Summary
Objective:
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare and potentially fatal adverse skin reactions that are most commonly triggered by certain medications. One class of medications that have been highly associated with SJS/TEN reactions are antiepileptic drugs (AEDs). We sought to quantify the risk of SJS/TEN associated with AEDs as a class, as well as individual AEDs, in the United States.
Methods:
An analysis was performed of the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) from July 2014 through December 2017. Rates of SJS/TEN were calculated for each AED compared with all other non-AEDs. Reporting odds ratios (ROR), proportional reporting ratios (PRR), and 95% confidence intervals (CI) were calculated using OpenEpi.
Results:
AEDs had the most reports of SJS/TEN than any other medication class with 198 reports. AEDs as a class had a ROR of 8.7 (CI 7.5–10.2) and a PRR of 8.7 (CI 7.5–10.2) compared with all other non-AEDs. The AEDs with the highest risk estimates were zonisamide (ROR: 70.2, CI 33.1–148.7; PRR: 68.7, CI 32.9–143.5), rufinamide (ROR: 60.0, CI 8.3–433.5; PRR: 58.9, CI 8.4–411.5), clorazepate (ROR: 56.0, CI 7.8–404.1; PRR: 55.1, CI 7.8–385.0), lamotrigine (ROR: 53.0, CI 43.2–64.9; PRR: 52.2, CI 42.7–63.7), phenytoin (ROR: 26.3, CI 15.5–44.7; PRR: 26.1, CI 15.4–44.2), and carbamazepine (ROR: 24.5, CI 16.0–37.5; PRR: 24.3, CI 16.0–37.1).
Keywords: Antiepileptic drugs, Stevens-Johnson syndrome, toxic epidermal necrolysis, pharmacovigilance, drug safety
Introduction
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare and life threatening adverse skin reactions often triggered by particular medications. Some medications that have been shown to trigger these reactions are antiepileptic drugs (AEDs), nonsteroidal anti-inflammatory drugs (NSAIDs), and certain antibiotics.1–3 Although these reactions initially were thought to be separate conditions, they are now considered part of the same continuum. SJS is defined as detachment of less than 10% of total body surface area (BSA).4 TEN is defined as detachment of greater than 30% of total BSA.4 Estimated incidence of these reactions is 1.5–8.3 cases per 1,000,000 person-years.5–7 Mortality from SJS is approximately 5% whereas mortality from TEN occurs in approximately 30% of patients.8 Mortality risk continues after presentation of SJS/TEN with studies showing mortality even 1 year after hospitalization.9, 10 Severity of disease appears to be the largest risk factor for mortality in the early phase of disease, and older age and comorbidities as the largest mortality risk factor at the 3–12 month timeframe.11 Patients who survive SJS/TEN reactions are at a high risk of developing long-term complications involving the skin, eye, mucosal, respiratory, renal, and/or hepatic systems.10, 11
Epilepsy is one of the most common neurologic disorders worldwide and is classified as a chronic disorder of recurrent unprovoked seizures.12 Approximately 3% of the population under the age of 75 is diagnosed with epilepsy.13 Some antiepileptic drugs have additional FDA approved indications outside of epilepsy including: neuropathic pain, migraines, anxiety, insomnia, bipolar disorder, among others.14 Therefore, their use extends beyond those with epilepsy, making them commonly prescribed. AEDs are associated with SJS/TEN reactions, in particular lamotrigine and carbamazepine as they have black boxed warnings regarding SJS/TEN reactions.15, 16 The black boxed warning for carbamazepine relates specifically to patients with the human leukocyte antigen (HLA)-B*1502 allele, as this has been found to increase the risk of developing SJS/TEN.15 The HLA-B*1502 allele is mostly found in patients of Asian ancestry. As such, genetic screening for the HLA-B*1502 allele is recommended for patients of Asian ancestry prior to initiating carbamazepine.15 Although not indicated with a black box warning, the package insert of oxcarbazepine also recommends genetic screening for the HLA-B*1502 allele due to the similar chemical structure of oxcarbazepine to carbamazepine along with clinical and non-clinical data suggesting an increased risk of SJS/TEN in these patients.17 In recent years, several case-control studies and analyses of adverse event reporting systems in Europe have explored the relationship between SJS/TEN and certain AEDs, specifically carbamazepine, lamotrigine, and phenytoin.18, 19 However, the class effect and impact of other AEDs are not well-described. As such, we sought to quantify the risk of SJS/TEN associated with AEDs as a class, as well as individual AEDs, in the United States (US).
