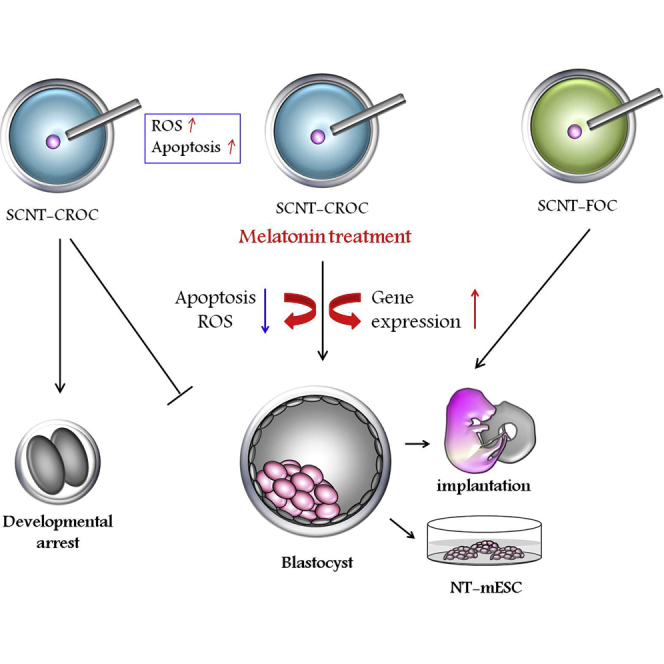
Summary
Cryopreservation has a negative effect on the quality of oocytes and may be closely associated with increased levels of reactive oxygen species (ROS) and apoptotic events. The purpose of the present study was to evaluate the detrimental effects on the developmental competence of somatic cell nuclear transferred (SCNT) mouse embryos using vitrified (cryopreserved) oocytes and to evaluate the recovery effects of melatonin on cryo-damage in cloned embryos. Development of SCNT embryos using cryopreserved oocyte cytoplasm (SCNT-CROC) was inferior to those using fresh cytoplasm (SCNT-FOC). Using RNA-sequencing analysis, we found upregulation of eight pro-apoptotic-related genes (Cyct, Dapk2, Dffb, Gadd45g, Hint2, Mien1, P2rx7, and Pmaip) in the SCNT-CROC group. Furthermore, the addition of melatonin, an agent that reduces apoptosis and ROS production, enhanced blastocyst formation rates in the SCNT-CROP group when compared with the melatonin-untreated group. Additionally, melatonin treatment increased the derivation efficiency of pluripotent stem cells from cloned embryos using cryopreserved oocyte.
Keywords: melatonin, cryopreserved oocytes, somatic cell nuclear transfer, embryonic development, cryo-damage
Graphical Abstract
Highlights
-
•
Cloned mouse embryos using cryopreserved oocytes have shown increased apoptosis
-
•
The addition of melatonin reduces apoptosis and ROS production
-
•
Melatonin enhances development of the SCNT embryos using cryopreserved oocytes
-
•
This system will be helpful in the derivation and application of human SCNT-ESC line
In this article, Lee and colleagues found that the low developmental efficiency of cloned mouse embryos using cryopreserved oocyte cytoplasm may be due to increased apoptosis and altered gene expression resulting from cryoinjury. Therefore, supplementation of melatonin, a scavenger agent, may positively affect the quality of cloned embryos by regulating gene expression and apoptotic processes.
Introduction
It has been suggested that somatic cell nuclear transfer (SCNT)-based reprogramming and subsequent derivation of embryonic stem cells (ESCs) can produce patient-specific stem cells for regenerative medicine (Lanza et al., 1999). Recently, three individual research groups have successfully derived several SCNT-ESC lines using good-quality human oocytes and fibroblast cells from various sources (Chung et al., 2014, Tachibana et al., 2013, Yamada et al., 2014). In addition, many of the developmental blocks in human SCNT embryos have been partially overcome by modulation of histone methylation (Chung et al., 2015) and are now an applicable technology for cell therapy.
Recently, oocyte vitrification has been used as a practical tool in human assisted reproductive technology as well as a popular cryopreservation method. In fact, it was reported that cytogenetic and developmental deficits in offspring born from vitrified (cryopreserved) oocytes were not increased when compared with conventional in vitro fertilization (Practice Committees of American Society for Reproductive Medicine and Society for Assisted Reproductive Technology, 2013). This technique provides a valuable opportunity to preserve fertility for infertile women, to treat fertile women at risk of age-induced fertility decline (Zhang et al., 2016) and cancer therapy-induced threats to fertility (Falcone et al., 2004). In addition, vitrification of surplus human oocytes could provide a steady source of eggs for research, such as SCNT, and its use also reduces ethical concerns (Baek et al., 2017). However, although cryopreserved mouse oocytes can support genomic reprogramming of the somatic cell nucleus to permit full-term development, developmental potential of the SCNT embryos was very poor (Hirata et al., 2011). In particular, production of cloned embryos using cryopreserved human oocytes and derivation of their SCNT-ESC lines was not achieved until recently. Even with a survival rate greater than 90%, clinical outcomes from vitrified oocytes are lower than from fresh oocytes in the human assisted reproductive technology program (Nakagata et al., 2013). This is suggested to be due to cytoskeletal damage (Hotamisligil et al., 1996), altered spindle structure (Joly et al., 1992), microtubules (Van der Elst et al., 1992), cortical granule distribution (Gook et al., 1993, Van Blerkom and Davis, 1994), and zonal hardening of oocytes (Chen et al., 2000, Kazem et al., 1995). Additionally, cryopreserved oocytes are particularly vulnerable to oxidative stress because of their high levels of lipids, and generate large amounts of reactive oxygen species (ROS) that influence the balance between oxidation and reduction reactions and the intracellular anti-oxidative system (Luberda, 2005, Nakamura et al., 2011).
Melatonin is a secretory product of the pineal gland and regulates circadian rhythmicity (Reiter et al., 2003), aging (Tamura et al., 2017), immune function (Calvo et al., 2013), and apoptosis (Wei et al., 2015). It has been increasingly recognized for its anti-oxidant capacity (Manchester et al., 2015, Reiter et al., 2016, Zhang and Zhang, 2014). In the field of reproductive biology, several recent studies have shown that melatonin improves age-induced fertility decline, attenuates ovarian mitochondrial oxidative stress (Song et al., 2016), and promotes oocyte maturation (Tian et al., 2014). Also, melatonin improves oocyte quality and embryonic development in sheep (Abecia et al., 2002), pigs (Shi et al., 2009), bovine species (Papis et al., 2007), mice (Ishizuka et al., 2000), and humans (Arjmand et al., 2016). Moreover, supplementation of melatonin enhanced embryonic development, improving the quality of SCNT blastocysts and reducing the apoptosis rate in porcine (Choi et al., 2008, Nakano et al., 2012, Pang et al., 2013), bovine (Su et al., 2015), and mouse embryos (Salehi et al., 2014).
In the present study, we primarily explored the effect of vitrified oocyte cytoplasm on the outcome of SCNT-mediated reprogramming. By modulation of histone methylation, a developmental block was overcome in cloned embryos derived from both cryopreserved and fresh oocytes; however, the developmental capacity was greater in those from fresh oocytes. This deficit in embryonic development can be partly recovered by addition of melatonin during cultivation in vitro, as shown in cloned embryos derived from fresh oocytes. In fact, supplementation of melatonin may positively affect the quality of cloned embryos by regulating gene expression and apoptotic processes.
