Abstract
The discovery of cell‐free microRNAs (miRNAs) in serum, plasma and other body fluids has yielded an invaluable potential source of non‐invasive biomarkers for cancer and other non‐malignant diseases. miRNAs in the blood and other body fluids are highly stable in biological samples and are resistant to environmental conditions, such as freezing, thawing or enzymatic degradation, which makes them convenient as potential biomarkers. In addition, they are more easily sampled than tissue miRNAs. Altered levels of cell‐free miRNAs have been found in every type of cancer analysed, and increasing evidence indicates that they may participate in carcinogenesis by acting as cell‐to‐cell signalling molecules. This review summarizes the biological characteristics and mechanisms of release of cell‐free miRNAs that make them promising candidates as non‐invasive biomarkers of cancer.
Introduction
MicroRNAs (miRNAs) are non‐coding RNAs that are ~21 nucleotides in length. They are synthesized in the cell nucleus 1, 2, processed by the Drosha enzyme complex 3 and then exported to the cytoplasm by GTP‐dependent exportin‐5 4, where they are further processed by the Dicer enzyme complex 5, 6 to yield mature miRNAs 7, 8. In the cytoplasm, a leader strand of mature miRNA is attached to the RNA‐induced silencing complex (RISC) 9, 10, and it recognizes messenger RNA (mRNA) with a partially complementary sequence 11, inducing degradation or inhibiting translation of the mRNA 12, 13, 14, 15. miRNAs are involved in post‐transcriptional gene regulation of virtually all cellular processes that have been investigated so far. These include proliferation, differentiation, apoptosis and haematopoiesis 7, 12, 16, 17, 18. It was soon discovered that cellular miRNA expression levels were altered in multiple pathological conditions, including cancer 12, 19, 20, 21, 22, 23. In cancer, miRNA expression profiles could distinguish malignant from non‐malignant tissue, leading to the search for potential cancer biomarkers. Several studies have suggested that tissue miRNAs could be used as biomarkers for the classification and diagnoses/prognoses of lung cancer, breast cancer, colorectal cancer, pancreatic cancer, hepatocarcinoma and neuroblastoma, among others 24, 25, 26, 27, 28, 29, 30, 31, 32. Moreover, experimentally manipulating the levels of specific miRNAs affects many carcinogenic properties of tumour cell lines, such as survival, invasion, metastasis and tumour progression 33, 34, 35, 36, 37. Tissue miRNAs have undeniable potential for clinical use, but accessing them in solid tumours remains problematic.
Later, it was discovered that miRNAs were also secreted by a variety of cell types under normal and pathological conditions. These secreted, or cell‐free, miRNAs are highly stable and can be delivered to recipient cells in a functional way. It has been hypothesized that secreted miRNAs are mediators of cell‐to‐cell communication and gene expression, and currently, there is experimental evidence that supports that hypothesis.
Cell‐free miRNAs have been found in peripheral blood (referred to as circulating miRNAs) and several other body fluids, such as tears, urine and pleural effusions, exhibiting distinctive expression patterns in healthy individuals 38, 39, 40. Similar to tissue miRNAs, altered profiles of cell‐free miRNAs were found to be associated with several pathological conditions including cancers 38, 41. The accessibility and stability of cell‐free miRNAs 38, 42, 43 makes them valuable non‐invasive biomarkers 44, and they have been actively investigated as such.
Cell‐free miRNAs in serum and plasma
In 2008, cell‐free miRNAs were isolated from the serum and plasma of healthy individuals in a stable form and were protected from endogenous ribonuclease (RNase) activity in the blood 38, 42.
Mitchell et al. 38 first confirmed the presence of small RNA species ranging 10–70 nt in size in human plasma by characterizing the size of total 32P‐labelled RNA extracted from healthy donor plasma. Then, they generated a small RNA cDNA library from the 18–24 nt RNA fraction, demonstrating that 91 of 98 sequences corresponded to known miRNAs and directly confirming that mature miRNAs are present in human plasma. Finally, they quantified three known mature miRNAs (miR‐15b, miR‐16 and miR‐24) in the plasma of three healthy donors using specific probes and RT‐qPCR. Additionally, Chen et al. 42 used semi‐quantitative RT‐PCR to detect six randomly selected mature miRNAs in human plasma and serum. They then sequenced the PCR products from serum and showed that 87 of 100 sequences corresponded to the appropriate miRNAs. Both studies demonstrated that endogenous miRNAs in serum and plasma are resistant to RNase digestion. Nevertheless, synthetic miRNAs added directly to serum and plasma samples were rapidly degraded, indicating that endogenous miRNAs exist in a protected form that is not related to their intrinsic short length or structure 38, 45, 46. In contrast, large RNAs from serum were rapidly degraded by ribonuclease digestion, which illustrated the potential biological relevance of cell‐free miRNAs in the blood 42. It was later shown that miRNAs are protected by encapsulation in exosomes and by protein‐binding complexes (see the Mechanisms of miRNA release section). In addition, miRNAs were shown to be resistant to environmental conditions, such as freezing, thawing, pH and enzymatic degradation 38, 42, which makes them to be used as potential biomarkers.
In addition to the high stability of cell‐free miRNAs, the first studies reported that miRNA levels are consistent among healthy individuals 42, 47. Chen et al. 42 analysed the levels of 14 randomly selected miRNAs in the serum of seven healthy Chinese individuals (22–25 years old; four men and three women) by reverse transcriptase (RT)‐PCR and found that the expression levels were consistent. Gilad et al. 47 analysed 18 highly expressed miRNAs in the serum of two unrelated healthy individuals (females; age not specified) by RT‐PCR and found that their levels were consistent. Mitchell et al. 38 measured the levels of three miRNAs that are found in the plasma at moderate to low levels from three healthy individuals (unspecified age or gender), and they found no differences in expression levels. Unfortunately, none of these studies assessed how individuals were determined to be healthy; only Gilad et al. mentioned that they were chosen by self‐reporting of good health. On the other hand, it was reported that serum miRNA levels vary with pregnancy in healthy women, suggesting that the levels of miRNAs differ according to physiological status. However, this study analysed pregnant women compared with unrelated non‐pregnant women rather than analysing the same subjects when pregnant and when not 47. In addition, it was reported that subjects with diabetes had altered levels of serum miRNAs compared with healthy subjects 42, showing that metabolic diseases could be associated with altered miRNA levels. Nevertheless, there are no studies that systematically analysed the levels of circulating miRNAs from healthy subjects of different ages, ethnicities or physiological states. In addition, the levels of miRNAs during subclinical pathologies or after the natural resolution of infectious diseases have not been investigated. Therefore, miRNA studies should include healthy controls who are matched in age, ethnicity and gender. Equally important, healthy status should be assessed by clinical and laboratory measurements. Additionally, recent reports have suggested that environmental contaminants can alter the expression profile of endogenous miRNAs in human and mouse cells 48, 49, 50. There are several studies of endogenous miRNA expression changes attributable to air pollutant exposure in human beings 50, 51, 52, 53, but only two studies regarding the effect of environmental contaminants on cell‐free miRNAs have been published 54, 55. Consequently, studies searching for miRNAs biomarkers may consider the influence of environmental pollution in the alterations of miRNAs levels: for example, people living in highly polluted regions compared with people living in regions without contamination or people exposed to a particular contaminant such as smokers versus non‐smokers.
Regarding total concentration of miRNAs from healthy subjects, the cell‐free miRNA concentration in serum and plasma is much lower than the miRNA concentration in blood cells from the same individual, although there is an enriched miRNA fraction within the small RNA fraction from serum 42. Moreover, miRNAs comprise >80% of the small RNA fraction from serum compared with <40% of the small RNA fraction from peripheral blood cells (Fig. 1). Consequently, total RNA extraction from the serum would not require the use of techniques to concentrate small RNAs. On the other hand, plasma contains a greater concentration of miRNAs than serum, although it was later demonstrated that this was due mainly to the miRNAs released by platelets during sample processing 56. To accurately analyse cell‐free miRNAs in plasma, the sample should be ultra‐centrifuged to eliminate platelets before storage (freezing) or RNA extraction 56. Additionally, serum obtained from haemolysed samples contains increased concentrations of certain miRNAs due to release from erythrocytes. Mature erythrocytes contain abundant miRNAs even when they lack ribosomal and large RNAs 57, 58. According to McDonald et al. 56, the levels of four miRNAs were not altered if serum samples contained <10 mg/dl of haemoglobin; therefore, this parameter could be used for the quality control of samples.
Figure 1.
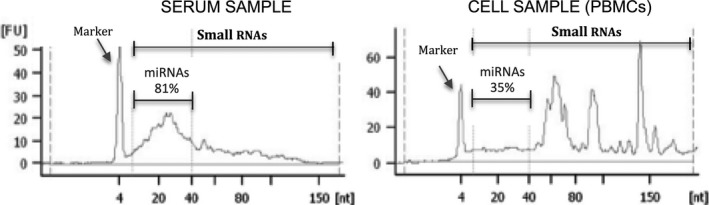
Small RNA profile in serum and peripheral blood mononuclear cells (PBMCs) assessed on the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and the Small RNA chip.
Early studies of circulating miRNAs revealed that while the levels of serum miRNAs in healthy individuals remained constant, the levels of miRNAs in patients with prostate cancer 38, lung cancer and colorectal cancer 42 were altered and distinguishable from healthy individuals. Moreover, it was shown that miRNAs originating from human prostate cancer cells were detected in the plasma of NOD/SCID mice after xenograft transplantation 38. All of this evidence prompted a search for circulating miRNAs in serum and plasma that could be potential non‐invasive biomarkers for cancer. Biomarkers that can be noninvasively sampled are particularly important for solid tumours because tissue samples for histopathological evaluation require invasive and sometimes dangerous procedures.
Altered levels of miRNAs have been found in serum and plasma from patients with every type of solid tumour analysed thus far, and miRNA signatures have been reported to be potentially useful for diagnoses and prognoses of lung, breast, prostate, ovarian, bladder, pancreatic, gastric, liver, colorectal, oral and oesophageal cancer 27, 38, 42, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89 (Table 1). Table 2 summarizes the candidate cell‐free miRNA tumour biomarkers that have been reported in the literature during the last 5 years.
Table 1.
Blood‐derived and other body fluids that contain cell‐free miRNAs
| Body fluid | Healthy subject | Cancer disease | References |
|---|---|---|---|
| Breast milk | Pregnant women | Not reported | 39 |
| Colostrum | Pregnant women | Not reported | 39 |
| Saliva | Yes | Oral cancer | 39, 92 |
| Tears | Yes | Not reported | 39 |
| Urine | Yes | Bladder and renal cancer | 39, 40, 96, 97, 99 |
| Seminal fluid | Yes | Not reported | 39 |
| Amniotic fluid | Yes | Not reported | 39 |
| Pleural fluid | Yes | Not reported | 39 |
| Bronchoalveolar lavage | Yes | Lung cancer | 39 |
| Pleural effusion | No | Lung and gastric cancer | 41, 103, 104 |
| Gastric juice | Yes | Gastric cancer | 91 |
| Pancreatic juice | No | Pancreatic cancer | 96 |
| Peritoneal fluid | Yes | Gastric cancer (metastasis) | 39, 105 |
| Cerebrospinal fluid | Yes | Brain cancer | 39, 107, 108 |
| Plasma | Yes | Lung, breast, prostate, ovarian, bladder, pancreatic, gastric, liver, colorectal, oral and oesophageal cancer | 27, 74, 75, 77, 78, 79, 80, 81, 83, 84, 86, 88 |
| Serum | Yes | Lung, breast, prostate, ovarian, gastric, liver and oesophageal cancer | 59, 60, 61, 62, 63, 65, 66, 67, 68, 69, 78 |
Table 2.
