not used)}
- Resnick B. Testing the effect of the WALC intervention on exercise adherence in older adults. Journal of Gerontological Nursing 2002;28(6):40‐9. [DOI] [PubMed] [Google Scholar]
Robertson 2001a {published data only}
- Gardner MM, Buchner DM, Robertson MC, Campbell AJ. Practical implementation of an exercise‐based falls prevention programme. Age and Ageing 2001;30(1):77‐83. [DOI] [PubMed] [Google Scholar]
- Robertson MC. Development of a falls prevention programme for elderly people: evaluation of efficacy, effectiveness, and efficiency [thesis]. Dunedin (NZ): University of Otago, 2001. [Google Scholar]
- Robertson MC, Campbell AJ, Gardner MM, Devlin N. Preventing injuries in older people by preventing falls: a meta‐analysis of individual‐level data. Journal of the American Geriatrics Society 2002;50(5):905‐11. [DOI] [PubMed] [Google Scholar]
- Robertson MC, Devlin N, Gardner MM, Campbell AJ. Effectiveness and economic evaluation of a nurse delivered home exercise programme to prevent falls. 1: Randomised controlled trial. BMJ 2001;322(7288):697‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubenstein 2000 {published data only}
- Rubenstein LZ, Josephson KR, Trueblood PR, Loy S, Harker JO, Pietruszka FM, et al. Effects of a group exercise program on strength, mobility, and falls among fall‐prone elderly men. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2000;55(6):M317‐21. [DOI] [PubMed] [Google Scholar]
Sakamoto 2013 {published data only}
- Sakamoto K, Endo N, Harada A, Sakada T, Tsushita K, Kita K, et al. A randomized, controlled trial of balance therapy to prevent falls and fractures for elderly people who can stand on one leg for ≤15 s. Journal of Orthopaedic Science 2013;18(1):110‐20. [DOI] [PubMed] [Google Scholar]
Sales 2017 {published and unpublished data}
- Sales M. Fall outcome data [personal communication]. Email to: N Fairhall. 23 May 2018.
- Sales M, Polman R, Hill KD, Levinger P. A novel exercise initiative for seniors to improve balance and physical function. Journal of Aging and Health 2017;29(8):1424–43. [DOI] [PubMed] [Google Scholar]
Sherrington 2014 {published data only}
- Sherrington C, Lord SR, Vogler CM, Close JC, Howard K, Dean CM, et al. A post‐hospital home exercise program improved mobility but increased falls in older people: a randomised controlled trial. PLoS One 2014;9(9):e104412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington C, Lord SR, Vogler CM, Close JC, Howard K, Dean CM, et al. Minimising disability and falls in older people through a post‐hospital exercise program: a protocol for a randomised controlled trial and economic evaluation. BMC Geriatrics 2009;26:8. [DOI: 10.1186/1471-2318-9-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shigematsu 2008 {published data only}
- Shigematsu R, Okura T, Nakagaichi M, Tanaka K, Sakai T, Kitazumi S, et al. Square‐stepping exercise and fall risk factors in older adults: a single‐blind, randomized controlled trial. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2008;63(1):76‐82. [DOI] [PubMed] [Google Scholar]
- Shigematsu R, Okura T, Sakai T, Rantanen T. Square‐stepping exercise versus strength and balance training for fall risk factors. Aging Clinical and Experimental Research 2008;20(1):19‐24. [DOI] [PubMed] [Google Scholar]
Siegrist 2016 {published and unpublished data}
- Siegrist M. Fall and injury data [personal communication]. Email to: N Fairhall. 2 February 2018.
- Siegrist M, Freiberger E, Geilhof B, Salb J, Hentschke C, Landendoerfer P, et al. Fall prevention in a primary care setting. Deutsches Ärzteblatt International 2016;113(21):365‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skelton 2005 {published and unpublished data}
- Skelton D. [Personal communication]. Personal communication reported by Gillespie 2012 February 1 2005.
- Skelton D, Dinan S, Campbell M, Rutherford O. Tailored group exercise (Falls Management Exercise ‐‐ FaME) reduces falls in community‐dwelling older frequent fallers (an RCT). Age and Ageing 2005;34(6):636‐9. [DOI] [PubMed] [Google Scholar]
- Skelton DA, Dinan SM. Exercise for falls management: Rationale for an exercise programme aimed at reducing postural instability. Physiotherapy Theory and Practice 1999;15(2):105‐20. [Google Scholar]
- Skelton DA, Dinan SM, Campbell M, Rutherford OM. FaME (Falls Management Exercise): An RCT on the effects of a 9‐month group exercise programme in frequently falling community dwelling women aged 65 and over. Journal of Aging and Physical Activity 2004;12(3):457‐8. [Google Scholar]
- Skelton DA, Stranzinger K, Dinan S, Rutherford OM. BMD improvements following FaME (Falls Management Exercise) in frequently falling women age 65 and over: an RCT. Journal of Aging and Physical Activity 2008;16 Suppl:S89‐90. [Google Scholar]
Smulders 2010 {published data only}
- NCT00432692. Falls prevention in osteoporosis. clinicaltrials.gov/show/NCT00432692 (first received 8 February 2007).
- Smulders E, Weerdesteyn V, Duysens J, Laan R, Lankveld W. Falls prevention in persons with osteoporosis: A randomized clinical trial. Arthritis and Rheumatism 2009;10:1883. [Google Scholar]
- Smulders E, Weerdesteyn V, Groen BE, Duysens J, Eijsbouts A, Laan R, et al. Efficacy of a short multidisciplinary falls prevention program for elderly persons with osteoporosis and a fall history: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2010;91(11):1705‐11. [DOI] [PubMed] [Google Scholar]
Steadman 2003 {published and unpublished data}
- Kalra L. [Personal communication]. Personal communication reported by Gillespie 2012 27 March 2006.
