Abstract
Biomaterials are key factors in regenerative medicine. Matrices used for cell delivery are especially important, as they provide support to transplanted cells that is essential for promoting cell survival, retention, and desirable phenotypes. Injectable matrices have become promising and attractive due to their minimum invasiveness and ease of use. Conventional injectable matrices mostly use hydrogel precursor solutions that form solid, cell-laden hydrogel scaffolds in situ. However, these materials are associated with challenges in biocompatibility, shear-induced cell death, lack of control over cellular phenotype, lack of macroporosity and remodeling, and relatively weak mechanical strength. This Progress Report provides a brief overview of recent progress in developing injectable matrices to overcome the limitations of conventional in-situ hydrogels. Biocompatible chemistry and shear-thinning hydrogels have been introduced to promote cell survival and retention. Emerging investigations of the effects of matrix properties on cellular function in 3D provide important guidelines for promoting desirable cellular phenotypes. Moreover, several novel approaches are combining injectability with macroporosity to achieve macroporous, injectable matrices for cell delivery.
Keywords: injectable, cell delivery, hydrogel, macroporous, tissue regeneration, cell therapy
1. Introduction
Tissue engineering and regenerative medicine aim to restore lost tissue structures and functions using interdisciplinary approaches from biology, engineering, material science and medicine.[1] Remarkable achievements in the past decade have led to tremendous progress in translating various cell-based therapies from bench to bedside. The reported clinical trials increased from 970 to over 7000 between 2010 to 2015, the number has recently increased to over 8000 after 2016, according to the database in clinicaltrials.gov. In contrast to using cells alone, biomaterials can serve as three-dimensional matrices for cell delivery to enhance the outcomes of tissue regeneration by protecting cells from undesirable shear forces during injection, providing structural support, and facilitating desirable cell fates.[2] In addition, biomaterials with optimized biochemical and mechanical properties may also promote cell survival and retention, and facilitate cell-mediated tissue-matrix production, vascularization, and integration with endogenous tissue[3] Biomaterials for cell delivery can be divided into two categories: implantable prefabricated matrices and injectable matrices. Implantable matrices are prefabricated solid scaffolds, which must be transplanted through invasive surgery. In contrast, injectable matrices can be transplanted in a minimally-invasive manner via injection, a procedure that is compatible with current surgical techniques, facilitates homogeneous cell encapsulation, and can polymerize in situ to fill defects with irregular shapes. Given these benefits, injectable matrices have been widely used for enhancing cell delivery and tissue regeneration. Hydrogels are the most commonly used injectable matrices for cell delivery, due to their intrinsic injectability, tissue-mimicking water content, and tunable biochemical and mechanical properties.[4] Despite extensive efforts and progress in developing in-situ hydrogels as injectable matrices, various challenges remain. In this progress report, we will first summarize key challenges associated with cell-based tissue regeneration, and then review recent progress in developing injectable matrices that specifically address those challenges.
2. Challenges associated with cell-based tissue regeneration
2.1. Poor cell survival and retention post-transplantation
Successful cell-based therapy and tissue regeneration are based on high cell survival after transplantation, which remain challenging. Although delivering cells via direct injection has been preferred in the clinic due to low invasiveness and facile handling, direct injection usually results in low cell survival and retention. Cell death after transplantation can be caused by cellular shearing during injection, by hypoxic microenvironments with limited nutrient supplies, and dynamic mechanical pressure in the recipient tissue. Emerging approaches use matrices as carriers to deliver cells, thus supporting transplanted cells and improving cell survival and retention, but current materials are usually associated with issues that need to be addressed.
Biocompatibility is a concern associated with various materials. For example, naturally derived materials such as collagen and Matrigel provide excellent cytocompatibility and biodegradation during cell delivery, but are associated with immunogenicity and inflammation. In contrast, synthetic in-situ hydrogels provide better control over physical and biochemical properties and low immune response. However, some of the chemical reactions involved in hydrogel crosslinking interfere with the biological processes of cells, which can cause acute or long-term decline in cell survival.
When injected directly without any matrices, cells suffer from undesirable shear force as they pass through submillimeter-sized needles. To protect the cells from the undesirable shear force-induced cell death, recent studies have examined using hydrogels as delivery matrices. [5] Importantly, these studies suggest that the viscoelastic property of the delivery matrices is an important parameter that impacts cell viability after injection. [5a] For example, injecting cells using uncrosslinked polymer solution with minimal viscosity often leads to acute death of majority of transplanted cells. [5a] In contrast, injecting cells with crosslinked matrices with optimized viscoelastic properties substantially improved cell survival after injection.[5]
In addition to acute cell death, long-term cell survival and retention can be compromised by the premature loss of integrity of matrices, which is influenced by the mechanical property and degradation profile of matrices. For example, when transplanted in tissues such as cartilage and heart that are subject to constant mechanical stimulation, matrices with poor mechanical strength and stability can lead to premature disintegration of the scaffolds and a loss of protection for cells. Most natural materials used for cell delivery lack mechanical strength and are prone to rapid biodegradation. To solve this issue, there remains a critical need to develop injectable matrices with enhanced mechanical strength.[6]
2.2. Lack of control in promoting desirable cell phenotypes
Control over cellular phenotype post-transplantation to promote desirable clinical outcomes is yet another challenge, partly due to poor understanding of the effects of matrix niche cues on cell phenotype, especially in 3D environments. For decades, phenotype of transplanted cells in cell-based therapies relied on soluble factors provided by the innate tissue environment or exogenous soluble factors delivered using various drug delivery systems.[7] However, recent studies have revealed that dimensionality and insoluble cues of extracellular matrix, including biochemical ligands and biophysical properties, modulate cell fate and behavior.[8] Identifying and designing cell-delivery matrices with optimal biochemical and physical cues can help maintain cell phenotype and promote desired cell functions.
Despite extensive efforts on developing biomaterials as cell-delivery matrices to modulate cell phenotype, the efficacy of most injectable matrices developed so far remains unsatisfactory. Conventional cell-delivery matrices consisting of natural or nature-derived materials could provide excellent biocompatibility and a rich and customizable set of biochemical ligands. However, the poor tunability of biophysical and biochemical properties of naturally-derived matrices makes it difficult to optimize them to favor the formation of specific tissues. These materials also suffer from batch-to-batch variation and poor mechanical strength and stability.[9] In contrast, pure or semi-synthetic materials have the advantage of better-defined and tunable properties. Nonetheless, efforts are still needed to identify the properties that promote desirable outcomes. For example, comprehensive validation of the effects of matrix properties such as mechanical properties[10], biodegradation[11], and molecular mobility[10a, 12] on cell phenotype is very limited. Further, it remains challenging to achieve synthetic materials that fully biomimic the properties of natural extracellular matrix (ECM), including physical properties such as stress relaxation[13], stress stiffening[14], and the organization of spatial zones [15].
2.3. Lack of macroporosity to facilitate tissue formation
Another bottleneck limiting the application of injectable matrices in tissue engineering is the lack of macroporosity. Macroporosity is an essential property of the scaffolds used for tissue regeneration, as it allows sufficient nutrient diffusion, promotes the growth of transplanted cells or the recruitment of host cells, and enhances tissue formation and vascularization.[16] However, most injectable matrices for cell delivery are based on hydrogels, yet conventional hydrogels are characterized by crosslinked molecular networks with mesh size on a nanoscale. Current methods of tuning hydrogel properties on changing the crosslinking density and concentration will change the mesh size, which can lead to subsequent changes in the diffusivity of soluble factors.[17] This can either affect the available nutrients and the signaling molecules secretion of the delivered cells, both of which will impact the biological microenvironment will further lead to changes in cell behaviors. Furthermore, cells are physically confined in the nano-sized meshes after being encapsulated in hydrogels. As such, the lack of macroporosity hinders the adoption of cellular fates that are desirable for tissue regeneration. Currently, macroporosity is introduced via porogen leaching[18], gas foaming[19], polymer phase separation[20], and 3D printing technologies[21], which can only be performed in vitro for scaffold fabrication. Thus, engineering approaches are required to introduce macroporosity to injectable matrices for cell delivery.
2.4. Insufficient remodeling capability of artificial matrices
Tissue regeneration is based on cell-mediated tissue reconstruction and remodeling, which are characterized by a balance of the synthesis, deposition, and degradation of tissue matrix.[22] Although cell-mediated remodeling is well supported by the ECM during tissue development and innate tissue repair, the matrices used currently for tissue regeneration remain suboptimal in this regard. For example, natural materials have advantageous biodegradation properties, but are poorly controllable; rapid clearance could lead to premature disintegration of the matrices before new tissue forms. In contrast, synthetic polymers are easily modified in order to tailor their degradation kinetics. In particular, cell-mediated degradation has been introduced to better mimic natural tissue remodeling in a controlled manner, which is achieved by incorporating native ECM-derived peptide.[23] However, this degradation is still cell- and enzyme-dependent[23a, 23b], and further efforts are required to identify an optimal degradation profile that synergizes with tissue formation.
2.5. Relatively weak mechanical strength
The relatively weak mechanical strength of in-situ hydrogels has limited their applications in regenerating some tissues, especially those bearing dynamic mechanical loading such as musculoskeletal tissues. Although the elastic modulus of synthetic hydrogels is tunable, the tunable range is narrower than the elastic modulus of native tissues, which spans from a few pascals in fat and brain to mega pascals in bone. In addition, current methods of introducing porosity and degradation in order to facilitate remodeling and tissue formation may further decrease the mechanical strength of the hydrogel. Depending on the tissue to be regenerated, the mechanical stiffness and strength required to bear the dynamic loading could vary substantially, which leads to different requirements of the delivery matrices. For example, brain and fat may need soft matrices, while muscle, cartilage, and bone need increasingly strong scaffolds to support transplanted cells and to maintain shape and integrity during remodeling. In contrast, musculoskeletal tissues bear dynamic loading and thus require elastic delivery matrices that resist deformation while protecting resident cells from pressure. As such, it is critical to improve and tailor the mechanical strength of matrices to be used for cell delivery and regeneration in such tissues.
2.6. Poor integration with host tissue
Poor integration of engineered tissue with host tissue can lead to poor function and clinical outcomes, a known challenge in tissue engineering. Tissue engraftment and integration is associated with a series of remodeling processes coordinated between host tissue and exogenous cells and matrices, including synergistic cell proliferation, migration, tissue formation, and vascularization. Unsatisfactory outcomes can be partly due to the poor integration of cell-carrying matrices into surrounding tissue as well as to suboptimal remodeling. Integration of the matrices with surrounding tissue is important for signaling by niche cues to transplanted cells, which leads to engraftment and integration. For this purpose, various bioadhesives are used to enhance the integration of implanted scaffolds with the defect sites. [24] While fibrin has been used as a popular sealant in surgery, there remains a great need for more injectable matrices that can facilitate and accelerate tissue integration. Although hydrogels can be modified to form covalent links with host tissue[25], the nanoporosity of the hydrogels still restricts cell migration, vascularization, and in-growth of host tissue.
