Abstract
A combination of genetic and environmental factors contributes to schizophrenia (SZ), a catastrophic psychiatric disorder with a hypothesized neurodevelopmental origin. Increases in the brain levels of the tryptophan metabolite kynurenic acid (KYNA), an endogenous antagonist of α7 nicotinic acetylcholine and NMDA receptors, have been implicated specifically in the cognitive deficits seen in persons with SZ. Here we evaluated this role of KYNA by adding the KYNA precursor kynurenine (100 mg/day) to chow fed to pregnant rat dams from embryonic day (ED) 15 to ED 22 (control: ECon; kynurenine treated: EKyn). Upon termination of the treatment, all rats received normal rodent chow until the animals were evaluated in adulthood (postnatal days 56–85). EKyn treatment resulted in increased extracellular KYNA and reduced extracellular glutamate in the hippocampus, measured by in vivo microdialysis, and caused impairments in hippocampus-dependent learning in adult rats. Acute administration of BFF816, a systemically active inhibitor of kynurenine aminotransferase II (KAT II), the major KYNA-synthesizing enzyme in the brain, normalized neurochemistry and prevented contextual memory deficits in adult EKyn animals. Collectively, these results demonstrate that acute inhibition of KYNA neosynthesis can overcome cognitive impairments that arise as a consequence of elevated brain KYNA in early brain development.
Cognitive dysfunctions, including deficits in hippocampus-mediated explicit memory and learning, are a core domain of the psychopathology of several mental disorders, including schizophrenia (SZ) (Tamminga et al., 2010). These impairments appear to be related to glutamatergic and nicotinergic abnormalities, and excessive inhibition of NMDA and α7 nicotinic acetylcholine (α7nACh) receptors are believed to play critical roles in these functional deficits (Levin et al., 2006; Robbins and Murphy, 2006). Kynurenic acid (KYNA), a metabolite of the kynurenine pathway (KP) of tryptophan degradation, antagonizes both these receptors and may thus modulate cognitive processes (Pocivavsek et al., 2016). As KYNA levels are significantly elevated in post-mortem brain tissue and cerebrospinal fluid of patients with SZ (Erhardt et al., 2001; Schwarcz et al., 2001), inhibition of the enzyme kynurenine aminotransferase (KAT) II, which is primarily responsible for the formation of neuroactive KYNA in the brain, has emerged as an attractive novel strategy to overcome cognitive deficits in the disease (Chang et al., 2018; Jayawickrama et al., 2015; Kozak et al., 2014; Pocivavsek et al., 2011; Wu et al., 2014).
In adult rats, even modest increases in hippocampal KYNA cause a reduction in the extracellular levels of glutamate via inhibition of presynaptic α7nACh receptors located on glutamatergic terminals, and may therefore contribute to the cognitive impairments that are observed in these animals (Pocivavsek et al., 2011). Notably, and in line with leading hypotheses of SZ etiology (Murray and Lewis, 1987; Weinberger, 1987), the influence of KYNA on neurochemistry and behavior may also have a developmental dimension (Notarangelo and Pocivavsek, 2017). Thus, we demonstrated that raising KYNA levels in the fetal brain during the last week of gestation leads to elevated brain KYNA levels and impairs hippocampus-dependent memory and other cognitive functions in adulthood (Notarangelo and Pocivavsek, 2017; Pershing et al., 2015;Pocivavsek et al., 2014). Conceptually similar results were obtained, and identical conclusions were reached, independently by Stone and his collaborators (Forrest et al., 2013; Forrest et al., 2015).
Based on these findings, the present study was designed to test the hypothesis that acute inhibition of cerebral KYNA formation in adulthood, which does not affect the production of related metabolites such as 3-hydroxykynurenine and quinolinic acid (Amori et al., 2009), may overcome the learning impairments seen in animals that had received excessive amounts of KYNA’s bioprecursor kynurenine in utero (“EKyn” rats; Pocivavsek et al., 2014). Reduction in KYNA levels was achieved pharmacologically by administering the orally active KAT II inhibitor BFF816 (Wu et al., 2014). The behavioral effects of this treatment were tested by applying the compound either prior to memory consolidation or prior to memory retrieval in the passive avoidance paradigm (PAP), a contextual memory task that relies on the functional integrity of the hippocampus (Dobryakova et al., 2015; Khakpai et al., 2016), a region known to be involved in the psychopathology of schizophrenia (Tamminga et al., 2010). The PAP was also selected because reductions in glutamate, as well as inhibition of α7nACh and NMDA receptors, worsen performance in the task (Roesler et al., 1998; Marubio and Paylor, 2004), and because our previous work had demonstrated that elevations in brain KYNA, either during prenatal development (Pocivavsek et al., 2014; Notarangelo and Pocivavsek, 2017) or induced acutely in adulthood (Pocivavsek et al., 2017), impairs performance in this paradigm.
