Abstract
Objective:
Apply the NIH Stage Model to design and test an intervention to prevent depression in breast cancer patients at risk for depression.
Methods:
We identified mindful emotion awareness, along with approach and avoidance strategies for cancer-related coping and emotion regulation, as targets for a preventive intervention adapted from the Unified Protocol for Transdiagnostic Treatment of Emotional Disorders. Patients’ preferences for individual, in-person, and time-efficient sessions informed the design. Patients at risk for depression received a six-week, five-hour intervention with daily exercises. Intervention targets were assessed at baseline, before each session, and four weeks post-intervention. Mixed effects ANOVA assessed change over the follow-up period, controlling for age, partnered status and disease stage.
Results:
Fifty-five percent (40/72) of women screened within 6 months of diagnosis had elevated depression risk. Of these, 24 (60%) signed consent. Sixteen received intervention after five were excluded for current depressive disorder, cognitive impairment or death. Three dropped out. Ninety-eight percent attendance and 77% practice days indicated feasibility. Effect sizes (Cohen’s d) corrected for regression to the mean (RTM) were 0.82 for cancer-related acceptance coping, 0.65 for cancer-related emotional expression, and 0.32 and 0.42 for decreased cancer-related avoidance coping and depressive symptoms, respectively. Effect sizes for variables lacking data to correct for RTM were 1.0, 0.7 and 0.5 for decreased rumination, experiential avoidance and fear of depression and 1.3, 0.6 and 0.4 for increased cognitive flexibility, distress tolerance and describing/not judging emotions.
Conclusions:
The feasibility of this intervention and malleability of its targets support its further investigation.
Keywords: breast cancer, depression, coping, prevention, intervention, oncology
Introduction
Women with breast cancer are three times more likely than their healthy counterparts to develop clinically significant depression in the year following diagnosis.1 They not only experience lower quality of life, but also are at greater risk for medical comorbidities, incur higher medical care costs2 and have shorter survival after diagnosis3 relative to non-depressed survivors. Although depression can be treated effectively in cancer patients4,5, prevention eliminates the substantially increased risk for recurrent depression after a first major depressive episode.6 In the research that served as the foundation for the present trial, 56% of depressive episodes in breast cancer patients were the patients’ first episodes.7,8
This paper describes the second phase of the My Year After Cancer (MYA) project. We aimed to design and test an intervention for the prevention of major depressive disorder and persistent depressive symptoms in recently diagnosed breast cancer patients who are at elevated risk for depression. The project activities were guided by the NIH Stage Model9 that incorporates basic science questions of mechanisms into every stage of clinical science. (https://www.nia.nih.gov/research/dbsr/stage-model-behavioral-intervention-development). Stage 0 of the NIH Model involves basic science and research to identify mutable targets for intervention. Accordingly, the MYA project developed and tested a biopsychosocial model of risk and protective factors and processes for depression as they unfold during the year after breast cancer diagnosis and used these data to create a brief depression risk screener.10,11
NIH Model Stage 1A:
Identifying Scientific Findings Relevant to the Refinement of an Intervention
The MYA longitudinal investigation included 460 women recently diagnosed with breast cancer assessed frequently for one year.7 Machine learning methods identified 7 questions that quantify loneliness, low acceptance of emotion and neuroticism as well as depression and anxiety symptoms at study entry to create a risk screening questionnaire with high sensitivity and specificity for persistent depressive symptoms and major depressive episodes (MDE) in the following year.10 The resulting Depression Risk Questionnaire-7 (DRQ-7) distinguishes 50% of women at substantial depression risk, from others who are not in need of a preventive intervention.
Risk for depression varies in part as a function of the strategies women use to pursue their goals while navigating around and through obstacles in their internal and external environments. Coping and emotion regulation (ER) processes are used to initiate, delay, terminate, modify the form or content, or modulate the amount or intensity of a person’s cognitive, emotional, behavioral or physiological reactions to obstacles to goal attainment.12 They include many of the same strategies but it is rare for both to be measured in the same study. Coping measures responses to particular stressful circumstances whereas emotion regulation quantifies a person’s response to the presence of an emotion whether or not the emotion arises in response to a stressor. As such, coping strategies aimed at emotions related to breast cancer could be considered a subset of the emotion regulation strategies used across a broader range of situations.13,14
Basic motivational systems of approach and avoidance shape both coping and ER processe15,16 and are known empirically to capture broad differences within both of them.17 Approach-oriented strategies involve active efforts to accept, manage, and/or confront a stressor or the emotions, thoughts or behaviors evoked by a specific stressor or general life circumstances. Avoidant strategies include cognitive and behavioral efforts to avoid a stressor or the emotions, thoughts or behaviors evoked by a specific stressor or general life circumstances.
