Abstract
Purpose of Review:
The present review discusses brain circuits that are engaged by negative emotions and possibly linked to cardiovascular disease risk. It describes recent human brain imaging studies that relate activity in these brain circuits to emotional processes, peripheral physiology, preclinical cardiovascular disease, as well as clinical outcomes.
Recent Findings:
Negative emotions and the regulation of negative emotions reliably engage several brain regions that cross-sectional and longitudinal brain imaging studies have associated with CVD risk markers and outcomes. These brain regions include the amygdala, anterior cingulate cortex, medial prefrontal cortex, and insula. Other studies have applied advanced statistical techniques to characterize multivariate patterns of brain activity and brain connectivity that associate with negative emotion and CVD-relevant peripheral physiology.
Summary:
Brain imaging studies on emotion and cardiovascular disease risk are expanding our understanding of the brain-body bases of psychosocial and behavioral risk for cardiovascular disease.
Keywords: amygdala, brain imaging, emotion, emotion regulation, medial prefrontal cortex, stress
Introduction
Cardiovascular disease (CVD) is a leading contributor to morbidity and mortality in the developed world. A large body of epidemiological research suggests that negative emotions and mood states may play a significant role in the development and progression of CVD. Moreover, it is thought that negative emotions, moods, and related dispositional traits (e.g., anxiety, anger, and depressive phenotypes) may impact CVD risk and progression via peripheral physiological changes that are evoked by stressful or otherwise adverse experiences. However, the brain mechanisms that link the experience, expression, and regulation of negative affective states and traits with downstream physiological changes are not fully understood.
To better understand these mechanisms, parallel lines of research using animal models, as well as human brain imaging studies, have attempted to identify brain circuits that are implicated in processing and responding to negative emotional stimuli, as well as the regulation of autonomic, neuroendocrine, immune, and cardiovascular physiology. Emerging human brain imaging studies have, moreover, begun to link functioning in these brain circuits to preclinical and clinical CVD endpoints. Accordingly, the present review summarizes findings from these brain imaging studies, which together point to localized activity as well as patterned and network-level responses within a brain circuitry encompassing specific brainstem, subcortical, and cortical structures. After this summary, we identify open questions for further research in this field.
Emotion, Stress, and Cardiovascular Disease
Although mortality due to cardiovascular disease (CVD) has declined in recent decades, it nonetheless remains a leading cause of death among adult men and women in developed countries [1]. In addition to traditional CVD risk factors, psychosocial factors are thought to influence the development and progression of CVD across the lifespan [2, 3]. Key among these psychosocial factors are processes involving the generation and regulation of negative emotions [4]. Negative emotions may relate to CVD within at least two contexts. First, chronic or prolonged experiences of negative emotional or mood states, such as clinical depression, as well as the trait-like tendency to experience negative emotion, each associate with preclinical CVD disease markers, clinical CVD incidence, as well as treatment outcomes [5–7]. Along these lines, increased CVD risk has been demonstrated in relation to several other clinical disorders (e.g., anxiety, post-traumatic stress), and personality characteristics (e.g., hostility) that involve negative emotions [8–10]. Second, acute emotional responses to negative events may trigger cardiac events in at-risk individuals or individuals with ongoing CVD [11]. Importantly, reported associations between CVD and experiences of chronic or acute negative emotion are often independent of conventional CVD risk factors, such as lipid levels, blood pressure, and tobacco use [12]. Similarly, the statistical effect size of associations between negative emotionality and CVD is comparable to these and other CVD risk factors [13]. We note that, in addition to the literature on negative emotions, a parallel line of research suggests a possible protective role for positive emotions in CVD risk and incidence [14, 15]; however, to our knowledge, none of the brain imaging studies described below have yet examined neural correlates of positive emotions as they relate to CVD risk. Hence, taken together, chronic and acute negative emotional states represent a substantial and potentially modifiable source of risk for CVD and other chronic diseases of aging.
Despite cumulative epidemiological and clinical evidence linking negative emotions to CVD, physiological mechanisms underlying this link are not fully understood. A key component of negative emotional responses is the generation of peripheral physiological changes involving the autonomic, immune, and neuroendocrine systems. Some of the most frequently documented physiological changes that accompany negative emotions and are jointly implicated in CVD pathogenesis include a suppression of parasympathetic cardiac control, an increase in sympathetic nervous system activity, an increase in systemic inflammation, and activation of the hypothalamic-pituitary-adrenal axis [16–19]. The role of these different peripheral physiological changes in the context of emotion and CVD is beyond the scope of the present review, yet we note that these peripheral physiological responses can vary substantially across different emotions, contexts, and individuals. Regarding the latter, observed inter-individual variability in peripheral physiological responses may correspond to specific phenotypes that forecast an individual’s CVD risk or prognosis [20].
In a separate line of research, the peripheral physiological responses listed above (and others) are considered to comprise biological aspects of the canonical stress response: they are evoked when environmental demands tax or exceed an individuals’ ability to cope in order to prepare or motivate the individual to respond to changing environmental contexts and life circumstances [21]. In the context of emotional responses, these stressor-evoked peripheral physiological adjustments are adaptive insofar as they provide the individual with energy and metabolic support to respond to changes in the environment. However, it is thought that prolonged or repeated experience of stress and negative emotions may induce pathological changes in the heart and vasculature via activation of these physiological pathways [22]. For example, there are appreciable individual differences in peripheral blood pressure responses to acute stressors; moreover, individuals with a tendency to show larger and perhaps more sustained (longer lasting) physiological stress responses have increased risk for future incident CVD [23]. Accordingly, these and other perspectives on psychological stress have the goal of identifying components of negative emotions that translate into peripheral physiological responses and hence CVD risk.
