Abstract
Introduction
With expanding indications for programmed death 1 (PD-1) axis inhibitors in non–small cell lung cancer (NSCLC), acquired resistance (AR) to these therapies is increasingly being encountered. We sought to characterize clinical patterns of AR to PD-1 axis inhibitors in patients with advanced NSCLC, and evaluate subsequent outcome and management strategies for such patients.
Methods
Patients with NSCLC who developed AR to PD-1 axis inhibitor therapy initiated between December 2009 and February 2016 at one institution were identified and examined by clinical and radiographic features. AR was defined as progressive disease after initial response by either Response Evaluation Criteria in Solid Tumors v1.1 or immune-related response criteria.
Results
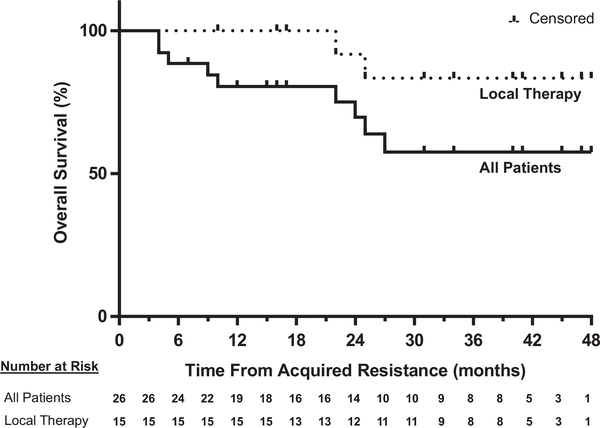
Twenty-six patients with AR to PD-1 axis inhibitor therapy were identified and evaluated. Median time to AR was 313 days; the 2-year survival rate from AR was 70% (95% confidence interval: 0.53–0.92). Twenty patients (77%) experienced AR in lymph nodes (LNs), including 11 patients with LN-only progression. Twenty-three (88%) patients had recurrence limited to one (54%) or two (35%) sites of disease. Fourteen patients (54%) continued PD-1 axis inhibitor therapy beyond progression. Three patients were re-challenged with the same PD-1 axis inhibitor after holiday from and progression off therapy, 2 again responded. Fifteen patients (58%) received local therapy to site(s) of AR, 11 continued respective PD-1 axis inhibitor after local therapy. The 2-year survival rate from AR among these 15 patients was 92% (95% confidence interval: 0.77–1).
Conclusions
Acquired resistance to PD-1 axis inhibitors is often limited to one or two sites when local therapy and continuation of PD-1 axis inhibitor therapy can result in prolonged benefit. LN metastases appear to be particularly susceptible sites to AR. When progression of disease following response occurs after holiday from PD-1 axis inhibitor, re-challenge can again lead to tumor regression.
Keywords: PD-1, PD-L1, acquired resistance, non–small cell lung cancer, immunotherapy
Introduction
Success of programmed death 1 (PD-1) axis inhibitors across solid tumors and hematologic malignances has ushered in a new era of cancer therapeutics, one which will increasingly harness the immune system to attack and control cancer. Currently, antagonist antibodies to PD-1 (anti–PD-1) or the programmed death ligand 1 (anti–PD-L1) are approved for use in advanced NSCLC, melanoma, head and neck cancer, renal cell carcinoma, bladder cancer, Merkel cell carcinoma, Hodgkin’s lymphoma, and colorectal cancer or other solid tumors with microsatellite instability or mismatch repair deficiency. Activity is being explored in virtually all other tumor types, with anticipated additional approvals in the coming years. Although responses to anti–PD-1 and anti–PD-L1 antibodies tend to be much more durable than responses to chemotherapy, resistance inevitably develops in most patients. Little is currently known about mechanisms of acquired resistance (AR), or clinical patterns and optimal management of such resistance.
NSCLC, once thought of as a non-immunogenic cancer, has been shown to be particularly susceptible to PD-1 axis inhibitors with up to 20% of unselected patients with advanced disease responding to therapy.1–6 When selected for high tumor PD-L1 expression using immunohistochemistry, defined as at least 50% of tumor cells staining for PD-L1, responses are seen in up to 45% of patients.2,7 Median duration of response in advanced NSCLC ranges from 12 to 25 months, 2 to 3 times as long as with traditional chemotherapy.3–8 A smaller percentage of patients will have durable responses lasting well beyond 2 years, with some patients from early trials without evidence of active disease now more than 7 years from initiating PD-1 axis inhibitor therapy (more than 5 years since completing course of therapy).9 Efforts are currently underway to understand which patients will have particularly durable responses, and how/why AR emerges. Here, we characterize clinical patterns of AR and share treatment strategies and outcomes for 26 patients with AR to PD-1 axis inhibitors.
