Abstract
The TSH receptor (TSHR) is the major autoantigen in Graves’ disease (GD). Bioinformatic analyses predict the existence of several human TSHR isoforms from alternative splicing, which can lead to the coexpression of multiple receptor forms. The most abundant of these is TSHRv1.3. In silico modeling of TSHRv1.3 demonstrated the structural integrity of this truncated receptor isoform and its potential binding of TSH. Tissue profiling revealed wide expression of TSHRv1.3, with a predominant presence in thyroid, bone marrow, thymus, and adipose tissue. To gain insight into the role of this v1.3 receptor isoform in thyroid pathophysiology, we cloned the entire open reading frame into a mammalian expression vector. Immunoprecipitation studies demonstrated that both TSHR-stimulating antibody and human TSH could bind v1.3. Furthermore, TSHRv1.3 inhibited the stimulatory effect of TSH and TSHR-Ab MS-1 antibody on TSHR-induced cAMP generation in a dose-dependent manner. To confirm the antigenicity of v1.3, we used a peptide ELISA against two different epitopes. Of 13 GD samples, 11 (84.6%) were positive for a carboxy terminal peptide and 10 (76.9%) were positive with a junction region peptide. To demonstrate that intracellular v1.3 could serve as an autoantigen and modulate disease, we used double-transfected Chinese hamster ovary cells that expressed both green fluorescent protein (GFP)-tagged TSHRv1.3 and full-length TSHR. We then induced cell stress and apoptosis using a TSHR monoclonal antibody and observed the culture supernatant contained v1.3-GFP protein, demonstrating the release of the intracellular receptor variant by this mechanism.
The TSH receptor (TSHR), a classical G protein–coupled receptor, is primarily expressed on the surface of thyroid follicular cells, although expression can be detected on a wide variety of cells, including fibroblasts, adipocytes, bone, and immune cells (1). In addition to its key regulatory function on thyroid growth and thyroid hormone production, the TSHR is also a primary autoantigen in autoimmune thyroid disease, especially autoimmune hyperthyroidism, better known as Graves’ disease (GD) (2, 3). Furthermore, the TSHR undergoes complex post-translational modifications, including proteolytic cleavage of the endogenous 50 amino acid region in the extracellular domain (4), and it also forms constitutive higher-order structures in heterologous and native thyroid membranes (5–7). Autoimmune thyroid disease is associated with the formation of TSHR autoantibodies with either stimulating, blocking, or neutral activity, and with stimulating antibodies exerting the predominant effect in GD (8, 9).
Several studies have clearly established that several genetic loci confer a small increase in the risk for GD developing, and many of these risks are shared with other autoimmune diseases (10, 11). However, the TSHR gene loci itself has been associated only with GD and has been linked to changes in expression of two TSHR variants resulting from alternate mRNA splicing (12). Hence, it is likely that genetic polymorphisms within the TSHR gene can influence its structure, expression, and/or post-transcriptional and translational processing, which may play a role in the autoimmune response against the TSHR. Beyond simply generating protein diversity, alternative splicing is one key to the complexity of post-translational regulation of gene expression that is observed with receptor and nonreceptor proteins (13). The cDNA for the TSHR is ∼4 kilobases long with 100 bp of 5′ untranslated region and a 1.6 Kb of 3′ untranslated region flanking the single open reading frame (ORF) of 2292 bp. The gene has 10 exons that encode the full-length receptor of 764 amino acid proteins, including the signal peptide. Early studies from our laboratory and others, using liquid hybridization analysis of TSHR mRNA in normal and abnormal human thyroid tissues, showed the presence of additional transcript bands besides the wild-type, full-length TSHR (TSHRfl) form, which was consistent with the formation of splice variants (14–16, 17). In addition, a soluble TSHR-like binding component compatible with a soluble splice variant of the receptor is present in human thyroid tissue (18, 19) and is reminiscent of the earlier soluble thyroid preparations used for measuring thyroid adsorbing activity (20–22). TSHR mRNA transcripts of ∼1.8 and 1.2 kb have been described, in addition to the 4-kb TSHRfl mRNA species in human thyroid (23, 24). We and others (14, 17) cloned the more abundant of these smaller TSHR transcripts (i.e., TSHRv1.3). However, to date, there is no clear consensus on the possible function of such intracellular isoforms.
We have shown that “neutral” TSHR antibodies can induce thyroid cell apoptosis in vitro and in vivo via induction of excessive cell stress and involving multiple organelles (25, 26). Hence, these antibodies can activate mitochondrial and endoplasmic reticulum stress in the absence of any traditional TSHR signal. Because neutral antibodies are present in patients with GD (25), it seems possible that cell stress and apoptosis are potential mechanisms for release of such receptor isoforms. The current study was designed, therefore, to examine the influence of this truncated receptor protein on the action of TSH and TSHR autoantibody and to gain insight into the possible mechanism by which this modified receptor isoform could be released as a pseudoreceptor as well as a novel antigenic source and thus contribute to the pathophysiology of the thyroid autoimmune process.
Materials and Methods
Bioinformatics
To identify the different splice variants of the TSHR, we used a Web-based open source site (Primer-Check) for analysis of different splice variants and their possible primer targets. By inputting a primer sequence and the gene symbol, the program scans the splice center database, which consists of transcripts from RefSeq and GenBank, and displays the different transcripts present in the database with their corresponding National Center for Biotechnology Information accession numbers (27).
Cloning of human TSHRv1.3
Because the entire coding sequence of the TSHRv1.3 was published (14), we synthesized the entire ORF through exon 8 along with the 63 divergent bases of intron 8 sequence tagged to either green fluorescent protein (GFP) or histidine (His) with a stop codon and polyadenylation signal of the vector. Because one of the vectors we used had a built-in N-terminal secretion signal of IgG κ, we made expression constructs with and without the endogenous TSHR signal peptide. The cDNA was flanked with restriction enzyme sites XhoI and BamH I and cloned into either pSecTag2His-Myc or pEGFP-N1 mammalian expression vectors and sequence verified.
Generation of stable clones
Human embryonic kidney (HEK) cells (HEK293) were grown in complete DMEM with 10% fetal bovine serum and 100 U of penicillin and of streptomycin. The aforementioned plasmids containing the TSHRv1.3 cDNA were used to generate stable clones in HEK293 cells, using a lipophilic reagent, Fugene (catalog no. E2691; Promega, Madison, WI) and subsequently selected with either hygromycin (600 μg/mL) or G418 (800 μg/mL) for 2 weeks.
Protein expression
HEK293 cells transfected with the TSHRv1.3 GFP construct were cultured in DMEM with 10% fetal bovine serum and 100 U each of penicillin and streptomycin. Protein expression in lysate was confirmed by the presence of the GFP fusion protein by flow cytometry or by Western blot using 1:10,000 diluted anti-His (28) and anti-TSHR monoclonal antibody (29) at 1:5000. The supernatant from these confluent cells was collected and concentrated 10 times with an Amicon filter (Ultracel 10K) before measuring GFP fluorescence with the microplate reader (ClarioStar, BMG LABTECH, Cary, NC). Cellular localization of the protein cells for GFP was carried out using a Nikon TE2000S epifluorescent microscope with a 20× objective with a numerical aperture of 0.45.
Purification of TSHRv1.3 protein
A stable clone of TSHRv1.3 HEK cells that was verified to express the protein strongly by immunoblot was used for purification. Total cell lysate was prepared using 1× RIPA buffer (catalog no. 9806; Cell Signaling, Danvers, MA) with protease inhibitors by holding the cells on ice for 10 minutes, followed by a brief sonication. The lysate was clarified by centrifugation at 12,000g at 4°C, followed by filtration through a 0.4-micron filter; protein presence was estimated using the Bradford method. Before purification, the lysate was diluted with binding buffer 1:1 and then loaded into a 1-mL packed Ni column (catalog no. 06781543001; Thermo Fisher Scientific, Waltham, MA) using a flow rate of 0.5 mL/min. The flow through was recycled four times through the column for optimum binding, then the column was washed with 10 volumes of PBS Tween buffer. The protein was eluted according to the manufacturer’s instructions, in the elution containing an imidazole gradient (0 to 250 mM). Fractions were collected and presence of protein in the fractions was measured at 280 nm.
Protein verification
The identity of the purified protein was verified by mass spectrometry. His-tagged purified protein was run on 10% SDS-PAGE, stained with Coomassie Blue for 2 hours, and destained by washing several times in water, per the manufacturer’s instructions. The bands corresponding to the protein of interest were excised and sent to MS Bioworks (Ann harbor, MI) for identification of the protein by mass spectrometry. An in-gel trypsin digestion was performed and analyzed by nano liquid chromatography–tandem mass spectrometry; the data were searched using Mascot and mascot data files were parsed into Scaffold (proteome software) for validation and filtering.