- Steadman J, Donaldson N, Kalra L. A randomized controlled trial of an enhanced balance training program to improve mobility and reduce falls in elderly patients. Journal of the American Geriatrics Society 2003;51(6):847‐52. [DOI] [PubMed] [Google Scholar]
Suzuki 2004 {published data only}
- Suzuki T, Kim H, Yoshida H, Ishizaki T. Randomized controlled trial of exercise intervention for the prevention of falls in community‐dwelling elderly Japanese women. Journal of Bone and Mineral Metabolism 2004;22(6):602‐11. [DOI] [PubMed] [Google Scholar]
Taylor 2012 {published data only}
- Taylor D, Hale L, Schluter P, Waters DL, Binns EE, McCracken H, et al. Effectiveness of tai chi as a community‐based falls prevention intervention: a randomized controlled trial. Journal of the American Geriatrics Society 2012;60(5):841‐8. [DOI] [PubMed] [Google Scholar]
Trombetti 2011 {published data only}
- NCT01107288. Effects of a music‐based multitask exercises program on gait, balance and fall risk in the elderly. clinicaltrials.gov/show/NCT01107288 (first received 20 April 2010).
- Trombetti A, Hars M, Herrmann F, Kressig R, Ferrari S, Rizzoli R. "Jaques‐Dalcroze eurhythmics" improves gait and prevents falls in the elderly [Prevention des chutes par une methode d'exercice en musique (rythmique Jaques‐Dalcroze)]. Revue Medicale Suisse 2011;7(299):1305‐8. [PubMed] [Google Scholar]
- Trombetti A, Hars M, Herrmann F, Kressig R, Ferrari S, Rizzoli R. A randomized controlled trial of music‐based multitask training on gait, balance and fall risk. Osteoporosis International 2011;22(Suppl 1):S119. [DOI] [PubMed] [Google Scholar]
- Trombetti A, Hars M, Herrmann FR, Kressig RW, Ferrari S, Rizzoli R. Effect of music‐based multitask training on gait, balance, and fall risk in elderly people: a randomized controlled trial. Archives of Internal Medicine 2011;171(6):525‐33. [DOI] [PubMed] [Google Scholar]
Uusi‐Rasi 2015 {published data only}
- Patil R, Kolu P, Raitanen J, Valvanne J, Kannus P, Karinkanta S, et al. Cost‐effectiveness of vitamin D supplementation and exercise in preventing injurious falls among older home‐dwelling women: findings from an RCT. Osteoporosis International 2016;27(1):193‐201. [DOI] [PubMed] [Google Scholar]
- Uusi‐Rasi K, Patil R, Karinkanta S, Kannus P, Tokola K, Lamberg‐Allardt C, et al. A 2‐year follow‐up after a 2‐year RCT with vitamin D and exercise: effects on falls, injurious falls and physical functioning among older women. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2017;72(9):1239‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusi‐Rasi K, Patil R, Karinkanta S, Kannus P, Tokola K, Lamberg‐Allardt C, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Internal Medicine 2015;175(5):703‐11. [DOI] [PubMed] [Google Scholar]
Verrusio 2017 {published data only}
- Verrusio W, Gianturco V, Cacciafesta M, Marigliano V, Troisi G, Ripani M. Fall prevention in the young old using an exoskeleton human body posturizer: a randomized controlled trial. Aging Clinical and Experimental Research 2017;29(2):207‐14. [DOI] [PubMed] [Google Scholar]
Vogler 2009 {published data only}
- Vogler CM, Sherrington C, Ogle SJ, Lord SR. Reducing risk of falling in older people discharged from hospital: a randomized controlled trial comparing seated exercises, weight‐bearing exercises, and social visits. Archives of Physical Medicine and Rehabilitation 2009;90(8):1317‐24. [DOI] [PubMed] [Google Scholar]
Voukelatos 2007 {published and unpublished data}
- Haas M. Economic analysis of tai chi as a means of preventing falls and related injuries among older adults. CHERE working paper 2006/4. Sydney, Australia: Centre for Health Economics Research and Evaluation, University of Technology. https://www.uts.edu.au/sites/default/files/wp2006_3.pdf (accessed 24 January 2019).
- Rissel C, Voukelatos A, Cumming B, Lord S. Central Sydney Tai Chi Trial. Canberra: Australian Resource Centre for Health Care Innovations, 2005. [Google Scholar]
- Voukelatos A. [Personal communication]. Personal communication reported by Gillespie 2012 25 July 2003.
- Voukelatos A, Cumming RG, Lord SR, Rissel C. A randomized, controlled trial of tai chi for the prevention of falls: the Central Sydney tai chi trial. Journal of the American Geriatrics Society 2007;55(8):1185‐91. [DOI] [PubMed] [Google Scholar]
- Voukelatos A, Metcalfe A. Central Sydney Tai Chi Trial: methodology. New South Wales Public Health Bulletin 2002;13(1‐2):19. [DOI] [PubMed] [Google Scholar]
- Voukelatos A, Rissel C, Cumming R, Lord S. The Central Sydney Tai Chi Trial: a randomised controlled trial of the effectiveness of tai chi in reducing risk of falls in older people. Sydney: NSW Department of Health, 2006 https://www.health.nsw.gov.au/research/Publications/2000‐tai‐chi.pdf (accessed 24 January 2019). [Google Scholar]
Voukelatos 2015 {published data only}
- Voukelatos A, Merom D, Sherrington C, Rissel C, Cumming RG, Lord SR. The impact of a home‐based walking programme on falls in older people: the Easy Steps randomised controlled trial. Age and Ageing 2015;44(3):377‐83. [DOI] [PubMed] [Google Scholar]
Weerdesteyn 2006 {published and unpublished data}
- Weerdesteyn V. [Personal communication]. Personal communication reported by Gillespie 2012 06 September 2006.
