Abstract
The key principle of gene delivery to articulations by direct intra-articular injection is to release complementary DNA (cDNA)-encoding medical products that will lead to maintained, endogenous production of the gene products within the articulation. In fact, this has been accomplished for both in vivo and ex vivo gene delivery, using several vectors, genes, and cells in some animal models. Some clinical trials for rheumatoid arthritis and osteoarthritis (OA) using retrovirus vectors for ex vivo gene delivery and adeno-associated virus (AAV) for in vivo delivery have been reported. AAV is of special attention because, contrary to other viral vectors, it can enter deep within joint cartilage and transduce chondrocytes in situ. This quality is of special significance in OA, in which modifications in chondrocyte metabolism are believed to be crucial to the pathophysiology of the disease. The clinical effectiveness of TissueGene-C (TG-C), a cell and gene therapy for OA consisting of nontransformed and transduced chondrocytes (3:1) retrovirally transduced to overexpress TGF-β1 has been reported in patients with knee OA. The most common complications of TG-C were peripheral edema (9%), arthralgia (8%), articular swelling (6%), and injection site pain (5%). TG-C was associated with relevant ameliorations in function and pain. Gene therapy appears to be a viable method for the management of articular cartilage defects and OA.
Key Words: Cartilage, Gene therapy, Injury, Repair
Introduction
Diverse articular pathologies such rheumatoid arthritis (RA), osteochondritis dissecans, osteonecrosis, and hemarthrosis following trauma or due to hemophilia can damage articular cartilage as well as other joint structures, resulting in small focal injuries leading to large amounts of joint degeneration (osteoarthritis [OA]) (1). The common target of the aforementioned pathological processes is the osteochondral unit. The stimulation of inflammatory processes within the joint causes a progressive degeneration of cartilaginous extracellular matrix (ECM) and activation of osteoclasts, with subsequent deterioration of subchondral bone (2). Localized small osteochondral defects usually undergo a natural healing process; however, this results in formation of fibrocartilaginous tissue, which is neither durable nor integrated into the adjacent cartilage. This is attributable to cartilage features, including the avascular and aneural nature of adult cartilage, reduced cartilage cell density, and a poorly organized ECM (2). The healing process is mediated by activated synoviocytes; however, their activity is frequently inappropriate, and more significant pathology can result (3). An archetypical management strategy for these injuries should produce restoration of hyaline articular cartilage, which is integrated into the surrounding healthy tissues, conferring mechanical resilience and longevity.
Diverse surgical methods aimed to enhance the delivery of circulating mesenchymal stem cells (MSCs) to the avascular cartilage, such as drilling of the subchondral osseous mater, abrasion arthroplasty, microfractures, transfer of an autologous osteochondral complex, mosaicplasty for small focal defects, autologous chondrocyte implantation (ACI), and matrix-induced ACI (MACI) for larger focal chondral and osteochondral injuries, have resulted in some successes; especially in younger patients. Despite these advances, no procedure to date has been able to repair joint cartilage to the (near) preinjury state (4, 5). In addition, these techniques have disadvantages, such as problems in donor identification, graft rejection, or intolerance, and additional degradative alterations attributable to two-stage surgery (6). The management of widespread OA injuries is even more complicated.
Following application of any of these techniques, as noted previously, the new cartilage that is produced has as its principal components type I collagen rather than type II collagen, and proteoglycans characteristic of the hyaline cartilage, giving it a fibrocartilaginous composition precluding long-lasting mechanical attributes and integration into the adjacent healthy cartilage (7). Therefore, new treatments are required. MSCs are multipotent stem cells found in the bone marrow, adipose tissue, and peripheral blood that are capable of differentiating into osteocytes, adipocytes, chondrocytes, and other cells native to the joint. As such, MSCs offer a tremendous potential for cartilage repair; however, over time, a fibrocartilage phenotype predominates over that of hyaline cartilage, limiting the benefits. Therefore, ameliorating this change in phenotype is one of the major hurdles to successful cartilage repair. Introduction of various transgenes into the MSCs might obviate this problem by promoting the chondrogenic phenotype.
Recently, encouraging reports of the success of gene therapy (GT) have appeared for sickle cell disease, hemophilia, and adrenoleukodystrophy, engendering growing enthusiasm for this technology to be applied to cartilage repair, especially for OA (8–10). The possibility of reprogramming cells present in the joint, and in particular the cartilage, through transduction of genes conferring favorable functions, such as modulating inflammation and conferring cellular longevity, or persistence of the hyaline cartilage phenotype by production of type II collagen and proteoglycans, is encouraging to address the complications of OA and other pathologies (11).
Several recent reports have focused on expressing therapeutic proteins following GT to enhance cartilage repair (12–14). These reports have used different vectors to deliver membrane repair proteins, transcription factors, and growth factors to augment cellular synthesis and control paracrine cascades [Table 1] (12, 15, 16).
Table 1.
Classes of expression vectors in gene therapy (GT)
| NON-VIRAL VECTORS |
|---|
| We need to transfer am expression vector (commonly a plasmid) in recipient cells. We can use physical methods (in vivo electroporation, ultrasounds) or chemical methods. The aforementioned methods are secure, easy to perform and cost-effective. However, their delivery is less efficient than in viral vectors. After their introduction into the cells, non-viral vectors commonly persist in the cytoplasm. There, they will express the specific gene protein. |
| VIRAL VECTORS |
| They are adenoviruses, recombinant adeno-associated viral (rAAV), retroviruses, and baculoviruses. Currently, viral vectors are preferred with most research focusing on the use of recombinant adeno-associated viral vectors to deliver all types of genes for all types of cartilage pathology. Currently there are about 50 adenovirus serotypes available for GT. Serotype 5 (Ad5) is the mostly used in all kind of studies (in vitro and in vivo). |
There are two main classes of expression vectors in GT: nonviral and viral. In nonviral vectors we need to transfer an expression vector (commonly a plasmid) to recipient cells. We can use physical methods (in vivo electroporation, ultrasounds) or chemical methods. The aforementioned methods are secure, easy to perform, and cost-effective. However, their delivery is less efficient than viral vectors. After their introduction into the cells, nonviral vectors commonly persist in the cytoplasm. There, they will express the specific gene protein. Viral vectors are adenoviruses, recombinant adeno-associated viral (rAAV), retroviruses, and baculoviruses. Currently, viral vectors are preferred, with most research focusing on the use of rAAV vectors to deliver all types of genes for all types of cartilage pathology. Currently, approximately 50 adenovirus serotypes are available for GT. Serotype 5 (Ad5) is the mostly frequently used serotype in all studies (in vitro and in vivo).
Despite these encouraging results, the principal limiting factor in the effectiveness of GT is the transitory expression of the gene product, without regard to the vector used. For example, one strategy of GT for cartilage repair has targeted augmented growth factor synthesis; yet, the gene product was expressed for up to 2 weeks, an insufficient amount of time to provide therapeutic benefit (17, 18). Furthermore, the maximal concentrations of growth factors are observed in the first days following transduction, which can lead to supratherapeutic or toxic levels of the growth factor (19). The aim of this article is to review current knowledge on the role of GT in cartilage repair and OA.
