Abstract
Purpose of the Review:
To summarize the potential interactions between obstructive sleep apnea (OSA), atrial fibrillation (AF), and connexins.
Recent Findings:
OSA is highly prevalent in patients with cardiovascular disease, and is associated with increased risk for end-organ substantial morbidities linked to autonomic nervous system imbalance, increased oxidative stress and inflammation, ultimately leading to reduced life expectancy. Epidemiological studies indicate that OSA is associated with increased incidence and progression of coronary heart disease, heart failure, stroke, as well as arrhythmias, particularly AF. Conversely, AF is very common among subjects referred for suspected OSA, and the prevalence of AF increases with OSA severity. The interrelationships between AF and OSA along with the well-known epidemiological links between these two conditions and obesity may reflect shared pathophysiological pathways, which may depend on the intercellular diffusion of signaling molecules into either the extracellular space or require cell-to-cell contact. Connexin signaling is accomplished via direct exchanges of cytosolic molecules between adjacent cells at gap membrane junctions for cell-to-cell coupling. The role of connexins in AF is now quite well established, but the impact of OSA on cardiac connexins has only recently begun to be investigated. Understanding the biology and regulatory mechanisms of connexins in OSA at the transcriptional, translational, and post-translational levels will undoubtedly require major efforts to decipher the breadth and complexity of connexin functions in OSA-induced AF.
Summary:
The risk of end-organ morbidities has initiated the search for circulating mechanistic biomarker signatures and the implementation of biomarker-based algorithms for precision-based diagnosis and risk assessment. Here we summarize recent findings in OSA as they relate to AF risk, and also review potential mechanisms linking OSA, AF and connexins.
Keywords: Connexins, obstructive sleep apnea (OSA), atrial fibrillation (AF), obesity, cardiac connexins, exosomes
Sleep-Disordered Breathing:
Sleep-disordered breathing (SDB) is a highly prevalent cluster of conditions that affects both genders and is frequently associated with a wide variety of co-morbid disorders affecting multiple organ systems. The major categories included in SDB consist of obstructive sleep apnea (OSA), central sleep apnea (CSA), obesity hypoventilation syndrome (OHS), and sleep-related hypoxemia such as in COPD and other lung parenchymal diseases. OSA affects at least 5-15% of the general population and possibly much more 1, 2 is characterized by recurrent collapse of the upper airway during sleep 3, and has been conclusively recognized as an independent cardiovascular disease (CVD) risk factor, as well as a major public health issue with society-wide adverse consequences involving motor vehicle accidents or work-related accidents, cognitive, mood and behavioral deficits impairing work performance, and metabolic and sexual dysfunction 4-7.
The cumulative evidence indicates that hypoxia, namely chronic intermittent hypoxia (CIH), generated during repetitive long apneic episodes is one of the major key factors linking SDB and CVD 8. Accordingly, OSA is an independent causally-associated factor in the development of hypertension, with the risk increasing as OSA severity increases 9, 10. Severe OSA (apnea– hypopnea index [AHI] ≥30 events/hour) has also been strongly associated with an increased risk of stroke, ischemic heart disease, atrial fibrillation (AF) and excess CVD and all-cause mortality 11-13.
To identify potential mechanisms of OSA morbidity, animal models have been developed to include not only the physiological perturbations that characterize SDB (i.e., CIH or sleep fragmentation (SF)), but also to incorporate disease aspects of human obesity and its co-morbidities that are important contributors to end-organ injury in the context of OSA. Here, we will particularly focus on the findings derived from studies in rodents who were exposed to chronic intermittent hypoxia (CIH). Most models induce environmental hypoxia using inspired oxygen concentrations in the 5–7% range, and aim to elicit nadir arterial oxyhemoglobin saturation levels of 75–80%, which closely correspond to the saturation levels seen in moderate to severe OSA in humans 14-16. Models of CIH in mice and rats, defined as intermittent hypoxia exposures during sleep periods for 2 weeks or longer, result in phenotypic manifestations that are strikingly similar to the clinical features of human OSA. Increased oxidative stress, evidence of autonomic nervous system dysregulation with increased sympathetic tonic and reflexive activity, and activation and propagation of tissue and systemic inflammatory pathways become all apparent, usually beginning within the first few days after initiation of CIH. These alterations result in cardiovascular derangements including hypertension and increased atherogenesis, along with metabolic perturbations that include insulin resistance and dyslipidemia even in non-obese animals fed with regular diet 16-21. These findings have been replicated in a very small number of experimental studies involving humans, usually involving relatively short 2–14 day exposures to intermittent hypoxia in young healthy volunteers, and have also resulted in measurable alterations in memory, systemic blood pressure, glucose disposition, and calculated sensitivity of peripheral tissues to insulin 22-25.
Considering that the prevalence of obesity is increasing worldwide, and that obesity represents one of the significant risk factors for OSA, as evidenced by the fact that more than 70% of patients being obese 26, 27, it is sometimes difficult to extricate the contributions of OSA and obesity to downstream morbidities. Obesity, a complex disorder, is most commonly caused by a combination of excessive food intake, lack of physical activity, and genetic susceptibility. Obese individuals are susceptible to co-morbidities such as type 2 diabetes mellitus, nonalcoholic fatty liver disease (NAFLD), asthma, cancers, cardiovascular, and neurodegenerative diseases 28-32. The prevalence of metabolic syndrome is on the rise due to the obesity epidemic. Evidence suggests that an abnormal metabolic syndrome is associated with higher risk of diabetes and CVD 33, 34. Obesity in turn is a well-recognized risk factor for OSA, and higher body mass index (BMI) is associated with greater severity of OSA for both genders 35.
Several attempts have been made to reproduce the pathological features of obesity-related OSA in animal models, and the more recent murine model is the New Zealand obese mouse (NZO/HlLtJ). These mice, which have anatomic and functional characteristics similar to those of obese OSA patients, also manifest obstructive respiratory events and may consequently be used as a pathophysiological model of OSA 36. It would be beneficial to study obesity and cardiovascular risk to identify OSA patients because several cardiovascular disease mechanisms in obese people can also be attributable to occult OSA 37.
It has been demonstrated that there is an independent association between OSA, insulin resistance, and type 2 diabetes mellitus (T2DM) by a number of cross-sectional studies, observational studies, and large population-based studies 38-40. Metabolic syndrome is a cluster of metabolic factors that increases the risk of cardiovascular disease (CVD) morbidity and mortality including AF, and also increases the risk of developing T2DM by three-fold, cardiovascular disease by two-fold, and is growing as a major public health challenge worldwide 41.
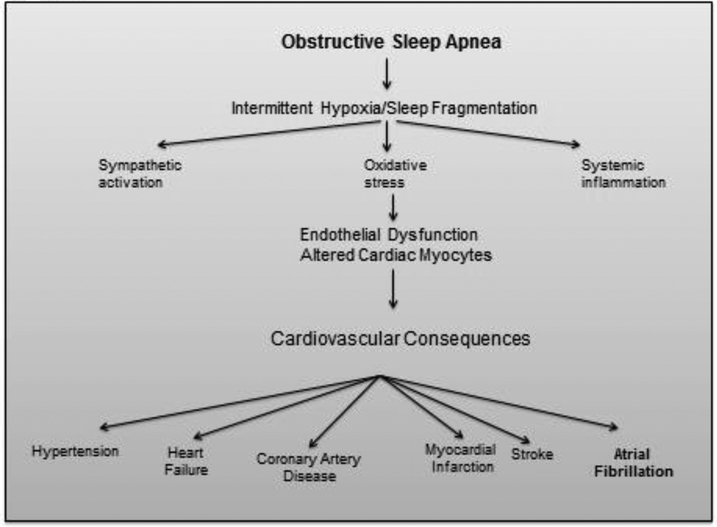
As indicated above, the physiological consequences of OSA include intermittent arterial hypoxemia, central and peripheral nervous system autonomic arousal, and large swings in intrathoracic pressures during sleep, which in turn are associated with enhanced sympathetic activation and parasympathetic withdrawal, a combination of neural inputs that clearly facilitates the induction of arrhythmias, such as AF in vulnerable individuals 42-48 Of note, these very same mechanisms have also been implicated in the pathogenesis of AF per se in the absence of OSA, either by triggering its initiation or by atrial remodeling so as to promote or maintain the AF arrhythmia 49-51. However, OSA can also induce and facilitate the occurrence of hypertension, myocardial infarction, and heart failure, and, in concert with obesity, these conditions can lead to cardiac remodeling, as well as arrhythmia, particularly AF 50, 52, 53. Therefore, as shown in Figure 1, there is strong biologic plausibility that OSA may predispose toward the development of AF 51.
