Abstract
Context
It is uncertain which osteoporosis therapy is more effective: bisphosphonates or denosumab.
Objective
To determine whether denosumab therapy increases bone mineral density (BMD) and reduces fracture risk more so than bisphosphonates in patients with low BMD or osteoporosis.
Methods
The PubMed, Embase, and the Cochrane Library databases were searched through November 2018 for head-to-head, randomized, controlled trials comparing denosumab and bisphosphonates among adult patients with low BMD or osteoporosis. Random-effects models were used.
Results
We identified 10 eligible trials including 5361 participants. Denosumab increased BMD more than bisphosphonate at 12 months (mean difference, 1.42%; 95% CI, 0.95% to 1.89%; P < 0.001) at lumbar spine, 1.11% (95% CI, 0.91% to 1.30%; P < 0.001) at total hip, and 1.00% (95% CI, 0.78% to 1.22%; P < 0.001) at femoral neck. At 24 months, the respective increase differences were 1.74% (95% CI, 1.05% to 2.43%; P < 0.001), 1.22% (95% CI, 0.66% to 1.77%; P < 0.001), and 1.19% (95% CI, 0.65% to 1.72%; P < 0.001). There was no difference in fracture end point at 12 months, but denosumab had a lower osteoporotic fracture incidence than alendronate at 24 months (risk ratio, 0.51; 95% CI, 0.27 to 0.97).
Conclusion
Denosumab improved BMD significantly more than bisphosphonate treatment at the lumbar spine, total hip, and femoral neck at 12 and 24 months. Only one study demonstrated greater osteoporotic fracture reduction with denosumab treatment. Longitudinal studies with longer follow-up and large sample size are needed to confirm the efficacy difference.
A meta-analysis was performed of 10 head-to-head trials comparing the efficacy of denosumab vs bisphosphonates in patients with low bone mineral density or osteoporosis.
Osteoporosis is a chronic, progressive skeletal condition characterized by decreased bone mass and microarchitectural deterioration, leading to increased risk of fracture (1). It is estimated that >9.9 million Americans have osteoporosis and an additional 43.1 million have low bone mineral density (BMD) (2). The annual direct costs of osteoporosis are estimated to reach $25.3 billion by 2025 (3).
Among the currently available osteoporosis therapeutics, bisphosphonates and denosumab are the most widely used (1, 4). Bisphosphonates are the most prescribed antiresorptive agents, which selectively adhere to and remain within bone. When internalized from the bone surface, bisphosphonates inactivate or promote apoptosis of osteoclasts (5). Denosumab is a fully human monoclonal IgG2 antibody that binds to the receptor activator of nuclear factor-κB ligand (RANKL) with high specificity and affinity. Denosumab impairs the development, activation, and survival of osteoclasts, thus inhibiting bone resorption (6). Because of their different mechanisms of action, bisphosphonates typically provide persistent antiresorptive effect after discontinuation, whereas the effect of denosumab on bone turnover is quickly reversible with discontinuation, leading to a transient rebound phenomenon (7). Previous phase 3 clinical trials found both drugs increased BMD and reduced the risk of fracture compared with placebo (8–12). However, the relative efficacy of bisphosphonates or denosumab remains uncertain (13, 14).
Five meta-analyses have compared denosumab and bisphosphonates in the treatment of osteoporosis (13–17) and although current evidence suggests denosumab might increase BMD and reduce fracture risk more than bisphosphonates do, these results are not conclusive. Two of these studies adopted a network meta-analysis design and reported the indirect treatment comparison of denosumab and bisphosphonates (14, 16). The other three meta-analyses included only head-to-head trials (13, 15, 17) and gave results of direct comparisons. However, several key issues remain unresolved. First, results from indirect comparison and direct comparison are inconsistent (16, 17), which deserves further clarification. Second, the efficacy comparison of BMD increase and fracture risk reduction were not well reported at 24 months. Third, direct-comparison meta-analyses did not include all key studies and require an update (15, 17).
Since 2014, five more pivotal head-to-head trials have been published. We performed a direct comparison of denosumab and bisphosphonate efficacy, incorporating these recent studies. In this meta-analysis of head-to-head randomized control trials (RCTs), we aimed to determine whether denosumab is more effective than bisphosphonates in increasing bone mass and reducing fracture risk in patients with low BMD or osteoporosis.
Materials and Methods
The current meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (18, 19). This study did not require ethical approval, because there was no human or animal experiment.
Study inclusion and exclusion criteria
Studies included in this meta-analysis were required to meet the following inclusion criteria for study design, subjects, and intervention: (i) RCT with a duration of at least 12 months; (ii) adult patients diagnosed with osteoporosis (T-score at or below −2.5) or low BMD (T-score between −1.0 and −2.5) receiving the study intervention (1); (iii) denosumab 60 mg subcutaneously every 6 months for at least 12 months (intervention group), or bisphosphonate treatment (comparator group), including alendronate (35 or 70 mg once weekly), ibandronate (150 mg once monthly), risedronate (150 mg once monthly), or zoledronic acid (5 mg infusion once yearly); and (iv) the outcome measurement included the mean percentage change in BMD measured by dual-energy X-ray absorptiometry of lumbar spine, total hip, or femoral neck. Trials were excluded if (i) they were animal studies or the study population included patients with cancer or glucocorticoid-induced osteoporosis, (ii) the same RCT was reanalyzed (i.e., we only included the most complete data of each trial once), (iii) subjects were not randomly allocated to treatments, and (iv) studies were published as abstracts, reviews articles, editorials, and letters.
Information sources and search strategy
We systematically searched PubMed, Embase, and the Cochrane Library from 1 January 1980 until 8 November 2018 with no language restrictions. In addition, relevant studies were obtained by searching references of articles identified in the initial searches, relevant meta-analyses, and systematic reviews. The literature search was performed independently by two authors (H.L., B.J.). Search strategies were developed using text words as well as medical subject headings associated with terms relevant to “osteoporosis,” “denosumab,” and “bisphosphonate,” together with “randomized controlled trial.” The full search strategies used in PubMed, Embase, and Cochrane Library are provided in an online repository (20).
Study selection
Our search records were imported into ENDNOTE X8 reference management software (Clarivate Analytics, Philadelphia, PA), and two authors (H.L., B.J.) independently reviewed the titles and abstracts of the literature searches. Trials that did not meet the eligibility criteria were excluded. After excluding the duplicated and irrelevant articles, the full text of the remaining studies was reviewed to ascertain whether they should be included according to the eligibility criteria. After completion, both authors met and reviewed their selections for agreement. Any disagreements were resolved by discussion or by seeking an independent third author (C.X.).
Data collection process
The available information and outcomes of all the eligible studies were independently extracted by two researchers (H.L., B.J.). The retained data included study characteristics, participant characteristics, type of intervention (type, dose, duration). If data were presented in figures, the GetData software (http://getdata-graph-digitizer.com/index.php) was used to extract data from the figures. The mean and SD, SE, and CI of percentage changes of BMD were extracted for calculation. Disagreements were resolved through discussion.
