Abstract
Agnostids (agnostinids and eodiscinids) are a widespread and biostratigraphically important group of Cambro-Ordovician euarthropods whose evolutionary affinities have been highly controversial. Their dumbbell-shaped calcified tergum was traditionally suggested to unite them with trilobites, but agnostinids have alternatively been interpreted as stem-crustaceans, based on Orsten larval material from the Cambrian of Sweden. We describe exceptionally preserved soft tissues from mature individuals of the agnostinids Peronopsis and Ptychagnostus from the middle Cambrian (Wuliuan Stage) Burgess Shale (Walcott Quarry and Marble Canyon, British Columbia, Canada), facilitating the testing of alternative hypotheses. The digestive tract includes conspicuous ramifying cephalic diverticulae. The cephalon carries one pair of elongate spinous antennules projecting to the front, two pairs of appendages with distally setose, oar-like exopods, and three pairs of presumably biramous appendages with endopods sporting club-shaped exites. The trunk bears five appendage pairs, at least the first two of which are similar to the posteriormost cephalic pairs. The combined evidence supports a nektobenthic and detritivorous lifestyle for agnostinids. A head with six appendiferous segments contrasts strikingly with the four known in trilobites and five typical of mandibulates. Agnostinids are retrieved as the sister group to polymeroid trilobites in our phylogeny, implying that crustacean-like morphologies evolved homoplastically. This result highlights the variability in segmental composition of the artiopodan head. Finally, our study emphasizes the continued role of Burgess Shale-type fossils in resolving the affinities of problematic biomineralizing taxa.
Keywords: Cambrian explosion, evo devo, phylogenetics, palaeoecology, Lagerstätte, body plan
1. Introduction
Agnostids (Order Agnostida Salter 1864) are a cosmopolitan group of extinct euarthropods whose calcified tergal elements are widespread in Cambro-Ordovician rocks [1]. They are diverse, but morphologically conservative, being characterized by cephalic and pygidial shields of similar size and shape, joined by two or three freely articulating (thoracic) tergites [2]. Two sub-orders of agnostids (Agnostina and Eodiscina) are currently defined, although the relationship of these two groups to each other has been debated [3]. As a whole, agnostids have classically been interpreted as a highly derived group of trilobites [4,5]. They notably share calcified tergites, conspicuous axial furrows and dorsal segmentation, and their mode of tergite articulation and enrolment [6] (see electronic supplementary material table S1 for a detailed review of similarities). Agnostids also share a number of characters with the larvae and juveniles of ‘typical’ (i.e. polymeroid) trilobites, notably their rounded cephalic shield, low number of free tergites, and in agnostinids, the lack of dorsal eyes and ecdysial sutures [7]. As such, they have been interpreted to have evolved via paedomorphosis (potentially progenesis; [8]). Eodiscinids exhibit a spectrum of putatively intermediate morphologies, from more polymeroid-like taxa with dorsal eyes and facial sutures to agnostinid-like taxa with trans-glabellar furrows and enlarged ‘basal lobes’ on the occipital margin [7].
The discovery of crustacean-like soft tissues in an early growth series of Agnostus pisiformis in the Orsten Lagerstätte [9] led some to question the hypothesized trilobite affinity of agnostinids and the monophyly of Agnostida [3,10–14]. Notably, reduced cephalic endopods, multisegmented stenopodous exopods, robust antennules and a prominent bilobed labral structure have been suggested to unite the agnostinids more closely with ‘crustaceans’ (or from a modern perspective, place them in the mandibulate stem group [15,16]) than with trilobites (see electronic supplementary material, table S1 for a comprehensive list). In addition, the Agnostus larvae lack characters associated with trilobites and related artiopodans (e.g. multipartite lamellate exopods, a protaspis larval stage [12]). Under this view, the characters that agnostinids share with trilobites are interpreted as symplesiomorphies or homoplasies and the lack of other mandibulate apomorphies is taken as a symptom of their basal position within the mandibulate stem-group.
Crucially, the soft tissues of Agnostus are only well known up until the ‘meraspid’ M1 ontogenetic stage [9], and these tiny individuals (less than 1 mm) could have differed morphologically and ecologically from mature agnostinids [7]. In addition, resolution of the ‘agnostid problem’ has been notably hindered by the lack of knowledge of soft tissues in eodiscinids, and in the larval stages of other critical taxa [17]. Besides controversy over their affinities, uncertainty remains over whether agnostids were pelagic or benthic, whether or not they lived permanently enrolled, and their feeding ecology [6,18]. Here, we describe new adult specimens (up to 13.4 mm in sagittal length; see electronic supplementary material, table S2) of the agnostinids Peronopsis and Ptychagnostus with exceptionally preserved soft tissues. The insight gleaned from this new material permits a re-evaluation of the various hypotheses of agnostid evolution and ecology.
2. Material and methods
The material studied comes from two Burgess Shale fossil localities in British Columbia, Canada and is deposited at the Royal Ontario Museum. The best-preserved specimens come from Marble Canyon (Peronopsis cf. columbiensis (Rasetti, 1951) and Ptychagnostus cf. praecurrens (Westergård, 1936)) in Kootenay National Park. Additional specimens come from the Walcott Quarry (Ptychagnostus praecurrens (Westergård, 1936)) in Yoho National Park. The species listed above might belong to different genera (Quadragnostus columbiensis and Pentagnostus praecurrens [19,20]) based on Russian material; however, we maintain the older nomenclature pending a thorough restudy of North American agnostinid material, in particular that from the Burgess Shale. Specimens were prepared with an air scribe to remove overlying matrix as needed. Microscopy and macrophotography were conducted using cross-polarized lighting conditions. Elemental maps were also generated for selected specimens using an environmental scanning electron microscope (FEI Quanta 200 FEG) equipped with an energy scanning spectroscopy (EDS) X-ray detector and octane plus silicon drift detector at the University of Windsor Great Lakes Institute for Environmental Research, Canada.
A time-calibrated Bayesian phylogenetic analysis was performed in MrBayes v. 3.2.6 [21], using the Mkv model [22] with gamma-distributed rate variation, and a modified version of the matrix and analysis of Vannier et al. [16], incorporating 104 taxa and 225 characters. We added the taxa Triarthrus, Hongshiyanaspis, Retifacies, Kodymirus, Cindarella, Cheloniellon, Kwanyinaspis, Tegopelte and Emucaris to better account for the morphological diversity of arachnomorphs. Taxa lacking preservation of soft tissue characters were not included to minimize the amount of missing information (see electronic supplementary material for full list of modifications and additional characters). We used four runs of 20 000 000 generations, sampling four parallel chains every 1000 generations and discarding the first 20% of samples as burn in. Character evolution was studied in Mesquite 3.4 [23].
3. Description
(a). Taphonomic considerations
The fossils are primarily preserved as carbon and aluminosilicate films. The strongest carbon traces occur in internal organs, including the digestive tract and appendages (electronic supplementary material, figure S1D,F). While such enhanced preservation of carbon is common in internal organs [16], the agnostinids lack the typical phosphatization of the gut seen in many other Burgess Shale taxa [24,25]. Rapid burial at various angles and partial decay has produced subtle asymmetries in many specimens (e.g. Figure 1a), including slight lateral shifting of the entire suite of appendages and hypostome relative to the dorsal exoskeleton, similar to that seen in the trilobite Olenoides [26], but displacement appears to have been minimal and does not significantly impact morphological interpretations.