Figure 1:
Cardiovascular consequences of obstructive sleep apnea.
OSA has not only been recognized as an independent risk factor for major postoperative cardiopulmonary complications in addition to increased consumption of economic resources and increased hospital stay duration 54, but such increased complication rates and associated hospitalization costs are accounted in a substantial proportion by underlying strong associations between OSA and AF 55, 56. Indeed, the prevalence of sleep apnea, particularly OSA, is 21% to 74% in patients with AF 57-59. Similar to OSA, AF prevalence is also expected to rise given the increased percentage of the aging population segment 60, 61.
Recently, several studies have shown a favorable effect of continuous positive airway pressure (CPAP) treatment on blood pressure, but this effect exhibits great variability 62. In fact, 25–30% of patients who use CPAP treatment for > 4 h/night do not experience a positive effect on blood pressure 63, 64. Application of precision medicine to these patients using circulating biomarkers such as microRNAs (miRNA) was reported as a first-line intervention to avoid the prescription of ineffective treatments and excessive consumption of pharmacological drugs that do not ameliorate the cardiovascular risk 62, 65. In parallel, Lim et al., 2017 presented a conceptual framework that provides the basis for a new P4 medicine approach to OSA, and should be considered more in depth: predict and prevent those at high risk for OSA and its morbid consequences, personalize the diagnosis and treatment of OSA, and build in patient participation to manage OSA, 66 thereby justifying its acronym of P4 (i.e., prediction, prevention, personalization, and participation). In this context, our effort to identify circulating biomarkers of personalized prediction to therapy employed identification and validation of plasma-based miRNA is the initial step in such direction 62, 65.
Atrial Fibrillation:
Atrial fibrillation (AF), is the most commonly sustained arrhythmia worldwide, is associated with significant morbidity and mortality, and impairs quality of life, while complicating the management of other chronic diseases 67-71. AF is driven by structural and functional alterations in the atria that consequently result in complex electrophysiological perturbations. Patterns that render the atrial conductive system and the autonomic system dysfunctional, lead to a vicious cycle of exacerbated atrial and ventricular remodeling events (electrical, structural, and autonomic) that promote and maintain AF 72, 73. Three hypotheses have been suggested that explain the mechanisms of atrial fibrillation: (i) multiple random propagating wavelets, (ii) focal electrical discharges, and (iii) localized re-entrant activity with fibrillary conduction 74.
A number of clinical conditions are associated with an increased incidence of AF. Most of these conditions contribute to a gradual and progressive process of atrial remodeling characterized by changes in (i) ion channel function, (ii) calcium homeostasis, (iii) atrial structure such as cellular hypertrophy, activation of fibroblasts, and (iv) tissue fibrosis. These alterations may favor the occurrence of “triggers” for AF that initiate the arrhythmia as well as enhance the formation of a “substrate for AF” that promotes its perpetuation 75. The major clinical risk factors for AF incidence include age, diabetes, hypertension, heart failure, and coronary artery disease 76. AF and the accompanying deterioration of atrial mechanical function is associated with considerable morbidity, including increased risk of cognitive impairments, a 3-fold increase in the risk of heart failure, and a 5-fold increase in the risk of stroke 77, 78. Consequently, AF leads to substantial health care resource use and economic burden. As would be expected from the above mentioned pathophysiology, AF is a complication of many cardiopulmonary disorders that lead to increased cardiac afterload, elevated filling pressures, and left atrial enlargement 79. Many of the conditions associated with such cardiac effects are increasing in prevalence, including hypertension, obesity, and of course OSA 80. Age-related declines in vascular compliance, increasing population longevity, and the increasing prevalence of cardiovascular disease in older persons has led to an expanding AF epidemic 81.
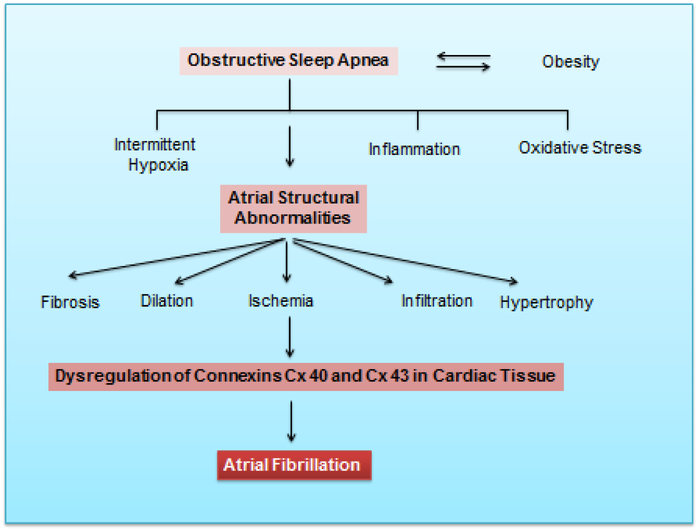
As discussed, obesity and OSA are both interactive risk factors for AF 82, 83. Obesity is commonly clustered with metabolic syndrome, diabetes, hypertension, and OSA, all of which may contribute to the development of AF. Obesity and OSA share multiple abnormalities implicated in the pathogenesis of AF, including hypoxia, negative intrathoracic pressure leading to increased atrial wall stress, sympathovagal imbalance, left ventricular diastolic dysfunction, systemic inflammation, and increased intravascular volume 82, 84-88,89, 90. Moreover, one-third of AF patients have at least three associated comorbidities, with a low percentage of AF patients presenting with presumably no heart disease or comorbidities 91. Figure 2 illustrates the link between obesity, OSA, and AF.
Figure 2:
Interactions between obstructive sleep apnea obesity and down-stream effects on atrial structure and function including down-regulation of connexins in atrial myocytes.
Notwithstanding the enormous advances in our understanding of the molecular pathophysiology of AF during the past decades, there are still numerous important gaps that need to be addressed. Structural remodeling seems a key for AF stabilization and therapy resistance 92. Notwithstanding, the genomics and proteomic features of AF require further investigation and clarification. Advanced bioinformatics and computational modeling approaches have the capacity to integrate and synthesize current insights to grapple with the complexity of AF. Bioinformatic tools will undoubtedly play a key translational role in understanding and combating the mechanisms of AF in vivo, due to sophisticated multiscale computational modeling that can integrate the cellular and molecular processes in the second and third dimensions, providing key insights into the impact of molecular events for AF at the multicellular tissue level 92.