Pregnant Wistar rats (gestational age: 2 days) were obtained from Charles River Laboratories. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Maryland School of Medicine and followed the ‘Principles of Laboratory Animal Care’ (NIH publication No. 86–23, 1996). Animals were housed in a temperature-controlled facility, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), at the Maryland Psychiatric Research Center. The rats were kept on a 12h/12h light dark cycle and had free access to food and water.
Beginning on embryonic day (ED) 15, animals were fed daily with 100 mg of kynurenine (L-kynurenine sulfate, “kynurenine”; purity 99.4%, Sai Advantium, Hyderabad, India), which was mixed with wet rodent chow (embryonic kynurenine: EKyn), as previously described (Pocivavsek et al., 2014). Control animals (embryonic control: ECon) received wet mash alone. Dams were fed the diet daily from ED 15 to ED 22. After giving birth, each dam received normal rodent chow pellets ad libitum. On postnatal day (PD) 2, each ECon and EKyn litter was cross-fostered such that half of the pups were cared for by a dam from the alternate treatment condition. Male offspring were weaned on PD 21, pair-housed, and given normal rodent chow pellets ad libitum. To minimize the contribution of individual prenatal litters, a maximum of 3 offspring per litter were used in the experiments. Offspring were tested from PD 56 to PD 85 by an experimenter who was blind to the treatment conditions.
In the first experiment, microdialysis was conducted in the dorsal hippocampus of awake, freely moving animals, as previously described (Wu et al., 2014). Briefly, rats underwent a survival surgery procedure to position a guide cannula (outer diameter: 0.65 mm) over the dorsal hippocampus (AP: −3.4 from bregma; L: ±2.3 mm from the midline; V: 1.5 mm below the dura). On the next day, a microdialysis probe (CMA/10, membrane length: 2 mm; Carnegie Medicin AB, Stockholm, Sweden) was inserted through the guide cannula, and the animals were perfused with Ringer solution (pH 6.7) at a flow rate of 1 μl/min. The first 30-min fraction was discarded, and the next four 30 min fractions were collected to obtain baseline values. BFF816 (30 mg/kg, dissolved in 10% cyclodextrin, adjusted to pH 8.0) (kindly provided by Dr. H. Yasumatsu, Mitsubishi-Tanabe Pharma Corporation, Yokohama, Japan) was then administered by oral gavage (p.o.), and an additional 12 consecutive 30-min fractions were collected. Extracellular concentrations of both KYNA (excitation: 344 nm; emission: 398 nm) and glutamate (excitation: 390; emission: 460 nm) in the microdialysates were determined fluorimetrically by high performance liquid chromatography (HPLC), according to previously published methods (Wu et al., 2014), using 15 μl of the microdialysate per analyte. Data were not corrected for recovery from the dialysis probe.
Basal extracellular KYNA levels were modestly but significantly elevated in adult EKyn rats (ECon: 2.3 ± 0.1 nM; EKyn: 2.8 ± 0.1 nM; t (13) = 3.72; Student’s t test, P < 0.01) (Figure 1A). In contrast, extracellular glutamate was decreased (ECon: 1.9 ± 0.03 μM; EKyn 1.6 ± 0.02 μM; t (7) = 7.47; Student’s t test, P < 0.001) (Figure 1B). BFF816 rapidly lowered extracellular KYNA levels in both ECon (to 29% below baseline) and EKyn (to 25% below baseline) rats (two-way repeated measures ANOVA, main effect of BFF816, F15,195 = 29.62, P < 0.0001) (Figure 2A). Notably, the treatment reduced KYNA in EKyn animals to 2.1 ± 0.1 nM, i.e. to levels that were not different from the baseline measured in ECon animals (P = 0.1). These results demonstrate that the elevation of KYNA seen in adult EKyn offspring can be overcome acutely with a single administration of a selective KAT II inhibitor. The reduction in KYNA lasted for only 4–5 hours after the administration of BFF816, and levels then reverted to their respective baseline in both ECon and EKyn animals. Measured in the same microdialysates as KYNA, glutamate levels were found to be elevated in both ECon (to 189% above baseline) and EKyn (to 169% above baseline) rats following the administration of BFF816 (two-way repeated measures ANOVA, main effect of BFF816: F15,105 = 43.83, P < 0.0001; BFF816 x prenatal condition interaction: F15,105 = 2.0, P < 0.05), and this effect, too, subsided after 4 hours (Figure 2B). Of note, the BFF816-induced increase in extracellular glutamate, though slightly larger in ECon than in EKyn animals, was not statistically different between the two groups (main effect of prenatal condition: F1,7 = 1.96, P = 0.20).
