Abstract
Macrophages serve as host cells, inflammatory disease drivers and drug runners for human immunodeficiency virus infection and treatments. Low-level viral persistence continues in these cells in the absence of macrophage death. However, the cellular microenvironment changes as a consequence of viral infection with aberrant production of pro-inflammatory factors and promotion of oxidative stress. These herald viral spread from macrophages to neighboring CD4+ T cells and end organ damage. Virus replicates in tissue reservoir sites that include the nervous, pulmonary, cardiovascular, gut, and renal organs. However, each of these events are held in check by antiretroviral therapy. A hidden and often overlooked resource of the macrophage rests in its high cytoplasmic nuclear ratios that allow the cell to sense its environment and rid it of the cellular waste products and microbial pathogens it encounters. These phagocytic and intracellular killing sensing mechanisms can also be used in service as macrophages serve as cellular carriage depots for antiretroviral nanoparticles and are able to deliver medicines to infectious disease sites with improved therapeutic outcomes. These undiscovered cellular functions can lead to reductions in persistent infection and may potentially facilitate the eradication of residual virus to eliminate disease.
Keywords: mononuclear phagocytes, monocyte-derived macrophages, human immunodeficiency virus, viral persistence, long acting slow effective release antiretroviral therapy, cell ontogeny, viral reservoirs
Introduction
Mononuclear phagocytes (MP; monocytes, macrophages and dendritic cells) serve as surveyors and protectors of the tissue microenvironment, secretors of inflammatory, growth and regulatory factors. Each and all enable intracellular killing, cell mobility, tissue and cellular repair and antigen presentation to maintain tissue homeostasis and affect T cell immunity for microbial and cancer surveillance (van Furth et al., 1972; Pistoia, 1991; Mindell, 2012). All serve in organ and body maintenance (Reviewed in Martinez and Gordon, 2014). However, the same MPs that protect the host can also, under pathological and infectious circumstances incite the very disease states that they were designed to thwart (Bocchino et al., 2001; Giralt et al., 2009). This is certainly true for a host of viral, bacterial and parasitic infections where MPs serve as reservoirs of infectious agents, sites for microbial persistence, and inducers of end organ disease (Koziel et al., 1999; Langford et al., 2002; Buckner et al., 2011). Over the past decade yet another role for the MP has been uncovered. That role rests in utilizing the cell’s own “high cytoplasmic content” to carry disease fighting drugs encased into stable nanoparticles (Edagwa et al., 2017). A discussion of the multifaceted MP in all its roles in health and disease has become the subject of this review with an eye towards how natural functions may be harnessed or even enhanced for the benefit of the host (Li et al., 2016). How the immune system affects its environment and vice versa characterize its multifaceted roles. When opportunities come available they can affect a functional change for the innate immune system (Moorjani et al., 1996; Kadiu and Gendelman, 2011b). The MP’s story is how a cell, when given opportunities, can protect and defend, elicit disease or be harnessed for therapeutic drug delivery (Edagwa et al., 2017).
The Monocyte, Macrophage and Dendritic Cell
Hematopoietic Origins
From their inception during embryonic development, MP exemplify the principal that ontogeny recapitulates function. Human hematopoiesis occurs at a series of sites including the yolk sac by 17 days post-conception, fetal liver and spleen, and finally within bones (Reviewed in Baron et al., 2012). Each of these locations supplies waves of primordial myeloid cell migration to the periphery, giving rise to the first tissue resident macrophages (McGrath et al., 2015). The pervasiveness of these amoeboid cells throughout the body led the early founder of cellular immunity, Ilya Metchnikoff, to postulate their utility in engulfing debris. Metchnikoff characterized macrophages’ phagocytic function as not only instrumental to tissue remodeling of tadpoles, but in taking up and killing foreign materials in starfish larvae as well (Reviewed in Gordon, 2016). Hematopoiesis thus establishes populations of long-lived and self-replenishing resident phagocytes (aka histiocytes) in the brain (microglia), liver (Kupffer cells), and lungs (alveolar macrophages). In contrast, the skin and intestine are continuously replaced by circulating macrophage precursors known as monocytes (Baron et al., 2012). Monocytes appear in early circulation several days after the first macrophages (Schulz et al., 2012). Early work by Ralph van Furth and Zanvil Cohn founded the paradigm wherein bone marrow promonocytes differentiate into monocytes that enter the bloodstream and become macrophages as they extravasate into tissue (van Furth and Cohn, 1968). Dendritic cells (DCs) represent the third of the mononuclear phagocyte trinity and act as immune sentinels capable of triggering adaptive immunity. DC may emerge from monocytes directly, a shared predecessor, or common lymphoid progenitors (Merad et al., 2013). MP contribute to a variety of processes during embryogenesis that reflect their ultimate functions (van Furth and Cohn, 1968). Examples include erythrocyte formation (Rhodes et al., 2008), reshaping limbs and central nervous system (CNS) (Hopkinson-Woolley et al., 1994; Squarzoni et al., 2014), production of angiogenic growth factors (DeFalco et al., 2014), and fetal immunity (Zaccheo et al., 1989; Li et al., 2014). MP in post-natal organisms similarly overlap in their roles as protectors, cytokine secretors, and linkages to adaptive immunity (Polin RA, 2017).
Innate Surveyors
MP vigilantly patrol the body for signs of damage or pathogenic invasion. Endocytosis ensues when MP take in foreign particles that may be trafficked throughout the cell via endosomes or degraded in the lysosome (Merad et al., 2013). A variety of membrane receptors aid in this process. Innate leukocytes have evolved numerous membrane-bound pattern recognition receptors (PRR) that directly bind motifs common amongst foreign pathogens (pathogen associated molecular patterns, PAMPs) or damaged cells in distress (DAMPs) (Davis et al., 2011). Pre-coating of pathogens with complement or antigen-specific antibodies enhances receptor-mediated endocytosis by MP. Monocytes migrate along chemical gradients in the blood stream towards tissue sites of inflammation. Monocyte chemotactic proteins 1 and 3 (MCP1 and 3 or CCL2 and CCL7, respectively) discharged from other monocytes/macrophages, N-formylmethionyl-leucyl-phenylalanine residues, and inflammatory arachidonic acid metabolites each recruit monocytes out of circulation (Obrist et al., 1983; Locati et al., 1994). Two monocytic subsets predominate (Reviewed in Boyette et al., 2017). Classical monocytes (CD14++CD16-) travel throughout the cardiovascular system in search of PAMPs. They express high levels of CD14, a co-receptor that aids in the recognition of lipopolysaccharide from gram-negative bacteria. Non-classical monocytes (CD14lowCD16+), in contrast, patrol local endothelium for nearby inflammation and recognize antibody-opsonized pathogen via CD16. Though naturally not abundantly endocytic (Steinman and Cohn, 2007), monocyte-derived DCs still exhibit complement- and C-type lectin opsonized phagocytosis. Much of DCs’ capacity for uptake of extracellular materials is relegated to subsequent antigen presentation to T lymphocytes. Macrophages endocytose as part of normal physiological processes such as removing reticulocytes and aging red blood cells from the blood stream (splenic red pulp macrophages), bone remodeling (osteoclasts), and in pruning neuronal synapses (microglia). They phagocytose free floating pathogens by recognizing common motifs via C type lectin-, scavenger-, and N-formylmethionyl membrane receptors. Specialized opsonin receptors for complement (Mac-1) or antibody (FcR) further facilitate ingestion of microorganisms by macrophages. Finally, macrophages surveil for early signs of bacterial sepsis. Hepcidin, an acute phase reactant, prompts iron sequestration in macrophages to halt bacterial growth (Reviewed in Michels et al., 2015). MP accordingly employ endocytosis to respond to physiological demands and guard from immunological insult.
Secretion and Homeostatic Regulation
MP control the balance between inflammation and repair by paracrine secretion of cytokines. Monocyte / macrophages promote their own replication by releasing granulocyte-monocyte colony stimulating factor (GM-CSF) (Pistoia, 1991). This growth factor prevents apoptosis, stimulates antifungal responses, and induces the differentiation of monocyte lineages (Chao et al., 1998; Hercus et al., 2012; Subramanian Vignesh et al., 2013). Dendritic cell subsets equilibrate between aggressive and defensive immune states. Plasmacytoid DCs produce abundant amounts of antiviral interferon-α when they sense viral nucleic acids in their cytoplasm (Santana-de Anda et al., 2013). Conventional DCs drive the maturation of anti-bacterial/fungal TH17 through interleukins 1, 6, and 23 (Espinosa and Rivera, 2012). Contrastingly, tolerogenic Langerhans cells (skin DCs) bolster peripheral T cell anergy and poor lymph node migration by constitutively releasing IL-10 (Kissenpfennig et al., 2005; Satpathy et al., 2012). Macrophages, too, tip the inflammatory scale in either direction with cytokines (Reviewed in Martinez and Gordon, 2014). Classically activated macrophages (M1) recruit additional leukocytes (TNFα and IL-1) or differentiate naïve CD4+ T cells to TH1 effectors (IL-12) in an effort to rid the host of intracellular bacteria or viruses. Classically activated macrophages predominate as sinus histiocytes (lymph node) and LysoMacs (Peyer’s Patches in gut associated lymphoid tissues). Alternative activation of macrophages (M2) by interleukins 4, 13, or 10 instigates adaptive immunity against extracellular pathogens or propagates tissue repair. M2 uniformly stifle differentiation of lymphocytes into TH1 effectors by extruding IL-10. M2s thematically convert arginine to cell-proliferative polyamines (M2a) or proline building blocks (M2c). This latter subtype signals the end of inflammatory responses with IL-10 and fosters rebuilding by synthesizing collagen, tumor growth factor beta (TGFβ), and matrix metalloproteinases. Thus, MP immunomodulate themselves and their surrounding stroma with growth factors, cytokines, and enzymes.
Innate Killers
MP make use of hydrolytic enzymes and reactive metabolites to destroy pathogens. Phagocytosed bacteria progress through a series of progressively acidified endosomes prior to fusing with the lysosome (Flannagan et al., 2009). In the phagolysosome, hydrogen ions (pH 4.5) denature invader proteins themselves and secondarily activate hydrolytic proenzymes cathepsin, alpha-glucosidase, and phospholipases (Mindell, 2012). The phagolysosomal membrane also houses phagocyte oxidases (Phox) and inducible nitric-oxide synthases (iNOS) that generate reactive-oxygen and -nitrogen species (Slauch, 2011). Impairment of monocyte macrophage NADPH oxidase exhibited increased infectivity by opportunistic parasites, Candidal yeasts, and Staphylococcal bacteria (Murray and Cohn, 1980). M1 characteristically upregulate synthesis of these intermediates to combat intracellular pathogens. Antibody-dependent phagocytosis (ADPh) couples debris clearance with adaptive immunity’s ability to target unique antigens. Activating membrane receptors present on macrophages / nonclassical monocytes that potentiate respiratory burst may tightly bind monomeric antibodies (FcɣRI) or loosely attach immune complexes (FcɣIII, FcɣRIIa). In contrast, ligands of FcɣRIIb abrogate phagocytosis in these cells (Gordan et al., 2015). Antibody-dependent cell-mediated cytotoxicity (ADCC) serves as another bridge between innate and adaptive immunity. ADCC effectors puncture plasma membranes with perforin and thereafter inject pro-apoptotic granzymes. Though macrophages are not the chief mediators of ADCC under physiological conditions, they retain their capacity to lyse malignant cells in the presence of antibody therapeutics (Beers et al., 2008; Hubert et al., 2011). To this end, monocyte / macrophages are vital in directly ridding hosts of hostile elements.
