ABSTRACT
Background
The ketogenic diet (KD) has gained interest as a potential therapy for numerous conditions; however, studies rarely report the food and micronutrient profile of the diet.
Objective
The aim of this study was to report changes in food selection and nutritional quality from the baseline diet (BD) to a KD therapy in participants with Alzheimer disease (AD).
Methods
Fifteen AD patients participated in a single-arm clinical trial to assess the feasibility of a 3-mo KD intervention. A registered dietitian instructed participant study partners to assist participants with a self-selected, nutritionally dense KD. We collected food and nutrient intake via monthly 3-d food records. Serum β-hydroxybutyrate was measured within 48 h of each 3-d food record to assess ketosis status. Food records before KD initiation characterized the BD. Food records during the intervention coincident with the most robust ketosis characterized the KD. Principal components analysis identified foods affiliated with the BD and KD. Mean food and nutrient intake change was tested by the Kruskal–Wallis test for variance with significance set at P ≤ 0.025.
Results
Ten participants adhered to the KD. Study partners provided complete food records for 6 KD-adherent individuals. The KD was characterized by increased medium-chain triglyceride (MCT) oil, nonstarchy vegetables, butter, eggs, olive oil, avocados, and nuts and seeds with practical elimination of potatoes, grains, red meat, sugar-sweetened beverages, and desserts. Fruit intake, including avocado, was similar between diets. Nonstarchy vegetable intake increased from 1.2 servings/d to 4.3 servings/d (P < 0.01) on the KD. Micronutrient intake was similar between diets, meeting Dietary Reference Intakes for most nutrients. Between diets, the KD was associated with increased intake of choline and vitamin K and decreased intake of manganese and fiber.
Conclusion
As a potential therapy in AD, the KD can be nutritionally dense with high intake of vegetables and substantial variety. This trial was registered at clinicaltrials.gov as NCT03690193.
Keywords: ketogenic diet, Alzheimer disease, fruits, vegetables, principal components analysis
Introduction
The ketogenic diet (KD) is a very high-fat, low-carbohydrate diet that restricts carbohydrate to ≤10% of consumed energy, starkly different from the 50–65% of energy from carbohydrate of the typical American diet. This macronutrient profile promotes a systemic shift from glucose metabolism toward conversion of fatty acids to ketone bodies as a substrate for energy. With a long history in the treatment of refractory childhood epilepsy (1), the KD has recently experienced a surge in mainstream popularity (2) as a diet for weight loss (3), athletic performance in competitive athletes (4), and perceived general health (5). Its utilization as a potential therapy for multiple neurological and metabolic conditions has piqued the interest of researchers and clinicians alike (6–11). Our recent publication suggests that the KD is feasible and may hold therapeutic efficacy in a population of patients with Alzheimer disease (AD) (12).
In the past decade, there have been a growing number of human trials involving the KD; however, these trials were generally most focused on achievement of a ketogenic macronutrient profile. Recently, nutrition experts have advocated for the application of “healthy” or “well-formulated” ketogenic diets (8, 13). Thus far, studies have described KD education principles, meal plans, and related biomarker outcomes well but often lacked reports of actual dietary intake (14–16). Ketone status measurement (i.e., blood or urine concentration) is a good indicator of adherence to the KD by definition, but these markers are ignorant of KD food group and micronutrient composition. Whether patients closely adhere to “healthy” prescribed dietary protocols is unknown. Absent this important information, many among the health care and scientific communities remain hesitant, believing the KD to be an unfeasible or merely short-term nutritional approach because of either long-term health concerns or perceptions that the KD is unpalatable and lacks variety (17).
The primary aim of this study was to describe the KD education principles and assess the dietary changes associated with KD adherence from the University of Kansas Ketogenic Diet Retention and Feasibility Trial (KDRAFT), a trial to assess the feasibility of the KD in patients with AD (12).
Methods
Study design
We examined the difference in dietary intake between the baseline diet (BD) and KD intervention from a single-arm, clinical trial (NCT03690193) involving patients diagnosed with AD.
Participants
Fifteen older adults meeting the McKhann et al. criteria (18) for AD were enrolled in the trial as described previously (12). Individuals were eligible to participate in the study if they had a clinical dementia rating (CDR) (19) of very mild AD (CDR 0.5), mild AD (CDR 1), or moderate AD (CDR 2); had an active study partner; BMI ≥21 kg/m2; normal electrolytes and liver function; and spoke English. Exclusion criteria included type 1 diabetes, ongoing or recent cancer, cardiac event in the past year, or other serious medical risks identified by research physicians. The study protocol was approved by the Institutional Review Board at the University of Kansas Medical Center. Informed consent was obtained from all study participants and study partners per institutional guidelines.