Figure 1:
Effects of prenatal kynurenine treatment on extracellular KYNA and glutamate levels in the dorsal hippocampus of adult animals. Pregnant rats were exposed to kynurenine (100 mg/day) from ED 15–22 (EKyn). ECon: control rats fed normal chow prenatally. In adulthood (PD 56–85), 30-min microdialysate samples were collected consecutively for 2 hours. Averaged individual data as well as the mean (± SEM) are shown for extracellular KYNA (N = 7–8 per group) (A) and glutamate (N = 4–5 per group) (B). Student’s t test. ** P < 0.01, *** P < 0.001.
Figure 2:
Kynurenine aminotransferase II inhibition by BFF816 (30 mg/kg, p.o.), administered 2 hours after baseline collection (arrow), results in an acute reduction in extracellular KYNA and an acute increase in extracellular glutamate levels in the rat hippocampus. Pregnant rats were exposed to kynurenine (100 mg/day) from ED 15–22 (EKyn). ECon: control rats fed normal chow prenatally. In adulthood (PD 56–85), 30-min microdialysate samples were collected from the dorsal hippocampus. Data (mean ± SEM) are shown for extracellular KYNA (N = 7–8 per group) (A) and glutamate (N = 4–5 per group) (B). Bonferroni corrected paired t test vs. basal levels. ECon: * P < 0.05; EKyn: # P < 0.05.
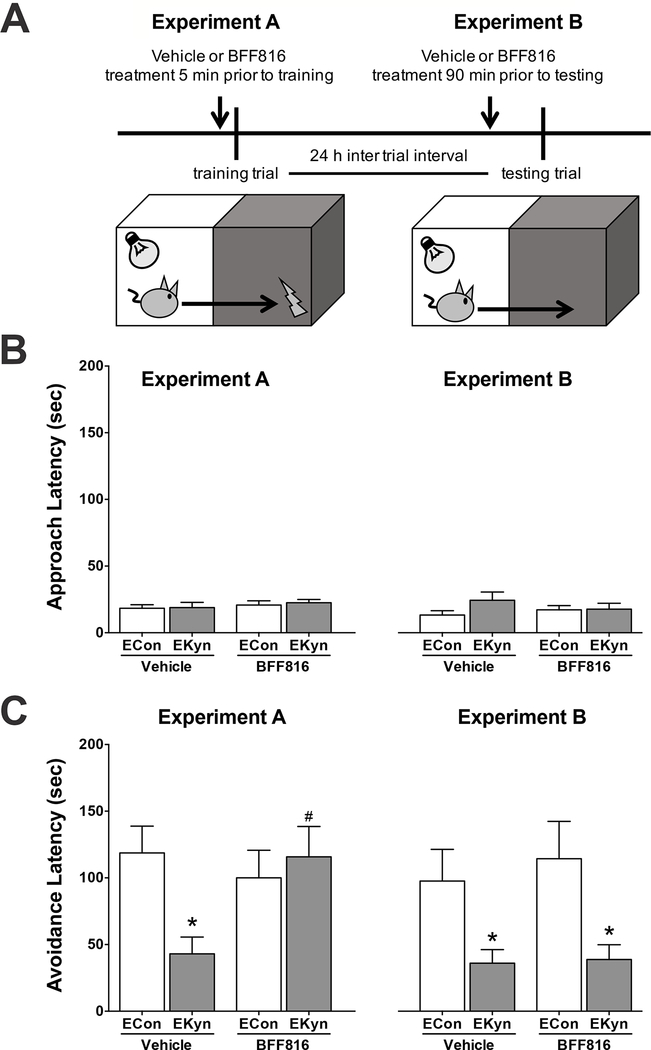
Behavior experiments were conducted in separate cohorts of adult offspring. Briefly, the PAP apparatus had two compartments of equal size (each 22 cm high, 18 cm wide and 16 cm deep), which were separated by a guillotine door. The right compartment was illuminated, and the left compartment remained dark. During the training trial, each animal was placed in the illuminated compartment, the door was opened, and the “approach latency” to enter the preferred dark compartment was recorded. Immediately upon entering, the door was closed, and an inescapable food shock (0.56 mA for 1 sec) was delivered through the grid floor (see cartoon; Figure 3A). Retention of the newly formed, one-trial, aversive foot shock memory was tested 24 hours later, when the rat was again placed in the illuminated compartment and the “avoidance latency”, i.e. the time to enter the dark compartment, was recorded.