Meta-analyses and systematic reviews demonstrate that coping and emotion regulation processes predict depression and anxiety over time in clinical and normative population samples.18 A meta-analysis of coping processes in breast cancer patients13 and other meta-analyses in adults with cancer indicate consistent relations of avoidant coping with higher depression19,20 whereas approach-oriented coping processes predict more favorable outcomes.21 In addition, onset and maintenance of depressive disorders is also associated with lower emotional awareness.22 Non-judgmental awareness of emotions that can be described and tolerated facilitates emotion regulation as it makes emotions more salient and available for reflection and response.23,24 Taken collectively, coping and emotion regulation research suggests strategies to decrease avoidance and to promote specific approach-oriented strategies are promising targets for preventing depression.25 Preliminary evidence shows changes in ER and coping processes mediate the effects of psychotherapy for resolution of depression, but more rigorously designed studies are needed to establish causal priority.22,25,26 To our knowledge, these mediating targets have not been tested in preventive interventions.
Based on this conceptual and empirical foundation, we selected three sets of mechanistic targets for an intervention to reduce the risk of depression: 1) Increased Mindful Awareness of Emotion (describe experience, non-judging stance toward emotions, distress tolerance); 2) Increased Approach-Oriented Strategies (cognitive skills, cancer-related coping through acceptance and expression of emotions); and 3) Decreased Avoidance-Oriented Strategies (cancer-related coping through avoidance, experiential avoidance, fear of depression, rumination).
NIH Model Stage IA: Adaptation of an Intervention
Breast Cancer Patient Interest in and Preferences for a Preventive Intervention
We conducted a small study of 36 women to assess their interest and preferences for intervention to prevent depression. We assessed consecutive breast cancer patients within four months of diagnosis at the MYA sites. Most patients (36/39; 92%) agreed to screening, and 18 (50%) of those had elevated Depression Risk Questionnaire-7 (DRQ-7)10 scores. Seventeen (94%) answered “yes” regarding their desire to talk with a professional about their experience of cancer, reporting that they would devote an average of 5 hours (from the range of 1–2 to 11–12 hours) to the program. The two most frequently endorsed modes of delivery were individual sessions in person or by phone (versus several other modes, including group format and reading material plus at-home and internet delivery).
Selection and Modification of an Intervention
The Unified Protocol for Transdiagnostic Treatment of Emotional Disorders (UP) was chosen as the foundation for this preventive intervention because it targets the awareness, coping and emotion regulation strategies identified as targets for change in Stage 0.27,28 In addition, it was documented to be effective for reducing clinically significant anxiety and depression in a large, comparative effectiveness trial.27 The consultant for adaptation of the UP was Shannon Sauer-Zavala of the Center for Anxiety and Related Disorders at Boston University, where she works with David Barlow to develop and test the UP.
The Unified Protocol for Prevention of Depression After Cancer (UP-PDAC) is an adaptation of the UP for breast cancer patients with elevated risk for depression. It includes an initial module in which the patient describes her emotional responses to breast cancer to the trained therapist. Table 1 describes seven additional modules in the UP-PDAC, six of which were directly adapted from the UP. Online Supplement 2 contains the table of contents for the Therapist Guide for the UP, which describes its modules. The other module unique to the UP-PDAC is an overall review of patients’ practice of approach and avoidance strategies between the third and the fourth (final) intervention sessions, to inform their plan for future use.
Table 1.
