Abstract
Background
The number of people living with dementia is increasing rapidly. Clinical dementia does not develop suddenly, but rather is preceded by a period of cognitive decline beyond normal age‐related change. People at this intermediate stage between normal cognitive function and clinical dementia are often described as having mild cognitive impairment (MCI). Considerable research and clinical efforts have been directed toward finding disease‐modifying interventions that may prevent or delay progression from MCI to clinical dementia.
Objectives
To evaluate the effects of at least 12 weeks of computerised cognitive training (CCT) on maintaining or improving cognitive function and preventing dementia in people with mild cognitive impairment.
Search methods
We searched to 31 May 2018 in ALOIS (www.medicine.ox.ac.uk/alois) and ran additional searches in MEDLINE, Embase, PsycINFO, CINAHL, ClinicalTrials.gov, and the WHO portal/ICTRP (www.apps.who.int/trialsearch) to identify published, unpublished, and ongoing trials.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs in which cognitive training via interactive computerised technology was compared with an active or inactive control intervention. Experimental computerised cognitive training (CCT) interventions had to adhere to the following criteria: minimum intervention duration of 12 weeks; any form of interactive computerised cognitive training, including computer exercises, computer games, mobile devices, gaming console, and virtual reality. Participants were adults with a diagnosis of mild cognitive impairment (MCI) or mild neurocognitive disorder (MND), or otherwise at high risk of cognitive decline.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias of the included RCTs. We expressed treatment effects as mean differences (MDs) or standardised mean differences (SMDs) for continuous outcomes and as risk ratios (RRs) for dichotomous outcomes. We used the GRADE approach to describe the overall quality of evidence for each outcome.
Main results
Eight RCTs with a total of 660 participants met review inclusion criteria. Duration of the included trials varied from 12 weeks to 18 months. Only one trial used an inactive control. Most studies were at unclear or high risk of bias in several domains. Overall, our ability to draw conclusions was hampered by very low‐quality evidence. Almost all results were very imprecise; there were also problems related to risk of bias, inconsistency between trials, and indirectness of the evidence.
No trial provided data on incident dementia. For comparisons of CCT with both active and inactive controls, the quality of evidence on our other primary outcome of global cognitive function immediately after the intervention period was very low. Therefore, we were unable to draw any conclusions about this outcome.
Due to very low quality of evidence, we were also unable to determine whether there was any effect of CCT compared to active control on our secondary outcomes of episodic memory, working memory, executive function, depression, functional performance, and mortality. We found low‐quality evidence suggesting that there is probably no effect on speed of processing (SMD 0.20, 95% confidence interval (CI) ‐0.16 to 0.56; 2 studies; 119 participants), verbal fluency (SMD ‐0.16, 95% CI ‐0.76 to 0.44; 3 studies; 150 participants), or quality of life (mean difference (MD) 0.40, 95% CI ‐1.85 to 2.65; 1 study; 19 participants).
When CCT was compared with inactive control, we obtained data on five secondary outcomes, including episodic memory, executive function, verbal fluency, depression, and functional performance. We found very low‐quality evidence; therefore, we were unable to draw any conclusions about these outcomes.
Authors' conclusions
Currently available evidence does not allow us to determine whether or not computerised cognitive training will prevent clinical dementia or improve or maintain cognitive function in those who already have evidence of cognitive impairment. Small numbers of trials, small samples, risk of bias, inconsistency between trials, and highly imprecise results mean that it is not possible to derive any implications for clinical practice, despite some observed large effect sizes from individual studies. Direct adverse events are unlikely to occur, although the time and sometimes the money involved in computerised cognitive training programmes may represent significant burdens. Further research is necessary and should concentrate on improving methodological rigour, selecting suitable outcomes measures, and assessing generalisability and persistence of any effects. Trials with long‐term follow‐up are needed to determine the potential of this intervention to reduce the risk of dementia.
Keywords: Aged; Humans; Middle Aged; Cognition; Cognitive Dysfunction; Cognitive Dysfunction/complications; Computer‐Assisted Instruction; Computer‐Assisted Instruction/methods; Dementia; Dementia/prevention & control; Disease Progression; Executive Function; Memory, Episodic; Quality of Life; Randomized Controlled Trials as Topic; Time Factors
Computerised cognitive training for preventing dementia in people with mild cognitive impairment
Background
The terms 'cognition' and 'cognitive function' describe all of the mental activities related to thinking, learning, remembering, and communicating. There are normal changes in cognition with age, There are also diseases that affect cognition, principally dementia, in which cognition is impaired to the point of affecting a person's ability to manage daily activities. More common than dementia is a condition often described as mild cognitive impairment (MCI), in which mild impairment of cognition, more than expected from age alone, can be detected on testing, but by which daily functioning is largely unaffected. For some people, MCI is a stage on the way to developing dementia. There is a lot of interest in anything that might prevent further decline in cognition in people with MCI. One thing that has been suggested as a means of doing this is computerised cognitive training (CCT). Cognitive training consists of a set of standardised tasks intended to 'exercise the brain' in various ways. These days, cognitive training exercises are often delivered via computers or mobile technology, so that people can do them on their own at home. We wanted to know whether CCT is an effective way for people with MCI to maintain their cognitive function and reduce their risk of going on to develop dementia.
What we did
We searched the medical literature up to 15 March 2018 for trials in which a group of people with MCI had participated in CCT for at least 12 weeks and had been compared with another group that had not received any CCT. This 'control' group could have taken part in an alternative activity instead, or group members could have received no intervention at all. For the comparison to be as fair as possible, it should have been decided at random whether people were in the CCT or control group. We were primarily interested in whether study participants developed dementia and in their overall cognitive function, but we also looked for evidence on particular cognitive skills, daily activities, quality of life, mood, or mental well‐being, and any harmful effects.
What we found
We found eight trials with 660 participants to include in the review. Seven of the trials (623 participants) compared CCT to an alternative activity. None of the included trials examined development of dementia, so this review presents no evidence on whether taking part in computerised cognitive training will help to prevent dementia. Our main finding in relation to all of the other outcomes in which we were interested was that the overall quality of the evidence was very low. This very low quality was mainly due to small sample sizes, problems with study methods, and differences between trials. Therefore, although we found some evidence for a few benefits of CCT for cognition, we were highly uncertain about study results and consider it likely that future research might lead to different results.
Our conclusions
Unfortunately, it is not yet possible to answer our review question with any certainty. We think it remains an important area for further study. We would like to see larger studies, which would be more able to detect effects of CCT, and longer studies, which are needed to show whether there are any benefits, whether benefits are long‐lasting, and whether there is a chance of preventing or delaying the development of dementia.
Summary of findings
Summary of findings for the main comparison.
| Computerised cognitive training compared with active control in people with mild cognitive impairment | ||||
|
Patient or population: patients with mild cognitive impairment Settings: general population Intervention: computerised cognitive training Comparison: active control | ||||
| Outcomes | Differences between CCT and control (95% CI)* | No. of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Global cognitive functioning (follow‐up ranging from 3 months up to 2 years) | SMD 0.53 lower (1.06 lower to 0.01 lower) | 407 participants (5 studies) | ⊕⊝⊝⊝ very lowb | It is uncertain whether CCT maintains global cognitive functioning better than active control |
| Episodic memory (follow‐up ranging from 3 months up to 2 years) | SMD 0.79 lower (1.54 lower to 0.04 lower) | 223 participants (5 studies) | ⊕⊝⊝⊝ very lowb | It is uncertain whether CCT improves episodic memory compared to active control |
| Speed of processing (follow‐up ranging from 3 months up to 2 years) |
SMD 0.20 higher (0.16 lower to 0.56 higher) | 119 participants (2 studies) | ⊕⊕⊝⊝ lowc | CCT may have little or no effect on speed of processing |
| Executive functioning (follow‐up ranging from 3 months up to 2 years) |
SMD 0.31 lower (0.90 lower to 0.28 higher) | 150 participants (3 studies) | ⊕⊝⊝⊝ very lowb | It is uncertain whether CCT improves executive functioning better than active control |
| Working memory (follow‐up ranging from 3 months up to 9 months) | SMD 0.88 lower (1.73 lower to 0.03 lower) | 72 participants (3 studies) | ⊕⊝⊝⊝ very lowd | It is uncertain whether CCT improves working memory compared to active control |
| Verbal fluency (follow‐up ranging from 3 months up to 18 months) | SMD 0.16 lower (0.76 lower to 0.44 higher) | 150 participants (3 studies) | ⊕⊕⊝⊝ lowc | CCT may have little or no effect on speed of processing |
| Quality of life (3 months of follow‐up) |
MD 0.40 higher (1.85 higher to 2.65 lower) | 19 participants (1 study) | ⊕⊕⊝⊝ lowc | CCT may have little or no effect on quality of life |
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CCT: computerised cognitive training; CI: confidence interval; MD: mean difference; RR: risk ratio; SMD: standardised mean difference. | ||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||
aThe direction of the difference in effect was standardised, so that lower values favour CCT and higher values favour control.
bDowngraded three levels for imprecision (confidence interval included effects that are not clinically relevant), inconsistency (high heterogeneity), and risk of bias.
cDowngraded two levels for imprecision (confidence interval included effects that are not clinically relevant) and risk of bias.
dDowngraded four levels for imprecision (confidence interval included effects that are not clinically relevant), inconsistency (high heterogeneity), indirectness, and risk of bias.
Summary of findings 2.
| Computerised cognitive training compared with inactive control in people with mild cognitive impairment | ||||
|
Patient or population: patients with mild cognitive impairment Settings: general population Intervention: computerised cognitive training Comparison: inactive control | ||||
| Outcomes | Difference between CCT and control (95% CI)* | No. of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Global cognitive functioning (measured at 12 months of follow‐up) |
MD 0.36 lower (0.30 lower to 1.02 higher) |
37 participants (1 study) |
⊕⊝⊝⊝ very lowb | It is uncertain whether CCT maintains global cognitive functioning better than inactive control |
| Episodic memory (measured at 12 months of follow‐up) |
MD 2.70 lower (5.00 lower to 0.40 lower) |
37 participants (1 study) |
⊕⊝⊝⊝ very lowb | It is uncertain whether CCT improves episodic memory compared to inactive control |
| Executive function (measured at 12 months of follow‐up) | MD 2.70 lower (6.21 lower to 0.81 higher) | 37 participants (1 study) | ⊕⊝⊝⊝ very lowb | It is uncertain whether CCT improves executive function compared to inactive control |
| Verbal fluency (measured at 12 months of follow‐up) | MD 1.90 higher (4.50 lower to 8.30 higher) | 37 participants (1 study) | ⊕⊝⊝⊝ very lowb | It is uncertain whether CCT improves verbal fluency compared to inactive control |
| Depression (measured at 12 months of follow‐up) | MD 1.30 lower (2.61 lower to 0.01 higher) | 37 participants (1 study) | ⊕⊝⊝⊝ very lowb | It is uncertain whether CCT improves depression compared to inactive control |
| Functional performance (measured at 12 months of follow‐up) | MD 0.00 lower (0.48 lower to 0.48 higher) | 37 participants (1 study) | ⊕⊝⊝⊝ very lowb | It is uncertain whether CCT improves functional performance compared to inactive control |
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CCT: computerised cognitive training; CI: confidence interval; MD: mean difference; RR: risk ratio. | ||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||
aThe direction of the difference in effect was standardised so that lower values favour CCT and higher values favour control
bDowngraded 3 levels for imprecision (confidence interval included effects that are not clinically relevant), risk of bias, and indirectness (cholinesterase inhibitors were included in the comparison which is not an approved medication for MCI patients)
Background
Description of the condition
Mild cognitive impairment
Normal ageing is associated with decline in many core cognitive functions (Salthouse 2003). When cognition deteriorates beyond normal age‐related change, but the ability to complete ordinary activities of daily function remains largely intact, the condition is described as mild cognitive impairment (MCI). In some people, MCI is an intermediate state on the pathway from normal cognition to dementia. When several cognitive domains are involved and function in daily activities has deteriorated significantly, the diagnosis is changed to that of dementia. However, there is no clear demarcation between normal cognition and mild cognitive impairment, or between mild cognitive impairment and dementia, and it is impossible to identify the specific points of conversion (Aisen 2011; Albert 2011).
One review identified 16 different classification and measurement approaches for MCI (Matthews 2008); there remains no standard definition of MCI accepted for use in clinical trials (Stephan 2013). The National Institute on Aging (NIA)‐Alzheimer’s Association published criteria for MCI in 2011 (Albert 2011), but the criteria suggested earlier by Petersen are still commonly used in clinical research (Petersen 1999). Clinical subtypes have been introduced based on the presence or absence of a primary memory impairment (amnestic or non‐amnestic MCI), and on the number of cognitive domains affected (single domain or multiple domains) (Petersen 2009; Winblad 2004). Further subdivisions can be made depending on the suspected underlying cause of cognitive deficits, for example, MCI due to Alzheimer's disease (MCI‐AD) and MCI due to vascular disease (also termed 'vascular cognitive impairment no dementia' (VCIND)). The term 'mild neurocognitive disorder' is broadly synonymous with MCI.
The prevalence of MCI is more than double than that of dementia (Petersen 2009). A recent review suggests a prevalence of MCI of 6.7% in those aged 60 to 64 years, increasing to 25.2% among those aged 80 to 84 (Petersen 2018). However prevalence rates vary depending on the diagnostic criteria used. When 18 different definitions of MCI were mapped, prevalence estimates were found to range from 0.1% to 42%, and 'conversion' rates to dementia were found to be generally low (Stephan 2007). Prevalence and conversion rates in specialist settings are higher than those observed in population‐based studies, with the adjusted annual conversion rate from MCI to dementia of 9.6% in specialist settings compared to 4.9% in the general population (Mitchell 2009). A large number of individuals with a diagnosis of MCI do not go on to develop dementia, and between 14% and 40% revert to normal cognitive function for their age (Koepsell 2012). Mild cognitive difficulties in themselves have functional and psychological ramifications for quality of life (Mitchell 2009).
Dementia
Dementia is usually a progressive syndrome of cognitive and functional decline. Although most commonly associated with 'forgetfulness', dementia, by definition, involves impairments in more than one cognitive domain, and impairments in language, executive function, complex attention, and social cognition are commonly identified. As the syndrome progresses, those affected become increasingly dependent on care from others for all activities of daily living (e.g. feeding, bathing, taking medication). Dementia is one of the principal causes of disease, disability, and decreased quality of life among older adults and is now identified as one of the biggest global health challenges. It may affect up to 135 million adults worldwide by 2050 (Prince 2013). The global economic cost of care for people with dementia is currently estimated at $315 billion (Wimo 2010).
Dementia is sometimes referred to as a neurocognitive disorder, as in the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM‐V; APA 2013); the two terms may be used interchangeably. Subtypes of dementia are distinguished by the underlying brain pathology. The four most common subtypes of dementia include:
dementia due to Alzheimer's disease (AD), which accounts for an estimated 60% to 70% of all dementia cases;
vascular dementia (VaD);
dementia with Lewy bodies (DLB); and
frontotemporal dementia (FTD).
Accurate diagnosis of subtypes can be difficult, especially when the clinical disease is severe. Mixed pathology is commonly reported, with more than 80% of cases having some features of AD (Jellinger 2006; WHO 2012).
Alzheimer's disease (AD), the most common cause of dementia, is now known to have a long prodromal period. In those with AD, MCI ‐ the symptomatic pre‐dementia phase ‐ offers an opportunity to introduce interventions that may prevent or postpone the onset of clinical dementia (Leifer 2003). Delaying progression from MCI to dementia would lead to a reduction in the incidence of dementia, with a significant reduction in associated costs to society and improved quality of life for individuals. Postponement of dementia onset by five years may reduce prevalence by 50% (Brookmeyer 1998). No drugs are currently available that can reduce the risk of progression from MCI to dementia (Russ 2012). As a result, investigations are focusing on non‐pharmacological interventions that may delay clinical progression (Acevedo 2007; Dresler 2013).
Risk and protective factors for MCI and dementia
Age is the strongest risk factor for dementia. However, research has identified several additional risk and protective factors linked with late‐onset dementia in general and with AD in particular (World Alzheimer Report 2014). The World Health Organization 2017 Dementia Action Plan reports that reducing such risks is a major health objective to reduce disability (who.int/mental_health/neurology/dementia/action_plan_2017_2025/en/). Epidemiological evidence suggests that AD shares many risk factors with vascular dementia; these include cerebrovascular disease, type 2 diabetes, midlife obesity, midlife hypertension, smoking, and physical inactivity (Pendlebury 2009; WHO 2012; World Alzheimer Report 2014). It has recently been suggested that, after non‐independence between risk factors is accounted for, around a third of AD cases worldwide might be attributable to potentially modifiable risk factors (Norton 2014), including alcohol intake, depression, diet, physical exercise, education, and mental activity (Barnes 2011; de Bruijn 2013; Diniz 2013; Erickson 2011; Jorm 2001). Lifestyle factors could increase or decrease risk of dementia (Amoyal 2012; Karp 2006).
Mental activity has been identified as a potentially important protective factor. Epidemiological studies indicate that lifelong cognitively stimulating experiences, including education and occupation and leisure activities, are linked to improved late‐life cognition, reduced risk of cognitive decline, and lower incidence of AD (Barnes 2011; Marioni 2014; Verghese 2003; Wilson 2002). Lack of education has been identified in meta‐analyses as a particularly strong predictor of dementia (Beydoun 2014). However, prospective studies indicate that even when mental activity is commenced late in life, it may have positive effects on cognition, with lowered rates of decline and lowered dementia incidence reported (Geda 2012; Wilson 2010; Wilson 2012). Cognitively stimulating activity may therefore offer an opportunity to maintain cognitive function, or to prevent or delay further deterioration, among those in early stages of cognitive decline.
Description of the intervention
This review focuses on randomised controlled trials (RCTs) investigating the effects of computerised cognitive training (CCT) interventions for maintenance of cognition and prevention of dementia in people with mild cognitive impairment. 'Cognitive training' has been operationally defined as an intervention consisting of repeated practice on standardised cognitive exercises targeting specific cognitive domains for the purpose of stimulating cognitive function (Gates 2010; Gates 2014; Kueider 2012). Although cognitive training may include traditional pen and paper tasks, it more commonly takes the form of computer‐based tasks, including exercises, games, and virtual reality. Computerised cognitive training may be delivered in individual sessions or within groups, with supervision or privately at home.
How the intervention might work
The underlying premise of cognitive training is that intensive cognitive exercises may build up or restore brain and cognitive reserve, providing greater resilience against neuropathology and maintaining function (Liberati 2012). 'Brain reserve' refers to structural tolerance of the brain to disease and may be evident in increased brain volume; 'cognitive reserve' refers to functional differences in neural activity and cognitive processes (Sterne 2012). Up to 33% of individuals functioning independently without clinical dementia have the same volume of disease pathology as those with clinical dementia (Neuropathology Group 2001). The concept of reserve provides a theoretical explanation for the differences between those who succumb to AD pathology and develop clinical dementia, and those who tolerate the disease and maintain function (Sterne 2012). It has been further suggested that cognitive stimulation may result in neural plasticity and neural compensation, that is, in the development of compensatory networks maintaining cognitive performance and potentially masking or preventing the clinical manifestation of neurocognitive disease (Grady 2012; Park 2013).
Although the evidence base is very limited, some human trials of cognitive training have suggested positive neuroplastic changes. Diverse changes have been reported, including neurochemical activation (Olesen 2004; Rosen 2011), altered fluorodeoxyglucose uptake (Belleville 2012), and reduced β‐amyloid burden (Landau 2012). Several diverse studies investigating neurophysiological changes seen on functional magnetic resonance imaging (fMRI) have identified increased prefrontal and parietal activity and hippocampal activation (Olesen 2004; Rosen 2011; Suo 2012a; Valenzuela 2003). Electroencephalography (EEG) and magnetic resonance spectrometry (MRS) studies of cognitive training support the concept of functional neural plasticity post training, with results indicating positive changes in brain metabolism, task‐dependent brain activation, and resting‐state networks (Belleville 2012; Berry 2010; Förster 2011). However, the research is limited, and significant further investigation is required.
Why it is important to do this review
The potential of CCT to be an effective intervention to maintain cognitive function, or to reduce the risk of clinical dementia, along with its low implementation costs and its high availability and accessibility, has led to the American Alzheimer's Association recommending rapid development and testing of such training (Alzheimer's Association 2014). However, the evidence base to date has been inconclusive, with mixed results reported. Several prior reviews exist, but these include mixed populations and varied interventions, and they need to be updated (Bahar‐Fuchs 2013; Martin 2011). Earlier reviews have been critical of clinical trials for poor specification of interventions, small sample sizes, failure to assign treatments randomly, and lack of longitudinal follow‐up ‐ all factors that may contribute to heterogeneous results (Gates 2010; Gates 2014; Kueider 2012; Mowszowski 2010; Papp 2009; Reijnders 2013; Walton 2014). Additional methodological criticisms with an impact upon valid evaluation of cognitive training include lack of differentiation between interventions, lack of adequate control conditions to isolate intervention benefit, a limited number of trials with active controls, and limited outcome measures to determine generalisation to non‐trained cognitive domains and persistence of benefits (Gates 2010; Green 2014; Mowszowski 2010; Park 2013; Walton 2014). Primary studies have identified that the benefits of cognitive training may depend upon several factors including age, cognitive level, and non‐cognitive factors (Lampit 2014; Stine‐Morrow 2014). Therefore a robust review is warranted to investigate the efficacy of computerised cognitive training for people with MCI on non‐trained cognitive domains, and to evaluate potential sources of bias and heterogeneity in the literature. If sufficient trials are identified, then it is important to examine the intervention characteristics and other factors that may affect outcomes.
There has been a proliferation of commercial brain training products purporting to improve cognitive function and reduce dementia risk. For older people, fear of cognitive decline and dementia may be a powerful motivator to seek such preventive interventions. However the development of such programmes has frequently outpaced thorough research into product benefits (Gates 2014; Lampit 2015). The World Alzheimer Report 2014 has reported that cognitively stimulating activities, including reading, playing musical instruments, and playing cards and board games, may be beneficial for improving and maintaining while preventing decline in cognitive functioning, although most of these activities have not been investigated in clinical trials. In this context of confusing and potentially misleading claims, this review is important to provide potential consumers with information on how best to spend time, effort, and money they might invest to prevent cognitive decline.
As well as informing individuals, the findings of this review may be useful to public health decision‐making bodies, healthcare practitioners, and researchers, providing them with a comprehensive synthesis of information about the current state of the evidence, and identifying research gaps and unanswered questions in the field.
We also refer readers to companion reviews on the effects of computerised cognitive training on healthy people at midlife and in late life (Gates 2019a; Gates 2019b).
Objectives
To evaluate the effects of at least 12 weeks of computerised cognitive training (CCT) on maintaining or improving cognitive function and preventing dementia in people with mild cognitive impairment.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs, published or unpublished, reported in any language. Full reports and other types of reports, such as conference abstracts, were eligible for inclusion. We included studies involving both randomised and non‐randomised trial arms but considered only results from the former. We included cross‐over studies but extracted and analysed data from the first treatment period only.
Types of participants
We included studies of people with a diagnosis of mild cognitive impairment (MCI) or mild neurocognitive disorder (MND), or from a population at high risk of cognitive decline.
We accepted diagnoses of MCI, MND, and risk of cognitive decline made by the authors of each clinical trial and recorded the definitions used. These could include diagnostic assessment and/or subjective memory complaints with reduced scores on cognitive tests such as the Mini Mental State Examination. In all cases, an attempt should have been made by the trial authors to exclude dementia, and it was acceptable for the purpose of excluding dementia for a study to have used a cognitive score cut‐off. Again, we accepted whatever cut‐off study authors used, and we explored this as a possible source of heterogeneity.
We excluded studies of adults with a diagnosis of dementia, any other neurological condition, or psychiatric illness.
We contacted study authors if we needed clarification to determine health status. If we received no response, clinical experts in our review group classified the trials or listed them as 'Studies awaiting classification'.
Types of interventions
We included studies that compared cognitive training interventions using interactive computerised technology versus active or inactive control interventions over at least 12 weeks.
Experimental interventions had to adhere to the following criteria: any form of interactive computerised cognitive intervention, including computer exercises, computer games, mobile devices, gaming console, and virtual reality, that involve repeated practice on standardised exercises including a specified cognitive domain or domains, for the purpose of enhancing cognitive function.
By 'active control', we mean all those control conditions that involve unguided computer‐ and/or screen‐based tasks that are not planned as interventions. These tasks can involve watching educational videos or playing computer games with no particular training component. By 'inactive control', we refer to control groups for which no intervention is applied that may be expected to have an effect on cognition.
The minimum treatment duration was set at 12 weeks, and all included trials had to report outcomes at a minimum of one time point 12 or more weeks after randomisation. To evaluate the effects of training on meaningful long‐term outcomes, it was necessary to make a judgement about the minimum 'dose' of training that may be required to effect an enduring change. Previous research suggests that acute brain changes can be seen following eight weeks of training (Engvig 2014), but we are unable to find any evidence that such brain changes persist. Most studies examining the benefits of brain and cognitive reserve identify long‐term cognitive stimulation from years of education. We therefore made an arbitrary judgement that at least 12 weeks of regular cognitive training would be required for intervention to have an enduring effect. Addtionally, this time frame is consistent with recommendations from reviews of clinical trials (Lampit 2014a). It is recognised that the relationship between short‐term cognitive training effects and maintenance of cognitive function over longer periods of time is unclear.
We excluded interventions that did not involve any form of computer delivery. We also excluded studies where researchers combined the experimental intervention with any other form of intervention, unless the added intervention was provided in a standardised manner to both experimental and control groups.
Types of outcome measures
Primary outcomes
Primary outcomes included the following.
Incidence of all‐cause dementia (measured as a dichotomous outcome).
Global cognitive function (measured as a continuous outcome).
Global cognitive functioning could be measured using any validated tests, for example (but not limited to):
Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (ADAS‐Cog);
Mini Mental State Examination (MMSE);
Repeatable Battery for Assessment of Neuropsychological Status (RBANS); and
Cambridge Cognition Examination (CAMCOG).
The main time point of interest was 'end of trial', defined as the time point with the longest period of follow‐up from randomisation (see also section Data collection and analysis). We also extracted and presented outcome data reported at other time points after randomisation.
Secondary outcomes
Secondary outcomes included the following.
-
Cognitive tests not included in the training programme, administered before and after training, that are any validated measure of:
episodic memory;
executive functioning;
speed of processing;
attention/working memory; or
verbal fluency.
Quality of life/psychological well‐being, either generic or disease‐specific.
Daily function, such as measures of instrumental activities of daily living.
Number of participants experiencing one or more serious adverse events.
If a trial provided data on more than one cognitive scale for a specific outcome, we applied a predetermined hierarchy of cognitive outcome scales and used data on the cognitive scale that was highest on this hierarchy. For example, if a trial reported results on both the Mini Mental State Examination and the Clinical Dementia Rating scale (CDR), we used outcome data from the MMSE in our quantitative analyses. The order of a scale in the hierarchy was determined by the frequency of its use in a large set of 79 trials, evaluating vitamin and mineral supplementation, dietary interventions, and physical exercise interventions.
Outcomes included in the 'Summary of findings' table
We addressed critical effectiveness outcomes in a 'Summary of findings' table for each comparison. We planned to include all outcomes related to cognitive function on non‐trained tasks and quality of life. For the comparison CCT versus active control, we were able to include the following outcomes: (1) global cognitive functioning, (2) episodic memory, (3) speed of processing, (4) executive functioning, (5) working memory, (6) verbal fluency, and (7) quality of life. For the comparison CCT versus inactive control, we were able to include the following outcomes: (1) global cognitive functioning, (2) episodic memory, (3) executive functioning, (4) verbal fluency, (5) depression, and (6) functional performance.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois) ‐ the specialised register of the Cochrane Dementia and Cognitive Improvement Group ‐ up to 31 May 2018.
The Information Specialist for the CDCIG maintained ALOIS, which contains studies that fall within the areas of dementia prevention, dementia treatment and management, and cognitive enhancement in the healthy elderly populations. These studies are identified through:
monthly searches of several major healthcare databases: MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, and Latin American Caribbean Health Sciences Literature (LILACS);
monthly searches of several trial registers: the University hospital Medical Information Network Clinical Trials Registry (Japan) (UMIN‐CTR) (www.umin.ac.jp/ctr/index.htm); the World Health Organization (WHO) portal (which covers ClinicalTrials.gov (clinicaltrials.gov/); International Standard Randomized Controlled Trials Number (ISRCTN) (www.isrctn.com/); the Chinese Clinical Trials Register (ChiCTR) (who.int/ictrp/network/chictr/en/); the German Clinical Trials Register (GermanCTR) (who.int/ictrp/network/drks2/en/); the Iranian Registry of Clinical Trials (IRCT) (who.int/ictrp/network/irct2/en/); and the Netherlands National Trials Register (NTR) (who.int/ictrp/network/ntr/en/), plus others);
quarterly searches of the Central Register of Controlled Trials, in the Cochrane Library (CENTRAL); and
six‐monthly searches of several grey literature sources: Institute for Scientific Information (ISI) Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS, see About ALOIS on the ALOIS website (www.medicine.ox.ac.uk/alois).
Details of the search strategies run in healthcare bibliographic databases, used for retrieval of reports of dementia, cognitive improvement, and cognitive enhancement trials, can be viewed in the ‘Methods used in reviews’ section within the editorial information about the Cochrane Dementia and Cognitive Improvement Group.
We conducted additional searches in MEDLINE, Embase, PsycINFO, CINAHL, ClinicalTrials.gov, and the WHO Portal/International Clinical Trials Registry Platform (ICTRP) (www.apps.who.int/trialsearch), to ensure that the searches were as comprehensive and as up‐to‐date as possible. The search strategies used are shown in Appendix 1.
Searching other resources
We screened the reference lists of all included trials. In addition, we screened the reference lists of recent systematic reviews, health technology assessment reports, and subject‐specific guidelines identified through www.guideline.gov. We restricted the search to those guidelines meeting National Guideline Clearinghouse (NGC) 2013 published inclusion criteria.
We contacted experts in the field and companies marketing included interventions to request additional randomised trial reports not identified by the search.
Data collection and analysis
We used the protocol for this review alongside instructions for data extraction, quality assessment, and statistical analyses generated by the editorial board of CDCIG, and based in part on a generic protocol approved by the Cochrane Musculoskeletal Group for another series of reviews (da Costa 2012; da Costa 2014; Reichenbach 2010; Rutjes 2009a; Rutjes 2009b; Rutjes 2010).
Selection of studies
If multiple reports described the same trial, we included all of them to allow extraction of complete trial details.
We used crowdsourcing to screen the search results. Details of this approach have been described at www.medicine.ox.ac.uk/alois/content/modifiable‐risk‐factors. In brief, teams of volunteers performed a 'first assess' on the search results. The crowd was recruited through the network called Students For Best Evidence (www.students4bestevidence.net). The crowd provided an initial screen of the results using an online tool developed for the Cochrane EMBASE project, but tailored for this programme of work. The crowd decided (based on reading of title and abstract) whether the citation was describing a randomised trial or a quasi‐randomised trial, irrespective of the citation topic. We then screened the remaining results (titles and abstracts). Four independent review authors (NG, EM, SK, RV) assessed the full text of studies for eligibility, with any disagreements resolved by a fifth independent review author.
We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table (Moher 2009). We did not impose any language restrictions.
