Abstract
G protein-coupled receptors (GPCRs) are the largest class of drug targets, largely owing to their druggability, diversity and physiological efficacy. Many drugs selectively target specific subtypes of GPCRs, but high specificity for individual GPCRs may not be desirable in complex multifactorial disease states in which multiple receptors may be involved. One approach is to target G protein subunits rather than the GPCRs directly. This approach has the potential to achieve broad efficacy by blocking pathways shared by multiple GPCRs. Additionally, because many GPCRs couple to multiple G protein signalling pathways, blocking specific G protein subunits can ‘bias’ GPCR signals by inhibiting only a subset of these signals. Molecules that target G protein α or βγ-subunits have been developed and show strong efficacy in multiple preclinical disease models and biased inhibition of G protein signalling. In this Review, we discuss the development and characterization of G protein α and βγ-subunit ligands and the preclinical evidence that this exciting new approach has potential for therapeutic efficacy in a number of indications, such as pain, thrombosis, asthma and heart failure.
G protein-coupled receptors (GPCRs) are important targets for current drugs and drug discovery largely owing to the wide range of physiologies and pathophysiologies in which GPCR targeting can have a major impact. GPCRs signal via direct interactions with heterotrimeric G proteins on the inner surface of the plasma membrane, where the GPCR acts as an exchange factor to enhance the release of GDP from the G protein, leading to the subsequent binding of GTP and conformational activation1,2. Heterotrimeric G proteins are composed of Gα, Gβ and Gγ subunits. The Gα subunit binds to either GTP or GDP; Gβ and Gγ subunits form a constitutive heterodimer that binds reversibly to the Gα subunit. GTP binding activates the Gα subunit, and the resulting conformational changes lead to dissociation from the receptor and from Gβγ subunits. These free subunits are now competent to interact with the downstream enzymes or channels to drive second messenger generation or changes in membrane potential that modulate cell physiology.
Following activation, most GPCRs are phosphorylated by GPCR kinases (GRKs), then bind to arrestin and become internalized. There are seven isoforms of GRKs, GRK1–7, with GRK1 and GRK7 strictly found in the retina, where they function to desensitize rhodopsin3,4. Of the remaining GRKs, GRK2 and GRK3 are cytosolic and are recruited to the membrane by binding to the free Gβγ subunits that are released upon G protein activation and by coincident association with phosphatidylinositol- 4,5-bisphosphate (PtdIns(4,5)P2, also known as PIP2) in the plasma membrane. GRK4, GRK5 and GRK6 are constitutively membrane associated via carboxy-terminal polybasic regions that interact with negatively charged phospholipids and/or post-translational palmitoylation. β-Arrestins bind to phosphorylated GPCRs to mediate internalization of the receptor, which was originally thought to turn off and desensitize the receptor5. It has been proposed that β-arrestins directly transmit GPCR signals. However, recent data indicate that downstream signalling previously attributed to β-arrestins is in fact dependent on classical G protein signalling6–9. Indeed, internalized GPCRs activate G protein signalling on endosomes10,11. This activation results in a second wave of longer-term GPCR-dependent signalling that could partially explain the effects of β-arrestin depletion on downstream signalling.
GPCRs bind to many known drugs and are important potential targets for drug discovery12. Recently, there has been interest in targeting G proteins downstream of the receptors themselves. This approach has multiple advantages. Many complex diseases result from dysregulation of multiple GPCRs, such that targeting a single GPCR may not achieve the desired effects13. Primary examples are the chronic inflammatory diseases in which multiple chemokines (the receptors for which are GPCRs) are dysregulated. Additionally, it has become appreciated that dysregulation of the G protein systems themselves can drive disease. The involvement of activating mutations of protein Gαq/11 subunits in driving uveal melanoma is discussed in detail below14,15.
A current approach to identifying new GPCR therapeutics has been to identify ligands that interact with GPCRs in binding modes that favour specific conformations of the receptor that activate only select downstream pathways16,17. The emphasis has been on finding ligands that lead either to preferential activation of G proteins or to β-arrestin binding by GPCRs. One example is the discovery of μ-opioid receptor (MOR) agonists that bias MORs towards G protein activation over β-arrestin recruitment to improve the safety of opioid analgesics. The underlying basis for this idea comes from data from β-arrestin-knockout mice, which show enhanced G protein-dependent analgesia upon opioid treatment with fewer adverse effects such as respiratory depression and development of tolerance18–20. Recent clinical trial data indicate that a new G protein-biased MOR agonist, oliceridine, is effective at relieving postoperative pain, with significantly less nausea and respiratory depression21,22.
Direct G protein targeting is an alternative approach to bias GPCRs by blocking selected post-receptor signalling pathways (FIG. 1). Targeting specific G protein sub units downstream of GPCRs can bias GPCR signals away from detrimental signalling pathways but leave pathways that are important for normal cell functioning intact. Targeting G protein subunits that are common to signalling downstream of receptor families may also improve therapeutic efficacy in complex disease such as heart failure, inflammation and asthma. Finally, targeting G proteins themselves could have efficacy in treating diseases driven by G protein dysregulation. This Review discusses the strategy behind targeting G proteins and provides examples in which G protein inhibition has shown therapeutic efficacy in preclinical models, thereby demonstrating the potential power of this new approach to therapeutics.
Figure 1. Diagrammatic representation of how G protein inhibition can bias GPCR signalling.
a| For G protein-coupled receptors (GPCRs) that couple to multiple G protein signalling pathways, inhibition of a specific G protein (inhibition of Gαq is shown as an example) will block some, but not all, pathways. b | Another way to bias GPCR signalling is to block of a subset of Gβγ targets. In this example, three downstream targets — phosphoinositide 3-kinase (PI3K), phospholipase Cβ (PLCβ) and GPCR kinase 2 (GRK2) — are selectively blocked. This strategy alters some Gβγ signals downstream of GPCRs but leaves other signals associated with these receptors intact. AC, adenylyl cyclase; GIRK, G protein-activated inward rectifier K+ channel.
Overview of G protein subunit families
The heterotrimeric G protein family consists of numerous, diverse individual subunit isoforms, with 20 different G protein α-subunits, 5 different β-subunits and 12 different γ-subunits that can associate combinatorially to produce a dizzying array of potential G protein heterotrimers23–26. GPCR coupling specificity and downstream target regulation are driven largely by the identity of the Gα subunit, and these can be classified into four different families, simplifying the problem to some degree27. For all of the G protein subunit classes discussed below, identification of direct regulatory interactions between specific G protein α-subunit family members and targets were defined through purified protein reconstitution experiments28–31. This approach has laid out a direct mechanistic biochemical basis for much, but not all, of the specificity observed in cellular physiology. Purified protein reconstitutions have also clearly defined direct interactions between Gβγ subunits and downstream effectors32–34. Gβ and Gγ diversity is important in physiology, but individual classification based on biochemical properties has not been successful26,35. For the purposes of pharmacological targeting, the Gβγ subunits will be discussed as a single class.
Gαs
The Gα subunits of the Gαs family were the first G proteins discovered and were purified on the basis of their ability to stimulate adenylyl cyclase28. Gαs–GTP directly binds to adenylyl cyclase, resulting in increased catalytic activity and cAMP production36. Although there are numerous modulators of the nine adenylyl cyclase isoforms, all are activatable by Gαs. There are three Gαs isoforms: two splice variants — Gαs short and Gαs long — and the distinct gene product Gαolf. Suramin and related small molecules inhibit activation of Gαs but have multiple targets and are not cell permeable; therefore, they are not useful for targeting Gαs in vivo37. There is potential interest in targeting members of the Gαs family because activating mutations in Gαs lead to numerous syndromes, including benign pituitary adenomas and pancreatic adenocarcinomas, and are found in 3.5% of all tumour sequences in the COSMIC database (see Related links).
Gαi
Gαi subunits were discovered as inhibitors of adenylyl cyclase, and one of them, Gαo, was found as a high-abundance Gα subunit in brain extracts38–41. The canonical function of Gαi family G proteins is to bind directly to and inhibit adenylyl cyclase isoforms, thereby leading to a decreased cAMP concentration in cells. The Gαi family consists of Gαo, Gαi1, Gαi2, Gαi3, Gαz and Gαt (REFS27,42). All three Gαi isoforms (1, 2 and 3) inhibit adenylyl cyclase in biochemical experiments with no clearly distinguishable isoform-specific functions36,43. Gαo also weakly inhibits adenylyl cyclase and has no other clearly defined function, although recent work suggests a role for Gαo in the Golgi apparatus regulating neurite outgrowth44. All of the Gαi family members, except for Gαz (REF.45), are inhibited by pertussis toxin (PTX) through ADP-ribose modification of a unique cysteine at the carboxyl terminus of Gαi subunits, which inhibits the interaction of Gαi with receptors, presumably by steric occlusion41,46. No specific small-molecule inhibitors of Gαi family subunits have been identified.
Gαq and Gα11
Gαq and its closely related homologue Gα11 (which are 90% identical) are responsible for activation of phospholipase Cβ (PLCβ) downstream of GPCR activation29,47,48. PLC hydrolyses the membrane lipid PtdIns(4,5)P2 to inositol-1,4,5-trisphosphate (Ins(1,4,5)P3, also known as IP3) and diacylglycerol (DAG), each of which initiates a signal transduction cascade. Ins(1,4,5)P3 causes the release of calcium into the cytoplasm and DAG activates protein kinase C (PKC); both of these pathways are ubiquitous regulators of cell physiology49–52. Gαq or Gα11 regulation of PLCβ and subsequent Ca2+ release are major drivers of cell function throughout the body. These include platelet aggregation, smooth muscle contraction and exocytosis, as well as many others53–57. Gαq family members in humans are Gαq, Gα11, Gα14 and Gα15, all of which can activate PLCβ58. These subunits have distinct tissue distributions, but no biochemical specificity for target interactions has yet been defined and most physiological data suggest that Gαq and Gα11 have overlapping functions47,53,59,60. However, one study has shown that Gαq and Gα11 have different roles in GPCR-mediated sensitization of mechanical and thermal nociception in mice that were often distinct and non-compensatory61.
Although PLCβ is recognized as the canonical target for Gαq/11, other direct targets of Gαq/11 have been identified. These include p63RhoGEF, which binds to Gαq/11 to initiate the activation of Rho by converting Rho–GDP to Rho–GTP. The canonical mechanism for GPCR-mediated activation of Rho downstream of GPCRs is through activation of the Gα12 or Gα13 family and direct activation of p115RhoGEF; however, depending on the cell type and the receptor, Gαq/11-dependent Rho activation may predominate. Examples of RhoGEFs that are activated by Gαq/11 include p63RhoGEF and TRIO62, which have been implicated in the regulation of vascular tone63,64 and uveal melanoma65, respectively. Another alternative Gαq/11-regulated pathway involves a direct interaction between Gαq and PKCζ66. The Ribas group has demonstrated a direct interaction between Gαq and PKCζ67 and showed that this interaction is important for extracellular- signal-regulated kinase 5 (ERK5) activation in cardiac myocytes and fibroblasts in response to angiotensin II (ATII)68.
Specific bioavailable inhibitors for Gαq family members have been identified and are currently the only Gα subunit inhibitors with therapeutic potential69,70. These will be discussed below and are a major subject of this Review.
Gα12 and Gα13
Gα12 and Gα13 were originally identified in a homology screen for novel G protein subunits23,71. Later, these subunits were found to directly interact with p115RhoGEF and increase its ability to activate Rho30,72. The Gα12 or Gα13 pathway is the major pathway through which GPCRs activate Rho.
Gβγ subunits
Early studies indicated that the Gα subunits were the only transducers of G protein signals, but seminal observations demonstrating regulation of inwardly rectifying K+ channels revealed that Gβγ subunits are bona fide signal transducers32. It is now well accepted that the Gβγ subunit heterodimer can activate a wide range of effector targets, the majority of which interact with Gβγ via a single hotspot interface on Gβ35,73–76. There are 5 different Gβ isoforms and 12 different Gγ isoforms in humans. Gβ1–4 are highly homologous, sharing ~80–90% sequence identity, and Gβ5 shares about 50% identity with those four. Gβ5 is unusual in that it is not thought to interact with Gγ subunits in vivo but rather forms dimers with regulator of G protein signalling (RGS) proteins of the R7 family77–79. No clear specific differences have been noted between Gβ1–4 in biochemical reconstitution or cell transfection experiments, although some subtle preferences have been noted80–84. Co-transfected Gβ5γ is often less effective at activating downstream targets, but because Gβ5 subunits are not thought to interact with Gγ or Gα subunits in the traditional sense, this apparent biochemical selectivity does not likely reflect physiological specificity85.
