Abstract
Despite years of research, we are still unraveling crucial stages of gene expression regulation in cancer. On the basis of major biological hallmarks, we hypothesized that there must be a uniform gene expression pattern and regulation across cancer types. Among non-coding genes, long non-coding RNAs (lncRNAs) are emerging as key gene regulators playing powerful roles in cancer. Using TCGA RNAseq data, we analyzed coding (mRNA) and non-coding (lncRNA) gene expression across 15 and 9 common cancer types, respectively. 70 significantly differentially expressed genes common to all 15 cancer types were enlisted. Correlating with protein expression levels from Human Protein Atlas, we observed 34 positively correlated gene sets which are enriched in gene expression, transcription from RNA Pol-II, regulation of transcription and mitotic cell cycle biological processes. Further, 24 lncRNAs were among common significantly differentially expressed non-coding genes. Using guilt-by-association method, we predicted lncRNAs to be involved in same biological processes. Combining RNA-RNA interaction prediction and transcription regulatory networks, we identified E2F1, FOXM1 and PVT1 regulatory path as recurring pan-cancer regulatory entity. PVT1 is predicted to interact with SYNE1 at 3′-UTR; DNAJC9, RNPS1 at 5′-UTR and ATXN2L, ALAD, FOXM1 and IRAK1 at CDS sites. The key findings are that through E2F1, FOXM1 and PVT1 regulatory axis and possible interactions with different coding genes, PVT1 may be playing a prominent role in pan-cancer development and progression.
Keywords: PVT1, E2F1, FOXM1, RNAseq, TCGA, NETWORK, Long non-coding RNAs, Pan-cancer
Significance
Our paper delves on the role and interplay of coding and long non-coding genes in almost all cancer types which are characterized by same biological hallmarks using systems biology. Analyzing the interplay, we have zeroed in on E2F1, FOXM1 (transcription factors) and PVT1 (lncRNA) regulatory path as recurring pan-cancer regulatory entity. E2F1 expression may be indirectly regulated by PVT1, with ETS1 being a powerful mediator between the two coding and non-coding genes. In addition to providing novel cancer drug targets, our results provide key insights into the interplay of these emerging novel class of gene regulators.
Introduction
Cancer, a hitherto-largely impregnable disease, is characterized by distinct hallmarks (Hanahan & Weinberg, 2011) which generalize their biological complexity. Transformation of normal cells into abnormal ones, i.e., cancer, is associated with profound changes in gene expression profile (Kopnin, 2000; Ismail et al., 2000; Herceg & Hainaut, 2007). Factors and causes involved range from genetic (somatic mutation and copy number variations) to epigenetic changes which in turn, lead to differential gene expression due to dysregulation (Sadikovic, Al-Romaih & Squire J.A, 2008; Du & Che, 2017). The dysregulated genes have been found to include both coding and noncoding RNAs (Ezkurdia et al., 2014; Yan et al., 2015). Among non-coding RNAs, long non-coding RNAs (LncRNAs), are found to be involved in epigenetic regulation, transcription and translation processes (Derrien et al., 2012; Mattick & Rinn, 2015; ENCODE Project Consortium et al., 2007; Mercer & Mattick, 2013; Patil et al., 2016). Several lncRNAs have been identified as a tumor suppressor or oncogene in most of the tumors and are aberrantly expressed in cancers (Yang et al., 2017b; Song et al., 2016b; Liu et al., 2016a; Chen et al., 2015; Pandey et al., 2014). Interacting with proteins and other RNAs, these may play an important role in signal transduction processes in cancer and normal cells in their capacity as signals, decoys, guides and scaffolds (Kung, Colognori & Lee, 2013). Many lncRNAs have been correlated with development and disease mainly due to the changes in their expression levels.
Studies are being carried out to understand their precise roles and molecular mechanisms of action. These act through diverse roles such as through down-regulation of gene expression at RNA level (Salameh et al., 2015), through Staufen 1 (STAU1)-mediated messenger RNA decay (SMD) (Gong & Maquat, 2011) and/or acting synergistically in regulating genes (Ma, Bajic & Zhang, 2013). LncRNAs have been demonstrated in several recent studies to be associated with cancer and role and mechanisms of several lncRNAs in cancer signaling pathways is detailed in a recent comprehensive review (Schmitt & Chang, 2016). Promoting cell proliferation in prostate cancer cells (Prensner et al., 2011), effects on the transcriptional and post-transcriptional regulation of cytoskeletal and extracellular matrix genes in lung adenocarcinoma cells (Tano et al., 2010), repressing tumor suppressors INK4a/p16 and INK4b/p15 (Yap et al., 2010; Kotake et al., 2011) are postulated to be some of the mechanisms. HOTAIR overexpression is associated with poor prognosis in several cancers and it has been suggested that it may increase tumor invasiveness and metastasis (Gupta et al., 2010). Thus, lncRNAs as master regulators have a strong potential to be used as biomarkers or drug targets. Several studies have been in place towards pan-cancer gene expression and lncRNA profiling (Bolha, Ravnik-Glavač & Glavač, 2017; Zhang et al., 2016; Wang et al., 2018), however, these studies have been performed separately for specific cancer types. An integrated approach towards identification of non-coding lncRNAs serving as likely master regulators of differentially expressed coding genes in cancer will fill the gap in our understanding of the regulatory role of these lncRNAs on mRNAs and proteins in cancer. Our studies will hopefully provide us with key novel molecule/s as common target/s involved in all the major and emerging hallmarks and possible one miracle drug intervention.
Using latest RNAseqV2 data from The Cancer Genome Atlas (TCGA), we performed several statistical analyses on global mRNA expression data from 15 types of cancers and lncRNA expression profiles from nine cancer types, these types are also present in above-mentioned 15 cancer types. Key findings from our studies have identified E2F1, FOXM1, and ETS1 as coding gene/s axes that are overexpressed and may show strong involvement in tumor development in almost all cancer types. Several lncRNA/s such as PVT1, SNHG-11, MIR22HG and UHRF-1 among others have been postulated to play a regulatory role for these protein-coding genes. It is intriguing to observe that the biological processes predicted for these lncRNA-mRNA modules fulfill all the six distinct cancer hallmarks. Validated by our systems-scale observations and literature evidence, we have attempted to put forward a working model of these genes’ regulation by lncRNAs that is uniform across cancer types.
Materials and Methods
Protein-coding genes (mRNA) analyses
Data acquisition
Gene expression data was obtained from TCGA data portal (https://cancergenome.nih.gov/ downloaded in the year 2016. We selected UNC_IlluminaHiseq_RNAseqV2 platform only to avoid heterogeneity of data and normalized level-3 sample data sets. A total number of samples was 5,601 from 15 different cancer types (primary cancers) and 601 corresponding normal samples. Each sample had associated expression values for 20,531 protein-coding genes.
Significant differential expression analysis of each gene
We analyzed significant differential expression by using unpaired t-test with unequal group variances (Welch approximation) in Multiple Experiment Viewer (MeV) version 4.9.0 (http://mev.tm4.org/) and Linear Model for Microarray (LIMMA) as a package in RStudio program (https://www.r-project.org/). For assessing significant differential expression in the t-test, the default cut-off was a critical p-value of 0.01 and in LIMMA, double filtration approach with the alpha value 0.01 and fold change >2 cut-offs was used. Combining these two results in order to increase accuracy and eliminate false positives, significantly differentially expressed common genes were enlisted using Venn diagram.
Identification of up- and down-regulated genes
Using our significantly differentially expressed common genes list, we applied Comparative Marker Selection version 10.1 (http://software.broadinstitute.org/cancer/software/genepattern/modules/docs/ComparativeMarkerSelection/10) to identify up- and down-regulated genes between the two classes of samples (cancer and normal) by t-test (median centered) at 10,000 permutations and a p-value cutoff of 0.01 with Bonferroni correction.
Correlation of protein and mRNA expression levels
We compared protein expression level (intensity obtained from immunohistochemistry results) published in “Human Protein Atlas (HPA)” (available from https://www.proteinatlas.org/, data accessed in 2016–2017) with mRNA expression level of each of the 70 significantly differentially expressed coding genes. As per Cancer Atlas in HPA, the staining intensity for each protein expression level from immunohistochemistry data is given in the range: high, medium, low and not detected, from the expert manual analysis. Side comparisons with normal samples are also presented.
Functional annotation
For functional annotation, we used GeneCoDis3 web server (http://genecodis.cnb.csic.es/). This web server takes a list of genes as inputs and annotates a gene set using Gene Ontology, KEGG Pathways, and other terms. Significance of annotations to gene set is determined by p-values computed using hypergeometric distribution or chi-square test. For our analyses, we examined only those annotations for which p-values obtained through hypergeometric analysis and corrected by the FDR method of Benjamini and Hochberg were provided for significance. Hypergeometric p-value of <or = 0.05 (default) was used. The results can be seen as the tags cloud where most significant terms that are enriched in the list of genes are presented as fonts of variable sizes. The font size of each tag depends on the number of genes enriched from our gene list that are present in a particular annotation.
Non Protein-coding genes (Long Noncoding RNA (lncRNA)) analyses
Data acquisition
LncRNAtor database (http://lncrnator.ewha.ac.kr/expression.htm) is an online database which integrates expression profile, interacting protein, integrated sequence curation, evolutionary scores, and coding potential. TCGA, GEO, ENCODE, and modENCODE databases provide the relevant datasets. We selected differentially expressed lncRNAs from TCGA RNAseq platform, level-3 count data. Searching for the same cancer types as present in TCGA database, we found nine cancer types which were the same as the cancer types used in coding genes’ work and for which data from TCGA for comparing normal vs tumor was present. In this way, a total of 2,900 samples with 403 normal and 2,497 tumor samples were investigated. Total number of lncRNAs in lncRNAtor was 24,154. Significantly differentially expressed lncRNAs identified by the database authors using DESeq2 package for TCGA level 3 data with adj p-value 0.01 were enlisted from the database. Bioconductor software specifies about DESeq2 as follows: “It estimates variance-mean dependence in count data from high-throughput sequencing assays and tests for differential expression based on a model using the negative binomial distribution” ( Love, Huber & Anders, 2014). We then used Comparative Marker Selection version 10.1 to discriminate up- and down-regulated lncRNA genes between two classes of samples by t-test (median centered) at 10,000 permutations and a p-value cutoff of 0.01. We used lncRNAtor and GeneCodis3 to predict co-expressed mRNAs and their role in biological processes and molecular function in order to assign putative functions to uncharacterized lncRNAs by “guilt-by-association”.