Antigen Presenting Cells (APC)
DC and monocyte / macrophages orchestrate adaptive immunity by activating T lymphocyte responses. T cells rely on two signals to mature from CD4+ naïve to T helper (TH) lymphocytes. First, antigen-specific T cell receptors (TCR) complementarily bind to digested peptides displayed in major histocompatibility complex II (MHC-II) on the surfaces of APC. CD4 co-receptor secures TCR / MHC-II attachment. B7–1 / B7–2 (CD80/86) immunoglobulins on APC must then serve as ligands for CD28 on T cells for activation to occur (Kindt et al., 2007). A landmark finding that paved the way for the 2011 Nobel Prize awarded to Ralph Steinman days after his death was made possible through his collaborations with Zanvil Cohn. The work established the presence of a large MP population with abundant pseudopodia in multiple secondary lymphoid organs (van Furth et al., 1972). DC, as they became known, stimulate T lymphocytes over 100 times more effectively than other APC subclasses (Steinman and Witmer, 1978). This is owed to their constitutive expression of MHC-II and costimulatory molecules (Kindt et al., 2007). Classical DCs (cDCs) capture antigen in tissue and migrate to draining lymph nodes where they stimulate T cells (Delamarre et al., 2005; Desch et al., 2014). Antigen cross presentation describes the loading of extracellular materials onto MHC class I molecules to prime CD8+ cytotoxic T lymphocytes. Monocytes, unlike at least one population of DCs, require toll-like-receptor (TLR) stimulation for cross-presentation to occur (Segura et al., 2012; Larson et al., 2016). Monocyte / macrophages also differ from DC in that surface MHC-II and B7–1 / B7–2 co-stimulators appear primarily only after phagocytosis (Kindt et al., 2007). LY6C+ monocytes migrate to murine secondary lymphoid organs, however debate persists whether they merely deliver antigen to lymph nodes for display by other APCs or activate T cells themselves (Reviewed in Jakubzick et al., 2017). Macrophages more closely parallel cDC in their capacity for antigen presentation. They successfully migrate to lymph nodes where they may induce TH1, TH2, or CD8 cytotoxic T lymphocyte responses (Desmedt et al., 1998; Moser, 2001; Pozzi et al., 2005). MP thus stand as essential intermediaries for launching T lymphocyte defenses against specific pathogens.
HIV MP Interactions
Human immunodeficiency virus (HIV)
HIV distinguishes itself as a unique lentivirus within the family Retroviridae. Retroviruses carry positive-sense single stranded RNA in roughly 100 nanometer envelopes. They reverse-transcribe genomic RNA using viral enzymes into DNA, prior to returning to the central dogma in which DNA transcribes RNA that in turn translates protein. Common features of the lentiviruses include their utilization of monocyte / macrophage lineages as cellular hosts, extended incubation periods prior to terminal disease, viral persistence in face of vigorous immunity, and ability to cause progressive health decline in the absence of treatment (Reviewed in Desport, 2010). HIV’s approximately 10kb genome encodes three canonical structures shared among all lentiviruses. Gag gives rise to core structural elements: matrix, capsid, and nucleocapsid. Enzymes necessary for survival that include reverse transcriptase, RNase-H, and integrase result from HIV’s pol sequence. Finally, the env gene produces surface and transmembrane proteins utilized in recognition and entry of virus into target cells. Six accessory proteins are transcribed from an open reading frame in the HIV genome. Vpx transports pre-integrated viral DNA into the nuclei of non-dividing cells. Tat (trans-activator of transcription) enhances transcriptional elongation by recognizing stem loops known as the Tat activation region (TAR). Rev, the only accessory protein common to all lentiviruses, carries full-length and singly spliced HIV transcripts from the nucleus to cytoplasm. Vif increases viral infectivity and assembly. Vpu, found only in HIV-1, and Nef decrease CD4 expression leading to enhanced release of virus.
HIV Life Cycle
HIV gene products facilitate eight major steps in the viral lifecycle of HIV (Reviewed in Desport, 2010). The process begins with (1) attachment of envelope glycoproteins that bind cellular primary CD4 receptors and chemokine co-receptors CCR5 or CXCR4. Next, (2) entry ensues as HIV transmembrane protein fuses with plasma membrane, followed by pore formation and deposition of viral core into the host cell. Uncoated viral products potentiate (3) reverse transcription and (4) integration of proviral DNA into the host’s chromosomes. The number of proviral DNA copies present in each HIV-1-infected cell varies by tissue type (Arainga et al., 2016) and viral strain ranging from 0–2 in PBMC (Shiramizu et al., 2004) to 3–4 in splenocytes (Jung et al., 2002). Gene expression and post-transcriptional regulation represent the fifth (5) stage of productive infection by HIV. Full-length or singly spliced RNA transcripts encode gag and pol, or env proteins, respectively. Proteases cleave Gag and Env polyproteins, which are each subsequently fatty acylated or N-glycosylated. Small multiply spliced RNAs comprise a minority of HIV transcripts that translate into the aforementioned accessory proteins. Complexes of envelope glycoproteins and gag-polyproteins classically thought to form at the plasma membrane or late endosomes signal (6) assembly of proviral elements. This also triggers the virus to regulate cellular endocytic trafficking of proteins and cytoskeletal rearrangement, which culminate in (7) budding from the host-cell. Finally, (8) maturation progresses as gag polyprotein is cleaved during the penultimate step and thereafter, spawning new progeny virions.
Four phases characterize the disease course of HIV in the absence of antiretroviral treatment (Le, 2017). During the first month post-infection, known as the window period, HIV RNA accumulates as CD4+ T lymphocyte counts begin to decline. Acute infection, marked by flu-like symptoms, presents in the following month. During this phase, viral RNA peaks while CD4+ counts reach a relative nadir, permitting for viral dissemination and seeding of lymphoid organs. HIV next enters clinical latency as cellular and humoral immune responses fight back against viremia. Symptoms subside from the third month to approximately five years after infection in concert with HIV RNA decline (<104 copies / mL) and CD4+ T lymphocyte rebound. HIV-1 is commonly latent, as marked by the absence of viral replication, however it differs from classical latency exemplified by herpes simplex virus that silence viral transcription by use of viral gene products (Desport, 2010). Resting memory CD4+ T cells (Williams and Greene, 2007) and monocytes (Gendelman et al., 1990) both harbor integrated HIV without demonstrating viral replication. Viral reactivation occurs once monocytes differentiate into macrophages upon reaching tissues (Meltzer et al., 1990). Final crisis results 8–11 years post-infection with HIV-1, but is vastly delayed in the case of HIV-2 (Marlink et al., 1994). Patients become progressively immunocompromised as viral replication depresses CD4+ counts below 400/mm3. Acquired Immunodeficiency Syndrome (AIDS) is diagnosed when exceedingly rare infections manifest that fewer than 200 CD4+ T lymphocytes are left circulating per milliliter blood.
Cell Tropism
HIV readily infects MP due to the presence of necessary receptors on their plasma membranes. HIV envelope glycoprotein 120 (gp120) initiates attachment to host cell CD4, prompting a conformational shift that exposes sites on gp120 for secondary connections to chemokine receptors, CCR5 or CXCR4. Subsequent binding of cell membranes to viral gp41 allows HIV fusion and entry. Viral strains (R5) that selectively recognize CCR5 can replicate in macrophages (M-Tropic) or CD4+ T lymphocytes (Alkhatib et al., 1996; Ancuta et al., 2006), while others (X4) that principally utilize the CXCR4 co-receptor produce higher number of viral progeny in CD4+ T cells and T-cell lines (Reviewed in Iordanskiy et al., 2013). Coupling of HIV to CCR5 increases the likelihood for replicative potential in monocyte / macrophages, though this feature is neither necessary nor sufficient for macrophage tropism (M-tropism). Some primary R5-HIV isolates fail to infect macrophages and X4-viruses may be capable of entering macrophage lineage cells (Gorry et al., 2001). Monocytes display basal amounts of all three HIV receptors in their resting states but growth factors alter this expression upon differentiation to macrophages or DC. Monocyte colony stimulating factor (M-CSF) induces macrophages to upregulate CCR5 by 10-fold whereas GM-CSF less potently increases CCR5 but markedly decreases CXCR4 among dendritic cells (Lee et al., 1999). M-tropic strains predominate in both early (Reviewed in Desport, 2010) and late (Li et al., 1999) disease states. This correlates with times of high transmissibility and terminal CD4+ T lymphocyte depletion, respectively. In contrast, X4-viruses appear most frequently in patients transitioning between early and intermediate phases (Reviewed in Desport, 2010). CCR5-mediated entry of HIV into macrophages indicates the underlying potential for disease state to progress.
The beta-chemokine receptor, CCR5, has long been recognized as important to HIV-1 infection. Mutations in CCR5 may confer HIV resistance in human hosts by halting entry of M-tropic HIV-1 clades (Dean et al., 1996; Cheng-Mayer et al., 1997) and delay AIDS progression in heterozygotes (Samson et al., 1996; Zimmerman et al., 1997). A G protein-coupled receptor, CCR5 serves as a docking site for CC motif chemokine ligands MIP-1α (CCL3), MIP-1β (CCL4), and RANTES (Struyf et al., 2001). High concentrations of these proteins correlated with low levels of viremia in patients, and were essential to natural killer (NK) cell cytotoxicity of infected CD4+ T lymphocytes (Kottilil et al., 2003). Furthermore, HIV-1 patients with low or undetectable plasma levels of virus, known as long-term non-potentiators (LTNPs), were found to maintain elevated blood concentrations of MIP-1α, MIP-1β, and RANTES (Walker et al., 2015). Thus, physiological blockade of CCR5 presents one adaptive means of interrupting HIV infection in macrophages.
HIV Persistence
One advantage offered by macrophages to HIV is their ability to survive for months to years. HIV ensures increased cellular longevity by making cells resistant to TNFα-induced apoptosis, increasing anti-apoptic proteins Bcl-2 and Bcl-XL, and decreasing pro-apoptotic Bax and Bad (Guillemard et al., 2004). Viral envelope glycoprotein itself induces M-CSF secretion, which upregulates anti-apoptotic genes bfl-1 and mcl-1 in macrophages (Swingler et al., 2007). HIV-1 also exerts biphasic control of Bcl-2 in monocytes to silence any early replication in preference for viral latency (Aillet et al., 1998). Finally, HIV can promote cell survival in MP. This occurs in microglia directly via Tat stimulation of PI-3-K/AKT signaling (Reviewed in Le Douce et al., 2010). HIV indirectly forces cell cycle arrest by activating cyclin-dependent kinase inhibitor, p21 (Allouch et al., 2014). MP therefore provide HIV with prime resources with which to establish persistent infection.
