Abstract
Precise synaptic connections among neurons in the neocortex generate the circuits that underlie a broad repertoire of cortical functions including perception, learning and memory, and complex problem solving. The specific patterns and properties of these synaptic connections are fundamental to the computations cortical neurons perform. How such specificity arises in cortical circuits has remained elusive. Here, we first consider the cell-type, subcellular and synaptic specificity required for generating mature patterns of cortical connectivity and responses. Next, we focus on recent progress in understanding how the synaptic connections among excitatory cortical projection neurons are established during development using the primary visual cortex of the mouse as a model.
Introduction
The neocortex is composed of many different types of neurons, each with distinct patterns of synaptic connectivity conferring different functions in vivo. The majority of these cell types are excitatory cortical projection neurons, with intracortical axons forming local synaptic connections within the cortex and long-range axons targeting distinct subsets of distant cortical and subcortical regions [1–3]. Although precise patterns of local intracortical synaptic connections are essential for proper cortical function, how local cortical circuits are established remains elusive. With the advent of two-photon in vivo imaging combined with whole-cell recordings of unitary synaptic connections among other techniques, recent work focused on mouse primary visual cortex (V1) has begun to shed light on the time course and mechanisms that generate the mature patterns of intracortical synaptic connections and resulting response properties of cortical neurons.
Mouse visual cortex as a model for cortical circuit development
The primary visual cortex of the mouse is traditionally divided into six layers. Following the canonical cortical microcircuit, incoming sensory information primarily enters layer 4 (L4), passes to layer 2/3 (L2/3) and then on to layers 5 and 6 (L5, L6) [4–6]. Although early work suggested that neurons with different response properties in mouse V1 were intermingled in a ‘salt-and-pepper’ pattern [7–9], recent studies have demonstrated more functional organization than previously appreciated [10,11••,12•,13•,14]. For instance, neurons that share orientation preferences are weakly clustered into vertical columns [12•,13•], and L5 pyramids with similar long-range projection patterns are also clustered into micro-columns in mouse V1 [10,11••]. In the horizontal plane, clusters of L2/3 neurons with distinct tuning preferences are aligned with patches of M2 muscarinic acetylcholine receptor expression and L1 patches, defined by the termination patterns of geniculocortical and feedback inputs into V1 [14]. Taken together, these studies indicate that the patterns of synaptic connectivity in mouse V1 exist within a columnar and tangential cortical organization.
Specificity of synaptic connections within the neocortex
Within this overarching organization, cortical projection neurons form precise synaptic connections defined at different scales. First, they establish cell-type specific patterns of synaptic connections. For example, the probability of forming synaptic connections among different classes of L5 cortical projection neurons defined by their long-range axonal targets depends on the identity of the presynaptic and postsynaptic cell types [15–17]. Similarly, L2/3 neurons defined by similar receptive field properties are preferentially connected [18,19•,20,21•]. Second, specific subcellular compartments of projection neurons receive distinct synaptic inputs. Inhibitory Chandelier cells, which synapse onto the axon initial segments of cortical projection neurons, are perhaps the most famous example [22]. However, Chandelier cells are not exceptional as other classes of inhibitory neuron similarly target particular dendritic compartments of cortical projection neurons [23,24]. Similarly, long-range inputs to the cortex also synapse onto specific dendritic compartments [25] as do local connections among cortical projection neurons [26–31]. Third, alongside specificity in target choice and location for synapse formation, developmental mechanisms must establish the appropriate synaptic properties for each connection (for review, see [32]). For example, in addition to being preferentially interconnected, L2/3 neurons with shared response properties also form stronger synaptic connections than average [19•,21•]. These studies highlight the many challenges in establishing the mature patterns of intra-cortical synaptic connectivity that shape the activity of adult cortex.