KD intervention and counseling
A registered dietitian (RD) counseled the study partners of each participant to assist in implementation of a self-selected, nutritionally dense KD at the baseline study visit. Participants were instructed to consume a 1:1 KD (ratio of lipid in grams to nonlipid in grams) or better, which was approximately comprised of 70% fat, 20% protein, and ≤10% carbohydrate as energy. The Mifflin-St Jeor equation (20) was used to estimate daily energy needs and target medium-chain TG (MCT) oil (Now Foods) dosage. Participants aimed for an MCT dosage of 40% of total targeted fat intake, starting at 1 tablespoon/d and adding ½ tablespoon on a weekly basis until the target daily dosage or maximum tolerance was met. Daily multivitamin, vitamin D, calcium, and phosphorus supplements were also provided to alleviate concerns of potential micronutrient deficiency. A 3-d example KD menu was provided at the beginning of the study as an aid for initiating the KD. After initiation of the KD, the RD made weekly phone calls to study partners to encourage compliance and address KD-related questions. Achievement of a ketosis-inducing macronutrient intake was the primary goal of the nutritional counsel, yet Table 1 describes additional key nutrition messages that were discussed with participants.
TABLE 1.
KDRAFT ketogenic diet education principles1
| Component | Guidelines |
|---|---|
| Macronutrient | |
| Fat | Consume ≥70% as energy |
| Focus: extra virgin olive oil, avocado, olives, nuts and seeds | |
| Moderate: nut and seed butters, butter, bacon | |
| Carbohydrate | Restrict to ≤10% of energy |
| Vegetables and small amount of fruit as primary source | |
| Protein | Not to exceed 100 g for most individuals |
| Focus: fatty fish, eggs, dark meat poultry, unprocessed red meat | |
| Limit: processed meat | |
| Food groups | |
| MCT oil | Titrate dosage weekly by 7.5 mL starting at 15 mL/d |
| Original aim: 40% of total energy as fat or until tolerance reached | |
| Feasible aim: 15–30 mL daily, as tolerated | |
| Best tolerated in coffee and blended with additional long-chain fat source | |
| Vegetables | Unlimited intake of nonstarchy vegetables |
| Eliminate starchy vegetables | |
| Use vegetable intake as opportunity for additional fat | |
| Fruit | Restrict to ½ cup of berries per day |
| Unlimited avocado intake | |
| Dairy | Use heavy cream as an additive to coffee or tea |
| 1–2 servings of full-fat cheese allowable | |
| Limit unsweetened, full-fat milk or yogurt to no more than ½ cup/d | |
| Grains | Eliminate refined and whole grains |
| Replace grain flours with almond or coconut flour | |
| Small amount of resistant starch may help MCT tolerance | |
| Sweetener | Eliminate sugar intake |
| Use sweeteners sparingly, primarily Stevia or Truvia | |
| Water | Drink ≥64 fluid ounces (1.89 L) of water daily |
| General | |
| Supplementation | Multivitamin, vitamin D, potassium, phosphorus (not included in this analysis) |
| Urinary ketones | Measure and record urinary ketones daily |
| If not producing ketones, reduce carbohydrate and increase fat |
KDRAFT, Ketogenic Diet Retention and Feasibility Trial; MCT, medium-chain TG.
Dietary intake
Dietary intake was measured via multiple self-reported 3-d food records completed by participant study partners at home, in real time. The RD provided study partners with written and verbal diet record instruction and reviewed recently completed records with study partners for detail and completeness upon their return at study visits. Records were collected at baseline, month 1, and month 2. Each record consisted of 3 consecutive days including 2 weekdays and 1 weekend day. Baseline records reflected intake before the initiation of the diet intervention. Records at months 1 and 2 reflected intake while following the KD. Food record data were entered into the Nutrition Data System for Research (version 2016) to quantify food and nutrient intake. To characterize intake that was truly ketogenic, dietary data that corresponded with a participant's most robust serum β-hydroxybutyrate (BHB) concentration were selected for presentation. All micronutrient intake was converted to percentage intake of RDA and Adequate Intake (AI). Estimated Average Requirement (EAR) is commonly used to assess group nutrient intake, but we elected to present RDA and AI based on the small sample size and our interest in the KD's ability to meet individual needs. We did calculate percentage intake of EAR for nutrient intake that fell below the RDA or AI. All dietary data represent intake from food, excluding micronutrient supplementation.
Ketone status
Ketone status was monitored via 2 measurement methods. Firstly, participants self-monitored urine ketones each evening using urine acetoacetate test strips (Ketostix, Bayer). Measurements were recorded as negative, trace (5–14.9 mg/dL), small (15–39.9 mg/dL), moderate (40–79.9 mg/dL), or large (≥80 mg/dL) in a provided diary. Daily ketone status served as immediate feedback to inform participants of their adherence to the KD. Negative to trace urine ketone status was used as an indication to decrease carbohydrate and increase fat intake as needed. Secondly, BHB was collected and measured after a 12-h fast within 48 h of the final day recorded in the 3-d food record for that study time point. Nursing staff at the KU Clinical and Translational Science Unit collected serum samples and BHB was analyzed in the KU Hospital Clinical Laboratory.
