Key Points
Question
Does augmentation of a nurse home visitation program with an intimate partner violence intervention, starting in pregnancy, compared with the home visitation program alone, lead to improved maternal quality of life at 24 months after infant delivery?
Findings
In this cluster randomized clinical trial that included 492 pregnant women, randomization to the augmented program compared with nurse home visitation alone resulted in maternal quality-of-life scores at 24 months postdelivery of 311.3 vs 316.2 (measured using the WHOQOL-BREF scale; range, 0-400)—a difference that was not statistically significant.
Meaning
These findings do not support augmenting a nurse home visitation program with this complex, multifaceted intimate partner violence intervention.
Abstract
Importance
Intimate partner violence (IPV) is a public health problem with significant adverse consequences for women and children. Past evaluations of a nurse home visitation program for pregnant women and first-time mothers experiencing social and economic disadvantage have not consistently shown reductions in IPV.
Objective
To determine the effect on maternal quality of life of a nurse home visitation program augmented by an IPV intervention, compared with the nurse home visitation program alone.
Design, Setting, and Participants
Cluster-based, single-blind, randomized clinical trial at 15 sites in 8 US states (May 2011-May 2015) enrolling 492 socially disadvantaged pregnant women (≥16 years) participating in a 2.5-year nurse home visitation program.
Interventions
In augmented program sites (n = 229 participants across 7 sites), nurses received intensive IPV education and delivered an IPV intervention that included a clinical pathway to guide assessment and tailor care focused on safety planning, violence awareness, self-efficacy, and referral to social supports. The standard program (n = 263 participants across 8 sites) included limited questions about violence exposure and information for abused women but no standardized IPV training for nurses.
Main Outcomes and Measures
The primary outcome was quality of life (WHOQOL-BREF; range, 0-400; higher score indicates better quality of life) obtained through interviews at baseline and every 6 months until 24 months after delivery. From 17 prespecified secondary outcomes, 7 secondary end points are reported, including scores on the Composite Abuse Scale, SPAN (Startle, Physiological Arousal, Anger, and Numbness), Prime-MD Patient Health Questionnaire, TWEAK (Tolerance/Worry About Drinking/Eye-Opener/Amnesia/C[K]ut Down on Drinking), Drug Abuse Severity Test, and the 12-Item Short-Form Health Survey (physical and mental health), version 2.
Results
Among 492 participants enrolled (mean age, 20.4 years), 421 (86%) completed the trial. Quality of life improved from baseline to 24 months in both groups (change in WHOQOL-BREF scores from 299.5 [SD, 54.4] to 308.2 [SD, 52.6] in the augmented program group vs from 293.6 [SD, 56.4] to 316.4 [SD, 57.5] in the standard program group). Based on multilevel growth curve analysis, there was no statistically significant difference between groups (modeled score difference, −4.9 [95% CI, −16.5 to 6.7]). There were no statistically significant differences between study groups in any of the secondary participant end points. There were no adverse events recorded in either group.
Conclusions and Relevance
Among pregnant women experiencing social and economic disadvantage and preparing to parent for the first time, augmentation of a nurse home visitation program with a comprehensive IPV intervention, compared with the home visitation program alone, did not significantly improve quality of life at 24 months after delivery. These findings do not support the use of this intervention.
Trial Registration
ClinicalTrials.gov Identifier: NCT01372098
This cluster randomized trial compares the effect of a nurse home visitation program augmented by an intimate partner violence (IPV) intervention vs nurse home visitation alone on quality of life 24 months after delivery among first-time mothers experiencing social and economic disadvantage.
Introduction
Nurse-Family Partnership is an evidence-based program of prenatal and infancy home visiting provided by nurses to socially and economically disadvantaged first-time mothers. In 3 randomized clinical trials conducted in the United States, this nurse home visitation program was shown to improve pregnancy outcomes, child health, and maternal life-course development.1 In the first trial, a notable finding was a reduction in state-verified reports of child abuse and neglect at the 15-year follow-up among the nurse-visited families.2 This reduction was not shown when mothers reported moderate to severe levels of intimate partner violence (IPV).
Across trials of this home visitation program in the United States as well as the Netherlands,3,4,5,6 there have been conflicting findings about program effects on IPV. While the standard program model included IPV screening questions as well as educational information for women disclosing IPV, this was not considered sufficient to meet women’s needs, nor did nurses perceive that they had sufficient knowledge or skills to address IPV.7,8 These findings indicated a need to develop a more comprehensive IPV intervention to augment the model and core curriculum of this nurse home visitation program.8
The aim of this trial was to compare the effectiveness of a nurse home visitation program augmented with a multicomponent IPV intervention compared with delivery of the standard nurse home visitation program. It was hypothesized that the augmented program would be more effective than the standard program in improving maternal quality of life among this group of women at high risk of IPV.
Methods
Trial Design and Oversight
This cluster-based, single-blind, randomized clinical trial was conducted from May 2011 to May 2015. A cluster was defined as a distinct site delivering the nurse home visitation program. A cluster randomized clinical trial was used to prevent contamination. Randomly assigning women within the nurse home visitation program sites to receive the augmented IPV intervention would have put nurses who had received the study-specific IPV training in a compromised position, to apply their new knowledge/skills or not, which was identified a priori as an undesirable condition for this study. The trial protocol is available in Supplement 1.
This study was conducted with approval from the Hamilton Integrated Research Ethics Board, McMaster University, as well as approvals from organizational and site-specific institutional review boards. A data and safety monitoring committee oversaw the ongoing safety of participants by monitoring primary outcomes. A systematic, formal process was instituted for this committee to review any reports of adverse events. Written informed consent was obtained from each study participant (for those aged 16 up to 18 years, participant assent and consent of a parent or guardian was required), as well as from each participating nurse, before enrollment in the study.