Results
Injection of Kdm4a mRNA Enhanced Embryonic Development of Cloned Embryos Using Vitrified/Warmed Oocytes, but Did Not Fully Reach that of Fresh Oocytes
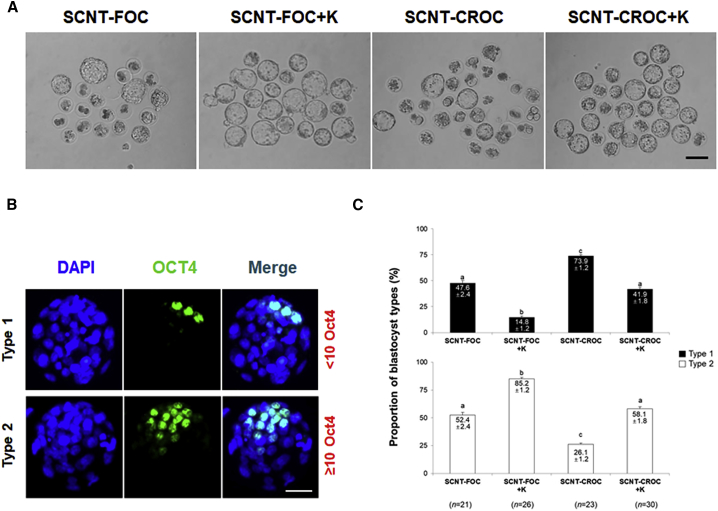
In our previous study, mRNA injection of Kdm4a encoding the H3K9me3 demethylase overcame a developmental block of SCNT mammalian eggs and improved their embryonic development (Chung et al., 2015). To analyze the effect of Kdm4a mRNA on the development of cloned embryos using cytoplasm of vitrified/warmed oocytes, we assessed their embryonic development after injections with or without Kdm4a mRNA. The injection of Kdm4a mRNA removed H3K9me3 activity (Figure S1) and overcame the 2-cell block in the cloned embryos (Table 1). In fact, downregulation of H3K9me3 activity highly improved blastocyst formation rates in both cloned embryos using fresh (SCNT-FOC) and cryopreserved oocyte cytoplasm (SCNT-CROC). Interestingly, although developmental block was nearly overcome after Kdm4a mRNA injection in both groups, embryonic development and the quality of blastocysts from the SCNT-CROC group was still lower than those of the SCNT-FOC group (Figure 1 and Table 1). As shown Figure 1C, the number of cloned embryos showing a high ratio of inner cell mass (ICM) number (more than 10 ICMs per blastocyst) was lower in the SCNT-CROC + K group than in the SCNT-FOC + K group (p < 0.05).
Table 1.
Effect of Kdm4a mRNA Injection on the Developmental Potential of Cloned Embryos from Cryopreserved Mouse Oocytes
| Group | mRNA (μg/μL) | No. of NT Oocytes | No. of 2-Cell Embryos (%)a | No. of 4-Cell Embryos (%)b | No. of 2-Cell Block Embryos (% ± SEM)b | No. of Blastocysts (% ± SEM)b |
|---|---|---|---|---|---|---|
| SCNT-FOC | – | 112 | 105 (94) | 70 (67) | 35 (33 ± 2.9)a | 27 (26 ± 3.4)a |
| SCNT-FOC + K | 2 | 109 | 103 (95) | 102 (99) | 1 (1 ± 1)b | 85 (83 ± 3.5)b |
| SCNT-CROC | – | 139 | 132 (95) | 93 (70) | 39 (30 ± 1.8)a | 30 (23 ± 3.1)a |
| SCNT-CROC + K | 2 | 130 | 125 (96) | 123 (98) | 2 (2 ± 1.2)b | 82 (66 ± 2.4)c |
SCNT-FOC, cloned embryos from somatic cell nuclear transfer using fresh oocyte cytoplasm; SCNT-CROC, cloned embryos from somatic cell nuclear transfer using cryopreserved (vitrified/warmed) oocyte cytoplasm; K, injection of lysine (K)-specific demethylase 4A (Kdm4a) mRNA. Within the same column, values with different superscript letters (a, b, c) are significantly different (p < 0.05; n = 5).
Based on the number of SCNT oocytes.
Based on the number of 2-cell embryos.
Figure 1.
Injection of Kdm4a mRNA Improved the Embryonic Development of SCNT Embryos Using Fresh Oocyte Cytoplasm and Cryopreserved Oocyte Cytoplasm
(A) Blastocyst formation in SCNT embryos using fresh oocyte cytoplasm (SCNT-FOC) and cryopreserved oocyte cytoplasm (SCNT-CROC) groups with and without Kdm4a mRNA injection (K). Scale bar, 20 μm.
(B) Expression of OCT4 and DAPI staining of blastocysts derived from SCNT-FOC and SCNT-CROC groups with or without Kdm4a mRNA injection (K). Scale bar, 20 μm.
(C) The proportion of SCNT-derived blastocysts based on the numbers of inner cell mass (ICM) and expressed immunoreactivity for OCT4. Type 1, the types of blastocysts containing more than 10 ICM cells; type II, the types of blastocysts containing fewer than 10 ICM cells. The results in the bars are presented as means ± SEM. The different letters on the bars indicate significantly different values (p < 0.05).
Analysis of Transcriptional Differences in Cloned 2-Cell Embryos between SCNT-FOC and SCNT-CROC Groups
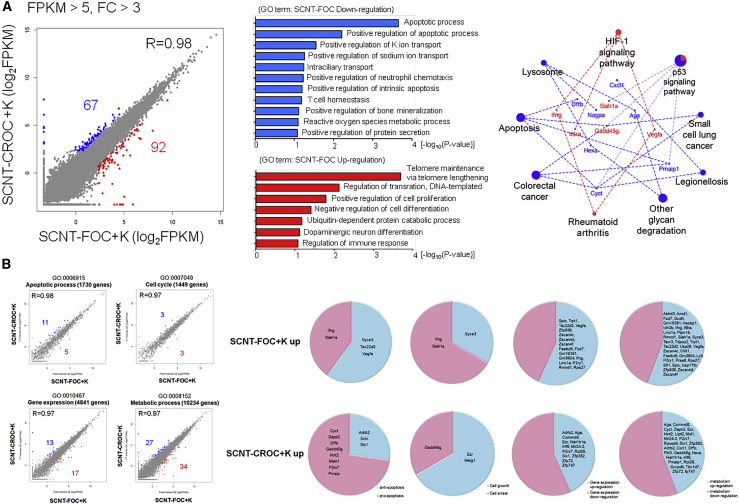
To identify the earliest embryonic transcriptional differences between SCNT-FOC and SCNT-CROC groups, we performed RNA-sequencing (RNA-seq) experiments with pooled 2-cell embryos (100 embryos per sample, repeated three times) of both groups 24 h after oocyte activation and Kdm4a mRNA injection. The expression of most genes was similar in both groups, and only a small number of genes (159 genes) were shown to have different expression patterns (Figure 2A). From gene ontology (GO) terms and KEGG analysis, we found that several pathways, including apoptosis and p53 signaling pathways, were markedly activated. When the differentially expressed genes (DEGs) in the GO-term database were analyzed, out of 1,730 apoptosis-related genes only 16 genes (0.92%) showed greater than a 3-fold difference between SCNT-FOC and SCNT-CROC groups. Interestingly, in the SCNT-CROC group, eight (50%) pro-apoptosis-related genes (Cyct, Dapk2, Dffb, Gadd45g, Hint2, Mien1, P2rx7, and Pmaip) and three (27.2%) anti-apoptotic genes (Adrb2, Scin, and Six1) were upregulated when compared with the SCNT-FOC group. Also, only two (18.2%) pro-apoptosis-related genes (Ifng and Siah1a) and three (27.2%) anti-apoptotic genes (Syce3, Tsc22d3, and Vegfa) were downregulated (Figure 2B).
Figure 2.
Analysis of Gene Expressions Between SCNT-CROC + K and SCNT-FOC + K Groups
(A) (Left) Scatterplot showing upregulated (red) and downregulated (blue) genes in SCNT-FOC. FPKM > 5 and FC > 3 is used as a cutoff value. (Center) Bar graph showing GO terms with upregulated (red) or downregulated (blue) genes in SCNT-FOC. (Right) KEGG pathway analysis using the DEGs. Red and blue color in pie charts represent ratio of genes repressed (blue) and enhanced (red) in SCNT-FOC.
(B) DEGs are compared with each GO dataset and then classified into four groups by apoptotic process, cell cycle, gene expression, and metabolic process.
In addition, only six cell-cycle-related genes (0.41% out of 1,449 genes) had different expression levels and only one cell arrest-related gene (Gadd45g) was upregulated in the SCNT-CROC group (Figure 2B).