Methods
We identified all patients with advanced NSCLC who developed AR to PD-1 axis inhibitor therapy across nine clinical trials conducted at Yale Cancer Center between September 1, 2009, and August 31, 2016. Only patients receiving PD-1 axis inhibitor alone, or in combination with either a cytotoxic T-lymphocyte associated protein 4 (CTLA-4) inhibitor or tyrosine kinase inhibitor (TKI) of the epidermal growth factor receptor (EGFR) were considered (in the latter case, only in patients who progressed on an EGFR-TKI as their last line of therapy). One additional patient developed AR to commercial use anti–PD-1 during this period and was included in our cohort. AR was defined as disease progression after partial or complete response by Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. criteria or immune-related response criteria.10 All living patients provided informed consent under Yale University Institutional Review Board approved protocols allowing collection and analysis of clinical, radiographic, and pathologic data. A wavier of HIPAA authorization was granted by the institutional review board for the inclusion of deceased patients.
Overall survival and median follow-up were calculated using the Kaplan Meier method. Survival curves were compared using the log-rank test and confidence intervals were calculated in R using the exact binomial Clopper-Pearson intervals.
Results
Twenty-eight patients with AR to PD-1 axis inhibitor therapy were identified including 27 among 216 patients treated on clinical trial and 1 who received commercial use PD-1 axis inhibitor. Two patients declined participation, leaving a cohort of 26 evaluable patients (Table 1, Fig. 1). Patients initiated PD-1 axis inhibitor therapy between December 29, 2009, and February 12, 2016, with data cutoff date of May 1, 2017. Eighteen patients received monotherapy with a PD-1 axis inhibitor, 5 received combination therapy with a CTLA-4 inhibitor, and 3 received combination therapy with erlotinib (the latter 3 patients had progressive disease [PD] while on an EGFR-TKI as their last line of therapy). Median follow-up was 43 months (range, 14 to 59 months among the 17 patients still alive). Twenty-five patients (96%) achieved partial response by RECIST v1.1, 1 had partial response only by immune-related response criteria (initial growth of non-target lesions followed by regression). Three patients had responses lasting longer than 2 years before developing resistance. Median survival from initiation of PD-1 axis inhibitor therapy was not reached; the 3-year survival rate was 70% (95% confidence interval [CI]: 0.53 – 0.92) (Supplementary Fig. 1).
Table 1.
Patient Characteristics
| Age, y | |
| Median | 64 |
| Range | 42–89 |
| Gender | |
| Female | 11 (42) |
| Male | 15 (58) |
| Smoking history | |
| <1 pack | 3 (11) |
| 1–10 pack | 2 (8) |
| >10 pack | 21 (81) |
| Histology | |
| Squamous | 6 (23) |
| Non-squamous | 19 (73) |
| Mixed | 1 (4) |
| EGFR mutant | 4 (15) |
| KRAS mutant | 5 (19) |
| HER2 mutant | 1 (4) |
| Prior therapy | |
| Irradiation | 16 (61) |
| Chemotherapy | 14 (54) |
| EGFR-TKI | 3 (11) |
| Type of immunotherapy | |
| Monotherapy | 18 (69) |
| Anti–PD-1 | 14 (54) |
| Anti–PD-L1 | 4 (15) |
| Combination | 4 (15) |
| Anti–PD-1 plus anti-CTLA4 | 4 (15) |
| Anti–PD-L1 plus anti-CTLA4 | 1 (4) |
| Anti–PD-1 plus erlotiniba | 3 (11) |
Values presented are n (%) unless otherwise noted.
After progression on an EGFR-TKI as last line of therapy.
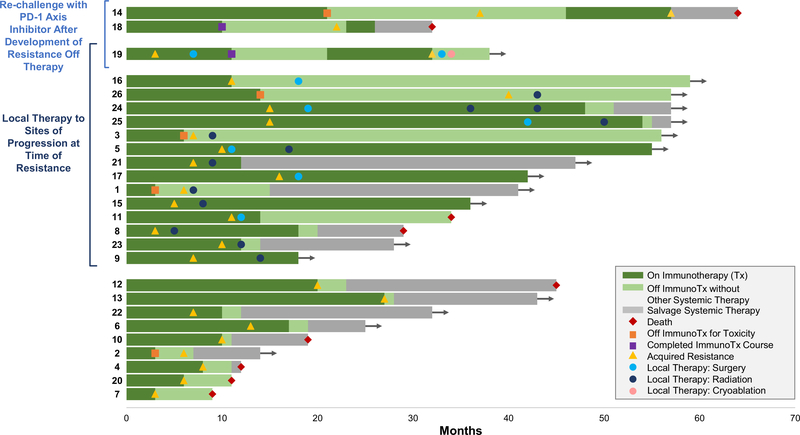
Figure 1.
