Abstract
Study Objectives
The Menopause Strategies: Finding Lasting Answers for Symptoms and Health network conducted three randomized clinical trials (RCTs) testing six interventions treating vasomotor symptoms (VMS), and also collected self-reported sleep outcomes. A fourth RCT assessed an intervention for insomnia symptoms among women with VMS. We describe these seven interventions’ effects relative to control in women with comparably severe insomnia symptoms and VMS.
Methods
We analyzed pooled individual-level data from 546 peri- and postmenopausal women with Insomnia Severity Index (ISI) ≥ 12, and ≥14 bothersome VMS/week across the four RCTs. Interventions included the following: escitalopram 10–20 mg/day; yoga; aerobic exercise; 1.8 g/day omega-3 fatty acids; oral 17-beta-estradiol 0.5-mg/day; venlafaxine XR 75-mg/day; and cognitive behavioral therapy for insomnia (CBT-I). Outcome measures were ISI and Pittsburgh Sleep Quality Index (PSQI) over 8–12 weeks of treatment.
Results
CBT-I produced the greatest reduction in ISI from baseline relative to control at −5.2 points (95% CI −7.0 to −3.4). Effects on ISI were similar for exercise at −2.1 and venlafaxine at −2.3 points. Comparably small decreases in ISI were observed with escitalopram, yoga, and estradiol. The largest reduction in PSQI from baseline was with CBT-I at −2.7 points (−3.9 to −1.5), although PSQI decreases of 1.2 to 1.6 points were significantly better than control with escitalopram, exercise, yoga, estradiol, and venlafaxine. Omega-3 supplements did not improve insomnia symptoms.
Conclusions
This study’s findings support current recommendations for CBT-I as a first line treatment in healthy midlife women with insomnia symptoms and moderately bothersome VMS.
Keywords: menopause, insomnia, vasomotor symptoms
Statement of Significance
Insomnia symptoms are common in perimenopausal and postmenopausal women with vasomotor symptoms (VMS) including hot flashes. This study uses a pooled analysis of sleep outcome data from four randomized trials for menopausal women with comparable VMS and insomnia symptoms to determine which of seven interventions had the greatest positive impact on sleep. Cognitive behavioral therapy for insomnia produced the greatest reduction in insomnia symptoms and increase in sleep quality ratings. Smaller improvements were found for physical exercise, venlafaxine, escitalopram, yoga, and estradiol. Omega-3 supplements did not improve insomnia symptoms. Findings from this study can help guide clinicians and patients in shared decision-making about the potential sleep benefits of medication and nonpharmacological interventions commonly recommended to menopausal women with VMS.
INTRODUCTION
Self-reported sleep problems are common in peri- and postmenopausal women and have been identified as a key symptom of the menopausal transition.1,2 Although not all women would meet criteria for a formal diagnosis of insomnia, many experience insomnia symptoms, particularly mid-night or early morning awakenings with inability to return to sleep, that they attribute to nocturnal hot flashes and night sweats. However, the relationship between these vasomotor symptoms (VMS) and sleep disturbances is complex.3 Elevated core body temperature and alterations in sex steroids and gonadotropins during the menopause transition and early postmenopausal years contribute to both sleep changes and hot flashes.4–10 Studies have also reported that the frequency and severity of hot flashes are associated in a graded manner with severity of insomnia symptoms11,12 and objective measures of nighttime wakefulness and sleep fragmentation.11,13 However, recent research has shown that although hot flashes are linked with nocturnal awakenings, there is not an exclusive one-to-one causal relationship. Rather, it appears that some underlying autonomic nervous system perturbation or other process is occurring in women with VMS that contributes to waking episodes.13–16 Furthermore, VMS and insomnia symptoms often cluster with other common symptoms, including depressed mood and pain, which can further exacerbate sleep disturbances.17 Regardless of etiology, once women are awake and aroused, they may experience typical insomnia symptoms such as difficulty returning to sleep and ruminating thoughts that lead them to engage in compensatory behaviors (e.g., napping or extending time in bed) that can perpetuate a true insomnia cycle. Because of the complexity of the relationship between VMS and sleep problems in menopause, women with comorbid VMS and insomnia symptoms may vary in their response to treatment for both hot flashes and sleep complaints.
The MsFLASH (Menopausal Strategies: Finding Lasting Answers to Symptoms and Health) Network has, to date, conducted four randomized clinical trials with healthy menopausal women experiencing moderately bothersome hot flashes. The first three of these trials (MsFLASH 01-03) targeted treatment of hot flashes; sleep measurements were collected as a secondary outcome because of prior literature suggesting that some of the interventions might improve sleep in menopausal women.18–20 The fourth trial (MsFLASH 04) enrolled women with both moderately severe insomnia symptoms and bothersome hot flashes; in this trial, improving sleep was the primary treatment goal. A motivating factor for the fourth MsFLASH trial was the observation that insomnia symptoms and poor self-reported sleep quality were common among women randomized to the first three trials. None of the women in any trial, however, were required to meet diagnostic criteria for insomnia disorder.
In total, the trials tested seven interventions in approximately 1,000 women, including a selective serotonin reuptake inhibitor (SSRI), a serotonin norepinephrine reuptake inhibitor (SNRI), oral low-dose estrogen, yoga, aerobic exercise and omega-3 fatty acid supplementation, and cognitive behavioral therapy for insomnia (CBT-I). Before the first trial was launched, MsFLASH investigators developed network standards for study design, eligibility and exclusion criteria, and study measures,21 with the intention that estimates of each intervention effect could be compared across trials and thus provide insight into their relative efficacy.
Despite standardized MsFLASH methodology, there were some design differences between studies that need to be accounted for when examining intervention effects. We published a novel pooled comparative effectiveness analysis to examine the effects of the MsFLASH 01-03 interventions on VMS frequency and bother, using individual-level data and adjusting for differences between studies.22 The current paper presents a similar analytic approach applied to data from four MsFLASH studies in order to describe the magnitude of insomnia treatment effects for multiple interventions relative to control within the same analysis. Only women who entered the four trials with comparably severe insomnia symptoms were included.