Importantly, however, for emotional or stressful stimuli to be translated into downstream physiological responses and hence CVD risk, they must first be processed by the brain [24]. Hence, the brain represents an integral yet relatively underappreciated component in the emotion-CVD link [25]. Drawing from preclinical animal models of stress and CVD, a growing line of research aims to delineate the brain circuits jointly involved in generating and regulating stress and negative emotions, as well as in generating and regulating subsequent peripheral physiological responses. These neurobiological accounts and their accompanying brain imaging studies, reviewed below, indicate there are overlapping neural circuits for negative emotion and CVD risk, respectively.
Neural Substrates of Negative Emotion and Psychological Stress
There is not a complete agreement of how negative emotions and mood states are generated and regulated in the brain. Nonetheless, many leading neurobiological models attribute emotional and stress processing to a core brain circuit comprising brainstem and subcortical regions including the amygdala, hypothalamus, periaqueductal gray (PAG), as well as cortical regions including the medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC), and insula (Figure 1) [26]. Hence, it is not plausible that there is any single brain region for negative emotions in particular. Rather, emotional and stressful experiences engage circuit-level patterns in the brain, and these patterns likely vary across contexts and individuals [27]. Moreover, these patterns of neural responses within and across the above brain regions may be most important for emotion and emotion associated risk for CVD.
Figure 1.
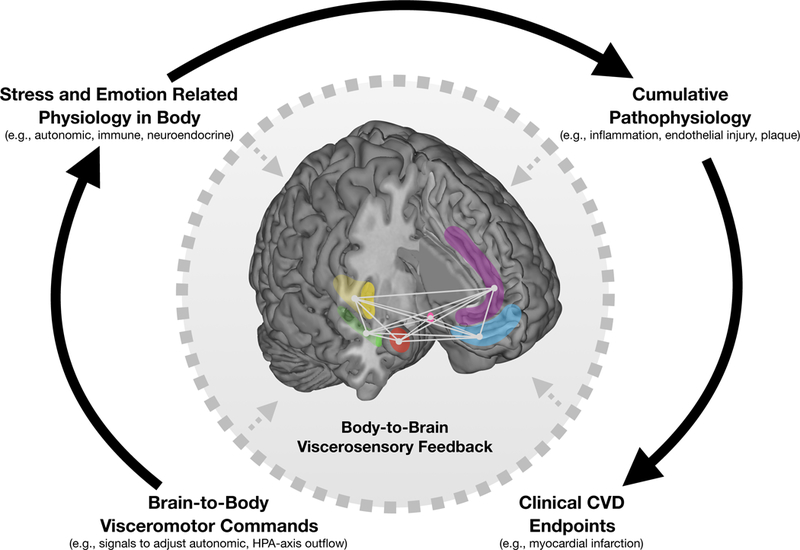
Neuroanatomically connected limbic and cortical brain regions linking negative emotion, psychological stress, and regulation of peripheral physiology. Highlighted are limbic regions including the amygdala (red), hippocampus (green), and hypothalamus (pink) as well as cortical regions including the insula (yellow), anterior cingulate cortex (purple), and ventromedial prefrontal cortex (blue). Negative emotion and psychological stress engage these regions via changes in (1) local (within-region) activity, (2) distributed and patterned (across-region) activity, and (3) network-level interactions between regions across over time. These responses issue brain-to-body visceromotor commands via specific brainstem nuclei to influence physiology in peripheral organs. Exaggerated or prolonged engagement of these responses promote cumulative pathophysiology and future clinical CVD endpoints. Along this pathway, the effect of stress and negative emotion on the body affects the brain via body-to-brain viscerosensory feedback (dotted arrows), including baroreceptor firing, recruitment of circulating mediators of systemic inflammation into the brain, and brain structural damage/remodeling following myocardial infarction.
Notwithstanding, of particular relevance to understanding the affective neurobiology of CVD risk is the role of the amygdala. The amygdala (Figure 1, red) is thought to be involved in assigning salience and relevance to environmental stimuli [28]. In particular, the amygdala is critical for pairing fearful stimuli and situations with appropriate responses [29]. Moreover, the amygdala issues neuroanatomical connections to the regions listed above, particularly the mPFC (Figure 1, blue) [30–32]. Animal models suggest that lesions to the amygdala associate with impaired physiological and behavioral responses to emotional stimuli [33]; in contrast, electrical stimulation of the amygdala in humans and animals results in an array of subjective, behavioral, and downstream physiological responses including feelings of fear and anxiety, altered respiration, and increased heart rate and blood pressure [34]. Finally, many, but not all, human brain imaging studies show that negative emotional states reliably evoke activity within the amygdala [35–37], and alterations in amygdala responsivity are consistently observed in clinical depression and other affective disorders [38]. Collectively, the amygdala is a major focus in neuroscience research linking emotion to peripheral physiology.
Parallel to subcortical and brainstem structures are regions within the cortex, particularly the mPFC, ACC, and insula, that are involved in negative emotion. Moreover, substantial human brain imaging evidence indicates these cortical regions represent and regulate downstream visceral physiological signals, especially those of the autonomic, vascular, neuroendocrine, and immune systems [39, 40].
Briefly, the mPFC and ACC (Figure 1, blue and purple, respectively) are thought to be involved in processes including executive control, conflict monitoring, and the expression and regulation of negative emotion [41–44]. Regarding the latter process, the mPFC and ACC are critically implicated in the regulation of negative emotion, in particular cognitive reappraisal [45]. Cognitive reappraisal is a major clinical focus of behavioral interventions for depression and other affective disorders [46], and individual differences in the tendency to use cognitive regulation of emotion associates with preclinical markers of CVD [47]; hence, the role of these cortical structures in emotion regulation and CVD risk is an emerging line of research. Separately, the mPFC and ACC issue ‘brain-to-body’ visceromotor commands in the form of autonomic and cardiovascular responses to environmental stimuli [48–50]. In particular, dorsal and ventral divisions of the ACC and mPFC may be involved in generating sympathetic and parasympathetic responses to stimuli, respectively [25]. Stimulation of the ventral mPFC (vmPFC) appears to reduce sympathetic tone and arterial pressure [51, 52]. Neurological patients with focal damage to areas in the dorsal ACC exhibit altered (i.e., “blunted”) autonomic and cardiovascular responses to effortful cognitive tasks [53], perhaps indicating that more dorsal and midline cortical territories participate in generating sympathetic nervous system responding.