Principal components analysis
We used the [psych] statistical package written for R statistical software to perform principal components analysis (PCA). The object of this analysis was to identify a single dietary pattern characterized by food groups with the greatest variability of intake between the BD and KD. To do this, Nutrition Data System for Research output of food group variables was consolidated into 33 food groups, centered and standardized, and entered into the PCA. Dietary intake data from all participants at both time points (BD and KD) were included together in a single PCA, resulting in a diet factor identifiable by opposing ends of a spectrum. The food items with large positive loading coefficients best described foods with the greatest change toward the KD, whereas foods with large negative loading coefficients best described intake of food items on the BD. Participant adherence to this dietary pattern was calculated for each time point by multiplying the standardized intake of each food group at that time point by its respective loading factor and summing all 33 of these values.
Statistical analyses
The primary aim of this research was to characterize the changes in food group and nutrient intake of the KD from the BD. Continuous variables were expressed as mean ± SD. ANOVA models utilized the nonparametric Kruskal–Wallis test for variance owing to small sample size, lack of normality through visualization of Q-Q plots, and no missing dietary data from each of the 6 individuals included in this analysis. Individual change in PCA diet pattern adherence and select food group intake changes are also presented. Statistical analyses were performed using R (version 3.4.3; R Foundation). To further account for the small sample size of this study, statistical tests were 2-tailed with significance set at P < 0.025.
Results
Ten of the 15 enrolled AD participants successfully adhered to the KD, evidenced by multiple days of urine ketone production and serum BHB elevated >0.3 mmol/L at ≥1 lab collection time point (12). Data from 6 KD-adherent participants who provided complete 3-d food records 1) at baseline and 2) during the KD therapy coincident with the most robust serum BHB were included in the analysis. Reported challenges to completeness of 3-d food records primarily involved caregiver burden associated with AD care responsibilities. Participant characteristics, cognition, and ketone production data for the entire study sample have been previously reported (12). The mean serum BHB of the 6 participants included in this analysis increased from 0.1 to 0.8 mmol/L, with a range of 0.3–1.4 mmol/L. Participant characteristics, macronutrient composition, and intake of nutrients or foods without DRI values of this analysis are reported in Table 2.
TABLE 2.
Participant characteristics and intake of food and nutrients without DRIs1
| Overall (n = 6) | Baseline diet (n = 6) | Ketogenic diet (n = 6) | P | |
|---|---|---|---|---|
| Sex (F/M) | 3/3 | |||
| Age, y | 71.7 ± 8.5 | |||
| Serum BHB, mmol/L | 0.1 ± 0.0 | 0.8 ± 0.5 | <0.01 | |
| Macronutrient intake | ||||
| Energy, kcal | 1950 ± 295 | 1860 ± 205 | 0.52 | |
| Fat, g | 91 ± 11 | 151 ± 20 | <0.01 | |
| Carbohydrate, g | 210 ± 57 | 46 ± 20 | <0.01 | |
| Protein, g | 81 ± 11 | 88 ± 11 | 0.26 | |
| Fatty acid intake | ||||
| MCT oil, mL | 0.0 ± 0.0 | 19.2 ± 3.0 | <0.001 | |
| SFAs, g | 30 ± 5 | 69 ± 18 | <0.01 | |
| MUFAs, g | 34 ± 4 | 50 ± 6 | <0.01 | |
| PUFAs, g | 20 ± 7 | 21 ± 7 | 0.87 | |
| Trans fatty acids, g | 2.6 ± 0.6 | 2.9 ± 1.0 | 0.42 | |
| ω-3 fatty acids, g | 2.2 ± 0.8 | 2.3 ± 0.6 | 0.87 | |
| Cholesterol, g | 264 ± 85 | 729 ± 155 | <0.01 | |
| Carbohydrate intake | ||||
| Sugar, g | 84 ± 33 | 25 ± 11 | <0.01 | |
| Glycemic load, glucose reference | 118 ± 33 | 18 ± 9 | <0.01 | |
| Fruits and vegetables2 | ||||
| Fruit | 0.8 ± 0.7 | 0.2 ± 0.4 | 0.10 | |
| Avocado | 0.0 ± 0.0 | 0.4 ± 0.6 | 0.16 | |
| Nonstarchy vegetables | 1.2 ± 0.9 | 4.3 ± 2.1 | <0.01 | |
Values are means ± SDs unless indicated otherwise. Group differences were assessed by the Kruskal–Wallis test for variance. Significance was set at P ≤ 0.025. All dietary data derived by 3-d food record at each time point. For both diets, nutrient data were derived by food only. BHB, β-hydroxybutyrate; DRI, Dietary Reference Intake; MCT, medium-chain TG.
Fruit and vegetable intake reported as ½-cup (118 mL) raw servings and 1-cup (237 mL) cooked servings.