Materials and Methods
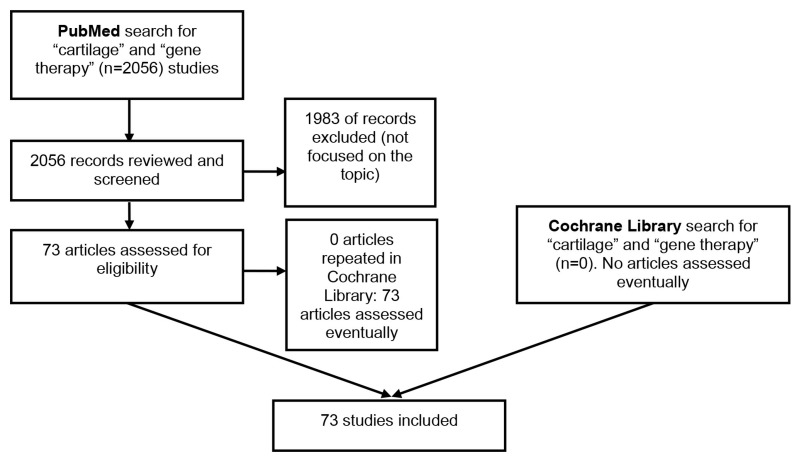
A Cochrane Library and PubMed (MEDLINE) search related to the role of GT in cartilage repair was performed and the output analyzed by both authors. Figure 1 shows our search strategy (PubMed/Medline and Cochrane Library). The search was limited to the English language. Search terms included the following: cartilage repair AND restoration and gene therapy AND transfer. The search resulted in 2056 articles, of which 73 citations were selected for further quantitative analysis. Scientific meeting abstracts and other sources of evidence were not considered. The main criteria for selection of articles to be included in this review were that they were focused on the role of GT in cartilage repair. The search parameters were from the beginning of the search engines (PubMed and Cochrane Library) until 18 June 2018.
Figure 1.
Flow chart of our search strategy regarding the role of gene therapy in cartilage injuries
Results
Recent data on transduction methods (nonviral, viral), genes of interest, and methods of delivery (direct, indirect)
The intent of GT is to treat human disease or illnesses by way of gene transfer to express the transgene in specific target cells in an effort to ameliorate the symptomatology (20). Compared with recombinant protein replacement treatment, in which the half-life of protein factors is very short, gene-based therapies conceivably permit longer-lasting, targeted, location-specific expression of a protein of interest, in a more physiologically relevant way and potentially with long-lasting consequences (21).
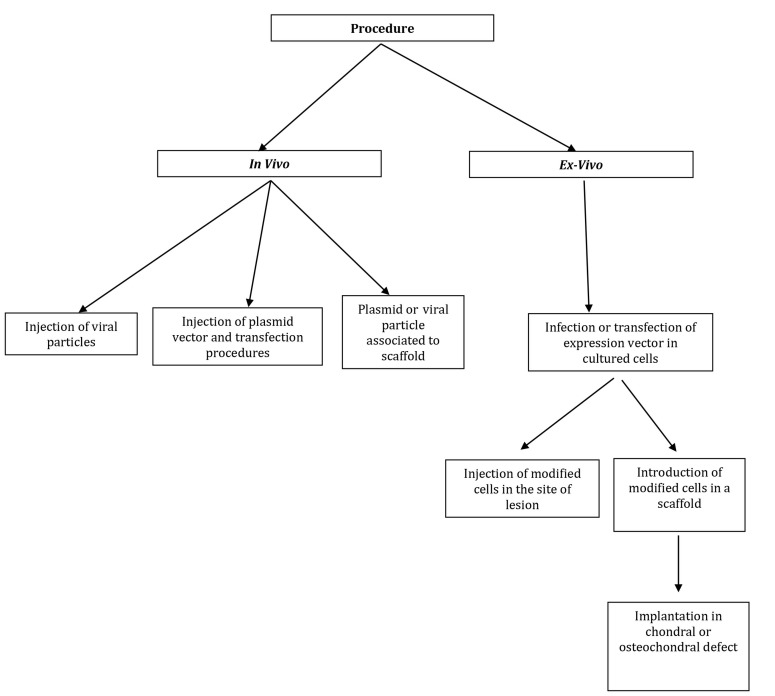
The concept of using GT for the regeneration of cartilaginous tissue began with the notion that the expression of certain genes in the lesion or defect might augment the repair cascade (22). Gene transfer to joint cartilage cells has recently made notable progress. Gene transfer can be performed in vivo (introduction of the vector and transgene directly into the joint) or ex vivo (introduction of the vector and transgene into explants of cells taken from the joint) then reintroduced into the joint. Such reintroduction methods can be associated with or without the use of a scaffold. In vitro repair of cartilage tissue can be achieved either by means of expression of genes that augment differentiation of the cartilaginous tissue or by genes that downregulate other deleterious pathways or adverse factors (22).
Despite many advances in the field of joint cartilage regeneration, there remains a lack of definitive evidence of clinical translation. The combination of freshly generated cartilaginous tissue and the repair of all cartilage-typical local architectural organizations are paramount for the proper functioning of the joint cartilage; however, until now, no method has been capable of generating a natural cartilaginous structure in the articulations (23).
Remaining obstacles that impede progress in GT for cartilage regeneration and osteoarthritis management include the influence of stem cells on chondrogenesis, which frequently results in new bone formation and bone hypertrophy, and the small number of cartilage cells that can be effectively transduced (24). The combination of novel expression vectors, newly designed genes targeting specific articular cells, and the provision of necessary scaffolds can result in the production of hyaline cartilage, although the nature of the injury (size, localization, structure) might require different combinations of the aforementioned factors (25).
The genes that can be transfected into and subsequently expressed by chondrocytes to ameliorate or reduce their phenotypic transition to that of fibrocartilage are numerous [Table 2] (11). The approach explored thus far consists of expression of genes that encode for growing factors (GFs) that lead to chondrogenic differentiation.
Table 2.
