Abstract
Background
Normal aging is associated with changes in cognitive function that are non‐pathological and are not necessarily indicative of future neurocognitive disease. Low cognitive and brain reserve and limited cognitive stimulation are associated with increased risk of dementia. Emerging evidence now suggests that subtle cognitive changes, detectable years before criteria for mild cognitive impairment are met, may be predictive of future dementia. Important for intervention and reduction in disease risk, research also suggests that engaging in stimulating mental activity throughout adulthood builds cognitive and brain reserve and reduces dementia risk. Therefore, midlife (defined here as 40 to 65 years) may be a suitable time to introduce cognitive interventions for maintaining cognitive function and, in the longer term, possibly preventing or delaying the onset of clinical dementia.
Objectives
To evaluate the effects of computerised cognitive training interventions lasting at least 12 weeks for maintaining or improving cognitive function in cognitively healthy people in midlife.
Search methods
We searched up to 31 March 2018 in ALOIS (www.medicine.ox.ac.uk/alois), the specialised register of the Cochrane Dementia and Cognitive Improvement Group (CDCIG). We ran additional searches in MEDLINE, Embase, PsycINFO, CINAHL, ClinicalTrials.gov, and the WHO Portal/ICTRP at www.apps.who.int/trialsearch, to ensure that the search was as comprehensive and as up‐to‐date as possible, to identify published, unpublished, and ongoing trials.
Selection criteria
We included randomised controlled trials (RCTs) or quasi‐RCTs, published or unpublished, reported in any language. Participants were cognitively healthy people between 40 and 65 years of age (80% of study population within this age range). Experimental interventions adhered to the following criteria: intervention was any form of interactive computerised cognitive intervention ‐ including computer exercises, computer games, mobile devices, gaming console, and virtual reality ‐ that involved repeated practice on standardised exercises of specified cognitive domain(s) for the purpose of enhancing cognitive function; duration of the intervention was at least 12 weeks; cognitive outcomes were measured; and cognitive training interventions were compared with active or inactive control interventions.
Data collection and analysis
For preliminary screening of search results, we used a 'crowd' method to identify RCTs. At least two review authors working independently screened remaining citations against inclusion criteria; independently extracted data; and assessed the quality of the included trial, using the Cochrane risk of bias assessment tool. We used GRADE to describe the overall quality of the evidence.
Main results
We identified one eligible study that examined the effect of computerised cognitive training (CCT) in 6742 participants over 50 years of age, with training and follow‐up duration of six months. We considered the study to be at high risk of attrition bias and the overall quality of the evidence to be low.
Researchers provided no data on our primary outcome. Results indicate that there may be a small advantage for the CCT group for executive function (mean difference (MD) ‐1.57, 95% confidence interval (CI) ‐1.85 to ‐1.29; participants = 3994; low‐quality evidence) and a very small advantage for the control group for working memory (MD 0.09, 95% CI 0.03 to 0.15; participants = 5831; low‐quality evidence). The intervention may have had little or no effect on episodic memory (MD ‐0.03, 95% CI ‐0.10 to 0.04; participants = 3090; low‐quality evidence).
Authors' conclusions
We found low‐quality evidence from only one study. We are unable to determine whether computerised cognitive training is effective in maintaining global cognitive function among healthy adults in midlife. We strongly recommend that high‐quality studies be undertaken to investigate the effectiveness and acceptability of cognitive training in midlife, using interventions that last long enough that they may have enduring effects on cognitive and brain reserve, and with investigators following up long enough to assess effects on clinically important outcomes in later life.
Keywords: Aged; Humans; Middle Aged; Cognition; Computer‐Assisted Instruction; Healthy Aging; Cognitive Dysfunction; Cognitive Dysfunction/prevention & control; Dementia; Dementia/prevention & control; Memory, Episodic; Time Factors
Computerised cognitive training for maintaining cognitive function in cognitively healthy people in midlife
Background
The terms 'cognition' and 'cognitive function' describe all the mental activities related to thinking, learning, remembering, and communicating. Normal changes in cognition become evident with aging. Also, diseases may affect cognition, principally dementia, which becomes increasingly common with increasing age from about 65 years onwards. Researchers have shown a great deal of interest in trying to prevent cognitive decline and dementia. It is known that being mentally active throughout life is associated with lower risk of dementia. Therefore, it is has been suggested that encouraging mental activity in midlife (which we define in this review as 40 to 65 years of age) might be an effective way of maintaining good cognitive function as people age. Cognitive training involves a set of standardised tasks intended to 'exercise the brain' in various ways. Programmes of cognitive training are often delivered by way of computers or mobile technology so that people can perform activities on their own at home. Increasingly, these are provided in commercial packages that are advertised to the general public. We wanted to know whether computerised cognitive training is an effective way for people between 40 and 65 years of age to maintain good cognitive function as they age.
What we did
We searched the medical literature up to 15 March 2018 for trials that compared the cognitive function of people 40 to 65 years of age who had taken part in computerised cognitive training lasting at least three months versus a control group that had not done so. For the comparison to be as fair as possible, it should have been decided randomly whether participants were assigned to the cognitive training group or the control group. We were primarily interested in overall measures of cognition. The choice of three months of intervention was somewhat arbitrary, but we thought it unlikely that shorter periods of training could have long‐lasting effects.
What we found
We found that a lot of shorter studies had been conducted, but only one study met our criteria for this review. It took place in the UK and included two different types of online cognitive training. The control group participated in an online game that was not expected to have cognitive effects. This training lasted six months, and study authors measured cognition at the end of the training period. Resarchers randomised 6742 people in the study, but the dropout rate was high. We thought this put the results at high risk of bias; therefore we considered the quality of evidence provided by this study to be low, meaning that further research might well lead to different results. This study did not measure overall cognitive functioning ‐ which we were most interested in ‐ but it did measure some subtypes of cognitive function. The cognitive training group did slightly better on a test of reasoning, and the control group did very slightly better on a test of working memory, which is a very short‐term type of memory. No evidence suggested that the groups differed in memory measured by a word‐learning test.
Our conclusions
We were not able to tell whether taking part in computerised cognitive training in midlife has any lasting effects on cognitive function. We think this is an important question that should be investigated further in trials that test cognitive training over three months or longer. It will also be important for researchers to try to find the best ways to keep people motivated to persist with training.
Summary of findings
Summary of findings for the main comparison.
| Computerised cognitive training compared with control intervention in cognitively healthy people in midlife | ||||
|
Patient or population: cognitively healthy people in midlife Settings: general population Intervention: computerised cognitive training Comparison: control intervention | ||||
| Outcomes | Difference between CCT and control (95% CI)1 | No. of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Global cognitive functioning | Not reported using a validated measure | |||
| Cognitive subdomain: episodic memory, 6 months of follow‐up | MD 0.03 lower (0.10 lower to 0.04 higher) | 3090 participants (1 study) | ⊕⊕⊝⊝ low2 | CCT may lead to little or no improvement in episodic memory |
| Cognitive subdomain: executive functioning, 6 months of follow‐up | MD 1.57 lower (1.85 lower to 1.29 lower) | 3994 participants (1 study) | ⊕⊕⊝⊝ low2 | CCT possibly improves executive function compared to active control |
| Cognitive subdomain: working memory, 6 months of follow‐up | MD 0.09 higher (0.03 higher to 0.15 higher) | 5831 participants (1 study) | ⊕⊕⊝⊝ low2 | CCT possibly maintains working memory worse than active control, but the difference is deemed negligible |
| Cognitive subdomain: speed of processing | Not reported using a validated measure | |||
| Quality of life | Not reported using a validated measure | |||
| One or more serious adverse events | Not reported using a validated measure | |||
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||
1The direction of the effect was standardised, so that lower values favour CCT and higher values favour control.
2Downgraded twice for attrition bias.
Background
Description of the condition
Cognitive health across the life span is essential for independent function and active aging. 'Active aging' refers to the process of optimising opportunities for health, participation, and security (WHO 2016). 'Cognitive health' broadly refers to absence of cognitive impairment and preservation of cognitive structure; this is necessary if older adults are to achieve active aging (Depp 2012; Hendrie 2006). Older adults fear cognitive decline and dementia, among other reasons, for the threat that they pose to active aging and independence (Deary 2009; Lustig 2009).
Cognitively healthy adults undergo normal age‐related changes in cognitive function, and they experience a reduction in neural resources as they age (Salthouse 2003; Shing 2008). A minor decline in some cognitive domains may be evident in adults in midlife. However, observers have noted considerable variability in cognitive function and brain structure between individuals and across the age span (Ronnlund 2015; Salthouse 2011). Large variations in cognitive health and function are seen at a population level, and lifetime trajectories of decline range from normal age‐related decline through to subjective complaint, mild cognitive impairment (MCI), and clinical dementia (World Alzheimer Report 2014). Advances in research technologies have increased our understanding of the pathophysiological changes linked to dementia and indicate that the brain changes underlying dementia develop over a period of at least 20 to 30 years before the onset of symptoms (World Alzheimer Report 2014). However, differentiating between normal age‐related changes and pathological changes due to slow progression of disease can be very difficult.
Differences in cognitive health and in individual susceptibility to the development of clinical dementia in late life may be due in part to variability in brain development and cognitive reserve (Barulli 2013; Stern 2009; Stern 2012). The concept of reserve can provide a theoretical explanation for differences between individuals with the same degree of brain pathology who present with a clinical dementia and are functionally impaired, and those who do not display any clinical symptoms and manage to maintain better levels of functioning (Stern 2012). Cognitive reserve is developed through educational attainment, occupation, and engagement in cognitive stimulating activities (Opedebeek 2016; World Alzheimer Report 2014). Lack of cognitive stimulation across the life span ‐ and by inference reduced reserve ‐ is a significant risk factor for reduced cognitive function and is associated with higher dementia risk (Norton 2014; World Alzheimer Report 2014).
Cross‐sectional and longitudinal comparisons indicate that acquired knowledge generally increases until about age 60 (Salthouse 2011). Therefore the introduction of mentally stimulating activity in midlife (40 to 65) offers cognitively healthy people an opportunity to improve or maintain cognitive function and potentially to build reserve (Gates 2014). Even small improvements in cognitive function may lead to important benefits for everyday functioning, and any delay or reduction in age‐related cognitive decline may substantially extend the period during which people can live independently (Hertzog 2008). Stimulating cognitive activity may improve cognitive function, leading to structural and functional neuroplasticity. Emerging research suggests that patterns of mental activity may influence the relationship between neuropathology and clinical dementia, with neural compensatory mechanisms the most likely mechanism, consistent with reserve models (Bennett 2014; Grady 2012).
Prospective epidemiological studies of cognitively stimulating leisure activities consistently report protective effects, including lower rates of cognitive decline and incident dementia (Marioni 2014; Verghese 2003; Wilson 2002). Prospective population and cohort studies also indicate benefits of mental activity, with lower rates of cognitive decline, less dementia pathology, and lower incidence of dementia reported (Beydoun 2014; Geda 2012; Landau 2012; Verghese 2003; Wilson 2012). For example, a meta‐analysis of 22 cohort studies of dementia incidence revealed that individuals with higher levels of lifetime mental activity almost halved their risk of developing dementia (Valenzuela 2003). A five‐year longitudinal cohort study, tracking more than 1000 cognitively healthy adults, indicated that the introduction of mental activities had a beneficial effect on cognition the following year, suggesting that intervention may be effective in countering age‐related cognitive decline (Wilson 2012).
Investigators are examining new non‐pharmacological interventions provided to build cognitive reserve, potentially maintaining better cognitive functioning with aging and delaying the onset of clinical dementia in later life (Acevedo 2007; Barnes 2011; Dresler 2013; Leifer 2003). Two models of cognitive enrichment have been developed, drawing on population studies of the benefits of mental activity and engagement: engagement through lifestyle within a complex environment, and engagement through instruction and practice interventions (Stine‐Morrow 2014). Both models introduce novel complex mental activities for improving cognitive function that may preserve cognitive health, build cognitive reserve, combat age‐related cognitive dysfunction, and promote active ageing (Amoyal 2012; Barnes 2011; Marquine 2012).
Description of the intervention
Cognitive training, frequently termed 'brain training' in commercial spheres, has been developed to provide mentally stimulating interventions to reduce age‐related decline (Gates 2014). Such programmes introduce participants to novel activities with the aim of stimulating cognitive change and slowing cognitive aging (Park 2007). Although cognitive training may include traditional pen and paper tasks, it now more commonly takes the form of computer‐based tasks, including exercises, games, and virtual reality (Gates 2010). Computerised cognitive training (CCT) programmes have been delivered in individual sessions and within groups, with supervision or privately at home; and studies show wide variation in the 'dose' or length of each training session, the frequency of sessions, and the duration of training programmes, leading to significant heterogeneity in the literature (Gates 2014).
How the intervention might work
The theoretical premise behind cognitive intervention to improve cognitive function or to minimise age‐related decline in cognitively healthy adults is that cognitively stimulating mental exercises will increase brain and cognitive reserve. Enhanced reserve may be associated with structural brain changes, such as increased brain volume, or with functional changes in neural activity (Stern 2012). Cognitive stimulation may lead to development of compensatory networks that work to maintain cognitive performance, and potentially to mask or prevent clinical manifestations of neurocognitive disease (Grady 2012). Evidence from animal studies indicates that new learning is associated with positive neuroplastic changes (Cotman 2007; Curlik 2013; Nithianantharajah 2006). Researchers have proposed a scaffold theory of compensatory activation to incorporate factors associated with age‐related cognitive decline and factors that may enhance function and reserve (Park 2013). Computerised cognitive training may stimulate positive neuroplastic changes (Valenzuela 2003), including increasing neural volume and neural activity (i.e. compensatory neural networks) (Grady 2012; Park 2013), brain metabolism (Forster 2011), neurochemistry activation (Olesen 2004; Rosen 2011), and fluorodeoxyglucose uptake (Belleville 2012). However, research findings have been limited, and significant further investigation is required.
Although the evidence base is very limited, human trials of cognitive training suggest positive neural changes, including reduced β‐amyloid burden (Landau 2012). Diverse studies investigating neurophysiological changes on functional magnetic resonance imaging have identified increased prefrontal and parietal activity and hippocampal activation (Olesen 2004; Rosen 2011; Suo 2012a; Valenzuela 2003). Electroencephalography and magnetic resonance spectrometry studies of cognitive training support the concept of functional neuroplasticity post training, with results showing positive changes in brain metabolism, task‐dependent brain activation, and resting‐state networks (Belleville 2012; Berry 2010; Forster 2011). Thus, emerging evidence suggests that cognitively stimulating activities might be stimulate neuroplasticity and build brain reserve.
Why it is important to do this review
The potential of computerised cognitive interventions for enhancing cognitive health, and even for helping to prevent clinical dementia, and their accessibility and low implementation costs have led the American Alzheimer's Association to make recommendations for rapid development and testing of computerised cognitive intervention programmes (Alzheimers Association 2014). Increasing consumer demand for interventions to maintain cognitive function has resulted in a multi‐billion dollar industry of commercial brain training computer software programmes that purport to maintain, and potentially enhance, cognitive function, yet often lack supportive data or independent research evaluation (Belleville 2012; Gates 2010; Sixsmith 2013).
Although research examining the effects of cognitive training in older adults is extensive and now spans several decades, results are inconclusive. The research literature has been characterised by significant variability in populations and interventions. Clinical trials have been criticised for poor specification of interventions, poor methodological rigour, small sample sizes, and failure to assign treatments randomly (Gates 2010; Kueider 2012; Papp 2009; Reijnders 2013; Walton 2014). Reviews have not always distinguished between the different types of cognitive interventions (Martin 2011). Results from studies in healthy adults have been inconsistent, with data showing negative findings from meta‐analyses (e.g. Papp 2009), and more recent meta‐analyses of computerised cognitive training in cognitively healthy adults, with defined intervention and clear eligibility criteria, have shown positive results on cognition (Kueider 2012; Lampit 2014a; Shao 2015). A significant limitation in the research, to be addressed by this review, is the paucity of studies examining cognitive interventions in midlife, with most studies focussing on young adults or older adults (over 60 years of age). For example, a comparative trial included younger (20 to 31 years) and older adults (65 to 80 years) but omitted those in midlife (Schmiedek 2010). Additionally, limited evidence shows generalisation and persistence of benefit over time (Park 2013). A robust review is therefore required to clarify the effects of cognitive training in midlife on global cognition, non‐trained cognitive domains, and general function (Green 2014; Park 2013). The present review aims to address these gaps in the evidence and to examine critically the current research literature, including an evaluation of potential sources of bias and heterogeneity.
For individuals, fear of cognitive decline and dementia may be powerful motivators to seek preventive interventions. The World Alzheimer Report 2014 indicates that cognitively stimulating activities, including reading, playing musical instruments, and playing cards and board games, may be beneficial for improving and maintaining cognition and potentially preventing decline in the future, although most of these activities have not been investigated in clinical trials. Technology and computerised 'brain training' games and cognitive training programmes are being more actively investigated (Alzheimers Association 2014; Peretz 2011; Sixsmith 2013). However, the proliferation of computer‐based commercial products purporting to improve cognitive function while reducing dementia risk is outpacing clinical research. In this context, this review will provide important information to the public so people can know whether the time, effort, and money they might invest to prevent cognitive decline is likely to be well spent.
From a research perspective, it is vital to review the evidence and to integrate clinical research into practice (Doody 2009). At this stage, reliable data are insufficient to provide clear guidelines for the implementation of intervention programmes. Recent primary studies have identified that the benefits of cognitive training may depend upon a number of factors including age, cognitive level, and non‐cognitive factors (Lampit 2014a; Stine‐Morrow 2014). Comparisons between single‐ and multiple‐domain training suggest that multiple‐domain training is better, and nascent evidence shows that different cognitive domains may respond differently to training, and hence may require specific interventions for different lengths of time (Lampit 2014a). Therefore, the present review of the effect size of interventions and stratification of data may highlight the ‘dose’, duration, and frequency of interventions necessary to achieve an effect.
Therefore, as well as informing consumers, this review may be useful to public health decision bodies, health practitioners, and researchers, providing them with a comprehensive synthesis of information about the current state of the evidence and identifying research gaps and unanswered questions in the field.
We also refer readers to companion reviews on the effects of computerised cognitive training on healthy people in late life and on people with MCI (Gates 2019a; Gates 2019b).
Objectives
To evaluate the effects of computerised cognitive training interventions lasting at least 12 weeks for maintaining or improving cognitive function in cognitively healthy people in midlife.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) or quasi‐RCTs, published or unpublished, reported in any language. Full reports and other types of reports, such as conference abstracts, were eligible for inclusion. We included studies involving both randomised and non‐randomised trial arms, but we considered results only from the former. We included cross‐over studies, but we extracted and analysed data from the first treatment period only.
Types of participants
We included studies of cognitively healthy people in midlife. Midlife is defined as ranging from 40 to 65 years of age. At least 80% of the study population had to be in this age range. We covered participants in late life (65 or older) in a separate review (Gates 2019a). If the age range of participants in a trial did not coincide with our categories, we used the median and range, or the mean and standard deviation (SD), to help place studies into the most appropriate review.
We determined the cognitive status of participants by using the trial authors' own definitions of ‘cognitively healthy’; we recorded these definitions. We excluded all studies reporting that more than 20% of participants had subjective memory complaints, or received a diagnosis, or were defined as having any cognitive, neurological, psychiatric, or medical condition.
We contacted study authors if we needed further clarification to determine health status. If we received no response, clinical experts in our review group classified trials, or listed them as 'Studies awaiting classification'.
Types of interventions
We included studies of cognitive training interventions using interactive computerised technology of 12 or more weeks' duration, compared with active or inactive control interventions.
Experimental interventions had to adhere to the following criteria: any form of interactive computerised cognitive intervention including computer exercises, computer games, mobile devices, gaming console, and virtual reality, which involves repeated practice on standardised exercises of specified cognitive domain/s, for the purpose of enhancing cognitive function.
By 'active control', we mean all control conditions that involve unguided computer‐ and/or screen‐based tasks that are not a planned intervention. These tasks can involve watching educational videos or playing computer games, with no particular training component. By 'inactive controls', we refer to controls for which no intervention is applied that may be expected to have an effect on cognition.
The minimum treatment duration was set at 12 weeks, and all included trials had to report outcomes at a minimum of one time point, 12 weeks or longer after randomisation. To evaluate the effects of training on meaningful long‐term outcomes, it was necessary to make a judgement about the minimum 'dose' of training that may be required to effect an enduring change. Previous research suggests that acute brain changes can be seen following eight weeks of training (Engvig 2014), but we are unable to find any evidence that such brain changes endure. Most studies examining the benefits of brain and cognitive reserve identify long‐term cognitive stimulation from years of education. We therefore made an arbitrary judgement that at least 12 weeks of regular cognitive training would be required for an enduring effect of the intervention. This time frame is consistent with recommendations received from reviews of clinical trials (Lampit 2014a). Trials in cognitively healthy people with a duration of intervention as short as 12 weeks typically investigate cognitive enhancement rather than maintenance of cognitive function. It is recognised that the relationship between short‐term cognitive training and maintenance of cognitive function over longer periods of time is unclear.
We excluded interventions that did not involve any form of computer delivery. We also excluded studies in which the investigator combined the experimental intervention with any other form of intervention, unless the added intervention was provided in a standardised manner to both experimental and control groups.
Types of outcome measures
Primary outcomes
-
Global cognitive functioning: measured using validated tests, for example (but not limited to)
Mini Mental State Examination (MMSE)
Alzheimer's Disease Assessment Scale (ADAS‐Cog)
Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)
Cambridge Cognition Examination (CAMCOG)
The main time point of interest was 'end of trial', defined as the time point with the longest follow‐up duration, as measured from randomisation (see also section Data collection and analysis). We also extracted and presented outcome data reported at other time points after randomisation.
Secondary outcomes
Secondary outcomes involved cognitive tests not included in the training programme, administered before and after training, that serve as any validated measure of:
specific cognitive functioning subdomain: episodic memory;
specific cognitive functioning subdomain: executive functioning;
specific cognitive functioning subdomain: speed of processing;
specific cognitive functioning subdomain: verbal fluency;
specific cognitive functioning subdomain: attention/working memory;
quality of life/psychological well‐being, either generic or health‐specific;
daily function, such as measures of instrumental activities of daily living; or
number of participants experiencing one or more serious adverse event(s).
If a trial provided data on more than one cognitive scale for a specific outcome, we applied a hierarchy of cognition‐related outcomes (manuscript in preparation) and used data from the cognitive scale that was highest in this hierarchy. For example, if a trial reported results on both the MMSE and the Clinical Dementia Rating scale (CDR), we used outcome data from MMSE in our quantitative analyses. The order of a scale in the hierarchy was determined by the frequency of its use in a large set of 79 trials undertaken to evaluate vitamin and mineral supplementation, dietary interventions, and physical exercise interventions.
Outcomes to be included in the 'Summary of findings' table
We addressed critical effectiveness outcomes in the 'Summary of findings' table for each review. We included all outcomes related to cognitive function on non‐trained tasks and quality of life.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois) ‐ the specialised register of the Cochrane Dementia and Cognitive Improvement Group (CDCIG) ‐ up to 31 March 2018.
