Abstract
Background
Patients with advanced lung cancer have a high symptom burden, which is often complicated by coexisting conditions. These issues, combined with the indirect effects of cancer treatment, can cumulatively lead patients to continued deconditioning and low exercise capacity. This is a concern as exercise capacity is considered a measure of whole body health, and is critical in a patient's ability to participate in life activities and tolerate difficult treatments. There is evidence that exercise training improves exercise capacity and other outcomes, such as muscle force and health‐related quality of life (HRQoL), in cancer survivors. However, the effectiveness of exercise training on these outcomes in people with advanced lung cancer is currently unclear.
Objectives
The primary aim of this review was to investigate the effects of exercise training on exercise capacity in adults with advanced lung cancer. Exercise capacity was defined as the six‐minute walk distance (6MWD; in meters) measured during a six‐minute walk test (6MWT; i.e. how far an individual can walk in six minutes on a flat course), or the peak oxygen uptake (i.e. VO₂peak) measured during a maximal incremental cardiopulmonary exercise test (CPET).
The secondary aims were to determine the effects of exercise training on the force‐generating capacity of peripheral muscles, disease‐specific global HRQoL, physical functioning component of HRQoL, dyspnoea, fatigue, feelings of anxiety and depression, lung function, level of physical activity, adverse events, performance status, body weight and overall survival in adults with advanced lung cancer.
Search methods
We searched CENTRAL, MEDLINE (via PubMed), Embase (via Ovid), CINAHL, SPORTDiscus, PEDro, and SciELO on 7 July 2018.
Selection criteria
We included randomised controlled trials (RCTs) which compared exercise training versus no exercise training in adults with advanced lung cancer.
Data collection and analysis
Two review authors independently screened the studies and selected those for inclusion. We performed meta‐analyses for the following outcomes: exercise capacity, disease‐specific global HRQoL, physical functioning HRQoL, dyspnoea, fatigue, feelings of anxiety and depression, and lung function (forced expiratory volume in one second (FEV1)). Two studies reported force‐generating capacity of peripheral muscles, and we presented the results narratively. Limited data were available for level of physical activity, adverse events, performance status, body weight and overall survival.
Main results
We identified six RCTs, involving 221 participants. The mean age of participants ranged from 59 to 70 years; the sample size ranged from 20 to 111 participants. Overall, we found that the risk of bias in the included studies was high, and the quality of evidence for all outcomes was low.
Pooled data from four studies demonstrated that, on completion of the intervention period, exercise capacity (6MWD) was significantly higher in the intervention group than the control group (mean difference (MD) 63.33 m; 95% confidence interval (CI) 3.70 to 122.96). On completion of the intervention period, disease‐specific global HRQoL was significantly better in the intervention group compared to the control group (standardised mean difference (SMD) 0.51; 95% CI 0.08 to 0.93). There was no significant difference between the intervention and control groups in physical functioning HRQoL (SMD 0.11; 95% CI ‐0.36 to 0.58), dyspnoea (SMD ‐0.27; 95% CI ‐0.64 to 0.10), fatigue (SMD 0.03; 95% CI ‐0.51 to 0.58), feelings of anxiety (MD ‐1.21 units on Hospital Anxiety and Depression Scale; 95% CI ‐5.88 to 3.45) and depression (SMD ‐1.26; 95% CI ‐4.68 to 2.17), and FEV1 (SMD 0.43; 95% CI ‐0.11 to 0.97).
Authors' conclusions
Exercise training may improve or avoid the decline in exercise capacity and disease‐specific global HRQoL for adults with advanced lung cancer. We found no significant effects of exercise training on dyspnoea, fatigue, feelings of anxiety and depression, or lung function. The findings of this review should be viewed with caution because of the heterogeneity between studies, the small sample sizes, and the high risk of bias of included studies. Larger, high‐quality RCTs are needed to confirm and expand knowledge on the effects of exercise training in this population.
Plain language summary
Exercise training for advanced lung cancer
Review question
We looked at the effect of exercise training on fitness level, muscle strength, quality of life, shortness of breath, tiredness, feelings of anxiety and depression, and lung function in patients with advanced lung cancer.
Background
Patients with advanced lung cancer often have many symptoms and accompanying diseases. This, combined with side‐effects of cancer treatment, leads patients to become less fit. This is concerning as fitness level is a measure of whole body health, and is critical in a patient's ability to participate in life activities and tolerate difficult treatments. Exercise training has been shown to improve fitness, muscle strength and quality of life in survivors of several types of cancers. However, the effect of exercise training on these outcomes in people with advanced lung cancer is not clear.
Study characteristics
We looked for all research studies (randomised controlled trials) published up to July 2018. We found six studies which included 221 participants, with an average age ranging from 59 to 70 years. These studies included different numbers of people, ranging from 20 to 111.
Key results
Our results showed that, compared to those who did not exercise, people with lung cancer who did exercise were fitter and had a better quality of life. We did not find any difference in muscle strength, shortness of breath, tiredness, feelings of anxiety and depression, or lung function. No serious harms were reported in people with lung cancer who exercised, but only three studies talked about harms.
Quality of the evidence
The results of this review are not clear, mainly because of the small number of studies found, the small numbers of people in those studies, and because the studies did not seem to have been carried out to a high standard.
Summary of findings
Summary of findings for the main comparison. Exercise training compared to no exercise training for advanced lung cancer.
| Exercise training compared to no exercise training for advanced lung cancer | ||||||
| Patient or population: adults with advanced lung cancer Setting: the studies were based in Australia, Germany, Taiwan, Poland, the UK and Cyprus, and Canada Intervention: exercise training (interventions ranged in length from six to twelve weeks) Comparison: no exercise training | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no exercise training | Risk with exercise training | |||||
| Exercise capacity (6MWD) | The mean change in exercise capacity (6MWD) in the control groups ranged from 6.6 to ‐47.5 metres. | MD 63.33 meters higher (3.7 higher to 122.96 higher) | ‐ | 59 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Exercise training appears to improve exercise capacity (6MWD) |
| Disease‐specific global health‐related quality of life, measured using various scales | The mean change in disease‐specific global health‐related quality of life in the control groups ranged from ‐6.41 to ‐3.1. | SMD 0.51 higher (0.08 higher to 0.93 higher) | ‐ | 90 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Exercise training appears to have a positive effect on disease‐specific health‐related quality of life |
| Physical functioning component of health‐related quality of life, measured using various scales | The mean change in the physical functioning component of health‐related quality of life in the control groups ranged from ‐7.18 to ‐2. | SMD 0.11 higher (0.36 lower to 0.58 higher) | ‐ | 73 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Exercise training does not appear to have an effect on the physical functioning component of health‐related quality of life. |
| Dyspnoea, measured using various scales | The mean change in dyspnoea in the control groups ranged from ‐12.82 to 0.7 | SMD 0.27 lower (0.64 lower to 0.1 higher) | ‐ | 121 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Exercise training does not appear to have an effect on dyspnoea. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; SMD: standardised mean difference; 6MWD: six‐metre walking distance | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Significant risk of bias across the studies
2 Small sample sizes across the studies, some with wide confidence intervals
Background
Description of the condition
Lung cancer is the leading cause of cancer worldwide; more than 1.6 million new cases are diagnosed each year. With 1.3 million deaths per year it is the most common cause of cancer‐related deaths, and accounts for one in every five cancer deaths (Ferlay 2015). The majority of lung cancers can be categorised as non‐small cell lung cancer (NSCLC, 70% to 85%), or small‐cell lung cancer (20% to 25%). The majority of patients (approximately 75%) have incurable locally advanced or metastatic cancers at the time of diagnosis (Govindan 2006), and a five‐year mortality rate of 85% to 90% (Siegel 2011). The treatment approach for lung cancer depends on type of lung cancer, stage of the disease, and the patient’s performance status (NCCN 2015). Therapeutic options include surgical resection, chemotherapy, radiation therapy, targeted therapy, immunotherapy, and palliative care, either alone or in combination (NCCN 2015).
In advanced lung cancer, treatment is aimed at prolonging life or improving the patient’s quality of life, or both (NCCN 2015). However, therapeutic options are limited in many patients because of poor functional status. Many factors may contribute to poor functional status including older age, high tumour burden, comorbidities (Aarts 2015), sedentary lifestyle (Lowe 2014), poor cardio‐respiratory fitness (Jones 2007), and muscle wasting (Baracos 2010). The direct effects of cancer progression such as fatigue, shortness of breath, weight loss, and pain (Lyer 2013), combined with the indirect effects of cancer treatment, can cumulatively lead to further deterioration in quality of life (Lyer 2013), reduced physical activity levels (Lin 2015), and continued loss of physical fitness (Kasymjanova 2009).
Exercise capacity, a term used to describe the aerobic fitness of an individual, is defined as "the maximal capacity of an individual to perform aerobic work or maximal oxygen consumption" (Fleg 2000). Measures of exercise capacity are particularly useful and relevant in this clinical population because they provide objective measures of an individual's overall functional capacity, and quantify the integrated functioning of numerous systems of the body. As such, exercise capacity is considered a measure of whole body health (Ross 2016), and is critical in a patient's ability to participate in life activities and tolerate difficult treatments. For patients with lung cancer, exercise capacity is typically measured by field‐based functional tests (e.g. the six‐minute walk test (6MWT)), or laboratory‐based exercise tests (e.g. cardiopulmonary exercise test (CPET) to measure peak oxygen uptake (VO₂peak)). Currently, in NSCLC, 6MWT is the most frequently reported assessment of exercise capacity (Granger 2013). In advanced NSCLC, VO₂peak exercise testing is a non‐invasive, safe and relatively inexpensive test that provides clinically relevant information (Jones 2007).
Poor exercise capacity, as measured by the 6MWT, is a predictor of poor prognosis in advanced NSCLC and chronic obstructive pulmonary disease (COPD) (Dajczman 2015; Jones 2012; Kasymjanova 2009). Exercise capacity, as measured by VO₂peak, has been identified as a strong predictor of risk of death among healthy populations (Myers 2002), is inversely associated with cancer mortality (Schmid 2015) and lung cancer mortality in men (Sui 2010), and has been identified as the strongest predictor of mortality independent of age and lung function in COPD (Oga 2003).
In those with lung cancer, increased exercise capacity and physical activity levels are associated with improved health‐related quality of life (HRQoL) (Sloan 2016), and reduced fatigue and inflammation (Jones 2008). Importantly, adequate exercise capacity is also critical for maintaining functional independence (Lakoski 2012). Functional independence, often assessed in an oncology setting as performance status, is a key consideration in most cancer treatment decisions. Patients with advanced lung cancer with borderline performance status (e.g. Eastern Cooperative Oncology Group rating of more than 2) experience greater treatment‐related toxicity, and are likely to derive little benefit from chemotherapy (NCCN 2015).
Description of the intervention
Exercise training was the intervention of interest for this systematic review. Exercise training is defined as "a subset of physical activity that is planned, structured and repetitive and has as a final or an intermediate objective the improvement or maintenance of physical fitness" (Caspersen 1985). In the context of this review, this includes aerobic, resistance, or respiratory muscle training, or a combination of these, in advanced lung cancer.
How the intervention might work
Patients with advanced lung cancer have a high symptoms burden. Some of the most frequently reported symptoms are fatigue, dyspnoea, reduced role function, insomnia, and pain (Johnsen 2009). Many patients with lung cancer have co‐existing lung diseases such as COPD, where cardio‐pulmonary limitations and muscle wasting contribute to reduced exercise tolerance (Ross 2003). In patients with advanced lung cancer, it is likely that the cause of poor exercise capacity is multifactorial. The disease itself, as well as conventional cancer treatments, can reduce exercise capacity because of weight loss, low haemoglobin, reduced lung function, and symptoms such as dyspnoea (Wang 2006). Levels of systemic inflammation, particularly proinflammatory cytokines, are also inversely related to exercise capacity in this population (Jones 2008).
There is good‐quality evidence that exercise training improves exercise capacity, muscular force, HRQoL, and physical functioning in cancer survivors (Cramp 2012; Gerritsen 2016; Schmitz 2010; Speck 2010). Exercise training works across multiple organ systems to improve cardiorespiratory fitness, offset treatment side effects, and improve HRQoL in individuals with cancer (Jones 2009). The specific mechanisms by which exercise training improves the patient condition in advanced lung cancer has not received attention in the literature. Exercise capacity is governed by the integrative capacity of the pulmonary and cardiovascular systems to transport oxygen, and the ability of skeletal muscles to use oxygen (Lakoski 2012). It is likely that adaptations in cardiac function (e.g. increased cardiac output), vascular function (e.g. increased anti‐inflammatory activity) and skeletal muscle (e.g. increased muscular strength and cellular respiration) contribute to improvements in exercise capacity following exercise training (Lakoski 2012).
Many of the limiting factors that reduce exercise capacity might be improved by exercise training. A Cochrane Review has shown that exercise training improves functional exercise capacity in patients after lung resection for early stage NSCLC (Cavalheri 2014). Preliminary cohort studies in advanced NSCLC suggest that exercise training improves exercise capacity and muscle force in a safe and effective manner (Kuehr 2014; Quist 2012; Quist 2015). However, the effect of exercise training on HRQoL in this population is unclear: some studies report a decline (Kuehr 2014), while others report no change (Quist 2015; Temel 2009). A Cochrane Review found that exercise training did not improve HRQoL in patients with early stage NSCLC following surgery (Cavalheri 2013). There is also some evidence that patients with advanced lung cancer have difficulty completing structured exercise interventions. Temel and colleagues have reported that fewer than half of those recruited were able to complete the exercise training intervention (Temel 2009). These patients could find it difficult to exercise due to a high burden of symptoms such as fatigue (Kartolo 2016). Finally, the effectiveness of exercise training in improving other outcomes, such as dyspnoea, fatigue, and anxiety and depression remains unknown.
Why it is important to do this review
This review identified the strengths, limitations, and gaps in the current knowledge base, which is important to inform future research. The results might also be a critical first step in promoting changes in clinical practice.
Objectives
The primary aim of this review was to investigate the effects of exercise training on exercise capacity in adults with advanced lung cancer. Exercise capacity was defined as the six‐minute walk distance (6MWD; in meters) measured during a six‐minute walk test (6MWT; i.e. how far an individual can walk in six minutes on a flat course), or the peak oxygen uptake (i.e. VO₂peak) measured during a maximal incremental cardiopulmonary exercise test (CPET).
The secondary aims were to determine the effects of exercise training on the force‐generating capacity of peripheral muscles, disease‐specific global health‐related quality of life (HRQoL), physical functioning component of HRQoL, dyspnoea, fatigue, feelings of anxiety and depression, lung function, level of physical activity, adverse events, performance status, body weight and overall survival in adults with advanced lung cancer.
Methods
Criteria for considering studies for this review
Types of studies
This systematic review included randomised controlled trials (RCTs) comparing exercise training with no exercise training in adults with advanced lung cancer. We considered studies and abstracts published in any language.
Types of participants
We included studies of adults diagnosed with advanced lung cancer, specifically stage IIIb to IV non‐small cell lung cancer (NSCLC) or extensive stage small cell lung cancer.
Types of interventions
We included studies that compared exercise training interventions and usual care, defined as no formal exercise intervention. The intervention needed to comprise more than four weeks of exercise training performed at least once a week. Exercise training could have been supervised or unsupervised, and included aerobic exercise, resistance exercise, respiratory muscle training, or a combination thereof. Aerobic training was defined as exercise that involves large muscle groups performing continuous or intermittent activity over an extended period of time (e.g. jogging or cycling; Newton 2008). Resistance training was defined as exercise that involves performing sets of repeated movements against a resistance (e.g. lifting weights; Newton 2008).
We recorded details of the exercise training programmes, including type of exercise, setting of exercise, level of supervision, as well as participant treatment status. Details of exercise prescription — including frequency, duration and intensity — were recorded. We also recorded information on exercise adherence (e.g. number of sessions attended, adherence rate).
Types of outcome measures
Primary outcomes
The primary outcome of our review was exercise capacity, measured as the six‐minute walk distance (6MWD; in metres) during the 6MWT, or as peak oxygen uptake (VO₂peak; in ml/Kg/min) during a maximal incremental cardiopulmonary exercise test (CPET). All outcomes were short term and were investigated immediately post‐intervention (i.e. on completion of the intervention).
Secondary outcomes
Force‐generating capacity of peripheral muscles (e.g. any measure of upper or lower limb muscle force).
Disease‐specific global health‐related quality of life (HRQoL) (e.g. the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC‐QLQ‐C30; Aaronson 1993); the Functional Assessment of Cancer Therapy – Lung scale (FACT‐L; Cella 1995); the Medical Outcomes Study Short Form 36 General Health Survey (SF‐36; Ware 1992).
Physical functioning HRQoL (e.g. the physical functioning subscale of the EORTC‐QLQ‐C30 (Aaronson 1993), or SF‐36 (Ware 1992)).