Gene expression
A human-tissue cDNA control was obtained from Biochain Institute (Newark, CA). Genomic DNA was removed from the RNA samples using RNase Free DNase (RNase-Free Dnase kit, catalog no. 79254; Qiagen, Germantown, MD). All RT-PCRs were performed with Hotstar Taq polymerase (catalog no. 1007837; Qiagen). Cycling conditions were as follows: 94°C for 3 minutes, 94°C denaturation for 1 minute, annealing at 57°C for 1 minute, 72°C elongation for 1 minute, and a final incubation at 72 C for 10 minutes. The amplified PCR products were separated on 2% agarose gels. The quantitative PCRs were performed using the StepOnePlus Real-time PCR system (Applied Biosystems, Foster City, CA). The reactions were established with 10 μL of SYBR Green Master Mix, 0.4 μL (2 μM) of sense/antisense gene-specific primers, 2 μL of cDNA, and diethyl pyrocarbonate–treated water to a final volume of 20 μL. The PCR reaction mix was denatured at 95°C for 60 seconds before the first PCR cycle. The thermal cycle profile was as follows: denaturizing for 30 seconds at 95°C, annealing for 30 seconds at 57°C to 60°C (depending on primers), and extension for 60 seconds at 72°C. A total of 40 PCR cycles were used. For each target gene, the relative gene expression was normalized to that of the glyceraldehyde-3-phosphate dehydrogenase housekeeping gene. Data presented as the means of two independent experiments in which all sample sets were analyzed in triplicate.
Binding studies
To examine the binding of the purified v1.3 protein to TSH and TSHR-stimulating antibody MS-1, we performed immunoprecipitation experiments. First, we labeled 50 μL of magnetic protein-G beads with 10 μg of monoclonal MS-1 or polyclonal anti-TSH antibodies (30). To prevent leaching of the bound IgG, we irreversibly cross-linked the antibodies to the beads by using 5 mM of bis(sulfosuccinimidyl) suberate cross-linker. For the TSH binding experiment, we incubated recombinant TSH (50 μg) with 100 μg of TSHRv1.3 protein for 30 minutes at room temperature with gentle shaking. This was followed by 50-μL of protein G–conjugated anti-TSH beads for 3 hours at 4°C.
For MS-1 binding studies, 100 μg of v1.3 protein was incubated with MS-1 conjugated protein-G beads for 3 hours at 4°C. The beads were washed three times with PBS Tween (0.05% Tween 20) and the complex was then diluted with sample buffer three times with dithiothreitol (100 mM) and examined on a 10% SDS-PAGE gel. The blots were probed with anti-His antibody (10:10,000). Nonspecific IgG conjugated to the beads and treated with sample identically, as described earlier in this section, was used as the internal control.
Inhibition studies
To study the ability of TSHRv1.3 protein to inhibit TSH and MS-1 cAMP signaling, we performed a dose-response assay by first incubating different concentrations of v1.3 purified protein or control protein with a fixed saturating concentration of bovine TSH (1000 μU/mL) or MS-1 (10 μg/mL) at 37°C for 30 minutes in serum-free F-12 medium. Then, 50 μL of this preincubated mixture of TSH and MS-1 with v1.3 protein was added to the wells of the plate containing the TSHR-Glo cells developed in our laboratory (31). After 4 hours’ incubation at 37°C, plates were developed by adding luciferase substrate (Bright Glow, catalog no. E2610; Promega) and luminescence was read using a microplate reader. TSH alone, medium alone, and medium with protein alone were used as the negative and positive controls. The control protein devoid of TSHRv1.3 was treated in an identical manner.
Molecular simulation studies
To assess the binding of TSHRv1.3 protein with TSH-β, we performed in silico modeling and molecular dynamics simulations for 20 ns, followed by a longer simulation of 200 ns. The structure of the TSHRv1.3 was generated using homology modeling based on the structure of the previously published ectodomain (ECD) (32, 33). The model was generated with the program Modeler (34, 35). The simulation setup was performed with the help of the CHARMM-GUI server (36) that was given a TSHR-ECD structure file, obtained by replacing the ECD structure in the TSHR-ECD complex by the TSHRv1.3 structure generated by the homology modeling and TSH-β model. The server generated the topology file, and water and counter ions (K+ and Cl−) were added to ensure neutrality, maintaining an ionic strength of 0.1 M. Contacts and hydrogen bonds between TSH and TSHRv1.3 ECD were calculated with the program Simulaid (37). Images of the variant complexed with TSH-β were generated by visual molecular dynamics (38). The molecular simulation indicated general stability and affinity of both the TSHβ and TSHRv1.3 for the binding pockets. The unique intronic sequence of TSHRv1.3 had no interaction sites with TSH.
Cell stress and protein release measurements
As previously reported (25), we studied the dose-dependent effect of a neutral antibody (Tab16) in inducing cell stress via mitochondrial damage by measuring increase in reactive oxygen species (ROS), using Mitosox dye (catalog no. M36008; Thermo Fisher Scientific), and release of the GFP-tagged TSHRv1.3 protein into culture supernatant by measuring GFP fluorescence in a microplate assay. Briefly, Chinese hamster ovary (CHO) cells coexpressing hemagglutinin-tagged TSHR and TSHRv1.3GFP were seeded at 30,000 cells per well in triplicate in a 96-well, black microtiter plate and allowed to grow in complete F-12 medium until 70% to 80% confluent. The cells were then weaned off serum containing medium for 2 days and then stained with Mitosox dye, per the manufacturer’s protocol. The Mitosox dye–stained cells were then treated with vehicle only, neutral antibody Tab16 IgG at 0.1, 1, and 10 μg/mL, and isotype control IgG in serum-free medium. The treated cells were then incubated at 37°C and the plate was read at 510 to 580 nm for mitochondrial ROS increase, and additional supernatant collected into a fresh plate was read at 417 to 515nM to detect GFP. The results are presented after background subtraction of untreated wells. Apoptosis in these cells was measured by estimating viability of treated cells and untreated cells by dye exclusion using trypan blue staining.
Peptide ELISA and specificity
The peptide ELISA was performed using two peptides synthesized against TSHRv1.3 protein: JNX peptide and carboxy terminal peptide (COOH) peptide. JNX peptide covers the junction bordering the exon 8–intron 8 sequence with corresponding amino acid sequence LPLGRKSLSFE. The COOH peptide covers the carboxyl end of the protein within intron 8 with an amino acid sequence of TQKAPSSSTPS. These peptides were selected because of their predicted immunogenicity and uniqueness to the splice variant. Briefly, the peptide ELISA was performed by coating 100 μL per well of peptides dissolved in bicarbonate buffer pH 9.6 at 10 μg/mL. The plates were blocked with 2% BSA for 2 hours at 25°C. Purified IgG (40 μg) previously prepared in the laboratory from various patients with GD was diluted 1:10 in antibody dilution buffer (PBS Tween with 2% BSA) and incubated for 1 hour at 25°C. After washing, 50 μL per well of anti-human IgG-Fab horseradish peroxidase was used as the detection antibody. The plate was developed using 3,3',5,5'-tetramethylbenzidine substrate and optical density (OD) was measured at 450 nm. The cutoff was specified by +2 SDs of the average of the normal tested in parallel. To verify the specificity of binding in the ELISA, two of the IgG samples that showed positivity in the ELISA were selected and diluted to a 4 μg final dilution and then premixed with 1 μg and 10 μg of the final concentration of the corresponding peptide (JNX or COOH) and incubated for 30 minutes at room temperature on a rotary shaker. A peptide directed to the transmembrane domain region of the receptor at 10 μg (final dilution) was used as the nonspecific control peptide in the assay. We used 50 μL of this preincubated mixture to perform the ELISA, as described previously.
Serum IgG for ELISA
The serum IgG used in this study, from patients with GD, was from deidentified stored samples in the laboratory that were originally collected with the full consent of patients (Table 1).
Table 1.
TSI and TBII Values of GD Samples
| Sample No. | TSI, %a | TBII, IU/Lb |
|---|---|---|
| 1 | 500 | 11.3 |
| 2 | 473 | 40 |
| 3 | 411 | 31.54 |
| 4 | 100 | 2.41 |
| 5 | NA | 5.35 |
| 6 | 118 | 14.87 |
| 7 | 407 | 31.49 |
| 8 | 154 | 4.58 |
| 9 | 366 | 17.11 |
| 10 | 200 | 3.47 |
| 11 | 275 | 6.7 |
| 12 | NA | 15.48 |
| 13 | 280 | NA |
Abbreviations: NA, not available; TBII, thyroid-binding inhibitory immunoglobulin; TSI, thyroid-stimulating immunoglobulin.
Cutoff for TSI, ≤122%.
Cutoff for TBII, ≤1.75 IU/L.
