Abstract
A library of thiosemicarbazide derivatives of isoniazid 3–27, was synthesized and evaluated for their anti-inflammatory and urease inhibition activities, by using in vitro bioassays. Among these compounds 9, 10, 12, 21, and 26 were identified as new derivatives. Prolonged use of non-steroidal anti-inflammatory drugs (NSAIDs) and infections caused by Helicobacter pylori (ureolytic bacteria), are the two most significant causes of gastric and peptic ulcers. We focused on the identification of the dual inhibitors of inflammation and urease enzyme. Compound 23 was identified as the best dual inhibitor of inflammation (ROS; IC50 = 12.3 µg/mL), and urease enzyme inhibition activity (IC50 = 22.4 µM). Many of these compounds showed comparable activities to the standard anti-inflammatory drug (ibuprofen, IC50 = 11.2 µg/mL) and urease inhibitor (thiourea/acetohydraoxamic acid, IC50 = 21.1/20.3 µM). Compound 12 was found to be the most potent urease inhibitor (IC50 = 12.3 µM) and good inhibitor of inflammation (IC50 = 27.7 µg/mL). Compounds 19, 11, 13, 9, 17, 10, and 16, were also found to be potent inhibitors of urease. Cytotoxicity was also evaluated and all the compounds were found to be non-cytotoxic, except compound 18 and the parent drug isoniazid (IC50 = 29.5 and 28.5 µM, respectively).
Subject terms: Drug discovery and development, Drug discovery and development
Introduction
Urease (urea amidohydrolase; E.C. 3.5.1.5) is a nickel-containing enzyme produced by plants, bacteria, fungi and parasites. Urease catalyzes the hydrolysis of urea into ammonia and carbon dioxide which increases the pH of stomach of the host1,2. The produced ammonia may cause several diseases, such as hepatic encephalopathy, gastric and peptic ulcers, atherosclerosis or rheumatoid arthritis. Urease produced by the Helicobacter pylori serve as a virulence factor through increasing the pH of the stomach, which helps the bacteria to colonize in the acidic environment of stomach and causes gastritis and peptic ulcers. Therefore, urease inhibitors serves as the anti-ulcer drugs3.
Inflammation is the host defense mechanism which protect the body from harmful stimuli and speeds up the restoration process4. The stimulus can be any microbial infection or chemicals. The inflammation is characterized with redness, pain, warmth, swelling and lack of function in the injured region5. The inadequate healing process of the wounds or any other dysfunction will result in a chronic inflammation which need to be treated6.
Currently Available Marketed Drugs and Their Side Effects
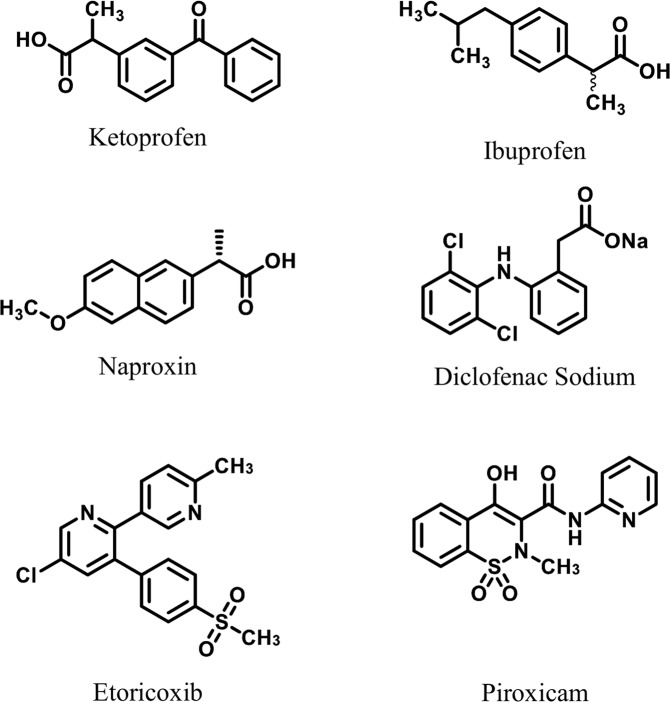
Globally used drugs for the treatment of inflammation and associated conditions, such as traumatic injuries, arthritis, fever, and pain, are non-steroidal anti-inflammatory drugs (NSAIDs), such as ketoprofen, ibuprofen, naproxin, diclofenac sodium, piroxicam, and etoricoxib7 (Fig. 1). These drugs are the selective inhibitors of cyclooxygenase-2 (COX-2) enzyme6. The major side effects caused by the NSAIDS are ulceration and gastrointestinal (GI) hemorrhage8. This has attracted the attention of the scientists towards the development of the new anti-inflammatory agents with no or less side effects9.
Figure 1.
Examples of Non-Steroidal Anti-inflammatory Drugs.
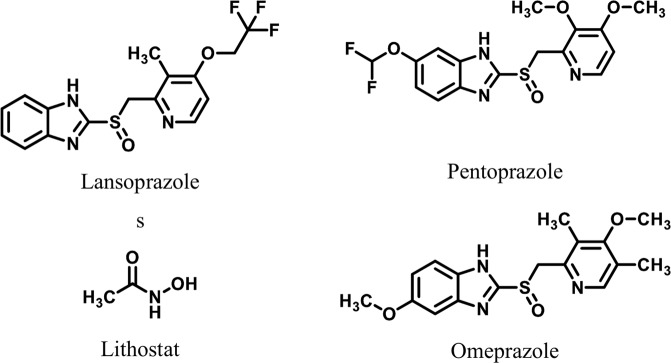
The drugs currently available for the treatment of ulceration and gsastrointestinal (GI) hemorrhage include pantoprazole, lansoprazole, lithostat, and omeprazole10 (Fig. 2). A study by Saniee.et al. (2015) showed that the proton pump inhibitors (PPI), such as omeprazole and lansoprazole, are also the urease inhibitors11. Due to the high prevalence of gastroesophageal reflux disease the use of PPIs has increased remarkably and now they are among the most frequently prescribed medicines globally12. Adverse effects of PPIs has also been observed such as nutritional deficiencies, visual impairment, chronic kidney disease13, dementia, infections, nervous system related abnormalities. The most adverse effect is the decreased in the bacterial richness and alteration in the gut microbiome. Approximately 65% increase in the risk of enteric infections development especially Clostridium difficile infection has been reported14, which limit clinical applications of PPIs15.
Figure 2.
Examples of Urease Inhibitors Used as Anti-Ulcer Drugs.
A Medicinal Chemistry Approach of Drug Discovery
Drug development is a time-intensive, costly, and high-risk process. One approach that has attracted a lot of attention in modern drug discovery is drug repositioning or repurposing16. Drug repurposing include cases in which a current drug, endorsed by an administrative organization for a particular disease, is found to have effect against another illness. Conversely, drug repositioning also depicts a condition where a drug that is in use for a disease is utilized as a template for the synthesis of new analogs possessing activity against another disease17. Drug repositioning thus essentially shorten the drug development process and thus decrease the discovery cost18.
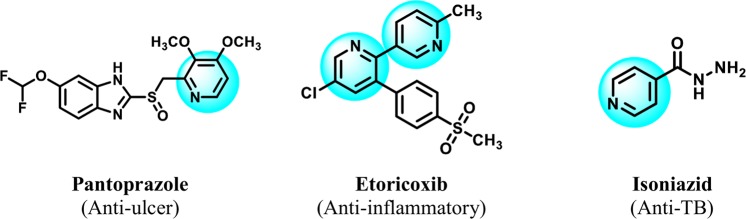
Current study describes the repositioning of isoniazid, an anti-bacterial agent. Isoniazid, was synthesized in 1952 for the treatment of tuberculosis19. The recommended daily dose of isoniazid is from 5–300 mg/day, which rarely causes side effects in individuals20. The use of isoniazid as the main scaffold for the synthesis of medicinally important compounds is well known as reported in the literature21–24 (Fig. 3). Therefore, we have randomly synthesized the library of compounds (3–27) followed by random screening against various biological targets. It was observed that some of these compounds are the significant dual inhibitors of inflammation, and urease. The structural similarity of synthesized compounds with the pyridine based anti-ulcer drug pantoprazole23, and anti-inflammatory drug etoricoxib25 may be the reason for the activities of these compounds (Fig. 4).
Figure 3.
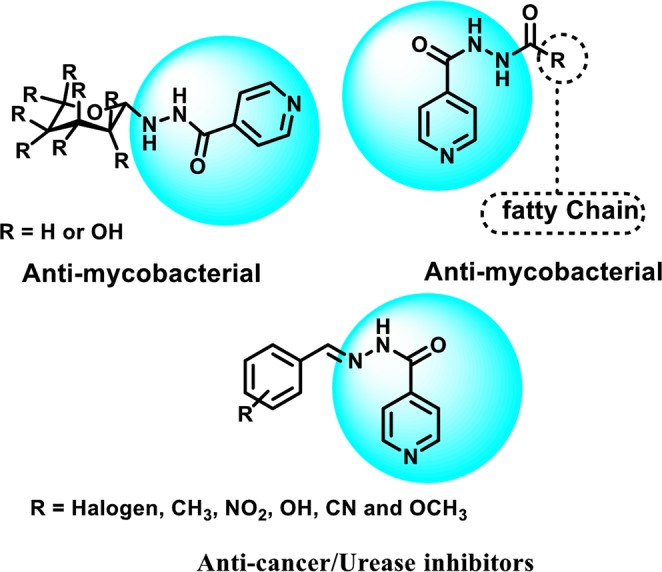
Some previously reported derivatives of isoniazid.
Figure 4.
Component structural similarities between isoniazid, etoricoxib, and pantoprazole.
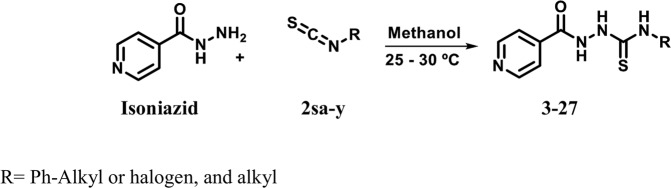
During the current study, we have synthesized thiosemicarbazide derivatives of isoniazid (3–27) through modification at terminal NH2 (Fig. 5) by reacting with different isothiocyanates. Thiosemicarbazide class of compounds possess the diverse biological activities, such as anti-cancer26, anti-fungal27, anti-helminthic28, anti-bacterial29 and anti-HIV30. Among synthesized compounds, all were identified as previously known31–38, except 9, 10, 12, 21, and 26. However, these compounds have not been reported as the dual inhibitor of inflammation and urease. Cytotoxicity of these compounds were also evaluated against 3T3 mouse fibroblast cell line.