Dyspnoea (e.g. the Borg scale (Borg 1970) or Medical Research Council scale (Fletcher 1960)).
Fatigue (e.g. the Functional Assessment of Chronic Illness Therapy ‐ Fatigue Subscale (Yellen 1997)).
Feelings of anxiety and depression (e.g. the Hospital Anxiety and Depression Scale (Zigmond 1983)).
Lung function (e.g. spirometry, lung volumes, and diffusing capacity).
Level of physical activity (e.g. physical activity questionnaires or objective measures of physical activity using accelerometers or motion sensors).
Adverse events. Serious adverse events (e.g. mortality) and minor adverse events (e.g. musculoskeletal pain) recorded during the intervention period.
Performance status (e.g. Eastern Cooperative Oncology Group (Oken 1982) or Karnofsky Performance Status Scale (Mor 1984)).
Body weight.
Overall survival.
Search methods for identification of studies
Electronic searches
We identified RCTs from searches of the following databases.
Cochrane Central Register of Controlled Trials (CENTRAL, Issue 7, 2018) in the Cochrane Library (searched 7 July 2018)
MEDLINE (via PubMed; from 1946 to 7 July 2018)
Embase OVID (from 1980 to 7 July 2018)
CINAHL EBSCO (from 1970 to 7 July 2018)
SPORTDiscus (via EBSCOhost) (from 1985 to 7 July 2018)
PEDro (Physiotherapy Evidence database) (from 1980 to 7 July 2018)
SciELO (The Scientific Electronic Library Online) (from 1978 to 7 July 2018)
The Cochrane Lung Cancer Group Information Specialists developed the search strategies for the three main databases: CENTRAL (Appendix 1), MEDLINE (Appendix 2) and Embase (Appendix 3). The search string for MEDLINE was developed according to the Cochrane Highly Sensitive Search Strategy, sensitivity maximising version (2008 version) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.b of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We adapted it for use in CINAHL, SPORTDiscus, PEDro, SciELO. We also conducted a search of ClinicalTrials.gov (ClinicalTrials.gov), and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/) in July 2018.
Searching other resources
We used additional techniques to search other resources including: 1) contacting authorities in the field for additional references, as well as unpublished or ongoing studies; 2) checking the list of references of the RCTs included in the review; and 3) manually searching abstracts from the American Thoracic Society, American Society of Clinical Oncology, Thoracic Society of Australia and New Zealand, European Respiratory Society, Clinical Oncology Society Australia, and the American College of Sports Medicine from 2014 to July 2018.
Data collection and analysis
Selection of studies
Two review authors (CPM and FS) independently reviewed all studies identified in the literature searches. Initially, the two review authors excluded unsuitable studies by reviewing the title and the abstract. We recorded the reasons for exclusion. The same two review authors independently reviewed and classified the full text of all remaining studies as: 1) 'include', 2) 'unclear', or 3) 'exclude', based on the criteria outlined in our review. We resolved disagreements by consensus. In the case where consensus could not be reached, a third review author (VC) made the final decision. The study selection process was performed using Covidence systematic review software (Covidence 2017; Higgins 2011).
Data extraction and management
Two review authors (CPM and VC) independently extracted data from all included studies using a standard form. Any discrepancies were resolved by either consensus or, where necessary, by a third review author (FS). In the case of missing data, we contacted study authors for the required data. One of the review authors (CPM) entered data into Review Manager 5 (Review Manager 2014), and a second review author (VC) checked that data were correctly entered into Review Manager 5.
Assessment of risk of bias in included studies
We used the Cochrane 'seven evidence‐based domains' tables for assessing risk of bias in all included studies. Two review authors (CPM and FS) independently assessed risk of bias. Any disagreements were resolved by consensus or, where necessary, by a third review author (VC). We judged the risk of bias as either low, high or unclear for: 1) selection (i.e. random sequence generation and allocation concealment); 2) performance (i.e. blinding of participants and personnel); 3) detection (i.e. blinding of outcome assessment); 4) attrition (i.e. incomplete outcome data); 5) reporting (i.e. selective outcome reporting); as well as 6) other potential sources of bias (Higgins 2011). We presented the decision in the ‘Risk of bias’ table with a direct quote, specific study details, or both. When necessary, we contacted authors of unpublished studies to obtain evidence regarding bias. In the ‘Risk of bias’ table we have documented the assessment process. We generated a ‘Risk of bias’ graph (i.e. bar chart) and ‘Risk of bias’ summary (i.e. traffic lights). We rated the level of quality of evidence using the GRADE approach (Atkins 2004; Guyatt 2008).
Measures of treatment effect
As both the primary outcome and secondary outcomes are continuous variables, we used mean difference (MD) or standardised mean difference (SMD) to report treatment effect. In addition, we calculated the 95% confidence intervals (CIs).
Unit of analysis issues
Where studies had data for specific outcomes at multiple time points (e.g. exercise capacity post‐intervention, and at six‐week follow‐up or at 12‐week follow‐up, or both), results from the different time points were not combined in a single meta‐analysis. We used the primary endpoint as based on the study power calculation.
Dealing with missing data
If we were unsuccessful in contacting a study author, we limited the presentation of the outcome(s) of that specific study to a narrative discussion.
Assessment of heterogeneity
To assess the consistency of the results of included studies we visually inspected the forest plots. We assessed statistical heterogeneity across the studies using the I² statistic. We considered heterogeneity to be substantial when the I² value was greater than 50%, and considerable when the I² value was greater than 75% (Higgins 2011). We planned to perform sensitivity analysis to investigate the potential causes of inconsistency in cases where statistically significant heterogeneity was evident.
Assessment of reporting biases
In order to investigate the risk of reporting bias we searched online trial registries. Given the number of included studies (i.e. less than 10), we did not examine funnel plots for signs of asymmetry.
Data synthesis
We entered data from the included studies into Review Manager 5 software to conduct the statistical analyses and generate forest plots (Review Manager 2014). Initially, a random‐effects model was used for calculating summary estimates. If the studies were clinically, methodologically, and statistically homogeneous, we then changed to a fixed‐effect model. We meta‐analysed the results of homogenous studies using the inverse variance DerSimonian and Laird method (DerSimonian 1986). Where data aggregation was not possible due to statistical heterogeneity, we used descriptive techniques.
GRADE and 'Summary of findings' table
We created a GRADE 'Summary of findings' table to aid interpretation of review findings (Atkins 2004; Guyatt 2008). The outcomes included in the 'Summary of findings' table were:
exercise capacity measured as 6MWD (in metres) during the 6MWT, or VO₂peak (in mL/Kg/min) measured during a cardiopulmonary exercise test;
disease‐specific global HRQoL;
Physical functioning component of HRQoL;
dyspnoea.
We used the five GRADE criteria to assess the quality of evidence for each outcome by downgrading or upgrading evidence according to the methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 12.2.1; Higgins 2011).
Subgroup analysis and investigation of heterogeneity
Where possible, we had planned to conduct subgroup analyses to make comparisons between effects of the intervention in different groups, specifically:
different types of exercise training interventions (e.g. aerobic exercise versus resistance exercise);
those undergoing treatment (e.g. chemotherapy/radiation therapy) versus no treatment;
different types of treatment (e.g. chemotherapy versus tyrosine kinase inhibitor therapy).
We assessed heterogeneity and the extent of inconsistency between studies by visual inspection of the forest plots, and by using the Chi² test and the I² statistic.
Sensitivity analysis
We used sensitivity analysis to assess if study findings were influenced by decisions made during the review. Methodological differences across the studies and quality indicators, such as concealment allocation, assessor blinding, intention‐to‐treat analysis, or some combination thereof were used to conduct the analyses.
Results
Description of studies
Six studies (11 records) met the criteria to be included in this review (Figure 1). Of these, we included five in meta‐analyses (Henke 2014; Hwang 2012; Jastrzebski 2015; Molassiotis 2015; Vanderbyl 2017); we described the data from the remaining study narratively (Dhillon 2017). For complete details of studies that were included or excluded, please refer to Characteristics of included studies and Characteristics of excluded studies.
1.
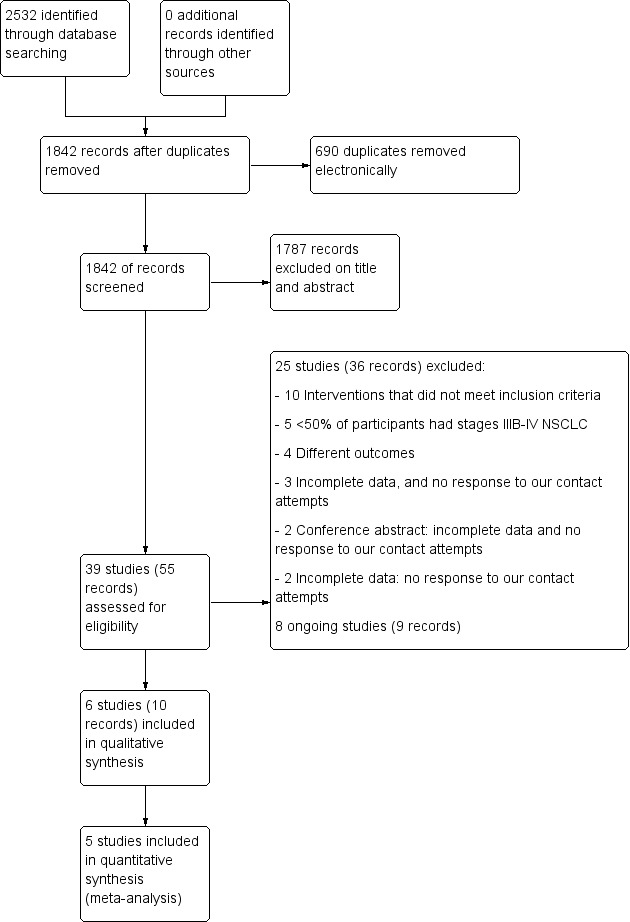
Study flow diagram.
Results of the search
The search of all the databases on 7 July 2018 resulted in a total of 2532 records: 792 from CENTRAL; 765 from MEDLINE; 568 from EMBASE; 266 from CINAHL EBSCO; 75 from SPORTDiscus; 50 from PEDro, and 6 from ScIELO, and 10 from ongoing trials registries. Following removal of duplicates, the total was 1842 records (Figure 1). We excluded 1781 records based on the titles and abstracts. Subsequently, 45 studies and conference abstracts (61 records in total) were assessed for eligibility. We excluded 31 studies (42 records), and the specific reasons for the exclusions are presented in Figure 1. Further, we identified eight ongoing studies (nine records) (ACTRN12614001268639; NCT03334071; NCT03482323; NCT03500393; CTRI/2015/01/005348; NCT01881906; NCT03066271; NCT02055508).
Included studies
Details of the included studies can be found in Characteristics of included studies. We were able to contact the authors of two studies eligible for this review to provide missing data.
Study
We included six studies in this review (Dhillon 2017; Henke 2014; Hwang 2012; Jastrzebski 2015; Molassiotis 2015; Vanderbyl 2017), which included a total of 221 participants with advanced lung cancer. The studies were published between 2012 and 2017.
Population
The sample size of the included studies ranged from 20 to 111. The mean age of the participants ranged from 59 to 70 years. Of the six studies, four reported fully on male/female ratios (n = 184 participants with known sex): 110 (60%) were male and 74 (40%) were female.
Setting
The studies were based in Australia, Germany, Taiwan, Poland, the United Kingdom and Cyprus, and Canada.
Intervention
There was considerable variation in the timing of commencement, type, frequency and intensity of the exercise programmes that were investigated. Regarding timing of exercise training commencement, in three studies participants were enrolled during treatment with either epidermal growth factor receptor inhibitors (Hwang 2012), or chemotherapy (Henke 2014; Jastrzebski 2015). In two studies, participants could be on palliative treatment (Dhillon 2017), or scheduled/eligible for anti‐cancer treatment (Vanderbyl 2017). In one study, participants could not have had chemotherapy within two weeks or chest radiotherapy within four weeks (Molassiotis 2015). The interventions ranged in length from six to twelve weeks (or three cycles of chemotherapy). All studies had a supervised component; three studies had a home‐based component (Dhillon 2017; Jastrzebski 2015; Vanderbyl 2017), and one study was largely home‐based (Molassiotis 2015). Interventions consisted of aerobic exercise alone (Hwang 2012), combined aerobic and resistance exercise (Henke 2014; Jastrzebski 2015; Vanderbyl 2017), physical activity and behavioural support (Dhillon 2017), or inspiratory muscle training alone (Molassiotis 2015). Three studies included some type of breathing exercises (Henke 2014; Jastrzebski 2015; Molassiotis 2015). The frequency of supervised training varied from one day a week to five days a week. The intensity of aerobic exercise was variably reported, and was most frequently based on age‐predicted heart rate calculations (Henke 2014; Jastrzebski 2015; Vanderbyl 2017). In three studies (Henke 2014; Hwang 2012; Vanderbyl 2017), the intervention delivery was supervised by a physiotherapist. The remaining studies did not report the credentials of those supervising the intervention (Dhillon 2017; Jastrzebski 2015; Molassiotis 2015). Adherence to the exercise interventions was reported in two studies (Dhillon 2017; Hwang 2012), and ranged from 69% to 71%. One study only included participants in the analysis who had at least 75% adherence to the exercise training intervention (Henke 2014).
Excluded studies
Of the 45 studies (61 records) for which we reviewed the full text, 31 (42 records) were excluded. The reasons for exclusions are summarised in Characteristics of excluded studies and Figure 1.
Risk of bias in included studies
Details of the risk of bias of the included studies can be found in the 'Risk of Bias' tables (Characteristics of included studies) as well as in Figure 2 and Figure 3. For all cases where the 'Risk of bias' rating was unclear, we contacted the study authors to request additional information. One author (Vanderbyl 2017) provided additional information which was incorporated into the 'Risk of bias' assessment.
2.
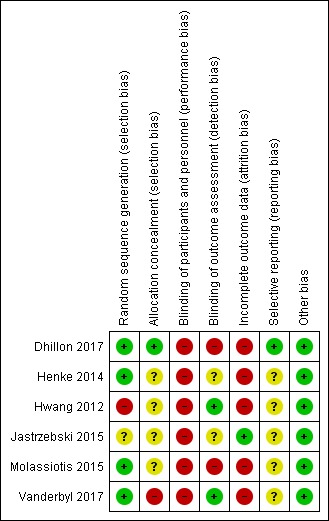
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
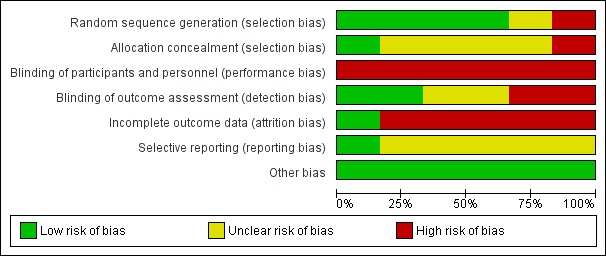
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We judged one study to be at high risk of selection bias (random sequence generation) because three participants were reallocated based on their group preference (Hwang 2012). We judged one study to be at unclear risk of bias since it failed to report sufficient information about the random sequence generation process to permit judgement (Jastrzebski 2015). We judged the remaining four studies to be at low risk of selection bias (random sequence generation) as they provided adequate descriptions of random sequence generation. Allocation concealment was only adequately reported in one study (Dhillon 2017). One study had a high risk of allocation concealment (Vanderbyl 2017), and we rated the remaining studies as having an unclear risk of selection bias (allocation concealment).
Blinding
No studies reported blinding participants and personnel. It is not practical to blind participants of the randomisation to an exercise intervention versus control. We assessed all the studies as being at high risk of performance bias.
Only two studies reported blinding of outcome assessment and we rated these as having low risk of detection bias (Hwang 2012; Vanderbyl 2017).
Incomplete outcome data
We rated five studies as having high risk of attrition bias. This judgement was due to differences reported between those that completed the intervention compared to those who did not (Dhillon 2017); disparities in dropout rates between intervention and control groups (Henke 2014; Hwang 2012; Vanderbyl 2017); or participants being excluded from analysis due to lack of symptoms at baseline (Molassiotis 2015).
Selective reporting
We judged two studies to be at low risk of reporting bias (Dhillon 2017; Vanderbyl 2017). Due to insufficient information, we judged the remaining studies to be at unclear risk of reporting bias.
Other potential sources of bias
All studies appeared to be free of other sources of bias.
