Abstract
Opioids were one of the earliest classes of medications used for pain across a variety of conditions, but morbidity and mortality have been increasingly associated with their chronic use. Despite these negative consequences, chronic opioid use is increasing worldwide, with the USA and Canada having the highest rates. Chronic opioid use for noncancer pain can have particularly negative effects in the gastrointestinal and central nervous systems, including opioid-induced constipation, narcotic bowel syndrome, worsening psychopathology and addiction. This Review summarizes the evidence of opioid misuse in gastroenterology, including the lack of evidence of a benefit from these drugs, as well as the risk of harm and negative consequences of opioid use relative to the brain–gut axis. Guidelines for opioid management and alternative pharmacological and nonpharmacological strategies for pain management in patients with gastrointestinal disorders are also discussed. As chronic pain is complex and involves emotional and social factors, a multimodal approach targeting both pain intensity and quality of life is best.
Prescription opioid use has become a global epidemic. Between 2011 and 2013, opioid use more than doubled worldwide, with substantial increases occurring in North America, Europe and Oceania1. Despite the increasing trends worldwide, opioid consumption rates per capita in the USA and Canada far outpace those in other countries2 (FIG. 1). One reason cited for this difference is that, in the rest of the world, opioids are not as frequently prescribed for chronic pain syndromes as they are in North America3. Similar trends are occurring in the UK4,5, and its rates of opioid consumption in 2010 are comparable to rates at the inception (1999) of the opioid crisis in the USA6. Postulated reasons for lower opioid consumption in countries with national health systems, which are publicly funded, are that doctors have less incentive to over-prescribe and that prescription records are stored centrally, enabling patient misuse to be detected more quickly7. The notable rise in opioid prescribing over the past 15 years in the USA relates in part to the report by The Joint Commission in 2000, which indicated that pain was inadequately treated and better pain management was needed8. Notably, the commission provided little guidance on how best to achieve pain relief and did not recommend that the approach should involve increased use of opioids. In addition, opioids are recommended treatments on the WHO’s three step ‘analgesic ladder’ for cancer pain9; however, this guideline has also been widely applied for managing chronic noncancer pain10.
Figure 1. Trends in total opioid consumption by country between 1994 and 2014.
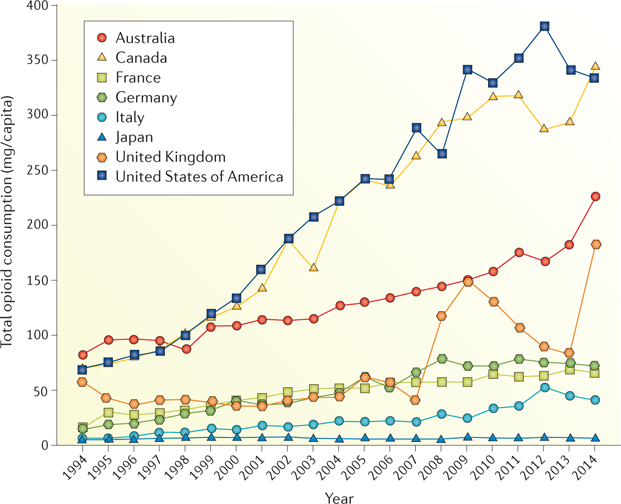
Graph indicates trends in total opioid consumption (mg per capita) per year among some of the world’s most industrialized countries. Figure adapted with permission from REF. 205, Pain & Policy Studies Group, University of Wisconsin–Madison.
Although debate about the effectiveness of opioids in treating chronic noncancer pain exists, opioid misuse and overdose-related deaths continue to increase11–13. In 2014, >240 million opioid prescriptions were written14, and nearly 19,000 deaths related to opioid prescriptions were reported in the US — a 3.4-fold increase in deaths since 2001 (REF. 15). In addition to mortality, chronic use of opioids is associated with serious medical morbidities, including addiction, and increased related medical resource utilization16–19. For example, in the USA, there was a nearly 300% increase in opioid-related emergency department visits between 2004 and 2011 (REF. 20), and >520,000 hospitalizations related to opioid abuse and dependence were reported in 201217. Australia also had a 2.4-fold increase in opioid-related hospitalizations between 1998 and 2009 (REF. 21).
In 2010, 15.5 million people had opioid dependence globally, with the highest rates in North America (292.1 per 100,000), Eastern Europe (288.4 per 100,000) and Australia (278.6 per 100,000)22. In China, although heroin addiction remains the largest opioid problem, polydrug use, including prescription opioids, is on the rise23. In the US, the prevalence of opioid dependence in patients receiving prescription opioids is as high as 26% in primary care24,25, the estimated rate of opioid misuse is 21–29%, and the rate of addiction is 8–12%18. The estimated rates of substance use disorder in patients with chronic pain seen in pain clinics are as high as 45% in the US26,27. In addition, nearly 53% of patients receiving treatment for chronic pain in two pain centres in Israel were found to have problematic opioid use28.
Chronic opioid use can be particularly detrimental to the gastrointestinal tract and central nervous system, particularly in patients with pre-existing gastrointestinal conditions19,29. As opioids are prescribed predominantly in primary care and pain clinics30,31, gastroenterologists often receive referral patients who are already on chronic opioid therapies. In fact, although gastroenterologists prescribe only 0.3% of opioids, the prevalence of pain is higher in individuals with gastrointestinal disorders than in the general public32–34. Estimates of chronic opioid use have ranged from 13% to nearly 50% in adults with IBD33,35,36 and as high as 70% in hospitalized adult patients with IBD37. Rates of opioid use have been reported at >50% in patients with chronic pancreatitis38 and at almost 20% in patients with IBS39. Among patients with chronic nonalcoholic pancreatitis, 39% had high scores on opioid misuse measures, with depressive symptoms, pain ratings, alcohol use and psychological quality of life being notable predictors40. Furthermore, prescription opioid use has been associated with increased hospitalization in patients with chronic pancreatitis41 and with increased risk for infection in patients with Crohn’s disease36.
As opioid use and misuse are continuing to gain recognition as a global problem, increased awareness of opioid-induced gastrointestinal problems is necessary 42,43. This Review summarizes the evidence of opioid misuse in gastroenterology, including the lack of evidence for a benefit from opioids and the risk of harm and negative consequences of opioid use relative to the brain–gut axis. Strategies to prevent opioid misuse, the treatment of pain for patients already on opioids and alternative intervention strategies for pain management in patients with gastrointestinal disorders are also discussed.
Opioids for gastrointestinal disorders
The evidence supporting the effectiveness of opioids for short-term use (<3 months) for chronic noncancer pain is largely focused on musculoskeletal pain44. Evidence for the efficacy of opioids for gastrointestinal pain is lacking. In chronic pancreatitis, one small randomized study assessed short-term (5 days) use of tramadol versus morphine and found that 67% of the tramadol group, compared with 20% of the morphine group, reported analgesia as “excellent” (REF. 45). There is a lack of randomized controlled trials showing the efficacy of opioids for chronic abdominal pain; additionally, gastrointestinal disease has been identified as a major cause of oral opioid ineffectiveness due to malabsorption46. Furthermore, existing evidence underscores concerns related to problematic opioid use in individuals with gastrointestinal disorders. In a study of chronic opioid users with Crohn’s disease, 37% of patients with a concurrent functional gastrointestinal disorder (FGID) diagnosis were identified to be misusing opioids, compared with 9.6% of those without an FGID diagnosis47. The increased rate of opioid misuse in these patients might be explained by the presence of prior psychiatric diagnoses37 and/or as part of a dissociative coping style for those patients with prior trauma history — a factor that has been independently linked to increased rates of IBS48. Additionally, a large epidemiological study indicates that ~5% of patients with IBD will become heavy users of opioids within 10 years after diagnosis49. This finding is of particular concern as the risk for addiction in patients with noncancer pain increases with higher dosing (>120 mg morphine equivalent) and chronic opioid therapy50.
Opioid-induced gastrointestinal effects
Substantial evidence links opioid use to compounding and deleterious gastrointestinal-related adverse effects, collectively known as opioid-induced bowel dysfunction (OIBD). Among the most common opioid-induced gastrointestinal effects are constipation, nausea, abdominal pain or discomfort, gas, ileus, gall bladder contraction and gastro-oesophageal reflux51,52. In addition, in a large observational study among 6,273 patients with Crohn’s disease, opioid use was significantly (P < 0.001) associated with serious infections and was also a significant predictor of death36. In a study of 2,055 patients taking opioids for noncancer pain, 57% reported constipation, 13% reported nausea, 11% reported abdominal pain and 10% reported increased gas as the most bothersome opioid-associated symptoms51. Opioid-induced constipation (OIC) is the most frequently reported gastrointestinal adverse effect, with estimates ranging from 15–90% of individuals taking opioids53,54. For instance, opioid users seeking care for abdominal pain in the emergency department were three times more likely than non-users to have constipation55. In addition, among 489 patients with chronic noncancer pain, OIC had a negative effect on quality of life, particularly on work performance and productivity (38%), performing activities of daily living (49%), social interactions (45%), sex lives (45%) and the ability to leave the house (43%)56. Other gastrointestinal symptoms reported with chronic opioid use are GERD (33%)19 and opioid-induced oesophageal dysfunction57. In pooled data from two randomized controlled trials evaluating teduglutide (a glucagon-like peptide-2 analogue) in short-bowel syndrome (n = 136), patients taking opioids (n = 52) had significantly (P = 0.0009) greater adverse gastrointestinal effects than those who were not (51% versus 21% for abdominal pain, 42% versus 11% for nausea, 17% versus 8% for abdominal distension and 19% versus 6% for vomiting)58.
The mechanisms underlying OIBD are complex but are primarily attributed to the high density of opioid receptors (μ-type, κ-type, δ-type) within the gastrointestinal tract, which, when activated, result in decreased motility and secretion59,60 (FIG. 2). The effects of opioids on gastrointestinal motility (for example, delayed gastric emptying and slowing of intestinal transit) are associated predominantly with the activation of μ-opioid receptors in neurons of the enteric nervous system via cellular second-messenger systems such as cyclic AMP, chloride and potassium channels and protein kinases29,61. Opioid receptor antagonists can modulate both excitatory and inhibitory neural inputs in the gastrointestinal tract62. This interaction involves neurotransmitters such as acetylcholine, vasoactive intestinal peptide (VIP), nitric oxide (NO) and 5-hydroxytryptamine (serotonin)60. Inhibition of excitatory pathways, such as those involving acetylcholine or substance P, leads to the blockade of distention-induced peristaltic contractions, whereas suppression of inhibitory pathways such as NO signalling results in disinhibition of smooth muscle activity, elevation of muscle tone and nonpropulsive motility (for example, spasms)60,63. Activation of δ-opioid receptors might also be involved in decreased gastrointestinal motility by altering pyloric tone29.