3. New strategies to overcome challenges associated with current injectable matrices for cell delivery
3.1. Introducing biorthogonal click chemistry to improve biocompatibility of in-situ hydrogel formation
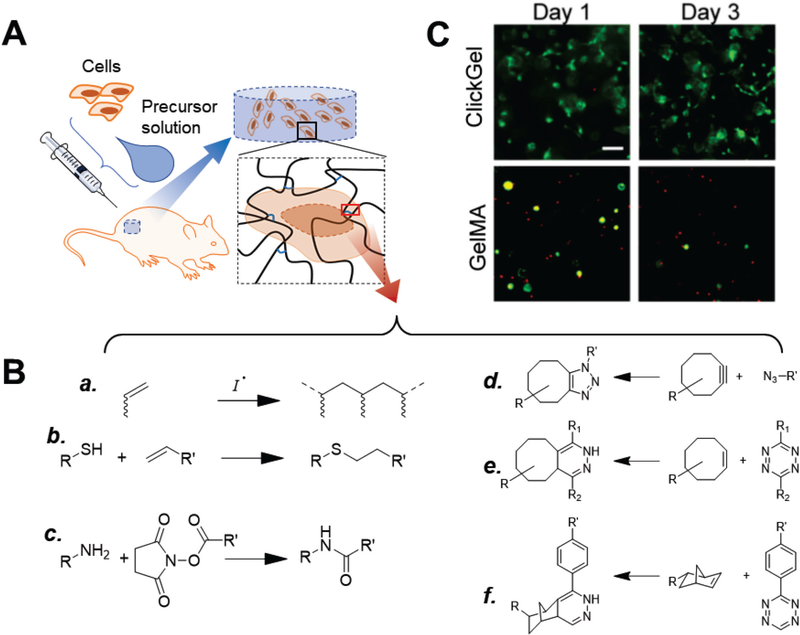
In-situ hydrogels are hydrogels that can be crosslinked under physiological conditions in vivo via physical interactions or chemical reactions (Fig. 1A). They are the most well-known and widely used matrices for cell delivery. The chemical reactions used for in-situ hydrogels include photo- and thermal-initiated radical polymerization, amine-N-hydroxylsuccimide ester coupling, and thiol-ene reaction through either radical addition or Michael-type addition (Fig. 1B a-c).[4a, 26] These reactions are highly efficient, but lack chemoselectivity. For example, the amine groups involved in crosslinking are abundant in natural ECM proteins and growth factors and thus these reactions may interfere with the activity of natural proteins[27]. Unpaired thiol groups, on the other hand, is not as common as amine groups. Free thiol groups usually present in the dynamic exchange of its oxidized form (paired disulfide) and reductive form during various biological processes, either on large protein or short peptide. For example, glutathione is a short peptide containing thiol from its cysteine residual plays as critical antioxidant to protect cells from oxygen species. Thiol-ene reaction may result in consumption of the thiols, and lead to insufficient glutathione that further impacts the cell survival and functions.[28]
Figure 1.
In-situ hydrogels and crosslinking chemistry. (A) Typical use of in-situ hydrogels for cell delivery. Cells are mixed with hydrogel precursor solution (usually a solution of functionalized polymers) and co-injected into the defect site, where the polymers crosslink into a polymer network as hydrogel scaffolds that support cells. (B) Crosslinking in-situ hydrogels. Conventional chemical reactions used for in-situ hydrogels include (a) photo- and thermal-initiated radical polymerization of (meth)acrylate groups, (b) thiol-ene reaction, and (c) amine-N-hydroxylsuccimide ester coupling.[26] To avoid interference with biological processes, recent studies have explored the use of biorthogonal click reactions, such as (d) strain-promoted azide-alkyne cycloaddition[29–30], (e) tetrazole-alkene[31], and (f) tetrazine-norbornene chemistry[32]. (C) Gelatin hydrogels crosslinked via tetrazine-norbornene click chemistry (ClickGel) led to significantly improved short and long-term cell viability versus radical-crosslinked methacrylated gelatin (GelMA). [32a] Reproduced with permission. [32a] Copyright 2016, John Wiley and Sons.
To address concerns that some chemical reactions may interfere with biological processes, recent studies have explored biorthogonal click chemistry[29] to improve the biocompatibility of crosslinking. These methods include strain-promoted azide-alkyne cycloaddition[29–30], tetrazole-alkene[31], and tetrazine-norbornene chemistry[32] (Fig. 1B d-f). For example, Koshy et al. compared injectable gelatin hydrogels crosslinked via biorthogonal tetrazine-norbornene click chemistry and photo-initiated radical polymerization; biorthogonal chemistry significantly increased the survival of cells encapsulated in 3D, with better supported cell spreading (Fig. 1C). [32a]
Although biorthogonal chemistry has shown promising results in recent reports [30b-d, 32], its application in matrices for cell-delivery is limited partly due to the relatively tedious synthesis versus conventional chemistries. In addition, despite the raised concerns and some reported drawbacks associated with the conventional chemistries, their application in current in-situ hydrogels can support reasonable cell viability and functions, when appropriately optimized. The long-term effects of current chemistries and the benefits of biorthogonal chemistry require further investigation.
3.2. Using shear-thinning hydrogels to enhance cell survival during injection
Delivering cells via injection takes advantage of easy handling and minimum invasiveness, but leads to undesirable shear force during injection. Recent studies have demonstrated that the rheology and viscoelastic properties of the medium carrying cells for injection can be optimized to improve cell survival. For example, Aguado et al. found that shifting from a Newtonian solution such as saline buffer to a physically crosslinked alginate hydrogel resulted in a substantial increase in the survival of injected cells.[5a] Interestingly, simply increasing the viscosity with a non-crosslinked alginate solution did not improve cell survival[5a], suggesting that the protective effect is due to the “plug flow” process, during which part of the hydrogel is reversibly transitioned to a shear-thinning fluid, while the rest remains hydrogels and protect cells from mechanical stress. Other shear-thinning hydrogels have also been explored as cell-delivery matrices and have yielded similar protective effects on cell survival.
Shear-thinning hydrogel is a term that has been widely used to describe hydrogels that can undergo reversible gel-sol transition when stressed or sheared, such as when pushed through a syringe needle.[5b, 33] These hydrogels are usually crosslinked by reversible bonds such as physical interactions including peptide amphiphile assembly[34], biorecognition of a protein domain with a ligand[35], and host-guest interactions.[5c, 5d] ... It should be noted that the so-called “shear-thinning” is different from the traditional definition describing a unique property of viscous liquid that the viscosity decreases under strain. Specifically, the shear-thinning liquid are not gels from the rheology prospect, while the “shear-thinning” hydrogels are hydrogels under static conditions, and they undergo reversible transitions under shear that transform them into viscous fluid.
3.2.1. Peptide amphiphiles
Peptide amphiphiles are peptides that can self-assemble into nanofibers mediated by hydrogen bonding and hydrophobic interactions.[34b, 36] The self-assembled nanofibers further form a 3D architecture that has been used to deliver therapeutic drugs and cells in regenerative medicine.[37] The hydrogels formed by self-assembled peptide-amphiphile nanofibers undergo shear-thinning, which can be tuned by changing the self-assembly kinetics. For example, Haines-Butterick et al. developed a 20-residue peptide (MAX8) that folds and self-assembles in response to cell culture medium, resulting in mechanically rigid hydrogels.[38] The MAX8 hydrogel encapsulated mesenchymal stem cells (MSCs) and was delivered via injection through a 20-gauge needle; majority of the delivered cells survived after injection, as shown by the qualitative live-dead staining.[38] Incorporating biochemical ligands could further promote cell survival in vivo. For example, when Webber et al. used peptide-amphiphile nanofibers assembled from C16-V3A3E3 derivatives with or without cell adhesion motif RGDS, the incorporation of RGDS substantially improved the survival and significantly enhanced the proliferation of bone marrow mononuclear cells.[39] Although the self-assembly of peptide amphiphiles has shown some promising outcomes, limitations include their weak mechanical strength and relatively low stability due to physical interactions between peptide molecules. While peptide-amphiphile nanofibers eliminate the use of whole protein, the immunogenicity of the peptide sequences may require long-term investigation.
3.2.2. Biorecognition-based hydrogels
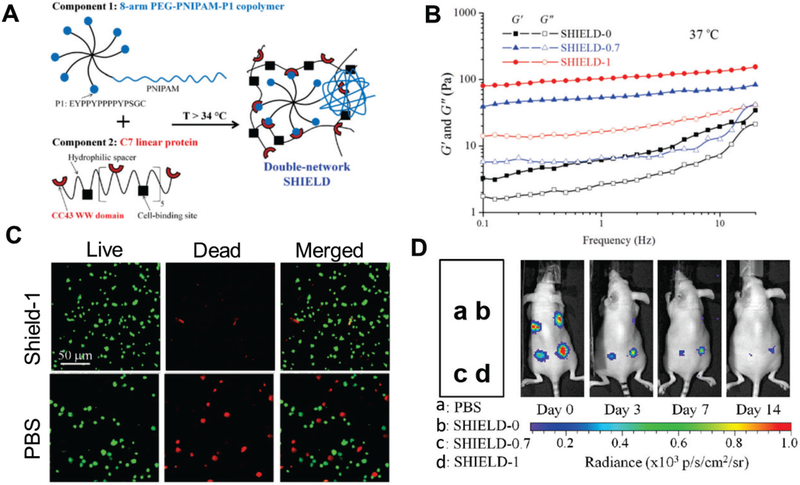
Instead of using peptides as building blocks for hydrogels, polymers can be incorporated with peptides and used to form hydrogels through the biorecognition of peptide domains and ligands. For example, Parisi-Amon et al. developed a two-component, molecular recognition physical hydrogel (MITCH) assembled from two polymers containing repeats of the CC43 WW domain and proline-rich peptide, respectively.[35] MITCH significantly improved the retention of transplanted cells after injection versus alginate, collagen, and saline buffer.[35a] Encouraged by this result, Cai et al. further improved the hydrogel by incorporating poly(N-isopropylacrylamide) (PNIPAM) (Fig. 2A), which undergoes secondary crosslinking in vivo, strengthening the scaffold and decreasing the degradation rate, which in turn prolonged cell retention in vivo.[40] The rheological and mechanical properties of this shear-thinning hydrogel for injectable encapsulation and long-term delivery (SHIELD) can be tuned by varying the incorporation of PNIPAM (Fig. 2B). While all SHIELD hydrogels with various levels of PNIPAM incorporation supported higher cell survival after injection than the control (Fig. 2C) [40a], hydrogels with higher PNIPAM incorporation led to significantly prolonged cell retention and survival over 14 days (Fig. 2D). These data highlight the importance of sustained mechanical support and protection for long-term cell survival in vivo.