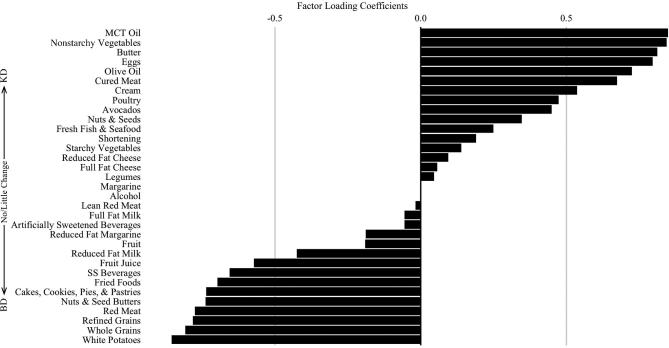
The PCA dietary pattern was distinguishable by food group loadings on opposite ends of the pattern's spectrum (Figure 1). Foods that loaded toward the positive end characterized the foods with the largest change in intake toward the KD, foods that loaded in the middle of the figure indicated foods that had little change from the BD to the KD, and foods that loaded toward the negative end indicated foods that were commonly consumed in the BD but not in the KD. Adherence to the KD was associated with increased intake of MCT oil, nonstarchy vegetables, butter, eggs, olive oil, cured meat, cream, poultry, avocados, nuts and seeds, and fresh fish and seafood. In addition, adherence to the KD essentially eliminated intake of potatoes, grains, red meat, desserts, fried foods, and sugar-sweetened beverages. Individual adherence to the PCA diet pattern at both time points is presented in Figure 2.
FIGURE 1.
Food group loadings for the principal components analysis diet pattern (n = 6). Rotated food group loading coefficients are represented as the bars. Foods located toward the positive terminal of the graph are foods that had the largest increase in consumption with adherence to the KD. Conversely, foods located toward the negative terminal are foods that were most associated with the BD and had the largest decrease in consumption with adherence to the KD. BD, baseline diet; KD, ketogenic diet; MCT, medium-chain TG; SS, sugar-sweetened.
FIGURE 2.
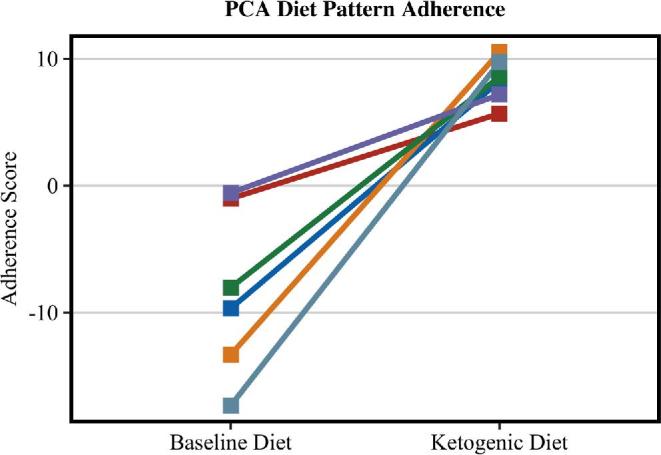
Individual change in adherence to the PCA diet pattern between the baseline and ketogenic diets. The lines indicate the individual change in PCA diet pattern adherence scores between diets (n = 6). Individual scores are calculated by multiplying the PCA factor loading score for each food group by the standardized intake of that food, then summing the products of all the food groups. Negative scores indicate high consumption of non-ketogenic diet–related foods. Positive scores indicate high consumption of ketogenic diet–related foods. PCA, principal components analysis.
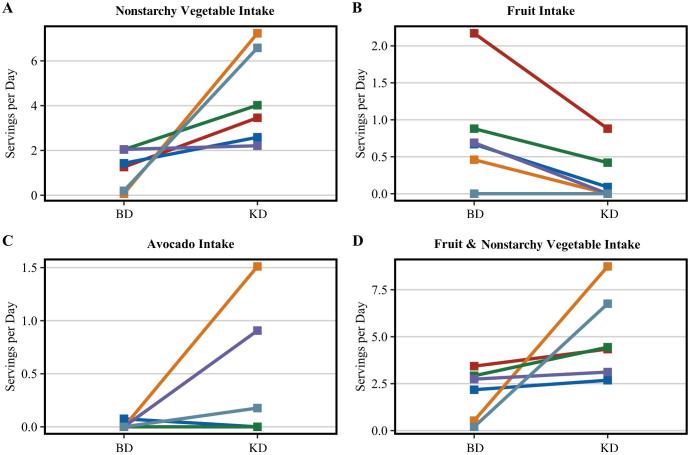
Daily nonstarchy vegetable intake increased by 3.1 servings on the KD (mean ± SD: 1.2 ± 0.9 compared with 4.3 ± 2.1 servings). Fruit servings on the KD decreased by 0.6 servings (mean ± SD: 0.8 ± 0.7 compared with 0.2 ± 0.4 servings), although nonsignificantly. Avocado servings increased nonsignificantly from BD to KD (mean ± SD: 0.0 ± 0.0 compared with 0.4 ± 0.6 servings). Considering intake of both fruits (including avocado) and nonstarchy vegetables, intake significantly increased from BD to KD (mean ± SD: 2.0 ± 1.3 compared with 5.0 ± 2.3 servings). Individual change in intake of fruits and vegetables is presented in Figure 3.