Pathophysiological Mechanisms of Atrial Fibrillation:
The pathophysiology of AF is complex, involving dynamic interactions among several factors, including substrate, triggers, and perpetuators, and the therapeutic approaches/strategies are informed by the disease progression from initiation of the abnormal electrical rhythm to its maintenance 93. It has been reported that inflammation is a key component of the pathophysiological processes that lead to the development of AF; and the amplification of inflammatory pathways triggers AF, as AF increases the inflammatory state 94. There are a number of risk factors and comorbidities that are common to both AF and OSA including age, male sex, hypertension, congestive heart failure and coronary artery disease 95. In addition, the intermittent hypoxia, recurrent arousals and increased negative intrathoracic pressures that characterize OSA and result in increased sympathetic nerve activity, oxidative stress, inflammation, and electrical and mechanical remodeling of both atria as well as the left ventricle are most likely to further aggravate the risk of AF or make AF more resistant to therapy while promoting recurrence 96, 97, 98,99. OSA induces deeply negative intrathoracic pressure, increases venous return, impairs LV filling, and diminishes stroke volume. Strongly negative intrathoracic pressures activate intrathoracic baroreceptors, inducing autonomic reflex responses that promote AF 100. AF onset tends to occur during sleep apnea episodes, suggesting that episodes of OSA acutely enhance the risk of AF 101.
Atrial Fibrillation and OSA:
There is now little doubt that the prevalence of OSA in AF is markedly higher than in the general population 95, 102, increasing the awareness of the potential relationships between AF and OSA 80, 103. As mentioned, OSA has been documented as a comorbidity with potential interaction and impact on progression and outcome of patients with cardiovascular disease 104, and several studies have indicated the presence of OSA as a predictor of AF in specific subgroups including post-cardiac surgery, post-electrical cardioversion, post-ablation, or in association with underlying congestive heart failure 105. It has been estimated that the risk of atrial fibrillation is four times higher in patients with OSA independent of obesity, age, hypertension, heart failure or other confounding variables. In addition, nearly 50% of patients of AF have OSA 55. The severity of OSA has been shown to influence the prevalence of AF. Patients with an AHI of ≥10/h had an AF prevalence rate of 58% compared with 42% in those with an AHI of ≤10/h (P<0.0001), and the frequency of AF was even higher (70%) in patients with severe OSA (AHI ≥40/h) 102.
Mechanisms of Atrial Fibrillation in OSA:
The common mechanisms linking OSA and AF are complex and mediated by multiple mechanisms. For example, human and animal studies have demonstrated that the pathophysiologic changes brought on by OSA, including changes in intrathoracic pressure, hypoxia, and hypercapnia may cause structural and electrical changes that predispose to arrhythmia including AF 82. It has been indicated that OSA increases atrial pressures, causing atrial stretch that could promote remodeling 106. Furthermore, animal data evaluating the impact of apnea on atrial electrophysiology demonstrated slowed atrial conduction and increased atrial refractoriness. Temporal differences of normalization of these factors after apnea cessation enable a window for heightened AF vulnerability 107-109. OSA induces repeated episodes of hypoxia that trigger chemoreflex and enhance sympathetic nerve activity, leading to tachycardia and blood pressure elevation, especially at the end of the apneic episodes 110. Also, hypoxia and reoxygenation cycles in OSA cause a change in oxidative balance, leading to the formation of reactive oxygen species capable of reacting with other organic molecules impairing their functions 111. Additionally, it has been indicated that hypoxia and hypercapnia associated with sleep apnea affect sympathetic nerve activity and cause vasoconstriction and, as a result, hypertension that is a known risk factor for AF 112, 113. There is also increasing evidence that OSA results in atrial electrical and structural remodeling. The atria of OSA patients were shown to have extensive areas of low voltage or electrical silence and conduction abnormalities, slower atrial conduction velocity, and sinus node recovery times 114. Very recently, it has been reported that mice exposed to CIH exhibit changes in the passive stiffness of the cardiac tissue extracellular matrix (ECM), a critical factor underlying conduction changes and predisposing to AF 115.
Understanding of AF mechanisms in the context of OSA may allow for more direct targeting of specific pathophysiological contributors. Furthermore, new insights into the molecular pathophysiology of AF open new opportunities in risk assessment and monitoring of therapeutic responses. Novel biomarkers under investigation include noninvasive indices of atrial fibrosis and plasma biomarkers reflecting underlying biochemical mechanisms or responses 116, 117. Genetic findings suggest that dysregulation of gene transcription and an imbalance in major regulatory pathways of cell function may contribute to the complex genesis of AF. Future challenges include the identification and investigation of the downstream components of these pathways and henceforth, the identification of therapeutic targets of AF, particularly in the context of OSA 118.
Connexins:
Connexins ubiquitous proteins that are highly expressed in the heart, brain, and liver, as well as in endothelial and smooth muscle of blood vessels 119, 120. Connexins are critical for the development, function, and homeostasis of tissues and organs. Dysregulations of connexins are linked to many diseases such as stroke, heart attack and cancer 119. Connexins compose of a large family of trans-membrane proteins that allow intercellular communication and the transfer of ions and small signaling molecules between cells. Their main function is to facilitate cell-cell communication by forming channels called gap junctions (GJs) that connect the cytoplasm of cells 120. It has been reported that 20 different connexin genes have been found in mice and 21 in humans 121. Disruption of adhesion complexes, mainly adherent junctions, tight junctions, and gap junctions, leads to interference with normal tissue function, and may eventually lead to tissue dysfunction 122. Connexins are commonly named according to their molecular weights, and three different connexins were documented in cardiac myocytes, namely Cx40, Cx43, and Cx45. These 3 connexins were found to be expressed between cardiomyocytes, whereas Cx37 and Cx40 are present between endothelial cells 123, 124. All connexin molecules have four membrane-spanning domains, two extracellular domains, and a cytoplasmic carboxy-terminal tail of varying length that has an important role in the regulation of the gating properties of the channel 125. Connexin signaling can be achieved via direct exchanges of cytosolic molecules between adjacent cells at gap junctions, for cell-to-cell coupling, and possibly also can involve the formation of membrane “hemichannels,” for the extracellular release of cytosolic signals, direct interactions between connexins and other cell proteins, coordinating influence on the expression of multiple genes 126. Among the various connexins, Cx43 is the most studied connexin protein due to its expression in a wide variety of different tissues. For example, Cx43-containing gap junctions couple cardiomyocytes with non-cardiomyocytes, which can then alter the electrophysiological properties of cardiomyocytes 127.
Connexins play a central role in the synchronized contraction of the heart muscle, as well as the essential physiological processes such as tissue inflammation and repair 128, 129. However, the loss of connexins or mutations affecting their normal functions such as embryonic development, morphogenesis, and cell differentiation, as well as in the control of adult cell proliferation, and migration, and therefore connexins deficits have been implicated in a variety of diseases 130, 131,132-135.
Cellular interaction in blood vessels is maintained by multiple communication pathways, including gap junctions and consist of intercellular channels ensuring direct interaction between endothelial and smooth muscle Cells 125. In general, Cx40 and Cx37 are abundantly expressed in elastic (aorta) and muscular (coronary) arteries of various species 136-138, whereas the expression of Cx43 is restricted to the endothelial cells at branch points of these arteries 137. Furthermore, two studies reported that Cx40 plays an important role in blood-pressure regulation, and deletion of the Cx40 gene in mice results in a marked, sustained form of systemic hypertension 139, 140. In addition, the major dysfunction in Cx40-deficient mice appeared to depend on local blood flow–induced signaling in the afferent arteriole, a concept that was elegantly confirmed in perfused kidney by using a gap-junction blocker 140. For example, Cx43 plays a role in the looping of the ascending limb of the heart tube and the development of the right ventricle and the outflow tract, while Cx43-knockout mice die at birth of severe cardiac malformations 141.