Figure 3.
Administration of the kynurenine aminotransferase II inhibitor BFF816 (30 mg/kg, p.o.) prior to contextual memory task training attenuates memory impairment in adult EKyn rats. Rats were exposed to kynurenine (100 mg/day) from ED 15–22. In adulthood (PD 56–85), animals were tested in the passive avoidance paradigm. (A) Schematic: in Experiment A, animals received vehicle or BFF816 5 min prior to the training trial; in Experiment B, animals received vehicle or BFF816 90 min prior to the test trial; (B) Approach latency during the training trial; (C) Avoidance latency during the testing trial. Data are the mean ± SEM. N = 10–17 per group. Holm-Sikad’s multiple comparison t test. * P < 0.05 vs. ECon. # P < 0.05 vs EKyn vehicle.
This PAP was used to evaluate whether KYNA synthesis inhibition affects the consolidation of newly formed memory and/or memory retrieval. To this end, animals received BFF816 (30 mg/kg) or vehicle (10% cyclodextrin), applied by oral gavage (p.o.) at a concentration of 10 mg BFF816/ml, either 5 min prior to the training trial (Experiment A) or 90 min prior to the testing trial (Experiment B). Based on the biochemical results (Figure 2), this experimental design assured that extracellular KYNA was reduced both during consolidation of newly formed memory in Experiment A and during the retention of memory in Experiment B. There were no differences in approach latency between the various experimental groups (Figure 3B), demonstrating identical baseline performance during the training trial and intact acquisition. Importantly, task performance and the ability to significantly learn were not different in ECon offspring treated with either vehicle or BFF816 in both Experiment A and Experiment B. However, in line with previous studies (Notarangelo and Pocivavsek, 2017; Pocivavsek et al., 2014), vehicle-treated EKyn offspring showed significantly lower avoidance latency than ECon offspring during the retention trial (Student’s t test, ECon vehicle vs. EKyn vehicle, P < 0.05) (Figure 3C). In contrast, EKyn offspring which had received BFF816 immediately prior to training demonstrated no such learning impairment during the retrieval test on Day 2 (two-way ANOVA, BFF816 x prenatal condition interaction: F1,60 = 5.25, P < 0.05), indicating that an acute reduction in KYNA during the memory acquisition phase was sufficient to overcome the cognitive deficit in these animals. Importantly, when KYNA levels were reduced only during the test trial, i.e. on Day 2, EKyn offspring remained significantly impaired in their PAP performance (two-way ANOVA, main effect of prenatal condition: F1,41 = 12.61, P < 0.01). These findings suggest a selective role for KYNA in the acquisition and consolidation of contextual memory.
Using the offspring of ECon and EKyn rats, the present study not only revealed that prenatal kynurenine treatment leads to a significant elevation in extracellular KYNA levels in the adult hippocampus, but also showed that extracellular glutamate levels are simultaneously reduced in the same samples. These results are in line with previous demonstrations that acute fluctuations in KYNA levels reciprocally and bi-directionally influence extracellular glutamate levels in the adult rat brain (Konradsson-Geuken et al., 2010; Pocivavsek et al., 2011). This regulation of glutamate by KYNA, which may be mainly related to KYNA’s inhibition of presynaptic α7nACh receptors on glutamatergic nerve terminals, has been repeatedly proposed to be causally linked to the various behavioral changes that are observed in animals when KYNA levels are manipulated experimentally (Alexander et al., 2012; Chess et al., 2007; Pocivavsek et al., 2011; Pocivavsek et al., 2017; Potter et al., 2010).