Effects of interventions
See: Table 1
The means and standard deviations for differences in outcome measures collected before and after intervention were available in three studies (Henke 2014; Hwang 2012; Jastrzebski 2015), and were provided by the authors of two studies (Molassiotis 2015; Vanderbyl 2017). The remaining study did not report the standard deviations or 95% confidence intervals (CIs) for outcome measures (Dhillon 2017). We contacted the study authors, however no additional data were provided for inclusion in the meta‐analysis. We performed the meta‐analyses using either the post‐intervention mean and standard deviation (for studies that reported no between‐group differences pre‐intervention); the mean differences and standard deviation of change, when available (Molassiotis 2015; Vanderbyl 2017); or the pre‐intervention standard deviation (Henke 2014; Hwang 2012; Jastrzebski 2015). We included data on the following outcomes in the meta‐analyses: exercise capacity, disease‐specific global health‐related quality of life (HRQoL), physical functioning component of HRQoL, dyspnoea, fatigue, feelings of anxiety and depression and lung function (forced expiratory volume in one second; FEV1) . We presented a narrative summary for force‐generating capacity of peripheral muscles, level of physical activity, adverse events, performance status, body weight, and overall survival. We did not conduct subgroup analysis due to the lack of clear subgroups. We did not perform sensitivity analyses due to there being low statistical heterogeneity or a limited number of studies in the analyses.
1. Primary outcome: exercise capacity
Data on exercise capacity were either available or provided by the authors for five studies. Four studies reported the six‐minute walk distance (6MWD) as their measure of exercise capacity (Dhillon 2017; Henke 2014; Jastrzebski 2015; Vanderbyl 2017), and three provided data for meta‐analysis. Analysis of these studies demonstrated that, on completion of the intervention period, the 6MWD was higher in the intervention group compared to the control group (mean difference (MD) 63.33 m; 95% CI 3.70 to 122.96; three studies, 59 participants) (Figure 4).
4.
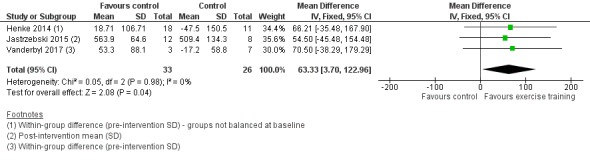
Forest plot of comparison: 1 Exercise training versus control, outcome: 1.1 Exercise capacity measured by Six‐Minute Walk Distance.
In Dhillon 2017, an improvement was reported in 6MWD from baseline to post‐intervention in the exercise group (234.9 m to 516.3 m) as well as the control group (251.0 m to 517.7 m). There was no significant between‐group difference (P = 0.972).
One study reported peak oxygen uptake (VO₂peak, in ml/Kg/min) during a cardiopulmonary exercise test (CPET) as their measure of exercise capacity (Hwang 2012). This study demonstrated a significant difference in VO₂peak (P < 0.005) between the intervention group (15.1 ± 3.4 to 16.8 ± 4.1 ml/Kg/min) and the control group (16.7 ± 4.8 to 16.3 ± 4.6 ml/Kg/min) on completion of the eight‐week intervention.
2. Secondary outcome: force‐generating capacity of peripheral muscles
One study, Hwang 2012, measured force‐generating capacity of a peripheral muscle as isokinetic quadriceps force (i.e. peak torque in Nm) using the Biodex isokinetic dynamometer (Biodex Medical Inc., Shirley, NY, USA). This study demonstrated no differences in peak torque (P = 0.97) between the intervention group (55.7 ± 18.1 to 61.2 ± 13.9 Nm) and the control group (61.4 ± 19.3 to 67.0 ± 20.2 Nm) on completion of the eight‐week intervention.
One study, Dhillon 2017, measured force‐generating capacity of a peripheral muscle group as handgrip strength (i.e. peak strength in kg; device not specified). This study demonstrated no difference in grip strength (P = 0.623) between the intervention group (32.2 kg to 30.9 kg) and the control group (32.9 kg to 31.9 kg) on completion of the eight‐week intervention.
3. Secondary outcome: disease‐specific global health‐related quality of life
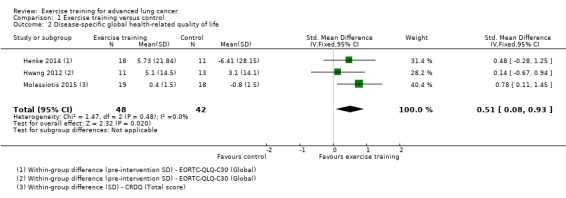
Data on disease‐specific global HRQoL were either available or provided by the authors for five studies, four of which could be included. Two studies used the disease‐specific global HRQoL European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC‐QLQ‐C30) (Henke 2014; Hwang 2012), and one used the total score of the Chronic Respiratory Disease Questionnaire (Molassiotis 2015). On completion of the intervention period, HRQoL was significantly better in the intervention group compared to the control group (standardised mean difference (SMD) 0.51; 95% CI 0.08 to 0.93; three studies, 90 participants) (Figure 5). We used data from an observational sample of advanced‐stage non‐small cell lung cancer (NSCLC) patients (Larsson 2012), in order to re‐express the pooled SMD as the original units of the EORTC‐QLQ‐C30 (this instrument was used in two of the three included studies). The estimated MD yielded a score of 11.22 units.
5.
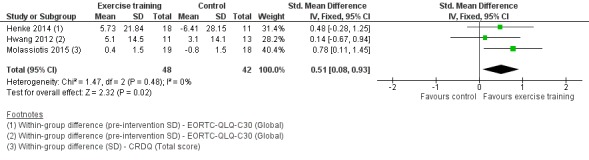
Forest plot of comparison: 1 Exercise training versus control, outcome: 1.2 Disease‐specific global health‐related quality of life
In Dhillon 2017, HRQoL was measured using the EORTC‐QLQ‐C30. This study demonstrated no difference in HRQoL score (P = 0.817) between the intervention group (63.8 to 63.2) and the control group (58.9 to 64.3) on completion of the eight‐week intervention.
4. Secondary outcome: physical functioning component of health‐related quality of life
Data on the physical functioning component of HRQoL were either available or provided by the authors for four studies, three of which could be included. Two studies used the physical functioning scale of the EORTC‐QLQ‐C30 (Henke 2014; Hwang 2012), and one used the physical functioning scale of the SF‐36 (Jastrzebski 2015). On completion of the intervention period, there was no difference in physical functioning HRQoL between the intervention and the control group (SMD 0.11; 95% CI ‐0.36 to 0.58; three studies, 73 participants) (Analysis 1.3).
1.3. Analysis.
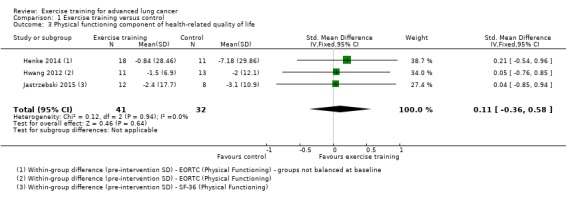
Comparison 1 Exercise training versus control, Outcome 3 Physical functioning component of health‐related quality of life.
5. Secondary outcome: dyspnoea
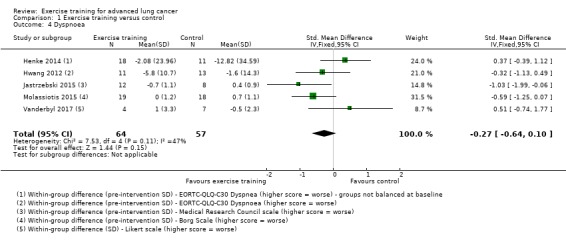
Data on dyspnoea were either available or provided by authors for five studies. Two studies used the EORTC‐QLQ‐C30 (Henke 2014; Hwang 2012); one used the Medical Research Council scale (Jastrzebski 2015); one used the Borg scale (Molassiotis 2015), and one used the Likert scale (Vanderbyl 2017). On completion of the intervention period, there was no significant difference in dyspnoea between the intervention and control group (SMD ‐0.27; 95% CI ‐0.64 to 0.10; five studies, 121 participants) (Figure 6).
6.
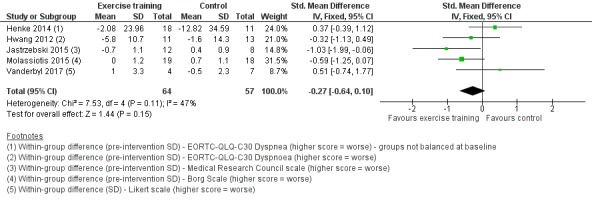
Forest plot of comparison: 1 Exercise training versus control, outcome: 1.4 Dyspnoea.
In Dhillon 2017, dyspnoea was assessed using the SanDiego Shortness of Breath Questionnaire. This study demonstrated no difference in dyspnoea scores (P = 0.281) between the intervention group (25.3 to 27.8) and the control group (20.6 to 22.7) on completion of the eight‐week intervention.
6. Secondary outcome: fatigue
Data on fatigue were either available or provided by the authors for three studies. Two studies used the EORTC‐QLQ‐C30 (Henke 2014; Hwang 2012) and one used the Chronic Respiratory Disease Questionnaire (Molassiotis 2015). On completion of the intervention period, there was no significant difference in fatigue between the intervention and control group (SMD 0.03; 95% CI ‐0.51 to 0.58; three studies, 90 participants) (Analysis 1.5).
1.5. Analysis.
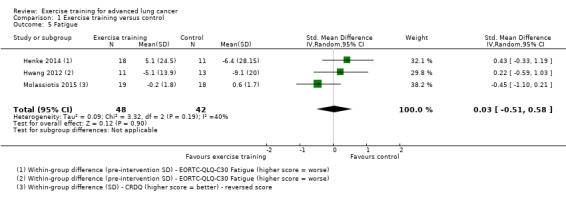
Comparison 1 Exercise training versus control, Outcome 5 Fatigue.
In Dhillon 2017, fatigue was assessed using the FACT‐Fatigue Questionnaire. This study demonstrated no difference in fatigue (P = 0.618) between the intervention group (38.4 to 37.5) and the control group (36.3 to 36.7) on completion of the eight‐week intervention.
7. Secondary outcome: feelings of anxiety and depression
Data on feelings of anxiety and depression were either available or provided by the authors for two studies (Molassiotis 2015; Vanderbyl 2017). The two studies used the Hospital Anxiety and Depression Scale (HADS). On completion of the intervention period, there was no significant difference in feelings of anxiety and depression between the intervention and control group (feelings of anxiety: MD ‐1.21; 95% CI ‐5.88 to 3.45 units on HADS; two studies, 38 participants, Analysis 1.6; feelings of depression: MD ‐1.26; 95% CI ‐4.68 to 2.17 units on HADS; two studies, 30 participants, Analysis 1.7).
1.6. Analysis.
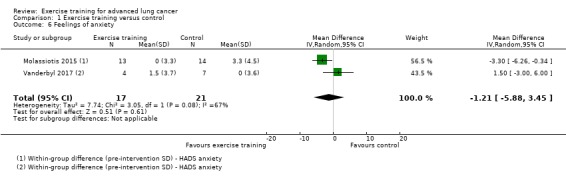
Comparison 1 Exercise training versus control, Outcome 6 Feelings of anxiety.
1.7. Analysis.
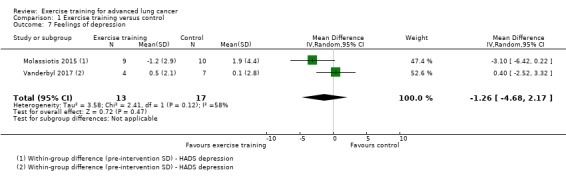
Comparison 1 Exercise training versus control, Outcome 7 Feelings of depression.
In Dhillon 2017, feelings of anxiety and depression were assessed using the Anxiety/Depression General Health Questionnaire. This study demonstrated no difference in feelings of anxiety and depression (P = 0.521) between the intervention group (25.1 to 22.7) and the control group (23.6 to 23.5) on completion of the 8‐week intervention.
8. Secondary outcome: lung function (forced expiratory volume in one second)
Three studies reported measures of lung function (Dhillon 2017; Jastrzebski 2015; Molassiotis 2015). The two studies which provided useable data for meta‐analysis demonstrated that, on completion of the intervention period, there was no significant difference in FEV1 between the intervention and control group (SMD 0.43; 95% CI ‐0.11 to 0.97; two studies, 55 participants) (Analysis 1.8).
1.8. Analysis.
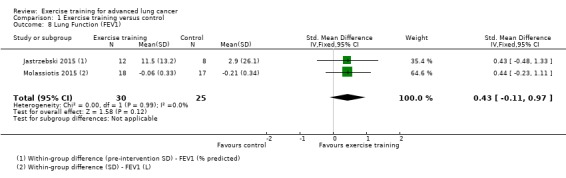
Comparison 1 Exercise training versus control, Outcome 8 Lung Function (FEV1).
In Dhillon 2017, lung function was reported using spirometry results of FEV1. This study demonstrated no difference in FEV1 (P = 0.699) between the intervention group (2.0 to 2.0) and the control group (2.2 to 2.1) on completion of the eight‐week intervention.
9. Secondary outcomes: level of physical activity, adverse events, performance status, body weight and overall survival
In Dhillon 2017, level of moderate and vigorous physical activity (MVPA) was measured using self‐report (Australian Active Questionnaire) as well as objective accelerometer (Actigraph, ActiGraph, LLC, Fort Walton Beach, FL). This study demonstrated no difference in self‐reported MVPA (P = 0.383) between the intervention group (34.5 min/day to 49.0 min/day) and the control group (37.5 min/day to 40.4 min/day) on completion of the eight‐week intervention. Similarly, this study demonstrated no difference in objectively measured MVPA (P = 0.289) between the intervention group (13.2 min/day to 18.1 minutes per day) and the control group (15.6 min/day to 13.2 min/day) on completion of the eight‐week intervention.
10. Secondary outcomes: adverse events
Three studies reported on the incidence of adverse events during the intervention period (Dhillon 2017; Hwang 2012; Molassiotis 2015). One study used the National Cancer Institute Common Terminology Criteria for Adverse Events Version 3, and reported eight minor adverse events (or musculoskeletal injuries) and no serious adverse events in the exercise group (Dhillon 2017). The second study reported that there were no 'reported' exercise‐related adverse events (Hwang 2012). The third study reported that 50% of the group undertaking inspiratory muscle training complained of fatigue after the inspiratory muscle training at baseline; there were also four reports of hypercapnia (e.g. headache), and one report of chest muscle soreness (Molassiotis 2015).
11. Secondary outcomes: performance status
Only one study, Dhillon 2017, reported on the effects of the intervention on performance status. It measured performance status using the Eastern Cooperative Oncology Group Scale. This study demonstrated no difference in performance status (P = 0.675) between the intervention group (0.52 to 0.99) and the control group (0.45 to 1.02) on completion of the eight‐week intervention.
12. Secondary outcomes: body weight
No study reported on changes in body weight following the exercise interventions.
13. Secondary outcomes: overall survival
One study reported the effects of exercise training on overall survival, rather than 12‐month survival (Dhillon 2017). This study demonstrated no difference in overall survival (P = 0.75) between the intervention group (15.4 months; 95% CI 11.3 to 24.1) and the control group (13.2 months, 95% CI 11.1 to 20.0) on completion of the eight‐week intervention.
Discussion
Summary of main results
The primary aim of this study was to investigate the effects of exercise training on exercise capacity in adults with advanced lung cancer. We included data from six randomised controlled trials, with a total of 221 participants. Our meta‐analysis found that compared with control, exercise training improved six‐minute walk distance (6MWD) and had a moderate positive effect on disease‐specific global health‐related quality of life (HRQoL) in people with advanced lung cancer. There were no significant effects of exercise training on dyspnoea, feelings of anxiety, depression, or lung function. Data on the effects of exercise training on force‐generating capacity of peripheral muscles were only available from two studies, neither of which demonstrated an effect for exercise training. Secondary outcomes of physical activity, performance status, and survival were only available from one study, that demonstrated no effect from exercise training. Limited evidence from three studies suggests that exercise training resulted in few adverse events, with no reported serious adverse events. Further, no studies reported the impact of exercise training on body weight. The findings of this review should be interpreted with caution as the overall quality of evidence was graded as low, based on the GRADE approach, because included studies had significant risks of bias, and most studies had small sample sizes. Limitations in the design and implementation of many available studies suggest that there is a high likelihood of bias. Further high‐quality, adequately‐powered randomised controlled trials are needed to fully understand the effects of exercise training in advanced lung cancer.
Exercise capacity in people with advanced lung cancer is adversely affected by disease burden, cachexia, comorbidities, advanced age, and treatment side effects (Jones 2009). In our review, we found low‐quality evidence that exercise training improved exercise capacity, with a mean between‐group difference of 63 metres in 6MWD. This mean change is above the threshold for a clinically meaningful difference in 6MWD in non‐small cell lung cancer (i.e. 42m; Granger 2015), and is in agreement with findings of improved 6MWD from previous systematic reviews of exercise training in people undergoing lung resection for non‐small cell lung cancer (Cavalheri 2013; Cavalheri 2017). Exercise capacity operationalises the integrative ability of many systems of the body to allow individuals to undertake activity, and as such is an important patient‐centred target for supportive care interventions.
