Abstract
Chronic stress is associated with the accelerated aging of the immune system and represents a potent risk factor for the development and progression of a wide range of physical and mental disorders. The elucidation of molecular pathways and mechanisms underlying the link between stress and cellular aging is an area of considerable interest and investigation. In this context, telomere biology has emerged as a particularly attractive candidate mechanism. Several studies have linked immune cell telomere length with stress-related conditions and states, and also with several physical and mental disorders. Because the cellular reverse transcriptase enzyme telomerase is the primary regulator of telomere length (by adding telomeric DNA to telomeres and thereby attenuating telomere shortening), the understanding of its regulation and regulatory functions constitutes a prime target for developing strategies to prevent, attenuate or reverse the adverse consequences of immune system aging (immunosenescence). In this review we provide an overview of the mechanistic pathways linking telomerase with stress and cellular aging, with an emphasis on the immune system. We summarize and synthesize the current state of the literature on immune cell telomerase in different stress- and aging-related disease states and provide recommendations for future research directions.
Keywords: Telomerase, Immunosenescence, hTERT, Inflammation, Oxidative stress, Stress hormones
1. Introduction
Chronic stress is a major contributor to the development and progression of a range of physical and mental disorders such as cardiovascular disease, type 2 diabetes, metabolic syndrome, autoimmune disease and depression (Cohen et al., 2007). Several lines of evidence converge to suggest that these pathophysiological effects of stress on health and disease risk are mediated in large part by rate of accelerated aging of the immune system (immunosenescence). This process is characterized by the inability to mount an appropriate and effective immune response to challenge, and it is associated with a low-grade chronic pro-inflammatory state (Fulop et al., 2017). Many of the immunological changes associated with aging resemble those observed following long-term or chronic stress exposure (Bauer, 2005), including “non-resolving” inflammation and elevated oxidative stress (Epel, 2009; Miller et al., 2014).
The telomere biology system plays a fundamental role in maintaining genomic and cellular and integrity. This system comprises two closely interlinked entities 1) telomeres, the tracts of non-coding tandem repeats of simple DNA sequences [5′-(TTAGGG)n-3′] and shelterin proteins that cap the ends of linear chromosomes, and 2) telomerase, the reverse transcriptase enzyme that adds telomeric DNA to telomeres (Blackburn et al., 2015). The loss of the integrity of telomeres affects the replicative capacity of cells and imposes a self-perpetuating pathway of global epigenetic changes that alter overall chromatin and transcriptional properties, ultimately resulting in cellular senescence and aging (Harrington and Pucci, 2018; Karlseder, 2009; O’Sullivan et al., 2010). A substantial and convergent body of evidence has linked shortened telomeres to several age-related risk factors, earlier mortality (Benetos et al., 2001; Cawthon et al., 2003; Kimura et al., 2008; Martin-Ruiz et al., 2006), non-communicable diseases (D’Mello et al., 2015; Georgin-Lavialle et al., 2010; Haycock et al., 2014; Lindqvist et al., 2015; Tamura et al., 2016) and psychological stress conditions (e.g., chronic stress in adults, childhood traumatic experiences and prenatal stress exposure (Coimbra et al., 2017; Entringer et al., 2018; Mathur et al., 2016; Oliveira et al., 2016)). Most of these associations have been documented in peripheral blood mononuclear cells (PBMCs), the leukocytes that are isolated from whole blood by density gradient centrifugation techniques. Because the enzyme telomerase can counteract telomere shortening and is essential for healthy cell and immune functioning and also is responsive to biological stress mediators, it constitutes a prime target for developing novel strategies to prevent, attenuate or even reverse the adverse consequences of imunosenescence.
In order to facilitate a better understanding of the mechanistic pathways linking telomerase, immunosenescence and stress, we first provide an overview of the regulation and regulatory functions of telomerase. Next, we review, summarize and comment on the current state of the literature concerning PBMC telomerase in different stress- and aging-related states and pathologies (the role of telomerase in the initiation and progression of cancer is beyond the scope of this review and has been extensively discussed elsewhere (Jafri et al., 2016)). We then describe intervention studies related to lifestyle changes that target telomerase, and discuss methodological issues related to the measurement of telomerase expression and activity (e.g., assessment of telomerase from non-stimulated vs. in vitro mitogen- stimulated immune cells) that might explain and reconcile some of the inconsistencies in the literature. Finally, we provide some recommendations for future research regarding immune cell telomerase in humans in the context of health and disease risk.
2. The role of telomerase in cell function
Telomeres are the DNA-protein complexes located at both ends of linear chromosomes (Blackburn et al., 2015). In humans, telomeres consist of double-stranded repeats of guanine-rich tandem DNA sequences [5’-(TTACGG)n-3’] that end in a single-stranded 3’-overhang and an attached shelterin protein complex that protects the single-stranded 3’-overhang by providing a secondary structure that facilitates the formation of a nucleotide loop (also referred to as the T-loop). Because conventional DNA polymerase machinery is unable to fully replicate the 3’-ends of the lagging strand, a phenomenon that is referred to as the “end-replication problem”, telomeres shorten between approximately 30 to 150 base pairs with each cell replication. After successive mitoses, telomeres reach a critically short length, which makes it more difficult to recruit shelterin proteins. This then results in chromosomal fusions, activation of DNA damage checkpoint responses and genomic instability, and thereby induces cellular apoptosis or activates senescence (Blackburn et al., 2015). Telomerase is a reverse transcriptase enzyme that consists of a RNA component (TR or TERC) and a catalytic protein domain (TERT). Both hTERT and hTERC are essential for the in vitro reverse transcriptase activity of the human telomerase enzyme. However, under in vivo conditions telomerase consists of additional subunits, including dyskerin, NHP2, NOP10, GAR1 and the ATPases reptin and pontin, all promoting the stability and assembly of a functional telomerase holoenzyme (Jafri et al., 2016; Ozturk et al., 2017). Telomerase is essential for the elongation and maintenance of telomeres, and it does so by adding telomeric repeat sequences to the chromosomal DNA ends and thereby compensating for losses that occur with each round of DNA replication (Artandi and DePinho, 2010; Blackburn, 2005). Telomerase expression varies during development and across cell types. In most somatic cells telomerase activity is almost completely inhibited, whereas in germ cells, stem cells of proliferating tissues and activated immune cells telomerase activity levels are maintained (Forsyth et al., 2002). Telomerase not only maintains and elongates telomere length but also preserves healthy cell function. Through its modulation of intracellular signaling pathways and its interaction with several transcription factors, telomerase promotes the proliferation of resting stem cells, modulates signaling pathways during embryogenesis and normal adult tissue genesis, and protects cellular proliferation capacity and survival under cellular stress conditions and after immune activation (Ale-Agha et al., 2014; Ozturk et al., 2017). The following section describes how telomerase is regulated and provides an overview of effects of telomerase on cellular and immune function that extend beyond those of telomere lengthening.
2.1. Regulation of telomerase activity
Telomerase is highly regulated by epigenetic, translational and posttranslational mechanisms. In humans, the expression and the activity of the telomerase holoenzyme are regulated through multiple pathways, including transcription, alternative splicing, phosphorylation, protein degradation and cellular localization. Several transcription factors directly influence the promoter region of the hTERT gene. For example, activation of the p53 tumor suppressor protein transcriptionally inhibits telomerase expression. p53 can be activated in response to DNA damage and telomere dysfunction, resulting in growth arrest, DNA repair, senescence or apoptosis (Sahin et al., 2011). Other transcription factors such as E2F-1 and Wilms’ tumor 1 tumor suppressor also inhibit hTERT expression (Crowe et al., 2001; Oh et al., 1999), whereas c-Myc, hypoxy-inducible factor-1 (HIF-1), nuclear factor- κB (NF-κB), β-catenin, signal transducer and activator of transcription(STAT)3 and STAT5 up-regulate hTERT expression (Anderson et al., 2006; Hoffmeyer et al., 2012; Sundin and Hentosh, 2012; Wu et al., 1999b; Yin et al., 2000; Zuo et al., 2011). For a complete overview of factors involved in the transcriptional regulation of hTERT we refer to the review by Ramlee et al. 2016 (Ramlee et al., 2016).
The hTERT protein can be phosphorylated at different sites by several kinases. Reversible phosphorylation of hTERT determines its cellular localization and activity level. In lymphocytes hTERT is transported into the nucleus, paralleled by increased nuclear telomerase activity upon activation by antigens (Liu et al., 2001). However, only the phosphorylated form of hTERT can be imported into the nucleus (Akiyama et al., 2004), where it can bind to the other components of the telomerase holoenzyme. Akt, an important kinase in the phosphoinositide 3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) signal transduction pathway, phosphorylates hTERT and up-regulates its activity. Heat shock protein 90 (Hsp90), a protein known to form a complex with hTERT, Akt and mTOR, is important in the regulation of telomerase activity and is necessary for its stability and activity (Haendeler et al., 2003; Kawauchi et al., 2005). The PI3K-Akt-mTOR signaling pathway is essential for immune cell proliferation and eliciting normal immune responses (Bu et al., 2007; Kawauchi et al., 2005; Sundin and Hentosh, 2012; Zhao et al., 2008; Zhou et al., 2003). This pathway is regulated by environmental cues in the form of nutrients, growth factors, cytokines and stress. Upon activation, it stimulates protein synthesis, cell growth and proliferation, regulates glucose and fat metabolism, and represses autophagy. Moreover, mTOR is considered an important regulator of immune function because of its role in sensing and integrating cues from the immune microenvironment and its involvement in the proliferation, activation and differentiation of immune cells, mainly by reprogramming metabolic pathways (Powell et al., 2012).
2.2. Telomerase function in mitochondria
Mitochondria are cellular organelles that convert oxygen and nutrients in adenosine triphosphate (ATP), the main source of cellular energy. Mitochondria are a major site for the generation of reactive oxygen species (ROS) and also the cell organelles most susceptible to ROS-induced damage. Cellular aging is characterized by a decline in mitochondrial respiratory function and ATP production and an increase in ROS-induced mitochondrial DNA mutations, thereby making mitochondrial DNA damage a good indicator of intracellular oxidative stress (Saretzki, 2009). Prolonged exposure to high(er) levels of oxidative stress is associated with shorter telomere length and reduced telomerase activity. Due to the high content of guanine residues, telomeres are highly sensitive to oxidative damage. Oxidative stress accelerates telomere shortening and induces senescence (or apoptosis) via DNA damage-induced activation of the p53 pathway, generating mitochondrial dysfunction, increased ROS concentrations, and consequently further DNA damage (Haendeler et al., 2004; Saretzki, 2009; von Zglinicki, 2002).
Telomerase is essential for normal mitochondrial production and function (Sahin et al., 2011). Several recent reports have examined the transport of hTERT into mitochondria, which appears to be mediated by a mitochondrial import sequence at the N-terminal of hTERT. Accumulating endogenous oxidative stress induces the nuclear export of hTERT to the cytosol and decreases nuclear and total telomerase activity, whereas cytosolic hTERT protein increases in cytosolic fractions. The addition of antioxidants decelerated this process (Haendeler et al., 2004). Mitochondrial hTERT has been shown to increase the membrane potential, reduce ROS production, protect mitochondrial DNA, and inhibit apoptosis induction (Ahmed et al., 2008; Ale-Agha et al., 2014; Haendeler et al., 2004; Santos et al., 2004), indicating an additional protective function of telomerase in mitochondria under conditions of increased oxidative stress. A positive correlation between mitochondrial hTERT and levels of oxidative stress also has been observed in cord blood mononuclear cells of newborns (Li et al., 2014), which suggests that this oxidative stress-induced accumulation of mitochondrial hTERT may protect the mitochondrial DNA from oxidative damage during gestation.
2.3. The role of telomerase activity in regulating the immune system
2.3.1. Telomerase is up-regulated upon immune activation
The immune system comprises primarily of two interacting components: innate and adaptive immunity. The innate immune system mounts rapid but unspecific responses, whereas the adaptive immune responses take longer to develop but are highly specific, and have the capacity for long-term memory. The innate immune response is mediated mainly by monocytes, natural killer cells, dendritic cells and the complement system, and it serves as the first line of defense against environmental pathogens. T- and B-lymphocytes are crucial in the development of an adaptive immune response and are activated upon antigen-presentation and recognition via unique and highly specific T-cell receptors (TCR), in combination with activating co-stimulatory signals, such as those delivered by the surface molecule CD28 (Chou and Effros, 2013). T-cells can further differentiate into various subtypes. CD8+ T- cells are mainly cytotoxic cells, which are important for the clearance of intracellular pathogens, whereas CD4+ T-cells primarily regulate the cellular and humoral immune responses. Additionally, T-cells can be naive or antigen-experienced, and can exist in a resting or activated state (Chaplin, 2010).
