Abstract
Background
Vitamins and minerals have many functions in the nervous system which are important for brain health. It has been suggested that various different vitamin and mineral supplements might be useful in maintaining cognitive function and delaying the onset of dementia. In this review, we sought to examine the evidence for this in people who already had mild cognitive impairment (MCI).
Objectives
To evaluate the effects of vitamin and mineral supplementation on cognitive function and the incidence of dementia in people with mild cognitive impairment.
Search methods
We searched ALOIS, the Cochrane Dementia and Cognitive Improvement Group’s (CDCIG) specialised register, as well as MEDLINE, Embase, PsycINFO, CENTRAL, CINAHL, LILACs, Web of Science Core Collection, ClinicalTrials.gov, and the WHO Portal/ICTRP, from inception to 25 January 2018.
Selection criteria
We included randomised or quasi‐randomised, placebo‐controlled trials which evaluated orally administered vitamin or mineral supplements in participants with a diagnosis of mild cognitive impairment and which assessed the incidence of dementia or cognitive outcomes, or both. We were interested in studies applicable to the general population of older people and therefore excluded studies in which participants had severe vitamin or mineral deficiencies.
Data collection and analysis
We sought data on our primary outcomes of dementia incidence and overall cognitive function and on secondary outcomes of episodic memory, executive function, speed of processing, quality of life, functional performance, clinical global impression, adverse events, and mortality. We conducted data collection and analysis according to standard Cochrane systematic review methods. We assessed the risk of bias of included studies using the Cochrane 'Risk of bias' assessment tool. We grouped vitamins and minerals according to their putative mechanism of action and, where we considered it to be clinically appropriate, we pooled data using random‐effects methods. We used GRADE methods to assess the overall quality of evidence for each comparison and outcome.
Main results
We included five trials with 879 participants which investigated B vitamin supplements. In four trials, the intervention was a combination of vitamins B6, B12, and folic acid; in one, it was folic acid only. Doses varied. We considered there to be some risks of performance and attrition bias and of selective outcome reporting among these trials. Our primary efficacy outcomes were the incidence of dementia and scores on measures of overall cognitive function. None of the trials reported the incidence of dementia and the evidence on overall cognitive function was of very low‐quality. There was probably little or no effect of B vitamins taken for six to 24 months on episodic memory, executive function, speed of processing, or quality of life. The evidence on our other secondary clinical outcomes, including harms, was very sparse or very low‐quality. There was evidence from one study that there may be a slower rate of brain atrophy over two years in participants taking B vitamins. The same study reported subgroup analyses based on the level of serum homocysteine (tHcy) at baseline and found evidence that B vitamins may improve episodic memory in those with tHcy above the median at baseline.
We included one trial (n = 516) of vitamin E supplementation. Vitamin E was given as 1000 IU of alpha‐tocopherol twice daily. We considered this trial to be at risk of attrition and selective reporting bias. There was probably no effect of vitamin E on the probability of progression from MCI to Alzheimer's dementia over three years (HR 1.02; 95% CI 0.74 to 1.41; n = 516; 1 study, moderate‐quality evidence). There was also no evidence of an effect at intermediate time points. The available data did not allow us to conduct analyses, but the authors reported no significant effect of three years of supplementation with vitamin E on overall cognitive function, episodic memory, speed of processing, clinical global impression, functional performance, adverse events, or mortality (five deaths in each group). We considered this to be low‐quality evidence.
We included one trial (n = 256) of combined vitamin E and vitamin C supplementation and one trial (n = 26) of supplementation with chromium picolinate. In both cases, there was a single eligible cognitive outcome, but we considered the evidence to be very low‐quality and so could not be sure of any effects.
Authors' conclusions
The evidence on vitamin and mineral supplements as treatments for MCI is very limited. Three years of treatment with high‐dose vitamin E probably does not reduce the risk of progression to dementia, but we have no data on this outcome for other supplements. Only B vitamins have been assessed in more than one RCT. There is no evidence for beneficial effects on cognition of supplementation with B vitamins for six to 24 months. Evidence from a single study of a reduced rate of brain atrophy in participants taking vitamin B and a beneficial effect of vitamin B on episodic memory in those with higher tHcy at baseline warrants attempted replication.
Plain language summary
Vitamin and mineral supplementation for preventing dementia or delaying cognitive decline in people with mild cognitive impairment
Review question
This review investigated whether people with mild cognitive impairment can reduce their risk of developing dementia, or can prevent their memory or other thinking skills from deteriorating further, by taking vitamin or mineral supplements.
Background
Slight changes in memory and thinking skills are common as people get older. When these changes are worse than can be expected in normal ageing, but are not bad enough to make a person's usual activities difficult to manage, then the person is said to have mild cognitive impairment (MCI). People with MCI are at increased risk of developing dementia in the future.
Vitamins and minerals are naturally occurring substances which are needed in the diet to maintain health. They have lots of different functions in the body and many are essential to keep the brain working properly. It has been suggested that supplementing a person's normal diet with extra doses of these vitamins or minerals might help to maintain thinking skills or prevent dementia.
Study characteristics
We found eight randomised controlled trials (RCTs), which investigated four different types of vitamin or mineral pills by comparing them to a placebo (a dummy pill). The vitamins tested were B vitamins (vitamin B6, vitamin B12 and folic acid), vitamin E, and vitamin E and C given together. The only mineral tested was chromium.
Key results and quality of the evidence
Vitamin B combination versus placebo
Five trials with a total of 879 participants compared B vitamins with placebo. Four used combinations of vitamin B6, vitamin B12, and folic acid; one small study tested folic acid on its own. None of these studies reported whether or not participants developed dementia. These studies did not find that memory or thinking skills differed between the group of people who took vitamin B supplements and those who took placebo after treatment lasting six months to two years. Our confidence in the results on different tests used in the studies varied from moderate to very low. Two years of vitamin B supplements did seem to help memory in a small subgroup of participants in one study who could be identified by a particular blood test at the start of the trial. One study found that there was probably no effect on participants' quality of life. One study scanned the brains of some participants and reported that B vitamins may slow the rate of brain shrinkage.
Harmful effects and deaths were reported in very few participants and we cannot conclude whether or not there are harms from taking these or similar combinations of B vitamins.
Vitamin E versus placebo.
One study with 516 participants compared a relatively high dose of vitamin E (2000 IU a day) to placebo in people who were also taking a multivitamin containing 15 IU of vitamin E (the daily requirement for vitamin E is approximately 30 IU). The risk of developing dementia due to Alzheimer’s disease (the commonest form of dementia) is probably not affected by three years of treatment with high‐dose vitamin E. The quality of the evidence for other outcomes was lower, but there may also be no effect of this dose of vitamin E on specific memory or thinking skills or on how well people could manage their daily activities.
Vitamin E and C versus placebo
One study with 256 participants compared a combination of vitamins C and E with placebo. It found no effect on overall memory and thinking skills, but we had little confidence in this result because of the quality of the evidence.
Chromium picolinate versus placebo
Only one very small study with 26 participants investigated the effect of chromium supplements. This study was too small for us to be able to draw any conclusions.
Conclusions
The amount and quality of research evidence about vitamin and mineral supplements for treating MCI in people without nutritional deficiency is limited. At the moment, it is not possible to identify any supplements which can reduce the risk of people with MCI developing dementia or which can effectively treat their symptoms. More research is needed before we can answer our review question.
Summary of findings
Background
Description of the condition
Mild cognitive impairment and dementia
Prior to the onset of dementia, there can be a prodromal (pre‐symptomatic) stage which is often termed 'mild cognitive impairment' (MCI). The category of MCI captures those individuals whose cognitive deficits are beyond those typically seen in normal ageing and who are at high risk of future dementia. Different criteria have been proposed to identify MCI, but, broadly speaking, MCI of the amnestic subtype is a state where individuals have subjective and objective memory impairment that is inconsistent with age, but normal global cognitive functioning, and normal performance in non‐memory cognitive domains. The main focus of these criteria is to detect memory problems due to prodromal Alzheimer’s disease (AD). However, not all forms of MCI evolve into AD dementia and, therefore, there have been calls for broader, more inclusive criteria. In 2003, an International Working Group (IWG) developed consensus criteria and expanded the definition of MCI to include objective and subjective impairments in any cognitive domain (Winblad 2004). The influential Petersen criteria have been similarly extended (Petersen 2004). In recent years, new criteria have been proposed for MCI due to AD, including the National Institute on Aging‐Alzheimer’s Association (NIA‐AA) criteria for preclinical/prodromal states (Albert 2011), updated NIA‐AA research criteria (Jack 2018), and updated versions of the IWG criteria (Dubois 2014).
Dementia is a syndrome of cognitive and functional decline which is usually progressive and which involves impairment in more than one cognitive function, memory being the most commonly affected in the early stages. Other higher cortical functions such as orientation, comprehension, learning, language, and judgement are also often affected. In most cases, the onset of dementia and its subsequent progression is gradual. The cognitive deficits in the early stages of the illness are relatively mild, but still have an impact on the ability to perform some normal daily activities. As the syndrome progresses, people with dementia eventually become increasingly dependent on others for support with all activities of daily living.
Types of MCI and dementia
There are numerous different definitions of MCI, with different focus (e.g. nature of the neuropsychological impairment, such as memory or non‐memory (Matthews 2007); prevalence (Stephan 2007); and risk of progression to dementia (Matthews 2008). Further subdivisions can be made depending on the suspected underlying cause of the cognitive deficits (e.g. MCI due to AD and MCI due to vascular disease, termed 'vascular cognitive impairment no dementia' (VCIND)). Moreover, attempts have been made to develop new criteria to capture even earlier preclinical states including, for example, 'pre‐MCI' that captures individuals with impaired executive function and language, higher apathy scores, and lower left hippocampal volumes on brain imaging compared to normal controls (Duara 2011). There is no standard definition of MCI universally accepted for use in clinical trials (Stephan 2013), but adaptations of the criteria suggested by Petersen are commonly used (Petersen 1999).
Subtypes of dementia are distinguished by the underlying pathology. The four most common subtypes are Alzheimer's disease dementia (AD) (accounting for an estimated 60% to 70% of all dementia cases); vascular dementia (VaD); dementia with Lewy Bodies (DLB); and frontotemporal dementia (FTD). Accurate diagnosis of the subtypes may be difficult. Mixed pathology is common, with more than 80% of cases having some features of Alzheimer’s disease (Jellinger 2006; WHO 2012). However, the proportion of dementia attributable to Alzheimer’s disease reduces with age (Savva 2009).
Prevalence of MCI and dementia
In the UK Medical Research Council's population‐based Cognitive Function and Ageing Study (CFAS), when 18 different definitions of MCI were mapped, the range of prevalence estimates was found to be highly variable (0.1% to 42.0%), and conversion rates to dementia generally low (Stephan 2007). In general, prevalence and conversion rates in specialist settings have been reported to be higher than in population‐based studies (adjusted conversion rate from MCI to dementia 9.6% versus 4.9%) (Mitchell 2009).
The risk of dementia increases with age; according to a World Health Organization (WHO) report, only 2% to 10% of cases start before the age of 65 (WHO 2012). The same report estimated that there were 35.6 million people with dementia in the world in 2010, and that this figure would double every 20 years to reach 65.7 million in 2030 (WHO 2012). However, there is a degree of uncertainty about the expected increase in prevalence of dementia. Recent research in the UK (Matthews 2013) and Denmark (Christensen 2013) suggests that the age‐specific prevalence of dementia may be falling in developed countries, supporting the idea that there may be modifiable risk factors. Nevertheless, because of population ageing, the overall prevalence continues to rise.
Risk factors
Generally, risk factors for dementia can be divided into modifiable and non‐modifiable factors. The non‐modifiable risk factors include age, genetic factors, family history, gender (females are at higher risk), and Down syndrome. The modifiable factors are smoking, high cholesterol, stroke, hypertension, lack of physical activity, diabetes mellitus, obesity, and low educational level. Among the non‐modifiable risk factors, age has the greatest effect. It has been calculated that in people older than 65, the risk of AD (the commonest cause of dementia) doubles every five years (Launer 1999; McCullagh 2001; van den Berg 2012; van der Flier 2005). A pooled analysis of four prospective studies in Europe found the incidence rate of AD among people aged 90 and over to be 63.5/1000 person‐years (Launer 1999). Genetics plays a major role in early onset AD, but a lesser role in the much commoner late onset disease. Epidemiological evidence suggests that many of the modifiable risk factors (diabetes, midlife obesity, midlife hypertension, smoking, and physical inactivity) are risk factors for both AD and vascular disease, including vascular dementia (The World Alzheimer Report 2014; WHO 2012).
At present, there is no cure for any subtype of dementia, but identifying and targeting modifiable risk factors may offer opportunities to modify its onset and course. Research has been reported suggesting that cognitive stimulation, exercise, diet, and the management of vascular risk factors such as hypertension, diabetes, obesity, smoking, and physical inactivity may have an important role in prevention of AD (Lindsay 2002; Lourida 2013; Norton 2014; Wilson 2002). There is also some evidence in support of vitamin supplementation as a preventive strategy. For example, vitamin B12 and folate lower levels of homocysteine, which is believed to be toxic to neurones. Protective effects of vitamin D and vitamin E against AD have also been proposed (Annweiler 2012; Dysken 2014; Llewellyn 2010). Many minerals might have antioxidant properties and may also be beneficial in protecting against oxidative stress and free radical damage. Hence, an evaluation of the role of vitamins and minerals as protective and preventive agents in cognitive impairment is warranted (see Appendix 1).
Description of the intervention
This review focusses on RCTs investigating the effect of vitamin and mineral supplementation for preventing dementia or delaying cognitive decline in people with mild cognitive impairment. Vitamins are organic compounds that are essential for the normal physiological process in the body and play important roles in growth and development (Kennedy 2011). Minerals are inorganic elements that come from the earth; as nutrients, they have similar essential roles in normal physiology (Centers for Disease Control and Prevention 2014). All of these essential nutrients are available naturally in food, although deficiencies can occur due to inadequate dietary intake or a variety of disease states. Dietary supplements are any consumed products that aim to provide additional nutrients to those obtained from the usual diet.
How the intervention might work
Vitamins and minerals have multiple important roles in the physiology of the human body at cellular and tissue levels. Putative biological mechanisms for each are summarised briefly in Appendix 1.
There is a complex array of micronutrients which protect the brain in a variety of ways, including protection against damaging oxygen‐free radicals, and which are important in neurogenesis, gene expression, and enzyme and receptor control (Powell 2000). Failure of these important systems appears to be implicated in the occurrence of neural damage (van der Schaft 2013). Therefore, ensuring adequate vitamin and mineral levels in the body might enhance cognitive function.
Oxidative stress has been shown to be a damaging process leading to an imbalance between oxygen‐free radicals, and the anti‐oxidative defences and repair of oxidative damage to proteins, lipids, RNA, and DNA (Halliwell 1992; Halliwell 1999; Tabet 2001; Tabet 2002). In addition, the central nervous system (CNS) contains high levels of unsaturated fatty acids that are substrates for peroxidation reactions (Ogawa 1994). An important defence mechanism in the brain involves enzymatic antioxidants which, if mediated through the supplementation of micronutrients, may replenish the brain with synthetic antioxidants providing a therapeutic approach to reduce oxidative stress (Reiter 1995). This may be a useful adjunct in modifying risk factors in the pathogenesis of neurodegenerative disorders (Packer 1997).
Vitamins:
Vitamins have a wide range of roles in the central nervous system and hence may affect the pathophysiological processes underlying the dementias in numerous different ways. Vitamin A may be involved in the stabilisation of beta amyloid fibrils (Ono 2012). Vitamin D is a precursor of hormones required for calcium and phosphorus metabolism and also has a possible role in cognition in older adults (Przybelski 2007). Vitamin E is an antioxidant which provides protection against free radical damage (Farina 2012; Takatsu 2009). B vitamins, particularly vitamin B12 and folic acid, have a role in energy production and metabolism within the CNS. B vitamins have also been implicated in the production of nucleic acids and production and maintenance of myelin essential for good neuronal health (Kühnast 2013; Osiezagha 2013; Pawlak 2014; Powers 2003; The World Alzheimer Report 2014). See Appendix 1 for more detail of possible mechanisms.
Minerals:
Minerals, similarly, have a very wide range of functions. For example, some may be involved in neuronal gene expression and the secretion of neurotransmitters (Ozawa 2012; Rossom 2012). Potassium, calcium, and magnesium were reported to be protective against cognitive decline in a cohort of Japanese participants (Ozawa 2012). Selenium is a critical component of the enzyme glutathione peroxidase and has been shown to protect the CNS and immune system from oxidative damage by harmful free radicals (Berr 2012; Mehdi 2013; Smorgon 2004). See Appendix 1 for more detail of possible mechanisms.
Micronutrients may not be maximally effective if supplemented in isolation. There are some patented formulas consisting of complex mixtures of micronutrients which are claimed to work synergistically. These are sometimes marketed as licensed medical foods. These licensed medical foods are not covered in this set of reviews.
Why it is important to do this review
The prevalence and financial implications of dementia are such that small effects on cognitive decline or on the incidence of dementia may have a large impact on healthcare costs and the overall burden of dementia. Robust assessments are needed of the effect size of interventions and of the ‘dose’ and duration of intervention necessary to achieve an effect.
For individuals, fear of cognitive decline and dementia may be a powerful motivator to seek preventive interventions. Nutritional supplements and cognitive activities (e.g. computerised 'brain training' games), in particular, are subject to promotion by those with commercial interests. It is important for people to know whether time, effort, and money they might invest to prevent cognitive decline is likely to be well spent. Information about adverse effects is also important. Although nutritional and behavioural interventions are often perceived to be ‘low risk’, they are not necessarily without the potential to cause harm. For example, trials have found high doses of vitamin E to be associated with more adverse effects than placebo (Bjelakovic 2012; Brigelius‐Flohe 2007; Miller 2005).
People with MCI are interested in interventions which could prevent or delay further cognitive decline. In addition, this review will be of interest to clinicians providing care for people with MCI and to policy makers.
Objectives
To evaluate the effects of vitamin and mineral supplementation on cognitive function and the incidence of dementia in people with mild cognitive impairment.
Methods
Criteria for considering studies for this review
Types of studies
We included in the review randomised or quasi‐randomised controlled trials, published or unpublished, reported in any language. We included studies involving both randomised and non‐randomised trial arms, but we only considered results from the former. We included cross‐over studies, but extracted and analysed data from the first treatment period only.
Types of participants
We included the following population: people diagnosed with mild cognitive impairment (MCI) according to internationally accepted and validated criteria. We recorded definitions. Participants should have been reported to be free of dementia at baseline. Consequently, we included only trials which assessed cognitive function or dementia status with internationally accepted and validated instruments at baseline and follow‐up.
We excluded trials of participants with severe vitamin or mineral deficiency where the intervention given could correct these deficiencies.
Types of interventions
We included studies comparing the effects of the described vitamin and mineral supplements with control interventions that were not expected to have specific risk‐modifying effects. The control arms typically involved placebo or no intervention/usual care. The minimum treatment duration was set at 12 weeks. Experimental interventions could be single vitamin or mineral supplements or combination treatments with any of the supplements listed in Appendix 1. We excluded trials of vitamins or minerals given in combination with other unrelated compounds (e.g. amino acids, fatty acids, or medications) unless the effects of the vitamins and minerals could be isolated. For example, a trial evaluating the effects of vitamin A and C versus methionine would have been excluded, whereas a trial evaluating vitamin A and C with methionine versus methionine only would have been included. We included only orally‐administered supplements. There were no restrictions on dose.
Types of outcome measures
Primary outcomes
1. The incidence of all‐cause dementia (assessed using internationally accepted and validated criteria).
The main time point of interest was end of trial, defined as the time point with the longest follow‐up duration as measured from randomisation (see also section, Data extraction and management). Outcome data reported at other time points after randomisation were extracted and presented. For this outcome, the minimum follow‐up period was 12 months.
2. Overall cognitive functioning, measured with, for example, Alzheimer's Disease Assessment Scale‐cognitive subscale (ADAS‐cog); the Mini Mental State Examination (MMSE); Repeatable Battery for the Assessment of Neuropsychological Status (RBANS); Cambridge Cognition Examination (CAMCOG).
Secondary outcomes
Secondary outcomes were any internationally accepted and validated measures of:
‐ specific cognitive functioning subdomain: episodic memory,
‐ specific cognitive functioning subdomain: executive functioning,
‐ specific cognitive functioning subdomain: speed of processing,
‐ quality of life, either generic or disease‐specific,
‐ clinical global impression,
‐ functional performance,
‐ number of participants experiencing one or more serious adverse events (SAE),
‐ mortality.
‐ biomarkers: where studies included validated biomarkers (e.g., beta‐amyloid or tau in cerebrospinal fluid, structural MRI or amyloid imaging) as well as cognitive outcomes, biomarker data were extracted.
Outcomes included in the 'Summary of findings' table
Critical effectiveness outcomes included in the 'Summary of findings' table for this review were incidence of dementia, all outcomes related to cognitive functioning, quality of life, and mortality.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group’s (CDCIG) specialised register on 25 January 2018.
ALOIS is maintained by the Information Specialists for the CDCIG, and contains studies that fall within the areas of dementia prevention, dementia treatment and management, and cognitive enhancement in healthy elderly populations. The studies are identified through:
Monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO and LILACS;
Monthly searches of a number of trial registers: ISRCTN; UMIN (Japan's Trial Register); the WHO portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials, and the Netherlands National Trials Register, plus others);
Quarterly search of the Cochrane Library's Central Register of Controlled Trials (CENTRAL);
Six‐monthly searches of a number of grey literature sources: ISI Web of Science Core Collection; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS, see 'About ALOIS' on the ALOIS website (www.medicine.ox.ac.uk/alois).
Details of the search strategies run in healthcare bibliographic databases, used for the retrieval of reports of dementia, cognitive improvement, and cognitive enhancement trials, can be viewed in the ‘methods used in reviews’ section within the editorial information about the Cochrane Dementia and Cognitive Improvement Group.
We ran additional searches in MEDLINE, Embase, PsycINFO, CENTRAL, CINAHL, Web of Science Core Collection, LILACs, ClinicalTrials.gov, and the WHO Portal/ICTRP to ensure that the searches for each suite of reviews was as comprehensive and as up‐to‐date as possible to identify published, unpublished, and ongoing trials. The search strategies used for the retrieval of reports of trials can be seen in Appendix 2.
Searching other resources
We screened reference lists of all included trials. In addition, we screened reference lists of recent systematic reviews, health technology assessment reports, and subject‐specific guidelines identified through www.guideline.gov. The search was restricted to those guidelines meeting the 2013 inclusion criteria of the National Guideline Centre (NGC), published in this year or later.
We contacted experts in the field and companies marketing included interventions, in order to provide additional randomised trial reports that were not identified by the search.
Data collection and analysis
We used this protocol, alongside instructions for data extraction, quality assessment, and statistical analyses which were based on a generic protocol generated by the editorial board of CDCIG to guide this and another 11 reviews on modifiable risk factors (see Acknowledgements).
Selection of studies
If multiple reports described the same trial, we included all to allow complete extraction of the trial details.
We used crowdsourcing to screen the search results. Details of this method have been described here (http://www.medicine.ox.ac.uk/alois/content/modifiable‐risk‐factors). In brief, teams of volunteers performed a ‘first assess’ on the search results. The volunteers were recruited through the author team’s institutions. They screened the results using an online tool developed for Cochrane Embase project but tailored for this programme of work. The crowd decided, based on a reading of title and abstract, whether the citation was describing a randomised or quasi‐randomised trial, irrespective of the citations topic. The citations identified as possibly relevant by the crowd were then screened by the author team.
Data extraction and management
Two review authors, working independently, extracted trial information using a standardised and piloted extraction method, referring also to a guidance document. Discrepancies were resolved by discussion, or by the involvement of a third reviewer. Where possible, we extracted (as a minimum) the following information related to characteristics of participants, intervention, and study design:
Participant characteristics
gender
baseline age (range, median, mean)
education (level and years of education)
baseline cognitive function
cognitive diagnostic status
duration of cognitive symptoms, if any
ethnicity
Apo‐E genotype
diabetes mellitus (yes/no)
physical activity (as defined by the trialists).
smoking (never/ever)
Intervention characteristics
nature of the intervention/generic and trade name of intervention
description of the control condition
duration of treatment
dosage and frequency
any concomitant treatments
treatment adherence
Methodological characteristics
trial design (individual or cluster randomisation; parallel group, factorial or cross‐over design)
number of participants
outcome measures used
duration of follow‐up, as measured from randomisation
duration of follow‐up, as measured from end of treatment
source of financial support
publication status
If secondary outcome data were available at multiple time‐points within a given trial, we grouped them as follows: immediate (up to 12 weeks), short‐term (up to one year), medium‐term (one to two years) and longer‐term results (more than two years). For the primary outcome (all‐cause dementia), we considered only outcome data at one year of follow‐up or longer. Within these time periods, we extracted the latest available data reported by the study. For example, if a study reported data at six months, nine months and one year, we extracted and analysed only the one‐year data for the one‐year (short‐term) time point.
For dichotomous outcomes (such as incident dementia or mortality), we extracted from each trial the number of participants with each outcome at each time point.
For continuous outcomes, we extracted the number of participants in whom the outcome was measured, and the mean and standard deviation of the change from baseline for each outcome at each time point. If change‐from‐baseline data were not available, we extracted the mean value at each time point. When necessary, means and measures of dispersion were approximated from figures in the reports.
Whenever possible, we extracted intention‐to‐treat data, i.e. analysing all participants according to the group randomisation; if this was not available, then we extracted and reported data from available case analyses. If neither of these types of data were available, we considered data from 'per protocol' analyses. We contacted the trialists if we were unable to obtain the necessary data from the trial report.