Figure 2. Summary of opioid-induced effects within the gastrointestinal system.

Opioid-induced adverse effects in the gastrointestinal system are primarily attributed to the activation of opioid receptors (μ-type, κ-type, δ-type) within the enteric nervous system, particularly in smooth muscle cells and in the terminals of sympathetic and sensory peripheral neurons in the gastrointestinal tract60. Consequences (symptoms) associated with opioid use include nausea, vomiting, constipation, abdominal distention, spasms and/or gastro-oesophageal reflux.
Gastrointestinal secretion is predominantly mediated by the three types of opioid receptors located in the gut wall60. Opioids activate the sympathetic nervous system and inhibit acetylcholine and VIP activity, resulting in reduced secretion60. Opioids also reduce epithelial secretion and promote the absorption of electrolytes and water, worsening constipation and abdominal distress or pain64. Morphine administration has been associated with increased sphincter contraction and reduction in the emptying of pancreatic juice and bile, causing delayed digestion65,66. However, the effects of opioids on μ-opioid receptors in the lower oesophageal sphincter significantly (P < 0.05) decrease motility and pressure, resulting in oesophageal reflux67,68. Collectively, these opioid-induced gastrointestinal effects lead to nausea, vomiting, delayed gastric emptying and constipation. In addition, sphincter of Oddi dysfunction via μ-opioid receptor stimulation might also result in pancreatitis, as reported in studies testing the mixed opioid drug eluxadoline in patients with IBS69,70.
Opioid use can also have deleterious effects for patients with liver diseases such as cirrhosis. Opioids are predominantly metabolized in the liver via oxidation, reduction or hydrolysis by the cytochrome P450 system (for example, CYP2D6, CYP2B6, CYP3A4); glucuronidation; or biliary excretion and elimination71. In a process known as the first-pass effect, orally ingested opioids, such as codeine, oxycodone and tramadol, are absorbed by the gastrointestinal tract and pass through the portal vein to undergo metabolism in the liver before the active metabolites of these medications reach the systemic circulation72. Hepatic dysfunction can result in reduced clearance of the drug from the blood or plasma and increased bioavailability and accumulation of the opioid73. A study in patients with cirrhosis who received an oral dose of morphine found that these patients had a mean elimination half-life of 5.5 h, compared with a mean of 3.3 h in a healthy control group (P < 0.05)74. The use of opioids in patients with liver disease can lead to, or aggravate, hepatic encephalopathy owing to the accumulation of opioid metabolites in the central nervous system72,75.
Opioid tolerance, defined as the state of adaptation in which exposure to a drug induces changes that result in diminution of one or more of the drug’s effects over time44, can develop at different rates for different opioid-related effects. For instance, it develops quickly for effects such as nausea, sedation and cognitive impairment, whereas other effects such as constipation can occur much more slowly76,77. There are also differences in tolerance between central and peripheral targets. For example, a lack of tolerance in the colon leads to opioid-induced chronic constipation, in contrast to more proximal regions of the gut and also the brain, in which adverse effects such as nausea, vomiting and sedation improve over time60. The rate of development of analgesic tolerance to opioids has not been as definitively determined, although some studies suggest that it might not be a pervasive problem in long-term opioid use for chronic pain78.
Opioid-induced constipation.
OIC is characterized as a change in baseline bowel habits, such as reduced bowel movement frequency, development or worsening of straining during bowel movements, a sense of incomplete rectal evacuation and/or a harder stool consistency79. Involvement of other areas of the gastrointestinal tract causing small bowel ileus or gastroparesis is part of the wider spectrum of OIBD, although the mechanism is the same as that of OIC29. The motility effects of opioids are mainly caused by μ-opioid receptors, which are found throughout the gastrointestinal tract, whereas μ-opioid receptors in the brain and spinal cord are involved in analgesia29. In OIC, the activation of μ-opioid receptors leads to reductions in gastric emptying, propulsive contractions of the intestines and secretions from the intestines, pancreas and gallbladder79. OIC is also partially mediated by changes in water absorption owing to the effects of opioids on chloride channels80. These processes, together with increased pyloric and anal sphincter tone, lead to the symptoms of constipation.
Narcotic bowel syndrome.
A growing number of studies report persistent abdominal pain in patients with chronic opioid use19,51. A specific type of persistent, yet under-recognized, abdominal pain termed narcotic bowel syndrome (NBS) has been identified in 6.4% of patients in the US with chronic opioid use19. NBS is defined as chronic or frequently recurring abdominal pain treated with high-dose opioids81, and diagnostic criteria for clinical research are now available82. The nature or intensity of pain is not entirely due to diagnosis of a gastrointestinal disease, such as IBD or chronic pancreatitis, and even with pain resulting from another underlying diagnosis, there is an opioid-induced amplification of the pain83. To meet the diagnostic criteria for NBS, a patient must have had at least two of the following symptoms for the past 3 months with symptom onset at least 6 months before diagnosis: pain worsens or incompletely resolves with escalating doses of narcotics; there is a marked worsening of pain when the narcotic dose wanes and improvement when narcotics are re-initiated; and there is a progression of the frequency, duration and intensity of the pain episodes84. In addition to visceral pain that worsens with long-term or increasing doses of opioids, the hallmark symptom of NBS, other symptoms can include nausea, periodic vomiting, abdominal distension and constipation83,84. The inclusion of NBS in the Rome IV diagnostic criteria of FGIDs as a centrally mediated abdominal pain disorder85 will increase its visibility among clinicians who manage these conditions.
The diagnosis of NBS can also be complicated by other physiological processes. For example, NBS, as a painful disorder, needs to be differentiated from OIC, which is characterized by bowel dysfunction, although pain might also be present. NBS is mediated by effects of opioids on the central nervous system resulting in central hyperalgesia, whereas OIC is caused by the peripheral effects of opioids on μ-opioid receptors affecting motility of the gastrointestinal tract83. Abdominal pain can also worsen during physiological withdrawal from opioids, but in this case, the pain occurs together with other symptoms of withdrawal such as lacrimation, yawning, rhinorrhoea, sweating, restlessness and diarrhoea86. Tolerance to opioids leading to escalating dosing is a concern but is not itself linked to worsening pain86.
The paradoxical increase in pain associated with opioid use that characterizes NBS has been explained through several putative mechanisms (FIG. 3), including inflammatory glial cell activation in the dorsal horn81,87, activation of excitatory pathways within a bimodal opioid regulation system81,88 and the descending facilitation of pain at the level of the rostral medulla along with pain facilitation via dynorphin or cholecystokinin activation81,89. These mechanisms have been described in detail elsewhere81–84,90. Evidence suggests that chronic opioid use has compounding effects on glial cell activation and induction of visceral hyperalgesic effects81. Opioids bind directly to the μ-opioid receptors, which activate glia through the release of pro-inflammatory cytokines that bind to glial cell receptors, subsequently potentiating hyperalgesia91. In addition, opioids have been shown to have an indirect effect on glial cell activation in rats through the release of dynorphin within the spinal cord92. Furthermore, findings from a rat model of NBS supported morphine-induced spinal microglial activation caused by peripheral neuroimmune activation, and spinal dynorphin release mediated visceral hyperalgesia93. The activation of glial cells through Toll-like receptors (specifically, TLR4) has also been shown to mediate neuropathic pain and other deleterious opioid effects such as hyperalgesia, tolerance and physical dependence94–96.
Figure 3. Putative mechanisms for narcotic bowel syndrome and other models of opioid-induced hyperalgesia.
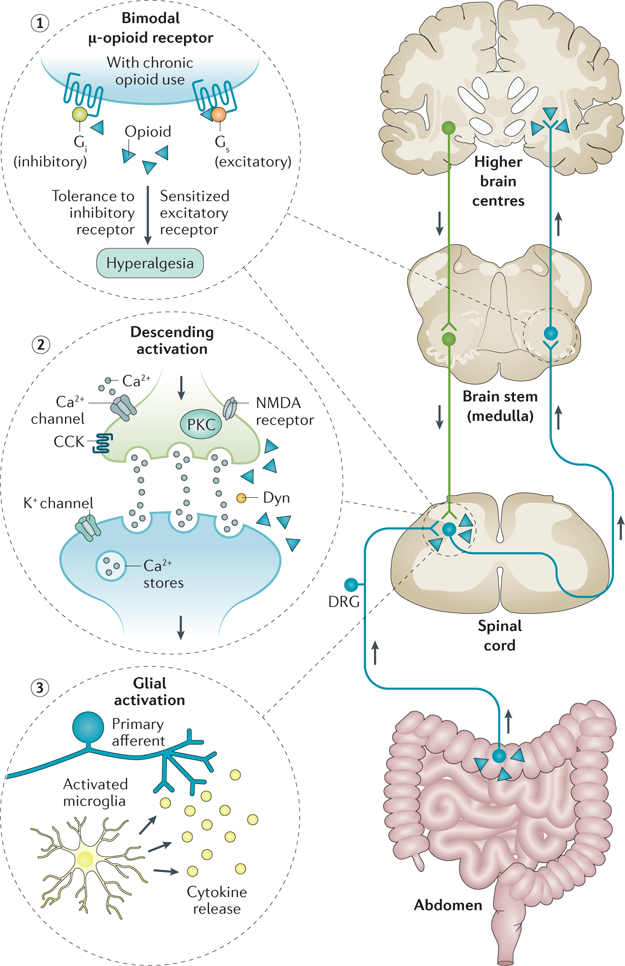
The bimodal opioid receptor switches from an inhibitory to an excitatory G protein state with chronic opioid exposure (1). Activation of descending pain pathways is mediated by several mechanisms including cholecystokinin (CCK) and release of dynorphin (Dyn), activation of calcium (Ca2+) and potassium (K+) channel-mediated membrane hyperexcitability, and protein kinase C (PKC)-induced increase in presynaptic N-methyl-D-aspartate (NMDA) receptor activation. On the presynaptic side, opioids paradoxically activate ‘on cells’ projecting from the rostral ventromedial medulla leading to increased dynorphin release. Opioids also activate NMDA receptors, which in turn can cause an influx in calcium and potassium through activated channels leading to increased PKC. These mechanisms sensitize the postsynaptic neuron to pain (2). Opioids induce the immune-system-related glial cells to release pro-inflammatory cytokines into dorsal horn of the spinal cord (3). DRG, dorsal root ganglion.