Antigen activated lymphocytes express higher levels of telomerase activity. In T-cells, crosslinking of the TCR and CD28 by antigens, lectins or antibodies initiates a signal transduction cascade that ultimately induce the translocation of transcription factors such as NF-kB to the nucleus, initiating IL-2 production, entry into the cell cycle, DNA synthesis, and telomerase activation (Buchkovich and Greider, 1996; Paul and Schaefer, 2013). The up-regulation of telomerase activity facilitates the immune response and prevents immune cell senescence during fast and profound (clonal) cell expansion. However, the level of telomerase expression is not sufficient to indefinitely prevent telomere shortening and senescence (Hathcock et al., 2005).
2.3.2. Telomerase activity is reduced in senescent T-cells
A considerable body of research has focused on the role of senescent T-cells during aging and disease. Senescent T-cells lack the CD28 co-stimulatory receptor. However, also other T-cell markers, like CD27, CD57, the killer cell-lectin-like receptor G1 and programmed death receptor 1 have been associated with senescence (Dedeoglu et al., 2017; Henson et al., 2009). The proportion of CD28+ cells decreases with age and is associated with decreased responses to vaccines in the elderly, reduction in the overall T-cell receptor repertoire, and diminished control over infections (Chou and Effros, 2013). In the total PBMC population senescent CD28- T-cells have the lowest telomerase activity and shortest telomere length (Lin et al., 2010). A higher percentage of CD8+CD28- T-cells is correlated with shorter PBMC telomere length. Moreover, PBMC telomerase activity seems to decline with age (Lin et al., 2015). CD28 signaling is required for optimal telomerase up-regulation, as indicated by the paralleled loss of telomerase activity and CD28 expression in T-cells after chronic antigen stimulation in vitro (Valenzuela and Effros, 2002). Senescent T-cells produce reduced levels of the antiviral cytokine, interferon(IFN)-γ, and increased levels of pro-inflammatory cytokines such as IL-6 and TNF-α (Effros, 2009). Interestingly, the loss of CD28 in a long-term stimulated CD8+ T cell culture is delayed by inhibition of TNF-α and is paralleled by increased telomerase activity (Parish et al., 2009). Prostaglandin E2, a lipid pro-inflammatory immune mediator, inhibits hTERT transcription and telomerase activity in a long term culture of activated CD8+ T cells. This reduction in telomerase activity is associated with telomere shortening, and is accompanied by increased intracellular ROS and loss of CD28 surface expression. It also appears that the effects of prostaglandin E2 on telomerase and CD28 expression and the progression of human CD8+ T cells towards replicative senescence are mediated by the cAMP-PKA pathway, potentially by inhibiting IL-2 signaling pathways (Chou et al., 2014). A more recent study measured T-cell telomerase activity at the single-cell level and observed a robust increase in telomerase activity in only a subset of CD28+ T-cells upon immune stimulation. Moreover, it was shown that solely the CD28+ T-cells that induced a robust telomerase response were capable of maintaining their telomere lengths during proliferation (Huang et al., 2017). The observation that telomerase can still be induced in a subset CD8+CD28- T cells, indicates that other co-stimulatory pathways may be involved in its upregulation. For example, CD8+CD28- T cells lacking the CD27 co-stimulatory receptor (CD8+CD28-CD27- T-cells) showed the shortest telomeres and extremely poor telomerase activity. The loss of telomerase up-regulation in this CD8+/CD28-/CD27- T-cell subset was associated with defective hTERT phosphorylation by Akt, but not by reduced expression of hTERT. (Plunkett et al., 2007).
Senescence of specific T-cells might result from chronic infection by viruses such as human herpes virus, human immunodeficiency virus (HIV), Cytomegalovirus (CMV), Epstein-Barr virus (EBV), Hepatitis B/C/D, human T-cell leukemia virus type 1, herpes simplex virus-1/2 and Varicella-Zoster virus (Bellon and Nicot, 2017), and several studies have examined telomerase activity in the context of virus exposure. For example, HIV-1 specific CD8+ T-cells from individuals that achieve spontaneous control of HIV-1 viremia show longer telomeres and higher levels of telomerase activity compared to virus specific CD8+ T-cells from HIV-1 progressors (Lichterfeld et al., 2008). Also, hTERT mRNA levels were lower in PBMCs of hepatitis B and C virus infected individuals, compared to healthy controls (Satra et al., 2005). Although high telomerase activity maintained telomere length in EBV-specific T-cells from individuals suffering a primary infection, later in life these specific T-cells displayed shorter telomere length and lower telomerase activity (Akbar and Vukmanovic-Stejic, 2007). Another study found that elevated CMV IgG among CMV seropositive individuals was related to lower telomerase activity, while CMV seropositivity was only associated with decreased telomerase activity among women but not men (Dowd et al., 2013). It has been shown that viruses like CMV and EBV can be reactivated during periods of chronic stress and disease, wherein the cellular immune response is impaired and virus replication can be enhanced (Rector et al., 2014). Therefore, the observation that telomerase activity is inhibited during periods of chronic stress and chronic virus exposure may be a reflection of impaired cell-mediated immunity. Chronic virus infection also induces the release of IFN-α by plasmacytoid dendritic cells, which inhibits telomerase activity by transcriptional and posttranscriptional mechanism and induces the loss of CD28 in human CD8+ T-cells in vitro (Lanna et al., 2013).
2.3.3. Telomerase and the regulation of immune function-related gene expression
Increasing evidence supports a regulatory role of telomerase in immune function related gene expression that is independent of telomere length. The exact mechanisms are still unknown, but several theories have been proposed. It is likely that telomerase influences gene expression by modulating chromatin structure, and/or by making epigenetic modifications via its interaction with transcription factors or chromatin modifying factors (Zhou et al., 2014). For example, hTERT interacts with transcription factors NF-κB and β-catenin, both of which are essential for proliferation and elicitation of an immune response (Akiyama et al., 2004; Ding et al., 2013; Ghosh et al., 2012; Zhou et al., 2014).
NF-κB is a key regulator of cell survival, proliferation, inflammatory responses and immune regulation. The continuous activation of the NF-κB pathway leads to chronic inflammation, which is causally linked to the development of many stress- and aging-related disorders (Miller et al., 2014). In lymphocytes, telomerase activity and the nuclear translocation of telomerase is induced by the PI3K-Akt- NF-κB signaling pathway in response to TNF-α (Akiyama et al., 2004). hTERT also regulates the expression of a subset of NF-κB-dependent genes (Ding et al., 2013; Ghosh et al., 2012). The observation that hTERT binds to the NF-κB p65 subunit and is recruited to a subset of NF-κB promoters (such as those of IL-6 and TNF-α, probably by binding to a T2G3 sequence near the NF-κB binding site) suggests that telomerase can provide a feed forward loop for the immune system by stimulating NF-κB-dependent gene expression (Ghosh et al., 2012).
Human TERT interacts with β-catenin and can act as a cofactor in the β-catenin transcription complex (Zhou et al., 2014). Vice versa, the Wnt/β-catenin pathway can directly regulate the transcription of hTERT (Hoffmeyer et al., 2012). The Wnt/β-catenin pathway controls many processes, including organ development, cell fate decision, and stem cell renewal. Moreover, it is essential for normal T-cell development, memory CD8+ T-cell formation and persistence, and it is required for CD4+ Th2 differentiation and CD4 re-expression on a subset of CD8+ T-cells involved in antiviral immune responses (Schenkel et al., 2010; Xue and Zhao, 2012). However, the effects of hTERT on Wnt signaling might be context and cell type specific, as other studies failed to find evidence that hTERT promotes Wnt signaling in multiple breast cancer cell lines (Listerman et al., 2014). Also, hTERC, the RNA component of the telomerase holoenzyme, appears to protect against dexamethasone-induced apoptosis in CD4+ T-cells (Gazzaniga and Blackburn, 2014). Taken together, it is apparent that telomerase plays an important role in pathways underlying immune activation, differentiation and immunosenescence by exerting effects on key immunoregulatory transcription factors. Figure 1 provides a schematic overview of the regulation and regulatory functions of hTERT/telomerase in immune cells.
Figure 1.
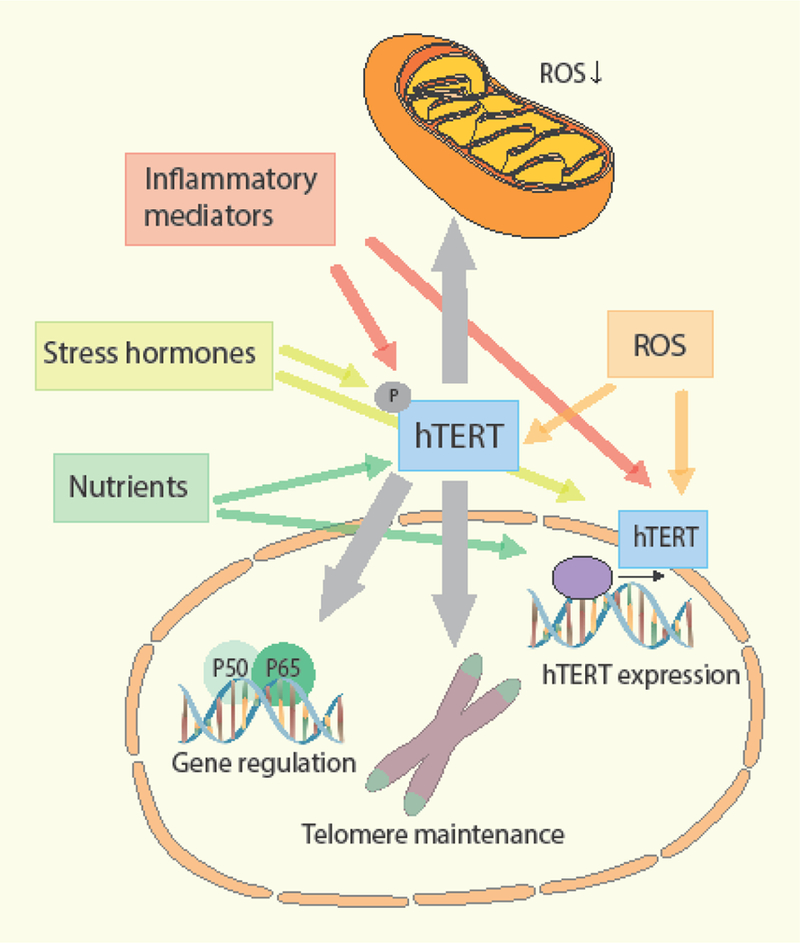
Overview of the regulation and regulatory functions of hTERT/telomerase in immune cells. Telomerase protects mitochondria against oxidative stress, regulates immune-related gene expression and maintains telomere length during immune activation. Inflammatory mediators, stress hormones, nutrients and ROS all influence telomerase expression, telomerase activity and its cellular localization by epigenetic, translational and posttranslational (e.g. phosphorylation) machanisms. ROS, Reactive oxygen species.
3. Telomerase in stress- and aging-related pathologies and conditions
Shorter leukocyte telomere length has been associated with psychological stress and also with several stress- and aging-related physical and mental disorders including cardiovascular disease (D’Mello et al., 2015; Haycock et al., 2014), type 2 diabetes (Tamura et al., 2016), autoimmune diseases (Georgin- Lavialle et al., 2010) and depression (Schutte and Malouff, 2015). Several studies on human telomerase in the context of acute and chronic stress exposure highlight the importance of stress and stress physiology as important regulators of the telomere biology system. In the following section, we first summarize findings about telomerase in the context of acute and chronic psychological stress (see also Table 1), and second, we discuss research on telomerase in the context of cardio-metabolic, autoimmune, and mental disorders (Table 2).
Table 1.