Gγ subunits are more diverse, with intrafamily identities as low as 25%. Similar to Gβ subunits, activation of most effectors by Gβγx combinations does not reveal any particular specificity either with purified proteins or upon transfection. Gγ1-containing Gβγ complexes are consistently less potent that other Gβγ subunits with respect to effector activation, but because Gγ1 is almost exclusively expressed in the retina (see Bgee database), this cannot be taken as evidence for physiological Gγ subunit target specificity80,81.
Most effectors bind to a highly conserved surface on Gβ subunits that roughly corresponds to the Gα–Gβγ interface. In GPCR–G protein crystal structures, interactions between receptors and Gγ subunits have not been observed. In two recent cryo-electron microscopy structures of calcitonin and glucagon-like peptide 1 (GLP1) receptors in complex with G proteins, some contacts between the receptor and Gβ were observed, but the importance of these interactions is not clear86–88. Gγ subunits are not directly involved in target interactions in cases for which this has been defined. These data likely explain why no clear, specific biochemical roles have been attributed to individual isoforms of Gβγ. On the other hand, a number of Gγ and Gβ subunit knockout or knockdown studies indicate exquisite specificity in vivo89–93. This finding strongly suggests specific roles for Gβ and Gγ subunits in physiological signalling, but the mechanistic nature of this specificity is not understood. An emerging concept is that different Gγ subunits may impart specific Gβγ plasma membrane affinities, but the physiological relevance of this is not yet clear94–96.
Because Gβγ subunits do not undergo major conformational changes upon G protein activation, their activities are regulated by the activity of the Gα subunits2. Activation of Gα leads to dissociation from Gβγ, which exposes a protein–protein interaction surface on Gβ that can bind to downstream targets35,74,97–99. Interestingly, PTX inhibits the activation of many and/or most Gβγ subunit signalling pathways in cells, although PTX does not have any direct actions on Gβγ35. PTX inhibits Gβγ signalling because it modifies Gαi in the Gαiβγ heterotrimer, preventing Gαi activation, thereby inhibiting release of Gβγ. This observation indicates that despite the requirement for Gβγ assembly with all Gα subunits for efficient GPCR coupling, and the presumed Gβγ release upon activation of all GPCRs, Gβγ signalling downstream seems to preferentially involve Gαi-coupled receptors, although some exceptions have been noted. One of the targets of downstream Gβγ signalling is GRK2, which functions in a negative feedback loop100. Cellular expression of the carboxy-terminal, Gβγ binding fragment from GRK2 selectively inhibits Gβγ signalling in cells in vivo and has been used to test for the specific involvement of Gβγ signalling in particular signalling pathways and pathologies101–104. Subsequently, prototypical small molecules that bind directly to Gβγ and inhibit Gβγ signalling were developed and used to validate Gβγ as a potential therapeutic target in a number of conditions75,105. The use of Gβγ as a potential therapeutic target will be discussed in detail later in this Review.
Pharmacological targeting of Gα
Gαq family inhibitors
To date, the only specific bioavailable inhibitors of Gα subunits that have a good level of validation are inhibitors of Gαq family members. YM-254890 is a cyclic peptide that was isolated from Chromobacterium spp. QS3666 as an inhibitor of ADP-dependent platelet aggregation70,106–108 (FIG. 2a). These and subsequent studies demonstrated that YM-254890 inhibits Gαq/11 signalling downstream of multiple Gαq-linked GPCRs in platelets and other native and heterologous systems without affecting other GPCR signalling pathways70. A highly related compound, FR900359 (FIG. 2a), was isolated from the ornamental primrose plant, Ardisia crenata, and also has potent and specific effects on Gαq/11 signalling69
Figure 2 |. Structures of inhibitors of G protein signalling.
a | The Gαq inhibitors YM-254890 (black only) and FR900359 (with red modifications) are shown. b | The Gβγ inhibitors, M119 and gallein, differ only in the saturation of the carboxyphenyl ring.
YM-254890 was co-crystallized with Gαq, which gave insight into its mechanism of action and suggests paths to the development of Gα subunit-specific inhibitors109. YM-254890 binds to a hinge between two independent domains of the Gα subunit, the α-helical domain and the Ras-like domain, and GDP and GTP bind at the interface between the domains110,111 (FIG. 3). GDP release and subsequent GTP binding require the interface between these domains to open and allow the nucleotide to diffuse into, and out of, the nucleotide binding site112,113. Binding of YM-254890 at the hinge between these domains is predicted to prevent domain opening and thereby prevent GDP release and GTP binding, leading to G protein inhibition. FR900359 is structurally very similar to YM-254890 and likely binds in a very similar mode. Indeed, FR900359 was demonstrated to be a potent inhibitor of GDP release in biochemical assays69.
Figure 3 |. Schematic of Gα subunit inhibition by YM‑254890 and FR900359.
Canonical G protein α-subunit signalling is initiated when GDP is released from the nucleotide-binding pocket, thereby allowing GTP to bind. The guanine nucleotide-free state is associated with separation of the α-helical domain (green) from the Ras-like domain (blue), which allows GDP to exit the nucleotide binding site. Gα returns to a closed state once GTP is bound, with minor structural perturbations in the hinge or switch region and around the nucleotide binding site. Signalling is terminated when the intrinsic GTPase activity of Gα hydrolyses GTP to GDP, returning Gα to its quiescent state. YM-254890 or FR900359 (purple) binds to the hinge region of Gαq, preventing the separation of the domains necessary for GDP release and Gα activation.
FR900359 has been extensively characterized in vitro to assess its specificity and efficacy for inhibition of Gαq/11 -mediated signal transduction69. These studies used cell-based assays to examine Gαq/11-stimulated PLC activity by monitoring inositol phosphate (IP) production, bioluminescence resonance energy transform (BRET) to monitor G protein subunit dissociation and dynamic mass redistribution (DMR) to measure cellular responses to GPCR activation. These data all showed strong specificity for inhibition of Gαq/11 but not for other G protein-mediated responses. In this same study, in a more physiologically relevant system, FR900359 relaxed phenylephrine/α1-adrenergic receptor (α1-AR)-dependent constriction of tail vein arteries. Interestingly, these effects were not diminished with FR900359 washout, suggesting a slowly reversible mechanism of action that is likely non-covalent. This property may improve the therapeutic utility of this compound in vivo and may partially explain its long duration of action in vivo as described below.
Specific inhibition of Gαq-mediated activation of downstream effectors has also been demonstrated using a 27mer peptide derived from a helix–turn–helix region of PLCβ3, which corresponds to a region critical for interaction with Gαq. This peptide inhibited PLC activation by Gαq in reconstituted lipid vesicles and inhibited Gαq signalling in cells when transfected as a fusion construct with attached fluorescent proteins114. Although it was effective, the peptide has not demonstrated bio-availability when applied exogenously.
Preclinical studies with Gαq/11 inhibitors in thrombosis.
YM-254890 was originally developed by Astellas Pharma as an antithrombotic agent. It was dropped as a therapeutic programme likely owing to concerns with systemic blood pressure effects that result from inhibiting Gαq in the vasculature. In humans, the receptors for thrombin — proteinase-activated receptors 1 (PAR1) and 4 (PAR4) — mediate platelet aggregation in part through Gαq/11-induced Ca2+ release from endoplasmic reticulum stores115. The purinergic receptors P2Y1 and P2Y12 are other important pharmacological targets in platelets. The P2Y1 receptor couples to Gαq/11 whereas P2Y12 is coupled to Gαi, with stimulation of either receptor resulting in activation of platelet aggregation116,117. YM-254890 was originally isolated in a screen to identify inhibitors of platelet aggregation in response to ADP detection by P2Y1 (REF.106). YM-254890 effectively and potently inhibited thrombus formation in a vascular carotid injury model in mice but also significantly increased bleeding time using the FeCl3 assay107,108. A substantial decrease in blood pressure was also observed with bolus injections in mice and rats. As a result of these side effects, it was suggested that YM-254890 and similar compounds are best utilized as locally delivered agents.
Preclinical studies with Gαq/11 inhibitors in asthma.
Because of the widely known role of Gαq-coupled receptors, such as M3 muscarinic receptors, ATII receptors and endothelin receptors, in mediating smooth muscle contraction, conditions involving excessive smooth muscle tone could be ameliorated by inhibiting Gαq/11 pathways. It is through these types of pathway that Gαq/11 inhibitors have hypotensive effects in vascular smooth muscle. More recent studies have focused on potential uses in the treatment of asthma. The standard of care for acute asthma is bronchodilators, such as β2-AR agonists, which produce cAMP and cause smooth muscle relaxation, or antagonists of GPCRs coupled to Gαq pathways, such as antagonists of the M3 muscarinic receptor or the leukotriene receptor, which may chronically mediate elevated bronchial tone.
The Benovic group first investigated the possibility of inhibiting Gαq/11 directly as a way to ameliorate bronchoconstriction with the idea that multiple Gαq-coupled receptors could be contributing to elevated tone and that targeting a single receptor may not achieve optimal efficacy118. In this study, FR900359 inhibited airway smooth muscle growth in vitro, which could prevent airway occlusion during airway remodelling in asthma. FR900359 also blocked constriction of lung slices stimulated with a muscarinic agonist, carbachol, or with histamine. These data suggested that targeting Gαq/11 could alleviate airway occlusion by blocking both smooth muscle remodelling and smooth muscle constriction mechanisms.
In a more recent study, a comprehensive analysis of inhibition of Gαq/11 signalling in vitro, ex vivo and in vivo was performed119. As suggested from previous work, Gαq/11 inhibition with FR900359 completely reversed methacholine-induced murine tracheal and lung tissue constriction. Methacholine is often used in humans to test for airway hyperresponsiveness. The same results were observed with histamine-induced porcine and human airway constriction ex vivo, with 80% relaxation observed after treatment with 1 μM FR900359.
Because of the blood pressure side effects with systemic administration of YM-254890 or FR900359, it was postulated that local delivery in the airways may be an ideal strategy to produce therapeutic efficacy. FR900359 was therefore delivered to mice via inhalation as an aerosol and airway resistance was measured. A single dose of FR900359 blocked acute methacholine-induced increases in airway resistance without affecting basal tone. This effect persisted for up to 24 hours, suggesting that FR900359 is stable in vivo and/or only slowly dissociates from Gαq/11 after binding. As postulated, aerosol delivery of FR900359 did not affect blood pressure or heart rate at doses that strongly inhibited airway constriction.
To further explore the utility of FR900359, it was tested in a murine allergic airway sensitization model that uses ovalbumin. This model results in airway inflammatory cell infiltration and hyperresponsiveness to the muscarinic agonist methacholine. FR900359 treatment completely blocked sensitized respiratory system resistance in response to methacholine. As a more physiologically relevant model, FR900359 application was tested in mice sensitized with an intratracheal house dust mite challenge. This model also results in inflammatory cell infiltration and mucin production, which was not inhibited by FR900359, but, again, FR900359 was very effective at inhibition of hyperresponsiveness to methacholine.
These studies are the first to demonstrate a potential therapeutic use for an inhibitor of a G protein α-subunit. Targeting G protein α-subunits has the potential for pleiotropic effects and could result in multiple side effects. These studies indicate that local application of Gα protein subunit inhibitors, in this case a Gαq/11 inhibitor, may circumvent these issues. Additionally, because multiple GPCRs that signal through Gαq/11 mediate airway hyperresponsiveness, a strategy that targets Gαq/11 has the potential to be more efficacious than a single selective GPCR antagonist.
Preclinical studies with Gαq/11 inhibitors in melanoma.
