Abstract
Circadian clocks play essential roles in the timing of events in the mammalian hypothalamo-pituitary-ovarian (HPO) axis. The molecular oscillator driving these rhythms has been localized to tissues of the HPO axis. It has been suggested that synchrony among these oscillators is a feature of normal reproductive function. The impact of fertility disorders on clock function and the role of the clock in the etiology of endocrine pathology remain unknown. Polycystic ovarian syndrome (PCOS) is a particularly devastating fertility disorder, affecting 5%–10% of women at childbearing age with features including a polycystic ovary, anovulation, and elevated serum androgen. Approximately 40% of these women have metabolic syndrome, marked by hyperinsulinemia, dyslipidemia, and insulin resistance. It has been suggested that developmental exposure to excess androgen contributes to the etiology of fertility disorders, including PCOS. To better define the role of the timing system in these disorders, we determined the effects of androgen-dependent developmental programming on clock gene expression in tissues of the metabolic and HPO axes. Female PERIOD2::luciferase (PER2::LUC) mice were exposed to androgen (dihydrotestosterone [DHT]) in utero (Days 16–18 of gestation) or for 9–10 wk (DHT pellet) beginning at weaning (pubertal androgen excess [PAE]). As expected, both groups of androgen-treated mice had disrupted estrous cycles. Analysis of PER2::LUC expression in tissue explants revealed that excess androgen produced circadian misalignment via tissue-dependent effects on phase distribution. In vitro treatment with DHT differentially affected the period of PER2::LUC expression in tissue explants and granulosa cells, indicating that androgen has direct and tissue-specific effects on clock gene expression that may account for the effects of developmental programming on the timing system.
Keywords: androgens/androgen receptors, circadian rhythm, clock genes, developmental origins of health and disease, female reproductive tract, fertility, mechanisms of hormone action, mouse, PCOS, PER2::luciferase, reproduction
Introduction
The circadian timing system drives daily rhythms of physiology and behavior [1]. Circadian oscillators play a major role in endocrine physiology, particularly in the timing of events in the female reproductive system [2]. The cell autonomous molecular oscillator has been localized to each tissue of the female hypothalamo-pituitary-ovarian (HPO) axis and implicated in the processes of folliculogenesis, ovulation, oocyte maturation, implantation, and steroid hormone synthesis [3]. The most salient feature of the mammalian reproductive cycle is the preovulatory rise in serum luteinizing hormone (LH) [4-6]. In rodents, the LH surge occurs during the evening of proestrus and dictates the timing of ovulation. The timing of the surge depends on the activity of pacemaker neurons in the central oscillator or suprachiasmatic nucleus (SCN) [7, 8] and positive feedback from ovarian estradiol [9]. Lesions that destroy the SCN or mutations that alter the timing or output of SCN neurons are known to affect the timing of LH secretion, block ovulation, and disrupt reproductive cycles [10, 11]. Recent evidence suggests that the timing of ovulation may also depend on a critical period of sensitivity to LH defined by the ovarian clock [12].
The substrate for circadian rhythms, often referred to as the molecular clock, is a transcriptional/translational autoregulatory feedback loop oscillator of interacting transcription factors or clock genes (for extensive and detailed review, see [1]). The activator BMAL1 forms a heterodimer with CLOCK and drives expression of period (period1, period2, and period3) and cryptochrome (cry1 and cry2) genes. PERIOD and CRY dimerize, undergo posttranslational modifications including phosphorylation by casein kinases (ε/δ), and translocate back to the nucleus wherein they repress the activity of BMAL1:CLOCK. A secondary loop including the activator RORα and the repressor REV-ERBα adds stability to the oscillator by regulating the timing and amplitude of bmal1 expression. In addition to their role as clock gene activator, BMAL1:CLOCK also regulate the expression of multiple clock-controlled genes (CCGs) that act downstream of the clock in a cell- and tissue-specific manner.
It is now widely accepted that the mammalian ovary contains cell autonomous circadian clocks [3]. Moreover, circadian clock gene expression has been reported at each level of the HPO axis, including the hypothalamic gonadotropin-releasing hormone neurons and the pituitary gonadotroph [13]. However, a functional role for the clock in these tissues, particularly in regard to ovarian physiology and fertility, has yet to be defined [3]. It has been reported that the ovarian clock regulates the timing of sensitivity to endocrine signals, including the rhythmic secretion of gonadotropins [14]. Others have linked circadian clock function to reproductive physiology, with particular emphasis on steroid hormone synthesis [15, 16]. Mutations that suppress clock function have dramatic impacts on fertility, including irregular LH secretion, abnormal cycles, and implantation failure [17, 18]. Clock function has also been described in tissues of both the digestive and cardiovascular systems, including the pancreas, adipose tissue, and liver [19]. Disrupting rhythms of clock gene expression in these tissues perturbs metabolic function, leading to impaired insulin secretion, glucose homeostasis, and lipid metabolism [20]. Chronodisruption, due to rotating shiftwork or chronic jet lag, is commonly experienced by medical professionals or long-haul flight crews and is associated with increased risk of preterm birth, irregular menstrual cycles, and subfertility in women [21]. Though less evidence supports a role for the molecular clock in women, data do suggest that the central clock controls the timing of LH secretion and that ovulation occurs consistently within 5–15 h after the LH surge [22]. Thus, whereas it appears that circadian dysfunction may be causative for metabolic and fertility disorders in rodents, a functional relationship between the timing system and the etiology of reproductive dysfunction in humans remains unconfirmed.
Polycystic ovarian syndrome (PCOS) is a commonly diagnosed endocrinopathy in women with primary symptoms, including amenorrhea or oligomenorrhea, a polycystic ovarian morphology, and excess circulating androgen [23]. According to recent clinical consensus, a diagnosis of PCOS requires confirmation of at least two of the three criteria [24]. PCOS is commonly comorbid with a metabolic syndrome characterized by hyperinsulinemia, dyslipidemia, reduced insulin sensitivity, obesity, and increased risk of type 2 diabetes and cardiovascular disease [25]. In addition to these reproductive and metabolic deficits, women with PCOS are more commonly diagnosed with sleep disorders, including abnormal REM sleep architecture, independent of obesity and metabolic disease [26, 27]. Excess testosterone of follicular origin (hyperandrogenemia) is a common feature of PCOS [25, 28]. The exact etiology of the disease as it relates to the timing of androgen exposure remains a topic of some debate [29]. Data from primate, sheep, and rodent models provide the strongest evidence that prenatal exposure to excess androgen may be causative [30]. Evidence also exists that excess androgen during late adolescence and puberty is a significant factor in the development of PCOS [31]. As a testament to these assertions, several animal models of androgen-induced PCOS, most often using the nonaromatizable androgen 5α-dihydrotestosterone (DHT), have been developed in rodents [32, 33]. These include the prenatally androgenized (PNA) mouse [34] and late-adolescent/pubertal exposure models (pubertal androgen excess [PAE]) applied in both rats and mice [35, 36]. Both models exhibit irregular reproductive cycles, disrupted gonadotropin secretion, and glucose intolerance without significant insulin resistance. Unlike PNA mice, PAE-exposed animals develop a clear polycystic ovarian morphology (fewer corpora lutea and more large unhealthy cystic follicles), abdominal obesity (characterized by increased inguinal and retroperitoneal fat mass), dyslipidemia, adipocyte hypertrophy, and elevated plasma cholesterol [33].
Mutations of the core clock genes have been linked to characteristics of the metabolic syndrome commonly associated with PCOS, including abdominal obesity [37]. However, the connection between fertility, metabolism, and clock function at the cellular level remains poorly understood. Metabolic syndrome and obesity are commonly associated with a decline in reproductive function, disrupted reproductive cycles, and attenuated gonadotropin secretion [38, 39]. Conversely, circadian disruption is known to increase the risk of obesity and metabolic disease [20, 40]. Given the comorbid sleep and metabolic disease prevalent among women with PCOS, it is reasonable to presume that circadian disruption may be a significant causative and/or permissive factor in the etiology of the disease. We hypothesize that developmental programming by androgen excess disrupts metabolism and reproductive physiology in part by altering the normal activity of the timing system. The ensuing internal circadian disorganization or misalignment contributes to the progression of disease and may underlie comorbidities, including sleep disorders.
