Abstract
Background
Several treatment options are available for stress urinary incontinence (SUI), including pelvic floor muscle training (PFMT), drug therapy and surgery. Problems exist such as adherence to PFMT regimens, side effects linked to drug therapy and the risks associated with surgery. We have evaluated an alternative treatment, electrical stimulation (ES) with non‐implanted devices, which aims to improve pelvic floor muscle function to reduce involuntary urine loss.
Objectives
To assess the effects of electrical stimulation with non‐implanted devices, alone or in combination with other treatment, for managing stress urinary incontinence or stress‐predominant mixed urinary incontinence in women. Among the outcomes examined were costs and cost‐effectiveness.
Search methods
We searched the Cochrane Incontinence Specialised Register, which contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE In‐Process, MEDLINE Epub Ahead of Print, CINAHL, ClinicalTrials.gov, WHO ICTRP and handsearches of journals and conference proceedings (searched 27 February 2017). We also searched the reference lists of relevant articles and undertook separate searches to identify studies examining economic data.
Selection criteria
We included randomised or quasi‐randomised controlled trials of ES with non‐implanted devices compared with any other treatment for SUI in women. Eligible trials included adult women with SUI or stress‐predominant mixed urinary incontinence (MUI). We excluded studies of women with urgency‐predominant MUI, urgency urinary incontinence only, or incontinence associated with a neurologic condition. We would have included economic evaluations had they been conducted alongside eligible trials.
Data collection and analysis
Two review authors independently screened search results, extracted data from eligible trials and assessed risk of bias, using the Cochrane 'Risk of bias' tool. We would have performed economic evaluations using the approach recommended by Cochrane Economic Methods.
Main results
We identified 56 eligible trials (3781 randomised participants). Eighteen trials did not report the primary outcomes of subjective cure, improvement of SUI or incontinence‐specific quality of life (QoL). The risk of bias was generally unclear, as most trials provided little detail when reporting their methods. We assessed 25% of the included trials as being at high risk of bias for a variety of reasons, including industry funding and baseline differences between groups. We did not identify any economic evaluations.
For subjective cure of SUI, we found moderate‐quality evidence that ES is probably better than no active treatment (risk ratio (RR) 2.31, 95% CI 1.06 to 5.02). We found a similar result for cure or improvement of SUI (RR 1.73, 95% CI 1.41 to 2.11), but the quality of evidence was lower. We are very uncertain if there is a difference between ES and sham treatment in terms of subjective cure because of the very low quality of evidence (RR 2.21, 95% CI 0.38 to 12.73). For subjective cure or improvement, ES may be better than sham treatment (RR 2.03, 95% CI 1.02 to 4.07). The effect estimate was 660/1000 women cured/improved with ES compared to 382/1000 with no active treatment (95% CI 538 to 805 women); and for sham treatment, 402/1000 women cured/improved with ES compared to 198/1000 with sham treatment (95% CI 202 to 805 women).
Low‐quality evidence suggests that there may be no difference in cure or improvement for ES versus PFMT (RR 0.85, 95% CI 0.70 to 1.03), PFMT plus ES versus PFMT alone (RR 1.10, 95% CI 0.95 to 1.28) or ES versus vaginal cones (RR 1.09, 95% CI 0.97 to 1.21).
Electrical stimulation probably improves incontinence‐specific QoL compared to no treatment (moderate quality evidence) but there may be little or no difference between electrical stimulation and PFMT (low quality evidence). It is uncertain whether adding electrical stimulation to PFMT makes any difference in terms of quality of life, compared with PFMT alone (very low quality evidence). There may be little or no difference between electrical stimulation and vaginal cones in improving incontinence‐specific QoL (low quality evidence). The impact of electrical stimulation on subjective cure/improvement and incontinence‐specific QoL, compared with vaginal cones, PFMT plus vaginal cones, or drugs therapy, is uncertain (very low quality evidence).
In terms of subjective cure/improvement and incontinence‐specific QoL, the available evidence comparing ES versus drug therapy or PFMT plus vaginal cones was very low quality and inconclusive. Similarly, comparisons of different types of ES to each other and of ES plus surgery to surgery are also inconclusive in terms of subjective cure/improvement and incontinence‐specific QoL (very low‐quality evidence).
Adverse effects were rare: in total nine of the women treated with ES in the trials reported an adverse effect. We identified insufficient evidence to compare the risk of adverse effects in women treated with ES compared to any other treatment. We were unable to identify any economic data.
Authors' conclusions
The current evidence base indicated that electrical stimulation is probably more effective than no active or sham treatment, but it is not possible to say whether ES is similar to PFMT or other active treatments in effectiveness or not. Overall, the quality of the evidence was too low to provide reliable results. Without sufficiently powered trials measuring clinically important outcomes, such as subjective assessment of urinary incontinence, we cannot draw robust conclusions about the overall effectiveness or cost‐effectiveness of electrical stimulation for stress urinary incontinence in women.
Plain language summary
Non‐invasive electrical stimulation for stress urinary incontinence in women
Review question
We investigated whether electrical stimulation was better than no treatment at all or better than other available treatments for curing or improving stress urinary incontinence (SUI) symptoms in women. We also investigated whether SUI was cured or improved by adding electrical stimulation to other treatments, compared to other treatments and to different types of electrical stimulation. Finally, we investigated whether electrical stimulation represented value for money.
Background
About 25% to 45% of women worldwide have problems with leaking urine involuntarily. Women with SUI often leak urine with physical exertion such as coughing or sneezing. SUI can be treated with pelvic floor muscle exercises, vaginal cones, drug therapy or surgery, but there are various problems with these treatments. A possible alternative is electrical stimulation with non‐implanted devices, whereby an electrical current is delivered through vaginal electrodes.
How up‐to‐date is this review?
We searched for studies that had been published up to 27 February 2017.
Study characteristics
We found 56 trials (involving a total of 3781 women, all with stress urinary incontinence but some with urgency urinary incontinence as well) comparing electrical stimulation to no treatment or to any other available treatment.
Key results
For cure or improvement of SUI, electrical stimulation was probably better than no active or sham treatment. There was not enough evidence to say whether it was any better than pelvic floor muscle training for curing or improving SUI, or for quality of life. Adding electrical stimulation to pelvic floor muscle training may not make much difference to cure or improvement of SUI. It is uncertain whether it offers any improvement in quality of life compared with pelvic floor muscle training.
We found that few women reported adverse effects with electrical stimulation, but there was not enough reliable evidence comparing electrical stimulation to other treatments to know more about its safety.
There was not enough evidence comparing electrical stimulation to other existing treatments such as drug therapy, pelvic floor muscle training plus vaginal cones, surgery, or different forms of electrical stimulation, to provide evidence‐based guidance on which would be better, and for which women, in curing or improving SUI or in improving quality of life. There was no information from these studies to judge value for money.
Quality of the evidence
There is some evidence to support the use of electrical stimulation for stress urinary incontinence in women, but we are still very uncertain about the full potential of this treatment because of the low quality of the existing evidence. While we found evidence indicating that electrical stimulation may be better than no treatment, we did not find enough well‐designed trials with enough women to fully answer our review questions, so we do not yet know if ES is better or worse than other treatments.
Summary of findings
Background
Description of the condition
Urinary incontinence (UI) affects 25% to 45% of women worldwide (ICI 2013). UI presents in the following forms.
Stress urinary incontinence (SUI): involuntary loss of urine through physical exertion or effort, coughing or sneezing.
Urgency urinary incontinence (UUI): involuntary loss of urine associated with a sudden and compelling desire (urgency) to urinate that is difficult to delay.
Mixed urinary incontinence (MUI): involuntary loss or urine associated with both stress and urgency.
Symptomatic diagnosis of SUI is typically based on whether urine leakage occurs with physical exertion or effort, as reported by women themselves.
In addition, urodynamically proven stress incontinence (USI) is diagnosed when an observer can see urine leakage on stress such as coughing during urodynamic examination, in the absence of a detrusor contraction (ICI 2013). Symptomatic diagnosis of MUI is based on self‐report of urine leakage through both physical exertion and urgency.
This review includes women with SUI, USI and stress‐predominant MUI.
Several mechanisms are thought to contribute to stress urinary incontinence.
Suboptimal pelvic floor muscle strength.
Hypermobility or significant displacement of the urethra and bladder neck during exertion.
Intrinsic urethral sphincter deficiency (ICI 2013).
In women, these mechanisms may coexist (Kursh 1994), but few clinical trials have distinguished between them as underlying causes. We will consider women whose incontinence may be due to any of these mechanisms together in this review.
Prevalence estimates of SUI range from 3% to 25% of adult women, with older women more likely to be affected (ICI 2013). Quality of life and sexual function are often substantially impaired by the fear of leakage, resulting in avoidance of social or physical activities which might cause it, embarrassment and poor sleep (Oh 2008). SUI can severely impact the ability to carry out daily activities, resulting in debilitating embarrassment, social isolation and considerably decreased health‐related quality of life (Bartoli 2010). Women with SUI may be less likely to participate in physical activity, which in turn has a detrimental impact on overall health because inactivity is a risk factor for many diseases (Bø 2004). Other evidence has shown that up to 50% of women with UI will avoid intimacy with their partners (Roos 2014).
Furthermore, SUI is associated with a considerable economic burden for women and for healthcare providers. For instance, routine care, such as sanitary pads, can entail considerable cost for each woman affected, while conservative treatment and surgery may cost the equivalent of several thousand GBP for each woman (ICI 2013).
Description of the intervention
In Europe and the USA, conservative interventions such as pelvic floor muscle training (PFMT), with or without biofeedback, are recommended as first‐line treatment for SUI (EAU 2015; NICE 2013; Qaseem 2014); however, many women may find it difficult to adhere to these methods in the long‐term (Bø 2005; Dumoulin 2014).
Surgery is usually suggested as a second‐line option where conservative treatment has not improved a woman's symptoms or she is unwilling or unable to continue the treatment. Synthetic mid‐urethral tape, open or laparoscopic colposuspension and autologous rectus fascia sling procedures are recommended by the National Institute for Health and Care Excellence (NICE), although the use of surgery with tapes in the management of UI remains controversial in terms of safety and adverse effects (Scottish Government 2015). Several Cochrane Reviews have investigated the effects of surgical management for SUI (Dean 2017; Lapitan 2017; Nambiar 2017; Rehman 2011). Other older surgical procedures, such as anterior repair or bladder neck needle suspension, have generally fallen out of use due to lower effectiveness (Glazener 2017a; Glazener 2017b).
Other less invasive second‐line treatment options available in some countries include bulking agents, where a substance is injected into the urethral wall to increase its size and allow it to remain closed, or pharmacological therapy, typically with duloxetine. The disadvantages of these treatments are that they are likely to be less effective than surgery, and, in the case of drug therapy, long‐term adherence is usually necessary and is associated with unpleasant side effects (Alhasso 2005; Mariappan 2005). Bulking agents can cause discomfort or bleeding when urinating, and their effectiveness decreases over time, requiring retreatment. Other available treatments for SUI include artificial urinary sphincters and complementary therapies such as acupuncture.
Electrical stimulation (ES) has emerged as a first‐line alternative to PFMT in women who are unable to contract their pelvic floor muscles voluntarily or as a second‐line treatment if PFMT alone is not sufficiently effective. It may also be beneficial to combine ES with the use of vaginal cones and drug therapy.
How the intervention might work
When a nerve is stimulated, signals travel both toward the periphery and toward the central nervous system. Electrical stimulation may elicit responses to these signals, which may come from the central nervous system or the innervated tissues, or the central nervous system may be modified to reinterpret some signals (Chancellor 2002; Fall 1994).
With respect to lower urinary tract dysfunctions, electrical stimulation is applied particularly to the pelvic floor muscles, bladder and sacral nerve roots. In the context of SUI, the aim of ES is to improve pelvic floor muscle function so that the pelvic floor muscles can be used when needed to occlude (close) the urethra (such as before a cough) and to increase muscle bulk, which may help reduce urine loss by closing up the urethral walls.
Direct ES of the pelvic floor is intended to stimulate motor‐efferent fibres of the pudendal nerve, which may elicit a direct contraction of the pelvic floor muscles or the striated peri‐urethral musculature, supporting the intrinsic part of the urethral sphincter‐closing mechanism (Fall 1991; Scheepens 2003). As such, ES might contribute to compensating for a weak intrinsic sphincter, but it is questionable whether or not ES in such cases would be the first‐choice treatment option or would have any additional value to pelvic floor muscle training (Ayeleke 2015).
Different authors have suggested that ES may restore continence in women with SUI by:
strengthening the structural support of the urethra and the bladder neck by increasing muscle bulk (Plevnik 1991);
securing the resting and active closure of the proximal urethra (Erlandson 1977);
strengthening the pelvic floor muscles and hence their ability to close the urethra (Sand 1995);
inhibiting reflex bladder contractions (Berghmans 2002; Fall 1994);
modifying the vascularity (improving blood supply) of the urethral and bladder neck tissues (Fall 1991; Fall 1994; Plevnik 1991).
In the context of conservative or non‐surgical, non‐medical therapy, ES can be applied using surface electrodes in the form of transcutaneous or percutaneous ES. Transcutaneous ES is administered via suprapubic or vulval surface electrodes, or vaginal/anorectal plug electrodes. Percutaneous ES uses needle electrodes that penetrate the skin in conjunction with a surface electrode placed close to the needle to act as a reference electrode (e.g. posterior tibial nerve stimulation, percutaneous nerve evaluation). Percutaneous ES is normally used for women with overactive bladder symptoms, not SUI, so we exclude it from this review (see companion review of ES for overactive bladder; Stewart 2016a).
The frequency, dosage and duration of treatment with ES varies considerably. Although authors have claimed success for a wide range of parameters, there is no agreement on the optimal set of parameters for each type of urinary incontinence. Clinical consensus from the International Consultation on Incontinence (ICI) underlines this uncertainty:
"EStim is provided by clinic‐based mains powered machines or portable battery powered stimulators with a seemingly infinite combination of current types, waveforms, frequencies, intensities, electrode types and placements. Without a clear biological rationale it is difficult to make choices about different ways of delivering EStim. Additional confusion is created by the relatively rapid developments in the area of EStim, and a wide variety of stimulation devices and protocols have been developed even for the same condition" (ICI 2013).
Evidence from a systematic review has suggested that, in men, ES with non‐implanted devices may be more effective than sham treatment for urinary incontinence and that ES might enhance the effectiveness of pelvic floor muscle training in the short term (Berghmans 2013). Other evidence suggests that ES is more effective than sham, placebo or no active intervention for treating overactive bladder and urgency urinary incontinence, but the quality of evidence identified was generally low (Stewart 2016a). It is not yet clear whether ES has similar effects in women with SUI.
Why it is important to do this review
ES has shown promise in the treatment of UUI, but the evidence base for its use in SUI is inconclusive (Schreiner 2013). Given the adherence issues with conservative treatment, the side effects of drug therapy and the safety concerns regarding some kinds of surgical intervention, it is important to investigate alternative options for women with SUI.
Many randomised controlled trials (RCTs) have been undertaken investigating ES for SUI, compared to a variety of conservative interventions for SUI such as pelvic floor muscle exercises, drug therapy, vaginal cones, sham ES and no active treatment. Some trials have found no evidence of a difference in treatment effect, while others have found ES to be more effective than a comparator intervention. Given the heterogeneity of ES treatments, it is important to attempt to synthesise the available evidence relating to the diverse ES devices and protocols. Previous publications have synthesised some of the earlier evidence relating to ES for SUI (ICI 2013; Imamura 2010), but with a growing number of trials addressing this question, an up‐to‐date and comprehensive systematic review is needed to obtain the best possible estimate of the effectiveness of ES.
Objectives
To assess the effects of electrical stimulation with non‐implanted devices, alone or in combination with other treatment, for managing stress urinary incontinence or stress‐predominant mixed urinary incontinence in women. Among the outcomes evaluated are costs and cost‐effectiveness.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel or cross‐over RCTs, quasi‐RCTs (RCTs in which allocation to treatment is by methods such as alternate medical records, date of birth, or other predictable methods) and cluster‐randomised trials.
To critically appraise and summarise current evidence on the cost effectiveness of ES, we included relevant health economics studies conducted alongside effectiveness studies that met the eligibility criteria for the effectiveness component of the review. This includes:
full economic evaluation studies of ES compared to other treatments (i.e. cost‐effectiveness analyses, cost‐utility analyses, cost‐benefit analyses);
partial economic evaluations of ES (i.e. cost analyses, cost‐description studies, cost‐outcome descriptions);
RCTs reporting more limited information, such as estimates of resource use or costs associated with ES.
Types of participants
Eligible studies included adult women (18 years or older, or according to study authors' definitions of adult) with SUI or stress‐predominant MUI on the basis of symptoms, signs or urodynamic diagnosis. We used the trialists' definitions to classify women with SUI or stress‐predominant MUI.
We excluded studies in women with urgency‐predominant MUI, UUI only, or incontinence associated with a neurologic condition or frailty. We also excluded studies in men and women that did not report data separately by sex and studies including only men or children. We included trials of participants with MUI, UUI and SUI only if the data for women with SUI were presented separately. We included trials in women with MUI if the condition was SUI‐predominant.
Types of interventions
Eligible interventions included any method of delivering electrical stimulation with non‐implanted devices (see Table 12 and Characteristics of included studies for details of methods used). These devices could be placed in the vagina or anus or on a skin surface, but we excluded those that penetrated the skin or had to be placed surgically, which a different Cochrane Review covers (Herbison 2009). Health professionals or participants themselves could administer the treatment in any setting.
1. Description of electrical stimulation interventions.
| Study | Current | Current intensity | Pulse shape & duration | Frequency (Hz) | Duty cycle | Electrodes | Treatment duration/supervision |
| Aaronson 1995 | Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal | Unclear |
| Abel 1997 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Alves 2011 | Unclear | Maximum tolerable intensity | Biphasic 2000 Hz 100 ms | 50 | 4 s on: 8 s off | Intravaginal | Twice a week for 6 weeks (12 sessions) |
| Biphasic 2000 Hz 700 ms | |||||||
| Bernardes 2000 | Unclear | 10‐30 mA up to maximum tolerable intensity | Symmetrical bidirectional 1 ms | 60 | 6 s on: 12 s off | Intravaginal | 20 min daily for 10 days (10 sessions) |
| Beuttenmuller 2010 | Unclear | Maximum tolerable intensity | 0.2‐0.5 ms | 50 | Rest time at least twice the time of current | Intravaginal | Two 20 min sessions per week for 6 weeks (12 sessions) |
| Bidmead 2002 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Bø 1999 | Unclear | 0‐120 mA up to maximum tolerable intensity | 0.2 ms | 50 | 0.5‐10 s on: 0‐30 s off, adapted on basis of ability to hold voluntary contraction | Intravaginal | 30 min daily |
| Bourcier 1994 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Twelve 30 min sessions over 6 weeks (20 min maximal ES, 10 min EMG/pressure biofeedback) |
| Bridges 1988 | Unclear | Maximum tolerable intensity | Unclear | 0‐100 | Unclear | Unclear | Three 15 min session per week for 4 weeks (12 sessions) |
| Brubaker 1997 | Bipolar | 0‐100 mA | Bipolar square wave 0.1 µs | 20 | 2 s on: 4 s off | Intravaginal | 20 min daily for 8 weeks (56 sessions) |
| Castro 2008 | Bipolar | 0‐100 mA up to maximum tolerable intensity | Bipolar square wave 0.5 milliseconds | 50 | 5 s on: 10 s off | Intravaginal | Three 20 min session per week under supervision of trained physical therapist |
| Correia 2013 | Unclear | Maximum tolerable intensity | 700 µs | 50 | Unclear | 4 surface electrodes: 2 in the suprapubic region and 2 medial to the ischial tuberosity | Two 20 min session per week for 3 weeks (6 sessions) |
| Intravaginal | |||||||
| Correia 2014 | Unclear | Maximum tolerable intensity | 700 µs | 50 | 4 s on: 8 s off | 4 surface electrodes: 2 in the suprapubic region and 2 medial to the ischial tuberosity | Two 20 min session per week for 6 weeks (12 sessions) |
| Intravaginal | |||||||
| Delneri 2000 | Unclear | "According to the patient's sensations" | Unclear | 15 min: 20 15 min: 50 |
4 s on: 8 s off | Unclear | 12 x 30 min sessions on consecutive days, excluding Saturdays and Sundays. |
| Demirturk 2008 | Unclear | Unclear | Unclear | 0‐100 Hz | Unclear | 4 vacuum electrodes: 2 in the suprapubic region, 2 near to the medial side of the ischial tuberosity, crosswise | 3 x 15 min session per week for 5 weeks (15 sessions) |
| Edwards 2000 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Eyjolfsdottir 2009 | Unclear | Unclear | 200 µs | 50 | Unclear | Unclear | Unclear |
| Firra 2013 | Unclear | Unclear current, intensity according to participant tolerance | Unclear | 12.5 | 5 s on: 10 s off | Intravaginal | 14 x 30 min sessions |
| Goode 2003 | Biphasic | According to participant tolerance, up to 100 mA | Biphasic, 1 millisecond | 20 | 1 s on: 1 s off1 | Intravaginal | Home use, 15 min every second day for 8 weeks |
| Hahn 1991 | Unclear | Unclear | Unclear | 50 | Unclear | Intravaginal | Home use, 6‐8 hours per night for 12 months |
| Haig 1995 | Unclear | Unclear | Unclear | 10‐40 | Intravaginal | 20 min sessions, treatment for 3 months (unclear how many sessions) | |
| Henalla 1989 | Unclear | According to participant tolerance | Unclear | 0‐100 | Unclear | Unclear | 1 x 20 min session per week for 10 weeks (10 sessions) |
| Hofbauer 1990 | Unclear | Intensity increased until participant felt a contraction | Unclear | Unclear | 10 ms on: 15 ms off | Unclear | 3 sessions per week for 6 weeks (18 sessions) |
| Huebner 2011 | Unclear | 20−80 mA | Unclear | 50 | 8 s on: 15 s off | Intravaginal | 2 x 15 min sessions per day for 12 weeks |
| Jeyaseelan 1999 | Unclear | Up to 90 mA | Balanced, asymmetrical biphasic pulse width 250 µs | Background low frequency (to target slow twitch fibres), and intermediate frequency with initial doublet (to target fast twitch fibres) | 10 s on: 50 s off | Intravaginal | Home use (portable device), 1 hour daily for 8 weeks (except when menstruating) |
| Jeyaseelan 2002 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 1 hour daily for 8 weeks (except when menstruating) |
| Jeyaseelan 2003 | Unclear | Unclear | Unclear | Unclear | A range of frequencies in conjunction with a longer duty cycle than is traditionally used | Unclear | Unclear |
| Knight 1998 | Unclear | Low intensity, barely perceptible tingling sensation | Pulse width 200 ms | Preset frequencies of 10 Hz with bursts of 35 Hz to maintain fast twitch fibre activity | 5 s on: 5 s off | Intravaginal | Home use (3 hours per day) for 6 months, except during menstruation (overnight) |
| According to maximum participant tolerance | 35 | 16 x 30 min sessions in clinic | |||||
| Laycock 1988 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 2‐3 30 min sessions per week for 4‐6 weeks |
| Laycock 1993a | Unclear | According to maximum participant tolerance | Unclear | Three different frequencies: 10 min 1 Hz, 10 min 10‐40 Hz, 10 min 40 Hz | Unclear | Transcutaneous: one medium electrode placed over perineal body and a small electrode positioned immediately inferior to the symphasis pubis | 10 sessions; 1 x 15 min, 9 x 30 min |
| Laycock 1993b | Unclear | According to maximum participant tolerance | Unclear | Three different frequencies: 10 min 1 Hz, 10 min 10‐40 Hz, 10 min 40 Hz | Unclear | Transcutaneous: one medium electrode placed over perineal body and a small electrode positioned immediately inferior to the symphasis pubis | 10 sessions; 1 x 15 min, 9 x 30 min |
| Lo 2003 | Unclear | According to maximum participant tolerance | Unclear | 0‐100 | Unclear | Transcutaneous: 2 anterior flat electrodes placed over obturator foramen 1.5‐2 cm lateral to symphasis, 2 posterior electrodes placed medial to ischial tuberosities, either side of anus | One 15 min session, 11 x 30 min sessions. 3 sessions per week for 4 weeks (12 sessions) |
| Lopes 2014 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 3 x 30 min sessions per week at home |
| Luber 1997 | Unclear | 10−100 mA | 2 ms | 50 | 2 s on: 4 s off | Intravaginal | 2 x 15 min sessions per day for 12 weeks |
| Maher 2009 | Unclear | Unclear | Unclear | Unclear | Unclear | External electrodes | Home use, at least 4 x 30 min sessions per week for 8 weeks |
| Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal | Home use, at least 4 x 30 min sessions per week for 8 weeks | |
| Min 2015 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Olah 1990 | Unclear | 0−100 mA, up to maximum participant tolerance | Unclear | Unclear | Unclear | Transcutaneous: 2 electrodes placed on abdomen and 2 on inner thighs | 3 x 15 min sessions per week for 4 weeks |
| Oldham 2013 | Unclear | Pre‐programmed to increase intensity over 24 s to reach therapeutic level and switch off automatically after 30 min. All devices same level of stimulation (average intensity considered comfortable and capable of producing contractions of pelvic floor muscles) | Unclear | During the 10 s 'on time' the device delivers 10 repeats of a short high intensity burst of 50 Hz stimulation immediately preceded by a doublet (125 Hz), superimposed on continuous low frequency 2 Hz stimulation | 10 s on: 10 s off | Intravaginal ‐ single use tampon‐like Pelviva device | One disposable device per day for 12 weeks except during menstruation |
| Parsons 2004 | Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal | Home use |
| Patil 2010 | Unclear | According to maximum participant tolerance | Unclear | 0‐100 | Unclear | Surface ES: 2 flat electrodes placed anteriorly over obturator foramen, 1.5‐2cm lateral to the symphysis; 2 electrodes placed posteriorly medial to ischial tuberosity on either side of the anus | 1st session 15 min, if no ill effects then 30 min for all subsequent sessions. 3 times a week, for 4 weeks (12 sessions) under supervision of a physiotherapist. Participants were asked to perform 8‐12 pelvic floor contractions 3 times a day at home |
| Pereira 2012 | Unclear | According to maximum participant tolerance | Pulse width 700 µs | 50 | 4 s on: 8 s off | Surface ES: 2 electrodes in suprapubic region, 2 medial to the ischial tuberosity | 2 x 20 min sessions per week for 6 weeks (12 sessions). "The women were not instructed to perform the contraction of the pelvic floor muscles in conjunction with electrical stimulation" |
| Pohl 2004 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Preisinger 1990 | Unclear | Surging faradic‐type current | Unclear | Unclear | Unclear | Unclear | 3 x 10 min sessions per week for 10‐12 weeks |
| Sand 1995 | Unclear | Gradually adjusted amperage to 60‐80 mA or highest tolerable level | Unclear | Unclear | First 2 weeks: 5 s on: 10 s off. Weeks 3‐4: 5s: 5s; weeks 5‐6: 5 s: 10 s; weeks 7‐12: 5 s: 5 s | Vaginal electrode ( 2.6 cm diameter, 6.35 cm length) with electrode resistance 85 Ω | Women instructed to use device twice daily for 12 weeks. First 4 weeks: 15 min sessions. Weeks 5‐12: 30 min |
| Santos 2009 | Unclear | 10‐100 mA | 1 ms | 50 | Unclear | Intravaginal: electrode: 10cm long, 3.5 cm wide with double metallic ring and cylindrical shape, positioned in medium third of the vagina | 2 x 20 min sessions per week for 4 months |
| Schmidt 2009 | Unclear | Unclear | 300 μs | 50 | Unclear | Unclear | 12 weeks (unclear how many sessions or duration of sessions) |
| Seo 2004 | Unclear | Unclear | Unclear | "Simultaneous electrical stimulation of 35 Hz and 50 Hz for 24 secs" | Unclear | Unclear | 2 x 20 min sessions per week for 6 weeks (12 sessions) (plus biofeedback) |
| Shepherd 1984 | Unclear | Up to 40 v | Unclear | 10‐50 | Unclear | Maximum perineal stimulation: Scott electrode in vagina, large indifferent electrode under buttocks | Single 20 min session |
| Shepherd 1985 | Unclear | Unclear | Unclear | 10 | Unclear | Intravaginal cushion attached to stimulator worn around waist | Cushion worn for 8/24 hours, day or night according to participant preference |
| Smith 1996 | Biphasic | 5 mA ‐ 10 mA, increased each month to 80 mA max (range 1‐100) | Asymmetric balanced biphasic pulse, 300 μs, | Channel 1: 50 Hz; channel 2: 12.5 Hz | 5 s contraction time (range 5‐15), duty cycle 1‐2 (range 1 to 1 to 2) | Unclear | 16 weeks (unclear how many sessions); increasing treatment time from 15, 30, 45, 60 minutes |
| Tapp 1987 | Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal. Faradic stimulation using vaginal probe | 2 sessions per week for 1 month (session duration not reported) |
| Tapp 1989 | Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal. Faradic stimulation using vaginal probe | 2 sessions per week for 1 month (session duration not reported) |
| Terlikowski 2013 | Unclear | Unclear | 200‐250 μs | 10‐40 | 15 s; 30 s | Intravaginal | 2 x 20 min sessions per day at home for 8 weeks |
| Whitmore 1995 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Wilson 1987 | Unclear | According to maximum participant tolerance | Unclear | Unclear | Groups of 12 surges/min, 2 min rest in between each group | Faradism. Surface electrodes. Saddle shaped indifferent electrode placed over the sacrum, active electrode applied to perineum | 6 weeks' treatment (session duration not reported) |
| Unclear | 20−25 mA | Unclear | Unclear | 15 pulses at pressure peak 0.25‐0.30 Pa/cmb | Interferential. 4 suction electrodes (2 on abdomen, 2 on adductor muscles) | First treatment 10 min, if no ill effects then duration increased to 15 min | |
| Wise 1993 | Unclear | 0‐90 mA, according to participant tolerance | Unclear | 20 | Unclear | Intravaginal | 1 session per day (at home) for 6 weeks |
EMG: electromyography; ES: electrical stimulation.
We excluded trials of magnetic stimulation and electro‐acupuncture.
Eligible comparators were no active treatment, placebo or sham treatment as well as drug therapy, surgery or any other intervention intended to decrease SUI, including conservative treatment (such as complementary therapies like acupuncture, pelvic floor muscle training (PFMT) and vaginal cones). We also included studies comparing different ES methods. There were no restrictions by type of device, stimulation parameters (such as continuous, interrupted, duration of stimulation), duration of treatment, route of administration (vaginal, rectal, skin, pretibial area, etc.), or other similar factors. We excluded trials of different combinations of treatments if it was not possible to identify the effect of the ES intervention (e.g. ES plus another treatment versus other combined treatments).
We made the following comparisons.
ES versus no active treatment.
ES versus placebo or sham treatment.
ES versus other conservative treatment (e.g. bladder training, PFMT, biofeedback, magnetic stimulation).
ES versus drugs (e.g. duloxetine).
ES versus surgery or injection of bulking agents.
ES plus another treatment versus the other treatment alone.
One type of ES versus another.
We did not include studies where the comparator interventions, alone or as a supplement to ES, were different in the intervention and control arms (i.e. ES plus treatment A versus treatment B, with or without ES).
Types of outcome measures
We extracted outcome data reported at the end of treatment and at the end of the longest available follow‐up period. We considered the following outcomes.
Primary outcomes
Cure: number of women with self‐reported continence (no urinary incontinence, as reported by women)
Cure or improvement: number of women with self‐reported cure or improvement in urinary incontinence
Incontinence‐specific quality of life (QoL) measures (however defined by authors or by any validated measurement scales such as the International Consultation on Incontinence Questionnaire)
Secondary outcomes
Satisfaction with treatment
Need for further treatment
QoL measures of general health status, e.g. the 36‐item Short Form Health Survey (SF‐36); QoL measures of sexual function or satisfaction; measures of psychological or emotional well‐being
Quantification of symptoms (e.g. number of incontinence episodes (every 24 hours), number of micturitions every 24 hours, pad tests)
Adverse effects (e.g. skin or tissue damage, pain or discomfort, vascular, visceral or nerve injury, voiding dysfunction)
Economic data (e.g. costs of interventions, resource implications, cost‐effectiveness of interventions in terms of incremental cost‐effectiveness ratios (ICERs), costs per quality‐adjusted life year (QALY) or cost‐benefit ratios)
Tertiary outcomes
We extracted data related to the following assessments as indirect measures of the physiological effect of treatment.
Clinicians' observations (e.g. objectively measured cure, improvement or incontinence, such as observation of leakage, leakage observed at urodynamics study, urodynamic measurement parameters).
Pelvic floor muscle function, strength or ability to contract the pelvic floor muscles.
Any other outcomes judged important when performing the review.
Search methods for identification of studies
We did not impose any restrictions, for example language or publication status, on the searches described below.
Electronic searches
We drew on the search strategy developed for Cochrane Incontinence. We identified relevant trials from the Cochrane Incontinence Specialised Register. For more details of the search methods used to build the Specialised Register, please see the Group's module in the Cochrane Library. The register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE In‐Process, MEDLINE Epub Ahead of Print, ClinicalTrials.gov, WHO ICTRP and handsearching of journals and conference proceedings. Many of the trials in the Cochrane Incontinence Specialised Register are also contained in CENTRAL. The date of the last search for this review was 27 Feburary 2017.
The terms that we used to search the Cochrane Incontinence Specialised Register are in Appendix 1.
Economic data searches
We also undertook separate searches to identify studies examining the economic data of ES for SUI. Using the search strategies presented in Appendix 1, we searched the following databases on 10 February 2016. No limits were applied. The first four databases were searched via OvidSP and the last two were searched on their own websites.
Ovid MEDLINE (1946 to January week 4 2016).
Ovid MEDLINE In‐Process & Other Non‐Indexed Citations (covering to 9 February 2016).
Embase (1974 to 9 February 2016).
Health Management Information Consortium (HMIC) (1983 to 9 February 2016).
Cost‐Effectiveness Analysis Registry (CEA Registry) (from inception to 9 February 2016).
Research Papers in Economics (RePEc) (from inception to 9 February 2016).
Searching other resources
We checked the reference lists of the identified relevant studies for additional citations. We consulted with clinical specialists and contacted the authors of included trials where appropriate to obtain unpublished data or to seek clarification on ambiguous data in published trial reports.
Data collection and analysis
We conducted the review in accordance with the methods outlined in the published protocol unless otherwise stated in the Differences between protocol and review section (Stewart 2016b).
Selection of studies
Two review authors independently screened the trials identified by the literature search, resolving any disagreements by discussion or by referring to a third party.
Data extraction and management
Two review authors extracted data independently, resolving any disagreements by discussion or by referring to a third party. We used a standard data extraction form to extract data on study characteristics (design, methods of randomisation), participants, interventions and outcomes.
We would have developed a data extraction form for economic evaluations based on the format and guidelines used to produce structured abstracts of economic evaluations for inclusion in the NHS Economic Evaluation Database (NHS EED), according to the specific requirements of this review.
Assessment of risk of bias in included studies
We assessed risks of bias with the Cochrane 'Risk of bias' tool (Higgins 2011), which addresses the following kinds of bias.
Selection bias (randomisation and allocation concealment).
Performance bias (blinding of participants, caregivers).
Detection bias (blinding of outcome assessors).
Attrition bias (incomplete outcome data or differential withdrawal).
Reporting bias (selective reporting of outcomes).
Other bias.
Two review authors independently carried out risk of bias assessments and resolved any disagreements by consulting a third author.
We would have assessed the overall methodological quality of included economic evaluations by applying a combination of Consolidated Health Economics Evaluation Reporting Standards (CHEERS) statement (Husereau 2013) and CHEC Criteria list for assessment of methodological quality of economic evaluations (Evers 2005).
Measures of treatment effect
For dichotomous data, we calculated the risk ratio (RR) with a 95% confidence interval (CI). For continuous data, we present the mean difference (MD) with a 95% CI. We calculated the standardised mean difference (SMD) to combine trials that measure the same outcome but using different methods such as different quality of life instruments.
Unit of analysis issues
We analysed studies with multiple treatment groups by splitting the 'shared' group to create independent comparisons. For instance, we would analyse a trial comparing one kind of ES versus another kind of ES versus PFMT by splitting the PFMT group to create two smaller groups.
We would have analysed studies with non‐standard designs, such as cross‐over trials and cluster‐randomised trials, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Had data from randomised cross‐over trials been incomplete, we would have included data from the first period of randomisation only.
The unit of analysis was each woman recruited into the trials.
Dealing with missing data
We followed an intention‐to‐treat (ITT) principle as far as possible, analysing data from all participants according to the groups to which they were randomised. Where participants were excluded after allocation or withdrew from the trial, we reported any available details in full.
Where trials reported mean values without standard deviations (SDs) but with P values or 95% CIs, we used the Review Manager 5 (RevMan 5) calculator to estimate the SD (RevMan 2014). Where trials reported mean values only, we assumed the outcome to have an SD equal to the highest SD from the other trials within the same analysis.
We made all reasonable attempts to contact authors for clarification of missing data.
Assessment of heterogeneity
We assessed clinical heterogeneity by examining the trial methods and tested for statistical heterogeneity between trial results using the Chi² test and the I² statistic (Higgins 2011). We considered that heterogeneity may not be important if less than 30%, may be moderate if valued at 30% to 50%, and may be substantial if above 50%.
Assessment of reporting biases
We intended to assess the likelihood of potential publication bias using funnel plots, provided that we identified 10 or more eligible trials contributing to an outcome, but there were insufficient trials.
Data synthesis
We used the fixed‐effect model to analyse data. Where there was significant heterogeneity (for example I² higher than 50%), we computed pooled estimates of the treatment effect for each outcome using a random‐effects model.
We would have summarised the characteristics and results of included economic evaluations using additional tables, supplemented by a narrative summary to compare and evaluate methods used and principal results between studies. Unit cost data were be tabulated.
Subgroup analysis and investigation of heterogeneity
If data permitted, we intended to carry out the following subgroup analyses.
Population: trials with participants with SUI only versus participants with MUI.
Different approaches to electrode placement (transcutaneous (e.g. perineal skin, sacral) versus vaginal or anorectal).
If we found substantial heterogeneity (I² more than 50%), we investigated the possible causes and would have carried out subgroup analyses if appropriate.
Sensitivity analysis
If data permitted, we intended to perform sensitivity analysis comparing trials at low risk of selection bias to those at high risk of selection bias to test the robustness of the results, but there were insufficient numbers of trials in the meta‐analyses.
'Summary of findings' table
We applied the principles of the GRADE system to assess the quality of the body of evidence (Guyatt 2008). This approach uses four categories (very low, low, moderate and high) to rate the quality of evidence available for selected outcomes; for instance, evidence from RCTs starts at a level of high quality but may be downgraded if there are other indications of low quality, such as small sample sizes or high risk of bias.
We included the following outcomes in 'Summary of findings' tables.
Cure: number of women with self‐reported continence.
Improvement: number of women with self‐reported improvement in SUI (cured or improved).
QoL measures due to SUI.
Adverse effects: pain or discomfort due to treatment.
Cost‐effectiveness of interventions.
We used GRADEpro GDT 2015 software to create the 'Summary of findings' tables.
We pre‐specified seven comparisons, but in this review we present 11 'Summary of findings' tables because several of our pre‐specified comparisons were broad categories encompassing heterogeneous interventions (e.g. one type of ES versus another), and we considered it to be more meaningful to present 'Summary of findings' tables separately for each subcomparison.
Results
Description of studies
Results of the search
The electronic searches yielded 622 records, 99 of which we selected for full‐text screening. Fifty‐six studies (79 reports), involving 3781 randomised women, met the eligibility criteria for inclusion in the review. Additionally, there were 10 reports of 8 ongoing studies (see the Characteristics of ongoing studies). Figure 1 shows the flow of literature through the assessment process.
1.
