Abstract
Obesity is associated with both increased cancer incidence and progression in multiple tumour types, and is estimated to contribute to up to 20% of cancer-related deaths. These associations are driven, in part, by metabolic and inflammatory changes in adipose tissue that disrupt physiological homeostasis both within local tissues and systemically. However, the mechanisms underlying the obesity–cancer relationship are poorly understood. In this Review, we describe how the adipose tissue microenvironment (ATME) evolves during body-weight gain, and how these changes might influence tumour initiation and progression. We focus on multiple facets of ATME physiology, including inflammation, vascularity and fibrosis, and discuss therapeutic interventions that have the potential to normalize the ATME, which might be translationally relevant for cancer prevention and therapy. Given that the prevalence of obesity is increasing on an international scale, translational research initiatives are urgently needed to provide mechanistic explanations for the obesity–cancer relationship, and how to best identify high-risk individuals without relying on crude measures, such as BMI.
The obesity pandemic is growing globally, representing a considerable cost to public health and a clinically urgent issue for a substantial proportion of the population1,2. On a global scale, more people are overweight or obese than underweight; in 2013, ~36% of men, ~38% of women and ~23% of children were overweight or obese3,4. In the United States alone (one of the most obese countries worldwide3), more than one-third of the adult population is obese and an additional one-third is overweight5, requiring ~$190 billion in annual health-care expenditure6. If these trends continue, the incidence of global obesity, excluding the population who are overweight, is expected to reach ~20% by 2025 (REF3). Given its prevalence, a critical need exists to evaluate the contribution of this comorbidity to cancer and other diseases in both clinical and preclinical research.
Associations between obesity and several pathological conditions are well-established, the most widely recognized being hypercholesterolaemia, hypertension, cardiovascular disease and type 2 diabetes mellitus. However, the link between obesity and cancer is relatively underappreciated among the general population. A retrospective analysis of >1,000 epidemiological studies reported that excess body fatness is correlated with increased risk of 13 distinct types of cancer in adults, out of the 24 that were evaluated7. Of the remaining 11 types of cancer, correlations were not necessarily refuted as data were too limited to draw definitive conclusions. In addition to being a risk factor for numerous cancers, obesity is associated with worse outcomes for a subset of tumour types. Indeed, a prospective study following up 1 million adults over a 16-year period reported that obesity is associated with an increased relative risk of death across 10 types of cancer in men and 12 in women8, including but not limited to tumours that are in direct contact with adipose tissue. Given these statistics and the prevalence of obesity worldwide, it is not surprising that obesity now competes with smoking tobacco as the leading preventable risk factor for cancer9, and is estimated to be responsible for ~14% and ~20% of all cancer-related deaths in men and women, respectively8,10. Thus, translational research initiatives are urgently needed to provide mechanistic explanations for these striking statistics in order to better serve this growing segment of the population11.
Although these epidemiological studies are critically informative, they do not provide the whole picture. Most studies use crude measures of obesity, such as BMI or waist circumference, which do not capture the diverse biology of ‘fatness’ (Box 1). For instance, epidemiological studies cannot easily uncouple the relative contributions of diet versus adiposity to cancer risk, yet these factors could act alone or together to affect dysbiosis (pathologic imbalance in the gut microbiota), inflammation and other factors that influence malignancy. In addition, although the volume of adipose tissue is associated with risk of disease, the quality of adipose tissue (for example, the presence of inflammation, adipocyte hypertrophy or hypoxia) is an important factor that is not accounted for with simple weight measurements, and is a major driver of metabolic aberrancies including insulin resistance, metabolic syndrome, all-cause mortality and cancer death12,13. The importance of adipose tissue quality is exemplified by metabolically obese normal-weight (MONW) individuals, who display metabolic abnormalities despite appearing lean. Finally, marked differences exist between the biology and physiological roles of different fat depots within the body (FIG. 1); both the type of adipose tissue (for example, thermogenically active brown fat versus energy-storing white fat)14 and the distribution of adipose tissue (such as visceral versus sub-cutaneous adipose tissue)15 differentially influence the risk of developing the metabolic syndrome. For example, during body-weight gain, accumulation of visceral white adipose tissue (WAT), particularly omental and mesenteric fat, is strongly associated with the development of insulin resistance and the metabolic syndrome16–18, compared with accumulation of subcutaneous WAT. Given the complex biology underlying adipose tissue and obesity, properly controlled preclinical studies are required to understand how obesity affects tumour biology at the molecular and cellular levels, and to define the driving forces behind the obesity–cancer relationship.
Box 1 |. Weighing the value of BMI.
High BMI has been associated with reduced mortality following certain health events, including heart failure, heart attack and some cancers173. This observation is known as the obesity paradox, and is debated widely owing to its clinical implications. Several competing explanations for the obesity paradox have been proposed.
Weight and the metabolic syndrome do not always correlate
BMI is used as a readily available cost-effective surrogate for predicting obesity comorbidities. Although this assumption is reasonable for population studies, ~25% of individuals who are obese are metabolically healthy174 and ~20% of individuals who are lean are metabolically obese175 (metabolically obese normal-weight; that is, they have qualitatively similar health risks as people who are obese despite having a healthy-range BMI).
BMi categories are not one-size-fits-all
BMI does not consider age or sex; therefore, certain demographics are frequently misclassified (for example, postmenopausal women)176. Additionally, BMI categories are based on morbidity and mortality statistics from white European populations, and do not universally predict adverse health conditions. For example, a high number of Asian Indians with a healthy-range BMI have type 2 diabetes mellitus. Therefore, BMI categories need to be adjusted between populations177.
BMi is not static
For some cancers, weight loss is the first symptom that prompts a medical evaluation. Although illness-induced weight loss is unlikely to push an individual into an entirely new BMI category, even modest weight loss can have biological consequences. For example, 10% acute weight loss in women who are morbidly obese leads to an improvement in some systemic markers of the metabolic syndrome and inflammation156, and reduced systemic predictors of metastasis based on preclinical models124.
A biological basis for the obesity paradox
Although excess body fat might increase the risk of cancer, it probably affects other biological processes that could suppress the progression of some malignancies (for example, immune function and clonal selection). Defining the basis for the obesity paradox is an active area of ongoing investigation.
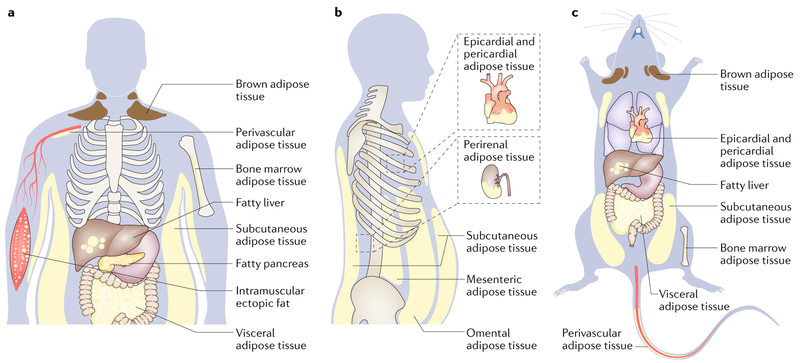
Fig. 1 |. Major adipose depots and anatomical locations in adult humans and mice.
There are two major types of adipose tissue, Lipid-rich white adipose tissue (WAT; energy storing) and mitochondria-rich brown adipose tissue (BAT; energy burning). Adipocytes from BAT and WAT emerge from distinct cell fate lineages; however, WAT can convert to metabolically active fat through the process of browning. WAT is found in many anatomical locations. The largest WAT depots are subcutaneous (under the skin; for example, inguinal, gluteal and femoral) and visceral (within the abdominal cavity, between the organs; for example, omental, mesenteric and epicardial). Smaller WAT depots are found around blood vessels (perivascular, which regulate vascular tone189), within the bone marrow (which regulate bone remodelling190), or as ectopic depots within specific organs (for example, non-alcoholic fatty liver disease, fatty pancreas or intramuscular fat). These adipose depots have unique molecular features; therefore, weight distribution has important biological consequences. For example, most visceral adipocytes have slightly higher mitochondrial density, are more lipolytic, are less sensitive to insulin, produce less leptin, and contribute more circulating free fatty acids compared to subcutaneous adipocytes15, and therefore have a stronger association with the metabolic syndrome. Part a depicts the general distribution of fat in humans from a forward view and part b depicts types of visceral fat depots from a side view.
Part c depicts the general distribution of fat in mice from a supine view.
Several excellent reviews have been published that highlight various aspects of a growing appreciation for the link between obesity and cancer9,19–23. This Review describes how local changes within the obese adipose tissue microenvironment (ATME) affect tumorigenesis, with a focus on WAT, and how the quality of adipose tissue influences cancer incidence and/or progression. We first consolidate preclinical findings that focus on different components of the ATME, such as inflammation, vascularity and fibrosis, to gain an overall picture of how these local changes might mechanistically influence cancer. We then focus on translational implications of these mechanisms, by discussing therapeutic intervention strategies that might be useful to normalize the obese ATME. Finally, we discuss some important considerations for future management of the obesity–cancer relationship, including how to best identify high-risk individuals.
Fuelling cancer with the obese ATME
In healthy individuals, WAT can comprise approximately a quarter of body mass24,25 and is a major physiological fuel source (FIG. 1). WAT consists of a variety of different cell types (for example, immune cells, fibroblasts and endothelial cells) that support tissue homeostasis and insulin sensitivity. Studies in mice and humans show that under normal-weight conditions (BMI of 18.5–24.9), the ATME is rich in type 2 (anti-inflammatory) cytokines, and as a consequence has an anti-inflammatory immune cell landscape26. However, during weight gain, adipocytes become hypertrophic and die, which is a key event during the evolution of the obese ATME. Adipocyte death triggers innate immune responses27, thus pivoting the immune repertoire towards a type 1 (pro-inflammatory) state. This shift is characterized by increased production and release of numerous cytokines into the microenvironment (for example, TNF, IFNγ, IL-1β and IL-6) and remodelling of the immune cell landscape26. These inflammatory changes coincide with chronic fibrosis and vascular inflammation, which feed-forward to constantly disrupt tissue homeostasis28. Of note, immune regulation of metabolic homeostasis has been covered by several excellent reviews26,28–31 and will not be the focus here. Instead, in the following section we discuss the ancillary consequences of these heterotypic relationships; namely, how local changes in the obese ATME affect tumour biology (FIGS 2,3), with a focus on tumour types that have direct exposure to WAT, most notably breast cancer.
