Abstract
Human pathogens that are transmitted by insects are a global problem, particularly those vectored by mosquitoes; for example, malaria parasites transmitted by Anopheles species, and viruses such as dengue, Zika and chikungunya that are carried by Aedes mosquitoes. Over the past 15 years, the prevalence of malaria has been substantially reduced and virus outbreaks have been contained by controlling mosquito vectors using insecticide-based approaches. However, disease control is now threat-ened by alarming rates of insecticide resistance in insect populations, prompting the need to develop a new generation of specific strategies that can reduce vector-mediated transmission. Here, we review how increased knowledge in insect biology and insect–pathogen interactions is stimulating new concepts and tools for vector control. We focus on strategies that either interfere with the development of pathogens within their vectors or directly impact insect survival, including enhancement of vector-mediated immune control, manipulation of the insect microbiome, or use of powerful new genetic tools such as CRISPR– Cas systems to edit vector genomes. Finally, we offer a perspective on the implementation hurdles as well as the knowledge gaps that must be filled in the coming years to safely realize the potential of these novel strategies to eliminate the scourge of vector-borne disease.
Pathogens transmitted by arthropod vectors have impacted humanity greatly throughout our evolutionary history. For example, Plasmodium parasites, the causative agents of the deadly disease malaria, have left multiple signatures of natural selection visible in our genome through their interactions with humans and our ancestors over millions of years1,2. Today, vector-borne pathogens endanger people mostly in tropical and subtropical regions around the globe, from South America and Africa to South East Asia and the Pacific, such that half of the world’s population lives at risk of the diseases that the pathogens cause (Table 1). Nearly 200 million people become infected every year with parasites including Plasmodium falciparum, Trypanosoma brucei and Leishmania donovani, viruses such as dengue (DENV), Zika (ZIKV) and chikungunya (CHIKV), and filarial nematodes such as Brugia malayi and Wuchereria bancrofti3,4, and hundreds of thousands, mostly young African children, die annually because of such pathogens. These pathogens show high specificity for their insect vectors, and while human malaria P. falciparum and Plasmodium vivax parasites are exclusively transmitted by species belonging to the Anopheles genus, viruses are generally transmitted by culicine species, which diverged from anophelines approximately 100 million years ago (Ma)5 (Box 1). Besides mosquitoes, a variety of other insects transmit important human pathogens, including glossinid tsetse flies that vector African trypanosomes, parasites that cause sleeping sickness in humans and nagana in cattle, phlebotomine sandflies that transmit Leishmania parasites, triatomine reduviid bugs that transmit American trypanosomes, the causative agents of Chagas disease, and simulid black flies that vector onchocerciasis (river blindness). Mosquitoes of the Anopheles and Culex genera also support the transmission of nematodes that cause lymphatic filariasis, commonly known as elephantiasis (Table 1). Additionally, some pathogens inflict severe lifelong disabilities that are associated with social stigma when visible (such as in the cases of elephantiasis or leishmaniasis)6,7. The costs of vector-borne diseases on the society, economy and health systems of the affected regions are immense, yet are difficult to quantify accurately, making policy decisions about resource allocation extremely challenging7.
Table 1 |.
The global distribution and burden of ten of the world’s most important vector-borne human diseases
| Human disease | Causative organism | Vector | Distribution | At risk (millions)a | Prevalence (millions)a | Deaths (thousands)a | DALYs192 (thousands) |
|---|---|---|---|---|---|---|---|
| Malaria | Protozoan: Plasmodium | Mosquito: Anopheles | Tropical and subtropical regions (91% malaria-ascribed deaths occur in sub-Saharan Africa (2016)) | 2016: 3,025.03 | Unknown asymptomatic reservoir | 2016: 445.03 | 46,486 |
| Lymphatic filariasis | Nematodes: Wuchereria bancrofti, Brugia malayi, Brugia timori | Mosquito: Culex, Aedes, Anopheles (in Africa), Mansonia | Tropical and subtropical regions of SE Asia, Africa W Pacific, parts of Central and S America | 2016: 856.4193 | 2015: 120.000194 (38.4 disfigured195) | N.R. | 5,777 |
| Sleeping sickness (human African trypanosomiasis) | Protozoan: Trypanosoma brucei | Tsetse fly: Glossina | Regions of subtropical and tropical Africa | 2010–2014: 61.018 | 2015: 0.011195 | 2015: 3.5196 | 1,525 |
| Chagas disease (American trypanosomiasis) | Protozoan: Trypanosoma cruzi | Reduviid bug: Rhodnius, Triatoma | Regions of the Americas | 25.0197 | 2015: 6.653195 | 2015: 8.0196 | 667 |
| Leishmaniasis | Protozoan: Leishmania | Sand fly: Phlebotomus, Lutzomyia | 87 countries in intertropical and temperate regions; E Africa, Mediterranean, S America, Middle East, S Asia | 1990: 350.0198 | 2015: 3.859195 | 2015: 24.2196 | 2,090 |
| River blindness (Onchocerciasis) | Nematode: Onchocerca volvulus | Black fly: Similium | 99% tropical and subtropical Africa; also Yemen, Brazil and Venezuela | 123.0199 | 2015: 15.500195 | N.R. | 484 |
| Dengue | Flavivirus: DENV | Mosquito: Aedes | 128 countries in tropical and subtropical regions around the globe | 2012: 3,970.0200 | 2015: 79.600195 | 2015: 18.4196 | 616 |
| Yellow fever | Flavivirus: YFV | Mosquito: Aedes, Haemogogus | Tropical and subtropical Africa and regions of S America (44 countries) | 2017: 912.5201 | 2015: 0.003195 | 2015: 5.1196 | N.R. |
| Zika | Flavivirus: ZIKV | Mosquito: Aedes | Tropical and subtropical regions around the globe (96 countries)202 | 2016: 2,170.0203 | 2013–2014: ~0.02910 (French Polynesia) 2015–2016: unknown (Brazil) | 2013–2014: 0.010 (French Polynesia) 2015–2016: unknown (Brazil) | N.R. |
| Chikungunya | Togavirus: CHIKV | Mosquito: Aedes | Tropical, subtropical and temperate regions around the globe (94 countries)204 | 2016: 1,300.0204 | 2013–Apr 2015: ~1.400205 (suspected, Americas) | 2013–Apr 2015: 191.0205 (Americas) | N.R. |
Data were collated from cited references and the World Health Organization and Centers for Disease Control websites. DALYs, disability-adjusted life years; N.R., not reported
estimated
Box 1 |. Key vectors.
Anopheles mosquito.
This is a brown mosquito characterized by the black speckled pattern on their wings and the length of their maxillary palps (sensory organs on the head are as long as the proboscis). There are ~430 species worldwide (except Antarctica), each adapted to different regions and habitats, but only 30–40 transmit human malaria parasites. In most of these transmitting species, females blood feed in the evening and during the night, making bed nets an effective strategy to prevent biting of humans. Other species within the genus are also able to transmit mammalian malaria parasites, filarial worms and some viruses.
Aedes mosquito.
This is a dark brown or black mosquito characterized by dark legs with distinctive white stripes. The most important human disease vector species are A. aegypti (the yellow fever mosquito) and Aedes albopictus (the Asian tiger mosquito). Aedes species belong to the Culicinae subfamily and are able to transmit many viruses (dengue, chikungunya, yellow fever and Zika, among others) but only transmit malaria parasites of birds, not mammals. Aedes have an excellent adaptability to their environment, and the same species can be found in both rural and urban settings. Their black eggs can desiccate and survive for several months, facilitating their global spread. Females can bite anytime during the day, making bed nets relatively ineffectual.
Culex mosquito.
This is a brown/grey mosquito with white patches on the abdomen, unpatterned wings and short maxillary palps. Culex mosquitoes also belong to the Culicinae subfamily and are responsible for several zoonotic infections in humans (West Nile virus and Japanese encephalitis) and can also transmit filarial worms and avian malaria parasites. Females bite during the evening and lay eggs in raft containing many eggs stuck together on the surface of the water.
Tsetse fly.
This is a large dipteran fly (0.5–1.5 cm in length) found only in tropical Africa with a unique reproductive biology, in that a female fly nurses a developing egg internally and gives birth to a live larva. Both males and females blood feed on humans and transmit trypanosomes, which cause human African trypanosomiasis (also known as sleeping sickness). Attracted to the colour blue, insecticide-treated blue-black traps have effectively controlled this vector across Africa. There are ~34 species, many of which also transmit trypanosomes in animals, causing a disease called nagana.
Sand fly.
This is a very small hairy brown fly (3 mm in length) that is found globally from tropical to temperate regions. There are two major genera that transmit Leishmania parasites: Lutzomyia in the Americas and Phlebotomus in Europe, Asia and Africa, together comprising many hundreds of species. Female sandflies tend to bite at night and use their mouthparts to break capillaries in the skin, drinking blood from the resulting wound (pool feeding).
Black fly.
This is a small black/grey fly in the genus Simulium and found globally as over 1,000 species. Black flies transmit the nematode Onchocerca volvulus, which causes onchocerciasis or ‘river blindness’. The female black fly lays her eggs in the clean flowing water of streams and rivers, rather than static pools, and this association to rivers gives the disease its common name.
Kissing bug.
These are true bugs from the subfamily Triatominae, and unusual in the reduviid genus because they feed on vertebrate blood. There are more than 130 species and are most widespread in the Americas. Nocturnally active, they live around people in low-quality housing (cracks in the floor and walls) and bite them when they are sleeping. They are known as kissing bugs because they often bite people on the lips. They vector Trypanosoma cruzi, the causative agent of Chagas disease, and the main protection against bites is improving the quality of housing to prevent vector access.
Arboviruses (a general name including all arthropod-borne viruses) have become an urgent public health priority, with a staggering four billion people in the world at risk of infection. Tropical and subtropical regions are affected the most, but more extreme latitudes are not exempt from these threats—in part due to a warming global climate predicted to extend the ecological niches of the mosquito vectors8,9. Indeed, transmission of flaviviruses such as DENV, ZIKV and yellow fever virus (YFV) is facilitated by the near-global presence of the two major Aedes vectors, Aedes aegypti and Aedes albopictus. Increasing travel, migration and global trade are expanding the original boundaries of these vectors and the pathogens they transmit, spreading disease to naive populations with devastating consequences. Two prominent examples are the recent ZIKV outbreaks triggered by infected travellers to French Polynesia and Brazil10,11, and the expansion of A. albopictus into Europe and the Americas by the trade in used tyres12,13. The Zika epidemic is a potent warning that new viruses may become significant health threats, as is potentially the case for Mayaro virus, which causes dengue-like illness. It was first identified in the Amazon rainforest and has now been reported in Peru, Venezuela and Haiti14,15.
Vector control is one of the most effective methods to stop these diseases. For example, the use of longlasting insecticide-treated nets (LLINs) and indoor residual spraying (IRS) to control the Anopheles mosquitoes that transmit malaria parasites has contributed to more than 75% of the averted malaria deaths since the beginning of this century16. Accordingly, the World Health Organization estimates that “mosquito control is the only intervention that can reduce [malaria] transmission from very high levels to close to zero”17. Moreover, strengthened vector control and surveillance have made significant gains against pathogens spread by other blood-feeding insects such as tsetse flies18 and sandflies19 through the use of insecticide-impregnated traps and aerial insecticide sprays, respectively. However, the increased application of chemical control programmes has led to the emergence and spread of insecticide resistance in natural Anopheles20, Aedes21 and Phlebotomus22 populations, and although the public health impact of this phenomenon is yet unknown, it raises strong concerns for the future of our best vector control methods.