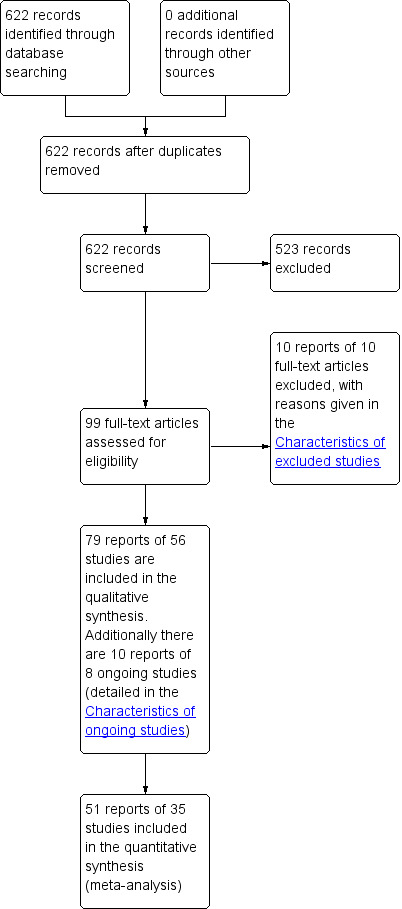
PRISMA study flow diagram
The searches for economic data yielded 215 records, 31 of which we selected for full‐text screening. However, none of these met our eligibility criteria.
Included studies
Design
All of the studies were randomised controlled trials.
Sample size
The sample sizes in the included trials ranged from 14 to 200 women (mean N = 67, median N = 56).
Setting
Most of the trials took place in hospital settings, with the exception of nine trials investigating types of ES for home or portable use (Goode 2003; Hahn 1991; Jeyaseelan 1999; Jeyaseelan 2002; Knight 1998; Lopes 2014; Maher 2009; Oldham 2013; Parsons 2004).
The included trials were based in the following countries.
Twenty in the UK (Bidmead 2002; Bridges 1988; Edwards 2000; Haig 1995; Henalla 1989; Jeyaseelan 1999; Jeyaseelan 2002; Jeyaseelan 2003; Knight 1998, Laycock 1988, Olah 1990; Oldham 2013; Parsons 2004; Patil 2010; Shepherd 1984; Shepherd 1985; Tapp 1987; Tapp 1989; Wilson 1987; Wise 1993).
Nine in Brazil (Alves 2011; Bernardes 2000; Beuttenmuller 2010; Castro 2008; Correia 2013; Correia 2014; Pereira 2012; Santos 2009; Schmidt 2009).
Seven in the USA (Brubaker 1997; Firra 2013; Goode 2003; Luber 1997; Sand 1995; Smith 1996; Whitmore 1995).
Two each in Austria (Hofbauer 1990; Preisinger 1990), France (Bourcier 1994; Lopes 2014), and Germany (Huebner 2011; Pohl 2004).
One each in Australia (Lo 2003), China (Min 2015), Denmark (Abel 1997), Iceland (Eyjolfsdottir 2009), Ireland (Maher 2009), Italy (Delneri 2000), Korea (Seo 2004), Norway (Bø 1999), Poland (Terlikowski 2013), Sweden (Hahn 1991), and Turkey (Demirturk 2008).
Three trials did not report any details on their setting (Aaronson 1995; Laycock 1993a; Laycock 1993b).
Participants
Almost all trials included only women with stress urinary incontinence.
Nine trials included women with other kinds of incontinence (Beuttenmuller 2010; Demirturk 2008; Goode 2003; Huebner 2011; Lo 2003; Lopes 2014; Schmidt 2009; Shepherd 1984; Shepherd 1985).
Three of these included some women with stress urinary incontinence alone and others with stress‐predominant MUI (Goode 2003; Huebner 2011; Lopes 2014).
Four trials did not separate data according to type of incontinence or excluded women with urgency urinary incontinence (Lo 2003; Schmidt 2009; Shepherd 1984; Shepherd 1985).
Two trials did not define the type of incontinence (Beuttenmuller 2010; Demirturk 2008).
One trial was restricted to women who had been referred for continence surgery (Hahn 1991).
Pereira 2012 and Goode 2003 restricted their inclusion criteria on the basis of age; over 60 years and over 40 years, respectively.
The mean age in the included trials ranged from 41 to 69 years. Fourteen trials did not report age (Bidmead 2002; Bourcier 1994; Jeyaseelan 1999; Jeyaseelan 2002; Jeyaseelan 2003; Knight 1998; Pohl 2004; Schmidt 2009; Shepherd 1984; Shepherd 1985; Tapp 1987; Tapp 1989; Whitmore 1995; Wise 1993).
Interventions
The included trials reported a range of different kinds of ES; most were intravaginal ES interventions, while others used surface electrodes. The intervention regimens were characterised by their wide diversity in terms of current, current intensity, pulse shape and duration, frequency (Hz), duty cycle, electrodes, and duration of treatment and its supervision. In most cases trialists failed to report at least one of these parameters. Table 12 shows the full details of the types, frequencies and parameters of the ES interventions.
Comparators included:
no active treatment (Correia 2013; Correia 2014; Bidmead 2002; Bø 1999; Castro 2008; Henalla 1989; Hofbauer 1990; Oldham 2013; Pereira 2012);
sham electrical stimulation (Abel 1997; Bidmead 2002; Brubaker 1997; Hofbauer 1990; Jeyaseelan 1999; Laycock 1993b; Luber 1997; Preisinger 1990; Sand 1995; Shepherd 1984; Shepherd 1985; Terlikowski 2013; Whitmore 1995); Table 13 presents details of the sham interventions;
placebo (Abel 1997);
pelvic floor muscle training (PFMT) (Aaronson 1995; Bernardes 2000; Bidmead 2002; Bø 1999; Bourcier 1994; Castro 2008; Demirturk 2008; Eyjolfsdottir 2009; Hahn 1991; Lo 2003; Hofbauer 1990; Huebner 2011; Jeyaseelan 2002; Jeyaseelan 2003; Pohl 2004; Preisinger 1990; Smith 1996).
vaginal cones (Bridges 1988; Bø 1999; Castro 2008; Delneri 2000; Olah 1990; Santos 2009; Seo 2004; Wise 1993);
PFMT plus vaginal cones (Bourcier 1994; Laycock 1993a; Wise 1993);
drug therapy (Abel 1997; Henalla 1989);
vaginal oestrogen cream (Henalla 1989).
2. Description of sham electrical stimulation interventions.
| Study | Description of sham interventiona |
| Brubaker 1997 | Identical device to the intervention group with disconnected wire so no electricity supplied |
| Hofbauer 1990 | Electrodes placed in the lumbar region |
| Jeyaseelan 1999 | One 250 μ impulse every minute for 60 min (proven to have no physiological effect on muscle) |
| Laycock 1993b; | Machine modified to bypass the patient circuit and divert the interferential current to a separate circuit within the machine so the participant received no current. Participants told to expect no sensation |
| Luber 1997 | Wiring from the unit to the probe was covertly discontinuous |
| Sand 1995 | Same system as intervention group but limited to maximum output 1 mA |
| Shepherd 1984 | Vaginal electrode but no current |
| Shepherd 1985 | Identical device to intervention group but not activated |
| Terlikowski 2013 | Women were provided with a placebo set to parameters proven to have no physiological effect |
aFour of 13 trials comparing ES with sham ES did not describe the sham intervention in detail.
Fifteen trials compared ES plus another treatment to the other treatment alone.
ES plus PFMT (Beuttenmuller 2010; Bidmead 2002; Edwards 2000; Firra 2013; Goode 2003; Haig 1995; Hofbauer 1990; Huebner 2011; Knight 1998; Jeyaseelan 2002; Jeyaseelan 2003; Parsons 2004; Patil 2010; Schmidt 2009; Tapp 1987; Tapp 1989).
ES plus behavioural training (Goode 2003).
ES plus surgery (Min 2015).
Six trials compared different types of ES to each other (Alves 2011; Correia 2013; Correia 2014; Knight 1998; Maher 2009; Wilson 1987).
The control group in Castro 2008 received a motivational phone call once a month for six months. In another trial, the control group received "any other therapy at the discretion of the investigator" (Lopes 2014). For the purposes of our review, we treated these two comparators as no active treatment.
Follow‐up
Five trials reported outcomes at more than one follow‐up point, usually once the end of the treatment period and again at a further follow‐up point.
Outcomes
Eleven of the included trials did not report any usable data suitable for analysis in this review (Aaronson 1995; Abel 1997; Bidmead 2002; Correia 2013; Lo 2003; Maher 2009; Parsons 2004; Shepherd 1984; Shepherd 1985; Whitmore 1995; Wise 1993).
Eighteen of the included trials did not report any usable data relating to our primary outcomes of woman‐reported cure or improvement, or incontinence‐specific quality of life. No trials provided information about sexual function or psychological or emotional well‐being. We did not identify any economic evaluations conducted alongside included trials.
The Characteristics of included studies provides further information.
Excluded studies
After full‐text screening, we excluded 43 trials from the review. The main reasons for exclusion were ineligible study design (i.e. non‐RCTs), ineligible population (i.e. participants did not have SUI) and ineligible interventions such as sacral neuromodulation with implanted devices or magnetic stimulation.
See the Characteristics of excluded studies for full details of the most important excluded studies.
Ongoing studies
We identified eight ongoing studies, investigating the following comparisons (two of the ongoing studies are three‐arm trials).
ES versus placebo (Robson 2013).
ES versus PFMT (Jha 2013; NCT02185235 2014).
ES versus vaginal cones (ACTRN12610000254099).
ES compared with kinesiotherapy (ACTRN12610000254099).
ES plus PFMT versus PFMT alone (Maher 2010).
ES version A plus PFMT compared with ES version B plus PFMT (Maher 2010).
One type of ES versus another (Maher 2010; NCT00762593 2006; NCT02423005 2015; Robson 2014).
Further details are available in the Characteristics of ongoing studies.
Risk of bias in included studies
Please see Figure 2 for a summary of the risk of bias in the included trials and Figure 3 for the results of the risk of bias assessment in each trial for each domain.
2.
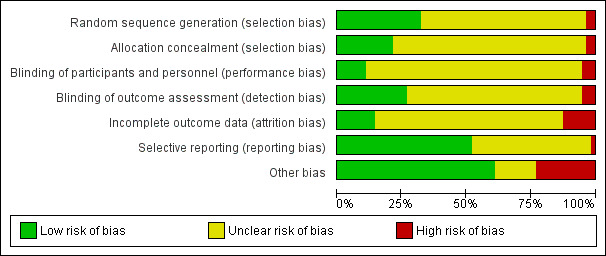
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
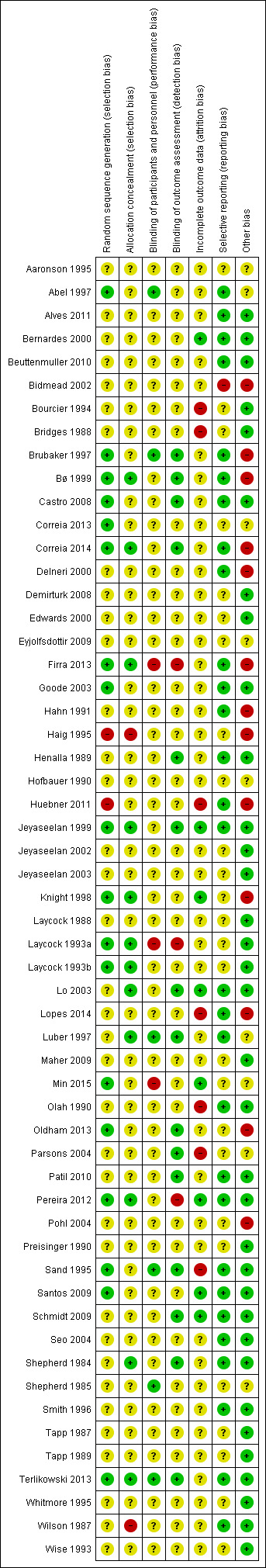
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Most trials (36/56) did not adequately report randomisation methods and so were at unclear risk of selection bias. Two trials were at high risk of selection bias because group allocation was not carried out on a truly randomised basis (Haig 1995; Huebner 2011). We judged the remaining trials (18/56) to have undertaken sufficiently robust randomisation procedures and considered them at low risk of selection bias.
Allocation concealment
We assessed two trials as being at high risk of selection bias because of inadequate allocation concealment (Haig 1995; Wilson 1987). Twelve trials reported adequate allocation concealment methods, meriting a judgment of low risk of selection bias, and the remainder did not report allocation methods in sufficient detail to make a clear determination.
Blinding
Blinding of participants and personnel
We judged three trials as being at high risk of performance bias because of inadequate blinding of participants (Firra 2013; Laycock 1993a; Min 2015). In many other cases, it was not possible to blind participants, and the risk of performance bias was unclear. Six trials reported adequate methods for blinding participants appropriately and were therefore at low risk of performance bias (Abel 1997; Brubaker 1997; Luber 1997; Sand 1995; Shepherd 1985; Terlikowski 2013).
Blinding of outcome assessment
Fifteen trials reported adequate blinding of outcome assessment (Brubaker 1997; Bø 1999; Castro 2008; Correia 2014; Henalla 1989; Jeyaseelan 1999; Lo 2003; Luber 1997; Oldham 2013; Parsons 2004; Patil 2010; Sand 1995; Schmidt 2009; Shepherd 1984; Terlikowski 2013). We considered three trials to be at high risk of detection bias because the outcome assessors were not blinded to the participants' group allocation (Firra 2013; Laycock 1993a; Pereira 2012). The remaining trials did not report blinding of outcome assessment in sufficient detail, and their risk of detection bias was therefore unclear.
Incomplete outcome data
Seven trials were at high risk of attrition bias for reasons such as differential withdrawal, unclear reporting of withdrawals per group and disparities between attrition data reported in the text and in the tables (Bourcier 1994; Bridges 1988; Huebner 2011; Lopes 2014; Olah 1990; Parsons 2004; Sand 1995). We judged eight trials to be at low risk of attrition bias because they had undertaken robust statistical methods for dealing with missing data, or they reported very low attrition in all groups (Bernardes 2000; Jeyaseelan 1999; Knight 1998; Lo 2003; Min 2015; Pereira 2012; Santos 2009; Schmidt 2009). The remaining trials did not report sufficient detail regarding attrition to make a clear determination on their risk of bias.
Selective reporting
We judged one trial to be at high risk of reporting bias because authors reported having collected data relating to symptom scores and quality of life outcomes, but the trial report did not include the details of the data (Bidmead 2002).
Twenty‐six trials did not report sufficient detail for us to judge their risk of reporting bias (Aaronson 1995; Bourcier 1994; Bridges 1988; Correia 2013; Demirturk 2008; Edwards 2000; Eyjolfsdottir 2009; Haig 1995; Hofbauer 1990; Jeyaseelan 2002; Jeyaseelan 2003; Knight 1998; Laycock 1988; Laycock 1993a; Laycock 1993b; Maher 2009; Min 2015; Oldham 2013; Parsons 2004; Pohl 2004; Preisinger 1990; Shepherd 1985; Tapp 1987; Tapp 1989; Whitmore 1995; Wise 1993). We judged the remaining trials to be at low risk of reporting bias because there was sufficient indication that they had reported all pre‐specified outcomes in full for each treatment group.
Other potential sources of bias
We judged 14 trials to be at high risk of bias for various reasons.
Technical problems with intervention equipment (Brubaker 1997).
Unclear role of funders likely to have vested interests in one of the interventions (Bø 1999; Eyjolfsdottir 2009; Hahn 1991; Lopes 2014; Oldham 2013).
Differences in intervention delivery procedures between the protocol and the trial report (Correia 2014).
Baseline differences between groups (Bidmead 2002; Delneri 2000; Firra 2013; Haig 1995; Huebner 2011; Knight 1998; Pohl 2004).
Some trials were reported only as abstracts with limited information, and we judged these to be at unclear risk of bias from other sources (Aaronson 1995; Abel 1997; Correia 2013; Parsons 2004; Shepherd 1985). The risk of other bias was also unclear in three non‐English language trials where only partial translation was available (Eyjolfsdottir 2009; Hofbauer 1990; Min 2015), plus one more that stopped early because interim analysis suggested no difference between groups (Luber 1997).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11
Summary of findings for the main comparison. Electrical stimulation versus no active treatment.
| Electrical stimulation versus no active treatment | ||||||
| Patient or population: women with stress urinary incontinence Setting: home and/or hospitals (Brazil, France, Norway, UK) Intervention: electrical stimulation Comparison: no active treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no active treatment | Risk with electrical stimulation | |||||
| Cure: number of women with self‐reported continence Follow‐up: mean 6 months | Study population | RR 2.31 (1.06 to 5.02) | 101 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | — | |
| 122 per 1000 | 283 per 1000 (130 to 615) | |||||
| Improvement: number of women with self‐reported improvement in SUI (cured or improved) Follow‐up: range 12 weeks to 9 months | Study population | RR 1.73 (1.41 to 2.11) | 347 (5 RCTs) | ⊕⊕⊝⊝ Lowb,c | — | |
| 382 per 1000 | 660 per 1000 (538 to 805) | |||||
| Incontinence‐specific quality of life (higher score = worse quality of life) assessed with: King's Health Questionnaire, Incontinence Severity Index, ICI‐Q Follow‐up: median 6 weeks | The mean incontinence‐specific quality of life score was 0.72 standard deviations lower (0.99 lower to 0.45 lower). | — | 250 (4 RCTs) | ⊕⊕⊕⊝ Moderated | A standard deviation of 0.80 represents a large difference between groups | |
| Adverse effects Follow‐up: range 6 weeks to 6 months | Study population | RR 5.96 (0.30 to 118.70) | 103 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,d | 2 trials reported no women with adverse effects in either group. 1 trial reported 2 women with adverse effects in the ES group (1 tenderness and bleeding, 1 discomfort) |
|
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Cost‐effectiveness | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ES: electrical stimulation; ICI‐Q: International Consultation on Incontience Questionnaire; RCT: randomised controlled trial; RR: risk ratio; SUI: stress urinary incontinence. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to very serious imprecision (small sample sizes, few events and wide confidence intervals around estimates of effect). bDowngraded one level due to serious risk of bias (manufacturer involved in some trials). cDowngraded one level due to serious imprecision (small sample sizes, few events and wide confidence intervals around estimates of effect). dDowngraded one level due to risk of serious risk of bias (detection, performance, attrition bias or manufacturers' involvement).
Summary of findings 2. Electrical stimulation versus sham treatment.
| Electrical stimulation versus sham treatment | ||||||
| Patient or population: women with stress urinary incontinence Setting: home and/or hospital (Austria, Denmark, Poland, UK, USA) Intervention: electrical stimulation Comparison: sham treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with sham treatment | Risk with electrical stimulation | |||||
| Cure: number of women with self‐reported continence Follow‐up: range 12 weeks to 6 months | Study population | RR 2.21 (0.38 to 12.73) | 158 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b | — | |
| 95 per 1000 | 210 per 1000 (36 to 1000) | |||||
| Improvement: number of women with self‐reported improvement in SUI (cured or improved) Follow‐up: range 12 weeks to 6 months | Study population | RR 2.03 (1.02 to 4.07) | 236 (5 RCTs) | ⊕⊕⊝⊝ Lowa | — | |
| 198 per 1000 | 402 per 1000 (202 to 805) | |||||
| Incontinence‐specific quality of life assessed with: IIQ, UDI, I‐QoL Follow‐up: range 8 weeks to 16 weeks | One trial found significantly better I‐QoL scores in the ES group, while another trial found no evidence of a difference between groups in IIQ or UDI scores. | — | 117 (2 RCTs) | ⊕⊕⊝⊝ Lowc | — | |
| Adverse effects Follow‐up: range 12 weeks to 6 months | Study population | RR 2.01 (0.52 to 7.67) | 233 (4 RCTs) | ⊕⊕⊝⊝ Lowc | 2 trials reported no women with adverse effects in either group. 2 trials reported vaginal irritation, bleeding and discomfort in the ES groups. |
|
| 23 per 1000 | 47 per 1000 (12 to 178) | |||||
| Cost‐effectiveness | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ES: electrical stimulation; IIQ: incontinence impact questionnaire; I‐QoL: Incontincence Quality of Life questionnaire; RCT: randomised controlled trial; RR: risk ratio; SUI: stress urinary incontinence; UDI: urogenital distress inventory. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to unclear risk of bias in most domains). bDowngraded one level due to serious imprecision (different directions of effect). cDowngraded two levels due to very serious imprecision (few trials and events, small sample sizes, wide confidence intervals around estimates of effect)
Summary of findings 3. Electrical stimulation versus pelvic floor muscle training.
| Electrical stimulation versus PFMT | ||||||
| Patient or population: women with stress urinary incontinence Setting: home and/or hospital (Austria, Brazil, France, Germany, Iceland, Norway, Sweden, Turkey, UK, USA) Intervention: electrical stimulation Comparison: pelvic floor muscle training (PFMT) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with PFMT | Risk with Electrical stimulation | |||||
| Cure: number of women with self‐reported continence Follow‐up: median 6 months | Study population | RR 0.51 (0.16 to 1.63) | 143 (4 RCTs) | ⊕⊕⊝⊝ Lowa,b | — | |
| 507 per 1000 | 259 per 1000 (81 to 826) | |||||
| Improvement: number of women with self‐reported improvement in SUI (cured or improved) Follow‐up: range 3 months to 4 years | Study population | RR 0.85 (0.70 to 1.03) | 244 (7 RCTs) | ⊕⊕⊝⊝ Lowa,b | — | |
| 669 per 1000 | 569 per 1000 (469 to 690) | |||||
| Incontinence‐specific quality of life assessed with: I‐QoL and unvalidated instrument Follow‐up: range 5 weeks to 6 months | None of the trials found any evidence of a difference between groups. | — | 93 (2 RCTs) | ⊕⊕⊝⊝ Lowa,c | — | |
| Adverse effects Follow‐up: range 5 weeks to 6 months | Study population | RR 5.00 (0.25 to 99.16) | 121 (3 RCTs) | ⊕⊕⊝⊝ Lowd | 2 trials reported no women with adverse effects in either group. 1 trial reported 2 women with adverse effects in the ES group (1 tenderness and bleeding, 1 discomfort) |
|
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Cost‐effectiveness | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ES: electrical stimulation; I‐QoL: Incontincence Quality of Life questionnaire; PFMT: pelvic floor muscle training; RCT: randomised controlled trial; RR: risk ratio; SUI: stress urinary incontinence. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to serious risk of bias (unclear risk of bias in most domains). bDowngraded one level due to serious imprecision (different directions of effect). cDowngraded one level due to serious imprecision (very small sample sizes). dDowngraded two levels due to serious imprecision (estimate based on single trial with very wide confidence intervals).
Summary of findings 4. Electrical stimulation versus vaginal cones.
| Electrical stimulation versus vaginal cones | ||||||
| Patient or population: women with stress urinary incontinence Setting: home and/or hospital (Brazil, Italy, Korea, Norway, UK) Intervention: electrical stimulation Comparison: vaginal cones | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with vaginal cones | Risk with electrical stimulation | |||||
| Cure: number of women with self‐reported continence Follow‐up: median 6 months | Study population | RR 1.04 (0.70 to 1.54) | 157 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | — | |
| 363 per 1000 | 454 per 1000 (341 to 606) | |||||
| Subjective cure or improvement Follow‐up: range 4 weeks to 6 months | Study population | RR 1.09 (0.97 to 1.21) | 331 (5 RCTs) | ⊕⊕⊝⊝ Lowb,c | — | |
| 685 per 1000 | 768 per 1000 (678 to 863) | |||||
| Incontinence‐specific quality of life assessed with: I‐QoL (range of possible scores: 0‐100) Follow‐up: range 4 months to 6 months | — | MD 1.59 higher (3.72 lower to 6.9 higher) | — | 96 (2 RCTs) | ⊕⊕⊝⊝ Lowc,d | Minimum clinically important difference between treatments is 2.5 points |
| Adverse effects Follow‐up: mean 6 months | Study population | RR 0.54 (0.11 to 2.70) | 52 (1 RCT) | ⊕⊕⊝⊝ Lowe,f | Adverse effects in the ES group:
Adverse effects in the vaginal cones group:
|
|
| 148 per 1000 | 80 per 1000 (16 to 400) | |||||
| Cost‐effectiveness | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ES: electrical stimulation; I‐QoL: Incontincence Quality of Life questionnaire; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to serious risk of attrition bias. bDowngraded one levels due to serious imprecision (few trials and events, small sample sizes, wide confidence intervals around estimates of effect). cDowngraded one level due to serious risk of bias (unclear risk of bias in most domains). dDowngraded one level due to serious imprevision (small sample sizes, wide confidence intervals around estimates of effect). eDowngraded one level due to serious risk of bias (manufacturer's funding and provision of intervention equipment). fDowngraded one level due to serious imprecision (single trial with small sample size).
Summary of findings 5. Electrical stimulation versus PFMT plus vaginal cones.
| Electrical stimulation versus PFMT plus vaginal cones | ||||||
| Patient or population: women with stress urinary incontinence Setting: home (UK) Intervention: electrical stimulation Comparison: PFMT plus vaginal cones | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with PFMT plus vaginal cones | Risk with electrical stimulation | |||||
| Cure: number of women with self‐reported continence Follow‐up: mean 6 weeks | Study population | RR 1.45 (0.96 to 2.20) | 123 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | — | |
| 333 per 1000 | 483 per 1000 (320 to 733) | |||||
| Subjective cure or improvement Follow‐up: mean 6 weeks | Study population | RR 1.53 (1.08 to 2.18) | 123 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | — | |
| 426 per 1000 | 652 per 1000 (460 to 929) | |||||
| Incontinence‐specific quality of life | Not reported | |||||
| Adverse effects | Not reported | |||||
| Cost‐effectiveness | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to serious risk of performance and detection bias. bDowngraded one level due to serious imprecision (small sample sizes, few events). cDowngraded one level due to serious inconsistency (different directions of effect).
Summary of findings 6. Electrical stimulation versus drug therapy.
| Electrical stimulation versus drug therapy | ||||||
| Patient or population: women with stress urinary incontinence Setting: home and hospital (UK) Intervention: electrical stimulation Comparison: drug therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with drug therapy | Risk with electrical stimulation | |||||
| Cure: number of women with self‐reported continence | Not reported | |||||
| Subjective cure or improvement Follow‐up: mean 9 months | Study population | RR 13.89 (0.84 to 230.82) | 50 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Incontinence‐specific quality of life | Not reported | |||||
| Adverse effects | Not reported | |||||
| Cost‐effectiveness | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to serious risk of bias (unclear risk of bias in most domains). bDowngraded two levels due to serious imprecision (single trial, small sample, wide confidence intervals around estimate of effect).
Summary of findings 7. Electrical stimulation plus PFMT versus PFMT.
| Electrical stimulation plus PFMT versus PFMT | ||||||
| Patient or population: women with stress urinary incontinence Setting: home and/or hospital (Australia, Brazil, UK, USA) Intervention: electrical stimulation plus PFMT Comparison: PFMT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with PFMT | Risk with Electrical stimulation plus PFMT | |||||
| Cure: number of women with self‐reported continence Follow‐up: range 9 weeks to 9 months | Study population | RR 0.76 (0.38 to 1.52) | 99 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | — | |
| 240 per 1000 | 182 per 1000 (91 to 365) | |||||
| Improvement: number of women with self‐reported improvement in SUI (cured or improved) Follow‐up: range 6 weeks to 9 months | Study population | RR 1.10 (0.95 to 1.28) | 308 (6 RCTs) | ⊕⊕⊝⊝ Lowa,c | — | |
| 639 per 1000 | 703 per 1000 (607 to 818) | |||||
| Incontinence‐specific quality of life (higher score = worse quality of life) assessed with: King's Health Questionnaire, IIQ−7, VAS for perceived effect of SUI on QoL Follow‐up: range 4 weeks to 6 months | The mean incontinence‐specific quality of life score was 0.35 standard deviations lower (0.64 lower to 0.05 lower) | — | 193 (4 RCTs) | ⊕⊝⊝⊝ Very lowc,d | A standard deviation of 0.20 represents a small difference between groups. 2 other trials found no evidence of a difference between groups (data unsuitable for meta‐analysis) |
|
| Adverse effects Follow‐up: mean 18 months | 1 trial reported 4/59 women in the ES + PFMT group with adverse effects (vaginal irritation) but did not report any data for the PFMT‐only group. | 133 (1 RCT) | ⊕⊕⊝⊝ Lowe,f | — | ||
| Cost‐effectiveness | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ES: electrical stimulation; IIQ: incontinence impact questionnaire; MD: mean difference; PFMT: pelvic floor muscle training; QoL: quality of life; RR: risk ratio; SUI: stress urinary incontinence; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to serious risk of bias (unclear risk of bias in all domains). bDowngraded one level due to serious imprecision (small sample sizes, wide confidence intervals around estimates of effect). cDowngraded one level: different directions of effect. dDowngraded two levels due to very serious risk of bias (high risk of selection bias and unclear risk in most other domains). eDowngraded one level due to serious risk of performance bias. fDowngraded one level due to serious imprecision (single trial, small sample size).
Summary of findings 8. Electrical stimulation plus surgery versus surgery.
| Electrical stimulation plus surgery versus surgery | ||||||
| Patient or population: women with stress urinary incontinence Setting: hospital (China) Intervention: electrical stimulation plus surgery Comparison: surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with surgery | Risk with electrical stimulation plus surgery | |||||
| Cure: number of women with self‐reported continence Follow‐up: mean 18 months | Study population | RR 1.19 (0.53 to 2.67) | 120 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — | |
| 750 per 1000 | 893 per 1000 (398 to 1000) | |||||
| Improvement: number of women with self‐reported improvement in SUI (cured or improved) Follow‐up: mean 18 months | Study population | RR 5.36 (0.61 to 47.36) | 120 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — | |
| 917 per 1000 | 1000 per 1000 (559 to 1000) | |||||
| Incontinence‐specific quality of life assessed with: I‐QoL and ICIQ‐SF Follow‐up: mean 18 months | Both I‐QoL and ICIQ‐SF scores suggested higher QoL when ES was added to surgery. | — | 120 (1 RCT) | ⊕⊕⊝⊝ Lowa,c | — | |
| Adverse effects Follow‐up: mean 18 months | Study population | RR 1.00 (0.37 to 2.72) | 120 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | 3 women in each group reported medial thigh pain. | |
| 150 per 1000 | 150 per 1000 (56 to 408) | |||||
| Cost‐effectiveness | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ES: electrical stimulation; ICIQ‐SF: International Consultation on Incontinence Questionnaire ‐ Short Form; I‐QoL: Incontincence Quality of Life questionnaire; PFMT: pelvic floor muscle training; RR: risk ratio; SUI: stress urinary incontinence. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to serious risk of performance bias. bDowngraded two levels due to very serious imprecision (single trial, wide confidence intervals around estimate of effect). cDowngraded one level due to serious imprecision (single trial).
Summary of findings 9. Surface ES versus intravaginal ES.
| Surface ES versus intravaginal ES | ||||||
| Patient or population: women with stress urinary incontinence Setting: hospital (Brazil) Intervention: surface ES Comparison: intravaginal ES | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with intravaginal ES | Risk with surface ES | |||||
| Cure: number of women with self‐reported continence | Not reported | |||||
| Improvement: number of women with self‐reported improvement in SUI (cured or improved) | Not reported | |||||
| Incontinence‐specific quality of life assessed with: King's Health Questionnaire (range of possible scores: 0‐100) Follow‐up: mean 6 weeks | — | MD 2.9 points higher (3.24 lower to 9.04 higher) | — | 30 (1 RCT) | ⊕⊕⊝⊝ Lowa | Lower score suggests greater quality of life in the intravaginal group |
| Adverse effects | Not reported | |||||
| Cost‐effectiveness | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ES: electrical stimulation; MD: mean difference; PFMT: pelvic floor muscle training; SUI: stress urinary incontinence. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to very serious imprecision (single trial, small sample, wide confidence intervals around estimate of effect).
Summary of findings 10. Low intensity ES plus PFMT versus maximal intensity ES plus PFMT.
| Low intensity ES plus PFMT versus maximal intensity ES plus PFMT | ||||||
| Patient or population: women with stress urinary incontinence Setting: home and hospital (UK) Intervention: low intensity ES plus PFMT Comparison: maximal intensity ES plus PFMT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with maximal intensity ES plus PFMT | Risk with low intensity ES plus PFMT | |||||
| Cure: number of women with self‐reported continence | Not reported | |||||
| Improvement: number of women with self‐reported improvement in SUI (cured or improved) Follow‐up: mean 12 months | Study population | RR 0.28 (0.09 to 0.91) | 49 (1 RCT) | ⊕⊕⊝⊝ Lowa | — | |
| 667 per 1000 | 187 per 1000 (60 to 607) | |||||
| Incontinence‐specific quality of life | Not reported | |||||
| Adverse effects ‐ not reported | Not reported | |||||
| Cost‐effectiveness ‐ not reported | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ES: electrical stimulation; MD: mean difference; PFMT: pelvic floor muscle training; SUI: stress urinary incontinence. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to very serious imprecision (single trial, small sample, wide confidence intervals around estimate of effect).
Summary of findings 11. Conventional ES plus PFMT versus dynamic ES plus PFMT.
| Conventional ES plus PFMT versus dynamic ES plus PFMT | ||||||
| Patient or population: women with stress urinary incontinence Setting: home (Germany) Intervention: conventional ES plus PFMT Comparison: dynamic ES plus PFMT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with dynamic ES plus PFMT | Risk with conventional ES plus PFMT | |||||
| Cure: number of women with self‐reported continence | Not reported | |||||
| Improvement: number of women with self‐reported improvement in SUI (cured or improved) (perception of bother of UI symptoms) assessed with: change in VAS Scale from: 0 to 10 Follow‐up: mean 12 weeks | — | MD 0.7 higher (0.83 lower to 2.23 higher) | — | 61 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | — |
| Incontinence‐specific quality of life assessed with: change in King's Health Questionnaire scores (range of possible scores: 0‐100) Follow‐up: mean 12 weeks | — | MD 4.1 points higher (1.43 higher to 6.77 higher) | — | 61 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | Scores indicate greater quality of life in the dynamic ES group |
| Adverse effects ‐ not reported | Not reported | |||||
| Cost‐effectiveness ‐ not reported | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ES: electrical stimulation; MD: mean difference; PFMT: pelvic floor muscle training; SUI: stress urinary incontinence; UI: urinary incontinence; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to very serious risk of bias (high risk of selection and attrition bias). bDowngraded one level due to serious indirectness (measures change in scores instead of actual scores). cDowngraded two levels due to very serious imprecision (single trial, small sample, wide confidence intervals around estimate of effect).
1. Electrical stimulation versus no active treatment
Nine trials (N = 903) compared electrical stimulation to no active treatment (Bidmead 2002; Bø 1999; Castro 2008; Correia 2013; Correia 2014; Henalla 1989; Oldham 2013; Parsons 2004; Pereira 2012). In addition, we considered Lopes 2014, which compared ES to "any other therapy at the discretion of the investigator" part of this comparison, bringing the total number of trials in this group to 10 (N = 1066).
Primary outcomes
Woman‐reported cure or improvement
In terms of women's assessment of SUI, there was moderate‐quality evidence to suggest that more women achieved cure with ES than with no active treatment (risk ratio (RR) 2.31, 95% confidence intervals (CI) 1.06 to 5.02; N = 101) (Bø 1999; Castro 2008; see Analysis 1.1, Table 1).
1.1. Analysis.
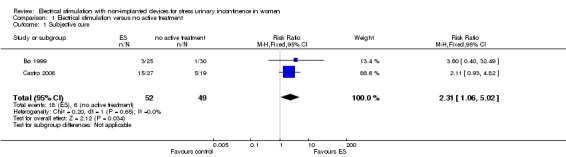
Comparison 1 Electrical stimulation versus no active treatment, Outcome 1 Subjective cure.
Similarly, ES was more effective than no active treatment when we considered self‐reported improvement together with cure (RR 1.73, 95% CI 1.41 to 2.11; 5 trials, N = 347 (Bø 1999; Castro 2008; Henalla 1989; Lopes 2014; Oldham 2013; see Analysis 1.2). However, the quality of evidence was low and heterogeneity high (I² = 83%; Table 1), possibly due to differences in participant population; one trial included participants who had either SUI or stress‐predominant MUI (Lopes 2014). Removing this trial from the analysis reduced heterogeneity to I² = 54%, and applying a random‐effects model changed the estimate of effect further in favour of ES (RR 4.66, 95% CI 1.50 to 14.45).
1.2. Analysis.
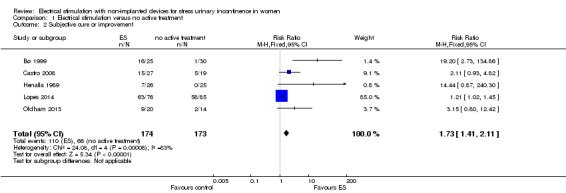
Comparison 1 Electrical stimulation versus no active treatment, Outcome 2 Subjective cure or improvement.
Furthermore, there were differences across the trials in terms of the participants' previous experiences of incontinence treatment; Lopes 2014 only included participants who had responded favourably to 10 to 15 sessions of perineal re‐education, whereas Oldham 2013 included participants who "do not seek or do not have access to supervised pelvic floor muscle exercise training with a health care professional". The remaining trials did not report this aspect of participant characteristics. If we remove both Lopes 2014 and Oldham 2013 from the analysis, heterogeneity remains high (I² = 70%). The result remains in favour of ES under a random‐effects model but with extremely wide 95% confidence intervals (RR 6.70, 95% CI 1.02, 43.84; N = 152).
Incontinence‐specific quality of life
Moderate‐quality evidence suggested that participants undergoing ES had higher incontinence‐specific QoL than those receiving no active treatment (standardised mean difference (SMD) ‐0.72, 95% CI ‐0.99 to ‐0.45; N = 230; Correia 2013; Correia 2014; Lopes 2014, Pereira 2012; see Analysis 1.3, Table 1). However, heterogeneity was high (I² = 80%), again probably due to the inclusion of Lopes 2014 (women with SUI or stress‐predominant MUI who had already responded favourably to perineal re‐education sessions). There was no heterogeneity after removing this trial from the analysis, and the estimate of effect was further in favour of ES, with relatively narrow confidence intervals (SMD −1.90, 95% CI −2.40 to −1.39).
1.3. Analysis.
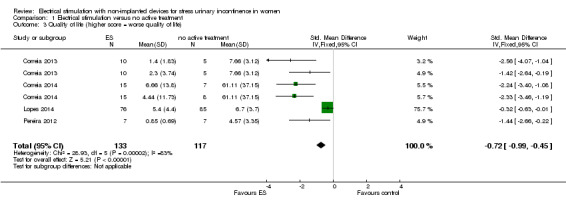
Comparison 1 Electrical stimulation versus no active treatment, Outcome 3 Quality of life (higher score = worse quality of life).
Additionally, another trial reporting data unsuitable for meta‐analysis found significantly higher incontinence‐specific QoL in the ES group than the no active treatment group (Castro 2008; N = 51; Table 14).
3. Electrical stimulation versus no active treatment.
| Study | Outcome | ES: mean (SD), N or n/N | No active treatment: mean (SD), N or n/N | Result |
| Primary outcomes | ||||
| Castro 2008 | I‐QoL scorea | 83.4 (12.1), 27 | 57.6 (28.2), 24 |
Favours ES MD 25.80 (95% CI 13.63 to 37.97) |
| Women with significant improvement in QoL | 9/27 | 0/24 |
Favours ES RR 16.96 (95% CI 1.04 to 276.81) |
|
| Secondary outcomes | ||||
| Bø 1999 | Women requesting further treatment in addition to the allocated intervention | 19/25 | 28/30 | RR 0.23 (95% CI 0.04 to 1.24) |
| Incontinence episodes per 24 hours | 0.3 (0.8), 27 | 1.3 (0.9), 24 |
Favours ES MD −1.00 (95% CI 1.47 to −0.53) |
|
| Tertiary outcomes | ||||
| Bø 1999 | Objective cure or improvement | 7/25 | 2/30 |
Favours ES Pooled RR 2.41 (95% CI 1.02 to 5.68) |
| Castro 2008 | 11/27 | 3/12 | ||
| Bø 1999 | Pelvic floor muscle strength (cmH2O) | 18.6 (13.52b), 25 | 16.0 (8.38b), 30 | MD 2.60 (95% CI −3.49 to 8.69) MD 4.73 (95% CI −3.92 to 13.38) Pooled MD 3.31 (95% CI −1.67 to 8.28) |
| Pereira 2012 | 14.57 (11.55), 7 | 9.84 (1.71), 7 | ||
| Castro 2008 | Women with negative urodynamic stress test | 11/27 | 3/12 | RR 2.79 (95% CI 0.62 to 12.60) |
| Pelvic floor muscle strength measured by PERFECTc | 2.9 (1.00), 27 | 2.3 (1.07), 24 |
Favours ES Pooled MD 0.84 (95% CI 0.55 to 1.14) MD 0.60 (95% CI 0.03 to 1.17) |
|
| Pereira 2012 | 1.71 (0.95), 7 | 1.14 (0.37), 7 | MD 0.57 (95% CI −0.19 to 1.33) | |
| Correia 2014 | Surface ES: 2.53 (0.83), 15 | 2.25 (0.86), 15 | MD 0.28 (95% CI −0.32 to 0.88) | |
| Intravaginal ES: 2.66 (0.81), 15 |
1.14 (0.37), 7 | MD 0.41 (95% CI −0.19 to 1.01) | ||
| Henalla 1989 | Maximum urethral closure pressure (cmH2O) | 26 (30.0b), 25 | 20 (19.60b), 24 | MD 6.00 (95% CI −8.13, 20.13) |
CI: confidence interval; ES: electrical stimulation; I‐QoL: Incontincence Quality of Life questionnaire; MD: mean difference; QoL: quality of life; RR: risk ratio; SD: standard deviation.
aHigher score = greater QoL. Range of possible scores: 0‐100. bImputed SD. cPower/pressure, Endurance, Repetitions, Fast contractions, Every Contraction Timed. Measure of vaginal muscle strength (higher score = stronger)
Secondary outcomes
Satisfaction with treatment
Not reported.