Prediction of lncRNA-mRNA interaction and function
We used the human lncRNA-mRNA interaction database (http://rtools.cbrc.jp/cgi-bin/RNARNA/index.pl) (Terai et al., 2016) which is validated. It contains lncRNA-mRNA and lncRNA-lncRNA interactions between 23,898 lncRNAs and 20,185 mRNA (human) sequences obtained from the GENCODE Project (http://www.gencodegenes.org/releases/19.html). The first step in this pipeline; RACCESS program is used for extracting accessible regions in each RNA sequence, Step 2: tandem repeats are removed using the TANTAN program, Step 3: reverse complementary ‘seed matches’ are detected using LAST, Step 4: the binding energies between query and target RNAs from the seed matches are evaluated using INTARNA. In Step 5, the candidate interaction pairs are ranked according to the interaction energies (SUMENERGY and MINENERGY). Finally, the interaction site secondary structure with minimum interaction energy is predicted in each pair of RNA sequence using RACTIP. Using this database, we analyzed interactions of common significantly differentially expressed mRNAs and lncRNAs enlisted from our studies. Interaction energy (ranking was done using SUMENERGY score which is adequate for long RNA sequences such as lncRNAs and mRNAs), location of the interaction site on mRNA and joint secondary structures of the binding site were predicted. We used Excel for generating plots of SUMENERGY.
Integrated Network analysis
For experimentally verified transcriptional regulatory network (TFs-gene interaction) analysis, we retrieved data from Open-Access Repository of Transcriptional Interactions (ORTI, URL: http://orti.sydney.edu.au/about.html) database, which apart from other databases on mammalian transcription factors (TF) and their regulated target gene (TG) interactions, harbors Human Transcriptional Regulatory Interaction database (HTRIdb). LncRNAs are also included in the network interactions. In another network, we annotated differentially expressed genes as co-expressed, physically interacting and pathway genes using GeneMania for further analysis (Fig. S1). These two types of interaction data were imported into Cytoscape 3.5.1 (http://www.cytoscape.org/download.php). The network analyzer tool—using the directed network with TF as the source and gene as the target (directed TF to gene network)—was used to produce quantitative data for node degree, betweenness centrality, clustering coefficient among others.
Result
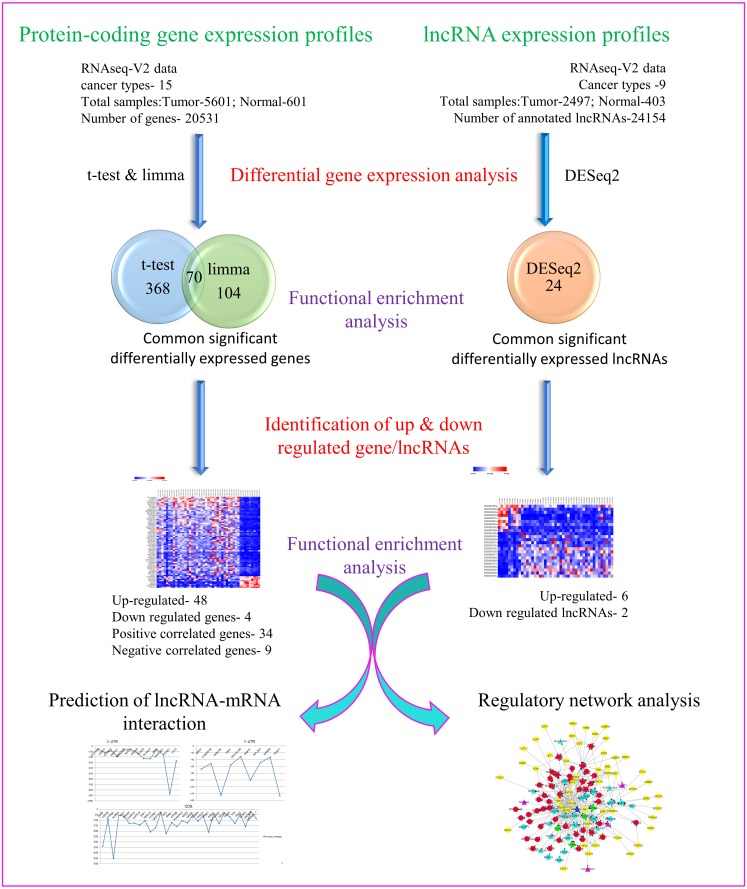
Towards our studies on understanding interrelationship between coding and non-coding genes playing possible essential roles in pan-cancer development, we have used several well-established and validated tools to generate hypothesis and gain useful insights. A concise summary of various methods used has been put together as a flowchart in Fig. 1.
Figure 1. Flowchart depicting different steps used and narrowing down genes as seen through corresponding number of coding and non-coding genes.
Differential expression analysis of coding RNAs (mRNA)
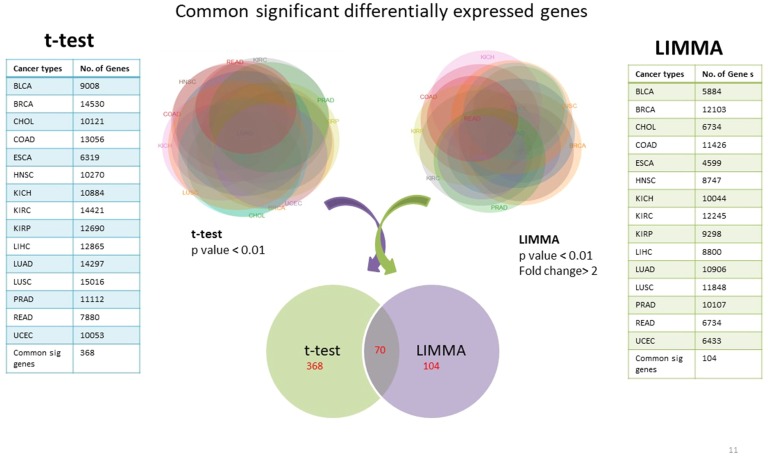
Using TCGA RNAseqv2 data, we analyzed a total of 6,202 samples from 15 cancer types out of which 5,601 were tumors and 601 were corresponding normal samples. Total number of coding genes analyzed was 20,531. The number of samples within each tumor type is enlisted in Table 1. 368 significantly differentially expressed genes common to all cancer types were identified using unpaired t-test with critical p-value <0.01 and 104 such genes were selected using LIMMA (Linear Model for Microarray Analysis) with p-value <0.01 and fold change >2 (Fig. 2). In order to narrow down to more significant genes list, we pooled together genes from both t-test and LIMMA results and constructed a Venn diagram. 70 common significantly differentially expressed genes were enlisted (Table 2).
Table 1. Tumor types and number of samples in each tumor in our study taken from TCGA RNAseq v2 data.
These cancer types are used in studies on protein-coding gene expression. Italicized cancer types are those used in non-protein coding lncRNA expression studies also.
| S. NO | Cancer type | Tumor samples | Normal samples | Total |
|---|---|---|---|---|
| 1 | Bladder Urothelial Carcinoma [BLCA] | 408 | 19 | 427 |
| 2 | Breast invasive carcinoma [BRCA] | 1,112 | 113 | 1,225 |
| 3 | Cholangiocarcinoma [CHOL] | 36 | 9 | 45 |
| 4 | Colon adenocarcinoma [COAD] | 286 | 41 | 327 |
| 5 | Esophageal carcinoma [ESCA] | 185 | 11 | 196 |
| 6 | Head and Neck squamous cell carcinoma [HNSC] | 522 | 44 | 566 |
| 7 | Kidney Chromophobe [KICH] | 66 | 25 | 91 |
| 8 | Kidney renal clear cell carcinoma [KIRC] | 534 | 72 | 606 |
| 9 | Kidney renal papillary cell carcinoma [KIRP] | 290 | 32 | 322 |
| 10 | Liver hepatocellular carcinoma [LIHC] | 374 | 50 | 424 |
| 11 | Lung adenocarcinoma [LUAD] | 517 | 59 | 576 |
| 12 | Lung squamous cell carcinoma [LUSC] | 502 | 51 | 553 |
| 13 | Prostate adenocarcinoma [PRAD] | 497 | 52 | 549 |
| 14 | Rectum adenocarcinoma [READ] | 95 | 10 | 105 |
| 15 | Uterine Corpus Endometrial Carcinoma [UCEC] | 177 | 13 | 190 |
| Total | 6,202 | |||
Figure 2. Common significantly differentially expressed protein-coding genes from t-test (at p-value <0.01) & LIMMA (at p-value <0.01, fold change >2.0).
Table 2. Common significantly differentially expressed genes in all 15 types of cancer.