Other factors also influence our ability to control insect vectors. For example, the adaptability of Aedes mosquitoes to new habitats poses a significant challenge, particularly in urban environments where high population densities of both humans and mosquitoes expose many more people to the risk of disease23. In the case of malaria, LLIN and IRS strategies only target Anopheles species that feed and rest indoors, and besides a limited use of larvicidal compounds, at present there are no tools to prevent transmission by outdoor biting and feeding mosquito populations. Control of residual malaria, that is, malaria transmission in the presence of universal bed net coverage24, could represent an insurmountable hurdle in the effort towards malaria eradication.
The development of alternative strategies to effectively reduce transmission of pathogens by insect vectors is therefore a high priority. Substantial efforts are underway to exploit genetic information provided by genome-sequencing projects5,25–27 and design effective genetic tools towards the development of novel vector control methods28–33. In parallel, studies on basic insect biology and insect–pathogen interactions are helping unravel the processes that regulate the insect’s ability to transmit deadly human pathogens, creating new concepts to stop transmission. Here, we review these efforts, focusing both on strategies that aim to reduce or even eliminate natural vector populations, and on strategies that target the pathogen within the insect.
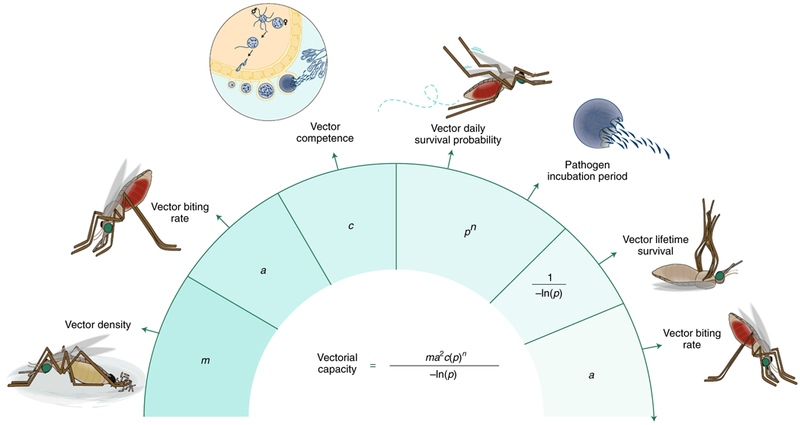
The key components driving vectorial capacity
Vector-borne pathogens have a high specificity for the arthropods that transmit them. As mentioned above, Plasmodium species that infect humans are transmitted exclusively by mosquitoes belonging to the Anopheles genus, but these vectors do not support the development and transmission of most arboviruses, including DENV, ZIKV34, CHIKV35 and YFV, which are transmitted by Aedes species (Box 1). Although the biological reasons behind this specificity mostly remain elusive, the parameters that shape the ability of an insect to act as a vector for human pathogens are understood and form the components of vectorial capacity, defined as the rate at which insects can transmit a pathogen from a currently infectious case36 (Box 2). Female insects that feed on vertebrate blood do so primarily to obtain nutrients to develop eggs for reproduction. Different species differ dramatically in their population density, and this parameter of the vectorial capacity equation is a function of both the reproduction rate and complex interactions with the local ecological environment (predation, nutritional resources and other density-dependent effects) (Box 2). For example, Anopheles gambiae mosquitoes, the most important vectors of malaria in sub-Saharan Africa, have a large reproductive output as they develop ~50–150 eggs each time they take a blood meal. Conversely, Glossina morsitans tsetse flies, which transmit the African sleeping sickness pathogen Trypanosoma brucei, may produce only 8 off-spring in their entire lives37. Females employ a costly but effective viviparous reproductive strategy whereby they develop a single egg in their ovary and hatch it in their uterus, nourishing the developing larva through several larval moults until they give birth to a mature larva38. Therefore, targeting Glossina densities through using odourbaited traps treated with insecticides has a dramatic effect on the transmission of African trypanosomes39. In contrast, for vectors with higher reproductive outputs such as A. gambiae, insecticide-based strategies may have a more limited impact unless coverage is high, and alternative interventions aimed at interrupting pathogen transmission, as discussed below, are needed.
Box 2 |. Vectorial capacity.
To estimate the ability of different vector species to transmit disease-causing pathogens, multiple biological factors can be combined into a simplified equation, which is helpful to understand which species may represent the most urgent targets for vector control, or which methods may be most immediately effective to reduce transmission36.
Vector density (m).
Logically, the more vectors per human, the more likely they can transmit pathogens. Vector density is regulated by the reproductive biology of each species (as well as other ecological and environmental factors) and different species can differ dramatically in their reproductive output.
Human-biting rate (a).
Vectors use olfactory and other sensory cues (such as CO2 and heat) to identify hosts for a blood meal, and can be either highly specific or plastic in their preferences206. To transmit a pathogen, a vector must bite humans twice—once to pick up the infection from an infected person and again after a few days to transmit the infection to another person. Since this occurs twice, the rate of human-biting is squared in the equation, making this parameter highly influential on vectorial capacity36. Therefore, many vector control strategies aim to reduce the human-biting rates, such as LLINs and repellents.
Competence (c).
Ingested pathogens must survive and develop within their insect hosts to be transmitted. The variation in competence to support pathogen development between vectors is due to many biological factors, including the internal physiology, nutritional status and immune responses, and possible interactions with other pathogens43.
Survival (p).
Pathogens ingested by competent vectors complete their development and migrate to specific tissues (such as the midgut and salivary glands) to be transmitted to the next host. This lag between being taken up and becoming infectious is known as the extrinsic incubation period (EIP) and can take a substantial fraction of the insect’s lifespan, making survival the most influential parameter in vectorial capacity36. In the equation, the infectious lifespan of the vector is represented by pn/−ln(p), where EIP is represented by n, the number of days the pathogen needs to develop in the vector before it can be transmitted, and the daily survival rate of mosquitoes represented by p. Strategies that shorten lifespan below the length of EIP yet allow vector reproduction would prevent transmission without causing intense selective pressures on the vector to evolve resistance.
Pathogens have evolved a variety of transmission strategies that exploit the intrinsic need of insects to feed on blood. While most are transmitted to a human host directly by an infectious bite, some others, such as the Chagas disease agent Trypanosoma cruzi, are mechanically transferred by bug defecation onto human skin during blood feeding, or ingestion of food contaminated with bug faeces. The parasite is then introduced into the body by scratching of the insect bite or through mucosal membranes of the eyes or mouth. Regardless of the mechanism, transmission depends on the frequency at which the insect vector bites humans as opposed to animals. Insects that have a higher preference for humans as a source for blood are more likely to transmit human pathogens40, so human-biting rates are a major component of vectorial capacity (Box 2). These rates are mostly determined by host feeding preferences, but also vary with the length of the insect reproductive cycle41 and energy reserves available42—factors that influence the frequency of blood feeding and therefore the chances of transmission. Because of its relevance for vectorial capacity, reducing the human-biting rate is a key component of malaria control programmes through using LLINs and IRS.
Once a pathogen has been ingested, the probability of transmission depends on the vector competence of the insect, defined as its ability to support pathogen development (Box 2). As discussed below, multiple novel control strategies aim at reducing this parameter by genetic and biological means. Whether because of strong immune responses or the lack of key factors needed for invasion or replication, most insects represent a dead end for pathogens, but competence is also modified by physiological and environmental factors, including nutritional status, interactions with the microbiota and coinfections with other pathogens43. Resident midgut bacteria may produce compounds that directly affect parasite development, or they may trigger an indirect stimulation of the mosquito immune response. For example, an Enterobacter species isolated from natural Anopheles populations in Zambia (Esp_Z) can block both human and rodent Plasmodium development if these bacteria colonize the mosquito midgut44. Dietary restrictions during larval development also negatively affect intensity and prevalence of Plasmodium infections45, as do coinfections with entomopathogenic fungi46.
Finally, most pathogens have a complex and lengthy developmental cycle within their insect hosts (Fig. 1). For example, the sporogonic journey of the deadliest malaria parasite P. falciparum within the Anopheles mosquito takes 12–14 days, from the earliest stages of development in the midgut to invasion of the salivary glands, from where it can be transmitted to the next human host. DENV also needs a minimum of 4–5 days at 25°C (refs 47,48) and multiple rounds of replication before the mosquito becomes infectious. Therefore, insect survival represents a crucial factor for vectorial capacity (Box 2), as many insects will not live long enough to ensure effective pathogen transmission49.
Fig. 1 |. The malaria and dengue transmission cycles.
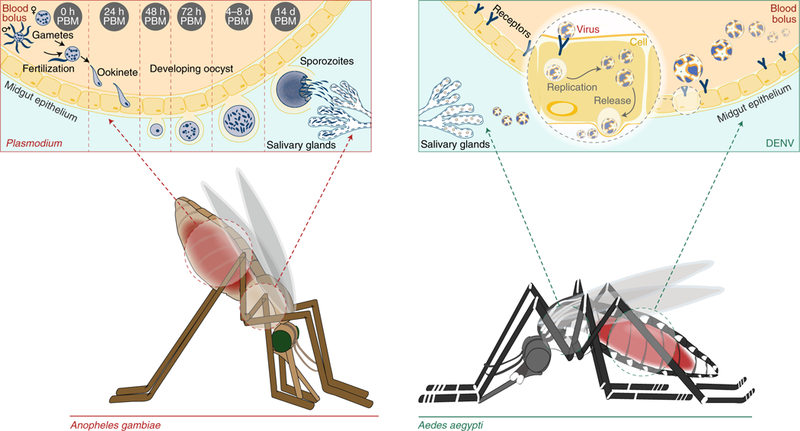
The developmental stages of P. falciparum (left) and DENV (right) are shown in A. gambiae and A. aegypti, respectively. Anopheles mosquitoes take up Plasmodium parasites as female and male gametocytes, which rapidly convert to gametes. The male gamete exflagellates to produce eight microgametes, which can fertilize the single female macrogamete. The formed zygote becomes a motile ookinete, traverses the midgut epithelium, and develops into an oocyst on the basal lamina of the outer midgut. Over several days, sporozoites develop in the oocyst, burst out and spread to the mosquito salivary glands by the haemolymph. Sporozoites are injected into the next host when the mosquito bites again. Similarly, in Aedes mosquitoes, DENV is taken up into midgut cells and, over several days, replicates its genome and expresses viral proteins. New virions are assembled and released into the mosquito haemolymph and invade the salivary glands for transmission to the next host. PBM, post blood meal.
Interventions for vector control
Insecticide-based strategies such as LLINs, IRS, aerial sprays and insecticide-treated traps are the mainstay of vector control, and until recently, with the absence of effective vaccines for most vector-borne pathogens, they represented the only conceivable tools that could achieve disease elimination. However, studies elucidating both the immune mechanisms of vector defence against pathogens and the impact of the microbiota for vector competence have expanded our prospects of disrupting pathogen transmission. Combined with the whole-genome sequencing of the most important disease vectors5,25–27 and the recent explosion of genetic engineering technologies to modify genomes28–33, unparalleled opportunities to prevent transmission by targeting the biological aspects of vectorial capacity are in view.
Chemical interventions.
