Abstract
Following traumatic brain injury (TBI), individuals over 65 years of age show increased mortality and worse functional outcomes compared to younger persons. As neuroinflammation is a key pathobiological mechanism of secondary injury after TBI, we examined how aging affects posttraumatic microglial responses and functional outcomes. Young (12-week-old) and aged (18-month-old) male C57Bl/6 mice were subjected to moderate-level controlled cortical impact (CCI) or sham surgery and neurological function was evaluated. At 72 hours post-injury, brain, blood, and spleen leukocyte counts were assessed ex vivo using flow cytometry. Aged mice demonstrated more severe deficits in forelimb grip strength, balance and motor coordination, spontaneous locomotor activity, and anxiety-like behavior. These animals also exhibited more robust microglial proliferation and significantly higher numbers of brain-infiltrating leukocytes. Microglia in aged mice showed impairments in phagocytic activity and higher production of interleukin-1β (IL-1β). Infiltrating myeloid cells in aged TBI mice also had deficits in phagocytosis, but showed diminished pro-inflammatory cytokine production and greater reactive oxygen species production. Expression of several senescence markers (Bcl-2, p16ink4a, p21cip1a, lipofuscin, and H2AX (pS139)) was increased with age and/or TBI in both microglia and injured cortex. Although there was no difference in the number of circulating blood neutrophils as a function of age, young mice exhibited more pronounced TBI-induced splenomegaly and splenic myeloid cell expansion. Thus, worse posttraumatic behavioral outcomes in aged animals are associated with exaggerated microglial responses, increased leukocyte invasion, and up-regulation of senescence markers.
Keywords: Aging, Traumatic Brain Injury, Neuroinflammation, Microglia, Behavior
Introduction
Although the young adult population is most at risk for traumatic brain injury (TBI), a second peak incidence occurs in those 65 years of age and older (Flanagan, et al., 2006). Older TBI patients have increased mortality and worse functional outcomes compared to younger persons (Thompson, et al., 2006). They show higher rates of intracerebral hemorrhage and poorer outcomes regardless of injury severity (Karibe, et al., 2017,Papa, et al., 2012,Susman, et al., 2002). Falls at ground level are the most common mechanism of TBI in the elderly, responsible for more than 60% of such injuries (Filer and Harris, 2015). In fact, fall-related TBIs have increased by 8% per year from 1998 to 2011 (Harvey and Close, 2012). Older TBI patients have longer hospital stays and greater need for rehabilitation- highlighting the high morbidity, resource usage, and costs of managing this patient group (Coronado, et al., 2005,Marin, et al., 2017). These observations underscore the need to better understand how aging affects the pathogenesis of TBI.
Recent clinical and pre-clinical research have demonstrated the importance of the post-traumatic inflammatory response to subsequent neurodegeneration and related functional deficits (Faden, et al., 2016, Simon, et al., 2017b). This inflammatory response is characterized by a stereotypic sequence of events involving the brain, its vasculature, and the peripheral immune system. Within minutes after TBI, tissue structure is disrupted, axons are stretched, and significant blood-brain barrier (BBB) breakdown occurs. Oxidative stress and release of pro-inflammatory mediators induce expression of adhesion molecules on brain endothelial cells, promoting leukocyte entry into the brain (Abdul-Muneer, et al., 2015). Microglia and infiltrating macrophages further amplify the immune response by secreting cytotoxic agents including cytokines (e.g. TNF, IL-1β), reactive oxygen species (ROS), and extracellular proteases (e.g. MMP-9). Importantly, aging alone chronically alters microglial phenotype and sensitivity to injury-induced stimuli (Wong, 2013). Early studies had suggested that aging primes microglia so that they produce exaggerated levels of inflammatory mediators, and that microglial responses to injury are generally amplified or dysregulated with age (Niraula, et al., 2017). Because elderly TBI patients are believed to have poorer outcomes, they are often treated less aggressively (Gardner, et al., 2018). However, recent data indicate that certain “younger elderly” people, aged 65-75 years, may have a comparable outcome to younger adults after mild-to-moderate TBI (Mak, et al., 2012,Wan, et al., 2016). These observations emphasize the need for more pre-clinical research to better understand age-related changes in TBI pathology and to develop age-specific targets for treatment.
Previous work has shown that the expression level of ionized calcium-binding adapter molecule 1 (Iba1), a microglia/macrophage marker, is chronically elevated in the hippocampus of aged mice following TBI (Sandhir, et al., 2008). Furthermore, our laboratory demonstrated that aged mice have altered gene expression of pro- and anti-inflammatory markers and increased numbers of reactive (bushy and hypertrophic) Iba1-positive microglia/macrophages in the cortex, hippocampus, and thalamus (Kumar, et al., 2013). More recently, Morganti, et al. (2016) reported that therapeutic targeting with a dual CCR2/5 antagonist significantly attenuated TBI-induced expression of inflammatory mediators and oxidative stress-related genes in aged mice. However, the understanding of cellular neuroimmune responses in the aged brain following TBI is very limited. Thus, we hypothesized that normal aging primes the microglial/macrophage response following TBI resulting in an age-related exacerbation of post-traumatic neuroinflammation. In this study, we subjected young and aged mice to moderate-level CCI and evaluated functional outcomes using a battery of neurobehavioral tests and inflammatory responses using ex vivo analyses.
METHODS & MATERIALS
Animals:
Young adult (3-month-old) and aged (18-month-old) male C57BL/6 mice bred in-house from the Jackson colony were housed on sawdust bedding in a specific pathogen free facility (12 hours light/dark cycle). All animals had access to chow and water ad libitium. Animal procedures were performed in accordance with NIH guidelines for the care and use of laboratory animals and approved by the Animal Care Committee of the University of Maryland School of Medicine.
Controlled cortical impact:
Our custom-designed controlled cortical impact (CCI) injury device consists of a microprocessor-controlled pneumatic impactor with a 3.5 mm diameter tip as previously described (Loane, et al., 2009). Briefly, mice were anesthetized with isoflurane evaporated in a gas mixture containing 70% N2O and 30% O2 and administered through a nose mask. Mice were placed on a heated pad and core body temperature was maintained at 37°C. The head was mounted in a stereotaxic frame, a 10 mm midline incision was made over the skull and the skin and fascia were reflected. A 5 mm craniotomy was made on the central aspect of the left parietal bone. The impounder tip of the injury device was then extended to its full stroke distance (44 mm), positioned to the surface of the exposed dura, and reset to impact the cortical surface. Moderate-level CCI was induced using an impactor velocity of 6 m/s, deformation depth of 1 mm, and a dwell time of 50 ms (Loane, et al., 2009). After injury, the incision was closed with interrupted 6-0 silk sutures, anesthesia was terminated, and the animal was placed into a heated cage to maintain normal core temperature for 45 minutes post-injury. Sham animals underwent the same procedure as CCI mice except for craniotomy and cortical impact.
Open Field Analysis:
The open field test was used to measure locomotor activity on post-injury day 2 as previously described (Zhao, et al., 2012). The apparatus consists of an open field (22.5 cm × 22.5 cm) with two adjacently located imaginary circular zones. Mice were individually placed in a corner facing the wall of the open-field chamber and allowed to freely explore the chamber for 5 minutes. The distance travelled was recorded by ANY-Maze software (Stoelting Co., Wood Dale, IL). Mice were baseline tested prior to CCI and on post-injury day 2.
Rotarod:
Motor function was assessed using a rotarod as previously described (Doran, et al., 2018). Prior to sham or CCI all animals were trained three trials per day for 2 consecutive days on the rotarod apparatus (IITC, Inc., Life Sciences, St. Petersburg, FL). Latency was assessed by measuring the length of time each mouse remained on the rotating drum as it accelerated from 4 to 40 rpm over a span of 2 minutes. The latency of each mouse to fall from the rotating drum was recorded for each trial (in seconds), and the average latency was used for further analysis. Animals were then tested on day 1, 2, and 3 post-injury, with three trials as in the training period, and the average latency was recorded.
Grip Strength:
Grip strength was measured using a digital grip strength meter (Bioseb BP, In Vivo Research Instruments, France) on day 1, 2, and 3 post-injury, as previously described (Jacotte-Simancas, et al., 2015). Forelimb grip strength was measured from the mouse using both the ipsilateral and contralateral forepaws together. The mouse was held by its tail, the forelimbs were placed on the grasping metal wire grid and the mouse gripped the wire grid attached to the force transducer. Once the grip was secured, the animal was slowly pulled away from the bar. The maximal average force exerted on the grip strength meter by both forepaws was averaged from 3 trials per day for each mouse, for 2 consecutive days.
Cylinder Test:
The cylinder test was used to assess asymmetry in forelimb usage as described previously (Venna, et al., 2012). Each animal was individually placed in a transparent plexiglass cylinder of 9 cm diameter and 15 cm height during the test. Forelimb use of the first contact against the cylinder wall after rearing and during lateral exploration was analyzed using the following criteria: a total of 20 limb placements on cylinder wall were recorded during the 10-min test session. A mirror was placed behind the cylinder with an angle to enable the rater to view forelimb movements when the mouse was turned to other side. The final score = (nonimpaired forelimb use (right) − impaired forelimb use (left))/(nonimpaired forelimb use + impaired forelimb use + both limbs movement). Mice were assessed prior to CCI and on day 2 post-injury. Of the 19 mice in each group that were tested, 32% of young mice (N=6) and 36% of aged mice (N=7) did not perform and were excluded from the analysis.