Clonally related neurons are connected via gap junctions in the first postnatal week
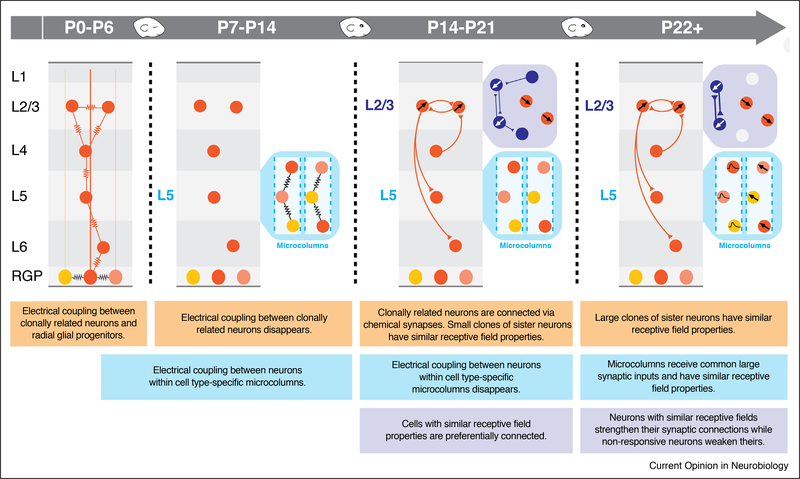
One possibility is that cell lineage seeds the initial synaptic organization of local cortical circuits. Radial glial progenitors (RGPs) in the ventricular zone generate cortical neurons in an inside-out fashion, such that L6 neurons are born first and L2 neurons last. This process produces clonally related sister neurons arising from the same RGP that may represent the basis of the cortical column [33]. Gap junctions, which mediate coordinated electrical activity and the passage of small molecules among connected cells (for review, see [34]), preferentially connect vertically aligned, clonally related neurons through the first postnatal week before disappearing by postnatal day (P)6 [35,36••] (Figure 1, P0–P6 panel). These clonally related sister neurons go on to preferentially form chemical synaptic connections after the initial electrical connections have been eliminated [35,37]. Strikingly, these chemical connections within ontogenetic columns reflect the flow of information through the canonical cortical microcircuit, from L4 to L2/3 to L5 and L6 [37]. The formation of these early gap junctions and the subsequent preferential chemical synaptic connections among sister neurons is disrupted when normal neuronal migration is disturbed either by abolishing Reelin signaling, essential for the normal inside-out development of the neocortex, or by altering the tangential migration of sister neurons through Ephrin-A signaling [36••].
Figure 1.
Development of intracortical synaptic connections in the visual cortex of the mouse. The formation of specific patterns of intracortical synaptic connectivity is regulated by activity-dependent and independent mechanisms. Because most studies sample only a subset of developmental time points, the precise biological start and end of the processes illustrated and their temporal relationships remain unclear. How these mechanisms act together to elaborate synaptic connections among clonally related cortical projection neurons (orange), among neurons in cortical microcolumns (cyan), and among neurons with related response properties (dark blue) at the cell-type, subcellular and synaptic level remains to be fully elucidated.
Projection neurons within cortical microcolumns are connected via gap junctions
In addition to clonal networks, small clusters of vertically aligned neuronal cell bodies form microcolumns within the cortex [10,11••,12•]. The neurons within microcolumns share long-range axonal projection patterns [10], and these cell-type specific columnar clusters in L5 tile the cortex in a hexagonal lattice, with a period of approximately 30 μm [10,11••]. Although most neurons in a microcolumn are not clonally related, they are also electrically coupled early in cortical development via cell-type specific gap junctions [11••] (Figure 1, P7–P14 panel). Unlike gap junctions among clonally related sister cells which have largely disappeared by P6–7 [35], the electrical coupling within L5 microcolumns persists longer, becoming undetectable around P10–14, before the time of eye opening [11••]. In contrast to clonally related cortical neurons, no preferential chemical synapses were found within microcolumns after gap junctions among neurons within a microcolumn had disappeared [11••,38]. However, neurons within microcolumns share strong, common synaptic inputs [11••]. The relationship between electrical coupling of clonally related neurons and elec- trical coupling of neurons within microcolumns remains unclear.