Figure 5.
Isoniazid (1).
Results
Chemistry
Thiosemicarbazide derivatives of isoniazid (3–27) were synthesized by its reaction with various isothiocyanates using method reported by Yahyazadeh. et al. (2013) with slight modification39 (Fig. 6). The resulting product was purified through solvent-solvent extraction with hexane and ethyl acetate, followed by recrystallization with methanol. Structures of synthesized compounds were elucidated by using different spectroscopic techniques, such as mass spectrometry, infrared spectrophotometry, and 1H- and 13C-NMR spectroscopy. Among synthesized compounds, 9, 10, 12, 21, and 26 were found to be new.
Figure 6.
Synthesis of thiosemicarbazide derivatives of isoniazid (3–27).
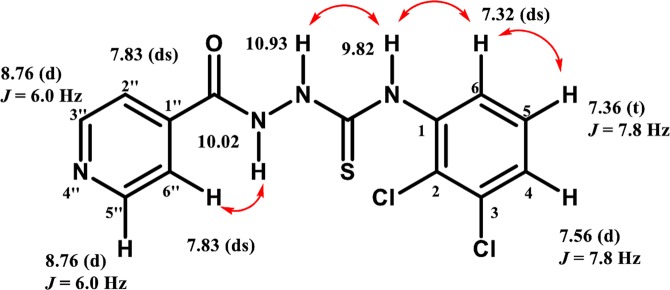
Structure Elucidation of Representative Compound 4
The structure elucidation of the synthesized thiosemicarbazide derivatives (3–27) was performed through various spectroscopic techniques (MS, NMR, and IR). Structure elucidation of compound 4, a representative member of the library, is presented in Fig. 7.
Figure 7.
NMR and Key 2D NOSEY correlations of N-(2, 3-Dichlorophenyl)−2-isonicotinoylhydrazinecarbothioamide (4).
Singlets of protons attached to nitrogen were at δ10.93, 10.02, and 9.82. A doublet at δ 8.76 showed two protons of pyridine ring i.e. H-3″, H-5″ which are coupled with H-2″, H-6″ (J3″,2″ = J5″,6″ = 6 Hz). A distorted singlet at δ 7.83 represented another two protons of pyridine ring i.e. H-2″, and H-6″. Doublet appeared at δ 7.56 showed H-4 of phenyl ring, coupled with the H-5, with ortho coupling (J4,5 = 7.8 Hz), while triplet at δ 7.36 showed a proton of phenyl ring i.e.H-5 which is coupled with H-4 and H-6 with coupling constant (J5(4–6) = 7.8 Hz). H-6 proton of phenyl ring showed a distorted singlet at δ 7.32.
The conformation of the synthesized compound was deduced based on key 2D NOESY correlations (Fig. 7). NH (δ9.82, singlet) showed correlations with the H-6 phenyl ring proton (δ7.32, distorted singlet), and NH (δ10.93, singlet). On the other hand, proton attached to nitrogen NH (δ10.02, singlet) showed correlation with pyridine ring H-6″ (δ7.83, distorted singlet).
13C NMR spectrum of compound 4 showed five characteristic peaks of quaternary carbons at δ182.0 (C=S), 165.0 (C=O), 140.0 (C-1″), 139.3 (C-1) and 132.1, 131.0 (C-2, C-3). C-3″, C-5″ and C-2″, C-6″ of pyridine ring were resonated at δ150.6 and 121.7, while C-4 and C-5 of phenyl ring were resonated at δ 128.6 and 127.5, respectively. C-6 of phenyl ring appeared at δ 129.9. The HRFAB-MS (+ve mode) was observed at m/z 341.0017 correlating with the molecular formula [(C13H10N4O1Cl2S1) + H]+ (341.0031).
Bioactivities
In Vitro Urease Inhibition Activity
Thiosemicarbazide derivatives, synthesized in the current study, were evaluated for their in vitro urease inhibitory activity. The standard inhibitor used in the assay was thiourea (IC50 = 21.1 ± 0.2 µM) and acetohydroxamic acid (IC50 = 20.3 ± 0.4 µM). Most of the synthesized thiosemicarbazides showed urease inhibition activity between the range of 63.2 ± 2.0 µM to 12.3 ± 1.04 µM (Table 1).
Table 1.
In vitro urease Inhibition, anti-inflammatory, and cytotoxicity of synthetic compounds 3–27.
| Compounds | R | Urease Inhibition Activity IC50 ± SEMa µM | Anti-Inflammatory Activity IC50 ± SDb µg/mL | Cytotoxicity (3T3 Cell line) IC50 ± SDb µM |
|---|---|---|---|---|
| 3 |
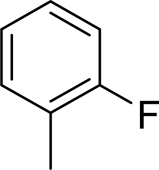
|
Inactivec | Inactive | >30 |
| 4 |
|
38.8 ± 0.35 | Inactive | >30 |
| 5 |
|
36.5 ± 0.14 | 18.5 ± 1.0 | >30 |
| 6 |
|
Insoluble | Inactive | >30 |
| 7 |
|
46.3 ± 0.43 | Inactive | >30 |
| 8 |
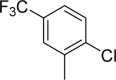
|
44.4 ± 0.43 | Inactive | >30 |
| 9 |
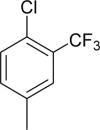
|
14.2 ± 1.38 | 26.7 ± 2.5 | >30 |
| 10 |
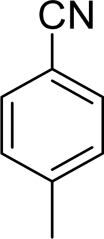
|
15.7 ± 1.32 | Inactive | >30 |
| 11 |
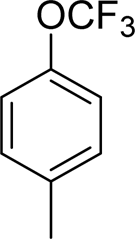
|
13.2 ± 1.64 | Inactive | >30 |
| 12 |
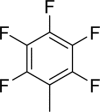
|
12.3 ± 1.04 | 27.7 ± 2.4 | >30 |
| 13 |
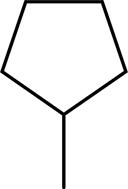
|
13.6 ± 1.61 | Inactive | >30 |
| 14 |
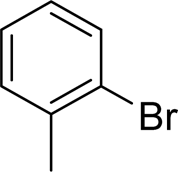
|
35.8 ± 1.89 | Inactive | >30 |
| 15 |
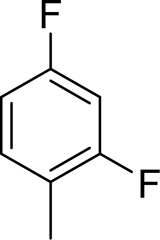
|
34.1 ± 0.45 | Inactive | >30 |
| 16 |
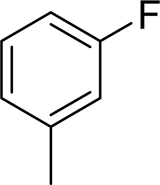
|
21.5 ± 1.3 | 29.7 ± 1.7 | >30 |
| 17 |
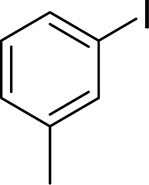
|
14.7 ± 1.01 | 36.9 ± 3.0 | >30 |
| 18 |
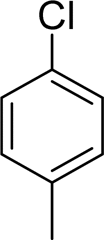
|
26.3 ± 1.59 | Inactive | 29.5 ± 1.9 |
| 19 |
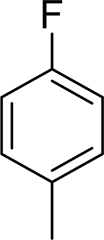
|
12.7 ± 0.8 | Inactive | >30 |
| 20 |
|
22.0 ± 1.15 | Inactive | >30 |
| 21 |
|
28.2 ± 1.48 | Inactive | >30 |
| 22 |
|
22.1 ± 1.91 | 25.1 ± 0.4 | >30 |
| 23 |
|
22.4 ± 1.83 | 12.3 ± 1.2 | >30 |
| 24 |
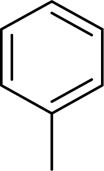
|
63.2 ± 2.0 | Inactive | >30 |
| 25 |
|
Insoluble | Inactive | >30 |
| 26 |
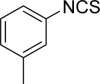
|
46.8 ± 1.8 | 25.4 ± 1.3 | >30 |
| 27 |
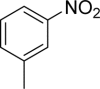
|
Insolubled | Inactive | >30 |
| 28 | Isoniazid(1) | Inactive | Inactive | 28.5 ± 1.2 |
| 29 | Thiourea (Standard) | 21.1 ± 0.2 (Observed) | — | — |
| 30 | Aetohydaoxamic acid (standard) | 20.3 ± 0.4 (Observed) 17.2 ± 0.9 (Reported) | ||
| 31 | Ibuprofen (Standard) | — | 11.2 ± 1.9 (observed) 12.97 ± 0.23 (reported) | — |
| 32 | Cyclohexanamide (Standard) | — | — | 0.8 ± 0.2 (observed) 0.26 ± 0.04 (reported) |
SEMa is the standard error of the mean, SDb is the standard deviation, and Insolubled refers to those analogue which are partially soluble in HPLC methanol used in the protocol of in vitro urease inhibition activity.
Compounds showed <50% inhibition were considered as inactive.
For Urease enzyme inhibition activity, screening concentration was 0.5 mM.
For ROS inhibition assay screening concentration was 25 µg/mL.
Note: All data were presented as mean ± standard deviation/standard error of the mean of three independent experiments where each sample was run in triplicate. The IC50 values were obtained using three concentrations of test compound, and were calculated using Excel Based Program.