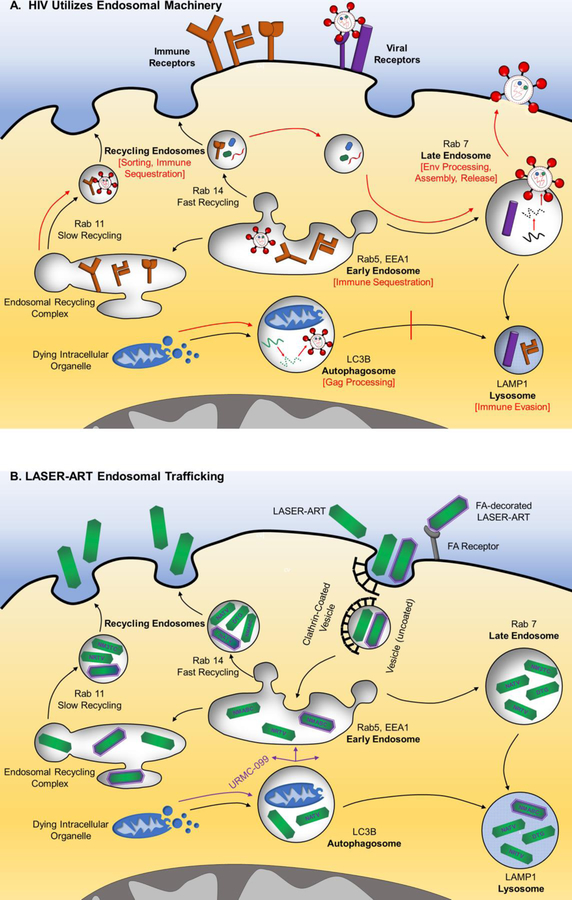
HIV manipulates normal sorting of endocytic vesicles to promote its own life-cycle (figure 1A). During HIV assembly, viral Gag polyprotein accumulates in a subset of late endosomes known as multivesicular bodies (Gousset et al., 2008). HIV also redirects late endosomes to carry viral metabolites to its assembly sites (Chertova et al., 2006; Gerber et al., 2015). Endosomes further provide viral progeny with material for envelope encasement, as evidenced by the abundance of late-endosomal markers HIV virion envelopes (Pelchen-Matthews et al., 2003). Additionally, HIV utilizes late endosome machinery in macrophages to aid in budding or direct spread amongst MP. High levels of host annexin II and ESCRT I in late endosome extracts collected from HIV-1-infected macrophages suggest that these membrane bending proteins play a role in viral budding (Chertova et al., 2006). Moreover, retrograde transport of HIV from late endosomes back to the endoplasmic reticulum and golgi complex enables these organelles to serve as staging locales for intercellular infection via bridging conduits (Kadiu and Gendelman, 2011a). HIV reorganizes myosin II to construct bridging conduits between macrophages that perpetuate viral spread without exposure to host immune defenses (Kadiu and Gendelman, 2011b). Thus, HIV capitalizes on late endosomes to advance viral assembly, budding, and direct spread to adjacent MP.
Fig 1.
Utilization of endocytic machinery in (A) HIV Infection: Endocytosis and autophagy direct extracellular materials or dying organelles to the lysosome, respectively (black arrows). HIV binds CD4 and CCR5 or CXCR4 to enter host cells. Provirus resides in early, recycling, and late endosomes in addition to within autophagosomes. HIV manipulates subcellular organelle trafficking to its advantage (red arrows). Early endosomes sequester MHC-I and immune checkpoint inhibitor, CTLA-4. Recycling endosomes aid in sorting proviral proteins prior to assembly, as well as in sequestration of CTLA-4 and co-stimulatory molecules, CD80/86. HIV promotes autophagosome formation, where Gag polyprotein processing occurs, and inhibits its own degradation in the lysosome. Late endosomes assist in envelope protein processing, proviral assembly in MP, and viral release. HIV evades adaptive immunity by degrading CD4 and MHC-I in the lysosome. (B) LASER-ART delivery is shown. Nanoformulated nanocrystals accumulate in high concentrations within recycling and late endosomes and to lesser levels in early endosomes or lysosomes. URMC-099, a mixed lineage kinase-3 inhibitor, enhances shuttling of antiretroviral particles from the autophagosome to each of these compartments, where they inhibit key HIV processes due to their proximity to virus. Recycling endosomes also provide for sustained release of free-drug from nanocrystals present in MP. N- nanoformulated; M- myristoylated; FA- folic acid; 3TC- lamivudine; ABC- abacavir; ATV- atazanavir; DTG- dolutegravir encased in europium doped cobalt-ferrite; RTV- ritonavir.
HIV and MP Function
Phagolysosomal degradation of antigen is also impaired at multiple stages in HIV-1-infected macrophages. Firstly, HIV-1 reduce antibody-dependent phagocytosis by post-transcriptionally downregulating FcɣR on monocyte-derived macrophages (MDM) (Leeansyah et al., 2007). HIV additionally weakens downstream Syk-phosphorylation and cytoskeletal rearrangements needed for ADPh (Reviewed in Kedzierska et al., 2003). HIV disrupts phagocytosis of C3b-opsonized materials by elevating MP cyclic AMP (Azzam et al., 2006). Secondly, viral Nef protein interferes with AP-1 mediated endosome formation at macrophages’ plasma membrane (Mazzolini et al., 2010). HIV pass directly from early endosomes to more hospitable recycling endosomes and multivesicular bodies, representing a tertiary means of lysosomal escape (Kadiu and Gendelman, 2011b). Fourthly, HIV impedes phagolysosomal fusion immediately upon gp120-CD4 attachment and post-entry with Vpr (Moorjani et al., 1996; Dumas et al., 2015). Each of these mechanisms diminish the chance that lysosomal protons, reactive oxygen / nitrogen species, and hydrolases will lethally maim HIV. Lysosomes are additionally central to autophagy and antigen presentation in MP (figure 1A). Autophagy describes the ability of cells to extend their lifespans by directing worn cytoplasmic organelles from autophagosomes to lysosomes. HIV-1 requires autophagy in macrophages but not CD4+ T lymphocytes for active replication (Espert et al., 2009). Gag polyprotein processing transpires in autophagosomes, however HIV Nef halts lysosomal fusion to protect proviral constituents (Kyei et al., 2009). Nef further sequesters MHC-I (Dirk et al., 2016), CTLA-4 (El-Far et al., 2013) immune checkpoint inhibitors, and CD80/86 (Chaudhry et al., 2008) costimulatory molecules in early / recycling endosomes. Lastly, HIV escapes adaptive immunity by augmenting MHC-II. Viral Nef protein accelerates endocytic removal of mature antigen presenting MHC-II as well as CD4 to lysosomes (Cenac et al., 1975; Chaudhry et al., 2009; Amorim et al., 2014). HIV also reduces lysosomal processing of antigen and MHC-II peptide loading in monocytes (Polyak et al., 1997). In this regard, HIV takes full advantage of a key organelle in MPs- the lysosome.
Secretory Responses
Secretion of inflammatory mediators by MP in response to HIV advances viral spread and facilitates immune exhaustion. Without antiretroviral therapy (ART), macrophages produce IL-12 and TNFα which skew differentiation of naïve CD4+ T cells to TH1 effectors. These lymphocytes, in turn, prompt macrophages to (1) produce reactive oxygen species (ROS), inducible nitric oxide synthase (iNOS), and phagolysosomal enzymes, (2) upregulate antigen presenting MHC-II and T cell costimulatory molecule B7, and (3) secrete additional cytokines that promote antiviral immunity. Macrophages from ART-treated patients produce immunosuppressive IL-10 while only expressing IL-12 and TNFα in response to appropriate stimuli such as LPS (Bocchino et al., 2001). Monocytes from off-therapy HIV-1 patients exhibit amplified signaling along the JAK-STAT1-IFNɣ axis, suggesting further enactment TH1 immune responses (Alhetheel et al., 2008). Peroxisome proliferator-activated receptor (PPAR)-gamma antagonism in macrophages during HIV-1-infection likewise tipped homeostatic balance in favor of inflammation and lipodystrophy (Giralt et al., 2009). Administration of rosiglitazone, a PPAR-gamma agonist, halted brain inflammation and HIV-1 replication in murine HIV-encephalitis subjects (Potula et al., 2008). Chemokines and growth factors contribute to viremia by recruiting CD4+ T lymphocytes and making cells more permissive for infection. CXCR3 expressed on the surface of CD4+ cells enables uninfected T cells to chemotax towards CXCL10 and CXCL11 released by infected macrophages in lymph nodes (Foley et al., 2005) and the central nervous system (Poluektova et al., 2001). HIV infection of MDMs induces HIV co-receptor expression, viral shedding, and differentiation of new macrophage hosts by M-CSF (Kutza et al., 2002). In summary, monocytic-lineage release of TH1 proinflammatory cytokines, lymphocyte recruiting chemokines, and growth factors assist in prolonged viral spread throughout the body.
HIV Disease Pathogenesis
Viral Reservoirs and Disseminators
Control of viral transcription in MP stations these cell types to distribute HIV to numerous organ systems. Monocytes in vivo serve as latent reservoirs devoid of active viral replication (Gendelman et al., 1990; Aillet et al., 1998). Monocytes suppress Cyclin T1, a component of positive transcription elongation factor, via micro RNA silencing (Sung and Rice, 2009). Infected monocytes may also therefore use this mechanism to quell Tat-driven expression of HIV genes. Microglia subdue HIV-1 transcription at the epigenetic level. CTIP2, a transcription factor abundant in microglia, establishes heterochromatic environments in the vicinities of HIV promoters (Marban et al., 2007). In contrast, HIV readily exits latency upon transitioning to macrophage states. Macrophages harbor abundant amounts of circular episomal viral DNA that is stable for up to two months (Marban et al., 2007). Persistent transcription of this unintegrated DNA has further been demonstrated in macrophages (Kelly et al., 2008). These represent but a few of the molecular means that situate MP as viral reservoirs.
Monocytes and their descendants are also instrumental in transmitting virus to and from anatomic sites. HIV penetrates gut mucosa early in disease. Infection of lamina propria macrophages leads to the loss of tight junctions in intestinal epithelia (Kotler, 2005). R5-tropic virus has the highest transmission efficiency in female genital mucosal tissue, another early site of pathogen introduction, causing HIV-positivity in macrophages by 96 hours post-infection (Gupta et al., 2002). The “Trojan Horse” paradigm posits that HIV-1-infected monocyte / macrophages traffic the virus into the central nervous system (CNS) across the blood-brain-barrier, which normally excludes most pathogens and host proteins. Macrophages, however, undergo diapedeses between choroid plexus epithelial- and brain-endothelial tight junctions, introducing virus to microglia (Reviewed in Meltzer et al., 1990). Distinct populations of HIV-ferrying monocytes have been classified immunophenotypically as CD14+CD16+CD11b+Mac387+ and capable of migration in response to a CCL2 concentration gradient (Buckner et al., 2011). Lectins on plasma membranes anchor cells together for adhesion. HIV-1 has been shown to induce myeloid cell sialic acid-binding immunoglobulin-type lectin-1 (siglec-1) expression, a receptor that binds viral membrane gangliosides, and enhances trans-infection of other cells (Pino et al., 2015). Langerhans (epithelial dendritic) cells transfer R5-tropic HIV to surrounding T lymphocytes in a CCR5 dependent manner, indicating that virus spreads locally upon occupational exposures that break skin (Kawamura et al., 2008). Monocyte lineages are thus critical in exposing gut, CNS, genital, and dermal leukocytes to HIV infection.
MP and HIV Neuropathogenesis
Macrophages, in the context of HIV-1, mount free radical stress and toxic responses that damage uninfected neurons. Normal microbicidal activity in phagocytes includes superoxide (O2-) and peroxide (HOOH) formation. Peroxide, in turn, degenerates to hydroxyl free radicals (HO.). Activated macrophages induce nitric oxide synthase that eventually leads to nitric oxide radicals (NO2.) from peroxynitrite (ONOO-) intermediates. Each of these radicals damage pathogen DNA. HIV dysregulates mitochondrial superoxide dismutase leading to sustained intracellular superoxide anions and peroxynitrite (Aquaro et al., 2007). HIV-1 further insults monocyte / macrophage natural responses to free radicals by limiting the production of heme oxygenase 1 (HO-1), prompting accumulation of heme (Cross et al., 2011) and other neurotoxins in the CNS. Glutamate, an excitatory neurotransmitter, damages neurons when high levels amass as has been shown to occur during neuroinflammation due to HIV-1-infected macrophages (Gill et al., 2015). Adoptive transfer of HIV-1-infected human MDM into basal ganglia of immunodeficient mice has provided a murine model by which to study HIV-encephalitis (HIVE) and neurocognitive dementia (HAND). Anti-inflammatory agents, platelet activating factors and TNFα inhibitors were applied to this system and successfully protected against neuronal injury (Persidsky and Gendelman, 2002). To this end, altered biochemical processes in HIV-1-infected macrophages negatively affect neuronal survival.