Inhibiting gap junctions in early development disrupts cortical circuit formation
These early electrical connections play an important role in establishing local cortical connections and cortical receptive field properties [35,39,40]. Connexin26 is a gap junction protein highly expressed in the developing cortex. Expressing a dominant negative form of Connexin26, for example, in L2/3 projection neurons starting at embryonic day (E) 12–13 reduced the subsequent formation of preferential chemical synaptic connections between related sister neurons in V1 of P12–17 mice [35] and also the similarity in response properties among sister neurons [40]. However, any differences in the contributions of gap junctions specifically among clonally related neurons, among neurons within a microcolumn, or among yet to be defined neurons to the initial establishment of cortical circuits, remains to be clarified.
How these gap junctions influence the later synaptic organization of cortical circuits also remains unclear. One possibility is that spontaneous activity before eye opening coordinates the activity of electrically coupled neurons [35] and contributes to the initial formation of preferential connectivity between neurons that share receptive field properties [18,19•,20,21•,41]. Modeling studies suggested that cell pairs are more likely to stabilize the same set of feedforward thalamocortical inputs and share similar receptive field properties if they were connected via gap junctions during the first postnatal week [41]. This prediction is consistent with the finding that clonally related sister neurons have more similar orientation preferences than unrelated cortical neurons in mature circuits [40,42].
Contributions of neural activity to early circuit formation
Early in development, the transmission frequency of dendritic spine responses that are poorly synchronized with their neighbors during spontaneous activity becomes reduced [43]. This process may contribute to functional clustering of spines within the dendritic arbor of cortical neurons [44•,45] and the formation of strong, shared, inputs between L5 neurons within a microcolumn [11••]. The resulting clustering of coordinately active inputs may also be reflected in the clustered distribution of synaptic inputs with particular receptive field properties within the dendritic arbors of mature L2/3 neurons: spines responding to the same location in visual space as a neuron’s receptive field preferentially cluster on neighboring spines of proximal dendrites while spines responding to regions beyond the receptive field are found on higher order branches [44•].
These findings have been interpreted to mean that mechanisms dependent on spontaneous activity prior to eye opening underlie the formation of early cortical circuits. However overexpression of the potassium channel Kir2.1, to suppress L2/3 neurons beginning at late embryonic stages, suppressed spontaneous activity before eye opening but did not affect the initial development of orientation and direction selectivity, suggesting that these receptive field properties develop in an activity-independent manner [46••]. Whether this manipulation affected the pattern of chemical synaptic connections among neurons with similar receptive field properties or among clonally related neurons akin to inhibition of gap junctions was not tested. Thus, the precise mechanisms by which gap junctions and spontaneous activity contribute to the patterns of synaptic connectivity in mouse V1 prior to visual experience remain to be fully elucidated.
Molecular contributions to early cortical development
Additional molecular mechanisms have also been implicated in establishing synaptic relationships prior to visual experience, but how they influence specificity in circuit formation is not well understood. For example, a recent study implicated Dnmt3b DNA methyltransferase in stabilizing reciprocal chemical connections among clonally related layer 4 sister neurons in somatosensory cortex [47]. The authors proposed that methylation patterns influence the expression patterns of clustered protocadherins, cell adhesion molecules thought to play roles in self-recognition and non-self-recognition [47]. Other molecular mechanisms involved in input-specific regulation of synapse formation in primary somatosensory cortex may also contribute to circuit formation in V1 [43,48], as may mechanisms implicated in the regulation of synapse formation in specific cortical cell types and dendritic compartments [49–52]. Together, these studies demonstrate that, before visual experience, multiple mechanisms likely work in concert to establish early patterns of synaptic connections that generate initial receptive field properties — including the spatial structure, orientation tuning, and direction preference of mouse V1 neurons [41,46••,53–55].
