Abstract
Rationale:
Dietary monounsaturated fatty acids (MUFAs) can come from both plant and animal sources with divergent nutrient profiles that may potentially obscure the associations of total MUFAs with chronic diseases.
Objective:
To investigate the associations of cis-MUFA intake from plant (MUFA-P) and animal (MUFA-A) sources with total and cause-specific mortality.
Methods and Results:
We followed 63,412 women from the Nurses’ Health Study (1990–2012) and 29,966 men from the Health Professionals Follow-Up Study (1990–2012). MUFA-Ps and MUFA-As were calculated based on data collected through validated food frequency questionnaires administered every 4-years and updated food composition databases. During 1,896,864 person-years of follow-up, 20,672 deaths occurred. Total MUFAs and MUFA-Ps were inversely associated with total mortality after adjusting for potential confounders, whereas MUFA-As were associated with higher mortality. When MUFA-Ps were modeled to iso-calorically replace other macronutrients, hazard ratios [HRs, 95% confidence intervals (95%CIs)] of total mortality were 0.84 (0.77, 0.92;P<0.001) for replacing saturated fatty acids (SFAs; 5% of energy); 0.86 (0.82, 0.91;P<0.001) for replacing refined carbohydrates (5% energy); 0.91 (0.85, 0.97;P<0.001) for replacing trans fats (2% energy), and 0.77 (0.71, 0.82; P<0.001) for replacing MUFA-As (5% energy). For iso-calorically replacing MUFA-As with MUFA-Ps, HRs (95% CIs) were 0.74 (0.64, 0.86; P<0.001) for cardiovascular mortality; 0.73 (0.65, 0.82; P<0.001) for cancer mortality, and 0.82 (0.73, 0.91; P<0.001) for mortality due to other causes.
Conclusions:
Higher intake of MUFA-Ps was associated with lower total mortality, and MUFA-As intake was associated with higher mortality. Significantly lower mortality risk was observed when SFAs, refined carbohydrates, or trans fats were replaced by MUFA-Ps, but not MUFA-As. These data suggest that other constituents in animal foods, such as SFAs, may confound the associations for MUFAs when they are primarily derived from animal products. More evidence is needed to elucidate the differential associations of MUFA-Ps and MUFA-As with mortality.
Subject Terms: Cardiovascular Disease, Diabetes, Type 2, Diet and Nutrition, Epidemiology, Mortality/Survival
Keywords: Monounsaturated fatty acid, plant fat, animal fat, total mortality, cause-specific mortality, cardiovascular disease prevention, nutrition, epidemiology, diet
INTRODUCTION
According to the World Health Organization, of the 56.4 million deaths worldwide in 2015, more than half (54%) were due to 10 top causes.1 Ischemic heart disease, stroke and cancer remain leading causes of deaths in the U.S. and several other developed countries.1 Many premature deaths are preventable by adopting a healthy lifestyle, including smoking cessation, increasing physical activity, and improving diet quality.1
Recommendations by international organizations and the 2015 USDA Dietary Guidelines for Americans have emphasized the importance of the quality of dietary fat rather than the quantity of fat for the primary prevention of chronic diseases.2 Specifically, the intake of plant oils and other fats from plant sources is encouraged while the intake of animal fats, and particularly those from red and processed meat and butter, is discouraged. Of the fatty acids rich in plant-based food sources, polyunsaturated fatty acids (PUFAs) were consistently associated with lower risk of cardiovascular disease (CVD) and mortality across observational studies and clinical trial,3–6 but the impact of monounsaturated fatty acids (MUFAs) on chronic disease risk and especially mortality is less clear.7,8
Existing studies regarding MUFA intake and mortality risk have largely reported inconsistent findings.3,5,9 One possible reason is that dietary MUFAs come from both plant and animal sources with divergent dietary components that may potentially obscure the associations for MUFAs and health outcomes. In a recent analysis, we found that MUFAs from plant-based foods (MUFA-Ps) were associated with a lower risk of coronary heart disease (CHD), whereas the opposite was observed for MUFAs from animal products (MUFA-As), suggesting that food sources may play an important role in the relation between MUFAs and human health.8 To our knowledge, potentially divergent associations of long-term intake of MUFA-Ps and MUFA-As with total and cause-specific mortality have never been evaluated. Moreover, no large cohort studies have examined the associations with cause-specific mortality when other nutrients are replaced by MUFAs from different sources.
In two large prospective cohorts of U.S. men and women, we examined the hypothesis that the intake of MUFA-Ps is associated with lower total and cause-specific mortality whereas MUFA-A intake is not. In addition, we estimated the risk of total and cause-specific mortality when substituting MUFAs for SFAs, refined carbohydrates, and trans fats, based on the current dietary guidelines that recommend replacing these nutrients with healthier alternatives.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study design and population.
The Nurses’ Health Study (NHS) is a prospective cohort study of 121,700 female registered nurses aged 30–55 years at enrollment in 1976. The Health Professionals Follow-up Study (HPFS) is a prospective cohort study of 51,529 male health professionals aged 40–75 years at enrollment in 1986. In both cohorts, information about medical history, lifestyle, and health conditions has been collected by self-administered biennial questionnaires since baseline. Detailed information on the cohorts has been described in previous publications.10–12
For this analysis, we used 1990 as study baseline when olive oil consumption was first asked as part of a validated food frequency questionnaire (FFQ) administered in the cohorts. At baseline, 80,332 women and 38,842 men completed the FFQ. Participants were excluded if: they reported physician-diagnosed cancer, diabetes, or CVD at study baseline; reported implausible energy intake (<600 or >3,500 kcal/day in NHS, and <800 or >4,200 kcal/day in HPFS); had missing values for age; or they answered the baseline questionnaire only. The final analyses included 63,412 women and 29,966 men. The institutional review boards of Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health approved the study protocol. The return of a completed questionnaire was considered as informed consent.
Dietary assessment.
Dietary intake was measured using the FFQ with >130 items administered every 4 years to assess and update habitual diet. The questionnaire inquires how often, on average, participants had consumed specific foods, as well as the types of fats, oils, and brand or type of margarines used for cooking and added at the table in the preceding year. Nutrient intakes were calculated based on the U.S. Department of Agriculture and Harvard University Food Composition Database, which is updated over time to reflect potential changes in the nutrient profile of food items and to incorporate new items13. We periodically analyzed the fatty acid composition of commonly consumed foods using gas chromatography during the follow-up period to account for changes in food processing. The nutrient database separated trans fats with one double bond from cis MUFAs, which are the main exposures of the present study. MUFA-As were the sum of MUFAs from animal foods, such as animal fats for cooking, dairy products, eggs, poultry, processed and unprocessed red meats, and fish; MUFA-Ps were calculated based on plant-based foods, such as plant-based cooking oils, salad dressing, margarines, bread and cereals, fruits, vegetables, legumes, nuts and seeds. For mixed food items, ingredients were identified according to manufacturer product labels or cookbooks for home-prepared items. We derived refined carbohydrates as the sum of added sugar and carbohydrates from refined grains (such as pasta, bread, white rice, pizza, and English muffins, among others).