The bimodal opioid regulation system encompasses the excitatory and inhibitory effects on sensory neurons caused by opioids. This system is mediated by the stimulatory G protein-coupled receptors within the dorsal root ganglia97. Although opioids are generally considered to have inhibitory effects on afferent neurons and their signalling, evidence also suggests that chronic opioid use can induce excitatory effects (mediated by glutamate) on stimulatory G protein-coupled receptors, causing tolerance and hyperalgesia81,88,97.
Prevention and treatment considerations
Patients with chronic pain who request opioids pose an ethical dilemma to clinicians who strive to find a balance between adequate pain relief and the risks of misuse and abuse of opioids. The basic ethical obligation of providers is to acquire current knowledge, skills and tools to assist in making prescribing decisions and uphold the ethical principles of beneficence, nonmaleficence, autonomy and justice98. In the absence of strong research evidence supporting the chronic use of opioids for abdominal pain, it is critical that prescribers use objective factors to conduct risk–benefit analyses99,100 and not succumb to pressures to prescribe opioids only to increase patient satisfaction metrics101,102. One study in 2016 revealed that emergency room physicians perceived pressure to prescribe opioids to avoid administrative criticism and poor patient satisfaction103. Primary prevention is the most effective way to control the opioid epidemic104. However, should opioid use be warranted, for example, in patients in which the therapeutic benefit outweighs the many risks, appropriate steps need to be taken to mitigate the risks associated with opioids and ensure that they are not used as a first-line approach for treatment of any chronic noncancer pain. An algorithm for cautious opioid prescribing has been previously published100, with key points highlighted in BOX 1. For patients who are already on opioids, recommended measures include repetitive monitoring of the risk–benefit ratio, optimizing non-opioid pain management strategies and tapering patients to the lowest possible opioid dose.
Box 1. Risk mitigation when prescribing opioids.
Screen for opioid misuse by using available validated measures and avoid using opioids or taper the dose for patients with untreated substance abuse and/or dependence.
Monitor appropriate use of opioids by using state or country prescription drug monitoring programmes and random toxicology screens.
Form a treatment contract with patients that includes informed consent about risks versus benefits, such as the risk of overdose, addiction or serious adverse effects and increased risk of death.
Discuss alternative treatment options with patients.
Work within a multidisciplinary team (for example, physicians, nurses, mental health counsellors or addiction specialists and social workers) with resources for treating addiction and comorbid mental conditions.
Provide access to naloxone as a rescue medication.
Provide the lowest possible dose of opioids, avoid ‘as-needed’ dosing and evaluate the risk–benefit ratio at least every 3 months. Doses >80 mg per day of morphine equivalent are best avoided owing to increased risks of adverse events.
Taper long-acting opioids when risks are greater than benefits, and supplement the taper with non-opioid analgesics and behavioural interventions.
Avoid concomitant use of benzodiazepines owing to increased risks of overdose and synergistic adverse effects.
Provide careful documentation in the medical record.
Opioid detoxification.
As a first step in evaluating a pain treatment plan that includes opioids, an objective analysis of the risk–benefit ratio of chronic opioid prescription should be performed, and whenever possible, an opioid detoxification process to taper narcotic use should be considered. Published protocols outlining algorithms that use non-opioid strategies can help with chronic abdominal pain. Drossman et al.105 demonstrated good short-term success with detoxification in patients with NBS, suggesting an opioid taper schedule of 10–33% reduction per day of morphine equivalent and attention to treating associated anxiety and withdrawal. During the opioid taper, short-term benzodiazepines for anxiety and agents such as clonidine for withdrawal symptoms were used105. This strategy should be used specifically for the purpose of detoxification, especially given an FDA warning in 2016 against prescribing opioids and benzodiazepines together on a chronic basis owing to synergistic brain-related adverse effects and an increased risk of mortality106. The protocol105 also follows the wisdom for the use of off-label non-opioid pain medication substitutions such as tricyclic antidepressants (TCAs) or serotonin–noradrenaline reuptake inhibitors (SNRIs) to manage chronic abdominal pain107,108. Gabapentin, a GABA analogue anticonvulsant, has shown promise in reducing opioid-induced hyperalgesia in patients with chronic pain by reducing central sensitization109. Preoperative gabapentin administration has been shown to significantly (P < 0.001) reduce postoperative opioid consumption in a meta-analysis of 1,793 patients who underwent abdominal, genitourinary, orthopaedic or thoracic surgery110. In a study of experimentally induced opioid withdrawal, co-administration of palonosetron (a 5-hydroxytryptamine receptor 3 (5-HT3) antagonist) with hydroxyzine reduced the severity of withdrawal111. In addition, a Cochrane review published in 2017 identified buprenorphine as more effective than alternatives (for example, clonidine or lofexidine) in terms of severity of withdrawal, duration of treatment and likelihood of completing treatments112.
Although developed for patients with NBS, this detoxification approach can be useful for moving from opioid to non-opioid pain management strategies for other patients with chronic abdominal pain, such as those with IBS, IBD or chronic pancreatitis. TCAs can improve the global symptoms of IBS113, and the noradrenergic modulatory action of TCAs might have anti-inflammatory effects114 and might also inhibit TLR4 and TLR2 activation94. These TCA-linked pathways might also be beneficial for patients with IBD115. In a double-blind placebo-controlled trial of patients with IBD, the SNRI duloxetine was more efficacious than placebo in reducing depression, anxiety and the severity of gastrointestinal symptoms and chronic pain116. In addition, a study evaluating the effects of antidepressant therapies on global symptoms in patients with functional dyspepsia found that a TCA (amitriptyline) significantly reduced dyspepsia symptoms, whereas the selective serotonin reuptake inhibitor (SSRI) escitalopram did not117. Although SSRIs have not been shown to directly reduce abdominal pain, they can have an indirect effect by reducing associated anxiety and depression118,119. Serotonergic psychoactive agents can affect other aspects of gastrointestinal functioning, such as accelerating small bowel transit, enhancing gastric accommodation, increasing colonic compliance and reducing sensations of distension120.
Alternative pharmacological treatments.
Off-label medications such as mirtazapine, a tetracyclic antidepressant, can be prescribed as an alternative, or in addition, to TCAs or SNRIs, particularly if there is persistent sleep disturbance or nausea. Low-dose quetiapine, an antipsychotic, has also been recommended as an adjunct medication for pain in patients with refractory FGIDs but requires monitoring for possible adverse effects, such as metabolic syndrome, sedation and involuntary movements121. Anticonvulsant medications such as gabapentin and pregabalin have shown efficacy in reducing pain in patients with pancreatitis, IBS or IBD, mediated via effects in the brain122–126. Several review articles published elsewhere cover the risks and benefits of using other psychotropic agents for the management of abdominal pain81,87,114,127,128. TABLE 1 summarizes the empirical evidence for the use of psychotropic agents for chronic abdominal pain113,116,129–133; however, the findings of these meta-analyses should be interpreted in light of several general limitations, including overlap of included studies, small sample sizes, diversity of medications within each drug class, lack of uniformity of study endpoints and substantial heterogeneity among the studies analysed. In clinical studies, antispasmodics, peppermint oil, 5-HT3 receptor antagonists and mixed opioid agents have also demonstrated efficacy for the treatment of pain and other symptoms in IBS69,134.
Table 1.
Evidence for antidepressant medication efficacy for treating abdominal pain
| Study (design) | Number of studies (number of participants) | Medication versus comparison groups | Duration of therapy (weeks) | Selected outcome assessment | Summary of evidence of efficacy |
|---|---|---|---|---|---|
| TCAs | |||||
| Ford et al.129 (meta-analysis) | 11 RCTs (744 patients with IBS) | TCAs (amitriptyline, desipramine, doxepin, imipramine, nortriptyline, trimipramine) versus placebo | 4–12 | Global IBS symptoms or abdominal pain |
|
| Ruepert et al.130 (meta-analysis) | 4 RCTs (320 patients with IBS) | TCAs (amitriptyline, desipramine, doxepin) versus placebo | 4–12 | Abdominal pain (dichotomous outcome) | Subgroup analyses of abdominal pain showed significant benefit from TCAs (RR 1.26, 95% CI 1.03–1.55) |
| Xie et al.113 (meta-analysis) | 5 RCTs (428 patients with IBS) | TCAs (amitriptyline, desipramine, imipramine, trimipramine) versus placebo | 4–12 | Global symptom relief, abdominal pain | Treatment with TCAs was associated with an improvement in global symptoms (RR 1.36, 95% CI 1.07–1.71) |
| SSRIs | |||||
| Ford et al.129 (meta-analysis) | 7 RCTs (356 patients with IBS) | SSRIs (citalopram, fluoxetine, imipramine, paroxetine) versus placebo | 6–12 | Global IBS symptoms or abdominal pain |
|
| Ruepert et al.130 (meta-analysis) | 4 RCTs (197 patients with IBS) | SSRIs (citalopram, fluoxetine, paroxetine) versus placebo | 6–12 | Abdominal pain | Subgroup analyses for abdominal pain showed no benefit from SSRIs (RR 2.29, 95% CI 0.79–6.68) |
| Xie et al.113 (meta-analysis) | 6 RCTs (371 patients with IBS) | Citalopram, fluoxetine, paroxetine versus placebo | 6–12 | Global symptom relief, abdominal pain | SSRIs produced no difference in improvement of symptoms compared with placebo (RR = 1.38, 95% CI 0.83–2.28) and produced no improvement in abdominal pain or quality of life |
| SNRIs | |||||
| Brennan et al.131 (open-label pilot study) | NA (14 patients with IBS) | Duloxetine* | 12 | CGI, IBS symptoms, anxiety, quality of life, disability | Duloxetine was associated with significantly improved quality of life and significantly reduced pain, severity of illness, loose stool, disability and anxiety (all P < 0.05) |
| Daghaghzadeh et al.116 (blind RCT) | NA (35 patients with IBD) | Duloxetine versus placebo | 12 | Anxiety, depression, colitis activity, quality of life |
|
| Kaplan et al.132 (Open-label pilot study) | NA (13 patients with GAD and IBS) | Duloxetine* | 12 | CGI, IBS symptoms, anxiety | Significant improvements in symptom severity (P < 0.001), anxiety (P < 0.01), and CGI improvement (P < 0.001) and severity scales (P < 0.001) |
| Lewis-Fernández et al.133 (open-label pilot study) | NA (17 patients with current MDD and IBS) | Duloxetine* | 12 | CGI, IBS symptoms, depression |
|
CGI, Clinical Global Impression scale; GAD, general anxiety disorder; MDD, major depressive disorder; NA, not applicable; RCT, randomized controlled trial; RR, relative risk; SNRI, serotonin–noradrenaline reuptake inhibitors; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
No comparison group.