Macrophages are central to many of the processes that precipitate dementia in adults with HIV. HAND severity has been known since the mid-1990s to correlate with the number of macrophages and microglia in the CNS and not HIV gp41 immunoreactivity, AIDS duration, or the use of antiretrovirals (Glass et al., 1995). Rapidity of dementia progression was also associated with macrophage activation (Bouwman et al., 1998). Peripherally circulating monocytes can cross the blood-brain barrier (BBB) and infect CNS microglia in addition to brain perivascular macrophages. Infected cells release inflammatory cytokines (IL-12, TNFα), chemokines (CXCL10, CXCL11), amino acids (glutamate), reactive oxygen / nitrogen species, and eicosinoids, all of which contribute to neuronal death (Reviewed in Ramesh et al., 2013). Moreover, aberrant conductance through voltage-gated potassium channels on microglia has been associated with some of the early symptoms of HAND (Reviewed in Keblesh et al., 2008). Monocyte / macrophages from seropositive patients with HAND also secrete less defense proteins, such as lysozyme, leaving the CNS open to concomitant infection that can advance cognitive decline (Sun et al., 2004). Some of these opportunistic infections will be noted below.
HIV can disrupt healthy brain parenchyma leading to behavioral symptoms that characterize HIV-associated encephalitis. The importance of HIV-1-infected MDM to abnormal behavior has been validated using the murine HIVE model (Persidsky and Gendelman, 2002). Post-mortem brain tissue from HIVE cases shows adherence of platelet-monocyte complexes (PMCs) in CNS postcapillary venules (Singh et al., 2014). It is hypothesized that PMCs precipitate neuroinflammation by secreting chemokines that further recruit activated monocytes to the CNS. Second, PMCs likely also damage the blood-brain-barrier during trans-endothelial migration. Viral gene product-, cytokine-, phospholipid-, and eicosanoid secretions by HIV-1-infected microglia are a third implication of MP in HIVE pathology (Reviewed in Gelbard and Epstein, 1995). CNS-permeable ART reduced monocyte chemotactic protein 1 (MCP-1) and viral RNA contained in HIV-patient cerebrospinal fluid (De Luca et al., 2002). The same was seen in rhesus macaques treated with minocycline, an antibiotic with anti-inflammatory and neuroprotective properties (Zink et al., 2005). In aggregate, these findings support early damage caused by activated monocytes as an etiology for HIVE, which can be pharmacologically combatted.
HIV infection of the MP potentiates opportunistic infection of the CNS as well as peripheral neuropathy. Several forms of damage to brain white matter have been linked to HIV infection of monocyte / macrophages. Severe demyelinating leukoencephalopathy transpires in ART-refractory patients as HIV-carrying monocytes damage brain capillaries, demyelinate axons, and inflame astrocytes (Langford et al., 2002). High MP chemokine levels in cerebrospinal fluid (CSF) samples also point to impaired immune clearance of AIDS-defining infections. Progressive multifocal leukoencephalopathy, brought on by JC polyomavirus reactivation in immunocompromised patients (CD4+ < 200/mm3), often presents with elevated cerebrospinal fluid MCP-1 concentrations (Marzocchetti et al., 2005). Undiagnosed AIDS individuals with symptomatic Cryptococcal meningitis (CD4+ < 100/mm3) similarly test high for macrophage secreted IL-13 and MIP-1α in CSF. This finding correlates with poor outcome of early intervention (Scriven et al., 2015). Newly recruited monocytes to the CNS become alternatively activated M2 under this cytokine milieu, thereby suppressing appropriate effector responses. Peripheral neuropathy is the most common neurological symptom in HIV-1 patients (Reviewed in McArthur et al., 2005), manifesting as weakness, numbness, and pain often in the hands in feet. HIV-1-infected macrophages in the endoneurial sheath generate cytokine-mediated autoimmunity against peripheral nerves (Dalakas and Cupler, 1996). Other causes of neuropathy in HIV patients relate to the degeneration of long axons, loss of unmyelinated fibers, macrophage infiltration into peripheral nerves and dorsal root ganglia, and terminal effects of cytomegalovirus and herpes zoster coinfections (Pardo et al., 2001). To this end, MP shuttle HIV into the nervous system where they damage neurons as well as generate permissive environments for opportunistic pathogens.
MP and the Lung
Pulmonary coinfections with HIV stem from altered immunity of lung resident macrophages. Alveolar macrophages from HIV-1-infected patients produce decreased TNFα in response to TLR2 and TLR4 stimulation, indicating that the virus diminishes innate immune responses to opportunistic infection (Nicol et al., 2008). Toll-like receptor 2 is critical for phagocyte recognition of gram-positive bacteria while TLR4 binds a broader variety of pathogenic motifs such as LPS on gram-negative bacteria, protozoal phospholipids, fungal mannan, and viral envelope proteins. Higher HIV-1 RNA was measured in bronchioalveolar lavage (BAL) fluid of patients coinfected with Pneumocystis carinii, Mycobacterium avium complex (MAC), Nocardia sp., and Aspergillus fumigatus than in matched HIV-1 patients, further demonstrating HIV’s ability to impair innate immunity (Koziel et al., 1999). A prime concern of clinicians caring for AIDS individuals is their susceptibility to progressive primary tuberculosis. Unlike in immunocompetent adults in whom Mycobacterium tuberculosis (Mtb) remains latently confined to the lung in the absence of reactivation, Mtb primary infection of AIDS patients hematogenously disseminates to reticuloendothelial organs, bone/vertebrae, and meninges. Alveolar macrophages collected from newly-treated ART patients displayed weakened ability for proteolytic cleavage of proteins, a necessary step in antigen presentation to T cells (Jambo et al., 2014). These macrophages not only failed to facilitate Mtb-specific TH1 effector responses, which are essential to clearance of tuberculosis, but were also defective in IL-10 attenuation of immune inflammation (Tomlinson et al., 2014). These observations together suggest that, in the context of HIV, alveolar macrophages promote chronic inflammation that hinders both innate and adaptive immune clearance of pathogens.
MP and the Gastrointestinal Tract
HIV upregulation of proinflammatory cytokines along the gastrointestinal (GI) system derails macrophage prevention of Kaposi’s sarcoma and liver fibrosis. Kaposi’s sarcoma, a neoplasm of vascular endothelial cells in immunocompromised patients (CD4+ < 500/mm3) who are co-infected with human herpesvirus 8 (HHV-8), presents as violaceous nodules on skin, lung parenchyma, and GI lining. HIV Tat protein promotes growth of macrophages, which are among the only HHV-8 infected cells until late disease (Reviewed in Gallo, 1998). HHV-8 itself is not regarded as oncogenic. Rather, endothelial cells perpetuate sustained microinflammation by secreting IL-6, IL-10, and IL-13. These cytokines polarize macrophages to differentiate into tumor associated macrophages (TAMs) that promote tumor formation instead of viral clearance (Bhaskaran et al., 2017). The prototypical ability of macrophages to aid in intracellular pathogen destruction is also lost in HIV-1-infected resident liver macrophages. HIV infection of Kupffer cells enhances expression of TLR4 and its co-receptor, CD14, leading to overproduction of IL-6 and TNFα. These cytokines were shown to bolster liver fibrosis (Mosoian et al., 2017). Moreover, fewer Kupffer cells in liver biopsies from HIV-hepatitis C coinfected patients correlated with progression to hepatic fibrosis (Balagopal et al., 2009). Thus, many of the GI pathologies seen in HIV-1 patients arise from macrophages’ inability to effectively clear coinfecting microorganisms.
MP and ART
ART is the standard of care for HIV-1 patients in the Western world, however its curative potential remains far from realized. Dosing of HIV-1 patients with nucleoside reverse-transcriptase inhibitors (NRTIs) plus protease inhibitors, together comprising the backbone of ART, was discovered in the late 1990s as a means of suppressing over 99% of free HIV-1 in plasma. Pharmacodynamic studies detail viral clearance kinetics as a two-phase system. ART first clears HIV from short-lived productively infected cells after several days (Perelson et al., 1997). HIV-1-infected macrophages or other MP require upwards of 3 weeks to 3 years of consistent ART treatment to reduce viral stores (Cavert et al., 1997; Perelson et al., 1997; Murray et al., 2007). As much as one-quarter of patients who attain the treatment goal of <50 HIV copies/mL plasma within one year of initiating ART enjoy only modest increases in CD4+ lymphocyte counts (Benveniste et al., 2005; Aiuti and Mezzaroma, 2006; Marziali et al., 2006; Taiwo et al., 2010). Furthermore, ART achieves little if any reduction of HIV replication in anatomic reservoirs such as the lymph node, spleen, GALT, CNS, and genital tissues (Lorenzo-Redondo et al., 2016; Edagwa et al., 2017). Drug resistance to highly active antiretroviral therapy emerges when patients fall below 95% adherence to designated regimens (Lima et al., 2008). Poor access to ART not only contributes to ART-resistance, but perpetuates viral transmission in endemic regions as well. The World Health Organization reports that as of 2016, fewer than 34% of HIV-1-infected individuals in eastern Mediterranean, western and central African countries receive ART (World Health Organization, 2016). Future therapeutic strategies must therefore address these issues along with the plethora of previously noted HIV-associated comorbidities.
Erythrocyte ART Delivery
Due to fact that macrophages readily phagocytose erythrocytes under physiological conditions, red blood cells (RBCs) became early vectors for antiretroviral delivery beginning in the 1990s. A barrier to the success of conventional nucleoside reverse-transcriptase inhibitors is the low level of phosphorylation in target cells needed to activate them. Erythrocyte encapsulation of the pre-phosphorylated nucleotide reverse-transcriptase inhibitor ddCTP, prompted IgG-mediated phagocytosis by macrophages in retrovirally infected mice along with nearly complete inhibition of HIV replication and acute infection symptoms (Magnani et al., 1992). This method also demonstrated increased drug stability and prophylactic protection of macrophages from HIV infection when the pre-phosphorylated zidovudine dinucleotide was loaded into RBCs (Magnani et al., 1996). Chemotherapeutic fludarabine embedded in erythrocytes has also been utilized to selectively kill HIV-1-infected monocyte / macrophage populations in vitro (Magnani et al., 2003), and delay in viral rebound after therapy cessation in primates (Cervasi et al., 2006). These observations provided early justification for the employment of macrophages as drug targets.
Long Acting Slow Effective Release ART (LASER-ART)
MPs position at the crossroads of immune defense and HIV hosting primes them as ideal conduits for novel treatment schemes. Long-acting slow effective release (LASER) antiretroviral particles tip macrophage biology in favor of viral clearance. LASER-ART are generated by chemically converting existing antiretrovirals to more hydrophobic prodrugs, which are then encased in lipophilic excipients. The increased hydrophobicity of these prodrugs better potentiates drug-crystal formation, while the raised lipophilicity of drug-excipient nanoparticles eases their passage across cell membranes. Together, these improvements boost the apparent half-lives of antiretrovirals by ensuring slow release of active drug from macrophages. LASER-ART also seek to enhance patient adherence, target anatomic sanctuaries of HIV, and serve as candidates for pre-exposure prophylaxis in medically underserved areas (Edagwa et al., 2017).