The cumulative average of food intake from all available FFQs was calculated to better represent long-term diet and to minimize within-person variation.14 To minimize the possibility of reverse causation bias, we stopped updating diet information after participants reported a diagnosis of stroke, heart disease, angina, diabetes, or cancer. We replaced missing values of MUFAs during follow-up with valid cumulative averages of prior assessments. Intakes of different dietary fats estimated by FFQs were validated at baseline and during follow-up.12,15,16 In the most recent validation study of the NHS, de-attenuated Spearman rank correlation coefficients (rs) of energy-adjusted nutrient data from FFQ and multiple 7-day dietary records were between 0.58 and 0.65 (both P<0.001) for total MUFAs and oleic acid, respectively.17
Ascertainment of deaths.
Deaths were identified through search of the vital records of states and of the National Death Index. This search was supplemented by reports from next of kin and postal authorities. Using these methods, we were able to ascertain >98% of the deaths in the cohorts.18 A physician who was blinded to data on food consumption data and other risk factors reviewed death certificates, medical records, or autopsy reports to classify the cause of deaths according to the eighth and ninth revisions of the International Classification of Diseases. Deaths were grouped into 5 major groups (CVD, cancer, respiratory disease, neurodegenerative disease and all other causes, including suicide, injury, infections, diabetes, kidney disease, and etc.) (Online Table I).
Statistical analysis.
Because the consumption of MUFA-Ps and MUFA-As changed during follow-up,8 we presented participants’ characteristics according to MUFA quintiles and the correlations among dietary fats at the mid-point of follow-up in 2002. Macronutrients were analyzed as percentages of energy by dividing the energy from specific macronutrients by total energy intake. We calculated each individual’s person-time from the return date of baseline questionnaire to the date of death, or the end of follow-up (June 2012 in NHS and January 2012 in HPFS), whichever came first. We used Cox proportional hazards regression models to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) of total and cause-specific mortality in each cohort with follow-up duration as the timescale. Multivariate models were stratified jointly by age in months and calendar year to better control for confounding by age, calendar time, and any possible two-way interactions between them. Multivariate models were adjusted for covariates that were updated in follow-up questionnaires. The following covariates were considered: ethnicity, smoking status, alcohol intake, family history of myocardial infarction, family history of diabetes, family history of cancer, menopausal status and post-menopausal hormone use (NHS only), physical activity, current aspirin use, multivitamin use, baseline hypertension, baseline hypercholesterolemia, body mass index (BMI), calorie intake, energy from trans fats, energy from SFAs, fruit and vegetables, and coffee intake (in quintiles). When modeling MUFA-Ps, we further included MUFA-As as a covariate, and vice versa. We calculated P values for trend with the use of the Wald test of a score variable based on the median of MUFAs in each category as a continuous variable. The proportional hazards assumption was tested by fitting a model that included interaction terms between MUFAs and duration of follow-up and by using a likelihood ratio test to examine the significance of the interaction terms. The assumption was unlikely to be violated (P > 0.05 for all tests).
We estimated the risk of total and cause-specific mortality when energy from SFAs was replaced by MUFA-Ps in an isocaloric energy density model that included total energy, energy from carbohydrates, energy from protein and energy from other fats (PUFAs, trans fats and MUFA-As). By leaving SFAs out of the model, regression coefficients for MUFA-Ps can be interpreted as the estimated effect of iso-calorically substituting MUFA-Ps for SFAs while holding other fats and total energy constant. Similar isocaloric substitution analyses were conducted for MUFA-As and for substituting MUFA fractions for trans fats and refined carbohydrates. In light of strong correlations of SFAs and MUFA-As and of PUFAs and MUFA-Ps, we further conducted substitution analyses replacing SFAs+MUFA-As with PUFAs+MUFA-Ps.
We performed sensitivity analyses to examine the robustness of findings by: 1) continuing updating diet after the diagnosis of intermediate outcomes; and 2) further adjusting for modified Alternate Healthy Eating Index (AHEI), to explore whether findings may be explained by underlying dietary pattern. Analyses were conducted in the two cohorts separately, and then results were pooled with the use of an inverse variance–weighted meta-analysis using a fixed-effect model. Analyses were performed with the SAS statistical package (version 9.4, SAS Institute). Statistical tests were 2 sided, and P values of <0.05 were considered to indicate statistical significance.
RESULTS
During 22 years of follow-up, we documented 20,672 deaths (12,774 in NHS and 7,898 in HPFS) in 1,896,864 person-years. Participants’ characteristics according to MUFA-P and MUFA-A quintiles at the midpoint of follow-up (2002) are shown in Table 1. Compared with participants with lower MUFA-P intake, those in the highest quintile were younger, less likely to have hypertension, more likely to take aspirin and multivitamins, and had a higher AHEI score. Participants with higher MUFA-A intake, were younger, more likely to smoke, and less likely to exercise. They also had higher BMI, a lower intake of fruits and vegetables, and a lower AHEI score (Table 1).
Table 1.
Age-standardized characteristics according to MUFA intake at the mid-point of follow-up (2002)