Intervention Sessions, Modules with Targeted Strategies and Measures
|
Session #1 Increase Mindful Awareness (phone) |
Intervention Module 1: Personal Story of Emotional Response to Breast Cancer 2: Psychoeducation and Monitoring Emotional Experiences |
Targeted Strategies and Measures Describe emotions and thoughts – FFMQ Non-judging stance toward emotions and thoughts –FFMQ Distress tolerance - DTS |
|
#2 Increase Approach (in person) |
3: Approach coping and mindfulness 4: Cognitive Flexibility |
Cancer-related acceptance – COPE ACCEPTANCE Cancer-related emotion expression – EAC Notice/reappraise thinking – UP CSQ |
|
#3 Reduce Avoidance (in person) |
5: Countering Emotion-Driven Behaviors 6. Emotional Exposure |
Cancer-related mental and behavioral disengagement & denial – COPE-AVOID Rumination - RRQ Experiential avoidance – MEAQ Reduce fear of depression – ACS |
|
#4 Retention of Changes (phone) |
7: Maintaining Emotional Approach and Low Avoidance 8: Anticipating Barriers and Moving Forward |
FFMQ = Five Factor Mindfulness Questionnaire: Describe and Non-judging Subscales33
DTS = Distress Tolerance Scale34
COPE Inventory = Cancer-related Acceptance and Avoidance Subscales35
EAC – Emotion Approach Coping: Emotion Expression Subscale36
UP CSQ = Unified Protocol Cognitive Skills Questionnaire37
RRQ = Rumination subscale of the Rumination and Reflection Questionnaire38
MEAQ = Multidimensional Experiential Avoidance: Avoidance and Repression Subscales39
ACS = Affective Control Scale: Depression Subscale40
Sixteen hourly sessions are provided in the original UP. UP-PDAC is a 5-hour intervention delivered to individual patients over a total of four sessions during a 6-week period, with two weeks between sessions. Two sessions are held in person and two by phone. The phone sessions last approximately 45 minutes. The in-person sessions last approximately two hours with a 10–15 minute break halfway, with the goal of efficiency, allowing more content in fewer sessions. The reduced number and duration of sessions were expected to be appropriate for a preventive intervention, compared to the UP, which addresses anxiety and depressive disorders.
Each UP-PDAC module includes psychoeducation, demonstration, practice and feedback on the use of each targeted coping and emotion regulation strategy. Prior to the initial intervention session, women received by mail a workbook containing worksheets for recording their practice each day in each of three, two-week homework intervals. The workbook is reviewed at the beginning of each session. The workbook also contains instructions and optional readings on content learned in the corresponding session.
Primary Targets and Measures
Table 1 shows the intervention modules delivered in sessions 1–3, along with the heuristic model of the strategies they target and the measures thereof. Session 4 consolidated all strategies for making a maintenance plan. Depressive symptoms were also measured; only modest effects were expected, however, as main depression outcomes would only be measurable in a larger and longer study in which onset or persistence of depression could be assessed.
Stage IB: Pilot Testing
Study Design
This was a single-arm intervention study in women within 6 months of breast cancer diagnosis. Cancer-related emotional distress is highest during this phase of the cancer trajectory7, so an intervention to prevent depression is expected to be most effective during this time. Measures of cancer-related coping and ER targets as well as depressive symptoms, were collected at baseline, before each session, and four weeks after the UP-PDAC.
The estimated sample size of 15–20 participants was based on estimated 90% power to detect moderate effect sizes of the intervention on targets, recognizing the single-arm study is not designed to draw conclusions about intervention efficacy. Based on previous experience,7,29 we anticipated: 1) 80% of patients would agree to be screened for depression risk, 2) 50% of those would score as high risk, 3) 10% of high-risk patients would have current MDE, 4) 50% of high-risk patients without current MDE would agree to participate, 5) patients who enrolled in the intervention would attend 90% of intervention sessions and complete 70% of recommended home practice, and 6) the intervention could be delivered with 90% fidelity to protocol. We anticipated low attrition once participants were engaged in UP-PDAC, as it was specifically designed to link participation to an important concern of the particular woman (her own emotional experience), and to accommodate this significantly burdened population by limiting in-person sessions to two occasions that could be scheduled in conjunction with the patients’ other visits to the oncology setting. The sample size was not adjusted for attrition but a larger study would need to take this into consideration.
Interventionist Training and Fidelity Assessment
Two masters-level therapists were trained to deliver the UP-PDAC by PI Weihs, who completed UP certification training at the Center for Anxiety and Related Disorders. Two-hour training sessions on each of the UP-PDAC modules included content review, practice delivering the psychoeducational material, and role-playing skill practice with a mock breast cancer patient. Dr. Weihs monitored fidelity to the protocol by listening to audio recordings of all intervention sessions and provided feedback to the interventionists on a weekly basis.
Eligibility Criteria
Eligibility required elevated depression risk indicated by a score of ≥ 6/23 on the DRQ-7. This 7-item screener has 0.68 positive predictive value, and 0.86 negative predictive value for clinically significant depression in the year after breast cancer diagnosis. Its C-statistic was 0.85 in a cross-validation sample.10
Other inclusion criteria were 1) ability to read and speak English at a 6th grade level, 2) no observable evidence of dementia, 3) no current MDE, 4) agreement not to initiate new depression treatment during the study, 5) agreement not to change antidepressant medication or regular psychotherapy during study participation. Other cancer support activities were allowed and patients with a previous diagnosis or treatment of MDE were eligible.