Need for further treatment
One trial found insufficient evidence to decide between the ES and control groups in terms of the number of women requesting further treatment (Bø 1999; see Table 14).
QoL measures of general health status
Not reported.
Quantification of symptoms
ES was better than no active treatment in terms of leakage measured by pad tests (SMD −0.71 g less urine lost with active treatment, 95% CI −1.11 to −0.31; N = 110; Analysis 1.4; Castro 2008; Correia 2013; Correia 2014; Pereira 2012), but it is unclear whether a difference of less than a gram of urine is of practical importance to women.
1.4. Analysis.
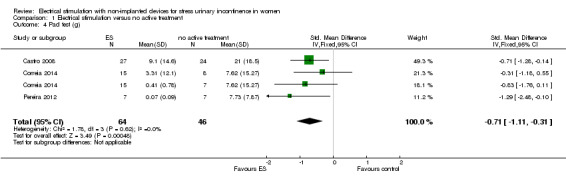
Comparison 1 Electrical stimulation versus no active treatment, Outcome 4 Pad test (g).
One trial found fewer incontinence episodes in the ES group than in the control group (Bø 1999; see Table 14).
Adverse effects
Very low‐quality evidence showed no difference between ES and no active treatment (RR 5.96, 95% CI 0.30 to 118.70; N = 103; Analysis 1.5; Table 1). Two of the three trials reporting adverse effects had no events in either group, so our meta‐analysis is based on a single small trial with two adverse events (1 tenderness and bleeding, 1 discomfort, both in women receiving active ES) in 25 women who had ES (Bø 1999). However, in total 2 of 103 (2%) women receiving active ES experienced an adverse event.
1.5. Analysis.
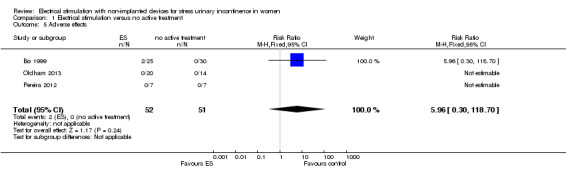
Comparison 1 Electrical stimulation versus no active treatment, Outcome 5 Adverse effects.
Economic data
Not reported.
Tertiary outcomes
Clinicians' observations
Pooled data from two trials suggested better rates of objective cure or improvement in women undergoing ES than no active treatment (Table 14).
There was insufficient evidence to decide between ES and no active treatment in terms of urodynamic stress tests or maximum urethral closure pressure.
Pelvic floor muscle outcomes
Pooled data from three trials suggested the ES groups had better pelvic floor muscle function than the no active treatment groups, measured by the PERFECT scale (Power/pressure, Endurance, Repetitions, Fast contractions, Every Contraction Timed) (Table 14).
2. Electrical stimulation versus sham treatment
Thirteen trials (N = 925) compared electrical stimulation to sham electrical stimulation (Abel 1997; Bidmead 2002; Brubaker 1997; Hofbauer 1990; Jeyaseelan 1999; Laycock 1993b; Luber 1997; Preisinger 1990; Sand 1995; Shepherd 1984; Shepherd 1985; Terlikowski 2013; Whitmore 1995).
Table 13 describes details of the sham interventions.
Primary outcomes
Woman‐reported cure or improvement
Very low‐quality evidence from three trials suggested a threefold increase in cure rates in the ES groups compared to sham ES (RR 3.14, 95% CI 1.28 to 7.68; analysis not shown). However, heterogeneity was high (I² = 62%), probably due to between‐group differences in direction of effect; one trial reported the unusual result of higher cure rates in the sham treatment group than in the active ES group (Luber 1997), so we applied a random‐effects model instead, which changed the overall estimate of effect to suggest there was little evidence of a difference (RR 2.21, 95% CI 0.38 to 12.73; see Analysis 2.1, Table 2).
2.1. Analysis.
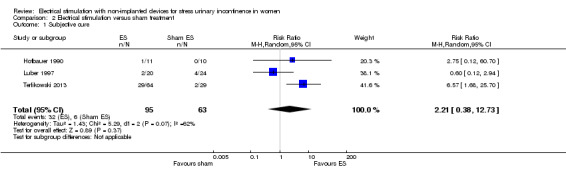
Comparison 2 Electrical stimulation versus sham treatment, Outcome 1 Subjective cure.
When we pooled data for improvement with data for cure, the result was less heterogeneous (I² = 42%) and favoured ES over sham ES (RR 2.03, 95% CI 1.02 to 4.07; 5 trials, N = 236). We deemed the quality of this evidence to be low (Hofbauer 1990; Laycock 1993b; Luber 1997; Terlikowski 2013; Whitmore 1995; see Analysis 2.2, Table 2).
2.2. Analysis.
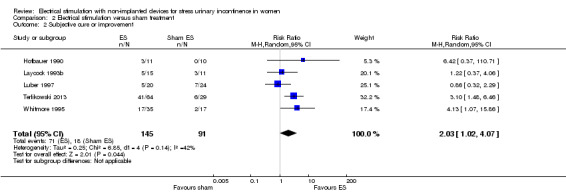
Comparison 2 Electrical stimulation versus sham treatment, Outcome 2 Subjective cure or improvement.
Three trials reported women's own assessment of a range of symptoms using visual analogue scales (Laycock 1993b; Sand 1995; Whitmore 1995). We could not include the data in a meta‐analysis, but overall the results favoured ES compared to sham ES (Table 15).
4. Electrical stimulation versus sham treatment.
| Study | Outcome | ES: mean (SD), N or n/N unless otherwise stated | Sham: mean (SD), N or n/N unless otherwise stated | Result |
| Primary outcomes | ||||
| Jeyaseelan 1999 | IIQ scorea | 28.42 (17.22), 12 | 30.11 (17.94), 12 | MD −1.69 (95% CI −15.76 to 12.38) |
| UDI scorea | 34.45 (25.25), 12 | 38.32 (11.75), 12 | MD −3.87 (95% CI −19.63 to 11.89) | |
| Terlikowski 2013 | I‐QoL scoreb | 80.8 (24.1), 64 | 50.6 (14.9), 29 |
Favours ES MD 30.20 (95% CI 22.18 to 38.22) |
| Sand 1995 | Subjective assessment of SUI severity (VAS) | 4.8 (NR), 28 | 6.3 (NR), 16 | Not estimable |
| Laycock 1993b | 2.9 (NR), 15 | 3.5 (NR), 11 | ||
| Whitmore 1995 | Subjective assessment of improvement in SUI (VAS) | NR | NR | Significantly greater improvement in group A (ES) than group B (sham ES) |
| Subjective assessment of improvement in frequency of urine loss (VAS) | NR | NR | ||
| Secondary outcomes | ||||
| Jeyaseelan 1999 | Incontinence episodes per week | Median (range), N: 0 (0 to 5), 7 | Median (range), N: 3 (0 to 17), 10 | Not estimable |
| 1 hour pad test (g) | Median (range), N: 5.0 (1 to 91.9), 12 | Median (range), N: 5.2 (0 to 75.0), 12 | Not estimable | |
| Tertiary outcomes | ||||
| Laycock 1993b | Objective cure or improvement | 11/13 | 5/9 |
Favours ES Pooled RR 3.32 (95% CI 1.89 to 5.84) |
| Luber 1997 | 3/20 | 3/24 | ||
| Preisinger 1990 | 3/11 | 0/10 | ||
| Terlikowski 2013 | 25/64 | 0/29 | ||
| Whitmore 1995 | 22/35 | 3/17 | ||
| Jeyaseelan 1999 | PFM strength (cmH2O) | 24 (13), 12 | 19 (6), 12 | MD −5.00 (95% CI −3.46 to 13.46) |
| Sand 1995 | Vaginal muscle strength (mmHg) | 15.5 (13.49c), 28 | 8.9 (5.75c), 16 |
Favours ES MD −6.60 (−12.34 to −0.86) |
| Terlikowski 2013 | Oxford scoreb | 8 weeks: 4.2 (NR), 64 16 weeks: 4.1 (NR) 64 |
8 weeks: 2.6 (NR), 29 16 weeks: 2.7 (NR), 29 |
Favours ES |
CI: confidence interval; ES: electrical stimulation; IIQ: incontinence impact questionnaire; I‐QoL: Incontincence Quality of Life questionnaire; MD: mean difference; NR: not reported; QoL: quality of life; PFM: pelvic floor muscle; RR: risk ratio; SD: standard deviation; SUI: stress urinary incontinence; UDI: urogenital distress inventory; VAS: visual analogue scale.
aHigher score = greater severity. IIQ range of possible scores: 0‐100. UDI range of possible scores: 0‐300. bHigher score = greater QoL.Range of possible scores: 0‐100. cImputed SD.
Incontinence‐specific quality of life
Low‐quality evidence from two trials provided inconclusive evidence regarding incontinence‐specific quality of life. Terlikowski 2013 found higher scores in the ES group than sham, measured by the Urinary Incontinence Quality of Life (I‐QoL) score, but Jeyaseelan 1999 found insufficient evidence to indicate an effect of ES, compared with sham ES, in terms of QoL, measured by the Incontinence Impact Questionnaire and Urogenital Distress Inventory (see Table 2, Table 15).
Secondary outcomes
Satisfaction with treatment
Not reported.
Need for further treatment
Not reported.
QoL measures of general health status
Not reported.
Quantification of symptoms
Women receiving ES had better outcomes than the sham ES groups in the following measurements.
Fewer incontinence episodes per 24 hours (MD −1.34 episodes, 95% CI −2.02 to −0.66; N = 181; Luber 1997; Sand 1995; Terlikowski 2013; see Analysis 2.3).
Fewer numbers of pads per week (MD −0.78 pads, 95% CI −1.23 to −0.33; N = 97; Sand 1995; Terlikowski 2013; see Analysis 2.5).
Pad tests (SMD −0.89 g less urine lost with active treatment, 95% CI −1.27 to −0.52; N = 137; Sand 1995; Terlikowski 2013; see Analysis 2.6).
2.3. Analysis.
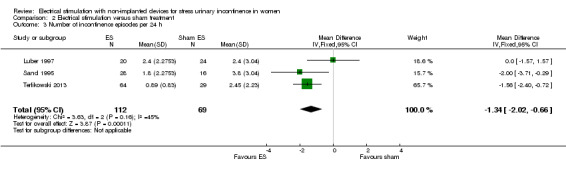
Comparison 2 Electrical stimulation versus sham treatment, Outcome 3 Number of incontinence episodes per 24 h.
2.5. Analysis.
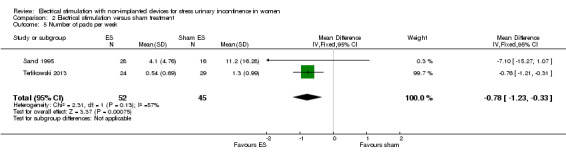
Comparison 2 Electrical stimulation versus sham treatment, Outcome 5 Number of pads per week.
2.6. Analysis.
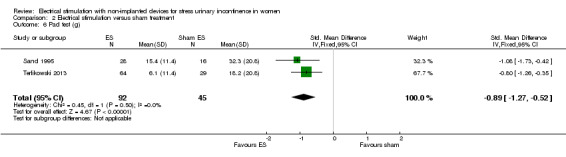
Comparison 2 Electrical stimulation versus sham treatment, Outcome 6 Pad test (g).
There was insufficient evidence to indicate an effect of ES, compared with sham ES, in terms of the number of micturitions per day (MD −0.46 micturitions, 95% CI −1.38 to 0.46; N = 163; Laycock 1993b; Sand 1995; Terlikowski 2013; see Analysis 2.4).
2.4. Analysis.
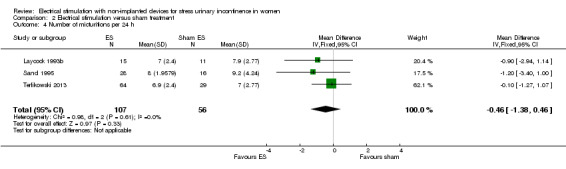
Comparison 2 Electrical stimulation versus sham treatment, Outcome 4 Number of micturitions per 24 h.
Data that we could not include in the meta‐analysis suggested fewer incontinence episodes per week in the ES group than the sham group and slightly less leakage measured by pad tests (Jeyaseelan 1999; Table 15).
Adverse effects
Very low‐quality evidence suggested there was no difference between the ES and sham ES groups in the number of women with adverse effects, but these were rare (nine with ES, two with sham treatment: RR 2.01, 95% CI 0.52 to 7.67; N = 233; Luber 1997; Sand 1995; Terlikowski 2013; Whitmore 1995; see Analysis 2.7, Table 2). Adverse effects in the ES groups included vaginal irritation, bleeding and discomfort.
2.7. Analysis.
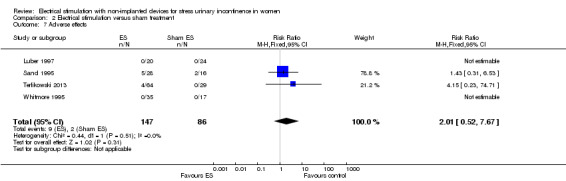
Comparison 2 Electrical stimulation versus sham treatment, Outcome 7 Adverse effects.
Economic data
Not reported.
Tertiary outcomes
Clinicians' observations
Pooled data from five trials reporting objective cure or improvement suggested ES was more effective than sham ES (RR 3.32, 95% CI 1.89 to 5.84, N = 232; Laycock 1993b; Luber 1997; Preisinger 1990; Terlikowski 2013; Whitmore 1995; see Table 15).
Pelvic floor muscle outcomes
Three trials reported various measures related to pelvic floor muscle function or strength, but the data were largely inconclusive (Jeyaseelan 1999; Terlikowski 2013; Sand 1995; see Table 15).
3. Electrical stimulation versus other conservative treatment
3.1 ES versus PFMT
Seventeen trials (N = 772) compared electrical stimulation to pelvic floor muscle training (PFMT) (Aaronson 1995; Bernardes 2000; Bidmead 2002; Bø 1999; Bourcier 1994; Castro 2008; Demirturk 2008; Eyjolfsdottir 2009; Hahn 1991; Lo 2003; Hofbauer 1990; Huebner 2011; Jeyaseelan 2002; Jeyaseelan 2003; Pohl 2004; Preisinger 1990; Smith 1996).
Primary outcomes
Woman‐reported cure or improvement
Low‐quality evidence from four trials indicated lower subjective cure rates reported by women in the ES group (21/72, 29%) compared with the PFMT group (36/71, 51%; RR 0.57, 95% CI 0.37 to 0.87; N = 143). However, heterogeneity was high (I² = 71%), possibly due to considerable variation in the PFMT treatment administered to the comparator groups, so we applied a random‐effects model, which altered the estimate of effect such that there was no longer any evidence of a difference (RR 0.51, 95% CI 0.16 to 1.63; N = 143; Bø 1999; Castro 2008; Hofbauer 1990; Smith 1996; see Analysis 3.1, Table 3).
3.1. Analysis.
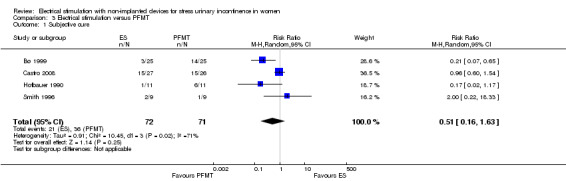
Comparison 3 Electrical stimulation versus PFMT, Outcome 1 Subjective cure.
Considering improvement together with cure reported by women, there was again low‐quality evidence suggesting no difference between ES and PFMT (RR 0.85, 95% CI 0.70 to 1.03; N = 244; Bø 1999; Castro 2008; Hahn 1991; Henalla 1989; Hofbauer 1990; Laycock 1988; Smith 1996). Again, heterogeneity was high (I² = 60%), probably due to differences in participant populations. Removing Smith 1996 and Hahn 1991, which included only women with SUI who were scheduled to undergo continence surgery, had little impact on heterogeneity (I² = 56%), nor did the estimate of effect alter significantly when applying a random‐effects model (RR 0.79, 95% CI 0.56 to 1.12; N = 206; see Analysis 3.2, Table 3).
3.2. Analysis.
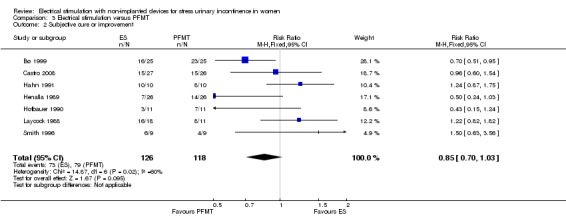
Comparison 3 Electrical stimulation versus PFMT, Outcome 2 Subjective cure or improvement.
One trial measured women's own assessment of symptoms on a 10‐point VAS, but the data were inconclusive (Pohl 2004; see Table 16).
5. Electrical stimulation versus PFMT.
| Study | Outcome | ES: mean (SD), N or n/N unless otherwise stated | PFMT: mean (SD), N or n/N unless otherwise stated | Result |
| Primary outcomes | ||||
| Castro 2008 | I‐QoL scorea | 83.4 (12.1), 27 | 82.2 (17.6), 26 | MD 1.20 (95% CI (−6.96 to 9.36) |
| Women with significant improvement in QoL | 9/27 | 7/26 | RR 1.24 (95% CI 0.54 to 2.83) | |
| Demirturk 2008 | Quality of life questionnaire score (non‐validated instrument)b | 22.5 (17.11c), 20 | 13.5 (11.41c), 20 | MD 9.00 (95% CI −0.01 to 18.01) |
| Pohl 2004 | Subjective assessment of SUI severity (VAS) | 4.81 (NR), 21 | 5.33 (NR), 10 | Not estimable |
| Secondary outcomes | ||||
| Castro 2008 | Incontinence episodes per 24 hours | 0.3 (0.8), 27 | 0.4 (0.5), 26 | MD −0.10 (95% CI −0.46 to 0.26) |
| Smith 1996 | Mean (range), N: 1.4 (0 to 5), 9 |
Mean (range), N: 2.4 (0 to 6), 9 |
Not estimable | |
| Bø 1999 | Women requesting further treatment in addition to the allocated intervention | 19/25 | 4/25 |
Favours PFMT RR 16.63 (95CI 4.06 to 68.04) |
| Smith 1996 | Women going on to have surgery | 2/9 | 3/9 | RR 0.57 (95% CI 0.07 to 4.64) |
| Pads per week | Mean (range), N: 4.0 (0−10), 9 | Mean (range), N: 5.4 (0−10), 9 | Not estimable | |
| Pohl 2004 | Pad test (g) (timescale not reported) | 6.21 (NR), 21 | 10.00 (NR), 10 | Not estimable |
| Tertiary outcomes | ||||
| Bernardes 2000 | Objective cure or improvement | 2/7 | 5/7 | Pooled RR 1.18 (95% CI 0.68 to 2.03 |
| Bø 1999 | 7/25 | 1/25 | ||
| Hahn 1991 | 4/10 | 1/10 | ||
| Preisinger 1990 | 3/11 | 7/11 | ||
| Smith 1996 | 4/9 | 3/9 | ||
| Bø 1999 | PFM strength (cmH2O) | 18.6 (13.52c), 25 | 19.2 (9.95c), 25 | MD −0.60 (95% CI −7.18 to 5.98) |
| Bernardes 2000 | Perineal contraction strength (0−5 scale; higher score = stronger contraction) | 1 (0.82), 7 | 8 (1.83), 7 |
Favours PFMT MD −2.00 (95% CI −3.49, −0.51) |
| Castro 2008 | Women with negative urodynamic stress test | 11/27 | 10/26 | RR 1.49 (95% CI 0.50 to 4.43) |
| Oxford scored | 2.9 (1.0), 27 | 3.6 (0.7), 26 |
Favours PFMT MD −0.70 (95% CI −1.16 to −0.24) |
|
| Pohl 2004 | 2.55 (NR), 21 | 2.7 (NR), 10 | Not estimable | |
| Jeyaseelan 2002 | Median (range): 13 (0−14) | Median (range): 11 (0−83 | ||
| Henalla 1989 | Maximum urethral closure pressure (cmH2O) | 26 (30.0c), 25 | 32 (20.40c), 26 | MD −6.00 (95% CI −20.13, 8.13) |
| Preisinger 1990 | Maximum urethral closure pressure (mmHg) | 42.6 (8.2), 11 | 41.4 (14.3), 11 | MD 1.20 (95% CI −8.54 to 10.94) |
CI: confidence interval; ES: electrical stimulation; I‐QoL: Incontincence Quality of Life questionnaire; MD: mean difference; NR: not reported; QoL: quality of life; PFM(T): pelvic floor muscle training; RR: risk ratio; SD: standard deviation; SUI: stress urinary incontinence; VAS: visual analogue scale.
aHigher score = greater QoL.Range of possible scores: 0‐100 bHigher score = worse QoL. cImputed data. dMeasure of vaginal muscle strength (higher score = stronger). Range of possible scores: 0‐5.
Incontinence‐specific quality of life
Low‐quality evidence, based on two trials reporting various measures relating to incontinence‐specific QoL, suggested there was no difference between the ES and PFMT groups (Castro 2008; Demirturk 2008; see Table 3, Table 16).
Secondary outcomes
Satisfaction with treatment
Not reported.
Need for further treatment
One trial found that more women in the ES group (19/25) than in the PFMT group (4/25) requested further treatment in addition to the allocated intervention (Bø 1999). Another trial reported the numbers of women going on to have continence surgery but found insufficient evidence to differentiate between the ES and PFMT groups (Smith 1996; see Table 16).
QoL measures of general health status
Not reported.
Quantification of symptoms
Pohl 2004 reported pad tests, and Smith 1996 reported the number of pads used per week. The data did not indicate any evidence of a difference between ES and PFMT in terms of quantification of symptoms (Table 16).
Adverse effects
Low‐quality evidence showed insufficient evidence of to indicate an effect of ES, compared with PFMT, in terms of the number of women with adverse effects (RR 5.00, 95% CI 0.25 to 99.16; N = 121). Two of the three trials reporting adverse effects had no events in either group (Demirturk 2008; Pohl 2004); our result is therefore based on two events (1 tenderness and bleeding, 1 discomfort, in 25 women having ES) in a single small trial (Bø 1999; see Analysis 3.3, Table 3). In total, 2/121 (2%) women had an adverse event.
3.3. Analysis.
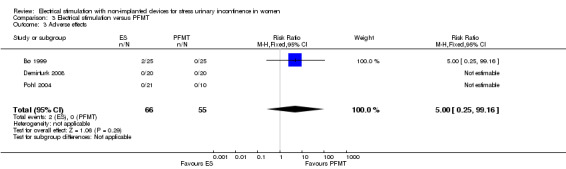
Comparison 3 Electrical stimulation versus PFMT, Outcome 3 Adverse effects.
Economic data
Not reported.
Tertiary outcomes
Clinicians' observations
Data from five trials suggested insufficient evidence of an effect of ES, compared with PFMT, in terms of the numbers of women cured or improved according to objective measures (Bernardes 2000; Bø 1999; Hahn 1991; Preisinger 1990; Smith 1996; see Table 16).
Pelvic floor muscle outcomes
Seven trials included a variety of pelvic floor assessments, most of which reported inconclusive data (Bø 1999; Bernardes 2000; Castro 2008; Pohl 2004; Jeyaseelan 2002; Henalla 1989; Preisinger 1990). Bernardes 2000 found that PFMT was better than ES in terms of perineal contraction strength, and Castro 2008 in terms of Oxford score (Table 16).
3.2 ES versus vaginal cones
Eight trials (N = 625) compared electrical stimulation to vaginal cones (Bridges 1988; Bø 1999; Castro 2008; Delneri 2000; Olah 1990; Santos 2009; Seo 2004; Wise 1993).
Primary outcomes
Woman‐reported cure or improvement
Low‐quality evidence from three trials suggested no difference in self‐reported cure rates in women receiving ES versus vaginal cones (RR 1.04, 95% CI 0.70 to 1.54; N = 157; Bø 1999; Castro 2008; Olah 1990; see Analysis 4.1, Table 4).
4.1. Analysis.
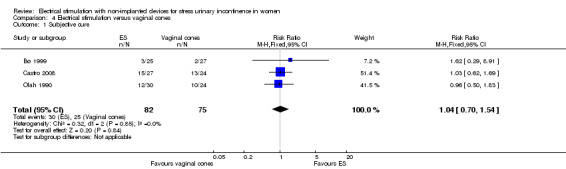
Comparison 4 Electrical stimulation versus vaginal cones, Outcome 1 Subjective cure.
Similarly, low‐quality evidence demonstrated little difference in numbers of women reporting cure or improvement with ES (171/218, 78%) compared with vaginal cones (135/197, 69%; RR 1.09, 95% CI 0.97 to 1.21; N = 331; Bridges 1988; Bø 1999; Castro 2008; Olah 1990; Seo 2004; see Analysis 4.2, Table 4).
4.2. Analysis.
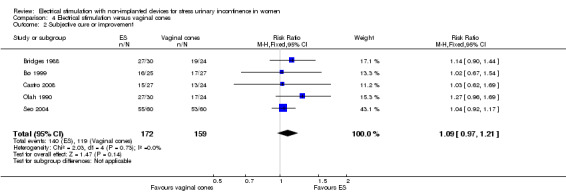
Comparison 4 Electrical stimulation versus vaginal cones, Outcome 2 Subjective cure or improvement.
Another trial measured women's assessment of SUI severity on a 10‐point VAS and found insufficient evidence to decide between ES and vaginal cones (Delneri 2000; see Table 17).
6. Electrical stimulation versus vaginal cones.
| Study | Outcome | ES: mean (SD), N or n/N | Vaginal cones: mean (SD), N or n/N | Result |
| Primary outcomes | ||||
| Castro 2008 | Women with significant improvement in QoL | 9/27 | 7/24 | RR 1.14 (95% CI 0.50 to 2.60) |
| Delneri 2000 | Subjective assessment of SUI severity (10 point VAS) | 5/10 | 5/10 | RR 1.00 (95% CI 0.17 to 5.77) |
| Secondary outcomes | ||||
| Olah 1990 | Women requiring continence surgery | 2/30 | 3/24 | RR 0.50 (95% CI 0.08 to 3.27) |
| Bø 1999 | Women requesting further treatment in addition to the allocated intervention | 19/25 | 23/27 | RR 0.55 (95% CI 0.14 to 2.24) |
| Adverse effects | 2/25 | 4/27 | RR 0.54 (95% CI 0.11 to 2.70) | |
| Olah 1990 | No leakage at 6 months | 11/28 | 10/19 | RR 0.58 (95% CI 0.18 to 1.89) |
| Weekly leakage (g) | 5.3 (9.2), 30 | 3.9 (9.4), 24 | MD 1.40 (95% CI −3.60 to 6.40) | |
| Tertiary outcomes | ||||
| Bø 1999 | Objective cure | 7/25 | 4/27 | RR 1.89 (95% CI 0.63 to 5.69) |
| Objective cure or improvement | 18/30 | 20/24 | Pooled RR 0.93 (95% CI 0.72 to 1.20) | |
| Bridges 1988 | 7/25 | 4/27 | ||
| Wise 1993 | 15/30 | 16/21 | ||
| Bø 1999 | PFM strength (cmH2O) | 18.6 (12.8a), 25 | 15.4 (11.40a), 27 | MD 3.20 (95% CI −3.62 to 10.02) |
| Castro 2008 | Women with negative urodynamic stress test | 11/27 | 9/24 | RR 1.55 (95% CI 0.51 to 4.74) |
| Oxford scoreb | 2.9 (1.0), 27 | 3.0 (0.8), 24 | MD −0.10 (95% CI −0.59 to 0.39) | |
| Seo 2004 | Maximum vaginal pressure (mmHg) | 33.64 (16.72), 60 | 27.20 (13.21), 60 |
Favours ES plus PFMT MD 6.44 (95% CI 1.05 to 11.83) |
| Maximum urethral pressure (mmH2O) | 77.93 (30.96), 60 | 78.38 (18.30), 60 | MD −0.45 (95% CI −9.55 to 8.65) | |
CI: confidence interval; ES: electrical stimulation; MD: mean difference; QoL: quality of life; PFM: pelvic floor muscle; RR: risk ratio; SD: standard deviation; SUI: stress urinary incontinence; VAS: visual analogue scale. aImputed SD. bMeasure of vaginal muscle strength (higher score = stronger).
Incontinence‐specific quality of life
Based on low‐quality evidence there appeared to be insufficient evidence to indicate an effect of ES, compared with vaginal cones, in terms of incontinence‐specific QoL, measured with I‐QoL (MD 1.59 points, 95% CI −3.72 to 6.90; N = 96; Castro 2008; Santos 2009; see Analysis 4.3, Table 4, Table 17). I‐QoL contains 22 items, whereby each item is rated on a 5‐point ordinal scale.
4.3. Analysis.
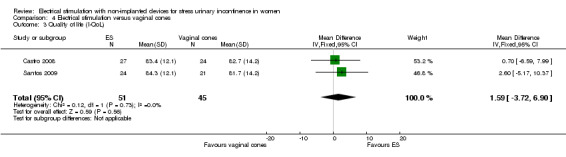
Comparison 4 Electrical stimulation versus vaginal cones, Outcome 3 Quality of life (I‐QoL).
Secondary outcomes
Satisfaction with treatment
Not reported.
Need for further treatment
Two trials reported insufficient evidence of an effect of ES, compared with vaginal cones, in terms of the numbers of women requiring continence surgery (RR 0.50, 95% CI 0.08 to 3.27; Olah 1990) or requesting further treatment in addition to the allocated intervention (RR 0.55, 95% CI 0.14 to 2.24; Bø 1999; see Table 17).
QoL measures of general health status
Not reported.
Quantification of symptoms
Pooled data from two trials indicated no difference between ES and vaginal cones in number of incontinence episodes per 24 hours (MD 0.10 episodes, 95% CI −0.13 to 0.33; N = 96; Castro 2008; Santos 2009; see Analysis 4.4).
4.4. Analysis.
Comparison 4 Electrical stimulation versus vaginal cones, Outcome 4 Number of incontinence episodes per 24 h.
Similarly, there was insufficient evidence to indicate an effect of ES, compared with vaginal cones, in terms of leakage measured by pad tests (MD 0.06 g of urine lost, 95% CI −0.20 to 0.31; N = 239; Delneri 2000; Olah 1990; Santos 2009; Seo 2004; see Analysis 4.5).
4.5. Analysis.
Comparison 4 Electrical stimulation versus vaginal cones, Outcome 5 Pad test (g).
Olah 1990 found insufficient evidence to indicate an effect of ES, compared with vaginal cones, in terms of the numbers of women with no leakage at all (RR 0.58, 95% CI 0.18 to 1.89) or in weekly leakage (MD 1.40 g of urine lost, 95% CI −3.60 g to 6.40 g; see Table 17).
Adverse effects
A single trial with low‐quality evidence reported insufficient evidence to indicate an effect of ES, compared with vaginal cones, with regard to adverse effects (Bø 1999; see Table 4, Table 17). Two of 25 women in the ES group reported adverse effects (1 tenderness and bleeding, 1 discomfort) compared with 4 of 27 in the vaginal cones group (1 abdominal pain, 2 vaginitis, 1 bleeding).
Economic data
Not reported.
Tertiary outcomes
Five trials reported a range of objective measures, including objective cure or improvement, pelvic floor muscle strength and Oxford score, but the data were inconclusive (Bø 1999; Bridges 1988; Castro 2008; Wise 1993; Seo 2004; see Table 17).
3.3 ES versus PFMT plus vaginal cones
Three trials (204 participants) compared electrical stimulation versus pelvic floor muscle training plus vaginal cones (Bourcier 1994; Laycock 1993a; Wise 1993).
Primary outcomes
Woman‐reported cure or improvement
Very low‐quality evidence from two trials found insufficient evidence to differentiate between ES and PFMT plus vaginal cones in terms of self‐reported cure alone (RR 1.45, 95% CI 0.96 to 2.20; N = 123; Bourcier 1994; Laycock 1993a; see Analysis 5.1; Table 5).
5.1. Analysis.
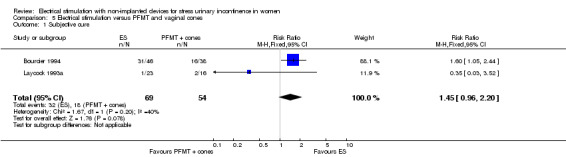
Comparison 5 Electrical stimulation versus PFMT and vaginal cones, Outcome 1 Subjective cure.
However, considering cure or improvement together, very low‐quality evidence from the same two trials suggested there may be better outcomes for women treated with ES than with PFMT plus vaginal cones (RR 1.53, 95% CI 1.08 to 2.18; N = 123; see Analysis 5.2, Table 5).
5.2. Analysis.
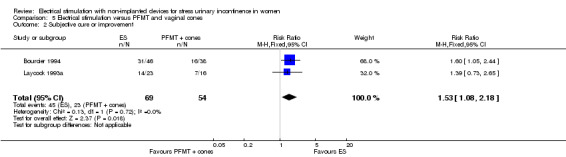
Comparison 5 Electrical stimulation versus PFMT and vaginal cones, Outcome 2 Subjective cure or improvement.
Incontinence‐specific quality of life
Not reported.
Secondary outcomes
Not reported.
Tertiary outcomes
One trial found insufficient evidence to differentiate between ES versus PFMT plus vaginal cones in terms of the numbers of women cured or improved according to pad tests (RR 0.80, 95% CI 0.45 to 1.43; Laycock 1993a; see Table 18).
7. Electrical stimulation versus PFMT plus vaginal cones.
| Study | Outcome | ES: mean (SD), N or n/N | PFMT plus vaginal cones: mean (SD), N or n/N | Result |
| Secondary outcomes | ||||
| Bourcier 1994 | Pad test (g) (timescale not reported) | 7.1 (NR), 52 | 11.5 (NR), 50 | Not estimable |
| Tertiary outcomes | ||||
| Bourcier 1994 | Urethral pressure profile (cmH2O) | 57 (NR), 38 | 45 (NR), 46 | Not estimable |
| Laycock 1993a | Objective cure or improvement | 10/20 | 10/16 | RR 0.80 (95% CI 0.45 to 1.43) |
CI: confidence interval; ES: electrical stimulation; MD: mean difference; NR: not reported; QoL: quality of life; PFMT: pelvic floor muscle training; RR: risk ratio; SD: standard deviation.
4. Electrical stimulation versus drug therapy
One trial (N = 100) compared electrical stimulation to oestrogen vaginal cream (Henalla 1989). A further trial testing the same comparison had too few participants with SUI (N = 9) for meaningful analysis (Abel 1997).
Primary outcomes
Woman‐reported cure or improvement
Very low‐quality evidence based on one trial suggested insufficient evidence to differentiate between ES and oestrogen vaginal cream in terms of cure or improvement reported by women (RR 13.89, 95% CI 0.84 to 230.082; N = 50; Henalla 1989; see Table 6, Table 19).
8. Electrical stimulation versus drug therapy.
| Study | Outcome | ES: mean (SD), N or n/N | Drug therapy: mean (SD), N or n/N | Result |
| Primary outcomes | ||||
| Henalla 1989 | Subjective cure or improvement | 7/26 | 0/24 | RR 13.89 (95% CI 0.84 to 230.82) |
| Tertiary outcomes | ||||
| Henalla 1989 | Maximum urethral closure pressure (cmH2O) | 26 (30.0a), 25 | 24 (19.60a), 24 | MD 2.00 (95% CI −12.13 to 16.13) |
CI: confidence interval; ES: electrical stimulation; MD: mean difference; RR: risk ratio; SD: standard deviation. aImputed SD.
Incontince‐specific quality of life
Not reported.
Secondary outcomes
Not reported.
Tertiary outcomes
Not reported.
5. Electrical stimulation versus surgery or injection of bulking agents
We did not identify any trials comparing ES to surgery or bulking agents.
6. Electrical stimulation plus another treatment versus the other treatment alone
6.1 ES plus PFMT versus PFMT
Sixteen trials (1248 participants) compared electrical stimulation plus PFMT to PFMT alone (Beuttenmuller 2010; Bidmead 2002; Edwards 2000; Firra 2013; Goode 2003; Haig 1995; Hofbauer 1990; Huebner 2011; Knight 1998; Jeyaseelan 2002; Jeyaseelan 2003; Parsons 2004; Patil 2010; Schmidt 2009; Tapp 1987; Tapp 1989).
Primary outcomes
Woman‐reported cure or improvement
Low‐quality evidence from three trials did not find that adding ES to PFMT resulted in higher self‐reported cure rates than PFMT alone (RR 0.76, 95% CI 0.38 to 1.52; N = 99; Eyjolfsdottir 2009, Hofbauer 1990; Tapp 1989; see Analysis 6.1, Table 7).
6.1. Analysis.
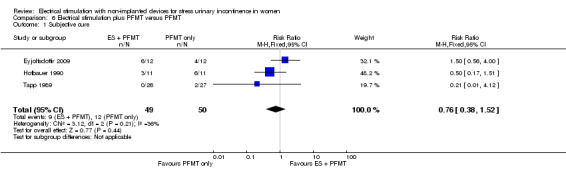
Comparison 6 Electrical stimulation plus PFMT versus PFMT, Outcome 1 Subjective cure.
Similarly, when comparing cure or improvement together, low‐quality evidence suggested little difference between ES plus PFMT versus PFMT alone (RR 1.10, 95% CI 0.95 to 1.28; N = 308; Eyjolfsdottir 2009, Goode 2003; Hofbauer 1990; Knight 1998; Tapp 1989; Wilson 1987; see Analysis 6.2, Table 7). Removing the one trial that included women with mixed urinary incontinence as well as SUI, Goode 2003, did not significantly alter the result (RR 1.20, 95% CI 0.92 to 1.57; N = 214).
6.2. Analysis.
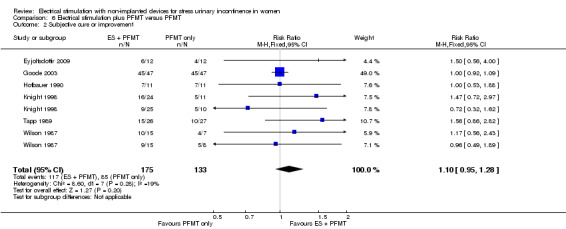
Comparison 6 Electrical stimulation plus PFMT versus PFMT, Outcome 2 Subjective cure or improvement.
In addition, three trials found that adding ES to PFMT was better than PFMT alone in terms of women's assessment of symptoms on visual analogue scales (SMD −0.57, 95% CI −0.90 to −0.24; N = 150; Haig 1995; Patil 2010; Tapp 1987; see Analysis 6.4).
6.4. Analysis.
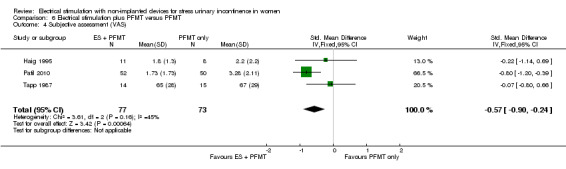
Comparison 6 Electrical stimulation plus PFMT versus PFMT, Outcome 4 Subjective assessment (VAS).