| Gene name | Protein name | Gene name | Protein name |
|---|---|---|---|
| ACACB | Acetyl-CoA Carboxylase Beta | NOP2 | NOP2 Nucleolar Protein |
| ALAD | Aminolevulinate Dehydratase | NR2C2AP | Nuclear Receptor 2C2 Associated Protein |
| ASF1B | Anti-Silencing Function 1B Histone Chaperone | NR3C2 | Nuclear Receptor Subfamily 3 Group C Member 2 |
| ATXN2L | Ataxin 2 Like | NSUN5 | NOP2/Sun RNA Methyltransferase Family Member 5 |
| AURKA | Aurora Kinase A | NUP85 | Nucleoporin 85 |
| AZI1(CEP13) | 5-Azacytidine-Induced Protein 1 (Centrosomal Protein 131) | NUTF2 | Nuclear Transport Factor 2 |
| C19orf48 | Chromosome 19 Open Reading Frame 48 | OBFC2B | Nucleic Acid Binding Protein 2 |
| C20orf20 | MRG/MORF4L Binding Protein | ORC6L | Origin Recognition Complex Subunit 6 |
| CCDC86 | Coiled-Coil Domain Containing 86 | PAQR4 | Progestin And AdipoQ Receptor Family Member 4 |
| CDCA5 | Cell Division Cycle Associated 5 | PCGF5 | Polycomb Group Ring Finger 5 |
| CHAF1A | Chromatin Assembly Factor 1 Subunit A | PLXNA3 | Plexin A3 |
| CHTF18 | Chromosome Transmission Fidelity Factor 18 | POLD1 | Polymerase (DNA) Delta 1, Catalytic Subunit |
| DHX37 | DEAH-Box Helicase 37 | PPIA | Peptidylprolyl Isomerase A |
| DNAJC9 | DnaJ Heat Shock Protein Family (Hsp40) Member C9 | PPP1R14B | Protein Phosphatase 1 Regulatory Inhibitor Subunit 14B |
| E2F1 | E2F Transcription Factor 1 | PTBP1 | Polypyrimidine Tract Binding Protein 1 |
| EHMT2 | Euchromatic Histone-Lysine N-Methyltransferase 2 | RACGAP1 | Rac GTPase Activating Protein 1 |
| FBXL19 | F-Box And Leucine-Rich Repeat Protein 19 | RNPS1 | RNA Binding Protein With Serine Rich Domain 1 |
| FEN1 | Flap Structure-Specific Endonuclease 1 | RPL36A | Ribosomal Protein L36a |
| FOXM1 | Forkhead Box M1 | SAAL1 | Serum Amyloid A Like 1 |
| FOXN3 | Forkhead Box N3 | SLBP | Stem-Loop Binding Protein |
| GPR146 | G Protein-Coupled Receptor 146 | SNRPB | Small Nuclear Ribonucleoprotein Polypeptides B And B1 |
| GPR172A | Solute Carrier Family 52 Member 2 | SUV420H2 | Lysine Methyltransferase 5C |
| GTPBP3 | GTP Binding Protein 3 | SYNE1 | SYNE1 |
| HN1L | Hematological And Neurological Expressed 1-Like | SYNJ2BP | Synaptojanin 2 Binding Protein |
| ILF3 | Interleukin Enhancer Binding Factor 3 | TCF3 | Transcription Factor 3 |
| IRAK1 | Interleukin 1 Receptor Associated Kinase 1 | TELO2 | Telomere Maintenance 2 |
| KAT2A | Lysine Acetyltransferase 2A | THOC4 | Aly/REF Export Factor |
| KAT2B | Lysine Acetyltransferase 2B | TMEM206 | Transmembrane Protein 206 |
| KIAA0415 | Adaptor Related Protein Complex 5 Zeta 1 Subunit | TRAIP | TRAF Interacting Protein |
| MAD2L1 | MAD2 Mitotic Arrest Deficient-Like 1 | TRMT1 | TRNA Methyltransferase 1 |
| MCM3 | Minichromosome Maintenance Complex Component 3 | TTK | TTK Protein Kinase |
| MND1 | Meiotic Nuclear Divisions 1 | WDR75 | WD Repeat Domain 75 |
| MRPL9 | Mitochondrial Ribosomal Protein L9 | YDJC | Not Available |
| MSH5 | MutS Homolog 5 | ZC3H3 | Zinc Finger CCCH-Type Containing 3 |
| MSL3L2 | Not Available | ZNF668 | Zinc Finger Protein 668 |
Identification of up- and down- regulated genes
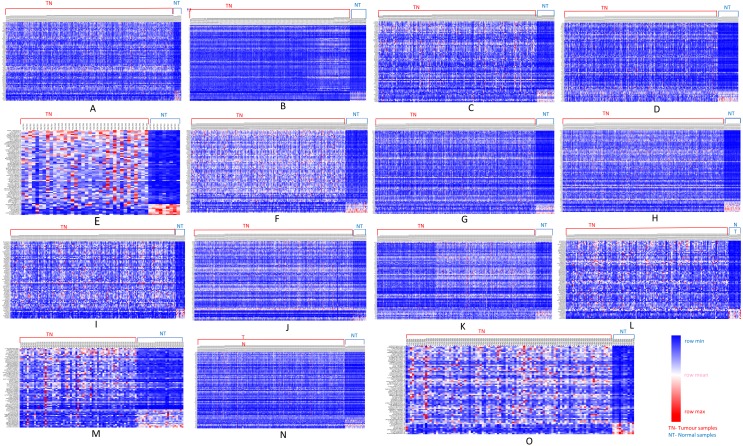
We further analyzed our data to identify which of these genes are up-regulated and which ones are down-regulated in cancers as compared to normal tissues. For this purpose, comparative marker selection in GenePattern suite of programs was used. We observed that out of these 70 commonly dysregulated genes, 48 were up-regulated and 4 genes down-regulated in all 15 types of tumors while remaining 18 were significantly differentially expressed genes that differed in their expression pattern in one or two tumor types only and therefore, still taken as showing a uniform pattern of expression (Fig. 3). These have been tabulated in Table 3.
Figure 3. Heatmap showing up- and down-regulated protein-coding genes in 15 types of tumors.
Rows refer to genes and columns are the samples. The red color bar indicates genes with higher expression levels and the blue color indicates genes with lower expression levels. N: Normal samples, T: Tumor samples. (A) BLCA, (B) BRCA, (C) KIRP, (D) LIHC, (E) CHOL, (F) COAD, (G) LUAD, (H) LUSC, (I) ESCA, (J) HNSC, (K) PRAD, (L) UCEC, (M) KICH, (N) KIRC, (O) READ.
Table 3. List of up-and down-regulated genes common across 15 cancer types.
| Genes | ||
|---|---|---|
| Up-regulated genes | ASF1B, ATXN2L, AURKA, AZI1, C19orf48, C20orf20, CCDC86, CDCA5, CHAF1A, CHTF18, DHX37, DNAJC9, E2F1, FBXL19, FEN1, FOXM1, GPR172A, GTPBP3, ILF3, IRAK1, KIAA0415, MAD2L1, MND1, NOP2, NR2C2AP, NSUN5, NUTF2, ORC6L, PAQR4, PLXNA3, POLD1, PPIA, PPP1R14B, PTBP1, RACGAP1, RNPS1, RPL36A, SAAL1, SNRPB, SUV420H2, TCF3, TELO2, TRAIP, TRMT1, TTK, YDJC, ZC3H3,ZNF668 | These genes are commonly up-regulated in all 15 types of cancers. |
| Down-regulated genes | ALAD, KAT2B, PCGF5, SYNE1. | These genes are down-regulated in 15 types of cancers. |
| Common significantly differentially expressed genes up-regulated or down-regulated in 1 or 2 tumors | ACACB, EHMT2, FOXN3, GPR146, HN1L, KAT2A, MCM3, MARPL9, MSH5, MSL3L2, NR3C2, NUP85, OBFC2B, SLBP, SYNJ2BP, THOC4, TMEM206, WDR75. |
Functional annotation
We annotated the functioning of these 70 common significantly differentially expressed genes using GeneCodis3 web-server implementing Gene Ontology (GO) resources for biological processes. Except two genes, these genes (Table 4) were found to be involved in prominent biological processes such as gene expression, DNA-dependent regulation of transcription, transcription from RNA polymerase-II, DNA replication, mitotic cell cycle and DNA repair in decreasing order of the number of genes involved in each biological process. These are all major processes widely implicated in tumor development and progression.
Table 4. Functional annotation of common significantly differentially expressed protein-coding genes.
| GO.id | No. of genes | Biological process | Hypergeometric p-value | Name of genes |
|---|---|---|---|---|
| GO:0010467 | 9 | gene expression (BP) | 1.18E–07 | RPL36A, NR2C2AP, PTBP1, KAT2B, SNRPB, RNPS1, SLBP, THOC4, NR3C2 |
| GO:0006355 | 10 | regulation of transcription, DNA-dependent (BP) | 0.00124323 | ZNF668, C20orf20, CHAF1A, ASF1B, PCGF5, FOXM1, TCF3, E2F1, SUV420H2, NR3C2 |
| GO:0000278 | 8 | mitotic cell cycle (BP) | 1.65E–07 | MAD2L1, AZI1, POLD1, FEN1, E2F1, AURKA, NUP85, MCM3 |
| GO:0006366 | 6 | transcription from RNA polymerase II promoter (BP) | 2.68E–05 | KAT2A, FOXM1, SNRPB, RNPS1, SLBP, THOC4 |
| GO:0006260 | 5 | DNA replication (BP) | 1.35E–05 | CHAF1A, POLD1, FEN1, MCM3, CHTF18 |
| GO:0006281 | 6 | DNA repair (BP) | 2.25E–05 | OBFC2B, KIAA0415, CHAF1A, POLD1, FOXM1, FEN1 |
Correlation with protein expression levels
It is widely known that expression levels of mRNA and protein may not correlate at times due to the stability issues of mRNA and corresponding protein and further post-transcriptional modifications and regulatory processes. Further, if proteins are to be used as targets, it is important that their levels correlate with their mRNA levels too. In our attempt to understand further if mRNA and protein levels correlate in our list of genes, we used Human Protein Atlas (HPA) database. In HPA, protein expression levels are scored based on staining intensity score from immunohistochemistry experiments in cancer tissues. When mRNAs were found to be up-regulated or down-regulated in tumors and corresponding proteins had a score of high and low immunoreactivity, respectively, it was deciphered to be a positive correlation. A negative correlation was understood to be present when protein intensity score was high while mRNA is downregulated and vice versa. In some cases, we were unable to establish a clear correlation and categorized these genes as ‘uncorrelated or not able to correlate’. Thus, out of 70 genes, 34 genes were positively correlated, 9 genes negatively correlated, and remaining 16 genes were found to be uncorrelated (Table 5 and Fig. S2). Data was not available for 11 of these. Of these 34 positively correlated genes, using GeneCodis3, we found that processes/annotations over-represented for majority of genes were: gene expression, transcription from RNA polymerase-II promoter and DNA repair. Mitotic cell cycle and DNA replication were also among the biological processes involved (Table 6).
Table 5. Correlation of common significantly differentially expressed genes with protein levels (Human Protein Atlas database).
| Correlation type | Genes | Description |
|---|---|---|
| Positively correlated genes | ALAD, ATXN2L, CCDC86, CDCA5, CHAF1A, DHX37, DNAJC9, E2F1, FEN1, FOXM1, GTPBP3, ILF3, IRAK1, KAT2A, MRPL9, NR3C2, NUP85, ORC6L PCGF5, POLD1, PPIA, PPP1R14B, PTBP1, RACGAP1, SAAL1, SNRPB, SLBP, SYNE1, TELO2, THOC4, TRAIP, TTK, WDR75, ZNF668 | Similar expression levels of both mRNA and protein (protein expression levels are calculated based on “colour intensity score”). |
| Negatively correlated genes | AURKA, AZI1, C19ORF48, MAD2L1, MND1, RNPS1, RPL36A, ZC3H3, NUTF2 | High mRNA expression values and negative color intensity score for proteins and vice versa. |
| Not able to establish clear correlation | ACACB, EHMT2, FOXN3, GRP172A, HN1L, KAT2B, MCM3, NR2C2AP, NOP2, NSUN5, OBFC2B, SYNJ2BP, SUV420H2, TCF3, TMEM206, TRMT1 | These genes are having unique expression pattern at mRNA and protein level. |
| Not available | ASF1B, C20ORF20, CHTF18, FBXL19, GPR146, KIAA0415, MSH5, MSL3L2, PAQR4, PLXNA3, YDJC, PLXNA3. | These genes are not available in “Human Protein Atlas database. |
Table 6. Functional annotation of positively correlated protein-coding genes.
| GO.id | No. of genes | Biological process | Hypergeometric p-value | Name of genes |
|---|---|---|---|---|
| GO:0006355 | 6 | regulation of transcription, DNA-dependent | 0.00398166 | ZNF668, CHAF1A, PCGF5, FOXM1, E2F1, NR3C2 |
| GO:0010467 | 5 | gene expression | 4.25E–05 | PTBP1, SNRPB, SLBP, THOC4, NR3C2 |
| GO:0006366 | 5 | transcription from RNA polymerase II promoter | 8.83E–06 | KAT2A, FOXM1, SNRPB, SLBP, THOC4 |
| GO:0007165 | 4 | signal transduction | 0.0257568 | RACGAP1, IRAK1, TRAIP, NR3C2 |
| GO:0007049 | 4 | cell cycle | 0.000788097 | RACGAP1, CHAF1A, FOXM1, CDCA5 |
| GO:0006281 | 4 | DNA repair | 0.000159569 | CHAF1A, POLD1, FOXM1, FEN1 |
| GO:0000278 | 4 | mitotic cell cycle | 0.000204197 | POLD1, FEN1, E2F1, NUP85 |
| GO:0008283 | 3 | cell proliferation | 0.00334808 | KAT2A, E2F1, TRAIP |
Involvement of these genes/proteins in cancer development and progression and their mechanistic actions have been detailed in a separate Supplemental File.