As several of the impairments observed in adult EKyn rats resemble neurochemical and behavioral changes seen in persons with SZ (Tamminga et al., 2012; Wijtenburg et al., 2017), these animals have considerable construct validity in SZ research (see Pocivavsek et al., 2016, for review). The present demonstration that acute inhibition of KYNA synthesis prior to training is sufficient to overcome a translationally relevant learning deficit in these animals substantiates a functionally significant role of KYNA in the disease. Interestingly, the two best-established targets of endogenous KYNA in the mammalian brain, NMDA and α7nACh receptors (Albuquerque and Schwarcz, 2013; Moroni et al., 2012; Stone et al., 2013), are both linked to the pathophysiology of SZ (Balu, 2016; Wallace and Bertrand, 2015) and are critically involved in hippocampal learning and memory (Levin et al., 2006; Robbins and Murphy, 2006). The BFF816-induced decrease in hippocampal KYNA levels shown here may therefore have improved memory consolidation by disinhibition of these receptors. The fact that a reduction in KYNA levels during the retention phase of the PAP failed to normalize the memory impairment in EKyn offspring provides further support for this hypothesis since intact cholinergic and glutamatergic activity is of critical importance specifically during the acquisition and consolidation of hippocampus-dependent memory (Dobryakova et al., 2015; Khakpai et al., 2016). Pharmacological agents targeting KAT II to reduce KYNA levels in the brain may therefore be a useful approach to treat cognitive dysfunctions in SZ (Chang et al., 2018; Jayawickrama et al., 2015; Kozak et al., 2014; Pocivavsek et al., 2011; Wu et al., 2014).
Acknowledgements
This work was supported by USPHS grants P50 MH103222, K12 HD43489-14, and R01 NS102209. We thank Dr. Hui-Qiu Wu for valuable technical contributions. The authors have no conflicts of interest.
References
- Albuquerque EX, Schwarcz R. 2013. Kynurenic acid as an antagonist of alpha7 nicotinic acetylcholine receptors in the brain: facts and challenges. Biochem Pharmacol 85(8):1027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KS, Wu HQ, Schwarcz R, Bruno JP. 2012. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology (Berl) 220(3):627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amori L, Guidetti P, Pellicciari R, Kajii Y, Schwarcz R. 2009. On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J Neurochem 109:316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT. 2016. The NMDA receptor and schizophrenia: from pathophysiology to treatment. Adv Pharmacol 76:351–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Fonseca KR, Li C, Horner W, Zawadzke LE, Salafia MA, Welch KA, Strick CA, Campbell BM, Gernhardt SS and others. 2018. Quantitative translational analysis of brain kynurenic acid modulation via irreversible kynurenine aminotransferase II inhibition. Mol Pharmacol 94(2):823–33. [DOI] [PubMed] [Google Scholar]
- Chess AC, Simoni MK, Alling TE, Bucci DJ. 2007. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull 33(3):797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobryakova YV, Gurskaya OY, Markevich VA. 2015. Administration of nicotinic receptor antagonists during the period of memory consolidation affects passive avoidance learning and modulates synaptic efficiency in the CA1 region in vivo. Neuroscience 284:865–71. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. 2001. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett 313(1–2):96–8. [DOI] [PubMed] [Google Scholar]
- Forrest CM, Khalil OS, Pisar M, McNair K, Kornisiuk E, Snitcofsky M, Gonzalez N, Jerusalinsky D, Darlington LG, Stone TW. 2013. Changes in synaptic transmission and protein expression in the brains of adult offspring after prenatal inhibition of the kynurenine pathway. Neuroscience 254:241–59. [DOI] [PubMed] [Google Scholar]
- Forrest CM, McNair K, Pisar M, Khalil OS, Darlington LG, Stone TW. 2015. Altered hippocampal plasticity by prenatal kynurenine administration, kynurenine-3-monoxygenase (KMO) deletion or galantamine. Neuroscience 310:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawickrama GS, Sadig RR, Sun G, Nematollahi A, Nadvi NA, Hanrahan JR, Gorrell MD, Church WB. 2015. Kynurenine aminotransferases and the prospects of inhibitors for the treatment of schizophrenia. Curr Med Chem 22(24):2902–18. [DOI] [PubMed] [Google Scholar]
- Khakpai F, Nasehi M, Zarrindast MR. 2016. The role of NMDA receptors of the medial septum and dorsal hippocampus on memory acquisition. Pharmacol Biochem Behav 143:18–25. [DOI] [PubMed] [Google Scholar]