| NHS | HPFS | |||||
|---|---|---|---|---|---|---|
| Variable* | MUFA-P | |||||
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| 12007 | 12008 | 12007 | 5497 | 5497 | 5497 | |
| Total MUFAs,% energy | 9.4±2.0 | 11.6±1.6 | 14.4±2.1 | 9.8±2.3 | 12.0±1.8 | 14.4±2.1 |
| MUFA-Ps,% energy | 3.7±0.6 | 5.9±0.3 | 9.2±1.7 | 4.0±0.7 | 6.2±0.3 | 9.2±1.5 |
| MUFA-As,% energy | 5.4±1.8 | 5.5±1.5 | 4.9±1.5 | 5.6±2.1 | 5.6±1.8 | 5.0±1.7 |
| Age, years | 68.9±7.1 | 67.4±7.1 | 67.0±6.9 | 68.4±9.0 | 66.9±8.9 | 67.7±8.7 |
| Caucasian, % | 97 | 98 | 98 | 94 | 96 | 97 |
| Alcohol intake, g/day | 5.7±11.5 | 5.6±10.1 | 7.2±11.2 | 13.3±17.5 | 12.5±15.6 | 12.4±16.0 |
| Current smoking, % | 8 | 8 | 8 | 4 | 4 | 4 |
| Physical activity, METs/week | 18.4±24.4 | 16.7±20.9 | 18.1±21.4 | 35.7±40.9 | 34.9±41.7 | 35.5±40.1 |
| BMI, kg/m2 | 26.7±5.4 | 26.6±5.3 | 26.1±5.1 | 23.4±8.8 | 24.2±7.9 | 23.9±7.9 |
| Hypertension at midpoint (2002) | 55 | 53 | 49 | 48 | 45 | 42 |
| Hypercholesterolemia at midpoint (2002) | 63 | 63 | 63 | 53 | 55 | 53 |
| Family history of myocardial infarction, % | 38 | 38 | 37 | 33 | 31 | 32 |
| Family history of diabetes, % | 25 | 25 | 24 | 21 | 21 | 20 |
| Family history of cancer, % | 44 | 46 | 45 | 27 | 27 | 28 |
| Multivitamin use,% | 60 | 62 | 62 | 55 | 58 | 57 |
| Current use of Aspirin, % | 32 | 34 | 34 | 45 | 48 | 47 |
| Any use of postmenopausal hormone, % | 67 | 71 | 72 | |||
| Total energy, kcal | 1614±423 | 1757±441 | 1811±470 | 1843±506 | 2010±534 | 2095±572 |
| Total fats,% energy | 24.3±4.9 | 28.9±4.2 | 32.9±4.8 | 24.8±5.6 | 29.5±4.6 | 33.3±4.9 |
| PUFAs,% energy | 4.6±0.8 | 5.7±0.9 | 6.6±1.4 | 4.7±0.9 | 5.8±0.9 | 6.9±1.4 |
| n-3 PUFAs,% energy | 0.6±0.2 | 0.6±0.1 | 0.7±0.2 | 0.6±0.2 | 0.7±0.2 | 0.7±0.3 |
| n-6 PUFAs,% energy | 3.9±0.7 | 5.0±0.8 | 5.8±1.3 | 4.1±1.0 | 5.2±1.0 | 6.2±1.6 |
| SFAs,% energy | 9.1±2.5 | 10.1±2.1 | 10.3±2.3 | 9.0±2.7 | 10.0±2.3 | 10.3±2.3 |
| Trans fats,% energy | 1.2±0.3 | 1.6±0.4 | 1.7±0.6 | 1.2±0.4 | 1.6±0.5 | 1.8±0.7 |
| Proteins,% energy | 19.4±3.0 | 17.9±2.4 | 16.8±2.4 | 19.1±3.1 | 17.7±2.4 | 16.5±2.4 |
| Fruit and vegetables, serving/day | 5.9±2.4 | 5.5±2.2 | 5.6±2.3 | 6.3±2.9 | 5.9±2.5 | 5.8±2.6 |
| Coffee intake, servings/day | 2.0±1.4 | 2.2±1.4 | 2.2±1.5 | 1.8±1.6 | 1.9±1.5 | 1.9±1.6 |
| Modified AHEI† | 31.6±7.1 | 31.3±7.0 | 34.6±7.5 | 31.6±8.3 | 30.9±7.7 | 34.0±8.2 |
| MUFA-A | ||||||
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| N | 12007 | 12007 | 12007 | 5497 | 5497 | 5497 |
| Total MUFAs,% energy | 9.9±2.5 | 11.6±1.9 | 13.7±1.9 | 9.8±2.5 | 12.0±1.8 | 14.3±1.8 |
| MUFA-Ps,% energy | 6.5±2.5 | 6.2±1.9 | 5.8±1.8 | 6.7±2.4 | 6.5±1.8 | 6.0±1.6 |
| MUFA-As,% energy | 3.2±0.7 | 5.3±0.2 | 7.6±1.0 | 3.0±0.8 | 5.4±0.3 | 8.2±1.1 |
| Age, years | 69.4±7.1 | 67.5±7.1 | 66.4±6.9 | 68.1±9.0 | 67.4±9.0 | 67.3±8.8 |
| Caucasian, % | 97 | 98 | 98 | 95 | 96 | 96 |
| Alcohol intake, g/day | 6.2±10.8 | 6.3±10.8 | 5.4±10.2 | 12.2±16.2 | 13.4±16.8 | 11.2±14.7 |
| Current smoking, % | 4 | 7 | 13 | 1 | 4 | 6 |
| Physical activity, METs/week | 23.3±26.0 | 17.1±22.2 | 13.3±18.4 | 42.7±45.4 | 33.9±38.7 | 30.7±41.5 |
| BMI, kg/m2 | 25.0±4.5 | 26.6±5.0 | 27.8±5.9 | 22.9±7.2 | 24.4±7.6 | 24.2±9.3 |
| Hypertension at midpoint (2002) | 48 | 53 | 56 | 41 | 44 | 47 |
| Hypercholesterolemia at midpoint (2002) | 67 | 64 | 58 | 57 | 54 | 48 |
| Family history of myocardial infarction, % | 38 | 38 | 36 | 35 | 31 | 28 |
| Family history of diabetes, % | 24 | 24 | 26 | 20 | 21 | 21 |
| Family history of cancer, % | 45 | 46 | 45 | 27 | 28 | 28 |
| Multivitamin use,% | 68 | 63 | 53 | 63 | 58 | 49 |
| Current use of Aspirin, % | 35 | 35 | 31 | 51 | 48 | 43 |
| Any use of postmenopausal hormone, % | 72 | 71 | 65 | |||
| Total energy, kcal | 1714±444 | 1754±445 | 1729±476 | 1923±526 | 1997±540 | 2057±590 |
| Total fats,% energy | 23.7±4.6 | 28.7±3.8 | 33.9±4.2 | 23.6±5.0 | 29.3±3.8 | 35.0±4.1 |
| PUFAs,% energy | 5.4±1.3 | 5.7±1.2 | 5.8±1.2 | 5.6±1.4 | 5.8±1.1 | 6.0±1.2 |
| n-3 PUFAs,% energy | 0.6±0.2 | 0.6±0.2 | 0.6±0.2 | 0.7±0.2 | 0.7±0.2 | 0.6±0.2 |
| n-6 PUFAs,% energy | 4.7±1.2 | 5.0±1.1 | 5.1±1.1 | 5.0±1.5 | 5.2±1.3 | 5.2±1.3 |
| SFAs,% energy | 7.3±1.4 | 9.8±1.2 | 12.6±1.8 | 7.0±1.5 | 9.9±1.3 | 12.8±1.8 |
| Trans fats,% energy | 1.2±0.4 | 1.5±0.4 | 1.8±0.5 | 1.2±0.5 | 1.6±0.5 | 1.9±0.5 |
| Proteins,% energy | 17.2±2.7 | 18.0±2.6 | 18.9±2.7 | 16.9±2.7 | 17.6±2.6 | 18.8±2.7 |
| Fruit and vegetables, serving/day | 6.7±2.6 | 5.6±2.1 | 4.7±2.0 | 7.3±3.0 | 5.8±2.4 | 4.9±2.1 |
| Coffee intake, serving/day | 1.9±1.4 | 2.2±1.4 | 2.3±1.5 | 1.6±1.4 | 1.9±1.5 | 2.2±1.7 |
| Modified AHEI† | 36.7±7.0 | 31.9±6.5 | 28.7±6.7 | 38.3±7.4 | 31.0±7.0 | 26.8±6.7 |
Abbreviations: NHS, Nurses’ Health Study; HPFS, Health Professional’s Follow-up Study; MUFA, monounsaturated fat; MUFA-P, MUFAs from plant sources; MUFA-A, MUFAs from animal sources; MET, metabolic equivalent task; BMI, body mass index; AHEI, alternative health eating index; SFA, saturated fatty acids; PUFA, polyunsaturated fatty acids.
Values are means (SD) or percentages and are standardized to the age distribution of the study population.
Modified by excluding fat components.
Major MUFA-P sources included olive oil, nuts, salad dressing, fried foods, baked products (chocolate chip cookies and homemade/ready-made pie), margarine, milk chocolate, and avocado.8 MUFA-As came mainly from red (beef and pork) and processed meats (41–42%), dairy products, butter, poultry, eggs, and fish. In both cohorts, mean percentage of energy from MUFA-Ps increased from 5.8–6.3% to 7.9% during the follow-up, whereas MUFA-As decreased from 5.4–5.5% to 4.2–4.4%8.
Intakes of MUFA-Ps and MUFA-As were weakly, inversely correlated (r =−0.07 for NHS and −0.10 HPFS). MUFA-Ps were positively correlated with total PUFAs and n-6 PUFAs (r≥0.59, P<0.001). MUFA-As were weakly correlated with total PUFAs and n-6 PUFAs (r≤0.16, P<0.001). SFA intake was strongly correlated with MUFA-As (r≥0.82, P<0.001) (Online Table II).