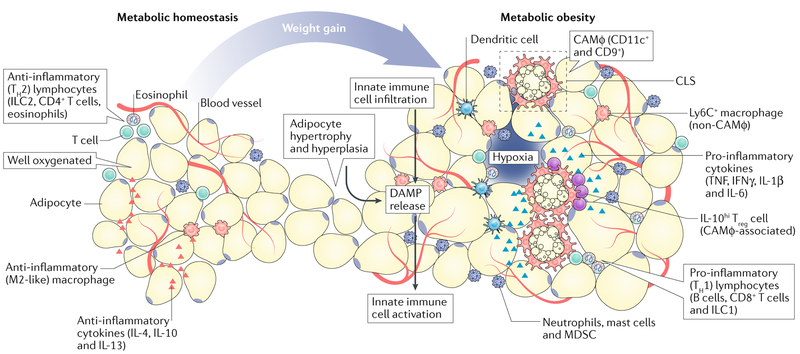
Fig. 2 |. Evolution of the adipose tissue microenvironment during obesity.
During healthy body-weight conditions (metabolic homeostasis), the adipose tissue microenvironment (ATME) is well-vascularized and rich in anti-inflammatory cytokines (such as IL-4, IL-10 and IL-13), and as a consequence, hosts a variety of type 2 immune cells, including alternatively activated (M2-like) macrophages, group 2 innate lymphoid cells, type 2 T helper (TH2) cells and IL-4-producing eosinophils. In response to body-weight gain or metabolic obesity, adipocytes undergo hyperplasia and hypertrophy; as the vascular supply becomes limited, these cells become stressed or die. This releases damage-associated molecular patterns (DAMP) into the microenvironment, which trigger the infiltration and activation of innate immunecells (for example, dendritic cells, macrophages and granulocytes). These effects promote the development of crown-like structures (CLS) and type 1 (pro-inflammatory) immune responses. This response includes accumulation of type 1 cytokines (for example TNF, IFNγ, IL-1β and IL-6), and pro-inflammatory immune cells, including various granulocytes, group 1 innate lymphoid cells, B cells and CD8+T cells, which perpetuate chronic inflammatory responses. Macrophages are highly diverse within the obese ATME; those associated with CLS (CLS-associated macrophage, CAMφ) proliferate and express the cell surface markers CD11c and CD9, while those that are further away (non-CAMφ) express lymphocyte antigen 6C (Ly6C). These inflammatory changes coincide with chronic fibrosis and vascular inflammation, which feed-forward to sustain inflammation. IL-10hi Treg cells, T regulatory cells producing high levels of IL-10; ILC1, group 1 innate lymphoid cells; MDSC, myeloid-derived suppressor cells.
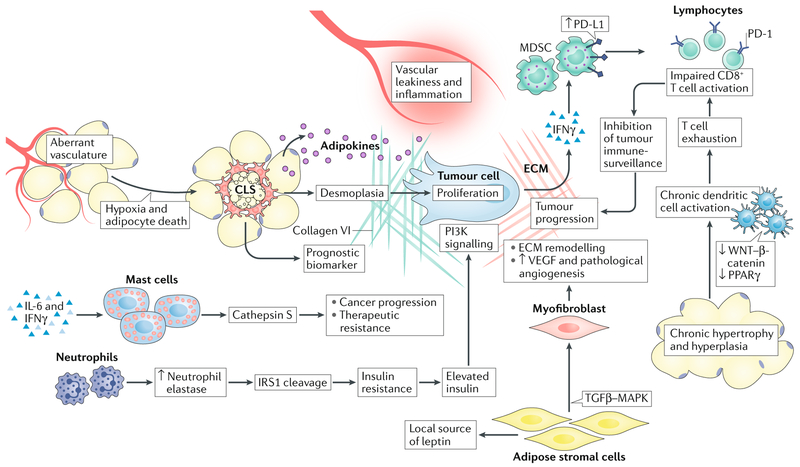
Fig. 3 |. Interactions between tumour cells and cells within the obese adipose tissue microenvironment.
Tumour cell biology is affected by multiple cellular players in the adipose tissue microenvironment (ATME). The enzyme neutrophil elastase cleaves insulin receptor substrate 1 (IRS1), leading to insulin resistance and elevated levels of free insulin, which can enhance phosphoinositide 3-kinase (PI3K) signalling within tumour cells. Mast cells produce various proteases such as cathepsin S, which can promote cancer progression and therapeutic resistance to cytotoxic therapies. Adipose stromal cells and myofibroblasts promote extracellular matrix (ECM) remodelling and facilitate tumour angiogenesis through vascular endothelial growth factor (VEGF) production. However, angiogenic vessels are dysfunctional, and when combined with adipocyte hypertrophy this triggers innate immune responses by macrophages and dendritic cells. Macrophages form crown-like structures (CLS) that not only serve as biomarkers for the metabolic syndrome, but also produce high levels of cytokines (promote vascular permeability) and cause desmoplasia, which together support tumour progression. At the same time, dendritic cells become chronically activated through the suppression of tolerogenic signalling pathways such as the canonical WNT and peroxisome proliferator-activated receptor-γ (PPARγ) pathways, eventually leading to T cell exhaustion. T cell activation is further suppressed by myeloid-derived suppressor cells (MDSCs), which engage co-inhibitory checkpoint molecules on T cells (such as programmed cell death 1; PD-1). Together, each of these cell types facilitates evolution of the tumour microenvironment and disease progression. MAPK, mitogen-activated protein kinase; TGFβ, transforming growth factor-β.
Crown-like structures and adipose tissue macrophages.
During weight gain, adipocytes accumulate lipids, become hypertrophic and eventually die. The mechanism of adipocyte death is still an open question in the field, although it could be through pyroptosis32 or necrosis33. Both processes involve rupture of the cell membrane and release of cellular contents into the microenvironment, such as lipids, cytokines and damage-associated molecular patterns (for example, fatty acids, ATP, reactive oxygen species, cholesterol and nucleic acids34–39). These signals are major triggers for the accumulation of phagocytic macrophages40 through enhanced recruitment (following peripheral myelopoiesis and monocytosis)41, as well as in situ proliferation42,43 (which is, paradoxically, a hallmark feature of type 2 inflammation43,44). Up to 90% of macrophages in the obese ATME are directly associated with adipocyte hypertrophy33, where they encircle the dying adipocyte to form crown-like structures (CLS), scavenge lipids and cellular debris, and sometimes evolve into multinucleated giant cells or foam cells45 (FIGS. 2,3). The outcomes of these interactions include activation of macrophage pattern recognition receptors (such as nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) or Toll-like receptors (TLRs)) and downstream signalling through inflammasome activation37,41,46, inhibitor of nuclear factor-κB (NF-κB) kinase subunit-β (IKKβ)-NF-κB47 and/or c-Jun N-terminal kinase 1 (JNK1; also known as MAPK8)48. Indeed, targeting these pathways using genetic or pharmacological approaches is sufficient to protect against insulin resistance during diet-induced obesity47,48. Therefore, the formation of CLS in the obese ATME is a principle inflammatory lesion and biomarker of adipose tissue inflammation, and is correlated with insulin resistance and metabolic syndrome. Furthermore, in individuals who have normal body weight, the presence of CLS in WAT correlates with metabolic dysfunction49, suggesting that these structures are a stronger indicator of metabo-inflammation and metabolic risk than BMI (Box 1).
In patients with cancer, CLS have been associated with worse outcomes, and interest is growing in using these structures as a prognostic biomarker. In breast cancer, histology reveals CLS of the breast in ~50% of women. A positive status for CLS of the breast is associated with higher BMI and systemic markers of the metabolic syndrome compared with negative CLS status, as well as increased breast cancer risk, distant recurrence and mortality13,50,51. Consistent with preclinical observations that overexpression of aromatase (an enzyme that converts androgens to oestrogens) in the mammary gland stimulates tumorigenesis52, CLS of the breast in humans are associated with elevated levels of aromatase and increased risk of breast cancer in women with a history of benign breast disease51. These relationships are similar in prostate cancer; in a prospective study of 169 men with newly diagnosed prostate cancer, CLS within periprostatic fat were found in 49.7% of men in association with higher BMI and tumour grade, concomitant with elevated circulating levels of insulin, triglycerides, leptin and other factors53. Of note, the association between CLS and tumorigenesis is not specific to hormone-driven cancers with close proximity to large fat depots; CLS are even found in the tongue, where they are correlated with increased BMI and reduced disease-specific and overall survival in patients with early-stage squamous cell carcinoma of the tongue54. These data provide clinical evidence that CLS can be used as a biomarker and might be an important biological driver of the obesity–cancer relationship, and suggest that the consequences of CLS are probably multifactorial.
Macrophages within CLS and surrounding adipose tissue are diverse, and probably affect tumour biology in different ways. In a variety of cancer types, macrophages are often the most abundant cell type in the tumour microenvironment, and their accumulation is frequently associated with worse outcomes and resistance to cytotoxic therapy55. Macrophages are highly plastic cells; compartmentalization of macrophages within the ‘M1–M2’ linear paradigm (in which macrophages are polarized towards a classical (M1 or pro-inflammatory) or alternative (M2 or anti-inflammatory) activation state) has been largely dismissed, as it is now known that macrophages can adopt myriad activation states that are much more diverse. Indeed, macrophages in the ATME are phenotypically and spatially heterogeneous. For instance, obese ATME macrophages can be functionally subdivided by expression of cluster of differentiation molecule 11c (CD11c; also known as integrin alpha X), a classical dendritic cell marker, whereby the CD11c+ fraction exclusively promotes insulin resistance in mouse models of diet-induced obesity56,57 and is frequently associated with CLS58. Specific ablation of CD11c+ cells normalizes insulin sensitivity, even when the population of CD11c− macrophages remains intact56,57. Interestingly, in preclinical models of breast cancer, tumour-associated macrophages similarly express high levels of canonical dendritic cell markers including MHC class II and CD11c, distinguishing them from normal mammary tissue macrophages59. The population of CD11c+ macrophages accelerates breast cancer growth by promoting T cell exhaustion, through progressive expansion of programmed cell death 1 (PD-1)+ granzyme B− CD8+ T cells59. It is conceivable that the increase in the number of CD11c+ macrophages in the ATME during obesity contributes to their availability in the mammary gland during breast tumour progression, thus contributing to an immunosuppressive microenvironment.
Functional heterogeneity in subpopulations of ATME macrophages has also been identified based on their spatial relationship with CLS. ATME macrophages that are not directly associated with CLS express the monocyte marker lymphocyte antigen 6C (Ly6C), whereas CLs-associated macrophages (CAMφ) lose the expression of Ly6C and instead express the canonical exosome marker CD9 (REF60). Both tumour-derived and stroma-derived exosomes have received widespread attention for their role in cancer progression and metastasis55,61. CD9+ CAMφ secrete exosomes, which could, in principle, facilitate direct communication between CLS and tumours; however, the content of these exosomes is unknown. Another unique feature of CAMφ in the ATME is that they proliferate42, which is typically a hallmark feature of type 2 inflammatory responses44, thus challenging the dogma that ATME macrophages are type 1 cells. Paralleling a growing appreciation for more complex macrophage diversity in tumour biology, it is conceivable that ATME macrophages are similarly capable of co-expressing both M1 and M2 markers leading to a blended phenotype, emphasizing the importance of moving away from the M1–M2 paradigm in this field. Supporting this notion, transcriptional profiling showed no statistically significant enrichment for traditional M1 or M2 signatures in either CD11c− or CD11c+ ATME macrophages in response to obesity62, including specifically in CD11c+ CAMφ58, and proteomics analyses have confirmed that obesity induces ‘metabolic activation’ in ATME macrophages that is phenotypically distinct from both M1 and M2 activation63.