Methods
We reviewed adverse event reports from the US Food and Drug Administration Adverse Event Reporting System (FAERS) for a 42-month time frame of July 2014 through December 2017.20 Follow-up reports were excluded, along with duplicate reports and reports missing event date, sex, and age. We conducted searches of the medical dictionary for regulatory activities (MedRA) terms “Stevens-Johnson syndrome” and “toxic epidermal necrolysis”. Subsequent listings of adverse events were reviewed for inclusion. We included AEDs with a FDA indication for epilepsy or seizures (Table 1). Reports using brand names were categorized under their generic names. Since SJS and TEN are part of the same continuum, these reactions were combined as SJS/TEN. The proportional reporting ratio (PRR), reporting odds ratio (ROR), and corresponding 95% confidence intervals (CI) for SJS/TEN were calculated for each AED medication compared to all other non-AEDs. The ROR, PRR, and corresponding 95% CI was also calculated for the entire class of AEDs versus all other medications. All analyses were conducted using OpenEpi (Version 3.01) which calculates Taylor series 95% CIs.21
Table 1:
Table of antiepileptic medications evaluated
| Antiepileptic Drugs | |
|---|---|
| Acetazolamide | Lamotrigine |
| Amobarbital | Levetiracetam |
| Brivaracetam | Lorazepam |
| Carbamazepine | Methsuximide |
| Clobazam | Oxcarbazepine |
| Clonazepam | Pentobarbital |
| Clorazepate | Perampanel |
| Diazepam | Phenobarbital |
| Divalproex sodium | Phenytoin |
| Eslicarbazepine | Pregabalin |
| Ethosuximide | Primidone |
| Ethotoin | Rufinamide |
| Ezogabine | Tiagabine |
| Felbamate | Topiramate |
| Fosphenytoin | Valproic acid/ valproate sodium |
| Gabapentin | Vigabatrin |
| Lacosamide | Zonisamide |
Results
During the study period, there were 2,886,886 total adverse reactions of any type reported with 1,035 (0.04%) SJS/TEN reactions (690 SJS, 66.7%; 345 TEN, 33.3%). There were 76,251 (2.6%) adverse event reports, of any kind, which included AEDs. Of these reports with AEDs, 198 SJS/TEN reactions were reported (Table 2). Among the SJS/TEN reactions, AEDs were the most common medications listed (19.1%) and which was more than 2 times higher than NSAIDs, with the second most SJS/TEN reactions (n=80, 7.7%). Of the 34 different AEDS evaluated, there were 17 AEDs (50.0%) that had at least one SJS/TEN reaction. The AEDs with the most reported SJS/TEN reactions were lamotrigine with 106 reactions (53.5%), carbamazepine with 22 reactions (11.1%), levetiracetam with 14 reactions (7.1%), phenytoin with 14 reactions (7.1%), valproic acid with 9 reactions (4.5%), clonazepam with 8 reactions (4.0%), and zonisamide with 7 reactions (3.5%). AEDs as a class had a ROR of 8.7 (95% CI 7.5–10.2) and a PRR of 8.7 (95% CI 7.5–10.2) compared with all other medications (Table 2, Figure 1, Figure 2). There were six AEDs with ROR/PRR risk estimates for SJS/TEN exceeding 20: zonisamide with a ROR of 70.2 (95% CI 33.1–148.7) and a PRR of 68.7 (95% CI 32.9–143.5), rufinamide with a ROR of 60.0 (95% CI 8.3–433.5) and a PRR of 58.9 (95% CI 8.4–411.5), clorazepate with a ROR of 56.0 (95% CI 7.8–404.1) and a PRR of 55.1 (95% CI 7.8–385.0), lamotrigine with a ROR of 53.0 (95% CI 43.2–64.9) and a PRR of 52.2 (95% CI 42.7–63.7), phenytoin with a ROR of 26.3 (95% CI 15.5–44.7) and a PRR of 26.1 (95% CI 15.4–44.2), and carbamazepine with a ROR of 24.5 (95% CI 16.0–37.5) and a PRR of 24.3 (95% CI 16.0–37.1). Significant associations were also observed for valproic acid, eslicarbazepine, oxcarbazepine, clonazepam, and levetiracetam (see Table 2, Figure 1, Figure 2).