On the other hand, 30 gene expression-related genes (0.62% out of 4,841 genes) and 61 metabolic process-related genes (0.60% out of 10,234 genes) showed different expression between SCNT-FOC and SCNT-CROC groups. However, most of them have no negative function on gene expression or metabolic process (Figure 2B).
Melatonin Enhances Embryonic Development of Cloned Embryos Using Vitrified/Warmed Oocytes
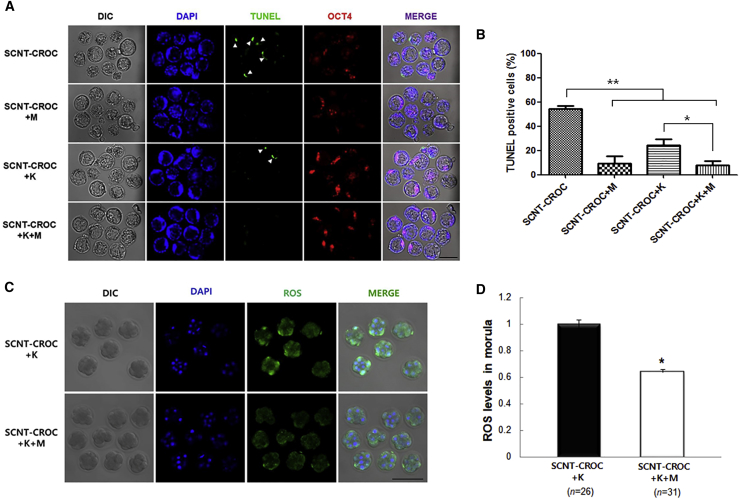
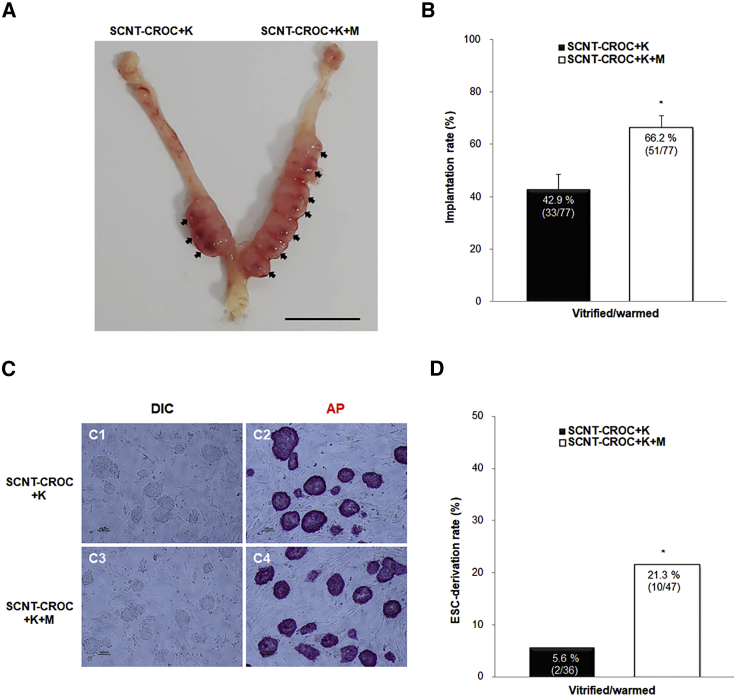
Due to the low rate of embryonic development up to the blastocyst stage and different expressions of apoptotic genes in the SCNT-CROC group, we applied 10 μM melatonin to the culture system of cloned embryos to improve their quality. The concentration of melatonin resulting in the best quality and a high blastocyst formation rate was selected from a preliminary experiment using conventionally fertilized mouse embryos (data not shown). As shown in several studies (Gao et al., 2012, Liang et al., 2017, Zhao et al., 2016), supplementation of melatonin during cultivation reduced ROS levels and the number of TUNEL-positive cells in blastocysts from the SCNT-CROC group (Figure 3). The overall percentage of TUNEL-positive embryos were significantly decreased in the melatonin-supplemented group (SCNT-CROC + K + M) compared with those of the non-supplemented group (SCNT-CROC and SCNT-CROC + K) (7.8% versus 54.3% and 24.1%, p < 0.05; Figures 3A and 3B). In addition, melatonin has a positive role on the reduction of ROS levels and in the embryonic development of the SCNT-CROC group, regardless of Kdm4a mRNA injection (Table 2). To compare the embryonic quality of cloned embryos with or without supplementation of melatonin, we analyzed the implantation rate after embryo transfer and the derivation rate of mouse ESCs (mESCs). The number of implantation rates in the melatonin-supplemented group (SCNT-CROC + K + M) was significantly increased compared with those of the non-supplemented group (SCNT-CROC + K) (66.2% [51/77] versus 42.9% [33/77], p < 0.05; Figures 4A and S2). Also, we obtained a higher derivation rate of mESCs in the melatonin-supplemented group compared with those in the non-supplemented group (21.3% [10/47] versus 5.6% [2/36], p < 0.05; Figure 4B).
Figure 3.
Effects of Kdm4a mRNA Injection (K) and/or Melatonin Treatment (M, 10 μM) on Apoptosis and ROS Levels in SCNT Embryos Using Cryopreserved Oocyte Cytoplasm
(A) Immunostaining of cloned blastocysts in the SCNT-CROC group after various treatments. The nuclei (blue) are stained with DAPI, and the TUNEL-positive apoptotic nuclei (green) are indicated by arrows. OCT4 staining (red) is indicated ICM. Scale bar, 100 μm.
(B) TUNEL-positive cells indicate each embryo with apoptotic cells. ∗∗p < 0.05, significantly different from SCNT-CROC group; ∗p < 0.05, significantly different from SCNT-CROC + K group (n = 4).
(C) ROS staining (green) of cloned morula in the SCNT-CROC group after various treatments. Scale bar, 100 μm.
(D) Quantification of ROS fluorescence intensity of cloned morula in the SCNT-CROC group after various treatments. The experiments were performed four times. In each replicate, n = 6–9 per group. The total number of embryos was 26 and 31 in the SCNT-CROC + K and SCNT-CROC + M + K groups, respectively. Asterisk indicates significantly different value (p < 0.05).
Table 2.
Effect of Kdm4a mRNA Injection and Melatonin Supplement on the Developmental Potential in Cloned Embryos of Cryopreserved Mouse Oocytes
| Group | Melatonin (μM) | No. of NT Oocytes | No. of 2-Cell Embryos (%)a | No. of 4-Cell Embryos (%)b | No. of 2-Cell Block Embryos (% ± SEM)b | No. of Blastocysts (% ± SEM)b |
|---|---|---|---|---|---|---|
| SCNT-CROC | – | 125 | 112 (90) | 66 (59) | 46 (42 ± 3.7)a | 23 (20 ± 1.5)a |
| SCNT-CROC + M | 10 | 125 | 114 (91) | 81 (71) | 33 (31 ± 3.7)a | 44 (39 ± 1.2)b |
| SCNT-CROC + K | – | 138 | 128 (98) | 124 (94) | 4 (3 ± 1.5)a | 76 (59 ± 3.3)a |
| SCNT-CROC + K + M | 10 | 138 | 123 (96) | 119 (96) | 4 (4 ± 1.8)a | 91 (75 ± 3.3)b |
SCNT-CROC, cloned embryos from somatic cell nuclear transfer using cryopreserved (vitrified/warmed) oocyte cytoplasm; M, treatment of melatonin; K, injection of lysine (K)-specific demethylase 4A (Kdm4a) mRNA (2 μg/μL). Within the same column, values with different superscript letters (a, b) are significantly different (p < 0.05; n = 5).
Based on the number of SCNT oocytes.
Based on the number of 2-cell embryos.
Figure 4.
Effects of Kdm4a mRNA Injection (K) and/or Melatonin Treatment (M, 10 μM) on Implantation and SCNT-ESC Derivation from SCNT Embryos Using Cryopreserved Oocyte Cytoplasm
(A) Photograph of representative uterus at day 7. The blastocysts from the SCNT-CROC + K were transferred into the left horn only and the blastocysts from the SCNT-CROC + K + M into the right horn. Embryo transfer was performed 11 times (see Figure S2).