Cell‐free microRNAs detected in serum and plasma as potential biomarkers for cancer
| Blood‐derived fluid | Cancer disease | Study design | Differentially expressed miRNAs | Method | References |
|---|---|---|---|---|---|
| Serum | Lung cancer | NSCLC versus healthy controls (HC) | 10 miRNA signature: miR‐20a, miR‐24, miR‐25, miR‐145, miR‐152, miR‐199a‐5p, miR‐221, miR‐222, miR‐223, miR‐320 | RT‐qPCR | 59 |
| NSCLC‐asymptomatic high‐risk individuals versus healthy smokers | 34‐miRNA signature | RT‐qPCR | 60 | ||
| NSCLC longer versus NSCLC shorter survival | miR‐486, miR‐30d, miR‐1 and miR‐499; overall survival | Solexa sequencing and RT‐qPCR | 62 | ||
| NSCLC versus healthy controls (HC) | miR‐21 | MicroArray and RT‐qPCR | 61 | ||
| NSCLC versus healthy controls (HC) | miR‐21 | RT‐qPCR | 65 | ||
| NSCLC versus healthy controls (HC) | miR‐15b and miR‐27b | RT‐qPCR | 66 | ||
| Breast cancer (BC) | Primary BC after surgery (M0), metastatic BC (M1) versus HC | miR‐17, miR‐34a, miR‐155 and miR‐373 | RT‐qPCR | 69 | |
| Primary BC (M0) versus metastatic BC (M1) versus HC | miR‐155 (M0 from HC), miR10b‐34a & ‐155 (M1 from HC and metastases) | RT‐qPCR | 68 | ||
| BC versus HC | miR‐195 | RT‐qPCR | 67 | ||
| BC versus HC | miR‐21, miR‐106a and miR‐155 | RT‐qPCR | 63 | ||
| Prostate cancer (PC) | Metastatic PC versus non‐recurrent PC | miR‐141 and miR‐375 (metastatic PC) | RT‐qPCR | 78 | |
| Gastric cancer (GC) | GC versus HC | miR‐1, miR‐20a, miR‐27a, miR‐34 and miR‐423‐5p | Solexa sequencing and RT‐qPCR | 64 | |
| Oesophageal cancer (ESCC) | ESCC versus HC | miR‐10a, miR‐22, miR‐100, miR‐148b, miR‐223, miR‐133a and miR‐127‐3p | Solexa sequencing and RT‐qPCR | 73 | |
| Plasma | Lung cancer | NSCLC versus COPD versus HC |
Signature (high miR‐155‐5p, high miR‐223‐3p, and low miR‐126‐3p) progression AD Signature (high miR‐20a‐5p, low miR‐152‐3p, and low miR‐199a‐5p) survival SCC |
RT‐qPCR | 74 |
| NSCLC versus HC |
let‐7f, miR‐20b and miR‐30e‐3p (NSCLC) let‐7f and ‐30e‐3p (poor survival) |
RT‐qPCR | 233 | ||
| NSCLC versus before and at the time of disease versus HC |
miR‐17, miR‐660, miR‐92a, miR‐106a, and miR‐19b (diagnosis) miR‐660, miR‐140‐5p, miR‐451, miR‐28‐3p, miR‐30c and miR‐92a (predictive) mir‐221, miR‐660, miR‐486‐5p, miR‐28‐3p, miR‐197, miR‐106a, miR‐451, miR‐140‐5p and miR‐16 (predictive aggressive cancer) |
RT‐qPCR | 27 | ||
| NSCLC versus HC | miRNA‐21, miRNA‐126, miRNA‐210, and miRNA‐486‐5p | RT‐qPCR | 75 | ||
| Breast cancer (BC) | BC early‐stage Caucasian (CA), African‐American (AA) versus HC | let‐7c, miR‐589 (CA); miR‐425* and let‐7d* (AA) | MicroArray and RT‐qPCR | 77 | |
| Prostate cancer (PC) | PC versus HC |
miR‐107 and miR‐574‐3p (PC versus HC) miR‐141, miR‐375 and miR‐200b (metastatic versus non‐metastatic) |
RT‐qPCR | 78 | |
| PC stage 2 and 3 | miR‐20a (stage 3); miR‐20a and miR‐21 (high‐risk CAPRA score) | RT‐qPCR | 79 | ||
| Ovarian cancer (OC) | Endometriosis (Ea) versus Ea‐associated OC (EAOC) versus HC versus and serous ovarian cancer (SOC) |
miR‐16, miR‐21 and miR‐191 (EAOC versus HC) miR‐21, miR‐362‐5p and miR‐127a (Ea versus EAOC) miR‐16, miR‐191 and miR‐4284 (Hc versus SOC) |
RT‐qPCR | 80 | |
| Bladder cancer (BdC) | Muscle invasive BdC (MIBdC) versus non‐muscle‐invasive BdC (NMIBdC) versus noncancerous controls | miR‐200b (MIBdC) | MicroArray | 81 | |
| Pancreatic cancer (Pc) | Pc versus HC | miR‐210 | RT‐qPCR | 82 | |
| Gastric cancer (GC) | GC versus HC | miR‐17‐5p, miR‐21, miR‐106a, miR‐106b and let‐7a | RT‐qPCR | 83 | |
| Liver cancer (LC) | LC versus HC | miR‐483‐5p | RT‐qPCR | 234 | |
| HBV‐related LC versus HC versus hepatitis B versus cirrhosis | miR‐122, miR‐192, miR‐21, miR‐223, miR‐26a, miR‐27a and miR‐801 (HBV‐related LC) | MicroArray and RT‐qPCR | 84 | ||
| Colorectal cancer (CRC) | CRC versus HC | miR‐601 and miR‐760 | PCR array and RT‐qPCR | 103 | |
| Advanced CRC versus HC | miR‐29a and miR‐92a | RT‐qPCR | 87 | ||
| Oesophageal cancer (ESCC) | ESCC versus HC |
miR‐16, miR‐21, miR‐185 and miR‐375 (ESCC) miR‐16 (T3‐4 versus T1‐2) miR‐16 and miR‐21 (survival) |
RT‐qPCR | 86 | |
| ESCC (3‐year follow‐up) | miR‐21 and miR‐375 (shorter survival) | RT‐qPCR | 88 |
NSCLC, non–small‐cell lung cancer; ESCC, Oesophageal squamous cell carcinoma; COPD, chronic obstructive pulmonary disease; AD, lung adenocarcinoma; SCC, squamous cell carcinoma; CAPRA, Cancer of the Prostate Risk Assessment score; T, stages of disease.
Cell‐free miRNAs in other body fluids
To date, cell‐free miRNAs have been detected in every body fluid analysed. Serum, plasma, tears, urine, amniotic fluid, colostrum, breast milk, bronchial lavage, cerebrospinal fluid, peritoneal fluid, pleural fluid, seminal fluid, saliva and gastric juices from healthy individuals all contain miRNAs (Table 1) 39, 40, 90, 91, 92. They have also been detected in pancreatic cyst fluids 93. Sadakari et al. 94 reported the presence of miRNAs in pancreatic fluid, but the total RNA was extracted from a cell pellet; therefore, they were not cell‐free or secreted miRNAs. It was reported that cell‐free miRNAs showed a distinctive expression pattern in each body fluid analysed 39, 95, suggesting a potential biological function associated with the surrounding tissues. These distinctive expression patterns include variations in miRNA concentrations in different body fluids, as well as differences in the species of miRNAs detected. For example, of 12 body fluids analysed, saliva, breast milk and seminal fluid had the highest number of detectable miRNA species (~400), whereas urine, cerebrospinal fluid, and pleural fluid had the fewest detectable miRNA species (~200) 39. Weber et al. 39 also reported variation in the concentration of total RNA obtained from 300 μl of different body fluids of 113–48 240 μg/l. In addition, it was reported that highly abundant miRNAs are detected in many different fluids, but several miRNAs are present exclusively in one body fluid, such as miR‐637 in tears and miR‐193b in breast milk 39. According to Hanson et al. 95, a panel of nine miRNAs allows for the identification of the body fluid of origin of forensic samples. In addition, it was shown that cell‐free miRNAs in urine could be used to detect physiological status such as pregnancy in normal individuals 39, 47.
Similar to cell‐free miRNAs in serum and plasma, there is evidence to indicate that alterations in the levels of cell‐free miRNAs in other body fluids might indicate diseases such as oral 92, bladder 40, 96, 97, 98, renal 99, lung 41, 100, 101, 102, 103, 104, gastric 91, 105, pancreatic 106 and brain cancer 107, 108 (Tables 1 and 3). In particular, saliva and urine are fluids that are easy to obtain and do not require invasive procedures. Table 3 summarizes the miRNAs that are candidates as biomarkers for cancer that have been reported in the literature to date.
Table 3.
Cell‐free microRNAs detected in other body fluids as potential biomarkers for cancer
| Body fluid | Cancer disease | Study design | Differentially expressed miRNAs | Method | Number of miRNAs analysed | Number of samples | References |
|---|---|---|---|---|---|---|---|
| Saliva | Oral cancer (OSCC) | OSCC versus healthy controls (HC) | miR‐125a and miR‐200a ↓ | RT‐qPCR | 314 | 50 OSCC, 50 HC | 92 |
| Urine | Bladder cancer (UCC) | Invasive UCC versus superficial UCC versus HC | Invasive UCC: miR‐618 and miR‐1255b‐5p ↑ | MicroArray | 754 | 4 versus 4 versus 4 | 96 |
| RT‐qPCR | 6 | 19 versus 16 versus 20 | |||||
| Bladder cancer (UCC) | UCC versus controls* | miR‐1224‐3p and miR‐15b↑ mR‐135b↓ | RT‐qPCR | 15 | 68 UCC, 53 controls | 97 | |
| Renal cancer (RCC) | RCC & benign cocytoma (Bco) versus controls ** | miR‐15a ↑ (RCC) | RT‐qPCR | 1 | 18 RCC, 5 Bco and 5 controls | 99 | |
| Bladder cancer (UCC) | UCC versus HC versus urinary tract infection (UTI) | miR‐96 and miR‐183 ↑ (UCC versus HC only) | RT‐qPCR | 3 | 100 UCC, 45 HC, 25 UTI | 98 | |
| Bladder cancer (UCC) | UCC hi, UCC lo versus HC versus UTI | miR‐126 and miR‐182 ↑ (UCC) | RT‐qPCR | 157 | 9 each | 40 | |
| RT‐qPCR | 3 | 47 each | |||||
| Bronchoalveolar lavage | Lung cancer (LC*) | LC versus benign lung diseases (BL*) | miR‐1285, miR‐1303, miR‐29a‐5p, 650 ↑ | PCR‐Array | 700 | 3 pools LC and BL (10 each) | 100 |
| RT‐qPCR | 8 | 30 LC, 30 BL | |||||
| Lung cancer (NSCLC) | NSCLC versus cancer‐free controls | miR‐21, miR‐143, miR‐155, miR‐210 and miR‐372 | RT‐qPCR | 5 | 21 NSCLC, 10 controls | 101 | |
| Pleural effusion | Lung cancer (AD) | AD versus benign pleural effusion (BPE) | miR‐134, miR‐185, miR‐22↓ | RT‐qPCR | 3 | 45 AD, 42 BPE | 102 |
| Lung cancer (AD) | AD versus BPE* | miR‐198 ↓ | MicroArray | 160 | 10 AD, 10 BPE | 41 | |
| RT‐qPCR | 1 | 42 AD4, 5 BPE | |||||
| Lung cancer (NSCLC) | NSCLC longer survival (LS) versus NSCLC shorter survival (SS) | miR‐93, miR‐100, miR‐134, miR‐151, miR‐345 (survival indicators) | MicroArray& RT‐qPCR | Not specified | 10 (5LS, 5SS) | 103 | |
| 5 | 184 (92LS, 92SS) | ||||||
| Lung + gastric cancer (malignant) | Malignant (Ma) versus benign(Be) | miR‐24, miR‐26a and miR‐30d ↑ | RT‐qPCR | 22 | Pools 18 Ma and 12 Be | 104 | |
| 3 | 18 Ma versus 11 Be | ||||||
| Gastric juice | Gastric cancer (GC) | GC versus gastric ulcer versus normal mucosa/gastritis | miR‐21 and miR‐106a ↓ | RT‐qPCR | 2 | 42 GC, 34 ulcer, 18 normal | 91 |
| Pancreatic juice | Pancreatic cancer (PDAC) | PDAC versus NPNH and chronic pancreatitis (CP) | miR‐205, miR‐210, miR‐492 and miR‐1247↑ (PDAC) | MicroArrays | 470 | 6 PDAC, 2 pools 6 NPNH each | 106 |
| RT‐qPCR | 4 | 44 PDAC, 19 CP and 13 NPNH | |||||
| Peritoneal fluid | Metastasis of GC | GC stage T4 versus GC T1‐T3 versus GC malignant ascites (MA) versus supernatant metastatic cell lines (Sn) | miR‐21, miR‐1225‐5p↑ (serosa‐invasive GC) | MicroArray | 1126 | 3 GCT4, 3 GCT1‐3, 4MA, 2 Sn | 105 |
| Cerebrospinal fluid | Brain cancer (glioblastoma) | Glioblastoma versus brain trauma non‐tumour control | miR‐21↑ (recurrence and poor prognosis) | RT‐qPCR | 1 | 70 glioblastoma, 25 controls | 107 |
| Brain cancer (Glioblastoma) | Glioblastoma versus non‐tumour control * (Ctl) | miR‐21↑ | RT‐qPCR | 1 | 13 glioblastoma, 14 Ctl. 2 ns set: 15 glioblastoma, 15 Ctl | 108 | |
| Cyst fluid | Pancreatic duct neoplasm (IPMN) | Cystic lesions: IPMNs hi versus IPMNs lo versus SCAs | miR‐24, miR‐30a‐3p, miR‐18a, miR‐92a, miR‐342‐3p, miR‐99b, miR‐106b, miR‐142‐3p, miR‐532‐3p, miR‐21 | PCR‐Array | 377 | 15 (5 versus 5 versus 5) | 93 |
| RT‐qPCR | 18 | 50 (6 versus 14 versus 20) |
LC*, mix SCC & AD; SCC, squamous carcinoma; AD, lung cancer adenocarcinoma; NSCLC, non–small‐cell lung cancer; BL*, fibrosis, pneumonia, chronic obstructive pulmonary disease or tracheal stenosis; BPE, benign pleural effusion (tuberculosis, pneumonia and transudate); BPE*, benign pleural effusion (tuberculous pleurisy or parapneumonic effusion); NSCLC, non–small‐cell lung cancer; Be, benign (pleural effusions from patients diagnosed with liver cirrhosis, tuberculosis, pneumonia, heart failure or injury); HC, healthy controls; OSCC, oral squamous cell carcinoma; UCC, urothelial cell carcinoma; UCC hi & lo, high and low grade; controls*, treated for benign urinary conditions and without urinary pathology; RCC, malignant renal cell carcinoma; controls **, urinary inflammatory conditions; PDA, pancreatic ductal adenocarcinoma; IPMN, intraductal papillary mucinous neoplasms; non‐tumour control *, head trauma, subarachnoid haemorrhage, normal pressure hydrocephalus, arteriovenous malformation; IPMN hi & lo, high‐grade & low‐grade IPMNs; SCAs, serous cystadenomas; PDAC, pancreatic ductal adenocarcinoma; NPNH, no pancreatic disease, non‐healthy controls; ↓, downregulated; ↑, upregulated.
Mechanisms of miRNA release
The potential usefulness of circulating miRNAs as reliable biomarkers in cancer may be based on their potential biological function. If cells actively secrete miRNAs as a mechanism of cell communication, then the levels and patterns of these miRNAs should be specific and would depend on the type of secretory cell and its metabolic activity. Because cancerous cells have higher metabolic activity than normal cells, it is feasible that certain miRNAs released from those cancerous cells can be used as biomarkers of cancer. Therefore, it is necessary to understand the release mechanisms of miRNAs.
Experimental evidence indicates that four potential forms of miRNAs are released from cells: miRNAs that are encapsulated within exosomes 109, complexed with Argonaute2 protein (Ago2) 45, 46, bound to high‐density lipoprotein (HDL) 110 or bound to the RNA‐binding protein nucleophosmin (NPM1) 111 (Fig. 2). Importantly, none of this evidence contradicts the existence of one or another extracellular form of miRNA in any cell line or biological sample analysed. In addition, it has been suggested that miRNAs are released from broken cells passively, due to injured tissue, chronic inflammation, apoptosis or necrosis 42, 46. These processes regularly occur in cancer, and it has been suggested that the high stability of these leaked miRNAs could be associated with their binding to Ago proteins 46. However, a systematic or quantitative analysis of the passive release of miRNAs has not been reported.
Figure 2.
