Abstract
Three-dimensional printing of composite materials such as polycaprolactone/beta-tricalcium phosphate (PCL/β-TCP) enables the design and manufacturing of scaffolds with advanced geometries, along with improved physical and biological properties for large bone defect repair. Terminal sterilization of the scaffolds is inevitable for clinical applications. Electron beam (E-beam) is nontoxic, and can be used for sterilizing heat-sensitive scaffolds by the use of high radiation dose in a short period of time. In this article, we assessed the influence of E-beam sterilization on the properties of 3D-printed PCL/β-TCP scaffolds, focusing on the key physical and biological properties for bone tissue engineering. More specifically, we characterized the effect of a single-dose E-beam sterilization (25 kGy, ISO 11137) on surface morphology, hydrophilicity, degradation, and mechanical properties of the scaffolds as well as in vitro biological responses. The results showed that E-beam irradiation did not alter the surface properties of scaffolds. A 14% increase in initial mechanical stiffness and strength of the scaffolds was observed after E-beam treatment. In addition, the E-beam-treated scaffolds had 25% faster degradation. The PCL chains within the scaffolds had larger polydispersity after the E-beam irradiation that was indicative of a concurrent cross-linking and chain scission. Moreover, in vitro cell studies showed no influence of E-beam sterilization on viability, attachment, proliferation, and osteogenic differentiation of cells seeded on the PCL/β-TCP scaffolds.
Impact Statement
Providing customized geometries and improved control in physical and biological properties, 3D-printed polycaprolactone/beta-tricalcium phosphate (PCL/β-TCP) composite constructs are of high interest for bone tissue engineering applications. A critical step toward the translation and clinical applications of these types of scaffolds is terminal sterilization, and E-beam irradiation might be the most relevant method because of PCL properties. Through in vitro experimental testing of both physical and biological properties, it is proven in this article that E-beam irradiation is relevant for sterilization of 3D-printed PCL/β-TCP scaffolds for bone tissue engineering applications.
Keywords: electron beam sterilization, bone tissue engineering, 3D printing, composite, PCL
Introduction
Arange of fabrication techniques, including molding, casting, electrospinning, and additive manufacturing (AM), have been explored in recent years for the manufacturing of scaffolds in bone tissue engineering.1–3 It is well established that optimal scaffold porosity plays a major role in promoting bone ingrowth, regardless of the fabrication technique; hence accelerating the bone healing process.4–6 AM, also known as 3D printing, has recently attracted extensive attention in the bone tissue engineering field because it enables the design and fabrication of porous constructs with a high level of control over the shape, geometry, and size of the pores.7–9 In addition, constructs manufactured by AM are highly reproducible and can be patient specific.
Biodegradable polymers, including polyglycolic acid (PGA), polylactic acid (PLA), or polycaprolactone (PCL), have been studied extensively for bone tissue engineering applications due to their biocompatibility, degradability, and Food and Drug Administration (FDA) clearance for clinical use, but the polymers lack osteoconductivity and their degradable products may cause inflammation due to acidic nature.1–4
Calcium phosphate bioceramics are osteoconductive and bioresorbable, but they are brittle in shear and tension.10–12 Composites based on degradable polymers and bioceramics have therefore been developed to take advantage of the properties of these two classes of materials.13–17 In particular, PCL and β-tricalcium phosphate (β-TCP) composites have attracted extensive attention due to their high biocompatibility, long-term degradation, appropriate mechanical performances, and osteoconductivity.18–21 Moreover, PCL/β-TCP composites can be processed using AM techniques such as fused deposition modeling (FDM).17,22
A critical step toward the translation and clinical applications of PCL/β-TCP-based 3D-printed constructs and devices is terminal sterilization.23 A number of methods including autoclave, dry heat, ethylene oxide (EtO), gamma irradiation (gamma), and electron-beam (E-beam) have been used for sterilization of grafts and medical devices.24,25 Heat-based sterilization methods such as autoclave and dry heat are the gold standards, because they are nontoxic, noncorrosive, highly effective, and inexpensive. However, heat-based methods cannot be used for sterilization of heat-sensitive materials such as PCL (melting temperature 60°C). Ethylene oxide treatment is a low temperature sterilization method with a large penetrating power, but EtO is toxic, and a long period of aeration is required after sterilization to remove secondary residues of materials.26,27
Low temperature ionizing radiation-based methods (gamma or E-beam) are highly suitable for sterilization of heat-sensitive materials and prepacked medical products.24 E-beam radiation uses a pulse technology that provides a higher dosing rate with a shorter exposure time, and is relevant for sterilizing radiation-sensitive materials.25,28 Several commercially available ceramic-based bone scaffold products are sterilized with gamma or E-beam irradiation.24,26 Therefore, E-beam treatment might be a relevant method for the sterilization of 3D-printed PCL/β-TCP scaffolds, even though it is known that radiation affects the properties of PCL and certain bioceramics.24
A number of studies have been conducted to test the effect of radiation-based sterilization methods on bioceramics or PCL.26,29–33 Di Foggia et al. reported that irradiation treatment changed carbonate/phosphate ratio of carbonated hydroxyapatite (HA), and resultantly their chemical and structural characteristics.29 Cottam et al.30 reported an increase in the tensile mechanical strength and a decrease in the degradation rate of PCL films after gamma irradiation.
Further, gamma irradiation caused both scission and cross-linking of the polymer chains, as well as an increase in the polydispersity of PCL chains. Filipczak et al.34 showed that E-beam irradiation did not affect the biocompatibility but improved the mechanical strength of PCL. Oláh et al.31 reported an increase in the apparent Young's modulus of porous PCL scaffolds with increasing E-beam radiation dose. To the best of our knowledge, the effect of E-beam on the characteristics of 3D-printed porous PCL/β-TCP scaffolds has not been reported elsewhere.
In this study, we investigated the effect of E-beam treatment on the physical and biological properties of 3D-printed PCL/β-TCP scaffolds. We used a single dose of radiation (25 kGy) as recommended for E-beam terminal sterilization in ISO 11137-2:2006 standard. The effect of E-beam irradiation on the physical and structural properties of the scaffolds was tested, including hydrophilicity, water retention, surface morphology, molecular weight distribution of PCL chains, degradation kinetics, and mechanical properties during degradation. Further, the effect of E-beam treatment on the cellular response to PCL/β-TCP constructs was evaluated with respect to cell viability, proliferation, and osteogenic differentiation of two different cell lines in vitro.