Study Setting and Participants
Eligible nurse home visitation program sites had no previous involvement in the development or pilot testing of the IPV intervention and no ongoing participation in other program-related research. Within each site, all women 16 years and older who spoke English and met the nurse home visitation program criteria (eg, enrolled before 28 weeks’ gestation, living in poverty, first live birth) were eligible. Before the fourth planned home visit, the nurse determined eligibility and obtained written permission to share contact information with a research assistant, who followed up to secure written informed consent to participate in the trial.
Sample Size and Randomization
Allowing for a trial refusal rate of 15% and losses to follow-up of 20%, initial funding for this study was sufficient to enlist and retain 90 participants per group (n = 180) in 10 sites that included 18 participants per site. With α set at P < .05 (2-tailed), 90 women per group provided 80% power (1-β) to detect a moderate improvement in the intervention group vs standard nurse home visitation group (∆ = 20.0 units—a standardized effect, d = 0.459) on the primary outcome (quality of life, as measured by the World Health Organization Quality of Life–BREF [WHOQOL-BREF] instrument).10,11 This estimate allowed for a design effect of 1.18 derived from [1 + (n − 1) × ρ], in which n = 18 (mean cluster size) and ρ = 0.01 (the between-cluster differences [intraclass correlation coefficient]). Additional funding made it possible to enlist 5 more sites, resulting in 225 participants per group in 15 sites that included 32 participants per site and enabled the study to detect a smaller effect size (∆ = 13.6 units—a standardized effect d = 0.30). Effect sizes were estimated12 by (Z(1 − α/2) + Z1-β)2 2σ2 [1 + (n − 1) ρ]/∆2, where Z(1 − α/2) = 1.96; Z(1 − β) = 0.84; σ2 = 2025 (the original variance associated with the WHOQOL-BREF); and the design effect increased to 1.31 based on [1 + (32 − 1) × 0.01].
For randomization, the sites were stratified by the number of nurses per site, either a small site (1-7 nurses) or a large site (≥8 nurses). In total, 10 small sites and 7 large sites were randomized. Within the strata, using a randomization table, each site was randomly assigned either to the intervention group or standard nurse home visitation group. Once half of the sites in each stratum had been allocated to one of the conditions (ie, 5 sites for the small and 4 sites for the larger sites), remaining sites were assigned to the other condition to ensure a balance in the number of sites per group. The randomization of all sites occurred in February 2011. Outcome assessors were blinded to group assignment, but it was not possible to blind program sites or researchers. Across sites, initiation of participant recruitment occurred at different times because of local logistical issues.
Intervention
In sites randomized to the intervention group, nurses delivered the standard nurse home visitation program augmented with an IPV intervention specifically developed to support nurses to identify women exposed to IPV and then respond with a tailored clinical response. This intervention8 (see eFigure in Supplement 2 for conceptual model) included a comprehensive program of nurse IPV education, guidelines for reflective supervision, a checklist to assist sites to implement the intervention, and a clinical pathway to guide decision making that included elements of assessment, diagnosis, care planning, and intervention tailored to the participant’s needs (component summary listed in the eTable in Supplement 2). This complex, multicomponent, tailored intervention consisted of (1) universal assessment of safety8 or case-finding assessment approaches to identify IPV exposure and (2) empathic response to IPV disclosure, followed by (3) risk assessment (administration of the Danger Assessment instrument13) plus an adapted brief empowerment intervention14 including immediate discussion of safety options and (4) assessment of mental health, substance use, and stage of readiness to address safety to plan a nursing response tailored to a woman’s needs that focuses on (5) safety, awareness of IPV health effects, self-efficacy, and system navigation. System navigation involves identifying, referring, and actively facilitating participant access to external domestic violence, legal, housing, or other health or social care services.
In the standard nurse home visitation group, nurses visited study participants regularly from early in pregnancy until the child’s second birthday (maximum, 64 home visits). In each visit, nurses addressed content from 6 domains: personal health, environmental health, life course development, maternal role, family and friends, and health and human services. Nurses did not receive program-specific IPV training as part of their orientation to the nurse home visitation program; however, the provision of professional development on IPV was provided at the discretion of supervisors at individual sites. The existing guidelines of the nurse home visitation program required that a relationship assessment based on a modified Abuse Assessment Screen15 be conducted by the nurse at 3 points in time. If a woman enrolled in the program disclosed current IPV, program guideline recommendations were to assess safety, provide information about IPV, and refer to appropriate services.
Measures
Study participants completed a baseline interview in person with a research assistant; subsequent interviews were conducted by telephone at 6 months postpartum and then every 6 months until 24 months postpartum. Research data continued to be collected for participants who dropped out of the program but remained in the study. As retention strategies, participants were contacted at 2-month intervals to update their contact information and compensated with a $25 gift card for the baseline interview and a $50 gift card for each subsequent interview.
Baseline demographic data were collected about all participants, including age, ethnicity, race, level of education, marital status, income, employment, presence of partner, and presence of children aged 0 to 16 years at least part-time. Ethnicity was included in this study to inform generalizability, given that many of the sites that participated were in the southern United States. Participants self-identified in response to fixed categories for ethnicity and race as outlined in Table 1.
Table 1. Characteristics of Sites and Participants by Allocation Status.