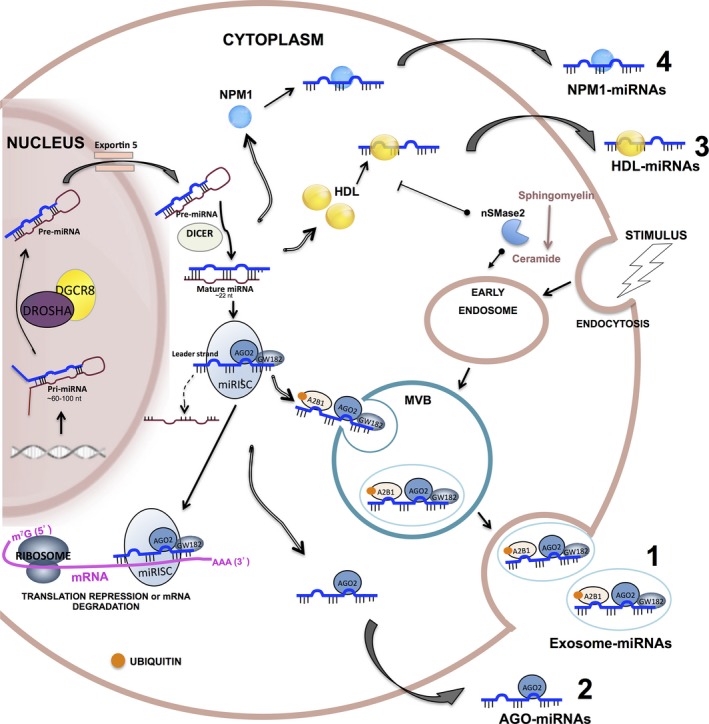
Biogenesis and proposed release mechanisms of free‐cell miRNAs. miRNA precursors (pri‐miRNAs) are transcribed in the nucleus and processed by the Drosha complex to generate pre‐miRNAs. Pre‐miRNAs are exported to the cytoplasm via exportin‐5 and are excised by DICER into a mature form of double‐stranded RNAs that are ~22 nucleotides long. One strand is loaded into the RISC along with Argonaut 2 (AGO2) and GW182 to form the miRISC. There, miRNAs can bind to complementary sequences on target mRNAs to repress translation or trigger mRNA cleavage. miRNAs can also be transported to the extracellular environment via four proposed mechanisms. (1) Encapsulated within exosomes: miRNAs are sorted into multivesicular bodies (MVBs), which are derived from early endosomes, through a mechanism that requires ceramide production on the cytosolic side by neutral sphingomyelinase 2 (nSMase2), the endosomal sorting complex required for transport (ESCRT) machinery and the sumoylated hnRNPA2B1 protein, which specifically binds mature miRNAs and controls their loading into MVBs. MVBs fuse with the plasma membrane and release exosomes into the extracellular medium. MVBs are enriched with GW182 and AGO2, the two main components of the RISC, which could be involved in the function of miRNAs. (2) Complexed with Argonaute2 protein (Ago2): miRNAs can be stably exported when they are associated with Ago2, a major component of the RISC. (3) Bound to high‐density lipoprotein (HDL): miRNA can also be stably exported in conjunction with HDL, via a mechanism that is repressed by nSMase2. (4) Bound to the RNA‐binding protein nucleophosmin (NPM1): NPM1 was shown to bind and protect exosome‐free miRNAs from RNase activity in blood circulation.
Apart from the leaked free miRNAs, it has been hypothesized that miRNAs are secreted as mediators of cell‐to‐cell communication and gene regulation. Consistent with this hypothesis, it has been shown that secretion of miRNAs via exosomes and HDL is energy dependent, as they are transported and delivered to other cells in a functional state 109, 110, 112, 113, 114, 115. There is still no direct evidence that miRNAs bound to AGO2 and NPM1 are actively released from cells or that they are transported to other cells.
Exosomes
In 2007, Valadi et al. 109 provided the first evidence that exosomes from human and mouse mast cell lines contained miRNAs that were transferred to other cells and maintained their functional capacity. Soon, it was shown that both normal and tumour cells secreted miRNAs contained in exosomes 116, 117, 118, 119. Moreover, evidence suggested that tumour cells are capable of influencing their microenvironment and promoting cancer development through exosomal miRNAs 114, 116, 120.
Exosomes are homogenous membrane vesicles of endocytic origin (20–90 nm) that are released into the extracellular medium by the merging of multivesicular bodies (MVB) with the plasma membrane using active secretion 121, 122. First, early endosomes are formed by the fusion of endocytic vesicles from the plasma membrane. These endosomes mature to late endosomes where intraluminal vesicles bud off into the lumen to form MVBs. MVBs directly fuse with the plasma membrane and release exosomes into the extracellular medium. These exosomes contain small RNAs, cytoplasmic proteins and cell receptors, which can be transferred to recipient cells 116, 118, 123 (Fig. 2). Exosome biogenesis requires the endosomal sorting complex required for transport (ESCRT complex) 124 as well as associated proteins, such as Alix 125 and Tsg 101 126. Evidence suggests that GW182 and AGO2, which are two main components of the RISC, are enriched in MVBs and could be involved in the function of miRNAs 127, 128 (Fig. 2). Li et al. 128 reported evidence that AGO2 selectively protects the miRNAs in exosomes, providing a second model of stability of circulating miRNAs in addition to the exosomal lipid bilayer. Recently, Lv et al. 129 showed that AGO2 facilitates the packaging of miR‐16 into MVBs secreted by HeLa cells. In addition, they reported that incubation of 293T cells with MVBs from HeLa cells co‐transfected with HA‐tagged‐AGO2 and miR‐16 induced a higher inhibition of Bcl2 (the putative target of miR‐16) expression in recipient 293T cells than incubation with MVBs containing only miR‐16. On the other hand, Villarroya‐Beltru et al. 130 showed that mature miRNAs contain specific motifs that are recognized by the sumoylated‐heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1), which controls their loading into MVBs. Likely, the mature miRNAs along with AGO2‐GW182 and hnRNPA2B1 are loaded into MVBs and released as exosomes into the extracellular medium. Although the mechanisms of exosome interactions with the receptor cells are not well known, labelling of purified exosomes with the green fluorescent lipid dye PKH67 showed that exosomes carrying miRNAs fuse with the plasma membrane of the target cells 113, 131.
Ago2‐associated miRNAs
The first studies that explored the possibility of extracellular miRNAs outside exosomes in clinical samples were reported in 2011 45, 46. Turchinovich et al. 46 reported that of 188 miRNA species detected in plasma, 97 miRNAs were detected in both exosomal and exosome‐free fractions (51%), while 69 were found exclusively in the exosome‐free fraction (37%) and 22 were detected exclusively in exosomes (11%). Arroyo et al. 45 reported that of 128 miRNAs detected in plasma, 66% were detected in the exosome‐free fraction and 43% were detected only in this fraction. In addition to showing the presence of exosome‐free miRNAs, these studies revealed that specific miRNAs were exclusively detected in one or another fraction, suggesting the existence of selective release mechanisms. More importantly, using differential centrifugation purification and immunoprecipitation methods, they showed that exosome‐free miRNAs were associated with Ago2, a major component of the RISC 9 (Fig. 2), and this association protects miRNAs from RNase A activity. This finding revealed an alternative mechanism of miRNA release and cell communication. However, there is no direct evidence that cells actively secrete miRNAs associated with Ago2, that they are taken up by recipient cells, or that there are specific Ago2‐miRNA complexes associated with cancer.
NPM1‐associated miRNAs
Wang et al. 111 first reported that cell‐free miRNAs were found in both exosomes and exosome‐free fractions obtained from the supernatants of tumour cell lines cultured after serum deprivation. Moreover, similar to the studies by Turchinovich et al. 46 and Arroyo et al. 45, they found that there are miRNA species exclusively in one or another fraction. These authors found important levels of the RNA‐binding protein nucleophosmin 1 (NPM1) in the exosome‐free fraction from fibroblast culture supernatants (Fig. 2), and they showed that NPM1 binds and protects miRNAs from RNase activity. They did not report the discovery of Ago2 in their study. However, this study did not provide direct evidence that NPM1‐associated miRNAs are taken up by recipient cells, that they are secreted via an energy‐dependent mechanism or that they are detected in clinical samples.
HDL‐associated miRNAs
High‐density lipoprotein‐associated miRNAs were found in plasma from healthy human beings and from patients with familial atherosclerosis, stable coronary artery disease and acute coronary syndrome 110, 132. Notably, it was reported that the HDL‐miRNA profile was distinctly different than the exosome‐miRNA profile from matched healthy individuals, which correlates with previously described data suggesting that more than one selective mechanism for miRNA release exists 110. Vickers et al. 110 also reported that HDL purified from human plasma transfers miRNAs into cultured hepatocytes (Huh7) with functional capacity, while Tabet et al. 115 showed that HDL suppresses the expression of intercellular adhesion molecule‐1 (ICAM‐1) through the transfer of miR‐223 to endothelial cells (HCAEC).
The exact mechanism by which HDL is loaded with miRNAs and which proteins could facilitate this association are not known. However, it was hypothesized that HDL could bind to extracellular plasma miRNAs through divalent cation bridging 110. Vickers et al. reported that the inhibition of nSMAse2 significantly increased the amount of miR‐223 exported to HDL using J774 macrophages, suggesting that nSMase2 and the ceramide pathway repress miRNA export to HDL (Fig. 2). Conversely, it was previously reported that overexpression of nSMase2 and activation of the ceramide pathway induced export of miRNAs by exosomes 112.
Distinct HDL‐miRNA plasma profiles were found in familial hypercholesterolaemia (FH) patients compared with healthy individuals 110, but none have been published so far regarding cancer.
Circulating and body‐fluid miRNAs as biomarkers in cancer
A biomarker is defined as any cellular, molecular or genetic component that can be measured and associated with a biological process, pathogenic process or pharmacologic response to treatment 44. Current studies aiming to identify circulating miRNAs with diagnostic value in cancer are mainly based on the different expression profiles of miRNAs in samples from patients with cancer compared with healthy individuals, as the first approach in the pre‐clinical exploratory phase. These differentially expressed miRNAs should be able to distinguish cancer patients from non‐cancer subjects, which requires measuring the sensitivity and specificity of the candidate biomarker in a second exploratory phase 133, preferably in an independent cohort. Finally, a biomarker for cancer diagnosis should be able to detect a specific type of cancer in a general population with a high specificity and sensitivity. Evidence has indicated that circulating and body‐fluid miRNAs are potential biomarkers for diagnosis, as well as biomarkers for prognosis and indicators of disease stage in several cancers.
Nevertheless, a suitable cancer biomarker has to be associated with the presence of tumour cells or the malignant process; therefore, the hypothesis that tumour cells are the main source of the secreted miRNAs with altered levels in cancer patients is relevant. Identifying actual cancer‐related miRNAs is crucial, considering that the levels of circulating miRNAs seem to depend on several normal and pathological physiological conditions, such as pregnancy, diabetes and hypertension 42, 47, 134, 135, 136.
We summarized two types of evidence that may indicate that certain upregulated or downregulated miRNAs in cancer patients are associated with malignant processes and, potentially, cancer diagnosis.
Before and after tumour removal
There are studies that have reported that highly expressed circulating miRNAs from cancer patients return to normal levels after tumour resection, suggesting that such miRNAs are of tumour origin. First, several studies designed screening strategies to identify candidate miRNAs with diagnostic value by comparing the serum or plasma of cancer patients to that of healthy controls. Then, they analysed the levels of the candidate miRNAs in pre‐ and post‐surgery samples from patients diagnosed with breast cancer, osteosarcoma, head and neck squamous cell carcinoma, hepatocellular carcinoma, tongue squamous cell carcinoma and gastric cancer 67, 83, 137, 138, 139, 140, 141.
In contrast, Konishi et al. 142 first used microarray analysis to compare the expression levels of miRNAs in pre‐ and post‐operative paired plasma samples from gastric patients. They then confirmed the levels of nine candidate miRNAs, which were markedly decreased in the post‐operative plasma, in a large cohort using RT‐qPCR. Finally, they compared the miRNA levels of two candidate miRNAs between 56 cancer patients and 30 healthy controls and found that they were significantly decreased in post‐operative plasma in 90 and 93% of patients (miR‐451 and miR‐486, respectively). Correspondingly, this strategy was used to identify serum and plasma miRNAs with diagnostic value in colorectal cancer, cervical squamous carcinoma and lung carcinoma 143, 144, 145. In addition, Li et al. 146 used this strategy to identify serum miRNAs to predict post‐operative disease recurrence for stage II/III colorectal cancer patients.
Similar to the findings in serum and plasma, the expression levels of two upregulated candidate miRNAs in the urine of urothelial carcinoma patients (miR‐96 and miR‐183) were significantly lower in urine collected after surgery from the same patients 98, suggesting tumour origin of such altered miRNAs.
Role of secreted miRNAs in oncogenesis
In cancer, tumour cells may deliver specific miRNAs to their surroundings to promote tumour survival. Those miRNAs could be associated with potential carcinogenic mechanisms that may overcome the normal cellular environment, making such miRNAs suitable biomarkers for the detection of tumour cells.
Correspondingly, the analysis of the potential roles of candidate miRNAs in oncogenesis is a good supportive approach for choosing reliable miRNAs with diagnostic potential during the exploratory phase of biomarker discovery.
First, the function of a miRNA depends on its target gene; therefore, a miRNA could function as either a tumour suppressor or an oncogene during cancer development. Accordingly, miRNAs functioning as oncogenes (also named oncomirs) promote tumour development by inhibiting tumour suppressor genes, mainly when they are upregulated. miRNAs functioning as tumour suppressors prevent tumour development by negatively inhibiting oncogenes or genes that control cell differentiation or apoptosis; however, downregulation of these miRNAs could lead to cancer development.
For example, miR‐10b is highly expressed in the metastatic breast cancer cell line MDA‐MB‐231 compared with the non‐metastatic breast cancer cell line HMLE or non‐malignant breast cells 147. This miRNA was also actively secreted via exosomes, and it was shown that treatment with miR‐10b‐enriched exosomes suppressed protein levels of its target genes HOXD10 and KLF4 in HMLE target cells. As a result of this suppression, HMLE cells acquired invasion ability 147. Other studies have also reported that low expression of HOXD10 and KLF4 induces cell migration and metastasis 148, 149; therefore, miR‐10b, which is found to be upregulated in breast and bladder cancer 147, 148, 150, 151, could be considered an oncogene.
Another example is miR‐105 152, which is expressed and secreted by highly metastatic breast cancer cells (MBC) isolated from pleural effusions. MBC‐secreted miR‐105 can be transferred to endothelial cells via exosomes and results in a significant decrease in the expression of its putative target gene zonula occludens (ZO‐1), which induces the downregulation of tight junctions and the destruction of the barrier function of endothelial monolayers. Transfecting the recipient cells with a miR‐105 inhibitor abolished this effect. It was also demonstrated that cancer‐secreted miR‐105 induces vascular permeability and promotes metastasis in vivo.