Recruitment
Procedures were approved by the Scientific Review Committee at the University of Arizona Cancer Center (UACC) and by the Human Participants Protection Program at the University of Arizona (Protocol Number 1706593027R00) which conforms to the Declaration of Helsinki standards. Recruitment was done in person by the interventionists at the clinic, providing a personal acquaintance prior to data collection and the first telephone intervention session. Patients with elevated depression risk provided written informed consent prior to data collection.
Enrollment Procedures and Measures
Women provided their cancer diagnosis and stage, mental health history, previous mental health services, and demographic information. Participants who agreed to complete internet-based questionnaires did so within two days prior to each intervention session and four weeks after the final session. Two women requested and completed paper questionnaires. Online Supplement 1 provides psychometrics for the questionnaires (See Table 1), as well as instructions and example items for each scale. The Mood Disorders section of the Structured Clinical Interview for the DSM-5 (SCID-5)30 was administered prior to the intervention, and referrals for treatment were made for those with depressive disorders.
At each intervention session, the therapist recorded the number of home practice days from the workbook and made a participative rating indicating the participant’s “Grasp” (How well did the participant grasp the concepts from the last session/homework?) and “Use” (How much did the participant use and integrate the concepts into her life?) on a 5-point Likert scale (1 = Not at all, 3 = Well enough, 5 = Very well/very much).
Outcomes
Tables 1 and 2 list the 10 targets of the intervention and their corresponding measures. The CES-D31 was used as a measure of depressive symptoms.
Table 2.
Change in Cancer-related Coping and Emotion Regulation Strategies Over Time in EMERGE
| Strategy | Measure | F value (df) | p value |
d | d RTM |
Baseline | Estimated Marginal Means (St Er) | Post Tx | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre S2 | Pre S3 | Pre S4 | |||||||||
| Awareness | Describe Emotions&Thoughts | FFMQ | 0.516 | 0.724 | 0.4 | NA | 29.53(1.88) | 30.00 (1.88) | 30.60 (1.88) | 31.52 (1.90) | 31.86(1.91) |
| (4/53.29) | |||||||||||
| Distress Tolerance | DTS | 1.183 | 0.33 | 0.6 | NA | 20.27 (1.49) | 22.4 (1.49) | 22.87 (1.49) | 23.97 (1.53) | 24.21 (1.54) | |
| (4/48.46) | |||||||||||
| Non-judging Stance | FFMQ | 0.967 | 0.434 | 0.43 | NA | 28.8 (1.63) | 30.0 (1.63) | 30.6 (1.63) | 29.70(1.67) | 31.90 (1.68) | |
| (4/49.52) | |||||||||||
| Approach | Cancer-related Acceptance** | COPE ACC | 4.099 | 0.006 | 1.31 | 0.82 | 3.15 (.13) | 3.48 (.12) | 3.47 (.13) | 3.72 (.13) | 3.75 (.13) |
| Hgjghkhui;puoi | |||||||||||
| Cancer-related Emo Express* | COPE EXP | :Kjl;u9oip; | 0.02 | 0.84 | 0.65 | 2.60 (.20) | 2.52 (.20) | 2.80 (.20) | 3.14 (.20) | 3.2 (.20) | |
| (4/52.98) | |||||||||||
| Cognitive Skills* | UP CSQ | 4.65 | 0.033 | 1.34 | NA | 22.93 (1.2) | 23.5 (1.17) | 26.9 (1.2) | 27.8 (1.2) | 29.4(1.2) | |
| (4/50.46) | |||||||||||
| Avoidance | Cancer-related Avoidance* | COPE AVD | 3.06 | 0.025 | 0.62 | 0.32 | 1.93 (.12) | 1.84 (.13) | 1.62 (.12) | 1.54 (.12) | 1.50 (.12) |
| (4/51.15) | |||||||||||
| Rumination | RRQ | 2.527 | 0.053 | 1.04 | NA | 3.74 (.22) | 3.57 (.22) | 3.11 (.22) | 3.05 (.23) | 2.89 (.23) | |
| (4/48.64) | |||||||||||
| Experiential Avoidance* | MEAQ | 2.763 | 0.037 | 0.46 | NA | 3.4 (.21) | 3.50 (.21) | 2.94 (.21) | 2.97 (.22) | 2.94 (.22) | |
| (4/51.38) | |||||||||||
| Fear of Depression | ACS | 10.16 | 0.007 | 0.69 | NA | 24.3 (2.39) | NA | NA | NA | 17.6 (2.50) | |
| (1/13.35) | |||||||||||
| Depressive Symptoms | CES-D | 1.953 | 0.116 | 0.95 | 0.42 | 15.73 (1.36) | 16.67 (1.36) | 13.87 (1.36) | 13.82 (1.39) | 12.31 (1.40) | |
| (4/50.67) | |||||||||||
p < .05.
p < .01.
p < .001
All models adjusted age, partnered status and disease stage.