In terms of determining the best strategy for treating abdominal pain in each unique patient, previous exposure to psychotropic medications, drug allergies or sensitivities and the presence of comorbid medical and psychiatric conditions need to be considered. In addition, psychological drivers of pain (for example, the unconscious meaning of pain, secondary gain, poor cognitive flexibility, catastrophizing and fear-based attentional bias) can be important measurable confounders of other pain reduction strategies if not addressed as part of a comprehensive treatment plan (see BOX 2 for definitions of these psychological terms)135,136.
Box 2. Definitions of psychological confounders of opioid management.
Cognitive flexibility: The ability to adapt and apply a broad range of coping strategies to face new or unexpected challenges.
Catastrophizing: A cognitive distortion or irrational thought that something is much worse than it actually is such that imagined worst-case scenarios are treated as inevitable.
Fear-based attentional bias: The differential attention towards one stimulus class (for example, fear of pain) over another (neutral emotion) such that this selective processing of threatening information leads to hypervigilance and avoidance behaviour instead of adaptive coping or acceptance.
Unconscious meaning of pain: A process by which an unbearable or unacceptable emotion (for example, rage) is converted to a more acceptable physical symptom; this process occurs outside of conscious awareness as a solution to avoid inner conflict.
Secondary gain: The advantage that occurs in different domains (for example, increased attention from others, disability benefits, or being excused from responsibilities) owing to a real or stated illness.
The management of OIC is also best achieved by stopping opioid use. Osmotic-type laxatives such as polyethylene glycol are most commonly used79. Alternatives to laxatives include more prolonged-release formulations that contain naloxone (an opioid antagonist), such as oxynal, which have also shown some benefit for improving bowel function137. Peripherally acting μ-opioid receptor antagonists (PAMORAs), including agents such as methylnaltrexone, alvimopan and naloxegol (NKTR-118), are efficacious for treating patients with more persistent OIC138,139. These agents selectively bind to μ-opioid receptors in the gastrointestinal tract to block the peripheral effects of opioids while sparing the central opioid receptors that contribute to the analgesic effects140. Newer laxatives such as lubiprostone and prucalopride have shown efficacy in treating OIC. Lubiprostone activates chloride channel protein 2 in gastrointestinal epithelial cells, resulting in the secretion of chloride and ultimately softened stool consistency141. Prucalopride alters motility by activating selective 5-HT4 receptors in the gut and has been shown to be effective in treating both chronic and opioid-induced constipation142,143. Other agents under development to treat OIC include the PAMORA naldemedine and the guanylate cyclase receptor agonist linaclotide144. Recommendations on initiating prescription therapies for OIC have previously been published and include the use of validated assessment tools to determine when treatments for OIC should be initiated145. Tools to assess for OIC, such as the Bowel Function Index146 and the Patient Assessment of Constipation Symptoms147, include the evaluation of patient-reported bowel symptoms (for example, incomplete evacuation, straining, stool consistency and ease of defecation).
Nonpharmacological treatments for pain.
In addition to non-opioid pharmacological management of pain, there is a growing body of literature supporting behavioural interventions as part of a comprehensive treatment plan for chronic abdominal pain, even when opioids are not involved148. In fact, there is a large body of literature supporting the use of various psychosocial interventions, including cognitive behavioural therapy (CBT) or mindfulness training, for other types of chronic pain149–151. Several meta-analyses of randomized controlled trials targeting pain in IBS showed that antidepressants and behavioural interventions such as CBT outperformed placebo and standard medical treatments129,152–155. Cognitive behavioural interventions in addition to usual care also show promise in treating chronic pain in patients with IBD156,157. However, pain in IBD studies is often measured as an item on another scale (for example, quality of life), and optimal efficacy of CBT has been hampered by study design issues and high attrition rates158. Other behavioural interventions, such as hypnosis and mindfulness meditation, have demonstrated efficacy in reducing psychiatric morbidity and chronic abdominal pain and improving quality of life across different gastrointestinal conditions (see TABLE 2 for a summary of meta-analyses evaluating the efficacy of behavioural interventions for chronic abdominal pain and associated symptoms)159–168.
Table 2.
Sample of meta-analyses evaluating behavioural interventions for abdominal pain and/or global gastrointestinal symptoms
| Study (design) | Number of studies included (number of participants) | Behavioural interventions included | Outcomes of interest | Summary of evidence for behavioural interventions |
|---|---|---|---|---|
| Altayar et al.154 (meta-analysis) | 15 RCTs (1,352 adults with IBS) | CBT, psychoeducational courses, mind–body therapy, psychodynamic interpersonal therapy and contingency management | IBS symptom severity, abdominal pain and quality of life |
|
| Aucoin et al.161 (meta-analysis) | 7 RCTs for FGIDs (592 participants with an FGID) | Mindfulness-based therapies | IBS symptom severity and quality of life |
|
| Laird et al.153 (meta-analysis) | 41 RCTs (2,290 adults with IBS) | CBT, mindfulness, relaxation, hypnosis and emotional awareness training | GI symptom severity |
|
| Lee et al.159 (meta-analysis) | 7 RCTs (374 adults with IBS) | Hypnotherapy | Abdominal pain, constipation and diarrhoea |
|
CBT, cognitive behavioural therapy; FGID, functional gastrointestinal disorder; GI, gastrointestinal; RCT, randomized controlled trial; SMD, standardized mean difference.
Behavioural interventions in patients with IBS appear to have their strongest effects on visceral pain via modulation of mood (anxiety, depression)169. Negative emotional states and stress have been shown to alter gut physiology, motility and sensitivity to nociceptive stimuli170. Neuroplastic changes in emotional and cognitive brain centres are known to amplify pain signals from the viscera148,171. The fear–avoidance model of chronic pain has the most support in explaining how behavioural interventions can alter multiple aspects of central processing of pain, such as the sensory–discriminative and motivational–affective domains (discussed in detail elsewhere148). These therapy-conditioned responses in the brain can influence the gastrointestinal tract by multiple pathways such as the autonomic nervous system, the hypothalamic–pituitary–adrenal stress axis, the immune system and the microbiome172.
Many barriers to patients receiving nonpharmacological pain management therapies still exist, including geographical distance, shortage of behavioural intervention providers and long waiting lists173. The availability and demonstrated benefits of scripted hypnosis protocols can make this treatment feasible by trained medical staff 174. The development of effective virtual behavioural therapy options (web-based approaches) to reduce pain has helped with the access problem, with patients now able to receive the adequate level of psychotherapy in the comfort of their homes, which is also associated with long-term efficacy175–177.
A strong physician–patient relationship to facilitate management of pain and other medical conditions is critical, and this relationship should include good communication, empathic engagement and addressing problems and treatment as a dyadic team178–181. This approach is particularly important as patients with pain often expect a quick fix without understanding the long-term consequences of opioid therapies180,182. Working in a setting in which a broader team (nurses, social workers, behavioural specialists) is involved can help patients learn new coping strategies to reduce pain perception and help them understand the limitations associated with chronic opioid use. For managing people with gastrointestinal disorders, different models of integrated behavioural medical care and transdisciplinary treatment might be more effective than collaborative or integrated models, in which team members from different disciplines might be co-located but still practising in their particular niche183.
Psychopathological considerations.
Psychopathology, especially mood and anxiety disorders, is commonly associated with opioid use. In a study of 34,653 people, nonmedical opioid use was associated with mood disorders, major depressive disorder, bipolar disorder and anxiety disorders184. Psychiatric comorbidity is also associated with reduced opioid analgesia and increased opioid misuse185. Depressive symptoms have been reported to moderate the relationship between pain levels and opioid misuse186. Depression and anxiety were identified to mediate the relationship between catastrophizing and opioid misuse in another chronic pain population187.
There can be serious centrally mediated consequences of opioids188, as opioid receptors are also found in the brain. These receptors also bind endogenous opioids (endorphins) that are involved in pain perception and modulation of reward mechanisms and mood (euphoria)188. These positive effects of opioids can be reinforcing, leading to repeated administration, which, in vulnerable individuals, becomes addiction. These risk factors for addiction include a biological predisposition for opioid-induced craving, loss of control or compulsive use189. It is important to distinguish between physical dependence (withdrawal syndrome following abrupt dose reduction or administration of an antagonist) and addiction (loss of control over use, continued use despite adverse consequences, preoccupation with obtaining and using the drug)18.
An area that warrants attention is that relapse rates for patients with chronic pain who have been tapered from opioids are high and require long-term treatment algorithms to be developed105. One under-recognized cause of such relapse is opioid addiction. In a small study evaluating detoxification in NBS, the patients who had high relapse rates were those who had high opioid misuse scores, indicating that those addicted to opioids need greater intervention105. For patients who show signs of opioid addiction (problematic behaviours consistent with craving, loss of control or compulsive use), it is necessary to refer them to appropriate substance abuse clinics where they can have access to agonists (methadone or partial agonists such as buprenorphine) and appropriate counselling. In 2015, transdiagnostic and integrative therapies such as Acceptance and Commitment Therapy were found to be more efficacious than traditional CBT or 12-step programmes for patients with substance abuse disorders190. Opioid addiction can also lead to intravenous drug abuse191, increasing the risks of contracting HIV and hepatitis C192,193.
For treatment-refractory patients with chronic pain and addiction, several nonpharmacological biological interventions are in development. These options include peripheral nerve stimulation, as well as brain stimulation procedures such as transcranial magnetic stimulation, transcranial direct current stimulation, high-definition transcranial direct current stimulation and deep brain stimulation194–198. All of these approaches are thought to influence the modulation of pain in the peripheral or central nervous system by inhibiting the transmission of pain signals194,196. For example, published case reports have indicated the subcutaneous placement of peripheral nerve stimulator electrodes in the location or dermatome associated with chronic abdominal pain improved pain severity and function and decreased the use of pain medications194. The risk–benefit ratio of these approaches for patients with abdominal pain has yet to be determined, and large controlled trials are needed197,198.