ALOIS was maintained by the Information Specialist for the CDCIG and contains studies that fall within the areas of dementia prevention, dementia treatment and management, and cognitive enhancement in healthy elderly populations. These studies are identified through:
monthly searches of several major healthcare databases: MEDLINE, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, and Latin American Caribbean Health Sciences Literature (LILACS);
monthly searches of several trial registers: University hospital Medical Information Network (UMIN) Clinical Trials Registry (Japan) (UMIN‐CTR) (www.umin.ac.jp/ctr/index.htm); the World Health Organization (WHO) Portal (which covers ClinicalTrials.gov (clinicaltrials.gov/); International Standard Randomized Controlled Trials Number (ISRCTN) (www.isrctn.com/); the Chinese Clinical Trials Register (ChiCTR) (who.int/ictrp/network/chictr/en/); the German Clinical Trials Register (GermanCTR) (who.int/ictrp/network/drks2/en/); the Iranian Registry of Clinical Trials (IRCT) (who.int/ictrp/network/irct2/en/); and the Netherlands National Trials Register (NTR) (who.int/ictrp/network/ntr/en/), plus others);
quarterly searches of the Cochrane Library’s Central Register of Controlled Trials (CENTRAL); and
six‐monthly searches of a number of grey literature sources, including Institute for Scientific Information (ISI) Web of Knowledge Conference Proceedings; Index to Theses; and Australasian Digital Theses.
To view a list of all sources searched for ALOIS, see About ALOIS on the ALOIS website (www.medicine.ox.ac.uk/alois).
Details of the search strategies used in healthcare bibliographic databases for retrieval of reports on dementia, cognitive improvement, and cognitive enhancement trials can be viewed in the ‘Methods used in reviews’ section within the editorial information about the Cochrane Dementia and Cognitive Improvement Group.
We conducted additional searches in MEDLINE, Embase, PsycINFO, CINAHL, ClinicalTrials.gov, and the WHO Portal/ICTRP at www.apps.who.int/trialsearch, to ensure that the searches for this review were as comprehensive and as up‐to‐date as possible in identifying published, unpublished, and ongoing trials. We used this search strategy to retrieve reports of trials from MEDLINE (via the Ovid search platform ‐ SP), as shown in Appendix 1.
Searching other resources
We screened the reference lists of all included trials. In addition, we screened the reference lists of recent systematic reviews, health technology assessment reports, and subject‐specific guidelines identified through www.guideline.gov. We restricted the search to guidelines meeting National Guideline Clearinghouse (NGC) 2013 published inclusion criteria.
We contacted experts in the field and companies marketing included interventions to request additional randomised trial reports not identified by the search.
Data collection and analysis
We used this protocol alongside instructions for data extraction, quality assessment, and statistical analyses generated by the editorial board of CDCIG, and based in part on a generic protocol approved by the Cochrane Musculoskeletal Group for another series of reviews (da Costa 2012; da Costa 2014; Reichenbach 2010; Rutjes 2009a; Rutjes 2009b; Rutjes 2010).
Selection of studies
If multiple reports described the same trial, we included all of them to allow complete extraction of trial details.
We used crowd‐sourcing to screen the search results. We have presented details of this at www.medicine.ox.ac.uk/alois/content/modifiable‐risk‐factors. In brief, teams of volunteers will perform a 'first assess' of the search results. We recruited the crowd through the network called Students 4 Best Evidence (www.students4bestevidence.net). The crowd performed an initial screen of search results using an online tool developed for the Cochrane Embase project, but tailored for this programme of work. The crowd decided (based on a reading of title and abstract) whether the citation is describing a randomised or a quasi‐randomised trial, irrespective of the citation topic. It is estimated that this removed 75% to 90% of the results retrieved. We then screened the remaining results (titles and abstracts). Four independent review authors (NG, EM, SK, RV) assessed the full text of studies for eligibility, with disagreements resolved by a fifth independent review author.
We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), along with a 'Characteristics of excluded studies' table. We imposed no language restrictions.
Data extraction and management
Four review authors (NG, MN, SK, RV), working independently, extracted trial information using a standardised and piloted extraction method, referring also to a guidance document, and resolving discrepancies by discussion, or by involvement of a fifth review author. When possible, we extracted the following information related to characteristics of participants, interventions, and study design.
Participant characteristics
Gender
Age (range, median, mean)
Education (level and years of education)
Baseline cognitive function
Cognitive diagnostic status
Duration of cognitive symptoms, if any
Ethnicity
Apo‐E genotype
Vascular risk factors (hypertension, diabetes, hyperlipidaemia)
Body mass index (BMI)
Depression and stress
Physical activity
Work status
Intervention characteristics
Type and description of computerised cognitive training
Type and description of the control intervention
Delivery mode (individualised, group sessions, supervised)
Length of training sessions (in minutes)
Frequency of sessions (per week)
Duration of treatment programme
Any concomitant treatments for which benefits can be isolated from the intervention
Methodological characteristics
Trial design (individual or cluster randomisation, parallel‐group, factorial or cross‐over design)
Number of participants
Allocation to trial (randomisation, blind allocation)
Outcome measures used
Duration of follow‐up (as measured from randomisation)
Duration of follow‐up (as measured from end of treatment)
Source of financial support
Publication status
If outcome data were available at multiple time points within a given trial, we extracted data at 12 weeks and obtained short‐term (up to one year), medium‐term (one to two years), and long‐term results (longer than two years). Within these time periods, we extracted the latest data reported by the study (e.g. if the study reports data at six months, nine months, and one year, we extracted only one‐year data and analysed these for the one‐year (short‐term) time point). For dichotomous outcomes (e.g. number of participants experiencing one or more serious adverse events), we extracted from each trial the number of participants with each outcome at each time point. For continuous outcomes, we extracted the number of participants for whom the outcome was measured, along with the mean and SD of the change from baseline for each outcome at each time point. If change from baseline data were not available, we extracted the mean value at each time point. When necessary and possible, we approximated means and measures of dispersion from figures in the reports. For cross‐over trials, we extracted data on the first treatment period only. Whenever possible, we extracted intention‐to‐treat data (i.e. analysing all patients according to the group randomisation); if these were not available, then we extracted and reported data from available case analyses. If neither of these data were available, we considered data from per‐protocol analyses. We contacted trial authors if we could not obtain the necessary data from the trial report.
Assessment of risk of bias in included studies
After completion of a standardised training session provided by AR, one member of the study author team and one experienced review author provided by the editorial team independently assessed the risk of bias in each of the included trials, using the Cochrane 'Risk of bias' tool (Higgins 2011), and resolved disagreements by consensus. We assessed the risk of bias potentially introduced by suboptimal design choices with respect to sequence generation, concealment of allocation, blinding of participants and caregivers, blinded outcome assessment, selective outcome reporting, and incomplete outcome data, including the type of statistical analysis used (true intention‐to‐treat vs other). Based on the aforementioned criteria, we rated studies as 'low risk', 'unclear risk', or 'high risk' of bias for each domain and provided a description of the reasoning for our rating. The general definitions used are reported in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We derived the review‐specific definitions in part from a previously published systematic review (Rutjes 2012), and we explained them in detail in Appendix 2.
Measures of treatment effect
The measure of treatment effect for continuous outcomes was an effect size with a 95% confidence interval (CI). If only one trial contributed data to a comparison, or if all studies used the same instrument, this was a mean difference (MD). If trials used different instruments to assess the same outcome, the effect size was a standardised mean difference (SMD) (the between‐group difference in mean values divided by the pooled SD). We expressed the treatment effect for dichotomous outcomes as a risk ratio (RR).
Unit of analysis issues
We included no cluster randomised or cross‐over trials.
Dealing with missing data
Missing data for individual trials may bias effect estimates and may lower the overall quality of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group (www.gradeworkinggroup.org). We dealt with missing data in our 'Risk of bias' assessments and evaluations of attrition bias via stratified analyses of the primary outcomes (Appendix 2). We analysed available information and did not contact study authors with a request to provide missing information. We did not impute missing data ourselves.
Assessment of heterogeneity
We aimed to inspect forest plots for the presence of heterogeneity and to calculate the variance estimate tau² as a measure of between‐trial heterogeneity (DerSimonian 1986). As we identified only a single trial, we could not perform such an analysis.
Assessment of reporting biases
We did not identify enough trials to construct funnel plots with appropriate statistics to explore reporting biases and other biases related to small‐study effects.
Data synthesis
We reported summary and descriptive statistics (means and SDs) for participant and intervention characteristics.
We planned to use standard inverse‐variance random‐effects meta‐analysis to combine outcome data across trials at the end of the trial (DerSimonian 1986); if possible, we planned to use at least one additional time point (see Primary outcomes and Data collection and analysis for definitions of time points). As we included only a single trial, we reported mean differences for the outcomes of interest in this trial. We conducted statistical analyses using Review Manager 5 (RevMan 2014), along with STATA, release 13 (Statacorp, College Station, Texas, USA). All P values are two‐sided.
GRADE and 'Summary of findings' table
We used GRADE to describe the quality of the overall body of evidence for each outcome in the 'Summary of findings' table (Guyatt 2008; Higgins 2011). We defined 'quality' as the degree of confidence that we can place in estimates of treatment benefits and harms. We assigned four possible ratings: high, moderate, low, and very low. Rating evidence as 'high quality' implies that we are confident in our estimate of the effect and further research is very unlikely to change this. A rating of 'very low' quality implies that we are very uncertain about the obtained summary estimate of the effect. The GRADE approach rates evidence from RCTs that do not have serious limitations as 'high quality'. However, several factors can lead to downgrading of the evidence to 'moderate', 'low', or 'very low'. We determined the degree of downgrading by noting the seriousness of these factors: study limitations (risk of bias); inconsistency; indirectness of evidence; imprecision; and publication bias (Guyatt 2008; Higgins 2011).
Subgroup analysis and investigation of heterogeneity
We did not identify enough trials to conduct protocol‐defined subgroup analyses.
Sensitivity analysis
We did not identify enough trials to conduct protocol‐defined sensitivity analyses.
Results
Description of studies
See Characteristics of included studies,Characteristics of excluded studies, Characteristics of studies awaiting classification, and Characteristics of ongoing studies.
Results of the search
We conducted searches in January 2015, July 2015, February 2016, July 2016, and March 2018. In total, we retrieved 7727 records from the five searches. After de‐duplication, 5832 remained. A crowd and the CDCIG Information Specialist assessed these studies at the title and abstract review level. In total, 1090 results remained after this assessment. The review author team then assessed these records. Of these, we assessed 317 full‐text articles for eligibility and found that one study met our inclusion criteria for this review (Corbett 2015). We have depicted this process in Figure 1.
Figure 1.
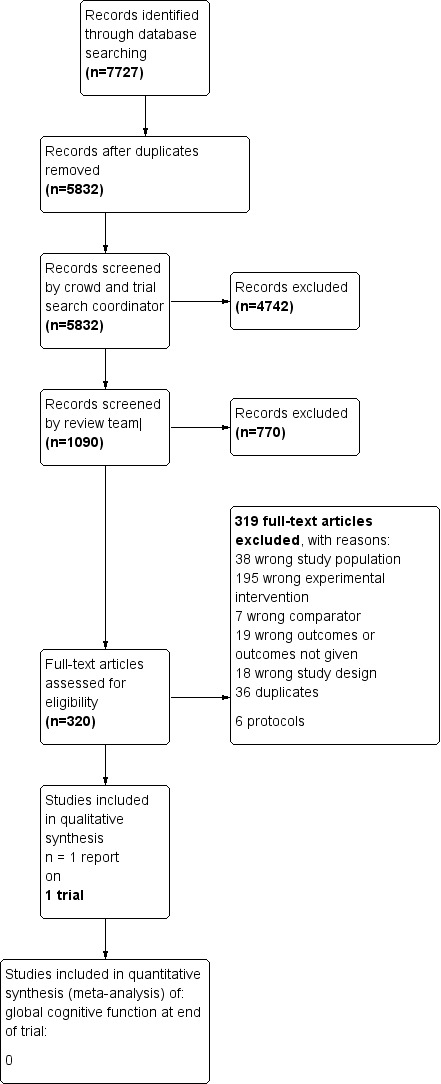
Study flow diagram.
Included studies
We have provided details of the included study in the Characteristics of included studies and have summarised them below.
Design
Corbett 2015 was a randomised controlled trial with three arms, consisting of two computerised cognitive training (CCT) interventions and an active control. Researchers assessed all outcomes after six months.
Sample size
Corbett 2015 randomised 6742 participants to three study arms.
Setting
The study took place in the United Kingdom (UK); all adults older than 50 were invited to take part in the study through a collaboration of the British Broadcasting Corporation (BBC), Alzheimer’s Society UK, and the Medical Research Council.
Interventions
The two CCT interventions were (1) reasoning training (ReaCT), involving six tasks related to executive function, and (2) general cognitive training (GCT), targeting multiple cognitive domains, including memory, attention, and visuospatial ability. The active control group engaged in an Internet game requiring the re‐ordering of statements. Study authors reported 2557 participants in the ReaCT group, 2432 participants in the GCT group, and 1753 participants in the control group. Those completing the study completed on average 112 training sessions over six months.
Participants
All participants were cognitively healthy. They had a mean age of 58.5 (SD 6.5) in the ReaCT arm, 59.1 (SD 6.4) in the GCT arm, and 59.1 (SD 6.6) in the control arm. More women than men participated in this study, accounting for 68.5% of all participants in the ReaCT experimental arm 1, 68.9% of all participants in the GCT experimental arm 2, and 62.4% of all participants in the control arm.
Outcomes
Researchers used five different outcome measures: (1) instrumental activities of daily living (IADLs), (2) Baddeley Grammatical Reasoning Test, (3) Spatial Working Memory (SWM), (4) digit span, and (5) verbal short‐term memory. These are secondary outcomes in this review, and no measure was consistent with our primary outcome.
Excluded studies
We excluded 319 articles after we examined them in full text. Of these, we excluded nine because they focused on cognitively healthy people in late life (Desjardins‐Crépeau 2016; Klusmann 2010; Lampit 2014; Lampit 2015; Legault 2011; Leung 2015; Peretz 2011; Shatil 2013; Van het Reve 2014), and we excluded eight because they included patients with MCI (Barnes 2013; Djabelkhir 2017; Fiatrone Singh 2014; Gooding 2016; Herrera 2012; Kwok 2013a; Optale 2010; Rozzini 2007). Two other Cochrane reviews have included these 17 studies (Gates 2019a; Gates 2019b). We excluded 195 studies because they investigated an intervention of less than 12 weeks' duration, or because they did not provide a computerised cognitive training intervention, and 18 because they used a study design that did not meet review criteria. We identified no ongoing trials in trial registers or conference proceedings. We have provided reasons for exclusion of the remainder in the Characteristics of excluded studies section.
Risk of bias in included studies
We have displayed graphically in Figure 2 risks of bias in the included study.
Figure 2.
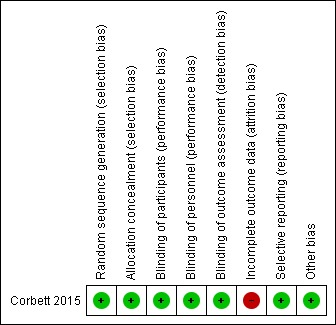
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Corbett 2015 described both sequence generation and allocation concealment adequately, and we judged the study to be at low risk of bias in this domain.
Blinding
Corbett 2015 provided adequate blinding of participants, personnel (home‐based intervention with no involvement of researchers), and outcome assessors (computer‐collected data). Therefore, we judged the study to be at low risk of performance and detection bias.
Incomplete outcome data
For outcomes of interest, we found that final outcome data were missing for between 14% and 66% of participants in individual intervention groups. Study authors imputed final outcome data using the last observation carried forward (LOCF) method. Study authors stated that "reasons for withdrawal are not known due to the online format of intervention and study design" (Corbett 2015). We judged the study to be at high risk of bias for all outcomes, as imputing results using LOCF is likely to yield biased estimates in the presence of observed fractions of participants with missing outcome data at six months.
Selective reporting
We did not identify a trial registration nor a trial protocol. Relying on the published report, we considered the risk of reporting bias to be low, as all outcomes mentioned in the methods section were fully addressed in the results section.
Other potential sources of bias
We identified no other sources of bias.
Effects of interventions
See: Table 1
See Table 1 for the comparison of pooled data from both CCT interventions (ReaCT and GCT) versus active control.
Primary outcome: global cognitive function
Corbett 2015 did not examine the effects of training on any measure of global cognitive function.
Secondary outcomes
For all outcomes, negative values favour CCT.
Cognitive subdomain
Episodic memory
We found low‐quality evidence on episodic memory measured as verbal learning (Analysis 1.1; Figure 3). We downgraded the level of evidence twice for very serious concern about the risk of attrition bias. There may be little or no difference in episodic memory performance between intervention and active control groups.(mean difference (MD) ‐0.03, 95% confidence interval (CI) ‐0.10 to 0.04; participants = 3090).
Analysis 1.1.
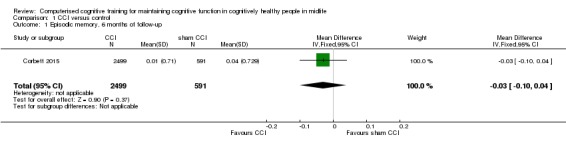
Comparison 1 CCI versus control, Outcome 1 Episodic memory, 6 months of follow‐up.
Figure 3.
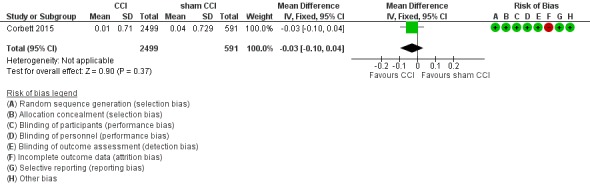
Forest plot of comparison: 1 CCI versus control, outcome: 1.1 Episodic memory, 6 months of follow‐up.
Executive function
We found low‐quality evidence on executive functioning measured by the Baddeley Grammatical Reasoning Test (Analysis 1.2; Figure 4). Again, we downgraded the level of evidence twice for very serious concern about the risk of attrition bias. The active intervention may provide benefit for executive function (MD ‐1.57, 95% CI ‐1.85 to ‐1.29; participants = 3994).
Analysis 1.2.

Comparison 1 CCI versus control, Outcome 2 Executive functioning, 6 months of follow‐up.
Figure 4.
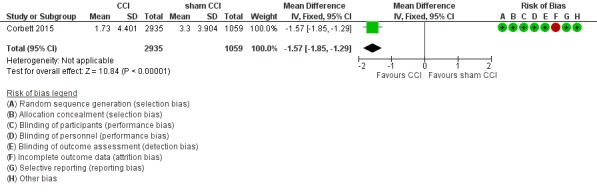
Forest plot of comparison: 1 CCI versus control, outcome: 1.2 Executive functioning, 6 months of follow‐up.
Working memory
Researchers provided low‐quality evidence on working memory measured by digit span (Analysis 1.3; Figure 5). Again, we downgraded the level of evidence twice for very serious concern about the risk of attrition bias. Results probably indicate a very small advantage of working memory for the control group (MD 0.09, 95% CI 0.03 to 0.15; participants = 5831).
Analysis 1.3.
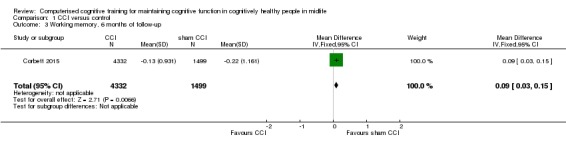
Comparison 1 CCI versus control, Outcome 3 Working memory, 6 months of follow‐up.
Figure 5.
Forest plot of comparison: 1 CCI versus control, outcome: 1.3 Working memory, 6 months of follow‐up.
Cognitive function subdomain: speed of processing
The included study did not report this outcome.
Quality of life
The included study did not report this outcome.
Functional performance
Investigators measured daily function with instrumental activities of daily living (IADLs) as the study primary outcome measure. We did not consider these data, as they were reported for only a subgroup of participants 60 years of age or older ‐ not for our age group of interest.
Number of participants experiencing one or more serious adverse events
The included study did not report serious adverse events.
Discussion
Summary of main results
This review identified only one randomised controlled trial (RCT) that was eligible for inclusion. In this trial, the intervention lasted for six months. This trial did not measure our primary outcome of interest: global cognitive functioning. We found low‐quality evidence of an advantage at the end of the intervention period for the active intervention group on measures of executive functioning and of a very small advantage for the control group on working memory. We found low‐quality evidence of little or no effect on episodic memory.
Overall completeness and applicability of evidence
The fundamental limitation of this review is that only one RCT was eligible for inclusion, and we caution against over‐interpretation. The included trial suggested a small improvement in executive functioning from six months of training, but without longer‐term follow‐up, it is not possible to determine whether the benefit is enduring and represents maintenance of cognitive function or increased brain reserve.
Quality of the evidence
We judged the included study, Corbett 2015, to have high risk of bias for all outcomes, as the imputation technique used to deal with missing outcome data is likely to yield biased estimates in the presence of the observed proportion of participants with missing data. Analyses were compromised by anomalies in the data provided for the control group. Specifically, given values for the control group were different in general cognitive training (GCT) and reasoning training (ReaCT) comparisons. Additionally, it was difficult to extrapolate data from the published material, and we had concerns regarding the imputation model.
Potential biases in the review process
We conducted a very thorough search to identify relevant trials. We searched multiple data sources for published, unpublished, and ongoing studies. We did not restrict our search by language or publication type. We attempted to avoid bias at the review level by following guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we used independent trial selection, data extraction, and quality assessment by at least two review authors. Nevertheless, our review is limited by the quality of the included trial, and overall, we have low confidence in the effect estimates reported here. Exclusion of interventions lasting less than 12 weeks ‐ a central criterion in this series of reviews ‐ led to the exclusion of 37% of identified studies (N = 123); as a result, extrapolating from these results to other computerised cognitive training (CCT) studies of shorter duration is inappropriate.
Agreements and disagreements with other studies or reviews
A limited number of trials of CCT in adults in midlife are available for comparison with our findings. Three meta‐analyses of cognitive training in cognitively healthy adults from 50 years of age into late life indicate improved performance on non‐trained measures of global cognition, executive function, and composite measures of cognitive function (Kueider 2012; Lampit 2014a; Shao 2015). However, evidence from clinical trials specifically in the midlife age range is limited, and results are contradictory. Regression analyses in Lee 2014, which we excluded because the intervention was too short, showed a positive impact of leisure physical and cognitive activities on episodic memory and executive function in adults in midlife (mean age 63), but contrary to what the reserve hypothesis would predict, employment did not have a positive influence. In contrast, Borness 2013, which we also excluded because duration of the intervention was too short, reported no benefit from training among employed adults with a mean age of 41 years.
Authors' conclusions
Our review shows that randomised controlled trials in this age cohort are too few to test the hypothesis that cognitive interventions in midlife may help to maintain cognitive function over time. No implications for practice can be drawn at this time.
