Abstract
Rapid Diagnostic Tests (RDTs) for malaria are restricted to a few biomarkers and antibody-mediated detection. However, the expression of commonly used biomarkers varies geographically and the sensibility of immunodetection can be affected by batch-to-batch differences or limited thermal stability. In this study we aimed to overcome these limitations by identifying a potential biomarker and by developing molecular sensors based on aptamer technology. Using gene expression databases, ribosome profiling analysis, and structural modeling, we find that the High Mobility Group Box 1 protein (HMGB1) of Plasmodium falciparum is highly expressed, structurally stable, and present along all blood-stages of P. falciparum infection. To develop biosensors, we used in vitro evolution techniques to produce DNA aptamers for the recombinantly expressed HMG-box, the conserved domain of HMGB1. An evolutionary approach for evaluating the dynamics of aptamer populations suggested three predominant aptamer motifs. Representatives of the aptamer families were tested for binding parameters to the HMG-box domain using microscale thermophoresis and rapid kinetics. Dissociation constants of the aptamers varied over two orders of magnitude between nano- and micromolar ranges while the aptamer-HMG-box interaction occurred in a few seconds. The specificity of aptamer binding to the HMG-box of P. falciparum compared to its human homolog depended on pH conditions. Altogether, our study proposes HMGB1 as a candidate biomarker and a set of sensing aptamers that can be further developed into rapid diagnostic tests for P. falciparum detection.
Introduction
Malaria is an infectious disease that affects animals and humans, caused by protozoans of the genus Plasmodium. Malaria remains the cause of 435,000 deaths worldwide, with 219 million cases reported during 2017 [1]. Accurate treatment requires the identification of the parasite of the genus Plasmodium, in addition to the species causing the disease. Currently, there are several methods to diagnose malaria, such as PCR-based, Giemsa microscopy, and Rapid Diagnostic Tests (RDTs). Among these options, the last two appear to be suitable for low-income and mostly affected countries; nonetheless, they also present certain limitations [2].
Giemsa microscopy is inexpensive to perform, can differentiate malaria species and stages, and can quantify the parasites [2]. However, well-trained personnel is required and, frequently unavailable in low-income countries [2]. RDTs, on the other hand, detect Plasmodium spp. biomarker antigens in a small amount of blood using immobilized antibodies. The two most used biomarkers are histidine-rich protein 2 (HRP-2) and lactate dehydrogenase (LDH), which have been shown of high sensitivity and specificity for detecting P. falciparum [3]. However, P. falciparum strains lacking the HRP-2 and HRP-3 genes have appeared, increasing false negative results [4,5]. Furthermore, PfLDH has a maximum peak of expression at 24 to 30 hours in the intraerythrocytic cycle but decreases to a minimum in the schizont stage. On the other hand, HRP-2 is exclusively synthesized during P. falciparum asexual erythrocytic development (reviewed in [6]). Using biomarkers that are present longer in the blood stages of P. falciparum can be advantageous to increase the sensibility of infection detection.
Additionally, the production of sensing antibodies requires advanced facilities to ensure both, reproducibility and consistency between batches. This limitation is aggravated by delivery logistics to the final user where transportation and temperature conditions affect the efficiency, sensibility, and specificity of RDTs. Although RDTs continue being the best alternative for rapid screening and detection of malaria in low-income countries, they require novel biomarkers for Plasmodium spp. to overcome the detection of false negatives and the development of sensing molecules that are more reproducible and thermostable.
Recent genomic, transcriptomic, and proteomic studies of Plasmodium spp. are a great source to identify potential new and abundant biomarkers [7–9]. In this context, ribosome profiling (RP) appears to fill the gap between transcriptomics and proteomics [10]. RP evaluates mRNA expression and estimates the ribosomal load for each particular mRNA [10]. Thus, RP allows the researcher to identify which genes are expressed at any given conditions and, at the same time, RP estimates the relative protein abundance potential. In the context of biomarker discovery, RP arrives as a valid alternative to identify proteins that are highly synthesized in the cell.
Aptamers offer a valid alternative for the development of biosensors as they overcome such immunogenic requirement of antibodies [11]. Additionally, aptamers are chemically synthetized which leads to the substantial reduction of variability between production batches [11]. Aptamers, identified through Systematic Evolution of Ligands by Exponential enrichment (SELEX) [12,13], have shown additional and significant advantages over antibodies without compromising binding and biochemical specificity parameters [11]. Aptamers for malaria detection have been successfully developed for the commonly used biomarkers HRP-2 and LDH [14–17]. Here, we use gene expression databases and recent RP reports to identify a potential new biomarker for the detection Plasmodium spp., recombinantly produce it to purity, and develop high-affinity aptamers as biosensors. Altogether, our study proposes a novel biomarker and a set of biosensors that can further be developed into RDTs for Plasmodium spp. detection.
Material and methods
Biomarker selection
Translational gene expression regulation, as assessed by RP on asexual blood stages, were primarily used to identify highly translated mRNAs [9]. RNA-seq on all blood stages of P. falciparum were compared using PlasmoDB search tools [9,18,19]. Genes whose transcription was homogenous and constant among all blood stages of P. falciparum were selected. The corresponding proteins were probed for the availability of three-dimensional structures or the potential to be predicted. Proteins without atomic resolution or predicted structures were excluded as available structural reports show that aptamers bind to tertiary folds of proteins [20]. Protein stability and solubility, as well as the presence of transmembrane domains, were estimated using the ExPASy ProtParam tool (Swiss Institute of Bioinformatics, Switzerland) and TMHMM 2.0 (Center for Biological Sequence Analysis, Technical University of Denmark, DK). The human homolog of HMGB1 was used for negative selection steps. HMGB1 contains the HMG-box, which is a conserved domain among eukaryotes [21]. Both conserved domains, from Plasmodium falciparum or Homo sapiens, are subsequently going to be called HMG-box Pf and HMG-box Hs, respectively. Clustal Omega (EMBL-EBI, UK) was used to compare HMG-box Pf and HMG-box Hs by multiple sequence alignment analysis between these two domains [22].
Oligonucleotides, cloning, expression, and purification of HMG-box domain
Oligonucleotides, unmodified or chemically modified with fluorescein, were synthesized by Macrogen (Korea). DNA sequences coding for HMG-box Pf and Hs were cloned into the plasmid pET-24c (+) (KanR) by GenScript (USA). The coding sequences of both domains were optimized for codon usage of the host (E. coli BL21) using OptimumGene (GenScript, USA) and a His-Tag was added at the carboxyl end for further purification. E. coli BL21 (DE3) were made competent and transformed using Mix & Go E. coli Transformation Kit & Buffer Set (Zymo Research, USA). Transformed E. coli were cultured in Terrific Broth (yeast extract 24 g/L, tryptone 20 g/L, KH2PO4 0.017 M, K2HPO4 0.072 M) medium at 37°C until the absorbance at 600 nm reached 0.5. Then, Isopropyl β-D-1-thiogalactopyranoside (IPTG) 1 mM was added to induce protein expression and incubation continued for three hours. After incubation, the culture was centrifuged at 6,000 g for 20 min at 4°C. Then, the supernatant was discarded, and the pellet was resuspended using HAKM10 Buffer (50 mM HEPES (pH 7.4), 70 mM ammonium acetate, 30 mM NaCl, 10 mM MgCl2, 6 mM 2-mercaptoethanol). Cells were pelleted again before lysis with using 5 mL BugBuster Master Mix (EMD Millipore, USA) for each gram of cells. After resuspension and incubation at room temperature for 20 minutes, the lysate was centrifuged at 12,000 g for 40 min at 4°C. The supernatant was collected for purification using a 1 mL His Trap FF Crude Column containing Ni-NTA (GE Healthcare, USA). Briefly, the column was equilibrated using five volumes of His-Tag Buffer with 10 mM imidazole (20 mM sodium phosphate (pH 7.4), 0.5 M NaCl). Then, the collected supernatant was loaded into the column followed by three volumes of His-Tag Buffer containing 30 mM imidazole to remove nonspecific bound proteins. Finally, HMG-box was eluted with five volumes of His-Tag Buffer containing 300 mM imidazole. 1 mL fractions were collected independently and analyzed by SDS-PAGE electrophoresis (18% acrylamide) and Coomassie blue staining. Eluted proteins were dialyzed using a 3 kDa membrane D-Tube Dialyzer (Merck, Germany) to remove the imidazole in Storage Buffer (200 mM ammonium acetate, 50 mM HEPES, 6 mM 2-Mercaptoethanol, 10% glycerol, pH 7.1). Protein purity was assessed by (18% acrylamide) SDS-PAGE electrophoresis and Coomassie blue staining.