Materials and Methods
Synthesis of PCL/β-TCP and scaffold manufacturing
Medical-grade polycaprolactone (Sigma-Aldrich) and β-TCP nanopowder with an average particle size of 100 nm (Berkeley Advanced Materials, Inc.) were weighed with a weight ratio of 80:20. Ten percent (wt/v) PCL solution and 5% (wt/v) β-TCP solution in dimethylformamide (DMF) were prepared at 80°C and stirred for 3 h. The PCL and β-TCP solutions were then mixed and thoroughly stirred for an hour. The PCL/β-TCP solution was then precipitated in 4 L of water and thoroughly rinsed to remove any trace of solvent. The precipitated composite was air dried for 24 h at room temperature, and then extruded using a screw-based filament extruder to make composite filaments.
Scaffolds were manufactured using an FDM 3D printer Lulzbot Mini (Aleph Objects, Inc.) with a nozzle diameter of 0.5 mm. The printing temperature was set to 160°C and the layer thickness to 200 μm. Each layer was constituted of parallel struts oriented at 90° compared with the previous layer. The porosity was defined by adjusting the distance between struts. The printing speed was set to 5 mm/s and calibrated to deposit struts with a width of 350–400 μm.
Two types of scaffolds were manufactured (Supplementary Fig. S1). For physical properties assessment, porous cylinders of 10 mm diameter and 5 mm height, with a strut distance of 1 mm in each layer, were manufactured. For biological evaluation, 100% infill disks of 10 mm diameter, 600 μm thickness, and 0.4 mm strut distance were manufactured to facilitate easy observation and image analysis of cellular response to the nontransparent samples.
The fabricated scaffolds were immersed in a 5M NaOH solution for surface treatment, centrifuged to ensure proper penetration of the solution into the scaffold, incubated for 12 h at room temperature, and then thoroughly rinsed. The scaffolds were then immersed in a 70% ethanol solution for 20 min, rinsed three times in phosphate-buffered saline (PBS) solution, and dried overnight. Half of the fabricated scaffolds were exposed to E-beam irradiation with a standard single dose of 25 kGy, following norm ISO 11137-2:2006. The samples were stored in the dark and tested 3 months after manufacturing.
Hydrophilicity measurement, water retention, and surface morphology
The hydrophilicity of PCL/β-TCP constructs was evaluated using a contact angle goniometer, Ramé-Hart 290 (Ramé-Hart instrument co.). In brief, a droplet (4 μL) was deposited on the disk scaffolds, and the contact angle was computed using image processing (n = 5). To measure the water retention, porous scaffolds were immersed in deionized water for 60 s, followed by removing the water on the outer surface using a wet paper towel as described by Shanjani et al.7,35
The water uptake was calculated as the weight ratio of the retained water over the dry scaffold in percentage. The surface morphology of scaffolds was analyzed using scanning electron microscopy (SEM) imaging. Porous or 100% infill scaffolds were sputter coated (SPI Sputter; SPI Supplier Division of Structure Prob, Inc., West Chester, PA) to form a gold layer of 10 nm thickness. They were then imaged using a scanning electron microscope (Zeiss Sigma FESEM, acceleration voltage: 3 kV), at magnifications 90, 5000, and 50,000.
Molecular weight dispersion
Gel permeation chromatography (GPC) was performed to quantify the change in molecular weight of the PCL chains (Dionex Ultimate 3000; Thermo Fischer Scientific), using DMF as the solvent. E-beam exposed and nonsterilized control samples were dissolved in DMF overnight (n = 3). The solutions were centrifuged at 7000 rpm for 3 min to separate the TCP particles, and the supernatants were filtered through 0.2 μm nylon filters. Filtered samples were injected to the GPC system at 1.0 mL/min flow rate. The number average molecular weight (Mn) and weight average molecular weight (Mw) were determined by comparing polyethylene glycol (PEG) standards.
Degradation kinetics and mechanical characterization
Degradation kinetics and mechanical properties of the porous scaffolds during degradation were measured using an accelerated degradation protocol as described elsewhere.36 Specimens (n = 4) were weighed, and then incubated in a 5M NaOH solution at 37°C. At each time point (0, 48, 96, and 120 h), specimens were rinsed with DI water, dried in an oven at 35°C for 45 min and in a ventilated hood at room temperature for 12 h, and weighed again. Degradation was then quantified through measuring the remaining mass; that is, the ratio of the mass after degradation over the initial mass. The samples were imaged using SEM following the protocol described previously, to observe the degradation process for both groups.
Apparent Young's modulus and the yield strength in compression of the scaffolds were tested using an Instron 5944 uniaxial testing system with a 2 kN load cell (Instron Corporation, Norwood, MA) as described elsewhere.36 A preload of 1N was applied at a rate of 1% strain/s up to 25% strain. Young's modulus was calculated as the initial slope of the stress–strain curve. The stress at which a line with a slope equal to Young's modulus and starting at 1% strain offset intersected with the stress–strain curve was defined as yield strength.
In vitro evaluation
Multipotent mouse C3H10T1/2 fibroblasts (ATCC) and mouse C2C12 myoblasts (ATCC) were cultured in Dulbecco's Modified Eagle's medium (DMEM) (Life Technologies) supplemented with 10% fetal bovine serum (FBS; Life Technologies) and 1% penicillin/streptomycin, and incubated in a 37°C, 5% CO2 humidified environment.
Cells were seeded on the surface of the disks at a density of 1.6 × 104 cells/cm2. The cell culture medium was changed at days 3, 5, and 7. The viability of the cells was assessed at days 1 and 3 using live/dead staining (Life Technologies, Carlsbad, CA), following the manufacturer's instructions. In brief, the samples were stained in a 2 μM Calcein AM and 4 μM ethidium homodimer-1 solution and imaged using a fluorescent microscope (Zeiss AxioObserver Z1). Live/dead ratios were obtained by counting the cells over three different regions for each sample. For cell proliferation measurement, at days 1, 3, and 7, cells were detached from the scaffolds using 0.05% trypsin-EDTA (Life Technologies), suspended in medium and counted using a Z2 particle counter (Beckman Coulter).
The effect of E-beam sterilization of scaffolds on osteogenic differentiation of seeded C3H10T1/2 and C2C12 cells was assessed over 7 days in two groups; the cell-seeded scaffolds in normal condition (NC) group, incubated in DMEM, and the cell-seeded scaffolds in osteogenic condition (OC) group, incubated in DMEM supplemented with 200 ng/mL BMP-2.
The alkaline phosphatase (ALP) activity of cells was assessed through semiquantitative staining (days 1, 3, and 7), using ALP stain (Sigma-Aldrich). Following manufacturer's instructions, cells were fixed in 3.7% formaldehyde, and samples were incubated with ALP stain (1 h for C3H10 cells, 40 min for C2C12). After staining, the samples were imaged, and the ALP levels were quantified through image processing (Matlab R2013; MathWorks). In brief, for each scaffold, red color features were extracted, quantified, and the pixel values were averaged over the entire surface of the scaffold. For each group, values were normalized using the average value at day 1.