Age-adjusted and multivariate-adjusted analyses showed a consistent, significant, inverse association between MUFA-Ps and total mortality, and a positive association between MUFA-As and total mortality (Table 2). The pooled multivariate-adjusted HRs (95% CIs) for participants in the highest quintile of MUFA-Ps and MUFA-As, as compared with those in the lowest quintile, were: 0.84 [0.80, 0.89; Ptrend < 0.001] and 1.16 [1.08, 1.24; Ptrend < 0.001], respectively (Table 2). In the model without SFAs, total MUFAs were not associated with total mortality. After adjustment for SFAs, which were highly correlated with MUFA-As, the HR (95%CI) of total mortality comparing extreme quintiles of total MUFAs was significant at 0.84 (0.79, 0.89; Ptrend<0.001).
Table 2.
Hazard Ratios (95% CIs) of total and cause-specific mortality according to MUFA intake in NHS and HPFS pooled
| Quintiles of MUFA intake (% energy) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P for trend | |||
| Total mortality | ||||||||
| Cases/person-years | 4574/379071 | 4037/379760 | 3939/379654 | 4030/379389 | 4092/378630 | |||
| Total MUFAs | Model 1 | 1 | 0.99 (0.95, 1.03) | 1.06 (1.01, 1.10) | 1.13 (1.08, 1.18) | 1.21 (1.16, 1.27) | <0.001 | |
| Model 2 | 1 | 0.99 (0.95, 1.04) | 1.01 (0.97, 1.06) | 1.04 (0.99, 1.09) | 1.00 (0.95, 1.06) | 0.50 | ||
| Model 3 | 1 | 0.93 (0.89, 0.98) | 0.92 (0.88, 0.97) | 0.91 (0.87, 0.96) | 0.84 (0.79, 0.89) | <0.001 | ||
| Cases/person-years | 5327/377503 | 4334/379286 | 3808/379871 | 3752/379897 | 3451/380306 | |||
| MUFA-Ps | Model 1 | 1 | 0.89 (0.85, 0.92) | 0.83 (0.79, 0.86) | 0.83 (0.79, 0.86) | 0.77 (0.73, 0.80) | <0.001 | |
| Model 2 | 1 | 0.94 (0.90, 0.98) | 0.88 (0.84, 0.92) | 0.86 (0.82, 0.90) | 0.79 (0.76, 0.83) | <0.001 | ||
| Model 3 | 1 | 0.95 (0.91, 1.00) | 0.90 (0.86, 0.95) | 0.90 (0.86, 0.94) | 0.84 (0.80, 0.89) | <0.001 | ||
| Cases/person-years | 3873/379974 | 3812/379939 | 3940/379823 | 4139/379262 | 4908/377885 | |||
| MUFA-As | Model 1 | 1 | 1.10 (1.05, 1.15) | 1.20 (1.15, 1.26) | 1.35 (1.29, 1.41) | 1.67 (1.60, 1.75) | <0.001 | |
| Model 2 | 1 | 1.10 (1.05, 1.15) | 1.15 (1.10, 1.21) | 1.21 (1.16, 1.27) | 1.32 (1.26, 1.39) | <0.001 | ||
| Model 3 | 1 | 1.05 (1.00, 1.11) | 1.08 (1.03, 1.14) | 1.11 (1.05, 1.18) | 1.16 (1.08, 1.24) | <0.001 | ||
| Cardiovascular mortality | ||||||||
| Cases/person-years | 1033/379071 | 885/379760 | 887/379654 | 889/379389 | 894/378630 | |||
| Total MUFAs | Model 1 | 1 | 0.97 (0.88, 1.06) | 1.09 (0.99, 1.19) | 1.10 (1.01, 1.21) | 1.22 (1.12, 1.34) | <0.001 | |
| Model 2 | 1 | 1.01 (0.92, 1.11) | 1.12 (1.01, 1.24) | 1.15 (1.04, 1.28) | 1.16 (1.04, 1.30) | <0.01 | ||
| Model 3 | 1 | 0.94 (0.85, 1.04) | 1.01 (0.91, 1.12) | 1.00 (0.89, 1.13) | 0.96 (0.84, 1.09) | 0.79 | ||
| Cases/person-years | 1129/377503 | 955/379286 | 831/379823 | 780/379897 | 793/380306 | |||
| MUFA-Ps | Model 1 | 1 | 0.87 (0.79, 0.94) | 0.82 (0.75, 0.90) | 0.77 (0.70, 0.84) | 0.79 (0.72, 0.87) | <0.001 | |
| Model 2 | 1 | 0.94 (0.86, 1.02) | 0.91 (0.83, 1.00) | 0.85 (0.77, 0.94) | 0.89 (0.80, 0.99) | <0.01 | ||
| Model 3 | 1 | 0.96 (0.88, 1.05) | 0.94 (0.85, 1.04) | 0.89 (0.81, 0.99) | 0.96 (0.86, 1.07) | 0.31 | ||
| Cases/person-years | 886/379974 | 831/379286 | 877/379823 | 930/379262 | 1064/377885 | |||
| MUFA-As | Model 1 | 1 | 1.07 (0.97, 1.18) | 1.19 (1.08, 1.31) | 1.37 (1.24, 1.50) | 1.62 (1.48, 1.78) | <0.001 | |
| Model 2 | 1 | 1.04 (0.94, 1.16) | 1.11 (0.99, 1.24) | 1.18 (1.05, 1.33) | 1.20 (1.04, 1.38) | <0.01 | ||
| Model 3 | 1 | 1.03 (0.93, 1.14) | 1.09 (0.97, 1.22) | 1.15 (1.01, 1.30) | 1.16 (1.00, 1.35) | 0.03 | ||
| Cancer mortality | ||||||||
| Cases/person-years | 1471/380877 | 1410/381180 | 1376/381046 | 1499/380710 | 1547/380248 | |||
| Total MUFAs | Model 1 | 1 | 1.04 (0.96, 1.12) | 1.08 (1.00, 1.16) | 1.21 (1.13, 1.31) | 1.29 (1.20, 1.39) | <0.001 | |
| Model 2 | 1 | 1.06 (0.98, 1.14) | 1.06 (0.98, 1.15) | 1.16 (1.07, 1.26) | 1.13 (1.04, 1.23) | <0.01 | ||
| Model 3 | 1 | 1.01 (0.94, 1.10) | 0.99 (0.91, 1.08) | 1.05 (0.96, 1.15) | 0.99 (0.90, 1.10) | 0.91 | ||
| Cases/person-years | 1735/3379452 | 1465/380886 | 1392/381219 | 1370/381159 | 1370/381342 | |||
| MUFA-Ps | Model 1 | 1 | 0.90 (0.84, 0.97) | 0.89 (0.83, 0.95) | 0.89 (0.82, 0.95) | 0.86 (0.80, 0.92) | <0.001 | |
| Model 2 | 1 | 0.95 (0.88, 1.02) | 0.94 (0.87, 1.02) | 0.93 (0.86, 1.00) | 0.89 (0.82, 0.96) | <0.01 | ||
| Model 3 | 1 | 0.97 (0.90, 1.04) | 0.98 (0.91, 1.06) | 0.98 (0.90, 1.06) | 0.96 (0.88, 1.05) | 0.43 | ||
| Cases/person-years | 1326/381444 | 1340/381296 | 1358/381176 | 1490/380643 | 1784/379500 | |||
| MUFA-As | Model 1 | 1 | 1.09 (1.01, 1.17) | 1.15 (1.07, 1.24) | 1.32 (1.23, 1.43) | 1.63 (1.51, 1.75) | <0.001 | |
| Model 2 | 1 | 1.09 (1.00, 1.18) | 1.11 (1.02, 1.21) | 1.21 (1.10, 1.33) | 1.32 (1.18, 1.47) | <0.001 | ||
