This article reports data from the OPTIMIZE‐2 study and two phase I studies that examined the pharmacokinetics of zoledronic acid in patients with bone metastasis.
Keywords: Zoledronic acid, Long‐term dosing, Bone saturation, Pharmacokinetics, OPTIMIZE‐2
Abstract
Background.
Zoledronic acid (ZA), a potent bisphosphonate used for treatment of bone metastasis, has high bone affinity. This post hoc analysis evaluated the effects of long‐term treatment and reduction in dosing frequency of ZA on bone saturation.
Materials and Methods.
Pharmacokinetic data from three independent studies, OPTIMIZE‐2 (patients receiving ≥9 doses of bisphosphonates) and two phase I studies, CZOL4460503 and CZOL4460506 (patients who were bisphosphonate naïve/bisphosphonate free for ≥1 year after previous dosing), were pooled. Serial urine and plasma samples were used as surrogate markers to determine ZA plasma area under the curve (AUC) over 6 hours (AUC0–6h) and dose excreted in urine over 6 hours (urine0–6h). Potential relationships between the number of years for which patients had been treated previously at time of study entry and AUC0–6h or urine0–6h were analyzed graphically.
Results.
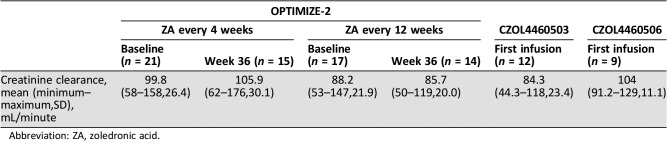
Creatinine clearances for patients were similar across the three studies and at all time points analyzed. The levels of AUC0–6h ZA in plasma at week 0 in every (q) 4 and q12 weekly arms of OPTIMIZE‐2 were 0.366 h × mg/L and 0.397 h × mg/L compared with 0.345 h × mg/L and 0.356 h × mg/L in CZOL4460503 and CZOL4460506, respectively. In OPTIMIZE‐2, the AUC0–6h ZA plasma levels were the same (0.428 h × mg/L) at week 36 in both q4 and q12 arms. The levels of ZA urine0–6h at week 36 in OPTIMIZE‐2 (q4 and q12 week arms), CZOL4460503, and CZOL4460506 were 36.6%, 30.8%, 26.5%, and 27.3%, respectively.
Conclusion.
Long‐term ZA treatment may not impact bone saturation, and ZA dosing frequency does not seem to influence drug retention rates.
Implications for Practice.
Zoledronic acid (ZA), used along with standard antineoplastic therapy to treat bone metastases associated with solid tumors and multiple myeloma, requires frequent (every 3–4 or every 12 weeks) long‐term administration. This may result in bone saturation and subsequently lead to a higher risk of adverse events such as osteonecrosis of the jaw and atypical fractures. This post hoc analysis used surrogate markers to demonstrate that prolonged ZA administration does not cause bone saturation. Furthermore, reduction in ZA dosing frequency does not affect its retention level in bones over time. These findings will help in addressing clinicians' concerns regarding prolonged ZA administration.
摘要
背景。唑来膦酸 (ZA) 是一种强效的二膦酸盐,用于治疗骨转移,具有较高的趋骨性。本事后分析评估了长期治疗和减少 ZA 给药频率对骨饱和的影响。
材料和方法。来自三项独立研究,包括 OPTIMIZE‐2(接受 ≥ 9 个剂量二磷酸盐的患者)和两项 I 期研究,即 CZOL4460503 和 CZOL4460506(既往给药后未使用二磷酸盐/无二磷酸盐 ≥ 1年的患者)的药代动力学数据汇集在一起。连续尿液和血浆样本用作替代指标,以测定 6 小时以上 ZA 血浆曲线下面积 (AUC)(AUC0–6 小时)和 6 小时以上尿液排出量(尿液 0–6 小时)。我们对研究开始时患者先前接受治疗的年数与 AUC0–6 小时或尿液 0–6 小时之间的潜在关系进行了图形分析。
结果。在三项研究中,以及在所分析的全部时间点上,患者的肌酐清除率相似。每 (q) 4 和 q12 周 OPTIMIZE‐2 治疗组在第 0 周的 AUC0–6 小时 ZA 血浆水平分别为 0.366 h × mg/L 和 0.397 h × mg/L,对比 CZOL4460503 和 CZOL4460506 为 0.345 h × mg/L 和 0.356 h × mg/L。OPTIMIZE‐2 中,q 4 和 q12 治疗组在第 36 周的 AUC0–6 小时 ZA 血浆水平相同 (0.428 h × mg/L)。第 36 周 OPTIMIZE‐2(q4 和 q12 周治疗组)、CZOL4460503 和 CZOL4460506 的 ZA 尿液 0–6 小时水平分别为 36.6%、30.8%、26.5% 和 27.3%。
结论。长期 ZA 治疗可能不会影响骨饱和,而 ZA 的给药频率似乎也不会影响药物保留率。
对实践的启示:唑来膦酸 (ZA) 与标准抗肿瘤疗法一起用于治疗与实体瘤和多发性骨髓瘤相关的骨转移,需要经常(每 3‐4 周或每 12 周)长期给药。这样可能会造成骨饱和,进而导致发生不良反应事件的较高风险,如颌骨坏死和非典型骨折。此项事后分析使用替代指标来证明延长 ZA 给药,其不会导致骨饱和。此外,降低 ZA 给药频率不会随着时间而影响其在骨骼中的吸药量水平。这些调查结果将有助于解决临床医生对长期服用 ZA 的担忧。
Introduction
Bone metastasis is one of the most common complications in patients with advanced solid tumors and affects up to 70% of patients with advanced breast cancer or prostate cancer and up to 40% of patients with other solid tumors [1], [2], [3], [4], [5]. It is associated with clinical sequelae, in particular skeletal‐related events (SREs) such as pathologic bone fracture, radiation therapy, or surgery to bone [4]. Enhanced osteoclastic bone resorption is central to the pathogenesis of SREs and hypercalcemia of malignancy associated with bone metastases [4].