Development and management of acquired resistance to programmed death 1 axis inhibitor therapy. Swimmer plot showing the time to resistance after initial response to PD-1 axis inhibitor therapy, and subsequent therapy, among 26 patients with advanced non–small cell lung cancer. Each bar represents one patient. Patient identification number preceding each bar corresponds to patient identification number provided in Table 2.
Median time to AR was 313 days (range, 104 to 1236 days). Among the 9 and 17 patients who received PD-1 axis inhibitor therapy as first line or salvage therapy, respectively, median time to AR was 232 days and 356 days (p = 0.017) (Supplementary Fig. 2). Median survival from the time of acquired resistance on PD-1 axis inhibitor therapy was not reached; the 2-year survival rate from AR was 70% (95% CI: 0.53 – 0.92) (Fig. 2). Fourteen patients continued PD-1 axis inhibitor therapy beyond progression, with a median duration of 452 days. Six patients discontinued PD-1 axis inhibitor therapy before progression of disease was encountered, including 5 for toxicity and 1 because the 1-year prescribed treatment course had been completed. Time from last dose of PD-1 axis inhibitor to PD was less than 4 months in 3 of these patients (40, 102, and 110 days, respectively).
Figure 2.
Kaplan-Meier curves of overall survival from acquired resistance to programmed death 1 axis inhibitor therapy for all patients and the subset of patients who received local therapy to all sites of acquired resistance.
Pathology Review
Twenty-three patients underwent biopsy or excision of site(s) of AR; all yielded malignant cells. Hematoxylin and eosin stained slides from tumor tissue sampled before initiation of immunotherapy and at the time of AR to immunotherapy were available for review from all 23 patients. No clear changes in lung cancer histology were appreciated (e.g., transformation from non–small cell to small-cell). Considering most specimens were obtained from lymph nodes (LNs), accurate characterization of infiltrating immune cells was limited. Furthermore, discordant non-LN sites and different methods of specimen collection (i.e., fine needle aspiration, core/forceps biopsy, and excision) limited comparison. Tumor PD-L1 status by immunohistochemistry (Dako 22C3 PharmDx) was available for paired specimens from patient 19. PD-L1 expression was not identified on tumor cells before or at AR to combination therapy with anti–PD-L1/anti–CTLA-4; however, it was newly appreciated on tumor immune cells at the time of AR. Tumor PD-L1 expression using either the 22C3 or E1L3N antibody was additionally available for resistance specimens from patients 2, 8, 9, 17, and 23; two specimens had no detectable PD-L1 expression (patients 2 and 8), two had between 1% and 5% (patients 9 and 23), and one had 10% to 25% tumor cell staining (patient 17).
Additional molecular/immunologic findings in a subset of these patients, and supportive laboratory studies (including tumor cell line and patient derived xenografts) are presented elsewhere.11
Re-Challenge
Three patients who developed progression after discontinuing PD-1 axis inhibitor therapy were re-challenged with the same treatment; two again responded.
One patient (patient 14) had previously received two chemotherapy regimens for advanced adenocarcinoma of the lung, and discontinued anti–PD-1 after 22 months due to renal toxicity, with sustained response for an additional 16 months off any systemic therapy. Upon progression (growth of a lung nodule and biopsy confirmed axillary LN metastasis), anti–PD-1 was resumed with response lasting 11 months when she developed new symptomatic brain metastases, progressive pulmonary nodules, and thoracic adenopathy.
The second patient (patient 19) with response to re-challenge was previously treated with two lines of chemotherapy for advanced squamous cell carcinoma of the lung. Four months after initiating combination therapy with an anti–PD-L1 antibody and anti–CTLA-4 antibody, imaging revealed a new site of disease (celiac mass) with ongoing response at known sites of disease (Supplementary Fig. 3). The celiac mass was resected, yielding squamous cell carcinoma, and she continued a 1-year prescribed course of combination therapy without further progression. After a 9-month period off any therapy, she developed PD at multiple sites, including both new sites and sites that previously regressed with anti–PD-L1/anti–CTLA-4. She restarted the same therapy, with the second response lasting 11 months at which time PD was appreciated in cervical and retroperitoneal LNs. The former was resected, again yielding squamous cell carcinoma, and the latter underwent cryoablation. As of the data cutoff date, she had not received any subsequent systemic or local therapy for her cancer, without evidence of other disease 6 months after her last dose of anti–PD-L1/anti–CTLA-4.