Adipose tissue leukocytosis—inflammatory reactions beyond macrophages.
Once CLS develop, the innate immune system is engaged, the mandate of which is to rapidly defend the body while enabling the slower developing adaptive immune response. In addition to the formation of CLS, obesity is associated with a variety of other inflammatory changes (FIG. 3). Within the myeloid compartment, even though the frequency of eosinophils is decreased64, this reduction is compensated by several other granulocytic cell types that become activated. Neutrophils, which have been heavily implicated in cancer65, are rapidly increased in WAT after just 3 days of a high-fat diet, where they produce the serine protease neutrophil elastase that cleaves insulin receptor substrate 1 (IRS1)66. This cleavage event prevents IRS1 from binding to phosphoinositide 3-kinase (PI3K), thus leading to insulin resistance66, which could in turn promote the proliferation of epithelial cells. Although activating mutations in the PI3K pathway represent one of the most common oncogenic events in cancer, obesity might act as a surrogate mechanism of PI3K activation in wild-type tumours via its inductive effects on insulin. The frequency of mast cells is also increased, which respond to IL-6 and IFNγ by producing cathepsin S67, a member of the cysteine cathepsin protease family with roles in cancer progression and therapeutic resistance68. Granulocytic myeloid-derived suppressor cells also become elevated as a defence mechanism in numerous tissues including the obese ATME, where they attempt to counter the development of inflammation and insulin resistance69. In breast cancer models, the immune checkpoint molecule programmed death-ligand 1 (PD-L1) is upregulated in myeloid-derived suppressor cells during obesity by IFNγ released from tumour cells, causing impaired CD8+ T cell surveillance, and accelerated tumour onset and progression70. Even though each of these granulocyte dynamics might initially serve as an attempt to keep inflammation in check during bodyweight gain, in the context of cancer, this effect might lower the barrier to malignant transformation of pre-cancerous cells by impairing immune surveillance, and ultimately supporting tumour proliferation and progression.
Supplementing these granulocytic responses, antigen-presenting myeloid cells such as classic dendritic cells (cDC) become involved in the obese ATME. In response to over-nutrition, adipocyte hyperplasia suppresses tolerogenic (anti-inflammatory) canonical WNT-β-catenin and peroxisome proliferator-activated receptor-γ (PPARγ) signalling pathways in cDC1 and cDC2 cells, respectively71. This effect enables the activation of cDC and T cell-mediated responses within visceral WAT, thus bridging innate and adaptive immunity to facilitate immune surveillance. However, perpetual antigen presentation by cDCs during sustained over-nutrition might eventually lead to T cell exhaustion and chronic inflammation. Indeed, obesity accelerates thymic ageing in conjunction with reduced frequency of peripheral naive T cells72, compromised T cell receptor diversity72,73 and increased expression of checkpoint molecules and molecular markers of exhaustion74. However, upon PD-1 blockade, obesity facilitates strong T cell effector functions74, which suggests that this process is partially reversible, and might explain the high efficacy of checkpoint inhibitors (for example, PD-1-blocking or PD-L1-blocking antibodies) in men who are obese and have melanoma75.
Several additional lymphoid cell types within the obese ATME might also contribute to malignancy. B cells accumulate in WAT during body-weight gain, and preclinical models show that genetic depletion of B cells protects against the metabolic syndrome in response to a high-fat diet even though mice still gain weight76, suggesting that B cells might regulate the development of CLS but not adipocyte hypertrophy or hyperplasia. Activated effector CD8+ T cells also infiltrate WAT in response to obesity; this event might even precede the increase in numbers of macrophages in the ATME as CD8-depletion prevents macrophage infiltration and improves insulin sensitivity77. In lean mice, CD4+ forkhead box protein P3 (FOXP3)+ regulatory T cells (Treg cells) are present in the ATME78,79, where they interact closely with CAMφ (which are rare in lean mice) and produce high levels of anti-inflammatory stimuli, such as IL-10 (REF80). These interactions might help keep CLS frequency low in lean adipose tissue. However, during obesity, these populations of Treg cells are reduced relative to the expansion of adipose tissue78–80, thus alleviating homeostatic safeguards. Despite the fact that Treg cells usually enable tumour outgrowth owing to their immunosuppressive properties, the loss of Treg cells in the obese ATME might enable the expansion of CLS and lead to a pro-tumorigenic microenvironment. Finally, IL-12-regulated group 1 innate lymphoid cells, with long-term residency in the ATME, proliferate and produce the type 1 cytokine IFNγ in response to obesity; however, these cells are distinct from both mature and immature natural killer cells in the ATME, which are principal members of the group 1 innate lymphoid cell family81. Similar to CAMφ, these studies collectively suggest an ATME-specific activation phenotype of these different populations of immune cells; how these unique populations affect tumour biology remains largely unclear.
Taken together, these data suggest that the obesity-inflammation axis is a central regulator of the metabolic syndrome that might also underlie many of the associated risks with cancer. Indeed, the link between chronic inflammatory conditions and cancer is widely appreciated82, both at the level of risk and progression; it is probable that obesity falls within this realm. Of note, although much of the discussion in this section is focused on solid tumours, the effect of obesity on haematological malignancies and the role of fat residing within the bone marrow is discussed in BOX 2.
Box 2 |. Obesity and haematological malignancies.
In older adults, bone marrow adipocytes comprise up to ~70% of bone marrow volume, and are therefore an important component of the microenvironment during haematopoiesis. Although adipocytes impair haematopoietic repopulation and expansion during infection178–181, obesity is associated with chronically increased lymphopoietic and haematopoietic processes during steady-state conditions182. Given the effect of adipocytes on normal haematopoietic responses, does obesity predispose individuals to haematological malignancies? Leptin, a hormone produced predominantly by adipocytes to help control appetite, might facilitate communication between adipocytes and bone marrow progenitor cells. In preclinical models, downregulation of the leptin receptor in haematopoietic progenitor cells is required for the initiation of B cell and T cell acute lymphoblastic leukaemia (ALL)183, and diet-induced obesity accelerates the onset of disease in association with elevated circulating levels of leptin184. Countering these effects, fasting prevents the initiationof ALL by upregulating the expression of the leptin receptor, thus supporting differentiation183. Within the bone marrow, acute myeloid leukaemia (AML) blast cells can metabolically reprogramme adipocytes to promote lipolysis, and this event in turn transfers fatty acids to tumour cells185. This bidirectional communication and exchange of fatty acids mirrors that of other solid tumours in larger fat depots, including breast and prostate cancer, for which associations between obesity and cancer are apparent. Indeed, epidemiological studies show that obesity is associated with an increased risk of multiple myeloma7 and AML186 in adults. In an epidemiological study of 5,420 children, obesity also increased the risk of relapse from ALL by 50%187; however, similar analyses in adults are based on smaller cohort sizes, and the data are variable and inconclusive. Although the role of obesity in additional haematological malignanciesin patients has not been formally established, these associations might exist.
Vascular inflammation.
A hallmark feature of cancer is the ability to recruit a vascular supply and engage the endothelium to permit the passage of inflammatory cells, circulating factors and metastatic tumour cells. Given the well-established association between obesity and the cardiovascular system, defining how obesity affects vascular function is relevant to understanding the obesity-cancer relationship. Indeed, the vascular demand in growing adipose tissue mimics that of a growing tumour; although both tissues are highly vascularized, vessel function is aberrant and often insufficient to meet metabolic demand in both cases (FIG. 4). In the obese ATME, vascular dysfunction leads to areas of hypoxia and adipocyte death — a triggering event for CLS formation. In mouse models of obesity, several studies showed that in mice, adipocyte-specific deletion of hypoxia-regulated genes that are necessary to stimulate angiogenesis, such as Vegfa83 or Hif1α84, reduces WAT vascularity, increases CLS formation and leads to insulin resistance, compared with mice with wild-type adipocytes. In pre-clinical models of breast cancer, angiogenesis within the obese mammary fat pad primes the ATME during early stages of tumour initiation85. However, vessels that are conditioned by obesity are highly dysregulated. Vascular inflammation resulting from adipokine exposure leadsto enhanced permeability (fig. 3), which might facilitate the robust inflammatory changes that ensue in the ATME. For example, the discordant interplay between lipopolysaccharide and adiponectin regulates vascular function; lipopolysaccharide causes increased production of leukocyte adhesion molecules and decreased endothelial adhesion molecules, whereas adiponectin reverses these effects86 (fig. 4). Exposure of microvascular cells to the free fatty acid palmitate induces activation of the NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome in endothelial cells, in concordance with weakened endothelial tight junctionsvia decreased expression of zonula occludens 1 (ZO1) and ZO2 (REf87) (fig. 4). Notably, patients with breast cancer who are obese are less sensitive to anti-vascular endothelial growth factor (VEGF) therapy; preclinical models suggest that this effect is attributable to elevated levels of IL-6 production by adipocytes and myeloid cells in the breast ATME, such that blocking IL-6 is sufficient to improve the response to anti-VEGF therapy88. Interestingly, treatment with the antidiabetic agent metformin could phenocopy these effects88. Given that obesity affects multiple aspects of vascular biology, it is probable that vascular normalization strategies are also an important consideration for patients with cancer who are obese89.
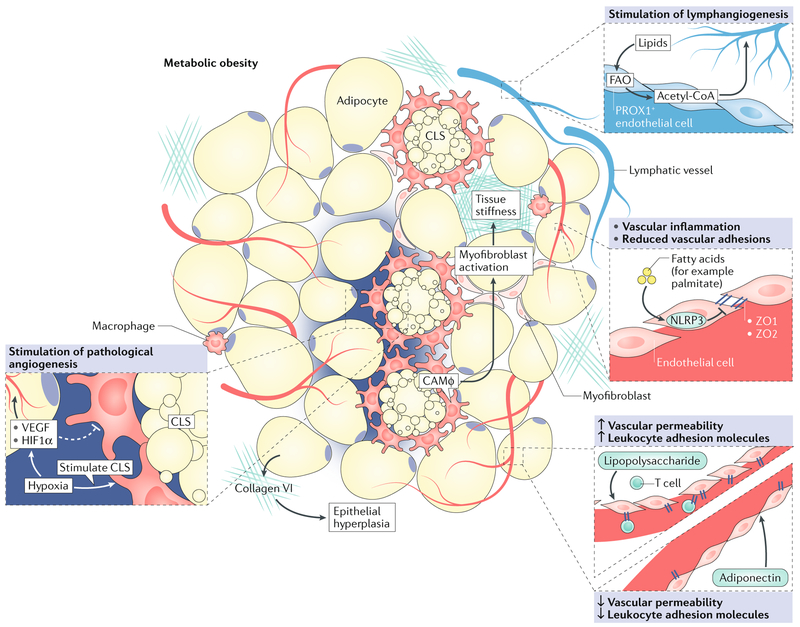
Fig. 4 |. Vascular inflammation and fibrosis in the obese adipose tissue microenvironment.