Figure 2.
Shear-thinning hydrogel for injectable encapsulation and long-term delivery (SHIELD), a modified mixing-induced two-component hydrogel (MITCH) with optimal shear moduli that mechanically protects cells during injection and allows injectable cell encapsulation and long-term delivery.[40a] (A) Schematic of SHIELD hydrogel components. A star-shaped poly(ethylene glycol)(PEG) copolymer incorporated with the proline-rich peptide EYPPYPPPPYPSGC (component 1) forms a weak physical hydrogel with a recombinant C7 linear protein copolymer bearing CC43 WW-domain (component 2) [35]. Poly(N-isopropylacrylamide) (PNIPAM) is also incorporated to component 1, and undergoes a second crosslinking at body temperature to strengthen the hydrogel and prolong cell retention in vivo. (B) Increasing PNIPAM incorporation increased the storage (G’) and loss moduli (G”) at body temperature (37 °C). SHIELD-0, SHIELD-0.7, and SHIELD-1 are hydrogels formed with 0, 0.7, and 1 wt% incorporated PNIPAM. (C) SHIELD hydrogels provide more protection to cells passed through a 28-G needle at 1.0 mL/min that does phosphate-buffered saline (PBS). Fluorescent LIVE/DEAD staining performed on human adipose derived stem cells injected with SHIELD-1 hydrogel and PBS. When delivered with the SHIELD hydrogel, cell survival was substantially increased. (D) Cell retention after transplantation via injection evaluated using bioluminescence imaging. SHIELD-1 and SHIELD-0.7 hydrogels significantly increased cell retention up to 14 days after injection compared to the PBS control and the MITCH hydrogel without PNIPAM.
(A–D) Reproduced with permission. [35] Copyright 2015, John Wiley and Sons.
3.2.3. Host-guest-based supramolecular hydrogels
Another example of shear-thinning hydrogels is host-guest-based supramolecular hydrogels. Rodell et al. exploited the guest-host interactions of adamantane and beta-cyclodextrin to modify hyaluronic acid into shear-thinning hydrogels.[41] These hydrogels displayed promising injectability, in vivo stability and retention, and support of in vitro cell survival. [41] To enhance in vivo stability and mechanical support, hyaluronic acid was functionalized with methacrylate group, which allows radical polymerization for secondary crosslinking.[5c, 42] However, the efficacy of this synthetic hydrogel on cell delivery and in vivo cell retention are yet to be determined.
Other materials possess shear-thinning properties, such as kappa-carrageenan, which can be crosslinked in the presence of potassium.[5e] Some improvements are needed to address the drawbacks of these materials, especially their low mechanical strength and stability. The shear-thinning feature of these materials lowers their mechanical strength under pressure, which is good for cell delivery via injection but not desirable for cell protection in vivo. While recent strategies that introduce secondary crosslinking provide a way to overcome some of these drawbacks[5c, 40a, 42], combining advanced, more biocompatible crosslinking mechanisms merits further investigation.
3.3. Engineering hydrogels to promote desirable cell phenotypes in 3D
Cell-based tissue regeneration relies on transplanted cells adopting desirable phenotypes; phenotype is known to be modulated by various cues, such as the surrounding cells, soluble factors, and microenvironment mechanics.[10c, 43] While extensive studies have elucidated how cell functions are influenced by the described cues, most of these studies employed 2-dimensional (2D) substrates. However, cells reside in 3D niches in vivo, which could be more spatially restricted than 2D models. The nanoporosity of hydrogels may significantly hinder cells from remodeling the surrounding niche and depositing new extracellular matrix. Recent studies have revealed the effects of microenvironment rigidity, ligand reorganization, hydrogel degradation, and molecular mobility on stem-cell differentiation in 3D.[10a, 11–12]
3.3.1. Harnessing matrix stiffness-regulated reorganization of adhesion ligands
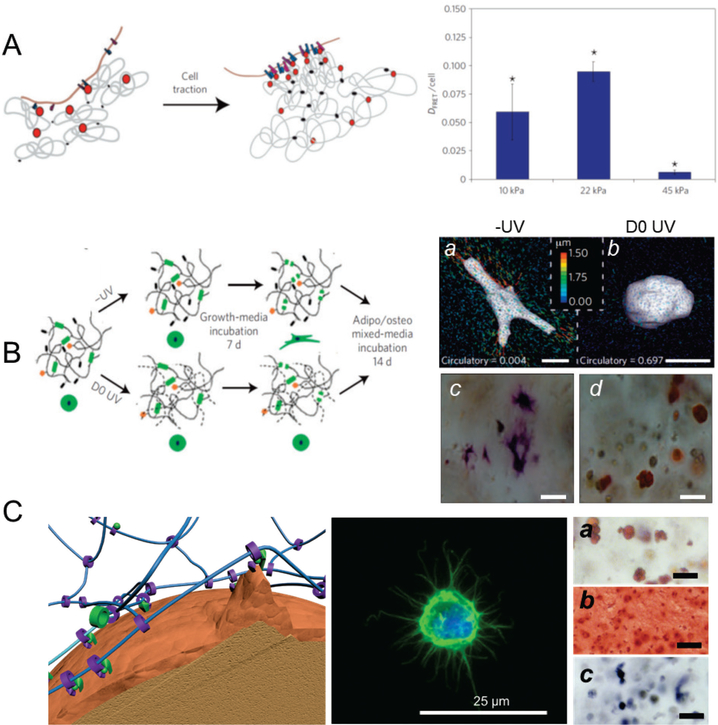
Tissue development is associated with reorganization of the microenvironment in which cells reside. Cell-delivery matrices must support this reorganization in order to promote cell-based tissue formation. In natural tissue environments, cells reorganize the niche easily, since the natural ECM is assembled through physical interactions and is flexible. As such, physically crosslinked hydrogels may better mimic the natural tissue microenvironment to facilitate niche reorganization. For example, Huebsch et al. reported that the osteogenic commitment of MSCs is regulated by matrix stiffness and by the nanoscale reorganization of adhesion ligands.[10a] They used fluorescence resonance energy transfer to assess matrix stiffness-dependent clustering of cell-adhesion ligands (RGD) (Fig. 3A), which peaked at a stiffness of 22 kPa; this peak was consistent with osteogenesis by MSCs in 3D.[10a] Although physically crosslinked hydrogels are advantageous in terms of flexibility, they are inherently less stable. As previously described in section 3.2, the mechanical strength of these hydrogels decreases under pressure due to shear-thinning, which does not protect cells during and after injection.
Figure 3.
Molecular mobility enables stem-cell functions in 3D environments. (A) Stem cells perform their functions in 3D in response to the mechanical properties of the matrix, mediated by the manipulation and nanoscale reorganization of surrounding adhesion ligands. Left, Schematic demonstrating cell traction mediated nanoscale clustering of surrounding adhesion ligands (RGD) incorporated in the calcium ion crosslinked alginate hydrogels enhanced cell–RGD bond formation. Right, alginate is incorporated with RGD-FITC and RGD-TAMRA to measure ligand clustering using fluorescence resonance energy transfer. Human MSCs encapsulated in hydrogels demonstrated cell traction-mediated and mechanical stiffness-dependent RGD clustering. Ligand clustering peaked at 22 kPa, consistent with the peak of osteogenic differentiation in this study. (n=3–5; *p<0:01 compared with other conditions, Holm–Bonferroni test) [10a] Reproduced with permission. [10a] Copyright 2010, Nature Publishing Group. (B) Degradation is essential for covalently crosslinked chemical hydrogels to rescue differentiation, since the lack of molecular mobility of covalent bonds restricts cells from reorganizing the niche matrix and experiencing traction. Left, schematic of crosslinking of hyaluronic acid hydrogels using MMP-cleavable peptide only (-UV) and UV initiated radical polymerization of methacrylate groups (D0 UV). Right, representative 3D TFM images (a,b) and bright-field images of hMSC staining for ALP (osteogenesis, c) and lipid droplets (adipogenesis, d). Hyaluronic acid hydrogels crosslinked by MMP-leavable peptide without UV (-UV) allowed encapsulated human MSCsto exert traction, and to change their morphology (right), leading to osteogenic differentiation favored by the matrix stiffness of 4.3 kPa. However, in the presence of the non-degradable UV crosslinked hydrogel counterpart (D0 UV), cells did not reorganize the matrix and remained spherical, with adipogenic differentiation predominating. (Scale bar: a,b 15 μm; c,d 20 μm) [11] Reproduced with permission. [11] Copyright 2013, Nature Publishing Group. (C) Mobile ligands and crosslinks in sliding hydrogels provide essential molecular mobility without introducing degradation. Left, ligands and crosslinks are covalently attached to cyclodextrin rings that are confined on the linear PEG chains, with the ability to slide and rotate. Molecular mobility enables encapsulated human MSCs to reorganize the surrounding ligands and (middle) to change their morphology to form protrusions. Right, representative images of hMSCs in a sliding hydrogel stained for (a) neutral lipid accumulation (Oil Red O; adipogenesis), (b) glycoaminoglycan deposition (Safranin O, chondrogenesis), and (c) ALP activity (Fast Blue; osteogenic) after culture in adipogenic, chondrogenic, and osteogenic medium. Without changing the mechanical properties of the sliding hydrogels, human MSCs differentiate toward multiple lineages including adipogenesis, chondrogenesis, and osteogenesis. (Scale bar a-c, 50 μm). [12] Reproduced with permission. [12] Copyright 2016, John Wiley and Sons.
3.3.2. Utilizing degradation-mediated cellular traction
In contrast to physical hydrogels, covalently crosslinked hydrogels, which are widely used and much more stable than physical hydrogels, are less flexible due to the fixed crosslinks that they contain. As such, cells are often restricted by the nanosized meshes and are incapable of reorganizing surrounding niche and ligands. For example, Khetan et al. reported that covalently crosslinked hyaluronic hydrogels could not support osteogenesis by encapsulated MSCs even with varied matrix stiffness.[11] Introducing proteolytic degradation enabled the cells to reorganize the matrix, to change their morphology, and to undergo tractions, which further directed the MSCs from adipogenesis toward osteogenesis (Fig. 3B).[11] Khetan et al. also found that secondary crosslinking (after the spread morphology appeared) inhibited osteogenic commitment[11], indicating that the permissive environment that allows cells to reorganize the niche is more important than mere morphological changes would suggest. While degradation of the hydrogels can rescue cell-mediated reorganization of chemically-crosslinked hydrogel matrices, attention should be paid to the extent and timing of degradation, as well as to the dependence on cell type and enzymatic activity.[23] Degradation can simultaneously impact hydrogel diffusivity and mechanical strength, requiring optimization to achieve the desired tissue formation.