The remaining patient (patient 18) who failed to respond to re-challenge had previously received three lines of chemotherapy, and completed a 1-year course of anti–PD-L1 with sustained response. Recurrent disease (progressive thoracic and new mesenteric LN, both confirmed to be NSCLC by biopsy) was appreciated 1 year after his last dose of anti–PD-L1, which progressed through re-challenge.
Sites of AR
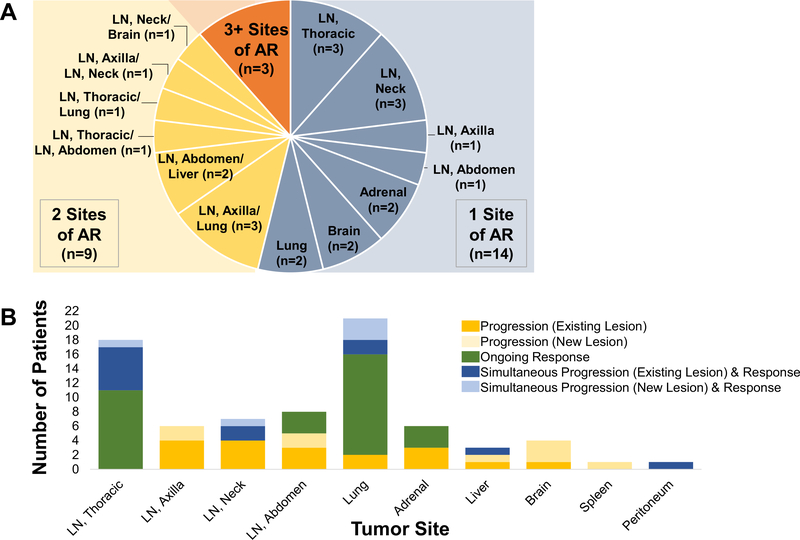
The most common site of AR was LNs, encountered in 20 patients (77%). Eleven (42%) patients had AR limited to LN sites. All 20 patients with AR in LN had other sites of disease with sustained response at the time of AR, including 13 with ongoing response in both non-LN sites and other LNs, 4 with ongoing response limited to other LNs, and 3 patients with ongoing response in only non-LN sites (Fig. 3). Fifteen of 20 patients with AR in LNs had initial regression of respective LN during PD-1 axis inhibitor therapy. Of the remaining 5 patients, 1 experienced initial stability of pre-existing LN, and 4 did not have appreciable respective LNs present before initiating PD-1 axis inhibitor therapy. (Additional details are provided in Table 2 and Fig. 4.)
Figure 3.
Sites of AR. (A) Pie chart showing number of patients with one site (grey), 2 sites (yellow), or three or more sites (orange) of progressive disease. Site location provided for patients with progression limited to one or two sites. (B) Progression and ongoing response at time of AR by tumor site. Bar graph showing both sites of tumor growth and sustained tumor response at the time of AR to PD-1 axis inhibitor therapy. Thirteen patients had both progressing and responding lesions in the same organ site at the time of AR. AR, acquired resistance to PD-1 axis inhibitor therapy; LN, lymph node; PD-1, programmed death 1.
Table 2.