Changes in synaptic connectivity following eye opening in the mouse
Significant synaptogenesis and maturation of receptive field properties occurs around eye opening (around P14), but little is known regarding the specific changes in cortical circuits during this time period [56,57]. The patterns of synaptic connectivity and selectivity in receptive field properties continue to be shaped after eye opening (Figure 1, P14–P21 and P22+ panels). However, only some of these changes depend on visual experience. For example, prior to eye opening, the preferential connectivity between L2/3 neurons with similar orientation preferences is immature. Only after eye opening does the probability of synaptic connection among L2/3 pyramids with similar receptive field properties and the proportion of bidirectionally connected L2/3 neurons sharing receptive field properties increase significantly, as does the synaptic strength of these connections [19•,41,58,59••]. This increase in preferential connectivity represents both an increase and strengthening of connections among L2/3 neurons with similar response properties as well as a decrease in the connectivity of non-responsive neurons [41,59••]. Interestingly, the emergence of reciprocal synaptic connections among neurons with shared response properties proceeds largely unaffected by the absence of visual experience [59••]. In contrast, the elimination of connections among visually non-responsive L2/3 neurons was inhibited by dark-rearing [59••]. The significant increases in the probability of connection and synaptic strength among randomly selected L2/3 neurons in rats following eye opening were also eliminated by dark-rearing but not by binocular eyelid suturing [58], suggesting a role for patterned visual input. Whether visual experience shapes the chemical connections formed among clonally related sister neurons has not been tested.
As with the patterns of intracortical connectivity, only some changes in receptive field properties following eye opening are dependent on visual experience. For example, the correspondence between ON and OFF subfields received from the two eyes and binocular matching of orientation preferences in mouse V1 is disrupted by dark-rearing, although experience-independent mechanisms generate the ON and OFF subregions and the overlap of the receptive fields in visual space [54,55]. The broadening of the orientation tuning of L2/3 fast-spiking neurons was also disrupted by dark-rearing [60]. In contrast, the elimination of initial biases in the distribution of preferred orientations and preferred directions in L2/3 excitatory neurons required neuronal activity but not visual experience [46••,53,60]. The sparsification of V1 neuronal responses in L2/3 also proceeded, although delayed, without visual experience [61]. How the evolution of response properties during the first weeks following eye opening relates to underlying changes in synaptic connectivity remains unclear. Furthermore, the contributions of molecular mechanisms implicated in shaping synaptic connections in visual and somatosensory cortex in later development, including specifying connections among cortical neurons at the cell-type or subcellular levels, remains to be fully elucidated [48,50,62,63••,64] (for reviews, see [65,66]). Nonetheless, together, these results indicate that experience-dependent, activity-dependent and activity-independent mechanisms contribute to cortical circuit development after eye opening.
Summary
The advent of two-photon imaging of calcium indicators in combination with recordings of unitary synaptic connections has begun to generate insights into how the patterns of synaptic connectivity in primary visual cortex of the mouse change during development. However, technical limits of these approaches have largely limited analyses to the supragranular layers of the cortex and to only a subset of time points and conditions. Many studies focus only on one level of specificity, making it challenging to understand how mechanisms may work in concert. Do, for example, molecular signals guide axons to a particular cortical layer to restrict the choice of available targets for synapse formation while additional mechanisms confer further cell-type or subcellular specificity? Whether all synaptic connections require each of these levels of specificity also remains unclear. For example, a recent study suggested that L4 spiny stellate cells target the apical tufts of L6 pyramids, while L4 star pyramids target basal and proximal dendrites, exhibiting compartment-specific targeting [31]. However, these connections did not distinguish between the type of L6 neurons targeted, L6 corticothalamic neurons or L6 corticocortical neurons, thus showing no specificity with regard to cell type. Generating a framework that integrates these distinct levels of specificity, and understanding how different mechanisms, including experience-dependent, activity-dependent and activity-independent processes, work in concert to produce mature cortical circuits remain important challenges.