Outcomes
The primary outcomes were the mean percentage change in BMD at lumbar spine, total hip, and femoral neck at 12 months. The secondary outcomes were: (i) mean percentage change in BMD at lumbar spine, total hip, and femoral neck at 24 months; (ii) overall incidence of vertebral fractures and overall incidence of nonvertebral fractures at 12 and 24 months; and (iii) total adverse events, severe adverse events, and selected adverse events of interest (i.e., severe infection, malignancy, death, adverse events leading to withdrawal, gastrointestinal disorders, and eczema) at 12 months.
Subgroup and sensitivity analyses
A priori specified and exploratory subgroup analyses were performed to examine potential sources of heterogeneity and explore the reasons for inconsistent results between indirect and direct meta-analyses. First, a priori–specified subgroup analysis was performed by grouping studies into those including alendronate vs those including any other bisphosphonates. Second, exploratory subgroup analysis was performed by grouping studies into those including patients who previously received bisphosphonate therapy vs those including patients who did not receive bisphosphonate therapy. And third, we exploratively assessed the route of bisphosphonate administration (oral vs intravenous). Two additional sensitivity analyses for the primary outcomes were performed to examine the heterogeneity by omitting two trials of small sample size from the overall analysis and by omitting five trials including osteopenic populations.
Risk of bias assessment
Two authors (H.L., B.J.) independently assessed the risk of bias using the Cochrane risk-of-bias tool (21). This tool assessed bias across the following seven domains: (i) random-sequence generation, (ii) allocation concealment, (iii) blinding of participants and personnel, (iv) blinding of outcome assessment, (v) incomplete outcome data, (vi) selective reporting, and (vii) other bias. Each domain was determined as low risk, unclear risk, or high risk. For the first four domains, if the trial clearly reported adequate methods, it was regarded as a low risk of bias. However, if the trial did not clearly report the methods, it was regarded as an unclear risk of bias; if the trial inadequately reported methods, it was regarded as a high risk of bias. For incomplete outcome data, we considered ≥20% loss to follow-up to represent a high risk of bias. We assessed selective reporting, by comparing each publication with its corresponding published protocol, when available. For other sources of bias, we considered major imbalances in key baseline characteristics to represent a high risk of bias. Any disagreements were resolved through discussion, adjudicated by another reviewer (C.X.) if necessary.
Statistical analysis
We used a random-effects model to calculate pooled estimates, because heterogeneity was anticipated (22). For the continuous variable (i.e., percentage changes in BMD), weighted mean difference and 95% CIs were calculated. For dichotomous variables (i.e., fracture and adverse events), risk ratio (RR) and 95% CIs were calculated. To assess the heterogeneity of the results from individual studies, Cochran Q statistic, the I2 statistic (I2 > 50% was regarded as substantial heterogeneity), and P values (P < 0.10 was considered as substantial heterogeneity) were used (23). The preplanned subgroup and sensitivity analyses were performed to examine the sources of heterogeneity. Publication bias was assessed visually with a funnel plot and the Egger weighted regression statistic, with P < 0.05 indicating significant publication bias. The meta-analysis was analyzed using the statistical environment R, version 3.4.3 (https://cran.r-project.org) with the “meta” and “metafor” packages (24). All the tests were two tailed and P < 0.05 was deemed statistically significant.
Results
Search results
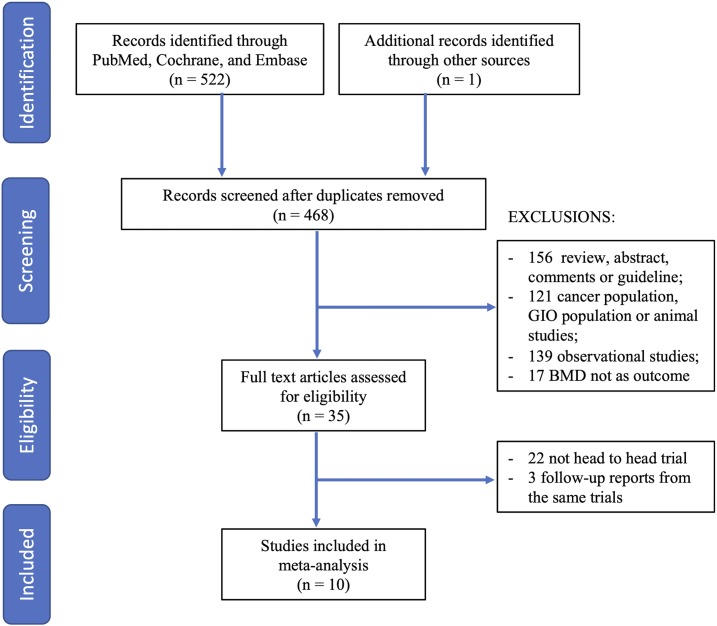
A total of 523 articles were obtained through electronic and manual searches. After 55 duplicates were removed, the titles and abstracts of 468 records were reviewed. Of these, 433 records were excluded for not meeting the inclusion criteria; the remaining 35 articles were retrieved for further assessment. Of the 35 articles, 25 were excluded because they were not head-to-head trials or were follow-up reports of same trial. Ten trials (9, 25–33) fulfilled criteria and were included in the meta-analysis (Fig. 1).
Figure 1.
Flow diagram of the process of literature selection. GIO, glucocorticoid-induced osteoporosis.
Characteristics of included trials
The main characteristics of the included trials are summarized in Table 1. These trials were published from 2006 to 2018 and involved a total of 5361 patients; the sample size ranged from 64 to 1189 patients. Mean age ranged from 63 to 78 years and 99.0% of patients were female. Of the 5361 patients, 1533 (28.6%; n = 3 studies) had no prior osteoporosis treatment, 714 (13.3%; n = 1 study) had previous fractures, 2914 (54.3%; n = 5 studies) received bisphosphonate treatment before the study, and 200 (3.7%; n = 1 study) received teriparatide before the study. The bisphosphonates included alendronate, ibandronate, risedronate, and zoledronic acid. The doses of bisphosphonates were alendronate 70 mg once weekly, ibandronate 150 mg once monthly, risedronate 150 mg once monthly, and zoledronic acid 5-mg infusion once yearly, except one study in which alendronate 35 mg once weekly was used (9.4%; 242 of 2562) (28). The study duration was 12 months for eight trials (9, 25, 26, 27, 29, 30, 31, 33) and 24 months for two trials (28, 32), although only the first 12 months results were used for one trial (32). Six of the 10 studies (9, 25, 26, 28, 32, 33) compared the efficacy of denosumab with alendronate, two studies (30, 31) compared denosumab with zoledronic acid, one study compared denosumab with ibandronate (27) and one study compared denosumab with risedronate (29). All studies reported concomitant administration of daily oral calcium and vitamin D supplements.
Table 1.