Our most effective weapon for controlling disease continues to be the use of neurological toxins to kill insect vectors. In the case of malaria, where widespread use of insecticides has successfully eliminated disease in many countries, chemical strategies remain the cornerstone of control efforts through LLINs and IRS. LLINs are aimed at those mosquito species that bite sleeping people during the night, while IRS targets insects that rest on house walls after blood feeding. These two methods have significantly reduced malaria cases16 but have limited use against Aedes or Culex species, which tend to bite outdoors during the day or early evening when people are unprotected. Insecticides against these mosquitoes are therefore applied more liberally to the environment using questionably effective airborne spraying methods50.
Limited diversity in the modes of action of the insecticides for public health, combined with widespread use for agricultural purposes, has strongly selected for resistance mutations in insect populations across the world. Despite heavy investment in vector control, these chemicals can now no longer control outbreaks of disease effectively51. This is particularly alarming in the case of pyrethroid insecticides, as these are the only compounds currently approved for use on LLINs due to their low toxicity to mammals. Analogous mutations in the voltage-gated sodium channel targeted by both pyrethroids and DDT have occurred independently in Anopheles and Culex mosquitoes as well as in sandflies22,52–54, or have been transferred between closely related vectors55. More generalized resistance mechanisms have also spread rapidly, including the upregulated expression of detoxification enzymes in A. gambiae56, A. aegypti57,58 and A. albopictus59, and the thickening of the insect cuticle in A. gambiae to prevent insecticide penetration60. Combined, these mechanisms are predicted to have a significant impact on our ability to kill insects and may limit our options for future insecticide development. However, the true public health impact of insecticide resistance needs to be better determined as lifetime costs to insect longevity and reproduction61,62 may partially offset the effects of reduced mortality.
Chemical control does not necessarily have to kill vectors on contact to achieve an end to pathogen transmission. Alternative strategies incorporating active ingredients with the ability to sterilize, repel or treat vectors could be equally effective in the long term. Research on mating and reproductive physiology has developed sterilizing compounds for mosquitoes based on the insect hormones JH (juvenile hormone) and 20E (20-hydroxyecdysone), which are needed for reproduction63–67. The JH analogue PPF (pyriproxifen) induces sterility andhas life-shortening activity in adult mosquitoes, and is currently being tested on LLINs for its effectiveness against pyrethroid-resistant mosquito populations68–72, and similar effects are produced by the 20E agonist DBH (methoxyfenozide)73. These compounds, when added to insecticide formulations, would sterilize females that are not killed, thereby limiting the spread of resistance73,74.
Interestingly, DBH compounds also reduce Plasmodium parasite development within the Anopheles mosquito by an as yet unknown mechanism. Infection prevalence was strongly reduced when females were exposed to DBH shortly before a P. falciparum-infected blood meal, an effect predicted to have a significant impact on malaria transmission73. This finding will hopefully spur interest in moving beyond insecticide-only strategies to block transmission. Anti-pathogen compounds could be incorporated within insecticide formulations to minimize the effects of insecticide resistance emerging in the insect, or could be deployed alone whenever the ecological costs of chemical control are significant.
Immune-enhancing strategies.
Even in the absence of control strategies, pathogens suffer large losses at the hands of the immune system as they pass through vectors. However, in competent insects, although immune responses do limit the number of pathogens that are effectively transmitted, they do not achieve complete elimination. Insects are not endowed with an adaptive immune response but possess an innate immunity system composed of both humoral and cell-mediated immune pathways. These pathways allow the insect to mount a generalized defence based on the production of antimicrobial peptides and/or antiviral proteins, which inhibit pathogen replication or promote pathogen lysis. Cellular immunity is mediated by midgut epithelial barriers and haemocytes, the circulating immune cells in the haemolymph, which can phagocytose invading pathogens. During infection in mosquitoes, an initial immune response is triggered by the binding of receptors to antigens produced by the invading pathogen. These receptors mostly recognize glycoprotein patterns that label particular classes of pathogens: the Toll pathway responds to Gram-positive bacteria, fungi and rodent malaria parasites75–78, while the immune deficiency (IMD) pathway targets Gram-negative bacteria and human malaria parasites78–81—although the two pathways do interact82. Once activated, these pathways trigger the release of antimicrobial peptides that lyse invading pathogens75,83.
In the Anopheles mosquito, anti-plasmodial responses occur both as malaria parasites attempt to cross the midgut epithelium and later as they encyst. At the ookinete stage (Fig. 1), parasite invasion into midgut cells triggers JNK-signalling-mediated production of reactive oxygen and nitrogen species (ROS and RNS), which nitrate the surface of the parasite. Ookinete nitration stimulates haemocytes to undergo apoptosis and release microvesicles that promote the activation of the mosquito complement system in the haemolymph84. The complement-like system consists of a complex of the thioester-containing protein TEP1 and two leucine-rich repeat proteins (LRIM1 and APL1), which binds to ookinetes and promotes their killing by either lysis or encapsulation by melanization. There is also growing evidence of a late-phase haemocyte-mediated immunity targeting oocysts and reducing their numbers85,86. This effect requires two transcription factors, LL3 (litaf-like factor 3)87 and STAT (signal transducer and activator of transcription)86, which mediate the proliferation and differentiation of haemocytes following Plasmodium infection, but the killing mechanism is unknown.
In the case of arboviruses, the primary defence in many vectors is the short interfering RNA (siRNA) silencing pathway, which degrades viral double-stranded RNA produced during replication.
The ~21 nucleotide fragments of the viral genome are incorporated into the RNA-induced silencing complex (RISC) and direct the cleavage of single-stranded RNA complementary to the viral fragment, removing additional genome copies to control infection88. The related PIWI-interacting RNA (piRNA) pathway that controls transposon activity in the germline may also be used in antiviral defence in the soma, where it produces viral-derived piRNAs by an unknown biogenesis89–91. Knockdown of the piRNA-binding proteins Ago3 or Piwi5 in A. aegypti cells reduced Sindbis virus piRNAs92, but the relative contribution of this pathway to antiviral defence requires further study.
The innate immune pathways (IMD, Toll and JAK–STAT) also contribute to antiviral immunity; it has been found that knock-down of pathway components affects DENV titres in A. aegypti93–96. Variation in transcriptional responses in different vector–virus combinations suggests that insects have evolved to tailor innate immune responses to be virus specific97.
Increased knowledge of the mechanisms that limit pathogen development can favour the generation of novel strategies to reduce vector competence (Fig. 2). The effectiveness of the immune system can be boosted by genetically engineering the overexpression of immune effectors76,81,98–101 or the repression of negative regulators of immunity77,78,94,102,103 in key transmission tissues such as the midgut, fat body and salivary glands. The final goal is to spread these immunity-enhancing factors in the wild using genetic elements known as gene drives (see below). Genetically engineered mosquito strains that successfully reduce vector competence for Plasmodium parasites81,101,104,105 and DENV100,106 have been generated in the laboratory, although none yet completely abort pathogen development. For example, transgenes expressing the transcriptional activator of the IMD pathway, Relish2, in the mid-gut and fat body effectively inhibited P. falciparum development in Anopheles stephensi mosquitoes by upregulation of genes in the complement-like system, TEP1 and APL1 (ref. 81). A similar strategy to activate the antiviral JAK–STAT pathway in A. aegypti mosquitoes by overexpressing the pathway’s receptor Dome or signal transducer Hop was effective against DENV2 and DENV4, but interestingly not against the closely related flavivirus ZIKV, nor CHIKV100. This difference again suggests that immune pathways may specialize to the level of particular viruses or, alternatively, effective immunity requires multiple pathways to be triggered together. Since immunity is stimulated only in specific tissues and/ or after a blood meal, the costs of additional immune function on mosquito fitness are contained, an important requirement if these strains are to compete in a natural environment.
Fig. 2 |. Immune control strategies.
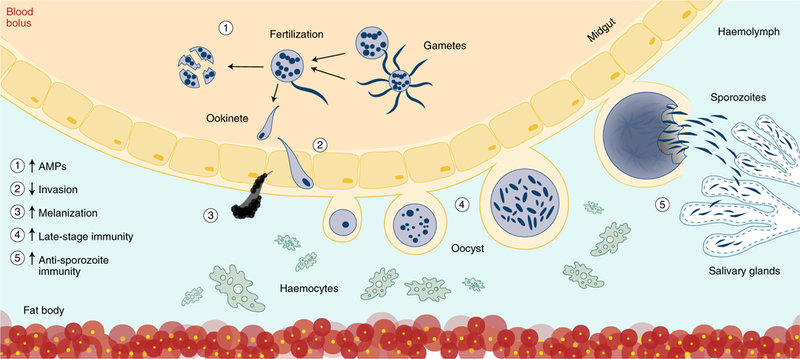
Illustrated are examples of how insect immunity can by modified at multiple life stages to combat parasite development. Increasing expression of anti-parasitic factors or antimicrobial peptides (AMPs) (1) when insects take a blood meal can induce lysis of ingested parasites. Ookinetes can be blocked from invasion by occluding ookinete ligands or midgut receptors required for successful invasion (2). Stronger, more effective immune responses to invading ookinetes (3), oocysts (4) and sporozoites (5) can be engineered by overexpressing immune effectors or reducing expression of negative regulators of mosquito immunity from the mosquito fat body and haemocytes circulating in the haemolymph. Invasion of sporozoites into the salivary glands can also be blocked by disrupting specific ligand–receptor interactions.
Besides curbing pathogen development, however, these immune strategies may also affect the natural insect microbiota81,104 and, as a result, modify mosquito behaviour unexpectedly. As an example, transgenes expressing Relish2 and AgDsPf (a variable pattern recognition receptor of A. gambiae) appeared to cause non-random mating between transgenic and wild-type individuals. Although this particular effect (possibly mediated by the gut microbiome) favoured the spread of each transgene in population cages of A. stephensi107, the introduction of assortative mating into field populations may lead to reproductive isolation and in turn speciation, thereby complicating control strategies and perturbing the natural ecosystem. Transgenes that block pathogen transmission without significantly affecting insect behaviour are therefore desirable.
While they do not modulate immune pathways per se, other strategies interfere with key parasite transitions in the mosquito such as invasion of the ookinete into the midgut epithelium or the sporozoite into the salivary glands. The first study demonstrating the feasibility of such approaches identified the SM1 peptide for its ability to block Plasmodium development at both these invasion steps when expressed in transgenic A. stephensi mosquitoes108. SM1 interferes with the binding of enolase on the ookinete’s surface to the midgut receptor EBP (enolase-binding protein) by mimicking the enolase epitope109,110, and it blocks saglin receptors on the salivary glands to prevent an interaction with the sporozoite TRAP (thrombospondin-related anonymous protein) invasion ligand111.
It is likely, however, that immune-based strategies will impose selective pressures on pathogens to adapt and ensure their transmission. Understanding mechanisms of immune evasion could nevertheless provide novel opportunities for disease control, as illustrated by studies showing how Plasmodium avoids immune recognition by the mosquito midgut. During invasion85,112, ookinetes express the protein Pfs47 on their surface, which in some way makes them ‘invisible’ to the immune system, preventing nitration and activation of the complement-like cascades described earlier113,114. The global diversity of Pfs47 in Plasmodium isolates suggests that this mechanism has been selected for on a local scale in different Anopheles– Plasmodium combinations, with potential consequences for the global spread of malaria115. Evasion could potentially be reversed using chemical sprays or transmission-blocking vaccines that prevent the function of Pfs47 or other pathogen factors required for successful midgut invasion such as those described above.