Human studies on stress and PBMC telomerase.
| Ref | Study design | Predictor | Basal TA | Stimulated TA |
Effects |
|---|---|---|---|---|---|
| (Epel et al.,2004) | 39 healthy caregiving mothers, 19 controls | Chronic stress | TA ↓ | Mean TA 48% lower in high-stress group. The group with short TL showed higher perceived stress levels and displayed higher oxidative stress and lower TA levels. |
|
| (Epel et al., 2006) | 62 healthy mothers | Chronic stress | TA ↓ | Lower TA associated with higher stress arousal, perceived stress and chronicity of stress. Lower TA associated with several risk factors for cardiovascular diseases. |
|
| (Damjanovic et al., 2007) | 41 Alzheimer caregivers, 41 controls |
Chronic stress | TA ↑ | No difference | Caregivers showed higher TA and shorter TL. Similar results in isolated T-cells. No difference in stimulated TA. Caregivers showed reduced stimulated proliferation and increased cytokine production of T cells. |
| (Epel et al., 2010) | 44 postmenopausal women: 22 caretakers, 22 controls |
Chronic stress Acute stress |
TA ↓ TA ↑ |
Caretakers lower TA activity at baseline. 18% higher TA in response to TSST in both groups. TA response higher in persons responding with higher cortisol. TA activity was associated with threat appraisal in controls. |
|
| (Zalli et al., 2014) | 333 middle aged and elderly healthy men and women |
Stress reactivity | TL ↓ + TA ↑ | Males with shorter TL and high TA showed blunted stress-reactivity compared to men with longer TL or men with shorter TL and low TA. | |
| Stress recovery | TL ↓ + TA ↑ | Males with shorter TL and high TA showed reduced post-stress recovery. Shorter TL in combination with high TA was associated with reduced social support, lower optimism, higher hostility, and greater early life adversity. | |||
| (de Punder etal., 2018) | 28 healthy participants |
Acute stress | No difference | No difference in stimulated TA levels before and after TSST exposure. |
|
| Stress-reactivity | TA ↓ | Inverse correlation between overall TA levels and the cortisol response. | |||
| Chronic stress | TA ↓ | Individuals exposed to high levels of chronic stress showed a 25% reduction in induced TA levels compared to individuals perceiving medium or low levels of stress. | |||
Abbreviations: BP, blood pressure; TA, telomerase activity; TL, telomere length; TSST, Trier Social Stress Test.
Table 2.
Human studies on PBMC telomerase in disease.
| Ref | Study design | Predictor | Basal TA | Stimulated TA |
Effects |
|---|---|---|---|---|---|
|
Cardio-metabolic disease | |||||
| (Rentoukas etal., 2012) | 39 patients with metabolic syndrome, 20 controls | Metabolic syndrome | TA ↑ | Higher TA, Il-6, TNF-α, ADMA and CD163 in patients compared to controls. Negative correlations between TA and HDL and waist circumference. |
|
| (Kroenke etal., 2012) | 440 black and white men | Atherosclerosis/Coronary artery calcification | TA ↑ | CRP and current smoking positively associated with TA. Low socioeconomic status associated with higher TA only in black men. TA positively associated with prevalent (15 years after follow up) and progressive (20 years after follow up) coronary artery calcification. Progression greater in individuals with short TL. |
|
| (Laish et al.,2016) | 22 NAFLD patients, 20 patients with cryptogenic cirrhosis, 20 healthy age-matched controls | NAFLD Cryptogenic cirrhosis |
hTERT ↑ No difference |
Higher hTERT mRNA expression in NAFLD patients compared to patients with cryptogenic cirrhosis. | |
|
Autoimmune disease | |||||
| (Wu et al., 1999a) | 15 atopic dermatitis patients, 13 healthy controls | Atopic dermatitis | TA ↑ | No difference | Higher TA in unstimulated PBMC after 72 hours in vitro. No difference in stimulated TA after 72 hours between patients and controls. The relative increase in TA was lower in patients compared to controls. |
| (Yudoh et al., 1999) | 18 RA patients, 9 PVS patients, 12 osteoarthritis patients, 10 controls with knee joint trauma | RA | TA ↑ | Higher TA levels in RA patients compared to the other groups | |
| (Wu et al., 2000) | 16 psoriasis and 32 atopic dermatitis patients, 30 controls | Psoriasis Atopic dermatitis |
TA ↑ | TA increased in both patients groups compared to controls. | |
| (Katayama and Kohriyama, 2001) | 9 Sc patients, 17 SLE patients, 11 MCTD patients, 11 Sj patients | Sc SLE MCTD Sj |
No difference TA ↑ TA ↑ TA ↑ |
No difference between Sc patients and controls TA higher in SLE, MCTD, and Sj patients compared to controls. Correlation between SLE disease activity index scores and TA. |
|
| (Kurosaka et al., 2003) | 55 SLE patients, 45 controls | SLE | TA ↑ | Higher TA in Sj patients with extraglandular manifestations. Higher TA in SLE patients with active disease. No difference in TA between inactive SLE patients in their 20s, 30s and 40s, but higher TA in inactive SLE patients in their 50s compared to controls. |
|
| (Thewissen et al., 2005) | 15 RA patients, 8 healthy controls, 7 MS patients, 8 flu patients |
Early RA, MS and Flu patients |
hTERT ↓ | Increase in TA related to increase in hTERT mRNA expression. PBMCs of early-untreated RA patients, MS patients and flu patients showed reduced hTERT mRNA levels after in vitro stimulation compared to controls and chronically treated RA patients. No decrease in stimulated hTERT mRNA level observed with age. |
|
| (Fritsch et al., 2006) | 37 SLE patients, 42 healthy controls | SLE | TA ↓ | Stimulated TA lower in naive CD4+ and CCR7+/CD27+ memory T cells from patients compared to controls. | |
| (Kurosaka et al., 2006) | 34 SLE patients (18 active disease, 16 inactive disease), 17 controls | SLE | TA ↓ | TA in T-cells higher in both active and inactive SLE patients compared to controls. TA in B-cells higher in active SLE patients compared to controls. |
|
| (Tarhan et al., 2008) | 19 Sc 15 SLE, 10 RA and 14 Sj patients, 29 | RA Sc SLE Sj |
hTERT ↑ hTERT ↓ No difference No difference |
hTERT mRNA higher in RA group compared to controls. In RA group hTERT mRNA levels higher in clinically active healthy controls compared to non-active group. Sc patients displayed lower hTERT mRNA compared to controls. |
|
| (Fujii et al., 2009) | 69 RA patients, 60 healthy age-matched controls |
RA | TA ↓ | Impaired induction of TA in stimulated naive CD4+ T-cells from RA patients compared to controls. No difference in memory T-cells. |
|
| (Qi et al., 2013) | 37 active chronic immune thrombocytopenia patients, 22 healthy controls | Immune thrombocytopenia | TA ↑ | TA increased in CD4+, CD8+ and CD19+ lymphocytes from patients compared to controls. | |
| (Invernizzi et al., 2014) | 30 women with primary biliary cirrhosis, 12 healthy women | primary biliary cirrhosis | TA ↑/TA ↓ | Patients with advanced disease less TA compared to those with early-stage disease | |
| (Zhang et al., 2016) | 30 oral lichen planus patients, 19 controls |
Oral lichen planus | hTERT ↓ | Lower hTERT mRNA levels in CD4+ T cells of patients compared to controls. No difference in CD8+ T cells. |
|
| (Vazirpanah et al., 2017) | 64 gout patients, 89 controls |
Gout | No difference |
No difference in hTERT mRNA levels between patients and controls. |
|
|
Mental disorders | |||||
| (Porton et al., 2008) | 53 schizophrenia patients, 31 unaffected relatives, 59 unrelated controls | Schizophrenia | TA ↓ | Lower TA in individuals with schizophrenia compared to unaffected individuals. |
|
| (Wolkowitz et al., 2012) | 20 unmedicated depressed individuals, 18 controls | MDD | TA ↑ | TA elevated in depressed versus controls. TA positively correlated with inventory of depressive symptomatology and perceived stress scale ratings. Depressed group: individuals with lower pretreatment TA + greater increase in TA during treatment showed better antidepressant responses. |
|
| (Chen et al., 2014) | 20 unmedicated depressed adults, 20 controls |
MDD + ACE exposure ACE exposure |
TA ↑ No difference |
In the depressed group higher ACE exposure was associated with higher TA and a higher TA: TL ratio. In healthy controls ACE exposure was associated with shorter TL, but not associated with TA. |
|
| (Jergovic et al., 2014) | 30 PTSD patients (middle-aged), 17 age-matched controls, 15 elderly controls 15 elderly controls |
PTSD Age |
No difference TA ↓ |
No difference in TA between PTSD patients and controls. Elderly group showed lower TA, increased spontaneous pro-inflammatory cytokine production and reduced T-cell proliferation compared to PTSD and control group. |
|
| (Jacobs et al., 2014) | 47 midlife women, including 19 with apolipoprtotein E-ε4 genotype (risk factor for accelerated aging) |
Hippocampal volume |
TA/TL↑ | A higher TA/TL ratio was associated with lower hippocampal volume. | |
| (Simon et al., 2015) | 166 individuals with MDD,166 age- and gender-matched controls |
MDD (males) MDD (females) |
TA ↑ No difference |
TA higher in depressed males versus controls. No difference in women. |
|
Abbreviations: ACE, adverse childhood experiences; ADMA, asymmetric dimethylarginine; HDL, high density lipoprotein; MCTD, mixed connective tissue disease; MDD, major depressive disorder; MS, multiple sclerosis; NAFLD, non-alcoholic fatty liver disease; PTSD, posttraumatic stress syndrome; PVS, pigmented villonodular synovitis; RA, rheumatoid arthritis; Sc, systemic sclerosis; Sj, Sjögren’s syndrome; SLE, systemic lupus erythematodes; TA, telomerase activity; TL, telomere length.
3.1. Stress and telomerase; differential effects of acute vs chronic stress
3.1.1. The effects of acute stress on telomerase expression
To the best of our knowledge, only two published studies to date have described the effects of acute stress on telomerase activity in humans (Table 1). The first study was conducted in a population of chronically stressed and healthy (control) women and found that PBMC telomerase activity increased by 18% after a standardized stress test (Trier Social Stress Test; TSST) across both groups. Although overall telomerase activity was influenced by blood immune cell composition, the increase in telomerase activity was independent of immune cell type. Interestingly, women responding with a higher cortisol response to the acute stress paradigm exhibited a greater increase in PBMC telomerase activity, indicating that telomerase activity may be responsive to acute changes in stress hormones (Epel et al., 2010). As discussed by these authors, telomerase activity levels may change dynamically in response to stress, probably to protect telomeric regions or mitochondria from the effects of stress-induced acute increases in biological stress mediators such as oxidative stress. Alternatively, the increase in telomerase activity could also indicate the anticipation or preparation of the immune system to immune activation.
The second study, conducted by our research program, examined the effect of acute stress exposure on a measure of in vitro stimulated telomerase activity in a population of healthy young adult women and men (de Punder et al., 2018). This measure of telomerase differs from the first study in that it captures the maximal capacity of immune cells to express telomerase using a mitogen challenge across a 3-day period of time. We observed no difference in induced telomerase activity levels in PBMCs obtained before and immediately after exposure to the standardized stress test (TSST). We did, however, find an inverse correlation between total telomerase production and the cortisol response to the stress test, suggesting that individuals exhibiting higher psychophysiological stress-reactivity exhibit a reduced capacity to induce telomerase activity upon mitogen challenge. Consistent with our findings, a previous study had reported that exogenous hydrocortisone inhibited telomerase production in human stimulated T-cells (Choi et al., 2008). Glucocorticoids place a limit on the maximal activity of the immune system, modulate inflammatory gene transcription (Barnes and Adcock, 2009), and can, either through direct action or through the modulation of cytokine release, inhibit lymphocyte proliferation and immune cell activity (Bauer, 2005), to thereby influence telomerase regulatory pathways (Buchkovich and Greider, 1996; Kawauchi et al., 2005). It is likely that individuals experiencing persistently exaggerated stress responses are exposed to greater concentrations of cortisol over longer periods of time, which may have consequences on the immune- and telomere system.
