Abstract
Background
Risky consumption of alcohol is a global problem. More than 3.3 million deaths annually are associated with risky use of alcohol, and global alcohol consumption continues to increase. People who have high alcohol consumption often require planned and emergency surgical procedures.
Risky drinking is associated with increased postoperative complications such as infections, cardiopulmonary complications, and bleeding episodes. Alcohol causes disorders of the liver, pancreas, and nervous system. Stopping consumption of alcohol can normalize these organ systems to some degree and may reduce the occurrence of complications after surgery.
This review was first published in 2012 and was updated in 2018.
Objectives
To assess the effects of perioperative alcohol cessation interventions on rates of postoperative complications and alcohol consumption.
Search methods
We searched the following databases up until 21 September 2018: Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library; MEDLINE; Embase; CINAHL via EBSCOhost; and two trials registers. We scanned the reference lists and citations of included trials and any identified relevant systematic reviews for further references to additional trials. When necessary, we contacted trial authors to ask for additional information.
Selection criteria
We included all randomized controlled trials (RCTs) that evaluated the effects of perioperative alcohol cessation interventions on postoperative complications and alcohol consumption. We included participants with risky consumption of alcohol who were undergoing all types of elective or acute surgical procedures under general or regional anaesthesia or sedation, who were offered a perioperative alcohol cessation intervention or no intervention.
We defined 'risky drinking' as alcohol consumption equivalent to more than 3 alcoholic units (AU)/d or 21 AU/week (with 1 AU containing 12 grams of ethanol) with or without symptoms of alcohol abuse or dependency. This corresponds to the amount of alcohol associated with increased postoperative complication rates in most clinical studies.
Data collection and analysis
We used guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions. We presented main outcomes as dichotomous variables in a meta‐analysis. When data were available, we conducted subgroup and sensitivity analyses to explore the risk of bias. Primary outcome measures were postoperative complications and in‐hospital and 30‐day mortality. Secondary outcomes were successful quitting at the end of the programme, postoperative alcohol use, and length of hospital stay. We assessed the quality of evidence using the GRADE approach.
Main results
We included in this updated review one new study (70 participants), resulting in a total of three RCTs (140 participants who drank 3 to 40 AU/d). All three studies were of moderate to good quality. All studies evaluated the effects of intensive alcohol cessation interventions, including pharmacological strategies for alcohol withdrawal symptoms, patient education, and relapse prophylaxis. We identified one ongoing study.
Overall, 53 of the 122 participants from three studies who underwent surgery developed any type of postoperative complication that required treatment. Of 61 participants in the intervention groups, 20 had complications, compared with 33 of 61 participants in the control groups (risk ratio (RR) 0.62, 95% confidence interval (CI) 0.40 to 0.96). Results show differences between the three clinical studies regarding outcome measurement and intensity of the interventions. However, all alcohol cessation programmes were intensive and included pharmacological therapy. The overall quality of evidence for this outcome is moderate.
In‐hospital and 30‐day postoperative mortality rates were low in the three studies. Researchers reported one death among 61 participants in the intervention groups, and three deaths among 61 participants in the control groups (RR 0.47, 95% CI 0.07 to 2.96). The quality of evidence for this outcome is low.
Investigators describe more successful quitters at the end of the intervention programme than among controls. Forty‐one out of 70 participants in the intervention groups successfully quit drinking compared with only five out of 70 participants in the control groups (RR 8.22, 95% CI 1.67 to 40.44). The quality of evidence for this outcome is moderate.
All three studies reported postoperative alcohol consumption (grams of alcohol/week) at the end of the programme as median and range values; therefore it was not possible to estimate the mean and the standard deviation (SD). We performed no meta‐analysis. All three studies reported length of stay, and none of these studies described a significant difference in length of stay. Data were insufficient for review authors to perform a meta‐analysis. No studies reported on the prevalence of participants without risky drinking in the longer term.
Authors' conclusions
This systematic review assessed the efficacy of perioperative alcohol cessation interventions for postoperative complications and alcohol consumption. All three studies showed a significant reduction in the number of participants who quit drinking alcohol during the intervention period. Intensive alcohol cessation interventions offered for four to eight weeks to participants undergoing all types of surgical procedures to achieve complete alcohol cessation before surgery probably reduced the number of postoperative complications. Data were insufficient for review authors to assess their effects on postoperative mortality. No studies reported an effect on length of stay, and no studies addressed the prevalence of risky drinking in the longer term.