A further trial reported inconclusive data on women's assessment of their symptoms using a five‐point scale (Knight 1998; see Table 20).
9. Electrical stimulation plus PFMT versus PFMT only.
| Study | Outcome | ES plus PFMT: mean (SD), N or n/N unless otherwise stated | PFMT: mean (SD), N or n/N unless otherwise stated | Result |
| Primary outcomes | ||||
| Firra 2013 | York Incontinence Perception Scale scorea | 46.4 (7.2), 9 | 44.8 (6.3), 12 | MD 0.23 (95% CI −0.64 to 1.10) |
| Huebner 2011 | Change in perception of bother of UI symptoms (1‐10 VASb) | Conventional ES: −2.2 (3.2), 33 | −2.5 (2.1), 27 | MD 0.30 (95% CI −1.18 to 1.78) |
| Dynamic ES: −2.9 (2.9), 28 | MD −0.40 (95% CI −1.73 to 0.93) | |||
| Change in King's Health Questionnaire scoreb | Conventional ES: −20.7 (5.3), 33 | −20.2 (5.4), 27 | MD −0.50 (95% CI −3.22 to 2.22) | |
| Dynamic ES: −24.8 (5.3), 28 |
Favours ES MD −4.60 (95% CI −7.43 to −1.77) |
|||
| Jeyaseelan 2003 | % change in UDI score | Median (range), N: −32 (−50 to −18), 6 | Median (range), N: 0 (−43 to 180), 7 | Not estimable |
| % change in IIQ score | Median (range), N: −27 (−63 to 0), 6 | Median (range), N: 0 (−67 to 200), 7 | ||
| Secondary outcomes | ||||
| Goode 2003 | Women 'somewhat' or 'completely' satisfied | 46/47 | 46/47 | RR 1.00 (95% CI 0.06 to 16.47) |
| Women whose incontinence no longer restricts activities | 37/47 | 33/47 | RR 1.57 (95% CI 0.61 to 4.01) | |
| Knight 1998 | Subjective assessment of symptoms (1‐5 scale, higher score = better) | Low intensity ES at home plus PFMT: Mean (range), N: 3.3 (1 to 5), 19 |
Mean (range), N: 3.5 (2−5), 18 | Not estimable |
| Maximal ES in clinic plus PFMT: mean (range), N: 105.6 (−55.9 to 3.9 (3−5), 20 | ||||
| Schmidt 2009 | Incontinence episodes | Median (IQR), N: 12 weeks: 0 (0, 1), 11 6 months: 0.5 (0, 1.25), 11 |
Median (IQR), N: 12 weeks: 2 (0, 3), 11 6 months: 0 (0, 5.25), 11 |
Not estimable |
| Daytime micturitions | Median (IQR), N: 12 weeks: 5 (5, 6), 11 6 months: 4.5 (4, 6), 11 |
Median (IQR), N: 12 weeks: 7 (5, 10), 11 6 months: 2 (1, 3), 11 |
||
| Goode 2003 | Women with adverse effects | 4/59 | Unclear | Not estimable |
| Bidmead 2002 | Pad test (g) | 4.3 (NR), 88 | 4.2 (NR), 40 | |
| Knight 1998 | Low intensity ES plus PFMT: Median (range), N: 2.9 (0.0‐50.9), 19 |
Median (range), N: 0.8 (0.0‐88.1), 18 | ||
| Maximal intensity ES plus PFMT: Median (range), N: 1.5 (0.0‐28.1), 20 | ||||
| Tertiary outcomes | ||||
| Knight 1998 | Objective cure or improvement | Low intensity ES plus PFMT: 10/25 | 13/21 | RR 0.41 (95% CI 0.12 to 1.35) |
| Maximal intensity ES plus PFMT: 16/24 | RR 1.23 (95% CI 0.36 to 4.18) | |||
| Firra 2013 | PFM strength (cmH2O) | 36.7 (14.1), 9 | 32.5 (18.5), 12 | MD 0.24 (95% CI −0.63 to 1.11) MD −7.03 (95% CI −26.20 to 12.14) Pooled MD −0.04 (95% CI −0.64 to 0.57) |
| Schmidt 2009 | 41.85 (26.1), 11 | 48.88 (19.25), 11 | ||
| Eyjolfsdottir 2009 | Oxford scorec | 4.1 (0.9), 12 | 3.8 (1.4), 12 | MD 0.25 (95% CI −0.56 to 1.05) MD 0.48 (95% CI −0.03 to 1.00] MD 0.42 (95% CO −0.12 to 0.96) Pooled MD 0.39 (95% CI −0.01 to 0.79) |
| Huebner 2011 | Conventional ES plus PFMT: 1.9 (0.9), 33 | 1.5 (0.7), 27 | ||
| Dynamic ES plus PFMT: 1.8 (0.7), 27 | ||||
| Preisinger 1990 | Maximum urethral closure pressure (mmHg) | 49.9 (12.0), 11 | 41.4 (14.3), 11 | MD 8.50 (95% CI −2.53 to 19.53) |
| Wilson 1987 | Maximum urethral closure pressure at rest (cmH2O) | ES (faradism): 58.0 (15.4), 14 | 51.5 (10.5), 9 | MD 6.50 (95% CI −4.09 to 17.09) |
| ES (interferential) 56.0 (16.7), 12 | MD 4.50 (95% CI −7.18 to16.18) | |||
| Beuttenmuller 2010 | Contraction of pelvic floor at rest | 30.79 (7.44), 25 | 32.28 (7.33), 15 | MD −1.49 (95% CI −6.21 to 3.23) |
CI: confidence interval; ES: electrical stimulation; MD: mean difference; QoL: quality of life; PFM(T): pelvic floor muscle training; RR: risk ratio; SD: standard deviation; SUI: stress urinary incontinence; CI: confidence interval; ES: electrical stimulation; MD: mean difference; PFMT: pelvic floor muscle training; RR: risk ratio; SD: standard deviation.VAS: visual analogue scale. aHigher score = greater QoL. Range of possible scores: 8‐56. bHigher score = worse QoL. Range of possible scores: 0‐100 cMeasure of vaginal muscle strength (higher score = stronger). Range of possible scores: 0‐5.
Incontince‐specific quality of life
Very low‐quality evidence suggested that adding ES to PFMT improved incontinence‐specific QoL more than PFMT alone (SMD −0.35, 95% CI −0.64 to −0.05; N = 193; see Analysis 6.3, Table 7). Heterogeneity was high (I² = 87%), probably due to the between‐group differences in direction of effect. Removing from the analysis Beuttenmuller 2010, which included women with incontinence other than SUI, eliminated all evidence of heterogeneity (I² = 0%) and did not substantially alter the estimate of effect (SMD −0.77, 95% CI −1.11 to −0.42; N = 141).
6.3. Analysis.
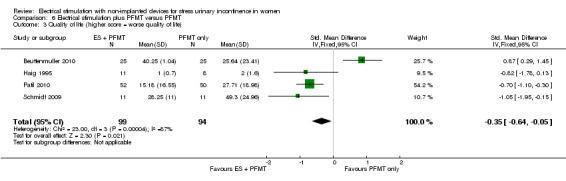
Comparison 6 Electrical stimulation plus PFMT versus PFMT, Outcome 3 Quality of life (higher score = worse quality of life).
Three trials reported other incontinence‐specific quality of life data, not suitable for meta‐analysis, but the data were largely inconclusive (Firra 2013; Huebner 2011; Jeyaseelan 2003; see Table 20).
Secondary outcomes
Satisfaction with treatment
One trial reported insufficient evidence of an effect of ES plus PFMT, compared with PFMT alone, in terms of numbers of women satisfied or whose incontinence no longer restricted their activities (RR 1.00, 95% CI 0.06 to 16.47; Goode 2003; see Table 20).
Need for further treatment
Pooled data from two trials suggested insufficient evidence of an effect of ES plus PFMT, compared with PFMT alone, in the number of women requesting surgery at the end of follow‐up (RR 0.91, 95% CI 0.59 to 1.41; N = 82; Tapp 1987; Tapp 1989; see Analysis 6.5).
6.5. Analysis.
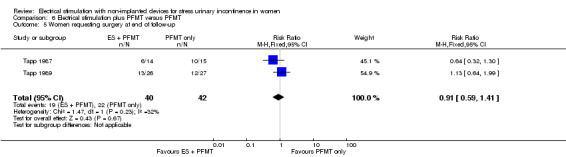
Comparison 6 Electrical stimulation plus PFMT versus PFMT, Outcome 5 Women requesting surgery at end of follow‐up.
QoL measures of general health status
Not reported.
Quantification of symptoms
Based on four trials, adding ES to PFMT resulted in fewer incontinence episodes than PFMT alone (MD −0.33 episodes, 95% CI −0.59 to −0.06; N = 275; Firra 2013; Goode 2003; Haig 1995; Patil 2010; see Analysis 6.6), but heterogeneity was high (I² = 59%), probably due to the inclusion in the analysis of a trial that included women with either SUI or stress‐predominant MUI (Goode 2003), in contrast to the other trials that included women with SUI only. When we removed this trial from the analysis there was no longer any evidence of heterogeneity (I² = 0%), and the effect size was greater, with narrower 95% confidence intervals (MD −0.52 episodes, 95% CI −0.82 to −0.21; N = 142). However, it is debatable whether this is a large enough reduction in frequency of incontinence to be clinically worthwhile.
6.6. Analysis.
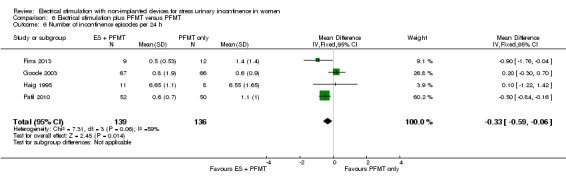
Comparison 6 Electrical stimulation plus PFMT versus PFMT, Outcome 6 Number of incontinence episodes per 24 h.
Furthermore, there was insufficient evidence of an effect of ES plus PFMT, compared with PFMT alone, in terms of:
micturitions per 24 hours (MD −0.13, 95% CI −1.46 to 1.20; N = 66; Firra 2013; Wilson 1987; see Analysis 6.7);
pad test (SMD −0.20, 95% CI −0.61 to 0.21; N = 346; Bidmead 2002; Haig 1995; Parsons 2004; Patil 2010; see Analysis 6.8).
6.7. Analysis.
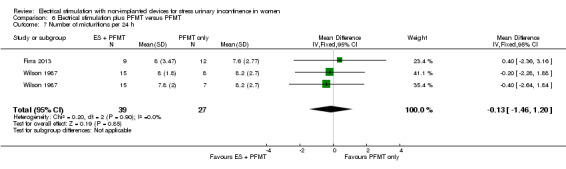
Comparison 6 Electrical stimulation plus PFMT versus PFMT, Outcome 7 Number of micturitions per 24 h.
6.8. Analysis.
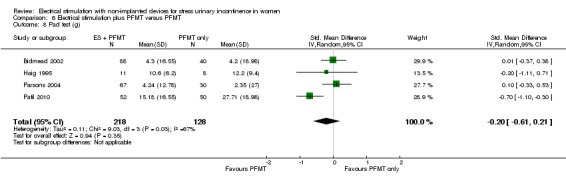
Comparison 6 Electrical stimulation plus PFMT versus PFMT, Outcome 8 Pad test (g).
Additionally, Schmidt 2009 reported inconclusive data, unsuitable for meta‐analysis, relating to incontinence episodes and micturitions per 24 hours, and Bidmead 2002 and Knight 1998 regarding pad tests (Table 20).
Adverse effects
One trial reported inconclusive data relating to the numbers of women with adverse effects (Goode 2003; see Table 20); 4/59 women who received both ES + PFMT experienced an adverse effect, but none in the group receiving PFMT alone did.
Economic data
Not reported.
Tertiary outcomes
Clinicians' observations
Data from one trial found insufficient evidence to differentiate between ES plus PFMT versus PFMT alone in terms of objective cure or improvement (Knight 1998; see Table 20).
Pelvic floor muscle outcomes
There was insufficient evidence to indicate an effect of ES plus PFMT, compared with PFMT alone, in terms of:
6.2 ES plus surgery versus surgery
One trial compared electrical stimulation added to surgery versus surgery alone (Min 2015).
Primary outcomes
Woman‐reported cure or improvement
Very low‐quality evidence from a single trial suggested no difference between ES plus surgery versus surgery alone in the numbers of women cured or improved according to their own assessment (RR 5.36, 95% CI 0.61 to 47.36; Min 2015; see Table 8, Table 21).
10. Electrical stimulation plus surgery versus surgery alone.
| Study | Outcome | ES plus surgery: mean (SD), N or n/N | Surgery: mean (SD), N or n/N | Result |
| Primary outcomes | ||||
| Min 2015 | Subjective cure | 43/60 | 45/60 | RR 1.19 (95% CI 0.53 to 2.67) |
| Subjective cure or improvement | 59/60 | 55/60 | RR 5.36 (95% CI 0.61 to 47.36) | |
| I‐QoL scorea | 96.0 (15.2), 60 | 89.0 (11.2), 60 |
Favours ES plus surgery MD 7.00 (95% CI 2.22 to 11.78) |
|
| ICIQ‐SF scoreb | 2.0 (2.5), 60 | 5.0 (3.1), 60 |
Favours ES plus surgery MD −3.00 (95% CI −4.01 to −1.99) |
|
| Secondary outcomes | ||||
| Min 2015 | Incontinence episodes per 24 hours | c4.3 (1), 60 | c3 (1.3), 60 |
Favours ES plus surgery MD −0.70 (95% CI −1.19 to −0.21) |
| Micturitions per 24 hours | c7.67 (1.67), 60 | c8 (1.3), 60 | MD −0.33 (95% CI −0.87 to 0.21) | |
| Pad test (g/h) | 1.8 (1.21), 60 | 3.0 (1.08), 60 |
Favours ES plus surgery MD −1.20 (95% CI −1.61 to −0.79) |
|
| Adverse effects: Urgency Dysuria Medial thigh pain |
d9/60 3/60 3/60 3/60 |
d9/60 4/60 2/60 3/60 |
RR 1.00 (95% CI 0.37 to 2.72) | |
CI: confidence interval; ES: electrical stimulation; ICIQ‐SF: International Consultation on Incontinence Questionnaire ‐ Short Form; I‐QoL: Incontincence Quality of Life questionnaire; MD: mean difference; SD: standard deviation.
aHigher score = greater QoL. Range of possible scores: 0‐100. bHigher score = worse QoL. Range of possible scores: 0‐21. cImputed from 72 hour data. dAssume one per woman.
Quality of life
Low‐quality evidence from a single trial suggested that adding ES to surgery resulted in higher incontinence‐specific quality of life, as measured with the I‐QoL and ICIQ‐SF instruments (Min 2015; see Table 8, Table 21).
Secondary outcomes
Satisfaction with treatment
Not reported.
Need for further treatment
Not reported.
QoL measures of general health status
Not reported.
Quantification of symptoms
Min 2015 found insufficient evidence to indicate an effect of ES plus surgery, compared with surgery alone, in terms of micturitions per 24 hours, pad tests or adverse effects. However, this trial also found that women receiving ES in addition to surgery had fewer incontinence episodes per 24 hours (Table 21).
Adverse effects
Not reported.
Economic data
Not reported.
Tertiary outcomes
Not reported.
7. One type of electrical stimulation versus another
Six trials (243 participants) compared different types of ES to each other (Alves 2011; Correia 2013; Correia 2014; Knight 1998; Maher 2009; Wilson 1987). Given the number of different comparisons made and the paucity of data for each comparison, we do not report further details here but do make them available in Appendix 2.
Discussion
Summary of main results
To the best of our knowledge, this is the first synthesis of the available evidence from randomised controlled trials investigating the effectiveness of electrical stimulation with non‐implanted devices compared to any other treatment for managing SUI in women.
Our results suggest that while ES shows promise in managing SUI compared to no active treatment or sham treatment, it may be no better than other conservative treatments in terms of clinical effectiveness or the risk of adverse effects. There was no information from the included trials on any of the prespecified economic data. There was not enough evidence about other comparators such as drugs or surgery, nor were the data sufficient to compare different methods of delivering ES.
Woman‐reported cure or improvement
Primary outcome data as well as evidence from symptom quantification and measures of pelvic floor function indicate that ES is likely to be more effective than no active treatment in self‐reported cure rates (Table 1).
ES may be more effective than sham treatment for cure or improvement reported by women (Table 2). Notwithstanding the low quality of the evidence comparing ES to sham treatment, data from measures relating to pelvic floor muscle function support this finding. However, the differences found in favour of ES compared with sham treatment in terms of symptom quantification outcomes, such as urine loss on pad tests and number of pads used, were so small that they are unlikely to have clinical importance to women.
We are uncertain if there is a difference between ES and PFMT (Table 3) or between ES vaginal cones (Table 4) for cure or improvement reported by women. The data from symptom quantification and pelvic floor muscle measures were similar to those relating to outcomes reported by women, that is, they were inconclusive regarding any differences between ES versus PFMT and between ES versus vaginal cones. Furthermore, the quality of evidence underlying these results is low. Overall, we are cautious in these conclusions because the evidence base largely consists of underpowered trials. Moreover, substantial heterogeneity in the PFMT protocols meant that it was difficult to compare trials of ES versus PFMT to each other in a meaningful way.
Similarly, low‐quality evidence indicates there may be little difference in cure or improvement rates reported by women with ES plus PFMT versus PFMT alone (Table 7). Again, secondary and tertiary outcomes, which indicate little evidence of a difference, support this conclusion.
We cannot draw any conclusions about women's assessment of cure or improvement from the following comparisons.
ES versus drug therapy.
ES versus PFMT plus vaginal cones.
ES plus surgery versus surgery alone.
Different types of ES versus each other.
Incontinence‐specific quality of life
Women receiving ES are likely to have better incontinence‐specific QoL than those not receiving any active treatment (Table 1). However, there may be little difference in incontinence‐specific QoL between women undergoing ES and those receiving sham ES or vaginal cones (Table 2; Table 4).
The low quality of evidence identified means we cannot be sure that adding ES to PFMT or surgery results in higher incontinence‐specific QoL than PFMT alone, nor can we conclude anything relating to incontinence‐specific QoL from the available evidence comparing ES to drug therapy or to other types of ES.
It is difficult to determine whether any improvement in QoL is directly attributed to reduced leakage, or whether there is a 'placebo' or psychological effect of having gone to seek treatment, being taken seriously by care providers, or being relieved at overcoming embarrassment in order to seek help.
Adverse effects
The low‐quality evidence means we cannot be certain about differences in adverse effects between ES and PFMT (Table 3); however, the paucity of data means we can draw no conclusions regarding adverse effects when comparing ES to:
no active treatment;
sham treatment;
vaginal cones;
PFMT plus vaginal cones; or
other types of ES.
Nor can we conclude anything about the risk of adverse effects when ES is added to PFMT or surgery.
Economic data
We did not identify any economic evaluations conducted alongside any of the included trials, so we cannot draw any conclusions relating to the prespecified economic outcomes from the evidence reviewed. Evidence from model‐based economic evaluations exists, but we did not include this in the review (see Potential biases in the review process).
Overall completeness and applicability of evidence
Insufficient evidence means we cannot draw any conclusions regarding the effectiveness or risk of adverse effects of the following.
ES compared to drug therapy.
ES compared to PFMT plus vaginal cones.
ES compared to surgery.
ES added to surgery compared to surgery alone.
Nor did we identify sufficient evidence regarding the effectiveness or safety of different types of ES. As noted above, we failed to identify any economic evidence.
Twenty trials did not report any data relating to our primary outcome of woman‐reported cure or improvement of stress urinary incontinence. We identified no evidence relating to the cost‐effectiveness of ES compared to any other treatments. Nine of the included trials met our inclusion criteria but did not report any usable data, often because they did not present results per treatment group. Some of these studies reported that their numbers of participants were too low to present any meaningful data, which is another indication of inadequately powered trials.
Readers should consider our analysis relating to woman‐reported cure or improvement of SUI with ES compared to no active treatment carefully. Because the comparator arm in Lopes 2014 received treatment at the discretion of the investigator but the treatment given was not reported, it is unclear how different this trial might be from the others included in the ES versus no active treatment comparison.
We considered pelvic floor muscle function as a tertiary outcome (an indirect measure of the physiological effects of treatment), but it was not possible to combine or summarise this information due to heterogeneity in the methods of measuring pelvic floor function (e.g. PERFECT, Oxford score, etc.). No trials addressed sexual function or psychological or emotional well‐being.
We have given descriptions of the exact ES intervention regimens in Table 12. Currently, there appears to be little agreement regarding the most effective ES parameters. Indeed, the active ES interventions were distinguished by their differences rather than similarities, and it was not possible to identify which, if any, of the ES regimens were more or less effective than any other. In general the trialists failed to specify one or more of the parameters they used; Yamoto 2016 have also drawn attention to the incomplete descriptions of the interventions in the context of physiotherapy trials.
Similarly, there was little agreement over what constituted a 'sham' intervention (Table 13). We chose to compare ES separately versus no active treatment and versus 'sham' treatment to differentiate between possible placebo effects. It is difficult to determine whether any differences are directly attributed to reduced leakage, or whether there is a placebo or psychological effect of having gone to seek treatment, being taken seriously by care providers, or being relieved at overcoming embarrassment in order to seek help.
A paucity of data meant that we were not able to conduct subgroup analyses amongst women with SUI only versus participants with MUI, nor could we evaluate different approaches to electrode placement (such as skin versus vaginal or rectal). The age of women included in the trials ranged from 41 to 69 years. Therefore the evidence may not be generalisable to other age groups.
Quality of the evidence
Despite the large number of trials identified and included in this review (56 trials in 3781 women), they were inadequately powered to detect clinically meaningful differences between interventions. Furthermore, the lack of detail on methods in the vast majority of the trials meant that when compiling the GRADE evidence profile we had to downgrade the evidence from many trials due to an unclear risk of bias. Additionally, we downgraded many trials as a result of their small sample sizes.
Overall, the relatively low quality of the body of evidence contributing to the results of this review does not lead to definitive answers to many of the research questions we set out to answer.
Potential biases in the review process
We made every attempt to minimise potential biases in our review processes, including our comprehensive search strategy, designed to minimise the risk of language and publication bias, and our system of independent screening for potentially eligible trials.
However, many of the included trials inadequately reported their methods and/or data, which made it difficult to judge the extent of potential biases and to make full use of the relevant data from the trials. This was especially problematic in instances where trialists inadequately described the comparator groups' treatment, most notably in Lopes 2014, which carried a considerable amount of weight in the meta‐analysis of ES versus no active treatment.
Agreements and disagreements with other studies or reviews
Our findings build on the recommendations from the International Continence Society, which found that ES may be more effective than no treatment but cautioned that there was insufficient evidence to draw any further conclusions (ICI 2013). The data we present indicate that future International Consultaions on Incontinence (ICI) may be able make further evidence‐based recommendations as a result of our findings.
The findings of the Schreiner 2013 review, investigating any kind of ES for any kind of urinary incontinence in women, were inconclusive with regard to non‐invasive ES for SUI (Schreiner 2013). Unlike Schreiner 2013, our review did not impose any language restrictions on the eligibility criteria, and we did not exclude studies based on the reporting of specific outcomes. Therefore we identified and assessed a more comprehensive evidence base, which lends weight to the conclusion that ES may be beneficial for SUI compared to no active treatment but that differences in effectiveness between ES and other treatments are still uncertain.
Imamura 2010 found little evidence of a difference in effectiveness between ES and PFMT, based on evidence from seven studies. We identified a further 11 trials and found similar results, that is, there may be no difference in effectiveness between ES and PFMT.
Authors' conclusions
Implications for practice.
We set out to answer the following questions:
Is electrical stimulation with non‐implanted devices clinically effective for stress urinary incontinence in women?
We found that ES is probably more effective than no treatment, placebo or sham in terms of women's assessment of cure or improvement.
Is electrical stimulation more effective than other conservative treatments?
Adding electrical stimulation to pelvic floor muscle training may not make much difference to cure or improvement of SUI. It is uncertain whether it offers any improvement in quality of life compared with pelvic floor muscle training. The evidence base is currently inadequate to draw conclusions regarding the effectiveness of ES versus drug therapy or ES versus PFMT plus vaginal cones.
Is electrical stimulation more effective than surgery?
We cannot tell from the available evidence if ES is more effective than surgery as there were no trials.
Is one kind of electrical stimulation more effective than other?
Again, we cannot tell from the evidence whether one kind of electrical stimulation is more effective than another.
Is electrical stimulation safe?
Gaps in the evidence base mean that we cannot say with any certainty what the risk of adverse effects may be, but no trials reported serious adverse effects.
Is electrical stimulation cost‐effective?
Given the lack of evidence we cannot say how electrical simulation compares to other therapies in terms of resources use, costs and cost‐effectiveness.
Implications for research.
Much larger trials are needed to ascertain whether there are clinically relevant differences between electrical stimulation and other treatments as the existing evidence is based on underpowered trials.
It is imperative that future trials measure clinically relevant outcomes, most importantly women's own assessment of symptomatic improvement and cure of urinary incontinence. Furthermore, future trials should collect data relating to adverse effects as we currently cannot even be sure if there is a lower risk with sham treatment than genuine electrical stimulation.
Although we did not identify any existing trials comparing ES to surgery, and only two trials comparing ES to drug therapy, it is vital to ascertain with confidence whether ES is more effective and safer than no treatment or sham/placebo treatment before progressing to more trials comparing ES to other active treatments. A large three‐arm trial could be considered, comparing ES to sham treatment and to adequate pelvic floor muscle training, ideally recording the level of adherence to PFMT regimens. Participants should be followed up beyond the end of the treatment period to ascertain whether a course of ES treatment has lasting effects. Cost‐effectiveness data on ES and its comparators should also be estimated to allow clinical decision‐makers to take economic data into account when considering treatment options.
However, of the eight ongoing trials we identified, comparing ES against various different interventions, including other types of ES with each other, very few have stated they are measuring women's subjective assessment of symptoms. Rather, the outcomes remain focused on objective measures such as stress tests or pad weights. Again, few trials have stated they will follow up participants after the end of treatment, and none have included any reference to cost‐effectiveness in their protocols. Given the improving evidence base for ES, investigators could consider performing an economic evaluation based on an updated systematic review and meta‐analysis. Target sample sizes appear to be generally higher than the completed trials we identified. Therefore, while these trials may help to clarify the effectiveness of ES compared to other treatments, they may not provide conclusive answers to the research questions posed in this review.
Acknowledgements
The authors are grateful to Jo Booth, Jean Hay‐Smith, Jennifer Hislop, Priya Kannan, Emily Karahalios, Mary Kilonzo, Doreen McClurg and Fiona Tito Wheatland for valuable comments on drafts of this review. We would also like to thank Suzanne Macdonald, Imran Omar, Sheila Wallace and Luke Vale for their support, and the author of one trial for extra information (Oldham 2013).
We would also like to thank Miriam Brazzelli, Mette Frahm Olsen, Beatriz Gualao, Anna Sierawska and Gavin Stewart for help with translations.
Appendices
Appendix 1. Search strategies
Cochrane Incontinence Group Specialised Register
The terms used to search the Cochrane Incontinence Specialised Register are given below:
(({DESIGN.CCT*} OR {DESIGN.RCT*}) AND {INTVENT.PHYS.ELECTSTIM*} AND {TOPIC.URINE.INCON*})
(All searches were of the keyword field of Reference Manager 2012). The date of the last search was 27 February 2017.
Cost‐effectiveness searches
The following databases were searched on 10 February 2016:
Ovid MEDLINE (1946 to January week 4 2016)
Ovid MEDLINE In‐Process & Other Non‐Indexed Citations (covering to 9 February 2016)
Embase (1974 to 2016 February 09)
Health Management Information Consortium (HMIC) (1983 to 9 February 2016)
Cost‐Effectiveness Analysis Registry (CEA Registry) (from inception to 9 February 2016)
Research Papers in Economics (RePEc) (from inception to 9 February 2016).
Ovid MEDLINE (covering 1946 to January week 4 2016), Ovid MEDLINE In‐Process & Other Non‐Indexed Citations (covering to February 09 2016) and Embase (covering 1974 to 2016 February 09) were searched using an OvidSP multifile search using the following search:
1. exp "costs and cost analysis"/
2. economics/
3. exp economics,hospital/
4. exp economics,medical/
5. economics,pharmaceutical/
6. exp budgets/
7. exp models, economic/
8. exp decision theory/
9. ec.fs.
10. monte carlo method/
11. markov chains/
12. exp health status indicators/
13. cost$.ti.
14. (cost$ adj2 (effective$ or utilit$ or benefit$ or minimis$)).ab.
15. economic$ model$.tw.
16. (economic$ or pharmacoeconomic$ or pharmaco‐economic$).tw.
17. (price$ or pricing).tw.
18. (financial or finance or finances or financed).tw.
19. ((value adj2 money) or monetary).tw.
20. markov$.tw.
21. monte carlo.tw.
22. (decision$ adj2 (tree? or analy$ or model$)).tw.
23. (standard adj1 gamble).tw.
24. trade off.tw.
25. or/1‐22
26. electrostimulation/
27. Electric Stimulation/
28. neuromodulation/
29. (electrical stimulation or neuromodulation or ((percutaneous or transcutaneous) adj4 stimulation)).tw.
30. or/26‐29
31. urine incontinence/ or mixed incontinence/ or stress incontinence/
32. urinary incontinence/ or urinary incontinence, stress/
33. ((stress or urinary) adj3 incontinence).tw.
34. or/31‐33
35. 25 and 30 and 34
36. 35 not (letter or comment$ or editorial or note).pt.
Health Management Information Consortium (HMIC), (1983 to 9 February 2016)
1. Incontinence/ Exp urinary incontinence
2. (neuromodulation or ((percutaneous or transcutaneous) adj4 stimulation)).tw.
3. Electr*.tw
4. (1 and 3) or 6
Cost‐Effectiveness Analysis Registry (CEA Registry) (from inception to 9 February 2016)
Basic search: incontinence
RePEc (http://repec.org/, from inception to 9 February 2016)
(incontinence AND electrical) or (incontinence AND stimulation)
Appendix 2. One type of electrical stimulation versus another
i) Surface ES versus intravaginal ES
Three trials investigated electrical stimulation with surface (skin) electrodes to ES with intravaginal electrodes (Correia 2013; Correia 2014; Maher 2009).
Primary outcomes
Woman‐reported cure or improvement
Not reported.
Incontinence‐related quality of life
Low‐quality evidence based on a single trial found insufficient evidence of a difference between surface and intravaginal ES in terms of QoL measured by the King's Health Questionnaire (Correia 2014; see Table 9, Table 28).
11. Surface ES versus intravaginal ES.
| Study | Outcome | Surface ES: mean (SD), N or n/N | Intravaginal ES: mean (SD), N or n/N | Result |
| Primary outcomes | ||||
| Correia 2014 | King's Health Questionnaire Incontinence impact scorea | 6.66 (13.80), 15 | 4.44 (11.73), 15 | MD 2.22 (95% CI −6.95, 11.39) |
| Secondary outcomes | ||||
| Correia 2014 | 1 hour pad test (g) | 3.31 (12.10), 15 | 0.41 (0.78), 15 | MD 2.90 (95% CI −3.24, 9.04) |
| Tertiary outcomes | ||||
| Correia 2014 | Pelvic floor muscle strength measured by PERFECTb | 2.53 (0.83), 15 | 2.66 (0.81), 15 | MD −0.13 (95% CI −0.72, 0.46) |
CI: confidence interval; ES: electrical stimulation; MD: mean difference; RR: risk ratio; SD: standard deviation.
aHigher score = worse QoL. Range of possible scores: 0‐100. bMeasure of vaginal muscle strength (higher score = stronger). Range of possible scores: 0‐5.
Assessment by women using visual analogue scale
Not reported.
Secondary outcomes
Satisfaction with treatment
Not reported.
Need for further treatment
Not reported.
QoL measures of general health status
Not reported.
Quantification of symptoms
The one‐hour pad test carried out by Correia 2014 found insufficient evidence of a difference between surface and intravaginal ES (Table 9). The identified trials reported no other symptom quantification measures.
Adverse effects
Not reported.
Economic data
Not reported.
Tertiary outcomes
Clinicians' observations
Not reported.
Pelvic floor muscle outcomes
Correia 2014 found insufficient evidence of a difference in pelvic floor muscle function between surface and intravaginal ES (Table 28).
ii) Low‐intensity home ES plus PFMT versus maximum intensity clinic ES plus PFMT
One trial compared low‐intensity ES carried out at home plus PFMT versus maximum intensity ES carried out in clinic plus PFMT (Knight 1998).
Primary outcomes
Woman‐reported cure or improvement
Low‐quality evidence, based on a single small trial, showed more women reporting cure or improvement in the low‐intensity home ES group (16/24) than maximum intensity clinic ES (9/25) (RR 0.28, 95% CI 0.09 to 0.91; Knight 1998; see Table 10, Table 29).
12. Low intensity ES plus PFMT versus maximal intensity ES plus PFMT.
| Study | Outcome | Low intensity ES plus PFMT: mean (SD), N or n/N, unless otherwise stated | Maximal intensity ES plus PFMT: mean (SD), N or n/N, unless otherwise stated | Result |
| Primary outcomes | ||||
| Knight 1998 | Subjective cure or improvement | 9/25 | 16/24 |
Favours maximal intensity RR 0.28 (95% CI 0.09 to 0.91) |
| Secondary outcomes | ||||
| Knight 1998 | Subjective assessment of symptoms (1‐5 scale, higher score = better) | Median (range), N: 3.3 (1 to 5), 19 | Median (range), N: 3.9 (3 to 5), 20 | Not estimable |
| Pad test (g) | Median (range), N: 2.9 (0.0‐50.9), 19) | Median (range), N: 1.5 (0.0 to 28.1), 20 | Not estimable | |
| Tertiary outcomes | ||||
| Knight 1998 | Objective cure | 6/19 | 11/20 | RR 0.38 (95% CI 0.10 to 1.40) |
| Objective cure or improvement | 10/25 | 16/24 | RR 0.33 (95% CI 0.10 to 1.07) | |
CI: confidence interval; ES: electrical stimulation; MD: mean difference; PFMT: pelvic floor muscle training; RR: risk ratio; SD: standard deviation.
Incontinence‐related quality of life
Not reported.
Assessment by women using visual analogue scale
Not reported.
Secondary outcomes
Satisfaction with treatment
Not reported.
Need for further treatment
Not reported.
QoL measures of general health status
Not reported.
Quantification of symptoms
One trial carried out pad tests, but the data were inconclusive (Knight 1998; Table 29).
Adverse effects
Not reported.
Economic data
Not reported.
Tertiary outcomes
There was insufficient evidence of a difference between low‐intensity home ES and maximum‐intensity clinic ES in terms of objective cure or improvement (Table 29).
iii) Low‐frequency ES versus medium‐frequency ES
One trial evaluated low‐frequency ES (50 Hz) compared to medium‐frequency ES (2000 Hz) (Alves 2011).
Primary outcomes
Woman‐reported cure or improvement
Not reported.
Incontinence‐related quality of life
Alves 2011 reported insufficient evidence of a difference between the groups in terms of SUI‐related discomfort measured on a 10 cm VAS (Table 30).
13. Low freqency ES versus medium frequency ES.
| Study | Outcome | Low frequency ES: mean (SD), N or n/N | Medium frequency ES: mean (SD), N or n/N | Result |
| Primary outcomes | ||||
| Alves 2011 | SUI‐related discomfort (10 cm VAS) | 0.5 (0.4), 10 | 0.6 (0.7), 10 | MD −0.10 (95% CI −0.60 to 0.40) |
| Secondary outcomes | ||||
| Alves 2011 | 1 hour pad test (g) | 1.2 (NR), 10 | 1 (NR), 10 | Not estimable |
| Tertiary outcomes | ||||
| Alves 2011 | Objective cure (Laycock and Green criteria) | 6 months: 4/10 12 months: 10/10 |
6 months: 9/10 12 months: 10/10 |
6 months: RR 0.07 (95% CI 0.01 to 0.84) 10 months: not estimable |
| Perineal pressure (mmHg) | 9.82 (2.87), 10 | 8.59 (5.47), 10 | MD 1.23 (95% CI −2.60, 5.06) | |
CI: confidence interval; ES: electrical stimulation; MD: mean difference; NR: not reported; RR: risk ratio; SD: standard deviation; SUI: stress urinary incontinence; VAS: visual analogue scale..
Secondary outcomes
Not reported.
Satisfaction with treatment
Not reported.
Need for further treatment
Not reported.
QoL measures of general health status
Not reported.
Quantification of symptoms
There was inconclusive evidence of a difference in pad tests between low‐frequency ES to medium‐frequency ES (Table 30).
Adverse effects
Not reported.
Economic data
Not reported.
Tertiary outcomes
Clinicians' observations
Alves 2011 reported objective cure at two time points, assessed against Laycock and Green criteria. At six months participants receiving medium frequency ES were slightly more likely to be cured according to objective assessment (RR 0.07, 95% CI 0.01 to 0.84; N = 20); however, at 12 months, all participants in both groups were cured according to objective assessment (Table 30). Treatment in this trial lasted six weeks.
Pelvic floor muscle outcomes
Alves 2011 found insufficient evidence of a difference between low‐ and medium‐frequency ES in terms of perineal pressure (Table 30).
iv) Faradic ES versus interferential ES
Wilson 1987 compared faradic versus interferential ES, both with surface electrodes and PFMT.
Primary outcomes
Woman‐reported cure or improvement
Very low‐quality evidence, based on a single trial, suggested insufficient evidence of a difference between faradic and interferential ES in terms of women's report of cure or improvement (Wilson 1987; Table 31).
14. ES (faradism) plus PFMT versus ES (interferential) plus PFMT.
| Study | Outcome | ES (faradism) plus PFMT: mean (SD), N or n/N | ES (interferential) plus PFMT: mean (SD), N or n/N | Result |
| Primary outcomes | ||||
| Wilson 1987 | Subjective cure or improvement | 10/15 | 9/15 | RR 1.33 (95% CI 0.30 to 5.91) |
| Secondary outcomes | ||||
| Wilson 1987 | Micturitions per 24 hours | 7.8 (2.0), 15 | 8.0 (1.8), 15 | MD (95% CI −0.20 −1.56 to 1.16) |
| Pads per 24 hours | 1.3 (1.4), 15 | 1.6 (2.3), 15 | MD −0.30 (95% CI −1.66 to 1.06) | |
| Tertiary outcomes | ||||
| Wilson 1987 | Maximum urethral closure pressure (cmH2O) | 58.0 (15.4), 14 | 56.0 (16.7), 12 | MD 2.00 (95% CI −10.42 to 14.42) |
CI: confidence interval; ES: electrical stimulation; MD: mean difference; PFMT: pelvic floor muscle training; RR: risk ratio; SD: standard deviation.
Incontinence‐related quality of life
Not reported.
Assessment by women using visual analogue scale
Not reported.
Secondary outcomes
Satisfaction with treatment
Not reported.
Need for further treatment
Not reported.
QoL measures of general health status
Not reported.
Quantification of symptoms
Wilson 1987 found insufficient evidence of a difference between groups in terms of micturitions per 24 hours or number of pads used per 24 hours (Table 31).
Adverse effects
Not reported.
Economic data
Not reported.
Tertiary outcomes
Wilson 1987 found insufficient evidence of a difference between groups in maximum urethral closure pressure (Table 31).
v) Conventional ES plus PFMT versus dynamic ES plus PFMT
One trial compared conventional intravaginal electrical stimulation versus dynamic intravaginal ES, both with EMG biofeedback‐assisted PFMT (Huebner 2011).
Primary outcomes
Woman‐reported cure or improvement
Huebner 2011 measured change in perception of symptoms but found insufficient evidence of a difference between the groups (very low‐quality evidence, see Table 11Table 32).