The Alzheimer’s Association has recommended the development and testing of cognitive training because of its potential as an effective and accessible cognitive intervention to delay and potentially prevent clinical dementia (AA National Plan Milestone Workgroup 2014). Interventions that can be shown to have even small effects on cognition at the individual level could be important at the population level (Andrieu 2015). This review highlights the need to establish a coherent research agenda for computerised cognitive training (CCT) in midlife, which could lead to recommendations for implementation (including type, dose, duration, and intensity of training). A secondary objective would be to develop guidance and regulation codes for commercial products as ‘medical devices’ (AA National Plan Milestone Workgroup 2014).
Computerised cognitive training (CCT) interventions in midlife have the potential to maintain cognitive function via the development of brain and cognitive reserve. However, whether the introduction of CCT at midlife does in practice maintain cognitive function, reduce age‐related cognitive decline, and ultimately prevent clinical dementia remains uncertain. High‐quality trials that adhere fully to CONSORT guidance are necessary to investigate efficacy and mechanisms of interventions.
One consideration for future research with adults in midlife is how to maintain engagement in the intervention when multiple competing demands are present at this time of life, including employment, family commitments, and other leisure activities. Dropout was a significant issue in the included study. Strategies to support motivation and compliance are necessary. Entertainment or gamification and other incentives to complete training may be helpful, along with structured training times and supervision. Outcome measures should be relevant to this age cohort, especially as the idea of investing in brain health decades in the future may be too abstract and removed from current life demands. It is important to compare CCT with various levels of occupational demand, given that occupation is a primary source of cognitive stimulation in this age cohort and across the life span generally. It also would be useful to compare CCT with rest and with other leisure or recreational pursuits.
It is very important to note that studies in midlife with the long‐term objective of maintaining cognitive function and ultimately of preventing or delaying clinical dementia must include longer‐term follow‐up to reveal whether any benefits are enduring and can be expected to have effects lasting into late life, when the risk of clinically important cognitive decline increases.
Acknowledgements
The review authors would like to thank the group’s Information Specialist, Anna Noel‐Storr, for designing and running the electronic searches, and for co‐ordinating the crowd‐sourcing component of the review. This review is part of a program grant in which 11 other reviews were produced using a protocol template (Abraham 2015; Al‐Assaf 2015; Denton 2015; Forbes 2015; Forbes 2015a; Forbes 2015b; Gates 2019a; Gates 2019b; Harrison 2015; Siervo 2015; Tang 2015). All authors participating in this review also acted as authors in several other reviews. As a consequence, wording chosen in the methods section may be identical across reviews, and concepts discussed and content of reviews may be similar.
We also thank the following members of the Cochrane crowd, who made significant contributions to screening of search results: Michael J. Arnatt, Soumyadeep Bhaumik, Mª Paz Campos Pérez, C Cartlidge, Daniel Casey, Mohamed Fawzy Abdelghafar, Cristi Francis, Pishoy Gouda, Dan Griffiths, Michael Haas, Shirley Hall, Jake Hartley, Michael Hull, Geanina Ilinoiu, Deborah Jackson, Sofia Jaramillo, Robert Kemp, Ivan Murrieta Alvarez, Shireen Rafeeq, Miriam Thiel, Robin Vernooij, Jennifer Ware, and Hakan Yaman.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| ALOIS (www.medicine.ox.ac.uk/alois) [Date of most recent search: 31 March 2018] |
Basic search: COG [Studies within ALOIS are coded COG if the intervention is a cognitive‐based intervention] |
Jan 2015: 31 Jul 2015: 4 Feb 2016: 2 Jul 2016: 0 Mar 2018: 0 |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) [Date of most recent search: 31 March 2018] |
1. “cognitive stimulation”.ti,ab. 2. cognitive ADJ3 train*.ti,ab. 3. “cognitive exercis*”.ti,ab. 4. “brain train*”.ti,ab. 5. (memory adj3 train*).ti,ab. 6. “memory rehab*”.ti,ab. 7. “memory enhance*”.ti,ab. 8. “poetry‐based stimulation”.ti,ab. 9. “cognitive flexibility”.ti,ab. 10. “brain exercis*”.ti,ab. 11. “cognitive rehab*”.ti,ab. 12. “mnemonic train*”.ti,ab. 13. CST.ti,ab. 14. (mental adj3 activit*).ti,ab. 15. “cognitive intervention*”.ti,ab. 16. “cognitive motor intervention*”.ti,ab. 17. “cognition based intervention*”.ti,ab. 18. “cognitive enrich*”.ti,ab. 19. Cognitive Therapy/ mt 20. or/1‐19 21. *aging/ 22. Aged 23. “Aged, 80 and over” 24. Middle Aged 25. Age Factors 26. *Cognition/ 27. *Cognition Disorders/ 28. Memory/ 29. Memory Disorders/ 30. Brain/ 31. Mild Cognitive Impairment/ 32. Executive Function/ 33. (cognit* ADJ3 (func* OR declin* OR reduc* OR impair* OR improve* OR deficit* OR progress* 34. OR perform*)).ti,ab. 35. “mental perform*”.ti,ab. 36. memory.ti,ab. 37. “executive function*”.ti,ab. 38. MCI.ti,ab. 39. AAMI.ti,ab. 40. ACMI.ti,ab. 41. ARCD.ti,ab. 42. CIND.ti,ab. 43. (nMCI OR aMCI OR mMCI OR MCIa).ti,ab. 44. Dementia/ 45. Alzheimer Disease/ 46. dement*.ti,ab. 47. alzheimer*.ti,ab. 48. “old* age*”.ti,ab. 49. elderly.ti,ab. 50. “middle age*”.ti,ab. 51. “old*adults”.ti,ab. 52. seniors.ti,ab. 53. “senior citizens”.ti,ab. 54. “community dwelling”.ti,ab. 55. pensioners.ti,ab. 56. or/21‐55 57. randomized controlled trial.pt. 58. controlled clinical trial.pt. 59. randomized.ab. 60. placebo.ab. 61. drug therapy.fs. 62. randomly.ab. 63. trial.ab. 64. groups.ab. 65. or/57‐64 66. exp animals/ not humans.sh. 67. 65 NOT 66 68. 67 AND 56 AND 20 [all results] 69. (“cognitive stimulation” OR “cognitive training”).ti. 70. *Cognition 71. *Aging/ 72. and/69‐71 73. 72 AND 57 [‘no brainer’ results ‐ directly sent to core author team] 74. 68 NOT 73 [results minus ‘no brainer’ results ‐ for the crowd to screen] |
Jan 2015: 1455 Jul 2015: 70 Feb 2016: 303 Jul 2016: 423 Mar 2018: 489 |
| EMBASE 1974‐24 January 2018 (Ovid SP) [Date of most recent search: 31 March 2018] |
1. aging/ 2. aged/ 3. middle aged/ 4. mild cognitive impairment/ 5. elderly.ti,ab. 6. MCI.ti,ab. 7. AAMI.ti,ab. 8. ACMI.ti,ab. 9. ARCD.ti,ab. 10. CIND.ti,ab. 11. (nMCI or aMCI or mMCI or MCIa).ti,ab. 12. "old* age*".ti,ab. 13. elderly.ti,ab. 14. "middle age*".ti,ab. 15. "old* aadults".ti,ab. 16. seniors.ti,ab. 17. "senior citizens".ti,ab. 18. "community dwelling".ti,ab. 19. pensioners.ti,ab. 20. ("aged sample" or "aged population" or "older sample" or "older population").ti,ab. 21. "CDR 0.5".ti,ab. 22. (cognit* adj3 (func* or declin* or reduc* or impair* or improve* or deficit* or progress* or perform* or abilit*)).ti,ab. 23. or/1‐22 24. *cognition/ 25. memory/ or episodic memory/ 26. executive function/ 27. attention/ 28. "mental perform*".ti,ab. 29. memory.ti,ab. 30. dementia/ 31. Alzheimer disease/ 32. dement*.ti,ab. 33. alzheimer*.ti,ab. 34. or/24‐33 35. randomized controlled trial/ 36. controlled clinical trial/ 37. (randomly adj2 allocat*).ab. 38. (randomly adj2 divide*).ab. 39. randomi?ed.ab. 40. (controlled adj7 (study or design or trial)).ti,ab. 41. "double‐blind*".ti,ab. 42. "single blind*".ti,ab. 43. groups.ab. 44. or/35‐43 45. "cognitive stimulation".ti,ab. 46. (cognitive adj3 train*).ti,ab. 47. "cognitive exercis*".ti,ab. 48. "brain train*".ti,ab. 49. (memory adj3 train*).ti,ab. 50. "memory enhance*".ti,ab. 51. "memory rehab*".ti,ab. 52. "brain exercis*".ti,ab. 53. "cognitive rehab*".ti,ab. 54. "cognitive rehab*".ti,ab. 55. "mnemonic train*".ti,ab. 56. CST.ti,ab. 57. (mental adj3 activit*).ti,ab. 58. "cognitive intervention*".ti,ab. 59. "cognitive motor intervention*".ti,ab. 60. "cognition based intervention*".ti,ab. 61. "cognitive enrich*".ti,ab. 62. "reality orientation".ti,ab. 63. (memory adj2 game*).ti,ab. 64. or/45‐63 65. 23 and 34 and 44 and 64 66. ("cognitive stimulation" or "cognitive training").ti,ab. 67. cognition/ 68. (MCI or "mild cognitive impairment" or elderly or "old* adults" or "middle age*").ti. 69. 66 and 67 and 68 70. 35 and 69 71. 65 not 70 |
Jan 2015: 1289 Jul 2015: 163 Feb 2016: 380 Jul 2016: 268 Mar 2018: 640 |
| PSYCINFO 1806‐January week 2 2018 (Ovid SP) [Date of most recent search: 31 March 2018] |
1. exp Aging/ 2. exp Cognitive Impairment/ 3. "cognit* impair*".ti,ab. 4. MCI.ti,ab. 5. AAMI.ti,ab. 6. ACMI.ti,ab. 7. ARCD.ti,ab. 8. CIND.ti,ab. 9. (nMCI or aMCI or mMCI or MCIa).ti,ab. 10. "old* age*".ti,ab. 11. elderly.ti,ab. 12. "middle age*".ti,ab. 13. "old* adults".ti,ab. 14. seniors.ti,ab. 15. "senior citizens".ti,ab. 16. "community dwelling".ti,ab. 17. pensioners.ti,ab. 18. or/1‐17 19. randomi?ed.ti. 20. (randomly adj2 allocat*).ab. 21. (randomly adj2 divide*).ab. 22. RCT.ti,ab. 23. "double‐blind*".ti,ab. 24. "single blind*".ti,ab. 25. "randomi?ed trial".ab. 26. "randomi?ed control* trial".ab. 27. "random allocation".ab. 28. "controlled clinical trial".ti,ab. 29. (controlled adj4 (study or design or trial)).ti,ab. 30. or/19‐29 31. "cognitive stimulation".ti,ab. 32. (cognitive adj3 train*).ti,ab. 33. "cognitive exercis*".ti,ab. 34. "brain train*".ti,ab. 35. (memory adj3 train*).ti,ab. 36. "memory enhance*".ti,ab. 37. "memory rehab*".ti,ab. 38. "brain exercis*".ti,ab. 39. "cognitive rehab*".ti,ab. 40. "cognitive rehab*".ti,ab. 41. "mnemonic train*".ti,ab. 42. CST.ti,ab. 43. (mental adj3 activit*).ti,ab. 44. "cognitive intervention*".ti,ab. 45. "cognitive motor intervention*".ti,ab. 46. "cognition based intervention*".ti,ab. 47. "cognitive enrich*".ti,ab. 48. "reality orientation".ti,ab. 49. (memory adj2 game*).ti,ab. 50. or/31‐49 51. 18 and 30 and 50 52. *Cognition/ 53. (MCI or "mild cognitive impairment" or elderly or "old* adults" or "middle age*").ti. 54. ("cognitive stimulation" or "cognitive training").ti,ab. 55. 19 or 20 or 21 56. 52 and 53 and 54 and 55 57. 51 not 56 |
Jan 2015: 166 Jul 2015: 20 Feb 2016: 25 Jul 2016: 12 Mar 2018: 70 |
| CINAHL (EBSCOhost) [Date of most recent search: 31 March 2018] |
Jan 2015: 390 Jul 2015: 13 Feb 2016: 57 Jul 2016: 12 Mar 2018: 125 |
|
| ISI Web of Science [includes: Web of Science (1945‐present); BIOSIS Previews (1926‐present); MEDLINE (1950‐present); Journal Citation Reports]; BIOSIS Previews [Date of most recent search: 31 March 2018] |
("mild cognitive impairment" OR elderly OR "age* subjects" OR "old* adult*" OR "middle age*" OR MCI) AND TOPIC: ("randomly allocated" OR "random allocation" OR randomised OR randomized OR RCT OR "controlled trial" OR "double blind" OR "single blind") AND TOPIC: ("cognit* stim*" OR "cognit* train*" OR puzzle OR "brain train*" OR "cognit* exercis*" OR "brain exercis*" OR "memory exercis*" OR "brain gam*" OR "cognit* gam*" OR "memory gam*" OR sudoku OR crossword* OR "reality orientation") AND TOPIC: (cognition OR dementia OR memory OR "executive function" OR alzheimer*) Timespan: All years. Search language=Auto |
Jan 2015: 333 Jul 2015: 44 Feb 2016: 108 Jul 2016: 35 Mar 2018: 268 |
| LILACS (BIREME) [Date of most recent search: 31 March 2018] |
Jan 2015: 4 Jul 2015: 0 Feb 2016: 0 Jul 2016: 0 Mar 2018: 0 |
|
| CENTRAL (via CRSO) [Date of most recent search: 31 March 2018] |
#1 MeSH descriptor: [Aged, 80 and over] explode all trees #2 MeSH descriptor: [Aged] explode all trees #3 MeSH descriptor: [Middle Aged] explode all trees #4 MeSH descriptor: [Mild Cognitive Impairment] explode all trees #5 "cognit* impair*" or MCI #6 elderly #7 "old* adults" #8 "old* age*" #9 "old* sample" #10 senior citizens #11 pensioners #12 seniors #13 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 #14 MeSH descriptor: [Cognition] explode all trees #15 MeSH descriptor: [Dementia] explode all trees #16 cognit* #17 memory #18 "executive function*" #19 processing #20 "mental perform*" #21 dement* #22 alzheimer* #23 #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 #24 "cognitive stimulation" #25 "cognitive training" #26 "brain train*" #27 "brain gam*" #28 "memory train*" or "memory game*" #29 puzzle* #30 crossword* #31 sudoku* #32 "mental game*" #33 "mental agil*" #34 "cognitive exercis*" #35 "mental exercis*" #36 #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 #37 #13 and #23 and #36 |
Jan 2015: 274 Jul 2015: 11 Feb 2016: 57 Jul 2016: 4 Mar 2018: 125 |
| Clinicaltrials.gov (www.clinicaltrials.gov) [Date of most recent search: 31 March 2018] |
Jan 2015: 17 Jul 2015: 4 Feb 2016: 2 Jul 2016: 0 Mar 2018: 4 |
|
| ICTRP Search Portal (http://apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] [Date of most recent search: 31 March 2018] |
Jan 2015: 22 Jul 2015: 3 Feb 2016: 1 Jul 2016: 0 Mar 2018: 4 |
|
| TOTAL before de‐duplication | Jan 2015: 3981 Jul 2015: 332 Feb 2016: 935 Jul 2016: 754 Mar 2018: 1725 TOTAL: 7727 |
|
| TOTAL after de‐duplication | TOTAL: 5832 | |
| TOTAL after first assessment by the Crowd and CDCIG Information Specialists | Jan 2015: 604Jul 2015: 60 Feb 2016: 164 Jul 2016: 73 Mar 2018: 189 TOTAL: 1090 |
|
Appendix 2. Definitions of design, participant and intervention characteristics for use in the stratified analyses exploring between‐trial variations in intervention effects
| Item | Definition |
| Design‐related characteristicsa | |
| Concealment of allocation (avoiding selection bias) | Guidance from the Cochrane Handbook for Systematic Reviews of Interventions will be used to judge bias related to sequence generation and concealment of allocation using the 2 Cochrane 'Risk of bias' items (Higgins 2011). From these, the statistician will derive a single variable to be used in the stratified analysis: allocation concealment will be judged at low risk of bias if the investigators responsible for patient selection were unable to suspect, before allocation, which treatment was next. Concealment will be downgraded to high risk of bias if there is evidence of inadequate sequence generation (Rutjes 2012) |
| Blinding of patients and personnel (avoiding performance bias) | Low risk of bias will be judged if:
|
| Blinding of outcome assessment (avoiding detection bias) |
For self‐reported/partner‐reported outcomes Low risk of bias will be judged if:
For other outcomes
|
| Statistical analyses (avoiding attrition bias) |
For continuous outcomes Low risk of bias will be judged if:
For binary outcomes of rare events Low risk of bias will be judged if:
For binary outcomes of non‐rare events Low risk of bias will be judged if:
|
| Trial size | The cut‐off to distinguish small from larger trials will be determined by a sample size calculation on the primary outcome |
| Publication status | Full journal article vs other type or unpublished material |
| Follow‐up duration | For cognitive outcomes, we will group studies according to these follow‐up cut‐offs to describe immediate (up to 12 weeks), short‐term (up to 1 year), medium‐term (1 to 2 years) and longer‐term results (more than 2 years) |
| Treatment‐related characteristics | |
| Treatment and control Treatment dose and duration |
Analyses will be stratified by:
Analyses will be stratified into session length > 30 minutes, frequency > 3 sessions per week, and total number of sessions. These cut‐offs are based upon previous findings (Lampit 2014a). Minimum treatment duration of 3 months is considered short‐term, 3 to 12 months as medium‐term, and 12 months as long‐term |
| Cognition and participant‐related criteria | Gender, level of education (in years) |
| aThe descriptions depicted in this table added to the guidance provided by Cochrane (Higgins 2011). Stratified analyses were performed only for the primary outcome, if about 10 randomised controlled trials contributed to the analyses. | |
Data and analyses
Comparison 1.
CCI versus control
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Episodic memory, 6 months of follow‐up | 1 | 3090 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.10, 0.04] |
| 2 Executive functioning, 6 months of follow‐up | 1 | 3994 | Mean Difference (IV, Fixed, 95% CI) | ‐1.57 [‐1.85, ‐1.29] |
| 3 Working memory, 6 months of follow‐up | 1 | 5831 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.03, 0.15] |
Differences between protocol and review
The protocol referred throughout to 'cognition‐based interventions'. It was subsequently agreed that the widely used term 'cognitive training' accurately described the interventions of interest and was preferred.
Due to the lack of trials, we could not perform any of the planned stratified analyses by trial, participant, and intervention to explore between‐trial heterogeneity (see also Appendix 2). Neither could we perform the protocol‐defined funnel plot analyses or sensitivity analyses.
Before we published our protocol, we decided to use a hierarchy to select instruments for which we would analyse outcome data in the event of an outcome being assessed with more than one instrument or scale. As the hierarchy was being developed, it was not yet described in the protocol. However, the hierarchy was established before the start of data extraction for this review and the other two reviews related to this topic (Gates 2019a; Gates 2019b)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Judgment: random sequence adequately generated Quote(s): "Participants were randomly assigned in equal proportions via simple randomization to receive ReaCT, GCT, or control. This was achieved by using a computer‐generated randomization sequence to eliminate allocation bias" |
| Allocation concealment (selection bias) | Low risk |
Judgment: adequate method of allocation concealment Quote(s): "The online format enabled complete allocation concealment from investigators" |
| Blinding of participants (performance bias) | Low risk |
Judgment: Study authors report that participants were blinded to treatment assigned Quote(s): "Participants were blind to which group they were allocated"; "This was a double‐blind 6‐month online randomised 3‐arm controlled trial" |
| Blinding of personnel (performance bias) | Low risk |
Judgment: home‐based; no involvement of therapists Quote(s): "The online format enabled complete allocation concealment from investigators" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Judgment: outcomes based on computer tests Quote(s): "The online format enabled complete allocation concealment from investigators" |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Judgment: we judged high risk of bias for all outcomes, as the imputation technique (last observation carried forward) is likely to yield biased estimates in the presence of observed fractions of participants with missing outcome data at 6 months Comparison ReaCT reasoning and planning vs computerised tasks
Comparison GCT multi‐domain vs computerised tasks
Comment: no data at 12 weeks were available for extraction Quote(s): "The primary analysis was intention‐to‐treat and involved all participants who were randomized"; "Missing values were imputed by last observation carried forward for the 6‐month outcome for individuals who completed the 3‐month outcome assessment" |
| Selective reporting (reporting bias) | Low risk | Judgment: all outcomes indicated in the methods are reported in the results |
| Other bias | Low risk | Judgment: no other sources of bias are important |
APOE: apolipoprotein E.
CT: computerised training.
GCT: general cognitive training.
IADL: instrumental activity of daily living.
PG: postgraduate.
ReaCT: reasoning training.
SD: standard deviation.