METHODS
MsFLASH Studies Design Overview
MsFLASH 01 was a randomized, placebo-controlled, double-blind clinical trial designed to determine the efficacy and tolerability of 10–20 mg/day of the SSRI escitalopram for reducing VMS frequency and severity compared with placebo.23 The study aimed to recruit approximately equal numbers of African-American and White women. Participants were randomized in a 1:1 ratio to receive escitalopram 10 mg/day or a matching placebo capsule for 8 weeks. If a woman did not report a reduction in VMS frequency of ≥50% or a decrease in VMS severity after 4 treatment weeks, her study medication dose was increased to 20 mg/day (or matched placebo) without unblinding the randomization.
MsFLASH 02 employed a 3 × 2 factorial, randomized controlled trial design to compare the effects of yoga and exercise separately to a usual activity control group, and simultaneously to compare omega-3 fatty acid capsules to placebo capsules, on VMS frequency and bother.24–26 Eligible women were randomized in a 3:3:4 ratio to 12 weeks of yoga, exercise, or usual activity, and simultaneously randomized in a 1:1 ratio to 1.8 g/day of omega-3 fish oil capsules or identical-looking placebo capsules. Details regarding specific yoga and exercise activities and doses are described in previous manuscripts.24,25 The 1.8 gm/day omega-3 fatty acids supplements were taken three times/day for 12 weeks and contained 425 mg ethyl eicosapentaenoic acid, 100 mg docosahexaenoic acid, and 90 mg of other omega-3s. The omega-3 component of the trial was double-blinded.
MsFLASH 03 was a randomized, placebo-controlled, double-blind, 8-week trial comparing the efficacy for reducing VMS frequency of low-dose oral 17-beta-estradiol 0.5 mg/day, the SNRI venlafaxine XR (37.5 mg/day for the first week, then 75 mg/day), or placebo in a 2:2:3 ratio.27
MsFLASH 04 was a placebo-controlled trial in which women were randomized to either telephone-delivered CBT-I or menopause education control.28 Six telephone sessions were conducted over 8 weeks; follow-up assessments were conducted at 8 and 24 weeks post-randomization. This was the only MsFLASH trial that focused specifically on treatment of insomnia symptoms rather than VMS, but VMS outcomes comparable to those collected in the other MsFLASH trials were measured. Participants were not told whether their intervention assignment was the experimental or control condition.
Design differences between trials are described in Table 1. For all studies, randomization was accomplished through a secure Web-based database, maintained by the Data Coordinating Center, utilizing a dynamic randomization algorithm.29 The randomization was stratified by clinical site and race for MsFLASH 01, and by clinical site only for MsFLASH 02 and 03. The analyses presented here were not pre-specified in study protocols, but the MsFLASH trials were designed to permit eventual pooled analysis. All MsFLASH studies were approved by the Institutional Review Boards of each clinical site and the Data Coordinating Center. All participants provided written informed consent.
Table 1.
MsFLASH Trial Designs.
| Trial | Total enrollment | Design | Hot flash + insomnia eligibility | Trial-specific exclusion criteria | Intervention length | Sleep assessment |
|---|---|---|---|---|---|---|
| 01 | 205; 98 eligible for this analysis |
2-arm: Escitalopram vs. placebo |
≥ 28 hot flashes/week | • Use of psychotropic medications (past 30 days) • Use of gabapentin, pregabalin, triptans, warfarin, or St. John’s Wort • Use of selective estrogen receptor modulators or aromatase inhibitors (past 60 days) • Suicide attempt in the past 3 years • History of endometrial or ovarian cancer |
8 weeks | Baseline Week 4 Week 8 |
| 02 | 355; 188 eligible for this analysis |
3 × 2 factorial: Aerobic exercise and yoga vs. usual activity, plus omega-3 supplementation vs. placebo |
≥ 14 hot flashes/week | • Body mass index > 37 kg/m2 • Contraindications to yoga, exercise training, or omega-3 • Current participation in yoga or regular exercise • Current use of omega-3 supplements • Consumption ≥ 4 servings of fish/week |
12 weeks | Baseline Week 12 |
| 03 | 339; 154 eligible for this analysis |
3-arm: Low dose oral estradiol and venlafaxine vs. placebo |
≥ 14 hot flashes/week | • Hypersensitivity or contraindication to study medications • Use of psychotropic medications (past 30 days) • Use of selective estrogen receptor modulators or aromatase inhibitors (past 60 days) • Suicide attempt in the past 3 years • History of thrombotic or endometrial disease, pre-breast cancer conditions, or breast cancer |
8 weeks | Baseline Week 4 Week 8 |
| 04 | 106; all eligible for this analysis |
2-arm: Telephone cognitive behavior therapy for insomnia vs. menopause education control | ≥ 14 hot flashes/week Insomnia Severity Index ≥ 12 (at both screening and baseline) |
• Known primary sleep disorder diagnosis • Works job with rotating shifts > 3 times per week • Routine use of prescription sleeping or sedating medication at night (> 3 times per week) |
8 weeks | Baseline Week 8 Week 24 |
Setting and Participants
Participants were recruited from July 2009 to August 2015, primarily by mass mailings to age-eligible women using purchased mailing lists and health-plan enrollment files. There were five MsFLASH network sites (Boston, Indianapolis, Oakland, Philadelphia, and Seattle). All sites participated in at least two trials and each trial was implemented at three or four sites, except MsFLASH 04 which was implemented only in Seattle (Table 2).
Table 2.