15. Conventional ES plus PFMT versus dynamic ES plus PFMT.
| Study | Outcome | Conventional ES: mean (SD), N or n/N | Dynamic ES: mean (SD), N or n/N | Result |
| Primary outcomes | ||||
| Huebner 2011 | Change in perception of bother of UI symptoms (VAS) | −2.2 (3.2), 33 | −2.9 (2.9), 28 | MD 0.70 (95% CI −0.83 to 2.23) |
| Change in King's Health Questionnaire score | −20.7 (5.3), 33 | −24.8 (5.3), 28 |
Favours dynamic ES MD 4.10 (95% CI 1.43 to 6.77) |
|
| Tertiary outcomes | ||||
| Huebner 2011 | Oxford scorea | 1.9 (0.9), 33 | 1.8 (0.7), 27 | MD 0.10 (95% CI −0.30 to 0.50) |
CI: confidence interval; ES: electrical stimulation; MD: mean difference; PFMT: pelvic floor muscle training; SD: standard deviation; UI: urinary incontinence; VAS: visual analogue scale.
aMeasure of vaginal muscle strength (higher score = stronger). Range of possible scores: 0‐5.
Incontinence‐specific quality of life
Very low‐quality evidence from a single trial suggested a greater change in QoL in the dynamic ES group than the conventional ES group, measured by the King's Health Questionnaire (Huebner 2011; see Table 11Table 32).
Assessment by women using visual analogue scale
Not reported.
Secondary outcomes
Not reported.
Tertiary outcomes
Huebner 2011 found insufficient evidence of a difference between groups in pelvic floor muscle function measured by Oxford score (Table 32).
Data and analyses
Comparison 1. Electrical stimulation versus no active treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective cure | 2 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [1.06, 5.02] |
| 2 Subjective cure or improvement | 5 | 347 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.41, 2.11] |
| 3 Quality of life (higher score = worse quality of life) | 4 | 250 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.72 [‐0.99, ‐0.45] |
| 4 Pad test (g) | 3 | 110 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.11, ‐0.31] |
| 5 Adverse effects | 3 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.96 [0.30, 118.70] |
Comparison 2. Electrical stimulation versus sham treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective cure | 3 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [0.38, 12.73] |
| 2 Subjective cure or improvement | 5 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.02, 4.07] |
| 3 Number of incontinence episodes per 24 h | 3 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐1.34 [‐2.02, ‐0.66] |
| 4 Number of micturitions per 24 h | 3 | 163 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐1.38, 0.46] |
| 5 Number of pads per week | 2 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐0.78 [‐1.23, ‐0.33] |
| 6 Pad test (g) | 2 | 137 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.89 [‐1.27, ‐0.52] |
| 7 Adverse effects | 4 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.52, 7.67] |
Comparison 3. Electrical stimulation versus PFMT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective cure | 4 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.16, 1.63] |
| 2 Subjective cure or improvement | 7 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.70, 1.03] |
| 3 Adverse effects | 3 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 99.16] |
Comparison 4. Electrical stimulation versus vaginal cones.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective cure | 3 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.70, 1.54] |
| 2 Subjective cure or improvement | 5 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.97, 1.21] |
| 3 Quality of life (I‐QoL) | 2 | 96 | Mean Difference (IV, Fixed, 95% CI) | 1.59 [‐3.72, 6.90] |
| 4 Number of incontinence episodes per 24 h | 2 | 96 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.13, 0.33] |
| 5 Pad test (g) | 4 | 239 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.20, 0.31] |
Comparison 5. Electrical stimulation versus PFMT and vaginal cones.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective cure | 2 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.96, 2.20] |
| 2 Subjective cure or improvement | 2 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.08, 2.18] |
Comparison 6. Electrical stimulation plus PFMT versus PFMT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective cure | 3 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.38, 1.52] |
| 2 Subjective cure or improvement | 6 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.95, 1.28] |
| 3 Quality of life (higher score = worse quality of life) | 4 | 193 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.64, ‐0.05] |
| 4 Subjective assessment (VAS) | 3 | 150 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.57 [‐0.90, ‐0.24] |
| 5 Women requesting surgery at end of follow‐up | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.59, 1.41] |
| 6 Number of incontinence episodes per 24 h | 4 | 275 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.59, ‐0.06] |
| 7 Number of micturitions per 24 h | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐1.46, 1.20] |
| 8 Pad test (g) | 4 | 346 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.61, 0.21] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aaronson 1995.
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: not reported Period: October 1992 to January 1994 Sample size: not reported Follow‐up:: unclear |
|
| Participants | N: 47 randomised and analysed Age: 24‐82 years Sex: women Inclusion criteria: genuine stress urinary incontinence or detrusor instability Exclusion criteria: not reported |
|
| Interventions | For detrusor overactivity women only (DI) A: probanthine B: electrical stimulation (ES) 2nd RCT in patients with GSUI C: PFMT D: ES Participant numbers unclear |
|
| Outcomes | Cure ‐ defined as cessation of incontinence Improvement ‐ defined as reduction in frequency of voids per 24 h by ≥ 50% or ≤ 10 voids per 24 h, or decrease number of pads per 24 h by ≥ 50% |
|
| Notes | No usable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Abel 1997.
| Methods | Study design: RCT Multicentre or single‐centre: Multicentre, "… conducted at the gynecologic‐obstetric dept at Hvidovre Hospital, Rigshospitalet and Amtshospitalet in Glostrup" (p 23) Setting: gynecologic‐obstetric dept at Hvidovre Hospital, Rigshospitalet and Amtshospitalet in Glostrup (p 23) Period: not reported Dates not reported Details of sample size calculation: not reported "The statistical power calculation is based on the 24 h pad weighing test. It was decided that there should be a decrease of at least 50% to be considered significant. With a power of 90% and a significance level of 5%, the calculation showed, that a minimum of 10 patients in each group needed to complete the study to enable results to be analysed statistically" (p 29) Follow‐up: unclear |
|
| Participants | N: 81 randomised, 65 analysed 65 completed the study, but of these, only data from 45 in group 1 (urge incontinence) are analysed. Data from group 2 (stress incontinence) is presented in table 9 and 15 but not analysed because of insufficient sample size. Mean age in group 1: 70 y (range: 57‐92 years) (table 3, p 30) Sex: women Inclusion criteria: postmenopausal women with stress or urgency incontinence Exclusion criteria: uncontrolled hypertension and/or diastolic BP ≥ 110 mmHg, took sex hormones within the last 6 months, previous treatment with implanted sex hormone, previous oestrogen‐related cancer, acute or chronic liver disease,disposed to thrombo‐embolic disease, untreated heart disorder, pace‐maker, extra‐urethral incontinence (fistel, ectopic) or ischuria paradoxa, untreated UTI, total genital prolapse, chronic neurologic disorder, participated in a clinical study with other treatment within the last 3 months (p 26) |
|
| Interventions | Group 1: UUI (n = 45) A (n = 11): electrical stimulation B (n = 11): sham electrical stimulation C (n = 13): 17‐β‐estradiol D (n = 10): placebo Group 2: SUI (n = 20) E (n = 6): electrical stimulation F (n = 6): sham electrical stimulation G (n = 3): 17‐β‐estradiol H (n = 5): placebo |
|
| Outcomes | Continence, defined as score of 10 on VAS and > 8 g leakage on 24 h pad test Improvement, according to subjective (VAS) and objective assessment (24 h pad test, number of micturitions per 24 h, number of incontinence episodes per 24 h) |
|
| Notes | Danish – need translation. Data obtained from English abstract SUI: no data reported because the number of patients was too small for valid analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[R]andomization was conducted by a computer list" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients randomised to oestrogen treatment were double‐blinded, while patients randomised to electrical stimulation were single blinded. Blinding in the oestrogen group was done by shaping the vagitories so they were identical and packing them in identical packages, where one half contained oestradiol and the other half were without oestradiol. Blinding in the electrical stimulation group was done by not connecting power to the electrodes at the patients who were blinded to placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported per group |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | Unclear risk | "The expectations to the treatment were high, since patients were familiar with the good international results. Factors which may be expected to facilitate placebo effect" |
Alves 2011.
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: Brazil Period: not reported Details of sample size calculation: "The sample size was previously calculated based on averages and standard deviations of perineal pressure obtained from a pilot study. The result demonstrated the need to include 20 volunteers for a test power of 80% with alpha significance level of 0.05." Follow‐up: 6 weeks |
|
| Participants | N: 24 eligible, 20 analysed, unclear how many randomised Mean (SD) age: A ‐ 55.4 (6.98) years; B ‐ 55.7 (7.17) years Sex: women Inclusion criteria: clinical diagnosis of SUI and urinary loss for at least three months Exclusion criteria: urogenital prolapse grade III or higher, urinary tract infection, instability of the detrusor muscle, cardiac pacemakers, devices implanted in the pelvis, vaginal inflammation/infections, pregnancy, intrinsic sphincter deficiency, use of hormone replacement therapy, pelvic or abdominal surgery within the last six months, cognitive impairment and non‐attendance of the number of sessions provided |
|
| Interventions | Both groups: pulse generator 961 Dualpex with intravaginal electrodes, 20 min at maximum tolerable intensity twice a week for 6 weeks (12 sessions) A (n = 10) ‐ medium frequency electrical stimulation. Current, biphasic 2000 Hz frequency, pulse width 100 ms. Time on:off 4:8 s. Modulation frequency 50 Hz B (n = 10) ‐ low frequency electrical stimulation. Current, biphasic, 50 Hz frequency, pulse width 700 ms. Time on:off 4:8 s |
|
| Outcomes | Participants cured (objectively measured according to Laycock and Green criteria) Discomfort caused by SUI measured on 10 cm VAS 1 hour pad test (g) Perineal pressure (mmHg) |
|
| Notes | Contacted authors to clarify allocation/randomisation methods on 24 February 2016 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The volunteers were randomly divided into two distinct groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Twenty four volunteers were included in this randomized controlled trial; however, only 20 were included in our results as 4 patients failed to complete treatments". No explanation given for withdrawal, no indication of whether attrition was equal across groups |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Bernardes 2000.
| Methods | Study design: RCT Multicentre or single‐centre: single Setting: Urogynecology and Midwifery, Santa Casa de Belo Brazil Period: February – August 1998 Details of sample size calculation: not reported Follow‐up: 10 days' treatment |
|
| Participants | N: 14 randomised and analysed. Mean age: A ‐ 53.3 years; B ‐ 44.1 years Inclusion criteria: women aged 31‐67 years with SUI with perineal contraction force 1‐3 Exclusion criteria: pregnancy, using intrauterine device, urological disease or orthopaedic disorders contraindicating kinesiotherapy, GSUI rating > 3 relating to pelvic floor muscle strength, previous physical therapy for SUI |
|
| Interventions | A (n = 7) ‐ ES Dualpex 961 Quark Medical Products with perineal intracavitary electrode with 2 metal rings. Symmetrical bidirectional current, frequency 60 Hz, pulse width 1 ms, 6 s: 12 s on:off cycle, intensity 10‐30 mA up to women's maximum tolerance. 20 min session every day for 10 days B (n = 7) ‐ kinesiotherapy/PFMT. One session per day for 10 days. Series of 6 exercises: 2 for specific abdominal muscles, 2 specifically for the pelvic floor, 2 for contractions of adductor and gluteal muscles. Exercise series done 3 times to achieve voluntary contraction of pelvic floor lasting 6 s. Additional home programme to be done daily for 10 days (micturition control and perineal reinforcement) |
|
| Outcomes | Perineal contraction strength (categories 0‐5, higher score = greater ability to contract) Objectively observed symptoms – leakage observed at cough test following treatment |
|
| Notes | At baseline 6/7 and 4/7 could not contract PFM | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomly" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Beuttenmuller 2010.
| Methods | Study design: RCT Multicentre or single‐centre: single Setting: Brazil Period: August 2008 – August 2009 Details of sample size calculation: not reported Follow‐up: 6 weeks |
|
| Participants | N: 75 randomised and analysed Mean (SD) age: A ‐ 52.17 (3.76) years; B ‐ 49.96 (23.4) years; C ‐ 44.82 (24.36) years Inclusion criteria: "female patients that had been diagnosed with UI" Exclusion criteria: not reported |
|
| Interventions | A (n = 25) ES and kinesitherapy. 12 sessions (2 sessions per week for 6 weeks) ES: 20 min sessions with Uro, Quark Medical Product with intravaginal electrodes, 50 Hz frequency, pulse width 0.2‐0.5 ms. Rest time at least twice the time of current, with a maximum tolerated intensity determined for each individual. Kinesitherapy: standing or sitting exercises using a Swiss ball of varying size, according to the height and weight of the patient. 20 min group sessions up to 4 people under supervision of a therapist. B (n = 25) ‐ kinesitherapy alone (as above) C (n = 25) ‐ control group, "not subjected to any physical therapy" |
|
| Outcomes | Subjective assessment of severity of stress urinary incontinence (SINT_5) (higher score indicates greater severity Impact of incontinence on quality of life (measured by King's Health Questionnaire (KHQ)*) Symptom severity (higher score indicates greater severity):
Contraction of pelvic floor at rest (cmH2O) |
|
| Notes | *Other KHQ domains reported in full | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were randomly divided into three groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition not reported |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias "No funds were received in support of this study. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript." |
Bidmead 2002.
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: UK Period: not reported Details of sample size calculation: not reported Follow‐up: 14 weeks |
|
| Participants | N: 170 randomised, unclear how many included in analysis Mean (SD) age: not reported Sex: women Inclusion criteria: urodynamically proven GSI Exclusion criteria: not reported |
|
| Interventions | A (n = 88) ‐ home ES plus supervised pelvic floor exercises B (n = 42) ‐ sham ES at home plus supervised pelvic floor exercises C (n = 40) ‐ supervised pelvic floor exercises alone D (n = 20) ‐ control: deferred treatment group |
|
| Outcomes | Pad tests (g) | |
| Notes | No details about treatment reported. No SDs. Data relating to symptoms and QoL collected but not reported: "Symptom scores and QoL scores also improved significantly in all treatment groups but not in the control group" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomised to one of four treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "[T]here were no statistical differences between women withdrawing and completing the study or between withdrawals across the treatment groups" |
| Selective reporting (reporting bias) | High risk | Data relating to symptoms and QoL collected but not reported: "Symptom scores and QoL scores also improved significantly in all treatment groups but not in the control group" |
| Other bias | High risk | No explanation for unequal numbers allocated to groups |
Bourcier 1994.
| Methods | Study design: prospective randomised study | |
| Participants | Women with mild genuine stress incontinence (USI) N: 102 randomised and analysed Mean (SD) age: not reported Inclusion criteria: women with SUI. Diagnosis based on history, urodynamics, pad test, pelvic floor grading, perineometry. Exclusion criteria: not reported |
|
| Interventions | A (n = 50) ‐ conservative treatment. Instructed to perform 20 maximal PFM contractions 3 times a day, use of cones twice a day, and one 30 min instructor‐led session per week doing a series of pelvic exercises B (n = 52) ‐ electrical stimulation. 12 × 30 min sessions over 6 weeks. 20 min maximal functional electrical stimulation and 10 min EMG/pressure biofeedback |
|
| Outcomes | Women reporting continence Pad tests (g) Urethral pressure profile (cmH2O) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[P]rospective randomised study" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Group A ‐ 12/50 and Group B ‐ 6/52 lost to follow‐up. No explanation. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine if planned outcomes are reported in full |
| Other bias | Low risk | Nothing to indicate other risk of bias |
Bridges 1988.
| Methods | Study design: RCT Multicentre or single‐centre: single centre Setting: UK Period: not reported Details of sample size calculation: not reported Follow‐up: 4 weeks' treatment |
|
| Participants | N: 69 randomised, 54 analysed Mean age: 38 years Inclusion criteria: women with symptoms of stress incontinence Exclusion criteria: not reported |
|
| Interventions | All participants shown how to perform pelvic floor exercises A (n = 36) ‐ ES. Interferential therapy 3 times a week for 4 weeks. 0‐100 Hz, maximum tolerable intensity for 15 min B (n = 33) ‐ cones. Participants attended physiotherapy department once a week for 4 weeks. Asked to retain same passive cone weight as on first testing, then asked to try retaining same cone twice day for up to 15 min. After achieving this on 2 consecutive occasions they moved onto next weight |
|
| Outcomes | Participants with subjective improvement in SUI Participants with no subjective improvement or worse Objective improvement No improvement according to objective measurement Perineometer test of pelvic floor |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/36 and 9/33 not included in analysis; no explanation |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Nothing to indicate any other risk of bias |
Brubaker 1997.
| Methods | Study design: RCT Multicentre or single‐centre: 4 centres Setting: Rush‐Presbyterian‐St.Luke´s Medical Center, Chicago; Methodist Hospital, Indianopolis; Greater Baltimore Medical Center; and the Oregon Health Science University, Portland Period: not reported Sample size: not reported Follow‐up: 8 weeks. |
|
| Participants | N: 148 enrolled, 121 randomised and analysed Mean (SD) age for all participants (not stratified by GSUI/DO): A ‐ 56 (11.9) years B ‐ 57.7 (12.4) years Sex: women Inclusion criteria: women with symptoms or urodynamic evidence of genuine stress incontinence or detrusor instability Exclusion criteria: urinary incontinence other than genuine stress incontinence, detrusor instability, or mixed incontinence. Age < 25 years, leakage episodes ≤ 3/weeks, inadequate cognitive ability investigator judgment), infected urine, anatomic defect that precluded use of device, postvoid residual > 100 mL, implanted electric device, genitourinary surgery < 6 months previously, medication alteration ≤ 3 months previously, anticipated geographic relocation during study |
|
| Interventions | For DO and mixed women only (n = 61): A (n = 33) ‐ transvaginal electric stimulation. Device: InCare Microgyn II. 20 Hz frequency, 2‐second 4‐second work‐rest cycle, pulse width 0.1 µs. Bipolar square wave could be delivered over a range of 0 to 100 mA. 20 min daily. B (n = 28) sham. Identical device with disconnected wire so no electricity supplied. 20 min daily |
|
| Outcomes | Objective cure, according to urodynamic diagnosis (of those who had pure SUI at beginning of treatment) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers, and used for stratified randomisation |
| Allocation concealment (selection bias) | Unclear risk | The study nurse at each site was responsible for carrying out the random assignment of patients in accordance with the randomisation scheme. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study nurse at each site was aware of the difference in probes; however the physician investigators were masked as to the type of vaginal probe provided to each participant. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data sent to centralised data manager |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "A total of 148 women were enrolled, 18% of whom withdrew from the study, leaving of a total 121 participants who completed the study. There was no statistically significant difference between the treatment groups with respect to withdrawal rates: 21% for the sham group and 14% for the stimulation group." No explanation reported for withdrawals. |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | High risk | One site had unusually high attrition, there were also technical problems with the devices: "there was concern that the stimulation group had subjects who were not receiving any stimulation" |
Bø 1999.
| Methods | Study design: RCT Multicentre or single‐centre: multicentre Setting: Norway Period: unclear Details of sample size calculation: based on previous study designed to detect differences between groups of 1 SD with 80% power and 5% α. In previous study significant differences were found in the same outcomes after the same training programme in groups of 23 and 29; 30 participants were recruited per group. Follow‐up: 6 months |
|
| Participants | N: 122 randomised, 107 analysed Mean (SD) age: A ‐ 51.7 (8.8) years; B ‐ 49.6 (10.0) years; C ‐ 47.2 (10.1) years; D ‐ 49.2 (10.6) years Sex: women Inclusion criteria: history of SUI, > 4 g leakage measured by pad test with standardised bladder volume Exclusion criteria: UI other than SUI, involuntary detrusor contractions exceeding 10 cmH2O on cystometry, abnormal bladder function (residual urine > 50 mL and maximal uroflow < 15 mL/s), previous SUI surgery, neurological/psychiatric disease, ongoing UTI, other diseases that could interfere with participation, use of concomitant treatments during the trial, inability to understand instructions in Norwegian. |
|
| Interventions | A (n = 30) ‐ no active treatment. Offered instruction in use of continence guard. B (n = 25) ‐ pelvic floor muscle exercises. 8‐12 high intensity (close to maximum) contractions 3 times daily at home. 1 × 45 min group training session per week with physical therapist. Group training was performed in lying, standing, kneeling, and sitting positions with legs apart to emphasise specific strength training of the pelvic floor muscles and relaxation of other muscles. Aim to hold each contraction for 6‐8 s, 3 or 4 fast contractions then added. 8‐12 contractions completed in each position with maximal contraction encouraged. Body awareness, breathing, relaxation exercises and strength training for the abdominal, back and thigh muscles were performed to music between positions. Participants encouraged to use their preferred position and perform equally intensive contractions at home. Audiotape with verbal guidance for 12 maximum contractions available for home training and a training diary was kept. C (n = 25) ‐ vaginal electrical stimulation with MS 106 Twin 30 min per day. Frequency 50 Hz, pulse width 0.2 ms, current intensity 0‐120 mA, individually adapted on‐off (duty) cycles on the basis of ability to hold voluntary contraction. On time 0.5‐10 s, off time 0‐30 s. Highest tolerable intensity. Treatment adherence electronically recorded and monitored D (n = 27) ‐ vaginal cones. Mabella cones used for 20 min per day according to manufacturer's instructions. Progressed through 3 cone weights: 20 g, 40 g, 70 g according to ability. Training diary kept. |
|
| Outcomes | Subjective cure (defined as women stating the condition was unproblematic on 5‐point scale of unproblematic, minimal problem, moderate problem, problematic, very problematic) Objective cure (defined as ≤ 2 g leakage on pad test with standardised bladder volume) Change in incontinence episodes over 3 days Change in stress pad test (g) Change in 24 hr pad tests (g) PFM strength (cm H2O) Adverse effects Women wanting treatment additional to allocation |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants stratified according to leakage (>20 g and ≤20 g), randomisation schemes stratified by degree of incontinence were constructed by computer generated random numbers. Information for decoding randomisation was kept locked in the statistician's office |
| Allocation concealment (selection bias) | Low risk | "[O]paque sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants. Personnel blinding unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The main investigator was not involved in any interventions and was blind to group allocation. Physicians evaluating the effect of interventions were also blind to allocation of treatments." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2/32 withdrew from control arm (1 excluded due to other ongoing treatments, 1 unclear) 4/29 withdrew from PFMT arm (1 lack of motivation, 1 time spent travelling, 2 unclear). 7/32 withdrew from ES arm (2 due to pain, 1 due to bleeding, 4 lack of motivation) 2/29 withdrew from vaginal cones arm (1 excluded due to other ongoing treatments, 1 unclear) "Results according to intention to treat analysis showed virtually the same results as the treatment analyses" – ITT analysis not shown |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to be reported in full |
| Other bias | High risk | Funding from Norwegian Fund for Postgraduate studies in Physiotherapy and Norwegian Research Council. Coloplast AS provided continence guards and Vitacon AS provided electrical stimulators and cones. They also gave financial support to seminars for the research group. |
Castro 2008.
| Methods | Study design: RCT Multicentre or single‐centre: single Setting: São Paulo, Brazil Period: not reported Details of sample size calculation: "based on the power estimate and results of a previous study designed to detect differences between groups of 1SD, with a 0.05 significance level and power of 0.8. In the previous study , significant differences were shown in groups of 23 and 31 subjects; therefore, 30 participants were recruited for each of the four groups" Follow‐up: 6 months |
|
| Participants | N: 118 randomised and analysed Mean (SD) age: A ‐ 56.2 (12.5) years; B ‐ 55.2 (12.8) years; C ‐ 52.6 (11.2) years; D ‐ 52.6 (11.2) years Inclusion criteria: proven urodynamic SUI with no detrusor overactivity, positive cough stress test, > 3 g leakage measured by pad test with standardised bladder volume (200 mL) Exclusion criteria: chronic degenerative disease affecting muscular and nerve tissues, advanced genital prolapse, pregnancy, active or recurrent UTI, vulvovaginitis, atrophic vaginitis, continence surgery within a year, cardiac pacemakers, intrinsic sphincteric deficiencies. |
|
| Interventions | All groups taught to contract pelvic floor muscles correctly, measured by vaginal palpation. A (n = 31) ‐ pelvic floor muscle exercises. Groups sessions lasting 45 min. 10 × 5 s contractions with 5 s recovery time; 20 × 1 s contractions with 1 s recovery; 20 × 2 s contractions with 2 s recovery; 20 × 1 s contractions with 1 s recovery; 5 × 10 s contractions with 10 s recovery, 5 × strong contractions + simulated cough with 1 min interval between sets. Warm up for joints at beginning of session, stretching at end. 3 sessions per week under supervision of trained physical therapist. B (n = 30) ‐ electrical stimulation. Transvaginal 10 cm long, 3.5 cm wide electrodes. 50 Hz frequency, on:off 5 s:10 s, pulse width 0.5 milliseconds. Bipolar square wave, 0‐100 mA range according to maximum tolerable intensity. 20 min treatment. 3 sessions per week under supervision of trained physical therapist. C (n = 27) ‐ vaginal cones. Nine cones of equal shape and volume, increasing in weight from 20 g to 100 g. Starting with lightest weight, women were taught to insert the cone into the vagina while standing. Heaviest weight that could be retained for 1 min without voluntarily contracting the pelvic floor was the passive cone. Then use the next heaviest weight that required voluntary contraction to prevent cone falling out. Heaviest weight retained was active cone. Instructed to exercises with cones for 45 min. 3 sessions per week under supervision of trained physical therapist. D (n = 30) control group, received motivational phone call once a month during the intervention period. |
|
| Outcomes | Subjective: dissatisfied = would want a different treatment Incontinence episodes per week Pad tests (200 mL) Objective cure (negative pad test (< 2 g) with standardised bladder volume) Muscle strength Oxford scale N with negative urodynamic SUI stress test Quality of life: I‐QoL score N with significant improvement in IQoL score |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[C]omputer‐generated random numbers prepared by the Biostatistics Centre of the Federal University of São Paulo" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "[T]he investigator responsible for assessing patients outcomes was not involved in administering any of the treatments and was blind to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Group A: 2/31 withdrew (lack of clinical improvement), 3/31 excluded (1 withdrew consent Group B: 3/30 withdrew (1 lack of clinical improvement, 2 withdrew consent) Group C: 1/27 withdrew (lack of clinical improvement). 2/27 excluded (withdrew consent) Group D: 4/30 withdrew (2 lack of clinical improvement), 2/30 excluded (3 withdrew consent) Text and figure do not match up regarding numbers of withdrawals/exclusions |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Correia 2013.
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: Brazil Period: not reported Details of sample size calculation: not reported Follow‐up: 3 weeks |
|
| Participants | N: 30 randomised and analysed Mean (SD) age: A ‐ 65.62 (13.71) years; B ‐ 60.90 (4.40) years; C ‐ 63.50 (9.51) years Inclusion criteria: women over the age of 50 years, with at least one episode of SUI during the previous month Exclusion criteria: previous treatment for SUI or hormone therapy, ongoing urinary tract infections, cognitive or neurological disorder, uncontrolled hypertension, inability to perform the proposed procedures, or use of pacemaker implantation or metal rods |
|
| Interventions | A + B ‐ 6 sessions, 2 weekly sessions of 20 min frequency: 50 Hz; pulse width: 700 μs; stimulation intensity: maximal level tolerable. In SESG and IESG the women were not instructed to perform the contraction of the PFM in conjunction with electrical stimulation A (n = 10) ‐ surface electrical stimulation. 4 surface electrodes, 2 placed in the suprapubic region and two medial to the ischial tuberosity B (n = 10) ‐ intravaginal electrical stimulation C (n = 10) ‐ control group. No treatment |
|
| Outcomes | Quality of life: Incontinence Severity Index (higher score = greater severity) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[C]omputer generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Correia 2014.
| Methods | Study design: RCT Multicentre or single‐centre: single Setting: Brazil Period: January 2012 to March 2013 Details of sample size calculation: "The sample size was calculated considering the values of pad test (in grams) from previous data on a pilot study of SES treatment. At a significance level of 5% and power of 90%, it was estimated to require a sample of at least 45 people." Follow‐up: 6 weeks? |
|
| Participants | N: 48 randomised, 45 analysed Mean (SD) age: A ‐ 64.46 (8.83) years; B ‐ 59.86 (4.82) years; C ‐ 60.13 (9.35) years Inclusion criteria: women aged over 50 years, who complained of urinary leakage on stress and who had not undergone physical therapy for UI. Exclusion criteria: women with symptoms of urgency UI and mixed UI were excluded, latex allergies, vaginal or urinary infections, pelvic organ prolapse greater than grade II, inability to perform voluntary PFM contraction, cognitive or neurological disorder, uncontrolled hypertension, inability to carry out the evaluation or treatment, hormone therapy, use of pacemaker or metal rod implantation |
|
| Interventions | A + B ‐ 12 individual sessions of ES, 2 weekly sessions of 20 min with Dualpex 961 (Quark Medical Products) equipment. The electric parameters used in both treatments were: current type: functional electrical stimulation; frequency: 50 Hz; pulse duration: 700 ms; time: 20 min; 4‐s on/8‐s off cycles; rise: 2 s fall: 2 s; stimulation intensity: maximal level tolerable. In the SESG and IESG the women were not instructed to perform the PFM contraction during the ES. The treatment of both groups was performed by another physical therapist that did not participate in the evaluations A (n = 15) ‐ surface electrical stimulation. The women were positioned supine, with 458 of hip and knee flexion. In this treatment, 4 surface electrodes of silicone (2.0 cm × 3.0 cm) were fixed with masking tape. Two electrodes were placed in the suprapubic region and the other two electrodes were crossed on the skin and fixed medial to the ischial tuberosity. During the treatment the women used panties. B (n = 16) ‐ intravaginal electrical stimulation. Participants were positioned supine with 458 of hip and knee flexion for the positioning of an intravaginal electrode. The intravaginal electrode used was the Dualpex 961 (Quark Medical Products) urogynecological electrode. During the treatment the volunteers were positioned supine with hip and knee in a neutral position. C (n = 17) ‐ control group. No treatment during the corresponding treatment time. Afterwards, participants were referred for physical therapy treatment |
|
| Outcomes | 1‐hour pad test (g) Pelvic floor muscle strength measured by PERFECT Quality of life: King's Health Questionnaire incontinence impact score |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[C]omputerized random numbers" – "A researcher who was not involved in the data collection or analyses created this randomization list." |
| Allocation concealment (selection bias) | Low risk | "A researcher who was not involved in the data collection or analyses created this randomization list." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants. "The treatment of groups A and B was performed by another physical therapist that did not participate in the evaluations" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "[O]ne blinded experienced physiotherapist performed all evaluation" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Group B 1/16 reported dysmenorrhea and was excluded from this treatment. Group C: 2/17 withdrew "due to a health problem" These 3 participants were "substituted by other participants" |
| Selective reporting (reporting bias) | Low risk | Main outcomes in trials registry record reported for all groups |
| Other bias | High risk | Clinical trials registry indicates 16 sessions of ES will be carried out for groups A and B but paper reports 12. |
Delneri 2000.
| Methods | Study design: randomised Multicentre or single‐centre: single Setting: Italy Period: not reported Details of sample size calculation: not reported Follow‐up: assume same as length of treatment, i.e. approx. 2.5 weeks for group A, 4 weeks group B |
|
| Participants | N: 20 randomised Mean (SD) age: A ‐ 49.5 (14.5) years; B ‐ 41.5 (7.4) years Inclusion criteria: women with GSI Exclusion criteria: detrusor instability, inversion of perineal command, absent contraction of the pubococcygeal muscle, neurological disease, unwillingness to collaborate |
|
| Interventions | A (n = 10) ‐ functional electrical stimulation. Lying in dorsal position, 12 × 30 min sessions on consecutive days, excluding Saturdays and Sundays. Pulse width 4 seconds, 8 seconds recovery phase, 15 min at frequency 20 Hz, 15 at 50 Hz, intensity according to the participant's sensations B (n = 10) ‐ vaginal cones. Femcon set of 5 cones, same size and volume, weight 20‐70 g. To select treatment cone, static and dynamic tests were carried out. Static test: standing patient required to hold cone in place for 1 min without any voluntary contractions. Dynamic test: series of exercises requiring voluntary contraction of PFM, e.g. up and down a step‐ladder, skipping With treatment cone women were taught a series of exercises starting from positions facilitating the holding of the cone in place to the upright position, then invited to practise with the heaviest cone they could hold in the vagina for 1 min, aiming to reach 20‐25 min, then begin with next heaviest cone. Once familiar with method, instructed to perform exercises at home 25‐35 min per day for 4 weeks. |
|
| Outcomes | Subjective assessment of SUI (10 point VAS) Pad test (g) (not specified if 1‐hour) Change in maximum urethral pressure (cmH2O) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomly divided into two equal groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Impossible to blind participants. No blinding reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Group B 2/10 refused to undergo follow‐up urethral pressure profile. Unclear if they were included in other analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | High risk | Groups not comparable at baselines in terms of age |
Demirturk 2008.
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: Turkey Period: 1998‐2005 Details of sample size calculation: not reported Follow‐up: 5 weeks |
|
| Participants | N: 41 randomised, 40 analysed Mean (SD) age: overall 50.4 (6.9) years; A ‐ 52 (7) years; B ‐ 47 (7) years Inclusion criteria: moderate intensity of incontinence as determined by a one‐hour pad test Exclusion criteria: urinary tract infections, detrusor over activity, cognitive problems and neoplasm |
|
| Interventions | A (n = 20) ‐ electrical stimulation with interferential current. Frequency of 0–100 Hz was applied for a duration of 15 min, 3 times a week for a total of 15 sessions. Two vacuum electrodes were placed in the suprapubic region, whilst another two were positioned near to the medial side of the ischial tuberosity, crosswise B (n = 21) ‐ biofeedback. Performed Kegel exercises using a BF device for 15 min, 3 times a week, for a total of 15 sessions. The treatment protocol was individually designed and all of the patients were instructed in the use of a BF device to obtain isolated pelvic floor muscle contraction. Before starting the treatment, duration of maintenance of maximum contraction of the pelvic floor muscles was determined for each patient. This duration was then taken as the working period in the initial treatment sessions, and increased as the capability to maintain the maximal contraction improved. A 10‐s resting period was given between the working periods |
|
| Outcomes | Adverse effects Pelvic floor muscle strength, measured with biofeedback device Change in PFM strength (hectoPascals hPa) Change in 1 hour pad test (g) Quality of life questionnaire score (non‐validated instrument) Change in quality of life questionnaire score |
|
| Notes | "As the follow‐up period of all cases is not completed, it is planned to present the long term results of these treatments in a further study. Subjects who successfully perform a home program will be compared with those who fail to sustain the exercise program" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomly assigned according to application order" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, no blinding reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Group B 1/21 withdrew (no explanation). No other withdrawals |
| Selective reporting (reporting bias) | Unclear risk | "As the follow‐up period of all cases is not completed, it is planned to present the long term results of these treatments in a further study." |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Edwards 2000.
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: UK Period: not reported Details of sample size calculation: not reported Follow‐up: 12 weeks |
|
| Participants | N: 20 randomised and analysed. Mean (range) age: 46 years (32‐51) Inclusion criteria: premenopausal women with urodynamic diagnosis of GSUI Exclusion criteria: previous pelvic surgery |
|
| Interventions | A (n = ?) conservative treatment; pelvic floor exercises and biofeedback B (n = ?) electrical stimulation; PFE plus electrical therapy |
|
| Outcomes | Objectively measured incontinence | |
| Notes | No usable data – numbers allocated per group not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomised" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals reported. Outcomes not reported by intervention group. |
| Selective reporting (reporting bias) | Unclear risk | No data reported per intervention group |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Eyjolfsdottir 2009.
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: Iceland Period: unclear Details of sample size calculation: unclear Follow‐up: 9 weeks |
|
| Participants | N: 24 randomised and analysed Mean (SD) age: A ‐ 56 (11) years; B 46 (14) years Inclusion criteria: diagnosis of SUI Exclusion criteria: pregnancy and urgency urinary incontinence |
|
| Interventions | A (n = 12) ‐ conservative treatment. "Traditional pelvic floor exercises." 15 min per day for 9 weeks B (n = 12) ‐ ES plus conservative treatment. PFE 15 min per day for 9 weeks plus intermittent electrical stimulation. 50 Hz frequency, 200 µs pulse width |
|
| Outcomes | Women cured Oxford scale (0‐5, higher is stronger) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[A]llocated into groups by random selection" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants who were randomised seem to be included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available from translator |
| Other bias | Unclear risk | Funded by Research Fund of the Association of Icelandic Physiotherapists and the Research Fund of the National Hospital of Iceland. Insufficient information available from translator to judge whether there was any other risk of bias. |
Firra 2013.
| Methods | Study design: RCT Multicentre or single‐centre: single Setting: USA Period: not reported Details of sample size calculation: "to achieve a power of 0.80 with an estimated conventional large effect size (f = 0.40), we sought a sample size of 66 women (33 with urge UI and 33 with stress UI) with 11 participants per treatment by diagnosis group." Follow‐up: 8 weeks |
|
| Participants | N: 63 randomised, 48 analysed Mean (SD) age: UUI overall 61.0 (12.4) years; A ‐ 57.3 (12.5) years; B ‐ 66.5 (12.4) years; C ‐ 63.0 (14.5) years SUI overall 55.1 (14.4), A ‐ 52.7 (15.0) years; B ‐ 63.6 (13.3) years; C ‐ 48.2 (16.2) years Sex: women Inclusion criteria: SUI or UUI diagnosed by urodynamics or Medical, Epidemiological and Social Aspects of Aging (MESA) questionnaire, parous or nulliparous women 21 years or older, manual dexterity to dial the Liberty Electrical Stimulation Unit, fluent English, ≥ 3 incontinent episodes in 3 days. Women on HRT to maintain same oestrogen intake throughout study, women not taking hormones were asked not to start an oestrogen regimen during study Exclusion criteria: zero score on Oxford pelvic floor muscle strength scale, denervation injury to the sphincters, anti‐incontinence surgery, vaginal extent to extent that middle finger could not be inserted into vagina, BMI > 50, stage III/IV prolapse, pregnancy, neurologic conditions, any potentially confounding prescriptions drugs |
|
| Interventions | UUI A (n = 7) intravaginal electrical stimulation plus PFMT. 14 sessions of 60 min PFMT exercises, then 30 min (12.5 Hz) at highest tolerable intensity. Tampon‐shaped Liberty electrical stimulation device. B (n = 8) PFMT alone. 60 min twice a week for 8 weeks C (n = 7) no active treatment SUI D (n = 14) as per group A E (n = 15) as per group B F (n = 12) as per group C |
|
| Outcomes | York Incontinence Perception Scale (YIPS) score (higher score is better) % change in YIPS score Pelvic floor muscle strength, cm H2O % change in pelvic floor muscle strength, cm H2O Incontinence episodes in 3 days *Incontinence episodes per day % change in incontinence episodes in 3 days Frequency of micturitions in 3 days *Frequency of micturitions per day % change in frequency of micturitions in 3 days |
|
| Notes | Different numbers of participants reported in thesis and journal article. *Mean (SD) per day calculated from 3‐day data: mean and SD divided by 3 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "2 containers were prepared representing diagnosis groups (urge or stress incontinence). Each container held 33 slips of paper with 11 reading "e‐stim," 11 reading "therapeutic exercise" and 11 reading "control." The office assistant offered the correct diagnostic container to the participant on the second visit." |
| Allocation concealment (selection bias) | Low risk | "2 containers were prepared representing diagnosis groups (urge or stress incontinence). Each container held 33 slips of paper with 11 reading "e‐stim," 11 reading "therapeutic exercise" and 11 reading "control." The office assistant offered the correct diagnostic container to the participant on the second visit." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The primary researcher performed the outcome measures and administered the exercise programs. She was blinded to the participants' diagnosis as determined by the MESA but was not blinded to group allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "The primary researcher performed the outcome measures and administered the exercise programs. She was blinded to the participants' diagnosis as determined by the MESA but was not blinded to group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "[O]f those who dropped out after randomization most (11/16) were in the exercise and stimulation group...there was no indication that discomfort was a factor." |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to be reported in full |
| Other bias | High risk | "This study was funded in part by the Texas Physical Therapy Foundation" |
Goode 2003.