Statistical analysis
For every experiment, five samples were tested per group, unless indicated otherwise. The t-test was used for statistical analysis between two groups. One-way analysis of variance (ANOVA) followed by ad hoc Tukey's test was used for statistical analysis between three or more groups (Matlab R2013; MathWorks). In figures, data are presented using mean ± standard deviation, and a statistical significant difference between groups (p < 0.05) is labeled by the symbol*.
Results
Hydrophilicity and water retention
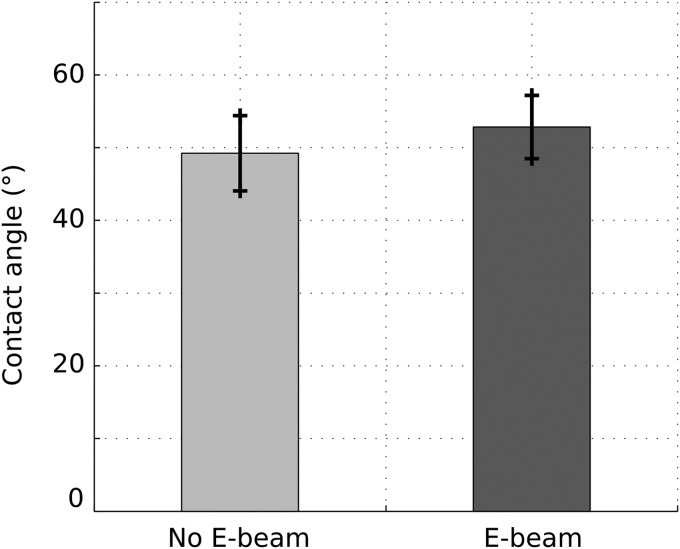
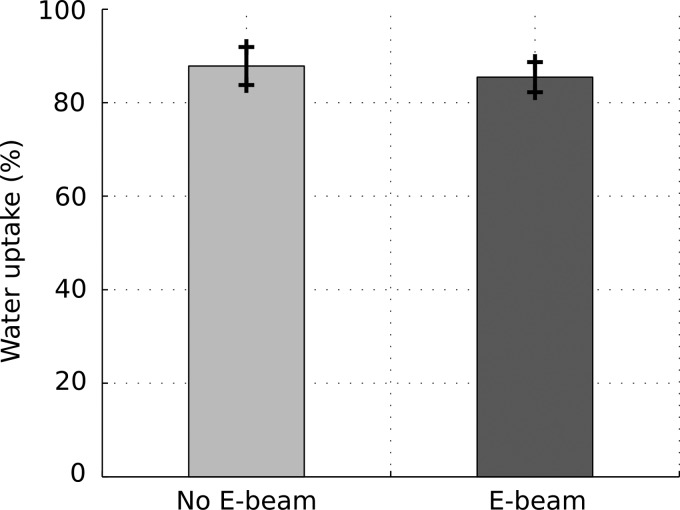
Figure 1 shows results of the contact angle measurements. The average values of contact angles were 49.2° and 52.9° for nonsterilized samples and E-beam sterilized samples, respectively. No significant difference was observed between the two groups. The contact angles for both groups were <90°, indicating a hydrophilic surface of the scaffolds regardless of the E-beam exposure. The water retention data for E-beam sterilized and nonsterilized scaffolds are shown in Figure 2. The values were 87.8% and 86.3% for nonsterilized samples and E-beam sterilized samples, respectively. E-beam sterilization did not significantly affect the water retention of scaffolds.
FIG. 1.
Contact angle measurement of 3D-printed scaffolds before and after E-beam sterilization (n = 5).
FIG. 2.
Results of water uptake experiment, comparing the ability of porous scaffold to retain water before and after E-beam sterilization (n = 5).
Surface morphology
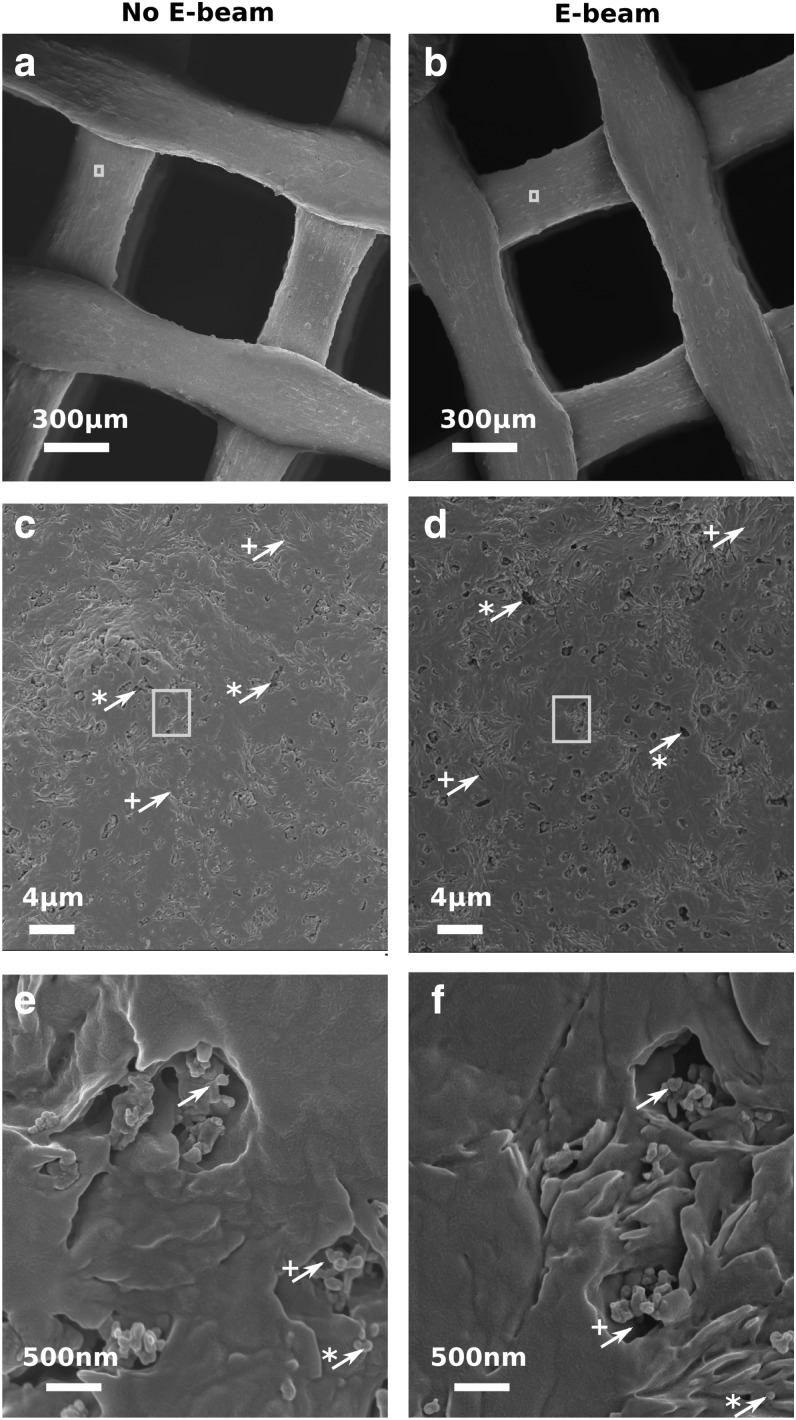
Figure 3 shows SEM images of the porous scaffolds at three different magnifications. The scaffolds in Figure 3b, d, and f were exposed to E-beam sterilization, while those in Figure 3a, c, and e were not E-beam sterilized. All scaffolds had uniform macropores (void space) separated by well-organized struts (Fig. 3a, b). Figure 3c and d show the surface morphology of a strut at higher magnification. The observed micropores and cracks on the surface in Figure 3c and d are attributed to the NaOH surface treatment (Supplementary Fig. S2). β-TCP particles with an average size of 100 nm or particle aggregates were observed within the micropores at the highest magnification (Fig. 3e, f). No significant change in the strut size, void size, or surface morphology was observed after the E-beam irradiation.