Table 2:
Adverse event report counts and risk estimates of Stevens-Johnson syndrome (SJS) / toxic epidermal necrolysis (TEN) for antiepileptic medications
| Generic | Total reports |
SJS | TEN | Total SJS/ TEN |
ROR | 95% CI | PRR | 95% CI |
|---|---|---|---|---|---|---|---|---|
| All antiepileptic medications* | 76,251 | 140 | 58 | 198 | 8.7 | 7.5–10.2 | 8.7 | 7.5–10.2 |
| Carbamazepine* | 3,038 | 13 | 9 | 22 | 24.5 | 16.0–37.5 | 24.3 | 16.0–37.1 |
| Clonazepam* | 3,896 | 7 | 1 | 8 | 6.9 | 3.4–13.9 | 6.9 | 3.4–13.8 |
| Clorazepate* | 61 | 0 | 1 | 1 | 56.0 | 7.8–404.1 | 55.1 | 7.9–385.0 |
| Diazepam | 2,895 | 1 | 0 | 1 | 1.2 | 0.2–8.3 | 1.2 | 0.2–8.2 |
| Divalproex | 2,585 | 1 | 0 | 1 | 1.3 | 0.2–9.2 | 1.3 | 0.2–9.2 |
| Eslicarbazepine* | 338 | 0 | 1 | 1 | 10.0 | 1.4–71.0 | 9.9 | 1.4–70.4 |
| Gabapentin | 10,949 | 2 | 2 | 4 | 1.2 | 0.5–3.3 | 1.2 | 0.5–3.3 |
| Lacosamide | 1,497 | 0 | 1 | 1 | 2.2 | 0.3–16.0 | 2.2 | 0.3–15.9 |
| Lamotrigine* | 6,826 | 81 | 25 | 106 | 53.0 | 43.2–64.9 | 52.2 | 42.7–63.7 |
| Levetiracetam* | 7,102 | 8 | 6 | 14 | 6.6 | 3.9–11.3 | 6.6 | 3.9–11.2 |
| Lorazepam | 2,282 | 1 | 0 | 1 | 1.5 | 0.2–10.5 | 1.5 | 0.2–10.5 |
| Oxcarbazepine* | 1,116 | 3 | 0 | 3 | 9.1 | 2.9–28.2 | 9.0 | 2.9–28.0 |
| Phenytoin* | 1,801 | 11 | 3 | 14 | 26.3 | 15.5–44.7 | 26.1 | 15.4–44.2 |
| Pregabalin | 19,029 | 3 | 1 | 4 | 0.7 | 0.3–1.9 | 0.7 | 0.3–1.9 |
| Rufinamide* | 57 | 1 | 0 | 1 | 60.0 | 8.3–433.5 | 58.9 | 8.4–411.5 |
| Valproic Acid* | 2,891 | 4 | 5 | 9 | 10.5 | 5.4–20.2 | 10.5 | 5.4–20.1 |
| Zonisamide* | 342 | 4 | 3 | 7 | 70.2 | 33.1–148.7 | 68.7 | 32.9–143.5 |
Estimates that are statistically significant
Reporting odds ratios (ROR) and proportional reporting ratios (PRR) were calculated for each drug compared with all other non-antiepileptic medications.