| Characteristic | Intervention | Standard Nurse Home Visitation |
|---|---|---|
| Nurse Home Visitation Program Sites | ||
| Location of sites by state (No.) | California (1), Nevada (1), Minnesota (1), New Jersey (1), Pennsylvania (1), Texas (2) | California (1), Colorado (2), Pennsylvania (1), Texas (3), Washington (1) |
| No. of all nurse home visitors (median No. per study site) | 77 (4) | 101 (7) |
| Study Participants, No./No. (%)a | ||
| All participants | 229 | 263 |
| Age, mean (SD), y | 20.3 (3.6) | 20.5 (3.7) |
| Ethnicity: Hispanic/Latina background | 121/196 (61.7) | 106/252 (42.1) |
| Race | ||
| White only | 135/197 (68.5) | 143/251 (57.0) |
| Black or African American only | 32/197 (16.2) | 71/251 (28.3) |
| Asian only | 0/197 (0) | 3/251 (1.2) |
| American Indian or Alaska Native only | 3/197 (1.5) | 3/251 (1.2) |
| Native Hawaiian or other Pacific Islander only | 0/197 (0) | 1/251 (0.4) |
| Multirace categories | 10/197 (5.1) | 16/251 (6.4) |
| Declined to self-identify | 17/197 (8.6) | 14/251 (5.6) |
| Graduated from high school or obtained vocational certificate | 121/221 (54.8) | 135/259 (52.1) |
| Single or never married | 178/229 (77.7) | 216/262 (82.4) |
| Dependent or no income | 82/221 (37.1) | 87/252 (34.5) |
| Employed or self-employedb | 45/222 (20.3) | 62/246 (25.2) |
| Main source of income was wages or salary | 46/220 (20.9) | 59/250 (23.6) |
| No current partner | 23/229 (10.0) | 36/261 (13.8) |
| Children (0-16 y) living at home at least part time | 78/225 (34.7) | 114/262 (43.5) |
Abbreviation: IPV, intimate partner violence.
No. of participants with a “yes” response on binary measures/No. of participants with a response.
Response to question, “What is your MAIN activity? (Please choose only one).” Response choices were employed or self-employed; helping with family business or farm; unemployed; student, in school, in training; retired; ill or disabled for a long time or permanently; maternity leave; looking after the home and/or family; other.
Primary Outcome
The primary end point, quality of life, was measured using the WHOQOL-BREF instrument, a 26-item tool that includes 4 quality-of-life domains: physical (7 items), psychological (6 items), social (3 items), and environmental (8 items).10,11 A recent meta-analysis of clinical trials using the WHOQOL-BREF to assess outcomes identified substantial between-study variability in sensitivity to change across the domains.16 Because of this variability and the multidimensional effect on life quality expected of the intervention, the 4 domains, each of which was scored on a standardized scale (0-100), were combined in this study to represent overall quality of life (0-400). All items are positively oriented, rated on a 5-point intensity or frequency continuum, and refer to the previous 2 weeks. Reliability of internal consistency (Cronbach α) in this study at baseline was 0.91. Based on Cohen d, a minimally clinically important and achievable standardized effect size is about d = 0.30.16
Secondary Outcomes
Data on secondary end points were collected every 6 months. IPV recurrence was measured using the Composite Abuse Scale.17,18 This 30-item instrument has 4 subscales: severe combined abuse, emotional abuse, physical abuse, and harassment (scored as the sum of 30 Likert items [range, 0 [never] to 5 [daily]; a score of 7 or more was used as the criterion for IPV exposure). Posttraumatic stress disorder was assessed using the 4-item SPAN (Startle, Physiological Arousal, Anger, and Numbness) screen, derived from the Davidson Trauma Scale (4 items with 5 response options each, ranging from 0 [“not at all”] to 4 [“extremely distressing”]; a score of ≥5 indicated presence of posttraumatic stress disorder).19 Depressive symptoms were assessed using the depression subscale from the PRIME-MD Patient Health Questionnaire20 (items are summed [range, 0-27], with a threshold score of ≥10 representing moderate depression; a 5-point change is considered to indicate clinical importance). Alcohol misuse was assessed with the 5-item TWEAK (Tolerance, Worry, Eye-Opener, Amnesia, C(K)ut Down on Drinking) screening tool (tolerance of ≥3 drinks scored 2 points; a “yes” response to “worry” scored 2 points; and the remaining items scored 1 point each; a total score of ≥2 points indicated an alcohol problem).21,22 Two questions from the Drug Abuse Severity Test assessed excess use of prescription drugs and use of street drugs23; endorsing either question indicated a drug problem. The 12-Item Short-Form-12 Health Survey, version 2, a valid and reliable short form of the widely used 36-Item Short Form Health Survey, was used to measure global mental and physical health and well-being.24
Prespecified secondary outcomes not reported here include Domestic Violence Survivor Assessment,25 the Intimate Partner Violence Strategies Index,26 the Childhood Experiences of Violence Questionnaire Short Form,27 the Childhood Trauma Questionnaire,28 a modified version of the Health and Social Service Utilization questionnaire,29 child health outcomes (eg, birth weight, length of gestation, injuries, emergency department visits, hospitalizations, immunizations, developmental delays), child protective services reports, adherence to the standard nurse home visitation program components (multiple program indicators as measured by the nurse home visitation program clinical implementation system, such as sociodemographic factors, maternal health behaviors, psychosocial characteristics, infant neonatal intensive care unit and hospital visits, patterns of welfare use), nurse performance, and additional sources assessing IPV.
Post hoc End Points
Nurses at intervention sites completed an intervention implementation log to document whether a procedure on the clinical pathway was completed (yes/no) and the date.
A mixed-methods evaluation of the education component of the intervention was conducted to measure changes in nurse knowledge and confidence (Public Health Nurses’ Responses to Women Who are Abused Scale).30 Additionally, a qualitative process evaluation to identify factors influencing implementation and uptake of the intervention was conducted that included focus groups with nurses (n = 46) at each of the 7 intervention sites at the end of the trial. Findings from these latter 2 post hoc end points are not reported in this article.
Data Analysis
Multilevel Models for Windows (MLwiN; Centre for Multilevel Modelling) version 2.1031 was used to model growth trajectories for quality of life (linear model) and IPV recurrence (logistic model). The growth curve analysis included 2 steps: first, repeat assessments for each participant were modeled as a function of time (number of months, in 6-month intervals since baseline) to estimate individual trajectories that include a starting point (baseline) and change per unit of time or growth (trajectory) for each person; and second, between-group differences were estimated at baseline and for rates of change.