- Konradsson-Geuken A, Wu HQ, Gash CR, Alexander KS, Campbell A, Sozeri Y, Pellicciari R, Schwarcz R, Bruno JP. 2010. Cortical kynurenic acid bi-directionally modulates prefrontal glutamate levels as assessed by microdialysis and rapid electrochemistry. Neuroscience 169(4):1848–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak R, Campbell BM, Strick CA, Horner W, Hoffmann WE, Kiss T, Chapin DS, McGinnis D, Abbott AL, Roberts BM and others. 2014. Reduction of brain kynurenic acid improves cognitive function. J Neurosci 34(32):10592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. 2006. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 184(3–4):523–39. [DOI] [PubMed] [Google Scholar]
- Marubio LM, Paylor R. 2004. Impaired passive avoidance learning in mice lacking central neuronal nicotinic acetylcholine receptors. Neuroscience 129(3):575–582. [DOI] [PubMed] [Google Scholar]
- Moroni F, Cozzi A, Sili M, Mannaioni G. 2012. Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery. J Neural Transm (Vienna) 119(2):133–9. [DOI] [PubMed] [Google Scholar]
- Murray RM, Lewis SW. 1987. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed) 295(6600):681–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarangelo FM, Pocivavsek A. 2017. Elevated kynurenine pathway metabolism during neurodevelopment: Implications for brain and behavior. Neuropharmacology 112(Pt B):275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pershing ML, Bortz DM, Pocivavsek A, Fredericks PJ, Jorgensen CV, Vunck SA, Leuner B, Schwarcz R, Bruno JP. 2015. Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: implications for schizophrenia. Neuropharmacology 90:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Baratta AM, Mong JA, Viechweg SS. 2017. Acute kynurenine challenge disrupts sleep-wake architecture and impairs contextual memory in adult rats. Sleep 40(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Notarangelo FM, Wu HQ, Bruno JP, Schwarcz R. 2016. Astrocytes as pharmacological targets in the treatment of schizohprenia: focus on kynurenic acid In: Pletnikov MV, Waddington JL, editors. Modeling the Psychophathological Dimensions of Schizophrenia - From Molecules to Behavior. San Diego: Elsevier; pp 423–43. [Google Scholar]
- Pocivavsek A, Thomas MA, Elmer GI, Bruno JP, Schwarcz R. 2014. Continuous kynurenine administration during the prenatal period, but not during adolescence, causes learning and memory deficits in adult rats. Psychopharmacology (Berl) 231(14):2799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Wu HQ, Potter MC, Elmer GI, Pellicciari R, Schwarcz R. 2011. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology 36(11):2357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter MC, Elmer GI, Bergeron R, Albuquerque EX, Guidetti P, Wu HQ, Schwarcz R. 2010. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology 35(8):1734–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Murphy ER. 2006. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol Sci 27(3):141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler T, Vianna M, Sant’Anna MK, Kuyyen CR, Kruel AV, Quevedo J, Ferreira MB. 1998. Intrahippocampal infusion of the NMDA receptor antagonist AP5 impairs retention of an inhibitory avoidance task: protection from impairment by pretraining or preexposure to the task apparatus. Neurobiol Learn Mem 69(2):87–91. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. 2001. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry 50(7):521–30. [DOI] [PubMed] [Google Scholar]
- Stone TW, Stoy N, Darlington LG. 2013. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol Sci 34(2):136–43. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Southcott S, Sacco C, Wagner AD, Ghose S. 2012. Glutamate dysfunction in hippocampus: relevance of dentate gyrus and CA3 signaling. Schizophr Bull 38(5):927–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. 2010. The hippocampal formation in schizophrenia. Am J Psychiatry 167(10):1178–93. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Bertrand D. 2015. Neuronal alpha7 nicotinic receptors as a target for the treatment of schizophrenia. Int Rev Neurobiol 124:79–111. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. 1987. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 44(7):660–9. [DOI] [PubMed] [Google Scholar]
- Wijtenburg SA, Wright SN, Korenic SA, Gaston FE, Ndubuizu N, Chiappelli J, McMahon RP, Chen H, Savransky A, Du X and others. 2017. Altered glutamate and regional cerebral blood flow levels in schizophrenia: A (1)H-MRS and pCASL study. Neuropsychopharmacology 42(2):562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HQ, Okuyama M, Kajii Y, Pocivavsek A, Bruno JP, Schwarcz R. 2014. Targeting kynurenine aminotransferase II in psychiatric diseases: promising effects of an orally active enzyme inhibitor. Schizophr Bull 40 Suppl 2:S152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]