Data Analysis
Feasibility outcomes were summarized using proportions. All outcome measures were continuous and analyzed using mixed effects repeated measures ANOVA to test change over the intervention course, controlling for age, partnered status and disease stage.
Effect sizes, calculated as standardized differences between baseline and follow-up means, were adjusted for regression to the mean where feasible, using the formula (1-r)*(μp-μs) where r is the intra-individual correlation, μp is the population mean and μs is the sample mean.32 Reliable population mean estimates for this specific population were available for the CES-D and the three COPE measures from our recently reported study. Such estimates were not available for other measures and thus unadjusted effect sizes are reported. Pearson correlations between CES-D and target measures at baseline and posttreatment were computed.
Results
Feasibility
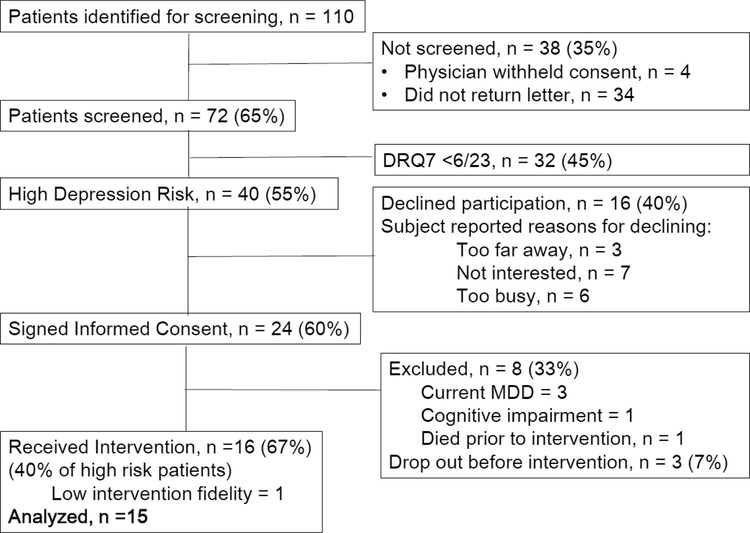
Figure 1 shows 65% (expected 80%) of eligible patients were screened, with 98% agreeing when approached in the clinic but only 40% responding by mail. Seventy-two patients were screened, of whom 40 (55%) met DRQ-7 scoring criteria, similar to the expected rate of 50%. Of these, 24 (60%; expected 50%) signed consent. Patients with current depressive disorder (3), cognitive impairment (1) and death (1) were excluded and three dropped out before receiving intervention, leaving 16 who received UP-PDAC. Data from the first participant were excluded due to the therapist’s low (75%) fidelity to protocol delivery criteria, resulting in 15 patients for the analytic sample.
Figure 1.
CONSORT Diagram for the EMERGE Pilot Study
Three of 15 high-risk patients had current MDE; 40% (16/40; expected 50%) of high-risk patients received UP-PDAC. Five of 15 intervention participants were receiving chemotherapy during the study, compared to 2 out of 3 excluded for MDE or death, and none of those who dropped out or had dementia. Patients who enrolled attended 98% (expected 90%) of sessions and recorded home practice on 71% (expected 70%) of days. Therapist fidelity was 90% for the 15 participants.
Participants
UP-PAC participants (N = 15) were predominantly Non-Hispanic White (81%), 57 (SD =9.8) years of age and 3.5 (SD =1.44) months post-diagnosis. Disease stages were: Stage 0 = 1, Stage 1 = 9, and Stage II = 3, Stage III = 3 and Stage IV = 2. Eight women had a past MDE. Nine (60%) were married/living as married. Nine patients reported receiving medication for depression, sleep problems, or anxiety since the cancer diagnosis, including antidepressants and benzodiazepines.