Patterns of Acquired Resistance to PD-1 Axis Inhibitor Therapy
| Pt | Histo (Mut) | Cig Pack Year | Prior Lines Tx (#) | Prior RT | TTR (d) | OS (m) | Sites of PD | Prior Response at PD sites | Sites of Ongoing Response at PD |
|---|---|---|---|---|---|---|---|---|---|
| Anti-PD-1 monotherapy, first line | |||||||||
| 01 | NS | 45 | – | No | 201 | 41+ | LN (thoracic) | Decrease | LN (thoracic) |
| 02 | NS (KRAS) | 10 | – | No | 201 | 14+ | 1. LN (neck) 2. LN (abdomen) 3. Brain |
1. Decrease 2. Decrease 3. Not present |
Lung, LN (thoracic) |
| 03 | NS | 35 | – | Yes | 236 | 56+ | Adrenal | Decrease | Lung |
| 04 | NS (KRAS) | 108 | – | No | 245 | 12 | >3: LN (thoracic), lung, adrenal, liver, peritoneum, spleen | All decreased, except spleen (not present) | Liver, peritoneum, LN (thoracic) |
| 05 | NS (KRAS) | 60 | – | Yes | 318 | 55+ | 1. LN (neck) 2. LN (axilla) |
1. Decrease 2. Decrease |
Lung, adrenal, LN (thoracic & abdomen) |
| 06 | NS (KRAS) | 15 | – | No | 418 | 25+ | 1. LN (axilla) 2. Lung |
1. Decrease 2. Decrease |
Lung |
| Anti-PD-1 monotherapy, salvage therapy | |||||||||
| 07 | S | 68 | 1 | Yes | 109 | 9 | 1. LN (abdomen) 2. Liver |
1. Decrease 2. Not present |
Lung, LN (thoracic) |
| 08 | NS (HER2) | <1 | 2 | No | 113 | 29 | 1. LN (neck) 2. Brain |
1. Stable 2. Decrease |
Lung, adrenal |
| 09 | NS | 36 | 1 | Yes | 225 | 18+ | LN (thoracic) | Not present | Lung, LN (thoracic) |
| 10 | NS (KRAS) | 25 | 5 | No | 309 | 19 | 1. LN (abdomen) 2. Liver |
1. Decrease 2. Decrease |
Lung |
| 11 | S (AKT1) | 50 | 4 | Yes | 347 | 34 | LN (neck) | Decrease | Lung, LN (thoracic & neck) |
| 12 | S | >100 | 3 | No | 637 | 45 | LN (axilla) | Decrease | Lung, LN (thoracic) |
| 13 | NS | 30 | 1 | Yes | 848 | 43+ | Lung | Decrease | Adrenal, LN (thoracic & abdomen) |
| 14a | NS (EGFR) | 15 | 2 | Yes | 1148 | 64 | 1. LN (axilla) 2. Lung |
1. Not present 2. Not present |
Lung, LN (thoracic) |
| 14- Re-challenge after progression off PD-1 axis inhibitor with second response | 328b | 18b | 1. LN (thoracic) 2. Brain 3. Lung |
1. Stable 2. Not present 3. Decrease |
Lung | ||||
| Anti-PD-L1 monotherapy, salvage therapy | |||||||||
| 15 | NS/S | 40 | 4 | Yes | 163 | 36+ | LN (neck) | Decrease | LN (neck) |
| 16 | NS | 20 | 6 | Yes | 356 | 59+ | LN (thoracic) | Decrease | LN (thoracic) |
| 17 | NS | 88 | 3 | No | 506 | 42+ | Adrenal | Decrease | Lung |
| 18a | NS | 60 | 3 | Yes | 676 | 32 | 1. LN (abdomen) 2. LN (thoracic) |
1. Not present 2. Decrease |
Lung, LN (thoracic) |
| Combination therapy, anti-PD-1/PD-L1 plus anti-CTLA-4 | |||||||||
| 19 | S | 30 | 2 | Yes | 104 | 38+ | LN (abdomen) | Not present | LN (thoracic) |
| 19- Re-challenge after progression off PD-1 axis inhibitor with second response | 330b | 17+b |
1. LN (neck) 2. LN (abdomen) |
1. Decrease 2. Not present |
LN (thoracic & neck) | ||||
| 20 | NS | 6 | – | Yes | 200 | 11 | 1. Brain | Not present | Lung, LN (thoracic) |
| 21 | S | 35 | – | No | 227 | 47+ | 1. LN (axilla) 2. LN (neck) 3. LN (thoracic) |
1. Decrease 2. Not present 3. Decrease |
Lung, LN (thoracic & neck) |
| 22 | NS | 35 | – | Yes | 232 | 32+ | 1. LN (thoracic) 2. Lung |
1. Decrease 2. Decrease |
Lung, LN (thoracic) |
| 23 | S | 46 | 1 | Yes | 322 | 28+ | Lung | Not present | Lung |
| Combination therapy, anti-PD-1 plus erlotinib (after progression on EGFR-TKI) | |||||||||
| 24 | NS (EGFR) | <1 | 1 | Yes | 483 | 57+ | LN (neck) | Decrease | Lung, LN (thoracic) |
| 25 | NS (EGFR) | – | 2 | No | 485 | 57+ | 1. LN (axilla) 2. Lung |
1. Not present 2. Not present |
Lung, LN (abdomen) |
| 26a | NS (EGFR) | 35 | 1 | Yes | 1236 | 57+ | Brain | Not present | Lung, LN (thoracic) |
Delayed resistance (developing at least 6 months after discontinuing PD-1 axis inhibitor).
From re-challenge.