Figure 1:
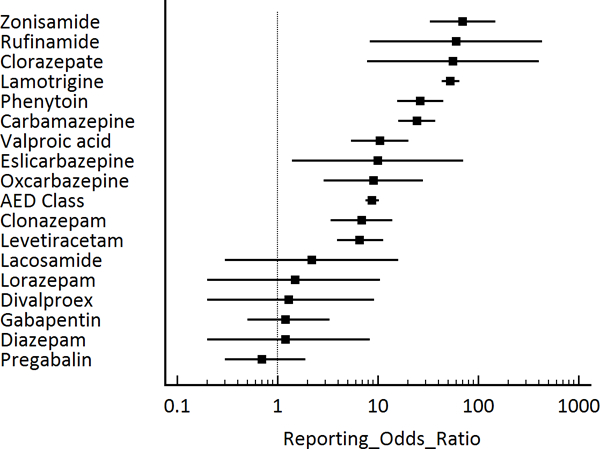
Antiepileptic medications and Stevens-Johnson syndrome (SJS) / toxic epidermal necrolysis (TEN), reporting odds ratios
Figure 2:
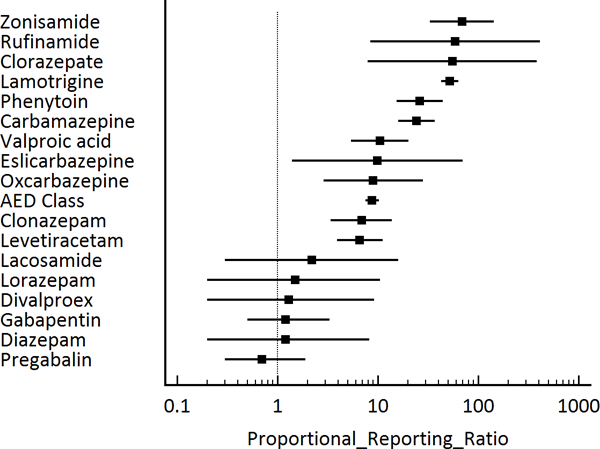
Antiepileptic medications and Stevens-Johnson syndrome (SJS) / toxic epidermal necrolysis (TEN), proportional reporting ratios
Discussion
The results of study show that SJS/TEN reporting rates were significantly higher with AEDs compared to all other medications (non-AEDs). In our study, zonisamide, rufinamide, clorazepate, lamotrigine, phenytoin, and carbamazepine demonstrated the highest increased risk of SJS/TEN. We identified 17 AEDs with reported SJS/TEN reactions, and significant associations were observed with 11 of these AEDs. While most listed SJS/TEN as possible adverse reactions in the package inserts, four did not (clonazepam, clorazepate, diazepam, and lorazepam).15–17, 22–35
Several European studies have explored the most common medications associated with SJS/TEN. One matched case-control study conducted in the United Kingdom, evaluated the risk of SJS/TEN specifically among new AED users.18 This study was conducted using the Clinical Practice Research Datalink (CPRD) and included validated SJS/TEN cases from 1995 to 2013. Each case of SJS/TEN was matched to 4 patients with same birth year, sex, general practice, and years of recorded history. Due to small numbers, the confidence intervals were wide, however, even at the low end of the confidence interval, the odds of exposure among cases was 5–20 times higher than control group, carbamazepine 92.57 (95% CI 19.89−∞), phenytoin 49.96 (95% CI 10.13−∞), and lamotrigine 26.90 (95% CI 4.88−∞).