Among the current available palliative therapies for bone metastasis, bisphosphonates have been established as an effective option for management of SREs [6]. Bisphosphonates have a high binding affinity for divalent cations, especially calcium, and consequently bind strongly to the hydroxyapatite of bone [7]. Zoledronic acid is the best‐in‐class amino bisphosphonate that has demonstrated efficacy in reducing SREs and delaying their onset in patients with advanced solid tumor cancer metastatic to bone [1], [6], [8]. It is structurally similar to pamidronate but is 100–850 times more potent and is associated with higher binding affinity to bone and higher antiresorptive potential [9], [10].
The major molecular target of zoledronic acid (ZA) is an enzyme in the mevalonate pathway, farnesyl pyrophosphate synthase (FPPS) in osteoclasts [11], [12], [13]. Inhibition of FPPS by ZA reduces the bone resorptive activity and promotes osteoclast apoptosis [12]. Following osteoclastic resorption of bone with ZA bound to it, ZA is internalized in osteoclasts, with subsequent FPPS inhibition [12]. ZA can persist for years in mineralized bone until it is released into systemic circulation once bone resorption occurs [14]. In various in vitro and in vivo studies, ZA has the largest therapeutic window between the desired inhibition of calcium resorption and the unwanted inhibition of mineralization [15]. Nevertheless, preclinical disposition studies of ZA show a high and persistent affinity for bone tissue, similar to that observed with other marketed bisphosphonates [16].
The pharmacokinetic profile of ZA demonstrated a multiphasic plasma disposition [17]. The peak plasma concentrations (Cmax) were reached at the end of the infusion period, followed by an early rapid decline (half‐lifet1 of 0.2 hours and half‐lifet2 of 1.4 hours) from the end of infusion to <1% of Cmax at 24 hours after the dose, and subsequent phases of very low concentrations between days 2 and 28 after the dose (half‐lifet3 of 39 hours and half‐lifet4 of 4,526 hours). The drug is primarily excreted by the kidney through glomerular filtration, and more than half of the injected dose of ZA binds to the hydroxyapatite of bone [4], [17].
ZA is administered intravenously at a dose of 4 mg infused over ≥15 minutes every (q) 3–4 weeks or q12 weeks over a prolonged duration, with many patients receiving continuous treatment for years and potentially decades [1], [18]. Due to ZA's high affinity to bone and rapid renal clearance, prolonged exposure to ZA may eventually result in accumulation of ZA in osteoclasts, which may subsequently lead to adverse events such as osteonecrosis of the jaw and atypical femur fracture [19], [20], [21], [22]. It was hypothesized that in case of bone saturation, a reduction in ZA dosing frequency would result in lower plasma and urine levels of ZA. This hypothesis was based on the theory that bisphosphonates have a very high affinity for bone mineral because they bind to hydroxyapatite crystals. Accordingly, bisphosphonate skeletal retention depends on the availability of hydroxyapatite binding sites [23]. Thus, the duration and optimal treatment schedule for patients receiving long‐term ZA is a topic of clinical interest, partly because of the potential for bone accumulation of ZA with prolonged dosing.
Here, we present a post hoc pharmacokinetic analysis of data from the OPTIMIZE‐2 (CZOL446E2352) study and data from two phase I studies (CZOL4460503 and CZOL4460506) that examined the pharmacokinetics of ZA in patients with bone metastasis [17], [18], [24]. Data included from OPTIMIZE‐2 was part of a substudy done to fulfill a U.S. Food and Drug Administration request to investigate the relationship between dose, pharmacokinetics, and rate of bone turnover. Because direct measurement of ZA levels in bone would require biopsy and would not be practical because of its invasive nature, we evaluated bone saturation levels using surrogate markers by measuring the levels of ZA in plasma and urine. The plasma and urine levels of ZA from patients who had received the very first dose of ZA (treatment‐naïve patients), or were bisphosphonate free for ≥1 year after previous dosing, and from patients who had been treated continuously with once‐monthly ZA for up to 7 years (OPTIMIZE‐2) were compared to evaluate the effect of long‐term ZA dosing on bone saturation. In addition, we evaluated the effect of reduction in dosing frequency (from q4 to q12 weeks in OPTIMIZE‐2) on retention of ZA.