Results
Bioinformatic studies of TSHR splice variants and surveying TSHRv1.3 expression in human tissue
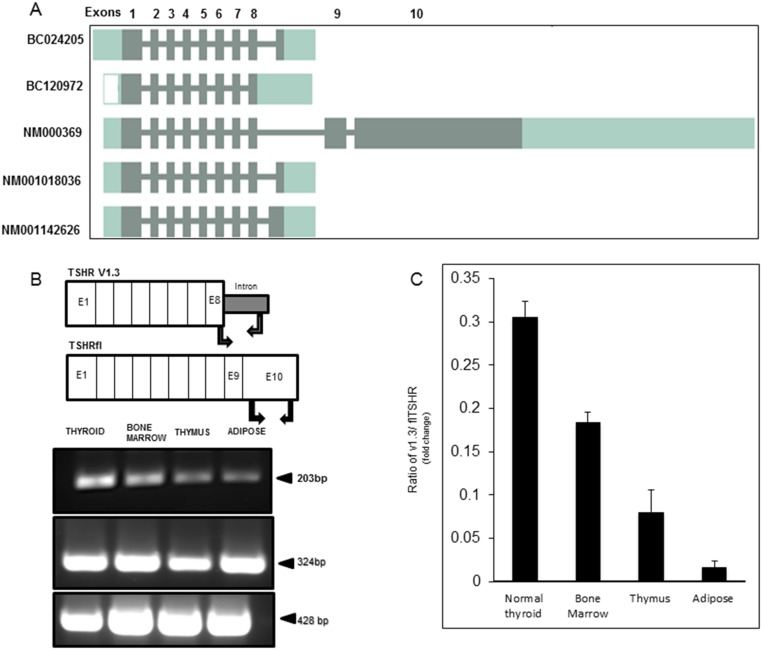
Figure 1A is a graphical representation of a bioinformatic analysis of the TSHR scanning for potential variants. In addition to the full-length receptor displayed by the accession number NM_000369, the tool also predicted a variety of possible transcripts of the TSHR gene, showing variable truncated forms. By this predictive analysis, we learned that the TSHR exists not only as a predominant single transcript but also may have multiple isoforms including TSHRv1.3 (14). BC120972 shown in this representation is the closest form to the TSHRv1.3 by nucleotide sequence identity.
Figure 1.
Predicted TSHR transcripts and assessment of TSHRv1.3 in human tissues. (A) Schematic representation of the different predicted transcripts of human TSHR. In addition to the TSHRfl (accession no. NM000369), the representation shows four other possible transcripts with the accession numbers, which are truncated before exon 9. It is not known if any of these encode a protein product. By sequence verification, BC120972 is similar to TSHRv1.3 though not identical. (B) Schematic representation of the exon distribution of the TSHR and the location of the specific primers used for the detection of TSHRv1.3 and TSHRfl. PCR products: 203-bp fragment specific to the TSHRv1.3 (top panel), 324-bp fragment corresponding to exon 10 of TSHRfl (middle panel), and 428-bp β-actin (bottom panel). (C) Quantitative PCR results of TSHRv1.3 in human tissues expressed as a ratio of v1.3 to TSHRfl. The v1.3 message is highly expressed in thyroid, bone marrow, and thymus, and at a lower level in adipose tissue. E, exon.
Because the expression of TSHR is ubiquitous (1), we measured the expression of splice variant TSHRv1.3 mRNA in some key human tissues by RT-PCR, using a forward primer starting at the boundary of exon 8 and a reverse primer culminating at the end of the 63-bp intron 8 sequence. The TSHRfl was examined using forward and reverse primers directed to regions within exon 10, which encodes the transmembrane region of the TSHRfl, as shown in the schematic representation in Fig. 1B. β-Actin was used as the housekeeping gene. Dnase I–treated human cDNA (1 μg) was amplified from thyroid, bone marrow, thymus, and adipose tissue for the presence of the 203 bp of v1.3 PCR product and the 324 bp of the TSHRfl exon 10 product. A 203-bp fragment corresponding to specific amplified v1.3 was observed in each of these tissues, although with variable expression (Fig. 1B and 1C). A 324-bp TSHR fragment was also amplified from these tissues, indicating the presence of TSHRfl (Fig. 1B, lower). To quantify expression of this truncated protein, we used quantitative PCR and expressed the data as a ratio of v1.3 divided by TSHRfl (Fig. 1C). These results indicated that the highest levels of v1.3 were observed in thyroid, bone marrow, and thymus.
Splicing analysis of TSHRv1.3 DNA
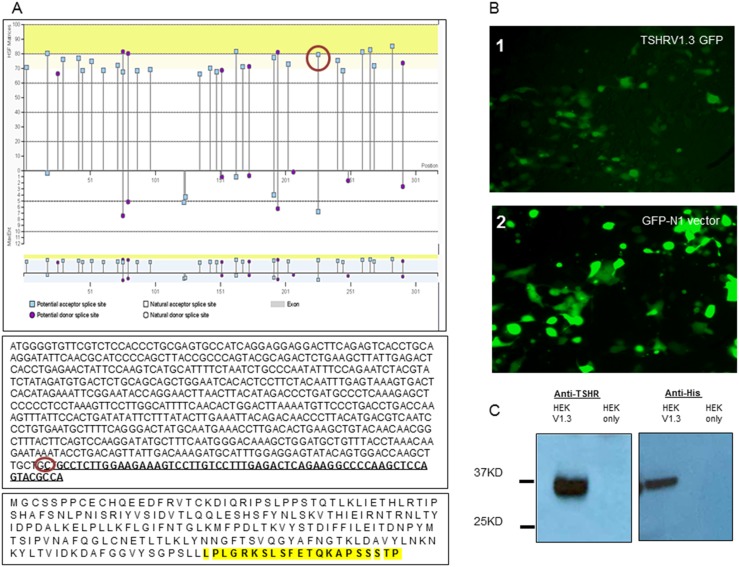
The 21 amino acid, unique sequence of TSHRv1.3 is derived from the N-terminus half of intron 8, which is ∼32,000 Kb. To understand what might lead to the splicing of this intron 8 sequence, which resulted in TSHRv1.3, we analyzed the partial N-terminus of intron 8 that contributed to the unique intronic region of TSHRv1.3. We looked for splice acceptor and donor sites using a Web-based Human Splicing Finder application (HSF; http://www.umd.be/HSF3/) (39). As indicated in Fig. 2A, at the junction of the unique intron 8 sequence (indicated by the red circle), there was a predicted acceptor splice site in the region of the intron that is an essential site to which splicing factors bind and, therefore, makes it susceptible to splicing. The HSF consensus score for this predicted acceptor site was 79.58, which was well above the threshold of 65, which qualifies it as a splicing site. Furthermore, the maximum entropy score, also a measure of a splice site, of 7, shown in the lower half of Fig. 2A, also qualifies it to be a potential splice site. By this analysis, we know that the intron 8 junction has a potential splice site and that this acceptor site might, by virtue of its location on the transcripted DNA strand, preferentially result in the formation of TSHRv1.3 in tissues.
Figure 2.
Predicted splice site and protein expression and purification of TSHRv1.3. (A) Graphical representation of the distribution of splice donor and acceptor sites as predicted by the HSF program. The DNA sequences are shown of v1.3 (minus the endogenous signal peptide). The nucleotides in bold and underlined correspond to the 21 bases derived from the unique intronic region. The HSF score that predicted a splice-acceptor site at the start of the unique sequence (circled on the graph and sequence) would suggest that splicing occurs at this site and possibly generates TSHRv1.3 transcripts in addition to the full-length receptor. Furthermore, the coding v1.3 protein sequence indicates the unique intronic segment encompassing the JNX and COOH peptides (bold and highlighted in the bottom-most box) used in Fig. 6A. The protein sequence represented here was used for our in silico modeling studies shown in Fig. 4B. (B) Fluorescent image (×200) of a representative clone of TSHRv1.3 GFP (panel 1) and GFP vector only (panel 2) transfected into HEK293 cells. The expression of the GFP-TSHRv1.3 within the cells as seen in the vector-alone transfected cells suggests they are expressed in the cytoplasm only. (C) Western blot performed on lysate from stable clones of TSHRv1.3 using (left panel) anti-TSHR (1:5000) recognizing the epitope from 22 to 41 amino acids and (right panel) anti-His monoclonal antibody (1:10,000) recognizing the six-histidine tag fused to the C-terminus of the expressed protein. The predicted protein band (∼37 kDa) was observed by both these antibodies but not in lysate from HEK cells transfected with the empty vector only.
Expression and characterization of TSHRv1.3 protein
These findings led us to examine in vitro the purified recombinant protein and its effects. To express this protein in heterologous cells, we synthesized the entire ORF of TSHRv1.3 and generated stable clones of TSHRv1.3 in HEK293, using two different mammalian expression vectors to study its intracellular localization and secretion and then to try to force secretion by N-terminus fusion of the ORF of TSHRv1.3 with an IgG κ leader sequence of pSecTag vector. A stable line of GFP-tagged TSHRv1.3 transfected into HEK cells was imaged for GFP-v1.3 fusion protein (Fig. 2B, panel 1), which localized to the cytoplasm in a similar way to the GFP vector alone in transfected cells (Fig. 2B, panel 2). However, immunoblots performed on total lysate and probed with anti-TSHR or anti-His showed an ∼37-kDa protein that was not seen in HEK cell lysates transfected with the empty vector (Fig. 2C).
