Abstract
Background
In people with chronic obstructive pulmonary disease (COPD), the use of neuromuscular electrostimulation (NMES) either alone, or together with conventional exercise training, might improve the condition of the peripheral muscles, increase exercise capacity and functional performance, reduce symptoms and improve health‐related quality of life (HRQoL).
Objectives
To determine the effects of NMES, applied in isolation or concurrently with conventional exercise training to one or more peripheral muscles, on peripheral muscle force and endurance, muscle size, exercise capacity, functional performance, symptoms, HRQoL and adverse events in people with COPD.
Search methods
We searched the Cochrane Airways Group Specialised Register, the Physiotherapy Evidence Database, clinical trial registries and conference abstracts on 14 March 2018.
Selection criteria
Randomised controlled trials that recruited adults with COPD if they had compared outcomes between a group that received NMES and a group that received usual care or compared outcomes between a group that received NMES plus conventional exercise training and a group that participated in conventional exercise training alone.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias using the Cochrane 'Risk of bias' tool. We expressed continuous data as either the standardised mean difference (SMD) or mean difference (MD) with the corresponding 95% confidence interval (CI). We assessed the quality of evidence using the GRADE approach.
Main results
Nineteen studies met the inclusion criteria of which 16 contributed data on 267 participants with COPD (mean age 56 to 76 years and 67% were men). Of these 16 studies, seven explored the effect of NMES versus usual care and nine explored the effect of NMES plus conventional exercise training versus conventional exercise training alone. Six studies utilised sham stimulation in the control group. When applied in isolation, NMES produced an increase in peripheral muscle force (SMD 0.34, 95% CI 0.02 to 0.65; low‐quality evidence) and quadriceps endurance (SMD 1.36, 95% CI 0.59 to 2.12; low‐quality evidence) but the effect on thigh muscle size was unclear (MD 0.25, 95% CI ‐0.11 to 0.61; low‐quality evidence). There were increases in six‐minute walk distance (6MWD) (MD 39.26 m, 95% CI 16.31 to 62.22; low‐quality evidence) and time to symptom limitation exercising at a submaximal intensity (MD 3.62 minutes, 95% CI 2.33 to 4.91). There was a reduction in the severity of leg fatigue on completion of an exercise test (MD ‐1.12 units, 95% CI ‐1.81 to ‐0.43). The increase in peak rate of oxygen uptake (VO2peak) was of borderline significance (MD 0.10 L/minute, 95% CI 0.00 to 0.19).
For NMES with conventional exercise training, there was an uncertain effect on peripheral muscle force (SMD 0.47, 95% CI ‐0.10 to 1.04; very low‐quality evidence) and there were insufficient studies to undertake a meta‐analysis on the effect on quadriceps endurance or thigh muscle size. However, there was an increase in 6MWD in favour of NMES combined with conventional exercise training (MD 25.87 m, 95% CI 1.06 to 50.69; very low‐quality evidence). In people admitted to either in an intensive care unit or a respiratory high dependency centre, NMES combined with conventional exercise reduced the time taken for participants to first sit out of bed by 4.98 days (95% CI ‐8.55 to ‐1.41; very low‐quality evidence), although the statistical heterogeneity for this analysis was high (I2 = 60%). For both types of studies (i.e. NMES versus usual care and NMES with conventional exercise training versus conventional exercise training alone), there was no risk difference for mortality or minor adverse events in participants who received NMES.
Authors' conclusions
NMES, when applied in isolation, increased quadriceps force and endurance, 6MWD and time to symptom limitation exercising at a submaximal intensity, and reduced the severity of leg fatigue on completion of exercise testing. It may increase VO2peak, but the true effect on this outcome measure could be trivial. However, the quality of evidence was low or very low due to risk of bias within the studies, imprecision of the estimates, small number of studies and inconsistency between the studies. Although there were no additional gains in quadriceps force with NMES plus conventional exercise training, there was evidence of an increase in 6MWD. Further, in people who were the most debilitated, the addition of NMES may have accelerated the achievement of a functional milestone, that is, the first time someone sits out of bed.
Plain language summary
Muscle stimulation for people with chronic obstructive pulmonary disease (COPD)
Review question
We reviewed the evidence for applying electrical stimulation to the thigh muscles of people with COPD (a long‐term lung condition characterised by cough, sputum production (fluids from the lungs, i.e. phlegm) and difficulty breathing). We looked at studies that used two groups; one receiving electrical stimulation by placing conductive pads over the muscle, the other receiving usual medical care. We also looked at studies that added electrical stimulation to an exercise programme and compared the results with a group that only undertook the exercise programme.
The studies measured muscle strength and endurance (how long the muscle could work), muscle size, exercise capacity, shortness of breath, leg fatigue and health‐related quality of life (HRQoL; a measure of a person's satisfaction with their life and health). We also looked to see if applying electrical stimulation to the muscles in the thigh caused any unwanted effects.
Background
People with COPD find exercise difficult and feel breathless. But exercise such as frequent brisk walking or stationary cycling reduces breathing difficulties and improves the ability to exercise. One way that exercise helps is by improving the condition (how well they work) of the thigh muscles.
However, for some people with COPD, exercising at a level that is high enough to improve the condition of the thigh muscles is difficult because they experience severe shortness of breath with exercise. In these people, it may be that using an electrical current to stimulate the thigh muscles will help to improve their condition. Because the electrical stimulation is applied to only a few muscles (in contrast to exercise, which involves several muscles), electrical stimulation can be completed without causing much shortness of breath. If electrical stimulation can improve the condition of the leg muscles, it might be a useful rehabilitation approach.
Search date
The evidence is current to March 2018.
Study characteristics
Nineteen studies met the inclusion criteria for the review, of which 16 had data on 267 participants that could be included in the analyses. The average age of people in each of the studies ranged from 56 to 76 years and 179 (67%) were men. Seven studies explored the effect of applying electrical stimulation alone and nine studies explored the effect of adding electrical stimulation to an exercise programme. Electrical stimulation was applied in a range of settings, such as at home, in an outpatient hospital department, on a hospital ward or in an intensive care unit. Most studies stimulated the thigh muscles once or twice a day for 30 to 60 minutes on four to seven days each week for four to eight weeks.
Key results
Studies that explored the effect of applying electrical stimulation alone showed an increase in strength and endurance of the thigh muscles. They showed an increase in some, but not all, measures of exercise capacity and a decrease in the severity of leg fatigue after exercise. Studies that explored the effect of adding electrical stimulation to an exercise programme showed a small increase in the distance walked in six minutes. In people who were most unwell (e.g. in an intensive care unit), adding electrical stimulation to an exercise programme helped people to spend fewer days confined to bed. Electrical stimulation did not increase the risk of side effects.
Quality of the evidence
The quality of evidence provided by this review was low. This is because most studies had design problems. The inclusion of future studies into this review is likely to change the results.
Summary of findings
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is a condition characterised by persistent expiratory airflow limitation (Vogelmeier 2017). It is also associated with several systemic manifestations, including profound deconditioning of the peripheral muscles (Maltais 2014). The prevalence of COPD that is of at least moderate severity among adults over 40 years of age is approximately 10% (Buist 2007). The cardinal complaint of people with this condition is dyspnoea (Vogelmeier 2017). One updated Cochrane Review suggested that, in people with COPD, pulmonary rehabilitation that includes conventional exercise training will increase exercise tolerance, reduce symptoms of dyspnoea and fatigue, and improve health‐related quality of life (HRQoL) (McCarthy 2015). This appears to relate to a reduction in the signs suggestive of muscle deconditioning, such as early lactic acid accumulation (Casaburi 1991). Nevertheless, amongst people with the most marked ventilatory limitation to exercise, intolerable dyspnoea may preclude the application of a training stimulus to the peripheral muscles that is of sufficient intensity to confer a training adaptation. For this reason, there is interest in the use of strategies to optimise the training load borne by the muscles of locomotion, particularly the quadriceps (Hill 2014). One such strategy is the use of neuromuscular electrostimulation (NMES). This involves eliciting a muscle contraction by applying an intermittent electrical current to a superficial peripheral muscle (Maffiuletti 2010).
Description of the intervention
NMES involves placing conductive pads over the muscle and using an intermittent electrical current to trigger action potentials, activate the intramuscular nerve branches and muscle fibres to generate a strong muscle contraction (Maffiuletti 2010). The conductive pads are attached to a preprogrammed stimulation unit. Stimulation parameters can be manipulated to favour a pattern of contractions that promote strength or endurance adaptations in the muscle. For example, protocols aimed at a strength adaptation may comprise few contractions, using high‐frequency stimulation to ensure highest possible force, performed at the highest tolerable current to maximise the number of muscle fibres recruited (Maffiuletti 2010). A relatively long contraction period followed by an even longer rest period may be advantageous (Filipovic 2011). These protocols, which commonly feature in studies undertaken in people with COPD, aim to create the greatest force during each and every contraction because the mechanical stress is likely to stimulate synthesis of the contractile proteins (Murton 2010). Protocols aimed at an endurance adaptation may comprise multiple relatively frequent and brief contractions over prolonged periods. Relatively short contractions interspersed with short rest periods may be advantageous (Nuhr 2004). These protocols aim to mimic repeated contractions to elevate metabolism and accumulation of products that stimulate mitochondrial biogenesis (and inhibit protein synthesis, i.e. strength adaptations) (Takahashi 1993). The highest intensity tolerated may be used for both strength and endurance protocols, because maximising the intensity of stimulation increases the number of fibres stimulated. This is important because, unlike with voluntary contractions, orderly recruitment of the muscle fibres does not occur with transcutaneous stimulation (Gregory 2005; Henneman 1985). The muscle group most commonly targeted by NMES is the quadriceps.
How the intervention might work
NMES may be used to target increases in peripheral muscle strength or endurance. Targeting gains in strength may be most appropriate for people who are very debilitated (e.g. intensive care unit ((ICU) survivors) and lack the strength required to undertake everyday activities (e.g. rise from sitting to standing). In contrast, targeting gains in endurance may be most appropriate for people who are unable to achieve adequate intensity during aerobic exercise due to the onset of intolerable dyspnoea (e.g. in people with severe disease or during exacerbations of the disease) (Parker 2005). Specifically, aerobic exercise training, such as brisk walking or cycling, involves many muscles, including postural muscles, which must be supported by the ventilatory system. In people with COPD, the ventilatory system is compromised and therefore the duration that an effective training stimulus can be sustained during aerobic exercise is often constrained by intolerable dyspnoea (Maltais 1997). In contrast, NMES isolates contracting muscle groups, thereby lessening the overall ventilatory load (Sillen 2011). For this reason, in people with COPD who experience dyspnoea so severe that it precludes them from participating in aerobic exercise training at sufficient intensity to condition the peripheral muscles, NMES might be an appropriate option. As impairments in exercise capacity have been related to decrements in quadriceps function (Maltais 2000; Saey 2003), it is likely that conditioning these muscles via NMES will increase exercise capacity.
Why it is important to do this review
The aim of this review was to determine the effects of NMES, applied in isolation or concurrently with conventional exercise training, on peripheral muscle force and endurance, muscle size, exercise capacity, functional performance, symptoms, HRQoL and adverse events in adults with COPD. The results of this review will provide clinicians who work in the area of pulmonary rehabilitation, as well as clinicians who treat people hospitalised with an acute exacerbation of COPD, with information to guide their decisions regarding whether or not to use this approach.
Objectives
To determine the effects of NMES, applied in isolation or concurrently with conventional exercise training to one or more peripheral muscles, on peripheral muscle force and endurance, muscle size, exercise capacity, functional performance, symptoms, HRQoL and adverse events in people with COPD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) only were eligible for inclusion, as they are the gold standard study design for determining the effectiveness of an intervention. We excluded randomised cross‐over trials.
Types of participants
Adults with a diagnosis of COPD regardless of their clinical stability; that is, studies that recruited people with stable COPD or people with an exacerbation of COPD. We included studies that recruited a sample of participants with a range of chronic respiratory diseases only if most participants (greater than 50%) had a diagnosis of COPD.
Types of interventions
NMES (of any peripheral muscle) compared with usual care (any aspect of usual medical care, with or without sham training NMES, but not conventional exercise training). This allowed us to determine the effects of NMES in isolation from other exercise rehabilitation strategies.
NMES (of any peripheral muscle) plus conventional exercise training (which included active limb movement if the participants were hospitalised) compared with conventional exercise training alone, with or without sham training NMES. This allowed us to determine the effects of using NMES as an adjunct to conventional exercise training.
Types of outcome measures
Primary outcomes
Peripheral muscle force (using any method): defined as the peak force (or torque) elicited during a maximum voluntary contraction or a twitch force elicited in response to stimulation of a peripheral nerve.
Peripheral muscle endurance/fatigability (using any method): defined as performance during any test that aimed to elicit a decline in muscle force over time using repeated muscle contractions.
Thigh muscle size (using any method).
For these muscle‐specific outcomes, we extracted measurements made before and after the intervention period.
Serious adverse events (e.g. mortality) recorded during the intervention period only.
Because NMES aims to condition the peripheral muscles, muscle‐specific outcomes were selected as the primary outcomes for this study. Serious adverse events were also selected as a primary outcome as information on this outcome will assist clinicians in determining whether or not NMES poses a risk to people with COPD.
Secondary outcomes
Exercise capacity (e.g. six‐minute walk distance (6MWD), incremental shuttle walk distance, performance during an endurance shuttle walk test (ESWT), peak rate of oxygen uptake (VO2peak), peak power, lactate threshold, time to symptom limitation during a constant submaximal power test, changes in cardiorespiratory measures taken at iso‐time).
Functional performance (e.g. Timed Up and Go test or capacity to get out of bed independently).
Symptoms of dyspnoea and fatigue (using any validated questionnaire or scale).
Health‐related quality of life (using any validated disease‐specific HRQoL questionnaire).
For outcomes of exercise capacity, functional performance, symptoms and HRQoL, we extracted measurements made before and after the intervention period.
Minor adverse events recorded during the intervention period only (e.g. discomfort, musculoskeletal pain, muscle soreness, skin irritation).
Exercise capacity, measures of functional performance, symptoms and HRQoL are outcomes that are perceived to be important by patients. That is, any improvement in muscle function following NMES is unlikely to be perceived as important by the patient, unless the effect translates into an improvement in exercise capacity, measures of functional performance, symptoms of dyspnoea and fatigue or HRQoL.
Reporting of one or more of the outcomes listed here was not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We searched the following databases:
the Cochrane Airways Trials Register;
the Physiotherapy Evidence Database (PEDro).
The Cochrane Airways Trials Register is maintained by the Information Specialist for the Group. It contains studies identified from several sources:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org);
weekly searches of MEDLINE OvidSP from 1946 to date of search;
weekly searches of Embase OvidSP from 1974 to date of search;
monthly searches of PsycINFO OvidSP from 1967 to date of search;
monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature) from 1937 to date of search;
monthly searches of AMED EBSCO (Allied and Complementary Medicine);
handsearches of the proceedings of major respiratory conferences.
Studies contained in the Cochrane Airways Trials Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, and a list of handsearched conference proceedings are in Appendix 1. Records in the Cochrane Airways Trials Register were searched using the strategy outlined in Appendix 2. This strategy was adapted to search PEDro. The most recent search was conducted on 14 March 2018.
We searched ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization trials portal (www.who.int/ictrp/en/). We searched all databases from their inception to the date of search, and imposed no restrictions on language of publication or publication status.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references and relevant manufacturers' websites for trial information. We contacted investigators who were prominent in this field to ask about unpublished or ongoing studies.
We handsearched abstracts presented at the World Confederation for Physical Therapy ‐ congress meetings from 2003, 2007, 2011, 2015 and 2017.
We searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Two groups of review authors (KH and SM or KH and VC) independently screened the titles and abstracts of all studies identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve.' We retrieved the full‐text study reports/publications, and two review authors (KH and SM or KH and VC) independently screened the full text and identified studies for inclusion. We recorded the reasons for exclusion of ineligible studies. We resolved disagreements through discussion. We identified and excluded duplicates and collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. The selection process was recorded in sufficient detail to complete a PRISMA flow diagram and a Characteristics of excluded studies table.
Studies reported as full text, those published as abstracts only and unpublished data were eligible for inclusion.
Data extraction and management
We used an electronic data collection form related to study characteristics and outcomes after it was piloted on two studies included in the review. We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, withdrawals, date of study and details to allow an assessment of the risk of bias.
Participants: number, mean age, age range, gender, severity of condition, diagnostic criteria, presence (or not) of a recent (four weeks or less) acute exacerbation of their disease, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
Interventions: NMES training and, where relevant, sham training parameters (including stimulation current, force of stimulated contraction, current ramp, pulse width, stimulation frequency, on time, duty cycle, frequency of exposure, duration of therapy and muscles stimulated).
Outcomes: data related to both primary and secondary outcomes assessed before (i.e. baseline) and after the intervention period. We extracted baseline data, postintervention data (i.e. measures of central tendency, measures of dispersion and sample size) and data pertaining to the change from baseline (or, where possible, calculated using baseline and postintervention data).
Notes: funding for trial and notable conflicts of interest of trial authors.
For each study, two review authors (SM, PR, TJF, MR or VC and KH) independently extracted data from included studies. We resolved disagreements by consensus. If outcome data were not reported in a usable way, we contacted the study authors to seek clarification. When we were unable to contact the authors, it was noted in the Characteristics of included studies table that data were not reported in a usable way. Once data extraction was complete, one review author (KH) transferred data into the Review Manager 5 (RevMan 2014). We double‐checked data to ensure that they had been entered correctly by comparing the data presented in the systematic review with that provided in the study reports. A second review author (VC) spot‐checked study characteristics for accuracy against the trial report.
We analysed measures of peripheral muscle force and endurance, muscle size, exercise capacity, functional performance, symptoms of dyspnoea and fatigue and HRQoL as continuous data. We reported adverse events as dichotomous outcomes (yes/no).
We presented data reported using scales (e.g. quadriceps endurance and HRQoL) with a consistent direction of effect.
Assessment of risk of bias in included studies
Two groups of review authors (KH and SM or KH and VC) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion. We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
In the 'Risk of bias' table, we graded each potential source of bias as high, low or unclear and provided a quote from the study report, together with a justification for our judgement. We summarised the risk of bias judgements across different studies for each of the domains listed. We described the implications of a lack of blinding separately for different key outcomes. When information on risk of bias related to unpublished data or correspondence with an investigator, we noted this in the 'Risk of bias' table.
When considering the quality of the evidence for treatment effects, we took into account the risk of bias for the studies that contributed to each outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Hill 2013), and reported deviations from it in the Differences between protocol and review section.
Measures of treatment effect
For dichotomous data, we calculated the risk difference (RD) and their 95% confidence intervals (CI). For continuous data that were reported using different units of measurement, we calculated the standardised mean differences (SMD) and their corresponding 95% CI using the change scores together with the standard deviation (SD) of the baseline measures in both groups. For continuous data that were reported using the same units of measurement, we calculated the mean differences (MD) and their corresponding 95% CI using the changes scores and the SD of the change scores in both groups. For studies that did not report the SD of the change scores, we used the SD of the baseline measures in both groups.
We undertook meta‐analyses only when meaningful (i.e. when treatments, participants and the underlying clinical question were similar enough for pooling to make sense).
We extracted skewed data as medians, interquartile ranges or range and converted them to mean and SDs using online software (Wen 2011), and used them in meta‐analyses that estimated MD. However, we did not included studies that reported outcome data as median, maximum and minimum values, or interquartile range in meta‐analyses that estimated SMD (Abdellaoui 2011; Akar 2017; Tasdemir 2015).
Unit of analysis issues
For studies that randomly assigned participants to groups (i.e. either NMES or control), the unit of analysis was the participant. For studies that randomly assigned one limb of a person to receive NMES and the other limb to receive control, the unit of analysis was the limb. We accept that inclusion of these studies may dampen our effect size for the results of NMES on muscle function because NMES may produce systemic effects such as improvement in microcirculation and increased heart rate response, which result in contralateral leg facilitation (Gerovasili 2009; Hortobágyi 1999). To address this issue, we undertook a sensitivity analysis, in which we excluded studies that used this design to see whether this changed our estimate of the effect.
Dealing with missing data
We contacted investigators to verify key study characteristics and to obtain missing outcome data (e.g. when a study was identified as an abstract only).
Assessment of heterogeneity
We used the I2 statistic to measure statistical heterogeneity among the trials in each analysis. We explored possible causes of substantial heterogeneity (I2 of 50% or greater) through sensitivity analyses.
Assessment of reporting biases
If we were able to pool more than 10 trials for any one meta‐analysis, we planned to create a funnel plot to examine possible publication and small‐study biases. Where available, we reviewed protocols published on clinical trial registries to explore reporting bias.
Data synthesis
We expected that some disparity would be present in the way NMES was applied between the studies, and that would introduce heterogeneity to the effects of the intervention. Therefore, we used a random‐effects model for the meta‐analyses.
'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: peripheral muscle force, peripheral muscle endurance, thigh muscle size, 6MWD, functional performance, dyspnoea, HRQoL and minor adverse events. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it related to the studies that contributed data to the meta‐analyses for the prespecified outcomes (Guyatt 2008). We also presented results of subgroup analyses. We applied methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions using GRADEpro software (Higgins 2011). We justified all decisions to downgrade or upgrade the quality of studies in the table to aid the reader's understanding of the review.
Subgroup analysis and investigation of heterogeneity
For each analysis (i.e. NMES versus usual care, and NMES plus conventional exercise training versus conventional exercise training alone), we planned the following subgroup analyses.
Studies that recruited participants who were clinically stable versus studies that recruited participants during an acute exacerbation of COPD. This allowed us to explore whether the effectiveness of NMES differed between people who were clinically stable versus those who were experiencing an acute exacerbation of their disease.
Studies that used stimulation frequencies less than 15 Hz versus studies that used stimulation frequencies of 15 Hz or greater (Sillen 2011). This separated studies that used frequencies more likely to result in pulse fusion, a tetanic muscle contraction and favour strength adaptations (i.e. 15 Hz or greater) from those that did not (i.e. less than 15 Hz) and may assist in determining the most effective stimulation parameters.
Studies that recruited participants with, on average, severe disease (i.e. forced expiratory volume in one second (FEV1) less than 50%) versus studies that recruited participants with, on average, less severe disease (FEV1 of 50% or greater). This allowed us to explore whether the effectiveness of NMES differed between people with mild and moderate disease versus people with severe or very severe disease.
Studies that used robust, reliable methods for quantifying peripheral muscle force (i.e. via a mechanical dynamometer, fixed strain gauge or a twitch force elicited in response to stimulation of a peripheral nerve and measured with a strain gauge) versus studies that used less robust measures (i.e. via a hand‐held or non‐fixed dynamometer, the one‐repetition maximum or by applying the Medical Research Council grading system for manual muscle testing) (Clarkson 2000). This was important, as some outcome measures (e.g. Medical Research Council grading system for manual muscle testing) were likely to be less responsive to change than others (e.g. a mechanical dynamometer) and may have been at higher risk of detection bias.
Studies in which a minimum of 10 training sessions were completed within a four‐week period versus studies in which fewer than 10 training sessions were completed over this period. This allowed us to explore whether 'dose' influenced effectiveness.
We used the following outcomes in the subgroup analyses.
Muscle‐specific outcome measures such as peripheral muscle force, muscle endurance and muscle size.
Exercise capacity.
Sensitivity analysis
We conducted sensitivity analyses by excluding studies that described the use of different methodologies (e.g. studies that randomly assigned one limb of a person to receive NMES and the other limb to receive control).
Results
Description of studies
Nineteen (28 records) studies met the criteria to be included in this review (Figure 1). Of these, 16 contributed data for the meta‐analyses (Abdellaoui 2011; Akar 2017; Akinlabi 2013; Bourjeily‐Habr 2002; Dang 2011; Giavedoni 2012; Kucio 2016; Latimer 2013; Maddocks 2016; Neder 2002; Tardif 2015; Tasdemir 2015; Vieira 2014; Vivodtzev 2006; Vivodtzev 2012; Zanotti 2003), and one had data that were included in the narrative discussion (Dolmage 2016).