To account for the study design, with its potential effects of clustering, site was included as a separate level in the analysis and time was allowed to vary between sites or respondents (specified as random effects) if there was empirical evidence of between-site or between-participant variation in response trajectories. Plotting trajectories of response showed some curvature (ie, an acceleration or deceleration of response with time). Accordingly, response was modeled in relation to a polynomial function of time that could include linear, quadratic (time squared) and cubic (time cubed) terms to improve model fit. Baseline assessments were included in the growth models. Baseline differences were treated as random and not adjusted for in the analyses. The intent-to-treat principle was followed as closely as possible, allowing for dropouts and losses to follow-up; sites and participants within these categories were analyzed as randomized. Growth curve models use all available assessments, so participants contributing assessments on any occasion were included in the analysis. The effect on the primary outcome of between-group, baseline differences in the sociodemographic characteristics of participants exceeding |15%| was investigated post hoc by statistically adjusting for them in separate growth models. Results are presented at 24 months as score differences with 95% CIs in multilevel linear growth models and as percentage differences and 95% CIs in logistic growth models.
Statistical significance was set at P < .05 (2-sided). Because of the potential for type 1 error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
Results
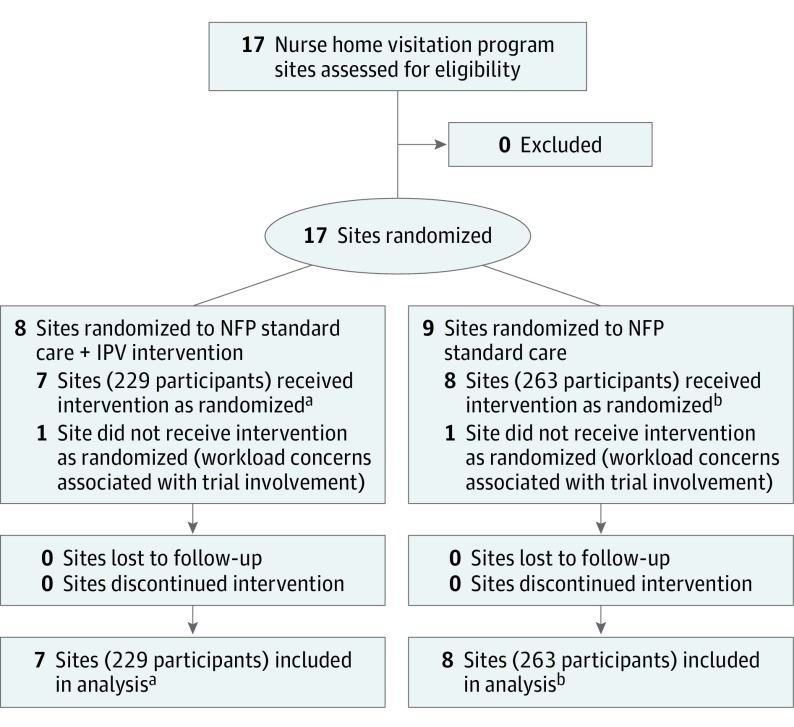
Seventeen sites initially expressed interest in participating in this trial, but after randomization 2 sites subsequently declined to participate because of workload concerns associated with trial involvement. A total of 15 sites delivering the nurse home visitation program across 8 US states were enrolled; Table 1 reports information about site locations. Home visitation nurses in the intervention (n = 77) and standard nurse home visitation (n = 101) groups were registered nurses employed to deliver this specific home visitation program. The mean cluster size (mean number of participants enrolled across sites) was 32.7 for sites allocated to receive the intervention and 32.9 for sites allocated to receive the standard nurse home visitation program. The median number of home visitation nurses per study site was 4 in the intervention group and 7 in the standard nurse home visitation group. The median number of nurse supervisors in both groups was 1. Figure 1 illustrates the site flow for the trial.
Figure 1. Enrollment, Randomization, and Follow-up of Sites Flow Diagram.
IPV indicates intimate partner violence; NFP, Nurse-Family Partnership.
aNumbers of participants at each site were 45, 19, 43, 38, 10, 36, and 38.
bNumbers of participants at each site were 45, 35, 13, 17, 33, 45, 30, and 45.
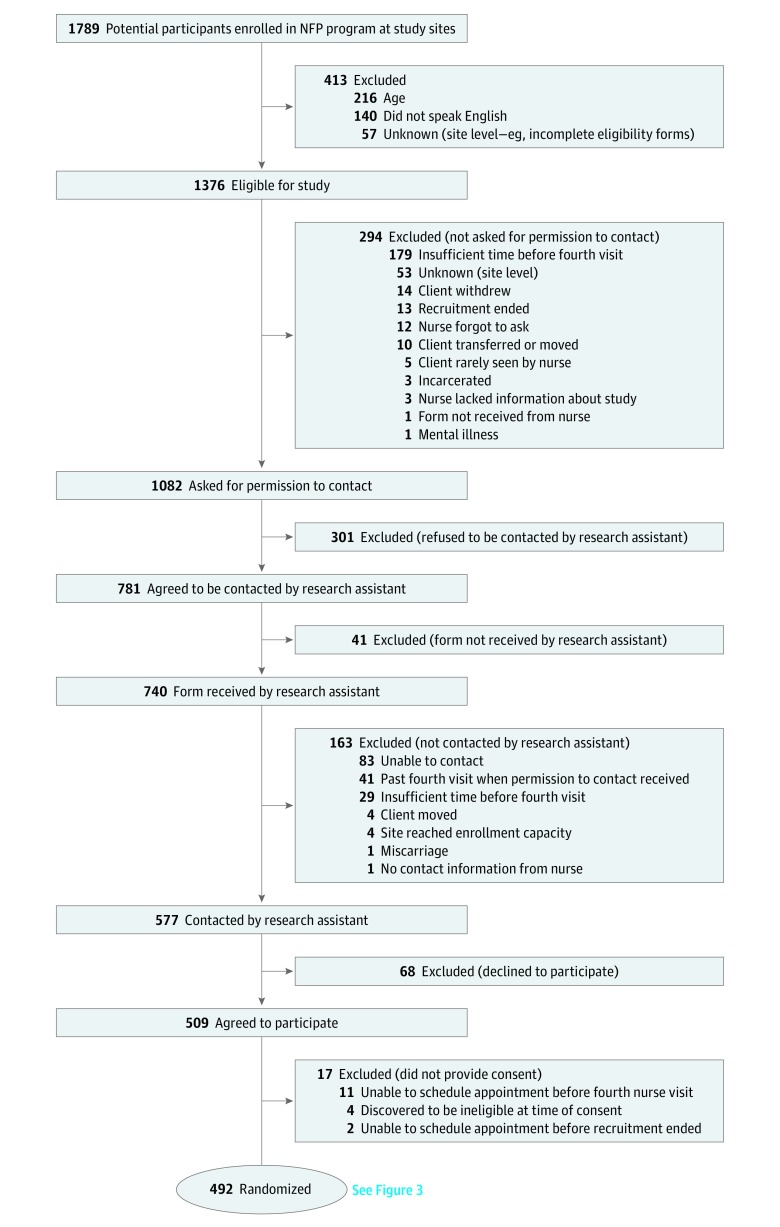
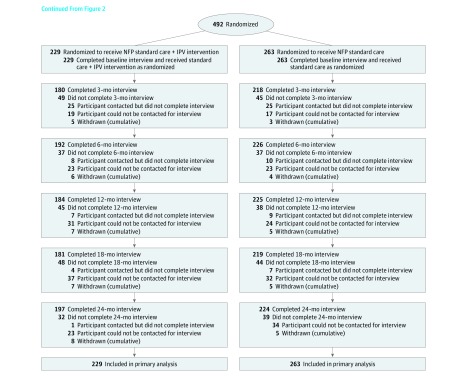