During obesity, adipose tissue expands rapidly, leading to high demand for a vascular supply. Similar to a growing tumour, this effect results in regions where vascular supply is insufficient, creating areas of hypoxia. Hypoxia triggers angiogenesis through induction of pro-angiogenic factors (such as vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1α (HIF1α)); however, resulting blood vessels are poorly functional, and thus low oxygen levels persist. Chronic hypoxia eventually contributes to adipocyte death, and supports the formation of crown-like structures (CLS). Vascular integrity is in part reduced by downregulation of endothelial adhesions (such as zonula occludens 1 (ZO1) and ZO2) in responseto obesity-derived factors (for example, lipopolysaccharide and palmitate), while leukocyte adhesion molecules are increased to facilitate infiltration of immune cells (such as T cells). Adiponectin can reverse these effects and improve vascular integrity. In addition, prospero homeobox protein 1 (PROX1)+ lymphatic endothelial cells use lipids to stimulate lymphangiogenesis through fatty acid oxidation (FAO) and production of acetyl-CoA. The extracellular matrix is also aberrant; CLS-associated macrophages (CAMφ) and myofibroblasts contribute to increased tissue stiffness in the obese adipose tissue microenvironment, and adipocytes produce high levels of collagen VI, which further support adipocyte hyperplasia.
In addition to understanding the effects of obesity on blood vessels, lymphatic vessels are also central regulators of inflammatory responses, and lipids are well-established mediators of lymphatic function90. Indeed, elevated BMI is associated with increased lymphoedema in humans91, including after postoperative lymph node dissection in patients with breast cancer92, suggesting that obesity affects lymphatic vessel physiology. Lymphatic vessels are regulated and defined by the expression of prospero homeobox protein 1 (PROX1); in lymphatic endothelial cells, PROX1 mediates fatty acid oxidation leading to elevated production of acetyl-CoA, which promotes epigenetic modifications that are critical for lymphangiogenesis93 (a key step during tumour metastasis94) (fig. 4). This effect demonstrates that remodelling of the lymphatic endothelium relies on fat catabolism. Interestingly, Prox1+/− mice develop late-onset obesity, characterized by abnormal lymphatic function and ruptured vessels95, and restoration of Prox1 expression specifically in lymphatic endothelial cells is sufficient to reverse this phenotype96. In the tumour microenvironment, lymphatic vessels not only provide a conduit for metastatic tumour cells, but also act as a mechano-biosensor for lymph flow and stiffness of the extracellular matrix (ECM), and have an active role in regulating the immune composition97. How obesity affects these complex lymphatic processes is unclear, particularly for tumours that are enveloped by adipose tissue such as breast cancer. Of note, lifestyle modifications, including exercise98 or diet-induced weight loss99, rescue some of the deleterious effects of obesity on lymphatic function, suggesting that these effects are reversible through non-pharmacological approaches (which might be relevant for patients with cancer once clearer mechanisms are defined). For a more detailed summary of the relationship between obesity and vascularity, see refs100,101.
Fibrosis and matrix composition.
With vascular inflammation comes changes in matrix composition within tissues (fig. 4). Remodelling of the ECM and stromal microenvironment, a process called desmoplasia, accompanies the disruption of tissue homeostasis including during the process of malignancy. Desmoplasia is regulated by myofibroblasts, which deposit stiff matrix components including fibrillar collagen and fibronectin, to alter the biomechanics and tensile properties of the microenvironment. Tumours tend to have stiffer matrices compared with normal tissues102,103. During obesity, CAMφ promote myofibroblast activation and tissue fibrosis in WAT104. In the mammary gland, the stroma also becomes enriched with myofibroblasts during obesity to promote ECM stiffness105 (Fig. 4). Matrices isolated from obesity-associated stromal cells promote the aggressiveness of breast cancer cells by stimulating mechano-signalling, and this effect is reversible with body-weight loss through caloric restriction105.
Adipocytes are also one of the highest sources of collagen VI, and deletion of Col6a1 in lean MMTV-PyMT mice (a mouse model of breast cancer) leads to reduced epithelial hyperplasia and breast tumour growth106 (fig. 4), suggesting a pro-tumorigenic role for collagen VI deposition. During obesity, the production of several collagens is upregulated in adipose tissue, including collagen VI, reflecting a broad shift towards adipose tissue fibrosis107, which can be further perpetuated by hypoxia108. In pancreatic ductal adenocarcinoma, of which desmoplasia is a hallmark histological feature, obesity promotes crosstalk between adipocytes, tumour-associated neutrophils and pancreatic stellate cells to promote matrix remodelling and impaired vascular perfusion, leading to tumour progression and ineffective delivery of chemotherapy109. Taken together, the robust changes to ECM composition that coincide with obesity provide a pro-tumorigenic niche by disrupting tissue mechano-homeostasis, and these changes probably contribute to some of the inflammatory effects within the obese ATME. In support of this notion, obese leptin-deficient ob/ob mice show improvements in ATME inflammation when Col6a1 is deleted, marked by reduced macrophage infiltration, adipocyte death and CLS formation107.
Transmitting signals between tumour cells and adipocytes.
One component of the ATME that cannot be overlooked is the adipocyte — the conductor of microenvironmental evolution during obesity. Aside from having indirect effects on tumour cells through inflammation, vascularity and fibrosis as discussed previously, adipocytes can also have direct effects on tumour cells through paracrine signalling110. In melanoma, adipocytes in subcutaneous WAT transfer lipids to tumour cells to induce metabolic reprogramming, growth and invasion111. In prostate cancer, adipocytes secrete CC-chemokine ligand 7 (CCL7), which stimulates the migration of CC-chemokine receptor 3 (CCR3)+ tumour cells to the periprostatic fat (a common region of cancer recurrence in patients112). In humans, CCR3 expression in prostate cancer cells is associated with more aggressive disease. Adipokines can also have autocrine effects that might indirectly affect tumour biology by regulating insulin resistance and inflammation. For example, adiponectin, which promotes ATME homeostasis, acts locally on adipocytes to regulate glucose uptake, adipogenesis and lipid storage113,114, whereas leptin (which promotes ATME dysfunction) regulates adipocyte lipolysis by engaging the central nervous system115. Downregulation of adiponectin, and upregulation of leptin in sera are both significantly correlated with CLS formation in breast tissue of women with early-stage breast cancer13. These studies suggest that targeting these cytokine axes might hold therapeutic value to disrupt both direct and indirect interactions between adipocytes and tumour cells within the ATME.
Adipose stromal cells (ASCs; commonly referred to as pre-adipocytes) are multipotent mesenchymal progenitor cells within adipose tissue that can also exert local effects on tumour cells. ASCs are recruited from WAT into tumours through chemokine gradients. For example, prostate cancer cells produce growth-related α-protein (CXCL1) and IL-8, which attract CXC-chemokine receptor 1 (CXCR1)+ and CXCR2+ ASCs, respectively116. In patients who are obese, these cytokines are overexpressed by prostate tumour cells, and IL-8 in particular is associated with high-grade disease116. Upon arrival, ASCs promote various hallmark cancer phenotypes, most notably vascularization117–119, and display phenotypic plasticity in response to cues from the tumour. For example, in breast cancer, the differentiation of ASCs into myofibroblasts can be induced by tumour extracellular vesicles through a transforming growth factor-β (TGFβ)–mitogen-activated protein kinase (MAPK) signalling axis to support ECM remodelling, increased VEGF production and angio-genesis120 (flG. 3). ASCs can also act as a local source of leptin in the mammary fat pad to promote oestrogen receptor (ER)+ breast tumour progression during obesity121. Given their presence within WAT, it is probable that ASCs are important contributors to the obesity–cancer relationship. Indeed, syngeneic mouse models of melanoma, breast, prostate and lung cancer showed that specific depletion of ASCs using pro-apoptotic peptides inhibits tumour vascularization and proliferation, and induces necrosis122. Interestingly, this effect was more pronounced in obese mice compared with lean mice, where ASCs are more abundant.
In addition to acting locally, adipocytes are experts at mediating endocrine effects, which facilitate inter-organ crosstalk between the ATME and distant tissues123. This ability has important implications for cancer, as it suggests that tumours with no direct contact with adipose tissue can still be affected by obesity, reflecting the epidemiological data that link obesity with diverse cancer types7,8. For instance, the ATME produces IL-5, which stimulates lung neutrophilia and breast cancer metastasis by increasing circulating levels of granulocyte-macrophage colony-stimulating factor (GM-CSF)124. The obese ATME also produces IL-6 and TNF, which regulate chronic inflammation within the liver and support malignancy by activating the oncogenic transcription factor, signal transducer and activator of transcription 3 (STAT3)125. Given the relationship between obesity and diet, it is not surprising that the gastrointestinal tract also has a close relationship with adipose tissue, which can facilitate early changes associated with tumour initiation. In the pre-malignant colon epithelium, high adiposity, but not diet, triggers epigenetic changes that mirror early alterations associated with tumour initiation, including activation of PI3K and RAS126. Obesity also exacerbates adipokine production by the ATME in response to Helicobacter felis infection in mice, marked by a statistically significant increase in the pro-inflammatory cytokines IL-6, CCL7 and leptin, which accelerate the onset of gastric cancer127. Importantly, this effect is not observed in lean control mice, demonstrating that these effects are specifically driven by the obese ATME. Together, these data demonstrate that the inflammatory response of the obese ATME has far-reaching effects on a variety of tumour types that can exist throughout the host, thus providing a rationale for targeting canonical adipokines even for tumours that are more distant from major adipose depots.
The elevation in serum levels of adipokines in response to obesity and the metabolic syndrome coincides with a spike in circulating levels of cholesterol, and hypercholesterolaemia is a hallmark consequence of obesity. In preclinical breast cancer models, the cholesterol metabolite 27-hydroxycholesterol can act as a selective ER modulator, and studies have shown that it can promote the growth of ER+ breast tumours128,129. Similar to the effects of the IL-5-GM-CSF axis124, 27-hydroxycholesterol can also affect lung inflammation and facilitate breast cancer metastasis by promoting lung neutrophilia130. Cholesterol metabolism also leads to the generation of the oncometabolite, 6-oxocholestan-3β,5α-diol. Unlike 27-hydroxycholesterol, 6-oxo-cholestan-3β,5α-diol has no effect on ER+ breast cancers, and instead stimulates growth of tumour cells within the mammary fat pad by acting through the glucocorticoid receptor131. These data suggest that controlling indirect consequences of obesity, for example, through the use of statins, might have beneficial effects on both cancer incidence and prognosis.