Protein labeling
HMG-box Pf and Hs preparations were labeled with NHS-Biotin for SELEX rounds or with NHS-Atto540Q essentially as described in [23]. Protein preparations were dialyzed using NHS Labeling Buffer (200 mM NaCl, 50 mM HEPES, 6 mM 2-Mercaptoethanol, 5% Glycerol, pH 7.8) to remove primary amines. Labeling reactions were performed using a 2:1 label to protein ratio. Reactions were incubated with moderated stirring at room temperature for 1 hour. After incubation, non-protein-bound labels were quenched using Glycine in a final concentration of 25 mM. Labeled proteins were purified using His Trap FF Crude Column containing Ni-NTA (as described above) and dialyzed in Storage Buffer.
SELEX
SELEX was performed using a 40-mer ssDNA random library (Trilink Biotechnologies, USA) which contained constant flanking regions: 5’ TAG GGA AGA GAA GGA CAT ATG AT (N40) TTG ACT AGT ACA TGA CCA CTT GA 3’. Amplification primers were: forward (5’ TAG GGA AGA GAA GGA CAT ATG AT 3’) and reverse (5’ TCA AGT GGT CAT GTA CTA GTC AA 3’). For transcription-mediated amplification of libraries, a T7 promoter sequence was added (T7 reverse primer: 5’ TTC AGG TAA TAC GAC TCA CTA TAG GGT CAA GTG GTC ATG TAC TAG TCA A 3’). Each selection round was composed of: i) Aptamer selection using biotinylated HGM-box that were immobilized on streptavidin-coated magnetic beads (Streptavidin Mag Sepharose from GE Healthcare, USA). The bead binding capacity for either HMG-box Pf or HMG-box Hs was estimated to19 pmol/μL beads slurry. Selections were performed in SELEX Buffer (100 mM NaCl, 1.5 mM KCl, 5 mM MgCl2, 6mM 2-Mercaptoethanol, 50 mM Tris HCl, pH 7.5) at 25°C with moderate and continuous shaking. ii) HGM-box-bound aptamers were separated from the solution using a magnet followed by resuspension using SELEX buffer and a second magnetic separation. The number of bead resuspensions (washes) varied along SELEX rounds (S2 Table). iii) Bound oligonucleotides were used for further regeneration of the ssDNA library by PCR using Trilink forward, and T7 Trilink-modified reverse primers using the Maxima Hot Start Green PCR Master Mix (2X) (Thermo Fisher Scientific, USA). iv) Resulting dsDNA were used for in vitro transcription using the TranscriptAid T7 High Yield Transcription Kit (Thermo Fisher Scientific, USA). v) in vitro reverse transcription was used to regenerate a ssDNA pool of enriched aptamers using the RevertAid Reverse transcriptase (Thermo Fisher Scientific, USA). Generally, the selection stringency was increased along cycles of SELEX by modifying ionic strength, time of incubation and the number of washes (S2 Table). Purity and size of dsDNA, RNA, and ssDNA was assessed by 8M UREA PAGE followed by methylene blue staining. Concentration was measured by absorbance at 260 nm in a Nanodrop One spectrophotometer (Thermo Fisher Scientific, USA).
Diversity Visualization by Endonuclease (DiVE) assay and library enrichment
DiVE protocol was standardized based on Lim et al. [24]. All the experiments were performed using the Thermo Scientific S1 endonuclease (Thermo Fisher Scientific, USA). For the reaction tubes, 200 ng of double-stranded DNA (dsDNA) from each SELEX cycle was used. The final volume of the reaction was 20 μL, and each sample contained S1 Reaction Buffer (40 mM sodium acetate (pH 4.5), 300 mM NaCl, 2 mM ZnSO4). Each tube reaction was subjected to denaturation at 95°C for 5 minutes and reannealing at 65°C for 5 minutes. After that, 1 μL of S1 endonuclease U/μL was added to the mixture followed by 30 minutes incubation at 65°C. Reactions were stopped by adding ethylene- diaminetetraacetic acid (EDTA) to a final concentration of 2 mM (pH 8.0). Finally, 5μL of each sample was mixed with 1 μL of RunSafe (Cleaver Scientific, UK) and visualized in a 2% agarose gel. Low diversity samples appeared as defined bands of the expected DNA length while high diversity samples appeared as smears or were absent.
Library sequencing
Cycles four, six, eight, ten, and fourteen were taken for Next Generation Sequencing as they showed low (cycle four), partial (cycles six and eight) and high (cycles ten and fourteen) enrichment as evaluated by DiVE. The dsDNA pools were processed as described in the 16S Metagenomic Sequencing Library Preparation protocol (Illumina, USA). Briefly, 5 ng of dsDNA from each selected cycle was amplified by PCR for eight cycles using forward (5’ TCG TCG GCA GCG TCA GAT GTG TAT AAG AGA CAG TAG GGA AGA GAA GGA CAT ATG AT 3’) and reverse (5’ GTC TCG TGG GCT CGG AGA TGT GTA TAA GAG ACA GTC AAG TGG TCA TGT ACT AGT CAA 3’) Trilink primers modified with overhang adapters. After amplification, PCR products were purified with the AMPure XP beads (Beckman Coulter, USA) protocol. Subsequently, sequencing indexes were added by PCR, provided by the Nextera XT Index Kit (Illumina, USA). Finally, indexed PCR products were purified with AMPure XP beads (Ampure, USA). Each indexed PCR product was quantified by absorbance at 260 nm and diluted to a final concentration of 4 nM using 10 mM Tris (pH 8.5). Five microliters of each indexed PCR were mixed to obtain the pooled library. Finally, the pooled library was denatured using an equal volume of 0.2 N NaOH, incubated and room temperature for 5 minutes and diluted again to a final concentration of 4 pM using HT1 Buffer (Illumina, USA) and loaded into the MiSeq Reagent Nano Kit v2 cartridge (Illumina, USA) for 300 reading cycles.
Bioinformatic analysis and aptamer selection
For a primary analysis of the sequences and a comparison between SELEX cycles, the bioinformatic toolkit FASTAptamer was employed [25]. Reverse data retrieved by sequencing was reverse complemented and mixed with the forward data to obtain a collection of sequences comprising both sequencing steps. The FASTAptamer Count command allowed to estimate the frequency for all sequences in each SELEX cycle. Subsequently, the FASTAptamer Enrich command was used to estimate the relative increase in abundance between subsequent SELEX cycles for each sequence analyzed. Candidate molecules were ranked depending on the enrichment between cycles and abundance. The MEME Suite and Clustal Omega (EMBL-EBI) was used to evaluate the presence of conserved motifs [22,26]. Mfold was used to determine the Gibbs free energy of potential secondary structures using the selection conditions, 25°C, 100 mM Na+, and 3 mM Mg+2 [27]. Conserved flanking regions of the aptamer library were included in the analysis.
Three-dimensional structure modeling and docking predictions
Modeling of three-dimensional (3D) structures of aptamers followed the principles described by Jeddi et al. [28]. Briefly, the Mfold web server was employed to predict secondary structures of the aptamers as described above. Output files were saved using the Vienna bracket format [27]. A 3D equivalent single-stranded RNA (ssRNA) model was built for each aptamer through the RNAComposer web server, using the bracket structures previously obtained [29]. The 3D ssRNA models were manually transformed into ssDNA structures using the molefacture and the autoPSF plugin of VMD software [30]. This was achieved by replacing the H5 atom in each uracil residue with a methyl moiety, manually replacing the uracil denomination with thymine in the PDB file, and changing the ribose to deoxyribose with the autoPSF builder [28]. Then, we used VMD and NAMD to refine the new 3D DNA structures in an ionized (100 mM NaCl) water box through 10,000 iterations of energy minimization [31,32]. The 3D structure of HMG-box Pf was built using homology modeling with the SWISS-MODEL Suit ([33]. Finally, the ZDOCK server was employed to predict the structural interaction between the aptamers and the HMG-box Pf [34]. We used the ZDOCK server with ZDOCK 3.0.2 without specifying specific interactions.
Determination of dissociation constants
Dissociation constants were measured using Microscale Thermophoresis (MST) on a Monolith NT.115 instrument (NanoTemper, Germany). Thermal migrations were monitored in real time by using a FAM-labeled probe (probeFAM) annealed to the 5' end of the aptamer. Pre-annealing of the probe to the aptamer was performed by mixing aptamers with probeFAM in 4:1 ratio followed by 5 min denaturation at 95°C and 20 min renaturation at room temperature. Reactions (20 μL) were prepared containing 50 nM of aptamers and varying concentrations of either HMG-box Pf or Hs. Before measuring, samples were centrifuged at 14,000 g for 5 min. The reactions were loaded into MO-K022 standard capillaries (NanoTemper, Germany) and measured. A preliminary fluorescence scan of capillaries was used to assess homogeneity within the titration. Reactions were excluded if a fluorescence difference between scans was higher than 10%. Time courses of fluorescence measurements were set 5 seconds with the infrared (IR) laser off, 30 seconds with the IR on, and 5 seconds off. A delay of 25 seconds between reads was used for thermal equilibration of the instrument. All reactions were performed in PBS at room temperature. IR laser power was set to 20% while fluorescence excitation power was set to 20% to 70% according to the dynamic of each aptamer.