Participant Compliance with Protocol Activities
Home practice was recorded on 77.1 ± 32.3% of days with a downward trend of 90.5 ± 28.4%, 81.9 ± 30.2%, and 57.6 ± 30.8% for sessions 2, 3 and 4 respectively. Interventionists’ ratings of participants’ “Grasp” and “Use” of the intervention were 3.83 ± 0.75 and 3.83 ± 1.06, respectively (1 = Not at all, 3 = Well enough, 5 = Very well/very much).
Change in Intervention Targets
Table 2 provides ANOVA results and estimated marginal means at each assessment. Effect sizes, adjusted for regression to the mean (RTM), showed a large intervention effect on cancer-related acceptance coping (d=0.82), a medium effect on cancer-related emotional expression coping (d =0.65) and smaller effects on cancer-related avoidance (d =0.32) and depressive symptoms (d =0.42). Adjustment for RTM reduced effect sizes between 23% and 56%. Effect sizes for measures that could not be adjusted for RTM, due to lack of reliable population mean estimates for this specific population, ranged from low-medium [describe /non-judging (d =0.40)] to large [rumination (d =1.04)] to very large [cognitive skills (d =1.34)].
Table 3 shows medium to large magnitudes for correlations of depressive symptoms with measures of awareness, coping and emotion regulation strategies.
Table 3.
Baseline and Post-treatment Correlations of Cancer-related Coping and Emotion Regulation Strategies with Depressive Symptoms
| Tolerate Distress | FFMQ | COPE-Accept | COPE - Express | Cognitive Skills | Rumination | COPE_avoid | Exp. Avoid | Fear of Depress | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post tx | Baseline | Post tx | Baseline | Post tx | Baselin | Post tx | Baseline | Post tx | Baseline | Post tx | Baselin | Post tx | Baselin | Post tx | Baseline | Post tx |
| −0.375 | −0.468 | −0.256 | −0.475 | −0.354 | −0.521 | 0.111 | −.721** | −0.486 | −0.491 | 0.573* | 0.623* | 0.169 | 0.246 | 0.334 | 0.601* | 0.361 | 0.675** |
p < .05.
p < .01.
p < .001
Discussion
Delivery of the UP-PDAC to 40% of recently diagnosed breast cancer patients at elevated depression risk, while one third were in active chemotherapy treatment and with 98% attendance at intervention sessions, demonstrates the feasibility of this individually-delivered preventive intervention. It also supports the use of patient preferences to design a parsimonious intervention that is low in use of resources and participant burden.
The low-moderate to large effects of the UP-PDAC on the empirically selected targets highlight the value of careful intervention design as guided by the NIH Stage Model. Measures of cancer-related coping received the strongest support as malleable targets in this single-arm pilot study of the UP-PDAC, which supports the use of measures that query participants’ experiences in the cancer context. Substantial intervention effects on cognitive skills and emotion regulation strategies supports the use of non-cancer specific assessments to capture intervention effects, as well.
Several discoveries from the project can be used to improve the next phases of intervention refinement and testing. First, successful screening of potentially eligible patients for depression risk required they be screened in clinic, as attempts to do so by mail were not successful. Second, therapists needed experience with delivery of the intervention in the clinical setting to achieve fidelity to the protocol, as skills demonstrated during role plays were not effectively delivered in the clinic until actual implementation and feedback occurred. Third, the plan for participants’ skill practice following the third intervention session could benefit from revision to boost practice during the subsequent two weeks, as it dropped considerably to 58% compared to 85% of days practiced after the first two intervention sessions in this pilot study.
Limitations
Although very promising, the size and single-arm design of this trial precludes causal conclusions regarding the efficacy of UP-PDAC. In addition, all measures were self-report and thus susceptible to bias and demand characteristics. The measures are previously validated and widely used, however.
Conclusions
The step-by-step approach of the NIH Stage Model produced the UP-PDAC and a single arm pilot study showed it is feasible for delivery during the stressful treatment phase shortly after breast cancer diagnosis. Substantial effects of this intervention on targets that are known to be related to the onset and maintenance of depression suggest that it warrants further development and testing. If further studies extend these results, the UP-PDAC may become an evidence-based approach to reduce the risk of clinical depression and its complications for cancer patients.