Included studies were few and reported small sample sizes; therefore one should be careful about drawing firm conclusions based on these study results. All three studies were conducted in Denmark, and most participants were men. The included participants may represent a selective group, as they could have been more motivated and/or more interested in participating in clinical research or otherwise different, and effects may have been overestimated for both intervention and control groups in these studies. Trial results indicate that these studies are difficult to perform, that strong research competencies are necessary for future studies, and that further evaluation of perioperative alcohol cessation interventions in high‐quality randomized controlled trials is needed. Once published and assessed, the one 'ongoing' study identified may alter the conclusions of this review.
Plain language summary
Effects of perioperative alcohol cessation interventions on postoperative complications following surgery
Review question
We assessed the evidence from randomized controlled trials to determine whether not drinking alcohol during the perioperative period reduces postoperative complications for people with risky alcohol consumption. These programmes supported participants in quitting drinking or in reducing their alcohol consumption before, during, and after surgery. 'Risky drinking' was defined as alcohol consumption equivalent to more than 3 alcoholic units (three small glasses of wine) per day or 21 units per week ‐ with or without alcohol abuse or dependency. Most clinical studies report that consuming this amount of alcohol increases postoperative complication rates.
Background
Risky consumption of alcohol is a global problem, and alcohol is an important threat to world health. More than 3.3 million deaths annually are associated with risky use of alcohol, and global alcohol consumption continues to increase. People who have a high level of alcohol consumption often require planned and emergency surgical procedures.
Risky drinking affects surgical outcomes ‐ even when the disease is not alcohol related. Typical surgical complications include infections, heart and breathing problems, and bleeding episodes. Alcohol causes disorders of the liver, pancreas, and nervous system. Stopping drinking of alcohol can normalize these organ systems to some degree and may reduce the occurrence of complications after surgery. Quitting drinking can result in mild to severe alcohol withdrawal symptoms and may lead to a change in lifestyle.
This review was first published in 2012 and was updated in 2018.
Search date
The evidence is current to 21 September 2018.
Study characteristics
We included three randomized controlled trials with a total of 140 participants. All three studies included participants with risky alcohol intake (3 to 40 AU daily) who were in need of surgery. These studies investigated intensive alcohol interventions aimed at complete alcohol cessation at the time of surgery compared with no intervention. Interventions included educational strategies for alcohol withdrawal and relapse prevention. Programmes were started three months before surgery, four weeks before surgery, and from the time of admission to surgery, and continued for six weeks after surgery, respectively.
Quality of the evidence
The quality of the evidence is of moderate to low quality.
Key results
In all three studies, intensive intervention programmes clearly increased the number of participants who quit drinking alcohol. The occurrence of postoperative complications appeared to be reduced as well. Of 61 participants in the intervention groups, 20 had complications requiring treatment, compared with 33 of 61 participants in the control groups (moderate‐quality evidence). Of 70 participants in the intervention groups, 41 successfully quit drinking, compared to five of 70 participants in the control groups (moderate‐quality evidence). Data were insufficient to show the effect of quitting drinking on the number of deaths (low‐quality evidence), and results show no effect on length of hospital stay. None of the included studies reported on the number of participants who continued to avoid risky drinking in the longer term (at three‐, six‐, nine‐, and 12‐month follow‐up).
Included studies were few and reported small sample sizes; therefore one should be careful about drawing firm conclusions based on these results. All three studies were conducted in Denmark, and most participants were men. The included participants may represent a selective group, as they could be more motivated and/or more interested in participating in clinical research or otherwise different, and effects may therefore have been overestimated for both intervention and control groups in these studies. More research is needed and new strategies are required to improve outcomes after surgery among risky drinkers.
Summary of findings
Summary of findings for the main comparison. Perioperative alcohol cessation intervention compared with treatment as usual in patients undergoing surgery.
| Perioperative alcohol cessation intervention compared with treatment as usual in patients undergoing surgery | ||||||
| Patient or population: adult surgical patients consuming 3 or more units of alcohol per day Setting: surgical departments (elective and acute) in Copenhagen, Denmark Intervention: perioperative alcohol cessation intervention Comparison: treatment as usual | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with treatment as usual | Risk with perioperative alcohol cessation intervention | |||||
| Postoperative complications after perioperative intervention (e.g. wound‐related complications, secondary surgery, cardiopulmonary complications, admission to intensive care) defined by need for treatment | Study population | RR 0.62 (0.40 to 0.96) | 122 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | ||
| 541 per 1000 | 335 per 1000 (216 to 519) | |||||
| In‐hospital and 30‐day mortality | Study population | RR 0.47 (0.07 to 2.96) | 122 (3 RCTs) | ⊕⊕⊝⊝ Lowb,c | ||
| 49 per 1000 | 23 per 1000 (3 to 146) | |||||
| Successful quitters (number of abstainers) at the end of the programme (with or without validation by interview, breath test, or alcohol markers) | Study population | RR 8.22 (1.67 to 40.44) | 140 (3 RCTs) | ⊕⊕⊕⊝ Moderated,e | ||
| 71 per 1000 | 587 per 1000 (119 to 1000) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded the quality of evidence by one level due to imprecision. The low numbers of participants and especially of events increase the width of the 95% CI.