To test this hypothesis, we exposed PER2::luciferase (PER::LUC) transgenic mice to excess androgen during sexual development. The goals of the present study were 1) to determine the impacts of androgen-dependent developmental programming on the circadian timing system in mice (both PAE and PNA) and 2) to isolate the potential influence of obesity by comparing and contrasting the impacts of excess androgen on the timing system in lean (PNA) and obese (PAE) models of PCOS [33]. To that end, we measured the timing of PER2::LUC expression in tissue explants of the reproductive tract from PNA and PAE mice, including the SCN, oviduct, pituitary, and ovarian follicle. Furthermore, we measured the same in liver, lung, and white adipose tissue (WAT) as components of the metabolic axis.
Materials and Methods
Animals
Female PER2::LUC transgenic mice (originally from Dr. Joseph Takahashi, University of Texas Southwestern Medical Center, Dallas, TX) from our local colony were used for all experiments. Unless otherwise noted, animals were reared under a standard 12L:12D photoperiod with constant temperature and humidity. Food (standard chow) and water were made available ad libitum. All handling and procedures were in compliance with the guidelines for the care and use of animals outlined by the University Committee on Animal Resources at the University of Rochester School of Medicine and Dentistry and in accord with the National Institutes of Health Guidelines for the Care and Use of Animals.
Generation of PAE Mice
Weanling (age, 4 wk; body wt, 11.49 ± 0.62 g) female PER2::LUC transgenic mice (congenic on a C57BL6/j background) were anesthetized with isoflurane gas, and a small incision was made in the intrascapular region. Animals received a subcutaneous placebo pellet (a chemical matrix containing cholesterol, stearates, lactose, cellulose, and phosphates minus the active steroid) or a 90-day continuous release pellet (Innovative Research of America) containing 5 mg of DHT (daily dose of 55 μg, or ∼5 μg/g/day) as described previously [36] with slight modification. This treatment is intended to produce androgen levels approximately 1.7- to 2-fold higher than normal, which is equivocal to the hyperandrogenemia in patients with PCOS [35, 41]. After recovery, animals were placed within our light-tight boxes in a 12L:12D photoperiod and provided food and water ad libitum. Body weight was measured weekly, and reproductive cycles were monitored by vaginal smear for a minimum of 17 days. Only those control animals showing at least two consecutive 5- to 7-day cycles were included in our experimental analyses and were euthanized on the afternoon of diestrus to avoid the potential impact of elevated ovarian steroids on circadian organization [42]. All animals were euthanized 9–10 wk after pellet surgery, and tissues were recovered for luminescence recording as described below.
Generation of PNA Mice
Adult (age, 3–6 mo) female PER2::LUC transgenic mice (congenic on a C57BL6/j background) were used in all experiments. Mice were housed under a standard 12L:12D photoperiod with food and water available ad libitum. Females were paired with congenic PER2::LUC homozygote males and checked for copulatory plugs daily. Pregnant dams were given daily subcutaneous injections of DHT (250 μg) in 50 μl of sesame oil or sesame oil vehicle on Days 16–18 of gestation [34]. Adult (age, 3–6 mo) female offspring from DHT- and oil- treated dams were used for experiments. Mice were transferred to our light-tight chambers, housed under a 12L:12D photoperiod, and provided food and water ad libitum. Body weight was measured weekly, and reproductive cycles were monitored by daily vaginal smear for a minimum of 17 days. Only those oil-treated control animals showing at least two consecutive 5- to 7-day estrous cycles were included in our experimental analyses and were euthanized on the day of diestrus to avoid the confounding effects of ovarian steroids on phase synchrony among oscillators. Both PNA and control mice were euthanized at 3–6 mo of age, and tissues were recovered for luminescence recording as described below.
Behavioral Analysis
Wheel-running activity by PNA, PAE, and control mice (placebo pellet or oil vehicle) was recorded using ClockLab (Actimetrics). Because we did not detect a significant difference in free-running period between control groups (pellet or oil), only placebo-pellet mice were included as controls in our behavioral analyses. To determine the potential effect of developmental programming on circadian rhythms of activity, PNA (n = 6), PAE (n = 4), and control (placebo pellet, n = 4) mice were kept under a 12L:12D photoperiod for at least 1 wk before being placed in constant darkness for 14 days. The period (τ) of locomotor activity was determined during the 2 wk in constant darkness using a chi-square periodogram (ClockLab). Behavioral analyses were done on a smaller cohort of mice from each group that were not included in subsequent analyses, including tissue luminescence recording, due to the potential confounding effect of wheel running on circadian organization.
Tissue Explant Culture and Bioluminescence Recording
Adult female PNA (n = 15), oil vehicle (n = 8), PAE (n = 17), and placebo (n = 8) mice of the PER2::LUC strain or untreated, random-cycling adult female PER2::LUC mice (for androgen treatment in vitro, n = 8) were euthanized 3 h before lights-off by excess CO2 exposure. Every effort was made to recover tissues on diestrus (PNA, PAE, and controls) to avoid the potential confounding effects of the estrous cycle on phase cohesion [42]. The brain, pituitary, ovary, and portions of the liver, lung, kidney, and WAT were collected in sterile Hanks balanced salt solution (HBSS). The brain was sectioned in the coronal plane with a vibrating microtome (WPI, Inc.) at a thickness of 300 μm. A section containing the bilateral SCN near the midpoint of the rostrocaudal extent was recovered, and a minimum of tissue including the paired SCN was removed. The ovary was isolated from the oviduct, and individual large follicles with a clear antrum were isolated. The pituitary was cultured intact. For all other tissues (liver, lung, kidney, WAT, and oviduct), individual 5- to 7-mm2 tissue explants were collected. Tissues were placed in 35-mm culture dishes with 1.2 ml of culture medium (Dulbecco modified Eagle medium supplemented with B27 [Gibco], 10 mM HEPES, 352.5 μg/ml of NaHCO3, 3.5 mg/ml of d-glucose, 25 U/ml of penicillin, 25 μg/ml of streptomycin, and 0.1 mM luciferin [Promega]). Our medium was serum-free and devoid of all but a minute level of progesterone (<10 fM) present in the B27 supplement. For steroid treatment experiments, DHT (in 100% EtOH) at the appropriate concentration (100 nM, 500 nM, 1 μM, and 10 μM) or EtOH vehicle was added to the culture medium (to a final EtOH concentration of 0.1%). Cultures were sealed with coverglass and maintained at 35°C in a light-tight incubator. Luminescence was recorded for 1 min every 10 min with an automated luminometer (LumiCycle; Actimetrics). Data are presented as luminescence counts per second.
Granulosa Cell Culture and Bioluminescence Recording
Weanling (age, 3–4 wk) female PER2::LUC mice maintained with food and water ad libitum and reared under a 12L:12D photoperiod were injected with equine chorionic gonadotropin (5 IU; Sigma) between 4 and 6 h after lights-on to induce follicular development. Forty-eight hours later, animals were euthanized with CO2, and the ovaries were quickly removed and placed in chilled HBSS supplemented with antibiotics. Ovaries were separated from peritoneal fat and oviducts, rinsed in clean HBSS, and placed in 0.6 ml of chilled culture medium (as above). Granulosa cells (GCs) were recovered as described previously [43] with slight modification. Cells were harvested by manual puncture of follicles with a 23-gauge needle followed by application of gentle pressure. Cells were dissociated using gentle trituration, washed once by centrifugation in fresh media at 4°C for 10 min (1000 × g), and resuspended for cell counting and viability assessment. Cells were diluted to a density of 5 × 105 cells/ml in our serum-free culture medium supplemented with 10 ng/ml of ovine follicle-stimulating hormone (Dr. Albert Parlow, National Institute of Diabetes and Digestive and Kidney Diseases) and plated in 35-mm cell cultures dishes (vacuum gas plasma-treated; BD Falcon). A total of three or four cultures were recovered from each mouse on average. Cultures were incubated at 37°C in a 95% O2/5% CO2 incubator for 48 h. On Day 3, cells were synchronized with fresh medium containing 200 nM dexamethasone (Sigma) for 30 min [44]. Following synchronization, fresh medium containing luciferin (0.1 mM; Promega), DHT (100 nM, 500 nM, 1 μM, or 10 μM; Sigma), DHT (1 μM), Bicalutamide (BIC; 100 nM; Sigma), or EtOH vehicle (0.1% final concentration) was added, and cultures were sealed with glass and sterile vacuum grease. Cultures were then placed in our luminometer (Lumicycle), and luminescence was recorded for a minimum of 6 days.