Genes whose overexpression can augment the generation of diverse proteins which are paramount to ameliorate healing of cartilaginous tissue
|
Growth Factors
IGF-1, TGF-b, BMPs, FGF-2, GDF-5, VEGF antagonist |
|
Transcription Factors
SOX genes (5, 6 and 9) ZNF145 |
|
Anti-Inflammatory Cytokines
IL10, IL1ra |
|
Mirna
MiR-23b, miR-140, miR-181b, miR221, miR-145, miR-335 |
|
Other Factors
Cell signaling protein iHH, ECM component (COMP), integrin b 1 |
| IGF-1=Insulin-like growth factor-1; TGF-b=Transforming growth factor b; BMPs=Bone morphogenetic proteins; FGF-2; Fibroblast growth factor 2; GDF-5=Growth and differentiation factor 5; VEGF=Vascular endothelial growth factor; SOX=Sex-determining Region Y-related High Mobility Group box; ZNF145=Zinc-finger protein 145; IL10=Interleukin 10; IL1ra=Interleukin 1 receptor antagonist; miRNA=Micro RNA; iHH=Indian hedgehog homolog; ECM=Extracellular matrix; COMP=Cartilage oligomeric matrix protein. |
Delivery of the transgene can be achieved by viral or nonviral methods. Currently, viral vectors are preferred, with most research focusing on the use of rAAV vectors to deliver all types of genes for all types of cartilage pathology. The clinical application of GT currently appears to be distant from the clinic due to questions of both safety and effectiveness. The risk benefit assessment of GT applied to cartilage restoration is an important area of debate. The nonlethal nature of illnesses of cartilaginous tissue and the potential adverse effects of GT highlight the importance of this debate (23). Some authors fear that the use of viral vectors might not be safe, primarily due to concerns for carcinogenesis and the development of severe adverse events such as leukemia. Patients with X-linked severe combined immunodeficiency who received a retroviral medicated gene therapy developed leukemia, and individuals with RA receiving intra-articular rAAV-2 injection to express an antagonist to transforming growth factor α (TNF-α) all had fatal outcomes (26). Although these deaths were not clearly associated with viral vectors, the concerns remain, and appropriate monitoring for carcinogenesis has been emphasized (26).
The expression of GFs and anti-inflammatory cytokines are of interest to treat cartilaginous disorders and are being studied in phase I and II GT trials to assess their potential toxicity, biological activity, and biological distribution. TGF-β expressed by cartilage cells following transduction with a retrovirus is being studied, as is expression of an interleukin 1 receptor antagonist (IL1ra) expressed in fibroblasts, either following transduction with adenoviruses, or by means of direct injection into the injuries. Retroviruses and adenoviruses or self-complementary adeno-associated virus (scAVV) are the only vectors that have been analyzed in clinical studies until now. To produce efficient GT procedures that lessen the risk of adverse consequences (e.g., host immune response) it is compulsory to increase our knowledge of several basic questions in molecular and cellular biology, immunology, and virology, all of which transcend cartilage repair and apply more generally to the field of GT. More work should focus on the identification of how scaffold transduced cells and the microenvironment interact. This information will provide the knowledge needed to ensure the success of GT for cartilage repair following cartilaginous and/or osteochondral injuries by creating an equilibrium between the treatment of transitory articular mechanical incompetence and affected metabolic and inflammatory homeostasis.
Vectors that can be used
A recent report has summarized the latest clinical trials on GT in RA and OA. These studies are using retroviral vectors for ex vivo gene delivery and adeno-associated virus (AAV) for in vivo delivery (27). Unlike other viral vectors, AAV has the advantage of penetrating deeply within articular cartilage to transduce chondrocytes in situ. In Korea, the first worldwide study on gene therapy in OA has been approved by regulatory authorities. This trial is aimed at expressing transforming growth factor-β1 in allogeneic chondrocytes after ex vivo transduction with a retrovirus. According to Evans et al., two phase III studies will begin soon in the US. Meanwhile, two additional phase I studies using AAV have been posted to the ClinicalTrials.gov website: the first trial, on RA, will examine the effects of interferon-β expression; the other, on OA, focuses on the role of IL1ra expression on the disease phenotype (27).
Lentiviral vectors and induced pluripotent stem cells
According to Ying et al., it is well-known that transforming growth factor-β1 (TGF-β1) is a chondrogenic factor that can augment chondrocyte differentiation from bone marrow mesenchymal stem cells (BMSCs) (28). In animal models of full thickness cartilage defects, these authors investigated the role of TGF-β1 in the repair of cartilage defects and the ability of TGF-β1 to prevent chondrocyte hypertrophy (28). In these preclinical experiments, TGF-β1 appeared to be beneficial in promoting chondrogenic differentiation from BMSCs via the canonical Smad pathway to facilitate repair of cartilage defects (28). Moreover, TGF-β1 was able to reduce chondrocyte hypertrophy evidenced by reduced expression of the cell hypertrophy marker gene, Hippo. This experiment supports the notion that long-term expression of TGF-β1 might be useful to repair cartilage through a regeneration pathway.
Table 1 summarizes the current knowledge of the genes to be expressed in chondrocytes. Plasmids (nonviral expression vectors) and adenoviral vectors are commonly used in preclinical research, mainly to express a transcription factor in an effort to increase anti-inflammatory cytokines. Other less commonly explored substances are proteins related to cell signaling, matrix proteins, and receptors.
The expression vector can be introduced into chondrocytes directly or indirectly, with or without the addition of scaffolds. Once transduced with specific genes, chondrocytes treated ex vivo can be implanted into the cartilage lesion.
The role of insulin-like growth factor-1
One of the proteins frequently examined for its effects on cartilage repair is insulin-like growth factor-1 (IGF-1) (18, 19, 29, 30). Viral and nonviral vectors have been used to transduce chondrocytes ex vivo to upregulate IGF-1 production before therapeutic cell delivery (18, 31–33). Although increased IGF-1 expression can persist for a month or more, its expression diminishes over time, and is frequently suboptimal by 2 weeks (19, 35, 36). This is a remarkably short time compared with the 6–12 months ordinarily required for effective cartilage repair and is not likely to produce beneficial cartilage repair in vivo (36). The time framework provided here has been reported for ACI.
Accordingly, there is a necessity to develop methods to prolong the expression of IGF-1 in cartilage cells. In the cartilage, IGF-1 is bound by a family of binding proteins termed insulin-like growth factor binding proteins (IGFBPs). IGFBPs are specific for IGF-1, with binding affinities of 1–10 nM that localize IGF-1 to cartilage matrix as well as acting as both a sink and a source for the growth factor required for chondrocyte health (37, 38). IGFBP-5 has demonstrated a small molecular domain, accounting for its high affinity binding to IGF-1, making this an attractive target for GT to promote cartilage repair (37).
Supplemental biomaterials to enhance gene therapy
Cell-based GT for articular cartilage repair requires a means for directing the potentially beneficial cells to the site of injury and therefore to the area in need of repair (39). Table 2 summarizes the type of biomaterials used for the different gene transfer vectors currently used in GT for cartilage repair (40–45).