Another cortical brain region implicated in emotion and CVD risk is the insula (Figure 1, yellow). In the context of emotion, the insula integrates ‘body-to-brain’ viscerosensory feedback into subjective emotional states [54, 55]. Specifically, viscerosensory feedback (e.g., autonomic, immune) conveying bodily states is sensed by the posterior insula and subsequently integrated and interpreted by the anterior insula. The process by which viscerosensory feedback is sensed and processed is known as interoception, and hence the insula is considered to be an ‘interoceptive cortex’ [56, 57]. In the context of emotion, viscerosensory feedback shapes feeling states (e.g., fatigue), and biases motivations and drives to maintain optimal functioning [58]. Separately, the insula is involved in regulating cardiac function [59]. Interestingly, several studies have found that stroke patients with infarctions localized to the insula, when compared to patients with infarctions in other brain regions, exhibit altered autonomic tone, elevated blood pressure, and more complex arrhythmias [60–62]. Moreover, stimulating the insula can induce cardiac arrhythmias as well as structural damage to cells of the heart (myocytolysis) [63]. Along these lines, it has been suggested that the insula may be involved in acute emotion-induced cardiac alterations, arrhythmias, and sudden death, including Takotsubo cardiomyopathy [64].
Taken together, brain substrates for emotion and CVD risk are not limited to evolutionarily ‘old’ subcortical and brainstem structures, but additionally implicate cortical and insular regions and networks involved in higher-order cognitive, emotional, and social behavioral processes [65].
Brain Imaging Studies of Emotion, Stress, and CVD
Drawing from the evidence linking emotional processes to CVD risk as described above, an emerging body of brain imaging research examines brain circuits jointly implicated in processing emotional and stressful experiences as well as regulating physiology that is involved in CVD [66, 67]. To this end, such brain imaging studies typically employ behavioral task paradigms in the scanner, requiring participants to view emotional or aversive pictures or film clips [68], complete difficult cognitive tasks under unpredictable time pressure and negative feedback [69], prepare a difficult speech before an unsupportive panel of judges [68], or respond to social exclusion during a computerized group interactions [70]. Further, these studies examine an array of peripheral physiological systems that are engaged by negative emotion and also relate to CVD pathophysiology, including heart rate [71], heart rate variability [72], cardiac contractility [73], baroreflex sensitivity [74], and blood pressure [75]. Broadly, these studies consistently report brain regions, particularly those described above, that relate to peripheral physiology during emotional and stressful experiences [74, 76, 77]. For example, a recent study observed an association between amygdala responses during the processing of threatening faces with circulating levels of C-Reactive protein (CRP) [78], a marker of systemic inflammation known to predict incident CVD independently of traditional CVD risk factors [79]. In another study, negative emotion inductions engaged areas in the mPFC, insula, and PAG, and responses in these areas associated with changes in high frequency heart rate variability[80], an indirect or surrogate index of cardiac parasympathetic autonomic nervous system function that is linked to CVD risk [81].
Other brain imaging studies examine associations of emotion processing with markers of preclinical CVD pathophysiology. In one study, amygdala responses during the processing of emotional faces associated with carotid intima-media thickness (cIMT), a preclinical marker of CVD risk [82]. In another study, activity in the dorsal subdivision of the ACC (dACC) during cognitive regulation of negative emotional stimuli associated with cIMT [83]. In the latter study, the observed association was statistically mediated by circulating levels of the pro-inflammatory cytokine interleukin(IL)-6. Several other regions in the mPFC, ACC, and insula associated with IL-6, but not with cIMT. Notably, however, this study failed to replicate the above association between amygdala activity during the processing of negative emotional stimuli and cIMT, indicating that single brain areas including the amygdala may not be uniformly associated with CVD risk across all contexts and subject populations.
While the above studies are promising insofar as they identify candidate brain regions linking emotion to CVD, nearly all are cross sectional, which limits generating causal interpretations. As mentioned previously, some of the candidate brain regions reviewed here are implicated in relaying and representing viscerosensory feedback to the brain; hence, it is plausible that preclinical changes in peripheral CVD risk factors (e.g., inflammatory or vascular state) could influence, in a body-to-brain manner, brain activity observed in response to emotion [39]. Accordingly, to better interrogate the directionality of these brain-body pathways, what is needed are longitudinal brain imaging studies demonstrating that functional activity in brain circuits involved in negative emotion precede the development of CVD, or otherwise predict clinical outcomes in CVD patients.
The only study so far to take a longitudinal approach to these questions used a brain imaging method called positron emission tomography to examine resting metabolism of the amygdala in a sample of nearly 300 individuals [84]. This study showed that higher resting activity in the amygdala at baseline was associated with a greater incidence of CVD events, defined as coronary death, myocardial infarction, coronary insufficiency, angina, cerebrovascular accidents, revascularization, peripheral artery disease, and heart failure, over a median follow-up period of 3.7 years. Importantly, the predictive utility of resting amygdala activity on these subsequent events remained following statistical adjustment for several traditional CVD risk factors at baseline. Finally, the relation between baseline amygdala activity and CVD event incidence was statistically mediated by bone marrow activity and arterial inflammation, and amygdala activity was positively associated with increased symptoms of perceived stress in a subset of participants. In summary, this study provided crucial longitudinal evidence in a clinical population linking neural substrates of negative emotion to objective CVD outcomes, and moreover identified physiological pathways that plausibly mediate this longitudinal risk.