Flow cytometry:
Body weights were recorded and immediately after mice were euthanized blood (200 μL) was drawn by cardiac puncture with heparinized needles. Following transcardial perfusion with 60 mL of ice-cold sterile PBS, the spleen was removed, weighed, and processed by mechanical disruption on a 70-μm-filter screen. Red blood cell lysis was achieved by successive 10-minute incubations with Tris-ammonium chloride (Stem Cell Technologies, Vancouver, Canada). Splenocytes were subsequently washed and resuspended in a total of 5 mL of RPMI (Lonza Group, Basel, Switzerland) from which 500 μL was then transferred into FACS tubes. Brain hemispheres were mechanically digested using a razor blade to mince tissue and were passed through a 70-μm-filter using RPMI. CNS tissue was then enzymatically digested using DNAse (10 mg/mL; Roche, Mannheim, Germany), Collagenase/Dispase (1 mg/mL; Roche), and Papain (25 U; Worthington Biochemical, Lakewood, NJ, USA) for 1 hour at 37°C in a shaking CO2 incubator (200 rpm). Tissue homogenates were centrifuged at 1500 rpm for 5 minutes at 4°C. The supernatant was discarded and the cells were resuspended in 70% Percoll™ (GE Healthcare, Pittsburgh, PA, USA) and underlayed in 30% Percoll™. This gradient was centrifuged at 500 g for 20 minutes at 21 °C. Myelin was removed by suction and cells at the interface were collected. Leukocytes were washed and blocked with mouse Fc Block (eBioscience, San Diego, CA, USA; clone 93) prior to staining with primary antibody-conjugated flourophores (CD45-eF450 (30-F11), CD11b-APCeF780 (M1/70), Ly6C-APC (HK1.4), and Ly6G-PE (1A8) were purchased from eBioscience, whereas CD45-PerCP-Cy5.5 (30-F11) and CD11b-PerCP-Cy5.5 (M/70) were purchased from Biolegend (San Diego, CA, USA). For live/dead cell discrimination, a fixable viability dye, Zombie Aqua™ (Biolegend), was dissolved in DMSO according to the manufacturer’s instructions and added to cells in a final concentration of 1:200. Data were acquired on a LSRII using FACsDiva 6.0 (BD Biosciences, San Jose, CA, USA) and analyzed using FlowJo (Tree Star, San Carlos, CA, USA). A standardized gating strategy was used to identify microglia (CD45intCD11b+Ly6C−) and brain-infiltrating myeloid cells (CD45hiCD11b+), including monocyte (CD45hiCD11b+Ly6C+Ly6G−) and neutrophil (CD45hiCD11b+Ly6C+Ly6G+) populations as previously described (Ritzel, et al., 2018b). Cell-specific fluorescence minus one (FMO) controls were used to determine the positivity of each antibody. Cell count estimations were performed using CountBright™ absolute counting beads (40 μL/test; Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
For intracellular cytokine staining, leukocytes were collected as described above, and 1 μL of GolgiPlug containing brefeldin A (BD Biosciences) was added to 500 μL complete RPMI. Cells were then resuspended in Fc Block, stained for surface antigens and washed in 100 μL of fixation/permeabilization solution (BD Biosciences) for 20 minutes. Cells were washed twice in 500 μL Permeabilization/Wash buffer (BD Biosciences) and resuspended in an intracellular antibody cocktail containing cytokine antibodies (MMP-9-R-PE, StressMarq Biosciences (SMC-396D); p16ink4a-APC, StressMarq Biosciences (SPC-1280D); p21cip1 FITC, StressMarq Biosciences (SPC-1281); H2AX pS139-PE, MACS Miltenyi Biotec (130-107-585); NOX2-AF647, Bioss Antibodies (bs-3889R); TNF-PE-Cy7, eBioscience (MP6-XT22); IL-1β-PerCP-eF710, eBioscience (NJTEN3); and Bcl-2-PE-Cy7, BioLegend (BCL/10C4)) and fixed.
For detection of reactive oxygen species, leukocytes were incubated with dihydrorhodamine (DHR) 123 (5mM; 1:500 in RPMI; Ex/Em: 500/536), a cell-permeable fluorogenic probe (Life Technologies/Invitrogen, Carlsbad, CA). Cells were loaded for 20 minutes at 37°C, washed three times with FACS buffer (without NaAz), and then stained for surface markers including viability stain.
Phagocytic activity of myeloid cells was performed as described (Ritzel, et al., 2015a) with minor modification. Briefly, red fluorescent carboxylate-modified polystyrene latex beads (0.5 μm mean diameter; Sigma) were added to freshly isolated cells in a final dilution of 1:100 (in RPMI). After 45 minutes of incubation at 37°C, the cells were washed twice, re-suspended in FACS buffer, stained for surface markers, and fixed in PFA.
RNA isolation and qPCR analysis:
Total RNA was extracted from ipsilateral cortex of young and aged sham and CCI mice using a RNeasy Mini kit (Qiagen, Valencia, CA) with on-column DNase treatment (Qiagen). cDNA was then made using a VERSO cDNA Reverse Transcription kit (Thermo Scientific, Pittsburg, PA). Real-time quantitative PCR analysis for target mRNAs was performed using TaqMan gene expression assays (GAPDH, Mm99999915_g1; p16ink4a Mm00494449_m1; and p21cip1 Mm04205640_g1). Samples were assayed in triplicate in one run (40 cycles), which was composed of 3 stages, 50°C for 2 minutes, 95°C for 10 seconds for each cycle (denaturation) and finally the transcription step at 60°C for 1 minute. Gene expression was normalized by GAPDH per sample and then compared to the control sample (young sham) to determine relative expression values by 2-ΔΔCt method.
Protein isolation and Western blot analysis:
Proteins from ipsilateral cortical tissue of young and aged mice were extracted using RIPA buffer, equalized, and loaded onto 5–20% gradient gels for SDS PAGE (Bio-Rad; Hercules, CA). Proteins were transferred onto nitrocellulose membranes using a dry transfer method and then blocked for 1 hour in 5% milk in 1 × TBS containing 0.05% Tween-20 (TBS-T) at room temperature. The membrane was incubated in rabbit Anti-CDKN2A/p16ink4a antibody [EPR20418] (ab211542) (1:1000; Abcam, Cambridge, UK), rabbit Anti-p21cip1a antibody [EPR18021] (ab188224) (1:1000; Abcam), or mouse anti-β-Actin (1:5000; Sigma-Aldrich) overnight at 4 °C, then washed three times in TBS-T, and incubated in appropriate HRP-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 hours at room temperature. Membranes were washed three times in TBS-T, and proteins were visualized using SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, Rockford, IL). Chemiluminescence was captured using ChemiDoc™ XRS+ System (Bio-Rad), and protein bands were quantified by densitometric analysis using BioRad Molecular Imaging Software. The data presented reflects the intensity of target protein band normalized based on the intensity of the endogenous control for each sample (expressed in arbitrary units).
Statistical analyses:
Data from individual experiments are presented as mean ± S.E.M. Group effects were determined by two-way ANOVA analysis with Sidak or Tukey post-hoc correction for multiple comparisons. For all behavioral and flow cytometry experiments an N= 7 for sham and N= 12 for CCI mice per age group were used. A separate cohort of N= 4 sham and N= 6 CCI mice per age group were used to perform all gene and protein analyses. Behavioral data were also analyzed by two-way repeated measures ANOVA. All behavioral and ex vivo studies were performed by an investigator blinded to surgical condition. Statistical analysis was performed using GraphPad Prism Software v. 6.0 (GraphPad Software, Inc., La Jolla, CA). p<0.05 was considered statistically significant.
RESULTS
Aging worsens functional outcomes following acute TBI
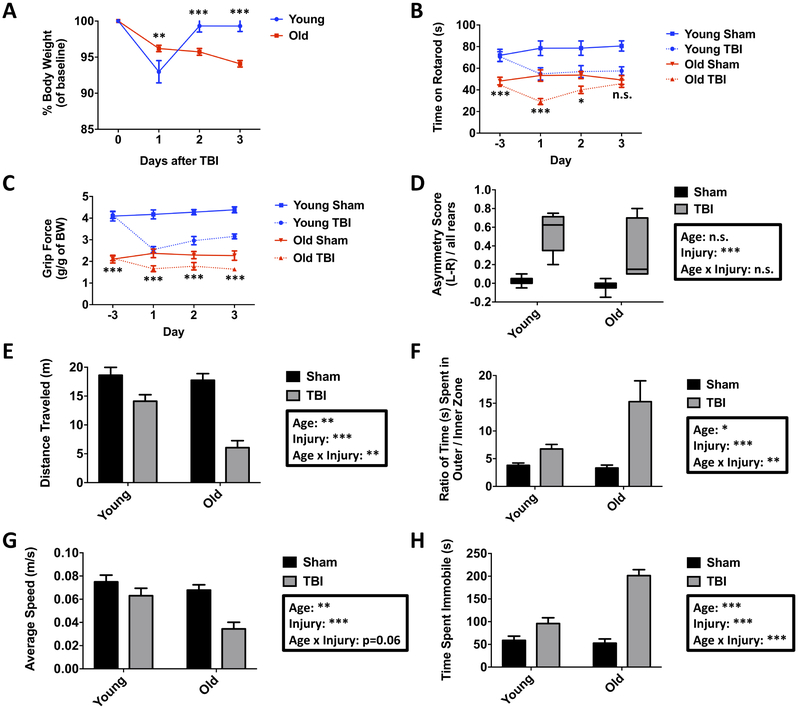
We induced moderate-level CCI in young adult (3-month-old) and aged (18-month-old) male C57Bl/6 mice and evaluated neurological function daily across the first 72 hours post-injury using a battery of behavioral tests to determine the extent of TBI-induced deficits in locomotor activity, motor coordination, grip strength, and contralateral forelimb impairment. We observed that the percent of body weight loss was initially higher in young TBI mice at day one post-injury, but subsequently returned to baseline weight by day two (Figure 1A). In contrast, aged TBI mice exhibited a gradual, but continued increase in body weight loss up to day three post-injury compared to baseline, age-matched sham, and young TBI mice.
Figure 1. Age-related differences in acute behavioral deficits following traumatic brain injury.
The percent change in body weight from baseline was recorded for young and aged mice for three days post-injury (A). Time spent on an accelerating rotarod was evaluated over the course of three days after TBI (B). Grip strength for young and old mice in sham and TBI groups is shown (C). Cylinder testing demonstrated significant asymmetric forelimb use in both age groups following TBI (D). Animals were then evaluated in an open field apparatus at three days after TBI to measure spontaneous locomotor activity. The total distance traveled in the open field apparatus for sham and TBI mice is shown (E). The ratio of the total time spent in the inner versus outer zones of the open field demonstrated significant effects of age and injury (F). The average speed (G) and time spent immobile (H) in the open field showed a robust interaction between age and injury. For all experiments, N=7 sham and N=12 TBI/group. Statistical comparisons between groups were determined by one- or two-way repeated measures ANOVA analysis with Tukey’s multiple comparisons test. For cylinder and open field analysis, analysis was performed using two-way ANOVA with Tukey’s test. Significant group effects of age, injury, and interaction between age and injury are shown in each box. Error bars show mean SEM. Abbreviation: TBI traumatic brain injury, g gram, BW body weight, L left, R right, n.s. not significant, m meters, s seconds, SEM standard error of mean. *p<0.05, **p<0.01, and ***p<0.001
Motor performance was evaluated using an accelerating rotarod. As previously reported (Shoji, et al., 2016), normal aging significantly decreased the latency to fall at baseline. Following CCI, both groups spent less time on the rotarod relative to baseline (Figure 1B). TBI-induced motor function deficits in young mice reached their maximum at the same latency to fall time observed in aged sham mice, whereas aged TBI mice spent significantly less time on the rotarod than age-matched controls. Recovery during this acute period appeared to favor aged mice, with young TBI mice exhibiting continued impairment in motor function by day three post-injury, whereas aged TBI mice had similar fall latencies as age-matched sham mice at this time point. We then measured grip strength using a handheld device. Consistent with other studies (Ge, et al., 2016), normal aging resulted in a decrease in grip strength, whereas TBI caused significant impairment in forelimb grip force in both young and aged mice (Figure 1C). Deficits in grip strength reached their lowest point in young TBI mice at levels that were similar to aged sham mice. Although the change in grip strength was not as pronounced in aged mice following TBI relative to young mice, the absolute grip force was significantly lower compared to both age-matched sham control and the young TBI group. Neither young nor aged TBI groups showed a complete restoration in grip strength during this acute time period. We then assessed for unilateral forelimb impairment using the cylinder test. TBI induced spontaneous forelimb-use asymmetry in both young and aged mice at three days post-injury, with greater preference for using the unimpaired right forepaw (Figure 1D). Although these changes were robust, age-related deficits in sensorimotor function could not be determined given the high incidence of mice unable to perform this task (32% of young TBI mice and 36% of aged TBI mice).