Compound 12 (IC50 = 12.3 ± 1.04 µM), 19 (IC50 = 12.7 ± 0.8 µM), 11 (IC50 = 13.2 ± 1.64 µM), 13 (IC50 13.6 ± 1.61 µM), 9 (IC50 = 14.2 ± 1.38 µM), 17 (IC50 = 14.7 ± 1.01 µM), 10 (IC50 = 15.7 ± 1.32 µM), and 16 (IC50 = 21.5 ± 1.3 µM) were found more potent than the thiourea (Standard: IC50 = 21.1 ± 0.2 µM)/acetohydroxamic acid (Standard: IC50 = 20.3 ± 0.4 µM). While compounds 20 (IC50 = 22.0 ± 1.1 µM), 22 (IC50 = 22.1 ± 1.91 µM), and 23 (IC50 = 22.4 ± 1.83 µM) showed comparable activity with standard thiourea (IC50 = 21.1 ± 0.2 µM)/acetohydroxamic acid (Standard: IC50 = 20.3 ± 0.4 µM). Compounds 18 (IC50 = 26.3 ± 1.59 µM), 21 (IC50 = 28.2 ± 1.48 µM), 15 (IC50 = 34.1 ± 0.45 µM), 14 (IC50 = 35.8 ± 1.8 µM), 4 (IC50 = 38.8 ± 0.35 µM) showed significant activity, while 24 (IC50 = 63.2 ± 2.0 µM) showed moderate activity. Whereas, compound 3 and the parent drug isoniazid (1) was found to be inactive.
Indeed, the inhibition showed by all the synthesized analogues is due to the mutual participation of all parts of the molecule. However, it is also true that there are some characteristic features, which play an important role in the biological activity. As depicted in the Fig. 8 the current library has only aryl part (R) which is varying. Therefore, only the limited structure activity relationship can be drawn by comparing the position and nature of the substituents present in the aryl part of thiosemicarbazide derivatives (3–27).
Figure 8.
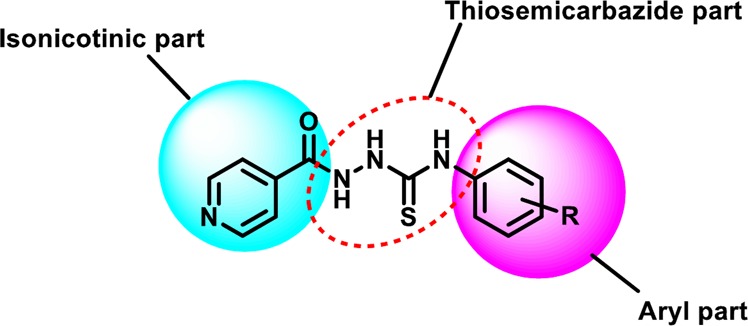
Rationale of the biological activity.
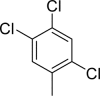
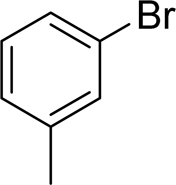
The compound 24 with no substituents on aryl part showed the lowest activity (IC50 = 63.2 ± 2.0 µM) as compared to other members of the library. Substitution of bromo group at the C-3 position as in compound 22 enhances the activity with IC50 = 22.1 ± 1.91 µM. While changing the position of bromo group to the C-2 position as in compound 14, slightly reduces the activity (IC50 = 35.8 ± 1.8 µM).
Substitution of chloro group at the C-4 position as in compound 18 showed almost similar activity with the (IC50 = 26.3 ± 1.59 µM). Compound 4 possess the two chloro groups at C-2 and C-3 positions and compound 5 with the chloro groups at C-2 and C-5 positions showed reduced activity with the IC50 values 38.8 ± 0.35 µM and 36.5 ± 0.14 µM, respectively. The compound with bromo substitution at C-3 position was found to be more efficient in the inhibitory potential as compared to the chloro substituted analogues, similar effect has also been reported by ali. et al.40.
Compound 12 (IC50 = 12.3 ± 1.04 µM), 19 (IC50 = 12.7 ± 0.8 µM), 16 (IC50 = 21.5 ± 1.3 µM), 15 (IC50 = 34.1 ± 0.45 µM), and 7 (IC50 = 46.3 ± 0.43 µM), possess fluoro group but they differ in the numbers and positions, compound 19 posses the fluoro group at C-4 positionis found to be the more active (IC50 = 12.7 ± 0.8 µM) than the compound 16 (IC50 = 21.5 ± 1.3 µM) having the fluoro group at C-3 position among, the disubstituted analogue 15 posses fluoro group at C-2 and C-4 showed inhibition with the IC50 value 34.1 ± 0.45 µM. Compound 7 posses the fluoro group at C-2, and C-6 exhibited decrease in the inhibitory potential with the IC50 value 46.3 ± 0.43 µM. Penta fluoro substituted compound 12 (IC50 = 12.3 ± 1.04 µM) showed the comparable activity as of mono substituted analogue 19. Similar SAR have been reported by taha. et al.41.
Incorporation of the chloro and trifluoromethyl group in compound 9 have further enhanced the activity with IC50 = 14.2 ± 1.38 µM.
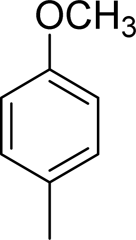
Compound 11 is a para substituted derivatives (IC50 = 13.2 ± 1.64 µM), which posses an electron withdrawing trifluoromethoxy group, showed comparable activity with compound 10 (IC50 = 15.7 ± 1.32 µM) having the cyano group, while compound 20 (IC50 = 22.0 ± 1.1 µM) having the electron donating methoxy group showed less activity than the compounds 11 and 10. It demonstrates that the electron withdrawing groups have the profound effect on inhibitory potential.
From the limited structure activity relationship, it can be summarized that the nature and position of the substituent both are equally responsible for demonstrating the inhibitory activity.
In Vitro Anti-inflammatory Activity
All synthesized derivatives of isoniazid (1) were also evaluated for their anti-inflammatory activity using oxidative burst assay. The standard inhibitor used was ibuprofen (IC50 = 11.2 ± 1.9 µg/mL). Among all the compounds, compounds 23, 5, 22, 26, 9, 12, 16, and 17 showed promising anti-inflammatory activities with IC50 value 12.3 ± 1.2 to 36.9 ± 3.0 µg/mL, while other compounds were found inactive (Table 1).
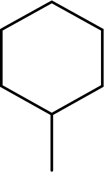
Among these compounds, compound 23 containing a cyclohexyl ring was found to be the most active inhibitor of ROS (IC50 = 12.3 ± 1.2 µg/mL). Compound 5 bearing chloro groups at C-2 and C-5 positions showed a good activity with an IC50 value 18.5 ± 1.0 µg/mL, while with a bromo group at C-3, compound 22 was also found to be a good inhibitor but less active than compound 5 (IC50 = 25.1 ± 0.4 µg/mL). On the other hand, compound 26 with a isothiocyanate group at C-3 showed the similar activity (IC50 = 25.4 ± 1.3 µg/mL) as that of compound 5. Compound 9 with a chloro group at C-4 and trifluoromethyl group at C-3 also showed a good activity (IC50 = 26.7 ± 2.5 µg/mL), along with this pentaflouro containing phenyl ring analogue 12 which resulted in decrease of activity from IC50 values 26.7 ± 2.5 to 27.7 ± 2.4 µg/mL. Interestingly the mono substituted analogue of fluorine at C-3 (compound 16) showed a decreased activity with IC50 value 29.7 ± 1.7 µg/mL, while the iodine containing analogue at C-3 (compound 17) showed further decreased activity (IC50 = 36.9 ± 3.0 µg/mL).
Evaluation of cytotoxicity on 3T3 normal cell line
Derivatives 3–27 and isoniazid (1) were evaluated for their cytotoxicity against 3T3 normal (mouse fibroblast) cell lines in which all derivatives were found to be inactive, except compound 18 and isoniazid (1) which showed IC50 values of 29.5 ± 1.9, and 28.5 ± 1.2 µM, respectively, in comparison to the standard cyclohexamide (IC50 = 0.8 ± 1.9 µM) (Table 1).
Discussion
The present research study indicates that thiosemicarbazides 3–27 possess promising urease inhibition activity, as well as significant anti-inflammatory activities. Compounds 5, 9, 12, 16, 17, 22, 23, and 26 were found to be the dual inhibitors of inflammation (ROS) and urease, few members of the current library have been reported as urease inhibitors by Ali. et al.34 but here we report the dual inhibitors of urease and inflammation along with its cytoxicity profile against 3T3 mouse fibroblast cell line. The limited SAR concludes that the compound 23 with the cyclohexyl ring was found to be the most significant dual inhibitor of urease and inflammation (IC50 = 22.4 ± 1.83 µM and 12.3 ± 1.2 µg/mL, respectively). This showed comparable activity to the clinically used anti-inflammatory drug ibuprofen (IC50 = 11.2 ± 1.9 µg/mL), and urease inhibitor acetohyroxamic acid (IC50 = 20.3 ± 1.9 µg/mL). Compound 12, is the pentafluoro substituted analogue. Compound 12 was identified as the most potent inhibitor of urease (IC50 = 12.3 ± 1.04 µM) and significant inhibitor of inflammation (IC50 = 27.7 ± 2.4 µg/mL).
Whereas compounds 10, 11, and 13 were identified as potent inhibitors of urease enzyme, however, these compounds were found to be inactive in anti-inflammatory assay. The SAR clearly shows that change in the substituents and its positions or changing the aryl ring by the alkyl ring greatly affects the biological activity of the synthesized compounds.
In conclusion, the compounds reported here may serve as the starting points for the designing and development of new and powerful dual inhibitors of inflammation and infections caused by ureolytic bacteria.
Experimental
The experimental section describes various methods and technical aspects of the current study including synthesis, purification, characterization of the synthesized analogues through different spectroscopic techniques and evaluation of the biological activities, such as inhibition of urease enzyme and inflammation.
Chemicals
Isoniazid (1) was purchased from Sigma Aldrich, India; 2-bromophenyl isothiocyanate, 3-bromophenyl isothiocyanate, 4-fluorophenyl isothiocyanate, and 3-nitrophenyl isothiocyanate from Aldrich, Poland. Cyclopentylisothiocyanate, cyclohexylisothiocyanate, 2,5-dichlorophenyl isothiocyanate, 3-fluorophenyl isothiocyanate, hexyl isothiocyanate, 3-iodophenyl isothiocyanate, and 4-methoxy phenyl isothiocyanate from Sigma Aldrich, USA; 2,4,5-trichloro phenyl isothiocyanate from Aldrich, USA. 4-Chlorophenylisothiocyanate, 2,4-difluorophenyl isothiocyanate, and 2,6-dichlorophenyl isothiocyanate, 3,4-dichlorophenyl isothiocyanate were obtained from Alfa Aesar, USA. 2-Fluorophenyl isothiocyanate from Sigma Aldrich Chemie, Germany; 2-chloro-5-trifluoromethyl)phenyl isothiocyanate, 4-chloro-3-(trifluoromethyl)phenyl isothiocyanate, 4-cyanophenyl isothiocyanate, and 4-(trifluoromethoxy)phenyl isothiocyanate, 1,3-phenylene diisothiocyanate were purchased from Oakwood Chemicals, USA, 4-Dimethylaminoazobenzene 4′-isothiocyanate, and pentafluorophenylisothiocyanate were bought from Fluka, Switzerland, phenyl isothiocyanate from Schuchardt, Hohenbrunn, Germany.