Toward Brain Networks and Multivariate Patterns for Predicting CVD Risk
Thus far, the majority of brain imaging research on negative emotion, stress reactivity, and CVD has focused on mean levels of activity within discrete regions of the brain. Two emerging lines of research aim to expand current knowledge using novel conceptual and statistical approaches: formulating the brain into networks of connections between brain regions, as well as examining patterns of activity across distinct brain regions.
First, brain circuits involved in emotional responses and physiological control may be conceptualized as networks. These networks comprise brain regions that are structurally connected via white matter fibers (i.e., structural connectivity networks [85]) as well as brain regions whose observed activities correlate with each other over time (i.e., functional connectivity networks [86]). The reformulation of brain regions into networks and their resulting connections attracts substantial interest, as metrics of communication between brain regions might estimate underlying neuronal processes more accurately than activity levels within brain regions [87].
As mentioned above, candidate brain regions involved in emotion and CVD risk are richly interconnected. Hence, metrics of structural and functional connectivity between these brain regions may more accurately estimate biobehavioral risk in the context of negative emotions and stress. Indeed, psychological stress consistently alters functional connectivity between the amygdala, insula, ACC and mPFC, and changes in these estimates of functional connectivity relate to downstream autonomic and cardiovascular reactivity (for review, see [66]). Several of these regions can be grouped into a corticolimbic circuit involving limbic (subcortical) regions such as the amygdala and hippocampus, as well as neuroanatomically connected cortical regions in the ACC and mPFC. This circuit is involved in emotion regulation, stress reactivity, and control over peripheral autonomic and cardiovascular physiology [88, 89]. Connections between these regions as well as the insula, periaqueductal gray matter, parabrachial complex, nucleus of the tractus solitarius, and ventrolateral medulla have moreover been historically described as comprising a central autonomic network [90]. Several brain imaging studies have linked connectivity across this network with peripheral physiology and CVD. For example, during a cognitive stressor task, individuals with exaggerated blood pressure responses also exhibited increased functional connectivity (i.e., cross-correlation) between the amygdala and other regions including the mPFC, insula, hippocampus, and pons [91]. Similarly, during a task in which participants received negative social feedback, individuals with greater IL-6 responses to the task also exhibited greater functional connectivity between the amygdala and mPFC [92]. Collectively, these and other studies suggest that functional connectivity between subcortical (i.e., amygdala) and cortical (e.g., mPFC) regions may link negative emotional states to peripheral physiological pathways as well as preclinical markers for CVD.
Parallel to the above are advances in statistical approaches to characterizing patterns of brain activity. These statistical approaches contend that complex psychological phenomena, such as negative emotions, may be expressed in the brain by distributed patterns, or signatures, of activity across multiple regions. Due to the multivariate nature of these brain patterns, studies typically employ machine learning algorithms to examine the predictive utility on unseen, out-of-sample observations. Similar to the above argument for brain networks, it is thought that patterns of neural responses across the entire brain may be important to consider above-and-beyond those observed in individual regions [93].
Indeed, several ‘brain signatures’ have recently been generated in the context of emotion and CVD. In one study, a brain signature encompassing the amygdala, insula, ACC, mPFC, as well as other regions, predicted subjective response to viewing negative affective pictures [94]. In the context of peripheral physiology, a separate recent study identified a brain signature comprising the dACC, ventral mPFC (vmPFC), and brainstem that predicted peripheral autonomic (i.e., heart rate, skin conductance) responses to a social stressor over time [95]. Moving towards individual differences in physiological reactivity, a recent study identified a brain signature for interindividual variability in cardiovascular responses to stress, which comprised similar regions within the ACC and vmPFC, as well as the insula [96]. Importantly, brain signatures identified in these studies were able to accurately predict emotional and physiological responses in participants who were not used to model and generate the brain signature, showing these brain signatures are generalizable. Moreover, these multivariate patterns predicted responses better than individual regions. To our knowledge, no studies have yet leveraged these statistical techniques toward predicting future preclinical CVD factors or clinical incidence; hence, this is a promising avenue for future research.
Open Questions and Future Directions
So far, brain imaging studies reviewed above suggest that brain regions involved in negative emotion and stress, as well as their network-level interactions and distributed patterns of activity, may associate with concurrent and future CVD risk. However, several open questions remain regarding the precise nature of these observed relationships, their reliability and generalizability, their relation to downstream physiology and health behaviors, as well as their clinical utility.
First, while the present review focuses on brain circuits for immediate emotional responses and downstream peripheral physiology, there are nonetheless other indirect pathways that could plausibly link emotion to CVD risk that implicate entirely independent sets of brain regions and circuits. One clear example points to behavioral evidence linking emotion to health behaviors that are known to elevate CVD risk, in particular diet, physical activity, alcohol intake, and smoking habits [97]. These behaviors and lifestyles are influenced by emotions but engage somewhat distinct brain circuits from the set reviewed here. Specifically, a corticostriatal network, including components of the basal ganglia and neuroanatomically connected divisions of the mPFC, is implicated in reward valuation, craving, reinforcement learning, and motor planning [98]. Alterations in this corticostriatal circuitry have been extensively documented in mood and stress-related disorders [99] and could plausibly be implicated in biasing individuals towards adopting unhealthy behaviors following negative emotional states [100]. Neural activity in these brain areas has also been linked to systemic inflammation [101]. However, to our knowledge, no brain imaging studies have yet examined this specific link in the context of emotion and CVD.