Materials and Methods
Data from three different previously carried out studies (OPTIMIZE‐2, CZOL4460503, and CZOL4460506) were pooled in this post hoc pharmacokinetic analyses [17], [18], [24]. OPTIMIZE‐2 (NCT00320710) was a multicenter, randomized, double‐blind, phase III clinical trial. The study included female patients (aged ≥18 years) with histologically confirmed breast cancer, ≥1 radiologically confirmed bone metastasis, and life expectancy of ≥1 year. Patients with severe renal impairment were excluded. The trial compared the standard dosing frequency (4 mg q4 weeks) with reduced dosing frequency (4 mg q12 weeks) of ZA in patients who had received ≥9 doses of bisphosphonates intravenously. Patients with evaluable plasma and urine pharmacokinetic data on ≥1 of 2 scheduled days (the day of first infusion of study drug [baseline] and the day of the week 36 infusion; n = 38) were included in the present analysis. The levels of ZA in plasma and urine were assessed during the first 6 hours after dosing. Plasma sampling was done at 0, 0.25, 2, and 6 hours after the start of the infusion at baseline and 36 weeks. The lower limit of quantification for ZA in plasma was 0.4 ng/mL. Values below the lower limit of quantification of ZA in plasma were stated as 0. Urine sampling was done at 0, 0–2, and 2–6 hours after infusion at weeks 0 and 36. The lower limit of quantification for ZA in urine was 10 ng/mL. Values below the lower limit of quantification were stated as 0.
In the CZOL4460503 study, pharmacokinetics, pharmacodynamics, and safety of ZA were evaluated in 36 patients with cancer, who were either bisphosphonate naïve or did not receive bisphosphonate for ≥1 year [17]. The patients were randomly allocated to one of the three parallel groups to receive ZA intravenously as follows: 4 mg as a 5‐minute or a 15‐minute infusion, 8 mg as a 15‐minute infusion, and 16 mg as a 15‐minute infusion. Eligible patients had a serum creatinine ≤3.0 mg/dL. In this study, data from patients receiving 4 mg of ZA for either 5 minutes (n = 5) or 15 minutes (n = 7) were considered for comparison purposes with OPTIMIZE‐2. The difference in infusion time (5 minutes vs. 15 minutes) did not affect ZA plasma area under the curve (AUC). For comparison with OPTIMIZE‐2, ZA levels in plasma and urine were assessed during the 24‐hour period after dosing. ZA plasma levels at sampling times of 0.15, 0.33, 0.58, 1, 2, 4, and 6 hours (for the 5‐minute infusion group) or 0.3, 0.5, 0.75, 1, 2, 4, and 6 hours (for the 15‐minute infusion group) following the start of the first infusion were used to calculate AUC0–6h. The dose excreted in urine was computed as the sum of the ZA content in the 0–3‐hour and 3–6‐hour urine collections and reported as a percentage of the infused dose.
The third study (CZOL4460506) included in this analysis measured the pharmacokinetics and pharmacodynamics of ZA (4 mg over 15 minutes) administered to 19 patients with varying levels of renal function [24]. These patients were bisphosphonate free or bisphosphonate naïve for ≥1 year. Because ZA is primarily excreted by the kidneys, patients with impaired renal function may have higher levels of ZA in plasma than those with normal renal function. Hence, pharmacokinetic data of patients with normal renal function (creatinine clearance >80 mL/minute; n = 9) were considered for comparison with the OPTIMIZE‐2 study. As with the CZOL4460503 study, the ZA levels in plasma and urine were assessed during the 24‐hour period after dosing. Samplings for plasma ZA levels done at 0.25, 0.33, 0.67, 1, 2, 4, and 8 hours (with 6 hours interpolated) were used for comparison with the OPTIMIZE‐2 study. Dose excreted in urine0–6h was interpolated from 0–2‐hour, 2–4‐hour, and 4–6‐hour urine collections.
The plasma ZA area under curve AUC0–6h and urine excretion over 6 hours (urine0–6h) were calculated in patients receiving the first ever ZA dose and in patients who were bisphosphonate free for ≥1 year after previous bisphosphonate treatment from CZOL4460503 and CZOL4460506. The concentration of ZA in the three studies was determined using a validated radioimmunoassay [25]. The area under the plasma concentration‐time curve from 0 to 6 hours (AUC0–6h) after the start of the infusion was calculated using the trapezoidal rule. The dose excreted in urine0–6h was computed as the sum of ZA content in urine at the respective time points and reported as a percentage of the infused dose. Creatinine clearance for the three studies was determined using the Cockcroft‐Gault equation. Statistical analyses performed were descriptive in nature, and the results presented here should be interpreted accordingly.
Study Approval
All three studies included in this post hoc analysis followed International Conference on Harmonisation harmonized tripartite guidelines for Good Clinical Practice, U.S. 21 Code of Federal Regulations dealing with clinical studies, and the declaration of Helsinki concerning medical research in humans. OPTIMIZE‐2 was reviewed by the independent ethics committee or institutional review board and the institutional review boards of each participating center. The other two studies (CZOL4460503 and CZOL4460506) were performed in compliance with the protocol and in accordance with Novartis standard operating procedures.
Results
The OPTIMIZE‐2 study, conducted between March 03, 2006, and July 25, 2013, demonstrated that an additional year of treatment with a reduced ZA dose schedule of 4 mg q12 weeks was noninferior to the standard 4 mg ZA q4‐week dose (using a noninferiority margin of 10% for the percentage of patients with at least one SRE) [18]. The median age of the patient population was 58.9 years. Other baseline characteristics of the full population in OPTIMIZE‐2 have been presented previously [18]. Patients included in this study received 9–20 doses of ZA over the prior 10–15 months.
In CZOL4460503, 12 patients with various types of advanced metastatic disease had received 4 mg of ZA for 5 minutes (n = 5) or 15 minutes (n = 7). The patient population consisted of five males and seven females with the mean age of 57.1 years. In CZOL4460506, ZA was administered as a 4‐mg intravenous infusion over 15 minutes q4 weeks to patients with varying levels of renal function. Data for patients with normal renal function, that is, creatinine clearance >80 mL/minute (n = 9; 8 males and 1 female), were considered for assessment in this study; the mean age of patients was 63.7 years.