Recruitment of participants in each site occurred between May 2011 and September 2012. At the 15 study sites, 1376 of 1789 participants (77%) met the inclusion criteria, and nurses asked 1082 of 1376 (79%) for permission to share contact information. Among the 1082 asked, 781 (72%) gave permission and 577 of 740 (78%) were contacted by a research assistant. Of the 577 contacted, 509 (88%) agreed to participate and 492 of 509 (97%) consented and completed a baseline interview. A total of 492 participants were followed up for a minimum of 24 months postpartum between May 2011 and May 2015. Figure 2 and Figure 3 illustrate the flow of participants through the phases of the trial.
Figure 2. Participant Flow.
NFP indicates Nurse-Family Partnership.
Figure 3. Participant Flow (Continued).
IPV indicates intimate partner violence; NFP, Nurse-Family Partnership.
At baseline, 229 participants were enrolled in the sites randomized to receive the intervention and 263 participants were enrolled in the sites that delivered the standard nurse home visitation program. Thirty-two of 229 participants (14.0%) in the intervention group and 39 of 263 (14.8%) in the standard nurse home visitation group missed the last assessment (24 months) but contributed assessments on some other occasions.
Table 1 presents participants’ characteristics. The mean age of participants was 20.3 years (SD, 3.7); 50.7% were Hispanic/Latina, 62.8% were white, 23.3% were black or African American, 0.7% Asian, 1.4% American Indian or Alaskan Native, 0.2% Native Hawaiian or other Pacific Islander, and 5.9% multiracial; 53.3% had graduated from high school or obtained a vocational certificate; 80% were single, and 35.7% were dependents or had no income. There was between-group balance on the characteristics of participants related to age, education, marital status, income, employment, presence of partner, and presence of children aged 0 to 16 years living at home at least part-time. However, proportionately more women of Hispanic/Latina background were allocated to the intervention group than to the standard nurse home visitation group (61.7% vs 42.1%). For race, proportionately more white women (68.5% vs 57.0%) and fewer black or African American women (16.2% vs 28.3%) were allocated to the intervention group than to the standard nurse home visitation group.
Primary Outcome
In both groups, there were improvements in quality of life. There were no statistically significant modeled score differences: women in the intervention group had low quality-of-life levels at 24 months compared with women in the standard nurse home visitation group (311.3 vs 316.2; modeled score difference, −4.9 [95% CI, −16.5 to 6.7]).
Secondary Outcomes
In both groups, there were improvements in all of the secondary outcomes (IPV, posttraumatic stress disorder, depression, use of alcohol and other drugs, physical health, and mental health). However, there were no statistically significant modeled score differences between the 2 groups on any of the 7 reported secondary outcomes. Table 2 shows the observed group mean values and percent at each measurement occasion for the primary and reported secondary outcomes. Table 3 shows between-group differences at 24 months based on the multilevel growth models.
Table 2. Observed Outcomes for the Intervention vs Standard Nurse Home Visitation Groups.