Precoated silica gel plates (ALUGRAM, SIL G/UV254) were used for thin layer chromatography (TLC). TLC chromatograms were viewed under the ultraviolet light of 254 and 365 nm. Electron impact mass spectroscopy (EI-MS) and Fast atom bombardment direct probe mass spectra (FAB-MS) were obtained through JEOLJMS-600H mass spectrometer (Japan). 1H and 13C-NMR spectra were performed on a 300, 400, and 100 MHz Bruker Avance spectrometers (Switzerland). Buchi M-560 apparatus was used for recording the melting point (Japan). I. R. Spectrophotometry of the compounds was performed on FTIR-8900 (Shimadzu, Japan) through KBr disc.
General Procedure of the Synthesis of Compounds 3–27
Isoniazid (1) (2 mmol) was refluxed with methanol (10 mL) for 15 minutes. Corresponding isothiocyanate (2 mmol) was added, and kept on stirring for 8–10 hours at room temperature (25 °C). Reaction progress was examined by TLC (7.9: 2: 0.1) (ethyl acetate, methanol and acetic acid) analysis. Disappearance of starting material from reaction mixture indicated the completion of reaction. The reaction mixture was concentrated under reduced pressure, solid product thus obtained was purified through solvent-solvent extraction with the help of hexane and ethyl acetate, and then recrystallized from methanol. Among these compounds, 9, 10, 12, 21, and 26 were identified as new derivatives. The known compounds were found to be spectroscopically similar to that reported in the literature.
N-(2-Fluorophenyl)-2-isonicotinoylhydrazinecarbothioamide (3)
Rf = 0.7, Mp: 241–242 °C; IR (KBr, cm–1): 3285, 3128 (N–H), 1679 (C=O), 1596, 1545, 1509 (C=C), 1226 (C=S), 1149 (C– F); 1H-NMR(300 MHz, DMSO-d6): δH 10.91 (s, 1 H, NH), 9.96 (s, 1 H, NH), 9.67 (s, 1 H, NH), 8.75 (d, J3″,2″ = J5″,6″ = 6.0 Hz, 2 H, H-3″, H-5″), 7.83 (d, J2″,3″ = J6″,5″ = 5.1 Hz, 2 H, H-2″, H-6″), 7.32–7.14 (m, 4 H, H-3, H-4, H-5, H-6); 13C-NMR (100 MHz, DMSO-d6): δ182.6 (C=S), 165.0 (C=O), 159.0 (d, JCF = 248.8 Hz, C-2), 140.0 (C-1″), 150.1 (C-3″,C-5″), 130.6 (C-5), 128.0 (C-4), 124.0 (C-6), 121.6 (C2″,C-6″), 116.1 (d, J CF= 19.9 Hz, C-1), 115.6 (d, JCF = 19.8 Hz,C-3); Positive FAB-MS m/z (rel. int. %): 291.1 [M + H]+ (56), 182.1 (100), 185.1 (27), 171.0 (16); HRFAB-MS (+ve mode) Calcd. for [(C13H11N4O1F1S1) + H]+: (m/z = 291.0716) Found 291.0712.
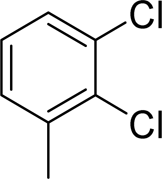
N-(2, 3-Dichlorophenyl)-2-isonicotinoylhydrazinecarbothioamide (4)
Rf = 0.76, Mp: 198–199 °C; IR (KBr, cm–1): 3289, 3137 (N–H), 1679 (C=O), 1546, 1512 (C=C), 1249 (C=S), 1058 (C–Cl). 1H-NMR (600 MHz, DMSO-d6): δH 10.93 (s, 1 H, NH), 10.02 (s, 1 H, NH), 9.82 (s, 1 H, NH), 8.76 (d, J3″,2″ = J5″,6″ = 6.0 Hz, 2 H, H-3″, H-5″), 7.83 (distorted singlet, 2 H, H-2″, H-6″), 7.56 (d, J4,5 = 7.8 Hz, 1 H, H-4), 7.36 (t, J5(4,6) = 7.8 Hz, 1 H, H-5), 7.32 (distorted singlet, 1 H, H-6); 13C-NMR (100 MHz, DMSO-d6): δ182.4 (C=S), 165.0(C=O), 150.6(C-3″,C-5″),139.9 (C-1″), 139.3(C-1), 132.1 (C-2), 131.0 (C-3), 129.9 (C-6), 128.6 (C-4), 127.5 (C-5), 121.7 (C-2″,C-6″); Positive FAB-MS m/z (rel. int. %): 342.0 [M + 2]+ (3), 341.0 [M + H]+ (6), 185.1 (100), 277.1 (16), 219.1 (11); HRFAB-MS (+ve mode) Calcd. For [(C13H10N4O1Cl2S1) + H]+: (m/z = 341.0031) Found 341.0017.
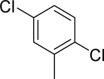
N-(2, 5-Dichlorophenyl)-2-isonicotinoylhydrazinecarbothioamide (5)
Rf = 0.73, Mp: 172–173 °C; IR (KBr, cm–1): 3285, 3130 (N–H), 1679 (C=O), 1546, 1512 (C=C), 1252 (C=S), 1092 (C–Cl), 1H-NMR (400 MHz, DMSO-d6): δH 10.93 (s, 1 H, NH), 10.07 (s, 1 H, NH), 9.76 (s,1 H, NH), 8.76 (d, J3″,2″ = J5″,6″ = 6.0 Hz, 2 H, H-3″, H-5″), 7.84(d, J2″,3″ = J6″,5″ = 5.0 Hz, 2 H, H-2″, H-6″), 7.54 (d, J5,4 = 8.4 Hz, 1 H, H-6), 7.38 (distorted t, 2 H, H-3, H-4); NegativeFAB-MS m/z (rel. int. %): 341.1[M + 2]+ (3), 339.1[M-H]+ (6), 275.2 (22) 183.0 (100), 164.0 (10), 150.9 (11); HRFAB-MS (+ve mode) Calcd. for [(C13H10N4O1Cl2S1) + H]+: (m/z = 341.0031) Found 341.0010.
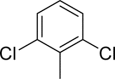
N-(2, 6-Dichlorophenyl)-2-isonicotinoylhydrazinecarbothioamide (6)
Rf = 0.73, Mp: 127–128 °C; IR (KBr, cm–1): 3287, 3164 (N–H), 1681 (C=O), 1513 (C=C), 1244 (C=S), 1064 (C–Cl). 1H-NMR (300 MHz, DMSO-d6): δH 10.91 (s, 1 H, NH), 10.0 (s, 1 H, NH), 9.75 (s, 1 H, NH), 8.76 (d, J3″,2″ = J5″,6″ = 5.1 Hz, 2 H, H-3″, H-5″), 7.83 (d, J2″,3″ = J6″,5″ = 4.5 Hz, 2 H, H-2″, H-6″), 7.51 (d, J3,4 = J5,4 = 8.1 Hz, 2 H, H-3, H-5), 7.39 (distorted t, 1 H, H-4); Negative FAB-MS m/z (rel. int. %): 341.0 [M + 2]+ (3), 339.2 [M-H]+ (8), 275.2 (18), 268.2 (14), 183.1(100), 176.1(44); HRFAB-MS (+ve mode) Calcd. for [(C13H10N4O1Cl2S1) + H]+: (m/z = 341.0031) Found 341.0010.
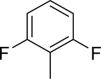
N-(2, 6-Difluorophenyl)-2-isonicotinoylhydrazinecarbothioamide (7)
Rf = 0.7, Mp: 248–249 °C; IR (KBr, cm–1): 3292, 3131 (N–H), 1681 (C=O), 1595, 1546 (C = C), 1246 (C=), 1151 (C–F), 1H-NMR (400 MHz, DMSO-d6): δH 10.95 (s, 1 H, NH), 10.11 (s, 1 H, NH), 9.47 (s, 1 H, NH), 8.75 (d, J3″,2″ = J5″,6″ = 4.8 Hz, 2 H, H-3″, H-5″), 7.84 (distorted singlet, 2 H, H-2″, H-6″), 7.34 (m, 1 H, H-4), 7.12 (t, J3,2/3,4 = J5,4/5,6 8.0 Hz, 2 H, H-3, H-5); 13C-NMR (125 MHz, DMSO-d6): δ182.6 (C=S), 164.4 (C=O), 159.8 (d, JCF = 247.8 Hz, C-2), 159.7 (d, JCF = 247 Hz, C-6), 150.1 (C-3″,C-5″), 139.3 (C-1″), 128.8 (t, JCF = 10 Hz, C-4), 121.7 (C2″,C-6″), 116.6 (t, JCF = 15.8 Hz, C-1), 111.7 (d, JCF = 21.8 Hz, C-3, C-5); Positive FAB-MS m/z (rel. int. %): 309.0 [M + H]+ (17), 277.0 (27), 219 (19), 185.0 (100), 171.1 (96), 157.0 (45); HRFAB-MS (+ve mode) Calcd. for [(C13H10F2N4OS) + H]+: (m/z = 309.0622) Found 309.0610.
N-(2-Chloro-5-(trifluoromethyl)phenyl)-2-isonicotinoylhydrazinecarbothioamide (8)
Rf = 0.8, Mp: 183–184 °C; IR (KBr, cm–1): 3304, 3134 (N–H), 1679 (C=O), 1548, 1513 (C=C), 1253 (C=S), 1080 (C–Cl). 1H-NMR (400 MHz, DMSO-d6): δH 10.96 (s, 1 H, NH), 10.14 (s, 1 H, NH), 9.83 (s, 1 H, NH), 8.76 (d, J3″,2″ = J5″,6″ = 5.6 Hz, 2 H, H-3″, H-5″), 7.84 (br s, 2 H, H-2″, H-6″), 7.76 (d, J6,4 = 8.8 Hz, 1 H, H-6), 7.67 (br s, 2 H, H-3, H-4); Positive FAB-MS m/z (rel. int. %): 376.1[M + 2]+ (13),375.1 [M + H]+ (19), 277.1 (8), 255.0 (16), 185.2 (83), 157.1 (100); HRFAB-MS (+ve mode) Calcd. for [(C14H10N4O1Cl1F3S1) + H]+: (m/z = 375.0294) Found 375.0307.