Data extraction and management
Five review authors (NG, MN, SK, RV, AR), working independently, extracted trial information using a standardised and piloted extraction method, referring also to a guidance document, and resolving discrepancies by discussion, or by involvement of an independent review author. Where possible, we extracted the following information related to characteristics of participants, interventions, and study design.
Participant characteristics
Gender
Age (range, median, mean)
Education (level and years of education)
Baseline cognitive function
Cognitive diagnostic status
Duration of cognitive symptoms
Ethnicity
Apo‐E genotype
Vascular risk factors (hypertension, diabetes, hyperlipidaemia)
Body mass index (BMI)
Depression and stress
Physical activity
Work status
Intervention characteristics
Type and description of cognition‐based intervention
Type and description of the control condition
Delivery mode (individualised, group intervention, supervision)
Length of training sessions (intensity)
Frequency of sessions per week (dose)
Duration of treatment programme
Presence of supervision
Group or individual
Any concomitant treatments
Methodological characteristics
Trial design (individual or cluster randomisation; parallel‐group, factorial, or cross‐over design)
Number of participants
Outcome measures used
Duration of follow‐up as measured from randomisation
Duration of follow‐up as measured from end of treatment
Source of financial support
Publication status
If outcome data were available at multiple time points within a given trial, we extracted data at 12 weeks, along with short‐term (up to one year), medium‐term (one to two years), and long‐term results (more than two years). Within these time periods, we extracted the latest data reported by the study (e.g. if the study reports data at six months, nine months, and one year, we extracted only the one‐year data, and we analysed these for the one‐year (short‐term) time point). For dichotomous outcomes (such as number of participants experiencing one or more serious adverse events), we extracted from each trial the number of participants with each outcome at each time point. For continuous outcomes, we extracted the number of participants for whom the outcome was measured, as well as the mean and standard deviation (SD) of the change from baseline for each outcome at each time point. If change from baseline data were not available, we extracted the mean value at each time point. When necessary and possible, we approximated means and measures of dispersion from figures in the reports. For cross‐over trials, we extracted data on the first treatment period only. Whenever possible, we extracted intention‐to‐treat data (i.e. analysing all participants according to the group randomisation); if this information was not available, we extracted and reported data from available case analyses. If none of these data were available, we considered data from per‐protocol analyses. We contacted the trial authors if we could not obtain necessary data from the trial report.
Assessment of risk of bias in included studies
After completion of a standardised training session provided by AR, one member of the review author team and one experienced review author provided by the editorial team independently assessed the risk of bias in each of the included trials, using Cochrane's 'Risk of bias' tool (Higgins 2011), and resolved disagreements by consensus. We assessed the risk of bias potentially introduced by suboptimal design choices with respect to sequence generation, concealment of allocation, blinding of participants and caregivers, blinded outcome assessment, selective outcome reporting, and incomplete outcome data, including the type of statistical analysis used (true intention‐to‐treat vs other). Based on the aforementioned criteria, we rated the studies as 'low risk', 'unclear risk', or 'high risk' of bias for each domain, including a description of the reasoning for our rating. The general definitions used are reported in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We derived review‐specific definitions in part from a previously published systematic review (Rutjes 2012), and we have explained them in detail in Appendix 2.
Measures of treatment effect
The measure of treatment effect for continuous outcomes was an effect size (standardised mean difference), defined as the between‐group difference in mean values divided by the pooled SD. In case a single trial contributed to a comparison, or if all studies used the same instrument, we used the mean difference to describe and analyse results. We expressed the treatment effect for dichotomous outcomes as a risk ratio (RR) with a 95% confidence interval (CI).
Unit of analysis issues
We identified no cluster‐randomised trials for inclusion. We included one cross‐over study, but we extracted and analysed data from the first treatment period only.
Dealing with missing data
Missing data in the individual trials may put study estimates of effects at high risk of bias and may lower the overall quality of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group (www.gradeworkinggroup.org). We dealt with missing data in our 'Risk of bias' assessments and planned evaluation of attrition bias in stratified analyses of the primary outcomes (Appendix 2;Differences between protocol and review). We analysed available information and did not contact study authors with a request to provide missing information, nor did we impute missing data ourselves.
Assessment of heterogeneity
We planned to examine between‐trial heterogeneity in stratified analyses by trial, participant, and intervention. As the number of trials identified was too small to permit meaningful analyses, we refrained from performing such analyses (Differences between protocol and review). We visually inspected forest plots for the presence of heterogeneity and calculated the variance estimate tau² as a measure of between‐trial heterogeneity (DerSimonian 1986). We prespecified a tau² of 0.04 to represent low heterogeneity, 0.09 to represent moderate heterogeneity, and 0.16 to represent high heterogeneity between trials (Spiegelhalter 2004). In addition, we used the I² statistic and the corresponding Chi² test to assist readers more familiar with these statistics (Higgins 2011). I² describes the percentage of variation across trials attributable to heterogeneity rather than to chance, with values of 25%, 50%, and 75% interpreted as low, moderate, and high (respectively) between‐trial heterogeneity. We preferred tau² over I² in interpreting between‐trial heterogeneity, as interpretation of I² can be largely affected by the precision of trials included in the meta‐analysis (Rücker 2008). All P values are two‐sided.
Assessment of reporting biases
We did not identify enough trials to construct funnel plots to explore reporting biases and other biases related to small‐study effects (Differences between protocol and review).
Data synthesis
We reported summary and descriptive statistics (means and SDs) for participant and intervention characteristics.
We used standard inverse‐variance random‐effects meta‐analysis to combine outcome data across trials at end of trial (DerSimonian 1986), and, if possible, at least one additional time point (see Primary outcomes and Data collection and analysis for definitions of time points). We conducted statistical analyses in Review Manager 5 (RevMan 2014) and in STATA, release 14 (Statacorp, College Station, Texas, USA).
GRADE and 'Summary of findings' tables
We used GRADE to describe the quality of the overall body of evidence for each outcome in the 'Summary of findings' tables (Guyatt 2008; Higgins 2011). We defined quality as the degree of confidence that we can place in the estimates of treatment benefits and harms. There were four possible ratings: high, moderate, low, and very low. Rating evidence as 'high quality' implies that we are confident in our estimate of the effect and further research is very unlikely to change this. A rating of 'very low' quality implies that we are very uncertain about the obtained summary estimate of the effect. The GRADE approach rates evidence from RCTs that do not have serious limitations as 'high quality'. However, several factors can lead to downgrading of the evidence to 'moderate', 'low', or 'very low'. The degree of downgrading is determined by the seriousness of these factors: study limitations (risk of bias); inconsistency; indirectness of evidence; imprecision; and publication bias (Guyatt 2008; Higgins 2011).
Subgroup analysis and investigation of heterogeneity
We did not identify enough trials to conduct subgroup analyses.
Sensitivity analysis
For the primary outcome, we performed one sensitivity analysis, including only those trials that used an internationally accepted definition of MCI.
Results
Description of studies
See Characteristics of included studies,Characteristics of excluded studies, Characteristics of studies awaiting classification, and Characteristics of ongoing studies.
Results of the search
We conducted searches in January 2015, July 2015, February 2016, July 2016, and May 2018. In total, we retrieved 8392 records through the five searches. After de‐duplication, 6233 records remained. A crowd and the CDCIG Information Specialist assessed these records at the title and abstract level. In total, 1091 results remained after this assessment. We then screened these records. Of these, we assessed 321 full‐text articles for eligibility, and we included eight studies in the review (Barnes 2013; Djabelkhir 2017; Fiatarone Singh 2014; Gooding 2016; Herrera 2012; Kwok 2013a; Optale 2010; Rozzini 2007). We have depicted this process in Figure 1.
Figure 1.
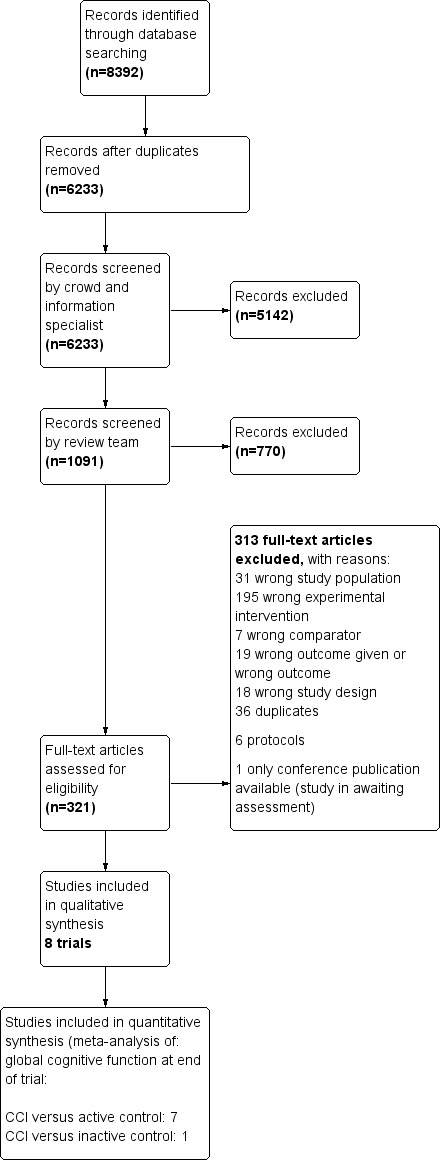
Study flow diagram.
Included studies
We have provided study details in the Characteristics of included studies section and have briefly summarised them below. We included in this review eight studies with a total of 660 participants.
Design
All studies are RCTs, with seven comparing CCT versus an active control and one versus an inactive control condition.
Study durations were 12 weeks (Kwok 2013a), three months (Barnes 2013; Djabelkhir 2017), four months (Gooding 2016), six months (Optale 2010), nine months (Herrera 2012), 12 months (Rozzini 2007), and 18 months (Fiatarone Singh 2014).
Sample size
Barnes 2013 randomised 126 participants to four different treatment arms (including one control arm), each with 31 or 32 participants. Djabelkhir 2017 randomised 10 participants to the experimental arm and 10 to the control arm. Fiatarone Singh 2014 randomised 51 participants to the experimental arms and 49 to the control arms. Gooding 2016 randomised 96 participants to the three arms of interest (the number of participants randomised to each arm is not reported). Herrera 2012 randomised 11 participants to both intervention and control groups. Kwok 2013a was the largest trial, with 111 participants randomised to the experimental arm and 112 to the control arm. Optale 2010 randomised 18 participants to each of the intervention and control groups. Finally, Rozzini 2007 randomised 15 participants to the intervention group and 22 to the control group.
Setting
Barnes 2013 was conducted at a single centre in the USA. Djabelkhir 2017 was conducted at a single centre in France. Fiatarone Singh 2014 was conducted in Australia. Gooding 2016 was conducted at four different sites in the USA; Herrera 2012 at a single centre in France; Kwok 2013a at six community centres randomly chosen from three districts in Hong Kong; Optale 2010 at a single centre; and Rozzini 2007 at two centres in Italy.
Participants
Four studies included participants with established MCI at baseline. Diagnostic criteria were consistent with Petersen criteria in Djabelkhir 2017, Herrera 2012 (Petersen 2004 criteria), Fiatarone Singh 2014 (Petersen 1999 criteria), and Rozzini 2007 (Petersen 2001 criteria). Optale 2010 included participants with a memory deficit defined by a corrected total score below 15.76 on the Verbal Story Recall (VSR) test. Barnes 2013, Gooding 2016, and Kwok 2013a included participants with self‐reported or informant‐reported cognitive complaints at baseline and satisfied our inclusion criteria, as participants had reduced scores on standardised dementia screening tests.
The mean age of participants in experimental and control groups ranged from 70 to 82 years. Rozzini 2007 gave an age range for participants (63 to 78 years), and Gooding 2016 gave only the median age for those who completed the study (76 years).
Interventions
Barnes 2013 used a 2 × 2 factorial design by which all participants received computerised training (Posit Science software) (MA‐I) or active mental control educational videos (MA‐C), along with an exercise regimen (EX‐I) or a sham exercise regimen (EX‐C) (Barnes 2013). We have included this study in comparison 1: computerised cognition‐based interventions versus active control.
Djabelkhir 2017 treated the intervention group with a computerised multi‐domain software programme (KODRO) and trained the control group to use a tablet PC and stiimulate social interactions among participants. We have included this study in comparison 1: computerised cognition‐based interventions versus active control.
Fiatarone Singh 2014 used a 2 × 2 factorial design involving cognitive training (CT) with Cogpack computer‐based exercises or sham cognitive training (watching educational videos followed by a set of questions), as well as progressive resistance training (PRT) or sham PRT (stretching and seated callisthenics exercises). We included all participants receiving CT (Cogpack) in the experimental group and all participants receiving sham CT in the active control group. We included these data in comparison 1: computerised cognition‐based interventions versus active control.
Gooding 2016 included three study arms. One arm received computerised cognitive training in the BrainFitness programme, another arm received the same BrainFitness programme and a motivational therapeutic milieu (not included in the analysis). The third arm played computer games. We have included this study in comparison 1: computerised cognition‐based interventions (BrainFitness programme only) versus active control.
Kwok 2013a provided 12 weekly sessions of computerised training focused on attention, memory, and reasoning as the experimental intervention. The control group received a series of health‐related educational lectures on prevention of mood disorder, heart disease, diabetes, and stroke. We have included this study in comparison 1: computerised cognition‐based interventions versus active control.
Herrera 2012 allocated the intervention group to computerised memory and attention task training programmed in Java, while the control group participated in activities such as finding names of countries and corresponding capitals, organising a list of purchases by categories, and finding similarities and differences. We have included this study in comparison 1: computerised cognition‐based interventions versus active control.
Optale 2010 provided virtual reality training as the experimental intervention and music therapy as the control intervention. We have included this study in comparison 1: computerised cognition‐based interventions versus active control.
Rozzini 2007 included three study arms. One arm received CT through a computerised multi‐domain software programme (TNP software) plus a cholinesterase inhibitor; another arm received a cholinesterase inhibitor only; and the third arm received neither CT nor cholinesterase inhibitor treatment (not included in the analysis). We have included data from the first two arms in comparison 2: computerised cognition‐based interventions versus inactive control.
Outcomes
Here we describe outcome measures addressing outcomes of interest to our review that we included in one or more meta‐analyses. We refer to the Characteristics of included studies table for other instruments reported by trial authors that we did not select for any meta‐analyses. We have described under Types of outcome measures the method used to select outcome measures for inclusion.
Primary outcomes
Global cognitive function
Eight studies measured global cognitive function as an outcome. Four studies measured global cognitive functioning using the MMSE (Djabelkhir 2017; Optale 2010; Rozzini 2007; with the modified MMSE (mMMSE) used in Gooding 2016); Kwok 2013a used the Chinese equivalent of the Mattis Dementia Rating Scale; and Fiatarone Singh 2014 used ADAS‐Cog.
Barnes 2013 used a composite score change at three months to measure global cognitive functioning. We could not include this outcome in the meta‐analyses (see Effects of interventions).
Secondary outcomes
Cognitive function subdomain: episodic memory
One study used the Rey Auditory Verbal Learning Test (RAVLT) to measure episodic memory (Barnes 2013). Fiatarone Singh 2014 used the Wechsler Memory Scale (WMS) Logical Memory I (immediate) at 6 months and 18 months; Gooding 2016 used the WMS Logical Memory II (delayed). Optale 2010, and Rozzini 2007 used non‐specified story recall. Herrera 2012, and Djabelkhir 2017 measured episodic memory using a list learning task: the 16‐Item free recall (FR) and cued recall (CR) test (16‐FR/CR test).
Cognitive function subdomain: executive functioning
Two studies used Trails B to measure executive functioning (Barnes 2013; Djabelkhir 2017).
Fiatarone Singh 2014 measured executive function on the Similarities subtest of the Wechsler Adult Intelligence Scale‐III (WAIS‐III) at 6 and 18 months; Optale 2010 used dual task performance to measure executive functioning; and Rozzini 2007 measured executive functioning using Raven's coloured matrices.
Cognitive function subdomain: speed of processing
Two studies used Trails A to measure speed of processing (Barnes 2013; Djabelkhir 2017).
Fiatarone Singh 2014 measured speed of processing using the Symbol Digit Modality Test (SDMT) at 6 months and 18 months.
Cognitive function subdomain: verbal fluency
Several studies measured verbal fluency using letter verbal fluency (number of words generated beginning with specified letters), including Barnes 2013, which measured in one minute all the words the attendee could remember, words not stated, one attempt; Djabelkhir 2017, which measured in two minutes all the words the attendee could remember, starting with the letter P, attempts not stated; Fiatarone Singh 2014, which used the Controlled Oral Words Association Test,(COWAT); Optale 2010, which measured in one minute all the words the attendee could remember, starting with the letters C, P, and S, attempts not stated; and Rozzini 2007, which measured in one minute all the words the attendee could remember, words not stated, attempts not stated.
Cognitive function subdomain: working memory
Three studies used the digit span to measure working memory: Djabelkhir 2017 (WAIS, 4th edition), Herrera 2012 (not stated), and Optale 2010 (WAIS procedure).
Quality of life/Psychological well‐being
Two studies measured depression using the Geriatric Depression Scale (Optale 2010; Rozzini 2007): Djabelkhir 2017 measured depression using the Goldberg Scale, and Gooding 2016 measured depression using the Beck Depression Inventory.
Djabelkhir 2017 measured quality of life using the quality of life scale for older French people.
Functional performance
Only three studies measured this outcome: Fiatarone Singh 2014 and Rozzini 2007 measured daily function with the BAYER ‐ Activities of Daily Living scale (B‐ADL), and Optale 2010 used the Activities of Daily Living ‐ Function scale.
Number of participants experiencing one or more serious adverse events
Optale 2010 reported mortality at six months.
Excluded studies
We excluded 312 full‐text articles during the full‐text screening. Of these, we excluded one because it focused on cognitively healthy people in midlife (Corbett 2015), and we excluded nine because they focused on cognitively healthy people in late life (Desjardins‐Crépeau 2016; Klusmann 2010; Lampit 2014; Lampit 2015; Legault 2011; Leung 2015; Peretz 2011; Shatil 2013; Van het Reve 2014). Two other Cochrane reviews have included these 10 studies (Gates 2019a; Gates 2019b). We excluded 195 reports that investigated an intervention because it was provided for less than 12 weeks or because it did not involve computerised cognitive training; and we excluded 18 because the study did not use an eligible study design. We identified no ongoing trials in the trial registers or conference proceedings. One study is awaiting classification because, at the time of the final search, it was available only as a conference abstract from which eligibility could not be determined (not clear how cognitive training was delivered). Reasons for exclusion of studies can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
For details, please see Characteristics of included studies. Figure 2 and Figure 3 display study level and aggregate results of the risk of bias assessments.
Figure 2.
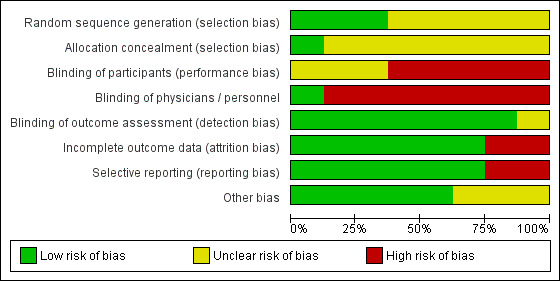
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
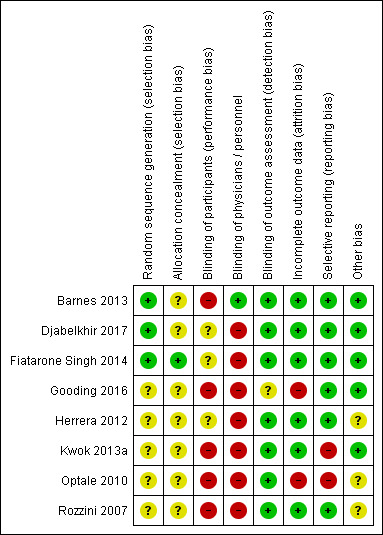
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
One study has low risk of selection bias due to adequate random sequence generation and allocation concealment (Fiatarone Singh 2014). Two studies have unclear risk of selection bias because allocation concealment was not described in sufficient detail, although the study authors described an adequate method for generating a random sequence (Barnes 2013; Djabelkhir 2017). The remaining studies did not describe any method for sequence generation nor allocation concealment (Gooding 2016; Herrera 2012; Kwok 2013a; Optale 2010; Rozzini 2007); we also judged these studies to be at unclear risk of selection bias.
Blinding
We considered Barnes 2013 to have high risk of performance bias because participants were not blinded to the type of intervention. However, both study personnel and outcome assessors were adequately blinded to the study treatment; therefore we judged the risk of detection bias to be low. We judged Fiatarone Singh 2014, Djabelkhir 2017, and Herrera 2012 to have unclear risk of performance bias for participants and high risk of performance bias for personnel, who were not blinded. However, study authors described adequate blinding of outcome assessors, giving these studies low risk of detection bias. We considered Kwok 2013a, Optale 2010, and Rozzini 2007 to be at high risk of performance bias due to lack of blinding for participants and personnel, but at low risk of detection bias as outcome assessors were adequately blinded. Gooding 2016 did not blind participants nor physicians (high risk of performance bias), and we identified unclear risk of detection bias due to lack of information regarding blinding of outcome assessors.
Incomplete outcome data
We considered six studies to be at low risk of attrition bias (Barnes 2013; Djabelkhir 2017; Fiatarone Singh 2014; Herrera 2012; Kwok 2013a; Rozzini 2007). We judged risk of attrition bias to be high in Gooding 2016 because 77% of randomised participants were analysed. In Optale 2010, 83% of participants randomised to the intervention arm and 89% randomised to the control arm were analysed; we judged this to put the study at high risk of attrition bias.
Selective reporting
We considered six studies to be at low risk of reporting bias (Barnes 2013; Djabelkhir 2017; Fiatarone Singh 2014; Gooding 2016; Herrera 2012; Rozzini 2007). We judged the remaining two studies to be at high risk of reporting bias. Optale 2010 did not report one outcome that was described as measured and Kwok 2013a incompletely reported outcome data described as non‐significant.
Other potential sources of bias
We identified no other sources of bias.
Effects of interventions
Comparison 1: computerised cognition‐based interventions versus active control
See Table 1 for the comparison CCT versus active control. Although Barnes 2013 reported eligible outcome data for all cognitive outcomes, we could not include these data in our meta‐analyses because the data were reported as standardised mean changes (z‐scores). Therefore, we report these results separately.
Primary outcomes
Incidence of dementia
We found no data on the incidence of dementia.
Global cognitive function
Evidence on global cognitive function at end of trial (Analysis 1.1; Figure 4) was very low quality, downgraded because of imprecision, inconsistency, and risk of bias. Therefore we are very uncertain of this result. Negative values favour the CCT group. Analysis of global cognitive function at end of follow‐up gives a standardised mean difference (SMD) of ‐0.53 (95% confidence interval (CI) ‐1.06 to ‐0.01; 5 studies; 407 participants). Results at individual time points are as follows: immediate time point (12 weeks) SMD ‐0.31 (95% CI ‐0.70 to 0.08; 4 studies; 356 participants); short‐term time point (12 weeks to one year) SMD ‐1.23 (95% CI ‐1.89 to ‐0.56; 2 studies; 82 participants); and medium‐term time point (one to two years) SMD 0.16 (95% CI ‐0.23 to 0.55; 1 study; 100 participants).
Analysis 1.1.
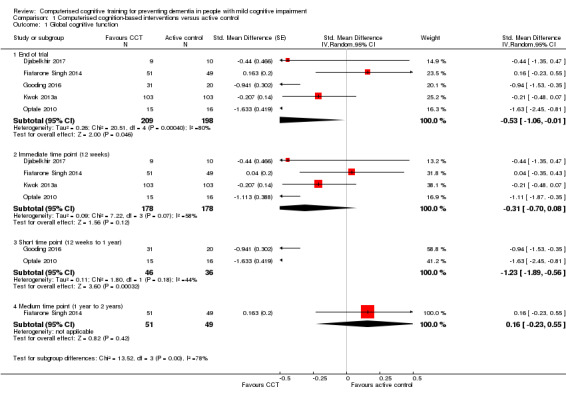
Comparison 1 Computerised cognition‐based interventions versus active control, Outcome 1 Global cognitive function.
Figure 4.
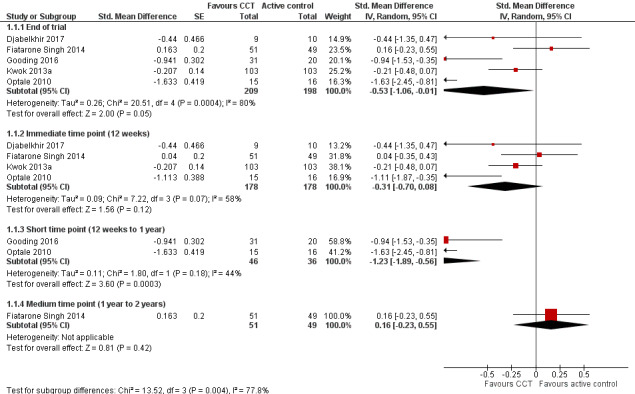
Forest plot of comparison: 1 Computerised cognition‐based interventions versus active control, outcome: 1.1 Global cognitive function.
Trial with outcome data not included in the meta‐analyses
Barnes 2013 derived a composite score from six distinct cognitive instruments at three months. Higher values indicated improvement. Study authors reported there were no significant differences between groups (P from interaction = 0.26). In the comparison between groups also receiving sham exercise, the mean change in z‐score was 0.17 in the CCT group (95% CI 0.03 to 0.31) and 0.16 in the educational DVD group (95% CI 0.05 to 0.26). In the comparison between groups also receiving aerobic exercise, the mean z‐score change was 0.22 in the CCT group (95% CI 0.12 to 0.33) and 0.08 in the educational DVD control group (95% CI ‐0.004 to 0.17). Overall we deemed the quality of this evidence to be very low (downgraded for imprecision, indirectness of the study population, and risk of bias).
Sensitivity analyses
We conducted a prespecified sensitivity analysis including only trials in which MCI was diagnosed on the basis of internationally accepted diagnostic criteria. Two studies with 119 participants contributed to this analysis (Djabelkhir 2017; Fiatarone Singh 2014). At our main time point of interest ‐ end of trial ‐ we found no clear evidence of an effect of training: SMD 0.01 (95% CI ‐0.51 to 0.52; Tau² = 0.05; I² = 29%). We considered this to be low‐quality evidence (downgraded for imprecision and risk of bias).
Secondary outcomes
Cognitive subdomain: episodic memory
Evidence regarding episodic memory at end of trial (Analysis 1.2; Figure 5) was very low quality, downgraded because of imprecision, inconsistency, and risk of bias. Therefore we are very uncertain about this result. Negative values favour the CCT group. Analysis at end of follow‐up gives an SMD of ‐0.79 (95% CI ‐1.54 to ‐0.04; 5 studies; 223 participants). Results at individual time points are as follows: immediate time point (12 weeks) SMD ‐0.99 (95% CI ‐1.80 to ‐0.19; 4 studies; 172 participants); short‐term time point (12 weeks to one year) SMD ‐1.39 (95% CI ‐2.35 to ‐0.44; 3 studies; 104 participants); and medium‐term time point (one to two years) SMD 0.02 (95% CI ‐0.37 to 0.41; 1 study; 100 participants).
Analysis 1.2.
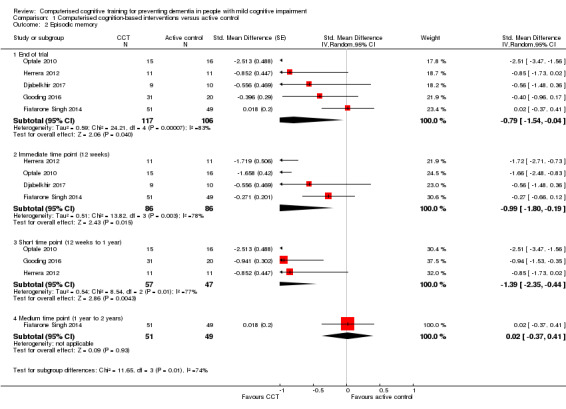
Comparison 1 Computerised cognition‐based interventions versus active control, Outcome 2 Episodic memory.
Figure 5.
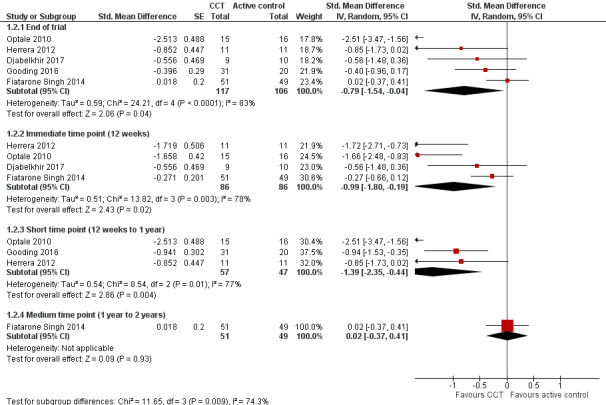
Forest plot of comparison: 1 Computerised cognition‐based interventions versus active control, outcome: 1.2 Episodic memory.
Trial with outcome data not included in the meta‐analyses
Barnes 2013 reported outcome data on verbal learning and memory (RAVLT), number of words learned, as standardised mean changes (z‐scores) at three months. Higher values indicated improvement. Study authors reported no significant differences between groups (P from interaction = 0.38). In the comparison between groups receiving sham exercise, the mean change in z‐score was 0.13 in the CCT group (95% CI ‐0.11 to 0.37) and 0.33 in the educational DVD group (95% CI 0.09 to 0.58). In the comparison between groups receiving aerobic exercise, the mean change in z‐score was ‐0.04 in the CCT group (95% CI ‐0.42 to 0.33) and 0.14 in the educational DVD control group (95% CI ‐0.14 to 0.43). We judged the quality of this evidence to be very low (downgraded for imprecision, indirectness of the study population, and risk of bias).
Cognitive subdomain: speed of processing
Evidence regarding speed of processing at end of trial (Analysis 1.3) was low quality, downgraded because of imprecision and risk of bias. Negative values favour the CCT group. Analysis at end of follow‐up gives an SMD of 0.20 (95% CI ‐0.16 to 0.56; 2 trials; 119 participants). This result is imprecise but indicates there may be little or no difference in the speed of processing between intervention and control groups. Results at individual time points are as follows: immediate time point (12 weeks) SMD 0.11 (95% CI ‐0.25 to 0.47; 2 studies; 119 participants) and medium‐term time point (one to two years) SMD 0.14 (95% CI ‐0.25 to 0.53; 1 study; 100 participants).
Analysis 1.3.
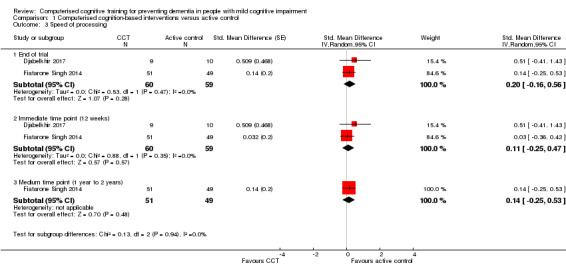
Comparison 1 Computerised cognition‐based interventions versus active control, Outcome 3 Speed of processing.