Assessment of risk of bias in included studies
After completion of a standardised training session provided by AR, one member of the author team and one experienced reviewer provided by the editorial team independently assessed the risk of bias in each of the included trials using the Cochrane's 'Risk of bias' tool (Higgins 2011). We resolved disagreements by consensus. We assessed the risk of bias potentially introduced by suboptimal design choices with respect to sequence generation, concealment of allocation, blinding of participants and caregivers, blinded outcome assessment, selective outcome reporting, and incomplete outcome data, including the type of statistical analyses used (true intention‐to‐treat versus other analyses). The general definitions that were used are reported in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
We expressed the measure of treatment effect for continuous outcomes as a mean difference if all included studies used the same outcome measure and as a standardised mean difference (SMD), defined as the between‐group difference in mean values divided by the pooled standard deviation (SD), if the same outcome was assessed with a variety of measurement scales. We expressed the treatment effect for dichotomous outcomes as a relative risk (RR).
Unit of analysis issues
We did not identify any cross‐over or cluster‐randomised trials for inclusion.
Dealing with missing data
Missing data in individual trials may put the study estimates of effects at a high risk of bias, and may lower the overall quality of the evidence according to GRADE (Higgins 2011). We dealt with missing data in our 'Risk of bias' assessments and evaluated attrition bias in stratified analyses of the primary outcomes (Appendix 2). We analysed the available information and did not contact authors with a request to provide missing information; nor did we impute missing data ourselves.
Assessment of heterogeneity
We examined heterogeneity in stratified analyses by trial, participant, and intervention characteristics, as outlined in the sections Data and analyses and Appendix 2.
Assessment of reporting biases
We identified too few trials to allow the use of funnel plots to explore reporting biases or other small study effects.
Data synthesis
We examined participants, interventions, and outcomes in the included trials in order to decide whether they were sufficiently similar for data to be pooled.
Where we considered it appropriate to pool data, we used standard inverse‐variance random‐effects meta‐analysis to combine outcome data across the trials at the end of trials (DerSimonian 1986); and, if possible, at least one additional time point (see Primary outcomes and Data extraction and management for definitions of time points). We visually inspected forest plots for the presence of heterogeneity and calculated the variance estimate tau² as a measure of between‐trial heterogeneity (DerSimonian 1986). We prespecifed a Tau² of 0.04 to represent low heterogeneity, 0.09 to represent moderate heterogeneity, and 0.16 to represent high heterogeneity between trials (Spiegelhalter 2004). We also presented the I² statistic and the corresponding Chi² test (Higgins 2003). I² describes the percentage of variation across trials attributable to heterogeneity rather than to chance, with values of 25%, 50%, and 75% typically being interpreted as low, moderate, and high between‐trial heterogeneity. We preferred Tau² over I² in the interpretation of between‐trial heterogeneity, as the interpretation of I² can be largely affected by the precision of trials included in the meta‐analysis (Rücker 2008). We did statistical analyses in Review Manager 5 (RevMan 2014) and in STATA, release 13 (StataCorp, College Station, Texas).
Subgroup analysis and investigation of heterogeneity
We had prespecified the following trial characteristics as of interest for exploring possible heterogeneity: concealment of allocation, blinding of participants, blinded outcome assessment, intention‐to‐treat analysis, trial size (based on power calculation for trial primary outcome), duration of treatment (<3, 3‐12, >12 months), and length of follow‐up from randomisation (<3 months, 3‐12 months, >1‐2 years, >2 years). We had also prespecified the following possible clinical effect modifiers: age (40‐65 or >65 years), comorbidities, concomittant medications, and ethnicity (Dawson‐Hughes 2004). However, too few studies were included to allow us to conduct subgroup analyses or explore the effect of these features. Because B vitamins may work by lowering homocysteine levels and therefore may be more effective in participants with high homocysteine levels at baseline, we decided to amend the protocol to report the effects of B vitamins in subgroups of participants distinguished by level of homocysteine at baseline, where this was reported in the included studies (see Differences between protocol and review).
Sensitivity analysis
We had prespecified a sensitivity analysis for the primary effectiveness outcome, including high‐quality trials only. However, too few trials were included for this to be done.
GRADE and summary of findings table
We used GRADE to describe the quality of the overall body of evidence for each outcome in the 'Summary of findings' table (Higgins 2011; Guyatt 2008).
Quality in GRADE is defined as the degree of confidence which can be placed in the estimates of treatment benefits and harms. There are four possible ratings: 'high', 'moderate', 'low' and 'very low'. Rating evidence as 'high‐quality' implies that we are confident in our estimate of the effect, and further research is very unlikely to change this. A rating of 'very‐low' quality implies that we are very uncertain about the obtained summary estimate of the effect.
The GRADE approach rates evidence from RCTs which do not have serious limitations as 'high‐quality'. However, several factors can lead to the downgrading of the evidence to 'moderate', 'low' or 'very low'. The degree of downgrading is determined by the seriousness of these factors: study limitations (risk of bias); inconsistency; indirectness of evidence; imprecision; and publication bias (Higgins 2011; Guyatt 2008; Chandra 2001).
Results
Description of studies
Results of the search
We conducted searches in December 2014, July 2015, March 2016, August 2016, March 2017, and January 2018. In total, we retrieved 7,257 records from the six searches. After de‐duplication, 5211 records remained. A Crowd and the CDCIG information specialist assessed these at title and abstract level. In total, 725 results remained after this assessment. The review team then screened these records. Of these, we assessed 110 full‐text articles describing 67 trials for eligibility and included eight trials in the review (one after the authors provided subgroup data). Three trials, one described in three papers, were placed in the section 'Awaiting classification'; we sought information from the authors of these trials but received none. We identified one ongoing study of vitamin D supplementation which was due to be completed in July 2018. This process is depicted in Figure 1.
1.
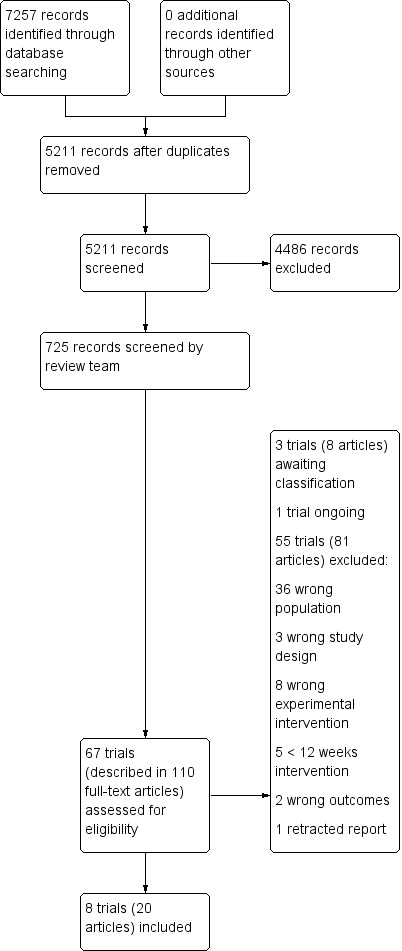
Study flow diagram.
Included studies
We identified eight studies eligible for inclusion in this review. For full details see Characteristics of included studies.
We grouped the studies into four comparisons. Five studies compared B vitamins to placebo (de Jager 2012, Eussen 2006, Fan 2017, Ting 2017, van Uffelen 2008). One study compared vitamin E to placebo (Petersen 2005), one compared vitamin E + vitamin C to placebo (Naeini 2014), and one compared chromium picolinate to placebo (Krikorian 2010).
Appendix 3 shows the supplement doses used in the studies in relation to the mean daily intake from food and the recommended daily intake for adults in the UK.
Comparison 1: B vitamins versus placebo ‐ description of studies
Five studies with 879 randomised participants contributed data to this comparison.
Setting
The studies were conducted in the UK, the Netherlands (2 studies), China and Singapore. Eussen 2006 included participants living in the community or in a care home; participants in all other studies were resident in the community.
Participants
All studies specifically excluded participants with dementia. de Jager 2012, Fan 2017 and van Uffelen 2008 used broadly similar criteria for MCI, which included a memory complaint and scores within a specified range on scales of cognition and daily functioning. Participants in Eussen 2006 had a Clinical Dementia Rating (CDR) global score of 0 or 0.5; for this review we, used data from the participants with a CDR score of 0.5. All participants in Ting 2017 had recent lacunar stroke and cognitive impairment ‐ no dementia (CIND); the cognitive impairment was defined as scoring at least 1.5 SDs below expected in at least one domain of a neuropsychological test battery.
All participants in Eussen 2006 met the authors' criteria for mild B12 deficiency.
Four studies had age‐based inclusion criteria: de Jager 2012 and Eussen 2006 only included participants aged 70 or older, Fan 2017 only included participants aged 60 to 75 years, and van Uffelen 2008 included participants aged 70 to 80 years. Across all studies, the mean age of participants ranged from approximately 66 years (Fan 2017) to approximately 80 years (Eussen 2006).
Interventions
All studies were placebo‐controlled. The experimental interventions varied in composition and dose.
Participants in de Jager 2012 received 0.5 mg B12 + 0.8 mg folic acid + 20 mg B6 once daily for two years.
Eussen 2006 was a three‐arm study. For this review, we combined the groups receiving 1 mg B12 and 1 mg B12 + 0.4 mg folic acid into a single experimental intervention group. Treatment was once daily for 24 weeks.
Participants in Fan 2017 received 0.4 mg folic acid once daily for six months.
Participants in Ting 2017 received 0.5 mg B12 + 2 mg folic acid + 25 mg B6 once daily for one to five years.
Participants in van Uffelen 2008 received 0.4 mg vitamin B12 + 5 mg folic acid + 50 mg vitamin B6 once daily for a year. This study also investigated the effect of aerobic exercise in a 2 x 2 factorial design. For the purposes of this review, we combined data for all participants receiving vitamin B supplementation or placebo (i.e. with or without aerobic exercise) into single experimental and control groups.
Outcomes
None of the studies reported on our primary outcome of incidence of all‐cause dementia (although diagnosis of dementia by DSM‐IV was listed as a secondary outcome in the protocol for de Jager 2012).
Four of the five studies measured overall cognitive function with the MMSE (de Jager 2012, Fan 2017, Ting 2017, van Uffelen 2008).
We were able to extract data on episodic memory from three studies, which assessed delayed recall on the Hopkins Verbal Learning Test (de Jager 2012), word learning (Eussen 2006) and the Auditory Verbal Learning Test (van Uffelen 2008). We extracted data on executive function from four studies, which used CLOX (de Jager 2012), the Stroop test (Eussen 2006; van Uffelen 2008) and the Frontal Assessment Battery (Ting 2017). We extracted data on speed of processing from three studies, which used Trail‐making Test A (Eussen 2006), digit cancellation (Ting 2017), and the Digit‐Symbol Substitution Test (van Uffelen 2008).
Only van Uffelen 2008 reported on quality of life, using the dementia‐specific D‐QOL scale.
de Jager 2012 reported overall clinical impression using global CDR scores.
Fan 2017 reported functional performance on a 14‐item Chinese ADL scale.
de Jager 2012 reported efficacy results separately for participants with high or low total homocysteine (tHCy) (based on the median values at baseline). However, it was possible to calculate results for the whole experimental intervention and control groups from the reported means and standard deviations. van Uffelen 2008 reported results for men and women separately in each group, but some of the outcomes were reported in enough detail to allow us to combine the data for men and women.
Data on adverse events were reported by de Jager 2012 and van Uffelen 2008.
Comparison 2: Vitamin E versus placebo ‐ description of study
One study with 769 participants investigated this comparison (Petersen 2005). The study also had a donepezil arm. The primary outcome of the study was time to development of possible or probable Alzheimer's disease.
Setting
The study took place at 69 Alzheimer's Disease Cooperative Study (ADCS) sites in the US and Canada.
Participants
Participants were aged 55 to 90 years and had amnestic MCI of a degenerative nature (insidious onset and gradual progression). Specific cognition‐related inclusion criteria were impaired memory, a logical memory delayed‐recall score approximately 1.5 to 2 SD below an education‐adjusted norm and a score of 24 to 30 on the MMSE, as well as a CDR global score of 0.5.
Intervention
The experimental group of interest to this review received 2000 IU vitamin E (1000 IU twice daily), placebo donepezil, and a multivitamin (containing 15 IU of vitamin E) for three years. The comparator group received placebo vitamin E, placebo donepezil and the same multivitamin. Any participant who met clinical criteria for Alzheimer's disease at any time in the study was offered open‐label donepezil until study completion.
Outcomes
The study assessed progression to possible or probable Alzheimer's disease. Overall cognitive function was assessed with the MMSE and with a composite score derived from a battery of individual neuropsychological tests. Composite scores of interest to this review were also derived for the domains of memory and executive function (and additional composites for language and visuospatial function). Clinical global impression was assessed using the Global Deterioration Scale (GDS) and the CDR. Functional performance was assessed using the ADCS Mild Cognitive Impairment ADL Scale. Data on individual adverse events were reported by treatment group if they occurred in at least 5% of subjects in the donepezil or vitamin E group and at least two times in the placebo group during the double‐blind phase. The number of deaths in each treatment group was also reported.
Comparison 3: Vitamin E + vitamin C versus placebo ‐ description of study
One study investigated this comparison (Naeini 2014). It reported data on the 256 participants who completed the study (out of 296 who were randomised).
Setting
The study took place at a single centre in Iran with participants recruited from community clubs for retired people.
Participants
Dementia was listed as an exclusion criterion, but there was no information on how this was applied. Participants were defined as having MCI and identified as eligible for inclusion on the basis of a score of 21 to 26 on the validated Iranian version of the MMSE. We considered this not to be an adequate definition of MCI. However, we decided to include the study, downgrading the result for indirectness in relation to our review question.
Intervention
The experimental intervention was 300 mg vitamin E (DL‐alpha‐tocopherol) + 400 mg vitamin C once daily for one year. The comparator was placebo.
Outcome
The only outcome of interest to this review was overall cognitive functioning assessed with the MMSE.
Comparison 4: Chromium picolinate versus placebo ‐ description of study
One small study with 26 participants contributed data to this comparison (Krikorian 2010).
Setting
This single centre study recruited participants via community advertisement.
Participants
Participants had a global rating of 0.5 on the CDR.
Intervention
The experimental intervention was chromium picolinate containing 1000 mcg elemental chromium once daily for 12 weeks. The comparator was placebo.
Outcome
The only outcome of interest to this review was episodic memory assessed with the California Verbal Learning Test (CVLT).
Excluded studies
The Characteristics of excluded studies table shows the reasons for exclusion of 55 studies which were assessed in full text. The most common reasons for exclusion were the wrong population (participants did not have MCI) or the wrong intervention (included additional components or was given for less than 12 weeks).
Three studies are awaiting classification. In all three cases, we have been unable to obtain additional information from the authors at the time of writing. No results were available from one study. Results from the other two trials have been published, but we considered that essential information on various details of the methods, sample sizes, or results was missing.
We identified one ongoing trial of vitamin D supplementation which, according to the trial register, was due to be completed in July 2018.
Risk of bias in included studies
We describe the risk of bias of the included studies in the table, Characteristics of included studies. Our 'Risk of bias' judgments are also depicted in the 'Risk of bias' summary and 'Risk of bias' graph (Figure 2 and Figure 3).
2.
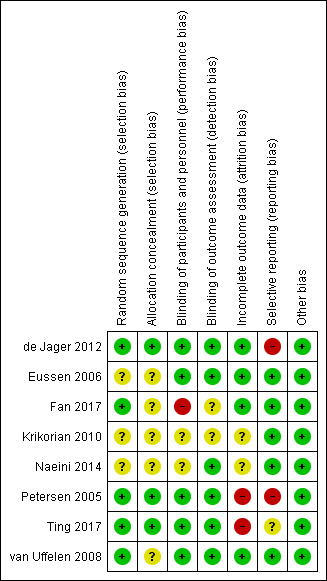
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
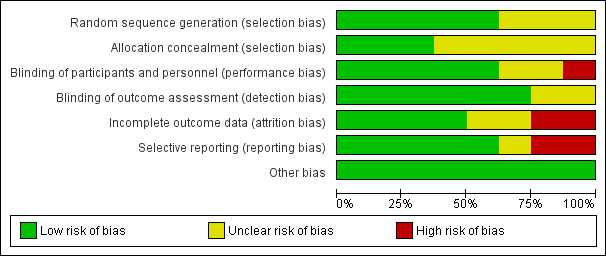
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Three studies (de Jager 2012, Petersen 2005, Ting 2017) provided enough information to judge the risk of selection bias to be low. The remaining five studies provided insufficient information on randomisation methods so we judged them to be at unclear risk of selection bias.
Blinding
Fan 2017 was an open‐label study which we judged to have a high risk of performance bias and an unclear risk of detection bias. There was also a lack of information about blinding from Krikorian 2010 and Naeini 2014. The other studies were at low risk of bias in this domain.
Incomplete outcome data
The longer studies ‐ Petersen 2005 and Ting 2017 ‐ lost high numbers of participants to follow‐up and we considered them to be at high risk of attrition bias by the end of the study. We judged the risk of attrition bias in Naeini 2014 to be unclear due to a lack of information on the group allocation of those who dropped out. The risk was also unclear in Krikorian 2010, where there was no information about whether or not there were any missing data. The remaining studies were at low risk of bias in this domain.
Selective reporting
We judged there to be a high risk of reporting bias in two studies. de Jager 2012 mentioned a number of outcomes in the protocol, including some relevant to this review, which were not reported. Petersen 2005 reported composite z‐scores rather than individual test results and did not report the number of participants in each analysis. Ting 2017 may have selected only some cognitive results from a larger neuropsychological test battery; we judged its risk of reporting bias to be unclear. We judged the risk in the other studies to be low, although for some studies there was no protocol and this judgement was based on the outcomes mentioned in the Methods sections of the papers being fully reported.
Other potential sources of bias
We found no other obvious sources of bias and rated this risk as low for all studies.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. B vitamins compared to placebo for MCI.
| B vitamins compared to placebo for MCI | |||||
| Patient or population: MCI Setting: community Intervention: B vitamins (B6, B12, folic acid) Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with B vitamins | ||||
| Incidence of dementia ‐ not measured | ‐ | ‐ | ‐ | ‐ | |
| Overall cognitive function assessed with: MMSE Scale from: 0 to 30 follow‐up: range 6 months to 24 months | MD with B vitamins was 0.44 MMSE points higher (0.23 lower to 1.12 higher) than with placebo * | 488 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | Due to the very low‐quality of the evidence, we cannot be sure of any effect of B vitamins on overall cognitive function. * 2 studies reported final score; 1 study reported change from baseline. From the 2 studies (n=150) which reported final scores, the mean MMSE with placebo was 26.97 points. |
|
| Episodic memory assessed with: various word list recall instrumentsa follow‐up: range 6 months to 24 months | SMD with B vitamins was 0.09 higher (0.1 lower to 0.29 higher) than with placebo | 397 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | B vitamins probably resulted in little to no difference in episodic memory. | |
| Executive function assessed with: various instrumentsb follow‐up: range 6 months to 24 months | SMD with B vitamins was 0.03 higher (0.23 lower to 0.29 higher) than with placebo | 392 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 4 | B vitamins probably resulted in little to no difference in executive function. | |
| Speed of processing assessed with: various instrumentsc follow‐up: range 6 months to 24 months | SMD with B vitamins was 0.04 higher (0.26 lower to 0.34 higher) than with placebo | 173 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 4 | B vitamins probably resulted in little to no difference in speed of processing. | |
| Quality of life assessed with: D‐QOL Scale from: 1 to 5 follow‐up: 12 months | The mean quality of life was 3.5 points | MD 0 points (0.1 lower to 0.1 higher) | 138 (1 RCT) | ⊕⊕⊕⊝ MODERATE 5 | B vitamins probably resulted in little to no difference in quality of life. |
| Mortality ‐ not reported | Reported by only one study (2/133 died in vitamin B group, 0/133 died in placebo group). | ‐ | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). MD: Mean difference; SMD: Standardised mean difference; CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded due to risk of bias. One study was at high risk of performance bias and at unclear risk of selection and detection bias.
2 Downgraded due to inconsistency. I2 = 87%.
3 Downgraded due to imprecision. 95% CI included little or no effect and small benefit of B vitamins.
4 Downgraded due to imprecision. 95% CI included small effects in either direction.
5 Downgraded due to imprecision. Result derived from one small study.
aEpisodic memory assessed with Hopkins Verbal Learning Test, word learning and the Auditory Verbal Learning Test.
bExecutive function assessed with CLOX, the Stroop test, and the Frontal Assessment Battery.
cSpeed of processing assessed with Trail‐making Test A, digit cancellation, and the Digit‐Symbol Substitution Test.
CLOX: Clock drawing executive test D‐QOL: Dementia quality of life questionnaire MCI: Mild cognitive impairment MMSE: Mini‐mental state examination
Summary of findings 2. Vitamin E compared to placebo for MCI.
| Vitamin E compared to placebo for MCI | |||
| Patient or population: MCI Setting: community Intervention: vitamin E Comparison: placebo | |||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE) |
| Incidence of dementia due to Alzheimer's disease follow‐up: 36 months | 76 cases in vitamin E group and 73 cases in placebo group (HR 1.02, 95% CI 0.74 to 1.41) | 516 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 |
| Overall cognitive functioning assessed with: MMSE and ADAS‐cog follow‐up: 36 months | Single study reported no significant difference between groups in changes from baseline of MMSE or ADAS‐cog. Sample sizes not reported. | (1 RCT) | ⊕⊕⊝⊝ LOW 2 3 |
| Episodic memory assessed with: z‐score incorporating various instrumentsa follow‐up: 36 months | Single study reported no significant difference between groups. Sample size not reported. | (1 RCT) | ⊕⊕⊝⊝ LOW 2 3 |
| Executive functioning assessed with: z‐score incorporating various instrumentsb follow‐up: 36 months | Single study reported no significant difference between groups. Sample size not reported. | (1 RCT) | ⊕⊕⊝⊝ LOW 2 3 |
| Quality of life ‐ not measured | ‐ | ‐ | |
| Mortality | No significant difference in deaths reported between vitamin E, donepezil, and placebo groups during double‐blind phase of trial. | (1 RCT) | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1 Downgraded due to imprecision. 95% CI around hazard ratio included possible effect in both directions.
2 Downgraded due to imprecision. Single study. Sample size for this outcome not reported.
3 Downgraded due to risk of bias. High risk of bias due to incomplete outcome data and selective reporting.
aEpisodic memory assessed using standardised composite z‐score incorporating ADAS immediate and delayed word‐recall scores and the New York University immediate and delayed paragraph‐recall scores.
bExecutive function assessed using standardised composite z‐score incorporating the digits‐backward test, Symbol Digit Modalities Test, and number‐cancellation test.
ADAS‐cog: Alzheimer's Disease Assessment Scale ‐ cognitive MCI: Mild cognitive impairment MMSE: Mini‐mental state examination
Summary of findings 3. Vitamin E + vitamin C compared to placebo for MCI.
| Vitamin E + vitamin C compared to placebo for MCI | |||||
| Patient or population: MCI Setting: community Intervention: vitamin E + vitamin C Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with vitamin E + vitamin C | ||||
| Incidence of dementia ‐ not measured | ‐ | ‐ | ‐ | ‐ | |
| Overall cognitive function assessed with: MMSE (Iranian version) Scale from: 0 to 30 follow‐up: 12 months | The mean overall cognitive function was 26.6 points | MD 0.23 points higher (0.25 lower to 0.71 higher) | 256 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | Due to very low‐quality evidence, we cannot be sure of any effect of vitamin C + vitamin E on overall cognitive function. |
| Episodic memory ‐ not measured | ‐ | ‐ | ‐ | ‐ | |
| Executive function ‐ not measured | ‐ | ‐ | ‐ | ‐ | |
| Speed of processing ‐ not measured | ‐ | ‐ | ‐ | ‐ | |
| Quality of life ‐ not measured | ‐ | ‐ | ‐ | ‐ | |
| Mortality ‐ not reported | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded due to risk of bias. Unclear risks of selection, performance, and attrition bias.
2 Downgraded due to indirectness. Inadequate definition of MCI.
3 Downgraded due to imprecision. Result from a single small study.
MCI: Mild cognitive impairment MMSE: Mini‐mental state examination
Comparison 1: B vitamins (folic acid, B12, B6) versus placebo
Five studies contributed data to this comparison (de Jager 2012; Eussen 2006; Fan 2017; Ting 2017; van Uffelen 2008).
Ting 2017 differed significantly from the other studies in recruiting only participants who had a recent history of lacunar stroke and in following them up for up to five years. In our primary analyses, in order to maximise comparability with the other studies, we included data from the one‐year outcome point from Ting 2017, but we considered the population as a potential source of heterogeneity. We also reported the results of this study at later time points, although these were all associated with substantial loss of participants from follow‐up.
de Jager 2012 reported the data for participants with higher (above median) baseline total homocysteine (tHcy) and lower baseline total homocysteine levels separately. For the primary analyses, we combined these groups. However, we also reported and commented on their subgroup data.
van Uffelen 2008 reported results separately for men and women; we have combined these data where we incorporated them in meta‐analyses.
Primary outcomes
Incidence of all‐cause dementia
No study reported the incidence of all‐cause dementia.
Overall cognitive functioning
Four studies used the MMSE to assess overall cognitive functioning (de Jager 2012; Fan 2017; Ting 2017; van Uffelen 2008). It is unknown what would constitute an important difference in MMSE score in this population. It is unlikely that MMSE is sensitive to small changes in cognition in people with MCI. We pooled data from three studies which reported MMSE in the form of mean score with standard deviation in each treatment group.
van Uffelen 2008 presented MMSE results as medians with interquartile ranges (IQR) for men and women separately. After 12 months of treatment, the median (IQR) MMSE score among men was 28 (27 to 30) in the vitamin group and 29 (28 to 29) in the placebo group. For women, the median (IQR) MMSE score was identical in both vitamin and placebo groups: 29 (27 to 30).
The pooled analysis of MMSE scores from the other three studies after six to 24 months was inconclusive due to imprecision; although the result slightly favoured B vitamins, we could not exclude the possibility of there being little or no effect (MD 0.44, 95%CI ‐0.23 to 1.12, 3 studies, 488 participants; Analysis 1.1, Figure 4). There was high heterogeneity in this analysis (I2 = 87%). This appeared to be due to a beneficial effect of B vitamins on MMSE score in Fan 2017, which was the only open‐label (unblinded) study. We considered the evidence behind this result to be very low‐quality because of the imprecision, study limitations, and inconsistency.
1.1. Analysis.
Comparison 1 B vitamins versus placebo, Outcome 1 Overall cognitive function (MMSE).