Supplementary Material
Funding:
Supported by NIH R01 CA133081 (Stanton & Weihs, co-PIs); Breast Cancer Research Foundation BCRF-18-153 (Stanton, PI); NCI-NIH P30CA023074 (Alberts, PI); NCI-NIH P30 CA 16042 (Crespi, PI: Teitel)
References
- 1.Suppli NP, Johansen C, Christensen J, Kessing LV, Kroman N, Dalton SO. Increased risk for depression after breast cancer: a nationwide population-based cohort study of associated factors in Denmark, 1998–2011. J. Clin. Oncol 2014;32(34):3831–3839. [DOI] [PubMed] [Google Scholar]
- 2.Mausbach BT, Yeung P, Bos T, Irwin SA. Health care costs of depression in patients diagnosed with cancer. Psychooncology 2018;27(7):1735–1741. [DOI] [PubMed] [Google Scholar]
- 3.Kanani R, Davies EA, Hanchett N, Jack RH. The association of mood disorders with breast cancer survival: an investigation of linked cancer registration and hospital admission data for South East England. Psychooncology 2016;25(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart SL, Hoyt MA, Diefenbach M, et al. Meta-analysis of efficacy of interventions for elevated depressive symptoms in adults diagnosed with cancer. J. Natl. Cancer Inst 2012;104(13):990–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker J, Hansen CH, Martin P, et al. Integrated collaborative care for major depression comorbid with a poor prognosis cancer (SMaRT Oncology-3): a multicentre randomised controlled trial in patients with lung cancer. Lancet Oncol 2014;15(10):1168–1176. [DOI] [PubMed] [Google Scholar]
- 6.Bifulco A, Bernazzani O, Moran PM, Ball C. Lifetime stressors and recurrent depression: preliminary findings of the Adult Life Phase Interview (ALPHI). Soc. Psychiatry Psychiatr. Epidemiol 2000;35(6):264–275. [DOI] [PubMed] [Google Scholar]
- 7.Stanton A, Wiley J, Krull J, et al. Depressive episodes, symptoms, and trajectories in women recently diagnosed with breast cancer. Breast Cancer Res. Treat 2015;154:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanton AL, Wiley JF, Krull JL, Crespi CM, Weihs KL. Cancer-related coping processes as predictors of depressive symptoms, trajectories, and episodes. J. Consult. Clin. Psychol 2018;86(10):820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onken L, Carroll K, Shoham V, Cuthbert B, Riddle M. Reenvisioning clinical science. Clinical Psychological Science 2014;2(1):22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weihs KL, Wiley JF, Crespi CM, Krull JL, Stanton AL. Predicting future major depression and persistent depressive symptoms: Development of a prognostic screener and PHQ4 cutoffs in breast cancer patients Psychooncology 2018;27:420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer MR, Wiley JF, Weihs KL, Stanton AL. Stuck in the spin cycle: Avoidance and intrusions following breast cancer diagnosis. Br. J. Health Psychol 2017;22(3):609–626. [DOI] [PubMed] [Google Scholar]
- 12.Compas BE, Jaser SS, Bettis AH, et al. Coping, emotion regulation, and psychopathology in childhood and adolescence: A meta-analysis and narrative review. Psychol. Bull 2017;143(9):939–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kvillemo P, Branstrom R. Coping with breast cancer: a meta-analysis. PLoS ONE [Electronic Resource] 2014;9(11):e112733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conley CC, Bishop BT, Andersen BL. Emotions and Emotion Regulation in Breast Cancer Survivorship. Healthcare 2016;4(3):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol. Bull 2000;126(6):890–909. [DOI] [PubMed] [Google Scholar]
- 16.Tobin DL, Holroyd KA, Reynolds RV, Wigal JK. The hierarchial factor structure of the coping strategies inventory 1989;13(4):343–361. [Google Scholar]
- 17.Roth S, Cohen LJ. Approach, avoidance, and coping with stress. American Psychologist, 41, 813.-. . 1986;41:813–819. [DOI] [PubMed] [Google Scholar]
- 18.S E, H K, Mouldin R, B S, M H, SP K. Emotion regulation as a transdiagnostic treatment construct across anxiety, depression, substance, eating and borderline personality disorders: A systematic review. Clin. Psychol. Rev 2017;57:141–163. [DOI] [PubMed] [Google Scholar]
- 19.Moskowitz J, Hult J, Bussolari C, Acree M. What works in coping with HIV? A meta-analysis with implications for coping with serious illness. Psychol. Bull 2009;135:121–141. [DOI] [PubMed] [Google Scholar]
- 20.Roesch S, Adams L, Hines A, et al. Coping with prostate cancer: A meta-analytic review. J. Behav. Med 2005;28(281–93). [DOI] [PubMed] [Google Scholar]