Kinetic experiments
To measure the kinetics of aptamer-protein interactions an SX20 stopped-flow apparatus was used (Applied Photophysics, UK). All reactions were performed in SELEX Buffer (pH 7.4). Aptamers, PfR6 and PfE3, were pre-annealed to the probeFAM as described above and called from herein, PfR6FAM and PfE3FAM. Fluorescent measurements were carried out at 22 ˚C using a monochromatic light source (470 nm) powered with 10 mA. Emitted fluorescence was measured with photomultipliers set at 320 V after passing a long-pass filter with a cut-off of 515 nm essentially as described in [35]. Equal volumes (60 μL) of each reactant were rapidly mixed and fluorescence changes over time were measured. Typically, one solution contained aptamer (0.25 μM), probeFAM (0.2 μM) while the second solution was composed of 1 μM HMG-box540Q Pf or Hs, quenching derivatives of their respective proteins labeled with Atto-540Q. Between 7 to 10 replicates were measured. Every single measurement acquired 1,000 data points in a logarithmic sampling mode. Apparent rate constants were estimated by non-linear regression with exponential equations using Prism 7.02 (Graphpad Sofware, USA). Averaged rate constants were calculated as described in [36].
Results
HMGB1 as a biomarker for Plasmodium detection
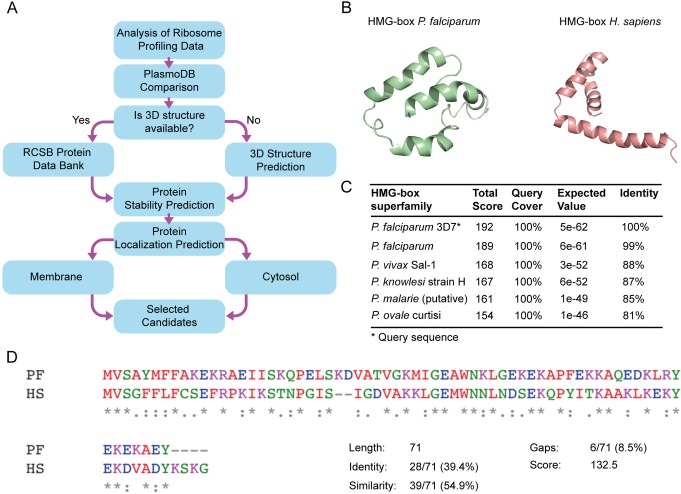
Recent advancements in the ‘omics field allow novel approaches to assess gene expression regulation at several stages, namely, gene transcription, mRNA translation and protein availability in the cell. Among these, translation regulation can be studied by RP, which provides unprecedented parameters, both qualitative and quantitative [37]. At any given cell growth state, RP can relatively estimate the ribosome load for each identified mRNA [37]. Recent applications of RP for P. falciparum identified more than 3,000 actively translated mRNAs at different stages of parasite life-cycle [9]. Actively translated mRNAs shall result in highly expressed proteins, an appealing feature to propose new potential biomarkers as parasite density can be estimated. Using the freely available RP databases, biomarker candidates were ranked according to their expression in the merozoite blood-stage, as being the stage with the most active protein synthesis apparatus (Fig 1A). Any gene whose expression was null during any blood stage was excluded since homogenous expression during all blood stages was desired as biomarkers that are present longer in the blood stages of P. falciparum can be advantageous increase the sensibility of infection detection. To assess the expression variability along blood-stages of P. falciparum the most expressed genes from RP datasets were validated by transcriptomic reports using PlasmoDB [18,19]. Candidates whose expression was not uniform during all blood stages were eliminated. Biomarker candidates that met both selection criteria, being abundant and showing similar expression patterns during all blood stages of the parasite, were pre-selected (Fig 1).
Fig 1. HMG-box as a potential biomarker of Plasmodium falciparum.
(A) Workflow for the identification of highly abundant proteins of Plasmodium falciparum as novel biomarker candidates. (B) Structure predictions by homology of HMG-box from P. falciparum and its human homolog using the Swiss-model suite [33]. (C) BLASTp suite of HMG-box Pf shows a high percentage of identity with other pathogenic Plasmodium species. (D) Clustal Omega (EMBL-EBI, UK) multiple sequence alignment for HMG-box Pf and HMG-box Hs. Both domains have 71 amino acids and share 39.4% of identity and 54.9% of similarity.
Further bioinformatic analysis allowed to investigate structural features of the potential biomarkers. From the top five biomarker candidates, only fructose-bisphosphate aldolase (FBPA) had a known 3D structure (PDB 1A5C) [38]. However, the tertiary structure of HMGB1, eIF1, Tetraubiquitin, 14-3-3 protein was available from modeling based on proteins from other species that shared at least 40% of their amino acid sequence (Plasmo DB, [18]). From the five potential biomarkers of P. falciparum, HMGB1 showed up as being abundant, present along all blood-stages of the parasite and, folding into a stable tertiary structure (Fig 1B). Therefore, HMGB1 displays substantial premises for the development of molecular sensors for Plasmodium detection. The HMGB1 protein from Plasmodium spp. has a human homolog, and both contain a DNA binding domain, HMG-box (Fig 1B) [21]. Furthermore, the HMG-box domain is highly conserved among Plasmodium species that infect humans, such as P. vivax (88% identity), P. knowlesi (87% identity), P. malarie (85% identity), and P. ovale (81% identity) (Fig 1C). Of particular importance, HMG-box Pf shows limited identity (39.4%) with HMG-box Hs (Fig 1D). Altogether, abundance, availability during all infectious stages of the parasite, and low identity with the human counterpart make the HMG-box an appealing biomarker to develop new biosensors.
Aptamers against HMG-box Pf
In order to develop molecular sensors that can specifically recognize the HMG-box Pf, we used Systematic Evolution of Ligands by Exponential Enrichment (SELEX) combined with next-generation sequencing (NGS). SELEX allows to in vitro select single-stranded oligonucleotides that strongly bind a given molecule [13]. These oligonucleotides are known as aptamers [39] and were shown to have significant advantages over antibodies such as better thermal stability and synthesis consistency between production batches without compromising their binding affinities [11,40]. These characteristics are very appealing for further development of aptamers into rapid diagnostic tests to be deployed in rural areas in low-income countries, the most affected by Malaria. We performed fourteen SELEX cycles (Fig 2A) starting from a library of ssDNA containing 40 randomized nucleotides. In order to enhance the selection of tight binders, HMG-box Pf concentration, incubation time and strength of washes varied, increasing the selection stringency along the SELEX cycles (S2 Table). Whereas, to increase the specificity of the potential aptamers, negative selections using the human counterpart, HMG-box Hs, were introduced at cycles five, eight, ten, and fourteen.
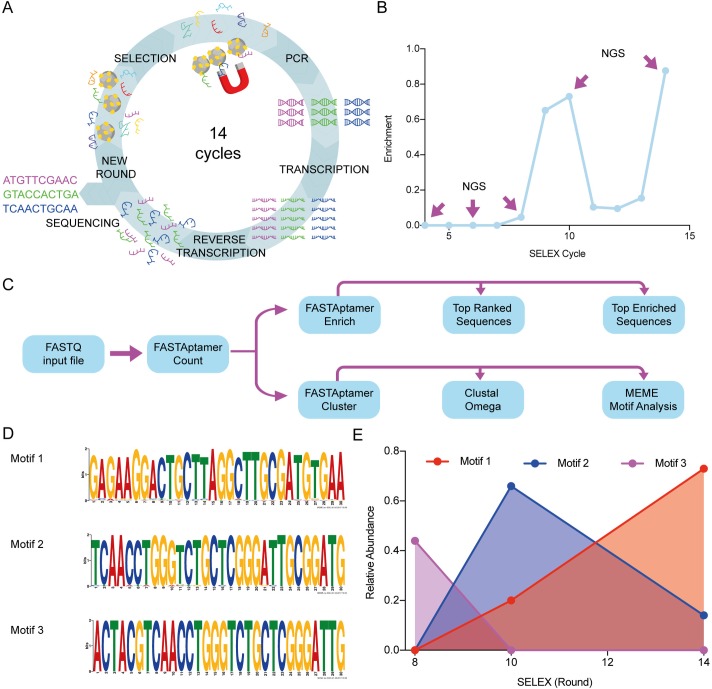
Fig 2. NGS allows the identification of aptamer motifs and their evolution during SELEX.