4.
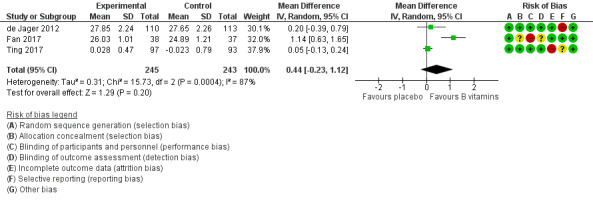
Forest plot of comparison: 1 B vitamins versus placebo, outcome: 1.1 Overall cognitive function (MMSE).
de Jager 2012 reported the data separately for participants with higher and lower baseline total homocysteine (tHcy). The results in these subgroups were also imprecise: higher baseline tHcy (MD 0.70, 95% CI ‐0.16 to 1.56; participants = 111); lower baseline tHcy (MD ‐0.30, 95% CI ‐1.10 to 0.50; participants = 112).
Ting 2017 did not find any significant effect of B vitamins on MMSE at any later time point (up to five years).
Secondary outcomes
Specific cognitive functioning subdomain: episodic memory
We pooled data on episodic memory from three studies (de Jager 2012; Eussen 2006; van Uffelen 2008). All used tests which involved delayed recall of word lists. There was probably little or no effect of six to 24 months of B vitamin supplementation on episodic memory (SMD 0.09, 95% CI ‐0.10 to 0.29; 3 studies, 397 participants; Analysis 1.2; Figure 5). Heterogeneity was low (I2 = 0%). We considered this to be moderate‐quality evidence, downgraded due to imprecision.
1.2. Analysis.
Comparison 1 B vitamins versus placebo, Outcome 2 Episodic memory.
5.
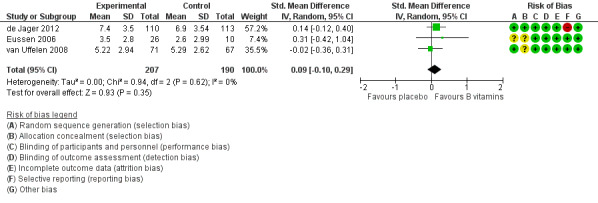
Forest plot of comparison: 1 B vitamins versus placebo, outcome: 1.2 Episodic memory.
In de Jager 2012, there was better episodic memory after 24 months in the group treated with vitamin B than in the group treated with placebo among participants with higher baseline tHCy (MD 1.30, 95% CI 0.02 to 2.58; participants = 111), but episodic memory did not differ significantly between intervention groups among participants with lower baseline tHcy (MD ‐0.30, 95% CI ‐1.58 to 0.98; participants = 112). The authors had analysed this outcome by logistic regression at five time points, starting from the 3rd month of the study, and estimated that after two years of the vitamin B intervention, participants taking vitamin B had a 69% higher likelihood of correct word‐recall than those taking placebo (OR 1.69, P = 0.001) (de Jager 2012).
Specific cognitive functioning subdomain: executive functioning
Four studies assessed executive functioning using three different measures (de Jager 2012; Eussen 2006; Ting 2017; van Uffelen 2008). We used CLOX‐1 data from the CLOX test and 'task 3' from the Stroop Colour‐Word Test. Ting 2017 reported only change‐from‐baseline data, so we were unable to pool these with data from the other studies. There was probably little or no effect of six to 24 months of B vitamin supplementation on executive functioning (SMD 0.03, 95% CI ‐0.23 to 0.29; 3 studies, 392 participants; Analysis 1.3; Figure 6). Heterogeneity was modest (I2 = 30%). We considered this to be moderate‐quality evidence, downgraded due to imprecision. Ting 2017 reported no significant difference between B vitamin and placebo groups on change from baseline in the Frontal Assessment Battery at any time point from one to five years.
1.3. Analysis.
Comparison 1 B vitamins versus placebo, Outcome 3 Executive function.
6.
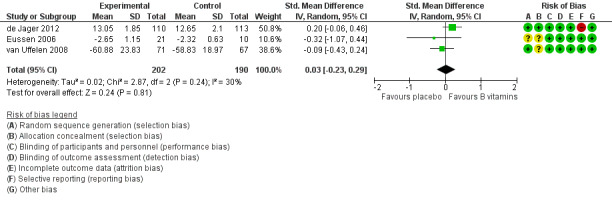
Forest plot of comparison: 1 B vitamins versus placebo, outcome: 1.3 Executive function.
From de Jager 2012, there was no evidence of a difference in CLOX‐1 score between intervention groups among participants with either higher baseline tHcy (MD 0.30, 95% CI ‐0.50 to 1.10; participants = 111) or lower baseline tHCy (MD 0.50, 95% CI ‐0.13 to 1.13, participants = 112).
Ting 2017 did not find any significant effect of B vitamins on executive functioning at any later time point (up to five years).
Specific cognitive functioning subdomain: speed of processing
Three studies assessed speed of processing using three different measures (Eussen 2006; Ting 2017; van Uffelen 2008). Again, we were unable to include data from Ting 2017 in the meta‐analysis because only change‐from‐baseline data were available. There was probably little or no effect of six to 24 months of B vitamin supplementation on speed of processing (SMD 0.04, 95% CI ‐0.26 to 0.34; 2 studies, 173 participants; Analysis 1.4; Figure 7). Heterogeneity was low (I2 = 0%). We considered this to be moderate‐quality evidence, downgraded due to imprecision. Ting 2017 reported no significant difference in change‐from‐baseline of speed of processing (digit cancellation) between B vitamin and placebo groups at any time point from one to five years.
1.4. Analysis.
Comparison 1 B vitamins versus placebo, Outcome 4 Speed of processing.
7.
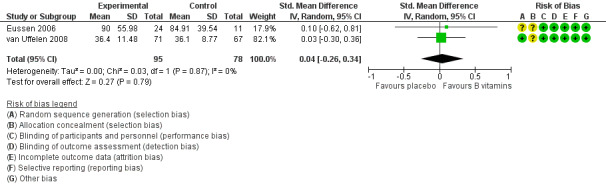
Forest plot of comparison: 1 B vitamins versus placebo, outcome: 1.4 Speed of processing.
Ting 2017 did not find any significant effect of B vitamins on speed of processing at any later time point (up to five years).
Quality of life, either generic or disease‐specific
One study (van Uffelen 2008) reported dementia‐specific quality of life using D‐QOL.There was no evidence of any effect of B vitamins after one year (MD 0, 95% CI ‐0.1 to 0.1; 1 study, 138 participants; Analysis 1.5). We considered this to be moderate‐quality evidence, downgraded due to imprecision.
1.5. Analysis.
Comparison 1 B vitamins versus placebo, Outcome 5 Quality of life (D‐QOL).
Clinical global impression
de Jager 2012 assessed overall clinical state using CDR. The authors reported that "(i)n the whole intention‐to‐treat cohort, there was no significant effect of B vitamins on CDR (P = 0.23)", nor was there a significant interaction with baseline tHcy split by median. However, when they stratified tHcy by quartiles, they noted a significant benefit of B vitamins on CDR in the quartile with the highest tHcy at baseline (P = 0.039, Fisher's exact test). In this subgroup, they calculated that "the odds of having CDR=0 at follow‐up is five times greater in the active‐treatment group compared with placebo (P = 0.02)." It was not clear whether or not this was a prespecified analysis.
Functional performance
One study (Fan 2017) assessed functional performance using a 14‐item ADL scale (range of possible scores 14 to 56, with a lower score representing a better outcome). There may be a small beneficial effect of B vitamins on functional performance after six months (MD ‐0.78, 95% CI ‐1.35 to ‐0.21; 1 study, 75 participants; Analysis 1.6). We considered this to be very low‐quality evidence, downgraded due to imprecision and very serious concern about study limitations.
1.6. Analysis.
Comparison 1 B vitamins versus placebo, Outcome 6 Functional performance (ADL).
Number of participants experiencing one or more serious adverse events (SAE)
Two papers reported adverse events. van Uffelen 2008 reported minor adverse events in 3/179 randomised participants (2 in B vitamins group, 1 in placebo group). de Jager 2012 reported 242 adverse events in total in the B vitamins group (n = 133) and 271 in the placebo group (n = 133) over two years.
Mortality
de Jager 2012 reported two deaths in the B vitamins group (2/133) but none in the placebo group (0/133). No deaths were reported by the other studies.
Biomarkers
de Jager 2012 reported the rate of brain atrophy measured by MRI. Out of 133 participants who started treatment in each group, 85 in the vitamin and 83 in the placebo group had serial MRI scans which were technically suitable for analysis. After adjustment for age, the rate of brain atrophy per year was reported to be 29.6% lower in the active treatment group (0.76%, 95% CI 0.63 to 0.90) than in the placebo group (1.08%, 95% CI 0.94 to 1.22) (P = 0.001).
Comparison 2: Vitamin E versus placebo
One study with 516 participants contributed data for this comparison (Petersen 2005).
Sample sizes for change scores were not reported for some outcomes, making it impossible to re‐analyse the data without imputation of sample sizes. It was difficult to tell how the study had treated the missing data arising from participants who discontinued the study during the double‐blind phase (reported as 72 in the vitamin E group and 66 from the placebo group), and participants who had developed Alzheimer’s Disease by 36 months and had therefore been transferred to an open‐label phase for treatment with donepezil (76 participants in the vitamin E group and 73 in the placebo group).
A z‐score was calculated for cognitive domain scores. Positive numbers on this score indicated better outcomes.
Primary outcomes
Incidence of all‐cause dementia
The study did not report the incidence of all‐cause dementia, but did report the incidence of Alzheimer's dementia. By 12 months, 33 participants in the vitamin E group and 38 in the placebo group had progressed to Alzheimer's dementia. By 36 months, these numbers were 76 and 73 respectively. There was no significant difference between vitamin E and placebo groups in the probability of progression from MCI to Alzheimer's dementia over 36 months based on Cox analysis (HR 1.02; 95% CI 0.74 to 1.41; n = 516; 1 study) (Petersen 2005). There was also no significant difference between groups at any of the six‐monthly time points between baseline and 36 months (prespecified analyses). We considered this to be moderate‐quality evidence, downgraded due to imprecision.
Overall cognitive functioning
This was measured using MMSE and ADAS‐Cog at a six‐monthly interval from baseline for three years. At 36 months, the change from baseline in MMSE score (range 0 to 30, a higher score was better) was ‐2.20 ± 3.64 in the vitamin E group and ‐2.75 ± 4.04 in the placebo group. This was reported not to be statistically significant. We were not able to calculate the mean differences since sample sizes were not reported. There was also reported to be no significant difference in the change from baseline for ADAS Cog (modified) score (range 0 to 85, a higher was worse); this was 3.98 ± 7.56 in the vitamin E group and 3.72 ± 8.54 in the placebo group. We considered this to be low‐quality evidence, downgraded due to risk of bias and imprecision (single study, estimated sample size).
Secondary outcomes
We considered the evidence on all of the secondary outcomes reported here to be low‐quality, downgraded due to risk of bias and imprecision (single study, estimated sample size in each analysis).
Specific cognitive functioning subdomain: episodic memory
The study reported the change from baseline in a standardised z‐score for a memory domain, incorporating ADAS‐cog immediate and delayed word‐recall scores and the New York University immediate and delayed paragraph‐recall scores. Positive numbers indicated improvement. At 36 months, the change from baseline was ‐0.31 ± 0.59 in the vitamin E group and ‐0.28 ± 0.62 in the placebo group. This was reported not to be statistically significant. Sample size was not reported.
Specific cognitive functioning subdomain: executive functioning
The study reported the change from baseline in a standardised z‐score for an executive function domain, incorporating the Digits‐Backward test, Symbol Digit Modalities test, and Number‐Cancellation test. Positive numbers indicated improvement. At 36 months, the change from baseline was ‐0.19 ± 0.48 in the vitamin E group and ‐0.19 ± 0.53 in the placebo group. This was reported not to be statistically significant. Sample size was not reported.
Specific cognitive functioning subdomain: speed of processing
Not reported in the study.
Quality of life, either generic or disease‐specific
Not reported in the study.
Clinical global impression
The study reported the change in Global Deterioration Scale (range 0 to 7, higher was worse) from baseline to 36 months (0.64 ± 0.96 in the vitamin E group, 0.56 ± 0.99 in the placebo group). The number of participants in the analysis was not reported. This difference was described as not statistically significant in the study report.
Functional performance
This was measured with the Activities of Daily Living Scale, which can range from 0 to 53, with higher scores indicating better function. The change from baseline at 36 months was ‐5.63 ± 8.75 in the vitamin E group and ‐6.39 ± 8.99 in the placebo group. The sample sizes used to calculate these values were not reported. The difference was described as not statistically significant.
Number of participants experiencing one or more serious adverse events (SAE)
This was not reported in the study. There was no statistically significant difference between vitamin E and placebo groups in the rate of ten individual adverse events which were reported because they occurred in at least 5% of participants receiving donepezil or vitamin E and at least twice among participants receiving placebo.
Mortality
Five subjects died in each of the vitamin E (n = 257) and placebo (n = 259) groups during the double‐blind phase.
Other validated biomarkers
Not reported in the review.
Comparison 3: Vitamin E and C versus placebo
Only one study Naeini 2014 contributed to this comparison.
Primary outcomes
Incidence of all‐cause dementia
Not assessed.
Overall cognitive functioning
This was measured using the Iranian version of the MMSE (validated for the local population, range 0 to 30) after one year of treatment. The mean and standard error of the mean at six and 12 months were reported. The difference between the groups was described as not statistically significant at either time point.
We assumed that all participants described as completing the study were analysed and estimated the mean difference at the end of the study. This indicated that there may be no little or no effect of supplementation with vitamins E and C (MD 0.23, 95% CI ‐0.25 to 0.71; 1 study, 256 participants; Analysis 3.1).We considered this to be very low‐quality evidence, downgraded due to indirectness (inadequate definition of MCI), risk of bias, and imprecision (result from a single study).
3.1. Analysis.
Comparison 3 Vitamins E and C versus placebo, Outcome 1 Overall cognitive function (MMSE).
Secondary outcomes
Mortality
There was one reported death, but the treatment allocation of this person was not reported.
None of our other secondary outcomes were reported.
Comparison 4: Chromium picolinate versus placebo
Only one study Krikorian 2010 contributed data to this comparison. This study was very small (n = 26). There were 15 participants in the intervention arm and 11 in the placebo arm.
Primary outcomes
The incidence of all‐cause dementia
Not assessed.
Overall cognitive functioning
Not assessed.
Secondary outcomes
Specific cognitive functioning subdomain: episodic memory
This was measured using the Californian Verbal Learning test (test for episodic verbal learning and memory) at 12 weeks. Group differences were not significant for performances on the CVLT learning trials (46.8 versus 45.8; P = 0.72), short‐delay recall (9.4 versus 8.4; P = 0.30), long‐delay recall (9.3 versus 9.5; P = 0.78), or recognition memory (14.4 versus 14.2; P = 0.77). We considered this to be very low‐quality evidence due to serious concern about study limitations and very serious concern about imprecision.
None of our other secondary outcomes were reported.
Discussion
Summary of main results
This review included eight RCTs which investigated the effect of vitamin or mineral supplements on the incidence of dementia or on cognitive function in participants with mild cognitive impairment at baseline. Five studies with 879 randomised participants investigated the effect of B vitamins; in four of these studies the intervention was a mixture of vitamins B12, B6 and folic acid and in one study it was folic acid only. The other supplements included were vitamin E (one study, 769 participants), vitamins E and C (one study, 256 participants) and chromium picolinate (one study, 26 participants).
None of the included studies assessed the incidence of all‐cause dementia, one of our primary outcomes. One study found that there was probably no effect of vitamin E on the probability of progression from MCI to dementia due to Alzheimer's disease over three years.
All of the studies reported on one or more measures of cognitive function.
For B vitamins, we found very low‐quality evidence on overall cognitive function. There was probably little or no effect on any of our specific cognitive subdomains of interest (episodic memory, executive functioning, and speed of processing). Only one study reported on each of global clinical impression and quality of life, and there was no evidence of any effect. There was only very low‐quality evidence on functional performance from one small, open‐label study. The very few data available on adverse events or mortality did not suggest any difference between groups. The only study reporting biomarker data found a reduction in the rate of brain atrophy over two years in the B vitamin group. We added to our protocol the reporting of subgroup data by baseline homocysteine (tHcy) level. The one study that reported such data found that there may be a significant benefit of B vitamins on episodic memory ‐ but not overall cognitive function or executive function ‐ of participants with tHcy above the median at baseline.
The study of vitamin E did not provide the data we needed to conduct analyses. The authors reported no significant effect of vitamin E supplementation on overall cognitive functioning, episodic memory, executive functioning, clinical global impression, functional performance, incidence of adverse events, or mortality.
The quality of evidence on vitamins E and C in combination (effect on overall cognitive function) and on chromium picolinate (effect on episodic memory) was very low so we had very little confidence in the results.
Overall completeness and applicability of evidence
The five trials all included community‐dwelling participants aged over 55 with mild cognitive impairment. In one of the studies of B vitamins, participants all had mild B12 deficiency at baseline at a level which is common in older people, but otherwise participants were not selected on the basis of nutritional status. Not all studies assessed nutritional status at baseline, but participants in all other studies had, or could be expected to have had, normal dietary intake for their population of origin and a low risk of specific vitamin or mineral deficiencies. The results of this review do not apply to people with significant nutritional deficiencies. In one study, participants had vascular cognitive impairment after a recent lacunar stroke; in the other studies, there was no selection for cause of cognitive impairment. Overall, the participants seemed to be quite well representative of people with MCI seen in clinical practice. The range of vitamin and mineral supplements tested was limited. Only B vitamins were investigated in more than one study and these studies all used different dose combinations so that it was not possible to isolate effects of individual B vitamins or to assess dose‐response relationships. The dose of folic acid used in some studies was similar to the maximum dietary intake in the UK population; doses of other vitamins were at least ten times more than the reference nutrient intake (see Appendix 3). All trials assessed different primary and secondary outcomes. Only one trial assessed progression to dementia due to Alzheimer's disease and none assessed progression to all‐cause dementia. Overall cognitive function was assessed mainly with the MMSE which has limited sensitivity to detect small changes in cognition in a population with MCI. There were few data on quality of life, global impression, functional performance, or adverse events. Only one study reported a biomarker outcome which has been widely used in MCI populations, namely, the rate of atrophy on structural MRI.
Quality of the evidence
There was some moderate‐quality evidence relating to the effect of B vitamins on specific cognitive domains and quality of life, and the effect of vitamin E on incidence of Alzheimer's dementia. The remaining evidence was of low‐ or very low‐quality. Some of the more serious concerns about risk of bias related to performance bias in one open‐label study, attrition bias in two studies and selective outcome reporting in two studies. For the comparisons of the vitamin E and C combination and chromium picolinate with placebo, the only evidence was of very low‐quality because of study limitations and very small sample sizes. The evidence concerning a possible differential effect on episodic memory in participants with higher or lower baseline homocysteine levels came from subgroups in a single study and must be regarded as preliminary.
Potential biases in the review process
We considered search biases to be unlikely. We used a standardised search strategy to identify articles, including unpublished studies. These search methods included a single‐concept search across multiple sources along with a search of the Cochrane trial register. This sensitive search approach may have identified studies that would have potentially been overlooked using less rigorous search methods. However, we found too few studies to conduct any formal tests to assess the likelihood of publication bias and this remains possible.
We selected only three specific cognitive subdomains as secondary outcomes. It is possible that effects on other specific cognitive subdomains could have been missed.
Where cognition is measured using several different instruments, it can be difficult to categorise instruments into specific cognitive subdomains and to make data pooling decisions. We tried to avoid bias by using a prespecified, hierarchical list of the most commonly used instruments, but it is possible that different categorisations could have led to different results.
Agreements and disagreements with other studies or reviews
We are not aware of other reviews of similar scope. The Collaboration of B‐vitamin Treatment Trialists have conducted a meta‐analysis of data on more than 22,000 older participants who were not selected for specific cognitive diagnoses (Clarke 2014). This review found no effect of B vitamins on overall or domain‐specific cognitive function despite lowering serum homocysteine levels by > 25%. Although not directly relevant to a population with mild cognitive impairment, this result does not support the hypotheses that B vitamin supplementation or homocysteine‐lowering are effective means to prevent cognitive decline.
Authors' conclusions
Implications for practice.
Currently, there is no evidence that any vitamin or mineral supplement is useful for preventing progression from MCI to dementia or for treating the symptoms of MCI.
Implications for research.
Research on vitamin and mineral supplements for the treatment of mild cognitive impairment should be driven by strong hypotheses based on preclinical research. Studies should investigate mechanisms by measuring putative mediating factors. Ultimately, longer and larger studies will be needed to investigate outcomes which are important to patients, including improved functioning and quality of life, but at the current stage of research, shorter term studies with surrogate outcomes (biomarkers or cognitive test scores) remain suitable for hypothesis testing. Outcomes should be measured across several cognitive domains using instruments which are known to be sensitive to small changes in the presence of mild impairments. Trials should include evaluation of baseline nutritional status and assess how this interacts with treatment. Other possible moderating factors, based on the hypotheses being tested, should also be measured.
Although there was no evidence of overall benefit from the studies included in this review, one study of B vitamins reported a slowing of brain atrophy in the whole study population and an attempt to replicate this result is needed. The same study reported that there may be a beneficial effect of B vitamins on episodic memory in the subgroup with higher homocysteine levels at baseline. However, the result of a very large individual patient meta‐analysis which found no effect of homocysteine‐lowering with B vitamins on cognitive ageing in 22,000 older participants without specific cognitive diagnoses at baseline makes this result less promising to pursue.
Feedback
Vitacog trial, 7 January 2019
Summary
Comment submitted by A David Smith (Department of Pharmacology, University of Oxford)
I have read with interest your Cochrane review and would like to congratulate you and the team on it. You have collected a lot of information and analysed it carefully. I do, however, have a few concerns ‐ some are general and some relate to how you report the VITACOG trial of B vitamins.
In general, I do not think it is really valid to combine trials of different durations (from 6 months to 24 months) since any effect on cognition is likely to be time‐dependent (e.g. see Fig. 1 in the De Jager paper). Also, in 6 months one is very unlikely to see any cognitive decline in the placebo group, so no measurable effect of treatment can be expected.
Secondly, I am not happy with the use of mean differences or SMD. In this way, you are combining measurements of different parameters, e.g. a change over 6 months or a change over 24 months; trials with different doses of vitamins, etc.. Cognitive outcomes are subject to many variables and rather than simply analysing the change from baseline to follow‐up, a better approach is to examine the effect of an intervention by modelling the score at follow‐up controlling for its baseline value and for variables suspected of influencing cognition (such as age, gender etc.). The model, for example GLM, should take into account the different type of outcome and use the appropriate distribution. I am not experienced in statistics but I suspect that it is very difficult, or impossible, to combine data from different trials using this kind of modelling which may be why you fall back on the MD or SMD approach.
Regarding VITACOG (De Jager et al 2012):
1. In Table 2 (page 18) and on pages 19 and 44 you say that our report has a high risk of reporting bias because we did not describe results from all the outcomes listed in the original Protocol. But in real life you have to select in order to produce a paper that fits the requirements of journals and we state in the paper “Tests reported here are representative of particular cognitive domains important in MCI”. I notice that you yourselves have been selective in which cognitive tests you include; for example, you did not cite our results on semantic memory or on CDR, which showed a beneficial effect of B vitamins in those with high homocysteine.
2. On page 44 you say that we have a high risk of reporting bias because we reported the results in subgroups with low and high baseline homocysteine that was not described in the published protocol in PLoS ONE (2010). You are correct: the Protocol we included as a Supplement of the 2010 paper was that written at the time of applications for regulatory approval and did not have details of the final analysis plan. On the other hand, we did state that we planned to measure homocysteine at baseline. Implicit here was the concept that the outcomes would be related to baseline homocysteine and vitamin levels, since this is standard practice in nutritional studies (see a cardinal principle of nutrition, Figure 2 in the review by Smith & Refsum 2016 (Smith 2016)). In the final agreed Analysis Plan (18.08.2009) we clearly specified that we would analyse the results according to the baseline levels of homocysteine, but I realise that this was not, at the time of writing, in the public domain; however, it is now freely available on https://doi.org/10.17605/OSF.IO/VMRZ5
3. Although you cite some of our subgroup analyses based upon baseline homocysteine, you ignored this in your pooled analyses, which correctly stated that there was no significant effect of B vitamins overall in VITACOG. For MMSE, you did not acknowledge that we found a protective effect of B vitamins in those with homocysteine in the top quartile. The major clinical finding in VITACOG is of a protective effect of B vitamins against decline in episodic and semantic memory and a decline in MMSE and IQCODE in those with MCI who have a high baseline homocysteine. But this is almost lost in your report, due to the use of MD and SMD in the tables, although you describe some of the findings in your text. But your final paragraph (page 31) seems to question even our positive results:
“Although there was no evidence of overall benefit from the studies included in this review, one study of B vitamins reported a slowing of brain atrophy in the whole study population and an attempt to replicate this result is needed. The same study reported that there may be a beneficial effect of B vitamins on episodic memory in the subgroup with higher homocysteine levels at baseline. However, the result of a very large individual patient meta‐analysis which found no effect of homocysteine‐lowering with B vitamins on cognitive ageing in 22,000 older participants without specific cognitive diagnoses at baseline makes this result less promising to pursue.”
This statement refers to a meta‐analysis (Clarke et al. 2014) that you correctly state on page 31 is “not directly relevant to a population with mild cognitive impairment” but you go on to say: “this result does not support the hypotheses that B vitamin supplementation or homocysteine‐lowering are effective means to prevent cognitive decline.” You should not have made that conclusion, neither should the original authors since they were not able to assess cognitive decline in their subjects as 76% of the participants only had a single measure of cognition. So no cognitive decline over time could be demonstrated in the placebo group. Without that information, there can be no measurable effect of B vitamin treatment. I am unhappy that your final conclusion is so strongly influenced by a flawed interpretation of a meta‐analysis. The standing of the Cochrane reviews is high and your report will be widely believed.