| Model 3 | 1 | 1.08 (0.99, 1.17) | 1.10 (1.00, 1.20) | 1.20 (1.09, 1.32) | 1.29 (1.15, 1.45) | <0.001 | ||
| Non-cardiovascular and non-cancer mortality | ||||||||
| Cases/person-years | 2073/381379 | 1741/381855 | 1673/381802 | 1640/381578 | 1647/381297 | |||
| Total MUFAs | Model 1 | 1 | 0.97 (0.91, 1.03) | 1.03 (0.96, 1.10) | 1.06 (0.99, 1.13) | 1.14 (1.07, 1.22) | <0.001 | |
| Model 2 | 1 | 0.95 (0.89, 1.02) | 0.94 (0.88, 1.01) | 0.91 (0.85, 0.98) | 0.86 (0.79, 0.93) | <0.001 | ||
| Model 3 | 1 | 0.88 (0.83, 0.95) | 0.84 (0.78, 0.90) | 0.78 (0.72, 0.85) | 0.70 (0.63, 0.77) | <0.001 | ||
| Case/person-years | 2360/380294 | 1913/381513 | 1583/381840 | 1599/381930 | 1319/382254 | |||
| MUFA-Ps | Model 1 | 1 | 0.89 (0.84, 0.95) | 0.80 (0.75, 0.85) | 0.82 (0.77, 0.88) | 0.69 (0.65, 0.74) | <0.001 | |
| Model 2 | 1 | 0.95 (0.89, 1.01) | 0.84 (0.79, 0.90) | 0.84 (0.79, 0.91) | 0.71 (0.66, 0.76) | <0.001 | ||
| Model 3 | 1 | 0.95 (0.89, 1.01) | 0.84 (0.78, 0.90) | 0.84 (0.78, 0.91) | 0.71 (0.65, 0.77) | <0.001 | ||
| Case/person-years | 1660/381966 | 1640/381940 | 1404/381887 | 1713/381561 | 2057/380556 | |||
| MUFA-As | Model 1 | 1 | 1.12 (1.04, 1.20) | 1.25 (1.16, 1.33) | 1.34 (1.25, 1.43) | 1.70 (1.59, 1.81) | <0.001 | |
| Model 2 | 1 | 1.09 (1.01, 1.17) | 1.14 (1.06, 1.24) | 1.13 (1.04, 1.23) | 1.24 (1.12, 1.37) | <0.001 | ||
| Model 3 | 1 | 1.05 (1.02, 1.07) | 1.03 (0.96, 1.11) | 1.05 (0.97, 1.14) | 1.01 (0.92, 1.10) | 0.05 | ||
Hazard ratios and 95% confidence intervals were calculated in Cox proportional hazards models; NHS, Nurses’ Health Study; HPFS, Health Professional’s Follow-up Study; MUFA, monounsaturated fat; MUFA-P, MUFAs from plant sources; MUFA-A, MUFAs from animal sources.
Model 1, adjusted for age; Model 2, further adjusted for ethnicity (Caucasian, and other ethnicity), smoking status (never, former, current (1–14, 15–24, or ≥25 cigarettes/day), alcohol intake (gram/day: 0, 0.1–4.9, 5.0–14.9, and >15.0 in women, 0, 0.1–4.9, 5.0–29.9, and >30.0 in men), family history of myocardial infarction (yes/no), family history of diabetes (yes/no), family history of cancer (yes/no), menopausal status and post-menopausal hormone use (pre-menopause, post-menopause (never, former, or current hormone use), for women), physical activity (<3, 3.0–8.9, 9.0–17.9, 18.0–26.9, ≥27.0 METs/week), current aspirin use (yes/no), multivitamin use (yes/no), baseline hypertension, baseline hypercholesterolemia, BMI (<23, 23–24.9, 25–29.9, 30–34.9, >35kg/m2), intakes of total energy, energy from trans fats, fruits and vegetables, and coffee intake (in quintiles). Model 3, further adjusted for percentage of energy from SFAs. MUFA-P models were further adjusted for MUFA-A, and vice versa. Study estimates from two cohorts were pooled using a fixed effects model.
MUFA-Ps were associated with significantly lower cardiovascular and cancer mortality after multivariate adjustments of covariates, although these associations were attenuated to non-significant when further adjusting for intake of MUFA-As and SFAs. In contrast, MUFA-As were associated with 16% and 29% higher risk of cardiovascular and cancer mortality, respectively, after adjustment for covariates and MUFA-Ps (Table 2). MUFA-Ps were inversely associated with mortality due to other causes while MUFA-As were not associated to these deaths after mutual adjustments. Cohort-specific HRs and 95% CIs of total and cause-specific mortality according to MUFA intake are presented in Online Table III. Furthermore, we observed inverse associations between MUFA-Ps and neurodegenerative and respiratory deaths (Online Table IV). After adjusting for potential confounders including MUFA-As, the HRs (95% CIs) comparing extreme quintiles of MUFA-Ps were 0.75 (0.63, 0.89, Ptrend <0.001) for neurodegenerative disease mortality and 0.65 (0.54, 0.78, Ptrend <0.001) for respiratory disease mortality.
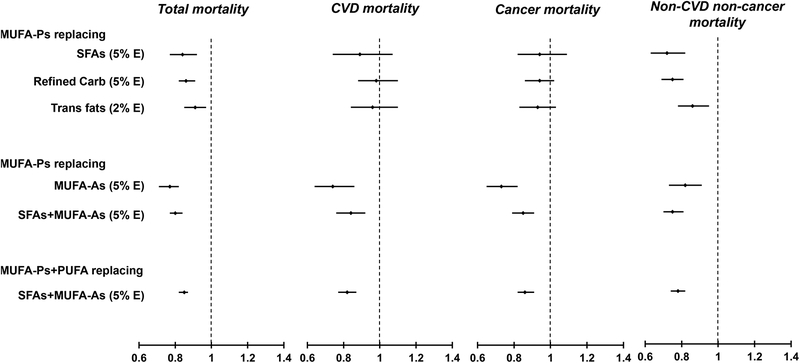
Figure 1 shows the pooled substitution analyses for total, CVD, cancer and non-CVD and non-cancer deaths. For MUFA-Ps, pooled HRs (95% CIs) of total mortality were 0.84 (0.77, 0.92; Ptrend <0.001) when replacing 5% energy of SFAs; 0.86 (0.82, 0.91; Ptrend <0.01) when replacing 5% energy of refined carbohydrates; and 0.91 (0.85, 0.97; Ptrend =0.003) when replacing 2% energy of trans fats. The relative risk of total mortality was 20% lower when 5% energy from MUFA-Ps iso-calorically replaced SFAs and MUFA-As (0.80 [0.77, 0.84]; Ptrend <0.001). A 15% to 27% lower risk of CVD, cancer and other deaths was observed when replacing MUFA-As or SFAs+MUFA-As with MUFA-Ps. Substituting MUFA-Ps for other nutrients was not significantly associated with CVD and cancer mortality. Cohort-specific and pooled HRs and 95% CIs for substitution analysis are shown in Online Table V.