SWM: spatial working memory.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adel 2013 | Wrong study design |
| Alves 2014 | Wrong intervention |
| Alves 2014a | Wrong intervention |
| Anderson 2014 | Intervention shorter than 12 weeks |
| Ann 2012 | Wrong patient population |
| Anon 2007 | Nature of intervention unclear |
| Anon 2007a | Nature of intervention unclear |
| Apostolo 2014 | Wrong patient population |
| Baglio 2011 | Nature of intervention unclear |
| Ball 2002 | Intervention shorter than 12 weeks |
| Ball 2002a | Duplicate |
| Ball 2006 | Intervention shorter than 12 weeks |
| Ball 2013 | Intervention shorter than 12 weeks |
| Ballesteros 2014 | Duplicate |
| Ballesteros 2014a | Duplicate |
| Ballesteros 2015 | Duplicate |
| Ballesteros 2015a | Duplicate |
| Ballesteros 2017 | Intervention shorter than 12 weeks |
| Bamidis 2015 | Wrong study design |
| Baniqued 2014 | Adult population |
| Baniqued 2015 | Aged under 30 |
| Barban 2012 | Duplicate |
| Barban 2016 | Wrong study design |
| Barbosa 2015 | Wrong intervention |
| Barcelos 2015 | Wrong intervention |
| Barnes 2006 | Intervention shorter than 12 weeks |
| Barnes 2009 | Duplicate |
| Barnes 2013 | Wrong patient population |
| Basak 2016 | Intervention shorter than 12 weeks |
| Beck 2013 | Wrong intervention |
| Belchior | Wrong outcomes |
| Belchior 2008 | Wrong outcomes |
| Belleville 2006 | Wrong intervention |
| Belleville 2014 | Wrong outcomes |
| Berry 2010 | Intervention shorter than 12 weeks |
| Bier 2015 | Wrong study design |
| Binder 2016 | Intervention shorter than 12 weeks |
| Bittner 2013 | Wrong study design |
| Borella 2010 | Intervention shorter than 12 weeks |
| Borella 2013 | Wrong intervention |
| Borella 2014 | Duplicate |
| Borella 2017 | Wrong intervention |
| Boripuntakul 2012 | Wrong intervention |
| Borness 2013 | Wrong study population: mean age is 41.3 years (SD 13.1), meaning that 46% were younger than 40 years (assuming a normal distribution of age) |
| Bottiroli 2009 | Duplicate |
| Bottiroli 2009a | Intervention shorter than 12 weeks |
| Bozoki 2013 | Intervention shorter than 12 weeks |
| Brehmer 2012 | Intervention shorter than 12 weeks |
| Brum 2013 | Duplicate |
| Buitenweg 2017 | Wrong intervention |
| Buiza 2008 | Wrong intervention |
| Bures 2016 | Intervention shorter than 12 weeks |
| Buschert 2011 | Wrong intervention |
| Buschert 2011a | Duplicate |
| Buschert 2012 | Wrong intervention |
| Buschert 2012a | Duplicate |
| Calkins 2011 | Wrong intervention |
| Cammarata 2011 | No outcome given |
| Cancela 2015 | Wrong patient population |
| Candela 2015 | Wrong intervention |
| Cantarella 2017 | Intervention shorter than 12 weeks |
| Cao 2016 | Wrong route of administration |
| Carretti 2013 | Wrong intervention |
| Casutt 2014 | Wrong outcomes |
| Chapman 2015 | Wrong intervention |
| Chapman 2016 | Wrong intervention |
| Chapman 2017 | Wrong intervention |
| Cheng 2012 | Wrong intervention |
| Cheng 2018 | Wrong patient population |
| Cho 2002 | Aged under 30 |
| Cleverley 2012 | Wrong intervention |
| Cohen‐Mansfield 2014 | Wrong intervention |
| Cohen‐Mansfield 2014a | Wrong intervention |
| Cohen‐Mansfield 2015 | Wrong intervention |
| Cohen‐Mansfield 2015a | Duplicate |
| Combourieu 2014 | Wrong outcomes |
| Costa 2015 | Wrong patient population |
| Danassi 2015 | Duplicate |
| Dannhauser 2014 | Wrong study design |
| de Almondes 2017 | Intervention shorter than 12 weeks |
| de Macedo 2015 | Wrong outcomes |
| De Vreese 1996 | Wrong intervention |
| Desjardins‐Crépeau 2016 | Wrong patient population |
| Diamond 2015 | Intervention shorter than 12 weeks |
| Dittmann‐Kohli 1991 | Wrong intervention |
| Djabelkhir 2017 | Wrong patient population |
| Duncan 2009 | Wrong intervention |
| Dwolatzky 2005 | Intervention shorter than 12 weeks |
| Eckroth‐Bucher 2009 | Wrong patient population |
| Edwards 2005 | Intervention shorter than 12 weeks |
| Edwards 2011 | Intervention shorter than 12 weeks |
| Edwards 2015 | Intervention shorter than 12 weeks |
| Edwards 2015a | Intervention shorter than 12 weeks |
| Efthymiou 2011 | Wrong comparator |
| Engvig 2014 | Wrong study design |
| Fabre 2002 | Wrong intervention |
| Faille 2007 | Nature of intervention unclear |
| Fairchild 2010 | Wrong intervention |
| Feng 2013 | Wrong intervention |
| Feng 2015 | Wrong intervention |
| Feng 2017 | Wrong patient population |
| Fiatrone Singh 2014 | Wrong patient population |
| Finn 2011 | Intervention shorter than 12 weeks |
| Finn 2015 | Intervention shorter than 12 weeks |
| Finn 2015a | Duplicate |
| Flak 2013 | Study protocol |
| Flak 2014 | Study protocol |
| Flak 2014a | Study protocol |
| Flak 2016 | Study protocol |
| Foerster 2009 | No outcome given |
| Forloni 2012 | No outcome given |
| Forster 2011 | Wrong intervention |
| Fortman 2013 | Wrong comparator |
| Gagnon 2012 | Wrong study design |
| Gagnon 2012a | Intervention shorter than 12 weeks |
| Gaitan 2013 | Wrong patient population |
| Gajewski 2012 | Intervention shorter than 12 weeks |
| Gajewski 2017 | Intervention shorter than 12 weeks |
| Garcia‐Campuzano 2013 | Nature of intervention unclear |
| Gates 2011 | Study protocol |
| Gill 2016 | Wrong intervention |
| Gillette 2009 | No outcome given |
| Giovannini 2015 | No outcome given |
| Giuli 2016 | Wrong intervention |
| Giuli 2017 | Wrong intervention |
| Golino 2017 | Wrong intervention |
| Gooding 2016 | Wrong patient population |
| Haesner 2015 | Wrong study design |
| Haesner 2015a | Intervention shorter than 12 weeks |
| Haimov 2013 | Intervention shorter than 12 weeks |
| Haimov 2013a | Intervention shorter than 12 weeks |
| Haimov 2013b | Intervention shorter than 12 weeks |
| Haimov 2013c | Duplicate |
| Haimov 2013d | Intervention shorter than 12 weeks |
| Haimov 2014 | Intervention shorter than 12 weeks |
| Haimov 2014a | Intervention shorter than 12 weeks |
| Hardy 2015 | Intervention shorter than 12 weeks |
| Hausmann 2012 | Wrong intervention |
| Hayashi 2012 | Wrong intervention |
| Hayslip B Jr 2016 | Intervention shorter than 12 weeks |
| Heinzel 2014 | Intervention shorter than 12 weeks |
| Herrera 2012 | Wrong patient population |
| Hudak 2013 | Intervention shorter than 12 weeks |
| Hötting 2013 | Intervention shorter than 12 weeks |
| Ignjatovic 2015 | Aged under 30 |
| Irigaray 2012 | Wrong intervention |
| Israel 1997 | Nature of intervention unclear |
| ISRCTN70130279 | Wrong intervention |
| Jackson 2012 | Nature of intervention unclear |
| Jansen 2012 | Wrong intervention |
| Jean 2010 | Intervention shorter than 12 weeks |
| Jeong 2016 | Wrong intervention |
| Jobe 2001 | Intervention shorter than 12 weeks |
| Jones 2013 | Intervention shorter than 12 weeks |
| Kampanaros 2010 | Wrong intervention |
| Kholin 2010 | Intervention shorter than 12 weeks |
| Kim 2012 | Wrong outcomes |
| Kim 2013 | Intervention shorter than 12 weeks |
| Kim 2013a | Wrong outcomes |
| Kim 2015 | Intervention shorter than 12 weeks |
| Kim 2015a | Intervention shorter than 12 weeks |
| Kim 2015b | Duplicate |
| Kivipelto 2014 | Wrong intervention |
| Klusmann 2009 | Duplicate |
| Klusmann 2010 | Wrong patient population |
| Klusmann 2010a | Duplicate |
| Klusmann 2011 | Aged under 30 |
| Kudelka 2014 | Intervention shorter than 12 weeks |
| Kwak 2015 | Nature of intervention unclear |
| Kwak 2017 | Nature of intervention unclear |
| Kwok 2013 | Intervention shorter than 12 weeks |
| Kwok 2013a | Intervention shorter than 12 weeks |
| Lampit 2013 | Wrong study design |
| Lampit 2014 | Wrong patient population |
| Lampit 2015 | Wrong patient population |
| Lavretsky 2016 | Nature of intervention unclear |
| Law 2014 | Intervention shorter than 12 weeks |
| Law 2014a | Duplicate |
| Lee 2013 | Intervention shorter than 12 weeks |
| Lee 2013a | Intervention shorter than 12 weeks |
| Lee 2013b | Intervention shorter than 12 weeks |
| Lee 2014 | Intervention shorter than 12 weeks |
| Lee 2015 | Intervention shorter than 12 weeks |
| Legault 2011 | Wrong patient population |
| Leung 2015 | Wrong patient population |
| León 2015 | Wrong comparator |
| Li 2010 | Intervention shorter than 12 weeks |
| Linde 2014 | Nature of intervention unclear |
| Mace 2015 | Intervention shorter than 12 weeks |
| Mahncke 2006 | Intervention shorter than 12 weeks |
| Man 2012 | Wrong comparator |
| Mann 2012 | Wrong patient population |
| Margrett 2006 | Wrong patient population |
| Mayas 2014 | Intervention shorter than 12 weeks |
| McAvinue 2013 | Intervention shorter than 12 weeks |
| McDaniel 2014 | Intervention shorter than 12 weeks |
| McDougall 2012 | Intervention shorter than 12 weeks |
| Middleton 2012 | Wrong intervention |
| Miller 2013 | Intervention shorter than 12 weeks |
| Mohs 1998 | Wrong intervention |
| Mombelli 2012 | No outcome given |
| Moon 2013 | Intervention shorter than 12 weeks |
| Mowszowski 2014 | Intervention shorter than 12 weeks |
| Mowszowski 2014a | Duplicate |
| Mozolic 2010 | Intervention shorter than 12 weeks |
| Mozolic 2011 | Intervention shorter than 12 weeks |
| Muller 2011 | Nature of intervention unclear |
| Na 2013 | Duplicate |
| Na 2014 | Nature of intervention unclear |
| Naismith 2014 | Duplicate |
| Navarro 2006 | Intervention shorter than 12 weeks |
| NCT02417558 2015 | Nature of intervention unclear |
| NCT02462135 2014 | No outcome given |
| NCT02480738 2012 | No outcome given |
| NCT02512627 2015 | No outcome given |
| NCT02747784 2016 | Wrong patient population |
| NCT02774083 2015 | Wrong comparator |
| NCT02785315 2016 | Wrong intervention |
| NCT02808676 2016 | Wrong intervention |
| Neely 2013 | Nature of intervention unclear |
| Ng 2015 | Wrong intervention |
| Ngandu 2015 | Wrong intervention |
| Ngandu 2015a | Wrong intervention |
| Nishiguchi 2015 | Wrong intervention |
| Nouchi 2012 | Intervention shorter than 12 weeks |
| Nouchi 2013 | Intervention shorter than 12 weeks |
| Nozawa 2015 | Intervention shorter than 12 weeks |
| O'Caoimh 2015 | Intervention shorter than 12 weeks |
| Oei 2013 | Intervention shorter than 12 weeks |
| Oliveira 2013 | Intervention shorter than 12 weeks |
| Optale 2010 | Wrong patient population |
| Otsuka 2015 | Wrong study design |
| Park 2009 | Nature of intervention unclear |
| Park 2014 | Intervention shorter than 12 weeks |
| Payne 2012 | Wrong intervention |
| Payne 2017 | Intervention shorter than 12 weeks |
| Peretz 2011 | Wrong patient population |
| Rahe 2015 | Intervention shorter than 12 weeks |
| Rahe 2015a | Intervention shorter than 12 weeks |
| Rebok 2013 | Intervention shorter than 12 weeks |
| Rebok 2014 | Intervention shorter than 12 weeks |
| Redick 2013 | Aged under 30 |
| Requena 2016 | Wrong intervention |
| Rizkalla 2015 | Intervention shorter than 12 weeks |
| Rojas 2013 | Wrong intervention |
| Rose 2015 | Intervention shorter than 12 weeks |
| Rosen 2011 | Intervention shorter than 12 weeks |
| Rozzini 2007 | Wrong patient population |
| Ryu 2013 | Wrong study design |
| Sakka 2015 | Wrong study design |
| Santos 2011 | Wrong comparator |
| Schoene 2015 | Duplicate |
| Schoene 2015a | Duplicate |
| Schumacher 2013 | Intervention shorter than 12 weeks |
| Shah 2012 | Wrong patient population |
| Shatil 2013 | Wrong patient population |
| Shatil 2014 | Intervention shorter than 12 weeks |
| Shatil 2014a | Duplicate |
| Sisco 2013 | Intervention shorter than 12 weeks |
| Slegers 2009 | Wrong intervention |
| Smith 2009 | Intervention shorter than 12 weeks |
| Smith‐Ray 2014 | Intervention shorter than 12 weeks |
| Smith‐Ray 2015 | Intervention shorter than 12 weeks |
| Smith‐Ray 2015a | Duplicate |
| Solomon 2014 | Wrong comparator |
| Song 2009 | Wrong intervention |
| Stepankova 2014 | Intervention shorter than 12 weeks |
| Stine‐Morrow 2014 | Intervention shorter than 12 weeks |
| Strenziok 2013 | Duplicate |
| Strenziok 2014 | Intervention shorter than 12 weeks |
| Sturz 2011 | Wrong patient population |
| Sturz 2011a | Nature of intervention unclear |
| Sturz 2015 | Duplicate |
| Styliadis 2015 | Intervention shorter than 12 weeks |
| Styliadis 2015a | Duplicate |
| Suo 2012 | Wrong outcomes |
| Szelag 2012 | Intervention shorter than 12 weeks |
| Talib 2008 | Intervention shorter than 12 weeks |
| Tappen 2014 | Wrong intervention |
| Tennstedt 2013 | Study protocol |
| Tesky 2012 | Wrong intervention |
| Tsai 2008 | Duplicate |
| Tsolaki 2013 | Nature of intervention unclear |
| Tucker‐Drob 2009 | Wrong study design |
| van den Berg 2016 | Intervention shorter than 12 weeks |
| van der Ploeg 2016 | Wrong study design |
| Van het Reve 2014 | Wrong patient population |
| Vance 2007 | Intervention shorter than 12 weeks |
| Vidovich 2009 | Intervention shorter than 12 weeks |
| Vidovich 2015 | Intervention shorter than 12 weeks |
| Vidovich 2015a | Duplicate |
| von Bastian 2013 | Intervention shorter than 12 weeks |
| Wadley 2007 | Wrong study design |
| Walton 2015 | Intervention shorter than 12 weeks |
| Wang 2013 | Wrong intervention |
| Weicker 2013 | Intervention shorter than 12 weeks |
| Wild‐Wall 2012 | Wrong outcomes |
| Williams 2014 | Intervention shorter than 12 weeks |
| Willis 1986 | Intervention shorter than 12 weeks |
| Willis 2006 | Intervention shorter than 12 weeks |
| Willis 2006a | Duplicate |
| Willis 2007 | Intervention shorter than 12 weeks |
| Willis 2013 | Intervention shorter than 12 weeks |
| Wojtynska 2011 | Intervention shorter than 12 weeks |
| Wolinsky 2006 | Intervention shorter than 12 weeks |
| Wolinsky 2006a | Intervention shorter than 12 weeks |
| Wolinsky 2010 | Intervention shorter than 12 weeks |
| Wolinsky 2010a | Intervention shorter than 12 weeks |
| Wolinsky 2013 | Intervention shorter than 12 weeks |
| Wolinsky 2015 | Intervention shorter than 12 weeks |
| Yam 2014 | Wrong intervention |
| Yassuda 2015 | Intervention shorter than 12 weeks |
| Yip 2012 | Intervention shorter than 12 weeks |
| Yoonmi 2012 | Intervention shorter than 12 weeks |
| Youn 2011 | Intervention shorter than 12 weeks |
| Zelinski 2011 | Wrong study design |
| Zelinski 2011a | Intervention shorter than 12 weeks |
| Zhuang 2013 | Wrong patient population |
| Zimmermann 2014 | Intervention shorter than 12 weeks |
Contributions of authors
Completion of the protocol: Nicola Gates, Anne Rutjes, Salman Karim, Jennifer Ware, Lee Yee Chong, Robin Vernooij. Screening of references: Students 4 Best Evidence (title/abstract screening), Nicola Gates, Salman Karim. Acquisition of data: Nicola Gates, Anne Rutjes, Marcello Di Nisio, Salman Karim, Evrim March, Robin Vernooij. 'Risk of bias' assessments: Nicola Gates, Anne Rutjes, Marcello Di Nisio, Salman Karim, Robin Vernooij. SoF and GRADE‐ing: Robin Vernooij. Statistical analysis: Anne Rutjes Overall interpretation of data: Nicola Gates, Anne Rutjes, Marcello Di Nisio, Salman Karim, Evrim March, Gabriel Martínez, Robin Vernooij. Manuscript preparation: Nicola Gates, Anne Rutjes, Gabriel Martínez, Robin Vernooij.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
This protocol was supported by the (NIHR), via a Cochrane Programme Grant to the Cochrane Dementia and Cognitive Improvement Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
-
SERI and Horizon 2020, Other.
The authors AR and MdN are partially funded by a grant for the project ‘OPERAM: OPtimising therapy to prevent Avoidable hospital admissions in the Multi‐morbid elderly’ supported by the European Union’s Horizon 2020 research and innovation programme under the grant agreement No 6342388, and by the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract number 15.0137. The opinions expressed and arguments employed herein are those of the authors and do not necessarily reflect the official views of the EC and the Swiss government.
Declarations of interest
Nicola J Gates ‐ none known Anne WS Rutjes ‐ Dr. Rutjes declares partial funding by a grant for the project 'OPERAM: OPtimising therapy to prevent Avoidable hospital admissions in the Multi‐morbid elderly' supported by the European Union's Horizon 2020 research and innovation programme under the grant agreement No 6342388, and by the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract number 15.0137. Marcello Di Nisio ‐ Di Nisio declares partial funding by a grant for the project 'OPERAM: OPtimising therapy to prevent Avoidable hospital admissions in the Multi‐morbid elderly' supported by the European Union's Horizon 2020 research and innovation programme under the grant agreement No 6342388. Di Nisio reports participation to Advisory Boards for Daiichi‐Sankyo, Aspen, and Pfizer, and consultancy fees for Daiichi‐Sankyo, Bayer Health Care, and Leo Pharma outside the submitted work. Salman Karim ‐ none known Lee‐Yee Chong ‐ none known Evrim March ‐ none known Gabriel Martínez ‐ none known Robin WM Vernooij ‐ none known
New
References
References to studies included in this review
- Corbett A, Owen A, Hampshire A, Grahn J, Stenton R, Dajani S, et al. The Effect of an Online Cognitive Training Package in Healthy Older Adults: An Online Randomized Controlled Trial. Journal of the American Medical Directors Association 2015;16(11):990‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Adel D, Boulanouar K, Chauveau N, Delrieu J, Voisin T, Vellas B, et al. Structural MRI and FDG‐PET modifications induced by one year multi‐domain intervention in elderly. Conference: 26th Annual Congress of the European Association of Nuclear Medicine, EANM 2013 Lyon France. 2013; Vol. Conference Start: 20131019 Conference End: 20131023:S208. [Google Scholar]
- Apóstolo JL, Cardoso DF, Rosa AI, Paúl C. The effect of cognitive stimulation on nursing home elders: a randomized controlled trial. Journal of Nursing Scholarship 2014;46(3):157‐66. [DOI] [PubMed] [Google Scholar]
- Alves J, Alves‐Costa F, Magalhães R, Gonçalves OF, Sampaio A. Cognitive stimulation for Portuguese older adults with cognitive impairment: a randomized controlled trial of efficacy, comparative duration, feasibility, and experiential relevance. American Journal of Alzheimer's Disease and Other Dementias 2014;29(6):503‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, White‐Schwoch T, Choi HJ, Kraus N. Partial maintenance of auditory‐based cognitive training benefits in older adults. Neuropsychologia 2014;62:286‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ann B, Eva E, Siv S, Elisabeth A. Effects of working memory training on functioning in daily life. Conference: 9th Annual Conference of the Special Interest Group in Neuropsychological Rehabilitation of the World Federation for NeuroRehabilitation, WFNR 2012 Bergen Norway. 2012;Conference Start: 20120702 Conference End: 20120703:182. [Google Scholar]
- Anon. Randomized prospective cognitive training study on elderly Japanese in Osaka. [unknown] 2007;[unknown]:[unknown]. [Google Scholar]
- Anon. Effects of a complex cognitive training in mild cognitive impairment and mild Alzheimer's disease. [unknown] 2007;[unknown]:[unknown]. [Google Scholar]
- Apóstolo JL, Cardoso DF, Rosa AI, Paúl C. The effect of cognitive stimulation on nursing home elders: a randomized controlled trial. Journal of Nursing Scholarship 2014;46(3):157‐66. [DOI] [PubMed] [Google Scholar]
- Baglio F, Griffanti L, Preti MG, Lagana MM, Alberoni M, Critelli R, et al. Cognitive training in outpatients affected by mild cognitive impairment: a longitudinal study with fMRI. Conference: 6th Sindem Meeting: Italian Association for the Study of Dementia linked to the Italian Neurological Society, SIN Milan Italy. 2011;Conference Start: 20110317 Conference End: 20110319.(var.pagings):S47‐8. [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. Journal of the American Medical Association 2002;288(18):2271‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults ‐ A randomized controlled trial. Journal of the American Medical Association 2002;288(18):2271‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Unverzagt F, Rebok G, Morris J, Tennstedt SL, Marsiske M. ACTIVE: advanced cognitive training for independent and vital elderly. NCT002985582006.