A small group of patients do benefit from chronic opioid use for pain management without developing tolerance, hyperalgesia or addiction78. For this group of patients, it is essential that prescribing physicians follow the legal recommendations in their state-run or national prescription drug monitoring programmes (PDMP), forming drug contracts with patients, including random toxicology screens, adhering to careful documentation in the electronic health records, using naloxone to treat opioid overdoses and continuing to explore non-opioid options with their patients. Several validated instruments for evaluating behaviours related to opioid misuse are available, including the Current Opioid Misuse Measure199 and the Screener and Opioid Assessment for Patients with Pain200, among others. Furthermore, almost all states in the USA have authorized PDMPs, and over half are permitted to share data with other state PDMPs201. In addition, Australia is seeking to establish a national PDMP202. PDMPs enable prescribers to assess aberrant behaviours (for example, ‘doctor shopping’) and have been associated with reductions in opioid prescribing201. The European Monitoring Centre for Drugs and Drug Addiction monitors trends in drug use for countries in the European Union and provides resources related to harm reduction interventions for drug use203,204.
Conclusions
Opioid misuse is a global epidemic and has led to substantial increases in opioid-related abuse and mortality. Given the lack of evidence supporting the effectiveness of opioids for managing chronic pain, as well as the debilitating effects of those drugs on both the gastrointestinal and central nervous systems, alternative approaches to pain management in patients with gastrointestinal conditions must be employed. Alternatives to prescribing opioids in this population should include non-opioid pharmacological agents, behavioural interventions, strong physician–patient relationships and transdisciplinary team approaches. This Review underscores the need for additional research (BOX 3) on non-opioid pharmacological and nonpharmacological interventions, as well as different formulations and/or delivery mechanisms of opioids for treating chronic abdominal pain in patients diagnosed with gastrointestinal disorders to reduce pain intensity, improve quality of life and avoid addiction.
Box 3. Outstanding research questions for managing chronic abdominal pain.
What are the optimal non-opioid pharmacological agents to treat abdominal pain in patients with gastrointestinal disorders?
What psychosocial, behavioural or self-management interventions are most beneficial for treating chronic abdominal pain?
Are peripheral nerve stimulation and brain stimulation procedures (for example, transcranial magnetic stimulation, transcranial direct current stimulation, high-definition transcranial direct current stimulation and deep brain stimulation) effective in treating chronic abdominal pain?
For patients already on opioids, what are the best approaches to achieve opioid detoxification? Specifically, how should opioids be tapered, and what adjuvant medications can be prescribed to aid in preventing symptoms of withdrawal?
How can opioid addiction be better prevented and treated?
Can different formulations of opioids or different delivery systems be developed that enable persistent analgesic effects without the development of dangerous side effects or addiction?
Key points.
Prescription opioid use is a global epidemic, with substantial increases in opioid-related morbidity and mortality around the world
There is a lack of evidence supporting the use of opioids for the management of chronic abdominal pain
Opioid use can have deleterious consequences on the gastrointestinal tract, including opioid-induced constipation and narcotic bowel syndrome
Many promising non-opioid pharmacological and nonpharmacological alternatives for treating abdominal pain exist; however, additional research is needed to identify best practices for treating abdominal pain in individuals with gastrointestinal disorders
If opioids are prescribed, it is essential to have strategies to monitor and manage opioid misuse, continually monitor risk–benefit clinical profiles, and prevent and treat addiction
Acknowledgements
M.K. is supported by NIH Award Number T32NR009759.
Footnotes
Competing interests
E.S. is a consultant for AbbVie and has received royalties from American Psychiatric Association Publishing, grant support from the NIH, the Crohn’s & Colitis Foundation and the Bruce and Cynthia Sherman Foundation, and honoraria for educational talks for Imedex and the American Academy of Child and Adolescent Psychiatry. D.D. is President of the Rome Foundation and has been on advisory boards for Allergan, AstraZeneca, Ironwood and Shionogi in the past year.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Berterame S et al. Use of and barriers to access to opioid analgesics: a worldwide, regional, and national study. Lancet 387, 1644–1656 (2016). [DOI] [PubMed] [Google Scholar]
- 2.University of Wisconsin–Madison Pain & Policy Studies Group. Opioid consumption data Pain & Policy Studies Group; http://www.painpolicy.wisc.edu/opioid-consumption-data (2017). [Google Scholar]
- 3.Denisco RA, Chandler RK & Compton WM Addressing the intersecting problems of opioid misuse and chronic pain treatment. Exp. Clin. Psychopharmacol 16, 417–428 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zin CS, Chen LC & Knaggs RD Changes in trends and pattern of strong opioid prescribing in primary care. Eur. J. Pain 18, 1343–1351 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruscitto A, Smith BH & Guthrie B Changes in opioid and other analgesic use 1995–2010: repeated cross-sectional analysis of dispensed prescribing for a large geographical population in Scotland. Eur. J. Pain 19, 59–66 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Weisberg DF, Becker WC, Fiellin DA & Stannard C Prescription opioid misuse in the United States and the United Kingdom: cautionary lessons. Int. J. Drug Policy 25, 1124–1130 (2014). [DOI] [PubMed] [Google Scholar]
- 7.The Economist. The problem of pain The Economist http://www.economist.com/news/international/21699363-americans-are-increasingly-addicted-opioids-meanwhile-people-poor-countries-die (2016).
- 8.Berry PH & Dahl JL The new JCAHO pain standards: implications for pain management nurses. Pain Manag. Nurs 1, 3–12 (2000). [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. WHO’s cancer pain ladder for adults World Health Organization; http://www.who.int/cancer/palliative/painladder/en/ (2016). [Google Scholar]
- 10.Manubay J, Muchow C & Sullivan M Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim. Care 38, 71–90 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang HY, Daubresse M, Kruszewski SP & Alexander GC Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am. J. Emerg. Med 32, 421–431 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Daubresse M et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000–2010. Med. Care 51, 870–878 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudd RA, Aleshire N, Zibbell JE & Gladden RM Increases in drug and opioid overdose deaths — United States, 2000–2014. MMWR Morb. Mortal. Wkly Rep 64, 1378–1382 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Department of Health and Human Services, USA. The opioid epidemic: by the numbers HHS.gov; http://www.hhs.gov/sites/default/files/Factsheet-opioids-061516.pdf (2016). [Google Scholar]
- 15.National Institute on Drug Abuse. Overdose death rates National Institute on Drug Abuse; https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates (2015). [Google Scholar]
- 16.Inocencio TJ, Carroll NV, Read EJ & Holdford DA The economic burden of opioid-related poisoning in the United States. Pain Med 14, 1534–1547 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Ronan MV & Herzig SJ Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002–2012. Health Aff 35, 832–837 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vowles KE et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 156, 569–576 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Tuteja AK, Biskupiak J, Stoddard GJ & Lipman AG Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol. Motil 22, 424–e96 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Substance Abuse and Mental Health Services Administration (SAMHSA). National estimates of drug-related emergency department visits, 2004–2011 Substance Abuse and Mental Health Services Administration (SAMHSA) http://www.samhsa.gov/data/emergency-department-data-dawn/reports (2014). [Google Scholar]
- 21.Blanch B, Pearson SA & Haber PS An overview of the patterns of prescription opioid use, costs and related harms in Australia. Br. J. Clin. Pharmacol 78, 1159–1166 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Degenhardt L et al. The global epidemiology and burden of opioid dependence: results from the global burden of disease 2010 study. Addiction 109, 1320–1333 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Marienfeld C Heroin addiction, methadone, and HIV in China. Lancet Psychiatry 3, 799–800 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Boscarino JA et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction 105, 1776–1782 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Fleming MF, Balousek SL, Klessig CL, Mundt MP & Brown DD Substance use disorders in a primary care sample receiving daily opioid therapy. J. Pain 8, 573–582 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz NP et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth. Analg 97, 1097–1102 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Passik SD, Kirsh KL, Donaghy KB & Portenoy RK Pain and aberrant drug-related behaviors in medically ill patients with and without histories of substance abuse. Clin. J. Pain 22, 173–181 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Feingold D, Goor-Aryeh I, Bril S, Delayahu Y & Lev-Ran S Problematic use of prescription opioids and medicinal cannabis among patients suffering from chronic pain. Pain Med 18, 294–306 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Hughes PA, Costello SP, Bryant RV & Andrews JM Opioidergic effects on enteric and sensory nerves in the lower GI tract; basic mechanisms and clinical implications. Am. J. Physiol. Gastrointest. Liver Physiol 311, G501–G513 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Levy B, Paulozzi L, Mack KA & Jones CM Trends in opioid analgesic-prescribing rates by specialty, U. S., 2007–2012. Am. J. Prev. Med 49, 409–413 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.IMS Institute for Healthcare Informatics. Medicines used and spending in the U. S.: A review of 2015 and outlook to 2020 (IMS Institute for Healthcare Informatics, 2016). [Google Scholar]