FIGURE 3.
Individual change in fruit and vegetable intake between the BD and KD. The lines indicate the individual change in standard USDA food group serving intake between diets (n = 6). (A) Change in nonstarchy vegetable intake from BD to KD. (B) Change in fruit intake (excludes avocado) from BD to KD. This was of interest owing to the message to limit fruit to ½ cup of berries per day. (C) Change in avocado intake from BD to KD. Two participants have overlapping lines as they did not consume avocados at either time point. (D) Change in total fruit (includes avocado) and nonstarchy vegetable intake from BD to KD. BD, baseline diet; KD, ketogenic diet.
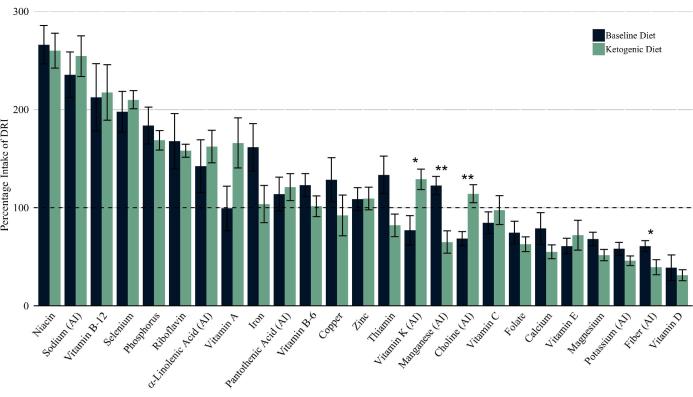
Figure 4 depicts the mean intake change for nutrients with established RDA and AI values. Both diets met the RDAs or AIs for α-linolenic acid, iron, niacin, pantothenic acid, phosphorus, riboflavin, selenium, vitamin B-12, vitamin B-6, and zinc. The KD also met the RDAs or AIs for choline, vitamin A, vitamin C, and vitamin K. The KD failed to meet the RDA or AI for calcium, folate, magnesium, manganese, potassium, thiamin, vitamin D, and vitamin E. On the KD, fiber intake was significantly lower (P = 0.025), whereas intakes of vitamin K (P = 0.025) and choline (P = 0.01) significantly increased.
FIGURE 4.
Comparison of mean nutrient intake with DRI values on the baseline and ketogenic diets. Nutrients are RDAs unless noted as AI. Intake is presented as percentage of the specified DRI. Mean differences were tested by the Kruskal–Wallis test for variance. n = 6. *,**Significant intake change: *P < 0.025, **P < 0.01. AI, Adequate Intake; DRI, Dietary Reference Intake.
Discussion
The findings of this study provide evidence that consumption of a nutrient-dense KD is attainable. Selection of 3-d food records at time points that coincide with robust serum ketone measures is justification that these data accurately represent ketosis-inducing dietary intake. We acknowledge that like any dietary approach focused on macronutrient parameters, KDs can have varying nutrient profiles; however, these data demonstrate that with appropriate messaging and adherence, the concerns regarding micronutrient deficiency with KDs may be unwarranted (21).
The PCA was intended to demonstrate the robust change in food group consumption by following the KD. This particular analysis was possible owing to the fundamental difference in food choices between a regular diet and KD, including practical elimination of specific food items. Because this particular analysis highlighted foods with contrasting intake variability dependent upon specific diet adherence, many of the food loadings were anticipated, yet there were unanticipated results. Toward the KD end, we expected MCT oil to have the highest factor loading as intake increased from 0 mL/d to a mean intake of 19.2 mL/d, exclusively unique to the KD. We did not anticipate that nonstarchy vegetables would load second highest in the PCA with a nearly identical loading to MCT oil, indicating that this food group dramatically increased owing to adherence to the KD. It is notable in addition that olive oil, avocados, nuts and seeds, and seafood loaded positively at the KD end, whereas sugar-sweetened beverages, fried foods, desserts, red meat, and grains were practically exclusive to the regular diet. It is unclear why red meat intake decreased because it was not restricted in the KD protocol. Misconceptions of KD
unpalatability may stem from the rigidity required to maintain a specific KD ratio and seizure control in many epilepsy cases (22); however, we have demonstrated that the KD for conditions with more flexibility can contain variety and be more palatable and nutrient dense than typical perceptions of the diet.