Trial with outcome data not included in the meta‐analyses
Barnes 2013 reported outcome data on Trail Making test part A as standardised mean changes (z‐scores) at three months. Lower values indicated improvement. Study authors reported no significant differences between groups (P from interaction = 0.24). In the comparison between groups receiving sham exercise, the mean change in z‐score was ‐0.03 in the CCT group (95% CI ‐0.50 to 0.44) and ‐0.36 in the educational DVD group (95% CI ‐0.58 to ‐0.15). In the comparison between groups receiving aerobic exercise, the mean change in z‐score was ‐0.36 in the CCT group (95% CI ‐0.63 to ‐0.08) and ‐0.12 in the educational DVD control group (95% CI ‐0.32 to 0.07). We judged the quality of this evidence to be very low (downgraded for imprecision, indirectness of the study population, and risk of bias).
Cognitive subdomain: executive function
Evidence regarding executive function at end of trial (Analysis 1.4) was very low quality, downgraded because of imprecision, inconsistency, and risk of bias. Therefore we are very uncertain about this result. Negative values favour the CCT group. Analysis at end of follow‐up gives SMD ‐0.31 (95% CI ‐0.90 to 0.28; 3 studies; 150 participants). Results at individual time points are as follows: immediate time point (12 weeks) SMD ‐0.18 (95% CI ‐0.50 to 0.14; 3 studies; 150 participants); short‐term time point (12 weeks to one year) SMD ‐0.81 (95% CI ‐1.54 to ‐0.07; 1 study; 31 participants); and medium‐term time point (one to two years) SMD 0.08 (95% CI ‐0.31 to 0.48; 1 study; 100 participants).
Analysis 1.4.
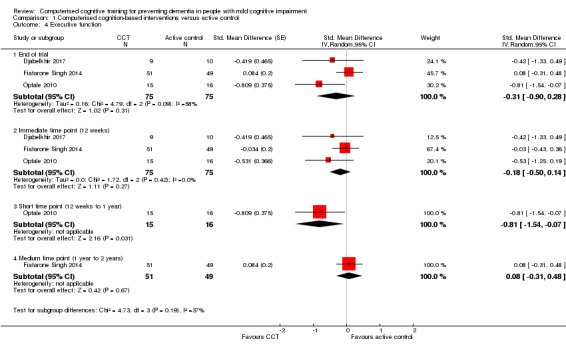
Comparison 1 Computerised cognition‐based interventions versus active control, Outcome 4 Executive function.
Trial with outcome data not included in the meta‐analyses
Barnes 2013 reported outcome data on Trail Making test part B as standardised mean changes (z‐scores) at three months. Lower values indicated improvement. No differences between groups were found (P from interaction = 0.31). In the comparison between groups receiving sham exercise, the mean change in z‐score was 0.13 in the CCT group (95% CI ‐0.21 to 0.48) and ‐0.22 in the educational DVD group (95% CI ‐0.45 to 0.002). In the comparison between groups receiving aerobic exercise, the mean change in z‐score was ‐0.25 in the CCT group (95% CI ‐0.51 to 0.01) and ‐0.18 in the educational DVD control group (95% CI ‐0.49 to 0.13). We judged the quality of this evidence to be very low (downgraded for imprecision, indirectness of the study population, and risk of bias).
Cognitive subdomain: working memory
Evidence regarding working memory at end of trial (Analysis 1.5) was very low quality, downgraded because of imprecision, inconsistency, indirectness, and risk of bias. Therefore we are very uncertain about this result. Negative values favour the CCT group. Analysis at end of follow‐up gives SMD ‐0.88 (95% CI ‐1.73 to ‐0.03; 3 studies; 72 participants). Results at individual time points are as follows: immediate time point (12 weeks) SMD ‐0.66 (95% CI ‐1.26 to ‐0.06; 3 studies; 72 participants) and short‐term time point (12 weeks to one year) SMD ‐1.29 (95% CI ‐1.88 to ‐0.69; 2 studies; 53 participants).
Analysis 1.5.
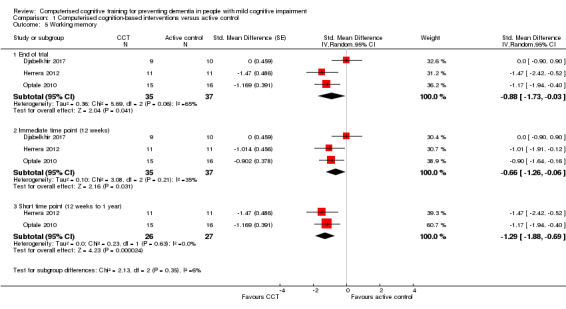
Comparison 1 Computerised cognition‐based interventions versus active control, Outcome 5 Working memory.
Cognitive subdomain: verbal fluency
Evidence regarding verbal fluency at end of trial (Analysis 1.6) was low quality, downgraded because of imprecision and risk of bias. Negative values favour the CCT group. Analysis at end of follow‐up gives SMD ‐0.16 (95% CI ‐0.76 to 0.44; 3 studies; 150 participants). Results at individual time points are as follows: immediate time point (12 weeks) SMD ‐0.02 (95% CI ‐0.46 to 0.42; 3 studies; 150 participants), short‐term time point (12 weeks to one year) SMD ‐0.78 (95% CI ‐1.51 to ‐0.04; 1 study; 31 participants), and medium‐term time point (one to two years) SMD 0.17 (95% CI ‐0.22 to 0.57; 1 study; 100 participants).
Analysis 1.6.
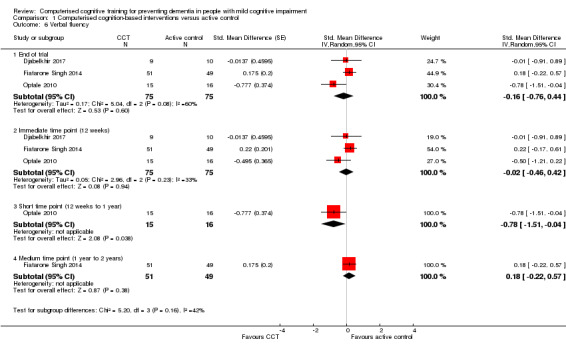
Comparison 1 Computerised cognition‐based interventions versus active control, Outcome 6 Verbal fluency.
Trial with outcome data not included in the meta‐analyses
Barnes 2013 reported outcome data on verbal fluency ‐ number of words, by letter, as standardised mean changes (z‐scores) at three months. Higher values indicated improvement. Researchers found no differences between groups (P from interaction = 0.57). In the comparison between groups receiving sham exercise, the mean change in z‐score was 0.24 in the CCT group (95% CI ‐0.11 to ‐0.58) and ‐0.05 in the educational DVD group (95% CI ‐0.33 to 0.24). In the comparison between groups receiving aerobic exercise, the mean change in z‐score was 0.22 in the CCT group (95% CI ‐0.15 to 0.58) and 0.08 in the educational DVD control group (95% CI ‐0.21 to 0.37). We judged the quality of this evidence to be very low (downgraded for imprecision, indirectness of the study population, and risk of bias).
Depression
Evidence regarding depression at end of trial (Analysis 1.7) was very low quality, downgraded because of imprecision, indirectness, and risk of bias. Negative values favour CCT. Analysis at end of follow‐up gives SMD of ‐0.77 (95% CI ‐2.07 to 0.52; 3 studies; 101 participants). Results at individual time points are as follows: immediate time point (12 weeks) SMD 0.22 (95% CI ‐0.68 to 1.13; 1 study; 19 participants) and short‐term time point (12 weeks to one year) SMD ‐1.26 (95% CI ‐3.11 to 0.59; 2 studies; 82 participants).
Analysis 1.7.
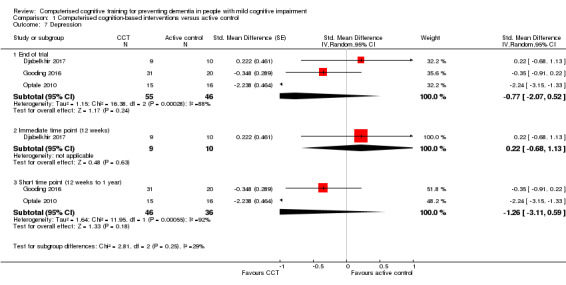
Comparison 1 Computerised cognition‐based interventions versus active control, Outcome 7 Depression.
Functional performance
Evidence regarding functional performance (Analysis 1.8) was very low quality, downgraded because of imprecision, indirectness, and risk of bias. Therefore we are very uncertain about this result. Negative values favour CCT. Analysis at end of follow‐up gives SMD 0.09 (95% CI ‐0.51 to 0.70; 2 studies; 131 participants). Results at individual time points are as follows: immediate time point (12 weeks) SMD 0.33 (95% CI ‐0.02 to 0.67; 2 studies; 131 participants), short‐term time point (12 weeks to one year) SMD ‐0.29 (95% CI ‐1.00 to 0.41; 1 study; 31 participants), and medium‐term time point (one to two years) SMD 0.34 (95% CI ‐0.06 to 0.73; 1 study; 100 participants).
Analysis 1.8.
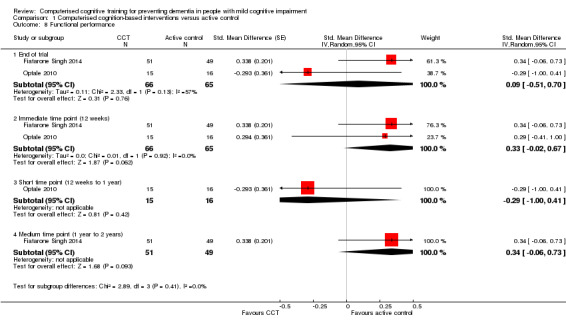
Comparison 1 Computerised cognition‐based interventions versus active control, Outcome 8 Functional performance.
Quality of life
Evidence regarding quality of life at end of trial (12 weeks) (Analysis 1.9) was low quality, downgraded because of imprecision and risk of bias. Negative values favour CCT. The mean difference (MD) was 0.40 (95% CI ‐1.85 to 2.65; 1 study; 19 participants). This result indicates that there may be little or no difference in quality of life between intervention and control groups.
Analysis 1.9.
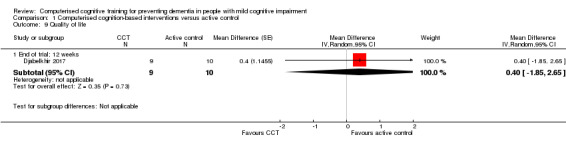
Comparison 1 Computerised cognition‐based interventions versus active control, Outcome 9 Quality of life.
Serious adverse events: mortality
Evidence regarding serious adverse events: mortality (Analysis 1.10) comes from a single study and was very low quality, downgraded because of imprecision (double downgrading) and risk of bias (Optale 2010). At short‐term follow‐up (12 weeks to one year), the risk ratio (RR) was 0.50 (95% CI 0.05 to 5.04; 1 study; 36 participants).
Analysis 1.10.
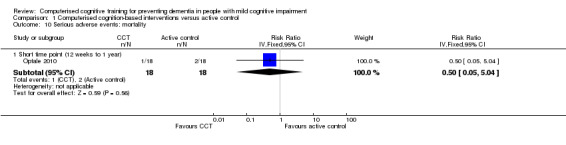
Comparison 1 Computerised cognition‐based interventions versus active control, Outcome 10 Serious adverse events: mortality.
Comparison 2: computerised cognition‐based interventions versus inactive control
See Table 2 for the comparison CCT versus inactive control. This comparison included only one study (Rozzini 2007). No data on incidence of dementia were available.
Primary outcomes
Global cognitive function
Evidence on global cognitive function at end of trial (12 months) (Analysis 2.1; Figure 6) was very low quality, downgraded because of imprecision, indirectness, and risk of bias. Therefore we are very uncertain about this result. The MD was 0.36, favouring the inactive control group (95% CI ‐0.30 to 1.02; 37 participants).
Analysis 2.1.

Comparison 2 Computerised cognition‐based interventions versus inactive control, Outcome 1 Global cognitive function.
Figure 6.
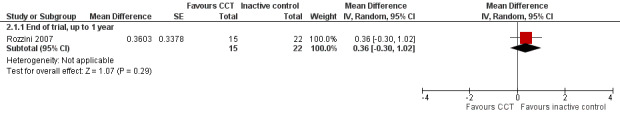
Forest plot of comparison: 2 Computerised cognition‐based interventions versus inactive control, outcome: 2.1 Global cognitive function.
Sensitivity analyses
As only a single trial contributed to the comparison, we performed no sensitivity analysis.
Secondary outcomes
Cognitive subdomain: episodic memory
Evidence regarding episodic memory at end of trial (12 months) (Analysis 2.2) was very low quality, downgraded because of imprecision, indirectness, and risk of bias. Therefore we are very uncertain about this result. The MD was ‐2.70, favouring CCT (95% CI ‐5.00 to ‐0.40; 37 participants).
Analysis 2.2.
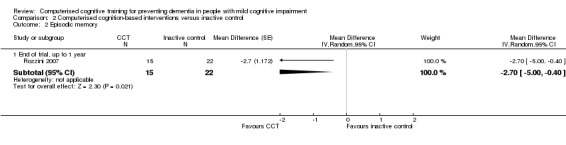
Comparison 2 Computerised cognition‐based interventions versus inactive control, Outcome 2 Episodic memory.
Cognitive subdomain: executive function
Evidence regarding executive function at end of trial (12 months) (Analysis 2.3) was very low quality, downgraded because of imprecision, indirectness, and risk of bias. Therefore we are very uncertain about this result. Negative values favour the CCT group. Analysis at end of follow‐up gives MD ‐2.70 (95% CI ‐6.21 to 0.81; 37 participants).
Analysis 2.3.
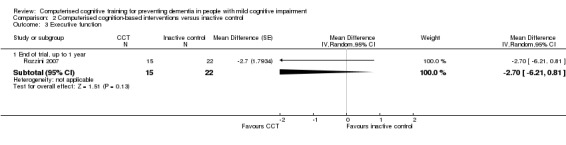
Comparison 2 Computerised cognition‐based interventions versus inactive control, Outcome 3 Executive function.
Cognitive subdomain: verbal fluency
Evidence regarding verbal fluency at end of trial (Analysis 2.4) was very low quality, downgraded because of imprecision, indirectness, and risk of bias. Negative values favour the CCT group. Therefore we are very uncertain about this result. Analysis at end of follow‐up gives MD 1.90 (95% CI ‐4.50 to 8.30; 37 participants).
Analysis 2.4.

Comparison 2 Computerised cognition‐based interventions versus inactive control, Outcome 4 Verbal fluency.
Depression
Evidence regarding depression at end of trial (Analysis 2.5) was very low quality, downgraded because of imprecision, indirectness, and risk of bias. Therefore we are very uncertain about this result. Negative values favour CCT. Analysis at end of follow‐up gives MD ‐1.30 (95% CI ‐2.61 to 0.01; 37 participants).
Analysis 2.5.

Comparison 2 Computerised cognition‐based interventions versus inactive control, Outcome 5 Depression.
Functional performance
Evidence regarding functional performance (Analysis 2.6) was very low quality, downgraded because of imprecision, indirectness, and risk of bias. Therefore we are very uncertain about this result. Negative values favour CCT. Analysis at end of follow‐up gives MD 0.00 (95% CI ‐0.48 to 0.48; 37 participants).
Analysis 2.6.
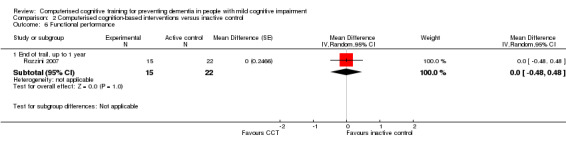
Comparison 2 Computerised cognition‐based interventions versus inactive control, Outcome 6 Functional performance.
Discussion
Summary of main results
This review examined the effects of computerised cognitive training (CCT), compared to active or inactive controls, on cognitive function in adults with mild cognitive impairment (MCI). Eight randomised controlled trials (RCTs) with a total of 660 participants were included. None of the studies reported on the incidence of dementia. All evidence was low or very low quality.
Seven trials compared CCT to a variety of active control interventions. Evidence was low quality (two outcomes) or very low quality (all other outcomes), and 95% confidence intervals (CIs) of the effect estimates were very wide, so we are very uncertain about all effect estimates. In our analyses, CCT appeared to improve performance on the primary outcome global cognition, and on secondary outcomes episodic memory and working memory, compared to active controls. However, these results are based on very low‐quality evidence. We found no evidence for effects on the cognitive subdomains of speed of processing, verbal fluency, and executive function, nor on functional performance, quality of life, depression, and serious adverse events, although, again, a high level of uncertainty is associated with all these results.
One small study compared CCT versus an inactive control intervention. Evidence for all outcomes was very low quality, so we were very uncertain about all results. With this caveat, CCT was favoured for episodic memory and executive function, but researchers found no evidence of effects on global cognition (primary outcome) nor on any of the secondary outcomes.
Overall completeness and applicability of evidence
The search was very broad including multiple data sources, all article forms, and publications in any language, so it is unlikely that relevant trials were missed. We searched for unpublished and ongoing data, but we had to rely on published data only to complete analyses. Although we did not detect publication bias, we could not formally assess this via funnel plot evaluations because of the small number of trials identified. Our objective was to measure treatment effects in participants with MCI at baseline, but we also included trials that sampled participants with cognitive deficits not meeting the MCI diagnosis (Barnes 2013; Optale 2010). We restricted inclusion to trials with a treatment duration of at least 12 weeks, and we excluded a significant number of trials with shorter periods of intervention. Although we think that a shorter treatment duration is less likely to result in treatment effects, our decision implies that our results may not be applicable to intervention programmes of shorter duration. An important limitation of this review is that we did not identify any trial with sufficiently long follow‐up to measure effects on the incidence of all‐cause dementia.
Quality of the evidence
We restricted inclusion to RCTs that we deemed to use the most valid approach in measuring treatment effects related to this topic. We identified several limitations of the included studies, and we classified none as having low risk of bias. We judged that only one study described adequate methods of both randomisation and allocation concealment and hence had low risk of selection bias (Fiatarone Singh 2014). We considered none of the included studies to have low risk of performance bias. Most studies had low risk of detection, attrition, and reporting bias.
Upon applying GRADE criteria, we considered the quality of evidence across outcomes to be very low or low, indicating that our confidence in the effect estimate is limited and, for most outcomes, very limited. Identified issues involving quality were due to imprecision, inconsistency, indirectness, and risk of bias.
Potential biases in the review process
We adhered to high standards in conducting our review, with at least two review authors independently performing trial selection, data extraction, and quality assessment to minimise bias and transcription errors. Tools used for quality assessment of trials and the overall body of evidence are those advised by the Cochrane Collaboration and the GRADE Working Group. We faced an important challenge in this and in our other Cochrane reviews evaluating CCT: the use of multiple instruments to measure a specific cognitive outcome within and across trials. Whereas others may have preferred to consider a single preferred instrument for each cognitive domain, using the mean difference to combine outcome data across trials, we preferred to use a hierarchy to select outcome data from a single validated instrument, employing the standardised mean difference (SMD) to combine outcome data across trials. Both strategies have advantages and disadvantages. For example, with the first approach, most trials will not be considered in the meta‐analyses, as studies reported large variation in the use of instruments. The advantage is that all outcome data can be easily interpreted on the natural scale. The advantage of using a hierarchy is that it allows for inclusion of all trials but makes interpretation of effect size (SMD) less intuitive. In addition, some claim that combining data derived from multiple instruments increases between‐trial heterogeneity. However, empirical evidence that supports such a claim is lacking in the field of cognitive functioning. Yet another method is to consider all reported outcome data for a specific cognitive domain, and to combine outcome data from all instruments within a trial before pooling across trials. Although this method may be valid if individual patient data are available, we deem the risk of ecological fallacy to be high when only group means are available. For this reason, we did not use such an approach. Some trials reported outcome data as z‐score changes, and even after we consulted several experienced statisticians, we were unable to transform these data to allow inclusion in the meta‐analyses. A future update of this review would benefit from clear author descriptions regarding the type of z‐score used and access to data supplements where estimates with confidence intervals are provided on the natural scale for each instrument.
In summary, our review is limited by the quality of included trials and the diversity of instruments reported to measure outcomes.
Agreements and disagreements with other studies or reviews
When we applied our rigorous quality assessment methods, we found only very low‐quality evidence for any beneficial effects of CCT. Two recent reviews have reported some positive results. In a recent review ‐ Hill 2016 ‐ review authors found an overall positive effect on cognition across 17 MCI trials (Hedges’ g = 0.35, 95% CI 0.20 to 0.51) and small to moderate effects for global cognition, attention, working memory, learning, memory, and psychosocial functioning, including depressive symptoms. In a meta‐analysis, Chandler 2016 examined the effects of cognitive interventions on more general outcome measures in MCI, including activities of daily living, mood, and quality of life; review authors identified only six computerised cognitive intervention studies and found that researchers reported benefits for mood (depression, anxiety, and apathy) among participants given the intervention compared to those given controls.
However, overall, the literature remains mixed. In adults with MCI or preclinical and early dementia, the number of clinical trials remains rather limited and studies show considerable differences between trial interventions and study methods (Gates 2014). Although multiple reviews of cognitive interventions in MCI have reported significant immediate and longer‐term benefits for cognitive function, they reported on different types of interventions such as CCT, along with cognitive stimulation and remediation, or they included mixed populations (e.g. Chandler 2016; Coyle 2014; Kurz 2009; Reijnders 2013; Simon 2012).
Subjective cognitive decline (SCD) is another cognitive category that includes healthy older adults who report concerns about a decline in cognitive function, although their performance on cognitive tests is within normal limits (Jessen 2014). Emerging evidence suggests that SCD may represent a preclinical phase of Alzheimer's disease. Therefore it is noteworthy that a recent meta‐analyses of interventions in SCD showed benefits for cognitive outcomes following cognitive training, even compared to active controls (Smart 2017).
Authors' conclusions
It is accepted that mild cognitive impairment (MCI) may represent a transitional state between normal aging and clinical dementia in some individuals; therefore it has been seen as an optimal period for intervention.
We were unable to draw any firm conclusions about the efficacy of computerised cognitive training (CCT) because of the quality of available evidence gathered for this review. However, our results suggest that CCT may have positive effects on global cognitive function, episodic memory, and working memory, when compared to involvement in other cognitively stimulating activities.
Adults with MCI and subjective cognitive decline (SCD) may possibly benefit from CCT in terms of improved cognitive function. This intervention therefore warrants longer‐term and larger‐scale trials of improved methodological quality to examine effects on cognition, conversion to dementia, daily functioning, mental well‐being and quality of life.
Key methodological considerations for future studies relate to selection of outcome measures, duration of follow‐up, and study design. First, greater attention must be paid to generalisation of benefits from trained tasks to other cognitive activities and daily function. For any programme of CCT to be useful, training must demonstrate transfer of benefits from trained to untrained tasks, and then generalisation to global function, real‐world skills, daily function, and mental health. Selected outcomes should be sensitive to subtle, and possibly non‐linear change; must have high reliability; are available in alternative forms or are psychometrically robust for repeated use; and are not affected by floor and ceiling effects.
Second, assessing the maintenance of any training gains is important. Studies with longer follow‐up are needed to measure change immediately after the intervention ends and then over time.
Third, improved reporting of study methods should be a matter of priority because of the high proportion of unclear risks of bias. Studies should adhere to CONSORT, improve data management to reduce reporting of incomplete data, and develop methods to facilitate blinding of participants and personnel. Blinding of participants is especially important given the commercialisation of CCT, advertisement, and widespread community exposure; an active control comparison arm may partially address this potential bias.
In summary, high‐quality longitudinal studies with appropriately selected outcome measures are required to determine whether CCT can contribute to maintaining cognitive function and preventing further cognitive decline and progression to clinical dementia in people with MCI.
Acknowledgements
The review authors would like to thank the group’s Information Specialist, Anna Noel‐Storr, for drafting and running electronic searches and for co‐ordinating the crowd effort. This review is part of a programme grant by which 11 other reviews were produced, using a protocol template (Abraham 2015; Al‐Assaf 2015; Denton 2015; Forbes 2015; Forbes 2015a; Forbes 2015b; Gates 2019a; Gates 2019b; Harrison 2015; Siervo 2015; Tang 2015). All authors participating in this review also acted as authors in several other reviews. As a consequence, wording chosen in the methods section may be identical across reviews, and concepts discussed, and as a result reviews, may be similar.