References
Clarke, R., D. Bennett, S. Parish, S. Lewington, M. Skeaff, et al. (2014). Effects of homocysteine lowering with B vitamins on cognitive aging: meta‐analysis of 11 trials with cognitive data on 22,000 individuals. Am J Clin Nutr 100: 657‐666
de Jager, C. A., A. Oulhaj, R. Jacoby, H. Refsum and A. D. Smith (2012). Cognitive and clinical outcomes of homocysteine‐lowering B‐vitamin treatment in mild cognitive impairment: a randomized controlled trial. Int J Geriatr Psychiatry 27: 592‐600
Smith, A. D. and H. Refsum (2016). Homocysteine, B vitamins, and cognitive impairment. Annu Rev Nutr 36: 211‐239
Conflict of Interest Statement
Do you have any affiliation with or involvement in any organisation with a financial interest in the subject matter of your comment?
Yes. I consult for Proctor & Gamble Personal Healthcare
Reply
We thank Professor Smith for his interest in our review. We think that some of the comments really relate to systematic reviewing in general, or more particularly to the validity of meta‐analysis as an approach. Differences among the studies included in a meta‐analysis are inevitable. In this case, we fully agree that the differences highlighted (constituents and doses of intervention, duration of treatment) are important. As in any SR with meta‐analysis, it was a matter of judgement whether differences were so great that data should not be pooled at all. In Cochrane reviews, if data are pooled, despite potentially important differences, the methods always include an assessment of heterogeneity and an investigation of its possible sources. We took the decision to pool data from trials with durations of 6‐24 months. Heterogeneity was low in all our B‐vitamin analyses except for overall cognitive function. There was no suggestion from the Forest plots that ‘positive’ effects in longer trials were being disguised by ‘negative’ effects in shorter ones. In fact, for overall cognitive function, the largest effect was from the shortest trial (Fan 2017), although we lacked confidence in this result from an open‐label study.
The MD/SMD approach is the standard Cochrane method for estimating a treatment effect from continuous data. We would agree that is not usually possible to construct more complex models across studies without access to individual participant data. As long as the studies are well‐randomised, potentially confounding factors such as age should not invalidate the analysis.
We regret any impression that we were unfair to VITACOG with our rating of high risk of bias in the selective outcome reporting domain and we understand that space limitations in a journal article may have influenced the selection of outcomes reported. We did look beyond single journal articles to determine whether all expected outcomes were reported in included studies (e.g. to secondary publications or results posted on trial registries). However, we have to be consistent in applying the risk of bias tool and in recording a risk if we are unable to find outcome data, or if the reporting differs from the publicly available analysis plan. We recognised the high quality of the VITACOG trial in our low risk of bias ratings in all other domains.
We agree that our review also selected particular outcomes to report; these were pre‐specified in the published review protocol. We did, in fact, report CDR data from VITACOG – including the data by homocysteine quartile – under our secondary outcome of Clinical global impression.
We would disagree with the suggestion that our review rather buries the possible effect found in VITACOG among patients with higher homocysteine at baseline. We recognised the importance of this finding to the field and actually amended our review methods from those in our protocol in order to be able to report it, which we do quite prominently, including it in the Abstract and in the Plain Language Summary, and making it the basis of our most concrete recommendation for future research.
Contributors
A David Smith ‐ Department of Pharmacology, University of Oxford Jenny McCleery ‐ Lead Author Naji Tabet ‐ Contact Author Sascha Köpke ‐ Deputy Co‐ordinating Editor CDCIG
What's new
| Date | Event | Description |
|---|---|---|
| 14 February 2019 | Feedback has been incorporated | Feedback contributor affiliation and reference added |
History
Protocol first published: Issue 10, 2015 Review first published: Issue 11, 2018
| Date | Event | Description |
|---|---|---|
| 14 February 2019 | Feedback has been incorporated | Feedback and reply incorporated |
Acknowledgements
The protocol was largely based on a general template constructed for the development of a larger series of protocols and reviews covered by a National Institute for Health Research (NIHR Systematic Reviews Programme Grant). The common protocol covered four types of intervention, for which some evidence exists that these may modify the risk of developing cognitive impairments or dementia. These include vitamin and mineral supplements, exercise, cognition, and dietary interventions. The general protocol was inspired by a generic protocol approved by the Cochrane Musculoskeletal Group for another series of reviews (Da Costa 2012; Da Costa 2014; Reichenbach 2010; Rutjes 2009a; Rutjes 2009b; Rutjes 2010).
We gratefully acknowledge the assistance of Professor Lisette de Groot and Dr Simone Eussen who generously provided subgroup data from their study (Eussen 2006).
We thank Wen Li Chow for her assistance in double checking the accuracy of some of the data.
We are very grateful to Dr Yoko Wong and Dr Charles Zheng of Cochrane Singapore, who kindly extracted and translated data from Fan 2017.
We also thank the following members of Cochrane Crowd who made significant contributions to screening the search results: Michael J. Arnatt, Soumyadeep Bhaumik, Mª Paz Campos Pérez, C Cartlidge, Daniel Casey, Mohamed Fawzy Abdelghafar, Cristi Francis, Pishoy Gouda, Dan Griffiths, Michael Haas, Shirley Hall, Jake Hartley, Michael Hull, Geanina Ilinoiu, Deborah Jackson, Sofia Jaramillo, Robert Kemp, Ivan Murrieta Alvarez, Shireen Rafeeq, Miriam Thiel, Robin Vernooij, Jennifer Ware, Hakan Yaman.
Appendices
Appendix 1. Biological plausibility of Vitamins and Minerals
| Supplement* | Summary of action | Biological plausibility |
| Vitamins | ||
| Vitamin A | antioxidant; anti‐inflammatory; anticholinesterase; beta‐amyloid inhibition |
Carboxylic form of Vitamin A known as all‐trans retinoic acid has been shown to have memory restorative function and it may be attributed to its anticholinesterase, antioxidative and antiinflammatory potential (Sodhi 2013). Vitamin A and beta‐carotene may also inhibit the formation, extension, and destabilising effects of beta‐amyloid fibrins. Plasma or cerebrospinal fluid concentrations of vitamin A and beta‐carotene have been reported to be lower in AD patients, and increased Vit A/beta‐carotene concentrations have been clinically shown to slow the progression of dementia (Ono 2012). |
| Vitamin D | neuronal activity | Vitamin D receptor (VDR) and 1, alpha‐hydroxylase, the terminal calcitriol‐activating enzyme, are distributed throughout both the foetal and adult brain. This is thought to play a role in brain development and critical brain functions (McCann 2008). Significant correlation between serum 25(OH)D levels and cognitive scores were reported in De Luca 1975 and Przybelski 2007. |
| Vitamin E | antioxidant; beta‐amyloid inhibition |
Vitamin E consists of a group of tocopherols and tocotrienols. Apart from lipid antioxidant activity, other functions include membrane stabilisation by forming complexes with the products of lipid hydrolysis (Wang 2000). It has been shown that the antioxidant and free radical scavenging activity of Vitamin E inhibits amyloid beta protein‐induced neuronal cell death and may have implication in prevention and treatment of Alzheimer’s dementia. (Behl 1992). |
| Vitamin K | neuronal activity | Vitamin K participates in the synthesis of sphingolipids. Sphingolipids participate in important cellular events such as proliferation, differentiation, senescence, and cell‐cell interactions. Sphingolipid metabolism has been linked to age‐related cognitive decline and neurodegenerative diseases such as Alzheimer's disease (Ferland 2012). A cross‐sectional study found correlations between higher serum phylloquinone concentration and better cognitive scores in tests evaluating episodic verbal memory among healthy older adults (Ferland 2013). |
| Thiamine (Vitamin B1) |
neuronal activity | Thiamine is required as a cofactor in the cellular production of energy and enhances normal neuronal activities (Osiezagha 2013). Rats with an episode of induced thiamine deficiency had cognitive, learning, and memory impairments (Langlais 1995). |
| Riboflavin (Vitamin B2) |
neuronal activity | Riboflavin (7,8‐dimethyl‐10‐ribityl‐isoalloxazine) is water‐soluble. Symptoms of neurodegeneration and peripheral neuropathy in riboflavin deficiency have been documented in animal studies, but not observed in humans. Subclinical riboflavin deficiency may contribute to increased concentrations of plasma homocysteine and may be associated with increased risk of cardiovascular disease and impaired handling of iron (Powers 2003). |
| Niacin (Vitamin B3) |
vascular: anti‐inflammatory |
Niacin is a water‐soluble precursor cofactor essential for the formation of dozens of enzymes. Niacin decreases atherosclerosis development mainly by reducing LDL cholesterol. It also has modest HDL‐cholesterol‐raising and anti‐inflammatory effects (Kühnast 2013). Niacin deficiency causes pellagra. Its neuropsychiatric symptoms are similar to those in Alzheimer's disease or vascular dementia (Amanullah 2010). |
| Vitamin B6 (Pyridoxine, pyridoxal, pyridoxamine, Pyridoxal 5' phosphate (PLP) and pyridoxamine 5' phosphate (PMP), Pyridoxine 5'‐phosphate (PNP)) |
homocysteine; neuronal activity |
Vitamin B6 is a group of water‐soluble compounds (vitamers). Pyridoxal 5' phosphate (PLP) and pyridoxamine 5' phosphate (PMP) are the active coenzyme forms of vitamin B6 (ODS 2014). Vitamin B6 has many important brain functions such as biosynthesis of neurotransmitters (GABA, dopamine, noradrenaline, serotonin), receptor binding, macronutrient metabolism, and gene expression. In a study looking at low plasma B6 levels predicting cognitive decline and depression in at‐risk individuals, low PLP status was seen as a risk factor for cognitive decline and depression in at‐risk populations (Scott 2013). |
| Folic Acid (Vitamin B9) |
antioxidant; homocysteine; neuronal activity |
Folate is a cofactor and promotes the remethylation of homocysteine ‐‐ an amino acid that can induce DNA strand breakage, oxidative stress, and apoptosis. Folate is required for normal development of the nervous system, playing important roles regulating neurogenesis and programmed cell death. Folate deficiency and its resultant increase in homocysteine levels has been linked to several neurodegenerative conditions, including stroke, Alzheimer's disease, and Parkinson's disease (Mattson 2003). |
| Vitamin B12 (cobalamins: cyanocobalamin, hydroxocobalamin, methylcobalamin, hydroxocobalamin) |
homocysteine; neuronal activity |
Vitamin B12 acts as a coenzyme in metabolism of amino acids and fatty acids required for the synthesis of nucleic acids, erythrocytes, and in the maintenance of myelin (Pawlak 2014). Lower vitamin B12 status has been associated with increased rates of cognitive decline and dementia (Clarke 2007; O'Leary 2012). |
| Pantothenic Acid (Vitamin B5) |
energy metabolism | Pantothenic acid (PA) is a component of coenzyme A, an essential cofactor in fatty acid oxidation, lipid elongation, and fatty acid synthesis (Kelly 2011). This may have an indirect effect in cognition. |
| Biotin (Vitamin H) |
energy metabolism | Biotin is also known as Vitamin H and is part of the B complex group of vitamins. They act as cofactors in carboxylase enzymes, fatty acid, and amino acid metabolism. This may have an indirect effect in cognition. |
| Vitamin C | antioxidant | Vitamin C has antioxidant functions and is required for the synthesis of noradrenaline from dopamine. It has been reported that Vitamin C levels have been lower than controls in patients with senile dementia of Alzheimer’s type (Jeandel 1989). In a longitudinal and cross‐sectional study, it was found that higher vitamin C levels were associated with better memory performance (Perrig 1997). |
| Minerals | ||
| Calcium | neuronal activity | Calcium ions regulate a number of physiological processes including neuronal gene expression and the neuronal secretion of neurotransmitters (Dolphin 2012; Linus Pauling Institute). Supplementation with calcium together with vitamin D was found to have no significant association with incident cognitive impairment (Rossom 2012). Ozawa 2012 concluded that, in the general Japanese population, higher self‐reported dietary intakes of potassium, calcium, and magnesium reduced the risk of all‐cause dementia, especially Vascular Dementia (VaD). The proposed mechanism was through the reduction of vascular risk factors. |
| Chromium | energy production; metabolism. | Chromium is needed for energy production and has been found to promote the effect of insulin involved in metabolism and storage of protein, carbohydrates and lipids within the CNS (IOM 2011; Ozawa 2012; Anderson 1997). Chromium is involved in metabolism of nucleic acid, which is needed to build DNA, the genetic material in cells and it promotes synthesis of cholesterol and fatty acids needed for brain function. It may lower LDL cholesterol and triglyceride levels, raise HDL cholesterol levels and reduce high blood pressure (Preuss 1997). Insulin resistance is implicated in the pathophysiological changes associated with Alzheimer's disease, and pharmaceutical treatments that overcome insulin resistance improve memory function in subjects with mild cognitive impairment (MCI) and early Alzheimer's disease. Chromium (Cr) supplementation improves glucose disposal in patients with insulin resistance and diabetes. A double‐blind RCT suggested that supplementation with chromium picolinate can enhance cognitive inhibitory control and cerebral function in older adults at risk for neurodegeneration (Krikorian 2010). A positive correlation between cognitive function and serum chromium levels was found in a study (Smorgon 2004). |
| Copper | antioxidant | Copper is a component of an antioxidant enzyme called superoxide dismutase that protects cells from damage by harmful free radicals. Copper is necessary for a healthy nerve system and taste sensitivity (IOM 2011). Copper may promote non‐amyloidogenic processing of amyloid precursor protein (APP) and thereby lowers the Aβ production in cell culture systems, and it increases lifetime and decreases soluble amyloid production in APP transgenic mice (Borchardt 1999). In Alzheimer patients, the decline of Aβ levels in CSF is diminished in the treatment group (Kaden 2011). |
| Iodine | neuronal activity | Iodine is needed for the synthesis of thyroid hormones, which, in turn, are needed for the myelination of the central nervous system. Iodine is necessary for the normal development of the brain. A deficiency of this mineral during critical periods of development in gestation can lead to intellectual disability and neurodevelopmental problems (Bath 2013a). Positive association was found between maternal iodine status and child IQ at age 8 years and reading ability at age 9 years (Bath 2013b). |
| Iron | neuronal activity | Iron is needed for development of oligodendrocytes and numerous enzymes that synthesise neurotransmitters such as noradrenaline, serotonin, and dopamine. It is important for production of the haemoglobin in red blood cells (Linus Pauling Institute; IOM 2011). Regression analysis showed that nonanaemic iron‐deficient adolescent girls who received iron performed better on a test of verbal learning and memory than girls in the control group (Bruner 1996). |
| Magnesium | energy, metabolism | Magnesium is involved in hundreds of enzyme reactions, including those for forming bone matrix and protein synthesis. It is vital for fat and carbohydrate metabolism, and so plays a role in energy production; can improve insulin sensitivity in diabetics and help regulate blood sugar level; regulates neuromuscular transmission and higher self‐reported dietary intakes of potassium, calcium, and magnesium reduce the risk of all‐cause dementia, especially VaD, in the general Japanese population (Ozawa 2012). |
| Manganese | metabolism | Manganese is needed to synthesise fatty acids and cholesterol, and metabolise carbohydrates and proteins. It is important for energy production. It promotes utilisation of other key nutrients like vitamin B1 (thiamine), biotin, choline, ascorbic acid, and vitamin E (Linus Pauling Institute;). Manganese is needed for glucose metabolism, which helps regulate blood glucose. It is needed to make manganese superoxide dismutase (MnSOD), one of the key antioxidants that protects cells from free radical damage, and so helps maintains healthy nerves. It works synergistically with the B‐complex vitamins to generate an overall feeling of well‐being (IOM 2011). |
| Molybdenum | metabolism | Molybdenum promotes normal cell function. It functions as a cofactor for three essential enzymes that play a vital role in carbohydrate metabolism, utilisation of iron, sulphite detoxification, and uric acid formation (Linus Pauling Institute; IOM 2011). |
| Phosphorus | metabolism, neuronal structure and function | Phosphorus is needed for metabolism of carbohydrates and fats to produce energy and is involved in the production of ATP required for growth and repair of cells and tissues; needed to make cell membranes. It helps the body utilise the B‐complex vitamins that support proper muscle and nerve function (Linus Pauling Institute; IOM 2011). |
| Potassium | nerve transmission | Potassium is involved in regulating nerve transmissions and muscle contractions. It helps the body handle sodium and so reduce the risk of high blood pressure (Berr 2012). It has been found to lower the risk of stroke and ischaemic heart disease. Potassium is needed for synthesis of protein from amino acids (Linus Pauling Institute; IOM 2011). Higher self‐reported dietary intakes of potassium, calcium, and magnesium reduce the risk of all‐cause dementia, especially VaD, in the general Japanese population (Ozawa 2012). |
| Selenium | antioxidant | Selenium is an important antioxidant especially in combination with vitamin E. It has been found to induce repair of DNA in damaged cells (Linus Pauling Institute; IOM 2011). Selenium is a major structural component of glutathione peroxidases which are important antioxidant enzymes in the central nervous system and other body tissues (Mehdi 2013; Rahman 2007). Low selenium levels were found to be related to a risk factor for cognitive function (Berr 2012; Smorgon 2004). Supplementation with selenium has been associated with an improved overall health, reducing oxidative stress and ameliorating risk factors for dementia (Mehdi 2013). |
| Sodium | neuronal activity | Sodium is essential for regulating muscle contractions, and nerve transmissions essential for normal CNS physiological mechanisms and homeostasis (Linus Pauling Institute; IOM 2011). |
| Zinc | anti‐oxidant neuronal activity |
Zinc is a constituent of the antioxidant enzyme superoxide dismutase that helps reduce the harm from harmful free radicals. Zinc regulates cell division and synthesis of genetic cell DNA. It is essential for reproduction, repair, and normal growth within the CNS (Linus Pauling Institute). Zinc is found in high levels in the brain where it performs catalytic, structural, and regulatory roles in cellular metabolism. In the brain, zinc is bound to proteins but free zinc is present in synaptic vesicles and performs a role in neurotransmission mediated by glutamate and gamma‐aminobutyric acid (GABA). Short‐term deficits of zinc have been shown to impair certain measures of mental and neurological function while long‐term deficits of zinc, especially during gestation, results in malformation or deficits in attention, learning, memory, and neuropsychological behaviour (IOM 2011) Zinc was found to be capable of reducing post‐ischaemic injury to a variety of tissues and organs through a mechanism that might involve the antagonism of copper reactivity. Although the evidence for the antioxidant properties of zinc is compelling, the mechanisms are still unclear (Powell 2000). |
| * Only orally‐administered supplements taken at any dose for at least 12 weeks will be included. Supplements that combine vitamins or minerals are eligible as well. | ||
Appendix 2. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| ALOIS (www.medicine.ox.ac.uk/alois) (Date of most recent search: 25 January 2018) |
Basic search: VIT (Studies within ALOIS are coded VIT if the intervention is a vitamin or mineral) |
Dec 2014: 254 Jul 2015: 0 Mar 2016: 2 Aug 2016: 0 Mar 2017: 3 Jan 2018: 1 |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950 ‐ present (Ovid SP) (Date of most recent search: 25 January 2018) |
1. exp *Vitamins/ 2. exp *Minerals/ 3. exp *Dietary Supplements/ 4. Calcium Carbonate/ 5. vitamin*.ti,ab. 6. cholecalciferol.ti,ab. 7. ergocalciferol.ti,ab. 8. toxiferol.ti,ab. 9. retinol.ti,ab. 10. "retinoic acid".ti,ab. 11. Vitamin A/ 12. Vitamin B 12/ 13. Vitamin D/ 14. Vitamin E/ 15. "beta‐carotene".ti,ab. 16. "alpha‐carotene".ti,ab. 17. "gamma‐carotene".ti,ab. 18. "beta‐cryptoanthin".ti,ab. 19. thiamine.ti,ab. 20. riboflavin.ti,ab. 21. niacin.ti,ab. 22. nicotinamide.ti,ab. 23. pantothenic.ti,ab. 24. pyridoxine.ti,ab. 25. pyridoxal.ti,ab. 26. pyridoxamine.ti,ab. 27. biotin.ti,ab. 28. "folic acid".ti,ab. 29. Folic Acid/ 30. cyanocobalamin.ti,ab. 31. methylcobalamin.ti,ab. 32. "l‐ascorbic acid".ti,ab. 33. "ascorbic acid".ti,ab. 34. ascorbate.ti,ab. 35. Ascorbic Acid/ 36. phylloquinone.ti,ab. 37. phytomeadione.ti,ab. 38. phytonadine.ti,ab. 39. mineral*.ti,ab. 40. multivitamin*.ti,ab. 41. "diet* supplement*".ti,ab. 42. calcium.ti,ab. 43. Calcium/ 44. iron.ti,ab. 45. zinc.ti,ab. 46. sodium.ti,ab. 47. potassium.ti,ab. 48. phosphorus.ti,ab. 49. magnesium.ti,ab. 50. chloride.ti,ab. 51. sulphur.ti,ab. 52. mangansese.ti,ab. 53. cobalt.ti,ab. 54. selenium.ti,ab. 55. copper.ti,ab. 56. iodine.ti,ab. 57. fluoride.ti,ab. 58. or/1‐57 59. *Aging/ 60. Aged/ 61. "Aged, 80 and over"/ 62. Middle Aged/ 63. Age Factors/ 64. "mild cognitive impairment".ti,ab. 65. Mild Cognitive Impairment/ 66. MCI.ti,ab. 67. AAMI.ti,ab. 68. ACMI.ti,ab. 69. ARCD.ti,ab. 70. CIND.ti,ab. 71. (nMCI or aMCI or mMCI or MCIa).ti,ab. 72. "old* adults".ti,ab. 73. elderly.ti,ab. 74. "old* age*".ti,ab. 75. "middle age*".ti,ab. 76. seniors.ti,ab. 77. "senior citizens".ti,ab. 78. "community dwelling".ti,ab. 79. pensioners.ti,ab. 80. "aged sample".ti,ab. 81. "aged population".ti,ab. 82. or/59‐81 83. 58 and 82 84. *Cognition/ 85. *Cognition Disorders/ 86. Memory/ 87. Memory Disorders/ 88. (cognit* adj3 (func* or declin* or reduc* or impair* or improve* or deficit* or progress* or perform* or abilit*)).ti,ab. 89. "mental perform*".ti,ab. 90. memory.ti,ab. 91. "executive function*".ti,ab. 92. Executive Function/ 93. Attention/ 94. (speed adj2 processing).ti,ab. 95. "episodic memory".ti,ab. 96. Memory, Episodic/ 97. or/84‐96 98. 83 and 97 99. randomized controlled trial.pt. 100. controlled clinical trial.pt. 101. randomized.ab. 102. placebo.ab. 103. drug therapy.fs. 104. randomly.ab. 105. trial.ab. 106. groups.ab. 107. or/99‐106 108. exp Animals/ not humans.sh. 109. 107 not 108 110. 98 and 109 [all results] 111. *Vitamins/ 112. *Cognition/ 113. "Aged, 80 and over"/ or Aged/ or Middle Aged/ 114. Mild Cognitive Impairment/ 115. "mild cognitive impairment".ti,ab. 116. 113 or 114 or 115 117. 111 and 112 and 116 118. 99 or 100 119. 117 and 118 [results sent directly to author team] 120. 110 not 119 [results minus those sent directly to author team. These results will be screened by the 'crowd'] | Dec 2014: 1320 Jul 2015: 53 Mar 2016: 111 Aug 2016: 103 Mar 2017: 166 Jan 2018: 120 |
| Embase 1974 ‐ 24 January 2018 (Ovid SP) (Date of most recent search: 25 January 2018) |