Baseline Demographic and Clinical Participant Characteristics by Trial.
| MsFLASH 01 (n = 98) | MsFLASH 02 (n = 188) | MsFLASH 03 (n = 154) | MsFLASH 04 (n = 106) | p* | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline characteristic | n | % | n | % | n | % | N | % | |
| Age at screening, mean (SD) | 53.9 | (4.1) | 54.4 | (3.8) | 54.3 | (3.8) | 54.8 | (4.2) | .39 |
| <50 | 14 | 14 | 12 | 6 | 18 | 12 | 7 | 4 | |
| 50–54 | 44 | 45 | 87 | 46 | 65 | 42 | 44 | 42 | |
| 55–59 | 31 | 32 | 69 | 37 | 57 | 37 | 41 | 39 | |
| ≥60 | 9 | 9 | 20 | 11 | 14 | 9 | 14 | 15 | |
| Race* | <.001 | ||||||||
| African-American | 51 | 52 | 44 | 23 | 58 | 38 | 1 | 1 | |
| White | 44 | 45 | 123 | 65 | 85 | 55 | 97 | 92 | |
| Other | 3 | 3 | 21 | 11 | 11 | 7 | 8 | 8 | |
| Hispanic | 0 | 0 | 4 | 2 | 0 | 0 | 3 | 3 | |
| American Indian | 0 | 0 | 7 | 4 | 1 | 1 | 1 | 1 | |
| Asian/Pacific Islander | 1 | 1 | 4 | 2 | 4 | 3 | 0 | 0 | |
| Undisclosed | 2 | 2 | 6 | 3 | 6 | 4 | 4 | 4 | |
| Education | <.001 | ||||||||
| ≤ High school diploma/GED | 19 | 19 | 8 | 4 | 30 | 20 | 5 | 5 | |
| Post-high school | 50 | 51 | 63 | 34 | 47 | 30 | 19 | 18 | |
| College graduate | 29 | 30 | 116 | 62 | 77 | 50 | 82 | 77 | |
| Smoking | <.001 | ||||||||
| Never | 51 | 52 | 120 | 64 | 75 | 49 | 82 | 77 | |
| Past | 18 | 18 | 49 | 26 | 48 | 31 | 23 | 22 | |
| Current | 29 | 30 | 17 | 9 | 30 | 20 | 1 | 1 | |
| BMI (m/kg2), mean (SD) | 29.8 | (7.2) | 27.3 | (4.3) | 28.5 | (7.0) | 24.9 | (5.1) | <.001 |
| <25 | 25 | 26 | 59 | 31 | 55 | 36 | 67 | 63 | |
| 25–<30 | 32 | 33 | 76 | 40 | 43 | 28 | 21 | 20 | |
| ≥30 | 41 | 42 | 53 | 28 | 52 | 34 | 18 | 17 | |
| Menopause status | .09 | ||||||||
| Postmenopausal | 69 | 70 | 136 | 72 | 107 | 70 | 68 | 64 | |
| Perimenopausal | 17 | 17 | 38 | 20 | 26 | 17 | 31 | 29 | |
| Indeterminate | 12 | 12 | 14 | 7 | 21 | 14 | 7 | 7 | |
| Site | — | ||||||||
| Boston | 17 | 17 | 0 | 0 | 44 | 29 | 0 | 0 | |
| Indianapolis | 13 | 13 | 72 | 38 | 0 | 0 | 0 | 0 | |
| Oakland | 28 | 29 | 49 | 26 | 0 | 0 | 0 | 0 | |
| Philadelphia | 40 | 41 | 0 | 0 | 59 | 38 | 0 | 0 | |
| Seattle | 0 | 0 | 67 | 36 | 51 | 33 | 106 | 100 | |
| HF frequency, mean (SD) | 9.8 | (6.2) | 7.7 | (3.8) | 8.4 | (5.8) | 7.6 | (4.3) | .003 |
| HF severity (1–3), mean (SD) | 2.3 | (0.5) | 2.0 | (0.4) | 2.1 | (0.5) | 1.8 | (0.4) | <.001 |
| PHQ depressiona, mean (SD) | 4.3 | (3.4) | 5.3 | (4.1) | 5.0 | (4.1) | 7.7 | (4.2) | <.001 |
| ISI, mean (SD)b | 16.7 | (3.8) | 16.0 | (3.3) | 16.3 | (3.5) | 16.3 | (3.5) | .46 |
| PSQI, mean (SD) | 10.4 | (3.4) | 9.8 | (2.9) | 9.7 | (2.9) | 9.2 | (2.8) | .04 |
SD = standard deviation; BMI = body mass index; HF = hot flash; PHQ = Patient Health Questionnaire; ISI = Insomnia Severity Index; PSQI = Pittsburgh Sleep Quality Index.
*Homogeneity across trials assessed via chi-squared or F-test, as appropriate. For race, assessment based on collapsed race categories (African-American, White, and Other).
aPHQ depression scores based on PHQ-9 for MsFLASH 01 and 03 and PHQ-8 in MsFLASH 02 and 04, but only the first 8 items were included in these scores.
bMinimum ISI score of 12 for inclusion in analysis.
Eligibility criteria common to all trials included the following: women aged 40–62 years; in the menopause transition (amenorrhea ≥ 60 days in the past year), or postmenopausal (≥12 months since last menstrual period or bi-lateral oophorectomy), or had a hysterectomy with one or both ovaries remaining and FSH >20 mIU/mL and estradiol ≤50 pg/mL; and in general good health as determined by medical history, a brief physical exam, and standard blood tests. In addition to the screening VMS frequency requirement (Table 1), for MsFLASH 01-03 trials, VMS had to be rated as bothersome or severe on at least 4 days or nights per week, and the frequency in screening week 3 could not decrease >50% from the mean weekly levels in screening weeks 1 and 2. Note that women were not selected based on insomnia symptoms in MsFLASH 01-03. For MsFLASH 04, women were eligible if the Insomnia Severity Index (ISI) score was 12 or higher at screening and at baseline, in addition to reporting ≥14 hot flashes over the 2-week screening period (Table 1). The current analysis includes only women from MsFLASH 01-03 who also had baseline ISI scores ≥12.
Exclusion criteria common to all trials included the following: pregnancy or breastfeeding; any current severe or unstable medical conditions; and drug or alcohol abuse (past year). The MsFLASH 01-03 trials also excluded participants with history of myocardial infarction, angina, or cerebrovascular events; major depressive episode (past 3 months); use of prescription or over-the-counter treatments for hot flashes (past 30 days), or use of exogenous sex steroid hormones or hormonal contraceptives (past 2 months). Women were excluded from MsFLASH 04 if they reported a prior primary sleep disorder diagnosis (e.g., sleep apnea), although none of the trials included polysomnography evaluation to rule out undiagnosed sleep disorders. Other exclusion criteria included consuming greater than three alcoholic drinks daily, presence of a current major illness interfering with sleep, having a job involving shift work (>3 times a week), or routinely (>3 times a week) using prescription sleeping medications. Additional trial-specific exclusion criteria are described in Table 1.