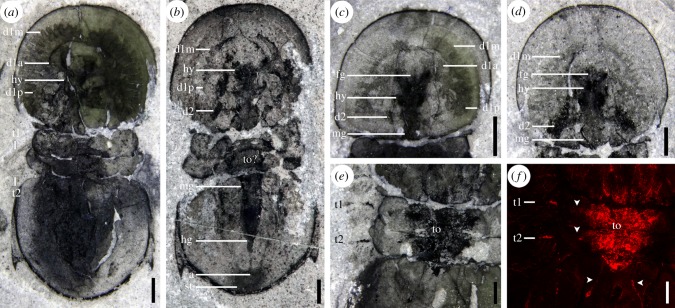
Figure 1.
Internal organs of Peronopsis cf. columbiensis from Marble Canyon. (a) ROMIP 64982, with detailed structure of the ramifying first pair of gut diverticulae; (b) ROMIP 64989, showing complete extent of digestive tract; (c) cephalon of ROMIP 64979, with the main branches of the ramifying pair of diverticulae inserting into the lateral notches in the hypostome complex; (d) cephalon of ROMIP 64994, with well-preserved second pair of cephalic diverticulae; (e,f) anterior trunk of ROMIP 64990 showing unknown triangular internal organ; (e), photograph; (f), carbon map, with arrows indicating carbon traces leading below the pygidium and towards the appendages. Bars = 1 mm. (a–e) photographed under polarized light, immersed in water. Abbreviations: a, anus; d#x, gut diverticulum # (branches of d1 labelled with suffix a, anterior; m, medial; p, posterior); fg, foregut; hg, hindgut; hy, hypostome; mg, midgut; st, staining from extruded material; to, triangular organ; t#, trunk appendage associated with free tergite #. (Online version in colour.)
(b). Digestive structures
The gut and associated digestive structures are best preserved in Peronopsis (figures 1 and 3; electronic supplementary material, figures S1D–F, S2DGH). The mouth was probably located at the posterior edge of the hypostome just below the transglabellar furrow as suggested by comparison with A. pisiformis ([9]; figure 1a–d). A small axial projection of soft tissues in front of the hypostome is interpreted to represent the foregut looping backwards from the posterior mouth (figure 1c,d). From the hypostome to approximately the point at which the pygidial axis starts to taper, the gut maintains a thickness of about one-third the width of the axis. Posterior to this, it constricts markedly towards the anus (figures 1b and 2i,j). We interpret this differentiated posteriormost section as the hindgut. Dark amorphous stains which do not preserve a carbon signal (figure 1a,b; electronic supplementary material, figure S1DE), also commonly occur around the anus. These are similar to dark stains observed preferentially around the mouth or anus in a number of Burgess Shale fossils, and probably represent decay fluids seeping out into the sediment [27]. The most conspicuous portion of the digestive system is a pair of large ramifying diverticulae originating near the anterior wings of the hypostome and occupying most of the extraglabellar space under the cephalic shield (figure 1a–d; electronic supplementary material, figures S1D–F, S2DGH). Each diverticulum divides into three main first-order branches (anterior, medial and posterior), each splitting further (at least once or twice) into a few relatively thick, blind-ending rami (figure 1a,c; electronic supplementary material, figures S1D–F, S2GH). The anterior branch is short and bends adaxially in front of the hypostome. The medial branch is the most extensive and sports eight second-order branches, along its outer surface only and decreasing in size anteromedially. The most adaxially positioned second-order branches nearly meet those of the opposing diverticulum. The main posterior branch splits into a small branch posteromedially, extending only about as far as the rear edge of the hypostome and ending in four undivided rami in a frog foot-shape (figure 1c). The remaining portion of the posterior branch extends posterolaterally, nearly to the occipital lobe of the cephalon and sports six second-order branches along its outer edge (figure 1a; electronic supplementary material, figures S1F, S2 H). Unlike the medial branch, it also bears a fringe of possibly undivided rami along its inner face.
Figure 3.
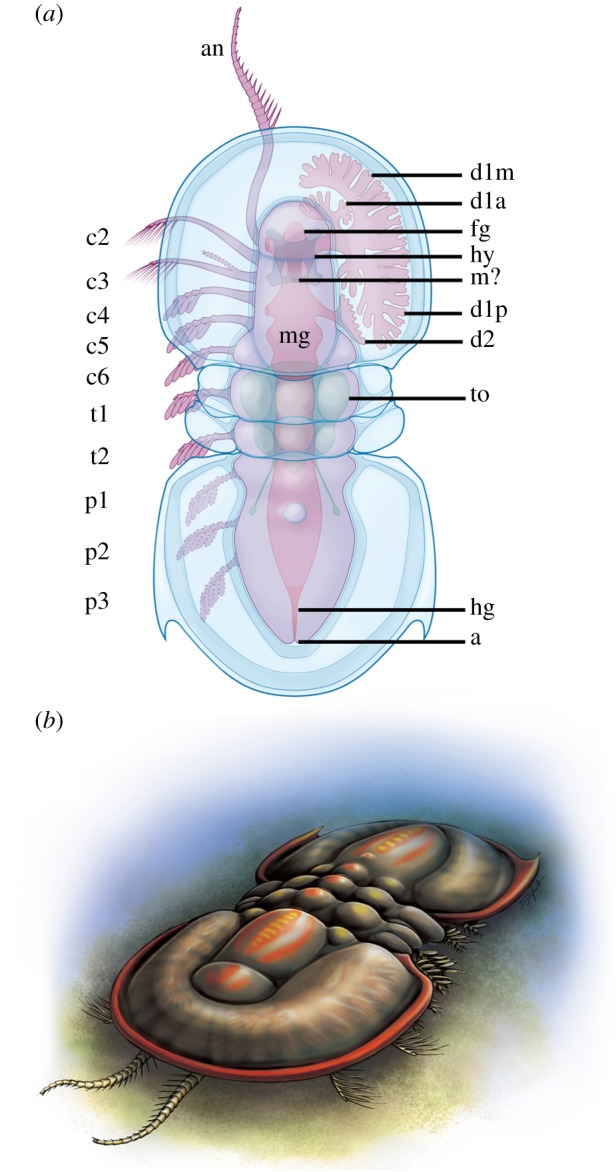
Reconstruction of Peronopsis. (a) Diagram showing appendages on the left and digestive structures on the right, dashed lines indicate features that are inferred; (b) life reconstruction, as the animal may have looked when unrolled. m? = inferred position of the mouth. See figures 1 and 2 for other abbreviations. Artwork by Danielle Dufault, Royal Ontario Museum. (Online version in colour.)