These data demonstrate that while there is great promise in immune-modulating intervention strategies, understanding local ecological, genetic and vector–pathogen interactions is paramount to their successful deployment in the diverse regions that mosquitoes inhabit.
Manipulating the microbiome.
Most insects possess rich and diverse microbiomes that regulate key physiological aspects of metabolism, reproduction, longevity and immunity, and play essential roles in the provision of nutrients that are limited or not provided by diet116–118. In blood-feeding insects, ingestion of a blood meal induces a significant expansion of the midgut microbiota, and when the blood is infected with pathogens, microorganism–pathogen interactions can affect the establishment of infection. Metagenomics sequencing projects have demonstrated the diversity of insect microbial communities119–125, and have led to the identification of several microorganisms that impair pathogen development and could be exploited as a means for blocking transmission122,123,125 (Fig. 3). For example, isolated Serratia marcescens strains have been shown to interfere with T. cruzi, Leishmania braziliensis and Plasmodium berghei in their respective vectors126–128, making these species possible transferable candidates for disease control. This search strategy follows the logic that resident microorganisms will not inflict severe fitness costs in their natural hosts and will readily colonize their tissues129. Interactions are likely to be pathogen and microorganism specific, and, in some cases, can lead to enhancing effects on pathogen development. While Serratia odorifera bacteria can increase DENV2 and CHIKV infections in A. aegypti130, the midgut microbiota is required for maximal infection by o’nyong-nyong virus (ONNV) in A. gambiae131. Alternatively, biocontrol can directly target the insect vector through entomopathogenic fungi such as Beauvaria bassiana and Metarhizium anisopliae, which kill insects slowly, allowing completion of early reproductive cycles and thus lessening selection for fungal resistance traits46,75,132.
Fig. 3 |. Manipulating the microbiome.
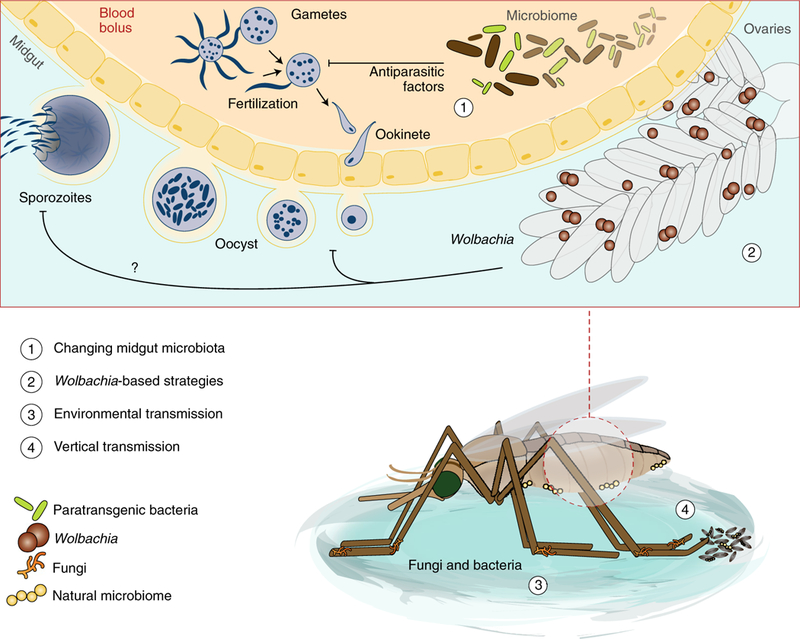
Several avenues for vector control through manipulations to the vector microbiome are possible, some of which are highlighted here for malaria mosquitoes. On parasite uptake, naturally occurring or modified paratransgenic bacteria in the mosquito could block infection by the secretion of antiparasitic factors (1). Similarly, infecting species with Wolbachia bacteria (which populate the germline, and occasionally other tissues) can block infection against a variety of parasites although the precise mechanisms of how this is achieved are unknown (2). Such strategies rely on spread of these blocking or entomopathogenic agents (fungi or bacteria) through the vector population, for instance by adding them to baited sugar traps or larval breeding sites for vectors to pick up from the environment (3). Additionally, biological agents could be spread from parents to offspring through vertical transmission to the egg or larva (4).
Besides unintended consequences for non-target organisms, one important caveat with the use of microorganisms to stop transmission is that the precise mechanism(s) of pathogen blocking are often unknown, and the potential for pathogen resistance has not been addressed. Resistance can be delayed or even avoided by engineering multiple blocking factors into one microbial species, in a strategy called paratransgenesis133,134. For example, antimicrobial peptides (such as apidaecin, cecropin A, magainin II and melittin) expressed in the Rhodnius prolixus symbiont Rhodococcus rhod-nii are not toxic to the paratransgenic bacteria but effectively kill T. cruzi parasites, and are synergistically effective133. Similar effectors have strong effects against P. falciparum when recombinantly expressed from the Serratia strain AS1 (ref. 134). Paratransgenic approaches are particularly appealing for insect species with outdoor biting and resting behaviour that cannot be effectively targeted by strategies such as LLINs and IRS. However, a major obstacle to their use is their delivery method, as currently there are no effective methods to disseminate desired microorganisms in natural populations. Without innovation in this area, paratransgenesis is unlikely to become a valuable asset in disease control.
The dissemination issue is partially alleviated when microorganisms are naturally spread from mother to offspring—a process known as vertical transmission, which can occur through multiple mechanisms. Some intracellular microorganisms such as Wolbachia are transovarially transmitted as they populate the female germline135,136, while the tsetse fly symbionts Sodalis and Wigglesworthia are provisioned in utero to the larva137. Asaia and Serratia bacteria can adhere to the surface of the egg and therefore can colonize oviposition sites (Fig. 3)134,138. Undoubtedly, Wolbachia, an α-proteobacterium that infects 66% of the insect species in the world139, is the leading candidate for the biological control of vector-borne pathogens. Some strains of this endosymbiont are ideally poised for transmission control because they combine pathogen-blocking effects with rapid spread through populations of their insect hosts. Besides generally high rates of mother–offspring inheritance, spread of Wolbachia is ensured by a number of manipulations of the host reproductive biology that impart a reproductive advantage to Wolbachia-infected females, namely biasing the sex ratio towards egg-producing females (male killing or feminization, parthenogenesis) and cytoplasmic incompatibility (CI)140. In CI, when uninfected females mate to Wolbachia-infected males, chromosomal segregation defects occur during the first cellular division in the offspring, leading to sterility. These effects are rescued when females are infected with the same or compatible Wolbachia strains. The discovery of two phage-derived proteins expressed in CI-causing Wolbachia strains141–143 may allow the development of synthetic CI mechanisms in other paratransgenic bacteria. Furthermore, in specific host–pathogen–Wolbachia combinations, these endosymbionts can block development of pathogens such as DENV and ZIKV136,144–151, probably through priming of the insect immune system or competition for host nutrients that the pathogen needs for replication145,146,149,152,153. Control strategies based on Wolbachia capitalize on one or both of these qualities. Where population suppression or elimination is desirable, large numbers of Wolbachia-infected males could be used to sterilize local uninfected females through CI (incompatible insect technique, IIT), causing a population crash154,155. Alternatively, spreading a pathogen-blocking Wolbachia strain would gradually replace the local permissive natural vectors with refractory insects. This latter strategy is now being evaluated in field trials aimed at reducing DENV transmission by A. aegypti mosquitoes in South America, South East Asia and Australia156. Released populations in Australia appear to maintain the Wolbachia infections over several years, although the infection is localized to the release area due to limited migration of adult mosquitoes157,158. Interestingly, stable Wolbachia infections were recently detected in natural Anopheles populations from West Africa, breaking the dogma that anopheline mosquitoes are resistant to colonization by these bacteria159,160. Moreover, Wolbachia infections appear to be negatively correlated to Plasmodium prevalence, opening up the possibility of harnessing these endosymbionts for the control of malaria transmission160,161.
As Wolbachia is poised to become an increasingly important component for the control of vector-borne diseases, a deeper under-standing of the molecular mechanisms mediating the Wolbachia–pathogen interactions is needed for effective and, above all, safe use of these bacteria. Moreover, as coinfection with Wolbachia can enhance development of some pathogens, as in the cases of West Nile virus in Culex tarsalis mosquitoes162 and the murine malaria model P. berghei in A. gambiae163, adequate testing of release strains against a multitude of known potential pathogens in the laboratory is strongly advisable.
Genetic manipulation of insect vectors.
The recent explosion in tools for the genetic modification of vectors has revolutionized our ability to create designer strains for genetic control strategies. To limit transmission, two principal strategies are envisaged that are either based on reducing the vector competence for pathogens (a strategy referred to as population replacement), or aim to suppress insect populations by spreading sterility (population suppression)164. In population replacement, effector molecules that can increase the efficacy of the immune system or provide other mechanisms for pathogen blocking can be engineered into the genome to reduce vector competence (Box 2). This strategy does not remove the existing vector species but rather replaces it with genetically modified insects that no longer transmit pathogens. Similarly, sterility can be engineered for population suppression in what could be considered an upgrade to the sterile insect technique (SIT). Traditionally, the SIT relies on releases of large numbers of insects (generally males) previously sterilized by exposure to high doses of radiation or chemicals. With sustained releases, this method can eliminate and prevent reinvasion of species from island nations or other geographically isolated areas, as successfully shown for agriculture pests such as the new world screwworm165. Multiple SIT trials against vectors of human disease have been carried out with rather limited success166–168—one problem being reduced male mating competitiveness caused by colonization, sterilization and mass-rearing conditions169. These issues are especially relevant in species where mating demands energy-intensive and behaviourally complex male swarming, as in many anopheline mosquitoes. As an alternative to radiation or chemical exposure, mosquitoes have been engineered to pass on a tetracycline-repressible dominant lethal transgene to their offspring, causing them to die during larval development. This strategy, called RIDL (release of insects carrying dominant lethal transgenes), is currently used in the field against A. aegypti mosquitoes170–172.
Ideally, the desired genetic changes—whether sterility-inducing genes or competence-reducing factors—should be spread through insect populations without having to rely on the unsustainable mass release of insects. This is the concept behind the development of gene drives, genetic elements that bias their own inheritance in a non-Mendelian fashion by copying themselves from one chromosome to its homologue within germline cells so that they can be carried through natural populations (Fig. 4)164. Multiple gene drive designs have been proposed173–175 but none so far is potentially as powerful as the one afforded by CRISPR editing31,32,176. In this system, the transgene encodes an endonuclease, generally Cas9, which is directed by a CRISPR guide RNA to cut the homologous chromosome at the insertion site. When the break is repaired by homology-directed repair using the original ‘transgenic’ chromosome as a template, all the offspring will inherit the gene drive.
Fig. 4 |. Gene drives bias inheritance to ensure their propagation.