LASER-ART modifications improve drug uptake and release by monocyte-derived macrophages (Singh et al., 2016; Guo et al., 2017; Sillman et al., 2018). Existing HIV therapies may be optimized through nanoformulation for macrophage phagocytosis. Particle size, surfactant coating, surface charge, and shape predicts uptake by MDM and antiretroviral efficacy (Nowacek et al., 2011). One advantage of nanoformulated ART lies in the ability to encapsulate multiple drugs in a single particle. Co-delivering lopinavir plus ritonavir together with tenofovir amplified intracellular drug concentrations 50-fold and sustained plasma concentrations 3.5 times longer than free drug (Freeling et al., 2015). Furthermore, conversion of native antiretroviral therapies to hydrophobic prodrugs enables prolonged host enzyme (e.g. carboxyesterase) cleavage. Nanoparticles of myristoylated abacavir (MABC) and lamivudine (M3TC) owed much of their improved efficacy to greater uptake by- and depot formation within MDM (Singh et al., 2016; Guo et al., 2017). To this end, LASER-ART utilizes macrophage predilection for phagocytosis to create cellular stores of drug.
LASER-ART further inhibits HIV by colocalizing to macrophage subcellular compartments used for viral assembly (figure 1B). Ritonavir-nanoparticles move from early to recycling endosomes, where particles’ antiretroviral activities are spared from lysosomal degradation (Kadiu et al., 2011). Atazanavir (ATV) and lamivudine nanoparticles traffic to recycling and late endosomes, where HIV claims sanctuary (Guo et al., 2014a; Guo et al., 2017). ART may also be pharmacologically modulated by induction of autophagy. URMC-099, a MLK-3 inhibitor and autophagy promoter, raised dolutegravir release from MDM 50-fold by extending cellular longevity (Gnanadhas et al., 2017). In this manner, URMC-099 exchanges an identical ploy used by HIV for survival in favor of extended drug release (Kyei et al., 2009). URMC-099 additionally boosted nanoATV levels in early, late, and recycling endosomes (Zhang et al., 2016). These tactics indicate that LASER-ART alone or with URMC-099 pinpoints HIV within infected cells.
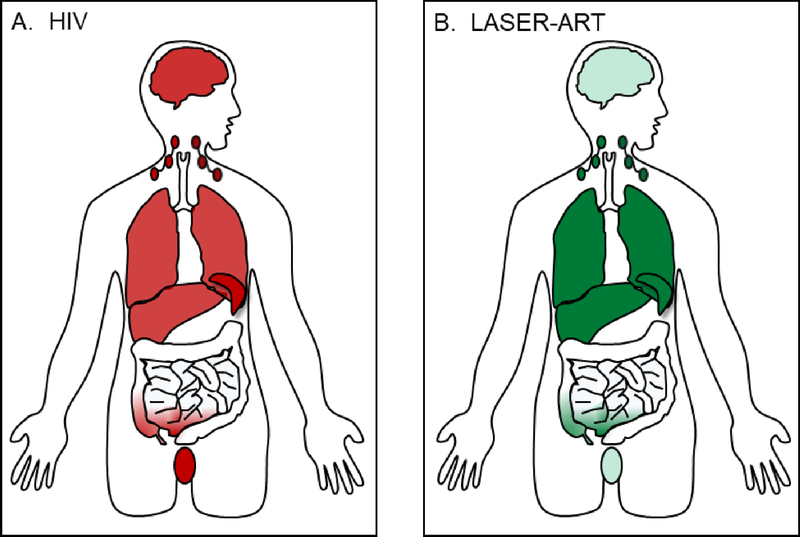
A last benefit conferred by MP rests in their ability to shuttle LASER-ART to anatomic HIV reservoirs (figure 2). Indinavir nanoparticle-loaded bone marrow-derived macrophages successfully mitigated the number of HIV-1-infected cells in murine lymph nodes, spleen, liver, lungs, and CNS (Dou et al., 2006; Dou et al., 2009). Injection of cell-free nanoformulations into humanized SCID mice demonstrated antiviral effects, neuroprotection, and that drug-carrying macrophages indeed pass drug through brain microvascular endothelial cells (Dash et al., 2012; Kanmogne et al., 2012). Nanoformulation affords the opportunity to attach targeting-ligands to antiretrovirals. Folate decoration of ATV/r and M3TC facilitated greater MDM uptake and delivery to liver, spleen, and lymph nodes of HIV-1-infected humanized mice (Puligujja et al., 2013; Singh et al., 2016). A final technical achievement in LASER-ART design rests in the ability to house nanoformulations in theranostic particles. Drug encapsulation by materials like magnetite (Guo et al., 2014b), 111 indium oxine (Gorantla et al., 2006), europium doped cobalt-ferrite (Kevadiya et al., 2017) permit real-time biodistribution tracking by MRI, SPECT, or gamma-scintigraphy. This methodology potentiates for the eventual correlation between radiographic signal and projected pharmacokinetics / pharmacodynamics profiles without the need to harvest tissue. To conclude, macrophages bring LASER-ART treatments and diagnostic particles to HIV-1-infected areas that conventional therapies may inadequately reach.
Fig 2.
LASER-ART drugs targets HIV reservoirs. (A) Key anatomic reservoirs of HIV include the liver, spleen, lymph nodes, lungs, central nervous system (CNS), gut-associated lymphoid tissue (GALT), and genitourinary tract (GU). (B) LASER-ART drugs accumulate at high concentrations within the liver, spleen, and lymph nodes. Moderate biodistribution of LASER-ART drugs has been observed in GALT. Low levels of drug are found in the CNS and GU.
Future Directions and Conclusions
HIV treatment has progressed significantly since the discovery of macrophages as viral hosts and drug targets. Though HIV promotes chronic-inflammatory programs in macrophages that drive CNS, pulmonary, cardiovascular, GI, and systemic pathologies, some of these aberrant processes serve as levers for therapeutic inhibition. Advances in bioengineering and medicinal chemistry, such as erythrocyte-loaded and nanoparticulated drug generation have allowed conventional antiretroviral therapies to become even more effective. Future developments in the field of macrophage-HIV eradication should apply CRISPR-Cas9 gene editing to splice out proviral sequences from host genomes. This approach has already met some success in vitro prophylactically in human embryonic stem cell-derived macrophages and primary CD4+ T cells (Liao et al., 2015), as well as therapeutically in infected-patient T lymphocytes (Kaminski et al., 2016). Adoptive transfer of antigen loaded antigen presenting cells capable of eliciting cellular and humoral immune responses (Cobb et al., 2011) is perhaps another exciting prospect on the horizon of HIV vaccinology. To this end, macrophages are paramount to the establishment of HIV infection and its eventual eradication.
Acknowledgments
We thank Drs. Benson Edagwa and Aditya Bade for their thoughtful discussions. This work was supported in part by NIH Grants R01 AG043530, P01 DA028555, P30 MH062261, R01 MH115860, R01 NS034249, R01 NS036126, and the Carol Swartz Emerging Neuroscience Fund.
Footnotes
Conflict of Interest: None
References
- Aillet F, Masutani H, Elbim C, Raoul H, Chene L, Nugeyre MT, Paya C, Barre-Sinoussi F, Gougerot-Pocidalo MA, Israel N (1998) Human immunodeficiency virus induces a dual regulation of Bcl-2, resulting in persistent infection of CD4(+) T- or monocytic cell lines. J Virol 72:9698–9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiuti F, Mezzaroma I (2006) Failure to reconstitute CD4+ T-cells despite suppression of HIV replication under HAART. AIDS Rev 8:88–97. [PubMed] [Google Scholar]
- Alhetheel A, Yakubtsov Y, Abdkader K, Sant N, Diaz-Mitoma F, Kumar A, Kryworuchko M (2008) Amplification of the signal transducer and activator of transcription I signaling pathway and its association with apoptosis in monocytes from HIV-infected patients. AIDS 22:1137–1144. [DOI] [PubMed] [Google Scholar]
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA (1996) CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955–1958. [DOI] [PubMed] [Google Scholar]
- Allouch A, David A, Amie SM, Lahouassa H, Chartier L, Margottin-Goguet F, Barre-Sinoussi F, Kim B, Saez-Cirion A, Pancino G (2014) Reply to Pauls et al. : p21 is a master regulator of HIV replication in macrophages through dNTP synthesis block. Proc Natl Acad Sci U S A 111:E1325–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim NA, da Silva EM, de Castro RO, da Silva-Januario ME, Mendonca LM, Bonifacino JS, da Costa LJ, daSilva LL (2014) Interaction of HIV-1 Nef protein with the host protein Alix promotes lysosomal targeting of CD4 receptor. J Biol Chem 289:27744–27756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancuta P, Kunstman KJ, Autissier P, Zaman T, Stone D, Wolinsky SM, Gabuzda D (2006) CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology 344:267–276. [DOI] [PubMed] [Google Scholar]
- Aquaro S, Muscoli C, Ranazzi A, Pollicita M, Granato T, Masuelli L, Modesti A, Perno CF, Mollace V (2007) The contribution of peroxynitrite generation in HIV replication in human primary macrophages. Retrovirology 4:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arainga M, Su H, Poluektova LY, Gorantla S, Gendelman HE (2016) HIV-1 cellular and tissue replication patterns in infected humanized mice. Sci Rep 6:23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam R, Kedzierska K, Leeansyah E, Chan H, Doischer D, Gorry PR, Cunningham AL, Crowe SM, Jaworowski A (2006) Impaired complement-mediated phagocytosis by HIV type-1-infected human monocyte-derived macrophages involves a cAMP-dependent mechanism. AIDS Res Hum Retroviruses 22:619–629. [DOI] [PubMed] [Google Scholar]
- Balagopal A, Ray SC, De Oca RM, Sutcliffe CG, Vivekanandan P, Higgins Y, Mehta SH, Moore RD, Sulkowski MS, Thomas DL, Torbenson MS (2009) Kupffer cells are depleted with HIV immunodeficiency and partially recovered with antiretroviral immune reconstitution. AIDS 23:2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron MH, Isern J, Fraser ST (2012) The embryonic origins of erythropoiesis in mammals. Blood 119:4828–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers SA, Chan CH, James S, French RR, Attfield KE, Brennan CM, Ahuja A, Shlomchik MJ, Cragg MS, Glennie MJ (2008) Type II (tositumomab) anti-CD20 monoclonal antibody out performs type I (rituximab-like) reagents in B-cell depletion regardless of complement activation. Blood 112:4170–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste O, Flahault A, Rollot F, Elbim C, Estaquier J, Pedron B, Duval X, Dereuddre-Bosquet N, Clayette P, Sterkers G, Simon A, Ameisen JC, Leport C (2005) Mechanisms involved in the low-level regeneration of CD4+ cells in HIV-1-infected patients receiving highly active antiretroviral therapy who have prolonged undetectable plasma viral loads. J Infect Dis 191:1670–1679. [DOI] [PubMed] [Google Scholar]
- Bhaskaran N, Ghosh SK, Yu X, Qin S, Weinberg A, Pandiyan P, Ye F (2017) Kaposi’s sarcoma-associated herpesvirus infection promotes differentiation and polarization of monocytes into tumor-associated macrophages. Cell Cycle 16:1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchino M, Ledru E, Debord T, Gougeon ML (2001) Increased priming for interleukin-12 and tumour necrosis factor alpha in CD64 monocytes in HIV infection: modulation by cytokines and therapy. AIDS 15:1213–1223. [DOI] [PubMed] [Google Scholar]
- Bouwman FH, Skolasky RL, Hes D, Selnes OA, Glass JD, Nance-Sproson TE, Royal W, Dal Pan GJ, McArthur JC (1998) Variable progression of HIV-associated dementia. Neurology 50:1814–1820. [DOI] [PubMed] [Google Scholar]
- Boyette LB, Macedo C, Hadi K, Elinoff BD, Walters JT, Ramaswami B, Chalasani G, Taboas JM, Lakkis FG, Metes DM (2017) Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One 12:e0176460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner CM, Calderon TM, Willams DW, Belbin TJ, Berman JW (2011) Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: implications for NeuroAIDS. Cell Immunol 267:109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavert W, Notermans DW, Staskus K, Wietgrefe SW, Zupancic M, Gebhard K, Henry K, Zhang ZQ, Mills R, McDade H, Schuwirth CM, Goudsmit J, Danner SA, Haase AT (1997) Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science 276:960–964. [DOI] [PubMed] [Google Scholar]
- Cenac A, Gaudeau S, Vernant P (1975) [Pericardial effusions and mediastinal radiotherapy. 4 cases]. Nouv Presse Med 4:185–187. [PubMed] [Google Scholar]
