Abstract
Nitric oxide (NO) is a small free radical with critical signaling roles in physiology and pathophysiology. The generation of sufficient NO levels to regulate the resistance of the blood vessels and hence the maintenance of adequate blood flow is critical to the healthy performance of the vasculature. A novel paradigm indicates that classical NO synthesis by dedicated NO synthases is supplemented by nitrite reduction pathways under hypoxia. At the same time, reactive oxygen species (ROS), which include superoxide and hydrogen peroxide, are produced in the vascular system for signaling purposes, as effectors of the immune response, or as byproducts of cellular metabolism. NO and ROS can be generated by distinct enzymes or by the same enzyme through alternate reduction and oxidation processes. The latter oxidoreductase systems include NO synthases, molybdopterin enzymes, and hemoglobins, which can form superoxide by reduction of molecular oxygen or NO by reduction of inorganic nitrite. Enzymatic uncoupling, changes in oxygen tension, and the concentration of coenzymes and reductants can modulate the NO/ROS production from these oxidoreductases and determine the redox balance in health and disease. The dysregulation of the mechanisms involved in the generation of NO and ROS is an important cause of cardiovascular disease and target for therapy. In this review we will present the biology of NO and ROS in the cardiovascular system, with special emphasis on their routes of formation and regulation, as well as the therapeutic challenges and opportunities for the management of NO and ROS in cardiovascular disease.
I. INTRODUCTION
Nitric oxide (NO) is a small free radical molecule with critical signaling roles. The discovery of the function of NO in the vascular endothelium as endothelium-derived relaxing factor led to the awarding of the 1998 Nobel Prize to Drs. Furchgott, Ignarro and Murad (36, 324, 449, 491, 716). The functions of NO in mammalian systems extend beyond vascular signaling and are relevant in all organ systems, including but not limited to neuronal signaling, and host defense (448, 659, 738).
A number of oxygen-related species of high chemical reactivity are referred to as reactive oxygen species (ROS). These include oxygen radicals and peroxides, such as superoxide (O2·−) and hydrogen peroxide (H2O2), nitrogen radical species, such as NO and nitrogen dioxide (NO2·), and other species, such as peroxynitrite (ONOO−) and hypochlorite (ClO−). The species containing nitrogen are often treated separately as reactive nitrogen species (RNS). It is worth indicating that despite being long considered toxic species, most of these molecules have been shown to exert important signaling functions (249, 778, 937, 960). Therefore, the role of many of these molecules in health and disease is related to their production rates, steady-state concentrations, and the ability of the cellular antioxidant systems to modulate their activity.
In general, dysregulated production of ROS/RNS, as is the case for NO, leads to oxidative stress and deleterious consequences for living systems. However, as pointed out above, these molecules often have important signaling roles at low concentrations. For instance, the differences in response to NO at varying concentrations have attracted considerable attention. It has been shown that low levels (pM/nM) are physiological and related to the activation of high affinity primary binding targets such as soluble guanylyl cyclase (sGC) and cytochrome c oxidase (433, 863). An emerging paradigm proposes that intermediate levels (50–300 nM) can activate a range of positive and negative responses from wound healing to oncogenic pathways (938). Higher concentrations of NO (>1 μM) can lead not only to oxidative stress but also nitrative and nitrosative stress via the generation of peroxynitrite and nitrosating species (411, 412, 938, 939), and in combination with oxygen, can trigger posttranslational modification of proteins, lipids, and DNA (277, 433, 938).
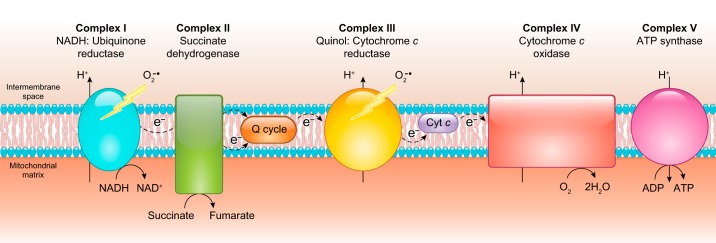
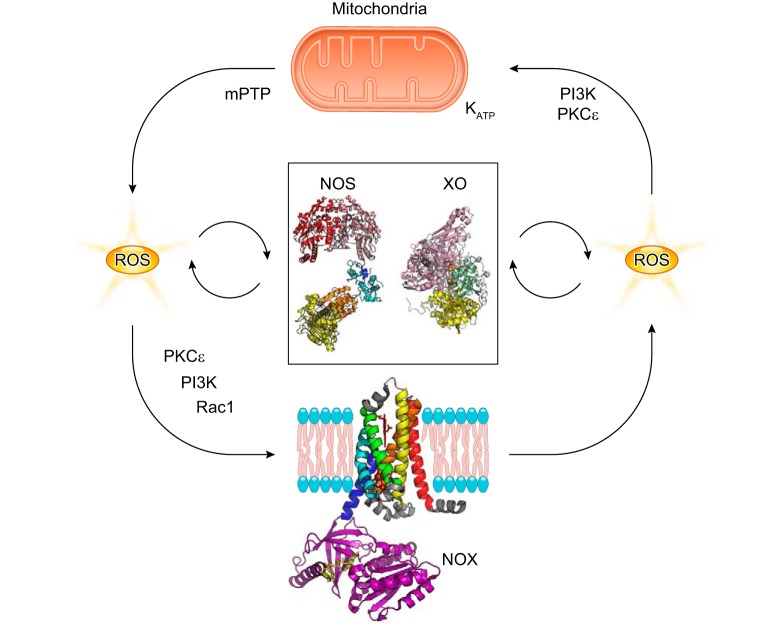
The production of adequate levels of NO in the vascular endothelium is critical for the regulation of blood flow and vasodilation, as will be discussed at length in this review (299, 565, 573, 600, 786). In this context, it has become increasingly appreciated that oxygen levels can impact the oxidation/reduction properties of different proteins and regulate NO levels (FIGURE 1) (367, 578, 595, 931). For example, nitric oxide synthases (NOSs) produce NO using l-arginine and molecular oxygen (O2) as substrates. Thus, under hypoxic or anoxic conditions, the generation of NO via NOS is compromised. However, a number of proteins that are involved in oxidative processes at basal oxygen levels can become de facto reductases as oxygen is depleted. The biological role of this transition is particularly prominent in the case of heme- and molybdopterin-containing proteins such as hemoglobin (Hb), myoglobin (Mb), and xanthine oxidase (XO) (185, 575, 578, 862, 880, 945, 990). Clinical intervention through these pathways continues attracting intense research.
FIGURE 1.
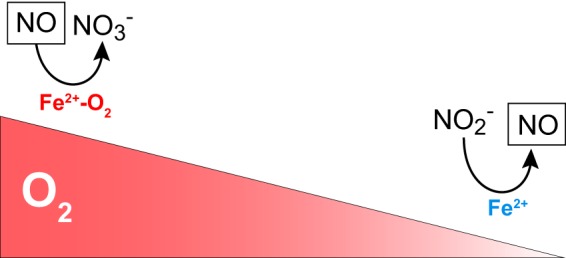
Oxygen and oxidoreductase enzymes regulate nitric oxide (NO) homeostasis. The gradient in the concentration of oxygen shifts the function of globins from oxidizing, NO-scavenging proteins to nitrite-reducing, NO-generating proteins.
The concept of oxygen-regulated oxidation and reduction processes in the metabolism of NO is not only relevant to NO generation but also to the scavenging of NO in the vasculature (FIGURE 1). In this regard, the role of globins like α-Hb and cytoglobin (Cygb) as catalytic NO dioxygenases that scavenge NO is a topic of current research (25, 593, 594, 898).
The translation of our knowledge about the biology of NO and ROS has encountered significant challenges. For instance, initial attempts to enhance NO levels using NO donors or supplementing with NOS substrates to reverse endothelial dysfunction have had limited success. The use of general antioxidants for the treatment of oxidative stress has also failed in most cases. Recent advances in the field have provided many clues on why these approaches have been unsuccessful. We will discuss these and other relevant physiological and pathophysiological issues and indicate how advances in basic biochemistry of the generation of NO/ROS have evolved our understanding and set new directions in the field.
In this review, we will present the biology of NO and ROS in the cardiovascular system with special emphasis on their routes of formation, chemistry, mode of action, and dysregulation in vascular disease. The formation pathways of NO and the mechanisms of NO signaling will be discussed in sect. II. The proteins and biological systems generating hydrogen peroxide and superoxide are treated in sect. III. Other ROS of particular relevance in the vascular system are discussed in sect. IV. Section V will study the cross-talk between NO- and ROS-generating systems. Finally, in sect. VI, we will discuss the current challenges and opportunities for the treatment of cardiovascular disease through the regulation of NO and ROS levels in pathological conditions.
II. NITRIC OXIDE GENERATION AND VASCULAR FUNCTION
The generation of sufficient NO levels to regulate the resistance of the blood vessels and hence the maintenance of an adequate blood flow is critical to the healthy performance of the vasculature (277, 299, 573, 600, 786). A number of mechanisms are involved in both the generation of NO and the response to NO signaling in the vasculature. In this section, we will overview the mammalian proteins involved in the generation and sensing of NO in the cardiovascular system. The production of NO in basal conditions is largely regulated by the activity of endothelial NOS (eNOS) in the vascular endothelium (324, 449, 716). Nevertheless, the contribution of other agents cannot be ignored. For instance, nitrite reduction by heme proteins can mediate hypoxic vasodilation and other physiological responses (367, 591, 968); neuronal NOS (nNOS) and inducible NOS (iNOS) can provide compensatory NO generation or exacerbated RNS synthesis (386, 444, 547, 704, 727). In this study, we will review NO synthases and other NO-generating biological systems.
A. Oxygen-Dependent Nitric Oxide Synthesis
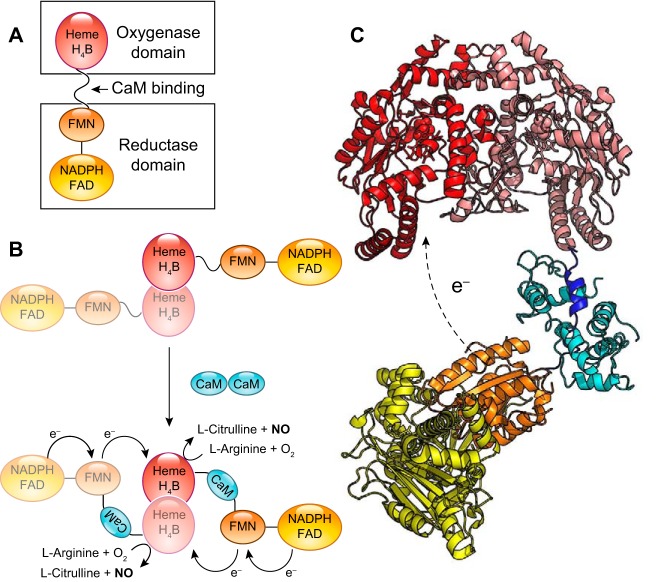
The canonical pathways of NO formation rely on the specialized NOS enzymes. NOS are dimeric, multidomain enzymes that synthesize NO from molecular oxygen and l-arginine and use iron protoporphyrin-IX (heme), tetrahydrobiopterin (BH4), FAD, flavin mononucleotide (FMN), and NADPH as cofactors (204, 904). The architecture of these proteins is complex, with a oxygenase/heme domain that binds BH4 and heme, in which the oxidation of arginine to NO using molecular oxygen takes place, and a reductase domain evolutionarily related to cytochrome P450 reductase (CYPOR) that binds the cofactors FAD and FMN (204, 377). The reductase domain uses NADPH as electron source to reduce the FAD and FMN and finally transfer the electrons to the oxygenase/heme domain (FIGURE 2). Between both domains, there is a calmodulin (CaM) binding domain that regulates the electron shuttling between the reductase and oxygenase domains. To add to this complexity, the electron transfer between domains occurs between the reductase domain of one monomer and the oxygenase domain of the other monomer; thus, only the dimer is able to generate NO catalytically.
FIGURE 2.
Architecture of nitric oxide synthases (NOS). A: the arrangement of the domains in the NOS monomer. The oxygenase/heme domain (red) is connected to the reductase domain by a flexible linker, containing a calmodulin (CaM) binding sequence. The reductase domain includes a flavin mononucleotide (FMN)-binding domain (orange) that shuttles electrons from NADPH/FAD to the heme group and a FAD-containing domain (yellow) that uses NADPH as an electron source. B: the binding of CaM (blue) to NOS promotes electron transfer from the FMN domain of one monomer to the heme domain of the other monomer. C: three-dimensional structure model of NOS. The figure is assembled from the separated structures of the human endothelial NOS oxygenase domain (PDB:4D1O) (574), the CaM binding peptide bound to CaM (PDB:2N8J) (740), and the structure of the neuronal NOS reductase (PDB:1TLL) (338).
The role of NOS enzymes in vascular function and pathobiology has been extensively studied (277, 299, 569, 790). In this study, we will summarize the most relevant concepts about the function and regulation of the NOS isoforms in health and disease states.
NOS enzymes comprise three main isoforms: nNOS (NOS I), iNOS (NOS II), and eNOS (NOS III). Among these isoforms, eNOS is the enzyme more abundant in vascular endothelial cells and has the most significant impact on vascular function, with a variety of pathologies related with eNOS dysfunction. However, the specific roles of iNOS and nNOS in vascular disease are not to be ignored and will be also discussed below.
1. eNOS
eNOS is the constitutive NOS form in endothelial cells (ECs), and as such, it is the main contributor to vascular NO levels in physiological conditions (446). For example, the infusion of a competitive inhibitor of eNOS into the human systemic or coronary circulation decreases basal blood flow by ~25% (146, 722). Basal levels of NO produced by eNOS not only regulate blood flow but tonically inhibit platelet activation and the expression of inflammatory adhesion molecules on endothelium. The role of eNOS in the maintenance of optimal cardiovascular function is also highlighted by the experiments with eNOS-deficient mice. eNOS deletion has some limited effects on mouse blood pressure and vasodilation, as adaptive processes increase the production of nNOS and prostaglandins (444, 913). However, eNOS−/− mice show impairments in angiogenesis and wound healing (563). In the apolipoprotein E (ApoE) knockout (KO) atherosclerosis model, addition of the eNOS deletion accelerates the atherosclerotic process (533). Notably, overexpression of eNOS is also detrimental (708), highlighting the delicate balance of eNOS function.
Although eNOS is predominantly expressed in the endothelium (Table 1), and the ECs are proposed to regulate NO signaling in the vasculature, a number of recent studies using chimeric cross bone marrow transplant mouse models have shown that the red blood cell also expresses a functional eNOS that contributes in part to systemic blood pressure responses (181, 536, 1025). This erythrocyte eNOS can contribute to endocrine NO signaling to modulate both blood pressure and myocardial injury and appears to be tonically regulated by arginase through the availability of the eNOS substrate l-arginine (1044).
Table 1.
Tissue distribution of NOS isoforms in the cardiovascular system
| Tissue | Cell Types | Cellular Location | References | |
|---|---|---|---|---|
| nNOS (NOS1) | Heart | ECs | Plasma membrane | 114, 126, 599, 841, 977, 1037 |
| Lung | VSMCs | Caveolae | ||
| Blood vessels | Adventitial fibroblasts | Sarcoplasmic reticulum | ||
| Cardiomyocytes | ||||
| iNOS (NOS2) | Heart | ECs | Plasma membrane | 126–128, 521, 525, 604, 688, 698, 867, 903, 984, 1064 |
| Lung | VSMCs | Phagosomes | ||
| Blood vessels | Adventitial fibroblasts | Golgi | ||
| Cardiomyocytes | Mitochondria | |||
| Macrophages and other leukocytes | ||||
| eNOS (NOS3) | Heart | ECs | Plasma membrane | 126, 181, 283, 358, 518, 819, 878, 1025 |
| Lung | VSMCs | Lipid rafts | ||
| Blood vessels | Cardiomyocytes | Caveolae | ||
| Erythrocytes | ||||
| Platelets |
EC, endothelial cell; eNOS, endothelial NOS; iNOS, inducible NOS; NOS, nitric oxide synthase; nNOS, neuronal NOS; VSMC, vascular smooth muscle cell.
eNOS is constitutively expressed; a number of posttranslational modifications impact the ability of eNOS to produce NO, from changes in the NO production rates to, in some cases, the complete blockade of NO synthesis (296). The relative presence of these modifications is critical to enzyme activity. General measurements of eNOS protein such as the determination of monomer/dimer ratio by Western blot or a single phosphorylation site status show a necessarily simplistic view of the activity of eNOS in cells. A deeper analysis of the main regulatory factors of eNOS activity, including individual phosphorylation sites, other posttranslational modifications, and the levels of the cofactors and substrates, with special interest on tetrahydropterin/dihydropterin (BH4/BH2) ratios and l-arginine/asymmetric dimethylarginine (ADMA) concentrations are necessary for a better assessment of the eNOS function in vivo and the assessment of endothelial dysfunction conditions. In this section, we will describe the regulation of eNOS activity by posttranslational modifications; the role of the cofactors BH4 and l-arginine and their counterparts BH2 and ADMA, and the role of arginases and l-arginine transport metabolons will be treated in sect. IIIB. A detailed study on other regulatory mechanisms, including protein/protein interactions, shear stress, and other mechanisms, has been provided by other reviews (59, 296, 299, 870).
a) phosphorylation.
A number of amino acids have been shown to be phosphorylated in human eNOS (296, 669). Because of their impact in eNOS activity, the more relevant phosphorylation sites are Thr495, Ser1177 (Ser1179 in bovine eNOS) and Tyr657. Other phosphorylation sites include Tyr81, Ser114, Ser615, and Ser633 (Table 2).
Table 2.
Posttranslational modification sites in human eNOS
| Site | Kinase | Effect of Modification | References |
|---|---|---|---|
| Phosphorylation | |||
| Thr495 | PKC | Impairs CaM binding | 297, 407, 646 |
| AMP kinase | Decreases NO synthesis rates | ||
| Constitutive | Can cause uncoupling through the reductase domain | ||
| Ser1177 | Akt | Activates electron transfer through the reductase domain | 74, 236, 321 |
| PKA | Increases NO synthesis rates | ||
| AMP kinase | |||
| CaMKII | |||
| Tyr81 | Src | Slight increase in activity | 320, 322 |
| May be involved in Ca2+/CaM sensitivity | |||
| Ser114 | Constitutive | 74, 327 | |
| Ser615 | PKA | No change in NO synthesis | 74, 647 |
| Akt | May modulate protein/protein binding interactions | ||
| Ser633 | PKA | No change in NO synthesis | 74, 647 |
| PKG | |||
| Tyr657 | PYK2 | Decrease in NO synthesis | 292 |
| Glutathionylation | |||
| Cys382 | Not determined | 160 | |
| Cys689 | Decrease flow through the reductase domain | 162 | |
| Decreased NO synthesis | |||
| Can cause uncoupling through the reductase domain | |||
| Cys908 | Decrease flow through the reductase domain | 162 | |
| Decreased NO synthesis | |||
| Can cause uncoupling through the reductase domain | |||
CaM, calmodulin; NO, nitric oxide
The activation of eNOS requires Ca2+ and CaM; however, changes in resting Ca2+ concentrations are not strictly necessary to regulate NO synthesis by eNOS. In turn, CaM affinity to eNOS is regulated via phosphorylation of Thr495. Thr495 is located in the CaM-binding site of eNOS, and the phosphorylation of Thr495 impairs CaM binding, thus blocking Ca2+/CaM-dependent activation of the enzyme (741). In resting endothelial cells, Thr495 is generally phosphorylated (297). The phosphorylation has been attributed to protein kinase C (PKC) (297, 646), and the residue is dephosphorylated by protein phosphatase 1 (PP1) (646). The equilibrium between phosphorylated and dephosphorylated Thr495 is mainly modulated by the changes in intracellular Ca2+ levels. Thus, bradykinin and Ca2+ ionophores promote Thr495 dephosphorylation and eNOS activation. Ser1177 phosphorylation activates electron flow through the reductase domain and, hence, increases NO synthesis in functional eNOS. Ser1177 is located in the C-terminal portion of eNOS in a helical C-terminal tail element that blocks flavin reduction and regulates the conformational equilibrium of the reductase domain (404, 631). The mechanism is similar to that described for nNOS Ser1412 (7, 947). In resting cells, Ser1177 is usually not phosphorylated. Phosphorylation is induced by a variety of signals, and the kinases involved are dependent on the inducing factor (Table 2). Some agonists like bradykinin and Ca2+ ionophores can induce Thr495 dephosphorylation via PP1 and Ser1177 phosphorylation via activation of CaMKII at the same time. Shear stress activates Ser1177 via PKA-dependent phosphorylation. VEGF, insulin, and estrogens promote Ser1177 phosphorylation via Akt kinase.
Tyr657 is located in the FMN domain of eNOS in close proximity to the FMN cofactor. The mutation of Tyr657 (292) causes complete loss of NO synthesis and l-citrulline formation, suggesting a blockade of the intramolecular electron transfer. Thus, Tyr657 phosphorylation effectively inactivates eNOS. The phosphorylation of Tyr657 is catalyzed by proline-rich tyrosine kinase 2 (PYK2). This kinase is activated in endothelial cells by several stimuli including angiotensin II, oxidative stress, and insulin (296). The detrimental role of this phosphorylation on NO synthesis has spurred interest in the pharmacological inhibition of PYK2 for the treatment of cardiovascular disease (94, 628, 869, 983).
b) glutathionylation.
Recent reports indicate that eNOS can also be modified posttranslationally by S-glutathionylation (162). At least three residues have been shown to be susceptible: Cys689, Cys908 (162), and Cys382 (160) (Table 2). The process can be reversed by glutaredoxin-1 and thioredoxin (160, 907). The reaction increases eNOS uncoupling and the formation of superoxide with diminished NO synthesis activity. Glutathionylation appears to be a detrimental modification caused by oxidative stress conditions (162, 522) and is very sensitive to the ratio of oxidized and reduced glutathione (160).
c) s-nitrosation.
S-nitrosation of eNOS has also been reported (777). This reaction is also dependent on eNOS myristoylation and Ser1177 phosphorylation and involves the nitrosation of the Zn-binding cysteines (Cys96 and Cys101 in the bovine eNOS) (271, 272). As a consequence of S-nitrosation, the formation of the eNOS dimer is blocked, resulting in the loss of NO synthesis. Denitrosation of Cys96-NO and Cys101-NO restores eNOS activity. Additional studies using mutant eNOS Cys96Ser and Cys101Ser, which cannot be S-nitrosated, indicate that the enzymatic activity is not altered by treatment with NO donors, confirming a role for the modification of these thiols in the regulation of eNOS activity (271).
d) other posttranslational modifications.
eNOS can be acylated at different residues. The myristoylation of the N-terminal glycine targets eNOS to the membrane (284), whereas the palmitoylation of Cys15 and Cys26 targets eNOS specifically to caveolae (82, 336). These modifications are not expected to change the intrinsic NOS activity but are important to modulate eNOS localization and function.
Hyperglycemia can result in N-acetyl glycosylation of Ser1177. This modification results in a nonphosphorylable Ser1177 and limits eNOS response to agonists and vasorelaxation (257, 280, 1035).
In summary, we want to remark that the generation of NO by eNOS in optimal conditions, at least as studied in vitro with saturating concentrations of coenzymes and substrates and absence of deleterious modifications, is a well-balanced process with a very limited production of superoxide as a side reaction. However, a number of circumstances can lead to changes in the NO generation cycle and trigger the production of superoxide instead of NO. These processes are generally termed “eNOS uncoupling” as the consumption of reducing equivalents by eNOS is no longer coupled to the formation of NO and is instead driven to the generation of superoxide from molecular oxygen. This process is largely deleterious and has been linked to endothelial dysfunction and other vascular pathologies. eNOS uncoupling will be discussed in sect. IIIB along with other mechanisms of superoxide generation.
2. iNOS
Unlike eNOS and nNOS, which are constitutively expressed enzymes regulated via CaM binding and posttranslational modifications, iNOS has a much higher CaM affinity, so it binds CaM at very low Ca2+ concentrations, and thus, its activity is not regulated by Ca2+/CaM but mainly at the level of gene transcription (169). The kinetic parameters of iNOS lead to a catalytic activity that generates higher NO levels than eNOS and nNOS, is less sensitive to NO-dependent autoinhibition, and generates higher levels of other nitrogen species, such as nitrate (816, 904).
The expression of iNOS is usually limited to airway epithelium and neuronal cells (Table 1), where it is highly expressed and active under basal conditions (604, 984, 1064), and activated macrophages and hepatocytes, where it is expressed in the setting of inflammatory stimuli (688).
Although healthy cells in the vasculature do not present significant levels of iNOS, several pathologic processes show increased iNOS activity in blood vessels (386, 704, 727). As iNOS can generate higher NO levels than eNOS, this iNOS activation leads to excess NO and severe impairment of vascular function. This effect is mediated by several pathways including continuous activation of sGC and competition for BH4 with eNOS (386). Overall, the excess NO limits the response of blood vessels to vasodilators and decreases NO sensitivity (263).
As pointed out, iNOS expression is prevalent in macrophages. In these cells, iNOS induction is necessary for the generation of high levels of NO in the phagosome and bacterial lysis and, therefore, is a basic tool for the innate immune response (519, 688). However, in pathological situations, increased NO synthesis can be deleterious (e.g., in macrophages recruited to the atherosclerotic plaque). The high rate of NO production by iNOS, together with superoxide formation from iNOS or other sources, can lead to the production of peroxynitrite (see sect. IVA) (222, 624, 981, 1034), a very toxic oxidant and nitrating agent (78, 709). Studies in which iNOS deletion was added to the ApoE−/− atherosclerosis model indicate improved cardiovascular function in the ApoE−/−iNOS−/− mice compared with ApoE−/− controls, indicating a detrimental role of iNOS in atherosclerosis progression (532).
Finally, it should be noted that iNOS-derived NO can also have positive effects in certain conditions. iNOS activation has been identified as a mechanism of protection against ischemia/reperfusion damage. This observation appears to be related to a preconditioning effect caused by increased levels of NO (109, 483, 579, 1009).
3. nNOS
Similar to eNOS, nNOS is a constitutively expressed form of NOS activated by CaM binding. NO synthesis rates for nNOS are generally higher than eNOS, but also tightly regulated by Ca2+/CaM (unlike iNOS), and nNOS is also very susceptible to feedback inhibition by NO (4, 902). This enables nNOS to produce NO in a pulsatile manner instead of generating sustained low levels. These features appear to be more related to its role in synaptic transmission (811, 812). Although nNOS is commonly found in neurons (120), it is also found in other tissues including vascular smooth muscle cells (VSMCs), adventitial fibroblasts, ECs, and cardiomyocytes (114, 126, 599, 841, 1037) (Table 1). Expression levels of nNOS in the vasculature are lower than for eNOS; however, there is increasing evidence of important functions for nNOS-derived NO in vascular physiology (188).
Early experiments in rodents indicated an important role of nNOS in the regulation of cerebral blood flow (731). Selective inhibition of nNOS causes increases in blood pressure and decreased response to acetylcholine in normotensive rats (139). In eNOS−/− mice, nNOS can be activated by shear stress, partially compensating the loss of eNOS in the vasculature (444, 547). Experiments with nNOS KO mice indicate increased neointima formation in carotid artery ligation and balloon injury models (666), indicating that nNOS appears to limit vascular injury independently of eNOS. To investigate the role of nNOS in atherosclerosis, the nNOS−/− deletion was incorporated in mice carrying the ApoE−/− deletion. The double deletion indicates a protective role for nNOS in the development of atherosclerosis, as the mice carrying both deletions showed accelerated progression of the disease (534).
The effects of nNOS in the human vascular system have been demonstrated by studies with nNOS-specific inhibitors. These works indicate that NO synthesis from nNOS has definite roles in the vasodilatory response, including, but not limited to, microvascular tone and coronary flow (498, 843, 844).
Another relevant pathway for nNOS-dependent NO signaling not mediated by sGC is the formation of S-nitrosothiols (462). This function has special relevance in brain function in health and neurodegenerative diseases (686). A more specific description of S-nitrosation and its signaling potential is presented in sect. IID.
4. Pharmacological regulation of NOS enzymes
There have been significant efforts to develop specific NOS inhibitors for research and pharmacological purposes (17, 752).
a) nonisozyme-specific nos inhibitors.
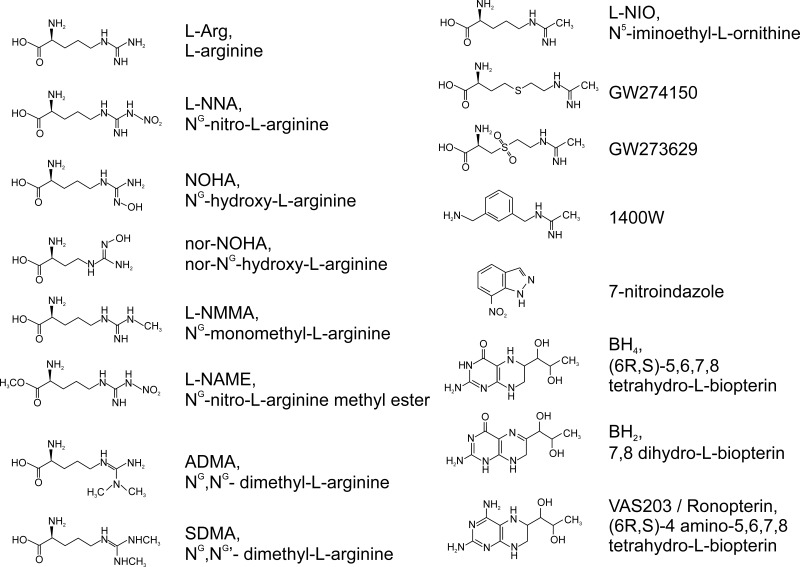
Early research indicated that analogs of the substrate l-arginine had inhibitory properties on the three NOS isoforms. Among these, NG-monomethyl-l-arginine (l-NMMA) and NG-nitro-l-arginine (l-NNA) and its precursor NG-nitro-l-arginine methyl ester (l-NAME) were characterized as general NOS inhibitors and continue to be broadly used in research, especially l-NAME (FIGURE 3).
FIGURE 3.
Chemical structure of the nitric oxide synthase (NOS) substrate l-arginine, tetrahydrobiopterin, and selected NOS inhibitors.
b) 7-nitroindazole.
7-Nitroindazole (7-NI) (FIGURE 3) is one of the earliest inhibitors showing isoform specificity. Although it was shown that all three isoforms can bind 7-NI with very similar affinity (17), in vivo studies showed inhibition of nNOS without significant effects in blood pressure, a surrogate of eNOS inhibition (664). It appears that 7-NI may have differential properties in cell permeability, with limited uptake in endothelial cells (402). Thus, although 7-NI cannot be accurately described as a specific nNOS inhibitor, it can behave as such in vivo.
c) 1400w.
N-[3-(aminomethyl)benzyl] acetamidine (FIGURE 3) is a specific inhibitor of iNOS (344). It remains one of the most widely used NOS inhibitors in research because of its cell and tissue permeability. Its selectivity seems related to the irreversible effect on the faster reacting iNOS, whereas the inhibition on nNOS and eNOS is reversible. A similar effect is observed in N(5)-(1-iminoethyl)-l-ornithine (l-NIO) (FIGURE 3) (279).
d) GW273629 and GW274150.
These sulfur-substituted acetamidine amino acids (FIGURE 3) are specific inhibitors for iNOS (16). With safer toxicity profiles than 1400W, GW273629 and GW274150 have been used in clinical trials for migraine (437, 965) (NCT00242866; NCT00319137). Although both compounds were ineffective, it is not clear if this is due to pharmacokinetic issues (965).
e) vaS203.
The pterin 4-amino-tetrahydrobiopterin (VAS203) (FIGURE 3) is a BH4 analog that can inhibit all NOS proteins by replacing the BH4 cofactor (1008). VAS203 has shown efficacy in the treatment of traumatic brain injury and has been used in phase II clinical trials (897) (NCT02012582).
The structural similarities between isoforms (and particularly between eNOS and nNOS) have made the development of specific eNOS and nNOS inhibitors particularly challenging. Structure-based inhibitors have allowed the development of new inhibitors with improved isoform specificity (337, 752). Cell permeability and other pharmacokinetic considerations have precluded the clinical use of novel inhibitors; however, newer compounds have been developed; for instance, novel-specific nNOS inhibitors have been tested in animal studies (29, 254, 255, 1051). The further development of new clinically available specific inhibitors for NOS, in particular for the iNOS and nNOS isoforms, is to be expected.
B. Nitrite-Dependent Nitric Oxide Synthesis
Despite early indications to the contrary (124, 329), nitrate and nitrite have been often overlooked as inert NO oxidation metabolites circulating in plasma and in cells. However, during the last two decades, a novel paradigm of nitrite as a source of NO, especially in hypoxia, has emerged (184, 185, 216, 364, 366, 369, 600, 603, 861, 968). Indeed, it has become increasingly accepted that the routes for the production of NO in vivo are not only oxidative (as in NOS) but also reductive (particularly by nitrite-reducing proteins such as globins and molybdopterin enzymes). These pathways can work synergistically to maintain NO levels in response to changes in oxygen tension. We foresee this theme of synergistic oxidative and reductive pathways to be potentially relevant to the generation of other reactive species in health and disease.
A few years after the discovery of the role of NO as the endothelial-derived relaxing factor, increasing evidence supported the presence of nonenzymatic NO generation (89, 605, 1075). These studies and observations of arterial-to-venous gradients of nitrite in the human circulation indicated a potential role for nitrite as a NO source in vivo (369). Subsequent studies have extended this notion to a more global paradigm that encompasses the role of nitrate and nitrite as part of a cycle of NO generation that includes both enzymatic and nonenzymatic processes.
Nitrate and nitrite can enter the circulation through the dietary intake of nitrate-rich foods, particularly leafy green vegetables (1002). In addition, NO generated from NOS enzymes is eventually oxidized to nitrate and nitrite as well. Mammals do not possess efficient systems for nitrate reduction, but oral commensal bacteria have enzymatic nitrate reduction pathways able to generate significant amounts of nitrite from nitrate reduction in the saliva (467, 602). Nitrate is not only acquired from dietary sources but is also concentrated from the blood into the saliva via the salivary glands, pumped through the sialin transporter (761). After consumption of nitrate-rich foods (beet root, spinach, kale, etc.), the concentration of nitrate and nitrite in saliva can reach levels as high as 10 mM and 2 mM, respectively (601). A portion of this nitrite is eventually absorbed into the circulation. Studies using antiseptic killing of the mouth microbiome, or systemic NOS inhibition, suggest that about half of basal plasma nitrite comes from the salivary reduction of dietary nitrate and the other half from the oxidation of NO produced mainly by eNOS (485, 557, 763, 865). NO oxidation to nitrite is proposed to occur via the oxidase activity of plasma ceruloplasmin (865).
Once nitrite accumulates in the plasma, the reduction of nitrite to NO in the vasculature is mediated by several proteins, including deoxygenated Hb (deoxyHb) (185). Increased physiological levels of nitrite in plasma from a nitrate-rich diet can lower blood pressure (554). The effect is dependent on the generation of nitrite from oral bacteria, as antiseptic mouthwash can eliminate both the increase in circulating nitrite and the blood pressure decrease (485). Subsequent studies have further validated the link between nitrite levels and improved cardiovascular function (486, 502).
Numerous metal-containing proteins have been shown to catalyze the reduction of nitrite to NO. These proteins generally contain a heme or molybdopterin cofactor. The presence of many of these proteins in diverse components of the vasculature, including blood cells, blood vessels, and heart tissue, makes them relevant for the generation of NO in vascular biology. In the subsequent sections, we will discuss the known mechanisms for nitrite reduction that are relevant for cardiovascular function. We also note that, to date, existing evidence indicates a role for Hb, Mb, and XO on biological NO generation, whereas the role of the other possible mechanisms in vivo are still a matter of discussion.
1. Inorganic nitrite reduction
Along with the role of different proteins in the reduction of nitrite, a number of nonenzymatic processes can reduce nitrite to NO in vivo. In general, these systems require concerted electron and proton donation, which is optimal at lower pH and hypoxic conditions, thus making them particularly relevant in ischemic events (1074, 1075). The simplest mechanism involves the disproportionation of nitrite, a process accelerated at acidic pH as it requires the protonation of nitrite to nitrous acid (HNO2). Two molecules of nitrous acid, then disproportionate, to form N2O3 and water (Eqs. 1–4)
| (1) |
| (2) |
| (3) |
| (4) |
This process is prevalent in very low pH conditions, as in the stomach (89, 605). In fact, this mechanism has been shown to be responsible for nitrite-dependent increases in gastric mucosal blood flow (98) and may relate to the role of nitrite in host defense as acidified nitrite is a potent antimicrobial agent (99, 261).
Several molecules present in cells have been shown to reduce nitrite to NO. Ascorbic acid (vitamin C) is a widely present cellular antioxidant that can catalyze the reduction of nitrite to NO in vitro (205). This effect is also observed with in vivo infusions of ascorbic acid and nitrite (217). Polyphenols can be assimilated through the diet and, like vitamin C, can reduce nitrite to NO (325, 733, 834).
Dietary interventions have indicated improved vascular function linked to the consumption of polyphenols from different sources, including cocoa and dealcoholized red wine (60, 168, 225, 417, 834). Combined use of polyphenols and nitrite appears to have additive effects in vascular protection (791). The effects of these dietary components can be, in part, due to the interaction with different enzymatic systems (30, 558, 824), but the link between these compounds and NO formation deserves further investigation (600, 789).
2. Heme proteins
Heme proteins have emerged as critical parts in the generation of NO under hypoxia. The specific role of globins in nitrite reduction and NO homeostasis has been studied in recent reviews (39, 770, 931). It should be noted that competing reactions, namely the scavenging of NO by the ferrous heme [rate constants in the order of 1.7 × 107 to 1.5 × 108 M−1s−1 (180, 967), Eq. 6] and the rate of NO dioxygenation [rate constants in the order of 3.4 × 107 to 8.9 × 107 M−1s−1 for mammalian Hb/Mb (264, 341), Eq. 8] greatly decrease the amount of bioavailable NO generated by the reactions of nitrite with heme proteins (931). However, nitrite-dependent NO generation in vivo catalyzed by Hb and Mb is well documented (183, 185, 365, 862, 975); it is very likely that the measurement of this NO generation is due to the fact that the high concentrations of Hb and Mb (in the mM range) can overcome the low efficiency of the nitrite reduction reaction.
a) hb.
The ability of deoxyHb to reduce nitrite has been extensively documented (124, 248, 366, 447). Initial reports by Brooks demonstrated that deoxyHb reacts with nitrite, generating nitrosyl Hb and methemoglobin, consistent with electron transfer from a ferrous Hb to nitrite (124). The studies by Huang et al. (445) confirmed the overall stoichiometry of two molecules of deoxyHb generating one molecule of nitrosyl Hb and one molecule of methemoglobin (Eq. 7), consistent with electron transfer from a ferrous Hb to nitrite and indicated the critical influence of pH in the process. By studying the same reaction using alkyl nitrites, which do not show a pH dependence in their reaction rates with deoxyHb, it was established that the reaction of nitrite requires a proton and thus formally requires nitrous acid (247, 248). This property also makes the reaction faster in low pH conditions. The overall process can be written as (Eqs. 5–7)
| (5) |
| (6) |
| (7) |
This scheme has been found to apply to virtually all the heme protein–mediated nitrite reduction reactions studied to date, including Hb (248, 447), Mb (247, 862), neuroglobin (Ngb) (932, 945), Cygb (182, 571, 780), cystathionine β-synthase (CBS) (149, 350), globin X (184), plant and cyanobacterial Hb (phytohemoglobins) (905, 946), flavohemoglobin (340), heme-albumin (43), cytochrome c (41), protoglobin (40), and microperoxidase-11 (42).
The reaction of deoxyHb with nitrite shows an increase in the instantaneous reaction rate as the reaction progresses (447). Studies with T-state and R-state Hb–stabilizing compounds have unambiguously identified the reason for this change is the shift from T-state to R-state during the course of the reaction, as the NO formed in the reaction binds to the unreacted deoxyHb to stabilize the R-state. This process is referred to as R-state autocatalysis. The rate constants for the reaction of Hb with nitrite change from 0.12 M−1s−1 for T-state Hb to 6.0 M−1s−1 for R-state Hb (Table 3) (447). As the distribution of R-state and T-state Hb in vivo are regulated by oxygen concentration, this phenomenon has important relevance for the reaction in vivo. In fact, the reaction of Hb with nitrite has been modeled mathematically at different oxygen tensions. The combination of a faster rate at higher oxygen, but increased availability of deoxyHb at low oxygen, leads to bell-shaped dependence of the observed rates versus the oxygen concentration with the maximal rates of NO generation around 50% Hb oxygen saturation, consistent with experimental data (367, 368, 794).
Table 3.
Rate constants for the reaction of nitrite with selected deoxygenated heme proteins
| Protein | K, M−1s−1 | Reference |
|---|---|---|
| Hemoglobin | ||
| Human (T-state)a | 0.12 | 447 |
| Human (R-state)a | 6 | 447 |
| Myoglobin | ||
| Horse hearta | 2.9 | 945 |
| Sperm whalea | 5.6 | 945 |
| Neuroglobin | ||
| Human (S-S)a | 0.12 | 945 |
| Human (SH)a | 0.062 | 945 |
| Cytoglobin | ||
| Humanb | 0.14 | 571 |
| Humanc | 1.14 | 182 |
| Cystathionine-β-synthase | ||
| Humand | 0.6 | 350 |
100 mM sodium phosphate, pH 7.4, 25°C; b100 mM sodium phosphate, pH 7.0, 25°C; c100 mM phosphate, pH 7.4, 37°C; d100 mM HEPES, pH 7.4, 37°C.
b) physiological implications of hb-dependent nitrite reduction.
The functional implications of the Hb-dependent nitrite reductase reaction remains a subject of intense research, particularly in the context of viable physiological models of erythrocytic NO transport (366, 424, 591). There is consensus in the field that NO-erythrocytic interactions regulate vascular function, particularly that oxygen desaturation of red blood cell Hb stimulates increased vascular NO bioavailability, leading to NO-dependent hypoxic vasodilation (266). However, the molecular mechanism of this regulation is more contentious. Three models by which this erythrocytic regulation of NO production occurs have been proposed: 1) hypoxic release of ATP from the red blood cell (882) that stimulates endothelial NO production by binding to endothelial purinergic receptors, 2) the S-nitrosation of cysteine 93 of the β-chain of Hb (SNO-Hb) (866), and 3) the reduction of nitrite by deoxyHb (as described above) (425).
Understanding the role of each of these potential mechanisms is an ongoing area of interest. The SNO-Hb hypothesis proposes that SNO-Hb is formed when Hb is oxygenated (R-state) in the lungs and remains stable. Once Hb becomes deoxygenated (T-state), the SNO-Hb reacts with existing thiols to release a vasodilatory signal (471, 728, 886). In the last 25 years since this proposal, numerous groups have debated key elements of the hypothesis including the mechanism of SNO-Hb formation and release and physiological levels of SNO-Hb. Additionally, a genetic murine model in which the cysteine 93 of β-Hb was replaced with alanine (and in which SNO-Hb formation was abolished) demonstrated no impairment in hypoxic vasodilation, questioning the role of SNO-Hb in physiologic hypoxic vasodilation (454). Importantly, more recent studies have shown that the β-93 cysteine KO mouse shows more cardiac injury in ischemic conditions, suggesting that SNO-Hb may play a more prominent role in cardiac rather than vascular function (1060).
With regards to the nitrite reduction hypothesis, human studies show a significant gradient of nitrite from the arterial to venous circulation, concomitant with the production of NO (detected as iron-nitrosyl Hb) in the venous circulation (185, 369). In vitro experiments in isolated aortic rings demonstrate that nitrite mediates vasodilation in conditions of Hb deoxygenation (185, 196). Consistent with these experiments, infusions of physiological nitrite concentrations mediate vasodilation in humans, which is enhanced during exercise and not affected by NOS inhibition. This effect is accompanied by iron-nitrosyl Hb formation, consistent with nitrite reductase chemistry (185).
A central controversy relevant to all theories of erythrocytic NO transport is the question of how NO generated can escape the red cell without reacting with Hb. Although theoretical calculations suggest that this type of NO escape should be limited, accumulating studies by many research groups now demonstrate the production of bioavailable NO generated from the incubation of erythrocytes with nitrite (185, 196, 883, 975). The production of NO gas has been measured by chemiluminescence in vitro from the reaction of deoxygenated Hb, Mb, or erythrocytes with nitrite. More physiological biosensor experiments demonstrate that incubation of nitrite with erythrocytes can induce cGMP production and inhibit activation in platelets coincubated with this reaction (591, 883, 975). The mechanism for NO release remains unclear but has been postulated to involve the formation of NO+ (684) or N2O3 as intermediates (69). Additionally, NO escape may relate to the localization of its formation, particularly at the surface of the erythrocyte (813) or via concerted reactions of NO and autoxidation of oxygen to form NO and NO2 (N2O3) at partial oxygen saturations (i.e., the reactions of nitrite with both oxygenated Hb and deoxyHb at around 50% Hb oxygen saturation) (384). More work is needed to understand the biophysics of NO signaling from the erythrocyte during nitrite reactions with deoxygenated red blood cells and Hb.
c) mb.
The physiological relevance of the reaction of Hb with nitrite suggested that similar processes could involve the closely related globin, Mb. The ability of Mb to catalyze nitrite reduction to NO was first confirmed in vitro. Consistent with the monomeric structure of Mb and its lack of allosteric regulation, the reaction rate is constant through the reaction, with a bimolecular rate constant similar to that of R-state Hb (Table 3) (447). In vitro studies of isolated mitochondria in the presence of Mb and nitrite have shown that Mb-dependent NO is bioavailable and can inhibit mitochondrial respiration in a similar manner to a conventional NO donor (862). In the cardiovascular system, the heart is the most relevant organ for the function of Mb as a nitrite reductase. In a murine myocardial infarction (MI) model, nitrite has been shown to decrease infarct size, and this effect was lost in Mb KO mice, which cannot reduce nitrite to NO (774). Through this mechanism, cardiac Mb is thought to be critical for the reduction of circulating nitrite accumulated through diet or through the therapeutic process of remote ischemic preconditioning, which mediates cardioprotection (267, 775).
d) ngb.
Ngb is a recently discovered six-coordinate globin that is evolutionarily related to Hb and Mb (134). The physiological function of Ngb is unknown, although because of its generally low concentration in cells (with the exception of the retina in the eye), it is probably not related to oxygen transport and storage as Hb and Mb are (38, 39, 133). It was recently demonstrated that, as observed with other globins, deoxygenated Ngb can also reduce nitrite to NO (932, 945). Furthermore, this process is redox regulated by the redox state of two surface thiols. Thus, oxidation of two cysteine residues to form an intramolecular disulfide bond doubles the rate of nitrite reduction (Table 3). This redox transition occurs in a physiologically relevant range and can be controlled by the cellular GSSG/GSH ratios (945). It appears that Ngb is not present in vascular cells, except for sympathetic nerves (898). The reaction of Ngb with nitrite may have implications for microcirculation in the brain, where several studies have found a vasoprotective effect of Ngb (140, 503, 917).
e) cygb.
Like Ngb, Cygb is a six-coordinate globin discovered in the early 2000s (132, 493, 951). Cygb is ubiquitous in human tissues, mainly found in fibroblasts and cells of related lineage such as osteoblasts and chondroblasts, but also in other cell types, including neurons (403, 687). Interestingly, it has been identified in VSMCs (398, 571, 594). Cygb can reduce nitrite to NO at rate constants similar or slightly higher than Ngb (182, 571) (Table 3). In addition, the oxygenated form of Cygb reacts with NO to form nitrate in a NO dioxygenation reaction that scavenges NO (339) (Eq. 8)
| (8) |
This reaction is common to most heme proteins (264, 341, 342, 931). In practice, this indicates that Cygb expression can regulate the diffusion of NO and possibly prevent excessive vasodilation at high levels of NO, not unlike the proposed role of α-Hb in vascular endothelial cells (898). This role has been proposed for Cygb in the vascular endothelium (398, 593, 594). It should be noted that the scavenging of NO is more efficient at high O2 tensions, which favor the formation of the ferrous/oxygenated heme species, whereas nitrite reduction would be prevalent when the oxygen concentration is low, and the ferrous/deoxygenated species is more abundant. Thus, both processes can synergize to regulate the concentration of NO (593, 594). The presence of an efficient reduction system for Cygb in smooth muscle cells via cytochrome b5 and cytochrome b5 reductase further supports the possible relevance of these processes in vivo (25, 593).
f) cbs.
CBS is a pivotal enzyme for the metabolism of homocysteine (649). Mutations in the CBS gene are associated with hereditary homocystinuria (531). CBS binds heme and 5′-pyridoxal phosphate. The 5′-pyridoxal phosphate group is critical for the reaction of homocysteine with serine or cysteine, whereas the role of the heme domain in the catalysis of CBS in unknown, but this heme appears to be functional and redox active (868). The CBS-mediated reduction of nitrite to NO by the CBS heme has been recently reported (149, 350). The reaction proceeds with rate constants similar to these of T-state Hb, Ngb, or Cygb (Table 3) (149, 350). The presence of CBS in endothelial cells (809) suggests that CBS could play a role in vascular nitrite reduction in vivo.
g) nos.
As NOS proteins contain a heme group, it is conceivable that they can catalyze the reduction of nitrite as observed in other heme enzymes. Indeed, in anoxic conditions, eNOS has been shown to catalyze nitrite reduction to NO (970). The contribution of eNOS to nitrite reduction could be significant in specific tissues, including red blood cells and kidney (654, 998).
3. Molybdoproteins
The relevance of molybdopterins in the generation of NO in the vasculature has been increasingly appreciated since this reaction was first described for XO (650, 1061). As the molybdopterin group does not react directly with oxygen or NO, these reactions that decrease NO release from heme proteins do not limit Mo-dependent nitrite reduction reactions. Thus, the reduction rates by molybdoproteins can be relevant in vivo at lower rate constant values as compared for heme protein reduction rates. The reduction of nitrite by all four mammalian molybdenum-containing enzymes has been characterized. Recent reviews on the nitrite metabolism by molybdenum enzymes are available (614, 616).
a) xo.
Mammalian molybdopterin-containing enzymes catalyze different oxidation-reduction reactions including, but not limited to, xanthine oxidation, sulfite oxidation, and drug metabolism processes (839, 840). To our knowledge, the ability of mammalian Mo-containing enzymes to reduce nitrite to NO was first described for XO (370, 578, 650, 1061). Earlier work had shown the ability of XO to also reduce nitrate to nitrite (50, 308). Subsequently, several reports have shown that all mammalian Mo-containing enzymes [XO, aldehyde oxidase (AO), sulfite oxidase, and the mitochondrial amidoxime-reducing component (mARC)] are able to reduce nitrite at different intrinsic rates (Table 4) (575, 880, 990).
Table 4.
Kinetic parameters for the reaction of nitrite with selected molybdopterin proteins
| Protein | kcat, s−1 | KM, mM | K, M−1s−1 | Reference |
|---|---|---|---|---|
| Xanthine oxidase | ||||
| Human, pH 7.4a | 0.41 | 2.2 | 186 | 616 |
| Human, pH 6.3b | 1.2 | 0.67 | 1800 | 616 |
| Rat, pH 7.4a | 0.55 | 1.9 | 289 | 616 |
| Rat, pH 6.3a | 0.58 | 0.25 | 2300 | 616 |
| Aldehyde oxidase | ||||
| Human, pH 7.4a | 0.47 | 4.1 | 115 | 616 |
| Rat, pH 7.4a | 0.67 | 3.6 | 186 | 616 |
| Rat, pH 6.3a | 0.66 | 0.43 | 1500 | 616 |
| Sulfite oxidase | ||||
| Human, pH 7.4c | 0.002 | 1.6 | 1.3 | 990 |
| Human, pH 6.5c | 0.004 | 1.7 | 2.4 | 990 |
| mARC-1 | ||||
| Human, pH 7.4d | 0.1 | 9.5 | 11 | 880 |
Reaction contains 50 μM of reductant (aldehyde); breaction contains 750 μM of reductant (aldehyde); creaction contains 5 μM of reductant (sulfite); dreaction contains 1 mM NADH, 0.2 μM cytochrome b5 reductase and 2 μM cytochrome b5.
Although the architecture of these enzymes is variable, existing evidence indicates that the reduction of nitrite takes place in the molybdopterin site, where Mo reduces nitrite to NO in a single electron transfer reaction similar to that of the heme proteins (615, 616) (Eqs. 9 and 10)
| (9) |
| (10) |
Depending on the redox potential of the enzyme, nitrite reduction may occur by either of the two reactions or only by Mo4+ (Eq. 9) pathways or one specific pathway (614, 616). The pH dependence of the reaction is not linear as in the case of the heme-dependent reactions, probably because of the protonation of important catalytic residues at low pH that offsets the effect of the increased proton concentration. These circumstances lead to a maximal rate for XO-dependent nitrite reduction around pH 6.3 (Table 4) (617). As noted above, unlike nitrite reduction by heme, the Mo cofactor does not bind NO. Thus, this reaction potentially has a higher yield of free NO.
In the vasculature, XO has been identified in the surface of epithelial cells, endothelial cells (5, 798, 979, 1010), and erythrocytes (357, 998). To date, XO is the most relevant nonheme nitrite reductase in vivo. Many studies have shown the relevance of XO in mammalian physiology (357, 568, 577, 578, 998). The use of specific XO inhibitors such as allopurinol and oxypurinol has allowed the determination of specific XO effects on nitrite reduction and NO metabolism (357, 568, 575, 998).
b) ao.
AO is a molybdenum-containing protein with high sequence and structural similarity to XO. Its function is yet unknown, although it has been related to the metabolism of retinoic acid, neurotransmitters, and a variety of xenobiotics (513, 756, 920). AO has been shown to reduce nitrite to NO, albeit a slower rate than XO (Table 4) (568, 575, 617, 1001). As observed for XO, the pH dependence of nitrite reduction shows a bell-shaped pattern with a maximal rate around pH 6.5 (617). The relevance of AO-dependent nitrite reduction has not been studied in so much detail as for XO, but depending on their tissue abundance and the availability of their reducing substrates, some studies suggest that the magnitude of AO activity in the vasculature can be similar to XO (568, 745, 1073).
c) sulfite oxidase.
The molydopterin-containg sulfite oxidase catalyzes the oxidation of sulfite to sulfate, preventing the accumulation of toxic levels of sulfite. Impaired sulfite oxidase function leads to severe neurological damage and death (510). Like XO and AO, the molybdopterin cofactor of sulfite oxidase is able to catalyze nitrite reduction to produce NO (Table 4) (990). Unlike other molydopterin proteins, in sulfite reductase only the Mo4+ center, and not the Mo5+ species, is able to catalyze the reaction (990). The reaction has a marked O2 dependence with very low NO formation in the presence of O2. This is most probably related to the fast oxidation of the Mo4+ species to Mo6+ by molecular oxygen as observed in other molybdopterin proteins (880, 990).
d) marc 1 and 2.