Finally, adipocytes sequester and catabolize chemotherapeutic agents, which might reduce the therapeutic efficacy of these compounds. In acute lymphoblastic leukaemia, adipocytes seem to protect tumour cells from vincristine132 and daunorubicin133. In addition to being able to absorb daunorubicin, adipocytes express carbonyl reductases and aldo-keto reductases, which metabolize daunorubicin to daunorubicinol (its largely inactive metabolite). These findings have potential implications not only for patients with cancer who are obese, but also for those patients who are lean with tumours that are in direct contact with adipose tissue, such as breast cancer. Of note, in vitro functional screens have shown that both cytotoxic and targeted therapies that effectively kill tumour cells in monoculture are often less effective when tumour cells are co-cultured with various stromal cell types134. One such cell type is macrophages, which are abundant in the obese ATME and are well-characterized for their ability to protect tumour cells from the cytotoxic effects of chemotherapy135. Adipocytes and macrophages in CLS might act synergistically to reduce the efficacy of cancer therapies during obesity, although this effect has not been formally tested. Taken together, although reduced efficacy of chemotherapy in patients who are obese has often been attributed to under-dosing, these data suggest that more complex adipose tissue biology might be involved.
For instance, adipose tissue biology and metabolic activity are strongly affected by circadian rhythms, which synchronize light-dark, fasting-feeding and sleep-wake cycles, leading to optimized host energy balance. Circadian rhythms regulate microbiome composition136, fatty acid and glucose metabolism and insulin release137; unsurprisingly, interest in the role of circadian rhythms during obesity and the metabolic syndrome is growing. Chronobiology studies in mice show that mutations in clock genes, such as Clock, Bmal1 (REF.138) or Per3 (REF.139), lead to enhanced development of obesity and the metabolic syndrome, and suppress adipogenesis. Interestingly, time-restricted feeding of mice without changing overall caloric intake or food content can attenuate metabolic aberrancies associated with a high-fat diet, including obesity, adipocyte hypertrophy and WAT inflammation140. How these anti-inflammatory effects might translate to cancer biology within the ATME is unknown. In cancer, pre-clinical studies have shown that agonists of REV-ERBα-related receptor (REV-ERBα; also known as NR1D1) or REV-ERBβ (also known as NR1D2), essential components of circadian rhythms and lipid metabolism, are selectively lethal in both malignant (for example, glioblastoma) and benign neoplasms (such as nevi), but not normal cells, by regulating autophagy and de novo lipogenesis141. Therefore, in light of these findings, it is plausible that ATME composition within humans can be fine-tuned by chronic behaviours that challenge our natural light–dark cycle, such as sleep disturbances and/or irregular eating behaviours (for example, night feeding).
Therapies against ATME dysfunction
In addition to conventional therapies that largely target tumour cells, targeting the tumour microenvironment is an effective strategy to treat cancer142. With growing interest in immunotherapy, many of these strategies are focused on targeting immune cells within the tumour microenvironment. We would similarly argue that therapeutic interventions that target the dysregulated ATME might be an effective strategy to improve the obesity-cancer relationship, not only for patients who are obese, but potentially also for those who are over-weight or MONW. In the following section, we review different intervention strategies that aim to normalize the obese ATME, which might be relevant to both cancer initiation and metastatic disease. A summary of ongoing and completed clinical trials can be found in TABLE 1. In BOX 3, we also discuss the possibility that some effects of obesity might be imprinted, and therefore might not be reversible.
Table 1 |.
Preclinical and clinical interventions that relate to the connection between obesity and cancer
| Trial type | Intervention | Details | Clinical trial IDs | Refs |
|---|---|---|---|---|
| Effects on cancer and related end points | ||||
| Clinical | Telephone-based consultation |
|
NCT02750826 (recruiting) | 154 |
| Clinical | Mediterranean-style diet |
|
ISRCTN35739639 | 162,191 |
| Clinical | Antidiabetic (metformin) |
|
|
192–195 |
| Clinical | Antidiabetic (pioglitazone) |
|
|
196–200 |
| Clinical | Exercise |
|
NA | 201 |
| Clinical | Exercise | Effects of exercise on biomarkers of the metabolic syndrome and sarcopenic obesity in survivors of breast cancer who are overweight and obese | NA | 168 |
| Clinical | Exercise | Effects of exercise on insulin resistance and circulating biomarkers in postmenopausal survivors of breast cancer | NA | 202 |
| Clinical | Bariatric surgery |
|
NCT01479452 | 152 |
| Preclinical | Caloric restriction mimetics | Effects of caloric restriction mimetics on anticancer immune surveillance | NA | 146 |
| Preclinical | Fasting-mimicking diet | Effects of fasting-mimicking diet in combination with chemotherapy in breast cancer and melanoma models | NA | 147 |
| Preclinical | Dietary restriction | Effects of dietary restriction (60% of normal daily food intake) on PI3K wild-type versus mutant tumour models. | NA | 159 |
| Effects on the ATME and related end points | ||||
| Clinical | VLCD | Effect of VLCD on inflammatory transcriptional profile of the ATME | NA | 155 |
| Clinical | VLCD | Effects of rapid weight loss on systemic and adipose tissue inflammation and metabolism in postmenopausal women who are obese | NA | 156 |
| Clinical | Mediterranean-style diet | Effect of Mediterranean-style diet on endothelial function in patients with the metabolic syndrome | NA | 160,161 |
| Clinical | Low-carbohydrate versus low-fat diet | Effect of a low-carbohydrate versus low-fat diet in patients with severe obesity | NCT00160108 | 163–165 |
| Clinical | Antidiabetic (pioglitazone) |
|
203 | |
| Preclinical | Caloric restriction | Effect of 30% caloric restriction on inflammation in the mammary fat pad of obese mice | NA | 158 |
| Preclinical | Antidiabetic (pioglitazone) | Effect of pioglitazone on periprostatic WAT inflammation in obese mice | NA | 143 |
| Preclinical | Oestrogen supplementation | Effect of oestrogen on mammary WAT inflammation in obese mice | NA | 149 |
The list of clinical trials is not exhaustive; only trials categorized as “Active, not recruiting” or “Completed” by ClinicalTrials.gov are shown, unless otherwise indicated. ATME, adipose tissue microenvironment; IDs, identifiers; NA, not applicable; PI3K, phosphoinositide 3-kinase; WAT, white adipose tissue; VLCD, very-low-calorie diet.
Box 3 |. Is weight loss the simple answer?
Given that excess body fat is associated with an increased risk of numerous malignancies as well as a worse clinical course for some cancers, it is reasonable to posit that body-weight loss might be beneficial. Although we have discussed several studies that suggest that body-weight loss can reduce the growth of experimental tumours and/or suppress metastasis, other preclinical studies have shown that weight normalization might be insufficient to reverse the effects of chronic obesity on haematopoietic function179, or inflammatory signals and epigenetic reprogramming in the microenvironment that are associated with tumour progression188. Currently, there are more clinically relevant questions than answers: will weight loss protect against the development of some or all of the obesity-related cancers? Will it reduce the risk of cancer regardless of the duration that a person has been obese? In patients who are obese and have cancer, how much weight loss will be necessary to improve prognosis? Will weight loss alter the efficacy of either targeted therapies or immunotherapy? If weight loss proves to be beneficial in reducing the risk of cancer or improving prognosis, can we identify blood biomarkers such as fasting levels of insulin or leptin? To begin addressing some of these questions, the Breast Cancer WEight Loss Study (BWEL Study) is a prospective phase III clinical trial testing whether weight loss in patients who are overweight or obese who have breast cancer will lead to a lower rate of recurrence compared with women who do not take part in the weight loss programme170. Given the importance of the link between obesity and cancer, an urgent need exists for additional studies to determine in what context body-weight loss will be clinically beneficial.
Pharmacological interventions to normalize the ATME.
Given the role of CLS in obesity-associated inflammation and insulin resistance, targeting these inflammatory structures during obesity is of considerable interest, for example, through specific macrophage-targeted therapies or vascular normalization strategies to optimize oxygenation. Although these treatment approaches have not been explored in great detail, pharmacological strategies against the metabolic syndrome have been tested. For example, treatment of obese mice with pioglitazone, a PPARγ ligand used to treat diabetes mellitus, induces adiponectin and reduces the expression of TNF, TGFβ and monocyte chemoattractant protein 1 (MCP1; also known as CCL2) in periprostatic adipose tissue, leading to reduced numbers of CLS143. Given the potential role of CLS within periprostatic fat in prostate cancer, whether this approach would be beneficial in patients is an important open question. Metformin is another antidiabetic agent that has received broad attention for its application in obesity-associated cancer (see TABLE 1). In addition to inducing modest weight loss, which can have anti-inflammatory effects, metformin can reduce the elevated levels of insulin that occur in association with inflamed adipose tissue144. Finally, gliptins are another class of hypoglycaemic agents that block dipeptidyl peptidase 4 (DPP4) and are prescribed for type 2 diabetes mellitus. One preclinical study showed that obesity-associated inflammatory pathways in ATME macrophages are regulated by liver-derived DPP4, such that targeting Dpp4 expression in hepatocytes, or its downstream effectors in adipose tissue macrophages, is sufficient to prevent WAT inflammation and insulin resistance145. However, treatment of mice with the oral DPP4 inhibitor sitagliptin did not reproduce these effects, suggesting that some of these mechanisms might not be reversible with pharmacological interventions.
Additional pharmacological interventions for obesity that have been explored in cancer include compounds that induce weight loss or control appetite, which might be more amenable to patient compliance compared with a low-calorie diet. Preclinical studies in lung cancer showed that caloric restriction mimetics that recapitulate the biochemical effects of nutrient deprivation, boost anticancer immune surveillance by depleting immune-suppressive Treg cells from the tumour microenvironment146. Similar effects can be achieved without pharmacological agents, by using a fasting-mimicking diet. This diet stimulates an increase in the infiltration of CD8+ cytotoxic T cells into the microenvironment of breast and melanoma tumours, leading to enhanced immune surveillance and delayed progression147. Although yet to be formally tested, it has been proposed that FDA-approved sympathomimetic compounds that suppress appetite (for example, phentermine or lorcaserin)27 or that activate thermogenesis (such as mira-begron)148 might likewise be valuable to reduce weight, WAT inflammation and cancer risk. Notably, these interventions might be relevant not only for obesity-associated cancers, but also for tumours that are particularly immunogenic, such as lung cancer or melanoma, which might benefit from improved anti-tumour immunity. The effect of combining these agents with immunotherapies in these tumours should be explored.