Characteristics of Randomized Controlled Trials Comparing Denosumab With Bisphosphonates for Osteoporosis Treatment
| Included Trials (Location) | Treatment Status | Basic Therapy a | Bisphosphonate Type | Denosumab Group (60 mg⁄6 mo) | Bisphosphonate Group | Duration (Mo) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age (SD), y | BMD TH (SD) b | BMD LS (SD) b | No. | Age (SD), y | BMD TH (SD) b | BMD LS (SD) b | Dosage | |||||
| McClung (9) (United States) | Untreated | Ca 1000 mg; Vit D 400 IU | Alendronate | 47 | 63.1 (8.1) | −1.4 (0.8) | −2.2 (0.7) | 47 | 62.8 (8.2) | −2.0 (0.9) | −2.0 (0.9) | 70 mg once weekly for 12 mo | 12 |
| Kendler (26) (international) | Bisphosphonate treated | Ca 1000 mg; Vit D 400 IU | Alendronate | 253 | 66.9 (7.8) | −1.8 (0.82) | −2.6 (0.75) | 251 | 68.2 (7.7) | −1.8 (0.7) | −2.6 (0.8) | 70 mg once weekly for 12 mo | 12 |
| Brown (25) (international) | Untreated | Ca 500 mg; Vit D 400 or 800 IU | Alendronate | 594 | 64.1 (8.6) | −1.8 (0.8) | −2.6 (0.8) | 595 | 64.6 (8.3) | −1.7 (0.8) | −2.6 (0.8) | 70 mg once weekly for 12 mo | 12 |
| Recknor (27) (international) | Bisphosphonate treated | Ca ≥500 mg; Vit D 800 IU | Ibandronate | 417 | 67.2 (8.1) | −1.8 (0.7) | −2.5 (0.9) | 416 | 66.2 (7.8) | −1.8 (0.7) | −2.5 (0.8) | 150 mg once monthly for 12 mo | 12 |
| Nakamura (28) (Japan)c | Previous fractures | Ca ≥600 mg; Vit D 400 IU | Alendronate | 472 | 69.9 (7.4) | −2.0 (0.8) | −2.8 (0.9) | 242 | 70.2 (7.3) | −2.0 (0.8) | −2.7 (0.9) | 35 mg once weekly for 24 mo | 24 |
| Roux (29) (international) | Bisphosphonate treated | Ca ≥1000 mg; Vit D 800 IU | Risedronate | 435 | 67.8 (7.0) | −1.6 (0.9) | −2.2 (1.2) | 435 | 67.7 (6.8) | −1.9 (0.7) | −2.3 (1.1) | 150 mg once monthly for 12 mo | 12 |
| Anastasilakis (30) (Greece)d | Bisphosphonate treated | Ca 1000 mg; Vit D 800 IU | Zoledronic acid | 34 | 63.2 (9.6) | — | −1.9 (1.3) | 30 | 63.3 (10.1) | — | −2.18 (0.9) | 5-mg Infusion once yearly for 12 mo | 12 |
| Miller (31) (international) | Bisphosphonate treated | Ca ≥1000 mg; Vit D ≥800 IU | Zoledronic acid | 321 | 68.5 (7.1) | −1.9 (0.7) | −2.7 (0.8) | 322 | 69.5 (7.7) | −1.9 (0.8) | −2.6 (0.9) | 5-mg Infusion once yearly for 12 mo | 12 |
| Kendler (32) (international) | Untreated | Ca 1000 mg; Vit D ≥400 IU | Alendronate | 126 | 65.1 (7.6) | −1.60 (0.74) | −2.04 (1.16) | 124 | 65.3 (7.7) | −1.60 (0.76) | −1.89 (1.13) | 70 mg once weekly for 12 mo | 12 |
| Niimi (33) (Japan)e | Teriparatide treated | Active or native Vit D in DMAb arm | Alendronate | 100 | 78.0 (8.0) | — | −1.7 (1.6) | 100 | 78.0 (9.0) | — | −1.7 (1.2) | 35 mg once weekly for 12 mo | 12 |
Abbreviations: —, no data; Ca, calcium; DMAb, denosumab; LS, lumbar spine; TH, total hip; Vit D, vitamin D.
The doses in basic therapy were given daily.
T-scores were used.
Included 95% women and 5% men; we used the data of the whole population.
Age and BMD were calculated based on patients who completed the study.
Included 90% women and 10% men; we used the data of the whole population.
Risk of bias assessment
Risk of bias was assessed with the Cochrane risk-of-bias tool (34). Two trials did not clearly report the random sequence generation (29, 31). Three trials (28–30) did not clearly report the allocation concealment. Five trials used an open-label design (27, 29, 30, 32, 33). Blinding of outcome assessment was inadequately reported in only three trials (27, 30, 32). There was a low risk of attrition bias, reporting bias, and other biases in all trials except for one that reported BMD only at one anatomic site (30). Two studies had a relatively small sample size (n = 64 and n = 94) (9, 30).
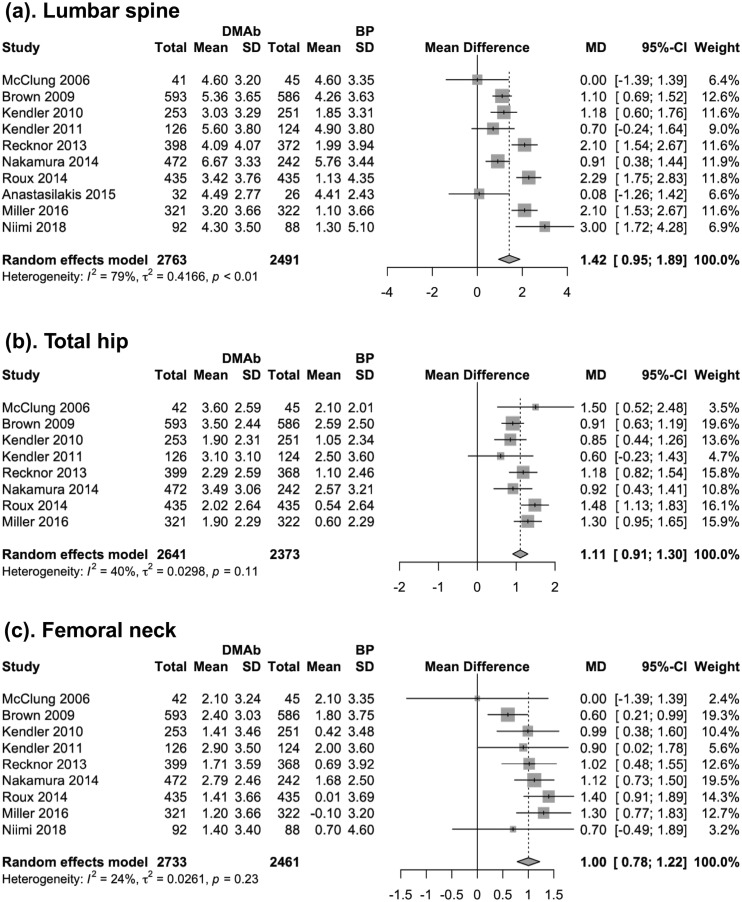
Primary analysis: mean percentage change in BMD at lumbar spine, total hip, and femoral neck at 12 months
The primary analysis involved 10 trials with a total of 5254 patients. Compared with bisphosphonates, the incremental 12-month increase in BMD with denosumab was greater by 1.42% (95% CI, 0.95% to 1.89%; P < 0.001) at lumbar spine, 1.11% (95% CI, 0.91% to 1.30%; P < 0.001) at total hip, and 1.00% (95% CI, 0.78% to 1.22%, P < 0.0001) at femoral neck (Fig. 2). Cumulative meta-analysis (35) showed that more BMD improvement with denosumab became evident in 2010, when 1769 patients had been randomly assigned in trials. At the end of 2010, the mean difference in BMD improvement was 1.05% (95% CI, 0.66% to 1.44%) at lumbar spine, 0.92% (95% CI, 0.70% to 1.15%) at total hip, and 0.68% (95% CI, 0.34% to 1.01%) at femoral neck. Subsequent trials brought the number of patients to 5254, resulting in slightly higher point estimates at the three anatomic sites.