Two isoforms of the mARC are expressed in mammals (705). Sequence homology suggests that mARC proteins belong to the sulfite oxidase family. The structure of the mARC proteins has not been elucidated, but electron paramagnetic resonance data seem to support these similarities (1043). mARC proteins have a not yet understood physiological function. Existing evidence suggests that they may be involved in the detoxification of N-hydroxylated substrates; mARC enzymes are required for the detoxification of some hydroxylamines (706). mARC can also catalyze the reduction of the NOS reaction-intermediate NG-hydroxy-l-arginine to l-arginine in a reaction that may be involved in NO biosynthesis (528).
Both mARC enzymes can reduce nitrite to NO in a process very sensitive to oxygen-mediated inhibition (Table 4) (880). As mARC enzymes can use the cytochrome b5 reductase/cytochrome b5 system as a source of electrons, the three proteins can form a mitochondrial metabolon for the reduction of nitrite to NO under hypoxic conditions (880).
4. Other proteins
a) carbonic anhydrase.
The reduction of nitrite by carbonic anhydrase has been described (1). Unlike heme- or molybdenum-containing proteins, the Zn2+ in the active site of the carbonic anhydrase is not redox active, thus invoking a nitrite anhydrase mechanism as described in Eqs. 1–4. Such reaction would be of notable interest in vascular physiology because of the ubiquitous presence of carbonic anhydrase in erythrocytes; however, this activity remains highly controversial (615).
b) cytochrome c.
The electron carrier cytochrome c has also been noted as a situational nitrite reductase. As cytochrome c is a six-coordinate heme protein with no distal site available for nitrite ligation, the reaction is only observable in conditions where the protein is partly unfolded, a situation that can be elicited by cardiolipin or other anionic phospholipids (477). In the presence of cardiolipin-containing liposomes, cytochrome c has been shown to catalyze nitrite reduction to NO in a reaction that may be prevalent in apoptotic processes (68).
c) cyp and cypor.
The heme P450 cytochromes (CYPs) are involved in a wide range of detoxification processes. CYP 2B4 and microsomal rat liver CYP fractions have been characterized as nitrite reductases (576). Notably, the associated CYP-reducing protein, CYPOR, can reduce nitrate to nitrite, forming a metabolon that can tentatively catalyze the complete process from nitrate to NO (576).
C. The NO Receptor sGC and the Modulation of Vascular Tone
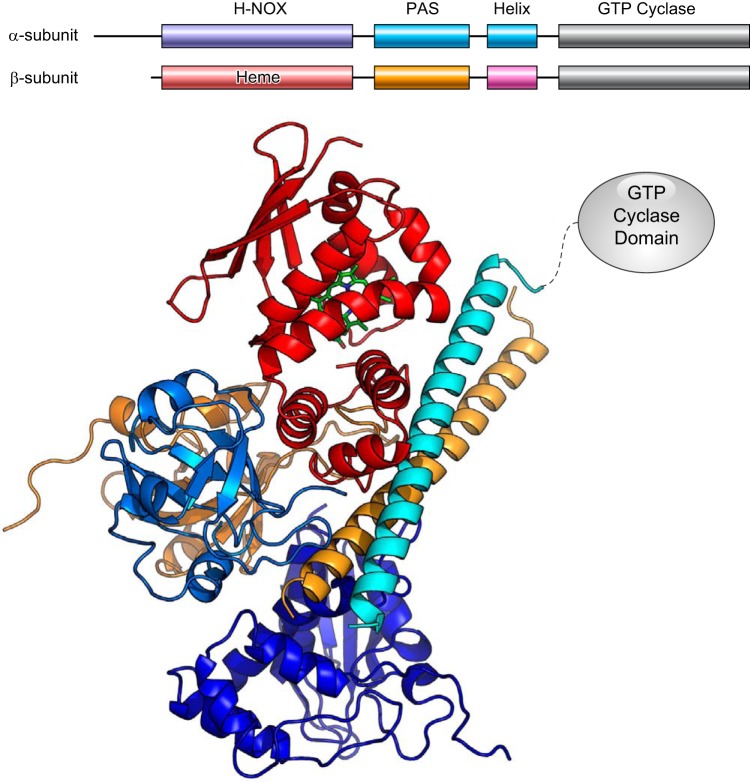
1. Structure and function of sGC
The effects of NO in the vasculature at concentrations in the nM/pM range (397) are mainly mediated through the activation of the canonical NO receptor sGC (223, 662, 711, 751). sGC is a cytosolic, heterodimeric protein comprising two homologous subunits, α and β. Although at least two isoforms for either α and β subunit exist (termed α1, α2 and β1, β2) with different tissue distributions (129), the α1β1 heterodimer is the most common form. Each monomer contains four domains: an N-terminal heme-binding domain, a Per-Arnt-Sim (PAS) domain, a helical/coiled coil motif, and a C-terminal, guanylyl cyclase domain that catalyzes the formation of cGMP from GTP (FIGURE 4). It should be noted that despite the homology between the N-terminal domains, only the N-terminal domain of the β subunit binds heme (489). The heme domain in the β subunit is the NO-sensing center of sGC. Remarkably, unlike most other hemoproteins that bind oxygen, CO and NO, this heme group shows a high selectivity toward NO and can also bind CO weakly but shows no affinity toward molecular oxygen (625). When NO binds to the heme, changes in the heme coordination cause a conformational change in the N-terminal heme domain that relieves an inhibitory interaction between the heme domain and the catalytic C-terminal domain, activating cGMP synthesis (1017). The exact details of the conformational changes that mediate enzyme activation are not yet known and are the focus of ongoing structural studies (145, 313, 958).
FIGURE 4.
Architecture of soluble guanylyl cyclase (sGC). Top: arrangement of sGC domains in the sequence of α and β subunits. Bottom: model for the interaction of the N-termini domains of the α and β subunits of human sGC. Domains are colored according to the top panel. Catalytic GTP cyclase domains are not shown. The model for the human sGC protein is based on the model for Manduca sexta sGC derived from chemical cross-linking, small-angle X-ray scattering, and homology modeling (312, 313, 662).
The activation of sGC by NO leads to an increase in cGMP synthesis of at least two orders of magnitude (895). cGMP exerts a number of downstream signaling effects on phosphodiesterases such as PDE5 and other targets including cGMP-dependent kinases and cGMP-gated ion channels, eliciting a vasodilatory response (673, 674, 993).
Apart from changes in NO levels or downstream signaling, the activity of sGC itself is compromised in a number of pathological conditions (355, 363, 600, 853, 888, 1022). Situations directly related to sGC that can account for a decrease in sGC activity include changes in the expression of sGC, defective incorporation or loss of sGC heme, a deficient reduction of the sGC heme iron, leading to an NO unresponsive ferric enzyme, posttranslational modifications, ubiquitination and proteosomal degradation, and defects in cGMP synthesis.
Changes in sGC related to the mRNA transcript have been described. For example, expression of a variant α2 subunit (α2i) containing a 31 amino acid insertion has been shown in different tissues. This subunit can form a dimer with β1 but produces an inactive sGC form (80). Alternatively, several splice variants of the α1 and β1 monomers have been identified in vivo (626, 787, 852). These splice forms can be related to decreased sGC activity and appear to be more abundant in disease conditions such as aortic aneurysm (626).
The incorporation of the heme group in the sGC β1 monomer is regulated by the chaperone heat shock protein 90 (Hsp90) (95, 354, 356, 818). This process is also regulated by NO, with low NO doses improving Hsp90/sGC interaction and heme insertion, whereas high NO blocks heme insertion (354, 985). The relevance of heme-free sGC in the context of cardiovascular disease is not well characterized. Several lines of evidence suggest the presence of pools of heme-free sGC in vivo (354, 356, 799, 943), and it is reasonable to expect that the exacerbated ROS conditions that exist in the origin and development of many cardiovascular pathologies can promote heme oxidation and subsequent heme loss (312, 363, 888). Thus, a plausible therapeutic strategy can target the inactive, heme-depleted sGC by use of agonists (activators) that can bind to the empty heme site activating the enzyme. Some examples of these drugs are discussed in the next section.
In addition to the presence of heme-free protein, it is increasingly appreciated that a pool of ferric sGC can also exist in smooth muscle cells (641, 769, 891). As noted, the oxidation of the heme iron is probably the intermediate step in the generation of free-heme sGC (312, 363, 888). The generation of ferric sGC is one of the mechanisms of sGC desensitization, and this form of the protein, whereas nonresponsive to stimulator drugs, can be rescued by sGC activators (302, 891). Recent work indicates that CYB5R3 (methemoglobin reductase) may mediate the reduction of ferric sGC to NO-responsive ferrous sGC in cells (769). These findings open the possibility of novel pathways to regulate sGC responses in blood flow regulation and for targeted therapy in diseases associated with oxidative stress and endothelial dysfunction.
Posttranslational modifications of sGC have been also described. Prolonged exposure to NO has been long known to cause a decrease in sGC activity, usually characterized as sGC desensitization (85, 671, 831). This process is related to some of the tolerance profiles observed for NO donor treatments (459). sGC desensitization is probably due to different concurrent mechanisms, including S-nitrosation and heme iron–nitrosylation. Recent reports indicate that several cysteine residues in sGC can undergo S-nitrosation, leading to decreased activity (93, 822, 823). The formation of a heme iron–nitrosylated form of sGC, which has decreased guanylyl cyclase activity, has been reported (953). Ubiquitination of sGC targets the protein for proteosomal degradation, a process that can be inhibited by sGC activators (643). Further research on these sGC modifications, and interventions to prevent their occurrence, is ongoing (92).
2. Pharmacological regulation of sGC
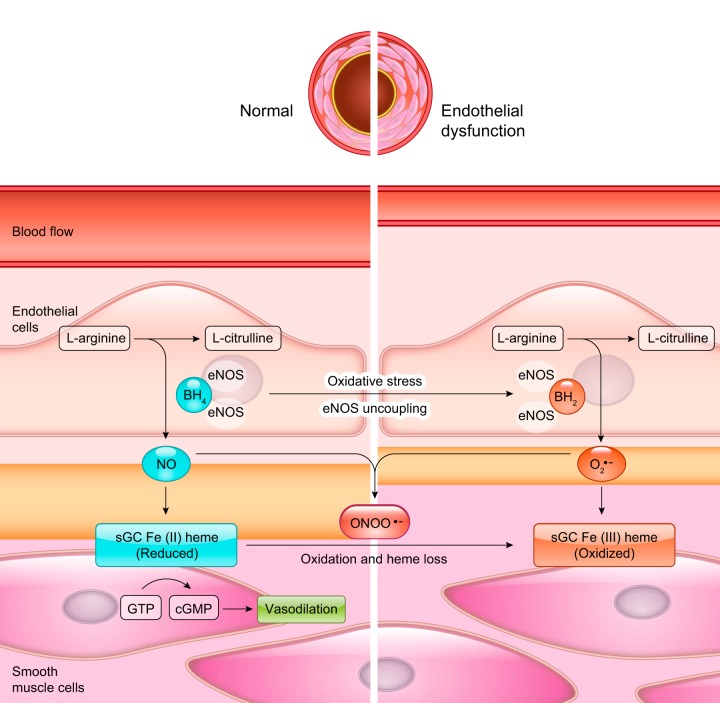
Because of the pivotal role of sGC in the regulation of the NO response, substantial efforts have been made to develop pharmacological regulators of sGC function. In theory, direct targeting of sGC offers notable advantages, bypassing the complex mechanisms that may limit NO generation and availability under pathological conditions (FIGURE 5). Two main groups of compounds, activators and stimulators, have been developed with different mechanisms of action. In both cases, the target of the drugs appears to be the NO-sensing, heme domain of the β subunit (662). A large number of sGC agonists have been developed over the last three decades; we discuss below a few of the more widely studied compounds. For a more complete view of the development of these and other sGC-related drugs, the reader is referred to more specific reviews (274, 302, 888).
FIGURE 5.
Soluble guanylyl cyclase (sGC) function in healthy and endothelial dysfunction states. Oxidative stress conditions cause oxidation of BH4 to BH2, superoxide production in the endothelial cells, and promote oxidation and heme loss in smooth muscle cell sGC.
a) stimulators.
Stimulators are compounds that enhance the activity of functional sGC and require the presence of ferrous (Fe2+) heme-bound sGC. These compounds can cooperate with endogenous NO-mediated activation.
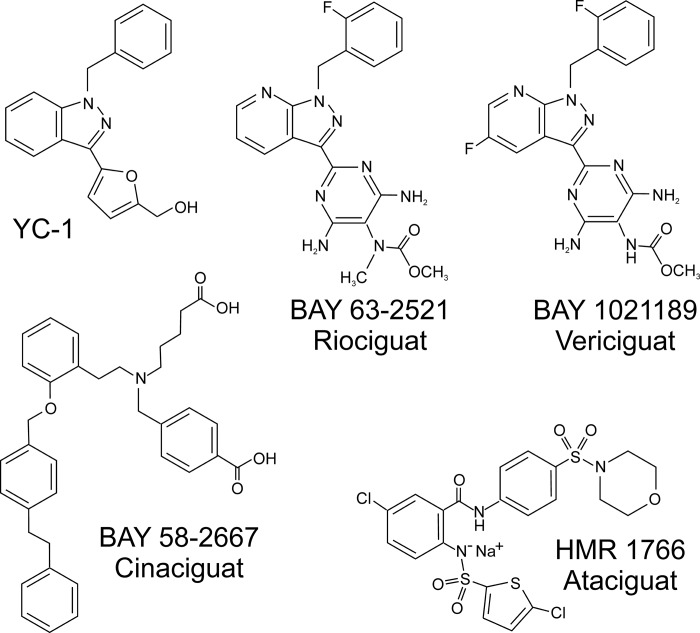
The benzyl indazole YC-1 (FIGURE 6) was the first developed sGC stimulator (524). The effects of YC-1 on sGC activation were found to be synergistic with NO donors and required heme-bound sGC (310, 436, 896). The compound presented some limitations and a lack of specificity with cGMP-independent effects and inhibition of PDE5 (309, 326, 991). Based on YC-1 structure, a number of optimized compounds have been developed such as BAY 41–2272 and BAY 41–8543 (889). These compounds are more specific than YC-1 and despite some poor pharmacokinetic properties, have been extensively used in research (105, 353, 670, 760, 764, 852, 889).
FIGURE 6.
Chemical structure of selected soluble guanylyl cyclase (sGC) stimulators and activators.
Further studies led to the development of another YC-1–related compound, BAY 63–2521 (riociguat) (83, 655) (FIGURE 6). Riociguat (commercialized as Adempas) was approved in 2013 by the Food and Drug Administration for the treatment of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension (351, 352).
Another second-generation stimulator compound is BAY 1021189 (vericiguat) (301) (FIGURE 6), which has completed Phase IIB trials (287, 349, 744) and is currently in Phase III clinical trials for the treatment of heart failure with reduced ejection fraction (301) (NCT02861534).
B) activators.
sGC activators are compounds that can trigger the catalytic activity of sGC independently of the redox state of the sGC heme or even in the absence of bound heme. In general, these compounds work as heme analogs, replacing the heme group and triggering a similar conformational change to that of NO-bound heme in the native enzyme, thus activating cGMP formation (302).
High-throughput screening assays led to the discovery of a novel series of sGC regulators that do not require the sGC heme. The first developed drug in this category was the compound BAY 582667 (cinaciguat) (890) (FIGURE 6). Apart from the recovery of sGC synthesis, it was noted that BAY 582667 could limit the degradation of heme-oxidized and heme-free sGC (643). Cinaciguat showed promising results in animal studies and acute decompensated heart failure (553), but the trials were stopped because of the development of low blood pressure (269).
Another sGC stimulator that has reached clinical use is HMR 1766 (ataciguat) (827) (FIGURE 6). Phase II clinical trials are currently evaluating the effect of ataciguat on aortic valve calcification (NCT02481258).
D. S-Nitrosothiols and the Modulation of Vascular Function
1. S-nitrosation and vascular function
Although activation of sGC is considered the predominant mechanism of NO-dependent signaling, it is now recognized that posttranslational modification of cysteine residues by S-nitrosation comprises a significant alternative mechanism of NO signaling. The first protein shown to be S-nitrosated was albumin (885), and its existence was first proposed to represent a reservoir of NO bioactivity. However, S-nitrosated albumin is now considered to be a product of more detrimental NO “scavenging” by the protein (332, 956). It is now well recognized that a plethora of proteins of various function and localization are S-nitrosated physiologically or in pathological conditions. For example, as described above in sect. IIB2, S-nitrosation of Hb is an active area of research and SNO-Hb has been considered a reservoir of NO activity. In this regard, decreased levels of SNO-Hb have been reported in conditions such as pulmonary hypertension and hypothesized to contribute to disease pathogenesis (586, 729).
Accumulating studies demonstrate that beyond simply representing a reservoir of NO activity, S-nitrosation is a mechanism of enzymatic regulation. S-nitrosation of specific cysteine residues can modulate enzymatic activity and function. One prototypical example of this paradigm is the type 2 ryanodine receptor (RyR2). The RyR2 is a Ca2+ release channel that releases Ca2+ from the sarcoplasmic reticulum to mediate cardiac excitation/contraction coupling. The magnitude and duration of Ca2+ release from through the RyR2 determines contractility of the myocyte. Although the RyR2 contains many surface-exposed thiols, physiological S-nitrosation of specific cysteine residues activate the protein and contribute to maintainance of healthy excitation contraction coupling (1038). A lack of this S-nitrosation has been associated with cardiac arrhythmias as well as heart failure (131, 372, 373). Similar to the RyR2, a multitude of enzymes have now been shown to be activated or inhibited by S-nitrosation. Although review of all these proteins is beyond the scope of this manuscript, the S-nitrosation of mitochondrial complex I and its cardioprotective signaling is discussed in sect. IIIC3, and the S-nitrosation of eNOS as a regulator of its activity is outlined in sect. IIA1. The role of S-nitrosation in regulating cardiovascular function has been reviewed elsewhere (586, 677, 914).
In the following section, we review the chemical mechanisms by which S-nitrosothiols are formed. It is important to note that physiological levels of S-nitrosothiols are determined not only by the rate of S-nitrosation but also by protein denitrosation. Although S-nitrosothiols degrade through transnitrosation reactions (outlined below) and interaction with other low molecular–weight thiols, the existence of denitrosating enzymes has been reported. The best characterized enzyme in this class is S-nitrosoglutathione reductase (GSNOR), a member of the aldehyde dehydrogenase enzyme group, which catalyzes the denitrosation of glutathione and thus regulates the equilibrium of S-nitrosation (592).
2. Formation of S-nitrosothiols
The formation of S-nitrosothiols is, on occasion, described as the reaction of NO with a thiol (R-SH) group to generate a protein-bound nitrosothiol (R-SNO). However, it should be noted that NO is not a nitrosating species and does not react directly with thiols. Formation of S-nitrosothiols involves the reaction of the deprotonated thiol (R-S−) and a formal nitrosonium ion (NO+) (Eq. 11)
| (11) |
In physiological conditions, the thiolate is generally provided by glutathione or cysteine, yielding GSNO or Cys-NO, respectively. The nature of the nitrosating species can vary, generating different possible mechanisms for the reaction. Most of these routes require the formation of the nitrogen dioxide radical intermediate (NO2·) (Eqs. 12a, 12b)
| (12a) |
| (12b) |
The reaction of NO2· with a thiolate in the presence of NO can generate the S-nitrosothiol via a thiyl (RS·) radical (Eqs. 13a, 13b)
| (13a) |
| (13b) |
The reaction of NO2· with another molecule of NO2· or NO generates dinitrogen tetroxide (N2O4) or dinitrogen trioxide (N2O3), respectively, which are strong nitrosating species and can react directly with the thiolate (691, 692, 1018) (Eqs. 14a–d)
| (14a) |
| (14b) |
| (14c) |
| (14d) |
Peroxynitrite (sect. IVA), formed by the reaction of NO and superoxide, is another source of NO2·, either through homolytic decomposition, via peroxynitrous acid, into NO2· and OH· radicals (Eqs. 15a–b) or via reactions with CO2 (Eq. 15c) or metal centers (Eq. 15d)
| (15a) |
| (15b) |
| (15c) |
| (15d) |
These reactions leading to the formation of NO2· are well characterized (123, 548, 585, 1018). Global kinetic analysis of the reactions involved suggests that the main source of NO2· in vivo may be the decomposition of peroxynitrite (585). This result is consistent with the unfavorable rates for the direct formation of NO2· from oxygen and NO in vivo, (1018) although the exact mechanisms of formation remain a topic of current research (123, 872).
An additional pathway involves the formation of a ferric nitrosyl heme which can be in transient equilibrium with a ferrous-nitrosonium species (Eqs. 16a–c)
| (16a) |
| (16b) |
| (16c) |
Finally, the reaction of an existing S-nitrosothiol with a thiolate group can result in transnitrosation (Eq. 17)
| (17) |
Transnitrosation is a fundamental reaction of S-nitrosothiols and allows for the formation of GSNO or S-nitrosated proteins to mediate the formation of other S-nitrosothiols and probably serve as a reservoir for S-nitrosothiols in the cellular environment. Altogether, we want to note that the formation of the S-nitrosothiols is a complex process, and existing evidence indicates the requirement of significant concentrations of NO and usually the presence of superoxide and/or peroxynitrite.
The formation of S-nitrosothiols and their functional roles in vivo is a growing field, with protein S-nitrosation identified in most physiological pathways. It should be considered that the stability and precision of this modification is under intense study to determine its parallels with other protein modifications such as phosphorylation (1023, 1024). Recent studies of the proteome of eNOS−/− mice compared with wild type mice identify a more limited set of S-nitrosated proteins (125, 378). Altogether, it appears that the detection of S-nitrosated proteins in the presence of exogenous NO or R-SNO species can lead to nonphysiological S-nitrosation and more sensitive methods, monitoring protein S-nitrosation levels in vivo are necessary for accurate detection of these modifications.
III. SUPEROXIDE AND HYDROGEN PEROXIDE GENERATION AND VASCULAR FUNCTION
The presence of ROS in the vasculature has historically been considered detrimental and a consequence of abnormal generation and/or an inability of the endogenous reductant and antioxidant systems to scavenge these reactive species. The excess of superoxide, or other ROS, in the cell causes the modification of different parts of the cellular machinery and is a known player in the genesis and progression of cardiovascular disease (227, 408, 409, 570, 672, 700, 893). Conversely, the signaling properties of many of these molecules are increasingly recognized. Indeed, the generation of superoxide and hydrogen peroxide, among others, has been shown to mediate critical processes such as angiogenesis, hypoxic adaptation, and energy homeostasis (96, 249, 291, 720, 778, 825, 960). In this section, we will discuss the main sources of superoxide and hydrogen peroxide generation in the vasculature and their endogenous and pharmacological regulation. Among these, NADPH oxidases (NOXs) appear to be the most important sources quantitatively. In healthy situations, the generation of ROS by eNOS, mitochondria, or XO is limited, and in the case of mitochondria, it is probably more related to signaling pathways (289, 290). However, there is significant evidence that not only NOXs but also the generation of ROS by uncoupled eNOS, mitochondria, or XO has particular relevance in the origin and development of cardiovascular disease.
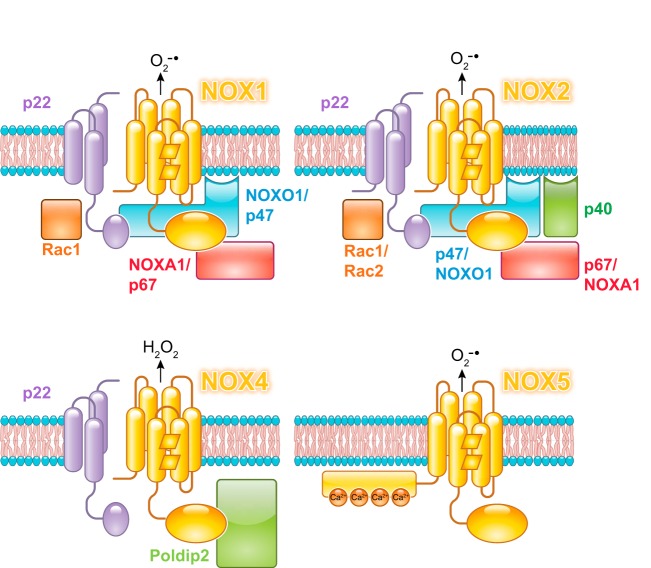
A. NOXs
NOXs are multiprotein enzyme complexes that catalyze the electron transfer to molecular oxygen to form ROS, more commonly superoxide, but also hydrogen peroxide (79, 252, 383, 546, 912). To date, seven isoforms have been described [NOX 1–5 and dual oxidases (DUOX) 1 and 2]. Each isoform consists of a core, catalytic subunit with a transmembrane domain and a variable number of regulatory subunits. The core subunit defines the name of the NOX complex. Thus, NOXs complexes show differences in their molecular architecture, activator proteins, localization, and function. NADPH is the preferred substrate for all NOX isoforms, with the NOX2 isoform being particularly NADPH specific (48, 63, 177, 1047).
The putative mechanism of all NOX proteins can be described as an electron transfer chain in which electrons are transferred from NADPH to the FAD cofactor in the flavoprotein/dehydrogenase domain and from FAD to the heme moieties (Eqs. 18, 19), where molecular oxygen is reduced to superoxide. The specific mechanism of superoxide generation is still unknown. According to one hypothesis, the reduced (Fe2+) heme moiety binds oxygen to form a ferrous/oxy species that decays to ferric heme and superoxide (Eq. 20) (245, 980). Routes that do not involve the formation of a stable heme/oxy complex are also possible (Eq. 21) (456). Notably, the reaction of NOX1–3 and 5 generates superoxide, whereas NOX4 and DUOX1/2 produce hydrogen peroxide (26, 936). Existing evidence suggests that the primary species generated is superoxide in all isoforms, and the formation of hydrogen peroxide is mediated by the peroxidase-like domain in DUOX or by the dismutation of superoxide molecules in NOX4 (Eq. 22). The mechanism of NOX4 is still controversial, but elegant work by Takac et al. indicates that the E-loop (sequence between helices V and VI) of NOX4 shows notable differences with the other NOX isoforms (925). This loop in NOX4 seems to provide steric hindrance to slow down superoxide release, forcing two superoxide molecules to react with each other in a reaction that appears to be catalyzed, in part, by a histidine residue (925).
| (18) |
| (19) |
| (20a) |
| (20b) |
| (21) |
| (22) |
The architecture of the NOX/DUOX core subunits has been described in detail elsewhere (13, 79, 383, 546) (FIGURE 7). NOX core subunits 1–4 are structurally similar with a N-terminal, membrane domain and a C-terminal flavoprotein, dehydrogenase domain with high sequence homology to the ferredoxin/NADP reductase family (490). NOX5 has a similar architecture but has an additional N-terminal domain before the transmembrane domain with four EF hand motifs that are responsive to Ca2+. The reductase domain of NOX5 also includes a CaM-binding motif not found in the NOX1–4 proteins. When Ca2+ is bound, the EF hands fold over the reductase CaM element to relieve autoinhibition (63, 944). DUOX1 and 2 have a similar architecture to NOX5 and include an additional transmembrane domain connected to a putative extracellular peroxidase domain (262). The ability of this domain to act as a peroxidase is still under debate (638, 639).
FIGURE 7.
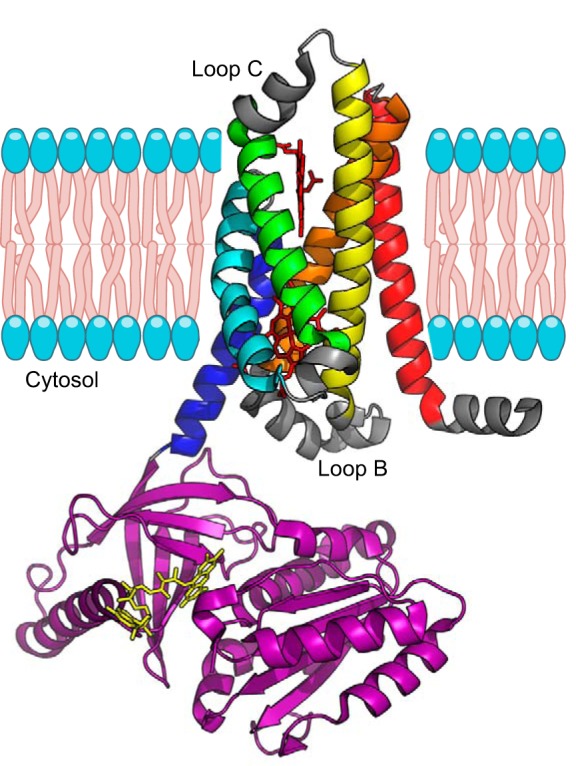
Structure of the NADPH oxidase (NOX) core protein membrane and cytosolic domains. The sequence comprises six transmembrane helices and a cytosolic flavoprotein/dehydrogenase domain. The transmembrane helices are indicated by colors: I, red; II, orange; III, yellow; IV, green; V, cyan; and VI, dark blue. The location of loops B and C is indicated. The dehydrogenase domain is shown in purple. The cofactors heme and FAD are shown as red and yellow sticks, respectively. Figure was drawn with PyMOL based on the structures for the transmembrane (PDB: 5O0T) and dehydrogenase (PDB: 5O0X) domains of NOX5 (613).
Recently, the crystal structure of a NOX core subunit (NOX5) has been determined (613). This structure is consistent with previous evidence suggesting the presence of six transmembrane α helices with five connecting loops (FIGURE 7) (613). The transmembrane domain includes four conserved histidines that serve as binding sites for two heme moieties bound between helices III and V (288, 613), and the flavoprotein domain binds the cofactor FAD and includes a binding site for NADPH (49, 480, 613, 980).
NOX isoforms are regulated by a large number of protein/protein interactions, and most isoforms are not functional without the assembly of the NOX protein with several additional proteins. NOX1–4 are associated to another membrane component, p22phox (24). Although there is no structural data available for p22phox, sequence analysis and computational models have been used to elaborate a consensus prediction of a three-helix transmembrane domain and a cytoplasmic C-terminal domain (637). Other models are consistent with the presence of two transmembrane helices instead of three (135, 451, 930).
In the human vasculature, the most relevant isoforms appear to be NOX1, NOX2, NOX4, and NOX5 (252, 253, 555). The relative architecture of these three proteins and their activator proteins are shown in FIGURE 8. NOX1 activation requires p22phox, NOX-organizer 1 (NOXO1), NOX-activator 1 (NOXA1), and the GTPase Rac1 (24, 108, 348). The activator protein p47phox can replace NOXO1 in a noncanonical mode (61). Recent studies indicate a possible role of EBP50 in NOX1 assembly (14). NOX2 activation requires p22phox, p47phox, p67phox, p40phox, and Rac1 (276). As described for NOX1, a noncanonical mode of activation can use NOXO1 instead of p47phox (927). The activation mechanisms of NOX4 are not completely understood. NOX4 appears to be constitutively active in the presence of p22phox without the participation of cytosolic proteins (24, 627). This, in part, can be explained by the constitutive activity of its dehydrogenase domain (694). However, a number of NOX4-interacting proteins have been recently identified. The protein polymerase delta–interacting protein 2 (poldip2) seems to exert regulatory effects on NOX4 (212, 607), although poldip2 is involved on many other pathways (316). The membrane protein Toll-like receptor 4 (TLR4) has been shown to increase NOX4 activity (86, 724), and Tks4/5, a homolog of NOXO1, also regulates NOX4 activity (231). Additional interactions with protein disulfide isomerase have also been reported (465). In addition, gene splicing also appears to modulate NOX4 activation and play important roles in cardiovascular disease (971).
FIGURE 8.
Putative assembly architecture of NADPH oxidase (NOX)1, NOX2, NOX4, and NOX5 and their regulatory proteins.
The regulation of NOX expression is modulated by a variety of agonist species and transcription factors. We will only highlight some of the main agonists for each isoform; a more detailed view is covered in recent reviews (79, 555, 737, 912).
1. NOX2
NOX2 is the main NOX isoform in neutrophils and macrophages where it mediates the oxidative burst (198, 845). Genetic defects in the main constituents of NOX2 can trigger an inability of the immune system cells to kill invading bacteria and fungi. This condition, characterized by an increased susceptibility to infections, is known as chronic granulomatous disease. Most mutations causing the disease (around 90%) are found in the membrane component NOX2 (also known historically as gp91phox) and the interacting protein p47phox. Mutations in p22phox, p67phox, and Rac2 have been also identified (428, 438).
NOX2 expression has been also reported in other cell types including endothelial cells and adventitial fibroblasts (376, 382, 657, 714, 715, 736, 876) (FIGURE 9, Table 5). Thus, the effects of NOX2 activity depend on different cell types and have important links to the immune response (392, 488). The current knowledge about NOX enzymes in the cardiovascular system indicates that NOX2 is usually the main player in the development of pathological conditions and increased NOX2 activity is linked to vascular disease (142, 252, 478).
FIGURE 9.
Cellular distribution of NADPH oxidase (NOX) species in the blood vessels. Predominant forms in each cell type are shown in bold.
Table 5.
Tissue distribution of NOX isoforms in the cardiovascular system
| Tissue | Cell Types | Cellular Location | References | |
|---|---|---|---|---|
| NOX1 | Heart | ECs | Plasma membrane | 9, 423, 431, 652, 876, 879, 909 |
| Blood vessels | VSMCs | Endosomes | ||
| Adventitial fibroblasts | Caveolae | |||
| Cardiomyocytes | ||||
| NOX2 | Heart | ECs | Plasma membrane | 9, 166, 376, 382, 427, 468, 473, 580, 657, 714, 715, 736, 742, 876, 950, 964 |
| Lung | VSMCs | Phagosomes | ||
| Blood vessels | Adventitial fibroblasts | Endoplasmic reticulum | ||
| Cardiomyocytes | ||||
| Hematopoietic stem cells | ||||
| Macrophages neutrophils, monocytes, dendritic cells | ||||
| NOX4 | Heart | ECs | Plasma membrane | 9, 10, 100, 118, 166, 179, 201, 228, 265, 347, 423, 431, 540, 541, 556, 736, 742, 876, 906, 964 |
| Lung | VSMCs | Focal adhesions | ||
| Blood vessels | Adventitial fibroblasts | Endoplasmic reticulum | ||
| Cardiomyocytes | Nucleus | |||
| Cardiac fibroblasts Hematopoietic stem cells | Mitochondria | |||
| NOX5 | Heart | ECs | Plasma membrane | 81, 166, 201, 396, 469, 494, 848 |
| Blood vessels | VSMCs | Endoplasmic reticulum | ||
| Cardiomyocytes | ||||
| Cardiac fibroblasts |
EC, endothelial cell; NOX, NADPH oxidase; VSMC, vascular smooth muscle cell.
In the vasculature, different effectors can increase NOX2 expression and activity (555, 737). A large number of cytokines and hormones have been shown to induce NOX2 expression, including angiotensin II (658, 714, 802), thrombin (725), and TNF-α (275, 964). Circulating lipids also increase NOX2 production in ECs in vitro (159, 208, 856, 1063). The role of endothelial NOX2 in the development of cardiovascular disease is controversial. Some studies have described that endothelial overexpression of NOX2 increases endothelial dysfunction (88, 676). However, in other cases, an increased expression of endothelial NOX2 has not been linked to pathological conditions even though an increased production of superoxide and macrophage recruitment was observed (243, 443).
Global KO studies in mice indicate a negative role of NOX2 in disease conditions. The deletion of the organizer subunit p47phox ameliorates the hypertensive response to angiotensin II, decreases endothelial superoxide formation and eNOS uncoupling, and improves survival after MI (238, 549, 550). The mice combining the mutation of p47phox−/− with ApoE−/− appear to be protected from atherosclerotic lesions (253) compared with ApoE−/− mice (64, 976), although some reports do not find significant differences in atherosclerosis progression or lesion size (443). It should be noted that the use of p47phox−/− as a surrogate for the NOX2 deletion is partly limited by the involvement of p47phox in other protein complexes, including not only the noncanonical activation of NOX1, as already mentioned above but also its interaction with protein disulfide isomerase (214, 559).
Other studies have directly targeted the NOX2 protein. Mouse models of NOX2 deletion have generally shown similar benefits as those using p47phox-deficient mice. The NOX2−/− mice are also protected against angiotensin II–dependent hypertension (151, 988). The combination of NOX2−/− with ApoE−/− can decrease the atherosclerotic lesions (475), but this is not always the case (508). Other works have found a positive effect of the NOX2 deletion in mice treated with high fat diet. In these studies, mice carrying the deletion gain weight but show no increase in ROS production or endothelial dysfunction as compared with the control animals (256, 609).
Finally, it is worth noting that the role of NOX2 in the immune system and in vascular endothelial cells will have, as noted, different impacts in cardiovascular disease (573). The relative contribution of these sources for the initiation and development of atherosclerotic lesions and other pathologies remains an issue of active research. The current paradigm indicates that macrophage NOX2 is more detrimental in the development of cardiovascular disease (395, 876), with endothelial NOX2 probably involved in the initiation, but not so much in the development of the disease, and a subsequent progress of the atherosclerotic lesion dependent on immune cells (243, 573, 976).
2. NOX1
The isoform NOX1 is expressed in VSMCs and in lower levels is also found in ECs (556, 909) (FIGURE 9, Table 5). NOX1 is found in caveolae and signalosomes of vascular cells (423, 431, 652). NOX1 expression is activated by several factors including angiotensin II, PDGF, and thrombin (382, 492, 630, 695, 999). The nature of the agonist can vary in different cellular compartments (651, 652, 1055).
Increased NOX1 activity is associated with vascular disease, and studies have shown links between high NOX1 levels and hypertension (1015), diabetes (163, 380, 1003), ischemia/reperfusion injury (479), and coronary artery disease (393). NOX1-dependent signaling has been linked to angiogenesis. For instance, knockdown of NOX1 can decrease angiogenesis (343), whereas increased NOX1 promotes tumor angiogenesis and growth (582). These findings indicate a critical role of NOX1-derived superoxide in angiogenesis signaling via PPARα-, AKT-, and ERK1/2-mediated pathways.
In agreement with these observations, the overexpression of NOX1 in VSMCs increases blood pressure and hypertrophy in mouse hypertension models (235). siRNA against NOX1 or NOXO1 decreased superoxide production and eNOS uncoupling in diabetic mice (1049). NOXA1 has been also associated with atherosclerotic injury, with overexpression of NOXA1 increasing ROS production and increased NOXA1 levels detected in atherosclerotic lesions (695).
Studies of NOX1 function have also taken advantage of the generation of several KO lines. Mice with deletions of NOX1 (345, 630), NOXO1 (511), and NOXA1 (293) have been developed. NOX1 deletion limits the blood pressure increase elicited by angiotensin II, although it is unclear if basal blood pressure levels are reduced (345, 346, 630).
The effect of NOX1 in atherosclerosis has been investigated similarly to NOX2, using a Nox1−/−ApoE−/− mouse model. The results of different studies are contradictory, with examples of improved outcomes for NOX1 deficient mice subjected to high fat diet (854) and diabetic mouse models (380), whereas in other cases, NOX1 deletion did not seem to have an effect (875).
The effects of the p47phox deletions, often used as a surrogate for the NOX2 deletion, can be partly due to changes in NOX1 activity. This notion is supported by a diminished effect of angiotensin II in the p47phox KO (549, 550) as compared with NOX1 (345, 630) or NOX2 deficient models (988).
Altogether, the role of NOX1 in cardiovascular disease seems secondary to NOX2, but still significant, as shown in several models (345, 380, 630, 854). It is important to consider that therapies focused on NOX2 can overlook compensatory effects of NOX1 in part by the sharing of a number of regulatory elements that can activate the production of superoxide in both proteins.
3. NOX4
NOX4 is the most abundant isoform in ECs, and it is also present in VSMCs and adventitial fibroblasts (10, 265, 736, 832, 876, 1036). In the heart, NOX4 has been identified in ECs, cardiomyocytes, and fibroblasts (682, 1058). The intracellular location of NOX4 suggests a role in signaling and may be involved in endoplasmic reticulum stress (817) (FIGURE 9, Table 5). The expression of NOX4 is upregulated by TGF-β (201, 906). Other regulators include TNF-α (70, 681, 884, 1048), hypoxia (455), and PDGF (487).
The role of NOX4 in vascular biology appears to be generally protective. Endothelial-targeted overexpression of NOX4 has beneficial effects and decreases blood pressure (779). Existing evidence supports a role of NOX4 in maintaining blood pressure levels (552, 723, 779, 832).
Endothelial-specific overexpression of NOX4 in mice promotes angiogenesis and eNOS function (193) and decreases blood pressure (779). Comparable results were achieved with NOX4 knockdown studies in cells (642). A beneficial effect of NOX4 in ischemia/reperfusion has also been observed (989).
Studies with NOX4−/− mice indicate protection from endothelial dysfunction in wild-type mice compared with the NOX4 deletion (832). In general, NOX4 shows protective effects in ApoE−/− mice (194, 229, 230, 379, 552, 838). One study has related NOX4 to hyperglycemia and insulin resistance in mice (1012). However, NOX4 deletion in the ApoE−/− mouse does not ameliorate diabetes-associated vasculopathy (380). Other studies have found no change in NOX4 levels in diabetic rat models (1003). Heart-specific NOX4 overexpression has protective effects in the response to chronic load stress in part due to increased HIF1α response and increased angiogenesis (1058). It should be noted that some aspects of NOX4 function in the cardiovascular system remain controversial. For instance, splicing of NOX4 appears to influence cardiovascular disease, with a shorter protein (NOX4D) exerting beneficial signaling functions in the nucleus, whereas full-length NOX4 is overexpressed in ischemia with detrimental effects (971).
NOX4 protective role may be due to its generation of hydrogen peroxide, and not superoxide (253). As hydrogen peroxide does not scavenge NO, changes in NOX4 activity do not directly impact NO levels. The signaling mechanisms of hydrogen peroxide in ECs (941) lead to the increased activity of eNOS and other enzymes (121, 141, 193, 251, 815, 940). Consistent with this role of NOX4-derived ROS in angiogenesis (642, 989), it has been shown that NOX4 can modulate the role of VEGF-induced dimerization of CD146 necessary for cell adhesion (1065), and NOX4-derived ROS can induce the reversible glutathionylation of SERCA and increase Ca2+ levels, thus regulating VEGF-signaling cell migration (273).
In a final note, it should be also considered that although NOX4 activity has been shown to reduce fibrosis in atherosclerosis (230), it can also promote fibrosis, particularly in the lung, but also in kidney and other tissues (110, 201, 416, 596, 820). The basis of these differences in NOX4 roles are not completely understood, but could be based on the specific roles of NOX4 in different cell types and/or detrimental effect of eNOS activation in conditions in which eNOS is uncoupled (323).
4. NOX5
NOX5 is the last discovered NOX isoform (62). In human blood vessels, NOX5 is expressed in ECs and VSMCs (81, 469) (FIGURE 9, Table 5).
NOX5 expression can be activated by most of the factors that activate other vascular NOX enzymes such as angiotensin II, TNF-α, PDGF, thrombin, and endothelin-1 (81, 660, 681). As indicated above, the particular architecture of NOX5 indicates a regulation of its activity through Ca2+ levels (62, 469). Phosphorylation of Ser475, Ser490, Thr494, and Ser498 within the flavoprotein domain increases Ca2+ sensitivity (463, 717, 718); this phosphorylation is mediated by different kinases, including PKC isoforms (164).
Unlike the rest of the NOX proteins, NOX5 does not have an homolog in rodents, which has hampered the research of this isoform (871). New approaches, such as mice strains incorporating the human NOX5 gene, may provide new information about NOX5 function (439, 517).
Despite these difficulties, increasing data suggest a role of NOX5 in cardiovascular disease. Higher NOX5 expression levels have been observed in patients with coronary artery disease (391), but causation has not been proven. NOX5 expression also increases in the human heart after MI (396). Expression of NOX5 in VSMCs and in macrophages and monocytes can contribute to the development of atherosclerotic lesions (622, 623). NOX5 also appears to modulate proliferation and cell migration processes (371, 719). Altogether, increased NOX5 activity seems to be involved in a pathological response (661). Further work is required to delineate the role of NOX5 in human cardiovascular disease.
5. Pharmacological regulation of NOX enzymes
A plethora of NOX inhibitors, including small molecules and peptides, have been developed (22). Most of the inhibitors have shown poor specificity between isoforms, hampering the progress in the field (174). In this section, we will present some of the most relevant inhibitors because of their wide use or high isoform specificity. A more comprehensive analysis of the available inhibitors for NOX enzymes can be found in recent reviews (22, 174, 175, 252).
a) diphenylene iodonium.
Diphenylene iodonium (DPI) (FIGURE 10) is a nonspecific inhibitor of all NOX isoforms (18, 197, 246). Its mechanism of action is based on the reaction with the FAD moiety of the NOX dehydrogenase domain (699). Unfortunately, this reaction is general for any flavin-containing protein, which means that DPI is also an inhibitor of many other ROS-producing enzymes, including, but not limited to, NOS, mitochondrial complex I and XO (18). Some reports indicate that the use of low levels of DPI may selectively target NOX in the presence of other flavoproteins (241); however, the lack of specificity makes unlikely the general use of DPI as a NOX inhibitor in vivo.
FIGURE 10.
Chemical structures of selected NADPH oxidase (NOX) inhibitors.
b) apocynin.
Apocynin (FIGURE 10) is a methoxy-substituted catechol generally considered to be a specific NOX2 isoform inhibitor. Apocynin inhibits NOX2 by reacting with cysteine residues in p47phox that appear to be necessary for enzyme assembly (894). It should be noted that this mechanism of action converts apocynin into a NOX1 inhibitor when NOX1 uses p47phox for activation in the noncanonical mode. Moreover, there are several factors that question the inhibitory effects of apocynin; notably, apocynin is an antioxidant and scavenger of ROS like HOCl and hydrogen peroxide (426, 735). Thus, although apocynin remains widely used in research and has led to important insights in the function of NOX enzymes (237, 413, 581), the results of experiments with apocynin need be considered with caution. In particular, effects in isoforms other than NOX1 and NOX2 should be validated with other approaches.
c) nox2 docking sequence-tat.
NOX2 docking sequence-tat (NOX2ds-tat), the first isoform-specific NOX inhibitor, is a peptide based on the sequence of the B-loop of NOX2 (199, 218, 782). NOX2ds blocks specifically the interaction between NOX2 and p47phox (199). The addition of the tat sequence (nine amino acids from HIV-tat) confers membrane penetration ability to the peptide (782). The inhibitor shows high specificity toward NOX2 (199). The chemical nature of the compound makes oral delivery unsuitable and has limited its pharmacological application, however, because of its isoform specificity and lack of off-target effects remains widely used in research (244, 461, 476, 762, 782, 910). Notably, a similar approach with NOX4 did not result in significant inhibition of NOX4 (200), which suggests a strong interaction between dehydrogenase and transmembrane domains in NOX4 and points out a critical function of the B-loop in this interaction (460), very probably linked to the constitutive activity of this NOX isoform.
d) noxa1 docking sequence.
NOXA1 docking sequence (NOXA1ds) is a peptide developed to block the interaction between NOX1 and NOXA1. The sequence contains 11 amino acids from the putative binding region of NOXA1 to NOX1. The selected sequence shows low similarity with p67phox to prevent cross reactivity with NOX2. The peptide prevents NOX1 assembly and does not inhibit NOX2, NOX4, or NOX5 as tested in in vitro assays (772, 792).
e) ml171.
ML171 (2-acetyl phenothiazine) (FIGURE 10) is one of the few small-molecule, NOX isoform–specific inhibitors reported to date. ML171 inhibits NOX1 at nanomolar concentrations, with IC50 values at least 20-fold higher for NOX2, NOX3, NOX4, and XO (359). The mechanism of action of ML171 seems to involve only interaction with the NOX1 protein and not with its activating subunits. ML171 showed off-target effects with the inhibition of serotonin and adrenergic receptors in the micromolar range (359). The study of differently substituted phenothiazines indicates some isoform selectivity that can represent a promising pathway to novel small-molecule, selective NOX inhibitors (847).
f) vas2870 and vas3947.
VAS2870 and VAS3947 (FIGURE 10) are recently described NOX inhibitors based on a triazolopyrimidine scaffold (892, 934, 1016). VAS2870 appears to inhibit all isoforms, with proven inhibitory effects at least for NOX1, NOX2, NOX4, and NOX5 isoforms (21, 520). The mode of action of these compounds is unknown, although for NOX2, they appear to block complex assembly (21). These inhibitors do not have a noticeable effect on XO- or eNOS-dependent ROS production, so they can be considered specific pan-NOX inhibitors. VAS3947, a more soluble derivative of VAS2870, shows similar inhibitory properties (1016). Off-target effects for VAS2870, namely nonspecific alkylation of thiols, have been reported (915).
g) gkt-136901 and gkt-137831.
GKT-136901 and GKT-137831 are pyrazolopyridines structurally related to VAS2870 and VAS3947. GKT-136901 and GKT-137831 (FIGURE 10) are the first inhibitors suitable for oral administration, and currently GKT-137831 is in phase 2 clinical trials (472). GKT-136901 was first characterized as a selective NOX1 and NOX4 inhibitor, although it can also inhibit NOX 2 at higher concentrations (545). Inhibitory effects against NOX5 have also been documented (680). No off-target effects were detected against a panel of 135 enzymes, including XO and eNOS (545). GKT-137831 shows similar inhibitory properties with potent inhibition of NOX1 and NOX4 and inhibitory effects on NOX2 and NOX5 at higher concentrations (35). The basis of the inhibition by these compounds is not known, although the activity of GKT-136901 as peroxynitrite scavengers has been recently reported (826).
h) gsk2795039.
The discovery of a novel, specific NOX2 inhibitor was recently reported (435). GSK2795039 (FIGURE 10) appears to be specific toward NOX2 and does not show off-target effects toward eNOS or XO. Existing evidence suggests that GSK2795039 inhibits NOX2 through competition with NADPH for the binding site of the NOX2 dehydrogenase domain (435).
B. Uncoupling of Endothelial NOS
NO production is critical for vascular function as described in previous sections (299, 573, 600, 786). In conditions in which the substrates and cofactors are present in saturating concentrations, NOS enzymes catalyze the synthesis of NO from molecular oxygen and l-arginine with high efficiency. The electrons transferred from NADPH are almost quantitatively used to catalyze l-arginine oxidation, and the ratio of NADPH to l-citrulline is close to the optimal value of 1.5 molecules of NADPH per l-citrulline (and NO) generated (3, 590). However, a number of factors can alter the electron transfer in NOS so the amount of NADPH required to generate NO becomes much larger. This is largely due to the reduction of other substrates, usually molecular oxygen, to generate superoxide. This situation when the consumption of NADPH is not correlated with a stoichiometric production of NO is generally termed eNOS uncoupling (488, 570, 870). Although the superoxide generation is the common manifestation of this NOS dysfunctional state, it is important to recognize and understand the causes of eNOS uncoupling to develop adequate interventions. In addition, a number of situations impinge NOS function and NO generation, but not necessarily cause increased superoxide production, despite being usually characterized as uncoupling. Factors that can trigger eNOS uncoupling are discussed below.
Initial reports indicated that nNOS had the ability to produce superoxide in a reaction dependent on NADPH and Ca2+/CaM (750). The effect of NOS inhibitors on this process was diverse, with some inhibitors efficiently suppressing superoxide production (e.g., l-NAME), whereas others had little effect (e.g., NG-monomethyl-l-arginine) (749). The reaction is observed in the three NOS isoforms (750, 973, 1034). The effect of cofactors and NOS inhibitors was thoroughly investigated (150, 250, 590, 749, 973, 1033). Superoxide can be generated by the oxygenase/heme domain, in a Ca2+/CaM-dependent way, or independently by the reductase domain from Ca2+/CaM (653, 1032). In conditions that promote uncoupling, the heme group is usually the main generator of superoxide species through the formation of a ferrous/oxy complex that decays to ferric heme and superoxide (Eq. 20) (FIGURE 11) (250, 901, 973, 1033).
FIGURE 11.
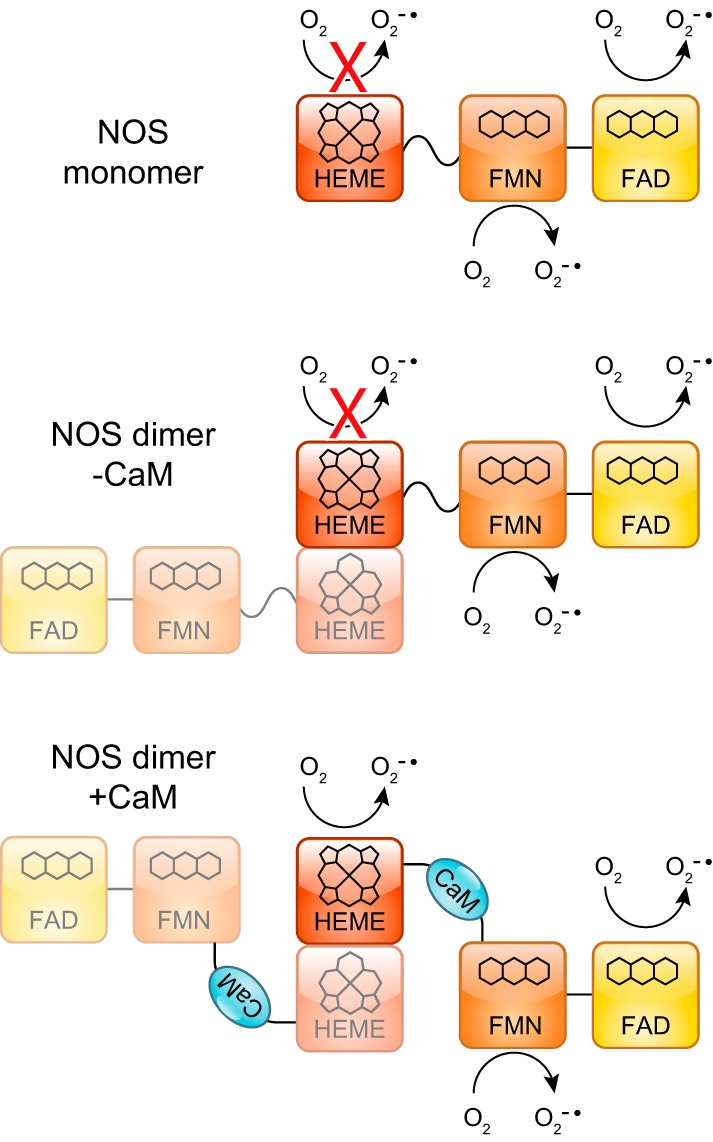
Superoxide generation by nitric oxide synthase (NOS) monomer and dimer in the presence or absence of calmodulin (CaM).
The loss of the dimer structure renders eNOS unable to produce NO, as the heme group of one monomer must receive electrons from the FMN domain of the other monomer. Thus, conditions that increase the monomer/dimer ratio decrease NO output and can increase superoxide production through the reductase domain but also decrease superoxide formation in the oxygenase domain (FIGURE 11). Therefore, it should be noted that measurement of dimer/monomer ratios as an indication of eNOS uncoupling is not a reliable and reproducible method, and more specific methods monitoring posttranslational modifications and substrate levels are preferable.