Data Analysis
Raw luminescence traces were detrended (24-h moving average) and smoothed (2-h moving average), and the peak of PER2::LUC expression on the first full 24 h in culture was recorded (Origin Pro 8.5; OriginLabs). The peak phase was recorded as relative Zeitgeber Time (ZT) with reference to the photoperiod before euthanization (ZT0 = lights-on during the day of euthanasia, and ZT12 = lights-off during the day of euthanasia). The peak phases of PER2::LUC expression for each tissue were converted from relative ZT to angles (e.g., ZT12 = 180°), plotted on circular Rayleigh plots, and analyzed using circular statistics (Oriana; Kovach Computing Services). Phase distribution among tissues within a group was analyzed using the Rayleigh test and considered to be “tight” at P < 0.05. A tight phase distribution is generally considered to be a group of oscillators that peak within 2–3 h on either side of the group mean, whereas dispersed tissues peak more than 3–4 h away from the mean. Phase synchrony is defined as the phase of an oscillator or group of tissues relative to the phase of a Zeitgeber (e.g., the photoperiod) or another oscillator (e.g., liver vs. SCN). Both are influenced by the number of tissues from a given animal peaking at a phase significantly different from the mean, such as a liver clock peaking in the middle of the day when the majority of liver tissues peak during the night. To visualize the impact of androgen on circadian organization, the phases of PER2::LUC expression in tissues from the same animal were plotted on horizontal phase “maps” with all those of other animals in that group (Origin Pro). To determine significant differences in mean phase between treatments, data were analyzed with a Watson-Williams F-test (Oriana), with a level for significance of P < 0.05.
The period of PER2::LUC expression was measured as described previously [42]. Briefly, 5 days of luminescence data, excluding the first 12 h to avoid the effects of culture preparation artifacts, were analyzed with a damped sin-fit using Lumicycle Analysis software (P < 0.001; Actimetrics). The percentage of time spent in each day of the estrous cycle was analyzed as a function of treatment using a one-way ANOVA followed by a Bonferroni post-hoc statistical test. The period of free-running locomotor activity in PNA, PAE, and control mice was analyzed with a two-tailed Student t-test. Body weight data were analyzed as a function of age and treatment using a two-factor ANOVA followed by a Bonferroni post-hoc analysis. The effects of DHT in vitro on the period of PER2::LUC expression were analyzed as a function of concentration using a one-factor ANOVA followed by Student-Newman-Keuls test for individual comparisons. For all analyses, results were considered to be significant at P < 0.05. Unless otherwise noted, all data are presented as mean ± SEM.
Results
Developmental Programming by Excess Androgen Differentially Affects Body Weight, Behavior, and Reproductive Cycles in Female Mice
As shown in Figure 1a, PAE resulted in an increase in body weight relative to that of mice given a placebo pellet (effect of treatment: F = 30.07, df = 1, P < 0.001; effects of age: F = 29.16, df = 10, P < 0.001, at 14 wk old or 10 wk after pellet implant). We did not detect a significant increase in body weight of PNA mice relative to controls (Fig. 1b). As we have reported in female rats [45], PAE did not affect the period of locomotor activity in mice ([controls: τ = 23.38 ± 0.08 (n = 4)] vs. [PAE: τ = 23.59 ± 0.05 (n = 4)], t = 2.24, df = 6, P > 0.05) (Supplemental Fig. S1, available online at www.biolreprod.org). We also did not detect an effect of PNA relative to controls on the period of wheel-running activity in mice (PNA: τ = 23.5 ± 0.07; n = 6 t = 1.099, df = 8, P > 0.05; data not shown). As expected, both methods of androgen exposure disrupted reproductive cycles in mice (Fig. 1, c and d). When analyzed as the percentage of time spent in each day of the cycle, it became clear that PNA and PAE mice spent a majority of their time in metestrus or diestrus (Fig. 1, e and f). PNA and PAE mice displayed on average less than one proestrous and estrous day during the 17-day recording period before euthanasia. In this analysis, a single proestrous day amounted to 5% of the total time period measured. As shown in Figure 1, e and f, both PNA and PAE mice exhibited one or less proestrous or estrous day on average (∼5%), whereas controls had 2–3 (∼10%–12%) on average. Though this effect was only statistically significant in PNA mice, the plots in Figure 1c are representative of the near-complete acyclicity detected in PAE mice.
Fig. 1.
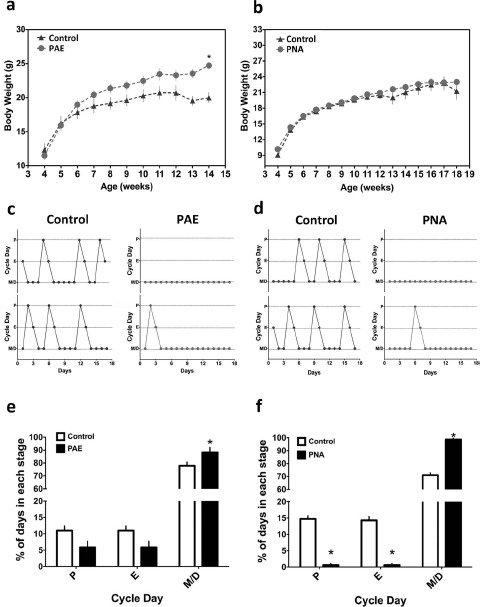
Developmental programming by excess androgen produces metabolic and reproductive features of PCOS in PER2::LUC mice. a) Weekly body weights of PER2::LUC mice exposed to PAE or placebo pellet and fed a standard diet ad libitum. PAE mice (n = 8) gained more weight over the course of treatment than controls (n = 8). b) Weekly body weights of PNA mice (n = 18) or controls (n = 14) fed a standard diet ad libitum. c and d) Estrous cycle patterns from representative PAE and controls (c) or PNA mice and vehicle-treated animals (d). PNA and PAE mice displayed intermittent or irregular cycles marked by persistent metestrus (M) or diestrus (D). e and f) Reproductive cycles from PNA (n = 18), oil control (n = 14), PAE (n = 8), and placebo pellet (n = 8) mice analyzed as a percentage of total time spent in each day of the 4-day cycle. Both PNA and PAE mice spent more time in M and D relative to controls. PNA mice, but not PAE mice, displayed a significant drop in the percent time spent in estrus (E) and proestrus (P; P < 0.05 for both). Data in a, b, e, and f are presented as the mean ± SEM. In a, *P < 0.001; in e and f, *P < 0.05.
Gestational Androgen Excess Differentially Affects PER2::LUC Expression in Central and Peripheral Oscillators, Resulting in Internal Circadian Misalignment
We have previously determined that PAE in rats reduces internal circadian organization marked by phase misalignment between the SCN and peripheral oscillators, including the liver, ovarian follicles, and WAT [45]. Because the PAE-treated rat model is an obese model of PCOS [35], we were unable to isolate the effects of androgen from the known impact of obesity on the timing system using this model. To address this concern we have now determined the influence of PNA, a model of lean PCOS, on the timing system in female mice. Adult female mice exposed to oil vehicle in utero showed characteristic phase coordination marked by a spread of 2–3 h around the mean (Fig. 2a, top). This phase clustering was apparent among ovarian follicles, oviduct, WAT, and liver explants (P < 0.05, Rayleigh test). In contrast, PNA mice showed reduced phase cohesion among ovarian follicles, WAT, and liver cultures (P > 0.05 for all three), with several explants peaking at least 3–4 h away from the mean (follicle, 8/15; WAT, 6/10; liver, 7/14). In comparison, only one of six follicles, one of five WAT explants, and none of the liver explants from control mice peaked more than 4 h away from the mean. Though phase cohesion among oviduct explants was significant (P < 0.05, Rayleigh test), several tissues peaked more than 3–4 h away from the mean phase (n = 5/15 vs. 1/8 in controls) (indicated by vector in Fig. 2a, bottom). This increase in phase distribution among tissues resulted in internal circadian misalignment (Fig. 2, b vs. c) [46]. We did not detect a significant affect of PNA on the phase distribution of pituitary, lung, or kidney cultures (Fig. 2c).
Fig. 2.