Gαq/11 signalling pathways have been implicated in oncogenic signalling in certain types of cancer. In melanoma, for example, metabotropic glutamate receptor 1 (mGluR1), which is a Gαq-coupled receptor, is highly elevated, and as such, FR900359 was tested in a variety of melanoma cell lines for inhibition of ERK signalling and cell proliferation69. In cell lines for which growth was sensitive to FR900359, a high basal level of inositol phosphates (IPs) was detected that was suppressed by FR900359, indicating high basal Gαq/11 activation. FR900359 caused the sensitive cells to change from a proliferative migrating phenotype to a differentiated, non-dividing and non-migratory state. This finding suggests that Gαq/11 inhibition is useful for treating melanoma and inhibiting its metastatic progression.
Constitutively active Gαq/11 mutations Gαq/11(Q209L) and Gαq/11(R183C) are prevalent in patients with uveal melanoma14,15. These mutations prevent hydrolysis of GTP, maintaining Gαq/11 in the GTP-bound state, and therefore do not require GDP–GTP exchange to become active. Thus, FR900359 or YM-254890 would not be predicted to inhibit Gαq/11(Q209L) activity in cells. However, FR900359 has been shown to suppress IP production and proliferation in a human melanoma cell line that carries a Gαq/11(Q209L) mutation69. One possibility is that in a cellular context some low level of nucleotide exchange is required to maintain Gαq/11(Q209L) in the active state. Indeed a recent study demonstrated that FR900359 drives constitutively active Gαq (Q209L) into the inactive, GDP-bound state by suppressing nucleotide exchange, which results in inhibition of downstream signalling pathways190. Additionally, treatment with FR900359 inhibited the proliferation and dedifferentiation of Gαq (Q209L)-driven uveal melanoma cell lines190, suggesting that FR900359 could be considered as a treatment for uveal melanoma.
For patients with activating Gαq/11 mutations in uveal melanoma, local application of the compound could bypass systemic effects of FR900359 administration. If topical application was possible, this could provide a useful route for delivery.
Other considerations for Gαq/11 inhibitors.
As discussed above, both YM-254890 and FR900359 are GDP dissociation inhibitors and would be predicted to inhibit GTP binding and activation of Gαq. Thus, this strategy would be expected to be effective under conditions in which the upstream GPCR is overexpressed but not under conditions in which the G protein is constitutively active; Gαq/11(Q209L) is predicted to have a negligible rate of GTP hydrolysis in vitro and is locked in the GTP-bound state. Thus, a mechanism that inhibits nucleotide exchange would not be expected to regulate these proteins. Nevertheless, the Kostenis group showed that FR900359 is able to inhibit IP accumulation driven by Gαq/11(Q209L) and Gαq/11(R183C) in HEK293 cells, although fairly high concentrations of FR900359 were used in these experiments69. A possible explanation for this observation is that although the GTP hydrolysis rate by Gαq/11(Q209L) is negligible in a purified system, in cells there may be factors such as RGS proteins that increase the hydrolysis rate enough that nucleotide exchange becomes a factor in its activation.
Although the detailed characterization of FR900359 by the Kostenis group provided strong evidence for the specificity of FR900359 for Gαq/11, subsequent analysis has revealed unexpected effects of FR900359 on Gαi-mediated signalling120. In some cases, GPCR-mediated PLC signalling proceeds through a Gαi -mediated pathway that is inhibited by PTX121–123. These PTX-sensitive responses are mediated by Gβγ-dependent PLC activation rather than Gαq/11 activity33,124,125. In these studies, FR900359 inhibited PTX-sensitive IP mobilization and ERK1–ERK2 activation stimulated by adenosine A1, M2 muscarinic and P2Y12 purinergic receptors expressed in CHO cells. FR900359 did not inhibit Gαi-mediated inhibition of cAMP production. One possible explanation is that FR900359 can bind directly to Gβγ and inhibit downstream signalling to PLC. Given the very high specificity of this compound for Gαq versus Gαs and Gαi in other respects, this seems unlikely but remains possible. Another potential explanation may reside in the cooperative nature of PLCβ3 activation by G protein subunits. PLCβ3 is prominently expressed in various cell lines and is likely the relevant PLC isoform in CHO cells124. PLCβ3 is synergistically activated by a combination of Gαq/11 and Gβγ126,127. It is possible that the Gβγ released upon activation of Gαi-coupled receptors in CHO cells is insufficient to activate PLC without a concomitant low-level activation by Gαq/11. Perhaps a low basal level of Gαq/11 signalling in these cells is required to observe Gβγ-dependent stimulation and FR900359 inhibits this low-level Gαq/11 basal activity.
A related issue is that FR900359 and YM-254890 stabilize the Gαq–GDP state, which has a high affinity for Gβγ. Thus, FR900359 and YM-254890 would inhibit signalling by both Gαq and Gβγ released from Gαq heterotrimers. As discussed above, Gβγ signalling is primarily associated with Gαi signalling, but some examples of Gβγ signalling are associated with release from Gαq heterotrimers128,129.
Prospects for Gα inhibitor development
FR900359 is a very powerful tool for dissecting Gαq-mediated signal transduction pathways, and the data discussed above suggest that this approach, and perhaps this compound, is an effective clinical lead. Total synthesis of FR900359 and YM-254890 analogues has been successfully achieved130,131, and some of the derivatives have half-maximal inhibitory concentration (IC50) values for Gαq/11 inhibition approaching that of FR900359 (REF.132). Synthetic production has allowed diversification of the YM-254890 scaffold, which could yield new Gα subunit subtype-specific inhibitors. Despite substantial divergence in sequence between Gα subunits in the YM-254890 and/or FR900359 binding site, all the YM-254890 derivatives synthesized thus far retain high selectivity for Gαq/11 inhibition132. Nevertheless, the structure–activity relationship of YM-254890 derivatives has only very recently begun to be explored, therefore the potential for development of G protein-specific inhibitors remains.
Pharmacological targeting of Gβγ
Rationale for targeting Gβγ
Initial data suggesting that Gβγ targeting is of clinical utility are based on in vivo expression of a protein-based inhibitor of Gβγ, the carboxy-terminal fragment of GRK2 (GRK2ct)102,103,133–135. This protein fragment contains the pleckstrin homology (PH) domain region of GRK2, which directly binds to Gβγ. Numerous cellular expression studies indicate that this domain can specifically inhibit signalling downstream of Gβγ without affecting other GPCR-initiated signalling pathways101.
The first utilization of this inhibitor by the Koch group showed that cardiomyocyte-specific expression of GRK2ct in mice improved cardiac function in an animal model of heart failure103. GRK2ct inhibits the recruitment of GRK2 to the β-AR, thereby preventing receptor desensitization, which is associated with heart failure103. This group and others have used expression of GRK2ct in various preclinical models that together provide evidence that targeting Gβγ could have therapeutic utility102–104,136,137. Other ideas for clinical utility come from the roles for Gβγ signalling in specific physiologies that will be discussed below.
Prototype small-molecule inhibitors of Gβγ subunit signalling have been identified that block a subset of Gβγ-dependent signals downstream of GPCR activation105. For example, some molecules block potentially detrimental Gβγ signals without affecting Gα signals, allowing potentially beneficial Gα signals and some Gβγ signals to proceed. As discussed earlier, oliceridine is a biased ligand for the MOR that preferentially activates G protein pathways over β-arrestin pathways138. As discussed in more detail below, preclinical data indicate that it is also possible to bias MOR signals with Gβγ inhibitors to favour pain relief while avoiding the detrimental effects of MOR activation. Developing biased ligands for each individual receptor involves painstaking screening and analysis to find appropriate ligands. Molecules that target Gβγ have the potential to bias any receptor that signals through Gβγ. A Gβγ-targeted drug could potentially be coadministered with a GPCR-targeted drug to improve the side-effect profile. This strategy would obviate the need for discovery and approval of biased ligands for every receptor. It is also likely that Gβγ-targeted compounds can bias GPCR signals in ways that cannot be achieved by GPCR-directed biased ligands, thus providing an alternative approach to altering pharmacological efficacy.
It is possible that virus-based strategies for delivery of protein inhibitors of Gβγ could have direct clinical application135. In addition to the potential of GRK2ct, the Palczewski laboratory has recently developed Gβγ-directed nanobodies that inhibit Gβγ signalling without interfering with Gα signalling139. Viral delivery of either of these proteins has potential for selective delivery to target tissues, but issues with developing therapeutics based on viral therapies must be overcome, and protein-based Gβγ inhibitors will not be discussed further.
Small-molecule Gβγ inhibitors
Prototypical Gβγ inhibitors were discovered in a competition screen with the peptide SIGK for binding to Gβγ5,140. The SIGK peptide has been co-crystallized with Gβγ and defines an interaction surface on Gβ that coordinates direct binding to downstream targets and Gα subunits73. Thus, compounds that inhibit binding of this peptide likely either bind directly to this surface or alter the surface in a way that interferes with peptide binding and potentially with Gβγ effectors. Gβ subunits are prototypical members of the WD40 repeat protein family and mediate downstream effector activation through direct protein–protein interactions141. Traditionally, protein–protein interactions are difficult to disrupt with small molecules, in part because protein interaction surfaces are often flat, making it difficult for small molecules to bind with sufficient energy to disrupt high-affinity interactions142 (BOX 1). The surface to which SIGK binds is not flat but is rather a concave surface present on the top face of all WD40 repeat proteins, and results from the toroid structure formed as a consequence of the circular folding of the seven blades of the WD40 repeat propeller. This motif is common for protein–protein interactions in biology and has been targeted successfully by small molecules, leading to multiple potentially clinically important drugs143–145.
Box 1 | Development of PPI inhibitors targeting WD40 repeat proteins.
In general, protein–protein interaction (PPI) inhibitors share features that seem logical or intuitive. PPI inhibitors tend to be larger than typical drugs (500–800 Da), which is unsurprising because PPIs are often expected to inhibit interactions that typically involve several-hundred to low-thousand Å2 (REF.2) interfaces of each protein. The size of these interfaces was perhaps the greatest barrier when envisioning efficacious and potent PPI inhibitors in the first place; however, one of the identified themes for PPI mechanisms is that blocking a few critical residues in protein hot spots is often sufficient to effectively inhibit the PPI. These regions often have unique flexibility, which allows for adaptability to structurally distinct partner proteins but also gives rise to the existence of small, transient pockets where PPI inhibitors can bind178. Gβγ has a hot spot that has been implicated in effector protein binding. Thus, Gβγ may have a PPI interface that is amenable to small-molecule binding.
A pattern noted for PPI inhibitors of WD40 repeat (WDR) proteins is a three-pronged or triradiate structure145. They often have substituents around a central ring that extend in three directions, one of which extends into the central core of the WDR protein. A rudimentary hypothesis is that such a structure is necessary to both imbue sufficient contact for high-affinity binding and cover sufficient surface area to disrupt the PPI. This observation should be interpreted with caution as the sample size for WDR PPI inhibitors is quite small; however, it is an interesting approach to keep in mind when developing Gβγ inhibitors. It could be argued that gallein has such a structure, with three rings extending from the central oxygen-containing heterocycle. This general structure can also be seen in other less well-characterized Gβγ binders105. Although no structural data have been generated for these compounds bound to Gβγ, they all displace binding of the SIGK peptide, which has been crystalized bound to the hot spot near the top of the central pore.
Two prototypical Gβγ-binding molecules have been extensively characterized in vitro and in vivo. These molecules — gallein and M119 — are structurally similar and are related to fluorescein (FIG. 2b). They bind to Gβγ with an apparent affinity of approximately 1 μM; fluorescein does not bind to Gβγ140,146. A potentially important property of these molecules is that the binding is very slowly reversible, which could explain their efficacy despite low apparent potency (koff = 0.0003 s−1)146,147 (BOX 2). These compounds are commercially available, are well tolerated in mice and have been widely used by the scientific community140,148–152. This profile has allowed for the characterization of the effects of inhibiting Gβγ subunits on GPCR signalling, the specificity of the approach and the evaluation of Gβγ as a potential therapeutic target in a number of animal models of disease.
Box 2 | Target residence time in therapeutic efficacy and specificity.