| Baseline | 6 moa | 12 moa | 18 moa | 24 moa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Standard Program | Intervention | Standard Program | Intervention | Standard Program | Intervention | Standard Program | Intervention | Standard Program | |
| All participants, No. | 229 | 263 | 192 | 225 | 183 | 223 | 181 | 219 | 197 | 224 |
| Primary Outcome | ||||||||||
| WHOQOL-BREF, No.b | 227 | 258 | 190 | 225 | 180 | 223 | 180 | 218 | 197 | 224 |
| Mean (SD) | 299.5 (54.4) |
293.6 (56.4) |
315.7 (52.7) |
310.2 (55.8) |
315.4 (58.7) |
310.7 (58.1) |
313.7 (50.5) |
316.2 (56.6) |
308.2 (52.6) |
316.4 (57.5) |
| Secondary Outcomes, No./No. (%)c | ||||||||||
| IPV (CAS)d | 43/224 (19.2) |
49/259 (18.9) |
30/176 (17.0) |
35/196 (17.9) |
28/166 (16.9) |
22/204 (10.8) |
18/157 (11.5) |
16/194 (8.2) |
19/167 (11.8) |
16/199 (8.0) |
| PTSD (SPAN)e | 62/221 (28.1) |
64/247 (25.9) |
23/163 (14.1) |
20/194 (10.3) |
20/144 (13.9) |
32/192 (16.7) |
17/136 (12.5) |
25/162 (15.4) |
21/113 (18.8) |
25/169 (14.8) |
| Depression (PHQ-9)f | 48/228 (21.1) |
56/263 (21.3) |
21/191 (11.0) |
23/224 (10.3) |
22/183 (12.0) |
25/221 (11.3) |
18/181 (9.9) |
28/219 (12.8) |
24/197 (12.2) |
29/223 (13.0) |
| Alcohol problem (TWEAK)g | 46/227 (20.3) |
48/263 (18.3) |
19/183 (10.4) |
33/222 (14.9) |
30/197 (15.2) |
22/223 (9.9) |
||||
| Drug problem (DAST)h | 51/226 (22.6) |
65/263 (24.7) |
11/183 (6.0) |
17/221 (7.7) |
17/197 (8.6) |
12/223 (5.4) |
||||
| SF-12 | ||||||||||
| Mental health, No.i | 226 | 259 | 186 | 221 | 177 | 221 | 179 | 217 | 195 | 222 |
| Mean (SD) | 48.8 (10.6) |
49.1 (10.9) |
49.7 (11.2) |
51.1 (10.4) |
50.8 (10.4) |
49.9 (10.9) |
49.8 (9.7) |
51.1 (10.7) |
50.0 (10.0) |
50.9 (9.6) |
| Physical health, No.i | 226 | 259 | 186 | 221 | 177 | 221 | 179 | 217 | 195 | 222 |
| Mean (SD) | 46.4 (8.5) |
46.9 (8.2) |
52.8 (7.5) |
52.5 (7.0) |
52.5 (7.9) |
53.2 (6.9) |
52.3 (7.9) |
52.6 (7.7) |
52.3 (7.7) |
52.5 (8.3) |
Abbreviations: CAS, Composite Abuse Scale; DAST, Drug Abuse Severity Test; IPV, intimate partner violence; PHD-9, Patient Health Questionnaire 9; PTSD, posttraumatic stress disorder; SF-12, 12-Item Short-Form Health Survey; SPAN, Startle, Physiological Arousal, Anger, and Numbness; TWEAK, Tolerance/WorryAbout Drinking/Eye-Opener/Amnesia/C[K]ut down on drinking; WHOQOL-BREF, World Health Organization Quality of Life–BREF.
Assessment occasions for each participant, rather than actual timing of assessment.
Range, 0-400; higher score indicates better quality of life. A score of 300 is a value similar in magnitude to the average score observed in the general population of young women based on combined individual domain scores.
No. of participants with a “yes” response on binary measures/No. of participants with a response.
Range, 0-150; 7 or higher considered “yes” for presence of IPV.
Range, 0-16; 5 or higher considered “yes” for presence of PTSD.
Range, 0-27; 10 or higher considered “yes” for presence of moderate depression.
Range, 0-7; 2 or higher considered “yes” for presence of alcohol problem; assessed at 12-month intervals.
Range, 0-2; 1 or higher considered “yes” for presence of drug problem; assessed at 12-month intervals.
Range, 0-100; scales positively scored. Scores were computed by multiplying each indicator variable by its respective regression weight (physical or mental health), and aggregated score was transformed with mean of 50 and SD of 10 in the general US population.
Table 3. Outcome Differences Between Intervention vs Standard Nurse Home Visitation Groups at 24 Months, Based on Multilevel Growth Models.
| Intervention (n = 229) |
Standard Program (n = 236) |
Modeled Score Difference, ∆ (95% CI)a | Wald χ2 | P Value | |
|---|---|---|---|---|---|
| Primary Outcomeb | |||||
| WHOQOL-BREF, mean | 311.3 | 316.2 | −4.9 (−16.5 to 6.7) | 0.69 | .41 |
| Secondary Outcomesb | |||||
| IPV (CAS), % | 13.3 | 8.9 | 4.3 (−0.5 to 11.3) | 3.01 | .08 |
| PTSD (SPAN), % | 13.8 | 14.8 | −1.0 (−6.7 to 6.2) | 0.08 | .78 |
| Depression (PHQ-9), % | 10.5 | 11.5 | −1.0 (−5.0 to 5.0) | 0.15 | .70 |
| Alcohol problem (TWEAK), % | 14.5 | 11.8 | 2.7 (−2.3 to 9.7) | 0.92 | .34 |
| Drug problem (DAST), % | 8.7 | 5.5 | 3.2 (−1.4 to 12.1) | 1.46 | .23 |
| Mental health (SF-12), mean | 49.9 | 51.0 | −1.0 (−2.9 to 0.9) | 1.07 | .30 |
| Physical health (SF-12), mean | 52.2 | 52.5 | −0.3 (−1.5 to 0.9) | 0.27 | .60 |
Abbreviations: CAS, Composite Abuse Scale; DAST, Drug Abuse Severity Test; IPV, intimate partner violence; PHD-9, Patient Health Questionnaire 9; PTSD, posttraumatic stress disorder; SF-12, 12-Item Short-Form Health Survey; SPAN, Startle, Physiological Arousal, Anger, and Numbness; TWEAK, Tolerance/Worry About Drinking/Eye-Opener/Amnesia/C[K]ut Down on Drinking; WHOQOL-BREF, World Health Organization Quality of Life–BREF.
Modeled between-group score difference with no control variables.
See Table 2 footnotes for scale definitions for primary and secondary outcomes.
Post hoc Outcomes
When between-group baseline differences in ethnic background (Hispanic/Latina vs other) are adjusted for statistically in the growth model, there was no significant difference in quality-of-life score at 24 months between the study groups (−7.5 [95% CI, −20.4 to 5.4]). There were no statistically significant between-group modeled score differences at 24 months based on the growth models for any of the secondary outcomes (Table 3).
Of the 229 participants in the 7 intervention sites, 216 returned implementation logs. Prenatally, the universal assessment of safety was completed with 154 of 216 participants (71%). Of the 100 participants who disclosed IPV to the nurse, only 26 (26%) completed the required Danger Assessment instrument.13 In addition, only 40 of 100 abused women (40%) received at least 1 component of the tailored intervention focused on safety, IPV awareness, self-efficacy, or system navigation.