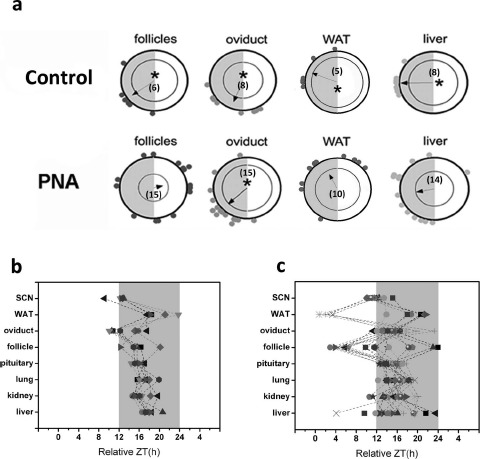
Gestational androgen excess has tissue-dependent effects on the phase of PER2::LUC expression in central and peripheral oscillators. a) Rayleigh plots showing the peak phase of PER2::LUC expression within individual explant cultures from control (top row) and PNA (bottom row) mice. Each gray dot plotted at the edge of the outer circle in a represents the peak phase of a different tissue explant. The outer circle represents the 24-h day, with ZT0 (lights-on) located at 1200 h and ZT12 (lights-off) located at 0600 h. The inner circle represents the statistical threshold for significant phase cohesion (P < 0.05). The vector in each plot represents the arithmetic mean phase of each group of tissues. Only when this vector crosses the significance threshold is the group of oscillators considered to have significant phase synchrony (indicated by asterisks). The numbers inside each plot indicate how many cultures for that tissue were included in the analysis. b and c) Phase distribution maps for individual tissues from control (b) and PNA (c) mice. Individual symbols represent tissues from different animals. The gray area indicates the 12-h dark phase. Dashed lines connect tissues from the same mouse.
Excess Androgen During Adolescence and Puberty Affects Phase Synchrony Among Central and Peripheral Oscillators, Resulting in Internal Circadian Misalignment
As previously stated, we have shown circadian misalignment in an obese rat model of PCOS. To accurately draw comparisons between the obese (PAE) and nonobese/lean (PNA) mouse models of PCOS, it is also necessary to examine the effects of PAE on the timing system in female mice. As shown in Figure 3, tissues recovered from mice receiving a placebo pellet showed significant phase clustering around the mean. This cohesion was significant among individual follicles (P < 0.05), oviduct (P < 0.05), WAT (P < 0.05), and liver (P < 0.05) explants (Fig. 3a, left). Exposure to excess androgen for 9–10 wk beginning at 4 wk of age resulted in a significant increase in phase distribution among follicles and WAT tissues (P > 0.05) (Fig. 3a, right) characterized by several explants (follicle, 11/15; WAT, 4/11) peaking 3–4 h away from the mean. In comparison, fewer follicle (1/7) and WAT (1/8) tissue explants from placebo-treated mice peaked more than 4 h away from the mean. We did not detect a significant increase in phase distribution among liver explants from PAE mice (Fig. 3a, bottom right). Surprisingly, the mean phase of peak PER2::LUC expression in ovarian follicles was significantly advanced (∼ZT7) in mice exposed to a placebo pellet compared with mice exposed to oil vehicle during gestation (∼ZT15; Watson-Williams F-test: F = 31.92, df = 20, P < 0.001) (Figs. 2a vs. 3a). Again, though statistically cohesive, an increase in the number of oviduct cultures peaking more than 4–6 h away from the mean was clearly observed (PAE, 5/17; control, 0/8) (Fig. 3a). As with PNA mice, phase dispersion among these oscillators resulted in internal circadian misalignment (Figs. 2c vs. 3c).
Fig. 3.
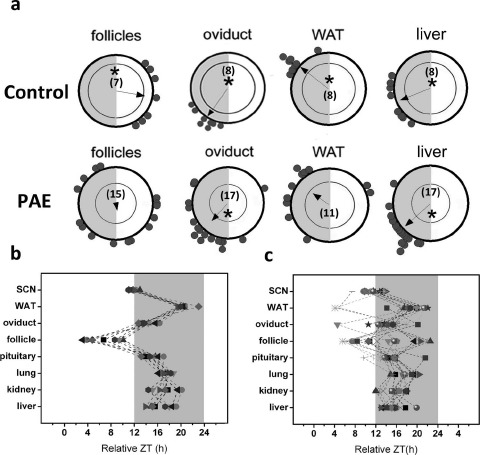
Excess androgen during late adolescence and puberty has tissue-dependent effects on the phase of PER2::LUC expression in central and peripheral oscillators. a) Rayleigh plots showing the peak phase of PER2::LUC expression within individual explant cultures from control (top row) and PAE (bottom row) mice. Each gray dot plotted at the edge of the outer circle in a represents the peak phase of a different tissue explant. The outer circle represents the 24-h day, with ZT0 (lights-on) located at 1200 h and ZT12 (lights-off) located at 0600 h. The inner circle represents the statistical threshold for significant phase cohesion (P < 0.05). The vector in each plot represents the arithmetic mean phase of each group of tissues. Only when this vector crosses the significance threshold is the group of oscillators considered to have significant phase synchrony (indicated by asterisks). The numbers inside each plot indicate how many cultures for that tissue were included in the analysis. b and c) Phase distribution maps for individual tissues from control (b) and PAE (c) mice. Individual symbols represent tissues from different animals. The gray area indicates the 12-h dark phase. Dashed lines connect tissues from the same mouse.
Androgen Exposure In Vitro Has Tissue-Specific Effects on the Period of PER2::LUC Expression in Isolated Central and Peripheral Oscillators
Both PNA and PAE differentially affect the timing of clock gene expression in select tissues, producing internal circadian misalignment that corresponds with reproductive dysfunction. The mechanism whereby excess androgen can alter the timing of the clock remains to be determined, though it likely results from a direct influence of ligand-bound androgen receptor (AR) on clock gene expression [47, 48]. To address this hypothesis, we treated tissue explant cultures of liver, lung, pituitary, ovarian follicles, oviduct, WAT, and SCN from adult female PER2::LUC mice (congenic on C57BL6/j) with several concentrations of DHT (100 nM to 1 μM). As shown in Figure 4, we observed tissue-dependent effects of DHT on the period of PER2::LUC expression in isolated tissue explant cultures. DHT had dose-dependent effects on the period of PER2::LUC expression (effect of DHT: F = 5.89, df = 15, P < 0.05) in the liver. Treatment with 100 nM DHT had no effect, whereas 500 nM DHT lengthened the period of PER2::LUC expression (P < 0.05 vs. control). This effect was not apparent at the highest concentration of DHT (1 μM) applied. Regardless of concentration applied, DHT did not affect the period of PER2::LUC expression in lung (F = 1.31, df = 15, P = 0.31) (Fig. 4b) and pituitary (F = 1.19, df = 16, P = 0.35) (Fig. 4c) explants.
Fig. 4.
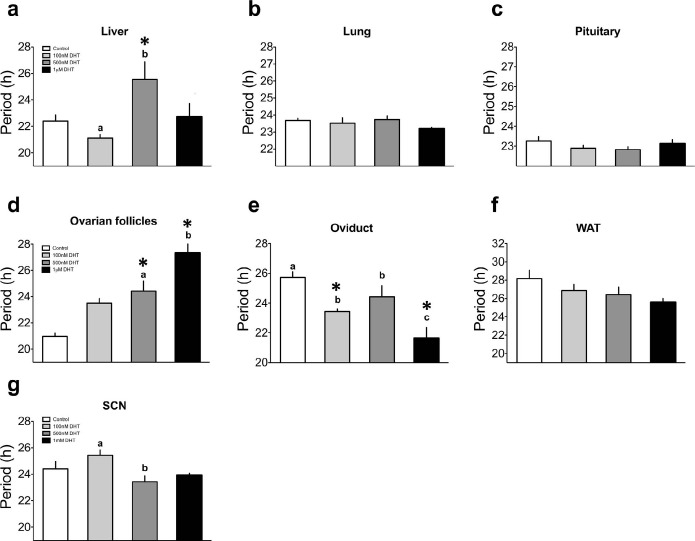
Treatment with DHT in vitro has tissue-specific and dose-dependent effects on the period of PER2::LUC expression in central and peripheral oscillators. The effects of DHT on the period of PER2::LUC expression in isolated tissue explants of the liver (a), lung (b), pituitary (c), ovarian follicles (d), oviduct (e), WAT (f), and SCN (g). Significant effects of DHT were detected in liver, follicle, and oviduct. A small but significant period shortening was also detected among SCN explants between 100 and 500 nM DHT. For a–g, n = 3–5 per treatment group. In each graph, differing letters above bars indicate differences between adjacent treatment groups (e.g., 100 nM DHT vs. 500 nM DHT), and an asterisk indicates differences between vehicle- and DHT-treated explants (both P < 0.05).