In our review, we found low‐quality evidence from three studies that exercise training had a moderate positive effect (Cohen 1988) on disease‐specific global HRQoL. To assist interpretation of the pooled SMD obtained from different sources of data, we translated SMD into an estimated mean difference of 11.22 on the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC‐QLQ‐C30). This is considered a moderate change (10 to 20 units) in people with advanced lung cancer, and is therefore a small but clinically meaningful effect (Osoba 1998). Multiple meta‐analyses have demonstrated small but significant improvements in quality of life following exercise training in people with chronic obstructive pulmonary disease (COPD) (McCarthy 2015), as well as cancer survivors (Sweegers, 2017; Buffart, 2017). Of note, those reviews of cancer survivors contained few participants with advanced lung cancer. Examining results of exercise training on HRQoL in people with advanced cancer, 60% of studies reviewed had a positive impact on HRQoL (Dittus 2017). In the current review, a variety of tools were used to assess HRQoL, and no studies reported HRQoL as a primary outcome. Larger, adequately‐powered randomised trials, which are designed to assess the impact of exercise on disease‐specific global HRQoL, are required to confirm our positive finding.
We found that exercise training appeared not to have an effect on measures of lung function, dyspnoea or fatigue in people with advanced lung cancer. However, for each of these outcomes, the evidence available was low‐quality, and limited by small sample sizes from a small number of studies that were limited by heterogenous interventions and high risk of bias. Of note, previously published systematic reviews support a lack of effect of exercise training on lung function in people following lung cancer surgery (Cavalheri 2014), and people with COPD (McCarthy 2015). Recent evidence suggests that exercise training improves dyspnoea in people with COPD (McCarthy 2015), and those with advanced cancer (Dittus 2017). The positive effect of exercise on fatigue has been demonstrated in cancer survivor populations following treatment (Cramp 2012). Yet, findings of the effects of exercise on fatigue in a cancer setting remain mixed, as another systematic review indicated that fatigue improved in 58% of studies (Dittus 2017). These disparate findings could be explained by differences in fatigue levels at baseline, measurement tools, intervention characteristics, and lack of power.
Limited data were available to assess adverse events of exercise training in adults with advanced lung cancer. Only three studies reported on adverse events and these limited data suggest that exercise training is safe: no serious adverse events were reported, and there were limited minor adverse events. However, these results should be interpreted cautiously. Future studies should rigorously monitor and carefully report adverse events and other measures of tolerability such as exercise adherence (Nilsen 2018).
Overall completeness and applicability of evidence
The current review included studies that employed a variety of exercise training interventions. The exercise training varied by the timing of exercise training commencement (i.e. before, during, or after treatment), type of exercise training (aerobic, aerobic and resistance, inspiratory muscle training alone, or a combination of all training modalities), length of intervention (six to 12 weeks), intensity of the intervention (unmonitored to interval training at 80% of peak oxygen uptake), frequency of contact (one to five supervised sessions per week), and the incorporation of behavioural support or unsupervised training. In most studies, measures of adherence were poorly reported. Collectively, these factors limit the conclusions we can draw about the benefits of these different types, intensities, or length of exercise training, or which aspect of exercise training is responsible for the effects we have found. These results must be interpreted cautiously due to the heterogeneity of exercise interventions assessed, differences in measures used to assess HRQoL, as well as high risk of bias in the majority of the included studies. Advanced lung cancer patients often suffer from poor HRQoL, high symptom burden (Lyer 2013), and reduced functional capacity (Jones 2007). Our review demonstrates the potential for exercise training to improve functional capacity and HRQoL. Given that improving quality of life is a primary aim in the treatment of lung cancer, exercise training programmes could be one component of comprehensive supportive care for those with advanced lung cancer.
Quality of the evidence
We rated the quality of the evidence as low. The sample size of most included studies was small; five out of the six included studies had a sample of less than 30 participants. In several studies we noted unclear reporting on allocation concealment, as well as unreported or poor random sequence generation. Only one study author responded to our request for additional information regarding unclear risk of bias. Blinding of participants and personnel is particularly challenging in randomised controlled trials investigating exercise training (as participants know if they are receiving exercise training or usual care). As a result, no studies blinded participants or personnel to group allocation, and only a small number reported blinding outcome assessments. The majority of studies had a high risk of attrition bias. Finally, a small number of studies were eligible to be included, not all outcomes were included in every study, and not all study authors provided additional requested data. Therefore, our meta‐analysis of primary outcomes included only three studies.
Potential biases in the review process
The strengths of this review include an extensive search strategy with no language limitation. Two authors reviewed and independently examined, selected, and assessed the bias in the studies. Two study authors provided additional data, which increased the number of studies we were able to include in the meta‐analysis. Our analysis was limited by selection bias due to the exclusion of two studies for which we were unable to access additional requested data required for inclusion in the review, and one study where we were unable to access additional information for inclusion in the meta‐analysis.
Agreements and disagreements with other studies or reviews
This is the first Cochrane Review of exercise training in advanced lung cancer. We found four other reviews that examined the impact of exercise in advanced lung cancer that identified similar conclusions to the present review (Bade 2015; Ozalevli 2013; Payne 2013; Rivas‐Perez 2015). Contrary to the current review, Lehto and colleagues reported benefits in depression and anxiety with exercise in patients with advanced lung cancer (Lehto 2017). However, this conclusion was based on single group study results, while we only included randomised controlled trials. Overall, these reviews included few studies, with a range of study designs (including non‐randomised studies), and did not conduct meta‐analyses. Our review is the first to complete meta‐analyses to demonstrate the positive impact of exercise training on exercise capacity and disease‐specific global HRQoL.
Authors' conclusions
Implications for practice.
Our meta‐analyses provide low‐quality evidence that exercise training can be completed by adults with advanced lung cancer with low risk of harm, and may increase exercise capacity (six‐minute walk distance) and disease‐specific global health‐related quality of life.
Implications for research.
This review clearly shows the need for larger high‐quality randomised controlled trials to be conducted to confirm and extend the current findings with higher quality evidence. The methodological quality of studies should be improved by addressing the limitations found in this review; in particular, they should report on allocation concealment, lack of blinding for study endpoints, and attrition bias. Comprehensive collection and reporting on safety, feasibility, and longer‐term outcome measures are important for future research.
History
Protocol first published: Issue 6, 2017 Review first published: Issue 2, 2019
| Date | Event | Description |
|---|---|---|
| 4 July 2017 | Amended | Citation names of the authors updated. No change in the contain of the protocol |
Acknowledgements
The authors would like to thank Corynne Marchal, Managing Editor of the Cochrane Lung Cancer Group, for her support. We also acknowledge Elisabeth Quoix, Bruno Degano, François Calais, Giorgio Maria Agazzi, Virginie Westeel, Fergus Macbeth, Nathalie Meneveau, Sophie Paget‐Bailly, and Tom Haswell for their input in the review process.
Appendices
Appendix 1. CENTRAL search strategy
#1MeSH descriptor: [Lung Neoplasms] explode all trees #2lung cancer* #3lung carcinoma* #4lung malignan* #5lung neoplasm* #6lung tumo* #7pulmonary cancer* #8pulmonary carcinom* #9pulmonary malignan* #10pulmonary neoplasm* #11pulmonary tumo* #12MeSH descriptor: [Carcinoma, Non‐Small‐Cell Lung] explode all trees #13nonsmall cell lung cancer* #14non small cell lung cancer* #15nonsmall cell lung carcinoma* #16non small cell lung carcinoma* #17NSCLC #18MeSH descriptor: [Carcinoma, Small Cell] explode all trees #19oat cell carcinoma* #20oat cell lung carcinoma* #21oat cell lung cancer* #22oat cell cancer* #23SCLC #24small cell lung cancer* #25small cell lung carcinom* #26#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 #27MeSH descriptor: [Breathing Exercises] explode all trees #28MeSH descriptor: [Exercise] explode all trees #29aerobic* #30exercise #31MeSH descriptor: [Exercise Therapy] explode all trees #32MeSH descriptor: [Physical Endurance] explode all trees #33endurance #34treadmill* #35MeSH descriptor: [Walking] explode all trees #36walking #37strength* #38MeSH descriptor: [Resistance Training] explode all trees #39resistance training #40weight training #41weight lifting #42MeSH descriptor: [Respiratory Muscles] explode all trees #43inspiratory muscle* #44expiratory muscle* #45MeSH descriptor: [Physical Therapy Modalities] explode all trees #46physiother* #47physical therap* #48MeSH descriptor: [Bicycling] explode all trees #49bicycling #50cycling #51#27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 #52#26 and #51
Appendix 2. MEDLINE search strategy
#1,"Search lung neoplasms[MeSH Terms]" #2,"Search lung cancer*[Title/Abstract]" #3,"Search lung carcinoma*[Title/Abstract]" #4,"Search lung malignan*[Title/Abstract]" #5,"Search lung neoplasm*[Title/Abstract]" #6,"Search lung tumo*[Title/Abstract]" #7,"Search pulmonary cancer*[Title/Abstract]" #8,"Search pulmonary carcinom*[Title/Abstract]" #9,"Search pulmonary malignan*[Title/Abstract]" #10,"Search pulmonary neoplasm*[Title/Abstract]" #11,"Search pulmonary tumo*" #12,"Search carcinoma, non small cell lung[MeSH Terms]" #13,"Search nonsmall cell lung cancer*[Title/Abstract]" #14,"Search non small cell lung cancer*[Title/Abstract]" #15,"Search nonsmall cell lung carcinoma*[Title/Abstract]" #16,"Search non small cell lung carcinoma*[Title/Abstract]" #17,"Search NSCLC[Title/Abstract]" #18,"Search carcinoma, small cell[MeSH Terms]" #19,"Search oat cell carcinoma*[Title/Abstract]" #20,"Search oat cell lung carcinoma*[Title/Abstract]" #21,"Search oat cell lung cancer*[Title/Abstract]" #22,"Search oat cell cancer*[Title/Abstract]" #23,"Search SCLC[Title/Abstract]" #24,"Search small cell lung cancer*" #25,"Search small cell lung carcinom*" #26,"Search #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25" #27,"Search breathing exercises[MeSH Terms]" #28,"Search exercise[MeSH Terms]" #29,"Search aerobic*[Title/Abstract]" #30,"Search exercise[Title/Abstract]" #31,"Search exercise therapy[MeSH Terms]" #32,"Search physical endurance[MeSH Terms]" #33,"Search endurance[Title/Abstract]" #34,"Search treadmill*[Title/Abstract]" #35,"Search walking[MeSH Terms]" #36,"Search walking[Title/Abstract]" #37,"Search strength*[Title/Abstract]" #38,"Search resistance training[MeSH Terms]" #39,"Search resistance training[Title/Abstract]" #40,"Search weight training[Title/Abstract]" #41,"Search weight lifting[Title/Abstract]" #42,"Search respiratory muscles[MeSH Terms]" #43,"Search inspiratory muscle*[Title/Abstract]" #44,"Search expiratory muscle*" #45,"Search physical therapy modalities[MeSH Terms]" #46,"Search physiother*[Title/Abstract]" #47,"Search physical therap*[Title/Abstract]" #48,"Search bicycling[MeSH Terms]" #49,"Search bicycling[Title/Abstract]" #50,"Search cycling[Title/Abstract]" #51,"Search #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50" #52,"Search #26 AND #51" #53,"Search randomized controlled trial[Publication Type]" #54,"Search controlled clinical trial[Publication Type]" #55,"Search randomized[Title/Abstract]" #56,"Search placebo[Title/Abstract]" #57,"Search drug therapy[MeSH Subheading]" #58,"Search randomly[Title/Abstract]" #59,"Search trial[Title/Abstract]" #60,"Search groups[Title/Abstract]" #61,"Search #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60" #62,"Search animals [MeSH Terms] NOT humans [MeSH Terms]" #63,"Search #61 not #62" #64,"Search #52 AND #63"
Appendix 3. Embase search strategy
#1 'lung tumor'/exp #2 'lung cancer*':ab,ti #3 'lung carcinoma*':ab,ti #4 'lung malignan*':ab,ti #5 'lung neoplasm*':ab,ti #6 'lung tumo*':ab,ti #7 'pulmonary cancer*':ab,ti #8 'pulmonary carcinom*':ab,ti #9 'pulmonary malignan*':ab,ti #10 'pulmonary neoplasm*':ab,ti #11 'pulmonary tumo*':ab,ti #12 'lung non small cell cancer'/exp #13 'nonsmall cell lung cancer*':ab,ti #14 'non small cell lung cancer*':ab,ti #15 'nonsmall cell lung carcinoma*':ab,ti #16 'non small cell lung carcinoma*':ab,ti #17 'nsclc':ab,ti47813 #18 'small cell lung cancer'/exp #19 'oat cell carcinoma*':ti,ab #20 'oat cell lung carcinoma*':ti,ab #21 'oat cell lung cancer*':ti,ab #22 'oat cell cancer*':ti,ab #23 'sclc':ti,ab #24 'small cell lung cancer*':ti,ab #25 'small cell lung carcinom*':ti,ab #26 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 #27 'breathing exercise'/exp #28 'exercise'/exp #29 'aerobic*':ti,ab #30 'exercise':ti,ab #31 'kinesiotherapy'/exp #32 'endurance'/exp #33 'endurance':ti,ab #34 'treadmill*':ti,ab #35 'walking'/exp #36 'walking':ti,ab #37 'strength*':ti,ab #38 'resistance training'/exp #39 'resistance training':ti,ab #40 'weight training':ti,ab #41 'weight lifting':ti,ab #42 'breathing muscle'/exp #43 'inspiratory muscle*':ti,ab #44 'expiratory muscle*':ti,ab #45 'physiotherapy'/exp #46 'physiother*':ti,ab #47 'physical therap*':ti,ab #48 'cycling'/exp #48 OR #49 #49 'cycling':ti,ab #50 #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #51 'crossover procedure'/exp OR 'double‐blind procedure'/exp OR 'randomized controlled trial'/exp OR 'single‐blind procedure'/exp OR random* OR factorial* OR crossover* OR cross NEXT/1 over* OR placebo* OR doubl* NEAR/1 blind* OR singl* NEAR/1 blind* OR assign* OR allocat* OR volunteer* #52 #26 AND #50 AND #
Data and analyses
Comparison 1. Exercise training versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exercise capacity (6MWD) | 3 | 59 | Mean Difference (IV, Fixed, 95% CI) | 63.33 [3.70, 122.96] |
| 2 Disease‐specific global health‐related quality of life | 3 | 90 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.51 [0.08, 0.93] |
| 3 Physical functioning component of health‐related quality of life | 3 | 73 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.36, 0.58] |
| 4 Dyspnoea | 5 | 121 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.64, 0.10] |
| 5 Fatigue | 3 | 90 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.51, 0.58] |
| 6 Feelings of anxiety | 2 | 38 | Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐5.88, 3.45] |
| 7 Feelings of depression | 2 | 30 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐4.68, 2.17] |
| 8 Lung Function (FEV1) | 2 | 55 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.11, 0.97] |
1.1. Analysis.
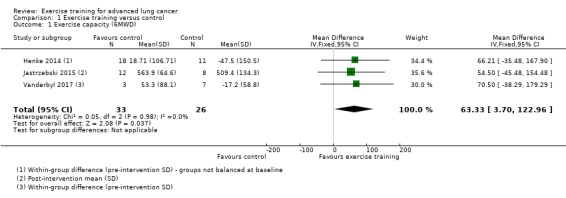
Comparison 1 Exercise training versus control, Outcome 1 Exercise capacity (6MWD).
1.2. Analysis.
Comparison 1 Exercise training versus control, Outcome 2 Disease‐specific global health‐related quality of life.