For example, chondrocytes have been cultured in fabricated chitosan and a plasmid DNA scaffold to promote cell proliferation, adherence, and transforming growth factor-β1 (TGF-β1) expression; and chitosan-plasmid-encoding green fluorescent protein nanoparticles have been used to transfer exogenous genes into primary chondrocytes for the management of articular disease (46, 47). Trimethylated chitosan (TMO) has been synthesized from oligometric chitosan to release luciferase plasmid DNA to epithelial cells (48). Poly-L-lactic acid has been used with MSCs transfected with adenoviral vector designed to express SOX-9 in an effort to differentiate monolayer MSCs into chondrocyte-like cells (49). Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds have been seeded with chondrocytes expressing SOX-9 (50). Modified polyethylenimine showed lower toxicity and higher gene expression following introduction of plasmid DNA into COS-7 cells and HepG2cells (51). Polyethylene glycol-grafted polyethylenimine has been tested with adipose stem cells to differentiate them from cartilage or osteoblast cells, and hydrogels, including alginate, have also been shown to achieve this objective (52, 53). There are many examples of short peptide sequences being grafted to materials to augment cell adhesion (19, 54, 55). In a similar manner, materials have been modified to have heparin-like carbohydrate components, which have been demonstrated to increase binding of growth factors such as fibroblast growth factor 2 (56). All the preceding materials have been tested for their ability to promote gene delivery and to prolong expression, but none have demonstrated the beneficial effect desired.
Aguilar et al. have recently developed a scaffold material which can bind the targeted gene product (57). This new scaffold material had a high affinity for IGF-1 due to the addition of a binding peptide sequence from IGFBP-5 onto alginate. This novel material greatly increased the availability of the growth factor during chondrocyte culture and augmented cartilage matrix biosynthesis up to 19-fold. These investigators showed that modifying alginate with the peptide sequence KPLHALL (K = lysine; P = proline; L = leucine; H = histidine; A = alanine) from the binding pocket of IGFBP-5 augmented IGF-1 binding affinity more than 10-fold; and in turn, prolonged IGF-1 availability over 30 days and augmented glucosamine glycans and hydroxyproline synthesis 7- and 20-fold, respectively. This work suggests a role for the addition of small peptides from growth factor binding proteins to biomaterials to enhance drug delivery and tissue engineering.
Transplantation of genetically modified peripheral blood aspirates
The articular cartilage facilitates the normal gliding of the joint surfaces in diarthrodial articulations (58). A key deficiency in repair of damaged hyaline cartilage following injury is the lack of vascularization of adult cartilage precluding the delivery of restorative MSCs (59). Interestingly, some recent evidence indicates the existence of competent peripheral blood MSCs (PB-MSCs) capable of repairing cartilage (60, 61). These PB-MSCs are a readily available source of cells capable of cartilage regeneration (60–63).
Although these studies have shown encouraging results, natural-appearing hyaline cartilage has not been achieved, which could be due to the low representation of MSCs in the blood (0.0002%) (64). One tactic to increase the effectiveness of PB-MSCs is to introduce bioactive proteins following GT as described previously, but also to artificially create a normal cellular and biological microenvironment using biochemical factors and other cell types, such as hematopoietic cells and fibroblasts, which have crosstalk with PB-MSCs (65).
According to Frisch et al., transplantation of genetically modified peripheral blood aspirates containing PB-MSCs could provide a novel opportunity to manage cartilage lesions (66). Such a method is easier than invasive methods (which use bone marrow concentrates or bone marrow-derived MSCs). Using rAAV to overexpress TGF-β in PB-MSCs augments their proliferative and metabolic characteristics and promotes chondrogenic differentiation as well as osteogenic differentiation and hypertrophy. These data support the possibility of a new method to manage cartilage injuries: direct modification of peripheral blood (66).
Transplantation of genetically modified bone marrow aspirates
In 2004 Pascher et al. studied gene delivery to cartilage defects using coagulated bone marrow aspirate (67). Their results suggested that coagulates formed from aspirated bone marrow can be useful as a means of gene delivery to cartilage. Cells within the fluid can be readily modified with an adenoviral vector, and the matrix formed from the clot is completely natural, native to the host, and is the fundamental platform on which healing and repair of mesenchymal tissues is based.
In 2017, Venkatesan et al. analyzed the impact of mechanical stimulation on the chondrogenic processes in human bone marrow aspirates modified to overexpress SOX-9 via rAAV vectors (68). Their findings showed the value of genetically modifying human bone marrow aspirates upon mechanical stimulation by rAAV SOX-9 as a promising strategy for future treatments to improve cartilage repair by implantation in lesions where the tissue is submitted to natural mechanical forces.
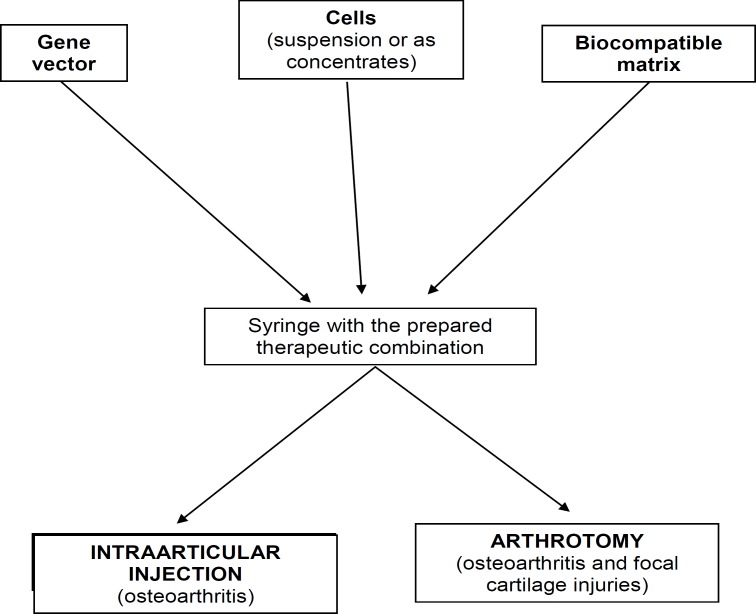
Table 3 summarizes the main advantages and limitations of common available vectors for GT; Figure 2 summarizes experimental methods for the delivery of therapeutic gene sequences in areas of joint cartilage lesions; and Figure 3 shows GT procedures to deliver genes into cartilaginous defects. Table 4 summarizes the advantages and disadvantages of current vectors for GT.
Table 3.
Types of biomaterials used for the different gene transfer vectors currently used in gene therapy for cartilage repair
| Author | Year | Biomaterial Used | Comments |
|---|---|---|---|
| Wang et al (43) | 2008 | PHBHHx | The results of this study demonstrated that PHBHHx is a useful material for cartilage tissue engineering. |
| Chen et al (44) | 2013 | PEI | 3.0-T MRI in vivo tracking of PEI/SPIO-labeled bone marrow-derived mesenchymal stem cells seeded in type II collagen gel on cartilage repair following transplantation is feasible in minipigs. |
| Shafiee (42) | 2016 | PLLA | Cells were seeded onto aligned electrospun PLLA/poly (ε-caprolactone) nanofibrous scaffolds. The aligned nanofibrous hybrid scaffolds could support the proliferation and chondrogenic differentiation of all cell types. |
| Dey et al (45) | 2016 | PEG | The chondrocyte viability in dPGS hydrogels is found to be higher than in pure PEG and alginate-based hydrogels after 21 d. The higher cell viability in the dPGS engineered hydrogels can be explained by the fact that dPGS can interact with different proteins responsible for cell growth and proliferation. |
| Chen et al (40) | 2017 | Chitosan | The combination of photo-crosslinked hydrogel and crizotinib-loaded chitosan microspheres might represent a promising strategy for osteoarthritis treatment. |
| Shi et al (41) | 2018 | Chitosan + PEG | Folate-PEG-CH-DEAE15 nanoparticles are a safe and effective platform for nonviral gene delivery of siRNA, and their potential clinical applications warrant further investigation. |
Figure 2.