The well‐known oncomir miR‐21 is highly expressed in several tumours, including breast, ovarian, colorectal, lung and leukaemia 153. miR‐21 targets tumour suppressors, such as PTEN 154 and TPM1 155.
An example of a miRNA functioning as a tumour suppressor is miR‐152. miR‐152 has been found to be downregulated in glioblastoma stem cells 156, non–small‐cell lung cancer (NSCLC) tissues 157, 158, gastric cancer tissues 159 and hepatocellular carcinoma cells 160. All of these studies reported that overexpression of miR‐152 reduced the proliferation, migration and invasion capacity of tumour cells. Although the authors reported different target genes in every study, the expression level of each target gene was associated with those common cellular functions. In addition, evidence indicates that the function of a miRNA depends on the type of cell that the miRNA acts on. miRNAs such as miR‐125b, miR‐29 and miR‐146 have been described as oncogenes or suppressors in different cell types 161, 162, 163, 164, 165. Consequently, identifying the potential roles of candidate miRNAs in oncogenesis may require the analysis of the specific expression/release patterns and the identification of specific target genes in each type of tumour cell.
Role of secreted miRNAs in cancer progression: metastasis
Cancer progression involves the regulation of the cellular and tissue microenvironment to promote carcinogenesis and spreading of cancerous cells to distant anatomic sites. This regulation involves cell–cell contact‐mediated signals and cell‐to‐cell signals mediated by secreted factors. miRNAs, in addition to functioning as oncogenes and oncosuppressors inside cancer and stromal cells, are secreted and taken up by nearby cells within the cancer microenvironment and by distant cells in other tissues or organs, thereby delivering regulatory signals that potentially participate in the spreading of those cancer cells. This section describes recent experimental evidence regarding the role of miRNAs in cancer progression, focusing on pivotal mechanisms involved in metastasis and on the secreted form of miRNAs.
Metastasis and epithelial‐mesenchymal transition
Metastasis is a multistep biological process that involves dissemination of cancer cells to surrounding and distant organs sites for the formation of new tumour lesions. This process is frequently referred to as the invasion‐metastasis cascade 166. During metastasis, tumour cells exit their primary sites of growth by breaching the basement membrane, intravasate into the blood and lymphatic vessels, disseminate through the lymphatic system and blood circulation, extravasate to nearby and distant organs and finally adapt to a new microenvironment for metastatic colonization 167. Metastasis, not the primary tumour, is the major cause of mortality in cancer patients, and it plays a critical role in tumour recurrence and poor prognosis; therefore, it is clinically relevant 168.
In carcinomas arising from epithelial tissues, the epithelial–mesenchymal transition (EMT) is an early step of cancer metastasis, which is characterized by the breaching of the basement membrane that separates epithelial cells from multiple layers of stroma. The EMT is a highly conserved process characterized by a transition from immotile epithelial cells to motile mesenchymal cells. The EMT is fundamental for normal embryonic development; however, it is aberrantly activated during cancer progression, providing cancer cells the ability to migrate and form distant metastases 169, 170. This transition process is characterized by a decrease in the expression of cell–cell contact proteins such as the epithelial marker E‐cadherin and by the loss of cell–cell junctions and apico‐basal cell polarity 169, 170, 171.
Several endogenous miRNAs associated with metastasis have been identified, and their roles in the EMT have been reported recently 172, 173. However, there is limited experimental evidence regarding the participation of the secreted forms of such miRNAs in metastasis‐EMT. Still, several circulating miRNAs have been identified as biomarkers for cancer metastasis.
Toiyama et al. 174 specifically analysed the diagnostic value of miR‐200 family members that were previously associated with the regulation of the EMT in cancer cells in the serum of colorectal cancer patients. They showed that high expression of serum miR‐200c was significantly associated with a metastatic phenotype, lymph node metastasis, liver metastasis and the development of distant metastasis in colorectal cancer patients. They analysed the levels of four candidate miRNAs (miR‐200b, miR‐200c, miR‐141 and miR‐429) in the serum samples of stage I (n = 12) and stage IV (n = 12) patients and further validated the increased levels of miR‐200c in stage IV patients in a larger independent cohort of 182 patients and 24 normal controls. The miR‐200 family (miR‐200a, miR‐200b, miR‐200c, miR‐141 and miR‐429) was the first group of endogenous miRNAs reported to regulate the EMT, and their roles in tumour progression have been associated with several cancers 175, 176, 177, 178. They inhibit the EMT by retaining the epithelial phenotype by targeting the E‐cadherin transcriptional repressors ZEB1 and ZEB2, which results in upregulation of E‐cadherin 179, 180. In this study, the authors also reported that miR‐200c was overexpressed in liver metastases compared with matched primary colorectal cancer tissues, which was in agreement with a previous study in which they demonstrated that miR‐200c was overexpressed in metastatic colorectal cancer tissue compared with matched primary colorectal cancer tissue 181. The authors speculated that the origin of serum miR‐200c could be from the metastatic site, but no further studies regarding the potential function of this circulating miRNA were performed. One hypothesis is that the low expression of miR‐200c in primary tumours facilitates the EMT and exit of cancer cells, but, after metastasis, high expression inhibits the EMT and facilitates the settlement and proliferation of cancer cells, which undergo the opposite process known as the mesenchymal‐to‐epithelial transition (MET) 182.
However, Imaoka et al. 183 also evaluated serum EMT‐associated miRNAs as metastatic biomarkers by analysing the expression levels of the miR‐200 family and miR‐203 in serum from stage I (n = 12) and stage IV (n = 12) gastric cancer (GC) patients. They performed a validation phase in serum samples from 130 patients and 22 controls. They found that serum miR‐203 expression was lower in GC patients with a higher T stage, vessel invasion, and lymph node and distant metastases; therefore, miR‐203 has the potential to serve as a noninvasive biomarker to predict metastasis. However, no further studies were performed. Regarding the endogenous expression of this miRNA, recent studies reported that forced expression of miR‐203 was associated with inhibited proliferation and reduced invasion and induction of MET in cancer cells from neck, laryngeal and tongue cancer. However, one study reported that miR‐203 promoted proliferation and invasion in pancreatic cancer cells 184. Perhaps, studies regarding miR‐203 expression in metastatic and matching primary tumour tissue, as well as in vitro studies in invasive and non‐invasive clones of cancer cells, would provide more clues regarding its participation in metastasis.
Another recent study by Stückrath et al. 185 reported that plasma levels of miR‐16, miR‐107, miR‐130a and miR‐146a were decreased in lymph node‐positive patients compared with lymph node‐negative breast cancer patients; therefore, they could be potential biomarkers of metastasis. The authors further examined the effect of miR‐107 expression on cell migration and invasion in breast cancer cell lines. They found that miR‐107 overexpression reduced migration and invasiveness of both non‐invasive MCF‐7 and invasive MDA‐MB‐231 cells, while miR‐107 inhibition increased migration of MCF‐7 cells and invasiveness of both cell lines. This study did not further analyse the molecular mechanisms involved in the observed effect. Nevertheless, another recent study regarding endogenous miR‐107 reported that its overexpression increased the migration of the hepatocarcinoma cell lines HepG2 and Huh7 through its target CPEB3 that acts via the EGFR pathway 186. This discrepancy could be an example of different miRNA functions that depend on the specific target gene and the specific type of tumour cell analysed. Moreover, observed miRNA function could depend on the type of miRNA expression: secreted or endogenous. Nevertheless, several circulating miRNAs have been recently identified as potential biomarkers for cancer metastasis (Table 4) 107, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198.
Table 4.
Cell‐free microRNAs as biomarkers for cancer metastasis
| Body fluid | Cancer | miRNAs | Biomarker for | References |
|---|---|---|---|---|
| Plasma | CRC | miR‐96, miR‐203, miR‐141 and miR‐200b | miR‐96 distinguished stage I–IV CRC from HC; miR‐203 distinguished stage III–IV CRC patients from stage I–II; miR‐141 differentiated stage IV CRC from stage I–III patients; miR‐96 and miR‐200b were independent prognostic factors for overall survival | 187 |
| Plasma | BC | miR‐200a, miR‐200b, miR‐200c, miR‐210, miR‐215 and miR‐486‐5p | Onset of metastasis | 188 |
| Plasma | Breast cancer (BC) | miR‐16, miR‐107, miR‐130a and miR‐146 | Predicts lymph node metastasis | 185 |
| Serum | GC | miR‐203 | Predicts metastases, early recurrence and poor prognosis | 183 |
| Serum | Small‐cell lung cancer (SCLC) | miR‐184, miR‐574‐5p, miR‐874, miR‐3074‐5p, miR‐4459, miR‐4685‐5p and miR‐4746‐3p | SCLC metastasis | 189 |
| Cerebrospinal fluid | Glioma | miR‐21 | Tumour spinal/ventricle metastasis | 107 |
| Plasma | Gastric cancer (GC) | miR‐214 | GC diagnosis and distant metastasis | 198 |
| Serum | Cholangiocarcinoma (CCA) | miR‐106a | Higher risk of metastasis to lymph node | 190 |
| Plasma | Hepatocellular carcinoma (HCC) | miR‐101 | Distant metastatic | 191 |
| Serum | Melanoma | miR‐210 | Early systemic melanoma recurrence | 192 |
| Serum | Colorectal cancer (CRC) | miR‐200c | Predicts metastatic phenotype, lymph node metastasis, liver metastasis and the development of distant metastasis | 174 |
| Serum | GC | miR‐218 | Metastasis | 193 |
| Plasma | Nasopharyngeal carcinoma (NPC) | miR‐9 | Metastasis | 194 |
| Serum | Cervical squamous cell carcinoma (CSCC) | miR‐1246, miR‐20a, miR‐2392, miR‐3147, miR‐3162‐5p and miR‐4484 | Predictive for lymph node metastasis in patients with early‐stage CSCC | 195 |
| Serum | GC | miR‐21, miR‐146a and miR‐148a | Metastasis to lymph node | 196 |
| Serum | Melanoma | miR‐29c and miR‐324‐3p | Metastasis | 197 |
The participation of cell‐free miRNAs in metastasis has also been analysed in in vitro models. As was previously mentioned, miR‐10b was highly secreted via exosomes by a metastatic breast cancer cell line compared with a non‐metastatic breast cancer cell line and non‐malignant breast cells 147. The authors showed that treatment with miR‐10b‐enriched exosomes suppressed protein levels of its target genes HOXD10 and KLF4 in the non‐metastatic breast cancer line HMLE, inducing acquired invasion ability. However, they did not report the levels of circulating or endogenous miR‐10b in clinical samples. Although, another study by Xu et al. 199 reported that serum mR‐10b could be used as a biomarker to distinguish oesophageal cancer patients from healthy subjects. Another example is the study by Ostenfeld et al. 200. The aim of the study was to determine whether exosomal miRNAs were associated with the metastatic properties of bladder carcinoma cells. Ostenfeld et al. 200 first found a relative increase in the secretion of exosomal miRNAs previously associated with tumour‐suppressor functions (including miR‐23b, miR‐224 and miR‐921) using SLT4 (metastatic) versus parenteral T24 (non‐metastatic) cells and LUL2 (high metastatic) versus UMUC3 (low metastatic) cells. Then, they ectopically expressed miR‐23b in T24 and FL3 cells and observed that invasion capacity was reduced for metastatic FL3 cells and increased for non‐metastatic T24 cells. They further examined early lung metastasis in NCr nude mice upon tail injection of FL3‐GFP‐miR23b and FL3‐GFP (control) cells, and they observed a reduced tumour cell burden for FL3‐GFP‐miR23b. The authors suggested that the tumour suppressor miR‐23b was secreted by metastatic carcinoma cells for disposal and to improve the metastatic capacity of parental cells as a secondary effect. Notably, this study provided evidence that the same miRNA can act differently in a specific type of cell with different metastatic abilities.
Angiogenesis
Meanwhile, there are little more studies that analysed the participation of circulating miRNAs in angiogenesis. Angiogenesis is another vital process associated with tumour progression and metastasis. Angiogenesis is the physiological process of the formation of new capillaries from pre‐existing vessels that is initiated by ischaemic and hypoxic conditions. Aberrant tumour angiogenesis occurs when rapidly proliferating cancer cells outgrow their blood supply and induce tumour hypoxia. Tumour hypoxia and excessive growth‐promoting signals produced by tumour cells induce persistent and unresolved angiogenesis. Therefore, angiogenesis is vital for the survival, proliferation and propagation of tumour cells.
Zhuang et al. 201 reported that miR‐9 was secreted by tumour cells and promoted endothelial cell migration and angiogenesis. They used co‐cultures of tumour cell lines and matching endothelial cells from normal tissue to discover that miRNAs packaged into microvesicles, and not known growth factors, promoted endothelial migration. Then, they found that miR‐9 was a relevant miRNA by inhibiting its expression in tumour cells and observed that the effect was retained using the conditional media from tumour cells. Overexpression of miR‐9 in endothelial cells reduced SOCS5 levels, leading to activation of the JAK‐STAT pathway, which promotes cell migration. In addition, they used a mouse model of subcutaneous implantation of the human colorectal carcinoma cell line HM7, which resulted in elevated levels of miR‐9 in the plasma, and found that intratumoural injection of miR‐9 antagomirs significantly decreased tumour angiogenesis.
However, Yamada et al. 202 reported that colorectal cancer cells secreted microvesicles containing miR‐1246 and TGF‐β that promoted angiogenesis by activating Smad 1/5/8 in human umbilical vein endothelial cells, inducing proliferation, migration and tube formation. Kosaka et al. 203 showed that exosomal miR‐210, which was released from metastatic breast cancer cells via an nSMase2‐dependent secretion mechanism, enhanced angiogenic activity in endothelial cells. These authors found that the known angiogenic miR‐210 was highly enriched in exosomes from the metastatic cell line (MDA‐MB‐231) compared with the non‐metastatic cell line (MCF7) and normal mammary epithelial cells (MCF10A). To show that exosomal miR‐210 contributed to the enhancement of endothelial angiogenic activity, they used miR‐210‐enriched exosomes from miR‐210 transiently transfected 4T1 cells to induce capillary formation and migration of HUVECs. Accordingly, the levels of plasma miR‐210 were found to be increased in breast cancer patients with lymph node metastasis in another study 204.
Meanwhile, Li et al. 205 implanted mouse sarcoma S‐180 cells into C57BL/6J mice and found that the plasma levels of exosomal miR‐150 of the tumour‐implanted mice were higher than those of the control mice. Using Matrigel plugs, they found that tumour implantation stimulated angiogenesis and that this angiogenic effect was suppressed in mice treated with microvesicles containing a miR‐150 inhibitor. They initially reported that microvesicles containing high levels of miR‐150 were secreted by the human monocyte cell line THP‐1 and enhanced the tube formation of endothelial HMEC‐1 cells.