1. exp *vitamin/ 2. exp *mineral/ 3. exp diet supplementation/ 4. calcium/ 5. vitamin*.ti,ab. 6. mineral*.ti,ab. 7. cholecalciferol.ti,ab. 8. ergocalciferol.ti,ab. 9. toxiferol.ti,ab. 10. retinol.ti,ab. 11. retinal.ti,ab. 12. "retinoic acid".ti,ab. 13. vitamin D/ 14. vitamin B complex/ or vitamin B group/ 15. vitamin D/ 16. vitamin K epoxide reductase/ or vitamin K group/ 17. colecalciferol/ or calcitriol/ or calcitriol derivative/ 18. ascorbic acid/ 19. vitamin supplementation/ 20. "beta‐carotene".ti,ab. 21. beta carotene/ 22. "alpha‐carotene".ti,ab. 23. alpha carotene/ 24. "gamma‐carotene".ti,ab. 25. gamma carotene/ 26. "beta‐cryptoanthin".ti,ab. 27. thiamine.ti,ab. 28. thiamine/ 29. riboflavin.ti,ab. 30. riboflavin/ 31. niacin.ti,ab. 32. nicotinic acid/ 33. nicotinamide.ti,ab. 34. pantothenic.ti,ab. 35. pyridoxamine.ti,ab. 36. pantothenic acid/ 37. pyridoxamine/ 38. biotin.ti,ab. 39. biotin/ 40. "folic acid".ti,ab. 41. folic acid/ 42. cyanocobalamin.ti,ab. 43. cyanocobalamin/ 44. methylcobalamin.ti,ab. 45. "l‐ascorbic acid".ti,ab. 46. "ascorbic acid".ti,ab. 47. phylloquinone.ti,ab. 48. phytonadine.ti,ab. 49. phytomeadione.ti,ab. 50. multivitamin*.ti,ab. 51. "vitamin* supple*".ti,ab. 52. "diet* supplement*".ti,ab. 53. calcium.ti,ab. 54. iron.ti,ab. 55. iron/ 56. zinc.ti,ab. 57. zinc/ 58. sodium.ti,ab. 59. sodium/ 60. potassium.ti,ab. 61. citrate potassium/ or potassium/ or clavulanate potassium/ or diclofenac potassium/ 62. phosphorus.ti,ab. 63. phosphorus/ 64. magnesium.ti,ab. 65. magnesium/ 66. chloride.ti,ab. 67. chloride/ 68. sulphur.ti,ab. 69. mangansese.ti,ab. 70. cobalt.ti,ab. 71. cobalt/ 72. selenium.ti,ab. 73. selenium/ 74. copper.ti,ab. 75. copper/ 76. iodine.ti,ab. 77. fluoride.ti,ab. 78. fluoride/ 79. or/1‐78 80. aging/ 81. aged/ 82. middle aged/ 83. mild cognitive impairment/ 84. "mild cognitive impairment".ti,ab. 85. MCI.ti,ab. 86. AAMI.ti,ab. 87. ACMI.ti,ab. 88. ARCD.ti,ab. 89. CIND.ti,ab. 90. (nMCI or aMCI or mMCI or MCIa).ti,ab. 91. "middle age*".ti,ab. 92. "old* age*".ti,ab. 93. "old* adults".ti,ab. 94. "community dwelling".ti,ab. 95. "senior citizens".ti,ab. 96. seniors.ti,ab. 97. pensioners.ti,ab. 98. "aged sample".ti,ab. 99. "aged population".ti,ab. 100. or/80‐99 101. exp cognition/ 102. cognition disorders/ 103. episodic memory/ or memory/ 104. memory disorder/ 105. dementia/ 106. Alzheimer disease/ 107. dement*.ti,ab. 108. alzheimer*.ti,ab. 109. cognition.ti,ab. 110. cognitive.ti,ab. 111. or/101‐110 112. 79 and 100 and 111 113. randomized controlled trial/ 114. controlled clinical trial/ 115. placebo.ab. 116. (random* adj2 divide*).ti,ab. 117. (random* adj2 allocate*).ti,ab. 118. trial.ab. 119. "double‐blind*".ti,ab. 120. "single blind*".ti,ab. 121. or/113‐120 122. 112 and 121 123. *Cognition/ 124. exp *vitamin/ or exp *vitamin supplementation/ 125. exp *mineral/ or exp *mineral supplementation/ 126. (vitamin* or mineral*).ti. 127. 124 or 125 or 126 128. exp *aging/ 129. (elderly or "middle age*" or "old* adults" or MCI or "mild cognitive impairment").ti. 130. exp *middle aged/ 131. 128 or 129 or 130 132. 123 and 127 and 131 133. 113 or 114 134. 132 and 133 135. 122 not 134 |
Dec 2014: 1275 Jul 2015: 114 Mar 2016: 184 Aug 2016: 94 Mar 2017: 257 Jan 2018: 250 |
| PsycINFO 1806 ‐ January week 2, 2018 (Ovid SP) (Date of most recent search: 25 January 2018) |
1. exp Aging/ 2. exp Cognitive Impairment/ 3. "cognit* impair*".ti,ab. 4. MCI.ti,ab. 5. AAMI.ti,ab. 6. ACMI.ti,ab. 7. ARCD.ti,ab. 8. CIND.ti,ab. 9. (nMCI or aMCI or mMCI or MCIa).ti,ab. 10. "old* age*".ti,ab. 11. elderly.ti,ab. 12. "middle age*".ti,ab. 13. "old* adults".ti,ab. 14. seniors.ti,ab. 15. "senior citizens".ti,ab. 16. "community dwelling".ti,ab. 17. pensioners.ti,ab. 18. or/1‐17 19. exp Cognition/ 20. exp Dementia/ 21. 19 or 20 22. randomi?ed.ti. 23. (randomly adj2 allocat*).ab. 24. (randomly adj2 divide*).ab. 25. RCT.ti,ab. 26. "double‐blind*".ti,ab. 27. "single blind*".ti,ab. 28. "randomi?ed trial".ab. 29. "randomi?ed control* trial".ab. 30. "random allocation".ab. 31. "controlled clinical trial".ti,ab. 32. or/22‐31 33. exp Vitamins/ 34. exp Dietary Supplements/ 35. vitamin*.ti,ab. 36. mineral*.ti,ab. 37. calcium.ti,ab. 38. Calcium/ 39. exp Ascorbic Acid/ 40. exp Folic Acid/ 41. "folic acid".ti,ab. 42. cholecalciferol.ti,ab. 43. ergocalciferol.ti,ab. 44. toxiferol.ti,ab. 45. retinol.ti,ab. 46. retinal.ti,ab. 47. "retinoic acid".ti,ab. 48. "beta‐carotene".ti,ab. 49. "alpha‐carotene".ti,ab. 50. "gamma‐carotene".ti,ab. 51. "beta‐cryptoanthin".ti,ab. 52. thiamine.ti,ab. 53. riboflavin.ti,ab. 54. niacin.ti,ab. 55. nicotinamide.ti,ab. 56. pantothenic.ti,ab. 57. pyridoxine.ti,ab. 58. pyridoxal.ti,ab. 59. pyridoxamine.ti,ab. 60. biotin.ti,ab. 61. "folic acid".ti,ab. 62. cyanocobalamin.ti,ab. 63. methylcobalamin.ti,ab. 64. "l‐ascorbic acid".ti,ab. 65. "ascorbic acid".ti,ab. 66. ascorbate.ti,ab. 67. phylloquinone.ti,ab. 68. phytomeadione.ti,ab. 69. phytonadine.ti,ab. 70. multivitamin*.ti,ab. 71. "diet* supplement*".ti,ab. 72. iron.ti,ab. 73. zinc.ti,ab. 74. sodium.ti,ab. 75. potassium.ti,ab. 76. phosphorus.ti,ab. 77. magnesium.ti,ab. 78. chloride.ti,ab. 79. sulphur.ti,ab. 80. mangansese.ti,ab. 81. cobalt.ti,ab. 82. selenium.ti,ab. 83. copper.ti,ab. 84. iodine.ti,ab. 85. fluoride.ti,ab. 86. 18 or 21 87. or/33‐85 88. 86 and 87 89. 32 and 88 90. exp *Vitamins/ 91. (vitamin* or mineral*).ti. 92. 90 or 91 93. exp *Cognition/ 94. (cognition or cognitive).ti. 95. 93 or 94 96. (elderly or "middle age*" or "old* adults" or MCI or "mild cognitive impairment").ti. 97. 92 and 95 and 96 98. (randomised or randomised or RCT or trial).ti. 99. 97 and 98 100. 89 not 99 |
Dec 2014: 202 Jul 2015: 15 Mar 2016: 10 Aug 2016: 0 Mar 2017: 14 Jan 2018: 9 |
| CINAHL (EBSCOhost) (Date of most recent search: 25 January 2018) |
S1 (MM “Vitamins+") S2 (MM “Minerals+") S3 (MH "Dietary Supplements") OR (MH "Dietary Supplementation") OR (MH "Dietary Carbohydrates") OR (MH "Dietary Fiber") OR (MH "Sodium, Dietary") OR (MH "Dietary Fats") OR (MH "Dietary Proteins") OR (MH "Dietary Sucrose”) S4 TX vitamin* S5 TX mineral* S6 TX “diet* supple*” S7 (MH "Fatty Acids") OR (MH "Fatty Acids, Omega‐6") OR (MH "Fatty Acids, Unsaturated") OR (MH "Trans Fatty Acids") OR (MH "Fatty Acids, Monounsaturated") OR (MH "Fatty Acids, Saturated") OR (MH "Fatty Acids, Essential”) S8 TX “fatty acid*” S9 (MH "Vitamin A”) S10 (MH "Vitamin B12") OR (MH "Vitamin B Complex") OR (MH "Thiamine") OR (MH "Riboflavin") OR (MH "Pyridoxine") OR (MH “Carnitine") S11 (MH "Folic Acid”) S12 (MH "Ascorbic Acid”) S13 (MH "Vitamin D") OR (MH "Cholecalciferol") OR (MH "Ergocalciferols") OR (MH “Calcitriol") S14 (MH "Vitamin E") OR (MH "Pantothenic Acid”) S15 (MH "Vitamin K") OR (MH “Osteocalcin") S16 TX “beta‐carotene" S17 TX “alpha‐carotene" S18 TX thiamine S19 TX riboflavin S20 TX niacin S21 TX pantothenic S22 TX nicotinamide S23 TX pyridoxine S24 TX pyridoxal S25 TX biotin S26 (MH “Calcium") S27 TX calcium S28 TX iron S29 (MH “iron”) S30 (MH “Zinc”) S31 TX zinc S32 (MH “Sodium”) S33 TX sodium S34 (MH “Potassium") S35 TX potassium S36 (MH “Phosphorus") S37 TX phosphorus S38 (MH “Magnesium") S39 TX magnesium S40 (MH "Sodium Chloride, Dietary”) S41 TX chloride S42 TX sulphur S43 TX cobalt S44 TX selenium S45 TX copper S46 TX iodine S47 TX flouride S48 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 S49 (MH “Aging") S50 (MH "Aged") OR (MH "Aged, 80 and Over”) S51 (MH "Middle Age”) S52 TX "Mild Cognitive Impairment” S53 TX MCI OR AAMI OR ACMI OR ARCD OR CIND S54 TX nMCI OR aMCI OR mMCI OR MCIa S55 TX elderly S56 TX "old* adults” S57 TX "old* age*” S58 TX pensioners S59 TX "community dwelling” S60 TX seniors S61 TX "senior citizen*” S62 TX "age* sample” S63 TX "age* population” S64 S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 S65 S48 AND S64 S66 (MH "Cognition") OR (MH "Cognition Disorders") OR (MH "Delirium, Dementia, Amnestic, Cognitive Disorders”) S67 TX cognition S68 TX memory S69 (MH "Memory") OR (MH "Memory Disorders") OR (MH "Memory, Short Term”) S70 TX "executive function” S71 TX "cognitive* declin*” S72 TX "cognitive* improv*” S73 TX "cognitive deficit*” S74 TX "mental perform*” S75 TX dementia S76 TX alzheimer* S77 (MH “Dementia+") S78 S66 OR S67 OR S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 OR S75 OR S76 OR S77 S79 S65 AND S78 S80 (MH "Randomized Controlled Trials”) S81 AB randomly S82 AB placebo S83 AB groups S84 AB RCT S85 TX "double blind*” S86 TX "single blind*” S87 TX "controlled clinical trial” S88 TI randomised S89 TI randomized S90 S80 OR S81 OR S82 OR S83 OR S84 OR S85 OR S86 OR S87 OR S88 S91 S79 AND S90 |
Dec 2014: 493 Jul 2015: 7 Mar 2016: 35 Aug 2016: 15 Mar 2017: 24 Jan 2018: 23 |
| ISI Web of Science (includes: Web of Science (1945 ‐ present); BIOSIS Previews (1926 ‐ present); MEDLINE (1950 ‐ present); Journal Citation Reports); BIOSIS Previews (Date of most recent search: 25 January 2018) |
("mild cognitive impairment" OR elderly OR "age* subjects" OR "old* adult*" OR "middle age*" OR MCI) AND TOPIC: ("randomly allocated" OR "random allocation" OR randomised OR randomized OR RCT OR "controlled trial" OR "double blind" OR "single blind") AND TOPIC: (vitamin* OR mineral* OR "diet* suppl*" OR "ascorbic acid" OR "folic acid" OR iron OR calcium OR sodium OR zinc OR potassium OR magnesium OR cobalt OR copper OR iodine) AND TOPIC: (cognition OR dementia OR memory OR "executive function" OR alzheimer*) Timespan: All years. Search language = Auto |
Dec 2014: 932 Jul 2015: 34 Mar 2016: 100 Aug 2016: 43 Mar 2017: 67 Jan 2018: 55 |
| LILACS (BIREME) (Date of most recent search: 25 January 2018) |
cognition OR "mild cognitive impairment" OR elderly OR "aged subjects" OR "older adults" OR "middle aged" [Words] and randomly OR randomised OR randomized OR RCT OR "controlled trial" [Words] and vitamin OR vitamins OR mineral OR minerals OR "fatty acid" OR "folic acid" [Words] | Dec 2014: 28 Jul 2015: 0 Mar 2016: 0 Aug 2016: 1 Mar 2017: 0 Jan 2018: 0 |
| CENTRAL (via CRSO) (Date of most recent search: 25 January 2018) |
#1 MeSH descriptor: [Aged, 80 and over] explode all trees #2 MeSH descriptor: [Aged] explode all trees #3 MeSH descriptor: [Middle Aged] explode all trees #4 MeSH descriptor: [Mild Cognitive Impairment] explode all trees #5 "cognit* impair*" or MCI #6 elderly #7 "old* adults" #8 "old* age*" #9 "old* sample" #10 senior citizens #11 pensioners #12 seniors #13 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 #14 MeSH descriptor: [Cognition] explode all trees #15 MeSH descriptor: [Dementia] explode all trees #16 cognit* #17 memory #18 "executive function*" #19 processing #20 "mental perform*" #21 dement* #22 alzheimer* #23 #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 #24 MeSH descriptor: [Vitamins] explode all trees #25 MeSH descriptor: [Minerals] explode all trees #26 vitamin* #27 mineral* #28 "ascorbic acid" #29 "folic acid" #30 MeSH descriptor: [Fatty Acids] explode all trees #31 zinc or iron or calcium or sodium or potassium or magnesium or cobalt or copper or selenium or iodine or flouride or chloride #32 "beta‐carotene" #33 "alpha‐carotene" #34 thiamine #35 riboflavin #36 niacin #37 biotin #38 pantothenic #39 nicotinamide #40 pyridoxal #41 "diet* suppl*" #42 #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 #43 #13 and #23 and #42 |
Dec 2014: 395 Jul 2015: 10 Mar 2016: 50 Aug 2016: 48 Mar 2017: 90 Jan 2018: 54 |
| Clinicaltrials.gov (www.clinicaltrials.gov) (Date of most recent search: 25 January 2018) |
In Intervention studies: [intervention] vitamin* OR mineral* OR "diet* suppl*" OR "ascorbic acid" OR "folic acid" OR iron OR calcium OR sodium OR zinc OR potassium OR magnesium OR cobalt OR copper OR iodine AND [condition]: cognition OR "mild cognitive impairment" OR elderly OR "aged subjects" OR "older adults" OR "middle aged" Trial Status: all |
Dec 2014: 147 Jul 2015: 0 Mar 2016: 2 Aug 2016: 0 Mar 2017: 6 Jan 2018: 8 |
| ICTRP Search Portal (http://apps.who.int/trialsearch) (includes: Australian New Zealand Clinical Trials Registry; ClinicalTrials.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register) (Date of most recent search: 25 January 2018) |
In Intervention studies: [intervention] vitamin* OR mineral* OR "diet* suppl*" OR "ascorbic acid" OR "folic acid" OR iron OR calcium OR sodium OR zinc OR potassium OR magnesium OR cobalt OR copper OR iodine AND [condition]: cognition OR "mild cognitive impairment" OR elderly OR "aged subjects" OR "older adults" OR "middle aged" Trial Status: all |
Dec 2014: 25 Jul 2015: 0 Mar 2016: 2 Aug 2016: 0 Mar 2017: 4 Jan 2018: 2 |
| TOTAL before de‐duplication | Dec 2014: 5071 Jul 2015: 233 Mar 2016: 496 Aug 2016: 304 Mar 2017: 631 Jan 2018: 522 TOTAL: 7257 |
|
| TOTAL after de‐duplication | Dec 2014: 3451 Jul 2015: 200 Mar 2016: 399 Aug 2016: 208 Mar 2017: 522 Jan 2018: 431 TOTAL: 5211 |
|
| TOTAL after first assessment by the Crowd and CDCIG information specialists | Dec 2014: 448 Jul 2015: 81 Mar 2016: 70 Aug 2016: 45 Mar 2017: 43 Jan 2018: 38 TOTAL: 725 |
|
Appendix 3. Dietary intake and recommended daily intake of included vitamins and minerals
| Vitamin or mineral | Daily exposure estimate from food sources, excluding supplements, for men and women in the UK (mg) (Gregory 1990) |
Reference Nutrient Intake (RNI) for adults (= the amount of a nutrient that is enough to ensure that the needs of nearly all a group (97.5%) are being met) set by UK Committee on Medical Aspects of Food and Nutrition Policy (COMA) in 1991 (Food Standards Agency 2003) |
Supplementary doses used in studies included in this review (mg) | |
| Mean | 95%ile | |||
| Folic acid | 0.26 | 0.49 | 0.20 | 0.4 ‐ 5.0 |
| Vitamin B6 | 2.0 | 3.9 | 1.4 (men), 1.2 (women) | 20 ‐ 50 |
| Vitamin B12 | 0.0062 | 0.020 | 0.0015 | 0.4 ‐ 1.0 |
| Vitamin C | 64 | 160 | 40 | 400 |
| Vitamin E | 8.5 (12.69 IU) | 18 (26.87 IU) | Requirement varies widely with diet, no fixed level of intake recommended. COMA concluded that daily intakes of 4mg and 3mg of α‐tocopherol equivalents (5.97 IU and 4.48 IU) could be adequate for men and women respectively (Food Standards Agency 2003) | 330 ‐ 2000 IU (International Units) |
| Chromium | 0.1 (source MAFF 1999) | 0.17 | COMA has set no RNI but suggests intake above 0.025mg/day is adequate for adults.US National Research Council specify an Estmated Safe and Adequate Daily Dietary Intake (ESADDI) of 0.05‐2.0mg/day for adults. |
1.0 |
Data and analyses
Comparison 1. B vitamins versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall cognitive function (MMSE) | 3 | 488 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.23, 1.12] |
| 2 Episodic memory | 3 | 397 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.10, 0.29] |
| 3 Executive function | 3 | 392 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.23, 0.29] |
| 4 Speed of processing | 2 | 173 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.26, 0.34] |
| 5 Quality of life (D‐QOL) | 1 | 138 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.10, 0.10] |
| 6 Functional performance (ADL) | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.35, ‐0.21] |
Comparison 3. Vitamins E and C versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall cognitive function (MMSE) | 1 | 256 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.25, 0.71] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
de Jager 2012.
| Methods | 2‐arm, placebo‐controlled, parallel group, randomised clinical trial, intervention for 2 years (trial short name = VITACOG) | |
| Participants |
Location: Oxford, United Kingdom. Single centre Setting of recruitment and treatment: University of Oxford Sample size:
Participant baseline characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: The B‐vitamin group received TrioBe PlusW containing 0.8 mg folic acid, 0.5 mg vitamin B12 and 20 mg vitamin B6 once daily. Comparator: Vitamin‐free tablets of similar appearance. |
|
| Outcomes |
Outcomes of interest in the review: Overall cognitive functioning: MMSE, (also measured: TICSm) Specific cognitive functioning subdomain: episodic memory: HVLT‐R (Hopkins Verbal Learning Test ‐ Revised with delayed recall) Specific cognitive functioning subdomain: executive functioning: CLOX, (also measured: category fluency) Clinical global impression: global CDR Functional performance: IQCODE Total adverse events Mortality Biomarker: rate of brain atrophy on volumetric MRI |
|
| Source of Funding | Charles Wolfson Charitable Trust, Medical Research Council, Alzheimer’s Research Trust, Henry Smith Charity, Thames Valley Dementias and Neurodegenerative Diseases Research Network of the UK National Institute for Health Research, John Coates Charitable Trust, and the Sidney and Elizabeth Corob Charitable Trust. Recip AB donated vitamins, Axis‐Shield provided assay equipment for homocysteine |
|
| Declaration of Interest | "AD Smith is named as an inventor on three patents held by the University of Oxford on the use of folic acid to treat AD or MCI (US6008221; US6127370; PCT/GB2010/051557); under the University’s rules, he could benefit financially if the patent is exploited. Drs Refsum and Smith report having in the past received speaking honoraria from Recip AB, the company that donated the vitamin tablets, and from Axis‐Shield, who make the equipment used to assay homocysteine." | |
| Notes | VITACOG study, ISRCTN 94410159, Eudract Number: 2004‐001527‐38 ISRCTN trial register entry refers to: Primary outcome measures:
Secondary outcome measures:
Protocol refers to: Primary efficacy endpoints: Clinical: rate of shrinkage of brain assessed by volumetric MRI, change in memory test score. Secondary efficacy endpoints: Diagnosis of dementia by DSM‐IV, IQCODE, change in any of the cognitive test scores. Cognitive tests further defined as MMSE, HVLT, paired associates learning (PAL), CLOX, Trailmaking, category fluency, SDMT, Map search/attention task, TICSm. Safety and other endpoints: Included adverse events, mortality, dropout rates, compliance. Protocol also stated that EQ‐5D (quality of life) and Geriatric Depression Scale will be administered at baseline and 24 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Centralized telephone randomization by independent statisticians was used with full allocation concealment and minimization for age, gender, baseline TICS‐M score and consent for MRI." Comment: Appropriately randomised. |
| Allocation concealment (selection bias) | Low risk | Quote: "Centralized telephone randomization by independent statisticians was used with full allocation concealment and minimization for age, gender, baseline TICS‐M score and consent for MRI." Comment: Appropriate allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants, study partners, and those assessing outcomes were blind to the assignment of intervention". "The placebo group received vitamin‐free tablets of similar appearance". Comment: Measures taken to blind participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Participants, study partners, and those assessing outcomes were blind to the assignment of intervention". Comment: Blinded outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 266 participants starting the intervention, 223 participants (83.8%) completed the second visit 2 years later". Total withdrawals of 20 participants (15%) from placebo group and 23 participants (17.3%) from active treatment group. "Fewer clinical measures at follow‐up (n = 191) compared with cognitive measures (n = 223) due to some study partners being unavailable to complete the CDR and IQCODE". Comment: Loss to follow‐up < 20% over 24 months and well balanced between groups. |
| Selective reporting (reporting bias) | High risk | Cognitive results reported were a subgroup of those specified in the protocol, considered by the authors to be "representative of particular cognitive domains important in MCI." Diagnosis of dementia by DSM‐IV and EQ‐5D (quality of life) not reported. Cognitive results were reported by subgroups of participants with high or low tHcy rather than as an overall effect; not specified in analysis plan in published protocol (journals.plos.org/plosone/article?id=10.1371/journal/pone.0012244#s5). |
| Other bias | Low risk | No other risks identified. |
Eussen 2006.
| Methods | 3‐arm, parallel group, randomised, placebo‐controlled trial, 24 week's duration. | |
| Participants |
Location: The Netherlands. Setting of recruitment and treatment: Free‐living older persons and older persons living in care‐facility homes, recruited via mailed health questionnaires. Sample size
"Randomization was stratified according to MMA concentration at the screening visit (< and > 0.45 µmol/L), age (< and > 80), sex and MMSE score (< and > 24 points)." Participant baseline characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: 1) Vitamin B12: 1000 µg vitamin B12 (cyanocobalamin) per day orally for 24 weeks. 2) Vitamin B12 and folic acid: 1000 µg vitamin B12 (cyanocobalamin) plus 400 µg folic acid per day orally for 24 weeks. Comparator: Placebo capsule. The placebo capsules contained AVICEL PH102 (Medipulp GmbH, Aschaffenburg, Germany) as a filler. Use of additional interventions (common to both treatment arm): not reported. |
|
| Outcomes |
Cognitive function Cognitive function was assessed before and after 24 wk of treatment with the use of an extensive neuropsychologic test battery that included the domains of attention, construction, sensorimotor speed, memory, and executive function. Cognitive function was assessed by 6 trained and registered neuropsychologists during the run‐in period (baseline) and at week 24 of the intervention during a 1.5 to 2 hour session. Neuropsychological test battery included:
Depression measured with Geriatric Depression Scale Biochemical measures: vitamin B12, methylmalonic acid, holotranscobalamin, homocysteine, red blood cell folate. |
|
| Source of Funding | Study supported by grants from ZON‐MW, The Hague, Netherlands; Kellogg's Benelux, Zaventem, Belgium; Foundation to Promote Research into Functional Vitamin B12 Deficiency and the European Union BIOMED Demonstration Project; Nutricia Health Foundation, Wageningen, Netherlands. | |
| Declaration of Interest | ||
| Notes | Compliance was checked by counting the number of unused capsules remaining in capsule dispensers and by verifying pill counts in the participants’ diaries. Mean compliance was 99%. Professor Simone Eussen and Professor Lisette de Groot kindly provided data separately on participants with CDR 0.5 at baseline for inclusion in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized", no further information. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: "The capsules given to the separate treatment groups were identical in appearance, smell and taste." "The study had a double‐blind design." Comment: participants and personnel blind to allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study had a double‐blind design." No specific mention of outcome assessors. Comment: outcome assessors probably blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data varied between cognitive tests. For included outcomes, data available for minimum of 82% of participants. In study as a whole, "16% ... were unable to complete the trial, mostly because of illness, and the dropout rate was slightly higher in the vitamin B12 + folic acid group than in the other groups." Dropout in whole study: 10/64 B12, 15/66 B12 + folic acid, 8/65 placebo. Comment: major effect of differential dropout unlikely. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methods fully reported. |
| Other bias | Low risk | No other biases identified. |
Fan 2017.
| Methods | 2‐arm, parallel group, randomised, controlled trial, 6 months duration. | |
| Participants |
Location: China, Shandong (Weifang), single centre. Setting of recruitment and treatment: community. Sample size
Participant baseline characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: 400 µg folic acid per day orally for 6 months. Comparator group: No intervention, maintenance of their usual lifestyle and eating habits. Use of additional interventions (common to both treatment arm): not reported. |
|
| Outcomes | Overall cognitive function: MMSE (also measured: MoCA). Functional performance: Activities of Daily Living (ADL) scale. |
|
| Source of Funding | Not reported. | |
| Declaration of Interest | No declarations. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table “按随机数字表法将受试对象随机分为对照组和干预组,每组40例。” (page 4161) |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. On the first day of each month, subjects in the experimental group were given a bottle of folic acid (400μg/tablet, 30 tablets/bottle) by research staff in community. They were instructed to take one tablet per day. Research staff contacted participants regularly and recorded the usage of folic acid in the intervention group. No mention of contact with comparator group. “干预组每月第1天由社区服务站相关人员统一发放规格为每片400μg的叶酸1瓶(30片),每天服用1片,持续6个月,定期回访,询问叶酸服用情况并记录。” “对照组不进行任何干预,维持原有的生活、饮食习惯。” (page 4161) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 75/80 (94%) completed trial. 3 dropped out in control arm, and 2 dropped out in intervention arm. 2 cases excluded for missing more than 5 doses, 3 cases voluntary dropout, group allocations not reported. Results reported only for the 75 participants who completed the trial. |
| Selective reporting (reporting bias) | Low risk | Results were reported for all outcomes mentioned in the Methods section of paper. No protocol identified. |
| Other bias | Low risk | No other sources of bias identified. |
Krikorian 2010.