Diets should be defined by more than just their caloric and macronutrient composition, as seems to be the trend with respect to the KD. The nutrient density of the individual foods consumed on the diet is an important consideration in relation to health. Interestingly, we observed an increase in vegetable and fruit intake associated with the KD, namely kale, spinach, Brussels sprouts, broccoli, bell pepper, and avocado. These data demonstrate that robust ketosis is achievable in the presence of high nonstarchy vegetable and moderate fruit intake. Nonstarchy vegetables are universally acknowledged for their wide array of health benefits (23). Their unique micronutrient density coupled with low energy and carbohydrate contribution make them an ideal component for the KD. The fruit, especially avocado (24), and vegetable intake of these participants accounted for changes in and maintenance of their micronutrient intake while following the KD.
Because the KD is a high-fat diet, some express health concerns due to inherent increases in SFA intake (25). Part of the SFA increase in our study can be explained by an average addition of 17 g of SFA from MCT oil. Even under normal metabolic circumstances, SFAs from MCT are metabolically different than short- and long-chain fatty acids. Fatty acids from MCT are easily shunted into hepatic mitochondria where they are β-oxidized and the resultant acetyl-CoA is converted to ketone bodies for nonhepatic energetics (26, 27). In a ketotic state, fat metabolism is the primary energy mechanism, indicating that SFA may be primarily destined for energy production, a contrasting metabolic fate to that in a glucose-rich state (28, 29). Even within KDs that double SFA intake, little change in circulating SFA has been observed (29). In this study, SFA accounted for 45% of total fat intake with negligible effect on cholesterol (12), considerably less than a SFA-centric KD found to have more detrimental effects on lipid status over the short term (30). Indeed, such high SFA intake may be of valid concern (31); however, we again assert that comprehensive reporting of food and nutrient intake on the KD has been mostly neglected in human trials. It is yet to be determined how KDs formulated with known protective foods (i.e., vegetables and avocado) may influence health outcomes. In addition, because the nutrigenetics of lipid metabolism are complex (32), there is evidence that genotype may be pertinent when considering KD applicability (33).
MUFAs coming from olive oil, avocados, and nuts and seeds also increased on the KD. MUFAs are considered vital fatty acids consistently linked to multiple improved health outcomes (34, 35), particularly those from plant sources (36), the primary source of MUFAs in this analysis. No change occurred in PUFAs, including anti-inflammatory omega-3 fatty acids. It should be noted that participants were already meeting recommendations for ω-3 as α-linolenic acid before the initiation of the KD intervention (37). Long-chain ω-3 intake, specifically DHA (22:6n–3) and EPA (20:5n–3), was low in the diet at baseline and KD. Within the context of a KD, increases in SFAs and MUFAs with no change in PUFAs may be a positive finding because PUFAs are anecdotally thought to have lower tolerance in high quantity on the KD and ω-6 PUFAs could have more proinflammatory properties than MUFAs, SFAs, and ω-3 PUFAs (38). In future studies, ω-3 intake could potentially be optimized with greater emphasis upon fatty fish, chia seed, and walnut consumption.
Although the KD was quite nutrient dense, some of the micronutrients that have previously been identified as nutrients of concern among older adults (39) fell below the RDA and AI. We calculated percentage intake of EAR for these nutrients. The KD met the following percentage intake of EAR: 90% vitamin E, 78% folate, 67% calcium, 62% magnesium, and 55% vitamin D. Supplement intake was not included in this analysis, but participants were provided and instructed to take multivitamin, vitamin D, calcium, phosphorus, and magnesium supplements while following the KD. This report suggests that, while following a KD, these may be nutrients to address from a food or supplementation perspective. It is noteworthy that the nutrients with low intake in the KD were also similarly low in the BD.
There are limitations to this study that should be discussed. Of the original 15 participants enrolled in the study, 5 participants withdrew within weeks of the start of the KD intervention. Completeness of the dietary intake information was also a factor that limited this analysis to a small sample of 6 participants. Discussed in our previous publication, the responsibility of the caregiver (study partner in our case) vastly increases as the patient's level of dementia also increases (40). Thus, caregiver burden was an obstacle to the collection of complete, informative 3-d food records from 4 of the study participants. Although one of our goals was to characterize patient intake on the KD, the primary feasibility aim was assessable through ketone production biomarkers, a much simpler measurement for study partners to collect. Three-day food records are commonly used as the “gold standard” in dietary assessment validation studies, yet they require high participant motivation and may have limitations related to self-report.
As KD research continues to grow, it will be inevitable that the nutrition field will embrace its practical use if it is found to benefit specific conditions. Going forward, it will be important to consider KD diet quality. Although the primary goal of the KD is to produce ketones, thought to have powerful messaging and potential therapeutic roles, it should be recognized that nutrient-dense versions of the diet may synergistically improve the efficacy, tolerability, and time capacity of KD therapies. Numerous diet quality indexes have been constructed to objectively differentiate nutritional quality between individuals (41–44). These indexes are inherently incapable of sensitively measuring the diet quality of the KD because they do not encompass foundational KD principles, primarily the expected significant increase in fatty acid consumption. We advocate for the formation of a hierarchical KD quality index that encompasses concepts such as KD ratio, nutrient-rich food intake, and optimal fatty acid profile/ratio that can be applied to future KD research.