Connexin signaling is regulated by several mechanisms at the transcriptional, posttranscriptional, translational, and posttranslational level 142, 143. Connexin expression may be related to epigenetic mechanisms, including reversible histone modifications, DNA methylation, and microRNA-related actions 144, 145, but is predominantly controlled by the conventional cis/trans machinery 142. A basal level of connexin gene transcription is maintained by general transcription factors, such as specificity protein 1 and activator protein 1. However, tissue-specific expression depends on cell type-specific repressors and activators, such as hepatocyte nuclear factor 1 alpha or Cx32 expression in the liver 146. In addition, epigenetic mechanisms, including histone modifications, DNA methylation, and microRNA-related control, are essential determinants of connexin gene transcription 144. In the vasculature there are several connexin (Cx) isoforms that were identified including Cx32, Cx37, Cx40, Cx43, and Cx45, which regulate the coordination of vessel contraction and relaxation. Generally, Cx32, Cx37, and Cx40 have been shown the most abundant in ECs with Cx43 and Cx45 routinely identified in the vascular SMCs 147. However, this expression pattern varies depending on vessel size and function with Cx43 found in the endothelium at arterial branch points and in smaller resistance vessels 148.
Connexin abnormalities have now been critically implicated in the pathophysiology of AF for quite some time. Generally, either mutations that reduce the expression or function of cardiac connexins or alternatively diseases that foster declines in the expression of connexins in atrium have been shown to increase the probability of AF and the susceptibility to AF recurrence 149-155. Initial experiments in rats indicated that induction of recurrent apneas was associated with increased probability of AF and that such susceptibility appeared to be dependent on connexins expression 109. More recently, work from our laboratory showed that CIH down regulates the expression of cardiac connexins traditionally implicated in the pathophysiology of AF, ie., Cx40 and Cx43, and that such reductions in expression are likely mediated by increased oxidative stress, since abrogation of NADPH oxidase was protective and preserved connexins expression in atrial myocytes 156.
As discussed above, the connexin gene family undergoes extensive regulation at the transcriptional and post-transcriptional level, and also undergoes numerous modifications at the protein level, including phosphorylation, which ultimately affects their trafficking, stability, and function. Recently, it has been indicated that intercellular communication can occur directly between neighbor cells via gap junctions (GJ), or indirectly at longer distances through soluble factors and extracellular vesicles (EVs) released into the environment 157, 158. Furthermore, Cx43, was able to modulate the interaction and communication between exosomes and cells 157. Extracellular vesicles (EVs) are membrane-bound, subcellular fragments that contain DNA, RNA, protein and lipids, and play an important role in intercellular communication. Currently, EVs are commonly classified based on their intracellular origin and size. Exosomes are class of EVs that were described, for the first time three decade ago, as very small vesicles of endosomal origin, and released as a result of the fusion of the multivesicular bodies (MVBs) with the plasma membrane in reticulocytes from rats and sheep 159, 160. Exosomes transfer biological information to neighboring cells, and through this cell-to-cell communication are involved not only in physiological functions such as cell-to-cell communication, but also in the pathogenesis of some diseases, including tumors and neurodegenerative conditions. They carry the large sized molecules such as RNA and proteins that influence gene expression.
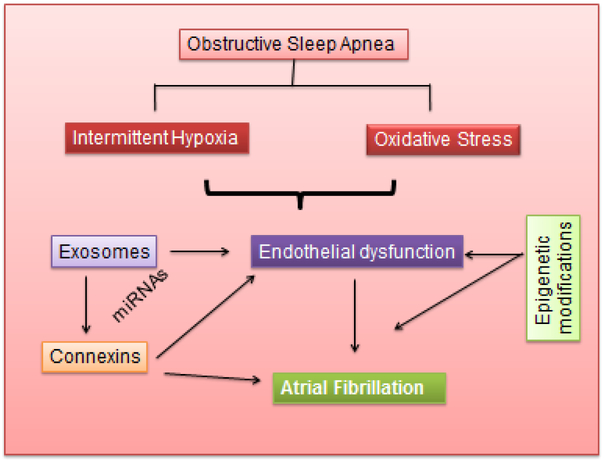
A major breakthrough was the demonstration that the cargo of EVs included both mRNA and miRNA and that EV-associated mRNAs could be translated into proteins by target cells 161. Later studies reported on the RNA contents of EV isolates from other cell cultures and body fluids 162-166. In both patients with OSA or in animal models using CIH or sleep fragmentation, we showed that exosomes carrying miRNAs can be internalized and transferred from one cell to another 164-169 as illustrated in Figure 3. In addition, initial work linking extracellular vesicle content and activity to AF has begun to emerge 170, suggesting that exosomes may play a contributory role in facilitating AF under specific circumstances such as OSA or obesity. Furthermore, therapeutic approaches involving exosome-based gene therapy are being explored on the ability of exosomes to cross biological barriers and their capacity to shuttle functional nucleic acids between cells 171-173. As such, the increased propensity and prevalence of AF in OSA may be not only detectable via identification of biomarkers within exosome cargo in plasma, but may also enable development of specific therapies that are selectively delivered to atrial tissue targets via exosomes.
Figure 3:
Hypothetical pathways involving coordinated activities of exosomes released in patients with obstructive sleep apnea, their effects on connexins in cardiac tissues, and the potential roles of epigenetic modifications on these elements to facilitate the occurrence of atrial fibrillation.
Summary:
OSA has now been extensively investigated as a condition influencing and adversely impacting the progression and outcomes of patients with cardiovascular disease. The awareness to the potential bidirectional interactions between AF and OSA has increased in the last decade. The mechanisms by which OSA predisposes to the development of AF, including sympathetic activation, intermittent hypoxia, transmural pressure changes, left atrial chamber enlargement, systemic inflammation, and endothelial dysfunction have been evoked, and the role of a third player, namely obesity has also emerged. It has been proposed that AF patients should be screened for OSA and therapy to alleviate OSA should be initiated as soon as it is diagnosed in patients with AF. In this context, the role of OSA in altering the expression and regulation of cardiac connexins which are mechanistically implicated in AF, and the need to identify at-risk patients with OSA or those with AF to facilitate more personalized approaches is prompting examination of circulating exosomes as both biomarkers and effectors of the OSA-AF dyad. If confirmed, exosomes may not only provide precise identification of AF risk in OSA patients, but may also constitute a precisely targeted therapeutic approach aimed at the atrial conduction system to abrogate the risk of AF in susceptible patients.
Acknowledgments
Funding Information This work was supported in part by National Institutes of Health grant HL130984 and the Marie M. and Harry L. Smith Endowed Chair in Child Health.
Footnotes
Conflict of Interest Abdelnaby Khalyfa and David Gozal declare no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- *1.Hnin K, Mukherjee S, Antic NA, et al. The impact of ethnicity on the prevalence and severity of obstructive sleep apnea. Sleep medicine reviews 2018. [DOI] [PubMed] [Google Scholar]; This is a recent review provding an overveiw about the impact of ethnicity on the severity of OSA pateints.