Acknowledgements
We thank Ulrich Mueller, Juhyun Kim, Su-Jeong Kim, Kihwan Lee and Seung-Chan Lee for critical reading of the manuscript. Work in SPB’s laboratory is supported by a Klingenstein-Simons Fellowship, a Johns Hopkins Science of Learning Grant, the Johns Hopkins Discovery Fund, the National Science Foundation (Grant 1656592) and the National Institute of Neurological Disorders and Stroke (R01NS085121). Maxime Chevée was supported by a Boerhinger-Ingelheim Fonds Fellowship.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Harris KD, Shepherd GM: The neocortical circuit: themes and variations. Nat Neurosci 2015, 18:170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han Y, Kebschull JM, Campbell RAA, Cowan D, Imhof F, Zador AM, Mrsic-Flogel TD: The logic of single-cell projections from visual cortex. Nature 2018, 556:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD: Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci 2007, 8:427–437. [DOI] [PubMed] [Google Scholar]
- 4.Wiesel TN, Gilbert CD: The Sharpey-Schafer lecture. Morphological basis of visual cortical function. Q J Exp Physiol 1983, 68:525–543. [DOI] [PubMed] [Google Scholar]
- 5.Douglas RJ, Martin KA: Neuronal circuits of the neocortex. Annu Rev Neurosci 2004, 27:419–451. [DOI] [PubMed] [Google Scholar]
- 6.Harris KD, Mrsic-Flogel TD: Cortical connectivity and sensory coding. Nature 2013, 503:51–58. [DOI] [PubMed] [Google Scholar]
- 7.Ohki K, Chung S, Ch’ng YH, Kara P, Reid RC: Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 2005, 433:597–603. [DOI] [PubMed] [Google Scholar]
- 8.Bonin V, Histed MH, Yurgenson S, Reid RC: Local diversity and fine-scale organization of receptive fields in mouse visual cortex. J Neurosci 2011, 31:18506–18521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M: Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron 2007, 54:961–972. [DOI] [PubMed] [Google Scholar]
- 10.Maruoka H, Kubota K, Kurokawa R, Tsuruno S, Hosoya T: Periodic organization of a major subtype of pyramidal neurons in neocortical layer V. J Neurosci 2011, 31:18522–18542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maruoka H, Nakagawa N, Tsuruno S, Sakai S, Yoneda T, Hosoya T: Lattice system of functionally distinct cell types in the neocortex. Science 2017, 358:610–615.•• Maruoka et al. describe cell-type specific microcolumns in layer 5, which are organized into a hexagonal lattice with a period of 30–45 μm. Neurons in these microcolumns show developmentally regulated gap junction connections that disappear by two weeks of age in the mouse. Although neurons within microcolumns do not form preferential chemical synapses, they were found to share strong synchronous synaptic inputs.
- 12.Kondo S, Yoshida T, Ohki K: Mixed functional microarchitectures for orientation selectivity in the mouse primary visual cortex. Nat Commun 2016, 7:13210.• Using three-dimensional two-photon imaging, Kondo et al. show that pairs of layer 2/3 and layer 4 neurons within 5–10 μm in the horizontal plane and within 60 μm in the vertical plane are similarly tuned to visual stimuli. They also show that bundles of apical dendrites of layer 5 neurons, originating from putative microcolumns, share similar visual response properties although these bundles lack a global organization across the visual cortex.
- 13.Ringach DL, Mineault PJ, Tring E, Olivas ND, Garcia-Junco-Clemente P, Trachtenberg JT: Spatial clustering of tuning in mouse primary visual cortex. Nat Commun 2016, 7:12270.• Using in vivo two-photon imaging of mouse layer 2/3 visual cortex, Ringach et al. show that neurons within 40 μm horizontally and up to 120 μm vertically share feature selectivity, as defined by their tuning in the joint orientation and spatial frequency domains, indicating that mouse primary visual cortex has more columnar and tangential organization than previously appreciated.
- 14.Ji W, Gamanut R, Bista P, D’Souza RD, Wang Q, Burkhalter A: Modularity in the organization of mouse primary visual cortex. Neuron 2015, 87:632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiritani T, Wickersham IR, Seung HS, Shepherd GM: Hierarchical connectivity and connection-specific dynamics in the corticospinal–corticostriatal microcircuit in mouse motor cortex. J Neurosci 2012, 32:4992–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown SP, Hestrin S: Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature 2009, 457:1133–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morishima M, Kawaguchi Y: Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. J Neurosci 2006, 26:4394–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko H, Hofer SB, Pichler B, Buchanan KA, Sjostrom PJ, Mrsic-Flogel TD: Functional specificity of local synaptic connections in neocortical networks. Nature 2011, 473:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cossell L, Iacaruso MF, Muir DR, Houlton R, Sader EN, Ko H, Hofer SB, Mrsic-Flogel TD: Functional organization of excitatory synaptic strength in primary visual cortex. Nature 2015, 518:399–403.• Cossell et al. use a combination of in vivo calcium imaging and whole-cell recordings of unitary synaptic connections in cortical slices to identify a functional rule of connectivity in mouse visual cortex. They demonstrate that the majority of connections between L2/3 neurons are weak and occur between neurons with non-correlated responses, but that neurons with highly correlated responses form rare, strong synaptic connections that dominate a neuron’s synaptic input.