In conclusion, the KD can be a diverse, nutrient-dense diet rich in nonstarchy vegetables and avocados.
Acknowledgments
The authors’ responsibilities were as follows—MKT: collected the data, was responsible for data analysis, and was the primary contributor of manuscript content; RHS, JMB, and DKS: oversaw data collection, and contributed content and edits to the manuscript; and all authors: were involved in study design, had full access to the data, and read and approved the final manuscript.
Notes
Supported by University of Kansas Alzheimer's Disease Center grant P30AG035982 (to RHS and JMB), the University of Kansas Heartland Institute for Clinical and Translational Science Award Center, the University of Kansas Department of Dietetics and Nutrition, and the Stop Alzheimer's Now Foundation.
Author disclosures: MKT and DKS, no conflicts of interest. JMB receives or has received research support in the last 2 y for clinical trials from Lilly, Avid Radiopharmaceuticals, Toyama Chemical Company, Merck, and Biogen. RHS within the last 2 y has received an honorarium from Accera and clinical trial support from Ausio Pharmaceuticals, LLC and the Alzheimer's Association.
Abbreviations used:
- AD
Alzheimer disease
- AI
Adequate Intake
- BD
baseline diet
- BHB
β-hydroxybutyrate
- CDR
clinical dementia rating
- EAR
Estimated Average Requirement
- KD
ketogenic diet
- MCT
medium-chain TG
- PCA
principal components analysis
- RD
registered dietitian
References
- 1. Wheless JW. History of the ketogenic diet. Epilepsia 2008;49(Suppl 8):3–5. [DOI] [PubMed] [Google Scholar]
- 2. Abbasi J. Interest in the ketogenic diet grows for weight loss and type 2 diabetes. JAMA 2018;319(3):215–17. [DOI] [PubMed] [Google Scholar]
- 3. Paoli A. Ketogenic diet for obesity: friend or foe? Int J Environ Res Public Health 2014;11(2):2092–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McSwiney FT, Wardrop B, Hyde PN, Lafountain RA, Volek JS, Doyle L. Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metabolism 2018;83:e1–e2. [DOI] [PubMed] [Google Scholar]
- 5. Urbain P, Strom L, Morawski L, Wehrle A, Deibert P, Bertz H. Impact of a 6-week non-energy-restricted ketogenic diet on physical fitness, body composition and biochemical parameters in healthy adults. Nutr Metab (Lond) 2017;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cunnane SC, Courchesne-Loyer A, Vandenberghe C, St-Pierre V, Fortier M, Hennebelle M, Croteau E, Bocti C, Fulop T, Castellano CA. Can ketones help rescue brain fuel supply in later life? Implications for cognitive health during aging and the treatment of Alzheimer's disease. Front Mol Neurosci 2016;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koppel SJ, Swerdlow RH. Neuroketotherapeutics: a modern review of a century-old therapy. Neurochem Int 2018;117:114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller VJ, Villamena FA, Volek JS. Nutritional ketosis and mitohormesis: potential implications for mitochondrial function and human health. J Nutr Metab 2018:5157645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gano LB, Patel M, Rho JM. Ketogenic diets, mitochondria, and neurological diseases. J Lipid Res 2014;55(11):2211–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McDonald TJW, Cervenka MC. Ketogenic diets for adult neurological disorders. Neurotherapeutics 2018;15(4):1018–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging 2012;33(2):425.e19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor MK, Sullivan DK, Mahnken JD, Burns JM, Swerdlow RH. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer's disease. Alzheimers Dement (N Y) 2018;4:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yuen AWC, Walcutt IA, Sander JW. An acidosis-sparing ketogenic (ASK) diet to improve efficacy and reduce adverse effects in the treatment of refractory epilepsy. Epilepsy Behav 2017;74:15–21. [DOI] [PubMed] [Google Scholar]
- 14. Perez-Guisado J, Munoz-Serrano A, Alonso-Moraga A. Spanish Ketogenic Mediterranean Diet: a healthy cardiovascular diet for weight loss. Nutr J 2008;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paoli A, Cenci L, Grimaldi KA. Effect of ketogenic Mediterranean diet with phytoextracts and low carbohydrates/high-protein meals on weight, cardiovascular risk factors, body composition and diet compliance in Italian council employees. Nutr J 2011;10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paoli A, Bianco A, Grimaldi KA, Lodi A, Bosco G. Long term successful weight loss with a combination biphasic ketogenic Mediterranean diet and Mediterranean diet maintenance protocol. Nutrients 2013;5(12):5205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Freeman JM, Kossoff EH. Ketosis and the ketogenic diet, 2010: advances in treating epilepsy and other disorders. Adv Pediatr 2010;57(1):315–29. [DOI] [PubMed] [Google Scholar]