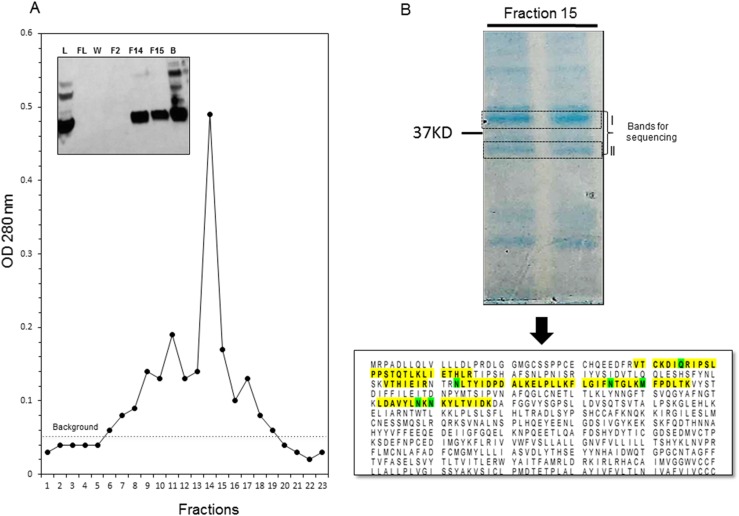
The protein was then purified from cell lysate, using nickel-chelating chromatography and a commercial His-trap column, as shown in Fig. 3A. Although the flow through did not contain any protein it was clearly observed in the eluate, although the beads retained some bound protein. Protein fractions with large ODs were pooled and a sample of it was resolved on 10% SDS-PAGE. Two bands corresponding to the indicated size were sent out for identification by mass spectrometry. The lower band corresponding to the predicted size identified unique peptides (highlighted in yellow in Fig. 3B) corresponding to the TSHR protein, thus confirming the identity of the purified protein also identified by immunoblot.
Figure 3.
Purification and protein identification of TSHRv1.3. (A) Chromatogram obtained from purification of TSHRv1.3 protein from the lysate prepared by 1× RIPA buffer on a HisTrap column. Fractions 11 to 17 correspond to the eluted protein. (Inset) An immunoblot developed with anti-His antibody staging the purification process. The fractions 14 and 15 strong bands of the 37-kDa protein are seen. Some protein in the beads was also observed, suggesting incomplete elution. (B) Fraction 15 was resolved on 10% SDS-PAGE and stained with Coomassie Blue gel stain, and the two bands observed near 37-kDa were cut out, washed with distilled water, and analyzed by mass spectrometry. In the mass spectrometry analysis, the Mascot software was set to search a human database. It identified the different peptides and assigned a probability of identification. Band II indicates peptide sequences (yellow highlighting, 90% probability; green highlighting, 95% to 100% probability), which corresponded to the TSHR protein in the database. Band I was not the protein of interest. B, beads postelution; F, fraction collected postelution; FL, flow through; L, lysate; W, wash.
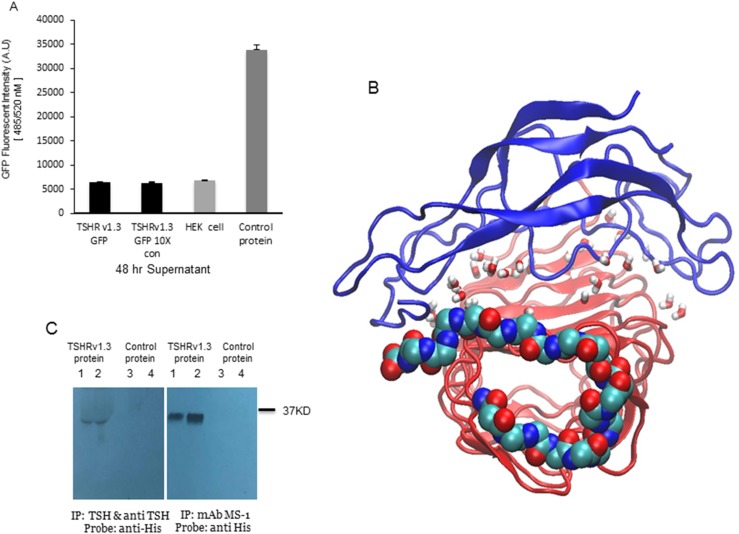
We examined if the TSHRv1.3-expressed protein is secreted by measuring the fluorescence of GFP in the culture supernatant from a stable clone overexpressing the TSHRv1.3–GFP fusion protein. The v1.3 protein was not secreted from intact cells, because there was a total lack of any measurable amount of GFP fluorescence in the supernatant even after 10-fold concentration of a 48-hour postculture supernatant (Fig. 4A). For additional characterization, and to try to induce secretion, we deleted the endogenous signal peptide of TSHRv1.3 and fused the ORF of TSHRv1.3 starting with the translation initiation site (i.e., ATG) with the IgG κ signal peptide sequence in the pSecTAG-His mammalian expression vector. There was still a lack of any measurable amount of secreted TSHRv1.3.
Figure 4.
TSH and TSHR antibody interaction with TSHRv1.3 protein. (A) Bar graph indicating quantitation of GFP fluorescent intensity for TSHRv1.3 protein in culture supernatants in unconcentrated and ×10 Amicon filter–concentrated supernatant from confluent cultures of stable HEK clones. The bar on the right is control protein GFP (1:100), which acted as the positive control. These data confirm the inability of the splice variant TSHRv1.3 protein to be secreted into the supernatant. (B) Molecular dynamic simulation (MD) of the TSHRv1.3 homology-modeled protein with TSH-β protein was performed using the CHARMM-GUI server for 200 ns. Shown here is a representative pose of the server-generated topology of the two complexed proteins. The β-pleated sheets and loops of α helices of TSH-β (blue) are seen to clasp the concave surface of the TSHRv1.3 ECD. Water molecules added to ensure neutrality are shown here as small stick structures within the complexed protein. The 21 amino acid intron 8 region of TSHRv1.3 is represented as continuous spheres of carbon (turquoise), nitrogen (blue), and oxygen (red). The MD simulation demonstrated that this truncated isoform of the receptor maintains the structural integrity to bind TSH with good stability. However, the affinity of binding was hard to predict in this modeling. (C) The binding of TSH and MS-1 to the purified protein was confirmed by immunoprecipitation (IP) with anti-TSH and anti-His antibodies as probes. The bound TSH-TSHRv1.3 complex formed by preincubation was precipitated with anti-TSH (10 μg per reaction) and the binding of MS-1 (10 μg per reaction) to TSHRv1.3 (10 and 50 μg protein per reaction, shown in lane 1 in the left-side blot and lane 2 in the right-side blot, respectively) was demonstrated with direct binding to purified protein followed by pull down of the complexed protein, using magnetic protein G beads. Normalized control protein lysate from vector-only transfected cells did not show any precipitation either with anti-TSH or MS-1 (lanes 3 and 4 in left and right blots, respectively). Therefore, this expressed receptor splice-variant protein has the ability to bind TSH and TSHR-stimulating antibody. mAb, monoclonal antibody.
Binding of TSHRv1.3 protein to TSH and stimulating TSHR antibody
In silico modeling and simulation studies confirmed the potential binding of TSH to the v1.3 protein. These interaction studies of TSHRv1.3 with the TSH-β subunit were carried out using the equilibrated ECD structure of the TSHRv1.3 protein with the unique 21 amino acid C-terminal extension via molecular dynamic stimulations (20 ns), as previously described (14). This in silico study showed TSHβ is capable of clasping the concave surface of the TSHRv1.3 protein in the leucine-rich domain, contacting the β-pleated loops L1 to L6 in the ECD (Fig. 4B). An even longer simulation of 200 ns with these complexed proteins showed them to be bound together with the unique intron 8 region not affecting the stability of the interaction.
To verify the binding of TSH and TSHR-stimulating antibody to the purified protein, we performed direct immunoprecipitation. Figure 4C (left panel) shows the binding of human TSH to v1.3 protein after probing the immunoprecipitate with a polyclonal anti-TSH; the binding yielded a ∼37-kDa band not present in control lysates. The binding of MS-1 to the v1.3 protein was also performed by directly incubating MS-1 (40) with v1.3, and the antibody-bound complexes were pulled down with magnetically conjugated protein G. This also yielded a clear protein band corresponding to the TSHRv1.3.