We also measured locomotor activity. Open field testing at 48 hours post-injury revealed a significant effect of TBI on spontaneous locomotor activity, with aged TBI mice traveling comparatively shorter distances than their younger counterparts (Figure 1E). Although both groups spent more time along the perimeter in the outer zone of the maze, aged TBI mice spent comparably less time in the inner zone, indicating that aging modified TBI-induced anxiety-like behaviors (Figure 1F). Despite spending more time in the outer zone following TBI, the average speed and time spent immobile was significantly decreased and increased, respectively, in an age-dependent manner (Figure 1G,H). These data indicate that TBI causes an age-related worsening of locomotor impairment, evidenced by less activity and slower movements. Aged TBI mice also showed a greater preference for the edges of the open field, indicating a more pronounced anxiety-driven aversion to being in open areas of the maze. Taken together, our findings demonstrate an important interaction between age and TBI that result in an age-related worsening of motor coordination and muscle strength.
Aging alters microglial sensitivity to TBI and increases neutrophil infiltration
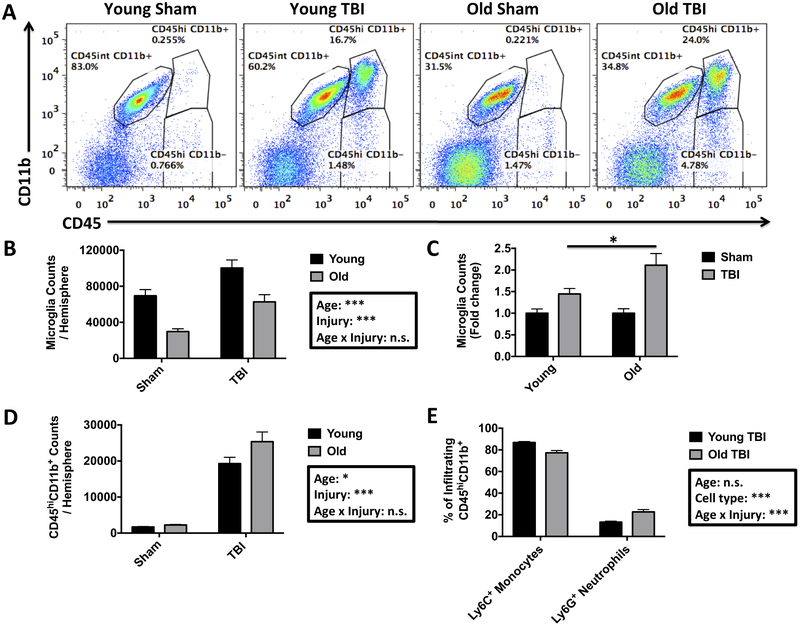
Inflammation is a key driver of TBI (Simon, et al., 2017). To better understand the cellular correlates of the observed age-related worsening in neurological outcomes, we next evaluated the inflammatory response in the brain using flow cytometry (Figure 2A). The absolute number of living CD45intCD11b+Ly6C− microglia was lower in aged brains (Figure 2B). Despite these baseline differences, TBI caused a significant increase in microglia counts in both age groups, with old mice displaying relatively more robust proliferation compared to their younger counterparts. Relative to each sham group, however, significantly greater rates of microglia proliferation were seen in old mice with respect to their younger counterparts (Figure 2C; 1.45±0.1-fold increase in young TBI versus 2.11±0.2-fold increase in aged TBI). Next we examined whether aging makes the brain more vulnerable to peripheral leukocyte invasion following TBI. The total number of infiltrating CD45hi leukocytes was dramatically increased in both young and aged TBI mice, however, aged TBI mice showed significantly greater numbers of peripheral immune cells than young (Figure 2D). The vast majority of these cells were of myeloid origin (CD45hCD11b+), whereas bulk lymphocyte (CD45hiCD11b−) infiltration was largely negligible at this acute time point. Compositional analysis of the infiltrating myeloid population revealed an age-related change in the relative proportion of Ly6C+Ly6G+ neutrophils and Ly6C+Ly6G− monocyte-derivatives, in that, aging increased the frequency of neutrophil extravasation in the TBI brain (Figure 2E). These findings suggest that aging alters the proliferative sensitivity of microglia and makes the brain more permeable to leukocyte invasion following TBI, with aged TBI mice showing a greater bias towards neutrophil recruitment.
Figure 2. Old age alters the microglial proliferation response and leukocyte extravasation following traumatic brain injury.
Representative dot plots show the identification of CD45int microglia and CD45hi infiltrating peripheral myeloid cells in the sham-injured and TBI brain of young and old mice at day 3 after surgery (A). The absolute number of microglia per ipsilateral hemisphere was determined and show a significant decrease in microglia number at baseline in old mice (B). Microglia counts expressed as a fold-change relative to each sham group demonstrated a greater TBI-induced microglial proliferative response in old mice (C). The number of brain-infiltrating myeloid cells is shown for young and old mice following injury (D). The relative composition of these CD45hi myeloid cells reveals a modest but significant neutrophilic shift in old mice after TBI (E). For all experiments, N=7 sham and N=12 TBI/group. Statistical comparisons between groups were determined by two-way ANOVA analysis with Tukey’s multiple comparisons test. Significant group effects of age, injury, and interaction between age and injury are shown in each box. Error bars show mean SEM. Abbreviation: n.s. not significant, TBI traumatic brain injury, SEM standard error of mean. *p<0.05, **p<0.01, and ***p<0.001
Microglial and infiltrating myeloid cell responses to TBI are functionally distinct and altered with age
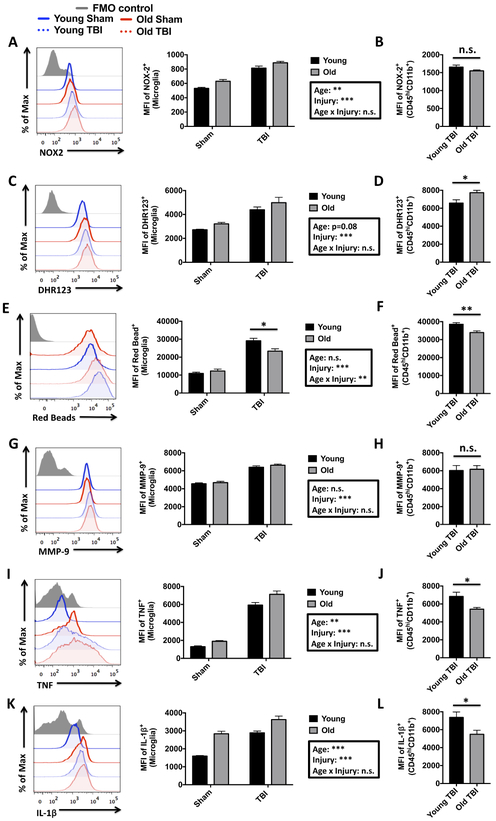
Neuroinflammation is characterized by changes in microglia/macrophage activation that lead to increased reactive oxygen species (ROS) generation, pro-inflammatory cytokine production, and phagocytic activity. We addressed these changes using an ex vivo flow cytometry approach. Oxidative stress was measured using NOX2 (NADPH oxidase 2) protein expression and dihydrorhodamine 123, a fluorogenic probe used to detect ROS, such as peroxide and peroxynitrite. A significant interaction between age and TBI was found for NOX2 expression and ROS production in microglia, as reflected by higher ROS levels in aged microglia and after TBI (Figure 3A,C). Infiltrating myeloid cells showed greater NOX2 and ROS production relative to resident microglia (813±27 vs. 1,550±26; p<0.001 and 4,399±224 vs. 6,845±394; p<0.01, respectively). Interestingly, infiltrating myeloid cells in the aged brain displayed less NOX2 expression and higher ROS production than those found in young TBI brains (Figure 3B,D), suggesting that NOX2 activity rather than its expression per se may be accelerated with age.
Figure 3. Age-related changes in the functional response of microglia and infiltrating myeloid cells after acute TBI.
A representative histogram depicts the relative expression level of NOX2 in brain-resident microglia (A). The MFI of NOX2-positive microglia is shown to the right, demonstrating an effect of age and injury in microglial expression. No change was seen in NOX2 MFI between young and aged CD45hiCD11b+ infiltrating myeloid populations after TBI (B). A representative histogram shows the relative production level of reactive oxygen species, as measured by DHR123 (C). MFI Quantification of DHR123-positive cells reveal a significant effect of injury on microglial-derived ROS production. A comparison of DHR123 MFI in young and old infiltrating myeloid cells is shown (D). A representative histogram illustrates the relative differences in the phagocytic uptake of fluorescent red beads in young and old microglia after TBI (E). To the right, the MFI quantification of red bead-positive microglia in young and old mice demonstrates that the relative level of phagocytosis induced by TBI is reduced in older microglia populations. The relative level phagocytic activity in young and old infiltrating myeloid cells is shown (F). A representative histogram depicts the relative expression level of MMP-9 in brain myeloid cells (G). MFI quantification of MMP-9-positive microglia is shown for young and aged mice at three days after TBI. No difference in relative MMP-9 expression between young and old infiltrating myeloid populations was found (H). A representative histogram shows the relative expression level of TNF (I). MFI Quantification of TNF-positive cells show a significant increase in microglia with both injury and age. An agerelated decrease in TNF production was in the infiltrating myeloid population after TBI (J). A representative histogram illustrates the relative expression level of IL-1β (K). To the right, MFI quantification of IL-1β-positive microglia demonstrates a significant effect of both age and injury. The relative production level of IL-1β is shown for infiltrating myeloid populations (L). For all experiments, N=4 sham and N=6 TBI/group. Statistical comparisons between groups were determined by two-way ANOVA analysis with Tukey’s multiple comparisons test. Significant group effects of age, injury, and interaction between age and injury are shown in each box. Error bars show mean SEM. Abbreviation: FMO fluorescence minus one, MFI mean fluorescence intensity, MMP-9 matrix metalloproteinase-9, NOX2 NADPH oxidase 2, DHR123 dihydrorhodamine 123, TNF tumor necrosis factor, IL-1β interleukin-1 beta, TBI traumatic brain injury, n.s. not significant, SEM standard error of mean. *p<0.05, **p<0.01, and ***p<0.001
Debris clearance and removal of apoptotic neurons is a critical function of microglia/macrophages following acute brain injury (Simon, et al., 2017). Therefore, we assessed phagocytic activity in leukocytes isolated from the TBI brain using a bead-engulfment assay. Microglial phagocytic activity was significantly increased after TBI in young and aged mice, but was significantly impaired in aged microglia (Figure 3E). Infiltrating myeloid cells were comparably better at engulfing beads than resident microglia (29,133±1,369 vs. 38,550±889; p<0.001), however engulfment was significantly impaired in both resident microglia and infiltrating myeloid cells in the aged TBI brain (Figure 3F). These data indicate that debris clearance mechanisms such as the phagocytic removal of damaged cells may be highly impaired in old immune cells.