An emerging idea in drug discovery is the concept that drug residence time — that is, the rate of dissociation of the drug from its target (koff) — can be an important determinant of therapeutic specificity and efficacy147. If a drug has a relatively short plasma half-life, its therapeutic utility could be limited unless target engagement is sustained after the compound is cleared from the plasma. Additionally, if a compound engages multiple targets but dissociates particularly slowly from the desired target, a long residence time could increase specificity. The effects of FR900359 in cells and in ex vivo systems were sustained after compound washout69 and airway relaxation was sustained in vivo for at least 24 hours after a single dose119. Binding of both gallein and M119 to Gβγ is slowly reversible, as measured by surface plasmon resonance146. The plasma half-life of gallein is 1–2 hours (A.S. unpublished data), and its affinity for Gβγ is in the high-nanomolar range, yet a dosing regimen of three times weekly was sufficient to ameliorate lupus symptoms over the course of the 20 weeks of disease development171. Thus, it appears likely that these compounds achieve therapeutic efficacy at least in part owing to their long residence times.
Another approach to identify small-molecule inhibitors of Gβγ was developed by the Hamm laboratory153. This laboratory identified an interaction between Gβγ and components of the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex, which is involved in regulating synaptic vesicle fusion. A surface plasmon resonance-based screen was used to identify molecules that would inhibit Gβγ–SNARE interactions. Several molecules were identified in this screen but have not yet been tested in physiological systems.
Downstream signalling specificity
One concern with targeting Gβγ is that Gβγ is a participant in every G protein signalling pathway because it is required for G protein activation by GPCRs, likely because Gβγ stabilizes the conformation of Gα that is required for GPCR interactions1,112. As such, completely blocking Gβγ subunit function would completely decouple the G protein system. Thus, the pharmacological approach to blocking Gβγ function must prevent downstream signalling without interfering with G protein activation in general. Proof of principle that this is achievable comes from the capacity of GRK2ct, M119 or gallein to block Gβγ signalling without affecting GPCR-dependent Gα activation101.
Another potential concern is the ubiquitous expression of Gβγ subunits in every cell downstream of every GPCR; consequently, inhibition of downstream signalling by Gβγ could have wide-ranging effects. In reality, Gβγ subunit signalling is highly restricted to cell types in which Gβγ-responsive effectors are expressed. An example of this is regulation of phosphoinositide 3-kinase (PI3K) signalling by Gβγ in immune cells in response to chemokines. Chemokine receptors couple primarily to Gαi and drive a number of immune cell responses by pathways that are Gβγ-dependent, including PI3K activation and subsequent phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P3, also known as PIP3) production, both of which are blocked by treatment with PTX. Chemokine-dependent PtdIns(3,4,5)P3 responses are very robust in immune cells such as neutrophils and macrophages121. The strength of this response is likely because they express high levels of the Gβγ-responsive PI3Kγ isoform, which consists of P110γ and P101 subunits34,154. By contrast, GPCR-dependent PTX-sensitive PtdIns(3,4,5)P3 responses are not readily observable in other cell types, likely because these cells do not express PI3Kγ. Thus, two levels of potential selectivity are present in this strategy. One level of selectivity is for Gαi-coupled receptors because Gβγ-dependent signalling responses arise primarily from Gαi, and the second level is due to the restricted expression of Gβγ-regulated downstream targets such as PI3Kγ.
As another source of specificity, small-molecule inhibitors of Gβγ that have been identified thus far inhibit a subset of protein–protein interactions without affecting interactions with Gα subunits105,140,146. Gβγ inter actions with downstream targets have been examined by both X-ray crystallography and mutagenesis74,155–157. One key feature of these interactions is that the targets share a common binding surface on Gβ but the details of the interactions are unique for each target75. This observation suggests that it is possible for small molecules to selectively interfere with downstream targets by binding to the subset of amino acids on Gβ that is required for binding to a subset of targets (FIG. 4). This contrasts with protein-based approaches with GRK2ct and Gβγ-directed nanobodies that occupy a large area on the Gβ protein interaction surface139. As yet, no X-ray crystal structure exists for a small molecule bound to Gβγ, but the empirical data in which small molecules selectivity block a subset of Gβγ targets support this idea105,158.
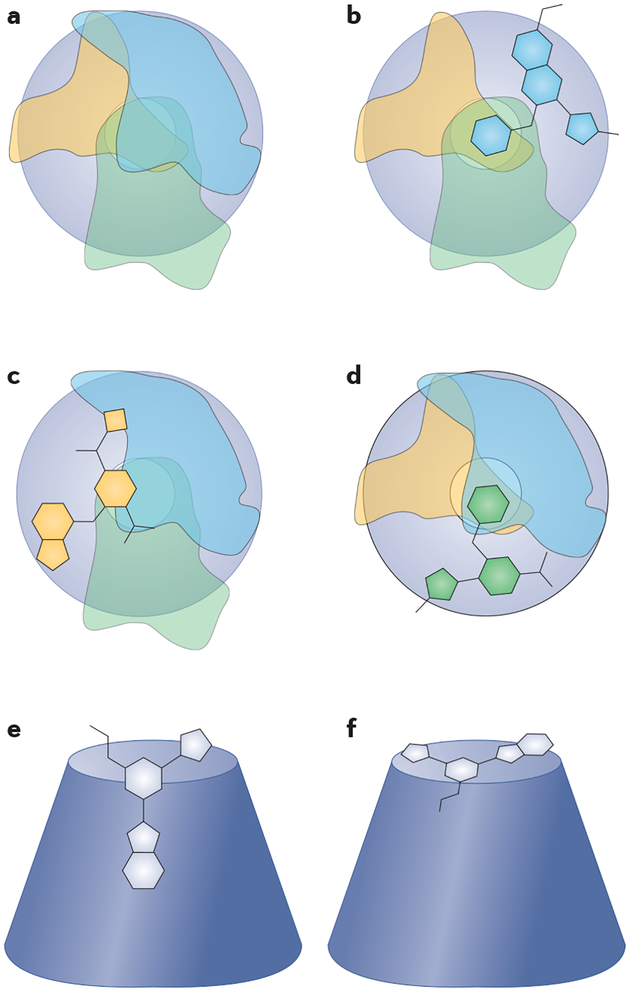
Figure 4 |. Mechanisms for selectively inhibiting signalling downstream of Gβγ.
Most downstream effector proteins (hypothetical footprints represented by orange, green and light blue shapes) interact with the hotspot surface on one face of the toroid structure of Gβ (dark blue). a | Downstream signalling proteins interact with Gβγ via distinct but overlapping binding sites. b–d | Compounds may anchor in the core but project into different interaction sites, thereby creating selective inhibition of specific binding partners. e,f | There are numerous potential ways in which small molecules could binding to the Gβγ hot spot. One possibility is a message–address-type pose (part e). One branch of the inhibitor would bind within the core of the toroid, mimicking a high-affinity binding pocket (address) and other branches would extend across the hotspot surface to disrupt protein binding (message). Alternatively, flat molecules may bind across the surface of the hot spot, disrupting large areas of protein binding (part f).
Still, some issues remain because even with restricted expression, inhibition of some targets would have major side effects. Particular targets of concern include ion channels such as the G protein-activated inward rectifier K+ channel (GIRK) and the N-type voltage-gated calcium channels32,159,160. Gβγ activates GIRK channels in neurons and in atria, leading to a hyperpolarization-induced decrease in action potential firing. Therefore, when considering the use of Gβγ inhibitors in cardiac or immune therapy, interfering with the regulation of action potentials would have highly undesirable side effects, such as arrhythmias. However, empirical data using prototypical Gβγ blockers indicate that these pathways are unaffected by Gβγ inhibitors, and animals treated with gallein show no signs of arrhythmias or alterations in heart rate161.
Another approach to generating specificity for Gβγ signalling would be to target specific Gβγ subtypes. As discussed, there are multiple subtypes of Gβ and Gγ subunits that have differential tissue distributions and signalling functions in cells and in vivo. Most of the diversity resides in the Gγ subunits, which have not been shown to interact with effectors, and the surface that has been targeted on Gβ is highly conserved among Gβ subunits (excep t Gβ5). These data suggest that direct, selective, small-molecule targeting of specific Gβγ subunit sub-types will be difficult.
Preclinical models of Gβγ targeting
Opioid analgesia.
Activation of MOR produces analgesia by virtue of its ability to inhibit the release of neurotransmitters in peripheral nociceptors as well as in the descending circuit in the periaqueductal grey of the brain. MOR is Gαi-coupled and inhibits neurotransmitter release by regulating a variety of systems in neurons. Gβγ activates GIRK channels to hyperpolarize presynaptic neurons and thus reduce excitability, and Gβγ inhibits the N-type calcium channels that are responsible for Ca2+ influx upon neuronal depolarization; both of these mechanisms inhibit neurotransmitter release. Gβγ also interacts with synaptosomal-associated protein 25 (SNAP25) to directly inhibit synaptic vesicle fusion162. Thus, complete inhibition of Gβγ signalling would be expected to eliminate opioid efficacy.
Chronic MOR activation and very-low-dose morphine have also been shown to promote hyperalgesia163,164. Several lines of evidence suggest that this phenomenon involves activation of PLCβ3 and subsequent downstream PKC activation163–165. Mice with global deletion of PLCβ3 show enhanced sensitivity to MOR activation, which results in a tenfold greater sensitivity to morphine in a 55°C tail flick assay of antinociception166. This finding indicates that PLCβ3 activation downstream of MOR inhibits MOR-dependent analgesia. PLCβ3 is responsive to both Gαq and Gβγ, and because MOR is coupled to Gαi, any mechanism for PLCβ3 activation must involve Gβγ subunits124,126,167. M119 inhibited Gβγ-dependent PLCβ2 and PLCβ3 activation without affecting K+ channel and Ca2+ channel inhibition by Gβ, which suggested that M119 or gallein could increase the analgesic potency of morphine105.
In initial experiments, M119 was administered through intracerebroventricular injection into mice and morphine dose-dependent analgesia was assessed in the tail flick antinociception test105. M119 injection indeed increased the potency of morphine by tenfold. M119 was ineffective when injected into mice lacking PLCβ3, supporting the idea that the effect of M119 is through inhibition of Gβγ regulation of PLCβ3. The observation that M119 injection into the periaqueductal grey inhibited the formation of Gβγ–PLCβ3 complexes in this tissue further supports this mechanism of action164.
Follow-up experiments showed that M119 was also effective when injected intraperitoneally, was specific for MOR activation and had no effect on δ-opioid or κ-opioid receptor-mediated antinociception158. M119 also blocked development of acute tolerance — co-injection of M119 and morphine prevented the rightward shift in dose dependence for antinociception that accompanies repeated morphine injection. In the same study, intracerebroventricular injection of M119 before intraperitoneal administration of high-dose morphine prevented the development of withdrawal symptoms upon naloxone injection. These experiments indicate that M119 acts on central MORs but when injected intraperitoneally it is possible that its actions may be mediated peripherally as well as centrally.
The ability of M119 to shift the dose–response curve for morphine suggests that, if administered in conjunction with morphine, considerably less opioid will be required to achieve the same level of pain relief. These studies indicate that M119 or other Gβγ inhibitors coadministered with μ-opioids would inhibit the development of tolerance and ameliorate withdrawal symptoms. On the other hand, if M119 potentiated all effects of morphine, including the side effects, such an approach would have limited utility. A subsequent study confirmed that gallein potentiated morphine-dependent antinociception when administered intraperitoneally. Gallein did not potentiate morphine-dependent respiratory depression, inhibition of gastrointestinal transit or conditioned place preference168. Together, these data indicate that gallein coadministration with opioids increases the therapeutic window for opioid use, which would increase the safety profile of well-characterized opioids and reduce their addictive potential.
New treatments for chronic pain are a major unmet need. Opioids are highly effective at treating acute pain such as postoperative pain, but prolonged usage results not only in tolerance but also in hyperalgesic sensitization of pain responses. This finding has also been shown to be dependent on PLCβ3 signalling, suggesting that M119 or gallein could prevent the hyper sensitization with chronic opioid use165. A recent study indeed showed that gallein inhibited hyperalgesic priming in response to repeated opioid administration in mice163. Thus, Gβγ inhibitors might be developed that would allow for safe longer-term use of opioids for chronic pain.
Chronic inflammatory disease.