Second, there is a lack of consensus on the generalizability and reliability of task paradigms that evoke stress and negative emotion in the above reviewed studies. For example, anger and hostility have long been proposed to confer CVD risk, but no studies have used anger provocation tasks during brain imaging in order to link brain activity to physiology and disease markers. It is unclear whether brain circuits engaged by anger would similarly associate with peripheral physiology and CVD risk markers. In contrast, a separate and emerging brain imaging method examines brain activity and functional connectivity networks at rest [102]. Connectivity across these networks at rest, also called intrinsic connectivity networks, has been linked to individual differences in emotion as well as peripheral markers of CVD risk (e.g., inflammation, heart rate variability) [103–105]. To this end, it is unclear whether individual differences in these brain circuits measured at rest, including the longitudinal study discussed above, are comparable or generalizable to individual differences evoked by emotions or stress in the context of CVD. Similarly, while the reliability of some of the above brain imaging tasks have been previously examined [106], there are open questions about whether other brain imaging studies of other emotion or emotion regulation tasks reliably reveal individual difference phenotypes that effectively stratify participants according to emotional responsivity and CVD risk.
Third, nearly all the extant brain imaging research on emotion and CVD focuses on risk factors and clinical occurrence, with little or no focus on functional or clinical prognosis for participants currently affected by chronic affective disorders or clinical CVD. Hence, future research is needed to characterize the role of these and other brain circuits in clinical samples. To this end, a recent study examined neural correlates of mental stress-induced myocardial ischemia (MI) in coronary heart disease patients [107]. This study found that patients exhibiting mental stress-induced MI also demonstrated exaggerated responsivity to stress in prefrontal cortical regions including the ACC. Whether these differences in stress-induced brain responsivity in this clinical sample relate to clinical prognosis is an exciting question for future study. Separately, as the majority of the above brain imaging studies was conducted on psychiatrically healthy community samples, it is relatively unknown whether these findings extend to individuals diagnosed with chronic psychiatric disorders. Along these lines, acute CVD events confer elevated risk for poor mental health outcomes (e.g., posttraumatic stress disorder) which in turn is associated with risk for recurrent CVD events [108]; hence, future studies may examine whether brain changes or remodeling following CVD events prospectively predict mental and physical health outcomes.
Finally, future studies might examine surrogate or intermediate markers in psychosocial interventions designed to reduce negative emotion or improve emotion regulation (e.g., cognitive behavioral therapy, mindfulness meditation) prior to later stage endpoints, particularly in patients with clinical depression or other affective disorders who are at elevated CVD risk [109].
Conclusions
The recent research presented in this review adds to a growing body of evidence indicating that activity within specific brain regions and circuits may link negative emotional and mood states and psychological stress to CVD risk. However, this evidence largely relies on cross-sectional studies on individuals without CVD, and does not systematically examine potential mediating pathways or moderating influences. Future studies that adopt longitudinal designs, employ advanced statistical techniques, and consider potential mediating pathways across diverse healthy and clinical samples stand to greatly increase our understanding of the brain-body pathways linking emotion with CVD [25].
Acknowledgements
This work was supported by National Institutes of Health grants T32 HL007560 and R01 HL089850.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Thomas E. Kraynak, Anna L. Marsland, and Peter J. Gianaros declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
** Of outstanding interest
* Of interest
- 1.GBD 2016 Causes of Death Collaborators (2017) Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390:1151–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Everson-Rose SA, Lewis TT (2005) Psychosocial factors and cardiovascular diseases. Annu Rev Public Health 26:469–500 [DOI] [PubMed] [Google Scholar]
- 3.Matthews KA (2013) Matters of the Heart Advancing Psychological Perspectives on Cardiovascular Diseases. Perspectives on Psychological Science 8:676–678 [DOI] [PubMed] [Google Scholar]
- 4.DeSteno D, Gross JJ, Kubzansky L (2013) Affective science and health: The importance of emotion and emotion regulation. Health Psychology 32:474–486 [DOI] [PubMed] [Google Scholar]
- 5.Musselman DL, Evans DL, Nemeroff CB (1998) The Relationship of Depression to Cardiovascular Disease: Epidemiology, Biology, and Treatment. Arch Gen Psychiatry 55:580–592 [DOI] [PubMed] [Google Scholar]
- 6.Rozanski A, Blumenthal JA, Kaplan J (1999) Impact of Psychological Factors on the Pathogenesis of Cardiovascular Disease and Implications for Therapy. Circulation 99:2192–2217 [DOI] [PubMed] [Google Scholar]
- 7.Gan Y, Gong Y, Tong X, et al. (2014) Depression and the risk of coronary heart disease: a meta-analysis of prospective cohort studies. BMC Psychiatry 14:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celano CM, Millstein RA, Bedoya CA, Healy BC, Roest AM, Huffman JC (2015) Association between anxiety and mortality in patients with coronary artery disease: A meta-analysis. Am Heart J 170:1105–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chida Y, Steptoe A (2009) The association of anger and hostility with future coronary heart disease: a meta-analytic review of prospective evidence. J Am Coll Cardiol 53:936–946 [DOI] [PubMed] [Google Scholar]
- 10.Edmondson D, Kronish IM, Shaffer JA, Falzon L, Burg MM (2013) Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J 166:806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang W (2015) Emotional Triggering of Cardiac Dysfunction: The Present and Future. Curr Cardiol Rep 17:91. [DOI] [PubMed] [Google Scholar]
- 12.Kivimäki M, Batty GD, Hamer M, Ferrie JE, Vahtera J, Virtanen M, Marmot MG, Singh-Manoux A, Shipley MJ (2011) Using additional information on working hours to predict coronary heart disease: a cohort study. Ann Intern Med 154:457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosengren A, Hawken S, Ounpuu S, et al. (2004) Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet 364:953–962 [DOI] [PubMed] [Google Scholar]
- 14.Cohen S, Pressman SD (2006) Positive Affect and Health. Curr Dir Psychol Sci 15:122–125 [Google Scholar]
- 15.Boehm JK, Kubzansky LD (2012) The heart’s content: The association between positive psychological well-being and cardiovascular health. Psychological Bulletin 138:655–691 [DOI] [PubMed] [Google Scholar]
- 16.Haensel A, Mills PJ, Nelesen RA, Ziegler MG, Dimsdale JE (2008) The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology 33:1305–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Libby P, Ridker PM, Maseri A (2002) Inflammation and Atherosclerosis. Circulation 105:1135–1143 [DOI] [PubMed] [Google Scholar]