N-(4-Chloro-3-(trifluoromethyl)phenyl)-2 isonicotinoylhydrazinecarbothioamide (9)
Rf = 0.76, Mp: 231–232 °C;IR (KBr, cm–1): 3279, 3125 (N–H), 1675 (C=O), 1551, 1516 (C=C), 1256 (C=S), 1065 (C–Cl). 1H-NMR (400 MHz, DMSO-d6): δH 10.92 (s, 1 H, NH), 10.14 (s, 1 H, NH), 10.02 (s, 1 H, NH), 8.77 (d, J3″,2″ = J5″,6″ = 5.6 Hz, 2 H, H-3″, H-5″), 7.99 (br s, 1 H, H-2), 7.85 (br.s, 3 H, H-6″, H-2″, H-5), 7.67 (d, J3,2 = 8.4 Hz, 1 H, H-6), 13C-NMR (125 MHz, DMSO-d6): δ180.8 (C=S), 164.4 (C=O), 150.2 (C-3″,C-5″), 139.2 (C-1″), 138.6 (C-1), 131.2 (C-6), 130.5 (C-5), 125.9 (m, CF3), 124.2 (C-2), 123.7 (C-3), 121.6 (C2″, C-6″), 119.4 (C-4); Positive FAB-MS m/z (rel. int. %): 377.2 [M + 2]+ (22), 375.1 [M + H]+ (32), 277.0 (27), 219 (19), 185.0 (100), 171.1 (96), 157.0 (45); HRFAB-MS (+ve mode) Calcd. for [(C14H10N4O1Cl1F3S1) + H]: (m/z = 375.0294) Found 375.0295.
N-(4-Cyanophenyl)-2-isonicotinoylhydrazinecarbothioamide (10)
Rf = 0.66, Mp: 162–163 °C; IR (KBr, cm–1): 3314, 3216 (N–H), 2224 (C≡N), 1675 (C=O), 1543, 1510 (C=C), 1217 (C=S). 1H-NMR (400 MHz, CD3OD): δH 8.72 (d, J3″,2″ = J5″,6″ = 6.0 Hz, 2 H, H-3″, H-5″), 7.88 (d, J2″,3″ = J6″,5″ = 6.0 Hz, 2 H, H-2″, H-6″), 7.82 (d, J2,3 = J6,5 = 8.4 Hz, 2 H, H-2, H-6), 7.67 (d, J3,2 = J5,6 = 8.4 Hz, 2 H, H-3, H-5), 13C-NMR (150 MHz, DMSO-d6): δ180.6 (C=S), 164.4 (C=O), 150.2 (C-3″,C-5″), 143.6 (C-1″), 139.3 (C-1), 132.5 (C-3), 132.1 (C-5), 125.4 (C-2), 122.6 (C-6), 118.9 (C-4), 106.8 (CN); Positive FAB-MS m/z (rel. int. %): 297.1 [M + H]+ (4), 277.1 (15), 219.2 (8), 185.0 (100), 171.0 (23), 157.0 (8); HRFAB-MS (+ve mode) Calcd. for [(C14H11N5O1S1) + H]+: (m/z = 298.0763) Found 298.0788.
2-Isonicotinoyl-N-(4-(trifluoromethoxy) phenyl) hydrazine carbothioamide (11)
Rf = 0.76, Mp: 192–193 °C; IR (KBr, cm–1): 3271, 3125 (N–H), 1678 (C=O), 1513 (C=C), 1267 (C=S). 1H-NMR (400 MHz, DMSO-d6): δH 10.86 (s, 1 H, NH), 9.92 (s, 2 H, NH), 8.76 (d, J3″,2″ = J5″,6″ = 5.6 Hz, 2 H, H-3″, H-5″), 7.83 (d, J2″,3″ = J6″,5″ = 5.6 Hz, 2 H, H-2″, H-6″), 7.54 (br s, 2 H, H-2, H-6), 7.32 (d, J3,2 = J5,6 = 8.4 Hz, 2 H, H-3, H-5), Positive FAB-MS m/z (rel. int. %): 357.1 [M + H]+ (38), 329 (8), 277.1 (15), 236.9 (58), 185.0 (100), 171.0 (19), 157.0 (8); HRFAB-MS (+ve mode) Calcd. for [(C14H11N4O2F3S1) + H]+: (m/z = 357.0633) Found 357.0619.
2-Isonicotinoyl-N-(perfluorophenyl) hydrazinecarbothioamide (12)
Rf = 0.7, Mp: 255–256 °C; IR (KBr, cm–1): 3303, 3150 (N–H), 1682 (C=O), 1528, 1505 (C=C), 1249 (C=S), 1153 (C–F). 1H-NMR (300 MHz, DMSO-d6): δH 11.06 (s, 1 H, NH), 10.44 (s, 1 H, NH), 9.72 (s, 1 H, NH), 8.77 (d, J3″,2″ = J5″,6″ = 5.1 Hz, 2 H, H-3″, H-5″), 7.84 (distorted singlet, 2 H, H-2″, H-6″), 13C-NMR (125 MHz, DMSO-d6): δ182.6 (C=S), 164.4 (C=O), 150.2 (C-3″,C-5″), 144.7 (d, J = 206 Hz, C-2, C-6), 140.5 (t, J = 11.4 Hz, C-1), 139.5 (C-1″), 138.9 (m, C-4), 137.8 (t, J = 11.9 Hz, C-3), 136.2 (t, J = 14.3 Hz, C-3), Positive FAB-MS m/z (rel. int. %): 363.0 [M + H]+ (15), 335.0 (4), 277.0 (17), 243.0 (7), 185.2 (100); HRFAB-MS (+ve mode) Calcd. for [(C13H7N4O1F5S1) + H]+ (m/z = 363.0339) Found 363.0323.
N-Cyclopentyl-2-isonicotinoylhydrazinecarbothioamide (13)
Rf = 0.7, Mp: 223–224 °C; IR (KBr, cm–1): 3268, 3142 (N–H), 1679 (C=O), 1552, 1527 (C=C), 1451 (CH2 bending), 1263 (C=S stretching). 1H-NMR (300 MHz, DMSO-d6): δH 10.54 (s, 1 H, NH), 9.26 (s, 1 H, NH), 8.74 (d, J3″,2″ = J5″,6″ = 5.7 Hz, 2 H, H-3″, H-5″), 7.80 (d, J2″,3″ = J6″,5″ = J3′,4′ = 5.7 Hz, 3 H, H-2″, H-6″, NH), 4.11 (br s, 1 H, H-1), 1.77–1.55 (m, 4 H, H-2a, H-2b, H-5a, H-5b), 1.27–1.03 (m, 4 H, H-3a, H-3b, H-4a, H-4b), Negative FAB-MS m/z (rel. int. %): 265.2 [M-H]+ (4), 219.9 (17), 207.0 (18), 183.0 (100), 164.0 (4); HRFAB-MS (+ve mode) Calcd. for. [C12H16N4OS + H]+: (m/z = 265.1045) Found 265.1043.
N-(2-Bromophenyl)-2-isonicotinoylhydrazinecarbothioamide (14)
Rf = 0.73, Mp: 156–157 °C;IR (KBr, cm–1): 3275, 3131 (N–H), 1679 (C=O), 1543, 1474 (C=C), 1251 (C=S), 1059 (C–Br). 1H-NMR (300 MHz, DMSO-d6): δH 10.89 (s, 1 H, NH), 9.90 (s, 1 H, NH), 9.70 (s, 1 H, NH), 8.75 (d,J3″,2″ = J5″,6″ = 6.3 Hz, 2 H, H-3″, H-5″), 7.84 (d,J2″,3″ = J6″,5″ = 5.1 Hz, 2 H, H-2″, H-6″), 7.65 (d, J3,4 = 7.8 Hz, 1 H, H-3), 7.38 (br s, 2 H, H-4, H-6), 7.19 (m, 1 H, H-5), Positive FAB-MS m/z (rel. int. %): 351.9 [(M + 2)]+ (7), 350.9 [M + H]+ (8), 277.1 (15), 185.2 (100), 171.2 (17), HRFAB-MS (+ve mode) Calcd. for [(C13H11N4O1Br1S1) + H]+ (m/z = 351.9993) Found 351.9999.
N-(2, 4-Difluorophenyl)-2-isonicotinoylhydrazinecarbothioamide (15)
Rf = 0.43, Mp 191–192 °C; IR (KBr, cm–1): 3300, 3141 (N–H), 1682 (C=O), 1550, 1514 (C=C), 1251 (C=S stretching), 1142 (C–F), 1H-NMR (400 MHz, DMSO-d6): δH 10.91 (s, 1 H, NH), 10.0 (s, 1 H, NH), 9.61 (s,1 H, NH), 8.75 (d, J3″,2″ = J5″,6″ = 4.8 Hz, 2 H, H-3″, H-5″), 7.83 (s, 2 H, H-2″, H-6″), 7.26 (t, J5,6 = J6,5 = 8.4 Hz, 2 H, H-6, H-5), 7.00 (t, J3(4,2) = 8.2 Hz, 1 H, H-3); Electron Ionization Mass spectrometry (direct probe) m/z 308.1 [M]+ (2.2), 274.1 (11.5), 171.0 (100), 106.0 (45.6), 78.0 (43.1); HREI-MS Calcd. For [C13H9N4O1F2S1]: (m/z = 308.0543) Found 308.0548.