As discussed above, Gβγ signalling is a major driver of signal transduction in immune cells because chemokine receptors signal through Gαi. The majority of the downstream processes driven by chemokine receptors are regulated by Gβγ, although some roles specific to Gαi have recently been identified169,170. Chemokine receptors are highly sought- after targets for the development of anti-inflammatory treatments for diseases such as rheumatoid arthritis and lupus. These chronic inflammatory diseases involve dysregulation of multiple chemokines and their receptors, so targeting individual GPCRs may have limited efficacy. Blockade of a common target such as Gβγ downstream of multiple chemokine receptors could have greater efficacy in these conditions.
Gallein delivered either intraperitoneally or orally was able to block acute inflammation in a carrageenan foot pad injection model140. More recently, gallein was tested in a mouse model of lupus nephritis. In those experiments, gallein was administered intraperitoneally three times a week during disease progression for 20 weeks, or after the animals developed active lupus171. Gallein prevented the clinically relevant end point of proteinuria, as well as glomerular inflammatory infiltration, either when given prophylactically or after disease development. Interestingly, gallein treatment also inhibited germinal centre formation in the spleen by reducing both their size and number. These data validate the idea that Gβγ inhibition may have therapeutic utility in chronic inflammatory diseases.
Heart failure.
Cardiac myocyte-specific expression of the protein-based Gβγ inhibitor GRK2ct increased cardiac performance in heart failure102,103. This observation suggests that gallein or M119 could be used to inhibit the development of heart failure. Initial studies demonstrated that intraperitoneal administration of gallein improved cardiac function and inhibited the development of cardiac hypertrophy and fibrosis stimulated by chronic β-AR stimulation with isoprenaline administration (for 1 week)172. Gallein also and blocked hypertrophy progression in a cardiac-specific calsequestrin transgenic mouse model of cardiac failure172.
Follow-up studies examined gallein in a pressure overload model of heart failure after establishment of hypertrophy161. Gallein administration at 10 mg/kg intraperitoneally preserved cardiac function and halted hypertrophic growth. One of the hallmarks of heart failure is increased plasma concentration of neurohumoral factors such as adrenaline and noradrenaline. Adrenaline release is inhibited by presynaptic Gαi-coupled α2-ARs that are in turn inhibited by GRK2 (REF.137) (FIG. 5). GRK2 is upregulated in heart failure and leads to desensitization of α2-ARs, reducing feedback inhibition of noradrenaline release. In a recent study, inhibition of Gβγ-regulated GRK2 activation, using adenoviruses to express GRK2ct, prevented α2-AR desensitization and reduced plasma adrenaline levels in a heart failure model. This finding suggested that systemic administration of a small molecule such as gallein achieves high therapeutic efficacy through a dual mechanism of action: through direct effects in cardiac myocytes and by lowering circulating catecholamine levels. Indeed, treatment with gallein normalized plasma adrenaline and noradrenaline levels and restored α2-AR-mediated feedback inhibition of catecholamine release161.
Figure 5 |. Possible targets of Gβγ inhibitors in heart failure.
Hypertrophic pathways in the myocardium and adrenal gland contribute to pathological hypertrophy and heart failure. Gallein or M119 is able to inhibit a number of Gβγ interactions in cardiomyocytes but also alters signalling in the adrenal medulla and thus demonstrates a multifactorial approach to the pharmacological treatment of cardiac hypertrophy and heart failure. In the adrenal medulla, inhibition of Gβγ increases the release of catecholamines (CAs), such as dopamine, adrenaline and noradrenaline. Adrenaline stimulates the β-adrenergic receptor (β-AR) on cardiomyocytes; therefore, inhibition of Gβγ could have numerous therapeutic effects in heart failure. DAG, diacylglycerol; ERK, extracellular-signal-regulated kinase; GRK2, G protein-coupled receptor kinase 2; PKD, protein kinase D; PLCε, phospholipase Cε.
Chronic heart failure can lead to complications, such as the development of secondary renal disease. This disease results in part from activation of the endothelin system, and renal damage is characterized by renal fibrosis and inflammation. A recent study examined the effects of gallein on renal damage caused indirectly by pressure overload-induced cardiac hypertrophy or directly by bilateral ischaemia–reperfusion in a mouse model of acute kidney injury (AKI)173. Gallein administration at 10 mg/kg per day prevented elevation of plasma creatinine and renal fibrosis in the transverse aortic constriction model and inhibited renal damage, apoptosis and endothelin elevation in the AKI model.
It has been proposed that Gβγ inhibition in cardiac myocytes inhibits progression of heart failure by preventing Gβγ-dependent recruitment of GRK2, thereby preventing desensitization of the β-AR (FIG. 5). However, treatment with Gβγ inhibitors likely blocks other pathways associated with Gβγ in the heart. For example, it was recently shown that Gβγ can directly bind to and activate ERK, leading to its phosphorylation and trans-location to the nucleus in cardiac myocytes; ERK trans-location is associated with the development of cardiac hypertrophy174. Another study identified Gβγ as a regulator of PLCε activation at the Golgi apparatus, which is also critical for endothelin 1 (ET1)-driven hypertrophy175,176. Gallein inhibits Gβγ-dependent regulation of PLCε in cardiac myocytes, and Gβγ inhibition has the potential to inhibit ERK activation. Thus, Gβγ inhibition has the potential to be highly efficacious by virtue of its ability to block activation of multiple prohypertrophic targets by Gβγ.
Fibrosis.
In both the heart failure model and the kidney damage model, Gβγ inhibition with gallein prevented the development of fibrosis173,177. It is possible that this is a direct cellular effect on fibrosis. Indeed, in mouse embryo fibroblasts, gallein at 10 μM inhibited fibroblast activation in vitro177.
Other effects of Gβγ blockade.
M119 and gallein have been widely used in cellular systems to assess the potential for blockade of Gβγ in particular disease-related systems. These include proliferation, migration and invasion of cancer cells, T cell regulation, Alzheimer disease models and other biological pathways. Some examples of these studies are listed in TABLE 1.
Table 1 |.
Selected gallein or M119 effects in ex vivo systems
| Ex vivo cellular or organ system | Signalling pathway | Indication | |
|---|---|---|---|
| Hippocampal neurons | Amyloid precursor protein-Gao | Blocks amyloid-p-dependent cell death. Reverses memory impairment in 3xTgAD mice | 148 |
| LNCaP prostate cancer cell line | Olfactory receptor 51E2 | Inhibits cell invasiveness in vitro and metastasis spread in a xenograft model | 149 |
| HEK293 cell line | DAT | Inhibits dopamine efflux through DAT | 179 |
| HEK293 and KNRK cell lines | PAR2-PKD | Trafficks PAR2 to the plasma membrane | 150 |
| T cells | TCR | Enhances TCR-dependent IL-2 production | 180 |
| MCF7 breast cancer cell line | PKC and PI3K | Decreases invasiveness but not proliferation | 181 |
| Rat mesenteric resistance arteries | CGRP-cAMP | Increases relaxation | 182 |
| HeLa cell line | PLC-PKD | Inhibits cargo transport from the Golgi to the plasma membrane | 183 |
| HeLa cell line | S1P-RAC-CDC42 | Alters cargo sorting into exosomal vesicles of multivesicular endosomes | 152 |
| MDA-MB-231 breast cancer cell line | CXCR4-RAC1 | Inhibits migration and invasion | 184 |
| MDA-MB-231 and MCF10A breast cancer cell lines | SDF1, LPA, PAR1 and PAR2 | Inhibits proliferation and migration | 185 |
| PC3 prostate cancer cell line | LPA, PAR1 and SDF1-AKT | Inhibits proliferation and tumour sphere formation, triggers apoptosis and enhances sensitivity to paclitaxel | 186 |
| HEK293 cell line | ADGRB2 and a disease-associated mutation | Inhibits downstream signalling to NFAT | 187 |
| NIH 3T3 cell line | SHH -SMO-PLA2 | Inhibits GLI activation | 188 |
| Primary human glioblastoma cells | PREX1 | Inhibits motility and invasion | 189 |
ADGRB2, adhesion G protein-coupled receptor B2; CGRP, calcitonin gene-related peptide; CXCR4, CXC-chemokine receptor 4; DAT, dopamine transporter; IL-2, interleukin 2; LPA, lysophosphatidic acid; NFAT, nuclear factor of activated T cells; PAR2, proteinase- activated receptor 2; PI3K, phosphoinositide 3-kinase; PKD, protein kinase D; PLA2, secretory phospholipase A2 receptor; PLC, phospholipase C; PREX1, phosphatidylinositol 3,4,5-trisphosphate-dependent Rac exchanger 1 protein; RAC1, Ras-related C3 botulinum toxin substrate 1; S1P, sphingosine 1-phosphate; SDF1, stromal cell-derived factor 1; SHH, sonic hedgehog protein; SMO, smoothened homologue; TCR, T cell receptor.
Prospects for Gβγ inhibitor development.
Gallein and M119 have been powerful tools to dissect Gβγ subunit signalling pathways and are widely available to the scientific community. These compounds are related and are flat, aromatic xanthene dye derivatives, and there are concerns with development of such compounds as human therapeutics owing to potential off-target effects. M119 and gallein seem to be fairly specific in vivo as no major side effects or toxic effects of these molecules were observed with chronic administration. This observation demonstrates that on-target-based toxicity is not likely to be a major problem. Nevertheless, new small-molecule screens should be pursued to identify novel Gβγ binding scaffolds that are more tractable for pharmaceutical development. Protein–protein interactions are notoriously difficult drug targets but, as has been discussed, WD40 proteins are unique in this regard145. Recent discoveries and observations have demonstrated that inhibition of WD40 proteins by small, drug-like molecules is possible and has also elucidated some basic themes about these drugs. Indeed, these WD40 protein–protein interaction inhibitors have been developed to the point of entering clinical trials, which suggests that further screening and the use of structure–activity relationship modelling to refine chemical leads has a reasonable chance of success. Furthermore, these results suggest that Gβγ is indeed a viable pharmacological target.
Outlook
Targeting G protein subunits has emerged as a viable approach to achieve pharmacological efficacy for a number of indications that perhaps would not be achieved by targeting individual GPCRs. Currently, there are specific inhibitors only for Gαq or general inhibitors of Gβγ subunits. Future work is likely to identify novel specific inhibitors for other classes of Gα subunits, and higher potency, selective inhibitors for Gβγ subunits. G proteins are involved in a wide range of physiologies and it is likely that they will emerge as targets for other unexplored therapeutic applications.
Acknowledgements
A.V.S. is funded by grants from the NIH (NIHR01GM81772 and R35GM127303).
Glossary
- Periaqueductal grey
An anatomical region in the brainstem that surrounds the cerebral aqueduct. This region is enriched in opioid receptors that are thought to mediate the central analgesic actions of opioid analgesics
- Hyperalgesia
Increased sensitivity to pain. Repeated opioid treatment for chronic pain can paradoxically increase sensitivity to pain stimuli over time
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
RELATED LINKS
Bgee database: https://bgee.org/?page=gene&gene_id=ENSMUSG00000029663
COSMIC database: https://cancer.sanger.ac.uk/cosmic
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Gilman AG G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem 56, 615–649 (1987). [DOI] [PubMed] [Google Scholar]
- 2.Oldham WM & Hamm HE Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell Biol 9, 60–71 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Komolov KE & Benovic JL G protein-coupled receptor kinases: past, present and future. Cell. Signal 41, 17–24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Homan KT & Tesmer JJG Structural insights into G protein-coupled receptor kinase function. Curr. Opin. Cell Biol 27, 25–31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson YK & Luttrell LM The diverse roles of arrestin scaffolds in G protein–coupled receptor signaling. Pharmacol. Rev 69, 256 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvarez-Curto E et al. Targeted elimination of G proteins and arrestins defines their specific contributions to both intensity and duration of G protein-coupled receptor signalling. J. Biol. Chem 291, 27147–27159 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eichel K, Jullie D & von Zastrow M β-Arrestin drives MAP kinase signalling from clathrin-coated structures after GPCR dissociation. Nat. Cell Biol 18, 303–310 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grundmann M et al. Lack of β-arrestin signaling in the absence of active G proteins. Nat. Commun 9, 341 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Hayre M et al. Genetic evidence that β-arrestins are dispensable for the initiation of β2adrenergic receptor signaling to ERK. Sci. Signal 10, eaal3395 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irannejad R et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature 495, 534–538 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrandon S et al. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat. Chem. Biol 5, 734–742 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos R et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov 16, 19 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fishman MC & Porter JA Pharmaceuticals: a new grammar for drug discovery. Nature 437, 491–493 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Van Raamsdonk CD et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 457, 599–602 (2009).This paper identifies constitutively activating mutations of Gαq at high prevalence in uveal melanoma and identifies Gαq as a potential therapeutic target for the treatment of uveal melanoma.