1.4. Analysis.
Comparison 1 Exercise training versus control, Outcome 4 Dyspnoea.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dhillon 2017.
| Methods | Design: randomised controlled trial Setting: outpatient clinic, Sydney, Australia Study duration: 8 weeks | |
| Participants | 232 participants with stage III or IV NSCLC, or SCLC were invited. 112 randomised, 1 became ineligible. 111 participants participated. Exercise group: n = 56 (29M), mean age 64 (38‐80) years, 70% were on palliative treatment, 33 were current or ex‐smokers, median 8.6 months following diagnosis. Control group: n = 55 (32M), mean age 64 (34‐76) years, 78% were on treatment, 95% stage IV, 36 were current or ex‐smokers, median 7.7 months post‐diagnosis. |
|
| Interventions |
Exercise (n = 56): 8‐week individualised PA programme. Supervised once weekly for 30‐45 minutes, for 8‐weeks; physical activity was individually tailored; walking mainly. The goal was to increase baseline PA over two months by 3 MET hours per week; no specifics provided on frequency of home‐base training. Behavioural support sessions, 15‐20 min/week. Guide book was provided for home exercise. Control (n = 55): usual care attended study assessments only. |
|
| Outcomes | Exercise capacity (6MWD), muscle force‐generating capacity (number of reps of arm curls; hand grip strength), HRQoL (EORTC Global), dyspnoea (SanDiego Shortness of Breath Questionnaire), fatigue (FACIT‐Fatigue), anxiety and depression (General Health Questionnaire‐12), lung function (FEV1, L; FEV1/FVC), Physical activity level (self‐report, Active Australia, physical activity minutes per day; objective accelerometer, Actigraph, moderate and vigorous physical activity, minutes), performance status (ECOG), body weight (kg), overall survival (months) | |
| Notes | Additional information was provided from authors on SD of within‐group changes (or baseline and post‐intervention scores). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment allocation was determined by minimisation". |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomised (1:1) via central Interactive Voice Response System,..." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Allocation was not blinded due to nature of the intervention". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "A comparison of baseline data for primary outcome between those with complete versus incomplete data, showed those with incomplete data were more ill, with poorer PS, more co morbidities, poorer QOL, worse ADL function, and shorter survival". |
| Selective reporting (reporting bias) | Low risk | Comment: all data outcome reporting matches the protocol paper. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Henke 2014.
| Methods | Design: Randomised controlled trial Setting: hospital inpatient, Germany Study duration: three cycles of chemotherapy (started day before first chemotherapy cycle, ended on the last day of third chemotherapy cycle) | |
| Participants | 70 diagnosed with non‐small cell lung cancer (NSCLC) or small cell lung cancer (SCLC) in stage IIIA/IIIB/IV, who received an inpatient palliative platinum‐based chemotherapy treatment. 44 were randomised, 29 completed participation. Exercise group: n = 18. Control group: n = 11. No medical or demographic information was provided. |
|
| Interventions |
Exercise (n = 18): intervention length was three cycles of chemotherapy. Aerobic exercise intervention: 6 min hallway walking, 2 min start walking at an intensity of 55% to 75% HRR dependent on their modified borg scale score (dependent on modified borg scale score), 5 days week. Resistance exercise training: 4 resistance exercises; bridge, abdominal exercise, bicep curl, tricep extension; intensity was an elastic band of medium resistance; intensity was increased via repetitions, performed every other day. Breathing exercises, physiotherapeutic breathing techniques included the active cycle of breathing, performed every other day. Conventional physiotherapy Control (n = 11): received conventional physiotherapy. |
|
| Outcomes | Exercise capacity (6MWD), muscle force‐generating capacity (number of reps of bicep curls), HRQoL (EORTC Global), dyspnoea (EORTC Dyspnoea), fatigue (EORTC Fatigue), | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomization took place after the patient had signed the informed consent". |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit a judgement of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit a judgement of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "When looking at the dropout rate in both groups, it is noticeable that more people in the CG dropped out due to noncompliance". Comment: only those with at least 75% adherence to the intervention were analysed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. Insufficient information to permit a judgement of 'low risk' or 'high risk' |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Hwang 2012.
| Methods | Design: Randomised controlled trial Setting: outpatient clinic, Taiwan Study duration: 8 weeks | |
| Participants | 44 participants with stage NSCLC being treated with epidermal growth factor receptor inhibitors were invited. 24 randomised, 18 completed. 111 participants participated. Exercise group: n = 13 (5 M), mean age 61 (6.3) years, BMI 22.6 (2.4), 77% stage IV, 0 were current or ex smokers, 2.6 (2.1) months following diagnosis. Control group: n = 11 (7 M), mean age 58.5 (8.2) years, 91% stage IV, 1 was current or ex‐smokers, 2.8 (2.4) months post diagnosis. |
|
| Interventions |
Exercise (n=13): eight weeks of aerobic exercise 30‐40min, 2‐5 min intervals; intervals 80% VO2peak or RPE 15‐17, 60% VO2peak RPE 11‐13; 10 min warm up, 5 min cool down, performed 3 times per week. Control (n=11): usual care, general patient education, and social phone calls every 2–3 weeks, without supervised exercise intervention. General exercise instructions with the Theraband® Elastic Band were given if the subjects in the control group specifically asked for exercise consultation. |
|
| Outcomes | Exercise capacity (VO2peak), muscle force‐generating capacity (peak torque; right quad; isokinetic Biodex Nm), HRQoL (EORTC Global), dyspnoea (EORTC dyspnoea), fatigue (EORTC Fatigue) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "A computer number generator was used to assign a random order..." Quote: "A minor reallocation was made at the beginning of the study..." Comment: three participants were reallocated based on patient preference. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "This allocation procedure was performed by an individual who was unaware of the purpose of this study." Comment: insufficient information to permit judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All tests were performed by a blinded assessor". Comment: blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there was higher dropout from the intervention group compared to the control group. Comment: potentially inappropriate application of simple imputation (baseline observation carried forward) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. Insufficient information to permit judgment of 'low risk' or 'high risk' |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Jastrzebski 2015.
| Methods | Design: Randomised controlled trial Setting: outpatient, Poland Study duration: 4‐12 weeks. | |
| Participants | 28 participants diagnosed with stage III or IV non‐small cell lung cancer (NSCLC) or small cell lung cancer currently receiving chemotherapy. 21 were randomised, 20 completed participation. Exercise group: n = 12 (10 M), mean age 59 (7) years, 100% on treatment, 10 NSCLC stage III/IV; 2 SCLC ED median 8.6 months following diagnosis. Control group: n = 8, 100% were on treatment, NSCLC stage III/IV, no other medical or demographic information provided |
|
| Interventions |
Exercise group (n=12): 8‐12 weeks of supervised physical rehabilitation was performed in 2‐week cycles interspersed with consecutive rounds of chemotherapy with the cytostatic Platidiam‐Vepesid. Intervention delivered was dependent on baseline Six‐Minute Walk Test results. If 6MWT > 200 m (n = 8): nordic walking for 45 min at least 5 days a week, with a heart rate (HR) target of 70% of predicted maximal HR (220 – age), aerobic exercises and respiratory exercises for 30 min, 5 times a week; resistance training, once a day for 30 min. If 6MWT < 200 m (n = 4), exercise of respiratory muscles and peripheral muscles of upper and lower extremities (cycle ergometer); the program was determined individually. Control group (n=8): observed without any physical rehabilitation. Participants were assessed before and after 8 weeks of chemotherapy alone (four consecutive rounds of the cytostatic). |
|
| Outcomes | Exercise capacity (6MWD), HRQoL (SF‐36, Physical Composite score), dyspnoea (MRCD), lung function (FEV1%) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "There were 12 patients randomly allocated to the pulmonary rehabilitation group and another 8 constituted the control group..." Comment: insufficient information to permit judgement of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Molassiotis 2015.
| Methods | Design: Randomised controlled trial Setting: outpatient, UK and Cyprus Study duration: 12 weeks. | |
| Participants | 104 participants with mesothelioma or NSCLC with refractory dyspnoea, who had no treatment with palliative radiotherapy to the chest received within 4 weeks or chemotherapy within 2 weeks were assessed for eligibility. 47 randomised, 27 of whom had advanced lung cancer (data provided by author). Exercise group: n = 13 (12 M), mean age 70 (10) years, 9 of 13 completed Control group: n = 14 (10 M), mean age 68 (8) years, 10 of 14 completed |
|
| Interventions |
Exercise intervention (n=13): 12‐week intervention. Supervised twice weekly, inspiratory muscle training 30 min/day five days/week. Intensity began at 40% of PImax, progressed as tolerated up to 5% per week to a maximum, of 70% PImax. Two initial sessions supervised. Home visits were conducted monthly in the IMT group for the duration of the trial for spirometry assessment and increasing the IMT’s resistance level. Control (n=14): 12 weeks usual care |
|
| Outcomes | HRQoL (CRDQ Mastery), dyspnoea (Borg Scale), fatigue (CRDQ Fatigue), anxiety and depression (Hospital Anxiety and Depression scale), lung function (FEV1). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned through a computer programme to IMT or a control group". |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "...non‐blinded..." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "...non‐blinded..." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Excluded from analysis (no dyspnea at baseline) (n=2)" Comment: participants were excluded from analysis of intervention group due to lack of dyspnoea at baseline. Otherwise, reasons for missing data are balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Vanderbyl 2017.
| Methods | Deign: Randomised controlled trial Setting: outpatient, Canada Study duration: 6‐weeks | |
| Participants | 301 participants with advanced lung or gastrointestinal cancer were assessed for eligibility, 36 randomised, 11 of whom had advanced lung cancer (data provided by author). Exercise group: n = 4 (1 M), mean age 64 (8) years, 3/4 completed, 100% on treatment, 100% stage IV. Control group: n = 7 (4 M), mean age 67 (12) years, 7/7 completed, 100% on treatment, 47% stage IV. |
|
| Interventions |
Exercise (n=4): 6 weeks of aerobic, supervised, 60% to 70% maximum heart rate of 2‐4 MET, 2 days/week; home‐based walking 60 min/day; resistance exercise supervised 2 days/week, frequency and intensity of the exercise prescription at each stage of the programme were calibrated to the individual’s ability and progress. 2‐week washout period before cross‐over. Control (n=7): 6 weeks of Qigong. Twice per week participants were led in a walking exercise programme that involved co‐ordinated arm movements while in a state of deep relaxation or meditation while performing a breathing pattern called “in, in, out” breathing. In addition, participants were instructed to practice QG for up to 1 h every day at home and refrain from independent resistance or cardiovascular training during the QG training period. |
|
| Outcomes | Exercise capacity (6MWD), HRQoL (FACT‐G Total), dyspnoea (Likert Scale, high worse), anxiety and depression (Hospital Anxiety and Depression Scale) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done using a computer‐generated number sequence..." |
| Allocation concealment (selection bias) | High risk | Comment: author provided information that allocation was not concealed. "There was no specific mechanism in place to stop the coordinator looking at this list for the next patient" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients were evaluated by a second physiotherapist who was not involved in training and who was blinded to group assignment". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comments: only complete cases were analysed. There was double the number of dropouts from the control condition. Therefore, there was imbalance in numbers for missing data across intervention groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Other bias | Low risk | Comment: the study appears to be free of other bias. |
6MWD: Six‐Minute Walk Test CRDQ: Chronic Respiartory Disease Questionnaire ED: Extensive Disease EORTC: European Orgnisation for Research and Treatment of Cancer FEV1: Forced expiratory volume in one second FVC: Forced vital capacity HRR: Heart rate reserve HRQoL: Health‐related quality of life IMT: Inspiratory muscle training M: Metres MET: Metabolic equivalent MRCD: Medical Research Council Dyspnoea NSCLC: Non‐small cell lung cancer PA: Phyiscal activity PImax: maximum inspiratory pressure QG: Qigong RPE: Rating of perceived exertion SCLC: Small cell lung cancer SD: Standard deviation VO2peak: Peak oxygen uptake
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aboda 2018 | Conference abstract: incomplete data and no response to our contact attempts |
| Adamsen 2012 | Different outcomes |
| Barton 2010 | Intervention did not meet inclusion criteria |
| Brocki 2016 | < 50% of participants had stages IIIB‐IV NSCLC |
| Chen 2015 | < 50% of participants had stages IIIB‐IV NSCLC |
| Chen 2016 | Different outcomes |
| Cheville 2013 | Incomplete data: no response to our contact attempts |
| Diepold 2016 | Different outcomes |
| Hung 2015 | Incomplete data: no response to our contact attempts |
| Jacobsen 2013 | < 50% of participants had stages IIIB‐IV NSCLC |
| Karvinen 2014 | Different outcomes |
| Khan 2016 | Intervention did not meet inclusion criteria |
| Maddocks 2009 | Intervention did not meet inclusion criteria |
| Maddocks 2013 | Intervention did not meet inclusion criteria |
| Molasiotis 2013 | Conference abstract of included study |
| Molassiotis 2014 | Duplicate of study |
| Oh 2008 | Intervention did not meet inclusion criteria |
| Oh 2010 | Intervention did not meet inclusion criteria |
| Oh 2012 | Intervention did not meet inclusion criteria |
| Oldervoll 2011 | < 50% of participants had stages IIIB‐IV NSCLC |
| Salhi 2014 | < 50% of participants had stages IIIB‐IV NSCLC |
| Salhi 2015 | < 50% of participants had stages IIIB‐IV NSCLC |
| Solheim 2017 | Intervention did not meet inclusion criteria |
| Uster 2016 | Intervention did not meet inclusion criteria |
| Zhang 2016 | Intervention did not meet inclusion criteria |
NSCLC: non‐small cell lung cancer RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12614001268639.
| Trial name or title | rehabiliTation In lunG cancER ‐ The TIGER trial |
| Methods | Randomised controlled trial Setting: home‐based, Australia Study duration: 6 months |
| Participants | Inclusion criteria: eligible patients will have a histologically confirmed diagnosis of inoperable, NSCLC; be scheduled to receive treatment other than surgery (radiotherapy, chemotherapy, targeted therapy); be aged 18 years or older; be able to read and write English; have an Eastern Cooperative Oncology Group performance status of 0‐2 and Clinical Frailty Scale score of less than 7 at study entry; have a physician rated estimated life expectancy of greater than or equal to 6 months; have primary attending oncologist approval; have been sedentary in the past month (i.e. patients not performing regular exercise on at least 5 days a week, for at least 30 minutes each session, at a moderate or vigorous intensity for the past month). Patients will be excluded if they have: pelvic or lower limb bony metastases, an unstable psychiatric or cognitive disorder; presence of a concurrent, actively treated other malignancy or history of other malignancy treated within the past one year (or three if treatment within the vicinity of the lung fields, e.g. radiotherapy for breast or oesophageal cancer), other than non‐melanoma skin cancer or in‐situ melanoma, or have any other comorbidities preventing participation in a land‐based exercise programme. |
| Interventions | Control: usual practice. All patients, including those allocated to usual care, will receive 2 booklets: on lung cancer diagnosis and treatment and exercise for people living with cancer, produced by Cancer Council Victoria. Participants will also report (via telephone calls from our research assistant every 4 weeks) their levels of exercise during the intervention. Intervention: in addition to usual care, participants will receive 8 weeks of a structured multidisciplinary intervention including a personalised aerobic and resistance exercise programme, goal setting and follow‐up in one‐to‐one phone and home visit consultations, and symptom self‐care education. At the completion of the 8‐week home‐based programme, a maintenance exercise programme will be prescribed for each participant individually, with monthly physiotherapy telephone contact to monitor adherence and progress to the final 6‐month time point. |
| Outcomes | Exercise capacity (6MWD), physical activity (7 days of accelerometry), muscle strength (quadriceps and hand‐grip assessed using hand held dynamometry), HRQoL (FACT‐L), dyspnoea (Likert Scale, high worse), anxiety and depression (Hospital Anxiety and Depression Scale) |
| Starting date | 1 December 2014 |
| Contact information | Ms Lara Edbrooke: larae@unimelb.edu.au |
| Notes |
CTRI/2015/01/005348.
| Trial name or title | Exercise for the management of the cancer‐related fatigue in advanced lung cancer. ‐ ExAL |
| Methods | Randomised controlled trial Setting: home‐based, India Study duration: 2 months |
| Participants | Inclusion criteria: diagnosis of advanced lung cancer (stage III–IV NSCLC), declared as unresectable by the multidisciplinary oncology team; aged 18 or above with WHO performance status 0–2; medically fit to undergo mixed type of exercise (aerobics and resistance); ability to fill the self‐reported questionnaire in any of the three languages (English, Hindi and Marathi); not being previously enrolled in a physiotherapy or exercise program; posted for or receiving chemotherapy or radiotherapy or combination of chemo‐radiotherapy. Exclusion criteria: life expectancy less than 6 months; inability to complete baseline physical fitness test; pregnancy; any neurological, orthopaedic condition that may impede ambulation; suffering from severe mental or cognitive impairment. |
| Interventions | Control: usual care — breathing exercises, incentive spirometer exercises and education regarding importance of physical activity. Patients will be reviewed with respiratory care program at equal number of visits that of intervention group. Intervention: progressive resistance exercises for 2 months. Resistance exercises will start with 60% to 70% of 1 Repetition Maximum (RM). Aerobic exercise training will be mainly walking; however, swimming, cycling and running may be considered as alternative aerobic activities. The aim is to achieve 30‐60 minutes of moderate‐intensity aerobic exercise for 5 days a week. However, considering many of the patients with advanced lung cancer may not be able to perform such intensity in the initial stages, intensity will be modified as per their functional status and their age adjusted predicted maximum heart rate. Initial intensity of light (%HRR 30‐39) to moderate (%HRR 40‐49) will be prescribed according to patients condition and during training participants will be encouraged to maintain their aerobic activity perceived exertion up to “Somewhat Hard” on the Modified Borg scale. |
| Outcomes | Cancer related fatigue (Functional Assessment of Chronic Illness Therapy‐ fatigue [FACIT‐F]), HRQoL (FACT‐L), exercise capacity (6MWD), muscle strength (hand‐grip assessed using hand held dynamometry), survival. |
| Starting date | 31 January 2015 |
| Contact information | Vincent Singh Paramanandam: vinsu24@gmail.com |
| Notes |
NCT01881906.