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: USA Period: October 1995 – May 2001 Details of sample size calculation: a sample size of 200 was selected to allow detection of 15% differences in reduction of episodes on bladder diary between treatment groups with 85% power and a significance level of 0.05, assuming a 2‐sided hypothesis test and a pooled within‐group SD of 20%. Follow‐up: 8 weeks' treatment |
|
| Participants | N: 200 randomised and analysed Mean (SD) age: A ‐ 57.7 (10.0) years; B ‐ 54.9 (9.4) years; C ‐ 55.9 (10.1) years Inclusion criteria: community‐dwelling women, 40 years or older, ambulatory, and describe a pattern of predominantly stress incontinence occurring at least twice per week and persisting for at least 3 months. Stress incontinence had to be the predominant pattern (i.e. the number of stress episodes had to exceed the number of urge and other episodes). Stress incontinence had to be objectively demonstrated during urodynamic testing. Exclusion criteria: continual leakage, postvoid residual urine volume greater than 150 mL, severe uterine prolapse (past the vaginal introitus), decompensated congestive heart failure, haemoglobin A1C ≥ 9, or impaired mental status (Mini‐Mental State Examination score > 24). |
|
| Interventions | A (n = 66) ‐ behavioural training. 4 clinic visits at 2‐week intervals. During visit 1, anorectal biofeedback (session lasting approx. 20 min) was used to help patients identify pelvic floor muscles and teach them how to contract and relax these muscles. Patients received verbal and written instructions for 3 sessions of pelvic floor muscle exercises daily. Each session consisted of 15 repetitions of 2‐to 4‐second contractions with equal periods of relaxation. The initial duration of each individual contraction was determined based on the ability demonstrated by the patient in the training session. Patients were advised to do 1 session lying, sitting, and standing, and whenever possible to integrate the exercises into other daily activities. Once daily they were to practice interruption or slowing of the urinary stream during voiding. During visits 2, 3, and 4, the home exercise regimen was adjusted by gradually increasing the duration of each contraction to a maximum of 10 seconds, with an equal period of relaxation between contractions. B (n = 67) ‐ electrical stimulation plus behavioural training. ES with home unit (Hollister InCare, Libertyville, Ill) programmed to deliver stimulation via vaginal probe with: biphasic pulses (frequency of 20 Hz), pulse width of 1 ms, and pulse train to rest period of 1:1. The current intensity was adjusted by the patient to the maximum level she could tolerate comfortably, up to 100 mA. Simultaneous with each muscle contraction induced by PFES, patients performed a voluntary pelvic floor muscle contraction. Patients were instructed to use the PFES unit for 15 min every other day. On alternate days, to keep the exercise time consistent between groups, patients were instructed to perform 3 sessions of pelvic floor muscle exercises (as in the behavioral training group). C (n = 67) ‐ control group. Self‐administered behavioural training. Booklet provided with written instructions for an 8‐week self‐help behavioral programme that was based on the behavioural training program described above but was completely self‐administered, without benefit of professional expertise or equipment. |
|
| Outcomes | N with improvement in SUI (subjective description of treatment outcome 'better' or 'much better') N 'somewhat' or 'completely' satisfied N with fewer incontinence episodes (subjective assessment) N reporting smaller episodes (subjective assessment) N wearing less protection Incontinence no longer restricts activities Incontinence episodes per week % reduction in incontinence episodes per week Objectively measured continence Adverse effects |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Within each stratum (stratified by self‐identified race), patients were randomized using a block size of 6 to ensure equity in group size. The randomization schedule was computer‐generated by the biostatistician and implemented by the nurse practitioners. "Stratification procedures were used at randomization to ensure that groups had similar types and severity of incontinence and race distribution (black or white)" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants or providers |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A: 12/66 withdrew (7 directly related to intervention) B 8/67 withdrew (2 directly related to intervention) C 25/67 withdrew (5 directly related to intervention) Adequate explanations for withdrawals. All participants who were randomised were included in primary analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Hahn 1991.
| Methods | Study design: RCT Multicentre or single‐centre: single Setting: Sweden Period: not reported Details of sample size calculation: not reported Follow‐up: 12 months' treatment, 4 year follow‐up |
|
| Participants | N: 20 randomised Mean (range) age: 47.2 (34‐64) years Inclusion criteria: pure GSUI, referred for surgery Exclusion criteria: previous continence surgery |
|
| Interventions | A (n = 10) ‐ conservative treatment. Pelvic floor training exercises performed in the supine, sitting and standing positions. Submaximal squeezing 2 s, relax 2 s; maximal squeezing 5 s, relax 5 s. "Repeated 5‐10 times, also against resistance and during different provocative situations like coughing." Also "submaximal squeezing 40secs or more, exercise during maneuvers causing stress incontinence, like running or jumping." Women instructed to use the exercise programme at home 6‐8 times per day. Weekly visits to physiotherapist for 4 weeks, then monthly for 5 months. B (n = 10) ‐ electrical stimulation. Intravaginal with Contelle device. Alternating pulses at repetition frequency of 50 Hz. Women instructed to use device 6‐8 hours per night |
|
| Outcomes | Subjective improvement Objective cure (defined as < 2 g pad test) at 6 months |
|
| Notes | "Patients not cured by the first treatment were offered the other one" Four year outcomes not usable because not reported by original intervention group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals reported in first period of treatment |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | High risk | The study was supported by Neurologiskt Handikappades Riksförbund (Neurological Disabilities National Association) and LIC Hygien |
Haig 1995.
| Methods | Study design: quasi‐RCT Multicentre or single‐centre: not reported Setting: UK Period: not reported Details of sample size calculation: not reported Follow‐up: 3 months' treatment |
|
| Participants | N: 58 randomised Mean (range) age: 50 (25‐72) Inclusion criteria: urodynamic diagnosis of uncomplicated GSI Exclusion criteria: not reported |
|
| Interventions | A (n = 20) ‐ conservative treatment. Pelvic floor muscle exercise programme. "Home exercise programme of thrice daily 'fast' and 'held' contractions in sitting and standing was given and patients were encouraged to perform these throughout the 3 months." B (n = 20) ‐ electrical stimulation plus PFME. Interferential therapy, 20 min 10‐40 Hz using vaginal electrodes C (n = 18) ‐ sham ES plus PFME. Placebo interferential therapy, 20 min A + B + C ‐ 5 appointments in month 1, 12 in month 2 (thrice weekly), 3 in month 3. Month 1 is identical for all three groups (i.e. ES and sham ES introduced in month 2) |
|
| Outcomes | Perceived severity of leakage (VAS) Micturitions per 48h 48hr pad tests (g) Quality of life Perceived effect on life (VAS) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "[A]ssigned consecutively to one of 3 treatment groups" |
| Allocation concealment (selection bias) | High risk | "[A]ssigned consecutively to one of 3 treatment groups" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind all participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A: 12/20 withdrew B: 9/20 withdrew C: 9/20 withdrew No explanation for withdrawals. Only participants completing the trial are included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Outcomes seem to be reported in full |
| Other bias | High risk | Groups not balanced at baseline in terms of objectively measured leakage |
Henalla 1989.
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: UK Period: not reported Details of sample size calculation: not reported Follow‐up: 3 months' treatment, 9 months' follow‐up |
|
| Participants | N: 104 randomised, 100 analysed Age range: 26‐74 years Inclusion criteria: urodynamic diagnosis of GSUI Exclusion criteria: complicated history of incontinence such as fistula or more than one previous surgical procedure for correction of incontinence, major degree of prolapse, absolute contraindication for oestrogen treatment |
|
| Interventions | A (n = 26) ‐ conservative treatment. Pelvic floor exercises: "they were asked to draw their pelvic floor muscles together for 5 seconds and repeat the manoeuvre 5 times every hour. Patients were seen weekly by the physiotherapist to monitor their progress." B (n = 26) ‐ electrical stimulation. One 20 min session of interferential therapy per week for 10 weeks. Current 0‐100 Hz, intensity adjusted according to participant's tolerance C (n = 24) ‐ oestrogen vaginal cream Premarin (containing equine oestrogens), 2 g inserted with applicator every night for 12 weeks D (n = 25) ‐ no treatment |
|
| Outcomes | Participants with no improvement in SUI at 3 months Cured or significant improvement at 9 months Change in pad tests (g) Maximum urethral closure pressure (cmH2O) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[A]llocated at random" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All patients who entered the study were seen and assessed clinically by one investigator" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals reported (mistake in text regarding 25 or 26 allocated to group B) |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported adequately for all groups |
| Other bias | Low risk | Nothing to indicate any other bias |
Hofbauer 1990.
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: Austria Period: not reported Details of sample size calculation: not reported Follow‐up: 6 weeks' treatment, 6 months' follow‐up |
|
| Participants | N: 43 randomised Mean (SD) age: 57.5 (12) Inclusion criteria: GSUI Exclusion criteria: not reported |
|
| Interventions | A (n = 11) ‐ electrical stimulation + physiotherapy. 3 times a week for 6 weeks (18 sessions): 10 ms impulse duration, 15 ms pause, intensity increased until patient felt a contraction. Patients were asked to actively contract the pelvic floor when a contraction was induced. Physiotherapy included pelvic floor, abdominal wall and adductor exercises, performed twice weekly for 20 min with a physiotherapist. Exercises to be continued at home after completion of the intervention. B (n = 11) ‐ physiotherapy alone C (n = 11) ‐ electrical stimulation alone D (n = 10) ‐ sham electrical stimulation. Electrodes placed in the lumbar region. |
|
| Outcomes | Participants cured (subjective) Participants cured or improved (subjective) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[P]laced prospectively and randomly in four groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Groups C and D blinded to each other, other blinding not possible. Personnel blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals reported |
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes reported by intervention group |
| Other bias | Unclear risk | Insufficient information |
Huebner 2011.
| Methods | Study design: RCT Multicentre or single‐centre: multicentre Setting: Germany; Department of Obstetrics and Gynecology, University Hospital of Tuebingen; Department of Urogynecology, German Pelvic Floor Center, St. Hedwig Hospitals, Berlin Period: August 2004 – December 2006 Details of sample size calculation: not reported Follow‐up: 12 weeks' treatment |
|
| Participants | N: 108 randomised* Mean (SD) age: 49.8 (12.9) years Inclusion criteria: age 18+ years with clinically verified SUI and mixed urinary incontinence with predominant SUI, both with leakage of urine on coughing and the ability to perform a voluntary pelvic floor muscle contraction between II–IV on the Oxford scale, ability to give consent and negative pregnancy test Exclusion criteria: cardiac pacemaker, non‐contracting and non‐functioning pelvic floor, pelvic organ prolapse, genital anomalies, urogynaecological surgery within the last 2 months, and participation in other studies, (OAB) symptoms, mixed urinary incontinence with predominant OAB. |
|
| Interventions | All participants received the same device with a vaginal electrode and were made familiar with it. A (n = 36) ‐ conventional intravaginal electrical stimulation plus EMG biofeedback‐assisted PMFT. 15 min twice a day. Frequency 50 Hz, 20‐80 mA current intensity. Stimulation 8 s, resting 15 s, active contracting 8 s, resting 15 s B (n = 36) ‐ dynamic intravaginal electrical stimulation plus EMG biofeedback‐assisted PMFT. 15 min twice a day. Frequency 50 Hz, 20‐80 mA current intensity. Active contracting 8 seconds. After reaching the maximum contraction, the electrical stimulation was added. Stimulation 8 s, resting 15 s C (n = 36) ‐ EMG biofeedback‐assisted pelvic floor muscle training. 15 min twice a day. Active contracting 8 s, resting 15 s |
|
| Outcomes | Change in psychological stress regarding UI ('How bothersome are the symptoms of urinary incontinence?' measured on 1‐10 VAS) Change in King's Health Questionnaire score Change in number of pads per 24 h Change in pad tests (g) Contractility of pelvic floor muscles (Oxford scale) Contractility of pelvic floor muscles measured by EMG (μV) |
|
| Notes | *Assume ITT analysis not carried out therefore 88/108 included in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Subjects were randomized into three groups according to their appearance" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "All subjects were examined by the same person" but unclear if this person was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals: A: 3/36; B 8/36; C 9/36. Explanations given for withdrawals but unclear which explanation relates to which intervention group. 13/20 withdrawals were due to 'motivation' problems. Unclear if all participants who were randomised were included in analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to be reported in full |
| Other bias | High risk | Groups not comparable at baseline: "there was a difference in the number of pads used per day prior to treatment." |
Jeyaseelan 1999.
| Methods | Study design: RCT Multicentre or single‐centre: multicentre Setting: UK Period: not reported Details of sample size calculation: sample size calculations based on another study with the 20 min pad test taken as primary outcome measure showed that a sample size of 12 in each group would have 90% chance of detecting a difference in means of −32.2 g (the difference between the treatment group mean of −29.9 g and the control group mean of 2.3 g) assuming that the common standard deviation is 23.048 using a two‐group t‐test with a 0.05 two‐sided significance level. Follow‐up: 8 weeks' treatment |
|
| Participants | N: 27 randomised, 24 analysed Mean (SD) age: not reported Inclusion criteria: urodynamically proven SUI, no neurological conditions diagnosed by consultant Exclusion criteria: previous ES for SUI, prolapse, pregnancy, pacemakers and cardiomyopathy, abnormal urological/gynaecological findings, UTI, vaginal infection, pelvic floor surgery within last 6 months |
|
| Interventions | A (n = 14) ‐ sham electrical stimulation. One 250 μ impulse every min for 60 min (proven to have no physiological effect on muscle) B (n = 13) ‐ electrical stimulation with portable stimulator PS1 (Dynamic Medical Instruments). One hour per day for 8 weeks. Background low frequency (to target slow twitch fibres) and intermediate frequency with initial doublet (to target fast twitch fibres). A low number of impulses within the high frequency component and adequate rest periods between stimulus trains were used to reduce premature fatigue |
|
| Outcomes | Change in incontinence episodes per week Change in pad tests (g) Change in IIQ score Change in UDI score PFM strength (cmH2O) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were allocated a number between 1 and 24 using a table of random numbers, if the patient was allocated a number between 1 and 12 they were to receive the sham stimulation whilst the remainder received the new pattern of stimulation" |
| Allocation concealment (selection bias) | Low risk | "[T]he investigator was responsible for randomly allocating all subjects to treatment groups, assigning stimulators and monitoring treatment" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and clinicians were blinded but investigator was not. "The study was double‐blind in that neither the subjects nor the clinician performing the outcome assessments knew what stimulation pattern was being administered." "Patients were instructed not to discuss their stimulation with the clinician conducting the assessments" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "[T]rained clinicians were responsible for performing all outcome assessment and were not aware of which group the patient was in" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 2/14 and B 1/13 withdrew because "stimulation protocol was too demanding". No differential attrition |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in full. Individual patient data reported |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Jeyaseelan 2002.
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: UK Period: not reported Details of sample size calculation: not reported Follow‐up: 8 weeks' treatment |
|
| Participants | N: 16 randomised Mean (SD) age: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | A (n = ?) ‐ electrical stimulation. One hour per day (except when menstruating) B (n = ?) ‐ PFMT. Individualised exercise regime, told to practise at least 3 times per day and to progress the exercises over the treatment period. Given information sheets and Periform probe as a means of biofeedback. C (n = ?) ‐ ES plus PFMT |
|
| Outcomes | % change in pad test (g) % change in leakage (mL) % change in IIQ score % change in UDI score % change in PFM strength (Oxford scale) |
|
| Notes | Numbers per intervention group not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomly allocated to one of three treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants. Other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers per intervention group not reported |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported as % change, not absolute numbers, but this applies across all groups |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Jeyaseelan 2003.
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: UK Period: not reported Details of sample size calculation: not reported Follow‐up: 8 weeks |
|
| Participants | N: 19 randomised and analysed Mean (SD) age: not reported Inclusion criteria: SUI with no contraindications for ES Exclusion criteria: not reported |
|
| Interventions | A (n = 6) ‐ electrical stimulation, with "a range of frequencies in conjunction with a longer duty cycle than is traditionally used" B (n = 7) ‐ PFMT, "as per usual physiotherapy practice" C (n = 6) ‐ ES plus PFMT |
|
| Outcomes | % change in pad test % change in leakage % change in IIQ score % change in UDI score % change in PFM strength |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals reported, outcomes seem to be reported for all participants who were randomised |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Knight 1998.
| Methods | Study design: RCT Multicentre or single‐centre: single Setting: Bradford Royal Infirmary, UK Period: 1992 to 1996 Details of sample size calculation: not reported Follow‐up: 6 months' treatment, 12 months' follow‐up |
|
| Participants | N: 70 randomised Mean (SD) age: not reported Inclusion criteria: age 16‐75 years, sterile urine, urodynamically proven GSI, written informed consent, English speaking Exclusion criteria: UTI, unstable bladder, unable to perform a voluntary pelvic floor contraction, pregnancy, breastfeeding, pelvic malignancy, cardia pacemaker, diagnosed neurological conditions, diabetes, HRT started with last 3 months |
|
| Interventions | A (n = 21) ‐ PFMT and biofeedback. Each subject was issued with a pelvic floor exerciser (PFX), for home biofeedback, and instructed to perform 1 of the 6 daily exercise sessions using this device. In‐clinic visual biofeedback with a computerised graphical display was performed weekly for 1st month of treatment, then on alternate weeks for remainder of 6‐month programme. This treatment was considered baseline exercise programme and an identical exercise regimen was issued to the 2 treatment groups. B (n = 25) ‐ low intensity vaginal electrical stimulation at home (except during menstruation) plus PFMT and biofeedback. In addition to baseline exercise programme, participants were instructed to use the battery‐operated stimulation units overnight at low intensity. Required current was described to participants as a barely perceptible tingling sensation. Stimulator had pre‐set frequencies of trains of 10 Hz with bursts of 35 Hz to try to maintain fast twitch fibre activity. Pulse width 200 ms. Duty cycle 5 s on/off. C (n = 24) ‐ maximal electrical stimulation in clinic plus PFMT and biofeedback. In addition to baseline exercise programme, 16 × 30 min sessions of maximal vaginal ES at 35Hz, pulse width 250 microseconds, duty cycle 5 s on/off. Pts instructed to perform a voluntary contraction with the stimulation |
|
| Outcomes | Participants with subjective cure or 'great improvement' at 6 and 12 months Pad tests at 6 months (g) Objectively measured cure or great improvement (defined as ≥75% reduction in urine loss at pad test) at 6 and 12 months % change in pelvic floor strength |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[T]able of random numbers" |
| Allocation concealment (selection bias) | Low risk | "[T]reatment groups were sealed in consecutively numbered envelopes. Neither subject nor examiner had prior knowledge of the treatment contained in the envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 14%, B 24%, C 17% withdrawal. ITT analysis carried out to give conservative estimate |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes not reported, e.g. "frequency/volume charts were poorly completed. Sets of data were incomplete and were unsuitable for statistical analysis" |
| Other bias | High risk | "[R]andomisation failed to produce an evenly matched population" |
Laycock 1988.
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: Bradford Royal Infirmary, UK Period: not reported Details of sample size calculation: not reported Follow‐up: different treatment periods for different groups, follow‐up 3 months |
|
| Participants | N: 36 randomised, 29 analysed Mean (SD) age: 44 (30‐74) years Inclusion criteria: urodynamically proven GSI Exclusion criteria: not reported |
|
| Interventions | A (n = 20) ‐ electrical stimulation. Interferential therapy: average of 11 (range 7‐13) 30 min sessions, 2‐3 times per week for 4‐6 weeks B (n = 16) ‐ PFMT course for 6‐8 weeks, attending 1/week and following a home exercise programme. |
|
| Outcomes | Participants with much or some improvement in SUI Participants with no improvement in SUI Change in urine loss measured by pad tests (g) |
|
| Notes | Length of time for pad test not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7/36 withdrew. Does not report withdrawals by intervention group or give reasons for withdrawal |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Laycock 1993a.
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: unclear Period: not reported Details of sample size calculation: not reported Follow‐up: approx. 6 weeks' treatment |
|
| Participants | N: 46 randomised Mean (range) age: A ‐ 41.8 (29‐59) years; B ‐ 39.5 (28‐53) years Inclusion criteria: women with GSUI Exclusion criteria: previous physiotherapy for GSUI, pregnancy, neurological dysfunction, present or previous pelvic malignancy, pacemaker |
|
| Interventions | A (n = 23) ‐ transcutaneous electrical stimulation. Interferential therapy using bipolar technique with Endomed 433 device. One medium electrode placed over perineal body and a small electrode positioned immediately inferior to the symphisis pubis. 10 treatments, first one 15 min, all others 30 min. Each patient was encouraged to accept her maximum current intensity. 3 different frequencies, 10 min each: 1 Hz, 10‐40 Hz (sweep), 40 Hz. Participants agreed not to practise PFM exercises so that ES could be evaluated in isolation. B (n = 17) ‐ PFMT. Patient‐specific exercise regimes, each patient being instructed to perform 5 maximum voluntary contractions every hour throughout the day, length of each contraction determined for each patient at assessment and revised during subsequent visits. Vaginal cones were supplied at the second visit and patients instructed to exercise with an appropriate cone for 10 min twice each day (except during menstruation). Patients seen once a week for 2 weeks, then once every 10 days for average of 6 weeks. Treatment incorporating digital biofeedback was given at each session. |
|
| Outcomes | Participants with SUI (worse or no change or improved) Participants with no improvement in SUI Women with improvement Objectively measured incontinence (according to pad test) No improvement in objectively measured incontinence (according to pad test) |
|
| Notes | Numbers in table 3 don't add up to n = 23 (group A) and n = 17 (group B) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[R]andom number tables" |
| Allocation concealment (selection bias) | Low risk | "[S]ealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants, "the same physiotherapist conducted the treatment sessions together with all pre‐ and post‐treatment assessments." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "[T]he same physiotherapist conducted the treatment sessions together with all pre‐ and post‐treatment assessments." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals reported during study period but numbers in text and table do not agree |
| Selective reporting (reporting bias) | Unclear risk | Data collected but not presented for frequency/volume |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Laycock 1993b.
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: unclear Period: not reported Details of sample size calculation: not reported Follow‐up: 8‐12 weeks |
|
| Participants | N: 30 randomised, 26 analysed Mean (range) age: A ‐ 43.7 (25‐62) years; B ‐ 46.2 (16‐66) years Inclusion criteria: women with GSUI Exclusion criteria: previous physiotherapy for GSUI, pregnancy, neurological dysfunction, present or previous pelvic malignancy, pacemaker |
|
| Interventions | A (n = 15) ‐ transcutaneous electrical stimulation. Interferential therapy using bipolar technique with Endomed 433 device. One medium electrode placed over perineal body and a small electrode positioned immediately inferior to the symphisis pubis. 10 treatments, first one 15 min, all others 30 min. Each patient was encouraged to accept her maximum current intensity. 3 different frequencies, 10 min each: 1 Hz, 10‐40 Hz (sweep), 40 Hz. Participants told to expect pins & needles sensation B (n = 15) ‐ sham electrical stimulation. Endomed 433 modified to bypass the patient circuit and divert the interferential current to a separate circuit within the machine so the patient received no current. Patients told to expect no sensation |
|
| Outcomes | Participants with SUI (worse or no change or improved) Participants with no improvement in SUI VAS score (subjective assessment of SUI severity; higher score indicates greater severity) % difference in VAS score Objectively measured incontinence (according to pad test)* Voids per day % decrease in pad test (g) Difference from baseline in voids per day |
|
| Notes | *Numbers in table 5 don't add up to n = 15 (group A) and n = 11 (group B) Length of time for pad test is unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[R]andom number tables" |
| Allocation concealment (selection bias) | Low risk | "[S]ealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants blinded "The same physiotherapist conducted the treatment sessions together with all pre‐ and post‐treatment assessments." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A 0/15, B 4/15 withdrew (no explanation) |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes not reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Lo 2003.
| Methods | Study design: RCT Single centre Setting: Department of a Regional Hospital in Perth, Western, Australia Period: not reported Sample size: 50 participants in each group would be sufficient to give 0.8 power at the 0.05 alpha level for for two‐sided alternative. Calculation of sample size was performed using the PASS statistical software (NCSS, Kaysville, Utah, USA). Follow‐up: 4 weeks |
|
| Participants | N: 24 randomised and analysed Sex: women Mean age (SD): A (n =12) ‐ 52.1 (17.5) years; B (n = 12) ‐ 55.1 (15.1) years Inclusion criteria: women aged 20 years or older with stress or urgency urinary incontinence Exclusion criteria: altered mental state, urinary incontinence caused by problems other than stress or urge, transient incontinence, or severe disability requiring full assistance with all acts of daily living |
|
| Interventions | A (n = 12) ‐ pelvic floor muscle exercises (PFME). 12 sessions (3 per week for 4 weeks): 10 sets of 5 contractions with 30 second rest between each set. Then repeated after an hour. B (n = 12) ‐ interferential therapy (ITT) plus PFME. 12 sessions (3 per week for 4 weeks) of 50 pelvic floor contractions followed by ITT with Nemectrodyne 5 stimulator then another 50 contractions. 2 anterior flat electrodes placed over obturator foramen 1.5 cm‐2 cm lateral to symphasis, 2 posterior electrodes placed medial to ischial tuberosities either side of anus. ITT was at highest tolerable frequency between 0‐100 Hz for 15 min (session 1), then 30 min for sessions 2‐12. |
|
| Outcomes | Pad test (g) Frequency (number of micturitions per day) Nocturia (number of nocturia episodes per night) Improvement in stop/start test, defined as change from unable to stop to being able to slow, or change from able to slow to able to stop |
|
| Notes | No usable data. Not stratified by stress/urgency incontinence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomly allocated as soon as they gave written consent, using the sealed envelope method". |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported. Outcomes seem to be reported for all participants who were randomised. |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Lopes 2014.
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: France Period: not reported Details of sample size calculation: 100 per group, based on the assumptions therapeutic benefit of 60% in the least effective group and a difference of 20% between the 2 groups Follow‐up: 6 months |
|
| Participants | N: 163 randomised Mean (SD) age: A ‐ 52.4 (13.5) years; B ‐ 50.2 (13.8) years Inclusion criteria: women with SUI or stress‐predominant MUI having responded to 10‐15 sessions of perineal re‐education (response defined as clinical improvement according to the specific criteria of the investigator and by a score ICIQ ≥ 12) Exclusion criteria: gave birth within last 6 months, pelvic surgery within last year, voiding disorder related to congenital malformation or neurological disorder, urinary incontinence treated surgically or medically treated in last 6 months, perineal hypoesthesia, conditions prohibiting the use of intravaginal probe (vaginal atrophy, prolapse degree > 2 , permanent metrorrhagia and patients with a pacemaker) |
|
| Interventions | A (n = 77) ‐ home intravaginal electrical stimulation with GYNEFFIK. 3 × 30 min session per week B (n = 86) ‐ usual care. Any other therapy at the discretion of the investigator |
|
| Outcomes | Participants with improvement in SUI Participants maintaining benefit of initial perineal re‐education (defined as no worsening in ICIQ and Ditrovie score) ICIQ score (higher score = greater severity) Ditrovie score (higher score = greater severity) |
|
| Notes | Unclear what treatment, if any, usual care group got | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by clusters of 30 gynaecologists and 20 GPs |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals: A ‐ 12/76 (1 adverse reaction, 1 due to ineffective treatment) B ‐ 3/85 (1 adverse reaction, 2 due to ineffective treatment) Other reasons: poor adherence to protocol, personal reasons, lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | High risk | 2 authors are employees of the device manufacturer |
Luber 1997.
| Methods | Study design: RCT Multicentre or single‐centre: single Setting: USA Period: June 1993 – January 1995 Details of sample size calculation: assumption of spontaneous recovery rate of 10% (control) and 50% (intervention). Detection of this difference with 0.05 significance, power 0.90 and 2 interim analyses would require 57 subjects Follow‐up: 12 weeks' treatment |
|
| Participants | N: 54 randomised, 44 analysed Mean (SD) age: overall 53.9 (10.3) years; A ‐ 54.1 (SD not reported) years; B ‐ 53.6 (SD not reported) years Inclusion criteria: GSUI diagnosis consistent with ICI criteria, ability to retain vaginal probe and to co‐operate with the study protocol, ability to understand randomisation and give informed consent. Exclusion criteria: POP ≥ grade II, detrusor instability, postvoid residual urine > 100 cc, extra‐urethral incontinence, history of vaginal intraepithelial neoplasia, evidence of vaginal or urinary tract infection, fixed immobile urethra, urodynamic evidence consistent with intrinsic sphincteric deficiency |
|
| Interventions | A (n = 26) ‐ electrical stimulation. Vaginal probes. 2 × 15 min sessions per day for 12 weeks. Pulse width 2msec, work:rest schedule 2 s:4 s, 50 Hz frequency, 10‐100 mA B (n = 28) ‐ sham electrical stimulation. Vaginal probes. 2 × 15 min sessions per day for 12 weeks. "Wiring from the unit to the probe was covertly discontinuous." |
|
| Outcomes | Subjective cure (5 on 1‐5 scale) Subjective improvement or cure (3‐4 on 1‐5 scale) Objective cure (negative stress test in repeat urodynamics with full bladder) Incontinence episodes per 24 h VLPP Valsalva leak point pressure, (cmH2O) Postvoid residual urine volume (cm³) Adverse effects |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomization using opaque envelopes" |
| Allocation concealment (selection bias) | Low risk | "[O]paque envelopes. Records of the randomization process were maintained by clerical staff without direct contact with the enrolled patients of study personnel." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded. Physical therapy and nursing personnel blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Principal investigator was not involved in initiation of therapy" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals: A 6/26 (1 death, 3 discomfort, 2 discouragement) B 4/28 (2 discomfort, 2 discouragement) ITT analysis not carried out |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full. |
| Other bias | Unclear risk | "[T]he study was discontinued after interim analysis revealed that after enrolment of 54 patients, no difference was observed in the outcomes between the two groups" |
Maher 2009.
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: School of Physiotherapy and Performance Science, University College Dublin, Dublin, Ireland Period: not reported Details of sample size calculation: not reported Follow‐up: 8 weeks' treatment |
|
| Participants | N: 18 randomised Age range: 36‐46 Inclusion criteria: diagnosis of stress urinary incontinence and a body mass index (BMI) of less than 30 (kg/m²) Exclusion criteria: not reported |
|
| Interventions | Both groups: 30 min of stimulation at least 4 times per week at home for 8 weeks. Subjects were blinded to sonography and were not instructed regarding pelvic floor contractions A (n = ?) ‐ electrical stimulation with external electrodes B (n = ?) ‐ electrical stimulation with vaginal electrodes |
|
| Outcomes | Self‐reported symptoms and pad use No usable data |
|
| Notes | No data reported. Conference abstract says "the study is still ongoing." Have not found any further publications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals reported |
| Selective reporting (reporting bias) | Unclear risk | No data reported |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Min 2015.
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: Chengdu, China Period: January 2012 to December 2013 Details of sample size calculation: not mentioned Follow‐up: 13 ˜ 24 months |
|
| Participants | N: randomised and analysed. 120 cases in total, Group A 60 cases, Group B 60 cases, no dropouts. Mean (SD) age: 48 (3) years Inclusion criteria: pad weight test positive; no abnormal in nerve examination; urinalysis found no urinary tract infection; normal in urodynamic studies Exclusion criteria: urge urinary incontinence; mixed urinary incontinence; neurogenic bladder |
|
| Interventions | A (n =60) ‐ tension‐free tape obturator technique (TVT‐O) B (n = 60) ‐ TVT‐O with biofeedback pelvic floor electrical stimulation |
|
| Outcomes | Participants cured Participants with improvement in SUI Incontinence episodes per 72 h Micturitions per 72 h Pad tests: g/h Objectively measured incontinence VLPP Valsalva leak point pressure (cmH2O) MFR Maximum flow rate (mL/s) RUV Residual urine volume (mL) Adverse effects Quality of life I‐QOL Urinary incontinence related quality of life questionnaire ICI‐Q‐SF International Advisory Committee on urinary incontinence urinary incontinence questionnaire short form |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised digital table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants, no blinding to personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Olah 1990.
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: UK Period: not reported Details of sample size calculation: not reported Follow‐up: 4 weeks' treatment, 6 months' follow‐up |
|
| Participants | N: 69 randomised, 47 analysed at 6 months Mean (SD) age: A ‐ 43.2 (8.9) years; B ‐ 47.9 (13.0) years Inclusion criteria: symptoms of UI (predominantly stress incontinence) Exclusion criteria: treated with pelvic floor physiotherapy within last 6 months |
|
| Interventions | All participants were taught pelvic floor exercises. A (n = 33) ‐ vaginal cones. Supervised physiotherapy appointment once a week for 4 weeks. 9 cones (20‐100 g). Instructed to train the PFM by actively retaining heaviest cone possible while contracting the PFM – twice a day for up to 15 min at home. When successful on two consecutive occasions move on to next weighted cone. B (n = 36) electrical stimulation with interferential therapy. 3 × 15 min sessions a week for 4 weeks. 2 electrodes placed on abdomen and 2 on inner thighs. Interferential current 0‐100 mA, maximum tolerable intensity. |
|
| Outcomes | Participants cured (subjective) Participants with improvement in SUI (subjective) Pad tests (g) at 4 weeks and 6 months Women requiring continence surgery Time spent with physiotherapist (min) No leakage at 6 months |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals: A ‐ 5/33, B ‐ 4/36 (inability to tolerate cones) A ‐ 6/33, B ‐ 0/36 (did not attend) A ‐ 3/33, B ‐ 2/36 (did not complete 6‐month assessment due to leakage serious enough to warrant surgery) A ‐ 1/33 developed psychiatric disorder B ‐ 1/36 non‐study related death A 15/33, B 7/36 withdrew "All patients included in the analysis of the % improvement between the groups": unclear what assumptions were made regarding missing data. |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Oldham 2013.
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: UK Period: not reported Details of sample size calculation: "the study was powered to detect a 3 point (common standard deviation of 6) between group difference on the ICIQ‐UI (scale of 0‐21) with 80% power at a 5% level of significance." Follow‐up: 12 weeks |
|
| Participants | N: 124 randomised, 97 analysed Mean (SD) age: A ‐ 47.9 (8.9) years; B ‐ 48.2 (8.6) years Sex: women Inclusion criteria: women, 18–65 years with self‐reported stress, urge, or mixed incontinence Exclusion criteria: pregnancy or a baby in the last 3 months. Recent abdominal surgery and previous or current active therapy for pelvic malignancy. Implanted pacemaker. Manual dexterity insufficient to place the device. Previous treatment for incontinence (including supervised pelvic floor muscle exercises. Presence of a neurological condition such as multiple sclerosis or Parkinson's disease |
|
| Interventions | A (n = 64) ‐ electrical stimulation. Pelviva device inserted like a tampon into the vagina. The stimulation programme is delivered using a duty cycle of 10 s stimulation followed by 10 s rest that runs for a period of 30 min, pre‐programmed to automatically gradually ramp‐up the intensity of stimulation over a 24‐s period to reach a therapeutic level and switch off automatically after 30 min. During the 10 s 'on time' the device delivers 10 repeats of a short high intensity burst of 50 Hz stimulation immediately preceded by a doublet (125 Hz), superimposed on continuous low frequency 2 Hz stimulation. Plus standardised advice about how and when to undertake pelvic floor muscle exercise. These included 10 slow and controlled squeezing and lifting contractions and 10 quick contractions each repeated 3–4 times a day B (n = 60) ‐ unsupervised conservative treatment (no active treatment). Standardised advice about how and when to undertake pelvic floor muscle exercise. These included 10 slow and controlled squeezing and lifting contractions and 10 quick contractions each repeated 3–4 times a day. |
|
| Outcomes | Participants with improvement (i.e. same or worse ICIQ score) Participants with urinary incontinence International Consultation on Incontinence Questionnaire – Urinary Incontinence (ICIQ‐UI) score (higher score is increased severity) Leak frequency (0‐5 scale, higher score is more leaks) Leak interference (0‐10 scale, higher score is more interference) Leak amount (0‐6 scale, higher score is greater amount) Adverse effects |
|
| Notes | Outcome data not separated by SUI/UUI/MUI – supplementary data received in personal communication from author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[S]ubjects were assigned by a simple computer generated AB randomization list to either the exercise or Pelviva group." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Participants could not be blinded to the treatment group and were aware of the study hypothesis. Every care was taken to ensure the assessor remained blind to treatment allocation and participants were advised not to discuss their treatment with them." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "[T]he assessor remained blind to treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No differential dropout. No explanations for withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Protocol says trial will be 18 weeks and that 200 women were recruited, but final report says 12 weeks and 124 women randomised. |
| Other bias | High risk | Femeda, the company responsible for developing and producing the Pelviva device was the trial sponsor. The sponsor was responsible for developing the Pelviva device, was the funder of the study, and was engaged in the development of the trial design. The sponsor has provided full access to the data and is fully informed of this publication process. The primary author (JO) takes full responsibility for the integrity of the data and accuracy of the data analysis. |
Parsons 2004.
| Methods | Study design: RCT Multicentre or single‐centre: single Setting: UK Period: not reported Details of sample size calculation: not reported Follow‐up: 14 weeks' treatment |
|
| Participants | N: 173 randomised Mean (SD) age: A ‐ 50.37 (11.46) years; B ‐ 51.5 (9.69) years; C ‐ 46.16 (8.53) years; D ‐ 47.47 (11.46) years Inclusion criteria: urodynamic stress incontinence, new diagnosis or no treatment within last 6 months Exclusion criteria: not reported |
|
| Interventions | Groups A, B and C: tailored individual lifestyle advice from experienced physiotherapist. Review at weeks 1, 3, 6, 10 and closing visit at week 14. A (n = 82) ES plus PFMT (Home ES with Unomax stimulator and Periform intra‐vaginal electrode). B (n = 42) sham ES plus PFMT C (n = 40) PFMT alone D (n = 20) no active treatment (deferred treatment) |
|
| Outcomes | Change in pad weight (g) at follow‐up calculated using baseline and change data | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomised into four groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Some participants blinded (i.e. sham v real ES), other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "[T]he investigator was blinded to treatment modality at the time of assessment" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential withdrawal (A 18.3%, B 28.5%, C 25%, D 35%). No explanations for withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | KHQ scores not presented in full |
| Other bias | Unclear risk | Insufficient information |
Patil 2010.
| Methods | Study design: RCT Multicentre or single‐centre: single Setting: India Period: not reported Details of sample size calculation: not reported Follow‐up: 4 weeks' treatment |
|
| Participants | N: 110 randomised, 102 analysed Mean (SD) age: A ‐ 45.17 (6.62) years; B ‐ 43.60 (6.75) years Inclusion criteria: GSI, 30–70 years old Exclusion criteria: urinary incontinence other than GSI, previous surgery for GSI, neurological or psychiatric diseases, ongoing urinary tract infections, use of concomitant treatment, were pregnant, were postnatal, within six weeks postpartum, were obese, diabetics |
|
| Interventions | Both groups: treatment was 3 times a week, for 4 weeks, making a total of 12 treatment sessions under the supervision of a physiotherapist. Patients were asked to perform 8–12 pelvic floor contractions 3 times per day at home A (n = 55) ‐ PFMT. "The patients were asked to perform 8–12 pelvic floor muscles contractions, each of which consisted of 1 contraction held for as long as possible, followed by 3 or 4 short contractions. This was done while observed by a physiotherapist" B (n = 55) ‐ interferential therapy plus PFMT. "Two flat electrodes were placed anteriorly over the obturator foramen, 1.5–2cm lateral to the symphysis; two electrodes were placed posteriorly medial to ischial tuberosity on either side of the anus. The frequency used ranged from 0–100 Hz. Patients received the maximum intensity that they could tolerate. The first treatment session lasted for 15 min. If no ill effects were reported, the duration of subsequent treatment sessions were increased to 30 minutes." PFMT as above |
|
| Outcomes | Incontinence episodes per week Subjective assessment of incontinence (VAS) 1 h pad test (g) IIQ‐7 (higher score = greater severity) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The participants were randomized, using an opaque sealed envelope method" |
| Allocation concealment (selection bias) | Unclear risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All recordings were taken by an independent observer and the examining therapist was kept blinded to the records." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals: Concomitant treatment: A ‐ 1/55. B ‐ 1/55 Motivation problems: A ‐ 4/55. B ‐ 2/55 |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Pereira 2012.
| Methods | Study design: RCT Multicentre or single‐centre: single Setting: Brazil Period: November 2010 to March 2011 Details of sample size calculation: not reported Follow‐up: 6 weeks' treatment |
|
| Participants | N: 14 randomised and analysed Mean (SD) age: A ‐ 68.57 (10.93) years; B ‐ 69.28 (6.94) years Inclusion criteria: > 60 years with at least one episode of stress urinary leakage during the previous month Exclusion criteria: UUI, previous treatment for UI or hormone therapy, ongoing urinary tract infections, cognitive or neurological disorder, uncontrolled hypertension, inability to perform the proposed procedures, or use of pacemaker implantation or metal rods |
|
| Interventions | A (n = 7) surface electrical stimulation. 2 × 20 min sessions per week for 6 weeks (12 sessions in total). The women were positioned in supine, with hip and knee flexion. 4 surface electrodes were used, 2 placed in the suprapubic region and 2 medial to the ischial tuberosity. Electric parameters were frequency at 50 Hz, a 4‐s to 8‐s work‐rest cycle, 700‐s pulse width, stimulation intensity gradually increasing up to the level of tolerable discomfort. The women were not instructed to perform the contraction of the pelvic floor muscles in conjunction with electrical stimulation. B (n = 7) control group. No active treatment |
|
| Outcomes | Participants satisfied (i.e. did not want a different treatment) Adverse effects 1 h pad tests (g) Pelvic floor muscle strength (cmH2O) Quality of life measured by King's Health Questionnaire (higher score = greater severity) Incontinence impact |
|
| Notes | Pilot study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A researcher not involved in data collection or analysis developed a randomization schedule", "computer generated randomization list" |
| Allocation concealment (selection bias) | Low risk | "A researcher not involved in data collection or analysis… produced 14 consecutively numbered sealed opaque envelopes containing each participant's allocation. Immediately after collecting baseline data, the evaluator opened the allocation envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, "one not blinded experienced physical therapist performed evaluations of the two groups" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "[O]ne not blinded experienced physical therapist performed evaluations of the two groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the women completed the treatment and were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Pohl 2004.