| Methods | 2‐arm, placebo‐controlled, randomised, controlled trial, duration 12 weeks | |
| Participants |
Location: Cincinnati, Ohio, USA. Single centre. Setting of recruitment and treatment: Recruitment via advertisement for volunteers with mild memory problems. Study conducted at Department of Psychiatry and Neuroscience, University of Cincinnati College of Medicine. Sample size:
Participant (Baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Active intervention: Chromium picolinate (CrPic) containing 1000 mcg elemental chromium once daily for 12 weeks. Comparator: Placebo once daily. |
|
| Outcomes |
Outcomes of interest in the review: Specific cognitive functioning subdomain: episodic memory: California Verbal Learning Test (CVLT) |
|
| Source of Funding | Funding and material support for this research was provided by Nutrition 21, Inc., Purchase, NY, USA. | |
| Declaration of Interest | No declarations provided. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Enrolled subjects were randomly assigned in a double‐blind manner to receive chromium picolinate (CrPic), containing 1000 mcg elemental chromium, or placebo for 12 weeks". Comment: Insufficient information about sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method of allocation concealment not described in the paper. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind". Comment: Insufficient information regarding the blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind" Comment: Insufficient information in the paper for judgement on the level of risk. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: The paper did not mention any participants lost to follow‐up or missing data. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes mentioned in Methods section reported. |
| Other bias | Low risk | No other risks identified. |
Naeini 2014.
| Methods | 2‐arm, parallel group, randomised, controlled trial, 1 year duration | |
| Participants |
Location: Iran. Single centre. Setting of recruitment and treatment: Tehran University, School of Nutritional Sciences and Dietetics. Sample size:
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Active intervention: 300 mg of vitamin E (Dl‐alpha‐ tocopherol acetate) plus 400 mg vitamin C (ascorbic acid) daily for a year. Comparator: Placebo. |
|
| Outcomes |
Outcomes of interest in the review: Overall cognitive function: MMSE. |
|
| Source of Funding | This study was supported by Institute of Nutritional Sciences, University of Vienna and the Vice‐chancellor for Research, Tehran University of Medical Sciences (TUMS), Iran, by a Grant (No. 11126). | |
| Declaration of Interest | "The authors declare that there are no conflicts of interest". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The MCI subjects were divided to two groups according to their gender (male or female), each of them were further divided to three age groups including 60‐65, 65‐70, and 70‐75 years. The subjects within each of these six groups were then further divided to two equally numbered supplemented or placebo subgroups by simple randomization". Comment: Insufficient information about sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information – allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as "double‐blind". Quote: “One group consumed 300 mg of vitamin E (Dl‐alpha‐ tocopherol acetate) plus 400 mg vitamin C (ascorbic acid) per day, and the second group consumed placebo with the identical condition over the 1‐year intervention period". Comment: Unclear from this whether appearance of supplements and placebo were identical. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Evaluation of cognitive function by MMMSE was blindly performed by an expert psychologist." Comment: Blinded outcome assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “Of the 761 volunteers, 296 were found to have MCI and selected for the next part of the study. From these, 40 did not continue with the study due to the problems tolerating the supplementations (14 subjects), 1 subject died, and 25 subjects abstained to continue participation due to personal reasons. 148 were randomised to each group and 256 subjects completed the study.” 21/148 in the vitamins group and 19/148 in the placebo group did not complete. Comment: The participants who did not complete the trial (40/296, 13.5%) were not included in the analysis. Reason for exclusion was not described based on the allocated group. |
| Selective reporting (reporting bias) | Low risk | Comment: Outcomes specified in the methods section were reported. No access to trial protocol. |
| Other bias | Low risk | No other risks identified. |
Petersen 2005.
| Methods | 3‐arm, parallel group, randomised, controlled trial, duration 3 years | |
| Participants |
Location: United States and Canada. Setting of recruitment and treatment: 69 ADCS sites in the United States and Canada, between March 1999 and January 2004. Sample size:
Participant baseline characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Active intervention: 2000 IU of vitamin E and multivitamin containing 15 IU of vitamin E daily. Intial vitamin E dose was 1000 IU daily, increased to 2000 IU (1000 IU twice daily) after 6 weeks. Comparator group: Placebo vitamin E and multivitamin containing 15 IU of vitamin E daily. Note: There was a third arm in which participants received donepezil. Both groups of interest in this review received placebo donepezil. |
|
| Outcomes |
Outcomes of interest in the review: Progression to possible or probable Alzheimer’s disease, defined according to the clinical criteria of the National Institute of Neurological and Communicative Diseases and Stroke and the Alzheimer’s Disease and Related Disorders Association. Overall cognitive function: MMSE (also measured: ADAS‐cog). Specific cognitive subdomain: episodic memory: standardised composite z‐score incorporating ADAS immediate and delayed word‐recall scores and the New York University immediate and delayed paragraph‐recall scores. Specific cognitive subdomain: executive function: standardised composite z‐score incorporating the digits‐backward test, Symbol Digit Modalities Test, and number‐cancellation test. Clinical global impression: CDR sum of boxes, Global Deterioration Scale. Functional performance: ADCS Mild Cognitive Impairment Activities of Daily Living Scale. Adverse events: rates of adverse events that occurred in at least 5% of subjects in the donepezil or vitamin E group and at least two times in the placebo group during the double‐blind phase. |
|
| Source of Funding | Supported by a grant from the National Institute on Aging (UO1 AG10483) (50% of funding), and by Pfizer and Eisai (50% of funding). DSM Nutritional Products donated the vitamin E. | |
| Declaration of Interest | Most authors were either employees of a pharmaceutical company funding the study, or had received fees for various engagements from pharmaceutical companies. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used an adaptive allocation scheme for the treatment assignment, with the MMSE score, age, and APOE ϵ4 status as balancing covariates. Comment: Adequate sequence generation in the study. |
| Allocation concealment (selection bias) | Low risk | Comment: Insufficient information given. Should be adequate in a large multicentre well designed study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “In this multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study, which was conducted between March 1999 and January 2004”. Comment: Insufficient information on the process of double‐blinding. Should be adequate in a large multicentre RCT. Placebos used and identical treatment regimens in all groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A data and safety monitoring board reviewed the blinded safety data every three months during the trial.".. "verification by a central review committee that a participant met these clinical criteria for Alzheimer’s disease..." Comment: Should be adequate in a large trial with safety and central review committees for main outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 185/257 (72%) in intervention and 193/259 (75%) completed review. Comment: More than 25% dropout from each arm. |
| Selective reporting (reporting bias) | High risk | No protocol identified. Cognitive results reported as composite z‐scores, individual test results not reported. Number of participants in each analysis not reported. |
| Other bias | Low risk | No other potential biases detected. |
Ting 2017.
| Methods | Substudy of the VITATOPS trial, a randomised, 2‐arm, parallel group, placebo‐controlled trial. | |
| Participants |
Location: VITATOPS was an international, multicentre (20 countries from 4 continents). This substudy took place in a single study centre of the VITATOPS trial in Singapore. Sample size Number randomised: 8164 participants with recent stroke or TIA randomised in VITATOPS. This substudy was of the 230 subjects with recent lacunar stroke and cognitive impairment no dementia (CIND). 118/230 allocated to B vitamins, 112/230 allocated to placebo. Participant baseline characteristics:
Inclusion criteria: "patients presenting within 7 months of stroke (ischaemic or haemorrhagic) or TIA (eye or brain), as defined by standard criteria, are eligible" for VITATOPS. For this substudy, which took place in a single VITATOPS centre, "patients with recent lacunar stroke and cognitive impairment no dementia (CIND) who were followed up every 6 months for 1 to 5 years as per VITATOPS trial protocol were included. CIND was defined as impairment in at least one domain of the neuropsychological test battery using education adjusted cut‐off values of 1.5 SDs below the established normal means on individual tests." Patients who consented for extended cognition study. Exclusion criteria:
|
|
| Interventions |
Intervention: Folic acid 2 mg, vitamin B6 25 mg, vitamin B12 500 μg once daily. Comparator: Placebo. |
|
| Outcomes | "Neuropsychological tests results focusing on attention and executive functions derived from a standardized cognitive assessment battery that validated for Singaporean elderly was analysed." Standardized MMSE; Digit span forward; Digit span backward; Visual memory span forward; Visual memory span backward; Category naming ‐ animals; Digit cancellation; Frontal assessment battery. Other: serum homocysteine. |
|
| Source of Funding | Singapore Biomedical Research Council and Singapore National Medical Research Council. | |
| Declaration of Interest | 6/8 authors: no disclosures. "Dr Chen has received support from the Biomedical Research Council, Singapore for current study of vitamin therapy in the prevention of dementia and cognitive deterioration following stroke from 2004 to 2008." "Dr EK Tan has received support from the National Medical Research Council, Duke NUS Graduate Medical school, and has received honoraria for duties as an editor of European Journal of Neurology (Wiley publisher) and Parkinsonism Related Disorders (Elsevier Publisher), and sponsorship from Novartis Pharmaceuticals, GSK Pharmaceuticals and Lundbeck Pharmaceuticals." | |
| Notes | 5 of 235 potentially eligible subjects did not consent to participate in this extended cognition substudy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Parent study (VITATOPS), quote: "a central 24‐hour telephone service or an interactive website ... uses random permuted blocks stratified by hospital to allocate a treatment pack number." |
| Allocation concealment (selection bias) | Low risk | Parent study (VITATOPS), quote: "a central 24‐hour telephone service or an interactive website ... uses random permuted blocks stratified by hospital to allocate a treatment pack number." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind". Parent study (VITATOPS), quote "the tablets being either vitamin supplements or matching placebo". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No specific information about site investigators. Likely blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "The number of the study participants at the end of each study year from year one till year five for active and placebo group was 97, 83, 73, 45, 33 and 93, 75, 59, 38, 27 respectively." |
| Selective reporting (reporting bias) | Unclear risk | Quote: "Neuropsychological tests (sic) results focusing on attention and executive functions derived from a standardized cognitive assessment battery that validated (sic) for Singaporean elderly was analysed." No protocol for substudy identified. Not clear if any outcomes were measured in addition to those reported. |
| Other bias | Low risk | No other sources of bias identified. |
van Uffelen 2008.
| Methods | The FACT study (Folate physical Activity Cognition Trial). 2 x 2 factorial, randomised controlled trial (RCT) comparing the effects of (1) a walking programme with a placebo activity programme, and (2) vitamin B supplementation with placebo supplementation over 1 year. |
|
| Participants |
Location: Netherlands. Setting of recruitment and treatment: Community setting in a Dutch town. Sample size:
Participant baseline characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Active intervention: 5 mg folic acid, 0.4 mg vitamin B‐12 (cyanocobalamin) and 50 mg vitamin B‐6 (pyridoxine hydrochloride) once daily for a year. Comparator: Placebo. |
|
| Outcomes |
Outcomes of interest in the review: Overall cognitive functioning: MMSE; Specific cognitive functioning subdomain: episodic memory: Auditory Verbal Learning Test (AVLT); Specific cognitive functioning subdomain: executive functioning: Stroop Colour Word Test‐Abridged (SCWT‐A), (also measured: letter fluency); Specific cognitive functioning subdomain: speed of processing: Digit Symbol Substitution Test (DSST); Quality of life: Dementia ‐ quality‐of‐life (D‐QOL), (also measured: health‐related QoL with SF‐12); Adverse events. |
|
| Source of Funding | Funded by Body@Work, Research Center Physical Activity, Work and Health, TNO‐VU University Medical Center. External financial support was obtained from the municipality of Alkmaar and the ‘‘Stichting Fonds voor het Hart’’. VIATRIS provided the FA/B12/B6 pills and placebo pills. |
|
| Declaration of Interest | "None of the external sources had input into protocol development, data collection, analyses, and interpretation or drafting this manuscript. | |
| Notes | International Standard Randomised Controlled Trial Number Register (ISCTRN) 19227688 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: After the baseline interview, participants were randomised using the option ‘‘random sample of cases’’ in SPSS. Comment: Computer software used for randomisation leading to adequate sequence generation in the study. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Participants and exercise instructors were blinded to group allocation by being left unaware of which exercise programme was supposed to be effective. The pills were coded as A or B by the manufacturer. The key was decoded after data analysis". Comment: Further information required. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Pills were coded A or B by manufacturer and then decoded only after data analysis". Comment: Participants and personnel were blinded to the intervention a participant received. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All cognitive outcome measures were assessed by trained examiners, who were also blinded to group allocation". Comment: Low risk of detection bias as the examiners were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "For both interventions, data were analysed on a modified intention‐to‐treat basis, including participants with at least one post‐baseline assessment". Comment: All participants accounted for and data analysed from all participants with an intention‐to‐treat method. |
| Selective reporting (reporting bias) | Low risk | Quote: "The walking program and/or FA/B12/B6 supplementation were not effective in improving cognition within one year". Comment: The only outcome measure tested was cognitive function and this was included in the results. |
| Other bias | Low risk | No other potential biases detected. |
AD: Alzheimer's disease ADAS‐cog: Alzheimer's Disease Assessment Scale ‐ cognitive ADCS: Alzheimer's Disease Cooperative Study ADL: Activities of Daily Living APOE ϵ4: Apolipoprotein‐E gene, ϵ4 allele AVLT: Auditory Verbal Learning Test CAMDEX: Cambridge Mental Disorders of the Elderly Examination CDR: Clinical Dementia Rating scale CIND: Cognitive Impairment ‐ no dementia CLOX: Clock drawing executive test CrPic: Chromium picolinate CT: Computerised tomography CVLT: California Verbal Learning Test D‐QOL: Dementia Quality of Life questionnaire DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition DSST: Digit Symbol Substitution Test EQ‐5D: Euroqol‐5D: a standardized instrument for use as a measure of health outcome FA: Folic acid GARS: Groningen Activity Restriction Scale HVLT: Hopkins Verbal Learning Test holoTC: Holotranscobalamin IQCODE: Informant Questionnaire on Cognitive Decline in the Elderly IQR: Interquartile range ISRCTN: International Standard Randomised Controlled Trial Number MCI: Mild cognitive impairment MMA: Methylmalonic acid MMSE: Mini‐Mental State Examination MoCA: Montreal Cognitive Assessment MRI: Magnetic resonance imaging PAL: Paired Associate Learning QoL: Quality of life RBC: Red blood cell SCWT‐A: Stroop Color‐Word Test ‐ Abridged SDMT: Symbol Digit Modalities Test SF‐12: 12‐item Short Form Survey TIA: Transient Ischaemic Attack TICS28: 28‐item Telephone Interview for Cognitive Status TICSm: Modified Telephone Interview for Cognitive Status vit: vitamin WAIS: Wechsler Adult Intelligence Scale wk: week 10 WLT: 10 Word Learning Test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbasi 2013 | No cognitive outcomes. |
| Anand 2011 | Wrong population. Participants cognitively healthy. |
| Andreeva 2011 | Wrong population. Participants cognitively healthy. |
| Anonymous 2008 | Wrong design. Not an RCT. |
| Arwert 2003 | Ineligible intervention. Duration of intervention three weeks. |
| Benton 1995 | Wrong population. Participants cognitively healthy. |
| Bryan 2002 | Ineligible intervention. Duration of intervention five weeks. |
| Chan 2010 | Ineligible intervention. Combined amino acids and vitamins. |
| Chandra 2001 | Other reason: retracted report. |
| Clarke 2003 | Wrong population. Approximately 2/3 of participants had a diagnosis of dementia. |
| Cockle 2000 | Wrong population. Participants cognitively healthy. |
| Corless 1987 | Wrong population. |
| Dangour 2011 | Wrong population. Participants were cognitively healthy. |
| Durga 2007 | Wrong population. Participants were cognitively healthy. |
| Ford 2008 | Wrong population. Participants were cognitively healthy. |
| Ford 2010 | Wrong population. Participants were cognitively healthy. |
| Grodstein 2007 | Wrong population. Participants were cognitively healthy. |
| Grodstein 2013 | Wrong population. Participants were cognitively healthy. |
| Hankey 2013 | Wrong population. Participants were cognitively healthy (after stroke). |
| Harris 2012 | Wrong intervention: included probiotics and herbal extracts. |
| Heart Protection Study Collaborative Group 1999 | Wrong population. Participants were cognitively healthy. |
| Hvas 2004 | Wrong intervention. Duration of intervention was four weeks; administered parenterally. |
| Kang 2006 | Wrong population. Participants were cognitively healthy. |
| Kang 2008 | Wrong population. Participants were cognitively healthy. |
| Kang 2009 | Wrong population. Participants were cognitively healthy. |
| Kesse‐Guyot 2011 (SUVIMAX trial) | Wrong population. Participants were cognitively healthy. |
| Kryscio 2017 | Wrong population. Participants were cognitively healthy. |
| Kwok 2011 | Wrong population. Participants had dementia. |
| Kwok 2017 | Wrong population: Participants were mixed population with CDR 0 or CDR 0.5 at baseline. |
| Lewerin 2005 | Wrong population. Participants were cognitively healthy. |
| Loriaux 1985 | Ineligible intervention. Duration of treatment was eight weeks. |
| Macpherson 2012 | Wrong population: elderly women who responded 'yes' to the single question "Do you feel like your memory is getting worse?" Wrong intervention: "multivitamin, antioxidant and mineral formula with added herbal and antioxidant plant extracts." |
| Maniam 2004 | Wrong population: probably healthy older people. Only an abstract has been published since 2004. |
| Maylor 2006 (ZENITH study) | Wrong population. Participants were cognitively healthy. |
| McMahon 2006 | Wrong population. Participants were cognitively healthy. |
| McNeill 2007 | Wrong population. Participants probably mainly cognitively healthy although no baseline assessment conducted. |
| Murray‐Kolb 2011 | Wrong population. Participants were cognitively healthy. |
| NCT00903695 | Wrong intervention. Intervention consisted of vitamins and amino acids. |
| NCT01095211 | Wrong intervention. Duration of Intervention 45 days. |
| NCT01317849 | Withdrawn (status posted on ClinicalTrials.gov on August 20, 2014) |
| NCT01708005 | Wrong intervention. Intervention contained PUFAs and grape extract as well as vitamins and minerals. |
| NCT02467153 | Wrong outcomes. |
| Pase 2015 | Wrong intervention. Intervention included fatty acids. |
| Pathansali 2006 | Wrong population. Participants were cognitively healthy. Wrong intervention. Duration of treatment four weeks. |
| Rietsema 2014 | Wrong design. Case report. |
| Rossom 2012 | Wrong population. Participants were cognitively healthy. |
| Sanchez 2011 | Wrong population. Participants were "apparently healthy", mean MMSE 26.7 ± 2.7. |
| Satalich 2014 | Wrong design. |
| Smith 1999 | Wrong population: excluded if MMSE < 18, but no information on mean MMSE; likely to have included healthy participants and participants with dementia. |
| Summers 2010 | Wrong intervention. Intervention included components other than vitamins and minerals. |
| van der Zwaluw 2014 | Wrong population. Participants were cognitively healthy. |
| Walker 2012 | Wrong population. Participants were cognitively healthy. |
| Wolters 2005 | Wrong population. Participants were cognitively healthy. |
| Wouters‐Wesseling 2005 | Wrong intervention. Intervention included components other than vitamins and minerals. |
| Yaffe 2004 | Wrong population. Participants were cognitively healthy. |
CDR: Clinical Dementia Rating scale MMSE: Mini‐Mental State Examination PUFA: Polyunsaturated Fatty Acid
Characteristics of studies awaiting assessment [ordered by study ID]
ACTRN12607000321448.
| Methods | Randomised placebo controlled trial. |
| Participants |
Location: Australia. Single centre. Setting of recruitment and treatment: WA Centre for Health & Ageing, University of Western Australia. Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention : 1000 IU vitamin D daily by oral tablet. Comparator group: Placebo (soyabean oil tablet indistinguishable from active tablet). |
| Outcomes |
Outcomes of interest in the review: Overall cognitive functioning: Cambridge Examination for Mental Disorders in the Elderly ‐ Cognitive section (CAMCOG); Specific cognitive functioning subdomain: executive function: digit‐symbol coding task; Specific cognitive functioning subdomain: episodic memory: California Verbal Learning Scale‐Revised; Development of dementia; Quality of life: SF‐12. |
| Reason awaiting classification | Only publication of baseline results and protocol found. No results available after contact with authors. |
| Notes | National Health and Medical Research Council Dementia Research Grants Program |
Jiang 2014.
| Methods | Randomised controlled trial. No information on randomisation methods. |
| Participants | "120 patients with VCIND complicated by HHcy were randomly selected". All were "patients with cerebral apoplexy that received treatment at the First Hospital Affiliated to the Chinese PLA General Hospital". |
| Interventions | Experimental intervention: "5 mg of extra folic acid per day and 500 mcg of mecobalamin thrice per day for 24 weeks, apart from conventional treatment." Control group: presumed conventional treatment only (not specified). |
| Outcomes | Montreal Cognitive Assessment (MoCA) |
| Reason awaiting classification | Multiple pieces of information sought from authors about methods and results. Unusual age profile of participants. First email sent 19/05/17. No response received by 23/05/18. |
| Notes |
Ma 2017.
| Methods | Randomised controlled trial. No information on randomisation methods other than "random cluster sampling." |
| Participants | 180 community‐dwelling people with MCI (modified Petersen's criteria) in the Binhai New District, Tainjin, China. |
| Interventions | Experimental intervention: Folic acid 400 mcg daily. Control intervention: conventional treatment. |
| Outcomes | Chinese version of Wechsler Adult Intelligence Scale ‐ Revised (WAIS‐RC). |
| Reason awaiting classification | Multiple pieces of information sought from authors about methods, sample size, and results. No response received by 23/05/18. |
| Notes | Outcomes at different time points (6, 12, and 24 months) are reported in three separate papers. |
CAMCOG: Cambridge Cognition Examination HHcy: high homocysteine MCI: Mild cognitive impairment MoCA: Montreal cognitive assessment SF‐12: 12‐item Short Form Survey VCIND: Vascular cognitive impairment (no dementia) WAIS‐RC: Wechsler Adult Intelligence Scale ‐ Revised (China)
Characteristics of ongoing studies [ordered by study ID]
NCT02185222.
| Trial name or title | Effect of vitamin D on cognitive decline of patients with memory complaint (trial short name = D‐COG). |
| Methods | RCT, triple‐blind. |
| Participants | Aged 60+; both genders; "report to a memory centre with symptoms of memory complaint"; MMSE score "> the 5th percentile for sociocultural level of the patient", no dementia. |
| Interventions | Experimental: Cholecalciferol 100 000 IU per month as a single dose, administered as oral solution. Control: placebo. Duration: 2 years. |
| Outcomes | Primary outcome: change from baseline on total recall test from Free and Cued Selective Reminding Test. Secondary outcomes: multiple general and domain‐specific cognitive tests; all adverse events; several biochemical outcomes. |
| Starting date | July 2014 |
| Contact information | Sponsored by University Hospital, Tours. Contact fanny.hennekinne@univ‐tours.fr |
| Notes | Estimated completion date July 2018. |
MMSE: Mini‐mental state examination
Differences between protocol and review
In the protocol, overall cognitive functioning was a secondary outcome. In the review, we have made it a primary outcome. This is because studies were selected for inclusion if they reported either the incidence of dementia or a continuous cognitive function measure at follow‐up. It was an objective of the review, reflected in the title, to assess both of these as the key outcomes.
After the publication of the protocol, we added the exclusion of study populations with severe vitamin or mineral deficiency where the intervention given could correct these deficiencies. However, we included studies of participants with mild vitamin deficiencies which are common in the older population.
In the case of studies of B vitamins, we added baseline serum homocysteine level as a potential effect modifier and decided to report effects in subgroups distinguished by baseline serum homocysteine.
Contributions of authors
Completion of the protocol: Rutjes AWS, Chong LY, Al‐Assaf AS, Malik MA, Tabet N, Abraham RP, Denton DA,
Completion of the searches: Noel‐Storr A
Screening references and full‐text assessment: Noel Storr A, Griffith D, Rafeeq S, Yaman H, Chong LY, Abraham RP, Denton DA, Al‐Assaf AS, Malik MA, Tabet N, McCleery J, Martinez Fuentes G
Acquisition of data: Abraham RP, Denton DA, Al‐Assaf AS, Malik MA, Di Nisio, M, Tabet N, LY Chong, McCleery J, Martinez Fuentes G
'Risk of bias' assessment: Abraham RP, Denton DA, Rutjes AWS, Chong LY, Al‐Assaf AS, Malik MA, Di Nisio, M, Tabet N, McCleery J, Martinez Fuentes G
Statistical analysis: McCleery J, Abraham RP, Denton DA, Rutjes AWS, Chong LY, Al‐Assaf AS, Malik MA
Overall interpretation of data: McCleery J, Abraham RP, Denton DA, Rutjes AWS, Chong LY, Al‐Assaf AS, Malik MA, Di Nisio, M, Tabet N
Manuscript preparation: McCleery J, Abraham RP, Denton DA, Chong LY, Rutjes AWS, Al‐Assaf AS, Malik MA, Di Nisio, M, Tabet N
Sources of support
Internal sources
No sources of support supplied
External sources
-
NHS, UK.