- 18. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R et al.. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7(3):263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43(11):2412–14. [DOI] [PubMed] [Google Scholar]
- 20. Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51(2):241–7. [DOI] [PubMed] [Google Scholar]
- 21. Christodoulides SS, Neal EG, Fitzsimmons G, Chaffe HM, Jeanes YM, Aitkenhead H, Cross JH. The effect of the classical and medium chain triglyceride ketogenic diet on vitamin and mineral levels. J Hum Nutr Diet 2012;25(1):16–26. [DOI] [PubMed] [Google Scholar]
- 22. Lee E, Kang HC, Kim HD. Ketogenic diet for children with epilepsy: a practical meal plan in a hospital. Clin Nutr Res 2016;5(1):60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Slavin JL, Lloyd B. Health benefits of fruits and vegetables. Adv Nutr 2012;3(4):506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mahmassani HA, Avendano EE, Raman G, Johnson EJ. Avocado consumption and risk factors for heart disease: a systematic review and meta-analysis. Am J Clin Nutr 2018;107(4):523–36. [DOI] [PubMed] [Google Scholar]
- 25. Vining EP. Long-term health consequences of epilepsy diet treatments. Epilepsia 2008;49(Suppl 8):27–9. [DOI] [PubMed] [Google Scholar]
- 26. Schonfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res 2016;57(6):943–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Courchesne-Loyer A, Lowry CM, St-Pierre V, Vandenberghe C, Fortier M, Castellano CA, Wagner JR, Cunnane SC. Emulsification increases the acute ketogenic effect and bioavailability of medium-chain triglycerides in humans: protein, carbohydrate, and fat metabolism. Curr Dev Nutr 2017;1(7):e000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Volek JS, Fernandez ML, Feinman RD, Phinney SD. Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog Lipid Res 2008;47(5):307–18. [DOI] [PubMed] [Google Scholar]
- 29. Forsythe CE, Phinney SD, Feinman RD, Volk BM, Freidenreich D, Quann E, Ballard K, Puglisi MJ, Maresh CM, Kraemer WJ et al.. Limited effect of dietary saturated fat on plasma saturated fat in the context of a low carbohydrate diet. Lipids 2010;45(10):947–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fuehrlein BS, Rutenberg MS, Silver JN, Warren MW, Theriaque DW, Duncan GE, Stacpoole PW, Brantly ML. Differential metabolic effects of saturated versus polyunsaturated fats in ketogenic diets. J Clin Endocrinol Metab 2004;89(4):1641–5. [DOI] [PubMed] [Google Scholar]
- 31. Leow ZZX, Guelfi KJ, Davis EA, Jones TW, Fournier PA. The glycaemic benefits of a very-low-carbohydrate ketogenic diet in adults with type 1 diabetes mellitus may be opposed by increased hypoglycaemia risk and dyslipidaemia. Diabet Med 2018;35(9):1258–63. [DOI] [PubMed] [Google Scholar]
- 32. Abdullah MM, Jones PJ, Eck PK. Nutrigenetics of cholesterol metabolism: observational and dietary intervention studies in the postgenomic era. Nutr Rev 2015;73(8):523–43. [DOI] [PubMed] [Google Scholar]
- 33. Freemantle E, Vandal M, Tremblay Mercier J, Plourde M, Poirier J, Cunnane SC. Metabolic response to a ketogenic breakfast in the healthy elderly. J Nutr Health Aging 2009;13(4):293–8. [DOI] [PubMed] [Google Scholar]
- 34. Gillingham LG, Harris-Janz S, Jones PJ. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011;46(3):209–28. [DOI] [PubMed] [Google Scholar]
- 35. Schwingshackl L, Hoffmann G. Monounsaturated fatty acids, olive oil and health status: a systematic review and meta-analysis of cohort studies. Lipids Health Dis 2014;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zong G, Li Y, Sampson L, Dougherty LW, Willett WC, Wanders AJ, Alssema M, Zock PL, Hu FB, Sun Q. Monounsaturated fats from plant and animal sources in relation to risk of coronary heart disease among US men and women. Am J Clin Nutr 2018;107(3):445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Institute of Medicine Food and Nutrition Board Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington (DC): National Academy Press; 2005. [Google Scholar]
- 38. Calder PC. Polyunsaturated fatty acids and inflammatory processes: new twists in an old tale. Biochimie 2009;91(6):791–5. [DOI] [PubMed] [Google Scholar]
- 39. ter Borg S, Verlaan S, Hemsworth J, Mijnarends DM, Schols JM, Luiking YC, de Groot LC. Micronutrient intakes and potential inadequacies of community-dwelling older adults: a systematic review. Br J Nutr 2015;113(8):1195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu H, Wang X, He R, Liang R, Zhou L. Measuring the caregiver burden of caring for community-residing people with Alzheimer's disease. PLoS One 2015;10(7):e0132168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guenther PM, Kirkpatrick SI, Reedy J, Krebs-Smith SM, Buckman DW, Dodd KW, Casavale KO, Carroll RJ. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr 2014;144(3):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr 2014;17(12):2769–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 2014;17(8):1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]