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: Germany Period: not reported Details of sample size calculation: not reported Follow‐up: 3 months |
|
| Participants | N: 70 randomised, 31 analysed Mean (SD) age: not reported Inclusion criteria: female SUI Exclusion criteria: not reported |
|
| Interventions | A (n = 21) electrical stimulation B (n = 10) PFMT with visual biofeedback. 10 min twice a day: patients asked to tighten the pelvic muscles and hold the contraction for 10 s followed by a 10 s rest. |
|
| Outcomes | VAS (higher score = greater severity) Pad test (g) (unclear time) PFM strength (Oxford scale) Adverse effects |
|
| Notes | No details given regarding treatment parameters. No SD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[P]rospective randomised study" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | High risk | No explanation for unequal numbers in groups |
Preisinger 1990.
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: Austria Period: not reported Details of sample size calculation: not reported Follow‐up: 10‐12 weeks' treatment |
|
| Participants | N: 43 randomised Mean (SD) age: 57.5 Inclusion criteria: women with SUI Exclusion criteria: any other kind of incontinence |
|
| Interventions | A (n = 11) surging faradic‐typ current plus PFMT. 3 × 10 min sessions per week B (n = 11) PFMT. 2 × 20 min sessions per week C (n = 11) surging faradic‐typ current D (n = 10) control group: sham ES |
|
| Outcomes | Cured participants (objective measure) Participants with improvement in SUI (objective measure) Participants with no improvement (objective measure) Maximum urethreal closure pressure (mmHg) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomly divided into 4 groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind all participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals reported. |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes not reported for group D |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Sand 1995.
| Methods | Study design: RCT Multicentre or single‐centre: multicentre Setting: USA Period: April 1992 – September 1993 Details of sample size calculation: designed to have 80% power to detect 40% difference in improvement rates (10% vs 50%) between groups for one‐sided hypothesis test with 5% type 1 error Follow‐up: 12 weeks' treatment, follow‐up at 14 weeks |
|
| Participants | N: 52 randomised, 44 analysed Mean (SD) age: 53.1 (11.4) Inclusion criteria: GSUI, ambulatory, community dwelling, understand questions, comply with visits, not seek other treatment, no current incontinence treatment, neurologically normal. Exclusion criteria: detrusor instability, pregnant, demand pacemaker, prior pelvic floor stimulation, pelvic implanted devices, active vaginal lesions or infections, UTI, hypermenorrhoea or menorrhaghia, urinary retention (> 100 mL), pelvic surgery in last 6 months, atrophic vaginitis, genital prolapse to introitus, pelvic irradiation, intrinsic sphincteric deficiency |
|
| Interventions | A (n = 35) ‐ electrical stimulation. Fully insertable vaginal electrode (1.025" diameter, 2.5" length) with electrode resistance 85 Ω. Women instructed to use device twice daily for 12 weeks, gradually adjusted amperage to 60‐80 mA or highest tolerable level. Treatment time and duty cycle (stimulation:rest ratio) progressed to allow for improvement in the resistance to muscle fatigue. First 2 weeks: 5 s on:10 s off for 15 min; weeks 3 and 4: 5 s:5 s for 15 min; weeks 5 and 6: 5 s:10 s for 30 min; weeks 7‐12: 5 s:5 s for 30 min B (n = 17) sham electrical stimulation. Same system but limited to maximum output 1 mA |
|
| Outcomes | Severity of stress incontinence measured by VAS Change in severity of stress incontinence measured on VAS Incontinence episodes per 24 h Incontinence episodes per week Micturitions per 24 h Micturitions per week Number of pads per week 20 min pad tests (g) Change in 20 min pad tests (g) Vaginal muscle strength (mm Hg) Change in vaginal muscle strength (mmHg) Adverse effects |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, 2:1 ratio favouring active over placebo; "randomization was established by Boston Biostatistics Inc from a list of computer‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, researchers and study co‐ordinator were all blinded. "The Principal Investigator at each site did not directly answer patient questions during the trial, and patients were instructed not to discuss whether they thought they were using an active device with the Principal Investigator in an attempt to maintain the double blind." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, researchers and study co‐ordinator were all blinded. "The Principal Investigator at each site did not directly answer patient questions during the trial, and patients were instructed not to discuss whether they thought they were using an active device with the Principal Investigator in an attempt to maintain the double blind." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A ‐ 7/35 (3 could not comply with protocol requirements and visit schedules, 2 persistent vaginal irritation, 1 had urgency after 2 weeks of treatment, 1 had resolution of SUI) B ‐ 1/17 (unable to comply with scheduled visits) "Analysis was done on an intent‐to‐treat basis" but not clear how missing data were dealt with. |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Santos 2009.
| Methods | Study design: RCT Multicentre or single‐centre: single Setting: Brazil Period: April 2003 – March 2005 Details of sample size calculation: not reported Follow‐up: 4 months' treatment |
|
| Participants | N: 45 randomised Mean (SD) age: A ‐ 55.2 (12.8) years; B ‐ 5.6 (11.2) years Inclusion criteria: SUI confirmed by urodynamics Exclusion criteria: any kind of chronic degenerative disease that could affect the muscular and nervous tissues; genital bleeding from any source; pregnant women; UTI; those who were with vulvovaginitis; genital dystopia that exceeded the vaginal opening; with atrophic vaginitis; cardiac pacemaker; overactive bladder, urethral sphincter deficiency |
|
| Interventions | A (n = 24) electrical stimulation. 2 × 20 min sessions per week for 4 months (32 sessions?). Electrode: 10 cm long, 3.5 cm wide with double metallic ring and a cylindrical shape, positioned in the medium third of the vagina. Intensity varying from 10‐100 mA and 50 Hz of fixed frequency, with pulse duration of 1 ms B (n = 21) vaginal cones. 2 × 45 min sessions per week for 4 months. Cone weights 20‐100 g |
|
| Outcomes | Incontinence episodes per week 1 h pad test Quality of life measured with I‐QoL (higher score = greater severity) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[T]able of random numbers generated by computer" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to be reported |
| Other bias | Low risk | Nothing to suggest any other source of bias |
Schmidt 2009.
| Methods | Study design: RCT Multicentre or single‐centre: single centre Setting: Urogynecology Clinic at the Hospital de Clínicas de Porto Alegre (HCPA), Brazil Period: January 2006 – May 2007 Details of sample size calculation: to detect a difference of one standard deviation in the study variables after 12 weeks of treatment, the sample size was established as 11 patients per group. This sample size assumes a significance level of 5%, power of 90%, and a correlation between measurements at the two different points of 0.5. Follow‐up: 6 months |
|
| Participants | N: 32 randomised Mean (SD) age: not reported Inclusion criteria: older than 30 years of age; had stress UI (SUI) or mixed UI (MUI) Exclusion criteria: any clinical or surgical treatment during the previous 6 months; significant genital prolapse (below stage 2 on the pelvic organ prolapse quantification [POP‐Q] system); urethral sphincter involvement (leak point pressure less than 60 cm H2O). |
|
| Interventions | All participants received identical specially designed equipment, providing real‐time information on the contraction waveform and information or guidance. Vaginal prove transducer for monitoring pelvic muscle contraction pressure during exercises. Programmable for either PFMT with or without biofeedback, or PFMT plus electrical stimulation. All participants: same exercise programme. Supine position with rapid contractions (2 s contraction, 4 s rest) then slow contractions (4 s contraction, 4 s rest), repeated 3 times with rest interval A (n = 10) ‐ PFMT plus biofeedback for 12 weeks. Device displays information on contraction intensity. B (n = 11) ‐ PFMT plus electrical stimulation for 12 weeks. Frequency 50 Hz and pulse duration 300 μs C (n = 11) ‐ PFMT alone for 12 weeks. Participants received no information from device on contraction intensity. |
|
| Outcomes | Outcomes assessed at 12 weeks and 6 months. Subjective evaluation:
Perineometric intensity (pelvic floor muscle strength) (Ic cmH2O) Daytime micturitions Nocturia episodes SUI episodes King's Health Questionnaire scores |
|
| Notes | No usable data because SUI and MUI women not separated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly selected" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The examiner who performed perineometry was blinded to the patients groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in the analysis. No dropouts reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias. |
Seo 2004.
| Methods | Study design: RCT Multicentre or single‐centre: multicentre Setting: South Korea Period: not reported Details of sample size calculation: not reported Follow‐up: 6 weeks |
|
| Participants | N: 120 randomised and analysed Mean (SD) age: A ‐ 42.7 (11.3) years; B ‐ 44.5 (12.1) years Inclusion criteria: SUI patients who required non‐surgical treatment Exclusion criteria: not reported |
|
| Interventions | A (n = 60) ‐ ES plus biofeedback. 2 × 20 min sessions per week for 6 weeks. Simultaneous electrical stimulation of 35 Hz and 50 Hz for 24 s, repeated for 20 min B (n = 60) ‐ vaginal cone. 150 g. In a supine position, the weight effect is approximately 0%, the oblique leaning position 50% and in the upright sitting position 100%. Patients were educated to start their PFM exercise in a position whereby the cone does not expulse when the PFM is contracted, and to change position gradually to an upright sitting position when they had developed enough contractile power to prevent cone expulsion. The PFM exercise with the cone consisted of 5 s of PFM contraction and 10 s of relaxation, repeating this cycle 3‐5 times for at least 5 min daily for 6 weeks. Patients instructed by specialist nurse. |
|
| Outcomes | Participants with improvement in SUI Pad test (g) Maximal urethral pressure (mmH2O) Maximal vaginal pressure (mmHg) Duration of PFM contraction (s) Change in subjective symptom scores Daytime frequency Leakage episodes Amount of leakage Difficulty in exercises due to incontinence Sexual life Daily life Avoiding places Difficulty in personal relationships Quality of life |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomly divided" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals reported |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Shepherd 1984.
| Methods | Study design: RCT Multicentre or single‐centre: single Setting: UK Period: not reported Details of sample size calculation: not reported Follow‐up: 12 weeks |
|
| Participants | N: 107 randomised, 94 analysed SUI 42 UUI 26 MUI 39 Mean (SD) age: not reported Inclusion criteria: SUI, UUI or MUI Exclusion criteria: not reported |
|
| Interventions | A (n = 53) electrical stimulation under general anaesthesia. Single session. Scott electrode in vagina, large indifferent electrode under buttocks. Current up to 40v, 10‐50 Hz for 20 min B (n = 54) sham treatment. Single session. Vaginal electrode but no current |
|
| Outcomes | Participants with no improvement in frequency of incontinence Participants not dry Participants with no improvement in pad changes Participants with no improvement in objectively measured pelvic floor control Participants with no improvement in incontinence |
|
| Notes | Not usable because data not presented by SUI/UUI/MUI groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Allocated at random into trial and control groups" |
| Allocation concealment (selection bias) | Low risk | "[A] sealed envelope was opened stating which group the patient was in" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants blinded. Other blinding not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients' subjective statements were recorded by a single observer who was unaware of the treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No differential dropout. No explanation reported for withdrawals. |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Shepherd 1985.
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: UK Period: not reported Details of sample size calculation: not reported Follow‐up: 6 months |
|
| Participants | N: 40 randomised, 15 analysed Mean (SD) age: not reported Inclusion criteria: genuine stress incontinence or detrusor overactivity (DO) Exclusion criteria: not reported |
|
| Interventions | A (n = 6 SUI, 4 DO) ‐ electrical stimulation. Intravaginal cushion attached to stimulator worn around the waist. Cushion worn for 8/24 h, night or day according to participant preference. Stimulation: 50 Hz (SUI participants), 10 Hz (DO participants) B (n = 3 SUI, 2 DO) sham electrical stimulation. Identical device to group A but not activated |
|
| Outcomes | Subjective and objective improvement in symptoms | |
| Notes | No usable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants given identical devices but unaware which were activated. "The code was held by the manufacturer and only broken when the trial was completed." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal per group not reported. Substantial withdrawal overall: 15/40 completed trial. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not reported in full |
| Other bias | Unclear risk | Nothing to indicate any other source of bias |
Smith 1996.
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre. Setting: Department of Urology, Lahey Clinic, Burlington, MA Period: October 1992 to January 1994 Sample size: not reported Follow‐up: 16 weeks |
|
| Participants | N: 57 randomised in total 18 with SUI randomised 38 with DO randomised and analysed Mean age (range): A ‐ 65 (45‐82) years; B ‐ 60 (44‐73) years Inclusion criteria: genuine stress urinary incontinence or detrusor instability Exclusion criteria: type 3 stress urinary incontinence, pregnancy, history of prolonged urinary retention, vaginal vault prolapse, diminished sensory perception or cardiac pacemaker |
|
| Interventions | For the 18 women with SUI: A (n = 9) Kegel exercises (PFMT). Given written materials and shown physically how to contract properly, and they were monitored during each examination. Instructed to repeat exercises approx. 60 times a day. Instructions included direction for slow and quick succession muscle exercise B (n = 9) electrical stimulation. 5‐s contraction time (range 5‐15), duty cycle 1:1‐2 s, and increasing treatment time from 15, 30, 45 and 60 min. Amplitude started at 5 mA to 10 mA, increased each month to 80 mA max (range 1‐100) Waveform current: asymmetric balanced biphasic pulsed Phase duration: 300 μs Pulse rate: channel 1: 50 Hz; channel 2: 12.5 Hz |
|
| Outcomes | Number of participants cured (defined as cessation of incontinence and no longer requiring pads) Number of participants with objective improvement (defined as reduction of ≥ 50% in episodes and pads, and ≤ 10 voiding episodes per 24 h) Number of participants with improvement Pads per week Number of leaks per 24 h Water Valsalva leak point pressure (cm) Adverse effects Women going on to have surgery |
|
| Notes | Unclear if numbers for adverse effects refer to women in both SUI and DO groups who received ES | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[P]atients were randomised to 1 of 2 treatment arms" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants. Blinding of others not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Tapp 1987.
| Methods | Study design: RCT Multicentre or single‐centre: single Setting: UK Period: not reported Details of sample size calculation: not reported Follow‐up: 3 months' treatment |
|
| Participants | N: 29 randomised Mean (SD) age: not reported Inclusion criteria: GSUI and no other significant urodynamic abnormality Exclusion criteria: previous incontinence or prolapse surgery |
|
| Interventions | A (n = 15) PFMT. "Comprehensive teaching about the mechanism of continence and the action of the pelvic floor." Women "saw the continence advisor regularly once a week for 3 months and were advised to perform exercises 4 times per hour every hour of the day B (n = 14) PFMT plus Faradic electrical stimulation. As per group A plus Faradic stimulation using vaginal probe twice weekly for 1 month |
|
| Outcomes | Symptom score (1‐100 VAS (higher score = greater severity)) Women requesting surgery after end of treatment |
|
| Notes | Other outcomes reported but not relevant to this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomised study" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals reported |
| Selective reporting (reporting bias) | Unclear risk | Cystometry and pad test outcomes not reported in full for either group |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Tapp 1989.
| Methods | Study design: RCT Multicentre or single‐centre: single Setting: UK Period: not reported Details of sample size calculation: not reported Follow‐up: 3 months' treatment, 9 months' follow‐up |
|
| Participants | N: 81 randomised Mean (SD) age: not reported Inclusion criteria: GSUI and no other significant urodynamic abnormality Exclusion criteria: previous incontinence or prolapse surgery |
|
| Interventions | A (n = 21) ‐ PFMT. "A continence advisor was trained to teach pelvic floor exercises and carried out 14 sessions over a 3‐month period with each patient." B (n = 23) ‐ ES and PFMT. As per group A plus Faradic stimulation using vaginal probe twice weekly for 1 month C (n = 24) ‐ Burch colposuspension |
|
| Outcomes | Outcomes assessed at 3 and 9 months. Women with subjective and objective cure Women with symptomatic improvement Women objectively cured Women with no improvement in SUI Requested surgery Women requesting surgery who were objectively cured at 6 months after treatment (i.e. 9 months' follow‐up) |
|
| Notes | Denominators are different in the 2 abstracts reporting this trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised into 3 groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals: A ‐ 6/27. B ‐ 3/26. C ‐ 4/27. No explanations for withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Terlikowski 2013.
| Methods | Study design: RCT Multicentre or single‐centre: single Setting: Poland Period: January 2008 to April 2012 Details of sample size calculation: sample size was calculated as 102 patients for a power of 80% and a 2:1 ratio (68 and 34, respectively) Follow‐up: 8 weeks' treatment, 16 weeks' follow‐up |
|
| Participants | N: 102 randomised, 93 analysed Mean (SD) age: A ‐ 46.9 (6.8) years; B ‐ 45.6 (7.9) years Inclusion criteria: women with urodynamic SUI Exclusion criteria: "chronic degenerative diseases that would affect muscular and nerve tissues, presence of any degree of pelvic organ prolapse (POP), active or recurrent urinary tract infections (UTI), vulvovaginitis, atrophic vaginitis, diabetes mellitus, neurological disease, psychiatric illness, use of medication affecting micturition, history of surgical or pharmaceutical treatment of SUI, chronic debilitating disease such as renal failure, and those with cardiac pacemakers, patients with intrinsic sphincteric deficiencies identified by the Valsalva leak‐point pressure ≤60 cmH20 measurement in the sitting position with a volume of 250 mL in the bladder and/or a urethral closure pressure ≤20 cmH20 in the sitting position at maximum cystometric capacity." |
|
| Interventions | A (n = 68) ‐ transvaginal electrical stimulation (TVES) with vaginal probe (VeriProbe), and sEMG (surface electromyography) biofeedback. Women "were provided with active TVES with sEMG. Parameters of muscle stimulation were adapted for each participant: frequency ranged from 10 to 40 Hz, impulse width from 200 to 250 μs, and runtime/decontraction in configuration of 15 s/30 s for 20 min. The treatment lasted for 8 weeks and was performed twice a day. The introduction took place in the clinic, and the actual treatment was performed by patients at home, with a gradual increase to a daily maximum of 40 min." EMG biofeedback assessment used a NeuroTrac ETS unit. "The device combines biofeedback and ES, with effective monitoring of compliance with treatment and performance. Patient position, accuracy of electrode placement, exact warmup period, and time of day were all recorded. The regimen included a warmup of five contractions and five relaxations, followed by a contraction/relaxation assessment. Participants were encouraged to selectively contract and relax their pelvic floor muscles with the assistance of visual and auditory feedback." B (n = 34) ‐ sham transvaginal ES with sEMG biofeedback. Women "were provided with a placebo set to parameters proven to have no physiological effect. The same type of electrode and hand‐held unit as described for TVES with sEMG biofeedback was used in the clinic and for home application. Preset parameters were a frequency of 2 Hz, a pulse width of 50 μs, 2 s of stimulation, and 60 s of no stimulation, with a ramp of 8 s. As with group 1, the introduction took place in the clinic, and patients used issued devices at home, with a gradual increase to a daily maximum of 40 min" "All participants were taught skills and strategies for preventing incontinence and suppressing urge. This included education about normal bladder control, lifestyle interventions such as weight reduction, relieving constipation, smoking cessation, caffeine reduction, fluid management, wearing non‐restrictive and easily removed clothing, reducing emotional stress, and correcting faulty habit patterns of frequent urination by suggesting distraction and avoidance techniques. Advice on good voiding position was also provided. In addition, an information booklet was provided to reinforce this information." |
|
| Outcomes | Outcomes assessed at 8 and 16 weeks. Subjective assessment of incontience Micturitions per 24 h and per week Frequency of urine loss per week Number of nocturia episodes per week Number of pads per week 20 min pad tests (g) Oxford score Quality of life measured with transformed I‐QoL score (higher score = greater QoL) Objectively cured according to standard pad test (≤ 1 g of leakage with a standardised bladder volume) Adverse effects |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized using a computer‐generated random sequence" |
| Allocation concealment (selection bias) | Low risk | "Group assignment was enclosed in sequentially numbered, sealed envelopes by a person not involved in the study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded. "The physiotherapist and physician carrying out the assessment were unaware of which treatment group the patient was in." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The physiotherapist and physician carrying out the assessment were unaware of which treatment group the patient was in. To minimize the likelihood of assessor bias, participants were asked not to discuss their treatment and/or reveal any information on group allocation to the principal investigator doing the assessments" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some withdrawals due to ES intervention: A ‐ 4/64 (2 protocol too demanding, 2 unable to use stimulator at home); B ‐ 5/29 (3 used other treatments, 1 change of work, 1 death in the family). No ITT analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Whitmore 1995.
| Methods | Study design: RCT Multicentre or single‐centre: multicentre Setting: USA Period: not reported Details of sample size calculation: not reported Follow‐up: 15 weeks' treatment |
|
| Participants | N: 52 randomised and analysed Mean (SD) age: not reported Inclusion criteria: GSUI Exclusion criteria: not reported |
|
| Interventions | A (n = 35) ‐ electrical stimulation B (n = 17) ‐ sham electrical stimulation |
|
| Outcomes | Women cured or improved by 50% according to pad test Women cured or improved by 50% according to voiding diaries Adverse effects Subjective improvement according to VAS Subjective frequency of urine loss Urine loss with sneezing, coughing or laughing |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomized" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants blinded, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many participants randomised. No withdrawals reported |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Wilson 1987.
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: UK Period: not reported Details of sample size calculation: not reported Follow‐up: 6 weeks' treatment, 6 months' follow‐up |
|
| Participants | N: 60 randomised and analysed Mean (SD range) age: 46.8 (19‐79) Inclusion criteria: GSUI Exclusion criteria: not reported |
|
| Interventions | A (n = 15) PFMT in hospital B (n = 15) PFMT plus faradism. Low frequency current to stimulate striated muscle contraction. Saddle shaped indifferent electrode placed over the sacrum, active electrode applied to perineum. Faradic battery provided surges at a repetition rate of 12 surges/min and as strong a current was used as the patient could tolerate comfortably. Patients instructed to contribute to muscle contraction when the current was felt so that later they could practice the contractions more easily. Groups of 12 surges were given with 2 min rest in between each group. C (n = 15) PFMT plus interferential therapy. Low frequency stimulating current. 2 medium‐frequency currents of around 4000 cycles/s applied to the body from different directions. Four medium‐sized suction electrodes (2 on abdomen, 2 on adductor muscles), 20‐25 mA current, 15 pulses at pressure peak 0.25‐0.30 Pa/cm2. First treatment 10 min, patient remained relaxed during stimulation. If no ill effects noted the duration increased to 15 min Groups B and C started each hospital session with ES then PFMT exercises were performed with the help of the perineometer. Vaginal perineometer used so that women were more easily aware of which muscles to contract (8 cm long, 3 cm diameter). Hold contraction for 5 s then rest 15 s. 3 series of 6 contractions were performed with 2 min rest in between. D (n = 15) PFMT at home. One session in physiotherapy department and given an instruction sheet for PFMT to be done at home. |
|
| Outcomes | Subjective assessment: improved or much improved, at 6 weeks and 6 months Subjective assessment: not improved, at 6 weeks and 6 months Micturitions per 24 h Number of pads per 24 h Perineometry reading (mmHg) Maximum urethral closure pressure (MUCP) at rest (cm H2O) MUCP plus pelvic floor contraction (cm H2O) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomly allocated" |
| Allocation concealment (selection bias) | High risk | "[A]ssigned consecutively" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A ‐ 1/15 withdrew. Different denominators reported for different outcomes without any explanation |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other bias |
Wise 1993.
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: UK Period: not reported Details of sample size calculation: not reported Follow‐up: 12 weeks' treatment |
|
| Participants | N: 62 many randomised and analysed Mean (SD) age: not reported Inclusion criteria: urodynamically proven GSUI Exclusion criteria: not reported |
|
| Interventions | A (n = 20) ‐ maximal electrical stimulation with CONMAX vaginal stimulator, impulse frequency 20 Hz, pulse duration 0.75 ms, variable pulse strength 0‐90 mA. Home treatment, 20 min per day B (n = 21) ‐ vaginal cones. Instructed to use cones for 15 min twice a day and to increase cone weight when successful on 2 occasions C (n = 21) ‐ vaginal cones plus PFMT. As per Group B, plus taught by vaginal examination to voluntarily contract pelvic floor. Instructed to do 10 sessions of 10 contractions per day |
|
| Outcomes | Pad test (40 min with standard bladder volume) Women with symptomatic improvement |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[W]omen were randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A 4/20, B 2/21, C 6/21 withdrew. No explanation for withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
BF: biofeedback; BMI: body mass index; DI: detrusor instability; DO: detrusor overactivity; EMG: electromyography; ES: electrical stimulation; GSI: genuine stress incontinence; GSUI: genuine stress urinary incontinence; HRT: hormone replacement therapy; ICIQ: International Consultation on Incontinence Questionnaire; IESG: intravaginal electrical stimulation group; IIQ: incontinence impact questionnaire; I‐QoL: Incontincence Quality of Life questionnaire; ITT: intention‐to‐treat; MUI: mixed urinary incontinence; PFM(T): pelvic floor muscle (training); POP: pelvic organ prolapse; RCT: randomised controlled trial; SD: standard deviation; SESG: surface electrical stimulation group; SUI: stress urinary incontinence; UDI: urogenital distress inventory; UI: urinary incontinence; UTI: urinary tract infection; UUI: urgency urinary incontinence.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bezerra 2009 | Ineligible comparison |
| Blowman 1991 | Ineligible comparison |
| Furst 2014 | Ineligible comparison |
| Kirschner‐Hermanns 1995 | Wrong study design |
| Kolbl 1989 | Ineligible population |
| NCT01763762 | Ineligible comparison |
| NCT02899520 | Ineligible comparison |
| Pennisi 1994 | Not randomised |
| RBR‐64s9ts | Ineligible population |
| Terry 1996 | Ineligible comparison |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12610000254099.
| Trial name or title | Physiotherapy for women with stress urinary incontinence: effects of kinesiotherapy, vaginal cones and electrical stimulation |
| Methods | Simple randomisation by dice‐rolling |
| Participants | Women with urine loss (stress urinary incontinence), > 35 years. Target 75 women Exclusion: prolapse ? Grade II; vaginal or urinary infection; uncontrolled hypertension; neurological or cognitive dysfunction |
| Interventions | A ‐ kinesiotherapy B ‐ vaginal cones C ‐ electrical stimulation |
| Outcomes | At baseline, 12 sessions after randomisation and 6 weeks, 3 months and 1 year after the end of treatment Digital evaluation of pelvic floor (PERFECT) using modified Oxford scale; evaluation of pressure contraction using perineometer (Quark) Urine loss measured by pad test and voiding diary Quality of life (King's Health Q); sexual function (Arizona Sexual Experiences Scale) Isometric and isokinetic evaluation for hip adductors and abductors (BIODEX dynamometer) |
| Starting date | 1 August 2008 |
| Contact information | Patricia Driusso, Brazil; Grasiela Nascimento Correia (grasiela_n_correia@yahoee.com.br) |
| Notes | ACTRN12610000254099 |
Jha 2013.
| Trial name or title | Impact of Physiotherapy on Sexual function in women with Stress Urinary Incontinence (SUI) and a comparison of electrical stimulation versus standard physiotherapy: a randomised controlled trial |
| Methods | RCT |
| Participants | Women with SUI (sample size 114) |
| Interventions | A ‐ electrical stimulation, B ‐ pelvic floor muscle training |
| Outcomes | At 4 (or 6) months. Pelvic floor symptoms, including incontinence severity before and after treatment will be assessed using the Electronic Pelvic Floor Assessment Questionnaire (ePAQ). Changes in sexual function will be assessed using the Prolapse and Incontinence Sexual function Questionnaire (PISQ). SF‐36 domain scores; EQ‐5D score; ePAQ urinary & sexual domain scores before and after physiotherapy |
| Starting date | No longer recruiting |
| Contact information | Dr Swati Jha (Swati.Jha@sth.nhs.uk) |
| Notes | www.isrctn.com/ISRCTN09586238; DOI 10.1186/ISRCTN09586238 |
Maher 2010.
| Trial name or title | Inko‐Outside multicentre, controlled, randomised, blinded study for the treatment of stress urinary incontinence |
| Methods | RCT (single‐blind multi centre, controlled, randomised, blinded comparative study) |
| Participants | Women with SUI (target 243) |
| Interventions | A ‐ 12 weeks of treatment with Inko‐Outside (external NMES; ESEX) and 14 weeks of Kegels B ‐ 12 weeks of treatment with conventional NMES using an internal vaginal probe (ESIN) and 14 weeks of Kegels (PFMT) C ‐ 26 weeks of Kegels (PFMT) |
| Outcomes | Change in continence scores compared to baseline:
Secondary outcome measures
Dryness will be defined as dry for 5 consecutive days as reported by participants on enquiry |
| Starting date | 12 April 2010 to 1 December 2010. No longer recruiting. |
| Contact information | Dr Ruth Maher (rmmaher@northgeorgia.edu) |
| Notes |
www.isrctn.com/ISRCTN32312996; DOI 10.1186/ISRCTN32312996; NCT01472068 Industry‐funded (Bio‐Medical Research Ltd (UK) ‐ provided devices) 8 April 2016: No publications found, verifying study status with principal investigator |
NCT00762593 2006.
| Trial name or title | A multicenter double blind randomized placebo controlled trial evaluating transvaginal electrical stimulation with a home use programmable device for urinary stress incontinence |
| Methods | Multicenter double‐blind randomised placebo‐controlled trial |
| Participants | Target 150 women with USI |
| Interventions | A ‐ transvaginal electrical stimulation with a home use programmable device used 30 min every day during 8 weeks B ‐ use of a transvaginal placebo home use programmable device used 30 min every day during 8 weeks |
| Outcomes | Number of urinary stress incontinence episodes measured by patients on a 7 days diary at 8 weeks
Assessment of the discomfort linked to urinary stress incontinence occurring the previous week assessed on a 0 ‐ 100 visual analog scale at 8 weeks Urodynamic investigation at 4 and 8 weeks Standardised Pad test at 4 and 8 weeks Number of severe urinary stress incontinence episodes at 4 and 8 weeks Number of sanitary napkins used at 4 and 8 weeks Leakage index at 4 and 8 weeks Subjective appreciation of patients at 4 and 8 weeks |
| Starting date | January 2006 to October 2008 |
| Contact information | Jacques Croissandeau, Akontis |
| Notes | clinicaltrials.gov/show/NCT00762593 |
NCT02185235 2014.
| Trial name or title | A randomized controlled trial of electrical stimulation to treat pelvic floor disorder |
| Methods | Randomised controlled trial |
| Participants | Women with pelvic organ prolapse and/or urinary incontinence and/or faecal incontinence. Target N 200. Age 20‐75 years |
| Interventions | A ‐ electrical stimulation + biofeedback B ‐ pelvic floor training + biofeedback |
| Outcomes | 1 hour pad test Quality of life (score) Vaginal pressure (mmHg) |
| Starting date | June 2014 to May 2015 |
| Contact information | Tsung‐Hsien Su, Mackay Memorial Hospital |
| Notes | clinicaltrials.gov/ct2/show/NCT02185235; 14MMHIS031 |
NCT02423005 2015.
| Trial name or title | Neurotech Vital Compact versus itouch Sure Pelvic Floor Exerciser US |
| Methods | Prospective, randomised, controlled, single‐blind, multi‐site clinical study |
| Participants | Women with SUI (target 180). Age 18‐65 years |
| Interventions | A ‐ Neurotech Vital Compact 5 days per week for 30 min per session for 12 weeks followed by 14 weeks of Kegel exercises (experimental) B ‐ itouch Sure Pelvic Floor Exerciser 7 days per week for 20 min per session for 12 weeks followed by 14 weeks of Kegel exercises (active comparator) |
| Outcomes | Proportion of subjects who have achieved > 50% improvement on the provocative pad weight test Between group comparison of mean change in urine leakage in a provocative pad weight test Within group comparison of mean change in urine leakage in the 1 hour pad weight test Between group comparison of the mean improvement in the Incontinence Quality of Life Questionnaire (I‐QoL) score Between‐group comparison of the proportion of subjects achieving dryness Number of incontinence episodes per day Mean change in urine leakage in the 24‐hour pad weight test Number of pads used Dryness measured on pad test Adverse events |
| Starting date | April 2015 to April 2017 |
| Contact information | Conor Minogue, PhD, Bio‐Medical Research, Ltd. |
| Notes | clinicaltrials.gov/ct2/show/NCT02423005 Sponsors and Collaborators: Bio‐Medical Research, Ltd. |
Robson 2013.
| Trial name or title | Neurotech Vital device for the treatment of stress urinary incontinence |
| Methods | Randomised crossover trial, double blind (Subject, Caregiver, Investigator) |
| Participants | Women with SUI. Target 50. |
| Interventions | A ‐ active comparator: Active Neurotech Vital Device 50% of 140 patients on a 12 week treatment programme with the device used 5 days out of 7 for 30 min over 12 weeks. B ‐ placebo comparator: Modified Neurotech Vital Device 50% of 140 patients on a 12 week treatment programme with the device used 5 days out of 7 for 30 min over 12 weeks. |
| Outcomes | Standardised 1‐min stress test Quality of life questionnaire (I‐QoL) 1 hour pad test weight test 24 hour pad weight test 3 day diary card 3 day voiding diary Modified Oxford Score Pelvic floor ultrasound Compliance (device compliance download) Device Ease of Use Questionnaire |
| Starting date | December 2012 to January 2015 |
| Contact information | R Tunn, Professor, St Hedwig Krankenhaus |
| Notes | clinicaltrials.gov/show/NCT02214784; Bio‐Medical Research, Ltd. |
Robson 2014.
| Trial name or title | A study to look at the safety and performance of Neuromuscular Electrical Stimulation (NMES) with the NeuroTech Vital device compared to the itouch Sure Pelvic Floor Exerciser for the treatment of stress urinary incontinence |
| Methods | Randomised, controlled, single‐blind, pilot clinical study |
| Participants | Women with SUI (target 10) |
| Interventions | A ‐ Neurotech Vital device (NTV) (external ES) B ‐ itouch Sure Pelvic Floor Exerciser (vaginal ES) |
| Outcomes | "[S]ignificant improvement" in incontinence following the 1‐hour pad weight test + numerous secondary outcomes. 6, 12 and 26 weeks after baseline |
| Starting date | May 2014 until May 2016 |
| Contact information | Mrs Karen Robson, krobson@bmr.ie; the Friarage Hospital, Northallerton, North Yorkshire, DL6 1JG, UK |
| Notes |
www.isrctn.com/ISRCTN27961345 Funded by Bio‐medical Research Ltd., Ireland |
ES: electrical stimulation; IIQ: incontinence impact questionnaire; I‐QoL: Incontincence Quality of Life questionnaire; NMES: neuromuscular electrical stimulation; PFMT: pelvic floor muscle training; SUI: stress urinary incontinence;
Differences between protocol and review
We added the following to the list of quality of life outcomes: "QoL measures of sexual function or satisfaction; measures of psychological or emotional well‐being".
Contributions of authors
FS: drafting of protocol, screening of search results, data extraction, data analysis and interpretation, draft manuscript, review manuscript. BB: review protocol, clinical advice, review analysis, review manuscript. KB: review protocol, review analysis, review manuscript. CG: screening of search results, data extraction, data analysis and interpretation, draft manuscript, review manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Incontinence Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
FS: none known. BB: none known. KB: lead author of an included trial (Bø 1999). She was not involved in extracting data or assessing risk of bias for this trial. CG: none known.