- 18.Girod JP, Brotman DJ (2004) Does altered glucocorticoid homeostasis increase cardiovascular risk? Cardiovasc Res 64:217–226 [DOI] [PubMed] [Google Scholar]
- 19.Wirtz PH, von Känel R (2017) Psychological Stress, Inflammation, and Coronary Heart Disease. Curr Cardiol Rep 19:111. [DOI] [PubMed] [Google Scholar]
- 20.Thayer JF, Yamamoto SS, Brosschot JF (2010) The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology 141:122–131 [DOI] [PubMed] [Google Scholar]
- 21.Cohen S, Gianaros PJ, Manuck SB (2016) A Stage Model of Stress and Disease. Perspect Psychol Sci 11:456–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krantz DS, Manuck SB (1984) Acute psychophysiologic reactivity and risk of cardiovascular disease: A review and methodologic critique. Psychological Bulletin 96:435–464 [PubMed] [Google Scholar]
- 23.Jennings JR, Kamarck TW, Everson-Rose SA, Kaplan GA, Manuck SB, Salonen JT (2004) Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation 110:2198–2203 [DOI] [PubMed] [Google Scholar]
- 24.McEwen BS, Gianaros PJ (2010) Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease: Central links between stress and SES. Annals of the New York Academy of Sciences 1186:190–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gianaros PJ, Wager TD (2015) Brain-body pathways linking psychological stress and physical health. Curr Dir Psychol Sci 24:313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF (2012) The brain basis of emotion: A meta-analytic review. Behavioral and Brain Sciences 35:121–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wager TD, Kang J, Johnson TD, Nichols TE, Satpute AB, Barrett LF (2015) A Bayesian Model of Category-Specific Emotional Brain Responses. PLOS Computational Biology 11:e1004066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeDoux J (2003) The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23:727–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips RG, LeDoux JE (1992) Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106:274–285 [DOI] [PubMed] [Google Scholar]
- 30.Dampney RA (1994) Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74:323–364 [DOI] [PubMed] [Google Scholar]
- 31.Price JL (2003) Comparative aspects of amygdala connectivity. Ann N Y Acad Sci 985:50–58 [DOI] [PubMed] [Google Scholar]
- 32.Amaral DG, Price JL (1984) Amygdalo-cortical projections in the monkey (Macaca fascicularis). J Comp Neurol 230:465–496 [DOI] [PubMed] [Google Scholar]
- 33.Goldstein LE, Rasmusson AM, Bunney BS, Roth RH (1996) Role of the Amygdala in the Coordination of Behavioral, Neuroendocrine, and Prefrontal Cortical Monoamine Responses to Psychological Stress in the Rat. J Neurosci 16:4787–4798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis M, Whalen PJ (2001) The amygdala: vigilance and emotion. Molecular Psychiatry 6:13–34 [DOI] [PubMed] [Google Scholar]
- 35.Lindquist KA, Satpute AB, Wager TD, Weber J, Barrett LF (2016) The Brain Basis of Positive and Negative Affect: Evidence from a Meta-Analysis of the Human Neuroimaging Literature. Cereb Cortex 26:1910–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF (2009) Brain mediators of cardiovascular responses to social threat: Part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage 47:821–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN (2009) Brain mediators of cardiovascular responses to social threat, Part II: Prefrontal-subcortical pathways and relationship with anxiety. NeuroImage 47:836–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH (2012) Functional Neuroimaging of Major Depressive Disorder: A Meta-Analysis and New Integration of Baseline Activation and Neural Response Data. AJP 169:693–703 [DOI] [PubMed] [Google Scholar]
- 39.Critchley HD, Harrison NA (2013) Visceral influences on brain and behavior. Neuron 77:624–638 [DOI] [PubMed] [Google Scholar]
- 40.Critchley HD (2005) Neural mechanisms of autonomic, affective, and cognitive integration. The Journal of Comparative Neurology 493:154–166 [DOI] [PubMed] [Google Scholar]
- 41.Etkin A, Egner T, Kalisch R (2011) Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci (Regul Ed) 15:85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004) The role of the medial frontal cortex in cognitive control. Science 306:443–447 [DOI] [PubMed] [Google Scholar]
- 43.Botvinick MM, Cohen JD, Carter CS (2004) Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences 8:539–546 [DOI] [PubMed] [Google Scholar]
- 44.Roy M, Shohamy D, Wager TD (2012) Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences 16:147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN (2013) Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cerebral Cortex. doi: 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gotlib IH, Joormann J (2010) Cognition and Depression: Current Status and Future Directions. Annual Review of Clinical Psychology 6:285–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Appleton AA, Loucks EB, Buka SL, Kubzansky LD (2014) Divergent Associations of Antecedent- and Response-Focused Emotion Regulation Strategies with Midlife Cardiovascular Disease Risk. ann behav med 48:246–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dum RP, Levinthal DJ, Strick PL (2016) Motor, cognitive, and affective areas of the cerebral cortex influence the adrenal medulla. PNAS 113:9922–9927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parvizi J, Rangarajan V, Shirer WR, Desai N, Greicius MD (2013) The Will to Persevere Induced by Electrical Stimulation of the Human Cingulate Gyrus. Neuron. doi: 10.1016/j.neuron.2013.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gianaros PJ, Derbtshire SWG, May JC, Siegle GJ, Gamalo MA, Jennings JR (2005) Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology 42:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verberne AJ (1996) Medullary sympathoexcitatory neurons are inhibited by activation of the medial prefrontal cortex in the rat. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 270:R713–R719 [DOI] [PubMed] [Google Scholar]
- 52.Resstel LBM, Fernandes KBP, Corrêa FMA (2004) Medial prefrontal cortex modulation of the baroreflex parasympathetic component in the rat. Brain Research 1015:136–144 [DOI] [PubMed] [Google Scholar]
- 53.Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar B-K, Cipolotti L, Shallice T, Dolan RJ (2003) Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain 126:2139–2152 [DOI] [PubMed] [Google Scholar]
- 54.Craig AD (2009) How do you feel — now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70 [DOI] [PubMed] [Google Scholar]
- 55.Harrison NA, Gray MA, Gianaros PJ, Critchley HD (2010) The Embodiment of Emotional Feelings in the Brain. J Neurosci 30:12878–12884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khalsa SS, Adolphs R, Cameron OG, et al. (2018) Interoception and Mental Health: A Roadmap. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 3:501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Craig AD (2003) Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology 13:500–505 [DOI] [PubMed] [Google Scholar]
- 58.Damasio A, Carvalho GB (2013) The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neurosci 14:143–152 [DOI] [PubMed] [Google Scholar]
- 59.Oppenheimer S, Cechetto D (2016) The insular cortex and the regulation of cardiac function. Compr Physiol 6:1081–1133** This comprehensive review provides extensive detail on animal and human brain imaging literatures linking the insula to cardiac function in health and disease.