The concomitant formation of NO and superoxide can be especially deleterious as both radicals can combine to form peroxynitrite at diffusion-limited rates. Peroxynitrite is a strong oxidant and nitrosating species (78). Therefore, the superoxide generated by the uncoupled eNOS not only induces toxic effects on its own but 1) acts as a NO scavenger, decreasing NO availability and 2) generates peroxynitrite, a more harmful species (78). Peroxynitrite can in turn oxidize BH4 to BH2, further promoting eNOS uncoupling (543). Notably, the deleterious effect of superoxide on the endothelium relaxing effect of NO was discovered even before the chemical nature of NO as endothelium-derived relaxing factor was elucidated (385). In the following sections, we dissect the different causes of NOS uncoupling and overview the current status of pharmacological approaches to prevent or reverse this dysfunctional state. Special emphasis is dedicated to the two most relevant aspects in pathophysiology, namely the dysregulation of BH4 and l-arginine levels.
1. BH4/BH2
The binding of the cofactor tetrahydrobiopterin (BH4) is a requisite for NO synthesis by NOS enzymes. BH4 binding synergistically enhances the affinity toward the substrate l-arginine. In addition, BH4 plays a critical role in the catalytic cycle of NOS (87, 204, 377, 904, 933, 1000, 1007). The ability of BH4 to support electron delivery to the heme group at critical steps, generating a transient radical species, is specific to BH4. Other pterins are, in general, unable to support NO synthesis in mammalian NOS enzymes, or can only support a NO synthesis rate much slower than BH4 (753). BH4 can be oxidized in vivo by molecular oxygen or ROS to generate dihydrobiopterin (BH2), a pterin very structurally similar to BH4 but unable to support NO synthesis (544, 753, 933).
Biological de novo synthesis of BH4 is mediated by three enzymes that catalyze the stepwise process from GTP to BH4, namely GTP cyclohydrolase I (GCH1), pyruvoyl tetrahydropterin synthase (PTPS), and sepiapterin reductase. In this route, the reaction of GTP with GCH1 is the limiting step. An additional salvage pathway can produce BH4 from sepiapterin and/or BH2. In this pathway, sepiapterin reductase reduces sepiapterin to BH2, whereas dihydrofolate reductase can reduce BH2 to BH4 (87, 1007).
The conversion of BH4 to BH2 in the setting of endothelial dysfunction has been widely reported (192, 258, 550). The accumulation of BH2 becomes deleterious as the binding site of BH4 in NOS can also bind this pterin. This is particularly problematic in the case of eNOS (190). iNOS and nNOS show higher affinity toward BH4 than for BH2 (around 10-fold in the absence of l-arginine) (6, 514, 753); however, eNOS shows almost identical binding affinities for BH4 and BH2, and it is thus very sensitive to imbalances in the concentrations of BH4 and BH2 (190). Thus, rather than the relative amount of BH2, the BH4/BH2 ratio gives a more precise estimation of the endothelial function (190, 192, 974).
Mechanistically, when NOS binds BH2 instead of BH4, the ferrous/oxy heme cannot hydroxylate l-arginine to NG-hydroxy-l-arginine, NO synthesis is blocked, and the oxygen is eventually oxidized to superoxide. Thus, not only is the NO synthesis completely abolished but the protein also becomes an oxidase, generating superoxide instead of NO (191, 972, 1007) (Table 6).
Table 6.
Relative effect of different factors on eNOS superoxide and NO production
| Superoxide Production | Pterin | Substrate | Other | NOSynthesis |
|---|---|---|---|---|
| +++ | None | l-arginine | None | |
| +++ | None | ADMA | None | |
| +++ | None | None | None | |
| ++ | BH2 | l-arginine | None | |
| ++ | BH2 | None | None | |
| + | BH4 | l-arginine | S1177p | + |
| = | BH4 | l-arginine | = | |
| = | BH4 | l-arginine | T495p | – |
2. l-Arginine/ADMA
In saturating conditions of the NOS cofactors, the synthesis of NO only requires l-Arginine and O2 as substrates (904). l-Arginine is also important for the dimerization of NOS (51, 515) and increases BH4 binding affinity (514, 753). The absence of l-arginine leads to the production of superoxide in an otherwise active form of NOS; thus, l-arginine availability can have an important effect on eNOS uncoupling (Table 6). Given the concentration of l-arginine in ECs [0.1–0.8 mM (1030)] and the high affinity of NOS enzymes toward l-arginine [eNOS KM = 2.9 μM (746)], it was long thought that l-arginine availability would not be a functional issue for NOS in vivo. However, in recent years, it has become clear that extracellular l-arginine and ADMA concentrations, compartmentalization, and membrane transporters are important for NOS function, as will be discussed below in sect. IIIB4 (167, 668, 1030).
ADMA (FIGURE 3) is mainly produced from the breakdown of proteins containing methylated arginine residues. The same processes also yield the methyl arginines NG-methyl monoarginine (FIGURE 3) and symmetric dimethylarginine (FIGURE 3) (668). Notably, ADMA and NG-monomethyl-l-arginine (but not symmetric dimethylarginine) are NOS inhibitors and can be important regulators of NOS activity in vivo (566, 963). The metabolism of ADMA and its effects on cardiovascular disease have been covered by recent reviews (668, 790, 962). Soon after its discovery in cells and tissue, ADMA was recognized as an important marker for cardiovascular disease progression (656, 829, 961, 1011, 1053, 1068). More recent observations indicate that the ADMA/l-arginine ratios in plasma may be a better marker than either metabolite assessed independently (75, 589, 606, 790, 966).
3. Other causes of eNOS uncoupling
a) disruption of the zn/s cluster.
The dimeric structure of NOS is held together by interactions between the oxygenase domains, and among these interactions, the formation of a Zn/4S cluster with the participation of two cysteines from each NOS monomer (195). The oxidation of the cysteine residues involved in the formation of the Zn/S cluster disrupts the dimer structure. Work by Zou et al. indicated that this cluster is particularly sensitive to peroxynitrite (1070). This observation is not unexpected, as metal-thiol bonds are some of the main targets of peroxynitrite (78, 526).
The breakdown of the Zn/S cluster is, in most cases, secondary to eNOS uncoupling. As discussed above, the monomeric eNOS cannot catalyze the NO synthesis reactions, and the monomerization renders the heme group inactive. The superoxide formation from the monomer is low as compared with the uncoupled dimer, and thus, this mechanism does not increase the rate of superoxide formation. However, the breakdown of the Zn/S cluster is a marker that indicates the generation of peroxynitrite in the cellular environment. Although this is not a 100% specific marker of eNOS uncoupling (the superoxide source may be different from NOS, for example, a NOX enzyme), the correlation of the Zn/S cluster breakdown and eNOS uncoupling is probably elevated (Table 6, FIGURE 12).
FIGURE 12.
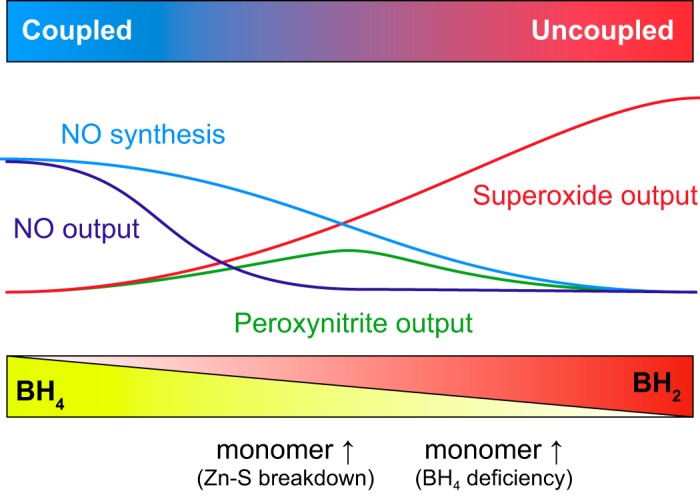
Progression of nitric oxide (NO) synthesis, superoxide, and peroxynitrite during the development of endothelial nitric oxide synthase (eNOS) uncoupling.
Another modification that disrupts the Zn/S cluster is S-nitrosation of the cysteine residues Cys96 and Cys101, as discussed in sect. IIA1.
b) glutathionylation of cysteine residues.
Recent reports indicate that eNOS can be modified posttranslationally by S-glutathionylation (162). At least three residues have been shown to be susceptible of modification: Cys 689, Cys908 (162), and Cys382 (160) (Table 2). The process can be reversed by glutaredoxin-1 (160). The glutathionylation of Cys689 and Cys908 causes eNOS uncoupling. These residues, located in the reductase domain, appear to disturb the electron transfer between FAD and FMN cofactors, and indirectly limit the rate of reduction of the oxygenase domain. Consistent with this hypothesis, the addition of the inhibitor l-NAME decreases superoxide production only in part, indicating that most of the superoxide production occurs in the reductase domain via electron transfer from the reduced FAD and FMN to molecular oxygen (162) (Table 6, Figs. 11, 12).
S-glutathionylation has been involved in the development of angiotensin II–mediated endothelial dysfunction (328) and nitroglycerin resistance (522). Both processes appear to be initiated by mitochondrial ROS. Consistent with this view, the increase in ROS levels, BH4 depletion, and glutathione oxidation, with subsequent eNOS S-glutathionylation, appears to be intimately related (189, 215).
Along with glutaredoxin-1, thioredoxin has also been proposed to deglutathionylate eNOS, and this process is independent of glutathione oxidation (907). This offers therapeutical opportunities in oxidative stress situations in which glutathione levels are low (432).
c) phosphorylation.
Phosphorylation of Thr495 is a negative regulator of CaM binding to eNOS (297, 407, 646). The Thr495-phosphorylated NOS shows decreased electron transfer from the reductase domain to the heme domain and decreased NO synthesis (588). The accumulation of electrons in the reductase domain causes an increased uncoupling from the reductase domain, which, as discussed previously, is less pronounced than when this occurs in the heme but can cause significant production of superoxide (Table 6).
4. Pharmacological interventions in eNOS uncoupling
The sensitivity of the eNOS system to oxidative stress converts eNOS into a main target in conditions of ROS imbalance. According to the kindling hypothesis of endothelial dysfunction, the initial formation of ROS by other sources can promote uncoupling of NOS, amplifying the initial excess of ROS (488). The uncoupling causes eNOS activity to change from healthy (producing NO) to toxic (generating superoxide) (303). As such, the uncoupling of eNOS has been noted as a target for therapeutic potential (488, 569, 570, 600, 784). Different interventions have been developed to address this misdirected reactivity. As the status of eNOS uncoupling changes with the severity of the cause, different treatments may prove more efficacious at different stages (FIGURE 12). Current strategies are discussed below.
a) bh4.
Many studies have shown that BH4 levels correlate with eNOS activity (796, 1006). Conversely, low levels of BH4 are found in endothelial dysfunction and cardiovascular disease (421, 429, 440, 550, 560, 858, 899). Spearheaded by these observations, interventions to reverse eNOS uncoupling via BH4 supplementation have been numerous, and in general, the supplementation of BH4 or positive modifications in the BH4 synthesis pathways have been positive. A large number of studies on mice and other animals have been described; for the sake of conciseness, we will only discuss some of the recent human studies. For a more complete overview of the BH4 supplementation experiments including studies in other organisms, the reader is referred to recent reviews (87, 828).
Acute supplementation of BH4, either orally or injected, generally improves vasodilatation. This effect has been observed in a number of human studies, including healthy individuals (450, 743, 987) and patients with hypertension (429), hypercholesterolemia (318, 899, 1031), chronic heart failure (849), coronary artery disease (618, 850), heart failure with reduced ejection fraction (178), or rheumatoid arthritis (619) or smokers (418, 929, 957). Studies, including the assessment of coronary artery function in patients with atherosclerosis, did not find an improvement after BH4 treatment (1028).
Several studies monitoring endothelial function after chronic BH4 administration have been conducted. In hypercholesterolemia patients, oral BH4 for 4 wk (400 mg twice daily) improved endothelial function (186). A treatment with oral BH4 for 2–6 wk in patients with coronary artery disease did not show improvement; and actually increased BH2 levels (202). In patients with hypertension, a 4–8-wk, 400 mg/day treatment was able to decrease blood pressure (747). Studies on pulmonary hypertension also show improvement in the 6-min walking distance (788). A recent study using 400 mg daily for 1 wk improved endothelial function in rheumatoid arthritis patients (619).
In most examples, BH4 is able to improve endothelial function, but the study by Cunnington et al. (202) clearly indicated that in some conditions, BH4 supplementation is not a practical therapeutic approach. In severe oxidative stress conditions, the BH4 will be rapidly oxidized to BH2, further increasing the BH2/BH4 ratio (FIGURE 12). As the treatment with antioxidants has been shown to improve endothelial function in certain conditions (56, 419, 420, 422, 572), it is possible that the coadministration of BH4 with ascorbic acid or other antioxidants can circumvent these issues. Of note, BH4 is also an antioxidant, and it is not clear if some of the observed effects at the supraphysiological concentrations used are just due to short-term antioxidant effects; related pterins, such as folate, can also produce similar effects (32, 33, 860). Techniques that can assess the oxidative stress conditions in tissues can certainly improve the use of BH4 therapy with a more personalized approach. Pleiotropic effects of some drugs can improve BH4 availability; this has been observed for statins such as atorvastatin (31).
As an alternative to BH4, supplementation using other related compounds such as sepiapterin has been also used. Sepiapterin can be converted into BH4 via the salvage pathway by sepiapterin reductase and dihydrofolate reductase as described in the previous sections. Sepiapterin supplementation improves endothelial function in several mouse models, including the atherosclerosis model ApoE−/− mice (560), diabetes models (721), or obesity models (527). Mouse GCH1 KO mice show abolished endothelial NO synthesis and increased superoxide production that can be reversed by sepiapterin supplementation (173). It should be noted that this pathway has BH2 as an intermediate, which could lead to detrimental effects. In conditions in which dihydrofolate reductase is inactive or absent, the recovery of eNOS coupling by sepiapterin supplementation is ineffective (452).
Folic acid has wide effects on the metabolism, impacting cysteine/homocysteine metabolism, but also pathways involving tetrahydrofolate and dihydrofolate and hence BH4 biosynthesis (598). The effects on eNOS uncoupling seem related to the antioxidant properties of the folic acid metabolites (900). Supplementation of folic acid (5-methyltetrahydrofolate) shows positive effects on eNOS function (900, 978) and has been shown to prevent nitroglycerin tolerance in short-term human studies (375). Folic acid supplementation studies in human blood vessel samples have shown increases in BH4 levels and BH4/BH2 ratios (33).
To improve endogenous BH4 synthesis, efforts have focused in GCH1, the rate limiting enzyme during de novo BH4 synthesis (143). Overexpression of GCH1 in cells and mouse models improves eNOS function (19, 143). Human variants of GCH1 show measurable effects in blood pressure, indicating the direct relationship between GCH1 and eNOS function (1057).
b) l-arginine.
Several lines of evidence indicate that the availability of l-arginine may be jeopardized under endothelial dysfunction, and the concentrations of inhibitory l-arginine metabolites, such as ADMA, can also increase in pathological conditions (107, 1053). In this regard, different studies have investigated alternatives to improve l-arginine supply either via direct l-arginine supplementation, the use of l-arginine precursors, or through the inactivation of l-arginine consuming arginases.
The direct supplementation of l-arginine was shown to increase NO production in cells. This finding was rather surprising, as most cells have intracellular l-arginine concentrations manyfold higher than the eNOS KM value, and thus, this observation has been termed the “arginine paradox” (12, 797). Further studies showed that l-arginine import via the CAT1 transporter is critical for eNOS activity (167, 634). A membrane metabolon including arginine succinate lyase, argininosuccinate synthetase, hsp90, and eNOS associates in the caveolae and is necessary for regular eNOS function (270, 294, 634). Another factor contributing to the arginine paradox, as will be discussed below, is the concentration of endogenous eNOS inhibitors, mainly ADMA. At high ADMA concentrations, l-arginine supplementation may not overcome eNOS inhibition, indicating the need to determine l-arginine/ADMA ratios to better assess l-arginine availability (102, 106, 954).
In animal models of MI, l-arginine supplementation shows mixed results. Some studies show improvement in functional recovery (712), whereas studies in l-arginine–treated dogs show detrimental outcomes (665). Further studies have explored l-arginine supplementation in human patients. Again, the results have been heterogeneous. The causes of this variability are still unclear. Many studies have reported a lack of effect on l-arginine in endothelial function markers, and on occasion, the supplementation of l-arginine has been associated with higher mortality effects, such as tests of l-arginine in patients after MI (835). Other studies show impaired recovery in peripheral artery disease patients (1014). In contrast, most studies detect decreases in blood pressure with supplementation. A recent meta-analysis indicates that doses between 4 and 24 g/day decrease blood pressure (systolic, 5 mmHg; diastolic, 3 mmHg) (240). Other recent studies show improved endothelial function and insulin sensitivity in patients with impaired glucose uptake and metabolic syndrome (6.6 g/day l-arginine for 2 wk) (663) and improved endothelial function and decreased homocysteine levels in patients with elevated blood pressure and hyperhomocysteinuria (2.4 g/day for 4 wk) (781).
Altogether, it is not clear if the levels of l-arginine are the best target for intervention. In some cases high, l-arginine levels have even been correlated with higher incidence of ischemic heart disease (1045). It seems that the involvement of l-arginine in different metabolic pathways makes the use of l-arginine levels as a biomarker a poor indicator of outcomes (668, 857, 1029). Studies of a panel of l-arginine metabolites indicate some correlations, particularly for ADMA, but also the need to consider other factors such as age, sex, and body mass index (75, 771). The interaction of l-arginine levels with ADMA and other metabolites is the focus of current studies. As pointed out above, the ratio of l-arginine to ADMA appears to be a better indicator of cardiovascular risk (28, 103, 104, 606, 1050). It has been noted that when ADMA levels are low, the effects of exogenous l-arginine are limited; however, the therapy seems to be more beneficial in patients with higher ADMA levels, in which the treatment increases the l-arginine/ADMA ratios and improves endothelial function (103).
Other l-arginine–related species may allow for interventions of l-arginine levels. For example, l-citrulline has also been used to increase l-arginine levels (842, 1029). This supplementation can avoid the formation of detrimental high ADMA levels (632). The studies of l-citrulline in humans show mixed results. In some cases, l-citrulline increased NO synthesis, but no changes in blood pressure were observed (506). Other studies have observed positive effects of l-citrulline including reduced blood pressure (20, 285, 286).
Finally, it must be noted that arginases play an important role in the regulation of l-arginine levels (144, 259, 734, 804). Exacerbated arginase activity can decrease l-arginine levels and cause eNOS uncoupling (91, 144, 667, 804, 1039). Thus, the use of arginase inhibitors is gaining increasing attention. Several human studies have used the arginase inhibitor nor-NG-hydroxy-l-arginine (FIGURE 3). In these cases, arginase inhibition showed protection against ischemia-reperfusion injury (530) and improved endothelial function in patients with coronary artery disease and diabetes (855) and in patients with hypercholesterolemia (529). Further studies in this field and the search for novel natural and synthetic arginase inhibitors are warranted (757).
C. Mitochondria
The mitochondrial electron transport chain (ETC) represents a physiologically significant generator of ROS within vascular cells. Mitochondria generate ATP through the transfer of electrons from ETC complexes I and II to complex IV, where oxygen binds and is reduced to water. Through electron transfer steps, protons are pumped from the matrix to the inner membrane space, establishing a proton-motive force that couples electron transfer to the phosphorylation of ADP to ATP at complex V (FIGURE 13). Although the majority of electrons entering the ETC ultimately reduce oxygen to water at complex IV, a small proportion (~2–11%) of electrons escape from the chain at complex I or III to generate superoxide (34, 115, 678). Although this superoxide, being a charged molecule, likely does not escape the mitochondrion, manganese superoxide dismutase (MnSOD) located within the mitochondria catalyzes the dismutation of superoxide to hydrogen peroxide, which can freely diffuse out of the organelle to mediate cellular signaling or contribute to oxidative stress.
FIGURE 13.
Mitochondrial electron transfer chain. Main sites of superoxide production are indicated.
1. Mechanisms of ROS production
Mitochondrial ROS production is regulated by a number of factors including oxygen levels, substrate availability, and mitochondrial morphology/dynamics, all of which ultimately modulate the redox state of the ETC. Complex I, a 1,000-kDa protein comprised of ~45 subunits, is the most significant site of superoxide generation within the ETC and produces superoxide by two distinct mechanisms depending on the reduction status of the ETC and strength of the proton-motive force across the inner mitochondrial membrane (FIGURE 13). A FMN center within the complex, which accepts electrons from NADH, serves as the entry point to the ETC. In conditions in which electron transport through the ETC is slow, this FMN can become reduced and react with oxygen, catalyzing superoxide production. This occurs in conditions of high NADH/NAD+ ratios, such as when cellular ATP demand is low or the ETC has sustained damage. In contrast, when electron supply is high through the ETC, coenzyme Q downstream of complex I becomes reduced. A high proton-motive force present in this condition can then push electrons in the reverse direction from coenzyme Q to the complex I FMN, generating superoxide by reverse electron transport. This type of ROS generation occurs when succinate, the substrate for complex II, is present in high concentration and is responsible in vivo for the ROS generated after ischemia upon reperfusion (171, 172).
Complex III also represents a site of superoxide production within the ETC, although the rate of generation at this site is significantly lower than by reverse electron transport through complex I. Complex III catalyzes the Q cycle in which electrons are accepted from ubiquinone to be used to reduce cytochrome c for subsequent transfer to complex IV (FIGURE 13). In this process, ubiquinol (QH2) binds to the Q0 site of complex III and undergoes a one-electron oxidation that produces an unstable semiquinone radical. Although in most cases this semiquinone is rapidly oxidized, its reaction with oxygen leads to superoxide production at this site (567, 678). Importantly, specific complex III inhibitors, such as Antimycin A, can stabilize this semiquinone radical, significantly enhancing superoxide production by complex III (678). Physiologically, it is unclear which factors stabilize this species to regulate superoxide production. However, a number of studies demonstrate that complex III–dependent ROS production is important in physiological signaling pathways, including hypoxic sensing (997).
Although complexes I and III are considered the predominant sources of ROS within the mitochondrion, it is important to note that mitochondrial enzymes beyond the ETC have been reported to generate ROS. Pyruvate dehydrogenase (887), α-ketoglutarate dehydrogenase (130, 887, 952), and monoamine oxygenase A and B (290) have all been demonstrated to serve as ROS sources. These sources, although lower in their ROS-generating capacity, potentially play a role in specific mitochondrial-dependent redox signaling pathways. Notably, other reactive species–producing enzymes have also been demonstrated to translocate into the mitochondrion in specific conditions. For example, eNOS has been shown to enter the mitochondrion of pulmonary smooth muscle cells upon stimulation with asymmetric dimethyl arginine or endothelin-1 (768, 916). Additionally, although controversial, NOX4 has been reported to be localized within the mitochondrion either constitutively or in specific pathological conditions (152, 306, 540).
2. Physiological role of mitochondrial ROS
Although it has been recognized since the 1960s that mitochondria produce ROS, the physiological role of this phenomenon remained unclear until decades later. Accumulating data now demonstrate that mitochondrial ROS both mediate essential vascular signaling pathways and can also contribute to the pathogenesis of cardiovascular disease. This section will provide an overview of what is known about the role of mitochondrial ROS in cardiovascular health and disease.
Early studies viewed mitochondrial ROS production as pathological and focused on the deleterious effects of mitochondrial superoxide production. Mice completely lacking MnSOD, leading to greater mitochondrial superoxide concentrations, died within weeks of birth (584), confirming the essentiality of mitochondrial superoxide scavenging. Further, mice partially or conditionally lacking MnSOD showed dilated cardiomyopathy characterized by left ventricle dilation, decreased wall thickness, and hypertrophy (584, 696). Notably, tissue from the left ventricle of heart failure patients showed an increase in superoxide production and a decrease in MnSOD protein levels and activity, consistent with a role for increased mitochondrial superoxide in the pathogenesis of heart failure (814). It is now evident that mitochondrial ROS production also plays a major role in the pathogenesis of MI. During myocardial ischemia, the ETC as well as the NADH and quinone pools become maximally reduced. Additionally, there is a significant buildup of succinate, the substrate for complex II, which further supports the reduction of the quinone pool. Together, these conditions promote reverse electron transfer upon reperfusion, which results in significant superoxide production from complex I (171). Pharmacological inhibitors of complex I, including classical inhibitors as well as NO donors and nitrite, decrease this reverse electron transfer–mediated ROS production, leading to attenuated infarct size and protection of myocardial function after ischemia/reperfusion in a number of animal models (discussed in the next section) (136, 165, 170, 864).
Beyond the heart, changes in mitochondrial redox status can also have detrimental effects in the vascular wall and circulating cells. Decreased MnSOD activity or increased mitochondrial superoxide generation causes endothelial dysfunction and impairs acetylcholine-dependent vasodilation, particularly with age (1005). This dysfunction occurs not only through the direct reaction of superoxide with NO but is also associated with the oxidative damage of mitochondrial DNA and proteins. Notably, specific polymorphisms in the gene for MnSOD have been established as an independent risk factor for coronary artery disease (317).
Importantly, although mitochondrial ROS can propagate cellular damage, low physiological concentrations of mitochondrial oxidants have been shown to mediate a number of essential cellular signaling pathways (290). For example, mitochondria have been proposed to serve as a cellular oxygen sensor, such that in hypoxic conditions, mitochondrial ROS production by complex III leads to the stabilization of HIF1α and downstream hypoxic adaptation (84, 997). A similar pathway exists in the pulmonary vasculature in which mitochondria-generated oxidants signal the conserved phenomenon of hypoxic vasoconstriction (995). On a cellular level, mitochondrial ROS have also been shown to be required for the differentiation of some hematopoietic stem cells as well as human mesenchymal stem cells (707, 948). Additionally, mitochondrial ROS have been implicated in pathways leading to cell proliferation and senescence (233, 1046) as well as in the activation of other major cell signaling molecules such as the MAP kinase JNK and NF-κB (330, 689).
Notably, mitochondrial ROS modulate mitochondrial function, number, and cellular metabolic balance through a number of mechanisms. Mitochondrial hydrogen peroxide can oxidize the catalytic subunit of AMP kinase, leading to its autophosphorylation and subsequent activation to modulate metabolism within the cell (481, 1067). Although activation of AMP kinase can lead to mitochondrial biogenesis and increased mitochondrial number, this pathway can also directly be stimulated by mitochondrial hydrogen peroxide-dependent activation of the MAP kinase Akt (911, 919). As a counterbalance, mitochondrial ROS have also been shown to induce organellar degradation (e.g., mitophagy) under specific conditions (810). Through these mechanisms, mitochondrial ROS potentially play a role as a mediator in elaborate feedback pathways to regulate mitochondrial energetics and redox signaling.
The role of mitochondrial oxidants in the pathogenesis of some disease processes remains controversial. For example, two competing hypotheses have been proposed for the role of mitochondrial ROS in the pathogenesis of pulmonary arterial hypertension. Studies by Schumacker and colleagues suggest that increases in pulmonary mitochondrial superoxide production leads to the release of intracellular Ca2+ stores and subsequent vasoconstriction (996). To the contrary, Archer and colleagues demonstrate in animal models of pulmonary arterial hypertension that mitochondrial ROS production in the pulmonary artery smooth muscle cells is decreased, leading to inhibition of the KV1.5 channel, which ultimately results in membrane depolarization, intracellular Ca2+ increases, and vasoconstriction (645). Although the role of mitochondrial ROS in pathogenesis remains controversial and a hot area of research, there is no doubt that changes in mitochondrial redox status play a role in disease progression.
3. Pharmacological interventions at the level of mitochondrial function
With the recognition that mitochondrial ROS play a role in disease pathogenesis, the development of antioxidants targeted to the mitochondrion is an active area of research. Several compounds have been developed with encouraging results in vascular disease and other pathologies.
a) mitoq.
The most extensively tested mitochondrial antioxidant is probably MitoQ (FIGURE 14). This small molecule consists of the antioxidant ubiquinol linked to the lipophilic cation triphenylphosphonium (500). The positive charge of the triphenylphosphonium cation is delocalized over its large hydrophobic area, which allows it to freely be taken into the mitochondria based on the mitochondrial membrane potential (873). MitoQ has been used extensively in animal models of disease and been shown to be protective against ischemia/reperfusion injury, cardiac hypertrophy, and hypertension (210, 211) among other pathologies.
FIGURE 14.
Structures of selected mitochondria-targeted compounds.
MitoQ has also been tested in phase II clinical trials for both Parkinson’s disease and hepatitis C (NCT00329056; NCT00433108). Although Parkinson’s patients taking MitoQ showed no difference in motor function compared with those on placebo treatment, this trial did establish that administration of MitoQ to humans for 1 yr is safe (874). In contrast, chronic hepatitis C patients treated with MitoQ for 28 days showed significant lowering of liver injury markers such as circulating alanine transaminase (333). These positive results have led to further phase IIB trials in hepatitis as well as the initiation of clinical trials to test MitoQ in other vascular pathologies.
b) szeto–schiller-31.
Szeto–Schiller peptides are another class of molecules that target antioxidant activity to the mitochondrion. Szeto–Schiller-31 (SS-31) (Bendavia/elamipretide; Stealth Peptides) is a small water-soluble tetrapeptide that accumulates within the mitochondrion and binds to cardiolipin in the inner mitochondrial membrane where it scavenges ROS and prevents lipid peroxidation (921) (FIGURE 14). These peptides have been shown to mediate protection in animal models of ischemia/reperfusion (922) and insulin resistance (27). Phase I clinical trials established that the molecule was safe and well tolerated (360). Multiple phase II trials are underway, examining the effects of the drug in conditions such as skeletal muscle disorders (NCT02245620), mitochondrial disorders (NCT02976038, NCT02805790, NCT02367014), and heart failure (NCT02814097, NCT02788747, NCT02914665).
c) mitosno and sodium nitrite.
In addition to pure mitochondrial-targeted oxidant scavengers, potential therapeutics that modulate ETC activity to decrease mitochondrial ROS production are being investigated. For example, MitoSNO (FIGURE 14), a mitochondrially targeted S-nitrosothiol, was shown in rodent models to significantly attenuate infarct size after MI (170). Mechanistically, this protection is dependent on MitoSNO transnitrosating a critical cysteine in the active site of complex I. This S-nitrosation of Cys39 in the ND3 subunit of complex I inhibits its catalytic activity and, hence, attenuates reverse electron transport and ROS production (170). Notably, other nitrogen-centered molecules, such as nitrite, have been shown to promote S-nitrosation of complex I and attenuate ischemia/reperfusion injury (864). Nitrite administered before or during cardiac ischemia has been shown to protect cardiac function and attenuate infarct size in a number of small and large animal models (226, 374, 864). These preclinical studies have led to ongoing clinical testing of nitrite as a therapeutic for MI as well as cardiac arrest.
D. XO
Xanthine oxidoreductase (XOR) is expressed as a xanthine dehydrogenase (XDH) but can become XO by posttranslational modifications (FIGURE 15). XOR is a dimeric protein containing one molybdopterin cofactor, two iron/sulfur clusters (2Fe/2S), and one molecule of FAD per 145-kDa subunit. The N-terminal domain contains the two Fe/S clusters, a central domain contains the FAD cofactor, and a NAD+/H binding site and the C-terminal domain contains the molybdopterin cofactor and a purine binding site (268). The enzyme has an important role in the catabolism of purines, catalyzing the stepwise oxidations of hypoxanthine to xanthine and xanthine to uric acid. Purine oxidation takes place in the molybdopterin site where the reaction leads to the reduction of the Mo6+ to Mo4+. The electrons are then transported within the enzyme, first to the Fe/S clusters and then to the FAD. The fully reduced FADH2 can reduce NAD+ to form NADH.
FIGURE 15.
Architecture of xanthine dehydrogenase (XDH)/zanthine oxidase. A and B indicate the arrangement of the domains in the XDH/xanthine oxidoreductase (XOR) monomer. The middle, FAD-containing domain (yellow), is connected to the N-terminal domain containing two iron/sulfur clusters (Fe2S2) (green) and the molybdopterin domain (pink) by two flexible linkers. Posttranslational changes modify the protein activity from a XDH (A), NADH-producing enzyme, to the xanthine oxidase (XO) (B), hydrogen peroxide/superoxide-generating enzyme. C: three-dimensional structure of the XDH monomer (PDB:1FO4) (268).
1. ROS generation by XOR
During normal operation, XDH shows a limited production of superoxide/hydrogen peroxide via electron leak through the reduced FAD cofactor to O2. This reaction only produces significant levels of ROS if NAD+ levels are low; however, these conditions can happen in the setting of vascular hypoxia (406, 562).
Oxidation of cysteine residues 535 and 992 in rat or bovine XDH or limited proteolysis turns XDH into XO (23, 72, 693, 773, 803, 994). XO shows a decreased affinity for NAD+ and increased O2 affinity. The comparison of the crystal structures for XDH and XO indicates the movement of a loop that partially blocks the NAD+ binding site and alters the electrostatics of the FAD environment (268, 542). Altogether, these changes in substrate affinity lead to an increased electron flow from the molybdopterin domain to oxygen instead of NAD+. Thus, XO catalyzes the oxidation of hypoxanthine to xanthine and subsequently xanthine to uric acid, generating hydrogen peroxide and superoxide in a reaction amplified under ischemia and hypoxia (147, 307, 496, 497, 499).
XO is expressed in ECs (393), and its activity is increased by angiotensin II (551), TNF-α, LPS (117, 311), IL-6, and hypoxia (410, 748). There are low-circulating levels of plasma XO in baseline conditions that can increase in disease conditions by the release from damaged cells in liver or other tissues (1010). The binding of plasma XO to ECs can lead to vascular dysfunction (45, 71, 562, 612, 1010).
2. Pharmacological interventions targeting XO
Extensive evidence supports a role of XO in the development of cardiovascular disease (137, 148, 157, 282, 430, 466, 561, 633). As XO has become an interesting pharmacological target in the treatment of heart and cardiovascular diseases, numerous studies have used different strategies for the inhibition of XO.
a) tungsten.
Tungsten can be used as a global inhibitor of molybdenum-containing enzymes as it replaces the Mo atom in the molybdopterin, rendering the enzymes that use this cofactor inactive. Because of this mechanism of action, it should be noted that effects independent of XO will also be observed because of inhibition of AO, sulfite oxidase, and mARC. Tungsten is a powerful tool for the study of molybdenum enzymes in cell and animal studies and has been found to alleviate atherosclerosis in ApoE−/− mice (833). However, because of its broad effects, the clinical use of tungsten is not feasible.
b) allopurinol.
Allopurinol is structurally similar to the XO substrate hypoxanthine and is metabolized to the active compound oxypurinol (FIGURE 16). Both allopurinol and oxypurinol have been used for the treatment of gout for decades (305, 710). Beyond its use for hyperuricemia and gout, more recent studies have used allopurinol and oxypurinol as XO inhibitors for the treatment of cardiovascular disease in human studies. In small trials, the pharmacological inhibition of XO with allopurinol/oxypurinol improved cardiovascular conditions in several pathologies, including heart failure (58, 278), dilated cardiomyopathy (148), and diabetes (137, 224). Amelioration of endothelial function in smokers was also observed (387). Despite these initial results, subsequent trials with larger number of patients have failed to report substantial positive effects as compared with the placebo groups (361, 405), although the meta-analysis of available studies indicates some improvement of cardiovascular function (430). Altogether, the effect of allopurinol in cardiovascular disease is still controversial. It has also been pointed out that allopurinol and oxypurinol have limited capacity to inhibit XO bound to glycosaminoglycans (620). It is very possible that the use of XO inhibitors needs to be complemented with other therapies targeting other sources of endothelial dysfunction (928).
FIGURE 16.
Structures of xanthine oxidase substrates and inhibitors.
c) febuxostat.
Febuxostat (2-(3-cyano-4-isobutoxyphenyl)-4-methyl-5-thiazole carboxylic acid) (TEI-6720, TMX-67) (FIGURE 16) is a XO inhibitor developed by Teijin, with higher selectivity and potency than allopurinol and oxypurinol (702). The selectivity is partly due to a lack of structural resemblance with purines; this also reduces side effects of purine and pyrimidine metabolism (1041). The higher affinity and specificity of febuxostat can have advantages over allopurinol and oxypurinol. Notably, the activity of febuxostat is not impaired by XO immobilization on the surface of ECs (620). Febuxostat was approved in 2008 in Europe and by the Food and Drug Administration in 2009 for the treatment of gout (commercial name Uloric in United States, Adenuric in Europe) (76, 77, 837). Studies in rodents have shown improvement in atherosclerosis and hypertension after treatment with febuxostat (697, 859). The safety of febuxostat for the treatment of cardiovascular disease in humans is still under investigation (331, 610).
d) topiroxostat.
Topiroxostat (FYX-051, 4-[5-pyridin-4-yl-1H-[1, 2, 4] triazol-3-yl]pyridine-2-carbonitrile) (FIGURE 16) is a novel XO inhibitor developed by Fuji Yahuhin Co. Ltd. Topiroxostat is a potent inhibitor of XO and induces a covalent modification in the enzyme that inactivates XO after complex dissociation (629, 703, 821). Topiroxostat is currently under study for the treatment of hyperuricemia in clinical trials (NCT02327754; NCT02837198) (441, 442).
e) nitrite.
Nitrite has also been used to inhibit XO-catalyzed ROS generation and to take advantage of its nitrite reductase activity to generate NO (reviewed in sect. IIB3) while decreasing superoxide production (1071). Recent work indicates that this treatment can be effective in the reduction of blood pressure in hypertensive patients (357).
E. Other Sources of ROS
Other proteins have been related to the direct or indirect production of ROS in the cardiovascular system, including, but not limited to, peroxidases, cyclooxygenases (COX), lipoxygenases, monoamine oxidase, heme oxygenase (HO), glucose oxidase, cytochrome b5 reductase, and CYPOR (209, 505, 790, 908). In this section, we will briefly describe some of these ROS sources.
1. Peroxidases and COX
Mammalian peroxidases are heme proteins that catalyze the oxidation of a wide range of substrates. These proteins are activated by their reaction with hydrogen peroxide to generate an oxidizing heme species that can hydroxylate or oxygenate a substrate. Mammalian peroxidases include thyroid peroxidase, myeloperoxidase (MPO), lactoperoxidase, eosinophil peroxidase, and COX. MPO, lactoperoxidase, and eosinophil peroxidase are integral components of the immune defense (516).
a) mpo.
MPO is found predominantly in neutrophils and monocytes and catalyzes the reaction of hydrogen peroxide to produce several reactive species, mainly hypochlorous acid (HOCl) (discussed in sect. IVB) (516, 1021). Macrophage localization in the atherosclerotic plaque leads to detectable MPO activity and products of HOCl oxidation (213, 414). Several studies have correlated low circulating MPO levels with better cardiovascular prognosis (239, 260, 644, 690, 1059).
b) cox.
COX are proteins from the peroxidase family, evolutionarily related to the peroxidase enzymes of the immune system (1052). They utilize lipid substrates, generally arachidonic acid, and produce a variety of prostaglandins, lipid oxidation products with important physiological effects (156). Two isoforms are expressed in mammals: COX-1 and COX-2, and both of them have been related to endothelial dysfunction (981, 982). Interestingly, some interplay between COX-2 and other ROS/RNS-generating proteins such as iNOS (52) and XO (701) have been described.
2. Lipoxygenases
Lipoxygenases catalyze the insertion of oxygen in polyunsaturated fatty acids (119, 535, 1040) and are also biological sources of superoxide (537). Several lipoxygenases have been related to inflammation and cardiovascular disease, in particular, 5-lipoxygenase and 12/15-lipoxygenase (203, 597, 636).
3. AO
The Mo-containing protein AO has also been described as a potential source of superoxide in vivo (538, 539). Although the function of AO in mammals is yet to be established (434, 935), AO appears to play an important role in drug metabolism and other metabolic pathways (335, 512, 513, 756, 920). Studies with rat AO have shown a significant production of superoxide when the enzyme uses NADH as a substrate (539). Recent work on the human enzyme shows that the production of superoxide by the human AOX1 isoform is lower than that of the rat enzymes, and the enzyme is not reactive toward NADH (304). However, a known single nucleotide polymorphism in the AOX1 gene was shown to produce a mutant enzyme with increased superoxide formation rates in vitro (304). The possible impact of these single nucleotide polymorphisms in vivo remains to be determined.
4. CYPOR and CYP
CYPOR is a diflavin protein, structurally and evolutionarily related to the reductase domain of the NOS enzymes (377) that functions as a general reductase of the many CYP proteins. The P450 system is conspicuous for its role in detoxification and hormone synthesis (675, 1013). CYPOR, either directly or in combination with the CYP proteins, has been described as a source of superoxide (758, 851). The presence of these proteins in endothelial cells makes them a significant source of superoxide in the vasculature (295, 298). Because of the functional similarities with the dehydrogenase domain of NOX, some NADPH-dependent superoxide measurements can be unable to differentiate CYPOR from NOX as the source of superoxide (783).
5. HO
HO mediates the degradation of heme in mammals. This enzyme catalyzes the degradation of the protoporphyrin IX (heme b) to biliverdin (805, 806). This process generates ferrous iron (Fe2+) and CO, which are well-known toxic species (122, 739, 795, 959), but CO can also be, in low concentrations, a signaling molecule (713, 739, 805, 807). Three HO isoforms exist; the inducible isoform HO-1 is, to date, the most relevant to vascular physiology. HO-1 expression in basal conditions is low or undetectable in most tissues. High levels of HO-1 are present in the spleen, consistent with an important role in the processing of heme from red blood cell turnover (713, 805). The interplay between HO-1, CO production, and NOX inactivation is a focus of current interest in cardiovascular research (685, 793, 805, 924).
IV. OTHER ROS
A. Peroxynitrite
Peroxynitrite (ONOO−) is a strong oxidizing and nitrating agent formed by the reaction of NO with superoxide (Eq. 23). This reaction occurs at a diffusion-limited rate and is governed by the flux of NO and superoxide production (78). Another physiological route by which peroxynitrite can be formed is through the reaction of hydrogen peroxide with nitrite in acidic conditions (Eq. 24) (808).
| (23) |
| (24a) |
| (24b) |
Despite its rapid rate of formation, peroxynitrite reacts relatively slowly in comparison with other RNS, with many biomolecules, including metal centers, proteins, DNA, and lipids (78, 526, 709). Additionally, studies show peroxynitrite can traverse cell membranes by both diffusion and through ion channels, suggesting that peroxynitrite can mediate oxidation/nitration distant from its site of formation (221, 611).
The reaction of peroxynitrite with biomolecules is generally considered as a detrimental event. Peroxynitrite can directly react with a variety of metal centers, resulting in their oxidation. In the cardiovascular system, peroxynitrite can oxidize heme proteins, such as Hb and Mb, resulting in the oxidation of their heme oxygen–carrying site from ferrous iron to ferric heme (101, 942). In the mitochondrion, a similar reaction oxidizes the electron transport protein cytochrome c (942). Reaction of peroxynitrite with Zn/S clusters such as that present in eNOS, causes dysfunction of the enzyme as outlined above (sect. IIIB3) (78, 526). Additionally, peroxynitrite reacts rapidly with Fe/S clusters leading to the inactivation of critical enzymes such as mitochondrial aconitase (155, 401).
Peroxynitrite is perhaps most well recognized for its reaction with cysteine and tyrosine residues in proteins. Peroxynitrite-dependent cysteine oxidation results in the formation of a sulfenic acid intermediate that can further be oxidized to a disulfide. This type of oxidation modulates the activity of a number of enzymes. For example, peroxynitrite-mediated oxidation of ETC components such as complex I and complex V results in inhibition of mitochondrial function (767). Oxidation of tyrosine phosphatases results in the inhibition of their enzymatic activity and the aberrant propagation of phosphorylative signaling (926). In contrast, oxidation of cysteine residues on metalloproteinases results in the activation of these enzymes, which may also be detrimental to tissue integrity and repair (992). In addition to proteins, peroxynitrite can directly oxidize reduced glutathione, depleting the antioxidant capacity of the cell (37).
Beyond cysteine oxidation, peroxynitrite can mediate the covalent addition of a nitro group (−NO2) to the aromatic ring of tyrosine, resulting in tyrosine nitration. This modification is measured as the hallmark of peroxynitrite presence and is traditionally viewed as a sign of nitrative or oxidative stress (78, 765). Importantly, protein nitration can also occur through the reaction of nitrite with heme proteins in the presence of hydrogen peroxide (153, 154). Thus, methodology beyond nitrotyrosine measurement is required to confirm the presence of peroxynitrite in biological systems.
Although a growing number of proteins have now been shown to be nitrated, multiple studies have shown that nitration is a selective process (47, 484). Tyrosines for which the aromatic ring is closer to the surface of a protein, in which there is a neighboring negative charge, and those in hydrophobic regions are more likely to be nitrated (66, 877). Several studies provide comprehensive lists of proteins identified to be nitrated in physiology and disease (44, 73, 381, 709). These proteins include, but are not limited to, enzymes involved in antioxidant defenses such as MnSOD (219, 220), detoxification enzymes such as CYP (587), nucleic acid regulatory proteins such as histone deacetylases (457) and histones (453, 504), and cell structural proteins such as tubulin (730, 732) and myosin heavy chain (648). Importantly, the nitration of these proteins decreases enzyme activity, disrupts metabolism and cellular detoxification, perturbs cytoskeletal organization, and ultimately contributes to the cytotoxic effects of peroxynitrite.
Although peroxynitrite is traditionally regarded as a harmful species, it should be noted that it may have some beneficial signaling properties. For example, lipids are also a significant biological target for peroxynitrite, and although the species can abstract a hydrogen atom to initiate lipid peroxidation, a detrimental process that can lead to cytotoxicity in many cases (65, 766), peroxynitrite can also mediate lipid nitration (54, 1027). Nitrated fatty acids are a class of electrophilic signaling molecules which have been shown to react with a number of cellular targets to mediate protective inflammatory and metabolic signaling (830). Primary mechanisms of nitrated fatty acid signaling include activation of PPARγ (55, 583) and Michael addition to thiol-containing proteins in a number of signaling pathways (53, 955). Through these signaling mechanisms, nitrated lipids mediate a number of physiological effects in animal models including attenuation of inflammatory bowel disease (113), protection after ischemia/reperfusion (683, 801), decreases in atherosclerosis (800), and reversal of obesity-induced pulmonary hypertension (495).
B. HOCl
Hypochlorous acid (HOCl) is generated by the enzyme MPO, localized within leukocytes by the following reaction (300) (Eq. 25)
| (25) |
This highly reactive oxidizing and chlorinating agent plays a major role in the microbicidal actions of neutrophils (15, 1021). Although HOCl reacts with a variety of biomolecule targets (755), in proteins, it predominantly oxidizes cysteine and methionine residues to disulfide and methionine sulfoxide, respectively (726). With regards to metal centers, HOCl can react with heme and Fe/S centers to mediate oxidative bleaching. Additionally, HOCl reacts more slowly with tyrosine residues to mediate the formation of 3-chlorotyrosine (1019). Notably, 3-chlorotyrosine is utilized as a marker of the presence of both MPO (as this is the only enzyme known to generate HOCl) and HOCl. Oxidation of amino acids and metal centers of enzymes results in the HOCl-dependent impairment of a number of critical enzymes.
The role of HOCl in the modification of proteins and lipids is particularly well studied and pertinent to the pathogenesis of vascular disease. In the context of atherosclerosis, HOCl has been shown to oxidize both high and low density lipoproteins (90, 470, 881, 1042). Furthermore, chlorinated proteins and lipids are present in atherosclerotic plaques (90, 415, 621). Hypochlorous acid decreases eNOS activity and NO bioavailability by a number of direct and indirect mechanisms, disrupting protective NO signaling (2, 464, 1054). The oxidation and chlorination of proteins, such as matrix metalloproteinases, leads to detrimental vascular remodeling (314).
C. Hydroxyl Radical
The hydroxyl radical (OH·) is one of the most reactive species formed in biological systems. In this context, OH· is generally formed as a product of Fenton reactions involving free iron or copper ions (Fe2+; Cu+) and hydrogen peroxide (Eq. 26) (281, 986) or from the reaction of hydrogen peroxide with superoxide (Haber–Weiss reaction) (Eq. 27) (394).
| (26a) |
| (26b) |
| (27) |
The process is generally more complex than indicated in Eq. 26 and includes the formation of a transient metal/peroxide complex that can react in a different fashion depending on the metal ligands and possible substrates. Therefore, in some cases, the reaction may not produce free hydroxyl radical but other metal-based oxidizing species (759, 1020).
Although the endogenous production of OH· in healthy conditions is apparently negligible (474), the excess of hydrogen peroxide and superoxide in oxidative stress conditions can yield an increased amount of this toxic species (1072).
In physiology, perhaps the most common source of Fenton chemistry is the presence of increased concentrations of free iron and/or copper (122, 458, 959). In the cardiovascular system, this can be related to aging (122) or hemolytic disease (1026). The source of this iron is most likely plasma-released Hb; whereas Hb or heme are not catalysts for the Fenton reaction, the reaction of plasma Hb with peroxides can cause the release of free iron (389, 759).
The hydroxyl radical reacts with multiple organic substrates including, but not limited to, DNA, lipids, and proteins at rates near the diffusion limits. This lack of specificity makes the use of antioxidants to prevent hydroxyl radical toxicity impractical. Ideally, the best strategies will be based on the chelation of the active metal ions in an environment that blocks their redox reactivity. Proteins like ceruloplasmin, transferrin, or lactoferrin limit the reaction (388, 1020). The binding of NO to heme iron can also limit heme reactivity and prevent iron release from heme (482).
D. Other Species
The reactions of NO can generate other reactive species that have a poorly characterized role in vivo. Among these species, we will mention the nitrogen dioxide radical (NO2·), dinitrogen trioxide (N2O3), and carbonate radical (CO3·−) (46, 692).
1. Nitrogen dioxide
Nitrogen dioxide (NO2·) is formed in the reaction of NO with oxygen (Eq. 28) (509, 1018). A more reactive species than NO, it is often used as a surrogate for the detection of NO. Other formation routes include the reactions of nitrite with oxygenated Hb (501) and peroxynitrite decomposition (46).
| (28a) |
| (28b) |
2. Dinitrogen trioxide
Dinitrogen trioxide (N2O3) is a strong nitrosating species also formed in the oxidation of NO (Eq. 29) (691, 692, 1018).
| (29) |
As discussed previously in section IIB2, the formation of N2O3 in the red blood cell via an anhydrase reaction involving Hb, NO, and nitrite (69) constitutes a possible pathway to preserve NO bioactivity (367).
3. Carbonate radical
The carbonate radical can be formed in vivo by the reaction of the hydroxyl radical with carbonate or bicarbonate (138) or via the reaction of peroxynitrite with carbon dioxide (Eq. 30) (112, 608, 640).
| (30a) |
| (30b) |
Several examples of biological oxidations mediated by the bicarbonate anion, in particular through interactions with peroxynitrite, have been reported in the literature [e.g., (46, 635, 918, 1056)]. In relation to cardiovascular biology, it is important to note that XO has also been characterized as a target and source of carbonate radical (111).
V. CROSS-TALK BETWEEN ROS AND RNS SOURCES
Our description of sources of NO and ROS may give the impression of a rigorous separation of roles for each protein or enzymatic system. This is certainly not the situation in physiological systems. We have discussed how some enzymes, in particular eNOS, can produce different species in response to posttranslational modifications or lack of cofactors or substrates (sects. IIA1 and IIIB). Current research has greatly advanced our knowledge on how different systems can influence the NO/ROS generation from other sources in which it has been termed ROS-induced ROS release (1066, 1069).
Mitochondria and NOX are the main effectors of downstream signaling to other sources and to each other. Mitochondrial uncoupling and increased ROS generation activates the mitochondrial permeability transition pore (mPTP), allowing the release of ROS to the cytosol (1069). In response to the increased levels of ROS, the PKC is activated, which in turn, can activate NOXs (319, 776). Other routes of mitochondrial triggered activation of NOX involve the activation of NOX1 via activation of PI3K and Rac1. The blockade of mitochondrial ROS by rotenone was shown to prevent NOX1 activation (FIGURE 17) (564).
FIGURE 17.
Cross-talk between mitochondria, NADPH oxidase (NOX), and other reactive oxygen species (ROS) sources. Mitochondrial ROS are released to the cytosol via the mitochondrial permeability transition pore (mPTP). These can cause activation of PKC and phosphatidylinositol 3-kinase (PI3K) kinases and Rac1 GTPase with subsequent activation of NOX enzymes and further ROS production. Alternatively, NOX can release ROS that activate protein kinase C, isoform ε (PKCε) and in turn activate the mitochondrial ATP-dependent potassium channels (KATP), increasing mitochondrial ROS. Cytosolic ROS can increase ROS-generating activity of endothelial nitric oxide synthase (eNOS) and xanthine oxidase (XO).
Indeed, activated NOX can in turn, increase ROS production by the mitochondria (11, 116). This process appears to require the function of mitochondrial ATP-dependent potassium channels (KATP), as shown by the ability of KATP inhibitors to block the increase of mitochondrial ROS production after angiotensin II–induced activation of NOX (242, 507). Of note, the activation of NOX via angiotensin II and the angiotensin II type 1 receptor is mediated by PKC; however, the isoform PKCε is also an activator of the KATP channel, which, as indicated, also stimulates mitochondrial ROS production (FIGURE 17) (187, 242).
Another case of significant cross-talk between NOX and mitochondria has been provided by studies of nitroglycerin resistance. In this case, the development of tolerance was mainly due to mitochondrial ROS release and was independent of NOX, whereas the activation of NOX, triggered by mitochondrial ROS, was the effector of the endothelial dysfunction associated to nitroglycerin tolerance (1004).
Secondary to the main role of mitochondria and NOX, but not to be ignored, XO and eNOS can also amplify the formation of ROS; for instance, XO is indirectly activated by angiotensin II, increasing cellular ROS production (551). A direct role of mitochondrial ROS on XO activation has also been described (362). As discussed in previous section, increased ROS eventually causes eNOS uncoupling via BH4 depletion, Zn/S cluster breakdown, and other routes, further perpetuating the generation of superoxide and other ROS (FIGURE 17) (488, 570).
The existence of many different routes interconnecting ROS/RNS sources may suggest that the normalization of dysfunctional conditions is a herculean task; however, it also presents the promise of normalizing a disease state by the direct inhibition of a single source of oxidative stress. Recent work indicates that this can be a feasible approach (206, 207, 234, 836). The development of novel, specific mitochondrial inhibitors (discussed in sect. IIIC3) is a promising step in this direction (8, 679, 923, 1062).
VI. CONCLUDING REMARKS: ADVANCES, CHALLENGES, AND THERAPEUTIC OPPORTUNITIES
Over the last decade, our knowledge about NO and ROS generation has increased substantially. From the initial ideas considering that oxidative species need to be suppressed and that oxidative stress could be combated with adequate antioxidant treatment, we now can appreciate the critical signaling properties of NO, hydrogen peroxide, or superoxide.
The initial approach to the treatment of oxidative stress was based on the supplementation with different antioxidants, in particular vitamins C and E and β-carotene. The use of antioxidants has been markedly ineffective when not damaging (97, 390, 400, 785). In fact, the situation has been termed the “antioxidant paradox” (399, 400). The failure of these studies, along with their limitations, has been discussed at length in this review, and a number of explanations for this phenomenon have been proposed (67, 158, 252, 400, 672, 949).