3.1.2. The effects of chronic stress on the immune system and telomerase expression
Chronic psychological stress has been linked to dysregulation of the immune system, for example by altering the cytokine balance from type-1 (cytotoxic) to type-2 cytokine (humoral) driven responses. The dysregulation of the immune system is accompanied by chronic low-grade inflammation, increased oxidative stress levels, delayed wound healing, poor responses to vaccine and increased susceptibility to infectious diseases (Glaser and Kiecolt-Glaser, 2005). In addition, chronic stress has been linked to shorter telomere length (Mathur et al., 2016; Oliveira et al., 2016). This association is also observed in the context of exposure to prenatal (Entringer et al., 2018) and early life stress (Coimbra et al., 2017). The effects in humans of chronic stress on telomerase activity have been described in six published studies to date (Damjanovic et al., 2007; de Punder et al., 2018; Epel et al., 2004; Epel et al., 2010; Epel et al., 2006; Zalli et al., 2014) (Table 1). Three of these studies (Epel et al., 2004; Epel et al., 2010; Epel et al., 2006) report a suppressive effect of chronic stress on basal telomerase activity. These effects may possibly be driven by chronic stress-induced increases in oxidative stress levels (Aschbacher et al., 2013), inducing the nuclear export of hTERT to the cytosol and decreasing nuclear and total telomerase activity (Haendeler et al., 2004). In addition, ROS causes telomeres to shorten more quickly, resulting in the accumulation of senescent immune cells expressing lower levels of telomerase activity. Alternatively, prolonged periods of psychological stress might render individuals more susceptible for virus-reactivation and continuous virus-exposure, which also induces senescence of specific T cells (Akbar and Vukmanovic-Stejic, 2007). Contrary findings were observed in a study of caretakers of patients with Alzheimer’ disease and age-matched controls. Caretakers displayed shorter telomere length in parallel with higher (basal) telomerase activity, while no differences in stimulated telomerase activity and the number of CD28- T-cells were seen between caretakers and controls (Damjanovic et al., 2007). Shorter telomere length in combination with higher (basal) telomerase activity was also associated with chronic psychological stress in older men (Zalli et al., 2014). The authors of this report suggest the finding may result from a potential increase in immune cell proliferation induced by continuous stress-induced immune stimulation (chronic low-grade inflammation), thereby increasing telomerase activity, but not high enough to maintain telomere length under conditions of chronic stress. In addition, elevations in telomerase activity in combination with shorter telomere length may reflect a counter-regulatory adaptation to states or conditions like a physiological stress exposure that have the potential to reduce telomere length. Finally, we have recently reported that a 25% reduction in the capacity of immune cells to mount a telomerase activity response after challenge was observed between subjects reporting high levels of chronic stress compared to individuals that perceived medium or low levels of chronic stress (de Punder et al., 2018). The protocol used in our study particularly stimulates cell types involved in cellular immunity, the component of the immune system that is initially affected by chronic stress exposure (Segerstrom and Miller, 2004). Chronic stress has been associated with blunted mitogen-induced lymphocyte proliferation and mitogen-induced IL-2 production (Bauer et al., 2000), both activators of signaling pathways stimulating telomerase activity (Buchkovich and Greider, 1996; Kawauchi et al., 2005). In addition, chronic stress exposure is associated with a higher level of oxidative stress (Aschbacher et al., 2013), eventually resulting in the accumulation of senescent (CD28-) T cells. Senescent T cells show impaired upregulation of telomerase activity after chronic antigen stimulation (Valenzuela and Effros, 2002) and could therefore inhibit telomerase activity induction capacity.
Taken together, contradictory results have been observed on chronic stress exposure and telomerase activity, which may be indicative of the dynamic regulation of telomerase activity by different biological mechanisms, like stress hormones, inflammatory mediators and oxidative stress. Until now, most research examining telomerase activity in the context of chronic stress exposure have not systematically studied these mechanisms and therefore further experimental investigations are needed to explain and reconcile some of the inconsistencies in the literature.
3.2. Cardiometabolic diseases and telomerase
The metabolic syndrome is associated with chronic systemic low-grade inflammation, oxidative stress and vascular endothelial dysfunction (Eckel et al., 2010). While the role of telomere length in the onset and progression of cardiovascular disease has been extensively studied (D’Mello et al., 2015; Haycock et al., 2014), only a few studies have assessed telomerase activity in this context (Table 2). In the CARDIA study, higher levels of PBMC telomerase activity predicted higher prevalence of coronary artery calcium (a measure of calcified atherosclerosis), especially in individuals with shorter telomeres (Kroenke et al., 2012). In parallel with elevated plasma levels of TNF-α, IL-6, sCD163 and asymmetric dimethyl arginine (a marker for endothelial dysfunction), PBMC telomerase activity was significantly elevated in patients with metabolic syndrome when compared to healthy volunteers. Significant negative correlations were found between telomerase activity and HDL and waist circumference, while telomerase activity was positively correlated with asymmetric dimethyl arginine. Yet, no associations were observed with TNF-α and IL-6 and telomerase activity (Rentoukas et al., 2012). Also, non-alcoholic fatty liver disease was associated with elevated hTERT mRNA expression (Laish et al., 2016). The elevated telomerase activity levels observed in patients with cardiometabolic disease could possibly be explained by a pro-inflammatory state, producing a feed-forward loop between NF-κB and hTERT (Ghosh et al., 2012). An alternative explanation could be that leptin, an adipokine important for energy homeostasis and associated with cardiometabolic risk factors (Ingelsson et al., 2008), induces expression of hTERT via STAT3 (Sundin and Hentosh, 2012). Finally, elevations in telomerase activity may reflect a counterregulatory adaptation to physiological stressors that reduce telomere length (Beery et al., 2012; Zalli et al., 2014).
3.3. Autoimmune disease and telomerase
During aging, the ability of the immune system to differentiate between pathogens and normal tissues diminishes, and the risk for immune responses against “self” body tissues increases. For example, autoantibody production increases with age, which in elderly individuals is inversely correlated with a decreased proliferation of T-cells in response to a mitogen challenge (Agarwal and Busse, 2010).
Most studies investigating telomerase in autoimmune diseases have found increased telomerase activity or hTERT gene expression in disease versus healthy states (Katayama and Kohriyama, 2001; Kurosaka et al., 2001; Kurosaka et al., 2003; Kurosaka et al., 2006; Qi et al., 2013; Tarhan et al., 2008; Wu et al., 2000; Wu et al., 1999a; Yudoh et al., 1999). However, one study observed no difference (Vazirpanah et al., 2017) and two studies reported lower hTERT mRNA expression in patients compared to healthy controls (Tarhan et al., 2008; Zhang et al., 2016). Interestingly, differences in telomerase activity were observed between active and inactive disease (Katayama and Kohriyama, 2001), probably due to changes in inflammatory and oxidative stress levels. Furthermore, differences in telomerase activity between early-and advanced-stage disease (Invernizzi et al., 2014) indicate that telomerase activity may fluctuate across the course of a disease (Table 2). A few studies assessing telomerase activity or hTERT expression following mitogen stimulation in vitro observed either a reduced capacity to induce telomerase activity (or hTERT expression) (Fritsch et al., 2006; Fujii et al., 2009; Thewissen et al., 2005) in patients, or observed no differences between the different disease- and control groups (Wu et al., 1999a) (Table 2). Immune dysregulations in combination with a reduced capacity to induce telomerase activity in response to challenge might explain accelerated telomere attrition rates observed in these pathologies.
3.4. Mental diseases and telomerase
Studies on the association of basal PBMC telomerase and mental disorders such as depression, posttraumatic stress disorder (PTSD) and schizophrenia have produced heterogeneous results (Chen et al., 2014; Jergovic et al., 2014; Porton et al., 2008; Simon et al., 2015; Wolkowitz et al., 2012) (Table 2). In the context of depression, a study of medication-free depressed individuals and healthy controls found that PBMC telomerase activity levels were elevated in depressed individuals compared to controls and were directly correlated with symptom severity (Wolkowitz et al., 2012). Also, higher PBMC telomerase activity was observed in depressed males versus controls, however, this association was not observed in women (Simon et al., 2015). Furthermore, in depressed patients, exposure to adverse childhood experiences (ACE) was associated with increased PBMC telomerase activity and a higher telomerase: telomere length ratio, while in healthy controls ACE exposure was associated with shorter telomeres and not associated with telomerase activity (Chen et al., 2014).
PBMCs from PTSD patients compared to those of healthy controls displayed shorter telomeres but comparable telomerase activity levels and in vitro immune responses. Yet, elderly participants with PTSD in the same study had shorter telomere length and lower telomerase activity levels, paralleled by increased in vitro (spontaneous) pro-inflammatory cytokine production (IL-6 and TNFα) and reduced T-cell proliferation (Jergovic et al., 2014). These findings may possibly be explained by the increased presence of senescent immune cells in the elderly population. Compared to healthy individuals, schizophrenia patients showed decreased PBMC telomerase activity levels that were accompanied by shorter telomeres (Porton et al., 2008). The precise mechanisms underlying the observed differences in PBMC telomerase activity between the abovementioned studies remain to be elucidated, but may be indicative of the dynamic regulation of telomerase activity in different stress- and disease states.
Finally, the ratio between PBMC telomerase activity and telomere length was associated with age-related structural brain changes in healthy adults, suggesting that changes in telomere biology can also reflect age-related structural brain alterations (Jacobs et al., 2014).
4. Lifestyle interventions targeting telomerase
Based on the consideration that the adverse effects of stress on the telomere system may be mediated, in part, via unhealthy behaviors that are associated with stress exposure (e.g., more sedentary behavior, unhealthy diet, smoking, substance abuse), it has been suggested and demonstrated in several studies that a healthy lifestyle with respect to nutrition, stress management and exercise may promote longer telomeres (Ornish et al., 2013; Puterman et al., 2013). This effect has been attributed to changes in telomerase activity (Boccardi et al., 2013; Ornish et al., 2008). Because intervention studies, in contrast to cross-sectional studies, provide stronger evidence for a causal role of stress-related processes on telomerase activity, we have reviewed here only those studies that have performed an intervention, but not those that have merely reported correlations. The effects of different lifestyle interventions on telomerase activity and or expression are summarized in Table 3.
Table 3.
Human intervention studies on lifestyle/meditation and PBMC telomerase.