1.
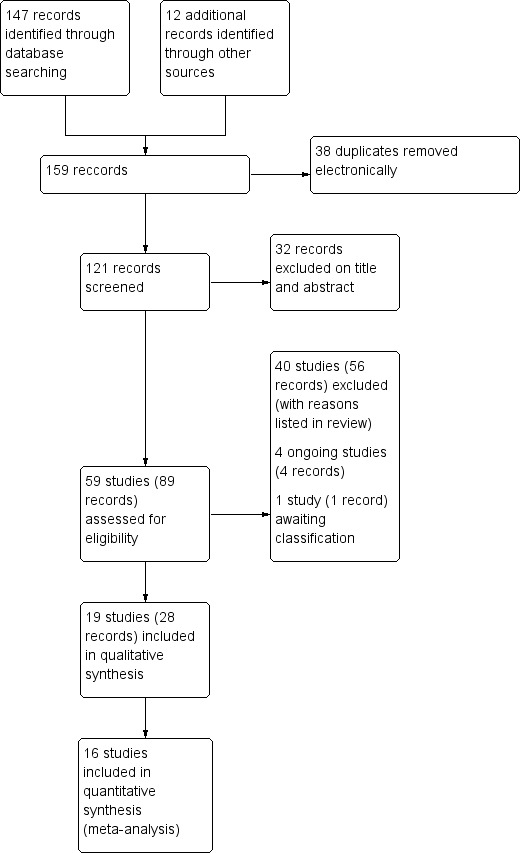
Study flow diagram.
Results of the search
The search strategy yielded 159 potential studies, of which 38 were duplicates that were removed as part of the electronic search process. Of the 121 remaining potential studies, 32 records were excluded based on title or abstract and 40 studies (56 records) were excluded after reading the full paper (Figure 1). We identified four ongoing studies (ChiCTR‐IPR‐16009845; JPRN‐UMIN000024443; NCT01799330; NCT02321163), and one study reported in a conference abstract that we need further information to assess for inclusion (Chen 2017). Of the 19 studies (28 records) that met the criteria for inclusion, three provided no data that could be included in any meta‐analyses (Dolmage 2016; Gigliotti 2004; Zanotti 2010). Of the 16 studies included in the meta‐analyses, seven explored the effect of NMES versus usual care (Bourjeily‐Habr 2002; Giavedoni 2012; Latimer 2013; Maddocks 2016; Neder 2002; Vieira 2014; Vivodtzev 2012; Table 3), and nine explored the effect of NMES plus conventional exercise training versus conventional exercise training alone (Abdellaoui 2011; Akar 2017; Akinlabi 2013; Dang 2011; Kucio 2016; Tardif 2015; Tasdemir 2015; Vivodtzev 2006; Zanotti 2003; Table 4). A total of 13 studies recruited participants who were clinically stable (Akinlabi 2013; Bourjeily‐Habr 2002; Dang 2011; Kucio 2016; Latimer 2013; Maddocks 2016; Neder 2002; Tardif 2015; Tasdemir 2015; Vieira 2014; Vivodtzev 2006; Vivodtzev 2012; Zanotti 2003), and three recruited participants during hospitalisation for an exacerbation (Abdellaoui 2011; Akar 2017; Giavedoni 2012). One study recruited participants who had recently spent a period in the ICU or had been hospitalised with acute exacerbation (or both), and were transferred to an inpatient rehabilitation facility (Vivodtzev 2006). However, at the time of recruitment, participants in this study were clinically stable (Vivodtzev 2006). One study recruited participants who were referred to a respiratory high dependency unit from surrounding ICUs and who were ventilated via a tracheostomy for chronic respiratory failure (Zanotti 2003). However, at the time of recruitment, participants in this study were also clinically stable (Zanotti 2003). One study randomised participants to three intervention arms, but we only extracted data on the groups that received NMES plus conventional exercise training or conventional exercise training alone (Akar 2017). Two studies randomly assigned one leg to receive NMES and used the other leg to receive control (Giavedoni 2012; Latimer 2013). The latest search was run on 14 March 2018.
1. Characteristics of studies that contributed data to meta‐analyses (NMES versus usual care).
| Study | Setting | Lower limb stimulation | Clinical stability | Dose | Frequency (Hz) | Intervention received by control group |
| Bourjeily‐Habr 2002 | Outpatient | Bilateral quadriceps, hamstrings and calf | Stable | 20 min per day, 3 days per week for 6 weeks at an intensity that elicited a muscle contraction, and increasing by 5 mA per week | 50 | Sham stimulation |
| Giavedoni 2012 | Hospital ward then at home | Unilateral quadriceps | Acute exacerbation | 30 min per day, once per day for 14 days at maximum tolerated current | 50 | Nil |
| Latimer 2013 | Combination of supervised and unsupervised home training | Unilateral quadriceps | Stable | 30 min per session, 5 times per week, for 6 weeks at maximum tolerated current | 50 | Nil |
| Maddocks 2016 | Home | Bilateral quadriceps | Stable | 30 min per day, 7 days per week for 6 weeks with current set to elicit a contraction equivalent to 15‐25% of a maximum voluntary contraction | 50 | Sham stimulation |
| Neder 2002 | First week as outpatient then home | Bilateral quadriceps | Stable | 15 min (to each leg) in the first week which increased to 30 min thereafter, for 5 days per week for 6 weeks at maximum tolerated current | 50 | Nil |
| Vieira 2014 | Presumably home | Bilateral quadriceps | Stable | 60 min per session, 2 times per day, 5 days per week, for 8 weeks at maximum tolerated current | 50 | Both groups received respiratory physical therapy (i.e. airway clearance) as indicated as well as stretching exercises for the upper limbs, lower limbs and back (control group also received sham stimulation). |
| Vivodtzev 2012 | Home | Bilateral quadriceps and calf | Stable | 60 minutes per session, 5 days per week for 6 weeks at maximum tolerated current | 50 | Sham stimulation |
min: minute; NMES: neuromuscular electrostimulation.
2. Characteristics of studies that contributed data to meta‐analyses (NMES + exercise versus exercise alone).
| Study | Setting | Lower limb stimulation | Clinical stability | Dose | Frequency (Hz) | Exercise intervention received by control group |
| Abdellaoui 2011 | Intensive care unit | Bilateral quadriceps and hamstrings | Acute exacerbation | 1 hour per day, 5 days per week for 6 weeks at maximum tolerated current | 35 | Both groups received education (once per week) and daily active‐passive mobilisation (control group also received sham stimulation). |
| Akar 2017 | Intensive care unit | Bilateral quadriceps | Respiratory failure | 5 days per week (total of 20 sessions) at maximum tolerated current | 50 | Both groups received active exercise, which comprised active joint range of motion exercise for upper and lower limbs. Participants who could not manage active exercise received active‐assisted or passive range of motion exercise. |
| Akinlabi 2013 | Home | Bilateral quadriceps and hamstrings | Stable | 2 days per week for 8 weeks (total of 16 sessions) | 10‐50 | Low‐intensity symptom‐limited exercise |
| Dang 2011 | Outpatient | Bilateral quadriceps | Stable | 36 min, 3 sessions per week for 12 weeks (total of 36 sessions) at maximum tolerated current | 8‐45 | Usual respiratory rehabilitation (no other details given) |
| Kucio 2016 | Inpatient rehabilitation | Bilateral quadriceps and calf | Stable | 36 min, presumably 6 supervised sessions per week for 3 weeks, intensity not specified | 35 | Both groups received breathing exercises, treadmill walking and resistance exercise. |
| Tardif 2015 | Home | Bilateral quadriceps | Stable | Presumably 30 min per day, 5 days per week, presumably for 8 weeks at maximum tolerated current | 35 | Both groups received pulmonary rehabilitation. |
| Tasdemir 2015 | Outpatient | Bilateral quadriceps | Stable | 20 min, 2 days per week for 10 weeks at maximum tolerated current | 50 | Both groups received pulmonary rehabilitation (control group also received sham stimulation). |
| Vivodtzev 2006 | Inpatient rehabilitation | Bilateral quadriceps | Stable, but shortly following acute illness | > 30 min per session, 4 times per week, for 4 weeks at maximum tolerated current | 5‐35 | Both groups received active limb exercises. The strongest participants also performed walking on a treadmill together with 5‐10 min of resistance arm exercises. They also completed health education sessions 1 day per week. |
| Zanotti 2003 | Respiratory high dependency unit for inpatient rehabilitation | Bilateral quadriceps and gluteals | Stable, but shortly following acute illness | Up to 30 min per session, 2 times per day, 5 days per week for 4 weeks presumably at maximum tolerated current | 8‐35 | Both groups received rehabilitation that comprised active limb exercises. |
min: minute; NMES: neuromuscular electrostimulation.
Included studies
With the exception of risk of bias, the results refer only to studies that provided data that could be incorporated into the review.
Participants
The 16 studies contributed data on 267 participants with COPD, of whom 150 (56%) received NMES. The mean age of the participants ranged from 56 to 76 years and 179 (67%) were men. The mean FEV1 of the participants ranged from 15% to 50% of the predicted value in healthy adults. Common inclusion criteria related to a diagnosis of severe or very severe COPD and severe functional limitation due to dyspnoea. Common exclusion criteria were the presence of an implanted cardiac pacemaker or comorbidities that may have interfered with participation in the study. For further description of the participants included in the studies in these meta‐analyses, refer to the Characteristics of included studies table.
Intervention
The intervention was undertaken:
at home by five studies (Akinlabi 2013; Maddocks 2016; Neder 2002; Tardif 2015; Vivodtzev 2012);
with supervision as well as in the home by one study (Latimer 2013);
at a pulmonary rehabilitation or outpatient centre by three studies (Bourjeily‐Habr 2002; Dang 2011; Tasdemir 2015);
on a hospital ward then at home following discharge by one study (Giavedoni 2012);
at an inpatient rehabilitation facility by two studies (Kucio 2016; Vivodtzev 2006);
in a high dependency unit by one study (Zanotti 2003); and
in the ICU by two studies (Abdellaoui 2011; Akar 2017).
One study did not state the location for the intervention (Vieira 2014), but it was likely that this study provided the intervention in the home.
The lower limb muscles stimulated were:
bilateral quadriceps by eight studies (Akar 2017; Dang 2011; Maddocks 2016; Neder 2002; Tardif 2015; Tasdemir 2015; Vieira 2014; Vivodtzev 2006);
bilateral quadriceps and the hamstrings by two studies (Abdellaoui 2011; Akinlabi 2013);
bilateral quadriceps, hamstrings and calf muscles by one study (Bourjeily‐Habr 2002);
bilateral quadriceps and calf muscles by two studies (Kucio 2016; Vivodtzev 2012); or
bilateral quadriceps and gluteals by one study (Zanotti 2003).
Both studies that randomly assigned one leg to receive NMES and used the other leg to receive control stimulated only unilateral quadriceps (Giavedoni 2012; Latimer 2013).
Regarding intensity, when described (either in the paper or through communication with the authors), most studies reported that stimulation was set to the maximum current that was perceived to be tolerable (Abdellaoui 2011; Akar 2017; Dang 2011; Giavedoni 2012; Latimer 2013; Neder 2002; Tardif 2015; Tasdemir 2015; Vieira 2014; Vivodtzev 2006; Vivodtzev 2012). One study described setting an intensity that elicited a muscle contraction, and increasing by 5 mA per week (Bourjeily‐Habr 2002), and another study described setting the intensity to produce a muscular contraction equivalent to 15% to 25% of force generated during a maximum voluntary contraction (Maddocks 2016). Waveforms were most commonly symmetric or biphasic (or both) (Abdellaoui 2011; Akar 2017; Akinlabi 2013; Dang 2011; Giavedoni 2012; Kucio 2016; Latimer 2013; Neder 2002; Tasdemir 2015; Vieira 2014; Vivodtzev 2006; Vivodtzev 2012; Zanotti 2003). Regarding frequency, nine studies stimulated using 50 Hz (Akar 2017; Bourjeily‐Habr 2002; Giavedoni 2012; Latimer 2013; Maddocks 2016; Neder 2002; Tasdemir 2015; Vivodtzev 2012). Other studies reported using frequencies that ranged between 8 Hz and 45 Hz (Dang 2011), 10 Hz and 50 Hz (Akinlabi 2013), 5 Hz and 35 Hz (Vivodtzev 2006), 8 Hz and 35 Hz (Zanotti 2003), or 35 Hz (Abdellaoui 2011; Kucio 2016; Tardif 2015). Regarding duration, most studies stimulated once or twice a day for 30 to 60 minutes on four to seven days per week for four to eight weeks (Abdellaoui 2011; Latimer 2013; Maddocks 2016; Neder 2002; Tardif 2015; Vieira 2014; Vivodtzev 2006; Vivodtzev 2012; Zanotti 2003).
Exercise programmes
For the four studies that were conducted in an ICU, high dependency area or inpatient rehabilitation facility, and compared the effect of NMES plus conventional exercise training versus conventional exercise training alone, exercise training comprised active movement, active‐assisted movement or passive range of motion if the participant was unable to perform active movement through full range (Abdellaoui 2011; Akar 2017; Vivodtzev 2006; Zanotti 2003). One study described a programme that also facilitated participants to walk on a treadmill and use light arm weights once able (Vivodtzev 2006). The one study that was conducted in participants who were hospitalised (but clinically stable), and compared the effect of NMES plus conventional exercise training versus conventional exercise training alone, offered breathing exercises together with treadmill walking and resistance exercises (Kucio 2016). For the four studies that were conducted at home or in a rehabilitation or outpatient centre, and compared the effect of NMES plus conventional exercise training versus conventional exercise training alone, exercise training was described as low intensity symptom‐limited exercise (Akinlabi 2013), or usual exercise training/pulmonary rehabilitation (Dang 2011; Tardif 2015; Tasdemir 2015).
Outcome measures
Regarding the assessment of peripheral muscle force, two studies assessed quadriceps force used an isokinetic chair‐mounted dynamometer (Bourjeily‐Habr 2002; Neder 2002), and six studies assessed quadriceps force using a strain gauge or digital load cell that was fixed to a chair or rig (Dang 2011; Giavedoni 2012; Latimer 2013; Maddocks 2016; Vivodtzev 2006; Vivodtzev 2012). For the one study that measured quadriceps force during both a maximal voluntary isometric contraction and as twitch force in response to supramaximal femoral nerve stimulation (Maddocks 2016), the former, but not the latter measure was used in a meta‐analysis. One study assessed quadriceps force using a device that appeared to be hanging scale (Abdellaoui 2011), and one measured the one‐repetition maximum (Tasdemir 2015). Two studies described performing manual muscle testing of the 'peripheral muscles' or 'lower extremity muscles,' and although it was not clear which muscles were included in this assessment, it seemed likely that this assessment would have included quadriceps as both studies stimulated this muscle (Akar 2017; Zanotti 2003).
Three studies reported the assessment of quadriceps endurance. One study described a fatigue index in which high values were indicative of worse endurance (Neder 2002). Two studies described muscle endurance as time to fatigue during a given task, in which high values were indicative of better endurance (Dang 2011; Vivodtzev 2012). However, one study provided incomplete data on this outcome (Dang 2011), and therefore neither a meta‐analysis nor narrative discussion of these data was possible. One study reported collecting measures of quadriceps endurance, but these measures were of functional performance, such as squat tests in which the participant was required to perform as many squats as possible in 30 seconds (Tasdemir 2015). Therefore, neither a meta‐analysis nor narrative discussion of these data were undertaken.
Five studies reported the assessment of thigh muscle size (Latimer 2013; Maddocks 2016; Vieira 2014; Vivodtzev 2006; Vivodtzev 2012). Maddocks 2016 obtained measures of rectus femoris cross‐sectional area by ultrasound and Vivodtzev 2012 obtained measures of mid‐thigh cross‐sectional area by computed tomography. Latimer 2013 obtained measures of thigh muscle mass by dual‐energy X‐ray absorptiometry (DEXA) and Vieira 2014 and Vivodtzev 2006 obtained measures of thigh circumference via anthropometry.
We collected mortality data as part of our assessment of serious adverse events. Twelve studies contributed mortality data (Abdellaoui 2011; Akinlabi 2013; Bourjeily‐Habr 2002; Dang 2011; Kucio 2016; Maddocks 2016; Neder 2002; Tardif 2015; Tasdemir 2015; Vieira 2014; Vivodtzev 2012; Zanotti 2003 ). For minor adverse events related specifically to the stimulation itself, all but five studies reported data (Akar 2017; Giavedoni 2012; Kucio 2016; Vieira 2014; Vivodtzev 2006).
The most common measure of assessment of exercise capacity was the 6MWD (Abdellaoui 2011; Akinlabi 2013; Dang 2011; Kucio 2016; Maddocks 2016; Tardif 2015; Vieira 2014; Vivodtzev 2006). Other field‐based walking tests were less common, such as the incremental shuttle walk test (ISWT) (Bourjeily‐Habr 2002; Tasdemir 2015), and ESWT (Tasdemir 2015; Vivodtzev 2012). Measures of exercise capacity also comprised peak rate oxygen consumption (VO2peak) expressed as litres per minute (Neder 2002; Vivodtzev 2012), millilitres per minute (Bourjeily‐Habr 2002; Vieira 2014), or millilitres per kilogram per minute (Dang 2011). However, one study that reported on VO2peak could not be included in the meta‐analyses (due to differences in the units of measurement) and narrative discussion was not possible as the data that were provided were incomplete (Dang 2011). Four studies expressed exercise capacity as peak power (Bourjeily‐Habr 2002; Dang 2011; Neder 2002; Tardif 2015), and two studies expressed time to symptom limitation cycling at a constant submaximal intensity (Neder 2002; Vieira 2014). However, two studies that reported peak power and compared NMES plus conventional exercise training with conventional exercise training alone provided incomplete data (Dang 2011; Tardif 2015). Therefore, neither a meta‐analysis nor a narrative discussion of these data was possible (Dang 2011; Tardif 2015).
Two studies reported functional performance giving the time required for the participants in both groups to achieve specific mobility milestones, such as sitting out of bed (Akar 2017; Zanotti 2003).
Six studies assessed symptoms and reported dyspnoea at the end of an exercise test or at iso‐time during an exercise test using the Borg 0 to 10 scale (Maddocks 2016; Neder 2002Tasdemir 2015; Vieira 2014; Vivodtzev 2006; Vivodtzev 2012). Five studies reported leg fatigue or leg effort at the end of an exercise test using the Borg 0 to 10 scale (Maddocks 2016; Neder 2002; Tasdemir 2015; Vieira 2014; Vivodtzev 2012), and one study reported general fatigue during daily life using the Fatigue Severity Scale (Tasdemir 2015). However, Maddocks 2016 reported dyspnoea and leg fatigue on completion of the 6MWT performed at baseline only (i.e. no postintervention period data reported) and therefore neither a meta‐analysis nor narrative discussion of these data was possible. Three studies reported dyspnoea during daily life using the dyspnoea domain of the Chronic Respiratory Disease Questionnaire (CRDQ) (Dang 2011; Maddocks 2016; Neder 2002), and one study used the Maugeri Foundation Respiratory Failure questionnaire (MRF‐28) (Vivodtzev 2006). However, one study that reported dyspnoea during daily life using the CRDQ presented these data at baseline only (i.e. no postintervention period data reported) and therefore neither a meta‐analysis nor narrative discussion of these data was possible (Maddocks 2016). Although two studies that compared NMES plus conventional exercise training with conventional exercise training alone reported dyspnoea during daily life (Dang 2011; Vivodtzev 2006), Vivodtzev 2006 did not report data in a way that could be included in a meta‐analysis and therefore a meta‐analysis for this outcome was not possible. Grades from the modified Medical Research Council Scale were not included in the assessment of dyspnoea, as this scale assesses functional limitation resulting from dyspnoea rather than the severity of dyspnoea itself.
Regarding the assessment of HRQoL, five studies used the St George's Respiratory Questionnaire (SGRQ) in which high values represented worse HRQoL (Akinlabi 2013; Maddocks 2016; Tardif 2015; Tasdemir 2015; Vieira 2014), three used the CRDQ in which high values represented better HRQoL (Dang 2011; Maddocks 2016; Neder 2002), and one used the MRF‐28 in which high values represented worse HRQoL (Vivodtzev 2006). However, one study did not provide data on total CRDQ scores and therefore neither a meta‐analysis nor narrative discussion of these data was possible (Neder 2002). As one study reported HRQoL using both the SGRQ and CRDQ, we used only data collected using the SGRQ in the meta‐analysis (Maddocks 2016).
Excluded studies
After the removal of duplicates and clinical trial registrations, we excluded 40 studies (56 records) with reasons provided in the Characteristics of excluded studies table. Common reasons for exclusion related to the: use of a cross‐over study design; provision of magnetic stimulation; stimulation of muscles that were not peripheral limb muscles; application of electrical stimulation as acu‐transcutaneous electrical nerve stimulation (acu‐TENS); lack of a suitable control group) or insufficient proportion of participants with COPD.
Risk of bias in included studies
See Figure 2 and Figure 3 for a summary of the risk of bias for the studies included in this review.
2.
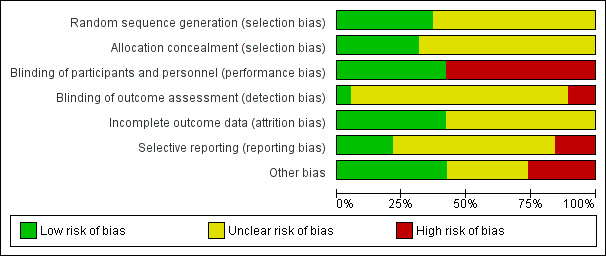
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
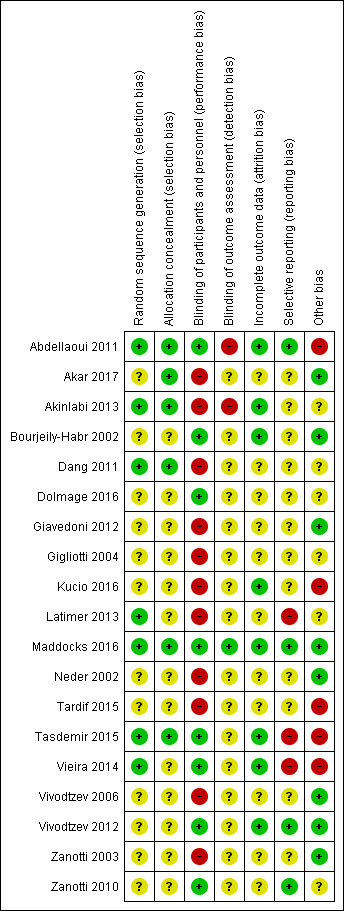
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Although all studies reported that participants were randomised to groups, 12 studies did not describe the method used to develop the randomisation sequence and we judged these studies at unclear risk of bias (Akar 2017; Bourjeily‐Habr 2002; Dolmage 2016; Giavedoni 2012; Gigliotti 2004; Kucio 2016; Neder 2002; Tardif 2015; Vivodtzev 2006; Vivodtzev 2012; Zanotti 2003; Zanotti 2010). We judged only studies that described concealment of the randomisation sequence as being at low risk of this bias (Abdellaoui 2011; Akar 2017; Akinlabi 2013; Dang 2011; Maddocks 2016; Tasdemir 2015).
Blinding
Eight studies utilised sham stimulation in the control group and we judged these at low risk of performance bias (Abdellaoui 2011; Bourjeily‐Habr 2002; Dolmage 2016; Maddocks 2016; Tasdemir 2015; Vieira 2014; Vivodtzev 2012; Zanotti 2010). Only one study reported using a blinded assessor to collect all outcome measures and therefore we judged this at low of risk detection bias (Maddocks 2016). Sixteen studies did not describe blinding procedures or used a blinded assessor to collect some, but not all, outcomes, and we judged these studies at unclear risk of bias (Akar 2017; Bourjeily‐Habr 2002; Dang 2011; Dolmage 2016; Giavedoni 2012; Gigliotti 2004; Kucio 2016; Latimer 2013; Neder 2002; Tardif 2015; Tasdemir 2015; Vieira 2014; Vivodtzev 2006; Vivodtzev 2012; Zanotti 2003; Zanotti 2010). We judged two studies that specifically stated that the outcome assessors were not blinded to group allocation as being at high risk of bias (Abdellaoui 2011; Akinlabi 2013).