The genes MSL3L2 or MSL3P1 and YDJC appear to be novel and their functions in cancer are unknown, to the best of our knowledge.
To further understand their regulation in cancers, we turned our focus on all of these 34 key essential genes. Apart from regular gene regulators, lncRNAs are among the emerging regulators involved in biological processes predominantly through epigenetic regulation and found to be powerful regulators in cancer as well. Further, epigenetic regulation is at the heart of almost all the biological processes over-represented in our gene set. Therefore, in order to gain further regulatory and functional insights, we proceeded for lncRNA studies.
Differential expression analysis of long non-coding RNAs (lncRNAs)
We analyzed and compared lncRNA expression profiles from 2,497 total samples in 9 types of cancer with their corresponding normal samples numbering 403, using lncRNAtor database. Our objective was not to compare mRNA and lncRNA expression profiles together but to enlist a list of significantly differentially expressed genes common to all cancer types studied. Among significantly aberrantly expressed lncRNA genes in each cancer type identified in lncRNAtor, 24 common lncRNA genes were enlisted (Fig. 4; Table 7).
Figure 4. Common significantly differentially expressed noncoding genes (lncRNAs) in nine types of tumors.
Table 7. Common significantly differentially expressed lncRNAs in nine types of cancers.
| lncRNA ID | lncRNA NAME | |
|---|---|---|
| Up-regulated lncRNAs in tumor |
ENSG00000249859
ENSG00000034063 ENSG00000236455 ENSG00000174365 ENSG00000246582 ENSG00000261373 |
PVT1 UHRF1 AC005154.5 SNHG11 RP11-1149O23.3 RP11-368I7.2 |
| Down-regulated lncRNAs in tumor |
ENSG00000234456
ENSG00000186594 |
MAGI2-AS3 MIR22HG |
| Tumor-specific differentially expressed lncRNAs |
ENSG00000214922
ENSG00000256594 ENSG00000137700 ENSG00000179818 ENSG00000214783 ENSG00000245025 ENSG00000251474 ENSG00000172965 ENSG00000234636 ENSG00000260917 ENSG00000255717 ENSG00000224078 ENSG00000257151 ENSG00000177410 ENSG00000234741 ENSG00000203499 |
HLA-F-AS1 RP11-705C15.2 SLC37A4 PCBP1-AS1 POLR2J4 RP11-875O11.1 RPL32P3 AC068491.1 MED14-AS1 RP11-57H14.4 SNHG1, SNHG14 RP11-701H24.2 ZNFX1-AS1 GAS5 RP11-429J17.6 |
Identification of up- and down-regulated lncRNAs
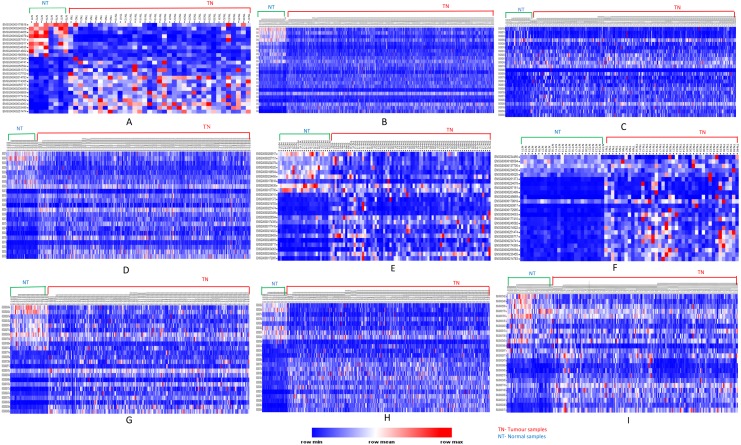
Analyses of up- and down-regulated status of these 24 common significantly differentially expressed lncRNAs, showed that lncRNAs PVT1, UHRF1, AC005154.5, SNHG11, RP11-1149O23.3, and RP11-368I7.2 were up-regulated in tumors; MAGI2-AS3, MIR22HG down-regulated in tumors and the remaining were tumor-specific differentially expressed lncRNAs (Fig. 5).
Figure 5. Heatmap showing common up- and down-regulated lncRNA genes in tumors.
Rows refer to lncRNA and columns are the samples. The red color bar indicates lncRNAs with higher expression levels and the blue color indicates lncRNAs with lower expression levels. N: Normal samples; T: Tumor samples. (A) BLCA, (B) BRCA, (C) HNSC, (D) KIRC, (E) KIRP, (F) LIHC, (G) LUAD, (H) LUSC, (I) PRAD.
Functional analyses of dysregulated lncRNAs
In light of the above studies and our own observations, we proceeded to study the function of lncRNA molecules in more detail. Function of a few lncRNAs such as Xist and NEAT1, both involved in cancer progression, have been experimentally validated (Patil et al., 2016; Qian et al., 2017). While Xist is involved in X-chromosome inactivation (Xiong et al., 2017; Zhang et al., 2017; Song et al., 2016a), NEAT1 plays a regulatory role in initiation and progression of cancers (Fang et al., 2017; Yang et al., 2017a; Li et al., 2016). PVT1 lncRNA functions as an oncogene by acting as a sponge for miRNAs (Li et al., 2018; Liu et al., 2016b; Li, Meng & Yang, 2017) as well as several other mechanisms as has been detailed above. Function of many lncRNAs remains to be discovered. One of the several approaches used to know the likely functions of lncRNAs is “guilt-by-association” with co-expressed protein-coding mRNAs, as these protein-coding genes are likely to be co-regulated by lncRNAs synergistically (Sun et al., 2015). In this manner, role of lncRNAs in specific biological processes and molecular function can be predicted.
We wanted to find out the function of lncRNAs in our narrowed down list. As no de-novo function prediction tool for lncRNAs is available as yet, we used lncRNAtor which predicts lncRNA function from co-expression of protein-coding mRNAs. We characterized each lncRNA for co-expressed mRNAs from lncRNAtor for each of the 9 cancer types. These co-expressed mRNAs were analyzed for their enrichment in biologicals processes and molecular function using GeneCodis3. Most prominent biological processes are common in which these lncRNAs may be involved through “guilt-by-association” are: signal transduction, gene expression, mitotic cell cycle, nerve growth factor receptor signaling pathway, apoptotic process and blood coagulation, in that order. DNA repair and DNA replication processes were also predicted. The predicted molecular functions are in protein binding, nucleotide-binding and ATP binding (Fig. 6 and Fig. S3 ). It is highly interesting to note from our observations that the major biological processes predicted for lncRNAs also correspond to the biological processes predicted for our significantly differentially expressed protein-coding gene list. This shows that these lncRNAs and protein-coding genes from our list may be interacting or regulating each other in one way or another.
Figure 6. Functional enrichment of PVT1 lncRNA using co-expressed protein coding genes from RNAseq data of bladder urothelial carcinoma (representative figure) using GeneCodis.

Hypergeometric p-value of < or = 0.05 (default) was used.
Prediction of interactions between dysregulated mRNAs and lncRNAs
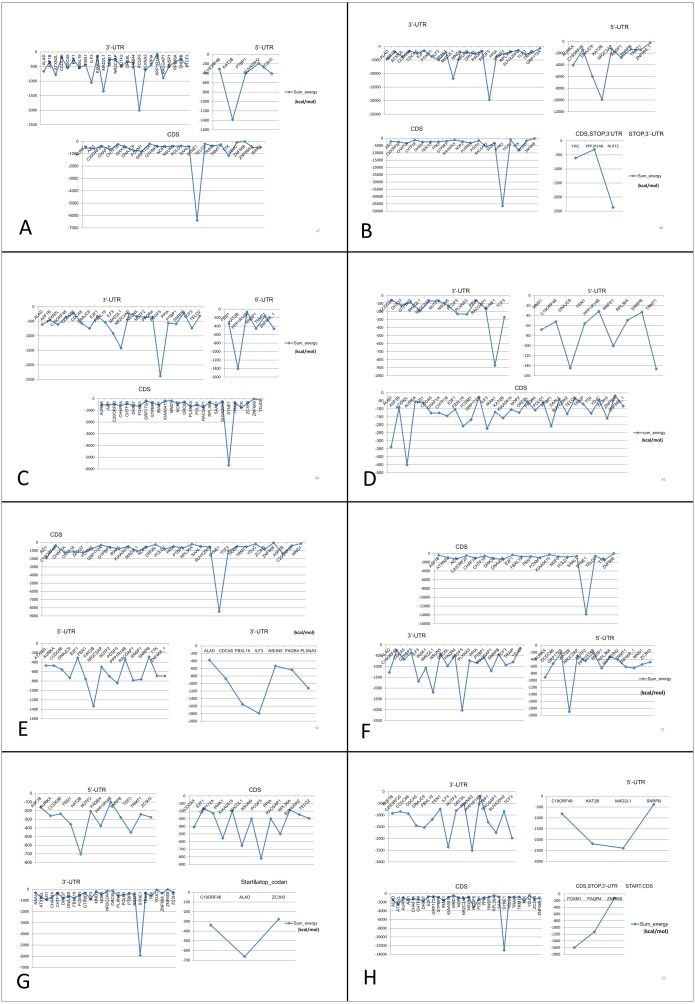
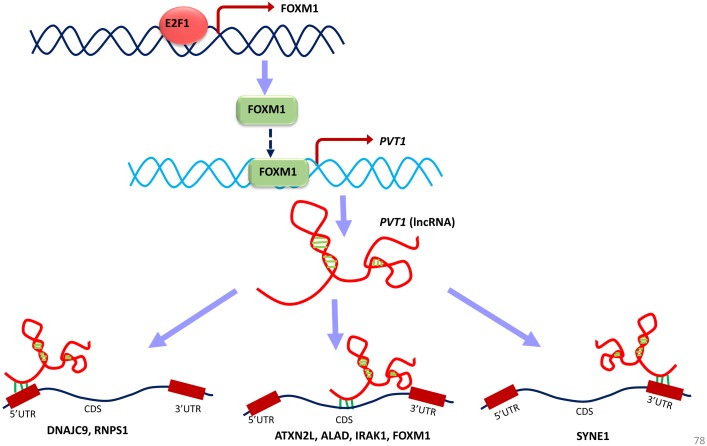
We predicted possible interactions between these eight common (both up- and down-regulated) lncRNAs and mRNAs using lncRNA-mRNA interaction database. Binding energy (sumenergy) values of each interaction were plotted (Fig. 7) and significant interactions based on lower binding energy values were enlisted (Table 8). From the patterns observed from our plots, we identified that SNHG11, AC005154.5, MAGI2-AS3, MIR22HG, RP11-368I7.2, RP11-1149O23.3, and UHRF1 lncRNAs interacted with KAT2B mRNA at 5′-UTR with higher SUMENERGY values except PVT1. Further, all lncRNAs except SNHG11 and PVT1 interacted with SYNE1 at CDS (Coding region). Both SNHG11 and PVT1 were predicted to interact with SYNE1 at 3′UTR. PVT1 had highest interactions with DNAJC9 at 5′-UTR, and with ATXN2L, ALAD, IRAK1 and FOXM1 at CDS sites.