(A) Workflow of the SELEX technique and variations applied in this work. The ssDNA library (N40) was incubated with the HMG-box proteins immobilized in magnetic beads. After incubation, a magnet is used to separate the ssDNA molecules that bound the proteins on the magnetic beads. To amplify the bound molecules a PCR using a modified reverse primer with the T7 promoter region is performed. After purification, in vitro transcription is carried out, followed by in vitro reverse transcription. Finally, an enriched ssDNA library is generated and used for a next cycle or for sequencing. (B) Progression of enrichment along steps of selection as evaluated by the DiVE technique [24]. The ratio between non-degraded DNA (enriched)/total DNA was calculated from image quantifications of the resulting agarose gel using the ImageJ software [41] (see material and methods). Rounds 4, 6, 8, 10 and 14 were chosen for NGS analysis. (C) Scheme depicting the workflow for sequence analysis. (D) MEME (The MEME Suite) was used to identify the most characteristic motifs in each sequenced SELEX cycle. Three motifs were the most represented in cycles 8 (motif 3), 10 (motif 2), and 14 (motif 1). Sequences from cycles 8 and 10 share a conserved region inside motifs 3 and 2, whereas this region is lost in motif 1. No representative motif was detected in cycle 4. (E) Dynamics of aptamer motifs along SELEX cycles.
It is expected that the initial variability of the starting library decreases along the SELEX cycles, enriching a sub-population that contains the potential aptamers. To assess the enrichment of aptamers over the high variability of the starting library, we used the DiVE assay [24] and bioinformatic analysis of sequencing data (see below). DiVE uses the S1 endonuclease to differentiate between homoduplex and heteroduplex dsDNA by cleaving short single-stranded ends, nicks, and heteroduplex loops. An enriched pool would have more dsDNA homoduplex compared with a non-enriched pool. Thus, the homoduplex would not be cleaved and could be visualized in an agarose gel. The DiVE assay allowed to confirm enrichment at cycles eight, ten, and fourteen with a loss of homogeneity from cycle eleven to thirteen (Fig 2B and S1 Fig). The dsDNA obtained from cycles four, six, eight, ten, and fourteen were used for NGS using the Illumina system.
Raw sequences (typically over 80,000 reads for each SELEX cycle) obtained from sequencing were processed using the FASTAptamer toolkit (Fig 2C) [25]. Briefly, a FASTQ file was processed using the algorithm FASTAptamer Count that ranks DNA sequences based on their abundance for each SELEX cycle (Refer to S1 Data for raw ranked sequences). Best ranked sequences in cycle 14 varied between 14,995 RPM (Reads per Million, a normalized index for frequency) and 27,6943 RPM. In cycle ten, the most abundant sequences ranged between 5,933 RPM and 103,658 RPM. Final rounds of selection provided fewer sequences with higher frequency, while earlier selection cycles did not show sequences over 190 RPM of representation (For raw data, refer to S2 Data). An important indicator of aptamer selection can be surmised from the enrichment of each sequence along the selection procedure. This can be estimated from the ratio of sequence frequencies belonging to successive SELEX cycles. Thus, we used the FASTAptamer Enrich algorithm to calculate the enrichment of all aptamer sequences over two SELEX cycles, i.e., fourteen over ten or ten over eight. Most enriched sequences showed a frequency ratio ranging from 82 to 566 in cycles fourteen over ten. The enrichment ratio for cycles ten over eight showed increased enrichment ranging between 24.1 and 16.5 (See S3 Data for all sequences). Combining the results from both, ranking and enrichment, nine potential aptamers were identified (Table 1).
Table 1. Top-ranked and enriched sequences as evaluated by NGS.
| Name | Sequencea | ΔG (Kcal/mol)b |
Rankingc | Enrichmentc |
Kd (nM)d |
Δ MST (a.u.)e |
|---|---|---|---|---|---|---|
| PfR1 | GAGAAGGACTGCTTAGGCTTGCGATGTGAAATCTGTAATC | -3.92 | 1(14) | 4.13(14/10) | 175 ± 54 | 7.6 ± 0.6 |
| PfR2 | AGATGCAAAGGACTGCTCAGGCTTGCGATGGCAGCTTCAT | -5.72 | 2(14) | 32.05(14/10) | 778 ± 1020 | 4 ± 2 |
| PfR3 | CACTAGGTCTGCTTAGGATTGCGTAAGTGAAAGTCATTTA | -6.43 | 3(14) | 566.11(14/10) | 1930 ± 560 | 18 ± 3 |
| PfR4 | ACCGACTACGTCAACCTGGGTCTGCTCGGGATTGCGGATG | -6.92 | 1(10) | 8.68(10/8) | 41 ± 11 | 8.6 ± 0.6 |
| PfR5 | AGCCGTGGATTGCAAAGGGCTGCTGTGGCTTAGTTGAATG | -5.33 | 3(10) | 18.38(10/8) | 560 ± 120 | 8.5 ± 0.6 |
| PfR6 | CGCGTGGGTTTGGCTCCGGGCCTGGCGGATGCACGTATAG | -6.11 | 5(10) | 2.18(10/8) | 65 ± 15 | 7 ± 0.6 |
| PfE1 | GCCGACTACGTCAACCTGGGTCTGCTCGGGATTGCGGATG | -7.53 | 4(10) | 24.10(10/8) | 81 ± 20 | 8.8 ± 0.5 |
| PfE2 | ACCGACTACGTCAACTTGGGTCTGCTCGGGATTGCGGATG | -6.92 | 14(10) | 18.58(10/8) | 55 ± 20 | 7.8 ± 0.7 |
| PfE3 | AGTTTACACCAAGGACTGCTTAGGATTGCGTTAGGGTATT | -2.93 | 10(14) | 301.81(14/10) | 815 ± 158 | 17 ± 0.6 |
a Top-ranked and enriched sequences obtained by bioinformatic analysis. Here, we show the N40 central region of the aptamers, excluding the flanking 5’ (TAGGGAAGAGAAGGACATATGAT) and 3’ (TTGACTAGTACATGACCACTTGA) regions.
b Gibbs free energy was calculated considering the flanking regions using the Mfold web server (See S3 Fig for secondary structure predictions). Ranking and enrichment values were taken from the FASTAptamer toolkit [25].
c The cycle in which the aptamer was obtained, whether by Ranking or Enrichment, is shown in parenthesis.
d Aptamer Kd values were obtained by Microscale Thermophoresis (Fig 3A and 3B).
e Thermophoretic amplitude changes as an indicator of the extent of binding. Mean values and standard error were calculated from three independent measurements.
Additionally, sequences from the sequential selection process were also used to select for families of aptamers sharing a common and conserved sequence with few substitutions within the sequence. The variability within each family can arise either from the original library or by synthesis errors of the Taq polymerase, T7 RNA polymerase, or retro transcriptase that were used to regenerate the pool of ssDNA. To further analyze how families are enriched along the SELEX cycles of selection, we used the FASTAptamer Cluster algorithm [25] together with MEME Suite Motif Analysis and the Clustal Omega [22,26]. Representative motifs in cycles eight, ten, and fourteen were identified (Fig 2D). Motif 3 was the most abundant in cycle eight with about 40% of share. However, motif 3 diminished in cycles ten and fourteen, concomitantly with an increase of share for motif 2. A relative abundance of 70% for this family was followed by a reduction to less than 20% in cycle fourteen. On the other hand, motif 1, with low representation in cycles eight and ten, appeared highly abundant in cycle fourteen, with 80% of share (Fig 2E). Motif 1 was represented by sequences PfR1, PfR2, PfR3, and PfE3. Whereas motif 2 was represented by sequences PfR4, PfR5, PfR6, PfE1, and PfE2. PfR4 and PfE1 shared motif 2 and 3 within their sequences (Table 1). Overall, the bioinformatic flow used here allowed to identify nine potential aptamers for the HMG-box Pf, with three representing families of aptamers that vary their relative abundances along the SELEX process (Table 1, Fig 2E).