Figure 1. Risk of total and cause-specific mortality for substitution analysis replacing other nutrients with MUFAs.
Abbreviations: MUFA, monounsaturated fatty acids; MUFA-P, MUFA from plant sources; MUFA-A, MUFA from animal sources; NHS, Nurses’ Health Study; HPFS, Health Professional’s Follow-up Study; SFA, saturated fatty acids.
Hazard ratios were adjusted for age, ethnicity (Caucasian, and other ethnicity), smoking status (never, former, current (1–14, 15–24, or ≥25 cigarettes/day, alcohol intake (grams/day: 0, 0.1–4.9, 5.0–14.9, and >15.0 in women, 0, 0.1–4.9, 5.0–29.9, and >30.0 in men), family history of myocardial infarction (yes/no), family history of diabetes (yes/no), family history of cancer (yes/no), menopausal status and post-menopausal hormone use (pre-menopause, post-menopause (never, former, or current hormone use), for women), physical activity (<3, 3.0–8.9, 9.0–17.9, 18.0–26.9, ≥27.0 METs/week), current aspirin use (yes/no), multivitamin use (yes/no), baseline hypertension, baseline hypercholesterolemia, BMI (<23, 23–24.9, 25–29.9, 30–34.9, >35kg/m2), intakes of total energy, fruits and vegetables and coffee intake (in quintiles); For refined carbohydrate substitution, models were further adjusted for energy from protein, whole grain carbohydrates, trans fats, PUFAs, and SFAs; For trans fats substitution, models were further adjusted for total fats, PUFAs, and SFAs; For SFA substitution, models were further adjusted for total fats, trans fats, and PUFAs; All MUFA-Ps models were further adjusted for MUFA-As, and vice versa.
† Study estimates from two cohorts were pooled using a fixed effects model.
The results for the substitution models remained largely unchanged when we continuously updated the diet regardless of the development of intermediate outcomes (Online Table VI) or when the models were adjusted for the AHEI score (Online Table VII).
DISCUSSION
In the present prospective investigation among men and women in two large U.S. cohorts, we observed that the association of MUFA intake with mortality was determined by food sources of these fatty acids. Higher intake of MUFA-Ps was associated with lower total mortality, whereas the opposite was true for higher intake of MUFA-As. Moreover, total mortality was 14–28% lower when SFAs, refined carbohydrates, or trans fats were isocalorically replaced by MUFA-Ps. Substituting MUFA-Ps for MUFA-As and SFAs combined was also associated with lower total and cause-specific mortality. To our knowledge, this is the first prospective study that examined MUFAs from plant and animal sources separately in relation to total and cause-specific mortality.
Previous data on the association between MUFA intake and mortality have been inconsistent. In some studies, non-significant associations were observed, while others showed positive associations.3,9,19 In a recent meta-analysis of 17 prospective cohort studies, Schwingshackl et al. found that MUFA intake was associated with 11% lower risk of all-cause mortality and 12% lower risk of CVD mortality.9 However, substantial between-study heterogeneity was observed, partly due to the inconsistent adjustment of covariates among individual studies.9 The other possible reason might be that MUFAs have diverse food sources, some of which may contain high amounts of unhealthful nutrients, such as SFAs or cholesterol in meats, dairy products, and partially hydrogenated oils, that may confound the associations for total MUFAs. In the NHS and HPFS, in the earlier FFQ, total MUFAs were strongly correlated with SFAs (r=0.8), which have been associated with higher mortality in previous analyses.5 Strong correlations of MUFAs with SFAs could likely explain the lack of associations observed between MUFAs and all-cause mortality when SFAs were not included in the model.
Our study findings generated novel evidence suggesting that MUFAs from plant and animal sources are differentially associated with total and cause-specific mortality. Existing studies that addressed MUFAs from different food sources in relation to mortality are sparse. In an ecological study from the Seven Countries Study, all-cause mortality rates were inversely correlated with the ratios of MUFAs/SFAs and (MUFAs + PUFAs)/(SFAs + trans fats), as well as vegetable oils,20 but this study did not explicitly examined MUFA-As and MUFA-Ps. In contrast, evidence is abundant for some major food sources of MUFAs. In our cohorts, olive oil, nuts, salad dressing, and fried foods were major sources of MUFA-Ps, while red and processed meats, dairy products, butter and poultry were leading contributors of MUFA-As.8 The meta-analysis by Schwingshackl et al. showed that higher intake of olive oil was associated with a 23% lower risk of all-cause mortality.9 In addition, higher intake of nuts was associated with a lower risk of all-cause and cause specific mortality in the NHS and HPFS cohorts21 and in a meta-analysis that included twenty prospective cohort studies.22 Specifically, this meta-analysis showed that per 28 g increase in nut consumption was associated with 22% (95% CI: 16%−28%) lower risk of all-cause mortality risk. In contrast, higher intake of red meat and processed meat has been associated with higher risk of mortality in prospective cohort studies.23,24
Specification of an explicit comparison is the cornerstone of isocaloric nutritional substitution analysis, which evaluates the effects of adding or subtracting a calorie-bearing macronutrient by changing intake of other macronutrients correspondingly while holding the total energy intake constant. In the NHS and HPFS cohorts, we previously reported that replacing 5% of energy from SFAs with equivalent energy from PUFAs and MUFAs was associated with 27% and 13% lower total mortality, respectively.5 In addition, the risk of CHD was significantly lower when SFAs, refined carbohydrates, or trans fats were isocalorically replaced by MUFA-Ps but not MUFA-As in our recent analysis.8 Findings from the present analysis also showed significantly lower CVD mortality when MUFA-Ps replaced MUFA-As and MUFA-As+SFAs, but not SFAs or refined carbohydrates.