In addition to using behavioural or pharmacological approaches to target the more obvious culprits (for example, calorie consumption), other methods have been proposed to disrupt the obesity-cancer relationship. Preclinical models using oestrogen replacement therapy in obese ovariectomized mice have shown that treatment with 17β-oestradiol is sufficient to mitigate weight gain, CLS formation and production of several inflammatory markers including cyclooxygenase 2, TNF, IL-1β and aromatase149. Whether oestrogen can be used as a monotherapy in carefully selected postmenopausal women to reduce WAT inflammation and potentially reduce the risk of breast cancer has yet to be explored.
Surgical intervention.
Bariatric surgical interventions such as gastric bypass, vertical-banded gastroplasty or banding are a method to rapidly induce weight loss, improve metabolic syndrome and normalize the obese ATME. In patients who are obese, gastric bypass surgery significantly reduces the number of infiltrating macrophages and the number of CLS in sub-cutaneous WAT, in concordance with downregulation of pro-inflammatory genes and upregulation of anti-inflammatory genes in the stromal-vascular fraction150. Consistent with these changes, a retrospective study of 15,850 participants (7,925 had undergone gastric bypass compared with 7,925 matched controls), followed up fora mean period of 7.1 years, reported that gastric bypass surgery significantly reduced all-cause mortality by 40% in individuals who were obese (HR = 0.60) and reduced cause-specific mortality resulting from coronary artery disease (56%), diabetes mellitus (92%) and cancer (60%)151. Similarly, the Swedish Obese Subjects study, which prospectively assessed 4,047 people who were obese (2,010 had undergone various forms of bariatric surgery compared with 2,037 matched controls), followed up for a mean period of 10.9 years, reported that bariatric surgery significantly reduced all-cause mortality by 29% (HR = 0.71) in people who were obese after adjustment for sex, age and risk factors152. For cause-specific mortality, cancer-related mortality was reduced by 37% in the surgery group versus control group (relative risk (RR) = 0.63), almost double that of cardiac-related mortality (19% reduced risk; RR = 0.81)152. Importantly, it is not always clear from these studies whether bariatric surgery leads to reduced cancer-related mortality as a consequence of reduced cancer incidence or progression (whether a patient has an undiagnosed tumour at the time of surgery cannot be ruled out). However, a multisite retrospective study of 88,625 patients (22,198 patients who had undergone surgery and 66,427 participants who had not undergone surgery) with follow up between 2–9 years reported a 33% reduction in cancer diagnosis following bariatric surgery (HR = 0.67)153, suggesting a probable role in cancer prevention.
Opportunities for lifestyle intervention.
The benefits of pharmacological or surgical interventions raise the possibility that lifestyle interventions might have similar effects. Ongoing clinical studies are testing the effects of body-weight loss on breast cancer recurrence through telephone-based coaching, which includes recommendations for calorie consumption and physical activity154. At the molecular level, additional clinical studies have evaluated the effect of weight loss on obesity-associated metabo-inflammation, commonly through diet adjustments such as caloric restriction. In patients, a very-low-calorie diet leads to transcriptional changes in subcutaneous WAT that are indicative of reduced inflammation155, as well as reduced levels of systemic biomarkers of inflammation and the metabolic syndrome156. However, in individuals who were morbidly obese and underwent acute body-weight loss, CLS formation in subcutaneous WAT increased156, suggesting inflammatory remodelling of the ATME. Of note, adipose inflammation is a physiological response to healthy adipose tissue remodelling157, which simply becomes dysregulated during obesity, suggesting that this increase in CLS might be an important intermediary during rapid body-weight loss in order to establish tissue homeostasis. Supporting this notion, in preclinical models in which long-term caloric restriction is associated with weight stabilization, CLS in the mammary gland are reduced158. How these effects influence cancer remains unknown; however, given that caloric restriction affects breast WAT, there are probably implications for tumour biology. Furthermore, whether all tumours will be equally responsive is unclear (BOX 3); for example, tumours might differentially respond to dietary restriction depending on the mutational status of the PI3K pathway159.
Of course, adherence to a very-low-calorie diet is not sustainable (or recommended) outside of a controlled trial setting. Therefore, more manageable forms of dietary intervention have been explored, such as the Mediterranean diet, which encourages healthier food choices, such as olive oil over butter, herbs over salt, fish and poultry over red meat, and plenty of plant-based foods. In prospective studies, the Mediterranean diet improved indicators of the metabolic syndrome in patients who were obese, including reduced circulating inflammatory factors, improved insulin sensitivity and improved vascular function, with durable responses of up to 2 years160,161. Importantly, in humans, a Mediterranean diet supplemented with extra-virgin olive oil led to reduced incidence of breast cancer compared with a control diet162. Whether the Mediterranean diet affects the composition of the breast ATME is unknown. Another sustainable dietary intervention that has been investigated is a low-carbohydrate diet compared with a low-fat diet. Several prospective clinical trials have reported that a low-carbohydrate diet is superior to induce weight loss compared with a low-fat diet, in association with improved lipid profiles and insulin sensitivity163–165. Whether these dietary interventions are sufficient to improve cancer outcomes remains unknown.
To complement these dietary interventions, physical activity is another important lifestyle factor that seems to affect cancer166. An analysis of pooled data from12 prospective trials, including a total of 1.44 million participants, demonstrated that self-reported leisure-time physical activity is associated with a reduced risk of 13 out of 26 different types of cancer, and in some cases these associations were independent of BMI167. In patients who are overweight or obese who have survived breast cancer, moderate-to-difficult exercise (65–85% maximum heart rate, 3 times weekly for 16 weeks) reduced systemic biomarkers of the metabolic syndrome, including reduced levels of insulin, insulin-like growth factor 1 (IGF1) and leptin, and increased adiponectin, and this effect was sustained for the 3 month follow-up period168. These data suggest that exercise might be beneficial not only for the general population, but also specifically for patients with breast cancer, in whom ≥5% weight gain post-diagnosis is associated with increased mortality169. Another appealing strategy is to combine a weight loss intervention with increased physical activity to stimulate body-weight loss while maintaining muscle mass. However, whether these solutions will be sustainable, or whether lifestyle interventions will be sufficient to completely erase the effects of obesity, remains unclear (BOX 3). Studies are underway to determine whether lifestyle interventions can improve cancer outcomes170.
Conclusions and future considerations
Given the striking associations between obesity, metabo-inflammation and cancer, preclinical and clinical studies that mechanically dissect these relationships are essential. We propose that patients who are obese will probably require special treatment considerations, such as diet and lifestyle counselling that is evidence-based, or adjuvant pharmacological interventions that target inflammation and the metabolic syndrome. These strategies will also be relevant to individuals who are not obese (BMI <30) who still display metabolic aberrancies, including adipose tissue dysfunction. Of note, an important consideration will be to develop cost-efficient, effective ways to identify individuals with excess body fat to supplement BMI measurements in routine medical practice, in order to more accurately identify those at risk. In particular, incorporating blood-based tests or body composition analyses in periodic medical examinations might help pinpoint individuals with inflamed adipose tissue who are within normal-range BMI171. Finally, disease management and therapeutic efficacy remain major obstacles for patients who are obese and have cancer, and experimental data suggest that this hurdle is not purely a dosing problem. This problem is particularly relevant for cancer immunotherapy, for which pre-existing chronic inflammatory conditions might trigger adverse effects. Although clinical studies in patients have reported an increased response to immuno-therapy in men who are obese and have melanoma75, preclinical work has suggested that adverse effects can occur, particularly when obesity is compounded by ageing172.
Obesity could account for up to 20% of cancer-related deaths, or 1.6 million deaths annually on an international scale. If this link is in fact causal, we have an urgent responsibility as a scientific community to respond to these statistics by increasing research initiatives focused on metabo-inflammation.
Key points.
Obesity is associated with increased cancer incidence and mortality.
Substantial changes occur within the adipose tissue microenvironment (ATME) with body-weight gain.
Metabolic and inflammatory changes related to the obese ATME contribute to cancer development and progression.
Targeting adipose tissue dysfunction through pharmacological or lifestyle interventions might be useful for the prevention and treatment of cancer.
Given the limitations of BMI as a measurement of adiposity, finding novel ways to identify individuals who are metabolically unhealthy with excess adipose tissue will be critical to pinpoint those at risk who might benefit from weight loss or other personalized interventions.
Acknowledgements
The authors thank Oakley C. Olson, Martin J. Richer and Azadeh Arabzadeh for their critical feedback on the manuscript. A.J.D. is supported by the Breast Cancer Research Foundation, the Botwinick-Wolfensohn Foundation (in memory of Mr and Mrs Benjamin Botwinick), NIH/NCI R01 CA215797 and NIH/NCI U54 CA210184. D.F.Q. is supported by Susan G. Komen CCR18548032 and Canadian Institutes of Health Research PJT-159742.
Glossary
- Adipocyte hypertrophy
Enlargement of adipocytes, which often occurs in association with obesity and increased numbers of crown-like structures in adipose tissue.
- Metabolically obese normal-weight
(MoNW). individuals within a normal-range BMi category (18.5–24.9), yet with a high body fat composition, leading to qualitatively similar health risks as individuals who are obese.
- Adipose tissue microenvironment
(ATME). The cellular and structural compartment of adipose tissue, including but not limited to the adipocyte.
- Pyroptosis
Inflammatory programmed cell death, in which an immune cell bursts to release intracellular contents into the microenvironment to trigger a rapid immune response.
- Myelopoiesis
Differentiation of haematopoetic progenitor cells within the bone marrow towards a myeloid lineage.
- Monocytosis
Expansion of monocytes within the peripheral blood, which are precursors for macrophages and dendritic cells.
- Crown-like structures
(CLS). A dying or dead adipocyte surrounded by a ‘crown’ of macrophages within adipose tissue; this structure is a histological biomarker of obesity-associated inflammation and the metabolic syndrome.
- Metabo-inflammation
inflammation within the adipose tissue microenvironment that has metabolic consequences irrespective of BMi status.
- Benign breast disease
Heterogeneous group of lesions within the breast that might increase the risk of developing breast cancer.
- CLS-associated macrophages
(CAMφ). Macrophages that are directly associated with crown-like structures (CLs) in inflamed adipose tissue; these cells are phenotypically and transcriptionally distinct from other macrophages within the adipose tissue microenvironment.
- Myofibroblasts
Contractile cells of the mesenchymal-fibroblast lineage that synthesize extracellular matrix and mediate tissue remodelling.
- Adipose stromal cells
(AsCs). Multipotent mesenchymal progenitor cells found in adipose tissue that can differentiate into mesoderm lineages (such as adipocytes, myofibroblasts, chondrocytes and osteoblasts); several terms have been used in the literature to refer to these cells (for example, adipose-derived stem cells, pre-adipocytes, adipose mesenchymal stem cells or lipoblasts).