- 32.Bharucha AE, Chakraborty S & Sletten CD Common functional gastroenterological disorders associated with abdominal pain. Mayo Clin. Proc 91, 1118–1132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buckley JP, Kappelman MD, Allen JK, Van Meter SA & Cook SF The burden of comedication among patients with inflammatory bowel disease. Inflamm. Bowel Dis 19, 2725–2736 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Buckley JP, Cook SF, Allen JK & Kappelman MD Prevalence of chronic narcotic use among children with inflammatory bowel disease. Clin. Gastroenterol. Hepatol 13, 310–315.e2 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Cross RK, Wilson KT & Binion DG Narcotic use in patients with Crohn’s disease. Am. J. Gastroenterol 100, 2225–2229 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Lichtenstein GR et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT registry. Am. J. Gastroenterol 107, 1409–1422 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long MD, Barnes EL, Herfarth HH & Drossman DA Narcotic use for inflammatory bowel disease and risk factors during hospitalization. Inflamm. Bowel Dis 18, 869–876 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nusrat S, Yadav D & Bielefeldt K Pain and opioid use in chronic pancreatitis. Pancreas 41, 264–270 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Dorn S et al. Patients with IBS commonly use narcotics [abstract]. Gastroenterology 138 (Suppl. 1), W1378 (2010). [Google Scholar]
- 40.Barth KS et al. Screening for current opioid misuse and associated risk factors among patients with chronic nonalcoholic pancreatitis pain. Pain Med 15, 1359–1364 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olesen SS, Poulsen JL, Broberg MC, Madzak A & Drewes AM Opioid treatment sand hypoalbuminemia are associated with increased hospitalisation rates in chronic pancreatitis outpatients. Pancreatology 16, 807–813 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Morley KI, Ferris JA, Winstock AR & Lynskey MT Polysubstance use and misuse or abuse of prescription opioid analgesics: a multi-level analysis of international data. Pain 158, 1138–1144 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Sharma A & Jamal MM Opioid induced bowel disease: a twenty-first century physicians’ dilemma. Considering pathophysiology and treatment strategies. Curr. Gastroenterol. Rep 15, 334 (2013). [DOI] [PubMed] [Google Scholar]
- 44.American Pain Society & American Academy of Pain Medicine. Guideline for the use of chronic opioid therapy in chronic noncancer pain: evidence review (American Pain Society, 2009). [Google Scholar]
- 45.Wilder-Smith CH, Hill L, Osler W & O’Keefe S Effect of tramadol and morphine on pain and gastrointestinal motor function in patients with chronic pancreatitis. Dig. Dis. Sci 44, 1107–1116 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Tennant F Why oral opioids may not be effective in a subset of chronic pain patients. Postgrad. Med 128, 18–22 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Crocker JA, Yu H, Conaway M, Tuskey AG & Behm BW Narcotic use and misuse in Crohn’s disease. Inflamm. Bowel Dis 20, 2234–2238 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Surdea-Blaga T, Baban A & Dumitrascu D Psychosocial determinants of irritable bowel syndrome. World J. Gastroenterol 18, 616–626 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Targownik LE, Nugent Z, Singh H, Bugden S & Bernstein CN The prevalence and predictors of opioid use in inflammatory bowel disease: a population-based analysis. Am. J. Gastroenterol 109, 1613–1620 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Edlund MJ et al. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin. J. Pain 30, 557–564 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cook SF et al. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment. Pharmacol. Ther 27, 1224–1232 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Kraichely RE, Arora AS & Murray JA Opiate-induced oesophageal dysmotility. Aliment. Pharmacol. Ther 31, 601–606 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaertner J et al. Definitions and outcome measures of clinical trials regarding opioid-induced constipation: a systematic review. J. Clin. Gastroenterol 49, 9–16 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Mearin F et al. Bowel disorders. Gastroenterology 150, 1393–1407.e5 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Khemani D et al. Opioid analgesic use among patients presenting with acute abdominal pain and factors associated with surgical diagnoses. Neurogastroenterol. Motil 29, e13000 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rauck RL, Hong KJ & North J Opioid-induced constipation survey in patients with chronic noncancer pain. Pain Pract 17, 329–335 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Ratuapli SK et al. Opioid-induced esophageal dysfunction (OIED) in patients on chronic opioids. Am. J. Gastroenterol 110, 979–984 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Fujioka K et al. Patients with short bowel on narcotics during 2 randomized trials have abdominal complaints independent of teduglutide. JPEN J. Parenter. Enteral. Nutr 10.1177/0148607116663481 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leppert W The impact of opioid analgesics on the gastrointestinal tract function and the current management possibilities. Contemp. Oncol 16, 125–131 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sobczak M, Sałaga M, Storr MA & Fichna J Physiology, signaling, and pharmacology of opioid receptors and their ligands in the gastrointestinal tract: current concepts and future perspectives. J. Gastroenterol 49, 24–45 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galligan JJ & Akbarali HI Molecular physiology of enteric opioid receptors. Am. J. Gastroenterol. Suppl 2, 17–21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holzer P New approaches to the treatment of opioid-induced constipation. Eur. Rev. Med. Pharmacol. Sci 12 (Suppl. 1), 119–127 (2008). [PMC free article] [PubMed] [Google Scholar]
- 63.Browning KN & Travagli RA Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol 4, 1339–1368 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barrett KE New insights into the pathogenesis of intestinal dysfunction: secretory diarrhea and cystic fibrosis. World J. Gastroenterol 6, 470–474 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou P, Li T, Su R & Gong Z Effects of thienorphine on contraction of the guinea pig sphincter of Oddi, choledochus and gall bladder. Eur. J. Pharmacol 737, 22–28 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Joehl RJ, Koch KL & Nahrwold DL Opioid drugs cause bile duct obstruction during hepatobiliary scans. Am. J. Surg 147, 134–138 (1984). [DOI] [PubMed] [Google Scholar]
- 67.Mittal RK, Frank EB, Lange RC & McCallum RW Effects of morphine and naloxone on esophageal motility and gastric emptying in man. Dig. Dis. Sci 31, 936–942 (1986). [DOI] [PubMed] [Google Scholar]
- 68.Penagini R, Bartesaghi B, Zannini P, Negri G & Bianchi PA Lower oesophageal sphincter hypersensitivity to opioid receptor stimulation in patients with idiopathic achalasia. Gut 34, 16–20 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schoenfeld PS Advances in IBS 2016: a review of current and emerging data. Gastroenterol. Hepatol 12 (Suppl. 3), 1–11 (2016). [PMC free article] [PubMed] [Google Scholar]
- 70.Cash BD, Lacy BE, Schoenfeld PS, Dove LS & Covington PS Safety of eluxadoline in patients with irritable bowel syndrome with diarrhea. Am. J. Gastroenterol 112, 365–374 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chandok N & Watt KD Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin. Proc 85, 451–458 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bosilkovska M, Walder B, Besson M, Daali Y & Desmeules J Analgesics in patients with hepatic impairment: pharmacology and clinical implications. Drugs 72, 1645–1669 (2012). [DOI] [PubMed] [Google Scholar]
- 73.Verbeeck RK Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur. J. Clin. Pharmacol 64, 1147–1161 (2008). [DOI] [PubMed] [Google Scholar]
- 74.Hasselstrom J et al. The metabolism and bioavailability of morphine in patients with severe liver cirrhosis. Br. J. Clin. Pharmacol 29, 289–297 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soleimanpour H, Safari S, Shahsavari Nia K, Sanaie S & Alavian SM Opioid drugs in patients with liver disease: a systematic review. Hepat. Mon 16, e32636 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Noble M et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst. Rev 1, CD006605 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ling GS, Paul D, Simantov R & Pasternak GW Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci 45, 1627–1636 (1989). [DOI] [PubMed] [Google Scholar]
- 78.Schneider JP & Kirsh KL Defining clinical issues around tolerance, hyperalgesia, and addiction: a quantitative and qualitative outcome study of long-term opioid dosing in a chronic pain practice. J. Opioid Manag 6, 385–395 (2010). [DOI] [PubMed] [Google Scholar]
- 79.Camilleri M et al. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol. Motil 26, 1386–1395 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harada Y et al. Mashiningan improves opioid-induced constipation in rats by activating cystic fibrosis transmembrane conductance regulator chloride channel. J. Pharmacol. Exp. Ther 362, 78–84 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Grunkemeier DM, Cassara JE, Dalton CB & Drossman DA The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin. Gastroenterol. Hepatol 5, 1126–1139 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Keefer L et al. Centrally mediated disorders of gastrointestinal pain. Gastroenterology 150, 1408–1419 (2016). [DOI] [PubMed] [Google Scholar]
- 83.Szigethy E, Schwartz M & Drossman D Narcotic bowel syndrome and opioid-induced constipation. Curr. Gastroenterol. Rep 16, 410 (2014). [DOI] [PubMed] [Google Scholar]
- 84.Drossman D & Szigethy E The narcotic bowel syndrome: a recent update. Am. J. Gastroenterol. Suppl 2, 22–30 (2014). [DOI] [PubMed] [Google Scholar]
- 85.Drossman DA Functional gastrointestinal disorders: history, pathophysiology, clinical features & Rome IV. Gastroenterology 150, 1262–1279.e2 (2016). [DOI] [PubMed] [Google Scholar]
- 86.Low Y, Clarke CF & Huh BK Opioid-induced hyperalgesia: a review of epidemiology, mechanisms and management. Singapore Med. J 53, 357–360 (2012). [PubMed] [Google Scholar]
- 87.Watkins LR & Maier SF Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol. Rev 82, 981–1011 (2002). [DOI] [PubMed] [Google Scholar]
- 88.Crain SM & Shen KF Antagonists of excitatory opioid receptor functions enhance morphine’s analgesic potency and attenuate opioid tolerance/dependence liability. Pain 84, 121–131 (2000). [DOI] [PubMed] [Google Scholar]
- 89.Ossipov MH, Lai J, King T, Vanderah TW & Porreca F Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers 80, 319–324 (2005). [DOI] [PubMed] [Google Scholar]
- 90.Kurlander JE & Drossman DA Diagnosis and treatment of narcotic bowel syndrome. Nat. Rev. Gastroenterol. Hepatol 11, 410–418 (2014). [DOI] [PubMed] [Google Scholar]
- 91.Watkins LR, Milligan ED & Maier SF Glial activation: a driving force for pathological pain. Trends Neurosci 24, 450–455 (2001). [DOI] [PubMed] [Google Scholar]
- 92.Rattan AK & Tejwani GA Effect of chronic treatment with morphine, midazolam and both together on dynorphin(1–13) levels in the rat. Brain Res 754, 239–244 (1997). [DOI] [PubMed] [Google Scholar]
- 93.Agostini S et al. Evidence of central and peripheral sensitization in a rat model of narcotic bowel-like syndrome. Gastroenterology 139, 553–563.e5 (2010). [DOI] [PubMed] [Google Scholar]