3.3.3. Engineering dynamic adaptable hydrogels to promote microenvironment reorganization
Recent approaches have explored the use of dynamic reversible covalent linkages to crosslink hydrogels, avoiding the drawbacks of attempting to match pre-engineered degradation to a biological process. Linkages formed by amine, oxyamine, or hydrazine with aldehyde (Schiff base)[44], thiol-disulfide exchange[45], and reversible thiol Michael type addition[46] are stable, providing better support to encapsulated cells. But due to their reversibility, these linkages are labile on a time scale that is pertinent to the stress exerted by cells, allowing the cells to reorganize the microenvironment. For example, McKinnon et al. developed an adaptable hydrogel crosslinked by aliphatic hydrazine with aldehyde-terminated poly(ethylene glycol) (PEG).[44] These hydrogels exhibited a frequency-dependent modulus, which indicates that they can relax the applied stress on a time scale on the order of tens of seconds and be deformed under pressures, highlighting the dynamic natures of the linkage and the hydrogel network. These dynamic changes allowed cells to reorganize the matrix niche, to change their morphology, and even to migrate[44]. While these adaptable hydrogels possess great potential for both fundamental and translational applications, further investigations into long-term cell survival and the modulation of cellular phenotype are needed.
3.3.4. Exploiting the molecular mobility of sliding hydrogels to enhance cell differentiation in 3D
Instead of using conventional physical or covalently crosslinked hydrogels, we recently reported the development of cell-compatible supramolecular sliding hydrogels with intrinsic molecular mobility, that yielded enhanced stem-cell differentiation in 3D.[12] Sliding gels are characterized by their topological architecture: the crosslinks and ligands are incorporated into a set of ring-shaped cyclodextrins that are confined on a linear PEG chain but can slide and rotate.[47] As such, the cells in sliding hydrogels utilize this mobility to reorganize ligands and to change their morphology (Fig. 3C). Most interestingly, without changing the stiffness or the degradation kinetics of the material, sliding hydrogels supported the differentiation of encapsulated MSCs into multiple lineages, including adipogenic, chondrogenic, and osteogenic lineages (Fig. 3C). This observation further suggests the importance of permitting cells to reorganize their microenvironment in 3D. Compared to covalent adaptable hydrogels, sliding hydrogels eliminate potential cross-reactions that may interfere with biological processes. For example, the use of aldehydes may cross-react with the amines in proteins and growth factors present in native cell niche, which is avoided in the stable sliding hydrogels.
3.4. Introducing macroporosity into injectable cell matrices
While several advanced engineering approaches have been applied to hydrogels used for cell delivery, nanoporosity remains an intrinsic limitation that restricts clinical applications. Conventional macroporous scaffolds are usually prefabricated from biodegradable polymers (such as poly(lactide-co-glycolide) (PLGA)) and inorganic ceramics, which have achieved notable success in clinical applications and have confirmed the importance of macroporosity for cell-based tissue regeneration. [48] However, none of these scaffolds are injectable; they require unfavorable invasive surgical intervention. Recent studies have explored various ways of achieving injectability with macroporous scaffolds.
3.4.1. Engineering injectable, preformed, macroporous cryo-hydrogels
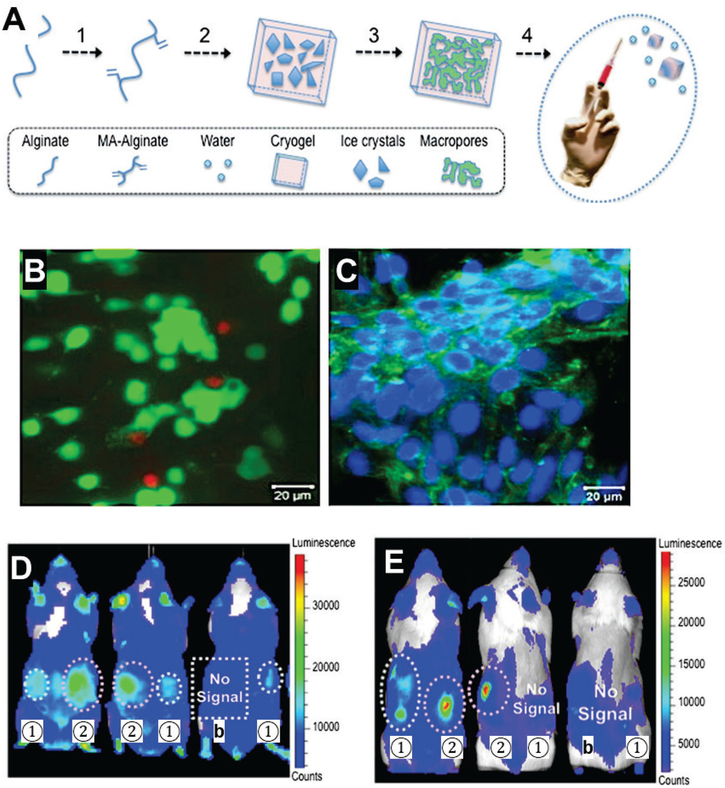
Novel approaches have been explored to fabricate macroporous but injectable scaffolds for cell delivery. The most notable progress has been achieved with cryo-hydrogels, which are used to create hydrogel scaffolds with interconnected pores.[49] Bencherif et al. developed sponge-like cryo-hydrogels with shape-memory properties that withstand reversible deformation and restore the original shape. As such, this cryo-hydrogel can be injected through a submillimeter-sized needle and recover into macroporous scaffolds after injection (Fig. 4A).[49a]] The pore size within the cryo-hydrogels is larger than 10 μm, which allows easy cell infiltration and migration. Human MSCs were loaded into the cryo-hydrogels, which supported robust cellular proliferation (Fig. 4B). These cryo-hydrogels enhanced cell survival and the retention of injected cells at day 2, while cryo-hydrogels with cell adhesion ligand (RGD) retained more cells (Fig. 4C) Furthermore, cryo-hydrogels itself led to high cell survival, and RGD incorporation further enhanced cell survival by day 15.[49a]
Figure 4.
Injectable preformed cryo-hydrogels with shape-memory properties. (A) Scheme of cryo-hydrogel fabrication. Alginate is modified with methacrylate (MA) groups to allow crosslinking at −20 °C. Ice crystals serve as porogens to introduce macroporosity inside cryo-hydrogels after thawing. Cryo-hydrogels can be mixed with cells and injected through a 16G needle, after which they recover their original shape. (B-C) Human MSCs can be seeded into cryo-hydrogels. (B) Live/dead staining of MSCs 1 day after injection showed high cell survival. (Green, live cells; red, dead cells) (C) Confocal live/dead image of cells in cryo-hydrogels cryogels after 6 days of incubation. The porosity of cryo-hydrogel scaffolds supports robust cell proliferation. (D) Luminescent images of C57BL/6J mice injected with bioluminescent reporter cells loaded in cryo-hydrogels without (1) or with (2) cell adhesion peptide (RGD) at day 2 post-injection. Cryo-hydrogels enhanced cell retention 2 days after injection, whereas the injection without delivery matrix (b) showed minimum signal, indicating low cell retention. RGD appeared to lead to more cell retention. (E) Cryo-hydrogels with RGD peptide supported better cell engraftment than cryo-hydrogels without RGD, as indicated by substantially higher cell signal at day 15. Labels are as in panel D.
(A-E) Reproduced with permission. [49a] Copyright 2012, National Academy of Sciences, USA.
One advantage of cryo-hydrogels is that cells can be preloaded into the macroporous cryo-hydrogels before injection, which protects the cells from the shear forces during injection. In addition, cryo-hydrogels are usually mechanically stronger and more stable than conventional hydrogels, thus providing better mechanical protection to transplanted cells after injection. For example, Zeng et al. used macroporous PEG microcryogels as microcarriers to preload MSCs that were injected into the intervertebral disk. [6b] Delivery using these microcryogels improved cell retention and survival versus the injection of free cells, leading to slower degeneration of the intervertebral disk.[6b] Some cryo-hydrogels can be used to co-deliver growth factors that prime implanted cells due to the excellent permeability of these materials.[49c, 50] Similar to most of the pre-fabricated macroporous scaffolds, cell infiltration into prefabricated cryo-hydrogels can be limited by the pore size and the loading method (i.e. static seeding vs. dynamic seeding using a bioreactor). [51] To facilitate cell infiltration and injectability, the diameter of cryo-hydrogels has to be small, ranging from hundreds of microns to a few millimeters.
3.4.2. Using pre-fabricated, spongy microspheres for cell delivery
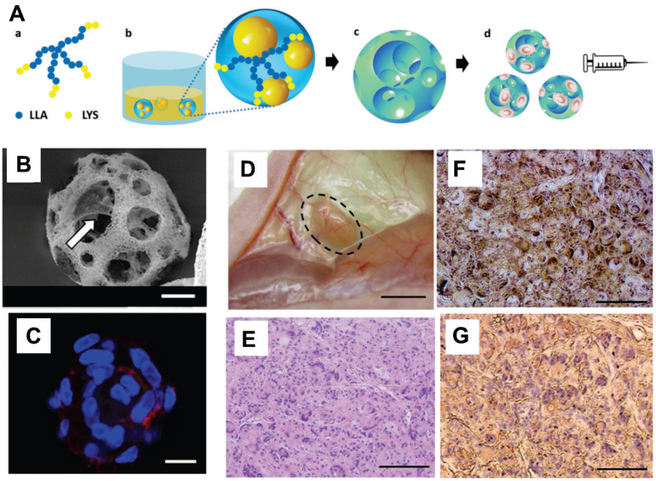
Biodegradable polymers such as poly(lactic acid) (PLA), poly(lactide-co-glycolide) (PLGA) and polycaprolactone have been widely used as cell carriers for tissue engineering applications, due to their high mechanical strength which is usually higher than most hydrogels. [48a, 52] The young’s modulus of most hydrogels typically ranges from 101 Pa to 105 Pa, while the modulus of scaffolds made of PL(G)A is orders of magnitude higher with a range of 106 Pa to 109 Pa. [52] Previous approaches fabricate these polymers directly into implantable tissue-scale scaffolds, which are not suitable for injection. To allow injectability of these polymers as cell carriers, recent approaches explored methods to fabricate these polymers into injectable microspheres, with diameter ranging from 30 to 100 μm, instead of tissue-scale scaffolds.[6a, 20a, 53] For example, Kuang et al. developed injectable, nanofibrous, hollow microspheres from biodegradable poly(L-lactic acid)-block-poly(L-lysine) (Fig 5A).[6a, 53b] These hollow structures allowed dental pulp stem cells to attach to the surface and the inner pores of the spheres (Fig. 5B). These nanofibrous spheres supported cell retention in the injected site and enhanced the odontogenic differentiation of these stem cells in vivo (Fig. 5D-G).[53b] One limitation of the injectable microspheres is the lack of control on the distribution of microspheres post-injection. As such, injectable microspheres may move away from the injection sites under mechanical stress. Furthermore, injectable microspheres do not form a tissue scale scaffold after injection, therefore cannot provide comparable structural support or mechanical strength as a conventional tissue-scale scaffold.