The European case-control surveillance of severe cutaneous adverse reactions (EuroSCAR) study identified hospitalized cases of SJS/TEN and matched hospital controls from April 1997 to December 2001.19 The study population consisted of patients from six countries (Austria, Italy, France, Germany, Netherlands, and Israel) among a network of 1,800 hospitals. For each case, patients were matched on age, sex, region, and date of interview to 3 hospital control patients. Odds ratios were adjusted for potential confounding factors, specifically concomitant medications that may affect the risk of SJS/TEN, as well as other medical conditions which may affect the risk of SJS/TEN, such as HIV/AIDS or recent cancer. They found that three of the top seven medications associated with SJS/TEN were AEDs: carbamazepine (adjusted OR [aOR] 72, 95% CI 23–225), phenobarbital (aOR 16, 95% CI 5–50), and phenytoin (aOR 17, 95% CI 4–68).
Our findings were similar to CPRD and EuroSCAR studies, although the magnitude of risk for carbamazepine was lower in our study (ROR 25 versus OR 93 in CPRD and aOR 72 in EuroSCAR). This may be explained by the difference in study time periods and implementation of genetic screening prior to initiating carbamazepine among those of Asian ancestry. While the CPRD and EuroSCAR studies demonstrated lower risk with lamotrigine compared to the other AED comparators, in our study, the ROR for lamotrigine was 53.0 (95% CI 43.2–64.9), more than 2-fold that of phenytoin (26.3, 95% CI 15.5–44.7) and carbamazepine (24.5, 95% CI 16.0–37.5). In our study, there were no reports of SJS/TEN reaction with phenobarbital. One possible reason for this is the declining utilization of phenobarbital which has become a nonpreferred therapy option in treatment guidelines due to (1) its less favorable side effect profile, (2) inclusion in the Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, (3) unfavorable pharmacokinetic properties, and (4) multiple drug-drug interactions.36–38
In two studies which utilized data from adverse event reporting systems, AEDs were among the top medications associated with SJS/TEN.39, 40 Lamotrigine and carbamazepine were among the top five most common medications listed in reports for SJS/TEN in the Japanese Adverse Drug Event Report database over a 12-year period from April 2004 through April 2015.39 Adjusted RORs were calculated for reporting year, gender, and age. The RORs were 5.7 (95% CI 5.2–6.3) and 11.5 (95% CI 10.3–12.8) for carbamazepine and lamotrigine, respectively. In a letter to the editor by the same authors, they describe a similar analysis of US FAERS data. From January 2004 to March 2013, two AEDs were among the top medications listed in reports of SJS/TEN (lamotrigine and phenytoin).40 The RORs were 20.4 (95% CI 19.2–21.8) and 12.0 (95% CI 11.3–12.7) for phenytoin and lamotrigine, respectively. As compared with our study, the risk associated with these AEDs were substantially lower than what we observed during a shorter and more recent time period (RORs, lamotrigine 53.0 versus 11.5 and 12.0, carbamazepine 24.5 versus 5.7, phenytoin 26.3 versus 20.4; time period, 2014–2017 versus 2004–2013/2015).
Greater than 90 percent of SJS/TEN reactions associated with AEDs occur within the first 2 months of treatment initiation,41 although some AEDs have been associated with such reactions during long term use (phenobarbital and valproic acid).1 Previous studies have indicated that SJS/TEN reactions may be associated with the starting dose and rate of titration for some AEDs.42, 43 An approach to preventing cutaneous reactions such as SJS/TEN in AEDs, is to use a low starting dose and slow titration schedule, as has been used with lamotrigine.44 Further research is needed to see which AEDs pose the highest risk for SJS/TEN, based on dose and titration schedule to determine whether lower starting doses and slow titration schedules would decrease occurrences of SJS/TEN.