Dampening of TSH and MS-1 bioactivity by TSHRv1.3 protein
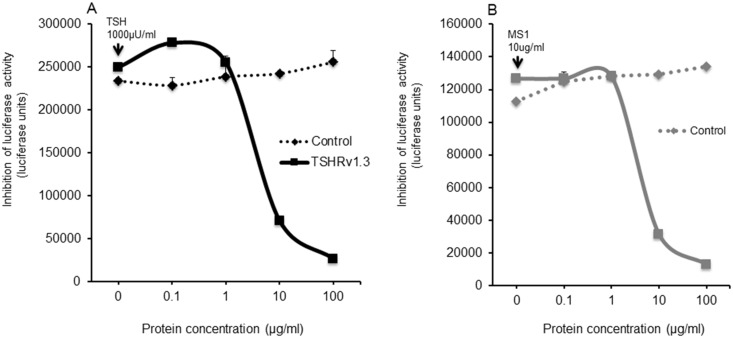
The potential dampening effect of TSHRv1.3 protein on TSH and MS-1 signaling was investigated by measuring inhibition of TSH- and MS-1–induced cAMP generation in the presence of increasing concentrations of purified v1.3 protein using TSHR-Glo cells (31). For controls, we used lysate from cells that did not express the v1.3 protein. As can be seen in Fig. 5A and 5B, the TSHRv1.3 protein induced a dose-dependent decrease in the stimulation induced by TSH and MS-1. These observations confirmed the ability of purified TSHRv1.3 protein to interact with TSH and autoantibody, and inhibit their activity
Figure 5.
Inhibition of TSH signaling by TSHRv1.3 protein. Inhibition of (A) TSH-stimulated cAMP generation and (B) MS-1–induced cAMP generation by increasing concentrations of purified TSHRv1.3 protein was measured using TSHR-Glo cells. For this assay, a fixed concentration of TSH corresponding to 1000 μU/mL was first preincubated with different concentrations of TSHRv1.3 purified protein or control protein, as indicated on the x-axis for 30 minutes at 37°C in a volume of 200 μL. This mixture was then equally divided and added in duplicate to 96-well microtiter plates seeded with 50,000 cells per well of TSHR-Glo cells and incubated overnight at 37°C. Control wells were either stimulated with medium alone or TSH 1000 μU/mL as negative and positive controls. TSHRv1.3 protein could almost totally dampen the induced stimulation of cAMP in this transcriptional-based bioassay. The data in these plots are the mean of two experiments.
The presence of specific antibodies to V1.3 epitopes in patients with GD
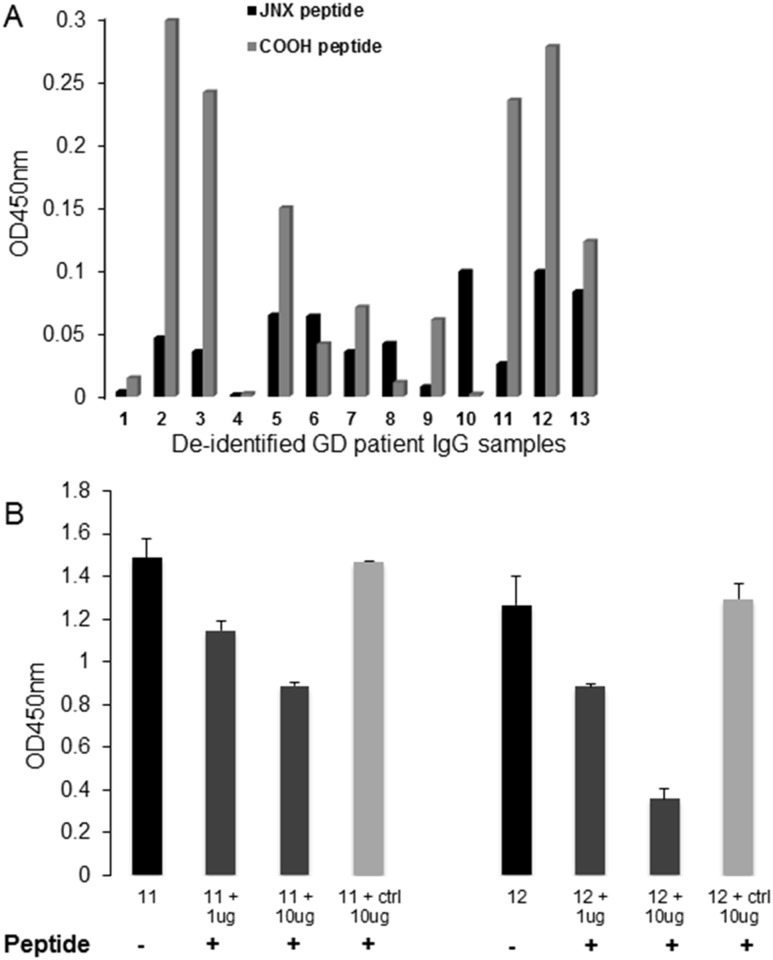
We examined antibodies to two different unique epitopes of TSHRv1.3 in purified IgG fractions from sera of patients with GD (n = 13) and known TSHR antibodies as well as normal controls (n = 10), using peptide-based ELISAs. The histogram in Fig. 6A shows the binding of the samples to the two peptides of TSHRv1.3 after subtraction of the background (mean plus 2 SD OD) obtained from the normal control samples. We found 84.6% of the GD samples were positive for the COOH peptide and 76.9% for the JNX peptide. The specificity of the response observed in these ELISA experiments was verified using the COOH peptide to block the IgG binding, using two different concentrations of specific peptide and a nonspecific peptide (Fig. 6B). A dose-dependent decrease in binding was observed in both the GD samples using the specific peptide, whereas no inhibition was observed with the control peptide. This confirmed the specificity of the binding of the GD samples to the TSHRv1.3 peptides, suggests the presence of autoantibodies to the v1.3 protein in the sera of patients with GD, and thus demonstrates the potential antigenicity of this receptor protein.
Figure 6.
Antibodies to TSHRv1.3 epitopes in patient sera. Peptides to the JNX and the COOH of TSHRv1.3 were used in our peptide ELISA. (A) The bar graph shows the binding response of 13 different GD IgGs (4 μg per well) indicated on the x-axis against the two peptides marked by black and light gray bars. Data represent OD values after subtraction of mean OD ±2 SD obtained from 10 control IgG samples. (B) Two IgG samples (samples 11 and 12) that were strongly positive were used to test for specificity of binding. The 6-μg IgG of these samples was first absorbed with 1 and 10 μg of the COOH peptide for 30 minutes at 37°C. An unrelated control peptide treated with the same concentrations of IgG was also used in the absorption, as indicated by the matrix below the x-axis. Addition of the specific peptide reduced the OD by >50%, suggesting specificity of patient IgG to the v1.3 peptides.
Release of TSHRv1.3 protein from double-transfected CHO cells by neutral TSHR antibody
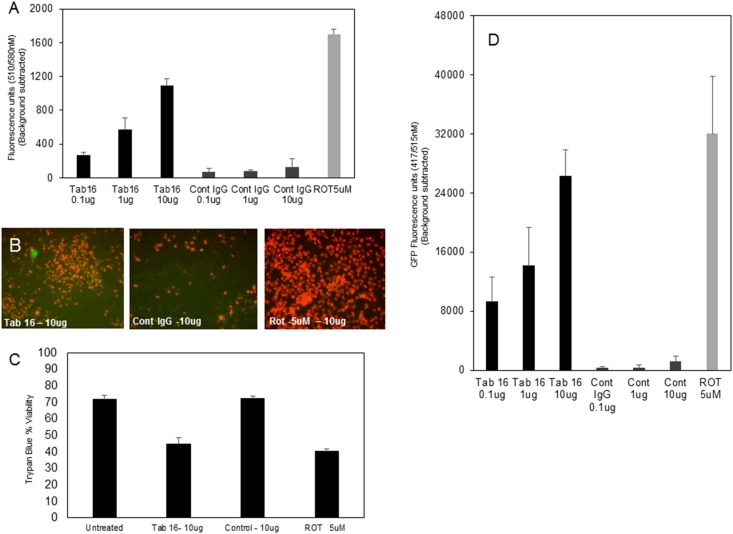
To examine the concept that a receptor isoform entrapped in the cells can be released as a source of antigen, we used CHO cells cotransfected with an N-terminus, hemagglutinin-tagged, TSHRfl and with TSHRv1.3 GFP. These cells, on treatment with neutral monoclonal antibody Tab16 at increasing concentration, showed increased mitochondrial ROS (mito-ROS) induction, as measured by MitoSox staining of cells and also as previously described (25) This increase in mito-ROS was not observed in the isotype IgG control (Fig. 7A and 7B). Furthermore, the viability of these neutral antibody-treated cells was decreased (Fig. 7C) and correlated well with increased mito-ROS. On measuring released v1.3 GFP protein in the supernatant of these apoptotic cells in the same experiment, we observed a dose-dependent increase in fluorescence in the supernatant of neutral antibody-treated cells and none in control IgG-treated cells (Fig. 7D). Rottlerin 5 μM, was used as the positive control for induction of mito-ROS and this corresponded with apoptosis of cells.