Incomplete outcome data
Eleven studies provided insufficient information regarding loss to follow‐up and so we judged these studies at unclear risk of bias (Akar 2017; Dang 2011; Dolmage 2016; Giavedoni 2012; Gigliotti 2004; Latimer 2013; Neder 2002; Tardif 2015; Vivodtzev 2006; Zanotti 2003; Zanotti 2010). All other studies reported minimal loss to follow‐up and so we judged these at low risk of bias (Abdellaoui 2011; Akinlabi 2013; Bourjeily‐Habr 2002; Kucio 2016; Maddocks 2016; Tasdemir 2015; Vieira 2014; Vivodtzev 2012).
Selective reporting
We judged the 12 studies that were published without having registered a study protocol as being at an unclear risk of bias (Akar 2017; Akinlabi 2013; Bourjeily‐Habr 2002; Dang 2011; Dolmage 2016; Giavedoni 2012; Gigliotti 2004; Kucio 2016; Neder 2002; Tardif 2015; Vivodtzev 2006; Zanotti 2003). We judged studies that were reported in a way that was generally consistent with a previously registered study protocol at low risk of bias (Abdellaoui 2011; Maddocks 2016; Vivodtzev 2012; Zanotti 2010). We judged studies that were reported in a way that was inconsistent with a previously registered study protocol as being a high risk of bias (Latimer 2013; Tasdemir 2015; Vieira 2014).
Other potential sources of bias
Five studies had a high proportion of men (Abdellaoui 2011; Kucio 2016; Tardif 2015; Tasdemir 2015; Vieira 2014), which may have increased the likelihood of a positive result as men have been demonstrated to tolerate high levels of stimulation when compared with women (Giavedoni 2012; Maffiuletti 2008), and gains in response to NMES appear to be dependent on the ability for participants to tolerate progressively higher current intensities (Vivodtzev 2012). Therefore we judged these at high risk of bias (Abdellaoui 2011; Kucio 2016; Tardif 2015; Tasdemir 2015; Vieira 2014). Seven studies were published in abstract form only (Akinlabi 2013; Dang 2011; Dolmage 2016; Gigliotti 2004; Latimer 2013; Tardif 2015; Zanotti 2010). Although additional information was obtained from the authors of four of these studies (Akinlabi 2013; Dang 2011; Latimer 2013; Tardif 2015), we judged these at unclear or high risk of 'other' bias. Regarding other potential sources of error, one study reported change scores as the difference in medians (not means) and the inclusion of these data in the meta‐analyses may have introduced error to the estimates of the effect (Akinlabi 2013).
Effects of interventions
Summary of findings for the main comparison. NMES compared to usual care (with or without sham NMES) for COPD.
| NMES compared to usual care (with or without sham NMES) for COPD | ||||||
|
Patient or population: COPD Setting: generally outpatient or home Intervention: NMES Comparison: usual care (with or without sham NMES) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care (with or without sham NMES) | Risk with NMES | |||||
|
Peripheral muscle force assessed with: any method |
— | SMD 0.34 SD higher (0.02 higher to 0.65 higher) | — | 159 (6 RCTs) | ⊕⊕⊝⊝ Lowa | In real terms, using data available in 1 study that reported changes in quadriceps force in kg (Maddocks 2016), an SMD of 0.34 was equivalent to a difference in force of 3.1 kg (from a baseline mean force of 23.1 kg). |
|
Peripheral muscle endurance/fatigability assessed with: any method |
— | SMD 1.36 SD higher (0.59 higher to 2.12 higher) | — | 35 (2 RCTs) | ⊕⊕⊝⊝ Lowb | — |
| Thigh muscle size assessed with: any method | — | SMD 0.25 SD higher (0.11 lower to 0.61 higher) | — | 124 (4 RCTs) | ⊕⊕⊝⊝ Lowc | — |
|
Exercise capacity assessed with: 6MWD (m) |
The mean change in 6MWD in the control group ranged from ‐5.70 m to 0.80 m | MD 39.26 m more (16.31 more to 62.22 more) | — | 72 (2 RCTs) | ⊕⊕⊝⊝ Lowd | — |
|
Functional performance assessed with: time (days) until first sit out of bed |
None of the studies reported on functional performance. | |||||
|
Symptoms of dyspnoea reported on completion of an exercise test assessed with: Borg score |
The mean change in dyspnoea reported on completion of an exercise test ranged from ‐0.50 to 0.40 | MD 1.03 less dyspnoea (2.13 less to 0.06 more) | — | 55 (3 RCTs) | ⊕⊝⊝⊝ Very lowe | — |
|
Health‐related quality of life assessed with: SGRQ |
The mean change in HRQoL ranged from ‐2.00 to 0.07 | MD 4.12 better (12.60 better to 4.35 worse) | — | 72 (2 RCTs) | ⊕⊝⊝⊝ Very lowf | — |
|
Minor adverse events assessed: related to intervention only (e.g. redness) |
5970 per 100,000 | 0 per 100,000 (‐418 to 418) | RD 0.00 (‐0.07 to 0.07) | 139 (5 RCTs) | ⊕⊕⊝⊝ Lowg | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 6MWD: 6‐minute walk distance; CI: confidence interval; COPD: chronic obstructive pulmonary disease; MD: mean difference; NMES: neuromuscular electrical stimulation; RCT: randomised controlled trials; RD: risk difference; SD: standard deviation; SGRQ: Saint George's Respiratory Questionnaire; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to risk of bias (three studies did not use sham stimulation) and one level due to imprecision (wide confidence intervals).
bDowngraded one level due to risk of bias (one study did not use sham stimulation) and one level due to small number of studies available for analyses.
cDowngraded one level due to risk of bias (one study did not use sham stimulation) and one level due imprecision (wide confidence intervals).
dDowngraded one level due to small number of studies available for analyses and one level due imprecision (wide confidence intervals).
eDowngraded one level due to risk of bias (one study did not use sham stimulation), one level for imprecision (wide confidence intervals) and one level for inconsistency.
fDowngraded one level due to small number of studies available for analyses, one level for imprecision (wide confidence intervals) and one level due to inconsistent findings.
gDowngraded one level due to risk of bias (two studies did not use sham stimulation) and one level for inconsistent findings.
Summary of findings 2. NMES and exercise compared to exercise (with or without sham NMES) for COPD.
| NMES and exercise compared to exercise (with or without sham NMES) for COPD | ||||||
|
Patient or population: COPD Setting: intensive care unit, inpatient rehabilitation, outpatient or home Intervention: NMES + exercise Comparison: exercise (with or without sham NMES) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with exercise (with or without sham NMES) | Risk with NMES and exercise | |||||
|
Peripheral muscle force assessed with: any method |
— | SMD 0.47 SD higher (‐0.10 higher to 1.04 higher) | — | 84 (4 RCTs) | ⊕⊝⊝⊝ Very lowa | — |
|
Peripheral muscle endurance/fatigability assessed with: any method |
— | — | — | — | — | None of the studies reported peripheral muscle endurance/fatigability. |
| Thigh muscle size assessed with: any method | — | — | — | — | — | None of the studies reported thigh muscle size. |
|
Exercise capacity assessed with: 6MWD (m) |
The mean change in 6MWD ranged from 10.30 m to 94.00 m | MD 25.87 m more (1.06 more to 50.69 more) | — | 138 (6 RCTs) | ⊕⊝⊝⊝ Very lowb | — |
|
Functional performance assessed with: time (days) until first sit out of bed |
The mean time until first sit out of bed ranged from 12.60 to 14.33 days | MD 4.98 fewer days (8.55 to 1.41 fewer) | — | 44 (2 RCTs) | ⊕⊝⊝⊝ Very lowc | — |
|
Symptoms of dyspnoea reported on completion of an exercise test assessed with: Borg score |
The mean change in dyspnoea reported on completion of an exercise test ranged from ‐0.62 units to 1.00 units | MD 0.44 less dyspnoea (2.27 less to 1.38 more) | — | 44 (2 RCTs) | ⊕⊝⊝⊝ Very lowd | — |
|
Health‐related quality of life assessed with: any validated questionnaire |
— | SMD 0.56 SD better (1.27 better to 0.15 worse) | — | 122 (5 RCTs) | ⊕⊝⊝⊝ Very lowe | — |
| Minor adverse events assessed: related to intervention only (e.g. redness) | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 144 (6 RCTs) | ⊕⊕⊝⊝ Lowf |
— |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 6MWD: 6‐minute walk distance; CI: confidence interval;COPD: chronic obstructive pulmonary disease; MD: mean difference; NMES: neuromuscular electrical stimulation; RCT: randomised controlled trials; SD: standard deviation; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to risk of bias (three studies did not use sham stimulation), one level due to imprecision (wide confidence intervals) and one level due to inconsistent findings.
bDowngraded one level due to risk of bias (five studies did not use sham stimulation), one level due to imprecision (wide confidence intervals) and one level due to inconsistent findings.
cDowngraded one level due to risk of bias (neither study used sham stimulation), one level due imprecision (wide confidence intervals) and one level due to small number of studies available for analyses.
dDowngraded one level due to risk of bias (one study did not use sham stimulation), one level due imprecision (wide confidence intervals) and small number of studies available for analyses, and one level for inconsistent findings.
eDowngraded one level due to risk of bias (four studies did not use sham stimulation), one level for imprecision (wide confidence intervals) and one level for inconsistency.
fDowngraded one level due to risk of bias (four studies did not use sham stimulation) and one level for imprecision (wide confidence intervals).
Primary outcomes
Peripheral muscle force
Six studies compared NMES with usual care and reported on measures of quadriceps muscle force (Bourjeily‐Habr 2002; Giavedoni 2012; Latimer 2013; Maddocks 2016; Neder 2002; Vivodtzev 2012). Meta‐analysis of these studies demonstrated a significant effect, however, the CIs were wide (SMD 0.34, 95% CI 0.02 to 0.65; participants = 159; low‐quality evidence; Analysis 1.1; Figure 4). One study included in this meta‐analysis also assessed quadriceps twitch force elicited in response to stimulation of the femoral nerve and demonstrated no significant between‐group difference (Maddocks 2016). As the meta‐analysis included the two studies that randomly assigned one leg to receive NMES and the other leg to receive control (Giavedoni 2012; Latimer 2013), we conducted a sensitivity analysis that excluded these studies. For this sensitivity analysis, the SMD from the remaining four studies was 0.39 (95% CI ‐0.00 to 0.78) (Bourjeily‐Habr 2002; Maddocks 2016; Neder 2002; Vivodtzev 2012). Regarding subgroup analyses based on whether or not the participants were clinically stable at the time of recruitment, of the six studies that compared NMES with usual care, only one recruited participants during a period of exacerbation (Giavedoni 2012). Therefore, rather than performing subgroup analyses based on the clinical stability of participants at the time of recruitment, we undertook a sensitivity analysis that excluded the study that recruited people during a period of exacerbation. The SMD for this subgroup analysis that included only participants with COPD who recruited during a period of clinical stability was 0.34 (95% CI 0.00 to 0.68) (Bourjeily‐Habr 2002; Latimer 2013; Maddocks 2016; Neder 2002; Vivodtzev 2012).
1.1. Analysis.
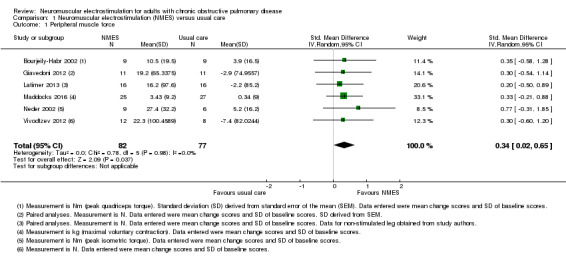
Comparison 1 Neuromuscular electrostimulation (NMES) versus usual care, Outcome 1 Peripheral muscle force.
4.
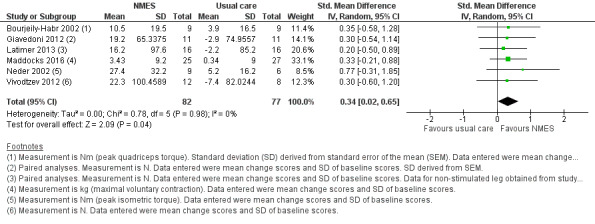
Forest plot of comparison: 1 Neuromuscular electrostimulation (NMES) versus usual care, outcome: 1.1 Peripheral muscle force.
One study reported a significant increase in hamstring force following NMES that was greater than any seen in the control group (Bourjeily‐Habr 2002). One additional study, published as an abstract with a small number of participants, that could not be included in the meta‐analysis, compared the effect of two NMES training protocols on quadriceps force (Dolmage 2016). This study suggested that the gains in muscle force may be greater following an NMES training programme designed to increase strength (i.e. high‐frequency, low‐duty cycle) when compared with an NMES training protocol designed to increase endurance (i.e. low ‐frequency, high‐duty cycle) (Dolmage 2016). There were insufficient studies to undertake planned subgroup analyses based on stimulation frequency, disease severity, method used to assess muscle force or number of training sessions.
Six studies compared NMES plus conventional exercise training with conventional exercise training alone and reported measures of quadriceps muscle force (Abdellaoui 2011; Dang 2011; Tasdemir 2015; Vivodtzev 2006), or used manual muscle testing to report on peripheral muscle strength (which was likely to include measures of quadriceps muscle force) (Akar 2017; Zanotti 2003). However, data from two studies reported data as median and interquartile range or minimum and maximum values, and therefore were not included in the meta‐analyses (Abdellaoui 2011; Akar 2017). Meta‐analysis of the remaining four studies produced an uncertain effect with an SMD of 0.47 (95% CI ‐0.10 to 1.04; participants = 84; very low‐quality evidence; Analysis 2.1; Figure 5). Regarding the subgroup analyses based on whether or not the method used to measure muscle force was deemed to be robust, two studies used robust methods (Dang 2011; Vivodtzev 2006), and the SMD for these studies was 0.44 (95% CI ‐0.25 to 1.14; participants = 33) and two studies used less robust methods (Tasdemir 2015; Zanotti 2003), and the SMD for these studies was 0.53 (95% CI ‐0.74 to 1.80; participants = 51; Analysis 2.2; Figure 6). The SMD for these subgroups was not different (Chi2 = 0.01; P = 0.91). Only the analysis for muscle force assessed using less robust methods had high statistical heterogeneity (I2 = 79%). Regarding subgroup analyses based on the minimum number of training sessions, of the four studies, only one completed fewer than 10 sessions in four weeks (Tasdemir 2015). Therefore, rather than performing a subgroup analysis based on the minimum number of training sessions, we undertook a sensitivity analysis that excluded the study where participants completed fewer than 10 sessions over four weeks. For this sensitivity analysis, the SMD from the remaining three studies was 0.73 (95% CI 0.19 to 1.28; participants = 57; Analysis 2.3) (Dang 2011; Vivodtzev 2006; Zanotti 2003). One study also undertook manual muscle testing of the upper limbs; however, it was unclear whether the improvement in this measure was different between the groups (Akar 2017). There were insufficient studies to undertake planned subgroup analyses based on clinical stability at the time of recruitment, stimulation frequency or disease severity.
2.1. Analysis.
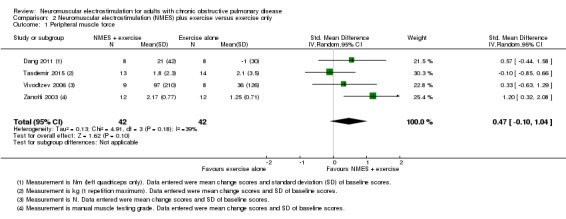
Comparison 2 Neuromuscular electrostimulation (NMES) plus exercise versus exercise only, Outcome 1 Peripheral muscle force.
5.
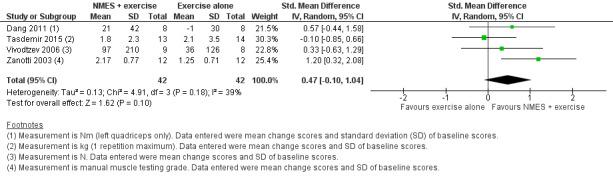
Forest plot of comparison: 2 Neuromuscular electrostimulation (NMES) plus exercise versus exercise only, outcome: 2.1 Peripheral muscle force.
2.2. Analysis.
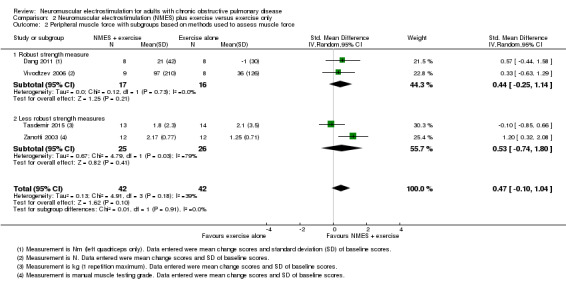
Comparison 2 Neuromuscular electrostimulation (NMES) plus exercise versus exercise only, Outcome 2 Peripheral muscle force with subgroups based on methods used to assess muscle force.
6.
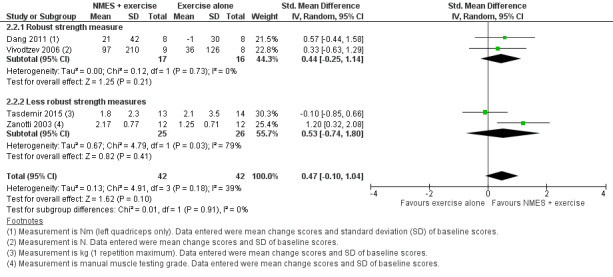
Forest plot of comparison: 2 Neuromuscular electrostimulation (NMES) plus exercise versus exercise only, outcome: 2.2 Peripheral muscle force with subgroups based on methods used to assess muscle force.
2.3. Analysis.
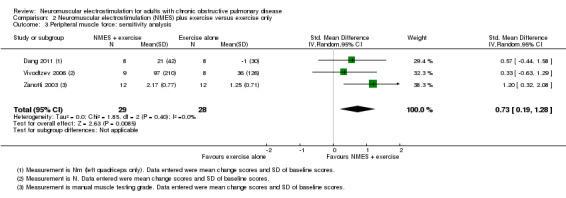
Comparison 2 Neuromuscular electrostimulation (NMES) plus exercise versus exercise only, Outcome 3 Peripheral muscle force: sensitivity analysis.
Peripheral muscle endurance/fatigability
Two studies compared NMES with usual care, and reported on measures of endurance of the quadriceps (Neder 2002; Vivodtzev 2012). Meta‐analysis of these studies demonstrated a significant effect in favour of NMES (SMD 1.36, 95% CI 0.59 to 2.12; participants = 35; low‐quality evidence; Analysis 1.2). One additional study, published as an abstract with a small numbers of participants, that could not be included in the meta‐analysis, compared the effect of two NMES training protocols on quadriceps endurance (Dolmage 2016). This study suggested that the gains in endurance may have been greater following an NMES training programme designed to increase endurance (i.e. low‐frequency, high‐duty cycle) when compared with an NMES training protocol designed the increase strength (i.e. high‐frequency, low‐duty cycle) (Dolmage 2016). There were insufficient studies to undertake planned subgroup analyses based on clinical stability at the time of recruitment, stimulation frequency, disease severity or the number of training sessions.
1.2. Analysis.
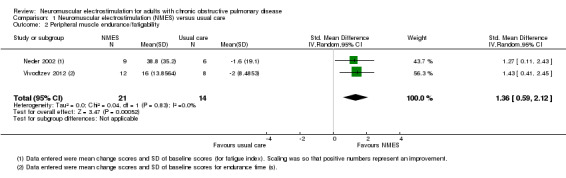
Comparison 1 Neuromuscular electrostimulation (NMES) versus usual care, Outcome 2 Peripheral muscle endurance/fatigability.
No studies compared NMES plus conventional exercise training with conventional exercise training alone, and reported on measures of quadriceps endurance.
Thigh muscle size
Four studies compared NMES with usual care, and reported on measures of thigh muscle size (Latimer 2013; Maddocks 2016; Vieira 2014; Vivodtzev 2012). Meta‐analysis of these studies produced an uncertain effect (SMD 0.25, 95% CI ‐0.11 to 0.61; participants = 124; low‐quality evidence; Analysis 1.3). As this analysis included the one study that randomly assigned one leg to receive NMES and the other leg to receive control (Latimer 2013), we conducted a sensitivity analysis that excluded this study. For this sensitivity analysis, the SMD from the remaining three studies was 0.32 (95% CI ‐0.09 to 0.74; participants = 92) (Maddocks 2016; Vieira 2014; Vivodtzev 2012). There were insufficient studies to undertake planned subgroup analyses based on clinical stability of the participants at the time of recruitment, stimulation frequency, disease severity or the number of training sessions.
1.3. Analysis.
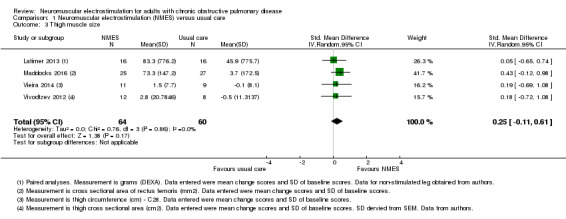
Comparison 1 Neuromuscular electrostimulation (NMES) versus usual care, Outcome 3 Thigh muscle size.
The one study that compared NMES plus conventional exercise training with conventional exercise training alone, and reported on measures of thigh circumference demonstrated no between‐group difference in this outcome (Vivodtzev 2006).
Serious adverse events
Although six studies compared NMES with usual care, and reported data on mortality (Bourjeily‐Habr 2002; Latimer 2013; Maddocks 2016; Neder 2002; Vieira 2014; Vivodtzev 2012), we excluded data from the study that randomly assigned one leg to receive NMES and the other leg to receive control (Latimer 2013). Meta‐analysis of the remaining five studies demonstrated no risk difference between groups (RD ‐0.02, 95% CI ‐0.08 to 0.05; participants = 131; Analysis 1.4). These studies were undertaken in participants who were clinically stable at the time of recruitment.
1.4. Analysis.
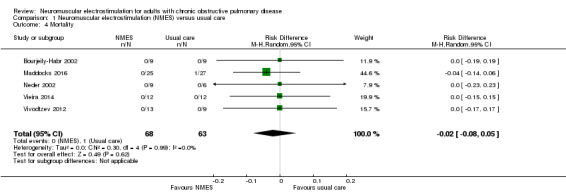
Comparison 1 Neuromuscular electrostimulation (NMES) versus usual care, Outcome 4 Mortality.
Seven studies compared NMES plus conventional exercise training with conventional exercise training alone, and provided information on mortality (Abdellaoui 2011; Akinlabi 2013; Dang 2011; Kucio 2016; Tardif 2015; Tasdemir 2015; Zanotti 2003). Meta‐analysis of these studies demonstrated no risk difference between groups (RD 0.00, 95% CI ‐0.05 to 0.05; participants = 183; Analysis 2.4).
2.4. Analysis.
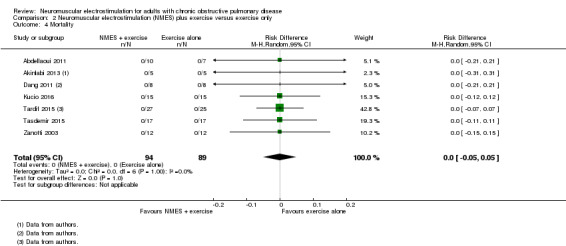
Comparison 2 Neuromuscular electrostimulation (NMES) plus exercise versus exercise only, Outcome 4 Mortality.