There are other studies that reported that exosomes affected tumour angiogenesis and metastasis 206, 207, but they did not analyse the participation of the miRNAs contained in such exosomes.
Evasion of immune response
The immune system is able to distinguish cancer cells from normal cells and eliminate them. Nevertheless, when cancer cells evade the anti‐tumour immune surveillance, they grow progressively and take advantage of the proliferative and angiogenic signals delivered during the immune response 208. Recent studies have reported that dysregulation of certain endogenous miRNAs in the tumour microenvironment may contribute to immune evasion 209, 210, 211, 212; however, only one study regarding secreted miRNAs has been published 120.
Ye et al. 120 examined the role of tumour‐derived exosomes in tumour progression in nasopharyngeal carcinoma (NPC). This study reported that tumour‐derived exosomes promote T‐cell dysfunction through the regulation of enriched exosomal miRNAs. However, the data showed that NPC‐derived exosomes impeded the proliferation of T cells and the differentiation of Th1 and Th17 and altered the cytokine profiles of stimulated lymphocytes without assessing that the enriched miRNAs found in exosomes participated in these effects. After obtaining experimental evidence of the effect of tumour‐derived exosomes on T cells, the authors found that five miRNAs, including miR‐24‐3p, miR‐891a, miR‐106a‐5p, miR‐20a‐5p and miR‐1908, were commonly enriched in the exosomes from the serum of NPC patients and in TW03 or TW03 NPC cells. Then, they performed a bioinformatics analysis that predicted that the mitogen‐activated protein kinase (MAPK) signalling pathway was associated with those miRNAs.
There are other publications regarding the role of tumour‐derived exosomes in immune evasion, but they did not analyse whether the miRNAs contained in those exosomes were responsible for the observed effect on the immune response 213, 214.
Regulation of miRNA expression
Alterations in the expression levels of miRNAs have been associated with the pathogenesis of several human diseases, including cancer. Therefore, understanding the regulation of the expression of miRNAs is relevant to elucidate which mechanisms are involved in dysregulation of miRNA expression. However, studies have only recently provided clues about this crucial topic. This section briefly describes some mechanisms of regulation of miRNA expression and the causes of their dysregulation in cancer. There are extensive reviews about this topic elsewhere 215, 216.
miRNA transcription and dysregulation by transcription factors
Most miRNAs are transcribed by RNA polymerase II 217; therefore, they are subjected to the same regulatory mechanisms as mRNA transcripts. miRNA promoters contain CpG islands, TATA boxes and TFIIB recognition sites, and histone modifications have been observed within them 217. Additionally, the expression of primary miRNA transcripts was found to be regulated by several transcription factors. For example, c‐Myc is an oncogene that is frequently dysregulated in cancer and was found to bind to E box elements in the miR‐17˜92 gene promoter, inducing the transcriptional activation of miR‐17~92 218. The cluster miR‐17~92 promotes cell‐cycle progression and proliferation, through the regulation of E2F transcription factors that regulate cell‐cycle progression 219, and the suppression of the tumour suppressor Pten and the pro‐apoptotic protein Bim 220. The miR‐17~92 is clustered within the region of C13orf25 transcript variant 2, which is a target for 13q31‐q32 amplification in malignant lymphoma 221. It was found that genomic amplification of this region correlated with overexpression of five members of the miR‐17~92 cluster in malignant lymphoma cell lines 222. Additionally, c‐Myc negatively regulates the transcriptional activity of tumour suppressor miRNAs, such as miR‐15a, miR‐34 and the let‐7 family of miRNAs 223. Another example is p53, which binds to the promoter of miR‐34a and miR‐34b/c and induces their expression 224. The miR‐34 family of miRNAs, including miR‐34a/b/c, promotes cell‐cycle arrest, cell senescence and apoptosis in cancer 225. Other regulatory pathways that affect the expression of miR‐34a in cancer include CEBPa, which when mutated induces decreased miR‐34a levels and enhanced expression of E2F3, resulting in acute myeloid leukaemia 226.
Methylation of miRNA promoters
MicroRNA genes can be epigenetically silenced through DNA methylation. Saito et al. 227 reported for the first time that miRNAs were strongly upregulated after treatment with the DNA methyltransferase inhibitor 5‐aza‐2′‐deoxycytidine in T24 bladder cancer cells. Among them, miR‐127 was later found to be downregulated in primary prostate, bladder and colon cancer tissues compared with matched normal tissues. Further evidence indicated that miR‐127 functions as a tumour suppressor by targeting the proto‐oncogene BCL6.
Another example of epigenetic silencing of tumour suppressor miRNA is the study by Toyota et al. 228. They found that miR‐34b and miR‐34c were epigenetically silenced in colorectal cancer by hypermethylation of the neighbouring CpG island. Methylation of the miR‐34b/c CpG Island was observed in colorectal cancer cell lines and primary tumours but not in normal colonic mucosa. Overexpression of miR‐34b/c suppressed colony formation of HCT116 cells.
MiRNA editing
RNA editing is the site‐specific modification of an RNA sequence to yield a product differing from that encoded by the DNA template 229. Most RNA editing in human cells is adenosine‐to‐inosine (A‐to‐I) RNA editing and is catalysed by the adenosine deaminases acting on RNA (ADARs). Blow et al. 229 identified novel A‐to‐I editing sites in 6 of 99 pri‐miRNAs, using an assay that detect relatively high levels of editing. Previously, Luciano et al. 230 had shown that the pri‐miRNA transcript of miR‐22 is subject to A‐to‐I RNA editing in a number of human and mouse tissue 230. Editing of pri‐miRNAs would have major effect for miRNA biogenesis and function, because DICER activity depends on proper base pairing within the stem region, and editing within the 20–22mer portion of the mature miRNA would alter the target mRNA repertoire for that particular miRNA. It was hypothesized that disruption to the A‐to‐I editing of the transcripts would lead to altered gene expression profiles in cancer that regulate tumour phenotypes and clinical behaviours. The study by Choudhury et al. 231 demonstrated that the A‐to‐I editing of the miR‐376 cluster miRNAs is significantly reduced in high‐grade human gliomas, resulting in accumulation of the unedited form in glioblastoma cells. Furthermore, authors showed that the overexpression of unedited miR‐376a* in glioblastoma cells promoted their migration and invasion while edited miR‐376a* suppressed this ability in vitro. It was shown that unedited miR‐376a* targeted RAP2A (a member of the RAS oncogene family), which produces a protein involved in both cancer cell migration and axomal branching, while the edited miR‐376a* targeted autocrine motility factor receptor (AMFR). For more information regarding miRNA processing, refer Schmittgen 232.
Conclusions
The potential usefulness of cell‐free miRNAs as reliable biomarkers in cancer may be based on their biological characteristics and potential functions. Evidence indicates that miRNAs are released from normal and tumour cells into the blood and other fluid in highly specific patterns and through mechanisms that depend on the physiology of each cell type. Such specificity suggests that miRNAs in fluids might not be the result of passive leakage from broken cells, but rather might be due to specific cellular transport mechanisms.
Currently, miRNAs are thought to be released to the outside environment for cell‐to‐cell communication. Like their intracellular counterparts, cell‐free miRNAs function as key regulators of gene expression, and disrupting or altering them could lead to carcinogenesis. The discovery that extracellular miRNAs are involved in carcinogenesis has led to potential applications to determine cancer diagnosis and prognosis and could even play a role in cancer therapy. Easy access to cell‐free miRNAs and their relative stability are important advantages for their use as biomarkers; however, reliable miRNA biomarkers still need to be identified, considering that their levels appear to depend on several normal and pathological physiological conditions. For their use as biomarkers, it is essential to determine the biological characteristics, release mechanisms/external forms and functions in oncogenesis of cell‐free miRNAs.
Acknowledgements
This work was supported by a grant from the Consejo Nacional de Ciencia y Tecnología, Project 261999, Mexico City, Mexico.
References
- 1. Lagos‐Quintana M, Rauhut R, Lendeckel W, Tuschl T (2001) Identification of novel genes coding for small expressed RNAs. Science 294, 853–858. [DOI] [PubMed] [Google Scholar]
- 2. Baskerville S, Bartel DP (2005) Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA 11, 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Han J, Lee Y, Yeom KH, Nam JW, Heo I, Rhee JK et al (2006) Molecular basis for the recognition of primary microRNAs by the Drosha‐DGCR8 complex. Cell 125, 887–901. [DOI] [PubMed] [Google Scholar]
- 4. Bohnsack MT, Czaplinski K, Gorlich D (2004) Exportin 5 is a RanGTP‐dependent dsRNA‐binding protein that mediates nuclear export of pre‐miRNAs. RNA 10, 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K et al (2005) TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 436, 740–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Provost P, Dishart D, Doucet J, Frendewey D, Samuelsson B, Radmark O (2002) Ribonuclease activity and RNA binding of recombinant human Dicer. EMBO J. 21, 5864–5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin S, Gregory RI (2015) MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer 15, 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olive V, Minella AC, He L. (2015) Outside the coding genome, mammalian microRNAs confer structural and functional complexity. Sci. Signal. 8, re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Azuma‐Mukai A, Oguri H, Mituyama T, Qian ZR, Asai K, Siomi H et al (2008) Characterization of endogenous human Argonautes and their miRNA partners in RNA silencing. Proc. Natl Acad. Sci. USA 105, 7964–7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zeng Y, Yi R, Cullen BR (2003) MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc. Natl Acad. Sci. USA 100, 9779–9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20. [DOI] [PubMed] [Google Scholar]
- 12. Carthew RW, Sontheimer EJ (2009) Origins and Mechanisms of miRNAs and siRNAs. Cell 136, 642–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Avila‐Moreno F, Urrea F, Ortiz‐Quintero B (2011) [MicroRNAs in diagnosis and prognosis in lung cancer]. Rev. Invest. Clin. 63, 516–535. [PubMed] [Google Scholar]
- 14. Petersen CP, Bordeleau ME, Pelletier J, Sharp PA (2006) Short RNAs repress translation after initiation in mammalian cells. Mol. Cell 21, 533–542. [DOI] [PubMed] [Google Scholar]
- 15. Guil S, Esteller M (2015) RNA‐RNA interactions in gene regulation: the coding and noncoding players. Trends Biochem. Sci. 40, 248–256. [DOI] [PubMed] [Google Scholar]
- 16. Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE et al (2000) The 21‐nucleotide let‐7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403, 901–906. [DOI] [PubMed] [Google Scholar]
- 17. Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM (2003) Bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113, 25–36. [DOI] [PubMed] [Google Scholar]
- 18. Chen CZ, Li L, Lodish HF, Bartel DP (2004) MicroRNAs modulate hematopoietic lineage differentiation. Science 303, 83–86. [DOI] [PubMed] [Google Scholar]
- 19. O'Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D (2007) MicroRNA‐155 is induced during the macrophage inflammatory response. Proc. Natl Acad. Sci. USA 104, 1604–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD et al (2006) A signature pattern of stress‐responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc. Natl Acad. Sci. USA 103, 18255–18260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang X, Tang G, Ozcan S (2008) Role of microRNAs in diabetes. Biochim. Biophys. Acta 1779, 697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Calin GA, Croce CM (2006) MicroRNA‐cancer connection: the beginning of a new tale. Cancer Res. 66, 7390–7394. [DOI] [PubMed] [Google Scholar]
- 23. Schetter AJ, Heegaard NH, Harris CC (2010) Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis 31, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schetter AJ, Leung SY, Sohn JJ, Zanetti KA, Bowman ED, Yanaihara N et al (2008) MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA 299, 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen Y, Stallings RL (2007) Differential patterns of microRNA expression in neuroblastoma are correlated with prognosis, differentiation, and apoptosis. Cancer Res. 67, 976–983. [DOI] [PubMed] [Google Scholar]
- 26. Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP et al (2007) MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA 297, 1901–1908. [DOI] [PubMed] [Google Scholar]
- 27. Boeri M, Verri C, Conte D, Roz L, Modena P, Facchinetti F et al (2011) MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc. Natl Acad. Sci. USA 108, 3713–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei L, Lian B, Zhang Y, Li W, Gu J, He X et al (2014) Application of microRNA and mRNA expression profiling on prognostic biomarker discovery for hepatocellular carcinoma. BMC Genomics 15(Suppl 1), S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang WC, Chan SH, Jang TH, Chang JW, Ko YC, Yen TC et al (2014) miRNA‐491‐5p and GIT1 serve as modulators and biomarkers for oral squamous cell carcinoma invasion and metastasis. Cancer Res. 74, 751–764. [DOI] [PubMed] [Google Scholar]
- 30. Raponi M, Dossey L, Jatkoe T, Wu X, Chen G, Fan H et al (2009) MicroRNA classifiers for predicting prognosis of squamous cell lung cancer. Cancer Res. 69, 5776–5783. [DOI] [PubMed] [Google Scholar]