- 2.Peppard PE, Hagen EW. The Last 25 Years of Obstructive Sleep Apnea Epidemiology-and the Next 25? Am J Respir Crit Care Med 2018;197:310–2. [DOI] [PubMed] [Google Scholar]
- 3.Levy P, Kohler M, McNicholas WT, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers 2015;1:15015. [DOI] [PubMed] [Google Scholar]
- 4.Leger D, Bayon V, Laaban JP, Philip P. Impact of sleep apnea on economics. Sleep medicine reviews 2012;16:455–62. [DOI] [PubMed] [Google Scholar]
- 5.Jehan S, Zizi F, Pandi-Perumal SR, et al. Obstructive Sleep Apnea and Obesity: Implications for Public Health. Sleep Med Disord 2017;1. [PMC free article] [PubMed] [Google Scholar]
- 6.Geovanini GR, Wang R, Weng J, et al. Association between Obstructive Sleep Apnea and Cardiovascular Risk Factors: Variation by Age, Sex, and Race. The Multi-Ethnic Study of Atherosclerosis. Ann Am Thorac Soc 2018;15:970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy P, Ryan S, Oldenburg O, Parati G. Sleep apnoea and the heart. Eur Respir Rev 2013;22:333–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ando SI. Influence of hypoxia induced by sleep disordered breathing in case of hypertension and atrial fibrillation. Journal of cardiology 2018;72:10–8. [DOI] [PubMed] [Google Scholar]
- 9.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA : the journal of the American Medical Association 2000;283:1829–36. [DOI] [PubMed] [Google Scholar]
- 10.Parati G, Lombardi C, Hedner J, et al. Recommendations for the management of patients with obstructive sleep apnoea and hypertension. Eur Respir J 2013;41:523–38. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-de-la-Torre M, Campos-Rodriguez F, Barbe F. Obstructive sleep apnoea and cardiovascular disease. The Lancet. Respiratory medicine 2013;1:61–72. [DOI] [PubMed] [Google Scholar]
- 12.Javaheri S, Campos-Rodriguez F. Outcomes of Positive Airway Pressure for Sleep Apnea. JAMA : the journal of the American Medical Association 2017;318:2042–3. [DOI] [PubMed] [Google Scholar]
- 13.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. American journal of epidemiology 2013;177:1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almendros I, Wang Y, Gozal D. The polymorphic and contradictory aspects of intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol 2014;307:L129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalyfa A, Qiao Z, Gileles-Hillel A, et al. Activation of the Integrated Stress Response and Metabolic Dysfunction in a Murine Model of Sleep Apnea. Am J Respir Cell Mol Biol 2017;57:477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khalyfa A, Youssefnia N, Foster GE, et al. Plasma Exosomes and Improvements in Endothelial Function by Angiotensin 2 Type 1 Receptor or Cyclooxygenase 2 Blockade following Intermittent Hypoxia. Front Neurol 2017;8:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unnikrishnan D, Jun J, Polotsky V. Inflammation in sleep apnea: an update. Rev Endocr Metab Disord 2015;16:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz R, Murzabekova G, Egemnazarov B, et al. Arterial hypertension in a murine model of sleep apnea: role of NADPH oxidase 2. J Hypertens 2014;32:300–5. [DOI] [PubMed] [Google Scholar]
- 19.Drager LF, Yao Q, Hernandez KL, et al. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. Am J Respir Crit Care Med 2013;188:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gileles-Hillel A, Almendros I, Khalyfa A, et al. Prolonged Exposures to Intermittent Hypoxia Promote Visceral White Adipose Tissue Inflammation in a Murine Model of Severe Sleep Apnea: Effect of Normoxic Recovery. Sleep 2017;40. [DOI] [PubMed] [Google Scholar]
- 21.Gharib SA, Khalyfa A, Abdelkarim A, et al. Intermittent hypoxia activates temporally coordinated transcriptional programs in visceral adipose tissue. Journal of molecular medicine 2012;90:435–45. [DOI] [PubMed] [Google Scholar]
- 22.Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. Journal of applied physiology 2009;106:1538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster GE, Hanly PJ, Ahmed SB, Beaudin AE, Pialoux V, Poulin MJ. Intermittent hypoxia increases arterial blood pressure in humans through a Renin-Angiotensin system-dependent mechanism. Hypertension 2010;56:369–77. [DOI] [PubMed] [Google Scholar]
- 24.Beaudin AE, Pun M, Yang C, et al. Cyclooxygenases 1 and 2 differentially regulate blood pressure and cerebrovascular responses to acute and chronic intermittent hypoxia: implications for sleep apnea. Journal of the American Heart Association 2014;3:e000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Champod AS, Eskes GA, Foster GE, et al. Effects of acute intermittent hypoxia on working memory in young healthy adults. Am J Respir Crit Care Med 2013;187:1148–50. [DOI] [PubMed] [Google Scholar]
- 26.Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care 2011;14:402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuomilehto H, Seppa J, Uusitupa M. Obesity and obstructive sleep apnea--clinical significance of weight loss. Sleep medicine reviews 2013;17:321–9. [DOI] [PubMed] [Google Scholar]
- 28.Hu Y, Bhupathiraju SN, de Koning L, Hu FB. Duration of obesity and overweight and risk of type 2 diabetes among US women. Obesity 2014;22:2267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 2010;51:679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol 2014;32:3568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006;444:875–80. [DOI] [PubMed] [Google Scholar]
- 32.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. The New England journal of medicine 2016;375:794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meigs JB, Wilson PW, Fox CS, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. The Journal of clinical endocrinology and metabolism 2006;91:2906–12. [DOI] [PubMed] [Google Scholar]
- 34.Matheus AS, Tannus LR, Cobas RA, Palma CC, Negrato CA, Gomes MB. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens 2013;2013:653789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thurnheer R, Wraith PK, Douglas NJ. Influence of age and gender on upper airway resistance in NREM and REM sleep. Journal of applied physiology 2001;90:981–8. [DOI] [PubMed] [Google Scholar]
- 36.Baum DM, Morales Rodriguez B, Attali V, et al. New Zealand Obese Mice as Translational Model of Obesity-related Obstructive Sleep Apnea Syndrome. Am J Respir Crit Care Med 2018. [DOI] [PubMed] [Google Scholar]
- 37.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. Journal of the American College of Cardiology 2007;49:565–71. [DOI] [PubMed] [Google Scholar]
- 38.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med 2005;172:1590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med 2009;122:1122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Togeiro SM, Carneiro G, Ribeiro Filho FF, et al. Consequences of obstructive sleep apnea on metabolic profile: a Population-Based Survey. Obesity 2013;21:847–51. [DOI] [PubMed] [Google Scholar]
- 41.Mongraw-Chaffin M, Foster MC, Anderson CAM, et al. Metabolically Healthy Obesity, Transition to Metabolic Syndrome, and Cardiovascular Risk. Journal of the American College of Cardiology 2018;71:1857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bober SL, Ciriello J, Jones DL. Atrial arrhythmias and autonomic dysfunction in rats exposed to chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol 2018;314:H1160–H8. [DOI] [PubMed] [Google Scholar]