We also thank the following members of the Cochrane Crowd, who made significant contributions to screening the search results: Michael J. Arnatt, Soumyadeep Bhaumik, María Paz Campos Pérez, C. Cartlidge, Daniel Casey, Mohamed Fawzy Abdelghafar, Cristi Francis, Pishoy Gouda, Dan Griffiths, Michael Haas, Shirley Hall, Jake Hartley, Michael Hull, Geanina Ilinoiu, Deborah Jackson, Sofia Jaramillo, Robert Kemp, Ivan Murrieta Alvarez, Shireen Rafeeq, Miriam Thiel, Jennifer Ware, and Hakan Yaman.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| ALOIS (www.medicine.ox.ac.uk/alois) [Date of most recent search: 31 May 2018] |
Basic search: COG [Studies within ALOIS are coded COG if the intervention is a cognitive‐based intervention] |
Jan 2015: 31 Jul 2015: 4 Feb 2016: 2 Jul 2016: 0 May 2018: 1 |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) [Date of most recent search: 31 May 2018] |
1. “cognitive stimulation”.ti,ab. 2. cognitive ADJ3 train*.ti,ab. 3. “cognitive exercis*”.ti,ab. 4. “brain train*”.ti,ab. 5. (memory adj3 train*).ti,ab. 6. “memory rehab*”.ti,ab. 7. “memory enhance*”.ti,ab. 8. “poetry‐based stimulation”.ti,ab. 9. “cognitive flexibility”.ti,ab. 10. “brain exercis*”.ti,ab. 11. “cognitive rehab*”.ti,ab. 12. “mnemonic train*”.ti,ab. 13. CST.ti,ab. 14. (mental adj3 activit*).ti,ab. 15. “cognitive intervention*”.ti,ab. 16. “cognitive motor intervention*”.ti,ab. 17. “cognition based intervention*”.ti,ab. 18. “cognitive enrich*”.ti,ab. 19. Cognitive Therapy/ mt 20. or/1‐19 21. *aging/ 22. Aged 23. “Aged, 80 and over” 24. Middle Aged 25. Age Factors 26. *Cognition/ 27. *Cognition Disorders/ 28. Memory/ 29. Memory Disorders/ 30. Brain/ 31. Mild Cognitive Impairment/ 32. Executive Function/ 33. (cognit* ADJ3 (func* OR declin* OR reduc* OR impair* OR improve* OR deficit* OR progress* 34. OR perform*)).ti,ab. 35. “mental perform*”.ti,ab. 36. memory.ti,ab. 37. “executive function*”.ti,ab. 38. MCI.ti,ab. 39. AAMI.ti,ab. 40. ACMI.ti,ab. 41. ARCD.ti,ab. 42. CIND.ti,ab. 43. (nMCI OR aMCI OR mMCI OR MCIa).ti,ab. 44. Dementia/ 45. Alzheimer Disease/ 46. dement*.ti,ab. 47. alzheimer*.ti,ab. 48. “old* age*”.ti,ab. 49. elderly.ti,ab. 50. “middle age*”.ti,ab. 51. “old*adults”.ti,ab. 52. seniors.ti,ab. 53. “senior citizens”.ti,ab. 54. “community dwelling”.ti,ab. 55. pensioners.ti,ab. 56. or/21‐55 57. randomized controlled trial.pt. 58. controlled clinical trial.pt. 59. randomized.ab. 60. placebo.ab. 61. drug therapy.fs. 62. randomly.ab. 63. trial.ab. 64. groups.ab. 65. or/57‐64 66. exp animals/ not humans.sh. 67. 65 NOT 66 68. 67 AND 56 AND 20 [all results] 69. (“cognitive stimulation” OR “cognitive training”).ti. 70. *Cognition 71. *Aging/ 72. and/69‐71 73. 72 AND 57 [‘no brainer’ results ‐ directly sent to core author team] 74. 68 NOT 73 [results minus ‘no brainer’ results ‐ for the crowd to screen] |
Jan 2015: 1455 Jul 2015: 70 Feb 2016: 303 Jul 2016: 423 May 2018: 703 |
| Embase 1974‐24 January 2018 (Ovid SP) [Date of most recent search: 31 May 2018] |
1. aging/ 2. aged/ 3. middle aged/ 4. mild cognitive impairment/ 5. elderly.ti,ab. 6. MCI.ti,ab. 7. AAMI.ti,ab. 8. ACMI.ti,ab. 9. ARCD.ti,ab. 10. CIND.ti,ab. 11. (nMCI or aMCI or mMCI or MCIa).ti,ab. 12. "old* age*".ti,ab. 13. elderly.ti,ab. 14. "middle age*".ti,ab. 15. "old* aadults".ti,ab. 16. seniors.ti,ab. 17. "senior citizens".ti,ab. 18. "community dwelling".ti,ab. 19. pensioners.ti,ab. 20. ("aged sample" or "aged population" or "older sample" or "older population").ti,ab. 21. "CDR 0.5".ti,ab. 22. (cognit* adj3 (func* or declin* or reduc* or impair* or improve* or deficit* or progress* or perform* or abilit*)).ti,ab. 23. or/1‐22 24. *cognition/ 25. memory/ or episodic memory/ 26. executive function/ 27. attention/ 28. "mental perform*".ti,ab. 29. memory.ti,ab. 30. dementia/ 31. Alzheimer disease/ 32. dement*.ti,ab. 33. alzheimer*.ti,ab. 34. or/24‐33 35. randomized controlled trial/ 36. controlled clinical trial/ 37. (randomly adj2 allocat*).ab. 38. (randomly adj2 divide*).ab. 39. randomi?ed.ab. 40. (controlled adj7 (study or design or trial)).ti,ab. 41. "double‐blind*".ti,ab. 42. "single blind*".ti,ab. 43. groups.ab. 44. or/35‐43 45. "cognitive stimulation".ti,ab. 46. (cognitive adj3 train*).ti,ab. 47. "cognitive exercis*".ti,ab. 48. "brain train*".ti,ab. 49. (memory adj3 train*).ti,ab. 50. "memory enhance*".ti,ab. 51. "memory rehab*".ti,ab. 52. "brain exercis*".ti,ab. 53. "cognitive rehab*".ti,ab. 54. "cognitive rehab*".ti,ab. 55. "mnemonic train*".ti,ab. 56. CST.ti,ab. 57. (mental adj3 activit*).ti,ab. 58. "cognitive intervention*".ti,ab. 59. "cognitive motor intervention*".ti,ab. 60. "cognition based intervention*".ti,ab. 61. "cognitive enrich*".ti,ab. 62. "reality orientation".ti,ab. 63. (memory adj2 game*).ti,ab. 64. or/45‐63 65. 23 and 34 and 44 and 64 66. ("cognitive stimulation" or "cognitive training").ti,ab. 67. cognition/ 68. (MCI or "mild cognitive impairment" or elderly or "old* adults" or "middle age*").ti. 69. 66 and 67 and 68 70. 35 and 69 71. 65 not 70 |
Jan 2015: 1289 Jul 2015: 163 Feb 2016: 380 Jul 2016: 268 May 2018: 796 |
| PSYCINFO 1806‐January week 2 2018 (Ovid SP) [Date of most recent search: 31 May 2018] |
1. exp Aging/ 2. exp Cognitive Impairment/ 3. "cognit* impair*".ti,ab. 4. MCI.ti,ab. 5. AAMI.ti,ab. 6. ACMI.ti,ab. 7. ARCD.ti,ab. 8. CIND.ti,ab. 9. (nMCI or aMCI or mMCI or MCIa).ti,ab. 10. "old* age*".ti,ab. 11. elderly.ti,ab. 12. "middle age*".ti,ab. 13. "old* adults".ti,ab. 14. seniors.ti,ab. 15. "senior citizens".ti,ab. 16. "community dwelling".ti,ab. 17. pensioners.ti,ab. 18. or/1‐17 19. randomi?ed.ti. 20. (randomly adj2 allocat*).ab. 21. (randomly adj2 divide*).ab. 22. RCT.ti,ab. 23. "double‐blind*".ti,ab. 24. "single blind*".ti,ab. 25. "randomi?ed trial".ab. 26. "randomi?ed control* trial".ab. 27. "random allocation".ab. 28. "controlled clinical trial".ti,ab. 29. (controlled adj4 (study or design or trial)).ti,ab. 30. or/19‐29 31. "cognitive stimulation".ti,ab. 32. (cognitive adj3 train*).ti,ab. 33. "cognitive exercis*".ti,ab. 34. "brain train*".ti,ab. 35. (memory adj3 train*).ti,ab. 36. "memory enhance*".ti,ab. 37. "memory rehab*".ti,ab. 38. "brain exercis*".ti,ab. 39. "cognitive rehab*".ti,ab. 40. "cognitive rehab*".ti,ab. 41. "mnemonic train*".ti,ab. 42. CST.ti,ab. 43. (mental adj3 activit*).ti,ab. 44. "cognitive intervention*".ti,ab. 45. "cognitive motor intervention*".ti,ab. 46. "cognition based intervention*".ti,ab. 47. "cognitive enrich*".ti,ab. 48. "reality orientation".ti,ab. 49. (memory adj2 game*).ti,ab. 50. or/31‐49 51. 18 and 30 and 50 52. *Cognition/ 53. (MCI or "mild cognitive impairment" or elderly or "old* adults" or "middle age*").ti. 54. ("cognitive stimulation" or "cognitive training").ti,ab. 55. 19 or 20 or 21 56. 52 and 53 and 54 and 55 57. 51 not 56 |
Jan 2015: 166 Jul 2015: 20 Feb 2016: 25 Jul 2016: 12 May 2018: 84 |
| CINAHL (EBSCOhost) [Date of most recent search: 31 May 2018] |
Jan 2015: 390 Jul 2015: 13 Feb 2016: 57 Jul 2016: 12 May 2018: 181 |
|
| ISI Web of Science [includes: Web of Science (1945‐present); BIOSIS Previews (1926‐present); MEDLINE (1950‐present); Journal Citation Reports]; BIOSIS Previews [Date of most recent search: 31 May 2018] |
("mild cognitive impairment" OR elderly OR "age* subjects" OR "old* adult*" OR "middle age*" OR MCI) AND TOPIC: ("randomly allocated" OR "random allocation" OR randomised OR randomized OR RCT OR "controlled trial" OR "double blind" OR "single blind") AND TOPIC: ("cognit* stim*" OR "cognit* train*" OR puzzle OR "brain train*" OR "cognit* exercis*" OR "brain exercis*" OR "memory exercis*" OR "brain gam*" OR "cognit* gam*" OR "memory gam*" OR sudoku OR crossword* OR "reality orientation") AND TOPIC: (cognition OR dementia OR memory OR "executive function" OR alzheimer*) Timespan: All years. Search language=Auto |
Jan 2015: 333 Jul 2015: 44 Feb 2016: 108 Jul 2016: 35 May 2018: 408 |
| LILACS (BIREME) [Date of most recent search: 31 May 2018] |
Jan 2015: 4 Jul 2015: 0 Feb 2016: 0 Jul 2016: 0 May 2018: 0 |
|
| CENTRAL (via CRSO) [Date of most recent search: 31 May 2018] |
#1 MeSH descriptor: [Aged, 80 and over] explode all trees #2 MeSH descriptor: [Aged] explode all trees #3 MeSH descriptor: [Middle Aged] explode all trees #4 MeSH descriptor: [Mild Cognitive Impairment] explode all trees #5 "cognit* impair*" or MCI #6 elderly #7 "old* adults" #8 "old* age*" #9 "old* sample" #10 senior citizens #11 pensioners #12 seniors #13 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 #14 MeSH descriptor: [Cognition] explode all trees #15 MeSH descriptor: [Dementia] explode all trees #16 cognit* #17 memory #18 "executive function*" #19 processing #20 "mental perform*" #21 dement* #22 alzheimer* #23 #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 #24 "cognitive stimulation" #25 "cognitive training" #26 "brain train*" #27 "brain gam*" #28 "memory train*" or "memory game*" #29 puzzle* #30 crossword* #31 sudoku* #32 "mental game*" #33 "mental agil*" #34 "cognitive exercis*" #35 "mental exercis*" #36 #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 #37 #13 and #23 and #36 |
Jan 2015: 274 Jul 2015: 11 Feb 2016: 57 Jul 2016: 4 May 2018: 210 |
| Clinicaltrials.gov (www.clinicaltrials.gov) [Date of most recent search: 31 May 2018] |
Jan 2015: 17 Jul 2015: 4 Feb 2016: 2 Jul 2016: 0 May 2018: 4 |
|
| ICTRP Search Portal (http://apps.who.int/trialsearch) [includes Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] [Date of most recent search: 31 May 2018] |
Jan 2015: 22 Jul 2015: 3 Feb 2016: 1 Jul 2016: 0 May 2018: 4 |
|
| TOTAL before de‐duplication | Jan 2015: 3981 Jul 2015: 332 Feb 2016: 935 Jul 2016: 754 May 2018: 2390 TOTAL: 8392 |
|
| TOTAL after de‐duplication | TOTAL: 6233 | |
| TOTAL after first assessment by the Crowd and CDCIG Information Specialists | Jan 2015: 604 Jul 2015: 60 Feb 2016: 164 Jul 2016: 73 May 2018: 190 TOTAL: 1091 |
|
Appendix 2. Definitions of design, patient, and intervention characteristics as applied in the stratified analyses exploring between‐trial variations in intervention effects
| ITEM | DEFINITION |
| Design‐related characteristics* | |
| Concealment of allocation (avoiding selection bias) | Guidance from the Cochrane Handbook for Systematic Reviews of Interventions will be used to judge bias related to sequence generation and concealment of allocation using the 2 Cochrane 'Risk of bias' items (Higgins 2011). From these, the statistician will derive a single variable to be used in the stratified analysis: allocation concealment will be judged at low risk of bias if the investigators responsible for patient selection were unable to suspect before allocation which treatment was next. Concealment will be downgraded to high risk of bias if there is evidence of inadequate sequence generation (Rutjes 2012) |
| Blinding of patients and personnel (avoiding performance bias) | Low risk of bias will be judged: ‐ if a credible sham procedure was used; or if a placebo supplement or pill was used that was reported to be identical in appearance to the experimental intervention and the specific outcome or group of outcomes is/are likely to be influenced by lack of blinding ‐ if blinding is absent or suboptimal and the specific outcome, such as mortality, is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (avoiding detection bias) |
For self‐reported/partner‐reported outcomes: Low risk of bias will be judged if self‐report outcomes were assessed AND blinding of patients was considered adequate AND there was no information to suggest that there was an investigator involved during the process of outcome assessment; OR if blinding of investigators performing the outcome assessment was reported AND an attempt to blind patients was reported For other outcomes: Outcome assessment was considered to be blinded if outcome assessment was reported to be blinded |
| Statistical analyses (avoiding attrition bias) |
For continuous outcomes: Low risk of bias will be judged: ‐ if at least 90% of the patients randomised were analysed AND the difference in percentage of participants not analysed was 5% or lower across trial arms ‐ for trials using imputations to handle missing data: the percentage of participants with missing data did not exceed 20% AND the difference in percentage of participants with imputed data was 5% or lower across trial arms AND applied imputation methods were judged to be appropriate. Multiple imputation techniques will be considered appropriate, simple methods such as 'last observation carried forward' or 'baseline carried forward' will be considered inappropriate For binary outcomes of rare events: Low risk of bias will be judged if the event rate is low (e.g. incidence of dementia) AND at least 95% of the patients randomised were analysed AND there is no evidence of differential reasons for missing data that may alter the estimate AND the rate of missing data does not exceed the expected event rates For binary outcomes of non‐rare events: Low risk of bias will be judged if at least 90% of the patients randomised were analysed AND the difference in percentage of participants not analysed was 5% or lower across trial arms AND there is no evidence of differential reasons for missing data that may alter the estimate AND the rate of missing data does not exceed the expected event rates |
| Trial size | The cut‐off to distinguish small from larger trials will be determined by a sample size calculation on the primary outcome |
| Publication status | Full journal article vs other type or unpublished material |
| Follow‐up duration | For the cognitive outcomes, we will group studies according to these follow‐up cut‐offs to describe immediate results (up to 12 weeks) and short‐term (up to 1 year), medium‐term (1 to 2 years), and longer‐term results (more than 2 years) |
| Treatment‐related characteristics | |
| Treatment and control Treatment duration |
Analyses will be stratified by
Analyses will be stratified into session length > 30 minutes (yes/no), frequency > 3 sessions per week (yes/no), based upon previous findings (Lampit 2014), and total number of sessions. The minimum treatment duration of 3 months is considered short term, 3 to 12 months as medium term, and 12 months as long term. For the outcome all‐cause dementia, only outcome data at 1 year of follow‐up or longer will be considered, and therefore the grouping will include short‐term (up to 1 year), medium‐term (1 to 2 years), and longer‐term results (more than 2 years) |
| Participant‐related characteristics | |
| Cognition and participant‐related criteria | Gender, level of education (in years), ApoE‐4 (yes/no), baseline age (mid‐life vs late‐life vs other), and time since diagnoses |
| *The descriptions given in this table are provided in addition to the guidance provided by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Stratified analyses are performed only for the primary outcome if about 10 RCTs contributed to the analyses. | |
Data and analyses
Comparison 1.
Computerised cognition‐based interventions versus active control
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global cognitive function | 5 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 End of trial | 5 | 407 | Std. Mean Difference (Random, 95% CI) | ‐0.53 [‐1.06, ‐0.01] |
| 1.2 Immediate time point (12 weeks) | 4 | 356 | Std. Mean Difference (Random, 95% CI) | ‐0.31 [‐0.70, 0.08] |
| 1.3 Short time point (12 weeks to 1 year) | 2 | 82 | Std. Mean Difference (Random, 95% CI) | ‐1.23 [‐1.89, ‐0.56] |
| 1.4 Medium time point (1 year to 2 years) | 1 | 100 | Std. Mean Difference (Random, 95% CI) | 0.16 [‐0.23, 0.55] |
| 2 Episodic memory | 5 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 2.1 End of trial | 5 | 223 | Std. Mean Difference (Random, 95% CI) | ‐0.79 [‐1.54, ‐0.04] |
| 2.2 Immediate time point (12 weeks) | 4 | 172 | Std. Mean Difference (Random, 95% CI) | ‐0.99 [‐1.80, ‐0.19] |
| 2.3 Short time point (12 weeks to 1 year) | 3 | 104 | Std. Mean Difference (Random, 95% CI) | ‐1.39 [‐2.35, ‐0.44] |
| 2.4 Medium time point (1 year to 2 years) | 1 | 100 | Std. Mean Difference (Random, 95% CI) | 0.02 [‐0.37, 0.41] |
| 3 Speed of processing | 2 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 End of trial | 2 | 119 | Std. Mean Difference (Random, 95% CI) | 0.20 [‐0.16, 0.56] |
| 3.2 Immediate time point (12 weeks) | 2 | 119 | Std. Mean Difference (Random, 95% CI) | 0.11 [‐0.25, 0.47] |
| 3.3 Medium time point (1 year to 2 years) | 1 | 100 | Std. Mean Difference (Random, 95% CI) | 0.14 [‐0.25, 0.53] |
| 4 Executive function | 3 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 4.1 End of trial | 3 | 150 | Std. Mean Difference (Random, 95% CI) | ‐0.31 [‐0.90, 0.28] |
| 4.2 Immediate time point (12 weeks) | 3 | 150 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.50, 0.14] |
| 4.3 Short time point (12 weeks to 1 year) | 1 | 31 | Std. Mean Difference (Random, 95% CI) | ‐0.81 [‐1.54, ‐0.07] |
| 4.4 Medium time point (1 year to 2 years) | 1 | 100 | Std. Mean Difference (Random, 95% CI) | 0.08 [‐0.31, 0.48] |
| 5 Working memory | 3 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 5.1 End of trial | 3 | 72 | Std. Mean Difference (Random, 95% CI) | ‐0.88 [‐1.73, ‐0.03] |
| 5.2 Immediate time point (12 weeks) | 3 | 72 | Std. Mean Difference (Random, 95% CI) | ‐0.66 [‐1.26, ‐0.06] |
| 5.3 Short time point (12 weeks to 1 year) | 2 | 53 | Std. Mean Difference (Random, 95% CI) | ‐1.29 [‐1.88, ‐0.69] |
| 6 Verbal fluency | 3 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 6.1 End of trial | 3 | 150 | Std. Mean Difference (Random, 95% CI) | ‐0.16 [‐0.76, 0.44] |
| 6.2 Immediate time point (12 weeks) | 3 | 150 | Std. Mean Difference (Random, 95% CI) | ‐0.02 [‐0.46, 0.42] |
| 6.3 Short time point (12 weeks to 1 year) | 1 | 31 | Std. Mean Difference (Random, 95% CI) | ‐0.78 [‐1.51, ‐0.04] |
| 6.4 Medium time point (1 year to 2 years) | 1 | 100 | Std. Mean Difference (Random, 95% CI) | 0.18 [‐0.22, 0.57] |
| 7 Depression | 3 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 7.1 End of trial | 3 | 101 | Std. Mean Difference (Random, 95% CI) | ‐0.77 [‐2.07, 0.52] |
| 7.2 Immediate time point (12 weeks) | 1 | 19 | Std. Mean Difference (Random, 95% CI) | 0.22 [‐0.68, 1.13] |
| 7.3 Short time point (12 weeks to 1 year) | 2 | 82 | Std. Mean Difference (Random, 95% CI) | ‐1.26 [‐3.11, 0.59] |
| 8 Functional performance | 2 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 8.1 End of trial | 2 | 131 | Std. Mean Difference (Random, 95% CI) | 0.09 [‐0.51, 0.70] |
| 8.2 Immediate time point (12 weeks) | 2 | 131 | Std. Mean Difference (Random, 95% CI) | 0.33 [‐0.02, 0.67] |
| 8.3 Short time point (12 weeks to 1 year) | 1 | 31 | Std. Mean Difference (Random, 95% CI) | ‐0.29 [‐1.00, 0.41] |
| 8.4 Medium time point (1 year to 2 years) | 1 | 100 | Std. Mean Difference (Random, 95% CI) | 0.34 [‐0.06, 0.73] |
| 9 Quality of life | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 9.1 End of trial; 12 weeks | 1 | 19 | Mean Difference (Random, 95% CI) | 0.4 [‐1.85, 2.65] |
| 10 Serious adverse events: mortality | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Short time point (12 weeks to 1 year) | 1 | 36 | Risk Ratio (IV, Fixed, 95% CI) | 0.5 [0.05, 5.04] |
Comparison 2.
Computerised cognition‐based interventions versus inactive control
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global cognitive function | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 End of trial, up to 1 year | 1 | 37 | Mean Difference (Random, 95% CI) | 0.36 [‐0.30, 1.02] |
| 2 Episodic memory | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 2.1 End of trial, up to 1 year | 1 | 37 | Mean Difference (Random, 95% CI) | ‐2.7 [‐3.00, ‐0.40] |
| 3 Executive function | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 End of trial, up to 1 year | 1 | 37 | Mean Difference (Random, 95% CI) | ‐2.7 [‐6.21, 0.81] |
| 4 Verbal fluency | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 4.1 End of trial, up to 1 year | 1 | 37 | Mean Difference (Random, 95% CI) | 1.90 [‐4.50, 8.30] |
| 5 Depression | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 5.1 End of the trial, up to 1 year | 1 | 37 | Mean Difference (Random, 95% CI) | ‐1.3 [‐2.61, 0.01] |
| 6 Functional performance | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 6.1 End of trail, up to 1 year | 1 | 37 | Mean Difference (Random, 95% CI) | 0.0 [‐0.48, 0.48] |
Differences between protocol and review
We planned stratified analyses to explore between‐trial heterogeneity according to the features outlined in Appendix 2, and we planned to prepare funnel plots to explore the impact of publication bias and other biases associated with small sample size. By protocol, we indicated that about 10 trials should contribute to the analysis for it to be meaningful. As the number of trials identified was substantially lower, we refrained from undertaking such analyses. We planned to perform one sensitivity analysis for the primary outcome, including high‐quality trials only. We aimed to define high quality by using results of the stratified analyses. As stratified analyses could not be performed, we refrained from conducting sensitivity analyses. Although not described in our published protocol, we made the decision to use a hierarchy to select outcome data before starting data extraction. The hierarchy itself was also established before any trial in this and two other Cochrane reviews had started (Gates 2019a; Gates 2019b).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Judgement: random sequence adequately generated Quote(s): "participants were randomized in blocks of 4. The randomization sequence was prepared in advance by using a random‐number generator on a computer" |
| Allocation concealment (selection bias) | Unclear risk |
Judgement: study authors state that allocation was concealed, although the method of allocation concealment is not reported Quote(s): "research staff involved with enrolment and outcome assessment were unaware of the randomization sequence and blinded to group assignment" |
| Blinding of participants (performance bias) | High risk |
Judgement: patients were not blinded to the type of intervention Quote(s): "study participants were unaware of study hypotheses and were told that the goal of the study was to compare the effects of different physical and mental activity programs" |
| Blinding of physicians / personnel | Low risk |
Judgement: therapists were blinded to study treatment Quote(s): "research staff involved with enrolment and outcome assessment were unaware of the randomization sequence and blinded to group assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Judgement: therapists were blinded to study treatment Quote(s): "research staff involved with enrolment and outcome assessment were unaware of the randomization sequence and blinded to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Judgement: 32 out of 32 (100%) randomised to the experimental group were analysed, and 31 out of 31 (100%) randomised to the control group were randomised; the statistical analyses were reported to be done according to the intent‐to‐treat principle; 9/32 in experimental and 3/31 in control withdrew from study but were included in the final analysis Quote(s): "all analyses were performed using intent‐to‐treat principles" |
| Selective reporting (reporting bias) | Low risk | Judgement: all outcomes mentioned in the methods section are reported in the results section |
| Other bias | Low risk | Judgement: no other sources of bias are apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | KODRO provided access to the software; study authors reported no conflict of interest in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Judgement: adequate method of random sequence generation Quote(s): "patients were assigned to either a computerized CS (CCS) group or a computerized cognitive engagement (CCE) group with a simple computerized randomization procedure |
| Allocation concealment (selection bias) | Unclear risk | Judgement: no description provided |
| Blinding of participants (performance bias) | Unclear risk |
Judgement: study described as single‐blinded; however, it is not clear if and how participants were blinded Quote(s): "we designed a randomized single‐blind study conforming to Consolidated Standards of Reporting Trials criteria for pilot and feasibility studies" |
| Blinding of physicians / personnel | High risk | Judgement: therapists could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement: blinded outcome assessment Quote(s): "these were carried out by an experienced neuropsychologist blinded to the intervention" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement: no participants were lost to follow‐up Quote(s): "none of the participants discontinued the intervention. Only one participant in the CCS group did not perform the M3 assessment for medical reasons (surgery), resulting in 19 subjects for the final analyses" |
| Selective reporting (reporting bias) | Low risk | Judgement: all outcomes indicated in the methods section are reported in the results section |
| Other bias | Low risk | Judgement: no other sources of bias are apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
*Our hierarchy did not indicate a preference for the delayed subscale over the immediate subscale. Whenever both immediate and delayed subscales were available, the delayed subscale was included in the meta‐analyses, as it was thought to be more clinically relevant |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Judgement: adequate method of random sequence generation Quote(s): "a concealed, computer‐generated sequence of randomly permuted blocks.. in a 1:1:1:1 ratio to each of the 4 intervention arms, stratified by sex and age (<75 and 75 þ years), was generated by a research assistant not otherwise involved in the study via a statistical website" |
| Allocation concealment (selection bias) | Low risk |
Judgement: adequate method of concealment allocation Quote(s): "assignments were then placed in sealed opaque envelopes and delivered to participants by the recruitment officer" |
| Blinding of participants (performance bias) | Unclear risk |
Judgement: study described as double‐blinded; however, it is not clear if patients were blinded Quote(s): "all training was fully supervised by research assistants from exercise physiology or physical therapy backgrounds" |
| Blinding of physicians / personnel | High risk |
Judgement: researchers supervising training were not blinded Quote(s): "all training was fully supervised by research assistants from exercise physiology or physical therapy backgrounds" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Judgement: blinded outcome assessment Quote(s): "blinded assessors administered all outcome measures at baseline, 6 and 18 months" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Judgement: Comparison 1: 24 out of 24 (100%) randomised were analysed in experimental group 1, and 27 out of 27 (100%) randomised were analysed in control group 1 Comparison 2: 27 out of 27 (100%) randomised were analysed in experimental group 2, and 22 out of 22 (100%) randomised were analysed in control group 2. Statistical analyses were reported to be done according to the intent‐to‐treat principle Quote(s): "all patients randomised were included in the analysis"; "n = 100 for all outcomes" |
| Selective reporting (reporting bias) | Low risk | Judgement: all outcomes indicated in the methods section are reported in the results section |
| Other bias | Low risk | Judgement: no other sources of bias are apparent |
| Methods |
|
|
| Participants |
Data provided only for 74 participants who completed the study:
|
|
| Interventions |
Type of concomitant treatment provided: not stated
|
|
| Outcomes |
|
|
| Notes | Funded by a grant from the Alzheimer’s Association (IIRG‐09–131861) and a Department of Veterans Affairs RR&D Career Development Award (RRD‐B4146V); study authors report no conflict of interest in the study The third arm (CVT) consisted of CCT plus a motivational therapeutic milieu and was not included in the analysis due to the ACG that did not receive the motivational therapeutic milieu intervention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement: no methods for randomisation described Quote(s): "this randomised clinical trial used a test–re‐test treatment controlled design with recruited patients randomly assigned to one of three research arms ‐ computerised cognitive training (CCT), cognitive vitality training (CVT), or an active control group (ACG)" |
| Allocation concealment (selection bias) | Unclear risk | Judgement: no methods for allocation concealment described |
| Blinding of participants (performance bias) | High risk |
Judgement: blinding not feasible Quote(s): none |
| Blinding of physicians / personnel | High risk |
Judgement: blinding not feasible Quote(s): none |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement: no methods for blinding the outcome assessor described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement: high proportion of participants were lost to follow‐up Quote(s): "a total of 96 participants were recruited for this study, and completed the baseline neuropsychological evaluation. Of those, 74 participants completed the full treatment, 7 completed a partial portion of the treatment, and 15 did not complete any portion of the assigned treatment. The overall study attrition rate was 23%" |
| Selective reporting (reporting bias) | Low risk | Judgement: all outcomes described in the methods section are adequately addressed in the results section |
| Other bias | Low risk | Judgement: no other sources of bias are apparent |
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Judgement: no methods for randomising participants have been described Quote(s): "the 22 patients were randomly assigned into two groups (11 patients per group): a group that performed training (Trained group) and a group that participated in stimulating cognitive activities (Control group)" |
| Allocation concealment (selection bias) | Unclear risk | Judgement: no description provided |
| Blinding of participants (performance bias) | Unclear risk | Judgement: blinding not reported and interventions are clearly different. Nevertheless, depending on the information participants received, blinding could have been successful. As trial authors did not measure this, we judged unclear risk of bias |
| Blinding of physicians / personnel | High risk |
Judgement: therapists could not be blinded Quote(s): "three trained neuropsychologists were involved in the study: one administered and scored the pre‐tests, post‐tests, and follow‐up tests (this person was kept blind to the group membership of patients), one supervised training, and one supervised cognitive activities" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Judgement: assessors were blinded to the treatment assigned, although the method of blinding is not described in detail Quote(s): "three trained neuropsychologists were involved in the study: one administered and scored the pre‐tests, post‐tests, and follow‐up tests (this person was kept blind to the group membership of patients), one supervised training, and one supervised cognitive activities" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement: 11 out of 11 (100%) randomised were analysed in the experimental group, and 11 out of 11 (100%) randomised were analysed in the control group. It is not clearly reported if all randomised participants were evaluated for this test, so for statistical analyses, we used the number randomised as the number analysed |
| Selective reporting (reporting bias) | Low risk | Judgement: all outcomes described in the methods section are adequately addressed in the results section |
| Other bias | Unclear risk | Judgement: the selection process for participants is not described in sufficient detail; few baseline characteristics are described, not allowing a judgement whether between‐group baseline imbalances occurred in this small trial |
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Although Kwok 2013a measured global cognitive function at 9 months of follow‐up, they did not report data for the entire study population | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Judgement: method of allocation not reported Quote(s): "single‐blind randomized placebo‐controlled trial" |
| Allocation concealment (selection bias) | Unclear risk |
Judgement: method of allocation concealment not reported Quote(s): none |
| Blinding of participants (performance bias) | High risk |
Judgement: blinding not feasible Quote(s): none |
| Blinding of physicians / personnel | High risk |
Judgement: blinding not feasible Quote(s): none |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Judgement: outcome assessor explicitly reported to be blind Quote(s): "trained research assistant who was blind to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Judgement: 103 out of 111 (93%) randomised in experimental group were analysed, and 103 out of 112 (92%) randomised in control group were analysed. Fraction with missing data below 10% Quote(s): none; "the authors did not mention analyses to be in line with intent‐to‐treat principles, neither did they report on imputation techniques" |
| Selective reporting (reporting bias) | High risk | Judgement: incomplete reporting of non‐significant outcome data for the overall group. For example, outcome data for the CDRS total score were not abstractable for the overall group but were reported for subgroups with low, moderate, or high educational baseline values |
| Other bias | Low risk | Judgement: none detected |
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Judgement: method for generating random sequence is not clearly reported Quote(s): "for each replicate, half of the participants were randomly allocated to the EG, whereas the remaining participants were allocated to the CG" |
| Allocation concealment (selection bias) | Unclear risk |
Judgement: method of allocation concealment is not reported Quote(s): "for each replicate, half of the participants were randomly allocated to the EG, whereas the remaining participants were allocated to the CG" |
| Blinding of participants (performance bias) | High risk |
Judgement: patients were not blinded Quote(s): "a randomized controlled single‐blind procedure was used, in which the examiner administrating the clinical and neuropsychological tests remained unaware of the participants’ allocations to the EG or CG" |
| Blinding of physicians / personnel | High risk |
Judgement: therapist supervising the training was not blinded Quote(s): "a randomized controlled single‐blind procedure was used, in which the examiner administrating the clinical and neuropsychological tests remained unaware of the participants’ allocations to the EG or CG" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Judgement: the outcome assessor was explicitly described to be blinded to the intervention assigned Quote(s): "the examiner administrating the clinical and neuropsychological tests remained unaware of the participants’ allocations to the EG or CG" |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Judgement: 15 out of 18 (83%) randomised in experimental group were analysed, and 16 out of 18 (89%) randomised in control group were analysed. We judged high risk of bias, as the percentage randomised but not analysed exceeded 10%; a complete case analyses was performed Quote(s): "one experimental group (EG) participant and 2 control group (CG) participants died before completing the booster training. Furthermore, 2 EG participants left the rest home and went back to their families before completing the booster phase. Because we aimed to investigate the effects of both the initial and the booster training phases, the 5 participants yielding incomplete data were not included in the analyses" |
| Selective reporting (reporting bias) | High risk |
Judgement: 1 out of 13 outcomes was not consistently performed for unclear reasons Quote(s): "the Trail Making Test was also part of the evaluation protocol but could not be administered to most participants and was not included in the final analysis" |
| Other bias | Unclear risk | Judgement: no other potential risks of bias detected. |
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Judgement: method of random sequence generation not reported Quote(s): "randomisation was made by a member of the research team" |
| Allocation concealment (selection bias) | Unclear risk |
Judgement: method of allocation not reported Quote(s): "randomisation was made by a member of the research team" |
| Blinding of participants (performance bias) | High risk |
Judgement: blinding not feasible Quote(s): none |
| Blinding of physicians / personnel | High risk |
Judgement: blinding not feasible Quote(s): none |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Judgement: blinded outcome assessors Quote(s): "the administration of the pre–post neuropsychological measures and the training program were conducted by two different experienced neuropsychologist, blinded to the subjects’ group status" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement: 15 out of 15 (100%) randomised in experimental group were analysed, and 22 out of 22 (100%) randomised in control group were analysed. From the Table, it seems that all included patients were considered for inclusion in the analysis, although this is not clearly reported in the text |
| Selective reporting (reporting bias) | Low risk | Judgement: all outcomes indicated in the methods section are reported in the results section. |
| Other bias | Unclear risk | Judgement: participants characteristics are not described and the selection process is not reported; it is unclear if participants were included consecutively |
16‐FR/CR test: 16‐item free and cued reminding test (also RI‐RI‐16: rappel libre / rappel indicé à 16 items)
3MS: Mini Mental State Examination.
ACG: active control group.
ADAS‐Cog: Alzheimer's Disease Assessment Scale Cognitive.
A‐MCImd: amnestic MCI multiple domains subtype.
APOE: apolipoprotein E.
BADL: Brief Activities of Daily Living.
BAYER‐ADL: Bayer Activities of Daily Living Scale.
BSRT: Buschke Selective Reminding Test.
BVRT: Benton Visual Retention Test.
CCE: computerised cognitive engagement.
CCS: computerised cognitive stimulation.
CG: control group.
ChEI: cholinesterase inhibitor.
CMMSE: Chinese version of Mini‐Mental State Examination.
CMSS: Chinese Memory Symptoms Scale
COWAT: Controlled Oral Word Association Test.
CT: cognitive training.
CVT: cognitive vitality training.
DSST: Digit Symbol Substitution Test.
EFT: Eriksen Flanker Test
EG: experimental group.
ILS: independent living scales.
LM: logical memory.
MCI: mild cognitive impairment.
mMMSE: modified Mini Mental State Examination.
MMSE: Mini Mental State Examination.
NEAR: Neuropsychological and Educational Approach to Remediation model of treatment
PRT: progressive resistance training.
RAVLT: Rey Auditory Verbal Learning Test.
RCT: randomised controlled test.
SD: standard deviation.
SDMT: Symbol Digit Modality Test.
TMT‐B and ‐A: Trail Making Test‐B and ‐A.
UFOV: useful field of view.
WAIS: Wechsler Adult Intelligence Scale.