We also detected a significant effect of DHT on the period of PER2::LUC expression in ovarian follicle cultures (effect of treatment: F = 11.61, df = 19, P < 0.001) (Fig. 4d). DHT at 500 nM (P < 0.05) and 1 μM (P < 0.05), but not at 100 nM, significantly lengthened the period of PER2:LUC expression in ovarian follicles. Among oviduct explants, DHT shortened the period of PER2::LUC expression (effect of treatment: F = 10.49, df = 15, P < 0.01), with the most significant effects observed at 100 nM (P < 0.05 vs. control) and 1 μM (P < 0.05 vs. control) (Fig. 4e). We did not detect a significant effect of DHT treatment on the period of PER2::LUC expression in cultures of WAT (F = 2.26, df = 15, P = 0.133) (Fig. 4f). In the SCN, we did not observe an overall effect of DHT treatment (F = 3.5, df = 13, P = 0.06). Though we did not detect a significant difference between vehicle and any of the DHT treatments, post-hoc tests did confirm a small shortening of the PER2::LUC rhythm in the SCN following treatment with 500 nM DHT when compared to treatment with 100 nM DHT (P < 0.05).
Androgen Dose-Dependently Alters the Period of PER2::LUC Expression in Cultured Ovarian GC
It is apparent that androgen can affect the timing of the clock in multiple peripheral oscillators, but whether the pathophysiological levels of androgen present in ovarian follicular fluid (FF) influences the timing of the clock in GCs remains to be seen. To address this issue, we exposed isolated GC monolayer cultures from primed, juvenile PER2::LUC mice to various concentrations of DHT. Our data revealed that DHT exposure has considerable dose-dependent effects on the timing of PER2::LUC expression in isolated mouse GCs (effect of treatment: F = 7.8, df = 4, P < 0.001) (Fig. 5, a and b). Treatment with DHT at 500 nM (P < 0.05), 1 μM (P < 0.01), and 10 μM (P < 0.001) significantly shortened the period of PER2::LUC expression in GC cultures (Fig. 5b).
Fig. 5.
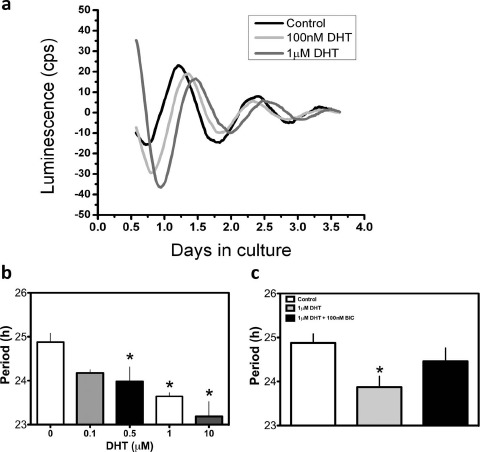
Dose-dependent effects of DHT on period of PER2::LUC expression in cultured ovarian GCs are attenuated by an AR antagonist. a) Representative luminescence traces of PER2::LUC expression in isolated GCs. The timing of peak PER2::LUC expression in both the 100 nM DHT- and 1 μM DHT-treated cultures peaked later on the first full day in culture but appeared to have an advancing peak, indicative of a short period, such that the treated cultures peaked at the same time as (or earlier, for 100 nM DHT) than the control. b) The period of PER2::LUC expression as a function of treatment. DHT dose-dependently shortened the period of PER2::LUC expression relative to vehicle-treated controls. c) The effects of DHT on the period of PER2::LUC expression were attenuated when DHT was added in the presence of the AR antagonist BIC (100 nM). In b and c, *P < 0.05. In b, n = 4–8 cultures per treatment group, and in c, n = 5–8 per treatment group.
These data revealed that DHT has dose-dependent effects on the period of PER2::LUC expression in isolated GCs (Fig. 5b). These effects likely are due to a direct impact of the activated AR on clock gene promoters, but it is possible the effects are indirect and/or unrelated to AR-mediated changes in clock gene expression. To address this issue, we repeated the experiment above in the presence of the AR antagonist BIC [49]. We treated a separate cohort of GC cultures with 1 μM DHT, a concentration shown above to shorten the period of PER2::LUC expression by approximately 1.5 h (Fig. 5b). As above, we detected a significant shortening of the PER2::LUC rhythm in the presence of 1 μM DHT (effect of treatment: F = 4.88, df = 2, P < 0.01) that was nearly reversed in the presence of 100 nM BIC (P > 0.05 vs. both vehicle control and DHT alone).
Discussion
The circadian timing system plays a significant role in reproductive physiology and metabolism. The molecular clock is localized to tissues of the female reproductive tract, including the ovary, and has been shown to regulate hormone secretion, follicular growth, and ovulation [3]. As in other physiological systems, synchrony between central and peripheral oscillators appears to be critical for reproductive function [46]. We hypothesized that developmental programming by abnormal hormone environments represents a novel and significant form of environmental circadian disruption (ECD) that, like other forms of ECD (e.g., shiftwork), leads to considerable deficits in physiological function. We have previously determined that synchrony among oscillators is disrupted in female rats following exposure to excess androgen during late adolescence and puberty [45]. These data imply that abnormally high androgen in circulation—a feature of several disorders of sexual development, including PCOS—disturbs fertility and metabolic function in part by reducing synchrony among oscillators. A caveat to this interpretation is that pubertal androgen produces a metabolic phenotype marked by abdominal obesity, dyslipidemia, and elevated serum cholesterol—a physiological landscape that by itself influences the timing system [50, 51]. In an attempt to isolate the impact of androgen, we measured the effects of developmental programming by either fetal (PNA mouse) or pubertal (PAE mouse) DHT exposure on the timing system of female mice. Our goals were to determine the impacts of androgen-dependent developmental programming on the circadian timing system in mice and to isolate the potential influence of marked abdominal obesity by comparing and contrasting the impacts of DHT in lean (PNA) and obese (PAE) mouse models of PCOS [33]. Neither PNA nor PAE affected the free-running circadian rhythm of locomotor activity, indicating that developmental programming did not influence certain aspects of central clock function. This result is consistent with our previous experiment [45]. The distribution of peak PER2::LUC expression in peripheral tissues across the 24-h day, synchronization between peripheral clocks, and the phase relationship between peak PER2::LUC expression in select tissues, the SCN, and the photoperiod cycle were affected by both PNA and PAE. Furthermore, treatment with androgen in vitro also affected the period of PER2::LUC expression in several tissues, including the liver, oviduct, and intact follicles. Treatment with DHT shortened the period of PER2::LUC expression in isolated GCs, an effect that was attenuated by cotreatment with a specific AR antagonist. These data suggest that circadian disruption arises due to androgen-dependent developmental programming. Furthermore, these effects are mediated by AR-dependent influence on tissue-specific patterns of clock and CCG expression.
Both PNA and PAE are commonly utilized as animal models of PCOS. Though each model recapitulates key features of the disease, both have been criticized for their deficiencies [32]. Some consider PNA mice to more accurately reflect the developmental origins of the disease, but these mice do not become obese and develop dyslipidemia, hypercholesterolemia, or a polycystic ovarian phenotype, all common features of clinical PCOS [33]. The PNA mouse can thus be considered a nonobese or “lean” model of PCOS. Because nearly 50% of women with PCOS do not present with comorbid obesity/metabolic disease, this model represents a large proportion of the patient population. Conversely, PAE mice develop more significant metabolic dysfunction (marked by dyslipidemia, hypercholesterolemia, and weight gain) and polycystic ovarian morphology [33] in parallel with marked circadian misalignment. These caveats aside, both models present with the fertility deficits, including irregular reproductive cycles, LH hypersecretion, and anovulation [52]. Our intent was to compare and contrast the effects of androgen in nonobese (PNA) and obese (PAE) models of PCOS to establish a common influence of androgen-dependent programming on the timing system. We hypothesized that the effects of androgen-dependent developmental programming on clock function are largely independent of obesity and/or dyslipidemia. While our results support the notion that obesity per se is not causative for misalignment, these data must be interpreted with caution, because we have not explicitly confirmed increased fat mass, dyslipidemia, or hypercholesterolemia in our PAE mice. That said, excess pubertal androgen in C57BL6/j mice is known to produce increased abdominal obesity, adipocyte hypertrophy, liver adiposity, and elevated serum cholesterol [33]. Though our PAE mice gained significant weight relative to controls (∼25%), we did not detect circadian desynchrony among liver explants, as we had previously observed in PAE rats [45]. This is surprising given that others have shown glucose intolerance, dyslipidemia, and liver steatosis in PAE mice and rats [33]. Considerable evidence suggests that obesity influences the timing of clock gene expression in central and peripheral oscillators [50, 53]. Unlike PAE, which can cause marked obesity and dyslipidemia [33], PNA mice fail to develop obesity and can be considered a “lean” model of PCOS. That said, PNA also produces circadian misalignment, with particular influence on oscillators associated with reproductive function (follicles) and metabolism (WAT and liver). In mice exposed to vehicle, we observed robust phase cohesion marked by a tight distribution of peak PER2::LUC expression around a central mean. The majority of tissues in PNA mice peaked within 2–3 h of the mean phase. In contrast, PNA reduced phase cohesion such that several animals had tissues peaking 4–8 h out of phase with the group mean. In the most dramatic instances, this resulted in peripheral oscillators that normally peaked during the middle of the relative night showing peak PER2::LUC expression at midday.