- 94.Hutchinson MR et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav. Immun 24, 83–95 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hutchinson MR et al. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J. Neurosci 32, 11187–11200 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mattioli TA et al. Toll-like receptor 4 mutant and null mice retain morphine-induced tolerance, hyperalgesia, and physical dependence. PLoS ONE 9, e97361 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Farmer AD, Ferdinand E & Aziz Q Opioids and the gastrointestinal tract — a case of narcotic bowel syndrome and literature review. J. Neurogastroenterol. Motil 19, 94–98 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kotalik J Controlling pain and reducing misuse of opioids: ethical considerations. Can. Fam. Physician 58, 381–385 (2012). [PMC free article] [PubMed] [Google Scholar]
- 99.Dowell D, Haegerich TM & Chou R CDC guideline for prescribing opioids for chronic pain — United States, 2016. JAMA 315, 1624–1645 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Murthy VH Ending the opioid epidemic — a call to action. N. Engl. J. Med 375, 2413–2415 (2016). [DOI] [PubMed] [Google Scholar]
- 101.Ballantyne JC & Fleisher LA Ethical issues in opioid prescribing for chronic pain. Pain 148, 365–367 (2010). [DOI] [PubMed] [Google Scholar]
- 102.Zgierska A, Miller M & Rabago D Patient satisfaction, prescription drug abuse, and potential unintended consequences. JAMA 307, 1377–1378 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kelly S, Johnson GT & Harbison RD “Pressured to prescribe”: the impact of economic and regulatory factors on South-Eastern ED physicians when managing the drug seeking patient. J. Emerg. Trauma Shock 9, 58–63 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kolodny A et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu. Rev. Publ. Health 36, 559–574 (2015). [DOI] [PubMed] [Google Scholar]
- 105.Drossman DA et al. Diagnosis, characterization, and 3-month outcome after detoxification of 39 patients with narcotic bowel syndrome. Am. J. Gastroenterol 107, 1426–1440 (2012). [DOI] [PubMed] [Google Scholar]
- 106.U.S. Food and Drug Administration. FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use U.S. Food and Drug Administration; https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm518697.htm (2016). [DOI] [PubMed] [Google Scholar]
- 107.Tornblom H & Drossman DA Centrally targeted pharmacotherapy for chronic abdominal pain. Neurogastroenterol. Motil 27, 455–467 (2015). [DOI] [PubMed] [Google Scholar]
- 108.Dekel R, Drossman DA & Sperber AD The use of psychotropic drugs in irritable bowel syndrome. Expert Opin. Investigat. Drugs 22, 329–339 (2013). [DOI] [PubMed] [Google Scholar]
- 109.Stoicea N et al. Opioid-induced hyperalgesia in chronic pain patients and the mitigating effects of gabapentin. Front. Pharmacol 6, 104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arumugam S, Lau CS & Chamberlain RS Use of preoperative gabapentin significantly reduces postoperative opioid consumption: a meta-analysis. J. Pain Res 9, 631–640 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Erlendson MJ et al. Palonosetron and hydroxyzine pre-treatment reduces the objective signs of experimentally-induced acute opioid withdrawal in humans: a double-blinded, randomized, placebo-controlled crossover study. Am. J. Drug Alcohol Abuse 43, 78–86 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gowing L, Ali R, White JM & Mbewe D Buprenorphine for managing opioid withdrawal. Cochrane Database Syst. Rev 2, CD002025 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xie C et al. Efficacy and safety of antidepressants for the treatment of Irritable Bowel Syndrome: a meta-analysis. PLoS ONE 10, e0127815 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thorkelson G, Bielefeldt K & Szigethy E Empirically supported use of psychiatric medications in adolescents and adults with IBD. Inflamm. Bowel Dis 22, 1509–1522 (2016). [DOI] [PubMed] [Google Scholar]
- 115.Cheng Y et al. Association between TLR2 and TLR4 gene polymorphisms and the susceptibility to Inflammatory Bowel Disease: a meta-analysis. PLoS ONE 10, e0126803 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Daghaghzadeh H et al. Efficacy of duloxetine add on in treatment of inflammatory bowel disease patients: a double-blind controlled study. J. Res. Med. Sci 20, 595–601 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Talley NJ et al. Effect of amitriptyline and escitalopram on functional dyspepsia: a multicenter, randomized controlled study. Gastroenterology 149, 340–349.e342 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Talley NJ et al. Antidepressant therapy (imipramine and citalopram) for irritable bowel syndrome: a double-blind, randomized, placebo-controlled trial. Dig. Dis. Sci 53, 108–115 (2008). [DOI] [PubMed] [Google Scholar]
- 119.Masand PS et al. A double-blind, randomized, placebo-controlled trial of paroxetine controlled-release in irritable bowel syndrome. Psychosomatics 50, 78–86 (2009). [DOI] [PubMed] [Google Scholar]
- 120.Grover M & Camilleri M Effects on gastrointestinal functions and symptoms of serotonergic psychoactive agents used in functional gastrointestinal diseases. J. Gastroenterol 48, 177–181 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grover M et al. Atypical antipsychotic quetiapine in the management of severe refractory functional gastrointestinal disorders. Dig. Dis. Sci 54, 1284–1291 (2009). [DOI] [PubMed] [Google Scholar]
- 122.Gurusamy KS, Lusuku C & Davidson BR Pregabalin for decreasing pancreatic pain in chronic pancreatitis. Cochrane Database Syst. Rev 2, CD011522 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Olesen SS et al. Randomised clinical trial: pregabalin attenuates experimental visceral pain through sub-cortical mechanisms in patients with painful chronic pancreatitis. Aliment. Pharmacol. Ther 34, 878–887 (2011). [DOI] [PubMed] [Google Scholar]
- 124.Gale JD & Houghton LA Alpha 2 delta (α2δ) Ligands, gabapentin and pregabalin: what is the evidence for potential use of these ligands in irritable bowel syndrome. Front. Pharmacol 2, 28 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Takemura Y et al. Effects of gabapentin on brain hyperactivity related to pain and sleep disturbance under a neuropathic pain-like state using fMRI and brain wave analysis. Synapse 65, 668–676 (2011). [DOI] [PubMed] [Google Scholar]
- 126.Saito YA et al. A placebo-controlled trial of pregabalin for irritable bowel syndrome. Am. J. Gastroenterol. Suppl 111, S236 (2016). [Google Scholar]
- 127.Paul SP & Basude D Non-pharmacological management of abdominal painrelated functional gastrointestinal disorders in children. World J. Pediatr 12, 389–398 (2016). [DOI] [PubMed] [Google Scholar]
- 128.Srinath AI, Walter C, Newara MC & Szigethy EM Pain management in patients with inflammatory bowel disease: insights for the clinician. Therap. Adv. Gastroenterol 5, 339–357 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ford AC et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am. J. Gastroenterol 109, 1350–1365 (2014). [DOI] [PubMed] [Google Scholar]
- 130.Ruepert L et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst. Rev 8, CD003460 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brennan BP et al. Duloxetine in the treatment of irritable bowel syndrome: an open-label pilot study. Hum. Psychopharmacol 24, 423–428 (2009). [DOI] [PubMed] [Google Scholar]
- 132.Kaplan A, Franzen MD, Nickell PV, Ransom D & Lebovitz PJ An open-label trial of duloxetine in patients with irritable bowel syndrome and comorbid generalized anxiety disorder. Int. J. Psychiatry Clin. Pract 18, 11–15 (2014). [DOI] [PubMed] [Google Scholar]
- 133.Lewis-Fernandez R et al. An open-label pilot study of duloxetine in patients with irritable bowel syndrome and comorbid major depressive disorder. J. Clin. Psychopharmacol 36, 710–715 (2016). [DOI] [PubMed] [Google Scholar]
- 134.Lacy BE, Chey WD & Lembo AJ New and emerging treatment options for Irritable Bowel Syndrome. Gastroenterol. Hepatol 11 (Suppl. 2), 1–19 (2015). [PMC free article] [PubMed] [Google Scholar]
- 135.Noyman-Veksler G et al. Role of pain-based catastrophizing in pain, disability, distress, and suicidal ideation. Psychiatry 80, 155–170 (2017). [DOI] [PubMed] [Google Scholar]
- 136.Cheng C, Hui W & Lam S Perceptual style and behavioral pattern of individuals with functional gastrointestinal disorders. Health Psych 19, 146–154 (2000). [DOI] [PubMed] [Google Scholar]
- 137.Sandner-Kiesling A et al. Long-term efficacy and safety of combined prolonged-release oxycodone and naloxone in the management of non-cancer chronic pain. Int. J. Clin. Pract 64, 763–774 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ford AC, Brenner DM & Schoenfeld PS Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and meta-analysis. Am. J. Gastroenterol 108, 1566–1574 (2013). [DOI] [PubMed] [Google Scholar]
- 139.Mehta N, O’Connell K, Giambrone GP, Baqai A & Diwan S Efficacy of methylnaltrexone for the treatment of opiod-induced constipation: a meta-analysis and systematic review. Postgrad. Med 128, 282–289 (2016). [DOI] [PubMed] [Google Scholar]
- 140.Kraft MD Methylnaltrexone, a new peripherally acting mu-opioid receptor antagonist being evaluated for the treatment of postoperative ileus. Expert Opin. Invest. Drugs 17, 1365–1377 (2008). [DOI] [PubMed] [Google Scholar]
- 141.Poulsen J, Brock C, Olesen AE, Nilsson M & Drewes AM Evolving paradigms in the treatment of opioid-induced bowel dysfunction. Therap. Adv. Gastroenterol 8, 360–372 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sloots CE, Rykx A, Cools M, Kerstens R & De Pauw M Efficacy and safety of prucalopride in patients with chronic noncancer pain suffering from opioid-induced constipation. Dig. Dis. Sci 55, 2912–2921 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sajid MS, Hebbar M, Baig MK, Li A & Philipose Z Use of prucalopride for chronic constipation: a systematic review and meta-analysis of published randomized, controlled trials. J Neurogastroenterol. Motil 22, 412–422 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nelson AD & Camilleri M Opioid-induced constipation: advances and clinical guidance. Ther. Adv. Chron. Dis 7, 121–134 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Argoff CE et al. Consensus recommendations on initiating prescription therapies for opioid-induced constipation. Pain Med 16, 2324–2337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ducrotte P & Causse C The Bowel Function Index: a new validated scale for assessing opioid-induced constipation. Curr. Med. Res. Opin 28, 457–466 (2012). [DOI] [PubMed] [Google Scholar]
- 147.Slappendel R, Simpson K, Dubois D & Keininger DL Validation of the PAC-SYM questionnaire for opioid-induced constipation in patients with chronic low back pain. Eur. J. Pain 10, 209–217 (2006). [DOI] [PubMed] [Google Scholar]
- 148.Keefer L & Mandal S The potential role of behavioral therapies in the management of centrally mediated abdominal pain. Neurogastroenterol. Motil 27, 313–323 (2015). [DOI] [PubMed] [Google Scholar]
- 149.Eccleston C, Morley SJ & Williams AC Psychological approaches to chronic pain management: evidence and challenges. Br. J. Anaesth 111, 59–63 (2013). [DOI] [PubMed] [Google Scholar]
- 150.Ehde DM, Dillworth TM & Turner JA Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am. Psychol 69, 153–166 (2014). [DOI] [PubMed] [Google Scholar]
- 151.Kerns RD, Sellinger J & Goodin BR Psychological treatment of chronic pain. Annu. Rev. Clin. Psychol 7, 411–434 (2011). [DOI] [PubMed] [Google Scholar]