Secondary outcomes
Exercise capacity
Two studies compared NMES with usual care, and reported on measures of 6MWD (Maddocks 2016; Vieira 2014). Meta‐analysis of these studies demonstrated a significant effect (MD 39.26 m, 95% CI 16.31 to 62.22; low‐quality evidence; participants = 72; Analysis 1.5). Both studies were undertaken in participants who were clinically stable at the time of recruitment. There were insufficient studies to undertake planned subgroup analyses for the effect on 6MWD based on clinical stability of the participants at the time of recruitment, stimulation frequency, disease severity or the number of training sessions. The one study that reported on measures of incremental shuttle walk distance (ISWD) demonstrated a significant between‐group increase in favour of NMES (Bourjeily‐Habr 2002). Four studies reported on measures of VO2peak (Bourjeily‐Habr 2002; Neder 2002; Vieira 2014; Vivodtzev 2012), and meta‐analysis of these studies demonstrated a significant effect in favour of NMES (MD 0.10 L/min, 95% CI 0.00 to 0.19; participants = 73; Analysis 1.6). These studies were undertaken in participants who were clinically stable at the time of recruitment. There were insufficient studies to undertake planned subgroup analyses for the effect on VO2peak based on clinical stability of the participants at the time of recruitment, stimulation frequency, disease severity or the number of training sessions. Two studies reported on measures of peak power (Bourjeily‐Habr 2002; Neder 2002), and meta‐analysis of these studies produced an uncertain effect (MD 5.77 W, 95% CI ‐6.00 to 17.53; participants = 33; Analysis 1.7). Both studies were undertaken in participants who were clinically stable at the time of recruitment. There were insufficient studies to undertake planned subgroup analyses for the effect on peak power based on clinical stability of the participants at the time of recruitment, stimulation frequency, disease severity or the number of training sessions. Three studies reported on endurance time during a constant power test on a bike (Neder 2002; Vieira 2014), or on the ESWT (Vivodtzev 2012), and meta‐analysis of these studies demonstrated a significant effect (MD 3.62 minutes, 95% CI 2.33 to 4.91; participants = 55; Analysis 1.8). These studies were undertaken in participants who were clinically stable at the time of recruitment. There were insufficient studies to undertake planned subgroup analyses for the effect on endurance time based on clinical stability of the participants at the time of recruitment, stimulation frequency, disease severity or the number of training sessions.
1.5. Analysis.
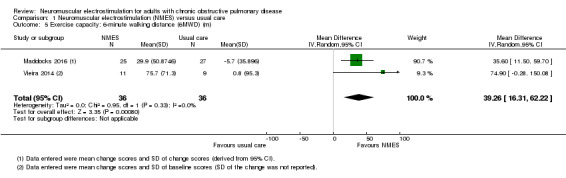
Comparison 1 Neuromuscular electrostimulation (NMES) versus usual care, Outcome 5 Exercise capacity: 6‐minute walking distance (6MWD) (m).
1.6. Analysis.
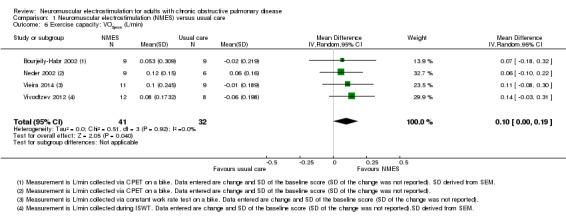
Comparison 1 Neuromuscular electrostimulation (NMES) versus usual care, Outcome 6 Exercise capacity: VO2peak (L/min).
1.7. Analysis.
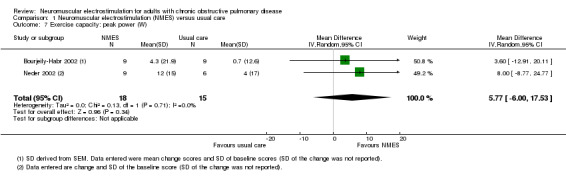
Comparison 1 Neuromuscular electrostimulation (NMES) versus usual care, Outcome 7 Exercise capacity: peak power (W).
1.8. Analysis.
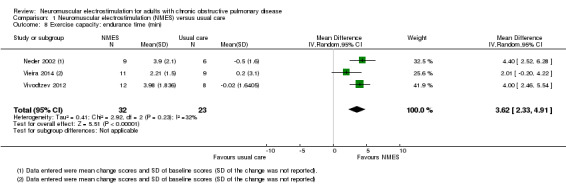
Comparison 1 Neuromuscular electrostimulation (NMES) versus usual care, Outcome 8 Exercise capacity: endurance time (min).
Six studies compared NMES plus conventional exercise training with conventional exercise training alone, and reported on measures of 6MWD (Abdellaoui 2011; Akinlabi 2013; Dang 2011; Kucio 2016; Tardif 2015; Vivodtzev 2006). Meta‐analysis of these studies demonstrated a significant effect (MD 25.87 m, 95% CI 1.06 to 50.69; participants = 138; very low‐quality evidence; Analysis 2.5). Regarding subgroup analyses based on whether or not the participants were clinically stable at the time of recruitment, of the six studies that compared NMES plus conventional exercise training with conventional exercise training alone, only one recruited participants during a period of exacerbation (Abdellaoui 2011). Therefore, rather than perform a subgroup analysis based on clinical stability, we undertook a sensitivity analysis that excluded the study that recruited participants during a period of exacerbation. The MD for this meta‐analysis that included only people with COPD who were clinically stable at the time of recruitment was 17.80 m (95% CI ‐6.81 to 42.41; participants = 123) (Akinlabi 2013; Dang 2011; Kucio 2016; Tardif 2015; Vivodtzev 2006). Regarding subgroup analyses based on the minimum number of training sessions, of the six studies, only one completed fewer than 10 sessions in four weeks (Akinlabi 2013). Therefore, rather than performing a subgroup analysis based on the minimum number of training sessions, we undertook a sensitivity analysis that excluded the study in which participants completed fewer than 10 sessions over four weeks. For this sensitivity analysis, the MD from the remaining five studies was 25.86 m (95% CI ‐3.17 to 54.89; participants = 128; Analysis 2.6) (Abdellaoui 2011; Dang 2011; Kucio 2016; Tardif 2015; Vivodtzev 2006). There were insufficient studies to undertake planned subgroup analyses for the effect on 6MWD based on stimulation frequency or disease severity. The one study that reported on measures of exercise capacity expressed as ISWD and performance on the ESWT demonstrated a significant between‐group difference in favour of NMES plus conventional exercise training on ISWD, but not performance on the ESWT (expressed in seconds) (Tasdemir 2015).
2.5. Analysis.
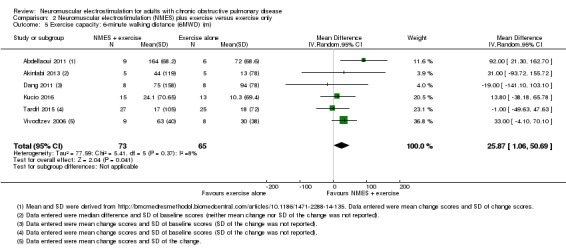
Comparison 2 Neuromuscular electrostimulation (NMES) plus exercise versus exercise only, Outcome 5 Exercise capacity: 6‐minute walking distance (6MWD) (m).
2.6. Analysis.
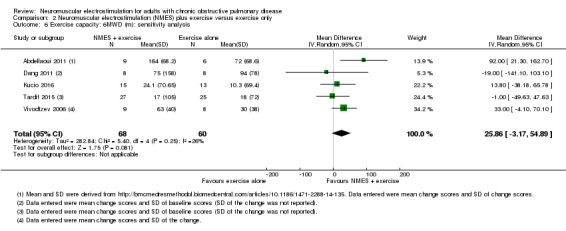
Comparison 2 Neuromuscular electrostimulation (NMES) plus exercise versus exercise only, Outcome 6 Exercise capacity: 6MWD (m): sensitivity analysis.
Functional performance
None of the studies comparing NMES with usual care reported on measures of functional performance.
Two studies compared NMES plus conventional exercise training with conventional exercise training alone (Akar 2017; Zanotti 2003), and reported on the same measure of functional performance; number of days between randomisation and when the participant first transferred out of bed. Meta‐analysis of these studies demonstrated a significant effect on when the participant first transferred out of bed in favour of NMES plus conventional exercise training (MD ‐4.98 days, 95% CI ‐8.55 to ‐1.41; participants = 44; very low‐quality evidence; Analysis 2.7). Although both studies included in this meta‐analysis reported the same direction of effect, this analysis had high statistical heterogeneity (I2 = 60%). One of these studies was undertaken in participants who were clinically stable at the time of recruitment (Zanotti 2003), and the other was undertaken in participants who were experiencing an exacerbation at the time of recruitment (Akar 2017).
2.7. Analysis.
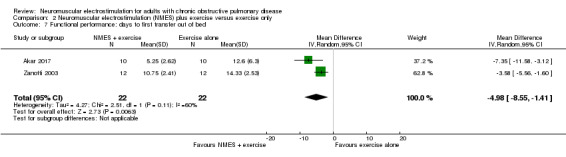
Comparison 2 Neuromuscular electrostimulation (NMES) plus exercise versus exercise only, Outcome 7 Functional performance: days to first transfer out of bed.
Symptoms of dyspnoea and fatigue
Three studies compared NMES with usual care, and reported on measures of dyspnoea reported on completion of a symptom‐limited exercise test (Neder 2002; Vieira 2014; Vivodtzev 2012). Meta‐analysis of these studies produced an uncertain effect (MD ‐1.03 units, 95% CI ‐2.13 to 0.06; participants = 55; very low‐quality evidence; Analysis 1.9). This analysis had high statistical heterogeneity (I2 = 59%). All studies were undertaken in participants who were clinically stable at the time of recruitment. The one study that reported on dyspnoea at iso‐time during the ESWT demonstrated no between‐group difference (Vivodtzev 2012). The one study that reported on measures of dyspnoea during daily life using the CRDQ demonstrated a significant between‐group difference in favour of NMES (Neder 2002). Three studies compared NMES with usual care, and reported on measures of leg fatigue reported on completion of an exercise test (Neder 2002; Vieira 2014; Vivodtzev 2006). Meta‐analysis of these studies demonstrated a significant effect (MD ‐1.12 units, 95% CI ‐1.81 to ‐0.43; participants = 55; Analysis 1.10). These studies were undertaken in participants who were clinically stable at the time of recruitment.
1.9. Analysis.
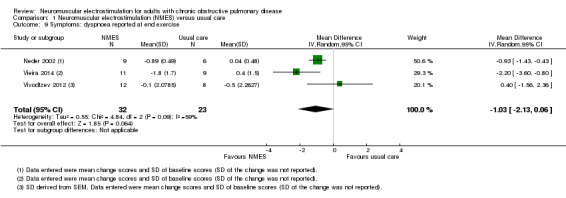
Comparison 1 Neuromuscular electrostimulation (NMES) versus usual care, Outcome 9 Symptoms: dyspnoea reported at end exercise.
1.10. Analysis.
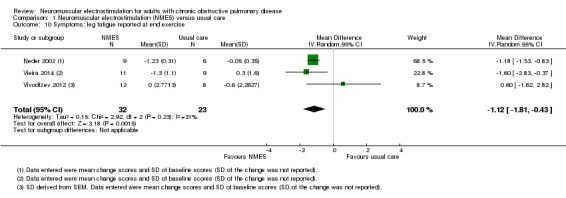
Comparison 1 Neuromuscular electrostimulation (NMES) versus usual care, Outcome 10 Symptoms: leg fatigue reported at end exercise.
Two studies compared NMES plus conventional exercise training with conventional exercise training alone (Tasdemir 2015; Vivodtzev 2006), and reported on measures of dyspnoea reported on completion of an exercise test. Meta‐analysis of these studies produced an uncertain effect (MD ‐0.44 units, 95% CI ‐2.27 to 1.38; participants = 44; very low‐quality evidence; Analysis 2.8). This analysis had high statistical heterogeneity (I2 = 69%). Both studies were undertaken in participants who were clinically stable at the time of recruitment. Two studies compared NMES plus conventional exercise training with conventional exercise training alone on measures of dyspnoea during daily life (Dang 2011; Vivodtzev 2006). However, for one study, it was unclear whether the improvement in this measure was different between the groups (Dang 2011), and the between‐group difference reported in the other study was of borderline significance (P = 0.05) (Vivodtzev 2006). The one study that compared NMES plus conventional exercise training with conventional exercise training alone and reported on measures of leg fatigue on completion of an exercise test and general fatigue during daily life using the Fatigue Severity Scale demonstrated no between‐group differences in either of these outcomes (Tasdemir 2015).
2.8. Analysis.
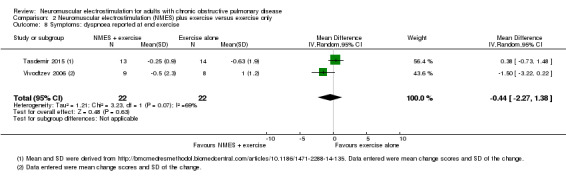
Comparison 2 Neuromuscular electrostimulation (NMES) plus exercise versus exercise only, Outcome 8 Symptoms: dyspnoea reported at end exercise.
Health‐related quality of life
Two studies compared NMES with usual care, and reported on measures of HRQoL measured using the SGRQ (Maddocks 2016; Vieira 2014). Meta‐analysis of these studies produced an uncertain effect (MD ‐4.12 %points, 95% CI ‐12.60 to 4.35; participants = 72; very low‐quality evidence; Analysis 1.11). This analysis had high statistical heterogeneity (I2 = 74%). One of these studies also demonstrated no between‐group difference in HRQoL assessed using the CRDQ (Maddocks 2016). These studies were undertaken in participants who were clinically stable at the time of recruitment.
1.11. Analysis.
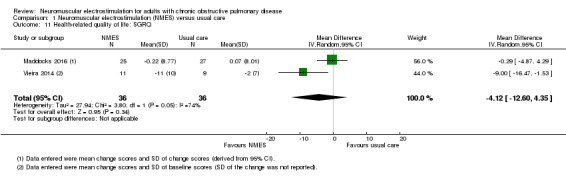
Comparison 1 Neuromuscular electrostimulation (NMES) versus usual care, Outcome 11 Health‐related quality of life: SGRQ.
Of the five studies that compared NMES plus conventional exercise training with conventional exercise training alone and reported on measures of HRQoL, three used the SGRQ (Akinlabi 2013; Tardif 2015; Tasdemir 2015), one used the CRDQ (Dang 2011), and one used the MRF‐28 (Vivodtzev 2006). Data from one study was reported as median and minimum and maximum and therefore were not included in the meta‐analysis (Tasdemir 2015). Meta‐analysis of the remaining four studies produced an uncertain effect (SMD ‐0.56, 95% CI ‐1.27 to 0.15; participants = 95; very low‐quality evidence; Analysis 2.9). This analysis had high statistical heterogeneity (I2 = 55%). These studies were undertaken in participants who were clinically stable at the time of recruitment.
2.9. Analysis.
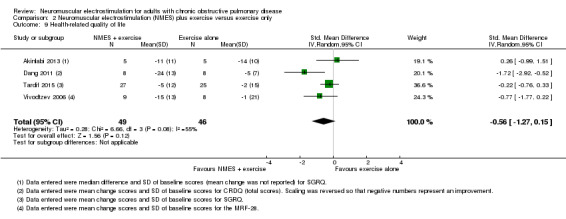
Comparison 2 Neuromuscular electrostimulation (NMES) plus exercise versus exercise only, Outcome 9 Health‐related quality of life.
Minor adverse events
Five studies compared NMES with usual care, and reported data on minor adverse events related to the intervention (Bourjeily‐Habr 2002; Latimer 2013; Maddocks 2016; Neder 2002; Vivodtzev 2012). Meta‐analysis of these studies demonstrated no risk difference between groups; however, there was inconsistency between individual studies (RD 0.00, 95% CI ‐0.07 to 0.07; participants = 139; low‐quality evidence; Analysis 1.12).
1.12. Analysis.
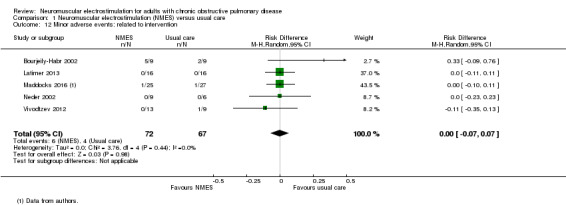
Comparison 1 Neuromuscular electrostimulation (NMES) versus usual care, Outcome 12 Minor adverse events: related to intervention.
Six studies compared NMES plus conventional exercise training with conventional exercise training alone and reported data on minor adverse events related to the intervention (Abdellaoui 2011; Akinlabi 2013; Dang 2011; Tardif 2015; Tasdemir 2015; Zanotti 2003). Meta‐analysis of these studies demonstrated no risk difference between groups (RD 0.00, 95% CI ‐0.05 to 0.05; participants = 144; low‐quality evidence; Analysis 2.10). Of the six studies that compared NMES plus conventional exercise training with conventional exercise training alone, only one recruited participants during a period of exacerbation (Abdellaoui 2011).
2.10. Analysis.
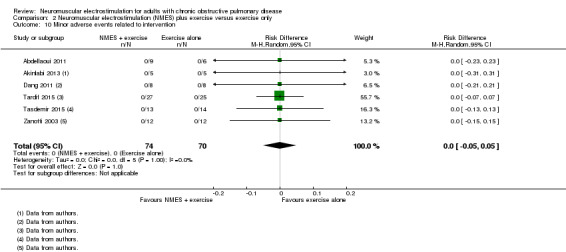
Comparison 2 Neuromuscular electrostimulation (NMES) plus exercise versus exercise only, Outcome 10 Minor adverse events related to intervention.
Discussion
Summary of main results
This review demonstrated an increase in quadriceps force following a programme of NMES applied in isolation, with an SMD of 0.34 (95% CI 0.02 to 0.65), suggesting a small to moderate effect (Cohen 1988). In real terms, using data available in one study that reported changes in quadriceps force in kilograms (Maddocks 2016), an SMD of 0.34 was equivalent to a difference in force of 3.1 kg (from a baseline mean force of 23.1 kg). Sensitivity analyses had minimal influence on the size of the SMD.
There was no increase in peripheral muscle force for those studies that applied NMES plus conventional exercise training (SMD 0.47, 95% CI ‐0.10 to 1.04). Subgroup analyses suggested that the size of the SMD was not influenced by the method used to quantify peripheral muscle force. Sensitivity analyses suggested that the effect of NMES on peripheral muscle force may have been influenced by the training dose applied. This is because the removal of the one study (Tasdemir 2015), which provided fewer than 10 training sessions over four weeks, resulted in a significant increase in the SMD to 0.73 (95% CI 0.19 to 1.28).
This review demonstrated that a programme of NMES applied in isolation produced a large increase in quadriceps muscle endurance, but no change in thigh muscle size. There were insufficient data to undertake a meta‐analysis of studies that explored the effect of applying NMES plus conventional exercise training on peripheral muscle endurance or thigh muscle size.
Regarding adverse events, there was no risk difference for mortality for participants who received NMES either in isolation or together with conventional exercise training. For the studies that reported data on mortality, of the 314 participants, there was only one death and this occurred in a control group participant who did not receive NMES (Maddocks 2016).
Regarding exercise capacity, studies that applied NMES in isolation demonstrated a small increase of VO2peak that was of borderline significance (MD 0.10 L/min, 95% CI 0.00 to 0.19). However, these studies demonstrated an increase in 6MWD that was statistically significant and exceeded the threshold for clinical importance suggested by Holland 2010 (MD 39.26 m, 95% CI 16.31 to 62.22). Further, an increase was also noted in the time to symptom limitation exercising at a submaximal intensity (i.e. endurance time). For studies that applied NMES plus conventional exercise training, there was a small increase in 6MWD, which was equivalent to the threshold for clinical importance (MD 25.87 m, 95% CI 1.06 to 50.69). This effect on 6MWD was no longer significant when the study that recruited participants during a period of exacerbation was excluded from the meta‐analysis (Abdellaoui 2011).
Regarding functional performance, there were insufficient data to undertake a meta‐analysis of studies that explored the effect of applying NMES in isolation. Studies that applied NMES plus conventional exercise training in people who were profoundly debilitated (i.e. either in an ICU or a respiratory high dependency unit following a prolonged admission to an ICU), demonstrated a reduction in the time taken to first sit out of bed by 4.98 days (95% CI ‐8.55 to ‐1.41). However, confidence in this result was reduced due to the high level of heterogeneity in this analysis (I2 = 60%). In addition to the gains in peripheral muscle function, improvements in exercise capacity and functional performance following NMES may also relate to the possible systemic effects of this intervention, such as increased microcirculation and heart rate response (Gerovasili 2009), and contralateral leg muscle facilitation (Hortobágyi 1999). Regarding symptoms measured on completion of exercise testing, studies that applied NMES in isolation demonstrated no difference in the severity of dyspnoea (MD ‐1.03, 95% CI ‐2.13 to 0.06). However, this analysis had high statistical heterogeneity (I2 = 59%). These studies demonstrated a decrease in the severity of leg fatigue on completion of exercise testing (MD ‐1.12 units, 95% CI ‐1.81 to ‐0.43). For studies that applied NMES plus conventional exercise training, there was no evidence of a decrease in the severity of dyspnoea on completion of exercise testing (MD ‐0.44, 95% CI ‐2.27 to 1.38). This analysis had high statistical heterogeneity (I2 = 69%).
Regarding HRQoL, studies that applied NMES in isolation demonstrated no difference in HRQoL, measured using the SGRQ. This analysis had high statistical heterogeneity (I2 = 74%). Similarly, studies that applied NMES plus conventional exercise training demonstrated no difference in HRQoL, measured using the SGRQ, CRDQ and MRF‐28.
Regarding minor adverse events related to the tolerance of the NMES, there was no risk difference for NMES when applied in isolation or together with conventional exercise training. For the studies that reported minor adverse events, data were available on 283 participants. Of these, six participants who received NMES and four participants who received sham NMES reported minor events.
Many of the planned subgroup analyses were not possible due to the limited number of studies available for inclusion in this review.
Overall completeness and applicability of evidence
Studies included in this review were undertaken at single centres and recruited modest sample sizes (fewer than 30 per group). Of the 16 studies included in the meta‐analyses, four were published only in abstract form. Several studies provided NMES in the home with little, if any, supervision. Therefore, poor adherence with NMES may have dampened the estimate of the effect of this intervention. Nevertheless, in clinical practice, offering NMES in the home is likely to be the most feasible and inexpensive approach and the inclusion of studies that described home‐based NMES increases the likelihood that the results of this review reflect the 'real life' effects of the intervention. In contrast to earlier reviews, we separated those studies that explored the effect of NMES applied in isolation (i.e. versus usual care) and those that explore the effect of adding NMES to a programme of conventional exercise training. In this way, the results of this review will assist clinicians to appreciate the possible effects of NMES applied in isolation from other rehabilitation strategies, as well as offering it as an adjunct to conventional exercise training.
Quality of the evidence
Using the GRADE approach, the quality of the evidence for most outcomes was low or very low. This was due, at least in part, to the risk of bias, especially detection and performance bias in most studies. Of note, only one study described blinding procedures for both participants and outcome assessors for every outcome (Maddocks 2016). Although in studies of interventions such as NMES, it is not possible to blind the person administering the intervention, the use of sham stimulation in the control group is critical to reduce the risk of performance bias, especially considering most outcomes reported in these studies were effort‐dependent or participant‐reported. For many of the meta‐analyses undertaken in this review, data were combined across only two or three studies, and the results of these studies were inconsistent, resulting in low levels of precision for our estimate of the effect. For outcomes related to functional performance, symptoms and HRQoL, most meta‐analyses showed high levels of heterogeneity. Finally, it is possible that the results of this review were influenced by the over‐representation of men in five studies (Abdellaoui 2011; Kucio 2016; Tardif 2015; Tasdemir 2015; Vieira 2014). Earlier work has demonstrated that men tolerate high levels of stimulation when compared with women (Giavedoni 2012; Maffiuletti 2008), and this may optimise the gains made in response to NMES (Vivodtzev 2012).