- Ball KK, Ross LA, Roth DL, Edwards JD. Speed of processing training in the ACTIVE study: how much is needed and who benefits?. Journal of Aging and Health 2013;25(8):65S‐84S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros S, Prieto A, Mayas J, Toril P, Pita C, Ponce de León L, et al. Brain training with non‐action video games enhances aspects of cognition in older adults: a randomized controlled trial. Frontiers in Aging Neuroscience 2014;6:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros S, Prieto A, Mayas J, Toril P, Pita C, Ponce de León L, et al. Brain training with non‐action video games enhances aspects of cognition in older adults: a randomized controlled trial. Frontiers in Aging Neuroscience. 2014;6:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros S, Mayas J, Prieto A, Toril P, Pita C, Laura Pde L, et al. A randomized controlled trial of brain training with non‐action video games in older adults: results of the 3‐month follow‐up. Frontiers in Aging Neuroscience 2015;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros S, Prieto A, Mayas J, Toril P, Pita C, Ponce de León L, et al. Corrigendum: brain training with non‐action video games enhances aspects of cognition in older adults: a randomized controlled trial. Frontiers in Aging Neuroscience 2015;7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros S, Mayas J, Prieto A, Ruiz‐Marquez E, Toril P, Reales JM. Effects of Video Game Training on Measures of Selective Attention and Working Memory in Older Adults: Results from a Randomized Controlled Trial. Frontiers in Aging Neuroscience 2017;9:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamidis PD, Fissler P, Papageorgiou SG, Zilidou V, Konstantinidis EI, Billis AS, et al. Gains in cognition through combined cognitive and physical training: the role of training dosage and severity of neurocognitive disorder. Frontiers in Aging Neuroscience 2015;7:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniqued PL, Kranz MB, Voss MW, Lee H, Cosman JD, Severson J, et al. Cognitive training with casual video games: points to consider. Frontiers in Psychology 2014;4:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniqued PL, Allen CM, Kranz MB, Johnson K, Sipolins A, Dickens C, et al. Working memory, reasoning, and task switching training: transfer effects, limitations, and great expectations?. PLoS One 2015;10(11):e0142169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barban F, Annicchiarico R, Perri R, Fadda L, Carlesimo GA, Pantelopoulos S, et al. Randomized clinical trial of a computer‐based cognitive treatment for healthy elderly, clinical and preclinical Alzheimer's disease. the SOCIABLE project. Conference: 7th Congresso Sindem: Italian Association for the Study of Dementia Linked to the Italian Neurological Society, SIN Naples Italy. 2012; Vol. Conference Start: 20120322. Conference End: 20120324:101. [Google Scholar]
- Barban F, Annicchiarico R, Pantelopoulos S, Federici A, Perri R, Fadda L, et al. Protecting cognition from aging and Alzheimer's disease: a computerized cognitive training combined with reminiscence therapy. International Journal of Geriatric Psychiatry 2016;31(4):340‐8. [DOI] [PubMed] [Google Scholar]; Barban F, Annicchiarico R, Pantelopoulos S, Federici A, Perri R, Fadda L, et al. Protecting cognition from aging and alzheimer's disease: a computerized cognitive training combined with reminiscence therapy. International Journal of Geriatric Psychiatry 2016;31(4):340‐8. [DOI] [PubMed] [Google Scholar]; Zaccarelli C, Benati G, Boschi R, Barban F, Annicchiarico R, Lymperopoulou O. Computer‐based cognitive training for dementia. Results from a randomized controlled trial on MCI, mild AD and healthy ageing. Journal of Alzheimer's Disease 2016;S56:52. [Google Scholar]; Zaccarelli C, Cirillo G, Passuti S, Annicchiarico R, Barban F. Computer‐based cognitive intervention for dementia: sociable motivating platform for elderly networking, mental reinforcement and social interaction. Proceedings of the 2013 7th international conference on pervasive computing technologies for healthcare and workshops (Pervasive Health 2013). 2013:430‐5. [Google Scholar]
- Barbosa AR, Guimaraes AV. Effects of exergames on cognitive performance and functional fitness in older adults: a pilot study. Conference: 2015 Annual Scientific Meeting of the American Geriatrics Society National Harbor, MD United States. 2015; Vol. Conference Start: 20150515. Conference End: 20150517:S176. [Google Scholar]
- Barcelos N, Shah N, Cohen K, Hogan MJ, Mulkerrin E, Arciero PJ, et al. Aerobic and cognitive exercise (ACE) pilot study for older adults: executive function improves with cognitive challenge while exergaming. Journal of the International Neuropsychological Society 2015;SI(10):768‐79. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Belfor N, Jagust WJ, DeCarli C, Reed BR, et al. Computer‐based cognitive training for mild cognitive impairment: results from a pilot randomized controlled trial. Alzheimer Disease and Associated Disorders 2006;66(5):A249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Belfor N, Jagust WJ, DeCarli C, Reed BR, et al. Computer‐based cognitive training for mild cognitive impairment: results from a pilot randomized, controlled trial. Alzheimer Disease and Associated Disorders 2009;23(3):205‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barness De, Santos‐Modesitt W, Poelke G, Kramer AF, Castro C, Middleton LE, et al. The Mental Activity and eXercise (MAX) trial: a randomized controlled trial to enhance cognitive function in older adults. JAMA Internal Medicine 2013;173(9):797‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak C, O'Connell MA. To switch or not to switch: role of cognitive control in working memory training in older adults. Frontiers in Psychology 2016;7:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C, Fausett JK, Krukowski RA, Cornell CE, Prewitt TE, Lensing S, et al. A randomized trial of a community‐based cognitive intervention for obese senior adults. Journal of Aging and Health 2013;25(1):97‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belchior PD. Cognitive training with video games to improve driving skills and driving safety among older adults. University of Florida 2007;Thesis:209. [Google Scholar]
- Belchior PD. Cognitive training with video games to improve driving skills and driving safety among older adults. Thesis 2008;68(9‐B):5897. [Google Scholar]
- Belleville S, Gilbert B, Fontaine F, Gagnon L, Manard E, Gauthier S. Improvement of episodic memory in persons with mild cognitive impairment and healthy older adults: evidence from a cognitive intervention program. Dementia and Geriatric Cognitive Disorders 2006;22(5‐6):486‐99. [DOI] [PubMed] [Google Scholar]
- Belleville S, Mellah S, Boysson C, Demonet JF, Bier B. The pattern and loci of training‐induced brain changes in healthy older adults are predicted by the nature of the intervention. PLoS One 2014;9(8):e102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Zanto TP, Clapp WC, Hardy JL, Delahunt PB, Mahncke HW, et al. The influence of perceptual training on working memory in older adults. PLoS One 2010;5(7):e11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bier N, Grenier S, Brodeur C, Gauthier S, Gilbert B, Hudon C, Lepage E, et al. Measuring the impact of cognitive and psychosocial interventions in persons with mild cognitive impairment with a randomized single‐blind controlled trial: rationale and design of the MEMO plus study. International Psychogeriatrics 2015;27(3):511‐25. [DOI] [PubMed] [Google Scholar]
- Binder JC, Martin M, Zollig J, Rocke C, Merillat S, Eschen A, et al. Multi‐domain training enhances attentional control. Psychology and Aging 2016;31(4):390‐408. [DOI] [PubMed] [Google Scholar]
- Bittner DM, Bittner V, Hausmann J, Reinhold D, Machts J, Westphal S, et al. Training intervention improves memory in mild cognitive impairment and healthy controls, but plasma BDNF acts differentially. Conference: International Conference "Aging and Cognition", IfADo 2013 Germany. 2013;Conference Start: 20130425 Conference End: 20130427 Sponsor: Brain Products ‐ Solutions for Neurophysiological Research, Dortmund Tourismus, DFG ‐ Deutsche Forschungsgemeinsch(var.pagings):49‐50. [Google Scholar]
- Borella E, Carretti B, Riboldi F, Beni R. Working memory training in older adults: evidence of transfer and maintenance effects. Psychology and Aging 2010;25(4):767‐78. [DOI] [PubMed] [Google Scholar]
- Borella E, Carretti B, Zanoni G, Zavagnin M, Beni R. Working memory training in old age: an examination of transfer and maintenance effects. Archives of Clinical Neuropsychology 2013;28(4):331‐47. [DOI] [PubMed] [Google Scholar]
- Borella E, Carretti B, Cantarella A, Riboldi F, Zavagnin M, Beni R. Benefits of training visuospatial working memory in young‐old and old‐old. Developmental Psychology 2014;50(3):714‐27. [DOI] [PubMed] [Google Scholar]
- Borella E, Carretti B, Sciore R, Capotosto E, Taconnat L, Cornoldi C, et al. Training working memory in older adults: is there an advantage of using strategies?. Psychology and Aging 2017;32(2):178‐91. [DOI] [PubMed] [Google Scholar]
- Boripuntakul S, Kothan S, Methapatara P, Munkhetvit P, Sungkarat S. Short‐term effects of cognitive training program for individuals with amnestic mild cognitive impairment: a pilot study. Physical & Occupational Therapy In Geriatrics 2012;30(2):138‐49. [Google Scholar]
- Borness C, Proudfoot J, Crawford J, Valenzuela M. Putting brain training to the test in the workplace: a randomized, blinded, multisite, active‐controlled trial. PLoS One 2013;8(3):e59982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottiroli S, Cavallini E. Can computer familiarity regulate the benefits of computer‐based memory training in normal aging? A study with an Italian sample of older adults. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition2009; Vol. 16, issue 4:401‐18. [DOI] [PubMed]
- Bottiroli S, Cavallini E. Can computer familiarity regulate the benefits of computer‐based memory training in normal aging? A study with an Italian sample of older adults. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition 2009;16(4):401‐18. [DOI] [PubMed] [Google Scholar]
- Bozoki A, Radovanovic M, Winn B, Heeter C, Anthony JC. Effects of a computer‐based cognitive exercise program on age‐related cognitive decline. Archives of Gerontology and Geriatrics 2013;57(1):1‐7. [DOI] [PubMed] [Google Scholar]
- Brehmer Y, Westerberg H, Bäckman L. Working‐memory training in younger and older adults: training gains, transfer, and maintenance. Frontiers in Human Neuroscience 2012;6:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum P, Yassuda M, Forlenza O. Memory training in healthy elderly and seniors with mild cognitive impairment: benefits on cognitive parameters. Conference: Alzheimer's Association International Conference 2013 Boston, MA United States. 2013;Conference Start: 20130713. Conference End: 20130718:P493. [Google Scholar]
- Buitenweg JI, Ven RM, Prinssen S, Murre JM, Ridderinkhof KR. Cognitive flexibility training: a large‐scale multimodal adaptive active‐control intervention study in healthy older adults. Frontiers in Human Neuroscience 2017;11:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiza C, Etxeberria I, Galdona N, González MF, Arriola E, López de Munain A, et al. A randomized, two‐year study of the efficacy of cognitive intervention on elderly people: the Donostia Longitudinal Study. International Journal of Geriatric Psychiatry 2008;23(1):85‐94. [DOI] [PubMed] [Google Scholar]
- Bures V, Cech P, Mikulecka J, Ponce D, Kuca K. The effect of cognitive training on the subjective perception of well‐being in older adults. PeerJ 2016;4:e2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschert V, Giegling I, Merensky W, Jolk S, Teipel S, Hampel H, et al. Long‐term effects of a multicomponent cognitive intervention in amnestic mild cognitive impairment (AMCI). Conference: Alzheimer's Association International Conference, AAIC 11 Paris, France. 2011;Conference Start: 20110716. Conference End: 20110721:S513‐4. [Google Scholar]
- Buschert VC, Friese U, Teipel SJ, Schneider P, Merensky W, Rujescu D, et al. Effects of a newly developed cognitive intervention in amnestic mild cognitive impairment and mild Alzheimer's disease: a pilot study. Journal of Alzheimer's Disease 2011;25(4):679‐94. [DOI] [PubMed] [Google Scholar]
- Buschert VC, Giegling I, Teipel SJ, Jolk S, Hampel H, Rujescu D, et al. Long‐term observation of a multicomponent cognitive intervention in mild cognitive impairment. Journal of Clinical Psychiatry 2012;73(12):e1492‐8. [DOI] [PubMed] [Google Scholar]
- Buschert VC, Giegling I, Teipel SJ, Jolk S, Hampel H, Rujescu D, et al. Long‐term observation of a multi component cognitive intervention in mild cognitive impairment. Journal of Clinical Psychiatry 2012;73(12):e1492‐8. [DOI] [PubMed] [Google Scholar]
- Calkins AW, Deveney CM, Weitzman ML, Hearon BA, Siegle GJ, Otto MW. The effects of prior cognitive control task exposure on responses to emotional tasks in healthy participants. Behavioural and Cognitive Psychotherapy 2011;39(2):205‐20. [DOI] [PubMed] [Google Scholar]
- Cammarata S, Novello C, Pollero V, Colucci M. Cognitive rehabilitation in patients with mild cognitive impairment. Conference: 6th Sindem Meeting: Italian Association for the Study of Dementia linked to the Italian Neurological Society, SIN Milan Italy. 2011;Conference Start: 20110317. Conference End: 20110319:S50. [Google Scholar]
- Cancela JM, Vila Suarez MH, Vasconcelos J, Lima A, Ayan C. Efficacy of brain gym training on the cognitive performance and fitness level of active older adults: a preliminary study. Journal of Aging and Physical Activity 2015;23(4):653‐8. [DOI] [PubMed] [Google Scholar]
- Candela F, Zucchetti G, Magistro D, Rabaglietti E. The effects of a physical activity program and a cognitive training program on the long‐term memory and selective attention of older adults: a comparative study. Activities, Adaptation & Aging 2015;39(1):77‐91. [Google Scholar]
- Cantarella A, Borella E, Carretti B, Kliegel M, Beni R. Benefits in tasks related to everyday life competences after a working memory training in older adults. International Journal of Geriatric Psychiatry 2017;32(1):86‐93. [DOI] [PubMed] [Google Scholar]
- Cao W, Cao X, Hou C, Li T, Cheng Y, Jiang L, et al. Effects of cognitive training on resting‐state functional connectivity of default mode, salience, and central executive networks. Frontiers in Aging Neuroscience 2016;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretti B, Borella E, Fostinelli S, Zavagnin M. Benefits of training working memory in amnestic mild cognitive impairment: specific and transfer effects. International Psychogeriatrics2013; Vol. 25, issue 4:617‐26. [DOI] [PubMed]
- Casutt G, Theill N, Martin M, Keller M, Jäncke L. The drive‐wise project: driving simulator training increases real driving performance in healthy older drivers. Frontiers in Aging Neuroscience 2014;13(6):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SB, Aslan S, Spence JS, Hart JJ Jr, Bartz EK, Didehbani N, et al. Neural mechanisms of brain plasticity with complex cognitive training in healthy seniors. Cerebral Cortex 2015;25(2):396‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SB, Aslan S, Spence JS, Keebler MW, DeFina LF, Didehbani N, et al. Distinct brain and behavioral benefits from cognitive vs. physical training: a randomized trial in aging adults. Frontiers in Human Neuroscience 2016;10:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SB, Spence JS, Aslan S, Keebler MW. Enhancing innovation and underlying neural mechanisms via cognitive training in healthy older adults. Frontiers in Aging Neuroscience 2017;9:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Wu W, Feng W, Wang J, Chen Y, Shen Y, et al. The effects of multi‐domain versus single‐domain cognitive training in non‐demented older people: a randomized controlled trial. BMC Medicine 2012;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CP, Chiu‐Wa Lam L, Cheng ST. The effects of integrated attention training for older Chinese adults with subjective cognitive complaints:. Journal of Applied Gerontology 2018;37(10):1195‐1214. [DOI] [PubMed] [Google Scholar]
- Cho BH, Ku J, Jang DP, Kim S, Lee YH, Kim IY, et al. The effect of virtual reality cognitive training for attention enhancement. Cyberpsychology & Behavior 2002;5(2):129‐37. [DOI] [PubMed] [Google Scholar]
- Cleverley M, Walker Z, Dannhauser T. Engaging patients at high risk of dementia in multimodal cognitive health promoting activities: the ThinkingFit study. Conference: Alzheimer's Association International Conference 2012 Vancouver, BC Canada. 2012;Conference Start: 20120714. Conference End: 20120719.(var.pagings):P220‐1. [Google Scholar]
- Cohen‐Mansfield J, Cohen R, Buettner L, Eyal N, Jakobovits H, Rebok G, et al. Interventions for older persons reporting memory difficulties: a randomized controlled pilot study. International Journal of Geriatric Psychiatry 2014;30(5):478‐86. [DOI] [PubMed] [Google Scholar]
- Cohen‐Mansfield J, Cohen R, Buettner L, Eyal N, Jakobovits H, Rebok G, et al. Interventions for older persons reporting memory difficulties: a randomized controlled pilot study. International Journal of Geriatric Psychiatry 2014;30(5):478‐86. [DOI] [PubMed] [Google Scholar]
- Cohen‐Mansfield J, Cohen R, Buettner L, Eyal N, Jakobovits H, Rebok G, et al. Interventions for older persons reporting memory difficulties: a randomized controlled pilot study. International Journal of Geriatric Psychiatry 2015;30(5):478‐86. [DOI] [PubMed] [Google Scholar]
- Cohen‐Mansfield J, Cohen R, Buettner L, Eyal N, Jakobovits H, Rebok G, et al. Interventions for older persons reporting memory difficulties: a randomized controlled pilot study. International Journal of Geriatric Psychiatry 2015;30(5):478‐86. [DOI] [PubMed] [Google Scholar]
- Combourieu L, Perrot A, Bloch F, Seux ML, Kemoun G. Effect of three different trainings on executive function and gait speed in MCI old adults. Conference: 19th European Congress of Physical and Rehabilitation Medicine Marseille France. 2014;Conference Start: 20140526. Conference End: 20140531.:e138. [Google Scholar]
- Costa NB, Aramaki F, Cecato J, Stella B, Araujo I, Aprahamian I, et al. Benefits of a computer‐based cognitive training program for elderly subjects with mild Alzheimer's disease. Conference: 17th IPA International Congress Berlin Germany. 2015;Conference Start: 20151013. Conference End: 20151016.(var.pagings):S119. [Google Scholar]
- Danassi E. SOCIABLE: a surface computing platform empowering effective cognitive training for healthy and cognitively impaired elderly. Advances in Experimental Medicine and Biology. 2015;821:129‐30. [DOI] [PubMed] [Google Scholar]
- Dannhauser TM, Cleverley M, Whitfield TJ, Fletcher BC, Stevens T, Walker Z. A complex multimodal activity intervention to reduce the risk of dementia in mild cognitive impairment ‐ ThinkingFit: pilot and feasibility study for a randomized controlled trial. BMC Psychiatry 2014;14:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almondes KM, Leonardo ME, Moreira AM. Effects of a cognitive training program and sleep hygiene for executive functions and sleep quality in healthy elderly. Dementia & Neuropsychologia 2017;11(1):69‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo LD, Oliveira TC, Soares FC, Bento‐Torres J, Bento‐Torres NV, Anthony DC, et al. Beneficial effects of multisensory and cognitive stimulation in institutionalized elderly: 12‐months follow‐up. Clinical Interventions in Aging 2015;10:1351‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins‐Crépeau L, Berryman N, Fraser SA, Vu TT, Kergoat MJ, Li KZ, et al. Effects of combined physical and cognitive training on fitness and neuropsychological outcomes in healthy older adults. Clinical Interventions in Aging 2017;11:1287‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreese LP, Neri M, Boiardi R, Ferrari P, Belloi L, Salvioli G. Memory training and drug therapy act differently on memory and metamemory functioning: evidence from a pilot study. Archives of Gerontology and Geriatrics 1996;23(Suppl 1):9‐22. [DOI] [PubMed] [Google Scholar]
- Diamond K, Mowszowski L, Cockayne N, Norrie L, Paradise M, Hermens DF, et al. Randomized controlled trial of a healthy brain ageing cognitive training program: effects on memory, mood, and sleep. Journal of Alzheimer's Disease 2015;44(4):1181‐91. [DOI] [PubMed] [Google Scholar]
- Dittmann‐Kohli F, Lachman ME, Kliegl R, Baltes PB. Effects of cognitive training and testing on intellectual efficacy beliefs in elderly adults. Journal of Gerontology 1991;46(4):162‐4. [DOI] [PubMed] [Google Scholar]
- Djabelkhir L, Wu YH, Vidal JS, Cristancho‐Lacroix V, Marlats F, Lenoir H, et al. Computerized cognitive stimulation and engagement programs in older adults with mild cognitive impairment: comparing feasibility, acceptability, and cognitive and psychosocial effects. Clinical Interventions in Aging 2017;12:1967‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan NL, Greenaway MC. The memory support system for mild cognitive impairment: emotional impacts of a cognitive rehabilitation program. Conference: 29th Annual Meeting of the National Academy of Neuropsychology New Orleans, LA United States. 2009;Conference Start: 20091111. Conference End: 20091114.(var.pagings):438. [Google Scholar]
- Dwolatzky T. The effect of computerized cognitive training on neuropsychological measures of cognitive function in the elderly. NCT001462632005.