At the same time, we are increasingly appreciative of the cross-talk between different radical species, generation pathways, and cellular compartments. Different examples of species interaction have been discussed throughout this review; for instance, the oxidation of BH4 by superoxide will trigger eNOS uncoupling with decreased NO production and increased superoxide production. In another example, NO, through its high affinity for heme groups or via S-nitrosation, can act as an important modulator of NOX (176, 315, 846) or can also modulate the insertion of heme in the receptor sGC. The generation of NO through NOS enzymes and oxidative pathways in normoxic conditions is supplemented by the actions of reductive, nitrite-dependent pathways in hypoxia (603, 968). We also note how some proteins are able to switch, in an oxygen-dependent manner, from NO scavengers into nitrite-reducing, NO-producing systems. The ability to interact with different oxidation states of sGC and use pharmacological tools to recover sGC-dependent signaling provides a new tool for the treatment of conditions in which NO signaling is compromised. Further research aimed to the understanding of these dynamics will be critical for the treatment of endothelial dysfunction and other redox imbalance-mediated pathologies.
The interactions between mitochondria and ROS-producing proteins, especially NOXs, are of particular current interest (116, 206, 234, 836). Mitochondria are a physiologically significant source of ROS with important implications in physiology, not only in cardiovascular disease but in many other conditions like aging (57, 84, 136, 165, 170, 290, 864, 997).
It is also significant that many of our advances have evolved not only from the clinical field but also from basic science. Chemical knowledge of NO and ROS reactivity has been available well before its biological relevance was established. This knowledge is critical for the understanding of the biology of ROS and can provide new tools and strategies for the study of ROS biology (232). The studies on purified protein systems, although necessarily simplistic, have provided insights on the reactivity of most proteins involved in NO and ROS biology that have been widely validated in vivo. Conversely, the study of genetic pathologies such as chronic granulomatous disease has helped to establish the relevance of NOX proteins even before these were thoroughly characterized.
In summary, we have progressed from a simpler approach leveraging antioxidant vitamins and ROS scavengers, which failed, to a more fundamental discovery of the enzymatic sources and reaction pathways of ROS. The future treatments need to address these issues. It is clearer now that specific inhibitors directed to the sources of ROS can yield more effective therapies. A better understanding of NOS function has allowed the development of new strategies to couple eNOS and target downstream effectors like sGC. Advances on NOX research have led to the development of novel inhibitors on their way to widespread use for the treatment of cardiovascular disease and other pathologies. New highly specific inhibitors of other ROS sources like the XO-targeted febuxostat and mitochondrial-targeted drugs provide additional pathways to modulate cellular ROS production. We foresee that the availability of these specific drugs and a better biomarker-based diagnosis of the dysregulated systems will allow for more personalized treatments.
Finally, antioxidants targeted to specific cells or organelles can still become relevant in the management of oxidative stress pathologies, in particular, but not limited to, mitochondria-targeted antioxidants (873). The development of these treatments directed toward specific organs, tissue, and cell types can be one of the next big advances in the field.
GRANTS
This work was supported by National Institutes of Health Grants R01 HL098032, R01 HL125886, P01 HL103455, T32 HL110849, and T32 HL007563 (to M.T. Gladwin), R21 ES027390 (to J. Tejero), and funding from the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania (to M.T. Gladwin and S. Shiva).
DISCLOSURES
M.T. Gladwin is a coinventor on a National Institutes of Health government patent application for the use of nitrite salts in the treatment of cardiovascular diseases. J. Tejero and S. Shiva do not declare any conflicts of interest, financial or otherwise.
ACKNOWLEDGMENTS
We thank Patrick Pagano (University of Pittsburgh), Maria Eugenia Cifuentes-Pagano (University of Pittsburgh), Eric E. Kelley (West Virginia University), Mauro Siragusa (Goethe University, Frankfurt am Main, Germany), and members of the Gladwin laboratory for critical reading of our manuscript. Coordinates for the sGC model were kindly provided by William Montfort (University of Arizona). Because of the immense volume of literature in the field, we are sure that many important works are not included in the reference list. We sincerely apologize to the authors for these unintentional omissions.
REFERENCES
- 1.Aamand R, Dalsgaard T, Jensen FB, Simonsen U, Roepstorff A, Fago A. Generation of nitric oxide from nitrite by carbonic anhydrase: a possible link between metabolic activity and vasodilation. Am J Physiol Heart Circ Physiol 297: H2068–H2074, 2009. doi: 10.1152/ajpheart.00525.2009. [DOI] [PubMed] [Google Scholar]
- 2.Abdo AI, Rayner BS, van Reyk DM, Hawkins CL. Low-density lipoprotein modified by myeloperoxidase oxidants induces endothelial dysfunction. Redox Biol 13: 623–632, 2017. doi: 10.1016/j.redox.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu-Soud HM, Presta A, Mayer B, Stuehr DJ. Analysis of neuronal NO synthase under single-turnover conditions: conversion of Nomega-hydroxyarginine to nitric oxide and citrulline. Biochemistry 36: 10811–10816, 1997. doi: 10.1021/bi971414g. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Soud HM, Wang J, Rousseau DL, Fukuto JM, Ignarro LJ, Stuehr DJ. Neuronal nitric oxide synthase self-inactivates by forming a ferrous-nitrosyl complex during aerobic catalysis. J Biol Chem 270: 22997–23006, 1995. doi: 10.1074/jbc.270.39.22997. [DOI] [PubMed] [Google Scholar]
- 5.Adachi T, Fukushima T, Usami Y, Hirano K. Binding of human xanthine oxidase to sulphated glycosaminoglycans on the endothelial-cell surface. Biochem J 289: 523–527, 1993. doi: 10.1042/bj2890523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adak S, Aulak KS, Stuehr DJ. Direct evidence for nitric oxide production by a nitric-oxide synthase-like protein from Bacillus subtilis. J Biol Chem 277: 16167–16171, 2002. doi: 10.1074/jbc.M201136200. [DOI] [PubMed] [Google Scholar]
- 7.Adak S, Santolini J, Tikunova S, Wang Q, Johnson JD, Stuehr DJ. Neuronal nitric-oxide synthase mutant (Ser-1412 --> Asp) demonstrates surprising connections between heme reduction, NO complex formation, and catalysis. J Biol Chem 276: 1244–1252, 2001. doi: 10.1074/jbc.M006857200. [DOI] [PubMed] [Google Scholar]
- 8.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RAJ, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J 19: 1088–1095, 2005. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 9.Ago T, Kitazono T, Kuroda J, Kumai Y, Kamouchi M, Ooboshi H, Wakisaka M, Kawahara T, Rokutan K, Ibayashi S, Iida M. NAD(P)H oxidases in rat basilar arterial endothelial cells. Stroke 36: 1040–1046, 2005. doi: 10.1161/01.STR.0000163111.05825.0b. [DOI] [PubMed] [Google Scholar]
- 10.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109: 227–233, 2004. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 11.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106: 1253–1264, 2010. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aisaka K, Gross SS, Griffith OW, Levi R. L-arginine availability determines the duration of acetylcholine-induced systemic vasodilation in vivo. Biochem Biophys Res Commun 163: 710–717, 1989. doi: 10.1016/0006-291X(89)92281-X. [DOI] [PubMed] [Google Scholar]
- 13.Al Ghouleh I, Khoo NKH, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med 51: 1271–1288, 2011. doi: 10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Ghouleh I, Meijles DN, Mutchler S, Zhang Q, Sahoo S, Gorelova A, Henrich Amaral J, Rodríguez AI, Mamonova T, Song GJ, Bisello A, Friedman PA, Cifuentes-Pagano ME, Pagano PJ. Binding of EBP50 to Nox organizing subunit p47phox is pivotal to cellular reactive species generation and altered vascular phenotype. Proc Natl Acad Sci USA 113: E5308–E5317, 2016. doi: 10.1073/pnas.1514161113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albrich JM, McCarthy CA, Hurst JK. Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc Natl Acad Sci USA 78: 210–214, 1981. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alderton WK, Angell AD, Craig C, Dawson J, Garvey E, Moncada S, Monkhouse J, Rees D, Russell LJ, Russell RJ, Schwartz S, Waslidge N, Knowles RG. GW274150 and GW273629 are potent and highly selective inhibitors of inducible nitric oxide synthase in vitro and in vivo. Br J Pharmacol 145: 301–312, 2005. doi: 10.1038/sj.bjp.0706168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 357: 593–615, 2001. doi: 10.1042/bj3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aldieri E, Riganti C, Polimeni M, Gazzano E, Lussiana C, Campia I, Ghigo D. Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr Drug Metab 9: 686–696, 2008. doi: 10.2174/138920008786049285. [DOI] [PubMed] [Google Scholar]
- 19.Alp NJ, McAteer MA, Khoo J, Choudhury RP, Channon KM. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler Thromb Vasc Biol 24: 445–450, 2004. doi: 10.1161/01.ATV.0000115637.48689.77. [DOI] [PubMed] [Google Scholar]
- 20.Alsop P, Hauton D. Oral nitrate and citrulline decrease blood pressure and increase vascular conductance in young adults: a potential therapy for heart failure. Eur J Appl Physiol 116: 1651–1661, 2016. doi: 10.1007/s00421-016-3418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altenhöfer S, Kleikers PWM, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, Schmidt HHHW. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci 69: 2327–2343, 2012. doi: 10.1007/s00018-012-1010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altenhöfer S, Radermacher KA, Kleikers PW, Wingler K, Schmidt HH. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid Redox Signal 23: 406–427, 2015. doi: 10.1089/ars.2013.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amaya Y, Yamazaki K, Sato M, Noda K, Nishino T, Nishino T. Proteolytic conversion of xanthine dehydrogenase from the NAD-dependent type to the O2-dependent type. Amino acid sequence of rat liver xanthine dehydrogenase and identification of the cleavage sites of the enzyme protein during irreversible conversion by trypsin. J Biol Chem 265: 14170–14175, 1990. [PubMed] [Google Scholar]
- 24.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem 279: 45935–45941, 2004. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 25.Amdahl MB, Sparacino-Watkins CE, Corti P, Gladwin MT, Tejero J. Efficient reduction of vertebrate cytoglobins by the cytochrome b5/cytochrome b5 reductase/NADH system. Biochemistry 56: 3993–4004, 2017. doi: 10.1021/acs.biochem.7b00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, Gnidehou S, Ohayon R, Noël-Hudson MS, Francon J, Lalaoui K, Virion A, Dupuy C. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem 280: 30046–30054, 2005. doi: 10.1074/jbc.M500516200. [DOI] [PubMed] [Google Scholar]
- 27.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW III, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderssohn M, Rosenberg M, Schwedhelm E, Zugck C, Lutz M, Lüneburg N, Frey N, Böger RH. The L-Arginine-asymmetric dimethylarginine ratio is an independent predictor of mortality in dilated cardiomyopathy. J Card Fail 18: 904–911, 2012. doi: 10.1016/j.cardfail.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Annedi SC, Maddaford SP, Ramnauth J, Renton P, Rybak T, Silverman S, Rakhit S, Mladenova G, Dove P, Andrews JS, Zhang D, Porreca F. Discovery of a potent, orally bioavailable and highly selective human neuronal nitric oxide synthase (nNOS) inhibitor, N-(1-(piperidin-4-yl)indolin-5-yl)thiophene-2-carboximidamide as a pre-clinical development candidate for the treatment of migraine. Eur J Med Chem 55: 94–107, 2012. doi: 10.1016/j.ejmech.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Anter E, Thomas SR, Schulz E, Shapira OM, Vita JA, Keaney JF Jr. Activation of endothelial nitric-oxide synthase by the p38 MAPK in response to black tea polyphenols. J Biol Chem 279: 46637–46643, 2004. doi: 10.1074/jbc.M405547200. [DOI] [PubMed] [Google Scholar]
- 31.Antoniades C, Bakogiannis C, Leeson P, Guzik TJ, Zhang M-H, Tousoulis D, Antonopoulos AS, Demosthenous M, Marinou K, Hale A, Paschalis A, Psarros C, Triantafyllou C, Bendall J, Casadei B, Stefanadis C, Channon KM. Rapid, direct effects of statin treatment on arterial redox state and nitric oxide bioavailability in human atherosclerosis via tetrahydrobiopterin-mediated endothelial nitric oxide synthase coupling. Circulation 124: 335–345, 2011. doi: 10.1161/CIRCULATIONAHA.110.985150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antoniades C, Shirodaria C, Crabtree M, Rinze R, Alp N, Cunnington C, Diesch J, Tousoulis D, Stefanadis C, Leeson P, Ratnatunga C, Pillai R, Channon KM. Altered plasma versus vascular biopterins in human atherosclerosis reveal relationships between endothelial nitric oxide synthase coupling, endothelial function, and inflammation. Circulation 116: 2851–2859, 2007. doi: 10.1161/CIRCULATIONAHA.107.704155. [DOI] [PubMed] [Google Scholar]
- 33.Antoniades C, Shirodaria C, Warrick N, Cai S, de Bono J, Lee J, Leeson P, Neubauer S, Ratnatunga C, Pillai R, Refsum H, Channon KM. 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation 114: 1193–1201, 2006. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- 34.Aon MA, Stanley BA, Sivakumaran V, Kembro JM, O’Rourke B, Paolocci N, Cortassa S. Glutathione/thioredoxin systems modulate mitochondrial H2O2 emission: an experimental-computational study. J Gen Physiol 139: 479–491, 2012. doi: 10.1085/jgp.201210772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aoyama T, Paik Y-H, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, Brenner DA. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56: 2316–2327, 2012. doi: 10.1002/hep.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci USA 74: 3203–3207, 1977. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arteel GE, Briviba K, Sies H. Protection against peroxynitrite. FEBS Lett 445: 226–230, 1999. doi: 10.1016/S0014-5793(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 38.Ascenzi P, di Masi A, Leboffe L, Fiocchetti M, Nuzzo MT, Brunori M, Marino M. Neuroglobin: From structure to function in health and disease. Mol Aspects Med 52: 1–48, 2016. doi: 10.1016/j.mam.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Ascenzi P, Gustincich S, Marino M. Mammalian nerve globins in search of functions. IUBMB Life 66: 268–276, 2014. doi: 10.1002/iub.1267. [DOI] [PubMed] [Google Scholar]
- 40.Ascenzi P, Leboffe L, Pesce A, Ciaccio C, Sbardella D, Bolognesi M, Coletta M. Nitrite-reductase and peroxynitrite isomerization activities of Methanosarcina acetivorans protoglobin. PLoS One 9: e95391, 2014. doi: 10.1371/journal.pone.0095391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ascenzi P, Marino M, Polticelli F, Santucci R, Coletta M. Cardiolipin modulates allosterically the nitrite reductase activity of horse heart cytochrome c. J Biol Inorg Chem 19: 1195–1201, 2014. doi: 10.1007/s00775-014-1175-9. [DOI] [PubMed] [Google Scholar]
- 42.Ascenzi P, Sbardella D, Fiocchetti M, Santucci R, Coletta M. NO2(-)-mediated nitrosylation of ferrous microperoxidase-11. J Inorg Biochem 153: 121–127, 2015. doi: 10.1016/j.jinorgbio.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Ascenzi P, Tundo GR, Fanali G, Coletta M, Fasano M. Warfarin modulates the nitrite reductase activity of ferrous human serum heme-albumin. J Biol Inorg Chem 18: 939–946, 2013. doi: 10.1007/s00775-013-1040-2. [DOI] [PubMed] [Google Scholar]
- 44.Aslan M, Dogan S. Proteomic detection of nitroproteins as potential biomarkers for cardiovascular disease. J Proteomics 74: 2274–2288, 2011. doi: 10.1016/j.jprot.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 45.Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci USA 98: 15215–15220, 2001. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Augusto O, Bonini MG, Amanso AM, Linares E, Santos CC, De Menezes SL. Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radic Biol Med 32: 841–859, 2002. doi: 10.1016/S0891-5849(02)00786-4. [DOI] [PubMed] [Google Scholar]
- 47.Aulak KS, Miyagi M, Yan L, West KA, Massillon D, Crabb JW, Stuehr DJ. Proteomic method identifies proteins nitrated in vivo during inflammatory challenge. Proc Natl Acad Sci USA 98: 12056–12061, 2001. doi: 10.1073/pnas.221269198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babior BM, Curnutte JT, Kipnes BS. Pyridine nucleotide-dependent superoxide production by a cell-free system from human granulocytes. J Clin Invest 56: 1035–1042, 1975. doi: 10.1172/JCI108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Babior BM, Kipnes RS. Superoxide-forming enzyme from human neutrophils: evidence for a flavin requirement. Blood 50: 517–524, 1977. [PubMed] [Google Scholar]
- 50.Bach A. Zur Kenntnis der Reduktionsfermente. I. Mitteilung über das Schardinger-Enzym (Perhydridase). Biochem Z 31: 443–449, 1911. [Google Scholar]
- 51.Baek KJ, Thiel BA, Lucas S, Stuehr DJ. Macrophage nitric oxide synthase subunits. Purification, characterization, and role of prosthetic groups and substrate in regulating their association into a dimeric enzyme. J Biol Chem 268: 21120–21129, 1993. [PubMed] [Google Scholar]
- 52.Baker CSR, Hall RJC, Evans TJ, Pomerance A, Maclouf J, Creminon C, Yacoub MH, Polak JM. Cyclooxygenase-2 is widely expressed in atherosclerotic lesions affecting native and transplanted human coronary arteries and colocalizes with inducible nitric oxide synthase and nitrotyrosine particularly in macrophages. Arterioscler Thromb Vasc Biol 19: 646–655, 1999. doi: 10.1161/01.ATV.19.3.646. [DOI] [PubMed] [Google Scholar]
- 53.Baker LM, Baker PR, Golin-Bisello F, Schopfer FJ, Fink M, Woodcock SR, Branchaud BP, Radi R, Freeman BA. Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction. J Biol Chem 282: 31085–31093, 2007. doi: 10.1074/jbc.M704085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baker PR, Schopfer FJ, Sweeney S, Freeman BA. Red cell membrane and plasma linoleic acid nitration products: synthesis, clinical identification, and quantitation. Proc Natl Acad Sci USA 101: 11577–11582, 2004. doi: 10.1073/pnas.0402587101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baker PRS, Lin Y, Schopfer FJ, Woodcock SR, Groeger AL, Batthyany C, Sweeney S, Long MH, Iles KE, Baker LMS, Branchaud BP, Chen YE, Freeman BA. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem 280: 42464–42475, 2005. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baker TA, Milstien S, Katusic ZS. Effect of vitamin C on the availability of tetrahydrobiopterin in human endothelial cells. J Cardiovasc Pharmacol 37: 333–338, 2001. doi: 10.1097/00005344-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 120: 483–495, 2005. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Baldus S, Müllerleile K, Chumley P, Steven D, Rudolph V, Lund GK, Staude HJ, Stork A, Köster R, Kähler J, Weiss C, Münzel T, Meinertz T, Freeman BA, Heitzer T. Inhibition of xanthine oxidase improves myocardial contractility in patients with ischemic cardiomyopathy. Free Radic Biol Med 41: 1282–1288, 2006. doi: 10.1016/j.freeradbiomed.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev 89: 481–534, 2009. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- 60.Balzer J, Rassaf T, Heiss C, Kleinbongard P, Lauer T, Merx M, Heussen N, Gross HB, Keen CL, Schroeter H, Kelm M. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J Am Coll Cardiol 51: 2141–2149, 2008. doi: 10.1016/j.jacc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 61.Bánfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem 278: 3510–3513, 2003. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 62.Bánfi B, Molnár G, Maturana A, Steger K, Hegedûs B, Demaurex N, Krause KHA. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem 276: 37594–37601, 2001. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 63.Bánfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnár GZ, Krause KH, Cox JA. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5). J Biol Chem 279: 18583–18591, 2004. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 64.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ETH, Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(−/−) mice. J Clin Invest 108: 1513–1522, 2001. doi: 10.1172/JCI200111927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bartesaghi S, Herrera D, Martinez DM, Petruk A, Demicheli V, Trujillo M, Martí MA, Estrín DA, Radi R. Tyrosine oxidation and nitration in transmembrane peptides is connected to lipid peroxidation. Arch Biochem Biophys 622: 9–25, 2017. doi: 10.1016/j.abb.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 66.Bartesaghi S, Valez V, Trujillo M, Peluffo G, Romero N, Zhang H, Kalyanaraman B, Radi R. Mechanistic studies of peroxynitrite-mediated tyrosine nitration in membranes using the hydrophobic probe N-t-BOC-L-tyrosine tert-butyl ester. Biochemistry 45: 6813–6825, 2006. doi: 10.1021/bi060363x. [DOI] [PubMed] [Google Scholar]
- 67.Bast A, Haenen GRMM. Ten misconceptions about antioxidants. Trends Pharmacol Sci 34: 430–436, 2013. doi: 10.1016/j.tips.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 68.Basu S, Azarova NA, Font MD, King SB, Hogg N, Gladwin MT, Shiva S, Kim-Shapiro DB. Nitrite reductase activity of cytochrome c. J Biol Chem 283: 32590–32597, 2008. doi: 10.1074/jbc.M806934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel R, King SB, Hogg N, Ghosh A, Gladwin MT, Kim-Shapiro DB. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat Chem Biol 3: 785–794, 2007. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 70.Basuroy S, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am J Physiol Cell Physiol 296: C422–C432, 2009. doi: 10.1152/ajpcell.00381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Battelli MG, Bolognesi A, Polito L. Pathophysiology of circulating xanthine oxidoreductase: new emerging roles for a multi-tasking enzyme. Biochim Biophys Acta 1842: 1502–1517, 2014. doi: 10.1016/j.bbadis.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 72.Battelli MG, Polito L, Bolognesi A. Xanthine oxidoreductase in atherosclerosis pathogenesis: not only oxidative stress. Atherosclerosis 237: 562–567, 2014. doi: 10.1016/j.atherosclerosis.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 73.Batthyány C, Bartesaghi S, Mastrogiovanni M, Lima A, Demicheli V, Radi R. Tyrosine-Nitrated Proteins: Proteomic and Bioanalytical Aspects. Antioxid Redox Signal 26: 313–328, 2017. doi: 10.1089/ars.2016.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bauer PM, Fulton D, Boo YC, Sorescu GP, Kemp BE, Jo H, Sessa WC. Compensatory phosphorylation and protein-protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric-oxide synthase. J Biol Chem 278: 14841–14849, 2003. doi: 10.1074/jbc.M211926200. [DOI] [PubMed] [Google Scholar]
- 75.Baum C, Johannsen SS, Zeller T, Atzler D, Ojeda FM, Wild PS, Sinning CR, Lackner KJ, Gori T, Schwedhelm E, Böger RH, Blankenberg S, Münzel T, Schnabel RB; Gutenberg Health Study investigators . ADMA and arginine derivatives in relation to non-invasive vascular function in the general population. Atherosclerosis 244: 149–156, 2016. doi: 10.1016/j.atherosclerosis.2015.10.101. [DOI] [PubMed] [Google Scholar]
- 76.Becker MA, Schumacher HR, MacDonald PA, Lloyd E, Lademacher C. Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with gout. J Rheumatol 36: 1273–1282, 2009. doi: 10.3899/jrheum.080814. [DOI] [PubMed] [Google Scholar]
- 77.Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, Streit J, Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 353: 2450–2461, 2005. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 78.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 271: C1424–C1437, 1996. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 79.Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 80.Behrends S, Harteneck C, Schultz G, Koesling D. A variant of the α 2 subunit of soluble guanylyl cyclase contains an insert homologous to a region within adenylyl cyclases and functions as a dominant negative protein. J Biol Chem 270: 21109–21113, 1995. doi: 10.1074/jbc.270.36.21109. [DOI] [PubMed] [Google Scholar]
- 81.BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C, Görlach A. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med 42: 446–459, 2007. doi: 10.1016/j.freeradbiomed.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 82.Belhassen L, Feron O, Kaye DM, Michel T, Kelly RA. Regulation by cAMP of post-translational processing and subcellular targeting of endothelial nitric-oxide synthase (type 3) in cardiac myocytes. J Biol Chem 272: 11198–11204, 1997. doi: 10.1074/jbc.272.17.11198. [DOI] [PubMed] [Google Scholar]
- 83.Belik J. Riociguat, an oral soluble guanylate cyclase stimulator for the treatment of pulmonary hypertension. Curr Opin Investig Drugs 10: 971–979, 2009. [PubMed] [Google Scholar]
- 84.Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GRS, Chandel NS. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol 177: 1029–1036, 2007. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bellamy TC, Wood J, Goodwin DA, Garthwaite J. Rapid desensitization of the nitric oxide receptor, soluble guanylyl cyclase, underlies diversity of cellular cGMP responses. Proc Natl Acad Sci USA 97: 2928–2933, 2000. doi: 10.1073/pnas.97.6.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ben Mkaddem S, Pedruzzi E, Werts C, Coant N, Bens M, Cluzeaud F, Goujon JM, Ogier-Denis E, Vandewalle A. Heat shock protein gp96 and NAD(P)H oxidase 4 play key roles in Toll-like receptor 4-activated apoptosis during renal ischemia/reperfusion injury. Cell Death Differ 17: 1474–1485, 2010. doi: 10.1038/cdd.2010.26. [DOI] [PubMed] [Google Scholar]
- 87.Bendall JK, Douglas G, McNeill E, Channon KM, Crabtree MJ. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid Redox Signal 20: 3040–3077, 2014. doi: 10.1089/ars.2013.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bendall JK, Rinze R, Adlam D, Tatham AL, de Bono J, Wilson N, Volpi E, Channon KM. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res 100: 1016–1025, 2007. doi: 10.1161/01.RES.0000263381.83835.7b. [DOI] [PubMed] [Google Scholar]
- 89.Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H. Stomach NO synthesis. Nature 368: 502, 1994. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 90.Bergt C, Pennathur S, Fu X, Byun J, O’Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci USA 101: 13032–13037, 2004. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 108: 2000–2006, 2003. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 92.Beuve A. Thiol-Based Redox Modulation of Soluble Guanylyl Cyclase, the Nitric Oxide Receptor. Antioxid Redox Signal 26: 137–149, 2017. doi: 10.1089/ars.2015.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beuve A, Wu C, Cui C, Liu T, Jain MR, Huang C, Yan L, Kholodovych V, Li H. Identification of novel S-nitrosation sites in soluble guanylyl cyclase, the nitric oxide receptor. J Proteomics 138: 40–47, 2016. doi: 10.1016/j.jprot.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bibli SI, Zhou Z, Zukunft S, Fisslthaler B, Andreadou I, Szabo C, Brouckaert P, Fleming I, Papapetropoulos A. Tyrosine phosphorylation of eNOS regulates myocardial survival after an ischaemic insult: role of PYK2. Cardiovasc Res 113: 926–937, 2017. doi: 10.1093/cvr/cvx058. [DOI] [PubMed] [Google Scholar]
- 95.Billecke SS, Bender AT, Kanelakis KC, Murphy PJM, Lowe ER, Kamada Y, Pratt WB, Osawa Y. hsp90 is required for heme binding and activation of apo-neuronal nitric-oxide synthase: geldanamycin-mediated oxidant generation is unrelated to any action of hsp90. J Biol Chem 277: 20504–20509, 2002. doi: 10.1074/jbc.M201940200. [DOI] [PubMed] [Google Scholar]
- 96.Bir SC, Shen X, Kavanagh TJ, Kevil CG, Pattillo CB. Control of angiogenesis dictated by picomolar superoxide levels. Free Radic Biol Med 63: 135–142, 2013. doi: 10.1016/j.freeradbiomed.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 297: 842–857, 2007. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 98.Björne H H, Petersson J, Phillipson M, Weitzberg E, Holm L, Lundberg JO. Nitrite in saliva increases gastric mucosal blood flow and mucus thickness. J Clin Invest 113: 106–114, 2004. doi: 10.1172/JCI19019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Björne H, Weitzberg E, Lundberg JO. Intragastric generation of antimicrobial nitrogen oxides from saliva–physiological and therapeutic considerations. Free Radic Biol Med 41: 1404–1412, 2006. doi: 10.1016/j.freeradbiomed.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 100.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci USA 106: 14385–14390, 2009. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boccini F, Herold S. Mechanistic studies of the oxidation of oxyhemoglobin by peroxynitrite. Biochemistry 43: 16393–16404, 2004. doi: 10.1021/bi0482250. [DOI] [PubMed] [Google Scholar]
- 102.Bode-Böger SM, Scalera F, Ignarro LJ. The L-arginine paradox: Importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol Ther 114: 295–306, 2007. doi: 10.1016/j.pharmthera.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 103.Böger RH. The pharmacodynamics of L-arginine. J Nutr 137, Suppl 2: 1650S–1655S, 2007. doi: 10.1093/jn/137.6.1650S. [DOI] [PubMed] [Google Scholar]
- 104.Böger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, Schulze F, Xanthakis V, Benndorf RA, Vasan RS. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation 119: 1592–1600, 2009. doi: 10.1161/CIRCULATIONAHA.108.838268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boerrigter G, Costello-Boerrigter LC, Cataliotti A, Tsuruda T, Harty GJ, Lapp H, Stasch JP, Burnett JC Jr. Cardiorenal and humoral properties of a novel direct soluble guanylate cyclase stimulator BAY 41-2272 in experimental congestive heart failure. Circulation 107: 686–689, 2003. doi: 10.1161/01.CIR.0000055737.15443.F8. [DOI] [PubMed] [Google Scholar]
- 106.Böger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr 134, Suppl: 2842S–2847S, 2004. doi: 10.1093/jn/134.10.2842S. [DOI] [PubMed] [Google Scholar]
- 107.Böger RH, Bode-Böger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation 98: 1842–1847, 1998. doi: 10.1161/01.CIR.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 108.Bokoch GM, Diebold BA. Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood 100: 2692–2696, 2002. doi: 10.1182/blood-2002-04-1149. [DOI] [PubMed] [Google Scholar]
- 109.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol 33: 1897–1918, 2001. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 110.Bondi CD, Manickam N, Lee DY, Block K, Gorin Y, Abboud HE, Barnes JL. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc Nephrol 21: 93–102, 2010. doi: 10.1681/ASN.2009020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bonini MG, Miyamoto S, Di Mascio P, Augusto O. Production of the carbonate radical anion during xanthine oxidase turnover in the presence of bicarbonate. J Biol Chem 279: 51836–51843, 2004. doi: 10.1074/jbc.M406929200. [DOI] [PubMed] [Google Scholar]
- 112.Bonini MG, Radi R, Ferrer-Sueta G, Ferreira AM, Augusto O. Direct EPR detection of the carbonate radical anion produced from peroxynitrite and carbon dioxide. J Biol Chem 274: 10802–10806, 1999. doi: 10.1074/jbc.274.16.10802. [DOI] [PubMed] [Google Scholar]
- 113.Borniquel S, Jansson EA, Cole MP, Freeman BA, Lundberg JO. Nitrated oleic acid up-regulates PPARgamma and attenuates experimental inflammatory bowel disease. Free Radic Biol Med 48: 499–505, 2010. doi: 10.1016/j.freeradbiomed.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boulanger CM, Heymes C, Benessiano J, Geske RS, Lévy BI, Vanhoutte PM. Neuronal nitric oxide synthase is expressed in rat vascular smooth muscle cells: activation by angiotensin II in hypertension. Circ Res 83: 1271–1278, 1998. doi: 10.1161/01.RES.83.12.1271. [DOI] [PubMed] [Google Scholar]
- 115.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 134: 707–716, 1973. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brandes RP. Triggering mitochondrial radical release: a new function for NADPH oxidases. Hypertension 45: 847–848, 2005. doi: 10.1161/01.HYP.0000165019.32059.b2. [DOI] [PubMed] [Google Scholar]
- 117.Brandes RP, Koddenberg G, Gwinner W, Kim D-y, Kruse H-J, Busse R, Mügge A. Role of increased production of superoxide anions by NAD(P)H oxidase and xanthine oxidase in prolonged endotoxemia. Hypertension 33: 1243–1249, 1999. doi: 10.1161/01.HYP.33.5.1243. [DOI] [PubMed] [Google Scholar]
- 118.Brar SS, Kennedy TP, Sturrock AB, Huecksteadt TP, Quinn MT, Whorton AR, Hoidal JR. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am J Physiol Cell Physiol 282: C1212–C1224, 2002. doi: 10.1152/ajpcell.00496.2001. [DOI] [PubMed] [Google Scholar]
- 119.Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem 274: 23679–23682, 1999. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 120.Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 347: 768–770, 1990. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 121.Bretón-Romero R, González de Orduña C, Romero N, Sánchez-Gómez FJ, de Álvaro C, Porras A, Rodríguez-Pascual F, Laranjinha J, Radi R, Lamas S. Critical role of hydrogen peroxide signaling in the sequential activation of p38 MAPK and eNOS in laminar shear stress. Free Radic Biol Med 52: 1093–1100, 2012. doi: 10.1016/j.freeradbiomed.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 122.Brewer GJ. Iron and copper toxicity in diseases of aging, particularly atherosclerosis and Alzheimer’s disease. Exp Biol Med (Maywood) 232: 323–335, 2007. [PubMed] [Google Scholar]
- 123.Broniowska KA, Hogg N. The chemical biology of S-nitrosothiols. Antioxid Redox Signal 17: 969–980, 2012. doi: 10.1089/ars.2012.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brooks J. The action of nitrite on haemoglobin in the absence of oxygen. Proc R Soc Med 123: 368–382, 1937. doi: 10.1098/rspb.1937.0057. [DOI] [Google Scholar]
- 125.Bruegger JJ, Smith BC, Wynia-Smith SL, Marletta MA. Comparative and integrative metabolomics reveal that S-nitrosation inhibits physiologically relevant metabolic enzymes. J Biol Chem 293: 6282–6296, 2018. doi: 10.1074/jbc.M117.817700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Buchwalow IB, Podzuweit T, Bocker W, Samoilova VE, Thomas S, Wellner M, Baba HA, Robenek H, Schnekenburger J, Lerch MM. Vascular smooth muscle and nitric oxide synthase. FASEB J 16: 500–508, 2002. doi: 10.1096/fj.01-0842com. [DOI] [PubMed] [Google Scholar]
- 127.Buchwalow IB, Schulze W, Karczewski P, Kostic MM, Wallukat G, Morwinski R, Krause E-G, Müller J, Paul M, Slezak J, Luft FC, Haller H. Inducible nitric oxide synthase in the myocard. Mol Cell Biochem 217: 73–82, 2001. doi: 10.1023/A:1007286602865. [DOI] [PubMed] [Google Scholar]
- 128.Buchwalow IB, Schulze W, Kostic MM, Wallukat G, Morwinski R. Intracellular localization of inducible nitric oxide synthase in neonatal rat cardiomyocytes in culture. Acta Histochem 99: 231–240, 1997. doi: 10.1016/S0065-1281(97)80046-3. [DOI] [PubMed] [Google Scholar]
- 129.Budworth J, Meillerais S, Charles I, Powell K. Tissue distribution of the human soluble guanylate cyclases. Biochem Biophys Res Commun 263: 696–701, 1999. doi: 10.1006/bbrc.1999.1444. [DOI] [PubMed] [Google Scholar]
- 130.Bunik VI, Sievers C. Inactivation of the 2-oxo acid dehydrogenase complexes upon generation of intrinsic radical species. Eur J Biochem 269: 5004–5015, 2002. doi: 10.1046/j.1432-1033.2002.03204.x. [DOI] [PubMed] [Google Scholar]
- 131.Burger DE, Lu X, Lei M, Xiang FL, Hammoud L, Jiang M, Wang H, Jones DL, Sims SM, Feng Q. Neuronal nitric oxide synthase protects against myocardial infarction-induced ventricular arrhythmia and mortality in mice. Circulation 120: 1345–1354, 2009. doi: 10.1161/CIRCULATIONAHA.108.846402. [DOI] [PubMed] [Google Scholar]
- 132.Burmester T, Ebner B, Weich B, Hankeln T. Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues. Mol Biol Evol 19: 416–421, 2002. doi: 10.1093/oxfordjournals.molbev.a004096. [DOI] [PubMed] [Google Scholar]
- 133.Burmester T, Hankeln T. Function and evolution of vertebrate globins. Acta Physiol (Oxf) 211: 501–514, 2014. doi: 10.1111/apha.12312. [DOI] [PubMed] [Google Scholar]
- 134.Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature 407: 520–523, 2000. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- 135.Burritt JB, Busse SC, Gizachew D, Siemsen DW, Quinn MT, Bond CW, Dratz EA, Jesaitis AJ. Antibody imprint of a membrane protein surface. Phagocyte flavocytochrome b. J Biol Chem 273: 24847–24852, 1998. doi: 10.1074/jbc.273.38.24847. [DOI] [PubMed] [Google Scholar]
- 136.Burwell LS, Nadtochiy SM, Brookes PS. Cardioprotection by metabolic shut-down and gradual wake-up. J Mol Cell Cardiol 46: 804–810, 2009. doi: 10.1016/j.yjmcc.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Butler R, Morris AD, Belch JJF, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension 35: 746–751, 2000. doi: 10.1161/01.HYP.35.3.746. [DOI] [PubMed] [Google Scholar]
- 138.Buxton GV, Elliot AJ. Rate-constant for reaction of hydroxyl radicals with bicarbonate ions. Radiat Phys Chem 27: 241–243, 1986. doi: 10.1016/1359-0197(86)90059-7. [DOI] [Google Scholar]
- 139.Cacanyiova S, Kristek F, Gerova M, Krenek P, Klimas J. Effect of chronic nNOS inhibition on blood pressure, vasoactivity, and arterial wall structure in Wistar rats. Nitric Oxide 20: 304–310, 2009. doi: 10.1016/j.niox.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 140.Cai B, Lin Y, Xue XH, Fang L, Wang N, Wu ZY. TAT-mediated delivery of neuroglobin protects against focal cerebral ischemia in mice. Exp Neurol 227: 224–231, 2011. doi: 10.1016/j.expneurol.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 141.Cai H, Davis ME, Drummond GR, Harrison DG. Induction of endothelial NO synthase by hydrogen peroxide via a Ca(2+)/calmodulin-dependent protein kinase II/janus kinase 2-dependent pathway. Arterioscler Thromb Vasc Biol 21: 1571–1576, 2001. doi: 10.1161/hq1001.097028. [DOI] [PubMed] [Google Scholar]
- 142.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 24: 471–478, 2003. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 143.Cai S, Alp NJ, McDonald D, Smith I, Kay J, Canevari L, Heales S, Channon KM. GTP cyclohydrolase I gene transfer augments intracellular tetrahydrobiopterin in human endothelial cells: effects on nitric oxide synthase activity, protein levels and dimerisation. Cardiovasc Res 55: 838–849, 2002. doi: 10.1016/S0008-6363(02)00460-1. [DOI] [PubMed] [Google Scholar]
- 144.Caldwell RW, Rodriguez PC, Toque HA, Narayanan SP, Caldwell RB. Arginase: A Multifaceted Enzyme Important in Health and Disease. Physiol Rev 98: 641–665, 2018. doi: 10.1152/physrev.00037.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Campbell MG, Underbakke ES, Potter CS, Carragher B, Marletta MA. Single-particle EM reveals the higher-order domain architecture of soluble guanylate cyclase. Proc Natl Acad Sci USA 111: 2960–2965, 2014. doi: 10.1073/pnas.1400711111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cannon RO III, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, Shelhamer JH, Gladwin MT. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest 108: 279–287, 2001. doi: 10.1172/JCI200112761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cantu-Medellin N, Kelley EE. Xanthine oxidoreductase-catalyzed reactive species generation: A process in critical need of reevaluation. Redox Biol 1: 353–358, 2013. doi: 10.1016/j.redox.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marbán E, Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation 104: 2407–2411, 2001. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- 149.Carballal S, Cuevasanta E, Yadav PK, Gherasim C, Ballou DP, Alvarez B, Banerjee R. Kinetics of Nitrite Reduction and Peroxynitrite Formation by Ferrous Heme in Human Cystathionine β-Synthase. J Biol Chem 291: 8004–8013, 2016. doi: 10.1074/jbc.M116.718734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai A-L, Berka V, Zweier JL. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem 282: 879–887, 2007. doi: 10.1074/jbc.M603606200. [DOI] [PubMed] [Google Scholar]
- 151.Carlström M, Lai EY, Ma Z, Patzak A, Brown RD, Persson AEG. Role of NOX2 in the regulation of afferent arteriole responsiveness. Am J Physiol Regul Integr Comp Physiol 296: R72–R79, 2009. doi: 10.1152/ajpregu.90718.2008. [DOI] [PubMed] [Google Scholar]
- 152.Case AJ, Li S, Basu U, Tian J, Zimmerman MC. Mitochondrial-localized NADPH oxidase 4 is a source of superoxide in angiotensin II-stimulated neurons. Am J Physiol Heart Circ Physiol 305: H19–H28, 2013. doi: 10.1152/ajpheart.00974.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cassina AM, Hodara R, Souza JM, Thomson L, Castro L, Ischiropoulos H, Freeman BA, Radi R. Cytochrome c nitration by peroxynitrite. J Biol Chem 275: 21409–21415, 2000. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 154.Castro L, Eiserich JP, Sweeney S, Radi R, Freeman BA. Cytochrome c: a catalyst and target of nitrite-hydrogen peroxide-dependent protein nitration. Arch Biochem Biophys 421: 99–107, 2004. doi: 10.1016/j.abb.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 155.Castro L, Rodriguez M, Radi R. Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J Biol Chem 269: 29409–29415, 1994. [PubMed] [Google Scholar]
- 156.Caughey GE, Cleland LG, Penglis PS, Gamble JR, James MJ. Roles of cyclooxygenase (COX)-1 and COX-2 in prostanoid production by human endothelial cells: selective up-regulation of prostacyclin synthesis by COX-2. J Immunol 167: 2831–2838, 2001. doi: 10.4049/jimmunol.167.5.2831. [DOI] [PubMed] [Google Scholar]
- 157.Chambers DE, Parks DA, Patterson G, Roy R, McCord JM, Yoshida S, Parmley LF, Downey JM. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J Mol Cell Cardiol 17: 145–152, 1985. doi: 10.1016/S0022-2828(85)80017-1. [DOI] [PubMed] [Google Scholar]
- 158.Chandel NS, Tuveson DA. The promise and perils of antioxidants for cancer patients. N Engl J Med 371: 177–178, 2014. doi: 10.1056/NEJMcibr1405701. [DOI] [PubMed] [Google Scholar]
- 159.Chen CY, Yi L, Jin X, Zhang T, Fu YJ, Zhu JD, Mi MT, Zhang QY, Ling WH, Yu B. Inhibitory effect of delphinidin on monocyte-endothelial cell adhesion induced by oxidized low-density lipoprotein via ROS/p38MAPK/NF-κB pathway. Cell Biochem Biophys 61: 337–348, 2011. doi: 10.1007/s12013-011-9216-2. [DOI] [PubMed] [Google Scholar]
- 160.Chen CA, De Pascali F, Basye A, Hemann C, Zweier JL. Redox modulation of endothelial nitric oxide synthase by glutaredoxin-1 through reversible oxidative post-translational modification. Biochemistry 52: 6712–6723, 2013. doi: 10.1021/bi400404s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen CA, Druhan LJ, Varadharaj S, Chen YR, Zweier JL. Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme. J Biol Chem 283: 27038–27047, 2008. doi: 10.1074/jbc.M802269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468: 1115–1118, 2010. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen F, Qian L-H, Deng B, Liu Z-M, Zhao Y, Le YY. Resveratrol protects vascular endothelial cells from high glucose-induced apoptosis through inhibition of NADPH oxidase activation-driven oxidative stress. CNS Neurosci Ther 19: 675–681, 2013. doi: 10.1111/cns.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen F, Yu Y, Haigh S, Johnson J, Lucas R, Stepp DW, Fulton DJR. Regulation of NADPH oxidase 5 by protein kinase C isoforms. PLoS One 9: e88405, 2014. doi: 10.1371/journal.pone.0088405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther 319: 1405–1412, 2006. doi: 10.1124/jpet.106.110262. [DOI] [PubMed] [Google Scholar]
- 166.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001. doi: 10.1016/S0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 167.Chin-Dusting JPF, Willems L, Kaye DM. L-arginine transporters in cardiovascular disease: a novel therapeutic target. Pharmacol Ther 116: 428–436, 2007. doi: 10.1016/j.pharmthera.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 168.Chiva-Blanch G, Urpi-Sarda M, Ros E, Arranz S, Valderas-Martínez P, Casas R, Sacanella E, Llorach R, Lamuela-Raventos RM, Andres-Lacueva C, Estruch R. Dealcoholized red wine decreases systolic and diastolic blood pressure and increases plasma nitric oxide: short communication. Circ Res 111: 1065–1068, 2012. doi: 10.1161/CIRCRESAHA.112.275636. [DOI] [PubMed] [Google Scholar]
- 169.Cho HJ, Xie QW, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Nathan C. Calmodulin is a subunit of nitric oxide synthase from macrophages. J Exp Med 176: 599–604, 1992. doi: 10.1084/jem.176.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, James AM, Cochemé HM, Reinhold J, Lilley KS, Partridge L, Fearnley IM, Robinson AJ, Hartley RC, Smith RAJ, Krieg T, Brookes PS, Murphy MP. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med 19: 753–759, 2013. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa ASH, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515: 431–435, 2014. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chouchani ET, Pell VR, James AM, Work LM, Saeb-Parsy K, Frezza C, Krieg T, Murphy MP. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab 23: 254–263, 2016. doi: 10.1016/j.cmet.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 173.Chuaiphichai S, McNeill E, Douglas G, Crabtree MJ, Bendall JK, Hale AB, Alp NJ, Channon KM. Cell-autonomous role of endothelial GTP cyclohydrolase 1 and tetrahydrobiopterin in blood pressure regulation. Hypertension 64: 530–540, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cifuentes-Pagano E, Meijles DN, Pagano PJ. The quest for selective nox inhibitors and therapeutics: challenges, triumphs and pitfalls. Antioxid Redox Signal 20: 2741–2754, 2014. doi: 10.1089/ars.2013.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cifuentes-Pagano ME, Meijles DN, Pagano PJ. Nox Inhibitors & Therapies: Rational Design of Peptidic and Small Molecule Inhibitors. Curr Pharm Des 21: 6023–6035, 2015. doi: 10.2174/1381612821666151029112013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Clancy RM, Leszczynska-Piziak J, Abramson SB. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest 90: 1116–1121, 1992. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Clark RA, Leidal KG, Pearson DW, Nauseef WM. NADPH oxidase of human neutrophils. Subcellular localization and characterization of an arachidonate-activatable superoxide-generating system. J Biol Chem 262: 4065–4074, 1987. [PubMed] [Google Scholar]
- 178.Clifton HL, Garten RS, Lee JF, Rossman MJ, Clifford JR, Hydren JR, Stehlik J, Richardson RS, Wray DW. The impact of acute oral tetrahydrobiopterin on vascular function in heart failure patients with reduced ejection fraction. FASEB J 30: 735.5, 2016. [Google Scholar]
- 179.Colston JT, de la Rosa SD, Strader JR, Anderson MA, Freeman GL. H2O2 activates Nox4 through PLA2-dependent arachidonic acid production in adult cardiac fibroblasts. FEBS Lett 579: 2533–2540, 2005. doi: 10.1016/j.febslet.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 180.Cooper CE. Nitric oxide and iron proteins. Biochim Biophys Acta 1411: 290–309, 1999. doi: 10.1016/S0005-2728(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 181.Cortese-Krott MM, Rodriguez-Mateos A, Sansone R, Kuhnle GG, Thasian-Sivarajah S, Krenz T, Horn P, Krisp C, Wolters D, Heiß C, Kröncke KD, Hogg N, Feelisch M, Kelm M. Human red blood cells at work: identification and visualization of erythrocytic eNOS activity in health and disease. Blood 120: 4229–4237, 2012. doi: 10.1182/blood-2012-07-442277. [DOI] [PubMed] [Google Scholar]
- 182.Corti P, Ieraci M, Tejero J. Characterization of zebrafish neuroglobin and cytoglobins 1 and 2: Zebrafish cytoglobins provide insights into the transition from six-coordinate to five-coordinate globins. Nitric Oxide 53: 22–34, 2016. doi: 10.1016/j.niox.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 183.Corti P, Tejero J, Gladwin MT. Evidence mounts that red cells and deoxyhemoglobin can reduce nitrite to bioactive NO to mediate intravascular endocrine NO signaling: commentary on “Anti-platelet effects of dietary nitrate in healthy volunteers: involvement of cGMP and influence of sex”. Free Radic Biol Med 65: 1518–1520, 2013. doi: 10.1016/j.freeradbiomed.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 184.Corti P, Xue J, Tejero J, Wajih N, Sun M, Stolz DB, Tsang M, Kim-Shapiro DB, Gladwin MT. Globin X is a six-coordinate globin that reduces nitrite to nitric oxide in fish red blood cells. Proc Natl Acad Sci USA 113: 8538–8543, 2016. doi: 10.1073/pnas.1522670113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO III, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505, 2003. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 186.Cosentino F, Hürlimann D, Delli Gatti C, Chenevard R, Blau N, Alp NJ, Channon KM, Eto M, Lerch P, Enseleit F, Ruschitzka F, Volpe M, Lüscher TF, Noll G. Chronic treatment with tetrahydrobiopterin reverses endothelial dysfunction and oxidative stress in hypercholesterolaemia. Heart 94: 487–492, 2008. doi: 10.1136/hrt.2007.122184. [DOI] [PubMed] [Google Scholar]
- 187.Costa AD, Garlid KD. Intramitochondrial signaling: interactions among mitoKATP, PKCepsilon, ROS, and MPT. Am J Physiol Heart Circ Physiol 295: H874–H882, 2008. doi: 10.1152/ajpheart.01189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Costa ED, Rezende BA, Cortes SF, Lemos VS. Neuronal Nitric Oxide Synthase in Vascular Physiology and Diseases. Front Physiol 7: 206, 2016. doi: 10.3389/fphys.2016.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Crabtree MJ, Brixey R, Batchelor H, Hale AB, Channon KM. Integrated redox sensor and effector functions for tetrahydrobiopterin- and glutathionylation-dependent endothelial nitric-oxide synthase uncoupling. J Biol Chem 288: 561–569, 2013. doi: 10.1074/jbc.M112.415992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am J Physiol Heart Circ Physiol 294: H1530–H1540, 2008. doi: 10.1152/ajpheart.00823.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Crabtree MJ, Tatham AL, Al-Wakeel Y, Warrick N, Hale AB, Cai S, Channon KM, Alp NJ. Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J Biol Chem 284: 1136–1144, 2009. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- 192.Crabtree MJ, Tatham AL, Hale AB, Alp NJ, Channon KM. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J Biol Chem 284: 28128–28136, 2009. doi: 10.1074/jbc.M109.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, Keaney JF Jr. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation 124: 731–740, 2011. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Craige SM, Kant S, Reif M, Chen K, Pei Y, Angoff R, Sugamura K, Fitzgibbons T, Keaney JF Jr. Endothelial NADPH oxidase 4 protects ApoE−/− mice from atherosclerotic lesions. Free Radic Biol Med 89: 1–7, 2015. doi: 10.1016/j.freeradbiomed.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Crane BR, Rosenfeld RJ, Arvai AS, Ghosh DK, Ghosh S, Tainer JA, Stuehr DJ, Getzoff ED. N-terminal domain swapping and metal ion binding in nitric oxide synthase dimerization. EMBO J 18: 6271–6281, 1999. doi: 10.1093/emboj/18.22.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD Jr, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood 107: 566–574, 2006. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cross AR, Jones OTG. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem J 237: 111–116, 1986. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cross AR, Segal AW. The NADPH oxidase of professional phagocytes–prototype of the NOX electron transport chain systems. Biochim Biophys Acta 1657: 1–22, 2004. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Csányi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egaña L, Lopes LR, Jackson HM, Kelley EE, Pagano PJ. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med 51: 1116–1125, 2011. doi: 10.1016/j.freeradbiomed.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Csányi G, Pagano PJ. Strategies aimed at Nox4 oxidase inhibition employing peptides from Nox4 B-loop and C-terminus and p22 (phox) N-terminus: an elusive target. Int J Hypertens 2013: 842827, 2013. doi: 10.1155/2013/842827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97: 900–907, 2005. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 202.Cunnington C, Van Assche T, Shirodaria C, Kylintireas I, Lindsay AC, Lee JM, Antoniades C, Margaritis M, Lee R, Cerrato R, Crabtree MJ, Francis JM, Sayeed R, Ratnatunga C, Pillai R, Choudhury RP, Neubauer S, Channon KM. Systemic and vascular oxidation limits the efficacy of oral tetrahydrobiopterin treatment in patients with coronary artery disease. Circulation 125: 1356–1366, 2012. doi: 10.1161/CIRCULATIONAHA.111.038919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cyrus T, Witztum JL, Rader DJ, Tangirala R, Fazio S, Linton MF, Funk CD. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice. J Clin Invest 103: 1597–1604, 1999. doi: 10.1172/JCI5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Daff S. NO synthase: structures and mechanisms. Nitric Oxide 23: 1–11, 2010. doi: 10.1016/j.niox.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 205.Dahn H, Loewe L, Bunton C. Über die Oxydation von Ascorbinsäure durch salpetrige Säure Teil VI: Übersicht und Diskussion der Ergebnisse. 18. Mitteilung über Reduktone und 1, 2, 3‐Tricarbonylverbindungen. Helv Chim Acta 43: 320–333, 1960. doi: 10.1002/hlca.19600430143. [DOI] [Google Scholar]