- Weerdesteyn V, Rijken H, Geurts AC, Smits‐Engelsman BC, Mulder T, Duysens J. A five‐week exercise program can reduce falls and improve obstacle avoidance in the elderly. Gerontology 2006;52(3):131‐41. [DOI] [PubMed] [Google Scholar]
Wolf 1996 {published data only (unpublished sought but not used)}
- Kutner NG, Barnhart H, Wolf SL, McNeely E, Xu T. Self‐report benefits of Tai Chi practice by older adults. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 1997;52(5):P242‐6. [DOI] [PubMed] [Google Scholar]
- McNeely E, Clements SD, Wolf SL. A program to reduce frailty in the elderly. In: Funk SG, Tornquist EM, Champagne MT, Weise RA editor(s). Key Aspects of Elder Care: Managing Falls, Incontinence, and Cognitive Impairment. New York: Springer, 1992:89‐96. [Google Scholar]
- O'Grady M, Wolf SL, Barnhart HX, Kutner N, McNeely E. Tai Chi effect on falls in frail older adults. Archives of Physical Medicine and Rehabilitation 1997; Vol. 78:1028. [CN‐00716674]
- Wolf SL, Barnhart HX, Ellison GL, Coogler CE. The effect of Tai Chi Quan and computerized balance training on postural stability in older subjects. Atlanta FICSIT Group. Frailty and Injuries: Cooperative Studies on Intervention Techniques. Physical Therapy 1997;77(4):371‐81; discussion 382‐4. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Barnhart HX, Kutner NG, McNeely E, Coogler C, Xu T. Reducing frailty and falls in older persons: an investigation of Tai Chi and computerized balance training. Atlanta FICSIT Group. Frailty and Injuries: Cooperative Studies of Intervention Techniques. Journal of the American Geriatrics Society 1996;44(5):489‐97. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Kutner NG, Green RC, McNeely E. The Atlanta FICSIT study: two exercise interventions to reduce frailty in elders. Journal of the American Geriatrics Society 1993;41(3):329‐32. [DOI] [PubMed] [Google Scholar]
Wolf 2003 {published data only}
- Greenspan AI, Wolf SL, Kelley ME, O'Grady M. Tai chi and perceived health status in older adults who are transitionally frail: a randomized controlled trial. Physical Therapy 2007;87(5):525‐35. [DOI] [PubMed] [Google Scholar]
- Sattin RW, Easley KA, Wolf SL, Chen Y, Kutner MH. Reduction in fear of falling through intense tai chi exercise training in older, transitionally frail adults. Journal of the American Geriatrics Society 2005;53(7):1168‐78. [DOI] [PubMed] [Google Scholar]
- Wolf SL, O'Grady M, Easley KA, Guo Y, Kressig RW, Kutner M. The influence of intense Tai Chi training on physical performance and hemodynamic outcomes in transitionally frail, older adults. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2006;61(2):184‐9. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Sattin RW, Kutner M, O'Grady M, Greenspan AI, Gregor RJ. Intense tai chi exercise training and fall occurrences in older, transitionally frail adults: a randomized, controlled trial. Journal of the American Geriatrics Society 2003;51(12):1693‐701. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Sattin RW, O'Grady M, Freret N, Ricci L, Greenspan A I, et al. A study design to investigate the effect of intense Tai Chi in reducing falls among older adults transitioning to frailty. Controlled Clinical Trials 2001;22(6):689‐704. [DOI] [PubMed] [Google Scholar]
Woo 2007 {published and unpublished data}
- Woo J. [Personal communication]. Personal communication reported by Gillespie 2012 May 6 2008.
- Woo J, Hong A, Lau E, Lynn H. A randomised controlled trial of Tai Chi and resistance exercise on bone health, muscle strength and balance in community‐living elderly people. Age and Ageing 2007;36(3):262‐8. [DOI] [PubMed] [Google Scholar]
Wu 2010 {published data only}
- Wu G, Keyes L, Callas P, Ren X, Bookchin B. Comparison of telecommunication, community, and home‐based Tai Chi exercise programs on compliance and effectiveness in elders at risk for falls. Archives of Physical Medicine and Rehabilitation 2010;91(6):849‐56. [DOI] [PubMed] [Google Scholar]
Yamada 2010 {published data only}
- Yamada M, Tanaka B, Nagai K, Aoyama T, Ichihashi N. Trail‐walking exercise and fall risk factors in community‐dwelling older adults: preliminary results of a randomized controlled trial. Journal of the American Geriatrics Society 2010;58(10):1946‐51. [DOI] [PubMed] [Google Scholar]
Yamada 2012 {published data only}
- Yamada M, Aoyama T, Arai H, Nagai K, Tanaka B, Uemura K, et al. Complex obstacle negotiation exercise can prevent falls in community‐dwelling elderly Japanese aged 75 years and older. Geriatrics and Gerontology International 2012;12(3):461‐7. [DOI] [PubMed] [Google Scholar]
Yamada 2013 {published data only}