Enrollment and Procedures
Following telephone screening, women completed a 2-week VMS diary and a questionnaire. Women in MsFLASH 01-03 who remained eligible attended an in-person visit that included a blood draw, physical measures, and another questionnaire. Following that visit, women completed the week-3 VMS diary and then returned to the clinic for determination of eligibility and randomization. Telephone contacts to encourage study compliance and assess adverse events were made 1 or 2 weeks after randomization, and then again midway through the intervention. Follow-up clinic visits were conducted at 8 weeks (MsFLASH 01 and 03) or 12 weeks (MsFLASH 02) post-randomization. Women in MsFLASH 04 completed the telephone screening, a 2-week sleep and VMS diary, and a questionnaire. Those who were remained eligible and returned their consent form were randomized and contacted by telephone by a study interventionist to schedule the first treatment session. Participants were invited to have their first session in person at a research office, but women were also permitted to have the first session by telephone. Follow-up occurred through telephone calls and mailed questionnaires.
Outcome Measures and Follow-up
The ISI is a valid and reliable self-administered instrument that measures perception of current (past 2 weeks) insomnia symptoms.30,31 The index consists of seven items assessing difficulty falling asleep, difficulty staying asleep, problems with early awakening, satisfaction with current sleep pattern, interference of sleep problem with daily functioning, noticeability of impairment attributed to the sleep problem, and degree of distress caused by the sleep problem. Each item is rated on a 0–4 point scale (total score 0–28), with higher scores suggesting more severe insomnia symptoms. The absence of insomnia is indicated by scores 0–7, subthreshold or mild insomnia by scores 8–14, clinical insomnia of moderate severity by scores 15–21, and severe clinical insomnia by scores 22–28. Trials of pharmacologic and behavioral interventions in patients with insomnia have suggested that the ISI is sensitive in measuring treatment response.32–34
The Pittsburgh Sleep Quality Index (PSQI) is a validated measure of self-reported sleep quality and sleep disturbances over a 1-month time period, including assessment of self-reported sleep quality, sleep-onset latency, duration, and efficiency; sleep disturbances; use of sleeping medication; and daytime dysfunction.35 Global PSQI scores range from 0 to 21 with higher scores indicating poorer sleep quality; a cutoff of 5 is indicative of poor sleep with sensitivity of 89.6% and specificity of 86.5%.36 The PSQI has been shown to be sensitive in measuring response to CBT in randomized trials conducted in patients with insomnia.37
Participants completed the ISI and PSQI at baseline and weeks 4 and 8 of treatment in MsFLASH 01 and 03; at baseline and week 12 of treatment in MsFLASH 02; and at baseline, week 8 of treatment, and an additional 24-week follow-up assessment in MsFLASH 04. In this paper, only the MsFLASH 04 baseline and week 8 data are included.
Statistical Analysis
For comparability across trials, the study cohort for this analysis included the subset of MsFLASH 01-03 participants whose baseline ISI ≥ 12, and all MsFLASH 04 participants. The outcomes of interest for the pooled analysis were changes in the ISI and PSQI at weeks 4, 8, and 12 relative to baseline means. In addition, we evaluated the proportion of participants with remission of insomnia symptoms (ISI < 8) at their 8- or 12-week visit. The intent-to-treat analysis included all randomized participants who provided follow-up ISI or PSQI data, regardless of adherence to treatment assignment. Baseline demographic and clinical characteristics including age, race, education level, smoking status, menopausal status, and body mass index (BMI) were summarized in combined treatment arms and compared across trials, with homogeneity assessed via chi-squared or F-test.
Linear regression models were applied to estimate differences between each intervention group and its corresponding control group in changes from baseline in ISI or PSQI at weeks 4, 8, and 12, with adjustment for trial number, clinical site, age, race, education level, smoking, and BMI. The models incorporated trial-specific effects of the baseline outcome measure and time to allow the relationships between the baseline and follow-up sleep measures, and sleep measures with study time, to vary across trials. Thus, the models estimate each intervention effect relative to its own trial’s control group, while adjusting for common factors across trials. Robust standard errors were calculated by generalized estimating equations. A logistic regression model was applied to estimate odds ratios for the occurrence of ISI < 8 at the end of intervention for each treatment group relative to its corresponding control group. This model was adjusted for trial number, clinical site, age, race, education level, smoking, and BMI and included trial-specific effects of the baseline ISI.
Analyses were conducted using SAS Version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Approximately half of the women randomized to MsFLASH 01-03 had baseline ISI scores of 12 or above. Trial participants meeting this insomnia symptom threshold were randomized as follows: 51 to escitalopram and 47 to placebo (MsFLASH 01); 54 to exercise, 58 to yoga, and 76 to usual activity, and simultaneously, 95 to omega-3 and 93 to placebo (MsFLASH 02); 45 to estradiol, 47 to venlafaxine, and 62 to placebo (MsFLASH 03); 53 to CBT-I and 53 to menopause education (MsFLASH 04). These sample sizes were proportionate to the overall randomization schema for each trial.
Post-treatment data collection retention was moderately high in all study subgroups: 96 (98%) of 98 participants provided ISI data and 94 (96%) provided PSQI data at follow-up in MsFLASH 01; 173 (92%) of 188 participants provided ISI data and 168 (89%) provided PSQI data at follow-up in MsFLASH 02; 151 (98%) of 154 participants provided ISI data and 143 (93%) provided PSQI data at follow-up in MsFLASH 03, and 91 (86%) of 106 participants provided ISI data and 89 (84%) provided PSQI data at 8 weeks post-treatment in MsFLASH 04.