Discussion
In this trial, augmenting a nurse home visitation program with a complex, multicomponent IPV intervention did not lead to additional benefits in the primary outcome or any of the secondary outcomes.
There are several possible reasons why the augmented program was not more effective than the standard program. It is possible that the standard program, given its potency, improved outcomes such as quality of life and IPV to such an extent that the augmented practice model did not provide any incremental benefits to participants. Similar outcomes observed in both groups may be related to receiving care from nurses experienced in working with abused women and providing IPV support through the establishment of a therapeutic relationship and addressing maternal needs related to housing, poverty, mental illness, and social support—issues that when addressed may also serve to improve the quality of life for all abused women. This possibility is supported by the results of a Dutch trial, which showed that use of the standard nurse home visitation program6 led to a significant reduction in IPV among nurse-visited mothers compared with a control group, and by results of a systematic review, which included the standard nurse home visitation program evaluated in this trial.32
At the time of this trial there was little evidence for interventions to address IPV in home visiting. Recent findings suggest that a brief brochure-based intervention focused on safety planning and empowerment in either nurse or paraprofessional home visiting programs may be of benefit to women who disclose IPV at baseline.33 In comparison, this study evaluated a complex intervention developed for nurses delivering a model of nurse home visitation that emphasized discussions of safe relationships and multiple strategies for IPV assessment with all enrolled women, followed by immediate risk assessments for participants experiencing abuse and then long-term tailored intervention for abused women, including support through the process of disengaging from the abusive relationship.
A potential limitation of cluster trials with a limited number of clusters is group imbalance attributable to between-site differences in the baseline characteristics of participants. In this study, there was a potentially important difference at baseline: proportionally more Hispanic/Latina women were allocated to and retained in the intervention group than the standard nurse home visitation group. Although controlling for Hispanic/Latina background increased the group differences in quality of life at 24 months, these differences were not statistically significant. While site differences in ability to retain participants can be a challenge for cluster trials, the proportion of women retained across sites was relatively high and showed no between-group differences.
Limitations
This study had several limitations. First, it was not possible to make previous IPV exposure an eligibility criterion; since the study intervention was applied to the overall program at a site level, all women eligible for the nurse home visitation program at each site were asked to participate in the trial. Although 19.1% of those entering the trial reported exposure to IPV, this meant that the opportunity for nurses to focus on assisting women exposed to IPV was limited to less than one-fifth of the overall sample. Second, only 36% of women deemed eligible for the study enrolled in the trial. The study participants may have had more stable living and health conditions compared with those who were eligible but not enrolled, and this may have limited the ability to assess potential intervention effects. Third, post hoc analysis indicated that there were limitations in the implementation of the intervention; among women who disclosed IPV to their nurse, a minority (26%) completed a core risk assessment and less than half received 1 or more tailored intervention components. The low level of fidelity to the intervention may have been related to the complexity of the intervention or issues such as challenges working in the home environment or insufficient supervisory support with implementation.
Conclusions
Among pregnant women experiencing social and economic disadvantage and preparing to parent for the first time, augmentation of a nurse home visitation program with an IPV intervention, compared with the home visitation program alone, did not significantly improve quality of life at 24 months after delivery. These findings do not support the use of this intervention.
Trial Protocol
eFigure. Conceptual Model of Intimate Partner Violence Intervention
eTable. Intimate Partner Violence Intervention Characteristics
eReferences
Data Sharing Statement
References
- 1.Olds DL, Sadler L, Kitzman H. Programs for parents of infants and toddlers: recent evidence from randomized trials. J Child Psychol Psychiatry. 2007;48(3-4):355-391. doi: 10.1111/j.1469-7610.2006.01702.x [DOI] [PubMed] [Google Scholar]
- 2.Olds DL, Eckenrode J, Henderson CR Jr, et al. Long-term effects of home visitation on maternal life course and child abuse and neglect: fifteen-year follow-up of a randomized trial. JAMA. 1997;278(8):637-643. doi: 10.1001/jama.1997.03550080047038 [DOI] [PubMed] [Google Scholar]
- 3.Eckenrode J, Ganzel B, Henderson CR Jr, et al. Preventing child abuse and neglect with a program of nurse home visitation: the limiting effects of domestic violence. JAMA. 2000;284(11):1385-1391. doi: 10.1001/jama.284.11.1385 [DOI] [PubMed] [Google Scholar]
- 4.Olds DL, Kitzman H, Cole R, et al. Effects of nurse home-visiting on maternal life course and child development: age 6 follow-up results of a randomized trial. Pediatrics. 2004;114(6):1550-1559. doi: 10.1542/peds.2004-0962 [DOI] [PubMed] [Google Scholar]
- 5.Olds DL, Robinson J, Pettitt L, et al. Effects of home visits by paraprofessionals and by nurses: age 4 follow-up results of a randomized trial. Pediatrics. 2004;114(6):1560-1568. doi: 10.1542/peds.2004-0961 [DOI] [PubMed] [Google Scholar]