- Yamada M, Higuchi T, Nishiguchi S, Yoshimura K, Kajiwara Y, Aoyama T. Multitarget stepping program in combination with a standardized multicomponent exercise program can prevent falls in community‐dwelling older adults: a randomized, controlled trial. Journal of the American Geriatrics Society 2013;61(10):1669‐75. [DOI] [PubMed] [Google Scholar]
Yang 2012 {published data only (unpublished sought but not used)}
- Yang XJ, Hill K, Moore K, Williams S, Dowson L, Borschmann K, et al. Effectiveness of a targeted exercise intervention in reversing older people's mild balance dysfunction: a randomized controlled trial. Physical Therapy 2012;92(1):24‐37. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alkan 2011 {published data only}
- Alkan H, Topuz O, Yildiz, Alkan S, Sarsan A, Ardıç F. Efficacy of home based exercise program and postural biofeedback therapy in reducing risk of falling among osteoporotic women over 65 years of age. Turkish Journal of Geriatrics 2011;14(1):26‐34. [Google Scholar]
Beling 2009 {published data only}
- Beling J, Roller M. Multifactorial intervention with balance training as a core component among fall‐prone older adults. Journal of Geriatric Physical Therapy 2009;32(3):125‐33. [DOI] [PubMed] [Google Scholar]
Clemson 2004b {published data only}
- Clemson L, Cumming RG, Kendig H, Swann M, Heard R, Taylor K. The effectiveness of a community‐based program for reducing the incidence of falls in the elderly: a randomized trial. Journal of the American Geriatrics Society 2004;52(9):1487‐94. [DOI] [PubMed] [Google Scholar]
DeSure 2013 {published data only}
- DeSure AR, Peterson K, Gianan FV, Pang L. An exercise program to prevent falls in institutionalized elderly with cognitive deficits: a crossover pilot study. Hawaii Journal of Medicine and Public Health 2013;72(11):391‐5. [PMC free article] [PubMed] [Google Scholar]
Fahlström 2017 {published data only}
Gianoudis 2014 {published data only}
- Gianoudis J, Bailey CA, Ebeling PR, Nowson CA, Sanders KM, Hill K, et al. Effects of a targeted multimodal exercise program incorporating high‐speed power training on falls and fracture risk factors in older adults: a community‐based randomized controlled trial. Journal of Bone and Mineral Research 2014;29(1):182‐91. [DOI] [PubMed] [Google Scholar]
Hinrichs 2016 {published and unpublished data}
- Hinrichs T. Outcome data [personal communication]. Email to: N Fairhall. 29 August 2017.
- Hinrichs T, Bücker B, Klaaßen‐Mielke R, Brach M, Wilm S, Platen P, et al. Home‐based exercise supported by general practitioner practices: Ineffective in a sample of chronically Ill, mobility‐limited older adults (the HOMEfit randomized controlled trial). Journal of the American Geriatrics Society 2016;64(11):2270‐9. [DOI] [PubMed] [Google Scholar]
Hsu 2017 {published data only}
- Hsu CL, Best JR, Wang S, Voss MW, Hsiung RG, Munkacsy M, et al. The impact of aerobic exercise on fronto‐parietal network connectivity and its relation to mobility: An exploratory analysis of a 6‐month randomized controlled trial. Frontiers in Human Neuroscience 2017;11:344. [DOI: 10.3389/fnhum.2017.00344] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iwamoto 2012 {published data only}
- Iwamoto J, Sato Y, Takeda T, Matsumoto H. Whole body vibration exercise improves body balance and walking velocity in postmenopausal osteoporotic women treated with alendronate: Galileo and Alendronate Intervention Trail (GAIT). Journal of Musculoskeletal & Neuronal Interactions 2012;12(3):136‐43. [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee HC, Chang KC, Tsauo JY, Hung JW, Huang YC, Lin SI. Effects of a multifactorial fall prevention program on fall incidence and physical function in community‐dwelling older adults with risk of falls. Archives of Physical Medicine and Rehabilitation 2013;94(4):606‐15. [DOI] [PubMed] [Google Scholar]
Leung 2014 {published data only}
- Leung KS, Li CY, Tse YK, Choy TK, Leung PC, Hung VW, et al. Effects of 18‐month low‐magnitude high‐frequency vibration on fall rate and fracture risks in 710 community elderly ‐ A cluster‐randomized controlled trial. Osteoporosis International 2014;25(6):1785‐95. [DOI] [PubMed] [Google Scholar]
Li 2018a {published data only}
- Li Z, Wang XX, Liang YY, Chen SY, Sheng J, Ma SJ. Effects of the visual‐feedback‐based force platform training with functional electric stimulation on the balance and prevention of falls in older adults: A randomized controlled trial. PeerJ 2018;6:e4244. [DOI: 10.7717/peerj.4244] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 2008 {published data only (unpublished sought but not used)}
- Morris D. An evaluation of yoga for the reduction of fall risk factors in older adults. purl.flvc.org/fsu/fd/FSU_migr_etd‐2257 (accessed 7 June 2018).