Pt, patient; Histo, histology; Mut, driver mutation; Cig, cigarette; Tx, number of prior systemic regimens for NSCLC; RT, radiation; TTR, time to acquired resistance; OS, overall survival; PD, progressive disease; PD-1, programmed death 1; NS, nonsquamous NSCLC; LN, lymph node; S, squamous NSCLC; PD-L1, programmed death ligand 1; +, ongoing.
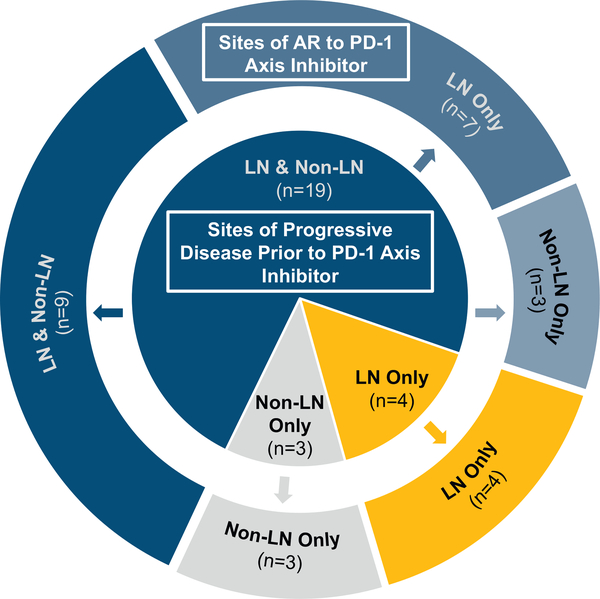
Figure 4.
Sites of AR by pre-treatment progressing sites. Donut chart showing sites of AR (LN and/or non-LN sites; outer ring) in patients grouped by pre–PD-1 axis inhibitor sites of progression (LN and/or non-LN sites; inner circle). AR, acquired resistance; PD-1, programmed death 1; LN, lymph node.
Fourteen (54%) patients had only one site of AR, including 8 with LN and 2 each with adrenal, brain, and lung progression. Of the remaining 12 patients with AR at more than one site, 9 (35%) and 2 patients had progression at two and three sites, respectively; one patient had progression involving more than three sites.
Management After AR
Other Systemic Therapy
Fifteen patients went on to receive salvage systemic therapy, with median time to initiation of 158 days from the development of AR to PD-1 axis inhibitor. Response to subsequent therapy was determined by treating physician, without formal RECIST measurements. Salvage therapies included first-line chemotherapy (n = 5, with tumor response in all five) and second- or later-line chemotherapy (n = 2, with tumor response in one), targeted therapy (n = 6, two responses, one prolonged stable disease lasting 210 days, and one ongoing stable disease at 48 days, among five evaluable patients), or other immune therapy (n = 2, stable disease in both, one lasting 163 days, the other ongoing 463 days after initiating salvage therapy) (Supplementary Table 1). The median survival time and 2-year survival rate from AR in these 15 patients were 27 months and 69% (95% CI: 0.48 – 1), respectively (Supplementary Fig. 4).
Local Therapy
Fifteen patients received local therapy to each site of AR without immediate initiation of salvage systemic therapy (Fig. 1). Local therapy did not result in unexpected adverse events. Median survival time from AR was not reached in these 15 patients, with a 2-year survival rate of 92% (95% CI: 0.77 – 1) (Fig. 2). Eleven continued respective PD-1 axis inhibitor therapy after local therapy. Six patients eventually received salvage systemic therapy (3, 5, 9, 16, 35, and 39 months, respectively, after AR). One patient died without other systemic therapy (22 months after AR). At the data cutoff date, 8 remaining patients had not received salvage systemic therapy (10, 16, 25, 31, 34, 45, 47, and 48 months, respectively, after development of AR); 4 continued respective PD-1 axis inhibitor.