Experimental methods for the delivery of theraoeutic gene sequences in areas of joint cartilage lesions
Figure 3.
Gene therapy procedures to deliver genes into cartilaginous defects
Table 4.
Advantages and disadvantages of current vectors for gene therapy
| Viral Vectors | ||
|---|---|---|
| Types | Advantages | Disadvantages |
| Adenovirus | High effectiveness | Immunogenic; toxic; short-run expression; replication competence |
| Retro-/Lentivirus | High effectiveness; long-run expression | Insertional mutagenesis; replication competence |
| Herpes Simplex Virus (HSV) | High effectiveness; substantial capacity | Cytotoxic; short-run expression |
| Recombinant adeno-associated virus (rAAV) | High effectiveness; long-run expression; low immunogenic | Difficult to produce; size limitation |
Nonviral Vectors
Advantages: Not toxic; not infections; easy to make; substantial capacity
Disadvantages: Low effectiveness; short-run expression
Discussion
Recent studies have focused on expressing therapeutic proteins using GT to augment repair of cartilage. These reports have focused on the delivery of membrane repair proteins, transcription factors, and growth factors. Much of the work has focused on the expression of IGF-1. Despite the impressive advances made to date, the principal limiting factor in the effectiveness of cartilage repair following GT has been the transient expression of the gene product, irrespective of the vector used. A second factor limiting the benefits of GT in cartilage repair has been the suboptimal concentrations of target proteins achieved, such as growth factors. On the other hand, in an effort to increase the expression of the target protein, supra-therapeutic or toxic concentrations are frequently observed in the first few days following GT.
Several viral vectors and nonviral methods have been used to directly introduce the gene of interest (for example, IGF-1) into chondrocytes. Irrespective of the method used to introduce the transgene, the duration of expression is insufficient (typically only 2–4 weeks) compared with the duration needed for cartilage repair (6–12 months). One way to circumvent this problem is the introduction of binding motifs for the target protein to prolong its residence time. For example, following the introduction of IGFBPs into scaffold materials such as alginate, the effective time of IGF-1 in cartilage after GT was prolonged for at least 30 days, and its biosynthesis increased up to 19-fold. Despite this improvement, the duration of expression remains insufficient to effect cartilage regeneration.
Cell-based GT for articular cartilage repair is an indirect method of expressing the target protein. This has been supported by the increase in knowledge of the role biomaterial scaffolds play to prolong the expression and increase the concentration of the gene product. Despite improvements, more work is needed to optimize this approach.
To date, no technique has demonstrated the ability to produce natural-appearing and functioning hyaline cartilage in the articulations. GT might offer the possibility to deliver on this goal of cartilage repair for OA and other cartilage pathologies; however, a number of hurdles persist and must be overcome. For instance, inducing stem cells into cartilage formation usually produces an insufficient amount of cartilage cells or chondral progenitors. Also, bone formation and bone hypertrophy are commonly found.
A recent report of Grol and Lee stated that in recent years, several therapeutic gene approaches (termed ‘monotherapies’) have demonstrated effectiveness in preclinical models of disease, and a number of them are being assessed in clinical trials (69). In particular, an ex vivo TGF-β1 gene therapy was approved in Korea in 2017 for the management of moderate-to-severe OA. The ability to use viral vectors for context-specific and combinatorial GT is also being investigated; these strategies are likely to be important in forthcoming studies addressing the complexities of tissue repair and regeneration in skeletal disease.
In February 2018, Bellavia et al. stated that GT might represent a promising strategy for chondral and osteochondral defect repair (70). They analyzed preclinical and clinical studies on GT for the repair of articular cartilage defects performed over the last 10 years, focusing on expression vectors (nonviral and viral), type of genes delivered, and GT procedures (direct or indirect). Plasmids (nonviral expression vectors) and adenoviruses (viral vectors) were the vectors most frequently used in preclinical studies. The genes delivered encoded mainly for growth factors, followed by transcription factors, anti-inflammatory cytokines, and less commonly by cell signaling proteins, matrix proteins, and receptors. Direct injection of the expression vector was used less than indirect injection of cells, with or without scaffolds, transduced with genes of interest, and then implanted into the lesion site. Clinical trials (phases I, II, or III) on safety, biological activity, efficacy, toxicity, and biodistribution used adenovirus viral vectors to deliver growth factors or anti-inflammatory cytokines for the management of OA, and tumor necrosis factor receptor or interferon for the treatment of inflammatory arthritis.
In 2018, Kim et al. reported two studies on the clinical efficacy of TissueGene-C (TG-C), a cell and gene therapy for knee OA in humans consisting of nontransformed and transduced chondrocytes (3:1) retrovirally transduced to overexpress TGF-β1 (71, 72). TG-C was associated with statistically significant improvements in function and pain in patients with knee OA. The most frequent adverse events in the TG-C group were peripheral edema (9%), arthralgia (8%), joint swelling (6%), and injection site pain (5%). Watson Levings et al. have recently reported the results of an equine model showing that scAAV.IL-1Ra administration is reasonably safe and capable of sustained therapeutic IL-1Ra production intra-articularly in joints of human scale. These data support consideration for human testing in OA (73).
Gene therapy involves the use of viral and nonviral vectors to deliver nucleic acids to tissues using direct (in vivo) or transduced cell-mediated (ex vivo) methods. In preclinical studies, gene therapy has been successfully used to manage cartilaginous injuries. Gene therapy is also being evaluated in clinical trials for its security and therapeutic power in OA. Thus far, a number of alternatives have been elected to express therapeutic transgenes at places of injury to favor or ease cartilage repair. Objectives of interest have essentially included secreted proteins such as growth factors and anti-inflammatory mediators; albeit, work has also started to focus on intracellularly on signaling components, transcription factors, and small, regulatory nucleic acids such as microRNAs. Lately, some single therapeutic gene methods (monotherapies) have shown effectiveness in preclinical models of disease, and some are being assessed in clinical trials. It is noteworthy that an ex vivo TGF-β1 gene therapy was authorized in Korea in 2017 for management of moderate-to-severe OA. This therapy, TissueGene-C (TG-C), is a cell and gene therapy for OA consisting of nontransformed and transduced chondrocytes (3:1) retrovirally transduced to overexpress transforming growth factor-β1; it has led to relevant ameliorations in function and pain in patients with knee OA. GT appears to be a viable therapeutic alternative for the management of articular cartilage defects and OA.