| Ref | Study design | Predictor | Basal TA | Effects |
|---|---|---|---|---|
|
Lifestyle, diet and nutrition supplementation | ||||
| (Omish et al., 2008) | 24 men with biopsy-diagnosed low-risk prostate cancer |
Lifestyle changes, 3 months | TA ↑ | TA increased almost 30% during the course of the 3 months intervention. Increases in TA associated with decreases in LDL cholesterol and psychological distress. |
| (Zhu et al., 2012) | 37 overweight African Americans (18 placebo, 19 vitamin D3) | Vitamin D3 2000 IU/day oral, 16 weeks | TA ↑ | TA increased by 19.2% from baseline in the vitamin D3 group. No difference in TA in the placebo group. |
| (Omish et al., 2013) | 10 men with biopsy-diagnosed low-risk prostate cancer, 25 controls | Lifestyle changes, 5 years | No difference | No association with lifestyle changes and TA. |
| (Balcerczyk et al., 2014) | 66 healthy women | Supplementation with an antioxidant formula, 12 weeks | TA ↑ | TA increased after 12-weeks of supplementation, paralleled by increases in vitamin D level, reductions in oxidative stress levels and increases in the antioxidant potential. |
| (Pawelczyk et al., 2018) | 71 schizophrenia patients | 2.2g/day of n-3 PUFA or olive oil placebo, 26 weeks | TA ↑ | Higher increases in TA in the intervention group compared to placebo group. Changes in TA were inversely correlated with improvement in depressive symptoms and severity of the illness. |
|
Exercise | ||||
| (Chilton et al., 2014). | 22 healthy males | 30 minutes of treadmill running at 80% of peak oxygen uptake | hTERT ↑ | hTERT mRNA expression significantly increased from pre- to 60 min post-exercise. |
| (Zietzer et al., 2016) | 26 young healthy participants 14 elderly peripheral artery disease patients |
Single session of treadmill running, 30 hrs individual shear rate therapy (ISRT) for 5 weeks |
TA ↑ TA ↑ |
TA increased after a single bout of aerobic exercise. TA increased after a 5-week period of ISRT therapy in elderly patients suffering from peripheral artery disease. |
|
Meditation | ||||
| (Jacobs et al., 2011) | 32 healthy subjects (17 intervention, 25 waitlist controls) | Meditation retreat, 3 months |
TA↑ | TA activity was significantly higher in intervention group compared to control group, no baseline measured. |
| (Daubenmier et al., 2012) | 47 overweight/obese women (24 intervention, 23 waitlist controls) | Mindfulness intervention on stress-eating, 4 months | TA ↑ | 18% higher TA increase in intervention group. Changes in TA were negatively correlated to changes in chronic stress, anxiety, dietary restraint, fat intake, cortisol and glucose. |
| (Ho et al., 2012) | 64 chronic fatigue syndrome patients (33 intervention, 31 waitlist controls) | Qigong exercise, 12 weeks | TA ↑ | TA increased in the intervention group. TA was higher in the intervention group compared to controls after 4 months. |
| (Lavretsky et al., 2013) | 39 dementia caregivers with mild depressive symptoms |
Kirtan Kriya meditation (n = 23) or relaxation music (n = 16), 12 min per day for 8 weeks |
TA ↑ | TA increased with 43.3% in the meditation group, compared to 3.7% in the relaxation group. Meditation group: correlation between TA and improved mental health score. |
| (Lengacher et al., 2014) | 142 breast cancer survivors (74 intervention, 68 waitlist controls) | Mindfulness-based stress reduction (MBSR), 6 weeks | TA↑ | TA increased with 17% over 12 weeks in the MBSR group. No increase in the control group. No difference in TL between both groups. |
| (Duraimani et al., 2015) | 48 hypertensive men and women | Transcendental Meditation + basic health education course (n = 24) or, Extensive health education program (n = 24), 16 weeks | hTERT ↑ hTERC ↑ |
Both groups showed increased hTERC and hTERT mRNA levels No differences between both groups. |
| (Epel et al., 2016) | 64 non-meditating women, 30 regular meditators | Vacation or meditation retreat, 1 week | TA↑ | Baseline TA lower in regular meditators TA increased only in the regular meditation group |
| (Tolahunase et al., 2017) | 96 men and women | Yoga- and meditation-based lifestyle intervention program, 12 weeks | TA↑ | TA increased, while levels of 8-OH2dG, ROS, cortisol, and IL-6 decreased after intervention. |
| (Tolahunase et al., 2018) | 58 depressed patients (29 intervention, 29 controls) | Yoga- and meditation-based lifestyle intervention program, 12 weeks | TA ↑ | TA increased, while depression severity decreased in intervention compared to control group. In addition, the intervention increased DHEAS and Sirtuin 1 levels, decreased cortisol, IL-6 and DNA damage and balanced oxidative stress. |
| (Conklin et al., 2018) | 28 retreat participants, 34 non-participating controls | Meditation retreat, 3 weeks | No difference | No difference in TA between retreat participants and control group. Pre-assessment TA levels were associated with greater TL increases across both groups. Post-assessment TA levels were negatively correlated with reports of practice diligence and the number of hours of practice time during the retreat. |
Abbreviations: DHEAS, Dehydroepiandrosteron sulfate; EEE, exercise energy expenditure; LDL, low-density lipoprotein; 8-OH2dG, 8-Oxo-2'- deoxyguanosine; PUFA, polyunsaturated fatty acid; ROS, Reactive oxygen species; TA, telomerase activity; TL, telomere length.
4.1. Lifestyle and diet/nutrition interventions
A three-months intensive lifestyle change intervention that included modifications in diet, exercise and stress management in men diagnosed with low-risk prostate cancer was found to increase PBMC telomerase activity (Ornish et al., 2008). However, no changes in telomerase activity were observed between controls and a small group of men that continued these changes over a 5 year period. Nevertheless, after 5 years, telomere length increased in the intervention group, while it declined in the control group (Ornish et al., 2013). Twelve weeks of supplementation with an antioxidant formula increased telomerase activity in healthy women. The supplement also improved vitamin D deficiencies, reduced oxidative stress, and increased the antioxidant potential (Balcerczyk et al., 2014). In schizophrenia patients, higher PBMC telomerase activity was observed after 26 weeks of n-3 PUFA (fish oil) supplementation added to antipsychotics compared to adding a placebo. Increases in telomerase activity were associated with improvements in depressive symptoms and disease severity (Pawelczyk et al., 2018). Higher serum vitamin D3 levels have been shown to be associated with longer telomere length in women (Richards et al., 2007). The effect of 16 weeks of vitamin D3 (60,000 IU/month) supplementation on telomerase activity was investigated in a randomized, double-blind, placebo-controlled clinical trial with overweight African American women (Zhu et al., 2012). In the vitamin D3 group PBMC telomerase activity increased by 19.2% from baseline, while in the control group no effect was observed. Vitamin D3 and N-3 PUFA are known to have immune modulatory effects (Calder, 2015; Kamen and Tangpricha, 2010) and can shape epigenetic changes in pathways involved in telomerase regulation (Lu et al., 2018; Tremblay et al., 2017). However, the exact mechanism underlying the upregulation of telomerase activity after Vitamin D3 and n-3 PUFA supplementation remains to be elucidated.
4.2. Exercise
Habitual physical exercise is associated with longer telomere length (Cherkas et al., 2008). Acute exercise up-regulated PBMC hTERT mRNA expression in healthy volunteers (Chilton et al., 2014). Similarly, a single session of treadmill running resulted in an acute increase in PBMC telomerase activity in healthy young individuals, again indicating the dynamic responses of telomerase to acute stimuli. Five weeks of regular individual shear rate therapy in elderly suffering from peripheral artery disease also increased post-assessment telomerase activity levels (Zietzer et al., 2016).
4.3. Meditation
The perception of and coping with stress can be altered by meditative practice. Most intervention studies examining the effect of meditation on telomerase activity observed elevations in PBMC telomerase activity or telomerase gene expression after an intervention period (Daubenmier et al., 2012; Duraimani et al., 2015; Epel et al., 2016; Ho et al., 2012; Jacobs et al., 2011; Lavretsky et al., 2013; Lengacher et al., 2014; Tolahunase et al., 2017; Tolahunase et al., 2018). A more recent study observed an increase in PBMC telomere length, but no difference in telomerase activity, in participants of a meditation retreat compared to non-participating controls. Interestingly, pre-assessment telomerase activity levels were associated with greater telomere length increases across both groups, while postassessment telomerase levels were negatively correlated with reports of practice diligence and the number of hours of practice time during the retreat (Conklin et al., 2018). Pre-assessment telomerase activity was also lower in regular meditators compared to non-meditators, but tended to increase only in the regular meditation group (Epel et al., 2016). It is not clear how practicing meditation increases telomerase activity, but a plausible mechanism is that decreases in psychological stress levels through regular meditative practice are associated with lower stress-related endocrine, immune and oxidative parameters.
Accumulating endogenous oxidative stress induces the nuclear export of hTERT to the cytosol and decreases nuclear and total telomerase activity (Haendeler et al., 2004). It could be possible that meditative practice and other positive life-style interventions reverse this process.
5. Methodological considerations in the assessment of telomerase
5.1. Baseline versus stimulated telomerase activity
Telomerase can be measured under several conditions. To date, most human studies have investigated telomerase activity in PBMCs isolated from whole blood and have measured basal telomerase activity in the total cell fraction or in different immune cell populations. However, we suggest that the interpretation of such data poses various challenges for the following reasons: Firstly, telomerase levels are normally expressed at very low levels in resting PBMCs, making it possible that expression can be missed or misinterpreted if it is below or close to the assay’s lower limit of detection. Secondly, telomerase levels may vary as a function of cell-cycle stage and other biological factors such as inflammatory mediators, stress hormones and oxidative stress. Thirdly, differences in telomerase levels may also reflect the secondary (counter-regulatory) adaptations to states and conditions that reduce telomere length (Zalli et al., 2014). Higher telomerase activity levels can be induced upon antigenic/mitogenic stimulation. Therefore, in some studies, immune cells were cultured and stimulated in vitro with an antigen or mitogen to induce maximal telomerase production (Choi et al., 2008; Damjanovic et al., 2007; Fritsch et al., 2006; Son et al., 2000; Thewissen et al., 2005). The potential advantage of this approach is that it may bypass the above-mentioned limitations to provide an indicator of individual differences of the overall capacity of immune cells to respond to an ecologically relevant challenge. For example, it has been shown in CD4+ T-cells that the level of telomerase activity induced by in vitro stimulation was correlated with changes in telomere length, while no decrease in the stimulated level of telomerase activity was observed with age (Son et al., 2000). However, contrasting findings have shown that stimulated T-cell (both CD8+ and CD4+) telomerase activity declines with age (Lin et al., 2015).
This discrepancy could possibly be explained by dissimilarities in the stimulation protocols and subsets of immune cells across these studies. We have recently advanced the use of an in vitro measure of maximal telomerase activity capacity (mTAC) of human PBMCs to a mitogen challenge (PHA+IL-2). We have determined that this measure empirically meets the criteria to represent a potentially useful individual difference construct, with adequate within-subject stability and between-subject variability. We have reported that mTAC does not appear to be influenced by situational factors including time of day, acute stress exposure, and immune cell distribution in the pre-stimulation blood sample. The mTAC measure is, however, associated with key stress-related states and traits, including perceived chronic stress and psychophysiological stress responsivity (de Punder et al., 2018). Based on the findings and considerations discussed above, we have advanced the concept that studies of the capacity of immune cells to mount a telomerase response to challenge may represent a useful individual difference measure, either independently or in combination with measures of basal telomerase activity and telomere length.
5.2. Immune cell distribution
Telomerase activity is known to vary by immune cell subtype. Biological factors such as stress hormones and inflammatory mediators and the circadian rhythm alter the trafficking of immune cells in and out of the circulation, thereby potentially influencing telomerase activity levels in isolated PBMCs. Early studies on telomerase expression in different subtypes of isolated immune cells reported detectable levels of telomerase activity in all T and B cell fractions, but undetectable levels in the monocytic fractions (Bodnar et al., 1996). Later findings in four lymphocyte subpopulation have suggested that senescent CD28- T-cells display the lowest telomerase activity, while B-cells have the highest telomerase activity, and that CD4+ T cells exhibit slightly higher telomerase activity than CD8+CD28+ T cells (Lin et al., 2010). However, it appears that within-individual telomerase activity across all four cell types is correlated, indicating that telomerase activity in these lymphocyte subpopulations may be regulated by common pathways (Lin et al., 2010). In whole blood, greater percentages of monocytes were associated with higher telomerase activity levels, while higher percentages of CD8+ T-cells were related to lower levels of telomerase activity (Epel et al., 2010). Our own study did not find significant alterations in stimulated telomerase activity (mTAC) as a function of immune cell type distributions present before in vitro stimulation. However, only the whole fraction of CD8+ cells was studied here, and no distinction was made between naïve, effector and memory CD8+ T-cells, or CD8+ CD28- T-cells. The percentage of senescent cells could influence mTAC, as it has been shown that CD28 signaling is required for optimal telomerase upregulation (Valenzuela and Effros, 2002),
5.3. Measurement of telomerase activity
Telomerase activity can be measured by a highly sensitive PCR-based method -- the telomerase repeat amplification protocol (TRAP) -- which was originally designed to measure telomerase in cancer cells (Counter et al., 1992; Kim and Wu, 1997; Morin, 1989). In this assay, endogenous telomerase adds a number of telomeric repeats on to the 3’ end of a substrate oligonucleotide. The products of telomerase-mediated extension of the substrate oligonucleotide are then amplified by PCR using reverse primers. This generates a ladder of bands with 6 base increments (the size of the telomeric repeat). In the first protocol a radioactive-labeled substrate oligonucleotide was used that could be detected by polyacrylamide electrophoresis followed by autoradiography. Later, fluorescein-labeled substrate primers or just a simple conventional in-gel staining of the products were used for detection. The use of energy transfer primers makes it possible to detect telomerase activity directly from reaction kinetics with real-time PCR. In addition, a very sensitive PCR ELISA kit is commercially available that combines a biotinylated substrate primer with a microplate detection format (extensively reviewed by Fajkus, 2006 (Fajkus, 2006)). When performing the TRAP assay it should be noted that isolating PBMCs from whole blood may result in different amounts of contaminating red blood cells and platelets, which may contribute to total protein in the extract. Therefore, more recent studies use viable cell numbers to normalize among different samples, instead of normalizing to total protein (Lin et al., 2010). Finally, a novel PCR technology, droplet digital PCR (ddPCR), has made it possible to quantify telomerase enzyme activity on a single-cell basis (ddTRAP) technique (Ludlow et al., 2014).
6. Conclusions and implication for further research
As reviewed above, telomerase is highly regulated in response to various biological factors and systems, such as stress hormones, inflammatory mediators, oxidative stress and the circadian rhythm. Therefore, we suggest that future studies of PBMC telomerase should include measures of these processes/biomarkers and should standardize potential confounding factors such as time of day of blood collection. Moreover, because, telomerase activity can vary by immune cell subtype, when characterizing total PBMC telomerase under conditions of stress and/or presence of disease states, it may be important to also assess possible changes in the distribution of various immune cell subpopulations, including senescent immune cells.