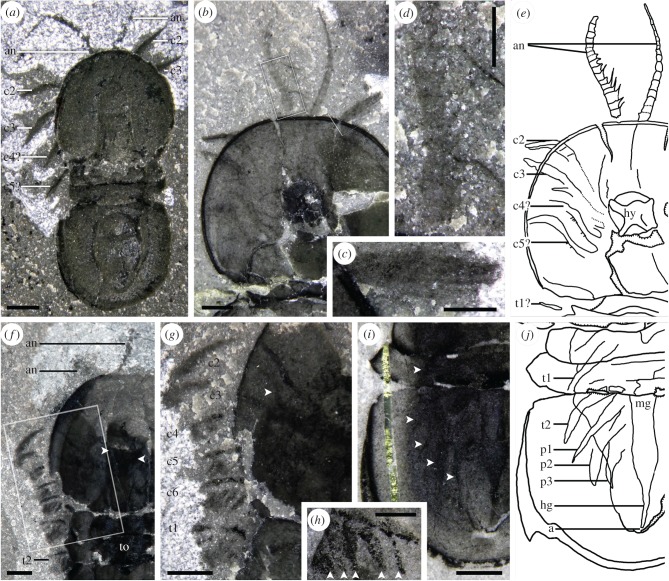
Figure 2.
Appendages of Ptychagnostus and Peronopsis. (a) Ptychagnostus praecurrens, ROMIP 65017 from the Walcott Quarry (WQ) showing cephalic appendages; (b,c,d,e) Peronopsis cf. columbiensis, ROMIP 64993 from Marble Canyon (MC); (b) cephalon showing appendages, boxed areas correspond to (c) and (d); (c) close-up of the setose distal tip of the second cephalic appendage; (d) close-up of part of the left antennule, with prominent medial spines; (e) interpretive diagram of left side of the cephalon indicating the appendages; (f,g) Peronopsis cf. columbiensis, ROMIP 64990 from MC; (f) composite image of part and counterpart showing the full complement of cephalic and the first two trunk appendages on the left side, as well as internal organs, arrows indicate anterior wings of hypostome; (g) close-up of appendages, showing cephalic appendages two to six and first trunk appendage, arrow indicates putative small endopod on c3; (h) closeup of cephalic appendage (c5?) of Peronopsis cf. columbiensis, ROMIP 64986 from MC, showing five club-shaped exites on the endopod; (i,j) Peronopsis cf. columbiensis, ROMIP 62941 from MC, with poorly preserved trunk appendages underlying the free tergites and the pygidium; (i) specimen, arrows indicating appendages; (j) interpretive diagram. Bars: a,b,f,g,i = 1 mm; c,d,h = 0.5 mm. All specimens photographed using polarized light, a–d,f,g, dry; h,i, immersed in water. an, antennule; c#, cephalic appendage #; p#, pygidial trunk appendage #; other abbreviations as in figure 1. (Online version in colour.)
In addition to the first pair of large anterior diverticulae, a second pair of diverticulae also occur below the cephalon (figures 1b–d and 3), posterior to the hypostome. These appear to have expanded posterolaterally, although rami cannot be distinguished. There is no evidence of diverticulae below the free tergites or the pygidium.
Some specimens preserve a symmetrical carbonaceous internal structure underlying the free tergites, anterior pygidium and possibly parts of the cephalon (figure 1b,e,f and 3; electronic supplementary material, figure S1C). This structure is sub-triangular and broadest anteriorly, as wide as the axis of the free tergites. A pair of thin extensions branch out posterolaterally ending in slightly enlarged terminations in one specimen (figure 1e,f; electronic supplementary material, figure S1C). In addition, short lateral extensions protrude towards the bases of the appendages below the free tergites. The presence of this structure across several specimens, its distinct bilateral shape and clear outline, the fine extensions, and the strong carbon signal detected by elemental mapping are inconsistent with it representing an internal decay stain. Whether the main triangular structure represents a differentially preserved portion of the gut or a combination of different organs (including at least the gut which this structure overlaps) is unknown. The short lateral extensions are possibly comparable to carbonaceous traces that extend into the limbs of other Burgess Shale panarthropod taxa [28].
(c). Appendages
The cephalon bears six pairs of appendages (figures 2 and 3). The first pair are uniramous and insert near the anterior portion of the hypostome (figure 2a,b,d–f; electronic supplementary material, figures S1A,B, S2A,B,D,H). Thus, we interpret these appendages as antennules. The antennules are longer than the cephalic shield and much longer than any other appendages, tapering from about one-fifth the width of the glabella to fine points distally. Each might have had at least 25–30 podomeres. At least the first 8 podomeres distal to the cephalic margin bear a robust, mediodistally projecting spine, of greater length than the supporting podomere. The second appendage consists of a single ramus and is usually preserved curving sigmoidally in an anterolateral direction (figure 2a,b,e–g; electronic supplementary material, figures S1A–C, S2A,B,E,F). This appendage originates from a proximal termination in front of the posterior margin of the hypostome and it bears at least 12 fine, backward pointing setae (figure 2c; electronic supplementary material, figure S2F). The third cephalic appendage (figure 2b,e–g; electronic supplementary material, figure S2E,F) has a similar morphology, but it may have a second small, poorly preserved ramus in addition to the larger main ramus. Appendage pair three appears to attach near the posterior of the hypostome. Posteriorly, the cephalon bears three more pairs of appendages (figure 2f,g; electronic supplementary material, figures S1A–C, S2A–E) which based on comparison with Orsten material should be biramous [9]. Only one ramus is well preserved in these appendages, often as a dense carbon film. Podomere boundaries are not visible, but their positions are suggested by four to five non-overlapping club-shaped outgrowths (figure 2f–h; electronic supplementary material, figures S1A–C, S2A–C), preserved as a dense carbon film and extending backwards from the appendage axis. The two free trunk tergites each additionally overlie an appendage of similar morphology to the posterior three of the cephalon (figures 1a and 2f,g; electronic supplementary material, figures S1A–D, S2C). Each appendage seems to be centred below its respective tergite. Appendages below the pygidium (figure 2i,j; electronic supplementary material, figure S2D) are poorly preserved in the available material and details of their morphology are undiscernible, although they were presumably similar to the preceding ones. Traces of three are visible, attaching along the axis, lateral to the gut. The posteriormost appendage originates near the point where the axis begins to constrict.
4. Discussion
(a). Digestive structures
The presence of a pair of ramifying cephalic diverticulae was previously suggested for some agnostinid species on the basis of characteristic patterns of ridges and furrows (‘rugae’ and ‘scrobiculae’) on the cephalic shield [29]. The digestive identity of such structures is confirmed by the specimens described here. Notably, rugae/scrobiculae are absent or indistinct in Peronopsis and Ptychagnostus, demonstrating that soft tissue structures cannot always be reliably inferred from tergal impressions. Based on comparison with rugae and scrobiculae in taxa where they exist [30], the diverticulae seem to have occupied a similar space below the cephalic shield, though the relative size and organization of the diverticular branches varies. Some agnostinids also possess rugae and scrobiculae on their pygidium [1], but we find no evidence for pygidial diverticulae in Peronopsis or Ptychagnostus.