Until gene therapy can be used as an effective technique in the treatment of idiopathic osteoarthritis (cartilage degeneration) of the knee, we will have to continue using the usual surgical treatments, namely high tibial osteotomy (HTO), UKA (unicompartmental knee arthroplasty) and TKA (total knee arthroplasty) (74-76).
Conflicts of Interest:
The authors have declared that they have no conflicts of interest. LAV is an employee and stock holder of Spark Therapeutics; however, this study was performed in an academic capacity as a member of the faculty of Rush University.
References
- 1.Rey-Rico A, Frisch J, Venkatesan JK, Schmitt G, Rial-Hermida I, Taboada P, et al. PEO-PPO-PEO carriers for rAAV-mediated transduction of human articular chondrocytes in vitro and in a human osteochondral defect model. ACS Appl Mater Interfaces. 2016;8(32):20600–13. doi: 10.1021/acsami.6b06509. [DOI] [PubMed] [Google Scholar]
- 2.Frisch J, Orth P, Venkatesan JK, Rey-Rico A, Schmitt G, Kohn D, et al. Genetic modification of human peripheral blood aspirates using recombinant adeno-associated viral vectors for articular cartilage repair with a focus on chondrogenic transforming growth factor-β gene delivery. Stem Cells Transl Med. 2017;6(1):249–60. doi: 10.5966/sctm.2016-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunziker EB. Articular cartilage repair: basic science and clinical progress A review of the current status and prospects. Osteoarthr Cartilage. 2002;10(6):432–63. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Merchan EC. The treatment of cartilage defects in the knee joint: Microfracture, mosaicplasty, and autologous chondrocyte implantation. Am J Orthop. 2012;41(5):236–9. [PubMed] [Google Scholar]
- 5.Rodriguez-Merchan EC. Regeneration of articular cartilage of the knee. Rheumatol Int. 2013;33(4):837–45. doi: 10.1007/s00296-012-2601-3. [DOI] [PubMed] [Google Scholar]
- 6.Veronesi F, Giavaresi G, Tschon M, Borsari V, Nicoli Aldini N, Fini M, et al. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 2013;22(2):181–92. doi: 10.1089/scd.2012.0373. [DOI] [PubMed] [Google Scholar]
- 7.Madry H, Grun UW, Knutsen G. Cartilage repair and joint preservation: Medical and surgical treatment options. Dtsch Arztebl Int. 2011;108(40):669–77. doi: 10.3238/arztebl.2011.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribeil JA, Hacein-Bey-Abina S, Payen E, Magnani A, Semeraro M, Magrin E, et al. Gene therapy in a patient with sickle cell disease. N Engl J Med. 2017;376(9):848–55. doi: 10.1056/NEJMoa1609677. [DOI] [PubMed] [Google Scholar]
- 9.George LA, Sullivan SK, Giermasz A, Rasko JE, Samelson-Jones BJ, Ducore J, et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med. 2017;377(23):2215–27. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713–22. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 11.Ondrésik M, Azevedo Maia FR, da Silva Morais A, Gertrudes AC, Dias Bacelar AH, Correia C, et al. Management of knee osteoarthritis Current status and future trends. Biotechnol Bioeng. 2017;114(4):717–39. doi: 10.1002/bit.26182. [DOI] [PubMed] [Google Scholar]
- 12.Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y, et al. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci Transl Med. 2012;4(139):139ra85. doi: 10.1126/scitranslmed.3003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med. 2009;15(3):331–7. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartus K, James ND, Didangelos A, Bosch KD, Verhaagen J, Yáñez-Muñoz RJ, et al. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci. 2014;34(14):4822–36. doi: 10.1523/JNEUROSCI.4369-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kafienah W, Al-Fayez F, Hollande AP, Barker MD. Inhibition of cartilage degradation: a combined tissue engineering and gene therapy approach. Arthritis Rheum. 2003;48(3):709–18. doi: 10.1002/art.10842. [DOI] [PubMed] [Google Scholar]
- 16.Evans CH, Gouze JN, Gouze E, Robbins PD, Ghivizzani SC. Osteoarthritis gene therapy. Gene Ther. 2004;11(4):379–89. doi: 10.1038/sj.gt.3302196. [DOI] [PubMed] [Google Scholar]
- 17.Nixon AJ, Saxer RA, Brower-Toland BD. Exogenous insulin-like growth factor-I stimulates an autoinductive IGF-I autocrine/paracrine response in chondrocytes. J Orthop Res. 2001;19(1):26–32. doi: 10.1016/S0736-0266(00)00013-9. [DOI] [PubMed] [Google Scholar]
- 18.Shi S, Mercer S, Trippel SB. Effect of transfection strategy on growth factor overexpression by articular chondrocytes. J Orthop Res. 2010;28(1):103–9. doi: 10.1002/jor.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu P, Wang X, Fu YX. Enhanced local delivery with reduced systemic toxicity: delivery, delivery, and delivery. Gene Ther. 2006;13(15):1131–2. doi: 10.1038/sj.gt.3302760. [DOI] [PubMed] [Google Scholar]
- 20.Bellavia D, Veronesi F, Carina V, Costa V, Raimondi L, De Luca A, et al. Gene therapy for chondral and osteoachndral regeneration: is the future now? Cell Mol Life Sci. 2018;75(4):649–67. doi: 10.1007/s00018-017-2637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi S, Chan AG, Mercer S, Eckert GJ, Trippel SB. Endogenous versus exogenous growth factor regulation of articular chondrocytes. J Orthop Res. 2014;32(1):54–60. doi: 10.1002/jor.22444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li KC, Hu YC. Cartilage tissue engineering: recent advances and perspectives from gene regulation/therapy. Adv Healthc Mater. 2015;4(7):948–68. doi: 10.1002/adhm.201400773. [DOI] [PubMed] [Google Scholar]
- 23.Steinert AF, Weissenberger M, Kunz M, Gilbert F, Ghivizzani SC, Göbel S, et al. Indian hedgehog gene transfer is a chondrogenic inducer of human mesenchymal stem cells. Arthritis Res Ther. 2012;14(4):R168. doi: 10.1186/ar3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somoza RA, Wleter JF, Correa D, Kaplan AI. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng Part B Rev. 2014;20(6):596–608. doi: 10.1089/ten.teb.2013.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu CH, Yeh TS, Yeh CL, Fang YH, Sung LY, Lin SY, et al. Regenerating cartilages by engineered ASCs: prolonged TGF-b3/BMP-6 expression improved articular cartilage formation and restored zonal structure. Mol Ther. 2014;22(1):186–95. doi: 10.1038/mt.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank KM, Hogarth DK, Miller JL, Mandal S, Mease PJ, Samulski RJ, et al. Investigation of the cause of death in a gene therapy trial. N Engl J Med. 2009;361(2):161–9. doi: 10.1056/NEJMoa0801066. [DOI] [PubMed] [Google Scholar]