(B) Comparison of implantation rate between blastocysts from the SCNT-CROC + K and SCNT-CROC + K + M groups.
(C) Photograph of ESCs from cloned embryos. C1 (bright-field picture) and C2 (alkaline phosphatase staining) represent colonies from melatonin-non-treated (SCNT-CROC + K) group. C3 (bright-field picture) and C4 (alkaline phosphatase staining) represent colonies from melatonin-treated (SCNT-CROC + K + M) group.
(D) Efficiency of SCNT-ESC derivation. The efficiency of SCNT-ESC derivation was analyzed based on the total number of blastocysts placed on mitotic inactivated MEF feeder cells. ESC derivation was performed three times.
Effect of Melatonin Treatment on Transcription in Cloned Blastocysts from the SCNT-CROC Group
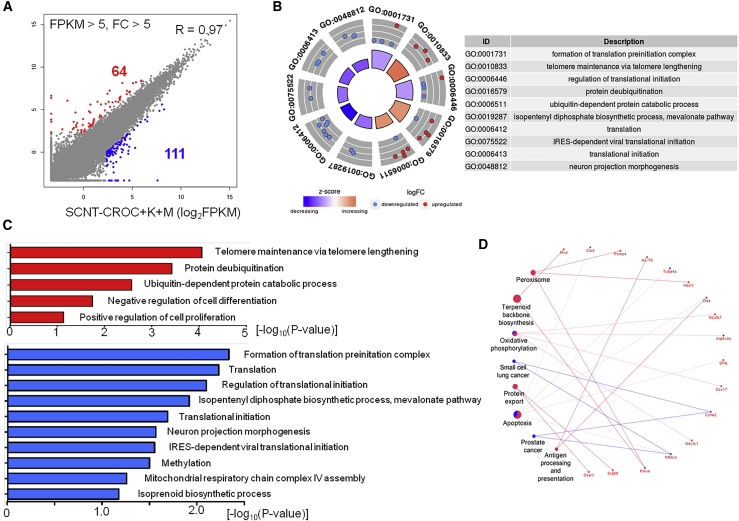
To find which genes are regulated by melatonin, we analyzed transcripts from 2-cell embryos and blastocysts (50 embryos or blastocysts per sample, repeated two times) in both melatonin-treated and -non-treated groups. In comparison of 2-cell embryos, 175 genes were considered differentially expressed at a fold change (FC) > 5 and fragments per kilobase per million reads (FPKM) > 5 (Figure 5 and Table S1). In the melatonin-treated (SCNT-CROC + K + M) group, 111 genes were upregulated compared with the melatonin-non-treated (SCNT-CROC + K) group. GO-term and KEGG pathway analysis revealed that the addition of melatonin ameliorates cell death-related pathways such as apoptosis, peroxisome, and oxidative phosphorylation (Figure 5). Notably, Deaf1, Fxn, Ppan, Rab10os, Sprr2d, Stag3, Tsen15, and Zfp335, which are involved in cell survival and tissue regeneration, were upregulated in 2-cell embryos from the SCNT-CROC + K + M group. In addition, Ctss, Dffa, Eif3f, Hacl1, Hspbp1, Mob2, Mrpl23, Mrpl33, Pmvk, Slc4a11, and Wbscr22, which are involved in anti-oxidant function, inflammation, and apoptosis, were also highly upregulated. In contrast, the expression of cell death and degeneration-related genes (Atp6v0c, Cd52, Dapk2, Ddit4l, Duoxa2, Nfkbia, Ptgds, Rdh12, and Rnd3) were downregulated in 2-cell embryos from the SCNT-CROC + K + M group (Table S1).
Figure 5.
Melatonin Confers a Beneficial Effect on Cell Survival to SCNT-CROC
(A) Scatterplot showing alteration of gene expression by melatonin treatment. Red and blue dots represent genes repressed and enhanced by melatonin, respectively. FPKM > 5 and FC > 5 is used as cutoff value.
(B) GO-term analysis using both up- and downregulated genes in melatonin-treated SCNT-CROCs. Each red and blue dot in the GO terms represents a gene repressed and enhanced by melatonin, respectively.
(C) Bar graph showing GO terms with up- or downregulated genes in SCNT-CROC + K + M group. Red bars, analysis with downregulated genes; blue bars, analysis with upregulated genes.
(D) KEGG pathway analysis using all DEGs. Red and blue color in pie charts represent ratio of genes repressed (red) and enhanced (blue) by melatonin.
As shown in the Table S2, in comparison of blastocysts, 81 genes were considered differentially expressed at a FC > 2 and FPKM > 5. In the melatonin-treated (SCNT-CROC + K + M) group, 20 genes were upregulated compared with the melatonin-non-treated (SCNT-CROC + K) group. Notably, Amd1, Fam46C, Oxt, and Ppt2, which are involved in cell survival and tissue regeneration, were upregulated in blastocysts from the SCNT-CROC + K + M group. In addition, Gulo and Txnip, which are involved in anti-oxidant function, inflammation, and apoptosis, were also highly upregulated.
In contrast, the expression of many oxidative stress-related genes (Adh1, Car2, Gsta3, Gstm2, Mb, Phlda2, and S100a1) and cell death and degeneration-related genes (Akr1c13, Amd2, Clu, Hist1h3a, Hspb1, Il1rn, and Nat8) were downregulated in blastocysts from the SCNT-CROC + K + M group. Also, many cell and tumor proliferation-related genes (Efemp1, Glipr1, Lgals4, Plac1, Snora15, Snora 21, Snora 34, and Anxa1) were shown to have decreased expression levels. Moreover, some immune response-related genes (Clec2f, Hrsp12, and Plat) were also shown to be downregulated in the melatonin-treated group (Table S2).
Discussion
During the last decade, three types of pluripotent stem cells (PSCs), including SCNT-ESCs, induced PSCs (iPSCs), and multipotent germline stem cells, have been established using mouse and human cells, and these technologies may provide a prominent approach for the treatment of patients with incurable diseases as well as for improving the quality of life for the aging population (Chung et al., 2014, Kanatsu-Shinohara et al., 2004, Tachibana et al., 2013, Takahashi et al., 2007). In addition, the establishment of PSC banks with various homozygous HLA types may accelerate the clinical application of stem cell therapy and the development of stem cell businesses (Lee et al., 2016, Taylor et al., 2012). Recently, several reports have suggested that the merits of SCNT-ESCs include improved genetic stability and non-transmission of mitochondrial disease compared with iPSCs (Kang et al., 2016, Ma et al., 2014). Beyond these promising applications, SCNT-ESC technologies may help solve the supply problem of human donated oocytes required for the establishment of PSCs. In the present study, we analyzed the developmental capacity of cloned embryos using vitrified oocytes in order to secure a supply of oocytes. The embryonic development of cloned mouse embryos using vitrified oocyte cytoplasm (SCNT-CROC) was decreased and the development was not fully recovered by recent SCNT technology. By RNA-seq analysis, we have evaluated that different expression in apoptosis- and cell-cycle-related genes were found in embryos from the SCNT-CROC group. Addition of melatonin, which has various positive anti-apoptotic and anti-oxidative stress effects during cultivation, was improved in the pre- and post-implantation development of cloned mouse embryos. Moreover, the expression of anti-apoptotic- and cell-survival-related genes was significantly upregulated in the melatonin-treated cloned embryos when compared with the non-treated group.