Figure 2.
Forest plot for the mean difference of BMD changes (%) at lumbar spine, total hip, and femoral neck at 12 mo. BP, bisphosphonate; DMAb, denosumab; MD, mean difference.
Sensitivity analyses
After removing five studies including osteopenic populations, the mean BMD increase difference was 1.47% (95% CI, 0.97% to 1.96%) at lumbar spine, 1.04% (95% CI, 0.85% to 1.12%) at total hip, and 0.97% (95% CI, 0.73% to 1.15%) at femoral neck. After removing the two small trials (9, 30), the mean difference increased from 1.42% (95% CI, 0.95% to 1.89%; P < 0.001) to 1.62% (95% CI, 1.14% to 2.09%) at lumbar spine (36). In addition, the mean difference at total hip and femoral neck also increased. After excluding one study in which 5% of study subjects were men (28), results were consistent with the primary analysis.
Subgroup analysis
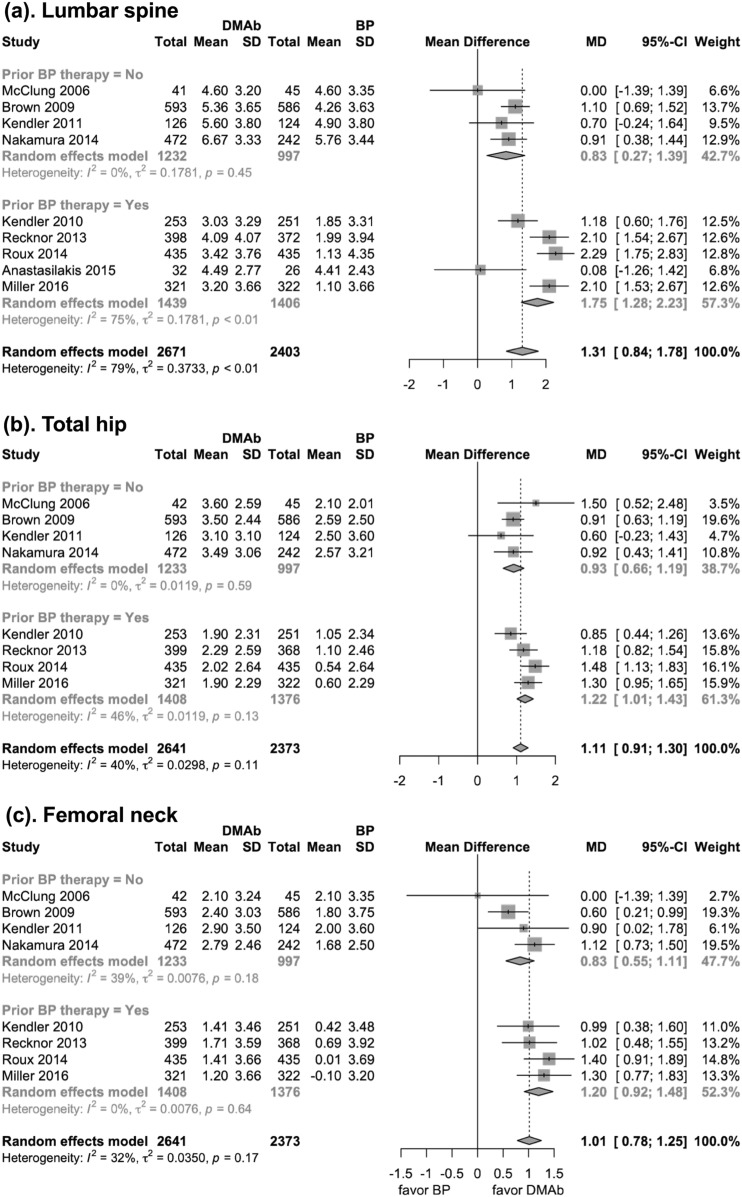
Subgroup analyses were performed on the basis of specific bisphosphonate and pretreatment status. The results showed that denosumab improved BMD more than each of the three oral bisphosphonates (i.e., alendronate, ibandronate and risedronate) at lumbar spine, total hip, and femoral neck. However, compared with zoledronic acid, denosumab only showed significant superiority in total hip and femoral neck BMD improvement (37).
In patients who did not previously receive bisphosphonate treatment, lumbar spine BMD was increased with denosumab treatment more than it was with use of bisphosphonates (mean difference, 0.83%; 95% CI, 0.27% to 1.39%; P < 0.001). In patients who received previous bisphosphonate treatment, denosumab still resulted in greater lumbar spine BMD improvement than they did with bisphosphonates (mean difference, 1.75%; 95% CI, 1.28% to 2.23%; P < 0.001). There was a significant interaction between the subgroups of different bisphosphonate-pretreatment status in lumbar spine BMD improvement (previously treated with bisphosphonates: 1.75% vs not previously treated: 0.83%; P for interaction = 0.014). Similar results were found at total hip (previously treated with bisphosphonates: 1.22% vs not previously treated: 0.93%; P for interaction = 0.089) and femoral neck (previously treated with bisphosphonates: 1.20% vs not previously treated: 0.83%; P for interaction = 0.069), but the subgroup difference was not significant (Fig. 3).
Figure 3.
Forest plot of the BMD changes at the following three anatomic sites at 12 mo (subgroup analysis stratified by previous treatment status): (a) lumbar spine, (b) total hip, and (c) femoral neck. BP, bisphosphonate; DMAb, denosumab; MD, mean difference.
Another subgroup analysis was performed on data on use of alendronate or non-alendronate bisphosphonates. The BMD increase difference was 1.91% (95% CI, 1.36% to 2.47%; P < 0.001) at lumbar spine for alendronate trials and 1.11% (95% CI, 0.63% to 1.58%; P < 0.001) for non-alendronate bisphosphonate use at 12 months (subgroup difference P = 0.031). Similar results were seen at total hip (subgroup difference P = 0.004) and femoral neck (subgroup difference P = 0.040) (38).