To further analyze the connection between aging and TBI-induced neuroinflammation, we examined protein production of inflammation-related molecules, including tumor necrosis factor (TNF), interleukin-1 beta (IL-1β), and matrix metalloproteinase-9 (MMP-9). MMP-9 expression and activity are upregulated acutely after TBI and are involved in the degradation of the extracellular matrix, resulting in endothelial dysfunction and blood-brain barrier permeability (Zhang, et al., 2016). Our data demonstrate that microglial MMP-9 production is significantly increased following TBI (Figure 3G). The mean fluorescence intensity of MMP-9 was diminished in infiltrating myeloid cells with age after TBI (Figure 3H). We next examined the production of the pro-apoptotic cytokine, TNF. There was a significant interaction of age for microglial TNF production, with aged microglia showing relatively higher TNF levels compared to young microglia (Figure 3I). Similarly, a significant interaction of TBI for microglial TNF production was observed, however, the interaction between age and TBI was not significant. Infiltrating myeloid cells produced similar levels of TNF relative to resident microglia; however, aging was associated with significantly lower expression in these cells (Figure 3J). IL-1β is a byproduct of inflammasome signaling that is involved in a variety of cellular activities, including cell proliferation, differentiation, and apoptosis. IL-1β also plays a key role in aging and disease (Goldberg and Dixit, 2015). Microglial production of IL-1β was significantly increased with both age and after TBI, although the interaction between age and TBI was not significant (Figure 3K). Young microglia produced higher levels of IL-1β after TBI, but did not exceed the levels detected in aged sham microglia. Notably, infiltrating myeloid cells were the predominant IL-1β producers in the TBI brain (2,890±102 vs. 7,369±611; p<0.001), while myeloid IL-1β production was significantly diminished with age (Figure 3L). Our data indicate that both resident microglia and infiltrating myeloid cells are important contributors of pro-inflammatory response following TBI. Notably, aging blunted the production of TNF and IL-1β in the infiltrating myeloid population, highlighting the important age-related alterations in the regulation of cytokine signaling pathways after TBI.
Microglial expression of senescence markers is increased with age and acutely following TBI
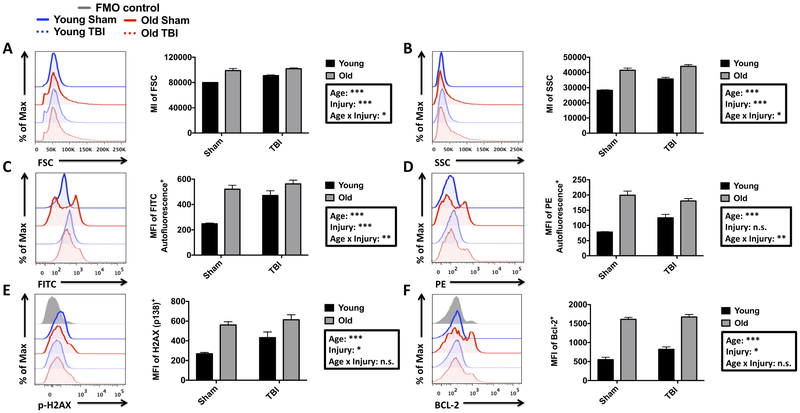
Aging is associated with an increase in inflammatory signaling, cellular dysfunction, and senescence (Fulop, et al., 2017). Although relatively low in number, senescent macrophages are believed to be significant contributors of inflamm-aging, a phenomenon characterized by an age-related elevation in chronic, low-grade inflammation (Prattichizzo, et al., 2016). To date, few studies have confirmed the expression of senescence markers (e.g., p-H2AX, BCL-2, p16ink4a, and p21cip1a) in the aged microglia population, or following TBI. First, we observed that the relative size and granularity (i.e., forward scatter (FSC) and side scatter (SSC), respectively) of microglia following TBI was significantly increased in both young and aged groups, with young TBI mice exhibiting a greater change from age-matched sham levels in these parameters (Figure 4A,B). The size and granularity of microglia in young TBI mice was significantly less than that observed with normal aging. Aging also results in increased lipofuscin accumulation in microglia that localize to lysosomal inclusions and may reflect impairment in autophagy function (Marschallinger, et al., 2017,von Bernhardi, et al., 2015). We found that microglial autofluorescence in the empty FITC or PE channels was more than doubled with normal aging (Figure 4C,D). TBI induced a significant increase in microglial autofluorescence in young TBI mice, but these levels did not exceed those of either the aged sham or TBI groups. At the protein level, our analysis reveals that two additional markers of senescence, the anti-apoptotic protein Bcl-2 (Hernandez-Segura, et al., 2018), and histone H2AX phosphorylation (pS139), indicative of double-stranded DNA breaks (Rogakou, et al., 1998), were significantly increased in microglia with both age and TBI (Figure 4E,F). However, the interaction between age and TBI was not significant for these senescence markers.
Figure 4. Evidence of age- and TBI-induced immune senescence in the resident microglia population.
A representative histogram is shown for the forward scatter intensity of microglia after TBI (A). MI quantification of this histogram revealed a significant effect of both age and injury, as well as a statistical interaction. A representative histogram is shown for the side scatter intensity of microglia (B). To the right, MI quantification demonstrated a significant effect of both age and injury, as well as a statistical interaction. A representative histogram illustrates the relative intensity of microglial autofluorescence in the FITC channel (C). MFI quantification of this data is shown. A representative histogram shows the relative intensity of microglial autofluorescence in the PE channel (D) and MFI quantification is shown to the right. A representative histogram depicts the relative expression level of phosphorylated (Ser139) H2AX in microglia populations (E). MFI quantification of p-H2AX-positive microglia reveals a significant group effect of both age and injury. A representative histogram shows the relative expression level of BCL-2 in microglia, and the MFI quantification of BCL-2-positive microglia is shown (F). For scatter data, N=7 sham and N=12 TBI/group. For all other experiments, N=3 sham and N=6 TBI/group. Statistical comparisons between groups were determined by two-way ANOVA analysis with Tukey’s multiple comparisons test. Significant group effects of age, injury, and interaction between age and injury are shown in each box. Error bars show mean SEM. Abbreviation: FMO fluorescence minus one, FITC fluorescein isothiocyanate, PE phycoerythrin, SSC side scatter, FSC forward scatter, H2AX histone 2a X, BCL-2 B cell lymphoma 2, MI mean intensity, MFI mean fluorescence intensity, TBI traumatic brain injury, n.s. not significant, SEM standard error of mean. *p<0.05, **p<0.01, and ***p<0.001
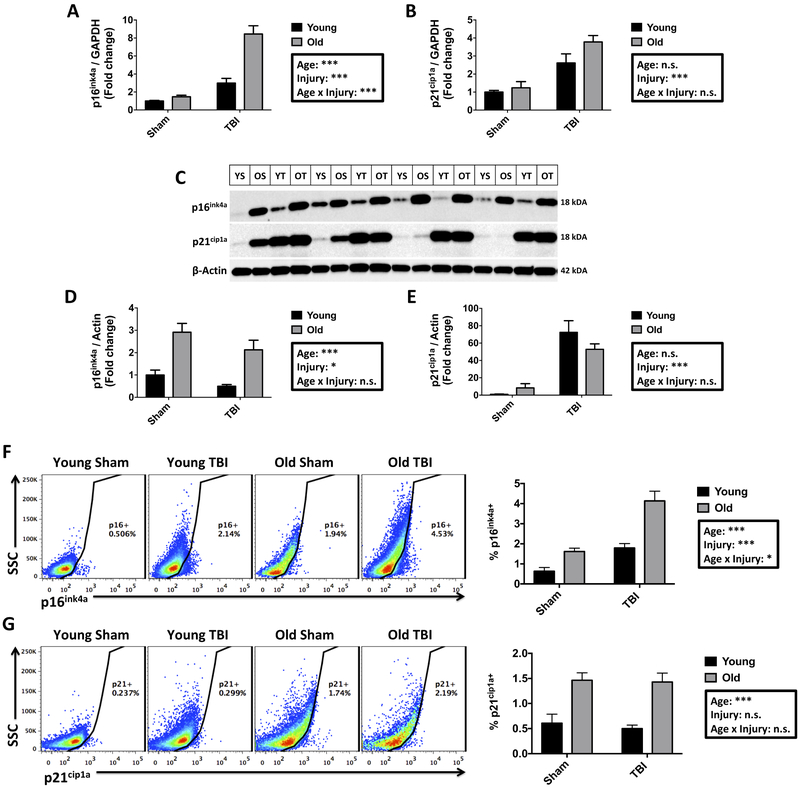
Finally, we examined for the up-regulation of the cellular senescence markers, p16ink4a and p21cip1a, tumor suppressor proteins involved in cell cycle arrest (Childs, et al., 2014,Herranz and Gil, 2018). Gene and protein expression were measured in cortical tissue and in microglia. A significant interaction between age and TBI was demonstrated for p16ink4a gene expression in the cortex, wherein TBI induced nearly two-fold higher expression in aged injured compared to young injured cortex (Figure 5A). There was a significant increase in p21cip1a transcription following TBI in both young and aged cortex (Figure 5B), but there was no interaction between age and TBI. Consistently, TBI also significantly increased the relative protein expression of both p16ink4a and p21cip1a in the cortex, as determined by Western blot analysis (Figure 5C-E). Expression of p16ink4a protein, but not p21cip1a, was significantly increased with normal aging. Notably, when we probed cellular senescence in microglia we identified a significant interaction between age and TBI in the percentage of p16ink4a-positive microglia, with the highest frequencies observed in aged TBI mice (Figure 5F). The percentage of p21cip1a-positive microglia was also significantly increased with age, but unaffected by TBI (Figure 5G). Taken together, these findings demonstrate that normal aging increases the expression of senescence markers within the microglia population and more widely, in the cerebral cortex, which can be differentially altered after TBI.