Figure 5.
Nanofibrous spongy microspheres are injectable, porous, cell-delivery matrices that enhance cell retention and proliferation in vivo. (A) Scheme of fabrication of these microspheres. Star-shaped poly(L-lactic acid)-block-polylysine (SS-PLLA-b-PLYS) copolymer (a) is used to form microspheres using a reversed emulsion method (b) and phase separation-freeze drying (c). These porous microspheres can be loaded with cells for delivery via injection (d). (B) Scanning electron microscopy reveals that the microporous structure of these spheres facilitates cell attachment to the surface of the inner pores (arrow). (C) Dental pulp stem cells were well distributed in porous microspheres, with high coverage of the inner surface. Cells were stained with Alexa Fluor 555 phalloidin (F-actin, red) and DAPI (nuclei, blue). (D) Odontogenic differentiation of human dental pulp stem cells in vivo 6 weeks after subcutaneous injection to demonstrate the dentin tissue regeneration using the nanofibrous spongy microspheres as carrier. Cell-loaded spheres were pre-cultured in vitro for 7 days. (E) Hematoxylin and eosin staining indicated substantial tissue formation and high cell density, with even distribution. (F) von Kossa staining demonstrated enhanced mineralization. (G) Nanofibrous spongy microspheres promoted odontogenic differentiation of human dental pulp stem cells, as indicated by immunohistological staining for dentin sialophosphoprotein. (Scale bar: B,C, 10 μm; D, 2 mm; E-G, 100 μm)
(A-G) Reproduced with permission. [53b] Copyright 2015, John Wiley and Sons.
3.4.3. Engineering void-forming hydrogels to achieve macroporosity in in-situ hydrogels
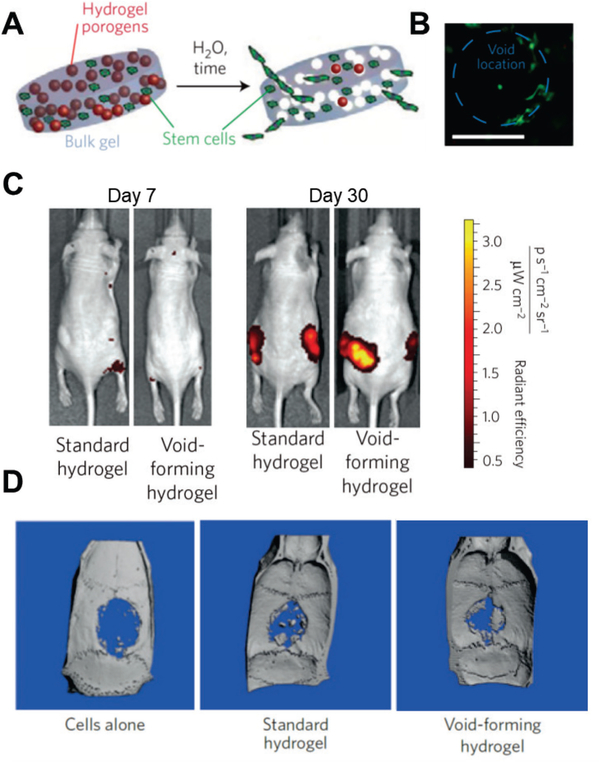
Inspired by the porogen leaching method used to fabricate conventional scaffolds[18], recent studies have explored the use of degradable hydrogels as porogens to introduce macroporosity in situ, such as gelatin hydrogels that undergo a gel-sol transition below 37 °C [54] and alginate hydrogels crosslinked by Ca2+ that are cleared by calcium chelators (e.g. EDTA).[54c] Degradable hydrogels are also used, such as hydrogels formed from oxidized alginate that undergo rapid hydrolytic degradation[55] and hyaluronic acid hydrogels that are degraded by hyaluronidase.[54c] Using these cell-compatible porogen leaching methods, cells and micron-sized porogens can be loaded into the hydrogels and injected. Macroporosity is introduced after ‘leaching’ of the porogens post-transplantation. Huebsch et al. developed an alginate-based void-forming hydrogel for cell delivery to study the effect of matrix elasticity on stem cell-based bone formation.[55] They used standard alginate to form the bulk hydrogel; oxidized alginate formed hydrogels as porogens, which rapidly degraded due to hydrolysis. [55] Cells were initially delivered using the nanoporous hydrogel with co-delivery of the degradable porogens. Pores form in situ after injection, yielding cell-laden macroporous hydrogel scaffolds (Fig. 6A). Huebsch et al. demonstrated that cells were homogenously distributed in the hydrogels and were attached to the inner pores after 10 days of in vitro culture (Fig. 6B). [55] When cells were delivered via void-forming alginate hydrogel, cell proliferation was significantly enhanced 30 days after transplantation due to the macroporsity of the material (Fig. 6C). [55] Further, significantly more formation of new bone was obtained 12 weeks after transplantation with void-forming alginate hydrogel than after transplantation with standard hydrogels and saline controls (Fig. 6D).[55] In void-forming hydrogels, cells are usually encapsulated in the bulk hydrogel fraction, which remains nanoporous. Although the introduction of macropores improved permeability, facilitating perfusion of nutrients and recruitment of endogenous cells, it takes time for cells to migrate out of void-forming alginate hydrogels (dependent on degradation of the bulk hydrogel).
Figure 6.
Void-forming hydrogels used to transplant MSCs for bone formation. (A) Scheme of the fabrication of a void-forming hydrogel. Cells (green) are loaded in bulk hydrogel precursor solution and co-delivered with porogens (red). Pores form after the porogens degrade; cells migrate to the pores and attach to the pore surface. (B) Image of mouse MSCs stained with calcein-AM (green) in void-forming hydrogels 10 days after encapsulation and cultured in vitro. (Scale bar: 100 μm) (C) Cells numbers post-injection revealed by tracking the fluorescence intensity of mCherry-labeled mouse MSCs in vivo. Void-forming hydrogels lead to more rapid proliferation (31-fold increase) from day 7 to day 30, versus standard hydrogels. (D) Void-forming hydrogels enhance bone formation. Human MSCs were delivered using saline, standard hydrogels, or void-forming hydrogels into cranial defects of nude rats. Bone formation was evaluated using micro-computed tomography 12 weeks after transplantation. Transplanted human MSCs within void-forming hydrogels led to significantly more formation of new bone than standard hydrogels and saline controls.
(A-D) Reproduced with permission. [55] Copyright 2015, Nature Publishing Group.
In contrast to standard void-forming hydrogels, other approaches load cells in cell-compatible porogens, so that the cells are released to the pores after the porogens leach. As a result, transplanted cells can quickly access the macropores and eliminate the physical restrictions of nanoporous hydrogels, leading to enhanced cell proliferation, migration, and faster tissue-matrix deposition.[54c, 56] By doing this, topographical features can also be introduced with cell-laden porogens with prefabricated shape. For example, fiber-like porogens can yield scaffolds with microchannel-like structure, which have been used to promote in vivo vascularization.[56] Porogen leaching methods are good for introducing pores in situ, but it is still challenging to obtain interconnected pores, which require highly packed porogens and substantially decrease the mechanical strength of the material after clearance of the porogens.
3.4.4. Engineering hydrogel scaffolds assembled from annealed microsphere building blocks
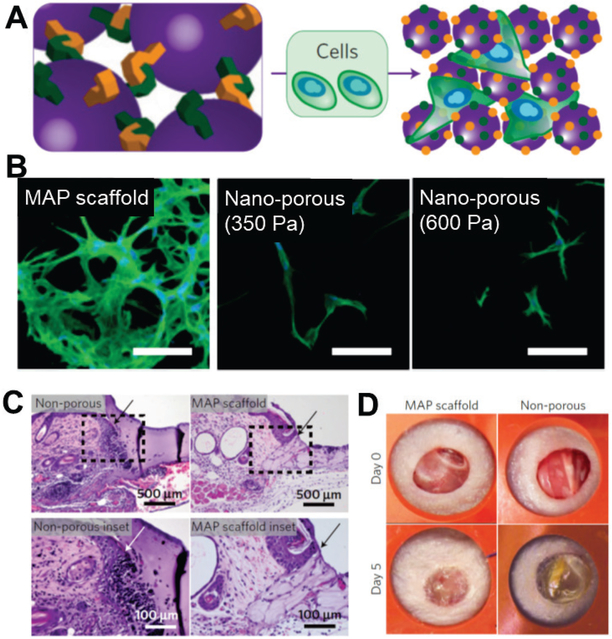
Top-down void-forming methods introduce macroporosity but are associated with low interconnectivity and poor mechanical strength. Thus, recent studies have explored bottom-up approaches to construct macroporous scaffolds in situ with injectable building blocks. Injectable micron-sized building blocks are pre-fabricated and then connected in the presence of cells. The advantages of such approaches include ensured and instant interconnectivity of the pores of the formed scaffolds, modulation of materials properties through building blocks with pre-designed biophysical and biochemical properties, and improved mechanical strength of the formed scaffolds by optimizing the mechanical properties and organization of the building blocks. For example, Griffin et al. developed injectable microporous scaffolds by annealing injectable PEG hydrogel microspheres to accelerate wound healing.[57] The microspheres were incorporated with peptides containing AcFKG and NQEQVSPL moieties, which are covalently bonded by FXIIIa.[58] Applying exogenous FXIIIa and thrombin activated the annealing of the microspheres, resulting in cell-laden microporous scaffolds (Fig. 7A).[57] The interconnected macroporosity substantially enhanced cell proliferation and cell connections, as compared to nanoporous and degradable standard hydrogels (Fig. 7B). [57] Moreover, the injectability of these materials helped with fitting to the lesion site, and their macroporosity further helped connect newly formed tissue with the host tissue. As a result, this injectable scaffold integrated seamlessly with the surrounding host tissue in the wound-healing model, (Fig. 7C). [57] The macroporosity of this system significantly accelerated the wound healing versus the non-porous hydrogel control (Fig. 7D).[57] Employing another approach, Rosales et al. reported macroporous scaffolds formed by crosslining PEG microspheres using the biorthogonal click reaction. These PEG microgels containing dibenzocyclooctyne or azide groups undergo coupling under physiological conditions to form microporous scaffolds in situ.[59].