Discussion
Although PD-1 axis inhibitors have had a tremendous impact on the lives of many lung cancer patients, most patients who initially benefit will develop resistance to therapy. It is now imperative to characterize patterns of such resistance, determine best management strategies, and dissect mechanisms of resistance that will enable more effective therapies. In our cohort of patients with AR, notable findings included a predilection for AR within LNs, often with involvement limited to one LN region. Several potential hypotheses explaining this finding can be offered. First, a LN may behave in some ways as an immunologically privileged site, limiting cytotoxic activity while maximizing conditions for antigen presentation. The malignant LN environment may further preferentially attract immunosuppressive cells and repulse cytotoxic T cells via cytokines, and favor further immunosuppressive evolution of cells within the LN. These hypotheses are currently being evaluated by our group by studying LNs with and without metastatic involvement using multiplexed quantitative immunofluorescence, and in select cases where fresh tissue was acquired, flow cytometry and/or mass cytometry. In our patients, most LNs regressed before growing, indicating that additional changes were induced by PD-1 axis inhibitor therapy and resultant tumor response promoting subsequent tumor outgrowth. Although it is unclear if similar initial reactive changes occurred at sites of disease other than LNs, one possibility is that the LN environment was more susceptible to immunosuppressive signals resulting from initial tumor attack. Additionally, considering PD-L1 is expressed on various immune cells found within an LN, continuous inhibition of PD-1 axis signaling in time may compromise ongoing tumor response within an LN.
Another notable observation in our AR cohort was that most patients experienced oligoprogressive disease, with 54% of patients having only one site of progression, and 35% having two sites of progression. Only one patient experienced simultaneous progression at more than three separate sites of disease. This pattern has also been seen in patients with EGFR mutant and ALK rearranged NSCLC at the time of AR to TKI therapy. Many thoracic oncologists will treat solitary sites of AR to EGFR and ALK TKIs with local therapy and continuation of respective TKI.12 This approach appeared promising in our cohort of patients with AR to PD-1 axis inhibitors as well, with long-term survival in the majority without alternative systemic therapy. Furthermore, with local radiation and other ablative therapies, there may be additional abscopal effects controlling occult disease at other sites. Ongoing clinical trials in advanced NSCLC are currently evaluating the abscopal potential of local tumor radiation with PD-1 axis inhibitor therapy.13
Additional observations from our patients include prolonged survival after development of AR, longer time to AR in previously treated than treatment-naïve patients, and re-response to re-challenge with PD-1 axis inhibitor after progression off therapy. The first observation suggests benefit from PD-1 axis inhibitors continues well beyond radiographic progression on such therapy. We additionally noted higher response rates than expected to subsequent chemotherapy in a handful of treated patients, suggesting immunotherapy may enhance tumor chemosensitivity. Of note, prolonged survival after the development of resistance in our cohort was not dependent on subsequent salvage therapy. The second finding of longer duration of response in those previously treated for advanced NSCLC parallels that seen with randomized trials evaluating nivolumab reporting median durations of response of 12 and 17 to 25 months, respectively, as first-line and salvage therapy.5,14 Although small numbers in our cohort limit interpretation, and formal statistical evaluation outside our cohort is lacking, it is conceivable that prior therapy effectively altered the tumor microenvironment, perhaps by depleting immunosuppressive cells or promoting antigen presentation leading to accumulation of tumor infiltrating lymphocytes, or, led to an increasingly immunogenic tumor due to molecular evolution of tumor cells. The final observation of response to re-induction with PD-1 axis inhibitor therapy in two of three patients is consistent with a prior report concerning a melanoma patient, and together support re-challenge with PD-1 axis inhibitor therapy after progression while on treatment holiday.15
Much work needs to be done to understand mechanisms of resistance to PD-1 axis inhibitor therapy across tumor types if we are to improve upon the success we have seen to date. Considering the complexities of the immune response, in addition to potential intrinsic changes and selection of tumor cells, discerning mechanisms of AR will be particularly challenging. Implicated mechanisms in a handful of patients to date include loss of beta 2 microglobulin preventing major histocompatibility complex – class I presentation of tumor antigen, loss of function JAK1 and JAK2 mutations resulting in insensitivity to interferon gamma, and longitudinal mutant neoantigen loss compromising immune recognition.11,16,17 Large sample sizes and organized efforts across cancer centers using consistent sample preparation and analysis will be required if we are to identify additional mechanisms of AR and primary resistance to immunotherapies, and develop more effective therapies for more patients with advanced cancer.
Limitations
Limitations to our study include the small number of patients, the different PD-1 axis inhibitors received, and different lines of therapy (first versus salvage). Furthermore, eight patients received combination therapy, five with an anti–CTLA4 antibody and three with erlotinib. Of note, the latter three patients had progressed on an EGFR-TKI as a last line of therapy. To limit potential selection bias in our cohort, all patients who received PD-1 axis inhibitor therapy across nine trials at one institution between December 2009 and February 2016 were considered, with only two patients declining participation. One additional patient who received commercial use PD-1 axis inhibitor during this period and developed AR was considered.