Potential biases in the review process
It is possible that our estimate of the effect of NMES on peripheral muscle force was influenced by including the study that measured 'peripheral muscle strength' using manual muscle testing (Zanotti 2003). We included this study because this review planned, a priori, to explore the effect of NMES on peripheral muscle force. Further, although it was not clear which muscles were tested in this study, it seemed reasonable to assume that these assessments included quadriceps force, as this was the muscle that was stimulated. Finally, the stimulation protocols described in the included studies varied considerably. For example, the setting varied from home‐based studies to those undertaken in the ICU. The number of muscles stimulated ranged from just the quadriceps to three separate lower limb muscles. The frequency used for stimulation ranged from 35 Hz to 50 Hz. The exposure to stimulation ranged from twice a day to twice a week, for 30 to 60 minutes per session, over three to 10 weeks. There were insufficient studies to attempt to determine the most effective protocol. We were unable to contact the authors of some studies that were published as abstracts and therefore, could not include the data from these studies in the review.
Agreements and disagreements with other studies or reviews
Data demonstrating that NMES increased peripheral muscle force when applied in isolation were consistent with previous reviews in this area (Chen 2016; Jones 2016; Roig 2009). Although one review reported no effect of NMES on maximal quadriceps muscle force, the estimate of the effect was of similar magnitude of that reported in the current study (SMD 0.38) and was likely not to have reached statistical significance as this earlier review included fewer studies in the meta‐analysis (Pan 2014).
Our data demonstrating an increase in quadriceps endurance were consistent with the only previous report to extract data on this outcome (Jones 2016). Our result of no change in thigh muscle size was consistent with one previous study that described equivocal evidence for the effect of NMES on this outcome (Roig 2009). Nevertheless, it contrasts with another review that demonstrated an effect on muscle mass measured using ultrasound or computed tomography (Jones 2016). The reasons for this disparity appeared to relate to the fact that this earlier review analysed studies grouped according to the method used to quantify thigh muscle size and also included data from a study undertaken in adults with severe chronic heart failure (Quittan 2001). Also, in contrast with the current review which used only measures of SD at baseline in the calculation of the SMD, the earlier review used the SD of the within‐group change to calculate the SMD. This reduced the variability of the estimate of the SMD and increased the likelihood of a 'significant' result.
Regarding adverse events, data demonstrating no risk difference between groups in major or minor adverse events were consistent with earlier reports (Jones 2016; Roig 2009).
Consistent with our findings of the effect of NMES on exercise capacity, earlier reviews reported changes in some, but not all, measures (Jones 2016; Chen 2016). Although consistent with earlier reviews that demonstrated a significant increase in 6MWD (Chen 2016; Jones 2016), our data extended this finding by suggesting that the effect of NMES on 6MWD was lessened when this intervention was combined with conventional exercise training. One review reported a significant increase in walking distance that was larger than seen in the current review or a previous review (MD 47.55 m) (Roig 2009). However, the earlier review combined data on exercise capacity measured via the 6MWT and the ISWT (Roig 2009). One previous review reported no difference in 6MWD (Pan 2014), but included different and fewer studies than the other reviews. Regarding other measures of exercise capacity, in contrast with the current review, an earlier review demonstrated no increase in VO2peak (Jones 2016). The reason for this disparity related to the inclusion of different studies in the meta‐analyses. Specifically, the earlier review included data from a randomised cross‐over study that was excluded from the current review (Napolis 2006). Also, we included measures of VO2peak collected on completion of the ISWT (Vivodtzev 2012), because in people with COPD, the VO2peak achieved on completion of an ISWT is similar to that achieved on completion of a laboratory‐based cycle ergometry test (Hill 2012). Finally, our finding of an increase in endurance time is consistent with earlier work (Chen 2016).
No earlier review has explored the effect of NMES on functional outcomes and so we were unable to comment on similarities or differences regarding this result.
Regarding the effect on symptoms, our data suggested that NMES applied in isolation reduced the severity of leg fatigue at the end of an exercise test. Earlier work suggested that NMES reduced dyspnoea, but did not separate the studies that applied NMES in isolation from those that applied NMES plus conventional exercise training (Pan 2014). One earlier review did not undertake a meta‐analysis of data on symptoms, but commented that data on this outcome were equivocal (Jones 2016).
Regarding changes in HRQoL, our finding of no effect was consistent with an earlier meta‐analysis on this outcome (Chen 2016), and supported the conclusion of equivocal evidence based on a narrative summary of these data (Jones 2016).
We acknowledge that the study criteria for this review meant that we were unable to include data reported in one of the largest trials of NMES in people with COPD (Sillen 2014). As this study compared two stimulation protocols with a programme of resistance training, it did not have an appropriate control group to be considered for inclusion in this review.
Authors' conclusions
Implications for practice.
When applied in isolation from other rehabilitation strategies, neuromuscular electrostimulation (NMES) applied to the quadriceps increases quadriceps force and quadriceps endurance. There are also improvements in six‐minute walk distance (6MWD), time to symptom limitation exercising at a submaximal intensity and the severity of leg fatigue on completion of an exercise test. It may increase peak rate of oxygen uptake (VO2peak), but the true effect on this outcome measure could be trivial. Therefore, in participants who are unable or unwilling to attend a pulmonary rehabilitation programme, consideration could be given to using NMES. Nevertheless, the quality of the evidence is low.
When applied with conventional exercise training, the effect of NMES on peripheral muscle force is uncertain. However, this result appears to have been influenced by the inclusion of a study that completed fewer than 10 sessions over four weeks. Exclusion of this study revealed a significant increase in peripheral muscle force. There was evidence for an increase in 6MWD, but this result appears to be influenced by the inclusion of a study that recruited people who were admitted to the intensive care unit (ICU) with an acute exacerbation of their disease. Evidence for additional benefit, over and above the effects seen with conventional exercise on symptoms or health‐related quality of life, was lacking. However, in people admitted to either an ICU or a respiratory high dependency unit, NMES combined with conventional exercise may accelerate the attainment of a functional milestone; that is, the time taken for participants to first sit out of bed. Therefore, it is likely that the addition of NMES strength protocol to a programme of exercise will be of most benefit to people who are experiencing or recovering from an exacerbation. However, again, the quality of the evidence is low.
Regardless of whether it was applied in isolation or together with conventional exercise training, NMES appears not to increase the risk of adverse events.
Implications for research.
In people with COPD, given the evidence that pulmonary rehabilitation, which includes an obligatory exercise training component, changes outcomes such as 6MWD, symptoms and HRQoL (McCarthy 2015), in clinical practice, there would seem to be little basis to offer NMES as an alternative to exercise training. Therefore, the most relevant question regarding the use of NMES in people with COPD is: does adding NMES to a programme of conventional exercise training produce additional benefits over and above those seen following conventional exercise training alone? The NMES protocols described in the studies included in this review were diverse and often did not use parameters that specifically targeted strength or endurance adaptations; a factor that may have dampened its effect. Future studies should give consideration to the type of adaptation (i.e. strength or endurance) that is most desirable in response to a programme of NMES (Dolmage 2016). In people who are most debilitated and lack the strength to complete everyday activities (such as moving from sit to stand), protocols that maximise strength adaptations are likely to be necessary before participation in an exercise training programme is appropriate. In contrast, in people who have adequate strength for everyday activities, but experience difficulty engaging in effective aerobic exercise due to intolerable dyspnoea (i.e. people referred to a pulmonary rehabilitation programme), protocols that maximise endurance adaptations are likely to be the most appropriate. The impact of these protocols on muscle endurance, rather than just muscle strength, needs to be explored. Regarding other outcome measures, studies should consider evaluating the effect of NMES on measures such as VO2peak, as well as measures that are known to be most responsive such as time to symptom limitation exercising at a high constant power and the progression of symptoms during exercise (e.g. iso‐time responses). Finally, the characteristics of 'responders' to NMES requires further exploration.
Acknowledgements
Anne Holland was the Editor for this review and commented critically on it.
The Background and Methods sections of this review were based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Trials Register
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (the Cochrane Library) | Monthly |
| MEDLINE (OvidSP) | Weekly |
| Embase (OvidSP) | Weekly |
| PsycINFO (OvidSP) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society (BTS) Winter Meeting | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
Handsearches: core physiotherapy conference abstracts
| Conference | Years searched |
| World Confederation for Physical Therapy ‐ Congress | 2003, 2007, 2011 |
MEDLINE search strategy used to identify trials for the Cochrane Airways Trials Register
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify randomised controlled trials
1. exp "clinical trial (publication type)"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and the RCT filter were adapted to identify trials in other electronic databases.
Appendix 2. Search strategy for the Cochrane Airways Group Register
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All
#2 MeSH DESCRIPTOR Bronchitis, Chronic
#3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)
#4 COPD:MISC1
#5 (COPD OR COAD OR COBD):TI,AB,KW
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 MeSH DESCRIPTOR Electric Stimulation Therapy Explode 1 2
#8 MeSH DESCRIPTOR Electric Stimulation Explode 1
#9 MeSH DESCRIPTOR Transcutaneous Electric Nerve Stimulation
#10 electrotherap*
#11 electrical NEAR stimulation
#12 electromyostimulation
#13 electrostimulation
#14 neuromuscular NEAR stimulation
#15 neuromuscular NEAR electric*
#16 functional NEAR electrical
#17 NMES
#18 TENS
#19 Electroshock
#20 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19
#21 #6 and #20
(In search line #4, MISC1 denotes the field in which the reference has been coded for condition, in this case, COPD.)
Data and analyses
Comparison 1. Neuromuscular electrostimulation (NMES) versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peripheral muscle force | 6 | 159 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [0.02, 0.65] |
| 2 Peripheral muscle endurance/fatigability | 2 | 35 | Std. Mean Difference (IV, Random, 95% CI) | 1.36 [0.59, 2.12] |
| 3 Thigh muscle size | 4 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.11, 0.61] |
| 4 Mortality | 5 | 131 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.08, 0.05] |
| 5 Exercise capacity: 6‐minute walking distance (6MWD) (m) | 2 | 72 | Mean Difference (IV, Random, 95% CI) | 39.26 [16.31, 62.22] |
| 6 Exercise capacity: VO2peak (L/min) | 4 | 73 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.00, 0.19] |
| 7 Exercise capacity: peak power (W) | 2 | 33 | Mean Difference (IV, Random, 95% CI) | 5.77 [‐4.00, 17.53] |
| 8 Exercise capacity: endurance time (min) | 3 | 55 | Mean Difference (IV, Random, 95% CI) | 3.62 [2.33, 4.91] |
| 9 Symptoms: dyspnoea reported at end exercise | 3 | 55 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐2.13, 0.06] |
| 10 Symptoms: leg fatigue reported at end exercise | 3 | 55 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐1.81, ‐0.43] |
| 11 Health‐related quality of life: SGRQ | 2 | 72 | Mean Difference (IV, Random, 95% CI) | ‐4.12 [‐12.60, 4.35] |
| 12 Minor adverse events: related to intervention | 5 | 139 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.07, 0.07] |
Comparison 2. Neuromuscular electrostimulation (NMES) plus exercise versus exercise only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peripheral muscle force | 4 | 84 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.10, 1.04] |
| 2 Peripheral muscle force with subgroups based on methods used to assess muscle force | 4 | 84 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.10, 1.04] |
| 2.1 Robust strength measure | 2 | 33 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.25, 1.14] |
| 2.2 Less robust strength measures | 2 | 51 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [‐0.74, 1.80] |
| 3 Peripheral muscle force: sensitivity analysis | 3 | 57 | Std. Mean Difference (IV, Random, 95% CI) | 0.73 [0.19, 1.28] |
| 4 Mortality | 7 | 183 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 5 Exercise capacity: 6‐minute walking distance (6MWD) (m) | 6 | 138 | Mean Difference (IV, Random, 95% CI) | 25.87 [1.06, 50.69] |
| 6 Exercise capacity: 6MWD (m): sensitivity analysis | 5 | 128 | Mean Difference (IV, Random, 95% CI) | 25.86 [‐3.17, 54.89] |
| 7 Functional performance: days to first transfer out of bed | 2 | 44 | Mean Difference (IV, Random, 95% CI) | ‐4.98 [‐8.55, ‐1.41] |
| 8 Symptoms: dyspnoea reported at end exercise | 2 | 44 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐2.27, 1.38] |
| 9 Health‐related quality of life | 4 | 95 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.27, 0.15] |
| 10 Minor adverse events related to intervention | 6 | 144 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdellaoui 2011.
| Methods | Randomised controlled trial | |
| Participants | 17 participants were enrolled. All participants had been admitted to the ICU with an exacerbation of their disease. 10 were allocated to the intervention group of whom 9 completed the study (7 men, median FEV1 % predicted = 25 (IQR 17 to 41) %, median age = 59 (IQR 57 to 69) yr). 7 were allocated to the control group of whom 6 completed the study (6 men, median FEV1 % predicted = 15 (IQR 10 to 27) %, median age = 67 (IQR 59 to 72) yr). | |
| Interventions | Both groups received education (once per week) and daily active‐passive mobilisation. Intervention: bilateral electrical stimulation of hamstrings and quadriceps using biphasic symmetric, constant current impulses with a pulse width of 400 µs and a frequency of 35 Hz for 1 hour per day, 5 days per week for 6 weeks. Intensity was set at the maximum that could be tolerated for each participant. Control: sham stimulation using identical stimulation parameters, except the stimulation did not cause contractions that were visible or palpable. |
|
| Outcomes | Quadriceps strength was measured (in kg) during a maximum voluntary contraction using a dynamometer Exercise capacity via the 6MWT Functional limitation resulting from dyspnoea via the MRC scale Muscle oxidation and fibre typology via biopsy analysis |
|
| Notes | Supported by patients' association APARD grant. Two study investigators were supported by a CIFRE grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed in block sizes of 5." Quote: "patients were randomly assigned to sham or NMES training." Randomisation sequence developed using a computer (information from authors) |
| Allocation concealment (selection bias) | Low risk | Quote: "….using blinded sealed envelopes prepared by an independent secretary." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "patients were blinded to the intervention groups." Quote: "the sham group had weekly therapeutic education sessions, daily active‐passive mobilisation and sham electrostimulation." Although it was unlikely that the investigators administering the electrical stimulation (and sham intervention) were blinded to group allocation, the risk of bias from this was low. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "tests were performed by researchers who were not blinded to the groups." This was unlikely to have affected outcomes related to muscle oxidative stress and structure, but may have affected measures of muscle strength, exercise capacity and functional limitation resulting from dyspnoea. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant withdrew in each group |
| Selective reporting (reporting bias) | Low risk | Generally reported as per protocol (except change in primary outcome) |
| Other bias | High risk | Of the 15 participants who completed the study, 13 were men. |
Akar 2017.
| Methods | Randomised controlled trial | |
| Participants | 30 participants were enrolled. All participants had been admitted to the ICU with respiratory failure. 10 were allocated to an intervention group that received NMES + exercise training (4 men, mean age = 70 (SD 12) yr). 10 were allocated to a control group that received exercise training only (5 men, mean age = 68 (SD 18) yr). 10 were allocated to a control group that received NMES only (these participants were not included in this review) (6 men, mean age = 63 (SD 7) yr). | |
| Interventions | All groups received positioning, postural drainage, bronchial hygiene techniques, tracheal aspiration as necessary and nutritional and psychological support. Intervention: bilateral electrical stimulation of deltoids and quadriceps using biphasic symmetric square waves with an amplitude of 20‐25 mA (determined by participant tolerance), at a frequency of 50 Hz for 6 s contractions, 5 days per week (total of 20 sessions). Both visible and palpable muscle contractions were obtained. This group also received active exercise that comprised active joint range of motion exercise for upper and lower limbs. Participants who could not manage active exercise received active‐assisted or passive range of motion exercise. Control: active exercise that comprised active joint range of motion exercise for upper and lower limbs. Participants who could not manage active exercise received active‐assisted or passive range of motion exercise. |
|
| Outcomes | Muscle strength measured via manual muscle testing Functional outcomes recorded (e.g. participant's capacity to sit up in bed, move from bed) Heart rate and respiratory rate before and after training programme Length of stay in ICU and weaning success Biomarkers (via venous blood samples) of CRP, IL‐6, IL‐8, IL‐10 and TNF‐alpha |
|
| Notes | Funding support not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...were randomized during the early intubation period." Details pertaining to the development of the randomisation sequence were not reported. |
| Allocation concealment (selection bias) | Low risk | Quote: "...were randomized during the early intubation period (the first workday following hospitalisation) in a blinded fashion." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No sham stimulation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Patients' pre‐ and post‐PR lower extremity and upper extremity muscle strength were scored manually by a single, experienced physician blinded to randomization with a scale of 5." No mention of blinding for other outcomes. This was unlikely to have affected outcomes related to blood biomarkers, but may have affected measures of functional outcomes, length of stay and weaning success. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details on attrition to comment on whether this was a source of bias. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | Study appeared free from other sources of bias. |
Akinlabi 2013.
| Methods | Randomised controlled trial | |
| Participants | 10 participants were enrolled (5 men, mean FEV1 % predicted = 25 (SD 8) %, mean age = 76 (presumed SD 8) yr). 5 were allocated to an intervention group that received NMES + low‐intensity exercise training (2 men, presume mean FEV1 % predicted = 24 (SD 9) %, mean age = 73 (SD 6) yr). 5 were allocated to a control group that received low‐intensity exercise training only (3 men, presume mean FEV1 % predicted = 26 (SD 8) %, mean age = 77 (SD 10) yr). All participants completed the study (information from authors). | |
| Interventions | Intervention: (information obtained from the author) NMES of hamstrings and quadriceps of 10‐120 mA at frequencies of 10‐50 Hz and a pulse duration of 200‐400 µs (using Physio‐Med EMS 9000D) 2 days per week for 8 weeks (total of 16 sessions). This group also received low‐intensity active exercises that comprised walking and upper limb resistance exercises using a 0.5 kg weight (aiming for Borg scores of 3 to 4). Control: low intensity active exercises only |
|
| Outcomes | Exercise capacity via the 6MWT HRQoL via the SGRQ Capacity to undertake activities of daily living via the LCADL Feelings of anxiety and depression via the HADS |
|
| Notes | Study was available in abstract form only. Funding support not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients with severe COPD (MRC 4 and 5) with a mean FEV1 of 25% predicted (± 7.8) were randomised into two 16‐session PR [ pulmonary rehabilitation] programmes." Information from authors: the randomisation sequence was developed using a SAS random number generator. |
| Allocation concealment (selection bias) | Low risk | Concealed using opaque envelopes (information from authors) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No sham stimulation. Although the investigators administering the electrical stimulation were not blinded to group allocation (information from authors), the risk of bias from this was low. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Investigator collecting the outcome measures was not blinded to group allocation (information from authors). This may have affected their outcomes related to exercise capacity and all outcomes assessed via questionnaires. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who were randomised also completed the study (information from authors). |
| Selective reporting (reporting bias) | Unclear risk | No publicly available protocol |
| Other bias | Unclear risk | Study was available in abstract form only. |
Bourjeily‐Habr 2002.
| Methods | Randomised, double‐blind controlled trial | |
| Participants | 18 participants were enrolled. All participants were medically stable. 9 were allocated to the intervention group (6 men, mean FEV1 % predicted = 36 (SEM 4) %, mean age = 58 (SEM 2) yr). 9 were allocated to the control group (4 men, mean FEV1 % predicted = 41 (SEM 4) %, mean age = 62 (SEM 2) yr). | |
| Interventions | Intervention: electrical stimulation of the hamstrings, quadriceps and calf muscles of both lower limbs, using an asymmetric, square‐wave pulse at a frequency of 50 Hz, pulse width of 200 ms every 1500 ms for 20 minutes per day, 3 days per week for 6 weeks (on an outpatient basis). Initial current 55‐120 mA and increased weekly by approximately 5 mA. Control: identical setup but they did not receive any active electrical stimulation. |
|
| Outcomes | Quadriceps and hamstring strength measured as maximum isokinetic torque via an isokinetic dynamometer Exercise capacity via the incremental shuttle walk test and a cardiopulmonary exercise test Respiratory muscle strength measured as maximum inspiratory and expiratory mouth pressures |
|
| Notes | Study supported in part by the departmental fund, Yale Section of Pulmonary and Critical Care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomised controlled double blind study was conducted." Quote: "After the initial evaluation, patients were randomised into two groups." Details pertaining to the development of the randomisation sequence were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The control group had the same electrode, stimulator step up and connection system with the unit on for identical time periods in the same setting but received no active electrical stimulation during the visits. Since patients did not know what, if any, sensations to expect during the electrical stimulation and were not in contact with each other, they remained blinded to randomisation. Both the patients and all but one investigator were blinded to the type of treatment…." Although it was unlikely that the investigator administering the electrical stimulation (and sham intervention) were blinded to group allocation, the risk of bias from this was low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only the measures of isokinetic strength were made by blinded outcome assessors. It was unclear if the measures of exercise capacity or respiratory muscle strength were made by an assessor who was blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All the subjects completed the study" |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | Study appeared free from other sources of bias. |
Dang 2011.
| Methods | Randomised controlled trial | |
| Participants | 16 participants were enrolled (8 men). All participants participated in a comprehensive 'ambulatory' respiratory rehabilitation programme for 12 weeks. 8 were allocated to the intervention group (information from authors; 3 men, mean FEV1 % predicted = 36 (SD 7) %, mean age = 63 (SD 4) yr). 8 were allocated to the control group (information from authors; 5 men, mean FEV1 % predicted = 40 (SD 6) %, mean age = 61 (SD 8) yr). | |
| Interventions | Intervention: respiratory rehabilitation + electrical stimulation of the quadriceps (information from authors bilateral electrical stimulation of quadriceps using symmetric rectangular impulses with pulse width of 0.35 ms at a frequency of 45 Hz for 4 s contractions followed by 8 Hz for 8 s for 36 minutes. Current intensity was titrated to the maximal tolerable. Participants completed 3 sessions per week for 12 weeks (total of 36 sessions). Control: respiratory rehabilitation only (no details of this programme were given). |
|
| Outcomes | Airflow obstruction via spirometry Exercise capacity via the 6MWT and cardiopulmonary exercise test (VO2peak) Quadriceps strength (using a strain gauge and a knee extensor chair) and endurance (time to task failure when asked to sustain a muscle contraction equal to 50% of that achieved during a maximum voluntary contraction) HRQoL (using CRDQ) |
|
| Notes | Study was available in abstract form only. Funding support not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…were randomised in two groups." Randomisation sequence was computer generated (information from authors). |
| Allocation concealment (selection bias) | Low risk | Randomisation was undertaken by someone external to the study (information from authors). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempt to blind participants. Control group did not appear to receive any sham intervention. Although the investigators administering the electrical stimulation were unaware of the aims of the study (information from authors), it was unlikely that they would have been blinded to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding for outcome assessors. This may have affected their outcomes related to exercise capacity, muscle strength, endurance and HRQoL. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details on attrition to comment on whether this was a source of bias. Information from authors was that 3 participants in each group were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Study was available in abstract form only. |
Dolmage 2016.
| Methods | Randomised controlled trial | |
| Participants | 11 participants were enrolled and all completed the study (mean FEV1 % predicted = 28 (SD 7) %, mean age = 62 (SD 9), number of men not reported). 4 were allocated to a group that received NMES to facilitate strength adaptations and 2 were allocated to a group that received NMES to facilitate endurance adaptations and 5 were allocated to a control group that received sham NMES. | |
| Interventions | Intervention group 1: electrical stimulation of the quadriceps using low‐frequency, high‐duty cycle (i.e. 15 Hz, duty cycle 0.33) Intervention group 2: electrical stimulation of the quadriceps using high‐frequency, low‐duty cycle (i.e. 75 Hz, duty cycle 0.17) Control: sham stimulation set using 1 of the above patterns, but with insufficient current to elicit a contraction Training in all groups took place at home, over 6 weeks |
|
| Outcomes | Isometric strength of the quadriceps Constant power cycle endurance |
|
| Notes | Data from this study could not be included in the meta‐analyses. Funding support not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...were randomly assigned..." |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The control group received sham stimulation. Although it was unlikely that the investigator administering the electrical stimulation (and sham intervention) were blinded to group allocation, the risk of bias from this was low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details on attrition to comment on whether this was a source of bias. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Study was available in abstract form only. |
Giavedoni 2012.