- 31. Avery‐Kiejda KA, Braye SG, Mathe A, Forbes JF, Scott RJ (2014) Decreased expression of key tumour suppressor microRNAs is associated with lymph node metastases in triple negative breast cancer. BMC Cancer 14, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vergho D, Kneitz S, Rosenwald A, Scherer C, Spahn M, Burger M et al (2014) Combination of expression levels of miR‐21 and miR‐126 is associated with cancer‐specific survival in clear‐cell renal cell carcinoma. BMC Cancer 14, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gordillo GM, Biswas A, Khanna S, Pan X, Sinha M, Roy S et al (2014) Dicer knockdown inhibits endothelial cell tumor growth via microRNA 21a‐3p targeting of Nox‐4. J. Biol. Chem. 289, 9027–9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Valeri N, Braconi C, Gasparini P, Murgia C, Lampis A, Paulus‐Hock V et al (2014) MicroRNA‐135b promotes cancer progression by acting as a downstream effector of oncogenic pathways in colon cancer. Cancer Cell 25, 469–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gururajan M, Josson S, Chu GC, Lu CL, Lu YT, Haga CL et al (2014) miR‐154* and miR‐379 in the DLK1‐DIO3 microRNA mega‐cluster regulate epithelial to mesenchymal transition and bone metastasis of prostate cancer. Clin. Cancer Res. 20, 6559–6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nasser MW, Datta J, Nuovo G, Kutay H, Motiwala T, Majumder S et al (2008) Down‐regulation of micro‐RNA‐1 (miR‐1) in lung cancer. Suppression of tumorigenic property of lung cancer cells and their sensitization to doxorubicin‐induced apoptosis by miR‐1. J. Biol. Chem. 283, 33394–33405. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37. Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I et al (2009) MiR‐21 is an EGFR‐regulated anti‐apoptotic factor in lung cancer in never‐smokers. Proc. Natl Acad. Sci. USA 106, 12085–12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova‐Agadjanyan EL et al (2008) Circulating microRNAs as stable blood‐based markers for cancer detection. Proc. Natl Acad. Sci. USA 105, 10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ et al (2010) The microRNA spectrum in 12 body fluids. Clin. Chem. 56, 1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hanke M, Hoefig K, Merz H, Feller AC, Kausch I, Jocham D et al (2010) A robust methodology to study urine microRNA as tumor marker: microRNA‐126 and microRNA‐182 are related to urinary bladder cancer. Urol. Oncol. 28, 655–661. [DOI] [PubMed] [Google Scholar]
- 41. Han HS, Yun J, Lim SN, Han JH, Lee KH, Kim ST et al (2013) Downregulation of cell‐free miR‐198 as a diagnostic biomarker for lung adenocarcinoma‐associated malignant pleural effusion. Int. J. Cancer 133, 645–652. [DOI] [PubMed] [Google Scholar]
- 42. Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K et al (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 18, 997–1006. [DOI] [PubMed] [Google Scholar]
- 43. Koberle V, Pleli T, Schmithals C, Augusto Alonso E, Haupenthal J, Bonig H et al (2013) Differential stability of cell‐free circulating microRNAs: implications for their utilization as biomarkers. PLoS ONE 8, e75184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Biomarkers Definitions Working Group (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 69, 89–95. [DOI] [PubMed] [Google Scholar]
- 45. Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF et al (2011) Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl Acad. Sci. USA 108, 5003–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Turchinovich A, Weiz L, Langheinz A, Burwinkel B (2011) Characterization of extracellular circulating microRNA. Nucleic Acids Res. 39, 7223–7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gilad S, Meiri E, Yogev Y, Benjamin S, Lebanony D, Yerushalmi N et al (2008) Serum microRNAs are promising novel biomarkers. PLoS ONE 3, e3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jardim MJ, Fry RC, Jaspers I, Dailey L, Diaz‐Sanchez D (2009) Disruption of microRNA expression in human airway cells by diesel exhaust particles is linked to tumorigenesis‐associated pathways. Environ. Health Perspect. 117, 1745–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang B, Pan X (2009) RDX induces aberrant expression of microRNAs in mouse brain and liver. Environ. Health Perspect. 117, 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vrijens K, Bollati V, Nawrot TS (2015) MicroRNAs as potential signatures of environmental exposure or effect: a systematic review. Environ. Health Perspect. 123, 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hou L, Barupal J, Zhang W, Zheng Y, Liu L, Zhang X et al (2016) Particulate air pollution exposure and expression of viral and human microRNAs in blood: The Beijing Truck Driver Air Pollution Study. Environ. Health Perspect. 124, 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yamamoto M, Singh A, Sava F, Pui M, Tebbutt SJ, Carlsten C (2013) MicroRNA expression in response to controlled exposure to diesel exhaust: attenuation by the antioxidant N‐acetylcysteine in a randomized crossover study. Environ. Health Perspect. 121, 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bollati V, Marinelli B, Apostoli P, Bonzini M, Nordio F, Hoxha M et al (2010) Exposure to metal‐rich particulate matter modifies the expression of candidate microRNAs in peripheral blood leukocytes. Environ. Health Perspect. 118, 763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Takahashi K, Yokota SI, Tatsumi N, Fukami T, Yokoi T, Nakajima M. (2013) Cigarette smoking substantially alters plasma microRNA profiles in healthy subjects. Toxicol. Appl. Pharmacol. 272, 154–160 [DOI] [PubMed] [Google Scholar]
- 55. Huang J, Wu J, Li Y, Li X, Yang T, Yang Q et al (2014) Deregulation of serum microRNA expression is associated with cigarette smoking and lung cancer. Biomed. Res. Int. 2014, 364316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McDonald JS, Milosevic D, Reddi HV, Grebe SK, Algeciras‐Schimnich A (2011) Analysis of circulating microRNA: preanalytical and analytical challenges. Clin. Chem. 57, 833–840. [DOI] [PubMed] [Google Scholar]
- 57. Kannan M, Atreya C (2010) Differential profiling of human red blood cells during storage for 52 selected microRNAs. Transfusion 50, 1581–1588. [DOI] [PubMed] [Google Scholar]
- 58. Chen SY, Wang Y, Telen MJ, Chi JT (2008) The genomic analysis of erythrocyte microRNA expression in sickle cell diseases. PLoS ONE 3, e2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen X, Hu Z, Wang W, Ba Y, Ma L, Zhang C et al (2012) Identification of ten serum microRNAs from a genome‐wide serum microRNA expression profile as novel noninvasive biomarkers for nonsmall cell lung cancer diagnosis. Int. J. Cancer 130, 1620–1628. [DOI] [PubMed] [Google Scholar]
- 60. Bianchi F, Nicassio F, Marzi M, Belloni E, Dall'olio V, Bernard L et al (2011) A serum circulating miRNA diagnostic test to identify asymptomatic high‐risk individuals with early stage lung cancer. EMBO Mol. Med. 3, 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang ZX, Bian HB, Wang JR, Cheng ZX, Wang KM, De W (2011) Prognostic significance of serum miRNA‐21 expression in human non‐small cell lung cancer. J. Surg. Oncol. 104, 847–851. [DOI] [PubMed] [Google Scholar]
- 62. Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu Y et al (2010) Serum microRNA signatures identified in a genome‐wide serum microRNA expression profiling predict survival of non‐small‐cell lung cancer. J. Clin. Oncol. 28, 1721–1726. [DOI] [PubMed] [Google Scholar]
- 63. Wang F, Zheng Z, Guo J, Ding X (2010) Correlation and quantitation of microRNA aberrant expression in tissues and sera from patients with breast tumor. Gynecol. Oncol. 119, 586–593. [DOI] [PubMed] [Google Scholar]
- 64. Liu R, Zhang C, Hu Z, Li G, Wang C, Yang C et al (2011) A five‐microRNA signature identified from genome‐wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur. J. Cancer 47, 784–791. [DOI] [PubMed] [Google Scholar]
- 65. Liu XG, Zhu WY, Huang YY, Ma LN, Zhou SQ, Wang YK et al (2012) High expression of serum miR‐21 and tumor miR‐200c associated with poor prognosis in patients with lung cancer. Med. Oncol. 29, 618–626. [DOI] [PubMed] [Google Scholar]
- 66. Hennessey PT, Sanford T, Choudhary A, Mydlarz WW, Brown D, Adai AT et al (2012) Serum microRNA biomarkers for detection of non‐small cell lung cancer. PLoS ONE 7, e32307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Heneghan HM, Miller N, Lowery AJ, Sweeney KJ, Newell J, Kerin MJ (2010) Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann. Surg. 251, 499–505. [DOI] [PubMed] [Google Scholar]
- 68. Roth C, Rack B, Muller V, Janni W, Pantel K, Schwarzenbach H (2010) Circulating microRNAs as blood‐based markers for patients with primary and metastatic breast cancer. Breast Cancer Res. 12, R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Eichelser C, Flesch‐Janys D, Chang‐Claude J, Pantel K, Schwarzenbach H (2013) Deregulated serum concentrations of circulating cell‐free microRNAs miR‐17, miR‐34a, miR‐155, and miR‐373 in human breast cancer development and progression. Clin. Chem. 59, 1489–1496. [DOI] [PubMed] [Google Scholar]
- 70. Lodes MJ, Caraballo M, Suciu D, Munro S, Kumar A, Anderson B (2009) Detection of cancer with serum miRNAs on an oligonucleotide microarray. PLoS ONE 4, e6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, Cohn DE (2009) The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real‐time PCR platform. Gynecol. Oncol. 112, 55–59. [DOI] [PubMed] [Google Scholar]
- 72. Yamamoto Y, Kosaka N, Tanaka M, Koizumi F, Kanai Y, Mizutani T et al (2009) MicroRNA‐500 as a potential diagnostic marker for hepatocellular carcinoma. Biomarkers 14, 529–538. [DOI] [PubMed] [Google Scholar]
- 73. Zhang C, Wang C, Chen X, Yang C, Li K, Wang J et al (2010) Expression profile of microRNAs in serum: a fingerprint for esophageal squamous cell carcinoma. Clin. Chem. 56, 1871–1879. [DOI] [PubMed] [Google Scholar]
- 74. Sanfiorenzo C, Ilie MI, Belaid A, Barlesi F, Mouroux J, Marquette CH et al (2013) Two panels of plasma microRNAs as non‐invasive biomarkers for prediction of recurrence in resectable NSCLC. PLoS ONE 8, e54596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shen J, Todd NW, Zhang H, Yu L, Lingxiao X, Mei Y et al (2011) Plasma microRNAs as potential biomarkers for non‐small‐cell lung cancer. Lab. Invest. 91, 579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rabinowits G, Gercel‐Taylor C, Day JM, Taylor DD, Kloecker GH (2009) Exosomal microRNA: a diagnostic marker for lung cancer. Clin. Lung Cancer 10, 42–46. [DOI] [PubMed] [Google Scholar]
- 77. Zhao H, Shen J, Medico L, Wang D, Ambrosone CB, Liu S (2010) A pilot study of circulating miRNAs as potential biomarkers of early stage breast cancer. PLoS ONE 5, e13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bryant RJ, Pawlowski T, Catto JW, Marsden G, Vessella RL, Rhees B et al (2012) Changes in circulating microRNA levels associated with prostate cancer. Br. J. Cancer 106, 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shen J, Hruby GW, McKiernan JM, Gurvich I, Lipsky MJ, Benson MC et al (2012) Dysregulation of circulating microRNAs and prediction of aggressive prostate cancer. Prostate 72, 1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Suryawanshi S, Vlad AM, Lin HM, Mantia‐Smaldone G, Laskey R, Lee M et al (2013) Plasma microRNAs as novel biomarkers for endometriosis and endometriosis‐associated ovarian cancer. Clin. Cancer Res. 19, 1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Adam L, Wszolek MF, Liu CG, Jing W, Diao L, Zien A et al (2013) Plasma microRNA profiles for bladder cancer detection. Urol. Oncol. 31, 1701–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ho AS, Huang X, Cao H, Christman‐Skieller C, Bennewith K, Le QT et al (2010) Circulating miR‐210 as a novel hypoxia marker in pancreatic cancer. Transl. Oncol. 3, 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T et al (2010) Circulating microRNAs in plasma of patients with gastric cancers. Br. J. Cancer 102, 1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhou J, Yu L, Gao X, Hu J, Wang J, Dai Z et al (2011) Plasma microRNA panel to diagnose hepatitis B virus‐related hepatocellular carcinoma. J. Clin. Oncol. 29, 4781–4788. [DOI] [PubMed] [Google Scholar]
- 85. Wang Q, Huang Z, Ni S, Xiao X, Xu Q, Wang L et al (2012) Plasma miR‐601 and miR‐760 are novel biomarkers for the early detection of colorectal cancer. PLoS ONE 7, e44398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li BX, Yu Q, Shi ZL, Li P, Fu S (2015) Circulating microRNAs in esophageal squamous cell carcinoma: association with locoregional staging and survival. Int. J. Clin. Exp. Med. 8, 7241–7250. [PMC free article] [PubMed] [Google Scholar]
- 87. Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X (2010) Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int. J. Cancer 127, 118–126. [DOI] [PubMed] [Google Scholar]
- 88. Komatsu S, Ichikawa D, Takeshita H, Konishi H, Nagata H, Hirajima S et al (2012) Prognostic impact of circulating miR‐21 and miR‐375 in plasma of patients with esophageal squamous cell carcinoma. Expert Opin. Biol. Ther. 12(Suppl 1), S53–S59. [DOI] [PubMed] [Google Scholar]
- 89. Komatsu S, Ichikawa D, Takeshita H, Tsujiura M, Morimura R, Nagata H et al (2011) Circulating microRNAs in plasma of patients with oesophageal squamous cell carcinoma. Br. J. Cancer 105, 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cortez MA, Calin GA (2009) MicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseases. Expert Opin. Biol. Ther. 9, 703–711. [DOI] [PubMed] [Google Scholar]
- 91. Cui L, Zhang X, Ye G, Zheng T, Song H, Deng H et al (2013) Gastric juice microRNAs as potential biomarkers for the screening of gastric cancer. Cancer 119, 1618–1626. [DOI] [PubMed] [Google Scholar]
- 92. Park NJ, Zhou H, Elashoff D, Henson BS, Kastratovic DA, Abemayor E et al (2009) Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin. Cancer Res. 15, 5473–5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Matthaei H, Wylie D, Lloyd MB, Dal Molin M, Kemppainen J, Mayo SC et al (2012) miRNA biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin. Cancer Res. 18, 4713–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sadakari Y, Ohtsuka T, Ohuchida K, Tsutsumi K, Takahata S, Nakamura M et al (2010) MicroRNA expression analyses in preoperative pancreatic juice samples of pancreatic ductal adenocarcinoma. JOP 11, 587–592. [PubMed] [Google Scholar]
- 95. Hanson EK, Lubenow H, Ballantyne J (2009) Identification of forensically relevant body fluids using a panel of differentially expressed microRNAs. Anal. Biochem. 387, 303–314. [DOI] [PubMed] [Google Scholar]
- 96. Tolle A, Jung M, Rabenhorst S, Kilic E, Jung K, Weikert S (2013) Identification of microRNAs in blood and urine as tumour markers for the detection of urinary bladder cancer. Oncol. Rep. 30, 1949–1956. [DOI] [PubMed] [Google Scholar]