N-(3-Fluorophenyl)-2-isonicotinoylhydrazinecarbothioamide (16)
Rf = 0.73, Mp 174–175 °C; IR (KBr, cm–1): 3266, 3112 (N–H), 1674 (C=O), 1601, 1515 (C=C), 1238 (C=S), 1142 (C–F); 1H-NMR (400 MHz, DMSO-d6): δH 10.86 (s, 1 H, NH), 9.93 (s, 2 H, NH), 8.76 (d, J3″,2″ = J5″,6″ = 6 Hz, 2 H, H-3″, H-5″), 7.83 (d, J2″,3″ = J6″,5″ = 5.6 Hz, 2 H, H-2″, H-6″), 7.45 (br.s, 1 H, H-2), 7.35 (q, J5(4,6) = 8.0 Hz, 1 H, H-5), 7.27 (d, J6,5 = 8.0 Hz, 1 H, H-6), 6.98 (t, J4(5,6) = 7.6 Hz, 1 H, H-4); Positive FAB-MS m/z (rel. int. %): 291.0 [M + H]+ (5), 185.1 (100), 277.1 (15), 219.1 (12); HRFAB-MS (+ve mode) Calcd. for [(C13H11FN4OS) + H]+ (m/z = 291.0716) Found 291.0721.
N-(3-Iodophenyl)-2-isonicotinoylhydrazinecarbothioamide (17)
Rf = 0.73, Mp 192–193 °C; IR (KBr, cm–1): 3266, 3112.3 (N–H), 1674.5 (C=O), 1601, 1515 (C=C), 1237.9 (C=S), 851.8 (C–I). 1H-NMR(400 MHz, DMSO-d6): δH 10.85 (s, 1 H, NH), 9.93 (s, 1 H, NH), 9.84 (s, 1 H, NH), 8.76 (d, J3″,2″ = J5″,6″ = 5.6 Hz, 2 H, H-3″, H-5″), 7.83 (d, J2″,3″ = J6″,5″ = J4,5 = 5.2 Hz, 3 H, H-2″, H-6″, H-4), 7.51 (t, J5,4/5,6) = J2,4/2,6) = 6.8 Hz, 2 H, H-2, H-5), 7.12 (t, J6,5 = 8.0 Hz, 1 H, H-6); Positive FAB-MS m/z (rel. int. %): 399.0 [M + H]+ (16), 183.2 (100), 275.2 (19), 243.0 (7); HRFAB-MS (+ve mode) Calcd. for [(C13H12N4O1I1S1) + H]+ (m/z = 398.9776) Found 398.9759.
N-(4-Chlorophenyl)-2-isonicotinoylhydrazine carbothioamide (18)
Rf = 0.73, Mp 150–151 °C; IR (KBr, cm–1): 3251.8, 3130.7 (N–H), 1676 (C=O), 1596, 1545 (C=C), 1256 (C=S), 1093.7 (C–Cl). 1H-NMR (300 MHz, DMSO-d6): δH 10.86 (s, 1 H, NH), 9.90 (s, 2 H, NH), 8.76 (dd, J3″,2″ = J5″,6″ = J1 = 1.8, J2 = 1.5 Hz, 2 H, H-3″,H-5″), 7.83 (dd, J2″,3″ = J6″,5″ = J1 = 1.5 Hz, J2 = 1.2 Hz, 2 H, H-2″, H-6″), 7.70 (s, 1 H, H-2), 7.47 (appear d, J3,2 = J5,6 = 7.3 Hz, 2 H, H-3, H-5), 7.37 (m, 2 H, H-2, H-6); Positive FAB-MS m/z (rel. int. %): 307.0 [M + 2]+ (5), 305 [M+] (15), 185.1(100), 277.1 (14), 219.2 (10); HRFAB-MS (+ve mode) Calcd. for [(C13H11N4O1Cl1S1) + H]+ (m/z = 307.0420) Found 307.0450.
N-(4-Fluorophenyl)-2-isonicotinoylhydrazinecarbothioamide (19)
Rf = 0.73, Mp 191–192 °C; IR (KBr, cm–1): 3201, 3155 (N–H), 1684 (C=O), 1544, 1512 (C=C), 1263 (C=S), 1223 (C–F). 1H-NMR (400 MHz, DMSO-d6): δH 10.84 (s, 1 H, NH), 9.82 (s, 2 H, NH), 8.76 (d, J3″,2″ = J5″,6″ = 6 Hz, 2 H, H-3″, H5″),7.83 (d, J2″,3″ = J6″,5″ = 5.6 Hz, 2 H, H-2″, H-6″), 7.39 (br.s, 2 H, H-2, H-6), 7.16 (t, J3,2/3,4 = J5,6/5,4 = 8.8 Hz, 2 H, H-3, H-5), Positive FAB-MS m/z (rel. int. %): 291.0 [M + H]+ (6), 185.1 (100), 277.1 (14), 219.2 (10); HRFAB-MS (+ve mode) Calcd. for [(C13H11N4O1F1S1) + H]+ (m/z = 291.0716) Found 291.0730.
2-Isonicotinoyl-N-(4-methoxyphenyl) hydrazinecarbothioamide (20)
Rf = 0.66, Mp: 171–172 °C; IR (KBr, cm–1): 3255, 3124 (N–H), 1675 (C=O), 1545, 1514 (C=C), 1253 (C=S). 1H-NMR (400 MHz, DMSO-d6): δH10.79 (s,1 H, NH), 9.72 (s,1 H, NH), 9.67 (s,1 H, NH), 8.75 (d, J3″,2″ = J5″,6″ = 5.6 Hz, 2 H, H-3″, H-5″), 7.83 (d, J2″,3″ = J6″,5″ = 5.6 Hz, 2 H, H-2″, H-6″), 7.25 (d, J3,2 = J5,6 = 7.6 Hz, 2 H, H-2, H-6), 6.88 (d, J2,3 = J6,5 = 9.2 Hz, 2 H, H-3, H-5), 3.11 (s, 3 H, H-7); Positive FAB-MS m/z (rel. int. %): 303.0 [M + H]+ (4), 185.1(100), 277.1(12), 219.1(8); HRFAB-MS (+ve mode) Calcd. for [(C14H14N4O2S1) + H]+ (m/z = 303.0916) Found 303.0924.
Isonicotinoyl-N-(2, 4, 5-trichlorophenyl) hydrazinecarbothioamide (21)
Rf = 0.73, Mp: 241–242 °C; IR (KBr, cm–1): 3282, 3127 (N–H), 1678 (C=O), 1561, 1510, (C=C), 1259 (C=S), 1079 (C–Cl). 1H-NMR (300 MHz, DMSO-d6): 10.94 (s, 1 H, NH), 10.14 (s, 1 H, NH), 9.79 (s,1 H, NH), 8.76 (d, J3″,2″ = J5″,6″ = 6 Hz, 2 H, H-3″, H-5″), 7.92 (s, 1 H, H-2), 7.83 (br s, 2 H, H-2″, H-6″), 7.61 (br s, 1 H, H-5); 13C-NMR (125 MHz, DMSO-d6): δ181.9 (C=S), 164.5 (C=O), 150.2 (C-3″,C-5″), 139.3 (C-1″), 137.0(C-1), 132.0 (C-2), 131.4 (C-4),130.4 (C-5), 129.9 (C-6), 129.2 (C-3)121.7 (C2″,C-6″); Positive FAB-MS m/z (rel. int. %): 377.0 [M + 2]+ (17),375.2 [M + H]+, (19), 275.3 (25), 183.1 (100), 136.1 (14), 127.1 (10). HRFAB-MS (+ve mode) Calcd. for [(C13H9N4O1Cl3S1) + H]+ (m/z = 374.9641) Found 374.9633.
N-(3-Bromophenyl)-2-isonicotinoylhydrazinecarbothioamide (22)
Rf = 0.73, Mp: 192–193 °C; IR (KBr, cm–1): 3301, 3143 (N–H), 1683 (C=O), 1589, 1548 (C=C), 1252 (C=S), 1066 (C–Br). 1H-NMR (400 MHz, DMSO-d6): δH 10.86 (s, 1 H, NH), 9.95 (s, 1 H, NH), 9.88 (s, 1 H, NH), 8.76 (d, J3″,2″ = J5″,6″ = 5.6 Hz, 2 H, H-3″, H-5″), 7.83 (d,J2″,3″ = J6″,5″ = 4.8 Hz, 2 H, H-2″, H-6″), 7.70 (s, 1 H, H-2), 7.49 (d, J4,5 = 7.6 Hz, 1 H, H-4),7.32(d,J6,5 = 7.6 Hz, 1 H, H-6),7.28 (t, J5,4/5,6 = 8.0 Hz, 1 H, H5); Positive FAB-MS m/z (rel. int. %): 353.0[(M + 2]+ (14), 351.0 [M + H]+ (15), 185.0 (100), 277.2 (14), 219.1 (4); HRFAB-MS (+ve mode) Calcd. for [(C13H11N4O1S1Br1) + H]+ (m/z = 350.9915) Found 350.9895.
N-Cyclohexyl-2-isonicotinoylhydrazinecarbothioamide (23)
Rf = 0.71, Mp: 212–213 °C; IR (KBr, cm–1): 3268, 3141 (N–H), 1680 (C=O), 1551, 1530 (C=C), 1452 (CH2 bending), 1266 (C=S). 1H-NMR (300 MHz, DMSO-d6): δH 10.56 (s, 1 H, NH), 9.28 (s, 1 H,NH), 8.76 (dd, J3″, 2″ = J5″, 6′ ‘ = 1.5 Hz, 2 H, H-3″, H-5″), 7.81 (d, J2″−3″ = J6″−5″ = 6.0 Hz, 3 H, H-2″, H-6″, NH), 4.11 (m, 1 H, H-1), 1.78 (m, 4 H, H-2a, H-2b, H-6a, H-6b), 1.60 (d, J5a,6 = 12.3 Hz, 1 H, H-5a), 1.28 (m, 4 H, H-3a, H-3b, H-4a, H-4b),1.07 (m, 1 H, H-5b); Positive FAB-MS m/z (rel. int. %): 279.1 [M + H]+ (25), 185.1 (100), 219.2 (6), 251.2 (4); HRFAB-MS (+ve mode) Calcd. for [(C13H18N4O1S1) + H]+ (m/z = 279.1280) Found 279.1273.