- 20.Wertz A, Trenholm S, Yonehara K, Hillier D, Raics Z, Leinweber M, Szalay G, Ghanem A, Keller G, Rozsa B et al. : Single-cell-initiated monosynaptic tracing reveals layer-specific cortical network modules. Science 2015, 349:70–74. [DOI] [PubMed] [Google Scholar]
- 21.Lee WC, Bonin V, Reed M, Graham BJ, Hood G, Glattfelder K, Reid RC: Anatomy and function of an excitatory network in the visual cortex. Nature 2016, 532:370–374.• By combining in vivo calcium imaging with electron microscopic reconstruction, Lee et al. show that layer 2/3 neurons with similar orientation preference are more likely to form synapses with each other than with neurons with different orientation preferences. This specificity arises at the level of spines, as they found that axons and dendrites of neurons with similar and different orientation preferences passed near each other similarly.
- 22.Inan M, Anderson SA: The chandelier cell, form and function. Curr Opin Neurobiol 2014, 26:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ: The organization of two new cortical interneuronal circuits. Nat Neurosci 2013, 16:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tremblay R, Lee S, Rudy B: GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 2016, 91:260–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petreanu L, Mao T, Sternson SM, Svoboda K: The subcellular organization of neocortical excitatory connections. Nature 2009, 457:1142–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lubke J, Markram H, Frotscher M, Sakmann B: Frequency and dendritic distribution of autapses established by layer 5 pyramidal neurons in the developing rat neocortex: comparison with synaptic innervation of adjacent neurons of the same class. J Neurosci 1996, 16:3209–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markram H, Lubke J, Frotscher M, Roth A, Sakmann B: Physiology and anatomy of synaptic connections between thick tufted pyramidal neurons in the developing rat neocortex. J Physiol 1997, 500:409–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gokce O, Bonhoeffer T, Scheuss V: Clusters of synaptic inputs on dendrites of layer 5 pyramidal cells in mouse visual cortex. Elife 2016, 5:e09222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sjostrom PJ, Hausser M: A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons. Neuron 2006, 51:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldmeyer D, Lubke J, Sakmann B: Efficacy and connectivity of intracolumnar pairs of layer 2/3 pyramidal cells in the barrel cortex of juvenile rats. J Physiol 2006, 575:583–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi G, Feldmeyer D: Dendritic target region-specific formation of synapses between excitatory layer 4 neurons and layer 6 pyramidal cells. Cereb Cortex 2016, 26:1569–1579. [DOI] [PubMed] [Google Scholar]
- 32.Nusser Z: Creating diverse synapses from the same molecules. Curr Opin Neurobiol 2018, 51:8–15. [DOI] [PubMed] [Google Scholar]
- 33.Rakic P: Specification of cerebral cortical areas. Science 1988, 241:170–176. [DOI] [PubMed] [Google Scholar]
- 34.Connors BW: Synchrony and so much more: diverse roles for electrical synapses in neural circuits. Dev Neurobiol 2017, 77:610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu YC He S, Chen S, Fu Y, Brown KN, Yao XH, Ma J, Gao KP, Sosinsky GE, Huang K et al. : Preferential electrical coupling regulates neocortical lineage-dependent microcircuit assembly. Nature 2012, 486:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He S, Li Z, Ge S, Yu YC, Shi SH: Inside-out radial migration facilitates lineage-dependent neocortical microcircuit assembly. Neuron 2015, 86:1159–1166.•• He et al. describe the development of electrical coupling among radial glia progenitors (RGPs) and among sister neurons, as well as between RGPs and their progeny. Using the Reeler mouse genetic model, knock down of Dab1 and overexpression of Efna5 and Epha7, they demonstrate that normal, columnar inside-out cortical development is required for the formation of preferential electrical and chemical connections between sister neurons.