The distributions of race, education levels, BMI, and smoking prevalence varied across studies, corresponding to the clinical site locations and goals of the trials (Table 2). For example, the MsFLASH 01 trial aimed to recruit African-American women as half of its study population. This choice was associated with other differences in demographics between 01 and the other trials. Also, the eligibility criterion for VMS frequency was lowered after MsFLASH 01 to facilitate recruitment in subsequent trials. Women recruited for MsFLASH 04 were more likely to be White, less likely to be smokers, had higher education levels, lower BMI, and higher depression scores compared with women in the first three trials. The baseline distributions of age, menopause status, and ISI varied little across trials (Table 2). The latter is not surprising since the sample of women from the MsFLASH 01-03 trials included only those with baseline ISI scores ≥12, which had been an eligibility requirement of MsFLASH04.
Mean reductions from baseline to follow-up within the control groups were fairly similar across trials, and ranged from 3.9 to 5.2 points for ISI and 1.4 to 2.0 points for PSQI (Table 3).
Table 3.
Changes From Baseline in ISI and PSQI Within MsFLASH Trial Control Groups.
| Week 4 – Baseline | Week 8 – Baseline | Week 12 – Baseline | ||||
|---|---|---|---|---|---|---|
| Outcome | Mean (95% CI) | Median % Change (IQR) |
Mean (95% CI) | Median % Change (IQR) |
Mean (95% CI) | Median % Change (IQR) |
| ISI | ||||||
| MsFLASH 01 | −3.9 (−5.3, −2.4) | −16% (−53, 0%) | −4.7 (−6.4, −3.1) | −24% (−50, −10%) | — | — |
| MsFLASH 02 | — | — | — | — | −5.2 (−6.7, −3.8) | −29% (−43, −16%) |
| MsFLASH 03 | −5.0 (−6.3, −3.6) | −28% (−53, −4%) | −4.8 (−6.1, −3.5) | −31% (−44, −9%) | — | — |
| MsFLASH 04 | — | — | −4.7 (−6.1, −3.3) | −29% (−44, −9%) | — | — |
| PSQI | ||||||
| MsFLASH 01 | −1.7 (−2.7, −0.8) | −17% (−31, 11%) | −2.0 (−2.9, −1.0) | −22% (−38, 6%) | — | — |
| MsFLASH 02 | — | — | — | — | −1.8 (−2.9, −0.6) | −19% (−39, 0%) |
| MsFLASH 03 | −1.4 (−2.3, −0.6) | −13% (−22, 0%) | −1.5 (−2.4, −0.6) | −13% (−34, 0%) | — | — |
| MsFLASH 04 | — | — | −1.4 (−2.1, −0.7) | −15% (−30, 0%) | — | — |
CI = confidence interval; IQR = inter-quartile range; ISI = Insomnia Severity Index; PSQI = Pittsburgh Sleep Quality Index.
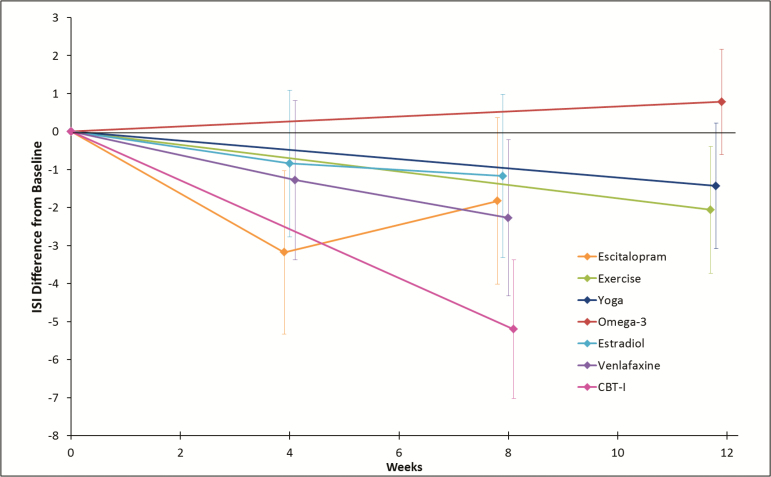
CBT-I provided substantially more relief from insomnia symptoms than the other active interventions. The mean ISI reduction from baseline to follow-up relative to control in the CBT-I group was 5.2 points in an adjusted model (Figure 1). Exercise and venlafaxine showed comparably moderate beneficial effects on ISI, with decreases of 2.1 at week 12 and 2.3 points at week 8 relative to control, respectively. Small reductions in ISI relative to control were observed in the escitalopram, yoga, and estradiol groups.
Figure 1.
Mean effect (95% confidence interval) of each intervention relative to control on changes from baseline in ISI, N = 511. CBT-I = cognitive behavioral therapy for insomnia; ISI = Insomnia Severity Index.
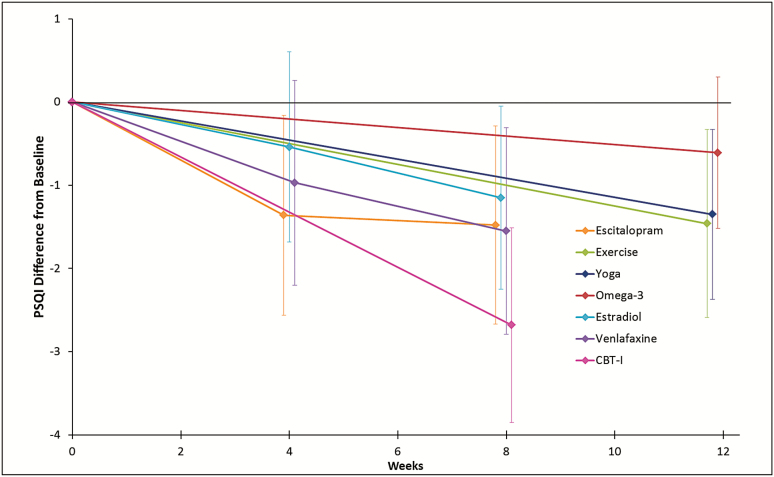
The impact of CBT-I on PSQI was again much greater than the effect of the other interventions, with a mean PSQI reduction from baseline to follow-up relative to control in the CBT-I group of 2.7 points (Figure 2). Reductions in PSQI from baseline of similar size were observed in the escitalopram, exercise, yoga, estradiol, and venlafaxine groups, relative to control. After 8 weeks of treatment with any of the three medications or 12 weeks of exercise or yoga, PSQI decreased by 1.2 to 1.6 points relative to control. As previously reported in the original published analyses,26 the omega-3 intervention showed little effect on ISI or PSQI relative to control.