2-Isonicotinoyl-N-phenylhydrazinecarbothioamide (24)
Rf = 0.7, Mp: 188–189 °C; IR (KBr, cm–1): 3262, 3120 (N–H), 1675 (C=O), 1547, 1512 (C=C), 1254 (C=S), 1H-NMR (300 MHz, DMSO-d6): δH 10.84 (s, 1 H, NH), 9.84 (s, 1 H, NH), 9.78 (s,1 H, NH), 8.76 (m, 2 H, H-3″, H-5″), 7.83 (d, J2″,3″ = J6″,5″ = 6.0 Hz, 2 H, H-2″, H-6″), 7.41 (d, J2,3 = J6,5 = 7.2 Hz, 2 H, H-2, H-6), 7.32 (t, J3,2 = J4,5 = J4,6 = 7.8 Hz, 2 H, H-3, H-5), 7.15 (t, J4,3 = J4,6 = 7.2 Hz, 1 H, H-4); Positive FAB-MS m/z (rel. int. %): 273.1 [M + H]+ (15), 185.1 (100), 219.1 (8), 263.1 (5), 191.1 (4); HRFAB-MS (+ve mode) Calcd. for [(C13H12N4O1S1) + H]+ (m/z = 273.0810) Found 273.0799.
N-Hexyl-2-isonicotinoylhydrazinecarbothioamide (25)
Rf = 0.46, Mp 215–216 °C; IR (KBr, cm–1): 3300.5, 3170.6 (N–H), 1677.4 (C=O), 1555, 1527 (C=C), 1245 (C=S), 753 (long chain band). 1H-NMR (400 MHz, DMSO-d6): δH 10.57 (s, 1 H, NH), 9.29 (s, 1 H, NH), 8.74 (d, J3″,2″ = J5″,6″ = 6.0 Hz 2 H, H-3″, H-5″), 8.12 (s,1 H, NH), 7.79 (d, J2″,3″ = J6″,5″ = 5.6 Hz, 2 H, H-2″, H-6″), 3.42 (m, 2 H, H-1a, H-1b), 1.46 (m, 2 H, H-2a, H-2b), 1.23 (m, 6 H, H-3a, H-3b, H-4a, H-4b, H5a, H-5b), 0.84 (m, 3 H, H-6a, H-6b, H-6c), Negative FAB-MS m/z (rel. int. %): 279 0 [M-H]+ (23), 275 (19), 255.1 (8), 183 (100), HRFAB-MS (−ve mode) Calcd. for [(C13H20N4O1S1) – H]: (m/z = 279.0811) Found 279.0810.
N-(3-Thiocyanophenyl)-2-isonicotinoylhydrazinecarbothioamide (26)
Rf = 0.39, Mp: 195–196 °C; IR (KBr, cm–1): 3283, 3139 (N–H), 2118 (N=C=S), 1678 (C=O), 1595, 1548 (C=C), 1241 (C=S). 1H-NMR (300 MHz, DMSO-d6): δH 10.89 (s, 1 H, NH), 9.91 (s, 2 H, NH), 8.76 (m, 2 H, H-3″, H-5″), 7.83 (d, J2″,3″ = J6″,5″ = 5.7 Hz, 2 H, H-2″, H-6″), 7.56 (s, 1 H, H-2), 7.47 (d, J6,5 = 8.4 Hz, 1 H, H-6),7.39 (m, 1 H, H-5), 7.21 (d, J4,5 = 7.8 Hz, 1 H, H-4), 13C-NMR (125 MHz, DMSO-d6): δ180.8 (C=S), 164.4 (C=O), 150.2 (C-3″,C-5″), 140.4 (C-1″), 139.5 (C-1), 139.4 (C-3), 134.1 (NCS), 129.4 (C-5), 125.2 (C-6), 122.8 (C-4), 122.4 (C-2) 121.6 (C2″, C-6″); Negative FAB-MS m/z (rel. int. %): 328.1 [M-H]+ (15), 275.1 (44), 183 (100), HRFAB-MS (−ve mode) Calcd. for [(C14H11N5OS2) – H]: (m/z = 328.0320) Found 328.0315.
2-Isonicotinoyl-N-(3-nitrophenyl) hydrazinecarbothioamide (27)
Rf = 0.7, Mp: 159–160 °C; IR (KBr, cm–1): 3284, 3124 (N–H), 1676 (C=O), 1597, 1531 (C=C), 1477 (N=O), 1247 (C=S). 1H-NMR (300 MHz, DMSO-d6): δH 10.94 (s, 1 H, NH), 10.15 (s, 2 H, NH), 8.78 (d, J3″,2″ = J5″,6″ = 6.0 Hz, 2 H, H-3″, H5″), 8.42 (bs, 1 H, H-2), 7.99 (t, J4,5 = J6,5 = 6.6 Hz, 2 H, H-4, H-6), 7.85 (d, J2″,3″ = J6″,5″ = 5.7 Hz, 2 H, H-2″, H-6″), 7.61 (t, J5,4/5,6) = 8.1 Hz, 1 H, H-5), Negative FAB-MS m/z (rel. int. %): 316 [M-H]+ (13), 275 (23), 183 (100), HRFAB-MS (−ve mode) Calcd. for [(C13H11N5O3S) – H]: (m/z = 316.1281) Found 316.1280.
Bioassays
Protocol for In Vitro Urease Inhibition Assay
The urease inhibition activity of compounds 3–27 was evaluated by using the method reported by Weatherburn. et al. (1967). Thiourea and acetohydraoxamic acid were used as standard compounds42–44. During the experiments all the compounds were evaluated at 0.5 mM each in triplicate. The compounds with >50% (greater than 50%) inhibition were further studied to determine their IC50 value in a separate experiment where, different concentration of the compound from 0.5–0.0078125 mM were tested in triplicates. On the other hand if compound showed <50% (less than 50%) inhibition at 0.5 mM then the compound was considered inactive.
Protocol for In Vitro Anti-Inflammatory Assay
Oxidative Burst Assay: Anti-inflammatory activity of the thiosemicarbazide derivatives 3–27, and isoniazid (1) was evaluated by following the method reported in Helfand. et al. (1982)45,46. In this experiment all the compounds were evaluated at 25 µg/mL, each in triplicate. To determine the IC50 values, the compounds with >50% inhibition were further evaluated on three different concentrations (1, 10 and 100 µg/mL). While, the compound failed to inhibit the production of ROS from zymosan activated whole blood cells at highest used dose (100 µg/mL) was considered as inactive.
All studies on human blood cells was carried out after an approval from independent ethics committee (Prof. Dr. Ghazala H. Rizwani Chair, IEC, Prof. Qamar Amin, Prof, Dr. Muddasir Uddin, Dr. Shahnaz Ghazi, Dr. SamiuzZaman, Prof. Dr. Ahsana Dar Farooq, Dr. M. Raza Shah member IEC), International Center for Chemical and Biological Sciences, University of Karachi, No: ICCBS/IEC-008-BC-2015/Protocol/1.0. Informed consents were obtained from the volunteers before drawing the blood. All the experiments were performed in accordance with relevant guidelines and regulations.
Protocol for Cytotoxicity (MTT assay) Assay
3T3 Cytotoxicity Assay: Cytotoxicity of the thiosemicarbazide derivatives 3–27, and isoniazid (1) was evaluated by the method reported by Pauwels. et al. (1988)47,48. In this experiment all the compounds were evaluated at 30 µM each in triplicate. If the compound showed >50% inhibition then for the determination of IC50 value different concentration of the compound from 30–0.9375 µM were tested. While if the compound showed <50% inhibition at 30 µM then the compound was considered to be inactive.
Supplementary information
Acknowledgements
We acknowledege the finnacial support of the Searle Company (Pakistan) for sponsoring the research project entitled, “Drug Repurposing and Repositioning Studies”. We are also thankful to the International Foundation for Science for the Individual Research Grant (I-1-F-5674-2) to Hina Siddiqui. We are thankful to Ms. Ayesha Fahim, Research Assistant, Dr. Pajnwani Center for Moelcular Medicine and Drug Research (International Center for Chemical and Biological Sciences), Universitry of Karachi, for conducting the anti-inflammatory assay.
Author Contributions
The concept of presented research was developed, and designed by M.I.C. and H.S.. All the data were analysed and interpreted by M.I.C., H.S. and F.R., M.K., A.J. F.R., M.K. and A.J. performed the experiments. M.I.C., H.S., F.R. wrote the manuscript. All the authors checked and revised the manuscript. The final version of manuscript was also approved by M.I.C. before submission.
Data Availability
All the supplementary mateial is available.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hina Siddiqui, Email: hinahej@gmail.com.