- 27.Evans CH, Ghivizzani SC, Robbins PD. Gene delivery to joints by intra-articular injection. Hum Gene Ther. 2018;29(1):2–14. doi: 10.1089/hum.2017.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ying J, Wang P, Zhang S, Xu T, Zhang L, Dong R, et al. Transforming growth factor-beta1 promotes articular cartilage repair through canonical Smad and Hippo pathways in bone mesenchymal stem cells. Life Sci. 2018;192(1):84–90. doi: 10.1016/j.lfs.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 29.Madry H, Zurakowski D, Trippel SB. Overexpression of human insuli-like growth factor-1 promotes new tissue formation in an ex vivo model of articular chondrocyte transplantation. Gene Ther. 2001;8(19):1443–9. doi: 10.1038/sj.gt.3301535. [DOI] [PubMed] [Google Scholar]
- 30.Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, et al. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor 1 (IGF-1) Gene Ther. 2005;12(15):1171–9. doi: 10.1038/sj.gt.3302515. [DOI] [PubMed] [Google Scholar]
- 31.Saxer RA, Bent SJ, Brower-Toland BD, Mi Z, Robbins PD, Evans CH, et al. Gene mediated insulin-like growth factor-1 delivery to the synovium. J Orthop Res. 2001;19(5):759–67. doi: 10.1016/S0736-0266(00)00077-2. [DOI] [PubMed] [Google Scholar]
- 32.Madry H, Cucchiarini M. Advances and challenges in gene-based approaches for osteoarthritis. J Gene Med. 2013;15(10):343–55. doi: 10.1002/jgm.2741. [DOI] [PubMed] [Google Scholar]
- 33.Hellgren I, Drvota V, Rieper R, Enoksson S, Blomberg P, Islam KB, et al. Highly efficient cell-mediated gene transfer using non-viral vectors and FuGene6: in vitro and in vivo studies. Cell Mol Life Sci. 2000;57(8-9):1326–33. doi: 10.1007/PL00000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodrich LR, Hidaka C, Robbins PD, Evans CH, Nixon AJ. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J Bone Joint Surg Br. 2007;89(5):672–85. doi: 10.1302/0301-620X.89B5.18343. [DOI] [PubMed] [Google Scholar]
- 35.Brower-Toland BD, Saxer RA, Goodrich LR, Mi Z, Robbins PD, Evans CH, et al. Direct adenovirus-mediated insulin-like growth factor 1 gene transfer enhances transplant chondrocyte function. Hum Gene Ther. 2001;12(2):117–29. doi: 10.1089/104303401750061186. [DOI] [PubMed] [Google Scholar]
- 36.Brigham . standard of care: autologous chondrocyte implantation (ACI) Massachusetts, US: Brigham & Women’s Hospital; 2007. pp. 1–8. [Google Scholar]
- 37.Kalus W, Zweckstetter M, Renner Y, Sanchez Y, Georgescu J, Grol M, et al. Structure of the IGF-binding domain of the insulin-like growth factor-binding protein-5 (IGFBP-5): implications for IGF and IGF-receptor interactions. EMBO J. 1998;17(22):6558–72. doi: 10.1093/emboj/17.22.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones JI, Gockerman A, Busby WH, Camacho-Hubner C, Clemmons DR. Extracellular matrix contains insulin-like growth factor binding protein-5: potentiation of the effects of IGF-1. J Cell Biol. 1993;121(3):679–87. doi: 10.1083/jcb.121.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ducheyne P, Mauck RL, Smith DH. Biomaterials on the repair of sports injuries. Nat Mater. 2012;11(8):652–4. doi: 10.1038/nmat3392. [DOI] [PubMed] [Google Scholar]
- 40.Chen P, Mei S, Xia C, Zhu R, Pang Y, Wang J, et al. The amelioration of cartilage degeneration by photo-crosslinked GelHA hydrogel and crizotinib encapsulated chitosan microspheres. Oncotarget. 2017;8(18):30235–51. doi: 10.18632/oncotarget.15750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Q, Rondon-Cavanzo EP, Dalla Picola IP, Tiera MJ, Zhang X, Dai K, et al. In vivo therapeutic efficacy of TNFα silencing by folate-PEG-chitosan-DEAE/siRNA nanoparticles in arthritic mice. Int J Nanomedicine. 2018;13(12):387–402. doi: 10.2147/IJN.S146942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shafiee A, Kabiri M, Langroudi L, Soleimani M, Ai J. Evaluation and comparison of the in vitro characteristics and chondrogenic capacity of four adult stem/progenitor cells for cartilage cell-based repair. J Biomed Mater Res A. 2016;104(3):600–10. doi: 10.1002/jbm.a.35603. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Bian YZ, Wu Q, Chen GQ. Evaluation of three-dimensional scaffolds prepared from poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) for growth of allogeneic chondrocytes for cartilage repair in rabbits. Biomaterials. 2008;29(19):2858–68. doi: 10.1016/j.biomaterials.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Wang F, Zhang Y, Jin X, Zhang L, Feng Y, et al. In vivo MRI tracking of polyethylenimine-wrapped superparamagnetic iron oxide nanoparticle-labeled BMSCs for cartilage repair: a minipig model. Cartilage. 2013;4(1):75–82. doi: 10.1177/1947603512455194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dey P, Schneider T, Chiappisi L, Gradzielski M, Schulze-Tanzil G, Haag R. Mimicking of chondrocyte microenvironment using in situ forming dendritic polyglycerol sulfate-based synthetic polyanionic hydrogels. Macromol Biosci. 2016;16(4):580–90. doi: 10.1002/mabi.201500377. [DOI] [PubMed] [Google Scholar]
- 46.Guo T, Zhao J, Chang Z, Ding Z, Hong H, Chen J, et al. Porous chitosan-gelatin scaffold containing plasmid DNA encoding transforming growth factor-771 for chondrocytes proliferation. Biomaterials. 2006;27(7):1095–103. doi: 10.1016/j.biomaterials.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 47.Zhao X, Yu SB, Wu FL, Mao ZB, Yu CL. Transfection of primary chondrocytes using chitosan-pEGFP nanoparticles. J Control Release. 2006;112(2):223–8. doi: 10.1016/j.jconrel.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 48.Thanou M, Florea BI, Geldof M, Junginger HE, Borchard G. Quaternized chitosan oligomers as novel gene delivery vectors in epithelial cell lines. Biomaterials. 2002;23(1):153–9. doi: 10.1016/s0142-9612(01)00090-4. [DOI] [PubMed] [Google Scholar]