This protocol/review was supported by the National Institute for Health Research, via a Cochrane Programme Grant to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
Declarations of interest
Jenny McCleery ‐ none known Rajesh P Abraham ‐ none known David A Denton ‐ none known Anne WS Rutjes ‐ none known Lee‐Yee Chong ‐ none known Aalya S Al‐Assaf ‐ none known Daniel J Griffith ‐ none known Shireen Rafeeq ‐ none known Hakan Yaman ‐ none known Muzaffar A Malik ‐ none known Marcello Di Nisio ‐ Di Nisio reports participation to Advisory Boards for Daiichi‐Sankyo, Aspen and Pfizer, and consultancy fees for Daiichi‐Sankyo and Bayer Health Care outside the submitted work. Gabriel Martínez ‐ none known Robin WM Vernooij ‐ none known Naji Tabet ‐ none known
This author contributed equally to this work
This author contributed equally to this work
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
de Jager 2012 {published data only}
- Douaud G, Refsum H, Jager CA, Jacoby R, Nichols TE, Smith SM, et al. Preventing Alzheimer's disease‐related gray matter atrophy by B‐vitamin treatment. Proceedings of the National Academy of Sciences of the United States of America 2013;110(23):9523‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD. Disease‐modification in mild cognitive impairment by lowering homocysteine. Neurobiology of Aging 2012;33(Suppl 1):S32. [Google Scholar]
- Smith AD, Smith SM, Jager CA, Whitbread P, Johnston C, Agacinski G, et al. Homocysteine‐lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLOS One 2010;5(9):e12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager CA, Oulhaj A, Jacoby R, Refsum H, Smith AD. Cognitive and clinical outcomes of homocysteine‐lowering B‐vitamin treatment in mild cognitive impairment: a randomized controlled trial. International Journal of Geriatric Psychiatry 2012;27(6):592‐600. [DOI] [PubMed] [Google Scholar]
Eussen 2006 {published data only}
- Eussen S, Ueland P, Clarke R, Blom H, Hoefnagels W, Staveren W, et al. The association of betaine, homocysteine and related metabolites with cognitive function in Dutch elderly people. British Journal of Nutrition 2007;98(5):960‐8. [DOI] [PubMed] [Google Scholar]
- Eussen SJ, Groot LC, Joosten LW, Bloo RJ, Clarke R, Ueland PM, et al. Effect of oral vitamin B‐12 with or without folic acid on cognitive function in older people with mild vitamin B‐12 deficiency: a randomized, placebo‐controlled trial. American Journal of Clinical Nutrition 2006;84(2):361‐70. [PUBMED: 16895884] [DOI] [PubMed] [Google Scholar]
- NCT00111267. Oral vitamin B12 supplementation and cognitive performance in elderly people. clinicaltrials.gov/ct2/show/NCT00111267 (first received 18 May 2005).
Fan 2017 {published data only}
- Fan J, He B, Sun F. Influence of folic acid intervention on cognitive function of community patients with mild cognitive impairment. Chinese Nursing Research 2017;31(32):4161‐3. [Google Scholar]
Krikorian 2010 {published data only}
- Krikorian R, Eliassen JC, Boespflug EL, Nash TA, Shidler MD. Improved cognitive‐cerebral function in older adults with chromium supplementation. Nutritional Neuroscience 2010;13(3):116‐22. [DOI] [PubMed] [Google Scholar]
Naeini 2014 {published data only}
- Naeini AM, Elmadfa I, Djazayeri A, Barekatain M, Aghayeghazvini MR, Jalali M, et al. Effect of vitamin E and C supplementation on elderly with mild cognitive impairment (MCI) in Isfahan, Iran. Annals of Nutrition & Metabolism 2013;63(Suppl 1):1145. [Google Scholar]
- Naeini AM, Elmadfa I, Djazayery A, Barekatain M, Ghazvini MR, Djalali M, et al. The effect of antioxidant vitamins E and C on cognitive performance of the elderly with mild cognitive impairment in Isfahan, Iran: a double‐blind, randomized, placebo‐controlled trial. European Journal of Nutrition 2014;53(5):1255‐62. [DOI] [PubMed] [Google Scholar]
Petersen 2005 {published data only}
- Blacker D. Neither vitamin E nor donepezil delays progression from amnestic mild cognitive impairment to Alzheimer's disease in the long term. Evidence‐Based Mental Health 2006;9(1):20. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Sowell BB, Taylor C, Gamst AC, Petersen RC, Thal LJ, et al. Clinical predictors of progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology 2007;68(19):1588‐95. [DOI] [PubMed] [Google Scholar]
- Grundman M. Vitamin E and Alzheimer disease: the basis for additional clinical trials. The American Journal of Clinical Nutrition February 1, 2000;71(2):630S‐636S. [DOI] [PubMed] [Google Scholar]
- Petersen R, Grundman M, Thomas R, Thal L. Donepezil and vitamin E as treatments for mild cognitive impairment. Neurobiology of Aging 2004;25(Supplement 2):S20. [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. The New England journal of medicine 2005;352(23):2379‐88. [DOI] [PubMed] [Google Scholar]
Ting 2017 {published data only}
- NCT00097669. VITATOPS: a study of vitamins to prevent stroke. clinicaltrials.gov/ct2/show/NCT00097669 (first received 24 November 2004).
- Ting S, Earnest A, Li H, Shahul H, Chen C, Tan E‐K. B vitamins and cognition in subjects with small vessel disease: a substudy of VITATOPS, a randomized, placebo‐controlled trial. Journal of the Neurological Sciences 2017;379:124‐6. [DOI] [PubMed] [Google Scholar]
- VITATOPS Trial Study Group. The VITATOPS (vitamins to prevent stroke) trial: rationale and design of an international, large, simple, randomized trial of homocysteine‐lowering multivitamin therapy in patients with recent transient ischaemic attack or stroke. Cerebrovascular Diseases 2002;13(2):120‐6. [DOI] [PubMed] [Google Scholar]
van Uffelen 2008 {published data only}
- Uffelen JG, Chinapaw MJ, Hopman‐Rock M, Mechelen W. The effect of walking and vitamin B supplementation on quality of life in community‐dwelling adults with mild cognitive impairment: a randomized, controlled trial. Quality of Life Research 2007;16(7):1137‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffelen JG, Chinapaw MJ, Mechelen W, Hopman‐Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. British Journal of Sports Medicine 2008;42(5):344‐51. [DOI] [PubMed] [Google Scholar]
- Uffelen JG, Hopman‐Rock M, Chinapaw MJ, Mechelen W. Protocol for project FACT: a randomised controlled trial on the effect of a walking program and vitamin B supplementation on the rate of cognitive decline and psychosocial wellbeing in older adults with mild cognitive impairment [ISRCTN19227688]. BMC Geriatrics 2005;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbasi 2013 {published data only}
- Abbasi B, Kimiagar M, Shahidi S, Shirazi MM, Sadeghniiat K, Payab M, et al. Effect of magnesium supplementation on mental health in elderly subjects with insomnia: a double‐blind randomized clinical trial. Iranian Journal of Psychiatry and Clinical Psychology 2013;19(1):9‐19. [Google Scholar]
Anand 2011 {published data only}
- Anand VP, Bhatt JK, Varghese JM, Das SK. Supplementation of vitamin E improves cognitive status and oxidative stress in type 2 diabetes mellitus. International Research Journal of Pharmacy 2011;2(11):169‐72. [Google Scholar]
Andreeva 2011 {published data only}
- Andreeva VA, Kesse‐Guyot E, Barberger‐Gateau P, Fezeu L, Hercberg S, Galan P. Cognitive function after supplementation with B vitamins and long‐chain omega‐3 fatty acids: ancillary findings from the SU.FOL.OM3 randomized trial. American Journal of Clinical Nutrition 2011;94(1):278‐86. [DOI] [PubMed] [Google Scholar]
Anonymous 2008 {published data only}
- Anonymous. High‐dose vitamin B does not slow cognitive decline in AD. Geriatric Psychopharmacology Update 2008;12:3. [Google Scholar]
Arwert 2003 {published data only}
- Arwert LI, Deijen JB, Drent ML. Effects of an oral mixture containing glycine, glutamine and niacin on memory, GH and IGF‐I secretion in middle‐aged and elderly subjects. Nutritional Neuroscience 2003;6(5):269‐75. [DOI] [PubMed] [Google Scholar]
Benton 1995 {published data only}
- Benton D, Fordy J, Haller J. The impact of long‐term vitamin supplementation on cognitive functioning. Psychopharmacology 1995;117(3):298‐305. [DOI] [PubMed] [Google Scholar]
Bryan 2002 {published data only}
- Bryan J, Calvaresi E, Hughes D. Short‐term folate, vitamin B‐12 or vitamin B‐6 supplementation slightly affects memory performance but not mood in women of various ages. Journal of Nutrition 2002;132(6):1345‐56. [DOI] [PubMed] [Google Scholar]
Chan 2010 {published data only}
- Chan A, Remington R, Kotyla E, Lepore A, Zemianek J, Shea TB. A vitamin/nutriceutical formulation improves memory and cognitive performance in community‐dwelling adults without dementia. The journal of nutrition, health & aging. 2010;14(3):224‐30. [DOI] [PubMed] [Google Scholar]
Chandra 2001 {published data only}
- Chandra RK. Effect of vitamin and trace‐element supplementation on cognitive function in elderly subjects. Nutrition 2001;17(9):709‐12 [Retraction in: Nutrition 2005; 21(2):286]. [DOI] [PubMed] [Google Scholar]
Clarke 2003 {published data only}
- Clarke R, Harrison G, Richards S, Vital Trial Collaborative Group. Effect of vitamins and aspirin on markers of platelet activation, oxidative stress and homocysteine in people at high risk of dementia. Journal of Internal Medicine 2003;254(1):67‐75. [DOI] [PubMed] [Google Scholar]
Cockle 2000 {published data only}
- Cockle S, Dawe R, Robinson L, Haller J, Hindmarch I. The effects of repeated doses of vitamins on cognitive function in healthy elderly volunteers. International Journal of Neuropsychopharmacology 1998;1(Suppl 1):245. [Google Scholar]
- Cockle SM, Haller J, Kimber S, Dawe RA, Hindmarch I. The influence of multivitamins on cognitive function and mood in the elderly. Aging & mental health 2000;4(4):339‐53. [Google Scholar]
Corless 1987 {published data only}
- Corless D, Ellis M, Dawson E, Fraser F, Evans S, Perry JD, et al. Using activities of daily living assessments to measure the effectiveness of vitamin D supplements in elderly long‐stay patients. British Journal of Occupational Therapy 1987;50(2):60‐2. [Google Scholar]
Dangour 2011 {published data only}
- Dangour AD, Allen E, Clarke R, Elbourne D, Fasey N, Fletcher AE, et al. A randomised controlled trial investigating the effect of vitamin B12 supplementation on neurological function in healthy older people: the older people and enhanced neurological function (OPEN) study protocol [ISRCTN54195799]. Nutrition Journal 2011;10(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
Durga 2007 {published data only}
- Durga J, Boxtel MP, Shouten EG. Folic acid supplementation improved cognitive function in older adults. Journal of Clinical Outcomes Management 2007;14(3):134‐5. [Google Scholar]
- Durga J, Boxtel MP, Schouten EG, Kok FJ, Jolles J, Katan MB, et al. Effect of 3‐year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet 2007;369(9557):208‐16. [DOI] [PubMed] [Google Scholar]
- Schiepers OJ, Boxtel MP, Groot RH, Jolles J, Kort WL, Swinkels DW, et al. Serum iron parameters, HFE C282Y genotype, and cognitive performance in older adults: results from the FACIT study. The journals of gerontology. Series A, Biological sciences and medical sciences 2010;65(12):1312‐21. [DOI] [PubMed] [Google Scholar]
Ford 2008 {published data only}
- Ford AH, Flicker L, Thomas J, Norman P, Jamrozik K, Almeida OP. Vitamins B12, B6, and folic acid for onset of depressive symptoms in older men: results from a 2‐year placebo‐controlled randomized trial. Journal of Clinical Psychiatry 2008;69(8):1203‐9. [DOI] [PubMed] [Google Scholar]
Ford 2010 {published data only}
- Ford AH, Flicker L, Alfonso H, Thomas J, Clarnette R, Martins R, et al. Vitamins B(12), B(6), and folic acid for cognition in older men. Neurology 2010;75(17):1540‐7. [DOI] [PubMed] [Google Scholar]
Grodstein 2007 {published data only}
- Grodstein F, Kang JH, Glynn RJ, Cook NR, Gaziano M. A randomized trial of beta‐carotene supplementation and cognitive function in men: the Physicians' Health study II. Archives of Internal Medicine 2007;167(20):2184‐90. [DOI] [PubMed] [Google Scholar]
Grodstein 2013 {published data only}
- Christen WG, Gaziano JM, Hennekens CH. Design of Physicians’ Health Study II — a randomized trial of beta‐carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Annals of Epidemiology 2000;10(2):125‐34. [DOI] [PubMed] [Google Scholar]
- Grodstein F, O'Brien J, Kang JH, Dushkes R, Cook NR, Okereke O, et al. Long‐term multivitamin supplementation and cognitive function in men: a randomized trial. Annals of Internal Medicine 2013;159(12):806‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hankey 2013 {published data only}
- Almeida OP, Marsh K, Alfonso H, Flicker L, Davis TM, Hankey GJ. B vitamins reduce the long‐term risk of depression after stroke: the VITATOPS‐DEP trial. Annals of Neurology 2010;68(4):503‐10. [DOI] [PubMed] [Google Scholar]
- Hankey GF, Ford AH, Qilong Yi, Eikelboom JW, Lees KR, Chen C, et al. Effect of B vitamins and lowering homocysteine on cognitive impairment in patients with previous stroke or transient ischemic attack. Stroke 2013;44(8):2232‐39. [DOI] [PubMed] [Google Scholar]
- NCT00097669. VITATOPS: a study of vitamins to prevent stroke. clinicaltrials.gov/ct2/show/NCT00097669 (first received 24 November 2004).
Harris 2012 {published data only}
Heart Protection Study Collaborative Group 1999 {published data only}
- Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol‐lowering therapy and of antioxidant vitamin supplementation in a wide range of patients at increased risk of coronary heart disease death: early safety and efficacy experience. European Heart Journal 1999;20(10):725‐41. [DOI] [PubMed] [Google Scholar]
Hvas 2004 {published data only}
- Hvas AM, Juul S, Lauritzen L, Nexo E, Ellegaard J. No effect of vitamin B‐12 treatment on cognitive function and depression: a randomized placebo controlled study. Journal of Affective Disorders 2004;81(3):269‐73. [DOI] [PubMed] [Google Scholar]
Kang 2006 {published data only}
- Kang JH, Cook N, Manson JA, Buring JE, Grodstein F. A randomized trial of vitamin E supplementation and cognitive function in women. Archives of Internal Medicine 2006;166(22):2462‐8. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomized trial of low‐dose aspirin in the primary prevention of cardiovascular disease in women. New England Journal of Medicine 2005;352(13):1293‐304. [DOI] [PubMed] [Google Scholar]
Kang 2008 {published data only}
- Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA 2008;299(17):2027‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassuk SS, Albert CM, Cook NR, Zaharris E, MacFadyen JG, Danielson E, et al. The women’s antioxidant cardiovascular study: design and baseline characteristics of participants. Journal of Womens Health 2004;13(1):99–117. [DOI] [PubMed] [Google Scholar]
- Kang JH, Cook N, Manson J, Buring JE, Albert CM, Grodstein F. A trial of B vitamins and cognitive function among women at high risk of cardiovascular disease. American Journal of Clinical Nutrition 2008;88(6):1602‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2009 {published data only}
- Kang JH, Cook NR, Manson JE, Buring JE, Albert CM, Grodstein F. Vitamin E, vitamin C, beta carotene, and cognitive function among women with or at risk of cardiovascular disease: the Women's Antioxidant and Cardiovascular Study. Circulation 2009;119(21):2772‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kesse‐Guyot 2011 (SUVIMAX trial) {published data only}
- Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, et al. The SU.VI.MAX study: a randomized, placebo‐controlled trial of the health effects of antioxidant vitamins and minerals. Archives of Internal Medicine 2004;164(21):2335‐42. [DOI] [PubMed] [Google Scholar]
- Hercberg S, Preziosi P, Briançon S, Galan P, Triol I, Malvy D, et al. A primary prevention trial using nutritional doses of antioxidant vitamins and minerals in cardiovascular diseases and cancers in a general population: the SU.VI.MAX study ‐ design, methods, and participant characteristics. Controlled Clinical Trials 1998;19(4):336‐51. [DOI] [PubMed] [Google Scholar]
- Kesse‐Guyot E, Fezeu L, Jeandel C, Ferry M, Andreeva V, Amieva H, et al. French adults' cognitive performance after daily supplementation with antioxidant vitamins and minerals at nutritional doses: a post hoc analysis of the SUpplementation in VItamins and Mineral AntioXidants (SU.VI.MAX) trial. American Journal of Clinical Nutrition 2011;94(3):892‐9. [DOI] [PubMed] [Google Scholar]
Kryscio 2017 {published data only}
- Kryscio RJ, Abner EL, Caban‐Holt A, Lovell M, Goodman P, Darke AK, et al. Association of antioxidant supplement use and dementia in the prevention of Alzheimer's disease by vItamin E and selenium trial (PREADVISE). JAMA Neurology 2017;74(5):567‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwok 2011 {published data only}
- Kwok T, Lee J, Law CB, Pan PC, Yung CY, Choi KC, et al. A randomized placebo controlled trial of homocysteine lowering to reduce cognitive decline in older demented people. Clinical Nutrition 2011;30(3):297‐302. [DOI] [PubMed] [Google Scholar]
Kwok 2017 {published data only}
- Kwok T, Lee J, Ma RC, Wong SY, Kung K, Lam A, et al. A randomized, placebo‐controlled trial of vitamin B12 supplementation to prevent cognitive decline in older diabetic patients with borderline low serum vitamin B12. Clinical Nutrition 2017;36(6):1509‐15. [DOI] [PubMed] [Google Scholar]
- NCT02457507. Vitamin B12 supplement to prevent cognitive decline. clinicaltrials.gov/ct2/show/NCT02457507 (first received 22 may 2015). [Google Scholar]
Lewerin 2005 {published data only}
- Lewerin C, Matousek M, Steen G, Johansson B, Steen B, Nilsson‐Ehle H. Significant correlations of plasma homocysteine and serum methylmalonic acid with movement and cognitive performance in elderly subjects but no improvement from short‐term vitamin therapy: a placebo‐controlled randomized study. American Journal of Clinical Nutrition 2005;81(5):1155‐62. [DOI] [PubMed] [Google Scholar]
- Lewerin C, Nilsson‐Ehle H, Matousek M, Lindstedt G, Steen B. Reduction of plasma homocysteine and serum methylmalonate concentrations in apparently healthy elderly subjects after treatment with folic acid, vitamin B12 and vitamin B6: a randomised trial. European Journal of Clinical Nutrition 2003;57(11):1426‐36. [DOI] [PubMed] [Google Scholar]
Loriaux 1985 {published data only}
- Loriaux SM, Deijen JB, Orlebeke JF, Swart JH. The effects of nicotinic acid and xanthinol nicotinate on human memory. Psychopharmacology 1985;87(4):390‐5. [DOI] [PubMed] [Google Scholar]
Macpherson 2012 {published data only}
- Macpherson H, Ellis KA, Sali A, Pipingas A. Memory improvements in elderly women following 16 weeks treatment with a combined multivitamin, mineral and herbal supplement: a randomized controlled trial. Psychopharmacology 2012;220(2):351‐65. [DOI] [PubMed] [Google Scholar]
Maniam 2004 {published data only}
- Maniam J, Krishnaswamy S, Mohamed J. Randomized double blind placebo controlled human clinical trial investigating effect of 1200 IU alpha‐tocopherol on lipid peroxidation, antioxidant status and cognitive function of elderly. Free Radical Biology & Medicine 2004;36(Suppl):S146. [Google Scholar]
Maylor 2006 (ZENITH study) {published and unpublished data}
- Andriollo‐Sanchez M, Hininger‐Favier I, Meunier N, Toti E, Zaccaria M, Brandolini‐Bunlon M, et al. Zinc intake and status in middle‐aged and older European subjects: the ZENITH study. European Journal of Clinical Nutrition 2005;59(Suppl 2):S37‐41. [PUBMED: 16254579] [DOI] [PubMed] [Google Scholar]
- Coudray C, O'Connor JM, Maiani G, Cashman KD, Simpson EE, Secker DL, et al. Introduction to the ZENITH study and summary of baseline results. European Journal of Clinical Nutrition 2005;59(Suppl 2):S5‐7. [PUBMED: 16254582] [DOI] [PubMed] [Google Scholar]
- Maylor EA, Simpson EEA, Secker DL, Meunier N, Andriollo‐Sanchez M, Polito A, et al. Effects of zinc supplementation on cognitive function in healthy middle‐aged and older adults: the ZENITH study. British Journal of Nutrition 2006;96(4):752‐60. [PubMed] [Google Scholar]
- Meunier N, O'Connor JM, Maiani G, Cashman KD, Secker DL, Ferry M, et al. Importance of zinc in the elderly: the ZENITH study. European Journal of Clinical Nutrition 2005;59(Suppl 2):S1‐4. [PUBMED: 16254574] [DOI] [PubMed] [Google Scholar]
- Simpson EE, Maylor EA, McConville C, Stewart‐Knox B, Meunier N, Andriollo‐Sanchez M, et al. Mood and cognition in healthy older European adults: the Zenith study. BMC Psychology 2014;2(1):11. [PUBMED: 25945252] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EE, Maylor EA, Rae G, Meunier N, Andriollo‐Sanchez M, Catasta G, et al. Cognitive function in healthy older European adults: the ZENITH study. European Journal of Clinical Nutrition 2005;59(Suppl 2):S26‐30. [PUBMED: 16254577] [DOI] [PubMed] [Google Scholar]
McMahon 2006 {published data only}
- Anonymous. Homocysteine and cognitive function in healthy elderly people. Geriatric Psychopharmacology Update 2006;10(11):4‐5. [Google Scholar]
- Cacabelos R. Homocysteine and cognition: from Galenic dogmatism to genetic relativism. Aging Health 2006;2(5):783–6. [Google Scholar]
- Clarke R. Homocysteine‐lowering vitamin B supplements do not improve cognitive performance in healthy older adults after two years. Evidence‐based Mental Health 2007;10(1):27. [DOI] [PubMed] [Google Scholar]
- Clarke R. Vitamin B12, folic acid, and the prevention of dementia. New England Journal of Medicine 2006;354(26):2817‐9. [DOI] [PubMed] [Google Scholar]
- McMahon JA, Green TJ, Skeaff CM, Knight RG, Mann JI, Williams SM. A controlled trial of homocysteine lowering and cognitive performance. New England Journal of Medicine 2006;354(26):2764‐72. [DOI] [PubMed] [Google Scholar]
McNeill 2007 {published data only}
- McNeill G, Avenell A, Campbell MK, Cook JA, Hannaford PC, Kilonzo MM, et al. Effect of multivitamin and multimineral supplementation on cognitive function in men and women aged 65 years and over: a randomised controlled trial. Nutrition Journal 2007;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray‐Kolb 2011 {published data only}
- Murray‐Kolb LE, Wenger MJ, Venkatramanan S, Marquis GS, Wesley AS, Haas JD. Effects of double‐fortified salt on perceptual and cognitive performance in women. FASEB Journal : official publication of the Federation of American Societies for Experimental Biology 2011;25(Suppl 1). [Google Scholar]
NCT00903695 {published data only}
- NCT00903695. Nutriceutical effects on cognitive status in mild cognitive impairment patients. clinicaltrials.gov/show/NCT00903695 (first received 18 May 2009).
NCT01095211 {published data only}
- NCT01095211. B‐vitamins treatment for improvement of cognitive function. clinicaltrials.gov/show/NCT01095211 (first received 30 March 2010).
NCT01317849 {published data only}
- Liu X, Shi M, Xia F, Han J, Liu Z, Wang B, et al. The China Stroke Secondary Prevention Trial (CSSPT) protocol: a double‐blinded randomized controlled trial of combined folic acid and B vitamins for secondary prevention of stroke. International Journal of Stroke 2015; Vol. 10, issue 2:264‐8. [DOI] [PubMed]
- NCT01317849. China stroke secondary prevention trial. clinicaltrials.gov/ct2/show/study/NCT01317849 (first received 17 March 2011).
NCT01708005 {published data only}
- NCT01708005. Dietary supplements, executive functions and vitamin D (DIET‐D): a double‐blind randomized controlled trial. clinicaltrials.gov/show/NCT01708005 (first received 16 October 2012).