A high incidence of developmental block has been observed in cloned mammalian embryos and is a major reason for the low efficiency of SCNT technology (Yang et al., 2007). Several recent reports have suggested that persistence of specific histone methylation interrupts cellular reprogramming of donor nuclei and that addition of histone demethylase enhanced the cloning efficiency in mouse and human SCNT procedures (Chung et al., 2015, Matoba et al., 2014, Matoba and Zhang, 2018). In addition, it has been generally accepted that the vitrification procedure can maintain the developmental capacity of mammalian oocytes after cryopreservation and contribute to the preservation of female fertility (Practice Committees of American Society for Reproductive Medicine and Society for Assisted Reproductive Technology, 2013). However, some effects of cryo-injury still must be overcome in conventional and cloned embryos, even if various technologies are applied (Baek et al., 2017, Yang et al., 2016). To test whether increased histone demethylation has an effect on the recovery of diminished reprogramming potential mediated by cryo-injury, we injected Kdm4a mRNAs into cloned embryos. Similar to our previous reports, Kdm4a mRNAs overcame the developmental block in embryos from the SCNT-FOC and SCNT-CROC groups (Table 1). It is well known that Oct4 is a specific gene marker for the ICM at the expanded blastocyst stage (Nichols et al., 1998, Strumpf et al., 2005). In the present study, to assess the quality of blastocysts, we evaluated ICM and total cell numbers (Kishigami et al., 2006). Although Kdm4a mRNA was injected, the number of ICM in the SCNT-FOC group (using fresh oocyte cytoplasm) was significantly higher than those in the SCNT-CROC group (Figure 1C). Also, a significant difference in developmental potential still remained in both SCNT-FOC and SCNT-CROC groups (Table 1), which suggests that cryo-injury is not fully overcome by this procedure.
We performed RNA-seq analysis to identify differential gene expression related to difference in embryonic development between the SCNT-FOC and SCNT-CROC groups. As shown in Figure 2, gene expression in both groups was similar except for a small number of genes. Of particular note were eight pro-apoptosis-related genes (Cyct, Dapk2, Dffb, Gadd45g, Hint2, Mien1, P2rx7, and Pmaip) and one cell-cycle arrest gene (Gadd45g) that were upregulated in the SCNT-CROC group (Figure 2). On the other hand, among the 30 gene expression-related genes (0.62% of 4,841 genes) and 61 metabolic process-related genes (0.60% of 10,234 genes) showing different expression in both SCNT-FOC and SCNT-CROC groups, we did not find any gene that downregulated their related functions. Based on these results, we hypothesized that cryo-injury during vitrification may upregulate apoptosis and downregulate embryonic development. Melatonin, well known as an anti-apoptotic and scavenging agent in mammalian embryology (Reiter et al., 2016, Salehi et al., 2014), was able to improve the developmental competence of cloned embryos in the SCNT-CROC group.
Supplementation of melatonin into culture medium increased embryonic development regardless of Kdm4a mRNA injection but showed no effect on overcoming the developmental block (Table 2). Also, melatonin supplementation decreased apoptosis in cloned mouse embryos (Figure 3). GO-term and KEGG pathway analysis revealed that the addition of melatonin results in altered expression of genes related to terpenoid backbone biosynthesis, apoptosis, peroxisome, and oxidative phosphorylation, among others. The data suggest that melatonin may confer a beneficial effect on cell survival to SCNT-CROC. Therefore, combining melatonin supplementation and Kdm4a mRNA injection can provide a synergistic effect on the embryonic development of cloned embryos in the SCNT-CROC group. In addition, we analyzed implantation and the ESC derivation of cloned embryos from the SCNT-CROC group after melatonin supplementation. As shown in Figures 4A and S2, the number of implantation sites at embryonic day 7.5 was significantly increased in the melatonin-treated group. We also found that the derivation efficiency of SCNT-ESCs from cloned embryos using cryopreserved oocytes was improved after melatonin supplementation (Figure 4B). More interestingly, we found that the expression of some immune response-related genes (Clec2f, Hrsp12, and Plat) was downregulated following melatonin treatment in in vitro culture, and this downregulation may be related to the increased implantation rate (Table S1).
In the present study, we have found that the low developmental efficiency of cloned mouse embryos using cryopreserved oocytes may be due to increased apoptosis and altered gene expression resulting from cryo-injury. Therefore, this decreased developmental competence should be alleviated by the addition of a scavenger agent such as melatonin. This system will also be helpful in the derivation and application of human SCNT-ESC lines by providing steady sources of oocytes, and may reduce the ethical concern related to oocyte donation.
Experimental Procedures
Animals
Eight- to 10-week-old female B6D2F1 mice (Orient-Bio, Gyunggi-do, Korea) were used for the collection of the recipient oocytes and as SCNT donors. Eight- to 10-week-old female ICR mice were used as the poster mothers of embryo transfer. To induce pseudopregnancy, these mice were mated with vasectomized male mice of the same strain. The protocols for the use of animals in these studies were approved by the Institutional Animal Care and Use Committee (IACUC) of CHA University (Project no. IACUC-170119) and all experiments were carried out in accordance with the approved protocols.
Oocyte Collection
Mice were superovulated by injecting them with 5 IU of pregnant mare serum gonadotropin (Sigma-Aldrich, St. Louis, MO), followed by 5 IU of human chorionic gonadotropin (hCG; Sigma-Aldrich) 48 h later. Oocytes were collected in M2 (Sigma-Aldrich) medium at 14 h after hCG injection, and cumulus cells were denuded with M2 medium containing 0.1% hyaluronidase (Sigma-Aldrich). The cumulus-free oocytes were then cultured in potassium simplex optimized medium (KSOM; Millipore, Darmstadt, Germany) for the experiment. Dispersed cumulus cells were removed by hyaluronidase treatment, diluted in M2 medium, and collected. The pellet was then resuspended in a small volume of 3% (v/v) polyvinylpyrrolidone (PVP) in M2 and kept at 4°C until use.
Oocyte Vitrification and Warming
Quinn's Advantage Medium with HEPES (Sage, Malov, Denmark) with 20% (v/v) Knockout Serum Replacement (KSR; Gibco, Grand Island, NY) was used as the base medium for preparation of all vitrification and warming solutions. Two cryoprotectant agents, a combination of ethylene glycol (EG; Sigma-Aldrich) and dimethylsulfoxide (DMSO; Sigma-Aldrich) were used for the vitrification procedure. MII oocytes were pre-equilibrated with HEPES medium containing 7.5% EG and 7.5% DMSO for 2 min 30 s. Pre-equilibrated oocytes were then placed and equilibrated in the same volume of HEPES medium supplemented with 15% EG, 15% DMSO, and 0.5 M sucrose (Sigma-Aldrich) for 20 s. Equilibrated oocytes were loaded onto electron microscopic (EM) copper grids (EM Grid; PELCO, Redding, CA) and plugged into slush nitrogen (SN2) using Vit-master (IMT; Ness Ziona, Israel). Vitrified oocytes were stored in an LN2 tank. For warming, vitrified oocytes were warmed by a four-step method. The EM grids were sequentially transferred to 0.5, 0.25, 0.125, and 0 M sucrose with an interval of 2 min 30 s at 37°C. The oocytes were washed three times with fresh modified HTF (Millipore) medium and cultured in HTF medium until the start of the experiments (Cha et al., 2011).
Preparation of Kdm4a mRNA
In vitro transcription was performed as described previously (Chung et al., 2015). In brief, full-length mouse Kdm4a/Jhdm3a cDNA was cloned into a pcDNA3.1 plasmid containing poly(A)83 at the 3′ end of the cloning site by using an In-Fusion Kit (Clonetech #638909). Messenger RNA was synthesized from the linearized template plasmids by in vitro transcription using a mMESSAGE mMACHINE T7 Ultra Kit (Life Technologies #AM1345). The synthesized mRNA was dissolved in nuclease-free water. The concentration of mRNA was measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies); aliquots of mRNA were stored at −80°C until use.