Figure 2.
Mean effect (95% confidence interval) of each intervention relative to control on changes from baseline in PSQI, N = 494. CBT-I = cognitive behavioral therapy for insomnia; PSQI = Pittsburgh Sleep Quality Index.
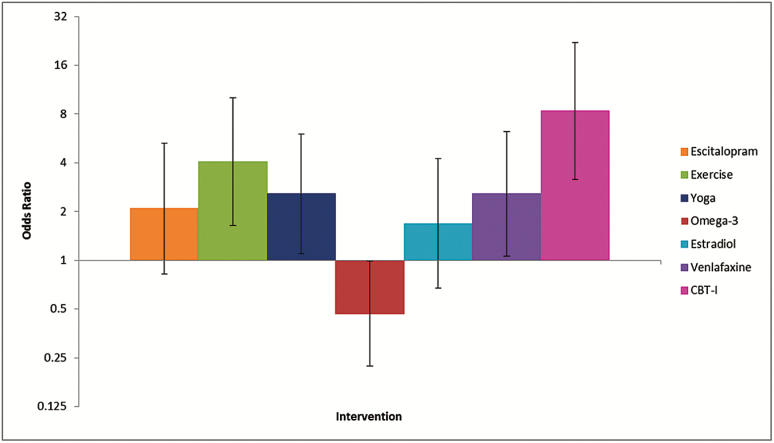
The odds ratio for remission of insomnia symptoms (ISI < 8) at the end of treatment relative to control was higher for CBT-I than for the other interventions (Figure 3), although gains in remission were also observed in the exercise, yoga, and venlafaxine groups compared with control. It is worth noting that CBT-I-related improvements on the ISI and PSQI in MsFLASH 04 were also sustained at 24 weeks,28 although these data are not shown in the tables and figures in this manuscript because none of the other MsFLASH trials collected participant outcomes beyond 12 weeks.
Figure 3.
Odds ratio (95% confidence interval) of insomnia symptoms remission (ISI < 8) relative to control at end of treatment by intervention, N = 503. CBT-I = cognitive behavioral therapy for insomnia; ISI = Insomnia Severity Index.
No serious adverse events due to study interventions were reported during the four trials of duration 8 to 12 weeks.23–27 All study medications were well-tolerated and no participants stopped the exercise, yoga, or CBT-I interventions due to side effects.
DISCUSSION
Compared with the first three MsFLASH clinical trials, pooled sleep treatment effects on both the ISI and PSQI were approximately two times larger for the MsFLASH 04 trial testing the efficacy of telephone-delivered CBT-I versus menopause education control in a study population selected on the basis of elevated insomnia symptoms.28 In this analysis of pooled individual-level data from MsFLASH clinical trials, the effects of escitalopram, low-dose estradiol, and low-dose venlafaxine on self-reported sleep outcomes were comparable in healthy midlife women experiencing hot flashes. Results also suggested that physical activity interventions such as aerobic exercise and yoga had small beneficial effects on sleep in midlife women. Our analysis confirms the lack of efficacy of omega-3 for self-reported sleep symptoms. Intervention effect estimates in these pooled analyses did not qualitatively diverge from the results of each individual trial,24–26,38,39 implying that the differences across trials in baseline characteristics, exclusion criteria, and follow-up times did not substantially affect the trials’ comparability.
Strengths of this pooled analysis include the use of standardized methods for measurement of participant characteristics, sleep and VMS, and similar inclusion and exclusion criteria. The MsFLASH trials were designed to be comparable, though not identical, making this multivariable analysis an important step in comparing estimates of the seven intervention effects on selected outcomes. A single trial designed to provide direct head-to-head comparisons of all interventions would be infeasible. The analyses presented here bridge the gap between the feasible, individual trials and an idealistic trial providing direct comparisons.
Although covering a wide range of intervention types, the treatments tested by MsFLASH do not encompass the full spectrum of potentially beneficial medicines for menopausal sleep disturbances. The recent Agency for Healthcare Research and Quality review40 “Menopausal Symptoms: Comparative Effectiveness of Therapies” concluded that eszopiclone, an FDA-approved drug for insomnia, produced greater sleep benefits compared with placebo than estrogen or other prescription or naturopathic agents. However, this eszopiclone finding was based on a single trial. In light of limited data and the potential for medications like eszopiclone to be addictive, caution is recommended when considering their use to improve sleep in women with menopausal symptoms.
The effects of the MsFLASH interventions on VMS frequency only partially aligned with their effects on sleep. In analyses of pooled individual-level data from the MsFLASH 01-03 trials, escitalopram, low-dose estradiol, and low-dose venlafaxine conveyed similarly moderate improvement in daily VMS frequency relative to the placebo group.22 No effects on VMS were seen with aerobic exercise, yoga, or omega-3 supplements. In a separate analysis, CBT-I also did not diminish VMS frequency relative to control.28
Recruitment procedures for the MsFLASH 04 trial were comparable to those in the earlier studies, but several eligibility criteria were different since the outcome focus was on insomnia treatment (Table 1). For example, in the MsFLASH 04 trial, eligible women scored 12 or higher on the ISI at both phone screening and on baseline questionnaires, in addition to reporting at least 14 hot flashes per week. That was our rationale for selecting only women in the MsFLASH 01-03 trials with ISI of 12 or higher. Most importantly, women randomized to MsFLASH 04 understood that the CBT-I intervention was designed primarily for insomnia symptoms and thus the expectancy for improvement could have affected our findings. However, such expectancy would have resulted in a higher placebo response in this trial and we did not observe this. It should be noted that in none of the MsFLASH trials were women included based upon a formal insomnia diagnosis. There have been few studies looking at the relationship between sleep and VMS in women with diagnosed insomnia;12 future studies are needed to examine whether the pattern of treatment effects described here would be comparable in a true insomnia population.