WMS‐R: Wechsler Memory Scale‐Revised.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adel 2013 | Wrong study design |
| Alves 2014 | Wrong intervention |
| Alves 2014a | Wrong intervention |
| Anderson 2014 | Intervention shorter than 12 weeks: 8‐week intervention period (2‐arm trial; n = 67; likely cognitive healthy; mean age 63 years; extension of earlier trial) |
| Ann 2012 | Wrong patient population |
| Apostolo 2014 | Wrong patient population |
| Baglio 2011 | Nature of intervention unclear |
| Ball 2002 | Intervention shorter than 12 weeks: 5‐ to 6‐week intervention period with 2‐ to 3‐week booster period at 11 and 35 months (4‐arm trial ACTIVE; n = 2832; cognitively healthy; mean age 74 years) |
| Ball 2002a | Duplicate |
| Ball 2006 | Intervention shorter than 12 weeks. Multiple reports for excluded trial: Ball 2002 (trial ACTIVE) |
| Ball 2013 | Intervention shorter than 12 weeks |
| Ballesteros 2014 | Duplicate |
| Ballesteros 2014a | Duplicate |
| Ballesteros 2015 | Duplicate |
| Ballesteros 2015a | Duplicate |
| Ballesteros 2017 | Intervention shorter than 12 weeks |
| Bamidis 2015 | Wrong study design |
| Baniqued 2014 | Adult population |
| Baniqued 2015 | Younger than 30 years of age |
| Barban 2012 | Duplicate |
| Barban 2016 | Wrong study design |
| Barbosa 2015 | Wrong intervention |
| Barcelos 2015 | Wrong intervention |
| Barnes 2006 | Intervention shorter than 12 weeks |
| Barnes 2009 | Duplicate |
| Basak 2016 | Intervention shorter than 12 weeks: 2 week intervention period (2‐arm trial; n = 46; cognitively healthy; mean age 69 years) |
| Beck 2013 | Wrong intervention |
| Belchior 2007 | Wrong outcomes |
| Belchior 2008 | Wrong outcomes |
| Belleville 2006 | Wrong intervention |
| Belleville 2014 | Wrong outcomes |
| Berry 2010 | Intervention shorter than 12 weeks: 3 to 5 weeks (2‐arm trial: n = 32; cognitively healthy; mean age 72 years) |
| Bier 2015 | Wrong study design |
| Binder 2016 | Intervention shorter than 12 weeks |
| Bittner 2013 | Wrong study design |
| Borella 2010 | Intervention shorter than 12 weeks: 2 weeks (2‐arm trial; n = 40; cognitively healthy; mean age 69 years) |
| Borella 2013 | Wrong intervention |
| Borella 2014 | Duplicate |
| Borella 2017 | Wrong intervention |
| Boripuntakul 2012 | Wrong intervention |
| Borness 2013 | Wrong patient population |
| Bottiroli 2009 | Duplicate |
| Bottiroli 2009a | Intervention shorter than 12 weeks: 3 training sessions (2‐arm trial; n = 44; cognitively healthy; mean age 66 years) |
| Bozoki 2013 | Intervention shorter than 12 weeks: 6‐week intervention period (2‐arm trial; n = 60; cognitively healthy; mean age 69 years) |
| Brehmer 2012 | Intervention shorter than 12 weeks: 5‐week intervention period (2‐arm trial stratified by younger and older age groups; n = 45 in old age groups, n = 55 in young age groups; cognitively healthy; mean age 64 years in old age groups, 26 in young age groups) |
| Brum 2013 | Duplicate |
| Buitenweg 2017 | Wrong intervention |
| Buiza 2008 | Wrong intervention |
| Bureš 2016 | Intervention shorter than 12 weeks |
| Buschert 2011 | Wrong intervention |
| Buschert 2011a | Duplicate |
| Buschert 2012 | Wrong intervention |
| Buschert 2012a | Duplicate |
| Calkins 2011 | Wrong intervention |
| Cammarata 2011 | No outcome given |
| Cancela 2015 | Wrong patient population |
| Candela 2015 | Wrong intervention |
| Cantarella 2017 | Intervention shorter than 12 weeks |
| Cao 2016 | Wrong route of administration |
| Carretti 2013 | Wrong intervention |
| Casutt 2014 | Wrong outcomes |
| Chapman 2015 | Wrong intervention |
| Chapman 2016 | Wrong intervention |
| Chapman 2017 | Wrong intervention |
| Cheng 2012 | Wrong intervention |
| Cheng 2018 | Wrong patient population |
| Cho 2002 | Younger than 30 years of age |
| Cleverley 2012 | Wrong intervention |
| Cohen‐Mansfield 2014 | Wrong intervention |
| Cohen‐Mansfield 2014a | Wrong intervention |
| Cohen‐Mansfield 2015 | Wrong intervention |
| Cohen‐Mansfield 2015a | Duplicate |
| Combourieu 2014 | Wrong outcomes |
| Corbett 2015 | Wrong patient population |
| Costa 2015 | Wrong patient population |
| Danassi 2015 | Duplicate |
| Dannhauser 2014 | Wrong study design |
| de Almondes 2017 | Intervention shorter than 12 weeks |
| de Macedo 2015 | Wrong outcomes |
| De Vreese 1996 | Wrong intervention |
| Desjardins‐Crépeau 2016 | Wrong patient population |
| Diamond 2015 | Intervention shorter than 12 weeks: 7‐week intervention period (2‐arm trial; n = 64; cognitively healthy; mean age 66 years) |
| Dittmann‐Kohli 1991 | Wrong intervention |
| Duncan 2009 | Wrong intervention |
| Dwolatzky 2005 | Intervention shorter than 12 weeks: multiple reports for excluded trial: Wolinsky 2015 (IHAMS study). This citation refers to the trial registration NCT01165463 |
| Eckroth‐Bucher 2009 | Wrong patient population |
| Edwards 2005 | Intervention shorter than 12 weeks: maximum 12 sessions (2‐arm SKILL trial; n = 126; participants with initial processing speed or processing difficulty; mean age 76 years) |
| Edwards 2011 | Intervention shorter than 12 weeks: multiple reports for Edwards 2005 (SKILL trial) |
| Edwards 2015 | Intervention shorter than 12 weeks: planned treatment duration 10 to 12 weeks, but less than 12 weeks provided on average |
| Edwards 2015a | Duplicate |
| Efthymiou 2011 | Wrong comparator. |
| Engvig 2014 | Wrong study design |
| Fabre 2002 | Wrong intervention |
| Faille 2007 | Nature of intervention unclear |
| Fairchild 2010 | Wrong intervention |
| Feng 2013 | Wrong intervention |
| Feng 2015 | Wrong intervention |
| Feng 2017 | Wrong patient population |
| Finn 2011 | Intervention shorter than 12 weeks |
| Finn 2015 | Intervention shorter than 12 weeks: 4‐week intervention period (2‐arm trial; n = 41; participants with MCI; mean age 75 years) |
| Finn 2015a | Duplicate |
| Flak 2013 | Study protocol |
| Flak 2014 | Study protocol |
| Flak 2014a | Study protocol |
| Flak 2016 | Study protocol |
| Foerster 2009 | No outcome given |
| Forloni 2012 | No outcome given |
| Forster 2011 | Wrong intervention |
| Fortman 2013 | Wrong comparator |
| Gagnon 2012 | Wrong study design |
| Gagnon 2012a | Intervention shorter than 12 weeks: 2‐week intervention period (2‐arm trial; n = 24; participants with MCI; mean age 68 years) |
| Gaitan 2013 | Wrong patient population |
| Gajewski 2012 | Intervention shorter than 12 weeks: cognitive training over 16 weeks, of which 12 concerned computerised cognitive training (4‐arm trial; n = 141; cognitively healthy; mean age 71 years) |
| Gajewski 2017 | Intervention shorter than 12 weeks |
| Garcia‐Campuzano 2013 | Nature of intervention unclear |
| Gates 2011 | Study protocol |
| Gill 2016 | Wrong intervention |
| Gillette 2009 | No outcome given |
| Giovannini 2015 | No outcome given |
| Giuli 2016 | Wrong intervention |
| Giuli 2017 | Wrong intervention |
| Golino 2017 | Wrong intervention |
| Haesner 2015 | Wrong study design |
| Haesner 2015a | Intervention shorter than 12 weeks: 8‐week intervention (2‐arm trial; n = 80, 40 cognitively healthy and 40 with subjective memory complaints; mean age 70 years) |
| Haimov 2013 | Duplicate |
| Haimov 2013a | Duplicate |
| Haimov 2013b | Intervention shorter than 12 weeks: 8‐week intervention period (2‐arm study; n = 51; likely cognitively healthy; mean age 72 years) |
| Haimov 2013c | Duplicate |
| Haimov 2013d | Intervention shorter than 12 weeks: multiple reports for Haimov 2013b |
| Haimov 2014 | Intervention shorter than 12 weeks: multiple reports for Haimov 2013b |
| Haimov 2014a | Intervention shorter than 12 weeks: multiple reports for Haimov 2013b |
| Hardy 2015 | Intervention shorter than 12 weeks: 10‐week intervention period (2‐arm trial; n = 9919; cognitively healthy; mean age 39 years; subgroup data by age can be analysed) |
| Hausmann 2012 | Wrong intervention |
| Hayashi 2012 | Wrong intervention |
| Hayslip B Jr 2016 | Intervention shorter than 12 weeks |
| Heinzel 2014 | Intervention shorter than 12 weeks: 4‐week intervention period (2‐arm trial; n = 60; 2‐arm trial stratified by younger and older age groups; n = 30 in old age groups, n = 30 in young age groups; cognitively healthy; mean age 66 years in old age groups, 26 in young age groups) |
| Hudak 2013 | Intervention shorter than 12 weeks: 10‐week intervention period (3‐arm trial; n = 53; cognitively healthy; mean age 82 years) |
| Hötting 2013 | Intervention shorter than 12 weeks: 6 sessions during 1 month (4‐arm trial; n = 33; cognitively healthy; mean age 49 years) |
| Ignjatovic 2015 | Younger than 30 years of age |
| Irigaray 2012 | Wrong intervention |
| Israel 1997 | Nature of intervention unclear |
| ISRCTN70130279 | Wrong intervention |
| Jackson 2012 | Nature of intervention unclear |
| Jansen 2012 | Wrong intervention |
| Jean 2010 | Intervention shorter than 12 weeks: 3‐week intervention period (2‐arm trial; n = 22; participants with MCI; mean age 69 years) |
| Jeong 2016 | Wrong intervention |
| Jobe 2001 | Intervention shorter than 12 weeks: multiple reports for excluded trial: Ball 2002 (trial ACTIVE) |
| Jones 2013 | Intervention shorter than 12 weeks: multiple reports for Ball 2002 (trial ACTIVE) |
| Kampanaros 2010 | Wrong intervention |
| Kholin 2010 | Intervention shorter than 12 weeks: 30‐day intervention period (2‐arm trial; n = 60; participants with MCI; age not reported; conference abstract) |
| Kim 2012 | Wrong outcomes |
| Kim 2013 | Intervention shorter than 12 weeks: 10‐week intervention period (2‐arm trial; n = 20; participants with MCI or dementia; mean age 69 years) |
| Kim 2013a | Wrong outcomes |
| Kim 2015 | Nature of intervention unclear |
| Kim 2015a | Intervention shorter than 12 weeks: 8‐week intervention period (2‐arm trial; n = 28; cognitively healthy; mean age 72 years) |
| Kim 2015b | Duplicate |
| Kivipelto 2014 | Wrong intervention |
| Klusmann 2009 | Duplicate |
| Klusmann 2010 | Wrong patient population |
| Klusmann 2010a | Duplicate |
| Klusmann 2011 | Younger than 30 years of age |
| Kudelka 2014 | Intervention shorter than 12 weeks: 8‐week intervention period (4‐arm trial; n = 96; cognitively healthy; mean age 65 years) |
| Kwak 2015 | Nature of intervention unclear |
| Kwak 2017 | Nature of intervention unclear |
| Kwok 2013 | Intervention shorter than 12 weeks: 8‐week intervention period (2‐arm trial; n = 194; mean MMSE score 25.92; mean age 75 years) |
| Lampit 2013 | Wrong study design |
| Lampit 2014 | Wrong patient population |
| Lampit 2015 | Wrong outcomes |
| Lavretsky 2016 | Nature of intervention unclear |
| Law 2014 | Intervention shorter than 12 weeks: 10‐week intervention period (2‐arm trial; n = 83; participants with MCI; mean age 74 years) |
| Law 2014a | Duplicate |
| Lee 2013 | Intervention shorter than 12 weeks: 6‐week intervention period (2‐arm trial; n = 30; mean MMSE‐K 26; mean age 72 years) |
| Lee 2013a | Intervention shorter than 12 weeks: 8‐week intervention period (2‐arm trial; n = 31; cognitively healthy; mean age 65 years) |
| Lee 2013b | Intervention shorter than 12 weeks: multiple reports for Lee 2013a |
| Lee 2014 | Intervention shorter than 12 weeks: 8‐week intervention period (2 2‐arm pilots trials; n = 31 & n = 39; likely cognitively healthy; age not reported; conference abstract that is part of multiple reports for Lee 2015) |
| Lee 2015 | Intervention shorter than 12 weeks: 8‐week intervention period (2‐arm trial; n = 39; cognitively healthy; mean age 65 years) |
| Legault 2011 | Wrong patient population |
| Leon 2015 | Wrong comparator |
| Leung 2015 | Wrong patient population |
| Li 2010 | Intervention shorter than 12 weeks: intervention period 5 weeks (2‐arm trial; n = 20; cognitively healthy; mean age 76 years) |
| Linde 2014 | Nature of intervention unclear |
| Mace 2015 | Intervention shorter than 12 weeks: 3‐week intervention period (2‐arm trial; n = 43; mild cognitive complaints; mean age 78 years) |
| Mahncke 2006 | Intervention shorter than 12 weeks: 8 to 10 weeks (2‐arm trial; n = 182; cognitively healthy; mean age 71 years) |
| Man 2012 | Wrong comparator |
| Mann 2012 | Wrong study population |
| Margrett 2006 | Wrong patient population |
| Mayas 2014 | Intervention shorter than 12 weeks: 20 sessions provided in 10‐ to 12‐week intervention period (n = 27; 2‐arm trial; cognitively healthy; mean age 69) |
| McAvinue 2013 | Intervention shorter than 12 weeks: 5‐week intervention period (n = 36; 2‐arm trial; likely cognitively healthy; mean age 70) |
| McDaniel 2014 | Intervention shorter than 12 weeks: 8‐week intervention period (n = 96; 4‐arm trial, cognitively healthy, mean age 65 years) |
| McDougall 2012 | Intervention shorter than 12 weeks: 6‐week intervention period (n = 41; 2‐arm trial; likely cognitively healthy; mean age 75) |
| Middleton 2012 | Wrong intervention |
| Miller 2013 | Intervention shorter than 12 weeks: 8‐week intervention period (n = 69; 2‐arm trial; cognitively healthy; mean age 81.8) |
| Mohs 1998 | Wrong intervention |
| Mombelli 2012 | No outcome given |
| Moon 2013 | Intervention shorter than 12 weeks: 10‐week intervention period (n = 38; likely participants with MCI; age not reported; conference abstract only) |
| Mowszowski 2014 | Intervention shorter than 12 weeks: 7‐week intervention period (n = 53; participants with memory complaints, MCI or late life depression; mean age 66) |
| Mowszowski 2014a | Duplicate |
| Mozolic 2010 | Intervention shorter than 12 weeks: 8‐week intervention period (n = 66; mean age 69; cognitively healthy participants) |
| Mozolic 2011 | Intervention shorter than 12 weeks: multiple reports for Mozolic 2010 |
| Muller 2011 | Nature of intervention unclear |
| Na 2013 | Duplicate |
| Na 2014 | Nature of intervention unclear |
| Naismith 2014 | Duplicate |
| Navarro 2006 | Intervention shorter than 12 weeks: 14 sessions; intervention duration not reported, but maximal follow‐up duration was 84 days (2‐arm trial; n = 80; likely cognitively healthy; mean age 66 years) |
| NCT00544856 | Nature of intervention unclear |
| NCT02417558 2015 | Nature of intervention unclear |
| NCT02462135 2014 | No outcome given |
| NCT02480738 2012 | No outcome given |
| NCT02512627 2015 | No outcome given |
| NCT02747784 2016 | Wrong patient population |
| NCT02774083 2015 | Wrong comparator |
| NCT02785315 2016 | Wrong intervention |
| NCT02808676 2016 | Wrong intervention |
| Neely 2013 | Nature of intervention unclear |
| Ng 2015 | Wrong intervention |
| Ngandu 2015 | Wrong intervention |
| Ngandu 2015a | Wrong intervention |
| Nishiguchi 2015 | Wrong intervention |
| Nouchi 2012 | Intervention shorter than 12 weeks: 4‐week intervention period (2‐arm trial; n = 32; cognitively healthy; mean age 69) |
| Nouchi 2013 | Intervention shorter than 12 weeks |
| Nozawa 2015 | Intervention shorter than 12 weeks: 8‐week intervention period (3‐arm trial; n = 37; cognitively healthy; mean age 68) |
| O'Caoimh 2015 | Intervention shorter than 12 weeks |
| Oei 2013 | Intervention shorter than 12 weeks: 4‐week intervention period (5‐arm trial; n = 75; cognitively healthy; mean age 21) |
| Oliveira 2013 | Intervention shorter than 12 weeks: 10‐week intervention period. (2‐arm cohort study; n = 182; subjective memory complaints; mean age not reported, all over 50 years of age, conference abstract only) |
| Otsuka 2015 | Wrong study design |
| Park 2009 | Nature of intervention unclear |
| Park 2014 | Intervention shorter than 12 weeks: 2‐week intervention period (2‐arm trial; n = 40; cognitively healthy; mean age 70) |
| Payne 2012 | Wrong intervention |
| Payne 2017 | Intervention shorter than 12 weeks |
| Peretz 2011 | Wrong patient population |
| R000001637 | Nature of intervention unclear |
| Rahe 2015 | Intervention shorter than 12 weeks: 6.5‐week intervention period (2‐arm trial; n = 30; cognitively healthy; mean age 67 years) |
| Rahe 2015a | Intervention shorter than 12 weeks: 7‐week intervention period (3‐arm trial; n = 81; cognitively healthy; mean age 68 years) |
| Rebok 2013 | Intervention shorter than 12 weeks: multiple reports for excluded trial: Ball 2002 (trial ACTIVE) |
| Rebok 2014 | Intervention shorter than 12 weeks: multiple reports for excluded trial: Ball 2002 (trial ACTIVE) |
| Redick 2013 | Younger than 30 years of age |
| Requena 2016 | Wrong intervention |
| Rizkalla 2015 | Intervention shorter than 12 weeks: 4‐week intervention period (2‐arm trial; n = 56; cognitively healthy; mean age 73 years) |
| Rojas 2013 | Wrong intervention |
| Rose 2015 | Intervention shorter than 12 weeks: 1‐month intervention period (2‐arm trial; n = 59; cognitively healthy; mean age 67 years) |
| Rosen 2011 | Intervention shorter than 12 weeks: 2‐month intervention period (2‐arm pilot trial; n = 12; participants with MCI; mean age 74) |
| Ryu 2013 | Wrong study design |
| Sakka 2015 | Wrong study design |
| Santos 2011 | Wrong comparator |
| Schoene 2015 | Duplicate |
| Schoene 2015a | Duplicate |
| Schumacher 2013 | Intervention shorter than 12 weeks: 10‐week intervention period (3‐arm trial; n = 63; cognitively healthy participants; mean age 72; conference abstract) |
| Shah 2012 | Wrong patient population |
| Shatil 2013 | Wrong patient population |
| Shatil 2014 | Intervention shorter than 12 weeks: 8‐week intervention period (2‐arm trial; n = 140; cognitively healthy; mean age 68) |
| Shatil 2014a | Duplicate citation |
| Sisco 2013 | Intervention shorter than 12 weeks: multiple reports for excluded trial: Ball 2002 (trial ACTIVE) |
| Slegers 2009 | Wrong intervention |
| Smith 2009 | Intervention shorter than 12 weeks: 8‐week intervention period (2‐arm trial IMPACT; n = 487; cognitively healthy; mean age 75 years) |
| Smith‐Ray 2014 | Intervention shorter than 12 weeks: 10‐week intervention period (2‐arm trial; n = 45; cognitively healthy; mean age 72) |
| Smith‐Ray 2015 | Intervention shorter than 12 weeks: 10‐week intervention period (2‐arm trial; n = 51; cognitively healthy; mean age 82) |
| Smith‐Ray 2015a | Duplicate |
| Solomon 2014 | Wrong comparator |
| Song 2009 | Wrong intervention |
| Stepankova 2014 | Intervention shorter than 12 weeks: 5‐week intervention period (3‐arm trial; n = 68; cognitively healthy; mean age 68 years) |
| Stine‐Morrow 2014 | Intervention shorter than 12 weeks: CCT intervention period 10 weeks (3‐arm trial; n = 461; cognitively healthy; mean age 73 years) |
| Strenziok 2013 | Duplicate |
| Strenziok 2014 | Intervention shorter than 12 weeks: 6‐week intervention period (3‐arm trial; n = 42; cognitively healthy; mean age 69 years) |
| Sturz 2011 | Wrong patient population |
| Sturz 2011a | Nature of intervention unclear |
| Sturz 2015 | Duplicate |
| Styliadis 2015 | Intervention shorter than 12 weeks: 8‐week intervention (5‐arm trial; n = 70; participants with MCI; mean age 71 years) |
| Styliadis 2015a | Duplicate |
| Suo 2012 | Wrong outcomes |
| Szelag 2012 | Intervention shorter than 12 weeks: 8‐week intervention period (3‐arm trial; n = 30; cognitively healthy; mean age 69 years) |
| Talib 2008 | Intervention shorter than 12 weeks: 4‐session intervention period (2‐arm trial; n = 23; cognitively healthy; mean age 68 years) |
| Tappen 2014 | Wrong intervention |
| Tennstedt 2013 | Study protocol: multiple reports for excluded trial: Ball 2002 (trial ACTIVE) |
| Tesky 2012 | Wrong intervention |
| Tsai 2008 | Wrong study design |
| Tsolaki 2013 | Nature of intervention unclear |
| Tucker‐Drob 2009 | Wrong study design |
| van den Berg 2016 | Intervention shorter than 12 weeks: 2‐week intervention period (2‐arm trial; n = 58; rehabilitation inpatients with MMSE ≥ 21 (mean MMSE 26 with SD = 3 in experimental and 27 with SD = 3 in control); mean age 80 years) |
| van der Ploeg 2016 | Wrong study design |
| Van het Reve 2014 | Wrong patient population |
| Vance 2007 | Intervention shorter than 12 weeks: 2 to 3 months (n = 159; cognitively healthy but with speed of processing impairment; mean age 75 years) |
| Vidovich 2009 | Intervention shorter than 12 weeks: multiple reports for excluded trial: Vidovich 2015 (PACE trial) |
| Vidovich 2015 | Intervention shorter than 12 weeks: 5‐week intervention period (2‐arm trial; n = 160; participants with MCI; mean age 75 years; PACE trial) |
| Vidovich 2015a | Duplicate |
| von Bastian 2013 | Intervention shorter than 12 weeks: 4‐week intervention period (2‐arm trial; n = 57 in the elderly subgroup; cognitively healthy; mean age 69 years in the elderly subgroup) |
| Wadley 2007 | Wrong study design |
| Walton 2015 | Intervention shorter than 12 weeks: 4‐week intervention period (2‐arm trial; n = 28; cognitively healthy; mean age 64 years) |
| Wang 2013 | Wrong intervention |
| Weicker 2013 | Intervention shorter than 12 weeks: 4‐week intervention period (2‐arm trial; n = not reported; cognitively healthy; age 60 to 75 years; conference abstract) |
| Wild‐Wall 2012 | Wrong outcomes |
| Williams 2014 | Intervention shorter than 12 weeks: 3‐week intervention period (3‐arm trial; n = 103; mild impairment in cognition, expressed concern about cognitive changes, or mild dementia ‐ mean MMSE = 25.3; mean age 86 years) |
| Willis 1986 | Intervention shorter than 12 weeks: 2‐week intervention period (2‐arm trial; n = 229; cognitively stable and cognitively declined participant subgroups; mean age 73 years) |
| Willis 2006 | Intervention shorter than 12 weeks: multiple reports for excluded trial: Ball 2002 (trial ACTIVE) |
| Willis 2006a | Duplicate |
| Willis 2007 | Duplicate |
| Willis 2013 | Intervention shorter than 12 weeks: multiple reports for excluded trial: Ball 2002 (trial ACTIVE) |
| Wojtynska 2011 | Intervention shorter than 12 weeks: 6‐week intervention period (2‐arm trial, stratified by 3 cognitive strata; n = 34 MCI, n = 29 AD, n = 12 cognitively healthy; participants with MCI and early dementia; mean age 69 years; conference abstract) |
| Wolinsky 2006 | Intervention shorter than 12 weeks: multiple reports for excluded trial: Ball 2002 (trial ACTIVE) |
| Wolinsky 2006a | Intervention shorter than 12 weeks: multiple reports for excluded trial: Ball 2002 (trial ACTIVE) |
| Wolinsky 2010 | Intervention shorter than 12 weeks: multiple reports for excluded trial: Ball 2002 (trial ACTIVE) |
| Wolinsky 2010a | Intervention shorter than 12 weeks: multiple reports for excluded trial: Ball 2002 (trial ACTIVE) |
| Wolinsky 2013 | Intervention shorter than 12 weeks: multiple reports for excluded trial: Wolinsky 2015 (IHAMS study) |
| Wolinsky 2015 | Intervention shorter than 12 weeks: 5‐ to 6‐week intervention period with booster at 11 months (4‐arm trial; n = 681; cognitively healthy; 50 to 64 years, n = 455; and 65 years and above, n = 226; Iowa Healthy and Active Minds Study (IHAMS study) |
| Yam 2014 | Wrong intervention |
| Yassuda 2015 | Intervention shorter than 12 weeks: 8‐session intervention period (2‐arm trial; n = 60; participants without depression/dementia; mean age not reported; conference abstract) |
| Yip 2012 | Intervention shorter than 12 weeks: 5‐week intervention period (3‐arm trial; n = 56; participants with acquired brain injury and subjective memory complaints; mean age 52 years) |
| Yoonmi 2012 | Intervention shorter than 12 weeks: 6‐week intervention period (2‐arm trial; n = 30; cognitively healthy; aged 65 to 80 years) |
| Youn 2011 | Intervention shorter than 12 weeks: 5‐week intervention period (2‐arm trial; n = 40; participants with subjective memory complaints; mean age 69 years) |
| Zelinski 2011 | Wrong study design |
| Zelinski 2011a | Intervention shorter than 12 weeks: multiple reports for excluded trial: Smith 2009 (IMPACT) |
| Zhuang 2013 | Wrong patient population |
| Zimmermann 2014 | Intervention shorter than 12 weeks: 6‐week intervention period (2‐arm trial; n = 20; cognitively healthy; mean age 68 years) |
MMSE: Mini Mental State Examination.
Contributions of authors
Completion of the protocol: NG, SK, AR, RV. Screening of references: Students For Best Evidence (title/abstract screening), NG, SK, GM, RV. Acquisition of data: NG, RV, MdN, SK, EM, AR, GV. 'Risk of bias' assessments and GRADE‐ing: NG, RV, MdN, SK, EM, AR, GM. Statistical analysis: AR. SoF & GRADE‐ing: RV. Overall interpretation of data: NG, RV, MdN, EM, AR, GM. Manuscript preparation: NG, AR, RV, EM, GM.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This protocol was supported by the National Institute for Health Research, via a Cochrane Programme Grant to the Cochrane Dementia and Cognitive Improvement Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
-
SERI and Horizon 2020, Other.
The review authors AR and MdN are partially funded by a grant for the project ‘OPERAM: OPtimising therapy to prevent Avoidable hospital admissions in the Multi‐morbid elderly’, supported by the European Union’s Horizon 2020 research and innovation programme, under the grant agreement No. 6342388, and by the Swiss State Secretariat for Education, Research, and Innovation (SERI), under contract number 15.0137. The opinions expressed and the arguments employed herein are those of the review authors and do not necessarily reflect the official views of the EC and the Swiss government.
Declarations of interest
Nicola J Gates ‐ none known Robin WM Vernooij ‐ none known Marcello Di Nisio ‐ Di Nisio declares partial funding by a grant for the project 'OPERAM: OPtimising therapy to prevent Avoidable hospital admissions in the Multi‐morbid elderly' supported by the European Union's Horizon 2020 research and innovation programme under the grant agreement No 6342388. Di Nisio reports participation to Advisory Boards for Daiichi‐Sankyo, Aspen, and Pfizer, and consultancy fees for Daiichi‐Sankyo, Bayer Health Care, and Leo Pharma outside the submitted work. Salman Karim ‐ none known Evrim March ‐ none known Gabriel Martínez ‐ none known Anne WS Rutjes ‐ Dr. Rutjes declares partial funding by a grant for the project 'OPERAM: OPtimising therapy to prevent Avoidable hospital admissions in the Multi‐morbid elderly' supported by the European Union's Horizon 2020 research and innovation programme under the grant agreement No 6342388, and by the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract number 15.0137.
New
References
References to studies included in this review
- Barnes DE, Santos‐Modesitt W, Poelke G, Kramer AF, Castro C, Middleton LE, et al. The Mental Activity and eXercise (MAX) trial: a randomized controlled trial to enhance cognitive function in older adults. JAMA Internal Medicine 2013;173(9):797‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djabelkhir L, Wu YH, Vidal JS, Cristancho‐Lacroix V, Marlats F, Lenoir H, et al. Computerized cognitive stimulation and engagement programs in older adults with mild cognitive impairment: comparing feasibility, acceptability, and cognitive and psychosocial effects. Clinical Interventions in Aging 2017;12:1967‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiatarone Singh MA, Gates N, Saigal N, Wilson GC, Meiklejohn J, Brodaty H, et al. The Study of Mental and Resistance Training (SMART) study—resistance training and/or cognitive training in mild cognitive impairment: a randomized, double‐blind, double‐sham controlled trial. Journal of the American Medical Directors Association 2014;15(12):873‐80. [DOI] [PubMed] [Google Scholar]
- Gooding AL, Choi J, Fiszdon JM, Wilkins K, Kirwin PD, Dyck CH, et al. Comparing three methods of computerised cognitive training for older adults with subclinical cognitive decline. Neuropsychological Rehabilitation 2016;26(5‐6):810‐21. [DOI] [PubMed] [Google Scholar]
- Herrera C, Chambon C, Michel BF, Paban V, Alescio‐Lautier B. Positive effects of computer‐based cognitive training in adults with mild cognitive impairment. Neuropsychologia 2012;50(8):1871‐81. [DOI] [PubMed] [Google Scholar]
- Kwok TC, Bai X, Li JC, Ho FK, Lee TM. Effectiveness of cognitive training in Chinese older people with subjective cognitive complaints: a randomized placebo‐controlled trial. International Journal of Geriatric Psychiatry 2013;28(2):208‐15. [DOI] [PubMed] [Google Scholar]
- Optale G, Urgesi C, Busato V, Marin S, Piron L, Priftis K, et al. Controlling memory impairment in elderly adults using virtual reality memory training: a randomized controlled pilot study. Neurorehabilitation and Neural Repair 2010;24(4):348‐57. [DOI] [PubMed] [Google Scholar]
- Rozzini L, Costardi D, Chilovi BV, Franzoni S, Trabucchi M, Padovani A. Efficacy of cognitive rehabilitation in patients with mild cognitive impairment treated with cholinesterase inhibitors. International Journal of Geriatric Psychiatry 2007;22(4):356‐60. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Adel D, Boulanouar K, Chauveau N, Delrieu J, Voisin T, Vellas B, et al. Structural MRI and FDG‐PET modifications induced by one year multidomain intervention in elderly. Conference: 26th Annual Congress of the European Association of Nuclear Medicine 2013, EANM, Lyon, France, 2013;Conference Start: 20131019 Conference End: 20131023:S208. [Google Scholar]
- Apóstolo JL, Cardoso DF, Rosa AI, Paúl C. The effect of cognitive stimulation on nursing home elders: a randomized controlled trial. Journal of Nursing Scholarship 2014;46(3):157‐66. [DOI] [PubMed] [Google Scholar]
- Alves J, Alves‐Costa F, Magalhães R, Gonçalves OF, Sampaio A. Cognitive stimulation for Portuguese older adults with cognitive impairment: a randomized controlled trial of efficacy, comparative duration, feasibility, and experiential relevance. American Journal of Alzheimer's Disease and Other Dementias 2014;29(6):503‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, White‐Schwoch T, Choi HJ, Kraus N. Partial maintenance of auditory‐based cognitive training benefits in older adults. Neuropsychologia 2014;62:286‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ann B, Eva E, Siv S, Elisabeth A. Effects of working memory training on functioning in daily life. Conference: 9th Annual Conference of the Special Interest Group in Neuropsychological Rehabilitation of the World Federation for NeuroRehabilitation 2012, WFNR, Bergen, Norway, 2012;Conference Start: 20120702 Conference End: 20120703:182. [Google Scholar]
- Apóstolo JL, Cardoso DF, Rosa AI, Paúl C. The effect of cognitive stimulation on nursing home elders: a randomized controlled trial. Journal of Nursing Scholarship 2014;46(3):157‐66. [DOI] [PubMed] [Google Scholar]
- Baglio F, Griffanti L, Preti MG, Lagana MM, Alberoni M, Critelli R, et al. Cognitive training in outpatients affected by mild cognitive impairment: a longitudinal study with fMRI. Conference: 6th Sindem Meeting: Italian Association for the Study of Dementia linked to the Italian Neurological Society 2011, SIN, Milan, Italy, 2011;Conference Start: 20110317 Conference End: 20110319:S47‐8. [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA 2002;288(18):2271‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults ‐ A randomized controlled trial. JAMA 2002;288(18):2271‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Unverzagt F, Rebok G, Morris J, Tennstedt SL, Marsiske M. ACTIVE: advanced cognitive training for independent and vital elderly. https://clinicaltrials.gov/ct2/show/NCT00298558,2006.