Figure 7.
Cell stress and v1.3 protein release. Intracellular release of v1.3 GFP-tagged protein was induced by treating double-transfected CHO (TSHRfl plus v1.3-GFP) cells with our neutral monoclonal antibody, Tab16, in a dose-dependent manner, which resulted in mito-ROS increase as measured by MitoSox staining. The released v1.3GFP from these cells was measured in the supernatant for GFP fluorescence. (A) The graph shows the dose-dependent increase of mito-ROS after treating the cells with Tab16 IgG. There was no ROS induction by the control antibody compared with rottlerin 5 μM, as a positive control, which induced significant ROS in these cells. The data shown here are after background ROS subtraction of untreated cells. (B) The images are representations of the ROS increase in these cells at the highest dose of Tab16. (C) The bar graph shows the level of apoptosis induced in these cells at the highest concentration, as measured by the viability test. (D) The bar graph shows the increase in GFP fluorescence observed in the supernatant collected from each of the treated cells. This showed that neutral TSHR antibodies can induce cell stress followed by cell death, thus leading to release of v1.3 protein. Cont, control; ROT, rottlerin.
Discussion
The human TSHR protein is encoded by a single gene located on chromosome 14q31 (24, 41, 42) and can transcribe multiple mRNA transcripts in addition to the single ORF that gives rise to the full-length receptor (43). Demonstration of the TSHRfl in human embryonic stem cells (44) and a variety of mature tissues, especially bone (45), fibroblasts, thymus, and adipocytes (46, 47), questions the relevance of multiple receptor messages transcribed and translated in these different tissue depots, which may reflect tissue-specific patterns of expression. This study has begun to characterize the most abundant alternatively spliced isoform of the TSHR, namely TSHRv1.3 (14).
Genomic studies have suggested that 40% to 60% of human genes are spliced (48, 49). This most likely contributes to the creation of a diverse proteome from relatively few mammalian genes (50), which, in turn, may influence human health and disease (13, 51). Comparative genomics, using orthologous genes in human, mouse, and rat, has suggested that the frequency of exon creation and/or loss is the most common splicing event associated with increased evolutionary change (52). Glycoprotein hormone receptors such as for LH/human chorionic gonadotropin, FSH, and TSH are not exempted from this molecular pre-mRNA processing. Analysis of 80 LH-receptor cDNA clones showed that 40% of clones revealed splice variants that had skipped transmembrane-encoding exons, leading to truncated receptor isoforms (53). Furthermore, studies using liquid hybridization (15) and Northern blot analysis (14), and other reports (17), have clearly shown that the TSHR has multiple isoforms arising from alternative splicing (43).
A bioinformatics method for identification of splice variants has been created for G protein–coupled receptors (54) on the basis of aligning the receptor nucleotide sequence and its expressed sequence tag and mRNA sequence from GenBank to the genome sequence using the BLAST-like alignment tool (55). Using a similar approach, we computationally examined the TSHR gene for its various existing splice variants (Fig. 1A). By this preliminary analysis, we learned that the TSHR exists in more than one isoform and v1.3 may be the most predominant form. This observation led us to perform a sampling of the cDNA obtained from human tissues to look for v1.3 mRNA (Fig. 1B). By quantitating the ratio of v1.3 to that of the full-length receptor in tissues such as thyroid, bone marrow, thymus, and adipose, we showed that the v1.3 transcript is actively transcribed in each of these tissue depots alongside the full-length receptor (Fig. 1C). These observations suggested that the alternatively spliced form of TSHR is not merely a transcriptional artifact, as previously stated (56), but that this receptor isoform might have causal effects in these tissue(s) by potentially encoding a protein molecule that is a pseudoreceptor and thus interacts with the cognate hormone and/or autoantibodies.
TSHRv1.3 mRNA has been reported in many tissues but especially in bone cells, thymus, adipocytes (47, 57), and orbital fibroblasts (46). Whether the protein product of this transcript could influence the full-length receptor or its ligand had not been studied, to our knowledge. Here we show that an intronic segment that contributes to the unique 63-bp (21 amino acids) of TSHRv1.3 is from intron 8, which harbors a predicted splice acceptor site that might perpetuate splicing events leading to the existence of this isoform. Hence, alternative splicing may be constitutive in the tissues that express the TSHR gene, although presently we do not fully understand the mechanism of its regulation. Although alternative spliced products of the TSHR might behave like “soluble” receptors that have been implicated as TSH-binding protein(s) in the peripheral circulation (58) and in crude thyroid tissue homogenates (59), direct evidence of a splice isoform interaction has been lacking. It has been suggested that such TSH-binding proteins can arise because of proteolytic cleavage and shedding of the full-length receptor (60, 61), but it is hard to comprehend that such a phenomenon would be a perpetual source of circulating antigen and ligand-bound protein, given the extremely low density of TSHRs (∼5000 receptors per cell) on the surface of these cells (23) and that cleavage of these cell-surface receptors never reaches 100%.
We expressed the entire ORF of TSHRv1.3 in heterologous cells and then examined the binding of the recombinant product to its cognate hormone and to TSHR autoantibody. Structurally, the TSHRv1.3 variant retains the crystallized concave ECD structure with the unique intronic region as a free-floating α helix. Because we were not sure if the endogenous signal peptide would bring the protein to the surface as a secreted molecule, we cloned it into a vector with a leader sequence from IgG κ, which we presumed would force secretion of any cDNA cloned to its C-terminus. However, the TSHRv1.3 protein did not secrete but was still retained within the cytoplasm (Fig. 4A). This observation points to the critical roles of the carboxyl tail and transmembrane domain of a receptor in proper trafficking to the plasma membrane.
Modeling the binding of TSHRv1.3 in silico using molecular dynamic simulation studies with TSH-β demonstrated that the variant protein retained TSH-binding potential despite the addition of the intronic region (Fig. 4B), suggesting structural integrity of this truncated isoform. The His-purified v1.3 protein expressed in HEK cells was thus able to bind both TSH and MS-1 during immunoprecipitation studies (Fig. 4C), suggesting that variant(s) of the TSHR may act as decoy proteins and can dampen TSHR signaling.
It has been reported that plasmid immunization of mice with a TSHR splice variant that lacks exon 5 (TSHR739) and that lacked TSH-binding activity induced a Graves-like disease in mice, which was proof that a novel splice variant may be a candidate neo-autoantigen (62). Entrapped protein formed by mutations of the gene, which alter cell physiology, have been described in congenital hypothyroidism (63). Similarly, cells with intracellular protein can act as an antigenic rheostat for immune stimulation if they are released by apoptosis. We have previously demonstrated that neutral TSHR antibodies are part of the pool of autoantibodies seen in patients with GD and, by virtue of their ability to bind to the receptor but inability to induce cAMP, can cause cell stress leading to increased intracellular ROS production and even apoptosis (25, 26). We have shown here as proof of principle that this can occur when using GFP-tagged v1.3 and showing its release from cells that were stressed by neutral TSHR antibody (Fig. 7A and 7B). Thus, TSHRv1.3 expressed in thyrocytes can also be a potential source of this neoantigen released on thyroid cell breakdown, and this may well occur in GD.
The demonstration of TSHRv1.3 expression in thymus tissue may be highly relevant when considering v1.3 as a novel autoantigen. An alteration in intrathymic expression of TSHRfl has been suggested as an inducer of aberrations in central tolerance, which can lead to thyroid autoimmunity (64, 65). For example, it has been shown that thymus glands from people homozygous for the GD-related single nucleotide polymorphism (SNP) rs179247 in intron 1 of the TSHR had significantly fewer thymic TSHR mRNA transcripts than did carriers of the protective allele (12). Furthermore, disease-associated SNP genotypes, such as rs179247 (AA) and rs12101255 (TT), are associated with changes in expression levels of TSHR splice variants, thus reinforcing the idea that a splice variant of the TSHR might have a role in disease pathogenesis (66). Furthermore, it has been proposed that GD risk alleles may determine increased expression of TSHR mRNA splice variants that could code for soluble receptor (67, 68). Released TSHRv1.3 after cell death would also retain the ability to attenuate TSH or stimulating antibody–induced responses by competing for ligand (Fig. 5). Antibody responses measured against v1.3 peptides in patients with GD would suggest the antigenic nature of such a splice protein.