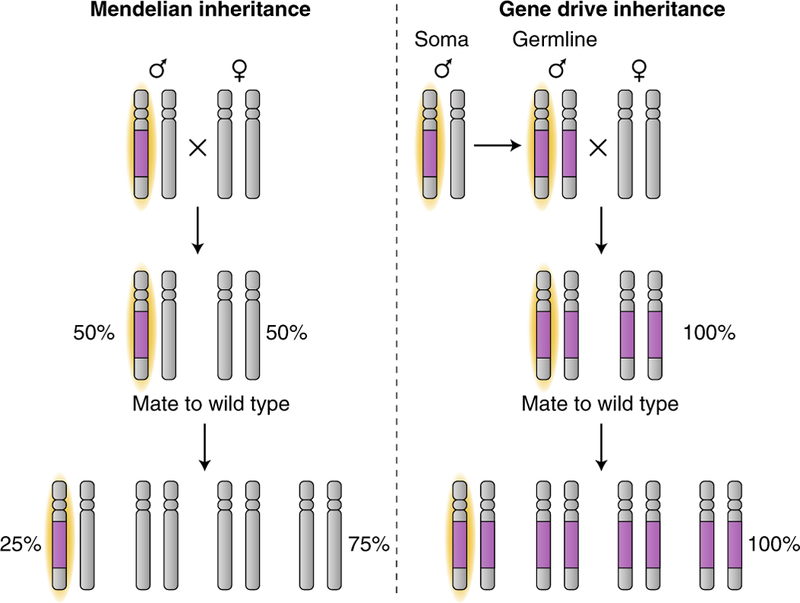
In normal Mendelian inheritance (left), a non-driving transgene (purple) that is on one chromosome (highlighted yellow) will be inherited by 50% of the offspring. Outcrossing to natural wild-type populations will halve the frequency of the transgene in the population with every generation. A gene drive transgene (right) biases transmission to over 50% of its offspring. Through a self-driven copying mechanism within the germline of heterozygotes, a gene drive transgene (purple) on one chromosome (highlighted yellow) will be copied to the other by cutting the other chromosome at the location of the insertion, and the cell uses the original chromosome containing the gene drive transgene as a template for repair by homology-directed repair. All the offspring inherit a copy of the gene drive transgene. Outcrossing to natural wild-type populations will cause spread of the gene drive transgene throughout the population.
The adaptation of CRISPR–Cas9 genome-editing technology to vector species, especially mosquitoes that carry malaria parasites, is occurring apace177, although technological problems in transgene architectures mean that no functional gene drives currently exist. Promising results have been obtained in two anophelines. In A. stephensi31, efficient gene conversion of a construct expressing short-chain anti-plasmodial antibodies was observed into a target locus in the kynurenine hydroxylase gene, required for eye pig-mentation. However, efficient gene conversion was observed only when the transgene was inherited from fathers, due to detrimental maternal effects of the Cas9 endonuclease in the early embryo. In A. gambiae, after four generations with an average observed homing efficiency of 85–98%, transgenes inserted into three different genes conferring a recessive female sterility phenotype32 began to decline in frequency as resistant mutations into which the gene drive could not insert itself were strongly selected178. The natural existence of drive-resistant alleles (or their drive-induced generation, for instance via chromosome repair by non-homologous end joining) represents a major hurdle to these strategies179. Given the extremely polymorphic nature of the Anopheles genome180, for instance, most genomic targets have naturally occurring mutations that will not be efficiently cleaved by CRISPR–Cas9, reducing the number of possible target genes to a handful. Theoretical designs such as those based on targeting multiple sites of the same target gene to slow down the emergence of drive resistant alleles exist176,181 but have yet to be tested.
Transgenic technologies, whether based on SIT or gene drives, would be extremely helpful for tackling the problem of residual malaria and in general for controlling insect populations that feed and rest outdoors or are not amenable to chemical approaches. A wealth of experience accumulated in a series of unsuccessful SIT attempts, however, suggests that without drastically improved knowledge of insect ecology and mating behaviour, and specifically of the determinants of male mating success182–186, the prospects of control approaches based on the release of genetically modified males are bleak.
Regardless of technical hurdles and objective constraints imposed by insect behaviour, the possibility of generating genetic systems that can spread effectively through natural populations poses unprecedented ethical, ecological and safety questions187. Effective gene drives would continue spreading until fixation in target populations, so that any unintended consequences would become permanently fixed. Moreover, insects carrying gene drives would not respect regional, national or international borders, complicating regulations and approvals. Importantly, the role of mosquitoes in maintaining their ecosystem is understudied, and the environmental impact of eliminating species that are deeply rooted in their habitats cannot be accurately predicted188. Efforts aimed at addressing these issues have started, and only when they are fully addressed will gene drives provide a credible weapon in our fight against vector-borne infectious diseases.
outlook
So far, our attempts to curb diseases caused by insect-borne pathogens rely intensely on the use of insecticides, applied in a variety of ways including on LLINs, IRS, traps or aerial sprays. However, in light of the spread of resistance towards these compounds, it is clear that insecticides alone cannot provide long-term solutions. We argue for the development of alternative and complementary approaches based on truly different concepts that are less likely to provoke resistance mechanisms. We identify three major areas that should be prioritized: (1) strategies to increase the effectiveness and sustainability of insecticide usage; (2) safe approaches for population suppression; and (3) effective methods to block pathogen development in the insect vector.
To extend the life span of current and future chemical formulations, the most promising strategy is to incorporate additional compounds that, such as PPF and DBH, act as chemosterilants. Given the dearth of new modes of action, which limits the combinatorial use of insecticides, preventing the inheritance of possible insecticide resistance traits becomes paramount. Although compounds that induce sterility would inevitably meet resistance in insects, the emergence of mechanisms to counteract both insecticides and chemosterilants would be considerably slower. Based on the unexpected reduction of P. falciparum numbers following exposure to DBH, other chemosterilants may also have additional beneficial effects in disrupting pathogen development.
Among strategies aimed at reducing insect populations, those using the sterilizing properties of Wolbachia are most developed and are already being implemented in the field against Aedes mosquitoes. Given these promising results, extending their use to insects such as the Anopheles mosquito that are less amenable to these endosymbiont infections should be considered a research priority worthy of substantially larger efforts. We maintain that the use of chemosterilants in LLINs and IRS to control Anopheles species is a safer option than the release of current designs for population suppression gene drives. Besides any ethical and ecological considerations, the latter will be extremely sensitive to the emergence of drive-resistant alleles that will halt the spread of the transgene. To tackle outdoor biting insect populations, the identification of powerful long-range attractants and repellents could favour the development of odour-baited insecticide traps to kill lured insects, or spatial repellents to drive them away from areas where people gather, respectively189.
Lastly, multiple alternative strategies based on anti-pathogenic gene drives or microorganisms are conceivable to block pathogen development within the insect. Issues in achieving widespread dissemination of pathogen-blocking microorganisms remain, requiring innovation in this area. The use of gene drives loaded with anti-pathogenic constructs also presents substantial technical and biological limitations. In theory, drive-resistant alleles should be less crucial for replacement drives than for suppression drives because selection against constructs targeting the pathogen specifically (and not the vector) is less likely. However, in reality, constructs tested so far either do not achieve complete parasite blockage or affect mosquito fitness and behaviour. Until these issues are resolved, these approaches are unlikely to be successful. As in the case of sterility-inducing strategies, Wolbachia-based methods show the most promise so far, although they are limited to Aedes species and are not thoroughly tested in disease-endemic countries.
Certainly, the wider rollout of any of the strategies described in this Review Article will require considerable testing in multiple field settings to gather reliable epidemiological data and assess their relative effectiveness. Computational modelling using parameters estimated from these trials is needed to pinpoint which strategies represent the stronger candidates, and we have summarized potential impacts on vectorial capacity parameters in Table 2. Field deployment should ultimately happen in consultation with and with the consent of affected communities, particularly for those strategies that may impose lasting changes to the ecological habitat190,191.
Table 2 |.
Comparative impact of vector control on vectorial capacity parameters
| Method | Control strategy | Vector density (m) | Human-biting rate (a) | Vector competence (c) | Adult survival (p) | Extrinsic incubation period (n) |
|---|---|---|---|---|---|---|
| LLINs | Suppression | Decrease: decrease in p reduces density, decrease in a causes longer reproductive cycle | Decrease: physical separation prevents biting, decrease in p | Possible: exposure may induce metabolic stress affecting pathogen | Decrease: kills vectors | Possible: exposure may induce metabolic stress affecting pathogen |
| IRS | Suppression | Decrease: decrease in p reduces density, decrease in a causes longer reproductive cycle | Decrease: repellence prevents home entry, decrease in p | Possible: exposure may induce metabolic stress affecting pathogen | Decrease: kills vectors | Possible: exposure may induce metabolic stress affecting pathogen |
| Larvicides | Suppression | Decrease: kills vectors at non-transmitting stages | Not likely | Not likely | Not likely | Not likely |
| Chemosterilant insecticides | Suppression | Decrease: sterilizes vectors, decrease in p reduces density, decrease in a causes longer reproductive cycle (bed net) | Decrease: physical separation prevents biting (bed net), decrease in p | Possible: reduction in infection prevalence in exposed vectors (DBH) | Possible: kills Vectors (DBH, PPF) | Possible: exposure may induce metabolic stress affecting pathogen |
| Entomopathogenic fungi | Suppression | Decrease: decrease in p reduces density, but kills after reproductive cycle is completed | Decrease: decrease in p | Decrease: reduction in infection prevalence in microorganism-infected vectors (pathogen blocking strain) | Decrease: kills vectors | Possible: exposure may induce metabolic stress affecting pathogen |
| Pathogen-blocking microorganisms (paratransgenic or Wolbachia) | Replacement | No change: transmitting population replaced (equal fitness of microorganisminfected vectors | Possible: by affecting length of reproductive cycle | Decrease: reduction in infection prevalence in microorganism-infected vectors | Possible: microorganism strain could decrease survival (efficient dissemination) | Possible: microorganism strain could increase EIP |
| Sterility-inducing microorganisms (IIT) or SIT | Suppression | Decrease: released males sterilize vectors | Not likely | Not likely | Not likely | Not likely |
| Sterility-inducing gene drives | Elimination | Decrease: released males sterilize vectors | Not likely | Not likely | Not likely | Not likely |
| Pathogen-blocking gene drives | Replacement | No change: transmitting population replaced (equal fitness of transgenic vectors) | Subject to design | Decrease: reduction in infection prevalence in transgenic vectors | Not likely | Subject to design |
Impacts on some parameters are dependent on particular conditions indicated in parentheses. Impacts on other parameters in surviving exposed vectors have not been determined but are theoretically possible. See the main text and Box 2 for full explanations of abbreviations
Are there additional alternatives? As suggested by epidemiological models, the use of compounds that can directly kill pathogens during their journey through the insect vector would also have a powerful impact on disease prevalence73, while affording little opportunity for resistance to arise. When identified, chemicals acting specifically against the pathogen would have a minimal impact on insect fitness, and preclude the evolution of insect resistance. These compounds could be incorporated directly in LLINs and IRS programmes, or in odour-baited traps. A deeper understanding of the biological processes regulating pathogen development within the insect vector should pave the way for the generation of new tools for safe and effective pathogen killing.
The diversity of approaches presented here is cause for cautious optimism that more targeted and effective control of vector-borne disease will be achievable in the near future. As some strategies have the potential to permanently change the environment, they pose unique risks to the ecological habitats of disease vectors. We believe an increased knowledge of the biological processes regulating the interactions between these genetic or biological elements and the insects that harbour them is essential, not least for successful implementation of these strategies, but also to anticipate and prevent possible negative consequences of their use. This involves careful evaluations of any released strains, even those meant for eventual population extinction, for their ability to support or suppress a range of disease pathogens, or even combinations of pathogens. Modifications designed and introduced to block the carriage of one pathogen may, for instance, inadvertently enhance the transmission of another. Moreover, as mentioned above, strategies relying on the release of modified vectors will require a solid understanding of mating biology and the determinants of mating success. This will ensure that manipulated organisms are able to compete in natural vector populations and avoid generating mechanisms of assortative mating that may lead to the unintended introduction of new species.