- 152.Tang QL, Lin GY & Zhang MQ Cognitive-behavioral therapy for the management of irritable bowel syndrome. World J. Gastroenterol 19, 8605–8610 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Laird KT, Tanner-Smith EE, Russell AC, Hollon SD & Walker LS Short-term and long-term efficacy of psychological therapies for Irritable Bowel Syndrome: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol 14, 937–947.e4 (2016). [DOI] [PubMed] [Google Scholar]
- 154.Altayar O, Sharma V, Prokop LJ, Sood A & Murad MH Psychological therapies in patients with irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Gastroenterol. Res. Pract 2015, 549308 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Palsson OS & Whitehead WE Psychological treatments in functional gastrointestinal disorders: a primer for the gastroenterologist. Clin. Gastroenterol. Hepatol 11, 208–216 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Regueiro M, Greer JB & Szigethy E Etiology and treatment of pain and psychosocial issues in patients with inflammatory bowel diseases. Gastroenterology 152, 430–439.e4 (2017). [DOI] [PubMed] [Google Scholar]
- 157.Knowles SR, Monshat K & Castle DJ The efficacy and methodological challenges of psychotherapy for adults with inflammatory bowel disease: a review. Inflamm. Bowel Dis 19, 2704–2715 (2013). [DOI] [PubMed] [Google Scholar]
- 158.Mikocka-Walus A, Andrews JM & Bampton P Cognitive behavioral therapy for IBD. Inflamm. Bowel Dis 22, E5–E6 (2016). [DOI] [PubMed] [Google Scholar]
- 159.Lee HH, Choi YY & Choi MG The efficacy of hypnotherapy in the treatment of Irritable Bowel Syndrome: a systematic review and meta-analysis. J. Neurogastroenterol. Motil 20, 152–162 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Webb AN, Kukuruzovic RH, Catto-Smith AG & Sawyer SM Hypnotherapy for treatment of irritable bowel syndrome. Cochrane Database Syst. Rev 4, CD005110 (2007). [DOI] [PubMed] [Google Scholar]
- 161.Aucoin M, Lalonde-Parsi MJ & Cooley K Mindfulness-based therapies in the treatment of functional gastrointestinal disorders: a meta-analysis. Evid. Based Complement. Alternat. Med 2014, 140724 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Goyal M et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern. Med 174, 357–368 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bawa FL et al. Does mindfulness improve outcomes in patients with chronic pain? Systematic review and meta-analysis. Br. J. Gen. Pract 65, e387–e400 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Palsson OS Hypnosis treatment of gastrointestinal disorders: a comprehensive review of the empirical evidence. Am. J. Clin. Hypn 58, 134–158 (2015). [DOI] [PubMed] [Google Scholar]
- 165.Keefer L et al. Gut-directed hypnotherapy significantly augments clinical remission in quiescent ulcerative colitis. Aliment. Pharmacol. Ther 38, 761–771 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jedel S et al. A randomized controlled trial of mindfulness-based stress reduction to prevent flare-up in patients with inactive ulcerative colitis. Digestion 89, 142–155 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Berrill JW, Sadlier M, Hood K & Green JT Mindfulness-based therapy for inflammatory bowel disease patients with functional abdominal symptoms or high perceived stress levels. J. Crohns Colitis 8, 945–955 (2014). [DOI] [PubMed] [Google Scholar]
- 168.Gerbarg PL et al. The effect of breathing, movement, and meditation on psychological and physical symptoms and inflammatory biomarkers in Inflammatory Bowel Disease: a randomized controlled trial. Inflamm. Bowel Dis 21, 2886–2896 (2015). [DOI] [PubMed] [Google Scholar]
- 169.Jones M, Koloski N, Boyce P & Talley NJ Pathways connecting cognitive behavioral therapy and change in bowel symptoms of IBS. J. Psychosom. Res 70, 278–285 (2011). [DOI] [PubMed] [Google Scholar]
- 170.Chang L The role of stress on physiological responses and clinical symptoms in irritable bowel syndrome. Gastroenterology 140, 761–765 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bonaz BL & Bernstein CN Brain-gut interactions in inflammatory bowel disease. Gastroenterology 144, 36–49 (2013). [DOI] [PubMed] [Google Scholar]
- 172.Carabotti M, Scirocco A, Maselli M & Severi C The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol 28, 203–209 (2015). [PMC free article] [PubMed] [Google Scholar]
- 173.Uc A et al. Chronic pancreatitis in the 21st century — research challenges and opportunities: summary of a National Institute of Diabetes and Digestive and Kidney Diseases Workshop. Pancreas 45, 1365–1375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Palsson OS & van Tilburg M Hypnosis and guided imagery treatment for gastrointestinal disorders: experience with scripted protocols developed at the University of North Carolina. Am. J. Clin. Hypn 58, 5–21 (2015). [DOI] [PubMed] [Google Scholar]
- 175.Ljotsson B et al. Internet-delivered exposure-based treatment versus stress management for irritable bowel syndrome: a randomized trial. Am. J. Gastroenterol 106, 1481–1491 (2011). [DOI] [PubMed] [Google Scholar]
- 176.Ljotsson B et al. Long-term follow-up of internet-delivered exposure and mindfulness based treatment for irritable bowel syndrome. Behav. Res. Ther 49, 58–61 (2011). [DOI] [PubMed] [Google Scholar]
- 177.Eccleston C et al. Psychological therapies (Internet-delivered) for the management of chronic pain in adults. Cochrane Database Syst. Rev 2, CD010152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Krupnick JL et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. J. Consult. Clin. Psychol 64, 532–539 (1996). [DOI] [PubMed] [Google Scholar]
- 179.Esquibel AY & Borkan J Doctors and patients in pain: conflict and collaboration in opioid prescription in primary care. Pain 155, 2575–2582 (2014). [DOI] [PubMed] [Google Scholar]
- 180.Bäckryd E “Professional Helper” or “Helping Professional?” The patient-physician relationship in the chronic pain setting, with special reference to the current opioid debate. J. Contin. Educ. Health Prof 36, 133–137 (2016). [DOI] [PubMed] [Google Scholar]
- 181.Drossman DA 2012 David Sun lecture: helping your patient by helping yourself — how to improve the patient-physician relationship by optimizing communication skills. Am. J. Gastroenterol 108, 521–528 (2013). [DOI] [PubMed] [Google Scholar]
- 182.Frantsve LM & Kerns RD Patient-provider interactions in the management of chronic pain: current findings within the context of shared medical decision making. Pain Med 8, 25–35 (2007). [DOI] [PubMed] [Google Scholar]
- 183.Reed-Knight B, Claar RL, Schurman JV & van Tilburg MA Implementing psychological therapies for functional GI disorders in children and adults. Expert Rev. Gastroenterol. Hepatol 10, 981–984 (2016). [DOI] [PubMed] [Google Scholar]
- 184.Martins SS et al. Mood and anxiety disorders and their association with non-medical prescription opioid use and prescription opioid-use disorder: longitudinal evidence from the National Epidemiologic Study on Alcohol and Related Conditions. Psychol. Med 42, 1261–1272 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wasan AD et al. Psychiatric comorbidity is associated prospectively with diminished opioid analgesia and increased opioid misuse in patients with chronic low back pain. Anesthesiology 123, 861–872 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mason MJ et al. Depression moderates the relationship between pain and the nonmedical use of opioid medication among adult outpatients. J. Addict. Med 10, 408–413 (2016). [DOI] [PubMed] [Google Scholar]
- 187.Arteta J, Cobos B, Hu Y, Jordan K & Howard K Evaluation of how depression and anxiety mediate the relationship between pain catastrophizing and prescription opioid misuse in a chronic pain population. Pain Med 17, 295–303 (2016). [DOI] [PubMed] [Google Scholar]
- 188.Rosenblum A, Marsch LA, Joseph H & Portenoy RK Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp. Clin. Psychopharmacol 16, 405–416 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cowan DT, Allan LG, Libretto SE & Griffiths P Opioid drugs: a comparative survey of therapeutic and “street” use. Pain Med 2, 193–203 (2001). [DOI] [PubMed] [Google Scholar]
- 190.Lee EB, An W, Levin ME & Twohig MP An initial meta-analysis of Acceptance and Commitment Therapy for treating substance use disorders. Drug Alcohol Depend 155, 1–7 (2015). [DOI] [PubMed] [Google Scholar]
- 191.Jones CM Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers — United States, 2002–2004 and 2008– 2010. Drug Alcohol Depend 132, 95–100 (2013). [DOI] [PubMed] [Google Scholar]
- 192.Des Jarlais DC et al. HIV incidence among injection drug users in New York City, 1990 to 2002: use of serologic test algorithm to assess expansion of HIV prevention services. Am. J. Publ. Health 95, 1439–1444 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hagan H et al. Attribution of hepatitis C virus seroconversion risk in young injection drug users in 5 US cities. J. Infect. Dis 201, 378–385 (2010). [DOI] [PubMed] [Google Scholar]
- 194.Paicius RM, Bernstein CA & Lempert-Cohen C Peripheral nerve field stimulation in chronic abdominal pain. Pain Physician 9, 261–266 (2006). [PubMed] [Google Scholar]
- 195.Kapural L & Jolly S Interventional pain management approaches for control of chronic pancreatic pain. Curr. Treat. Opt. Gastroenterol 14, 360–370 (2016). [DOI] [PubMed] [Google Scholar]
- 196.Simis M et al. Investigation of central nervous system dysfunction in chronic pelvic pain using magnetic resonance spectroscopy and noninvasive brain stimulation. Pain Pract 15, 423–432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Moreno-Duarte I et al. Targeted therapies using electrical and magnetic neural stimulation for the treatment of chronic pain in spinal cord injury. Neuroimage 85, 1003–1013 (2014). [DOI] [PubMed] [Google Scholar]
- 198.Gray AM et al. Deep brain stimulation as a treatment for neuropathic pain: a longitudinal study addressing neuropsychological outcomes. J. Pain 15, 283–292 (2014). [DOI] [PubMed] [Google Scholar]
- 199.Butler SF, Budman SH, Fanciullo GJ & Jamison RN Cross validation of the Current Opioid Misuse Measure (COMM) to monitor chronic pain patients on opioid therapy. Clin. J. Pain 26, 770–776 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Butler SF, Fernandez K, Benoit C, Budman SH & Jamison RN Validation of the Revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R). J. Pain 9, 360–372 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bao Y et al. Prescription drug monitoring programs are associated with sustained reductions in opioid prescribing by physicians. Health Aff 35, 1045–1051 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kovitwanichkanont T & Day CA Prescription opioid misuse and public health approach in Australia. Subst. Use Misuse 10.1080/10826084.2017.1305415 (2017). [DOI] [PubMed] [Google Scholar]
- 203.European Monitoring Centre for Drugs and Drug Addiction. Our activities — ongoing projects and programmes European Monitoring Centre for Drugs and Drug Addiction; http://www.emcdda.europa.eu/activities (2017). [Google Scholar]
- 204.Novak SP et al. Nonmedical use of prescription drugs in the European Union. BMC Psychiatry 16, 274 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.University of Wisconsin–Madison Pain & Policy Studies Group. Custom consumption graphs for opioid medicines Pain & Policy Studies Group; https://ppsg-chart.medicine.wisc.edu/ (2017). [Google Scholar]