In summary, our data continue to indicate that truncated TSHR isoforms, even expressed intracellularly, have the potential to act as pseudoreceptors and autoantigens when released from the cells by antibody-mediated cellular stress, thus retaining potential to modulate the pathogenesis of GD. Furthermore, the demonstration that epitopes within the splice variant are antigenic and that patients with GD can mount an antibody response would also suggest that TSHRv1.3 is not an inert receptor protein (Fig. 6). These results, therefore, show that the TSHRv1.3, which is globally transcribed in several human tissues, has TSH and autoantibody-binding ability, and this reflects its antigenicity. Understanding the regulatory signals and effect of disease-associated SNPs in modulating TSH signaling and immune reactivity during disease pathogenesis would give us stronger insight into the role of these isoforms in thyroid-disease pathogenesis.
Acknowledgments
We thank Dr. Ramkumarie Baliram and Dr. Simeng Sun for their helpful suggestions and reading through the manuscript.
Financial Support: This work was supported in part by the National Institutes of Health (Grant DK069713 to T.F.D.), the David Owen Segal Endowment, and the VA Merit Review Program (Merit Number BX000800 to T.F.D.).
Disclosure Summary: T.F.D. is a member of the Kronus Inc. board. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- CHO
Chinese hamster ovary
- COOH
carboxy terminal peptide
- ECD
ectodomain
- GD
Graves’ disease
- GFP
green fluorescent protein
- HEK
human embryonic kidney
- His
histidine
- mito-ROS
mitochondrial reactive oxygen species
- HSF
Human Splicing Finder
- OD
optical density
- ORF
open reading frame
- ROS
reactive oxygen species
- SNP
single nucleotide polymorphism
- TSHR
TSH receptor
- TSHRfl
full-length TSH receptor
References
- 1. Davies TF, Ando T, Lin RY, Tomer Y, Latif R. Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J Clin Invest. 2005;115(8):1972–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM. The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr Rev. 1998;19(6):673–716. [DOI] [PubMed] [Google Scholar]
- 3. Vassart G, Dumont JE. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev. 1992;13(3):596–611. [DOI] [PubMed] [Google Scholar]
- 4. Chazenbalk GD, Tanaka K, McLachlan SM, Rapoport B. On the functional importance of thyrotropin receptor intramolecular cleavage. Endocrinology. 1999;140(10):4516–4520. [DOI] [PubMed] [Google Scholar]
- 5. Latif R, Graves P, Davies TF. Oligomerization of the human thyrotropin receptor: fluorescent protein-tagged hTSHR reveals post-translational complexes. J Biol Chem. 2001;276(48):45217–45224. [DOI] [PubMed] [Google Scholar]
- 6. Urizar E, Montanelli L, Loy T, Bonomi M, Swillens S, Gales C, Bouvier M, Smits G, Vassart G, Costagliola S. Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. EMBO J. 2005;24(11):1954–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graves PN, Vlase H, Bobovnikova Y, Davies TF. Multimeric complex formation by the thyrotropin receptor in solubilized thyroid membranes. Endocrinology. 1996;137(9):3915–3920. [DOI] [PubMed] [Google Scholar]
- 8. Ando T, Latif R, Davies TF. Thyrotropin receptor antibodies: new insights into their actions and clinical relevance. Best Pract Res Clin Endocrinol Metab. 2005;19(1):33–52. [DOI] [PubMed] [Google Scholar]
- 9. Smith BR, Sanders J, Furmaniak J. TSH receptor antibodies. Thyroid. 2007;17(10):923–938. [DOI] [PubMed] [Google Scholar]
- 10. Davies TF, Yin X, Latif R. The genetics of the thyroid stimulating hormone receptor: history and relevance. Thyroid. 2010;20(7):727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee HJ, Li CW, Hammerstad SS, Stefan M, Tomer Y. Immunogenetics of autoimmune thyroid diseases: a comprehensive review. J Autoimmun. 2015;64:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colobran R, Armengol MP, Faner R, Gärtner M, Tykocinski LO, Lucas A, Ruiz M, Juan M, Kyewski B, Pujol-Borrell R. Association of an SNP with intrathymic transcription of TSHR and Graves’ disease: a role for defective thymic tolerance. Hum Mol Genet. 2011;20(17):3415–3423. [DOI] [PubMed] [Google Scholar]
- 13. Faustino NA, Cooper TA. Pre-mRNA splicing and human disease. Genes Dev. 2003;17(4):419–437. [DOI] [PubMed] [Google Scholar]
- 14. Graves PN, Tomer Y, Davies TF. Cloning and sequencing of a 1.3 KB variant of human thyrotropin receptor mRNA lacking the transmembrane domain. Biochem Biophys Res Commun. 1992;187(2):1135–1143. [DOI] [PubMed] [Google Scholar]
- 15. Tomer Y, Graves PN, Jin A, Schwartz AE, Friedman EW, Davies TF. Liquid hybridization analysis of TSH receptor mRNA in normal and abnormal human thyroid tissues. Thyroid. 1993;3(3):219–224. [DOI] [PubMed] [Google Scholar]
- 16. Grossman RF, Ban T, Duh QY, Tezelman S, Jossart G, Soh EY, Clark OH, Siperstein AE. Immunoprecipitation isolates multiple TSH receptor forms from human thyroid tissue. Thyroid. 1995;5(2):101–105. [DOI] [PubMed] [Google Scholar]
- 17. Takeshita A, Nagayama Y, Fujiyama K, Yokoyama N, Namba H, Yamashita S, Izumi M, Nagataki S. Molecular cloning and sequencing of an alternatively spliced form of the human thyrotropin receptor transcript. Biochem Biophys Res Commun. 1992;188(3):1214–1219. [DOI] [PubMed] [Google Scholar]
- 18. Hunt N, Willey KP, Abend N, Balvers M, Jähner D, Northemann W, Ivell R. Novel splicing variants of the human thyrotropin receptor encode truncated polypeptides without a membrane-spanning domain. Endocrine. 1995;3(3):233–240. [DOI] [PubMed] [Google Scholar]
- 19. Willey KP, Hunt N, Abend N, Northemann W, Ivell R, Leidenberger F. Serum unmasks the binding of thyroid-stimulating hormone to endogenous and transfected receptors: evidence for a soluble form of the receptor in human thyroid. J Endocrinol. 1993;139(2):317–328. [DOI] [PubMed] [Google Scholar]
- 20. Adams DD, Dirmikis S, Doniach D, El Kabir DJ, Hall R, Ibbertson HK, Irvine WJ, Kendall-Taylor P, Manley SW, Mehdi SQ, Munro DS, Purves HD, Smith BR, Stewart RD. Letter: nomenclature of thyroid-stimulating antibodies. Lancet. 1975;305(7917):1201. [DOI] [PubMed] [Google Scholar]
- 21. Adams DD, Kennedy TH, Purves HD, Siret NE. Failure of TSH antisera to neutralize long-acting thyroid stimulator. Endocrinology. 1962;70(6):801–805. [DOI] [PubMed] [Google Scholar]
- 22. Adams DD, Purves HD. The assessment of thyroid function by tracer tests with radioactive iodine. N Z Med J. 1956;55(305):36–41. [PubMed] [Google Scholar]
- 23. Libert F, Lefort A, Gerard C, Parmentier M, Perret J, Ludgate M, Dumont JE, Vassart G. Cloning, sequencing and expression of the human thyrotropin (TSH) receptor: evidence for binding of autoantibodies. Biochem Biophys Res Commun. 1989;165(3):1250–1255. [DOI] [PubMed] [Google Scholar]
- 24. Misrahi M, Loosfelt H, Atger M, Sar S, Guiochon-Mantel A, Milgrom E. Cloning, sequencing and expression of human TSH receptor. Biochem Biophys Res Commun. 1990;166(1):394–403. [DOI] [PubMed] [Google Scholar]
- 25. Morshed SA, Ma R, Latif R, Davies TF. How one TSH receptor antibody induces thyrocyte proliferation while another induces apoptosis. J Autoimmun. 2013;47:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morshed SA, Ma R, Latif R, Davies TF. Biased signaling by thyroid-stimulating hormone receptor-specific antibodies determines thyrocyte survival in autoimmunity. Sci Signal. 2018;11(514):eaah4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ryan MC, Zeeberg BR, Caplen NJ, Cleland JA, Kahn AB, Liu H, Weinstein JN. SpliceCenter: a suite of web-based bioinformatic applications for evaluating the impact of alternative splicing on RT-PCR, RNAi, microarray, and peptide-based studies. BMC Bioinformatics. 2008;9(1):313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. RRID: AB_444306, https://scicrunch.org/resolver/AB_444306.
- 29. RRID: AB_793069, https://scicrunch.org/resolver/AB_793069.
- 30. RRID: AB_2208240, https://scicrunch.org/resolver/AB_2208240.