New
References
References to studies included in this review
Aaronson 1995 {published data only}
- Aaronson PS, Loehner D, Bingham W, Smith JJ. Intravaginal electrical stimulation in the treatment of genuine stress urinary incontinence and detrusor instability: a controlled study [abstract]. Journal of Urology 1995;153(4 Suppl):491A. Abstract no. 1051. [sr‐incont15469 ] [Google Scholar]
Abel 1997 {published data only}
- Abel I. Elektrostimulation og lokal ostrogenterapi til behandling af urininkontinens hos postmenopausale kvinder (PhD thesis). Elektrostimulation og Lokal Ostrogenterapi til Behandling af Urininkontinens hos Postmenopausale Kvinder (PhD thesis). Copenhagen: University of Copenhagen, 1997:1‐73. [sr‐incont21489] [Google Scholar]
Alves 2011 {published data only}
- ACTRN12610000820000. Comparison of neuromuscular electrical stimulation procedures used in treatment of female stress urinary incontinence [A two group, single‐blind, randomised controlled study to assess the effectiveness of two intravaginal neuromuscular electrical stimulation procedures on perineal pressure and voiding in women with stress urinary incontinence]. anzctr.org.au/ACTRN12610000820000.aspx (first received 26 September 2010). [ACTRN12610000820000; sr‐incont645655]
- Alves PG, Nunes FR, Guirro EC. Comparison between two different neuromuscular electrical stimulation protocols for the treatment of female stress urinary incontinence: a randomized controlled trial. Revista Brasileira de Fisioterapia (Sao Carlos (Sao Paulo, Brazil)) 2011;15(5):393‐8. [sr‐incont42667] [DOI] [PubMed] [Google Scholar]
Bernardes 2000 {published data only}
- Bernardes NO, Peres FR, Souza ELBL, Souza OL. Methods of treatment of genuine stress incontinence: a comparative study between a pelvic floor exercise program and a pelvic floor electrical stimulation. Revista Brasileira de Ginecologia e Obstetricia 2000;22(1):49‐54. [sr‐incont 23741] [Google Scholar]
Beuttenmuller 2010 {published data only}
- Beuttenmuller L, Cader S A, Macena R H M, Araujo N D S, Nunes E F C, Dantas E H M. Muscle contraction of the pelvic floor and quality of life of women with stress urinary incontinence who underwent kinesitherapy. Physiotherapy (Fizjoterapia) 2010;18(1):35‐41. [sr‐incont43326] [Google Scholar]
- Beuttenmuller L, Cader SA, Macena RHM, Araujo NDS, Nunes EFC, Dantas EHM. Floor muscles contraction in women with stress urinary incontinence underwent to exercises and electric stimulation therapy: a randomized study. Fisioterapia e Pesquisa 2011;18(3):210‐6. [sr‐incont47117] [Google Scholar]
Bidmead 2002 {published data only}
- Bidmead J, Mantle J, Cardozo L, Hextall A, Boos K. Home electrical stimulation in addition to conventional pelvic floor exercises: a useful adjunct or expensive distraction? [abstract]. Neurourology and Urodynamics 2002;21(4):372‐3. Abstract no. 68. [sr‐incont14546] [Google Scholar]
Bourcier 1994 {published data only}
- Bourcier A, Juras J. Randomised study comparing physiotherapy and pelvic floor rehabilitation [abstract]. Proceedings of the International Continence Society (ICS), 24th Annual Meeting, 1994 Aug 30‐Sep 2; Prague, Czech Republic 1994:146. [sr‐incont10940]
Bridges 1988 {published data only}
- Bridges N, Denning J, Olah KS, Farrar DJ. A prospective trial comparing interferential therapy and treatment using cones in patients with symptoms of stress incontinence [abstract]. Neurourology and Urodynamics 1988;7(3):267‐8. [sr‐incont3819] [Google Scholar]
Brubaker 1997 {published data only}
- Brubaker L, Benson JT, Bent A, Clark A. Transvaginal electrical stimulation is effective for treatment of detrusor overactivity. Neurourology and Urodynamics 1996;15(4):282‐3. [sr‐incont4612] [Google Scholar]
- Brubaker L, Benson T, Bent A, Clark A, Shott S. Transvaginal electrical stimulation for female urinary incontinence. American Journal of Obstetrics & Gynecology 1997;177(3):536‐40. [sr‐incont5526] [DOI] [PubMed] [Google Scholar]
Bø 1999 {published data only}
- Bo K, Talseth T. Single blinded randomized controlled trial on the effect of pelvic floor muscle strength training, electrical stimulation, cones or control on severe genuine stress incontinence [abstract]. Neurourology and Urodynamics 1998;17(4):421‐2. [sr‐incont5675] [Google Scholar]
- Bø K, Talseth T, Holme I. Single blind, randomised controlled trial of pelvic floor exercises, electrical stimulation, vaginal cones, and no treatment in management of genuine stress incontinence in women. BMJ 1999; Vol. 318, issue 7182:487‐93. [sr‐incont6654] [DOI] [PMC free article] [PubMed]
Castro 2008 {published data only}
- Castro RA, Arruda RM, Zanetti MR, Santos PD, Sartori MG, Girao MJ. Single‐blind, randomized, controlled trial of pelvic floor muscle training, electrical stimulation, vaginal cones, and no active treatment in the management of stress urinary incontinence. Clinics (Sao Paulo, Brazil) 2008;63(4):465‐72. [sr‐incont27760] [DOI] [PMC free article] [PubMed] [Google Scholar]
Correia 2013 {published data only}
- Correia GN, Pereira VS, Bastos AM, Hirakawa HS, Driusso P. Surface and intravaginal electrical stimulation versus no treatment in severity of stress urinary incontinence: Randomized controlled study [abstract]. International Urogynecology Journal and Pelvic Floor Dysfunction 2013;24(Suppl 1):S23‐S24. Abstract number 23. [sr‐incont61899] [Google Scholar]
Correia 2014 {published data only}
- Correia G, Driusso P. Comparison of the effects of intravaginal electrical stimulation and surface electrical stimulation in women with stress urinary incontinence. http://www.ensaiosclinicos.gov.br/rg/RBR‐7gt9pb/ 2012. [RBR‐7gt9pb; sr‐incont64553] [DOI] [PubMed]
- Correia GN, Pereira VS, Hirakawa HS, Driusso P. Effects of surface and intravaginal electrical stimulation in the treatment of women with stress urinary incontinence: randomized controlled trial. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2014;173(1):113‐8. [sr‐incont49365] [DOI] [PubMed] [Google Scholar]
Delneri 2000 {published data only}
- Delneri C, Benedetto P. Pelvic floor rehabilitation. A comparison of two methods of treatment: vaginal cones versus functional electrical stimulation. Europa Medicophysica 2000;36(1):45‐8. [sr‐incont12117] [Google Scholar]
Demirturk 2008 {published data only}
- Demirturk F, Akbayrak T, Karakaya IC, Yuksel I, Kirdi N, Demirturk F, et al. Interferential current versus biofeedback results in urinary stress incontinence. Swiss Medical Weekly 2008;138(21‐22):317‐21. [sr‐incont27485] [DOI] [PubMed] [Google Scholar]
Edwards 2000 {published data only}
- Edwards G J, Wines H, Barrington J W. A comparison between pelvic floor exercises and pelvic floor exercises and electrical therapy with respect to urethral pressure profiles (Abstract number IDP50). International Urogynecology Journal and Pelvic Floor Dysfunction 2000;11(Suppl 1):S89. [sr‐incont11914] [Google Scholar]
Eyjolfsdottir 2009 {published data only}
- Eyjolfsdottir H, Ragnarsdottir M, Geirsson G. Pelvic floor muscle training with and without functional electrical stimulation as treatment for stress urinary incontinence. Laeknabladid 2009;95(9):575‐80; quiz 581. [sr‐incont34265] [PubMed] [Google Scholar]
Firra 2013 {published data only}
- Firra J, Thompson M, Smith SS. Paradoxical findings in the treatment of predominant stress and urge incontinence: a pilot study with exercise and electrical stimulation. Journal of Women's Health Physical Therapy 2013;37(3):113‐23. [sr‐incont61836] [Google Scholar]
- Firra JC. Effects of Treatment of Urinary Incontinence in Women: Exercise or Electrical Stimulation or Both [PhD thesis]. Denton, Texas: Texas Woman's University, 2008. [sr‐incont47108] [Google Scholar]
Goode 2003 {published data only}
- Burgio KL, Goode PS, Richter HE, Locher JL, Roth DL. Global ratings of patient satisfaction and perceptions of improvement with treatment for urinary incontinence: validation of three global patient ratings. Neurourology and Urodynamics 2006;25(5):411‐7. [sr‐incont22346] [DOI] [PubMed] [Google Scholar]
- Burgio Kathryn L, Goode Patricia S, Locher Julie L, Richter Holly E, Roth David L, Wright Kate Clark, et al. Predictors of outcome in the behavioral treatment of urinary incontinence in women. Obstetrics & Gynecology 2003;102(5 (Pt 1)):940‐947. [sr‐incont17367] [DOI] [PubMed] [Google Scholar]
- Goode PS, Burgio KL, Locher JL, Roth DL, Umlauf MG, Richter HE, et al. Effect of behavioral training with or without pelvic floor electrical stimulation on stress incontinence in women: a randomized controlled trial. JAMA 2003;290(3):345‐52. [sr‐incont16305] [DOI] [PubMed] [Google Scholar]
Hahn 1991 {published data only}
- Hahn I, Sommar S, Fall M. A comparative study of pelvic floor training and electrical stimulation for the treatment of genuine female stress urinary incontinence. Neurourology and Urodynamics 1991;10(6):545‐54. [sr‐incont2635] [Google Scholar]
Haig 1995 {published data only}
- Haig L, Mantle J, Versi E. Does interferential therapy (IFT) confer added benefit over a pelvic floor muscle exercise programme (PFMEP) for genuine stress incontinence (GSI)?. Proceedings of the International Continence Society (ICS), 25th Annual Meeting; 1995 Oct 17‐20; Sydney, Australia 1995:36‐7. Abstract no. 111. [sr‐incont10873]
Henalla 1989 {published data only}
- Henalla SM, Hutchins CJ, Castleden CM. Conservative management of urethral sphincter incompetence [abstract]. Neurourology and Urodynamics 1987;6(3):191‐2. [sr‐incont2689] [Google Scholar]
- Henalla SM, Hutchins CJ, Robinson P, MacVicar J. Non‐operative methods in the treatment of female genuine stress incontinence of urine. Journal of Obstetrics and Gynaecology 1989;9(3):222‐5. [sr‐incont2637] [Google Scholar]
Hofbauer 1990 {published data only}
- Hofbauer J, Preisinger F, Nurnberger N. The value of physical therapy in genuine female stress incontinence. Zeitschrift fur Urologie und Nephrologie 1990;83(5):249‐54. [sr‐incont350] [PubMed] [Google Scholar]
Huebner 2011 {published data only}
- Huebner M, Riegel K, Hinninghofen H, Wallwiener D, Tunn R, Reisenauer C. Pelvic floor muscle training for stress urinary incontinence: a randomized, controlled trial comparing different conservative therapies. Physiotherapy Research International 2011;16(3):133‐40. [sr‐incont60641] [DOI] [PubMed] [Google Scholar]
- Reisenauer C, Riegel K, Huebner M, Hinninghofen H, Wallwiener D, Tunn R. The effectiveness of pelvic floor exercise in patients with stress‐ urinary incontinence three‐armed prospective randomized comparison study [abstract]. Geburtshilfe und Frauenheilkunde 2008;68(Suppl 1):S20. [sr‐incont62355] [Google Scholar]
Jeyaseelan 1999 {published data only}
- ISRCTN56654882. An evaluation of pelvic floor muscle exercises and electrical muscle stimulation in patients with stress incontinence: a randomised, double‐blind, controlled trial. isrctn.org/ISRCTN56654882 (first received 1 March 2001). [ISRCTN56654882; sr‐incont47894]
- Jeyaseelan SM. A Pilot Evaluation of a New Pattern of Electrical Muscle Stimulation as a Treatment for Genuine Stress Incontinence: a Randomised, Double Blind Controlled Trial [PhD thesis]. Manchester: University of Manchester, 1999. [sr‐incont26926] [Google Scholar]
- Jeyaseelan SM, Haslam EJ, Winstanley J, Roe BH, Oldham JA. An evaluation of a new pattern of electrical stimulation as a treatment for urinary stress incontinence: a randomized, double‐blind, controlled trial. Clinical Rehabilitation 2000;14(6):631‐40. [sr‐incont11793] [DOI] [PubMed] [Google Scholar]
- Jeyaseelan SM, Haslam J, Roe B, Winstanley J, Oldham JA. The evaluation of a new pattern of electrical muscle stimulation as a treatment for genuine stress incontinence: a randomised, double‐blind, controlled trial [abstract]. Proceedings of the International Continence Society (ICS), 29th Annual Meeting, 1999 Aug 23‐26; Denver, Colorado 1999:74. [Abstract no. 522; sr‐incont9914]
Jeyaseelan 2002 {published data only}
- Jeyaseelan S, Haslam J, Oldham J. Can the effects of pelvic floor muscle exercises be enhanced with a new pattern of electrical stimulation in women with stress incontinence? Pilot data. Proceedings of the International Continence Society (ICS), 32nd Annual Meeting, 2002 Aug 28‐30; Heidelberg, Germany 2002:66‐7. Abstract no. 135. [sr‐incont14499]
Jeyaseelan 2003 {published data only}
- Jeyaseelan S, Oldham JA. Can the effects of pelvic floor muscle exercises be enhanced with a new pattern of electrical stimulation in women with stress incontinence. Proceedings of the World Confederation for Physical Therapy (WCPT), 14th International Congress; 2003 Jun 7‐12; Barcelona. 2003:Abstract no. RR‐PL‐1818. [sr‐incont26927]
Knight 1998 {published data only}
- Knight S. Evaluation of neuromuscular electrical stimulation in the treatment of genuine stress incontinence. Physiotherapy 1998;84(2):61‐71. [Google Scholar]
Laycock 1988 {published data only}
- Laycock J. Interferential therapy in the treatment of genuine stress incontinence [abstract]. Neurourology and Urodynamics 1988;7(3):268‐9. [sr‐incont3820] [Google Scholar]
Laycock 1993a {published data only}
- Laycock J, Jerwood D. Does pre‐modulated interferential therapy cure genuine stress incontinence?. Physiotherapy 1993;79(8):553‐60. [sr‐incont2320] [Google Scholar]
Laycock 1993b {published data only}
- Laycock J, Jerwood D. Does pre‐modulated interferential therapy cure genuine stress incontinence?. Physiotherapy 1993;79(8):553‐60. [sr‐incont2320] [Google Scholar]
Lo 2003 {published data only}
- Lo SK, Naidu J, Cao Y. Additive effect of interferential therapy over pelvic poor exercise alone in the treatment of female urinary stress and urge incontinence: a randomized controlled trial. Hong Kong Physiotherapy Journal 2003;21:37‐42. [sr‐incont19176] [Google Scholar]
Lopes 2014 {published data only}
- Lopes P, Levy‐Toledano R, Chiarelli P, Rimbault F, Mares P. Multicentric prospective randomized study evaluating the interest of intravaginal electro‐stimulation at home for urinary incontinence after prior perineal reeducation. Interim analysis. Gynecologie, Obstetrique & Fertilite 2014;42(3):155‐9. [sr‐incont60615] [DOI] [PubMed] [Google Scholar]
- Lopes P, Rimbault F, Scheffler M, Andre C, Cappelletti MC, Mares P. Multicentric prospective randomized and controlled study assessing effectiveness of intravaginal electrostimulation at home compared to usual care in female patients with urinary incontinence and prior perineal reeducation. Gynecologie, Obstetrique & Fertilite 2014;42(11):779‐86. [sr‐incont64887] [DOI] [PubMed] [Google Scholar]
- NCT02029027. Interest of intravaginal electro‐stimulation at home by GYNEFFIK compared to usual care in incontinent patients with prior perineal reeducation [Evaluation de l'intérêt de l'électrostimulation Intra‐vaginale à Domicile Par Rapport à Une Prise en Charge Habituelle Dans la Prise en Charge de Patientes Incontinentes Ayant bénéficié d'Une rééducation périnéale]. clinicaltrials.gov/show/NCT02029027 (first received 7 January 2014). [NCT02029027; sr‐incont64490]
Luber 1997 {published data only}
- Luber KM, Wolde‐Tsadik G. Efficacy of functional electrical stimulation in treating genuine stress incontinence: a randomized clinical trial. Neurourology and Urodynamics 1997;16(6):543‐51. [sr‐incont5523] [DOI] [PubMed] [Google Scholar]
Maher 2009 {published data only}
- Maher RM, Crowe L, Caulfield B. Comparison of two methods of electrical muscle stimulation training of pelvic floor musculature in the treatment of stress urinary incontinence [abstract]. Journal of Women's Health Physical Therapy 2009;33(1):24. [sr‐incont47080] [Google Scholar]
Min 2015 {published data only}
- Min L, Zhao X. Comparison of the efficacy and safety between TVT‐O and TVT‐O with biofeedback pelvic floor electrical stimulation on female stress urinary incontinence. Sichuan da Xue Xue Bao. Yi Xue Ban [Journal of Sichuan University. Medical Science Edition] 2015;46(1):149‐52. [sr‐incont66970] [PubMed] [Google Scholar]
Olah 1990 {published data only}
- Olah KS, Bridges N, Denning J, Farrar DJ. The conservative management of patients with symptoms of stress incontinence: a randomized, prospective study comparing weighted vaginal cones and interferential therapy. American Journal of Obstetrics & Gynecology 1990;162(1):87‐92. [sr‐incont381] [DOI] [PubMed] [Google Scholar]
Oldham 2013 {published and unpublished data}
- ISRCTN74508432. Evaluation of a new technology to treat urinary incontinence in women: a single blind randomised controlled trial. isrctn.org/ISRCTN74508432 (date first received 10 August 2011). [ISRCTN74508432; sr‐incont64555]
- Oldham J. Supplemental data from our trial [personal communication]. Email to: F Stewart. 13 June 2016.
- Oldham J, Herbert J, McBride K. Evaluation of a new disposable "tampon like" electrostimulation technology (Pelviva) for the treatment of urinary incontinence in women: a 12‐week single blind randomized controlled trial. Neurourology and Urodynamics 2013;32(5):460‐6. [ISRCTN74508432; sr‐incont48076] [DOI] [PubMed] [Google Scholar]
- Oldham J, McBride K, Herbert J. Evaluation of a new electrostim technology for the treatment of urinary incontinence in women: a randomized controlled trial [abstract]. Neurourology and Urodynamics 2010;29(6):1067. Abstract no. 182. [ISRCTN74508432; sr‐incont40151] [DOI] [PubMed] [Google Scholar]
Parsons 2004 {published data only}
- Parsons M, Mantle J, Cardozo L, Hextall A, Boos K, Bidmead J. A single blind, randomised, controlled trial of pelvic floor muscle training with home electrical stimulation in the treatment of urodynamic stress incontinence. Proceedings of the Joint Meeting of the International Continence Society (ICS) (34th Annual Meeting) and the International UroGynecological Association (IUGA); 2004 Aug 23‐27; Paris, France 2004. [Abstract no. 296; sr‐incont19054]
Patil 2010 {published data only}
- Patil SP Nagrale AV Ganvir SD. Additive effect of interferential therapy over pelvic floor exercises. International Journal of Therapy & Rehabilitation 2010;17(11):596‐602. [sr‐incont47058] [Google Scholar]
Pereira 2012 {published data only}
- Correia G, Pereira V, Bonioti L, Driusso P. Decrease of severity of stress urinary incontinence after surface electrical stimulation in older women [abstract]. Neurourology and Urodynamics 2012;31(6):878‐9. Abstract no. 119. [sr‐incont46720] [Google Scholar]
- Pereira VS, Bonioti L, Correia GN, Driusso P. Effects of surface electrical stimulation in older women with stress urinary incontinence: a randomized controlled pilot study. Actas Urologicas Españolas 2012;36(8):491‐6. [sr‐incont45933] [DOI] [PubMed] [Google Scholar]
Pohl 2004 {published data only}
- Pohl K, Jundt K, Greulich T, Drinovac V, Peschers U. Biofeedback versus electrostimulation in treatment of female stress urinary incontinence. Proceedings of the Joint Meeting of the International Continence Society (ICS) (34th Annual Meeting) and the International UroGynecological Association (IUGA); 2004 Aug 23‐27; Paris, France 2004. [Abstract no. 564; sr‐incont19073]
Preisinger 1990 {published data only}
- Preisinger E, Hofbauer J, Nurnberger N, Sadil S, Schneider B. Possibilities of physiotherapy for urinary stress incontinence. Zeitschrift Physische Medizin, Balneologische Medizin Klimatologie 1990;19(2):75‐9. [sr‐incont6314] [Google Scholar]
Sand 1995 {published data only}
- Sand PK, Richardson DA, Staskin DR, Swift SE, Appell RA, Whitmore KE, et al. Pelvic floor electrical stimulation in the treatment of genuine stress incontinence: a multicenter, placebo‐controlled trial. American Journal of Obstetrics & Gynecology 1995;173(1):72‐9. [sr‐incont2961] [DOI] [PubMed] [Google Scholar]
- Sand PK, Richardson DA, Staskin DR, Swift SE, Appell RA, Whitmore KE, et al. Pelvic floor stimulation in the treatment of genuine stress incontinence: a multicentre placebo‐controlled trial [abstract]. Neurourology and Urodynamics 1994;13(4):356‐7. [sr‐incont2679] [DOI] [PubMed] [Google Scholar]
- Sand PK, Richardson DA, Staskin DR, Swift SE, Appell RA, Whitmore KE, et al. Pelvic floor stimulation in the treatment of genuine stress incontinence: a multicentrer placebo controlled trial. Proceedings of the American Urogynecology Society (AUGS), 15th Annual Meeting; 1994 Sep 21‐24; Toronto, Canada 1994. [Abstract no. 27; sr‐incont14586]
Santos 2009 {published data only}
- Santos PF, Oliveira E, Zanetti MR, Arruda RM, Sartori MG, Girao MJ, et al. Electrical stimulation of the pelvic floor versus vaginal cone therapy for the treatment of stress urinary incontinence. Revista Brasileira de Ginecologia e Obstetricia 2009;31(9):447‐52. [sr‐incont34336] [DOI] [PubMed] [Google Scholar]
Schmidt 2009 {published data only}
- Schmidt AP, Sanches PR, Silva DP Jr, Ramos JG, Nohama P. A new pelvic muscle trainer for the treatment of urinary incontinence. International Journal of Gynaecology & Obstetrics 2009;105(3):218‐22. [sr‐incont31994] [DOI] [PubMed] [Google Scholar]
Seo 2004 {published data only}
- Seo JT, Yoon H, Kim YH. A randomized prospective study comparing new vaginal cone and FES‐Biofeedback. Yonsei Medical Journal 2004;45(5):879‐84. [sr‐incont19426] [DOI] [PubMed] [Google Scholar]
Shepherd 1984 {published data only}
- Shepherd AM, Tribe E, Bainton D. Maximum perineal stimulation. A controlled study. British Journal of Urology 1984;56(6):644‐6. [sr‐incont657] [DOI] [PubMed] [Google Scholar]
Shepherd 1985 {published data only}
- Shepherd AM, Blannin JP, Winder A. The English experience of intra‐vaginal electrical stimulation in urinary incontinence ‐ a double blind trial. Proceedings of the International Continence Society (ICS), 15th Annual Meeting; 1985 Sep 3‐6; London 1985:224‐5. [sr‐incont10923]
Smith 1996 {published data only}
- Smith J J 3rd. Intravaginal stimulation randomized trial. Journal of Urology 1996;155(1):127‐130. [sr‐incont2900] [PubMed] [Google Scholar]
- Smith JJ, Loehner D, Bingham W. Intravaginal electrical stimulation in the treatment of GSUI and DI: a controlled study [abstract number 25]. Proceedings of the American Urogynecology Society (AUGS), 15th Annual Meeting, 1994 Sep 21‐24, Toronto, Canada 1994. [sr‐incont14584]
Tapp 1987 {published data only}
- Tapp AJS, Williams S, Hills B, Cardozo LD. The role of physiotherapy in the treatment of genuine stress incontinence [abstract]. Proceedings of the International Continence Society (ICS), 17th Annual Meeting, 1987 Sep 2‐5, Bristol, UK, 1987:204‐5. [sr‐incont9038]
Tapp 1989 {published data only}
- Tapp AJS, Hills B, Cardozo L. Pelvic floor physiotherapy compared with the Burch colposuspension in the treatment of genuine stress incontinence. Proceedings of the Silver Jubilee British Congress of Obstetrics and Gynaecology; 1989 Jul 4‐7; London 1989:65. [sr‐incont8015]
- Tapp AJS, Hills B, Cardozo L. Randomised study comparing pelvic floor physiotherapy with the Burch colposuspension [abstract]. Neurourology and Urodynamics 1989;8(4):356‐7. [sr‐incont4515] [Google Scholar]
Terlikowski 2013 {published data only}
- Terlikowski R, Dobrzycka B, Kinalski M, Kuryliszyn‐Moskal A, Terlikowski SJ. Transvaginal electrical stimulation with surface‐EMG biofeedback in managing stress urinary incontinence in women of premenopausal age: a double‐blind, placebo‐controlled, randomized clinical trial. International Urogynecology Journal 2013;24(10):1631‐8. [sr‐incont48459] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitmore 1995 {published data only}
- Whitmore KE, Staskin DR, Grigoriev VE, Appell RA, Sand PK, Ostergaard DR. Pelvic floor stimulation in the treatment of genuine stress incontinence: a multicenter placebo controlled trial [abstract]. Journal of Urology 1995;153(4 Suppl):491A. Abstract no. 1050. [sr‐incont15467] [DOI] [PubMed] [Google Scholar]
Wilson 1987 {published data only}
- Wilson PD, Al Samarrai T, Deakin M, Kolbe E, Brown AD. An objective assessment of physiotherapy for female genuine stress incontinence. British Journal of Obstetrics and Gynaecology 1987;94(6):575‐82. [sr‐incont2656] [DOI] [PubMed] [Google Scholar]
- Wilson PD, Al Samarrai T, Deakin M, Kolbe E, Brown ADG. The value of physiotherapy in female genuine stress incontinence. Proceedings of the International Continence Society (ICS), 14th Annual Meeting; 1984 Sep 13‐15; Innsbruck, Austria 1984:156‐8. [sr‐incont10954]
Wise 1993 {published data only}
- Wise BG, Haken J, Cardozo LD, Plevnik S. A comparative study of vaginal cone therapy, cones + Kegel exercises, and maximal electrical stimulation in the treatment of female genuine stress incontinence [abstract]. Neurourology and Urodynamics 1993;12(4):436‐7. Abstract no. 76. [sr‐incont4589] [Google Scholar]
References to studies excluded from this review
Bezerra 2009 {published data only}
- Bezerra CA, Wroclawski ER, Rodrigues AO, Tristao R, Borrelli M. Randomized clinical trial do not show that PFMT plus vaginal electrical stimulation is better than electrical stimulation alone for SUI in women [abstract]. Journal of Urology 2009;181(4 Suppl):561. Abstract no. 1561. [sr‐incont62404] [Google Scholar]
Blowman 1991 {published data only}
- Blowman C, Pickles C, Emery S, Creates V, Towell L, Blackburn N, et al. Prospective double blind controlled trial of intensive physiotherapy with and without stimulation of the pelvic floor in treatment of genuine stress incontinence. Physiotherapy 1991;77(10):661‐4. [sr‐incont2338] [Google Scholar]
Furst 2014 {published data only}
- Furst MC, Mendonca RR, Rodrigues AO, Matos LL, Pompeo AC, Bezerra CA. Long‐term results of a clinical trial comparing isolated vaginal stimulation with combined treatment for women with stress incontinence. Einstein 2014;12(2):168‐174. [sr‐incont62563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirschner‐Hermanns 1995 {published data only}
- Kirschner‐Hermanns R, Niehaus S, Schafer W, Jakse G. Pelvic floor re‐education in female stress‐incontinence I. and II. follw‐up results (mean 43 months). Proceedings of the International Continence Society (ICS), 25th Annual Meeting; 1995 Oct 17‐20; Sydney, Australia 1995:230‐1. [Abstract no. 216; sr‐incont10891]
Kolbl 1989 {published data only}
- Kolbl H, Riss P. Percutaneous electrotherapy for micturition complications after bladder surgery. Archives of Gynecology and Obstetrics 1989;245(1‐4):788‐90. [sr‐incont25929] [Google Scholar]
NCT01763762 {published data only}
- NCT01763762. A comparison of the efficacy of electrical pudendal nerve stimulation to pelvic floor muscle training with transvaginal electrical stimulation in treating female stress incontinence. clinicaltrials.gov/show/NCT01763762 (date first received 9 January 2013). [NCT01763762; sr‐incont47834]
NCT02899520 {published data only}
- NCT02899520. Evaluation of programs of reeducation for urinary incontinence in woman. clinicaltrials.gov/show/NCT02899520 (date first received 14 September 2016). [NCT02899520; sr‐incont73997]
Pennisi 1994 {published data only}
- Pennisi M, Grasso‐Leanza F, Panella P, Pepe P. Rehabilitation therapy in the treatment of female urinary incontinence. Our experience with 121 patients. Minerva Urologica e Nefrologica [Italian Journal of Urology and Nephrology] 1994;46(4):245‐9. [PubMed] [Google Scholar]
RBR‐64s9ts {published data only}
- RBR‐64s9ts. Tibial nerve electrical stimulation for urinary incontinence in older women. www.ensaiosclinicos.gov.br/rg/RBR‐64s9ts (date first received 30 January 2014). [RBR‐64s9ts; sr‐incont74032]
Terry 1996 {published data only}
- Terry PB, Whyte SM. Randomised trial comparing Enhance with physiotherapy for the treatment of GSI. Proceedings of the International Continence Society (ICS), 26th Annual Meeting; 1996 Aug 27‐3; Athens, Greece 1996:248‐9. [sr‐incont5133]
References to ongoing studies
ACTRN12610000254099 {published data only}
- ACTRN12610000254099, Driusso P, Correia GN, Pereira VS, Aveiro MC, Melo MV, et al. Physiotherapy for women with stress urinary incontinence: effects of kinesiotherapy, vaginal cones and electrical stimulation. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=335280 (date first received 19 March 2010). [ACTRN12610000254099; sr‐incont41378]
Jha 2013 {published data only}
- Jha S, Pye C. IPSU study: Impact of Physiotherapy on Sexual function in women with Stress Urinary Incontinence (SUI) and a comparison of electrical stimulation versus standard physiotherapy: a randomised controlled trial. UK Clinical Research Network (UKCRN) (available at: https://www.ukctg.nihr.ac.uk/) (date first received 3 May 2013). [UKCRN11623; sr‐incont60901]
- Jha S, Pye C, ISRCTN09586238. Impact of Physiotherapy on sexual function in women with Stress Urinary Incontinence (SUI) [IPSU study: Impact of Physiotherapy on Sexual function in women with Stress Urinary Incontinence (SUI) and a comparison of electrical stimulation versus standard physiotherapy: a randomised controlled trial]. isrctn.org/ISRCTN09586238 (date first received 3 May 2013). [ISRCTN09586238; sr‐incont64533]
Maher 2010 {published data only}
- Maher R, ISRCTN32312996. Inko‐Outside multicentre, controlled, randomised, blinded study for the treatment of stress urinary incontinence [A single‐blind multicentre, controlled, randomised, blinded comparative study of three forms of pelvic floor muscle training: kegel exercises (KE), electrical stimulation using external skin‐electrodes (ESEX) and electrical stimulation using an intra‐vaginal electrode (ESIN), in the treatment of stress urinary incontinence]. isrctn.org/ISRCTN32312996 (date first received 23 March 2010). [ISRCTN32312996; NCT01472068; sr‐incont47887]
NCT00762593 2006 {published data only}
- NCT00762593, Croissandeau J. Trial Evaluating Transvaginal Electrical Stimulation With a Home Use Programmable Device for Urinary Stress Incontinence (KEAT F1) [A Multicenter Double Blind Randomized Placebo Controlled Trial Evaluating Transvaginal Electrical Stimulation With a Home Use Programmable Device for Urinary Stress Incontinence]. clinicaltrials.gov/show/NCT00762593 (date first received 30 September 2008). [NCT00762593; sr‐incont61579]
NCT02185235 2014 {published data only}
- NCT02185235, Su T‐H. A randomized controlled trial of electrical stimulation to treat pelvic floor disorder [To evaluate the efficacy of electrical stimulation and biofeedback treatment for pelvic floor disorder women]. clinicaltrials.gov/show/NCT02185235 (first received 9 July 2014). [NCT02185235; sr‐incont63818]
NCT02423005 2015 {published data only}
- NCT02423005, Minogue C, Nunez M, Lynch C, Jacobs BE, Swift S, et al. Neurotech Vital Compact versus Itouch Sure Pelvic Floor Exerciser US [A Single‐blind, Multi‐centre, Randomised, Controlled, Non‐inferiority, Clinical Study to Assess the Safety and Performance of the Neurotech Vital Compact Device Compared to the Itouch Sure Pelvic Floor Exerciser for the Treatment of Stress Urinary Incontinence in Female Patients]. clinicaltrials.gov/show/NCT02423005 (first received 22 April 2015). [NCT02423005; sr‐incont67529]
Robson 2013 {published data only}
- NCT02214784, O'farrell S, Tunn R. Neurotech Vital device for the treatment of stress urinary incontinence [A Randomised Controlled Double‐Blind Clinical Study To Evaluate The Safety And Performance Of Neuromuscular Electrical Stimulation (NMES) With The Neurotech Vital Device For The Treatment Of Stress Urinary Incontinence]. clinicaltrials.gov/show/NCT02214784 (first received 12 August 2014). [ISRCTN68358784; NCT02214784; sr‐incont64489]
- Robson K, ISRCTN68358784. Safety and performance of Neuromuscular Electrical Stimulation (NMES) with the Neurotech Vital device for the treatment of stress urinary incontinence (SUI) [A Randomised Controlled double‐blind clinical Trial to evaluate the safety and performance of Neuromuscular Electrical Stimulation (NMES) with the NeuroTech Vital device for the treatment of stress urinary incontinence (SUI)]. isrctn.org/ISRCTN68358784 (first received 23 July 2013). [ISRCTN68358784; sr‐incont64527]
Robson 2014 {published data only}
- Robson K, ISRCTN27961345. A study to look at the safety and performance of Neuromuscular Electrical Stimulation (NMES) with the NeuroTech Vital device compared to the itouch Sure Pelvic Floor Exerciser for the treatment of stress urinary incontinence [A randomised, controlled, single‐blind, pilot clinical study to evaluate the safety and performance of Neuromuscular Electrical Stimulation (NMES) with the NeuroTech Vital device compared to the itouch Sure Pelvic Floor Exerciser for the treatment of stress urinary incontinence in female patients]. isrctn.org/ISRCTN27961345 (first received 24 February 2014). [BMR‐14‐1001; ISRCTN27961345; sr‐incont64516]
Additional references
Alhasso 2005
- Alhasso A, Glazener CMA, Pickard R, N'Dow JMO. Adrenergic drugs for urinary incontinence in adults. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD001842.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ayeleke 2015
- Ayeleke RO, Hay‐Smith EJC, Omar MI. Pelvic floor muscle training added to another active treatment versus the same active treatment alone for urinary incontinence in women. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD010551.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bartoli 2010
- Bartoli S, Aguzzi G, Tarricone R. Impact on quality of life of urinary incontinence and overactive bladder: a systematic literature review. Urology 2010;75(3):491‐500. [DOI] [PubMed] [Google Scholar]
Berghmans 2002
- Berghmans BA, Waalwijk van Doorna E, Nieman F, Bie R, Brandt P, Kerrebroeck P. Efficacy of physical therapeutic modalities in women with proven bladder overactivity. European Urology 2002;41(6):581‐7. [DOI] [PubMed] [Google Scholar]
Berghmans 2013
- Berghmans B, Hendriks E, Bernards A, Bie R, Omar MI. Electrical stimulation with non‐implanted electrodes for urinary incontinence in men. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD001202.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bø 2004
- Bø K. Urinary incontinence, pelvic floor dysfunction, exercise and sport. Sports Medicine 2004;34(7):451‐64. [DOI] [PubMed] [Google Scholar]
Bø 2005
- Bø K, Kvarstein B, Nygaard I. Lower urinary tract symptoms and pelvic floor muscle exercise adherence after 15 Years. Obstetrics and Gynecology 2005;105(5):999‐1005. [DOI] [PubMed] [Google Scholar]
Chancellor 2002
- Chancellor MB, Leng W. The mechanism of action of sacral nerve stimulation in the treatment of detrusor overactivity and urinary retention. In: Jonas U, Grunewald V editor(s). New Perspectives in Sacral Nerve Stimulation: For Control of Lower Urinary Tract Dysfunction. London: Martin Dunitz, 2002. [Google Scholar]
Dean 2017
- Dean N, Ellis G, Herbison GP, Wilson D, Mashayekhi A. Laparoscopic colposuspension for urinary incontinence in women. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD002239.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dumoulin 2014
- Dumoulin C, Hay‐Smith EJC, Mac Habée‐Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD005654.pub3] [DOI] [PubMed] [Google Scholar]
EAU 2015
- Lucas MG, Bedretdinova D, Berghmans LC, Bosch JLHR, Burkhard FC, Cruz F, et al. Guidelines on Urinary Incontinence (partial update March 2015). Arnhem, the Netherlands: European Association of Urology (EAU), 2015. Available at: uroweb.org/wp‐content/uploads/20‐Urinary‐Incontinence_LR1.pdf (accessed 21 June 2016). [Google Scholar]
Erlandson 1977
- Erlandson BE, Fall M. Intravaginal electrical stimulation in urinary incontinence. An experimental and clinical study. Scandinavian Journal of Urology and Nephrology. Supplementum 1977;44:1. [PubMed] [Google Scholar]
Evers 2005
- Evers S, Goossens M, Vet H, Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. International Journal of Technology Assessment in Health Care 2005 Spring;21(2):240‐5. [PubMed] [Google Scholar]
Fall 1991
- Fall M, Lindstrom S. Electrical stimulation: a physiologic approach to the treatment of urinary incontinence. Urologic Clinics of North America 1991;18(2):393‐407. [PubMed] [Google Scholar]
Fall 1994
- Fall, M, Lindstrom S. Functional electrical stimulation: physiological basis and clinical principles. International Urogynecology Journal 1994;5(5):296‐304. [Google Scholar]
Glazener 2017a
- Glazener CMA, Cooper K, Mashayekhi A. Anterior vaginal repair for urinary incontinence in women. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD001755.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glazener 2017b
- Glazener CMA, Cooper K, Mashayekhi A. Bladder neck needle suspension for urinary incontinence in women. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD003636.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime Inc). GRADEpro Guideline Development Tool (GDT). Version accessed 21 November 2016. Hamilton, Ontario, Canada: McMaster University (developed by Evidence Prime Inc), 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Yitter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herbison 2009
- Herbison GP, Arnold EP. Sacral neuromodulation with implanted devices for urinary storage and voiding dysfunction in adults. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004202.pub2] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Husereau 2013
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BJOG 2013;120:765‐70. [DOI] [PubMed] [Google Scholar]
ICI 2013
- Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence: 5th International Consultation on Incontinence. Recommendations of the International Scientific Committee: evaluation and treatment of urinary incontinence, pelvic organ prolapse and faecal incontinence; 2012 Feb 23‐25; Paris. Belgium: International Consultation on Urological Diseases (ICUD‐EAU), 2013. [Google Scholar]
Imamura 2010
- Imamura M, Abrams P, Bain C, Buckley B, Cardozo L, Cody J, et al. Systematic review and economic modelling of the effectiveness and cost‐effectiveness of non‐surgical treatments for women with stress urinary incontinence. Health Technology Assessment 2010;14(40):1‐506. [DOI] [PubMed] [Google Scholar]
Kursh 1994
- Kursh ED, McGuire EJ. Female Urology. Philadelphia: Lippincott, 1994. [Google Scholar]
Lapitan 2017
- Lapitan MCM, Cody JD, Mashayekhi A. Open retropubic colposuspension for urinary incontinence in women. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD002912.pub7; CD002912] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mariappan 2005
- Mariappan P, Alhasso AA, Grant A, N'Dow JMO. Serotonin and noradrenaline reuptake inhibitors (SNRI) for stress urinary incontinence in adults. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004742.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nambiar 2017
- Nambiar A, Cody JD, Jeffery ST, Aluko P. Single‐incision sling operations for urinary incontinence in women. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD008709.pub3; CD008709] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2013
- National Collaborating Centre for Women's and Children's Health, NHS National Institute for Health and Clinical Excellence (NICE). Urinary incontinence: the management of urinary incontinence in women. NICE Clinical Guideline (CG171). London: National Institute for Health and Care Excellence, 2013. Available from: www.nice.org.uk/guidance/cg171. London: National Institute for Health and Care Excellence.
Oh 2008
- Oh SJ, Ku JH, Choo MS, Yun JM, Kim DY, Park WH. Health‐related quality of life and sexual function in women with stress urinary incontinence and overactive bladder. International Journal of Urology 2008;15(1):62‐7. [DOI] [PubMed] [Google Scholar]
Plevnik 1991
- Plevnik S, Janež J, Vodušek DB. Electrical stimulation. In: Krane RJ, Siroky MB editor(s). Clinical Neuro‐Urology. 2nd Edition. Boston: Little, Brown and Company, 1991:559‐71. [Google Scholar]
Qaseem 2014
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, Shekelle P. Nonsurgical management of urinary incontinence in women: A clinical practice guideline from the American College of Physicians. Annals of Internal Medicine 2014;161(6):429‐40. [DOI] [PubMed] [Google Scholar]
Reference Manager 2012 [Computer program]
- Thomson Reuters. Reference Manager Professional Edition. Version 12. New York: Thomson Reuters, 2012.
Rehman 2011
- Rehman H, Bezerra Carlos CB, Bruschini H, Cody JD. Traditional suburethral sling operations for urinary incontinence in women. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD001754.pub3; CD001754] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roos 2014
- Roos AM, Thakar R, Sultan AH, Burger CW, Paulus AT. Pelvic floor dysfunction: women's sexual concerns unraveled. Journal of Sexual Medicine 2014;11(3):743‐52. [DOI] [PubMed] [Google Scholar]
Scheepens 2003
- Scheepens WA, Koeveringe GA, Bie RA, Weil EHJ, Kerrebroeck PhEV. Urodynamic results of sacral neuromodulation correlate with subjective improvement in patients with an overactive bladder. European Urology 2003;43(3):282‐7. [DOI] [PubMed] [Google Scholar]
Schreiner 2013
- Schreiner L, Dos Santos TG, Souza ABA, Nygaard CC, Silva Filho IG. Electrical stimulation for urinary incontinence in women: a systematic review. International Brazilian Journal of Urology 2013;39(4):454‐64. [DOI] [PubMed] [Google Scholar]
Scottish Government 2015
- Scottish Government. The Scottish independent review of the use, safety and efficacy of transvaginal mesh implants in the treatment of stress urinary incontinence and pelvic organ prolapse in women. Edinburgh: the Scottish Government; 2015 October. Interim report. Available at: www.gov.scot/Resource/0048/00486661.pdf.
Stewart 2016a
- Stewart F, Gameiro LF, Dib R, Gameiro MO, Kapoor A, Amaro JL. Electrical stimulation with non‐implanted electrodes for overactive bladder in adults. Cochrane Database of Systematic Reviews 2016, Issue 12. [DOI: 10.1002/14651858.CD010098.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamoto 2016
- Yamoto TP, Maher CG, Saragioti BT, Hoffmann TC, Moseley AM. How completely are physiotherapy interventions described in reports of randomised trials?. Physiotherapy 2016;102(2):121‐6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Stewart 2016b
- Stewart F, Berghmans B, Bø K, Glazener CMA. Electrical stimulation with non‐implanted devices for stress urinary incontinence in women. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD012390] [DOI] [PMC free article] [PubMed] [Google Scholar]