- 15.Van Raamsdonk CD et al. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med 363, 2191–2199 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenakin T Signaling bias in drug discovery. Expert Opin. Drug Discov 12, 321–333 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Smith JS, Lefkowitz RJ & Rajagopal S Biased signalling: from simple switches to allosteric microprocessors. Nat. Rev. Drug Discov 17, 243–260 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bohn LM et al. Enhanced morphine analgesia in mice lacking β-arrestin 2. Science 286, 2495–2498 (1999).These authors demonstrate that genetic deletion of β-arrestin 2 enhances the analgesic potency of morphine. This and subsequent work provide the basis for the idea of development of G protein‑biased opioid analgesics.
- 19.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ & Caron MG μ-Opioid receptor desensitization by β arrestin 2 determines morphine tolerance but not dependence. Nature 408, 720–723 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Schmid CL et al. Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell 171, 1165–1175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koblish M et al. TRV0109101, a G protein-biased agonist of the μ-opioid receptor, does not promote opioid-induced mechanical allodynia following chronic administration. J. Pharmacol. Exp. Ther 362, 254–262 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Singla N et al. A randomized, phase IIb study investigating oliceridine (TRV130), a novel μ receptor G protein pathway selective (μ-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty. J. Pain Res 10, 2413–2424 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon MI, Strathmann MP & Gautam N Diversity of G proteins in signal transduction. Science 252, 802–808 (1991). [DOI] [PubMed] [Google Scholar]
- 24.Robishaw JD & Berlot CH Translating G protein subunit diversity into functional specificity. Curr. Opin. Cell Biol 16, 206–209 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Oldham WM & Hamm E Structural basis of function in heterotrimeric G proteins. Q. Rev. Biophys 39, 117–166 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Khan SM et al. The expanding roles of Gβγ subunits in G protein-coupled receptor signaling and drug action. Pharmacol. Rev 65, 545–577 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Hepler JR & Gilman AG G proteins. Trends Biochem. Sci 17, 383–387 (1992). [DOI] [PubMed] [Google Scholar]
- 28.Northup JK et al. Purification of the regulatory component of adenylate cyclase. Proc. Natl Acad. Sci. USA 77, 6516–6520 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smrcka AV, Hepler JR, Brown KO & Sternweis PC Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science 251, 804–807 (1991). [DOI] [PubMed] [Google Scholar]
- 30.Hart MJ et al. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Gα13. Science 280, 2112–2114 (1998). [DOI] [PubMed] [Google Scholar]
- 31.Taussig R, Iniguez-Lluhi JA & Gilman AG Inhibition of adenylyl cyclase by Giα. Science 261, 218–221 (1993). [DOI] [PubMed] [Google Scholar]
- 32.Logothetis DE, Kurachi Y, Galper J, Neer EJ & Clapham DE The βγ subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 325, 321–326 (1987). [DOI] [PubMed] [Google Scholar]
- 33.Camps M et al. Isozyme-selective stimulation of phospholipase Cβ2 by G protein βγ subunits. Nature 360, 684–686 (1992). [DOI] [PubMed] [Google Scholar]
- 34.Stephens L et al. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein βγ subunits. Cell 77, 83–93 (1994). [DOI] [PubMed] [Google Scholar]
- 35.Smrcka AV G protein βγ subunits: central mediators of G protein-coupled receptor signaling. Cell. Mol. Life Sci 65, 2191–2214 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sunahara RK, Dessauer CW & Gilman AG Complexity and diversity of mammalian adenylyl cyclases. Ann. Rev. Pharmacol. Toxicol 36, 461–480 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Hohenegger M et al. Gsα-selective G protein antagonists. Proc. Natl Acad. Sci. USA 95, 346–351 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sternweis PC & Robishaw JD Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J. Biol. Chem 259, 13806–13813 (1984). [PubMed] [Google Scholar]
- 39.Bokoch GM, Katada T, Northup JK, Ui M & Gilman AG Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J. Biol. Chem 259, 3560–3567 (1984). [PubMed] [Google Scholar]
- 40.Katada T, Bokoch GM, Northup JK, Ui M & Gilman AG The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Properties and function of the purified protein. J. Biol. Chem 259, 3568–3577 (1984). [PubMed] [Google Scholar]
- 41.Codina J et al. Pertussis toxin substrate, the putative N i component of adenylyl cyclases, in an αβ heterodimer regulated by guanine nucleotide and magnesium. Proc. Natl Acad. Sci. USA 80, 4276–4280 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strathmann M, Wilkie TM & Simon MI Diversity of the G protein family: sequences from five additional α subunits in the mouse. Proc. Natl Acad. Sci. USA 86, 7407–7409 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linder ME, Ewald DA, Miller RJ & Gilman AG Purification and characterization of G oα and three types of G iα after expression in Escherichia coli. J. Biol. Chem 265, 8243–8251 (1990). [PubMed] [Google Scholar]
- 44.Solis GP et al. Golgi-resident Gαo promotes protrusive membrane dynamics. Cell 170, 939–955 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Casey PJ, Fong HKW, Simon MI & Gilman AG Gz, a guanine nucleotide-binding protein with unique biochemical properties. J. Biol. Chem 265, 2383–2390 (1990). [PubMed] [Google Scholar]
- 46.Murayama T & Ui M Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. J. Biol. Chem 258, 3319–3326 (1983). [PubMed] [Google Scholar]
- 47.Lee CH, Park D, Wu D, Rhee SG & Simon MI Members of the Gαq subunit gene family activate phospholipase Cβ isozymes. J. Biol. Chem 267, 16044–16047 (1992). [PubMed] [Google Scholar]
- 48.Taylor SJ, Chae HZ, Rhee SG & Exton JH Activation of the β1 isozyme of phospholipase C by α subunits of the G q class of G proteins. Nature 350, 516–518 (1991). [DOI] [PubMed] [Google Scholar]
- 49.Rhee SG Regulation of phospho-specific phospholipase C. Annu. Rev. Biochem 70, 281–312 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singer WD, Brown HA & Sternweis PC Regulation of eukaryotic phosphatidylinositol-specific phospholipase C and phospholipase D. Ann. Rev. Biochem 66, 475–509 (1997). [DOI] [PubMed] [Google Scholar]
- 51.Kadamur G & Ross EM Mammalian phospholipase C. Annu. Rev. Physiol 75, 127–154 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Harden TK, Waldo GL, Hicks SN & Sondek J Mechanism of activation and inactivation of Gq/phospholipase C β signaling nodes. Chem. Rev 111, 6120–6129 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wettschureck N & Offermanns S Mammalian G proteins and their cell type specific functions. Physiol. Rev 85, 1159–1204 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Chagin AS et al. G Protein stimulatory subunit alpha and Gq/11α G proteins are both required to maintain quiescent stem-like chondrocytes. Nat. Commun 5, 3673 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S et al. P2Y(2) and Gq/G(1)(1) control blood pressure by mediating endothelial mechanotransduction. J. Clin. Invest 125, 3077–3086 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sivaraj KK et al. Endothelial Gαq/11 is required for VEGF-induced vascular permeability and angiogenesis. Cardiovasc. Res 108, 171–180 (2015). [DOI] [PubMed] [Google Scholar]
- 57.John AE et al. Loss of epithelial Gq and G11 signaling inhibits TGFbeta production but promotes IL 33 mediated macrophage polarization and emphysema. Sci. Signal 9, ra104 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Wilkie TM, Scherly PA, Strathmann MP, Slepak VZ & Simon MI Characterization of G protein α subunits in the G q class: expression in murine tissues and in stromal and hematopoietic cell lines. Proc. Natl Acad. Sci. USA 88, 10049–10053 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hepler JR et al. Purification from Sf9 cells and characterization of recombinant Gq α and G11 α. Activation of purified phospholipase C isozymes by G α subunits. J. Biol. Chem 268, 14367–14375 (1993). [PubMed] [Google Scholar]
- 60.Kozasa T et al. Purification and characterization of recombinant G16 α from Sf9 cells: activation of purified phospholipase C isozymes by G protein α subunits. Proc. Natl Acad. Sci. USA 90, 9176–9180 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wirotanseng LN, Kuner R & Tappe-Theodor A Gq rather than G11 preferentially mediates nociceptor sensitization. Mol. Pain 9, 54 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aittaleb M, Boguth CA & Tesmer JJG Structure and function of heterotrimeric G protein-regulated Rho guanine nucleotide exchange factors. Mol. Pharmacol 77, 111–125 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Momotani K et al. p63RhoGEF couples Gα(q/11)-mediated signaling to Ca2+ sensitization of vascular smooth muscle contractility. Circ. Res 109, 993–1002 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Momotani K & Somlyo AV p63RhoGEF: a new switch for G(q)-mediated activation of smooth muscle. Trends Cardiovasc. Med 22, 122–127 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vaque JP et al. A genome-wide RNAi screen reveals a Trio-regulated Rho GTPase circuitry transducing mitogenic signals initiated by G protein-coupled receptors. Mol. Cell 49, 94–108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanchez-Fernandez G et al. Gαq signalling: the new and the old. Cell. Signal 26, 833–848 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Hoz C et al. G α(q) acts as an adaptor protein in protein kinase C ζ (PKCζ)-mediated ERK5 activation by G protein-coupled receptors (GPCR). J. Biol. Chem 285, 13480–13489 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia-Hoz C et al. Protein kinase C (PKC)ζ mediated Gαq stimulation of ERK5 protein pathway in cardiomyocytes and cardiac fibroblasts. J. Biol. Chem 287, 7792–7802 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schrage R et al. The experimental power of FR900359 to study Gq regulated biological processes. Nat. Commun 6, 10156 (2015).This comprehensive analysis reviews the specificity and efficacy of FR900359 as an inhibitor of Gαq in vitro and in cell models.