- 206.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta 1797: 897–906, 2010. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 207.Daiber A, Steven S, Weber A, Shuvaev VV, Muzykantov VR, Laher I, Li H, Lamas S, Münzel T. Targeting vascular (endothelial) dysfunction. Br J Pharmacol 174: 1591–1619, 2017. doi: 10.1111/bph.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dandapat A, Hu C, Sun L, Mehta JL. Small concentrations of oxLDL induce capillary tube formation from endothelial cells via LOX-1-dependent redox-sensitive pathway. Arterioscler Thromb Vasc Biol 27: 2435–2442, 2007. doi: 10.1161/ATVBAHA.107.152272. [DOI] [PubMed] [Google Scholar]
- 209.Dao VT, Casas AI, Maghzal GJ, Seredenina T, Kaludercic N, Robledinos-Anton N, Di Lisa F, Stocker R, Ghezzi P, Jaquet V, Cuadrado A, Schmidt HH. Pharmacology and Clinical Drug Candidates in Redox Medicine. Antioxid Redox Signal 23: 1113–1129, 2015. doi: 10.1089/ars.2015.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dare AJ, Bolton EA, Pettigrew GJ, Bradley JA, Saeb-Parsy K, Murphy MP. Protection against renal ischemia-reperfusion injury in vivo by the mitochondria targeted antioxidant MitoQ. Redox Biol 5: 163–168, 2015. doi: 10.1016/j.redox.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dare AJ, Logan A, Prime TA, Rogatti S, Goddard M, Bolton EM, Bradley JA, Pettigrew GJ, Murphy MP, Saeb-Parsy K. The mitochondria-targeted anti-oxidant MitoQ decreases ischemia-reperfusion injury in a murine syngeneic heart transplant model. J Heart Lung Transplant 34: 1471–1480, 2015. doi: 10.1016/j.healun.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Datla SR, McGrail DJ, Vukelic S, Huff LP, Lyle AN, Pounkova L, Lee M, Seidel-Rogol B, Khalil MK, Hilenski LL, Terada LS, Dawson MR, Lassègue B, Griendling KK. Poldip2 controls vascular smooth muscle cell migration by regulating focal adhesion turnover and force polarization. Am J Physiol Heart Circ Physiol 307: H945–H957, 2014. doi: 10.1152/ajpheart.00918.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest 94: 437–444, 1994. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.de A Paes AM, Veríssimo-Filho S, Guimarães LL, Silva AC, Takiuti JT, Santos CX, Janiszewski M, Laurindo FR, Lopes LR. Protein disulfide isomerase redox-dependent association with p47(phox): evidence for an organizer role in leukocyte NADPH oxidase activation. J Leukoc Biol 90: 799–810, 2011. doi: 10.1189/jlb.0610324. [DOI] [PubMed] [Google Scholar]
- 215.De Pascali F, Hemann C, Samons K, Chen CA, Zweier JL. Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and S-glutathionylation. Biochemistry 53: 3679–3688, 2014. doi: 10.1021/bi500076r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dejam A, Hunter CJ, Schechter AN, Gladwin MT. Emerging role of nitrite in human biology. Blood Cells Mol Dis 32: 423–429, 2004. doi: 10.1016/j.bcmd.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 217.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO III, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation 116: 1821–1831, 2007. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 218.DeLeo FR, Yu L, Burritt JB, Loetterle LR, Bond CW, Jesaitis AJ, Quinn MT. Mapping sites of interaction of p47-phox and flavocytochrome b with random-sequence peptide phage display libraries. Proc Natl Acad Sci USA 92: 7110–7114, 1995. doi: 10.1073/pnas.92.15.7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Demicheli V, Moreno DM, Jara GE, Lima A, Carballal S, Ríos N, Batthyany C, Ferrer-Sueta G, Quijano C, Estrín DA, Martí MA, Radi R. Mechanism of the Reaction of Human Manganese Superoxide Dismutase with Peroxynitrite: Nitration of Critical Tyrosine 34. Biochemistry 55: 3403–3417, 2016. doi: 10.1021/acs.biochem.6b00045. [DOI] [PubMed] [Google Scholar]
- 220.Demicheli V, Quijano C, Alvarez B, Radi R. Inactivation and nitration of human superoxide dismutase (SOD) by fluxes of nitric oxide and superoxide. Free Radic Biol Med 42: 1359–1368, 2007. doi: 10.1016/j.freeradbiomed.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 221.Denicola A, Souza JM, Radi R. Diffusion of peroxynitrite across erythrocyte membranes. Proc Natl Acad Sci USA 95: 3566–3571, 1998. doi: 10.1073/pnas.95.7.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Depre C, Havaux X, Renkin J, Vanoverschelde JLJ, Wijns W. Expression of inducible nitric oxide synthase in human coronary atherosclerotic plaque. Cardiovasc Res 41: 465–472, 1999. doi: 10.1016/S0008-6363(98)00304-6. [DOI] [PubMed] [Google Scholar]
- 223.Derbyshire ER, Marletta MA. Structure and regulation of soluble guanylate cyclase. Annu Rev Biochem 81: 533–559, 2012. doi: 10.1146/annurev-biochem-050410-100030. [DOI] [PubMed] [Google Scholar]
- 224.Desco MC, Asensi M, Márquez R, Martínez-Valls J, Vento M, Pallardó FV, Sastre J, Viña J. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes 51: 1118–1124, 2002. doi: 10.2337/diabetes.51.4.1118. [DOI] [PubMed] [Google Scholar]
- 225.Desideri G, Kwik-Uribe C, Grassi D, Necozione S, Ghiadoni L, Mastroiacovo D, Raffaele A, Ferri L, Bocale R, Lechiara MC, Marini C, Ferri C. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the Cocoa, Cognition, and Aging (CoCoA) study. Hypertension 60: 794–801, 2012. doi: 10.1161/HYPERTENSIONAHA.112.193060. [DOI] [PubMed] [Google Scholar]
- 226.Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovasc Res 75: 327–338, 2007. doi: 10.1016/j.cardiores.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens 18: 655–673, 2000. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 228.Dhaunsi GS, Paintlia MK, Kaur J, Turner RB. NADPH oxidase in human lung fibroblasts. J Biomed Sci 11: 617–622, 2004. doi: 10.1007/BF02256127. [DOI] [PubMed] [Google Scholar]
- 229.Di Marco E, Gray SP, Chew P, Kennedy K, Cooper ME, Schmidt HH, Jandeleit-Dahm KA. Differential effects of NOX4 and NOX1 on immune cell-mediated inflammation in the aortic sinus of diabetic ApoE−/− mice. Clin Sci (Lond) 130: 1363–1374, 2016. doi: 10.1042/CS20160249. [DOI] [PubMed] [Google Scholar]
- 230.Di Marco E, Gray SP, Kennedy K, Szyndralewiez C, Lyle AN, Lassègue B, Griendling KK, Cooper ME, Schmidt HHHW, Jandeleit-Dahm KAM. NOX4-derived reactive oxygen species limit fibrosis and inhibit proliferation of vascular smooth muscle cells in diabetic atherosclerosis. Free Radic Biol Med 97: 556–567, 2016. doi: 10.1016/j.freeradbiomed.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Diaz B, Shani G, Pass I, Anderson D, Quintavalle M, Courtneidge SA. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal 2: ra53, 2009. doi: 10.1126/scisignal.2000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol 7: 504–511, 2011. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Diebold L, Chandel NS. Mitochondrial ROS regulation of proliferating cells. Free Radic Biol Med 100: 86–93, 2016. doi: 10.1016/j.freeradbiomed.2016.04.198. [DOI] [PubMed] [Google Scholar]
- 234.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51: 1289–1301, 2011. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Dikalova A, Clempus R, Lassègue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 112: 2668–2676, 2005. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 236.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 237.Dodd-O JM, Pearse DB. Effect of the NADPH oxidase inhibitor apocynin on ischemia-reperfusion lung injury. Am J Physiol Heart Circ Physiol 279: H303–H312, 2000. doi: 10.1152/ajpheart.2000.279.1.H303. [DOI] [PubMed] [Google Scholar]
- 238.Doerries C, Grote K, Hilfiker-Kleiner D, Luchtefeld M, Schaefer A, Holland SM, Sorrentino S, Manes C, Schieffer B, Drexler H, Landmesser U. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ Res 100: 894–903, 2007. doi: 10.1161/01.RES.0000261657.76299.ff. [DOI] [PubMed] [Google Scholar]
- 239.Dominguez-Rodriguez A, Samimi-Fard S, Abreu-Gonzalez P, Garcia-Gonzalez MJ, Kaski JC. Prognostic value of admission myeloperoxidase levels in patients with ST-segment elevation myocardial infarction and cardiogenic shock. Am J Cardiol 101: 1537–1540, 2008. doi: 10.1016/j.amjcard.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 240.Dong J-Y, Qin L-Q, Zhang Z, Zhao Y, Wang J, Arigoni F, Zhang W. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J 162: 959–965, 2011. doi: 10.1016/j.ahj.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 241.Doroshow JH, Gaur S, Markel S, Lu J, van Balgooy J, Synold TW, Xi B, Wu X, Juhasz A. Effects of iodonium-class flavin dehydrogenase inhibitors on growth, reactive oxygen production, cell cycle progression, NADPH oxidase 1 levels, and gene expression in human colon cancer cells and xenografts. Free Radic Biol Med 57: 162–175, 2013. doi: 10.1016/j.freeradbiomed.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 243.Douglas G, Bendall JK, Crabtree MJ, Tatham AL, Carter EE, Hale AB, Channon KM. Endothelial-specific Nox2 overexpression increases vascular superoxide and macrophage recruitment in ApoE−/− mice. Cardiovasc Res 94: 20–29, 2012. doi: 10.1093/cvr/cvs026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Dourron HM, Jacobson GM, Park JL, Liu J, Reddy DJ, Scheel ML, Pagano PJ. Perivascular gene transfer of NADPH oxidase inhibitor suppresses angioplasty-induced neointimal proliferation of rat carotid artery. Am J Physiol Heart Circ Physiol 288: H946–H953, 2005. doi: 10.1152/ajpheart.00413.2004. [DOI] [PubMed] [Google Scholar]
- 245.Doussière J, Gaillard J, Vignais PV. Electron transfer across the O2- generating flavocytochrome b of neutrophils. Evidence for a transition from a low-spin state to a high-spin state of the heme iron component. Biochemistry 35: 13400–13410, 1996. doi: 10.1021/bi960916b. [DOI] [PubMed] [Google Scholar]
- 246.Doussière J, Vignais PV. Diphenylene iodonium as an inhibitor of the NADPH oxidase complex of bovine neutrophils. Factors controlling the inhibitory potency of diphenylene iodonium in a cell-free system of oxidase activation. Eur J Biochem 208: 61–71, 1992. doi: 10.1111/j.1432-1033.1992.tb17159.x. [DOI] [PubMed] [Google Scholar]
- 247.Doyle MP, LePoire DM, Pickering RA. Oxidation of hemoglobin and myoglobin by alkyl nitrites inhibition by oxygen. J Biol Chem 256: 12399–12404, 1981. [PubMed] [Google Scholar]
- 248.Doyle MP, Pickering RA, DeWeert TM, Hoekstra JW, Pater D. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J Biol Chem 256: 12393–12398, 1981. [PubMed] [Google Scholar]
- 249.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 250.Druhan LJ, Forbes SP, Pope AJ, Chen CA, Zweier JL, Cardounel AJ. Regulation of eNOS-derived superoxide by endogenous methylarginines. Biochemistry 47: 7256–7263, 2008. doi: 10.1021/bi702377a. [DOI] [PubMed] [Google Scholar]
- 251.Drummond GR, Cai H, Davis ME, Ramasamy S, Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res 86: 347–354, 2000. doi: 10.1161/01.RES.86.3.347. [DOI] [PubMed] [Google Scholar]
- 252.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 10: 453–471, 2011. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Drummond GR, Sobey CG. Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends Endocrinol Metab 25: 452–463, 2014. doi: 10.1016/j.tem.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 254.Drury PP, Davidson JO, Mathai S, van den Heuij LG, Ji H, Bennet L, Tan S, Silverman RB, Gunn AJ. nNOS inhibition during profound asphyxia reduces seizure burden and improves survival of striatal phenotypic neurons in preterm fetal sheep. Neuropharmacology 83: 62–70, 2014. doi: 10.1016/j.neuropharm.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Drury PP, Davidson JO, van den Heuij LG, Tan S, Silverman RB, Ji H, Blood AB, Fraser M, Bennet L, Gunn AJ. Partial neuroprotection by nNOS inhibition during profound asphyxia in preterm fetal sheep. Exp Neurol 250: 282–292, 2013. doi: 10.1016/j.expneurol.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Du J, Fan LM, Mai A, Li J-M. Crucial roles of Nox2-derived oxidative stress in deteriorating the function of insulin receptors and endothelium in dietary obesity of middle-aged mice. Br J Pharmacol 170: 1064–1077, 2013. doi: 10.1111/bph.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest 108: 1341–1348, 2001. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, Zweier JL. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc Natl Acad Sci USA 104: 15081–15086, 2007. doi: 10.1073/pnas.0702986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol 34: 906–911, 2007. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Düzgünçinar O, Yavuz B, Hazirolan T, Deniz A, Tokgözoğlu SL, Akata D, Demirpençe E. Plasma myeloperoxidase is related to the severity of coronary artery disease. Acta Cardiol 63: 147–152, 2008. doi: 10.2143/AC.63.2.2029520. [DOI] [PubMed] [Google Scholar]
- 261.Dykhuizen RS, Frazer R, Duncan C, Smith CC, Golden M, Benjamin N, Leifert C. Antimicrobial effect of acidified nitrite on gut pathogens: importance of dietary nitrate in host defense. Antimicrob Agents Chemother 40: 1422–1425, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Edens WA, Sharling L, Cheng G, Shapira R, Kinkade JM, Lee T, Edens HA, Tang X, Sullards C, Flaherty DB, Benian GM, Lambeth JD. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J Cell Biol 154: 879–891, 2001. doi: 10.1083/jcb.200103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Eguchi D, D’Uscio LV, Wambi C, Weiler D, Kovesdi I, O’Brien T, Katusic ZS. Inhibitory effect of recombinant iNOS gene expression on vasomotor function of canine basilar artery. Am J Physiol Heart Circ Physiol 283: H2560–H2566, 2002. doi: 10.1152/ajpheart.00415.2002. [DOI] [PubMed] [Google Scholar]
- 264.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN Jr, Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry 35: 6976–6983, 1996. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 265.Ellmark SHM, Dusting GJ, Fui MNT, Guzzo-Pernell N, Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res 65: 495–504, 2005. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 266.Ellsworth ML. The red blood cell as an oxygen sensor: what is the evidence? Acta Physiol Scand 168: 551–559, 2000. doi: 10.1046/j.1365-201x.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 267.Elrod JW, Calvert JW, Gundewar S, Bryan NS, Lefer DJ. Nitric oxide promotes distant organ protection: evidence for an endocrine role of nitric oxide. Proc Natl Acad Sci USA 105: 11430–11435, 2008. doi: 10.1073/pnas.0800700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Enroth C, Eger BT, Okamoto K, Nishino T, Nishino T, Pai EF. Crystal structures of bovine milk xanthine dehydrogenase and xanthine oxidase: structure-based mechanism of conversion. Proc Natl Acad Sci USA 97: 10723–10728, 2000. doi: 10.1073/pnas.97.20.10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Erdmann E, Semigran MJ, Nieminen MS, Gheorghiade M, Agrawal R, Mitrovic V, Mebazaa A. Cinaciguat, a soluble guanylate cyclase activator, unloads the heart but also causes hypotension in acute decompensated heart failure. Eur Heart J 34: 57–67, 2013. doi: 10.1093/eurheartj/ehs196. [DOI] [PubMed] [Google Scholar]
- 270.Erez A, Nagamani SC, Shchelochkov OA, Premkumar MH, Campeau PM, Chen Y, Garg HK, Li L, Mian A, Bertin TK, Black JO, Zeng H, Tang Y, Reddy AK, Summar M, O’Brien WE, Harrison DG, Mitch WE, Marini JC, Aschner JL, Bryan NS, Lee B. Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat Med 17: 1619–1626, 2011. doi: 10.1038/nm.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Erwin PA, Lin AJ, Golan DE, Michel T. Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem 280: 19888–19894, 2005. doi: 10.1074/jbc.M413058200. [DOI] [PubMed] [Google Scholar]
- 272.Erwin PA, Mitchell DA, Sartoretto J, Marletta MA, Michel T. Subcellular targeting and differential S-nitrosylation of endothelial nitric-oxide synthase. J Biol Chem 281: 151–157, 2006. doi: 10.1074/jbc.M510421200. [DOI] [PubMed] [Google Scholar]
- 273.Evangelista AM, Thompson MD, Bolotina VM, Tong X, Cohen RA. Nox4- and Nox2-dependent oxidant production is required for VEGF-induced SERCA cysteine-674 S-glutathiolation and endothelial cell migration. Free Radic Biol Med 53: 2327–2334, 2012. doi: 10.1016/j.freeradbiomed.2012.10.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Evgenov OV, Pacher P, Schmidt PM, Haskó G, Schmidt HHHW, Stasch J-P. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov 5: 755–768, 2006. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Fan J, Frey RS, Rahman A, Malik AB. Role of neutrophil NADPH oxidase in the mechanism of tumor necrosis factor-alpha -induced NF-kappa B activation and intercellular adhesion molecule-1 expression in endothelial cells. J Biol Chem 277: 3404–3411, 2002. doi: 10.1074/jbc.M110054200. [DOI] [PubMed] [Google Scholar]
- 276.Fan LM, Teng L, Li JM. Knockout of p47 phox uncovers a critical role of p40 phox in reactive oxygen species production in microvascular endothelial cells. Arterioscler Thromb Vasc Biol 29: 1651–1656, 2009. doi: 10.1161/ATVBAHA.109.191502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Farah C, Michel LYM, Balligand JL. Nitric oxide signalling in cardiovascular health and disease. Nat Rev Cardiol 15: 292–316, 2018. doi: 10.1038/nrcardio.2017.224. [DOI] [PubMed] [Google Scholar]
- 278.Farquharson CA, Butler R, Hill A, Belch JJF, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation 106: 221–226, 2002. doi: 10.1161/01.CIR.0000022140.61460.1D. [DOI] [PubMed] [Google Scholar]
- 279.Fast W, Nikolic D, Van Breemen RB, Silverman RB. Mechanistic studies of the inactivation of inducible nitric oxide synthase by N-5-(1-iminoethyl)-L-ornithine (L-NIO). J Am Chem Soc 121: 903–916, 1999. doi: 10.1021/ja982318l. [DOI] [Google Scholar]
- 280.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 106: 466–472, 2002. doi: 10.1161/01.CIR.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 281.Fenton HJH. LXXIII. Oxidation of tartaric acid in presence of iron. J Chem Soc Trans 65: 899–910, 1894. doi: 10.1039/CT8946500899. [DOI] [Google Scholar]
- 282.Feoli AM, Macagnan FE, Piovesan CH, Bodanese LC, Siqueira IR. Xanthine oxidase activity is associated with risk factors for cardiovascular disease and inflammatory and oxidative status markers in metabolic syndrome: effects of a single exercise session. Oxid Med Cell Longev 2014: 587083, 2014. doi: 10.1155/2014/587083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem 271: 22810–22814, 1996. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- 284.Feron O, Michel JB, Sase K, Michel T. Dynamic regulation of endothelial nitric oxide synthase: complementary roles of dual acylation and caveolin interactions. Biochemistry 37: 193–200, 1998. doi: 10.1021/bi972307p. [DOI] [PubMed] [Google Scholar]
- 285.Figueroa A, Alvarez-Alvarado S, Jaime SJ, Kalfon R. l-Citrulline supplementation attenuates blood pressure, wave reflection and arterial stiffness responses to metaboreflex and cold stress in overweight men. Br J Nutr 116: 279–285, 2016. doi: 10.1017/S0007114516001811. [DOI] [PubMed] [Google Scholar]
- 286.Figueroa A, Trivino JA, Sanchez-Gonzalez MA, Vicil F. Oral L-citrulline supplementation attenuates blood pressure response to cold pressor test in young men. Am J Hypertens 23: 12–16, 2010. doi: 10.1038/ajh.2009.195. [DOI] [PubMed] [Google Scholar]
- 287.Filippatos G, Maggioni AP, Lam CSP, Pieske-Kraigher E, Butler J, Spertus J, Ponikowski P, Shah SJ, Solomon SD, Scalise AV, Mueller K, Roessig L, Bamber L, Gheorghiade M, Pieske B. Patient-reported outcomes in the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED ejection fraction (SOCRATES-PRESERVED) study. Eur J Heart Fail 19: 782–791, 2017. doi: 10.1002/ejhf.800. [DOI] [PubMed] [Google Scholar]
- 288.Finegold AA, Shatwell KP, Segal AW, Klausner RD, Dancis A. Intramembrane bis-heme motif for transmembrane electron transport conserved in a yeast iron reductase and the human NADPH oxidase. J Biol Chem 271: 31021–31024, 1996. doi: 10.1074/jbc.271.49.31021. [DOI] [PubMed] [Google Scholar]
- 289.Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol 10: 248–253, 1998. doi: 10.1016/S0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 290.Finkel T. Signal transduction by mitochondrial oxidants. J Biol Chem 287: 4434–4440, 2012. doi: 10.1074/jbc.R111.271999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 194: 7–15, 2011. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Fisslthaler B, Loot AE, Mohamed A, Busse R, Fleming I. Inhibition of endothelial nitric oxide synthase activity by proline-rich tyrosine kinase 2 in response to fluid shear stress and insulin. Circ Res 102: 1520–1528, 2008. doi: 10.1161/CIRCRESAHA.108.172072. [DOI] [PubMed] [Google Scholar]
- 293.Flaherty JP, Spruce CA, Fairfield HE, Bergstrom DE. Generation of a conditional null allele of NADPH oxidase activator 1 (NOXA1). Genesis 48: 568–575, 2010. doi: 10.1002/dvg.20655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Flam BR, Hartmann PJ, Harrell-Booth M, Solomonson LP, Eichler DC. Caveolar localization of arginine regeneration enzymes, argininosuccinate synthase, and lyase, with endothelial nitric oxide synthase. Nitric Oxide 5: 187–197, 2001. doi: 10.1006/niox.2001.0340. [DOI] [PubMed] [Google Scholar]
- 295.Fleming I. Cytochrome p450 and vascular homeostasis. Circ Res 89: 753–762, 2001. doi: 10.1161/hh2101.099268. [DOI] [PubMed] [Google Scholar]
- 296.Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch 459: 793–806, 2010. doi: 10.1007/s00424-009-0767-7. [DOI] [PubMed] [Google Scholar]
- 297.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88: E68–E75, 2001. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 298.Fleming I, Michaelis UR, Bredenkötter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-derived hyperpolarizing factor synthase (Cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res 88: 44–51, 2001. doi: 10.1161/01.RES.88.1.44. [DOI] [PubMed] [Google Scholar]
- 299.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 33: 829–837, 2012. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Folkes LK, Candeias LP, Wardman P. Kinetics and mechanisms of hypochlorous acid reactions. Arch Biochem Biophys 323: 120–126, 1995. doi: 10.1006/abbi.1995.0017. [DOI] [PubMed] [Google Scholar]
- 301.Follmann M, Ackerstaff J, Redlich G, Wunder F, Lang D, Kern A, Fey P, Griebenow N, Kroh W, Becker-Pelster EM, Kretschmer A, Geiss V, Li V, Straub A, Mittendorf J, Jautelat R, Schirok H, Schlemmer KH, Lustig K, Gerisch M, Knorr A, Tinel H, Mondritzki T, Trübel H, Sandner P, Stasch JP. Discovery of the Soluble Guanylate Cyclase Stimulator Vericiguat (BAY 1021189) for the Treatment of Chronic Heart Failure. J Med Chem 60: 5146–5161, 2017. doi: 10.1021/acs.jmedchem.7b00449. [DOI] [PubMed] [Google Scholar]
- 302.Follmann M, Griebenow N, Hahn MG, Hartung I, Mais F-J, Mittendorf J, Schäfer M, Schirok H, Stasch J-P, Stoll F, Straub A. The chemistry and biology of soluble guanylate cyclase stimulators and activators. Angew Chem Int Ed Engl 52: 9442–9462, 2013. doi: 10.1002/anie.201302588. [DOI] [PubMed] [Google Scholar]
- 303.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714, 2006. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 304.Foti A, Dorendorf F, Leimkühler S. A single nucleotide polymorphism causes enhanced radical oxygen species production by human aldehyde oxidase. PLoS One 12: e0182061, 2017. doi: 10.1371/journal.pone.0182061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Fravel MA, Ernst ME. Management of gout in the older adult. Am J Geriatr Pharmacother 9: 271–285, 2011. doi: 10.1016/j.amjopharm.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 306.Frazziano G, Al Ghouleh I, Baust J, Shiva S, Champion HC, Pagano PJ. Nox-derived ROS are acutely activated in pressure overload pulmonary hypertension: indications for a seminal role for mitochondrial Nox4. Am J Physiol Heart Circ Physiol 306: H197–H205, 2014. doi: 10.1152/ajpheart.00977.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem 245: 4053–4057, 1970. [PubMed] [Google Scholar]
- 308.Fridovich I, Handler P. Xanthine oxidase. V. Differential inhibition of the reduction of various electron acceptors. J Biol Chem 237: 916–921, 1962. [PubMed] [Google Scholar]
- 309.Friebe A, Müllershausen F, Smolenski A, Walter U, Schultz G, Koesling D. YC-1 potentiates nitric oxide- and carbon monoxide-induced cyclic GMP effects in human platelets. Mol Pharmacol 54: 962–967, 1998. doi: 10.1124/mol.54.6.962. [DOI] [PubMed] [Google Scholar]
- 310.Friebe A, Schultz G, Koesling D. Sensitizing soluble guanylyl cyclase to become a highly CO-sensitive enzyme. EMBO J 15: 6863–6868, 1996. [PMC free article] [PubMed] [Google Scholar]
- 311.Friedl HP, Till GO, Ryan US, Ward PA. Mediator-induced activation of xanthine oxidase in endothelial cells. FASEB J 3: 2512–2518, 1989. doi: 10.1096/fasebj.3.13.2806779. [DOI] [PubMed] [Google Scholar]
- 312.Fritz BG, Hu X, Brailey JL, Berry RE, Walker FA, Montfort WR. Oxidation and loss of heme in soluble guanylyl cyclase from Manduca sexta. Biochemistry 50: 5813–5815, 2011. doi: 10.1021/bi200794c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Fritz BG, Roberts SA, Ahmed A, Breci L, Li W, Weichsel A, Brailey JL, Wysocki VH, Tama F, Montfort WR. Molecular model of a soluble guanylyl cyclase fragment determined by small-angle X-ray scattering and chemical cross-linking. Biochemistry 52: 1568–1582, 2013. doi: 10.1021/bi301570m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem 276: 41279–41287, 2001. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 315.Fujii H, Ichimori K, Hoshiai K, Nakazawa H. Nitric oxide inactivates NADPH oxidase in pig neutrophils by inhibiting its assembling process. J Biol Chem 272: 32773–32778, 1997. doi: 10.1074/jbc.272.52.32773. [DOI] [PubMed] [Google Scholar]
- 316.Fujii M, Amanso A, Abrahão TB, Lassègue B, Griendling KK. Polymerase delta-interacting protein 2 regulates collagen accumulation via activation of the Akt/mTOR pathway in vascular smooth muscle cells. J Mol Cell Cardiol 92: 21–29, 2016. doi: 10.1016/j.yjmcc.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Fujimoto H, Taguchi J, Imai Y, Ayabe S, Hashimoto H, Kobayashi H, Ogasawara K, Aizawa T, Yamakado M, Nagai R, Ohno M. Manganese superoxide dismutase polymorphism affects the oxidized low-density lipoprotein-induced apoptosis of macrophages and coronary artery disease. Eur Heart J 29: 1267–1274, 2008. doi: 10.1093/eurheartj/ehm500. [DOI] [PubMed] [Google Scholar]
- 318.Fukuda Y, Teragawa H, Matsuda K, Yamagata T, Matsuura H, Chayama K. Tetrahydrobiopterin restores endothelial function of coronary arteries in patients with hypercholesterolaemia. Heart 87: 264–269, 2002. doi: 10.1136/heart.87.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Q IV, Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res 80: 45–51, 1997. doi: 10.1161/01.RES.80.1.45. [DOI] [PubMed] [Google Scholar]
- 320.Fulton D, Church JE, Ruan L, Li C, Sood SG, Kemp BE, Jennings IG, Venema RC. Src kinase activates endothelial nitric-oxide synthase by phosphorylating Tyr-83. J Biol Chem 280: 35943–35952, 2005. doi: 10.1074/jbc.M504606200. [DOI] [PubMed] [Google Scholar]
- 321.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999. doi: 10.1038/21218. Erratum at: Nature 399: 597–601, 1999. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Fulton D, Ruan L, Sood SG, Li C, Zhang Q, Venema RC. Agonist-stimulated endothelial nitric oxide synthase activation and vascular relaxation. Role of eNOS phosphorylation at Tyr83. Circ Res 102: 497–504, 2008. doi: 10.1161/CIRCRESAHA.107.162933. [DOI] [PubMed] [Google Scholar]
- 323.Fulton DJ, Barman SA. Clarity on the Isoform-Specific Roles of NADPH Oxidases and NADPH Oxidase-4 in Atherosclerosis. Arterioscler Thromb Vasc Biol 36: 579–581, 2016. doi: 10.1161/ATVBAHA.116.307096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 325.Gago B, Lundberg JO, Barbosa RM, Laranjinha J. Red wine-dependent reduction of nitrite to nitric oxide in the stomach. Free Radic Biol Med 43: 1233–1242, 2007. doi: 10.1016/j.freeradbiomed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 326.Galle J, Zabel U, Hübner U, Hatzelmann A, Wagner B, Wanner C, Schmidt HH. Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity. Br J Pharmacol 127: 195–203, 1999. doi: 10.1038/sj.bjp.0702495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Gallis B, Corthals GL, Goodlett DR, Ueba H, Kim F, Presnell SR, Figeys D, Harrison DG, Berk BC, Aebersold R, Corson MA. Identification of flow-dependent endothelial nitric-oxide synthase phosphorylation sites by mass spectrometry and regulation of phosphorylation and nitric oxide production by the phosphatidylinositol 3-kinase inhibitor LY294002. J Biol Chem 274: 30101–30108, 1999. doi: 10.1074/jbc.274.42.30101. [DOI] [PubMed] [Google Scholar]
- 328.Galougahi KK, Liu CC, Gentile C, Kok C, Nunez A, Garcia A, Fry NA, Davies MJ, Hawkins CL, Rasmussen HH, Figtree GA. Glutathionylation mediates angiotensin II-induced eNOS uncoupling, amplifying NADPH oxidase-dependent endothelial dysfunction. J Am Heart Assoc 3: e000731, 2014. doi: 10.1161/JAHA.113.000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Gamgee A. Researches on the blood. On the action of nitrites on blood. Philos Trans R Soc Lond 158: 589–625, 1868. doi: 10.1098/rstl.1868.0025. [DOI] [Google Scholar]
- 330.Gan P, Gao Z, Zhao X, Qi G. Surfactin inducing mitochondria-dependent ROS to activate MAPKs, NF-κB and inflammasomes in macrophages for adjuvant activity. Sci Rep 6: 39303, 2016. doi: 10.1038/srep39303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Gandhi PK, Gentry WM, Bottorff MB. Cardiovascular thromboembolic events associated with febuxostat: investigation of cases from the FDA adverse event reporting system database. Semin Arthritis Rheum 42: 562–566, 2013. doi: 10.1016/j.semarthrit.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 332.Gandley RE, Tyurin VA, Huang W, Arroyo A, Daftary A, Harger G, Jiang J, Pitt B, Taylor RN, Hubel CA, Kagan VE. S-nitrosoalbumin-mediated relaxation is enhanced by ascorbate and copper: effects in pregnancy and preeclampsia plasma. Hypertension 45: 21–27, 2005. doi: 10.1161/01.HYP.0000150158.42620.3e. [DOI] [PubMed] [Google Scholar]
- 333.Gane EJ, Weilert F, Orr DW, Keogh GF, Gibson M, Lockhart MM, Frampton CM, Taylor KM, Smith RA, Murphy MP. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int 30: 1019–1026, 2010. doi: 10.1111/j.1478-3231.2010.02250.x. [DOI] [PubMed] [Google Scholar]
- 334.Gao YT, Roman LJ, Martásek P, Panda SP, Ishimura Y, Masters BS. Oxygen metabolism by endothelial nitric-oxide synthase. J Biol Chem 282: 28557–28565, 2007. doi: 10.1074/jbc.M704890200. [DOI] [PubMed] [Google Scholar]
- 335.Garattini E, Terao M. The role of aldehyde oxidase in drug metabolism. Expert Opin Drug Metab Toxicol 8: 487–503, 2012. doi: 10.1517/17425255.2012.663352. [DOI] [PubMed] [Google Scholar]
- 336.García-Cardeña G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci USA 93: 6448–6453, 1996. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Garcin ED, Arvai AS, Rosenfeld RJ, Kroeger MD, Crane BR, Andersson G, Andrews G, Hamley PJ, Mallinder PR, Nicholls DJ, St-Gallay SA, Tinker AC, Gensmantel NP, Mete A, Cheshire DR, Connolly S, Stuehr DJ, Aberg A, Wallace AV, Tainer JA, Getzoff ED. Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat Chem Biol 4: 700–707, 2008. doi: 10.1038/nchembio.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Garcin ED, Bruns CM, Lloyd SJ, Hosfield DJ, Tiso M, Gachhui R, Stuehr DJ, Tainer JA, Getzoff ED. Structural basis for isozyme-specific regulation of electron transfer in nitric-oxide synthase. J Biol Chem 279: 37918–37927, 2004. doi: 10.1074/jbc.M406204200. [DOI] [PubMed] [Google Scholar]
- 339.Gardner AM, Cook MR, Gardner PR. Nitric-oxide dioxygenase function of human cytoglobin with cellular reductants and in rat hepatocytes. J Biol Chem 285: 23850–23857, 2010. doi: 10.1074/jbc.M110.132340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Gardner AM, Gardner PR. Flavohemoglobin detoxifies nitric oxide in aerobic, but not anaerobic, Escherichia coli. Evidence for a novel inducible anaerobic nitric oxide-scavenging activity. J Biol Chem 277: 8166–8171, 2002. doi: 10.1074/jbc.M110470200. [DOI] [PubMed] [Google Scholar]
- 341.Gardner PR. Nitric oxide dioxygenase function and mechanism of flavohemoglobin, hemoglobin, myoglobin and their associated reductases. J Inorg Biochem 99: 247–266, 2005. doi: 10.1016/j.jinorgbio.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 342.Gardner PR, Gardner AM, Martin LA, Salzman AL. Nitric oxide dioxygenase: an enzymic function for flavohemoglobin. Proc Natl Acad Sci USA 95: 10378–10383, 1998. doi: 10.1073/pnas.95.18.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Garrido-Urbani S, Jemelin S, Deffert C, Carnesecchi S, Basset O, Szyndralewiez C, Heitz F, Page P, Montet X, Michalik L, Arbiser J, Rüegg C, Krause KH, Imhof BA. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARα mediated mechanism. PLoS One 6: e14665, 2011. doi: 10.1371/journal.pone.0014665. Erratum at: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJ, Knowles RG. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem 272: 4959–4963, 1997. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- 345.Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett 580: 497–504, 2006. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 346.Gavazzi G, Deffert C, Trocme C, Schäppi M, Herrmann FR, Krause K-H. NOX1 deficiency protects from aortic dissection in response to angiotensin II. Hypertension 50: 189–196, 2007. doi: 10.1161/HYPERTENSIONAHA.107.089706. [DOI] [PubMed] [Google Scholar]
- 347.Geiszt M, Kopp JB, Várnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA 97: 8010–8014, 2000. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Geiszt M, Lekstrom K, Witta J, Leto TL. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J Biol Chem 278: 20006–20012, 2003. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- 349.Gheorghiade M, Greene SJ, Butler J, Filippatos G, Lam CS, Maggioni AP, Ponikowski P, Shah SJ, Solomon SD, Kraigher-Krainer E, Samano ET, Müller K, Roessig L, Pieske B; SOCRATES-REDUCED Investigators and Coordinators . Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients With Worsening Chronic Heart Failure and Reduced Ejection Fraction: The SOCRATES-REDUCED Randomized Trial. JAMA 314: 2251–2262, 2015. doi: 10.1001/jama.2015.15734. [DOI] [PubMed] [Google Scholar]
- 350.Gherasim C, Yadav PK, Kabil O, Niu WN, Banerjee R. Nitrite reductase activity and inhibition of H2S biogenesis by human cystathionine ß-synthase. PLoS One 9: e85544, 2014. doi: 10.1371/journal.pone.0085544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Ghofrani H-A, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, Weimann G, Wang C; CHEST-1 Study Group . Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 369: 319–329, 2013. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 352.Ghofrani H-A, Galiè N, Grimminger F, Grünig E, Humbert M, Jing Z-C, Keogh AM, Langleben D, Kilama MO, Fritsch A, Neuser D, Rubin LJ; PATENT-1 Study Group . Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 369: 330–340, 2013. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 353.Ghosh A, Koziol-White CJ, Asosingh K, Cheng G, Ruple L, Groneberg D, Friebe A, Comhair SA, Stasch JP, Panettieri RA Jr, Aronica MA, Erzurum SC, Stuehr DJ. Soluble guanylate cyclase as an alternative target for bronchodilator therapy in asthma. Proc Natl Acad Sci USA 113: E2355–E2362, 2016. doi: 10.1073/pnas.1524398113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Ghosh A, Stasch JP, Papapetropoulos A, Stuehr DJ. Nitric oxide and heat shock protein 90 activate soluble guanylate cyclase by driving rapid change in its subunit interactions and heme content. J Biol Chem 289: 15259–15271, 2014. doi: 10.1074/jbc.M114.559393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Ghosh A, Stuehr DJ. Regulation of sGC via hsp90, Cellular Heme, sGC Agonists, and NO: New Pathways and Clinical Perspectives. Antioxid Redox Signal 26: 182–190, 2017. doi: 10.1089/ars.2016.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Ghosh A, Stuehr DJ. Soluble guanylyl cyclase requires heat shock protein 90 for heme insertion during maturation of the NO-active enzyme. Proc Natl Acad Sci USA 109: 12998–13003, 2012. doi: 10.1073/pnas.1205854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Ghosh SM, Kapil V, Fuentes-Calvo I, Bubb KJ, Pearl V, Milsom AB, Khambata R, Maleki-Toyserkani S, Yousuf M, Benjamin N, Webb AJ, Caulfield MJ, Hobbs AJ, Ahluwalia A. Enhanced vasodilator activity of nitrite in hypertension: critical role for erythrocytic xanthine oxidoreductase and translational potential. Hypertension 61: 1091–1102, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00933. [DOI] [PubMed] [Google Scholar]
- 358.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 333: 214–221, 1995. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 359.Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L, Roush WR, Brown SJ, Bokoch GM, Rosen H. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem Biol 5: 981–993, 2010. doi: 10.1021/cb100219n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Gibson CM, Giugliano RP, Kloner RA, Bode C, Tendera M, Jánosi A, Merkely B, Godlewski J, Halaby R, Korjian S, Daaboul Y, Chakrabarti AK, Spielman K, Neal BJ, Weaver WD. EMBRACE STEMI study: a Phase 2a trial to evaluate the safety, tolerability, and efficacy of intravenous MTP-131 on reperfusion injury in patients undergoing primary percutaneous coronary intervention. Eur Heart J 37: 1296–1303, 2016. doi: 10.1093/eurheartj/ehv597. [DOI] [PubMed] [Google Scholar]
- 361.Givertz MM, Anstrom KJ, Redfield MM, Deswal A, Haddad H, Butler J, Tang WH, Dunlap ME, LeWinter MM, Mann DL, Felker GM, O’Connor CM, Goldsmith SR, Ofili EO, Saltzberg MT, Margulies KB, Cappola TP, Konstam MA, Semigran MJ, McNulty SE, Lee KL, Shah MR, Hernandez AF; NHLBI Heart Failure Clinical Research Network . Effects of Xanthine Oxidase Inhibition in Hyperuricemic Heart Failure Patients: The Xanthine Oxidase Inhibition for Hyperuricemic Heart Failure Patients (EXACT-HF) Study. Circulation 131: 1763–1771, 2015. doi: 10.1161/CIRCULATIONAHA.114.014536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Gladden JD, Zelickson BR, Wei CC, Ulasova E, Zheng J, Ahmed MI, Chen Y, Bamman M, Ballinger S, Darley-Usmar V, Dell’Italia LJ. Novel insights into interactions between mitochondria and xanthine oxidase in acute cardiac volume overload. Free Radic Biol Med 51: 1975–1984, 2011. doi: 10.1016/j.freeradbiomed.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Gladwin MT. Deconstructing endothelial dysfunction: soluble guanylyl cyclase oxidation and the NO resistance syndrome. J Clin Invest 116: 2330–2332, 2006. doi: 10.1172/JCI29807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Gladwin MT. Haldane, hot dogs, halitosis, and hypoxic vasodilation: the emerging biology of the nitrite anion. J Clin Invest 113: 19–21, 2004. doi: 10.1172/JCI20664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Gladwin MT. Hemoglobin as a nitrite reductase regulating red cell-dependent hypoxic vasodilation. Am J Respir Cell Mol Biol 32: 363–366, 2005. doi: 10.1165/rcmb.F294. [DOI] [PubMed] [Google Scholar]
- 366.Gladwin MT, Grubina R, Doyle MP. The new chemical biology of nitrite reactions with hemoglobin: R-state catalysis, oxidative denitrosylation, and nitrite reductase/anhydrase. Acc Chem Res 42: 157–167, 2009. doi: 10.1021/ar800089j. [DOI] [PubMed] [Google Scholar]
- 367.Gladwin MT, Kim-Shapiro DB. The functional nitrite reductase activity of the heme-globins. Blood 112: 2636–2647, 2008. doi: 10.1182/blood-2008-01-115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Gladwin MT, Raat NJ, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, Patel RP. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol 291: H2026–H2035, 2006. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 369.Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, Waclawiw MA, Panza JA, Ognibene FP, Cannon RO III. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci USA 97: 11482–11487, 2000. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Godber BL, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem 275: 7757–7763, 2000. doi: 10.1074/jbc.275.11.7757. [DOI] [PubMed] [Google Scholar]
- 371.Gole HK, Tharp DL, Bowles DK. Upregulation of intermediate-conductance Ca2+-activated K+ channels (KCNN4) in porcine coronary smooth muscle requires NADPH oxidase 5 (NOX5). PLoS One 9: e105337, 2014. doi: 10.1371/journal.pone.0105337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci USA 104: 20612–20617, 2007. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem 285: 28938–28945, 2010. doi: 10.1074/jbc.M110.154948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu LY, Hon YY, Aletras AH, Cannon RO III, Gladwin MT, Arai AE. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation 117: 2986–2994, 2008. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Gori T, Burstein JM, Ahmed S, Miner SES, Al-Hesayen A, Kelly S, Parker JD. Folic acid prevents nitroglycerin-induced nitric oxide synthase dysfunction and nitrate tolerance: a human in vivo study. Circulation 104: 1119–1123, 2001. doi: 10.1161/hc3501.095358. [DOI] [PubMed] [Google Scholar]
- 376.Görlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res 87: 26–32, 2000. doi: 10.1161/01.RES.87.1.26. [DOI] [PubMed] [Google Scholar]
- 377.Gorren AC, Mayer B. Nitric-oxide synthase: a cytochrome P450 family foster child. Biochim Biophys Acta 1770: 432–445, 2007. doi: 10.1016/j.bbagen.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 378.Gould NS, Evans P, Martínez-Acedo P, Marino SM, Gladyshev VN, Carroll KS, Ischiropoulos H. Site-Specific Proteomic Mapping Identifies Selectively Modified Regulatory Cysteine Residues in Functionally Distinct Protein Networks. Chem Biol 22: 965–975, 2015. doi: 10.1016/j.chembiol.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Gray SP, Di Marco E, Kennedy K, Chew P, Okabe J, El-Osta A, Calkin AC, Biessen EA, Touyz RM, Cooper ME, Schmidt HH, Jandeleit-Dahm KA. Reactive Oxygen Species Can Provide Atheroprotection via NOX4-Dependent Inhibition of Inflammation and Vascular Remodeling. Arterioscler Thromb Vasc Biol 36: 295–307, 2016. doi: 10.1161/ATVBAHA.115.307012. [DOI] [PubMed] [Google Scholar]
- 380.Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, de Haan JB, Koulis C, El-Osta A, Andrews KL, Chin-Dusting JPF, Touyz RM, Wingler K, Cooper ME, Schmidt HHHW, Jandeleit-Dahm KA. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 127: 1888–1902, 2013. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- 381.Greenacre SA, Ischiropoulos H. Tyrosine nitration: localisation, quantification, consequences for protein function and signal transduction. Free Radic Res 34: 541–581, 2001. doi: 10.1080/10715760100300471. [DOI] [PubMed] [Google Scholar]
- 382.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74: 1141–1148, 1994. doi: 10.1161/01.RES.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 383.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000. doi: 10.1161/01.RES.86.5.494. [DOI] [PubMed] [Google Scholar]
- 384.Grubina R, Huang Z, Shiva S, Joshi MS, Azarov I, Basu S, Ringwood LA, Jiang A, Hogg N, Kim-Shapiro DB, Gladwin MT. Concerted nitric oxide formation and release from the simultaneous reactions of nitrite with deoxy- and oxyhemoglobin. J Biol Chem 282: 12916–12927, 2007. doi: 10.1074/jbc.M700546200. [DOI] [PubMed] [Google Scholar]
- 385.Gryglewski RJ, Palmer RMJ, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320: 454–456, 1986. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 386.Gunnett CA, Lund DD, McDowell AK, Faraci FM, Heistad DD. Mechanisms of inducible nitric oxide synthase-mediated vascular dysfunction. Arterioscler Thromb Vasc Biol 25: 1617–1622, 2005. doi: 10.1161/01.ATV.0000172626.00296.ba. [DOI] [PubMed] [Google Scholar]
- 387.Guthikonda S, Sinkey C, Barenz T, Haynes WG. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation 107: 416–421, 2003. doi: 10.1161/01.CIR.0000046448.26751.58. [DOI] [PubMed] [Google Scholar]
- 388.Gutteridge JM. Inhibition of the Fenton reaction by the protein caeruloplasmin and other copper complexes. Assessment of ferroxidase and radical scavenging activities. Chem Biol Interact 56: 113–120, 1985. doi: 10.1016/0009-2797(85)90043-2. [DOI] [PubMed] [Google Scholar]
- 389.Gutteridge JM. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett 201: 291–295, 1986. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- 390.Gutteridge JMC, Halliwell B. Antioxidants: Molecules, medicines, and myths. Biochem Biophys Res Commun 393: 561–564, 2010. doi: 10.1016/j.bbrc.2010.02.071. [DOI] [PubMed] [Google Scholar]
- 391.Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, Harrison DG. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 52: 1803–1809, 2008. doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, Wierzbicki K, Korbut R, Harrison DG, Channon KM. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol 26: 333–339, 2006. doi: 10.1161/01.ATV.0000196651.64776.51. [DOI] [PubMed] [Google Scholar]
- 394.Haber F, Weiss J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc R Soc Lond A Math Phys Sci 147: 332–351, 1934. doi: 10.1098/rspa.1934.0221. [DOI] [Google Scholar]
- 395.Haendeler J, Eckers A, Lukosz M, Unfried K, Altschmied J. Endothelial NADPH oxidase 2: when does it matter in atherosclerosis? Cardiovasc Res 94: 1–2, 2012. doi: 10.1093/cvr/cvs106. [DOI] [PubMed] [Google Scholar]
- 396.Hahn NE, Meischl C, Kawahara T, Musters RJ, Verhoef VM, van der Velden J, Vonk AB, Paulus WJ, van Rossum AC, Niessen HW, Krijnen PA. NOX5 expression is increased in intramyocardial blood vessels and cardiomyocytes after acute myocardial infarction in humans. Am J Pathol 180: 2222–2229, 2012. doi: 10.1016/j.ajpath.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 397.Hall CN, Garthwaite J. What is the real physiological NO concentration in vivo? Nitric Oxide 21: 92–103, 2009. doi: 10.1016/j.niox.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Halligan KE, Jourd’heuil FL, Jourd’heuil D. Cytoglobin is expressed in the vasculature and regulates cell respiration and proliferation via nitric oxide dioxygenation. J Biol Chem 284: 8539–8547, 2009. doi: 10.1074/jbc.M808231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Halliwell B. The antioxidant paradox. Lancet 355: 1179–1180, 2000. doi: 10.1016/S0140-6736(00)02075-4. [DOI] [PubMed] [Google Scholar]
- 400.Halliwell B. The antioxidant paradox: less paradoxical now? Br J Clin Pharmacol 75: 637–644, 2013. doi: 10.1111/j.1365-2125.2012.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Han D, Canali R, Garcia J, Aguilera R, Gallaher TK, Cadenas E. Sites and mechanisms of aconitase inactivation by peroxynitrite: modulation by citrate and glutathione. Biochemistry 44: 11986–11996, 2005. doi: 10.1021/bi0509393. [DOI] [PubMed] [Google Scholar]
- 402.Handy RL, Moore PK. A comparison of the effects of L-NAME, 7-NI and L-NIL on carrageenan-induced hindpaw oedema and NOS activity. Br J Pharmacol 123: 1119–1126, 1998. doi: 10.1038/sj.bjp.0701735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Hankeln T, Wystub S, Laufs T, Schmidt M, Gerlach F, Saaler-Reinhardt S, Reuss S, Burmester T. The cellular and subcellular localization of neuroglobin and cytoglobin–a clue to their function? IUBMB Life 56: 671–679, 2004. doi: 10.1080/15216540500037794. [DOI] [PubMed] [Google Scholar]
- 404.Haque MM, Ray SS, Stuehr DJ. Phosphorylation Controls Endothelial Nitric-oxide Synthase by Regulating Its Conformational Dynamics. J Biol Chem 291: 23047–23057, 2016. doi: 10.1074/jbc.M116.737361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Hare JM, Mangal B, Brown J, Fisher C Jr, Freudenberger R, Colucci WS, Mann DL, Liu P, Givertz MM, Schwarz RP; OPT-CHF Investigators . Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J Am Coll Cardiol 51: 2301–2309, 2008. doi: 10.1016/j.jacc.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 406.Harris CM, Massey V. The reaction of reduced xanthine dehydrogenase with molecular oxygen. Reaction kinetics and measurement of superoxide radical. J Biol Chem 272: 8370–8379, 1997. doi: 10.1074/jbc.272.13.8370. [DOI] [PubMed] [Google Scholar]
- 407.Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, Chen ZP, Kemp BE, Venema RC. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem 276: 16587–16591, 2001. doi: 10.1074/jbc.M100229200. [DOI] [PubMed] [Google Scholar]
- 408.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol 91: 7A–11A, 2003. doi: 10.1016/S0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 409.Harrison DG, Ohara Y. Physiologic consequences of increased vascular oxidant stresses in hypercholesterolemia and atherosclerosis: implications for impaired vasomotion. Am J Cardiol 75: 75B–81B, 1995. doi: 10.1016/0002-9149(95)80018-N. [DOI] [PubMed] [Google Scholar]
- 410.Hassoun PM, Yu F-S, Cote CG, Zulueta JJ, Sawhney R, Skinner KA, Skinner HB, Parks DA, Lanzillo JJ. Upregulation of xanthine oxidase by lipopolysaccharide, interleukin-1, and hypoxia. Role in acute lung injury. Am J Respir Crit Care Med 158: 299–305, 1998. doi: 10.1164/ajrccm.158.1.9709116. [DOI] [PubMed] [Google Scholar]
- 411.Hausladen A, Gow AJ, Stamler JS. Nitrosative stress: metabolic pathway involving the flavohemoglobin. Proc Natl Acad Sci USA 95: 14100–14105, 1998. doi: 10.1073/pnas.95.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Hausladen A, Privalle CT, Keng T, DeAngelo J, Stamler JS. Nitrosative stress: activation of the transcription factor OxyR. Cell 86: 719–729, 1996. doi: 10.1016/S0092-8674(00)80147-6. [DOI] [PubMed] [Google Scholar]
- 413.Hayashi T, Juliet PAR, Kano-Hayashi H, Tsunekawa T, Dingqunfang D, Sumi D, Matsui-Hirai H, Fukatsu A, Iguchi A. NADPH oxidase inhibitor, apocynin, restores the impaired endothelial-dependent and -independent responses and scavenges superoxide anion in rats with type 2 diabetes complicated by NO dysfunction. Diabetes Obes Metab 7: 334–343, 2005. doi: 10.1111/j.1463-1326.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- 414.Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E, Stocker R. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest 97: 1535–1544, 1996. doi: 10.1172/JCI118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest 99: 2075–2081, 1997. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15: 1077–1081, 2009. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Heiss C, Keen CL, Kelm M. Flavanols and cardiovascular disease prevention. Eur Heart J 31: 2583–2592, 2010. doi: 10.1093/eurheartj/ehq332. [DOI] [PubMed] [Google Scholar]
- 418.Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Münzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res 86: E36–E41, 2000. doi: 10.1161/01.RES.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 419.Heitzer T, Finckh B, Albers S, Krohn K, Kohlschütter A, Meinertz T. Beneficial effects of alpha-lipoic acid and ascorbic acid on endothelium-dependent, nitric oxide-mediated vasodilation in diabetic patients: relation to parameters of oxidative stress. Free Radic Biol Med 31: 53–61, 2001. doi: 10.1016/S0891-5849(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 420.Heitzer T, Just H, Münzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation 94: 6–9, 1996. doi: 10.1161/01.CIR.94.1.6. [DOI] [PubMed] [Google Scholar]
- 421.Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia 43: 1435–1438, 2000. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- 422.Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem 276: 40–47, 2001. doi: 10.1074/jbc.M004392200. [DOI] [PubMed] [Google Scholar]
- 423.Helmcke I, Heumüller S, Tikkanen R, Schröder K, Brandes RP. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal 11: 1279–1287, 2009. doi: 10.1089/ars.2008.2383. [DOI] [PubMed] [Google Scholar]
- 424.Helms C, Kim-Shapiro DB. Hemoglobin-mediated nitric oxide signaling. Free Radic Biol Med 61: 464–472, 2013. doi: 10.1016/j.freeradbiomed.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Helms CC, Gladwin MT, Kim-Shapiro DB. Erythrocytes and vascular function: oxygen and nitric oxide. Front Physiol 9: 125, 2018. doi: 10.3389/fphys.2018.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HHHW, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 427.Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, Shah AM. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol 41: 2164–2171, 2003. doi: 10.1016/S0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 428.Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol 15: 578–584, 2003. doi: 10.1016/S0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 429.Higashi Y, Sasaki S, Nakagawa K, Fukuda Y, Matsuura H, Oshima T, Chayama K. Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals. Am J Hypertens 15: 326–332, 2002. doi: 10.1016/S0895-7061(01)02317-2. [DOI] [PubMed] [Google Scholar]