Interaction parameters between aptamers and HMG-box Pf
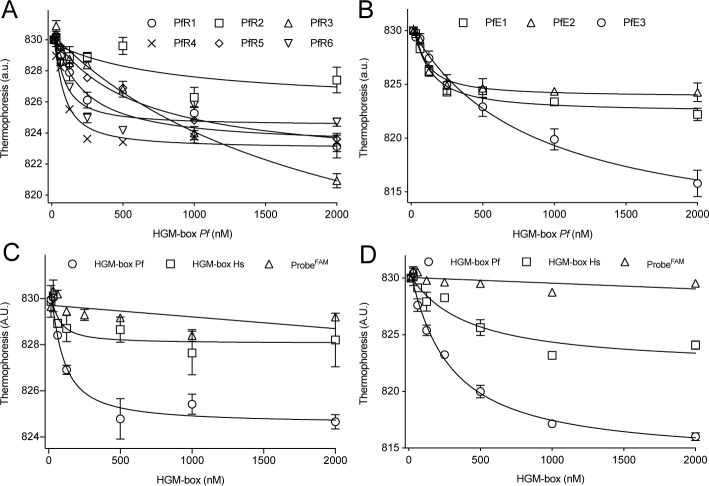
Nine aptamers, among the best, ranked or enriched, and belonging to the three most represented motifs were chemically synthesized and tested for binding to the HMG-box Pf using Microscale Thermophoresis (MST). MST allows the analysis of molecular interactions by measuring drifts of fluorescent reporters that are induced by small temperature changes, named thermophoresis [42,43]. The degree of thermophoresis change depends on the bound and unbound state of the fluorescent reporter with the ligand. Therefore, the thermophoresis changes as a function of ligand concentration allow calculating dissociation constants (Kd) [42]. Several MST applications for aptamer research have been reported (see [44] for detailed methods). Here, we label the aptamer with a fluorescent probe (probeFAM) and use it at a constant concentration and increased the concentrations of HMG-box Pf, ranging from 0.1 nM to 2 μM. The measured affinities ranged from 50 nM to 2 μM, with aptamers PfR1, PfR4, PfR6, PfE1, and PfE2 being in the low nanomolar range and PfR2, PfR5, and PfE3 in the micromolar range of dissociation constants (Table 1) (Fig 3A and 3B). PfR6 was representative of the group showing high affinities, while aptamer PfE3 showed the greatest MST amplitude at a lower affinity.
Fig 3. Aptamers efficiently bind the HMG-box from P. falciparum.
MST analysis of the interaction between aptamers and HMG-box. (A) Thermophoresis changes of best ranked aptamers as a function of HMG-box Pf concentration. (B) Thermophoresis changes of the most enriched aptamers as a function of HMG-box Pf concentration. Specificity assessment for PfR6 (C) and PfE3 (D). The binding of each aptamer to HMG-box Pf (open circles) was compared to the human homolog (open squares) or in the absence of the aptamer (open triangles). All reactions were measured in triplicates. Error bars indicate standard deviations. Continuous lines show the non-linear regression using a quadratic equation, allowing to estimate a Kd values (Table 1).
Another parameter to consider for optimal aptamers lays on specificity determinants. Thus, we probed PfR6 and PfE3 binding to HMG-box Hs by MST. The extent of binding PfR6 to the human homolog of HMG-box was lower when compared to the Plasmodium counterpart (Fig 3C). However, the PfE3 aptamer showed residual capacity to interact with HGM-box Hs, however with a 10-fold reduced affinity (Fig 3D). In brief, PfR6 shows better specificity than PfE3 as less binding to the Hs HGM-box1 was observed (Fig 3C and 3D) while aptamer PfE3 showed the greatest MST amplitude but a lower affinity (Fig 3A and 3B).
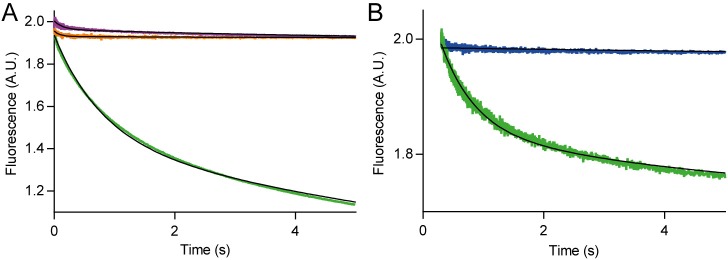
In order to further analyze the binding determinants of the PfR6 interaction with HMG-box Pf, we studied the kinetics of the reaction by the Stopped-flow (SF) technique. SF allows monitoring changes of fluorescence or absorbance in time and upon rapid mixing of two reactants. Here we used Föster Resonance Energy Transfer (FRET) between HMG-box (Pf or Hs) modified with the acceptor dye Atto540Q (from herein, HMG-box540Q Pf or Hs) and the PfR6 aptamer pre-annealed with a small complementary probe labeled with Fluorescein (PfR6FAM) (see material and methods). The interaction between the fluorescent aptamer and the quenching HMG-box resulted in a fluorescence decrease in time, due to the quenching effect induced by the vicinity of the non-emitting acceptor Atto540Q to the fluorescein reporter in the aptamer. To assess the specificity of the signal, first, we mixed HMG-box540Q Pf with the fluorescent probe only (in the absence of aptamer PfR6) and resulted in a negligible fluorescence change in time, indicating low or no interaction between the reactants (Fig 4A). Consistently, the mixing of PfR6FAM with the unlabeled HMG-box Pf did not result in any fluorescence change in time. Mixing HMG-box540Q Pf with PfR6FAM resulted in a decrease of fluorescence over time, consistent with a rapid binding of PfR6FAM with HMG-box540Q Pf. Nonlinear analysis with an exponential function allowed estimating the apparent average rate of the interaction, kAV = 0.58 ± 0.01 s–1.
Fig 4. Kinetics of PfR6 interaction with HMG-box.
HMG-box540Q and PfR6FAM were used to monitor the interaction between the aptamer and the protein in real-time using a stopped-flow apparatus (Applied Photophysics, UK). (A) FRET between HMG-box540Q and PfR6FAM. Mixing the labeled protein with the probe-bound aptamer (green) resulted in a decrease of fluorescence over time, indicating that the binding of both components results in the quenching of fluorescein due to its vicinity with the quencher in the protein. FRET assessment controls lacking the aptamer or the quencher Atto-540 in the HMG-box are shown in orange and purple traces respectively. (B) HMG-box 540Q Pf (green) or HMG-box 540Q Hs (blue) comparison during the binding with PfR6FAM at pH 5.5. 7–10 replicates for each reaction were measured and averaged. Continuous lines show the fitting with exponential functions.
Similar experiments using the human counterpart of the biomarker, HMG-box540Q Hs, showed variable degrees of binding as a function of pH. At pH = 5.5, PfR6 did not show any binding to HMG-box540Q Hs while the binding to the plasmodium counterpart was maintained (Fig 4B). Thus, PfR6 appears to bind specifically to HMG-box540Q Pf at the given conditions. However, the specificity of the binding was found to be perturbed by increasing the pH (S2 Fig). Nonlinear regression showed an average rate constant kAV = 0.18 ± 0.02 s–1 for PfR6 interaction with HMG-box540Q Pf. This rate increased with pH, reaching kAV = 1.12 s–1. Ion concentrations and pH have been shown to influence the tertiary structure of aptamers, therefore affecting their binding properties [45]. Altogether, our results indicate that PfR6 interacts efficient- and rapidly with the HMG-box Pf domain and provide experimental conditions where the specificity of the interaction is greatly enhanced.
Structural model of the PfR6– HMG-box Pf complex
In order to evaluate the theoretical molecular interactions between aptamers and HMG-box Pf, 3D models of the aptamers were generated followed by a molecular docking approximation. Secondary structures of the aptamers were predicted by MFold (S3 Fig) [27]. Then, the RNAComposer tool was used to generate 3D RNA models of the aptamers. The resulting models were converted to DNA structures with VMD and then stabilized through 10,000 iterations of energy minimization as described by Jeddi I. and Saiz L. [28]. 2D and 3D predictions indicated that all nine aptamers showed unstructured regions at the 5’ end. The length of the unstructured regions varied between 10 and 26 bases (S3 and S5 Figs). On the other hand, the 3’ end contained highly structured domains that included the 3’ flanking regions of the aptamer library (S5 Fig). PfR6 showed 21 (out of 86) unstructured nucleotides at the 5' end. The structured 3' region showed three segments with short double-stranded helixes and several helix-to-helix interactions (Fig 5A). Thus, PfR6 appears to fold into significantly structured regions, at the same time, provides a long unstructured segment that could be exploited to further develop diagnostic tests.
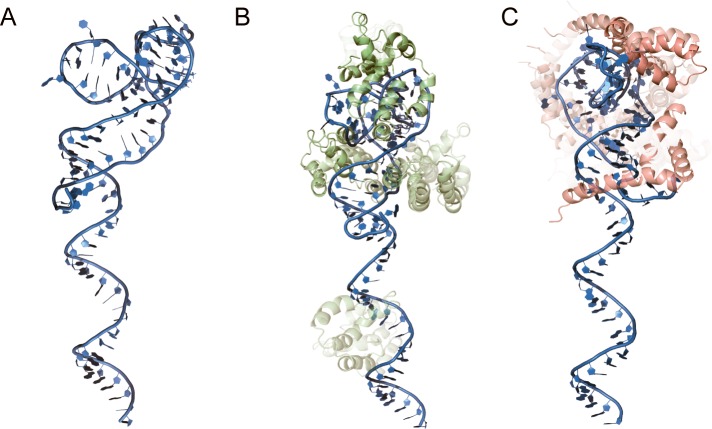
Fig 5. Structural modeling of aptamers and the PfR6–HMG-box Pf complex.