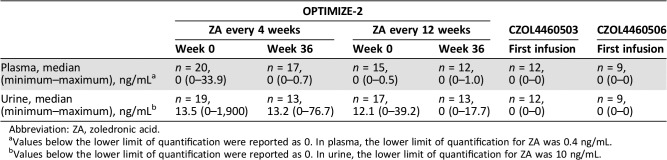
The mean and minimum creatinine clearance at baseline across the three studies ranged from 84.3 mL/minute to 104 mL/minute and 44.3 mL/minute to 91.2 mL/minute, respectively. For OPTIMIZE‐2, the creatinine clearance at week 36 did not change significantly from baseline (week 0; Table 1). The median preinfusion plasma and urine concentrations were zero or close to zero in OPTIMIZE‐2 for both q4 and q12 weekly treatment arms (Table 2). In CZOL4460503 and CZOL4460506, the preinfusion plasma and urine concentrations were all below their lower limits of quantification (Table 2).
Table 1. Creatinine clearance at baseline (and at week 36 for OPTIMIZE‐2).
Abbreviation: ZA, zoledronic acid.
Table 2. Preinfusion ZA plasma and urine concentrations.
Abbreviation: ZA, zoledronic acid.
Values below the lower limit of quantification were reported as 0. In plasma, the lower limit of quantification for ZA was 0.4 ng/mL.
Values below the lower limit of quantification were reported as 0. In urine, the lower limit of quantification for ZA was 10 ng/mL.
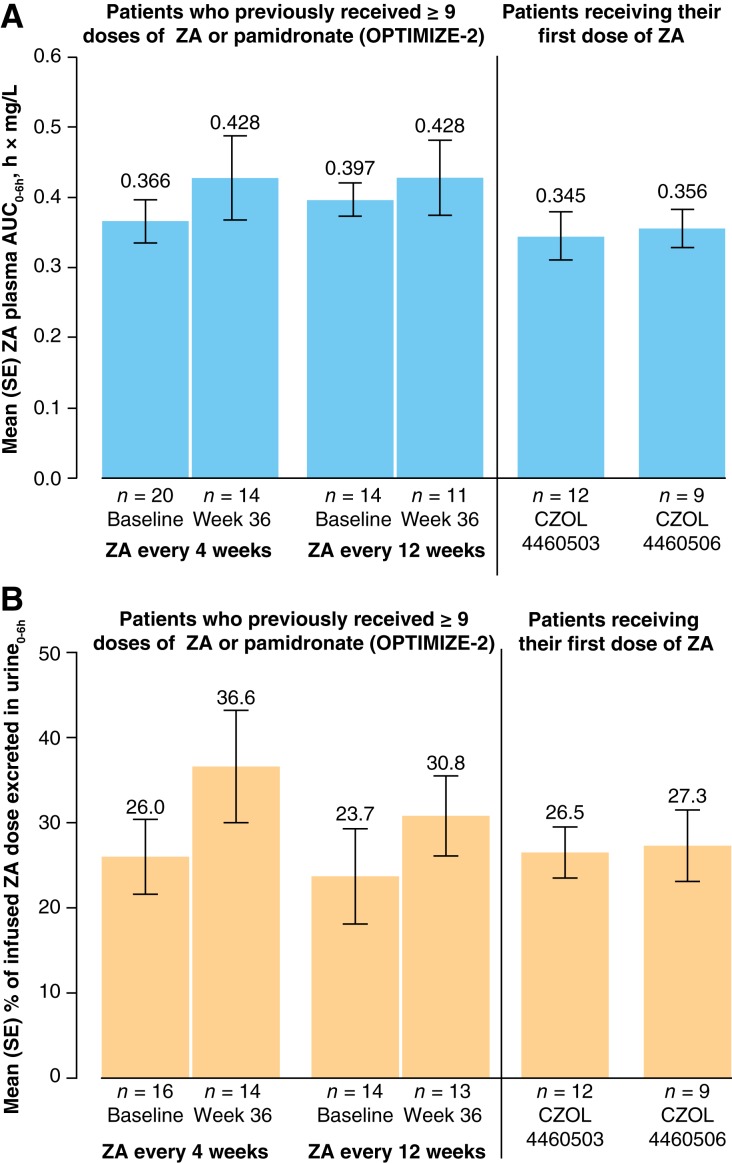
Based on the pharmacokinetic data from two previously conducted phase I studies (CZOL4460503 and CZOL4460506), plasma ZA AUC0–6h and urine0–6h were calculated in patients receiving the first‐ever ZA dose and in patients who were bisphosphonate free for ≥1 year after previous bisphosphonate treatment. Although the areas under curve data were from different trials, the AUC0–6h for ZA in plasma was similar between OPTIMIZE‐2 (at either the week 0 [baseline] or week 36) and the other two studies (Fig. 1A). The number of prestudy doses for patients included in the plasma AUC0–6h analysis ranged from 9 to 64 doses (week 0, ZA every 4 weeks), 10 to 64 doses (week 36, ZA every 4 weeks), 9 to 55 doses (week 0, ZA every 12 weeks), and 9 to 54 doses (week 0, ZA every 12 weeks). The values of AUC0–24h in the CZOL4460503 and CZOL4460506 studies were 0.345 h·mg/L and 0.356 h·mg/L, respectively.