- 430.Higgins P, Dawson J, Lees KR, McArthur K, Quinn TJ, Walters MR. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta-analysis. Cardiovasc Ther 30: 217–226, 2012. doi: 10.1111/j.1755-5922.2011.00277.x. [DOI] [PubMed] [Google Scholar]
- 431.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24: 677–683, 2004. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 432.Hilgers RH, Kundumani-Sridharan V, Subramani J, Chen LC, Cuello LG, Rusch NJ, Das KC. Thioredoxin reverses age-related hypertension by chronically improving vascular redox and restoring eNOS function. Sci Transl Med 9: eaaf6094, 2017. doi: 10.1126/scitranslmed.aaf6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Hill BG, Dranka BP, Bailey SM, Lancaster JR Jr, Darley-Usmar VM. What part of NO don’t you understand? Some answers to the cardinal questions in nitric oxide biology. J Biol Chem 285: 19699–19704, 2010. doi: 10.1074/jbc.R110.101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Hille R, Hall J, Basu P. The mononuclear molybdenum enzymes. Chem Rev 114: 3963–4038, 2014. doi: 10.1021/cr400443z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Hirano K, Chen WS, Chueng AL, Dunne AA, Seredenina T, Filippova A, Ramachandran S, Bridges A, Chaudry L, Pettman G, Allan C, Duncan S, Lee KC, Lim J, Ma MT, Ong AB, Ye NY, Nasir S, Mulyanidewi S, Aw CC, Oon PP, Liao S, Li D, Johns DG, Miller ND, Davies CH, Browne ER, Matsuoka Y, Chen DW, Jaquet V, Rutter AR. Discovery of GSK2795039, a Novel Small Molecule NADPH Oxidase 2 Inhibitor. Antioxid Redox Signal 23: 358–374, 2015. doi: 10.1089/ars.2014.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Hoenicka M, Becker EM, Apeler H, Sirichoke T, Schröder H, Gerzer R, Stasch JP. Purified soluble guanylyl cyclase expressed in a baculovirus/Sf9 system: stimulation by YC-1, nitric oxide, and carbon monoxide. J Mol Med (Berl) 77: 14–23, 1999. doi: 10.1007/s001090050292. [DOI] [PubMed] [Google Scholar]
- 437.Høivik HO, Laurijssens BE, Harnisch LO, Twomey CK, Dixon RM, Kirkham AJ, Williams PM, Wentz AL, Lunnon MW. Lack of efficacy of the selective iNOS inhibitor GW274150 in prophylaxis of migraine headache. Cephalalgia 30: 1458–1467, 2010. doi: 10.1177/0333102410370875. [DOI] [PubMed] [Google Scholar]
- 438.Holland SM. Chronic granulomatous disease. Clin Rev Allergy Immunol 38: 3–10, 2010. doi: 10.1007/s12016-009-8136-z. [DOI] [PubMed] [Google Scholar]
- 439.Holterman CE, Thibodeau JF, Towaij C, Gutsol A, Montezano AC, Parks RJ, Cooper ME, Touyz RM, Kennedy CR. Nephropathy and elevated BP in mice with podocyte-specific NADPH oxidase 5 expression. J Am Soc Nephrol 25: 784–797, 2014. doi: 10.1681/ASN.2013040371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Hong HJ, Hsiao G, Cheng TH, Yen MH. Supplemention with tetrahydrobiopterin suppresses the development of hypertension in spontaneously hypertensive rats. Hypertension 38: 1044–1048, 2001. doi: 10.1161/hy1101.095331. [DOI] [PubMed] [Google Scholar]
- 441.Hosoya T, Ogawa Y, Hashimoto H, Ohashi T, Sakamoto R. Comparison of topiroxostat and allopurinol in Japanese hyperuricemic patients with or without gout: a phase 3, multicentre, randomized, double-blind, double-dummy, active-controlled, parallel-group study. J Clin Pharm Ther 41: 290–297, 2016. doi: 10.1111/jcpt.12391. [DOI] [PubMed] [Google Scholar]
- 442.Hosoya T, Sasaki T, Ohashi T. Clinical efficacy and safety of topiroxostat in Japanese hyperuricemic patients with or without gout: a randomized, double-blinded, controlled phase 2b study. Clin Rheumatol 36: 649–656, 2017. doi: 10.1007/s10067-016-3474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Hsich E, Segal BH, Pagano PJ, Rey FE, Paigen B, Deleonardis J, Hoyt RF, Holland SM, Finkel T. Vascular effects following homozygous disruption of p47(phox): An essential component of NADPH oxidase. Circulation 101: 1234–1236, 2000. doi: 10.1161/01.CIR.101.11.1234. [DOI] [PubMed] [Google Scholar]
- 444.Huang A, Sun D, Shesely EG, Levee EM, Koller A, Kaley G. Neuronal NOS-dependent dilation to flow in coronary arteries of male eNOS-KO mice. Am J Physiol Heart Circ Physiol 282: H429–H436, 2002. doi: 10.1152/ajpheart.00501.2001. [DOI] [PubMed] [Google Scholar]
- 445.Huang KT, Keszler A, Patel N, Patel RP, Gladwin MT, Kim-Shapiro DB, Hogg N. The reaction between nitrite and deoxyhemoglobin. Reassessment of reaction kinetics and stoichiometry. J Biol Chem 280: 31126–31131, 2005. doi: 10.1074/jbc.M501496200. [DOI] [PubMed] [Google Scholar]
- 446.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377: 239–242, 1995. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 447.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest 115: 2099–2107, 2005. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Ignarro LJ. Nitric Oxide: Biology and Pathobiology. Cambridge, MA: Academic, 2009. [Google Scholar]
- 449.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 84: 9265–9269, 1987. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Ihlemann N, Rask-Madsen C, Perner A, Dominguez H, Hermann T, Køber L, Torp-Pedersen C. Tetrahydrobiopterin restores endothelial dysfunction induced by an oral glucose challenge in healthy subjects. Am J Physiol Heart Circ Physiol 285: H875–H882, 2003. doi: 10.1152/ajpheart.00008.2003. [DOI] [PubMed] [Google Scholar]
- 451.Imajoh-Ohmi S, Tokita K, Ochiai H, Nakamura M, Kanegasaki S. Topology of cytochrome b558 in neutrophil membrane analyzed by anti-peptide antibodies and proteolysis. J Biol Chem 267: 180–184, 1992. [PubMed] [Google Scholar]
- 452.Ionova IA, Vásquez-Vivar J, Whitsett J, Herrnreiter A, Medhora M, Cooley BC, Pieper GM. Deficient BH4 production via de novo and salvage pathways regulates NO responses to cytokines in adult cardiac myocytes. Am J Physiol Heart Circ Physiol 295: H2178–H2187, 2008. doi: 10.1152/ajpheart.00748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Irie Y, Saeki M, Kamisaki Y, Martin E, Murad F. Histone H1.2 is a substrate for denitrase, an activity that reduces nitrotyrosine immunoreactivity in proteins. Proc Natl Acad Sci USA 100: 5634–5639, 2003. doi: 10.1073/pnas.1131756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Isbell TS, Sun CW, Wu LC, Teng X, Vitturi DA, Branch BG, Kevil CG, Peng N, Wyss JM, Ambalavanan N, Schwiebert L, Ren J, Pawlik KM, Renfrow MB, Patel RP, Townes TM. SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation. Nat Med 14: 773–777, 2008. doi: 10.1038/nm1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-beta1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol 296: L489–L499, 2009. doi: 10.1152/ajplung.90488.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Isogai Y, Iizuka T, Shiro Y. The mechanism of electron donation to molecular oxygen by phagocytic cytochrome b558. J Biol Chem 270: 7853–7857, 1995. doi: 10.1074/jbc.270.14.7853. [DOI] [PubMed] [Google Scholar]
- 457.Ito K, Hanazawa T, Tomita K, Barnes PJ, Adcock IM. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem Biophys Res Commun 315: 240–245, 2004. doi: 10.1016/j.bbrc.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 458.Iyamu EW, Perdew H, Woods GM. Cysteine-iron promotes arginase activity by driving the Fenton reaction. Biochem Biophys Res Commun 376: 116–120, 2008. doi: 10.1016/j.bbrc.2008.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Jabs A, Oelze M, Mikhed Y, Stamm P, Kröller-Schön S, Welschof P, Jansen T, Hausding M, Kopp M, Steven S, Schulz E, Stasch JP, Münzel T, Daiber A. Effect of soluble guanylyl cyclase activator and stimulator therapy on nitroglycerin-induced nitrate tolerance in rats. Vascul Pharmacol 71: 181–191, 2015. doi: 10.1016/j.vph.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 460.Jackson HM, Kawahara T, Nisimoto Y, Smith SME, Lambeth JD. Nox4 B-loop creates an interface between the transmembrane and dehydrogenase domains. J Biol Chem 285: 10281–10290, 2010. doi: 10.1074/jbc.M109.084939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Jacobson GM, Dourron HM, Liu J, Carretero OA, Reddy DJ, Andrzejewski T, Pagano PJ. Novel NAD(P)H oxidase inhibitor suppresses angioplasty-induced superoxide and neointimal hyperplasia of rat carotid artery. Circ Res 92: 637–643, 2003. doi: 10.1161/01.RES.0000063423.94645.8A. [DOI] [PubMed] [Google Scholar]
- 462.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol 3: 193–197, 2001. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 463.Jagnandan D, Church JE, Banfi B, Stuehr DJ, Marrero MB, Fulton DJ. Novel mechanism of activation of NADPH oxidase 5. calcium sensitization via phosphorylation. J Biol Chem 282: 6494–6507, 2007. doi: 10.1074/jbc.M608966200. [DOI] [PubMed] [Google Scholar]
- 464.Jaimes EA, Sweeney C, Raij L. Effects of the reactive oxygen species hydrogen peroxide and hypochlorite on endothelial nitric oxide production. Hypertension 38: 877–883, 2001. [PubMed] [Google Scholar]
- 465.Janiszewski M, Lopes LR, Carmo AO, Pedro MA, Brandes RP, Santos CXC, Laurindo FRM. Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J Biol Chem 280: 40813–40819, 2005. doi: 10.1074/jbc.M509255200. [DOI] [PubMed] [Google Scholar]
- 466.Jankov RP, Kantores C, Pan J, Belik J. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. Am J Physiol Lung Cell Mol Physiol 294: L233–L245, 2008. doi: 10.1152/ajplung.00166.2007. [DOI] [PubMed] [Google Scholar]
- 467.Jansson EA, Huang L, Malkey R, Govoni M, Nihlén C, Olsson A, Stensdotter M, Petersson J, Holm L, Weitzberg E, Lundberg JO. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol 4: 411–417, 2008. doi: 10.1038/nchembio.92. [DOI] [PubMed] [Google Scholar]
- 468.Javeshghani D, Hussain SN, Scheidel J, Quinn MT, Magder SA. Superoxide production in the vasculature of lipopolysaccharide-treated rats and pigs. Shock 19: 486–493, 2003. doi: 10.1097/01.shk.0000054374.88889.37. [DOI] [PubMed] [Google Scholar]
- 469.Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassègue B, Griendling KK. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med 45: 329–335, 2008. doi: 10.1016/j.freeradbiomed.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Jerlich A, Horakova L, Fabjan JS, Giessauf A, Jürgens G, Schaur RJ. Correlation of low-density lipoprotein modification by myeloperoxidase with hypochlorous acid formation. Int J Clin Lab Res 29: 155–161, 1999. doi: 10.1007/s005990050083. [DOI] [PubMed] [Google Scholar]
- 471.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 380: 221–226, 1996. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 472.Jiang JX, Chen X, Serizawa N, Szyndralewiez C, Page P, Schröder K, Brandes RP, Devaraj S, Török NJ. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free Radic Biol Med 53: 289–296, 2012. doi: 10.1016/j.freeradbiomed.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Jones SA, O’Donnell VB, Wood JD, Broughton JP, Hughes EJ, Jones OTG. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol 271: H1626–H1634, 1996. [DOI] [PubMed] [Google Scholar]
- 474.Josephson RA, Silverman HS, Lakatta EG, Stern MD, Zweier JL. Study of the mechanisms of hydrogen peroxide and hydroxyl free radical-induced cellular injury and calcium overload in cardiac myocytes. J Biol Chem 266: 2354–2361, 1991. [PubMed] [Google Scholar]
- 475.Judkins CP, Diep H, Broughton BRS, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, Drummond GR. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am J Physiol Heart Circ Physiol 298: H24–H32, 2010. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 476.Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, Brandes RP. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation 109: 1795–1801, 2004. doi: 10.1161/01.CIR.0000124223.00113.A4. [DOI] [PubMed] [Google Scholar]
- 477.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol 1: 223–232, 2005. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 478.Kahles T, Brandes RP. Which NADPH oxidase isoform is relevant for ischemic stroke? The case for nox 2. Antioxid Redox Signal 18: 1400–1417, 2013. doi: 10.1089/ars.2012.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Kahles T, Kohnen A, Heumueller S, Rappert A, Bechmann I, Liebner S, Wittko IM, Neumann-Haefelin T, Steinmetz H, Schroeder K, Brandes RP. NADPH oxidase Nox1 contributes to ischemic injury in experimental stroke in mice. Neurobiol Dis 40: 185–192, 2010. doi: 10.1016/j.nbd.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 480.Kakinuma K, Kaneda M, Chiba T, Ohnishi T. Electron spin resonance studies on a flavoprotein in neutrophil plasma membranes. Redox potentials of the flavin and its participation in NADPH oxidase. J Biol Chem 261: 9426–9432, 1986. [PubMed] [Google Scholar]
- 481.Kamga Pride C, Mo L, Quesnelle K, Dagda RK, Murillo D, Geary L, Corey C, Portella R, Zharikov S, St Croix C, Maniar S, Chu CT, Khoo NK, Shiva S. Nitrite activates protein kinase A in normoxia to mediate mitochondrial fusion and tolerance to ischaemia/reperfusion. Cardiovasc Res 101: 57–68, 2014. doi: 10.1093/cvr/cvt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Kanner J, Harel S, Granit R. Nitric oxide as an antioxidant. Arch Biochem Biophys 289: 130–136, 1991. doi: 10.1016/0003-9861(91)90452-O. [DOI] [PubMed] [Google Scholar]
- 483.Kanno S, Lee PC, Zhang Y, Ho C, Griffith BP, Shears LL II, Billiar TR. Attenuation of myocardial ischemia/reperfusion injury by superinduction of inducible nitric oxide synthase. Circulation 101: 2742–2748, 2000. doi: 10.1161/01.CIR.101.23.2742. [DOI] [PubMed] [Google Scholar]
- 484.Kanski J, Behring A, Pelling J, Schöneich C. Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: effects of biological aging. Am J Physiol Heart Circ Physiol 288: H371–H381, 2005. doi: 10.1152/ajpheart.01030.2003. [DOI] [PubMed] [Google Scholar]
- 485.Kapil V, Haydar SMA, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med 55: 93–100, 2013. doi: 10.1016/j.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension 65: 320–327, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Kaplan-Albuquerque N, Bogaert YE, Van Putten V, Weiser-Evans MC, Nemenoff RA. Patterns of gene expression differentially regulated by platelet-derived growth factor and hypertrophic stimuli in vascular smooth muscle cells: markers for phenotypic modulation and response to injury. J Biol Chem 280: 19966–19976, 2005. doi: 10.1074/jbc.M500917200. [DOI] [PubMed] [Google Scholar]
- 488.Karbach S, Wenzel P, Waisman A, Munzel T, Daiber A. eNOS uncoupling in cardiovascular diseases–the role of oxidative stress and inflammation. Curr Pharm Des 20: 3579–3594, 2014. doi: 10.2174/13816128113196660748. [DOI] [PubMed] [Google Scholar]
- 489.Karow DS, Pan D, Davis JH, Behrends S, Mathies RA, Marletta MA. Characterization of functional heme domains from soluble guanylate cyclase. Biochemistry 44: 16266–16274, 2005. doi: 10.1021/bi051601b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Karplus PA, Daniels MJ, Herriott JR. Atomic structure of ferredoxin-NADP+ reductase: prototype for a structurally novel flavoenzyme family. Science 251: 60–66, 1991. doi: 10.1126/science.1986412. [DOI] [PubMed] [Google Scholar]
- 491.Katsuki S, Arnold W, Mittal C, Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res 3: 23–35, 1977. [PubMed] [Google Scholar]
- 492.Katsuyama M, Fan C, Yabe-Nishimura C. NADPH oxidase is involved in prostaglandin F2alpha-induced hypertrophy of vascular smooth muscle cells: induction of NOX1 by PGF2alpha. J Biol Chem 277: 13438–13442, 2002. doi: 10.1074/jbc.M111634200. [DOI] [PubMed] [Google Scholar]
- 493.Kawada N, Kristensen DB, Asahina K, Nakatani K, Minamiyama Y, Seki S, Yoshizato K. Characterization of a stellate cell activation-associated protein (STAP) with peroxidase activity found in rat hepatic stellate cells. J Biol Chem 276: 25318–25323, 2001. doi: 10.1074/jbc.M102630200. [DOI] [PubMed] [Google Scholar]
- 494.Kawahara T, Lambeth JD. Phosphatidylinositol (4,5)-bisphosphate modulates Nox5 localization via an N-terminal polybasic region. Mol Biol Cell 19: 4020–4031, 2008. doi: 10.1091/mbc.e07-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Kelley EE, Baust J, Bonacci G, Golin-Bisello F, Devlin JE, St Croix CM, Watkins SC, Gor S, Cantu-Medellin N, Weidert ER, Frisbee JC, Gladwin MT, Champion HC, Freeman BA, Khoo NK. Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high-fat diet-induced obesity. Cardiovasc Res 101: 352–363, 2014. doi: 10.1093/cvr/cvt341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Kelley EE, Hock T, Khoo NK, Richardson GR, Johnson KK, Powell PC, Giles GI, Agarwal A, Lancaster JR Jr, Tarpey MM. Moderate hypoxia induces xanthine oxidoreductase activity in arterial endothelial cells. Free Radic Biol Med 40: 952–959, 2006. doi: 10.1016/j.freeradbiomed.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 497.Kelley EE, Khoo NK, Hundley NJ, Malik UZ, Freeman BA, Tarpey MM. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic Biol Med 48: 493–498, 2010. doi: 10.1016/j.freeradbiomed.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Kellogg DL Jr, Zhao JL, Wu Y. Neuronal nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. J Physiol 586: 847–857, 2008. doi: 10.1113/jphysiol.2007.144642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Kellogg EW III, Fridovich I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem 250: 8812–8817, 1975. [PubMed] [Google Scholar]
- 500.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem 276: 4588–4596, 2001. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 501.Keszler A, Piknova B, Schechter AN, Hogg N. The reaction between nitrite and oxyhemoglobin: a mechanistic study. J Biol Chem 283: 9615–9622, 2008. doi: 10.1074/jbc.M705630200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Khambata RS, Ghosh SM, Rathod KS, Thevathasan T, Filomena F, Xiao Q, Ahluwalia A. Antiinflammatory actions of inorganic nitrate stabilize the atherosclerotic plaque. Proc Natl Acad Sci USA 114: E550–E559, 2017. doi: 10.1073/pnas.1613063114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Khan AA, Wang Y, Sun Y, Mao XO, Xie L, Miles E, Graboski J, Chen S, Ellerby LM, Jin K, Greenberg DA. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc Natl Acad Sci USA 103: 17944–17948, 2006. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Khan MA, Alam K, Zafaryab M, Rizvi MMA. Peroxynitrite-modified histone as a pathophysiological biomarker in autoimmune diseases. Biochimie 140: 1–9, 2017. doi: 10.1016/j.biochi.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 505.Kietzmann T, Petry A, Shvetsova A, Gerhold JM, Görlach A. The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system. Br J Pharmacol 174: 1533–1554, 2017. doi: 10.1111/bph.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Kim IY, Schutzler SE, Schrader A, Spencer HJ, Azhar G, Deutz NE, Wolfe RR. Acute ingestion of citrulline stimulates nitric oxide synthesis but does not increase blood flow in healthy young and older adults with heart failure. Am J Physiol Endocrinol Metab 309: E915–E924, 2015. doi: 10.1152/ajpendo.00339.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Suzuki T, Maeta H, Abe Y. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension 45: 860–866, 2005. doi: 10.1161/01.HYP.0000163462.98381.7f. [DOI] [PubMed] [Google Scholar]
- 508.Kirk EA, Dinauer MC, Rosen H, Chait A, Heinecke JW, LeBoeuf RC. Impaired superoxide production due to a deficiency in phagocyte NADPH oxidase fails to inhibit atherosclerosis in mice. Arterioscler Thromb Vasc Biol 20: 1529–1535, 2000. doi: 10.1161/01.ATV.20.6.1529. [DOI] [PubMed] [Google Scholar]
- 509.Kirsch M, Korth HG, Sustmann R, de Groot H. The pathobiochemistry of nitrogen dioxide. Biol Chem 383: 389–399, 2002. doi: 10.1515/BC.2002.043. [DOI] [PubMed] [Google Scholar]
- 510.Kisker C, Schindelin H, Pacheco A, Wehbi WA, Garrett RM, Rajagopalan KV, Enemark JH, Rees DC. Molecular basis of sulfite oxidase deficiency from the structure of sulfite oxidase. Cell 91: 973–983, 1997. doi: 10.1016/S0092-8674(00)80488-2. [DOI] [PubMed] [Google Scholar]
- 511.Kiss PJ, Knisz J, Zhang Y, Baltrusaitis J, Sigmund CD, Thalmann R, Smith RJH, Verpy E, Bánfi B. Inactivation of NADPH oxidase organizer 1 results in severe imbalance. Curr Biol 16: 208–213, 2006. doi: 10.1016/j.cub.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 512.Kitamura S, Sugihara K. Current status of prediction of drug disposition and toxicity in humans using chimeric mice with humanized liver. Xenobiotica 44: 123–134, 2014. doi: 10.3109/00498254.2013.868062. [DOI] [PubMed] [Google Scholar]
- 513.Kitamura S, Sugihara K, Ohta S. Drug-metabolizing ability of molybdenum hydroxylases. Drug Metab Pharmacokinet 21: 83–98, 2006. doi: 10.2133/dmpk.21.83. [DOI] [PubMed] [Google Scholar]
- 514.Klatt P, Schmid M, Leopold E, Schmidt K, Werner ER, Mayer B. The pteridine binding site of brain nitric oxide synthase. Tetrahydrobiopterin binding kinetics, specificity, and allosteric interaction with the substrate domain. J Biol Chem 269: 13861–13866, 1994. [PubMed] [Google Scholar]
- 515.Klatt P, Schmidt K, Lehner D, Glatter O, Bächinger HP, Mayer B. Structural analysis of porcine brain nitric oxide synthase reveals a role for tetrahydrobiopterin and L-arginine in the formation of an SDS-resistant dimer. EMBO J 14: 3687–3695, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol 77: 598–625, 2005. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 517.Kleikers PW, Dao VT, Göb E, Hooijmans C, Debets J, van Essen H, Kleinschnitz C, Schmidt HH. SFRR-E Young Investigator AwardeeNOXing out stroke: Identification of NOX4 and 5as targets in blood-brain-barrier stabilisation and neuroprotection. Free Radic Biol Med 75, Suppl 1: S16, 2014. doi: 10.1016/j.freeradbiomed.2014.10.593. [DOI] [PubMed] [Google Scholar]
- 518.Kleinbongard P, Schulz R, Rassaf T, Lauer T, Dejam A, Jax T, Kumara I, Gharini P, Kabanova S, Ozüyaman B, Schnürch HG, Gödecke A, Weber AA, Robenek M, Robenek H, Bloch W, Rösen P, Kelm M. Red blood cells express a functional endothelial nitric oxide synthase. Blood 107: 2943–2951, 2006. doi: 10.1182/blood-2005-10-3992. [DOI] [PubMed] [Google Scholar]
- 519.Kleinert H, Schwarz PM, Förstermann U. Regulation of the expression of inducible nitric oxide synthase. Biol Chem 384: 1343–1364, 2003. doi: 10.1515/BC.2003.152. [DOI] [PubMed] [Google Scholar]
- 520.Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, Klopstock T, de Angelis MH, Jandeleit-Dahm K, Shah AM, Weissmann N, Schmidt HHHW. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol 8: e1000479, 2010. doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Kleschyov AL, Muller B, Schott C, Stoclet JC. Role of adventitial nitric oxide in vascular hyporeactivity induced by lipopolysaccharide in rat aorta. Br J Pharmacol 124: 623–626, 1998. doi: 10.1038/sj.bjp.0701916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Knorr M, Hausding M, Kröller-Schuhmacher S, Steven S, Oelze M, Heeren T, Scholz A, Gori T, Wenzel P, Schulz E, Daiber A, Münzel T. Nitroglycerin-induced endothelial dysfunction and tolerance involve adverse phosphorylation and S-Glutathionylation of endothelial nitric oxide synthase: beneficial effects of therapy with the AT1 receptor blocker telmisartan. Arterioscler Thromb Vasc Biol 31: 2223–2231, 2011. doi: 10.1161/ATVBAHA.111.232058. [DOI] [PubMed] [Google Scholar]
- 524.Ko FN, Wu CC, Kuo SC, Lee FY, Teng CM. YC-1, a novel activator of platelet guanylate cyclase. Blood 84: 4226–4233, 1994. [PubMed] [Google Scholar]
- 525.Kobzik L, Bredt DS, Lowenstein CJ, Drazen J, Gaston B, Sugarbaker D, Stamler JS. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol 9: 371–377, 1993. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- 526.Koppenol WH. Peroxynitrite: the basics. In: Peroxynitrite Detection in Biological Media: Challenges and Advances, edited by Peteu SFS, Bayachou SM. London: Royal Society of Chemistry, 2015, p. 1–11. doi: 10.1039/9781782622352-00001. [DOI] [Google Scholar]
- 527.Korda M, Kubant R, Patton S, Malinski T. Leptin-induced endothelial dysfunction in obesity. Am J Physiol Heart Circ Physiol 295: H1514–H1521, 2008. doi: 10.1152/ajpheart.00479.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Kotthaus J, Wahl B, Havemeyer A, Kotthaus J, Schade D, Garbe-Schönberg D, Mendel R, Bittner F, Clement B. Reduction of N(ω)-hydroxy-L-arginine by the mitochondrial amidoxime reducing component (mARC). Biochem J 433: 383–391, 2011. doi: 10.1042/BJ20100960. [DOI] [PubMed] [Google Scholar]
- 529.Kovamees O, Shemyakin A, Eriksson M, Angelin B, Pernow J. Arginase inhibition improves endothelial function in patients with familial hypercholesterolaemia irrespective of their cholesterol levels. J Intern Med 279: 477–484, 2016. doi: 10.1111/joim.12461. [DOI] [PubMed] [Google Scholar]
- 530.Kövamees O, Shemyakin A, Pernow J. Effect of arginase inhibition on ischemia-reperfusion injury in patients with coronary artery disease with and without diabetes mellitus. PLoS One 9: e103260, 2014. doi: 10.1371/journal.pone.0103260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Kraus JP, Janosík M, Kozich V, Mandell R, Shih V, Sperandeo MP, Sebastio G, de Franchis R, Andria G, Kluijtmans LA, Blom H, Boers GH, Gordon RB, Kamoun P, Tsai MY, Kruger WD, Koch HG, Ohura T, Gaustadnes M. Cystathionine beta-synthase mutations in homocystinuria. Hum Mutat 13: 362–375, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 532.Kuhlencordt PJ, Chen J, Han F, Astern J, Huang PL. Genetic deficiency of inducible nitric oxide synthase reduces atherosclerosis and lowers plasma lipid peroxides in apolipoprotein E-knockout mice. Circulation 103: 3099–3104, 2001. doi: 10.1161/01.CIR.103.25.3099. [DOI] [PubMed] [Google Scholar]
- 533.Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation 104: 448–454, 2001. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 534.Kuhlencordt PJ, Hötten S, Schödel J, Rützel S, Hu K, Widder J, Marx A, Huang PL, Ertl G. Atheroprotective effects of neuronal nitric oxide synthase in apolipoprotein e knockout mice. Arterioscler Thromb Vasc Biol 26: 1539–1544, 2006. doi: 10.1161/01.ATV.0000223143.88128.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Kuhn H, Banthiya S, van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta 1851: 308–330, 2015. doi: 10.1016/j.bbalip.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Kuhn V, Diederich L, Keller TCS IV, Kramer CM, Lückstädt W, Panknin C, Suvorava T, Isakson BE, Kelm M, Cortese-Krott MM. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid Redox Signal 26: 718–742, 2017. doi: 10.1089/ars.2016.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Kukreja RC, Kontos HA, Hess ML, Ellis EF. PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ Res 59: 612–619, 1986. doi: 10.1161/01.RES.59.6.612. [DOI] [PubMed] [Google Scholar]
- 538.Kundu TK, Hille R, Velayutham M, Zweier JL. Characterization of superoxide production from aldehyde oxidase: an important source of oxidants in biological tissues. Arch Biochem Biophys 460: 113–121, 2007. doi: 10.1016/j.abb.2006.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Kundu TK, Velayutham M, Zweier JL. Aldehyde oxidase functions as a superoxide generating NADH oxidase: an important redox regulated pathway of cellular oxygen radical formation. Biochemistry 51: 2930–2939, 2012. doi: 10.1021/bi3000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA 107: 15565–15570, 2010. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells 10: 1139–1151, 2005. doi: 10.1111/j.1365-2443.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- 542.Kuwabara Y, Nishino T, Okamoto K, Matsumura T, Eger BT, Pai EF, Nishino T. Unique amino acids cluster for switching from the dehydrogenase to oxidase form of xanthine oxidoreductase. Proc Natl Acad Sci USA 100: 8170–8175, 2003. doi: 10.1073/pnas.1431485100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278: 22546–22554, 2003. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 544.Kwon NS, Nathan CF, Stuehr DJ. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem 264: 20496–20501, 1989. [PubMed] [Google Scholar]
- 545.Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, Page P. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem 53: 7715–7730, 2010. doi: 10.1021/jm100773e. [DOI] [PubMed] [Google Scholar]
- 546.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189, 2004. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 547.Lamping KG, Nuno DW, Shesely EG, Maeda N, Faraci FM. Vasodilator mechanisms in the coronary circulation of endothelial nitric oxide synthase-deficient mice. Am J Physiol Heart Circ Physiol 279: H1906–H1912, 2000. doi: 10.1152/ajpheart.2000.279.4.H1906. [DOI] [PubMed] [Google Scholar]
- 548.Lancaster JR., Jr Nitroxidative, nitrosative, and nitrative stress: kinetic predictions of reactive nitrogen species chemistry under biological conditions. Chem Res Toxicol 19: 1160–1174, 2006. doi: 10.1021/tx060061w. [DOI] [PubMed] [Google Scholar]
- 549.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40: 511–515, 2002. doi: 10.1161/01.HYP.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003. doi: 10.1172/JCI200314172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Landmesser U, Spiekermann S, Preuss C, Sorrentino S, Fischer D, Manes C, Mueller M, Drexler H. Angiotensin II induces endothelial xanthine oxidase activation: role for endothelial dysfunction in patients with coronary disease. Arterioscler Thromb Vasc Biol 27: 943–948, 2007. doi: 10.1161/01.ATV.0000258415.32883.bf. [DOI] [PubMed] [Google Scholar]
- 552.Langbein H, Brunssen C, Hofmann A, Cimalla P, Brux M, Bornstein SR, Deussen A, Koch E, Morawietz H. NADPH oxidase 4 protects against development of endothelial dysfunction and atherosclerosis in LDL receptor deficient mice. Eur Heart J 37: 1753–1761, 2016. doi: 10.1093/eurheartj/ehv564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Lapp H, Mitrovic V, Franz N, Heuer H, Buerke M, Wolfertz J, Mueck W, Unger S, Wensing G, Frey R. Cinaciguat (BAY 58-2667) improves cardiopulmonary hemodynamics in patients with acute decompensated heart failure. Circulation 119: 2781–2788, 2009. doi: 10.1161/CIRCULATIONAHA.108.800292. [DOI] [PubMed] [Google Scholar]
- 554.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med 355: 2792–2793, 2006. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 555.Lassègue B, San Martín A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110: 1364–1390, 2012. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Lassègue B, Sorescu D, Szöcs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res 88: 888–894, 2001. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 557.Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA 98: 12814–12819, 2001. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Laurent C, Chabi B, Fouret G, Py G, Sairafi B, Elong C, Gaillet S, Cristol JP, Coudray C, Feillet-Coudray C. Polyphenols decreased liver NADPH oxidase activity, increased muscle mitochondrial biogenesis and decreased gastrocnemius age-dependent autophagy in aged rats. Free Radic Res 46: 1140–1149, 2012. doi: 10.3109/10715762.2012.694428. [DOI] [PubMed] [Google Scholar]
- 559.Laurindo FRM, Fernandes DC, Amanso AM, Lopes LR, Santos CXC. Novel role of protein disulfide isomerase in the regulation of NADPH oxidase activity: pathophysiological implications in vascular diseases. Antioxid Redox Signal 10: 1101–1113, 2008. doi: 10.1089/ars.2007.2011. [DOI] [PubMed] [Google Scholar]
- 560.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103: 1282–1288, 2001. doi: 10.1161/01.CIR.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 561.Lee BE, Toledo AH, Anaya-Prado R, Roach RR, Toledo-Pereyra LH. Allopurinol, xanthine oxidase, and cardiac ischemia. J Investig Med 57: 902–909, 2009. doi: 10.2310/JIM.0b013e3181bca50c. [DOI] [PubMed] [Google Scholar]
- 562.Lee MC, Velayutham M, Komatsu T, Hille R, Zweier JL. Measurement and characterization of superoxide generation from xanthine dehydrogenase: a redox-regulated pathway of radical generation in ischemic tissues. Biochemistry 53: 6615–6623, 2014. doi: 10.1021/bi500582r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Lee PC, Salyapongse AN, Bragdon GA, Shears LL II, Watkins SC, Edington HDJ, Billiar TR. Impaired wound healing and angiogenesis in eNOS-deficient mice. Am J Physiol 277: H1600–H1608, 1999. [DOI] [PubMed] [Google Scholar]
- 564.Lee SB, Bae IH, Bae YS, Um HD. Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J Biol Chem 281: 36228–36235, 2006. doi: 10.1074/jbc.M606702200. [DOI] [PubMed] [Google Scholar]
- 565.Lei J, Vodovotz Y, Tzeng E, Billiar TR. Nitric oxide, a protective molecule in the cardiovascular system. Nitric Oxide 35: 175–185, 2013. doi: 10.1016/j.niox.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 566.Leiper J, Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res 43: 542–548, 1999. doi: 10.1016/S0008-6363(99)00162-5. [DOI] [PubMed] [Google Scholar]
- 567.Lenaz G. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life 52: 159–164, 2001. doi: 10.1080/15216540152845957. [DOI] [PubMed] [Google Scholar]
- 568.Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL. Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase. J Biol Chem 283: 17855–17863, 2008. doi: 10.1074/jbc.M801785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Li H, Forstermann U. Pharmacological prevention of eNOS uncoupling. Curr Pharm Des 20: 3595–3606, 2014. doi: 10.2174/13816128113196660749. [DOI] [PubMed] [Google Scholar]
- 570.Li H, Förstermann U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr Opin Pharmacol 13: 161–167, 2013. doi: 10.1016/j.coph.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 571.Li H, Hemann C, Abdelghany TM, El-Mahdy MA, Zweier JL. Characterization of the mechanism and magnitude of cytoglobin-mediated nitrite reduction and nitric oxide generation under anaerobic conditions. J Biol Chem 287: 36623–36633, 2012. doi: 10.1074/jbc.M112.342378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Li H, Horke S, Förstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol Sci 34: 313–319, 2013. doi: 10.1016/j.tips.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 573.Li H, Horke S, Förstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 237: 208–219, 2014. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 574.Li H, Jamal J, Plaza C, Pineda SH, Chreifi G, Jing Q, Cinelli MA, Silverman RB, Poulos TL. Structures of human constitutive nitric oxide synthases. Acta Crystallogr D Biol Crystallogr 70: 2667–2674, 2014. doi: 10.1107/S1399004714017064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Li H, Kundu TK, Zweier JL. Characterization of the magnitude and mechanism of aldehyde oxidase-mediated nitric oxide production from nitrite. J Biol Chem 284: 33850–33858, 2009. doi: 10.1074/jbc.M109.019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Li H, Liu X, Cui H, Chen YR, Cardounel AJ, Zweier JL. Characterization of the mechanism of cytochrome P450 reductase-cytochrome P450-mediated nitric oxide and nitrosothiol generation from organic nitrates. J Biol Chem 281: 12546–12554, 2006. doi: 10.1074/jbc.M511803200. [DOI] [PubMed] [Google Scholar]
- 577.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the effects of oxygen on xanthine oxidase-mediated nitric oxide formation. J Biol Chem 279: 16939–16946, 2004. doi: 10.1074/jbc.M314336200. [DOI] [PubMed] [Google Scholar]
- 578.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem 276: 24482–24489, 2001. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 579.Li J, Billiar TR. Nitric Oxide. IV. Determinants of nitric oxide protection and toxicity in liver. Am J Physiol 276: G1069–G1073, 1999. [DOI] [PubMed] [Google Scholar]
- 580.Li JM, Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem 277: 19952–19960, 2002. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- 581.Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, Chen AF. Endothelin-1 increases vascular superoxide via endothelin(A)-NADPH oxidase pathway in low-renin hypertension. Circulation 107: 1053–1058, 2003. doi: 10.1161/01.CIR.0000051459.74466.46. [DOI] [PubMed] [Google Scholar]
- 582.Li Q, Fu GB, Zheng JT, He J, Niu XB, Chen QD, Yin Y, Qian X, Xu Q, Wang M, Sun AF, Shu Y, Rui H, Liu LZ, Jiang BH. NADPH oxidase subunit p22(phox)-mediated reactive oxygen species contribute to angiogenesis and tumor growth through AKT and ERK1/2 signaling pathways in prostate cancer. Biochim Biophys Acta 1833: 3375–3385, 2013. doi: 10.1016/j.bbamcr.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 583.Li Y, Zhang J, Schopfer FJ, Martynowski D, Garcia-Barrio MT, Kovach A, Suino-Powell K, Baker PRS, Freeman BA, Chen YE, Xu HE. Molecular recognition of nitrated fatty acids by PPAR gamma. Nat Struct Mol Biol 15: 865–867, 2008. doi: 10.1038/nsmb.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet 11: 376–381, 1995. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 585.Lim CH, Dedon PC, Deen WM. Kinetic analysis of intracellular concentrations of reactive nitrogen species. Chem Res Toxicol 21: 2134–2147, 2008. doi: 10.1021/tx800213b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res 106: 633–646, 2010. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Lin HL, Kenaan C, Zhang H, Hollenberg PF. Reaction of human cytochrome P450 3A4 with peroxynitrite: nitrotyrosine formation on the proximal side impairs its interaction with NADPH-cytochrome P450 reductase. Chem Res Toxicol 25: 2642–2653, 2012. doi: 10.1021/tx3002753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Lin MI, Fulton D, Babbitt R, Fleming I, Busse R, Pritchard KA Jr, Sessa WC. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of L-arginine metabolism to efficient nitric oxide production. J Biol Chem 278: 44719–44726, 2003. doi: 10.1074/jbc.M302836200. [DOI] [PubMed] [Google Scholar]
- 589.Lind L, Larsson A, Teerlink T. L-Arginine is related to endothelium-dependent vasodilation in resistance and conduit arteries in divergent ways-The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Atherosclerosis 203: 544–549, 2009. doi: 10.1016/j.atherosclerosis.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 590.List BM, Klösch B, Völker C, Gorren AC, Sessa WC, Werner ER, Kukovetz WR, Schmidt K, Mayer B. Characterization of bovine endothelial nitric oxide synthase as a homodimer with down-regulated uncoupled NADPH oxidase activity: tetrahydrobiopterin binding kinetics and role of haem in dimerization. Biochem J 323: 159–165, 1997. doi: 10.1042/bj3230159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Liu C, Wajih N, Liu X, Basu S, Janes J, Marvel M, Keggi C, Helms CC, Lee AN, Belanger AM, Diz DI, Laurienti PJ, Caudell DL, Wang J, Gladwin MT, Kim-Shapiro DB. Mechanisms of human erythrocytic bioactivation of nitrite. J Biol Chem 290: 1281–1294, 2015. doi: 10.1074/jbc.M114.609222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410: 490–494, 2001. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 593.Liu X, El-Mahdy MA, Boslett J, Varadharaj S, Hemann C, Abdelghany TM, Ismail RS, Little SC, Zhou D, Thuy LT, Kawada N, Zweier JL. Cytoglobin regulates blood pressure and vascular tone through nitric oxide metabolism in the vascular wall. Nat Commun 8: 14807, 2017. doi: 10.1038/ncomms14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Liu X, Follmer D, Zweier JR, Huang X, Hemann C, Liu K, Druhan LJ, Zweier JL. Characterization of the function of cytoglobin as an oxygen-dependent regulator of nitric oxide concentration. Biochemistry 51: 5072–5082, 2012. doi: 10.1021/bi300291h. [DOI] [PubMed] [Google Scholar]
- 595.Liu X, Tong J, Zweier JR, Follmer D, Hemann C, Ismail RS, Zweier JL. Differences in oxygen-dependent nitric oxide metabolism by cytoglobin and myoglobin account for their differing functional roles. FEBS J 280: 3621–3631, 2013. doi: 10.1111/febs.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Liu XH, Zhang QY, Pan LL, Liu SY, Xu P, Luo XL, Zou SL, Xin H, Qu LF, Zhu YZ. NADPH oxidase 4 contributes to connective tissue growth factor expression through Smad3-dependent signaling pathway. Free Radic Biol Med 94: 174–184, 2016. doi: 10.1016/j.freeradbiomed.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 597.Lötzer K, Funk CD, Habenicht AJR. The 5-lipoxygenase pathway in arterial wall biology and atherosclerosis. Biochim Biophys Acta 1736: 30–37, 2005. doi: 10.1016/j.bbalip.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 598.Lucock M. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab 71: 121–138, 2000. doi: 10.1006/mgme.2000.3027. [DOI] [PubMed] [Google Scholar]
- 599.Lührs H, Papadopoulos T, Schmidt HH, Menzel T. Type I nitric oxide synthase in the human lung is predominantly expressed in capillary endothelial cells. Respir Physiol 129: 367–374, 2002. doi: 10.1016/S0034-5687(01)00323-1. [DOI] [PubMed] [Google Scholar]
- 600.Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov 14: 623–641, 2015. doi: 10.1038/nrd4623. [DOI] [PubMed] [Google Scholar]
- 601.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med 37: 395–400, 2004. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 602.Lundberg JO, Weitzberg E, Cole JA, Benjamin N. Nitrate, bacteria and human health. Nat Rev Microbiol 2: 593–602, 2004. doi: 10.1038/nrmicro929. Erratum at: doi:. [DOI] [PubMed] [Google Scholar]
- 603.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7: 156–167, 2008. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 604.Lundberg JO, Farkas-Szallasi T, Weitzberg E, Rinder J, Lidholm J, Anggåard A, Hökfelt T, Lundberg JM, Alving K. High nitric oxide production in human paranasal sinuses. Nat Med 1: 370–373, 1995. doi: 10.1038/nm0495-370. [DOI] [PubMed] [Google Scholar]
- 605.Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut 35: 1543–1546, 1994. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Lüneburg N, Xanthakis V, Schwedhelm E, Sullivan LM, Maas R, Anderssohn M, Riederer U, Glazer NL, Vasan RS, Böger RH. Reference intervals for plasma L-arginine and the L-arginine:asymmetric dimethylarginine ratio in the Framingham Offspring Cohort. J Nutr 141: 2186–2190, 2011. doi: 10.3945/jn.111.148197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassègue B, Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res 105: 249–259, 2009. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Lymar SV, Hurst JK. Rapid reaction between peroxonitrite ion and carbon-dioxide - implications for biological-activity. J Am Chem Soc 117: 8867–8868, 1995. doi: 10.1021/ja00139a027. [DOI] [Google Scholar]
- 609.Lynch CM, Kinzenbaw DA, Chen X, Zhan S, Mezzetti E, Filosa J, Ergul A, Faulkner JL, Faraci FM, Didion SP. Nox2-derived superoxide contributes to cerebral vascular dysfunction in diet-induced obesity. Stroke 44: 3195–3201, 2013. doi: 10.1161/STROKEAHA.113.001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.MacDonald TM, Ford I, Nuki G, Mackenzie IS, De Caterina R, Findlay E, Hallas J, Hawkey CJ, Ralston S, Walters M, Webster J, McMurray J, Perez Ruiz F, Jennings CG, MacDonald T, Ford I, Nuki G, Mackenzie I, Hallas J, Webster J, Walters M, Ralston S, Hawkey C, De Caterina R, Perez-Ruiz F, Findlay E, McMurray J, Maseri A, Murray G, Bird H, McMurray J, Petrie M, MacDonald M, Jhund P, Saywood W, Flynn R, Ford I, Kean S; Members of the FAST Study Group . Protocol of the Febuxostat versus Allopurinol Streamlined Trial (FAST): a large prospective, randomised, open, blinded endpoint study comparing the cardiovascular safety of allopurinol and febuxostat in the management of symptomatic hyperuricaemia. BMJ Open 4: e005354, 2014. doi: 10.1136/bmjopen-2014-005354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Macfadyen AJ, Reiter C, Zhuang Y, Beckman JS. A novel superoxide dismutase-based trap for peroxynitrite used to detect entry of peroxynitrite into erythrocyte ghosts. Chem Res Toxicol 12: 223–229, 1999. doi: 10.1021/tx980253u. [DOI] [PubMed] [Google Scholar]
- 612.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25: 29–38, 2005. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 613.Magnani F, Nenci S, Millana Fananas E, Ceccon M, Romero E, Fraaije MW, Mattevi A. Crystal structures and atomic model of NADPH oxidase. Proc Natl Acad Sci U S A 114: 6764–6769, 2017. doi: 10.1073/pnas.1702293114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Maia LB, Moura JJ. Nitrite reduction by xanthine oxidase family enzymes: a new class of nitrite reductases. J Biol Inorg Chem 16: 443–460, 2011. doi: 10.1007/s00775-010-0741-z. [DOI] [PubMed] [Google Scholar]
- 615.Maia LB, Moura JJG. How biology handles nitrite. Chem Rev 114: 5273–5357, 2014. doi: 10.1021/cr400518y. [DOI] [PubMed] [Google Scholar]
- 616.Maia LB, Moura JJG. Nitrite reduction by molybdoenzymes: a new class of nitric oxide-forming nitrite reductases. J Biol Inorg Chem 20: 403–433, 2015. doi: 10.1007/s00775-014-1234-2. [DOI] [PubMed] [Google Scholar]
- 617.Maia LB, Pereira V, Mira L, Moura JJ. Nitrite reductase activity of rat and human xanthine oxidase, xanthine dehydrogenase, and aldehyde oxidase: evaluation of their contribution to NO formation in vivo. Biochemistry 54: 685–710, 2015. doi: 10.1021/bi500987w. [DOI] [PubMed] [Google Scholar]
- 618.Maier W, Cosentino F, Lütolf RB, Fleisch M, Seiler C, Hess OM, Meier B, Lüscher TF. Tetrahydrobiopterin improves endothelial function in patients with coronary artery disease. J Cardiovasc Pharmacol 35: 173–178, 2000. doi: 10.1097/00005344-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 619.Mäki-Petäjä KM, Day L, Cheriyan J, Hall FC, Östör AJ, Shenker N, Wilkinson IB. Tetrahydrobiopterin supplementation improves endothelial function but does not alter aortic stiffness in patients with rheumatoid arthritis. J Am Heart Assoc 5: e002762, 2016. doi: 10.1161/JAHA.115.002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Malik UZ, Hundley NJ, Romero G, Radi R, Freeman BA, Tarpey MM, Kelley EE. Febuxostat inhibition of endothelial-bound XO: implications for targeting vascular ROS production. Free Radic Biol Med 51: 179–184, 2011. doi: 10.1016/j.freeradbiomed.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Malle E, Waeg G, Schreiber R, Gröne EF, Sattler W, Gröne HJ. Immunohistochemical evidence for the myeloperoxidase/H2O2/halide system in human atherosclerotic lesions: colocalization of myeloperoxidase and hypochlorite-modified proteins. Eur J Biochem 267: 4495–4503, 2000. doi: 10.1046/j.1432-1327.2000.01498.x. [DOI] [PubMed] [Google Scholar]
- 622.Manea A, Manea SA, Florea IC, Luca CM, Raicu M. Positive regulation of NADPH oxidase 5 by proinflammatory-related mechanisms in human aortic smooth muscle cells. Free Radic Biol Med 52: 1497–1507, 2012. doi: 10.1016/j.freeradbiomed.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 623.Manea A, Manea SA, Gan AM, Constantin A, Fenyo IM, Raicu M, Muresian H, Simionescu M. Human monocytes and macrophages express NADPH oxidase 5; a potential source of reactive oxygen species in atherosclerosis. Biochem Biophys Res Commun 461: 172–179, 2015. doi: 10.1016/j.bbrc.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 624.Marfella R, Di Filippo C, Esposito K, Nappo F, Piegari E, Cuzzocrea S, Berrino L, Rossi F, Giugliano D, D’Amico M. Absence of inducible nitric oxide synthase reduces myocardial damage during ischemia reperfusion in streptozotocin-induced hyperglycemic mice. Diabetes 53: 454–462, 2004. doi: 10.2337/diabetes.53.2.454. [DOI] [PubMed] [Google Scholar]
- 625.Martin E, Berka V, Bogatenkova E, Murad F, Tsai A-L. Ligand selectivity of soluble guanylyl cyclase: effect of the hydrogen-bonding tyrosine in the distal heme pocket on binding of oxygen, nitric oxide, and carbon monoxide. J Biol Chem 281: 27836–27845, 2006. doi: 10.1074/jbc.M601078200. [DOI] [PubMed] [Google Scholar]
- 626.Martin E, Golunski E, Laing ST, Estrera AL, Sharina IG. Alternative splicing impairs soluble guanylyl cyclase function in aortic aneurysm. Am J Physiol Heart Circ Physiol 307: H1565–H1575, 2014. doi: 10.1152/ajpheart.00222.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 628.Matsui A, Okigaki M, Amano K, Adachi Y, Jin D, Takai S, Yamashita T, Kawashima S, Kurihara T, Miyazaki M, Tateishi K, Matsunaga S, Katsume A, Honshou S, Takahashi T, Matoba S, Kusaba T, Tatsumi T, Matsubara H. Central role of calcium-dependent tyrosine kinase PYK2 in endothelial nitric oxide synthase-mediated angiogenic response and vascular function. Circulation 116: 1041–1051, 2007. doi: 10.1161/CIRCULATIONAHA.106.645416. [DOI] [PubMed] [Google Scholar]
- 629.Matsumoto K, Okamoto K, Ashizawa N, Nishino T. FYX-051: a novel and potent hybrid-type inhibitor of xanthine oxidoreductase. J Pharmacol Exp Ther 336: 95–103, 2011. doi: 10.1124/jpet.110.174540. [DOI] [PubMed] [Google Scholar]
- 630.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation 112: 2677–2685, 2005. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 631.McCabe TJ, Fulton D, Roman LJ, Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J Biol Chem 275: 6123–6128, 2000. doi: 10.1074/jbc.275.9.6123. [DOI] [PubMed] [Google Scholar]
- 632.McCarty MF. Asymmetric dimethylarginine is a well established mediating risk factor for cardiovascular morbidity and mortality-should patients with elevated levels be supplemented with citrulline? Healthcare (Basel) 4: E40, 2016. doi: 10.3390/healthcare4030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312: 159–163, 1985. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 634.McDonald KK, Zharikov S, Block ER, Kilberg MS. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “arginine paradox”. J Biol Chem 272: 31213–31216, 1997. doi: 10.1074/jbc.272.50.31213. [DOI] [PubMed] [Google Scholar]
- 635.Medinas DB, Cerchiaro G, Trindade DF, Augusto O. The carbonate radical and related oxidants derived from bicarbonate buffer. IUBMB Life 59: 255–262, 2007. doi: 10.1080/15216540701230511. [DOI] [PubMed] [Google Scholar]
- 636.Mehrabian M, Allayee H, Wong J, Shi W, Wang XP, Shaposhnik Z, Funk CD, Lusis AJ. Identification of 5-lipoxygenase as a major gene contributing to atherosclerosis susceptibility in mice. Circ Res 91: 120–126, 2002. doi: 10.1161/01.RES.0000028008.99774.7F. [DOI] [PubMed] [Google Scholar]
- 637.Meijles DN, Howlin BJ, Li JM. Consensus in silico computational modelling of the p22phox subunit of the NADPH oxidase. Comput Biol Chem 39: 6–13, 2012. doi: 10.1016/j.compbiolchem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 638.Meitzler JL, Ortiz de Montellano PR. Caenorhabditis elegans and human dual oxidase 1 (DUOX1) “peroxidase” domains: insights into heme binding and catalytic activity. J Biol Chem 284: 18634–18643, 2009. doi: 10.1074/jbc.M109.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Meitzler JL, Ortiz de Montellano PR. Structural stability and heme binding potential of the truncated human dual oxidase 2 (DUOX2) peroxidase domain. Arch Biochem Biophys 512: 197–203, 2011. doi: 10.1016/j.abb.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Meli R, Nauser T, Koppenol WH. Direct observation of intermediates in the reaction of peroxynitrite with carbon dioxide. Helv Chim Acta 82: 722–725, 1999. [Google Scholar]
- 641.Mendes-Silverio CB, Leiria LOS, Morganti RP, Anhê GF, Marcondes S, Mónica FZ, De Nucci G, Antunes E. Activation of haem-oxidized soluble guanylyl cyclase with BAY 60-2770 in human platelets lead to overstimulation of the cyclic GMP signaling pathway. PLoS One 7: e47223, 2012. doi: 10.1371/journal.pone.0047223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Meng D, Mei A, Liu J, Kang X, Shi X, Qian R, Chen S. NADPH oxidase 4 mediates insulin-stimulated HIF-1α and VEGF expression, and angiogenesis in vitro. PLoS One 7: e48393, 2012. doi: 10.1371/journal.pone.0048393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Meurer S, Pioch S, Pabst T, Opitz N, Schmidt PM, Beckhaus T, Wagner K, Matt S, Gegenbauer K, Geschka S, Karas M, Stasch J-P, Schmidt HHHW, Müller-Esterl W. Nitric oxide-independent vasodilator rescues heme-oxidized soluble guanylate cyclase from proteasomal degradation. Circ Res 105: 33–41, 2009. doi: 10.1161/CIRCRESAHA.109.198234. [DOI] [PubMed] [Google Scholar]
- 644.Meuwese MC, Stroes ESG, Hazen SL, van Miert JN, Kuivenhoven JA, Schaub RG, Wareham NJ, Luben R, Kastelein JJP, Khaw K-T, Boekholdt SM. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol 50: 159–165, 2007. doi: 10.1016/j.jacc.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 645.Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res 90: 1307–1315, 2002. doi: 10.1161/01.RES.0000024689.07590.C2. [DOI] [PubMed] [Google Scholar]
- 646.Michell BJ, Chen Zp, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp BE. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem 276: 17625–17628, 2001. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 647.Michell BJ, Harris MB, Chen ZP, Ju H, Venema VJ, Blackstone MA, Huang W, Venema RC, Kemp BE. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem 277: 42344–42351, 2002. doi: 10.1074/jbc.M205144200. [DOI] [PubMed] [Google Scholar]