| Methods | Randomised controlled trial in which 1 leg received electrical stimulation and 1 leg received no stimulation. The choice of leg to receive stimulation was randomised. | |
| Participants | 11 participants were enrolled and all completed the study (5 men, mean FEV1 % predicted = 41 (SEM 6) %, mean age = 72 (SEM 3) yr). They were recruited within 48 hours of admission to hospital with an acute exacerbation of COPD. | |
| Interventions | Intervention: electrical stimulation of the quadriceps of 1 leg using an asymmetric, biphase pulse wave at a frequency of 50 Hz, pulse width of 400 ms for 30 minutes per day, once per day for 14 days. Duty cycle was 8 s on, 20 s off. Intensity was set at the maximum that could be tolerated for each participant. 4 sessions were supervised (3 in hospital and 1 at home) with the rest performed unsupervised either in hospital or at home (after discharge). Adherence was optimised with 2 telephone calls following discharge and extra supervised sessions were offered if needed. Control: no stimulation |
|
| Outcomes | BMI (descriptive data only) Airflow obstruction via spirometry (descriptive data only) Quadriceps strength was measured as force generated during a maximum isometric voluntary contraction using a strain gauge |
|
| Notes | Study supported by the BLF. Investigator supported by ERS long‐term research fellowship | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…the dominant leg was randomly allocated to treatment with NMES or no stimulation (control)." Details pertaining to the development of the randomisation sequence were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither participants nor investigators administering the intervention were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding for outcome assessors. This may have affected their outcome related to muscle strength. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details on attrition to comment on whether this was a source of bias. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | Study appeared free from other sources of bias. |
Gigliotti 2004.
| Methods | Randomised controlled trial | |
| Participants | 20 participants with severe COPD and MRC dyspnoea score of 4 and 5 were enrolled in the study. No details given of the characteristics of the participants or on how many were allocated to the 2 groups. | |
| Interventions | Participants in both groups underwent exercise training Intervention: NMES of peripheral muscles (no other details of stimulation parameters provided) Control: no stimulation |
|
| Outcomes | Exercise capacity via the 6MWT and cycling endurance via a cycle‐ergometer Dyspnoea via the Borg scale HRQoL via the SGRQ Quadriceps muscle force via isokinetic dynamometer |
|
| Notes | Study was available in abstract form only. No usable data were available. Funding support not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details pertaining to the development of the randomisation sequence were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No sham stimulation. Neither participants nor investigators administering the intervention were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding for outcome assessors. This may have affected their outcome related to muscle strength. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details on attrition to comment on whether this was a source of bias. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Study was available in abstract form only. |
Kucio 2016.
| Methods | Randomised controlled trial | |
| Participants | 30 hospitalised participants were enrolled (21 men). All participants entered a traditional pulmonary rehabilitation programme for 3 weeks. 15 were allocated to the intervention group (11 men, mean FEV1 = 1.66 (SD 0.69) L, mean age = 68 (SD 6) yr). 15 were allocated to the control group (10 men, mean FEV1 = 1.78 (SD 0.78) L, mean age = 61 (SD 8) yr). | |
| Interventions | Participants in both groups received pulmonary rehabilitation (3 weeks, 6 supervised sessions per week that comprised breathing exercises, treadmill walking and resistance exercise). Intervention: NMES of the quadriceps and gastrocnemius using symmetric rectangular impulses with pulse width of 0.30 ms at a frequency of 35 Hz for 2 s on and 4 s off for 36 min. Details of stimulation duration and intensity were not provided. Control: no stimulation |
|
| Outcomes | Exercise capacity via the 6MWT Airflow obstruction via spirometry Arterial oxygen and carbon dioxide concentrations via arterialised capillary samples |
|
| Notes | Funding support not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients that fulfilled the inclusion criteria were randomly assigned to one of the two groups." Details pertaining to the development of the randomisation sequence were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No sham stimulation. Neither participants nor investigators administering the intervention were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding for outcome assessors. This may have affected their outcome related to muscle strength. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 30 participants randomised, 2 withdrew from the control group |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | Disproportionate number of men |
Latimer 2013.
| Methods | Randomised controlled trial in which 1 leg received NMES and 1 leg received no stimulation. The choice of leg to receive stimulation was randomised (information from authors). | |
| Participants | 16 medically stable participants were enrolled (8 men, mean FEV1 % predicted = 50 (SD 22) %, mean age = 64 (SD 9) yr). | |
| Interventions | Intervention: NMES of the quadriceps of 1 leg for 30 minutes per session, 5 times per week (3 sessions were supervised, 2 were unsupervised) for 6 weeks. Information from authors: the stimulation parameters were biphasic pulses at a frequency of 50 Hz, pulse duration of 300 µs with a duty cycle of 15 s on and 5 s off. Intensity was set at the maximum that could be tolerated for each participant. Control: no stimulation |
|
| Outcomes | Thigh lean muscle mass via DEXA (information obtained from authors) Quadriceps strength was measured as force generated during a maximum isometric voluntary contraction (information from authors: using a load cell and a fixed chair). |
|
| Notes | Study was available in abstract form only. Funding support not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The leg to be trained (i.e. dominant vs non‐dominant) was randomised using a computer‐generated randomisation sequence (information from authors). |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither participants nor investigators administering the intervention were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Measures of muscle mass were made by a blinded assessor, but measures of strength were not (information from authors). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details on attrition to comment on whether this was a source of bias. |
| Selective reporting (reporting bias) | High risk | Abstract did not report on several outcomes that were stated in the study protocol. |
| Other bias | Unclear risk | Study was available in abstract form only. |
Maddocks 2016.
| Methods | Randomised controlled trial | |
| Participants | 52 medically stable participants were randomised. 25 were allocated to the intervention group (11 men, mean FEV1 % predicted = 31 (SD 11) %, mean age = 70 (SD 11) yr). 27 were allocated to the control group (10 men, mean FEV1 % predicted = 31 (SD 13) %, mean age = 69 (SD 9) yr) | |
| Interventions | Intervention: self‐administered electrical stimulation of the quadriceps of both lower limbs, at a frequency of 50 Hz, pulse width of 350 µs for 30 minutes per day, 7 days per week for 6 weeks. Current was set to elicit a contraction equivalent to 15‐25% of a maximum voluntary contraction. Duty cycle increased on a weekly basis from (on:off) 2:15 s to 5:20 s to 10:15 s, and thereafter remained unchanged. Control: identical setup but had a current range of 0‐20 mA and produced a sensory stimulus that was detectable by the participant, but was insufficient to elicit a tetanic contraction. |
|
| Outcomes | Exercise capacity via the 6MWT Quadriceps twitch tension via supramaximal femoral nerve stimulation Quadriceps force via isometric maximum voluntary contraction using a chair‐mounted strain gauge Rectus femoris cross‐sectional area via ultrasonography Fat‐free mass via bioimpedance Physical activity via the activPAL HRQoL via the EuroQoL 5 dimension, SGRQ and CRDQ Formal and informal care via the Client Service Receipt Inventory (to assess health, voluntary and social care services and career support) |
|
| Notes | Study supported by UK National Institute for Health Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...participants were randomly assigned (1:1) at the individual level, using an independent web‐based randomisation system within the independent UK Clinical Research Collaboration..." |
| Allocation concealment (selection bias) | Low risk | Quote: "Following randomisation to active of placebo NMES, the Clinical Trials Unit informed trial staff via secure email." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants were not informed of group allocation." Placebo (sham) NMES was provided to the control group. Although the investigators administering the electrical stimulation (and sham intervention) were not blinded to group allocation, the risk of bias from this was low. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The trial coordinator (masked to group allocation) undertook physical assessments." Quote: "Questionnaires were self‐completed independently by the participants" (who were blinded to group allocation). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 participants from each group did not complete all follow‐up assessments. The reasons for withdrawal were similar and missing data were imputed |
| Selective reporting (reporting bias) | Low risk | Reported as per protocol |
| Other bias | Low risk | Study appeared free from other sources of bias. |
Neder 2002.
| Methods | Randomised controlled trial | |
| Participants | 15 medically stable participants (9 men) were enrolled. 9 were allocated to the intervention group (mean FEV1 % predicted = 38 (SD 10) %, mean age = 67 (SD 8) yr). 6 were allocated to the control group (mean FEV1 % predicted = 40 (SD 13) %, mean age = 65 (SD 5) yr) | |
| Interventions | Intervention: electrical stimulation of both quadriceps using a symmetric, biphasic square‐pulsed wave at a frequency of 50 Hz. Duty cycle was 2 s on, 18 s off for the first week, then 5 s on, 25 s off for the second week and then 10 s on 30 s off for the rest of the training period. The pulse width was 300‐400 µs and the intensity was titrated the maximum tolerable. Training was applied for 15 minutes (to each leg) in the first week and increased to 30 minutes thereafter, for 5 days per week for 6 weeks. The first week of training was supervised (in an outpatient department) and thereafter, training was undertaken at home with weekly visits by the therapist. Control: no stimulation |
|
| Outcomes | HRQoL via the CRDQ Fat‐free mass via bioimpedance (descriptive data only) Airflow obstruction via spirometry (descriptive data only) Lung volumes via nitrogen washout and single breath diffusing capacity for carbon monoxide (descriptive data only) Exercise capacity via a cardiopulmonary exercise test Quadriceps strength measured as torque and force during a maximum isokinetic contraction using an isokinetic dynamometer Quadriceps endurance measured using an isokinetic dynamometer |
|
| Notes | Investigator supported by a long‐term ERS fellowship | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This was a prospective randomised controlled study." Details pertaining to the development of the randomisation sequence were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempt to blind participants or personnel. Control group did not receive any sham intervention. Unlikely that the investigators administering the NMES were blinded to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding for outcome assessors. A lack of blinding of may have affected outcomes such as muscle strength, muscle endurance, exercise capacity and HRQoL. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details on attrition to comment on whether this was a source of bias. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | Study appeared free from other sources of bias. |
Tardif 2015.
| Methods | Randomised controlled trial | |
| Participants | 52 participants were enrolled in the study (unclear what measures of central tendency and variability were reported, 45 men, FEV1 % predicted = 24‐59 %, mean age = 59 (SD 9) yr). 27 were allocated to the intervention group. 25 were allocated to the control group. Characteristics of the participants grouped as intervention vs group not provided. | |
| Interventions | Participants in both groups received PR (either outpatient or home‐based, over 8 weeks, total number of sessions 18‐24). Intervention: home‐based NMES (presumably to the quadriceps as reported in an earlier abstract (Roy 2013) at a frequency of 35 Hz for 30 minutes per day, 5 days per week for 8 weeks) Control: no stimulation |
|
| Outcomes | Exercise capacity via 6MWT and maximum power achieved during an exercise test HRQoL via SGRQ BODE index Functional limitation resulting from dyspnoea by MRC scale |
|
| Notes | Study was available in abstract form only. Funding support not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were randomly assigned..." Details pertaining to the development of the randomisation sequence were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempt to blind participants or personnel. Control group did not receive any sham intervention. Unlikely that the investigators administering the electrical stimulation were blinded to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding for outcome assessors. A lack of blinding of may have affected outcomes such as 6MWD, maximal power during an exercise test and HRQoL. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details on attrition to comment on whether this was a source of bias. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | Study was available in abstract form only. Disproportionate number of men |
Tasdemir 2015.
| Methods | Randomised controlled trial | |
| Participants | 34 medically stable participants were enrolled. 17 were allocated to the intervention group and of these, data were available on 13 (11 men, median FEV1 % predicted = 29 (range 16 to 71) %, mean age = 62 (SD 8) yr). 17 were allocated to the control group and of these, data were available on 14 (13 men, median FEV1 % predicted = 42 (range 23 to 66) %, mean age = 63 (SD 8) yr). | |
| Interventions | Participants in both groups completed a pulmonary rehabilitation programme for 2 days per week over 10 weeks. Intervention: NMES of both quadriceps using a symmetric, biphasic constant current impulse with a pulse width of 300 µs, at a frequency of 50 Hz. Duty cycle was 10 s on, 20 s off for 20 min (administered during each pulmonary rehabilitation session). Intensity was titrated to the maximum tolerable. Control: sham stimulation using a similar protocol, except that stimulation frequency was 5 Hz and the intensity was sufficient to cause a visible twitch. |
|
| Outcomes | Exercise capacity via the incremental shuttle walk test and the endurance shuttle walk test HRQoL via the SGRQ Quadriceps strength using a 1‐repetition maximum and 30‐s chair up test Quadriceps endurance using a squat test and a 2‐min step in place test Quadriceps fatigue using a visual analogue scale Activities of daily living using the LCADLS Functional limitation resulting from dyspnoea using the MRC Dyspnoea scale Fatigue using the Fatigue Severity Scale Feelings of anxiety and depression using the HADS |
|
| Notes | Funding support not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "prospective randomized controlled study" Quote: "using a computer‐generated randomization list" |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization sequence was concealed using a sealed envelope." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Control group received sham intervention. Unlikely that the investigators administering the electrical stimulation were blinded to group allocation, but the risk of bias from this was low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The evaluation team was blinded to the patients' treatment. They were not blinded to the final evaluations, although the psychologist who administered the St. George Respiratory Questionnaire and the Hospital Anxiety and Depression scale was blinded." The physiotherapists involved in collecting all other measures may not have been blinded and this may have affected measures of exercise capacity, quadriceps strength and endurance, dyspnoea, fatigue and activities of daily living |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar loss to follow‐up in both groups (4 participants in the intervention group and 3 participants in the control group). Reason for loss to follow‐up were similar in both groups. |
| Selective reporting (reporting bias) | High risk | Reported on several outcomes that were not stated in the study protocol. |
| Other bias | High risk | Disproportionate number of men |
Vieira 2014.
| Methods | Randomised, double‐blind controlled trial | |
| Participants | 24 medically stable men were enrolled. 12 were allocated to the intervention group of whom 11 completed the study (mean FEV1 % predicted = 36 (SD 10) %, mean age = 56 (SD 11) yr). 12 were allocated to the control group of whom 9 completed the study (mean FEV1 % predicted = 40 (SD 14) %, mean age = 56 (SD 13) yr). | |
| Interventions | All participants received respiratory physical therapy (i.e. airway clearance) as indicated as well as stretching exercises for the upper limbs, lower limbs and back. Intervention: bilateral electrical stimulation of the quadriceps using a biphasic, symmetric, square pulse at a frequency of 50 Hz. Duty cycle was 2 s on, 18 s off for the first week, then 5 s on, 25 s off for the second week and then 10 s on, 30 s off for the rest of the training period. The pulse width was 300‐400 µs and the intensity was titrated to the maximum tolerable. Stimulation was applied 5 days per week, 2 times per day, 60 minutes per session for 8 weeks. Control: identical setup for the electrodes, but no current was provided. |
|
| Outcomes | Fat‐free mass via bioimpedance Thigh circumference was measured at 14 cm, 21 cm and 28 cm below the iliac crest Airflow obstruction via spirometry Respiratory muscle strength measured as maximum inspiratory and expiratory mouth pressures Exercise capacity via a constant power cycle test (which included VO2peak and endurance time) and 6MWT Muscle activity via electromyography during a maximum voluntary contraction Mechanical efficiency was measured as the ratio of work accomplished during the cardiopulmonary exercise test to the energy expended (calculated using measures of VO2 and respiratory exchange ratio) Blood biomarkers via venous sampling HRQoL via the SGRQ |
|
| Notes | Study supported by a research grant from the Hospital de Clinicas de Porto Alegre, FIPE/HCPA, Porto Alegre, Brazil. Investigator supported by postdoctoral fellowship for the Coordination for the Improvement of Higher Education Personnel (CAPES), Brasilia, Brazil | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by the Graphpad StatMate computerized program." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were referred from a Private Physical Therapy Clinic by two investigators who were blind to the order of patient allocation." Unclear if this means that the sequence was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Sham stimulation was provided to the control group. Although it was unlikely that the investigators administering the electrical stimulation (and sham intervention) were blinded to group allocation, the risk of bias from this was low |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "A prospective double‐blind, randomized, pilot study was conducted…" However, process used to blind outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 24 participants randomised, 1 withdrew from the intervention group and 3 withdrew from the control group (all for exacerbations). |
| Selective reporting (reporting bias) | High risk | Discordance between outcomes stated in protocol and those reported in paper. |
| Other bias | High risk | All participants were men. |
Vivodtzev 2006.
| Methods | Randomised controlled trial | |
| Participants | 17 participants were enrolled. All participants were admitted to a pulmonary rehabilitation centre for 1 month following hospitalisation. 9 were allocated to the intervention group (6 men, mean FEV1 = 27 (SD 3) % predicted, mean age = 59 (SD 15) yr). 8 were allocated to the control group (5 men, mean FEV1 = 34 (SD 11) % predicted, mean age = 68 (SD 12) yr). | |
| Interventions | All participants received rehabilitation 4 days per week for 4 weeks, which comprised active limb exercises. The strongest participants also performed walking on a treadmill together with 5‐10 min of resistance arm exercises. They also completed health education sessions 1 day per week. Intervention: rehabilitation + bilateral electrical stimulation of both quadriceps for > 30 min, 4 times per week, for 4 weeks using a biphasic, symmetric, square pulse. Each session commenced with a 5 min warm‐up at frequency of 5 Hz, and a pulse width of 400 µs using continuous current. This was followed by 25 min of stimulation at 35 Hz for 400 µs lasting 7 s alternating with a resting current of 5 Hz for 400 µs lasting 8 s. The intensity was titrated to the maximum tolerable and was increased by 5 mA each day. Control: rehabilitation without any stimulation |
|
| Outcomes | Airflow obstruction via spirometry BMI Quality of life and dyspnoea during daily life via the Maugeri Foundation Respiratory Failure Questionnaire Quadriceps muscle strength measured during a maximum voluntary contraction via a strain gauge Quadriceps muscle composition via thigh circumference and skin fold measures Exercise capacity via the 6MWT |
|
| Notes | Study supported by grants from the Association pour le Traitement, la Reeducation et la Readaptation des Insuffisants Respiratoires (ATRIR), Nyons, France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomized into two different rehabilitation groups." Details pertaining to the development of the randomisation sequence were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempt to blind participants or personnel. Control group did not receive any sham intervention. Unlikely that the investigators administering the electrical stimulation were blinded to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding for outcome assessors. A lack of blinding of the outcome assessors was unlikely to have affected outcomes related to lung function, BMI or quadriceps muscle composition, but may have affected outcomes such as HRQoL, muscle strength and exercise capacity. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details on attrition to comment on whether this was a source of bias. Quadriceps muscle composition data available on 11/17 participants who completed the study. Reasons for missing data not provided |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | Study appeared free from other sources of bias. |
Vivodtzev 2012.
| Methods | Randomised, double‐blind controlled trial | |
| Participants | 22 medically stable participants were enrolled. 13 were allocated to the intervention group of whom 12 completed the study (8 men, mean FEV1 % predicted = 34 (SEM 3) %, mean age = 70 (SEM 1) yr). 9 were allocated to the control group of whom 8 completed the study (5 men, mean FEV1 % predicted = 30 (SEM 4) %, mean age = 68 (SEM 3) yr). | |
| Interventions | Intervention: bilateral electrical stimulation of the quadriceps (35 min) followed by bilateral stimulation of the calf muscles (25 min) using biphasic symmetric, square‐pulsed current of 50 Hz, with a pulse width of 400 µs for 6 s alternating with a frequency of 5 Hz for 10 s. Training took place 5 days per week for 6 weeks. Intensity was set at the maximum that could be tolerated for each participant. Training was completed at home after a practice session at the hospital. Participants were visited at home each week to monitor progress. Control: identical stimulation programme except undertaken at 5 Hz in the continuous mode |
|
| Outcomes | Biomarkers via venous sampling Muscle cross‐sectional area via computerised tomography Quadriceps strength measured via a maximum voluntary contraction using a strain gauge Quadriceps endurance measured as time to fatigue during a contraction at 60% of a participant's maximum voluntary contraction Exercise capacity via the incremental and endurance shuttle walk test with cardiorespiratory monitoring Muscle signalling pathways, enzyme activity, fibre type and size, and capillarisation via biopsy |
|
| Notes | Study supported by a grant from the Canadian Institute of Health Research. Investigator holds a GSK/CIHR Research Chair on COPD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This was a randomized, double‐blind, controlled and parallel group study." Details pertaining to the development of the randomisation sequence were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Sham training was delivered and monitored in the same fashion except for the stimulation frequency, which was set to 5 Hz in the continuous mode with a 100 µs pulse duration." Although it was unlikely that the investigators administering the electrical stimulation (and sham intervention) were blinded to group allocation, the risk of bias from this was low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "This was a randomized, double‐blind, controlled and parallel group study." Quote: "All muscle analyses were done without knowledge of clinical data." However, unclear if other assessments (e.g. muscle strength, endurance, exercise capacity) were undertaken by an assessor who was blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant did not complete in each group. The reasons for withdrawal were similar. |
| Selective reporting (reporting bias) | Low risk | Reported as per protocol |
| Other bias | Low risk | Study appeared free from other sources of bias. |
Zanotti 2003.
| Methods | Randomised controlled trial | |
| Participants | 24 participants were enrolled. All participants had chronic hypercapnic respiratory failure due to COPD, needed mechanical ventilation via a tracheostomy and had marked muscle atrophy. They had been referred to a high dependency unit from an ICU and had been confined to bed for ≥ 30 days. However, they were clinically stable. 12 were allocated to the intervention group (9 men, mean age = 66 (SD 8) yr). 12 were allocated to the control group (8 men, mean age = 64 (SD 4) yr). | |
| Interventions | All participants received rehabilitation, which comprised active limb exercises 5 days per week, twice per day for 4 weeks. Each session was conducted for the maximum time that could be tolerated, up to a maximum of 30 minutes. Intervention: rehabilitation + bilateral electrical stimulation of the quadriceps and vastus glutei for 30 min using a bipolar, biphasic, asymmetric, rectangular pulse. Each session comprised 5 min at frequency of 8 Hz, and a pulse width of 250 µs. This was followed by 25 min of stimulation at 35 Hz for 350 µs. Control: rehabilitation without any stimulation |
|
| Outcomes | Quadriceps strength measured via manual muscle testing Cardiorespiratory variables such as heart rate and arterial oxygen saturation at the beginning and end of treatment Functional outcomes such as the number of days needed to transfer from bed to chair |
|
| Notes | Funding support not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned…" Details pertaining to the development of the randomisation sequence were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although the rehabilitation (provided to both groups) was overseen by a therapist who was unaware of the aim of the study, there was no mention of any attempt to blind participants or other study personnel. The control group did not receive a sham intervention. Unlikely that the investigators administering the electrical stimulation were blinded to group allocation, but the risk of bias from this was low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding for outcome assessors. A lack of blinding of the outcome assessors was unlikely to have affected outcomes related to cardiorespiratory function collected at the beginning and the end of treatment, but may have affected outcomes such as muscle strength and attainment of functional milestones. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details on attrition to comment on whether this was a source of bias. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | Study appeared free from other sources of bias. |
Zanotti 2010.