SCNT and Embryonic Development
After incubation at 37°C, the cumulus of fresh or vitrified/warmed oocytes was denuded and fresh cumulus cells were also collected as nuclear donors. The fresh and vitrified/warmed oocytes were enucleated in M2 medium containing 5 μg/mL cytochalasin B. For nuclear transfer, cumulus cells were injected into enucleated oocytes in M2 medium using a piezo-driven micromanipulator (Primetech, Tsuchiura-shi, Japan). After nuclear transfer, the reconstructed embryos were activated for 6 h by 10 mM SrCl2, 2 mM EGTA, and 5 μg/mL cytochalasin B in M16 (Millipore) medium and then cultured in KSOM in a humidified atmosphere of 5% CO2 at 37°C. The group of cloned embryos from SCNT using fresh oocyte cytoplasm (FOC) was referred as SCNT-FOC, and the group using vitrified/warmed (cryopreserved/thawed) oocyte cytoplasm was referred to as SCNT-CROC. In the first-round experiment, cloned embryos of both groups were injected with ∼10 pL of water (control) or 2 μg/μL Kdm4a mRNA using a piezo-driven micromanipulator. In the second-round experiment, cloned embryos from the SCNT-CROC group were first injected with Kdm4a (SCNT-CROC + K) and then cultured in KSOM medium with or without 10 μM melatonin (Sigma). The concentration of melatonin was chosen from our previous report (Kim et al., 2013) and comparison test (data not shown). The embryonic development of cloned embryos was assessed for 5 days (120 h) after activation.
Immunofluorescence
Cloned embryos were washed in PBS containing 0.1% polyvinyl alcohol (PVA) and then fixed in 4% (w/v) paraformaldehyde at room temperature for 30 min. The oocytes were then washed in PBS/PVA and incubated overnight in PBS containing 1% BSA and 0.1% Triton X-100 at 4°C. Following this, the oocytes were washed three times in PBS-0.1% BSA and incubated with a 1:200 dilution of H3K9me3 antibody (07–442, Millipore) at the 1- and 2-cell stages and purified mouse anti-OCT-3/4 (611203, BD Bioscience) at the blastocyst stage for 2 h at room temperature. The cloned embryos were then washed three times in PBS-0.1% BSA and incubated with a 1:200 dilution of goat anti-mouse antibody (ab150113, Abcam) for 1 h at room temperature. Following this, they were washed in PBS-0.1% BSA three additional times. The DNA was visualized by staining the oocytes with 4′,6-diamidino-2-phenylindole (DAPI; D5942, Sigma-Aldrich). The embryos were mounted on glass slides with a drop of fluorescent mounting medium and then observed using fluorescence confocal microscopy (Zeiss LSM880; Zeiss, Jena, Germany).
Detection of DNA Fragmentation by TUNEL
DNA fragmentation was detected using TUNEL staining (in situ Cell Death Detection Kit, Roche, Indianapolis, IN). According to the instruction manual, SCNT-derived blastocysts were washed three times in 0.1% PVA in Dulbecco’s PBS. The embryos were then incubated in TUNEL reaction medium at 37°C in the dark for 1 h. DNA staining with 1 μg/mL Hoechst 33342 (bis-benzimide, Sigma) was used for nuclear counterstaining. Signals in embryos were observed with a confocal microscope (Zeiss LSM880).
Analysis of ROS Level
In the SCNT-CROC + K group, embryos at the morula or blastocyst stages were treated with or without melatonin and incubated in culture medium containing 5 μM CellROX Oxidative Stress Reagents (Molecular Probes, Eugene, OR) for 30 min, then washed two times with 0.1% PVA-D-PBS. Embryos were examined under a fluorescence confocal microscope (Zeiss LSM880). The recorded fluorescence intensities were analyzed using ImageJ software. The fluorescence pixel value of the embryos was measured within a constant area from different embryos' cytoplasmic regions. Background fluorescent values were subtracted from the final values before analysis of statistically significant differences between the groups. The experiment was replicated three times with 5–10 embryos in each replicate.
RNA-Seq Analysis of Cloned Embryos and Blastocysts
We performed two rounds of RNA-seq analysis. In the first round, gene expression was assessed in 2-cell embryos between SCNT-FOC + K and SCNT-CROC + K (100 embryos per sample, repeated three times). In the second round, gene expression was assessed in 2-cell embryos and blastocysts between SCNT-CROC + K and SCNT-CROC + K + M (50 2-cell embryos and 50 blastocysts per sample, repeated two times). Complementary DNAs (cDNAs) were amplified using a SMARTer Ultra Low Input RNA cDNA preparation kit (Takara, 634890) according to the manufacturer's instructions. The cDNAs were then fragmented into approximately 200-bp fragments using an M220 sonicator (Covaris). The fragmented cDNAs were end-repaired and adapter-ligated. The sequencing libraries were prepared using a ScriptSeq v2 kit (Illumina) according to the manufacturer's instructions. Single-end sequencing was performed on a HiSeq2500 (Illumina), and reads were mapped to the mm9 mouse genome using STAR (v2.5.2b, https://github.com/alexdobin/STAR). After mapping, FPKM was calculated by Cufflinks (v2.2.1) using a default option. Genes were considered differentially expressed at FC >2–5 and FPKM > 5. KEGG pathway analysis was visualized by ClueGO package. Gene set enrichment analysis was also used to identify biological function of the DEGs. An R (v3.3.2) package was used for statistical analyses and scatterplot generation.
Embryo Transfer and Implantation Monitoring
Reconstructed embryos cultured to the blastocyst stage under melatonin treatment or non-treatment conditions (SCNT-CROC + K and SCNT-CROC + K + M groups) were transferred into the uteri of 2.5-day pseudopregnant female ICR mice that had been mated with vasectomized male ICR mice. Each groups are transplanted into the left (SCNT-CROC + K) and right (SCNT-CROC + K + M) uterus in the same mouse. Females were subsequently checked for implantation rate at 7.5 days post coitus. For visualization of implantation, mice were euthanized, the intact uterus was excised into normal saline, adhering fat was dissected away, and the tissue was photographed.
Derivation of Mouse Embryonic Stem Cells from SCNT Blastocysts
Hatched blastocysts obtained from both groups (SCNT-CROC + K and SCNT-CROC + K + M) were placed on mitotic inactivated mouse embryonic fibroblast (MEF) feeder cells in mESC culture medium to form outgrowths. We used DMEM/F12 containing 20% KSR, 0.1 mM β-mercaptoethanol, 1% non-essential amino acids, 100 units/mL penicillin, 100 μg/mL streptomycin (all products from Gibco/Invitrogen, Grand Island, NY), and 1.5 × 103 units/mL recombinant mouse leukemia inhibitory factor (Chemicon, Temecula, CA) for mESC culture medium. Outgrowths were transferred onto new MEF feeder cells, mechanically at first, then passaged using trypsin-EDTA. All of the established mESC lines were monitored and then characterized by morphology and alkaline phosphatase staining. Alkaline phosphatase activity was assessed by histochemical staining. Colonies were fixed in 4% paraformaldehyde at room temperature for 1 min, washed twice with PBS, and stained with an alkaline phosphatase substrate solution (10 mL of FRV-alkaline solution, 10 mL of naphthol AS-BI alkaline solution; alkaline phosphatase kit, Sigma-Aldrich) for 30 min at room temperature. Alkaline phosphatase activity was detected colorimetrically by light microscopy.
Statistical Analysis
All the experiments were repeated at least three times. The results are presented as mean or mean ± SEM. Embryonic developments were analyzed by one-way ANOVA by Duncan's test using SAS software and implantation, and ESC-derivation rates were analyzed by the Chi-square test. p < 0.05 was regarded as statically significant.
Author Contributions
A.R.L. and K.H. designed and performed the experiments, analyzed data, and wrote the manuscript. S.H.C., C.P., J.K.P., J.I.L., J.I.B., D.-W.S., and J.E.L. performed the experiments and analyzed data. D.R.L. designed and directed the experiments, analyzed and assembled data, and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This research was supported partly by the Bio & Medical Technology Development Program of the National Research Foundation and funded by the Korean government MSIT (2015M3A9C6028961, 2017M3A9C6061284, and 2017M3A9C8029318). All authors state that they have no conflicts of interest to disclose.
Published: February 21, 2019
Footnotes
Supplemental Information includes two figures and two tables and can be found with this article online at https://doi.org/10.1016/j.stemcr.2019.01.019.
Accession Numbers
The GEO accession number for the RNA-seq data reported in this paper is GEO: GSE125389.