(A) Tertiary structure prediction of the PfR6 aptamer. (B) Alternative models for the PfR6–HMG-box Pf complex as evaluated by ZDOCK [34]. The degree of opacity is proportional to the ZDOCK Score (10% opacity corresponding to a score of 1350, maximum opacity corresponds to a score of 1850) (S6 Fig). (C) as B for the PfR6–HMG-box Hs. The PfR6 aptamer is depicted in sky blue, the HMG-box Pf in pale green, and HMG-box Hs in pink.
Modeling of the PfR6–HMG-box Pf complex was performed using the predicted tertiary structures of the aptamer and the protein (see above) using the ZDOCK tool [34]. We generated 2,000 complexes for each aptamer and evaluated their respective docking score based on rigid bodies interactions (S4 Data, S6 Fig). The average value of the docking scores did not indicate substantial differences between the aptamers. However, the highest score varied for each aptamer, ranging from 1500 to 1850, with PfE3 and PfR6 among the highest ones (S6 Fig). Thus, in silico docking results appear to be consistent with the biochemical data as measured by MST (Fig 3, Table 1). A comparison among the best ten PfR6–HMG-box Pf complexes, as evaluated by the ZDOCK score, indicates that in eight out ten models HMG-box Pf interacts with the structured region of PfR6 (Fig 5B). As a comparison, the PfR6–HMG-box Hs complex (human counterpart) showed much lower ZDOCK scores; however, the first ten models show an interaction of the protein with the structured region of the aptamer (Fig 5C).
Discussion
The identification of potential biomarkers is a complex process where many factors must be considered. In the case of Plasmodium species, an ideal biomarker should establish the presence or absence of infection, determine the species involved, be detectable at low concentrations, and be proportional to parasite density [46]. To account for these characteristics, the successful entrepreneurship requires several experimental stages, from basic research towards final clinical validations. Initial steps for biomarker selection can be pursued using bioinformatic strategies, with large databases of the -omics providing valid alternatives [47,48]. Genome-wide translation dynamics studies as revealed by RP, and in contrast to transcriptomic approaches, offer a precise approximation for the P. falciparum proteome [9]. Secondary analysis of RP datasets allowed selecting HGMB1 as a highly expressed protein during all the blood stages of P. falciparum (Fig 1, S1 Table). Structural stability, conservation among Plasmodium spp., and diversity to human homologs provided additional properties to cope for an ideal biomarker (Fig 1). Conserved regions of proteins from Plasmodium could be exploited to generate biosensors for the genus. Conversely, non-conserved regions allow generating species-specific biosensors [49]. Alternative approaches for the selection of biomarkers for malaria have been described [50], leading to different candidates for biomarkers, however, considering biomarker abundance shall increase the detection likelihood of the corresponding biosensors. As a comparison, during merozoite blood-stage, HGMB1 showed 88.45 folds more expression than the commonly used PfLDH biomarker, while HRP-2 expression is null.
HMGB1 is a highly conserved non-histone protein among eukaryotes that binds the minor groove of DNA and enhances the activity of transcription factors [51]. HMGB1 of H. sapiens can be exported to the extracellular medium and can fulfill a role as an alarmin by being involved in infectious and non-infectious inflammatory conditions [52]. In vivo assays showed that HMGB1 Pf to be an inducing factor for potent pro-inflammatory processes, leading to the induction of TNF-α and Nitric Oxide Synthase [51]. Both, transcriptional enhancer and extracellular activities, add additional features to HMGB1 as a biomarker. One can expect that Plasmodium spp. exploit the natural regulatory network of the human HMGB1 by exporting HMGB1 Pf, although, to our knowledge, experimental evidence is still lacking. Deletion of the HMGB2 homolog from Plasmodium resulted in attenuated pathogenicity in mouse models [53], indicating an involvement on severe inflammatory responses. HMGB1 and HMGB2 are differentially expressed in the Plasmodium cycle, with HMGB1 Pf preferentially expressed during erythrocytic stages and HMGB2 Pf during gametocytes [54]. Although HMGB1 and HMGB2 could to be implicated in the inflammatory response of the host, they are more likely involved in proliferation (HMGB1 Pf) and differentiation (HMGB2 Pf) of P. falciparum[55]; making the HMGB family a candidate for directed inhibitory drugs. Backing up this notion, aptamers targeting a wide spectrum of diseases are currently being evaluated in clinical trials (reviewed in [56]). Here, we showed that PfR6 tightly bind the conserved domain HMG-box displaying affinities that are comparable to antibodies used as biological drugs. Besides their potential for pharmacological administration, PfR6 may further be modified as a biosensor of HMGB1 from P. falciparum.
Aptamers for the detection of infectious diseases are of remarkably flexibility for the development of diagnostic assays. Such versatility arises from uncountable chemical modifications that are commercially available. Aptamer-tethered enzymes have been shown of great sensibility and to differentiate P. falciparum from P. vivax in blood samples [57]. Additionally, the development of aptamers using species specific epitopes allowed to isolate an aptamer that could accurately discriminate between the above species [49]. Aptamers against PfLDH were used in impedance spectroscopy setups allowing to propose electrochemical sensors that recognize the biomarker at a wide range of conditions [58]. Measurements of intramolecular FRET between aptamer linked dyes allowed the development of aptamer beacons [59]. In this work we show that fluorescently modified aptamers PfR6 and PfE3 allowed to monitor the binding to HGM-box Pf using two different techniques, providing substantial premises to develop aptamer beacons. Indeed, stopped-flow assays resulted in large FRET changes that, in principle, are compatible with further developing of detection assays. Alternatively, aptamers can be enzyme-linked and used for rapid colorimetric detection of Malaria as recently shown for aptamers against PfLDH, offering a quantitative test that is both, sensitive and specific [60,61]. PfR6, developed here, interacts with HMG-box Pf rapidly, taking place in less than a minute. Both, high affinity and rapid binding, can allow the development RDTs with low detection times, and could be integrated into microfluidic devices as shown for aptamers against PfLDH.
The chemical flexibility of aptamers can furthermore be exploited for binding optimization and stability in complex samples such as blood, plasma, saliva, etc. Indeed, the introduction of unnatural modifications can result in increased diversity, higher affinity as well as providing inhibitory capabilities [62]. The introduction of a single phosphorodithioate substitution resulted in a 1000-fold increase of affinity for an RNA aptamer against thrombin, leading to picomolar dissociation constants [63]. PfR6 showed binding affinities in the nanomolar range which could be further increased by targeted substitutions.
RDTs are the best alternative for rapid screening and detection of malaria in emerging countries, and the present research allows to cope with two current limitations. First, HMG-box Pf, is proposed as an alternative biomarker to the mostly available HRP and LDH. Second, the development of aptamers and their fluorescent derivatives as potentially new biosensors of HMG-box Pf. The latter can be further investigated in order to develop alternative detection methods, i.e. enzyme-tethered, surface immobilized or molecular beacons.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
See materials and methods for further details.
(PDF)
HMG-box540Q and PfR6FAM were used to monitor the interaction between the aptamer and the protein in real-time using a stopped-flow apparatus (Applied Photophysics, UK). HMG-box Pf Atto540Q labeled (green) or HMG-box Hs Atto540Q labeled (blue) comparison during the binding with PfR6FAM. Seven to ten replicates for each reaction were measured and averaged. Continuous lines show the fitting with exponential functions.
(PDF)
Here, we show the N40 variable region of the aptamers, including the flanking 5’ (TAGGGAAGAGAAGGACATATGAT) and 3’ (TTGACTAGTACATGACCACTTGA) regions. Secondary structures were predicted considering the flanking regions, at 25oC, 100 mM Na+, and 3 mM Mg2+, using the Mfold web server.
(PDF)
(A) Thermophoresis changes of PfR6 as a function of HMG-box Pf concentration. Continuous line shows the non-linear regression using a quadratic equation, allowing to estimate a Kd of 64 ± 20 nM and a thermophoretic amplitude of 7 AU. (B) As A for the PfE3 aptamer. The estimated Kd was 740 ± 120 nM and an amplitude of 16 AU. Specificity assessment for PfR6.
(PDF)
Here, we show the N40 variable region of the aptamers, including the flanking 5’ (TAGGGAAGAGAAGGACATATGAT) and 3’ (TTGACTAGTACATGACCACTTGA) regions. Secondary structures were predicted considering the flanking regions, at 25oC, 100 mM Na+, and 3 mM Mg+2, using the Mfold web server. A 3-D ssRNA model was built through RNAcomposer server and using VMD molefacture and PSF builder the ssRNA structure was transformed into a ssDNA structure. Finally, the structure was minimized using Namd software.