Figure 7.
Injectable hydrogel microspheres used as building blocks to deliver cells and to build macroporous scaffolds in situ, improving tissue integration and accelerating wound healing. (A) Scheme of hydrogel microspheres incorporated with peptides that can be linked via factor XIII under physiological conditions in the presence of cells, yielding cell-loaded macroporous scaffolds in situ. (B) Human dermal fibroblasts cultured in microporous annealed particle (MAP) scaffolds (350 Pa) and in standard non-porous hydrogels (bulk moduli indicated) for 6 days. Cells continue to proliferate over 6 days and the cell network increased in size and complexity, which cannot be achieved with non-porous hydrogels with identical chemical and mechanical properties. (Scale bar: 100 μm) (C) Injectability and in-situ formation of MAP scaffolds lead to seamless interfaces with the surrounding tissue, indicated by the hematoxylin and eosin staining. (D) MAP scaffolds substantially accelerated wound closure versus the non-porous hydrogel control (60% versus 100% remaining wound area after 5 days, respectively).</p>(A-D) Reproduced with permission. [57] Copyright 2015, Nature Publishing Group.
3.4.5. Engineering microribbon-based, in-situ macroporous hydrogels
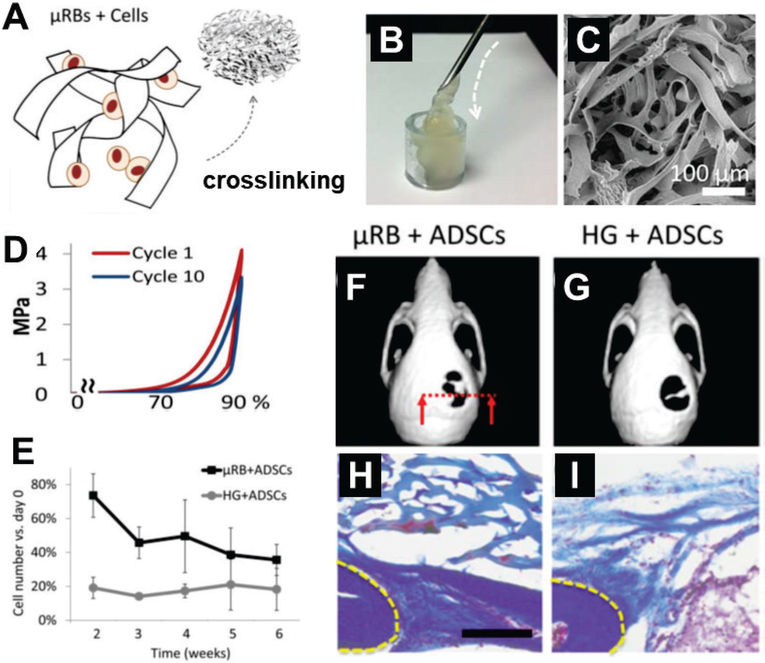
Although microspheres are easily fabricated from building blocks, they do not yield porous scaffolds with sufficient surface area to support cell seeding and growth because spheres have low ratios of surface area to volume. Further, this geometry limits contact between spheres; scaffolds of these microspheres require high packing density, leading to inefficient formation of voids. We recently developed approaches to build macroporous scaffolds using injectable microribbon-like hydrogels as building blocks (Fig. 8A-C). [60] Compared to microspheres, fiber-like or ribbon-like geometries provide higher surface areas for cells and more void-space. In addition, the microribbon-like hydrogels have higher diameter-to-length aspect ratio and higher connectivity between the hydrogel blocks.[6c, 61] Thus, macroporosity can be tuned by varying the density of the blocks, yielding varying levels of cell proliferation and mechanical strength of the bulk scaffolds.[6c] Microribbon-shaped hydrogel building blocks exhibits a higher aspect ratio of the cross-section than microfibers, which dramatically enhance the shock-absorbing capacity of the resulting macroporous scaffolds upon crosslinking. For example, scaffolds made of gelatin-based microribbons revert to their original shape and maintain their mechanical strength after receiving 90% cyclic-strain, while their microfiber counterparts fell apart when strain exceeded 60% (Fig. 8D).[6c, 60] This mechanical stability is especially important for the repair and regeneration of skeletal tissues, which are usually under high mechanical load. With the help of macroporosity, microribbion scaffolds led to more cell retention up to 6 weeks after delivery than the conventional nanoporous control (Fig. 8E). [60] Strikingly, new bone formation and integration with endogenous tissue were both enhanced by microribbon scaffolds, due to their macroporosity (Fig. 8F-I).[60]
Figure 8.
Injectable microribbon (μRB) hydrogels as carriers of stem cells for bone repair. (A) μRB hydrogel building blocks are fabricated using wet-spinning [ref] and can be crosslinked into microporous scaffolds in the presence of cells in situ. (B) Cell-laden μRBs are injected through a 16G needle to form a cell-laden, intact macroporous scaffold. (C) Scanning electron microscopy reveals the interconnected macroporosity of μRB scaffolds. (D) μRB scaffolds exhibit excellent mechanical flexibility and stability, withstanding cycles of compression up to 90% strain. (E) μRB scaffolds led to significantly higher cell survival than conventional hydrogels, with consistently higher cell numbers over 6 weeks. (n=4 per group, error-bars: standard deviations, p<0.05) (F-I) μRB-based scaffolds with adipose-derived stem cells (ADSCs) promote bone healing and bone-scaffold integration. Micro-computed tomography reveals that (F) stem cell-seeded uRB scaffolds led to significantly more mineralized bone formation in critical-size cranial defects than (G) conventional hydrogel controls. (H) Macroporosity in μRB scaffolds promoted more endogenous tissue integration than (I) nanoporous hydrogel scaffolds.
(A-I) Reproduced with permission. [60] Copyright 2016, John Wiley and Sons.
4. Conclusions and future directions
In summary, recent progress in developing injectable matrices have resulted in significant progress in enhancing biocompatibility, cell survival and retention, controlling cell phenotype, introducing desirable macroporosity, and improving scaffold mechanical strength. Further optimization will be required to achieve matrices with properties ideal for specific applications. In-situ hydrogels remain the mostly widely used matrices for cell delivery, due to their ease of handling and versatile modification of physical and biochemical properties. Novel approaches have been explored to overcome concerns associated with conventional in-situ hydrogels. Advanced biorthogonal click chemistry has been utilized to improve biocompatibility; shear-thinning mechanisms have been employed to improve cell survival during and after injection. It should be noted that current studies usually report relatively short-term cell survival (from a few days to a few weeks). Longer-term survival and proliferation of transplanted cells remains challenging. Moreover, the phenotypes and tissue-formation by these cells require further investigation. To enable clinical translation of injectable polymers for tissue engineering applications, considerations must be taken to optimize the biocompatibility of the injectable matrices in vivo to ensure safety. Various natural and synthetic polymers have been approved for acellular applications including collagen, fibronectin, hyaluronic acid, alginate and PEG. However, there is currently no FDA approved hydrogel products for delivering stem cells. [3d, 62] Future studies will need to assess the degradation kinetics and potential toxicity of the degraded by-product using relevant large animal models.
Increasing interest has been paid to elucidating the effects of biophysical and biochemical niche cues in 3D versus conventional 2D culture on cell functions, such as stem-cell proliferation and differentiation. Recognition of the important roles of ligand reorganization, matrix degradation, and molecular mobility in 3D provides more comprehensive criteria for designing matrices for cell delivery. In addition, it is important to consider different aspects of the biomechanics of the tissue, such as viscoelastic properties, elastic property, and dynamic mechanical properties. For example, recent studies have revealed that extracellular matrix-based hydrogels possess unique stress stiffening [14a, 63] and stress relaxation [13, 64] properties, which were not be reproduced using synthetic materials. These findings highlight the importance of comprehensive characterization of different biomechanical properties of new materials.
Injectable macroporous scaffolds for cell delivery and tissue engineering have been developed via top-down porogen leaching methods and bottom-up construction from building blocks. Given the importance of macroporisity to tissue regeneration, these approaches have opened important new prospects for the application of injectable matrices to regenerative medicine. Although the injectable macroporous scaffolds described here provide better mechanical strength than standard in-situ hydrogels, they are still not as strong as conventional macroporous plastic polymer materials (e.g. PLGA, PCL) and inorganic ceramics. Combining macroporous scaffolds with stronger building blocks may be necessary to achieve the regeneration of load-bearing tissues such as bone.
Bottom-up approaches assemble macroporous scaffolds in situ from pre-fabricated injectable building blocks, which can be designed with varying geometries and biophysical and biochemical properties. These approaches can be used to better recapitulate the highly complex physical and biochemical properties, as well as the heterogeneous microstructures, of the natural tissues. Bottom-up assembly approaches could also be used for post-injection scaffold formation to a desired organization and architecture, for example by introducing reorganizable building blocks [65]. These approaches enable the use of zonal, organized scaffolds in situ to promote desirable cell phenotypes and matrix production, better mimicking the organization of natural tissue. Nonetheless, future efforts will be needed to achieve the goal of fully mimicking the comprehensive structure and composition of tissue with injectable matrices.
Table 1.
Recent developed approaches to address challenges associated with the conventional injectable matrices for cell delivery and tissue regeneration.
| Major challenges | Approaches | Examples | ref |
|---|---|---|---|
| Biocompatibility of in-situ hydrogels | Introducing bio-orthogonal click chemistry | Strain-promoted azide-alkyne cycloaddition (SPAAC) | [29–30] |
| Tetrazole-alkene | [31] | ||
| Tetrazine-norbornene | [32] | ||
| Shear induced cell death | Developing shear-thinning hydrogels | Peptide amphiphiles | [38][39] |
| Bio-recognition based hydrogels | [35][40] | ||
| Host-guest supramolecular hydrogels | [41] | ||
| Ionic crosslinked hydrogels | [5a,5e] | ||
| Lack of support on desirable cell phenotype in 3D | Engineering hydrogels with supporting properties | Matrix stiffness regulated reorganization of adhesion ligands | [10a] |
| Degradation mediated cell traction | [11] | ||
| Dynamic covalent linkage permitted microenvironment reorganization | [44–46] | ||
| Molecular mobility supporting multiple lineage differentiation | [12] | ||
| Lack of macroporisity | Engineering pre-fabricated injectable scaffolds | Injectable preformed macroporous cryo-hydrogels | [49a] |
| Injectable pre-fabricated spongy micro-carriers | [6a,6b,53b] | ||
| Top-down strategies to introduce macroporisity in situ | Void-forming hydrogels | [55][56] | |
| Bottom-up strategies to construct macroporous scaffolds using injectable building blocks | Assembled and annealed microspheres | [57][59] | |
| Microribbon based in-situ macroporous hydrogels | [6c,60–61] | ||
Acknowledgements
This work was supported by the following grants: NIH R01DE024772 (F.Y.), NSF CAREER award (CBET-1351289) (F.Y.), and California Institute for Regenerative Medicine Tools and Technologies Award (RT3–07804) (F.Y.). The authors also acknowledge funding from the Stanford Chem-H Institute (F.Y.), Stanford Bio-X Interdisciplinary Initiative Program (F.Y.), the Stanford Child Health Research Institute Faculty Scholar Award (F.Y.), and Alliance for Cancer Gene Therapy Young Investigator award grant (F.Y.).