Euarthropods possessing ramifying cephalic diverticulae akin to those in agnostinids include naraoiids, Burgessia, the poorly known Notchia, limulids and several extant mandibulate taxa [31–34]. Of these, the comparison with naraoiids is of note, as the ramifying diverticulae are similarly organized and emerge laterally from the hypostome complex ([35]; contra [31]). By contrast, the digestive tracts known in polymeroid trilobites possess small, simple secondary digestive structures, undifferentiated in the head and trunk ([36]; but Cisne [37] reconstructed putative ramifying digestive diverticulae in Triarthrus, though fossil material was never figured). Small tubules, sometimes called ‘genal caecae’ in some trilobites have been interpreted as homologous to the rugae of agnostinids [7], but they are quite structurally different and are more likely to be related to circulation and respiration [38–40].
(b). Appendages
Peronopsis and Ptychagnostus share similar appendage morphology with Agnostus from the Orsten Lagerstätte [9]. The spinous antennules are clearly equivalent between all three, though it is notable that they are much shorter in Agnostus. This difference could potentially be ontogenetic, although the exact length of Agnostus' antennules is unknown as their distal ends were broken in all published specimens. The two pairs of appendages following the antennules, with oar-like, distally setose rami, are comparable to the exopods of the second and third cephalic appendages of Agnostus. The smaller ramus on the third appendage is probably a reduced endopod, like that present in Agnostus. The relatively homonomous morphology of more posterior biramous appendages, with endopods bearing distinctive club-shaped outgrowths, is also shared by the three taxa. The non-overlap of the outgrowths, their low number and their preservation as a relatively dense carbon film, is not consistent with an affinity with the exopod lamellae of other euarthropods, which are thinner, more numerous and extensively imbricated [41]. Instead, we interpret them as equivalent to the four to five bloated, digitiform exites that occur on the endopods of Agnostus.
The most surprising finding is the presence of six pairs of appendages in the head of Peronopsis and probably also in Ptychagnostus. Müller & Walossek [9] interpreted Agnostus to have had only four pairs. Such a difference in the number of appendiferous segments in the head, either ontogenetically or between closely related and otherwise morphologically similar taxa would be highly unusual. As such, a preservational and interpretational explanation may be more likely. Müller & Walossek's interpretation of the number of cephalic segments was based primarily on counting sternites below the cephalic shield, as most of their specimens were enrolled with densely clustered appendages that are difficult to associate with certainty with a given segment. They identified only two post-hypostomal sternites as belonging to the head (with the first post-hypostomal sternite apparently being absent). However, several of the best-preserved specimens clearly show that additional cephalic space existed behind these two sternites (e.g. their Plates 12 : 5, 14 : 1, 16 : 1). One specimen even shows one or two additional sternites in this position, interpreted by Müller & Walossek as ‘anteriorly displaced’ trunk sternites (their Plate 15 : 1). As such, the number of head appendages in Agnostus may have exceeded four and should be considered uncertain, pending restudy of the Orsten material.
Prior to the discovery of the Orsten specimens, Öpik [42] suggested that the agnostinid head likely bore five pairs of appendages, on the basis of glabellar segmentation and putative muscle scars. In fact, some agnostinid cephala bear as many as six pairs of scars [43]. This evidence is compatible with our discovery of a head with six appendiferous segments. Pygidia of many agnostinid taxa bear three prominent pairs of muscle scars (sometimes followed by a series of smaller scars, or ‘notulae’; e.g. [2]), which also matches the three appendage pairs observed in Peronopsis and the immature pygidium of Agnostus [9].
(c). Evolutionary implications
Although the possession of six appendiferous head segments is an apomorphy of euchelicerates, a close relationship between agnostinids and this clade is unlikely given the absence of other key characters like chelicerae or the reduction of trunk endopods [44]. A close relationship between agnostinids and crustaceans, or even mandibulates, is similarly unlikely. Firstly, agnostinids lack convincing mandibulate apomorphies such as mandibles, maxillae and coxae (or, for that matter any form of sclerotized prebasal limb elements; [45,46]). Secondly, many of the characters initially proposed to place agnostinids in a basal position in the mandibulate stem-group have since been recognized as widespread among euarthropods (i.e. probable symplesiomorphies) or are found only in a few other Orsten taxa, themselves of problematic affinity (see electronic supplementary material, table S1). Notably, Oelandocaris, which has been considered as likely close to agnostinids in the ‘crustacean’ stem group [13,14,47], has been alternatively interpreted as a leanchoiliid megacheiran larva [28,46]. Similarly, the elongate, antenniform antennules of Peronopsis and Ptychagnostus are no more similar to those of Orsten larval taxa than they are to those of some polymeroid trilobites [48], questioning the significance of this putative link with crustaceans (contra [10,49]). Finally, the compliment of six appendiferous cephalic segments in agnostinids contrasts with the inferred plesiomorphic state of four [28,50] and the derived state of five characterizing the ground plan of at least the mandibulate crown group [14,16,51].
Alternatively, the cephalic condition in Peronopsis and Ptychagnostus departs strikingly from that considered typical of trilobites. Evidence from the best-preserved taxa (particularly Triarthrus) suggests that four appendiferous cephalic segments are characteristic of polymeroid trilobites [37,52,53]. The precise number of head appendages is well constrained only in a few taxa, and a complete set of head appendages from a trilobite taxon with five rather than four pairs of glabellar furrows has yet to be documented, leaving open the possibility that some species bore an additional cephalic segment (contra the suggestion of [54], that the fifth furrow is intersegmental). Even with that considered, the agnostinid head represents a clear deviation from this pattern.
In spite of this, our phylogenetic analysis (figure 4; electronic supplementary material, figure S3) finds strong support for a clade containing agnostinids as the sister group to the polymeroid trilobites included in our analysis (94% posterior probability), all contained within a grouping of artiopodans. This clade is mainly supported by the calcified tergites, the form of tergal articulation, externally segmented cephalic shield and occipital lobe. Agnostus resolved as sister to Arachnomorpha in ([16], electronic supplementary material 22), but this result was predominantly due to the coding of gnathobases as absent. However, we think the strong differentiation of the robust and spinous basipod of Agnostus justifies coding this character as present. Some of the subgroupings of artiopodans are similar to the results of previous analyses, (e.g. resolving the close relationship of Tegopelte, Naraoia and xandarellids [35,55], Sidneyia, Emeraldella and Kodymirus [56], and Emucaris, Arthroaspis and Kuamaia [35]). The unexpected position of Marrella with Cheloniellon and Aglaspis, all in a basally branching stem chelicerate clade, and the resulting paraphyly of Artiopoda sensu [57] seems to be driven by their lack of endopodal endites and the differentiated post-antennular appendage in the former two. Aglaspidids and cheloniellids have been recovered in a similar position before [58], but considering the low support in our tree and the controversy over the affinities of marrellomorphs in particular, this result should be considered with caution. The relationships between major artiopodan subclades seem to be strongly driven by exopod morphology, but posterior probabilities are low, preventing a confident evaluation of the sequence of character evolution. This does not impact the conclusion that agnostinids and polymeroid trilobites are closely related.
Figure 4.