Assuming the oscillator driving PER2 expression (the BMAL1:CLOCK complex, REV-ERBα, RORα, etc.) is also consistently out of alignment, all clock-dependent processes likely are similarly misaligned. Transgenic reporter models, like our PER2::LUC mice, have been successfully used to interrogate the effects of environmental perturbations on circadian clock function and internal circadian organization [46]. It is possible that the effects we observed in both PNA and PAE mice are limited to the phase of PER2 expression alone. It will be critical to examine the pattern of other clock genes and CCGs in each of these tissues to confirm the full impact of androgenization on the timing system. Though evidence is limited, adaptive phase synchrony among oscillators is widely believed to be a critical facet of physiological homeostasis and a defining feature of the timing system [1, 8, 54]. ECD leads to dissociation of oscillators (misalignment) primarily due to variable tissue-specific rates of resynchronization or disruption of clock gene expression rhythms in target tissues [55]. It has been suggested that a persistent condition of oscillator desynchrony in the presence of a normal photoperiod underlies the pathophysiology of circadian disruption [55]. Internal circadian disorganization is implicated in the negative effects of ECD on immune, cognitive, and metabolic function [55, 56]. Circadian disruption can also negatively influence reproductive function in women [46, 57]. In mammals, phase coordination among central and peripheral oscillators is mediated by a complex network of humoral and neural cues driven by the pacemaker in the SCN [54]. Considerable evidence supports a role for the timing of adrenal glucocorticoid secretion in maintaining circadian organization [58]. Reports also suggest that dynamic titers of ovarian steroid hormone secretion during the reproductive cycle can affect the timing of clock gene expression in target tissues [42]. Thus, any disorder that alters or abolishes normal patterns of hormone secretion might be expected to have significant detrimental effects on circadian organization. The inverse relationship has already been established—that is, mutations affecting the circadian clock have been shown to alter steroid hormone synthesis and secretion [15, 59]. Our results provide further evidence that internal circadian misalignment may be a critical factor in the etiology of complex disorders affecting both reproduction and metabolism. While our intent was to systematically analyze the effects of developmental programming by excess androgen on the timing system, many endocrine disorders, including obesity, metabolic syndrome, and diabetes, negatively affect both metabolic function and fertility. It remains to be seen if reduced internal circadian organization is a common feature of these conditions.
Though data from PNA and PAE mice suggest androgen-dependent influence on the timing of clock gene expression, we endeavored to assess the direct impact of androgen on the clock in select tissues. Serum levels of androgen in patients with PCOS can be roughly 2-fold higher than normal, amounting to a level of free androgen ranging between 1.7 and 2 nM [60]. However, the level of testosterone and testosterone metabolites in FF isolated from women with PCOS are reportedly as high as 5–10 μM [61, 62]. The level of testosterone or its metabolites (e.g., dihydroepiandrosterone) and the threshold for their effects in other peripheral tissue (e.g., liver and oviduct) are unknown. Furthermore, the threshold for the effects of androgens on clock gene expression have not been established, though some evidence suggests a potent effect in the nanomolar range [47, 48]. We attempted to determine if androgen can affect the timing of PER2::LUC expression at a level several-fold higher than that found in serum from women with PCOS. We did not detect a significant effect of DHT on the period of PER2::LUC expression in SCN explants (DHT vs. vehicle) with the exception of a small shortening of the period between explants treated with 100 nM DHT and 500 nM DHT (Fig. 4g). We also did not observe a significant effect of DHT, regardless of concentration, on PER2::LUC expression in lung and pituitary explants. This result is unsurprising given that none of these tissues responded to either PNA or PAE exposure. A significant dose-dependent effect of DHT on PER2::LUC expression was detected in liver, oviduct, and ovarian follicular cultures. Interestingly, the directionality of the effect varied as a function of tissue. In both liver and follicle cultures, it appears that DHT lengthens the period of PER2::LUC expression (follicles, from ∼21.5 to 28 h; liver, from ∼23 to 26 h), albeit at a lower concentration in the liver. In contrast, DHT shortened the period of the oscillator in oviduct tissue. As described above, we detected the most appreciable effects of PNA and PAE on liver, follicles, oviduct, and WAT explants. Thus, our in vitro experiments largely corroborate our in vivo experiments and suggest that internal circadian misalignment following androgen-dependent developmental programming is in fact due to the direct effect of AR signaling on the timing of clock gene expression in target tissues. Though our culture medium was serum-free, the neurobasal B27 supplement did contain a minute (<10 fM) amount of progesterone. Thus, we cannot rule out an influence of this steroid on the response to DHT in vitro, particularly in light of the known interaction between progesterone and the AR [63].
Because we determined that circadian alignment among ovarian follicles was consistently affected by androgen-dependent developmental programming, we measured the direct effect of DHT on ovarian GC cultures. Our goal was to examine the response of the clock in GCs to androgen levels similar to those reported in the FF of women with PCOS. As detailed above, the measured concentration of androgen in serum varies greatly in the literature [64, 65], but the level of testosterone metabolites in FF (primarily androstenedione) isolated from women with PCOS is reportedly as high as 5–10 μM [61, 62]. DHT dose-dependently shortened the period of PER2::LUC expression in isolated GCs, and this effect could be attenuated by the addition of the AR antagonist BIC. The effects of DHT on the period of PER2::LUC expression were apparent at DHT concentrations as low as 500 nM. As mentioned above, androgen levels in FF can approach 5–10 μM in anovulatory women with PCOS [61, 62]. Our data suggest that androgen at 5- to 10-fold lower concentrations can alter the timing of clock gene expression in GCs. It is interesting to note that the direction of DHT effects on period were opposite between intact follicles (lengthened) and isolated GCs (shortened). Though we cannot provide evidence to explain this contrast, other cells in the intact ovary (e.g., stromal cells and thecal cells) and/or paracrine influences lost after isolation of GCs in monolayer culture likely account for this discrepancy. To that end, evidence indicates that paracrine interactions between cells in the follicle are critical for maintaining robust oscillator function [44]. Whereas BIC was able to reverse the effects of DHT, suggesting that AR actively binds to and regulates transcription of PER2, we have yet to define the molecular interaction between AR and the PER2 promoter. Reports suggest that AR interacts with PER1 and regulates the expression of the per1 mRNA in prostate cancer cell lines [47]. It is possible that AR influences PER2 expression indirectly by influencing other clock genes. Ligand-bound AR may interact with the BMAL1:CLOCK enhancer complex or auxiliary loop members like REV-ERBα. Though the molecular dynamics of ligand-bound AR interactions with clock genes and clock gene promoters are unknown, our data reveal a decline in circadian organization due to developmental programming that may depend in part on ligand-dependent activation of the AR.
Neither PNA nor PAE affected the free-running rhythm of wheel-running activity, though we did observe a modest (<1-h), dose-dependent effect of DHT on the period of PER2::LUC expression in SCN explants. ARs are expressed in SCN pacemaker neurons, and testicular androgens affect circadian rhythms of locomotor activity in males [66-68]. Gonadectomy reduces activity levels and produces fragmented activity with variable onset. Each of these effects of gonadectomy can be reversed by androgen replacement [66-68]. ARs are only sparsely expressed in the SCN of female mice, and testosterone or DHT treatment following ovariectomy increased activity levels and restored onset precision but had no effect on the period of the free-running activity rhythm [69]. These data, along with the present results, suggest that the amplitude of activity, light sensitivity of the pacemaker, and behavioral precision may be influenced by androgen but that the period of the oscillator and the timing of its output are not. This conclusion is also based on the observation that phase synchrony is robust among SCN explants from PNA and PAE mice. One caveat to our interpretation relates to the potential difference between in vitro and in vivo DHT exposure. Comparisons between in vivo exposure (PNA and PAE) during various stages of sexual development (organizational vs. activational) and in vitro exposure must be considered in light of possible developmental effects (e.g., epigenetic modifications) that might not be expected following acute DHT exposure.