| Methods | Randomised controlled trial | |
| Participants | 83 participants were enrolled (mean FEV1 % predicted = 58 (SD 12) %, mean age = 62 (SD 8) yr, number of men not reported). All participants completed a pulmonary rehabilitation programme. | |
| Interventions | Pulmonary rehabilitation comprised treadmill walking 5 days per week for 5 weeks Intervention: pulmonary rehabilitation + NMES Control: pulmonary rehabilitation + sham electrical stimulation |
|
| Outcomes | Exercise capacity (methods not described) Rating of dyspnoea (methods not described) HRQoL (methods not described) Quadriceps strength (methods not described) Airflow obstruction (methods not described) BODE index |
|
| Notes | Study was available in abstract form only. No usable data were available. Funding support not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…were randomly assigned." Details pertaining to the development of the randomisation sequence were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Sham stimulation was provided to the control group. Although it was unlikely that the investigators administering the electrical stimulation (and sham intervention) were blinded to group allocation, the risk of bias from this was low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding for outcome assessors. A lack of blinding of the outcome assessors was unlikely to have affected outcomes related to airflow obstruction, but may have affected outcomes such as exercise capacity, dyspnoea, HRQoL and muscle strength. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details on attrition to comment on whether this was a source of bias. |
| Selective reporting (reporting bias) | Low risk | Reported as per protocol |
| Other bias | Unclear risk | Study was available in abstract form only. |
6MWT: six‐minute walk test;BLF: British Lung Foundation; BMI: body mass index; BODE: Body mass index, airflow Obstruction, Dyspnea and Exercise capacity; CIFRE: Conventions Industrielles de Formation par la Recherche; COPD: chronic obstructive pulmonary disease; CRP: C‐reactive protein; CRDQ: Chronic Respiratory Disease questionnaire; DEXA: dual‐energy x‐ray absorptiometry; ERS: European Respiratory Society; FEV1: forced expiratory volume in one second; HADS: Hospital Anxiety and Depression questionnaire; HRQoL: health‐related quality of life; ICU: intensive care unit; IL: interleukin; IQR: interquartile range; LCADL: London Chest Activity of Daily Living Scale; min: minute; MRC: Medical Research Council; NMES: neuromuscular electrostimulation; s: second; SAS: Statistical Analysis System; SD: standard deviation; SEM: standard error of the mean; SGRQ: St George's Respiratory Questionnaire; TNF: tumour necrosis factor; VO2peak: peak rate oxygen consumption; yr: year.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alexandrov 1990 | No stimulation applied to a peripheral muscle |
| Bustamante 2008 | Magnetic stimulation used |
| Bustamante 2013 | Magnetic stimulation used |
| Chaplin 2013 | No control group (compared electrical stimulation using 2 different frequencies) |
| Cox 2014 | TENS used |
| Dal Corso 2005 | Cross‐over trial |
| Dall'Aqua 2012 | No stimulation applied to a peripheral muscle |
| Davis 2001 | Acu‐TENS used |
| Fauge 2014 | Compared 2 frequencies |
| Feschenko 2003 | No stimulation applied to a peripheral muscle |
| Flaugher 2014 | Compared 2 frequencies |
| Gagnon 2009 | Used quadriceps twitch force as an outcome measure only. Not a study of electrical stimulation |
| Greening 2014 | No appropriate control |
| Gupta 2012 | Acu‐TENS used |
| Han 2010 | Acu‐TENS used |
| Hatipoglu 1999 | Drug study that used diaphragm contractility as an outcome measure |
| Ito 2012 | No stimulation of a peripheral muscle |
| Jones 2011 | Acu‐TENS used |
| Kaymaz 2015 | Non‐randomised and no appropriate control |
| Klock 2001 | Not a study of electrical stimulation |
| Kurtoglu 2011 | No stimulation of a peripheral muscle |
| Lau 2008 | Acu‐TENS used |
| Lewith 2004 | Acu‐TENS used |
| Liu 2015 | Acu‐TENS used |
| Lopez 2017 | No suitable control group |
| Lotvall 1994 | Not conducted on people with COPD |
| Malaguti 2009 | Comparing stimulation protocols |
| Martin‐Salvador 2016 | No suitable control group |
| Medrinal 2016 | Protocol paper |
| Meesen 2010 | < 50% of the participants had COPD |
| Napolis 2006 | Cross‐over trial |
| Ngai 2010 | Acu‐TENS used |
| Oncu 2016 | Acu‐TENS |
| Sanchez 2016 | No suitable control group |
| Sillen 2008 | No appropriate control |
| Sillen 2013 | Review article |
| Valenza 2017 | No suitable control group |
| Vivodtzev 2014 | Not an RCT |
| Wen 2011 | Acu‐TENS |
| Yang 2005 | Explored the use of a diaphragm pacemaker. Not a study of electrical stimulation of a peripheral muscle |
COPD: chronic obstructive pulmonary disease; RCT: randomised controlled trial; TENS: transcutaneous electrical nerve stimulation.
Characteristics of studies awaiting assessment [ordered by study ID]
Chen 2017.
| Methods | RCT |
| Participants | People who had been mechanically ventilated for > 21 days. Unclear what proportion had COPD. |
| Interventions | Randomised to 1 of 3 groups:
Stimulation took place for 30 min per session, 2 sessions per day for 10 days. |
| Outcomes | Muscle strength, muscle size, level of activity of daily life (via the Functional Independence Measure), weaning rate, length of stay and mortality |
| Notes | Available as abstract only |
COPD: chronic obstructive pulmonary disease; min: minute; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐IPR‐16009845.
| Trial name or title | Clinical Trial of NMES of the Lower Extremities in Acute Exacerbated Patients with Chronic Obstructive Pulmonary Disease |
| Methods | Randomised controlled trial |
| Participants | People with COPD who are experiencing an acute exacerbation of their disease |
| Interventions | NMES vs sham stimulation |
| Outcomes | Muscle function, exercise capacity, biomarkers (CRP, IL‐8, TNF‐alpha, hospital length of stay, antibiotic use, dyspnoea, D‐dimer, cross‐sectional area of the quadriceps, lung function, arterial blood gases, adverse events |
| Starting date | 16 November 2016 |
| Contact information | Lei Pan; bzyxy2013@163.com |
| Notes |
JPRN‐UMIN000024443.
| Trial name or title | Effect of Belt Electrode ‐ Skeletal Muscle Electrical Stimulation (B‐SES) on Physical Performance in COPD Patients |
| Methods | Randomised controlled trial |
| Participants | People with COPD |
| Interventions | NMES together with home‐based exercise training vs home‐based exercise training |
| Outcomes | Muscle strength, gait speed, 6MWD, dyspnoea, ADL score, CAT, BODE |
| Starting date | Unknown |
| Contact information | Marcio Makoto Nishida (marcio.nishida@gmail.com) |
| Notes | upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000027824 |
NCT01799330.
| Trial name or title | Rehabilitation of Patients with COPD using Electrical Muscle Stimulation |
| Methods | Randomised controlled trial |
| Participants | People with COPD |
| Interventions | NMES vs sham stimulation |
| Outcomes | Incremental shuttle walk distance, body composition, body mass index, pulmonary function, muscle strength, 6MWD, cardiopulmonary exercise testing, functional limitation resulting from dyspnoea, HRQoL |
| Starting date | Unknown, but estimated completion date was June 2016 |
| Contact information | Carolyn L Rochester, MD |
| Notes |
NCT02321163.
| Trial name or title | A New Paradigm of Neuromuscular Electrical Stimulation in Attenuating Muscle Atrophy: a Randomised Controlled Trial |
| Methods | Randomised controlled trial |
| Participants | People with COPD |
| Interventions | NMES (2 different stimulation protocols) vs sham stimulation |
| Outcomes | Muscle cross‐sectional area, muscle force, muscle activation, 6MWD, protocol acceptability, discomfort during stimulation |
| Starting date | February 2016 |
| Contact information | Contact: Simon S Yeung, PhD; + 852 27666705; simon.yeung@polyu.edu.hk Contact: Ella W Yeung, PhD; 852 27666748; ella.yeung@polyu.edu.hk |
| Notes |
6MWD: six‐minute walk test; ADL: activities of daily living; BODE: Body mass index, airflow Obstruction, Dyspnea and Exercise capacity; CAT: COPD Assessment Test; CRP: C‐reactive protein; COPD: chronic obstructive pulmonary disease; HRQoL: health‐related quality of life; IL: interleukin; NMES: neuromuscular electrostimulation; TNF: tumour necrosis factor.
Differences between protocol and review
We excluded studies that used a randomised cross‐over design. We modified the wording of our primary outcomes to clarify that the muscle‐specific measures (i.e. strength and endurance) needed to relate to a peripheral muscle and that muscle size measures needed to relate to the thigh muscles only.
We defined serious adverse events as mortality only. We excluded studies that randomly assigned one leg to receive NMES and the other leg to receive control from the meta‐analyses on mortality.
An additional author joined the team (VC) and assisted with screening of the studies, assessing the risk of bias, extracting data, entering the data into Revman, completing the meta‐analyses and writing the manuscript . Additional authors assisted with data extraction.
Due to the very low number of adverse events, we reported dichotomous data (for adverse events) as RD rather than odds ratio. To optimise the number of studies that could be included in the calculation of MDs, we extracted data as medians, interquartile ranges or range and converted them to mean and SDs using online software (Wan 2014).
Two studies randomly assigned one limb of a person to receive NMES and the other limb to receive control (Giavedoni 2012; Latimer 2013). Therefore, rather than undertaking a separate analysis of these studies, we undertook a sensitivity analysis that excluded studies that used this design to see whether this changed our estimate of the effect.
The search found one abstract that compared different NMES interventions versus a control condition (e.g. NMES protocol A versus NMES protocol B versus control) (Dolmage 2016), but we were unable to include these data in the meta‐analyses as the small sample size precluded us from dividing the control group data evenly, in accordance with Section 16.5.4 in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The limited number of studies available for this review precluded the planned subgroup analyses based on clinical stability, stimulation frequency, disease severity or minimal training dose. When a meta‐analysis included data from all but one study that recruited participants who were clinically stable, we conducted sensitivity analyses by excluding the one study that recruited participants during an exacerbation to ascertain the influence this study had on the estimate of the effect. Similarly, when a meta‐analysis included all but one study that provided 10 or more training sessions in four weeks, we conducted sensitivity analyses by excluding the one study that did not provide this minimum training dose to determine the influence of this study on the estimate of the effect.
The planned sensitivity analysis in which quality indicators such as using a fixed‐effect model, missing data, concealed allocation, assessor blinding, intention‐to‐treat analysis, or a combination of these were to be used, were not undertaken.
Contributions of authors
KH drafted the protocol, screened the studies, assessed the risk of bias, extracted the data, entered the data into Review Manager 5, undertook the meta‐analyses and drafted the manuscript.
VC assisted with screening of the studies, assessing the risk of bias, extracting data, entering the data into Review Manager 5, completing the meta‐analyses and writing the manuscript.
SM assisted with screening the studies, assessing the risk of bias, extracting the data, editing and refining the protocol, and reviewed the final manuscript.
MR assisted with editing and refining the protocol, extracting the data and reviewed the final manuscript.
TJF assisted with editing and refining the protocol, extracting the data and reviewed the final manuscript.
PR assisted with editing and refining the protocol, extracting the data and reviewed the final manuscript.
TED assisted with providing critical appraisal, editing and refining the protocol, and writing the paper.
RG assisted with editing and refining the protocol, and reviewed the final manuscript.
Sources of support
Internal sources
School of Physiotherapy and Exercise Science, Faculty of Health Sciences, Curtin University, Australia.
External sources
-
Cancer Council Western Australia, Australia.
Vinicius Cavalheri is supported by a Cancer Council Western Australia Postdoctoral Fellowship
Declarations of interest
KH declared no conflict of interest.
VC declared no conflict of interest.
SM declared no conflict of interest.
MR declared no conflict of interest.
TJF declared no conflict of interest.
PR declared no conflict of interest.
TED declared no conflict of interest.
RG declared no conflict of interest.
New
References
References to studies included in this review
Abdellaoui 2011 {published and unpublished data}
- Abdellaoui A, Prefaut C, Gouzi F, Couillard A, Coisy‐Quivy M, Hugon G, et al. Skeletal muscle effects of electrostimulation after COPD exacerbation: a pilot study. European Respiratory Journal 2011;38(4):781‐8. [] [DOI] [PubMed] [Google Scholar]
Akar 2017 {published and unpublished data}
Akinlabi 2013 {published and unpublished data}
- Akinlabi K, Main E, Garrod R, Harvey A. Neuromuscular electrical stimulation (NMES), a new strategy in the pulmonary rehabilitation of patients with severe and very severe MRC 4 and 5 chronic obstructive pulmonary disease (COPD) [Abstract]. Thorax 2013;68(Suppl 3):A17 [S28]. [Google Scholar]
Bourjeily‐Habr 2002 {published and unpublished data}
- Bourjeily G, Palermo F, Rochester C, Mohsenin V. Transcutaneous electrical stimulation of the lower extremities improves muscle mass, strength and exercise endurance in severe chronic obstructive pulmonary disease (COPD). American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A21. [; 4900100000012381] [Google Scholar]
- Bourjeily‐Habr G, Rochester CL, Palermo F, Snyder P, Mohsenin V. Randomised controlled trial of transcutaneous electrical muscle stimulation of the lower extremities in patients with chronic obstructive pulmonary disease. Thorax 2002;57(12):1045‐9. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dang 2011 {published and unpublished data}
- Dang DN, Gluck M, Pirnay F, Louis R, Vanderthommen M. Interest of neuromuscular electro‐stimulation (NMES) in COPD patients during an ambulatory comprehensive respiratory rehabilitation program [Abstract]. European Respiratory Journal 2011;38(55):P4807. [] [Google Scholar]
Dolmage 2016 {published data only}
- Dolmage TE, Brooks D, Goldstein RS. The effects of high and low frequency transcutaneous electrical muscle stimulation (TCEMS) on exercise in patients with COPD: a pilot study. American Journal of Respiratory and Critical Care Medicine 2016; Vol. 193:A3990.
Giavedoni 2012 {published data only (unpublished sought but not used)}
- Giavedoni S, Deans A, McCaughey P, Drost E, MacNee W, Rabinovich RA. Neuromuscular electrical stimulation prevents muscle function deterioration in exacerbated COPD: a pilot study. Respiratory Medicine 2012;106(10):1429‐34. [] [DOI] [PubMed] [Google Scholar]
- Giavedoni S, MacNee W, Rabinovich R. Effect of neuro‐muscular electrical stimulation on muscle dysfunction in exacerbations of COPD [Abstract]. European Respiratory Journal 2010:3655. [] [Google Scholar]
Gigliotti 2004 {published data only (unpublished sought but not used)}
- Gigliotti F, Ballini M, Bertolini MG, Bianchi R, Romagnoli I, Grazzini M, et al. Effect of neuromuscular electrical stimulation (NMES) as an adjunct to an exercise training program (EXT) in patients with severe COPD [Abstract]. European Respiratory Journal 2004;24(Suppl 48):256s. [] [Google Scholar]
Kucio 2016 {published data only}
- Kucio C, Niesporek J, Kucio E, Narloch D, Wegrzyn B. Evaluation of the effects of neuromuscular electrical stimulation of the lower limbs combined with pulmonary rehabilitation on exercise tolerance in patients with chronic obstructive pulmonary disease. Journal of Human Kinetics 2016;54:75‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Latimer 2013 {published and unpublished data}
- Latimer L, Greening N, Morgan M, Singh S, Bradding P, Steiner M. Unilateral neuromuscular electrical stimulation (NMES) of the quadriceps muscles in stable COPD. European Respiratory Journal 2013;42:P3571. [Google Scholar]
Maddocks 2016 {published and unpublished data}
- Maddocks M, Nolan CM, Man WD, Polkey MI, Hart N, Gao W, et al. Neuromuscular electrical stimulation to improve exercise capacity in patients with severe COPD: a randomised double‐blind, placebo‐controlled trial. Lancet Respiratory Medicine 2016;4:27‐36. [DOI] [PubMed] [Google Scholar]
Neder 2002 {published data only (unpublished sought but not used)}
- Neder JA, Sword D, Cochrane LK, Mackay E, Ward SA, Clark CJ. A new rehabilitative strategy for severely‐disabled patients with advanced COPD: neuromuscular electrical stimulation. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A967. [] [Google Scholar]
- Neder JA, Sword D, Ward SA, Mackay E, Cochrane LM, Clark CJ. Home based neuromuscular electrical stimulation as a new rehabilitative strategy for severely disabled patients with chronic obstructive pulmonary disease (COPD). Thorax 2002;57(4):333‐7. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tardif 2015 {published and unpublished data}
- Roy AL, Dupuis J, Viacroze C, Debeaumont D, Quieffin J, Marques MH, et al. Effects of neuromuscular electrical stimulation in addition to a rehabilitation program in patients with chronic obstructive pulmonary disease [Abstract]. Annals of Physical and Rehabilitation Medicine 2013;56:e327. [Google Scholar]
- Tardif C, Roy AL, Viacroze C, Debeaumont D, Quieffin J, Marques MH, et al. Does neuromuscular electrical stimulation of the quadriceps during rehabilitation in COPD outpatients adds a benefit?. European Respiratory Journal 2015;46:OA1755. [DOI: 10.1183/13993003.congress-2015.OA1755] [DOI] [Google Scholar]
Tasdemir 2015 {published and unpublished data}
- Tasdemir F, Inal‐Ince D, Ergun P, Kaymaz D, Demir N, Demirci E, et al. Effects of exercise training and neuromuscular electrical stimulation on symptoms, muscle strength, exercise capacity, activities of daily living, and quality of life in COPD [Abstract]. European Respiratory Journal 2012;40:P2835. [] [Google Scholar]
- Tasdemir F, Inal‐Ince D, Ergun P, Kaymaz D, Demir N, Demirci E, et al. Neuromuscular electrical stimulation as an adjunct to endurance and resistance training during pulmonary rehabilitation in stable chronic obstructive pulmonary disease. Expert Review of Respiratory Medicine 2015;9:493‐502. [DOI] [PubMed] [Google Scholar]
Vieira 2014 {published data only (unpublished sought but not used)}
- Neves L, Vieira P, Chiappa A, Cipriano G, Umpierre D, Arena R, et al. Neuromuscular electrical stimulation improves clinical and physiological function in COPD patients [Abstract]. European Respiratory Journal 2014;44:P1289. [DOI] [PubMed] [Google Scholar]
- Vieira PJ, Chiappa AM, Cipriano G Jr, Umpierre D, Arena R, Chiappa GR. Neuromuscular electrical stimulation improves clinical and physiological function in COPD patients. Respiratory Medicine 2014;108(4):609‐20. [DOI: 10.1016/j.rmed.2013.12.013] [DOI] [PubMed] [Google Scholar]
Vivodtzev 2006 {published and unpublished data}
- Vivodtzev I, Pepin J‐L, Vottero G, Mayer V, Porsin B, Levy P, et al. Improvement in quadriceps strength and dyspnea in daily tasks after 1 month of electrical stimulation in severely deconditioned and malnourished COPD. Chest 2006;129(6):1540‐8. [] [DOI] [PubMed] [Google Scholar]
- Vivodtzev I, Pepin JL, Vottero G, Mayer V, Porsin B, Levy P, et al. Improvement in quadriceps strength and dyspnea in daily tasks after 1‐month of electrical stimulation in severely deconditioned and malnourished COPD [Abstract]. Proceedings of the American Thoracic Society 2006:A495. [] [DOI] [PubMed] [Google Scholar]
Vivodtzev 2012 {published and unpublished data}
- Vivodtzev I, Debigare R, Dube A, Pare M‐E, Gagnon P, Mainguy V. Molecular mechanisms of muscle hypertrophy after neuromuscular electrical stimulation in patients with severe COPD [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181:A3762. [] [Google Scholar]
- Vivodtzev I, Debigare R, Gagnon P, Mainguy V, Saey D, Dube A, et al. Functional and muscular effects of neuromuscular electrical stimulation in patients with severe COPD: a randomized clinical trial. Chest 2012;141(3):716‐25. [] [DOI] [PubMed] [Google Scholar]
- Vivodtzev I, Gagnon P, Mainguy V, Belanger M, Maltais F. Mechanisms of improvement in exercise tolerance after neuromuscular electrical stimulation training in patients with severe COPD [Abstract]. European Respiratory Society 19th Annual Congress; 2009 Sept 12‐15; Vienna. 2009. []
Zanotti 2003 {published and unpublished data}
- Zanotti E, Felicetti G, Maini M, Fracchia C. Peripheral muscle strength training in bed‐bound patients with COPD receiving mechanical ventilation: effect of electrical stimulation. Chest 2003;124(1):292‐6. [] [DOI] [PubMed] [Google Scholar]
Zanotti 2010 {published data only (unpublished sought but not used)}
- Zanotti E, Grasso R, Zocchi L, Biglieri M, Bagliani S, Mombaruzzo Petal. Combination of pulmonary rehabilitation and neuromuscular electrical stimulation in moderately impaired COPD patients [Abstract]. European Respiratory Society 20th Annual Congress; 2010 Sept 18‐22; Barcelona. 2010:3654. []
References to studies excluded from this review
Alexandrov 1990 {published data only}
- Alexandrov O, Manakova E, Sevrunova O. Transcutaneous electrostimulation of diaphragm (TESD) in complex treatment of chronic obstructive pulmonary diseases (COPD). European Respiratory Journal 1990;3(Suppl 10):208S. [] [Google Scholar]
Bustamante 2008 {published data only}
- Bustamante V, Casanova J, Lopez de Santamaria E, Mas S, Sellares J, Gea J, et al. Redox balance following magnetic stimulation training in the quadriceps of patients with severe COPD. Free Radical Research 2008;42(11‐12):939‐48. [] [DOI] [PubMed] [Google Scholar]
- Bustamante V, Lopez de Santa Maria E, Gorostiza A, Jimenez U, Galdiz JB. Muscle training with repetitive magnetic stimulation of the quadriceps in severe COPD patients. Respiratory Medicine 2010;104(2):237‐45. [] [DOI] [PubMed] [Google Scholar]
Bustamante 2013 {published data only}
- Bustamante V, López de Santamaría E, Marina N, Gorostiza A, Fernández Z, Gáldiz J. Neuromuscular magnetic stimulation of the quadriceps muscle after COPD exacerbations [Abstract]. European Respiratory Journal 2013;42(Suppl 57):734s. [Google Scholar]
Chaplin 2013 {published data only}
- Chaplin EJ, Houchen L, Greening NJ, Harvey‐Dunstan T, Morgan MD, Steiner MC, et al. Neuromuscular stimulation of quadriceps in patients hospitalised during an exacerbation of COPD: a comparison of low (35 Hz) and high (50 Hz) frequencies. Physiotherapy Research International 2013;18(3):148‐56. [] [DOI] [PubMed] [Google Scholar]
Cox 2014 {published data only}
- Cox PD, Bartsch S, Haouzi P. Sensory distraction via cutaneous stimulation reduces dyspnea in patients with obstructive lung disease [Abstract]. American Journal of Respiratory and Critical Care Medicine 2014;189:A6723. [Google Scholar]
Dal Corso 2005 {published data only}
- Dal Corso S, Malaguti C, Napolis LM, Setta JH, Fuccio MB, Nery LE, et al. Predictors of functional improvement after neuromuscular electrical stimulation in patients with COPD, a randomized controlled study [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, CA. 2005:B89. []
- Dal Corso S, Napolis L, Malaguti C, Gimenes AC, Albuquerque A, Nogueira CR, et al. Skeletal muscle structure and function in response to electrical stimulation in moderately impaired COPD patients. Respiratory Medicine 2007;101(6):1236‐43. [] [DOI] [PubMed] [Google Scholar]