- Cervasi B, Paiardini M, Serafini S, Fraternale A, Menotta M, Engram J, Lawson B, Staprans SI, Piedimonte G, Perno CF, Silvestri G, Magnani M (2006) Administration of fludarabine-loaded autologous red blood cells in simian immunodeficiency virus-infected sooty mangabeys depletes pSTAT-1-expressing macrophages and delays the rebound of viremia after suspension of antiretroviral therapy. J Virol 80:10335–10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao JR, Wang JM, Lee SF, Peng HW, Lin YH, Chou CH, Li JC, Huang HM, Chou CK, Kuo ML, Yen JJ, Yang-Yen HF (1998) mcl-1 is an immediate-early gene activated by the granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling pathway and is one component of the GM-CSF viability response. Mol Cell Biol 18:4883–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Das SR, Jameel S, George A, Bal V, Mayor S, Rath S (2008) HIV-1 Nef induces a Rab11-dependent routing of endocytosed immune costimulatory proteins CD80 and CD86 to the Golgi. Traffic 9:1925–1935. [DOI] [PubMed] [Google Scholar]
- Chaudhry A, Verghese DA, Das SR, Jameel S, George A, Bal V, Mayor S, Rath S (2009) HIV-1 Nef promotes endocytosis of cell surface MHC class II molecules via a constitutive pathway. J Immunol 183:2415–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C, Liu R, Landau NR, Stamatatos L (1997) Macrophage tropism of human immunodeficiency virus type 1 and utilization of the CC-CKR5 coreceptor. J Virol 71:1657–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertova E, Chertov O, Coren LV, Roser JD, Trubey CM, Bess JW Jr., Sowder RC 2nd, Barsov E, Hood BL, Fisher RJ, Nagashima K, Conrads TP, Veenstra TD, Lifson JD, Ott DE (2006) Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J Virol 80:9039–9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross SA, Cook DR, Chi AW, Vance PJ, Kolson LL, Wong BJ, Jordan-Sciutto KL, Kolson DL (2011) Dimethyl fumarate, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and macrophage-mediated neurotoxicity: a novel candidate for HIV neuroprotection. J Immunol 187:5015–5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalakas MC, Cupler EJ (1996) Neuropathies in HIV infection. Baillieres Clin Neurol 5:199–218. [PubMed] [Google Scholar]
- Dash PK, Gendelman HE, Roy U, Balkundi S, Alnouti Y, Mosley RL, Gelbard HA, McMillan J, Gorantla S, Poluektova LY (2012) Long-acting nanoformulated antiretroviral therapy elicits potent antiretroviral and neuroprotective responses in HIV-1-infected humanized mice. AIDS 26:2135–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BK, Wen H, Ting JP (2011) The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 29:707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Ciancio BC, Larussa D, Murri R, Cingolani A, Rizzo MG, Giancola ML, Ammassari A, Ortona L (2002) Correlates of independent HIV-1 replication in the CNS and of its control by antiretrovirals. Neurology 59:342–347. [DOI] [PubMed] [Google Scholar]
- Dean M, Carrington M, Winkler C, Huttley GA, Smith MW, Allikmets R, Goedert JJ, Buchbinder SP, Vittinghoff E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, O’Brien SJ (1996) Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science 273:1856–1862. [DOI] [PubMed] [Google Scholar]
- DeFalco T, Bhattacharya I, Williams AV, Sams DM, Capel B (2014) Yolk-sac-derived macrophages regulate fetal testis vascularization and morphogenesis. Proc Natl Acad Sci U S A 111:E2384–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES (2005) Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 307:1630–1634. [DOI] [PubMed] [Google Scholar]
- Desch AN, Gibbings SL, Clambey ET, Janssen WJ, Slansky JE, Kedl RM, Henson PM, Jakubzick C (2014) Dendritic cell subsets require cis-activation for cytotoxic CD8 T-cell induction. Nat Commun 5:4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt M, Rottiers P, Dooms H, Fiers W, Grooten J (1998) Macrophages induce cellular immunity by activating Th1 cell responses and suppressing Th2 cell responses. J Immunol 160:5300–5308. [PubMed] [Google Scholar]
- Desport M (2010) Lentiviruses and macrophages : molecular and cellular interactions Norkfolk, UK: Caister Academic Press. [Google Scholar]
- Dirk BS, Pawlak EN, Johnson AL, Van Nynatten LR, Jacob RA, Heit B, Dikeakos JD (2016) HIV-1 Nef sequesters MHC-I intracellularly by targeting early stages of endocytosis and recycling. Sci Rep 6:37021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou H, Grotepas CB, McMillan JM, Destache CJ, Chaubal M, Werling J, Kipp J, Rabinow B, Gendelman HE (2009) Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. J Immunol 183:661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou H, Destache CJ, Morehead JR, Mosley RL, Boska MD, Kingsley J, Gorantla S, Poluektova L, Nelson JA, Chaubal M, Werling J, Kipp J, Rabinow BE, Gendelman HE (2006) Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood 108:2827–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas A, Le-Bury G, Marie-Anais F, Herit F, Mazzolini J, Guilbert T, Bourdoncle P, Russell DG, Benichou S, Zahraoui A, Niedergang F (2015) The HIV-1 protein Vpr impairs phagosome maturation by controlling microtubule-dependent trafficking. J Cell Biol 211:359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edagwa B, McMillan J, Sillman B, Gendelman HE (2017) Long-acting slow effective release antiretroviral therapy. Expert Opin Drug Deliv 14:1281–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Far M, Isabelle C, Chomont N, Bourbonniere M, Fonseca S, Ancuta P, Peretz Y, Chouikh Y, Halwani R, Schwartz O, Madrenas J, Freeman GJ, Routy JP, Haddad EK, Sekaly RP (2013) Down-regulation of CTLA-4 by HIV-1 Nef protein. PLoS One 8:e54295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espert L, Varbanov M, Robert-Hebmann V, Sagnier S, Robbins I, Sanchez F, Lafont V, Biard-Piechaczyk M (2009) Differential role of autophagy in CD4 T cells and macrophages during X4 and R5 HIV-1 infection. PLoS One 4:e5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa V, Rivera A (2012) Cytokines and the regulation of fungus-specific CD4 T cell differentiation. Cytokine 58:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannagan RS, Cosio G, Grinstein S (2009) Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol 7:355–366. [DOI] [PubMed] [Google Scholar]
- Foley JF, Yu CR, Solow R, Yacobucci M, Peden KW, Farber JM (2005) Roles for CXC chemokine ligands 10 and 11 in recruiting CD4+ T cells to HIV-1-infected monocyte-derived macrophages, dendritic cells, and lymph nodes. J Immunol 174:4892–4900. [DOI] [PubMed] [Google Scholar]
- Freeling JP, Koehn J, Shu C, Sun J, Ho RJ (2015) Anti-HIV drug-combination nanoparticles enhance plasma drug exposure duration as well as triple-drug combination levels in cells within lymph nodes and blood in primates. AIDS Res Hum Retroviruses 31:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo RC (1998) Some aspects of the pathogenesis of HIV-1-associated Kaposi’s sarcoma. J Natl Cancer Inst Monogr:55–57. [DOI] [PubMed] [Google Scholar]
- Gelbard HA, Epstein LG (1995) HIV-1 encephalopathy in children. Curr Opin Pediatr 7:655–662. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Baca LM, Turpin J, Kalter DC, Hansen B, Orenstein JM, Dieffenbach CW, Friedman RM, Meltzer MS (1990) Regulation of HIV replication in infected monocytes by IFN-alpha. Mechanisms for viral restriction. J Immunol 145:2669–2676. [PubMed] [Google Scholar]
- Gerber PP, Cabrini M, Jancic C, Paoletti L, Banchio C, von Bilderling C, Sigaut L, Pietrasanta LI, Duette G, Freed EO, Basile Gde S, Moita CF, Moita LF, Amigorena S, Benaroch P, Geffner J, Ostrowski M (2015) Rab27a controls HIV-1 assembly by regulating plasma membrane levels of phosphatidylinositol 4,5-bisphosphate. J Cell Biol 209:435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill AJ, Kovacsics CE, Vance PJ, Collman RG, Kolson DL (2015) Induction of Heme Oxygenase-1 Deficiency and Associated Glutamate-Mediated Neurotoxicity Is a Highly Conserved HIV Phenotype of Chronic Macrophage Infection That Is Resistant to Antiretroviral Therapy. J Virol 89:10656–10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt M, Domingo P, Villarroya F (2009) HIV-1 infection and the PPARgamma-dependent control of adipose tissue physiology. PPAR Res 2009:607902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC (1995) Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol 38:755–762. [DOI] [PubMed] [Google Scholar]
- Gnanadhas DP, Dash PK, Sillman B, Bade AN, Lin Z, Palandri DL, Gautam N, Alnouti Y, Gelbard HA, McMillan J, Mosley RL, Edagwa B, Gendelman HE, Gorantla S (2017) Autophagy facilitates macrophage depots of sustained-release nanoformulated antiretroviral drugs. J Clin Invest 127:857–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorantla S, Dou H, Boska M, Destache CJ, Nelson J, Poluektova L, Rabinow BE, Gendelman HE, Mosley RL (2006) Quantitative magnetic resonance and SPECT imaging for macrophage tissue migration and nanoformulated drug delivery. J Leukoc Biol 80:1165–1174. [DOI] [PubMed] [Google Scholar]
- Gordan S, Biburger M, Nimmerjahn F (2015) bIgG time for large eaters: monocytes and macrophages as effector and target cells of antibody-mediated immune activation and repression. Immunol Rev 268:52–65. [DOI] [PubMed] [Google Scholar]
- Gordon S (2016) Elie Metchnikoff, the Man and the Myth. J Innate Immun 8:223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorry PR, Bristol G, Zack JA, Ritola K, Swanstrom R, Birch CJ, Bell JE, Bannert N, Crawford K, Wang H, Schols D, De Clercq E, Kunstman K, Wolinsky SM, Gabuzda D (2001) Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J Virol 75:10073–10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gousset K, Ablan SD, Coren LV, Ono A, Soheilian F, Nagashima K, Ott DE, Freed EO (2008) Real-time visualization of HIV-1 GAG trafficking in infected macrophages. PLoS Pathog 4:e1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemard E, Jacquemot C, Aillet F, Schmitt N, Barre-Sinoussi F, Israel N (2004) Human immunodeficiency virus 1 favors the persistence of infection by activating macrophages through TNF. Virology 329:371–380. [DOI] [PubMed] [Google Scholar]
- Guo D, Zhang G, Wysocki TA, Wysocki BJ, Gelbard HA, Liu XM, McMillan JM, Gendelman HE (2014a) Endosomal trafficking of nanoformulated antiretroviral therapy facilitates drug particle carriage and HIV clearance. J Virol 88:9504–9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Li T, McMillan J, Sajja BR, Puligujja P, Boska MD, Gendelman HE, Liu XM (2014b) Small magnetite antiretroviral therapeutic nanoparticle probes for MRI of drug biodistribution. Nanomedicine (Lond) 9:1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Zhou T, Arainga M, Palandri D, Gautam N, Bronich T, Alnouti Y, McMillan J, Edagwa B, Gendelman HE (2017) Creation of a Long-Acting Nanoformulated 2’,3’-Dideoxy-3’-Thiacytidine. J Acquir Immune Defic Syndr 74:e75–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Collins KB, Ratner D, Watkins S, Naus GJ, Landers DV, Patterson BK (2002) Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol 76:9868–9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercus TR, Broughton SE, Ekert PG, Ramshaw HS, Perugini M, Grimbaldeston M, Woodcock JM, Thomas D, Pitson S, Hughes T, D’Andrea RJ, Parker MW, Lopez AF (2012) The GM-CSF receptor family: mechanism of activation and implications for disease. Growth Factors 30:63–75. [DOI] [PubMed] [Google Scholar]