It is likely that within the next decade we will see the field implementation of some of the tools that are presently being tested in the laboratory, as well as the generation of additional powerful ideas and methods for controlling the transmission of vector-borne diseases. We argue that future vector control strategies will need to be rooted in basic biology research in insect ecology, behaviour and vector–pathogen–microbiome interactions. In this way, we will ensure an effective and responsible use of tools to mitigate the tremendous burden imposed on societies by vector-borne pathogens, without inflicting negative and irreversible long-term consequences on our environment.
Acknowledgements
The authors would like to thank M. Bernardi for graphic assistance and D. Abernathy, P. Marcenac and D. Paton for careful reading of this manuscript. F.C. is funded on research related to the topic discussed here by a Faculty Research Scholar Award by the Howard Hughes Medical Institute (HHMI) and the Bill & Melinda Gates Foundation (BMGF) (grant ID OPP1158190), and by the National Institutes of Health (NIH) (R01 AI124165). The findings and conclusions within this publication are those of the authors and do not necessarily reflect positions or policies of the HHMI, the BMGF or the NIH.
Footnotes
Competing interests
Harvard University has filed a patent application on behalf of the investigators related to this research.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kwiatkowski DP How malaria has affected the human genome and what human genetics can teach us about malaria. Am. J. Hum. Genet 77, 171–192 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwiatkowski D Malaria genomics: tracking a diverse and evolving parasite population. Int. Health 7, 82–84 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Malaria Report 196 (WHO, Geneva, 2017). [Google Scholar]
- 4.The malERA Consultative Group on Vector Control. A research agenda for malaria eradication: vector control. PLoS Med 8, e1000401 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neafsey DE et al. Mosquito genomics. Highly evolvable malaria vectors: the genomes of 16 Anopheles mosquitoes. Science 347, 1258522 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeldenryk LM, Gray M, Speare R, Gordon S & Melrose W The emerging story of disability associated with lymphatic filariasis: a critical review. PLoS Negl. Trop. Dis 5, e1366 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bern C, Maguire JH & Alvar J Complexities of assessing the disease burden attributable to leishmaniasis. PLoS Negl. Trop. Dis 2, e313 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garza M et al. Projected future distributions of vectors of Trypanosoma cruzi in North America under climate change scenarios. PLoS Negl. Trop. Dis 8, e2818 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gascon J, Bern C & Pinazo MJ Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop 115, 22–27 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Ioos S et al. Current Zika virus epidemiology and recent epidemics. Med. Mal. Infect 44, 302–307 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Faria NR et al. Zika virus in the Americas: early epidemiological and genetic findings. Science 352, 345–349 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiter P & Sprenger D The used tire trade: a mechanism for the worldwide dispersal of container breeding mosquitoes. J. Am. Mosq. Control Assoc 3, 494–501 (1987). [PubMed] [Google Scholar]
- 13.Rezza G et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 370, 1840–1846 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Lednicky J et al. Mayaro virus in child with acute febrile illness, Haiti, 2015. Emerg. Infect. Dis 22, 2000–2002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotez PJ & Murray KO Dengue, West Nile virus, chikungunya, Zika—and now Mayaro? PLoS Negl. Trop. Dis 11, e0005462 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatt S et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO Malaria fact sheet #94, December 2011. (Innovative Vector Control Consortium); www.ivcc.com/who-malaria-fact-sheet [Google Scholar]
- 18.Franco JR et al. Monitoring the elimination of human African trypanosomiasis: update to 2014. PLoS Negl. Trop. Dis 11, e0005585 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Investing to Overcome the Global Impact of Neglected Tropical Diseases—Third WHO Report on Neglected Tropical Diseases 191 (WHO, Geneva, 2015). [Google Scholar]
- 20.Ranson H & Lissenden N Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol 32, 187–196 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Moyes CL et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis 11, e0005625 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomes B et al. Knockdown resistance mutations predict DDT resistance and pyrethroid tolerance in the visceral leishmaniasis vector Phlebotomus argentipes. PLoS Negl. Trop. Dis 11, e0005504 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison AC, Zielinski-Gutierrez E, Scott TW & Rosenberg R Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med 5, e68 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Killeen GF Characterizing, controlling and eliminating residual malaria transmission. Malar. J 13, 330 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nene V et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316, 1718–1723 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudchenko O et al. De novo assembly of the Aedes aegypti genome using Hi-C yields chromosome-length scaffolds. Science 356, 92–95 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen XG et al. Genome sequence of the Asian Tiger mosquito, Aedes albopictus, reveals insights into its biology, genetics, and evolution. Proc. Natl Acad. Sci. USA 112, E5907–5915 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smidler AL, Terenzi O, Soichot J, Levashina EA & Marois E Targeted mutagenesis in the malaria mosquito using TALE nucleases. PLoS ONE 8, e74511 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong S et al. Heritable CRISPR/Cas9-mediated genome editing in the yellow fever mosquito, Aedes aegypti. PLoS ONE 10, e0122353 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itokawa K, Komagata O, Kasai S, Ogawa K & Tomita T Testing the causality between CYP9M10 and pyrethroid resistance using the TALEN and CRISPR/Cas9 technologies. Sci. Rep 6, 24652 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gantz VM et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl Acad. Sci. USA 112, E6736–6743 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammond A et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol 34, 78–83 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li M et al. Germline Cas9 expression yields highly efficient genome engineering in a major worldwide disease vector, Aedes aegypti. Proc. Natl Acad. Sci. USA 114, E10540–E10549 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dodson BL & Rasgon JL Vector competence of Anopheles and Culex mosquitoes for Zika virus. PeerJ 5, e3096 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanlandingham DL et al. Differential infectivities of o’nyong-nyong and chikungunya virus isolates in Anopheles gambiae and Aedes aegypti mosquitoes. Am. J. Trop. Med. Hyg 72, 616–621 (2005). [PubMed] [Google Scholar]
- 36.Macdonald G Epidemiological basis of malaria control. Bull. World Health Organ 15, 613–626 (1956). [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan AM & Curtis CF Productivity of Glossina morsitans morsitans Westwood maintained in the laboratory, with particular reference to the sterile-insect release method. Bull. World Health Organ 46, 33–38 (1972). [PMC free article] [PubMed] [Google Scholar]
- 38.Tobe SS & Langley PA Reproductive physiology of Glossina. Ann. Rev. Entomol 23, 283–307 (1978). [DOI] [PubMed] [Google Scholar]
- 39.Knols BG, Willemse L, Flint S & Mate A A trial to control the tsetse fly, Glossina morsitans centralis, with low densities of odour-baited targets in west Zambia. Med. Vet. Entomol 7, 161–169 (1993). [DOI] [PubMed] [Google Scholar]
- 40.Gurtler RE et al. Domestic animal hosts strongly influence human-feeding rates of the Chagas disease vector Triatoma infestans in Argentina. PLoS Negl. Trop. Dis 8, e2894 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beach R Mosquitoes: biting behavior inhibited by ecdysone. Science 205, 829–831 (1979). [DOI] [PubMed] [Google Scholar]
- 42.Takken W, Klowden MJ & Chambers GM Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): the disadvantage of being small. J. Med. Entomol 35, 639–645 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Lefèvre T, Vantaux A, Dabiré KR, Mouline K & Cohuet A Non-genetic determinants of mosquito competence for malaria parasites. PLoS Pathog 9, e1003365 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cirimotich CM et al. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 332, 855–858 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takken W et al. Larval nutrition differentially affects adult fitness and Plasmodium development in the malaria vectors Anopheles gambiae and Anopheles stephensi. Parasites Vectors 6, 345 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanford S et al. Fungal pathogen reduces potential for malaria transmission. Science 308, 1638–1641 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Chan M & Johansson MA The incubation periods of Dengue viruses. PLoS ONE 7, e50972 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye YH et al. Wolbachia reduces the transmission potential of Dengue-infected Aedes aegypti. PLoS Negl. Trop. Dis 9, e0003894 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindsay SW et al. Ability of Anopheles gambiae mosquitoes to transmit malaria during the dry and wet seasons in an area of irrigated rice cultivation in The Gambia. J. Trop. Med. Hyg 94, 313–324 (1991). [PubMed] [Google Scholar]
- 50.Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition (WHO, 2009). [PubMed] [Google Scholar]
- 51.Achee NL et al. A critical assessment of vector control for dengue prevention. PLoS Negl. Trop. Dis 9, e0003655 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinez-Torres D et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol. Biol 7, 179–184 (1998). [DOI] [PubMed] [Google Scholar]
- 53.Martinez-Torres D et al. Voltage-dependent Na+ channels in pyrethroid-resistant Culex pipiens L mosquitoes. Pestic. Sci 55, 1012–1020 (1999). [Google Scholar]
- 54.Ranson H et al. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol. Biol 9, 491–497 (2000). [DOI] [PubMed] [Google Scholar]
- 55.Norris LC et al. Adaptive introgression in an African malaria mosquito coincident with the increased usage of insecticide-treated bed nets. Proc. Natl Acad. Sci. USA 112, 815–820 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toe KH et al. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg. Infect. Dis 20, 1691–1696 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poupardin R, Srisukontarat W, Yunta C & Ranson H Identification of carboxylesterase genes implicated in temephos resistance in the dengue vector Aedes aegypti. PLoS Negl. Trop. Dis 8, e2743 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bariami V, Jones CM, Poupardin R, Vontas J & Ranson H Gene amplification, ABC transporters and cytochrome P450s: unraveling the molecular basis of pyrethroid resistance in the dengue vector, Aedes aegypti. PLoS Negl. Trop. Dis 6, e1692 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grigoraki L et al. Transcriptome profiling and genetic study reveal amplified carboxylesterase genes implicated in temephos resistance, in the Asian Tiger mosquito Aedes albopictus. PLoS Negl. Trop. Dis 9, e0003771 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balabanidou V et al. Cytochrome P450 associated with insecticide resistance catalyzes cuticular hydrocarbon production in Anopheles gambiae. Proc. Natl Acad. Sci. USA 113, 9268–9273 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Viana M, Hughes A, Matthiopoulos J, Ranson H & Ferguson HM Delayed mortality effects cut the malaria transmission potential of insecticide-resistant mosquitoes. Proc. Natl Acad. Sci. USA 113, 8975–8980 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martins AJ et al. Effect of insecticide resistance on development, longevity and reproduction of field or laboratory selected Aedes aegypti populations. PLoS ONE 7, e31889 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baldini F et al. The interaction between a sexually transferred steroid hormone and a female protein regulates oogenesis in the malaria mosquito Anopheles gambiae. PLoS Biol 11, e1001695 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gabrieli P et al. Sexual transfer of the steroid hormone 20E induces the postmating switch in Anopheles gambiae. Proc. Natl Acad. Sci. USA 111, 16353–16358 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitchell SN et al. Mosquito biology. Evolution of sexual traits influencing vectorial capacity in anopheline mosquitoes. Science 347, 985–988 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pitts RJ, Liu C, Zhou X, Malpartida JC & Zwiebel LJ Odorant receptor-mediated sperm activation in disease vector mosquitoes. Proc. Natl Acad. Sci. USA 111, 2566–2571 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clifton ME, Correa S, Rivera-Perez C, Nouzova M & Noriega FG Male Aedes aegypti mosquitoes use JH III transferred during copulation to influence previtellogenic ovary physiology and affect the reproductive output of female mosquitoes. J. Insect Physiol 64, 40–47 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harris C et al. Sterilising effects of pyriproxyfen on Anopheles arabiensis and its potential use in malaria control. Parasit. Vectors 6, 144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lwetoijera DW et al. Comprehensive sterilization of malaria vectors using pyriproxyfen: a step closer to malaria elimination. Am. J. Trop. Med. Hyg 90, 852–855 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawada H et al. A small-scale field trial of pyriproxyfen-impregnated bed nets against pyrethroid-resistant Anopheles gambiae s.s. in western Kenya. PLoS ONE 9, e111195 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohashi K et al. Efficacy of pyriproxyfen-treated nets in sterilizing and shortening the longevity of Anopheles gambiae (Diptera: Culicidae). J. Med. Entomol 49, 1052–1058 (2012). [DOI] [PubMed] [Google Scholar]