MsFLASH 04 participants were also predominantly White, more highly educated, and had lower BMI than women in the other MsFLASH trials. Although several studies have shown that demographics are not highly predictive of CBT-I treatment response in adults with medical comorbidities,41–43 BMI and sociodemographic characteristics do impact risk for development of insomnia44,45 and thus may have played some role in between-trial differences observed in this study. However, subgroup analyses in both MsFLASH 01 and 03 showed little evidence of differential treatment effects on self-reported sleep outcomes by race.38,39
MsFLASH 04 participants also entered the trial with significantly higher levels of depression symptoms than MsFLASH 01-03 participants, although they were still under the mild/sub-clinical depression range on average. VMS and insomnia symptoms often cluster with other common symptoms, including depressed mood and pain, which can further exacerbate sleep disturbances.17 A recent post hoc analysis showed that CBT-I reductions relative to control in both the ISI and PSQI were significantly greater at 8 weeks in women with total scores ≥ 10 on the 8-item Patient Health Questionnaire (i.e., women with clinical signs of depression) compared with women with lower depression scores.46 However, the observed interaction with depression was not maintained at 24-week follow-up, and no other demographic variable (including baseline VMS) showed a significant statistical interaction with sleep outcome. Also, the treatment effects of escitalopram, estradiol, and venlafaxine on ISI and PSQI did not significantly differ according to baseline depressive symptoms.38,39
Regardless of the etiology of their sleep problems, once women are awake and aroused, they may experience typical insomnia symptoms such as difficulty returning to sleep and ruminating thoughts that lead them to engage in compensatory behaviors (e.g., napping or extending time in bed) that can perpetuate an insomnia cycle. The American College of Physicians (ACP)47 has recommended that CBT-I be offered as the initial treatment for chronic insomnia, and that clinicians take a shared decision-making approach in evaluating whether pharmacological therapy should be considered when CBT-I is ineffective. Future studies are needed to explore the complex relationship between CBT-I response and comorbid VMS and insomnia symptoms in menopausal women. Although women may vary in their treatment response, findings from this current study support the ACP recommendations for CBT-I as a first line of treatment in menopausal population.
In summary, findings from these pooled analyses suggest that telephone-delivered CBT-I is more effective for reducing moderate-to-severe insomnia symptoms in menopausal women with hot flashes than other commonly used pharmacologic or lifestyle modification options. In settings where CBT-I is not available, data suggest that exercise and venlafaxine may produce moderate improvements in both self-reported insomnia symptoms and sleep quality ratings. The relative benefits of escitalopram, yoga, and estradiol varied by sleep outcome, and there was no evidence of sleep improvement from the omega-3 intervention on either ISI or PSQI relative to control. Such results may provide the foundation for conversations between women and their health care providers about their best treatment options for insomnia symptoms during the menopausal transition.
FUNDING
The MsFLASH studies were supported by a cooperative agreement issued by the National Institute of Aging (NIA), in collaboration with the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD), the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Research and Women’s Health (ORWH), and NIA grants U01AG032659, U01AG032669, U01AG032682, U01AG032699, and U01AG032700. In Indiana, the project was supported by the Indiana Clinical and Translational Sciences Institute, funded in part by grant UL1 RR025761 from the National Institutes of Health, National Center for Research Resources, Clinical and Translational Sciences Award. Escitalopram and matching placebo pills for the MsFLASH 01 trial were provided by Forest Research Institute. Omega-3 supplements and matching placebo pills for the MsFLASH 02 trial were provided by Nordic Naturals, Watsonville, CA.
CLINICALTRIALS.GOV IDENTIFIERS
NCT00894543 (MsFLASH 01), NCT01178892 (MsFLASH 02), NCT01418209 (MsFLASH 03), and NCT01936441 (MsFLASH 04).
DISCLOSURE STATEMENT
HJ has received grant funding from NIH, Merck, and SAGE and has been a consultant for NeRRe, Merck, SAGE, and Mitsubishi Tanabe. SDR has received research funding from Bayer Pharmaceuticals. CMM has received fees for consultancy from Merck and Cereve.
REFERENCES
- 1. Shaver JL, Woods NF. Sleep and menopause: a narrative review. Menopause. 2015; 22(8): 899–915. [DOI] [PubMed] [Google Scholar]
- 2. Xu Q, Lang CP. Examining the relationship between subjective sleep disturbance and menopause: a systematic review and meta-analysis. Menopause. 2014; 21(12): 1301–1318. [DOI] [PubMed] [Google Scholar]
- 3. Kravitz HM, Joffe H. Sleep during the perimenopause: a SWAN story. Obstet Gynecol Clin North Am. 2011; 38(3): 567–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005; 23(2): 117–125. [DOI] [PubMed] [Google Scholar]
- 5. Freeman EW, Sammel MD, Gross SA, Pien GW. Poor sleep in relation to natural menopause: a population-based 14-year follow-up of midlife women. Menopause. 2015; 22(7): 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kravitz HM, Janssen I, Santoro N, et al. Relationship of day-to-day reproductive hormone levels to sleep in midlife women. Arch Intern Med. 2005; 165(20): 2370–2376. [DOI] [PubMed] [Google Scholar]
- 7. Lack LC, Gradisar M, Van Someren EJ, Wright HR, Lushington K. The relationship between insomnia and body temperatures. Sleep Med Rev. 2008; 12(4): 307–317. [DOI] [PubMed] [Google Scholar]
- 8. Murphy PJ, Campbell SS. Sex hormones, sleep, and core body temperature in older postmenopausal women. Sleep. 2007; 30(12): 1788–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Freedman RR. Hot flashes: behavioral treatments, mechanisms, and relation to sleep. Am J Med. 2005; 118Suppl 12B: 124–130. [DOI] [PubMed] [Google Scholar]
- 10. Guidozzi F. Sleep and sleep disorders in menopausal women. Climacteric. 2013; 16(2): 214–219. [DOI] [PubMed] [Google Scholar]
- 11. Ensrud KE, Stone KL, Blackwell TL, et al. Frequency and severity of hot flashes and sleep disturbance in postmenopausal women with hot flashes. Menopause. 2009; 16(2): 286–292. [DOI] [PubMed] [Google Scholar]