Subgroup analyses indicated that heterogeneity could be assumed because of two studies of small sample size (Q = 5.37; P = 0.021) and the difference in the population characteristics, such as different types of bisphosphonate and pretreatment status.
Secondary outcomes
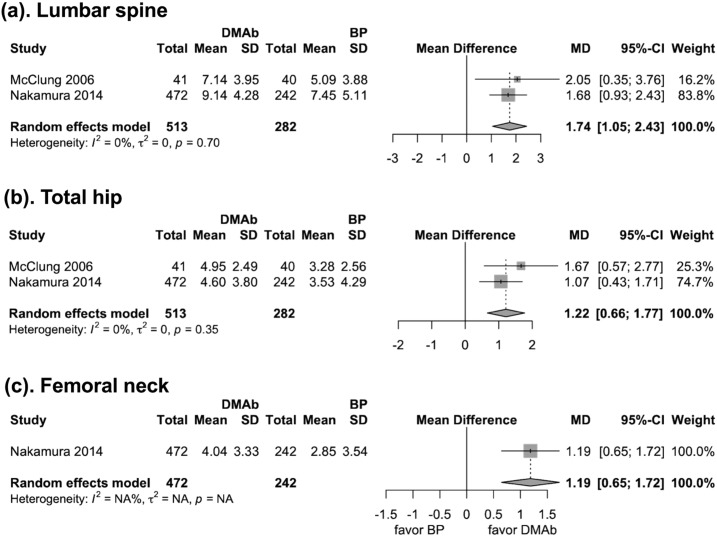
Two trials (n = 795 patients) reported changes in BMD at 24 months. The pooled results showed BMD increase difference between denosumab and bisphosphonate was 1.74% at spine, 1.22% at total hip, and 1.19% at femoral neck, which was slightly higher than the 12-month BMD increase difference (Fig. 4).
Figure 4.
Forest plot for the mean difference of BMD changes (%) at lumbar spine, total hip, and femoral neck at 24 mo. BP, bisphosphonate; DMAb, denosumab; MD, mean difference.
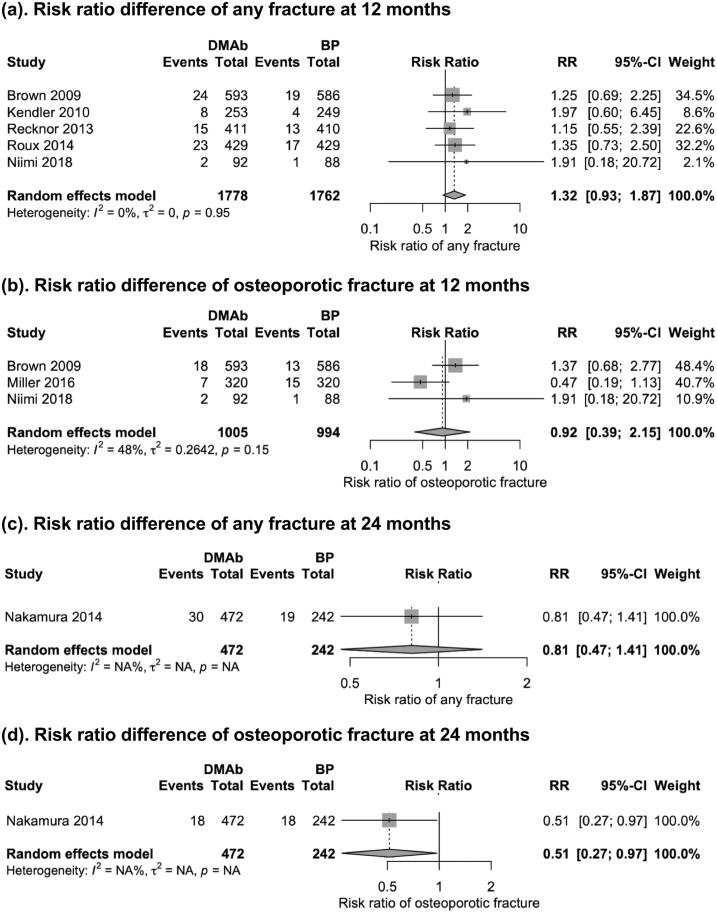
Five trials (25, 26, 29, 31, 33) (n = 3540 patients) reported fracture data at 12 months, and one trial (28) (n = 714 patients) reported fracture data at 24 months. Because of the sparse report of vertebral fractures and nonvertebral fractures, we report the pooled fracture end points: the incidence of any fractures and osteoporotic fractures. Denosumab therapy did not demonstrate significant difference in reducing the risk of any type of fracture (RR, 1.32; 95% CI, 0.93 to 1.87) or osteoporotic fracture (RR, 0.92; 95% CI, 0.39 to 2.15) at 12 months (Fig. 5). Denosumab therapy showed no significant difference in reducing the risk of any type of fracture (RR, 0.81; 95% CI, 0.47 to 1.41) at 24 months. However, denosumab showed better performance in reducing risk of osteoporotic fracture than did alendronate (RR, 0.51; 95% CI, 0.27 to 0.97; Fig. 5).
Figure 5.
Forest plot of any fracture and osteoporotic fractures at 12 and 24 mo. Denosumab therapy did not demonstrate a significant difference in reducing the risk of (a) any type of fracture, (b) osteoporotic fracture at 12 mo, or (c) the risk of any type of fracture at 24 mo, but (d) denosumab was associated with a lower osteoporotic fracture incidence than bisphosphonate. BP, bisphosphonate; DMAb, denosumab; RR, risk ratio.
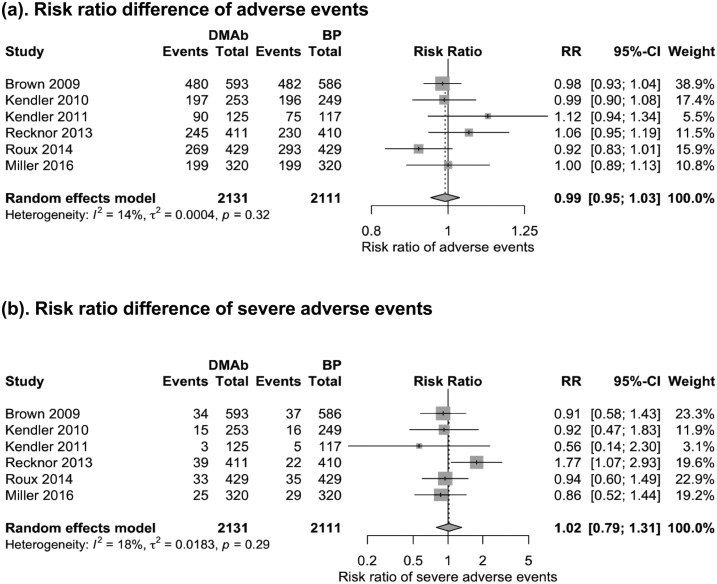
Six trials (25–27, 29, 31, 32) with 4242 patients reported adverse events and severe adverse events at 12 months. Denosumab therapy did not demonstrate a higher risk for adverse events (RR, 0.99; 95% CI, 0.95 to 1.03) or risk for severe adverse events (RR, 1.02; 95% CI, 0.79 to 1.31) than did bisphosphonates therapy (Fig. 6). Risk of selected adverse events of interest, including severe infection (RR, 1.05; 95% CI, 0.61 to 1.80), malignancy (RR, 0.99; 95% CI, 0.66 to 1.50), death (RR, 0.66; 95% CI, 0.16 to 2.63), adverse events leading to withdrawal (RR, 0.62; 95% CI, 0.38 to 1.03), gastrointestinal disorders (RR, 0.99; 95% CI, 0.75 to 1.31), and eczema (RR, 2.01; 95% CI, 0.63 to 6.42), were also similar for denosumab and bisphosphonates (39).