- 43.Estrada JA, Barlow MA, Yoshishige D, et al. delta-Opioid receptors: Pivotal role in intermittent hypoxia-augmentation of cardiac parasympathetic control and plasticity. Auton Neurosci 2016;198:38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferreira CB, Schoorlemmer GH, Rossi MV, et al. Brainstem areas activated by intermittent apnea in awake unrestrained rats. Neuroscience 2015;297:262–71. [DOI] [PubMed] [Google Scholar]
- 45.Dergacheva O, Dyavanapalli J, Pinol RA, Mendelowitz D. Chronic intermittent hypoxia and hypercapnia inhibit the hypothalamic paraventricular nucleus neurotransmission to parasympathetic cardiac neurons in the brain stem. Hypertension 2014;64:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dyavanapalli J, Jameson H, Dergacheva O, Jain V, Alhusayyen M, Mendelowitz D. Chronic intermittent hypoxia-hypercapnia blunts heart rate responses and alters neurotransmission to cardiac vagal neurons. The Journal of physiology 2014;592:2799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chalacheva P, Thum J, Yokoe T, O'Donnell CP, Khoo MC. Development of autonomic dysfunction with intermittent hypoxia in a lean murine model. Respir Physiol Neurobiol 2013;188:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu Z, Nie L, He B, et al. Increase in vulnerability of atrial fibrillation in an acute intermittent hypoxia model: importance of autonomic imbalance. Auton Neurosci 2013;177:148–53. [DOI] [PubMed] [Google Scholar]
- 49.Dimitri H, Ng M, Brooks AG, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm 2012;9:321–7. [DOI] [PubMed] [Google Scholar]
- 50.Shah RV, Abbasi SA, Heydari B, et al. Obesity and sleep apnea are independently associated with adverse left ventricular remodeling and clinical outcome in patients with atrial fibrillation and preserved ventricular function. Am Heart J 2014;167:620–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Todd K, McIntyre WF, Baranchuk A. Obstructive sleep apnea and atrial fibrillation. Nature and science of sleep 2010;2:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. The New England journal of medicine 2000;342:1378–84. [DOI] [PubMed] [Google Scholar]
- 53.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 2001;163:19–25. [DOI] [PubMed] [Google Scholar]
- 54.Memtsoudis SG, Stundner O, Rasul R, et al. The impact of sleep apnea on postoperative utilization of resources and adverse outcomes. Anesth Analg 2014;118:407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med 2006;173:910–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehra R, Wang L, Andrews N, et al. Dissociation of Objective and Subjective Daytime Sleepiness and Biomarkers of Systemic Inflammation in Sleep-Disordered Breathing and Systolic Heart Failure. J Clin Sleep Med 2017;13:1411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. The Lancet. Respiratory medicine 2015;3:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Costa LE, Uchoa CH, Harmon RR, Bortolotto LA, Lorenzi-Filho G, Drager LF. Potential underdiagnosis of obstructive sleep apnoea in the cardiology outpatient setting. Heart (British Cardiac Society) 2015;101:1288–92. [DOI] [PubMed] [Google Scholar]
- 59.Zhao LP, Kofidis T, Lim TW, et al. Sleep apnea is associated with new-onset atrial fibrillation after coronary artery bypass grafting. J Crit Care 2015;30:1418 e1–5. [DOI] [PubMed] [Google Scholar]
- 60.Ghias M, Scherlag BJ, Lu Z, et al. The role of ganglionated plexi in apnea-related atrial fibrillation. Journal of the American College of Cardiology 2009;54:2075–83. [DOI] [PubMed] [Google Scholar]
- 61.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. The American journal of cardiology 2013;112:1142–7. [DOI] [PubMed] [Google Scholar]
- 62.Sapina E, Torres G, Barbe F, Sanchez-de-la-Torre M. The Use of Precision Medicine to Manage Obstructive Sleep Apnea Treatment in Patients with Resistant Hypertension: Current Evidence and Future Directions. Current hypertension reports 2018;20:60. [DOI] [PubMed] [Google Scholar]
- 63.Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA : the journal of the American Medical Association 2013;310:2407–15. [DOI] [PubMed] [Google Scholar]
- 64.Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA : the journal of the American Medical Association 2012;307:2161–8. [DOI] [PubMed] [Google Scholar]
- **65.Sanchez-de-la-Torre M, Khalyfa A, Sanchez-de-la-Torre A, et al. Precision Medicine in Patients With Resistant Hypertension and Obstructive Sleep Apnea: Blood Pressure Response to Continuous Positive Airway Pressure Treatment. Journal of the American College of Cardiology 2015;66:1023–32. [DOI] [PubMed] [Google Scholar]; This study provides an evidence about how miRNA signature can be used in a precision medicine in OSA pateints with with hybertension.
- 66.Lim DC, Sutherland K, Cistulli PA, Pack AI. P4 medicine approach to obstructive sleep apnoea. Respirology 2017;22:849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dorian P, Jung W, Newman D, et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. Journal of the American College of Cardiology 2000;36:1303–9. [DOI] [PubMed] [Google Scholar]
- 68.Linssen GC, Rienstra M, Jaarsma T, et al. Clinical and prognostic effects of atrial fibrillation in heart failure patients with reduced and preserved left ventricular ejection fraction. European journal of heart failure 2011;13:1111–20. [DOI] [PubMed] [Google Scholar]
- 69.Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. European heart journal 2012;33:2719–47. [DOI] [PubMed] [Google Scholar]
- 70.Marrouche NF, Brachmann J, Andresen D, et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. The New England journal of medicine 2018;378:417–27. [DOI] [PubMed] [Google Scholar]
- 71.Karayiannides S, Lundman P, Friberg L, Norhammar A. High overall cardiovascular risk and mortality in patients with atrial fibrillation and diabetes: A nationwide report. Diab Vasc Dis Res 2018;15:31–8. [DOI] [PubMed] [Google Scholar]
- 72.Pellman J, Sheikh F. Atrial fibrillation: mechanisms, therapeutics, and future directions. Compr Physiol 2015;5:649–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002;415:219–26. [DOI] [PubMed] [Google Scholar]
- 74.Jalife J, Berenfeld O, Mansour M. Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc Res 2002;54:204–16. [DOI] [PubMed] [Google Scholar]
- 75.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiological reviews 2011;91:265–325. [DOI] [PubMed] [Google Scholar]
- 76.Wasmer K, Eckardt L, Breithardt G. Predisposing factors for atrial fibrillation in the elderly. J Geriatr Cardiol 2017;14:179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920–5. [DOI] [PubMed] [Google Scholar]
- 78.Ko YJ, Kim S, Park K, et al. Impact of the Health Insurance Coverage Policy on Oral Anticoagulant Prescription among Patients with Atrial Fibrillation in Korea from 2014 to 2016. Journal of Korean medical science 2018;33:e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McManus DD, Xanthakis V, Sullivan LM, et al. Longitudinal tracking of left atrial diameter over the adult life course: Clinical correlates in the community. Circulation 2010;121:667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004;110:364–7. [DOI] [PubMed] [Google Scholar]
- 81.Camm AJ, Savelieva I, Potpara T, Hindriks G, Pison L, Blomstrom-Lundqvist C. The changing circumstance of atrial fibrillation - progress towards precision medicine. Journal of internal medicine 2016;279:412–27. [DOI] [PubMed] [Google Scholar]
- *82.Marulanda-Londono E, Chaturvedi S. The Interplay between Obstructive Sleep Apnea and Atrial Fibrillation. Front Neurol 2017;8:668. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review provides details about the interplay between OSA and artirial fibrillation.