Figure 5. Gene and protein expression of senescence markers in the aging cortex and microglia following acute TBI.
Relative gene expression was determined by qPCR and expressed as fold change to young naïve levels. A significant interaction was seen between age and injury in tissue expression of p16ink4a (A). A significant effect of injury was seen in the gene expression level of p21cip1a (B). Representative Western blot images are shown for p16ink4a, P21cip1a, and Actin expression using whole cell lysate from cortical tissue of young and aged sham and TBI mice at 72 hrs after injury (C). Quantification of densitometric analysis revealed an effect of both age and injury on the relative expression of p16ink4a protein (D). The relative expression level of p21cip1a protein was increased with injury in both groups (E). Representative dot plots show the percentage of p16ink4a-positive microglia (F). Percent quantification of p16ink4a-positive microglia shows a significant interaction, including a group effect of both age and injury. Representative dot plots show the percentage of p21cip1a-positive microglia (G). A significant group effect of age, but not injury was seen in the percent quantification of p21cip1a-positive microglia. For all experiments, N= 4-6/group. Statistical comparisons between groups were determined by two-way ANOVA analysis with Tukey’s multiple comparisons test. Significant group effects of age, injury, and interaction between age and injury are shown in each box. Error bars show mean SEM. Abbreviation: TBI traumatic brain injury, SEM standard error of mean. *p<0.05, **p<0.01, and ***p<0.001
Aging alters TBI-induced changes in the systemic immune response
Systemic immune activation shapes brain injury outcomes and is an important predictor of TBI severity and recovery (Hazeldine, et al., 2015). We explored the possibility that aging alters the systemic response to TBI. Blood and spleen from young and aged sham and TBI mice were collected to evaluate changes in leukocyte composition and counts by flow cytometry. Blood neutrophilia is a hallmark of acute TBI and is an indirect measure of bone marrow activation (Ritzel, et al., 2018a). We found that total CD11b+ myeloid, Ly6C+ monocyte, and Ly6G+ neutrophil counts were similarly increased in blood from young and aged TBI mice at 72 hours post-injury (Supplemental Figure 1A). We then examined the splenic response to TBI. Spleen weights were significantly increased in young TBI mice as previously reported (Supplemental Figure 1B) (Ritzel, et al., 2018a). However, TBI-induced splenomegaly was less pronounced in aged TBI mice, and spleen mass was not significantly different between sham and injured aged mice. An analysis of leukocyte counts revealed that the increase in spleen size in young TBI mice was associated with greater accumulation of myeloid cells (Supplemental Figure 1C). No such increase in splenic myeloid cells was observed in aged TBI mice. Collectively, these data suggest that, despite aged mice having greater behavioral deficits and an exacerbated neuroinflammatory response, the systemic response to TBI is either attenuated with age, or similarly impacted.
DISCUSSION
TBI is associated with increased mortality and poorer functional outcome in older patients, yet the underlying molecular and cellular mechanisms of secondary injury in the aged brain are poorly understood. The present study provides a comprehensive examination of how aging impacts phenotypic and functional markers of post-traumatic neuroinflammation. We demonstrate that aging increases microglial sensitivity to TBI, altering their proliferation potential, oxidative stress response, phagocytic behavior, and cytokine profile. Aging also increased the number and compositional makeup of brain-invading myeloid cells, differentially affecting their functional response to TBI. In addition, we provide the first evidence that aging increases the expression of senescence markers in the resident microglia population. These senescence markers can also be induced by TBI. We report that age-related impairments in neuroimmune function were accompanied by deficits in muscle strength, locomotor activity, endurance level, and motor coordination. Thus, our data identify several distinguishing features of the neuroinflammatory response to TBI in the aged brain, including the functional resolution of resident and infiltrating macrophage populations, further underscoring the need for more in-depth analysis of co-existing central and systemic immune pathway dysfunction that occur in the aged brain following TBI.
Perhaps surprisingly, our flow cytometry analysis showed that the total number of hemispheric microglia were substantially lower in old mice. The reason for this decrease is unclear, but appears to parallel the transition of microglia from a ramified morphology to a dystrophic state, indicating an age-related impairment in both cell turnover/repopulation and cellular resilience pathways (Streit and Xue, 2014). These findings are consistent with those from several other studies using various methods to enumerate microglia in old mice (Ritzel, et al., 2018b,Sharaf, et al., 2013,Sun, et al., 2013,Zoller, et al., 2018). A global (or region-specific) reduction in microglia could have profound effects on CNS homeostasis. Moreover, the increased conversion of healthy microglia into dysfunctional dystrophic cells could greatly impact the brain’s ability to maintain and repair itself. Aging also altered the microglial response to TBI, and microglial proliferation was significantly increased in old mice following TBI. Thus, whereas the TBI-induced proliferation potential was increased, basal homeostatic proliferation rates in microglia appeared to be inhibited or impaired. Others have demonstrated a comparable increase in the global tissue expression level of CD11b/Iba1 (Kumar, et al., 2013,Sandhir, et al., 2008), and our data provide confirmation at the cellular level that the increase in these myeloid-specific proteins are driven not only by the extensive influx of migrating leucocytes, but also by microglial proliferation. Although these myeloid populations were clearly delineated in our flow cytometry dot plots, it is important to note that while the CD45intCD11b+Ly6C− microglia gating strategy employed in this study is extremely precise under homeostatic conditions, after TBI the gating may be altered by increased CD45 expression in activated microglia or down-regulated Ly6C expression in newly-resident bone marrow-derived monocytes (Greter, et al., 2015). Nevertheless, the majority of CD45int and CD45hi myeloid cells exhibited striking differences with age following cortical injury.
How aged microglia respond to acute brain trauma is not well understood, and may vary depending on the type of injury or the disease models employed. Aging impacts many cellular processes in microglia/macrophages, with some being potentially detrimental and others adaptive and neuroprotective (Mattson and Arumugam, 2018). Although IL-1β induction was highly pronounced with both age and injury, suggesting an age-related increase in microglial inflammasome activity, pro-inflammatory cytokine production was generally decreased in peripheral myeloid populations in the aged TBI brain. Age-related changes in macrophage/macrophage polarization and transition states have been described (Kumar, et al., 2013). However, peripheral macrophage reductions in TNF, IL-1β, and MMP-9 were not associated with better outcomes, and may suggest that injury severity is also driven by cytokine-independent pathways, as several studies have the highlighted the importance of the dual nature of inflammation in the brain’s response to injury (Chaturvedi and Kaczmarek, 2014,Clausen, et al., 2016,Patel, et al., 2013). We also demonstrated marked changes in other cellular immune functions that could profoundly alter outcomes, such as phagocytosis and ROS production. TBI-induced phagocytosis was blunted in aged microglia and also infiltrating populations. Given that our laboratory, and others, have demonstrated that old rodents have greater lesion volumes following TBI (Itoh, et al., 2013,Kumar, et al., 2013), such changes may reflect an inability to clear neuronal debris which consequently impedes the recovery process. However, other studies have reported that the greater loss of neurological function in aged TBI mice cannot be fully explained by acute lesion size (i.e., tissue loss at the impact site) alone, and demonstrate that prolonged edema and BBB disruption in aged mice underscore the importance of secondary processes in age-related TBI outcomes (Onyszchuk, et al., 2008,Timaru-Kast, et al., 2012). Vascular dysfunction also promotes neuroinflammation and contributes to secondary injury in TBI and chronic neurodegeneration (Erturk, et al., 2016,Faden, et al., 2016). Thus, our finding of phagocytic impairment could have important implications with regard to the chronic effects of TBI, and may be one overlapping or contributing factor to the higher rates of amyloid plaque deposition in Alzheimer’s disease or phosphorylated tau accumulation in chronic traumatic encephalopathy (Ramos-Cejudo, et al., 2018,Streit and Xue, 2014). The current study is limited to the acute injury period; therefore, it remains to be seen how aging intersects with TBI to impact key microglial functions such as phagocytosis during the chronic phase of recovery. Normal aging itself has been shown to decrease beta-amyloid uptake in microglia (Njie, et al., 2012,Ritzel, et al., 2015b). Although not physiological cargo, fluorescent latex beads are inert and ensure that the TBI response being measured is largely unadulterated. The 0.5μm latex beads used in this study are more easily ingested than larger 1.0μm sized beads used in previous studies, and likely explains the lack of baseline change previously observed with age (Rejman, et al., 2004).
Marked increases in TBI-induced ROS production were also observed in old mice, both in resident microglia and infiltrating myeloid populations. Whereas the compositional shift to a more neutrophilic brain response in aged mice could account for the higher level of oxidative stress, similar increases have been observed in other age-related disease and injury models, including Parkinson’s and Alzheimer’s diseases and stroke (Cheignon, et al., 2018,Qin, et al., 2013,Radak, et al., 2011,Rosenzweig and Carmichael, 2013). Although global brain levels of ROS were not measured, the higher numbers of microglia and peripheral myeloid cells in the injured brains of older mice suggest that tissue levels are likely similarly higher. The consequences of higher oxidative stress levels in the brain are not just hypothetical because several studies have demonstrated that aging increases neuronal sensitivity to ROS, leading to greater cell death (Foster, et al., 2008,Wang and Michaelis, 2010,Xu, et al., 2007). NOX2 protein expression levels were significantly higher in the infiltrating myeloid population but did not parallel intracellular ROS production, suggesting that NOX2 activity may be differentially affected in these cells. Nonetheless, the NOX2 expression pattern largely mirrored ROS production in microglia, potentially making it a more reliable surrogate marker of ROS in these cells. Higher levels of NADPH oxidase (including NOX2) and reduced antioxidant enzyme expression were previously found in the brain of aged TBI mice, including in enriched microglia/macrophage populations (Kumar, et al., 2013,Morganti, et al., 2015). Thus, the present data imply that age-related elevations in microglia/myeloid-mediated oxidative stress may be a major contributor to neuronal injury in older mice.