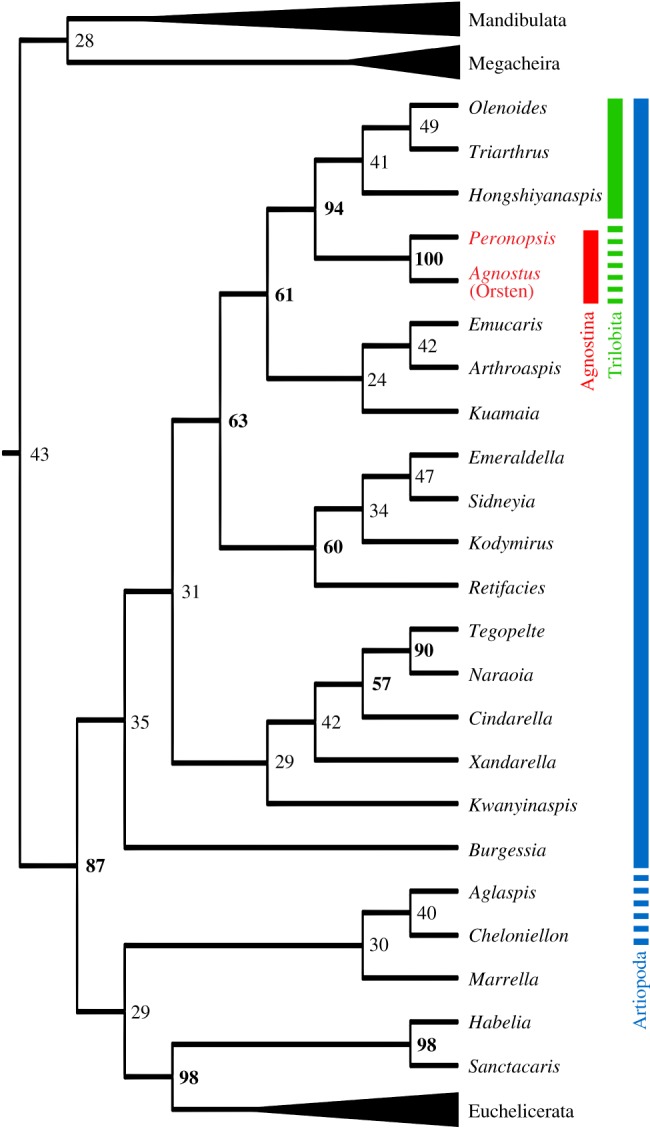
Evolutionary affinities of agnostinids. Simplified cladogram showing the position of Agnostina, cut from a time-calibrated Bayesian phylogeny incorporating 225 characters and 104 taxa. Numbers denote posterior probabilities (indicated in bold when over 50%). See the electronic supplementary material for complete analysis results, character list and nexus file. (Online version in colour.)
Our phylogenetic topology is not strictly consistent with the traditional hypothesis that agnostinids are a derived ingroup of trilobites [7], as we find agnostinids as a sister group to polymeroid trilobites. Under the hypothesis that their morphology is derived, agnostinids might still be regarded as trilobites, though in light of the highly differentiated head tagmata of agnostinids and polymeroids, such an evolutionary scenario would have been more complex than has traditionally been envisioned. Assuming an ancestor with four appendiferous head segments, as documented in polymeroids, a transition to the agnostinid state would have required the acquisition of two new pairs of head appendages, either by fusion from the trunk or by de novo development in the head tagma. This could entail the non-homology of the occipital rings in agnostinids and polymeroids. An additional implication is that morphological differences between agnostinids and polymeroid trilobites can no longer be attributed to paedomorphosis alone, instead requiring a considerable deviation from the standard trilobite ontogenetic trajectory. It is still possible that ‘localized’ paedomorphic shifts played a role in the evolution of some agnostinid characters, but testing this hypothesis will require knowledge of larval polymeroid trilobite soft anatomy which is currently unavailable. Similar cases of the incorporation of additional segments into the head tagma have probably occurred a number of times during the evolution of major euarthropod clades, for example prior to the radiation of crown euchelicerates [44] and mandibulates [16] as well as several times among various crustacean groups that evolved a cephalothorax [50].
There is an alternative hypothesis, which may have more radical implications. By comparison with polymeroid trilobites, most artiopodans have been assumed to have had four pairs of cephalic appendages [35,53,55], though some may have had five (Emeraldella [41,44], Naraoia [59], Cindarella [60] and aglaspidids [56,61]). Xandarella is distinct in probably possessing seven pairs of cephalic appendages [57]. In many artiopodan taxa, the number remains poorly constrained (to name just a few included in our phylogeny: Sidneyia, [49]; Retifacies, [57]; Kuamaia, [57], Kwanyinaspis [62]) and it is possible that an even greater diversity existed in the segmental composition of the head tagma in this group than is currently appreciated. Similar diversity in head composition is potentially paralleled in other extinct groups like marrellomorphs [63]. Considering the variability in head segmentation among non-trilobite artiopodans, the ancestral condition in this group is unclear. It is therefore possible that the four appendiferous segments that characterize the head tagma of polymeroid trilobites represent a derived condition, having been reduced from an ancestrally higher number. The loss of head appendages also has precedent among euarthropods, for example in the intercalary segment of hexapods, myriapods and hymenocarines [16], the missing second maxillae of diplopods and pauropods [64], and the loss of chilaria in arachnids [65]. If this hypothesis is correct, agnostinids could represent a plesiomorphic sister group to polymeroid trilobites. This could potentially be supported in our analysis by their possession of the probable plesiomorphic state for several characters compared to polymeroids (absence of eyes and ophthalmic ridges within the cephalic shield, effaced segmental impressions in the tergum and differentiation of cephalic secondary digestive structures).
The precise relationship of agnostinids with polymeroids and whether they should be considered members of Trilobita sensu stricto is thus critically dependent on the polarity of character evolution among artiopodans. The inclusion of taxa lacking soft tissue characters such as eodiscinids might help to resolve the phylogeny of basally diverging trilobite groups, however, it would do nothing to polarize soft tissue characters, which are responsible for much of the morphological differentiation between these groups. Additional studies, focussing on the segmental composition of the heads of other artiopodans, will be required to test the hypotheses outlined above. New discoveries of Burgess Shale-type fossils will be critical in this regard.
(d). Palaeoecology
Given the ontogenetically conserved morphologies of agnostinids, their mode of life was probably similar across known ontogenetic stages despite considerable changes in size, arguing against the idea that the Agnostus ‘meraspid’ represents a true larval stage. It has been posited that agnostinids could be representative of a crustacean-like larval niche in early euarthropods, from which crown mandibulate characters arose heterochronously [46]. In the light of our findings, the body plan of agnostinids may be better interpreted as the result of convergent adaptation to a niche analogous to that occupied by small extant crustaceans.
A nektobenthic ecology for agnostinids was proposed based on meraspid soft tissue morphology [9] and is further supported by that of the adult individuals presented here. At all stages, agnostinids are clearly highly adapted for enrolment, however, the abundance of articulated and outstretched individuals at the Burgess Shale and many other sites, sometimes with preserved soft tissue (as presented here), strongly suggests that these animals did not live permanently enrolled (contra [6,9]) and that they lived in or close to the benthos [66]. The specialized second and third appendages of agnostinids are reminiscent of the anterior appendages of small crustaceans, such as some ostracods and branchiopods (particularly larvae), which are used for swimming and food gathering [67]. The projection of these exopods from between the cephalic and pygidial shields could likely have permitted swimming while partially enrolled and may have enabled a more nektonic lifestyle than in most polymeroid trilobites [9].