Moreover, we also observed lower mortality of cancer, and non-CVD and non-cancer causes when MUFA-Ps replaced MUFA-As and MUFA-As+SFAs. Existing data on specific types of dietary fats and non-CVD mortality are sparse. One prospective study showed an inverse association between MUFAs and breast cancer incidence in women aged 50 years or more, while other studies reported non-significant associations.25 Some studies have suggested that olive oil could be beneficial in the prevention of certain cancers, such as breast cancer.26 The consumption of nuts, an important source of MUFAs, has also been inversely related with the incidence of colorectal cancer, endometrial cancer, pancreatic cancer, and total cancer.27 Nut consumption was not associated with a lower risk of prostate cancer incidence and mortality,27,28 although frequent nut consumption was associated with better survival among prostate cancer patients.28 In addition, several lines of evidence suggested that high intakes of MUFAs and PUFAs were associated with slower cognitive decline.29 An analysis in the Rotterdam Study that prospectively followed 5289 participants ≥55 years old showed that the intakes of total fats, cis-MUFAs and PUFAs were significantly associated with a lower risk of Parkinson disease.30
The cardiovascular effects of different fatty acids have been extensively examined. Results from clinical trials showed that higher MUFA intake improves blood lipid profile,31 decreases blood pressure,32 and modulates insulin resistance and glycemic control.33 In a meta-analysis of RCTs comparing high- versus low-MUFA diets in patients with abnormal glucose metabolism, high MUFA intake was associated with lower HbA1c but other parameters of insulin resistance were unaffected.32 However, whether these effects can be entirely ascribed to MUFA-Ps deserves further investigations. Nevertheless, controlled feeding studies that examined vegetable oils rich in MUFAs, including olive oil, high-oleic-acid sunflower oil, high-oleic acid canola oil and nuts, have consistently demonstrated beneficial effects of higher intake of these oils on reducing cardiovascular risk.6,33,34 These findings may underlie the associations that we observed between MUFA-P intake and mortality risk. The observed inverse associations between plant food sources of MUFAs and mortality can also be explained by the potential synergic effects with other bioactive components such as polyphenols, dietary fiber, vitamins and minerals.35,36 Meanwhile, recent clinical trials comparing vegetable oils that differed in the composition of fatty acids only showed that MUFAs significantly improved blood lipid profile and reduced central obesity, independent of the other components in the vegetable oils.37–39
Observational studies have suggested that higher consumption of red meat and processed meat, sources of MUFA-As and SFAs, is associated with a higher risk of developing type 2 diabetes,40 CVD,41 and certain cancers.42,43 In addition, many controlled feeding studies have shown that dietary cholesterol and SFAs increase total and LDL concentrations, especially when compared with unsaturated fatty acids.34 Because dietary cis-MUFAs, including those from animal sources, are mostly oleic acid, the positive associations of MUFA-As with total and CVD mortality are likely explained by confounding of other components in animal foods, especially SFAs. Importantly, our results indicated that replacement of the combination of MUFA-As and SFAs by MUFA-Ps was significantly associated with lower risk of total, CVD, cancer and non-CVD and non-cancer mortality. Given that MUFA-As and SFAs cannot be easily separated in the diet, they shall be replaced together by MUFAs from plant foods as a preferable source of fats.
Even though the intake of cis-MUFAs has been associated with beneficial effects on health, there are still no consistent dietary recommendations regarding MUFAs. However, most dietary guidelines recommend higher intake of healthy plant foods, including mainly unsaturated vegetable oils, nuts and seeds, which are high in MUFAs and PUFAs, and lower intake of animal foods to prevent chronic diseases2. Our results provide further epidemiological evidence supporting the recommendation of increasing the intake of unsaturated fats from plant-based food sources instead of fats from animal food sources, as well as replacing SFAs with unsaturated fatty acids. This evidence may also assist individuals in identifying healthy dietary choices for reducing animal fat intake.
The present study has several strengths including using data from two large, longitudinal cohorts with long follow-up and repeated measurements of diet. As in any observational study, the possibility of residual or unmeasured confounding cannot be excluded. Although we adjusted for many dietary factors in our analysis, confounding by other dietary components in the same food sources of MUFAs cannot be fully ruled out. In addition, synergistic effects of MUFAs with other nutrients are also possible,8 although a larger sample size is needed to examine such interactions. Our study population was relatively homogeneous (predominantly Caucasian health professionals), and thus caution shall be exercised when extrapolating our findings to other populations with different demographic characteristics. However, there is no reason to believe that the biological mechanisms would differ in other populations. We cannot entirely rule out reverse causation bias, because people with chronic diseases might change their habitual diet. However, we excluded participants with known major chronic diseases at baseline, used cumulative averages of diet to reduce short-term variability, and stopped updating diet after the development of certain major chronic diseases.
In conclusion, we found divergent associations between MUFA-Ps and MUFA-As with total and cause-specific mortality. Higher MUFA-P intake was associated with lower mortality whereas MUFA-A intake was associated with higher mortality. Significantly lower mortality was observed when SFAs, trans fats, or refined carbohydrates were replaced by MUFA-Ps. Overall, these data support current dietary recommendations to replace animal fats with unsaturated plant oils for the prevention of chronic diseases and premature deaths.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Existing studies regarding monounsaturated fatty acid (MUFA) intake and mortality risk have reported inconsistent findings.
MUFAs share animal foods as major dietary sources with saturated fatty acids (SFAs), cholesterol and other constituents, therefore the expected benefits of MUFAs could be masked in observational studies.
What New Information Does This Article Contribute?
Total and plant MUFAs were associated with lower mortality risk, whereas MUFA-As were associated with higher risk.
Replacing saturated fats, refined carbohydrates, trans fats, or MUFA-As with MUFA-Ps was associated with lower mortaltity risk.
In this study we report that MUFAs from animal and plant sources are largely different with respect to their associations with the risk of mortality. These data support current dietary recommendations to replace animal fats with unsaturated plant oils for the prevention of chronic diseases and premature deaths.
ACKNOWLEDGEMENTS
We would like to thank the participants and staff of the NHS and HPFS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
SOURCES OF FUNDING
Supported by research grants UM1 CA186107, P01 CA87969, U01 CA167552, P30 DK046200, HL034594, HL088521, HL35464 and HL60712 from the National Institutes of Health. MG-F is supported by American Diabetes Association grant #1–18-PMF-029. GZ is supported by the National Key Research and Development Program of China (Project No. 2018YFC604404) and the Key deployment project of Chinese Academy of Sciences (ZDBS-SSW-DQC-01). Geng Zong is supported by 100 Talents Program of The Chinese Academy of Sciences.
Nonstandard Abbreviations and Acronyms:
- AHEI
Alternate Healthy Eating Index
- CHD
coronary heart disease
- CI
confidence interval
- CVD
cardiovascular disease
- FFQ
food frequency questionnaire
- HPFS
Health Professionals Follow-up Study
- HR
hazard ratio
- MUFA
monounsaturated fatty acids
- MUFA-As
monounsaturated fatty acids from animal sources
- MUFA-Ps
monounsaturated fatty acids from plant sources
- NHS
Nurses’ Health Study
- PUFA
polyunsaturated fatty acids
- SFA
saturated fatty acids
Footnotes
In December 2018, the average time from submission to first decision for all original research papers submitted to Circulation Research was 14.99 days.
DISCLOSURES
GZ is supported by a postdoctoral fellowship funded by Unilever R&D. Peter L. Zock and Anne Wanders are employees of Unilever Research and Development. Unilever is a producer of food consumer products. FBH has received research support from California Walnut Commission and honoraria for lectures from Metagenics and Standard Process and honoraria from Diet Quality Photo Navigation, outside the submitted work. QS reports receiving ad hoc consulting fees from Emavant Solutions GmbH. All other authors declare no conflict of interest.
REFERENCES
- 1.World Health Organization. Global health risks: mortality and burden of disease. 2016.