Figure 1.
ZA levels in plasma and urine. (A) ZA plasma AUC0–6h. (B) Percentage of ZA dose excreted in urine0–6h. Abbreviations: AUC, area under the curve; ZA, zoledronic acid.
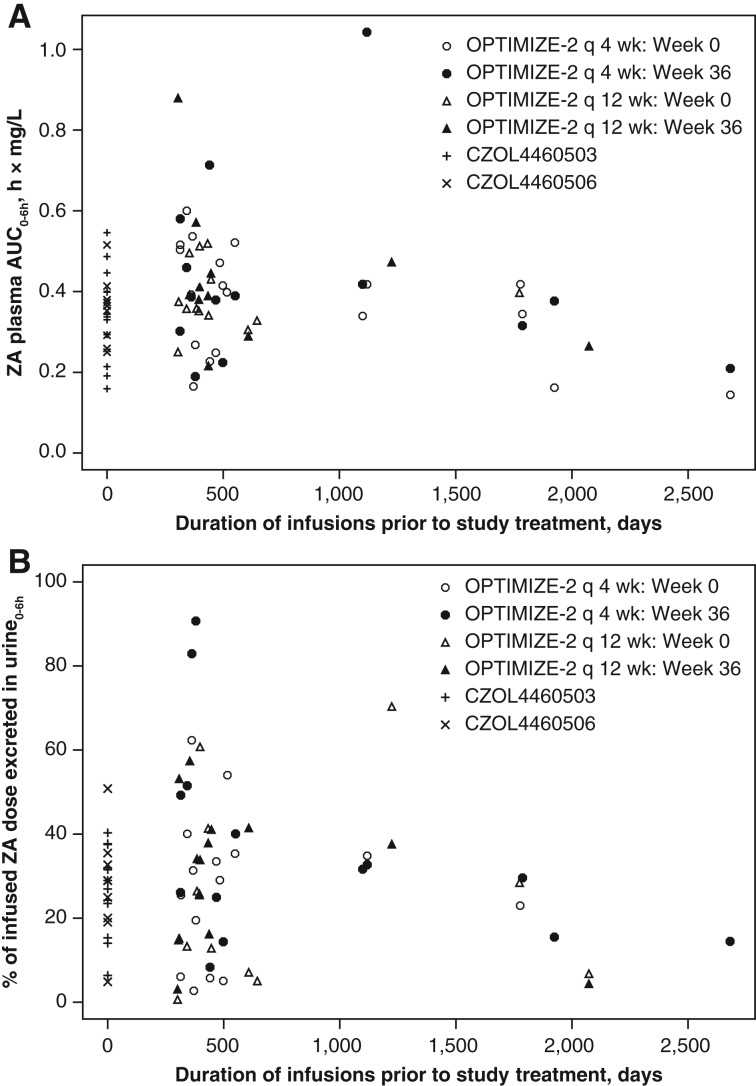
The doses excreted in urine0–6h in CZOL4460503 and CZOL4460506 were 26.5% and 27.3%, respectively. The amounts of ZA excreted in urine were similar between OPTIMIZE‐2 (at either week 0 [baseline] or week 36) and CZOL4460503 and CZOL4460506 (Fig. 1B). The ZA plasma AUC0–6h represented around 85% of the plasma AUC0–24h. The concentration of ZA in plasma declined rapidly after the end of infusion, and very low concentrations were detectable 24 hours after dosing. Scatter plots of plasma and urine exposure against duration of prior once‐monthly treatment with ZA (or pamidronate) indicated no association between exposure and duration of prior administration of ZA (Fig. 2A, 2B).
Figure 2.
ZA levels in plasma and urine versus number of years that the patient received standard once‐monthly ZA dosing. (A) ZA plasma AUC0–6h versus number of years that the patient received standard once‐monthly ZA dosing. (B) Percentage of infused ZA dose excreted in urine versus the number of years that the patient received standard once‐monthly ZA dosing. Abbreviations: AUC, area under the curve; q, every; ZA, zoledronic acid.