- Hopkinson-Woolley J, Hughes D, Gordon S, Martin P (1994) Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci 107 ( Pt 5):1159–1167. [DOI] [PubMed] [Google Scholar]
- Hubert P, Heitzmann A, Viel S, Nicolas A, Sastre-Garau X, Oppezzo P, Pritsch O, Osinaga E, Amigorena S (2011) Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy. Cancer Res 71:5134–5143. [DOI] [PubMed] [Google Scholar]
- Iordanskiy S, Santos S, Bukrinsky M (2013) Nature, nurture and HIV: The effect of producer cell on viral physiology. Virology 443:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubzick CV, Randolph GJ, Henson PM (2017) Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol 17:349–362. [DOI] [PubMed] [Google Scholar]
- Jambo KC, Banda DH, Afran L, Kankwatira AM, Malamba RD, Allain TJ, Gordon SB, Heyderman RS, Russell DG, Mwandumba HC (2014) Asymptomatic HIV-infected individuals on antiretroviral therapy exhibit impaired lung CD4(+) T-cell responses to mycobacteria. Am J Respir Crit Care Med 190:938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung A, Maier R, Vartanian JP, Bocharov G, Jung V, Fischer U, Meese E, Wain-Hobson S, Meyerhans A (2002) Recombination: Multiply infected spleen cells in HIV patients. Nature 418:144. [DOI] [PubMed] [Google Scholar]
- Kadiu I, Gendelman HE (2011a) Macrophage bridging conduit trafficking of HIV-1 through the endoplasmic reticulum and Golgi network. J Proteome Res 10:3225–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiu I, Gendelman HE (2011b) Human immunodeficiency virus type 1 endocytic trafficking through macrophage bridging conduits facilitates spread of infection. J Neuroimmune Pharmacol 6:658–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiu I, Nowacek A, McMillan J, Gendelman HE (2011) Macrophage endocytic trafficking of antiretroviral nanoparticles. Nanomedicine (Lond) 6:975–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmogne GD, Singh S, Roy U, Liu X, McMillan J, Gorantla S, Balkundi S, Smith N, Alnouti Y, Gautam N, Zhou Y, Poluektova L, Kabanov A, Bronich T, Gendelman HE (2012) Mononuclear phagocyte intercellular crosstalk facilitates transmission of cell-targeted nanoformulated antiretroviral drugs to human brain endothelial cells. Int J Nanomedicine 7:2373–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Koyanagi Y, Nakamura Y, Ogawa Y, Yamashita A, Iwamoto T, Ito M, Blauvelt A, Shimada S (2008) Significant virus replication in Langerhans cells following application of HIV to abraded skin: relevance to occupational transmission of HIV. J Immunol 180:3297–3304. [DOI] [PubMed] [Google Scholar]
- Keblesh JP, Reiner BC, Liu J, Xiong H (2008) Pathogenesis of Human Immunodeficiency Virus Type-1 (HIV-1)-Associated Dementia: Role of Voltage-Gated Potassium Channels. Retrovirology (Auckl) 2:1–10. [PMC free article] [PubMed] [Google Scholar]
- Kedzierska K, Azzam R, Ellery P, Mak J, Jaworowski A, Crowe SM (2003) Defective phagocytosis by human monocyte/macrophages following HIV-1 infection: underlying mechanisms and modulation by adjunctive cytokine therapy. J Clin Virol 26:247–263. [DOI] [PubMed] [Google Scholar]
- Kelly J, Beddall MH, Yu D, Iyer SR, Marsh JW, Wu Y (2008) Human macrophages support persistent transcription from unintegrated HIV-1 DNA. Virology 372:300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevadiya BD, Bade AN, Woldstad C, Edagwa BJ, McMillan JM, Sajja BR, Boska MD, Gendelman HE (2017) Development of europium doped core-shell silica cobalt ferrite functionalized nanoparticles for magnetic resonance imaging. Acta Biomater 49:507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt TJ, Goldsby RA, Osborne BA, Kuby J (2007) Kuby immunology, 6th Edition. New York: W.H. Freeman. [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B (2005) Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 22:643–654. [DOI] [PubMed] [Google Scholar]
- Kotler DP (2005) HIV infection and the gastrointestinal tract. AIDS 19:107–117. [DOI] [PubMed] [Google Scholar]
- Kottilil S, Chun TW, Moir S, Liu S, McLaughlin M, Hallahan CW, Maldarelli F, Corey L, Fauci AS (2003) Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J Infect Dis 187:1038–1045. [DOI] [PubMed] [Google Scholar]
- Koziel H, Kim S, Reardon C, Li X, Garland R, Pinkston P, Kornfeld H (1999) Enhanced in vivo human immunodeficiency virus-1 replication in the lungs of human immunodeficiency virus-infected persons with Pneumocystis carinii pneumonia. Am J Respir Crit Care Med 160:2048–2055. [DOI] [PubMed] [Google Scholar]
- Kutza J, Fields K, Grimm TA, Clouse KA (2002) Inhibition of HIV replication and macrophage colony-stimulating factor production in human macrophages by antiretroviral agents. AIDS Res Hum Retroviruses 18:619–625. [DOI] [PubMed] [Google Scholar]
- Kyei GB, Dinkins C, Davis AS, Roberts E, Singh SB, Dong C, Wu L, Kominami E, Ueno T, Yamamoto A, Federico M, Panganiban A, Vergne I, Deretic V (2009) Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J Cell Biol 186:255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford TD, Letendre SL, Marcotte TD, Ellis RJ, McCutchan JA, Grant I, Mallory ME, Hansen LA, Archibald S, Jernigan T, Masliah E, Group H (2002) Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. AIDS 16:1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson SR, Atif SM, Gibbings SL, Thomas SM, Prabagar MG, Danhorn T, Leach SM, Henson PM, Jakubzick CV (2016) Ly6C(+) monocyte efferocytosis and cross-presentation of cell-associated antigens. Cell Death Differ 23:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douce V, Herbein G, Rohr O, Schwartz C (2010) Molecular mechanisms of HIV-1 persistence in the monocyte-macrophage lineage. Retrovirology 7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T (2017) First aid for the USMLE step 1 2017 New York: McGraw-Hill Medical. [Google Scholar]
- Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW (1999) Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci U S A 96:5215–5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeansyah E, Wines BD, Crowe SM, Jaworowski A (2007) The mechanism underlying defective Fcgamma receptor-mediated phagocytosis by HIV-1-infected human monocyte-derived macrophages. J Immunol 178:1096–1104. [DOI] [PubMed] [Google Scholar]
- Li S, Juarez J, Alali M, Dwyer D, Collman R, Cunningham A, Naif HM (1999) Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates. J Virol 73:9741–9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Tong HI, Gorantla S, Poluektova LY, Gendelman HE, Lu Y (2016) Neuropharmacologic Approaches to Restore the Brain’s Microenvironment. J Neuroimmune Pharmacol 11:484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Esain V, Teng L, Xu J, Kwan W, Frost IM, Yzaguirre AD, Cai X, Cortes M, Maijenburg MW, Tober J, Dzierzak E, Orkin SH, Tan K, North TE, Speck NA (2014) Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes Dev 28:2597–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima VD, Harrigan R, Murray M, Moore DM, Wood E, Hogg RS, Montaner JS (2008) Differential impact of adherence on long-term treatment response among naive HIV-infected individuals. AIDS 22:2371–2380. [DOI] [PubMed] [Google Scholar]
- Locati M, Zhou D, Luini W, Evangelista V, Mantovani A, Sozzani S (1994) Rapid induction of arachidonic acid release by monocyte chemotactic protein-1 and related chemokines. Role of Ca2+ influx, synergism with platelet-activating factor and significance for chemotaxis. J Biol Chem 269:4746–4753. [PubMed] [Google Scholar]
- Lorenzo-Redondo R, Fryer HR, Bedford T, Kim EY, Archer J, Pond SLK, Chung YS, Penugonda S, Chipman J, Fletcher CV, Schacker TW, Malim MH, Rambaut A, Haase AT, McLean AR, Wolinsky SM (2016) Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 530:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani M, Rossi L, Brandi G, Schiavano GF, Montroni M, Piedimonte G (1992) Targeting antiretroviral nucleoside analogues in phosphorylated form to macrophages: in vitro and in vivo studies. Proc Natl Acad Sci U S A 89:6477–6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani M, Balestra E, Fraternale A, Aquaro S, Paiardini M, Cervasi B, Casabianca A, Garaci E, Perno CF (2003) Drug-loaded red blood cell-mediated clearance of HIV-1 macrophage reservoir by selective inhibition of STAT1 expression. J Leukoc Biol 74:764–771. [DOI] [PubMed] [Google Scholar]
- Magnani M, Casabianca A, Fraternale A, Brandi G, Gessani S, Williams R, Giovine M, Damonte G, De Flora A, Benatti U (1996) Synthesis and targeted delivery of an azidothymidine homodinucleotide conferring protection to macrophages against retroviral infection. Proc Natl Acad Sci U S A 93:4403–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marban C, Suzanne S, Dequiedt F, de Walque S, Redel L, Van Lint C, Aunis D, Rohr O (2007) Recruitment of chromatin-modifying enzymes by CTIP2 promotes HIV-1 transcriptional silencing. EMBO J 26:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlink R, Kanki P, Thior I, Travers K, Eisen G, Siby T, Traore I, Hsieh CC, Dia MC, Gueye EH, et al. (1994) Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science 265:1587–1590. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marziali M, De Santis W, Carello R, Leti W, Esposito A, Isgro A, Fimiani C, Sirianni MC, Mezzaroma I, Aiuti F (2006) T-cell homeostasis alteration in HIV-1 infected subjects with low CD4 T-cell count despite undetectable virus load during HAART. AIDS 20:2033–2041. [DOI] [PubMed] [Google Scholar]
- Marzocchetti A, Cingolani A, Giambenedetto SD, Ammassari A, Giancola ML, Cauda R, Antinori A, Luca AD (2005) Macrophage chemoattractant protein-1 levels in cerebrospinal fluid correlate with containment of JC virus and prognosis of acquired immunodeficiency syndrome--associated progressive multifocal leukoencephalopathy. J Neurovirol 11:219–224. [DOI] [PubMed] [Google Scholar]
- Mazzolini J, Herit F, Bouchet J, Benmerah A, Benichou S, Niedergang F (2010) Inhibition of phagocytosis in HIV-1-infected macrophages relies on Nef-dependent alteration of focal delivery of recycling compartments. Blood 115:4226–4236. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Brew BJ, Nath A (2005) Neurological complications of HIV infection. Lancet Neurol 4:543–555. [DOI] [PubMed] [Google Scholar]
- McGrath KE, Frame JM, Palis J (2015) Early hematopoiesis and macrophage development. Semin Immunol 27:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer MS, Skillman DR, Gomatos PJ, Kalter DC, Gendelman HE (1990) Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol 8:169–194. [DOI] [PubMed] [Google Scholar]
- Merad M, Sathe P, Helft J, Miller J, Mortha A (2013) The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 31:563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels K, Nemeth E, Ganz T, Mehrad B (2015) Hepcidin and Host Defense against Infectious Diseases. PLoS Pathog 11:e1004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell JA (2012) Lysosomal acidification mechanisms. Annu Rev Physiol 74:69–86. [DOI] [PubMed] [Google Scholar]
- Moorjani H, Craddock BP, Morrison SA, Steigbigel RT (1996) Impairment of phagosome-lysosome fusion in HIV-1-infected macrophages. J Acquir Immune Defic Syndr Hum Retrovirol 13:18–22. [DOI] [PubMed] [Google Scholar]
- Moser M (2001) Regulation of Th1/Th2 development by antigen-presenting cells in vivo. Immunobiology 204:551–557. [DOI] [PubMed] [Google Scholar]