| Trial name or title | Exercise in Advanced Stage Lung Cancer Patients ‐ EXHALE |
| Methods | Randomised controlled trial Setting: outpatient, Denmark Study duration: 12 weeks |
| Participants | Inclusion criteria: non‐small cell lung cancer stage IIIb‐IV; small cell lung cancer extensive disease; patients > 18 years; WHO performance status 0‐2; undergoing chemotherapy. Exclusion criteria: brain or bone metastases; prolonged bone marrow suppression; anti‐coagulant treatment; symptomatic heart disease congestive heart failure; arrhythmia; myocardial infarction diagnosed within the last three months; inability to provide informed consent. |
| Interventions | Control: usual care. The patients randomised to the control group will receive no training but are offered the chance to participate in the supervised training after they have completed their antineoplastic treatment, at least after twelve weeks. Patients in early second‐line treatment ("switch maintenance") will be offered training after 12 weeks, although they have not completed chemotherapy. Intervention: each session has a duration of 1.5 hours and occurs twice weekly, supervised by a research physiotherapist. The training comprises warm‐up exercises, strength and fitness training, as well as stretching. Strength training will be carried out using 6 machines (Technogym: leg press, chest press, lateral machine, leg extension, abdominal crunch, and lower back). The practical aim of strength training was to complete 3 series of 5‐8 sets, with 70% to 90% of 1RM. Cardiovascular training will be carried out as interval training on stationery bikes. Intensity will be equivalent to 85% to 95% of each patient's maximum HR and will last approximately 10‐15 minutes. |
| Outcomes | Exercise capacity (VO2peak and 6MWD), muscle strength (1RM), lung function (FEV1); HRQoL (SF‐36, FACT‐L), anxiety and depression (HADS) |
| Starting date | February 2012 |
| Contact information | Morten Quist: morten.quist@regionh.dk |
| Notes |
NCT02055508.
| Trial name or title | POSITIVE ‐ Physical Exercise Program in Lung Cancer Patients With Non‐operable Disease Undergoing Palliative Treatment |
| Methods | Randomised controlled trial Setting: inpatient and outpatient, Germany Study duration: 24 weeks |
| Participants | Inclusion criteria: NSCLC stage IIIB/IV; receiving systemic treatment (palliative radiotherapy accepted); BMI > 18
ECOG (Eastern Cooperative Oncology Group) performance status ≤ 2; signed informed consent. Exclusion criteria: serious active infection (i.e. requiring an intravenous antibiotic, antifungal or antiviral agent); inability to walk; immobility (more than two days); previously untreated (non‐irradiated or non‐resected) symptomatic brain metastases; permitted are: 1) previously treated brain metastases (radiotherapy, surgery, dexamethasone dosage 8 mg per day, anti‐epileptic therapy); 2) asymptomatic brain metastases without additional therapy requirement; severe neurologic impairment (e.g. apoplectic insult, Morbus Parkinson, pareses of extremities); severe cardiac impairment (e.g. cardiac insufficiency NYHA (New York Heart Association) > III, myocardial infarction within the last three months, unexplained syncope events, severe cardiac arrhythmias, high grade aortic stenosis); severe respiratory insufficiency; uncontrolled pain abuse of alcohol or drugs reducing compliance to the study; bone metastasis inducing skeletal fragility; any circumstance that would impede ability to give informed consent or adherence to study requirements. |
| Interventions | Control: weekly "care‐management‐phone‐call" (CMPC), performed by an advanced practice nurse (APN). The CMPCs are based on a structured questionnaire, reflecting pain, shortness of breath, disturbed sleep, exhaustion and distress and potentially treatment related side effects (e.g. infections, polyneuropathy, etc.). In case of demanding management of symptoms or complaints (e.g. uncontrolled pain or breathlessness) the treating physician is contacted by the APN to facilitate improvement. Intervention: combined resistance and endurance program consisting of free weight and rubber band training for major upper and lower body muscle groups respectively of cycling/walking on an ergometer/treadmill 3 times a week. Outpatient periods (3 times a week at least two/one supervised training sessions): supervised training sessions in the local outpatient training centre will comprise of resistance exercise on machines and endurance training on an ergometer/treadmill. For non‐supervised training session during the outpatient period, participants will receive an exercise manual for individualised home‐based exercising. In weekly phone calls, the advanced practice nurse will review adherence to the intervention and identify problems. |
| Outcomes | HRQoL (FACT‐L), general fatigue (Multidimensional Fatigue Inventory), exercise capacity (6MWD), muscle strength (hand‐grip assessed using hand held dynamometry), survival |
| Starting date | December 2013 |
| Contact information | Joachim Wiskemann: joachim.wiskemann@nct‐heidelberg.de |
| Notes |
NCT03066271.
| Trial name or title | Pre Radiotherapy Daily Exercise Training in Non‐Small Cell Lung Cancer (PRIME) |
| Methods | Randomised controlled trial Setting: Denmark Study duration: 6 weeks |
| Participants | Inclusion criteria: non‐small cell lung cancer treated with concomitant chemo‐ and radiotherapy; age: > 18 year; WHO performance status 0‐1. Exclusion criteria: patients with any symptoms or circumstances that advise against physical activity; symptomatic heart disease; congestive heart failure; inability to read and speak Danish; brain or bone metastases; prolonged bone marrow suppression; anti‐coagulant treatment; inability to provide informed consent. |
| Interventions | Control: patients randomised to the control group received no exercise training but were equipped with a Garmin vivo‐smart HR® activity tracker every day in 24h during the course of radiotherapy treatment. Intervention: patients randomised to the intervention group received supervised daily exercise training (Monday to Friday) on a cycle ergometer for 20 minutes prior to radiotherapy treatment. The training comprised a warm‐up phase followed by 3 exercise phases. Warm‐up consisted of 5 mins light stationary cycling, adjusted to 50% to 60% of the patient's peak power output determined at the incremental cycle test (iPPO). The first exercise phase comprised of 5 mins interval training consisting of 5 x 30 sec intervals at 80% to 95% of the patient's iPPO. Between each interval, there was a 30 sec pause. The second exercise phase consisted of 5 mins continuous cycling at an intensity equalling 80% of the patient's iPPO. The third exercise phase was similar to the first exercise phase. Intensities increased progressively from the first week to the last week (from 50%, 80% and 70% of iPPO according to the three different phases, to 60%, 95% and 80% of iPPO respectively). Furthermore, patients were equipped with a Garmin vivo‐smart HR® activity tracker every day in 24h during the course of radiotherapy treatment. |
| Outcomes | Exercise capacity (VO2peak and 6MWD), lung function (FEV1, ventilation and tidal volume measured by indirect calorimetry), hypoxia in tumor (dynamic contrast‐enhanced magnetic resonance imaging), stroke volume, cardiac output, systemic vascular resistance, safety (sports‐injury, pain, neuropathies, nausea/vomiting, fatigue etc.), respiratory exchange ration, rated perceived exertion, overall survival rate, physical activity (International Physical Activity Questionnaire Long), quality of life and well‐being (Functional Assessment of Cancer Therapy ‐ Lung), anxiety and depression (Hospital Anxiety and Depression Scale), activity data (steps, distance and intensity minutes measured by Garmin vivo‐smart HR® activity tracker). |
| Starting date | 3 April 2017 |
| Contact information | Morten Quist: morten.quist@regionh.dk |
| Notes |
NCT03334071.
| Trial name or title | Exercise Intervention During Chemotherapy in Advanced Lung Cancer Patients (EMBRACE) |
| Methods | Pilot randomised controlled trial Setting: inpatient, home‐based, United Kingdom Study duration: 12 weeks |
| Participants | Inclusion criteria: male or female patients, aged over 18 years old; histologically or cytologically confirmed NSCLC (adenocarcinoma, squamous cell carcinoma, large cell carcinoma, undifferentiated carcinoma or other); stage IIIb/IV disease; patients being treated with first‐line gemcitabine and platinum based chemotherapy; performance status 0‐2. Exclusion criteria: unable to consent; unable to perform CPET; significant cardiac ischaemia of > 1.5 mm symptomatic and > 2 mm asymptomatic observed on the baseline ECG. |
| Interventions | Control: no exercise Intervention: supervised in‐hospital, exercise training programme on a cycle ergometer before and during chemotherapy. At week 5‐6 there will be a transition period of in‐hospital to home‐based exercise training (at this point we will perform the exercises that they will perform at home in the in‐hospital environment to ensure that the patient understands the home‐based exercise training programme) and then week 7‐12 will be home‐based exercise training only with telephone support. |
| Outcomes | Adverse events and number of participants completing exercise sessions as a function of the whole programme. Exercise capacity (VO2peak), muscle strength (hand‐grip assessed using hand held dynamometry), HRQoL (no details of questionnaire reported), physical activity (no details reported). |
| Starting date | 4 April 2014 |
| Contact information | Samantha Legett: Samantha.leggett@uhs.nhs.uk |
| Notes |
NCT03482323.
| Trial name or title | Aerobic Exercise and Tai‐chi Interventions for Improving Survival in Lung Cancer Patients |
| Methods | Randomised controlled trial Setting: Hong Kong Study duration: 12 weeks |
| Participants | Inclusion criteria: stage IIIB, or IV non‐small‐cell lung cancer confirmed by pathology; not currently engaged in other research or participant in any other exercise or mind‐body classes; age at least 18 years old; able to communicate in Cantonese, Mandarin or English; no other cancer diagnosis within the previous 1 year; report not doing regular exercises (defined < 150 min of moderate‐intensity exercise weekly) in daily living, but are able to attend either exercise or tai‐chi classes at scheduled times; being conscious and alert. Exclusion criteria: suffering from a diagnosed active neurological, substance abuse and/or psychiatric disorders (i.e. depression, chronic insomnia). |
| Interventions | Control: receive written information on health levels of physical activity, which they can participate in at home (self‐management) and continue to receive their usual care. At the end of the evaluation stage of the study control participants are invited to take part in an intervention of their choice. Exercise intervention: supervised exercise classes twice a week for 12 weeks. Classes includes aerobic exercises of walking (on treadmill, or out‐doors, at a moderate intensity of exercise, determined by baseline physical functioning assessment and modified based on Rated Perceived Exertion) or cycling on a stationary bike. Four moderate‐intensity strengthening exercises (legs, arms, abdomen, trunk) are included in one of the exercise classes each week. Tai‐chi intervention: supervised classes (taught by an experienced tai‐chi master) twice a week for 12 weeks (˜60 minutes/class). Supervised sessions include a warm up, self‐massage and a guided run‐through of the movements, breathing techniques, and relaxation in tai‐chi. The tai‐chi master will guide participants to practice the tai‐chi they learn in the classes at home each day. |
| Outcomes | One‐year survival rate, levels of physical activity (Actigraph accelerometer), circadian rhythms (Actigraph accelerometer, melatonin rhythms and cortisol rhythms will be measured using saliva samples), functional capacity (6‐minute walking test), physical functioning (timed up and go test, sit to stand test, single leg standing test, Get Active Questionnaire), immune functions (cytotoxic activity of natural killer cells, and spontaneous or phytohemagglutinin (PHA)‐stimulated T‐lymphocyte proliferation), health‐related quality of life (EORTC QLQC30, EORTC QLQ LC13), psychological distress (Hospital Anxiety and Depression Score), sleep quality (Pittsburgh Sleep Quality Index), fatigue (Brief Fatigue Inventory). |
| Starting date | 10 May 2018 |
| Contact information | Chia‐Chin Lin, cclin@hku.hk |
| Notes |
NCT03500393.
| Trial name or title | A Remotely Supervised Exercise Program for Lung Cancer Patients Undergoing Chemoradiation (REM) |
| Methods | Randomised controlled trial Setting: home‐based, United States of Americia Study duration: from at least two‐weeks prior to beginning chemoradiation to one‐month post‐chemoradiation. |
| Participants | Inclusion criteria: over the age of 18 and diagnosed with Stage IIa to IIIb lung cancer; definitive treatment with chemoradiation with weekly carboplatin and paclitaxel concurrent with radiation is planned to begin in no less than 2 weeks; have an Apple or Android device with capacity to install a fitness device app and access to either WiFi or cellular service; English‐speaking and able to provide voluntary, written consent; able to tolerate chemoradiation as indicated by Zubrod/ECOG Performance Status 0‐1; CBC/differential obtained within 14 days prior to registration on study, with adequate bone marrow function defined as follows: absolute neutrophil count (ANC) ≥ 1,500 cells/mm3; platelets ≥ 100,000 cells/mm3; haemoglobin ≥ 8.0 g/dl; adequate renal function within 14 days prior to registration, defined as creatinine clearance must be at least 35 ml/min; Adequate hepatic function within 14 days prior to registration, defined as total bilirubin ≤ 1.5 x upper limit of normal (ULN) for the institution and ALT, AST, and alkaline phosphatase ≤ 2.5 x ULN for the institution; no prior thoracic radiation therapy. Exclusion criteria: life expectancy of < 12 months or are receiving hospice services; psychiatric diagnosis that would require significant study modification to meet their needs such as uncontrolled severe mental illness, substance abuse, or active suicidal ideation; exhibit American College of Sports Medicine contraindications to exercise (includes a resting heart rate of > 120 bpm, blood pressure > 180/100 mmHg or unstable angina 40 or musculoskeletal issue preventing exercise; are unable to walk 100 meters); less than 2 weeks to the beginning of chemoradiation; physician discretion; are unable to walk or to complete the 6‐minute walk test. |
| Interventions | Active comparator: Unsupervised Exercise (UNSUP). The control condition represents a minimalist intervention that could occur in any setting: 1) enthusiastic provision on an exercise prescription, and 2) provision of a fitness device (i.e. the Garmin VivioActive) that can help participants track their exercise engagement. Participants are instructed in how to use the device to track their adherence to the exercise prescription. Experimental: Remotely Supervised Exercise (REM) designed to function as an acceptance‐based health coaching intervention and will utilise theory‐based behaviour change techniques (i.e. goal setting/action planning, self‐monitoring, receiving feedback, and reviewing relevant goals in the light of feedback) to promote adoption and adherence to the exercise prescription. |
| Outcomes | Recruitment and retention statistics (number of participants enrolled/number of patients eligible; number completing all data collection/number enrolled; number adhering to randomisation/number enrolled; number withdrawn/number enrolled), minutes spent in exercise (collected from fitness device), functional capacity (6MWD), physical functioning (timed up‐and‐go test, five times sit‐to‐stand test), lung function (FEV1, DLCO, FVC), Physical Function Scale of the Patient Reported Outcomes Measurement information System, Pittsburgh Sleep Quality Index, Functional Assessment of Cancer Therapy‐fatigue scale, Dose reductions (determine the degree to which participants received the prescribed regimen of chemoradiation), grip strength (JAMAR Handheld Dynamometer). |
| Starting date | 22 June 2018 |
| Contact information | Kayla Fay: Kayla.A.Fay@hitchcock.org |
| Notes |
6MWD: Six‐minute walk distance 6MWT: Six‐minute walk test BMI: Body mass index bpm: Beats per minute CPET: Cardiopulmonary exercise test DLCO: Diffusing capacity of the lungs for carbon monoxide EORTC QLQC30: European Orgnisation for Research and Treatment of Cancer Core Quality of Life Questionnaire EORTC QLQ LC13: European Orgnisation for Research and Treatment of Cancer lung cancer‐specific questionnaire module
FEV1: Forced expiratory volume in one second FVC: Forced vital capacity HRQoL: Health‐related quality of life NSCLC: Non‐small cell lung cancer VO2peak: Peak oxygen uptake
Differences between protocol and review
There were four main differences between our review and the previously published protocol (Peddle‐McIntyre 2017). First, we changed the outcome measure "health‐related quality of life (HRQoL)" to "disease‐specific global HRQoL" to more accurately reflect the construct being analysed. Further, we added the outcome measure of physical functioning component of HRQoL. This was added to examine the effects of exercise training and the specific physical functioning aspects of HRQoL. Second, as we included less than 10 studies in our meta‐analyses, we did not examine funnel plots for signs of asymmetry. Third, force‐generating capacity of peripheral muscles was not included in the GRADE 'Summary of findings' table due to lack of data. We included dyspnoea instead. Finally, while we had identified 12‐month overall survival as a secondary outcome measure, only one study reported a survival outcome of overall survival. Therefore we have included overall survival as our secondary outcome rather than 12‐month overall survival.
Contributions of authors
CPM initiated, wrote and organised the protocol and review into Review Manager, selected studies, rated risk of bias, extracted data from studies, conducted the analysis and wrote the final review paper.