Ohtake 2013 {published data only}
- Ohtake M, Morkiagi Y, Suzuki I, Kanoya Y, Sato C. Effects of exercise on the prevention of conditions leading to the need for long‐term care. Aging Clinical and Experimental Research 2013;25(1):49–57. [DOI] [PubMed] [Google Scholar]
Olsen 2014 {published data only}
- Olsen CF, Bergland A. The effect of exercise and education on fear of falling in elderly women with osteoporosis and a history of vertebral fracture: results of a randomized controlled trial. Osteoporosis International 2014;25(8):2017‐25. [DOI] [PubMed] [Google Scholar]
Pai 2014 {published data only}
- Pai YC, Bhatt T, Yang F, Wang E. Perturbation training can reduce community‐dwelling older adults' annual fall risk: a randomized controlled trial. Journals of Gerontology Series A, Biological Sciences and Medical Sciences 2014;69(12):1586‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pereira 1998 {published data only}
- Kriska AM, Bayles C, Cauley JA, LaPorte RE, Sandler RB, Pambianco G. A randomized exercise trial in older women: increased activity over two years and the factors associated with compliance. Medicine and Science in Sports and Exercise 1986;18(5):557‐62. [PubMed] [Google Scholar]
- Pereira MA. Ten year follow‐up of a randomized exercise trial in post‐menopausal women [thesis]. Pittsburgh (USA): Univ. of Pittsburgh, 1996. [Proquest Digital Dissertations Publication Number AAT 97 16627] [Google Scholar]
- Pereira MA, Kriska AM, Day RD, Cauley JA, LaPorte RE, Kuller LH. A randomized walking trial in postmenopausal women: effects on physical activity and health 10 years later. Archives of Internal Medicine 1998;158(15):1695‐701. [DOI] [PubMed] [Google Scholar]
Rossi‐Izquierdo 2017 {published data only}
- Rossi‐Izquierdo M, Gayoso‐Diz P, Santos‐Pérez S, Del‐Río‐Valeiras M, Faraldo‐García A, Vaamonde‐Sanchez‐Andrade I, et al. Short‐term effectiveness of vestibular rehabilitation in elderly patients with postural instability: a randomized clinical trial. European Archives of Oto‐Rhino‐Laryngology 2017;274(6):2395‐403. [DOI] [PubMed] [Google Scholar]
Steinberg 2000 {published data only}
- Steinberg M, Cartwright C, Peel N, Williams G. A sustainable programme to prevent falls and near falls in community dwelling older people: results of a randomised trial. Journal of Epidemiology and Community Health 2000;54(3):227‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Swanenburg 2007 {published data only}
- Swanenburg J, Bruin ED, Stauffacher M, Mulder T, Uebelhart D. Effects of exercise and nutrition on postural balance and risk of falling in elderly people with decreased bone mineral density: randomized controlled trial pilot study. Clinical Rehabilitation 2007;21(6):523‐34. [DOI] [PubMed] [Google Scholar]
Ueda 2017 {published data only}
- Ueda T, Higuchi Y, Imaoka M, Todo E, Kitagawa T, Ando S. Tailored education program using home floor plans for falls prevention in discharged older patients: A pilot randomized controlled trial. Archives of Gerontology and Geriatrics 2017;71:9‐13. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Jagdhane 2016 {published data only}
- Jagdhane S, Kanekar N, Aruin AS. The effect of a four‐week balance training program on anticipatory postural adjustments in older adults: a pilot feasibility study. Current Aging Science 2016;9(4):295‐300. [DOI] [PubMed] [Google Scholar]
Li 2018b {published data only}
- Li F, Harmer P, Fitzgerald K, Eckstrom E, Akers L, Chu L, et al. Effectiveness of a therapeutic Tai Ji Quan intervention vs a multimodal exercise intervention to prevent falls among older adults at high risk of falling. JAMA Internal Medicine 2018; Vol. 178, issue 10:1301‐10. [DOI: 10.1001/jamaintemmed.2018.3915] [DOI] [PMC free article] [PubMed]
References to ongoing studies
ACTRN 12613001161718 {published data only}
- ACTRN12613001161718. Effects of dual‐task functional power training on falls in the elderly? An 18‐month community‐based randomised controlled trial. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364361 (first received 21 October 2013).
ACTRN 12615000138583 {published data only}
- ACTRN12615000138583. Standing Tall ‐ a home‐based exercise program using mobile technology for preventing falls in older people [Evaluating the effect of a home‐based exercise program delivered through mobile technology for preventing falls in older community‐dwelling people over 2 years, compared to a health promotion education ‘control’ program. The 'Standing Tall' randomized control trial]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367746 (first received 13 February 2015).
ACTRN 12615000865516 {published data only}
- ACTRN12615000865516. Balance Exercise and Strength Training (BEST) program for older people living at home [Lower limb home‐based exercise program compared with upper limb home‐based exercise program to prevent falls and upper limb dysfunction in older community‐dwelling people: a randomised controlled trial]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367886 (first received 19 August 2015).
CTRI/2018/01/011214 {published data only}
- CTRI/2018/01/011214. Role of physiotherapy in prevention of falls and fractures in elder population [Falls & fractures a physiotherapy approach to prediction & prevention in Healthcare]. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=22175 (first received 9 January 2018).
ISRCTN71002650 {published data only}
- ISRCTN71002650. Prevention of fall injury trial [Prevention of fall injury trial: a parallel group cluster randomised controlled trial and economic evaluation]. www.isrctn.com/ISRCTN71002650 (first received 13 April 2010).
NCT01029171 {published data only}
- NCT01029171. Action Seniors! Exercise to prevent falls [Action Seniors!: A 12 month randomized controlled trial of a home based strength and balance retraining program in reducing falls]. clinicaltrials.gov/ct2/show/NCT01029171 (first received 9 December 2009).
NCT02126488 {published data only}
- NCT02126488. Effect of adaptive training for balance recovery [Perturbation training for fall‐risk reduction among older adults]. clinicaltrials.gov/ct2/show/NCT02126488 (first received 30 April 2014).
NCT02287740 {published data only}
- NCT02287740. Prevention of falls among older adults in community settings [Comparative effectiveness and cost‐effectiveness of a fall prevention intervention: Tai Ji Quan: moving for better balance]. clinicaltrials.gov/ct2/show/NCT02287740 (first received 11 November 2014).
NCT02617303 {published data only}
- NCT02617303. Prevention of falls and its consequences in elderly people [Effectiveness of an intervention through physical exercise for the prevention of falls and its consequences in elderly people (75‐89 years) performed in primary care: study protocol for a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT02617303 (first received 30 November 2015).
NCT02926105 {published data only}
- NCT02926105. Comparison of home‐based exercise programmes for falls prevention and quality of life in older adults [Comparison of the effects of three home‐based exercise programmes regarding falls, quality of life and exercise‐adherence in older adults at risk of falling: protocol for a randomised controlled trial]. clinicaltrials.gov/ct2/show/NCT02926105 (first received 6 October 2016).