There were several limitations to our study. Incidence and prevalence cannot be calculated as the total number of patients using these medications is undetermined.45 Due to the voluntary nature of reporting to FAERS, underreporting is expected.46 A reporting bias that may have influenced the number of reactions reported is the Weber effect,47 in which adverse event reporting starts to decline in the 2 years after market introduction.48 All of the AEDs included in our study with reports of SJS/TEN have been on the market for greater than two years with the exception of eslicarbazepine, and therefore decreases in reporting likely occurred over time. Another known limitation of the FAERS database is that causality between the adverse event and medication does not have to be established in order for a report to be submitted, which may be further complicated by concomitant medications and patient comorbidities.46 Additionally, drugs with frequent side effects can increase the number of overall adverse effects and therefore decrease ROR and PRR.49 This may have occurred in our study since the two medications with the most adverse reaction reports (pregabalin and gabapentin) were tied for the eighth most SJS/TEN reactions but had two of the three lowest RORs and PRRs. Whereas, two of the three highest RORs and PRRs were among medications with only one SJS/TEN reaction (rufinamide and clorazepate) and they had the two lowest total adverse reaction reports. As there was only one report of SJS/TEN with clorazepate and rufinamide, the confidence intervals of the ROR and PRR were large and though statistically significant, the magnitude of the lower end of the confidence interval was closer to that of the overall class. The magnitude and statistical significance of these associations may change as more adverse event reports are submitted for clorazepate and rufinamide.
Conclusion
Our study supports previous evidence that certain AEDs are associated with a higher risk of SJS/TEN, in particular, lamotrigine, phenytoin, and carbamazepine. Further, we identified 8 other AEDs significantly associated with SJS/TEN: zonisamide, rufinamide, clorazepate, valproic acid, eslicarbazepine, oxcarbazepine, clonazepam, and levetiracetam. While AEDs as a class were associated with 9 times the risk of SJS/TEN compared with non-AEDs, there were six AEDs with risk estimates greater than 20. Increased awareness of this risk among both prescribers and patients, particularly variations in risk among different AEDs, along with education on early recognition of SJS/TEN signs/symptoms may help mitigate the number and severity of these adverse events.
Key Point Box:
While several antiepileptic drugs (AEDs) have been associated with (SJS) and toxic epidermal necrolysis (TEN), the class effect and impact of other AEDs are not well-described.
We sought to quantify the risk of SJS/TEN with AEDs using the Food and Drug Administration Adverse Event Reporting System (FAERS).
For AEDs as a class, the reporting odds ratio for SJS/TEN was 8.7 (95% confidence interval 7.5–10.2), respectively, compared to non-AEDs.
A third of AEDs assessed (11/34) were associated with a significantly increased risk of SJS/TEN (zonisamide, rufinamide, clorazepate, lamotrigine, phenytoin, carbamazepine, valproic acid, eslicarbazepine, oxcarbazepine, clonazepam, and levetiracetam).
Significance:
While AEDs as a class were associated with 9 times the risk of SJS/TEN compared with non-AEDs, there were six AEDs with risk estimates greater than 20. Increased awareness of this risk among both prescribers and patients, particularly variations in risk among different AEDs, along with education on early recognition of SJS/TEN signs/symptoms may help mitigate the number and severity of these adverse events.
Acknowledgements
Contents of this study were presented as a poster presentation and a podium presentation at the Academy of Managed Care Pharmacy (AMCP) 2017 Nexus; October 16–19, 2017; Grapevine, Texas, where it received the platinum ribbon award for best professionally peer reviewed abstract. The views expressed are those of the authors and do not necessarily reflect the position or policy of the United States Department of Veterans Affairs. This material is based upon work supported, in part, by the Office of Research and Development, Department of Veterans Affairs.