- 72.Ngufor C et al. Olyset Duo(R) (a pyriproxyfen and permethrin mixture net): an experimental hut trial against pyrethroid resistant Anopheles gambiae and Culex quinquefasciatus in Southern Benin. PLoS ONE 9, e93603 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Childs LM et al. Disrupting mosquito reproduction and parasite development for malaria control. PLoS Pathog 12, e1006060 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tiono AB et al. The AvecNet Trial to assess whether addition of pyriproxyfen, an insect juvenile hormone mimic, to long-lasting insecticidal mosquito nets provides additional protection against clinical malaria over current best practice in an area with pyrethroid-resistant vectors in rural Burkina Faso: study protocol for a randomised controlled trial. Trials 16, 113 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shin SW et al. REL1, a homologue of Drosophila dorsal, regulates toll antifungal immune pathway in the female mosquito Aedes aegypti. J. Biol. Chem 280, 16499–16507 (2005). [DOI] [PubMed] [Google Scholar]
- 76.Bian G, Shin SW, Cheon HM, Kokoza V & Raikhel AS Transgenic alteration of Toll immune pathway in the female mosquito Aedes aegypti. Proc. Natl Acad. Sci. USA 102, 13568–13573 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frolet C, Thoma M, Blandin S, Hoffmann JA & Levashina EA Boosting NF-κB-dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity 25, 677–685 (2006). [DOI] [PubMed] [Google Scholar]
- 78.Garver LS, Dong Y & Dimopoulos G Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog 5, e1000335 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meister S et al. Anopheles gambiae PGRPLC-mediated defense against bacteria modulates infections with malaria parasites. PLoS Pathog 5, e1000542 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mitri C et al. Fine pathogen discrimination within the APL1 gene family protects Anopheles gambiae against human and rodent malaria species. PLoS Pathog 5, e1000576 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dong Y et al. Engineered anopheles immunity to Plasmodium infection. PLoS Pathog 7, e1002458 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zou Z et al. Transcriptome analysis of Aedes aegypti transgenic mosquitoes with altered immunity. PLoS Pathog 7, e1002394 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Antonova Y, Alvarez KS, Kim YJ, Kokoza V & Raikhel AS The role of NF-κB factor REL2 in the Aedes aegypti immune response. Insect Biochem. Mol. Biol 39, 303–314 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Castillo JC, Ferreira ABB, Trisnadi N & Barillas-Mury C Activation of mosquito complement antiplasmodial response requires cellular immunity. Sci. Immunol 2, eaal1505 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith RC & Barillas-Mury C Plasmodium oocysts: overlooked targets of mosquito immunity. Trends Parasitol 32, 979–990 (2016). [DOI] [PubMed] [Google Scholar]
- 86.Gupta L et al. The STAT pathway mediates late-phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host Microbe 5, 498–507 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith RC, Barillas-Mury C & Jacobs-Lorena M Hemocyte differentiation mediates the mosquito late-phase immune response against Plasmodium in Anopheles gambiae. Proc. Natl Acad. Sci. USA 112, E3412–3420 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olson KE & Blair CD Arbovirus-mosquito interactions: RNAi pathway. Curr. Opin. Virol 15, 119–126 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morazzani EM, Wiley MR, Murreddu MG, Adelman ZN & Myles KM Production of virus-derived ping-pong-dependent piRNA-like small RNAs in the mosquito soma. PLoS Pathog 8, e1002470 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vodovar N et al. Arbovirus-derived piRNAs exhibit a ping-pong signature in mosquito cells. PLoS ONE 7, e30861 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hess AM et al. Small RNA profiling of Dengue virus-mosquito interactions implicates the PIWI RNA pathway in anti-viral defense. BMC Microbiol 11, 45 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miesen P, Girardi E & van Rij RP Distinct sets of PIWI proteins produce arbovirus and transposon-derived piRNAs in Aedes aegypti mosquito cells. Nucleic Acids Res 43, 6545–6556 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xi Z, Ramirez JL & Dimopoulos G The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog 4, e1000098 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Souza-Neto JA, Sim S & Dimopoulos G An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc. Natl Acad. Sci. USA 106, 17841–17846 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ramirez JL & Dimopoulos G The Toll immune signaling pathway control conserved anti-dengue defenses across diverse Ae. aegypti strains and against multiple dengue virus serotypes. Dev. Comp. Immunol 34, 625–629 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sim S et al. Transcriptomic profiling of diverse Aedes aegypti strains reveals increased basal-level immune activation in dengue virus-refractory populations and identifies novel virus-vector molecular interactions. PLoS Negl. Trop. Dis 7, e2295 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Palmer WH, Varghese FS & van Rij RP Natural variation in resistance to virus infection in Dipteran insects. Viruses 10, 118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kokoza V et al. Engineering blood meal-activated systemic immunity in the yellow fever mosquito, Aedes aegypti. Proc. Natl Acad. Sci. USA 97, 9144–9149 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim W et al. Ectopic expression of a cecropin transgene in the human malaria vector mosquito Anopheles gambiae (Diptera: Culicidae): effects on susceptibility to Plasmodium. J. Med. Entomol 41, 447–455 (2004). [DOI] [PubMed] [Google Scholar]
- 100.Jupatanakul N et al. Engineered Aedes aegypti JAK/STAT pathway-mediated immunity to Dengue virus. PLoS Negl. Trop. Dis 11, e0005187 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Isaacs AT et al. Transgenic Anopheles stephensi coexpressing single-chain antibodies resist Plasmodium falciparum development. Proc. Natl Acad. Sci. USA 109, E1922–1930 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clayton AM, Cirimotich CM, Dong Y & Dimopoulos G Caudal is a negative regulator of the Anopheles IMD pathway that controls resistance to Plasmodium falciparum infection. Dev. Comp. Immunol 39, 323–332 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garver LS, de Almeida Oliveira G & Barillas-Mury C The JNK pathway is a key mediator of Anopheles gambiae antiplasmodial immunity. PLoS Pathog 9, e1003622 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dong Y, Cirimotich CM, Pike A, Chandra R & Dimopoulos G Anopheles NF-κB-regulated splicing factors direct pathogen-specific repertoires of the hypervariable pattern recognition receptor AgDscam. Cell Host Microbe 12, 521–530 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smith RC, Kizito C, Rasgon JL & Jacobs-Lorena M Transgenic mosquitoes expressing a phospholipase A(2) gene have a fitness advantage when fed Plasmodium falciparum-infected blood. PLoS ONE 8, e76097 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Franz AW et al. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc. Natl Acad. Sci. USA 103, 4198–4203 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pike A et al. Changes in the microbiota cause genetically modified Anopheles to spread in a population. Science 357, 1396–1399 (2017). [DOI] [PubMed] [Google Scholar]
- 108.Ito J, Ghosh A, Moreira LA, Wimmer EA & Jacobs-Lorena M Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature 417, 452–455 (2002). [DOI] [PubMed] [Google Scholar]
- 109.Ghosh AK, Coppens I, Gardsvoll H, Ploug M & Jacobs-Lorena M Plasmodium ookinetes coopt mammalian plasminogen to invade the mosquito midgut. Proc. Natl Acad. Sci. USA 108, 17153–17158 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vega-Rodriguez J et al. Multiple pathways for Plasmodium ookinete invasion of the mosquito midgut. Proc. Natl Acad. Sci. USA 111, E492–500 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ghosh AK et al. Malaria parasite invasion of the mosquito salivary gland requires interaction between the Plasmodium TRAP and the Anopheles saglin proteins. PLoS Pathog 5, e1000265 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clayton AM, Dong Y & Dimopoulos G The Anopheles innate immune system in the defense against malaria infection. J. Innate Immun 6, 169–181 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Molina-Cruz A et al. The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science 340, 984–987 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith RC & Jacobs-Lorena M Malaria parasite Pfs47 disrupts JNK signaling to escape mosquito immunity. Proc. Natl Acad. Sci. USA 112, 1250–1251 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Molina-Cruz A et al. Plasmodium evasion of mosquito immunity and global malaria transmission: the lock-and-key theory. Proc. Natl Acad. Sci. USA 112, 15178–15183 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dillon RJ & Dillon VM The gut bacteria of insects: nonpathogenic interactions. Annu. Rev. Entomol 49, 71–92 (2004). [DOI] [PubMed] [Google Scholar]
- 117.Dale C & Moran NA Molecular interactions between bacterial symbionts and their hosts. Cell 126, 453–465 (2006). [DOI] [PubMed] [Google Scholar]
- 118.Douglas AE Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol 43, 17–37 (1998). [DOI] [PubMed] [Google Scholar]
- 119.Dong Y, Manfredini F & Dimopoulos G Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog 5, e1000423 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Y, Gilbreath TM 3rd, Kukutla P, Yan G & Xu J Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS ONE 6, e24767 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boissière A et al. Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog 8, e1002742 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.da Mota FF et al. Cultivation-independent methods reveal differences among bacterial gut microbiota in triatomine vectors of Chagas disease. PLoS Negl. Trop. Dis 6, e1631 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aksoy E et al. Analysis of multiple tsetse fly populations in Uganda reveals limited diversity and species-specific gut microbiota. Appl. Environ. Microbiol 80, 4301–4312 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Segata N et al. The reproductive tracts of two malaria vectors are populated by a core microbiome and by gender-and swarm-enriched microbial biomarkers. Sci. Rep 6, 24207 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fraihi W et al. An integrated overview of the midgut bacterial flora composition of Phlebotomus perniciosus, a vector of zoonotic visceral leishmaniasis in the Western Mediterranean Basin. PLoS Negl. Trop. Dis 11, e0005484 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Azambuja P, Feder D & Garcia ES Isolation of Serratia marcescens in the midgut of Rhodnius prolixus: impact on the establishment of the parasite Trypanosoma cruzi in the vector. Exp. Parasitol 107, 89–96 (2004). [DOI] [PubMed] [Google Scholar]
- 127.Moraes CS et al. Prodigiosin is not a determinant factor in lysis of Leishmania (Viannia) braziliensis after interaction with Serratia marcescens D-mannose sensitive fimbriae. Exp. Parasitol 122, 84–90 (2009). [DOI] [PubMed] [Google Scholar]