- Mosoian A, Zhang L, Hong F, Cunyat F, Rahman A, Bhalla R, Panchal A, Saiman Y, Fiel MI, Florman S, Roayaie S, Schwartz M, Branch A, Stevenson M, Bansal MB (2017) Frontline Science: HIV infection of Kupffer cells results in an amplified proinflammatory response to LPS. J Leukoc Biol 101:1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray HW, Cohn ZA (1980) Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med 152:1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JM, Emery S, Kelleher AD, Law M, Chen J, Hazuda DJ, Nguyen BY, Teppler H, Cooper DA (2007) Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS 21:2315–2321. [DOI] [PubMed] [Google Scholar]
- Nicol MQ, Mathys JM, Pereira A, Ollington K, Ieong MH, Skolnik PR (2008) Human immunodeficiency virus infection alters tumor necrosis factor alpha production via Toll-like receptor-dependent pathways in alveolar macrophages and U1 cells. J Virol 82:7790–7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacek AS, Balkundi S, McMillan J, Roy U, Martinez-Skinner A, Mosley RL, Kanmogne G, Kabanov AV, Bronich T, Gendelman HE (2011) Analyses of nanoformulated antiretroviral drug charge, size, shape and content for uptake, drug release and antiviral activities in human monocyte-derived macrophages. J Control Release 150:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrist R, Reilly R, Leavitt T, Knapp RC, Bast RC, Jr. (1983) Monocyte chemotaxis mediated by formyl-methionyl-leucyl-phenylalanine conjugated with monoclonal antibodies against human ovarian carcinoma. Int J Immunopharmacol 5:307–314. [DOI] [PubMed] [Google Scholar]
- Pardo CA, McArthur JC, Griffin JW (2001) HIV neuropathy: insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Syst 6:21–27. [DOI] [PubMed] [Google Scholar]
- Pelchen-Matthews A, Kramer B, Marsh M (2003) Infectious HIV-1 assembles in late endosomes in primary macrophages. J Cell Biol 162:443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho DD (1997) Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387:188–191. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Gendelman HE (2002) Murine models for human immunodeficiency virus type 1-associated dementia: the development of new treatment testing paradigms. J Neurovirol 8 Suppl 2:49–52. [DOI] [PubMed] [Google Scholar]
- Pino M, Erkizia I, Benet S, Erikson E, Fernandez-Figueras MT, Guerrero D, Dalmau J, Ouchi D, Rausell A, Ciuffi A, Keppler OT, Telenti A, Krausslich HG, Martinez-Picado J, Izquierdo-Useros N (2015) HIV-1 immune activation induces Siglec-1 expression and enhances viral trans-infection in blood and tissue myeloid cells. Retrovirology 12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistoia V (1991) Granulocyte-macrophage colony stimulating factor (GM-CSF); sources, targets and mechanism of action. Leukemia 5 Suppl 1:114–118. [PubMed] [Google Scholar]
- Polin RA AS, Rowitch D, and Benitz WE (2017) Fetal and Neonatal Physiology (Fifth Edition). 2:1208–1216. [Google Scholar]
- Poluektova L, Moran T, Zelivyanskaya M, Swindells S, Gendelman HE, Persidsky Y (2001) The regulation of alpha chemokines during HIV-1 infection and leukocyte activation: relevance for HIV-1-associated dementia. J Neuroimmunol 120:112–128. [DOI] [PubMed] [Google Scholar]
- Polyak S, Chen H, Hirsch D, George I, Hershberg R, Sperber K (1997) Impaired class II expression and antigen uptake in monocytic cells after HIV-1 infection. J Immunol 159:2177–2188. [PubMed] [Google Scholar]
- Potula R, Ramirez SH, Knipe B, Leibhart J, Schall K, Heilman D, Morsey B, Mercer A, Papugani A, Dou H, Persidsky Y (2008) Peroxisome proliferator-activated receptor-gamma activation suppresses HIV-1 replication in an animal model of encephalitis. AIDS 22:1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi LA, Maciaszek JW, Rock KL (2005) Both dendritic cells and macrophages can stimulate naive CD8 T cells in vivo to proliferate, develop effector function, and differentiate into memory cells. J Immunol 175:2071–2081. [DOI] [PubMed] [Google Scholar]
- Puligujja P, McMillan J, Kendrick L, Li T, Balkundi S, Smith N, Veerubhotla RS, Edagwa BJ, Kabanov AV, Bronich T, Gendelman HE, Liu XM (2013) Macrophage folate receptor-targeted antiretroviral therapy facilitates drug entry, retention, antiretroviral activities and biodistribution for reduction of human immunodeficiency virus infections. Nanomedicine 9:1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh G, MacLean AG, Philipp MT (2013) Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm 2013:480739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Conway S, Orkin SH, Yoder MC, Mikkola HK (2008) The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell 2:252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson M et al. (1996) Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722–725. [DOI] [PubMed] [Google Scholar]
- Santana-de Anda K, Gomez-Martin D, Soto-Solis R, Alcocer-Varela J (2013) Plasmacytoid dendritic cells: key players in viral infections and autoimmune diseases. Semin Arthritis Rheum 43:131–136. [DOI] [PubMed] [Google Scholar]
- Satpathy AT, Wu X, Albring JC, Murphy KM (2012) Re(de)fining the dendritic cell lineage. Nat Immunol 13:1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F (2012) A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336:86–90. [DOI] [PubMed] [Google Scholar]
- Scriven JE, Rhein J, Hullsiek KH, von Hohenberg M, Linder G, Rolfes MA, Williams DA, Taseera K, Meya DB, Meintjes G, Boulware DR, Team C (2015) Early ART After Cryptococcal Meningitis Is Associated With Cerebrospinal Fluid Pleocytosis and Macrophage Activation in a Multisite Randomized Trial. J Infect Dis 212:769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura E, Valladeau-Guilemond J, Donnadieu MH, Sastre-Garau X, Soumelis V, Amigorena S (2012) Characterization of resident and migratory dendritic cells in human lymph nodes. J Exp Med 209:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu B, Gartner S, Cho M, Liu Y, Pyron N, Valcour V, Shikuma C (2004) Assessment of HIV-1 DNA copies per cell by real-time polymerase chain reaction. Front Biosci 9:255–261. [DOI] [PubMed] [Google Scholar]
- Sillman B, Bade AN, Dash PK, Bhargavan B, Kocher T, Mathews S, Su H, Kanmogne GD, Poluektova LY, Gorantla S, McMillan J, Gautam N, Alnouti Y, Edagwa B, Gendelman HE (2018) Creation of a long-acting nanoformulated dolutegravir. Nat Commun 9:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, McMillan J, Hilaire J, Gautam N, Palandri D, Alnouti Y, Gendelman HE, Edagwa B (2016) Development and characterization of a long-acting nanoformulated abacavir prodrug. Nanomedicine (Lond) 11:1913–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MV, Davidson DC, Jackson JW, Singh VB, Silva J, Ramirez SH, Maggirwar SB (2014) Characterization of platelet-monocyte complexes in HIV-1-infected individuals: possible role in HIV-associated neuroinflammation. J Immunol 192:4674–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slauch JM (2011) How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol Microbiol 80:580–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, Bessis A, Ginhoux F, Garel S (2014) Microglia modulate wiring of the embryonic forebrain. Cell Rep 8:1271–1279. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Witmer MD (1978) Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci U S A 75:5132–5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Cohn ZA (2007) Pillars Article: Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med 1973 137: 1142–1162. J Immunol 178:5–25. [PubMed] [Google Scholar]
- Struyf S, Menten P, Lenaerts JP, Put W, D’Haese A, De Clercq E, Schols D, Proost P, Van Damme J (2001) Diverging binding capacities of natural LD78beta isoforms of macrophage inflammatory protein-1alpha to the CC chemokine receptors 1, 3 and 5 affect their anti-HIV-1 activity and chemotactic potencies for neutrophils and eosinophils. Eur J Immunol 31:2170–2178. [DOI] [PubMed] [Google Scholar]
- Subramanian Vignesh K, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS Jr. (2013) Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity 39:697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Rempel HC, Pulliam L (2004) Loss of macrophage-secreted lysozyme in HIV-1-associated dementia detected by SELDI-TOF mass spectrometry. AIDS 18:1009–1012. [DOI] [PubMed] [Google Scholar]
- Sung TL, Rice AP (2009) miR-198 inhibits HIV-1 gene expression and replication in monocytes and its mechanism of action appears to involve repression of cyclin T1. PLoS Pathog 5:e1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swingler S, Mann AM, Zhou J, Swingler C, Stevenson M (2007) Apoptotic killing of HIV-1-infected macrophages is subverted by the viral envelope glycoprotein. PLoS Pathog 3:1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiwo B, Hicks C, Eron J (2010) Unmet therapeutic needs in the new era of combination antiretroviral therapy for HIV-1. J Antimicrob Chemother 65:1100–1107. [DOI] [PubMed] [Google Scholar]
- Tomlinson GS, Bell LC, Walker NF, Tsang J, Brown JS, Breen R, Lipman M, Katz DR, Miller RF, Chain BM, Elkington PT, Noursadeghi M (2014) HIV-1 infection of macrophages dysregulates innate immune responses to Mycobacterium tuberculosis by inhibition of interleukin-10. J Infect Dis 209:1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R, Cohn ZA (1968) The origin and kinetics of mononuclear phagocytes. J Exp Med 128:415–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL (1972) The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ 46:845–852. [PMC free article] [PubMed] [Google Scholar]
- Walker WE, Kurscheid S, Joshi S, Lopez CA, Goh G, Choi M, Barakat L, Francis J, Fisher A, Kozal M, Zapata H, Shaw A, Lifton R, Sutton RE, Fikrig E (2015) Increased Levels of Macrophage Inflammatory Proteins Result in Resistance to R5-Tropic HIV-1 in a Subset of Elite Controllers. J Virol 89:5502–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Greene WC (2007) Regulation of HIV-1 latency by T-cell activation. Cytokine 39:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2016) People receiving ART by region, 2000–2016 [Google Scholar]
- Zaccheo D, Pistoia V, Castellucci M, Martinoli C (1989) Isolation and characterization of Hofbauer cells from human placental villi. Arch Gynecol Obstet 246:189–200. [DOI] [PubMed] [Google Scholar]
- Zhang G, Guo D, Dash PK, Arainga M, Wiederin JL, Haverland NA, Knibbe-Hollinger J, Martinez-Skinner A, Ciborowski P, Goodfellow VS, Wysocki TA, Wysocki BJ, Poluektova LY, Liu XM, McMillan JM, Gorantla S, Gelbard HA, Gendelman HE (2016) The mixed lineage kinase-3 inhibitor URMC-099 improves therapeutic outcomes for long-acting antiretroviral therapy. Nanomedicine 12:109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman PA et al. (1997) Inherited resistance to HIV-1 conferred by an inactivating mutation in CC chemokine receptor 5: studies in populations with contrasting clinical phenotypes, defined racial background, and quantified risk. Mol Med 3:23–36. [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Uhrlaub J, DeWitt J, Voelker T, Bullock B, Mankowski J, Tarwater P, Clements J, Barber S (2005) Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA 293:2003–2011. [DOI] [PubMed] [Google Scholar]