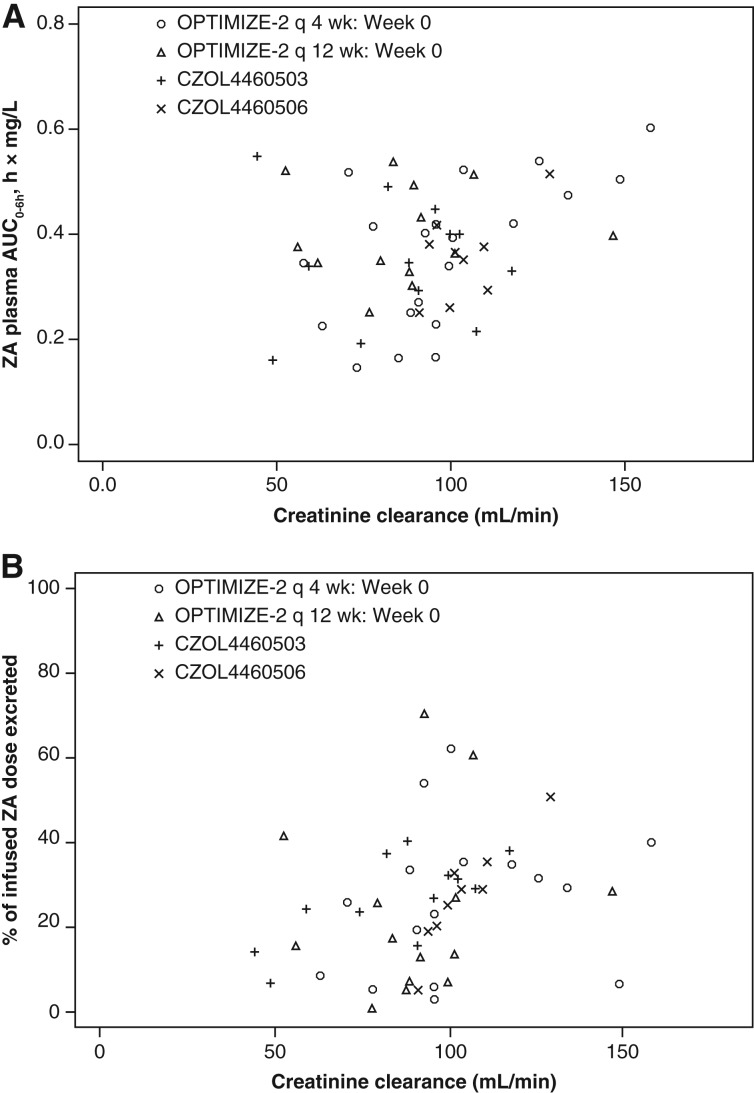
Taking into consideration the slight differences in the minimum creatinine clearance values between the three studies, we evaluated if there was an effect of creatinine clearance on ZA plasma AUC0–6h or urine0–6h. Two patients from an earlier study had the lowest creatinine clearances at baseline—just below 50 mL/minute. However, there was no consistent increase in ZA exposure for these patients compared with those with creatinine clearance above 50 mL/minute. Furthermore, the plasma and urine levels also did not increase with a decrease in creatinine clearance (Fig. 3A, 3B). Thus, at least in these two patients, creatinine clearance did not correlate with plasma and urine AUC over the range in this study and was unlikely to contribute much weight to the overall conclusions.
Figure 3.
ZA levels versus creatinine clearance at baseline. (A) ZA plasma AUC0–6h versus creatinine clearance at baseline. (B) Percentage of ZA dose excreted in urine0–6h versus creatinine clearance at baseline. Abbreviations: AUC, area under the curve; q, every; ZA, zoledronic acid.
Discussion
ZA is considered to be a standard of care for the treatment of bone metastases in patients with advanced solid tumors such as breast cancer and prostate cancer [8]. In clinical practice, ZA is often used for periods longer than 1 year, which has raised some concerns among clinicians because of the potential of ZA to accumulate in bone tissue, or to saturate bone tissue with its prolonged use [26], [27], [28]. Although ZA has the largest therapeutic window between the desired inhibition of calcium resorption and the unwanted inhibition of mineralization compared with other bisphosphonates, it still has a high binding affinity for divalent cations, especially calcium, and consequently binds strongly to the hydroxyapatite of bone. This prolonged exposure of ZA may cause excessive inhibition of bone turnover, subsequently leading to adverse events such as bone fractures and osteonecrosis of the jaw [4]. This post hoc analysis was undertaken to address the question of whether long‐term ZA administration would lead to ZA bone saturation. Pharmacokinetic data from three independent studies (OPTIMIZE‐2, CZOL4460503, and CZOL4460506) representing ZA exposure data were analyzed. The levels of ZA in bone were determined indirectly using surrogate markers, by measuring the ZA plasma and urine levels.
Because ZA is primarily cleared by the renal route, it was important to establish that renal function (as measured by creatinine clearance) was comparable between the different studies analyzed. Taking this into consideration, patients with impaired renal function were excluded from this analysis. The results indicated that the patient population generally had normal renal function or, at most, mild impairment. Thus, creatinine clearance does not seem to be a confounding factor in the interpretation of ZA plasma AUC0–6h or urine0–6h across the three different studies. However, these data need to be interpreted with caution because this is based on the observation of two patients.
One of the key findings in this study was that the levels of plasma and urine ZA in patients who were ZA naïve were comparable to those in patients who had previous long exposure to ZA (1–7 years). Because ZA levels in urine and plasma provide an indirect indication of ZA bone saturation, these findings suggest that prolonged treatment with once‐monthly ZA does not lead to bone saturation. It was anticipated that in case of bone saturation, the levels of ZA in plasma and urine would increase with time, and a positive correlation would be seen between the number of years that a patient had been on ZA treatment and ZA plasma AUC0–6h or urine0–6h. However, in the present analysis, no increase was observed, indicating that prolonged dosing of ZA did not lead to saturation of bone.
We also analyzed preinfusion ZA plasma and urine concentrations in the OPTIMIZE‐2 study. Because ZA is continuously released from bone, there is a theoretical possibility that preinfusion ZA values might increase with the long‐term ZA treatment, in case the bone compartment was saturated with ZA. The patients in this study had taken ZA for a duration ranging from 1 to 7 years. Our analysis showed that both plasma and urine preinfusion values were near or below the lower limit of quantification, regardless of duration of ZA treatment.