FIG. 3.
SEM imaging of 3D-printed PCL/β-TCP scaffolds before (a, c, e) and after (b, d, f) E-beam sterilization at magnification × 90 (a, b), × 5000 (c, d), and × 50,000 (e, f). In (c, d), arrows indicate features created by the surface treatment: * for micropores, + for cracks. Rectangles specify the location of the images (c, d). In (e, f), simple, *, and + arrows are, respectively, indicating β-TCP particle aggregates, single β-TCP particles, and pores around them. Rectangles specify the location of the images (e, f). PCL/β-TCP, polycaprolactone/beta-tricalcium phosphate.
Molecular weight dispersion
The weight average molecular weight (Mw), number average molecular weight (Mn), and polydispersity index (PI = Mw/Mn) of PCL polymer chains in the PCL/β-TCP scaffolds before and after E-beam treatment are shown in Table 1. The Mw increased from 71.2 ± 1.2 (kDa) to 73.3 ± 0.5 (kDa), but the Mn decreased from 54.7 ± 2.7 (kDa) to 47.5 ± 1.7 (kDa) with E-beam treatment. The PI of PCL chains increased 18% from 1.3 to 1.54 when scaffolds were sterilized by E-beam.
Table 1.
Weight Average Molecular Weight (Mw), Number Average Molecular Weight (Mn), and Polydispersity Index of Polycaprolactone/Beta-Tricalcium Phosphate Scaffolds Not Exposed and Exposed to E-Beam Sterilization
| Mw | Mn | PI | |
|---|---|---|---|
| No E-beam | 71.2 × 103 (1.2 × 103) | 54.7 × 103 (2.7 × 103) | 1.3 |
| E-beam | 73.3 × 103 (0.5 × 103) | 47.5 × 103 (1.7 × 103) | 1.54 |
| p value | 0.042 | 0.016 | 0.029 |
The value presented is the mean value, with the standard deviation in parentheses (n = 3).
Degradation
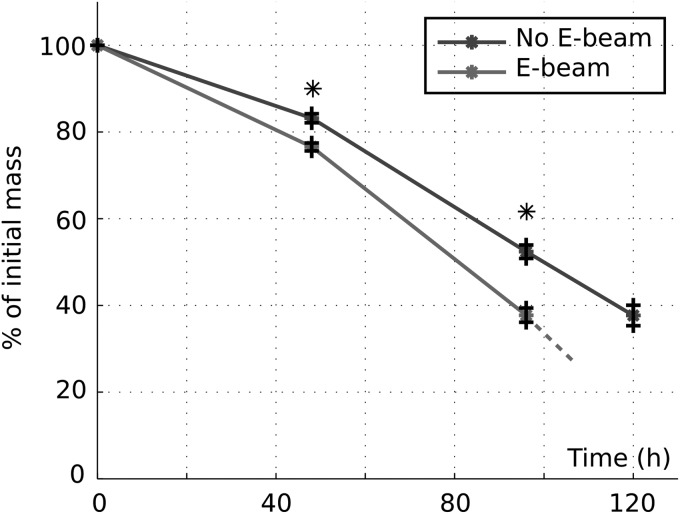
Figure 4 shows the mass loss of porous scaffolds over the course of accelerated degradation. The scaffolds exposed to E-beam had a significantly larger mass loss after 48 h or 96 h compared with those scaffolds that were not irradiated by E-beam. The remaining mass of E-beam irradiated and nonirradiated scaffolds after 48 h was 40% and 50%, respectively. The nonirradiated scaffolds had 40% remaining mass after 120 h of accelerated degradation, while the E-beam irradiated scaffolds could not be retrieved after 120 h due to the loss of structural integrity of scaffolds.
FIG. 4.
Evolution of the mass of porous scaffolds exposed and not exposed to E-beam sterilization during degradation in alkaline conditions (n = 4). *p < 0.05.
SEM images of the samples during degradation are presented in Supplementary Figure S3. During degradation, PCL is hydrolytically severed, leading to the dislocation of the ceramic particles. As observed in the images, it results in voids in the bulk material. No significant visual differences could be observed on the scaffold surface at both scales ( × 90 and × 5000), confirming the similarity of the degradation process of nonirradiated and irradiated scaffolds in alkaline environment. The SEM images of scaffolds confirmed the faster degradation of E-beam irradiated scaffolds after 4 days of incubation in the alkaline solution, compared with the nonirradiated scaffold.