- Eckroth‐Bucher M, Siberski J. Preserving cognition through an integrated cognitive stimulation and training program. American Journal of Alzheimer's Disease and Other Dementias 2009;24(3):234‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JD, Wadley VG, Vance DE, Wood K, Roenker DL, Ball KK. The impact of speed of processing training on cognitive and everyday performance. Aging and Mental Health 2005;9(3):262‐71. [DOI] [PubMed] [Google Scholar]
- Edwards JD. Cognitive speed of processing training transfers to improved functional performance. Conference: International Conference "Aging and Cognition" 2010 Dortmund Germany.. 2011; Vol. Conference Start: 20101014. Conference End: 20101016:10. [Google Scholar]
- Edwards JD, Valdés EG, Peronto C, Castora‐Binkley M, Alwerdt J, Andel R, et al. The efficacy of InSight cognitive training to improve useful field of view performance: a brief report. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 2015;70(3):417‐22. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Valdés EG, Peronto C, Castora‐Binkley M, Alwerdt J, Andel R, et al. The efficacy of InSight cognitive training to improve useful field of view performance: a brief report. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2015;70(3):417‐22. [DOI] [PubMed] [Google Scholar]
- Efthymiou A, Konstantinidis V, Tryfonopoulos E, Karpathiou N, Dimakopoulou E, Nikolaou C, et al. Non‐pharmacological intervention: effectiveness of a multi‐component rehabilitation program on cognitive functions of people with mild cognitive impairment. Conference: Alzheimer's Association International Conference, AAIC 11 Paris, France. 2011;Conference Start: 20110716. Conference End: 20110721:S643. [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Skaane NV, Dale AM, Holland D, et al. Effects of cognitive training on gray matter volumes in memory clinic patients with subjective memory impairment. Journal of Alzheimer's Disease 2014;41(3):779‐91. [DOI] [PubMed] [Google Scholar]
- Fabre C, Chamari K, Mucci P, Masse‐Biron J, Prefaut C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. International Journal of Sports Medicine 2002;23(6):415‐21. [DOI] [PubMed] [Google Scholar]
- Faille L. Performance on a brain‐plasticity‐based memory‐training computer program for the elderly as influenced by cognitive functioning and gender. Thesis 2007;68(3‐B):1922. [Google Scholar]
- Fairchild JK, Scogin FR. Training to Enhance Adult Memory (TEAM): an investigation of the effectiveness of a memory training program with older adults. Aging and Mental Health 2010;14(3):364‐73. [DOI] [PubMed] [Google Scholar]
- Feng W, Li CB, Chen Y, Cheng Y, Wu WY. Integrative cognitive training for healthy elderly Chinese in community: a controlled study. Biomedical Research 2013;24(2):223‐9. [Google Scholar]
- Feng W, Yokoyama JS, Yu S, Chen Y, Cheng Y, Bonham LW, et al. APOE genotype affects cognitive training response in healthy Shanghai community‐dwelling elderly individuals. Journal of Alzheimer's Disease 2015;47(4):1035‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Li G, Xu C, Ju C, Qiu X. Training rehabilitation as an effective treatment for patients with vascular cognitive impairment with no dementia. Rehabilitation Nursing 2017;42(5):290‐7. [DOI] [PubMed] [Google Scholar]
- Fiatarone Singh MA, Gates N, Saigal N, Wilson GC, Meiklejohn J, Brodaty H, et al. The study of Mental and Resistance Training (SMART) study ‐ resistance training and/or cognitive training in mild cognitive impairment: a randomized, double‐blind, double‐sham controlled trial. Journal of the American Medical Directors Association 2014;15(12):873‐80. [DOI] [PubMed] [Google Scholar]
- Finn M, McDonald S. Computerised Cognitive Training for Older Persons With Mild Cognitive Impairment: A Pilot Study Using a Randomised Controlled Trial Design. Brain Impairment 2011;12(3):187‐199. [Google Scholar]
- Finn M, McDonald S. Repetition‐lag training to improve recollection memory in older people with amnestic mild cognitive impairment. A randomized controlled trial. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition 2015;22(2):244‐58. [DOI] [PubMed] [Google Scholar]
- Finn M, McDonald S. Repetition‐lag training to improve recollection memory in older people with amnestic mild cognitive impairment. A randomized controlled trial. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition 2015;22(2):244‐58. [DOI] [PubMed] [Google Scholar]
- Flak M, Hernes SS, Skranes J, Lohaugen GC. Memory aid‐computer based working memory training in elderly with mild cognitive impairment (MCI). A randomized, controlled trial. Conference: 21st World Congress of Neurology Vienna Austria. 2013;Conference Start: 20130921. Conference End: 20130926:e322‐3. [Google Scholar]
- Flak MM, Hernes SS, Chang L, Ernst T, Douet V, Skranes J, et al. The Memory Aid study: protocol for a randomized controlled clinical trial evaluating the effect of computer‐based working memory training in elderly patients with mild cognitive impairment (MCI). Trials 2014;15:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak MM, Hernes SS, Chang L, Ernst T, Douet V, Skranes J, et al. The Memory Aid study: protocol for a randomized controlled clinical trial evaluating the effect of computer‐based working memory training in elderly patients with mild cognitive impairment (MCI). Trials 2014;15:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak MM, Hernes SS, Chang L, Ernst T, Douet V, Skranes J, et al. Erratum to: 'The Memory Aid study: protocol for a randomized controlled clinical trial evaluating the effect of computer‐based working memory training in elderly patients with mild cognitive impairment (MCI)'.[Erratum for Trials. 2014;15:156 Note: Chang, Linda; Ernst, Thomas; and Douet, Vanessa [Added]; PMID: 24886034]. Trials 2016;17:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster S, Buschert VC, Buchholz HG, Teipel SJ, Zach C, Hampel H, et al. Positive effects of a 6‐month stage‐specific cognitive intervention program on brain metabolism in subjects with amnestic mild cognitive impairment (AMCI) and mild Alzheimer's disease (AD). Conference: Alzheimer's Association International Conference on Alzheimer's Disease Vienna Austria. 2009; Vol. Conference Start: 20090711. Conference End: 20090716:38. [Google Scholar]
- Forloni G, Polito L, Davin A, Abbondanza S, Vaccaro R, Valle E. Cognitive stimulation and APOE genotype in non‐demented elderly subjects: a randomized controlled study (RCT). Conference: 5th Conference Clinical Trials on Alzheimer's Disease Monte Carlo Monaco. 2012; Vol. Conference Start: 20121029. Conference End: 20121031:841‐2. [Google Scholar]
- Förster S, Buschert VC, Teipel SJ, Friese U, Buchholz HG, Drzezga A, et al. Effects of a 6‐month cognitive intervention on brain metabolism in patients with amnestic MCI and mild Alzheimer's disease. Journal of Alzheimer's Disease 2011;26(3):605‐16. [DOI] [PubMed] [Google Scholar]
- Fortman J. Computer‐based cognitive training for age‐related cognitive decline and mild cognitive impairment. Thesis 2013;74(5‐B(E)):No Pagination Specified. [Google Scholar]
- Gagnon LG, Belleville S. Training of attentional control in mild cognitive impairment with executive deficits: results from a double‐blind randomised controlled study. Neuropsychological Rehabilitation 2012;22(6):809‐35. [DOI] [PubMed] [Google Scholar]
- Gagnon L. Working memory in Alzheimer's disease and mild cognitive impairment (MCI): assessment and intervention. Thesis 2012;73(5‐B):3262. [Google Scholar]
- Gaitán A, Garolera M, Cerulla N, Chico G, Rodriguez‐Querol M, Canela‐Soler J. Efficacy of an adjunctive computer‐based cognitive training program in amnestic mild cognitive impairment and Alzheimer's disease: a single‐blind, randomized clinical trial. International Journal of Geriatric Psychiatry 2013;28(1):91‐9. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Falkenstein M. Training‐induced improvement of response selection and error detection in aging assessed by task switching: effects of cognitive, physical, and relaxation training. Frontiers in Human Neuroscience 2012;6:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski PD, Freude G, Falkenstein M. Cognitive training sustainably improves executive functioning in middle‐aged industry workers assessed by task switching: a randomized controlled ERP study. Frontiers in Human Neuroscience 2017;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Campuzano MT, Virues‐Ortega J, Smith S, Moussavi Z. Effect of cognitive training targeting associative memory in the elderly: a small randomized trial and a longitudinal evaluation. Journal of the American Geriatrics Society 2013;61(12):2252‐4. [DOI] [PubMed] [Google Scholar]
- Gates NJ, Valenzuela M, Sachdev PS, Singh NA, Baune BT, Brodaty H, et al. Study of Mental Activity and Regular Training (SMART) in at risk individuals: a randomised double blind, sham controlled, longitudinal trial. BMC Geriatrics 2011;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill DP, Gregory MA, Zou G, Liu‐Ambrose T, Shigematsu R, Hachinski V, et al. The Healthy Mind, Healthy Mobility Trial: a novel exercise program for older adults. Medicine and Science in Sports and Exercise 2016;48(2):297‐306. [DOI] [PubMed] [Google Scholar]
- Gillette S. The multidomain Alzheimer preventive trial (MAPT): a new approach for the prevention of Alzheimer's disease. Conference: Alzheimer's Association International Conference on Alzheimer's Disease Vienna Austria. 2009;Conference Start: 20090711. Conference End: 20090716:145. [Google Scholar]
- Giovannini E, Borso E, Benso F, Carabelli E, Sette M, Ciarmiello A. FDG‐PET in the evaluation of brain metabolic changes induced by cognitive stimulation in aMCI subjects. Conference: 28th Annual Congress of the European Association of Nuclear Medicine, EANM 2015 Hamburg, Germany. 2015; Vol. Conference Start: 20151010. Conference End: 20151014:S552‐3. [Google Scholar]
- Giuli C, Papa R, Lattanzio F, Postacchini D. The effects of cognitive training for elderly: results from My Mind project. Rejuvenation Research 2016;19(6):485‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuli C, Fattoretti P, Gagliardi C, Mocchegiani E, Venarucci D, Balietti M, et al. My Mind Project: the effects of cognitive training for elderly ‐ the study protocol of a prospective randomized intervention study. Aging Clinical and Experimental Research 2017;29(3):353‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golino MT, Flores Mendoza C, Golino HF. Effects of cognitive training on cognitive performance of healthy older adults. Spanish Journal of Psychology 2017;20:E39. [DOI] [PubMed] [Google Scholar]
- Gooding AL, Choi J, Fiszdon JM, Wilkins K, Kirwin PD, Dyck CH, et al. Comparing three methods of computerised cognitive training for older adults with subclinical cognitive decline. Neuropsychological Rehabilitation 2016;26(5‐6):810‐21. [DOI] [PubMed] [Google Scholar]
- Haesner M, O'Sullivan JL, Gövercin M, Steinhagen‐Thiessen E. Requirements of older adults for a daily use of an internet‐based cognitive training platform. Informatics for Health and Social Care 2015;40(2):139‐53. [DOI] [PubMed] [Google Scholar]
- Haesner M, Steinert A, O'Sullivan JL, Weichenberger M. Evaluating an online cognitive training platform for older adults: user experience and implementation requirements. Journal of Gerontological Nursing 2015;41(8):22‐31. [DOI] [PubMed] [Google Scholar]
- Haimov I, Shatil E. Protocol_S1.doc. Figshare2013.
- Haimov I, Shatil E. Checklist_S1.pdf. Figshare2013.
- Haimov I, Shatil E. Cognitive training improves sleep quality and cognitive function among older adults with insomnia. PLoS One 2013;8(4):e61390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimov I, Shatil E. Cognitive training improves sleep quality and cognitive function among older adults with insomnia. PLoS One 2013;8(4):e61390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimov I, Shatil E. Cognitive training improves sleep quality and cognitive function among older adults with insomnia. Conference: 5th International World Association of Sleep Medicine Congress and the 22nd Annual Congress of the Spanish Sleep Society Valencia, Spain. 2013; Vol. Conference Start: 20130928. Conference End: 20131002. Conference Publication::e61390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimov I, Shatil E. Cognitive training improves sleep quality and cognitive function among older adults with insomnia. Conference: 22nd Annual Meeting of the Israel Society for Neuroscience, ISFN and the 2nd Bi national Italy‐Israel Neuroscience Meeting Eilat Israel. 2014; Vol. Conference Start: 20131214. Conference End: 20131217. Conference Publication::S60. [Google Scholar]
- Haimov I, Shatil E. Cognitive training improves sleep quality and cognitive function among older adults with insomnia. Conference: 22nd Congress of the European Sleep Research Society, Tallinn, Estonia. 2014; Vol. Conference Start: 20140916. Conference End: 20140920. Conference Publication::137. [Google Scholar]
- Hardy JL, Nelson RA, Thomason ME, Sternberg DA, Katovich K, Farzin F, et al. Enhancing cognitive abilities with comprehensive training: a large, online, randomized, active‐controlled trial. PLoS One 2015;10(9):e0134467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann J, MacHts J, Bittner V, Mueller N, Heinze H‐J, Bittner D. No title provided. Conference: Alzheimer's Association International Conference 2012 Vancouver, BC Canada. 2012; Vol. Conference Start: 20120714. Conference End: 20120719. Conference Publication::P393. [Google Scholar]
- Hayashi N. Cognitive training and occupational recreational therapy on elderly Japanese in Osaka: major outcome (ADAS) from prospective, randomized, open, blind‐endpoint trial. Alzheimer's & Dementia 2012;7(4):S644. [Google Scholar]
- Hayslip B Jr, Paggi K, Caballero D. The impact of mental aerobics training on older adults. Journal of Applied Gerontology 2016;35(11):1130‐53. [DOI] [PubMed] [Google Scholar]
- Heinzel S, Schulte S, Onken J, Duong QL, Riemer TG, Heinz A, et al. Working memory training improvements and gains in non‐trained cognitive tasks in young and older adults. Neuropsychology, Development and Cognition. Section B: Aging, Neuropsychology, and Cognition 2014;21(2):146‐73. [DOI] [PubMed] [Google Scholar]
- Herrera C, Chambon C, Michel BF, Paban V, Alescio‐Lautier B. Positive effects of computer‐based cognitive training in adults with mild cognitive impairment. Neuropsychologia 2012;50(8):1871‐81. [DOI] [PubMed] [Google Scholar]
- Hötting K, Holzschneider K, Stenzel A, Wolbers T, Röder B. Effects of a cognitive training on spatial learning and associated functional brain activations. BMC Neuroscience 2013;14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak EM. The effects of cognitive stimulation and computerized memory training among older adults residing in independent‐living facilities. Thesis 2013;74(1‐B(E)):No Pagination Specified. [Google Scholar]
- Ignjatovic VB, Kalabic S, Batic S, Zikic M. Improvement of cognitive efficiency through cognitive training in healthy subjects. Acta Clinica Croatica 2015;54(2):169‐78. [PubMed] [Google Scholar]
- Irigaray TQ, Filho IG, Schneider RH. Effects of an attention, memory and executive functions training on the cognition of healthy elderly people. Psicologia: Reflexão e Crítica 2012;25(1):188‐202. [Google Scholar]
- Israël L, Myslinski M, Dubos G, Mélac M. [Combined therapies in family practice and hospitals. A controlled clinical study of a population of 162 patients with criteria of age‐related memory disorders]. [French]. Presse Médicale 1997;26(25):1186‐91. [PubMed] [Google Scholar]
- ISRCTN70130279. Effects of the six‐month training on cognitive, physical performance, and daily physical activity in older adults. http://www.isrctn.com/ISRCTN701302792013.
- Jackson JJ, Hill PL, Payne BR, Roberts BW, Stine‐Morrow EA. Can an old dog learn (and want to experience) new tricks? Cognitive training increases openness to experience in older adults. Psychology and Aging 2012;27(2):286‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen P, Dahmen‐Zimmer K. Effects of cognitive, motor, and karate training on cognitive functioning and emotional well‐being of elderly people. Frontiers in Psychology 2012;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean L, Simard M, Wiederkehr S, Bergeron ME, Turgeon Y, Hudon C, et al. Efficacy of a cognitive training programme for mild cognitive impairment: results of a randomised controlled study. Neuropsychological Rehabilitation 2010;20(3):377‐405. [DOI] [PubMed] [Google Scholar]
- Jeong JH, Na HR, Choi SH, Kim J, Na DL, Seo SW, et al. Group‐ and home‐based cognitive intervention for patients with mild cognitive impairment: a randomized controlled trial. Psychotherapy and Psychosomatics 2016;85(4):198‐207. [DOI] [PubMed] [Google Scholar]
- Jobe JB, Smith DM, Ball K, Tennstedt SL, Marsiske M, Willis SL, et al. ACTIVE: A Cognitive Intervention Trial to Promote Independence in Older Adults. Controlled Clinical Trials 2001;22(4):453‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RN, Marsiske M, Ball K, Rebok G, Willis SL, Morris JN, et al. The ACTIVE cognitive training interventions and trajectories of performance among older adults. Journal of Aging and Health 2013;25(8 Suppl):186S‐208S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampanaros D, Weber IL, Endler PC. Conventional and complementary interventions and cognitive performance in old age. Conference: 3rd European Congress for Integrative Medicine, ECIM 2010 Berlin, Germany. 2010; Vol. Conference Start: 20101203. Conference End: 20101204:264. [Google Scholar]
- Kholin V. Cognitive‐emotional stimulation in mild cognitive impairment. Conference: 14th Congress of the European Federation of Neurological Societies, EFNS Geneva Switzerland. 2010; Vol. Conference Start: 20100925. Conference End: 20100928:362. [Google Scholar]
- Kim GH, Jeon S, Lee BH, Kim HS, Chin JH, Kim GY. Robot assisted cognitive training can change the brain in the elderly: a single blind, randomized controlled trial of clinical efficacy. Conference: 5th Conference Clinical Trials on Alzheimer's Disease Monte Carlo, Monaco. 2012; Vol. Conference Start: 20121029. Conference End: 20121031:865‐6. [Google Scholar]
- Kim HJ, Yang YS, Choi KH, Kim TY. The effect of computer‐based cognitive training program on cognition. Dementia and Neurocognitive Disorders 2013;12(4):87‐93. [Google Scholar]
- Kim GH, Jeon S, Im K, Seo SW, Cho H, Noh Y, et al. Structural brain changes after robot assisted cognitive training in the elderly: a single‐blind randomized controlled trial. Alzheimer's and Dementia 2013;9(4 Suppl 1):P476‐7. [Google Scholar]
- Kim GH, Jeon S, Im K, Kwon H, Lee BH, Kim GY, et al. Structural brain changes after traditional and robot‐assisted. PLoS One 2015;10(4):e0123251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Choi Y, You H, Na DL, Yoh MS, Park JK, et al. Effects of a serious game training on cognitive functions in older adults. Journal of the American Geriatrics Society 2015;63(3):603‐5. [DOI] [PubMed] [Google Scholar]
- Kim GH, Jeon S, Im K, Kwon H, Lee BH, Kim GY, et al. Structural brain changes after traditional and robot‐assisted multi‐domain cognitive training in community‐dwelling healthy elderly. PLoS One 2015;10(4):e0123251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Lehtisalo J, Hanninen T, Jula A, Laatikainen T, et al. A multidomain two‐year randomized controlled trial to prevent cognitive impairment ‐ The FINGER study. Conference: 10th International Congress of the European Union Geriatric Medicine Society ‐ Geriatric Medicine Crossing Borders, EUGMS 2014 Rotterdam, Netherlands 2014;Conference Start: 20140917. Conference End: 20140919(var.pagings):S69. [Google Scholar]
- Klusmann V, Evers A, Schwarzer R, Dimeo FC, Reischies FM, Heuser I. Complex mental and physical activity in older women maintains episodic memory and working memory: a 6‐month randomized controlled trial. Conference: 64th Annual Scientific Convention and Meeting of the Society of Biological Psychiatry Vancouver, BC, Canada 2009;Conference Start: 20090514. Conference End: 20090516:106S. [Google Scholar]
- Klusmann V, Evers A, Schwarzer R, Schlattmann P, Reischies FM, Heuser I, et al. Complex mental and physical activity in older women and cognitive performance: a 6‐month randomized controlled trial. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2010;65(6):680‐8. [DOI] [PubMed] [Google Scholar]
- Klusmann V, Evers A, Schwarzer R, Schlattmann P, Reischies FM, Heuser I, et al. Complex mental and physical activity in older women and cognitive performance: a 6‐month randomized controlled trial. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2010;65(6):680‐8. [DOI] [PubMed] [Google Scholar]
- Klusmann V, Evers A, Heuser I. Cognitive benefits from mental and physical activity in older women: results from the Berlin Stays Fit study. Conference: International Conference "Aging and Cognition" 2010 Dortmund, Germany 2011;Conference Start: 20101014. Conference End: 20101016:18. [Google Scholar]
- McDaniel MA, Binder EF, Bugg JM, Waldum ER, Dufault C, Meyer A, et al. Effects of cognitive training with and without aerobic exercise on cognitively demanding everyday activities. Psychology and Aging 2014;29(3):717‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak KP, Lee S, Kim T, Bae N. Cognitive training programs for very old lone adults in a Korean rural community. Conference: Alzheimer's Association International Conference 2015 Washington, DC, United States. 2015; Vol. Conference Start: 20150718. Conference End: 20150723:P590. [Google Scholar]
- Kwak K, Kim T. Cognitive stimulation intervention improves BDNF peripheral levels in older adults with non‐amnestic mild cognitive impairment. Alzheimer's & Dementia. 2017; Vol. Conference: Alzheimer's Association International Conference, AAIC 2017. United Kingdom:P860‐P1. [Google Scholar]
- Kwok T, Wong A, Chan G, Shiu YY, Lam KC, Young D, et al. Effectiveness of cognitive training for Chinese elderly in Hong Kong. Clinical Interventions in Aging 2013;8:213‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok TC, Bai X, Li JC, Ho FK, Lee TM. Effectiveness of cognitive training in Chinese older people with subjective cognitive complaints: a randomized placebo‐controlled trial. International Journal of Geriatric Psychiatry 2013;28(2):208‐15. [DOI] [PubMed] [Google Scholar]
- Lampit A, Hallock H, Moss R, Kwok S, Rosser M, Lukjanenko M, et al. A dose‐response relationship between computerized cognitive training and global cognition in older adults. Conference: 6th Conference Clinical Trials on Alzheimer's Disease San Diego, CA, United States 2013;Conference Start: 20131114. Conference End: 20131116:803‐4. [Google Scholar]
- Lampit A, Hallock H, Moss R, Kwok S, Rosser M, Lukjanenko M, et al. The timecourse of global cognitive gains from supervised computer‐assisted cognitive training: a randomised, active‐controlled trial in elderly with multiple dementia risk factors. Journal of Prevention of Alzheimer's Disease 2014;1(1):33‐9. [DOI] [PubMed] [Google Scholar]
- Lampit A, Hallock H, Suo C, Naismith SL, Valenzuela M. Cognitive training‐induced short‐term functional and long‐term structural plastic change is related to gains in global cognition in healthy older adults: a pilot study. Frontiers in Aging Neuroscience 2015;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H. Changes in the functional brain connectivity and cognitive performance following yoga or memory training in older adults with subjective memory complaints. Conference: 71st Annual Scientific Convention and Meeting of the Society of Biological Psychiatry, SOBP 2016 Atlanta, GA, United States 2016;Conference Start: 20160512. Conference End: 20160514:209S. [Google Scholar]
- Law LL, Barnett F, Yau MK, Gray MA. Effects of functional tasks exercise on older adults with cognitive impairment at risk of Alzheimer's disease: a randomised controlled trial. Age Ageing 2014;43(6):813‐20. [DOI] [PubMed] [Google Scholar]
- Law LL, Barnett F, Yau MK, Gray MA. Effects of functional tasks exercise on older adults with cognitive impairment at risk of Alzheimer's disease: a randomised controlled trial. Age Ageing 2014;43(6):813‐20. [DOI] [PubMed] [Google Scholar]
- Lee YM, Jang C, Bak IH, Yoon JS. Effects of computer‐assisted cognitive rehabilitation training on the cognition and static balance of the elderly. Journal of Physical Therapy Science 2013;25(11):1475‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TS, Goh SJ, Quek SY, Phillips R, Guan C, Cheung YB, et al. A brain‐computer interface based cognitive training system for healthy elderly: a randomized control pilot study for usability and preliminary efficacy. PLoS One 2013;8(11):e79419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TS, Goh SJ, Quek SY, Guan C, Cheung YB, Krishnan KR. Efficacy and usability of a brain computer interface system in improving cognition in the elderly. Conference: Alzheimer's Association International Conference 2013 Boston, MA, United States 2013;Conference Start: 20130713. Conference End: 20130718(var.pagings):P296. [Google Scholar]
- Lee TS, Goh ASJ, Quek SY, Phillips R, Guan C, Cheung YB, et al. Pilot trials of EEG‐based brain‐computer interface (BCI) training system for improving cognitive performance in older persons. Conference: NUHS Academic Psychiatry Conference 2014 Singapore, Singapore 2014;Conference Start: 20141031. Conference End: 20141101(var.pagings):S27. [Google Scholar]
- Lee TS, Quek SY, Goh SJ, Phillips R, Guan C, Cheung YB, et al. A pilot randomized controlled trial using EEG‐based brain‐computer interface training for a Chinese‐speaking group of healthy elderly. Clinical Interventions in Aging 2015;10:217‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legault C, Jennings JM, Katula JA, Dagenbach D, Gaussoin SA, Sink KM, et al. Designing clinical trials for assessing the effects of cognitive training and physical activity interventions on cognitive outcomes: the Seniors Health and Activity Research Program Pilot (SHARP‐P) study, a randomized controlled trial. BMC Geriatrics 2011;11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León J, Ureña A, Bolaños MJ, Bilbao A, Oña A. A combination of physical and cognitive exercise improves reaction time in persons 61‐84 years old. Journal of Aging and Physical Activity 2015;23(1):72‐7. [DOI] [PubMed] [Google Scholar]
- Leung NT, Tam HM, Chu LW, Kwok TC, Chan F, Lam LC, et al. Neural plastic effects of cognitive training on aging brain. Neural Plasticity 2015;2015:535618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KZ, Roudaia E, Lussier M, Bherer L, Leroux A, McKinley PA. Benefits of cognitive dual‐task training on balance performance in healthy older adults. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2010;65(12):1344‐52. [DOI] [PubMed] [Google Scholar]
- Linde K, Alfermann D. Single versus combined cognitive and physical activity: effects on fluid cognitive abilities of healthy older adults: a 4‐month randomized controlled trial with follow‐up. Journal of Aging and Physical Activity 2014;22(3):302‐13. [DOI] [PubMed] [Google Scholar]
- Mace RA, Mansbach WE. The efficacy of a computer‐assisted cognitive rehabilitation program for patients with mild cognitive deficits: a pilot study. Conference: Alzheimer's Association International Conference 2015 Washington, DC, United States 2015;Conference Start: 20150718. Conference End: 20150723(var.pagings):P783. [DOI] [PubMed] [Google Scholar]
- Mahncke HW, Connor BB, Appelman J, Ahsanuddin ON, Hardy JL, Wood RA, et al. Memory enhancement in healthy older adults using a brain plasticity‐based training program: a randomized, controlled study. Proceedings of the National Academy of Sciences of the United States of America 2006;103(33):12523‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man DW Chung JC, Lee GY. Evaluation of a virtual reality‐based memory training programme for Hong Kong Chinese older adults with questionable dementia: a pilot study. International Journal of Geriatric Psychiatry 2012;27(5):513‐20. [DOI] [PubMed] [Google Scholar]
- Mann D, Szwanki VL, Mistry JJ. The effect of brain training on cognitive assessment: a pilot investigation. Conference: 10th Annual Conference on Brain Injury of the North American Brain Injury Society's, NABIS 2012 Miami, FL, United States 2012;Conference Start: 20120912. Conference End: 20120915:E39‐40. [Google Scholar]
- Margrett JA, Willis SL. In‐home cognitive training with older married couples: individual versus collaborative learning. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition. 2006;13(2):173‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayas J, Parmentier FB, Andres P, Ballesteros S. Plasticity of attentional functions in older adults after non‐action video game training: a randomized controlled trial. PLoS One 2014;9(3):e92269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvinue LP, Golemme M, Castorina M, Tatti E, Pigni FM, Salomone S, et al. An evaluation of a working memory training scheme in older adults. Frontiers in Aging Neuroscience 2013;23(5):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA, Binder EF, Bugg JM, Waldum ER, Dufault C, Meyer A, et al. Effects of cognitive training with and without aerobic exercise on cognitively demanding everyday activities. Psychology and Aging 2014;29(3):717‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall S, House B. Brain training in older adults: evidence of transfer to memory span performance and pseudo‐Matthew effects. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition. 2012;19(1‐2):195‐221. [DOI] [PubMed] [Google Scholar]
- Middleton LE, Poelke G, Santos WM, Yaffe K, Barnes DE, Goodson W. Impact of a 12‐week exercise intervention on non‐cognitive outcomes in sedentary elders with cognitive complaints or mild cognitive impairment: findings from the MAX trial. Conference: Alzheimer's Association International Conference 2012 Vancouver, BC, Canada 2012;Conference Start: 20120714. Conference End: 20120719(var.pagings):P146. [Google Scholar]
- Miller KJ, Dye RV, Kim J, Jennings JL, O'Toole E, Wong J, et al. Effect of a computerized brain exercise program on cognitive performance in older adults. American Journal of Geriatric Psychiatry 2013;21(7):655‐63. [DOI] [PubMed] [Google Scholar]
- Mohs RC, Ashman TA, Jantzen K, Albert M, Brandt J, Gordon B, et al. A study of the efficacy of a comprehensive memory enhancement program in healthy elderly persons. Psychiatry Research 1998;77(3):183‐95. [DOI] [PubMed] [Google Scholar]
- Mombelli G, Riva M, Cerea E, Zanetti M, Rozzini L, Padovani A. Neuropsychological training (TNP) in MCI subjects: one year follow‐up study. Conference: 7th Sindem Meeting: Italian Association for the Study of Dementia linked to the Italian Neurological Society, SIN Napoli Italy. 2012;Conference Start: 20120322. Conference End: 20120324:77. [Google Scholar]
- Moon SK, Chung S, Han MI. The effectiveness of self‐efficacy based memory training program for the elderly with mild cognitive impairment. Conference: 16th International Congress of the International Psychogeriatric Association, IPA 2013 Seoul, South Korea 2013;Conference Start: 20131001. Conference End: 20131004:S141‐2. [Google Scholar]
- Mowszowski L, Hermens DF, Diamond K, Norrie L, Cockayne N, Ward PB, et al. Cognitive training enhances pre‐attentive neurophysiological responses in older adults 'at risk' of dementia. Journal of Alzheimer's Disease 2014;41(4):1095‐108. [DOI] [PubMed] [Google Scholar]
- Mowszowski L, Hermens DF, Diamond K, Norrie L, Cockayne N, Ward PB, et al. Cognitive training enhances pre‐attentive neurophysiological responses in older adults 'at risk' of dementia. Journal of Alzheimer's Disease 2014;41(4):1095‐108. [DOI] [PubMed] [Google Scholar]
- Mozolic JL, Hayasaka S, Laurienti PJ. A cognitive training intervention increases resting cerebral blood flow in healthy older adults. Frontiers in Human Neuroscience 2010;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozolic JL, Long AB, Morgan AR, Rawley‐Payne M, Laurienti PJ. A cognitive training intervention improves modality‐specific attention in a randomized controlled trial of healthy older adults. Neurobiology of Aging 2011;32(4):655‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller NG, Bittner V, Hausmann J, Bittner DM. The effect of a combined motor and cognitive training on cognitive function, structural and functional MRI and BDNF plasma levels in MCI patients. Conference: International Conference "Aging and Cognition" 2010 Dortmund, Germany 2011;Conference Start: 20101014. Conference End: 20101016:22‐3. [Google Scholar]
- Na HR, Choi S, Jeong JH, Na D, Park SA, Kim EJ, et al. A multicenter, randomized trial to assess efficacy of home‐based and group cognitive intervention programs in amnestic mild cognitive impairment. Conference: Alzheimer's Association International Conference 2013 Boston, MA, United States 2013;Conference Start: 20130713. Conference End: 20130718:P495. [Google Scholar]
- Na HR, Choi SH, Jeong JH, Kim JE, Na DL, Seo SW, et al. A multicenter, randomized trial to assess efficacy of home‐based and group cognitive intervention programs for amnestic mild cognitive impairment. Conference: Alzheimer's Association International Conference 2014 Copenhagen, Denmark 2014;Conference Start: 20140712. Conference End: 20140717:P916. [Google Scholar]
- Mowszowski L, Hermens DF, Diamond K, Norrie L, Cockayne N, Ward PB, et al. Cognitive training enhances pre‐attentive neurophysiological responses in older adults 'at risk' of dementia. Journal of Alzheimer's Disease 2014;41(4):1095‐108. [DOI] [PubMed] [Google Scholar]
- Navarro JI, Menacho I, Alcalde C, Marchena E, Simon Velez R, Aguilar M. Comparative study of two cognitive training procedures for elderly people. [Spanish]. Geriátrika 2006;22(6):36‐42. [Google Scholar]
- NCT02417558. Study to Evaluate the Effectiveness of Personalized Brain Network Activation Technology in a Cognitive/Physical Computer‐Game Blended Training of Elderly (Alterniity AR). clinicaltrials.gov2015.