- 648.Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, Bauer JA. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation 104: 174–180, 2001. doi: 10.1161/01.CIR.104.2.174. [DOI] [PubMed] [Google Scholar]
- 649.Miles EW, Kraus JP. Cystathionine beta-synthase: structure, function, regulation, and location of homocystinuria-causing mutations. J Biol Chem 279: 29871–29874, 2004. doi: 10.1074/jbc.R400005200. [DOI] [PubMed] [Google Scholar]
- 650.Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett 427: 225–228, 1998. doi: 10.1016/S0014-5793(98)00430-X. [DOI] [PubMed] [Google Scholar]
- 651.Miller FJ Jr, Chu X, Stanic B, Tian X, Sharma RV, Davisson RL, Lamb FS. A differential role for endocytosis in receptor-mediated activation of Nox1. Antioxid Redox Signal 12: 583–593, 2010. doi: 10.1089/ars.2009.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Miller FJ Jr, Filali M, Huss GJ, Stanic B, Chamseddine A, Barna TJ, Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res 101: 663–671, 2007. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 653.Miller RT, Martásek P, Roman LJ, Nishimura JS, Masters BSS. Involvement of the reductase domain of neuronal nitric oxide synthase in superoxide anion production. Biochemistry 36: 15277–15284, 1997. doi: 10.1021/bi972022c. [DOI] [PubMed] [Google Scholar]
- 654.Milsom AB, Patel NSA, Mazzon E, Tripatara P, Storey A, Mota-Filipe H, Sepodes B, Webb AJ, Cuzzocrea S, Hobbs AJ, Thiemermann C, Ahluwalia A. Role for endothelial nitric oxide synthase in nitrite-induced protection against renal ischemia-reperfusion injury in mice. Nitric Oxide 22: 141–148, 2010. doi: 10.1016/j.niox.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 655.Mittendorf J, Weigand S, Alonso-Alija C, Bischoff E, Feurer A, Gerisch M, Kern A, Knorr A, Lang D, Muenter K, Radtke M, Schirok H, Schlemmer K-H, Stahl E, Straub A, Wunder F, Stasch J-P. Discovery of riociguat (BAY 63-2521): a potent, oral stimulator of soluble guanylate cyclase for the treatment of pulmonary hypertension. ChemMedChem 4: 853–865, 2009. doi: 10.1002/cmdc.200900014. [DOI] [PubMed] [Google Scholar]
- 656.Miyazaki H, Matsuoka H, Cooke JP, Usui M, Ueda S, Okuda S, Imaizumi T. Endogenous nitric oxide synthase inhibitor: a novel marker of atherosclerosis. Circulation 99: 1141–1146, 1999. doi: 10.1161/01.CIR.99.9.1141. [DOI] [PubMed] [Google Scholar]
- 657.Mohazzab KM, Kaminski PM, Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol Heart Circ Physiol 266: H2568–H2572, 1994. [DOI] [PubMed] [Google Scholar]
- 658.Mollnau H, Wendt M, Szöcs K, Lassègue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Förstermann U, Meinertz T, Griendling K, Münzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 90: E58–E65, 2002. doi: 10.1161/01.RES.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 659.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43: 109–142, 1991. [PubMed] [Google Scholar]
- 660.Montezano AC, Burger D, Paravicini TM, Chignalia AZ, Yusuf H, Almasri M, He Y, Callera GE, He G, Krause K-H, Lambeth D, Quinn MT, Touyz RM. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ Res 106: 1363–1373, 2010. doi: 10.1161/CIRCRESAHA.109.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Montezano AC, Tsiropoulou S, Dulak-Lis M, Harvey A, Camargo LL, Touyz RM. Redox signaling, Nox5 and vascular remodeling in hypertension. Curr Opin Nephrol Hypertens 24: 425–433, 2015. doi: 10.1097/MNH.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Montfort WR, Wales JA, Weichsel A. Structure and Activation of Soluble Guanylyl Cyclase, the Nitric Oxide Sensor. Antioxid Redox Signal 26: 107–121, 2017. doi: 10.1089/ars.2016.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Monti LD, Casiraghi MC, Setola E, Galluccio E, Pagani MA, Quaglia L, Bosi E, Piatti P. L-arginine enriched biscuits improve endothelial function and glucose metabolism: a pilot study in healthy subjects and a cross-over study in subjects with impaired glucose tolerance and metabolic syndrome. Metabolism 62: 255–264, 2013. doi: 10.1016/j.metabol.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 664.Moore PK, Babbedge RC, Wallace P, Gaffen ZA, Hart SL. 7-Nitro indazole, an inhibitor of nitric oxide synthase, exhibits anti-nociceptive activity in the mouse without increasing blood pressure. Br J Pharmacol 108: 296–297, 1993. doi: 10.1111/j.1476-5381.1993.tb12798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Mori E, Haramaki N, Ikeda H, Imaizumi T. Intra-coronary administration of L-arginine aggravates myocardial stunning through production of peroxynitrite in dogs. Cardiovasc Res 40: 113–123, 1998. doi: 10.1016/S0008-6363(98)00146-1. [DOI] [PubMed] [Google Scholar]
- 666.Morishita T, Tsutsui M, Shimokawa H, Horiuchi M, Tanimoto A, Suda O, Tasaki H, Huang PL, Sasaguri Y, Yanagihara N, Nakashima Y. Vasculoprotective roles of neuronal nitric oxide synthase. FASEB J 16: 1994–1996, 2002. doi: 10.1096/fj.02-0155fje. [DOI] [PubMed] [Google Scholar]
- 667.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM Jr, Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA 294: 81–90, 2005. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Morris SM., Jr Arginine Metabolism Revisited. J Nutr 146: 2579S–2586S, 2016. doi: 10.3945/jn.115.226621. [DOI] [PubMed] [Google Scholar]
- 669.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 42: 271–279, 2007. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 670.Mullershausen F, Russwurm M, Friebe A, Koesling D. Inhibition of phosphodiesterase type 5 by the activator of nitric oxide-sensitive guanylyl cyclase BAY 41-2272. Circulation 109: 1711–1713, 2004. doi: 10.1161/01.CIR.0000126286.47618.BD. [DOI] [PubMed] [Google Scholar]
- 671.Mülsch A, Busse R, Bassenge E. Desensitization of guanylate cyclase in nitrate tolerance does not impair endothelium-dependent responses. Eur J Pharmacol 158: 191–198, 1988. doi: 10.1016/0014-2999(88)90066-0. [DOI] [PubMed] [Google Scholar]
- 672.Münzel T, Camici GG, Maack C, Bonetti NR, Fuster V, Kovacic JC. Impact of Oxidative Stress on the Heart and Vasculature: Part 2 of a 3-Part Series. J Am Coll Cardiol 70: 212–229, 2017. doi: 10.1016/j.jacc.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Münzel T, Feil R, Mülsch A, Lohmann SM, Hofmann F, Walter U. Physiology and pathophysiology of vascular signaling controlled by guanosine 3′,5′-cyclic monophosphate-dependent protein kinase [corrected]. Circulation 108: 2172–2183, 2003. doi: 10.1161/01.CIR.0000094403.78467.C3. [DOI] [PubMed] [Google Scholar]
- 674.Murad F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med 355: 2003–2011, 2006. doi: 10.1056/NEJMsa063904. [DOI] [PubMed] [Google Scholar]
- 675.Murataliev MB, Feyereisen R, Walker FA. Electron transfer by diflavin reductases. Biochim Biophys Acta 1698: 1–26, 2004. doi: 10.1016/j.bbapap.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 676.Murdoch CE, Alom-Ruiz SP, Wang M, Zhang M, Walker S, Yu B, Brewer A, Shah AM. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res Cardiol 106: 527–538, 2011. doi: 10.1007/s00395-011-0179-7. Erratum at: doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Murphy E, Kohr M, Menazza S, Nguyen T, Evangelista A, Sun J, Steenbergen C. Signaling by S-nitrosylation in the heart. J Mol Cell Cardiol 73: 18–25, 2014. doi: 10.1016/j.yjmcc.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Murphy MP, Smith RAJ. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol 47: 629–656, 2007. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 680.Musset B, Clark RA, DeCoursey TE, Petheo GL, Geiszt M, Chen Y, Cornell JE, Eddy CA, Brzyski RG, El Jamali A. NOX5 in human spermatozoa: expression, function, and regulation. J Biol Chem 287: 9376–9388, 2012. doi: 10.1074/jbc.M111.314955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Muzaffar S, Jeremy JY, Angelini GD, Shukla N. NADPH oxidase 4 mediates upregulation of type 4 phosphodiesterases in human endothelial cells. J Cell Physiol 227: 1941–1950, 2012. doi: 10.1002/jcp.22922. [DOI] [PubMed] [Google Scholar]
- 682.Nabeebaccus A, Zhang M, Shah AM. NADPH oxidases and cardiac remodelling. Heart Fail Rev 16: 5–12, 2011. doi: 10.1007/s10741-010-9186-2. [DOI] [PubMed] [Google Scholar]
- 683.Nadtochiy SM, Zhu QM, Urciuoli W, Rafikov R, Black SM, Brookes PS. Nitroalkenes confer acute cardioprotection via adenine nucleotide translocase 1. J Biol Chem 287: 3573–3580, 2012. doi: 10.1074/jbc.M111.298406. Erratum at: doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Nagababu E, Ramasamy S, Rifkind JM. Intermediates detected by visible spectroscopy during the reaction of nitrite with deoxyhemoglobin: the effect of nitrite concentration and diphosphoglycerate. Biochemistry 46: 11650–11659, 2007. doi: 10.1021/bi700364e. [DOI] [PubMed] [Google Scholar]
- 685.Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, Wang X, Sasidhar M, Nabel EG, Takahashi T, Lukacs NW, Ryter SW, Morita K, Choi AMK. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med 203: 2377–2389, 2006. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Nakamura T, Lipton SA. Protein S-Nitrosylation as a Therapeutic Target for Neurodegenerative Diseases. Trends Pharmacol Sci 37: 73–84, 2016. doi: 10.1016/j.tips.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Nakatani K, Okuyama H, Shimahara Y, Saeki S, Kim DH, Nakajima Y, Seki S, Kawada N, Yoshizato K. Cytoglobin/STAP, its unique localization in splanchnic fibroblast-like cells and function in organ fibrogenesis. Lab Invest 84: 91–101, 2004. doi: 10.1038/labinvest.3700013. [DOI] [PubMed] [Google Scholar]
- 688.Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest 100: 2417–2423, 1997. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.NaveenKumar SK, Hemshekhar M, Sundaram MS, Kemparaju K, Girish KS. Cell-free methemoglobin drives platelets to apoptosis via mitochondrial ROS-mediated activation of JNK and p38 MAP kinase. Biochem Biophys Res Commun 491: 183–191, 2017. doi: 10.1016/j.bbrc.2017.07.073. [DOI] [PubMed] [Google Scholar]
- 690.Ndrepepa G, Braun S, Mehilli J, von Beckerath N, Schömig A, Kastrati A. Myeloperoxidase level in patients with stable coronary artery disease and acute coronary syndromes. Eur J Clin Invest 38: 90–96, 2008. doi: 10.1111/j.1365-2362.2007.01908.x. [DOI] [PubMed] [Google Scholar]
- 691.Nedospasov A, Rafikov R, Beda N, Nudler E. An autocatalytic mechanism of protein nitrosylation. Proc Natl Acad Sci USA 97: 13543–13548, 2000. doi: 10.1073/pnas.250398197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Nedospasov AA. Is N2O3 the main nitrosating intermediate in aerated nitric oxide (NO) solutions in vivo? If so, where, when, and which one? J Biochem Mol Toxicol 16: 109–120, 2002. doi: 10.1002/jbt.10029. [DOI] [PubMed] [Google Scholar]
- 693.Nishino T, Nishino T. The conversion from the dehydrogenase type to the oxidase type of rat liver xanthine dehydrogenase by modification of cysteine residues with fluorodinitrobenzene. J Biol Chem 272: 29859–29864, 1997. doi: 10.1074/jbc.272.47.29859. [DOI] [PubMed] [Google Scholar]
- 694.Nisimoto Y, Jackson HM, Ogawa H, Kawahara T, Lambeth JD. Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochemistry 49: 2433–2442, 2010. doi: 10.1021/bi9022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Niu X-L, Madamanchi NR, Vendrov AE, Tchivilev I, Rojas M, Madamanchi C, Brandes RP, Krause K-H, Humphries J, Smith A, Burnand KG, Runge MS. Nox activator 1: a potential target for modulation of vascular reactive oxygen species in atherosclerotic arteries. Circulation 121: 549–559, 2010. doi: 10.1161/CIRCULATIONAHA.109.908319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Nojiri H, Shimizu T, Funakoshi M, Yamaguchi O, Zhou H, Kawakami S, Ohta Y, Sami M, Tachibana T, Ishikawa H, Kurosawa H, Kahn RC, Otsu K, Shirasawa T. Oxidative stress causes heart failure with impaired mitochondrial respiration. J Biol Chem 281: 33789–33801, 2006. doi: 10.1074/jbc.M602118200. [DOI] [PubMed] [Google Scholar]
- 697.Nomura J, Busso N, Ives A, Matsui C, Tsujimoto S, Shirakura T, Tamura M, Kobayashi T, So A, Yamanaka Y. Xanthine oxidase inhibition by febuxostat attenuates experimental atherosclerosis in mice. Sci Rep 4: 4554, 2014. doi: 10.1038/srep04554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Nunokawa Y, Ishida N, Tanaka S. Cloning of inducible nitric oxide synthase in rat vascular smooth muscle cells. Biochem Biophys Res Commun 191: 89–94, 1993. doi: 10.1006/bbrc.1993.1188. [DOI] [PubMed] [Google Scholar]
- 699.O’Donnell BV, Tew DG, Jones OTG, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J 290: 41–49, 1993. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest 91: 2546–2551, 1993. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Ohtsubo T, Rovira II, Starost MF, Liu C, Finkel T. Xanthine oxidoreductase is an endogenous regulator of cyclooxygenase-2. Circ Res 95: 1118–1124, 2004. doi: 10.1161/01.RES.0000149571.96304.36. [DOI] [PubMed] [Google Scholar]
- 702.Okamoto K, Eger BT, Nishino T, Kondo S, Pai EF, Nishino T. An extremely potent inhibitor of xanthine oxidoreductase. Crystal structure of the enzyme-inhibitor complex and mechanism of inhibition. J Biol Chem 278: 1848–1855, 2003. doi: 10.1074/jbc.M208307200. [DOI] [PubMed] [Google Scholar]
- 703.Okamoto K, Matsumoto K, Hille R, Eger BT, Pai EF, Nishino T. The crystal structure of xanthine oxidoreductase during catalysis: implications for reaction mechanism and enzyme inhibition. Proc Natl Acad Sci USA 101: 7931–7936, 2004. doi: 10.1073/pnas.0400973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Oller J, Méndez-Barbero N, Ruiz EJ, Villahoz S, Renard M, Canelas LI, Briones AM, Alberca R, Lozano-Vidal N, Hurlé MA, Milewicz D, Evangelista A, Salaices M, Nistal JF, Jiménez-Borreguero LJ, De Backer J, Campanero MR, Redondo JM. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat Med 23: 200–212, 2017. doi: 10.1038/nm.4266. [DOI] [PubMed] [Google Scholar]
- 705.Ott G, Havemeyer A, Clement B. The mammalian molybdenum enzymes of mARC. J Biol Inorg Chem 20: 265–275, 2015. doi: 10.1007/s00775-014-1216-4. [DOI] [PubMed] [Google Scholar]
- 706.Ott G, Plitzko B, Krischkowski C, Reichmann D, Bittner F, Mendel RR, Kunze T, Clement B, Havemeyer A. Reduction of sulfamethoxazole hydroxylamine (SMX-HA) by the mitochondrial amidoxime reducing component (mARC). Chem Res Toxicol 27: 1687–1695, 2014. doi: 10.1021/tx500174u. [DOI] [PubMed] [Google Scholar]
- 707.Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461: 537–541, 2009. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Ozaki M, Kawashima S, Yamashita T, Hirase T, Namiki M, Inoue N, Hirata K, Yasui H, Sakurai H, Yoshida Y, Masada M, Yokoyama M. Overexpression of endothelial nitric oxide synthase accelerates atherosclerotic lesion formation in apoE-deficient mice. J Clin Invest 110: 331–340, 2002. doi: 10.1172/JCI0215215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Pacher P, Nivorozhkin A, Szabó C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 58: 87–114, 2006. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Padayatti PS, Pattanaik P, Ma X, van den Akker F. Structural insights into the regulation and the activation mechanism of mammalian guanylyl cyclases. Pharmacol Ther 104: 83–99, 2004. doi: 10.1016/j.pharmthera.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 712.Padilla F, Garcia-Dorado D, Agulló L, Inserte J, Paniagua A, Mirabet S, Barrabés JA, Ruiz-Meana M, Soler-Soler J. L-Arginine administration prevents reperfusion-induced cardiomyocyte hypercontracture and reduces infarct size in the pig. Cardiovasc Res 46: 412–420, 2000. doi: 10.1016/S0008-6363(00)00048-1. [DOI] [PubMed] [Google Scholar]
- 713.Pae H-O, Lee YC, Chung H-T. Heme oxygenase-1 and carbon monoxide: emerging therapeutic targets in inflammation and allergy. Recent Pat Inflamm Allergy Drug Discov 2: 159–165, 2008. doi: 10.2174/187221308786241929. [DOI] [PubMed] [Google Scholar]
- 714.Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc Natl Acad Sci USA 94: 14483–14488, 1997. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 715.Pagano PJ, Ito Y, Tornheim K, Gallop PM, Tauber AI, Cohen RA. An NADPH oxidase superoxide-generating system in the rabbit aorta. Am J Physiol 268: H2274–H2280, 1995. [DOI] [PubMed] [Google Scholar]
- 716.Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 717.Pandey D, Fulton DJ. Molecular regulation of NADPH oxidase 5 via the MAPK pathway. Am J Physiol Heart Circ Physiol 300: H1336–H1344, 2011. doi: 10.1152/ajpheart.01163.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.Pandey D, Gratton JP, Rafikov R, Black SM, Fulton DJ. Calcium/calmodulin-dependent kinase II mediates the phosphorylation and activation of NADPH oxidase 5. Mol Pharmacol 80: 407–415, 2011. doi: 10.1124/mol.110.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Pandey D, Patel A, Patel V, Chen F, Qian J, Wang Y, Barman SA, Venema RC, Stepp DW, Rudic RD, Fulton DJ. Expression and functional significance of NADPH oxidase 5 (Nox5) and its splice variants in human blood vessels. Am J Physiol Heart Circ Physiol 302: H1919–H1928, 2012. doi: 10.1152/ajpheart.00910.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Panieri E, Santoro MM. ROS signaling and redox biology in endothelial cells. Cell Mol Life Sci 72: 3281–3303, 2015. doi: 10.1007/s00018-015-1928-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Pannirselvam M, Simon V, Verma S, Anderson T, Triggle CR. Chronic oral supplementation with sepiapterin prevents endothelial dysfunction and oxidative stress in small mesenteric arteries from diabetic (db/db) mice. Br J Pharmacol 140: 701–706, 2003. doi: 10.1038/sj.bjp.0705476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Panza JA, García CE, Kilcoyne CM, Quyyumi AA, Cannon RO III. Impaired endothelium-dependent vasodilation in patients with essential hypertension. Evidence that nitric oxide abnormality is not localized to a single signal transduction pathway. Circulation 91: 1732–1738, 1995. doi: 10.1161/01.CIR.91.6.1732. [DOI] [PubMed] [Google Scholar]
- 723.Paravicini TM, Chrissobolis S, Drummond GR, Sobey CG. Increased NADPH-oxidase activity and Nox4 expression during chronic hypertension is associated with enhanced cerebral vasodilatation to NADPH in vivo. Stroke 35: 584–589, 2004. doi: 10.1161/01.STR.0000112974.37028.58. [DOI] [PubMed] [Google Scholar]
- 724.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol 173: 3589–3593, 2004. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 725.Patterson C, Ruef J, Madamanchi NR, Barry-Lane P, Hu Z, Horaist C, Ballinger CA, Brasier AR, Bode C, Runge MS. Stimulation of a vascular smooth muscle cell NAD(P)H oxidase by thrombin. Evidence that p47(phox) may participate in forming this oxidase in vitro and in vivo. J Biol Chem 274: 19814–19822, 1999. doi: 10.1074/jbc.274.28.19814. [DOI] [PubMed] [Google Scholar]
- 726.Pattison DI, Davies MJ. Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem Res Toxicol 14: 1453–1464, 2001. doi: 10.1021/tx0155451. [DOI] [PubMed] [Google Scholar]
- 727.Pautz A, Art J, Hahn S, Nowag S, Voss C, Kleinert H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide 23: 75–93, 2010. doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 728.Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature 409: 622–626, 2001. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- 729.Pawloski JR, Hess DT, Stamler JS. Impaired vasodilation by red blood cells in sickle cell disease. Proc Natl Acad Sci USA 102: 2531–2536, 2005. doi: 10.1073/pnas.0409876102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Peinado MA, Hernández R, Peragón J, Ovelleiro D, Pedrosa JA, Blanco S. Proteomic characterization of nitrated cell targets after hypobaric hypoxia and reoxygenation in rat brain. J Proteomics 109: 309–321, 2014. doi: 10.1016/j.jprot.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 731.Pelligrino DA, Koenig HM, Albrecht RF. Nitric oxide synthesis and regional cerebral blood flow responses to hypercapnia and hypoxia in the rat. J Cereb Blood Flow Metab 13: 80–87, 1993. doi: 10.1038/jcbfm.1993.10. [DOI] [PubMed] [Google Scholar]
- 732.Peluffo H, Shacka JJ, Ricart K, Bisig CG, Martìnez-Palma L, Pritsch O, Kamaid A, Eiserich JP, Crow JP, Barbeito L, Estèvez AG. Induction of motor neuron apoptosis by free 3-nitro-L-tyrosine. J Neurochem 89: 602–612, 2004. doi: 10.1046/j.1471-4159.2004.02363.x. [DOI] [PubMed] [Google Scholar]
- 733.Peri L, Pietraforte D, Scorza G, Napolitano A, Fogliano V, Minetti M. Apples increase nitric oxide production by human saliva at the acidic pH of the stomach: a new biological function for polyphenols with a catechol group? Free Radic Biol Med 39: 668–681, 2005. doi: 10.1016/j.freeradbiomed.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 734.Pernow J, Jung C. Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal? Cardiovasc Res 98: 334–343, 2013. doi: 10.1093/cvr/cvt036. [DOI] [PubMed] [Google Scholar]
- 735.Petrônio MS, Zeraik ML, Fonseca LM, Ximenes VF. Apocynin: chemical and biophysical properties of a NADPH oxidase inhibitor. Molecules 18: 2821–2839, 2013. doi: 10.3390/molecules18032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736.Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Görlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal 8: 1473–1484, 2006. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- 737.Petry A, Weitnauer M, Görlach A. Receptor activation of NADPH oxidases. Antioxid Redox Signal 13: 467–487, 2010. doi: 10.1089/ars.2009.3026. [DOI] [PubMed] [Google Scholar]
- 738.Pfeiffer S, Mayer B, Hemmens B. Nitric oxide: Chemical puzzles posed by a biological messenger. Angew Chem Int Ed Engl 38: 1714–1731, 1999. [DOI] [PubMed] [Google Scholar]
- 739.Piantadosi CA. Carbon monoxide, reactive oxygen signaling, and oxidative stress. Free Radic Biol Med 45: 562–569, 2008. doi: 10.1016/j.freeradbiomed.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Piazza M, Dieckmann T, Guillemette JG. Structural Studies of a Complex Between Endothelial Nitric Oxide Synthase and Calmodulin at Physiological Calcium Concentration. Biochemistry 55: 5962–5971, 2016. doi: 10.1021/acs.biochem.6b00821. [DOI] [PubMed] [Google Scholar]
- 741.Piazza M, Taiakina V, Guillemette SR, Guillemette JG, Dieckmann T. Solution structure of calmodulin bound to the target peptide of endothelial nitric oxide synthase phosphorylated at Thr495. Biochemistry 53: 1241–1249, 2014. doi: 10.1021/bi401466s. [DOI] [PubMed] [Google Scholar]
- 742.Piccoli C, Ria R, Scrima R, Cela O, D’Aprile A, Boffoli D, Falzetti F, Tabilio A, Capitanio N. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. Novel evidence of the occurrence of NAD(P)H oxidase activity. J Biol Chem 280: 26467–26476, 2005. doi: 10.1074/jbc.M500047200. [DOI] [PubMed] [Google Scholar]
- 743.Pierce GL, Jablonski KL, Walker AE, Seibert SM, DeVan AE, Black SM, Sharma S, Seals DR. Tetrahydrobiopterin supplementation enhances carotid artery compliance in healthy older men: a pilot study. Am J Hypertens 25: 1050–1054, 2012. doi: 10.1038/ajh.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Pieske B, Maggioni AP, Lam CSP, Pieske-Kraigher E, Filippatos G, Butler J, Ponikowski P, Shah SJ, Solomon SD, Scalise AV, Mueller K, Roessig L, Gheorghiade M. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J 38: 1119–1127, 2017. doi: 10.1093/eurheartj/ehw593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 745.Pinder AG, Pittaway E, Morris K, James PE. Nitrite directly vasodilates hypoxic vasculature via nitric oxide-dependent and -independent pathways. Br J Pharmacol 157: 1523–1530, 2009. doi: 10.1111/j.1476-5381.2009.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Pollock JS, Förstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci USA 88: 10480–10484, 1991. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 747.Porkert M, Sher S, Reddy U, Cheema F, Niessner C, Kolm P, Jones DP, Hooper C, Taylor WR, Harrison D, Quyyumi AA. Tetrahydrobiopterin: a novel antihypertensive therapy. J Hum Hypertens 22: 401–407, 2008. doi: 10.1038/sj.jhh.1002329. [DOI] [PubMed] [Google Scholar]
- 748.Poss WB, Huecksteadt TP, Panus PC, Freeman BA, Hoidal JR. Regulation of xanthine dehydrogenase and xanthine oxidase activity by hypoxia. Am J Physiol 270: L941–L946, 1996. [DOI] [PubMed] [Google Scholar]
- 749.Pou S, Keaton L, Surichamorn W, Rosen GM. Mechanism of superoxide generation by neuronal nitric-oxide synthase. J Biol Chem 274: 9573–9580, 1999. doi: 10.1074/jbc.274.14.9573. [DOI] [PubMed] [Google Scholar]
- 750.Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem 267: 24173–24176, 1992. [PubMed] [Google Scholar]
- 751.Poulos TL. Soluble guanylate cyclase. Curr Opin Struct Biol 16: 736–743, 2006. doi: 10.1016/j.sbi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 752.Poulos TL, Li H. Nitric oxide synthase and structure-based inhibitor design. Nitric Oxide 63: 68–77, 2017. doi: 10.1016/j.niox.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 753.Presta A, Siddhanta U, Wu C, Sennequier N, Huang L, Abu-Soud HM, Erzurum S, Stuehr DJ. Comparative functioning of dihydro- and tetrahydropterins in supporting electron transfer, catalysis, and subunit dimerization in inducible nitric oxide synthase. Biochemistry 37: 298–310, 1998. doi: 10.1021/bi971944c. [DOI] [PubMed] [Google Scholar]
- 755.Prütz WA. Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates. Arch Biochem Biophys 332: 110–120, 1996. doi: 10.1006/abbi.1996.0322. [DOI] [PubMed] [Google Scholar]
- 756.Pryde DC, Dalvie D, Hu Q, Jones P, Obach RS, Tran TD. Aldehyde oxidase: an enzyme of emerging importance in drug discovery. J Med Chem 53: 8441–8460, 2010. doi: 10.1021/jm100888d. [DOI] [PubMed] [Google Scholar]
- 757.Pudlo M, Demougeot C, Girard-Thernier C. Arginase Inhibitors: A Rational Approach Over One Century. Med Res Rev 37: 475–513, 2017. doi: 10.1002/med.21419. [DOI] [PubMed] [Google Scholar]
- 758.Puntarulo S, Cederbaum AI. Production of reactive oxygen species by microsomes enriched in specific human cytochrome P450 enzymes. Free Radic Biol Med 24: 1324–1330, 1998. doi: 10.1016/S0891-5849(97)00463-2. [DOI] [PubMed] [Google Scholar]
- 759.Puppo A, Halliwell B. Formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Is haemoglobin a biological Fenton reagent? Biochem J 249: 185–190, 1988. doi: 10.1042/bj2490185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760.Purohit R, Fritz BG, The J, Issaian A, Weichsel A, David CL, Campbell E, Hausrath AC, Rassouli-Taylor L, Garcin ED, Gage MJ, Montfort WR. YC-1 binding to the β subunit of soluble guanylyl cyclase overcomes allosteric inhibition by the α subunit. Biochemistry 53: 101–114, 2014. doi: 10.1021/bi4015133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 761.Qin L, Liu X, Sun Q, Fan Z, Xia D, Ding G, Ong HL, Adams D, Gahl WA, Zheng C, Qi S, Jin L, Zhang C, Gu L, He J, Deng D, Ambudkar IS, Wang S. Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc Natl Acad Sci USA 109: 13434–13439, 2012. doi: 10.1073/pnas.1116633109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Quesada IM, Lucero A, Amaya C, Meijles DN, Cifuentes ME, Pagano PJ, Castro C. Selective inactivation of NADPH oxidase 2 causes regression of vascularization and the size and stability of atherosclerotic plaques. Atherosclerosis 242: 469–475, 2015. doi: 10.1016/j.atherosclerosis.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Raat NJ, Noguchi AC, Liu VB, Raghavachari N, Liu D, Xu X, Shiva S, Munson PJ, Gladwin MT. Dietary nitrate and nitrite modulate blood and organ nitrite and the cellular ischemic stress response. Free Radic Biol Med 47: 510–517, 2009. doi: 10.1016/j.freeradbiomed.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764.Raat NJ, Tabima DM, Specht PA, Tejero J, Champion HC, Kim-Shapiro DB, Baust J, Mik EG, Hildesheim M, Stasch JP, Becker EM, Truebel H, Gladwin MT. Direct sGC activation bypasses NO scavenging reactions of intravascular free oxy-hemoglobin and limits vasoconstriction. Antioxid Redox Signal 19: 2232–2243, 2013. doi: 10.1089/ars.2013.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765.Radi R. Peroxynitrite, a stealthy biological oxidant. J Biol Chem 288: 26464–26472, 2013. doi: 10.1074/jbc.R113.472936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 288: 481–487, 1991. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 767.Radi R, Rodriguez M, Castro L, Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys 308: 89–95, 1994. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 768.Rafikov R, Rafikova O, Aggarwal S, Gross C, Sun X, Desai J, Fulton D, Black SM. Asymmetric dimethylarginine induces endothelial nitric-oxide synthase mitochondrial redistribution through the nitration-mediated activation of Akt1. J Biol Chem 288: 6212–6226, 2013. doi: 10.1074/jbc.M112.423269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 769.Rahaman MM, Nguyen AT, Miller MP, Hahn SA, Sparacino-Watkins C, Jobbagy S, Carew NT, Cantu-Medellin N, Wood KC, Baty CJ, Schopfer FJ, Kelley EE, Gladwin MT, Martin E, and Straub AC. Cytochrome b5 Reductase 3 Modulates Soluble Guanylate Cyclase Redox State and cGMP Signaling. Circulation Research 121: 137–148, 2017. doi: 10.1161/CIRCRESAHA.117.310705. [DOI] [PMC free article] [PubMed]
- 770.Rahaman MM, Straub AC. The emerging roles of somatic globins in cardiovascular redox biology and beyond. Redox Biol 1: 405–410, 2013. doi: 10.1016/j.redox.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 771.Ramuschkat M, Appelbaum S, Atzler D, Zeller T, Bauer C, Ojeda FM, Sinning CR, Hoffmann B, Lackner KJ, Böger RH, Wild PS, Münzel T, Blankenberg S, Schwedhelm E, Schnabel RB; Gutenberg Health Study Investigators . ADMA, subclinical changes and atrial fibrillation in the general population. Int J Cardiol 203: 640–646, 2016. doi: 10.1016/j.ijcard.2015.05.102. [DOI] [PubMed] [Google Scholar]
- 772.Ranayhossaini DJ, Rodriguez AI, Sahoo S, Chen BB, Mallampalli RK, Kelley EE, Csanyi G, Gladwin MT, Romero G, Pagano PJ. Selective recapitulation of conserved and nonconserved regions of putative NOXA1 protein activation domain confers isoform-specific inhibition of Nox1 oxidase and attenuation of endothelial cell migration. J Biol Chem 288: 36437–36450, 2013. doi: 10.1074/jbc.M113.521344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773.Rasmussen JT, Rasmussen MS, Petersen TE. Cysteines involved in the interconversion between dehydrogenase and oxidase forms of bovine xanthine oxidoreductase. J Dairy Sci 83: 499–506, 2000. doi: 10.3168/jds.S0022-0302(00)74909-5. [DOI] [PubMed] [Google Scholar]
- 774.Rassaf T, Flögel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ Res 100: 1749–1754, 2007. doi: 10.1161/CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- 775.Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res 114: 1601–1610, 2014. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- 776.Rathore R, Zheng Y-M, Niu C-F, Liu Q-H, Korde A, Ho Y-S, Wang Y-X. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 45: 1223–1231, 2008. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 777.Ravi K, Brennan LA, Levic S, Ross PA, Black SM. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci USA 101: 2619–2624, 2004. doi: 10.1073/pnas.0300464101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 778.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24: 981–990, 2012. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 779.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol 31: 1368–1376, 2011. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- 780.Reeder BJ, Ukeri J. Strong modulation of nitrite reductase activity of cytoglobin by disulfide bond oxidation: Implications for nitric oxide homeostasis. Nitric Oxide 72: 16–23, 2018. doi: 10.1016/j.niox.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 781.Reule CA, Goyvaerts B, Schoen C. Effects of an L-arginine-based multi ingredient product on endothelial function in subjects with mild to moderate hypertension and hyperhomocysteinemia - a randomized, double-blind, placebo-controlled, cross-over trial. BMC Complement Altern Med 17: 92, 2017. doi: 10.1186/s12906-017-1603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ Res 89: 408–414, 2001. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 783.Rezende F, Prior KK, Löwe O, Wittig I, Strecker V, Moll F, Helfinger V, Schnütgen F, Kurrle N, Wempe F, Walter M, Zukunft S, Luck B, Fleming I, Weissmann N, Brandes RP, Schröder K. Cytochrome P450 enzymes but not NADPH oxidases are the source of the NADPH-dependent lucigenin chemiluminescence in membrane assays. Free Radic Biol Med 102: 57–66, 2017. doi: 10.1016/j.freeradbiomed.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 784.Risbano MG, Gladwin MT. Therapeutics targeting of dysregulated redox equilibrium and endothelial dysfunction. Handb Exp Pharmacol 218: 315–349, 2013. doi: 10.1007/978-3-662-45805-1_13. [DOI] [PubMed] [Google Scholar]
- 785.Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106: 8665–8670, 2009. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786.Ritchie RH, Drummond GR, Sobey CG, De Silva TM, Kemp-Harper BK. The opposing roles of NO and oxidative stress in cardiovascular disease. Pharmacol Res 116: 57–69, 2017. doi: 10.1016/j.phrs.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 787.Ritter D, Taylor JF, Hoffmann JW, Carnaghi L, Giddings SJ, Zakeri H, Kwok PY. Alternative splicing for the alpha1 subunit of soluble guanylate cyclase. Biochem J 346: 811–816, 2000. doi: 10.1042/bj3460811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 788.Robbins IM, Hemnes AR, Gibbs JS, Christman BW, Howard L, Meehan S, Cabrita I, Gonzalez R, Oyler T, Zhao L, Du RH, Mendes LA, Wilkins MR. Safety of sapropterin dihydrochloride (6r-bh4) in patients with pulmonary hypertension. Exp Lung Res 37: 26–34, 2011. doi: 10.3109/01902148.2010.512972. [DOI] [PubMed] [Google Scholar]
- 789.Rocha BS, Nunes C, Pereira C, Barbosa RM, Laranjinha J. A shortcut to wide-ranging biological actions of dietary polyphenols: modulation of the nitrate-nitrite-nitric oxide pathway in the gut. Food Funct 5: 1646–1652, 2014. doi: 10.1039/C4FO00124A. [DOI] [PubMed] [Google Scholar]
- 790.Rochette L, Lorin J, Zeller M, Guilland JC, Lorgis L, Cottin Y, Vergely C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets? Pharmacol Ther 140: 239–257, 2013. doi: 10.1016/j.pharmthera.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 791.Rodriguez-Mateos A, Hezel M, Aydin H, Kelm M, Lundberg JO, Weitzberg E, Spencer JP, Heiss C. Interactions between cocoa flavanols and inorganic nitrate: additive effects on endothelial function at achievable dietary amounts. Free Radic Biol Med 80: 121–128, 2015. doi: 10.1016/j.freeradbiomed.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 792.Rodríguez AI, Csányi G, Ranayhossaini DJ, Feck DM, Blose KJ, Assatourian L, Vorp DA, Pagano PJ. MEF2B-Nox1 signaling is critical for stretch-induced phenotypic modulation of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 35: 430–438, 2015. doi: 10.1161/ATVBAHA.114.304936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 793.Rodriguez AI, Gangopadhyay A, Kelley EE, Pagano PJ, Zuckerbraun BS, Bauer PM. HO-1 and CO decrease platelet-derived growth factor-induced vascular smooth muscle cell migration via inhibition of Nox1. Arterioscler Thromb Vasc Biol 30: 98–104, 2010. doi: 10.1161/ATVBAHA.109.197822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794.Rong Z, Wilson MT, Cooper CE. A model for the nitric oxide producing nitrite reductase activity of hemoglobin as a function of oxygen saturation. Nitric Oxide 33: 74–80, 2013. doi: 10.1016/j.niox.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 795.Rose JJ, Wang L, Xu Q, McTiernan CF, Shiva S, Tejero J, Gladwin MT. Carbon Monoxide Poisoning: Pathogenesis, Management, and Future Directions of Therapy. Am J Respir Crit Care Med 195: 596–606, 2017. doi: 10.1164/rccm.201606-1275CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796.Rosenkranz-Weiss P, Sessa WC, Milstien S, Kaufman S, Watson CA, Pober JS. Regulation of nitric oxide synthesis by proinflammatory cytokines in human umbilical vein endothelial cells. Elevations in tetrahydrobiopterin levels enhance endothelial nitric oxide synthase specific activity. J Clin Invest 93: 2236–2243, 1994. doi: 10.1172/JCI117221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 797.Rossitch E Jr, Alexander E III, Black PM, Cooke JP. L-arginine normalizes endothelial function in cerebral vessels from hypercholesterolemic rabbits. J Clin Invest 87: 1295–1299, 1991. doi: 10.1172/JCI115132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 798.Rouquette M, Page S, Bryant R, Benboubetra M, Stevens CR, Blake DR, Whish WD, Harrison R, Tosh D. Xanthine oxidoreductase is asymmetrically localised on the outer surface of human endothelial and epithelial cells in culture. FEBS Lett 426: 397–401, 1998. doi: 10.1016/S0014-5793(98)00385-8. [DOI] [PubMed] [Google Scholar]
- 799.Roy B, Mo E, Vernon J, Garthwaite J. Probing the presence of the ligand-binding haem in cellular nitric oxide receptors. Br J Pharmacol 153: 1495–1504, 2008. doi: 10.1038/sj.bjp.0707687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 800.Rudolph TK, Rudolph V, Edreira MM, Cole MP, Bonacci G, Schopfer FJ, Woodcock SR, Franek A, Pekarova M, Khoo NKH, Hasty AH, Baldus S, Freeman BA. Nitro-fatty acids reduce atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 30: 938–945, 2010. doi: 10.1161/ATVBAHA.109.201582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Rudolph V, Rudolph TK, Schopfer FJ, Bonacci G, Woodcock SR, Cole MP, Baker PRS, Ramani R, Freeman BA. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovasc Res 85: 155–166, 2010. doi: 10.1093/cvr/cvp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 802.Rueckschloss U, Quinn MT, Holtz J, Morawietz H. Dose-dependent regulation of NAD(P)H oxidase expression by angiotensin II in human endothelial cells: protective effect of angiotensin II type 1 receptor blockade in patients with coronary artery disease. Arterioscler Thromb Vasc Biol 22: 1845–1851, 2002. doi: 10.1161/01.ATV.0000035392.38687.65. [DOI] [PubMed] [Google Scholar]
- 803.Ryan MG, Balendran A, Harrison R, Wolstenholme A, Bulkley GB. Xanthine oxidoreductase: dehydrogenase to oxidase conversion. Biochem Soc Trans 25: 530S, 1997. doi: 10.1042/bst025530s. [DOI] [PubMed] [Google Scholar]
- 804.Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, Lim HK, Sohi J, Santhanam L, Soucy K, Tuday E, Baraban E, Ilies M, Gerstenblith G, Nyhan D, Shoukas A, Christianson DW, Alp NJ, Champion HC, Huso D, Berkowitz DE. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res 102: 923–932, 2008. doi: 10.1161/CIRCRESAHA.107.169573. [DOI] [PubMed] [Google Scholar]
- 805.Ryter SW, Alam J, Choi AMK. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86: 583–650, 2006. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 806.Ryter SW, Morse D, Choi AMK. Carbon monoxide and bilirubin: potential therapies for pulmonary/vascular injury and disease. Am J Respir Cell Mol Biol 36: 175–182, 2007. doi: 10.1165/rcmb.2006-0333TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem 234-235: 249–263, 2002. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 808.Saha A, Goldstein S, Cabelli D, Czapski G. Determination of optimal conditions for synthesis of peroxynitrite by mixing acidified hydrogen peroxide with nitrite. Free Radic Biol Med 24: 653–659, 1998. doi: 10.1016/S0891-5849(97)00365-1. [DOI] [PubMed] [Google Scholar]
- 809.Saha S, Chakraborty PK, Xiong X, Dwivedi SK, Mustafi SB, Leigh NR, Ramchandran R, Mukherjee P, Bhattacharya R. Cystathionine β-synthase regulates endothelial function via protein S-sulfhydration. FASEB J 30: 441–456, 2016. doi: 10.1096/fj.15-278648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 810.Sakellariou GK, Pearson T, Lightfoot AP, Nye GA, Wells N, Giakoumaki II, Vasilaki A, Griffiths RD, Jackson MJ, McArdle A. Mitochondrial ROS regulate oxidative damage and mitophagy but not age-related muscle fiber atrophy. Sci Rep 6: 33944, 2016. doi: 10.1038/srep33944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 811.Salerno JC. Neuronal nitric oxide synthase: prototype for pulsed enzymology. FEBS Lett 582: 1395–1399, 2008. doi: 10.1016/j.febslet.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 812.Salerno JC, Ghosh DK. Space, time and nitric oxide–neuronal nitric oxide synthase generates signal pulses. FEBS J 276: 6677–6688, 2009. doi: 10.1111/j.1742-4658.2009.07382.x. [DOI] [PubMed] [Google Scholar]
- 813.Salgado MT, Cao Z, Nagababu E, Mohanty JG, Rifkind JM. Red blood cell membrane-facilitated release of nitrite-derived nitric oxide bioactivity. Biochemistry 54: 6712–6723, 2015. doi: 10.1021/acs.biochem.5b00643. [DOI] [PubMed] [Google Scholar]
- 814.Sam F, Kerstetter DL, Pimental DR, Mulukutla S, Tabaee A, Bristow MR, Colucci WS, Sawyer DB. Increased reactive oxygen species production and functional alterations in antioxidant enzymes in human failing myocardium. J Card Fail 11: 473–480, 2005. doi: 10.1016/j.cardfail.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 815.Sánchez-Gómez FJ, Calvo E, Bretón-Romero R, Fierro-Fernández M, Anilkumar N, Shah AM, Schröder K, Brandes RP, Vázquez J, Lamas S. NOX4-dependent Hydrogen peroxide promotes shear stress-induced SHP2 sulfenylation and eNOS activation. Free Radic Biol Med 89: 419–430, 2015. doi: 10.1016/j.freeradbiomed.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 816.Santolini J, Meade AL, Stuehr DJ. Differences in three kinetic parameters underpin the unique catalytic profiles of nitric-oxide synthases I, II, and III. J Biol Chem 276: 48887–48898, 2001. doi: 10.1074/jbc.M108666200. [DOI] [PubMed] [Google Scholar]
- 817.Santos CXC, Tanaka LY, Wosniak J Jr, Laurindo FRM. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal 11: 2409–2427, 2009. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 818.Sarkar A, Dai Y, Haque MM, Seeger F, Ghosh A, Garcin ED, Montfort WR, Hazen SL, Misra S, Stuehr DJ. Heat Shock Protein 90 Associates with the Per-Arnt-Sim Domain of Heme-free Soluble Guanylate Cyclase: Implications for Enzyme Maturation. J Biol Chem 290: 21615–21628, 2015. doi: 10.1074/jbc.M115.645515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 819.Sase K, Michel T. Expression of constitutive endothelial nitric oxide synthase in human blood platelets. Life Sci 57: 2049–2055, 1995. doi: 10.1016/0024-3205(95)02191-K. [DOI] [PubMed] [Google Scholar]
- 820.Sato N, Takasaka N, Yoshida M, Tsubouchi K, Minagawa S, Araya J, Saito N, Fujita Y, Kurita Y, Kobayashi K, Ito S, Hara H, Kadota T, Yanagisawa H, Hashimoto M, Utsumi H, Wakui H, Kojima J, Numata T, Kaneko Y, Odaka M, Morikawa T, Nakayama K, Kohrogi H, Kuwano K. Metformin attenuates lung fibrosis development via NOX4 suppression. Respir Res 17: 107, 2016. doi: 10.1186/s12931-016-0420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 821.Sato T, Ashizawa N, Matsumoto K, Iwanaga T, Nakamura H, Inoue T, Nagata O. Discovery of 3-(2-cyano-4-pyridyl)-5-(4-pyridyl)-1,2,4-triazole, FYX-051 - a xanthine oxidoreductase inhibitor for the treatment of hyperuricemia. Corrigendum at: doi: 10.1016/j.bmcl.2010.02.026. Bioorg Med Chem Lett 19: 6225–6229, 2009. doi:. [DOI] [PubMed] [Google Scholar]
- 822.Sayed N, Baskaran P, Ma X, van den Akker F, Beuve A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc Natl Acad Sci USA 104: 12312–12317, 2007. doi: 10.1073/pnas.0703944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 823.Sayed N, Kim DD, Fioramonti X, Iwahashi T, Durán WN, Beuve A. Nitroglycerin-induced S-nitrosylation and desensitization of soluble guanylyl cyclase contribute to nitrate tolerance. Circ Res 103: 606–614, 2008. doi: 10.1161/CIRCRESAHA.108.175133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 824.Schewe T, Steffen Y, Sies H. How do dietary flavanols improve vascular function? A position paper. Arch Biochem Biophys 476: 102–106, 2008. doi: 10.1016/j.abb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 825.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 24: R453–R462, 2014. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 826.Schildknecht S, Weber A, Gerding HR, Pape R, Robotta M, Drescher M, Marquardt A, Daiber A, Ferger B, Leist M. The NOX1/4 inhibitor GKT136901 as selective and direct scavenger of peroxynitrite. Curr Med Chem 21: 365–376, 2014. doi: 10.2174/09298673113209990179. [DOI] [PubMed] [Google Scholar]
- 827.Schindler U, Strobel H, Schönafinger K, Linz W, Löhn M, Martorana PA, Rütten H, Schindler PW, Busch AE, Sohn M, Töpfer A, Pistorius A, Jannek C, Mülsch A. Biochemistry and pharmacology of novel anthranilic acid derivatives activating heme-oxidized soluble guanylyl cyclase. Mol Pharmacol 69: 1260–1268, 2006. doi: 10.1124/mol.105.018747. [DOI] [PubMed] [Google Scholar]
- 828.Schmidt TS, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 113: 47–63, 2007. doi: 10.1042/CS20070108. [DOI] [PubMed] [Google Scholar]
- 829.Schnabel R, Blankenberg S, Lubos E, Lackner KJ, Rupprecht HJ, Espinola-Klein C, Jachmann N, Post F, Peetz D, Bickel C, Cambien F, Tiret L, Münzel T. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ Res 97: e53–e59, 2005. doi: 10.1161/01.RES.0000181286.44222.61. [DOI] [PubMed] [Google Scholar]
- 830.Schopfer FJ, Cipollina C, Freeman BA. Formation and signaling actions of electrophilic lipids. Chem Rev 111: 5997–6021, 2011. doi: 10.1021/cr200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 831.Schröder H, Leitman DC, Bennett BM, Waldman SA, Murad F. Glyceryl trinitrate-induced desensitization of guanylate cyclase in cultured rat lung fibroblasts. J Pharmacol Exp Ther 245: 413–418, 1988. [PubMed] [Google Scholar]
- 832.Schröder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 110: 1217–1225, 2012. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 833.Schröder K, Vecchione C, Jung O, Schreiber JG, Shiri-Sverdlov R, van Gorp PJ, Busse R, Brandes RP. Xanthine oxidase inhibitor tungsten prevents the development of atherosclerosis in ApoE knockout mice fed a Western-type diet. Free Radic Biol Med 41: 1353–1360, 2006. doi: 10.1016/j.freeradbiomed.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 834.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA 103: 1024–1029, 2006. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 835.Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, Ernst KV, Kelemen MD, Townsend SN, Capriotti A, Hare JM, Gerstenblith G. L-arginine therapy in acute myocardial infarction: the Vascular Interaction With Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. JAMA 295: 58–64, 2006. doi: 10.1001/jama.295.1.58. [DOI] [PubMed] [Google Scholar]
- 836.Schulz E, Wenzel P, Münzel T, Daiber A. Mitochondrial redox signaling: Interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid Redox Signal 20: 308–324, 2014. doi: 10.1089/ars.2012.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837.Schumacher HR Jr, Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J, Lademacher C, Joseph-Ridge N. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum 59: 1540–1548, 2008. doi: 10.1002/art.24209. [DOI] [PubMed] [Google Scholar]
- 838.Schürmann C, Rezende F, Kruse C, Yasar Y, Löwe O, Fork C, van de Sluis B, Bremer R, Weissmann N, Shah AM, Jo H, Brandes RP, Schröder K. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur Heart J 36: 3447–3456, 2015. doi: 10.1093/eurheartj/ehv460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 839.Schwarz G. Molybdenum cofactor and human disease. Curr Opin Chem Biol 31: 179–187, 2016. doi: 10.1016/j.cbpa.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 840.Schwarz G, Mendel RR, Ribbe MW. Molybdenum cofactors, enzymes and pathways. Nature 460: 839–847, 2009. doi: 10.1038/nature08302. [DOI] [PubMed] [Google Scholar]
- 841.Schwarz PM, Kleinert H, Förstermann U. Potential functional significance of brain-type and muscle-type nitric oxide synthase I expressed in adventitia and media of rat aorta. Arterioscler Thromb Vasc Biol 19: 2584–2590, 1999. doi: 10.1161/01.ATV.19.11.2584. [DOI] [PubMed] [Google Scholar]
- 842.Schwedhelm E, Maas R, Freese R, Jung D, Lukacs Z, Jambrecina A, Spickler W, Schulze F, Böger RH. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol 65: 51–59, 2008. doi: 10.1111/j.1365-2125.2007.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 843.Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P, Shah AM. Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation 119: 2656–2662, 2009. doi: 10.1161/CIRCULATIONAHA.108.822205. [DOI] [PubMed] [Google Scholar]
- 844.Seddon MD, Chowienczyk PJ, Brett SE, Casadei B, Shah AM. Neuronal nitric oxide synthase regulates basal microvascular tone in humans in vivo. Circulation 117: 1991–1996, 2008. doi: 10.1161/CIRCULATIONAHA.107.744540. [DOI] [PubMed] [Google Scholar]
- 845.Segal AW, Jones OTG. Novel cytochrome b system in phagocytic vacuoles of human granulocytes. Nature 276: 515–517, 1978. doi: 10.1038/276515a0. [DOI] [PubMed] [Google Scholar]
- 846.Selemidis S, Dusting GJ, Peshavariya H, Kemp-Harper BK, Drummond GR. Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovasc Res 75: 349–358, 2007. doi: 10.1016/j.cardiores.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 847.Seredenina T, Chiriano G, Filippova A, Nayernia Z, Mahiout Z, Fioraso-Cartier L, Plastre O, Scapozza L, Krause KH, Jaquet V. A subset of N-substituted phenothiazines inhibits NADPH oxidases. Free Radic Biol Med 86: 239–249, 2015. doi: 10.1016/j.freeradbiomed.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 848.Serrander L, Jaquet V, Bedard K, Plastre O, Hartley O, Arnaudeau S, Demaurex N, Schlegel W, Krause K-H. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie 89: 1159–1167, 2007. doi: 10.1016/j.biochi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 849.Setoguchi S, Hirooka Y, Eshima K, Shimokawa H, Takeshita A. Tetrahydrobiopterin improves impaired endothelium-dependent forearm vasodilation in patients with heart failure. J Cardiovasc Pharmacol 39: 363–368, 2002. doi: 10.1097/00005344-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 850.Setoguchi S, Mohri M, Shimokawa H, Takeshita A. Tetrahydrobiopterin improves endothelial dysfunction in coronary microcirculation in patients without epicardial coronary artery disease. J Am Coll Cardiol 38: 493–498, 2001. doi: 10.1016/S0735-1097(01)01382-1. [DOI] [PubMed] [Google Scholar]
- 851.Sevanian A, Nordenbrand K, Kim E, Ernster L, Hochstein P. Microsomal lipid peroxidation: the role of NADPH–cytochrome P450 reductase and cytochrome P450. Free Radic Biol Med 8: 145–152, 1990. doi: 10.1016/0891-5849(90)90087-Y. [DOI] [PubMed] [Google Scholar]
- 852.Sharina IG, Jelen F, Bogatenkova EP, Thomas A, Martin E, Murad F. Alpha1 soluble guanylyl cyclase (sGC) splice forms as potential regulators of human sGC activity. J Biol Chem 283: 15104–15113, 2008. doi: 10.1074/jbc.M710269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 853.Sharina IG, Martin E. The Role of Reactive Oxygen and Nitrogen Species in the Expression and Splicing of Nitric Oxide Receptor. Antioxid Redox Signal 26: 122–136, 2017. doi: 10.1089/ars.2016.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 854.Sheehan AL, Carrell S, Johnson B, Stanic B, Banfi B, Miller FJ Jr. Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis 216: 321–326, 2011. doi: 10.1016/j.atherosclerosis.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 855.Shemyakin A, Kövamees O, Rafnsson A, Böhm F, Svenarud P, Settergren M, Jung C, Pernow J. Arginase inhibition improves endothelial function in patients with coronary artery disease and type 2 diabetes mellitus. Circulation 126: 2943–2950, 2012. doi: 10.1161/CIRCULATIONAHA.112.140335. [DOI] [PubMed] [Google Scholar]
- 856.Shin HK, Kim YK, Kim KY, Lee JH, Hong KW. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: prevention by cilostazol. Circulation 109: 1022–1028, 2004. doi: 10.1161/01.CIR.0000117403.64398.53. [DOI] [PubMed] [Google Scholar]
- 857.Shin S, Thapa SK, Fung HL. Cellular interactions between L-arginine and asymmetric dimethylarginine: Transport and metabolism. PLoS One 12: e0178710, 2017. doi: 10.1371/journal.pone.0178710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 858.Shinozaki K, Kashiwagi A, Nishio Y, Okamura T, Yoshida Y, Masada M, Toda N, Kikkawa R. Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2- imbalance in insulin-resistant rat aorta. Diabetes 48: 2437–2445, 1999. doi: 10.2337/diabetes.48.12.2437. [DOI] [PubMed] [Google Scholar]
- 859.Shirakura T, Nomura J, Matsui C, Kobayashi T, Tamura M, Masuzaki H. Febuxostat, a novel xanthine oxidoreductase inhibitor, improves hypertension and endothelial dysfunction in spontaneously hypertensive rats. Naunyn Schmiedebergs Arch Pharmacol 389: 831–838, 2016. doi: 10.1007/s00210-016-1239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 860.Shirodaria C, Antoniades C, Lee J, Jackson CE, Robson MD, Francis JM, Moat SJ, Ratnatunga C, Pillai R, Refsum H, Neubauer S, Channon KM. Global improvement of vascular function and redox state with low-dose folic acid: implications for folate therapy in patients with coronary artery disease. Circulation 115: 2262–2270, 2007. doi: 10.1161/CIRCULATIONAHA.106.679084. [DOI] [PubMed] [Google Scholar]