Footnotes
Declarations of interest
Aisling Caffrey has received research funding from Pfizer, Merck (Cubist), and The Medicines Company. There are no other conflicts to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Roujeau JC, Kelly JP, Naldi L, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995; 333: 1600–7. [DOI] [PubMed] [Google Scholar]
- 2.Levi N, Bastuji-Garin S, Mockenhaupt M, et al. Medications as risk factors of Stevens-Johnson syndrome and toxic epidermal necrolysis in children: a pooled analysis. Pediatrics. 2009; 123: e297–304. [DOI] [PubMed] [Google Scholar]
- 3.Raucci U, Rossi R, Da Cas R, et al. ; Italian Multicenter Study Group For Vaccine Safety In Drug And Children. Stevens-Johnson syndrome associated with drugs and vaccines in children: a case-control study. PLoS One. 2013; 8: e68231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Letko E, Papaliodis DN, Papaliodis GN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of the literature. Ann Allergy Asthma Immunol. 2005; 94: 419–36. [DOI] [PubMed] [Google Scholar]
- 5.Chan HL, Stern RS, Arndt KA, et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol 1990; 126: 43–7. [PubMed] [Google Scholar]
- 6.Yang M-S, Lee JY, Kim J, et al. Incidence of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Nationwide Population-Based Study Using National Health Insurance Database in Korea. Picardo M, ed. PloS ONE. 2016; 11: e0165933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong A, Malvestiti AA, Hafner Mde F. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review. Rev Assoc Med Bras (1992). 2016; 62: 468–73. [DOI] [PubMed] [Google Scholar]
- 8.Hsu DY, Brieva J, Silverberg NB, et al. Morbidity and Mortality of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in United States Adults. J Invest Dermatol. 2016;136: 1387–1397. [DOI] [PubMed] [Google Scholar]
- 9.Sekula P, Dunant A, Mockenhaupt M, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol 2013; 133: 1197–204. [DOI] [PubMed] [Google Scholar]
- 10.Yang CW, Cho YT, Chen KL, et al. Long-term Sequelae of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Acta Derm Venereol. 2016; 96: 525–9. [DOI] [PubMed] [Google Scholar]
- 11.Lee HY, Walsh SA, Creamer D. Long-term complications of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): the spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow-up. Br J Dermatol. 2017; 177: 924–935. [DOI] [PubMed] [Google Scholar]
- 12.Sander JW. The epidemiology of epilepsy revisted. Curr Opin Neurol. 2003; 16: 165–170. [DOI] [PubMed] [Google Scholar]
- 13.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993; 34: 453–68. [DOI] [PubMed] [Google Scholar]
- 14.Mula M Investigating psychotropic properties of antiepileptic drugs. Expert Rev Neurother. 2013; 13: 639–46. [DOI] [PubMed] [Google Scholar]
- 15.Carbamazepine [package insert]. Teva Pharmaceuticals USA Inc., North Wales, PA; 2015. [Google Scholar]
- 16.Lamotrigine [package insert]. Jubilant Cadista Pharmaceuticals Inc., Salisbury, MD; 2017. [Google Scholar]
- 17.Oxcarbazepine [package insert]. Taro Pharmaceutics U.S.A. Inc., Hawthorne, NY; 2014. [Google Scholar]
- 18.Frey N, Bodmer M, Bircher A, et al. The risk of Stevens-Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptic drugs. Epilepsia. 2017; 58: 2178–2185. [DOI] [PubMed] [Google Scholar]
- 19.Halevy S, Ghislain PD, Mockenhaupt M, et al. ; EuroSCAR Study Group. Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J Am Acad Dermatol. 2008; 58: 25–32. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration. FDA adverse event reporting system (FAERS). U.S. 162 Food and Drug Administration, Silver Spring (MD); 2017. 163 http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/Adve164rseDrugEffects/default.htm. Accessed May 26, 2017 [Google Scholar]
- 21.Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version. www.OpenEpi.com, updated 2013/04/06. Accessed June 21, 2018.