- 37.Yu YC, Bultje RS, Wang X, Shi SH: Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature 2009, 458:501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krieger P, Kuner T, Sakmann B: Synaptic connections between layer 5B pyramidal neurons in mouse somatosensory cortex are independent of apical dendrite bundling. J Neurosci 2007, 27:11473–11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su X, Chen JJ, Liu LY, Huang Q, Zhang LZ, Li XY, He XN, Lu W, Sun S, Li H et al. : Neonatal CX26 removal impairs neocortical development and leads to elevated anxiety. Proc Natl Acad Sci U S A 2017, 114:3228–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Lu H, Cheng PL, Ge S, Xu H, Shi SH, Dan Y: Clonally related visual cortical neurons show similar stimulus feature selectivity. Nature 2012, 486:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ko H, Cossell L, Baragli C, Antolik J, Clopath C, Hofer SB, Mrsic-Flogel TD: The emergence of functional microcircuits in visual cortex. Nature 2013, 496:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohtsuki G, Nishiyama M, Yoshida T, Murakami T, Histed M, Lois C, Ohki K: Similarity of visual selectivity among clonally related neurons in visual cortex. Neuron 2012, 75:65–72. [DOI] [PubMed] [Google Scholar]
- 43.Winnubst J, Cheyne JE, Niculescu D, Lohmann C: Spontaneous activity drives local synaptic plasticity in vivo. Neuron 2015, 87:399–410. [DOI] [PubMed] [Google Scholar]
- 44.Iacaruso MF, Gasler IT, Hofer SB: Synaptic organization of visual space in primary visual cortex. Nature 2017, 547:449–452.• Using two-photon imaging of layer 2/3 visual cortex neurons, Iacaruso et al. show that synaptic inputs with overlapping spatial receptive fields (RFs) cluster onto neighboring spines. They further show that synapses with RFs outside of the postsynaptic neuron’s RF preferentially target dendrites further away from the cell body, and that the spatial location of their RFs tends to align with the orientation preference of the postsynaptic neuron.
- 45.Scholl B, Wilson DE, Fitzpatrick D: Local order within global disorder: synaptic architecture of visual space. Neuron 2017, 96:1127–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hagihara KM, Murakami T, Yoshida T, Tagawa Y, Ohki K: Neuronal activity is not required for the initial formation and maturation of visual selectivity. Nat Neurosci 2015, 18:1780–1788.•• Hagihara et al. used expression of the inwardly rectifying potassium channel, Kir2.1, in a subset of layer 2/3 neurons through development to suppress both spontaneous and evoked activity in the mouse visual cortex. They show that the formation of orientation selectivity does not depend on spontaneous or evoked activity, while refinement of the distribution of preferred orientations following eye opening depends on spontaneous, but not evoked, activity.