NCT03211429 {published data only}
- NCT03211429. Effectiveness of three interventions to reduce fear of falling and improve functionality in the elderly [Randomized clinical trial evaluating the effectiveness of three interventions programs to improve functionality and decrease fear of falling]. clinicaltrials.gov/ct2/show/NCT03211429 (first received 7 July 2017).
NCT03320668 {published data only}
- NCT03320668. Efficacy of the Otago Exercise Program delivered as group training versus individually tailored training [Efficacy of the Otago Exercise Program (OEP) delivered as group training versus individually tailored training in community‐dwelling adults between 65 and 80 years old]. clinicaltrials.gov/ct2/show/NCT03320668 (first received 25 October 2017).
NCT03404830 {published data only}
- NCT03404830. High intensity training to reduce the risk of falls in older people [Effects of a program of high intensity exercise by intervals on the risk of falls the physical condition and the state of health in people over 60 years]. clinicaltrials.gov/ct2/show/NCT03404830 (first received 19 January 2018).
NCT03417531 {published data only}
- NCT03417531. Sarcopenia prevention with a targeted exercise and protein supplementation program. clinicaltrials.gov/ct2/show/NCT03417531 (first received 31 January 2018).
NCT03455179 {published data only}
- NCT03455179. Effects of slow‐speed traditional resistance training, high‐speed resistance training and multicomponent training with variable resistances on molecular, body composition, neuromuscular, physicalfFunction and quality of life variables in older adults. clinicaltrials.gov/ct2/show/NCT03455179 (first received 6 March 2018).
NCT03462654 {published data only}
- NCT03462654. Comparison of a group‐delivered vs. individually delivered 'LiFE' Program LiFE‐is‐LiFE [Comparison of a group‐delivered and individually delivered lifestyle‐integrated functional exercise (LiFE) program in older persons]. clinicaltrials.gov/ct2/show/NCT03462654 (first received 12 March 2018).
Additional references
AIHW 2018
- Australian Institute of Health and Welfare: Pointer S. Trends in Hospitalised Injury due to Falls in Older People, 2002‐03 to 2014‐15. Injury and Statistics Series, no 111.. Canberra: AIWH, 2018. [Google Scholar]
Anonymous 1987
- Anonymous. The prevention of falls in later life. A report of the Kellogg International Work Group on the Prevention of Falls by the Elderly. Danish Medical Bulletin 1987;34 Suppl 4:1‐24. [PubMed] [Google Scholar]
Burns 2016
- Burns ER, Stevens JA, Lee R. The direct costs of fatal and non‐fatal falls among older adults ‐ United States. Journal of Safety Research 2016;58:99‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 1990
- Campbell AJ, Borrie MJ, Spears GF, Jackson SL, Brown JS, Fitzgerald JL. Circumstances and consequences of falls experienced by a community population 70 years and over during a prospective study. Age and Ageing 1990;19(2):136‐41. [DOI] [PubMed] [Google Scholar]
Campbell 1999
- Campbell AJ, Robertson MC, Gardner MM, Norton RN, Buchner DM. Falls prevention over 2 years: a randomized controlled trial in women 80 years and older. Age and Ageing 1999;28(6):513‐8. [DOI] [PubMed] [Google Scholar]
Canning 2015
- Canning CG, Allen NE, Bloem BR, Keus SH, Munneke M, Nieuwboer A, et al. Interventions for preventing falls in Parkinson's disease. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD011574] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carande‐Kulis 2015
- Carande‐Kulis V, Stevens JA, Florence CS, Beattie BL, Arias I. A cost‐benefit analysis of three older adult fall prevention interventions. Journal of Safety Research 2015;52:65‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpenter 2018
Caspersen 1985
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health‐related research. Public Health Reports 1985;100(2):126‐31. [PMC free article] [PubMed] [Google Scholar]
Clemson 2004a
- Clemson L, Cumming RG, Kendig H, Swann M, Heard R, Taylor K. The effectiveness of a community‐based program for reducing the incidence of falls in the elderly: a randomized trial. Journal of the American Geriatrics Society 2004;52(9):1487‐94. [DOI] [PubMed] [Google Scholar]
Cochrane Norway 2017
- Cochrane Norway. How to write a plain language summary of a Cochrane intervention review. www.cochrane.no/sites/cochrane.no/files/public/uploads/checklist_for_cochrane_pls_28th_feb_2017_0.pdf (accessed 15 November 2018).
Davis 2009
- Davis J, Guy P, Liu‐Ambrose T, Donaldson M, Robertson M, Khan K. Cost‐effectiveness analysis of the Otago home‐based strength and balance retraining in senior fallers. Journal of Nutrition, Health & Aging 2009;13(1):S436. [Google Scholar]
Davis 2010
- Davis JC, Robertson MC, Ashe MC, Liu‐Ambrose T, Khan KM, Marra CA. Does a home‐based strength and balance programme in people aged > or =80 years provide the best value for money to prevent falls? A systematic review of economic evaluations of falls prevention interventions. British Journal of Sports Medicine 2010;44(2):80‐9. [DOI] [PubMed] [Google Scholar]
Deandrea 2010
- Deandrea S, Lucenteforte E, Bravi F, Foschi R, La VC, Negri E. Risk factors for falls in community‐dwelling older people: a systematic review and meta‐analysis. Epidemiology 2010;21(5):658‐68. [DOI] [PubMed] [Google Scholar]
Farag 2015
- Farag I, Howard K, Ferreira ML, Sherrington C. Economic modelling of a public health programme for fall prevention. Age and Ageing 2015;44(3):409‐14. [DOI] [PubMed] [Google Scholar]
Farag 2015a
- Farag I, Howard K, Hayes A J, Ferreira M L, Lord S R, Close J T, et al. Cost‐effectiveness of a home‐exercise program among older people after hospitalization. Journal of the American Medical Directors Association 2015;16(6):490‐6. [DOI] [PubMed] [Google Scholar]
Fitzharris 2010
- Fitzharris MP, Day L, Lord SR, Gordon I, Fildes B. The Whitehorse NoFalls trial: effects on fall rates and injurious fall rates. Age and Ageing 2010;39(6):728‐33. [DOI] [PubMed] [Google Scholar]
Fried 2001
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2001;56(3):M146‐56. [DOI] [PubMed] [Google Scholar]
Gillespie 2012
- Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database of Systematic Reviews 2012, issue 9. [DOI: 10.1002/14651858.CD007146.pub3] [DOI] [PMC free article] [PubMed]
GRADEPro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Haas 2006
- Haas M. Economic analysis of tai chi as a means of preventing falls and falls related injuries among older adults. CHERE working paper 2006/4. Sydney, Australia: Centre for Health Economics Research and Evaluation, University of Technology. https://www.uts.edu.au/sites/default/files/wp2006_3.pdf. ., (accessed 24 January 2019).