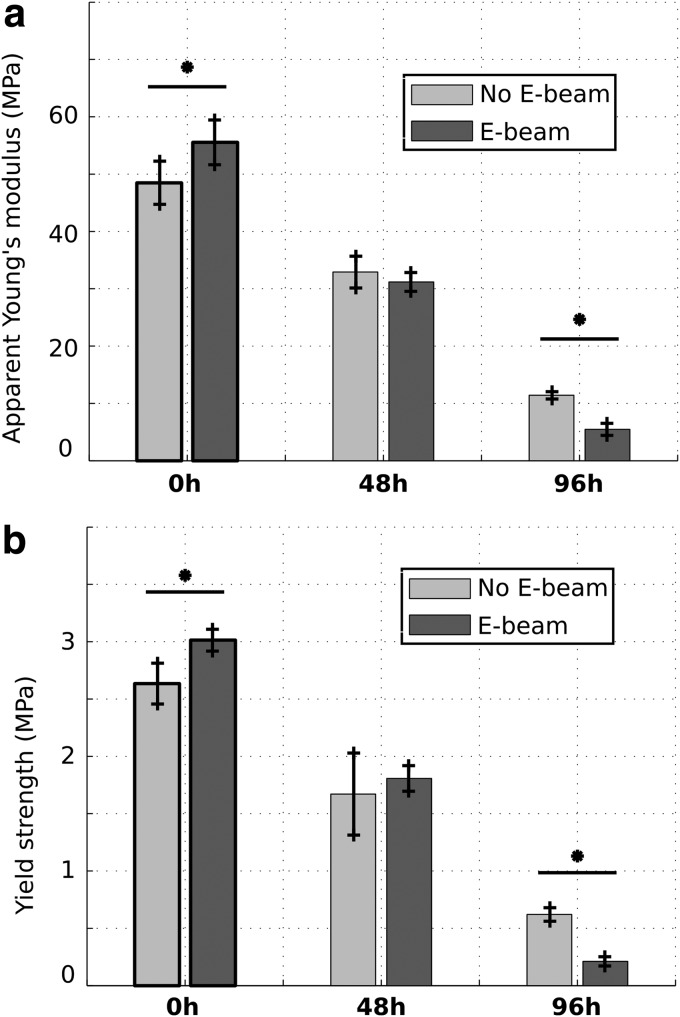
Mechanical properties
Figure 5 shows Young's modulus and yield strength of the scaffolds before and during degradation. Before degradation (0 h), Young's modulus and yield strength of nonirradiated scaffolds were 48.5 and 2.63 MPa, whereas those of E-beam irradiated scaffolds were 55.5 and 3.01 MPa, respectively. After 48 h of degradation, there was no significant difference between either Young's modulus or yield strength of E-beam irradiated and nonirradiated scaffolds. Young's modulus and yield strength of nonirradiated scaffolds were 11.4 and 0.62 MPa, whereas those of E-beam irradiated scaffolds were 5.6 and 0.21 MPa, respectively, after 96 h of degradation. E-beam irradiation significantly increased both stiffness and strength of scaffolds before degradation.
FIG. 5.
Evolution of apparent Young's modulus (a) and yield strength (b) during degradation for scaffolds submitted and not submitted to radiations (n = 4). *p < 0.05.
However, the stiffness and strength of E-beam irradiated scaffolds were significantly lower than those of nonirradiated scaffolds after 96 h of degradation. Therefore, the effect of degradation on mechanical properties of E-beam irradiated scaffolds was more pronounced compared with nonirradiated scaffolds.
In vitro evaluation
Cell viability and proliferation
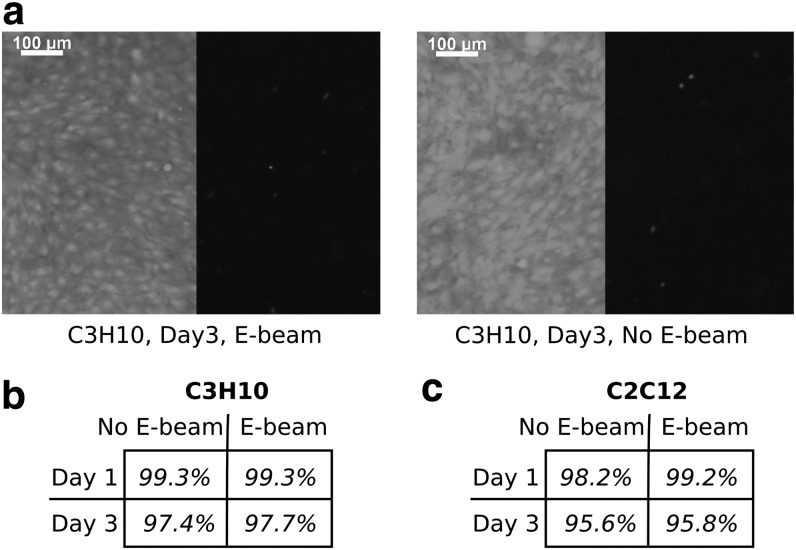
Figure 6 shows the viability of C3H10T1/2 and C2C12 cells seeded on PCL/β-TCP disks after 1 and 3 days. Representative live/dead images of two cell lines are shown in Figure 6a. The live/dead cell number ratios for C3H10T1/2 and C2C12 cells are shown in Figure 6b and c, respectively. The viability of cells was >95%, regardless of cell type or E-beam irradiation before cell seeding. A slight decrease in cell viability was noted for both cell lines between days 1 and 3. Although not statistically significant, it could be attributed to the fact that the cell culture medium was not changed over the course of 3 days. In the end, the viability of both C3H10T1/2 and C2C12 cells was not affected by E-beam sterilization of PCL/β-TCP substrates.
FIG. 6.
Viability of multipotent mouse C3H10 fibroblasts (b) and mouse C2C12 myoblasts (c) seeded onto scaffolds exposed and not exposed to E-beam radiations, after 1 and 3 days incubation periods. Example of fluorescent images resulting from the live/dead experiment are presented in (a) for C3H10 cells at day 3 (n = 5).