- 21.Greer J, Jacobs J, El-Jawahri A, et al. Role of patient coping strategies in understanding the effects of early palliative care on quality of life and mood. J. Clin. Oncol 2018;36(53–60). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Visted E, Vollestad J, Nielsen MB, Schanche E. Emotion regulation in current and remitted depression: A systematic review and meta-analysis Front. Psychol 2018;9:756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.V P, D C, S T, P E, B. J Neural effects of mindfulness-based interventions on patients with major depressive disorder: A systematic review. Neurosci. Biobehav. Rev 2018;88:98–105. [DOI] [PubMed] [Google Scholar]
- 24.Naragon-Gainey K, McMahon T, Chacko T. The structure of common emotion regulation strategies: A meta-analytic examination. Psychol. Bull 2017;143(4):384–427. [DOI] [PubMed] [Google Scholar]
- 25.Harvey PD, Jacobson W, Zhong W, et al. Determination of a clinically important difference and definition of a responder threshold for the UCSD performance-based skills assessment (UPSA) in patients with major depressive disorder. J. Affect. Disord 2017;213:105–111. [DOI] [PubMed] [Google Scholar]
- 26.van der Velden AM, Kuyken W, Wattar U, et al. A systematic review of mechanisms of change in mindfulness-based cognitive therapy in the treatment of recurrent major depressive disorder. Clin. Psychol. Rev 2015;37:26–39. [DOI] [PubMed] [Google Scholar]
- 27.Barlow D, Farchione T, Bullis J, et al. The Unified Protocol for Transdiagnostic Treatment of Emotional Disorders compared to diagnosis-specific protocols for anxiety disorders: A randomized clinical trial. JAMA Psychiatry 2017;74:875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barlow D, Sauer-Zavala S, Farchione T, et al. The Unified Protocol for Transdiagnostic Treatment of Emotional Disorders:Therapist Guide 2nd ed. New York, NY: Oxford University Press; 2018. [Google Scholar]
- 29.Dodds SE, Pace TW, Bell ML, et al. Feasibility of Cognitively-Based Compassion Training (CBCT) for breast cancer survivors: a randomized, wait list controlled pilot study.[Erratum appears in Support Care Cancer. 2015 Dec;23(12):3609–11; PMID: 26349773] Support. Care Cancer 2015;23(12):3599–3608. [DOI] [PubMed] [Google Scholar]
- 30.MB F, JBW W, RS K, RL S Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV) Arlington, VA: American Psychiatric Association; 2015. [Google Scholar]
- 31.Radloff L, Locke B. The community mental health assessment survey and the CES-D scale In: Weissman MMM JK; and Ross CE, ed. Community Surveys of Psychiatric Disorders New Brunswick, NJ: Rutgers University Press; 1986:177–189. [Google Scholar]
- 32.Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annu. Rev. Clin. Psychol 2007;3:1–27. [DOI] [PubMed] [Google Scholar]
- 33.Baer RA, Smith GT, Lykins E, et al. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment 2008;15(3):329–342. [DOI] [PubMed] [Google Scholar]
- 34.McHugh K, Otto M. Refining the Measurement of Distress Intolerance. Behav. Ther 2012;43:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int. J. Behav. Med 1997;4(1):92–100. [DOI] [PubMed] [Google Scholar]
- 36.Stanton AL, Kirk SB, Cameron CL, et al. Coping through emotional approach: scale construction and validation. J. Pers. Soc. Psychol 2000;78(6):1150–1169. [DOI] [PubMed] [Google Scholar]
- 37.Wood B, Conklin L, Cassiello-Robbins C, Sauer-Zavala S. Assessment of skill acquisition in the Unified Protocol: Validation of measures of cognitive flexibility and emotional avoidance. (under review) [Google Scholar]
- 38.Trapnell PD, Campbell JD. Private self-consciousness and the five-factor model of personality: distinguishing rumination from reflection. J. Pers. Soc. Psychol 1999;76(2):284–304. [DOI] [PubMed] [Google Scholar]
- 39.Sahdra BK, Ciarrochi J, Parker P, Scrucca L. Using genetic algorithms in a large nationally representative American sample to abbreviate the multidimensional experiential avoidance questionnaire. Front. Psychol 2016;7:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Treanor M, Erisman SM, Salters-Pedneault K, Roemer L, Orsillo SM. Affect Control Scale norms Acceptance-based behavioral therapy for GAD: effects on outcomes from three theoretical models. Depress. Anxiety 2011;28(2):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.