Supplemental Information
References
- Abecia J.A., Forcada F., Zuniga O. The effect of melatonin on the secretion of progesterone in sheep and on the development of ovine embryos in vitro. Vet. Res. Commun. 2002;26:151–158. doi: 10.1023/a:1014099719034. [DOI] [PubMed] [Google Scholar]
- Arjmand F., Khanmohammadi M., Arasteh S., Mohammadzadeh A., Kazemnejad S., Akhondi M.M. Extended culture of encapsulated human blastocysts in alginate hydrogel containing decidualized endometrial stromal cells in the presence of melatonin. Mol. Biotechnol. 2016;58:684–694. doi: 10.1007/s12033-016-9968-4. [DOI] [PubMed] [Google Scholar]
- Baek J.I., Seol D.W., Lee A.R., Lee W.S., Yoon S.Y., Lee D.R. Maintained MPF level after oocyte vitrification improves embryonic development after IVF, but not after somatic cell nuclear transfer. Mol. Cells. 2017;40:871–879. doi: 10.14348/molcells.2017.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo J.R., Gonzalez-Yanes C., Maldonado M.D. The role of melatonin in the cells of the innate immunity: a review. J. Pineal Res. 2013;55:103–120. doi: 10.1111/jpi.12075. [DOI] [PubMed] [Google Scholar]
- Cha S.K., Kim B.Y., Kim M.K., Kim Y.S., Lee W.S., Yoon T.K., Lee D.R. Effects of various combinations of cryoprotectants and cooling speed on the survival and further development of mouse oocytes after vitrification. Clin. Exp. Reprod. Med. 2011;38:24–30. doi: 10.5653/cerm.2011.38.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.U., Lien Y.R., Chen H.F., Chao K.H., Ho H.N., Yang Y.S. Open pulled straws for vitrification of mature mouse oocytes preserve patterns of meiotic spindles and chromosomes better than conventional straws. Hum. Reprod. 2000;15:2598–2603. doi: 10.1093/humrep/15.12.2598. [DOI] [PubMed] [Google Scholar]
- Choi J., Park S.M., Lee E., Kim J.H., Jeong Y.I., Lee J.Y., Park S.W., Kim H.S., Hossein M.S., Jeong Y.W. Anti-apoptotic effect of melatonin on preimplantation development of porcine parthenogenetic embryos. Mol. Reprod. Dev. 2008;75:1127–1135. doi: 10.1002/mrd.20861. [DOI] [PubMed] [Google Scholar]
- Chung Y.G., Eum J.H., Lee J.E., Shim S.H., Sepilian V., Hong S.W., Lee Y., Treff N.R., Choi Y.H., Kimbrel E.A. Human somatic cell nuclear transfer using adult cells. Cell Stem Cell. 2014;14:777–780. doi: 10.1016/j.stem.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Chung Y.G., Matoba S., Liu Y., Eum J.H., Lu F., Jiang W., Lee J.E., Sepilian V., Cha K.Y., Lee D.R. Histone demethylase expression enhances human somatic cell nuclear transfer efficiency and promotes derivation of pluripotent stem cells. Cell Stem Cell. 2015;17:758–766. doi: 10.1016/j.stem.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Falcone T., Attaran M., Bedaiwy M.A., Goldberg J.M. Ovarian function preservation in the cancer patient. Fertil. Steril. 2004;81:243–257. doi: 10.1016/j.fertnstert.2003.06.031. [DOI] [PubMed] [Google Scholar]
- Gao C., Han H.B., Tian X.Z., Tan D.X., Wang L., Zhou G.B., Zhu S.E., Liu G.S. Melatonin promotes embryonic development and reduces reactive oxygen species in vitrified mouse 2-cell embryos. J. Pineal Res. 2012;52:305–311. doi: 10.1111/j.1600-079X.2011.00944.x. [DOI] [PubMed] [Google Scholar]
- Gook D.A., Osborn S.M., Johnston W.I. Cryopreservation of mouse and human oocytes using 1,2-propanediol and the configuration of the meiotic spindle. Hum. Reprod. 1993;8:1101–1109. doi: 10.1093/oxfordjournals.humrep.a138201. [DOI] [PubMed] [Google Scholar]
- Hirata S., Fukasawa H., Wakayama S., Wakayama T., Hoshi K. Generation of healthy cloned mice using enucleated cryopreserved oocytes. Cell Reprogram. 2011;13:7–11. doi: 10.1089/cell.2010.0059. [DOI] [PubMed] [Google Scholar]
- Hotamisligil S., Toner M., Powers R.D. Changes in membrane integrity, cytoskeletal structure, and developmental potential of murine oocytes after vitrification in ethylene glycol. Biol. Reprod. 1996;55:161–168. doi: 10.1095/biolreprod55.1.161. [DOI] [PubMed] [Google Scholar]
- Ishizuka B., Kuribayashi Y., Murai K., Amemiya A., Itoh M.T. The effect of melatonin on in vitro fertilization and embryo development in mice. J. Pineal Res. 2000;28:48–51. doi: 10.1034/j.1600-079x.2000.280107.x. [DOI] [PubMed] [Google Scholar]
- Joly C., Bchini O., Boulekbache H., Testart J., Maro B. Effects of 1,2-propanediol on the cytoskeletal organization of the mouse oocyte. Hum. Reprod. 1992;7:374–378. doi: 10.1093/oxfordjournals.humrep.a137654. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Inoue K., Lee J., Yoshimoto M., Ogonuki N., Miki H., Baba S., Kato T., Kazuki Y., Toyokuni S. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Kang E., Wang X., Tippner-Hedges R., Ma H., Folmes C.D., Gutierrez N.M., Lee Y., Van Dyken C., Ahmed R., Li Y. Age-related accumulation of somatic mitochondrial DNA mutations in adult-derived human iPSCs. Cell Stem Cell. 2016;18:625–636. doi: 10.1016/j.stem.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Kazem R., Thompson L.A., Srikantharajah A., Laing M.A., Hamilton M.P., Templeton A. Cryopreservation of human oocytes and fertilization by two techniques: in-vitro fertilization and intracytoplasmic sperm injection. Hum. Reprod. 1995;10:2650–2654. doi: 10.1093/oxfordjournals.humrep.a135761. [DOI] [PubMed] [Google Scholar]
- Kim M.K., Park E.A., Kim H.J., Choi W.Y., Cho J.H., Lee W.S., Cha K.Y., Kim Y.S., Lee D.R., Yoon T.K. Does supplementation of in-vitro culture medium with melatonin improve IVF outcome in PCOS? Reprod. Biomed. Online. 2013;26:22–29. doi: 10.1016/j.rbmo.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Kishigami S., Mizutani E., Ohta H., Hikichi T., Thuan N.V., Wakayama S., Bui H.T., Wakayama T. Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem. Biophys. Res. Commun. 2006;340:183–189. doi: 10.1016/j.bbrc.2005.11.164. [DOI] [PubMed] [Google Scholar]
- Lanza R.P., Cibelli J.B., West M.D. Prospects for the use of nuclear transfer in human transplantation. Nat. Biotechnol. 1999;17:1171–1174. doi: 10.1038/70709. [DOI] [PubMed] [Google Scholar]
- Lee J.E., Chung Y.G., Eum J.H., Lee Y., Lee D.R. An efficient SCNT technology for the establishment of personalized and public human pluripotent stem cell banks. BMB Rep. 2016;49:197–198. doi: 10.5483/BMBRep.2016.49.4.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S., Jin Y.X., Yuan B., Zhang J.B., Kim N.H. Melatonin enhances the developmental competence of porcine somatic cell nuclear transfer embryos by preventing DNA damage induced by oxidative stress. Sci. Rep. 2017;7:11114. doi: 10.1038/s41598-017-11161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luberda Z. The role of glutathione in mammalian gametes. Reprod. Biol. 2005;5:5–17. [PubMed] [Google Scholar]
- Ma H., Morey R., O'Neil R.C., He Y., Daughtry B., Schultz M.D., Hariharan M., Nery J.R., Castanon R., Sabatini K. Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature. 2014;511:177–183. doi: 10.1038/nature13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchester L.C., Coto-Montes A., Boga J.A., Andersen L.P., Zhou Z., Galano A., Vriend J., Tan D.X., Reiter R.J. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015;59:403–419. doi: 10.1111/jpi.12267. [DOI] [PubMed] [Google Scholar]