- 22.Clonazepam [package insert]. Accord Healthcare Inc., Durham, NC; 2017. [Google Scholar]
- 23.Clorazepate dipotassium [package insert]. Ranbaxy Pharmaceuticals Inc., Jacksonville, FL; 2012. [Google Scholar]
- 24.Diazepam [package insert]. Mylan Pharmaceuticals Inc., Morgantown, WV; 2018. [Google Scholar]
- 25.Divalproex sodium [package insert]. Mylan Pharmaceuticals Inc., Morgantown, WV; 2017. [Google Scholar]
- 26.Aptiom (eslicarbazepine acetate) [package insert]. Sunovion Pharmaceuticals Inc., Marlborough, MA; 2017. [Google Scholar]
- 27.Gabapentin [package insert]. Greenstone LLC, Peapack, NJ; 2017. [Google Scholar]
- 28.Lacosamide [package insert]. Breckenridge Pharmaceuticals Inc., Boca Raton, FL; 2016. [Google Scholar]
- 29.Levetiracetam [package insert]. Lupin Pharmaceuticals Inc., Baltimore, MD; 2017. [Google Scholar]
- 30.Lorazepam [package insert]. Mylan Pharmaceuticals Inc., Morgantown, WV; 2017. [Google Scholar]
- 31.Phenytoin sodium [package insert]. Mylan Pharmaceuticals Inc., Morgantown, WV; 2015. [Google Scholar]
- 32.Lyrica (pregabalin) [package insert]. Parke-Davis Div of Pfizer Inc., New York, NY; 2017. [Google Scholar]
- 33.Banzel (Rufinamide) [package insert]. Eisai Inc., Woodcliff Lake, NJ; 2015. [Google Scholar]
- 34.Valproic acid [package insert]. Upsher-Smith Laboratories Inc., Maple Grove, MN; 2017. [Google Scholar]
- 35.Zonisamide [package insert]. Camber Pharmaceuticals, Piscataway, NJ; 2012. [Google Scholar]
- 36.Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: Management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015; 84: 1705–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015; 63: 2227–46. [DOI] [PubMed] [Google Scholar]
- 38.Marsot A, Brevaut-Malaty V, Vialet R, et al. Pharmacokinetics and absolute bioavailability of phenobarbital in neonates and young infants, a population pharmacokinetic modelling approach. Fundam Clin Pharmacol. 2014; 28: 465–71. [DOI] [PubMed] [Google Scholar]
- 39.Abe J, Umetsu R, Mataki K, et al. Analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis using the Japanese Adverse Drug Event Report database. J Pharm Health Care Sci. 2016; 2: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abe J, Mataki K, Umetsu R, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: the Food and Drug Administration adverse event reporting system, 2004–2013. Allergol Int. 2015; 64: 277–9. [DOI] [PubMed] [Google Scholar]
- 41.Trivedi BS, Darji NH, Malhotra SD, et al. Antiepileptic Drugs-induced Stevens–Johnson syndrome: A case Series. J Basic Clin Pharm. 2016; 8: 42–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chadwick D, Shaw MD, Foy P, et al. Serum anticonvulsant concentrations and the risk of drug induced skin eruptions. J Neurol Neurosurg Psychiatry. 1984; 47: 642–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Culy CR, Goa KL. Lamotrigine. A review of its use in childhood epilepsy. Paediatr Drugs. 2000; 2: 299–330. [DOI] [PubMed] [Google Scholar]
- 44.Messenheimer JA, Mullens EL, Giorgi L. Safety review of adult clinical trial experience with lamotrigine. Drug Saf. 1998; 18: 281–296. [DOI] [PubMed] [Google Scholar]
- 45.Sakaeda T, Tamon A, Kadoyama K, et al. Data mining of the public version of the FDA Adverse Event Reporting System. Int J Med Sci. 2013; 10: 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta AK, Carviel J, MacLeod MA, et al. Assessing finasteride-associated sexual dysfunction using the FAERS database. J Eur Acad Dermatol Venereol. 2017; 31: 1069–1075. [DOI] [PubMed] [Google Scholar]
- 47.McAdams MA, Governale LA, Swartz L, et al. Identifying patterns of adverse event reporting for four members of the angiotensin II receptor blockers class of drugs: revisiting the Weber effect. Pharmacoepidemiol Drug Saf. 2008; 17: 882–889. [DOI] [PubMed] [Google Scholar]
- 48.Hartnell NR, Wilson JP. Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy. 2004; 24: 743–749. [DOI] [PubMed] [Google Scholar]
- 49.Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001; 10: 483–6. [DOI] [PubMed] [Google Scholar]