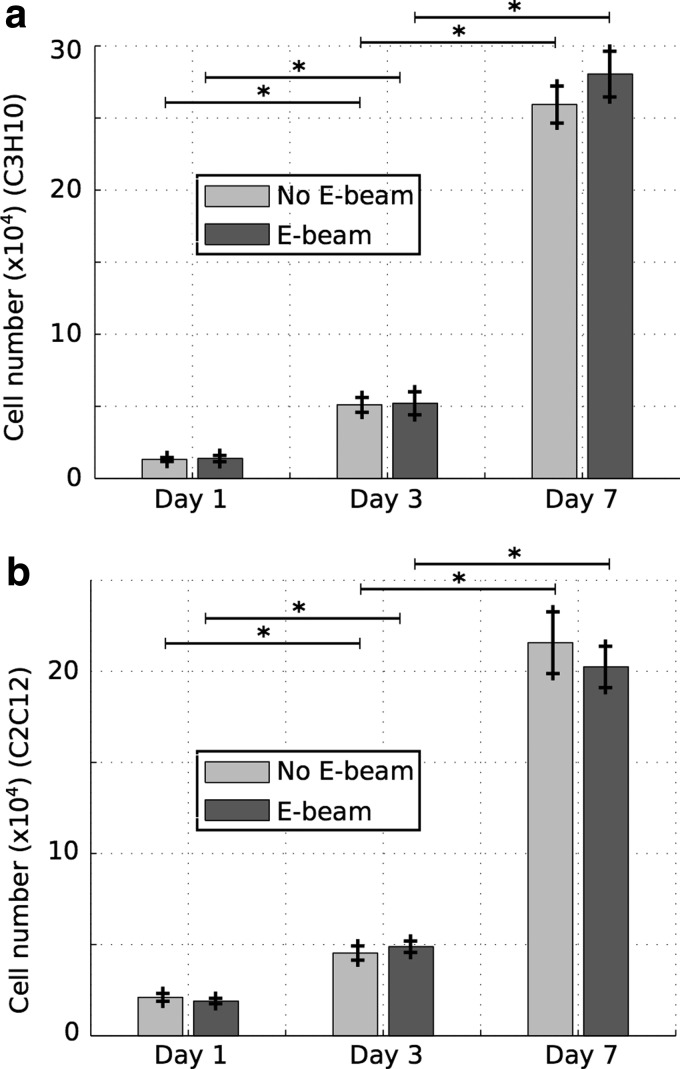
Results of the cell proliferation experiments are presented in Figure 7. The number of cells on the scaffolds increased significantly from days 1 to 7 for both cell lines. The proliferation of C3H10T1/2 or C2C12 cells at all time points was unaffected by E-beam sterilization of scaffolds before cell seeding. Therefore, PCL/β-TCP constructs supported cell growth regardless of E-beam sterilization or cell line.
FIG. 7.
Results of the cell proliferation experiment, showing the number of cells (C3H10 (a), C2C12 (b)) at the surface of scaffolds exposed and not exposed to radiations, at days 1, 3, and 7 (n = 5). *p < 0.05.
Osteogenic differentiation
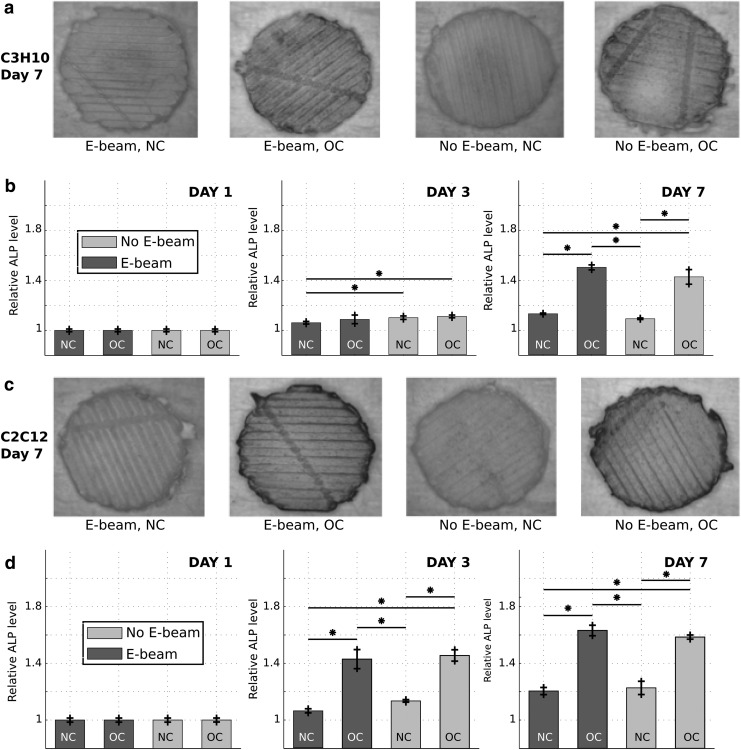
Representative images of ALP-stained C3H10 and C2C12 cells seeded on E-beam irradiated or nonirradiated PCL/β-TCP substrates and incubated under NC or OCs are shown in Figure 8a and c, respectively. An intense blue color spanned the entire surface of the scaffold in OC groups, indicative of a larger ALP activity of cells compared with NC groups (Fig. 8a, c). Relative ALP levels of C3H10 and C2C12 cells, for all groups in Figure 8a and c, were measured using image processing, and the semiquantitative results are reported in Figure 8b and d, respectively.
FIG. 8.
Semiquantitative measurement of the ALP enzyme activity of multipotent mouse C3H10 fibroblasts (a, b) and mouse C2C12 myoblasts (c, d) at the surface of 3D-printed PCL/β-TCP scaffolds exposed and not exposed to E-beam, at days 1, 3, and 7, in NC (DMEM) and OCs (DMEM supplemented with 100 ng/mL BMP-2). (a, c) show images of the scaffolds after ALP staining at day 7. (b, d) present the evolution of the ALP level for each group at days 1, 3, and 7 (n = 5). ALP, alkaline phosphatase; NC, normal conditions; OC, osteogenic condition. *p < 0.05.
For all groups, the ALP level increased significantly from days 1 to 3 and from days 3 to 7. The ALP level of both cell types was significantly higher in OC groups compared with NC groups at day 7. In addition, the ALP level of cells in all groups, after 7 days of incubation, was unaffected by E-beam sterilization of scaffolds.
Discussion
Influence of E-beam sterilization on the surface physical properties
The formation of micropores and cracks on the surface of PCL/β-TCP scaffolds (Fig. 3) was attributed to the cleavage of the ester groups on PCL polymer chains during NaOH surface treatment, hence polymer degradation.33,35 The hydrolytic degradation of PCL was augmented by the imperfections around β-TCP particles. As a result, micropores were formed primarily around β-TCP particles (Fig. 3e, f). The cracks on the surface might be formed during the extrusion of the PCL/β-TCP filament in the process of 3D printing. The formation of large bumps on the surface of scaffolds (Fig. 3a, b) was associated with β-TCP particle aggregates. The β-TCP particle aggregates within the scaffolds (Fig. 3e, f) were formed in the process of PCL/β-TCP composite synthesis.
The surface morphology of scaffolds was not affected by the E-beam sterilization. A change in surface morphology could potentially alter the hydrophilicity as well as cell attachment to the surface.37 The fact that the hydrophilicity and cell attachment were not changed by the E-beam irradiation (Figs. 1 and 7) indirectly confirmed the insignificant effect of E-beam sterilization on surface physical characteristics of PCL/β-TCP scaffolds.