(PDF)
Binding of the selected aptamers 3D ssDNA structures to the HMG-box Pf were predicted using ZDOCK server. A) The ZDOCK score of 2,000 iterations were averaged using Graphpad Prism 7.02. B) Highest ZDOCK score of each aptamer.
(PDF)
(PDF)
(PDF)
Acknowledgments
We are very thankful to Andrey Konevega and Nanotemper RUS for kindly providing access to the Microscale Thermophoresis technology. We are also thankful to Luis Carlos Poggi for expert support on the artwork and graphics.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was funded by grants from Grand Challenges Canada, 0692-01-10 (https://www.grandchallenges.ca/), Fondecyt, 038-2014-Fondecyt and 136-2016-Fondecyt (http://www.fondecyt.gob.pe/), and InnóvatePerú. 297-INNOVATEPERU-EC-2016 (http://www.innovateperu.gob.pe/) to PM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. World malaria report 2018 [Internet]. 2018 Nov pp. 1–210.
- 2.Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH. A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT). Am J Trop Med Hyg. 2007;77: 119–127. [PubMed] [Google Scholar]
- 3.Li B, Sun Z, Li X, Li X, Wang H, Chen W, et al. Performance of pfHRP2 versus pLDH antigen rapid diagnostic tests for the detection of Plasmodium falciparum: a systematic review and meta-analysis. Arch Med Sci. 2017;13: 541–549. 10.5114/aoms.2017.67279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng Q, Gatton ML, Barnwell J, Chiodini P, McCarthy J, Bell D, et al. Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malar J. BioMed Central; 2014;13: 283 10.1186/1475-2875-13-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menegon M, L'Episcopia M, Nurahmed AM, Talha AA, Nour BYM, Severini C. Identification of Plasmodium falciparum isolates lacking histidine-rich protein 2 and 3 in Eritrea. Infect Genet Evol. 2017;55: 131–134. 10.1016/j.meegid.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 6.Jain P, Chakma B, Patra S, Goswami P. Potential biomarkers and their applications for rapid and reliable detection of malaria. BioMed Research International. 2014;2014: 852645–20. 10.1155/2014/852645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Roch KG, Johnson JR, Florens L, Zhou Y, Santrosyan A, Grainger M, et al. Global analysis of transcript and protein levels across the Plasmodium falciparum life cycle. Genome Res. 2004;14: 2308–2318. 10.1101/gr.2523904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozdech Z, Mok S, Hu G, Imwong M, Jaidee A, Russell B, et al. The transcriptome of Plasmodium vivax reveals divergence and diversity of transcriptional regulation in malaria parasites. Proc Natl Acad Sci USA. 2008;105: 16290–16295. 10.1073/pnas.0807404105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caro F, Ahyong V, Betegon M, DeRisi JL. Genome-wide regulatory dynamics of translation in the Plasmodium falciparum asexual blood stages. eLife. 2014;3: 568 10.7554/eLife.04106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michel AM, Baranov PV. Ribosome profiling: a Hi-Def monitor for protein synthesis at the genome-wide scale. Wiley Interdiscip Rev RNA. 2013;4: 473–490. 10.1002/wrna.1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiviyanathan V, Gorenstein DG. Aptamers and the next generation of diagnostic reagents. Stastna M, Van Eyk JE, editors. Proteomics Clin Appl. 2012;6: 563–573. 10.1002/prca.201200042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliphant AR, Brandl CJ, Struhl K. Defining the sequence specificity of DNA-binding proteins by selecting binding sites from random-sequence oligonucleotides: analysis of yeast GCN4 protein. Molecular and Cellular Biology. 1989;9: 2944–2949. 10.1128/mcb.9.7.2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249: 505–510. [DOI] [PubMed] [Google Scholar]
- 14.Cheung Y-W, Kwok J, Law AWL, Watt RM, Kotaka M, Tanner JA. Structural basis for discriminatory recognition of Plasmodium lactate dehydrogenase by a DNA aptamer. Proc Natl Acad Sci USA. 2013;110: 15967–15972. 10.1073/pnas.1309538110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song H-O, Lee B, Bhusal RP, Park B, Yu K, Chong C-K, et al. Development of a novel fluorophore for real-time biomonitoring system. Carvalho LH, editor. PLoS ONE. 2012;7: e48459 10.1371/journal.pone.0048459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W-X, Cheung Y-W, Dirkzwager RM, Wong W-C, Tanner JA, Li H-W, et al. Specific and sensitive detection of Plasmodium falciparum lactate dehydrogenase by DNA-scaffolded silver nanoclusters combined with an aptamer. Analyst. 2017;142: 800–807. 10.1039/c6an02417c [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Manjunatha DH, Jeon W, Ban C. Cationic surfactant-based colorimetric detection of Plasmodium lactate dehydrogenase, a biomarker for malaria, using the specific DNA aptamer. Schallig HDFH, editor. PLoS ONE. 2014;9: e100847 10.1371/journal.pone.0100847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aurrecoechea C, Brestelli J, Brunk BP, Dommer J, Fischer S, Gajria B, et al. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Research. 2009;37: D539–43. 10.1093/nar/gkn814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otto TD, Wilinski D, Assefa S, Keane TM, Sarry LR, Böhme U, et al. New insights into the blood-stage transcriptome of Plasmodium falciparum using RNA-Seq. Molecular Microbiology. 2010;76: 12–24. 10.1111/j.1365-2958.2009.07026.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelinas AD, Davies DR, Janjic N. Embracing proteins: structural themes in aptamer-protein complexes. Curr Opin Struct Biol. 2016;36: 122–132. 10.1016/j.sbi.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 21.Štros M, Launholt D, Grasser KD. The HMG-box: a versatile protein domain occurring in a wide variety of DNA-binding proteins. Cell Mol Life Sci. 2007;64: 2590–2606. 10.1007/s00018-007-7162-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7: 539–539. 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milon P, Konevega AL, Peske F, Fabbretti A, Gualerzi CO, Rodnina MV. Transient kinetics, fluorescence, and FRET in studies of initiation of translation in bacteria. Meth Enzymol. 2007;430: 1–30. 10.1016/S0076-6879(07)30001-3 [DOI] [PubMed] [Google Scholar]
- 24.Lim TS, Schütze T, Lehrach H, Glökler J, Konthur Z. Diversity visualization by endonuclease: a rapid assay to monitor diverse nucleotide libraries. Anal Biochem. 2011;411: 16–21. 10.1016/j.ab.2010.12.024 [DOI] [PubMed] [Google Scholar]
- 25.Alam KK, Chang JL, Burke DH. FASTAptamer: A Bioinformatic Toolkit for High-throughput Sequence Analysis of Combinatorial Selections. Mol Ther Nucleic Acids. 2015;4: e230 10.1038/mtna.2015.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Research. 2009;37: W202–8. 10.1093/nar/gkp335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Research. 2003;31: 3406–3415. 10.1093/nar/gkg595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeddi I, Saiz L. Three-dimensional modeling of single stranded DNA hairpins for aptamer-based biosensors. Sci Rep. 2017;7: 1178 10.1038/s41598-017-01348-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popenda M, Szachniuk M, Antczak M, Purzycka KJ, Lukasiak P, Bartol N, et al. Automated 3D structure composition for large RNAs. Nucleic Acids Research. 2012;40: e112–e112. 10.1093/nar/gks339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14: 33–8–27–8. [DOI] [PubMed] [Google Scholar]
- 31.MacKerell AD, Banavali N, Foloppe N. Development and current status of the CHARMM force field for nucleic acids. Biopolymers. 2000;56: 257–265. [DOI] [PubMed] [Google Scholar]
- 32.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26: 1781–1802. 10.1002/jcc.20289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Research. 2018;46: W296–W303. 10.1093/nar/gky427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierce BG, Wiehe K, Hwang H, Kim B-H, Vreven T, Weng Z. ZDOCK server: interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics. 2014;30: 1771–1773. 10.1093/bioinformatics/btu097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chulluncuy R, Espiche C, Nakamoto JA, Fabbretti A, Milon P. Conformational Response of 30S-bound IF3 to A-Site Binders Streptomycin and Kanamycin. Antibiotics (Basel). Multidisciplinary Digital Publishing Institute; 2016;5: 38 10.3390/antibiotics5040038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milon P, Konevega AL, Gualerzi CO, Rodnina MV. Kinetic checkpoint at a late step in translation initiation. Molecular Cell. 2008;30: 712–720. 10.1016/j.molcel.2008.04.014 [DOI] [PubMed] [Google Scholar]