- 60.Nagai M, Hoshide S, Kario K (2010) The insular cortex and cardiovascular system: a new insight into the brain-heart axis. Journal of the American Society of Hypertension 4:174–182 [DOI] [PubMed] [Google Scholar]
- 61.Colivicchi F, Bassi A, Santini M, Caltagirone C (2004) Cardiac Autonomic Derangement and Arrhythmias in Right-Sided Stroke With Insular Involvement. Stroke 35:2094–2098 [DOI] [PubMed] [Google Scholar]
- 62.Meyer S, Strittmatter M, Fischer C, Georg T, Schmitz B (2004) Lateralization in autonomic dysfunction in ischemic stroke involving the insular cortex. Neuroreport 15:357–361 [DOI] [PubMed] [Google Scholar]
- 63.Oppenheimer SM, Wilson JX, Guiraudon C, Cechetto DF (1991) Insular cortex stimulation produces lethal cardiac arrhythmias: a mechanism of sudden death? Brain Res 550:115–121 [DOI] [PubMed] [Google Scholar]
- 64.Ueyama T Emotional Stress-Induced Tako-tsubo Cardiomyopathy: Animal Model and Molecular Mechanism. Annals of the New York Academy of Sciences 1018:437–444 [DOI] [PubMed] [Google Scholar]
- 65.Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, Quigley KS, Dickerson BC, Barrett LF (2017) Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nature Human Behaviour 1:0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ginty AT, Kraynak TE, Fisher JP, Gianaros PJ (2017) Cardiovascular and autonomic reactivity to psychological stress: Neurophysiological substrates and links to cardiovascular disease. Autonomic Neuroscience 207:2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gianaros PJ, Sheu LK (2009) A review of neuroimaging studies of stressor-evoked blood pressure reactivity: Emerging evidence for a brain-body pathway to coronary heart disease risk. NeuroImage 47:922–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hermans EJ, Marle HJF van, Ossewaarde L, et al. (2011) Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science 334:1151–1153 [DOI] [PubMed] [Google Scholar]
- 69.Bush G, Shin LM (2006) The Multi-Source Interference Task: an fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. Nat Protocols 1:308–313 [DOI] [PubMed] [Google Scholar]
- 70.Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD (2007) Neural pathways link social support to attenuated neuroendocrine stress responses. NeuroImage 35:1601–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ginty AT, Gianaros PJ, Derbyshire SWG, Phillips AC, Carroll D (2013) Blunted cardiac stress reactivity relates to neural hypoactivation. Psychophysiol 50:219–229 [DOI] [PubMed] [Google Scholar]
- 72.Gianaros PJ, Van der Veen FM, Jennings JR (2004) Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology 41:521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dalton KM, Kalin NH, Grist TM, Davidson RJ (2005) Neural-cardiac coupling in threat-evoked anxiety. J Cogn Neurosci 17:969–980 [DOI] [PubMed] [Google Scholar]
- 74.Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD (2012) Brain systems for baroreflex suppression during stress in humans. Human Brain Mapping 33:1700–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ (2000) Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. The Journal of Physiology 523:259–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beissner F, Meissner K, Bär K-J, Napadow V (2013) The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci 33:10503–10511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ruiz Vargas E, Sörös P, Shoemaker JK, Hachinski V (2016) Human cerebral circuitry related to cardiac control: A neuroimaging meta-analysis. Ann Neurol 79:709–716 [DOI] [PubMed] [Google Scholar]
- 78.Swartz JR, Prather AA, Hariri AR (2017) Threat-related amygdala activity is associated with peripheral CRP concentrations in men but not women. Psychoneuroendocrinology 78:93–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Libby P, Ridker PM (2004) Inflammation and atherosclerosis: role of C-Reactive protein in risk assessment. The American Journal of Medicine 116:9–16 [DOI] [PubMed] [Google Scholar]
- 80.Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF (2009) Neural correlates of heart rate variability during emotion. NeuroImage 44:213–222 [DOI] [PubMed] [Google Scholar]
- 81.Tsuji H, Larson MG, Venditti FJ, Manders ES, Evans JC, Feldman CL, Levy D (1996) Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 94:2850–2855 [DOI] [PubMed] [Google Scholar]
- 82.Gianaros PJ, Hariri AR, Sheu LK, Muldoon MF, Sutton-Tyrrell K, Manuck SB (2009) Preclinical Atherosclerosis Covaries with Individual Differences in Reactivity and Functional Connectivity of the Amygdala. Biological Psychiatry 65:943–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gianaros PJ, Marsland AL, Kuan DC-H, Schirda BL, Jennings JR, Sheu LK, Hariri AR, Gross JJ, Manuck SB (2014) An inflammatory pathway links atherosclerotic cardiovascular disease risk to neural activity evoked by the cognitive regulation of emotion. Biol Psychiatry 75:738–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tawakol A, Ishai A, Takx RA, et al. (2017) Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. The Lancet 389:834–845** This brain imaging study demonstrated that resting metabolism in the amygdala significantly predicted future CVD events, independent of traditional CVD risk factors, in a large sample. This study also identified arterial inflammation and bone marrow activity as potential physiological mediators between brain activity and CVD events.