Hannan 2010
- Hannan MT, Gagnon MM, Aneja J, Jones RN, Cupples LA, Lipsitz LA, et al. Optimizing the tracking of falls in studies of older participants: comparison of quarterly telephone recall with monthly falls calendars in the MOBILIZE Boston Study. American Journal of Epidemiology 2010;171(9):1031‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harper 2017
- Harper KJ, Barton AD, Arendts G, Edwards DG, Petta AC, Celenza A. Controlled clinical trial exploring the impact of a brief intervention for prevention of falls in an emergency department. Emergency Medicine Australasia 2017;29:524‐30. [DOI] [PubMed] [Google Scholar]
Hawley‐Hague 2017
- Hawley‐Hague H, Roden A, Abbott J. The evaluation of a strength and balance exercise program for falls prevention in community primary care. Physiotherapy Theory and Practice 2017;33(8):611‐621. [DOI] [PubMed] [Google Scholar]
Hektoen 2009
- Hektoen LF, Aas E, Luras H. Cost‐effectiveness in fall prevention for older women. Scandanavian Journal of Public Health 2009;37(6):584‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hopewell 2018
- Hopewell S, Adedire O, Copsey BJ, Sherrington C, Clemson LM, Close JCT, et al. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database of Systematic Reviews 2018, Issue 7. [DOI: 10.1002/14651858.CD012221.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howe 2011
- Howe TE, Rochester L, Neil F, Skelton DA, Ballinger C. Exercise for improving balance in older people. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD004963] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iliffe 2014
- Iliffe S, Kendrick D, Morris R, Masud T, Gage H, Skelton D, et al. Multicentre cluster randomised trial comparing a community group exercise programme and home‐based exercise with usual care for people aged 65 years and over in primary care. Health Technology Assessment 2014;18(49):1‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kendrick 2014
- Kendrick D, Kumar A, Carpenter H, Zijlstra GA, Skelton DA, Cook JR, et al. Exercise for reducing fear of falling in older people living in the community. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD009848.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamb 2005
- Lamb SE, Jorstad‐Stein EC, Hauer K, Becker C, Prevention of Falls Network Europe and Outcomes Consensus Group. Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. Journal of the American Geriatrics Society 2005;53(9):1618‐22. [DOI] [PubMed] [Google Scholar]
Lamb 2011
- Lamb SE, Becker C, Gillespie LD, Smith JL, Finnegan S, Potter R, et al. Reporting of complex interventions in clinical trials: development of a taxonomy to classify and describe fall‐prevention interventions. Trials 2011;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Liu 2009
- Liu CJ, Latham N. Progressive resistance strength training for improving physical function in older adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002759.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu‐Ambrose 2010
- Liu‐Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12‐month randomized controlled trial. Archives of Internal Medicine 2010;170(2):170‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lord 2011
- Lord SR, Sherrington C, Menz HB, Close J. Falls in Older People: Risk Factors and Strategies for Prevention. 2nd Edition. Cambridge University Press, 2011. [Google Scholar]
Lusardi 2017
- Lusardi MM, Fritz S, Middleton A, Allison L, Wingood M, Phillips E, et al. Determining risk of future falls in community‐dwelling older adults: a systematic review and meta‐analysis using posttest probability. Journal of Geriatric Physical Therapy 2017; Vol. 40, issue 1:1‐36. [DOI] [PMC free article] [PubMed]
Mahoney 1994
Matchar 2017
- Matchar DB, Duncan PW, Lien CT, Ong MEH, Lee M, Gao F, et al. Randomized controlled trial of screening, risk modification, and physical Tterapy to prevent falls among the elderly recently discharged from the emergency department to the community: The Steps to Avoid Falls in the Elderly Study. Archives of Physical Medicine and Rehabilitation 2017;98(6):1086‐96. [DOI] [PubMed] [Google Scholar]
McLean 2015
- McLean K, Day L, Dalton A. Economic evaluation of a group‐based exercise program for falls prevention among the older community‐dwelling population. BMC Geriatrics 2015;15(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menant 2017
NICE 2013
- National Institute for Health and Care Excellence. Falls in older people: assessing risk and prevention CG161. www.nice.org.uk/guidance/cg161/resources/falls‐in‐older‐people‐assessing‐risk‐and‐prevention‐pdf‐35109686728645 (accessed 10 August 2018). [PubMed]
NICE 2018
- National Institute for Health and Care Excellence. NICEimpact falls and fragility fractures. www.nice.org.uk/media/default/about/what‐we‐do/into practice/measuring‐uptake/nice‐impact‐falls‐and‐fragility‐fractures.pdf (accessed 5 August 2018).