To date, basal telomerase activity has not been found to be associated with telomere length when measured in total PBMC. This might be explained by the possibility that changes in telomerase are only related to telomere length in a subset of the total PBMC population and are therefore not revealed in total PBMC measurements. Also, it is important to note that changes in telomerase could be related to only a subset of the total PBMC population and could be independent of telomere length. Thus, future studies including telomerase and telomere length measurements on the single cell level are clearly warranted to fill in these research gaps. Future studies should also consider the telomere-independent functions of telomerase, for example, its role in protecting mitochondria against oxidative stress, to gain a broader knowledge of the role of telomerase in health and stress-related disease risk. Moreover, longitudinal studies on telomerase activity and change in telomere length are warranted. Only a relatively small number of studies have investigated the characteristics of stimulated telomerase responses. A reduced capacity for induction of telomerase might explain why telomeres shorten faster under pathological conditions. As reviewed above, stimulated telomerase activity levels and hTERT mRNA expression levels are reduced in several disease states (Fritsch et al., 2006; Fujii et al., 2009; Thewissen et al., 2005), associated with stress-related states and traits, and appear to be independent of situational factors such as acute stress exposure and time of day of blood collection (de Punder et al., 2018). For these reasons, the characterization of individual differences in the capacity of the telomere biology system to respond to challenge, in addition to measures of basal telomerase activity and telomere length, may represent a promising approach in future studies of telomere biology and stress- and aging-related disease risk.
Highlights.
Telomerase preserves healthy cell function and influences immune function-related gene expression.
Stress and stress physiology are important regulators of telomerase.
The telomerase response to challenge may represent a useful individual difference measure.
8.
Funding
Funding was provided by grants from Neurocure (Innovation Projects), Berlin, Germany, the German Federal Ministry of Education and Research (01KR1311A), the European Research Counsil ERC-STG-67073 and US PHS (NIH) grants R01 HD-065825, R01 HD-060628 and R01 AG-050455.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
10. References
- Agarwal S, Busse PJ, 2010. Innate and adaptive immunosenescence. Ann Allergy Asthma Immunol 104, 183–190; quiz 190–182, 210. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Passos JF, Birket MJ, Beckmann T, Brings S, Peters H, Birch-Machin MA, von Zglinicki T, Saretzki G, 2008. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. Journal of cell science 121, 1046–1053. [DOI] [PubMed] [Google Scholar]
- Akbar AN, Vukmanovic-Stejic M, 2007. Telomerase in T lymphocytes: use it and lose it? Journal of immunology 178, 6689–6694. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Yamada O, Hideshima T, Yanagisawa T, Yokoi K, Fujisawa K, Eto Y, Yamada H, Anderson KC, 2004. TNFalpha induces rapid activation and nuclear translocation of telomerase in human lymphocytes. Biochemical and biophysical research communications 316, 528–532. [DOI] [PubMed] [Google Scholar]
- Ale-Agha N, Dyballa-Rukes N, Jakob S, Altschmied J, Haendeler J, 2014. Cellular functions of the dual-targeted catalytic subunit of telomerase, telomerase reverse transcriptase - Potential role in senescence and aging. Experimental gerontology. [DOI] [PubMed] [Google Scholar]
- Anderson CJ, Hoare SF, Ashcroft M, Bilsland AE, Keith WN, 2006. Hypoxic regulation of telomerase gene expression by transcriptional and post-transcriptional mechanisms. Oncogene 25, 61–69. [DOI] [PubMed] [Google Scholar]
- Artandi SE, DePinho RA, 2010. Telomeres and telomerase in cancer. Carcinogenesis 31, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschbacher K, O’Donovan A, Wolkowitz OM, Dhabhar FS, Su Y, Epel E, 2013. Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology 38, 1698–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcerczyk A, Gajewska A, Macierzynska-Piotrowska E, Pawelczyk T, Bartosz G, Szemraj J, 2014. Enhanced antioxidant capacity and anti-ageing biomarkers after diet micronutrient supplementation. Molecules 19, 14794–14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ, Adcock IM, 2009. Glucocorticoid resistance in inflammatory diseases. Lancet 373, 1905–1917. [DOI] [PubMed] [Google Scholar]
- Bauer ME, 2005. Stress, glucocorticoids and ageing of the immune system. Stress 8, 69–83. [DOI] [PubMed] [Google Scholar]
- Bauer ME, Vedhara K, Perks P, Wilcock GK, Lightman SL, Shanks N, 2000. Chronic stress in caregivers of dementia patients is associated with reduced lymphocyte sensitivity to glucocorticoids. J Neuroimmunol 103, 84–92. [DOI] [PubMed] [Google Scholar]
- Beery AK, Lin J, Biddle JS, Francis DD, Blackburn EH, Epel ES, 2012. Chronic stress elevates telomerase activity in rats. Biology letters 8, 1063–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellon M, Nicot C, 2017. Telomere Dynamics in Immune Senescence and Exhaustion Triggered by Chronic Viral Infection. Viruses 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, Labat C, Bean K, Aviv A, 2001. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension 37, 381–385. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, 2005. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS letters 579, 859–862. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Epel ES, Lin J, 2015. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 350, 1193–1198. [DOI] [PubMed] [Google Scholar]
- Boccardi V, Esposito A, Rizzo MR, Marfella R, Barbieri M, Paolisso G, 2013. Mediterranean diet, telomere maintenance and health status among elderly. PloS one 8, e62781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar AG, Kim NW, Effros RB, Chiu CP, 1996. Mechanism of telomerase induction during T cell activation. Experimental cell research 228, 58–64. [DOI] [PubMed] [Google Scholar]
- Bu X, Jia F, Wang W, Guo X, Wu M, Wei L, 2007. Coupled down-regulation of mTOR and telomerase activity during fluorouracil-induced apoptosis of hepatocarcinoma cells. BMC cancer 7, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchkovich KJ, Greider CW, 1996. Telomerase regulation during entry into the cell cycle in normal human T cells. Mol Biol Cell 7, 1443–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC, 2015. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta 1851, 469–484. [DOI] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA, 2003. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361, 393–395. [DOI] [PubMed] [Google Scholar]
- Chaplin DD, 2010. Overview of the immune response. J Allergy Clin Immunol 125, S3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Epel ES, Mellon SH, Lin J, Reus VI, Rosser R, Kupferman E, Burke H, Mahan L, Blackburn EH, Wolkowitz OM, 2014. Adverse childhood experiences and leukocyte telomere maintenance in depressed and healthy adults. J Affect Disord 169, 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, Kimura M, Lu X, Spector TD, Aviv A, 2008. The association between physical activity in leisure time and leukocyte telomere length. Archives of internal medicine 168, 154–158. [DOI] [PubMed] [Google Scholar]
- Chilton WL, Marques FZ, West J, Kannourakis G, Berzins SP, O’Brien BJ, Charchar FJ, 2014. Acute Exercise Leads to Regulation of Telomere-Associated Genes and MicroRNA Expression in Immune Cells. PloS one 9, e92088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Fauce SR, Effros RB, 2008. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav. Immun. 22, 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JP, Effros RB, 2013. T cell replicative senescence in human aging. Curr Pharm Des 19, 1680–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JP, Ramirez CM, Ryba DM, Koduri MP, Effros RB, 2014. Prostaglandin E2 promotes features of replicative senescence in chronically activated human CD8+ T cells. PloS one 9, e99432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE, 2007. Psychological stress and disease. JAMA 298, 1685–1687. [DOI] [PubMed] [Google Scholar]
- Coimbra BM, Carvalho CM, Moretti PN, Mello MF, Belangero SI, 2017. Stress-related telomere length in children: A systematic review. J Psychiatr Res 92, 47–54. [DOI] [PubMed] [Google Scholar]
- Conklin QA, King BG, Zanesco AP, Lin J, Hamidi AB, Pokorny JJ, Jesus Alvarez-Lopez M, Cosin-Tomas M, Huang C, Kaliman P, Epel ES, Saron CD, 2018. Insight Meditation and Telomere Biology: The Effects of Intensive Retreat and the Moderating Role of Personality. Brain, behavior, and immunity. [DOI] [PubMed] [Google Scholar]
- Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S, 1992. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J 11, 1921–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe DL, Nguyen DC, Tsang KJ, Kyo S, 2001. E2F-1 represses transcription of the human telomerase reverse transcriptase gene. Nucleic acids research 29, 2789–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello MJ, Ross SA, Briel M, Anand SS, Gerstein H, Pare G, 2015. Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circulation. Cardiovascular genetics 8, 82–90. [DOI] [PubMed] [Google Scholar]
- Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou Y, Beversdorf DQ, Weng NP, 2007. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. Journal of immunology 179, 4249–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier J, Lin J, Blackburn E, Hecht FM, Kristeller J, Maninger N, Kuwata M, Bacchetti P, Havel PJ, Epel E, 2012. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology 37, 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Punder K, Heim C, Przesdzing I, Wadhwa PD, Entringer S, 2018. Characterization in humans of in vitro leucocyte maximal telomerase activity capacity and association with stress. Philosophical Transactions of the Royal Society B: Biological Sciences 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeoglu B, de Weerd AE, Huang L, Langerak AW, Dor FJ, Klepper M, Verschoor W, Reijerkerk D, Baan CC, Litjens NH, Betjes MG, 2017. Lymph node and circulating T cell characteristics are strongly correlated in end-stage renal disease patients, but highly differentiated T cells reside within the circulation. Clin Exp Immunol 188, 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Xi P, Zhou J, Wang M, Cong YS, 2013. Human telomerase reverse transcriptase regulates MMP expression independently of telomerase activity via NF-kappaB-dependent transcription. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 27, 4375–4383. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Bosch JA, Steptoe A, Blackburn EH, Lin J, Rees-Clayton E, Aiello AE, 2013. Cytomegalovirus is associated with reduced telomerase activity in the Whitehall II cohort. Experimental gerontology 48, 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraimani S, Schneider RH, Randall OS, Nidich SI, Xu S, Ketete M, Rainforth MA, Gaylord-King C, Salerno JW, Fagan J, 2015. Effects of Lifestyle Modification on Telomerase Gene Expression in Hypertensive Patients: A Pilot Trial of Stress Reduction and Health Education Programs in African Americans. PloS one 10, e0142689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Alberti KG, Grundy SM, Zimmet PZ, 2010. The metabolic syndrome. Lancet 375, 181–183. [DOI] [PubMed] [Google Scholar]
- Effros RB, 2009. Kleemeier Award Lecture 2008--the canary in the coal mine: telomeres and human healthspan. The journals of gerontology. Series A, Biological sciences and medical sciences 64, 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, de Punder K, Buss C, Wadhwa PD, 2018. The fetal programming of telomere biology hypothesis: an update. Philosophical Transactions of the Royal Society B: Biological Sciences 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, 2009. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones 8, 7–22. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM, 2004. Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the United States of America 101, 17312–17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Dhabhar FS, Wolkowitz OM, Puterman E, Karan L, Blackburn EH, 2010. Dynamics of telomerase activity in response to acute psychological stress. Brain, behavior, and immunity 24, 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, Dolbier C, Mendes WB, Blackburn EH, 2006. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology 31, 277–287. [DOI] [PubMed] [Google Scholar]