Increased oxidative stress levels are critical for the induction and maintenance of cell senescence processes (Davalli, et al., 2016). In response to chronic stress, cells can incur DNA, lipid, and protein damage as a result of increased oxidative stress (Maes, et al., 2011). Exogenous and endogenous ROS can pose a significant threat to cellular integrity leading to a state of dysregulation and homeostatic dysfunction. Senescence is characterized by the induction of a secretory phenotype, increased release of inflammatory factors, and is accompanied by the errant up-regulation of tumor suppressor proteins which prevents self-targeted destruction by programmed cell death (Campisi, 2013). Aging increases cellular senescence, which has been proposed to be the cause of many age-related pathologies, including neurodegeneration. Historical studies and more recent work describing ‘dark microglia’ have identified older microglia as having very high granular content and electron-dense cytoplasm (Bisht, et al., 2016,Samorajski, 1976,Vaughan and Peters, 1974). These dark inclusions reflect a pathological state that can also be found following a TBI or during chronic neurodegeneration. In this study, we examined senescence markers in microglia and the injured cortex. Changes in the physical characteristics of microglia were detected by their unique light scatter properties on the flow cytometer (SSC and FSC). Notably, the effects of normal aging on microglial size and granularity were more pronounced than the changes induced by TBI. Although subtle increases in microglia size and granularity were detected in aged mice following TBI, there appeared to be a ceiling effect, which was not observed in young TBI mice. Because age-related granules present in non-dividing cells often indicate lipofuscin, material in the lysosomal compartment that cannot be degraded, we examined signs of autofluorescence. With normal aging the accumulation of lipofuscin-like material occurs in neurons and glia and may affect all aspects of cellular physiology and possibly reflect CNS pathological states (Moreno-Garcia, et al., 2018). The effect of aging alone was far more profound than the effect of TBI in increasing the level of autofluorescence in the FITC or PE channels. However, the relatively greater increase in autofluorescence in young microglia following TBI was noteworthy, and may reflect acute changes in lysosomal degradation or autophagy in microglia. Further, it may pose an otherwise underappreciated challenge in accurately detecting changes using FITC- and PE-labeled antibodies by standard immunofluorescence microscopy and flow cytometry. Although it is not clear to what extent injury-induced autofluorescence interferes with typical immune-based fluorescence staining, it does suggest that negative controls using surgery-matched (sham-sham, TBI-TBI) tissues are needed to discern real from false positive fluorescence signals. A recent study suggested that injury-induced microglial autofluorescence in young mice is indicative of newly phagocytosed material (i.e., CNS debris, myelin, etc.) (Greenhalgh and David, 2014), which is consistent with our findings. It is unknown whether autofluorescent microglia in young TBI mice retain this phenotype with age or if they subsequently degrade this material and return to a normal state. With age, a high proportion of rodent and human microglia exhibit a so called dystrophic phenotype, characterized by high granularity, gnarled morphology, increased activation markers, and lipofuscin accumulation (Lopes, et al., 2008,Streit, et al., 2004,Wong, 2013). Senescent macrophages and microglia are also characterized by lysosomal dysfunction, which has been demonstrated to impair phagocytic clearance (Majumdar, et al., 2007,Safaiyan, et al., 2016). In Alzheimer’s disease, balanced microglia activity contributes to amyloid-beta clearance, while unbalanced or dysregulated activity can result in bystander neuronal injury (ElAli and Rivest, 2016). Interestingly, in humans, degenerating neurons more closely co-localize with severely fragmented microglia than activated microglia (Streit, et al., 2009). Furthermore, emerging data suggest that dystrophic microglia respond to ischemic injury in a dysregulated manner, exhibiting an exaggerated increase in ROS production and blunted TNF expression compared to their non-dystrophic counterparts (Ritzel, et al., 2018b). Whether this subpopulation of cells responds to TBI in a similar fashion requires further investigation. The temporal manifestation of these cells may also be important in understanding the onset of age-related neurological decline, as some studies suggest that microglial dystrophy precedes the spread of tau-dependent neurodegeneration (Streit, et al., 2009). In the present study, we used 18 month-old animals as our ‘old’ cohort of C57Bl/6 mice. Mice have a lifespan of around 24 months, wherein ‘old’ is defined by a minimum age of at least 18 months, at which time biomarkers of old age can be detected (Dutta and Sengupta, 2016). It is unknown how 18 month-old mice translate to human aging years, but some estimates correlate this age to 56–69 human years (Flurkey, et al., 2007). Thus, while the age of the old mice used in our study may translate to a minimum of 56 human years, is it reasonable to assume that the microglial response to TBI becomes even more dysregulated with increasing age.
Cellular senescence is a well-established phenomenon that has been widely characterized in vitro and in vivo, however, senescent cells are considered rare even in aged tissues. Despite their low frequencies, senescent cells are highly dysregulated, decrease tissue function, and are resistant to apoptosis, thereby enabling them to continually produce pro-inflammatory factors (He and Sharpless, 2017). Furthermore, studies have demonstrated that cellular senescence is transmissible and can spread to neighboring cells via secretory molecules (Chen, et al., 2018,Nelson, et al., 2012). Unlike in other tissues, relatively few studies have examined the expression of senescence markers in the brain, let alone within the resident microglial population. Thus, evidence of microglial senescence, using accepted markers, remains scarce. Our study demonstrated that normal aging increases protein expression of p16ink4a, p21cip1a, BCL-2, and H2AX phosphorylation in microglia. Tissue-level gene expression of p16ink4a and p21cip1a, and protein expression of p16ink4a were significantly increased in older mice. These data are consistent with other studies citing higher expression of p16ink4a in normal aged brain and in other cell types in vivo, including neurons, neuronal progenitors, and astrocytes (Al-Mashhadi, et al., 2015,Bhat, et al., 2012,Jurk, et al., 2012,Krishnamurthy, et al., 2004,Molofsky, et al., 2006,Salminen, et al., 2011). Interestingly, tau and beta-amyloid proteins have been shown to associate with and aggravate neuronal senescence in post-mortem human tissue and rodent models, respectively (Musi, et al., 2018,Wei, et al., 2016). A quantitative reference for the stereotypical number or frequency of senescent microglia/macrophages in the aged brain is currently lacking, due in part to the complexity of defining specific criteria for glial senescence in vivo. Although the percentage of p16ink4a-positive microglia ranged from 1-4% of total population in our study, this is consistent with senescent cell numbers in other tissues, and reflects a significant number of dysfunctional senescent cells. Indeed, very small numbers of transplanted senescent cells are enough to cause lasting physical dysfunction (Hall, et al., 2016,Xu, et al., 2017,Xu, et al., 2018). It is yet to be determined whether this microglial senescent phenotype is more prevalent in specific regions of the brain or if they are more uniformly distributed throughout the CNS. Recent reports indicate that stress and injury can induce a senescent phenotype in vulnerable cells (Chiche, et al., 2017,Hornsby, 2010). Notably, the stereotypical marker of senescence, p16ink4a, may be more highly expressed in long-term cultured microglia compared to acutely isolated microglia from normal aged mice (Stojiljkovic, et al., 2019). Our data show significant TBI-induced expression of p16ink4a, p21cip1a, and H2AX phosphorylation in microglia, with p16ink4a showing the most striking change. The discord between gene and protein level expression may reflect changes in transcriptional regulation, whereas the apparent discrepancy at the cell (i.e., microglia) and tissue level likely reflects heterogeneous expression patterns in various cell types. Given their multi-functional nature, these data highlight the need to measure senescence markers using more than one method of detection. Nevertheless, we have identified a subpopulation of microglia in the aged brain that express senescence markers. Follow up studies are required to determine the unique functional properties of senescent microglia and how they contribute to acute and chronic brain injury.
In summary, our data show that aging alters both the peripheral immune response and microglial sensitivity to TBI, as demonstrated by changes in proliferation, extravasation, oxidative stress, phagocytosis, and cytokine production. The identification of age-related and TBI-induced microglial senescence suggests that these pathways may be viable therapeutic targets.
Supplementary Material
Supplemental Figure 1. Age-related changes in circulating blood and spleen leukocyte counts.
Leukocyte counts were measured by flow cytometric analysis. Blood leukocyte counts did not differ between young and old mice at three days post-injury (A). Spleen weights were normalized to body weight and then expressed as a fold change relative to each respective sham group (B). Spleen leukocyte counts revealed significant age-related changes in the number of myeloid cells at day three following TBI. Error bars show mean SEM. Abbreviation: g gram, BW body weight, μL microliter, TBI traumatic brain injury, n.s. not significant, SEM standard error of mean. *p<0.05, **p<0.01, and ***p<0.001
Highlights.
Aging alters phenotypic and functional markers of neuroinflammation after TBI
Aging increases microglial sensitivity to TBI and numbers of brain-infiltrating leukocytes
Aged microglia have impaired phagocytic function and higher levels of IL-1β
Aging and TBI increase expression of senescence markers in microglia
Age-related impairments in neuroimmune function correlate with neurological deficits
ACKNOWLEDGEMENTS
We would like to acknowledge Nicholas Braganca, B.Sc., and Wesley Shoap, B.Sc. for their technical assistance. We thank Xiaoxuan Fan, Ph.D., and Karen Underwood, B.Sc., of the University of Maryland Greenebaum Comprehensive Cancer Center Flow Cytometry Facility for support with flow cytometry studies. This work was supported by National Institutes of Health grants R01NS082308 (D.J. Loane), R01NS037313 (A.I. Faden), F32NS105355 (R.M. Ritzel), and T32AI095190 (S.J. Doran), and The National Institute on Aging (NIA) Claude D. Pepper Older Americans Independence Center P30-AG028747 (D.J. Loane).
Footnotes
Co-author e-mail information: Rodney M. Ritzel (rritzel@som.umaryland.edu); Sarah J. Doran (sarah.doran@som.umaryland.edu); Ethan P. Glaser (eglaser@som.umaryland.edu); Victoria E. Meadows (vmeadows@som.umaryland.edu); Alan I. Faden (afaden@som.umaryland.edu); Bogdan A. Stoica (bstoica@som.umaryland.edu).