- Neutrophilia
High number of mature neutrophils within the peripheral blood or within tissues, resulting from neutrophil leukocytosis.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher's Disclaimer: Publisher’s note
Publisher's Disclaimer: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.James WPT & McPherson K The costs of overweight. Lancet Public Health 2, e203–e204 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Malik VS, Willett WC & Hu FB Global obesity: trends, risk factors and policy implications. Nat. Rev. Endocrinol 9, 13–27 (2013). [DOI] [PubMed] [Google Scholar]
- 3.NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 387, 1377–1396 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng M et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hales CM, Fryar CD, Carroll MD, Freedman DS& Ogden CL Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA 319, 1723–1725 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cawley J & Meyerhoefer C The medical care costs of obesity: an instrumental variables approach. J. Health Econ 31, 219–230 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Lauby-Secretan B et al. Body fatness and cancer — viewpoint of the IARC Working Group. N. Engl. J. Med 375, 794–798 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calle EE, Rodriguez C, Walker-Thurmond K & Thun MJ Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U. S. adults. N. Engl. J. Med 348, 1625–1638 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Ligibel JA et al. American Society of Clinical Oncology position statement on obesity and cancer.J. Clin. Oncol 32, 3568–3574 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calle EE & Kaaks R Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 4, 579–591 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Tao W & Lagergren J Clinical management of obese patients with cancer. Nat. Rev. Clin. Oncol 10, 519–533 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Rosenquist KJ et al. Fat quality and incident cardiovascular disease, all-cause mortality, and cancer mortality. J. Clin. Endocrinol. Metab 100, 227–234 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyengar NM et al. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin. Cancer Res 22, 2283–2289 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen ED & Spiegelman BM What we talk about when we talk about fat. Cell 156, 20–44 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wajchenberg BL Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr. Rev 21, 697–738 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Neeland IJ et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity 21, E439–E447 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLaughlin T, Lamendola C, Liu A & Abbasi F Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J. Clin. Endocrinol. Metab 96, E1756–E1760 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox CS et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116, 39–48 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Park J, Morley TS, Kim M, Clegg DJ & Scherer PE Obesity and cancer — mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol 10, 455–465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khandekar MJ, Cohen P & Spiegelman BM Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer 11, 886–895 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Font-Burgada J, Sun B & Karin M Obesity and cancer: the oil that feeds the flame. Cell Metab. 23, 48–62 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Lengyel E, Makowski L, DiGiovanni J & Kolonin MG Cancer as a matter of fat: the crosstalk between adipose tissue and tumors. Trends Cancer 4, 374–384 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson OC, Quail DF & Joyce JA Obesity and the tumor microenvironment. Science 358, 1130–1131 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Borrud LG et al. Body composition data for individuals 8 years of age and older: U.S. population, 1999–2004. Vital Health Stat. 250, 1–87 (2010). [PMC free article] [PubMed] [Google Scholar]
- 25.Flegal KM et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am. J. Clin. Nutr 89,500–508 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brestoff JR & Artis D Immune regulation of metabolic homeostasis in health and disease. Cell 161, 146–160 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howe LR, Subbaramaiah K, Hudis CA & Dannenberg AJ Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin. Cancer Res 19, 6074–6083 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crewe C, An YA & Scherer PE The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J. Clin. Invest 127, 74–82 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osborn O & Olefsky JM The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med 18, 363–374 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Reilly SM & Saltiel AR Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol 13, 633–643 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Kanneganti TD & Dixit VD Immunological complications of obesity. Nat. Immunol 13, 707–712 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Giordano A et al. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J. Lipid Res 54, 2423–2436 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cinti S et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res 46, 2347–2355 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Wen H et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol 12, 408–415 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou R, Tardivel A, Thorens B, Choi I & Tschopp J Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol 11, 136–140 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Mariathasan S et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Vandanmagsar B et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med 17, 179–188 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duewell P et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youm YH et al. The Nlrp3 inflammasome promotes age-related thymic demise and immunosenescence. Cell Rep. 1, 56–68 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weisberg SP et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest 112, 1796–1808 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagareddy PR et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 19, 821–835 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amano SU et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 19, 162–171 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braune J et al. IL-6 regulates M2 polarization and local proliferation of adipose tissue macrophages in obesity. J. Immunol 198, 2927–2934 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Jenkins SJ et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332, 1284–1288 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haka AS et al. Exocytosis of macrophage lysosomes leads to digestion of apoptotic adipocytes and foam cell formation. J. Lipid Res 57, 980–992 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolb R et al. Obesity-associated NLRC4 inflammasome activation drives breast cancer progression. Nat. Commun 7, 13007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arkan MC et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med 11, 191–198 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Solinas G et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 6, 386–397 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Iyengar NM et al. Metabolic obesity, adipose inflammation and elevated breast aromatase in women with normal body mass index. Cancer Prev. Res 10, 235–243 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koru-Sengul T et al. Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians. Breast Cancer Res. Treat 158, 113–126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carter JM et al. Macrophagic “crown-like structures” are associated with an increased risk of breast cancer in benign breast disease. Cancer Prev. Res 11, 113–119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diaz-Cruz ES, Sugimoto Y, Gallicano GI, Brueggemeier RW & Furth PA Comparison of increased aromatase versus ERalpha in the generation of mammary hyperplasia and cancer. Cancer Res. 71, 5477–5487 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gucalp A et al. Periprostatic adipose inflammation is associated with high-grade prostate cancer. Prostate Cancer Prostatic Dis. 20, 418–423 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iyengar NM et al. White adipose tissue inflammation and cancer-specific survival in patients with squamous cell carcinoma of the oral tongue. Cancer 122, 3794–3802 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quail DF & Joyce JA Microenvironmental regulation of tumor progression and metastasis. Nat. Med 19, 1423–1437 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patsouris D et al. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 8, 301–309 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen MT et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem 282, 35279–35292 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Shaul ME, Bennett G, Strissel KJ, Greenberg AS & Obin MS Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet–induced obesity in mice. Diabetes 59, 1171–1181 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franklin RA et al. The cellular and molecular origin of tumor-associated macrophages. Science 344, 921–925 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hill DA et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc. Natl Acad. Sci. USA 115, E5096–E5105 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tkach M & Thery C Communication by extracellular vesicles: where we are and where we need to go. Cell 164, 1226–1232 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Xu X et al. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 18, 816–830 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kratz M et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 20, 614–625 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu D et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coffelt SB, Wellenstein MD & de Visser KE Neutrophils in cancer: neutral no more. Nat. Rev. Cancer 16, 431–446 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Talukdar S et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med 18, 1407–1412 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu J et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med 15, 940–945 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olson OC & Joyce JA Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nat. Rev. Cancer 15, 712–729 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Xia S et al. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J. Biol. Chem 286, 23591–23599 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clements VK et al. Frontline science: high fat diet and leptin promote tumor progression by inducing myeloid-derived suppressor cells. J. Leukoc. Biol 103, 395–407 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Macdougall CE et al. Visceral adipose tissue immune homeostasis is regulated by the crosstalk between adipocytes and dendritic cell subsets. Cell Metab. 27, 588–601 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang H et al. Obesity accelerates thymic aging. Blood 114, 3803–3812 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang H et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J. Immunol 185, 1836–1845 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Canter RJ et al. Obesity results in higher PD-1-mediated T cell suppression but greater T cell effector functions following blockade. J. Clin. Oncol 36, S65 (2018). [Google Scholar]
- 75.McQuade JL et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 19, 310–322 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Winer DA et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med 17, 610–617 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nishimura S et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med 15, 914–920 (2009). [DOI] [PubMed] [Google Scholar]
- 78.Cipolletta D et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 486, 549–553 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kolodin D et al. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 21, 543–557 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feuerer M et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med 15, 930–939 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O’Sullivan TE et al. Adipose-resident group 1 innate lymphoid cells promote obesity-associated insulin resistance. Immunity 45, 428–441 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Elinav E et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 13, 759–771 (2013). [DOI] [PubMed] [Google Scholar]
- 83.Sung HK et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 17, 61–72 (2013). [DOI] [PubMed] [Google Scholar]
- 84.Lee YS et al. Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell 157, 1339–1352 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arendt LM et al. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res. 73, 6080–6093 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shah D et al. Obesity-induced adipokine imbalance impairs mouse pulmonary vascular endothelial function and primes the lung for injury. Sci. Rep 5, 11362 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Wang L et al. Enhancement of endothelial permeability by free fatty acid through lysosomal cathepsin B-mediated Nlrp3 inflammasome activation. Oncotarget 7, 73229–73241 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Incio J et al. Obesity promotes resistance to anti-VEGF therapy in breast cancer by up-regulating IL-6 and potentially FGF-2. Sci. Transl Med 10, eaag0945 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carmeliet P & Jain RK Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov 10, 417–427 (2011). [DOI] [PubMed] [Google Scholar]
- 90.Tammela T & Alitalo K Lymphangiogenesis: molecular mechanisms and future promise. Cell 140, 460–476 (2010). [DOI] [PubMed] [Google Scholar]
- 91.Greene AK, Grant FD & Slavin SA Lower-extremity lymphedema and elevated body-mass index. N. Engl. J. Med 366, 2136–2137 (2012). [DOI] [PubMed] [Google Scholar]
- 92.McLaughlin SA et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J. Clin. Oncol 26, 5213–5219 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wong BW et al. The role of fatty acid beta-oxidation in lymphangiogenesis. Nature 542, 49–54 (2017). [DOI] [PubMed] [Google Scholar]
- 94.Stacker SA et al. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 14, 159–172 (2014). [DOI] [PubMed] [Google Scholar]
- 95.Harvey NL et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet 37, 1072–1081 (2005). [DOI] [PubMed] [Google Scholar]
- 96.Escobedo N et al. Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight 1, e85096 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lund AW Rethinking lymphatic vessels and antitumor immunity. Trends Cancer 2, 548–551 (2016). [DOI] [PubMed] [Google Scholar]