- Ball KK, Ross LA, Roth DL, Edwards JD. Speed of processing training in the ACTIVE study: how much is needed and who benefits?. Journal of Aging and Health 2013;25(8):65S‐84S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros S, Prieto A, Mayas J, Toril P, Pita C, Ponce de León L, et al. Brain training with non‐action video games enhances aspects of cognition in older adults: a randomized controlled trial. Frontiers in Aging Neuroscience 2014;6:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros S, Prieto A, Mayas J, Toril P, Pita C, Ponce de León L, et al. Brain training with non‐action video games enhances aspects of cognition in older adults: a randomized controlled trial. Frontiers in Aging Neuroscience 2014;6:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros S, Mayas J, Prieto A, Toril P, Pita C, Laura Pde L, et al. A randomized controlled trial of brain training with non‐action video games in older adults: results of the 3‐month follow‐up. Frontiers in Aging Neuroscience 2015;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros S, Prieto A, Mayas J, Toril P, Pita C, Ponce de León L, et al. Corrigendum: brain training with non‐action video games enhances aspects of cognition in older adults: a randomized controlled trial. Frontiers in Aging Neuroscience 2015;7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros S, Mayas J, Prieto A, Ruiz‐Marquez E, Toril P, Reales JM. Effects of video game training on measures of selective attention and working memory in older adults: results from a randomized controlled trial. Frontiers in Aging Neuroscience 2017;9:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamidis PD, Fissler P, Papageorgiou SG, Zilidou V, Konstantinidis EI, Billis AS, et al. Gains in cognition through combined cognitive and physical training: the role of training dosage and severity of neurocognitive disorder. Frontiers in Aging Neuroscience 2015;7:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniqued PL, Kranz MB, Voss MW, Lee H, Cosman JD, Severson J, et al. Cognitive training with casual video games: points to consider. Frontiers in Psychology 2014;4:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniqued PL, Allen CM, Kranz MB, Johnson K, Sipolins A, Dickens C, et al. Working memory, reasoning, and task switching training: transfer effects, limitations, and great expectations?. PLoS One 2015;10(11):e0142169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barban F, Annicchiarico R, Perri R, Fadda L, Carlesimo GA, Pantelopoulos S, et al. Randomized clinical trial of a computer‐based cognitive treatment for healthy elderly, clinical and preclinical Alzheimer's disease. the SOCIABLE project. Conference: 7th Congresso Sindem: Italian Association for the Study of Dementia Linked to the Italian Neurological Society 2012, SIN, Naples, Italy, 2012;Conference Start: 20120322 Conference End: 20120324:101. [Google Scholar]
- Barban F, Annicchiarico R, Pantelopoulos S, Federici A, Perri R, Fadda L, et al. Protecting cognition from aging and Alzheimer's disease: a computerized cognitive training combined with reminiscence therapy. International Journal of Geriatric Psychiatry 2016;31(4):340‐8. [DOI] [PubMed] [Google Scholar]
- Barbosa AR, Guimaraes AV. Effects of exergames on cognitive performance and functional fitness in older adults: a pilot study. Conference: 2015 Annual Scientific Meeting of the American Geriatrics Society National Harbor, MD, United States, 2015;Conference Start: 20150515 Conference End: 20150517:S176. [Google Scholar]
- Barcelos N, Shah N, Cohen K, Hogan MJ, Mulkerrin E, Arciero PJ, et al. Aerobic and cognitive exercise (ACE) pilot study for older adults: executive function improves with cognitive challenge while exergaming. Journal of the International Neuropsychological Society 2015;21(10):768‐79. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Belfor N, Jagust WJ, DeCarli C, Reed BR, et al. Computer‐based cognitive training for mild cognitive impairment: results from a pilot randomized controlled trial. Alzheimer Disease and Associated Disorders 2006;66(5):A249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Belfor N, Jagust WJ, DeCarli C, Reed BR, et al. Computer‐based cognitive training for mild cognitive impairment: results from a pilot randomized, controlled trial. Alzheimer Disease and Associated Disorders 2009;23(3):205‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak C, O'Connell MA. To switch or not to switch: role of cognitive control in working memory training in older adults. Frontiers in Psychology 2016;7:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C, Fausett JK, Krukowski RA, Cornell CE, Prewitt TE, Lensing S, et al. A randomized trial of a community‐based cognitive intervention for obese senior adults. Journal of Aging and Health 2013;25(1):97‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belchior PD. Cognitive training with video games to improve driving skills and driving safety among older adults. http://etd.fcla.edu/UF/UFE0021218/belchior_p.pdf. University of Florida, 2007:209. [Google Scholar]
- Belchior PD. Cognitive training with video games to improve driving skills and driving safety among older adults. Dissertation Abstracts International: Section B: The Sciences and Engineering. (9‐B). Vol. 68, APA PsycNET, 2008. [Google Scholar]
- Belleville S, Gilbert B, Fontaine F, Gagnon L, Ménard E, Gauthier S. Improvement of episodic memory in persons with mild cognitive impairment and healthy older adults: evidence from a cognitive intervention program. Dementia and Geriatric Cognitive Disorders 2006;22(5‐6):486‐99. [DOI] [PubMed] [Google Scholar]
- Belleville S, Mellah S, Boysson C, Demonet JF, Bier B. The pattern and loci of training‐induced brain changes in healthy older adults are predicted by the nature of the intervention. PLoS One 2014;9(8):e102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Zanto TP, Clapp WC, Hardy JL, Delahunt PB, Mahncke HW, et al. The influence of perceptual training on working memory in older adults. PLoS One 2010;5(7):e11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bier N, Grenier S, Brodeur C, Gauthier S, Gilbert B, Hudon C, et al. Measuring the impact of cognitive and psychosocial interventions in persons with mild cognitive impairment with a randomized single‐blind controlled trial: rationale and design of the MEMO plus study. International Psychogeriatrics 2015;27(3):511‐25. [DOI] [PubMed] [Google Scholar]
- Binder JC, Martin M, Zöllig J, Röcke C, Mérillat S, Eschen A, et al. Multi‐domain training enhances attentional control. Psychology and Aging 2016;31(4):390‐408. [DOI] [PubMed] [Google Scholar]
- Bittner DM, Bittner V, Hausmann J, Reinhold D, Machts J, Westphal S, et al. Training intervention improves memory in mild cognitive impairment and healthy controls, but plasma BDNF acts differentially. Conference: International Conference "Aging and Cognition", IfADo 2013, Germany, 2013;Conference Start: 20130425 Conference End: 20130427 Sponsor: Brain Products ‐ Solutions for Neurophysiological Research, Dortmund Tourismus, DFG ‐ Deutsche Forschungsgemeinsch:49‐50. [Google Scholar]
- Borella E, Carretti B, Riboldi F, Beni R. Working memory training in older adults: evidence of transfer and maintenance effects. Psychology and Aging 2010;25(4):767‐78. [DOI] [PubMed] [Google Scholar]
- Borella E, Carretti B, Zanoni G, Zavagnin M, Beni R. Working memory training in old age: an examination of transfer and maintenance effects. Archives of Clinical Neuropsychology 2013;28(4):331‐47. [DOI] [PubMed] [Google Scholar]
- Borella E, Carretti B, Cantarella A, Riboldi F, Zavagnin M, Beni R. Benefits of training visuospatial working memory in young‐old and old‐old. Developmental Psychology 2014;50(3):714‐27. [DOI] [PubMed] [Google Scholar]
- Borella E, Carretti B, Sciore R, Capotosto E, Taconnat L, Cornoldi C, et al. Training working memory in older adults: is there an advantage of using strategies?. Psychology and Aging 2017;32(2):178‐91. [DOI] [PubMed] [Google Scholar]
- Boripuntakul S, Kothan S, Methapatara P, Munkhetvit P, Sungkarat S. Short‐term effects of cognitive training program for individuals with amnestic mild cognitive impairment: a pilot study. Physical & Occupational Therapy In Geriatrics 2012;30(2):138‐49. [Google Scholar]
- Borness C, Proudfoot J, Crawford J, Valenzuela M. Putting brain training to the test in the workplace: a randomized, blinded, multisite, active‐controlled trial. PLoS ONE 2013;8(3):e59982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottiroli S, Cavallini E. Can computer familiarity regulate the benefits of computer‐based memory training in normal aging? A study with an Italian sample of older adults. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition2009; Vol. 16, issue 4:401‐18. [DOI] [PubMed]
- Bottiroli S, Cavallini E. Can computer familiarity regulate the benefits of computer‐based memory training in normal aging? A study with an Italian sample of older adults. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition 2009;16(4):401‐18. [DOI] [PubMed] [Google Scholar]
- Bozoki A, Radovanovic M, Winn B, Heeter C, Anthony JC. Effects of a computer‐based cognitive exercise program on age‐related cognitive decline. Archives of Gerontology and Geriatrics 2013;57(1):1‐7. [DOI] [PubMed] [Google Scholar]
- Brehmer Y, Westerberg H, Bäckman L. Working‐memory training in younger and older adults: training gains, transfer, and maintenance. Frontiers in Human Neuroscience 2012;6:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum P, Yassuda M, Forlenza O. Memory training in healthy elderly and seniors with mild cognitive impairment: benefits on cognitive parameters. Conference: Alzheimer's Association International Conference 2013, Boston, MA, United States, 2013;Conference Start: 20130713 Conference End: 20130718:P493. [Google Scholar]
- Buitenweg JI, Ven RM, Prinssen S, Murre JM, Ridderinkhof KR. Cognitive flexibility training: a large‐scale multimodal adaptive active‐control intervention study in healthy older adults. Frontiers in Human Neuroscience 2017;11:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiza C, Etxeberria I, Galdona N, González MF, Arriola E, López de Munain A, et al. A randomized, two‐year study of the efficacy of cognitive intervention on elderly people: the Donostia longitudinal study. International Journal of Geriatric Psychiatry 2008;23(1):85‐94. [DOI] [PubMed] [Google Scholar]
- Bureš V, Čech P, Mikulecká J, Ponce D, Kuca K. The effect of cognitive training on the subjective perception of well‐being in older adults. PeerJ 2016;4:e2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschert V, Giegling I, Merensky W, Jolk S, Teipel S, Hampel H, et al. Long‐term effects of a multicomponent cognitive intervention in amnestic mild cognitive impairment (AMCI). Conference: Alzheimer's Association International Conference 2011, AAIC 11, Paris, France, 2011;Conference Start: 20110716 Conference End: 20110721:S513‐4. [Google Scholar]
- Buschert VC, Friese U, Teipel SJ, Schneider P, Merensky W, Rujescu D, et al. Effects of a newly developed cognitive intervention in amnestic mild cognitive impairment and mild alzheimer's disease: a pilot study. Journal of Alzheimer's Disease 2011;25(4):679‐94. [DOI] [PubMed] [Google Scholar]
- Buschert VC, Giegling I, Teipel SJ, Jolk S, Hampel H, Rujescu D, et al. Long‐term observation of a multicomponent cognitive intervention in mild cognitive impairment. Journal of Clinical Psychiatry 2012;73(12):e1492‐8. [DOI] [PubMed] [Google Scholar]
- Buschert VC, Giegling I, Teipel SJ, Jolk S, Hampel H, Rujescu D, et al. Long‐term observation of a multicomponent cognitive intervention in mild cognitive impairment. Journal of Clinical Psychiatry 2012;73(12):e1492‐8. [DOI] [PubMed] [Google Scholar]
- Calkins AW, Deveney CM, Weitzman ML, Hearon BA, Siegle GJ, Otto MW. The effects of prior cognitive control task exposure on responses to emotional tasks in healthy participants. Behavioural and Cognitive Psychotherapy 2011;39(2):205‐20. [DOI] [PubMed] [Google Scholar]
- Cammarata S, Novello C, Pollero V, Colucci M. Cognitive rehabilitation in patients with mild cognitive impairment. Conference: 6th Sindem Meeting: Italian Association for the Study of Dementia linked to the Italian Neurological Society 2011, SIN, Milan, Italy, 2011;Conference Start: 20110317 Conference End: 20110319:S50. [Google Scholar]
- Cancela JM, Vila Suarez MH, Vasconcelos J, Lima A, Ayan C. Efficacy of brain gym training on the cognitive performance and fitness level of active older adults: a preliminary study. Journal of Aging and Physical Activity 2015;23(4):653‐8. [DOI] [PubMed] [Google Scholar]
- Filippo C, Zucchetti G, Magistro D, Rabaglietti E. The effects of a physical activity program and a cognitive training program on the long‐term memory and selective attention of older adults: a comparative study. Activities, Adaptation & Aging 2015;39(1):77‐91. [Google Scholar]
- Cantarella A, Borella E, Carretti B, Kliegel M, Beni R. Benefits in tasks related to everyday life competences after a working memory training in older adults. International Journal of Geriatric Psychiatry 2017;32(1):86‐93. [DOI] [PubMed] [Google Scholar]
- Cao W, Cao X, Hou C, Li T, Cheng Y, Jiang L, et al. Effects of cognitive training on resting‐state functional connectivity of default mode, salience, and central executive networks. Frontiers in Aging Neuroscience 2016;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretti B, Borella E, Fostinelli S, Zavagnin M. Benefits of training working memory in amnestic mild cognitive impairment: specific and transfer effects. International Psychogeriatrics2013; Vol. 25, issue 4:617‐26. [DOI] [PubMed]
- Casutt G, Theill N, Martin M, Keller M, Jäncke L. The drive‐wise project: driving simulator training increases real driving performance in healthy older drivers. Frontiers in Aging Neuroscience 2014;6:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SB, Aslan S, Spence JS, Hart JJ Jr, Bartz EK, Didehbani N, et al. Neural mechanisms of brain plasticity with complex cognitive training in healthy seniors. Cerebral Cortex 2015;25(2):396‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SB, Aslan S, Spence JS, Keebler MW, DeFina LF, Didehbani N, et al. Distinct brain and behavioral benefits from cognitive vs. physical training: a randomized trial in aging adults. Frontiers in Human Neuroscience 2016;10:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SB, Spence JS, Aslan S, Keebler MW. Enhancing innovation and underlying neural mechanisms via cognitive training in healthy older adults. Frontiers in Aging Neuroscience 2017;9:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Wu W, Feng W, Wang J, Chen Y, Shen Y, et al. The effects of multi‐domain versus single‐domain cognitive training in non‐demented older people: a randomized controlled trial.. BMC Medicine 2012;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CP, Chiu‐Wa Lam L, Cheng ST. The effects of integrated attention training for older Chinese adults with subjective cognitive complaints: a randomized controlled study. Journal of Applied Gerontology 2018;37(10):1195‐214. [DOI] [PubMed] [Google Scholar]
- Cho BH, Ku J, Jang DP, Kim S, Lee YH, Kim IY, et al. The effect of virtual reality cognitive training for attention enhancement. Cyberpsychology and Behavior 2002;5(2):129‐37. [DOI] [PubMed] [Google Scholar]
- Cleverley M, Walker Z, Dannhauser T. Engaging patients at high risk of dementia in multimodal cognitive health promoting activities: the Thinkingfit study. Conference: Alzheimer's Association International Conference 2012, Vancouver, BC, Canada, 2012;Conference Start: 20120714 Conference End: 20120719:P220‐1. [Google Scholar]
- Cohen‐Mansfield J, Cohen R, Buettner L, Eyal N, Jakobovits H, Rebok G, et al. Interventions for older persons reporting memory difficulties: a randomized controlled pilot study. International Journal of Geriatric Psychiatry 2015;30(5):478‐86. [DOI] [PubMed] [Google Scholar]
- Cohen‐Mansfield J, Cohen R, Buettner L, Eyal N, Jakobovits H, Rebok G, et al. Interventions for older persons reporting memory difficulties: a randomized controlled pilot study. International Journal of Geriatric Psychiatry 2015;30(5):478‐86. [DOI] [PubMed] [Google Scholar]
- Cohen‐Mansfield J, Cohen R, Buettner L, Eyal N, Jakobovits H, Rebok G, et al. Interventions for older persons reporting memory difficulties: a randomized controlled pilot study. International Journal of Geriatric Psychiatry 2015;30(5):478‐86. [DOI] [PubMed] [Google Scholar]
- Cohen‐Mansfield J, Cohen R, Buettner L, Eyal N, Jakobovits H, Rebok G, et al. Interventions for older persons reporting memory difficulties: a randomized controlled pilot study. International Journal of Geriatric Psychiatry 2015;30(5):478‐86. [DOI] [PubMed] [Google Scholar]
- Combourieu L, Perrot A, Bloch F, Seux ML, Kemoun G. Effect of three different trainings on executive function and gait speed in MCI old adults. Conference: 19th European Congress of Physical and Rehabilitation Medicine 2014, Marseilles, France, 2014;Conference Start: 20140526 Conference End: 20140531:e138. [Google Scholar]
- Corbett A, Owen A, Hampshire A, Grahn J, Stenton R, Dajani S, et al. The effect of an online cognitive training package in healthy older adults: an online randomized controlled trial. Journal of the American Medical Directors Association 2015;16(11):990‐7. [DOI] [PubMed] [Google Scholar]
- Costa NB, Aramaki F, Cecato J, Stella B, Araujo I, Aprahamian I, et al. Benefits of a computer‐based cognitive training program for elderly subjects with mild Alzheimer's disease. Conference: 17th IPA International Congress 2015, Berlin, Germany, 2015;Conference Start: 20151013 Conference End: 20151016:S119. [Google Scholar]
- Danassi E. SOCIABLE: a surface computing platform empowering effective cognitive training for healthy and cognitively impaired elderly. Advances in Experimental Medicine and Biology 2015;821:129‐30. [DOI] [PubMed] [Google Scholar]
- Dannhauser TM, Cleverley M, Whitfield TJ, Fletcher BC, Stevens T, Walker Z. A complex multimodal activity intervention to reduce the risk of dementia in mild cognitive impairment ‐ ThinkingFit: pilot and feasibility study for a randomized controlled trial. BMC Psychiatry 2014;14:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almondes KM, Leonardo ME, Moreira AM. Effects of a cognitive training program and sleep hygiene for executive functions and sleep quality in healthy elderly. Dementia & Neuropsychologia 2017;11(1):69‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo LD, Oliveira TC, Soares FC, Bento‐Torres J, Bento‐Torres NV, Anthony DC. Beneficial effects of multisensory and cognitive stimulation in institutionalized elderly: 12‐month follow‐up. Clinical Interventions in Aging 2015;10:1351‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins‐Crépeau L, Berryman N, Fraser SA, Vu TT, Kergoat MJ, Li KZ, et al. Effects of combined physical and cognitive training on fitness and neuropsychological outcomes in healthy older adults. Clinical Interventions in Aging 2016;11:1287‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreese LP, Neri M, Boiardi R, Ferrari P, Belloi L, Salvioli G. Memory training and drug therapy act differently on memory and metamemory functioning: evidence from a pilot study. Archives of Gerontology and Geriatrics 1996;23(Suppl 5):9‐22. [DOI] [PubMed] [Google Scholar]
- Diamond K, Mowszowski L, Cockayne N, Norrie L, Paradise M, Hermens DF, et al. Randomized controlled trial of a healthy brain ageing cognitive training program: effects on memory, mood, and sleep. Journal of Alzheimer's Disease 2015;44(4):1181‐91. [DOI] [PubMed] [Google Scholar]
- Dittmann‐Kohli F, Lachman ME, Kliegl R, Baltes PB. Effects of cognitive training and testing on intellectual efficacy beliefs in elderly adults. Journal of Gerontology 1991;46(4):P162‐4. [DOI] [PubMed] [Google Scholar]
- Duncan NL, Greenaway MC. The memory support system for mild cognitive impairment: emotional impacts of a cognitive rehabilitation program. Conference: 29th Annual Meeting of the National Academy of Neuropsychology 2009, New Orleans, LA, United States, 2009;Conference Start: 20091111 Conference End: 20091114:438. [Google Scholar]
- Dwolatzky T. The effect of computerized cognitive training on neuropsychological measures of cognitive function in the elderly. NCT00146263,2005.
- Eckroth‐Bucher M, Siberski J. Preserving cognition through an integrated cognitive stimulation and training program. American Journal of Alzheimer's Disease and Other Dementias 2009;24(3):234‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JD, Wadley VG, Vance DE, Wood K, Roenker DL, Ball KK. The impact of speed of processing training on cognitive and everyday performance. Aging and Mental Health 2005;9(3):262‐71. [DOI] [PubMed] [Google Scholar]
- Edwards JD. Cognitive speed of processing training transfers to improved functional performance. Conference: International Conference "Aging and Cognition" 2011, Dortmund, Germany, 2011;Conference Start: 20101014 Conference End: 20101016:10. [Google Scholar]
- Edwards JD, Valdés EG, Peronto C, Castora‐Binkley M, Alwerdt J, Andel R, et al. The efficacy of InSight cognitive training to improve useful field of view performance: a brief report. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition 2015;70(3):417‐22. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Valdés EG, Peronto C, Castora‐Binkley M, Alwerdt J, Andel R, et al. The efficacy of InSight cognitive training to improve useful field of view performance: a brief report. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition 2015;70(3):417‐22. [DOI] [PubMed] [Google Scholar]
- Efthymiou A, Konstantinidis V, Tryfonopoulos E, Karpathiou N, Dimakopoulou E, Nikolaou C, et al. Non‐pharmacological intervention: effectiveness of a multi‐component rehabilitation program on cognitive functions of people with mild cognitive impairment. Conference: Alzheimer's Association International Conference 2011, AAIC 11, Paris, France, 2011. 2011; Vol. Conference Start: 20110716 Conference End: 20110721:S643. [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Skaane NV, Dale AM, Holland D, et al. Effects of cognitive training on gray matter volumes in memory clinic patients with subjective memory impairment. Journal of Alzheimer's Disease 2014;41(3):779‐91. [DOI] [PubMed] [Google Scholar]
- Fabre C, Chamari K, Mucci P, Massé‐Biron J, Préfaut C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. International Journal of Sports Medicine 2002;23(6):415‐21. [DOI] [PubMed] [Google Scholar]
- Faille L. Performance on a brain‐plasticity‐based memory‐training computer program for the elderly as influenced by cognitive functioning and gender. Thesis 2007;68(3‐B):1922. [Google Scholar]
- Fairchild JK, Scogin FR. Training to enhance adult memory (TEAM): an investigation of the effectiveness of a memory training program with older adults. Aging and Mental Health 2010;14(3):364‐73. [DOI] [PubMed] [Google Scholar]
- Feng W, Li CB, Chen Y, Cheng Y, Wu WY. Integrative cognitive training for healthy elderly Chinese in community: a controlled study. Allied Academics 2013;24(2):223‐9. [Google Scholar]
- Feng W, Yokoyama JS, Yu S, Chen Y, Cheng Y, Bonham LW, et al. APOE genotype affects cognitive training response in healthy Shanghai community‐dwelling elderly individuals. Journal of Alzheimer's Disease 2015;47(4):1035‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Li G, Xu C, Ju C, Qiu X. Training rehabilitation as an effective treatment for patients with vascular cognitive impairment with no dementia. Rehabilitation Nursing 2017;42(5):290‐7. [DOI] [PubMed] [Google Scholar]
- Finn M, McDonald S. Computerised cognitive training for older persons with mild cognitive impairment: a pilot study using a randomised controlled trial design. Brain Impairment 2011;12(3):187‐99. [Google Scholar]
- Finn M, McDonald S. Repetition‐lag training to improve recollection memory in older people with amnestic mild cognitive impairment. A randomized controlled trial. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition 2015;22(2):244‐58. [DOI] [PubMed] [Google Scholar]
- Finn M, McDonald S. Repetition‐lag training to improve recollection memory in older people with amnestic mild cognitive impairment. A randomized controlled trial. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition 2015;22(2):244‐58. [DOI] [PubMed] [Google Scholar]
- Flak M, Hernes SS, Skranes J, Lohaugen GC. Memory aid‐computer based working memory training in elderly with mild cognitive impairment (MCI). A randomized, controlled trial. Conference: 21st World Congress of Neurology 2013, Vienna, Austria, 2013;Conference Start: 20130921 Conference End: 20130926:e322‐3. [Google Scholar]
- Flak MM, Hernes SS, Chang L, Ernst T, Douet V, Skranes J, et al. The Memory Aid study: protocol for a randomized controlled clinical trial evaluating the effect of computer‐based working memory training in elderly patients with mild cognitive impairment (MCI). Trials 2014;15:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak MM, Hernes SS, Chang L, Ernst T, Douet V, Skranes J, et al. The Memory Aid study: protocol for a randomized controlled clinical trial evaluating the effect of computer‐based working memory training in elderly patients with mild cognitive impairment (MCI). Trials 2014;15:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak MM, Hernes SS, Chang L, Ernst T, Douet V, Skranes J, et al. Erratum to: 'The Memory Aid study: protocol for a randomized controlled clinical trial evaluating the effect of computer‐based working memory training in elderly patients with mild cognitive impairment (MCI)'.[Erratum for Trials. 2014;15:156 Note: Chang, Linda; Ernst, Thomas; and Douet, Vanessa [Added]; PMID: 24886034]. Trials 2016;17:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster S, Buschert VC, Buchholz HG, Teipel SJ, Zach C, Hampel H, et al. Positive effects of a 6‐month stage‐specific cognitive intervention program on brain metabolism in subjects with amnestic mild cognitive impairment (AMCI) and mild Alzheimer's disease (AD). Conference: Alzheimer's Association International Conference on Alzheimer's Disease 2009, Vienna, Austria. 2009; Vol. Conference Start: 20090711 Conference End: 20090716:38. [Google Scholar]
- Forloni G, Polito L, Davin A, Abbondanza S, Vaccaro R, Valle E. Cognitive stimulation and APOE genotype in non‐demented elderly subjects: a randomized controlled study (RCT). Conference: 5th Conference Clinical Trials on Alzheimer's Disease 2012, Monte Carlo, Monaco. 2012; Vol. Conference Start: 20121029 Conference End: 20121031:841‐2. [Google Scholar]
- Förster S, Buschert VC, Teipel SJ, Friese U, Buchholz HG, Drzezga A, et al. Effects of a 6‐month cognitive intervention on brain metabolism in patients with amnestic MCI and mild Alzheimer's disease. Journal of Alzheimer's Disease 2011;26(3):337‐48. [DOI] [PubMed] [Google Scholar]
- Fortman J. Computer‐based cognitive training for age‐related cognitive decline and mild cognitive impairment. Thesis 2013;74(5‐B(E)):No Pagination Specified. [Google Scholar]
- Gagnon LG, Belleville S. Training of attentional control in mild cognitive impairment with executive deficits: results from a double‐blind randomised controlled study. Neuropsychological Rehabilitation 2012;22(6):809‐35. [DOI] [PubMed] [Google Scholar]
- Gagnon L. Working memory in Alzheimer's disease and mild cognitive impairment (MCI): assessment and intervention. Thesis 2012;73(5‐B):3262. [Google Scholar]
- Gaitán A, Garolera M, Cerulla N, Chico G, Rodriguez‐Querol M, Canela‐Soler J. Efficacy of an adjunctive computer‐based cognitive training program in amnestic mild cognitive impairment and Alzheimer's disease: a single‐blind, randomized clinical trial. International Journal of Geriatric Psychiatry 2013;28(1):91‐9. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Falkenstein M. Training‐induced improvement of response selection and error detection in aging assessed by task switching: effects of cognitive, physical, and relaxation training. Frontiers in Human Neuroscience 2012;6:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski PD, Freude G, Falkenstein M. Cognitive training sustainably improves executive functioning in middle‐aged industry workers assessed by task switching: a randomized controlled ERP study. Frontiers in Human Neuroscience 2017;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Campuzano MT, Virues‐Ortega J, Smith S, Moussavi Z. Effect of cognitive training targeting associative memory in the elderly: a small randomized trial and a longitudinal evaluation. Journal of the American Geriatrics Society 2013;61(12):2252‐4. [DOI] [PubMed] [Google Scholar]
- Gates NJ, Valenzuela M, Sachdev PS, Singh NA, Baune BT, Brodaty H, et al. Study of mental activity and regular training (SMART) in at risk individuals: a randomised double blind, sham controlled, longitudinal trial. BMC Geriatrics 2011;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill DP, Gregory MA, Zou G, Liu‐Ambrose T, Shigematsu R, Hachinski V, et al. The healthy mind, healthy mobility trial: a novel exercise program for older adults. Medicine and Science in Sports and Exercise 2016;48(2):297‐306. [DOI] [PubMed] [Google Scholar]
- Gillette S. The multidomain Alzheimer preventive trial (MAPT): a new approach for the prevention of Alzheimer's disease. Conference: Alzheimer's Association International Conference on Alzheimer's Disease 2009, Vienna, Austria, 2009;Conference Start: 20090711 Conference End: 20090716:145. [Google Scholar]
- Giovannini E, Borso E, Benso F, Carabelli E, Sette M, Ciarmiello A. FDG‐PET in the evaluation of brain metabolic changes induced by Cognitive stimulation in aMCI subjects. Conference: 28th Annual Congress of the European Association of Nuclear Medicine 2015, EANM, Hamburg, Germany, 2015;Conference Start: 20151010 Conference End: 20151014:S552‐3. [Google Scholar]
- Giuli C, Papa R, Lattanzio F, Postacchini D. The effects of cognitive training for elderly: results from my mind project. Rejuvenation Research 2016;19(6):485‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuli C, Fattoretti P, Gagliardi C, Mocchegiani E, Venarucci D, Balietti M, et al. My Mind Project: the effects of cognitive training for elderly ‐ the study protocol of a prospective randomized intervention study. Aging Clinical and Experimental Research 2017;29(3):353‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golino MT, Flores Mendoza C, Golino HF. Effects of cognitive training on cognitive performance of healthy older adults. Spanish Journal of Psychology 2017;20:E39. [DOI] [PubMed] [Google Scholar]
- Haesner M, O'Sullivan JL, Gövercin M, Steinhagen‐Thiessen E. Requirements of older adults for a daily use of an internet‐based cognitive training platform. Informatics for Health and Social Care 2015;40(2):139‐53. [DOI] [PubMed] [Google Scholar]
- Haesner M, Steinert A, O'Sullivan JL, Weichenberger M. Evaluating an online cognitive training platform for older adults: user experience and implementation requirements. Journal of Gerontological Nursing 2015;41(8):22‐31. [DOI] [PubMed] [Google Scholar]
- Haimov I, Shatil E. Protocol_S1.doc. PLoS One 2013;8(4):e61390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimov I, Shatil E. Checklist_S1.pdf. PLoS One 2013;8(4):e61390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimov I, Shatil E. Cognitive training improves sleep quality and cognitive function among older adults with insomnia. PLoS One 2013;8(4):e61390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimov I, Shatil E. Cognitive training improves sleep quality and cognitive function among older adults with insomnia. PLoS One 2013;8(4):e61390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimov I, Shatil E. Cognitive training improves sleep quality and cognitive function among older adults with insomnia. Conference: 5th International World Association of Sleep Medicine Congress and the 22nd Annual Congress of the Spanish Sleep Society 2013, Valencia, Spain, 2013;Conference Start: 20130928 Conference End: 20131002:e61390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimov I, Shatil E. Cognitive training improves sleep quality and cognitive function among older adults with insomnia. Conference: 22nd Annual Meeting of the Israel Society for Neuroscience, ISFN and the 2nd Bi national Italy‐Israel Neuroscience Meeting 2014, Eilat, Israel, 2014;Conference Start: 20131214 Conference End: 20131217:S60. [Google Scholar]
- Haimov I, Shatil E. Cognitive training improves sleep quality and cognitive function among older adults with insomnia. Conference: 22nd Congress of the European Sleep Research Society 2014, Tallinn, Estonia, 2014;Conference Start: 20140916 Conference End: 20140920:137. [Google Scholar]
- Hardy JL, Nelson RA, Thomason ME, Sternberg DA, Katovich K, Farzin F, et al. Enhancing cognitive abilities with comprehensive training: a large, online, randomized, active‐controlled trial. PLoS One 2015;10(9):e0134467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann J, Machts J, Bittner V, Mueller N, Heinze HJ, Bittner D. No title provided. Conference: Alzheimer's Association International Conference 2012, Vancouver, BC, Canada, 2012;Conference Start: 20120714 Conference End: 20120719:P393. [Google Scholar]
- Hayashi N, Kazui H, Morhora T, Yokokoji K, Kono A, Hata Y, et al. Cognitive training and occupational recreational therapy on elderly Japanese in Osaka: major outcome (ADAS) from prospective, randomized, open, blind‐endpoint trial. Alzheimer's and Dementia 2012;P3:219. [Google Scholar]
- Hayslip B Jr, Paggi K, Caballero D. The impact of mental aerobics training on older adults. Journal of Applied Gerontology 2016;35(11):1130‐53. [DOI] [PubMed] [Google Scholar]
- Heinzel S, Schulte S, Onken J, Duong QL, Riemer TG, Heinz A, et al. Working memory training improvements and gains in non‐trained cognitive tasks in young and older adults. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition 2014;21(2):146‐73. [DOI] [PubMed] [Google Scholar]
- Hötting K, Holzschneider K, Stenzel A, Wolbers T, Röder B. Effects of a cognitive training on spatial learning and associated functional brain activations. BMC Neuroscience 2013;14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak EM. The effects of cognitive stimulation and computerized memory training among older adults residing in independent‐living facilities. Thesis 2013;74(1‐B(E)):No Pagination Specified. [Google Scholar]
- Ignjatović VB, Kalabić S, Batić S, Žikić M. Improvement of cognitive efficiency through cognitive training in healthy subjects. Acta Clinica Croatica 2015;54(2):169‐78. [PubMed] [Google Scholar]
- Irigaray TQ, Gomes Filho I, Schneider RH. Effects of an attention, memory and executive functions training on the cognition of healthy elderly people. Psicologia: Reflexão e Crítica 2012;25(1):188‐202. [Google Scholar]
- Israël L, Myslinski M, Dubos G, Mélac M. [Combined therapies in family practice and hospitals. A controlled clinical study of a population of 162 patients with criteria of age‐related memory disorders]. [French]. Presse Médicale 1997;26(25):1186‐91. [PubMed] [Google Scholar]
- ISRCTN70130279. Effects of the six‐month training on cognitive, physical performance, and daily physical activity in older adults. clinicaltrials.gov.