NCT02467153 {published data only}
- NCT02467153. Influence of combined vitamin D supplementation and resistance exercise training on musculoskeletal health in frail older men and women (EXVITD). clinicaltrials.gov/show/NCT02467153 (first received 9 June 2015). [Google Scholar]
Pase 2015 {published data only}
- Pase MP, Grima N, Cockerell R, Stough C, Scholey A, Sali A, et al. The effects of long‐chain omega‐3 fish oils and multivitamins on cognitive and cardiovascular function: a randomized, controlled clinical trial. Journal of the American College of Nutrition 2015;34(1):21‐31. [DOI] [PubMed] [Google Scholar]
Pathansali 2006 {published data only}
- Pathansali R, Mangoni AA, Creagh‐Brown B, Lan Z, Ngow G, Yuan X, et al. Effects of folic acid supplementation on psychomotor performance and hemorheology in healthy elderly subjects. Archives of Gerontology and Geriatrics 2006;43(1):127‐37. [DOI] [PubMed] [Google Scholar]
Rietsema 2014 {published data only}
- Rietsema WJ. Unexpected recovery of moderate cognitive impairment on treatment with oral methylcobalamin. Journal of the American Geriatrics Society 2014;62(8):1611‐2. [DOI] [PubMed] [Google Scholar]
Rossom 2012 {published data only}
- Rossom RC, Espeland MA, Manson JE, Dysken MW, Johnson KC, Lane DS, et al. Calcium and vitamin D supplementation and cognitive impairment in the Women's Health Initiative. Journal of the American Geriatrics Society 2012;60(12):2197‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanchez 2011 {published data only}
- Brito A, Verdugo R, Hertrampf E, Miller JW, Green R, Fedosov SN, et al. Vitamin B‐12 treatment of asymptomatic, deficient, elderly Chileans improves conductivity in myelinated peripheral nerves, but high serum folate impairs vitamin B‐12 status response assessed by the combined indicator of vitamin B‐12 status. American Journal of Clinical Nutrition 2016;103(1):250‐7. [PUBMED: 26607937] [DOI] [PubMed] [Google Scholar]
- Sanchez H, Albala C, Lera L, Castillo JL, Verdugo R, Lavados M, et al. Comparison of two modes of vitamin B12 supplementation on neuroconduction and cognitive function among older people living in Santiago, Chile: a cluster randomized controlled trial. a study protocol. Nutrition Journal 2011;10:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Satalich 2014 {published data only}
- Satalich TA, Shankle WR, Alexander GE, Batchelder WH. Markov models detect vitamin E and donepezil treatment effects in ADCS MCI trial. Alzheimer's & Dementia 2014;10(4 suppl):P776. [Google Scholar]
Smith 1999 {published data only}
- Smith A, Clark R, Nutt D, Haller J, Hayward S, Perry K. Anti‐oxidant vitamins and mental performance of the elderly. Human Psychopharmacology 1999;14(7):459‐71. [Google Scholar]
Summers 2010 {published data only}
- Summers WK, Martin RL, Cunningham M, Deboynton VL, Marsh GM. Complex antioxidant blend improves memory in community‐dwelling seniors. Journal of Alzheimer's Disease 2010;19(2):429‐39. [DOI] [PubMed] [Google Scholar]
van der Zwaluw 2014 {published data only}
- Zwaluw N, Wijngaarden J, Dhonukshe‐Rutten R, Brouwer‐Brolsma E, In't Veld P, Kessels R, et al. The impact of 2y B‐vitamin supplementation on cognitive performance: the B‐PROOF study. Alzheimer's & Dementia 2013;9(4):P880‐1. [Google Scholar]
- Zwaluw NL, Dhonukshe‐Rutten RAM, Wijngaarden JP, Brouwer‐Brolsma EM, Rest O, In't Veld PH, et al. Results of 2‐year vitamin B treatment on cognitive performance: secondary data from an RCT. Neurology 2014;83(23):2158‐66. [DOI] [PubMed] [Google Scholar]
Walker 2012 {published data only}
- Walker JG, Batterham PJ, Mackinnon AJ, Jorm AF, Hickie I, Fenech M, et al. Oral folic acid and vitamin B‐12 supplementation to prevent cognitive decline in community‐dwelling older adults with depressive symptoms ‐ the Beyond Ageing Project: a randomized controlled trial. American Journal of Clinical Nutrition 2012;95(1):194‐203. [DOI] [PubMed] [Google Scholar]
- Walker JG, Mackinnon AJ, Batterham P, Jorm AF, Hickie I, McCarthy A, et al. Mental health literacy, folic acid and vitamin B12, and physical activity for the prevention of depression in older adults: randomised controlled trial. British Journal of Psychiatry 2010;197(1):45‐54. [DOI] [PubMed] [Google Scholar]
Wolters 2005 {published data only}
- Wolters M, Hickstein M, Flintermann A, Tewes U, Hahn A. Cognitive performance in relation to vitamin status in healthy elderly German women ‐ the effect of 6‐month multivitamin supplementation. Preventive Medicine 2005;41(1):253‐9. [DOI] [PubMed] [Google Scholar]
Wouters‐Wesseling 2005 {published data only}
- Wouters‐Wesseling W, Wagenaar LW, Rozendaal M, Deijen JB, Groot LC, Bindels JG, et al. Effect of an enriched drink on cognitive function in frail elderly persons. Journals of Gerontology. Series A: Biological Sciences and Medical Sciences 2005;60(2):265‐70. [DOI] [PubMed] [Google Scholar]
Yaffe 2004 {published data only}
- Clemons TE, Rankin MW, McBee WL. Cognitive impairment in the age‐related eye disease study: AREDS report no. 16. Archives of Ophthalmology 2006;124(4):537‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Clemons TE, McBee WL, Lindblad AS, Bressler SB. A randomized, controlled trial of antioxidants and zinc and the impact on cognition in the elderly: the AREDS ancillary trial. Annals of Neurology 2003;54(Suppl 7):S28‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Clemons TE, McBee WL, Lindblad AS, Age‐Related Eye Disease Study Research Group. Impact of antioxidants, zinc, and copper on cognition in the elderly ‐ a randomized, controlled trial. Neurology 2004;63(9):1705‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN12607000321448 {published data only}
- ACTRN12607000321448. Vitamin D and cognition trial/a randomised, placebo controlled trial of Vitamin D in older adults with mild cognitive impairment and low vitamin D concentration to prevent cognitive decline and delay progression of cognitive decline. www.anzctr.org.au/TrialSearch.aspx?searchTxt=12607000321448 (first received 13 Jun 2007).
- Flicker L, Greenop K, Almeida O, Alfonso H, Beer C, Hill K, et al. Effect of vitamin D supplementation on cognitive function in older adults with mild cognitive impairment ‐ a randomized trial. Australasian Journal on Ageing 2011;30(1):11. [Google Scholar]
- Torres SJ, Lautenschlager NT, Wattanapenpaiboon N, Greenop KR, Beer C, Flicker L, et al. Dietary patterns are associated with cognition among older people with mild cognitive impairment. Nutrients 2012;4(11):1542‐51. [PUBMED: 23201831] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiang 2014 {published data only}
- Jiang B, Ding C, Yao G, Yao C, Zhang Y, Ge J, et al. Intervention effect of folic acid and vitamin B12 on vascular cognitive impairment complicated with hyperhomocysteinemia. Journal of Medical Biochemistry 2014;33(2):169‐174. [Google Scholar]
- Jiang Y, Cheng D, Kong H, Pang W, Yang H, Sun S, et al. Supplementation with B vitamins improves cognitive function in the middle‐aged and elderly with hyperhomocysteinemia. Annals of Nutrition & Metabolism 2013;63(Suppl 1):713. [Google Scholar]
Ma 2017 {published data only (unpublished sought but not used)}
- Ma F, Li Q, Zhou X, Zhao J, Song A, Li W, et al. Effects of folic acid supplementation on cognitive function and Abeta‐related biomarkers in mild cognitive impairment: a randomized controlled trial. European Journal of Nutrition 2017 Dec 18 [Epub ahead of print]. [DOI: ] [DOI] [PubMed]
- Ma F, Wu T, Zhao J, Han F, Marseglia A, Liu H, et al. Effects of 6‐month folic acid supplementation on cognitive function and blood biomarkers in mild cognitive impairment: a randomized controlled trial in China. Journals of Gerontology. Series A: Biological Sciences and Medical Sciences 2016;71(10):1376‐83. [DOI] [PubMed] [Google Scholar]
- Ma F, Wu T, Zhao J, Song A, Liu H, Xu W, et al. Folic acid supplementation improves cognitive function by reducing the levels of peripheral inflammatory cytokines in elderly Chinese subjects with MCI. Scientific Reports 2016;6(37486). [DOI: 10.1038/srep37486] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT02185222 {published data only}
- NCT02185222. Effect of vitamin D on cognitive decline of patients with memory complaint. clinicaltrials.gov/show/NCT02185222 2014; Vol. (first received 9 July 2014).
Additional references
Albert 2011
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia 2011;7(3):270‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amanullah 2010
- Amanullah S, Seeber C. Niacin deficiency resulting in neuropsychiatric symptoms: a case study and review of literature. Clinical Neuropsychiatry 2010;7(1):10‐4. [Google Scholar]
Anderson 1997
Annweiler 2012
- Annweiler C, Rolland Y, Schott AM, Blain H, Vellas B, Herrmann FR, et al. Higher vitamin D dietary intake is associated with lower risk of alzheimer's disease: a 7‐year follow‐up. Journals of Gerontology: Series A Biological Sciences and Medical Sciences 2012;67(11):1205‐11. [DOI] [PubMed] [Google Scholar]
Bath 2013a
- Bath SC, Rayman MP. Iodine deficiency in the U.K.: an overlooked cause of impaired neurodevelopment?. Proceedings of the Nutrition Society 2013;72(2):226‐35. [DOI] [PubMed] [Google Scholar]
Bath 2013b
- Bath SC, Steer CD, Golding J, Emmett P, Rayman MP. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon longitudinal study of parents and children (ALSPAC). Lancet 2013;382(9889):331‐7. [DOI] [PubMed] [Google Scholar]
Behl 1992
- Behl C, Davis J, Cole G, Schubert D. Vitamin E protects nerve cells from amyloid β protein toxicity. Biochemical and Biophysical Research Communications 1992;182(2):944‐50. [DOI] [PubMed] [Google Scholar]
Berr 2012
Bjelakovic 2012
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD007176.pub2] [DOI] [PubMed] [Google Scholar]
Borchardt 1999
- Borchardt T, Camakaris J, Cappai R, Masters CL, Beyreuther K, Multhaup G. Copper inhibits beta‐amyloid production and stimulates the non‐amyloidogenic pathway of amyloid‐precursor‐protein secretion. Biochemical Journal 1999;344(2):461‐7. [PUBMED: 10567229] [PMC free article] [PubMed] [Google Scholar]
Brigelius‐Flohe 2007
- Brigelius‐Flohe R. Adverse effects of vitamin E by induction of drug metabolism. Genes & Nutrition 2007;2(3):249‐56. [PUBMED: 18850180] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruner 1996
- Bruner AB, Joffe A, Duggan AK, Casella JF, Brandt J. Randomised study of cognitive effects of iron supplementation in non‐anaemic iron‐deficient adolescent girls. Lancet 1996;348(9033):992‐6. [DOI] [PubMed] [Google Scholar]
Centers for Disease Control and Prevention 2014
- Centers for Disease Control and Prevention. Vitamins and minerals. https://www.cdc.gov/nutrition/index.html (accessed prior to 28 September 2018).
Christensen 2013
- Christensen K, Thinggaard M, Oksuzyan A, Steenstrup T, Andersen‐Ranberg K, Jeune B, et al. Physical and cognitive functioning of people older than 90 years: a comparison of two Danish cohorts born 10 years apart. Lancet 2013;382(9903):1507‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2007
- Clarke R, Birks J, Nexo E, Ueland PM, Schneede J, Scott J, et al. Low vitamin B‐12 status and risk of cognitive decline in older adults. American Journal of Clinical Nutrition 2007;86(5):1384‐91. [DOI] [PubMed] [Google Scholar]
Clarke 2014
- Clarke R, Bennett D, Parish S, Lewington S, Skeaff M, et al. Effects of homocysteine lowering with B vitamins on cognitive aging: meta‐analysis of 11 trials with cognitive data on 22,000 individuals. American Journal of Clinical Nutrition 2014;100(2):657‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Da Costa 2012
- Costa BR, Nüesch E, Reichenbach S, Jüni P, Rutjes AW. Doxycycline for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD007323.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Da Costa 2014
- Costa BR, Nüesch E, Kasteler R, Husni E, Welch V, Rutjes AW, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2014;17(9). [DOI: 10.1002/14651858.CD003115.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Luca 1975
- Luca HF. Function of the fat‐soluble vitamins. American Journal of Clinical Nutrition 1975;28(4):339‐45. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dolphin 2012
Duara 2011
- Duara R, Loewenstein DA, Greig MT, Potter E, Barker W, Raj A, et al. Pre‐MCI and MCI: neuropsychological, clinical, and imaging features and progression rates. American Journal of Geriatric Psychiatry 2011;19(11):951‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dubois 2014
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer'sdisease: the IWG‐2 criteria. Lancet Neurology 2014;13(6):614‐29. [DOI] [PubMed] [Google Scholar]
Dysken 2014
- Dysken MW, Sano M, Asthana S, Vertrees JE, Pallaki M, Llorente M, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM‐AD VA cooperative randomized trial. JAMA 2014;311(1):33‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farina 2012
- Farina N, Isaac MG, Clark AR, Rusted J, Tabet N. Vitamin E for Alzheimer's dementia and mild cognitive impairment. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD002854.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferland 2012
- Ferland G. Vitamin K, an emerging nutrient in brain function. BioFactors 2012;38(2):151‐7. [DOI] [PubMed] [Google Scholar]
Ferland 2013
- Ferland G, Presse N, Belleville S, Gaudreau P, Greenwood CE, Kergoat MJ, et al. Vitamin K and cognitive function in healthy older adults. The nuage study. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2013;27:840.13. [Google Scholar]
Food Standards Agency 2003
- Expert group on vitamins and minerals. Safe Upper Levels for Vitamins and Minerals. cot.food.gov.uk/sites/default/files/vitmin2003.pdf. Food Standards Agency, 2003. [ISBN 1‐904026‐11‐7]
Gregory 1990
- Gregory J, Foster K, Tyler H, Wiseman M. The dietary and nutritional survey of British adults. The Dietary and Nutritional Survey of British Adults. London: HMSO Publications Centre, 1990. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halliwell 1992
- Halliwell B. Reactive oxygen species and the central nervous system. Journal of Neurochemistry 1992;59(5):1609‐23. [DOI] [PubMed] [Google Scholar]
Halliwell 1999
- Halliwell B. Antioxidant defence mechanisms: from the beginning to the end (of the beginning). Free Radical Research 1999;31(4):261‐72. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
IOM 2011
- Institute of Medicine (US) Committee on Nutrition, Trauma, the Brain. Nutrition and Traumatic Brain Injury: Improving Acute and Subacute Health Outcomes in Military Personnel. Washington (DC): National Academies Press, 2011. [PubMed] [Google Scholar]
Jack 2018
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimer's & Dementia 2018;14(4):535‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeandel 1989
- Jeandel C, Nicolas MB, Dubois F, Nabet‐Belleville F, Penin F, Cuny G. Lipid peroxidation and free radical scavengers in Alzheimer's disease. Gerontology 1989;35(5‐6):275‐82. [DOI] [PubMed] [Google Scholar]
Jellinger 2006
- Jellinger KA. Clinicopathological analysis of dementia disorders in the elderly ‐ an update. Journal of Alzheimer's Disease 2006;9(Suppl 3):61‐70. [DOI] [PubMed] [Google Scholar]
Kaden 2011
- Kaden D, Bush AI, Danzeisen R, Bayer TA, Multhaup G. Disturbed copper bioavailability in Alzheimer's disease. International Journal of Alzheimer's Disease 2011;Article ID 345614. [DOI: 10.4061/2011/345614] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2011
- Kelly GS. Pantothenic acid. Monograph. Alternative Medicine Review 2011;16(3):263‐74. [PubMed] [Google Scholar]
Kennedy 2011
- Kennedy DO, Haskell CF. Vitamins and cognition: what is the evidence?. Drugs 2011;71(15):1957‐71. [DOI] [PubMed] [Google Scholar]
Kühnast 2013
- Kühnast S, Louwe MC, Heemskerk MM, Pieterman EJ, Klinken JB, Berg SA, et al. Niacin reduces atherosclerosis development in APOE*3Leiden. CETP mice mainly by reducing nonHDL‐cholesterol. PLoS One 2013;8(6):e66467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langlais 1995
- Langlais PJ, Savage LM. Thiamine deficiency in rats produces cognitive and memory deficits on spatial tasks that correlate with tissue loss in diencephalon, cortex and white matter. Behavioural Brain Research 1995;68(1):75‐89. [DOI] [PubMed] [Google Scholar]
Launer 1999
- Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, et al. Rates and risk factors for dementia and Alzheimer's disease: results from EURODEM pooled analyses. EURODEM Incidence Research Group and Work Groups. European studies of dementia. Neurology 1999;52(1):78‐84. [DOI] [PubMed] [Google Scholar]
Lindsay 2002
- Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, et al. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. American Journal of Epidemiology 2002;156(5):445‐53. [DOI] [PubMed] [Google Scholar]
Linus Pauling Institute
- Linus Pauling Institute, Oregon State University. Minerals. lpi.oregonstate.edu/mic/minerals (accessed 8 September 2015).
Llewellyn 2010
- Llewellyn DJ, Lang IA, Langa KM, Muniz‐Terrera G, Phillips CL, Cherubini A, et al. Vitamin D and risk of cognitive decline in elderly persons. Archives of Internal Medicine 2010; Vol. 170, issue 13:1135‐41. [DOI] [PMC free article] [PubMed]
Lourida 2013
- Lourida I, Soni M, Thompson‐Coon J, Purandare N, Lang IA, Ukoumunne OC, et al. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology 2013;24(4):479‐89. [DOI] [PubMed] [Google Scholar]
MAFF 1999
- Ministry of Agriculture, Fisheries, Food. 1997 Total Diet Study: Aluminium, arsenic, cadmium, chromium, copper, lead, mercury, nickel, selenium, tin, zinc. London (UK): Joint Food Safety and Standards Group; 1999. Food Surveillance Information Sheet No. 191.
Matthews 2007
- Matthews FE, Stephan BC, Bond J, McKeith I, Brayne C. Operationalization of mild cognitive impairment: a graphical approach. PLoS medicine 2007;4(10):1615‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthews 2008
- Matthews FE, Stephan BC, McKeith IG, Bond J, Brayne C. Two‐year progression from mild cognitive impairment to dementia: to what extent do different definitions agree?. Journal of the American Geriatrics Society 2008;56(8):1424‐33. [DOI] [PubMed] [Google Scholar]
Matthews 2013
- Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, et al. A two‐decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet 2013;382(9902):1405‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattson 2003
- Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends in Neurosciences 2003;26(3):137‐46. [DOI] [PubMed] [Google Scholar]
McCann 2008
- McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction?. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2008;22(4):982‐1001. [DOI] [PubMed] [Google Scholar]
McCullagh 2001
- McCullagh CD, Craig D, McIlroy SP, Passmore AP. Risk factors for dementia. Advances in Psychiatric Treatment 2001;7(1):24‐31. [Google Scholar]
Mehdi 2013
- Mehdi Y, Hornick JL, Istasse L, Dufrasne I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013;18(3):3292–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2005
- Miller ER 3rd, Pastor‐Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta‐analysis: high‐dosage vitamin E supplementation may increase all‐cause mortality. Annals of Internal Medicine 2005;142(1):37‐46. [DOI] [PubMed] [Google Scholar]
Mitchell 2009
- Mitchell AJ, Shiri‐Feshki M. Rate of progression of mild cognitive impairment to dementia‐‐meta‐analysis of 41 robust inception cohort studies. Acta Psychiatrica Scandinavica 2009;119(4):252‐65. [DOI] [PubMed] [Google Scholar]
Norton 2014
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population‐based data. Lancet Neurology 2014;13(8):788‐94. [DOI] [PubMed] [Google Scholar]
O'Leary 2012
- O'Leary F, Allman‐Farinelli M, Samman S. Vitamin B₁₂ status, cognitive decline and dementia: a systematic review of prospective cohort studies. British Journal of Nutrition 2012;108(11):1948‐61. [DOI] [PubMed] [Google Scholar]
ODS 2014
- National Institute of Health: Office of Dietary Supplements. Vitamin B6; Dietary Supplement Fact Sheet. http://ods.od.nih.gov/factsheets/VitaminB6‐HealthProfessional/ 25April 2016.
Ogawa 1994
- Ogawa N. Free radicals and neural cell damage. Rinsho Shinkeigaku 1994;34(12):1266‐8 . [PubMed] [Google Scholar]
Ono 2012
- Ono K, Yamada M. Vitamin A and Alzheimer's disease. Geriatrics & Gerontology International 2012;12(2):180‐8. [DOI] [PubMed] [Google Scholar]
Osiezagha 2013
- Osiezagha K, Ali S, Freeman C, Barker N C, Jabeen S, Maitra S, et al. Thiamine deficiency and delirium. Innovations in Clinical Neuroscience 2013;10(4):26‐32. [PMC free article] [PubMed] [Google Scholar]
Ozawa 2012
- Ozawa M, Ninomiya T, Ohara T, Hirakawa Y, Doi Y, Hata J, et al. Self‐reported dietary intake of potassium, calcium, and magnesium and risk of dementia in the Japanese: the Hisayama study. Journal of the American Geriatrics Society 2012;60(8):1515‐20. [DOI] [PubMed] [Google Scholar]
Packer 1997
- Packer L, Tritschler H J, Wessel K. Neuroprotection by the metabolic antioxidant a‐lipoic acid. Free Radical Biology and Medicine 1997;22(1‐2):359‐78 . [DOI] [PubMed] [Google Scholar]
Pawlak 2014
- Pawlak R, Lester SE, Babatunde T. The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: a review of literature. European Journal of Clinical Nutrition 2014;68(5):541‐8. [DOI] [PubMed] [Google Scholar]
Perrig 1997
- Perrig WJ, Perrig P, Stahelin HB. The relation between antioxidants and memory performance in the old and very old. Journal of the American Geriatrics Society 1997;45(6):718‐24. [DOI] [PubMed] [Google Scholar]
Petersen 1999
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology 1999;56(3):303‐8. [DOI] [PubMed] [Google Scholar]
Petersen 2004
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine 2004;256(3):183‐94. [DOI] [PubMed] [Google Scholar]
Powell 2000
- Powell SR. The antioxidant properties of zinc. Journal of Nutrition 2000;130(Suppl 55):1447S‐54S. [DOI] [PubMed] [Google Scholar]
Powers 2003
- Powers HJ. Riboflavin (vitamin B‐2) and health. American Journal of Clinical Nutrition 2003;77(6):1352‐60. [DOI] [PubMed] [Google Scholar]
Preuss 1997
- Preuss HG, Grojec PL, Lieberman S, Anderson R A. Effects of different chromium compounds on blood pressure and lipid peroxidation in spontaneously hypertensive rats. Clinical Nephrology 1997 May;47(5):325‐30. [PubMed] [Google Scholar]
Przybelski 2007
- Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25‐hydroxyvitamin D concentration with cognitive function. Archives of Biochemistry and Biophysics 2007;460(2):202‐5. [DOI] [PubMed] [Google Scholar]
Rahman 2007
- Rahman K. Studies on free radicals, antioxidants, and co‐factors. Clinical Interventions in Aging 2007 Jun; Vol. 2, issue 2:219–236. [PMC free article] [PubMed]
Reichenbach 2010
- Reichenbach S, Rutjes AW, Nuesch E, Trelle S, Juni P. Joint lavage for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD007320.pub2] [DOI] [PubMed] [Google Scholar]
Reiter 1995
- Reiter RJ. Oxidative processes and antioxidative defence mechanisms in the ageing brain. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 1995;9(7):526‐33. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rutjes 2009a
- Rutjes AW, Nuesch E, Reichenbach S, Juni P. S‐Adenosylmethionine for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007321.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutjes 2009b
- Rutjes AW, Nuesch E, Sterchi R, Kalichman L, Hendriks E, Osiri M, et al. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD002823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutjes 2010
- Rutjes AW, Nuesch E, Sterchi R, Juni P. Therapeutic ultrasound for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003132.pub2] [DOI] [PubMed] [Google Scholar]
Rücker 2008
- Rücker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I2 in assessing heterogeneity may mislead. BMC Medical Research Methodology 2008;8(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Savva 2009
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. New England Journal of Medicine 2009;360(22):2302‐9. [DOI] [PubMed] [Google Scholar]
Scott 2013
- Scott TM, Tucker KL. Low plasma vitamin B6 predicts cognitive decline and depression in at‐risk individuals. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2013;27(Meeting abstract supplement):346.6. [Google Scholar]
Smorgon 2004
- Smorgon C, Mari E, Atti AR, Dalla Nora E, Zamboni PF, Calzoni F, et al. Trace elements and cognitive impairment: an elderly cohort study. Archives of Gerontology and Geriatrics 2004;38(Suppl 9):393‐402. [DOI] [PubMed] [Google Scholar]
Sodhi 2013
- Sodhi RK, Singh N. All‐trans retinoic acid rescues memory deficits and neuropathological changes in mouse model of streptozotocin‐induced dementia of Alzheimer's type. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2013;40:38‐46. [DOI] [PubMed] [Google Scholar]
Spiegelhalter 2004
- Spiegelhalter DJ, Abrams KJ, Myles JP. Bayesian Approaches to Clinical Trials and Health‐Care Evaluation. Chichester, UK: J Wiley, 2004. [Google Scholar]
Stephan 2007
- Stephan BC, Matthews FE, McKeith IG, Bond J, Brayne C. Early cognitive change in the general population: how do different definitions work?. Journal of the American Geriatrics Society 2007;55(10):1534‐40. [PUBMED: 17908056] [DOI] [PubMed] [Google Scholar]
Stephan 2013
- Stephan BCM, Minett T, Pagett E, Siervo M, Brayne C, McKeith IG. Diagnosing mild cognitive impairment (MCI) in clinical trials: a systematic review. BMJ Open 2013;3(2):e001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tabet 2001
- Tabet N, Mantle D, Walker Z, Orrel M. Vitamins, trace elements, and antioxidant status in dementia disorders. International Psychogeriatrics 2001;13(3):265‐75. [DOI] [PubMed] [Google Scholar]
Tabet 2002
- Tabet N, Mantle D, Walker Z, Orrell M. Endogenous antioxidant activities in relation to concurrent vitamins A, C, and E intake in dementia. International Psychogeriatrics 2002;14(1):7‐15. [DOI] [PubMed] [Google Scholar]
Takatsu 2009
- Takatsu H, Owada K, Abe K, Nakano M, Urano S. Effect of vitamin E on learning and memory deficit in aged rats. Journal of Nutritional Science and Vitaminology 2009;55(5):389‐93. [DOI] [PubMed] [Google Scholar]
The World Alzheimer Report 2014
- The World Alzheimer Report. Dementia and Risk Reduction: An analysis of protective and modifiable factors. London: Alzheimer’s Disease International (ADI), 2014. [Google Scholar]
van den Berg 2012
- Berg S, Splaine M. Policy brief risk factors for dementia. Alzheimer's Disease International 2012 Apr [accessed 2014 Sep 19].
van der Flier 2005
- Flier WM, Scheltens P. Epidemiology and risk factors of dementia. Journal of Neurology, Neurosurgery, and Psychiatry 2005;76(Suppl 5):v2–v7. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Schaft 2013
- Schaft J, Koek HL, Dijkstra E, Verhaar HJ, Schouw YT, Emmelot‐Vonk MH. The association between vitamin D and cognition: A systematic review. Ageing Research Reviews 2013;12(4):1013‐23. [DOI] [PubMed] [Google Scholar]
Wang 2000
- Wang X, Quinn PJ. The location and function of vitamin E in membranes. Molecular Membrane Biology 2000;17(3):143‐56. [DOI] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. Dementia: a public health priority. apps.who.int/iris/bitstream/handle/10665/75263/9789241564458_eng.pdf 2012.
Wilson 2002
- Wilson RS, Bennett DA, Bienias JL, Aggarwal NT, Mendes De Leon CF, Morris MC, et al. Cognitive activity and incident AD in a population‐based sample of older persons. Neurology 2002;59(12):1910‐4. [DOI] [PubMed] [Google Scholar]
Winblad 2004
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment‐‐beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine 2004;256(3):240‐6. [DOI] [PubMed] [Google Scholar]