Figure 7. Prediction of lncRNA-mRNA interaction and their binding sites.
Their corresponding binding energies (sumenergy) show the intensity of interactions with higher negative binding energy meaning tighter association leading to more favorable or stable interaction. (A) AC005154.5, (B) MAGI2-AS3, (C) MIR22HG, (D) PVT1, (E) RP11-368I7.2, (F) RP11-1149O23.3, (G) SNHG11, (H) UHRF1.
Table 8. Prediction of lncRNA-mRNA interactions and binding sites.
| lncRNA | Protein-coding gene | Binding site | lncRNA | Protein-coding gene | Binding site |
|---|---|---|---|---|---|
| AC005154.5 |
KAT2B SYNE1 PCGF5 MAD2L1 ILF3 |
5′UTR CDS 3′UTR 3′-UTR 3′-UTR |
RP11-368I7.2 |
KAT2B DNAJC9 PCGF5 SYNE1 ILF3 PLXNA3 |
5′-UTR 5′-UTR 5′UTR CDS 3′-UTR 3′-UTR |
| MAGI2-AS3 |
KAT2B SYNE1 PCGF5 MAD2L1 |
5′-UTR CDS 3′-UTR 3′-UTR |
UHRF1 |
MAD2L1 KAT2B SYNE1 ILF3 PLXNA3 RACGAP1 |
5′-UTR 5′-UTR CDS 3′-UTR 3′-UTR 3′-UTR |
| MIR22HG |
KAT2B SYNE1 PCGF5 MAD2L1 |
5′-UTR CDS 3′-UTR 3′-UTR |
SNHG11 |
KAT2B TCF3 PCGF5 SYNE1 ALAD |
5′-UTR 5′-UTR CDS 3′-UTR START & STOP |
| PVT1 |
DNAJC9 TRMT1 RNPS1 ATXN2L ALAD SYNE1 |
5′-UTR 5′-UTR 5′-UTR CDS CDS 3′UTR |
RP11-1149O23.3 |
KAT2B AURKA RNPS1 SYNE1 PCGF5 NSUN5 IRAK1 ALAD |
5′-UTR 5′-UTR 5′-UTR CDS 3′-UTR 3-UTR 3′UTR 3′-UTR |
MAGI2-AS3 and MIR22HG down-regulated lncRNAs both interact with same genes KAT2B, SYNE1, PCGF5 and MAD2L1 at same site. PVT1 interacts with ATXN2L at CDS, ATXN2L is a positively correlated gene interacting with PVT1 only. RP11-1149O23.3 interacts with AURKA at 5′-UTR, IRAK1 at 3′-UTR which are also positively correlated genes and NSUN5 at 3′-UTR, these genes interact specifically with RP11-1149O23.3 lncRNA. SNHG11 and UHRF1 have unique interactions with positively correlated genes TCF3 and RACGAP1 at 5′-UTR and at 3′-UTR, respectively.
Network analysis of significantly differentially expressed mRNAs and lncRNAs
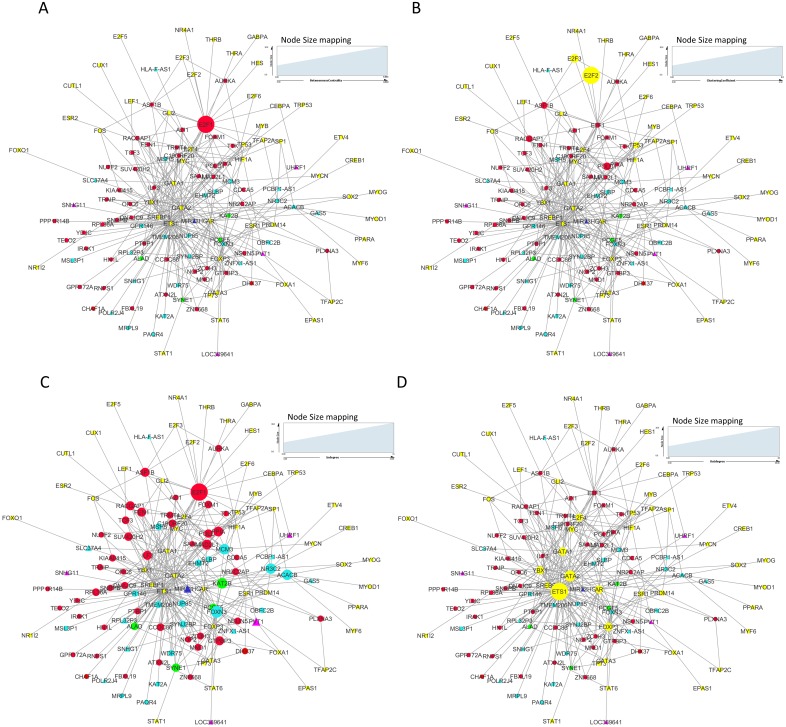
We constructed and analyzed context-specific regulatory network comprising of all the mRNAs/proteins and lncRNAs from our list of significantly differentially expressed molecules (master network). These networks constitute interaction signatures of transcription factors (TFs) and their regulated target genes. A total of 130 nodes (genes/proteins) and 311 edges (the interactions between these nodes) were taken from ORTI database to produce this master regulatory network. The network was analyzed on the basis of several parameters such as node degree, the degree to which one node is connected to other nodes in the network; betweenness centrality, which characterizes nodes that have many shortest paths going through them and clustering coefficient which refers to the extent to which nodes cluster together as a tight unit (Mishra, 2014; Albert & Barabasi, 2002). In a directed TF-gene network, node out-degree refers to number of genes regulated by a TF, while node in-degree refers to number of TFs that target/regulate a gene.
In our master network, analyses of betweenness centrality, clustering coefficient and node degree parameters (Table 9) showed that the node with highest betweenness centrality was E2F1 (Fig. 8A), the one with highest clustering coefficient was E2F2 (Fig. 8B), highest degree node was E2F1 for in-degree (Fig. 8C) and ETS1 for out-degree (Fig. 8D). Nodes E2F3, POLD1, RACGAP1 and ASF1B were also the nodes with higher clustering coefficient, some of which are again in our significantly differentially expressed and positively correlated with proteins list. From our network, we observed that E2F1 regulates POLD1, ASF1B, FOXM1 and RACGAP1 which are up-regulated in all 15 cancer types (Subnetwork, Fig. 9A). ETS1 regulates E2F1 apart from POLD1, ASF1B and RACGAP1 (Subnetwork, Fig. 9B). The direct interactions of these two essential genes with other genes shows that these directly interacting genes may also be playing a role in cancer development and progression by virtue of their association, and provide further insights.
Table 9. Regulatory network analysis of dysregulated protein coding and noncoding genes.
| Genes | Betweenness centrality | Clustering coefficient | Indegree | Outdegree |
|---|---|---|---|---|
| E2F1 | 0.00272529 | 0.02597403 | 19 | 3 |
| E2F2 | 0 | 0.5 | 0 | 2 |
| E2F3 | 0 | 0.16666667 | 0 | 3 |
| POLD1 | 0 | 0.13333333 | 6 | 0 |
| RACGAP1 | 0 | 0.0952381 | 7 | 0 |
| ASF1B | 0 | 0.0952381 | 7 | 0 |
| FOXN3 | 0 | 0 | 11 | 0 |
| KAT2B | 0 | 0 | 9 | 0 |
| NR3C2 | 0 | 0 | 9 | 0 |
| MAD2L1 | 0 | 0 | 8 | 0 |
| PVT1 | 0 | 0 | 6 | 0 |
| FOXM1 | 0 | 0 | 5 | 0 |
| ETS1 | 0 | 6.58E–04 | 0 | 68 |
| GATA2 | 0 | 0.00168067 | 0 | 35 |
| HIF1A | 0 | 0 | 0 | 5 |
Figure 8. Master regulatory network parameters.
(A) Betweenness centrality: E2F1 showing highest betweenness centrality; (B) Clustering coefficient: E2F2, E2F3, ASF1B, POLD1 and RACGAP1 genes are clustered together; (C) Indegree: Number of inward directed nodes, E2F1 has highest number of in-degree nodes; (D) Outdegree: Number of outward directed nodes, ETS1 has highest out degree node. The node size increases with values of the respective topological parameter.
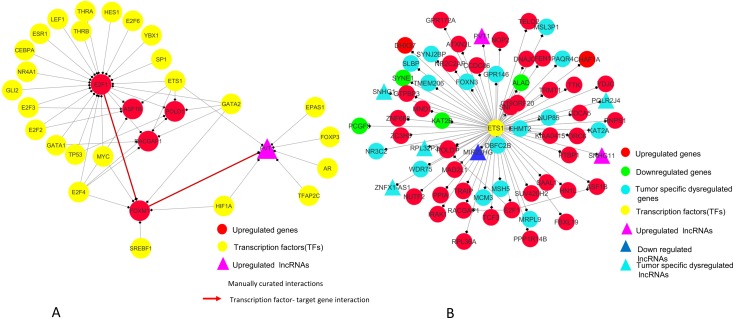
Figure 9. E2F1 and ETS1 subnetworks generated from master regulatory network.
(A) E2F1 subnetwork (generated from master regulatory network). E2F1 is seen directly connected to tumor-specific dysregulated transcription factors and common significantly differentially expressed genes POLD1, RACGAP1 and ASF1B. The red color edges are manually curated TF-TG interactions from the literature (Millour et al., 2011; De Olano et al., 2012). (B) ETS1 subnetwork (generated from master regulatory network). ETS1 is a tumor-specific TF in at least one cancer type, directly connected with most of the common significantly differentially expressed coding and non-coding genes in all types of cancers studied.
On the basis of quantitative data, E2F1 can be a hub bottleneck gene while ETS1 can be a hub gene. Among lncRNAs, PVT1 was found to be the node with highest degree thereby acting as a hub molecule. The other lncRNA with high node degree was MIR22HG. So, for analyzing subnetworks, we selected E2F1 and ETS1 among coding genes and PVT1 among all lncRNAs genes for further deeper understanding based on the following criteria:
-
a.
E2F1 is a gene positively correlated with protein expression levels and is up-regulated in all tumor types studied. It also has highest node in-degree and highest betweenness centrality in the network.
-
b.