Contributor Information
Xinming Tong, Department of Orthopaedic Surgery, Stanford University School of Medicine, CA, 94305, United States. xinming@stanford.edu.
F. Yang, Department of Orthopaedic Surgery and Bioengineering, Stanford University School of Medicine, 300 Pasteur Dr., Edwards R105, CA, 94305, United States. fanyang@stanford.edu
References
- [1].a) Witten CM, McFarland RD, Simek SL, Stem cells translational medicine 2015, 4, 1495; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Daar AS, Transplant Proc 2013, 45, 3450; [DOI] [PubMed] [Google Scholar]; cMason C, Dunnill P, Regen Med 2008, 3, 1. [DOI] [PubMed] [Google Scholar]
- [2].a) Khademhosseini A, Langer R, Nat. Protocols 2016, 11, 1775; [DOI] [PubMed] [Google Scholar]; b) Langer R, Vacanti J, Science 1993, 260, 920. [DOI] [PubMed] [Google Scholar]
- [3].a)Drury JL, Mooney DJ, Biomaterials 2003, 24, 4337; [DOI] [PubMed] [Google Scholar]; b) Hollister SJ, Nature Materials 2006, 5, 590; [Google Scholar]; c) Griffith LG, Naughton G, Science 2002, 295, 1009; [DOI] [PubMed] [Google Scholar]; d) Lutolf MP, Hubbell JA, Nature biotechnology 2005, 23, 47; [DOI] [PubMed] [Google Scholar]; e) Place ES, Evans ND, Stevens MM, Nat Mater 2009, 8, 457. [DOI] [PubMed] [Google Scholar]
- [4].a) Seliktar D, Science 2012, 336, 1124; [DOI] [PubMed] [Google Scholar]; b) Ko DY, Shinde UP, Yeon B, Jeong B, Progress in Polymer Science 2013, 38, 672; [Google Scholar]; c) Van Tomme SR, Storm G, Hennink WE, International journal of pharmaceutics 2008, 355, 1; [DOI] [PubMed] [Google Scholar]; d) Yang J-A, Yeom J, Hwang BW, Hoffman AS, Hahn SK, Progress in Polymer Science 2014, 39, 1973; [Google Scholar]; eYu L, Ding J, Chem Soc Rev 2008, 37, 1473. [DOI] [PubMed] [Google Scholar]
- [5].a) Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC, Tissue Eng Part A 2012, 18, 806; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Guvendiren M, Lu HD, Burdick JA, Soft Matter 2012, 8, 260; [Google Scholar]; c) Rodell CB, MacArthur JW, Dorsey SM, Wade RJ, Wang LL, Woo YJ, Burdick JA, Advanced Functional Materials 2015, 25, 636; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Highley CB, Rodell CB, Burdick JA, Adv Mater 2015, 27, 5075; [DOI] [PubMed] [Google Scholar]; e) Thakur A, Jaiswal MK, Peak CW, Carrow JK, Gentry J, Dolatshahi-Pirouz A, Gaharwar AK, Nanoscale 2016, 8, 12362. [DOI] [PubMed] [Google Scholar]
- [6].a) Kuang R, Zhang Z, Jin X, Hu J, Shi S, Ni L, Ma PX, Acta Biomaterialia 2016, 33, 225; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zeng Y, Chen C, Liu W, Fu Q, Han Z, Li Y, Feng S, Li X, Qi C, Wu J, Wang D, Corbett C, Chan BP, Ruan D, Du Y, Biomaterials 2015, 59, 53; [DOI] [PubMed] [Google Scholar]; c) Han L-H, Yu S, Wang T, Behn AW, Yang F, Advanced Functional Materials 2013, 23, 346. [Google Scholar]
- [7].a) Kamata H, Li X, Chung U.-i., Sakai T, Advanced healthcare materials 2015, DOI: 10.1002/adhm.201500076n/a; [DOI] [PubMed] [Google Scholar]; b) Vermonden T, Censi R, Hennink WE, Chemical Reviews 2012, DOI: 10.1021/cr200157d. [DOI] [PubMed] [Google Scholar]
- [8].Caliari SR, Vega SL, Kwon M, Soulas EM, Burdick JA, Biomaterials 2016, 103, 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Atrah HI, Bmj 1994, 308, 933; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Leach J. Baier, Bivens KA, Patrick CW Jr., Schmidt CE, Biotechnology and bioengineering 2003, 82, 578; [DOI] [PubMed] [Google Scholar]; c) Litwiniuk M, Krejner A, Speyrer MS, Gauto AR, Grzela T, Wounds 2016, 28, 78. [PubMed] [Google Scholar]
- [10].a) Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ, Nat Mater 2010, 9, 518; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dingal PCDP, Discher DE, Nature Materials 2014, 13, 532; [DOI] [PubMed] [Google Scholar]; c) Discher DE, Mooney DJ, Zandstra PW, Science 2009, 324, 1673; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Discher DE, Janmey P, Wang YL, Science 2005, 310, 1139. [DOI] [PubMed] [Google Scholar]
- [11].Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA, Nat Mater 2013, 12, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tong X, Yang F, Adv Mater 2016, 28, 7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee H.-p., Lippens E, Duda GN, Mooney DJ, Nat Mater 2016, 15, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].a) Das RK, Gocheva V, Hammink R, Zouani OF, Rowan AE, Nat Mater 2016, 15, 318; [DOI] [PubMed] [Google Scholar]; b) Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA, Nature 2005, 435, 191. [DOI] [PubMed] [Google Scholar]
- [15].a) Rienks M, Papageorgiou A-P, Frangogiannis NG, Heymans S, Circulation research 2014, 114, 872; [DOI] [PubMed] [Google Scholar]; b) Huh D, Hamilton GA, Ingber DE, Trends in Cell Biology 2011, 21, 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hollister SJ, Nat Mater 2005, 4, 518. [DOI] [PubMed] [Google Scholar]
- [17].a) Lee S, Tong X, Yang F, Biomaterials Science 2016, 4, 405; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lee S, Tong X, Yang F, Acta Biomaterialia 2014, 10, 4167. [DOI] [PubMed] [Google Scholar]
- [18].Wei G, Ma PX, Journal of biomedical materials research. Part A 2006, 78, 306. [DOI] [PubMed] [Google Scholar]
- [19].a) Harris LD, Kim B-S, Mooney DJ, Journal of Biomedical Materials Research 1998, 42, 396; [DOI] [PubMed] [Google Scholar]; b) Shastri VP, Martin I, Langer R, Proceedings of the National Academy of Sciences 2000, 97, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].a) Liu XH, Jin XB, Ma PX, Nature Materials 2011, 10, 398; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu X, Ma PX, Biomaterials 2009, 30, 4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].a) Kirchmajer DM, Gorkin Iii R, in het Panhuis M, J. Mater. Chem. B 2015, 3, 4105; [DOI] [PubMed] [Google Scholar]; b) Billiet T, Gevaert E, De Schryver T, Cornelissen M, Dubruel P, Biomaterials 2014, 35, 49. [DOI] [PubMed] [Google Scholar]
- [22].Thiriet M, in Tissue Functioning and Remodeling in the Circulatory and Ventilatory Systems, DOI: 10.1007/978-1-4614-5966-8_11, Springer New York, New York, NY: 2013, p. 675. [DOI] [Google Scholar]
- [23].a) Patterson J, Hubbell JA, Biomaterials 2011, 32, 1301; [DOI] [PubMed] [Google Scholar]; b) Patterson J, Hubbell JA, Biomaterials 2010, 31, 7836; [DOI] [PubMed] [Google Scholar]; c) Lutolf MP, Raeber GP, Zisch AH, Tirelli N, Hubbell JA, Adv Mater 2003, 15, 888. [Google Scholar]
- [24].Khanlari S, Dubé MA, Macromolecular Reaction Engineering 2013, 7, 573. [Google Scholar]
- [25].a) Ballios BG, Cooke MJ, Donaldson L, Coles BL, Morshead CM, van der Kooy D, Shoichet MS, Stem Cell Reports 2015, 4, 1031; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, Fairbrother DH, Cascio B, Elisseeff JH, Nat Mater 2007, 6, 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].a) Albrecht K, Moeller M, Groll J, 2011, 234, 65; [Google Scholar]; b) Zhu J, Biomaterials 2010, 31, 4639; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Liu SQ, Tay R, Khan M, Rachel Ee PL, Hedrick JL, Yang YY, Soft Matter 2010, 6, 67. [Google Scholar]
- [27].McCall JD, Anseth KS, Biomacromolecules 2012, 13, 2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].a) Palmer JT, Rasnick D, Klaus JL, Bromme D, Journal of Medicinal Chemistry 1995, 38, 3193; [DOI] [PubMed] [Google Scholar]; b) Rosenthal PJ, Olson JE, Lee GK, Palmer JT, Klaus JL, Rasnick D, Antimicrobial Agents and Chemotherapy 1996, 40, 1600; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Yarbrough JW, Schultz TW, Chem Res Toxicol 2007, 20, 558. [DOI] [PubMed] [Google Scholar]
- [29].Sletten EM, Bertozzi CR, Angewandte Chemie 2009, 48, 6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].a) Gordon CG, Mackey JL, Jewett JC, Sletten EM, Houk KN, Bertozzi CR, Journal of the American Chemical Society 2012, 134, 9199; [DOI] [PMC free article] [PubMed] [Google Scholar]; bJiang H, Qin S, Dong H, Lei Q, Su X, Zhuo R, Zhong Z, Soft Matter 2015, 11, 6029; [DOI] [PubMed] [Google Scholar]; c) Takahashi A, Suzuki Y, Suhara T, Omichi K, Shimizu A, Hasegawa K, Kokudo N, Ohta S, Ito T, Biomacromolecules 2013, 14, 3581; [DOI] [PubMed] [Google Scholar]; d) Hermann CD, Wilson DS, Lawrence KA, Ning X, Olivares-Navarrete R, Williams JK, Guldberg RE, Murthy N, Schwartz Z, Boyan BD, Biomaterials 2014, 35, 9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].a) Oneto J. M. Mejia, Khan I, Seebald L, Royzen M, ACS Cent Sci 2016, 2, 476; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fan Y, Deng C, Cheng R, Meng F, Zhong Z, Biomacromolecules 2013, 14, 2814. [DOI] [PubMed] [Google Scholar]