- 12. Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006; 166(12): 1262–1268. [DOI] [PubMed] [Google Scholar]
- 13. Joffe H, Crawford S, Economou N, et al. A gonadotropin-releasing hormone agonist model demonstrates that nocturnal hot flashes interrupt objective sleep. Sleep. 2013; 36(12): 1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baker FC, Willoughby AR, Sassoon SA, Colrain IM, de Zambotti M. Insomnia in women approaching menopause: beyond perception. Psychoneuroendocrinology. 2015; 60: 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bianchi MT, Kim S, Galvan T, White DP, Joffe H. Nocturnal hot flashes: relationship to objective awakenings and sleep stage transitions. J Clin Sleep Med. 2016; 12(7): 1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Zambotti M, Colrain IM, Javitz HS, Baker FC. Magnitude of the impact of hot flashes on sleep in perimenopausal women. Fertil Steril. 2014; 102(6): 1708–15.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woods NF, Hohensee C, Carpenter JS, et al. Symptom clusters among MsFLASH clinical trial participants. Menopause. 2016; 23(2): 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Afonso RF, Hachul H, Kozasa EH, et al. Yoga decreases insomnia in postmenopausal women: a randomized clinical trial. Menopause. 2012; 19(2): 186–193. [DOI] [PubMed] [Google Scholar]
- 19. Mansikkamäki K, Raitanen J, Nygård CH, et al. Sleep quality and aerobic training among menopausal women–a randomized controlled trial. Maturitas. 2012; 72(4): 339–345. [DOI] [PubMed] [Google Scholar]
- 20. Soares CN, Arsenio H, Joffe H, et al. Escitalopram versus ethinyl estradiol and norethindrone acetate for symptomatic peri- and postmenopausal women: impact on depression, vasomotor symptoms, sleep, and quality of life. Menopause. 2006; 13(5): 780–786. [DOI] [PubMed] [Google Scholar]
- 21. Newton KM, Carpenter JS, Guthrie KA, et al. Methods for the design of vasomotor symptom trials: the menopausal strategies: finding lasting answers to symptoms and health network. Menopause. 2014; 21(1): 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guthrie KA, LaCroix AZ, Ensrud KE, et al. Pooled analysis of six pharmacologic and nonpharmacologic interventions for vasomotor symptoms. Obstet Gynecol. 2015; 126(2): 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011; 305(3): 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sternfeld B, Guthrie KA, Ensrud KE, et al. Efficacy of exercise for menopausal symptoms: a randomized controlled trial. Menopause. 2014; 21(4): 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Newton KM, Reed SD, Guthrie KA, et al. Efficacy of yoga for vasomotor symptoms: a randomized controlled trial. Menopause. 2014; 21(4): 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cohen LS, Joffe H, Guthrie KA, et al. Efficacy of omega-3 for vasomotor symptoms treatment: a randomized controlled trial. Menopause. 2014; 21(4): 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med. 2014; 174(7): 1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCurry SM, Guthrie KA, Morin CM, et al. Telephone based cognitive-behavioral therapy for insomnia in peri- and postmenopausal women with vasomotor symptoms: A MsFLASH randomized trial. JAMA Intern Med. 2016; 176(7): 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975; 31(1): 103–115. [PubMed] [Google Scholar]
- 30. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001; 2(4): 297–307. [DOI] [PubMed] [Google Scholar]
- 31. Morin CM. Measuring outcomes in randomized clinical trials of insomnia treatments. Sleep Med Rev. 2003; 7(3): 263–279. [DOI] [PubMed] [Google Scholar]
- 32. Krystal AD, McCall WV, Fava M, et al. Eszopiclone treatment for insomnia: Effect size comparisons in patients with primary insomnia and insomnia with medical and psychiatric comorbidity. Prim Care Companion CNS Disord. 2012; 14(4):pii: PCC.11m01296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011; 34(5): 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu JQ, Appleman ER, Salazar RD, Ong JC. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015; 175(9): 1461–1472. [DOI] [PubMed] [Google Scholar]
- 35. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28(2): 193–213. [DOI] [PubMed] [Google Scholar]
- 36. Buysse DJ, Hall ML, Strollo PJ, et al. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008; 4(6): 563–571. [PMC free article] [PubMed] [Google Scholar]
- 37. Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011; 171(10): 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ensrud KE, Guthrie KA, Hohensee C, et al. Effects of estradiol and venlafaxine on insomnia symptoms and sleep quality in women with hot flashes. Sleep. 2015; 38(1): 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ensrud KE, Joffe H, Guthrie KA, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: a randomized controlled trial. Menopause. 2012; 19(8): 848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grant MD, Marbella A, Wang AT, et al. Menopausal Symptoms: Comparative Effectiveness of Therapies. Comparative effectiveness review No. 147. Rockville, MD: Agency for Healthcare Research and Quality, March 2015. 147. [PubMed] [Google Scholar]
- 41. Currie SR, Wilson KG, Curran D. Clinical significance and predictors of treatment response to cognitive-behavior therapy for insomnia secondary to chronic pain. J Behav Med. 2002; 25(2): 135–153. [DOI] [PubMed] [Google Scholar]
- 42. Gagne A, Morin CM. Predicting treatment response in older adults with insomnia. J Clin Geropsychol. 2001; 7(2): 131–143. [Google Scholar]
- 43. Troxel WM, Conrad TS, Germain A, Buysse DJ. Predictors of treatment response to brief behavioral treatment of insomnia (BBTI) in older adults. J Clin Sleep Med. 2013; 9(12): 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gamaldo AA, Beydoun MA, Beydoun HA, et al. Sleep disturbances among older adults in the United States, 2002–2012: Nationwide inpatient rates, predictors, and outcomes. Front Aging Neurosci. 2016;15(8):266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kaufmann CN, Mojtabai R, Hock RS, et al. Racial/ethnic differences in insomnia trajectories among U.S. older adults. Am J Geriatr Psychiatry. 2016; 24(7): 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McCurry SM, Guthrie KA, Larson JD, et al. Effects of telephone-delivered CBT-I on sleep: Do outcomes differ by baseline demographic, VMS, or mood symptoms? In: North American Menopause Society 27th Annual Meeting; October 5–8, 2016; Orlando, FL. [Google Scholar]
- 47. Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016; 165(2): 125–133. [DOI] [PubMed] [Google Scholar]