- 37.Ingolia NT, Ghaemmaghami S, Newman JRS, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. American Association for the Advancement of Science; 2009;324: 218–223. 10.1126/science.1168978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H, Certa U, Döbeli H, Jakob P, Hol WG. Crystal structure of fructose-1,6-bisphosphate aldolase from the human malaria parasite Plasmodium falciparum. Biochemistry. 1998;37: 4388–4396. 10.1021/bi972233h [DOI] [PubMed] [Google Scholar]
- 39.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346: 818–822. 10.1038/346818a0 [DOI] [PubMed] [Google Scholar]
- 40.Stoltenburg R, Reinemann C, Strehlitz B. FluMag-SELEX as an advantageous method for DNA aptamer selection. Anal Bioanal Chem. 2005;383: 83–91. 10.1007/s00216-005-3388-9 [DOI] [PubMed] [Google Scholar]
- 41.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9: 671–675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duhr S, Braun D. Why molecules move along a temperature gradient. Proceedings of the National Academy of Sciences. 2006;103: 19678–19682. 10.1073/pnas.0603873103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhont JKG, Wiegand S, Duhr S, Braun D. Thermodiffusion of charged colloids: single-particle diffusion. Langmuir. 2007;23: 1674–1683. 10.1021/la062184m [DOI] [PubMed] [Google Scholar]
- 44.Breitsprecher D, Schlinck N, Witte D, Duhr S, Baaske P, Schubert T. Aptamer Binding Studies Using MicroScale Thermophoresis. Methods Mol Biol. New York, NY: Springer New York; 2016;1380: 99–111. 10.1007/978-1-4939-3197-2_8 [DOI] [PubMed] [Google Scholar]
- 45.Hianik T, Ostatná V, Sonlajtnerova M, Grman I. Influence of ionic strength, pH and aptamer configuration for binding affinity to thrombin. Bioelectrochemistry. 2007;70: 127–133. 10.1016/j.bioelechem.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 46.Bell D, Peeling RW, WHO-Regional Office for the Western Pacific/TDR. Evaluation of rapid diagnostic tests: malaria. Nature reviews. Microbiology. 2006. pp. S34–8. 10.1038/nrmicro1524 [DOI] [PubMed] [Google Scholar]
- 47.Kaur H, Sehgal R, Kumar A, Sehgal A, Bansal D, Sultan AA. Screening and identification of potential novel biomarker for diagnosis of complicated Plasmodium vivax malaria. Journal of Translational Medicine. BioMed Central; 2018;16: 42 10.1186/s12967-018-1646-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meerstein-Kessel L, van der Lee R, Stone W, Lanke K, Baker DA, Alano P, et al. Probabilistic data integration identifies reliable gametocyte-specific proteins and transcripts in malaria parasites. Sci Rep. Nature Publishing Group; 2018;8: 410 10.1038/s41598-017-18840-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frith K-A, Fogel R, Goldring JPD, Krause RGE, Khati M, Hoppe H, et al. Towards development of aptamers that specifically bind to lactate dehydrogenase of Plasmodium falciparum through epitopic targeting. Malar J. 2018;17: 191 10.1186/s12936-018-2336-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rousu J, Agranoff DD, Sodeinde O, Shawe-Taylor J, Fernandez-Reyes D. Biomarker discovery by sparse canonical correlation analysis of complex clinical phenotypes of tuberculosis and malaria. Price ND, editor. PLoS Comput Biol. 2013;9: e1003018 10.1371/journal.pcbi.1003018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar K, Singal A, Rizvi MMA, Chauhan VS. High mobility group box (HMGB) proteins of Plasmodium falciparum: DNA binding proteins with pro-inflammatory activity. Parasitol Int. 2008;57: 150–157. 10.1016/j.parint.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 52.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14: 476–484. 10.2119/2008-00034.Klune [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Briquet S, Lawson-Hogban N, Boisson B, Soares MP, Péronet R, Smith L, et al. Disruption of Parasite hmgb2 Gene Attenuates Plasmodium berghei ANKA Pathogenicity. Infection and Immunity. 2015;83: 2771–2784. 10.1128/IAI.03129-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, et al. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301: 1503–1508. 10.1126/science.1087025 [DOI] [PubMed] [Google Scholar]
- 55.Briquet S, Boschet C, Gissot M, Tissandié E, Sevilla E, Franetich J-F, et al. High-mobility-group box nuclear factors of Plasmodium falciparum. Eukaryotic Cell. 2006;5: 672–682. 10.1128/EC.5.4.672-682.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nimjee SM, White RR, Becker RC, Sullenger BA. Aptamers as Therapeutics. Annu Rev Pharmacol Toxicol. 2017;57: 61–79. 10.1146/annurev-pharmtox-010716-104558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheung Y-W, Dirkzwager RM, Wong W-C, Cardoso J, D'Arc Neves Costa J, Tanner JA. Aptamer-mediated Plasmodium-specific diagnosis of malaria. Biochimie. 2018;145: 131–136. 10.1016/j.biochi.2017.10.017 [DOI] [PubMed] [Google Scholar]
- 58.Miranda GF, Feng L, Shiu SC-C, Dirkzwager RM, Cheung Y-W, Tanner JA, et al. Aptamer-Based Electrochemical Biosensor for Highly Sensitive and Selective Malaria Detection with Adjustable Dynamic Response Range and Reusability. Sensors & Actuators: B Chemical. Elsevier B.V; 2017;: 1–23. 10.1016/j.snb.2017.07.117 [DOI] [Google Scholar]
- 59.Hamaguchi N, Ellington A, Stanton M. Aptamer beacons for the direct detection of proteins. Anal Biochem. 2001;294: 126–131. 10.1006/abio.2001.5169 [DOI] [PubMed] [Google Scholar]
- 60.Dirkzwager RM, Kinghorn AB, Richards JS, Tanner JA. APTEC: aptamer-tethered enzyme capture as a novel rapid diagnostic test for malaria. Chem Commun (Camb). 2015;51: 4697–4700. 10.1039/c5cc00438a [DOI] [PubMed] [Google Scholar]
- 61.Fraser LA, Kinghorn AB, Dirkzwager RM, Liang S, Cheung Y-W, Lim B, et al. A portable microfluidic Aptamer-Tethered Enzyme Capture (APTEC) biosensor for malaria diagnosis. Biosensors and Bioelectronics. 2018;100: 591–596. 10.1016/j.bios.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 62.Gawande BN, Rohloff JC, Carter JD, Carlowitz von I, Zhang C, Schneider DJ, et al. Selection of DNA aptamers with two modified bases. Proc Natl Acad Sci USA. 2017;114: 2898–2903. 10.1073/pnas.1615475114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abeydeera ND, Egli M, Cox N, Mercier K, Conde JN, Pallan PS, et al. Evoking picomolar binding in RNA by a single phosphorodithioate linkage. Nucleic Acids Research. 2016;44: 8052–8064. 10.1093/nar/gkw725 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
See materials and methods for further details.
(PDF)
HMG-box540Q and PfR6FAM were used to monitor the interaction between the aptamer and the protein in real-time using a stopped-flow apparatus (Applied Photophysics, UK). HMG-box Pf Atto540Q labeled (green) or HMG-box Hs Atto540Q labeled (blue) comparison during the binding with PfR6FAM. Seven to ten replicates for each reaction were measured and averaged. Continuous lines show the fitting with exponential functions.
(PDF)
Here, we show the N40 variable region of the aptamers, including the flanking 5’ (TAGGGAAGAGAAGGACATATGAT) and 3’ (TTGACTAGTACATGACCACTTGA) regions. Secondary structures were predicted considering the flanking regions, at 25oC, 100 mM Na+, and 3 mM Mg2+, using the Mfold web server.
(PDF)
(A) Thermophoresis changes of PfR6 as a function of HMG-box Pf concentration. Continuous line shows the non-linear regression using a quadratic equation, allowing to estimate a Kd of 64 ± 20 nM and a thermophoretic amplitude of 7 AU. (B) As A for the PfE3 aptamer. The estimated Kd was 740 ± 120 nM and an amplitude of 16 AU. Specificity assessment for PfR6.
(PDF)
Here, we show the N40 variable region of the aptamers, including the flanking 5’ (TAGGGAAGAGAAGGACATATGAT) and 3’ (TTGACTAGTACATGACCACTTGA) regions. Secondary structures were predicted considering the flanking regions, at 25oC, 100 mM Na+, and 3 mM Mg+2, using the Mfold web server. A 3-D ssRNA model was built through RNAcomposer server and using VMD molefacture and PSF builder the ssRNA structure was transformed into a ssDNA structure. Finally, the structure was minimized using Namd software.
(PDF)
Binding of the selected aptamers 3D ssDNA structures to the HMG-box Pf were predicted using ZDOCK server. A) The ZDOCK score of 2,000 iterations were averaged using Graphpad Prism 7.02. B) Highest ZDOCK score of each aptamer.
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.