- 70.Takasaki J et al. A novel Gαq/11-selective inhibitor. J. Biol. Chem 279, 47438–47445 (2004). [DOI] [PubMed] [Google Scholar]
- 71.Strathmann MP & Simon MI Gα12 and Gα13 subunits define a fourth class of G protein α subunits. Proc. Natl Acad. Sci. USA 88, 5582–5586 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kozasa T et al. p115 RhoGEF, a GTPase activating protein for Gα12 and Gα13. Science 280, 2109–2111 (1998). [DOI] [PubMed] [Google Scholar]
- 73.Davis TL, Bonacci TM, Sprang SR & Smrcka AV Structural and molecular characterization of a preferred protein interaction surface on G protein βγ subunits. Biochemistry 44, 10593–10604 (2005). [DOI] [PubMed] [Google Scholar]
- 74.Ford CE et al. Molecular basis for interactions of G protein βγ subunits with effectors. Science 280, 1271–1274 (1998). [DOI] [PubMed] [Google Scholar]
- 75.Lin Y & Smrcka AV Understanding molecular recognition by G protein βγ subunits on the path to pharmacological targeting. Mol. Pharmacol 80, 551–557 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scott JK et al. Evidence that a protein-protein interaction ‘hot spot’ on heterotrimeric G protein βγ subunits is used for recognition of a subclass of effectors. EMBO J 20, 767–776 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cabrera JL, de Freitas F, Satpaev DK & Slepak VZ Identification of the Gβ5-RGS7 protein complex in the retina. Biochem. Biophys. Res. Commun 249, 898–902 (1998). [DOI] [PubMed] [Google Scholar]
- 78.Witherow DS et al. Complexes of the G protein subunit gβ5 with the regulators of G protein signaling RGS7 and RGS9. Characterization in native tissues and in transfected cells. J. Biol. Chem 275, 24872–24880 (2000). [DOI] [PubMed] [Google Scholar]
- 79.Snow BE et al. A G protein γ subunit-like domain shared between RGS11 and other RGS proteins specifies binding to Gβ5 subunits. Proc. Natl Acad. Sci. USA 95, 13307–13312 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ueda N et al. G protein βγ subunits. Simplified purification and properties of novel isoforms. J. Biol. Chem 269, 4388–4395 (1994). [PubMed] [Google Scholar]
- 81.Wickman KD et al. Recombinant G-protein βγ subunits activate the muscarinic-gated atrial potassium channel. Nature 368, 255–257 (1994). [DOI] [PubMed] [Google Scholar]
- 82.Diverse-Pierluissi M et al. Selective coupling of G protein βγ complexes to inhibition of Ca2+ channels. J. Biol. Chem 275, 28380–28385 (2000). [DOI] [PubMed] [Google Scholar]
- 83.Mayeenuddin LH, McIntire WE & Garrison JC Differential sensitivity of P-Rex1 to isoforms of G protein βγ dimers. J. Biol. Chem 281, 1913–1920 (2006). [DOI] [PubMed] [Google Scholar]
- 84.Gibson SK & Gilman AG Giα and Gβ subunits both define selectivity of G protein activation by α2-adrenergic receptors. Proc. Natl Acad. Sci. USA 103, 212–217 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lindorfer MA et al. Differential activity of the G protein β5 γ2 subunit at receptors and effectors. J. Biol. Chem 273, 34429–34436 (1998). [DOI] [PubMed] [Google Scholar]
- 86.Liang YL et al. Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature 546, 118–123 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liang YL et al. Phase-plate cryo-EM structure of a biased agonist-bound human GLP-1 receptor-Gs complex. Nature 555, 121–125 (2018). [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y et al. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 546, 248–253 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kleuss C, Scherübl H, Hescheler J, Schultz G & Wittig B Selectivity in signal transduction determined by γ subunits of heterotrimeric G proteins. Science 259, 832–834 (1993). [DOI] [PubMed] [Google Scholar]
- 90.Wang Q, Mullah B, Hansen C, Asundi J & Robishaw JD Ribozyme-mediated suppression of the G Protein γ 7 subunit suggests a role in hormone regulation of adenylylcyclase activity. J. Biol. Chem 272, 26040–26048 (1997). [DOI] [PubMed] [Google Scholar]
- 91.Schwindinger WF et al. Loss of G protein γ7 alters behavior and reduces striatal α(olf) level and cAMP production. J. Biol. Chem 278, 6575–6579 (2003). [DOI] [PubMed] [Google Scholar]
- 92.Schwindinger WF et al. Mice with deficiency of G protein γ3 are lean and have seizures. Mol. Cell. Biol 24, 7758–7768 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schwindinger WF et al. Synergistic roles for G-protein γ3 and γ7 subtypes in seizure susceptibility as revealed in double knock-out mice. J. Biol. Chem 287, 7121–7133 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cho JH, Saini DK, Karunarathne WK, Kalyanaraman V & Gautam N Alteration of Golgi structure in senescent cells and its regulation by a G protein γ subunit. Cell. Signal 23, 785–793 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O’Neill PR, Karunarathne WKA, Kalyanaraman V, Silvius JR & Gautam N G-Protein signaling leverages subunit-dependent membrane affinity to differentially control βγ translocation to intracellular membranes. Proc. Natl Acad. Sci. USA 109, E3568–E3577 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ajith Karunarathne WK, O’Neill PR, Martinez-Espinosa PL, Kalyanaraman V & Gautam N All G protein βγ complexes are capable of translocation on receptor activation. Biochem. Biophys. Res. Commun 421, 605–611 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lambright DG et al. The 2.0 Å crystal structure of a heterotrimeric G protein. Nature 379, 311–319 (1996). [DOI] [PubMed] [Google Scholar]
- 98.Sondek J, Bohm A, Lambright DG, Hamm HE & Sigler PB Crystal structure of a G-protein βγ dimer at 2.1Å resolution. Nature 379, 369–374 (1996). [DOI] [PubMed] [Google Scholar]
- 99.Clapham DE & Neer EJ G protein βγ subunits. Annu. Rev. Pharmacol. Toxicol 37, 167–203 (1997). [DOI] [PubMed] [Google Scholar]
- 100.Pitcher JA et al. Role of βγ subunits of G proteins in targeting the β-adrenergic receptor kinase to membrane-bound receptors. Science 257, 1264–1267 (1992). [DOI] [PubMed] [Google Scholar]
- 101.Koch WJ, Hawes BE, Inglese J, Luttrell LM & Lefkowitz RJ Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates Gβγ-mediated signaling. J. Biol. Chem 269, 6193–6197 (1994). [PubMed] [Google Scholar]
- 102.Koch WJ et al. Cardiac function in mice overexpressing the β-adrenergic receptor kinase or a βARK inhibitor. Science 268, 1350–1353 (1995).This study shows that cardiac expression of a protein that binds to Gβγ improves cardiac function, which is evidence that targeting Gβγ could be useful in the treatment of heart failure.
- 103.Rockman HA et al. Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc. Natl Acad. Sci. USA 95, 7000–7005 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eckhart AD, Ozaki T, Tevaearai H, Rockman HA & Koch WJ Vascular-targeted overexpression of G protein-coupled receptor kinase-2 in transgenic mice attenuates β-adrenergic receptor signaling and increases resting blood pressure. Mol. Pharmacol 61, 749–758 (2002). [DOI] [PubMed] [Google Scholar]
- 105.Bonacci TM et al. Differential targeting of Gβγ-subunit signaling with small molecules. Science 312, 443–446 (2006).This study identifies prototypical small‑molecule inhibitors of Gβγ subunit signalling. In this work, the concept of selective blockage of Gβγ downstream signalling by small molecules is introduced.
- 106.Taniguchi M et al. YM-254890, a novel platelet aggregation inhibitor produced by Chromobacterium sp. QS3666. J. Antibiot. (Tokyo) 56, 358–363 (2003). [DOI] [PubMed] [Google Scholar]
- 107.Kawasaki T et al. Antithrombotic and thrombolytic efficacy of YM-254890, a G q/11 inhibitor, in a rat model of arterial thrombosis. Thromb. Haemost 90, 406–413 (2003).This study demonstrates the utility of small-molecule Gαq inhibition in the treatment of thrombosis.
- 108.Kawasaki T et al. Pharmacological properties of YM-254890, a specific Gαq/11 inhibitor, on thrombosis and neointima formation in mice. Thromb. Haemost 94, 184–192 (2005). [DOI] [PubMed] [Google Scholar]
- 109.Nishimura A et al. Structural basis for the specific inhibition of heterotrimeric Gq protein by a small molecule. Proc. Natl Acad. Sci. USA 107, 13666–13671 (2010).These investigators solve the X-ray crystal co-structure of YM-254890 bound to Gαq, which provides a mechanism of action and a potential starting point for the development of Gα subunit-selective inhibitors.
- 110.Wall MA et al. The structure of the G protein heterotrimer Giα1β1γ2 Cell 83, 1047–1058 (1995). [DOI] [PubMed] [Google Scholar]
- 111.Lambright DG, Noel JP, Hamm HE & Sigler PB Structural determinants for activation of the α-subunit of a heterotrimeric G protein. Nature 369, 621–628 (1994). [DOI] [PubMed] [Google Scholar]
- 112.Rasmussen SGF et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477, 549–555 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dror RO et al. Structural basis for nucleotide exchange in heterotrimeric G proteins. Science 348, 1361–1365 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Charpentier TH et al. Potent and selective peptide-based inhibition of the G protein Gαq. J. Biol. Chem 291, 25608–25616 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Coughlin SR Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost 3, 1800–1814 (2005). [DOI] [PubMed] [Google Scholar]
- 116.Leon C et al. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y(1) receptor-null mice. J. Clin. Invest 104, 1731–1737 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gachet C P2Y(12) receptors in platelets and other hematopoietic and non-hematopoietic cells. Purinerg. Signal 8, 609–619 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carr R 3rd et al. Interdicting Gq activation in airway disease by receptor-dependent and receptor-independent mechanisms. Mol. Pharmacol 89, 94–104 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Matthey M et al. Targeted inhibition of Gq signaling induces airway relaxation in mouse models of asthma. Sci. Transl Med 9, eaag2288 (2017).This thorough study demonstrates the utility of FR900359 in the treatment of asthma. The authors use an aerosol-based approach to selectively deliver the compound to the lungs, thereby avoiding the global effects of Gαq inhibition.
- 120.Gao ZG & Jacobson KA On the selectivity of the Gαq inhibitor UBO-QIC: A comparison with the Gαi inhibitor pertussis toxin. Biochem. Pharmacol 107, 59–66 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ohta H, Okajima F & Ui M Inhibition by islet-activating protein of a chemotactic peptide-induced early breakdown of inositol phospholipids and Ca2+ mobilization in guinea pig neutrophils. J. Biol. Chem 260, 15771–15780 (1985). [PubMed] [Google Scholar]
- 122.Baldassare JJ, Henderson PA & Fisher GJ Plasma membrane associated phospholipase C from human platelets: synergistic stimulation of phosphatidylinositol 4,5-bisphosphate hydrolysis by thrombin and guanosine 5ʹ-O-(3-thiotriphosphate). Biochemistry 28, 56–60 (1989). [DOI] [PubMed] [Google Scholar]
- 123.Portilla D, Morrissey J & Morrison AR Bradykinin-activated membrane-associated phospholipase C in Madin-Darby canine kidney cells. J. Clin. Invest 81, 1896–1902 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smrcka AV & Sternweis PC Regulation of purified subtypes of phosphatidylinositol specific phospholipase Cβ by G protein α and βγ subunits. J. Biol. Chem 268, 9667–9674 (1993). [PubMed] [Google Scholar]
- 125.Boyer JL, Waldo GL & Harden TK βγ-Subunit activation of G-protein-regulated phospholipase C. J. Biol. Chem 267, 25451–25456 (1992). [PubMed] [Google Scholar]
- 126.Philip F, Kadamur G, Silos R. G. l., Woodson J & Ross EM Synergistic activation of phospholipase C-β3 by Gαq and Gβγ describes a simple two-state coincidence detector. Curr. Biol 20, 1327–1335 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rebres RA et al. Synergistic Ca2+ responses by Gβγ and Gαq-coupled G-protein-coupled receptors require a single PLCβ isoform that is sensitive to both Gβγ and Gαq. J. Biol. Chem 286, 942–951 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stehno-Bittel L, Krapivinsky G, Krapivinsky L, Perez-Terzic C & Clapham DE The G protein βγ subunit transduces the muscarinic receptor signal for Ca2+ release in Xenopus oocytes. J. Biol. Chem 270, 30068–30074 (1995). [DOI] [PubMed] [Google Scholar]
- 129.Maruko T et al. Involvement of the βγ subunits of G proteins in the cAMP response induced by stimulation of the histamine H1 receptor. Naunyn Schmiedebergs Arch. Pharmacol 372, 153–159 (2005). [DOI] [PubMed] [Google Scholar]
- 130.Rensing DT, Uppal S, Blumer KJ & Moeller KD Toward the selective inhibition of G proteins: total synthesis of a simplified YM-254890 analog. Org. Lett 17, 2270–2273 (2015). [DOI] [PubMed] [Google Scholar]
- 131.Xiong XF et al. Total synthesis and structure-activity relationship studies of a series of selective G protein inhibitors. Nat. Chem 8, 1035–1041 (2016).This paper synthesizes a series of compounds related to FR900359 demonstrating total synthesis of these natural products and a possible route to designing new selective inhibitors of Gα subunits.