ETS1 is directly connected to E2F1. It is also the node with highest out-degree directly connected to PVT1, SNHG11 and MIR22HG. Though it is not in our list of common genes (it was connected by ORTI database while generating networks using our genes list), by dint of its direct connections to all other key molecules from our data and by being a proto-oncogene, it is also studied further to discern patterns in regulation.
-
c.
PVT1 lncRNA gene is up-regulated in all tumor types studied.
-
d.
It is predicted to interact with SYNE1 at 3′-UTR, with DNAJC9 and RNPS1 at 5′-UTR and FOXM1, IRAK1, ATXN2L and ALAD at CDS sites.
-
e.
PVT1 function is known, i.e., it is an oncogene. Its localization is both cytoplasmic and nuclear in SiHa (cervical cancer cell line) (Iden et al., 2016) while in 85% of SK-BR-3 breast cancer cells, a nuclear co-localization of MYC and PVT1 was observed (Tseng et al., 2014).
While function of SNHG11 is unknown, we hypothesize that primarily by its expression, interaction and network patterns similar to PVT1, its function can be predicted by extrapolation. Further, the only other coding gene interacting with it in the network is ETS1, the proto-oncogene as mentioned above, so its importance cannot be overlooked.
ETS1 and GATA2 transcription factors regulate most of the common significant differentially expressed genes/lncRNAs as seen from the node degree (especially out-degree) regulatory network (Fig. 8D). However, these two genes are not in our list of significantly differentially expressed gene across all tumor types, and were added by ORTI database during regulatory network construction. ETS1 showed negative immunoreactivity in all types of cancers while GATA2 showed moderate to strong positive immunoreactivity in all tumor types.
Discussion
From our studies, we identified common coding and non-coding (lncRNAs) genes from various cancer types. Correlation pattern of mRNA with corresponding protein expression levels was also observed. Most of these genes were found to be involved in cancer progression-associated pathways such as gene expression, cell cycle regulation and nerve growth factor signaling. We hypothesized that the differential correlation between mRNA and protein expression levels may be due to the critical role of lncRNAs in regulating mRNA stability, degradation and role in protein synthesis.
Analysis of lncRNA-mRNA interactions of our dysregulated coding and non-coding genes list showed that in particular, PVT1 lncRNA interacting genes were among common coding genes. PVT1 is a widely studied lncRNA. SYNE1 (Nesprin 1), one of the PVT1 interacting genes, is a nuclear envelope protein, residing in outer nuclear membrane, and has been shown to play a role in cancers, the mode being speculated to be through DNA damage response pathway (Sur, Neumann & Noegel, 2014). Interactions of all of the lncRNAs from our dataset with SYNE1 mRNA shows its major prominent role. We hypothesize that since SYNE1 is found to be a part of complex linking nucleoskeleton and cytoskeleton to maintain spatial organization, its mRNA interactions with lncRNAs may be somehow involved in regulation/dysregulation of cancer cell migration and metastasis through possible nuclear membrane rupture and repairing processes (Denais et al., 2016). The nuclear membrane rupture may also drive genomic instability in cancers (Lim, Quinton & Ganem, 2016). Even altered nuclear shape and size may be a cancer driver. Further studies with SYNE1 protein expression and lncRNA interactions will throw more light into its role in cancer development and progression in all cancer types.
KAT2B interactions with most of the lncRNAs from our list shows that these lncRNAs may play a major role in epigenetic regulation in cancers, since KAT2B protein is involved in lysine acetyltransferase activity in case of histone molecules. However, KAT2B is not in our list of molecules with positive correlation between mRNA and protein levels. From our analysis, RNPS1 and AURKA mRNA expression level is negatively correlated with protein expression level. Because PVT1 is predicted to interact with 5′-UTR region of RNPS1 mRNA, and is found localized in both nucleus and cytoplasm of cervical cancer cells (Iden et al., 2016), this may be one possible mechanism of regulating RNPS1 mRNA translation in cytoplasm. Similar case may hold for RP11-1149O23.3 lncRNA in AURKA translation regulation. In case of other coding genes which are positively correlated with protein expression levels, the lncRNA interaction may play a role in mRNA stability.
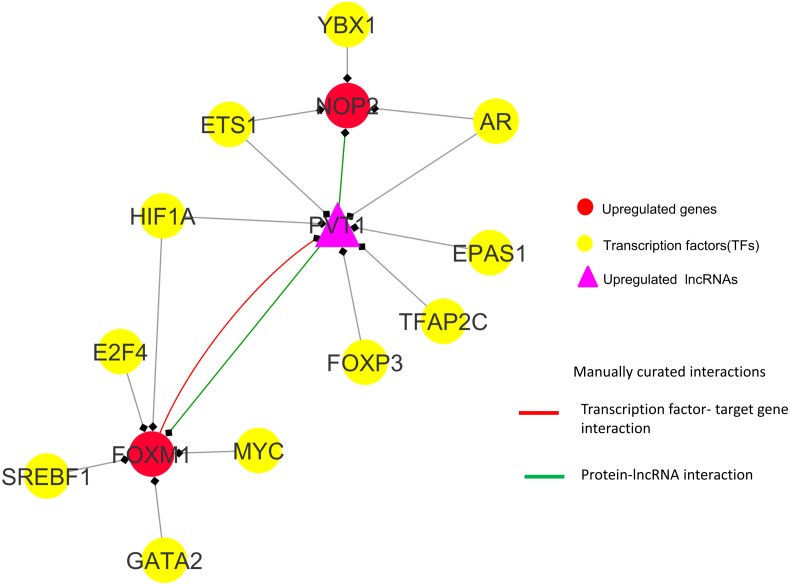
Observations from the connectivity patterns in the network show that E2F1 may be indirectly regulated by PVT1, and possibly also SNHG11, with ETS1 being a mediator between the two coding and non-coding genes. Further, PVT1 transcription is regulated by FOXM1, another significantly differentially expressed coding gene from our results (Fig. 9A). PVT1 also binds and stabilizes FOXM1 and NOP2 protein (Millour et al., 2011; Wang et al., 2014) (Fig. 10). This experimental conclusion from literature is validated by our prediction of lncRNA-mRNA interactions (Fig. 7).
Figure 10. FOXM1, NOP2 and PVT1 subnetwork generated from master regulatory network.
TF-TG interaction between FOXM1 and PVT1 (red colored edge), lncRNA-protein interactions between FOXM1, NOP2 and PVT1 (green colored edges) were taken from the literature and manually added as extra information (Xu et al. (2017) and Wang et al. (2014), respectively).
Converging our varied set of results into one unified summary, we put forward a working model of the coding and non-coding genes’ regulation in all cancer types (Fig. 11). In the model, E2F1 regulates the FOXM1 transcription (Millour et al., 2011; De Olano et al., 2012), and when FOXM1 protein is translated, it regulates PVT1 transcription. This E2F1-FOXM1-PVT1 regulatory path was identified from our network studies. PVT1 promotes cell progression, growth, invasion, and acquisition of stem cell-like properties by stabilizing FOXM1 and NOP2 proteins in breast cancer and hepatocellular carcinoma, respectively (Millour et al., 2011; Wang et al., 2014). Our studies show that this may be the case in multiple cancer types as well. PVT1 after being transcribed further binds to 5′-UTR, CDS and 3′-UTR of the genes in our results, specifically, DNAJC9 (at 5′-UTR), ATXN2L, ALAD, FOXM1, IRAK1 at CDS and SYNE1 at 3′-UTR and may be regulating these genes through processes such as mRNA splicing, stability and degradation. ETS1, though not in our list of significantly differentially expressed gene across all tumor types, regulates at multiple levels: at the level of E2F1, FOXM1 and PVT1, but due to its negative immunoreactivity in all tumor types, it may be doing so at gene/mRNA level. Or it may be even working with non-coding genes to establish the regulatory orders. It remains to be seen, whether it may be involved in regulation in multiple cancer types. Further investigations will be required to understand if multiple lncRNAs can regulate the same gene or a set of genes by binding to the same or a different location. From a look at Table 8, from the given binding patterns, we surmise that multiple lncRNAs may regulate a single gene by binding to the same location at different stages in regulatory process. Several key genes may be missed because of limitations such as the p-value cutoff used and need for other cancer types to be included in case of lncRNA genes. The hypotheses generated here require further validation by qPCR and other molecular biology techniques.
Figure 11. A unified model of pan-cancer regulation of common coding genes’ (mRNA) by non-coding genes (lncRNAs).
E2F1, FOXM1 and PVT1 genes are identified as common significantly differentially expressed coding and non-coding genes from our analysis. E2F1 up-regulates FOXM1 expression and FOXM1 up-regulates PVT1 lncRNA expression. PVT1 may play a role in regulation of mRNA splicing, stability and degradation of target genes to which it is predicted to bind such as DNAJC9, RNPS1, ATXN2L, ALAD, IRAK1, FOXM1 and SYNE1.
Conclusions
In this study, we have analyzed pan-cancer gene expression using RNASeq data and the mechanistic role and interplay of both coding and long non-coding RNA genes in pan-cancer gene regulation. Analyzing their cross-interacting patterns, among a total of about 20,531 protein-coding and as many non-coding RNA genes, we have zeroed in on the major players: E2F1, FOXM1 (transcription factors) and PVT1 (lncRNA). The regulatory path defined by these key genes is a pan-cancer recurrent pattern. Our studies show that E2F1 transcription factor may be indirectly regulated by PVT1 lncRNA. Transcription factor ETS1 may be playing a powerful mediator role between these two coding and non-coding genes. Several key insights gained into the interplay of these emerging novel class of gene regulators may help us understand pan-cancer gene regulation and provide novel cancer drug targets.
Supplemental Information
First row shows co-expressed gene networks, second row as pathway gene networks and third row as physically intercating gene networks from our list of significantly differentially expressed genes.
For each protein, protein expression levels are scored based on staining intensity score from immunohistochemistry experiments in several cancer tissues. The protein names are mentioned on the top right corner of the figures.
Biological processes and molecular functions predicted by GeneCodis for each lncRNA are shown.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Asanigari Saleembhasha performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Seema Mishra conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The research in this article did not generate any raw data or code. It uses data available in public domain. The raw data is included in the manuscript (in the ‘Materials and Methods’ section and in Supplemental Information) and has been duly credited by naming the source.