- 47.Tarusawa E, Sanbo M, Okayama A, Miyashita T, Kitsukawa T, Hirayama T, Hirabayashi T, Hasegawa S, Kaneko R, Toyoda S et al. : Establishment of high reciprocal connectivity between clonal cortical neurons is regulated by the Dnmt3b DNA methyltransferase and clustered protocadherins. BMC Biol 2016, 14:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li MY, Miao WY, Wu QZ, He SJ, Yan G, Yang Y, Liu JJ, Taketo MM, Yu X: A critical role of presynaptic cadherin/catenin/p140Cap complexes in stabilizing spines and functional synapses in the neocortex. Neuron 2017, 94:1155–1172. [DOI] [PubMed] [Google Scholar]
- 49.Cubelos B, Sebastian-Serrano A, Beccari L, Calcagnotto ME, Cisneros E, Kim S, Dopazo A, Alvarez-Dolado M, Redondo JM, Bovolenta P et al. : Cux1 and Cux2 regulate dendritic branching, spine morphology, and synapses of the upper layer neurons of the cortex. Neuron 2010, 66:523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demyanenko GP, Mohan V, Zhang X, Brennaman LH, Dharbal KE, Tran TS, Manis PB, Maness PF: Neural cell adhesion molecule NrCAM regulates Semaphorin 3F-induced dendritic spine remodeling. J Neurosci 2014, 34:11274–11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harwell CC, Parker PR, Gee SM, Okada A, McConnell SK, Kreitzer AC, Kriegstein AR: Sonic hedgehog expression in corticofugal projection neurons directs cortical microcircuit formation. Neuron 2012, 73:1116–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, Huganir RL, Ginty DD, Kolodkin AL: Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature 2009, 462:1065–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rochefort NL, Narushima M, Grienberger C, Marandi N, Hill DN, Konnerth A: Development of direction selectivity in mouse cortical neurons. Neuron 2011, 71:425–432. [DOI] [PubMed] [Google Scholar]
- 54.Sarnaik R, Wang BS, Cang J: Experience-dependent and independent binocular correspondence of receptive field subregions in mouse visual cortex. Cereb Cortex 2014, 24:1658–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang BS, Sarnaik R, Cang J: Critical period plasticity matches binocular orientation preference in the visual cortex. Neuron 2010, 65:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lohmann C, Kessels HW: The developmental stages of synaptic plasticity. J Physiol 2014, 592:13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seabrook TA, Burbridge TJ, Crair MC, Huberman AD: Architecture, function, and assembly of the mouse visual system. Annu Rev Neurosci 2017, 40:499–538. [DOI] [PubMed] [Google Scholar]
- 58.Ishikawa AW, Komatsu Y, Yoshimura Y: Experience-dependent emergence of fine-scale networks in visual cortex. J Neurosci 2014, 34:12576–12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ko H, Mrsic-Flogel TD, Hofer SB: Emergence of feature-specific connectivity in cortical microcircuits in the absence of visual experience. J Neurosci 2014, 34:9812–9816.•• Ko et al. performed paired recordings of neurons whose responses to natural movies were previously determined using in vivo two-photon imaging in dark-reared mice. They show that visual experience is not necessary for the initial formation of preferential connectivity between neurons that share response properties. They found that visual experience was, however, required for the pruning of connections between non-visually responsive neurons that normally occurs after eye-opening.
- 60.Kuhlman SJ, Tring E, Trachtenberg JT: Fast-spiking interneurons have an initial orientation bias that is lost with vision. Nat Neurosci 2011, 14:1121–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rochefort NL, Garaschuk O, Milos RI, Narushima M, Marandi N, Pichler B, Kovalchuk Y, Konnerth A: Sparsification of neuronal activity in the visual cortex at eye-opening. Proc Natl Acad Sci U S A 2009, 106:15049–15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel AB, Loerwald KW, Huber KM, Gibson JR: Postsynaptic FMRP promotes the pruning of cell-to-cell connections among pyramidal neurons in the L5A neocortical network. J Neurosci 2014, 34:3413–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rajkovich KE, Loerwald KW, Hale CF, Hess CT, Gibson JR, Huber KM: Experience-dependent and differential regulation of local and long-range excitatory neocortical circuits by postsynaptic Mef2c. Neuron 2017, 93:48–56.•• Using sparse genetic deletion of Mef2c in mouse somatosensory cortex, Rajkovich et al. show that Mef2c and sensory experience interact to differentially modulate the synaptic input onto layer 2/3 neurons in a cell-autonomous manner. Deletion of Mef2c reduced the strength of local synaptic inputs while increasing the strength of long-range synaptic inputs from contralateral cortex.
- 64.Bian WJ, Miao WY, He SJ, Qiu Z, Yu X: Coordinated spine pruning and maturation mediated by inter-spine competition for cadherin/catenin complexes. Cell 2015, 162:808–822. [DOI] [PubMed] [Google Scholar]
- 65.Yogev S, Shen K: Cellular and molecular mechanisms of synaptic specificity. Annu Rev Cell Dev Biol 2014, 30:417–437. [DOI] [PubMed] [Google Scholar]
- 66.de Wit J, Ghosh A: Specification of synaptic connectivity by cell surface interactions. Nat Rev Neurosci 2016, 17:22–35. [DOI] [PubMed] [Google Scholar]