- 85.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O (2008) Mapping the Structural Core of Human Cerebral Cortex. PLOS Biology 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yeo BTT, Krienen FM, Sepulcre J, et al. (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology 106:1125–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hutchison RM, Womelsdorf T, Allen EA, et al. (2013) Dynamic functional connectivity: Promise, issues, and interpretations. NeuroImage 80:360–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Phillips ML, Drevets WC, Rauch SL, Lane R (2003) Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry 54:504–514 [DOI] [PubMed] [Google Scholar]
- 89.Myers B (2017) Corticolimbic regulation of cardiovascular responses to stress. Physiol Behav 172:49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Benarroch EE (1993) The Central Autonomic Network: Functional Organization, Dysfunction, and Perspective. Mayo Clinic Proceedings 68:988–1001 [DOI] [PubMed] [Google Scholar]
- 91.Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR (2008) Individual Differences in Stressor-Evoked Blood Pressure Reactivity Vary with Activation, Volume, and Functional Connectivity of the Amygdala. J Neurosci 28:990–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE, Irwin MR, Eisenberger NI (2015) Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav Immun 43:46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Woo C-W, Chang LJ, Lindquist MA, Wager TD (2017) Building better biomarkers: brain models in translational neuroimaging. Nat Neurosci 20:365–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang LJ, Gianaros PJ, Manuck SB, Krishnan A, Wager TD (2015) A Sensitive and Specific Neural Signature for Picture-Induced Negative Affect. PLoS Biol 13:e1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eisenbarth H, Chang LJ, Wager TD (2016) Multivariate brain prediction of heart rate and skin conductance responses to social threat. J Neurosci 36:11987–11998* This brain imaging study used machine learning techniques to generate a multivariate brain signature, comprising regions such as the medial prefrontal cortex, anterior cingulate cortex, and brainstem, that predicted peripheral autonomic (heart rate, skin conductance) responses during a stress task.
- 96.Gianaros PJ, Sheu LK, Uyar F, Koushik J, Jennings JR, Wager TD, Singh A, Verstynen TD (2017) A brain phenotype for stressor‐evoked blood pressure reactivity. J Am Heart Assoc 6:e006053.* Using a large, representative community sample, this brain imaging study used machine learning techniques to generate a multivariate brain signature, comprising regions such as the medial prefrontal cortex, anterior cingulate cortex, and insula, that predicted individual differences in stressor-evoked blood pressure reactivity, a potential biobehavioral risk factor for CVD.
- 97.Rozanski A (2014) Behavioral Cardiology: Current Advances and Future Directions. Journal of the American College of Cardiology 64:100–110 [DOI] [PubMed] [Google Scholar]
- 98.Pauli WM, O’Reilly RC, Yarkoni T, Wager TD (2016) Regional specialization within the human striatum for diverse psychological functions. PNAS 113:1907–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Russo SJ, Nestler EJ (2013) The brain reward circuitry in mood disorders. Nat Rev Neurosci 14:609–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dallman MF (2010) Stress-induced obesity and the emotional nervous system. Trends in Endocrinology & Metabolism 21:159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kraynak TE, Marsland AL, Wager TD, Gianaros PJ (2018) Functional neuroanatomy of peripheral inflammatory physiology: A meta-analysis of human neuroimaging studies. Neuroscience & Biobehavioral Reviews 94:76–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Buckner RL, Krienen FM, Yeo BTT (2013) Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci 16:832–837 [DOI] [PubMed] [Google Scholar]
- 103.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ (2011) Anxiety Dissociates Dorsal and Ventral Medial Prefrontal Cortex Functional Connectivity with the Amygdala at Rest. Cereb Cortex 21:1667–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marsland AL, Kuan DC-H, Sheu LK, Krajina K, Kraynak TE, Manuck SB, Gianaros PJ (2017) Systemic inflammation and resting state connectivity of the default mode network. Brain Behav Immun 62:162–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jennings JR, Sheu LK, Kuan DC-H, Manuck SB, Gianaros PJ (2016) Resting state connectivity of the medial prefrontal cortex covaries with individual differences in high‐frequency heart rate variability. Psychophysiol 53:444–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sheu LK, Jennings JR, Gianaros PJ (2012) Test–retest reliability of an fMRI paradigm for studies of cardiovascular reactivity. Psychophysiol 49:873–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bremner JD, Campanella C, Khan Z, et al. (2018) Brain Correlates of Mental Stress-Induced Myocardial Ischemia. Psychosom Med 80:515–525*This recent brain imaging study is one of the first to examine stressor-evoked brain activity and myocardial ischemia in patients with coronary artery disease, finding that patients with stress-induced ischemia exhibited increased activity in regions including the anterior cingulate cortex.
- 108.Edmondson D, Richardson S, Falzon L, Davidson KW, Mills MA, Neria Y (2012) Posttraumatic Stress Disorder Prevalence and Risk of Recurrence in Acute Coronary Syndrome Patients: A Meta-analytic Review. PLOS ONE 7:e38915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dutcher JM, Creswell JD Behavioral interventions in health neuroscience. Annals of the New York Academy of Sciences. doi: 10.1111/nyas.13913 [DOI] [PMC free article] [PubMed] [Google Scholar]