- 97. Miah S, Dudziec E, Drayton RM, Zlotta AR, Morgan SL, Rosario DJ et al (2012) An evaluation of urinary microRNA reveals a high sensitivity for bladder cancer. Br. J. Cancer 107, 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yamada Y, Enokida H, Kojima S, Kawakami K, Chiyomaru T, Tatarano S et al (2011) MiR‐96 and miR‐183 detection in urine serve as potential tumor markers of urothelial carcinoma: correlation with stage and grade, and comparison with urinary cytology. Cancer Sci. 102, 522–529. [DOI] [PubMed] [Google Scholar]
- 99. von Brandenstein M, Pandarakalam JJ, Kroon L, Loeser H, Herden J, Braun G et al (2012) MicroRNA 15a, inversely correlated to PKCalpha, is a potential marker to differentiate between benign and malignant renal tumors in biopsy and urine samples. Am. J. Pathol. 180, 1787–1797. [DOI] [PubMed] [Google Scholar]
- 100. Rehbein G, Schmidt B, Fleischhacker M (2015) Extracellular microRNAs in bronchoalveolar lavage samples from patients with lung diseases as predictors for lung cancer. Clin. Chim. Acta 450, 78–82. [DOI] [PubMed] [Google Scholar]
- 101. Kim JO, Gazala S, Razzak R, Guo L, Ghosh S, Roa WH et al (2015) Non‐small cell lung cancer detection using microRNA expression profiling of bronchoalveolar lavage fluid and sputum. Anticancer Res. 35, 1873–1880. [PubMed] [Google Scholar]
- 102. Shin YM, Yun J, Lee OJ, Han HS, Lim SN, An JY et al (2014) Diagnostic value of circulating extracellular miR‐134, miR‐185, and miR‐22 levels in lung adenocarcinoma‐associated malignant pleural effusion. Cancer Res. Treat. 46, 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang T, Lv M, Shen S, Zhou S, Wang P, Chen Y et al (2012) Cell‐free microRNA expression profiles in malignant effusion associated with patient survival in non‐small cell lung cancer. PLoS ONE 7, e43268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Xie L, Chen X, Wang L, Qian X, Wang T, Wei J et al (2010) Cell‐free miRNAs may indicate diagnosis and docetaxel sensitivity of tumor cells in malignant effusions. BMC Cancer 10, 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tokuhisa M, Ichikawa Y, Kosaka N, Ochiya T, Yashiro M, Hirakawa K et al (2015) Exosomal miRNAs from peritoneum lavage fluid as potential prognostic biomarkers of peritoneal metastasis in gastric cancer. PLoS ONE 10, e0130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wang J, Raimondo M, Guha S, Chen J, Diao L, Dong X et al (2014) Circulating microRNAs in pancreatic juice as candidate biomarkers of pancreatic cancer. J. Cancer 5, 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shi R, Wang PY, Li XY, Chen JX, Li Y, Zhang XZ et al (2015) Exosomal levels of miRNA‐21 from cerebrospinal fluids associated with poor prognosis and tumor recurrence of glioma patients. Oncotarget 6, 26971–26981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Akers JC, Ramakrishnan V, Kim R, Skog J, Nakano I, Pingle S et al (2013) MiR‐21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): a platform for glioblastoma biomarker development. PLoS ONE 8, e78115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659. [DOI] [PubMed] [Google Scholar]
- 110. Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT (2011) MicroRNAs are transported in plasma and delivered to recipient cells by high‐density lipoproteins. Nat. Cell Biol. 13, 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wang K, Zhang S, Weber J, Baxter D, Galas DJ (2010) Export of microRNAs and microRNA‐protective protein by mammalian cells. Nucleic Acids Res. 38, 7248–7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T (2010) Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 285, 17442–17452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Pegtel DM, Cosmopoulos K, Thorley‐Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL et al (2010) Functional delivery of viral miRNAs via exosomes. Proc. Natl Acad. Sci. USA 107, 6328–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Webber J, Steadman R, Mason MD, Tabi Z, Clayton A (2010) Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 70, 9621–9630. [DOI] [PubMed] [Google Scholar]
- 115. Tabet F, Vickers KC, Cuesta Torres LF, Wiese CB, Shoucri BM, Lambert G et al (2014) HDL‐transferred microRNA‐223 regulates ICAM‐1 expression in endothelial cells. Nat. Commun. 5, 3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena‐Esteves M et al (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB et al (2009) Transfer of microRNAs by embryonic stem cell microvesicles. PLoS ONE 4, e4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pigati L, Yaddanapudi SC, Iyengar R, Kim DJ, Hearn SA, Danforth D et al (2010) Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS ONE 5, e13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Goldie BJ, Dun MD, Lin M, Smith ND, Verrills NM, Dayas CV et al (2014) Activity‐associated miRNA are packaged in Map1b‐enriched exosomes released from depolarized neurons. Nucleic Acids Res. 42, 9195–9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS, Zhang XS et al (2014) Tumor‐derived exosomes promote tumor progression and T‐cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget 5, 5439–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. van Niel G, Porto‐Carreiro I, Simoes S, Raposo G (2006) Exosomes: a common pathway for a specialized function. J. Biochem. 140, 13–21. [DOI] [PubMed] [Google Scholar]
- 122. Lee Y, El Andaloussi S, Wood MJ (2012) Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 21, R125–R134. [DOI] [PubMed] [Google Scholar]
- 123. Segura E, Nicco C, Lombard B, Veron P, Raposo G, Batteux F et al (2005) ICAM‐1 on exosomes from mature dendritic cells is critical for efficient naive T‐cell priming. Blood 106, 216–223. [DOI] [PubMed] [Google Scholar]
- 124. Raiborg C, Stenmark H (2009) The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458, 445–452. [DOI] [PubMed] [Google Scholar]
- 125. Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A et al (2012) Syndecan‐syntenin‐ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 14, 677–685. [DOI] [PubMed] [Google Scholar]
- 126. Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q (2012) Formation and release of arrestin domain‐containing protein 1‐mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc. Natl Acad. Sci. USA 109, 4146–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O (2009) Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat. Cell Biol. 11, 1143–1149. [DOI] [PubMed] [Google Scholar]
- 128. Li L, Zhu D, Huang L, Zhang J, Bian Z, Chen X et al (2012) Argonaute 2 complexes selectively protect the circulating microRNAs in cell‐secreted microvesicles. PLoS ONE 7, e46957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lv Z, Wei Y, Wang D, Zhang CY, Zen K, Li L (2014) Argonaute 2 in cell‐secreted microvesicles guides the function of secreted miRNAs in recipient cells. PLoS ONE 9, e103599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Villarroya‐Beltri C, Gutierrez‐Vazquez C, Sanchez‐Cabo F, Perez‐Hernandez D, Vazquez J, Martin‐Cofreces N et al (2013) Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 4, 2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Iguchi H, Kosaka N, Ochiya T (2010) Secretory microRNAs as a versatile communication tool. Commun. Integr. Biol. 3, 478–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wagner J, Riwanto M, Besler C, Knau A, Fichtlscherer S, Roxe T et al (2013) Characterization of levels and cellular transfer of circulating lipoprotein‐bound microRNAs. Arterioscler. Thromb. Vasc. Biol. 33, 1392–1400. [DOI] [PubMed] [Google Scholar]
- 133. Pepe MS (2000) An interpretation for the ROC curve and inference using GLM procedures. Biometrics 56, 352–359. [DOI] [PubMed] [Google Scholar]
- 134. Yang Q, Jia C, Wang P, Xiong M, Cui J, Li L et al (2014) MicroRNA‐505 identified from patients with essential hypertension impairs endothelial cell migration and tube formation. Int. J. Cardiol. 177, 925–934. [DOI] [PubMed] [Google Scholar]
- 135. Cengiz M, Karatas OF, Koparir E, Yavuzer S, Ali C, Yavuzer H et al (2015) Differential expression of hypertension‐associated microRNAs in the plasma of patients with white coat hypertension. Medicine (Baltimore) 94, e693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Prabu P, Rome S, Sathishkumar C, Aravind S, Mahalingam B, Shanthirani CS et al (2015) Circulating MiRNAs of ‘Asian Indian Phenotype’ identified in subjects with impaired glucose tolerance and patients with type 2 diabetes. PLoS ONE 10, e0128372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lian F, Cui Y, Zhou C, Gao K, Wu L (2015) Identification of a plasma four‐microRNA panel as potential noninvasive biomarker for osteosarcoma. PLoS ONE 10, e0121499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hou B, Ishinaga H, Midorikawa K, Shah SA, Nakamura S, Hiraku Y et al (2015) Circulating microRNAs as novel prognosis biomarkers for head and neck squamous cell carcinoma. Cancer Biol. Ther. 16, 1042–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hsu CM, Lin PM, Wang YM, Chen ZJ, Lin SF, Yang MY (2012) Circulating miRNA is a novel marker for head and neck squamous cell carcinoma. Tumour Biol. 33, 1933–1942. [DOI] [PubMed] [Google Scholar]
- 140. Qi P, Cheng SQ, Wang H, Li N, Chen YF, Gao CF (2011) Serum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. PLoS ONE 6, e28486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wong TS, Ho WK, Chan JY, Ng RW, Wei WI (2009) Mature miR‐184 and squamous cell carcinoma of the tongue. ScientificWorldJournal 9, 130–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Konishi H, Ichikawa D, Komatsu S, Shiozaki A, Tsujiura M, Takeshita H et al (2012) Detection of gastric cancer‐associated microRNAs on microRNA microarray comparing pre‐ and post‐operative plasma. Br. J. Cancer 106, 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nonaka R, Miyake Y, Hata T, Kagawa Y, Kato T, Osawa H et al (2015) Circulating miR‐103 and miR‐720 as novel serum biomarkers for patients with colorectal cancer. Int. J. Oncol. 47, 1097–1102. [DOI] [PubMed] [Google Scholar]
- 144. Wang WT, Zhao YN, Yan JX, Weng MY, Wang Y, Chen YQ et al (2014) Differentially expressed microRNAs in the serum of cervical squamous cell carcinoma patients before and after surgery. J. Hematol. Oncol. 7, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Le HB, Zhu WY, Chen DD, He JY, Huang YY, Liu XG et al (2012) Evaluation of dynamic change of serum miR‐21 and miR‐24 in pre‐ and post‐operative lung carcinoma patients. Med. Oncol. 29, 3190–3197. [DOI] [PubMed] [Google Scholar]
- 146. Li J, Liu Y, Wang C, Deng T, Liang H, Wang Y et al (2015) Serum miRNA expression profile as a prognostic biomarker of stage II/III colorectal adenocarcinoma. Sci. Rep. 5, 12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Singh R, Pochampally R, Watabe K, Lu Z, Mo YY (2014) Exosome‐mediated transfer of miR‐10b promotes cell invasion in breast cancer. Mol. Cancer. 13, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Xiao H, Li H, Yu G, Xiao W, Hu J, Tang K et al (2014) MicroRNA‐10b promotes migration and invasion through KLF4 and HOXD10 in human bladder cancer. Oncol. Rep. 31, 1832–1838. [DOI] [PubMed] [Google Scholar]
- 149. Hakami F, Darda L, Stafford P, Woll P, Lambert DW, Hunter KD (2014) The roles of HOXD10 in the development and progression of head and neck squamous cell carcinoma (HNSCC). Br. J. Cancer 111, 807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tian Y, Luo A, Cai Y, Su Q, Ding F, Chen H et al (2010) MicroRNA‐10b promotes migration and invasion through KLF4 in human esophageal cancer cell lines. J. Biol. Chem. 285, 7986–7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ma L, Teruya‐Feldstein J, Weinberg RA (2007) Tumour invasion and metastasis initiated by microRNA‐10b in breast cancer. Nature 449, 682–688. [DOI] [PubMed] [Google Scholar]
- 152. Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR et al (2014) Cancer‐secreted miR‐105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 25, 501–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Krichevsky AM, Gabriely G (2009) miR‐21: a small multi‐faceted RNA. J. Cell Mol. Med. 13, 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Meng F, Henson R, Wehbe‐Janek H, Ghoshal K, Jacob ST, Patel T (2007) MicroRNA‐21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 133, 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zhu S, Si ML, Wu H, Mo YY (2007) MicroRNA‐21 targets the tumor suppressor gene tropomyosin 1 (TPM1). J. Biol. Chem. 282, 14328–14336. [DOI] [PubMed] [Google Scholar]
- 156. Ma J, Yao Y, Wang P, Liu Y, Zhao L, Li Z et al (2014) MiR‐152 functions as a tumor suppressor in glioblastoma stem cells by targeting Kruppel‐like factor 4. Cancer Lett. 355, 85–95. [DOI] [PubMed] [Google Scholar]
- 157. Su Y, Wang Y, Zhou H, Lei L, Xu L (2014) MicroRNA‐152 targets ADAM17 to suppress NSCLC progression. FEBS Lett. 588, 1983–1988. [DOI] [PubMed] [Google Scholar]
- 158. Cheng Z, Ma R, Tan W, Zhang L (2014) MiR‐152 suppresses the proliferation and invasion of NSCLC cells by inhibiting FGF2. Exp. Mol. Med. 46, e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zhai R, Kan X, Wang B, Du H, Long Y, Wu H et al (2014) miR‐152 suppresses gastric cancer cell proliferation and motility by targeting CD151. Tumour Biol. 35, 11367–11373. [DOI] [PubMed] [Google Scholar]
- 160. Dang YW, Zeng J, He RQ, Rong MH, Luo DZ, Chen G (2014) Effects of miR‐152 on cell growth inhibition, motility suppression and apoptosis induction in hepatocellular carcinoma cells. Asian Pac. J. Cancer Prev. 15, 4969–4976. [DOI] [PubMed] [Google Scholar]
- 161. Le MT, Teh C, Shyh‐Chang N, Xie H, Zhou B, Korzh V et al (2009) MicroRNA‐125b is a novel negative regulator of p53. Genes Dev. 23, 862–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Zhang Y, Yan LX, Wu QN, Du ZM, Chen J, Liao DZ et al (2011) miR‐125b is methylated and functions as a tumor suppressor by regulating the ETS1 proto‐oncogene in human invasive breast cancer. Cancer Res. 71, 3552–3562. [DOI] [PubMed] [Google Scholar]
- 163. Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M et al (2011) miR‐146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J. Exp. Med. 208, 1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Garcia AI, Buisson M, Bertrand P, Rimokh R, Rouleau E, Lopez BS et al (2011) Down‐regulation of BRCA1 expression by miR‐146a and miR‐146b‐5p in triple negative sporadic breast cancers. EMBO Mol. Med. 3, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Pekarsky Y, Croce CM (2010) Is miR‐29 an oncogene or tumor suppressor in CLL? Oncotarget 1, 224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- 168. Steeg PS (2006) Tumor metastasis: mechanistic insights and clinical challenges. Nat. Med. 12, 895–904. [DOI] [PubMed] [Google Scholar]