Reduction in dosing frequency from once q4 weeks to once q12 weeks was found to be noninferior for efficacy in OPTIMIZE‐2 (noninferiority margin of 10%) [18]. Further evaluation of the effect of reduction of dosing frequency on the retention of ZA in bone was carried out in this study. However, results from this analysis showed that there was no change in plasma and urine ZA concentrations when the dosing frequency was changed from q4 weeks to q12 weeks. Thus, a reduction in dosing frequency of ZA did not have any effect on bone saturation. Based on this observation, it can be implied that existing binding capacity in the bone compartment is so extensive that even a threefold change in the ZA dose administered, that is, reducing dosing frequency from q4 to q12, did not affect the plasma or urine concentration of ZA. This might also explain the relative lack of extraosseous toxicity of bisphosphonate therapy over many years, although it would not explain the cumulative dose‐dependent increase in osteonecrosis of the jaw and renal toxicity [18].
A limitation of this study is that we evaluated the potential of bone saturation with long‐term ZA dosing using the indirect method of measuring plasma and urine concentrations. Bone saturation with ZA is best assessed by directly measuring ZA concentrations in bone biopsies. This, however, is not feasible to conduct. Hence, we measured ZA concentration in bone indirectly using plasma and urine ZA levels as surrogate markers. In short, the approach taken in this study provides the best possible way to address the important question of potential bone saturation with ZA following long‐term standard once‐monthly dosing. Because ZA levels in urine and plasma provide an indication of ZA bone saturation, we used plasma and urine ZA levels as surrogate markers for measurement of ZA in bone tissue.
Overall, the results from this study suggest that prolonged treatment with q4 or q12 weekly ZA does not lead to bone saturation. Furthermore, a reduction in dosing frequency of ZA from q4 to q12 weeks does not have any impact on ZA bone saturation. These findings are important from a clinical perspective as they will aid in addressing concerns regarding the toxic effects of drug accumulation due to its prolonged use. Studies have reported that prolonged use of bisphosphonates such as ZA may change the normal functioning of osteoclasts and disrupt normal bone homeostasis [21], [22], [29]. As a result, exposure of the bone to any future trauma may result in impaired healing and necrosis. Osteonecrosis of the jaw and atypical femur fracture are two such serious adverse events that have been reported in patients receiving ZA [21], [22], [29]. Thus, awareness of prolonged drug efficacy and the potential harm of ZA is important for patients who have been on continuous treatment. Longer duration and greater frequency of ZA infusion were found to have a significant effect on the incidence of these adverse events [21], [22]. Theoretically, these effects could be attributed to saturation of the bone tissue with ZA, which would impair the function of the osteoclasts. Results from our study, however, demonstrate that prolonged exposure of bones to ZA does not lead to saturation. This in turn may reduce the risk of adverse events associated with exposure of bone to prolonged dosing of ZA.
Conclusion
Results from this post hoc analysis suggest that the longer duration of ZA therapy does not lead to bone saturation and the dosing frequency does not impact drug retention rates. Although the use of surrogate markers to measure levels of ZA should be considered while interpreting the results from this study, these results are important from the clinical perspective in that clinicians can now make more informed judgment with regard to duration and frequency of ZA administration to patents with bone metastases. Because dosing frequency did not have any impact on efficacy or bone saturation, ZA can be safely administered less frequently, thereby improving clinical benefits in terms of convenience, reduced toxicity, improved compliance, and cost.
Acknowledgments
We thank William Sallas for his significant contribution toward data analyses and drafting of this manuscript. This study was sponsored by Novartis Pharmaceuticals Corporation, who also provided financial support for medical editorial assistance. We thank Sweta Rathore from Novartis Healthcare Pvt Ltd for medical editorial assistance with this manuscript. R.M. is currently affiliated with AstraZeneca and BeyondSpring Pharmaceuticals.
Footnotes
For Further Reading: Ryota Tanaka, Kan Yonemori, Akihiro Hirakawa et al. Risk Factors for Developing Skeletal‐Related Events in Breast Cancer Patients with Bone Metastases Undergoing Treatment with Bone‐Modifying Agents. The Oncologist 2016;21:508–513
Implications for Practice: Retrospectively, risk factors were identified for skeletal‐related events (SREs) in breast cancer patients with bone metastasis who were treated with bone‐modifying agents (BMAs). For the time to the first SRE and for the SRE frequency, presence of extraskeletal metastases and BMA initiation ≥6 months after the detection of bone metastases were risk factors, respectively. Luminal B type disease, a history of palliative radiation therapy, BMA treatment within 2 years, and elevated serum calcium levels at initial BMA dose were risk factors for both first SRE and SRE frequency. More vigilant observation should be considered for patients with these risk factors.