- 861.Shiva S, Gladwin MT. Nitrite mediates cytoprotection after ischemia/reperfusion by modulating mitochondrial function. Basic Res Cardiol 104: 113–119, 2009. doi: 10.1007/s00395-009-0009-3. [DOI] [PubMed] [Google Scholar]
- 862.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res 100: 654–661, 2007. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 863.Shiva S, Oh JY, Landar AL, Ulasova E, Venkatraman A, Bailey SM, Darley-Usmar VM. Nitroxia: the pathological consequence of dysfunction in the nitric oxide-cytochrome c oxidase signaling pathway. Free Radic Biol Med 38: 297–306, 2005. doi: 10.1016/j.freeradbiomed.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 864.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med 204: 2089–2102, 2007. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 865.Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol 2: 486–493, 2006. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 866.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol 67: 99–145, 2005. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 867.Singh K, Balligand JL, Fischer TA, Smith TW, Kelly RA. Glucocorticoids increase osteopontin expression in cardiac myocytes and microvascular endothelial cells. Role in regulation of inducible nitric oxide synthase. J Biol Chem 270: 28471–28478, 1995. doi: 10.1074/jbc.270.47.28471. [DOI] [PubMed] [Google Scholar]
- 868.Singh S, Madzelan P, Banerjee R. Properties of an unusual heme cofactor in PLP-dependent cystathionine beta-synthase. Nat Prod Rep 24: 631–639, 2007. doi: 10.1039/B604182P. [DOI] [PubMed] [Google Scholar]
- 869.Siragusa M, Fisslthaler B. Insulin Keeps PYK-ing on eNOS: Enhanced Insulin Receptor Signaling Induces Endothelial Dysfunction. Circ Res 120: 748–750, 2017. doi: 10.1161/CIRCRESAHA.117.310576. [DOI] [PubMed] [Google Scholar]
- 870.Siragusa M, Fleming I. The eNOS signalosome and its link to endothelial dysfunction. Pflugers Arch 468: 1125–1137, 2016. doi: 10.1007/s00424-016-1839-0. [DOI] [PubMed] [Google Scholar]
- 871.Sirokmány G, Donkó Á, Geiszt M. Nox/Duox Family of NADPH Oxidases: Lessons from Knockout Mouse Models. Trends Pharmacol Sci 37: 318–327, 2016. doi: 10.1016/j.tips.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 872.Smith BC, Marletta MA. Mechanisms of S-nitrosothiol formation and selectivity in nitric oxide signaling. Curr Opin Chem Biol 16: 498–506, 2012. doi: 10.1016/j.cbpa.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 873.Smith RA, Hartley RC, Murphy MP. Mitochondria-targeted small molecule therapeutics and probes. Antioxid Redox Signal 15: 3021–3038, 2011. doi: 10.1089/ars.2011.3969. [DOI] [PubMed] [Google Scholar]
- 874.Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, Smith RA, Murphy MP, Taylor KM; Protect Study Group . A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov Disord 25: 1670–1674, 2010. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- 875.Sobey CG, Judkins CP, Rivera J, Lewis CV, Diep H, Lee HW, Kemp-Harper BK, Broughton BR, Selemidis S, Gaspari TA, Samuel CS, Drummond GR. NOX1 deficiency in apolipoprotein E-knockout mice is associated with elevated plasma lipids and enhanced atherosclerosis. Free Radic Res 49: 186–198, 2015. doi: 10.3109/10715762.2014.992893. [DOI] [PubMed] [Google Scholar]
- 876.Sorescu D, Weiss D, Lassègue B, Clempus RE, Szöcs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105: 1429–1435, 2002. doi: 10.1161/01.CIR.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 877.Souza JM, Daikhin E, Yudkoff M, Raman CS, Ischiropoulos H. Factors determining the selectivity of protein tyrosine nitration. Arch Biochem Biophys 371: 169–178, 1999. doi: 10.1006/abbi.1999.1480. [DOI] [PubMed] [Google Scholar]
- 878.Sowa G, Pypaert M, Sessa WC. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc Natl Acad Sci USA 98: 14072–14077, 2001. doi: 10.1073/pnas.241409998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 879.Spallarossa P, Altieri P, Garibaldi S, Ghigliotti G, Barisione C, Manca V, Fabbi P, Ballestrero A, Brunelli C, Barsotti A. Matrix metalloproteinase-2 and -9 are induced differently by doxorubicin in H9c2 cells: The role of MAP kinases and NAD(P)H oxidase. Cardiovasc Res 69: 736–745, 2006. doi: 10.1016/j.cardiores.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 880.Sparacino-Watkins CE, Tejero J, Sun B, Gauthier MC, Thomas J, Ragireddy V, Merchant BA, Wang J, Azarov I, Basu P, Gladwin MT. Nitrite reductase and nitric-oxide synthase activity of the mitochondrial molybdopterin enzymes mARC1 and mARC2. J Biol Chem 289: 10345–10358, 2014. doi: 10.1074/jbc.M114.555177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 881.Spickett CM, Jerlich A, Panasenko OM, Arnhold J, Pitt AR, Stelmaszyńska T, Schaur RJ. The reactions of hypochlorous acid, the reactive oxygen species produced by myeloperoxidase, with lipids. Acta Biochim Pol 47: 889–899, 2000. [PubMed] [Google Scholar]
- 882.Sprague RS, Stephenson AH, Ellsworth ML. Red not dead: signaling in and from erythrocytes. Trends Endocrinol Metab 18: 350–355, 2007. doi: 10.1016/j.tem.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 883.Srihirun S, Sriwantana T, Unchern S, Kittikool D, Noulsri E, Pattanapanyasat K, Fucharoen S, Piknova B, Schechter AN, Sibmooh N. Platelet inhibition by nitrite is dependent on erythrocytes and deoxygenation. PLoS One 7: e30380, 2012. doi: 10.1371/journal.pone.0030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 884.St Hilaire C, Koupenova M, Carroll SH, Smith BD, Ravid K. TNF-alpha upregulates the A2B adenosine receptor gene: The role of NAD(P)H oxidase 4. Biochem Biophys Res Commun 375: 292–296, 2008. doi: 10.1016/j.bbrc.2008.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 885.Stamler JS, Jaraki O, Osborne J, Simon DI, Keaney J, Vita J, Singel D, Valeri CR, Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci USA 89: 7674–7677, 1992. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 886.Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276: 2034–2037, 1997. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 887.Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci 24: 7779–7788, 2004. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 888.Stasch J-P, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123: 2263–2273, 2011. doi: 10.1161/CIRCULATIONAHA.110.981738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 889.Stasch JP, Dembowsky K, Perzborn E, Stahl E, Schramm M. Cardiovascular actions of a novel NO-independent guanylyl cyclase stimulator, BAY 41-8543: in vivo studies. Br J Pharmacol 135: 344–355, 2002. doi: 10.1038/sj.bjp.0704483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 890.Stasch JP, Schmidt P, Alonso-Alija C, Apeler H, Dembowsky K, Haerter M, Heil M, Minuth T, Perzborn E, Pleiss U, Schramm M, Schroeder W, Schröder H, Stahl E, Steinke W, Wunder F. NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br J Pharmacol 136: 773–783, 2002. doi: 10.1038/sj.bjp.0704778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 891.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, H S AK, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Müller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Müller-Esterl W, Schmidt HH. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 116: 2552–2561, 2006. doi: 10.1172/JCI28371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 892.Stielow C, Catar RA, Muller G, Wingler K, Scheurer P, Schmidt HH, Morawietz H. Novel Nox inhibitor of oxLDL-induced reactive oxygen species formation in human endothelial cells. Biochem Biophys Res Commun 344: 200–205, 2006. doi: 10.1016/j.bbrc.2006.03.114. [DOI] [PubMed] [Google Scholar]
- 893.Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev 84: 1381–1478, 2004. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 894.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol 11: 95–102, 1994. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 895.Stone JR, Marletta MA. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry 33: 5636–5640, 1994. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 896.Stone JR, Marletta MA. Synergistic activation of soluble guanylate cyclase by YC-1 and carbon monoxide: implications for the role of cleavage of the iron-histidine bond during activation by nitric oxide. Chem Biol 5: 255–261, 1998. doi: 10.1016/S1074-5521(98)90618-4. [DOI] [PubMed] [Google Scholar]
- 897.Stover JF, Belli A, Boret H, Bulters D, Sahuquillo J, Schmutzhard E, Zavala E, Ungerstedt U, Schinzel R, Tegtmeier F; NOSTRA Investigators . Nitric oxide synthase inhibition with the antipterin VAS203 improves outcome in moderate and severe traumatic brain injury: a placebo-controlled randomized Phase IIa trial (NOSTRA). J Neurotrauma 31: 1599–1606, 2014. doi: 10.1089/neu.2014.3344. [DOI] [PubMed] [Google Scholar]
- 898.Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, Bortz PS, Best AK, Columbus L, Gaston B, Isakson BE. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature 491: 473–477, 2012. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 899.Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Lüscher T, Rabelink T. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest 99: 41–46, 1997. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 900.Stroes ES, van Faassen EE, Yo M, Martasek P, Boer P, Govers R, Rabelink TJ. Folic acid reverts dysfunction of endothelial nitric oxide synthase. Circ Res 86: 1129–1134, 2000. doi: 10.1161/01.RES.86.11.1129. [DOI] [PubMed] [Google Scholar]
- 901.Stuehr D, Pou S, Rosen GM. Oxygen reduction by nitric-oxide synthases. J Biol Chem 276: 14533–14536, 2001. doi: 10.1074/jbc.R100011200. [DOI] [PubMed] [Google Scholar]
- 902.Stuehr DJ, Abu-Soud HM, Rousseau DL, Feldman PL, Wang J. Control of electron transfer in neuronal nitric oxide synthase by calmodulin, substrate, substrate analogs, and nitric oxide. Adv Pharmacol 34: 207–213, 1995. doi: 10.1016/S1054-3589(08)61087-X. [DOI] [PubMed] [Google Scholar]
- 903.Stuehr DJ, Cho HJ, Kwon NS, Weise MF, Nathan CF. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc Natl Acad Sci USA 88: 7773–7777, 1991. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 904.Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem 279: 36167–36170, 2004. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- 905.Sturms R, DiSpirito AA, Hargrove MS. Plant and cyanobacterial hemoglobins reduce nitrite to nitric oxide under anoxic conditions. Biochemistry 50: 3873–3878, 2011. doi: 10.1021/bi2004312. [DOI] [PubMed] [Google Scholar]
- 906.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290: L661–L673, 2006. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 907.Subramani J, Kundumani-Sridharan V, Hilgers RH, Owens C, Das KC. Thioredoxin Uses a GSH-independent Route to Deglutathionylate Endothelial Nitric-oxide Synthase and Protect against Myocardial Infarction. J Biol Chem 291: 23374–23389, 2016. doi: 10.1074/jbc.M116.745034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 908.Sugamura K, Keaney JF Jr. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med 51: 978–992, 2011. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 909.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature 401: 79–82, 1999. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 910.Sukumar P, Viswambharan H, Imrie H, Cubbon RM, Yuldasheva N, Gage M, Galloway S, Skromna A, Kandavelu P, Santos CX, Gatenby VK, Smith J, Beech DJ, Wheatcroft SB, Channon KM, Shah AM, Kearney MT. Nox2 NADPH oxidase has a critical role in insulin resistance-related endothelial cell dysfunction. Diabetes 62: 2130–2134, 2013. doi: 10.2337/db12-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 911.Suliman HB, Carraway MS, Welty-Wolf KE, Whorton AR, Piantadosi CA. Lipopolysaccharide stimulates mitochondrial biogenesis via activation of nuclear respiratory factor-1. J Biol Chem 278: 41510–41518, 2003. doi: 10.1074/jbc.M304719200. [DOI] [PubMed] [Google Scholar]
- 912.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J 275: 3249–3277, 2008. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 913.Sun D, Huang A, Smith CJ, Stackpole CJ, Connetta JA, Shesely EG, Koller A, Kaley G. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ Res 85: 288–293, 1999. doi: 10.1161/01.RES.85.3.288. [DOI] [PubMed] [Google Scholar]
- 914.Sun J, Murphy E. Protein S-nitrosylation and cardioprotection. Circ Res 106: 285–296, 2010. doi: 10.1161/CIRCRESAHA.109.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 915.Sun Q-A, Hess DT, Wang B, Miyagi M, Stamler JS. Off-target thiol alkylation by the NADPH oxidase inhibitor 3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo[4,5-d]pyrimidine (VAS2870). Free Radic Biol Med 52: 1897–1902, 2012. doi: 10.1016/j.freeradbiomed.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 916.Sun X, Kumar S, Sharma S, Aggarwal S, Lu Q, Gross C, Rafikova O, Lee SG, Dasarathy S, Hou Y, Meadows ML, Han W, Su Y, Fineman JR, Black SM. Endothelin-1 induces a glycolytic switch in pulmonary arterial endothelial cells via the mitochondrial translocation of endothelial nitric oxide synthase. Am J Respir Cell Mol Biol 50: 1084–1095, 2014. doi: 10.1165/rcmb.2013-0187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 917.Sun Y, Jin K, Peel A, Mao XO, Xie L, Greenberg DA. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci USA 100: 3497–3500, 2003. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 918.Surmeli NB, Litterman NK, Miller AF, Groves JT. Peroxynitrite mediates active site tyrosine nitration in manganese superoxide dismutase. Evidence of a role for the carbonate radical anion. J Am Chem Soc 132: 17174–17185, 2010. doi: 10.1021/ja105684w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 919.Suzuki J, Kaziro Y, Koide H. Synergistic action of R-Ras and IGF-1 on Bcl-xL expression and caspase-3 inhibition in BaF3 cells: R-Ras and IGF-1 control distinct anti-apoptotic kinase pathways. FEBS Lett 437: 112–116, 1998. doi: 10.1016/S0014-5793(98)01213-7. [DOI] [PubMed] [Google Scholar]
- 920.Swenson TL, Casida JE. Aldehyde oxidase importance in vivo in xenobiotic metabolism: imidacloprid nitroreduction in mice. Toxicol Sci 133: 22–28, 2013. doi: 10.1093/toxsci/kft066. [DOI] [PubMed] [Google Scholar]
- 921.Szeto HH. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J 8: E277–E283, 2006. doi: 10.1007/BF02854898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 922.Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal 10: 601–619, 2008. doi: 10.1089/ars.2007.1892. [DOI] [PubMed] [Google Scholar]
- 923.Szeto HH. Mitochondria-targeted peptide antioxidants: novel neuroprotective agents. AAPS J 8: E521–E531, 2006. doi: 10.1208/aapsj080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 924.Taillé C, El-Benna J, Lanone S, Boczkowski J, Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem 280: 25350–25360, 2005. doi: 10.1074/jbc.M503512200. [DOI] [PubMed] [Google Scholar]
- 925.Takac I, Schröder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286: 13304–13313, 2011. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 926.Takakura K, Beckman JS, MacMillan-Crow LA, Crow JP. Rapid and irreversible inactivation of protein tyrosine phosphatases PTP1B, CD45, and LAR by peroxynitrite. Arch Biochem Biophys 369: 197–207, 1999. doi: 10.1006/abbi.1999.1374. [DOI] [PubMed] [Google Scholar]
- 927.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem 278: 25234–25246, 2003. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 928.Tamariz L, Hare JM. Xanthine oxidase inhibitors in heart failure: where do we go from here? Circulation 131: 1741–1744, 2015. doi: 10.1161/CIRCULATIONAHA.115.016379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 929.Taylor BA, Zaleski AL, Dornelas EA, Thompson PD. The impact of tetrahydrobiopterin administration on endothelial function before and after smoking cessation in chronic smokers. Hypertens Res 39: 144–150, 2016. doi: 10.1038/hr.2015.130. [DOI] [PubMed] [Google Scholar]
- 930.Taylor RM, Burritt JB, Baniulis D, Foubert TR, Lord CI, Dinauer MC, Parkos CA, Jesaitis AJ. Site-specific inhibitors of NADPH oxidase activity and structural probes of flavocytochrome b: characterization of six monoclonal antibodies to the p22phox subunit. J Immunol 173: 7349–7357, 2004. doi: 10.4049/jimmunol.173.12.7349. [DOI] [PubMed] [Google Scholar]
- 931.Tejero J, Gladwin MT. The globin superfamily: functions in nitric oxide formation and decay. Biol Chem 395: 631–639, 2014. doi: 10.1515/hsz-2013-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 932.Tejero J, Sparacino-Watkins CE, Ragireddy V, Frizzell S, Gladwin MT. Exploring the mechanisms of the reductase activity of neuroglobin by site-directed mutagenesis of the heme distal pocket. Erratum at: doi: 10.1021/asc.biochem.5b00579. Biochemistry 54: 722–733, 2015. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 933.Tejero J, Stuehr D. Tetrahydrobiopterin in nitric oxide synthase. IUBMB Life 65: 358–365, 2013. doi: 10.1002/iub.1136. [DOI] [PubMed] [Google Scholar]
- 934.ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Bäumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res 71: 331–341, 2006. doi: 10.1016/j.cardiores.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 935.Terao M, Romão MJ, Leimkühler S, Bolis M, Fratelli M, Coelho C, Santos-Silva T, Garattini E. Structure and function of mammalian aldehyde oxidases. Arch Toxicol 90: 753–780, 2016. doi: 10.1007/s00204-016-1683-1. [DOI] [PubMed] [Google Scholar]
- 936.Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem 270: 30334–30338, 1995. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 937.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028, 2000. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 938.Thomas DD, Heinecke JL, Ridnour LA, Cheng RY, Kesarwala AH, Switzer CH, McVicar DW, Roberts DD, Glynn S, Fukuto JM, Wink DA, Miranda KM. Signaling and stress: The redox landscape in NOS2 biology. Free Radic Biol Med 87: 204–225, 2015. doi: 10.1016/j.freeradbiomed.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 939.Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, Colton CA, Harris CC, Roberts DD, Wink DA. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med 45: 18–31, 2008. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 940.Thomas SR, Chen K, Keaney JF Jr. Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem 277: 6017–6024, 2002. doi: 10.1074/jbc.M109107200. [DOI] [PubMed] [Google Scholar]
- 941.Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal 10: 1713–1765, 2008. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 942.Thomson L, Trujillo M, Telleri R, Radi R. Kinetics of cytochrome c2+ oxidation by peroxynitrite: implications for superoxide measurements in nitric oxide-producing biological systems. Arch Biochem Biophys 319: 491–497, 1995. doi: 10.1006/abbi.1995.1321. [DOI] [PubMed] [Google Scholar]
- 943.Thoonen R, Cauwels A, Decaluwe K, Geschka S, Tainsh RE, Delanghe J, Hochepied T, De Cauwer L, Rogge E, Voet S, Sips P, Karas RH, Bloch KD, Vuylsteke M, Stasch JP, Van de Voorde J, Buys ES, Brouckaert P. Cardiovascular and pharmacological implications of haem-deficient NO-unresponsive soluble guanylate cyclase knock-in mice. Nat Commun 6: 8482, 2015. doi: 10.1038/ncomms9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 944.Tirone F, Radu L, Craescu CT, Cox JA. Identification of the binding site for the regulatory calcium-binding domain in the catalytic domain of NOX5. Biochemistry 49: 761–771, 2010. doi: 10.1021/bi901846y. [DOI] [PubMed] [Google Scholar]
- 945.Tiso M, Tejero J, Basu S, Azarov I, Wang X, Simplaceanu V, Frizzell S, Jayaraman T, Geary L, Shapiro C, Ho C, Shiva S, Kim-Shapiro DB, Gladwin MT. Human neuroglobin functions as a redox-regulated nitrite reductase. J Biol Chem 286: 18277–18289, 2011. doi: 10.1074/jbc.M110.159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 946.Tiso M, Tejero J, Kenney C, Frizzell S, Gladwin MT. Nitrite reductase activity of nonsymbiotic hemoglobins from Arabidopsis thaliana. Biochemistry 51: 5285–5292, 2012. doi: 10.1021/bi300570v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 947.Tiso M, Tejero J, Panda K, Aulak KS, Stuehr DJ. Versatile regulation of neuronal nitric oxide synthase by specific regions of its C-terminal tail. Biochemistry 46: 14418–14428, 2007. doi: 10.1021/bi701646k. [DOI] [PubMed] [Google Scholar]
- 948.Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab 14: 537–544, 2011. doi: 10.1016/j.cmet.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 949.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension 44: 248–252, 2004. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 950.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 90: 1205–1213, 2002. doi: 10.1161/01.RES.0000020404.01971.2F. [DOI] [PubMed] [Google Scholar]
- 951.Trent JT III, Hargrove MS. A ubiquitously expressed human hexacoordinate hemoglobin. J Biol Chem 277: 19538–19545, 2002. doi: 10.1074/jbc.M201934200. [DOI] [PubMed] [Google Scholar]
- 952.Tretter L, Adam-Vizi V. Generation of reactive oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. J Neurosci 24: 7771–7778, 2004. doi: 10.1523/JNEUROSCI.1842-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 953.Tsai A-L, Berka V, Sharina I, Martin E. Dynamic ligand exchange in soluble guanylyl cyclase (sGC): implications for sGC regulation and desensitization. J Biol Chem 286: 43182–43192, 2011. doi: 10.1074/jbc.M111.290304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 954.Tsikas D, Böger RH, Sandmann J, Bode-Böger SM, Frölich JC. Endogenous nitric oxide synthase inhibitors are responsible for the L-arginine paradox. FEBS Lett 478: 1–3, 2000. doi: 10.1016/S0014-5793(00)01686-0. [DOI] [PubMed] [Google Scholar]
- 955.Turell L, Vitturi DA, Coitiño EL, Lebrato L, Möller MN, Sagasti C, Salvatore SR, Woodcock SR, Alvarez B, Schopfer FJ. The Chemical Basis of Thiol Addition to Nitro-conjugated Linoleic Acid, a Protective Cell-signaling Lipid. J Biol Chem 292: 1145–1159, 2017. doi: 10.1074/jbc.M116.756288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 956.Tyurin VA, Liu SX, Tyurina YY, Sussman NB, Hubel CA, Roberts JM, Taylor RN, Kagan VE. Elevated levels of S-nitrosoalbumin in preeclampsia plasma. Circ Res 88: 1210–1215, 2001. doi: 10.1161/hh1101.092179. [DOI] [PubMed] [Google Scholar]
- 957.Ueda S, Matsuoka H, Miyazaki H, Usui M, Okuda S, Imaizumi T. Tetrahydrobiopterin restores endothelial function in long-term smokers. J Am Coll Cardiol 35: 71–75, 2000. doi: 10.1016/S0735-1097(99)00523-9. [DOI] [PubMed] [Google Scholar]
- 958.Underbakke ES, Iavarone AT, Chalmers MJ, Pascal BD, Novick S, Griffin PR, Marletta MA. Nitric oxide-induced conformational changes in soluble guanylate cyclase. Structure 22: 602–611, 2014. doi: 10.1016/j.str.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 959.Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol 90: 1–37, 2016. doi: 10.1007/s00204-015-1579-5. [DOI] [PubMed] [Google Scholar]
- 960.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84, 2007. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 961.Valkonen VP, Päivä H, Salonen JT, Lakka TA, Lehtimäki T, Laakso J, Laaksonen R. Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet 358: 2127–2128, 2001. doi: 10.1016/S0140-6736(01)07184-7. [DOI] [PubMed] [Google Scholar]
- 962.Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol 24: 1023–1030, 2004. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- 963.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 339: 572–575, 1992. doi: 10.1016/0140-6736(92)90865-Z. [DOI] [PubMed] [Google Scholar]
- 964.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal 7: 308–317, 2005. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 965.Van der Schueren BJ, Lunnon MW, Laurijssens BE, Guillard F, Palmer J, Van Hecken A, Depré M, Vanmolkot FH, de Hoon JN. Does the unfavorable pharmacokinetic and pharmacodynamic profile of the iNOS inhibitor GW273629 lead to inefficacy in acute migraine? J Clin Pharmacol 49: 281–290, 2009. doi: 10.1177/0091270008329548. [DOI] [PubMed] [Google Scholar]
- 966.van der Zwan LP, Scheffer PG, Dekker JM, Stehouwer CD, Heine RJ, Teerlink T. Systemic inflammation is linked to low arginine and high ADMA plasma levels resulting in an unfavourable NOS substrate-to-inhibitor ratio: the Hoorn Study. Clin Sci (Lond) 121: 71–78, 2011. doi: 10.1042/CS20100595. [DOI] [PubMed] [Google Scholar]
- 967.Van Doorslaer S, Dewilde S, Kiger L, Nistor SV, Goovaerts E, Marden MC, Moens L. Nitric oxide binding properties of neuroglobin. A characterization by EPR and flash photolysis. J Biol Chem 278: 4919–4925, 2003. doi: 10.1074/jbc.M210617200. [DOI] [PubMed] [Google Scholar]
- 968.van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, Kozlov AV, Li H, Lundberg JO, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin AF, Weitzberg E, Zweier J, Gladwin MT. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev 29: 683–741, 2009. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 970.Vanin AF, Bevers LM, Slama-Schwok A, van Faassen EE. Nitric oxide synthase reduces nitrite to NO under anoxia. Cell Mol Life Sci 64: 96–103, 2007. doi: 10.1007/s00018-006-6374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 971.Varga ZV, Pipicz M, Baán JA, Baranyai T, Koncsos G, Leszek P, Kuśmierczyk M, Sánchez-Cabo F, García-Pavía P, Brenner GJ, Giricz Z, Csont T, Mendler L, Lara-Pezzi E, Pacher P, Ferdinandy P. Alternative splicing of NOX4 in the failing human heart. Front Physiol 8: 935, 2017. doi: 10.3389/fphys.2017.00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 972.Vásquez-Vivar J. Tetrahydrobiopterin, superoxide, and vascular dysfunction. Free Radic Biol Med 47: 1108–1119, 2009. doi: 10.1016/j.freeradbiomed.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 973.Vásquez-Vivar J, Kalyanaraman B, Martásek P, Hogg N, Masters BSS, Karoui H, Tordo P, Pritchard KA Jr. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA 95: 9220–9225, 1998. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 974.Vásquez-Vivar J, Martásek P, Whitsett J, Joseph J, Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J 362: 733–739, 2002. doi: 10.1042/bj3620733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 975.Velmurugan S, Kapil V, Ghosh SM, Davies S, McKnight A, Aboud Z, Khambata RS, Webb AJ, Poole A, Ahluwalia A. Antiplatelet effects of dietary nitrate in healthy volunteers: involvement of cGMP and influence of sex. Free Radic Biol Med 65: 1521–1532, 2013. doi: 10.1016/j.freeradbiomed.2013.06.031. Erratum at: doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 976.Vendrov AE, Hakim ZS, Madamanchi NR, Rojas M, Madamanchi C, Runge MS. Atherosclerosis is attenuated by limiting superoxide generation in both macrophages and vessel wall cells. Arterioscler Thromb Vasc Biol 27: 2714–2721, 2007. doi: 10.1161/ATVBAHA.107.152629. [DOI] [PubMed] [Google Scholar]
- 977.Venema VJ, Ju H, Zou R, Venema RC. Interaction of neuronal nitric-oxide synthase with caveolin-3 in skeletal muscle. Identification of a novel caveolin scaffolding/inhibitory domain. J Biol Chem 272: 28187–28190, 1997. doi: 10.1074/jbc.272.45.28187. [DOI] [PubMed] [Google Scholar]
- 978.Verhaar MC, Wever RM, Kastelein JJ, van Dam T, Koomans HA, Rabelink TJ. 5-methyltetrahydrofolate, the active form of folic acid, restores endothelial function in familial hypercholesterolemia. Circulation 97: 237–241, 1998. doi: 10.1161/01.CIR.97.3.237. [DOI] [PubMed] [Google Scholar]
- 979.Vickers S, Schiller HJ, Hildreth JE, Bulkley GB. Immunoaffinity localization of the enzyme xanthine oxidase on the outside surface of the endothelial cell plasma membrane. Surgery 124: 551–560, 1998. doi: 10.1016/S0039-6060(98)70102-3. [DOI] [PubMed] [Google Scholar]
- 980.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci 59: 1428–1459, 2002. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 981.Virdis A, Colucci R, Fornai M, Blandizzi C, Duranti E, Pinto S, Bernardini N, Segnani C, Antonioli L, Taddei S, Salvetti A, Del Tacca M. Cyclooxygenase-2 inhibition improves vascular endothelial dysfunction in a rat model of endotoxic shock: role of inducible nitric-oxide synthase and oxidative stress. J Pharmacol Exp Ther 312: 945–953, 2005. doi: 10.1124/jpet.104.077644. [DOI] [PubMed] [Google Scholar]
- 982.Virdis A, Colucci R, Fornai M, Duranti E, Giannarelli C, Bernardini N, Segnani C, Ippolito C, Antonioli L, Blandizzi C, Taddei S, Salvetti A, Del Tacca M. Cyclooxygenase-1 is involved in endothelial dysfunction of mesenteric small arteries from angiotensin II-infused mice. Hypertension 49: 679–686, 2007. doi: 10.1161/01.HYP.0000253085.56217.11. [DOI] [PubMed] [Google Scholar]
- 983.Viswambharan H, Yuldasheva NY, Sengupta A, Imrie H, Gage MC, Haywood N, Walker AM, Skromna A, Makova N, Galloway S, Shah P, Sukumar P, Porter KE, Grant PJ, Shah AM, Santos CX, Li J, Beech DJ, Wheatcroft SB, Cubbon RM, Kearney MT. Selective Enhancement of Insulin Sensitivity in the Endothelium In Vivo Reveals a Novel Proatherosclerotic Signaling Loop. Circ Res 120: 784–798, 2017. doi: 10.1161/CIRCRESAHA.116.309678. [DOI] [PubMed] [Google Scholar]
- 984.Voraphani N, Gladwin MT, Contreras AU, Kaminski N, Tedrow JR, Milosevic J, Bleecker ER, Meyers DA, Ray A, Ray P, Erzurum SC, Busse WW, Zhao J, Trudeau JB, Wenzel SE. An airway epithelial iNOS-DUOX2-thyroid peroxidase metabolome drives Th1/Th2 nitrative stress in human severe asthma. Mucosal Immunol 7: 1175–1185, 2014. doi: 10.1038/mi.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 985.Waheed SM, Ghosh A, Chakravarti R, Biswas A, Haque MM, Panda K, Stuehr DJ. Nitric oxide blocks cellular heme insertion into a broad range of heme proteins. Free Radic Biol Med 48: 1548–1558, 2010. doi: 10.1016/j.freeradbiomed.2010.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 986.Walling C. Fenton’s reagent revisited. Acc Chem Res 8: 125–131, 1975. doi: 10.1021/ar50088a003. [DOI] [Google Scholar]
- 987.Walter R, Kaufmann PA, Buck A, Berthold T, Wyss C, von Schulthess GK, Schaffner A, Schoedon G. Tetrahydrobiopterin increases myocardial blood flow in healthy volunteers: a double-blind, placebo-controlled study. Swiss Med Wkly 131: 91–94, 2001. [DOI] [PubMed] [Google Scholar]
- 988.Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res 88: 947–953, 2001. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 989.Wang J, Hong Z, Zeng C, Yu Q, Wang H. NADPH oxidase 4 promotes cardiac microvascular angiogenesis after hypoxia/reoxygenation in vitro. Free Radic Biol Med 69: 278–288, 2014. doi: 10.1016/j.freeradbiomed.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 990.Wang J, Krizowski S, Fischer-Schrader K, Niks D, Tejero J, Sparacino-Watkins C, Wang L, Ragireddy V, Frizzell S, Kelley EE, Zhang Y, Basu P, Hille R, Schwarz G, Gladwin MT. Sulfite Oxidase Catalyzes Single-Electron Transfer at Molybdenum Domain to Reduce Nitrite to Nitric Oxide. Antioxid Redox Signal 23: 283–294, 2015. doi: 10.1089/ars.2013.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 991.Wang JP, Chang LC, Huang LJ, Kuo SC. Inhibition of extracellular Ca(2+) entry by YC-1, an activator of soluble guanylyl cyclase, through a cyclic GMP-independent pathway in rat neutrophils. Biochem Pharmacol 62: 679–684, 2001. doi: 10.1016/S0006-2952(01)00725-0. [DOI] [PubMed] [Google Scholar]
- 992.Wang W, Sawicki G, Schulz R. Peroxynitrite-induced myocardial injury is mediated through matrix metalloproteinase-2. Cardiovasc Res 53: 165–174, 2002. doi: 10.1016/S0008-6363(01)00445-X. [DOI] [PubMed] [Google Scholar]
- 993.Warner TD, Mitchell JA, Sheng H, Murad F. Effects of cyclic GMP on smooth muscle relaxation. Adv Pharmacol 26: 171–194, 1994. doi: 10.1016/S1054-3589(08)60054-X. [DOI] [PubMed] [Google Scholar]
- 994.Waud WR, Rajagopalan KV. The mechanism of conversion of rat liver xanthine dehydrogenase from an NAD+-dependent form (type D) to an O2-dependent form (type O). Arch Biochem Biophys 172: 365–379, 1976. doi: 10.1016/0003-9861(76)90088-6. [DOI] [PubMed] [Google Scholar]
- 995.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res 88: 1259–1266, 2001. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 996.Waypa GB, Marks JD, Guzy RD, Mungai PT, Schriewer JM, Dokic D, Ball MK, Schumacker PT. Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation. Am J Respir Crit Care Med 187: 424–432, 2013. doi: 10.1164/rccm.201207-1294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 997.Waypa GB, Smith KA, Schumacker PT. O2 sensing, mitochondria and ROS signaling: The fog is lifting. Mol Aspects Med 47-48: 76–89, 2016. doi: 10.1016/j.mam.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 998.Webb AJ, Milsom AB, Rathod KS, Chu WL, Qureshi S, Lovell MJ, Lecomte FMJ, Perrett D, Raimondo C, Khoshbin E, Ahmed Z, Uppal R, Benjamin N, Hobbs AJ, Ahluwalia A. Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia: role for xanthine oxidoreductase and endothelial nitric oxide synthase. Circ Res 103: 957–964, 2008. doi: 10.1161/CIRCRESAHA.108.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 999.Weber DS, Taniyama Y, Rocic P, Seshiah PN, Dechert MA, Gerthoffer WT, Griendling KK. Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res 94: 1219–1226, 2004. doi: 10.1161/01.RES.0000126848.54740.4A. [DOI] [PubMed] [Google Scholar]
- 1000.Wei CC, Crane BR, Stuehr DJ. Tetrahydrobiopterin radical enzymology. Chem Rev 103: 2365–2383, 2003. doi: 10.1021/cr0204350. [DOI] [PubMed] [Google Scholar]
- 1001.Weidert ER, Schoenborn SO, Cantu-Medellin N, Choughule KV, Jones JP, Kelley EE. Inhibition of xanthine oxidase by the aldehyde oxidase inhibitor raloxifene: implications for identifying molybdopterin nitrite reductases. Nitric Oxide 37: 41–45, 2014. doi: 10.1016/j.niox.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1002.Weitzberg E, Lundberg JO. Novel aspects of dietary nitrate and human health. Annu Rev Nutr 33: 129–159, 2013. doi: 10.1146/annurev-nutr-071812-161159. [DOI] [PubMed] [Google Scholar]
- 1003.Wendt MC, Daiber A, Kleschyov AL, Mülsch A, Sydow K, Schulz E, Chen K, Keaney JF Jr, Lassègue B, Walter U, Griendling KK, Münzel T. Differential effects of diabetes on the expression of the gp91phox homologues nox1 and nox4. Free Radic Biol Med 39: 381–391, 2005. doi: 10.1016/j.freeradbiomed.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 1004.Wenzel P, Mollnau H, Oelze M, Schulz E, Wickramanayake JM, Müller J, Schuhmacher S, Hortmann M, Baldus S, Gori T, Brandes RP, Münzel T, Daiber A. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid Redox Signal 10: 1435–1447, 2008. doi: 10.1089/ars.2007.1969. [DOI] [PubMed] [Google Scholar]
- 1005.Wenzel P, Schuhmacher S, Kienhöfer J, Müller J, Hortmann M, Oelze M, Schulz E, Treiber N, Kawamoto T, Scharffetter-Kochanek K, Münzel T, Bürkle A, Bachschmid MM, Daiber A. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc Res 80: 280–289, 2008. doi: 10.1093/cvr/cvn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1006.Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Schmidt K, Weiss G, Wachter H. Pteridine biosynthesis in human endothelial cells. Impact on nitric oxide-mediated formation of cyclic GMP. J Biol Chem 268: 1842–1846, 1993. [PubMed] [Google Scholar]
- 1007.Werner ER, Blau N, Thöny B. Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem J 438: 397–414, 2011. doi: 10.1042/BJ20110293. [DOI] [PubMed] [Google Scholar]
- 1008.Werner ER, Pitters E, Schmidt K, Wachter H, Werner-Felmayer G, Mayer B. Identification of the 4-amino analogue of tetrahydrobiopterin as a dihydropteridine reductase inhibitor and a potent pteridine antagonist of rat neuronal nitric oxide synthase. Biochem J 320: 193–196, 1996. doi: 10.1042/bj3200193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1009.West MB, Rokosh G, Obal D, Velayutham M, Xuan Y-T, Hill BG, Keith RJ, Schrader J, Guo Y, Conklin DJ, Prabhu SD, Zweier JL, Bolli R, Bhatnagar A. Cardiac myocyte-specific expression of inducible nitric oxide synthase protects against ischemia/reperfusion injury by preventing mitochondrial permeability transition. Circulation 118: 1970–1978, 2008. doi: 10.1161/CIRCULATIONAHA.108.791533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1010.White CR, Darley-Usmar V, Berrington WR, McAdams M, Gore JZ, Thompson JA, Parks DA, Tarpey MM, Freeman BA. Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits. Proc Natl Acad Sci USA 93: 8745–8749, 1996. doi: 10.1073/pnas.93.16.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1011.Willeit P, Freitag DF, Laukkanen JA, Chowdhury S, Gobin R, Mayr M, Di Angelantonio E, Chowdhury R. Asymmetric dimethylarginine and cardiovascular risk: systematic review and meta-analysis of 22 prospective studies. J Am Heart Assoc 4: e001833, 2015. doi: 10.1161/JAHA.115.001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1012.Williams CR, Lu X, Sutliff RL, Hart CM. Rosiglitazone attenuates NF-κB-mediated Nox4 upregulation in hyperglycemia-activated endothelial cells. Am J Physiol Cell Physiol 303: C213–C223, 2012. doi: 10.1152/ajpcell.00227.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1013.Williams PA, Cosme J, Sridhar V, Johnson EF, McRee DE. Mammalian microsomal cytochrome P450 monooxygenase: structural adaptations for membrane binding and functional diversity. Mol Cell 5: 121–131, 2000. doi: 10.1016/S1097-2765(00)80408-6. [DOI] [PubMed] [Google Scholar]
- 1014.Wilson AM, Harada R, Nair N, Balasubramanian N, Cooke JP. L-arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation 116: 188–195, 2007. doi: 10.1161/CIRCULATIONAHA.106.683656. [DOI] [PubMed] [Google Scholar]
- 1015.Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AHS, Janowitz D, Neff C, Shah AM, Wingler K, Schmidt HHHW. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension 56: 490–497, 2010. doi: 10.1161/HYPERTENSIONAHA.109.149187. [DOI] [PubMed] [Google Scholar]
- 1016.Wind S, Beuerlein K, Eucker T, Müller H, Scheurer P, Armitage ME, Ho H, Schmidt HHHW, Wingler K. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br J Pharmacol 161: 885–898, 2010. doi: 10.1111/j.1476-5381.2010.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1017.Winger JA, Marletta MA. Expression and characterization of the catalytic domains of soluble guanylate cyclase: interaction with the heme domain. Biochemistry 44: 4083–4090, 2005. doi: 10.1021/bi047601d. [DOI] [PubMed] [Google Scholar]
- 1018.Wink DA, Darbyshire JF, Nims RW, Saavedra JE, Ford PC. Reactions of the bioregulatory agent nitric oxide in oxygenated aqueous media: determination of the kinetics for oxidation and nitrosation by intermediates generated in the NO/O2 reaction. Chem Res Toxicol 6: 23–27, 1993. doi: 10.1021/tx00031a003. [DOI] [PubMed] [Google Scholar]
- 1019.Winterbourn CC. Biological reactivity and biomarkers of the neutrophil oxidant, hypochlorous acid. Toxicology 181-182: 223–227, 2002. doi: 10.1016/S0300-483X(02)00286-X. [DOI] [PubMed] [Google Scholar]
- 1020.Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett 82-83: 969–974, 1995. doi: 10.1016/0378-4274(95)03532-X. [DOI] [PubMed] [Google Scholar]
- 1021.Winterbourn CC, Kettle AJ. Redox reactions and microbial killing in the neutrophil phagosome. Antioxid Redox Signal 18: 642–660, 2013. doi: 10.1089/ars.2012.4827. [DOI] [PubMed] [Google Scholar]
- 1022.Wobst J, Kessler T, Dang TA, Erdmann J, Schunkert H. Role of sGC-dependent NO signalling and myocardial infarction risk. J Mol Med (Berl) 93: 383–394, 2015. doi: 10.1007/s00109-015-1265-3. [DOI] [PubMed] [Google Scholar]
- 1023.Wolhuter K, Eaton P. How widespread is stable protein S-nitrosylation as an end-effector of protein regulation? Free Radic Biol Med 109: 156–166, 2017. doi: 10.1016/j.freeradbiomed.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 1024.Wolhuter K, Whitwell HJ, Switzer CH, Burgoyne JR, Timms JF, Eaton P. Evidence against stable protein S-nitrosylation as a widespread mechanism of post-translational regulation. Mol Cell 69: 438–450.e5, 2018. doi: 10.1016/j.molcel.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1025.Wood KC, Cortese-Krott MM, Kovacic JC, Noguchi A, Liu VB, Wang X, Raghavachari N, Boehm M, Kato GJ, Kelm M, Gladwin MT. Circulating blood endothelial nitric oxide synthase contributes to the regulation of systemic blood pressure and nitrite homeostasis. Arterioscler Thromb Vasc Biol 33: 1861–1871, 2013. doi: 10.1161/ATVBAHA.112.301068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1026.Wood KC, Hsu LL, Gladwin MT. Sickle cell disease vasculopathy: a state of nitric oxide resistance. Free Radic Biol Med 44: 1506–1528, 2008. doi: 10.1016/j.freeradbiomed.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 1027.Woodcock SR, Bonacci G, Gelhaus SL, Schopfer FJ. Nitrated fatty acids: synthesis and measurement. Free Radic Biol Med 59: 14–26, 2013. doi: 10.1016/j.freeradbiomed.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1028.Worthley MI, Kanani RS, Sun YH, Sun Y, Goodhart DM, Curtis MJ, Anderson TJ. Effects of tetrahydrobiopterin on coronary vascular reactivity in atherosclerotic human coronary arteries. Cardiovasc Res 76: 539–546, 2007. doi: 10.1016/j.cardiores.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 1029.Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc Rhoads J, Carey Satterfield M, Smith SB, Spencer TE, Yin Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37: 153–168, 2009. doi: 10.1007/s00726-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1030.Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J 336: 1–17, 1998. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1031.Wyss CA, Koepfli P, Namdar M, Siegrist PT, Luscher TF, Camici PG, Kaufmann PA. Tetrahydrobiopterin restores impaired coronary microvascular dysfunction in hypercholesterolaemia. Eur J Nucl Med Mol Imaging 32: 84–91, 2005. doi: 10.1007/s00259-004-1621-y. [DOI] [PubMed] [Google Scholar]
- 1032.Xia Y, Roman LJ, Masters BSS, Zweier JL. Inducible nitric-oxide synthase generates superoxide from the reductase domain. J Biol Chem 273: 22635–22639, 1998. doi: 10.1074/jbc.273.35.22635. [DOI] [PubMed] [Google Scholar]
- 1033.Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem 273: 25804–25808, 1998. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 1034.Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci USA 94: 6954–6958, 1997. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1035.Xu B, Chibber R, Ruggiero D, Kohner E, Ritter J, Ferro A. Impairment of vascular endothelial nitric oxide synthase activity by advanced glycation end products. FASEB J 17: 1289–1291, 2003. doi: 10.1096/fj.02-0490fje. [DOI] [PubMed] [Google Scholar]
- 1036.Xu H, Goettsch C, Xia N, Horke S, Morawietz H, Förstermann U, Li H. Differential roles of PKCalpha and PKCepsilon in controlling the gene expression of Nox4 in human endothelial cells. Free Radic Biol Med 44: 1656–1667, 2008. doi: 10.1016/j.freeradbiomed.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 1037.Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci USA 96: 657–662, 1999. doi: 10.1073/pnas.96.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1038.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science 279: 234–237, 1998. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 1039.Xu W, Kaneko FT, Zheng S, Comhair SAA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 18: 1746–1748, 2004. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- 1040.Yamamoto S. Mammalian lipoxygenases: molecular structures and functions. Biochim Biophys Acta 1128: 117–131, 1992. doi: 10.1016/0005-2760(92)90297-9. [DOI] [PubMed] [Google Scholar]
- 1041.Yamamoto T, Moriwaki Y, Fujimura Y, Takahashi S, Tsutsumi Z, Tsutsui T, Higashino K, Hada T. Effect of TEI-6720, a xanthine oxidase inhibitor, on the nucleoside transport in the lung cancer cell line A549. Pharmacology 60: 34–40, 2000. doi: 10.1159/000028344. [DOI] [PubMed] [Google Scholar]
- 1042.Yang CY, Gu ZW, Yang M, Lin SN, Garcia-Prats AJ, Rogers LK, Welty SE, Smith CV. Selective modification of apoB-100 in the oxidation of low density lipoproteins by myeloperoxidase in vitro. J Lipid Res 40: 686–698, 1999. [PubMed] [Google Scholar]
- 1043.Yang J, Giles LJ, Ruppelt C, Mendel RR, Bittner F, Kirk ML. Oxyl and hydroxyl radical transfer in mitochondrial amidoxime reducing component-catalyzed nitrite reduction. J Am Chem Soc 137: 5276–5279, 2015. doi: 10.1021/jacs.5b01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1044.Yang J, Gonon AT, Sjöquist P-O, Lundberg JO, Pernow J. Arginase regulates red blood cell nitric oxide synthase and export of cardioprotective nitric oxide bioactivity. Proc Natl Acad Sci USA 110: 15049–15054, 2013. doi: 10.1073/pnas.1307058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1045.Au Yeung SL, Lin SL, Lam HSHS, Schooling CM. Effect of l-arginine, asymmetric dimethylarginine, and symmetric dimethylarginine on ischemic heart disease risk: A Mendelian randomization study. Am Heart J 182: 54–61, 2016. doi: 10.1016/j.ahj.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 1046.Yoboue ED, Mougeolle A, Kaiser L, Averet N, Rigoulet M, Devin A. The role of mitochondrial biogenesis and ROS in the control of energy supply in proliferating cells. Biochim Biophys Acta 1837: 1093–1098, 2014. doi: 10.1016/j.bbabio.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 1047.Yoshida LS, Nishida S, Shimoyama T, Kawahara T, Kondo-Teshima S, Rokutan K, Kobayashi T, Tsunawaki S. Superoxide generation by Nox1 in guinea pig gastric mucosal cells involves a component with p67(phox)-ability. Biol Pharm Bull 27: 147–155, 2004. doi: 10.1248/bpb.27.147. [DOI] [PubMed] [Google Scholar]
- 1048.Yoshida LS, Tsunawaki S. Expression of NADPH oxidases and enhanced H(2)O(2)-generating activity in human coronary artery endothelial cells upon induction with tumor necrosis factor-alpha. Int Immunopharmacol 8: 1377–1385, 2008. doi: 10.1016/j.intimp.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 1049.Youn JY, Gao L, Cai H. The p47phox- and NADPH oxidase organiser 1 (NOXO1)-dependent activation of NADPH oxidase 1 (NOX1) mediates endothelial nitric oxide synthase (eNOS) uncoupling and endothelial dysfunction in a streptozotocin-induced murine model of diabetes. Diabetologia 55: 2069–2079, 2012. doi: 10.1007/s00125-012-2557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1050.Yu E, Ruiz-Canela M, Hu FB, Clish CB, Corella D, Salas-Salvadó J, Hruby A, Fitó M, Liang L, Toledo E, Ros E, Estruch R, Gómez-Gracia E, Lapetra J, Arós F, Romaguera D, Serra-Majem L, Guasch-Ferré M, Wang DD, Martínez-González MA. Plasma Arginine/Asymmetric Dimethylarginine Ratio and Incidence of Cardiovascular Events: A Case-Cohort Study. J Clin Endocrinol Metab 102: 1879–1888, 2017. doi: 10.1210/jc.2016-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1051.Yu L, Derrick M, Ji H, Silverman RB, Whitsett J, Vásquez-Vivar J, Tan S. Neuronal nitric oxide synthase inhibition prevents cerebral palsy following hypoxia-ischemia in fetal rabbits: comparison between JI-8 and 7-nitroindazole. Dev Neurosci 33: 312–319, 2011. doi: 10.1159/000327244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1052.Zamocky M, Jakopitsch C, Furtmüller PG, Dunand C, Obinger C. The peroxidase-cyclooxygenase superfamily: Reconstructed evolution of critical enzymes of the innate immune system. Proteins 72: 589–605, 2008. doi: 10.1002/prot.21950. [DOI] [PubMed] [Google Scholar]
- 1053.Zeller M, Korandji C, Guilland J-C, Sicard P, Vergely C, Lorgis L, Beer J-C, Duvillard L, Lagrost A-C, Moreau D, Gambert P, Cottin Y, Rochette L. Impact of asymmetric dimethylarginine on mortality after acute myocardial infarction. Arterioscler Thromb Vasc Biol 28: 954–960, 2008. doi: 10.1161/ATVBAHA.108.162768. [DOI] [PubMed] [Google Scholar]
- 1054.Zhang C, Reiter C, Eiserich JP, Boersma B, Parks DA, Beckman JS, Barnes S, Kirk M, Baldus S, Darley-Usmar VM, White CR. L-arginine chlorination products inhibit endothelial nitric oxide production. J Biol Chem 276: 27159–27165, 2001. doi: 10.1074/jbc.M100191200. [DOI] [PubMed] [Google Scholar]
- 1055.Zhang G, Zhang F, Muh R, Yi F, Chalupsky K, Cai H, Li PL. Autocrine/paracrine pattern of superoxide production through NAD(P)H oxidase in coronary arterial myocytes. Am J Physiol Heart Circ Physiol 292: H483–H495, 2007. doi: 10.1152/ajpheart.00632.2006. [DOI] [PubMed] [Google Scholar]
- 1056.Zhang H, Andrekopoulos C, Joseph J, Crow J, Kalyanaraman B. The carbonate radical anion-induced covalent aggregation of human copper, zinc superoxide dismutase, and alpha-synuclein: intermediacy of tryptophan- and tyrosine-derived oxidation products. Free Radic Biol Med 36: 1355–1365, 2004. doi: 10.1016/j.freeradbiomed.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 1057.Zhang L, Rao F, Zhang K, Khandrika S, Das M, Vaingankar SM, Bao X, Rana BK, Smith DW, Wessel J, Salem RM, Rodriguez-Flores JL, Mahata SK, Schork NJ, Ziegler MG, O’Connor DT. Discovery of common human genetic variants of GTP cyclohydrolase 1 (GCH1) governing nitric oxide, autonomic activity, and cardiovascular risk. J Clin Invest 117: 2658–2671, 2007. doi: 10.1172/JCI31093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1058.Zhang M, Brewer AC, Schröder K, Santos CXC, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci USA 107: 18121–18126, 2010. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1059.Zhang R, Brennan M-L, Fu X, Aviles RJ, Pearce GL, Penn MS, Topol EJ, Sprecher DL, Hazen SL. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA 286: 2136–2142, 2001. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 1060.Zhang R, Hess DT, Reynolds JD, Stamler JS. Hemoglobin S-nitrosylation plays an essential role in cardioprotection. J Clin Invest 126: 4654–4658, 2016. doi: 10.1172/JCI90425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1061.Zhang Z, Naughton DP, Blake DR, Benjamin N, Stevens CR, Winyard PG, Symons MC, Harrison R. Human xanthine oxidase converts nitrite ions into nitric oxide (NO). Biochem Soc Trans 25: 524S, 1997. doi: 10.1042/bst025524s. [DOI] [PubMed] [Google Scholar]
- 1062.Zhao K, Luo G, Giannelli S, Szeto HH. Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines. Biochem Pharmacol 70: 1796–1806, 2005. doi: 10.1016/j.bcp.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 1063.Zhao R, Ma X, Xie X, Shen GX. Involvement of NADPH oxidase in oxidized LDL-induced upregulation of heat shock factor-1 and plasminogen activator inhibitor-1 in vascular endothelial cells. Am J Physiol Endocrinol Metab 297: E104–E111, 2009. doi: 10.1152/ajpendo.91023.2008. [DOI] [PubMed] [Google Scholar]
- 1064.Zhao XJ, Wang L, Shiva S, Tejero J, Myerburg MM, Wang J, Frizzell S, Gladwin MT. Mechanisms for cellular NO oxidation and nitrite formation in lung epithelial cells. Free Radic Biol Med 61: 428–437, 2013. doi: 10.1016/j.freeradbiomed.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1065.Zhuang J, Jiang T, Lu D, Luo Y, Zheng C, Feng J, Yang D, Chen C, Yan X. NADPH oxidase 4 mediates reactive oxygen species induction of CD146 dimerization in VEGF signal transduction. Free Radic Biol Med 49: 227–236, 2010. doi: 10.1016/j.freeradbiomed.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 1066.Zinkevich NS, Gutterman DD. ROS-induced ROS release in vascular biology: redox-redox signaling. Am J Physiol Heart Circ Physiol 301: H647–H653, 2011. doi: 10.1152/ajpheart.01271.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1067.Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem 285: 33154–33164, 2010. doi: 10.1074/jbc.M110.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1068.Zoccali C, Bode-Böger S, Mallamaci F, Benedetto F, Tripepi G, Malatino L, Cataliotti A, Bellanuova I, Fermo I, Frölich J, Böger R. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet 358: 2113–2117, 2001. doi: 10.1016/S0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- 1069.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94: 909–950, 2014. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1070.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest 109: 817–826, 2002. doi: 10.1172/JCI0214442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1071.Zuckerbraun BS, Shiva S, Ifedigbo E, Mathier MA, Mollen KP, Rao J, Bauer PM, Choi JJ, Curtis E, Choi AM, Gladwin MT. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation 121: 98–109, 2010. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]
- 1072.Zweier JL, Duke SS, Kuppusamy P, Sylvester JT, Gabrielson EW. Electron paramagnetic resonance evidence that cellular oxygen toxicity is caused by the generation of superoxide and hydroxyl free radicals. FEBS Lett 252: 12–16, 1989. doi: 10.1016/0014-5793(89)80881-6. [DOI] [PubMed] [Google Scholar]
- 1073.Zweier JL, Li H, Samouilov A, Liu X. Mechanisms of nitrite reduction to nitric oxide in the heart and vessel wall. Nitric Oxide 22: 83–90, 2010. doi: 10.1016/j.niox.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1074.Zweier JL, Samouilov A, Kuppusamy P. Non-enzymatic nitric oxide synthesis in biological systems. Biochim Biophys Acta 1411: 250–262, 1999. doi: 10.1016/S0005-2728(99)00018-3. [DOI] [PubMed] [Google Scholar]
- 1075.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med 1: 804–809, 1995. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]