- NCT02462135. The Development and Evaluation of the Effectiveness of a Virtual Interactive Memory Training Program for Older Adults With Mild Cognitive Impairment: Protocol of a Randomized Controlled Study. clinicaltrials.gov2014.
- NCT02480738. Effectiveness of Computerized Cognitive Training Apparatus (CoCoTA) in the Elderly With Normal Cognition, Subjective Cognitive Impairment, Mild Cognitive Impairment. clinicaltrials.gov2012.
- NCT02512627. Evolving Methods to Combine Cognitive and Physical Training for Individuals With Mild Cognitive Impairment: An Efficacy Study. clinicaltrials.gov2015. [DOI] [PMC free article] [PubMed]
- NCT02747784. Randomized Evaluation to Assess Cognitive Training for the Prevention of Post‐operative Cognitive Decline (REACT) ‐ a Pilot Study. clinicaltrials.gov2016.
- NCT02774083. An Evaluation of the Feuerstein Instrumental Enrichment Program for the Cognitive Enhancement of Older People With Mild Cognitive Impairment (MCI) Living in the Community. clinicaltrials.gov2015.
- NCT02785315. Cognitive Intervention for Persons With Amnestic Mild Cognitive Impairment: The Efficacy in Enhancement of Cognition and Complex Activities of Daily Living Function. clinicaltrials.gov2016.
- NCT02808676. SYNchronizing Exercises, Remedies in GaIt and Cognition (SYNERGIC): A Randomized Controlled Double Blind Trial. Clinicaltrials.gov2016. [DOI] [PMC free article] [PubMed]
- Neely AS, Sehlstedt I, Ekman U, Eriksson J, Sandberg P, Qwillbard T, et al. Working memory updating training in older adults: is level of performance after training related to transfer?. Conference: International Conference "Aging and Cognition", IfADo 2013, Germany 2013;Conference Start: 20130425. Conference End: 20130427 Sponsor: Brain Products ‐ Solutions for Neurophysiological Research, Dortmund Tourismus, DFG ‐ Deutsche Forschungsgemeinsch(var.pagings):69‐70. [Google Scholar]
- Ng TP, Feng L, Nyunt MS, Feng L, Niti M, Tan BY, et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: a randomized controlled trial. American Journal of Medicine 2015;128(11):1225‐36. [DOI] [PubMed] [Google Scholar]
- Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet 2015;385(9984):2255‐63. [DOI] [PubMed] [Google Scholar]
- Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet 2015;385(9984):2255‐63. [DOI] [PubMed] [Google Scholar]
- Nishiguchi S, Yamada M, Tanigawa T, Sekiyama K, Kawagoe T, Suzuki M, et al. A 12‐week physical and cognitive exercise program can improve cognitive function and neural efficiency in community‐dwelling older adults: a randomized controlled trial. Journal of the American Geriatrics Society 2015;63(7):1355‐63. [DOI] [PubMed] [Google Scholar]
- Nouchi R, Taki Y, Takeuchi H, Hashizume H, Akitsuki Y, Shigemune Y, et al. Brain training game improves executive functions and processing speed in the elderly: a randomized controlled trial. PLoS One 2012;7(1):e29676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouchi R, Taki Y, Takeuchi H, Hashizume H, Nozawa T, Kambara T, et al. Brain training game boosts executive functions, working memory and processing speed in the young adults: a randomized controlled trial. PLoS One 2013;8(2):e55518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa T, Taki Y, Kanno A, Akimoto Y, Ihara M, Yokoyama R, et al. Effects of different types of cognitive training on cognitive function, brain structure, and driving safety in senior daily drivers: a pilot study. Behavioral Neurology 2015;2015:525901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Caoimh R, Sato S, Wall J, Igras E, Foley MJ, Timmons S, et al. Potential for a memory Gymâ intervention to delay conversion of mild cognitive impairment to dementia. Journal of the American Medical Directors Association 2015;16(11):998‐9. [DOI] [PubMed] [Google Scholar]
- Oei AC, Patterson MD. Enhancing cognition with video games: a multiple game training study. PLoS One 2013;8(3):e58546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira de Lima Queiroz L, Junqueira AX, Fontana AM, Oliveira ER, Lima VC, Guarienti VC. Prevention of cognitive impairment through a cognitive stimulation and rehabilitation program mediated by computers and internet. Conference: 21st World Congress of Neurology Vienna, Austria. 2013; Vol. Conference Start: 20130921. Conference End: 20130926:e537. [Google Scholar]
- Optale G, Urgesi C, Busato V, Marin S, Piron L, Priftis K, et al. Controlling memory impairment in elderly adults using virtual reality memory training: a randomized controlled pilot study. Neurorehabilitation and Neural Repair 2010;24(4):348‐57. [DOI] [PubMed] [Google Scholar]
- Otsuka T, Tanemura R, Noda K, Nagao T, Sakai H, Luo ZW. Development of computer‐aided cognitive training program for elderly and its effectiveness through a 6 months group intervention study. Current Alzheimer Research 2015;12(6):553‐62. [DOI] [PubMed] [Google Scholar]
- Park MH, Kwon DY, Seo WK, Lim KS, Song MS. The effects of cognitive training on community‐dwelling elderly Koreans. Journal of Psychiatric and Mental Health Nursing 2009;16(10):904‐9. [DOI] [PubMed] [Google Scholar]
- Park SH, Seo JH, Kim YH, Ko MH. Long‐term effects of transcranial direct current stimulation combined with computer‐assisted cognitive training in healthy older adults. Neuroreport 2014;25(2):122‐6. [DOI] [PubMed] [Google Scholar]
- Payne BR, Jackson JJ, Hill PL, Gao X, Roberts BW, Stine‐Morrow EA. Memory self‐efficacy predicts responsiveness to inductive reasoning training in older adults. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 2012;67(1):27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BR, Stine‐Morrow EA. The effects of home‐based cognitive training on verbal working memory and language comprehension in older adulthood. Frontiers in Aging Neuroscience 2017;9:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz C, Korczyn AD, Shatil E, Aharonson V, Birnboim S, Giladi N. Computer‐based, personalized cognitive training versus classical computer games: a randomized double‐blind prospective trial of cognitive stimulation. Neuroepidemiology 2011;36(2):91‐9. [DOI] [PubMed] [Google Scholar]
- Rahe J, Petrelli A, Kaesberg S, Fink GR, Kessler J, Kalbe E. Effects of cognitive training with additional physical activity compared to pure cognitive training in healthy older adults. Clinical Interventions in Aging 2015;19(10):297‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahe J, Becker J, Fink GR, Kessler J, Kukolja J, Rahn A, et al. Cognitive training with and without additional physical activity in healthy older adults: cognitive effects, neurobiological mechanisms, and prediction of training success. Frontiers in Aging Neuroscience 2015;7:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebok GW, Langbaum JB, Jones RN, Gross AL, Parisi JM, Spira AP, et al. Memory training in the ACTIVE study: how much is needed and who benefits?. Journal of Aging and Health 2013;25(8):21S‐42S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebok GW, Ball K, Guey LT, Jones RN, Kim HY, King JW, et al. Ten‐year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatrics Society 2014;62(1):16‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redick TS, Shipstead Z, Harrison TL, Hicks KL, Fried DE, Hambrick DZ, et al. No evidence of intelligence improvement after working memory training: a randomized, placebo‐controlled study. Journal of Experimental Psychology. General 2013;142(2):359‐79. [DOI] [PubMed] [Google Scholar]
- Requena C, Turrero A, Ortiz T. Six‐year training improves everyday memory in healthy older people. Randomized controlled trial. Frontiers in Aging Neuroscience 2016;8:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizkalla M. Cognitive training in the rural elderly: a randomized trial to evaluate the efficacy and accessibility of a new approach. Thesis 2015;75(11‐B(E)):No Pagination Specified. [Google Scholar]
- Rojas GJ, Villar V, Iturry M, Harris P, Serrano CM, Herrera JA, et al. Efficacy of a cognitive intervention program in patients with mild cognitive impairment. International Psychogeriatrics 2013;25(5):825‐31. [DOI] [PubMed] [Google Scholar]
- Rose NS, Rendell PG, Hering A, Kliegel M, Bidelman GM, Craik FI. Cognitive and neural plasticity in older adults' prospective memory following training with the Virtual Week computer game. Frontiers in Human Neuroscience 2015;9:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AC, Sugiura L, Kramer JH, Whitfield‐Gabrieli S, Gabrieli JD. Cognitive training changes hippocampal function in mild cognitive impairment: a pilot study. Journal of Alzheimer's Disease 2011;26(Suppl 3):349‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzini L, Costardi D, Chilovi BV, Franzoni S, Trabucchi M, Padovani A. Efficacy of cognitive rehabilitation in patients with mild cognitive impairment treated with cholinesterase inhibitors. International Journal of Geriatric Psychiatry 2007;22(4):356‐60. [DOI] [PubMed] [Google Scholar]
- Ryu SH, Kim S, Youn JH, Lee JY. Improvement cognitive functions in the elderly with mild cognitive impairment and subjective memory complaints. Conference: 16th International Congress of the International Psychogeriatric Association, IPA 2013 Seoul, South Korea 2013;Conference Start: 20131001. Conference End: 20131004(var.pagings):S165. [Google Scholar]
- Sakka P, Ntanasi E, Zoi P, Kalligerou F, Pantelopoulos S. Sociable: a comprehensive ICT cognitive training programme for healthy and cognitively impaired elderly. Neurology 2015;84(14):P6.188. [Google Scholar]
- Santos G, Ortega L, Yassuda M, Forlenza O, Nunes P. The effects of a multi‐professional cognitive and functional rehabilitation program for patients with Alzheimer's disease and mild cognitive impairment. Conference: Alzheimer's Association International Conference, AAIC 11 Paris, France 2011;Conference Start: 20110716. Conference End: 20110721(var.pagings):S800. [Google Scholar]
- Schoene D, Valenzuela T, Toson B, Delbaere K, Severino C, Garcia J, et al. Interactive cognitive‐motor step training improves cognitive risk factors of falling in older adults ‐ a randomized controlled trial. PLoS One 2015;10(12):e0145161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoene D, Valenzuela T, Toson B, Delbaere K, Severino C, Garcia J, et al. Interactive cognitive‐motor step training improves cognitive risk factors of falling in older adults ‐ A randomized controlled trial. PLoS One 2015;10(12):e0145161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher V, Theill N, Martin M. Improving cognitive performance and motor‐cognition adaptability of older adults using an integrative motor‐cognitive training approach. Conference: International Conference "Aging and Cognition", IfADo 2013 Germany. 2013; Vol. Conference Start: 20130425. Conference End: 20130427 Sponsor: Brain Products ‐ Solutions for Neurophysiological Research, Dortmund Tourismus, DFG ‐ Deutsche Forschungsgemeinsch:68‐9. [Google Scholar]
- Shah T, Verdile G, Sohrabi H, Martins R. Cross‐training of auditory and visual brain training software program improves cognition and alters plasma BDNF levels in healthy older adults. Alzheimer's and Dementia 2012;8(4):P99. [Google Scholar]; Shah T, Verdile G, Sohrabi H, Martins R. Physical activity and cognitive stimulation improve cognition and alter levels of plasma beta‐amyloid in healthy elderly. Alzheimer's and Dementia. 2012:151. [Google Scholar]
- Shatil E. Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone? A four‐condition randomized controlled trial among healthy older adults. Frontiers in Aging Neuroscience 2013;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatil E, Mikulecká J, Bellotti F, Bureš V. Novel television‐based cognitive training improves working memory and executive function. PLoS One 2014;9(7):e101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatil E, Mikulecká J, Bellotti F, Bureš V. Novel television‐based cognitive training improves working memory and executive function. PLoS One 2014;9(7):e101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisco SM, Marsiske M, Gross AL, Rebok GW. The influence of cognitive training on older adults' recall for short stories. Journal of Aging and Health 2013;25(8):230S‐48S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slegers K, Boxtel M, Jolles J. Effects of computer training and internet usage on cognitive abilities in older adults: a randomized controlled study. Aging Clinical and Experimental Research 2009;21(1):43‐54. [DOI] [PubMed] [Google Scholar]
- Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, et al. A cognitive training program based on principles of brain plasticity: results from the improvement in memory with plasticity‐based adaptive cognitive training (IMPACT) study. Journal of the American Geriatrics Society 2009;57(4):594‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith‐Ray RL, Makowski‐Woidan B, Hughes SL. A randomized trial to measure the impact of a community‐based cognitive training intervention on balance and gait in cognitively intact Black older adults. Health Education and Behavior 2014;41(1 Suppl):62S‐9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith‐Ray RL, Hughes SL, Prohaska TR, Little DM, Jurivich DA, Hedeker D. Impact of cognitive training on balance and gait in older adults. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 2015;70(3):357‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith‐Ray RL, Hughes SL, Prohaska TR, Little DM, Jurivich DA, Hedeker D. Impact of cognitive training on balance and gait in older adults. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 2015;70(3):357‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A, Levalahti E, Soininen H, Tuomilehto J, Lindstrom J, Lehtisalo J, et al. A multidomain, two‐year, randomized controlled trial to prevent cognitive impairment: the finger study. Conference: Alzheimer's Association International Conference 2014 Copenhagen, Denmark. 2014; Vol. Conference Start: 20140712. Conference End: 20140717:P137‐8. [Google Scholar]
- Park MH, Kwon DY, Seo WK, Lim KS, Song MS. The effects of cognitive training on community‐dwelling elderly Koreans. Journal of Psychiatric and Mental Health Nursing 2009;16(10):904‐9. [DOI] [PubMed] [Google Scholar]
- Stepankova H, Lukavsky J, Buschkuehl M, Kopecek M, Ripova D, Jaeggi SM. The malleability of working memory and visuospatial skills: a randomized controlled study in older adults. Developmental Psychology 2014;50(4):1049‐59. [DOI] [PubMed] [Google Scholar]
- Stine‐Morrow EA, Payne BR, Roberts BW, Kramer AF, Morrow DG, Payne L, et al. Training versus engagement as paths to cognitive enrichment with aging. Psychology and Aging 2014;29(4):891‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strenziok M, Parasuraman R, Clarke E, Cisler DS, Thompson JC, Greenwood PM. Neurocognitive enhancement in older adults: comparison of three cognitive training tasks to test a hypothesis of training transfer in brain connectivity. Neuroimage 2013;85:1027‐39. [DOI] [PubMed] [Google Scholar]
- Strenziok M, Parasuraman R, Clarke E, Cisler DS, Thompson JC, Greenwood PM. Neurocognitive enhancement in older adults: comparison of three cognitive training tasks to test a hypothesis of training transfer in brain connectivity. Neuroimage 2014;85(3):1027‐39. [DOI] [PubMed] [Google Scholar]
- Stürz K, Hartmann S, Eder‐Pelzer B, Günther V. [Computer assisted cognitive training advances mood and psychological wellbeing ‐ a comparison to paper pencil training relating to neuropsychological parameters, mood and cognitions]. Neuropsychiatrie 2011;25(2):85‐92. [PubMed] [Google Scholar]
- Sturz K, Hartmann S, Eder‐Pelzer B, Gunther V. [Computer assisted cognitive training advances mood and psychological wellbeing ‐ a comparison to paper pencil training relating to neuropsychological parameters, mood and cognitions]. [German]. Neuropsychiatrie 2011;25(2):85‐92. [PubMed] [Google Scholar]
- Sturz K, Hartmann S, Kemmler G, Gunther V. Influence of a relaxation program, cognitive training and a combination of both intervention forms on neuropsychological and affective parameters in elderly care home residents. Conference: 23rd European Congress of Psychiatry, EPA 2015 Vienna, Austria. 2015; Vol. Conference Start: 20150328. Conference End: 20150331:1447. [Google Scholar]
- Styliadis C, Kartsidis P, Paraskevopoulos E, Ioannides AA, Bamidis PD. Neuroplastic effects of combined computerized physical and cognitive training in elderly individuals at risk for dementia: an eLORETA controlled study on resting states. Neural Plasticity 2015;2015:172192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styliadis C, Kartsidis P, Paraskevopoulos E, Ioannides AA, Bamidis PD. Neuroplastic effects of combined computerized physical and cognitive training in elderly individuals at risk for dementia: an eLORETA controlled study on resting states. Neural Plasticity 2015;2015:172192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo C, Fiatarone Singh MA, Sachdev PS, Gates NJ, Valenzuela M. Resting state network adaptation in older adults with MCI in the SMART trial: unique effects of combined cognitive training and physical exercise. Conference: 3rd Biennial Conference on Resting State Brain Connectivity Magdeburg, Germany 2012;Conference Start: 20120905. Conference End: 20120907(var.pagings):A90‐1. [Google Scholar]
- Szelag E, Skolimowska J. Cognitive function in elderly can be ameliorated by training in temporal information processing. Restorative Neurology and Neuroscience 2012;30(5):419‐34. [DOI] [PubMed] [Google Scholar]
- Talib LL, Yassuda MS, Diniz BS, Forlenza OV, Gattaz WF. Cognitive training increases platelet PLA2 activity in healthy elderly subjects. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2008;78(4‐5):265‐9. [DOI] [PubMed] [Google Scholar]
- Tappen RM, Hain D. The effect of in‐home cognitive training on functional performance of individuals with mild cognitive impairment and early‐stage Alzheimer's disease. Research in Gerontological Nursing 2014;7(1):14‐24. [DOI] [PubMed] [Google Scholar]
- Tennstedt SL, Unverzagt FW. The ACTIVE study: study overview and major findings. Journal of Aging and Health 2013;25(8):3S‐20S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesky V, Pantel J. Cognitively stimulating leisure activities: a new approach for patients with mild cognitive impairment (MCI). Conference: Alzheimer's Association International Conference 2012 Vancouver, BC, Canada 2012;Conference Start: 20120714. Conference End: 20120719(var.pagings):P571. [Google Scholar]
- Tsai AY, Yang MJ, Lan CF, Chen CS. Evaluation of effect of cognitive intervention programs for the community‐dwelling elderly with subjective memory complaints. International Journal of Geriatric Psychiatry 2008;23(11):1172‐4. [DOI] [PubMed] [Google Scholar]
- Tsolaki M, Poptsi E, Kounti F, Christina A, Evaggelia B, Aikaterini S, et al. Longitudinal cognitive training in people with mild cognitive impairment. Conference: Alzheimer's Association International Conference 2013 Boston, MA, United States. 2013; Vol. Conference Start: 20130713. Conference End: 20130718:P491‐2. [Google Scholar]
- Tucker‐Drob EM, Johnson KE, Jones RN. The cognitive reserve hypothesis: a longitudinal examination of age‐associated declines in reasoning and processing speed. Developmental Psychology 2009;45(2):431‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D, Dawson J, Wadley V, Edwards J, Roenker D, Rizzo M, et al. The accelerate study: the longitudinal effect of speed of processing training on cognitive performance of older adults. Rehabilitation Psychology 2007;52(1):89‐96. [Google Scholar]
- Berg M, Sherrington C, Killington M, Smith S, Bongers B, Hassett L, et al. Video and computer‐based interactive exercises are safe and improve task‐specific balance in geriatric and neurological rehabilitation: a randomised trial. Journal of Physiotherapy 2016;62(1):20‐8. [DOI] [PubMed] [Google Scholar]
- Ploeg ES, Hoorweg A, Lee J. User friendliness of computer‐based cognitive training for psychogeriatric patients with mild to moderate cognitive impairments [Gebruiksvriendelijkheid van computerondersteunde cognitive training bij psychogeriatrische patiënten met lichte tot matige cognitieve functiestoornissen]. Tijdschrift voor Gerontologie en Geriatrie 2016;47(2):58‐67. [DOI] [PubMed] [Google Scholar]
- het Reve E, Bruin ED. Strength‐balance supplemented with computerized cognitive training to improve dual task gait and divided attention in older adults: a multicenter randomized‐controlled trial. BMC Geriatrics 2014;14:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidovich MR, Lautenschlager NT, Flicker L, Clare L, Almeida OP. The PACE study: a randomised clinical trial of cognitive activity (CA) for older adults with mild cognitive impairment (MCI). Trials 2009;10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidovich MR, Lautenschlager NT, Flicker L, Clare L, McCaul K, Almeida OP. The PACE study: a randomized clinical trial of cognitive activity strategy training for older people with mild cognitive impairment. American Journal of Geriatric Psychiatry 2015;23(4):360‐72. [DOI] [PubMed] [Google Scholar]
- Vidovich MR, Lautenschlager NT, Flicker L, Clare L, McCaul K, Almeida OP. The PACE study: a randomized clinical trial of cognitive activity strategy training for older people with mild cognitive impairment. American Journal of Geriatric Psychiatry 2015;23(4):360‐72. [DOI] [PubMed] [Google Scholar]
- Bastian CC, Langer N, Jancke L, Oberauer K. Effects of working memory training in young and old adults. Memory & Cognition 2013;41(4):611‐24. [DOI] [PubMed] [Google Scholar]