Figure 6.
Forest plot of adverse events at 12 mo. Risk ratio of (a) any adverse events and (b) severe adverse events. BP, bisphosphonate; DMAb, denosumab; RR, risk ratio.
Publication bias
The publication bias of the primary outcomes was assessed using visual examination of funnel plots (40) and the Egger weighted regression statistic (P = 0.671, 0.863, and 0.514 for mean difference of BMD change at lumbar spine, total hip, and femoral neck, respectively); no significant publication bias was indicated. Trim-and-fill results suggested only zero to three missing studies were needed to achieve a symmetrical funnel plot.
Discussion
Main findings
This meta-analysis of head-to-head trials provided evidence of direct comparison between denosumab and bisphosphonates in treatment of low BMD or osteoporosis. Our study provides moderately strong evidence (41) that denosumab is more effective in increasing BMD at lumbar spine, total hip, and femoral neck than are bisphosphonates at 12 and 24 months. However, fracture risk reductions were similar at 12 months. Only one study reported denosumab to have lower osteoporotic fracture incidence than that of alendronate at 24 months. Safety profiles between denosumab and bisphosphonates were similar.
Clinical meaning
The association between BMD increase and fracture risk reduction related to use of antiresorptive agents is very important for osteoporosis treatment initiation and monitoring. In our study, the difference in the 12-month BMD increase between denosumab and bisphosphonate was 1.42% at spine, 1.11% at total hip, and 1.00% at femoral neck. Although these numbers appear small, such differences would translate into clinically important fracture differences if observed in large-enough populations. BMD change, especially hip BMD, is the most important surrogate for evaluation of therapeutic response (42). According to a recent meta-analysis using individual patient data from 21 randomized, placebo-controlled osteoporosis trials of >83,395 subjects, changes in total hip and femoral neck BMD over 2 years explained 60% to 65% of the treatment-related reduction in fracture risk (42). More specifically, for patients who received antiresorptive agents, a treatment-related 6% increase of lumbar spine BMD at 1 year was associated with a 39% reduction in nonvertebral fracture risk, and a 3% increase of hip BMD was associated with a 46% reduction in nonvertebral fracture risk (43). In our study, denosumab and bisphosphonate had different BMD increase profiles. A difference of 1.42% at the lumbar spine may not be associated with clinically significant reduction in fracture risk. However, a difference of 1.11% at total hip BMD and a difference of 1.00% at femoral neck would be expected to yield a 15% to 21% fracture risk reduction difference at 12 months, and larger number would be expected for longer treatment duration (≥24 months).
Comparison with previous studies
A network meta-analysis by Mandema et al. (16) showed that denosumab resulted in greater BMD improvement in lumbar spine and total hip from 12 months to 36 months when compared with alendronate and zoledronic acid. The point estimates of spine BMD–improvement difference for denosumab was only 0.4% greater than for zoledronic acid and 0.8% greater than for alendronate at 12 months. In a head-to-head meta-analysis, Wu et al. (17) reported a pooled estimate of BMD improvement difference of 1.55% at lumbar spine, 1.05% at total hip, and 1.06% at femoral neck. In our study, we included three more studies and excluded two studies from the same trial; the point estimates of the BMD improvement difference were similar to those of the Wu et al. study (17): 1.31% at lumbar spine, 1.11% at total hip, and 1.01% at femoral neck. But the inconsistent results between indirect and direct meta-analyses deserve further clarification. Here, we propose a possible explanation from the aspect of denosumab and bisphosphonates mechanisms.
Denosumab treatment results in increased BMD progressively, as long as the treatment is continued (44). On the other hand, bisphosphonates have a persistent but not progressive antiresorptive effect; bisphosphonate treatment results in increased BMD over the first few years, but then change in BMD plateaus (35). When comparing the effect of denosumab and bisphosphonates, prior treatment with bisphosphonates attenuates the efficacy of subsequent bisphosphonate treatment. This phenomenon may potentially inflate the efficacy difference between denosumab and bisphosphonates, as noted in our exploratory subgroup analysis performed by grouping patients with and without prior bisphosphonate therapy. In our current meta-analysis, the point estimates of BMD improvement difference in treatment-naïve patients was 0.83% at spine, 0.93% at total hip, and 0.83% at femoral neck, which may reflect the true efficacy difference.
In patients previously treated with a bisphosphonate, the difference was 1.75% at spine, 1.22% at total hip, and 1.20% at femoral neck. The larger efficacy difference in the latter subgroup may help us make clinical decisions regarding the sequential use of osteoporosis medication. In clinical practice, the most commonly used antiosteoporosis medication is bisphosphonates. When treatment with the first bisphosphonate is ineffective (e.g., due to unsatisfactory response or fractures), a different medication should be considered. Results of this study suggest that, in patients treated with a prior bisphosphonate, switching to denosumab would result in greater BMD increase than would switching to another bisphosphonate.
Current evidence on the difference in fracture risk reduction between denosumab and bisphosphonates is still limited. A previous network meta-analysis (14) reported that denosumab was more effective than bisphosphonates in preventing new vertebral fractures (RR, 0.62; 95% CI, 0.44 to 0.87) but not in preventing nonvertebral fracture, hip fracture, or wrist fracture. Only one trial reported that denosumab was more effective than alendronate in preventing osteoporotic fractures at 24 months (RR, 0.51; 95% CI, 0.27 to 0.97) (28). A recent observational data analysis showed denosumab was associated with a 23% lower risk of vertebral fracture than was alendronate (HR, 0.77; 95% CI, 0.57 to 1.03) (45). As there is only one RCT demonstrating greater osteoporotic fracture reduction using denosumab (28), future longitudinal studies with longer follow-up and large sample size are needed to confirm the efficacy difference.
The safety profiles were not significantly different between denosumab and bisphosphonates at 12 months. Denosumab does not demonstrate a higher rate of adverse events or severe adverse events than bisphosphonates (Fig. 6).