- Jackson JJ, Hill PL, Payne BR, Roberts BW, Stine‐Morrow EA. Can an old dog learn (and want to experience) new tricks? Cognitive training increases openness to experience in older adults. Psychology and Aging 2012;27(2):286‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen P, Dahmen‐Zimmer K. Effects of cognitive, motor, and karate training on cognitive functioning and emotional well‐being of elderly people. Frontiers in Psychology 2012;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean L, Simard M, Wiederkehr S, Bergeron ME, Turgeon Y, Hudon C, et al. Efficacy of a cognitive training programme for mild cognitive impairment: results of a randomised controlled study. Neuropsychological Rehabilitation 2010;20(3):377‐405. [DOI] [PubMed] [Google Scholar]
- Jeong JH, Na HR, Choi SH, Kim J, Na DL, Seo SW, et al. Group‐ and home‐based cognitive intervention for patients with mild cognitive impairment: a randomized controlled trial. Psychotherapy and Psychosomatics 2016;85(4):198‐207. [DOI] [PubMed] [Google Scholar]
- Jobe JB, Smith DM, Ball K, Tennstedt SL, Marsiske M, Willis SL, et al. ACTIVE: a cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials 2001;22(4):453‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RN, Marsiske M, Ball K, Rebok G, Willis SL, Morris JN, et al. The ACTIVE cognitive training interventions and trajectories of performance among older adults. Journal of Aging and Health 2013;25(8):186S‐208S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampanaros D, Weber IL, Endler PC. Conventional and complementary interventions and cognitive performance in old age. Conference: 3rd European Congress for Integrative Medicine, ECIM, 2010, Berlin, Germany, 2010;Conference Start: 20101203 Conference End: 20101204:264. [Google Scholar]
- Kholin V. Cognitive‐emotional stimulation in mild cognitive impairment. Conference: 14th Congress of the European Federation of Neurological Societies 2010, EFNS, Geneva, Switzerland, 2010;Conference Start: 20100925 Conference End: 20100928:362. [Google Scholar]
- Kim GH, Jeon S, Lee BH, Kim HS, Chin JH, Kim GY. Robot assisted cognitive training can change the brain in the elderly: a single blind, randomized controlled trial of clinical efficacy. Conference: 5th Conference Clinical Trials on Alzheimer's Disease 2012, Monte Carlo, Monaco, 2012;Conference Start: 20121029 Conference End: 20121031:865‐6. [Google Scholar]
- Kim HJ, Sun Yang Y, Choi KH, Kim TY. The effect of computer‐based cognitive training program on cognition. Dementia and Neurocognitive Disorders 2013;12(4):87‐93. [Google Scholar]
- Kim GH, Jeon S, Im K, Seo SW, Cho H, Noh Y, et al. Structural brain changes after robot assisted cognitive training in the elderly: a single‐blind randomized controlled trial. Alzheimer's & Dementia 2013;9(4):P476‐7. [Google Scholar]
- Kim GH, Jeon S, Im K, Kwon H, Lee BH, Kim GY, et al. Structural brain changes after traditional and robot‐assisted. PLoS One 2015;10(4):e0123251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Choi Y, You H, Na DL, Yoh MS, Park JK, et al. Effects of a serious game training on cognitive functions in older adults. Journal of the American Geriatrics Society 2015;63(3):603‐5. [DOI] [PubMed] [Google Scholar]
- Kim GH, Jeon S, Im K, Kwon H, Lee BH, Kim GY, et al. Structural brain changes after traditional and robot‐assisted multi‐domain cognitive training in community‐dwelling healthy elderly. PLoS One 2015;10(4):e0123251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Lehtisalo J, Hanninen T, Jula A, Laatikainen T, et al. A multidomain two‐year randomized controlled trial to prevent cognitive impairment ‐ the FINGER study. Conference: 10th International Congress of the European Union Geriatric Medicine Society ‐ Geriatric Medicine Crossing Borders, EUGMS, 2014, Rotterdam, Netherlands, 2014;Conference Start: 20140917 Conference End: 20140919:S69. [Google Scholar]
- Klusmann V, Evers A, Schwarzer R, Dimeo FC, Reischies FM, Heuser I. Complex mental and physical activity in older women maintains episodic memory and working memory: a 6‐month randomized controlled trial. Conference: 64th Annual Scientific Convention and Meeting of the Society of Biological Psychiatry 2009, Vancouver, BC, Canada, 2009;Conference Start: 20090514 Conference End: 20090516:106S. [Google Scholar]
- Klusmann V, Evers A, Schwarzer R, Schlattmann P, Reischies FM, Heuser I, et al. Complex mental and physical activity in older women and cognitive performance: a 6‐month randomized controlled trial. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2010;65A(6):680‐8. [DOI] [PubMed] [Google Scholar]
- Klusmann V, Evers A, Schwarzer R, Schlattmann P, Reischies FM, Heuser I, et al. Complex mental and physical activity in older women and cognitive performance: a 6‐month randomized controlled trial. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2010;65(6):680‐8. [DOI] [PubMed] [Google Scholar]
- Klusmann V, Evers A, Heuser I. Cognitive benefits from mental and physical activity in older women: results from the Berlin Stays Fit study. Conference: International Conference "Aging and Cognition" 2010, Dortmund, Germany, 2011;Conference Start: 20101014 Conference End: 20101016:18. [Google Scholar]
- McDaniel MA, Binder EF, Bugg JM, Waldum ER, Dufault C, Meyer A, et al. Effects of cognitive training with and without aerobic exercise on cognitively demanding everyday activities. Psychology and Aging 2014;29(3):717‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak KP, Lee S, Kim T, Bae N. Cognitive training programs for very old lone adults in a Korean rural community. Conference: Alzheimer's Association International Conference 2015, Washington, DC, United States, 2015;Conference Start: 20150718 Conference End: 20150723:P590. [Google Scholar]
- Kwak K, Kim T. Cognitive stimulation intervention improves BDNF peripheral levels in older adults with non‐amnestic mild cognitive impairment. Alzheimer's & Dementia. 2017; Vol. Conference: Alzheimer's Association International Conference, AAIC 2017:P860‐1. [Google Scholar]
- Kwok T, Wong A, Chan G, Shiu YY, Lam KC, Young D, et al. Effectiveness of cognitive training for Chinese elderly in Hong Kong. Clinical Interventions in Aging 2013;8:213‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampit A, Hallock H, Moss R, Kwok S, Rosser M, Lukjanenko M, et al. A dose‐response relationship between computerized cognitive training and global cognition in older adults. Conference: 6th Conference Clinical Trials on Alzheimer's Disease 2013, San Diego, CA, United States, 2013;Conference Start: 20131114 Conference End: 20131116:803‐4. [Google Scholar]
- Lampit A, Hallock H, Moss R, Kwok S, Rosser M, Lukjanenko M, et al. The timecourse of global cognitive gains from supervised computer‐assisted cognitive training: a randomised active‐controlled trial in elderly with multiple dementia risk factors. Journal of Prevention of Alzheimer's Disease 2014;1(1):33‐9. [DOI] [PubMed] [Google Scholar]; Lampit A, Hallock H, Suo C, Naismith SL, Valenzuela M. Cognitive training‐induced short‐term functional and long‐term structural plastic change is related to gains in global cognition in healthy older adults: a pilot study. Frontiers in Aging Neuroscience 2015;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampit A, Hallock H, Suo C, Naismith SL, Valenzuela M. Cognitive training‐induced short‐term functional and long‐term structural plastic change is related to gains in global cognition in healthy older adults: a pilot study. Frontiers in Aging Neuroscience 2015;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H. Changes in the functional brain connectivity and cognitive performance following yoga or memory training in older adults with subjective memory complaints. Conference: 71st Annual Scientific Convention and Meeting of the Society of Biological Psychiatry, SOBP 2016, Atlanta, GA, United States, 2016;Conference Start: 20160512 Conference End: 20160514:209S. [Google Scholar]
- Law LL, Barnett F, Yau MK, Gray MA. Effects of functional tasks exercise on older adults with cognitive impairment at risk of Alzheimer's disease: a randomised controlled trial. Age Ageing 2014;43(6):813‐20. [DOI] [PubMed] [Google Scholar]
- Law LL, Barnett F, Yau MK, Gray MA. Effects of functional tasks exercise on older adults with cognitive impairment at risk of Alzheimer's disease: a randomised controlled trial. Age Ageing 2014;43(6):813‐20. [DOI] [PubMed] [Google Scholar]
- Lee YM, Jang C, Bak IH, Yoon JS. Effects of computer‐assisted cognitive rehabilitation training on the cognition and static balance of the elderly. Journal of Physical Therapy Science 2013;25(11):1475‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TS, Goh SJ, Quek SY, Phillips R, Guan C, Cheung YB, et al. A brain‐computer interface based cognitive training system for healthy elderly: a randomized control pilot study for usability and preliminary efficacy. PLoS One 2013;8(11):e79419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TS, Goh SJ, Quek SY, Guan C, Cheung YB, Krishnan KR. Efficacy and usability of a brain computer interface system in improving cognition in the elderly. Conference: Alzheimer's Association International Conference 2013, Boston, MA, United States, 2013;Conference Start: 20130713 Conference End: 20130718:P296. [Google Scholar]
- Lee TS, Goh ASJ, Quek SY, Phillips R, Guan C, Cheung YB, et al. Pilot trials of EEG‐based brain‐computer interface (BCI) training system for improving cognitive performance in older persons. Conference: NUHS Academic Psychiatry Conference 2014, Singapore, Singapore, 2014;Conference Start: 20141031 Conference End: 20141101:S27. [Google Scholar]
- Lee TS, Quek SY, Goh SJ, Phillips R, Guan C, Cheung YB, et al. A pilot randomized controlled trial using EEG‐based brain‐computer interface training for a Chinese‐speaking group of healthy elderly. Clinical Interventions in Aging 2015;10:217‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legualt C, Jennings JM, Katula JA, Dagenbach D, Gaussoin SA, Sink KM, et al. Designing clinical trials for assessing the effects of cognitive training and physical activity interventions on cognitive outcomes: the Seniors Health and ACtivity Research PRogram Pilot (SHARP‐P) study, a randomized controlled trial. BMC Geriatrics 2011;11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León J, Ureña A, Bolaños MJ, Bilbao A, Oña A. A combination of physical and cognitive exercise improves reaction time in persons 61‐84 years old. Journal of Aging and Physical Activity 2015;23(1):72‐7. [DOI] [PubMed] [Google Scholar]
- Leung NT, Tam HM, Chu LW, Kwok TC, Chan F, Lam LC, et al. Neural plastic effects of cognitive training on aging brain. Neural Plasticity 2015;2015:535618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KZ, Roudaia E, Lussier M, Bherer L, Leroux A, McKinley PA. Benefits of cognitive dual‐task training on balance performance in healthy older adults. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2010;65(12):1344‐52. [DOI] [PubMed] [Google Scholar]
- Linde K, Alfermann D. Single versus combined cognitive and physical activity effects on fluid cognitive abilities of healthy older adults: a 4‐month randomized controlled trial with follow‐up. Journal of Aging and Physical Activity 2014;22(3):302‐13. [DOI] [PubMed] [Google Scholar]
- Mace RA, Mansbach WE. The efficacy of a computer‐assisted cognitive rehabilitation program for patients with mild cognitive deficits: a pilot study. Conference: Alzheimer's Association International Conference 2015, Washington, DC, United States, 2015;Conference Start: 20150718 Conference End: 20150723:P783. [DOI] [PubMed] [Google Scholar]
- Mahncke HW, Connor BB, Appelman J, Ahsanuddin ON, Hardy JL, Wood RA, et al. Memory enhancement in healthy older adults using a brain plasticity‐based training program: a randomized, controlled study. Proceedings of the National Academy of Sciences of the United States of America 2006;103(33):12523‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man DW, Chung JC, Lee GY. Evaluation of a virtual reality‐based memory training programme for Hong Kong Chinese older adults with questionable dementia: a pilot study. International Journal of Geriatric Psychiatry 2012;27(5):513‐20. [DOI] [PubMed] [Google Scholar]
- Mann D, Szwanki VL, Mistry JJ. The effect of brain training on cognitive assessment: a pilot investigation. Conference: 10th Annual Conference on Brain Injury of the North American Brain Injury Society's, NABIS 2012, Miami, FL, United States. 2012; Vol. Conference Start: 20120912 Conference End: 20120915:E39‐40. [Google Scholar]
- Margrett JA, Willis SL. In‐home cognitive training with older married couples: individual versus collaborative learning. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition 2006;13(2):173‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayas J, Parmentier FB, Andrés P, Ballesteros S. Plasticity of attentional functions in older adults after non‐action video game training: a randomized controlled trial. PLoS One 2014;9(3):e92269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvinue LP, Golemme M, Castorina M, Tatti E, Pigni FM, Salomone S, et al. An evaluation of a working memory training scheme in older adults. Frontiers in Aging Neuroscience 2013;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA, Binder EF, Bugg JM, Waldum ER, Dufault C, Meyer A, et al. Effects of cognitive training with and without aerobic exercise on cognitively demanding everyday activities. Psychology and Aging 2014;29(3):717‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall S, House B. Brain training in older adults: evidence of transfer to memory span performance and pseudo‐Matthew effects. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition 2012;19(1‐2):195‐221. [DOI] [PubMed] [Google Scholar]
- Middleton LE, Poelke G, Santos W‐M, Yaffe K, Barnes DE, Goodson W. Impact of a 12‐week exercise intervention on non‐cognitive outcomes in sedentary elders with cognitive complaints or mild cognitive impairment: findings from the max trial. Conference: Alzheimer's Association International Conference 2012, Vancouver, BC, Canada, 2012;Conference Start: 20120714 Conference End: 20120719:P146. [Google Scholar]
- Miller KJ, Dye RV, Kim J, Jennings JL, O'Toole E, Wong J, et al. Effect of a computerized brain exercise program on cognitive performance in older adults. American Journal of Geriatric Psychiatry 2013;21(7):655‐63. [DOI] [PubMed] [Google Scholar]
- Mohs RC, Ashman TA, Jantzen K, Albert M, Brandt J, Gordon B, et al. A study of the efficacy of a comprehensive memory enhancement program in healthy elderly persons. Psychiatry Research 1998;77(3):183‐95. [DOI] [PubMed] [Google Scholar]
- Mombelli G, Riva M, Cerea E, Zanetti M, Rozzini L, Padovani A. Neuropsychological training (TNP) in MCI subjects: one year follow‐up study. Journal of Alzheimer's disease. 2012:77. [Google Scholar]
- Moon SK, Chung S, Han M‐I. The effectiveness of self‐efficacy based memory training program for the elderly with mild cognitive impairment. Conference: 16th International Congress of the International Psychogeriatric Association, IPA 2013, Seoul, South Korea. 2013; Vol. Conference Start: 20131001 Conference End: 20131004:S141‐2. [Google Scholar]
- Mowszowski L, Hermens DF, Diamond K, Norrie L, Cockayne N, Ward PB, et al. Cognitive training enhances pre‐attentive neurophysiological responses in older adults 'at risk' of dementia. Journal of Alzheimer's Disease 2014;41(4):1095‐108. [DOI] [PubMed] [Google Scholar]
- Mowszowski L, Hermens DF, Diamond K, Norrie L, Cockayne N, Ward PB, et al. Cognitive training enhances pre‐attentive neurophysiological responses in older adults 'at risk' of dementia. Journal of Alzheimer's Disease 2014;41(4):1095‐108. [DOI] [PubMed] [Google Scholar]
- Mozolic JL, Hayasaka S, Laurienti PJ. A cognitive training intervention increases resting cerebral blood flow in healthy older adults. Frontiers in Human Neuroscience 2010;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozolic JL, Long AB, Morgan AR, Rawley‐Payne M, Laurienti PJ. A cognitive training intervention improves modality‐specific attention in a randomized controlled trial of healthy older adults. Neurobiology of Aging 2011;32(4):655‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller NG, Bittner V, Hausmann J, Bittner DM. The effect of a combined motor and cognitive training on cognitive function, structural and functional MRI and BDNF plasma levels in MCI patients. Conference: International Conference "Aging and Cognition" 2010, Dortmund, Germany, 2011;Conference Start: 20101014 Conference End: 20101016:22‐3. [Google Scholar]
- Na HR, Choi S, Jeong JH, Na D, Park SA, Kim EJ, et al. A multicenter, randomized trial to assess efficacy of home‐based and group cognitive intervention programs in amnestic mild cognitive impairment. Conference: Alzheimer's Association International Conference 2013, Boston, MA, United States, 2013;Conference Start: 20130713 Conference End: 20130718:P495. [Google Scholar]
- Na HR, Choi SH, Jeong JH, Kim JE, Na DL, Seo SW, et al. A multicenter, randomized trial to assess efficacy of home‐based and group cognitive intervention programs for amnestic mild cognitive impairment. Conference: Alzheimer's Association International Conference 2014, Copenhagen, Denmark, 2014;Conference Start: 20140712 Conference End: 20140717:P916. [Google Scholar]
- Mowszowski L, Hermens DF, Diamond K, Norrie L, Cockayne N, Ward PB, et al. Cognitive training enhances pre‐attentive neurophysiological responses in older adults 'at risk' of dementia. Journal of Alzheimer's Disease 2014;41(4):1095‐108. [DOI] [PubMed] [Google Scholar]
- Navarro JI, Menacho I, Alcalde C, Marchena E, Simon Velez R, Aguilar M. Comparative study of two cognitive training procedures for elderly people. [Spanish]. Geriátrika 2006;22(6):36‐42. [Google Scholar]
- NCT00544856. Effects of a complex cognitive training in mild cognitive impairment and mild Alzheimer's disease. https://clinicaltrials.gov/ct2/show/NCT005448562007.
- NCT02417558. Study to evaluate the effectiveness of personalized brain network activation technology in a cognitive/physical computer‐game blended training of elderly (Alterniity AR). clinicaltrials.gov2015.
- NCT02462135. The development and evaluation of the effectiveness of a virtual interactive memory training program for older adults with mild cognitive impairment: protocol of a randomized controlled study. clinicaltrials.gov2014.
- NCT02480738. Effectiveness of computerized cognitive training apparatus (CoCoTA) in the elderly With normal cognition, subjective cognitive impairment, mild cognitive impairment. clinicaltrials.gov2012.
- NCT02512627. Evolving methods to combine cognitive and physical training for individuals with mild cognitive impairment: an efficacy study. clinicaltrials.gov2015. [DOI] [PMC free article] [PubMed]
- NCT02747784. Randomized evaluation to assess cognitive training for the prevention of post‐operative cognitive decline (REACT) ‐ a pilot study. clincaltrials.gov2016.
- NCT02774083. An Evaluation of the Feuerstein instrumental enrichment program for the cognitive enhancement of older people with mild cognitive impairment (MCI) living in the community. clinicaltrials.gov2015.
- NCT02785315. Cognitive intervention for persons with amnestic mild cognitive impairment: the efficacy in enhancement of cognition and complex activities of daily living function. clinicaltrials.gov2016.
- NCT02808676. SYNchronizing Exercises, Remedies in GaIt and Cognition (SYNERGIC): a randomized controlled double blind trial. clinicaltrials.gov2016. [DOI] [PMC free article] [PubMed]
- Neely AS, Sehlstedt I, Ekman U, Eriksson J, Sandberg P, Qwillbard T, et al. Working memory updating training in older adults: is level of performance after training related to transfer?. Conference: International Conference "Aging and Cognition", IfADo 2013, Germany, 2013;Conference Start: 20130425 Conference End: 20130427 Sponsor: Brain Products ‐ Solutions for Neurophysiological Research, Dortmund Tourismus, DFG ‐ Deutsche Forschungsgemeinsch:69‐70. [Google Scholar]
- Ng TP, Feng L, Nyunt MS, Feng L, Niti M, Tan BY, et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: a randomized controlled trial. American Journal of Medicine 2015;128(11):1225‐36. [DOI] [PubMed] [Google Scholar]
- Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet 2015;385(9984):2255‐63. [DOI] [PubMed] [Google Scholar]
- Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet 2015;385(9984):2255‐63. [DOI] [PubMed] [Google Scholar]
- Nishiguchi S, Yamada M, Tanigawa T, Sekiyama K, Kawagoe T, Suzuki M, et al. A 12‐week physical and cognitive exercise program can improve cognitive function and neural efficiency in community‐dwelling older adults: a randomized controlled trial. Journal of the American Geriatrics Society 2015;63(7):1355‐63. [DOI] [PubMed] [Google Scholar]
- Nouchi R, Taki Y, Takeuchi H, Hashizume H, Akitsuki Y, Shigemune Y, et al. Brain training game improves executive functions and processing speed in the elderly: a randomized controlled trial. PLoS One 2012;7(1):e29676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouchi R, Taki Y, Takeuchi H, Hashizume H, Nozawa T, Kambara T, et al. Brain training game boosts executive functions, working memory and processing speed in the young adults: a randomized controlled trial. PLoS One 2013;8(2):e55518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa T, Taki Y, Kanno A, Akimoto Y, Ihara M, Yokoyama R, et al. Effects of different types of cognitive training on cognitive function, brain structure, and driving safety in senior Daily drivers: a pilot study. Behavioral Neurology 2015;2015:525901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Caoimh R, Sato S, Wall J, Igras E, Foley MJ, Timmons S, et al. Potential for a “memory gym” Intervention to delay conversion of mild cognitive impairment to dementia. Journal of the American Medical Directors Association 2015;16(11):998‐99. [DOI] [PubMed] [Google Scholar]
- Oei AC, Patterson MD. Enhancing cognition with video games: a multiple game training study. PLoS One 2013;8(3):e58546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira de Lima Queiroz L, Junqueira AX, Fontana AM, Oliveira ER, Lima VC, Guarienti VC. Prevention of cognitive impairment through a cognitive stimulation and rehabilitation program mediated by computers and internet. Conference: 21st World Congress of Neurology, Vienna, Austria, 2013;Conference Start: 20130921 Conference End: 20130926:e537. [Google Scholar]
- Otsuka T, Tanemura R, Noda K, Nagao T, Sakai H, Luo ZW. Development of computer‐aided cognitive training program for elderly and its effectiveness through a 6 months group intervention study. Current Alzheimer Research 2015;12(6):553‐62. [DOI] [PubMed] [Google Scholar]
- Park MH, Kwon DY, Seo WK, Lim KS, Song MS. The effects of cognitive training on community‐dwelling elderly Koreans. Journal of Psychiatric and Mental Health Nursing 2009;16(10):904‐9. [DOI] [PubMed] [Google Scholar]
- Park SH, Seo JH, Kim YH, Ko MH. Long‐term effects of transcranial direct current stimulation combined with computer‐assisted cognitive training in healthy older adults. Neuroreport 2014;25(2):122‐6. [DOI] [PubMed] [Google Scholar]
- Payne BR, Jackson JJ, Hill PL, Gao X, Roberts BW, Stine‐Morrow EA. Memory self‐efficacy predicts responsiveness to inductive reasoning training in older adults. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition 2012;67(1):27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BR, Stine‐Morrow EA. The effects of home‐based cognitive training on verbal working memory and language comprehension in older adulthood. Frontiers in Aging Neuroscience 2017;9:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz C, Korczyn Ad, Shatil E, Aharonson V, Birnboim S, Giladi N. Computer‐based, personalized cognitive training versus classical computer games: a randomized double‐blind prospective trial of cognitive stimulation. Neuroepidemiology 2011;36(2):91‐9. [DOI] [PubMed] [Google Scholar]
- R000001637. Randomized prospective cognitive training study on elderly Japanese in Osaka. https://upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R0000016372007.
- Rahe J, Petrelli A, Kaesberg S, Fink GR, Kessler J, Kalbe E. Effects of cognitive training with additional physical activity compared to pure cognitive training in healthy older adults. Clinical Interventions in Aging 2015;10:297‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahe J, Becker J, Fink GR, Kessler J, Kukolja J, Rahn A, et al. Cognitive training with and without additional physical activity in healthy older adults: cognitive effects, neurobiological mechanisms, and prediction of training success. Frontiers in Aging Neuroscience 2015;7:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebok GW, Langbaum JB, Jones RN, Gross AL, Parisi JM, Spira AP, et al. Memory training in the ACTIVE study: how much is needed and who benefits?. Journal of Aging and Health 2013;25(8):21S‐42S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebok GW, Ball K, Guey LT, Jones RN, Kim HY, King JW, et al. Ten‐year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatrics Society 2014;62(1):16‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redick TS, Shipstead Z, Harrison TL, Hicks KL, Fried DE, Hambrick DZ, et al. No evidence of intelligence improvement after working memory training: a randomized, placebo‐controlled study. Journal of Experimental Psychology. General 2013;142(2):359‐79. [DOI] [PubMed] [Google Scholar]
- Requena C, Turrero A, Ortiz T. Six‐year training improves everyday memory in healthy older people. Randomized controlled trial. Frontiers in Aging Neuroscience 2016;8:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizkalla M. Cognitive training in the rural elderly: a randomized trial to evaluate the efficacy and accessibility of a new approach. Thesis2015; Vol. 75, issue 11‐B(E).