- 6.Mejdoubi J, van den Heijkant SC, van Leerdam FJ, Heymans MW, Hirasing RA, Crijnen AA. Effect of nurse home visits vs. usual care on reducing intimate partner violence in young high-risk pregnant women: a randomized controlled trial. PLoS One. 2013;8(10):e78185. doi: 10.1371/journal.pone.0078185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack SM, Ford-Gilboe M, Davidov D, MacMillan HL; NFP IPV Research Team . Identification and assessment of intimate partner violence in nurse home visitation. J Clin Nurs. 2017;26(15-16):2215-2228. doi: 10.1111/jocn.13392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack SM, Ford-Gilboe M, Wathen CN, et al. ; NFP IPV Research Team . Development of a nurse home visitation intervention for intimate partner violence. BMC Health Serv Res. 2012;12:50. doi: 10.1186/1472-6963-12-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen J. A power primer. Psychol Bull. 1992;112(1):155-159. doi: 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 10.WHOQOL Group Development of the World Health Organization WHOQOL-BREF quality-of-life assessment. Psychol Med. 1998;28(3):551-558. doi: 10.1017/S0033291798006667 [DOI] [PubMed] [Google Scholar]
- 11.Bonomi AE, Patrick DL, Bushnell DM, Martin M. Validation of the United States’ version of the World Health Organization Quality of Life (WHOQOL) instrument. J Clin Epidemiol. 2000;53(1):1-12. doi: 10.1016/S0895-4356(99)00123-7 [DOI] [PubMed] [Google Scholar]
- 12.Rutterford C, Copas A, Eldridge S. Methods for sample size determination in cluster randomized trials. Int J Epidemiol. 2015;44(3):1051-1067. doi: 10.1093/ije/dyv113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell JC, Webster DW, Glass N. The Danger Assessment: validation of a lethality risk assessment instrument for intimate partner femicide. J Interpers Violence. 2009;24(4):653-674. doi: 10.1177/0886260508317180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McFarlane J, Parker B, Moran BA. Abuse During Pregnancy: A Protocol for Prevention and Intervention. 2nd ed White Plains, NY: March of Dimes; 2007. [Google Scholar]
- 15.Norton LB, Peipert JF, Zierler S, Lima B, Hume L. Battering in pregnancy: an assessment of two screening methods. Obstet Gynecol. 1995;85(3):321-325. doi: 10.1016/0029-7844(94)00429-H [DOI] [PubMed] [Google Scholar]
- 16.Skevington SM, Epton T. How will the sustainable development goals deliver changes in well-being? a systematic review and meta-analysis to investigate whether WHOQOL-BREF scores respond to change. BMJ Glob Health. 2018;3(suppl 1):e000609. doi: 10.1136/bmjgh-2017-000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegarty K, Sheehan M, Schonfeld C. A multidimensional definition of partner abuse: development and preliminary validation of the Composite Abuse Scale. J Fam Violence. 1999;14(4):399-415. doi: 10.1023/A:1022834215681 [DOI] [Google Scholar]
- 18.Hegarty K, Fracgp, Bush R, Sheehan M. The composite abuse scale: further development and assessment of reliability and validity of a multidimensional partner abuse measure in clinical settings. Violence Vict. 2005;20(5):529-547. doi: 10.1891/vivi.2005.20.5.529 [DOI] [PubMed] [Google Scholar]
- 19.Davidson JR, Book SW, Colket JT, et al. Assessment of a new self-rating scale for post-traumatic stress disorder. Psychol Med. 1997;27(1):153-160. doi: 10.1017/S0033291796004229 [DOI] [PubMed] [Google Scholar]
- 20.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. JAMA. 1999;282(18):1737-1744. doi: 10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- 21.Bradley KA, Boyd-Wickizer J, Powell SH, Burman ML. Alcohol screening questionnaires in women: a critical review. JAMA. 1998;280(2):166-171. doi: 10.1001/jama.280.2.166 [DOI] [PubMed] [Google Scholar]
- 22.Russell M, Martier SS, Sokol RJ, et al. Screening for pregnancy risk-drinking. Alcohol Clin Exp Res. 1994;18(5):1156-1161. doi: 10.1111/j.1530-0277.1994.tb00097.x [DOI] [PubMed] [Google Scholar]
- 23.El-Bassell N, Schilling R, Schinke S, Orlandi M, Sun W, Back S. Assessing the utility of the Drug Abuse Screening Test in the workplace. Res Soc Work Pract. 1997;7(1):99-114. doi: 10.1177/104973159700700106 [DOI] [Google Scholar]
- 24.Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 25.Dienemann J, Campbell J, Landenburger K, Curry MA. The domestic violence survivor assessment: a tool for counseling women in intimate partner violence relationships. Patient Educ Couns. 2002;46(3):221-228. doi: 10.1016/S0738-3991(01)00216-6 [DOI] [PubMed] [Google Scholar]
- 26.Goodman L, Dutton MA, Weinfurt K, Cook S. The Intimate Partner Violence Strategies Index: development and application. Violence Against Women. 2003;9(2):163-186. doi: 10.1177/1077801202239004 [DOI] [Google Scholar]
- 27.Walsh CA, MacMillan HL, Trocmé N, Jamieson E, Boyle MH. Measurement of victimization in adolescence: development and validation of the Childhood Experiences of Violence Questionnaire. Child Abuse Negl. 2008;32(11):1037-1057. doi: 10.1016/j.chiabu.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 28.Bernstein DP, Stein JA, Newcomb MD, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27(2):169-190. doi: 10.1016/S0145-2134(02)00541-0 [DOI] [PubMed] [Google Scholar]
- 29.Browne GB, Arpin K, Corey P, Fitch M, Gafni A. Individual correlates of health service utilization and the cost of poor adjustment to chronic illness. Med Care. 1990;28(1):43-58. doi: 10.1097/00005650-199001000-00006 [DOI] [PubMed] [Google Scholar]
- 30.Dickson F, Tutty LM. The development of a measure of public health nurses’ practice responses to women who are abused. J Nurs Meas. 1998;6(1):87-103. doi: 10.1891/1061-3749.6.1.87 [DOI] [PubMed] [Google Scholar]
- 31.Rasbash J, Steele F, Browne W, Goldstein H. A Users Guide to MLwiN, Version 2.10. London, United Kingdom: Centre for Multilevel Modelling; 2009. [Google Scholar]
- 32.Prosman GJ, Lo Fo Wong SH, van der Wouden JC, Lagro-Janssen ALM. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32(3):247-256. doi: 10.1093/fampra/cmu091 [DOI] [PubMed] [Google Scholar]
- 33.Sharps PW, Bullock LF, Campbell JC, et al. Domestic violence enhanced perinatal home visits: the DOVE randomized clinical trial. J Womens Health (Larchmt). 2016;25(11):1129-1138. doi: 10.1089/jwh.2015.5547 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure. Conceptual Model of Intimate Partner Violence Intervention
eTable. Intimate Partner Violence Intervention Characteristics
eReferences
Data Sharing Statement