VC wrote the protocol and review, developed the protocol and methodological topics, extracted data from the studies, contacted authors for additional information, conducted the analysis and critically appraised the final review paper.
FS wrote and developed the protocol, selected studies, and rated risk of bias.
RT, RN and DG critically appraised the protocol versions and the final review paper.
Sources of support
Internal sources
Exercise Medicine Research Institute, Edith Cowan University, Joondalup, Australia.
School of Medical and Health Sciences, Edith Cowan University, Joondalup, Australia.
School of Physiotherapy and Exercise Science, Faculty of Health Sciences, Curtin University, Bentley, Australia.
Sir Charles Gairdner Hospital, Nedlands, Australia.
Institute for Respiratory Health, Sir Charles Gairdner Hospital, Nedlands, Australia.
External sources
-
Cancer Council Western Australia Postdoctoral Research Fellowships, Australia.
Carolyn McIntyre, Rajesh Thomas, and Vinicius Cavalheri
-
Cancer Council Western Australia Research Fellowship, Australia.
Daniel A. Galvão
Declarations of interest
Carolyn Peddle‐McIntyre: none known.
Favil Singh: none known.
Rajesh Thomas: none known.
Robert Newton: none known.
Daniel Galvão: none known.
Vinicius Cavalheri: none known.
New
References
References to studies included in this review
Dhillon 2017 {published data only (unpublished sought but not used)}
- Dhillon H, Bell M, Ploeg H, Turner J, Kabourakis M, Spencer L, et al. The impact of physical activity on fatigue and quality of life in lung cancer patients: A randomised controlled trial. Asia‐Pacific Journal of Clinical Oncology. 2015; Vol. 11:70. [DOI: 10.1111/ajco.12432] [DOI]
- Dhillon HM, Bell ML, Ploeg HP, Turner JD, Kabourakis M, Spencer L, et al. Impact of physical activity on fatigue and quality of life in people with advanced lung cancer: a randomized controlled trial. Annals of Oncology 2017;28(8):1889‐1897. [DOI] [PubMed] [Google Scholar]
Henke 2014 {published data only}
- Henke C, Cabri J, Fricke L, Kandilakis G, Wulf P, Feyer PC, Wit M. Enhanced physical activity intervention in lung cancer patients during palliative chemotherapy‐a randomized controlled trial. Onkologie 2012;35:139. [DOI: 10.1159/000258182] [DOI] [Google Scholar]
- Henke CC, Cabri J, Fricke L, Pankow W, Kandilakis G, Feyer PC, et al. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Support Care Cancer 2014;22(1):95‐101. [DOI] [PubMed] [Google Scholar]
- Henke CC, Cabri J, Fricke L, Pankow W, Kandilakis G, Feyer PC, et al. Strength and endurance training in the treatment of lung cancer patients staged IIIA/IIIB/IV. Journal of Clinical Oncology. 2012; Vol. 30, issue 15 suppl. 1:9033.
Hwang 2012 {published data only}
- Hwang CL, Yu CJ, Shih JY, Yang PC, Wu YT. Effects of exercise training on exercise capacity in patients with non‐small cell lung cancer receiving targeted therapy. Supportive Care in Cancer 2012;20(12):3169‐3177. [DOI] [PubMed] [Google Scholar]
Jastrzebski 2015 {published data only}
- Jastrzebski D, Maksymiak M, Kostorz S, Bezubka B, Osmanska I, Mlynczak T, et al. Pulmonary rehabilitation in advanced lung cancer patients during chemotherapy. Advances in Experimental Medicine and Biology 2015;14:57‐64. [DOI] [PubMed] [Google Scholar]
Molassiotis 2015 {published and unpublished data}
- Molassiotis A, Charalambous A, Taylor P, Stamataki Z, Summers Y. The effect of resistance inspiratory muscle training the management of breathlessness in patients with thoracic malignancies: a feasibility randomised trial. Supportive Care in Cancer 2015;23(6):1637‐45. [DOI] [PubMed] [Google Scholar]
Vanderbyl 2017 {published and unpublished data}
- Vanderbyl BL, Mayer MJ, Nash C, Tran AT, Windholz T, Swanson T, et al. A comparison of the effects of medical Qigong and standard exercise therapy on symptoms and quality of life in patients with advanced cancer. Supportive Care in Cancer 2017;25(6):1749‐58. [DOI] [PubMed] [Google Scholar]
- Vanderbyl BL, Nash C, Tran AT, Windholz T, Swanson T, Kasymjanova G, et al. Standard exercise training offers advantages over Qigong for patients with advanced lung and gastrointestinal cancer. Supportive Care in Cancer. 2012; Vol. 20:S200. [DOI: 10.1007/s00520-012-1479-7] [DOI] [PubMed]
References to studies excluded from this review
Aboda 2018 {published data only}
- Aboda A, Taha W, Elkady A, Kanwar Jr. A pilot study for randomized controlled trial of the benefits of a physical exercise program for cancer cachexia patients. Journal of Cachexia, Sarcopenia and Muscle. Conference: 10th International Conference on Cachexia, Sarcopenia and Muscle Wasting. Italy. 2018; Vol. 9, issue 1:211.
Adamsen 2012 {published data only}
- Adamsen L, Stage M, Laursen J, Rorth M, Quist M. Exercise and relaxation intervention for patients with advanced lung cancer: a qualitative feasibility study. Scandinavian Journal of Medicine and Science in Sports 2012;22(6):804‐15. [DOI: 10.1111/j.1600-0838.2011.01323.x] [DOI] [PubMed] [Google Scholar]
Barton 2010 {published data only}
- Barton, R, English, A, Nabb S, Rigby AS, Johnson MJ. A randomised trial of high vs low intensity training in breathing techniques for breathless patients with malignant lung disease: a feasibility study. Lung Cancer 2010;70(3):313‐9. [DOI: 10.1016/j.lungcan.2010.03.007] [DOI] [PubMed] [Google Scholar]
Brocki 2016 {published data only}
- Brocki BC, Andreasen JJ, Langer D, Souza DS, Westerdahl E. Postoperative inspiratory muscle training in addition to breathing exercises and early mobilization improves oxygenation in high‐risk patients after lung cancer surgery: a randomized controlled trial. European Journal of Cardio‐thoracic Surgery 2016;49(5):1483‐91. [DOI: 10.1093/ejcts/ezv359] [DOI] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen HM, Tsai CM, Wu YC, Lin KC, Lin CC. Randomised controlled trial on the effectiveness of home‐based walking exercise on anxiety, depression and cancer‐related symptoms in patients with lung cancer. British Journal of Cancer 2015;112(3):438‐45. [DOI: 10.1038/bjc.2014.612] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HM, Tsai CM, Wu YC, Lin KC, Lin CC. Randomised controlled trial on the effectiveness of home‐based walking exercise on anxiety, depression and cancer‐related symptoms in patients with lung cancer. Palliative Medicine. 2016; Vol. 30, issue 6:Np15‐np16. [DOI: 10.1177/0269216316646056] [DOI] [PMC free article] [PubMed]
Chen 2016 {published data only}
- Chen HM, Tsai CM, Wu YC, Lin KC, Lin CC. Effect of walking on circadian rhythms and sleep quality of patients with lung cancer: a randomised controlled trial. British Journal of Cancer 2016;115(11):1304‐12. [DOI: 10.1038/bjc.2016.356] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheville 2013 {published data only (unpublished sought but not used)}
- Cheville AL, Kollasch J, Vandenberg J, Shen T, Grothey A, Gamble G, et al. A home‐based exercise program to improve function, fatigue, and sleep quality in patients with stage IV lung and colorectal cancer: A randomized controlled trial. Journal of Pain and Symptom Management 2013;45(5):811‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diepold 2016 {published data only}
- Diepold C, Wiskemann J, Hummler S, Steins M, Thomas M. Physical exercise program in non‐operable lung cancer patients undergoing palliative treatment‐preliminary report of recruitment rates and feasibility of the POSITIVE study (part III). Oncology Research and Treatment 2016;39:26. [DOI: 10.1159/000444354] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hung 2015 {published data only}
- Hung SH, Huang HL, Kou FS, Fan CI, Liao SC, Liou SG, et al. The effect of specific home‐based exercise program to cancer related fatigue and symptoms disturbance in late staged lung cancer patients. Cancer Nursing. 2015; Vol. 38, issue 4 suppl. 1:S29‐s30. [DOI: 10.1097/NCC.0000000000000287] [DOI]
Jacobsen 2013 {published data only}
- Jacobsen PB, Phillips KM, Jim HSL, Small BJ, Faul LA, Meade CD, et al. Effects of self‐directed stress management training and home‐based exercise on quality of life in cancer patients receiving chemotherapy: A randomized controlled trial. Psycho‐Oncology 2013;22(6):1229‐35. [DOI: 10.1002/pon.3122] [DOI] [PubMed] [Google Scholar]
Karvinen 2014 {published data only}
- Karvinen KH, Esposito D, Raedeke TD, Vick J, Walker PR. Effect of an exercise training intervention with resistance bands on blood cell counts during chemotherapy for lung cancer: a pilot randomized controlled trial. Springerplus 2014;3:15. [DOI: 10.1186/2193-1801-3-15] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2016 {published data only}
- Khan I, Manna A, Sinha S, Sarkar Sk. Randomized controlled trial of yoga among non small cell lung cancer patients: effects on pulmonary function. European Journal of Cancer 2016;54:S8. [Google Scholar]
Maddocks 2009 {published data only}
- Maddocks M, Lewis M, Chauhan A, Manderson C, Hocknell J, Wilcock A. Randomized controlled pilot study of neuromuscular electrical stimulation of the quadriceps in patients with non‐small cell lung cancer. Journal of Pain and Symptom Management 2009;38(6):950‐6. [DOI: 10.1016/j.jpainsymman.2009.05.011] [DOI] [PubMed] [Google Scholar]
Maddocks 2013 {published data only}
- Byrne A, Maddocks M, Halliday V, Chauhan A, Taylor V, Nelson A, et al. Is neuromuscular electrical stimulation (NMES) an acceptable and feasible exercise intervention for patients with non‐small cell lung cancer (NSCLC) receiving palliative chemotherapy? Preliminary results from the NCRI NMES trial. Lung Cancer 2013;79:S46‐7. [Google Scholar]
- Maddocks M, Halliday V, Chauhan A, Taylor V, Nelson A, Sampson C, et al. Neuromuscular electrical stimulation of the quadriceps in patients with non‐small cell lung cancer receiving palliative chemotherapy: A randomized phase II study. PLoS ONE 2013;8(12):e86059. [DOI: 10.1371/journal.pone.0086059] [DOI] [PMC free article] [PubMed] [Google Scholar]
Molasiotis 2013 {published data only}
- Molassiotis A, Charalambous A, Taylor P, Stamataki Z, Summers YJ. The effects of inspiratory muscle training in the management of breathlessness in patients with lung cancer: A pilot feasibility randomized trial. Journal of Thoracic Oncology 2013;8:S176‐S177. [DOI: 10.1097/01.JTO.0000438438.14562.c8] [DOI] [PubMed] [Google Scholar]
- Molassiotis A, Charalambous A, Taylor P, Summers Y, Stamataki Z. The effects of inspiratory muscle training in the management of breathlessness in patients with lung cancer: A pilot feasibility randomized trial. Supportive Care in Cancer 2014;22(1):S219‐S220. [DOI: 10.1007/s00520-014-2222-3] [DOI] [PubMed] [Google Scholar]
Molassiotis 2014 {published data only}
- Charalambous A, Molassiotis A, Summers Y, Stamataki Z, Taylor P. Use of inspiratory muscle training in managing dyspnoea in lung cancer patients. Journal of Thoracic Oncology. 2017; Vol. 12, issue 1:S206‐S208.
Oh 2008 {published data only}
- Oh B, Butow P, Mullan B, Clarke S. Medical Qigong for cancer patients: Pilot study of impact on quality of life, side effects of treatment and inflammation. American Journal of Chinese Medicine 2008;36(3):459‐72. [DOI: 10.1142/S0192415X08005904] [DOI] [PubMed] [Google Scholar]
Oh 2010 {published data only}
- Oh B, Butow P, Mullan B, Clarke S, Beale P, Pavlakis N, et al. Impact of Medical Qigong on quality of life, fatigue, mood and inflammation in cancer patients: A randomized controlled trial. Annals of Oncology 2010;21(3):608‐614. [DOI: 10.1093/annonc/mdp479] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oh 2012 {published data only}
- Oh B, Butow PN, Mullan BA, Clarke SJ, Beale PJ, Pavlakis N, et al. Effect of medical Qigong on cognitive function, quality of life, and a biomarker of inflammation in cancer patients: A randomized controlled trial. Supportive Care in Cancer 2012;20(6):1235‐42. [DOI: 10.1007/s00520-011-1209-6] [DOI] [PubMed] [Google Scholar]
Oldervoll 2011 {published data only}
- Oldervoll LM, Loge JH, Lydersen S, Paltiel H, Asp MB, Nygaard UV, et al. Physical exercise for cancer patients with advanced disease: A randomized controlled trial. Oncologist 2011;16(11):1649‐57. [DOI: 10.1634/theoncologist.2011-0133] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salhi 2014 {published data only}
- Salhi B, Huysse W, Maele G, Surmont VF, Derom E, Meerbeeck JP. The effect of radical treatment and rehabilitation on muscle mass and strength: a randomized trial in stages I‐III lung cancer patients. Lung Cancer 2014;84(1):56‐61. [DOI: 10.1016/j.lungcan.2014.01.011] [DOI] [PubMed] [Google Scholar]
Salhi 2015 {published data only}
- Meerbeeck JP, Salhi B, Haenebalcke C, Perez‐Bogerd S, Nguyen MD, Malfait TLA, et al. Resistance training in patients with radically treated respiratory cancer: Mature results of a multi‐centre randomised phase 3 trial (reinforce). Journal of Thoracic Oncology. 2013; Vol. 8:S245. [DOI: 10.1097/01.JTO.0000438438.14562.c8] [DOI]
- Salhi B, Haenebalcke C, Perez‐Bogerd S, Nguyen Dang DM, Colman R, Vermaelen KY, et al. Effect of resistance training (RT) on maximal exercise capacity (MEC) and muscle force (MF) in patients (pts) with radically treated (RaT) intrathoracic cancer. European Respiratory Journal. 2013; Vol. 42:P5070.
- Salhi B, Haenebalcke C, Perez‐Bogerd S, Nguyen M, Ninane V, Malfait T, et al. A randomized trial of rehabilitation in patients with radically treated intrathoracic cancer (reinforce). American Journal of Respiratory and Critical Care Medicine. 2013; Vol. 187:A1807.
- Salhi B, Haenebalcke C, Perez‐Bogerd S, Nguyen MD, Ninane V, Malfait TL, et al. Rehabilitation in patients with radically treated respiratory cancer: a randomised controlled trial comparing two training modalities. Lung Cancer 2015;89(2):167‐74. [DOI: 10.1016/j.lungcan.2015.05.013] [DOI] [PubMed] [Google Scholar]
- Salhi B, Thysebaert G, Malfait TL, Vermaelen K, Surmont VF, Huysse W, et al. The effect of radical treatment and pulmonary rehabilitation on muscle mass as measured with CT‐scan: A randomised trial in patients (pts) with lung cancer and mesothelioma. European Respiratory Journal. 2012; Vol. 40:2840.
- Surmont VF, Salhi B, Haenebalcke C, Perez Bogerd S, Nguyen Dang DM, Colman R, et al. REINFORCE: A randomized trial of resistance training in patients with radically treated respiratory cancer. Journal of Clinical Oncology. 2013; Vol. 31, issue 15 suppl. 1:7541.
- Meerbeeck J, Salhi B, Haenebalcke C, Perez‐Bogerd S, Nguyen, MD, Malfait, T, et al. Resistance training in patients with radically treated respiratory cancer: A prospective randomized multi‐center study (REINFORCE). European Journal of Cancer 2013;49:S796‐7. [DOI: 10.1016/S0959-8049(13)70065-0] [DOI] [Google Scholar]
Solheim 2017 {published data only}
- Kaasa S, Solheim T, Laird BJA, Balstad T, Stene GB, Bye A, et al. A randomised, open‐label trial of a Multimodal Intervention (Exercise, Nutrition and Anti‐inflammatory Medication) plus standard care versus standard care alone to prevent/attenuate cachexia in advanced cancer patients undergoing chemotherapy. Journal of Clinical Oncology. 2015; Vol. 33, issue 15_suppl:9628.