AUTHOR DISCLOSURES
No competing financial interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul-Muneer PM, Chandra N, Haorah J 2015. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol 51(3), 966–79. doi: 10.1007/s12035-014-8752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mashhadi S, Simpson JE, Heath PR, Dickman M, Forster G, Matthews FE, Brayne C, Ince PG, Wharton SB, Medical Research Council Cognitive, F., Ageing, S. 2015. Oxidative Glial Cell Damage Associated with White Matter Lesions in the Aging Human Brain. Brain Pathol 25(5), 565–74. doi: 10.1111/bpa.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, Johnson FB, Trojanowski JQ, Sell C, Torres C 2012. Astrocyte senescence as a component of Alzheimer's disease. PLoS One 7(9), e45069. doi: 10.1371/journal.pone.0045069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht K, Sharma KP, Lecours C, Sanchez MG, El Hajj H, Milior G, Olmos-Alonso A, Gomez-Nicola D, Luheshi G, Vallieres L, Branchi I, Maggi L, Limatola C, Butovsky O, Tremblay ME 2016. Dark microglia: A new phenotype predominantly associated with pathological states. Glia 64(5), 826–39. doi: 10.1002/glia.22966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J 2013. Aging, cellular senescence, and cancer. Annu Rev Physiol 75, 685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi M, Kaczmarek L 2014. Mmp-9 inhibition: a therapeutic strategy in ischemic stroke. Mol Neurobiol 49(1), 563–73. doi: 10.1007/s12035-013-8538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F 2018. Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol 14, 450–64. doi: 10.1016/j.redox.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NC, Partridge AT, Tuzer F, Cohen J, Nacarelli T, Navas-Martin S, Sell C, Torres C, Martin-Garcia J 2018. Induction of a Senescence-Like Phenotype in Cultured Human Fetal Microglia During HIV-1 Infection. J Gerontol A Biol Sci Med Sci. doi: 10.1093/gerona/gly022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiche A, Le Roux I, von Joest M, Sakai H, Aguin SB, Cazin C, Salam R, Fiette L, Alegria O, Flamant P, Tajbakhsh S, Li H 2017. Injury-Induced Senescence Enables In Vivo Reprogramming in Skeletal Muscle. Cell Stem Cell 20(3), 407–14 e4. doi: 10.1016/j.stem.2016.11.020. [DOI] [PubMed] [Google Scholar]
- Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM 2014. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep 15(11), 1139–53. doi: 10.15252/embr.201439245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen BH, Degn M, Sivasaravanaparan M, Fogtmann T, Andersen MG, Trojanowsky MD, Gao H, Hvidsten S, Baun C, Deierborg T, Finsen B, Kristensen BW, Bak ST, Meyer M, Lee J, Nedospasov SA, Brambilla R, Lambertsen KL 2016. Conditional ablation of myeloid TNF increases lesion volume after experimental stroke in mice, possibly via altered ERK1/2 signaling. Sci Rep 6, 29291. doi: 10.1038/srep29291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado VG, Thomas KE, Sattin RW, Johnson RL 2005. The CDC traumatic brain injury surveillance system: characteristics of persons aged 65 years and older hospitalized with a TBI. J Head Trauma Rehabil 20(3), 215–28. [DOI] [PubMed] [Google Scholar]
- Davalli P, Mitic T, Caporali A, Lauriola A, D'Arca D 2016. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid Med Cell Longev 2016, 3565127. doi: 10.1155/2016/3565127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran SJ, Ritzel RM, Glaser EP, Henry RJ, Faden AI, Loane DJ 2018. Sex Differences in Acute Neuroinflammation after Experimental Traumatic Brain Injury Are Mediated by Infiltrating Myeloid Cells. J Neurotrauma. doi: 10.1089/neu.2018.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Sengupta P 2016. Men and mice: Relating their ages. Life Sci 152, 244–8. doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- ElAli A, Rivest S 2016. Microglia in Alzheimer's disease: A multifaceted relationship. Brain Behav Immun 55, 138–50. doi: 10.1016/j.bbi.2015.07.021. [DOI] [PubMed] [Google Scholar]
- Erturk A, Mentz S, Stout EE, Hedehus M, Dominguez SL, Neumaier L, Krammer F, Llovera G, Srinivasan K, Hansen DV, Liesz A, Scearce-Levie KA, Sheng M 2016. Interfering with the Chronic Immune Response Rescues Chronic Degeneration After Traumatic Brain Injury. J Neurosci 36(38), 9962–75. doi: 10.1523/JNEUROSCI.1898-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden AI, Wu J, Stoica BA, Loane DJ 2016. Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. Br J Pharmacol 173(4), 681–91. doi: 10.1111/bph.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filer W, Harris M 2015. Falls and traumatic brain injury among older adults. N C Med J 76(2), 111–4. [DOI] [PubMed] [Google Scholar]
- Flanagan SR, Hibbard MR, Riordan B, Gordon WA 2006. Traumatic brain injury in the elderly: diagnostic and treatment challenges. Clin Geriatr Med 22(2), 449–68; x. doi: 10.1016/j.cger.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Currer JM, Harrison D 2007. Mouse models in aging research The Mouse in Biomedical Research (Second Edition). Elsevier, pp 637–72. [Google Scholar]
- Foster KA, Margraf RR, Turner DA 2008. NADH hyperoxidation correlates with enhanced susceptibility of aged rats to hypoxia. Neurobiol Aging 29(4), 598–613. doi: 10.1016/j.neurobiolaging.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C 2017. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front Immunol 8, 1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Dams-O'Connor K, Morrissey MR, Manley GT 2018. Geriatric Traumatic Brain Injury: Epidemiology, Outcomes, Knowledge Gaps, and Future Directions. J Neurotrauma. doi: 10.1089/neu.2017.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Cho A, Ciol MA, Pettan-Brewer C, Snyder J, Rabinovitch P, Ladiges W 2016. Grip strength is potentially an early indicator of age-related decline in mice. Pathobiol Aging Age Relat Dis 6, 32981. doi: 10.3402/pba.v6.32981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EL, Dixit VD 2015. Drivers of age-related inflammation and strategies for healthspan extension. Immunol Rev 265(1), 63–74. doi: 10.1111/imr.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh AD, David S 2014. Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J Neurosci 34(18), 6316–22. doi: 10.1523/JNEUROSCI.4912-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greter M, Lelios I, Croxford AL 2015. Microglia Versus Myeloid Cell Nomenclature during Brain Inflammation. Front Immunol 6, 249. doi: 10.3389/fimmu.2015.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BM, Balan V, Gleiberman AS, Strom E, Krasnov P, Virtuoso LP, Rydkina E, Vujcic S, Balan K, Gitlin I, Leonova K, Polinsky A, Chernova OB, Gudkov AV 2016. Aging of mice is associated with p16(Ink4a)- and beta-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging (Albany NY) 8(7), 1294–315. doi: 10.18632/aging.100991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey LA, Close JC 2012. Traumatic brain injury in older adults: characteristics, causes and consequences. Injury 43(11), 1821–6. doi: 10.1016/j.injury.2012.07.188. [DOI] [PubMed] [Google Scholar]
- Hazeldine J, Lord JM, Belli A 2015. Traumatic Brain Injury and Peripheral Immune Suppression: Primer and Prospectus. Front Neurol 6, 235. doi: 10.3389/fneur.2015.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Sharpless NE 2017. Senescence in Health and Disease. Cell 169(6), 1000–11. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Segura A, Nehme J, Demaria M 2018. Hallmarks of Cellular Senescence. Trends Cell Biol 28(6), 436–53. doi: 10.1016/j.tcb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Herranz N, Gil J 2018. Mechanisms and functions of cellular senescence. J Clin Invest 128(4), 1238–46. doi: 10.1172/JCI95148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsby PJ 2010. Stress-Induced Senescence in: Adams PD, Sedivy JM (Eds.). Cellular Senescence and Tumor Suppression. Springer New York, New York, NY, pp 85–106. [Google Scholar]
- Itoh T, Imano M, Nishida S, Tsubaki M, Mizuguchi N, Hashimoto S, Ito A, Satou T 2013. Increased apoptotic neuronal cell death and cognitive impairment at early phase after traumatic brain injury in aged rats. Brain Struct Funct 218(1), 209–20. doi: 10.1007/s00429-012-0394-5. [DOI] [PubMed] [Google Scholar]
- Jacotte-Simancas A, Costa-Miserachs D, Coll-Andreu M, Torras-Garcia M, Borlongan CV, Portell-Cortes I 2015. Effects of voluntary physical exercise, citicoline, and combined treatment on object recognition memory, neurogenesis, and neuroprotection after traumatic brain injury in rats. J Neurotrauma 32(10), 739–51. doi: 10.1089/neu.2014.3502. [DOI] [PubMed] [Google Scholar]
- Jurk D, Wang C, Miwa S, Maddick M, Korolchuk V, Tsolou A, Gonos ES, Thrasivoulou C, Saffrey MJ, Cameron K, von Zglinicki T 2012. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell 11(6), 996–1004. doi: 10.1111/j.1474-9726.2012.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karibe H, Hayashi T, Narisawa A, Kameyama M, Nakagawa A, Tominaga T 2017. Clinical Characteristics and Outcome in Elderly Patients with Traumatic Brain Injury: For Establishment of Management Strategy. Neurol Med Chir (Tokyo) 57(8), 418–25. doi: 10.2176/nmc.st.2017-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE 2004. Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114(9), 1299–307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ 2013. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging 34(5), 1397–411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Pocivavsek A, Moussa CE, Thompson R, Matsuoka Y, Faden AI, Rebeck GW, Burns MP 2009. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat Med 15(4), 377–9. doi: 10.1038/nm.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes KO, Sparks DL, Streit WJ 2008. Microglial dystrophy in the aged and Alzheimer's disease brain is associated with ferritin immunoreactivity. Glia 56(10), 1048–60. doi: 10.1002/glia.20678. [DOI] [PubMed] [Google Scholar]
- Maes M, Galecki P, Chang YS, Berk M 2011. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry 35(3), 676–92. doi: 10.1016/j.pnpbp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Cruz D, Asamoah N, Buxbaum A, Sohar I, Lobel P, Maxfield FR 2007. Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol Biol Cell 18(4), 1490–6. doi: 10.1091/mbc.e06-10-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak CH, Wong SK, Wong GK, Ng S, Wang KK, Lam PK, Poon WS 2012. Traumatic Brain Injury in the Elderly: Is it as Bad as we Think? Curr Transl Geriatr Exp Gerontol Rep 1, 171–8. doi: 10.1007/s13670-012-0017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin JR, Weaver MD, Mannix RC 2017. Burden of USA hospital charges for traumatic brain injury. Brain Inj 31(1), 24–31. doi: 10.1080/02699052.2016.1217351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschallinger J, Mosher KI, Wyss-Coray T 2017. Microglial Dysfunction in Brain Aging and Neurodegeneration in: Fulop T, Franceschi C, Hirokawa K, Pawelec G (Eds.). Handbook of Immunosenescence: Basic Understanding and Clinical Implications. Springer International Publishing, Cham, pp 1–15. [Google Scholar]