Strengths and limitations
Although several meta-analyses on this topic have been published, our study has several distinct strengths relative to those. First, we incorporated BMD and fracture data at 24 months and demonstrated better performance of denosumab in reducing osteoporotic fractures at 24 months. Previous meta-analyses either missed key studies [the study with fracture as end point for 2 years (28) and recently published trials (33)] or only focused on 12-month data. Second, all prior meta-analyses overlooked important profiles of the study population, and important subgroup analysis were not performed (15–17), especially prior bisphosphonate treatment status. Our study demonstrated the efficacy difference between denosumab and bisphosphonates in patients who had prior bisphosphonate treatment relative to treatment-naïve patients. This result suggested that if the prior use of bisphosphonate was ineffective, switching to denosumab would improve BMD more so than switching to another bisphosphonate.
However, several limitations should also be noted. There were some methodological limitations in some of the included trials, such as the inadequate concealment of treatment allocation. The quality of evidence for reduced incidence of fracture was only moderate, and only one study reported fracture end points at 24 months. Last, there was significant heterogeneity in some outcomes, because of the various types of bisphosphonates and patient characteristics. Given these limitations, results of this meta-analysis should be interpreted cautiously.
Implications for future studies
Several knowledge gaps remain regarding the comparative effectiveness of denosumab and bisphosphonates. First, evidence of long-term BMD benefit of denosumab compared with bisphosphonates was very limited. Second, only one study reported the efficacy difference on fracture end point between denosumab and bisphosphonates at 2 years; more studies are needed to clarify the fracture risk reduction benefit of denosumab compared with bisphosphonate. Third, whether there would be a response difference to treatment with denosumab between patients previously treated with bisphosphonates and treatment-naïve patients also remains unclear. Studies are needed to address these knowledge gaps.
Conclusion
Denosumab significantly improved the BMD at lumbar spine, total hip, and femoral neck more so than bisphosphonates at 12 and 24 months. Only one study demonstrated greater osteoporotic fracture reduction using denosumab at 24 months. The better performance of denosumab relative to that of bisphosphonates in increasing BMD was found in treatment-naïve patients and patients who previously had received bisphosphonate treatment.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health [Grants NIH-P30-AR072577 (VERITY) and NIH-K24AR055989 to D.H.S.].
Disclosure Summary: H.L. received a scholarship from Chinese PLA General Hospital and received support from Young Scientists Fund of the National Natural Science Foundation of China (Grant 81702176). K.Y. received financial support for his doctoral study from Harvard T.H. Chan School of Public Health (partially supported by training grants from Takeda, Pfizer, Bayer, and ASISA) and Honjo International Scholarship Foundation. D.H.S. receives salary support from the National Institutes of Health (Grant NIH-K24AR055989) and has research contracts with Amgen focused on rheumatoid arthritis. S.K.T. received support from the Lupus Foundation of America Career Development Award.
Glossary
Abbreviations:
- BMD
bone mineral density
- RCT
randomized control trial
- RR
risk ratio
References
- 1. Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R; National Osteoporosis Foundation . Clinician’s guide to prevention and treatment of osteoporosis [published correction appears in Osteoporos Int. 2015;26(7):2045–2047] Osteoporos Int. 2014;25(10):2359–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17(Suppl 6):S164–S169. [PubMed] [Google Scholar]
- 4. Qaseem A, Forciea MA, McLean RM, Denberg TD; Clinical Guidelines Committee of the American College of Physicians . Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(11):818–839. [DOI] [PubMed] [Google Scholar]
- 5. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, San Martin J, Dansey R. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11(5):401–419. [DOI] [PubMed] [Google Scholar]
- 7. Baron R, Ferrari S, Russell RGG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48(4):677–692. [DOI] [PubMed] [Google Scholar]
- 8. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C; FREEDOM Trial . Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. [DOI] [PubMed] [Google Scholar]
- 9. McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, Peacock M, Miller PD, Lederman SN, Chesnut CH, Lain D, Kivitz AJ, Holloway DL, Zhang C, Peterson MC, Bekker PJ; AMG 162 Bone Loss Study Group . Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354(8):821–831. [DOI] [PubMed] [Google Scholar]
- 10. Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J, Favus M; The Alendronate Phase III Osteoporosis Treatment Study Group . Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med. 1995;333(22):1437–1443. [DOI] [PubMed] [Google Scholar]
- 11. Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE; Fracture Intervention Trial Research Group . Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348(9041):1535–1541. [DOI] [PubMed] [Google Scholar]
- 12. Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280(24):2077–2082. [DOI] [PubMed] [Google Scholar]
- 13. Anastasilakis AD, Toulis KA, Goulis DG, Polyzos SA, Delaroudis S, Giomisi A, Terpos E. Efficacy and safety of denosumab in postmenopausal women with osteopenia or osteoporosis: a systematic review and a meta-analysis. Horm Metab Res. 2009;41(10):721–729. [DOI] [PubMed] [Google Scholar]
- 14. Freemantle N, Cooper C, Diez-Perez A, Gitlin M, Radcliffe H, Shepherd S, Roux C. Results of indirect and mixed treatment comparison of fracture efficacy for osteoporosis treatments: a meta-analysis. Osteoporos Int. 2013;24(1):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin T, Wang C, Cai X-Z, Zhao X, Shi M-M, Ying Z-M, Yuan F-Z, Guo C, Yan S-G. Comparison of clinical efficacy and safety between denosumab and alendronate in postmenopausal women with osteoporosis: a meta-analysis. Int J Clin Pract. 2012;66(4):399–408. [DOI] [PubMed] [Google Scholar]
- 16. Mandema JW, Zheng J, Libanati C, Perez Ruixo JJ. Time course of bone mineral density changes with denosumab compared with other drugs in postmenopausal osteoporosis: a dose-response-based meta-analysis. J Clin Endocrinol Metab. 2014;99(10):3746–3755. [DOI] [PubMed] [Google Scholar]
- 17. Wu J, Zhang Q, Yan G, Jin X. Denosumab compared to bisphosphonates to treat postmenopausal osteoporosis: a meta-analysis. J Orthop Surg Res. 2018;13(1):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyu H, Jundi B, Xu C, Tedeschi SK, Yoshida K, Zhao S, Nigwekar SU, Leder BZ, Solomon DH. Data from: Comparison of denosumab and bisphosphonates in patients with osteoporosis: a meta-analysis of randomized controlled trials. Figshare 2018. Deposited 30 November 2018. 10.6084/m9.figshare.7406585. [DOI] [PMC free article] [PubMed]