Given the established and significant influence of androgenization on the HPO axis, we were surprised to find that the effects of PNA and PAE were rather limited, consistently affecting only the ovarian follicle and WAT. Equally surprising is that circadian misalignment among ovarian follicles does not necessarily correlate with polycystic ovarian morphology. That is, whereas we endeavored to recover only the largest antral follicles, large cystic follicles may have been recovered from PAE mice [36]. The ovarian cysts common to patients with PCOS are fluid filled, with a hypertrophied thecal cell layer and reduced GC layer [70]. Though certainly possible, this alone does not explain the circadian disorganization we observed, as we have detected similar misalignment among follicles in PNA mice that fail to develop a polycystic ovarian phenotype. Unlike ovarian follicles, we recorded only minor, if any, effects of PAE and PNA on the distribution of circadian clocks in the pituitary gland. This is surprising given that both forms of developmental programming are known to affect the rhythm and amplitude of LH secretion [35, 36, 52], though this has largely been attributed to neuroendocrine deficits. Clock function has been described in several pituitary cells, being implicated in the control of cell signaling, gene expression, and hormone secretory activity [71]. Because the pituitary was cultured intact, we cannot with any clarity define the effects of androgenization on individual hormone-producing pituitary cell types.
Taken together, our data reveal a significant decline in internal circadian organization associated with the development of a complex condition affecting both reproduction and metabolism. These findings extend our previous work in rats, further strengthening the view that developmental programming by excess androgen, a critical factor in the etiology of PCOS, disrupts internal circadian organization by altering phase synchrony among select tissues of the metabolic and reproductive axes. Moreover, our comparison between PNA and PAE models suggests that the timing of androgen-dependent developmental programming disrupts reproductive function and differentially affects circadian organization independent of significant weight gain. Internal circadian misalignment is unlikely to be a unique feature of androgenic programming. Rather, it may represent a common feature of endocrine disease, particularly arising from exposure to an abnormal hormonal milieu of environmental or genetic origin during sexual development. If this presumption holds, it would suggest an exciting new avenue for translational medicine: the clinical application of clock-modifying compounds or chronobiotics to improve reproductive function in those suffering from these debilitating and costly diseases.
Supplementary Material
Acknowledgment
The authors gratefully acknowledge the technical assistance of Lindsay Marchetti, Drew Phillips, Londyn Cullifer, Susan Butler, Christopher Plunkett, and Natalie Tjota.
References
- 1. Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron 2012; 74:246–260. [DOI] [PubMed] [Google Scholar]
- 2. Boden MJ, Varcoe TJ, Kennaway DJ.. Circadian regulation of reproduction: from gamete to offspring. Prog Biophys Mol Biol 2013; 113:387–397. [DOI] [PubMed] [Google Scholar]
- 3. Sellix MT. Circadian clock function in the mammalian ovary. J Biol Rhythms 2015; 30:7–19. [DOI] [PubMed] [Google Scholar]
- 4. Bronson FH. Vom Saal FS. Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology 1979; 104:1247–1255. [DOI] [PubMed] [Google Scholar]
- 5. Goldman BD. The circadian timing system and reproduction in mammals. Steroids 1999; 64:679–685. [DOI] [PubMed] [Google Scholar]
- 6. Moenter SM, DeFazio AR, Pitts GR, Nunemaker CS.. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol 2003; 24:79–93. [DOI] [PubMed] [Google Scholar]
- 7. de la Iglesia HO, Schwartz WJ.. Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology 2006; 147:1148–1153. [DOI] [PubMed] [Google Scholar]
- 8. Mohawk JA, Green CB, Takahashi JS.. Central and peripheral circadian clocks in mammals. Annu Rev Neuro 2012; 35:445–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Legan SJ, Coon GA, Karsch FJ.. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology 1975; 96:50–56. [DOI] [PubMed] [Google Scholar]
- 10. Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS.. Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and Clock mutant mice. Biol Reprod 2006; 75:778–784. [DOI] [PubMed] [Google Scholar]
- 11. Wiegand SJ, Terasawa E, Bridson WE, Goy RW.. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology 1980; 31:147–157. [DOI] [PubMed] [Google Scholar]
- 12. Sellix MT, Yoshikawa T, Menaker M.. A circadian egg timer gates ovulation. Curr Biol 2010; 20:R266–R267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sellix MT. Clocks underneath: the role of peripheral clocks in the timing of female reproductive physiology. Front Endocrinol 2013; 4:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoshikawa T, Sellix M, Pezuk P, Menaker M.. Timing of the ovarian circadian clock is regulated by gonadotrophins. Endocrinology 2009; 150:4338–4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Johnson BP, Shen AL, Wallisser JA, Krentz KJ, Moran SM, Sullivan R, Glover E, Parlow AF, Drinkwater NR, Schuler LA, Bradfield CA.. Loss of BMAL1 in ovarian steroidogenic cells results in implantation failure in female mice. Proc Natl Acad Sci U S A 2014; 111:14295–14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakao N, Yasuo S, Nishimura A, Yamamura T, Watanabe T, Anraku T, Okano T, Fukada Y, Sharp PJ, Ebihara S, Yoshimura T.. Circadian clock gene regulation of steroidogenic acute regulatory protein gene expression in preovulatory ovarian follicles. Endocrinology 2007; 148:3031–3038. [DOI] [PubMed] [Google Scholar]
- 17. Boden MJ, Kennaway DJ.. Circadian rhythms and reproduction. Reproduction 2006; 132:379–392. [DOI] [PubMed] [Google Scholar]
- 18. Ratajczak CK, Asada M, Allen GC, McMahon DG, Muglia LM, Smith D, Bhattacharyya S, Muglia LJ.. Generation of myometrium-specific Bmal1 knockout mice for parturition analysis. Reprod Fertil Dev 2012; 24:759–767. [DOI] [PubMed] [Google Scholar]
- 19. Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS.. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A 2004; 101:5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marcheva B, Ramsey KM, Peek CB, Affinati A, Maury E, Bass J.. Circadian clocks and metabolism. Handbook Exp Pharmacol 2013;127–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chau YM, West S, Mapedzahama V.. Night work and the reproductive health of women: an integrated literature review. J Midwifery Womens Health 2013; 59:113–126. [DOI] [PubMed] [Google Scholar]
- 22. Sellix MT, Menaker M.. Circadian clocks in the ovary. Trends Endocrinol Metab 2010; 21:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ehrmann DA. Polycystic ovary syndrome. N Engl J Med 2005; 352:1223–1236. [DOI] [PubMed] [Google Scholar]
- 24. Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, Boivin J, Petraglia F et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril 2012; 97:28–38. [DOI] [PubMed] [Google Scholar]
- 25. Dumesic DA, Abbott DH, Padmanabhan V.. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord 2007; 8:127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Sousa G, Schluter B, Buschatz D, Menke T, Trowitzsch E, Andler W, Reinehr T.. A comparison of polysomnographic variables between obese adolescents with polycystic ovarian syndrome and healthy, normal-weight and obese adolescents. Sleep Breath 2010; 14:33–38. [DOI] [PubMed] [Google Scholar]
- 27. Nandalike K, Strauss T, Agarwal C, Coupey SM, Sin S, Rajpathak S, Cohen HW, Arens R.. Screening for sleep-disordered breathing and excessive daytime sleepiness in adolescent girls with polycystic ovarian syndrome. J Pediatr 2011; 159:591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Padmanabhan V, Manikkam M, Recabarren S, Foster D.. Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Mol Cell Endocrinol 2006; 246:165–174. [DOI] [PubMed] [Google Scholar]
- 29. Franks S, Berga SL.. Does PCOS have developmental origins? Fertil Steril 2012; 97:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R.. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nature Rev Endocrinol 2011; 7:219–231. [DOI] [PubMed] [Google Scholar]