Dall'Aqua 2012 {published data only}
- Dall'Aqua AM, Dohnert MB, Santos LJ. Neuromuscular electrical stimulation with Russian current for expiratory muscle training in patients with chronic obstructive pulmonary disease. Journal of Physical Therapy Science 2012;24(10):955‐9. [] [Google Scholar]
Davis 2001 {published data only}
- Davis CL, Lewith GT, Broomfield J, Prescott P. A pilot project to assess the methodological issues involved in evaluating acupuncture as a treatment for disabling breathlessness. Journal of Alternative and Complementary Medicine 2001;7(6):633‐9. [] [DOI] [PubMed] [Google Scholar]
Fauge 2014 {published data only}
- Fauge A, Alexandre F, Tremey E, Oliver N, Varray A, Heraud N. High vs low‐frequency neuromuscular electrical stimulation in COPD patients: what happens when current quantity is matched? [Abstract]. European Respiratory Journal 2014;44:P626. [Google Scholar]
Feschenko 2003 {published data only}
- Feschenko Y, Yashyna L, Dzhavad I, Gumenuk G, Milheeva K. Influence of electrostimulation of pharyngeal and laryngeal muscles in complex with traditional treatment of COPD on functional signs of obstructive sleep apnea syndrome in patients with combined pathology obstructive sleep apnea syndrome COPD [Abstract]. European Respiratory Journal 2007;30(Suppl 51):506s. [] [Google Scholar]
- Feschenko YI, Yashyna LA, Dzhavad IV, Ignatieva VI, Gumenuk GL. Influence of electrostimulation of pharyngeal and laryngeal muscles with application of prolonged beta 2 agonist on functional signs of obstructive sleep apnea syndrome in patients with obstructive sleep apnea syndrome combined with chronic obstructive bronchitis or bronchial asthma [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P2953. [] [Google Scholar]
Flaugher 2014 {published data only}
- Flaugher M, Callaghan M, Nouri B. Performance of thermal‐enhanced electrical muscle stimulation in challenging subjects: a randomized controlled trial [Abstract]. American Journal of Respiratory and Critical Care Medicine 2014;189:A6664. [Google Scholar]
Gagnon 2009 {published data only}
- Gagnon P, Saey D, Vivodtzev I, Laviolette L, Mainguy V, Milot J, et al. Impact of preinduced quadriceps fatigue on exercise response in chronic obstructive pulmonary disease and healthy subjects. Journal of Applied Physiology 2009;107(3):832‐40. [] [DOI] [PubMed] [Google Scholar]
Greening 2014 {published data only}
- Greening NJ, Williams JE, Hussain SF, Harvey‐Dunstan TC, Bankart MJ, Chaplin EJ, et al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trial. BMJ 2014;349:g4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2012 {published data only}
- Gupta Charu S. Effect of Acu‐TENS on pulmonary functions in patients with acute exacerbation of chronic obstructive pulmonary disease. Indian Journal of Physiotherapy and Occupational Therapy 2012;6(4):115‐9. [] [Google Scholar]
Han 2010 {published data only}
- Han Y, Zhang Y, Xie DP. Effect of Chinese medicine intestine adjusting therapy on patients with respiratory failure caused by acute exacerbation of chronic obstructive pulmonary disease and undergoing noninvasive ventilation [Chinese]. Zhongguo Zhong Xi Yi Jie He Xue Hui, Zhongguo Zhong Yi Yan Jiu Yuan Zhu Ban 2010;30(8):814‐8. [] [PubMed] [Google Scholar]
Hatipoglu 1999 {published data only}
- Hatipoglu U, Laghi F, Tobin MJ. Does inhaled albuterol improve diaphragmatic contractility in patients with chronic obstructive pulmonary disease?. American Journal of Respiratory and Critical Care Medicine 1999;160(6):1916‐21. [] [DOI] [PubMed] [Google Scholar]
Ito 2012 {published data only}
- Ito K, Nozoe T, Okuda M, Nonaka K, Horie J, Kawamura H. Effects of phasic electrical stimulation during expiration in elderly chronic obstructive pulmonary disease: a pilot study [Abstract]. Respirology 2012;17(Suppl 2):146. [] [Google Scholar]
- Ito K, Nozoe T, Okuda M, Nonaka K, Horie J, Kawamura H. Effects of phasic electrical stimulation during expiration in elderly patients with chronic obstructive pulmonary disease: a randomised control trial [Abstract]. European Respiratory Journal 2013;42(Suppl 57):448s. [Google Scholar]
- Ito K, Nozoe T, Okuda M, Nonaka K, Yamahara J, Horie J, et al. Electrically stimulated ventilation feedback improves the ventilation pattern in patients with COPD. Journal of Physical Therapy Science 2015;27:325‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2011 {published data only}
- Jones AY, Ngai SP, Hui‐Chan CW, Yu HP. Acute effects of Acu‐TENS on FEV1 and blood [beta]‐endorphin level in chronic obstructive pulmonary disease. Alternative Therapies in Health and Medicine 2011;17(5):8‐13. [] [PubMed] [Google Scholar]
- Ngai SP, Jones AY, Hui‐Chan CW, Ko FW, Hui DS. Effect of 4 weeks of Acu‐TENS on functional capacity and beta‐endorphin level in subjects with chronic obstructive pulmonary disease: A randomized controlled trial. Respiratory Physiology and Neurobiology 2010;173(1):29‐36. [] [DOI] [PubMed] [Google Scholar]
Kaymaz 2015 {published data only}
- Kaymaz D, Ergun P, Demirci E, Demir N. Comparison of the effects of neuromuscular electrical stimulation and endurance training in patients with severe chronic obstructive pulmonary disease. Tuberkuloz ve Toraks 2015;63:1‐7. [DOI] [PubMed] [Google Scholar]
Klock 2001 {published data only}
- Klock PA, Czeslick EG, Klafta JM, Ovassapian A, Moss J. The effect of sevoflurane and desflurane on upper airway reactivity. Anesthesiology 2001;94(6):963‐7. [] [DOI] [PubMed] [Google Scholar]
Kurtoglu 2011 {published data only}
- Kurtoglu DK, Tastekin N, Birtane M, Sut N, Tabakotlu E. The effectiveness of neuromuscular electrical stimulation applications on accessory respiratory muscles in patients with chronic obstructive pulmonary disease in intensive care unit. Turkish Journal of Physical Medicine and Rehabilitation 2011;57(Suppl 2):275. [] [Google Scholar]
Lau 2008 {published data only}
- Lau KS, Jones AY. A single session of Acu‐TENS increases FEV1 and reduces dyspnoea in patients with chronic obstructive pulmonary disease: a randomised, placebo‐controlled trial. Australian Journal of Physiotherapy 2008;54(3):179‐84. [] [DOI] [PubMed] [Google Scholar]
Lewith 2004 {published data only}
- Lewith GT, Prescott P, Davis CL. Can a standardized acupuncture technique palliate disabling breathlessness: a single‐blind, placebo‐controlled crossover study. Chest 2004;125(5):1783‐90. [] [DOI] [PubMed] [Google Scholar]
Liu 2015 {published data only}
- Liu X, Fan T, Lan Y, Dong S, Fu J, Mao B. Effects of transcutaneous electrical acupoint stimulation on patients with stable chronic obstructive pulmonary disease: a prospective, single‐blind, randomized, placebo‐controlled study. Journal of Alternative and Complementary Medicine 2015;21:610‐6. [DOI] [PubMed] [Google Scholar]
- Liu X, Fan T, Lan Y, Dong S, Fu J, Mao B. Effects of transcutaneous electrical acupoint stimulation on patients with stable chronic obstructive pulmonary disease: a prospective, single‐blind, randomized, placebo‐controlled study. Journal of Alternative and Complementary Medicine 2015;21:610‐6. [DOI] [PubMed] [Google Scholar]
Lopez 2017 {published data only}
- Lopez LL, Valenza MC, Sanchez IT, Martos IC, Ramirez MPM, Rubio AO. Results between two electrostimulation modalities in AECOPD patients. European Respiratory Journal 2017;50:PA2562. [DOI: 10.1183/1393003.congress-2017.PA2562] [DOI] [Google Scholar]
Lotvall 1994 {published data only}
- Lotvall J, Lunde H, Augustinson LE, Hedner T, Svedmyr N, Ben‐Menachem E. Airway effects of direct left‐sided cervical vagal stimulation in patients with complex partial seizures. Epilepsy Research 1994;18(2):149‐54. [] [DOI] [PubMed] [Google Scholar]
Malaguti 2009 {published data only}
- Malaguti C, Rondelli RR, Paz JR, Reis LC, Dal Corso S. Constant or adjustable intensity of electrical current does not elicit different level of quadriceps femoris fatigue during neuromuscular electrical stimulation sessions in patients with COPD [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20; San Diego, CA. 2009:A3402. []
Martin‐Salvador 2016 {published data only}
- Martin‐Salvador A, Colodro‐Amores G, Torres‐Sanchez I, Moreno‐Ramirez MP, Cabrera‐Martos I, Valenza MC. Physical therapy intervention during hospitalization in patients with acute exacerbation of chronic obstructive pulmonary disease and patients with pneumonia: a randomized clinical trial. Medicina Clinica 2016;146:301‐4. [DOI] [PubMed] [Google Scholar]
Medrinal 2016 {published data only}
- Medrinal C, Prieur G, Combret Y, Quesada AR, Debeaumont D, Quieffin J, et al. Oxygen uptake during cycle ergometry with and without functional electrical stimulation in COPD patients: a randomised placebo‐controlled cross‐over trial. European Respiratory Journal 2017;50(Suppl 61):OA2920. [DOI: 10.1183/1393003.congress-2017.OA2920] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrinal C, Prieur G, Debeaumont D, Quesada A R, Combret Y, Quieffin J, et al. Comparison of oxygen uptake during cycle ergometry with and without functional electrical stimulation in patients with COPD: protocol for a randomised, single‐blind, placebo‐controlled, cross‐over trial. British Medical Journal Open Respiratory Research 2016;3:e000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meesen 2010 {published data only}
- Meesen RL, Dendale P, Cuypers K, Berger J, Hermans A, Thijs H, et al. Neuromuscular electrical stimulation as a possible means to prevent muscle tissue wasting in artificially ventilated and sedated patients in the intensive care unit: a pilot study. Neuromodulation 2010;13(4):315‐20; discussion 321. [] [DOI] [PubMed] [Google Scholar]
Napolis 2006 {published data only}
- Napolis LM, Corso SD, Gimenes AC, Albuquerque AL, Villaca DS, Nogueira CR, et al. Neuromuscular electrical simulation can improve whole‐body exercise capacity and quality of life in patients with COPD: a randomized and controlled study [Abstract]. European Respiratory Journal 2006;28(Suppl 50):299s. [] [Google Scholar]
- Napolis LM, Dal Corso S, Neder JA, Malaguti C, Gimenes AC, Nery LE. Neuromuscular electrical stimulation improves exercise tolerance in chronic obstructive pulmonary disease patients with better preserved fat‐free mass. Clinics (Sao Paulo, Brazil) 2011;66(3):401‐6. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ngai 2010 {published data only}
- Ngai S, Alison J, Spencer L, Jones A. Acu‐TENS reduces breathlessness during exercise in people with COPD [Abstract]. European Respiratory Journal 2012;40(Suppl 56):96s. [] [Google Scholar]
- Ngai SP, Spencer LM, Jones AY, Alison JA. Acu‐TENS reduces breathlessness during exercise in people with chronic obstructive pulmonary disease. Evidence‐based Complementary and Alternative Medicine 2017;5:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai SP‐C. Effect of Acu‐TENS on Airway Obstructive Disease [Dissertation]. Hung Hom (Hong Kong): Hong Kong Polytechnic University, 2010. [] [Google Scholar]
Oncu 2016 {published data only}
- Oncu E, Zincir H. The effect of transcutaneous electrical nerve stimulation in patients with acute exacerbation of chronic obstructive pulmonary disease: randomized controlled trial. Journal of Clinical Nursing 2016;26:1834‐44. [DOI] [PubMed] [Google Scholar]
Sanchez 2016 {published data only}
- Sanchez IT, Valenza MC, Corral Nunez‐Flores T, Garcia CM, Torres JR, Demet GV. Home‐based rehabilitation program with superimposed neuromuscular electrical stimulation in stable COPD patients. European Respiratory Journal 2016;48:PA532. [Google Scholar]
Sillen 2008 {published data only}
- Sillen M, Franssen F, Delbressine J, Vaes A, Wouters E, Spruit M. Effects of 8‐wk muscle training in dyspneic COPD patients: results from the DICES study [Abstract]. European Respiratory Journal 2013;42(Suppl 57):392s. [Google Scholar]
- Sillen MJ, Franssen FM, Delbressine JM, Vaes AW, Wouters EF, Spruit MA. Efficacy of lower‐limb muscle training modalities in severely dyspnoeic individuals with COPD and quadriceps muscle weakness: results from the DICES trial. Thorax 2014;69(6):525‐31. [DOI] [PubMed] [Google Scholar]
- Sillen MJ, Franssen FM, Vaes AW, Delbressine JM, Wouters EF, Spruit MA. Metabolic load during strength training or NMES in individuals with COPD: results from the DICES trial. BMC Pulmonary Medicine 2014;14:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillen MJ, Janssen PP, Akkermans MA, Wouters EF, Spruit MA. The metabolic response during resistance training and neuromuscular electrical stimulation (NMES) in patients with COPD: a pilot study. Respiratory Medicine 2008;102:786‐9. [DOI] [PubMed] [Google Scholar]
- Sillen MJ, Wouters EF, Franssen FM, Meijer K, Stakenborg KH, Spruit MA. Oxygen uptake, ventilation, and symptoms during low‐frequency versus high‐frequency NMES in COPD: a pilot study. Lung 2011;189(1):21‐6. [] [DOI] [PubMed] [Google Scholar]
Sillen 2013 {published data only}
- Sillen MJ, Franssen FM, Gosker HR, Wouters EF, Spruit MA. Metabolic and structural changes in lower‐limb skeletal muscle following neuromuscular electrical stimulation: a systematic review. PloS One 2013;8:e69391. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Valenza 2017 {published data only}
- Valenza MC, Torres‐Sanchez I, Lopez‐Lopez L, Cabrera‐Martos I, Ortiz‐Rubio A, Valenza‐Demet G. Effects of home‐based superimposed neuromuscular electrical stimulation in severe chronic obstructive pulmonary disease patients: a randomized controlled clinical trial. European Journal of Physical and Rehabilitation Medicine 2017 Nov 16 [Epub ahead of print]. [DOI: 10.23736/S1973-9087.17.04745-1] [DOI] [PubMed]
Vivodtzev 2014 {published data only}
- Vivodtzev I, Rivard B, Gagnon P, Mainguy V, Dube A, Belanger M, et al. Tolerance and physiological correlates of neuromuscular electrical stimulation in COPD: a pilot study. PloS One 2014;9:e94850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wen 2011 {published data only}
- Wen Q, Li N, Yu PM. Randomized controlled trial on the effect of transcutaneous electrical nerve stimulation at Dingchuan (EX‐B1) on the pulmonary function of patients with COPD at acute stage [Chinese]. Zhongguo Zhen Jiu [Chinese Acupuncture & Moxibustion] 2011;31(2):97‐100. [] [PubMed] [Google Scholar]
Yang 2005 {published data only}
- Yang S‐Y, Feng E‐Z, Shen J‐L, Zhang Y, Zhao L‐H, Wu X‐M, et al. Effect of suitable electrostimulation on phrenic nerve in ameliorating diaphragmatic fatigue and pulmonary function in patients with stable chronic cor pulmonale at high altitude area [Chinese]. Zhongguo Linchuang Kangfu 2005;9(23):28‐30. [] [Google Scholar]
References to studies awaiting assessment
Chen 2017 {published data only}
- Chen Y‐H, Kao K‐C, Li L‐F, Hsiao H‐F, Chen N‐H, Huang C‐C. Effects of electrical stimulation on hospitalization outcomes in patients with prolong mechanical ventilator. American Journal of Respiratory and Critical Care Medicine 2017:A2337. [Google Scholar]
References to ongoing studies
ChiCTR‐IPR‐16009845 {published data only}
- ChiCTR‐IPR‐16009845. Clinical trial of neuromuscular electrical stimulation of the lower extremities in acute exacerbated patients with chronic obstructive pulmonary disease. www.chictr.org.cn/showprojen.aspx? Proj=16588 (accessed prior to 9 April 2018).
JPRN‐UMIN000024443 {published data only}
- JPRN‐UMIN000024443. Effect of belt electrode ‐ skeletal muscle electrical stimulation (B‐SES) on physical performance in COPD patients. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000027824 (accessed prior to 9 April 2018).
NCT01799330 {published data only}
- NCT01799330. Rehabilitation of patients with COPD using electrical muscle stimulation. clinicaltrials.gov/show/NCT01799330 (accessed prior to 9 April 2018). []
NCT02321163 {published data only}
- NCT02321163. A new paradigm of neuromuscular electrical stimulation in attenuating muscle atrophy: a randomised controlled trial. clinicaltrials.gov/ct2/show/NCT02321163 (accessed prior to 9 April 2018).
Additional references
Buist 2007
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD study): a population‐based prevalence study. Lancet 2007;370:741‐50. [DOI] [PubMed] [Google Scholar]
Casaburi 1991
- Casaburi R, Patessio A, Ioli F, Zanaboni S, Donner CF, Wasserman K. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. American Review of Respiratory Disease 1991;143:9‐18. [DOI] [PubMed] [Google Scholar]
Chen 2016
- Chen RC, Li XY, Guan LL, Guo BP, Wu WL, Zhou ZQ, et al. Effectiveness of neuromuscular electrical stimulation for the rehabilitation of moderate‐to‐severe COPD: a meta‐analysis. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:2965‐75. [PUBMED: 27932876] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarkson 2000
- Clarkson HM. Musculoskeletal assessment: joint range of motion and manual muscle strength. 2nd Edition. Sydney: Lippincott Williams and Wilkins, 2000. [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioural Sciences. Mahwah (NJ): Lawrence Erlbaum Associates, 1988. [Google Scholar]
Filipovic 2011
- Filipovic A, Kleinoder H, Dormann U, Mester J. Electromyostimulation ‐ a systematic review of the influence of training regimens and stimulation parameters on effectiveness in electromyostimulation training of selected strength parameters. Journal of Strength and Conditioning Research 2011;25:3218‐38. [DOI] [PubMed] [Google Scholar]
Gerovasili 2009
- Gerovasili V, Tripodaki E, Karatzanos E, Pitsolis T, Markaki V, Zervakis D, et al. Short‐term systemic effect of electrical muscle stimulation in critically ill patients. Chest 2009;136:1249‐56. [DOI] [PubMed] [Google Scholar]
Gregory 2005
- Gregory CM, Bickel CS. Recruitment patterns in human skeletal muscle during electrical stimulation. Physical Therapy 2005;85:358‐64. [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henneman 1985
- Henneman E. The size‐principle: a deterministic output emerges from a set of probabilistic connections. Journal of Experimental Biology 1985;115:105‐12. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). The Cochrane Collaboration. Available from handbook.cochrane.org 2011.
Hill 2012
- Hill K, Dolmage TE, Woon L, Coutts D, Goldstein R, Brooks D. Comparing peak and submaximal cardiorespiratory responses during field walking tests with incremental cycle ergometry in COPD. Respirology 2012;17(2):278‐84. [PUBMED: 22008290] [DOI] [PubMed] [Google Scholar]
Hill 2014
- Hill K, Holland AE. Strategies to enhance the benefits of exercise training in the respiratory patient. Clinics in Chest Medicine 2014;35(2):323‐36. [PUBMED: 24874128] [DOI] [PubMed] [Google Scholar]
Holland 2010
- Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six‐minute walk distance in patients with chronic obstructive pulmonary disease. Archives of Physical Medicine and Rehabilitation 2010;91(2):221‐5. [PUBMED: 20159125] [DOI] [PubMed] [Google Scholar]
Hortobágyi 1999
- Hortobágyi T, Scott K, Lambert J, Hamilton G, Tracy J. Cross‐education of muscle strength is greater with stimulated than voluntary contractions. Motor Control 1999;2(3):205‐19. [DOI] [PubMed] [Google Scholar]
Jones 2016
- Jones S, Man WD, Gao W, Higginson IJ, Wilcock A, Maddocks M. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD009419.pub3; PUBMED: 27748503] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maffiuletti 2008
- Maffiuletti NA, Herrero AJ, Jubeau M, Impellizzeri FM, Bizzini M. Differences in electrical stimulation thresholds between men and women. Annals of Neurology 2008;63:507‐12. [DOI] [PubMed] [Google Scholar]
Maffiuletti 2010
- Maffiuletti NA. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. European Journal of Applied Physiology 2010;110:223‐34. [DOI] [PubMed] [Google Scholar]
Maltais 1997
- Maltais F, LeBlanc P, Jobin J, Berube C, Bruneau J, Carrier L, et al. Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1997;155(2):555‐61. [PUBMED: 9032194] [DOI] [PubMed] [Google Scholar]
Maltais 2000
- Maltais F, LeBlanc P, Whittom F, Simard C, Marquis K, Belanger M, et al. Oxidative enzyme activities of the vastus lateralis muscle and the functional status in patients with COPD. Thorax 2000;55:848‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maltais 2014
- Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigare R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2014;189(9):e15‐62. [PUBMED: 24787074] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2015
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD003793.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murton 2010
- Murton AJ, Greenhaff PL. Physiological control of muscle mass in humans during resistance exercise, disuse and rehabilitation. Current Opinion in Clinical Nutrition and Metabolic Care 2010;13:249‐54. [DOI] [PubMed] [Google Scholar]
Nuhr 2004
- Nuhr MJ, Pette D, Berger R, Quittan M, Crevenna R, Huelsman M, et al. Beneficial effects of chronic low‐frequency stimulation of thigh muscles in patients with advanced chronic heart failure. European Heart Journal 2004;25:136‐43. [DOI] [PubMed] [Google Scholar]
Pan 2014
- Pan L, Guo Y, Liu X, Yan J. Lack of efficacy of neuromuscular electrical stimulation of the lower limbs in chronic obstructive pulmonary disease patients: a meta‐analysis. Respirology 2014;19(1):22‐9. [PUBMED: 24256183] [DOI] [PubMed] [Google Scholar]
Parker 2005
- Parker CM, Voduc N, Aaron SD, Webb KA, O'Donnell DE. Physiological changes during symptom recovery from moderate exacerbations of COPD. European Respiratory Journal 2005;26:420‐8. [DOI] [PubMed] [Google Scholar]
Quittan 2001
- Quittan M, Wiesinger GF, Sturm B, Puig S, Mayr W, Sochor A, et al. Improvement of thigh muscles by neuromuscular electrical stimulation in patients with refractory heart failure: a single‐blind, randomized, controlled trial. American Journal of Physical Medicine and Rehabilitation 2001; Vol. 80, issue 3:206‐14; quiz 215‐6, 224. [DOI] [PubMed]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roig 2009
- Roig M, Reid WD. Electrical stimulation and peripheral muscle function in COPD: a systematic review. Respiratory Medicine 2009;103(4):485‐95. [PUBMED: 19091537] [DOI] [PubMed] [Google Scholar]
Roy 2013
- Roy AL, Dupuis J, Viacroze C, Debeaumont D, Quieffin J, Marques MH, et al. Effects of neuromuscular electrical stimulation in addition to a rehabilitation program in patients with chronic obstructive pulmonary disease [Abstract]. Annals of Physical and Rehabilitation Medicine 2013;56:e327. [Google Scholar]
Saey 2003
- Saey D, Debigare R, LeBlanc P, Mador MJ, Cote CH, Jobin J, et al. Contractile leg fatigue after cycle exercise: a factor limiting exercise in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2003;168:425‐30. [DOI] [PubMed] [Google Scholar]
Sillen 2011
- Sillen MJ, Wouters EF, Franssen FM, Meijer K, Stakenborg KH, Spruit MA. Oxygen uptake, ventilation, and symptoms during low‐frequency versus high‐frequency NMES in COPD: a pilot study. Lung 2011;189:21‐6. [DOI] [PubMed] [Google Scholar]
Sillen 2014
- Sillen MJ, Franssen FM, Delbressine JM, Vaes AW, Wouters EF, Spruit MA. Efficacy of lower‐limb muscle training modalities in severely dyspnoeic individuals with COPD and quadriceps muscle weakness: results from the DICES trial. Thorax 2014;69:525‐31. [DOI] [PubMed] [Google Scholar]
Takahashi 1993
- Takahashi M, Hood DA. Chronic stimulation‐induced changes in mitochondria and performance in rat skeletal muscle. Journal of Applied Physiology (Bethesda, Md. : 1985) 1993;74:934‐41. [DOI] [PubMed] [Google Scholar]
Vogelmeier 2017
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. American Journal of Respiratory and Critical Care Medicine 2017;195(5):557‐82. [PUBMED: 28128970] [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Hill 2013
- Hill K, Mathur S, Roig M, Janaudis‐Ferreira T, Robles P, Dolmage TE, et al. Neuromuscular electrostimulation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010821] [DOI] [PMC free article] [PubMed] [Google Scholar]