- 20.Lyu H, Jundi B, Xu C, Tedeschi SK, Yoshida K, Zhao S, Nigwekar SU, Leder BZ, Solomon DH. Data from: Comparison of denosumab and bisphosphonates in patients with osteoporosis: a meta-analysis of randomized controlled trials. Figshare 2018. Deposited 30 November 2018. 10.6084/m9.figshare.7406579. [DOI] [PMC free article] [PubMed]
- 21. Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC, Cochrane Bias Methods Group, Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–114. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwarzer G. Meta: an R package for meta-analysis. R News. 2007;7(3):40–45. [Google Scholar]
- 25. Brown JP, Prince RL, Deal C, Recker RR, Kiel DP, de Gregorio LH, Hadji P, Hofbauer LC, Alvaro-Gracia JM, Wang H, Austin M, Wagman RB, Newmark R, Libanati C, San Martin J, Bone HG. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2009;24(1):153–161. [DOI] [PubMed] [Google Scholar]
- 26. Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, Man HS, San Martin J, Bone HG. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2010;25(1):72–81. [DOI] [PubMed] [Google Scholar]
- 27. Recknor C, Czerwinski E, Bone HG, Bonnick SL, Binkley N, Palacios S, Moffett A, Siddhanti S, Ferreira I, Ghelani P, Wagman RB, Hall JW, Bolognese MA, Benhamou CL. Denosumab compared with ibandronate in postmenopausal women previously treated with bisphosphonate therapy: a randomized open-label trial. Obstet Gynecol. 2013;121(6):1291–1299. [DOI] [PubMed] [Google Scholar]
- 28. Nakamura T, Matsumoto T, Sugimoto T, Hosoi T, Miki T, Gorai I, Yoshikawa H, Tanaka Y, Tanaka S, Sone T, Nakano T, Ito M, Matsui S, Yoneda T, Takami H, Watanabe K, Osakabe T, Shiraki M, Fukunaga M. Clinical Trials Express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (DIRECT). J Clin Endocrinol Metab. 2014;99(7):2599–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roux C, Hofbauer LC, Ho PR, Wark JD, Zillikens MC, Fahrleitner-Pammer A, Hawkins F, Micaelo M, Minisola S, Papaioannou N, Stone M, Ferreira I, Siddhanti S, Wagman RB, Brown JP. Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open-label study. Bone. 2014;58:48–54. [DOI] [PubMed] [Google Scholar]
- 30. Anastasilakis AD, Polyzos SA, Gkiomisi A, Saridakis ZG, Digkas D, Bisbinas I, Sakellariou GT, Papatheodorou A, Kokkoris P, Makras P. Denosumab versus zoledronic acid in patients previously treated with zoledronic acid. Osteoporos Int. 2015;26(10):2521–2527. [DOI] [PubMed] [Google Scholar]
- 31. Miller PD, Pannacciulli N, Brown JP, Czerwinski E, Nedergaard BS, Bolognese MA, Malouf J, Bone HG, Reginster JY, Singer A, Wang C, Wagman RB, Cummings SR. Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J Clin Endocrinol Metab. 2016;101(8):3163–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kendler DL, McClung MR, Freemantle N, Lillestol M, Moffett AH, Borenstein J, Satram-Hoang S, Yang YC, Kaur P, Macarios D, Siddhanti S; DAPS Investigators . Adherence, preference, and satisfaction of postmenopausal women taking denosumab or alendronate. Osteoporos Int. 2011;22(6):1725–1735. [DOI] [PubMed] [Google Scholar]
- 33. Niimi R, Kono T, Nishihara A, Hasegawa M, Kono T, Sudo A. Efficacy of switching from teriparatide to bisphosphonate or denosumab: a prospective, randomized, open-label trial. JBMR Plus. 2018;2(5):289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyu H, Jundi B, Xu C, Tedeschi SK, Yoshida K, Zhao S, Nigwekar SU, Leder BZ, Solomon DH. Data from: Comparison of denosumab and bisphosphonates in patients with osteoporosis: a meta-analysis of randomized controlled trials. Figshare 2018. Deposited 30 November 2018. 10.6084/m9.figshare.7406510. [DOI] [PMC free article] [PubMed]
- 35.Lyu H, Jundi B, Xu C, Tedeschi SK, Yoshida K, Zhao S, Nigwekar SU, Leder BZ, Solomon DH. Data from: Comparison of denosumab and bisphosphonates in patients with osteoporosis: a meta-analysis of randomized controlled trials. Figshare 2018. Deposited 30 November 2018. 10.6084/m9.figshare.7406537. [DOI] [PMC free article] [PubMed]
- 36.Lyu H, Jundi B, Xu C, Tedeschi SK, Yoshida K, Zhao S, Nigwekar SU, Leder BZ, Solomon DH. Comparison of denosumab and bisphosphonates in patients with osteoporosis: a meta-analysis of randomized controlled trials. Figshare 2018. Deposited 30 November 2018. 10.6084/m9.figshare.7406540. [DOI] [PMC free article] [PubMed]
- 37.Lyu H, Jundi B, Xu C, Tedeschi SK, Yoshida K, Zhao S, Nigwekar SU, Leder BZ, Solomon DH. Data from: Comparison of denosumab and bisphosphonates in patients with osteoporosis: a meta-analysis of randomized controlled trials. Figshare 2018. Deposited 30 November 2018. 10.6084/m9.figshare.7406543. [DOI] [PMC free article] [PubMed]
- 38.Lyu H, Jundi B, Xu C, Tedeschi SK, Yoshida K, Zhao S, Nigwekar SU, Leder BZ, Solomon DH. Data from: Comparison of denosumab and bisphosphonates in patients with osteoporosis: a meta-analysis of randomized controlled trials. Figshare 2018. Deposited 30 November 2018. 10.6084/m9.figshare.7406549. [DOI] [PMC free article] [PubMed]
- 39.Lyu H, Jundi B, Xu C, Tedeschi SK, Yoshida K, Zhao S, Nigwekar SU, Leder BZ, Solomon DH. Data from: Comparison of denosumab and bisphosphonates in patients with osteoporosis: a meta-analysis of randomized controlled trials. Figshare 2018. Deposited 30 November 2018. 10.6084/m9.figshare.7406552. [DOI] [PMC free article] [PubMed]
- 40.Lyu H, Jundi B, Xu C, Tedeschi SK, Yoshida K, Zhao S, Nigwekar SU, Leder BZ, Solomon DH. Data from: Comparison of denosumab and bisphosphonates in patients with osteoporosis: a meta-analysis of randomized controlled trials. Figshare 2018. Deposited 30 November 2018. 10.6084/m9.figshare.7406564. [DOI] [PMC free article] [PubMed]
- 41. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Black D, Vittinghoff E, Eastell R Change in BMD as a surrogate for fracture risk reduction in osteoporosis trials: results from pooled, individual-level patient data from the FNIH Bone Quality Project. Summary 1070. Annual meeting of the American Bone and Mineral Research Society. September 28 – 1 October 2018. Montreal, Quebec, Canada. [Google Scholar]
- 43. Miller PD. Bone density and markers of bone turnover in predicting fracture risk and how changes in these measures predict fracture risk reduction. Curr Osteoporos Rep. 2005;3(3):103–110. [DOI] [PubMed] [Google Scholar]
- 44. Anastasilakis AD, Polyzos SA, Makras P. Therapy of endocrine disease: denosumab vs bisphosphonates for the treatment of postmenopausal osteoporosis. Eur J Endocrinol. 2018;179(1):R31–R45. [DOI] [PubMed] [Google Scholar]
- 45. Pedersen AB, Heide-Jørgensen U, Sørensen HT, Prieto-Alhambra D, Ehrenstein V. Risk of osteoporotic fractures in new users of denosumab compared with new users of alendronate: a Danish population-based cohort study. Pharmacoepidemiol Drug Saf. 2018;27:406–407. [Google Scholar]