Although the elongate antennules presumably performed a sensory function, their large medial spines (widespread among euarthropods, including notably in other artiopodans such as Kuamaia [57] and Emeraldella [41]) might additionally suggest a role in sweeping food particles towards the mouth, as originally posited by Müller & Walossek [9]. The bulbous endopodal exites of agnostinids have been compared functionally with the epipodites of crustaceans and may have similarly been involved in gas exchange [9,68], implying functional divergence with polymeroid trilobites and possibly other artiopodans, whose ventral body cuticle arguably served as the primary respiratory surface [40].
In modern deep-water environments, small benthic detritivorous crustaceans, such as ostracods and copepods, are among the most abundant organisms [69]. The similar abundance of agnostinids in relatively deep-water palaeoenvironments is consistent with a comparable mode of life [66]. In addition to feeding on detritus, agnostinids may have taken advantage of larger food items when available. Extensively ramifying digestive diverticulae like those in agnostinids have been associated with sporadic, opportunistic feeding habits in extant and fossil marine euarthropods, as they can serve as storage organs which enable engorgement on ephemeral food sources [31]. This could more speculatively align with observations of agnostinids clustering around putative carcasses or moult remains of other organisms at the Burgess Shale and other sites, potentially to feed opportunistically on carrion or encrusting microbial films [70–72].
5. Conclusion
The weight of evidence currently available supports an artiopodan affinity for agnostinids and suggests that they form a clade with polymeroid trilobites. More precise resolution of their relationship with polymeroid trilobites will be facilitated by a better understanding of the polarity of character evolution of the artiopodan cephalon and the phylogeny of the earliest trilobite lineages. Whether agnostinids are regarded as an early diverging trilobite ingroup or as a distinct sister clade to polymeroid trilobites, they testify to the high degree of morphological differentiation achieved among the Cambrian members of this group. Among artiopodans, trilobites are notably characterized by a release from canalization in their trunk development, especially among basally diverging lineages [73]. Agnostinids conform to this pattern with their highly reduced trunk [74], but additionally point to the possibility that the segmental composition of the cephalon was labile in the early evolutionary history of the trilobite-agnostinid clade—a trait potentially inherited from a deeper artiopodan ancestry.
In combination with the material from the Orsten Lagerstätte, our study contributes towards the most completely known ontogenetic sequence of any artiopodan. The similarity between adult and juvenile agnostinids implies that their distinctive morphology is not simply attributable to ontogenetic changes or a differentiated larval ecology. Instead, the morphological differentiation of agnostinids from polymeroid trilobites is likely a product of both the plesiomorphically variable head tagma and adaptation to a specialized mode of life. Agnostinids, as small nektobenthic detritus feeders and opportunists, present an example of a clade of artiopodans occupying a niche in the Early Palaeozoic which was subsequently overtaken by mandibulates. Taken together, these findings hint that considerable morphological and ecological diversity among artiopodans may still await discovery, particularly with continued study of Burgess Shale-type material.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Javier Ortega-Hernández and two anonymous reviewers for their constructive remarks which greatly improved the current manuscript. We thank Danielle Dufault for the technical drawing and life reconstruction, Sharon Lackie for elemental maps and Maryam Akrami and Peter Fenton for collections assistance. J.M. thanks Cédric Aria and Karma Nanglu for their patient mentorship, and Robert Gaines, Alejandro Izquierdo López, Luke Parry and Martin Smith for their helpful comments. The material from the Royal Ontario Museum was collected under several Parks Canada Research and Collections permits to Desmond Collins and J.-B.C.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
Funding comes from a NSERC Discovery grant (no. 341944) to J.-B.C. and the Dorothy Strelsin Foundation. This is Royal Ontario Museum Burgess Shale project number 82.
References
- 1.Peng S, Robison RA. 2000. Agnostoid biostratigraphy across the middle-Upper Cambrian boundary in Hunan, China. Mem. (Paleontol. Soc.) 53, 1–104. [Google Scholar]
- 2.Shergold JH, Laurie JR, Sun X. 1990. Classification and review of the Trilobite Order, Agnostida Salter, 1864: An Australian perspective. Report 296. Canberra, Australia: Australian Government Publishing Service.
- 3.Shergold JH. 1991. Protaspid and early meraspid growth stages of the eodiscoid trilobite Pagetia ocellata Jell, and their implications for classification. Alcheringa 15, 65–86. ( 10.1080/03115519108619010) [DOI] [Google Scholar]
- 4.Fortey RA. 2001. Trilobite systematics: The last 75 years. J. Paleontol. 75, 1141–1151. ( 10.1017/S0022336000017194) [DOI] [Google Scholar]
- 5.Cotton TJ, Braddy SJ. 2003. The phylogeny of arachnomorph arthropods and the origin of the Chelicerata. Trans. R. Soc. Edinb. Earth Sci. 94, 169–193. ( 10.1017/S0263593300000596) [DOI] [Google Scholar]
- 6.Bruton D, Nakrem H. 2005. Enrolment in a Middle Ordovician agnostoid trilobite. Acta Palaeontol. Pol. 50, 441–448. [Google Scholar]
- 7.Cotton TJ, Fortey RA. 2005. Comparative morphology and relationships of the agnostida. In Crustacea and arthropod relationships (eds Koenemann S, Jenner R), pp. 95–136. Boca Raton, FL: CRC Press. [Google Scholar]
- 8.Fortey RA, Theron JN. 1995. A new Ordovician arthropod, Soomaspis, and the agnostid problem. Palaeontology 37, 841–862. [Google Scholar]
- 9.Müller KJ, Walossek D. 1987. Morphology, ontogeny, and life habit of Agnostus pisiformis from the Upper Cambrian of Sweden. Foss. Strat. 19, 1–124. [Google Scholar]
- 10.Waloszek D, Müller KJ. 1990. Upper Cambrian stem-lineage crustaceans and their bearing upon the monophyletic origin of Crustacea and the position of Agnostus. Lethaia 23, 409–427. ( 10.1111/j.1502-3931.1990.tb01373.x) [DOI] [Google Scholar]
- 11.Bergström J. 1992. The oldest arthropods and the origin of the Crustacea. Acta Zool. 73, 287–291. ( 10.1111/j.1463-6395.1992.tb01093.x) [DOI] [Google Scholar]
- 12.Bergström J, Hou X. 2005. Early Palaeozoic non-lamellipedian arthropods. Crustac. Arthropod Relationships 16, 73–94. ( 10.1201/9781420037548.ch4) [DOI] [Google Scholar]
- 13.Stein M, Waloszek D, Maas A. 2005. Oelandocaris oelandica and the stem lineage of Crustacea. In Crustacea and arthropod relationships (eds Koenemann S, Jenner R), pp. 55–72. Boca Raton, FL: CRC Press. [Google Scholar]
- 14.Haug JT, Maas A, Waloszek D. 2009. Henningsmoenicaris scutula, †Sandtorpia vestrogothiensis gen. et sp. nov. and heterochronic events in early crustacean evolution. Earth Environ. Sci. Trans. R. Soc. Edinburgh 100, 311–350. ( 10.1017/S1755691010008145) [DOI] [Google Scholar]
- 15.Edgecombe GD. 2017. Inferring arthropod phylogeny: Fossils and their interaction with other data sources. Integr. Comp. Biol. 57, 467–476. ( 10.1093/icb/icx061) [DOI] [PubMed] [Google Scholar]
- 16.Vannier J, Aria C, Taylor RS, Caron JB. 2018. Waptia fieldensis Walcott, a mandibulate arthropod from the middle Cambrian Burgess Shale. R. Soc. open sci. 5, 172206 ( 10.1098/rsos.172206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boxshall GA. 2007. Crustacean classification: On-going controversies and unresolved problems. Zootaxa 1668, 313–1325. [Google Scholar]
- 18.Eriksson ME, Horn E. 2017. Agnostus pisiformis—a half a billion-year old pea-shaped enigma. Earth-Science Rev. 173, 65–76. ( 10.1016/j.earscirev.2017.08.004) [DOI] [Google Scholar]
- 19.Naimark EB, Pegel TV. 2017. Revision of the Cambrian Agnostina (Trilobita?) from Russia. Paleontol. J. 51, 1167–1248. ( 10.1134/S0031030117110016) [DOI] [Google Scholar]
- 20.Naimark EB. 2012. Hundred species of the genus Peronopsis Hawle et Corda, 1847. Paleontol. J. 46, 945–1057. ( 10.1134/S0031030112090018) [DOI] [Google Scholar]
- 21.Ronquist F, et al. 2012. Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. ( 10.1093/sysbio/sys029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis PO. 2001. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 50, 913–925. ( 10.1080/106351501753462876) [DOI] [PubMed] [Google Scholar]
- 23.Maddison WP, Maddison DR. 2018. Mesquite: a modular system for evolutionary analysis. Version 3.51.