- 98.Hespe GE et al. Exercise training improves obesity-related lymphatic dysfunction. J. Physiol 594, 4267–4282 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nitti MD et al. Obesity-induced lymphatic dysfunction is reversible with weight loss. J. Physiol 594, 7073–7087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Escobedo N & Oliver G The lymphatic vasculature: its role in adipose metabolism and obesity. Cell Metab. 26, 598–609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cao Y Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 18, 478–489 (2013). [DOI] [PubMed] [Google Scholar]
- 102.Levental KR et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paszek MJ et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005). [DOI] [PubMed] [Google Scholar]
- 104.Tanaka M et al. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nat. Commun 5, 4982 (2014). [DOI] [PubMed] [Google Scholar]
- 105.Seo BR et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci. Transl Med 7, 301ra130 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iyengar P et al. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J. Clin. Invest 115, 1163–1176 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khan T et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol 29, 1575–1591 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Halberg N et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol 29, 4467–4483 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Incio J et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 6, 852–869 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Z & Scherer PE Adipose tissue: the dysfunctional adipocyte - a cancer cell’s best friend. Nat. Rev. Endocrinol 14, 132–134 (2018). [DOI] [PubMed] [Google Scholar]
- 111.Zhang M et al. Adipocyte-derived lipids mediate melanoma progression via FATP proteins. Cancer Discov 8, 1006–1025 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Laurent V et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat. Commun 7, 10230 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim JY et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue.J. Clin. Invest 117, 2621–2637 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yamauchi T et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med 7, 941–946 (2001). [DOI] [PubMed] [Google Scholar]
- 115.Zeng W et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163, 84–94 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang T et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nat. Commun 7, 11674 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang Y et al. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 69, 5259–5266 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Y et al. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 72, 5198–5208 (2012). [DOI] [PubMed] [Google Scholar]
- 119.Klopp AH et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin. Cancer Res 18, 771–782 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Song YH et al. Breast cancer-derived extracellular vesicles stimulate myofibroblast differentiation and pro-angiogenic behavior of adipose stem cells. Matrix Biol. 60–61, 190–205 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Strong AL et al. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 17, 112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Daquinag AC et al. Targeted proapoptotic peptides depleting adipose stromal cells inhibit tumor growth. Mol. Ther 24, 34–40 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stern JH, Rutkowski JM & Scherer PE Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 23, 770–784 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Quail DF et al. Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF. Nat. Cell Biol 19, 974–987 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Park EJ et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140, 197–208 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li R et al. Obesity, rather than diet, drives epigenomic alterations in colonic epithelium resembling cancer progression. Cell Metab. 19, 702–711 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ericksen RE et al. Obesity accelerates Helicobacter felis-induced gastric carcinogenesis by enhancing immature myeloid cell trafficking and TH17 response. Gut 63, 385–394 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nelson ER et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science 342, 1094–1098 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu Q et al. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 5, 637–645 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Baek AE et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat. Commun 8, 864 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Voisin M et al. Identification of a tumor-promoter cholesterol metabolite in human breast cancers acting through the glucocorticoid receptor. Proc. Natl Acad. Sci. USA 114, E9346–E9355 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Behan JW et al. Adipocytes impair leukemia treatment in mice. Cancer Res. 69, 7867–7874 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sheng X et al. Adipocytes sequester and metabolize the chemotherapeutic daunorubicin. Mol. Cancer Res 15, 1704–1713 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Straussman R et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 487, 500–504 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Olson OC, Kim H, Quail DF, Foley EA & Joyce JA Tumor-associated macrophages suppress the cytotoxic activity of antimitotic agents. Cell Rep. 19, 101–113 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zarrinpar A, Chaix A, Yooseph S & Panda S Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 20, 1006–1017 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Panda S Circadian physiology of metabolism. Science 354, 1008–1015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Marcheva B et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466, 627–631 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Costa MJ et al. Circadian rhythm gene period 3 is an inhibitor of the adipocyte cell fate. J. Biol. Chem 286, 9063–9070 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hatori M et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 15, 848–860 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sulli G et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 553, 351–355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Joyce JA Therapeutic targeting of the tumor microenvironment. Cancer Cell 7, 513–520 (2005). [DOI] [PubMed] [Google Scholar]
- 143.Miyazawa M et al. Pioglitazone inhibits periprostatic white adipose tissue inflammation in obese mice. Cancer Prev. Res 11, 215–226 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Klil-Drori AJ, Azoulay L & Pollak MN Cancer, obesity, diabetes, and antidiabetic drugs: is the fog clearing? Nat. Rev. Clin. Oncol 14, 85–99 (2017). [DOI] [PubMed] [Google Scholar]
- 145.Ghorpade DS et al. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature 555, 673–677 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pietrocola F et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 30, 147–160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Di Biase S et al. Fasting-mimicking diet reduces HO-1 to promote T cell-mediated tumor cytotoxicity. Cancer Cell 30, 136–146 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cypess AM et al. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab. 21, 33–38 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bhardwaj P et al. Estrogen protects against obesity-induced mammary gland inflammation in mice. Cancer Prev. Res 8, 751–759 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cancello R et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 54, 2277–2286 (2005). [DOI] [PubMed] [Google Scholar]
- 151.Adams TD et al. Long-term mortality after gastric bypass surgery. N. Engl. J. Med 357, 753–761 (2007). [DOI] [PubMed] [Google Scholar]
- 152.Sjostrom L et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med 357, 741–752 (2007). [DOI] [PubMed] [Google Scholar]
- 153.Schauer DP et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann. Surg 10.1097/SLA0000000000002525 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ligibel JA et al. Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): study design. NPJ Breast Cancer 3, 37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Clement K et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 18, 1657–1669 (2004). [DOI] [PubMed] [Google Scholar]
- 156.Aleman JO et al. Effects of rapid weight loss on systemic and adipose tissue inflammation and metabolism in obese postmenopausal women. J. Endocr. Soc 1, 625–637 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wernstedt Asterholm I et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 20, 103–118 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bhardwaj P et al. Caloric restriction reverses obesity-induced mammary gland inflammation in mice. Cancer Prev. Res 6, 282–289 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 159.Kalaany NY & Sabatini DM Tumours with PI3K activation are resistant to dietary restriction. Nature 458, 725–731 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Esposito K et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA 292, 1440–1446 (2004). [DOI] [PubMed] [Google Scholar]
- 161.Esposito K et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA 289, 1799–1804 (2003). [DOI] [PubMed] [Google Scholar]
- 162.Toledo E et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: a randomized clinical trial. JAMA Intern. Med 175, 1752–1760 (2015). [DOI] [PubMed] [Google Scholar]
- 163.Samaha FF et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N. Engl. J. Med 348, 2074–2081 (2003). [DOI] [PubMed] [Google Scholar]
- 164.Foster GD et al. A randomized trial of a low-carbohydrate diet for obesity. N. Engl. J. Med 348, 2082–2090 (2003). [DOI] [PubMed] [Google Scholar]
- 165.Shai I et al. Weight loss with a low-carbohydrate, mediterranean, or low-fat diet. N. Engl. J. Med 359, 229–241 (2008). [DOI] [PubMed] [Google Scholar]
- 166.Koelwyn GJ, Quail DF, Zhang X, White RM & Jones LW Exercise-dependent regulation of the tumour microenvironment. Nat. Rev. Cancer 17, 620–632 (2017). [DOI] [PubMed] [Google Scholar]
- 167.Moore SC et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern. Med 176, 816–825 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dieli-Conwright CM et al. Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: a randomized controlled trial. J. Clin. Oncol 36, 875–883 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Playdon MC et al. Weight gain after breast cancer diagnosis and all-cause mortality: systematic review and meta-analysis. J. Natl Cancer Inst 107, djv275 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Demark-Wahnefried W et al. Weight management and physical activity throughout the cancer care continuum. CA Cancer J. Clin 68, 64–89 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Iyengar NM et al. Body fat and risk of breast cancer in postmenopausal women with normal body mass index. JAMA Oncol. (in the press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mirsoian A et al. Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice. J. Exp. Med 211, 2373–2383 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gupta S Obesity: the fat advantage. Nature 537, S100–S102 (2016). [DOI] [PubMed] [Google Scholar]
- 174.Denis GV & Obin MS ‘Metabolically healthy obesity’: origins and implications. Mol. Aspects Med 34, 59–70 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stefan N, Schick F & Haring HU Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab. 26, 292–300 (2017). [DOI] [PubMed] [Google Scholar]
- 176.Rubin R Postmenopausal women with a “normal” BMI might be overweight or even obese. JAMA 319, 1185–1187 (2018). [DOI] [PubMed] [Google Scholar]
- 177.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363, 157–163 (2004). [DOI] [PubMed] [Google Scholar]
- 178.Naveiras O et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460, 259–263 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee JM et al. Obesity alters the long-term fitness of the hematopoietic stem cell compartment through modulation of Gfi1 expression. J. Exp. Med 215, 627–644 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Masamoto Y et al. Adiponectin enhances antibacterial activity of hematopoietic cells by suppressing bone marrow inflammation. Immunity 44, 1422–1433 (2016). [DOI] [PubMed] [Google Scholar]
- 181.Liu A et al. Bone marrow lympho-myeloid malfunction in obesity requires precursor cell-autonomous TLR4. Nat. Commun 9, 708 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Trottier MD, Naaz A, Li Y & Fraker PJ Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proc. Natl Acad. Sci. USA 109, 7622–7629 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lu Z et al. Fasting selectively blocks development of acute lymphoblastic leukemia via leptin-receptor upregulation. Nat. Med 23, 79–90 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yun JP et al. Diet-induced obesity accelerates acute lymphoblastic leukemia progression in two murine models. Cancer Prev. Res 3, 1259–1264 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shafat MS et al. Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood 129, 1320–1332 (2017). [DOI] [PubMed] [Google Scholar]
- 186.Poynter JN et al. Obesity over the life course and risk of acute myeloid leukemia and myelodysplastic syndromes. Cancer Epidemiol. 40, 134–140 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Butturini AM et al. Obesity and outcome in pediatric acute lymphoblastic leukemia. J. Clin. Oncol 25, 2063–2069 (2007). [DOI] [PubMed] [Google Scholar]
- 188.Rossi EL et al. Obesity-associated alterations in inflammation, epigenetics, and mammary tumor growth persist in formerly obese mice. Cancer Prev. Res 9, 339–348 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Greenstein AS et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119, 1661–1670 (2009). [DOI] [PubMed] [Google Scholar]
- 190.Rosen CJ & Bouxsein ML Mechanisms of disease: is osteoporosis the obesity of bone? Nat. Clin. Pract. Rheumatol 2, 35–43 (2006). [DOI] [PubMed] [Google Scholar]
- 191.Estruch R et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med 378, e34 (2018). [DOI] [PubMed] [Google Scholar]
- 192.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01697566 (2018).
- 193.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01101438 (2018).
- 194.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02065687 (2018).
- 195.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02040376 (2017).
- 196.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01655719 (2017).
- 197.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00780234 (2016).
- 198.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01838317 (2018).
- 199.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00099021 (2016).
- 200.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00427999 (2016).
- 201.Irwin ML et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiol. Biomarkers Prev 18, 306–313 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fairey AS et al. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol. Biomarkers Prev 12, 721–727 (2003). [PubMed] [Google Scholar]
- 203.Spencer M et al. Pioglitazone treatment reduces adipose tissue inflammation through reduction of mast cell and macrophage number and by improving vascularity. PLOS ONE 9, e102190 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]