- 128.Bando H et al. Intra-specific diversity of Serratia marcescens in Anopheles mosquito midgut defines Plasmodium transmission capacity. Sci. Rep 3, 1641 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bahia AC et al. Exploring Anopheles gut bacteria for Plasmodium blocking activity. Environ. Microbiol 16, 2980–2994 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Apte-Deshpande A, Paingankar M, Gokhale MD & Deobagkar DN Serratia odorifera a midgut inhabitant of Aedes aegypti mosquito enhances its susceptibility to dengue-2 virus. PLoS ONE 7, e40401 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carissimo G et al. Antiviral immunity of Anopheles gambiae is highly compartmentalized, with distinct roles for RNA interference and gut microbiota. Proc. Natl Acad. Sci. USA 112, 176–185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Scholte EJ et al. An entomopathogenic fungus for control of adult African malaria mosquitoes. Science 308, 1641–1642 (2005). [DOI] [PubMed] [Google Scholar]
- 133.Fieck A, Hurwitz I, Kang AS & Durvasula R Trypanosoma cruzi: synergistic cytotoxicity of multiple amphipathic anti-microbial peptides to T. cruzi and potential bacterial hosts. Exp. Parasitol 125, 342–347 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang S et al. Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria. Science 357, 1399–1402 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xi Z, Khoo CC & Dobson SL Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310, 326–328 (2005). [DOI] [PubMed] [Google Scholar]
- 136.Bian G et al. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science 340, 748–751 (2013). [DOI] [PubMed] [Google Scholar]
- 137.Attardo GM et al. Analysis of milk gland structure and function in Glossina morsitans: milk protein production, symbiont populations and fecundity. J. Insect Physiol 54, 1236–1242 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Favia G et al. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc. Natl Acad. Sci. USA 104, 9047–9051 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A & Werren JH How many species are infected with Wolbachia?A statistical analysis of current data. FEMS Microbiol. Lett 281, 215–220 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Werren JH, Baldo L & Clark ME Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Micro 6, 741–751 (2008). [DOI] [PubMed] [Google Scholar]
- 141.LePage DP et al. Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature 543, 243–247 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Beckmann JF, Ronau JA & Hochstrasser M A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat. Microbiol 2, 17007 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lindsey ARI et al. Evolutionary genetics of cytoplasmic incompatibility genes cifA and cifB in prophage WO of Wolbachia. Genome Biol. Evol 10, 434–451 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hughes GL, Koga R, Xue P, Fukatsu T & Rasgon JL Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog 7, e1002043 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kambris Z et al. Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae. PLoS Pathog 6, e1001143 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Moreira LA et al. A Wolbachia symbiont in Aedes aegypti limits infection with Dengue, Chikungunya, and Plasmodium. Cell 139, 1268–1278 (2009). [DOI] [PubMed] [Google Scholar]
- 147.Glaser RL & Meola MA The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE 5, e11977 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.van den Hurk AF et al. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl. Trop. Dis 6, e1892 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Blagrove MSC, Arias-Goeta C, Failloux AB & Sinkins SP Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc. Natl Acad. Sci. USA 109, 255–260 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dutra HL et al. Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe 19, 771–774 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Aliota MT, Peinado SA, Velez ID & Osorio JE The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci. Rep 6, 28792 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Caragata EP et al. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog 9, e1003459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Geoghegan V et al. Perturbed cholesterol and vesicular trafficking associated with dengue blocking in Wolbachia-infected Aedes aegypti cells. Nat. Commun 8, 526 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Brelsfoard CL, Sechan Y & Dobson SL Interspecific hybridization yields strategy for South Pacific filariasis vector elimination. PLoS Negl. Trop. Dis 2, e129 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Atyame CM et al. Cytoplasmic incompatibility as a means of controlling Culex pipiens quinquefasciatus mosquito in the islands of the south-western Indian Ocean. PLoS Negl. Trop. Dis 5, e1440 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.World Mosquito Program (2017); www.worldmosquitoprogram.org
- 157.Frentiu FD et al. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl. Trop. Dis 8, e2688 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schmidt TL et al. Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol 15, e2001894 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Baldini F et al. Evidence of natural Wolbachia infections in field populations of Anopheles gambiae. Nat. Commun 5, 3985 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shaw WR et al. Wolbachia infections in natural Anopheles populations affect egg laying and negatively correlate with Plasmodium development. Nat. Commun 7, 11772 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gomes FM et al. Effect of naturally occurring Wolbachia in Anopheles gambiae s.l. mosquitoes from Mali on Plasmodium falciparum malaria transmission. Proc. Natl Acad. Sci. USA 114, 12566–12571 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dodson BL et al. Wolbachia enhances West Nile virus (WNV) infection in the mosquito Culex tarsalis. PLoS Negl. Trop. Dis 8, e2965 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hughes GL, Rivero A & Rasgon JL Wolbachia can enhance Plasmodium infection in mosquitoes: implications for malaria control? PLoS Pathog 10, e1004182 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Burt A Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proc. Biol. Sci 270, 921–928 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lindquist DA, Abusowa M & Hall MJ The New World screwworm fly in Libya: a review of its introduction and eradication. Med. Vet. Entomol 6, 2–8 (1992). [DOI] [PubMed] [Google Scholar]
- 166.Oliva CF et al. The sterile insect technique for controlling populations of Aedes albopictus (Diptera: Culicidae) on Reunion Island: mating vigour of sterilized males. PLoS ONE 7, e49414 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bellini R, Medici A, Puggioli A, Balestrino F & Carrieri M Pilot field trials with Aedes albopictus irradiated sterile males in Italian urban areas. J. Med. Entomol 50, 317–325 (2013). [DOI] [PubMed] [Google Scholar]
- 168.Vreysen MJ et al. Glossina austeni (Diptera: Glossinidae) eradicated on the island of Unguja, Zanzibar, using the sterile insect technique. J. Econ. Entomol 93, 123–135 (2000). [DOI] [PubMed] [Google Scholar]
- 169.Dame DA, Curtis CF, Benedict MQ, Robinson AS & Knols BG Historical applications of induced sterilisation in field populations of mosquitoes. Malar. J 2, S2 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Harris AF et al. Field performance of engineered male mosquitoes. Nat. Biotechnol 29, 1034–1037 (2011). [DOI] [PubMed] [Google Scholar]
- 171.Harris AF et al. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotechnol 30, 828–830 (2012). [DOI] [PubMed] [Google Scholar]
- 172.Carvalho DO et al. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl. Trop. Dis 9, e0003864 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen CH et al. A synthetic maternal-effect selfish genetic element drives population replacement in Drosophila. Science 316, 597–600 (2007). [DOI] [PubMed] [Google Scholar]
- 174.Windbichler N et al. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature 473, 212–215 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Simoni A et al. Development of synthetic selfish elements based on modular nucleases in Drosophila melanogaster. Nucleic Acids Res 42, 7461–7472 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Esvelt KM, Smidler AL, Catteruccia F & Church GM Concerning RNA-guided gene drives for the alteration of wild populations. eLife 3, e03401 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hsu PD, Lander ES & Zhang F Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hammond AM et al. The creation and selection of mutations resistant to a gene drive over multiple generations in the malaria mosquito. PLoS Genet 13, e1007039 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Champer J et al. Novel CRISPR/Cas9 gene drive constructs reveal insights into mechanisms of resistance allele formation and drive efficiency in genetically diverse populations. PLoS Genet 13, e1006796 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.The Anopheles gambiae 1000 Genomes Consortium. Genetic diversity of the African malaria vector Anopheles gambiae. Nature 552, 96–100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Marshall JM, Buchman A, Sanchez CH & Akbari OS Overcoming evolved resistance to population-suppressing homing-based gene drives. Sci. Rep 7, 3776 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sawadogo SP et al. Effects of age and size on Anopheles gambiae s.s. male mosquito mating success. J. Med. Entomol 50, 285–293 (2013). [DOI] [PubMed] [Google Scholar]
- 183.Sawadogo SP et al. Targeting male mosquito swarms to control malaria vector density. PLoS ONE 12, e0173273 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Diabate A & Tripet F Targeting male mosquito mating behaviour for malaria control. Parasit. Vectors 8, 347 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cator LJ, Arthur BJ, Harrington LC & Hoy RR Harmonic convergence in the love songs of the dengue vector mosquito. Science 323, 1077–1079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cator LJ & Harrington LC The harmonic convergence of fathers predicts the mating success of sons in Aedes aegypti. Anim. Behav 82, 627–633 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Oye KA et al. Biotechnology. Regulating gene drives. Science 345, 626–628 (2014). [DOI] [PubMed] [Google Scholar]
- 188.Ferguson HM et al. Ecology: a prerequisite for malaria elimination and eradication. PLoS Med 7, e1000303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Norris EJ & Coats JR Current and future repellent technologies: the potential of spatial repellents and their place in mosquito-borne disease control. Int. J. Environ. Res. Public Health 14, 124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ernst KC et al. Awareness and support of release of genetically modified “sterile” mosquitoes, Key West, Florida, USA. Emerg. Infect. Dis 21, 320–324 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Adalja A, Sell TK, McGinty M & Boddie C Genetically modified (gm) mosquito use to reduce mosquito-transmitted disease in the US: a community opinion survey. PLoS Curr 10.1371/currents.outbreaks.1c39ec05a743d41ee39391ed0f2ed8d3 (2016). [DOI] [PMC free article] [PubMed]
- 192.Mathers CD, Ezzati M & Lopez AD Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl. Trop. Dis 1, e114 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Global Program to Eliminate Lymphatic Filariasis Progress Report 594–608 (WHO, Geneva, 2017). [Google Scholar]
- 194.Lymphatic filariasis (WHO, Geneva, 2017); www.who.int/mediacentre/factsheets/fs102/en/ [Google Scholar]
- 195.GBD 2015 Disease and Injury Incidence Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1545–1602 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.GBD 2015 Mortality and Cause of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1459–1544 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.What is Chagas disease? (WHO, Geneva, 2017); www.who.int/chagas/disease/en/ [Google Scholar]
- 198.Control of the leishmaniases — Report of a WHO Expert Committee 158 (World Health Organization, Geneva, 1990). [PubMed] [Google Scholar]
- 199.Parasites—Onchocerciasis (also known as River Blindness) (Centers for Disease Control and Prevention, 2017); www.cdc.gov/parasites/onchocerciasis/epi.html [Google Scholar]
- 200.Brady OJ et al. Refi g the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis 6, e1760 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shearer FM et al. Global yellow fever vaccination coverage from 1970 to 2016: an adjusted retrospective analysis. Lancet Infect. Dis 17, 1209–1217 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.CDC: Zika Travel Information (2017); wwwnc.cdc.gov/travel/page/zika-information
- 203.Messina JP et al. Mapping global environmental suitability for Zika virus. eLife 5, e15272 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nsoesie EO et al. Global distribution and environmental suitability for chikungunya virus, 1952 to 2015. Euro. Surveill 10.2807/1560-7917.ES.2016.21.20.30234 (2016). [DOI] [PMC free article] [PubMed]
- 205.Chikungunya (WHO, Geneva, 2017); www.who.int/mediacentre/factsheets/fs327/en/ [Google Scholar]
- 206.Gibson G & Torr SJ Visual and olfactory responses of haematophagous Diptera to host stimuli. Med. Vet. Entomol 13, 2–23 (1999). [DOI] [PubMed] [Google Scholar]