- Rojas GJ, Villar V, Iturry M, Harris P, Serrano CM, Herrera JA, et al. Efficacy of a cognitive intervention program in patients with mild cognitive impairment. International Psychogeriatrics 2013;25(5):825‐31. [DOI] [PubMed] [Google Scholar]
- Rose NS, Rendell PG, Hering A, Kliegel M, Bidelman GM, Craik FI. Cognitive and neural plasticity in older adults' prospective memory following training with the Virtual Week computer game. Frontiers in Human Neuroscience 2015;9:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AC, Sugiura L, Kramer JH, Whitfield‐Gabrieli S, Gabrieli JD. Cognitive training changes hippocampal function in mild cognitive impairment: a pilot study. Journal of Alzheimer's Disease 2011;26(Suppl 3):349‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu SH, Kim S, Youn JH, Lee JY. Improvement cognitive functions in the elderly with mild cognitive impairment and subjective memory complaints. Conference: 16th International Congress of the International Psychogeriatric Association, IPA 2013, Seoul, South Korea, 2013;Conference Start: 20131001 Conference End: 20131004:S165. [Google Scholar]
- Sakka P, Ntanasi E, Zoi P, Kalligerou F, Pantelopoulos S. Sociable: a comprehensive ICT cognitive training programme for healthy and cognitively impaired elderly. Neurology 2015;84(14):P6.188. [Google Scholar]
- Santos G, Ortega L, Yassuda M, Forlenza O, Nunes P. The effects of a multiprofessional cognitive and functional rehabilitation program for patients with Alzheimer's disease and mild cognitive impairment. Conference: Alzheimer's Association International Conference, AAIC 11, Paris, France, 2011;Conference Start: 20110716 Conference End: 20110721:S800. [Google Scholar]
- Schoene D, Valenzuela T, Toson B, Delbaere K, Severino C, Garcia J, et al. Interactive cognitive‐motor step training improves cognitive risk factors of falling in older adults ‐ a randomized controlled trial. PLoS One 2015;10(12):e0145161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoene D, Valenzuela T, Toson B, Delbaere K, Severino C, Garcia J, et al. Interactive cognitive‐motor step training improves cognitive risk factors of falling in older adults ‐ a randomized controlled trial. PLoS One 2015;10(12):e0145161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher V, Theill N, Martin M. Improving cognitive performance and motor‐cognition adaptability of older adults using an integrative motor‐cognitive training approach. Conference: International Conference "Aging and Cognition", IfADo 2013, Germany, 2013;Conference Start: 20130425 Conference End: 20130427 Sponsor: Brain Products ‐ Solutions for Neurophysiological Research, Dortmund Tourismus, DFG ‐ Deutsche Forschungsgemeinsch:68‐9. [Google Scholar]
- Shah T, Verdile G, Sohrabi H, Martins R. Cross‐training of auditory and visual brain training software program improves cognition and alters plasma BDNF levels in healthy older adults. Alzheimer's & Dementia. 2012:99. [Google Scholar]; Shah T, Verdile G, Sohrabi H, Martins R. Physical activity and cognitive stimulation improve cognition and alters levels of plasma beta‐amyloid in healthy elderly. Alzheimer's & Dementia. 2012:151. [Google Scholar]
- Shatil E. Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone? A four‐condition randomized controlled trial among healthy older adults. Frontiers in Aging Neuroscience 2013;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatil E, Mikulecká J, Bellotti F, Bureš V. Novel television‐based cognitive training improves working memory and executive function. PLoS One 2014;9(7):e101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatil E, Mikulecká J, Bellotti F, Bureš V. Novel television‐based cognitive training improves working memory and executive function. PLoS One 2014;9(7):e101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisco SM, Marsiske M, Gross AL, Rebok GW. The influence of cognitive training on older adults' recall for short stories. Journal of Aging and Health 2013;25(8):230S‐48S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slegers K, Boxtel M, Jolles J. Effects of computer training and internet usage on cognitive abilities in older adults: a randomized controlled study. Aging Clinical and Experimental Research 2009;21(1):43‐54. [DOI] [PubMed] [Google Scholar]
- Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, et al. A cognitive training program based on principles of brain plasticity: results from the improvement in memory with plasticity‐based adaptive cognitive training (IMPACT) study. Journal of the American Geriatrics Society 2009;57(4):594‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith‐Ray RL, Makowski‐Woidan B, Hughes SL. A randomized trial to measure the impact of a community‐based cognitive training intervention on balance and gait in cognitively intact Black older adults. Health Education and Behavior 2014;41(1):62S‐9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith‐Ray RL, Hughes SL, Prohaska TR, Little DM, Jurivich DA, Hedeker D. Impact of cognitive training on balance and gait in older adults. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition 2015;70(3):357‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith‐Ray RL, Hughes SL, Prohaska TR, Little DM, Jurivich DA, Hedeker D. Impact of cognitive training on balance and gait in older adults. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition 2015;70B(3):357‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A, Levalahti E, Soininen H, Tuomilehto J, Lindstrom J, Lehtisalo J, et al. A multidomain, two‐year, randomized controlled trial to prevent cognitive impairment: the finger study. Conference: Alzheimer's Association International Conference 2014, Copenhagen, Denmark, 2014;Conference Start: 20140712 Conference End: 20140717:P137‐8. [Google Scholar]
- Park MH, Kwon DY, Seo WK, Lim KS, Song MS. The effects of cognitive training on community‐dwelling elderly Koreans. Journal of Psychiatric and Mental Health Nursing 2009;16(10):904‐9. [DOI] [PubMed] [Google Scholar]
- Stepankova H, Lukavsky J, Buschkuehl M, Kopecek M, Ripova D, Jaeggi SM. The malleability of working memory and visuospatial skills: a randomized controlled study in older adults. Developmental Psychology 2014;50(4):1049‐59. [DOI] [PubMed] [Google Scholar]
- Stine‐Morrow EA, Payne BR, Roberts BW, Kramer AF, Morrow DG, Payne L, et al. Training versus engagement as paths to cognitive enrichment with aging. Psychology and Aging 2014;29(4):891‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strenziok M, Parasuraman R, Clarke E, Cisler DS, Thompson JC, Greenwood PM. Neurocognitive enhancement in older adults: comparison of three cognitive training tasks to test a hypothesis of training transfer in brain connectivity. Neuroimage 2013;85:1027‐39. [DOI] [PubMed] [Google Scholar]
- Strenziok M, Parasuraman R, Clarke E, Cisler DS, Thompson JC, Greenwood PM. Neurocognitive enhancement in older adults: comparison of three cognitive training tasks to test a hypothesis of training transfer in brain connectivity. Neuroimage 2014;85:1027‐39. [DOI] [PubMed] [Google Scholar]
- Stürz K, Hartmann S, Eder‐Pelzer B, Günther V. [Computer assisted cognitive training advances mood and psychological wellbeing ‐ a comparison to paper pencil training relating to neuropsychological parameters, mood and cognitions] [German]. Neuropsychiatrie 2011;25(2):85‐92. [PubMed] [Google Scholar]
- Stürz K, Hartmann S, Eder‐Pelzer B, Günther V. [Computer assisted cognitive training advances mood and psychological wellbeing ‐ a comparison to paper pencil training relating to neuropsychological parameters, mood and cognitions]. [German]. Neuropsychiatrie 2011;25(2):85‐92. [PubMed] [Google Scholar]
- Sturz K, Hartmann S, Kemmler G, Gunther V. Influence of a relaxation program, cognitive training and a combination of both intervention forms on neuropsychological and affective parameters in elderly care home residents. Conference: 23rd European Congress of Psychiatry, EPA 2015, Vienna, Austria, 2015;Conference Start: 20150328 Conference End: 20150331:1447. [Google Scholar]
- Styliadis C, Kartsidis P, Paraskevopoulos E, Ioannides AA, Bamidis PD. Neuroplastic effects of combined computerized physical and cognitive training in elderly individuals at risk for dementia: an eLORETA controlled study on resting states. Neural Plasticity 2015;2015:172192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styliadis C, Kartsidis P, Paraskevopoulos E, Ioannides AA, Bamidis PD. Neuroplastic effects of combined computerized physical and cognitive training in elderly individuals at risk for dementia: an eLORETA controlled study on resting states. Neural Plasticity 2015;2015:172192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo C, Fiatarone Singh MA, Sachdev PS, Gates NJ, Valenzuela M. Resting state network adaptation in older adults with MCI in the SMART trial: unique effects of combined cognitive training and physical exercise. Conference: 3rd Biennial Conference on Resting State Brain Connectivity, Magdeburg, Germany, 2012;Conference Start: 20120905 Conference End: 20120907:A90‐1. [Google Scholar]
- Szelag E, Skolimowska J. Cognitive function in elderly can be ameliorated by training in temporal information processing. Restorative Neurology and Neuroscience 2012;30(5):419‐34. [DOI] [PubMed] [Google Scholar]
- Talib LL, Yassuda MS, Diniz BS, Forlenza OV, Gattaz WF. Cognitive training increases platelet PLA2 activity in healthy elderly subjects. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2008;78(4‐5):265‐9. [DOI] [PubMed] [Google Scholar]
- Tappen RM, Hain D. The effect of in‐home cognitive training on functional performance of individuals with mild cognitive impairment and early‐stage Alzheimer's disease. Research in Gerontological Nursing 2014;7(1):14‐24. [DOI] [PubMed] [Google Scholar]
- Tennstedt SL, Unverzagt FW. The ACTIVE study: study overview and major findings. Journal of Aging and Health 2013;25(8):3S‐20S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesky V, Pantel J. Cognitively stimulating leisure activities: a new approach for patients with mild cognitive impairment (MCI). Conference: Alzheimer's Association International Conference 2012 Vancouver, BC, Canada, 2012;Conference Start: 20120714 Conference End: 20120719:P571. [Google Scholar]
- Tsai AY, Yang MJ, Lan CF, Chen CS. Evaluation of effect of cognitive intervention programs for the community‐dwelling elderly with subjective memory complaints. International Journal of Geriatric Psychiatry 2008;23(11):1172‐4. [DOI] [PubMed] [Google Scholar]
- Tsolaki M, Poptsi E, Kounti F, Christina A, Evaggelia B, Aikaterini S, et al. Longitudinal cognitive training in people with mild cognitive impairment. Conference: Alzheimer's Association International Conference 2013, Boston, MA, United States, 2013;Conference Start: 20130713 Conference End: 20130718:P491‐2. [Google Scholar]
- Tucker‐Drob EM, Johnson KE, Jones RN. The cognitive reserve hypothesis: a longitudinal examination of age‐associated declines in reasoning and processing speed. Developmental Psychology 2009;45(2):431‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Dawson J, Wadley VG, Edwards JD, Roenker D, Rizzo M, et al. The accelerate study: the longitudinal effect of speed of processing training on cognitive performance of older adults. Rehabilitation Psychology 2007;52(1):89‐96. [Google Scholar]
- Berg M, Sherrington C, Killington M, Smith S, Bongers B, Hassett L, et al. Video and computer‐based interactive exercises are safe and improve task‐specific balance in geriatric and neurological rehabilitation: a randomised trial. Journal of Physiotherapy 2016;62(1):20‐8. [DOI] [PubMed] [Google Scholar]
- Ploeg ES, Hoorweg A, Lee J. User friendliness of computer‐based cognitive training for psychogeriatric patients with mild to moderate cognitive impairments [Gebruiksvriendelijkheid van computerondersteunde cognitieve training bij psychogeriatrische patiënten met lichte tot matige cognitieve functiestoornissen]. Tijdschrift Voor Gerontologie en Geriatrie 2016;47(2):58‐67. [DOI] [PubMed] [Google Scholar]
- het Reve E, Bruin ED. Strength‐balance supplemented with computerized cognitive training to improve dual task gait and divided attention in older adults: a multicenter randomized‐controlled trial. BMC Geriatrics 2014;14:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidovich MR, Lautenschlager NT, Flicker L, Clare L, Almeida OP. The PACE study: a randomised clinical trial of cognitive activity (CA) for older adults with mild cognitive impairment (MCI). Trials 2009;10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidovich MR, Lautenschlager NT, Flicker L, Clare L, McCaul K, Almeida OP. The PACE study: a randomized clinical trial of cognitive activity strategy training for older people with mild cognitive impairment. American Journal of Geriatric Psychiatry 2015;23(4):360‐72. [DOI] [PubMed] [Google Scholar]
- Vidovich MR, Lautenschlager NT, Flicker L, Clare L, McCaul K, Almeida OP. The PACE study: a randomized clinical trial of cognitive activity strategy training for older people with mild cognitive impairment. American Journal of Geriatric Psychiatry 2015;23(4):360‐72. [DOI] [PubMed] [Google Scholar]
- Bastian CC, Langer N, Jäncke L, Oberauer K. Effects of working memory training in young and old adults. Memory & Cognition 2013;41(4):611‐24. [DOI] [PubMed] [Google Scholar]
- Wadley VG, Crowe M, Marsiske M, Cook SE, Unverzagt FW, Rosenberg AL, et al. Changes in everyday function in individuals with psychometrically defined mild cognitive impairment in the advanced cognitive training for independent and vital elderly study. Journal of the American Geriatrics Society 2007;55(8):1192‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton CC, Kavanagh A, Downey LA, Lomas J, Camfield DA, Stough C. Online cognitive training in healthy older adults: a preliminary study on the effects of single versus multi‐domain training. Translational Neuroscience 2015;6(4):13‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JR, Hsieh S. Neurofeedback training improves attention and working memory performance. Clinical Neurophysiology 2013;124(12):2406‐20. [DOI] [PubMed] [Google Scholar]
- Weicker J, Hudl N, Marichal E, Muller K, Lepsien J, Trapp S, et al. Training of working memory in healthy elderly subjects ‐ a randomized controlled trial. Conference: Joint Meeting of the FESN/GNP 2013, Berlin, Germany, 2013;Conference Start: 20130912 Conference End: 20130914:371. [Google Scholar]
- Wild‐Wall N, Falkenstein M, Gajewski PD. Neural correlates of changes in a visual search task due to cognitive training in seniors. Neural Plasticity 2012;2012:529057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Herman R, Bontempo D. Reasoning exercises in assisted living: a cluster randomized trial to improve reasoning and everyday problem solving. Clinical Interventions in Aging 2014;9:981‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL, Schaie KW. Training the elderly on the ability factors of spatial orientation and inductive reasoning. Psychology and Aging 1986;1(3):239‐47. [DOI] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Long‐term effects of cognitive training on everyday functional outcomes in older adults. JAMA 2006;296(23):2805‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Long‐term effects of cognitive training on everyday functional outcomes in older adults. JAMA 2006;296(23):2805‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Long‐term effects of cognitive training on everyday functional outcomes in older adults. American Journal of Health Prevention 2007;21(5):469‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL, Caskie GI. Reasoning training in the ACTIVE study: how much is needed and who benefits?. Journal of Aging and Health 2013;25(8):43S‐64S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtynska R, Wlazlo A, Trypka E, Zimny A, Frydecka D. The evaluation of the effectiveness of the program of the cognitive rehabilitation of patients with MCI and early dementia of Alzheimer's type. European Psychiatry 2011;26(1):504. 21398097 [Google Scholar]
- Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Wright E, Tennstedt SL. The effects of the ACTIVE cognitive training trial on clinically relevant declines in health‐related quality of life. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 2006;61(5):S281‐7. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Stoddard A, Tennstedt SL. The ACTIVE cognitive training trial and health‐related quality of life: protection that lasts for 5 years. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2006;61(12):1324‐9. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Vander Weg MW, Martin R, Unverzagt FW, Willis SL, Marsiske M, et al. Does cognitive training improve internal locus of control among older adults?. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 2010;65B(5):591‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Mahncke H, Vander Weg MW, Martin R, Unverzagt FW, Ball KK, et al. Speed of processing training protects self‐rated health in older adults: enduring effects observed in the multi‐site ACTIVE randomized controlled trial. International Psychogeriatrics2010; Vol. 22, issue 3:470‐8. [DOI] [PMC free article] [PubMed]
- Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM. A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PLoS One 2013;8(5):e61624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM. The effect of cognitive speed of processing training on the development of additional IADL difficulties and the reduction of depressive symptoms: results from the IHAMS randomized controlled trial. Journal of Aging and Health 2015;27(2):334‐54. [DOI] [PubMed] [Google Scholar]
- Yam A, Gross AL, Prindle JJ, Marsiske M. Ten‐year longitudinal trajectories of older adults' basic and everyday cognitive abilities. Neuropsychology 2014;28(6):819‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassuda MS, Camargo MC, Brum PS, Bento T, Silva L, Spindola L. Working memory training: effects on cognition and psychological wellbeing of seniors without dementia and depression. Conference: Alzheimer's Association International Conference 2015, Washington, DC, United States, 2015;Conference Start: 20150718 Conference End: 20150723:P462. [Google Scholar]
- Yip CB. An intelligent rehabilitation system for cognitive rehabilitation. Thesis 2012;73(3‐B):1524. [Google Scholar]
- Lee Y, Lee CR, Hwang B. Effects of computer‐aided cognitive rehabilitation training and balance exercise on cognitive and visual perception ability of the elderly. Journal of Physical Therapy Science 2012;24(9):885‐7. [Google Scholar]
- Youn JH, Lee JY, Kim S, Ryu SH. Multistrategic memory training with the metamemory concept in healthy older adults. Psychiatry Investigation 2011;8(4):354‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski EM, Dalton SE, Smith GE. Consumer‐based brain fitness programs. Enhancing Cognitive Fitness in Adults: A Guide to the Use and Development of Community‐Based Programs. Philadelphia, PA: Springer, 2011:45‐66. [Google Scholar]
- Zelinski EM, Spina LM, Yaffe K, Ruff R, Kennison RF, Mahncke HW, et al. Improvement in memory with plasticity‐based adaptive cognitive training: results of the 3‐month follow‐up. Journal of the American Geriatrics Society 2011;59(2):258‐65. [DOI] [PubMed] [Google Scholar]
- Zhuang JP, Fang R, Feng X, Xu XH, Liu LH, Bai QK, et al. The impact of human‐computer interaction‐based comprehensive training on the cognitive functions of cognitive impairment elderly individuals in a nursing home. Journal of Alzheimer's Disease 2013;36(2):245‐51. [DOI] [PubMed] [Google Scholar]
- Zimmermann N, Netto TM, Amodeo MT, Ska B, Fonseca RP. Working memory training and poetry‐based stimulation programs: are there differences in cognitive outcome in healthy older adults?. NeuroRehabilitation 2014;35(1):159‐70. [DOI] [PubMed] [Google Scholar]
Additional references
- Abraham RP, Denton DA, Al‐Assaf AS, Rutjes AW, Chong LY, Malik MA, et al. Vitamin and mineral supplementation for prevention of dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011905] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo A, Loewenstein DA. Nonpharmacological cognitive interventions in aging and dementia. Journal of Geriatric Psychiatry and Neurology 2007;20(4):239–49. [DOI] [PubMed] [Google Scholar]
- Aisen PS, Andrieu S, Sampaio C, Carrillo M, Khachaturian ZS, Dubois B, et al. Report of the task force on designing clinical trials in early (pre‐dementia) AD. Neurology 2011;76(3):280‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Assaf AS, Denton DA, Abraham RP, Rutjes AW, Chong LY, Anderson JL, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in late life. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011906] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia 2011;7(3):270‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer's Association National Plan Milestone Workgroup, Fargo KN, Aisen P, Albert M, Au R, Corrada MM, DeKosky S, et al. Report on the milestones for the US national plan to address Alzheimer's disease. Alzheimers Dementia 2014;10(5 Suppl):S430‐52. [DOI] [PubMed] [Google Scholar]
- Amoyal N, Fallon E. Physical exercise and cognitive training clinical interventions used in slowing degeneration associated with mild cognitive impairment. A review of the literature. Topics in Geriatric Rehabilitation 2012;28(3):208‐16. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- Bahar‐Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for persons with mild to moderate dementia of the Alzheimer's or vascular type: a review. Alzheimer's Research and Therapy 2013;5(4):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurology 2011;10(9):819‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleville S, Bherer L. Biomarkers of cognitive training effects in aging. Current Translational Geriatrics and Experimental Gerontology Reports 2012;1(2):104‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta‐analysis. BMC Public Health 2014;14(1):643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Johnson E, Ziegler‐Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimer's & Dementia 2007;3(3):186‐91. [DOI] [PubMed] [Google Scholar]
- Costa BR, Nuesch E, Reichenbach S, Juni P, Rutjes AW. Doxycycline for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD007323.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa BR, Nüesch E, Kasteler R, Husni E, Welch V, Rutjes AWS, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD003115.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn RF, Schrijvers EM, Groot KA, Witteman JC, Hofman A, Franco OH, et al. The association between physical activity and dementia in an elderly population: the Rotterdam Study. European Journal of Epidemiology 2013;28(3):277‐83. [DOI] [PubMed] [Google Scholar]
- Denton DA, Abraham RP, Al‐Assaf AS, Rutjes AW, Chong LY, Anderson JL, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid life. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011904] [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
- Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF 3rd. Late‐life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta‐analysis of community‐based cohort studies. British Journal of Psychiatry: Journal of Mental Science 2013;202(5):329‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresler M, Sandberg A, Ohla K, Bublitz C, Trenado C, Mroczko‐Wąsowicz A, et al. Non‐pharmacological cognitive enhancement. Neuropharmacology 2013;64:529‐43. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America 2011;108(7):3017‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes SC, Forbes D, Forbes S, Blake CM, Chong LY, Thiessen EJ, et al. Exercise interventions for preventing dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011706] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes SC, Forbes D, Forbes S, Blake CM, Chong LY, Thiessen EJ, et al. Exercise interventions for maintaining cognitive function in cognitively healthy people in mid life. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011705] [DOI] [Google Scholar]
- Forbes SC, Forbes D, Forbes S, Blake CM, Chong LY, Thiessen EJ, et al. Exercise interventions for maintaining cognitive function in cognitively healthy people in late life. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011704] [DOI] [Google Scholar]
- Förster S, Buschert VC, Teipel SJ, Friese U, Buchholz HG, Drzezga A, et al. Effects of a 6‐month cognitive intervention on brain metabolism in patients with amnestic MCI and mild Alzheimer's disease. Journal of Alzheimer's Disease 2011;26(Suppl 3):337‐48. [DOI] [PubMed] [Google Scholar]
- Gates NJ, Sachdev P. Is cognitive training an effective treatment for preclinical and early Alzheimer's disease?. Journal of Alzheimer's Disease 2014;42(Suppl 4):S551–9. [DOI] [PubMed] [Google Scholar]
- Gates NJ, Rutjes AWS, Nisio M, Salman K, Chong L, March E, Vernooij RWM. Computerised cognitive training for maintaining cognitive function in cognitively healthy people in late life. Cochrane Database of Systematic Reviews [under submission], Issue [under submission]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates NJ, Rutjes AWS, Nisio M, Karim S, Chong L, March E, Vernooij RWM. Computerised cognitive training for maintaining cognitive function in cognitively healthy people in midlife. Cochrane Database of Systematic Reviews [under submission], Issue [under submission]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geda YE, Silber TC, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, et al. Computer activities, physical exercise, aging, and mild cognitive impairment: a population‐based study. Mayo Clinic Proceedings 2012;87(5):437‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C. The cognitive neuroscience of ageing. Nature Reviews. Neuroscience 2012;13(7):491‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CS, Strobach T, Schubert T. On methodological standards in training and transfer experiments. Psychological Research 2014;78(6):756‐72. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, Schünemann HJ, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SL, Birdi R, Smart CO, Brittain K, Rutjes AW, Siervo M, et al. Dietary interventions for maintaining cognitive function in cognitively healthy people in mid life. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2015, issue 10. [DOI: 10.1002/14651858.CD011911; CD011911] [DOI]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. Available from http://handbook.cochrane.org., Updated March 2011. [Google Scholar]
- Jellinger KA. Clinicopathological analysis of dementia disorders in the elderly ‐ an update. Journal of Alzheimer's Disease 2006;9(3 Suppl):61‐70. [DOI] [PubMed] [Google Scholar]
- Jorm AF. History of depression as a risk factor for dementia: an updated review. Australian and New Zealand Journal of Psychiatry 2001;35(6):776–81. [DOI] [PubMed] [Google Scholar]
- Karp A, Paillard‐Borg S, Wang HX, Silverstein M, Winblad B, Fratiglioni L. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dementia and Geriatric Cognitive Disorders 2006;21(2):65‐73. [DOI] [PubMed] [Google Scholar]
- Koepsell TD, Monsell SE. Reversion from mild cognitive impairment to near‐normal cognition: risk factors and prognosis. Neurology 2012;79(15):1591‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueider AM, Parisi JM, Gross AL, Rebok GW. Computerized cognitive training with older adults: a systematic review. PLoS One 2012;7(7):e40588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampit A, Hallock H, Valenzuela M. Computerized cognitive training in cognitively healthy older adults: a systematic review and meta‐analysis of effect modifiers. PLoS Medicine 2014;11(11):e1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Marks SM, Mormino EC, Rabinovici GD, Oh H, O'Neil JP, et al. Association of lifetime cognitive engagement and low B‐amyloid deposition. Archives of Neurology 2012;69(5):623‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifer BP. Early diagnosis of Alzheimer's disease: clinical and economic benefits. Journal of the American Geriatrics Society 2003;51(5 Suppl):S281‐8. [DOI] [PubMed] [Google Scholar]
- Liberati G, Raffone A, Olivetti Belardinelli M. Cognitive reserve and its implications for rehabilitation and Alzheimer's disease. Cognitive Processing 2012;13(1):1‐12. [DOI] [PubMed] [Google Scholar]
- Marioni RE, Proust‐Lima C, Amieva H, Brayne C, Matthews FE, Dartigues JF, et al. Cognitive lifestyle jointly predicts longitudinal cognitive decline and mortality risk. European Journal of Epidemiology 2014;29(3):211‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Clare L, Altgassen AM, Cameron MH, Zehnder F. Cognition‐based interventions for healthy older people and people with mild cognitive impairment. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD006220.pub2] [DOI] [PubMed] [Google Scholar]
- Matthews FE, Stephan BC, McKeith IG, Bond J, Brayne C, Medical Research Council Cognitive Function and Ageing Study. Two‐year progression from mild cognitive impairment to dementia: to what extent do different definitions agree?. Journal of the American Geriatrics Society 2008;56(8):1424‐33. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Shiri‐Feshki M. Rate of progression of mild cognitive impairment to dementia ‐ meta‐analysis of 41 robust inception cohort studies. Acta Psychiatrica Scandinavica 2009;119(4):252‐65. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009;339(2535):332‐6. [PMC free article] [PubMed] [Google Scholar]
- Mowszowski L, Batchelor J, Naismith S. Early intervention for cognitive decline: can cognitive training be used as a selective prevention technique?. International Psychogeriatrics 2010;22(4):537‐48. [DOI] [PubMed] [Google Scholar]
- Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study. Pathological correlates of late‐onset dementia: a multi‐centre community based population in England and Wales. The Lancet 2001;357(9251):169‐75. [DOI] [PubMed] [Google Scholar]
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population‐based data. Lancet Neurology 2014;13(8):788‐94. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nature Neurosciences 2004;7(1):75‐9. [DOI] [PubMed] [Google Scholar]
- Papp KV, Walsh SJ, Snyder PJ. Immediate and delayed effects of cognitive interventions in healthy elderly: a review of current literature and future directions. Alzheimer's and Dementia 2009;5(1):50‐60. [DOI] [PubMed] [Google Scholar]
- Park DC, Bischof GN. The aging mind: neuroplasticity in response to cognitive training. Dialogues in Clinical Neuroscience 2013;15:109‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre‐stroke and post‐stroke dementia: a systematic review and meta‐analysis. Lancet Neurology 2009;8(11):1006–18. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology 1999;56(3):303‐8. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Archives of Neurology 2001;58:1985–92. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine 2004;256:183–94. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, et al. Mild cognitive impairment: ten years later. Archives of Neurology 2009;66(12):1447‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Lopez O, Armstrong MS, Getchius TS, Ganguli M. Practice guideline update summary: mild cognitive impairment. Neurology 2018;90(3):126‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and meta‐analysis. Alzheimer's and Dementia 2013;9(1):63‐75. [DOI] [PubMed] [Google Scholar]
- Reichenbach S, Rutjes AW, Nuesch E, Trelle S, Juni P. Joint lavage for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD007320.pub2] [DOI] [PubMed] [Google Scholar]
- Reijnders J, van Heugten, Boxtel M. Comparative interventions in healthy adults and those with mild cognitive impairment: a systematic review. Ageing Research Reviews 2013;12:263‐75. [DOI] [PubMed] [Google Scholar]
- Russ TC, Morling JR. Cholinesterase inhibitors for mild cognitive impairment. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD009132.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutjes AW, Nüesch E, Sterchi R, Kalichman L, Hendriks E, Osiri M, et al. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD002823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutjes AW, Nuesch E, Reichenbach S, Juni P. S‐Adenosylmethionine for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007321.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutjes AW, Nuesch E, Sterchi R, Juni P. Therapeutic ultrasound for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003132.pub2] [DOI] [PubMed] [Google Scholar]
- Rutjes AW, Juni P, Costa BR, Trelle S, Nuesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta‐analysis. Annals of Internal Medicine 2012;157(3):180‐91. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Memory aging from 18 to 80. Alzheimer Disease and Associated Disorders 2003;17(3):162‐7. [DOI] [PubMed] [Google Scholar]
- Siervo M, Lara J, Munro A, Tang EY, Rutjes AW, Stephan B. Dietary interventions for maintaining cognitive function in cognitively healthy people in late life. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011910] [DOI] [Google Scholar]
- Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian Approaches to Clinical Trials and Health‐care Evaluation. Chichester, UK: John Wiley, 2004. [Google Scholar]
- Stephan BC, Matthews FE, McKeith IG, Bond J, Brayne C, Medical Research Council Cognitive Function and Aging Study. Early cognitive change in the general population: how do different definitions work?. Journal of the American Geriatrics Society 2007;55(10):5534‐40. [DOI] [PubMed] [Google Scholar]
- Stephan CMB, Minett T, Pagett E, Siervo M, Brayne C, McKeith IG. Diagnosing mild cognitive impairment (MCI) in clinical trials: a systematic review. BMJ Open 2013;3(2):e001909. [PubMed: 23386579] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurology 2012;11(11):1006‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo C, Valenzuela MJ. Neuroimaging outcomes of brain training trials. In: Bright P editor(s). Neuro‐imaging‐Cognitive and Clinical Neurosciences. Croatia: InTech, 2012. [Google Scholar]
- Tang EY, Harrison SL, Albanese E, Gorman TJ, Rutjes AW, Siervo M, et al. Dietary interventions for prevention of dementia in people with mild cognitive impairment. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011909] [DOI] [Google Scholar]
- Valenzuela MJ, Jones M, Wen W, Rae C, Graham S, Shnier R, et al. Memory training alters hippocampal neurochemistry in healthy elderly. Neuroreport 2003;14(10):1333‐7. [DOI] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, et al. Leisure activities and the risk of dementia in the elderly. New England Journal of Medicine 2003;348(25):2508‐16. [DOI] [PubMed] [Google Scholar]
- Walton CC, Mowszowski L, Lewis SJ, Naismith SL. Stuck in the muck: time for change in the implementation of cognitive training research in ageing?. Frontiers in Aging Neuroscience 2014;6:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Dementia: A Public Health Priority. Dementia: A Public Health Priority. Geneva, Switzerland: WHO, 2012. [Google Scholar]
- Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, et al. Participation in cognitive stimulating activities and risk of incidence of Alzheimer’s Disease. Journal of the American Medical Association 2002;287(6):742‐8. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Aggarwal NT, Boyle PA, Hebert LE, Mendes de Leon CF, et al. Cognitive activity and the cognitive morbidity of Alzheimer disease. Neurology 2010;75(11):990‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Segawa E, Boyle PA, Bennett DA. Influence of late‐life cognitive activity on cognitive health. Neurology 2012;78(15):1123‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimo A, Winbald B, Johnsson L. The worldwide societal costs of dementia: estimates for 2009. Alzheimer's and Dementia 2010;6(2):98‐103. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment ‐ beyond controversies, towards consensus: report of the international working group on mild cognitive impairment. Journal of International Medicine 2004;256(3):240‐6. [DOI] [PubMed] [Google Scholar]
- The World Alzheimer Report 2014. Dementia and Risk Reduction: An Analysis of Protective and Modifiable Factors. London: Alzheimer’s Disease International (ADI), 2014. [Google Scholar]