- Matoba S., Liu Y., Lu F., Iwabuchi K.A., Shen L., Inoue A., Zhang Y. Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell. 2014;159:884–895. doi: 10.1016/j.cell.2014.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba S., Zhang Y. Somatic cell nuclear transfer reprogramming: mechanisms and applications. Cell Stem Cell. 2018;23:471–485. doi: 10.1016/j.stem.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagata N., Takeo T., Fukumoto K., Kondo T., Haruguchi Y., Takeshita Y., Nakamuta Y., Matsunaga H., Tsuchiyama S., Ishizuka Y. Applications of cryopreserved unfertilized mouse oocytes for in vitro fertilization. Cryobiology. 2013;67:188–192. doi: 10.1016/j.cryobiol.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Nakamura B.N., Fielder T.J., Hoang Y.D., Lim J., McConnachie L.A., Kavanagh T.J., Luderer U. Lack of maternal glutamate cysteine ligase modifier subunit (Gclm) decreases oocyte glutathione concentrations and disrupts preimplantation development in mice. Endocrinology. 2011;152:2806–2815. doi: 10.1210/en.2011-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M., Kato Y., Tsunoda Y. Effect of melatonin treatment on the developmental potential of parthenogenetic and somatic cell nuclear-transferred porcine oocytes in vitro. Zygote. 2012;20:199–207. doi: 10.1017/S0967199411000190. [DOI] [PubMed] [Google Scholar]
- Nichols J., Zevnik B., Anastassiadis K., Niwa H., Klewe-Nebenius D., Chambers I., Scholer H., Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Pang Y.W., An L., Wang P., Yu Y., Yin Q.D., Wang X.H., Xin Z., Qian Z., Yang M.L., Min G. Treatment of porcine donor cells and reconstructed embryos with the antioxidant melatonin enhances cloning efficiency. J. Pineal Res. 2013;54:389–397. doi: 10.1111/jpi.12024. [DOI] [PubMed] [Google Scholar]
- Papis K., Poleszczuk O., Wenta-Muchalska E., Modlinski J.A. Melatonin effect on bovine embryo development in vitro in relation to oxygen concentration. J. Pineal Res. 2007;43:321–326. doi: 10.1111/j.1600-079X.2007.00479.x. [DOI] [PubMed] [Google Scholar]
- Practice Committees of American Society for Reproductive Medicine. Society for Assisted Reproductive Technology Mature oocyte cryopreservation: a guideline. Fertil. Steril. 2013;99:37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- Reiter R.J., Mayo J.C., Tan D.X., Sainz R.M., Alatorre-Jimenez M., Qin L. Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 2016;61:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- Reiter R.J., Tan D.X., Mayo J.C., Sainz R.M., Leon J., Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003;50:1129–1146. [PubMed] [Google Scholar]
- Salehi M., Kato Y., Tsunoda Y. Effect of melatonin treatment on developmental potential of somatic cell nuclear-transferred mouse oocytes in vitro. Zygote. 2014;22:213–217. doi: 10.1017/S0967199413000336. [DOI] [PubMed] [Google Scholar]
- Shi J.M., Tian X.Z., Zhou G.B., Wang L., Gao C., Zhu S.E., Zeng S.M., Tian J.H., Liu G.S. Melatonin exists in porcine follicular fluid and improves in vitro maturation and parthenogenetic development of porcine oocytes. J. Pineal Res. 2009;47:318–323. doi: 10.1111/j.1600-079X.2009.00717.x. [DOI] [PubMed] [Google Scholar]
- Song C., Peng W., Yin S., Zhao J., Fu B., Zhang J., Mao T., Wu H., Zhang Y. Melatonin improves age-induced fertility decline and attenuates ovarian mitochondrial oxidative stress in mice. Sci. Rep. 2016;6:35165. doi: 10.1038/srep35165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumpf D., Mao C.A., Yamanaka Y., Ralston A., Chawengsaksophak K., Beck F., Rossant J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- Su J., Wang Y., Xing X., Zhang L., Sun H., Zhang Y. Melatonin significantly improves the developmental competence of bovine somatic cell nuclear transfer embryos. J. Pineal Res. 2015;59:455–468. doi: 10.1111/jpi.12275. [DOI] [PubMed] [Google Scholar]
- Tachibana M., Amato P., Sparman M., Gutierrez N.M., Tippner-Hedges R., Ma H., Kang E., Fulati A., Lee H.S., Sritanaudomchai H. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228–1238. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tamura H., Kawamoto M., Sato S., Tamura I., Maekawa R., Taketani T., Aasada H., Takaki E., Nakai A., Reiter R.J. Long-term melatonin treatment delays ovarian aging. J. Pineal Res. 2017;62 doi: 10.1111/jpi.12381. [DOI] [PubMed] [Google Scholar]
- Taylor C.J., Peacock S., Chaudhry A.N., Bradley J.A., Bolton E.M. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell. 2012;11:147–152. doi: 10.1016/j.stem.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Tian X., Wang F., He C., Zhang L., Tan D., Reiter R.J., Xu J., Ji P., Liu G. Beneficial effects of melatonin on bovine oocytes maturation: a mechanistic approach. J. Pineal Res. 2014;57:239–247. doi: 10.1111/jpi.12163. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J., Davis P.W. Cytogenetic, cellular, and developmental consequences of cryopreservation of immature and mature mouse and human oocytes. Microsc. Res. Tech. 1994;27:165–193. doi: 10.1002/jemt.1070270209. [DOI] [PubMed] [Google Scholar]
- Van der Elst J., Van den Abbeel E., Nerinckx S., Van Steirteghem A. Parthenogenetic activation pattern and microtubular organization of the mouse oocyte after exposure to 1,2-propanediol. Cryobiology. 1992;29:549–562. doi: 10.1016/0011-2240(92)90060-f. [DOI] [PubMed] [Google Scholar]
- Wei J.Y., Li W.M., Zhou L.L., Lu Q.N., He W. Melatonin induces apoptosis of colorectal cancer cells through HDAC4 nuclear import mediated by CaMKII inactivation. J. Pineal Res. 2015;58:429–438. doi: 10.1111/jpi.12226. [DOI] [PubMed] [Google Scholar]
- Yamada M., Johannesson B., Sagi I., Burnett L.C., Kort D.H., Prosser R.W., Paull D., Nestor M.W., Freeby M., Greenberg E. Human oocytes reprogram adult somatic nuclei of a type 1 diabetic to diploid pluripotent stem cells. Nature. 2014;510:533–536. doi: 10.1038/nature13287. [DOI] [PubMed] [Google Scholar]
- Yang N.J., Seol D.W., Jo J., Jang H.M., Yoon S.Y., Lee W.S., Lee D.R. Supplementation with cell-penetrating peptide-conjugated estrogen-related receptor beta improves the formation of the inner cell mass and the development of vitrified/warmed mouse embryos. Reprod. Sci. 2016;23:1509–1517. doi: 10.1177/1933719116643594. [DOI] [PubMed] [Google Scholar]
- Yang X., Smith S.L., Tian X.C., Lewin H.A., Renard J.P., Wakayama T. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat. Genet. 2007;39:295–302. doi: 10.1038/ng1973. [DOI] [PubMed] [Google Scholar]
- Zhang H.M., Zhang Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014;57:131–146. doi: 10.1111/jpi.12162. [DOI] [PubMed] [Google Scholar]
- Zhang L., Xue X., Yan J., Yan L.Y., Jin X.H., Zhu X.H., He Z.Z., Liu J., Li R., Qiao J. L-proline: a highly effective cryoprotectant for mouse oocyte vitrification. Sci. Rep. 2016;6:26326. doi: 10.1038/srep26326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.M., Hao H.S., Du W.H., Zhao S.J., Wang H.Y., Wang N., Wang D., Liu Y., Qin T., Zhu H.B. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal Res. 2016;60:132–141. doi: 10.1111/jpi.12290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.