- [32].a) Koshy ST, Desai RM, Joly P, Li J, Bagrodia RK, Lewin SA, Joshi NS, Mooney DJ, Advanced healthcare materials 2016, 5, 541; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hong S, Carlson J, Lee H, Weissleder R, Advanced healthcare materials 2016, 5, 421; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Desai RM, Koshy ST, Hilderbrand SA, Mooney DJ, Joshi NS, Biomaterials 2015, 50, 30. [DOI] [PubMed] [Google Scholar]
- [33].Barnes HA, Journal of Non-Newtonian Fluid Mechanics 1997, 70, 1. [Google Scholar]
- [34].a) Zhao X, Pan F, Xu H, Yaseen M, Shan H, Hauser CA, Zhang S, Lu JR, Chem Soc Rev 2010, 39, 3480; [DOI] [PubMed] [Google Scholar]; b) Lowik DW, van Hest JC, Chem Soc Rev 2004, 33, 234. [DOI] [PubMed] [Google Scholar]
- [35].a) Parisi-Amon A, Mulyasasmita W, Chung C, Heilshorn SC, Advanced healthcare materials 2013, 2, 428; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wong Po Foo CT, Lee JS, Mulyasasmita W, Parisi-Amon A, Heilshorn SC, Proc Natl Acad Sci U S A 2009, 106, 22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ulijn RV, Smith AM, Chem Soc Rev 2008, 37, 664. [DOI] [PubMed] [Google Scholar]
- [37].a) Cui H, Webber MJ, Stupp SI, Biopolymers 2010, 94, 1; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Branco MC, Schneider JP, Acta Biomater 2009, 5, 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Haines-Butterick L, Rajagopal K, Branco M, Salick D, Rughani R, Pilarz M, Lamm MS, Pochan DJ, Schneider JP, Proc Natl Acad Sci U S A 2007, 104, 7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Webber MJ, Tongers J, Renault MA, Roncalli JG, Losordo DW, Stupp SI, Acta Biomater 2010, 6, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].a) Cai L, Dewi RE, Heilshorn SC, Advanced Functional Materials 2015, 25, 1344; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Cai L, Dewi RE, Goldstone AB, Cohen JE, Steele AN, Woo YJ, Heilshorn SC, Advanced healthcare materials 2016, 5, 2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rodell CB, Kaminski AL, Burdick JA, Biomacromolecules 2013, DOI: 10.1021/bm401280z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rodell CB, Dusaj NN, Highley CB, Burdick JA, Adv Mater 2016, 28, 8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tenney RM, Discher DE, Curr Opin Cell Biol 2009, 21, 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].a) McKinnon DD, Domaille DW, Cha JN, Anseth KS, Chemistry of Materials 2014, 26, 2382; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) McKinnon DD, Domaille DW, Cha JN, Anseth KS, Adv Mater 2014, 26, 865; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wu Y, Wang L, Zhao X, Hou S, Guo B, Ma PX, Biomaterials 2016, 104, 18; [DOI] [PubMed] [Google Scholar]; d) McKinnon DD, Domaille DW, Brown TE, Kyburz KA, Kiyotake E, Cha JN, Anseth KS, Soft Matter 2014, 10, 9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].a) Yu H, Wang Y, Yang H, Peng K, Zhang X, J. Mater. Chem. B 2017, 5, 4121; [DOI] [PubMed] [Google Scholar]; b) Gok O, Erturk P, Sumer Bolu B, Gevrek TN, Sanyal R, Sanyal A, Biomacromolecules 2017, DOI: 10.1021/acs.biomac.7b00619. [DOI] [PubMed] [Google Scholar]
- [46].a) Zhang B, Digby ZA, Flum JA, Chakma P, Saul JM, Sparks JL, Konkolewicz D, Macromolecules 2016, 49, 6871; [Google Scholar]; b) Baldwin AD, Kiick KL, Polymer Chemistry 2013, 4, 133; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kharkar PM, Kloxin AM, Kiick KL, J Mater Chem B Mater Biol Med 2014, 2, 5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].a) Harada A, Hashidzume A, Yamaguchi H, Takashima Y, Chem Rev 2009, 109, 5974; [DOI] [PubMed] [Google Scholar]; b) H. A, Acta Polymerica 1998, 49, 3; [Google Scholar]; c) Ito K, Current Opinion in Solid State and Materials Science Polymers 2010, 14, 28; [Google Scholar]; d) Karino T, Shibayama M, Ito K, Phys. B 2006, 385–386, 692; [Google Scholar]; e) Okumura Y, Ito K, Adv. Mater 2001, 13, 485. [Google Scholar]
- [48].a) Pan Z, Ding J, Interface Focus 2012, 2, 366; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dorozhkin SV, Biomaterials 2010, 31, 1465; [DOI] [PubMed] [Google Scholar]; c) Ma PX, Choi JW, Tissue engineering 2001, 7, 23. [DOI] [PubMed] [Google Scholar]
- [49].a) Bencherif SA, Sands RW, Bhatta D, Arany P, Verbeke CS, Edwards DA, Mooney DJ, Proc Natl Acad Sci U S A 2012, 109, 19590; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Cheng L, Ji K, Shih TY, Haddad A, Giatsidis G, Mooney DJ, Orgill DP, Nabzdyk CS, Tissue Eng Part A 2017, 23, 243; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Koshy ST, Ferrante TC, Lewin SA, Mooney DJ, Biomaterials 2014, 35, 2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].a) Bencherif SA, Warren Sands R, Ali OA, Li WA, Lewin SA, Braschler TM, Shih T-Y, Verbeke CS, Bhatta D, Dranoff G, Mooney DJ, Nat Commun 2015, 6; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu Y, Xu K, Chang Q, Darabi MA, Lin B, Zhong W, Xing M, Adv Mater 2016, 28, 7758; [DOI] [PubMed] [Google Scholar]; c) Zeng Y, Feng S, Liu W, Fu Q, Li Y, Li X, Chen C, Huang C, Ge Z, Du Y, J Biomed Mater Res B Appl Biomater 2017, 105, 507. [DOI] [PubMed] [Google Scholar]
- [51].a) Loh QL, Choong C, Tissue engineering. Part B, Reviews 2013, 19, 485; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Thevenot P, Nair A, Dey J, Yang J, Tang L, Tissue Eng Part C Methods 2008, 14, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].a) Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR, Biomaterials 2006, 27, 3413; [DOI] [PubMed] [Google Scholar]; b) Agrawal CM, Ray RB, Journal of Biomedical Materials Research 2001, 55, 141. [DOI] [PubMed] [Google Scholar]
- [53].a) Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS, Jhunjhunwala S, Chiu A, Siebert S, Tang K, Hollister-Lock J, Aresta-Dasilva S, Bochenek M, Mendoza-Elias J, Wang Y, Qi M, Lavin DM, Chen M, Dholakia N, Thakrar R, Lacik I, Weir GC, Oberholzer J, Greiner DL, Langer R, Anderson DG, Nat Mater 2015, 14, 643; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kuang R, Zhang Z, Jin X, Hu J, Gupte MJ, Ni L, Ma PX, Advanced healthcare materials 2015, 4, 1993; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ma C, Jing Y, Sun H, Liu X, Advanced healthcare materials 2015, 4, 2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].a) Hwang CM, Sant S, Masaeli M, Kachouie NN, Zamanian B, Lee SH, Khademhosseini A, Biofabrication 2010, 2, 035003; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Leong W, Lau TT, Wang DA, Acta Biomater 2013, 9, 6459; [DOI] [PubMed] [Google Scholar]; c) Han L-H, Lai JH, Yu S, Yang F, Biomaterials 2013, 34, 4251; [DOI] [PubMed] [Google Scholar]; d) Wang L, Lu S, Lam J, Kasper FK, Mikos AG, Tissue Eng Part C Methods 2015, 21, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Huebsch N, Lippens E, Lee K, Mehta M, Koshy Sandeep T., Darnell Max C., Desai RM, Madl Christopher M., Xu M, Zhao X, Chaudhuri O, Verbeke C, Kim Woo S., Alim K, Mammoto A, Ingber Donald E., Duda Georg N., Mooney David J., Nature Materials 2015, 14, 1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].a) Madl CM, Keeney M, Li X, Han LH, Yang F, Tissue Eng Part C Methods 2014, 20, 798; [DOI] [PubMed] [Google Scholar]; b) Hammer J, Han L-H, Tong X, Yang F, Tissue Engineering Part C-Methods 2014, 20, 169. [DOI] [PubMed] [Google Scholar]
- [57].Griffin DR, Weaver WM, Scumpia PO, Di Carlo D, Segura T, Nat Mater 2015, 14, 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].a) Schense JC, Hubbell , Bioconjugate Chemistry 1998, 10, 75; [DOI] [PubMed] [Google Scholar]; b) Mosiewicz KA, Kolb L, van der Vlies AJ, Martino MM, Lienemann PS, Hubbell JA, Ehrbar M, Lutolf MP, Nat Mater 2013, 12, 1072. [DOI] [PubMed] [Google Scholar]
- [59].Caldwell AS, Campbell GT, Shekiro KMT, Anseth KS, Advanced healthcare materials 2017, DOI: 10.1002/adhm.201700254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Han LH, Conrad B, Chung MT, Deveza L, Jiang X, Wang A, Butte MJ, Longaker MT, Wan D, Yang F, Journal of biomedical materials research. Part A 2016, 104, 1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Han L-H, Tong X, Yang F, Advanced Materials Adv. Mater. 2014, 26, 1757. [DOI] [PubMed] [Google Scholar]
- [62].a) I T, P W, K S, 2011, DOI: 10.5772/19775; [DOI] [Google Scholar]; b) Prestwich GD, Organogenesis 2014, 4, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Winer JP, Oake S, Janmey PA, PloS one 2009, 4, e6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chaudhuri O, Gu L, Darnell M, Klumpers D, Bencherif SA, Weaver JC, Huebsch N, Mooney DJ, Nat Commun 2015, 6, 6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].a) Zamanian B, Masaeli M, Nichol JW, Khabiry M, Hancock MJ, Bae H, Khademhosseini A, Small 2010, 6, 937; [DOI] [PMC free article] [PubMed] [Google Scholar]; bHarada A, Kobayashi R, Takashima Y, Hashidzume A, Yamaguchi H, Nature Chem. 2010, DOI: 10.1038/nchem.893. [DOI] [PubMed] [Google Scholar]