- Epel ES, Puterman E, Lin J, Blackburn EH, Lum PY, Beckmann ND, Zhu J, Lee E, Gilbert A, Rissman RA, Tanzi RE, Schadt EE, 2016. Meditation and vacation effects have an impact on disease-associated molecular phenotypes. Transl Psychiatry 6, e880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajkus J, 2006. Detection of telomerase activity by the TRAP assay and its variants and alternatives. Clinica chimica acta; international journal of clinical chemistry 371, 25–31. [DOI] [PubMed] [Google Scholar]
- Forsyth NR, Wright WE, Shay JW, 2002. Telomerase and differentiation in multicellular organisms: turn it off, turn it on, and turn it off again. Differentiation; research in biological diversity 69, 188–197. [DOI] [PubMed] [Google Scholar]
- Fritsch RD, Shen X, Illei GG, Yarboro CH, Prussin C, Hathcock KS, Hodes RJ, Lipsky PE, 2006. Abnormal differentiation of memory T cells in systemic lupus erythematosus. Arthritis and rheumatism 54, 2184–2197. [DOI] [PubMed] [Google Scholar]
- Fujii H, Shao L, Colmegna I, Goronzy JJ, Weyand CM, 2009. Telomerase insufficiency in rheumatoid arthritis. Proceedings of the National Academy of Sciences of the United States of America 106, 4360–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C, 2017. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Frontiers in immunology 8, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaniga FS, Blackburn EH, 2014. An antiapoptotic role for telomerase RNA in human immune cells independent of telomere integrity or telomerase enzymatic activity. Blood 124, 3675–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgin-Lavialle S, Aouba A, Mouthon L, Londono-Vallejo JA, Lepelletier Y, Gabet AS, Hermine O, 2010. The telomere/telomerase system in autoimmune and systemic immune-mediated diseases. Autoimmunity reviews 9, 646–651. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Saginc G, Leow SC, Khattar E, Shin EM, Yan TD, Wong M, Zhang Z, Li G, Sung WK, Zhou J, Chng WJ, Li S, Liu E, Tergaonkar V, 2012. Telomerase directly regulates NF-kappaB-dependent transcription. Nature cell biology 14, 1270–1281. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, 2005. Stress-induced immune dysfunction: implications for health. Nature reviews. Immunology 5, 243–251. [DOI] [PubMed] [Google Scholar]
- Haendeler J, Hoffmann J, Diehl JF, Vasa M, Spyridopoulos I, Zeiher AM, Dimmeler S, 2004. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circulation research 94, 768–775. [DOI] [PubMed] [Google Scholar]
- Haendeler J, Hoffmann J, Rahman S, Zeiher AM, Dimmeler S, 2003. Regulation of telomerase activity and anti-apoptotic function by protein-protein interaction and phosphorylation. FEBS letters 536, 180–186. [DOI] [PubMed] [Google Scholar]
- Harrington L, Pucci F, 2018. In medio stat virtus: unanticipated consequences of telomere dysequilibrium. Philos Trans R Soc Lond B Biol Sci 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathcock KS, Jeffrey Chiang Y, Hodes RJ, 2005. In vivo regulation of telomerase activity and telomere length. Immunological reviews 205, 104–113. [DOI] [PubMed] [Google Scholar]
- Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P, 2014. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. Bmj 349, g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson SM, Franzese O, Macaulay R, Libri V, Azevedo RI, Kiani-Alikhan S, Plunkett FJ, Masters JE, Jackson S, Griffiths SJ, Pircher HP, Soares MV, Akbar AN, 2009. KLRG1 signaling induces defective Akt (seR473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood 113, 6619–6628. [DOI] [PubMed] [Google Scholar]
- Ho RT, Chan JS, Wang CW, Lau BW, So KF, Yuen LP, Sham JS, Chan CL, 2012. A randomized controlled trial of qigong exercise on fatigue symptoms, functioning, and telomerase activity in persons with chronic fatigue or chronic fatigue syndrome. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine 44, 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeyer K, Raggioli A, Rudloff S, Anton R, Hierholzer A, Del Valle I, Hein K, Vogt R, Kemler R, 2012. Wnt/beta-catenin signaling regulates telomerase in stem cells and cancer cells. Science 336, 1549–1554. [DOI] [PubMed] [Google Scholar]
- Huang EE, Tedone E, O’Hara R, Cornelius C, Lai TP, Ludlow A, Wright WE, Shay JW, 2017. The Maintenance of Telomere Length in CD28+ T Cells During T Lymphocyte Stimulation. Scientific reports 7, 6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelsson E, Larson MG, Yin X, Wang TJ, Meigs JB, Lipinska I, Benjamin EJ, Keaney JF Jr., Vasan RS, 2008. Circulating ghrelin, leptin, and soluble leptin receptor concentrations and cardiometabolic risk factors in a community-based sample. J Clin Endocrinol Metab 93, 3149–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invernizzi P, Bernuzzi F, Lleo A, Pozzoli V, Bignotto M, Zermiani P, Crosignani A, Battezzati PM, Zuin M, Podda M, Raggi C, 2014. Telomere dysfunction in peripheral blood mononuclear cells from patients with primary biliary cirrhosis. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 46, 363–368. [DOI] [PubMed] [Google Scholar]
- Jacobs EG, Epel ES, Lin J, Blackburn EH, Rasgon NL, 2014. Relationship between leukocyte telomere length, telomerase activity, and hippocampal volume in early aging. JAMA neurology 71, 921–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs TL, Epel ES, Lin J, Blackburn EH, Wolkowitz OM, Bridwell DA, Zanesco AP, Aichele SR, Sahdra BK, MacLean KA, King BG, Shaver PR, Rosenberg EL, Ferrer E, Wallace BA, Saron CD, 2011. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology 36, 664–681. [DOI] [PubMed] [Google Scholar]
- Jafri MA, Ansari SA, Alqahtani MH, Shay JW, 2016. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med 8, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jergovic M, Tomicevic M, Vidovic A, Bendelja K, Savic A, Vojvoda V, Rac D, Lovric-Cavar D, Rabatic S, Jovanovic T, Sabioncello A, 2014. Telomere shortening and immune activity in war veterans with posttraumatic stress disorder. Progress in neuro-psychopharmacology & biological psychiatry 54, 275–283. [DOI] [PubMed] [Google Scholar]
- Kamen DL, Tangpricha V, 2010. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. Journal of molecular medicine 88, 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlseder J, 2009. Chromosome end protection becomes even more complex. Nat Struct Mol Biol 16, 1205–1206. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Kohriyama K, 2001. Telomerase activity in peripheral blood mononuclear cells of systemic connective tissue diseases. The Journal of rheumatology 28, 288–291. [PubMed] [Google Scholar]
- Kawauchi K, Ihjima K, Yamada O, 2005. IL-2 increases human telomerase reverse transcriptase activity transcriptionally and posttranslationally through phosphatidylinositol 3’-kinase/Akt, heat shock protein 90, and mammalian target of rapamycin in transformed NK cells. Journal of immunology 174, 5261–5269. [DOI] [PubMed] [Google Scholar]
- Kim NW, Wu F, 1997. Advances in quantification and characterization of telomerase activity by the telomeric repeat amplification protocol (TRAP). Nucleic acids research 25, 2595–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Hjelmborg JV, Gardner JP, Bathum L, Brimacombe M, Lu X, Christiansen L, Vaupel JW, Aviv A, Christensen K, 2008. Telomere length and mortality: a study of leukocytes in elderly Danish twins. American journal of epidemiology 167, 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CH, Pletcher MJ, Lin J, Blackburn E, Adler N, Matthews K, Epel E, 2012. Telomerase, telomere length, and coronary artery calcium in black and white men in the CARDIA study. Atherosclerosis 220, 506–512. [DOI] [PubMed] [Google Scholar]
- Kurosaka D, Ozawa Y, Yasuda J, Yamada A, Akiyama M, Saito S, Tajima N, 2001. Telomerase activity in peripheral blood mononuclear cells from patients with SLE. Annals of the rheumatic diseases 60, 1158–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaka D, Yasuda J, Yoshida K, Yokoyama T, Ozawa Y, Obayashi Y, Kingetsu I, Saito S, Yamada A, 2003. Telomerase activity and telomere length of peripheral blood mononuclear cells in SLE patients. Lupus 12, 591–599. [DOI] [PubMed] [Google Scholar]
- Kurosaka D, Yasuda J, Yoshida K, Yoneda A, Yasuda C, Kingetsu I, Toyokawa Y, Yokoyama T, Saito S, Yamada A, 2006. Abnormal telomerase activity and telomere length in T and B cells from patients with systemic lupus erythematosus. The Journal of rheumatology 33, 1102–1107. [PubMed] [Google Scholar]
- Laish I, Mannasse-Green B, Hadary R, Biron-Shental T, Konikoff FM, Amiel A, Kitay-Cohen Y, 2016. Telomere Dysfunction in Nonalcoholic Fatty Liver Disease and Cryptogenic Cirrhosis. Cytogenet Genome Res 150, 93–99. [DOI] [PubMed] [Google Scholar]
- Lanna A, Coutavas E, Levati L, Seidel J, Rustin MH, Henson SM, Akbar AN, Franzese O, 2013. IFN-alpha inhibits telomerase in human CD8(+) T cells by both hTERT downregulation and induction of p38 MAPK signaling. Journal of immunology 191, 3744–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H, Epel ES, Siddarth P, Nazarian N, Cyr NS, Khalsa DS, Lin J, Blackburn E, Irwin MR, 2013. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. International journal of geriatric psychiatry 28, 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengacher CA, Reich RR, Kip KE, Barta M, Ramesar S, Paterson CL, Moscoso MS, Carranza I, Budhrani PH, Kim SJ, Park HY, Jacobsen PB, Schell MJ, Jim HS, Post-White J, Farias JR, Park JY, 2014. Influence of Mindfulness-Based Stress Reduction (MBSR) on Telomerase Activity in Women With Breast Cancer (BC). Biological research for nursing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Tong Y, Yang H, Zhou S, Xiong F, Huo T, Mao M, 2014. Mitochondrial translocation of human telomerase reverse transcriptase in cord blood mononuclear cells of newborns with gestational diabetes mellitus mothers. Diabetes research and clinical practice 103, 310–318. [DOI] [PubMed] [Google Scholar]
- Lichterfeld M, Mou D, Cung TD, Williams KL, Waring MT, Huang J, Pereyra F, Trocha A, Freeman GJ, Rosenberg ES, Walker BD, Yu XG, 2008. Telomerase activity of HIV-1-specific CD8+ T cells: constitutive up-regulation in controllers and selective increase by blockade of PD ligand 1 in progressors. Blood 112, 3679–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, Wolkowitz O, Mellon S, Blackburn E, 2010. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. Journal of immunological methods 352, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Damjanovic A, Metter EJ, Nguyen H, Truong T, Najarro K, Morris C, Longo DL, Zhan M, Ferrucci L, Hodes RJ, Weng NP, 2015. Age-associated telomere attrition of lymphocytes in vivo is co-ordinated with changes in telomerase activity, composition of lymphocyte subsets and health conditions. Clinical science 128, 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Epel ES, Mellon SH, Penninx BW, Revesz D, Verhoeven JE, Reus VI, Lin J, Mahan L, Hough CM, Rosser R, Bersani FS, Blackburn EH, Wolkowitz OM, 2015. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neuroscience and biobehavioral reviews 55, 333–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listerman I, Gazzaniga FS, Blackburn EH, 2014. An investigation of the effects of the core protein telomerase reverse transcriptase on Wnt signaling in breast cancer cells. Molecular and cellular biology 34, 280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Hodes RJ, Weng N, 2001. Cutting edge: telomerase activation in human T lymphocytes does not require increase in telomerase reverse transcriptase (hTERT) protein but is associated with hTERT phosphorylation and nuclear translocation. Journal of immunology 166, 4826–4830. [DOI] [PubMed] [Google Scholar]
- Lu M, Taylor BV, Korner H, 2018. Genomic Effects of the Vitamin D Receptor: Potentially the Link between Vitamin D, Immune Cells, and Multiple Sclerosis. Frontiers in immunology 9, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow AT, Robin JD, Sayed M, Litterst CM, Shelton DN, Shay JW, Wright WE, 2014. Quantitative telomerase enzyme activity determination using droplet digital PCR with single cell resolution. Nucleic acids research 42, e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, Von Zglinicki T, 2006. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol 60, 174–180. [DOI] [PubMed] [Google Scholar]
- Mathur MB, Epel E, Kind S, Desai M, Parks CG, Sandler DP, Khazeni N, 2016. Perceived stress and telomere length: A systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain, behavior, and immunity 54, 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Murphy ML, Cashman R, Ma R, Ma J, Arevalo JM, Kobor MS, Cole SW, 2014. Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain, behavior, and immunity 41, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin GB, 1989. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell 59, 521–529. [DOI] [PubMed] [Google Scholar]