- 31. Latif R, Lau Z, Cheung P, Felsenfeld DP, Davies TF. The “TSH Receptor Glo Assay” – a high-throughput detection system for thyroid stimulation. Front Endocrinol (Lausanne). 2016;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Latif R, Ali MR, Mezei M, Davies TF. Transmembrane domains of attraction on the TSH receptor. Endocrinology. 2015;156(2):488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanders J, Chirgadze DY, Sanders P, Baker S, Sullivan A, Bhardwaja A, Bolton J, Reeve M, Nakatake N, Evans M, Richards T, Powell M, Miguel RN, Blundell TL, Furmaniak J, Smith BR. Crystal structure of the TSH receptor in complex with a thyroid-stimulating autoantibody. Thyroid. 2007;17(5):395–410. [DOI] [PubMed] [Google Scholar]
- 34. Šali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234(3):779–815. [DOI] [PubMed] [Google Scholar]
- 35. Šali A, Overington JP. Derivation of rules for comparative protein modeling from a database of protein structure alignments. Protein Sci. 1994;3(9):1582–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jo S, Kim T, Iyer VG, Im W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J Comput Chem. 2008;29(11):1859–1865. [DOI] [PubMed] [Google Scholar]
- 37. Mezei M. Simulaid: a simulation facilitator and analysis program. J Comput Chem. 2010;31(14):2658–2668. [DOI] [PubMed] [Google Scholar]
- 38.Humphrey W, Dalke A, Schulten K. doi: 10.1016/0263-7855(96)00018-5. VMD: visual molecular dynamics. J Mol Graph. 1996;14(1):33–38. [DOI] [PubMed] [Google Scholar]
- 39. Desmet FO, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, Béroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37(9):e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ando T, Latif R, Pritsker A, Moran T, Nagayama Y, Davies TF. A monoclonal thyroid-stimulating antibody. J Clin Invest. 2002;110(11):1667–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nagayama Y, Kaufman KD, Seto P, Rapoport B. Molecular cloning, sequence and functional expression of the cDNA for the human thyrotropin receptor. Biochem Biophys Res Commun. 1989;165(3):1184–1190. [DOI] [PubMed] [Google Scholar]
- 42. Parmentier M, Libert F, Maenhaut C, Lefort A, Gérard C, Perret J, Van Sande J, Dumont JE, Vassart G. Molecular cloning of the thyrotropin receptor. Science. 1989;246(4937):1620–1622. [DOI] [PubMed] [Google Scholar]
- 43. Kakinuma A, Nagayama Y. Multiple messenger ribonucleic acid transcripts and revised gene organization of the human TSH receptor. Endocr J. 2002;49(2):175–180. [DOI] [PubMed] [Google Scholar]
- 44. Ma R, Latif R, Davies TF. Human embryonic stem cells form functional thyroid follicles. Thyroid. 2015;25(4):455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, Iqbal J, Eldeiry L, Rajendren G, Blair HC, Davies TF, Zaidi M. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115(2):151–162. [DOI] [PubMed] [Google Scholar]
- 46. Bahn RS. TSH receptor expression in orbital tissue and its role in the pathogenesis of Graves’ ophthalmopathy. J Endocrinol Invest. 2004;27(3):216–220. [DOI] [PubMed] [Google Scholar]
- 47. Crisp MS, Lane C, Halliwell M, Wynford-Thomas D, Ludgate M. Thyrotropin receptor transcripts in human adipose tissue. J Clin Endocrinol Metab. 1997;82(6):2003–2005. [PubMed] [Google Scholar]
- 48. Croft L, Schandorff S, Clark F, Burrage K, Arctander P, Mattick JS. ISIS, the intron information system, reveals the high frequency of alternative splicing in the human genome. Nat Genet. 2000;24(4):340–341. [DOI] [PubMed] [Google Scholar]
- 49. Modrek B, Resch A, Grasso C, Lee C. Genome-wide detection of alternative splicing in expressed sequences of human genes. Nucleic Acids Res. 2001;29(13):2850–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Black DL. Protein diversity from alternative splicing: a challenge for bioinformatics and post-genome biology. Cell. 2000;103(3):367–370. [DOI] [PubMed] [Google Scholar]
- 51. Bracco L, Kearsey J. The relevance of alternative RNA splicing to pharmacogenomics. Trends Biotechnol. 2003;21(8):346–353. [DOI] [PubMed] [Google Scholar]
- 52. Graveley BR. Alternative splicing: increasing diversity in the proteomic world. Trends Genet. 2001;17(2):100–107. [DOI] [PubMed] [Google Scholar]
- 53. Loosfelt H, Misrahi M, Atger M, Salesse R, Vu Hai-Luu Thi MT, Jolivet A, Guiochon-Mantel A, Sar S, Jallal B, Garnier J, et al. . Cloning and sequencing of porcine LH-hCG receptor cDNA: variants lacking transmembrane domain. Science. 1989;245(4917):525–528. [DOI] [PubMed] [Google Scholar]
- 54. Kan Z, Rouchka EC, Gish WR, States DJ. Gene structure prediction and alternative splicing analysis using genomically aligned ESTs. Genome Res. 2001;11(5):889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kent WJ. BLAT--the BLAST-like alignment tool. Genome Res. 2002;12(4):656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Paschke R, Metcalfe A, Alcalde L, Vassart G, Weetman A, Ludgate M. Presence of nonfunctional thyrotropin receptor variant transcripts in retroocular and other tissues. J Clin Endocrinol Metab. 1994;79(5):1234–1238. [DOI] [PubMed] [Google Scholar]
- 57. Ludgate M, Crisp M, Lane C, Costagliola S, Vassart G, Weetman A, Daunerie C, Many MC. The thyrotropin receptor in thyroid eye disease. Thyroid. 1998;8(5):411–413. [DOI] [PubMed] [Google Scholar]
- 58. Lee G, Aloj SM, Beguinot F, Kohn LD. Existence of a soluble thyrotropin binding component in normal human sera. J Biol Chem. 1977;252(22):7967–7970. [PubMed] [Google Scholar]
- 59. Adlkofer F, Kotulla P, Schleusener H. Binding of thyrotrophin to low molecular weight fragments of human thyroid membranes. Acta Endocrinol (Copenh). 1980;94(1):58–63. [DOI] [PubMed] [Google Scholar]
- 60. de Bernard S, Misrahi M, Huet JC, Beau I, Desroches A, Loosfelt H, Pichon C, Pernollet JC, Milgrom E. Sequential cleavage and excision of a segment of the thyrotropin receptor ectodomain. J Biol Chem. 1999;274(1):101–107. [DOI] [PubMed] [Google Scholar]
- 61. Quellari M, Desroches A, Beau I, Beaudeux E, Misrahi M. Role of cleavage and shedding in human thyrotropin receptor function and trafficking. Eur J Biochem. 2003;270(17):3486–3497. [DOI] [PubMed] [Google Scholar]
- 62. Endo T, Kobayashi T. Immunization of mice with a newly identified thyroid-stimulating hormone receptor splice variant induces Graves’-like disease. J Autoimmun. 2013;43:18–25. [DOI] [PubMed] [Google Scholar]
- 63. Calebiro D, de Filippis T, Lucchi S, Covino C, Panigone S, Beck-Peccoz P, Dunlap D, Persani L. Intracellular entrapment of wild-type TSH receptor by oligomerization with mutants linked to dominant TSH resistance. Hum Mol Genet. 2005;14(20):2991–3002. [DOI] [PubMed] [Google Scholar]
- 64. McLachlan SM, Aliesky HA, Banuelos B, Lesage S, Collin R, Rapoport B. High-level intrathymic thyrotrophin receptor expression in thyroiditis-prone mice protects against the spontaneous generation of pathogenic thyrotrophin receptor autoantibodies. Clin Exp Immunol. 2017;188(2):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stefan M, Wei C, Lombardi A, Li CW, Concepcion ES, Inabnet WB III, Owen R, Zhang W, Tomer Y. Genetic-epigenetic dysregulation of thymic TSH receptor gene expression triggers thyroid autoimmunity. Proc Natl Acad Sci USA. 2014;111(34):12562–12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brand OJ, Barrett JC, Simmonds MJ, Newby PR, McCabe CJ, Bruce CK, Kysela B, Carr-Smith JD, Brix T, Hunt PJ, Wiersinga WM, Hegedüs L, Connell J, Wass JA, Franklyn JA, Weetman AP, Heward JM, Gough SC. Association of the thyroid stimulating hormone receptor gene (TSHR) with Graves’ disease. Hum Mol Genet. 2009;18(9):1704–1713. [DOI] [PubMed] [Google Scholar]
- 67. Pujol-Borrell R, Álvarez-Sierra D, Jaraquemada D, Marín-Sánchez A, Colobran R. Central Tolerance Mechanisms to TSHR in Graves’ disease: contributions to understand the genetic association. Horm Metab Res. 2018;50(12):863–870. [DOI] [PubMed] [Google Scholar]
- 68. Pujol-Borrell R, Giménez-Barcons M, Marín-Sánchez A, Colobran R. Genetics of Graves’ disease: special focus on the role of TSHR gene. Horm Metab Res. 2015;47(10):753–766. [DOI] [PubMed] [Google Scholar]