- 24.Butterfield NJ. 2002. Leanchoilia guts and the interpretation of three-dimensional structures in Burgess Shale-type fossils. Paleobiology 28, 155–171. () [DOI] [Google Scholar]
- 25.Aria C, Caron JB, Gaines R. 2015. A large new leanchoiliid from the Burgess Shale and the influence of inapplicable states on stem arthropod phylogeny. Palaeontology 58, 629–660. ( 10.1111/pala.12161) [DOI] [Google Scholar]
- 26.Whittington HB. 1975. Exoskeleton, moult stage, appendage morphology, and habits of the Middle Cambrian trilobite Olenoides serratus. Palaeontology 23, 171–204. [Google Scholar]
- 27.Whittington HB. 1977. The Middle Cambrian trilobite, Naraoia, Burgess Shale, British Columbia. Phil. Trans. R. Soc. Lond. B 280, 409–443. ( 10.1098/rstb.1977.0117) [DOI] [Google Scholar]
- 28.Aria C, Caron JB. 2015. Cephalic and limb anatomy of a new isoxyid from the Burgess Shale and the role of ‘stem bivalved arthropods' in the disparity of the frontalmost appendage. PLoS ONE 10, e0124979 ( 10.1371/journal.pone.0124979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Öpik AA. 1959. Genal caeca of agnostids. Nature 183, 1750 ( 10.1038/1831750a0) [DOI] [Google Scholar]
- 30.Laurie JR. 1988. Revision of some Australian Ptychagnostinae (Agnostida, Cambrian). Alcheringa 12, 169–205. ( 10.1080/03115518808619132) [DOI] [Google Scholar]
- 31.Vannier J, Chen JY. 2002. Digestive system and feeding mode in Cambrian naraoiid arthropods. Lethaia 35, 107–120. ( 10.1080/002411602320183971) [DOI] [Google Scholar]
- 32.Bergström J, Hou XG, Hålenius U. 2007. Gut contents and feeding in the Cambrian arthropod Naraoia. GFF 129, 71–76. ( 10.1080/11035890701292071) [DOI] [Google Scholar]
- 33.Vannier J, Liu J, Lerosey-Aubril R, Vinther J, Daley AC. 2014. Sophisticated digestive systems in early arthropods. Nat. Commun. 5, 3641 ( 10.1038/ncomms4641) [DOI] [PubMed] [Google Scholar]
- 34.Lerosey-Aubril R. 2015. Notchia weugi gen. et sp. nov.: A new short-headed arthropod from the Weeks Formation Konservat-Lagerstatte (Cambrian; Utah). Geol. Mag. 152, 351–357. ( 10.1017/S0016756814000375) [DOI] [Google Scholar]
- 35.Mayers B, Aria C, Caron JB. 2018. Three new naraoiid species from the Burgess Shale, with a morphometric and phylogenetic reinvestigation of Naraoiidae. Palaeontology 62, 1–32. [Google Scholar]
- 36.Lerosey-Aubril R, Hegna TA, Kier C, Bonino E, Habersetzer J, Carré M. 2012. Controls on gut phosphatisation: The trilobites from the Weeks Formation Lagerstätte (Cambrian; Utah). PLoS ONE 7, e32934 ( 10.1371/journal.pone.0032934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cisne J. 1975. Anatomy of Triarthrus and the relationships of the Trilobita. Foss. Strat. 4, 45–63. [Google Scholar]
- 38.Fortey RA. 1974. The Ordovician trilobites of Spitsbergen I. Olenidae. Nor. Polarinstitutt Skr. Oslo 160, 1–129. [Google Scholar]
- 39.Jell PA. 1978. Trilobite respiration and genal caeca. Alcheringa 2, 251–260. ( 10.1080/03115517808527783) [DOI] [Google Scholar]
- 40.Suzuki Y, Bergström J. 2008. Respiration in trilobites: a reevaluation. GFF 130, 211–229. ( 10.1080/11035890809452774) [DOI] [Google Scholar]
- 41.Stein M, Selden PA. 2012. A restudy of the Burgess Shale (Cambrian) arthropod Emeraldella brocki and reassessment of its affinities. J. Syst. Palaeontol. 10, 361–383. ( 10.1080/14772019.2011.566634) [DOI] [Google Scholar]
- 42.Öpik AA. 1979. Middle Cambrian agnostids: systematics and biostratigraphy. Canberra, Australia: Bureau of Mineral Resources.