- 169. Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial‐mesenchymal transitions in development and disease. Cell 139, 871–890. [DOI] [PubMed] [Google Scholar]
- 170. Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial‐mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Nieto MA (2013) Epithelial plasticity: a common theme in embryonic and cancer cells. Science 342, 1234850. [DOI] [PubMed] [Google Scholar]
- 172. Ceppi P, Peter ME (2014) MicroRNAs regulate both epithelial‐to‐mesenchymal transition and cancer stem cells. Oncogene 33, 269–278. [DOI] [PubMed] [Google Scholar]
- 173. Lamouille S, Subramanyam D, Blelloch R, Derynck R (2013) Regulation of epithelial‐mesenchymal and mesenchymal‐epithelial transitions by microRNAs. Curr. Opin. Cell Biol. 25, 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Toiyama Y, Hur K, Tanaka K, Inoue Y, Kusunoki M, Boland CR et al (2014) Serum miR‐200c is a novel prognostic and metastasis‐predictive biomarker in patients with colorectal cancer. Ann. Surg. 259, 735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Chung VY, Tan TZ, Tan M, Wong MK, Kuay KT, Yang Z et al (2016) GRHL2‐miR‐200‐ZEB1 maintains the epithelial status of ovarian cancer through transcriptional regulation and histone modification. Sci. Rep. 6, 19943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zhang HF, Alshareef A, Wu C, Li S, Jiao JW, Cao HH et al (2015) Loss of miR‐200b promotes invasion via activating the Kindlin‐2/integrin beta1/AKT pathway in esophageal squamous cell carcinoma: an E‐cadherin‐independent mechanism. Oncotarget 6, 28949–28960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Rhodes LV, Martin EC, Segar HC, Miller DF, Buechlein A, Rusch DB et al (2015) Dual regulation by microRNA‐200b‐3p and microRNA‐200b‐5p in the inhibition of epithelial‐to‐mesenchymal transition in triple‐negative breast cancer. Oncotarget 6, 16638–16652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Cong N, Du P, Zhang A, Shen F, Su J, Pu P et al (2013) Downregulated microRNA‐200a promotes EMT and tumor growth through the wnt/beta‐catenin pathway by targeting the E‐cadherin repressors ZEB1/ZEB2 in gastric adenocarcinoma. Oncol. Rep. 29, 1579–1587. [DOI] [PubMed] [Google Scholar]
- 179. Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G et al (2008) The miR‐200 family and miR‐205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 10, 593–601. [DOI] [PubMed] [Google Scholar]
- 180. Feng X, Wang Z, Fillmore R, Xi Y (2014) MiR‐200, a new star miRNA in human cancer. Cancer Lett. 344, 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Hur K, Toiyama Y, Takahashi M, Balaguer F, Nagasaka T, Koike J et al (2013) MicroRNA‐200c modulates epithelial‐to‐mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut 62, 1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Polyak K, Weinberg RA (2009) Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat. Rev. Cancer 9, 265–273. [DOI] [PubMed] [Google Scholar]
- 183. Imaoka H, Toiyama Y, Okigami M, Yasuda H, Saigusa S, Ohi M et al (2015) Circulating microRNA‐203 predicts metastases, early recurrence, and poor prognosis in human gastric cancer. Gastric Cancer Aug. 2 [Epub 2015/08/04] [DOI] [PubMed] [Google Scholar]
- 184. Ren ZG, Dong SX, Han P, Qi J (2016) miR‐203 promotes proliferation, migration and invasion by degrading SIK1 in pancreatic cancer. Oncol. Rep. 35, 1365–1374. [DOI] [PubMed] [Google Scholar]
- 185. Stuckrath I, Rack B, Janni W, Jager B, Pantel K, Schwarzenbach H (2015) Aberrant plasma levels of circulating miR‐16, miR‐107, miR‐130a and miR‐146a are associated with lymph node metastasis and receptor status of breast cancer patients. Oncotarget 6, 13387–13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Zou CD, Zhao WM, Wang XN, Li Q, Huang H, Cheng WP et al (2016) MicroRNA‐107: a novel promoter of tumor progression that targets the CPEB3/EGFR axis in human hepatocellular carcinoma. Oncotarget 7, 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Sun Y, Liu Y, Cogdell D, Calin GA, Sun B, Kopetz S et al (2016) Examining plasma microRNA markers for colorectal cancer at different stages. Oncotarget 7, 11434–11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Madhavan D, Peng C, Wallwiener M, Zucknick M, Nees J, Schott S et al (2016) Circulating miRNAs with prognostic value in metastatic breast cancer and for early detection of metastasis. Carcinogenesis 2016 Jan 19. pii: bgw008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 189. Zhou R, Zhou X, Yin Z, Guo J, Hu T, Jiang S et al (2015) Tumor invasion and metastasis regulated by microRNA‐184 and microRNA‐574‐5p in small‐cell lung cancer. Oncotarget 6, 44609–44622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Cheng Q, Feng F, Zhu L, Zheng Y, Luo X, Liu C et al (2015) Circulating miR‐106a is a novel prognostic and lymph node metastasis indicator for cholangiocarcinoma. Sci. Rep. 5, 16103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Zheng F, Liao YJ, Cai MY, Liu TH, Chen SP, Wu PH et al (2015) Systemic delivery of microRNA‐101 potently inhibits hepatocellular carcinoma in vivo by repressing multiple targets. PLoS Genet. 11, e1004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Ono S, Oyama T, Lam S, Chong K, Foshag LJ, Hoon DS (2015) A direct plasma assay of circulating microRNA‐210 of hypoxia can identify early systemic metastasis recurrence in melanoma patients. Oncotarget 6, 7053–7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Xin SY, Feng XS, Zhou LQ, Sun JJ, Gao XL, Yao GL (2014) Reduced expression of circulating microRNA‐218 in gastric cancer and correlation with tumor invasion and prognosis. World J. Gastroenterol. 20, 6906–6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Lu J, Xu X, Liu X, Peng Y, Zhang B, Wang L et al (2014) Predictive value of miR‐9 as a potential biomarker for nasopharyngeal carcinoma metastasis. Br. J. Cancer 110, 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Chen J, Yao D, Li Y, Chen H, He C, Ding N et al (2013) Serum microRNA expression levels can predict lymph node metastasis in patients with early‐stage cervical squamous cell carcinoma. Int. J. Mol. Med. 32, 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kim SY, Jeon TY, Choi CI, Kim DH, Kim DH, Kim GH et al (2013) Validation of circulating miRNA biomarkers for predicting lymph node metastasis in gastric cancer. J. Mol. Diagn. 15, 661–669. [DOI] [PubMed] [Google Scholar]
- 197. Greenberg E, Besser MJ, Ben‐Ami E, Shapira‐Frommer R, Itzhaki O, Zikich D et al (2013) A comparative analysis of total serum miRNA profiles identifies novel signature that is highly indicative of metastatic melanoma: a pilot study. Biomarkers 18, 502–508. [DOI] [PubMed] [Google Scholar]
- 198. Zhang KC, Xi HQ, Cui JX, Shen WS, Li JY, Wei B et al (2015) Hemolysis‐free plasma miR‐214 as novel biomarker of gastric cancer and is correlated with distant metastasis. Am. J. Cancer Res. 5, 821–829. [PMC free article] [PubMed] [Google Scholar]
- 199. Xu H, Yao Y, Meng F, Qian X, Jiang X, Li X et al (2015) Predictive value of serum miR‐10b, miR‐29c, and miR‐205 as promising biomarkers in esophageal squamous cell carcinoma screening. Medicine (Baltimore) 94, e1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Ostenfeld MS, Jeppesen DK, Laurberg JR, Boysen AT, Bramsen JB, Primdal‐Bengtson B et al (2014) Cellular disposal of miR23b by RAB27‐dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 74, 5758–5771. [DOI] [PubMed] [Google Scholar]
- 201. Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y et al (2012) Tumour‐secreted miR‐9 promotes endothelial cell migration and angiogenesis by activating the JAK‐STAT pathway. EMBO J. 31, 3513–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Yamada N, Tsujimura N, Kumazaki M, Shinohara H, Taniguchi K, Nakagawa Y et al (2014) Colorectal cancer cell‐derived microvesicles containing microRNA‐1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down‐regulation in endothelial cells. Biochim. Biophys. Acta 1839, 1256–1272. [DOI] [PubMed] [Google Scholar]
- 203. Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T (2013) Neutral sphingomyelinase 2 (nSMase2)‐dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J. Biol. Chem. 288, 10849–10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Jung EJ, Santarpia L, Kim J, Esteva FJ, Moretti E, Buzdar AU et al (2012) Plasma microRNA 210 levels correlate with sensitivity to trastuzumab and tumor presence in breast cancer patients. Cancer 118, 2603–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Li J, Zhang Y, Liu Y, Dai X, Li W, Cai X et al (2013) Microvesicle‐mediated transfer of microRNA‐150 from monocytes to endothelial cells promotes angiogenesis. J. Biol. Chem. 288, 23586–23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC et al (2011) Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 71, 5346–5356. [DOI] [PubMed] [Google Scholar]
- 207. Hood JL, San RS, Wickline SA (2011) Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 71, 3792–3801. [DOI] [PubMed] [Google Scholar]
- 208. Gajewski TF, Schreiber H, Fu YX (2013) Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 14, 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Gaziel‐Sovran A, Segura MF, Di Micco R, Collins MK, Hanniford D, Vega‐Saenz de Miera E et al (2011) miR‐30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell 20, 104–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Xu H, Cheung IY, Guo HF, Cheung NK (2009) MicroRNA miR‐29 modulates expression of immunoinhibitory molecule B7‐H3: potential implications for immune based therapy of human solid tumors. Cancer Res. 69, 6275–6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Gao F, Zhao ZL, Zhao WT, Fan QR, Wang SC, Li J et al (2013) miR‐9 modulates the expression of interferon‐regulated genes and MHC class I molecules in human nasopharyngeal carcinoma cells. Biochem. Biophys. Res. Commun. 431, 610–616. [DOI] [PubMed] [Google Scholar]
- 212. Yang P, Li QJ, Feng Y, Zhang Y, Markowitz GJ, Ning S et al (2012) TGF‐beta‐miR‐34a‐CCL22 signaling‐induced Treg cell recruitment promotes venous metastases of HBV‐positive hepatocellular carcinoma. Cancer Cell 22, 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Muller L, Mitsuhashi M, Simms P, Gooding WE, Whiteside TL (2016) Tumor‐derived exosomes regulate expression of immune function‐related genes in human T cell subsets. Sci. Rep. 6, 20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Lundholm M, Schroder M, Nagaeva O, Baranov V, Widmark A, Mincheva‐Nilsson L et al (2014) Prostate tumor‐derived exosomes down‐regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PLoS ONE 9, e108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Croce CM (2009) Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 10, 704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Suzuki HI, Miyazono K (2011) Emerging complexity of microRNA generation cascades. J. Biochem. 149, 15–25. [DOI] [PubMed] [Google Scholar]
- 217. Ozsolak F, Poling LL, Wang Z, Liu H, Liu XS, Roeder RG et al (2008) Chromatin structure analyses identify miRNA promoters. Genes Dev. 22, 3172–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT (2005) c‐Myc‐regulated microRNAs modulate E2F1 expression. Nature 435, 839–843. [DOI] [PubMed] [Google Scholar]
- 219. Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I et al (2008) E2F1‐regulated microRNAs impair TGFbeta‐dependent cell‐cycle arrest and apoptosis in gastric cancer. Cancer Cell 13, 272–286. [DOI] [PubMed] [Google Scholar]
- 220. Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J et al (2008) Lymphoproliferative disease and autoimmunity in mice with increased miR‐17‐92 expression in lymphocytes. Nat. Immunol. 9, 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Ota A, Tagawa H, Karnan S, Tsuzuki S, Karpas A, Kira S et al (2004) Identification and characterization of a novel gene, C13orf25, as a target for 13q31‐q32 amplification in malignant lymphoma. Cancer Res. 64, 3087–3095. [DOI] [PubMed] [Google Scholar]
- 222. Tagawa H, Seto M (2005) A microRNA cluster as a target of genomic amplification in malignant lymphoma. Leukemia 19, 2013–2016. [DOI] [PubMed] [Google Scholar]
- 223. Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM et al (2008) Widespread microRNA repression by Myc contributes to tumorigenesis. Nat. Genet. 40, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Yamakuchi M, Lowenstein CJ (2009) MiR‐34, SIRT1 and p53: the feedback loop. Cell Cycle 8, 712–715. [DOI] [PubMed] [Google Scholar]
- 225. Hermeking H (2010) The miR‐34 family in cancer and apoptosis. Cell Death Differ. 17, 193–199. [DOI] [PubMed] [Google Scholar]
- 226. Pulikkan JA, Peramangalam PS, Dengler V, Ho PA, Preudhomme C, Meshinchi S et al (2010) C/EBPalpha regulated microRNA‐34a targets E2F3 during granulopoiesis and is down‐regulated in AML with CEBPA mutations. Blood 116, 5638–5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Saito Y, Liang G, Egger G, Friedman JM, Chuang JC, Coetzee GA et al (2006) Specific activation of microRNA‐127 with downregulation of the proto‐oncogene BCL6 by chromatin‐modifying drugs in human cancer cells. Cancer Cell 9, 435–443. [DOI] [PubMed] [Google Scholar]
- 228. Toyota M, Suzuki H, Sasaki Y, Maruyama R, Imai K, Shinomura Y et al (2008) Epigenetic silencing of microRNA‐34b/c and B‐cell translocation gene 4 is associated with CpG island methylation in colorectal cancer. Cancer Res. 68, 4123–4132. [DOI] [PubMed] [Google Scholar]
- 229. Blow MJ, Grocock RJ, van Dongen S, Enright AJ, Dicks E, Futreal PA et al (2006) RNA editing of human microRNAs. Genome Biol. 7, R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Luciano DJ, Mirsky H, Vendetti NJ, Maas S (2004) RNA editing of a miRNA precursor. RNA 10, 1174–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Choudhury Y, Tay FC, Lam DH, Sandanaraj E, Tang C, Ang BT et al (2012) Attenuated adenosine‐to‐inosine editing of microRNA‐376a* promotes invasiveness of glioblastoma cells. J. Clin. Invest. 122, 4059–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Schmittgen TD (2008) Regulation of microRNA processing in development, differentiation and cancer. J. Cell Mol. Med. 12, 1811–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Silva J, Garcia V, Zaballos A, Provencio M, Lombardia L, Almonacid L et al (2011) Vesicle‐related microRNAs in plasma of nonsmall cell lung cancer patients and correlation with survival. Eur. Respir. J. 37, 617–623. [DOI] [PubMed] [Google Scholar]
- 234. Shen J, Wang A, Wang Q, Gurvich I, Siegel AB, Remotti H et al (2013) Exploration of genome‐wide circulating microRNA in hepatocellular carcinoma: MiR‐483‐5p as a potential biomarker. Cancer Epidemiol. Biomark. Prev. 22, 2364–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]