Influence on the degradation, mechanical properties, and molecular weight distribution
Degradation of the PCL/β-TCP scaffolds happens through the hydrolytic cleavage of ester groups on PCL polymer chains, and is therefore guided by the polymer degradation. E-beam irradiation is known to affect PCL chains' architecture, by either scission or cross-linking mechanisms. A decrease in Mn and increase in Mw of PCL chains in the PCL/β-TCP scaffolds after E-beam sterilization (Table 1) was indicative of a concurrent scission and cross-linking of PCL chains. As a result, the PCL chains had greater polydispersity after E-beam treatment. The effect of E-beam treatment on increasing the polydispersity of PCL was consistent with the effect of gamma irradiation on PCL reported by Cottam et al.30
Typically, chain scission facilitates the scaffold degradation, while cross-linking strengthens the material and slows down the degradation. However, the mechanical characteristics and degradation kinetics of the PCL/β-TCP scaffolds were affected by relative degree of chain scission versus cross-linking as well as by the presence of nonhydrolytically degradable β-TCP and the complex 3D layout.
Therefore, the individual effects of E-beam sterilization on mechanical characteristics and degradation kinetics of PCL/β-TCP were studied (Figs. 4 and 5). A 25% increase in degradation rate of scaffolds after E-beam sterilization (Fig. 4) was attributed to the PCL chains scission. On the contrary, a 14% increase in the apparent Young's modulus and yield strength of scaffolds, following E-beam treatment, was due to the PCL chains cross-linking. A similar increase in both stiffness and strength of pure PCL scaffolds after E-beam treatment has been reported elsewhere.29
Lower Young's modulus of the E-beam irradiated scaffolds was associated with a faster degradation compared with nonirradiated scaffolds. The mass loss of E-beam irradiated scaffolds was larger than that of nonirradiated scaffolds after 4 days of degradation (Fig. 4 and Supplementary Fig. S3). It has been shown elsewhere that the stiffness of PCL-based scaffolds is correlated with the remaining mass of scaffolds during the degradation.36,38 The effect of E-beam sterilization on the degradation kinetics of scaffolds in vivo remains to be evaluated in the future.
Influence on the biological performances
The unaltered viability and proliferation of cells on PCL/β-TCP constructs after E-beam sterilization regardless of cell type (Figs. 6 and 7) showed that E-beam irradiated constructs were biocompatible. Osteogenic differentiation was assessed through staining of ALP, an early osteogenic marker, followed by image processing. Both C3H10T1/2 and C2C12 cells underwent osteogenic differentiation when they were incubated in a medium supplemented with BMP-2 protein, and E-beam sterilization of scaffolds did not affect the differentiation process. The osteogenic differentiation of both cell types in the presence of osteoinductive BMP-2 has been shown elsewhere.39,40
The fact that E-beam sterilization did not affect cell viability, proliferation, and osteogenic differentiation showed that E-beam treatment is safe and appropriate for sterilization of PCL/β-TCP composite scaffolds for bone tissue engineering applications. The effect of E-beam sterilization on the biological response of the scaffolds in vivo remains to be determined in the future though some studies have shown biocompatibility of PCL scaffolds sterilized using radiation-based methods.41,42
Conclusions
In this article, we studied the effect of E-beam sterilization on the properties of 3D-printed PCL/β-TCP scaffolds for bone tissue engineering applications. SEM imaging and contact angle measurements showed no significant impact on hydrophilicity and surface morphology of scaffolds with E-beam treatment. E-beam sterilization increased Young's modulus and strength in compression of the scaffolds by 14% and the degradation rate of scaffolds by 25%. The molecular weight distribution of PCL polymer measured through GPC showed that the concurrent increase in scaffold stiffness and degradation rate was attributed to simultaneous cross-linking and scission of PCL chains by E-beam irradiation.
Further, the viability, attachment, and proliferation of seeded C3H10 fibroblasts and mouse C2C12 myoblasts were not affected by E-beam irradiation of PCL/β-TCP constructs. Moreover, E-beam sterilization of scaffolds did not impact the osteogenic differentiation of seeded C3H10 and C2C12 cells. Overall, results of this study were indicative of the relevance of E-beam treatment for sterilization of 3D-printed PCL/β-TCP scaffolds for bone tissue engineering applications.
Supplementary Material
Acknowledgments
We acknowledge the financial support of the following agencies and donors: NIH R01AR057837 (NIAMS), NIH 1U01AR069395 (NIAMS/NIBIB), Stanford Coulter Translational Seed Grant, Boswell Foundation, Foundation of Orthopedic Trauma, and Kent Thiry and Denise O'Leary.
Disclosure Statement
No competing financial interests exist.
References
- 1. Brian S., Yang Y., Mohandas A., Stucker B., and Nguyen K.T. A review of materials, fabrication methods, and strategies used to enhance bone regeneration in engineered bone tissues. J Biomed Mater Res B Appl Biomater 85B, 573, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Liu X., and Ma P.X. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng 32, 477, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Lichte P., Pape H.C., Pufe T., Kobbe P., and Fischer H. Scaffolds for bone healing: concepts, materials and evidence. Injury 42, 569, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Hutmacher D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 21, 2529, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Hollister S.J. Porous scaffold design for tissue engineering. Nat Mater 4, 518, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Kawai T., Shanjani Y., Fazeli S., et al. Customized, degradable, functionally graded scaffold for potential treatment of early stage osteonecrosis of the femoral head. J Orthop Res 36, 1002, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shanjani Y., Kang Y., Zarnescu L., et al. Endothelial pattern formation in hybrid constructs of additive manufactured porous rigid scaffolds and cell-laden hydrogels for orthopedic applications. J Mech Behav Biomed Mater 65, 356, 2017 [DOI] [PubMed] [Google Scholar]
- 8. Leong K.F., Cheah C.M., and Chua C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 24, 2363, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Mota C., Puppi D., Chiellini F., and Chiellini E. Additive manufacturing techniques for the production of tissue engineering constructs. J Tissue Eng Regen Med 9, 174, 2015 [DOI] [PubMed] [Google Scholar]
- 10. Stevens M.M. Biomaterials for bone tissue engineering. Mater Today 11, 18, 2008 [Google Scholar]