- 49.Richardson SM, Curran JM, Chen R, Vaughan-Thomas A, Hunt JA, Freemont AJ, et al. The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on ply-l-lactic acid (PLLA) scaffolds. Biomaterials. 2006;27(22):4069–78. doi: 10.1016/j.biomaterials.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 50.Yao Y, He Y, Guan Q, Wu Q. A tetracycline expression system in combination with Sox9 for cartilage tissue engineering. Biomaterials. 2014;35(6):1898–906. doi: 10.1016/j.biomaterials.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 51.Liu C, Zhang P, Zhai X, Tian F, Li W, Yang J, et al. Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials. 2012;33(13):3604–13. doi: 10.1016/j.biomaterials.2012.01.052. [DOI] [PubMed] [Google Scholar]
- 52.Chen XA, Zhang LJ, He ZJ, Wang WW, Xu B, Zhong Q, et al. Plasmid-encapsulated polyethylene glycol-grafted polythylenimine nanoparticles for gene delivery into rat mesenchymal stem cells. In J Nanomed. 2011;6(1):843–53. doi: 10.2147/IJN.S17155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madry H, Cucchiarini M, Stein U, Remberger K, Menger MD, Kohn D, et al. Sustained transgene expression in cartilage defects in vivo after transplantation of articular chondrocytes modified by lipid-mediated gene transfer in a gel suspension delivery system. J Gene Med. 2003;5(6):502–9. doi: 10.1002/jgm.368. [DOI] [PubMed] [Google Scholar]
- 54.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 55.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39(2):266–76. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 56.Marcum JA, Rosenberg RD. Anticoagulopathy active heparin-like molecules from vascular tissue. Biochemistry. 1984;23(8):1730–7. doi: 10.1021/bi00303a023. [DOI] [PubMed] [Google Scholar]
- 57.Aguilar IN, Trippel S, Shi S, Bonasar LJ. Customized biomaterials to augment chondrocyte gene therapy. Acta Biomater. 2017;53(1):260–7. doi: 10.1016/j.actbio.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shopia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461–8. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buckwalter JA. Articular cartilage: Injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28(4):192–202. doi: 10.2519/jospt.1998.28.4.192. [DOI] [PubMed] [Google Scholar]
- 60.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2(6):477–88. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chong PP, Selvaratnam L, Abbas M, Kamarul T. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow mesenchymal stem cells. J Orthop Res. 2012;30(4):634–42. doi: 10.1002/jor.21556. [DOI] [PubMed] [Google Scholar]
- 62.Skowronski J, Rutka M. Osteochondral lesions of the knee reconstructed with mesenchymal stem cells-results. Ortop Traumatol Rehabil. 2013;15(3):195–204. doi: 10.5604/15093492.1058409. [DOI] [PubMed] [Google Scholar]
- 63.Saw KY, Anz A, Merican S, Tay YG, Ragavanaidu K, Jee CS, et al. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. Arthroscopy. 2011;27(4):493–506. doi: 10.1016/j.arthro.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 64.Saw KY, Anz A, Siew-Yoke Jee C, Merican S, Ching-Soong Ng R, Roohi SA, et al. Articular cartilage regeneration with autologous peripheral blood progenitor cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29(4):684–94. doi: 10.1016/j.arthro.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 65.Skowronski J, Skowronski R, Rutka M. Cartilage lesions of the knee treated with blood mesenchymal cells – results. Ortop Traumatol Rehabil. 2012;14(6):569–77. doi: 10.5604/15093492.1012404. [DOI] [PubMed] [Google Scholar]
- 66.Frisch J, Orth P, Venkatesan JK, Rey-Rico A, Schmitt G, Kohn D, et al. Genetic modification of human peripheral blood aspirates using recombinant adeno-associated viral vectors for articular cartilage repair with a focus on chondrogenic transforming growth factor-β gene delivery. Stem Cells Transl Med. 2017;6(1):249–60. doi: 10.5966/sctm.2016-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pascher A, Palmer GD, Steinert A, Oligino T, Gouze E, Gouze JN, et al. Gene delivery to cartilage defects using coagulated bone marrow aspirate. Gene Ther. 2004;11(2):133–41. doi: 10.1038/sj.gt.3302155. [DOI] [PubMed] [Google Scholar]
- 68.Venkatesan JK, Frisch J, Rey-Rico A, Schmitt G, Madry H, Cucchiarini M. Impact of mechanical stimulation on the chondrogenic processes in human bone marrow aspirates modified to overexpress sox9 via rAAV vectors. J Exp Orthop. 2017;4(1) doi: 10.1186/s40634-017-0097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grol MW, Lee BH. Gene therapy for repair and regeneration of bone and cartilage. Curr Opin Pharmacol. 2018;40(1):59–66. doi: 10.1016/j.coph.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Bellavia D, Veronesi F, Carina V, Costa V, Raimondi L, De Luca A, et al. Gene therapy for chondral and osteochondral regeneration: is the future now? Cell Mol Life Sci. 2018;75(4):649–67. doi: 10.1007/s00018-017-2637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim MK, Ha CW, In Y, Cho SD, Choi ES, Ha JK, et al. A multicenter, double-blind, phase III clinical trial to evaluate the efficacy and safety of a cell and gene therapy in knee osteoarthritis patients. Hum Gene Ther Clin Dev. 2018;27(1) doi: 10.1089/humc.2017.249. [DOI] [PubMed] [Google Scholar]
- 72.Kim MK, Ha CW, In Y, Cho SD, Choi ES, Ha JK, et al. A multicenter, double-blind, phase III clinical trial to evaluate the efficacy and safety of a cell and gene therapy in knee osteoarthritis patients. Hum Gene Ther Clin Dev. 2018;29(1):48–59. doi: 10.1089/humc.2017.249. [DOI] [PubMed] [Google Scholar]
- 73.Watson Levings R, Broome TA, Smith AD, Rice BL, Gibbs EP, Myara DA, et al. Gene therapy for osteoarthritis: pharmacokinetics of intra-articular scAAVIL-1Ra delivery in an equine model. Hum Gene Ther Clin Dev. 2018;29(2):90–100. doi: 10.1089/humc.2017.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodriguez-Merchan EC. Medial unicompartmental osteoarthritis (MUO) of the knee: Unicompartmental knee replacement (UKR) or total knee replacement (TKR) Arch Bone Jt Surg. 2014;2(3):137–40. [PMC free article] [PubMed] [Google Scholar]
- 75.Rodriguez-Merchan EC. Unicompartmental knee osteoarthritis (UKOA): unicompartmental knee arthroplasty (UKA) or high tibial osteotomy (HTO)? Arch Bone Jt Surg. 2016;4(4):307–13. [PMC free article] [PubMed] [Google Scholar]
- 76.Rodriguez-Merchan EC. Does a previous high tibial osteotomy (HTO) influence the long-term function or survival of a total knee arthroplasty (TKA)? Arch Bone Jt Surg. 2018;6(1):19–22. [PMC free article] [PubMed] [Google Scholar]