- 31. Franks S. Polycystic ovary syndrome in adolescents. Int J Obes 2008; 32:1035–1041. [DOI] [PubMed] [Google Scholar]
- 32. Shi D, Vine DF.. Animal models of polycystic ovary syndrome: a focused review of rodent models in relationship to clinical phenotypes and cardiometabolic risk. Fertil Steril 2012; 98:185–193. [DOI] [PubMed] [Google Scholar]
- 33. Caldwell AS, Middleton LJ, Jimenez M, Desai R, McMahon AC, Allan CM, Handelsman DJ, Walters KA.. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology 2014; 155:3146–3159. [DOI] [PubMed] [Google Scholar]
- 34. Sullivan SD, Moenter SM.. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci U S A 2004; 101:7129–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Manneras L, Cajander S, Holmang A, Seleskovic Z, Lystig T, Lonn M, Stener-Victorin E.. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology 2007; 148:3781–3791. [DOI] [PubMed] [Google Scholar]
- 36. van Houten EL, Kramer P, McLuskey A, Karels B, Themmen AP, Visser JA.. Reproductive and metabolic phenotype of a mouse model of PCOS. Endocrinology 2012; 153:2861–2869. [DOI] [PubMed] [Google Scholar]
- 37. Arble DM, Ramsey KM, Bass J, Turek FW.. Circadian disruption and metabolic disease: findings from animal models. Best Pract Res Clin Endocrinol Metab 2010; 24:785–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Franks S. Genetic and environmental origins of obesity relevant to reproduction. Reprod Biomed Online 2006; 12:526–531. [DOI] [PubMed] [Google Scholar]
- 39. Balasubramanian P, Jagannathan L, Mahaley RE, Subramanian M, Gilbreath ET, Mohankumar PS, Mohankumar SM.. High fat diet affects reproductive functions in female diet-induced obese and dietary resistant rats. J Neuroendocrinol 2012; 24:748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang W, Ramsey KM, Marcheva B, Bass J.. Circadian rhythms, sleep, and metabolism. J Clin Invest 2011; 121:2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Silfen ME, Denburg MR, Manibo AM, Lobo RA, Jaffe R, Ferin M, Levine LS, Oberfield SE.. Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab 2003; 88:4682–4688. [DOI] [PubMed] [Google Scholar]
- 42. Murphy ZC, Pezuk P, Menaker M, Sellix MT.. Effects of ovarian hormones on internal circadian organization in rats. Biol Reprod 2013; 89:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ratoosh SL, Richards JS.. Regulation of the content and phosphorylation of RII by adenosine 3′,5′-monophosphate, follicle-stimulating hormone, and estradiol in cultured granulosa cells. Endocrinology 1985; 117:917–927. [DOI] [PubMed] [Google Scholar]
- 44. Chen H, Zhao L, Chu G, Kito G, Yamauchi N, Shigeyoshi Y, Hashimoto S, Hattori MA.. FSH induces the development of circadian clockwork in rat granulosa cells via a gap junction protein Cx43-dependent pathway. Am J Physiol Endocrinol Metab 2013; 304:E566–E575. [DOI] [PubMed] [Google Scholar]
- 45. Sellix MT, Murphy ZC, Menaker M.. Excess androgen during puberty disrupts circadian organization in female rats. Endocrinology 2013; 154:1636–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Amaral FG, Castrucci AM, Cipolla-Neto J, Poletini MO, Mendez N, Richter HG, Sellix MT.. Environmental control of biological rhythms: effects on development, fertility and metabolism. J Neuroendocrinol 2014; 26:603–612. [DOI] [PubMed] [Google Scholar]
- 47. Cao Q, Gery S, Dashti A, Yin D, Zhou Y, Gu J, Koeffler HP.. A role for the clock gene Per1 in prostate cancer. Cancer Res 2009; 69:7619–7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kawamura M, Tasaki H, Misawa I, Chu G, Yamauchi N, Hattori MA.. Contribution of testosterone to the clock system in rat prostate mesenchyme cells. Andrology 2014; 2:225–233. [DOI] [PubMed] [Google Scholar]
- 49. Rathkopf D, Scher HI.. Androgen receptor antagonists in castration-resistant prostate cancer. Cancer J 2013; 19:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J.. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 2007; 6:414–421. [DOI] [PubMed] [Google Scholar]
- 51. Barclay JL, Shostak A, Leliavski A, Tsang AH, Johren O, Muller-Fielitz H, Landgraf D, Naujokat N, van der Horst GT, Oster H.. High-fat diet-induced hyperinsulinemia and tissue-specific insulin resistance in Cry-deficient mice. Am J Physiol Endocrinol Metab 2013; 304:E1053–E1063. [DOI] [PubMed] [Google Scholar]
- 52. Walters KA, Allan CM, Handelsman DJ.. Rodent models for human polycystic ovary syndrome. Biol Reprod 2012; 86:149. [DOI] [PubMed] [Google Scholar]
- 53. Bass J, Takahashi JS.. Circadian integration of metabolism and energetics. Science 2010; 330:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Menaker M, Murphy ZC, Sellix MT.. Central control of peripheral circadian oscillators. Curr Opin Neurobiol 2013; 23:741–746. [DOI] [PubMed] [Google Scholar]
- 55. Evans JA, Davidson AJ.. Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Trans Sci 2013; 119:283–323. [DOI] [PubMed] [Google Scholar]
- 56. Shi SQ, Ansari TS, McGuinness OP, Wasserman DH, Johnson CH.. Circadian disruption leads to insulin resistance and obesity. Curr Biol 2013; 23:372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mahoney MM. Shift work, jet lag, and female reproduction. Int J Endocrinol 2010; 2010:813764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pezuk P, Mohawk JA, Wang LA, Menaker M.. Glucocorticoids as entraining signals for peripheral circadian oscillators. Endocrinology 2012; 153:4775–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ratajczak CK, Boehle KL, Muglia LJ.. Impaired steroidogenesis and implantation failure in Bmal1−/− mice. Endocrinology 2009; 150:1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Glintborg D, Hermann AP, Hagen C, Jensen LT, Frystyk J, Bennett P, Flyvbjerg A, Andersen M.. A randomized placebo-controlled study on the effects of pioglitazone on cortisol metabolism in polycystic ovary syndrome. Feril Steril 2009; 91:842–850. [DOI] [PubMed] [Google Scholar]
- 61. Michael AE, Glenn C, Wood PJ, Webb RJ, Pellatt L, Mason HD.. Ovarian 11β-hydroxysteroid dehydrogenase (11βHSD) activity is suppressed in women with anovulatory polycystic ovary syndrome (PCOS): apparent role for ovarian androgens. J Clin Endocrinol Metab 2013; 98:3375–3383. [DOI] [PubMed] [Google Scholar]
- 62. Mason HD, Willis DS, Beard RW, Winston RM, Margara R, Franks S.. Estradiol production by granulosa cells of normal and polycystic ovaries: relationship to menstrual cycle history and concentrations of gonadotropins and sex steroids in follicular fluid. J Clin Endocrinol Metab 1994; 79:1355–1360. [DOI] [PubMed] [Google Scholar]
- 63. Moore NL, Hickey TE, Butler LM, Tilley WD.. Multiple nuclear receptor signaling pathways mediate the actions of synthetic progestins in target cells. Mol Cell Endocrinol 2012; 357:60–70. [DOI] [PubMed] [Google Scholar]
- 64. Agarwal SK, Judd HL, Magoffin DA.. A mechanism for the suppression of estrogen production in polycystic ovary syndrome. J Clin Endocrinol Metab 1996; 81:3686–3691. [DOI] [PubMed] [Google Scholar]
- 65. Robinson S, Kiddy D, Gelding SV, Willis D, Niththyananthan R, Bush A, Johnston DG, Franks S.. The relationship of insulin insensitivity to menstrual pattern in women with hyperandrogenism and polycystic ovaries. Clin Endocrinol 1993; 39:351–355. [DOI] [PubMed] [Google Scholar]
- 66. Daan S, Damassa D, Pittendrigh CS, Smith ER.. An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus). Proc Natl Acad Sci U S A 1975; 72:3744–3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Karatsoreos IN, Wang A, Sasanian J, Silver R.. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology 2007; 148:5487–5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Morin LP, Cummings LA.. Effect of surgical or photoperiodic castration, testosterone replacement or pinealectomy on male hamster running rhythmicity. Physiol Behav 1981; 26:825–838. [DOI] [PubMed] [Google Scholar]
- 69. Iwahana E, Karatsoreos I, Shibata S, Silver R.. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Horm Behav 2008; 53:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xita N, Tsatsoulis A.. Review: fetal programming of polycystic ovary syndrome by androgen excess: evidence from experimental, clinical, and genetic association studies. J Clin Endocrinol Metab 2006; 91:1660–1666. [DOI] [PubMed] [Google Scholar]
- 71. Resuehr HE, Resuehr D, Olcese J.. Induction of mPer1 expression by GnRH in pituitary gonadotrope cells involves EGR-1. Mol Cell Endocrinol 2009; 311:120–125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