Author Contributions
Conception/design: Gabriel N. Hortobagyi, Ming Zheng, Ramon Mohanlal
Provision of study material or patients: Gabriel N. Hortobagyi
Collection and/or assembly of data: Gabriel N. Hortobagyi, Ming Zheng, Ramon Mohanlal
Data analysis and interpretation: Gabriel N. Hortobagyi, Ming Zheng, Ramon Mohanlal
Manuscript writing: Gabriel N. Hortobagyi, Ming Zheng, Ramon Mohanlal
Final approval of manuscript: Gabriel N. Hortobagyi, Ming Zheng, Ramon Mohanlal
Disclosures
Gabriel N. Hortobagyi: Novartis (RF), Novartis, F. Hoffman‐La Roche, Ltd., Lilly Research Laboratories, Peregrine Pharmaceuticals, Inc., Merck & Co., Bayer HealthCare Pharmaceuticals, Celgene, Pfizer, Antigen Express (H); Ming Zheng: Novartis (E, OI); Ramon Mohanlal: Novartis (E).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
References
- 1.Van Poznak C, Somerfield MR, Barlow WE et al. Role of bone‐modifying agents in metastatic breast cancer: An American Society of Clinical Oncology‐Cancer Care Ontario focused guideline update. J Clin Oncol 2017;35:3978–3986. [DOI] [PubMed] [Google Scholar]
- 2.Cecchini MG, Wetterwald A, van der Pluijm G et al. Molecular and biological mechanisms of bone metastasis. EAU Update Series 2005;3:214–226. [Google Scholar]
- 3.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:6243s–6249s. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RE. Risks and benefits of bisphosphonates. Br J Cancer 2008;98:1736–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macedo F, Ladeira K, Pinho F et al. Bone metastases: An overview. Oncol Rev 2017;11:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holen I, Coleman RE. Bisphosphonates as treatment of bone metastases. Curr Pharm Des 2010;16:1262–1271. [DOI] [PubMed] [Google Scholar]
- 7.Senaratne SG, Pirianov G, Mansi JL et al. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer 2000;82:1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polascik TJ, Mouraviev V. Zoledronic acid in the management of metastatic bone disease. Ther Clin Risk Manag 2008;4:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Body JJ. Clinical research update: Zoledronate. Cancer 1997;80:1699–1701. [DOI] [PubMed] [Google Scholar]
- 10.Gnant M. Zoledronic acid in breast cancer: Latest findings and interpretations. Ther Adv Med Oncol 2011;3:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Idrees AS, Sugie T, Inoue C et al. Comparison of gammadelta T cell responses and farnesyl diphosphate synthase inhibition in tumor cells pretreated with zoledronic acid. Cancer Sci 2013;104:536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coxon FP, Thompson K, Rogers MJ. Recent advances in understanding the mechanism of action of bisphosphonates. Curr Opin Pharmacol 2006;6:307–312. [DOI] [PubMed] [Google Scholar]
- 13.van Beek E, Pieterman E, Cohen L et al. Farnesyl pyrophosphate synthase is the molecular target of nitrogen‐containing bisphosphonates. Biochem Biophys Res Commun 1999;264:108–111. [DOI] [PubMed] [Google Scholar]
- 14.Drake MT, Clarke BL, Khosla S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin Proc 2008;83:1032–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Body JJ, Lortholary A, Romieu G et al. A dose‐finding study of zoledronate in hypercalcemic cancer patients. J Bone Miner Res 1999;14:1557–1561. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee S, Huang C, Guerra F et al. Thermodynamics of bisphosphonates binding to human bone: A two‐site model. J Am Chem Soc 2009;131:8374–8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen T, Berenson J, Vescio R et al. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol 2002;42:1228–1236. [DOI] [PubMed] [Google Scholar]
- 18.Hortobagyi GN, Van Poznak C, Harker WG et al. Continued treatment effect of zoledronic acid dosing every 12 vs 4 weeks in women with breast cancer metastatic to bone: The OPTIMIZE‐2 randomized clinical trial. JAMA Oncol 2017;3:906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown JE, Ellis SP, Lester JE et al. Prolonged efficacy of a single dose of the bisphosphonate zoledronic acid. Clin Cancer Res 2007;13:5406–5410. [DOI] [PubMed] [Google Scholar]
- 20.Coxon FP, Helfrich MH, Van't Hof R et al. Protein geranylgeranylation is required for osteoclast formation, function, and survival: Inhibition by bisphosphonates and GGTI‐298. J Bone Miner Res 2000;15:1467‐1476. [DOI] [PubMed] [Google Scholar]
- 21.Kajizono M, Sada H, Sugiura Y et al. Incidence and risk factors of osteonecrosis of the jaw in advanced cancer patients after treatment with zoledronic acid or denosumab: A retrospective cohort study. Biol Pharm Bull 2015;38:1850–1855. [DOI] [PubMed] [Google Scholar]
- 22.Kim YS, Park WC. Atypical subtrochanteric femur fracture in patient with metastatic breast cancer treated with zoledronic acid. J Breast Cancer 2012;15:261–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drug Absorption Tracy TS. and Distribution. Philadelphia: Lippincott Williams and Wilkins, 2004. [Google Scholar]
- 24.Skerjanec A, Berenson J, Hsu C et al. The pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with varying degrees of renal function. J Clin Pharmacol 2003;43:154–162. [DOI] [PubMed] [Google Scholar]
- 25.Legay F, Gauron S, Deckert F et al. Development and validation of a highly sensitive ria for zoledronic acid, a new potent heterocyclic bisphosphonate, in human serum, plasma and urine. J Pharm Biomed Anal 2002;30:897–911. [DOI] [PubMed] [Google Scholar]
- 26.Amadori D, Aglietta M, Alessi B et al. Efficacy and safety of 12‐weekly versus 4‐weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (zoom): A phase 3, open‐label, randomised, non‐inferiority trial. Lancet Oncol 2013;14:663–670. [DOI] [PubMed] [Google Scholar]
- 27.Brufsky AM, Sereika SM, Mathew A et al. Long‐term treatment with intravenous bisphosphonates in metastatic breast cancer: A retrospective study. Breast J 2013;19:504–511. [DOI] [PubMed] [Google Scholar]
- 28.Carteni G, Bordonaro R, Giotta F et al. Efficacy and safety of zoledronic acid in patients with breast cancer metastatic to bone: A multicenter clinical trial. The Oncologist 2006;11:841–848. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg MS. Intravenous bisphosphonates and osteonecrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;98:259–260. [DOI] [PubMed] [Google Scholar]