References
- Albert & Barabasi (2002).Albert R, Barabasi A-L. Statistical mechanics of complex networks. Reviews of Modern Physics. 2002;74:47–97. doi: 10.1103/RevModPhys.74.47. [DOI] [Google Scholar]
- Bolha, Ravnik-Glavač & Glavač (2017).Bolha L, Ravnik-Glavač M, Glavač D. Long noncoding RNAs as biomarkers in cancer. Disease Markers. 2017;2017:7243968. doi: 10.1155/2017/7243968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2015).Chen T, Xie W, Xie L, Sun Y, Zhang Y, Shen Z, Sha N, Xu H, Wu Z, Hu H, Wu C. Expression of long noncoding RNA lncRNA-n336928 is correlated with tumor stage and grade and overall survival in bladder cancer. Biochemical and Biophysical Research Communications. 2015;468(4):666–670. doi: 10.1016/j.bbrc.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Denais et al. (2016).Denais CM, Gilbert RM, Isermann P, McGregor AL, teLindert M, Weigelin B, Davidson PM, Friedl P, Wolf K, Lammerding J. Nuclear envelope rupture and repair during cancer cell migration. Science. 2016;352(6283):353–358. doi: 10.1126/science.aad7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Olano et al. (2012).De Olano N, Koo CY, Monteiro LJ, Pinto PH, Gomes AR, Aligue R, Lam EW. The p38 MAPK-MK2 axis regulates E2F1 and FOXM1 expression after epirubicin treatment. Molecular Cancer Research. 2012;10(9):1189–1202. doi: 10.1158/1541-7786.MCR-11-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien et al. (2012).Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, Lagarde J, Veeravalli L, Ruan X, Ruan Y, Lassmann T, Carninci P, Brown JB, Lipovich L, Gonzalez JM, Thomas M, Davis CA, Shiekhattar R, Gingeras TR, Hubbard TJ, Notredame C, Harrow J, Guigó R. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Research. 2012;22(9):1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du & Che (2017).Du H1, Che G. Genetic alterations and epigenetic alterations of cancer-associated fibroblasts. Oncology Letters. 2017;13(1):3–12. doi: 10.3892/ol.2016.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium et al. (2007).ENCODE Project Consortium. Birney E, Stamatoyannopoulos JA, Dutta A, Guigó R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE, Kuehn MS, Taylor CM, Neph S, Koch CM, Asthana S, Malhotra A, Adzhubei I, Greenbaum JA, Andrews RM, Flicek P, Boyle PJ, Cao H, Carter NP, Clell GK, Davis S, Day N, Dhami P, Dillon SC, Dorschner MO, Fiegler H, Giresi PG, Goldy J, Hawrylycz M, Haydock A, Humbert R, James KD, Johnson BE, Johnson EM, Frum TT, Rosenzweig ER, Karnani N, Lee K, Lefebvre GC, Navas PA, Neri F, Parker SC, Sabo PJ, Sandstrom R, Shafer A, Vetrie D, Weaver M, Wilcox S, Yu M, Collins FS, Dekker J, Lieb JD, Tullius TD, Crawford GE, Sunyaev S, Noble WS, Dunham I, Denoeud F, Reymond A, Kapranov P, Rozowsky J, Zheng D, Castelo R, Frankish A, Harrow J, Ghosh S, Sandelin A, Hofacker IL, Baertsch R, Keefe D, Dike S, Cheng J, Hirsch HA, Sekinger EA, Lagarde J, Abril JF, Shahab A, Flamm C, Fried C, Hackermüller J, Hertel J, Lindemeyer M, Missal K, Tanzer A, Washietl S, Korbel J, Emanuelsson O, Pedersen JS, Holroyd N, Taylor R, Swarbreck D, Matthews N, Dickson MC, Thomas DJ, Weirauch MT, Gilbert J, Drenkow J, Bell I, Zhao X, Srinivasan KG, Sung WK, Ooi HS, Chiu KP, Foissac S, Alioto T, Brent M, Pachter L, Tress ML, Valencia A, Choo SW, Choo CY, Ucla C, Manzano C, Wyss C, Cheung E, Clark TG, Brown JB, Ganesh M, Patel S, Tammana H, Chrast J, Henrichsen CN, Kai C, Kawai J, Nagalakshmi U, Wu J, Lian Z, Lian J, Newburger P, Zhang X, Bickel P, Mattick JS, Carninci P, Hayashizaki Y, Weissman S, Hubbard T, Myers RM, Rogers J, Stadler PF, Lowe TM, Wei CL, Ruan Y, Struhl K, Gerstein M, Antonarakis SE, Fu Y, Green ED, Karaöz U, Siepel A, Taylor J, Liefer LA, Wetterstrand KA, Good PJ, Feingold EA, Guyer MS, Cooper GM, Asimenos G, Dewey CN, Hou M, Nikolaev S, Montoya-Burgos JI, Löytynoja A, Whelan S, Pardi F, Massingham T, Huang H, Zhang NR, Holmes I, Mullikin JC, Ureta-Vidal A, Paten B, Seringhaus M, Church D, Rosenbloom K, Kent WJ, Stone EA, NISC Comparative Sequencing Program. Baylor College of Medicine Human Genome Sequencing Center. Washington University Genome Sequencing Center. Broad Institute. Children’s Hospital Oakland Research Institute. Batzoglou S, Goldman N, Hardison RC, Haussler D, Miller W, Sidow A, Trinklein ND, Zhang ZD, Barrera L, Stuart R, King DC, Ameur A, Enroth S, Bieda MC, Kim J, Bhinge AA, Jiang N, Liu J, Yao F, Vega VB, Lee CW, Ng P, Shahab A, Yang A, Moqtaderi Z, Zhu Z, Xu X, Squazzo S, Oberley MJ, Inman D, Singer MA, Richmond TA, Munn KJ, Rada-Iglesias A, Wallerman O, Komorowski J, Fowler JC, Couttet P, Bruce AW, Dovey OM, Ellis PD, Langford CF, Nix DA, Euskirchen G, Hartman S, Urban AE, Kraus P, Van Calcar S, Heintzman N, Kim TH, Wang K, Qu C, Hon G, Luna R, Glass CK, Rosenfeld MG, Aldred SF, Cooper SJ, Halees A, Lin JM, Shulha HP, Zhang X, Xu M, Haidar JN, Yu Y, Ruan Y, Iyer VR, Green RD, Wadelius C, Farnham PJ, Ren B, Harte RA, Hinrichs AS, Trumbower H, Clawson H, Hillman-Jackson J, Zweig AS, Smith K, Thakkapallayil A, Barber G, Kuhn RM, Karolchik D, Armengol L, Bird CP, deBakker PI, Kern AD, Lopez-Bigas N, Martin JD, Stranger BE, Woodroffe A, Davydov E, Dimas A, Eyras E, Hallgrímsdóttir IB, Huppert J, Zody MC, Abecasis GR, Estivill X, Bouffard GG, Guan X, Hansen NF, Idol JR, Maduro VV, Maskeri B, McDowell JC, Park M, Thomas PJ, Young AC, Blakesley RW, Muzny DM, Sodergren E, Wheeler DA, Worley KC, Jiang H, Weinstock GM, Gibbs RA, Graves T, Fulton R, Mardis ER, Wilson RK, Clamp M, Cuff J, Gnerre S, Jaffe DB, Chang JL, Lindblad-Toh K, Lander ES, Koriabine M, Nefedov M, Osoegawa K, Yoshinaga Y, Zhu B, De Jong PJ. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447(7146):799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezkurdia et al. (2014).Ezkurdia I, Juan D, Rodriguez JM, Frankish A, Diekhans M, Harrow J, Vazquez J, Valencia A, Tress ML. Multiple evidence strands suggest that there may be as few as 19, 000 human protein-coding genes. Human Molecular Genetics. 2014;23(22):5866–5878. doi: 10.1093/hmg/ddu309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang et al. (2017).Fang L, Sun J, Pan Z, Song Y, Zhong L, Zhang Y, Liu Y, Zheng X, Huang P. Long non-coding RNA NEAT1 promotes hepatocellular carcinoma cell proliferation through the regulation of miR-129-5p-VCP-I κB. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2017;313(2):G150–G156. doi: 10.1152/ajpgi.00426.2016. [DOI] [PubMed] [Google Scholar]
- Gong & Maquat (2011).Gong C, Maquat LE. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′UTRs via Alu elements. Nature. 2011;470(7333):284–288. doi: 10.1038/nature09701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta et al. (2010).Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, Van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan & Weinberg (2011).Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Herceg & Hainaut (2007).Herceg Z, Hainaut P. Genetic and epigenetic alterations as biomarkers for cancer detection, diagnosis and prognosis. Molecular Oncology. 2007;1(1):26–41. doi: 10.1016/j.molonc.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iden et al. (2016).Iden M, Fye S, Li K, Chowdhury T, Ramchandran R, Rader JS. The lncRNA PVT1 contributes to the cervical cancer phenotype and associates with poor patient prognosis. PLOS ONE. 2016;11(5):e0156274. doi: 10.1371/journal.pone.0156274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail et al. (2000).Ismail RS, Baldwin RL, Fang J, Browning D, Karlan BY, Gasson JC, Chang DD. Differential gene expression between normal and tumor-derived ovarian epithelial cells. Cancer Research. 2000;60(23):6744–6749. [PubMed] [Google Scholar]
- Kopnin (2000).Kopnin BP. Targets of oncogenes and tumor suppressors: key for understanding basic mechanisms of carcinogenesis. Biochemistry. 2000;65(1):2–27. [PubMed] [Google Scholar]
- Kotake et al. (2011).Kotake Y, Nakagawa T, Kitagawa K, Suzuki S, Liu N, Kitagawa M, Xiong Y. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene. 2011;30(16):1956–1962. doi: 10.1038/onc.2010.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung, Colognori & Lee (2013).Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics. 2013;193(3):651–669. doi: 10.1534/genetics.112.146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2018).Li H, Chen S, Liu J, Guo X, Xiang X, Dong T, Ran P, Li Q, Zhu B, Zhang X, Wang D, Xiao C, Zheng S. Long non-coding RNA PVT1-5 promotes cell proliferation by regulating miR-126/SLC7A5 axis in lung cancer. Biochemical and Biophysical Research Communications. 2018;495(3):2350–2355. doi: 10.1016/j.bbrc.2017.12.114. [DOI] [PubMed] [Google Scholar]
- Li, Meng & Yang (2017).Li T, Meng XL, Yang WQ. Long noncoding RNA PVT1 acts as a sponge to inhibit microRNA-152 in Gastric Cancer Cells. Digestive Diseases and Sciences. 2017;62(11):3021–3028. doi: 10.1007/s10620-017-4508-z. [DOI] [PubMed] [Google Scholar]
- Li et al. (2016).Li Z, Wei D, Yang C, Sun H, Lu T, Kuang D. Overexpression of long noncoding RNA, NEAT1 promotes cell proliferation, invasion and migration in endometrial endometrioid adenocarcinoma. Biomedicine and Pharmacotherapy. 2016;84:244–251. doi: 10.1016/j.biopha.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Lim, Quinton & Ganem (2016).Lim S, Quinton RJ, Ganem NJ. Nuclear envelope rupture drives genome instability in cancer. Molecular Biology of the Cell. 2016;27(21):3210–3213. doi: 10.1091/mbc.e16-02-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2016b).Liu HT, Fang L, Cheng YX, Sun Q. LncRNA PVT1 regulates prostate cancer cell growth by inducing the methylation of miR-146a. Cancer Medicine. 2016b;5(12):3512–3519. doi: 10.1002/cam4.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2016a).Liu B, Pan CF, He ZC, Wang J, Wang PL, Ma T, Xia Y, Chen YJ. Long Noncoding RNA-LET suppresses tumor growth and emt in lung adenocarcinoma. BioMed Research International. 2016a;2016:4693471. doi: 10.1155/2016/4693471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, Huber & Anders (2014).Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Bajic & Zhang (2013).Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biology. 2013;10(6):925–933. doi: 10.4161/rna.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick & Rinn (2015).Mattick JS, Rinn JL. Discovery and annotation of long noncoding RNAs. Nature Structural & Molecular Biology. 2015;22:5–7. doi: 10.1038/nsmb.2942. [DOI] [PubMed] [Google Scholar]