- 2.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. 2015. [DOI] [PMC free article] [PubMed]
- 3.Jakobsen MU, O’Reilly EJ, Heitmann BL, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89:1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michas G, Micha R, Zampelas A. Dietary fats and cardiovascular disease: Putting together the pieces of a complicated puzzle. Atherosclerosis. 2014;234:320–328. [DOI] [PubMed] [Google Scholar]
- 5.Wang DD, Li Y, Chiuve SE, Stampfer MJ, Manson JE, Rimm EB, Willett WC, Hu FB. Association of Specific Dietary Fats With Total and Cause-Specific Mortality. JAMA Intern Med. 2016;176:1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estruch R, Ros E, Salas-Salvadó J, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. 2018;378:e34. [DOI] [PubMed] [Google Scholar]
- 7.Guasch-Ferré M, Babio N, Martínez-González MA, et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am J Clin Nutr. 2015; 102(6):1563–73. [DOI] [PubMed] [Google Scholar]
- 8.Zong G, Li Y, Sampson L, Dougherty LW, Willett WC, Wanders AJ, Alssema M, Zock PL, Hu FB, Sun Q. Monounsaturated fats from plant and animal sources in relation to risk of coronary heart disease among US men and women. Am J Clin Nutr. 2018;107:445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwingshackl L, Hoffmann G. Monounsaturated fatty acids, olive oil and health status: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr. 2018;107:445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belanger C, Speizer FE, Hennekens CH, Rosner B, Willett W, Bain C. The nurses’ health study: current findings. Am J Nurs. 1980;80:1333. [DOI] [PubMed] [Google Scholar]
- 11.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. [DOI] [PubMed] [Google Scholar]
- 12.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–6. [DOI] [PubMed] [Google Scholar]
- 13.Harvard TH Chan School of Public Health Nutrition Department. Food composition table; https://regepi.bwh.harvard.edu/health/nutrition/. [Google Scholar]
- 14.Hu FB, Stampfer MJ, Manson JE, Ascherio A, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr. 1999;70:1001–8. [DOI] [PubMed] [Google Scholar]
- 15.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 16.Garland M, Sacks FM, Colditz GA, Rimm EB, Sampson LA, Willett WC, Hunter DJ. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am J Clin Nutr. 1998;67:25–30. [DOI] [PubMed] [Google Scholar]
- 17.Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a Dietary Questionnaire Assessed by Comparison With Multiple Weighed Dietary Records or 24-Hour Recalls. Am J Epidemiol. 2017;185:570–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, Hennekens CH. Test of the National Death Index. Am J Epidemiol. 1984;119:837–9. [DOI] [PubMed] [Google Scholar]
- 19.Skeaff CM, Miller J. Dietary fat and coronary heart disease: summary of evidence from prospective cohort and randomised controlled trials. Ann Nutr Metab. 2009;55:173–201. [DOI] [PubMed] [Google Scholar]
- 20.Menotti A, Kromhout D, Puddu PE, Alberti-Fidanza A, Hollman P, Kafatos A, Tolonen H, Adachi H, Jacobs DR. Baseline fatty acids, food groups, a diet score and 50-year all-cause mortality rates. An ecological analysis of the Seven Countries Study. Ann Med. 2017;49:718–727. [DOI] [PubMed] [Google Scholar]
- 21.Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Association of nut consumption with total and cause-specific mortality. N Engl J Med. 2013;369:2001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016;14:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;172:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsson SC, Orsini N. Red Meat and Processed Meat Consumption and All-Cause Mortality: A Meta-Analysis. Am J Epidemiol. 2014;179:282–289. [DOI] [PubMed] [Google Scholar]
- 25.Schwab U, Lauritzen L, Tholstrup T, Haldorssoni T, Riserus U, Uusitupa M, Becker W. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: a systematic review. Food Nutr Res. 2014;58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelucchi C, Bosetti C, Negri E, Lipworth L, La Vecchia C. Olive oil and cancer risk: an update of epidemiological findings through 2010. Curr Pharm Des. 2011;17:805–812. [DOI] [PubMed] [Google Scholar]
- 27.Wu L, Wang Z, Zhu J, Murad AL, Prokop LJ, Murad MH. Nut consumption and risk of cancer and type 2 diabetes: a systematic review and meta-analysis. Nutr Rev. 2015;73:409–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Yang M, Kenfield SA, Hu FB, Stampfer MJ, Willett WC, Fuchs CS, Giovannucci EL, Bao Y. Nut consumption and prostate cancer risk and mortality. Br J Cancer. 2016;115:371–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solfrizzi V, Custodero C, Lozupone M, et al. Relationships of Dietary Patterns, Foods, and Micro- and Macronutrients with Alzheimer’s Disease and Late-Life Cognitive Disorders: A Systematic Review. J Alzheimer’s Dis. 2017;59:815–849. [DOI] [PubMed] [Google Scholar]
- 30.de Lau LML, Bornebroek M, Witteman JCM, Hofman A, Koudstaal PJ, Breteler MMB. Dietary fatty acids and the risk of Parkinson disease: The Rotterdam Study. Neurology. 2005;64:2040–2045. [DOI] [PubMed] [Google Scholar]
- 31.Mensink RP, Zock PL, Kester ADM, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–55. [DOI] [PubMed] [Google Scholar]
- 32.Schwingshackl L, Strasser B, Hoffmann G. Effects of monounsaturated fatty acids on cardiovascular risk factors: a systematic review and meta-analysis. Ann Nutr Metab. 2011;59:176–86. [DOI] [PubMed] [Google Scholar]
- 33.Imamura F, Micha R, Wu JHY, de Oliveira Otto MC, Otite FO, Abioye AI, Mozaffarian D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016;13:e1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mensink R Effects of saturated fatty acids on serum lipids and lipoproteins: a systematic review and regression analysis. Geneva: World Health Organization; 2016. [Google Scholar]
- 35.Huang T, Yang B, Zheng J, Li G, Wahlqvist ML, Li D. Cardiovascular disease mortality and cancer incidence in vegetarians: a meta-analysis and systematic review. Ann Nutr Metab. 2012;60:233–40. [DOI] [PubMed] [Google Scholar]
- 36.Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q, Hu FB. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLOS Med. 2016;13:e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lichtenstein AH, Matthan NR, Jalbert SM, Resteghini NA, Schaefer EJ, Ausman LM. Novel soybean oils with different fatty acid profiles alter cardiovascular disease risk factors in moderately hyperlipidemic subjects. Am J Clin Nutr. 2006;84:497–504. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Kris-Etherton PM, West SG, Lamarche B, Jenkins DJA, Fleming JA, McCrea CE, Pu S, Couture P, Connelly PW, Jones PJH. Effects of canola and high-oleic-acid canola oils on abdominal fat mass in individuals with central obesity. Obesity (Silver Spring). 2016;24:2261–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones PJH, MacKay DS, Senanayake VK, Pu S, Jenkins DJA, Connelly PW, Lamarche B, Couture P, Kris-Etherton PM, West SG, Liu X, Fleming JA, Hantgan RR, Rudel LL. High-oleic canola oil consumption enriches LDL particle cholesteryl oleate content and reduces LDL proteoglycan binding in humans. Atherosclerosis. 2015;238:231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan A; Sun Q; Bernstein AM; Manson JE; Willet WC; Hu FB. Changes in red meat consumption and subsequent risk of type 2 diabetes mellitus: three cohorts of US men and women. Jama Intern Med. 2013;173:1328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Micha R, Michas G, Mozaffarian D. Unprocessed Red and Processed Meats and Risk of Coronary Artery Disease and Type 2 Diabetes – An Updated Review of the Evidence. Curr Atheroscler Rep. 2012;14:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J, Zeng R, Huang J, Li X, Zhang J, Ho J, Zheng Y. Dietary Protein Sources and Incidence of Breast Cancer: A Dose-Response Meta-Analysis of Prospective Studies. Nutrients. 2016;8:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lippi G, Mattiuzzi C, Cervellin G. Meat consumption and cancer risk: a critical review of published meta-analyses. Crit Rev Oncol Hematol. 2016;97:1–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.