- 43.Fortey RA. 1980. The Ordovician trilobites of Spitsbergen. III. Remaining trilobites of the Valhallfonna Formation. Nor. Polarinstitutt Skr. Oslo 171, 1–113. [Google Scholar]
- 44.Aria C, Caron JB. 2017. Mandibulate convergence in an armoured Cambrian stem chelicerate. BMC Evol. Biol. 17, 261 ( 10.1186/s12862-017-1088-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maas A, Waloszek D. 2005. Phosphatocopina: Ostracode-like sister group of Eucrustacea. Hydrobiologia 538, 139–152. ( 10.1007/s10750-004-4944-6) [DOI] [Google Scholar]
- 46.Aria C, Caron JB. 2017. Burgess Shale fossils illustrate the origin of the mandibulate body plan. Nature 545, 89–92. ( 10.1038/nature22080) [DOI] [PubMed] [Google Scholar]
- 47.Stein M, Waloszek D, Maas A, Haug JT, Müller KJ. 2008. The stem crustacean Oelandocaris oelandica re-visited. Acta Palaeontol. Pol. 53, 461–484. ( 10.4202/app.2008.0308) [DOI] [Google Scholar]
- 48.Zeng H, Zhao F, Yin Z, Zhu M. 2017. Appendages of an early Cambrian metadoxidid trilobite from Yunnan, SW China support mandibulate affinities of trilobites and artiopods. Geol. Mag. 154, 1306–1328. ( 10.1017/S0016756817000279) [DOI] [Google Scholar]
- 49.Stein M. 2013. Cephalic and appendage morphology of the Cambrian arthropod Sidneyia inexpectans Walcott, 1911. Zool. Anz. 253, 164–178. ( 10.1016/j.jcz.2013.05.001) [DOI] [Google Scholar]
- 50.Scholtz G, Edgecombe GD. 2006. The evolution of arthropod heads: Reconciling morphological, developmental and palaeontological evidence. Dev. Genes Evol. 216, 395–415. ( 10.1007/s00427-006-0085-4) [DOI] [PubMed] [Google Scholar]
- 51.Zhang XG, Siveter DJ, Waloszek D, Maas A. 2007. An epipodite-bearing crown-group crustacean from the Lower Cambrian. Nature 449, 595–599. ( 10.1038/nature06138) [DOI] [PubMed] [Google Scholar]
- 52.Hughes NC. 2003. Trilobite tagmosis and body patterning from morphological and developmental perspectives. Integr. Comp. Biol. 43, 185–206. ( 10.1093/icb/43.1.185) [DOI] [PubMed] [Google Scholar]
- 53.Scholtz G, Edgecombe GD. 2005. Heads, hox and the phylogenetic position of trilobites. In Crustacea and arthropod relationships (eds Koenemann S, Jenner R), pp. 139–166. Boca Raton, FL: CRC Press. [Google Scholar]
- 54.Park TYS, Kihm JH. 2017. Head segmentation of trilobites. Lethaia 50, 1–6. ( 10.1111/let.12187) [DOI] [Google Scholar]
- 55.Ortega-Hernández J, Legg DA, Braddy SJ. 2013. The phylogeny of aglaspidid arthropods and the internal relationships within Artiopoda. Cladistics 29, 15–45. ( 10.1111/j.1096-0031.2012.00413.x) [DOI] [PubMed] [Google Scholar]
- 56.Lerosey-Aubril R, Zhu X, Ortega-Hernández J. 2017. The Vicissicaudata revisited: insights from a new aglaspidid arthropod with caudal appendages from the Furongian of China. Sci. Rep. 7, 11117 ( 10.1038/s41598-017-11610-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hou X, Bergström J. 1997. Arthropods of the Lower Cambrian Chengjiang fauna, southwest China. Oslo, Norway: Scandinavian University Press. [Google Scholar]
- 58.Legg DA, Sutton MD, Edgecombe GD. 2013. Arthropod fossil data increase congruence of morphological and molecular phylogenies. Nat. Commun. 4, 3485 ( 10.1038/ncomms3485) [DOI] [PubMed] [Google Scholar]
- 59.Zhang X-L, Shu D-G, Erwin DH. 2007. Cambrian naraoiids (Arthropoda): morphology, ontogeny, systematics, and evolutionary relationships. Paleontol. Soc. Mem. 81, 1–52. ( 10.1666/06-082.1) [DOI] [Google Scholar]
- 60.Ramsköld L, Junyuan C, Edgecombe GD, Guiqing Z. 1997. Cindarella and the arachnate clade Xandarellida (Arthropoda, Early Cambrian) from China. Trans. R. Soc. Edinburgh, Earth Sci. 88, 19–38. ( 10.1017/S0263593300002297) [DOI] [Google Scholar]
- 61.Briggs DEG, Bruton DL, Whittington HB. 1979. Appendages of the arthropod Aglaspis spinifer (Upper Cambrian, Wisconsin) and their significance. Palaeontology 22, 167–179. [Google Scholar]
- 62.Zhang X, Shu D. 2005. A new arthropod from the Chengjiang Lagerstätte, early Cambrian, southern China. Alcheringa 29, 185–194. ( 10.1080/03115510508619300) [DOI] [Google Scholar]
- 63.Kühl G, Rust J. 2010. Re-investigation of Mimetaster hexagonalis: a marrellomorph arthropod from the Lower Devonian Hunsrück Slate (Germany). Palaontologische Zeitschrift 84, 397–411. ( 10.1007/s12542-009-0049-x) [DOI] [Google Scholar]
- 64.Shear WA, Edgecombe GD. 2010. The geological record and phylogeny of the Myriapoda. Arthropod Struct. Dev. 39, 174–190. ( 10.1016/j.asd.2009.11.002) [DOI] [PubMed] [Google Scholar]
- 65.Dunlop JA, Lamsdell JC. 2017. Segmentation and tagmosis in Chelicerata. Arthropod. Struct. Dev. 46, 395–418. ( 10.1016/j.asd.2016.05.002) [DOI] [PubMed] [Google Scholar]
- 66.Fortey RA, Owens RM. 1999. Feeding habits in trilobites. Palaeontology 42, 429–465. ( 10.1111/1475-4983.00080) [DOI] [Google Scholar]
- 67.Martin JW, Olesen J, Høeg JT, Høeg J (eds). 2014. Atlas of crustacean larvae. Baltimore, MD: JHU Press. [Google Scholar]
- 68.Maas A, Haug C, Haug JT, Olesen J, Zhang X-GX, Waloszek D. 2009. Early crustacean evolution and the appearance of epipodites and gills. Arthropod Syst. Phylogeny 67, 273. [Google Scholar]
- 69.Rex MA. 1981. Community structure in the deep-sea benthos. Annu. Rev. Ecol. Syst. 12, 331–353. ( 10.1146/annurev.es.12.110181.001555) [DOI] [Google Scholar]
- 70.Chatterton BDE, Collins DH, Ludvigsen R. 2003. Cryptic behaviour in trilobites: Cambrian and Silurian examples from Canada, and other related occurrences. Special Papers in Palaeontology 70, 157–174. [Google Scholar]
- 71.Fatka O, Vokáč V, Moravec J, Šinágl M, Valent M. 2009. Agnostids entombed in hyolith conchs. Mem. Assoc. Australas. Palaeontol. 37, 481–489. [Google Scholar]
- 72.Mángano MG, Bromley RG, Harper DAT, Nielsen AT, Smith MP, Vinther J. 2012. Nonbiomineralized carapaces in Cambrian seafloor landscapes (Sirius Passet, Greenland): opening a new window into early Phanerozoic benthic ecology. Geology 40, 519–522. ( 10.1130/G32853.1) [DOI] [Google Scholar]
- 73.Webster M. 2015. Ontogeny and intraspecific variation of the early Cambrian trilobite Olenellus gilberti, with implications for olenelline phylogeny and macroevolutionary trends in phenotypic canalization. J. Syst. Palaeontol. 13, 1–74. ( 10.1080/14772019.2013.852903) [DOI] [Google Scholar]
- 74.Hughes NC. 2007. The evolution of trilobite body patterning. Annu. Rev. Earth Planet. Sci. 35, 401–434. ( 10.1146/annurev.earth.35.031306.140258) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.