- 83.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity--results of a meta-analysis. Am Heart J 2008;155:310–5. [DOI] [PubMed] [Google Scholar]
- 84.Zhang L, Hou Y, Po SS. Obstructive Sleep Apnoea and Atrial Fibrillation. Arrhythm Electrophysiol Rev 2015;4:14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maan A, Mansour M, Anter E, et al. Obstructive Sleep Apnea and Atrial Fibrillation: Pathophysiology and Implications for Treatment. Crit Pathw Cardiol 2015;14:81–5. [DOI] [PubMed] [Google Scholar]
- 86.Goyal SK, Sharma A. Atrial fibrillation in obstructive sleep apnea. World J Cardiol 2013;5:157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Latina JM, Estes NA 3rd, Garlitski AC. The Relationship between Obstructive Sleep Apnea and Atrial Fibrillation: A Complex Interplay. Pulm Med 2013;2013:621736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fung JW, Li TS, Choy DK, et al. Severe obstructive sleep apnea is associated with left ventricular diastolic dysfunction. Chest 2002;121:422–9. [DOI] [PubMed] [Google Scholar]
- 89.Di Pasquale G, Mathieu G, Maggioni AP, et al. Current presentation and management of 7148 patients with atrial fibrillation in cardiology and internal medicine hospital centers: the ATA AF study. Int J Cardiol 2013;167:2895–903. [DOI] [PubMed] [Google Scholar]
- 90.Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol 2014;6:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wyse DG, Van Gelder IC, Ellinor PT, et al. Lone atrial fibrillation: does it exist? Journal of the American College of Cardiology 2014;63:1715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circulation research 2014;114:1483–99. [DOI] [PubMed] [Google Scholar]
- 93.Calvo D, Filgueiras-Rama D, Jalife J. Mechanisms and Drug Development in Atrial Fibrillation. Pharmacol Rev 2018;70:505–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zacharia E, Papageorgiou N, Ioannou A, et al. Inflammatory biomarkers in atrial fibrillation. Curr Med Chem 2017. [DOI] [PubMed] [Google Scholar]
- 95.Wolk R, Kara T, Somers VK. Sleep-disordered breathing and cardiovascular disease. Circulation 2003;108:9–12. [DOI] [PubMed] [Google Scholar]
- 96.Digby GC, Baranchuk A. Sleep apnea and atrial fibrillation; 2012 update. Curr Cardiol Rev 2012;8:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rossi VA, Stradling JR, Kohler M. Effects of obstructive sleep apnoea on heart rhythm. Eur Respir J 2013;41:1439–51. [DOI] [PubMed] [Google Scholar]
- 98.Gutierrez A, Van Wagoner DR. Oxidant and Inflammatory Mechanisms and Targeted Therapy in Atrial Fibrillation: An Update. J Cardiovasc Pharmacol 2015;66:523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Youn JY, Zhang J, Zhang Y, et al. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. Journal of molecular and cellular cardiology 2013;62:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Linz D, Schotten U, Neuberger HR, Bohm M, Wirth K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm 2011;8:1436–43. [DOI] [PubMed] [Google Scholar]
- 101.Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. Journal of the American College of Cardiology 2009;54:1797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lavergne F, Morin L, Armitstead J, Benjafield A, Richards G, Woehrle H. Atrial fibrillation and sleep-disordered breathing. Journal of thoracic disease 2015;7:E575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. European heart journal 2008;29:1662–9. [DOI] [PubMed] [Google Scholar]
- 104.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046–53. [DOI] [PubMed] [Google Scholar]
- 105.Bitter T, Nolker G, Vogt J, Prinz C, Horstkotte D, Oldenburg O. Predictors of recurrence in patients undergoing cryoballoon ablation for treatment of atrial fibrillation: the independent role of sleep-disordered breathing. J Cardiovasc Electrophysiol 2012;23:18–25. [DOI] [PubMed] [Google Scholar]
- 106.Kannel WB, Benjamin EJ. Current perceptions of the epidemiology of atrial fibrillation. Cardiol Clin 2009;27:13–24, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010;7:1263–70. [DOI] [PubMed] [Google Scholar]
- 108.Guasch E, Benito B, Qi X, et al. Atrial fibrillation promotion by endurance exercise: demonstration and mechanistic exploration in an animal model. Journal of the American College of Cardiology 2013;62:68–77. [DOI] [PubMed] [Google Scholar]
- 109.Iwasaki YK, Kato T, Xiong F, et al. Atrial fibrillation promotion with long-term repetitive obstructive sleep apnea in a rat model. Journal of the American College of Cardiology 2014;64:2013–23. [DOI] [PubMed] [Google Scholar]
- 110.Pepin JL, Levy P. [Pathophysiology of cardiovascular risk in sleep apnea syndrome (SAS)]. Rev Neurol (Paris) 2002;158:785–97. [PubMed] [Google Scholar]
- 111.Lira AB, de Sousa Rodrigues CF. Evaluation of oxidative stress markers in obstructive sleep apnea syndrome and additional antioxidant therapy: a review article. Sleep Breath 2016;20:1155–60. [DOI] [PubMed] [Google Scholar]
- 112.Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. Journal of the American Heart Association 2013;2:e000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation-Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J 2015;169:647–54 e2. [DOI] [PubMed] [Google Scholar]
- 114.Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm 2013;10:331–7. [DOI] [PubMed] [Google Scholar]
- 115.Farre N, Otero J, Falcones B, et al. Intermittent Hypoxia Mimicking Sleep Apnea Increases Passive Stiffness of Myocardial Extracellular Matrix. A Multiscale Study. Frontiers in physiology 2018;9:1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Daccarett M, McGann CJ, Akoum NW, MacLeod RS, Marrouche NF. MRI of the left atrium: predicting clinical outcomes in patients with atrial fibrillation. Expert Rev Cardiovasc Ther 2011;9:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Latini R, Masson S, Pirelli S, et al. Circulating cardiovascular biomarkers in recurrent atrial fibrillation: data from the GISSI-atrial fibrillation trial. Journal of internal medicine 2011;269:160–71. [DOI] [PubMed] [Google Scholar]
- 118.Riley G, Syeda F, Kirchhof P, Fabritz L. An introduction to murine models of atrial fibrillation. Frontiers in physiology 2012;3:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *119.Aasen T, Johnstone S, Vidal-Brime L, Lynn KS, Koval M. Connexins: Synthesis, Post-Translational Modifications, and Trafficking in Health and Disease. International journal of molecular sciences 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a recent review about connexins in health and diseases conditions.
- 120.Leybaert L, Lampe PD, Dhein S, et al. Connexins in Cardiovascular and Neurovascular Health and Disease: Pharmacological Implications. Pharmacol Rev 2017;69:396–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res 2004;62:228–32. [DOI] [PubMed] [Google Scholar]
- 122.Liu H, Radisky DC, Bissell MJ. Proliferation and polarity in breast cancer: untying the Gordian knot. Cell Cycle 2005;4:646–9. [DOI] [PubMed] [Google Scholar]
- 123.Gros DB, Jongsma HJ. Connexins in mammalian heart function. BioEssays : news and reviews in molecular, cellular and developmental biology 1996;18:719–30. [DOI] [PubMed] [Google Scholar]
- 124.Desplantez T. Cardiac Cx43, Cx40 and Cx45 co-assembling: involvement of connexins epitopes in formation of hemichannels and Gap junction channels. BMC Cell Biol 2017;18:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brisset AC, Isakson BE, Kwak BR. Connexins in vascular physiology and pathology. Antioxidants & redox signaling 2009;11:267–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bosco D, Haefliger JA, Meda P. Connexins: key mediators of endocrine function. Physiological reviews 2011;91:1393–445. [DOI] [PubMed] [Google Scholar]
- 127.Ongstad E, Kohl P. Fibroblast-myocyte coupling in the heart: Potential relevance for therapeutic interventions. Journal of molecular and cellular cardiology 2016;91:238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Beauchamp P, Yamada KA, Baertschi AJ, et al. Relative contributions of connexins 40 and 43 to atrial impulse propagation in synthetic strands of neonatal and fetal murine cardiomyocytes. Circulation research 2006;99:1216–24. [DOI] [PubMed] [Google Scholar]
- 129.Severs NJ, Dupont E, Coppen SR, et al. Remodelling of gap junctions and connexin expression in heart disease. Biochimica et biophysica acta 2004;1662:138–48. [DOI] [PubMed] [Google Scholar]
- 130.Avanzo JL, Mesnil M, Hernandez-Blazquez FJ, et al. Altered expression of connexins in urethane-induced mouse lung adenomas. Life Sci 2006;79:2202–8. [DOI] [PubMed] [Google Scholar]