- 132.Zhang H et al. Structure-activity relationship studies of the cyclic depsipeptide natural product YM-254890, targeting the Gq protein. ChemMedChem 12, 830–834 (2017). [DOI] [PubMed] [Google Scholar]
- 133.Iaccarino G, Smithwick LA, Lefkowitz RJ & Koch WJ Targeting Gβγ signaling in arterial vascular smooth muscle proliferation: a novel strategy to limit restenosis. Proc. Natl Acad. Sci. USA 96, 3945–3950 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Iaccarino G & Koch WJ Transgenic mice targeting the heart unveil G protein-coupled receptor kinases as therapeutic targets. Assay. Drug Dev. Technol 1, 347–355 (2003). [DOI] [PubMed] [Google Scholar]
- 135.Rengo G, Lymperopoulos A, Leosco D & Koch WJ GRK2 as a novel gene therapy target in heart failure. J. Mol. Cell Cardiol 50, 785–792 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bookout AL et al. Targeting Gβγ signaling to inhibit prostate tumor formation and growth. J. Biol. Chem 278, 37569–37573 (2003). [DOI] [PubMed] [Google Scholar]
- 137.Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD & Koch WJ Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat. Med 13, 315 (2007). [DOI] [PubMed] [Google Scholar]
- 138.Siuda ER, Carr R 3rd, Rominger DH & Violin JD Biased μ-opioid receptor ligands: a promising new generation of pain therapeutics. Curr. Opin. Pharmacol 32, 77–84 (2017). [DOI] [PubMed] [Google Scholar]
- 139.Gulati S et al. Targeting G protein-coupled receptor signaling at the G protein level with a selective nanobody inhibitor. Nat. Commun 9, 1996 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lehmann DM, Seneviratne AMPB & Smrcka AV Small molecule disruption of G protein βγ subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol. Pharmacol 73, 410–418 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Neer EJ, Schmidt CJ, Nambudripad R & Smith TF The ancient regulatory-protein family of WD-repeat proteins. Nature 371, 297–300 (1994). [DOI] [PubMed] [Google Scholar]
- 142.Arkin MR & Wells JA Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat. Rev. Drug Discov 3, 301–317 (2004). [DOI] [PubMed] [Google Scholar]
- 143.Bolshan Y et al. Synthesis, optimization, and evaluation of novel small molecules as antagonists of WDR5-MLL interaction. ACS Med. Chem. Lett 4, 353–357 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grebien F et al. Pharmacological targeting of the Wdr5-MLL interaction in C/EBPα N-terminal leukemia. Nat. Chem. Biol 11, 571–578 (2015).This paper describes the development and the structural basis for binding of a therapeutically relevant compound that binds to a WD40 repeat protein to block protein–protein interactions, which suggests that development of novel Gβγ inhibitors that bind with high affinity is possible.
- 145.Schapira M, Tyers M, Torrent M & Arrowsmith CH WD40 repeat domain proteins: a novel target class? Nat. Rev. Drug Discov 16, 773–786 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Seneviratne AM, Burroughs M, Giralt E & Smrcka AV Direct-reversible binding of small molecules to G protein βγ subunits. Biochim. Biophys. Acta 1814, 1210–1218 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tonge PJ Drug–target kinetics in drug discovery. ACS Chem. Neurosci 9, 29–39 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bignante EA et al. APP/Go protein Gβγ-complex signaling mediates Aβ degeneration and cognitive impairment in Alzheimer’s disease models. Neurobiol. Aging 64, 44–57 (2018). [DOI] [PubMed] [Google Scholar]
- 149.Sanz G et al. Gallein, a Gβγ subunit signalling inhibitor, inhibits metastatic spread of tumour cells expressing OR51E2 and exposed to its odorant ligand. BMC Res. Notes 10, 541 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jensen DD et al. Protein kinase D and Gβγ subunits mediate agonist-evoked translocation of protease-activated receptor-2 from the golgi apparatus to the plasma membrane. J. Biol. Chem 291, 11285–11299 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gautam J et al. 4-Hydroxynonenal-induced GPR109A (HCA2 receptor) activation elicits bipolar responses, Gαi -mediated anti-inflammatory effects and Gβγ -mediated cell death. Br. J. Pharmacol 175, 2581–2598 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kajimoto T et al. Involvement of Gβγ subunits of Gi protein coupled with S1P receptor on multivesicular endosomes in F-actin formation and cargo sorting into exosomes. J. Biol. Chem 293, 245–253 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wells CA et al. Gβγ inhibits exocytosis via interaction with critical residues on soluble N-ethylmaleimide-sensitive factor attachment protein-25. Mol. Pharmacol 82, 1136–1149 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stephens LR et al. The Gβγ sensitivity of a PI3K is dependent upon a tightly associated adaptor, 101. Cell 89, 105–114 (1997). [DOI] [PubMed] [Google Scholar]
- 155.Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T & Tesmer JJG Snapshot of activated G proteins at the membrane: the Gαq-GRK2-Gβγ complex. Science 310, 1686–1690 (2005). [DOI] [PubMed] [Google Scholar]
- 156.Whorton MR & MacKinnon R X-Ray structure of the mammalian GIRK2-βγ G-protein complex. Nature 498, 190–197 (2013).These authors solve the X‑ray structure of the Gβγ subunit bound to one of its effector molecules, GIRK. This structure reveals why small molecules that bind to the top of Gβγ do not inhibit GIRK channel regulation.
- 157.Gaudet R, Bohm A & Sigler PB Crystal structure at 2.4 angstroms resolution of the complex of transducin βγ and its regulator, phosducin. Cell 87, 577–588 (1996). [DOI] [PubMed] [Google Scholar]
- 158.Mathews JL, Smrcka AV & Bidlack JMA Novel Gβγ subunit inhibitor selectively modulates μ-opioid-dependent antinociception and attenuates acute morphine-induced antinociceptive tolerance and dependence. J. Neurosci 28, 12183–12189 (2008).This study shows that small-molecule inhibition of Gβγ potentiates opioid analgesia and prevents the development of acute tolerance and dependence, which suggests this as a strategy for improving the safety of opioid analgesics.
- 159.Bourinet E, Soong TW, Stea A & Snutch TP Determinants of the G protein-dependent opioid modulation of neuronal calcium channels. Proc. Natl Acad. Sci. USA 93, 1486–1491 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ikeda SR Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature 380, 255–258 (1996). [DOI] [PubMed] [Google Scholar]
- 161.Kamal FA et al. Simultaneous adrenal and cardiac GPCR-Gβγ inhibition halts heart failure progression. J. Am. Coll. Cardiol 63, 2549–2557 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yoon EJ, Gerachshenko T, Spiegelberg BD, Alford S & Hamm HE Gβγ interferes with Ca2+-dependent binding of synaptotagmin to the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex. Mol. Pharmacol 72, 1210–1219 (2007). [DOI] [PubMed] [Google Scholar]
- 163.Araldi D, Ferrari LF & Levine JD Repeated μ-opioid exposure induces a novel form of the hyperalgesic priming model for transition to chronic pain. J. Neurosci 35, 12502–12517 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bianchi E, Norcini M, Smrcka A & Ghelardini C Supraspinal Gβγ-dependent stimulation of PLCβ originating from G inhibitory protein-μ opioid receptor-coupling is necessary for morphine induced acute hyperalgesia. J. Neurochem 111, 171–180 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Joseph EK, Bogen O, Alessandri-Haber N & Levine JD PLC-β3 signals upstream of PKC ε in acute and chronic inflammatory hyperalgesia. Pain 132, 67–73 (2007). [DOI] [PubMed] [Google Scholar]
- 166.Xie W et al. Genetic alteration of phospholipase Cβ3 expression modulates behavioral and cellular responses to μ opioids. Proc. Natl Acad. Sci. USA 96, 10385–10390 (1999).This study demonstrates that deletion of PLCβ3 in mice potentiates morphine‑dependent opioid analgesia, which suggests a mechanism by which Gβγ inhibitors potentiate opioid analgesia through blockade of Gβγ‑dependent regulation of PLCβ3.
- 167.Park D, Jhon DY, Lee CW, Lee KH & Goo Rhee S Activation of phospholipase C isozymes by G protein βγ subunits. J. Biol. Chem 268, 4573–4576 (1993). [PubMed] [Google Scholar]
- 168.Hoot MR et al. Inhibition of Gβγ-subunit signaling potentiates morphine-induced antinociception but not respiratory depression, constipation, locomotion, and reward. Behav. Pharmacol 24, 144–152 (2013). [DOI] [PubMed] [Google Scholar]
- 169.Xu J et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 114, 201–214 (2003). [DOI] [PubMed] [Google Scholar]
- 170.Surve CR, To JY, Malik S, Kim M & Smrcka AV Dynamic regulation of neutrophil polarity and migration by the heterotrimeric G protein subunits Gαi-GTP and Gβγ. Sci. Signal 9, ra22 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rangel-Moreno J et al. Inhibition of G protein βγ subunit signaling abrogates nephritis in lupus-prone mice. Arthritis Rheumatol. 68, 2244–2256 (2016).This study shows that chronic Gβγ inhibition with gallein prevents the development of lupus, which shows the utility of this approach in chronic inflammatory disease and that chronic administration of gallein is well tolerated in mice.
- 172.Casey LM et al. Small molecule disruption of Gβγ signaling inhibits the progression of heart failure. Circ. Res 107, 532–539 (2010).These authors demonstrate that small‑molecule Gβγ inhibition prevents the development of heart failure.
- 173.Kamal FA et al. G protein-coupled receptor-G-protein βγ-subunit signaling mediates renal dysfunction and fibrosis in heart failure. J. Am. Soc. Nephrol 28, 197–208 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lorenz K, Schmitt JP, Schmitteckert EM & Lohse MJ A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat. Med 15, 75–83 (2009). [DOI] [PubMed] [Google Scholar]
- 175.Zhang L et al. Phospholipase Cε hydrolyzes perinuclear phosphatidylinositol 4-phosphate to regulate cardiac hypertrophy. Cell 153, 216–227 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Malik S et al. G protein βγ subunits regulate cardiomyocyte hypertrophy through a perinuclear Golgi phosphatidylinositol 4-phosphate hydrolysis pathway. Mol. Biol. Cell 26, 1188–1198 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Travers JG et al. Pharmacological and activated fibroblast targeting of Gβγ-GRK2 after myocardial ischemia attenuates heart failure progression. J. Am. Coll. Cardiol 70, 958–971 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wells JA & McClendon CL Reaching for high-hanging fruit in drug discovery at protein–protein interfaces. Nature 450, 1001 (2007). [DOI] [PubMed] [Google Scholar]
- 179.Garcia-Olivares J et al. Gβγ subunit activation promotes dopamine efflux through the dopamine transporter. Mol. Psychiatry 22, 1673–1679 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yost EA, Hynes TR, Hartle CM, Ott BJ & Berlot CH Inhibition of G-protein βγ signaling enhances T cell receptor-stimulated interleukin 2 transcription in CD4+ T helper cells. PLOS One 10, e0116575 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ouellaa-Benslama R et al. Identification of a GαGβγ, AKT and PKCα signalome associated with invasive growth in two genetic models of human breast cancer cell epithelial-to-mesenchymal transition. Int. J. Oncol 41, 189–200 (2012). [DOI] [PubMed] [Google Scholar]
- 182.Meens MJ et al. G-Protein βγ subunits in vasorelaxing and anti-endothelinergic effects of calcitonin gene-related peptide. Br. J. Pharmacol 166, 297–308 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Irannejad R & Wedegaertner PB Regulation of constitutive cargo transport from the trans-Golgi network to plasma membrane by Golgi-localized G protein betagamma subunits. J. Biol. Chem 285, 32393–32404 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kirui JK et al. Gβγ signaling promotes breast cancer cell migration and invasion. J. Pharmacol. Exp. Ther 333, 393–403 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tang X et al. A critical role of Gbetagamma in tumorigenesis and metastasis of breast cancer. J. Biol. Chem 286, 13244–13254 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Paudyal P, Xie Q, Vaddi PK, Henry MD & Chen S Inhibiting G protein βγ signaling blocks prostate cancer progression and enhances the efficacy of paclitaxel. Oncotarget 8, 36067–36081 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Purcell RH, Toro C, Gahl WA & Hall RA Hum. Mutat 38, 1751–1760 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Arensdorf AM et al. Sonic Hedgehog activates phospholipase A2 to enhance smoothened ciliary translocation. Cell Rep 19, 2074–2087 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gont A, Daneshmand M, Woulfe J, Lavictoire SJ & Lorimer IA PREX1 integrates G protein-coupled receptor and phosphoinositide 3-kinase signaling to promote glioblastoma invasion. Oncotarget 8, 8559–8573 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Onken MD et al. Targeting nucleotide exchange to inhibit constitutively active G protein α subunits in cancer cells. Sci. Signal 11, eaao6852 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]