- Mattson MP, Arumugam TV 2018. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab 27(6), 1176–99. doi: 10.1016/j.cmet.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ 2006. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 443(7110), 448–52. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Garcia A, Kun A, Calero O, Medina M, Calero M 2018. An Overview of the Role of Lipofuscin in Age-Related Neurodegeneration. Front Neurosci 12, 464. doi: 10.3389/fnins.2018.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti JM, Jopson TD, Liu S, Riparip LK, Guandique CK, Gupta N, Ferguson AR, Rosi S 2015. CCR2 antagonism alters brain macrophage polarization and ameliorates cognitive dysfunction induced by traumatic brain injury. J Neurosci 35(2), 748–60. doi: 10.1523/JNEUROSCI.2405-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti JM, Riparip LK, Chou A, Liu S, Gupta N, Rosi S 2016. Age exacerbates the CCR2/5- mediated neuroinflammatory response to traumatic brain injury. J Neuroinflammation 13(1), 80. doi: 10.1186/s12974-016-0547-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Zapata A, Shen Q, Orr ME 2018. Tau protein aggregation induces cellular senescence in the brain. bioRxiv. doi: 10.1101/369074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G, Wordsworth J, Wang C, Jurk D, Lawless C, Martin-Ruiz C, von Zglinicki T 2012. A senescent cell bystander effect: senescence-induced senescence. Aging Cell 11(2), 345–9. doi: 10.1111/j.1474-9726.2012.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niraula A, Sheridan JF, Godbout JP 2017. Microglia Priming with Aging and Stress. Neuropsychopharmacology 42(1), 318–33. doi: 10.1038/npp.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ 2012. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging 33(1), 195 e1–12. doi: 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyszchuk G, He YY, Berman NE, Brooks WM 2008. Detrimental effects of aging on outcome from traumatic brain injury: a behavioral, magnetic resonance imaging, and histological study in mice. J Neurotrauma 25(2), 153–71. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- Papa L, Mendes ME, Braga CF 2012. Mild Traumatic Brain Injury among the Geriatric Population. Curr Transl Geriatr Exp Gerontol Rep 1(3), 135–42. doi: 10.1007/s13670-012-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AR, Ritzel R, McCullough LD, Liu F 2013. Microglia and ischemic stroke: a double-edged sword. Int J Physiol Pathophysiol Pharmacol 5(2), 73–90. [PMC free article] [PubMed] [Google Scholar]
- Prattichizzo F, Bonafe M, Olivieri F, Franceschi C 2016. Senescence associated macrophages and "macroph-aging": are they pieces of the same puzzle? Aging (Albany NY) 8(12), 3159–60. doi: 10.18632/aging.101133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Y, Hong JS, Crews FT 2013. NADPH oxidase and aging drive microglial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia 61(6), 855–68. doi: 10.1002/glia.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, Zhao Z, Goto S, Koltai E 2011. Age-associated neurodegeneration and oxidative damage to lipids, proteins and DNA. Mol Aspects Med 32(4-6), 305–15. doi: 10.1016/j.mam.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Ramos-Cejudo J, Wisniewski T, Marmar C, Zetterberg H, Blennow K, de Leon MJ, Fossati S 2018. Traumatic Brain Injury and Alzheimer's Disease: The Cerebrovascular Link. EBioMedicine 28, 21–30. doi: 10.1016/j.ebiom.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejman J, Oberle V, Zuhorn IS, Hoekstra D 2004. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J 377(Pt 1), 159–69. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Doran SJ, Barrett JP, Henry RJ, Ma EL, Faden AI, Loane DJ 2018a. Chronic Alterations in Systemic Immune Function after Traumatic Brain Injury. J Neurotrauma 35(13), 1419–36. doi: 10.1089/neu.2017.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Lai YJ, Crapser JD, Patel AR, Schrecengost A, Grenier JM, Mancini NS, Patrizz A, Jellison ER, Morales-Scheihing D, Venna VR, Kofler JK, Liu F, Verma R, McCullough LD 2018b. Aging alters the immunological response to ischemic stroke. Acta Neuropathol. doi: 10.1007/s00401-018-1859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Patel AR, Grenier JM, Crapser J, Verma R, Jellison ER, McCullough LD 2015a. Functional differences between microglia and monocytes after ischemic stroke. J Neuroinflammation 12, 106. doi: 10.1186/s12974-015-0329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Patel AR, Pan S, Crapser J, Hammond M, Jellison E, McCullough LD 2015b. Age- and location-related changes in microglial function. Neurobiol Aging 36(6), 2153–63. doi: 10.1016/j.neurobiolaging.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM 1998. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273(10), 5858–68. [DOI] [PubMed] [Google Scholar]
- Rosenzweig S, Carmichael ST 2013. Age-dependent exacerbation of white matter stroke outcomes: a role for oxidative damage and inflammatory mediators. Stroke 44(9), 2579–86. doi: 10.1161/STROKEAHA.113.001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S, Edinger AL, Jung S, Rossner MJ, Simons M 2016. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci 19(8), 995–8. doi: 10.1038/nn.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H 2011. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci 34(1), 3–11. doi: 10.1111/j.1460-9568.2011.07738.x. [DOI] [PubMed] [Google Scholar]
- Samorajski T 1976. How the human brain responds to aging. J Am Geriatr Soc 24(1), 4–11. [DOI] [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NE 2008. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol 213(2), 372–80. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharaf A, Krieglstein K, Spittau B 2013. Distribution of microglia in the postnatal murine nigrostriatal system. Cell Tissue Res 351(3), 373–82. doi: 10.1007/s00441-012-1537-y. [DOI] [PubMed] [Google Scholar]
- Shoji H, Takao K, Hattori S, Miyakawa T 2016. Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol Brain 9, 11. doi: 10.1186/s13041-016-0191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DW, McGeachy MJ, Bayir H, Clark RS, Loane DJ, Kochanek PM 2017. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol 13(3), 171–91. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojiljkovic MR, Ain Q, Bondeva T, Heller R, Schmeer C, Witte OW 2019. Phenotypic and functional differences between senescent and aged murine microglia. Neurobiol Aging 74, 56–69. doi: 10.1016/j.neurobiolaging.2018.10.007. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Braak H, Xue QS, Bechmann I 2009. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathol 118(4), 475–85. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL 2004. Dystrophic microglia in the aging human brain. Glia 45(2), 208–12. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Xue QS 2014. Human CNS immune senescence and neurodegeneration. Curr Opin Immunol 29, 93–6. doi: 10.1016/j.coi.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Sun D, McGinn M, Hankins JE, Mays KM, Rolfe A, Colello RJ 2013. Aging- and injury-related differential apoptotic response in the dentate gyrus of the hippocampus in rats following brain trauma. Front Aging Neurosci 5, 95. doi: 10.3389/fnagi.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman M, DiRusso SM, Sullivan T, Risucci D, Nealon P, Cuff S, Haider A, Benzil D 2002. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J Trauma 53(2), 219–23; discussion 23-4. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, McCormick WC, Kagan SH 2006. Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. J Am Geriatr Soc 54(10), 1590–5. doi: 10.1111/j.1532-5415.2006.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timaru-Kast R, Luh C, Gotthardt P, Huang C, Schafer MK, Engelhard K, Thal SC 2012. Influence of age on brain edema formation, secondary brain damage and inflammatory response after brain trauma in mice. PLoS One 7(8), e43829. doi: 10.1371/journal.pone.0043829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan DW, Peters A 1974. Neuroglial cells in the cerebral cortex of rats from young adulthood to old age: an electron microscope study. J Neurocytol 3(4), 405–29. [DOI] [PubMed] [Google Scholar]
- Venna VR, Weston G, Benashski SE, Tarabishy S, Liu F, Li J, Conti LH, McCullough LD 2012. NF-kappaB contributes to the detrimental effects of social isolation after experimental stroke. Acta Neuropathol 124(3), 425–38. doi: 10.1007/s00401-012-0990-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bernhardi R, Eugenin-von Bernhardi L, Eugenin J 2015. Microglial cell dysregulation in brain aging and neurodegeneration. Front Aging Neurosci 7, 124. doi: 10.3389/fnagi.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X, Liu S, Wang S, Zhang S, Yang H, Ou Y, Zhao M, James L, Shu K, Chen J, Lei T 2016. Elderly Patients with Severe Traumatic Brain Injury Could Benefit from Surgical Treatment. World Neurosurg 89, 147–52. doi: 10.1016/j.wneu.2016.01.084. [DOI] [PubMed] [Google Scholar]
- Wang X, Michaelis EK 2010. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 2, 12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Chen XC, Song Y, Pan XD, Dai XM, Zhang J, Cui XL, Wu XL, Zhu YG 2016. Amyloid beta Protein Aggravates Neuronal Senescence and Cognitive Deficits in 5XFAD Mouse Model of Alzheimer's Disease. Chin Med J (Engl) 129(15), 1835–44. doi: 10.4103/0366-6999.186646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WT 2013. Microglial aging in the healthy CNS: phenotypes, drivers, and rejuvenation. Front Cell Neurosci 7, 22. doi: 10.3389/fncel.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Sun X, Puchowicz MA, LaManna JC 2007. Increased sensitivity to transient global ischemia in aging rat brain. Adv Exp Med Biol 599, 199–206. [DOI] [PubMed] [Google Scholar]
- Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T, Curran GL, Ogrodnik M, Jurk D, Johnson KO, Lowe V, Tchkonia T, Westendorf JJ, Kirkland JL 2017. Transplanted Senescent Cells Induce an Osteoarthritis-Like Condition in Mice. J Gerontol A Biol Sci Med Sci 72(6), 780–5. doi: 10.1093/gerona/glw154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL 2018. Senolytics improve physical function and increase lifespan in old age. Nat Med 24(8), 1246–56. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Kojic L, Tsang M, Grewal P, Liu J, Namjoshi D, Wellington CL, Tetzlaff W, Cynader MS, Jia W 2016. Distinct roles for metalloproteinases during traumatic brain injury. Neurochem Int 96, 46–55. doi: 10.1016/j.neuint.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Loane DJ, Murray MG 2nd, Stoica BA, Faden AI 2012. Comparing the predictive value of multiple cognitive, affective, and motor tasks after rodent traumatic brain injury. J Neurotrauma 29(15), 2475–89. doi: 10.1089/neu.2012.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller T, Attaai A, Potru PS, Russ T, Spittau B 2018. Aged Mouse Cortical Microglia Display an Activation Profile Suggesting Immunotolerogenic Functions. Int J Mol Sci 19(3). doi: 10.3390/ijms19030706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Age-related changes in circulating blood and spleen leukocyte counts.
Leukocyte counts were measured by flow cytometric analysis. Blood leukocyte counts did not differ between young and old mice at three days post-injury (A). Spleen weights were normalized to body weight and then expressed as a fold change relative to each respective sham group (B). Spleen leukocyte counts revealed significant age-related changes in the number of myeloid cells at day three following TBI. Error bars show mean SEM. Abbreviation: g gram, BW body weight, μL microliter, TBI traumatic brain injury, n.s. not significant, SEM standard error of mean. *p<0.05, **p<0.01, and ***p<0.001