- 131.Laird DW. Life cycle of connexins in health and disease. The Biochemical journal 2006;394:527–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Firouzi M, Bierhuizen MF, Kok B, et al. The human Cx40 promoter polymorphism −44G-->A differentially affects transcriptional regulation by Sp1 and GATA4. Biochimica et biophysica acta 2006;1759:491–6. [DOI] [PubMed] [Google Scholar]
- 133.Firouzi M, Kok B, Spiering W, et al. Polymorphisms in human connexin40 gene promoter are associated with increased risk of hypertension in men. J Hypertens 2006;24:325–30. [DOI] [PubMed] [Google Scholar]
- 134.Firouzi M, Ramanna H, Kok B, et al. Association of human connexin40 gene polymorphisms with atrial vulnerability as a risk factor for idiopathic atrial fibrillation. Circulation research 2004;95:e29–33. [DOI] [PubMed] [Google Scholar]
- 135.Richard G. Connexin disorders of the skin. Clinics in dermatology 2005;23:23–32. [DOI] [PubMed] [Google Scholar]
- 136.Bruzzone R, Haefliger JA, Gimlich RL, Paul DL. Connexin40, a component of gap junctions in vascular endothelium, is restricted in its ability to interact with other connexins. Molecular biology of the cell 1993;4:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circulation research 1998;83:636–43. [DOI] [PubMed] [Google Scholar]
- 138.van Kempen MJ, Jongsma HJ. Distribution of connexin37, connexin40 and connexin43 in the aorta and coronary artery of several mammals. Histochem Cell Biol 1999;112:479–86. [DOI] [PubMed] [Google Scholar]
- 139.de Wit C, Roos F, Bolz SS, et al. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circulation research 2000;86:649–55. [DOI] [PubMed] [Google Scholar]
- 140.Wagner C, de Wit C, Kurtz L, Grunberger C, Kurtz A, Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circulation research 2007;100:556–63. [DOI] [PubMed] [Google Scholar]
- 141.Reaume AG, de Sousa PA, Kulkarni S, et al. Cardiac malformation in neonatal mice lacking connexin43. Science 1995;267:1831–4. [DOI] [PubMed] [Google Scholar]
- 142.Oyamada M, Oyamada Y, Takamatsu T. Regulation of connexin expression. Biochimica et biophysica acta 2005;1719:6–23. [DOI] [PubMed] [Google Scholar]
- 143.D'Hondt C, Iyyathurai J, Vinken M, et al. Regulation of connexin- and pannexin-based channels by post-translational modifications. Biology of the cell / under the auspices of the European Cell Biology Organization 2013;105:373–98. [DOI] [PubMed] [Google Scholar]
- 144.Oyamada M, Takebe K, Oyamada Y. Regulation of connexin expression by transcription factors and epigenetic mechanisms. Biochimica et biophysica acta 2013;1828:118–33. [DOI] [PubMed] [Google Scholar]
- 145.Vinken M, De Rop E, Decrock E, et al. Epigenetic regulation of gap junctional intercellular communication: more than a way to keep cells quiet? Biochimica et biophysica acta 2009;1795:53–61. [DOI] [PubMed] [Google Scholar]
- 146.Piechocki MP, Toti RM, Fernstrom MJ, Burk RD, Ruch RJ. Liver cell-specific transcriptional regulation of connexin32. Biochimica et biophysica acta 2000;1491:107–22. [DOI] [PubMed] [Google Scholar]
- 147.Johnstone S, Isakson B, Locke D. Biological and biophysical properties of vascular connexin channels. Int Rev Cell Mol Biol 2009;278:69–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Straub AC, Johnstone SR, Heberlein KR, et al. Site-specific connexin phosphorylation is associated with reduced heterocellular communication between smooth muscle and endothelium. J Vasc Res 2010;47:277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nagibin V, Egan Benova T, Viczenczova C, et al. Ageing related down-regulation of myocardial connexin-43 and up-regulation of MMP-2 may predict propensity to atrial fibrillation in experimental animals. Physiol Res 2016;65 Suppl 1:S91–S100. [DOI] [PubMed] [Google Scholar]
- 150.Tribulova N, Egan Benova T, Szeiffova Bacova B, Viczenczova C, Barancik M. New aspects of pathogenesis of atrial fibrillation: remodelling of intercalated discs. J Physiol Pharmacol 2015;66:625–34. [PubMed] [Google Scholar]
- 151.Santa Cruz A, Mese G, Valiuniene L, Brink PR, White TW, Valiunas V. Altered conductance and permeability of Cx40 mutations associated with atrial fibrillation. J Gen Physiol 2015;146:387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bruegmann T, Beiert T, Vogt CC, Schrickel JW, Sasse P. Optogenetic termination of atrial fibrillation in mice. Cardiovasc Res 2018;114:713–23. [DOI] [PubMed] [Google Scholar]
- 153.Zhang F, Bian Y, Huang L, Fan W. Association between connexin 40 and potassium voltage-gated channel subfamily A member 5 expression in the atrial myocytes of patients with atrial fibrillation. Experimental and therapeutic medicine 2017;14:5170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shu C, Huang W, Zeng Z, et al. Connexin 43 is involved in the sympathetic atrial fibrillation in canine and canine atrial myocytes. Anatol J Cardiol 2017;18:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kanthan A, Fahmy P, Rao R, et al. Human Connexin40 Mutations Slow Conduction and Increase Propensity for Atrial Fibrillation. Heart, lung & circulation 2018;27:114–21. [DOI] [PubMed] [Google Scholar]
- 156.Gemel J, Su Z, Gileles-Hillel A, Khalyfa A, Gozal D, Beyer EC. Intermittent hypoxia causes NOX2-dependent remodeling of atrial connexins. BMC cell biology 2017;18:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Soares AR, Martins-Marques T, Ribeiro-Rodrigues T, et al. Corrigendum: Gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Scientific reports 2015;5:14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ribeiro-Rodrigues TM, Martins-Marques T, Morel S, Kwak BR, Girao H. Role of connexin 43 in different forms of intercellular communication - gap junctions, extracellular vesicles and tunnelling nanotubes. Journal of cell science 2017;130:3619–30. [DOI] [PubMed] [Google Scholar]
- 159.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. The Journal of cell biology 1983;97:329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. The Journal of cell biology 1985;101:942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology 2007;9:654–9. [DOI] [PubMed] [Google Scholar]
- 162.Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nature cell biology 2008;10:1470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Michael A, Bajracharya SD, Yuen PS, et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis 2010;16:34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Khalyfa A, Khalyfa AA, Akbarpour M, et al. Khalyfa A, Khalyfa AA, Akbarpour M, Connes P, Romana M, Laping-Carr G, Zhang C, Andrade J, Gozal D. Extracellular microvesicle microRNAs in children with sickle cell anemia with divergent clinical phenotypes. British J. Haematol 2016 (in press). Extracellular microvesicle microRNAs in children with sickle cell anemia with divergent clinical phenotypes. 2016;In press. [DOI] [PubMed] [Google Scholar]
- 165.Khalyfa A, Kheirandish-Gozal L, Khalyfa AA, et al. Circulating plasma extracellular microvesicle miRNA cargo and endothelial dysfunction in OSA children. . Am J. Resp Crit Care Med 2016;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Khalyfa A, Almendros I, Gileles-Hillel A, et al. Circulating exosomes potentiate tumor malignant properties in a mouse model of chronic sleep fragmentation. Oncotarget 2016;7:54676–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Almendros I, Khalyfa A, Trzepizur W, et al. Tumor Cell Malignant Properties Are Enhanced by Circulating Exosomes in Sleep Apnea. Chest 2016;150:1030–41. [DOI] [PubMed] [Google Scholar]
- 168.Khalyfa A, Kheirandish-Gozal L, Gozal D. Circulating exosomes in obstructive sleep apnea as phenotypic biomarkers and mechanistic messengers of end-organ morbidity. Respir Physiol Neurobiol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **169.Khalyfa A, Gozal D, Masa JF, et al. Sleep-disordered breathing, circulating exosomes, and insulin sensitivity in adipocytes. International journal of obesity 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the most recent study shows using in vitro adipocyte-based functional reporter assays, alterations in plasma exosomal cargo occur in SDB, and appear to contribute to adipocyte metabolic dysfunction.
- 170.Jesel L, Abbas M, Toti F, Cohen A, Arentz T, Morel O. Microparticles in atrial fibrillation: a link between cell activation or apoptosis, tissue remodelling and thrombogenicity. Int J Cardiol 2013;168:660–9. [DOI] [PubMed] [Google Scholar]
- 171.Sundararajan V, Sarkar FH, Ramasamy TS. The versatile role of exosomes in cancer progression: diagnostic and therapeutic implications. Cell Oncol (Dordr) 2018;41:223–52. [DOI] [PubMed] [Google Scholar]
- 172.Barile L, Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacology & therapeutics 2017;174:63–78. [DOI] [PubMed] [Google Scholar]
- 173.Urbanelli L, Buratta S, Sagini K, Ferrara G, Lanni M, Emiliani C. Exosome-based strategies for Diagnosis and Therapy. Recent Pat CNS Drug Discov 2015;10:10–27. [DOI] [PubMed] [Google Scholar]