- Solheim TS, Laird BJA, Balstad TR, Stene GB, Bye A, Johns N, et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. Journal of Cachexia, Sarcopenia and Muscle 2017;8(5):778‐88. [DOI: 10.1002/jcsm.12201] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uster 2016 {published data only}
- Uster A, Ruhlin M, Mey S, Gisi D, Knols R, Imoberdorf R, et al. Combined nutritional and physical intervention leads to a sustained increase of dietary intake in palliative cancer patients ‐a randomised controlled study. Clinical nutrition. 2016; Vol. 35:S148.
- Uster A, Ruhlin M, Mey S, Gisi D, Knols R, Imoberdorf R, et al. Effect of nutrition and physical exercise intervention on quality of life and treatment related complications in advanced cancer patients: a randomised controlled trial. Clinical nutrition. 2016; Vol. 35:S148. [DOI] [PubMed]
Zhang 2016 {published data only}
- Zhang LL, Wang SZ, Chen HL, Yuan AZ. Tai Chi exercise for cancer‐related fatigue in patients with lung cancer undergoing chemotherapy: a randomized controlled trial. Journal of Pain and Symptom Management 2016;51(3):504‐11. [DOI: 10.1016/j.jpainsymman.2015.11.020] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12614001268639 {published data only (unpublished sought but not used)}
- ACTRN12614001268639. In people with inoperable non small cell lung cancer, what is the effect of home‐based multi‐disciplinary rehabilitation compared to usual care, on physical function, symptoms and health related quality of life?. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367489&isReview=true (first received 23 November 2014).
CTRI/2015/01/005348 {published data only (unpublished sought but not used)}
- CTRI/2015/01/005348. Exercise for the management of the cancer‐related fatigue in advanced lung cancer planned for systemic palliative therapy: randomized controlled trial. ‐ ExAL. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=10425 (first received 05 January 2015).
NCT01881906 {published data only (unpublished sought but not used)}
- NCT01881906. "EXHALE" A randomized clinical trial comparing the effects of a 12 week supervised exercise intervention versus usual care for advanced stage lung cancer patients. https://clinicaltrials.gov/show/NCT01881906 (first received 20 June 2013). [DOI] [PMC free article] [PubMed]
- Quist M. Exercise improves functional capacity in patients with advance stage lung cancer. Journal of Thoracic Oncology. 2017; Vol. 12, issue 11 Supplement 2:S1774 (11.02).
NCT02055508 {published data only (unpublished sought but not used)}
- NCT02055508. Physical Exercise Program in Lung Cancer Patients With Non‐operable Disease Undergoing Palliative Treatment. https://clinicaltrials.gov/show/NCT02055508 (first received 5 February 2014).
NCT03066271 {published data only (unpublished sought but not used)}
- NCT03066271. Pre radiotherapy daily exercise training in non‐small cell lung cancer (PRIME). https://clinicaltrials.gov/ct2/show/record/NCT03066271?term=Pre+Radiotherapy+Daily+Exercise+Training+in+Non‐Small+Cell+Lung+Cancer&rank=1 (first received 28 February 2017).
NCT03334071 {published data only (unpublished sought but not used)}
- NCT03334071. Exercise regimens before and during advanced cancer therapy: a pilot study to investigate improvements in physical fitness with exercise training programme before and during chemotherapy in advanced lung cancer patients. https://clinicaltrials.gov/show/NCT03334071 (first received 7 November 2017).
NCT03482323 {published data only (unpublished sought but not used)}
- NCT03482323. Aerobic exercise and tai‐chi interventions for improving survival in lung cancer patients. https://clinicaltrials.gov/ct2/show/record/NCT03482323?term=Aerobic+Exercise+and+Tai‐chi+Interventions+for+Improving+Survival+in+Lung+Cancer+Patients&rank=1 (first received 29 March 2018).
NCT03500393 {published data only (unpublished sought but not used)}
- NCT03500393. A remotely supervised exercise program for lung cancer patients undergoing chemoradiation (REM). https://clinicaltrials.gov/ct2/show/record/NCT03500393?term=A+Remotely+Supervised+Exercise+Program+for+Lung+Cancer+Patients+Undergoing+Chemoradiation+%28REM%29&rank=1 (first received 17 April 2018).
Additional references
Aaronson 1993
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. Journal of National Cancer Institute 1993;85(5):365‐76. [DOI] [PubMed] [Google Scholar]
Aarts 2015
- Aarts MJ, Aerts JG, Borne BE, Biesma B, Lemmens VE, Kloover JS. Comorbidity in patients with small‐cell lung cancer: trends and prognostic impact. Clinical Lung Cancer 2015;16(4):282‐91. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Services Research 2004; Vol. 4, issue 1:38. [DOI] [PMC free article] [PubMed]
Bade 2015
Baracos 2010
- Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non‐small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. American Journal of Clinical Nutrition 2010;91(4):1133S‐7S. [DOI] [PubMed] [Google Scholar]
Borg 1970
- Borg G. Perceived exertion as an indicator of somatic stress. Scandinavian journal of rehabilitation medicine 1970;2(2):92‐8. [PubMed] [Google Scholar]
Buffart, 2017
Caspersen 1985
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health‐related research. Public Health Reports (Washington, D.C. : 1974) 1985;100(2):126‐31. [PMC free article] [PubMed] [Google Scholar]
Cavalheri 2013
- Cavalheri V, Tahirah F, Nonoyama M, Jenkins S, Hill K. Exercise training undertaken by people within 12 months of lung resection for non‐small cell lung cancer. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD009955.pub2] [DOI] [PubMed] [Google Scholar]
Cavalheri 2014
- Cavalheri V, Tahirah F, Nonoyama M, Jenkins S, Hill K. Exercise training for people following lung resection for non‐small cell lung cancer ‐ a Cochrane systematic review. Cancer Treatment Reviews 2014;40(4):585‐94. [DOI: 10.1002/14651858.CD009955.pub2] [DOI] [PubMed] [Google Scholar]
Cavalheri 2017
- Cavalheri V, Granger C. Preoperative exercise training for patients with non‐small cell lung cancer. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD012020.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cella 1995
- Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy‐Lung (FACT‐L) quality of life instrument.. Lung Cancer 1995;12(3):199‐220. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988. [Google Scholar]
Covidence 2017 [Computer program]
- Veritas Health Innovation. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, (accessed 4 April 2017).
Cramp 2012
- Cramp F, Byron‐Daniel J. Exercise for the management of cancer‐related fatigue in adults. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD006145.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dajczman 2015
- Dajczman E, Wardini R, Kasymjanova G, Prefontaine D, Baltzan MA, Wolkove N. Six minute walk distance is a predictor of survival in patients with chronic obstructive pulmonary disease undergoing pulmonary rehabilitation. Canadian Respiratory Journal [Revue Canadienne de Pneumologie] 2015;22(4):225‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Dittus 2017
Ferlay 2015
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136(5):E359‐86. [DOI] [PubMed] [Google Scholar]
Fleg 2000
- Fleg JL, Pina IL, Balady GJ, Chaitman BR, Fletcher B, Lavie C, et al. Assessment of functional capacity in clinical and research applications: An advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association. Circulation 2000;102(13):1591‐7. [DOI] [PubMed] [Google Scholar]
Fletcher 1960
- Fletcher CM, Clifton M, Fairbain AS, Fry J, Gilson JC, Higgins ITT, et al. Standardised questionnaire on respiratory symptoms: a statement prepared and approved by the MRC Committee on the Aetiology of Chronic Bronchitis (MRC breathlessness score). British Medical Journal 1960;2:1665. [Google Scholar]
Gerritsen 2016
- Gerritsen JK, Vincent AJ. Exercise improves quality of life in patients with cancer: a systematic review and meta‐analysis of randomised controlled trials. British Journal of Sports Medicine 2016;50(13):796‐803. [DOI] [PubMed] [Google Scholar]
Govindan 2006
- Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small‐cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 2006;24(28):4539‐44. [DOI] [PubMed] [Google Scholar]
Granger 2013
- Granger CL, McDonald CF, Parry SM, Oliveira CC, Denehy L. Functional capacity, physical activity and muscle strength assessment of individuals with non‐small cell lung cancer: a systematic review of instruments and their measurement properties. BMC Cancer 2013;13:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Granger 2015
- Granger CL, Holland AE, Gordon IR, Denehy L. Minimal important difference of the 6‐minute walk distance in lung cancer. Chronic Respiratory Diseases March 6;12(2):146‐154. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; Vol. 336, issue 7650:924‐6. [DOI] [PMC free article] [PubMed]
Higgins 2011
- Higgins JP, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Johnsen 2009
- Johnsen AT, Petersen MA, Pedersen L, Groenvold M. Symptoms and problems in a nationally representative sample of advanced cancer patients. Palliative Medicine 2009;23(6):491‐501. [DOI] [PubMed] [Google Scholar]
Jones 2007
- Jones LW, Eves ND, Mackey JR, Peddle CJ, Haykowsky M, Joy AA, et al. Safety and feasibility of cardiopulmonary exercise testing in patients with advanced cancer. Lung Cancer 2007;55(2):225‐32. [DOI] [PubMed] [Google Scholar]
Jones 2008
- Jones LW, Eves ND, Mackey JR, Peddle CJ, Haykowsky M, Joy AA, et al. Systemic inflammation, cardiorespiratory fitness, and quality of life in patients with advanced non‐small cell lung cancer. Journal of Thoracic Oncology 2008;3(2):194‐5. [DOI] [PubMed] [Google Scholar]
Jones 2009
- Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncology 2009;10(6):598‐605. [DOI] [PubMed] [Google Scholar]
Jones 2012
- Jones LW, Hornsby WE, Goetzinger A, Forbes LM, Sherrard EL, Quist M, et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non‐small cell lung cancer. Lung Cancer 2012;76(2):248‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kartolo 2016
- Kartolo A, Cheng S, Petrella T. Motivation and preferences of exercise programmes in patients with inoperable metastatic lung cancer: a need assessment. Supportive Care in Cancer 2016;24(1):129‐37. [DOI] [PubMed] [Google Scholar]
Kasymjanova 2009
- Kasymjanova G, Correa JA, Kreisman H, Dajczman E, Pepe C, Dobson S, et al. Prognostic value of the six‐minute walk in advanced non‐small cell lung cancer. Journal of Thoracic Oncology 2009;4(5):602‐7. [DOI] [PubMed] [Google Scholar]
Lakoski 2012
- Lakoski SG, Eves ND, Douglas PS, Jones LW. Exercise rehabilitation in patients with cancer. Nature Reviews. Clinical Oncology 2012;9(5):288‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larsson 2012
- Larsson M, Ljung L, Johansson BB. Health‐related quality of life in advanced non‐small cell lung cancer: correlates and comparisons to normative data. European Journal of Cancer Care 2012;21(5):642‐9. [DOI] [PubMed] [Google Scholar]
Lehto 2017
- Lehto RH. Psychosocial challenges for patients with advanced lung cancer: interventions to improve well‐being. Lung Cancer 2017; Vol. 8:79‐90. [DOI] [PMC free article] [PubMed]
Lin 2015
- Lin YY, Rau KM, Lin CC. Longitudinal study on the impact of physical activity on the symptoms of lung cancer survivors. Supportive Care in Cancer 2015;23(12):3545‐53. [DOI] [PubMed] [Google Scholar]
Lowe 2014
- Lowe SS, Danielson B, Beaumont C, Watanabe SM, Baracos VE, Courneya KS. Associations between objectively measured physical activity and quality of life in cancer patients with brain metastases. Journal of Pain and Symptom Management 2014;48(3):322‐32. [DOI] [PubMed] [Google Scholar]
Lyer 2013
- Lyer S, Taylor‐Stokes G, Roughley A. Symptom burden and quality of life in advanced non‐small cell lung cancer patients in France and Germany. Lung Cancer 2013;81(2):288‐93. [DOI] [PubMed] [Google Scholar]
McCarthy 2015
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD003793.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mor 1984
- Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky performance status scale. An examination of its reliability and validity in a research setting.. Cancer 1984;53(9):2002‐7. [DOI] [PubMed] [Google Scholar]
Myers 2002
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. New England Journal of Medicine 2002;346(11):793‐801. [DOI] [PubMed] [Google Scholar]
NCCN 2015
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Non‐Small Cell Lung Cancer NCCN Evidence BlocksTM Guidelines for patients Non‐Small Cell Lung Cancer (Version 1). www.nccn.org/professionals/physician_gls/f_guidelines.asp#nscl (accessed 23 May 2017).
Newton 2008
- Newton RU, Galvao DA. Exercise in prevention and management of cancer. Current Treatment Options in Oncology 2008;9(2‐3):135‐46. [DOI] [PubMed] [Google Scholar]
Nilsen 2018
- Nilsen TS, Scott JM, Michalski M, Capaci C, Thomas S, Herndonx JE, et al. Novel methods for reporting of exercise dose and adherence: an exploratory analysis. Medicine and Science in Sports Exercise 2018. [DOI: 10.1249/MSS.0000000000001545] [DOI] [PMC free article] [PubMed]
Oga 2003
- Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. American Journal of Respiratory and Critical Care Medicine 2003;167(4):544‐9. [DOI] [PubMed] [Google Scholar]
Oken 1982
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology 1982;5(6):649‐55. [PubMed] [Google Scholar]
Osoba 1998
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health‐related quality‐of‐life scores. Journal of Clinical Oncology 1998;16(1):139‐44. [DOI] [PubMed] [Google Scholar]
Ozalevli 2013
- Ozalevli S. Impact of physiotherapy on patients with advanced lung cancer. Chronic Respiratory Disease 2013;10(4):223‐32. [DOI] [PubMed] [Google Scholar]
Payne 2013
- Payne C, Larkin PJ, McIlfatrick S, Dunwoody L. Gracey JH. Exercise and nutrition interventions in advanced lung cancer: a systematic review. Current Oncology 2013;20(4):e321‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peddle‐McIntyre 2017
- Peddle‐McIntyre CJ, Singh F, Thomas R, Newton RU, Galvão DA, Cavalheri V. Exercise training for advanced lung cancer. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD012685] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rivas‐Perez 2015
- Rivas‐Perez, H, Nana‐Sinkam, P. Integrating pulmonary rehabilitation into the multidisciplinary management of lung cancer: a review. Respiratory Medicine 2015;109(4):437‐42. [DOI] [PubMed] [Google Scholar]
Ross 2003
- Ross RM. ATS/ACCP statement on cardiopulmonary exercise testing. American Journal of Respiratory and Critical Care Medicine 2003;167:1451; author reply 1451. [DOI] [PubMed] [Google Scholar]
Ross 2016
- Ross R, Blair SN, Arena R, Church TS, Despres JP, Franklin BA, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation 2016;134(24):e653‐e99. [DOI] [PubMed] [Google Scholar]
Schmid 2015
- Schmid D, Leitzmann MF. Cardiorespiratory fitness as predictor of cancer mortality: a systematic review and meta‐analysis. Annals of Oncology: Official Journal of the European Society for Medical Oncology 2015;26(2):272‐8. [DOI] [PubMed] [Google Scholar]
Schmitz 2010
- Schmitz KH, Courneya KS, Matthews C, Demark‐Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine and Science in Sports and Exercise 2010;42(7):1409‐26. [DOI] [PubMed] [Google Scholar]
Siegel 2011
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: a Cancer Journal for Clinicians 2011;61(4):212‐36. [DOI] [PubMed] [Google Scholar]
Sloan 2016
- Sloan JA, Cheville AL, Liu H, Novotny PJ, Wampfler JA, Garces YI, et al. Impact of self‐reported physical activity and health promotion behaviors on lung cancer survivorship. Health and Quality of Life Outcomes 2016;14(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Speck 2010
- Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta‐analysis. Journal of Cancer Survivorship 2010;4(2):87‐100. [DOI] [PubMed] [Google Scholar]
Sui 2010
- Sui X, Lee DC, Matthews CE, Adams SA, Hebert JR, Church TS, et al. Influence of cardiorespiratory fitness on lung cancer mortality. Medicine and Science in Sports and Exercise 2010;42(5):872‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sweegers, 2017
- Sweegers MG, Altenburg TM, Chinapaw MJ, Kalter J, Verdonck‐de Leeuw IM, Courneya KS, et al. Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? A systematic review and meta‐analysis of randomised controlled trials. British Journal of Sports Medicine 2017; Vol. 10.1136/bjsports‐2017‐097891. [DOI] [PubMed]
Temel 2009
- Temel JS, Greer JA, Goldberg S, Vogel PD, Sullivan M, Pirl WF, et al. A structured exercise program for patients with advanced non‐small cell lung cancer. Journal of Thoracic Oncology 2009; Vol. 4, issue 5:595‐601. [DOI] [PMC free article] [PubMed]
Wang 2006
- Wang JS, Abboud RT, Wang LM. Effect of lung resection on exercise capacity and on carbon monoxide diffusing capacity during exercise. Chest 2006;129(4):863‐72. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection.. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Yellen 1997
- Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia‐related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. Journal of Pain and Symptom Management 1997;13(2):63‐74. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [DOI] [PubMed] [Google Scholar]