M. Iqbal Choudhary, Email: hej@cyber.net.pk.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43082-0.
References
- 1.Hanif, M. et al. Synthesis, urease inhibition, anti-oxidant, anti-bacterial, and molecular docking studies of 1,3,4-oxadiazole derivatives. Int. Sch. Res. Notices (2012). [DOI] [PMC free article] [PubMed]
- 2.Perveen S, Khan KM, Lodhi MA, Choudhary MI, Voelter W. Urease and α-chymotrypsin inhibitory effects of selected urea derivatives. Lett. Drug Des. Discov. 2008;5:401–405. doi: 10.2174/157018008785777315. [DOI] [Google Scholar]
- 3.Sadat A, Uddin G, Alam M, Ahmad A, Siddiqui BS. Structure -activity relationship of bergenin, p-hydroxybenzoyl bergenin, 11-O-galloylbergenin as potent anti-oxidant and urease inhibitor isolated from bergenia ligulata. Nat. Prod. Res. 2015;29:2291–2294. doi: 10.1080/14786419.2015.1004173. [DOI] [PubMed] [Google Scholar]
- 4.Krishnaraju AV, Rao CB, Sundararaju D, Sengupta K, Trimurtulu G. Anti-inflammatory activity of Vitex leucoxylonl. bark extracts against fremiti’s complete adjuvant induced arthritis in Sprague Daw ley Rat. Am. J. Infect. Dis. 2009;5:68–73. doi: 10.3844/ajidsp.2009.68.73. [DOI] [Google Scholar]
- 5.Leelaprakash G, Dass SM. Invitro anti-inflammatory activity of methanol extract of Enicostemma axillare. Int. J. Drug Dev. Res. 2011;3:189–196. [Google Scholar]
- 6.Ho KJ, et al. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am. J. Pathol. 2010;177:2116–2123. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suleyman H, Demircan B, Karagoz Y. Anti-inflammatory and side effects of cyclo-oxygenase inhibitors. Pharmacol. Rep. 2007;59:247. [PubMed] [Google Scholar]
- 8.Bjorkman DJ. Current status of nonsteroidal anti-inflammatory drug (NSAID) use in the United States: risk factors and frequency of complications. Am. J. Med. 1999;107:3–8. doi: 10.1016/S0002-9343(99)00362-9. [DOI] [PubMed] [Google Scholar]
- 9.Ulbrich H, Fiebich B, Dannhardt G. Cyclooxygenase-1/2 (COX-1/COX-2) and 5-lipoxygenase (5-LOX) inhibitors of the 6, 7-diaryl-2, 3-1H-dihydropyrrolizine type. Eur. J. Med. Chem. 2002;37:953–959. doi: 10.1016/S0223-5234(02)01418-6. [DOI] [PubMed] [Google Scholar]
- 10.Miner P, Jr, Katz PO, Chen Y, Sostek M. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five-way crossover study. Am. J. Gastroenterol. 2003;98:2616–2620. doi: 10.1111/j.1572-0241.2003.08783.x. [DOI] [PubMed] [Google Scholar]
- 11.Saniee P, Shahreza S, Siavoshi F. Negative effect of proton‐pump inhibitors (PPI s) on Helicobacter pylori growth, morphology, and urease test and recovery after PPI removal–an in vitro study. Helicobacter. 2016;21:143–152. doi: 10.1111/hel.12246. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita Y, Ishimura N, Ishihara S. Advantages and disadvantages of long-term proton pump inhibitor use. J. Neurogastroenterol. Motil. 2018;24:182. doi: 10.5056/jnm18001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu D, et al. Systematic toxicity mechanism analysis of proton pump inhibitors: an in silico study. Chem. Res. Toxicol. 2015;28:419–430. doi: 10.1021/tx5003782. [DOI] [PubMed] [Google Scholar]
- 14.Imhann F, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modolo LV, de Souza AX, Horta LP, Araujo DP, de Fátima Â. An overview on the potential of natural products as ureases inhibitors: A review. J. Adv. Res. 2015;6:35–44. doi: 10.1016/j.jare.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, et al. Ceftriaxone, an FDA-approved cephalosporin antibiotic, suppresses lung cancer growth by targeting Aurora B. Carcinogenesis. 2012;33:2548–2557. doi: 10.1093/carcin/bgs283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kigondu EM, Wasuna A, Warner DF, Chibale K. Pharmacologically active metabolites, combination screening and target identification-driven drug repositioning in anti-tuberculosis drug discovery. Bioorg. Med. Chem. 2014;22:4453–4461. doi: 10.1016/j.bmc.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Sun P, Guo J, Winnenburg R, Baumbach J. Drug repurposing by integrated literature mining and drug–gene–disease triangulation. Drug Discov. Today. 2017;22:615–619. doi: 10.1016/j.drudis.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Elhakeem MA, Taher AT, Abuel-Maaty SM. Synthesis and anti-mycobacterial evaluation of some new isonicotinylhydrazide analogues. Bull. Fac. Pharm. 2015;53:45–52. [Google Scholar]
- 20.Arbex MA, Varella MDCL, Siqueira HRD, Mello FAFD. Anti-tuberculosis drugs: drug interactions, adverse effects, and use in special situations-part 1: first-line drugs. J. Bras. Pneumol. 2010;36:626–640. doi: 10.1590/S1806-37132010000500016. [DOI] [PubMed] [Google Scholar]
- 21.Cardoso SH, et al. Synthesis and anti-tubercular activity of isoniazid condensed with carbohydrate derivatives. Quím. Nova. 2009;32:1557–1560. doi: 10.1590/S0100-40422009000600038. [DOI] [Google Scholar]
- 22.Rodrigues MO, et al. Synthesis and anti-mycobacterial activity of isoniazid derivatives from renewable fatty acids. Bioorg. Med. Chem. 2013;21:6910–6914. doi: 10.1016/j.bmc.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues FAR, et al. Biological evaluation of isoniazid derivatives as an anti-cancer class. Sci. Pharm. 2013;82:21–28. doi: 10.3797/scipharm.1307-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habala L, et al. Anti-microbial activity and urease inhibition of schiff bases derived from isoniazid and fluorinated benzaldehydes and of their copper (II) complexes. Molecules. 2016;21:1742. doi: 10.3390/molecules21121742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dammann HGerd, et al . Eradication of H. pyloriwith pantoprazole, clarithromycin, and metronidazole in duodenal ulcer patients: A head‐to‐head comparison between two regimens of different duration. Helicobacter. 2000;5:41–51. doi: 10.1046/j.1523-5378.2000.00006.x. [DOI] [PubMed] [Google Scholar]
- 26.Jakobsson JG. Pain management in ambulatory surgery- A review. Pharmaceuticals. 2014;7:850–865. doi: 10.3390/ph7080850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H-J, Qian Y, Zhu D-D, Yang X-G, Zhu H-L. Synthesis, molecular modeling and biological evaluation of chalcone thiosemicarbazide derivatives as novel anti-cancer agents. Eur. J. Med. Chem. 2011;46:4702–4708. doi: 10.1016/j.ejmech.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Siwek A, Stefańska J, Dzitko K, Ruszczak A. Anti-fungal effect of 4-arylthiosemicarbazides against Candida species. search for molecular basis of anti-fungal activity of thiosemicarbazide derivatives. J. Mole. Model. 2012;18:4159–4170. doi: 10.1007/s00894-012-1420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haraguchi SK, et al. Anti-trypanosomal activity of novel benzaldehyde-thiosemicarbazone derivatives from kaurenoic acid. Molecules. 2011;16:1166–1180. doi: 10.3390/molecules16021166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biot C, et al. Design, synthesis, and anti-malarial activity of structural chimeras of thiosemicarbazone and ferroquine analogues. Bioorg. Med. Chem. Lett. 2007;17:6434–6438. doi: 10.1016/j.bmcl.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Some AAO, Thiosemicarbazides N-S. Anti-bacterial activity of some 4-N-substituted thiosemicarbazides and thiosemicarbazones. Indian J. Pharm. Sci. 2003;65:423. [Google Scholar]
- 32.Pitucha M, et al. Synthesis, anti-bacterial and anti-proliferative potential of some new 1-pyridinecarbonyl-4-substituted thiosemicarbazide derivatives. Med. Chem. Res. 2016;25:1666–1677. doi: 10.1007/s00044-016-1599-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hearn MJ, Chen MF, Cynamon MH, Wang’ondu R, Webster ER. Preparation and properties of new anti-tubercularthioureas and thiosemicarbazides. J. Sulfur Chem. 2006;27:149–164. doi: 10.1080/17415990600576826. [DOI] [Google Scholar]
- 34.Ali B, et al. Synthetic nicotinic/isonicotinicthiosemicarbazides: In vitro urease inhibitory activities and molecular docking studies. Bioorg. Chem. 2018;79:34–45. doi: 10.1016/j.bioorg.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Sriram D, Yogeeswari P, Priya DY. Anti-mycobacterial activity of novel N-(substituted)-2-isonicotinoylhydrazinocarbothioamide endowed with high activity towards isoniazid resistant tuberculosis. Biomed. Pharmacother. 2009;63:36–39. doi: 10.1016/j.biopha.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Zhang HH, Hu XQ, Fan GF, Xu PF. Synthesis and molecular structure of new S‐nucleosides of 5‐(4‐Pyridyl)‐4‐Aryl‐4H‐1, 2, 4‐triazole‐3‐thiols. J. Chin. Chem. Soc. 2008;55:834–841. doi: 10.1002/jccs.200800124. [DOI] [Google Scholar]
- 37.Nadeem H, et al. Synthesis and in vitro Biological Activities of 4, 5-Disubstituted 1, 2, 4-Triazole-3-Thiols. J. Adv. Microbiol. 2013;3:366. doi: 10.4236/aim.2013.34050. [DOI] [Google Scholar]
- 38.Yıldırım SÖ, et al. 4-Phenyl-3-(pyridin-4-yl)-1H-1,2,4-triazole-5 (4H)-thione. Acta. Cryst. E: Structure Reports Online. 2005;61:o619–o621. doi: 10.1107/S1600536805003983. [DOI] [Google Scholar]
- 39.Yahyazadeh A, Ghasemi Z. Synthesis of unsymmetrical thiourea derivatives. Eur. Chem. Bull. 2013;2:573–575. [Google Scholar]
- 40.Ali B, et al. 1-[(4′-Chlorophenyl) carbonyl-4-(aryl) thiosemicarbazide derivatives as potent urease inhibitors: Synthesis, in vitro and in silico studies. Bioorg. Chem. 2018;79:363–371. doi: 10.1016/j.bioorg.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 41.Taha M, et al. Bisindolylmethane thiosemicarbazides as potential inhibitors of urease: Synthesis and molecular modeling studies. Bioorg. Med. Chem. 2018;26:152–160. doi: 10.1016/j.bmc.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 42.Lodhi, M A et al. Structural basis of binding and rationale for the potent urease inhibitory activity of biscoumarins. Biomed. Res. Int (2014). [DOI] [PMC free article] [PubMed]
- 43.Weatherburn M. Phenol-hypochlorite reaction for determination of ammonia. Ana. Chem. 1967;39:971–974. doi: 10.1021/ac60252a045. [DOI] [Google Scholar]
- 44.Xiao ZP, et al. The synthesis, structure and activity evaluation of pyrogallol and catechol derivatives as Helicobacter pylori urease inhibitors. Eur. J. Med. Chem. 2010;45:5064–5070. doi: 10.1016/j.ejmech.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 45.Helfand SL, Werkmeister J, Roder JC. Chemiluminescence response of human natural killer cells. I. The relationship between target cell binding, chemiluminescence, and cytolysis. J. Exp. Med. 1982;156:492–505. doi: 10.1084/jem.156.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mbiantcha M, Almas J, Shabana SU, Nida D, Aisha F. Anti-arthritic property of crude extracts of Piptadeniastrum africanum (Mimosaceae) in complete Freund’s adjuvant-induced arthritis in rats. BMC Complement. Altern. Med. 2017;17:111. doi: 10.1186/s12906-017-1623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choudhary MI, et al. cis-Clerodane-type furanoditerpenoids from Tinospora crispa. J. Nat. Prod. 2010;73:541–547. doi: 10.1021/np900551u. [DOI] [PubMed] [Google Scholar]
- 48.Pauwels R, et al. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods. 1988;20:309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the supplementary mateial is available.