Oliver 2017
- Oliver D. Fighting pyjama paralysis in hospital wards. BMJ 2017;357:j2096. [DOI] [PubMed] [Google Scholar]
Patil 2016
- Patil R, Karinkanta S, Tokola K, Kannus P, Sievanen H, Uusi‐Rasi K. Effects of vitamin D and exercise on the wellbeing of older community‐dwelling women: a randomized controlled trial. Gerontology. 2016;62(4):401‐8. [DOI] [PubMed] [Google Scholar]
Peel 2002
- Peel NM, Kassulke DJ, McClure RJ. Population based study of hospitalised fall related injuries in older people. Injury Prevention 2002;8(4):280‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Public Health England 2018
- Public Health England. Guidance Falls prevention: cost‐effective commissioning. A resource to help commissioners and communities provide cost‐effective falls prevention activities. www.gov.uk/government/publications/falls‐prevention‐cost‐effective‐commissioning (accessed 15 November 2018).
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robertson 2001b
- Robertson MC, Gardner MM, Devlin N, McGee R, Campbell AJ. Effectiveness and economic evaluation of a nurse delivered home exercise programme to prevent falls. 2: Controlled trial in multiple centres. BMJ 2001;322(7288):701‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robertson 2002
- Robertson MC, Campbell AJ, Gardner MM, Devlin N. Preventing injuries in older people by preventing falls: a meta‐analysis of individual‐level data. Journal of the American Geriatrics Society 2002;50(5):905‐11. [DOI] [PubMed] [Google Scholar]
Robertson 2005
- Robertson MC, Campbell AJ, Herbison P. Statistical analysis of efficacy in falls prevention trials. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2005;60(4):530‐4. [DOI] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group (formerly Applicability and Recommendations Methods Group) and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Sherrington 2017
- Sherrington C, Michaleff ZA, Fairhall N, Paul SS, Tiedemann A, Whitney J, et al. Exercise to prevent falls in older adults: an updated systematic review and meta‐analysis. British Journal of Sports Medicine 2017;51(24):1750‐8. [DOI] [PubMed] [Google Scholar]
Skelton 2001
- Skelton DA. Effects of physical activity on postural stability. Age and Ageing 2001;30 Suppl 4:33‐9. [PUBMED: 11769787] [DOI] [PubMed] [Google Scholar]
Smeeth 2002
- Smeeth L, Ng ES. Intraclass correlation coefficients for cluster randomized trials in primary care: data from the MRC Trial of the Assessment and Management of Older People in the Community. Controlled Clinical Trials 2002;23(4):409‐21. [DOI] [PubMed] [Google Scholar]
Speechley 1991
- Speechley M, Tinetti M. Falls and injuries in frail and vigorous community elderly persons. Journal of the American Geriatrics Society 1991;39(1):46‐52. [DOI] [PubMed] [Google Scholar]
Stenhagen 2014
- Stenhagen M, Ekström H, Nordell E, Elmståhl S. Accidental falls, health‐related quality of life and life satisfaction: A prospective study of the general elderly population. Archives of Gerontology and Geriatrics 2014;58(1):95‐100. [DOI] [PubMed] [Google Scholar]
Sterne 2011
Thompson 2013
- Thompson PD, Arena R, Riebe D, Pescatello LS. ACSM's new preparticipation health screening recommendations from ACSM's guidelines for exercise testing and prescription, 9th edition. Current Sports Medicine Reports 2013;12(4):215‐7. [DOI] [PubMed] [Google Scholar]
Tinetti 1988
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. New England Journal of Medicine 1988;319(26):1701‐7. [DOI] [PubMed] [Google Scholar]
Tinetti 1997
- Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. New England Journal of Medicine 1997;337(18):1279‐84. [DOI] [PubMed] [Google Scholar]
Tricco 2017
- Tricco AC, Thomas SM, Veroniki AA, Hamid JS, Cogo E, Strifler L, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta‐analysis. Journal of the American Medical Association 2017; Vol. 318, issue 7:1687‐99. [DOI] [PMC free article] [PubMed]
Verheyden 2013
- Verheyden GS, Weerdesteyn V, Pickering RM, Kunkel D, Lennon S, Geurts ACH, et al. Interventions for preventing falls in people after stroke. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD008728.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2009
- Walker DG, Aedo C, Albala C, Allen E, Dangour AD, Elbourne D, et al. Methods for economic evaluation of a factorial‐design cluster randomised controlled trial of a nutrition supplement and an exercise programme among healthy older people living in Santiago, Chile: the CENEX study. BMC Health Service Research 2009;9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 2003
- Walters SJ, Brazier JE. What is the relationship between the minimally important difference and health state utility values? The case of the SF‐6D. Health and Quality of Life Outcomes 2003;1(4):10.1186/1477‐7525‐1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 2005
- Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ‐5D and SF‐6D. Quality of Life Research 2005;14(6):1523‐32. [DOI] [PubMed] [Google Scholar]
WHO 2015
- World Health Organization. World Report on Ageing and Health. www.who.int/ageing/events/world‐report‐2015‐launch/en/. World Health Organization, 2015 (accessed Jaunuary 25 2018).
Yardley 2002
- Yardley L, Smith H. A prospective study of the relationship between feared consequences of falling and avoidance of activity in community‐living older people. Gerontologist 2002;42(1):17‐23. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sherrington 2016
- Sherrington C, Tiedemann A, Fairhall NJ, Hopewell S, Michaleff ZA, Howard K, Clemson L, Lamb SE. Exercise for preventing falls in older people living in the community. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD012424] [DOI] [PMC free article] [PubMed] [Google Scholar]