- Wadley VG, Crowe M, Marsiske M, Cook SE, Unverzagt FW, Rosenberg AL, et al. Changes in everyday function in individuals with psychometrically defined mild cognitive impairment in the Advanced Cognitive Training for Independent and Vital Elderly Study. Journal of the American Geriatrics Society 2007;55(8):1192‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton CC, Kavanagh A, Downey LA, Lomas J, Camfield DA, Stough C. Online cognitive training in healthy older adults: a preliminary study on the effects of single versus multi‐domain training. Translational Neuroscience 2015;6(1):13‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JR, Hsieh S. Neurofeedback training improves attention and working memory performance. Clinical Neurophysiology 2013;124(12):2406‐20. [DOI] [PubMed] [Google Scholar]
- Weicker J, Hudl N, Marichal E, Muller K, Lepsien J, Trapp S, et al. Training of working memory in healthy elderly subjects ‐ a randomized controlled trial. Conference: Joint Meeting of the FESN/GNP 2013 Berlin, Germany 2013;Conference Start: 20130912. Conference End: 20130914(var.pagings):371. [Google Scholar]
- Wild‐Wall N, Falkenstein M, Gajewski PD. Neural correlates of changes in a visual search task due to cognitive training in seniors. Neural Plasticity 2012;2012:529057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Herman R, Bontempo D. Reasoning exercises in assisted living: a cluster randomized trial to improve reasoning and everyday problem solving. Clinical Interventions in Aging 2014;9:981‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL, Schaie KW. Training the elderly on the ability factors of spatial orientation and inductive reasoning. Psychology and Aging 1986;1(3):239‐47. [DOI] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Long‐term effects of cognitive training on everyday functional outcomes in older adults. JAMA 2006;296(23):2805‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Long‐term effects of cognitive training on everyday functional outcomes in older adults. JAMA 2006;296(23):2805‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Mann Koepke K, Morris JN, Rebok GW, Unverzagt FW, Stoddard AM, Wright E, ACTIVE Study Group. Long‐term Effects of Cognitive Training on Everyday Functional Outcomes in Older Adults. American Journal of Health Promotion 2007;21(5):469‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL, Caskie GI. Reasoning training in the ACTIVE study: how much is needed and who benefits?. Journal of Aging and Health 2013;25(8 Suppl):43S‐64S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtynska R, Wlazlo A, Trypka E, Zimny A, Frydecka D. The evaluation of the effectiveness of the program of the cognitive rehabilitation of patients with MCI and early dementia of Alzheimer's type. European Psychiatry 2011;26(1):504. 21398097 [Google Scholar]
- Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Wright E, Tennstedt SL. The effects of the ACTIVE cognitive training trial on clinically relevant declines in health‐related quality of life. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 2006;61B(5):S281‐7. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Stoddard A, Tennstedt SL. The ACTIVE cognitive training trial and health‐related quality of life: protection that lasts for 5 years. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 2006;61(12):1324‐9. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Vander Weg MW, Martin R, Unverzagt FW, Willis SL, Marsiske M, et al. Does cognitive training improve internal locus of control among older adults?. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 2010;65(5):591‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Mahncke H, Vander Weg MW, Martin R, Unverzagt FW, Ball KK, et al. Speed of processing training protects self‐rated health in older adults: enduring effects observed in the multi‐site ACTIVE randomized controlled trial. International Psychogeriatrics2010; Vol. 22, issue 3:470‐8. [DOI] [PMC free article] [PubMed]
- Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM. A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PLoS One 2013;8(5):e61624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM. The effect of cognitive speed of processing training on the development of additional IADL difficulties and the reduction of depressive symptoms: results from the IHAMS randomized controlled trial. Journal of Aging and Health 2015;27(2):334‐54. [DOI] [PubMed] [Google Scholar]
- Yam A, Gross AL, Prindle JJ, Marsiske M. Ten‐year longitudinal trajectories of older adults' basic and everyday cognitive abilities. Neuropsychology 2014;28(6):819‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassuda MS, Camargo MC, Brum PS, Bento T, Silva L, Spindola L. Working memory training: effects on cognition and psychological wellbeing of seniors without dementia and depression. Conference: Alzheimer's Association International Conference 2015 Washington, DC, United States 2015;Conference Start: 20150718. Conference End: 20150723:P462. [Google Scholar]
- Yip CB. An intelligent rehabilitation system for cognitive rehabilitation. Thesis 2012;73(3‐B):1524. [Google Scholar]
- Yoonmi L, Chang‐Ryeol L, Byeongjun H. Effects of computer‐aided cognitive rehabilitation training and balance exercise on cognitive and visual perception ability of the elderly. Journal of Physical Therapy Science 2012;24(9):885‐7. [Google Scholar]
- Youn JH, Lee JY, Kim S, Ryu SH. Multistrategic memory training with the metamemory concept in healthy older adults. Psychiatry Investigation 2011;8(4):354‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski EM, Dalton SE, Smith GE. Consumer‐based brain fitness programs. Enhancing cognitive fitness in adults: a guide to the use and development of community‐based programs. Springer, 2011:45‐66. [Google Scholar]
- Zelinski EM, Spina LM, Yaffe K, Ruff R, Kennison RF, Mahncke HW, et al. Improvement in memory with plasticity‐based adaptive cognitive training: results of the 3‐month follow‐up. Journal of the American Geriatrics Society 2011;59(2):258‐65. [DOI] [PubMed] [Google Scholar]
- Zhuang JP, Fang R, Feng X, Xu XH, Liu LH, Bai QK, et al. The impact of human‐computer interaction‐based comprehensive training on the cognitive functions of cognitive impairment in elderly individuals in a nursing home. Journal of Alzheimer's Disease 2013;36(2):245‐51. [DOI] [PubMed] [Google Scholar]
- Zimmermann N, Netto TM, Amodeo MT, Ska B, Fonseca RP. Working memory training and poetry‐based stimulation programs: are there differences in cognitive outcome in healthy older adults?. NeuroRehabilitation 2014;35(1):159‐70. [DOI] [PubMed] [Google Scholar]
Additional references
- Alzheimer's Association National Plan Milestone Workgroup, Fargo KN, Aisen P, Albert M, Au R, Corrada MM, et al. 2014 Report on the Milestones for the US National Plan to Address Alzheimer's Disease. Alzheimer's and Dementia 2014;10(5):S430‐52. [DOI] [PubMed] [Google Scholar]
- Abraham RP, Denton DA, Al‐Assaf AS, Rutjes AWS, Chong LY, Malik Muzaffar A, et al. Vitamin and mineral supplementation for prevention of dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011905] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo A, Loewenstein DA. Nonpharmacological cognitive interventions in aging and dementia. Journal of Geriatric Psychiatry and Neurology 2007;20(4):239‐49. [DOI] [PubMed] [Google Scholar]
- Al‐Assaf AS, Denton DA, Abraham RP, Rutjes AW, Chong LY, Anderson JL, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in late life. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011906] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargo KN, Aisen P, Albert M, Au R, Corrada MM, DeKosky S, et al. 2014 Report on the Milestones for the US National Plan to Address Alzheimer's Disease. Alzheimer's and Dementia 2014;10(5 Suppl):S430‐52. [DOI] [PubMed] [Google Scholar]
- Amoyal N, Fallon E. Physical exercise and cognitive training clinical interventions used in slowing degeneration associated with mild cognitive impairment. A review of the literature. Topics in Geriatric Rehabilitation 2012;28(3):208‐16. [Google Scholar]
- Andrieu S, Coley N, Lovestone S, Aisen P, Vellas B. Prevention of sporadic Alzheimer’s disease: lessons learned from clinical trials and future directions. Lancet Neurology 2015;14:926. [DOI] [PubMed] [Google Scholar]
- Barnes D, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurology 2011;10(9):819‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barulli D, Stren Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends in Cognitive Sciences 2013;17(10):502‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleville S, Bherer L. Biomarkers of cognitive training effects in aging. Current Translational Geriatrics and Experimental Gerontology Reports 2012;1(2):104‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Arnold SE, Valenzuela MJ, Brayne C, Schneider JA. Cognitive and social lifestyle: links with neuropathology and cognition in late life. Acta Neuropathologica 2014;127(1):137‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta‐analysis. BMC Public Health 2014;14(1):643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold N, Christie LA. Exercise builds brain health; key roles of growth factor cascades and inflammation. Trends in Neurosciences 2007;30(9):464‐72. [DOI] [PubMed] [Google Scholar]
- Curlik DM, Shors TJ. Training your brain: do mental and physical (MAP) training enhance cognition through the process of neurogenesis in the hippocampus?. Neuropharmacology 2013;64:506‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa BR, Nuesch E, Reichenbach S, Juni P, Rutjes AWS. Doxycycline for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD007323.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa BR, Nüesch E, Kasteler R, Husni E, Welch V, Rutjes AWS, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD003115.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE. Age‐associated cognitive decline. BMJ 2009;92:135‐52. [DOI] [PubMed] [Google Scholar]
- Denton DA, Abraham RP, Al‐Assaf AS, Rutjes AW, Chong LY, Anderson JL, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid life. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011904] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depp CA, Harmell A, Vahia IV. Successful cognitive aging. Current Topics in Behavioral Neurosciences 2012;10:35‐50. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [PUBMED: 3802833] [DOI] [PubMed] [Google Scholar]
- Doody RS. Commentary on "A roadmap for the prevention of dementia II. Leon Thal Symposium 2008." Centers of Excellence in Alzheimer's disease: it is time to better integrate patient care and clinical research to improve the prevention and treatment of Alzheimer's disease. Alzheimer's and Dementia 2009;5(2):133‐6. [DOI] [PubMed] [Google Scholar]
- Dresler M, Sandberg A, Ohla K, Bublitz C, Trenado C, Mroczko‐Wasowicz A, et al. Non‐pharmacological cognitive enhancement. Neuropharmacology 2013;64:529‐43. [DOI] [PubMed] [Google Scholar]
- Forbes SC, Forbes D, Forbes S, Blake CM, Chong LY, Thiessen EJ, et al. Exercise interventions for preventing dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011706] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes SC, Forbes D, Forbes S, Blake CM, Chong LY, Thiessen EJ, et al. Exercise interventions for maintaining cognitive function in cognitively healthy people in mid life. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011705] [DOI] [Google Scholar]
- Forbes SC, Forbes D, Forbes S, Blake CM, Chong LY, Thiessen EJ, et al. Exercise interventions for maintaining cognitive function in cognitively healthy people in late life. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011704] [DOI] [Google Scholar]
- Gates NJ, Valenzuela MJ. Cognitive exercise and its role in cognitive functions in older adults. Current Psychiatry Reports 2010;12(1):20‐7. [DOI] [PubMed] [Google Scholar]
- Gates NJ, Sachdev P. Is cognitive training an effective treatment for preclinical and early Alzheimer’s disease?. Journal of Alzheimer's Disease 2014;42(Suppl 4):S551‐9. [DOI] [PubMed] [Google Scholar]
- Gates NJ, Rutjes AWS, Nisio M, Karim S, Chong L, March E, Vernooij RWM. Computerised cognitive training for maintaining cognitive function in cognitively healthy people in late life. Cochrane Database of Systematic Reviews [under submission], Issue [under submission]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates NJ, Vernooij RWM, Nisio M, Karim S, March E, Rutjes AWS. Computerised cognitive training for preventing dementia in people with mild cognitive impairment. Cochrane Database of Systematic Reviews [under submission], Issue [under submission]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geda YE, Silber TC, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, et al. Computer activities, physical exercise, aging, and mild cognitive impairment: a population‐based study. Mayo Clinic Proceedings 2012;87(5):437‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C. The cognitive neuroscience of ageing. Nature Reviews. Neuroscience 2012;13(7):491‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CS, Strobach T, Schubert T. On methodological standards in training and transfer experiments. Psychological Research 2014;78(6):756‐72. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SL, Birdi R, Smart Chris O, Brittain K, Rutjes AW, Siervo M, et al. Dietary interventions for maintaining cognitive function in cognitively healthy people in mid life. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011911] [DOI] [Google Scholar]
- Hendrie HC, Albert MS, Butters MA, Gao S, Knopman DS, Launer LJ, et al. The NIH Cognitive and Emotional Health Project. Report of the Critical Evaluation Study Committee. Alzheimer's and Dementia 2006;2(1):12‐32. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development: can the functional capacity of older adults be preserved and enhanced?. Psychological Science in the Public Interest 2008;9(1):1‐65. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Kueider AM, Parisi JM, Gross AL, Rebok GW. Computerized cognitive training with older adults: a systematic review. PLoS ONE 2012;7(7):e40588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampit A, Hallock H, Valenzuela M. Computerized cognitive training in healthy older adults: a systematic review and meta‐analysis of effect modifiers. PLoS Medicine 2014;11(11):e1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Marks SM, Mormino EC, Rabinovici GD, Oh H, O'Neil JP, et al. Association of lifetime cognitive engagement and low β‐amyloid deposition. Archives of Neurology 2012;69(5):623‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifer BP. Early diagnosis of Alzheimer's disease: clinical and economic benefits. Journal of the American Geriatrics Society 2003;51(5 Suppl Dementia):S281‐8. [DOI] [PubMed] [Google Scholar]
- Lustig C, Shah P, Seidle R, Reuter‐Lorenz PA. Aging, training, and the brain and future directions. Neuropsychology Review 2009;19(4):504‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE, Proust‐Lima C, Amieva H, Brayne C, Matthews FE, Dartigues JF, et al. Cognitive lifestyle jointly predicts longitudinal cognitive decline and mortality risk. European Journal of Epidemiology 2014;29(3):211‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquine MJ, Segawa E, Wilson RS, Bennett DA, Barnes LL. Association between cognitive activity and cognitive function in older Hispanics. Journal of the International Neuropsychological Society 2012;18(6):1041‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Clare L, Altgassen AM, Cameron MH, Zehnder F. Cognition‐based interventions for healthy older people and people with mild cognitive impairment. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD006220.pub2] [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA Statement. BMJ 2009;339(2535):332‐6. [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan A. Enriched environments, experience dependent plasticity and disorders of the nervous system. Nature Reviews. Neuroscience 2006;7(9):697‐709. [DOI] [PubMed] [Google Scholar]
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population‐based data. Lancet Neurology 2014;13(8):788‐94. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nature Neurosciences 2004;7(1):75‐9. [DOI] [PubMed] [Google Scholar]
- Opdebeeck C, Martyr A, Clare L. Cognitive reserve and cognitive function in healthy older people: a meta‐analysis. Aging, Neuropsychology, and Cognition 2016;23(1):40‐60. [DOI] [PubMed] [Google Scholar]
- Papp KV, Walsh SJ, Snyder PJ. Immediate and delayed effects of cognitive interventions in healthy elderly: a review of current literature and future directions. Alzheimer's and Dementia 2009;5(1):50‐60. [DOI] [PubMed] [Google Scholar]
- Park, DC, Gutchess AH, Meade ML, Stine‐Morrow EA. Improving cognitive function in older adults: nontraditional approaches. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 2007;62:45‐52. [DOI] [PubMed] [Google Scholar]
- Park DC, Bischof GN. The ageing mind: neuroplasticity in response to cognitive training. Dialogues in Clinical Neuroscience 2013;15(1):109‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach S, Rutjes AW, Nüesch E, Trelle S, Jüni P. Joint lavage for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD007320.pub2] [DOI] [PubMed] [Google Scholar]
- Reijnders J, van Heugten, Boxtel M. Cognitive interventions in healthy older adults and people with mild cognitive impairment: a systematic review. Ageing Research Reviews 2013;12(1):263‐75. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Rutjes AW, Nüesch E, Sterchi R, Kalichman L, Hendriks E, Osiri M, et al. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD002823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutjes AW, Nuesch E, Reichenbach S, Juni P. S‐Adenosylmethionine for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007321.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutjes AW, Nuesch E, Sterchi R, Juni P. Therapeutic ultrasound for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003132.pub2] [DOI] [PubMed] [Google Scholar]
- Rutjes AW, Juni P, Costa BR, Trelle S, Nuesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta‐analysis. Annals of Internal Medicine 2012;157(3):180‐91. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Memory aging from 18 to 80. Alzheimer Disease Associated Disorders 2003;17(3):162‐7. [DOI] [PubMed] [Google Scholar]
- Salthouse T. Neuroanatomical substrates of age‐related cognitive decline. Psychological Bulletin 2011;137(5):753‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedek F, Lövdén M, Lindenberger U. Hundred days of cognitive training enhance broad cognitive abilities in adulthood: findings from the COGITO study. Frontiers in Aging Neuroscience 2010;2(27):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Mang J, Li P, Wang J, Deng T, Xu Z. Computer‐based cognitive programs for improvement of memory, processing speed and executive function during age‐related cognitive decline: a meta‐analysis. PLoS One 2015;10(6):e0130831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing YL, Werkle‐Bergner M, Li SC, Lindenberger U. Associative and strategic components of episodic memory: a life‐span dissociation. Journal of Experimental Psychology. General 2008;137(3):495‐513. [DOI] [PubMed] [Google Scholar]
- Siervo M, Lara J, Munro A, Tang EY, Rutjes AW, Stephan B. Dietary interventions for maintaining cognitive function in cognitively healthy people in late life. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011910] [DOI] [Google Scholar]
- Sixsmith A, Carrillo M, Phillips D, Lansley P, Woolrych R. International initiatives in technology and aging. In: Sixsmith A, Gutman G editor(s). Technologies for Active Aging (International Perspectives on Aging). Vol. 9, New York: Springer, 2013:201‐22. [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia 2009;47(10):2015‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurology 2012;11(11):1006‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo C, Valenzuela MJ. Neuroimaging outcomes of brain training trials. In: Bright P editor(s). Neuroimaging Cognitive and Clinical Neuroscience. INTECH Open Access Publisher, 2012. [Google Scholar]
- Tang EY, Harrison SL, Albanese E, Gorman TJ, Rutjes AW, Siervo M, et al. Dietary interventions for prevention of dementia in people with mild cognitive impairment. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011909] [DOI] [Google Scholar]
- Valenzuela MJ, Jones M, Wen W, Rae C, Graham S, Shnier R, et al. Memory training alters hippocampal neurochemistry in healthy elderly. Neuroreport 2003;14(10):1333‐7. [DOI] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, et al. Leisure activities and the risk of dementia in the elderly. New England Journal of Medicine 2003;348(25):2508‐16. [DOI] [PubMed] [Google Scholar]
- Walton CC, Mowszowski L, Lewis SJ, Naismith SL. Stuck in the muck: time for change in the implementation of cognitive training research in ageing?. Frontiers in Aging Neuroscience 2014;6:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Ageing and life‐course. www.who.int/ageing/active_ageing/en/ (accessed 18 Jan 2016).
- Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, et al. Participation in cognitively stimulating activities and risk of incidence of Alzheimer’s disease. JAMA 2002;287(6):742‐8. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Segawa E, Boyle PA, Bennett DA. Influence of late‐life cognitive activity on cognitive health. Neurology 2012;78(15):1123‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The World Alzheimer Report 2014. Dementia and Risk Reduction: An Analysis of Protective and Modifiable Factors. London: Alzheimer’s Disease International (ADI), 2014. [Google Scholar]