- 11. El-Ghannam A. Bone reconstruction: from bioceramics to tissue engineering. Expert Rev Med Devices 2, 87, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Yang Y., Kang Y., Sen M., and Park S. Bioceramics in tissue engineering. In: Buroick J.A., and Mauck R.L., eds. Biomaterials for Tissue Engineering Applications. Springer, Vienna, 2001, pp. 179–207 [Google Scholar]
- 13. Bose S., Roy M., and Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol 30, 546, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jakus A.E., Rutz A.L., Jordan S.W., et al. Hyperelastic “bone”: a highly versatile, growth factor–free, osteoregenerative, scalable, and surgically friendly biomaterial. Sci Transl Med 8, 358ra127, 2016 [DOI] [PubMed] [Google Scholar]
- 15. Rezwan K., Chen Q.Z., Blaker J.J., and Boccaccini A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 27, 3413, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Kim S.S., Sun Park M., Jeon O., Yong Choi C., and Kim B.S. Poly (lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 27, 1399, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Huang B., Caetano G., Vyas C., Blaker J.J., Diver C., and Bártolo P. Polymer-Ceramic Composite Scaffolds: the Effect of Hydroxyapatite and β-tri-Calcium Phosphate. Materials 11, 129, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lei Y., Rai B., Ho K.H., and Teoh S.H. In vitro degradation of novel bioactive polycaprolactone—20% tricalcium phosphate composite scaffolds for bone engineering. Mater Sci Eng C 27, 293, 2007 [Google Scholar]
- 19. Yeo A., Rai B., Sju E., Cheong J.J., and Teoh S.H. The degradation profile of novel, bioresorbable PCL–TCP scaffolds: an in vitro and in vivo study. J Biomed Mater Res A 84, 208, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Lim H.C., Bae J.H., Song H.R., Teoh S.H., Kim H.K., and Kum D.H. High tibial osteotomy using polycaprolactone-tricalcium phosphate polymer wedge in a micro pig model. Bone Joint J 93-B, 120, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Macedo F.A., Nunes E.H.M., Vasconcelos W.L., Santos R.A., Sinisterra R.D., and Cortés M.E. A biodegradable porous composite scaffold of PCL/BCP containing Ang-(1–7) for bone tissue engineering. Cerâmica 58, 481, 2012 [Google Scholar]
- 22. Khojasteh A., Behnia H., Hosseini F.S., Dehghan M.M., Abbasnia P., and Abbas F.M. The effect of PCL‐TCP scaffold loaded with mesenchymal stem cells on vertical bone augmentation in dog mandible: a preliminary report. J Biomed Mater Res B Appl Biomater 101, 848, 2013 [DOI] [PubMed] [Google Scholar]
- 23. Lewis S., and McIndoe A.K. Cleaning, disinfection and sterilization of equipment. Anaesth Intensive Care Med 5, 360, 2004 [Google Scholar]
- 24. Baume A.S., Boughton P.C., Coleman N.V., and Ruys A.J. Sterilization of tissue scaffolds. In: Tomlins P., ed. Characterisation and Design of Tissue Scaffolds. Cambridge: Woodhead Publishing, 2016, pp. 225–244 [Google Scholar]
- 25. Silindir M., and Ozer A.Y. Sterilization methods and the comparison of e-beam sterilization with gamma radiation sterilization. Fabad J Pharm Sci 34, 43, 2009 [Google Scholar]
- 26. Plikk P., Odelius K., Hakkarainen M., and Albertsson A.C. Finalizing the properties of porous scaffolds of aliphatic polyesters through radiation sterilization. Biomaterials 27, 5335, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Gilding D.K., Reed A.M., and Baskett S.A. Ethylene oxide sterilization: effect of polymer structure and sterilization conditions on residue levels. Biomaterials 1, 145, 1980 [DOI] [PubMed] [Google Scholar]
- 28. Dai Z., Ronholm J., Tian Y., Sethi B., and Cao X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J Tissue Eng 7, 2041731416648810, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Di Foggia M., Corda U., Plescia E., Taddei P., and Torreggiani A. Effects of sterilisation by high-energy radiation on biomedical poly-(ɛ-caprolactone)/hydroxyapatite composites. J Mater Sci Mater Med 21, 1789, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Cottam E., Hukins D.W., Lee K., Hewitt C., and Jenkins M.J. Effect of sterilisation by gamma irradiation on the ability of polycaprolactone (PCL) to act as a scaffold material. Med Eng Phys 31, 221, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Oláh L., Filipczak K., Czvikovszky T., Czigány T., and Borbás L. Changes of porous poly (ɛ-caprolactone) bone grafts resulted from e-beam sterilization process. Radiat Phys Chem 76, 1430, 2007 [Google Scholar]
- 32. Odelius K., Plikk P., and Albertsson A.C. The influence of composition of porous copolyester scaffolds on reactions induced by irradiation sterilization. Biomaterials 29, 129, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Kodama Y., Oishi A., Nagasawa N., et al. Effect of Sterilization Dose on Electron Beam Irradiated Biodegradable Polymers and Coconut Fiber Based Composites. In: International Nuclear Atlantic Conference, Rio de Janeiro, Brazil: INAC, 2009 [Google Scholar]
- 34. Filipczak K., Wozniak M., Ulanski P., et al. Poly (ɛ‐caprolactone) Biomaterial Sterilized by E‐Beam Irradiation. Macromol Biosci 6, 261, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Yeo M., Simon C.G., and Kim G. Effects of offset values of solid freeform fabricated PCL–β-TCP scaffolds on mechanical properties and cellular activities in bone tissue regeneration. J Mater Chem 22, 21636, 2012 [Google Scholar]
- 36. Lam C.X., Teoh S.H., and Hutmacher D.W. Comparison of the degradation of polycaprolactone and polycaprolactone–(β‐tricalcium phosphate) scaffolds in alkaline medium. Polym Int 56, 718, 2007 [Google Scholar]
- 37. Samavedi S., Whittington A.R., and Goldstein A.S. Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behavior. Acta Biomater 9, 8037, 2013 [DOI] [PubMed] [Google Scholar]
- 38. Felfel R.M., Poocza L., Gimeno-Fabra M., et al. In vitro degradation and mechanical properties of PLA-PCL copolymer unit cell scaffolds generated by two-photon polymerization. Biomed Mater 11, 015011, 2016 [DOI] [PubMed] [Google Scholar]
- 39. Ker D.F., Wang D., Behn A.W., et al. Functionally-Graded, Bone- and Tendon-Like Polyurethane for Rotator Cuff Repair, Adv Funct Mater 28, 1707107, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ker D.F., Sharma R., Wang E.T., and Yang Y.P. Development of mRuby2-Transfected C3H10T1/2 Fibroblasts for Musculoskeletal Tissue Engineering. PLoS One 10, e0139054, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bhaskar P., Bosworth L.A., Wong R., et al. Cell response to sterilized electrospun poly (ɛ‐caprolactone) scaffolds to aid tendon regeneration in vivo. J Biomed Mater Res A 105, 389, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Valence S., Tille J.C., Mugnai D., et al. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials 33, 38, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.