Although the majority of our patients developed PD while still receiving PD-1 axis inhibitor therapy, six patients discontinued PD-1 axis inhibitor therapy before resistance was appreciated, including three with progressive disease occurring more than 6 months after last treatment. All six patients did have sites of sustained response at the time of progression. Among the three with late progression off therapy, two resumed same PD-1 axis inhibitor with subsequent progression while receiving therapy (one after initial regression).
Conclusions
AR to PD-1 axis inhibitor therapy in patients with advanced NSCLC is often limited to one or two sites of disease, when local therapy (i.e., surgery, radiation/radiosurgery, or thermal ablation) to oligoprogressive disease with continuation of PD-L1 axis inhibitor should be considered. LNs may be particularly susceptible sites to AR. Further studies are needed to verify and understand such vulnerability. Re-challenge with PD-1 axis inhibitor after holiday from and progression off therapy can again result in tumor response.
Supplementary Material
Acknowledgments
Supported by the Yale SPORE in Lung Cancer (P50CA196530) and Melissa Marottoli Hogan Foundation. The authors thank Michelle DeVeaux for statistical support; Ryan Sowell for data review; and Emily Duffield, Heather Gerrish, Elin Rowen, and Kelly Derosier for data gathering and patient management.
Disclosures: Dr. Gettinger has received consulting fees from Bristol-Myers Squibb, Alexion, and ARIAD/Takeda; and has received research funding from Bristol-Myers Squibb, Genentech, and Celldex. Dr. Goldberg has received grants from Astra Zeneca; and has received personal fees from Astra Zeneca, Bristol-Myers Squibb, Lilly, and Boehringer Ingelheim. Dr. Rimm has received personal fees from Astra Zeneca, Bristol-Myers Squibb, and Merck; and has received research funding from Astra Zeneca and Navigate/Novartis. Dr. Schalper has received research funding from Genoptix (Novartis), Tesaro, and Vasculox. Dr. Kaech has received research funding from Roche and Astra Zeneca. Dr. Chiang has received consulting fees from Astra Zeneca. Dr. Lilenbaum has received consulting fees from Genetech and Astra Zeneca. Dr. Politi has received consulting fees from Astra Zeneca, Novartis, Merck, and Tocagen; and has received royalties as co-investigator on a patent for Molecular MD/MSKCC; and has received grants from Astra Zeneca, Roche, Koltan, and Symphogen. Dr. Herbst has received consultant fees from Merck, Bristol-Myers Squibb, Astra Zeneca, Genentech/Roche, Pfizer, and Eli Lilly; and has received grants from Genentech/Roche and Merck. The remaining authors declare no conflicts of interest.
Footnotes
Supplementary Data
Note: To access the supplementary material accompanying this article, visit the online version of the Journal of Thoracic Oncology at www.jto.org and at https://doi.org/10.1016/j.jtho.2018.03.008.
References
- 1.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 2.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non–small-cell lung cancer. J Clin Oncol. 2015;33:2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barlesi F, Steins M, Horn L, et al. Long-term outcomes with nivolumab (Nivo) vs docetaxel (Doc) in patients (Pts) with advanced (Adv) NSCLC: CheckMate 017 and CheckMate 057 2-y update. Ann Oncol. 2016;27 (Suppl 6; Abstract 1215PD). [Google Scholar]
- 6.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non–small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2016;389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non–small-cell lung cancer. N Engl J Med. 2016;375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 8.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non–small-cell lung cancer (KEYNOTE-010): a randomized controlled trial. Lancet. 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 9.Brahmer JR, Horn L, Jackman D, et al. Five-year follow-up from the CA209–003 study of nivolumab in previously treated advanced non–small cell lung cancer: clinical characteristics of long-term survivors. AACR Annual Meeting. 2017; [Google Scholar]
- 10.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. [DOI] [PubMed] [Google Scholar]
- 11.Gettinger SG, Choi J, Hastings K, et al. Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov. 2017;7:1420–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non–small-cell lung cancer. J Thorac Oncol. 2012;7:1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decker R, Goldberg S, Nath S, et al. A phase I/II trial evaluating the combination of stereotactic body radiotherapy and pembrolizumab in metastatic NSCLC. J Thorac Oncol. 2017;12:S1303S13–04 (Abstract P3. 02c-048). [Google Scholar]
- 14.Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage iv or recurrent non–small-cell lung cancer. N Engl J Med. 2017;376:2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipson EJ, Sharfman WH, Drake CG, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to pd-1 blockade in melanoma. N Engl J Med. 2016;375:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anagnostou V, Smith KN, Forde PM, et al. Evolution of neoantigen landscape during immune checkpoint blockade in non–small cell lung cancer. Cancer Discov. 2017;7:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.