- Mercer & Mattick (2013).Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nature Structural & Molecular Biology. 2013;20:300–307. doi: 10.1038/nsmb.2480. [DOI] [PubMed] [Google Scholar]
- Millour et al. (2011).Millour J, De Olano N, Horimoto Y, Monteiro LJ, Langer JK, Aligue R, Hajji N, Lam EW. ATM and p53 regulate FOXM1 expression via E2F in breast cancer epirubicin treatment and resistance. Molecular Cancer Therapeutics. 2011;10(6):1046–1058. doi: 10.1158/1535-7163.MCT-11-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra (2014).Mishra S. CSNK1A1 and Gli2 as novel targets identified through an integrative analysis of gene expression data, protein-protein interaction and pathways networks in glioblastoma tumors: can these two be antagonistic proteins? Cancer Informatics. 2014;13:93–108. doi: 10.4137/CIN.S18377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey et al. (2014).Pandey GK, Mitra S, Subhash S, Hertwig F, Kanduri M, Mishra K, Fransson S, Ganeshram A, Mondal T, Bandaru S, Ostensson M, Akyürek LM, Abrahamsson J, Pfeifer S, Larsson E, Shi L, Peng Z, Fischer M, Martinsson T, Hedborg F, Kogner P, Kanduri C. The risk-associated long noncoding RNA NBAT-1 controls neuroblastoma progression by regulating cell proliferation and neuronal differentiation. Cancer Cell. 2014;26(5):722–737. doi: 10.1016/j.ccell.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Patil et al. (2016).Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, Jaffrey SR. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537(7620):369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prensner et al. (2011).Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, Laxman B, Asangani IA, Grasso CS, Kominsky HD, Cao X, Jing X, Wang X, Siddiqui J, Wei JT, Robinson D, Iyer HK, Palanisamy N, Maher CA, Chinnaiyan AM. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nature Biotechnology. 2011;29(8):742–749. doi: 10.1038/nbt.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian et al. (2017).Qian K, Liu G, Tang Z, Hu Y, Fang Y, Chen Z, Xu X. The long non-coding RNA NEAT1 interacted with miR-101 modulates breast cancer growth by targeting EZH2. Archives of Biochemistry and Biophysics. 2017;615:1–9. doi: 10.1016/j.abb.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Sadikovic, Al-Romaih & Squire J.A (2008).Sadikovic B, Al-Romaih K, Squire J.A Zielenska, M. Cause and consequences of genetic and epigenetic alterations in human cancer. Current Genomics. 2008;9(6):394–408. doi: 10.2174/138920208785699580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salameh et al. (2015).Salameh A, Lee AK, Cardó-Vila M, Nunes DN, Efstathiou E, Staquicini FI, Dobroff AS, Marchiò S, Navone NM, Hosoya H, Lauer RC, Wen S, Salmeron CC, Hoang A, Newsham I, Lima LA, Carraro DM, Oliviero S, Kolonin MG, Sidman RL, Do KA, Troncoso P, Logothetis CJ, Brentani RR, Calin GA, Cavenee WK, Dias-Neto E, Pasqualini R, Arap W. PRUNE2 is a human prostate cancer suppressor regulated by the intronic long noncoding RNA PCA3. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(27):8403–8408. doi: 10.1073/pnas.1507882112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt & Chang (2016).Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452–463. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song et al. (2016b).Song W, Liu YY, Peng JJ, Liang HH, Chen HY, Chen JH, He WL, Xu JB, Cai SR, He YL. Identification of differentially expressed signatures of long non-coding RNAs associated with different metastatic potentials in gastric cancer. Journal of Gastroenterology. 2016b;51(2):119–129. doi: 10.1007/s00535-015-1091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song et al. (2016a).Song P, Ye LF, Zhang C, Peng T, Zhou XH. Long non-coding RNA XIST exerts oncogenic functions in human nasopharyngeal carcinoma by targeting miR-34a-5p. Gene. 2016a;592(1):8–14. doi: 10.1016/j.gene.2016.07.055. [DOI] [PubMed] [Google Scholar]
- Sun et al. (2015).Sun M, Gadad SS, Kim DS, Kraus WL. Discovery, annotation, and functional analysis of long noncoding RNAs controlling cell-cycle gene expression and proliferation in breast cancer cells. Molecular Cell. 2015;59(4):698–711. doi: 10.1016/j.molcel.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur, Neumann & Noegel (2014).Sur I, Neumann S, Noegel AA. Nesprin-1 role in DNA damage response. Nucleus. 2014;5(2):173–191. doi: 10.4161/nucl.29023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tano et al. (2010).Tano K, Mizuno R, Okada T, Rakwal R, Shibato J, Masuo Y, Ijiri K, Akimitsu N. MALAT-1 enhances cell motility of lung adenocarcinoma cells by influencing the expression of motility-related genes. FEBS Letters. 2010;584(22):4575–4580. doi: 10.1016/j.febslet.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Terai et al. (2016).Terai G, Iwakiri J, Kameda T, Hamada M, Asai K6. Comprehensive prediction of lncRNA-RNA interactions in human transcriptome. BMC Genomics. 2016;17(Suppl 1):12. doi: 10.1186/s12864-015-2307-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng et al. (2014).Tseng YY, Moriarity BS, Gong W, Akiyama R, Tiwari A, Kawakami H, Ronning P, Reul B, Guenther K, Beadnell TC, Essig J, Otto GM, O’Sullivan MG, Largaespada DA, Schwertfeger KL, Marahrens Y, Kawakami Y, Bagchi A. PVT1 dependence in cancer with MYC copy-number increase. Nature. 2014;512(7512):82–86. doi: 10.1038/nature13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2014).Wang F, Yuan JH, Wang SB, Yang F, Yuan SX, Ye C, Yang N, Zhou WP, Li WL, Li W, Sun SH. Oncofetal long noncoding RNA PVT1 promotes proliferation and stem cell-like property of hepatocellular carcinoma cells by stabilizing NOP2. Hepatology. 2014;60(4):1278–1290. doi: 10.1002/hep.27239. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2018).Wang Z, B Yang, Zhang M, Guo W, Wu Z, Wang Y, Jia L, Li S, Cancer Genome Atlas Research Network . andXie, W, Yang D. lncRNA epigenetic landscape identifies EPIC1 as an oncogenic lncRNA that interacts with MYC and promotes cell cycle progression in cancer. Cancer Cell. 2018;33(4):706–720. doi: 10.1016/j.ccell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong et al. (2017).Xiong Y, Wang L, Li Y, Chen M, He W, Qi L. The long non-coding RNA XIST interacted with MiR-124 to modulate bladder cancer growth, invasion and migration by targeting androgen receptor (AR) Cellular Physiology and Biochemistry. 2017;43(1):405–418. doi: 10.1159/000480419. [DOI] [PubMed] [Google Scholar]
- Xu et al. (2017).Xu MD, Wang Y, Weng W, Wei P, Qi P, Zhang Q, Tan C, Ni SJ, Dong L, Yang Y, Lin W, Xu Q, Huang D, Huang Z, Ma Y, Zhang W, Sheng W8, Du X. A positive feedback loop of lncRNA-PVT1 and FOXM1 facilitates gastric cancer growth and invasion. Clinical Cancer Research. 2017;23(8):2071–2080. doi: 10.1158/1078-0432.CCR-16-0742. [DOI] [PubMed] [Google Scholar]
- Yan et al. (2015).Yan X, Hu Z, Feng Y, Hu X, Yuan J, Zhao SD, Zhang Y, Yang L, Shan W, He Q, Fan L, Kandalaft LE, Tanyi JL, Li C, Yuan CX, Zhang D, Yuan H, Hua K, Lu Y, Katsaros D, Huang Q, Montone K, Fan Y, Coukos G, Boyd J, Sood AK, Rebbeck T, Mills GB, Dang CV, Zhang L. Comprehensive genomic characterization of long non-coding RNAs across human cancers. Cancer Cell. 2015;28(4):529–540. doi: 10.1016/j.ccell.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2017a).Yang X, Xiao Z2, Du X, Huang L, Du G. Silencing of the long non-coding RNA NEAT1 suppresses glioma stem-like properties through modulation of the miR-107/CDK6 pathway. Oncology Reports. 2017a;37(1):555–562. doi: 10.3892/or.2016.5266. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2017b).Yang ZY, Yang F, Zhang YL, Liu B, Wang M, Hong X, Yu Y, Zhou YH, Zeng H. LncRNA-ANCR down-regulation suppresses invasion and migration of colorectal cancer cells by regulating EZH2 expression. Cancer Biomark. 2017b;18(1):95–104. doi: 10.3233/CBM-161715. [DOI] [PubMed] [Google Scholar]
- Yap et al. (2010).Yap KL, Li S, Muñoz Cabello AM, Raguz S, Zeng L, Mujtaba S, Gil J, Walsh MJ, Zhou MM. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Molecular Cell. 2010;38(5):662–674. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2017).Zhang YL, Li XB, Hou YX, Fang NZ, You JC, Zhou QH. The lncRNA XIST exhibits oncogenic properties via regulation of miR-449a and Bcl-2 in human non-small cell lung cancer. (This article has been corrected since Advanced Online Publication, and an erratum is also printed in this issue) Acta Pharmacologica Sinica. 2017;38(3):371–381. doi: 10.1038/aps.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2016).Zhang Y, Xu Y, Feng L, Li F, Sun Z, Wu T, Shi X, Li J, Li X. Comprehensive characterization of lncRNA-mRNA related ceRNA network across 12 major cancers. Oncotarget. 2016;7(39):64148–64167. doi: 10.18632/oncotarget.11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
First row shows co-expressed gene networks, second row as pathway gene networks and third row as physically intercating gene networks from our list of significantly differentially expressed genes.
For each protein, protein expression levels are scored based on staining intensity score from immunohistochemistry experiments in several cancer tissues. The protein names are mentioned on the top right corner of the figures.
Biological processes and molecular functions predicted by GeneCodis for each lncRNA are shown.
Data Availability Statement
The following information was supplied regarding data availability:
The research in this article did not generate any raw data or code. It uses data available in public domain. The raw data is included in the manuscript (in the ‘Materials and Methods’ section and in Supplemental Information) and has been duly credited by naming the source.