- O’Sullivan RJ, Kubicek S, Schreiber SL, Karlseder J, 2010. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat Struct Mol Biol 17, 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Song Y, Yim J, Kim TK, 1999. The Wilms’ tumor 1 tumor suppressor gene represses transcription of the human telomerase reverse transcriptase gene. The Journal of biological chemistry 274, 37473–37478. [DOI] [PubMed] [Google Scholar]
- Oliveira BS, Zunzunegui MV, Quinlan J, Fahmi H, Tu MT, Guerra RO, 2016. Systematic review of the association between chronic social stress and telomere length: A life course perspective. Ageing research reviews 26, 37–52. [DOI] [PubMed] [Google Scholar]
- Ornish D, Lin J, Chan JM, Epel E, Kemp C, Weidner G, Marlin R, Frenda SJ, Magbanua MJ, Daubenmier J, Estay I, Hills NK, Chainani-Wu N, Carroll PR, Blackburn EH, 2013. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. The lancet oncology 14, 1112–1120. [DOI] [PubMed] [Google Scholar]
- Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, Kemp C, Magbanua MJ, Marlin R, Yglecias L, Carroll PR, Blackburn EH, 2008. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. The lancet oncology 9, 1048–1057. [DOI] [PubMed] [Google Scholar]
- Ozturk MB, Li Y, Tergaonkar V, 2017. Current Insights to Regulation and Role of Telomerase in Human Diseases. Antioxidants (Basel) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish ST, Wu JE, Effros RB, 2009. Modulation of T lymphocyte replicative senescence via TNF-{alpha} inhibition: role of caspase-3. Journal of immunology 182, 4237–4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Schaefer BC, 2013. A new look at T cell receptor signaling to nuclear factor-kappaB. Trends in immunology 34, 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelczyk T, Grancow-Grabka M, Trafalska E, Szemraj J, Zurner N, Pawelczyk A, 2018. Telomerase level increase is related to n-3 polyunsaturated fatty acid efficacy in first episode schizophrenia: Secondary outcome analysis of the OFFER randomized clinical trial. Progress in neuro-psychopharmacology & biological psychiatry 83, 142–148. [DOI] [PubMed] [Google Scholar]
- Plunkett FJ, Franzese O, Finney HM, Fletcher JM, Belaramani LL, Salmon M, Dokal I, Webster D, Lawson AD, Akbar AN, 2007. The loss of telomerase activity in highly differentiated CD8+CD28-CD27- T cells is associated with decreased Akt (SeR473) phosphorylation. Journal of immunology 178, 7710–7719. [DOI] [PubMed] [Google Scholar]
- Porton B, Delisi LE, Bertisch HC, Ji F, Gordon D, Li P, Benedict MM, Greenberg WM, Kao HT, 2008. Telomerase levels in schizophrenia: a preliminary study. Schizophrenia research 106, 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JD, Pollizzi KN, Heikamp EB, Horton MR, 2012. Regulation of immune responses by mTOR. Annual review of immunology 30, 39–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, Epel ES, Lin J, Blackburn EH, Gross JJ, Whooley MA, Cohen BE, 2013. Multisystem resiliency moderates the major depression-telomere length association: findings from the Heart and Soul Study. Brain, behavior, and immunity 33, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi A, Zhou H, Zhou Z, Huang X, Ma L, Wang H, Yang Y, Zhang D, Li H, Ren R, Yang R, 2013. Telomerase activity increased and telomere length shortened in peripheral blood cells from patients with immune thrombocytopenia. Journal of clinical immunology 33, 577–585. [DOI] [PubMed] [Google Scholar]
- Ramlee MK, Wang J, Toh WX, Li S, 2016. Transcription Regulation of the Human Telomerase Reverse Transcriptase (hTERT) Gene. Genes (Basel) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector JL, Dowd JB, Loerbroks A, Burns VE, Moss PA, Jarczok MN, Stalder T, Hoffman K, Fischer JE, and Bosch JA Consistent associations between measures of psychological stress and CMV antibody levels in a large occupational sample, Brain Behav Immun 38, 2014, 133–141. [DOI] [PubMed] [Google Scholar]
- Rentoukas E, Tsarouhas K, Kaplanis I, Korou E, Nikolaou M, Marathonitis G, Kokkinou S, Haliassos A, Mamalaki A, Kouretas D, Tsitsimpikou C, 2012. Connection between telomerase activity in PBMC and markers of inflammation and endothelial dysfunction in patients with metabolic syndrome. PloS one 7, e35739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Valdes AM, Gardner JP, Paximadas D, Kimura M, Nessa A, Lu X, Surdulescu GL, Swaminathan R, Spector TD, Aviv A, 2007. Higher serum vitamin D concentrations are associated with longer leukocyte telomere length in women. The American journal of clinical nutrition 86, 1420–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA, 2011. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470, 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JH, Meyer JN, Skorvaga M, Annab LA, Van Houten B, 2004. Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging cell 3, 399–411. [DOI] [PubMed] [Google Scholar]
- Saretzki G, 2009. Telomerase, mitochondria and oxidative stress. Experimental gerontology 44, 485–492. [DOI] [PubMed] [Google Scholar]
- Satra M, Dalekos GN, Kollia P, Vamvakopoulos N, Tsezou A, 2005. Telomerase reverse transcriptase mRNA expression in peripheral lymphocytes of patients with chronic HBV and HCV infections. J Viral Hepat 12, 488–493. [DOI] [PubMed] [Google Scholar]
- Schenkel JM, Zloza A, Li W, Narasipura SD, Al-Harthi L, 2010. Beta-catenin signaling mediates CD4 expression on mature CD8+ T cells. Journal of immunology 185, 2013–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM, 2015. The association between depression and leukocyte telomere length: a meta-analysis. Depression and anxiety 32, 229–238. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE, 2004. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 130, 601–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NM, Walton ZE, Bui E, Prescott J, Hoge E, Keshaviah A, Schwarz N, Dryman T, Ojserkis RA, Kovachy B, Mischoulon D, Worthington J, DeVivo I, Fava M, Wong KK, 2015. Telomere length and telomerase in a well-characterized sample of individuals with major depressive disorder compared to controls. Psychoneuroendocrinology 58, 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son NH, Murray S, Yanovski J, Hodes RJ, Weng N, 2000. Lineage-specific telomere shortening and unaltered capacity for telomerase expression in human T and B lymphocytes with age. Journal of immunology 165, 1191–1196. [DOI] [PubMed] [Google Scholar]
- Sundin T, Hentosh P, 2012. InTERTesting association between telomerase, mTOR and phytochemicals. Expert reviews in molecular medicine 14, e8. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Takubo K, Aida J, Araki A, Ito H, 2016. Telomere attrition and diabetes mellitus. Geriatr Gerontol Int 16 Suppl 1, 66–74. [DOI] [PubMed] [Google Scholar]
- Tarhan F, Vural F, Kosova B, Aksu K, Cogulu O, Keser G, Gunduz C, Tombuloglu M, Oder G, Karaca E, Doganavsargil E, 2008. Telomerase activity in connective tissue diseases: elevated in rheumatoid arthritis, but markedly decreased in systemic sclerosis. Rheumatology international 28, 579–583. [DOI] [PubMed] [Google Scholar]
- Thewissen M, Linsen L, Geusens P, Raus J, Stinissen P, 2005. Impaired activation-induced telomerase activity in PBMC of early but not chronic rheumatoid arthritis patients. Immunol Lett 100, 205–210. [DOI] [PubMed] [Google Scholar]
- Tolahunase M, Sagar R, Dada R, 2017. Impact of Yoga and Meditation on Cellular Aging in Apparently Healthy Individuals: A Prospective, Open-Label Single-Arm Exploratory Study. Oxid Med Cell Longev 2017, 7928981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolahunase MR, Sagar R, Faiq M, Dada R, 2018. Yoga- and meditation-based lifestyle intervention increases neuroplasticity and reduces severity of major depressive disorder: A randomized controlled trial. Restor Neurol Neurosci 36, 423–442. [DOI] [PubMed] [Google Scholar]
- Tremblay BL, Guenard F, Rudkowska I, Lemieux S, Couture P, Vohl MC, 2017. Epigenetic changes in blood leukocytes following an omega-3 fatty acid supplementation. Clin Epigenetics 9, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela HF, Effros RB, 2002. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol 105, 117–125. [DOI] [PubMed] [Google Scholar]
- Vazirpanah N, Kienhorst LBE, Van Lochem E, Wichers C, Rossato M, Shiels PG, Dalbeth N, Stamp LK, Merriman TR, Janssen M, Radstake T, Broen JC, 2017. Patients with gout have short telomeres compared with healthy participants: association of telomere length with flare frequency and cardiovascular disease in gout. Annals of the rheumatic diseases 76, 1313–1319. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, 2002. Oxidative stress shortens telomeres. Trends in biochemical sciences 27, 339–344. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Reus VI, Rosser R, Burke H, Compagnone M, Nelson JC, Dhabhar FS, Blackburn EH, 2012. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Mol Psychiatry 17, 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Higashi N, Hansen ER, Lund M, Bang K, Thestrup-Pedersen K, 2000. Telomerase activity is increased and telomere length shortened in T cells from blood of patients with atopic dermatitis and psoriasis. Journal of immunology 165, 4742–4747. [DOI] [PubMed] [Google Scholar]
- Wu K, Volke A, Lund M, Bang K, Thestrup-Pedersen K, 1999a. Telomerase activity is spontaneously increased in lymphocytes from patients with atopic dermatitis and correlates with cellular proliferation. Journal of dermatological science 22, 24–30. [DOI] [PubMed] [Google Scholar]
- Wu KJ, Grandori C, Amacker M, Simon-Vermot N, Polack A, Lingner J, Dalla-Favera R, 1999b. Direct activation of TERT transcription by c-MYC. Nature genetics 21, 220–224. [DOI] [PubMed] [Google Scholar]
- Xue HH, Zhao DM, 2012. Regulation of mature T cell responses by the Wnt signaling pathway. Annals of the New York Academy of Sciences 1247, 16–33. [DOI] [PubMed] [Google Scholar]
- Yin L, Hubbard AK, Giardina C, 2000. NF-kappa B regulates transcription of the mouse telomerase catalytic subunit. The Journal of biological chemistry 275, 36671–36675. [DOI] [PubMed] [Google Scholar]
- Yudoh K, Matsuno H, Nezuka T, Kimura T, 1999. Different mechanisms of synovial hyperplasia in rheumatoid arthritis and pigmented villonodular synovitis: the role of telomerase activity in synovial proliferation. Arthritis and rheumatism 42, 669–677. [DOI] [PubMed] [Google Scholar]
- Zalli A, Carvalho LA, Lin J, Hamer M, Erusalimsky JD, Blackburn EH, Steptoe A, 2014. Shorter telomeres with high telomerase activity are associated with raised allostatic load and impoverished psychosocial resources. Proceedings of the National Academy of Sciences of the United States of America 111, 4519–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wei MH, Lu R, Du GF, Zhou G, 2016. Declined hTERT expression of peripheral blood CD4(+) T cells in oral lichen planus correlated with clinical parameter. J Oral Pathol Med 45, 516–522. [DOI] [PubMed] [Google Scholar]
- Zhao YM, Zhou Q, Xu Y, Lai XY, Huang H, 2008. Antiproliferative effect of rapamycin on human T-cell leukemia cell line Jurkat by cell cycle arrest and telomerase inhibition. Acta pharmacologica Sinica 29, 481–488. [DOI] [PubMed] [Google Scholar]
- Zhou C, Gehrig PA, Whang YE, Boggess JF, 2003. Rapamycin inhibits telomerase activity by decreasing the hTERT mRNA level in endometrial cancer cells. Molecular cancer therapeutics 2, 789–795. [PubMed] [Google Scholar]
- Zhou J, Ding D, Wang M, Cong YS, 2014. Telomerase reverse transcriptase in the regulation of gene expression. BMB reports 47, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Guo D, Li K, Pedersen-White J, Stallmann-Jorgensen IS, Huang Y, Parikh S, Liu K, Dong Y, 2012. Increased telomerase activity and vitamin D supplementation in overweight African Americans. International journal of obesity 36, 805–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietzer A, Buschmann EE, Janke D, Li L, Brix M, Meyborg H, Stawowy P, Jungk C, Buschmann I, Hillmeister P, 2016. Acute physical exercise and long-term individual shear rate therapy increase telomerase activity in human peripheral blood mononuclear cells. Acta Physiol (Oxf). [DOI] [PubMed] [Google Scholar]
- Zuo QP, Liu SK, Li ZJ, Li B, Zhou YL, Guo R, Huang LH, 2011. NF-kappaB p65 modulates the telomerase reverse transcriptase in the HepG(2) hepatoma cell line. European journal of pharmacology 672, 113–120. [DOI] [PubMed] [Google Scholar]