bWe downgraded the quality of evidence by one level due to indirectness related to the risk of non‐generalizability between the population of interest and those who participated in the studies.
cWe downgraded the quality of evidence by one level due to imprecision. The 95% CI is wide and the results are not significant.
dWe downgraded the quality of evidence by one level due to imprecision. The 95% CI is really wide.
eWe did not downgrade success for heterogeneity as all studies showed results favouring intervention.
Background
Description of the condition
Evidence is sufficient to show that alcohol is a significant threat to world health (GBD 2015; WHO 2014). Worldwide, harmful use of alcohol is linked to 3.3 million deaths per annum, and global alcohol consumption continues to increase (WHO 2014). In Europe, the prevalence of patients in surgical settings with a high level of alcohol consumption has been reported to range from 7% to 49% for patients undergoing elective surgical procedures, and from 14% to 38% for those undergoing emergency surgical procedures (Kip 2008; Tønnesen 2003).
Risky drinking affects human physiology in several ways, even in the absence of end‐stage disease. In addition to the well‐known alcohol‐induced disorders of the liver, pancreas, and nervous system, risky drinking affects cardiac function, immune capacity, haemostasis, and endocrine stress responses (Spies 2001). Subclinical cardiac insufficiency and arrhythmias are common among risky drinkers (Tønnesen 1992b), and both are important risk factors for the development of postoperative complications. Reduced immune capacity is found in most patients drinking 3 or more alcohol units (AU) per day (Tønnesen 2003). This has been explained by suppressed cellular elements of the immune system and suppression of delayed‐type hypersensitivity reaction (DHT) (Tønnesen 2009). For surgical patients, the poor DHT response is further suppressed by surgical trauma per se, and the result may be a compromised postoperative immune system (Spies 2004). Prolonged bleeding time and an increased endocrine stress response during surgery are other pathophysiological mechanisms that may contribute to increased complication rates among patients with high consumption of alcohol (Tønnesen 1999). Increased endocrine stress can be identified by increased epinephrine, norepinephrine, and cortisol blood levels (Spies 2004; Tønnesen 1999a).
Preoperative alcohol‐induced organ dysfunction adds to the burden of the disease requiring surgery, and to the stress response caused by the surgical procedure itself. This may result in a poor surgical outcome. The postoperative complication rate is increased by about 50% at an intake of more than 2 to 3 AU/d (28 grams/unit per day) (Eliasen 2013; Rubinsky 2013). The complication rate for patients drinking more than 5 AU/d is increased by 300%. Typical postoperative complications include infections, cardiopulmonary complications, and bleeding episodes (Tønnesen 2003). To some extent, abstinence may reverse alcohol‐induced pathophysiological processes, and postoperative complications might be preventable with perioperative alcohol cessation (Tønnesen 1999a; Tønnesen 2003).
Unhealthy alcohol intake comprises a continuum ranging from risky drinking over alcohol abuse and moderate addiction to complicated addiction with alcohol hallucinations, delirium, and withdrawal seizures. This continuum is also reflected in the clinical setting and in the literature. Definitions have changed several times during the last decades, and it is a challenge to categorize high alcohol intake according to different criteria. The International Classification of Diseases and Related Health Problems, Tenth Edition (ICD‐10), defines dependency as three of six symptoms within 12 months (The ICD‐10 Classification, 1993); the previous Diagnostic and Statistical Manual of Mental Disorders (DSM) requires three of seven symptoms (DSM‐4 1994); and the updated version of the DSM has changed the terminology to 'addiction' and has included it in a broader definition of substance or alcohol use disorder defined by two of 11 symptoms (DSM‐5 2013). In October 2017, the ICD‐10 Clinical Modification was updated with new coding for a new classification including modifiers (mild, moderate, and severe, as well as early and sustained remission). Furthermore, risky drinking could be defined as consumption just above the limits defined by health authorities, but these limits have also changed over time and differ from country to country. In addition, questionnaires such as the Alcohol Use Disorders Identification Test (AUDIT) (Babor 2001), the CAGE questionnaire (acronym for four questions ‐ Cut down, Annoyed, Guilty, and Eye opener) (Ewing 1984), and the Michigan Alcoholism Screening Test (MAST) have provided their own definitions (Selzer 1971).
As a surgical risk factor and in this review, 'risky drinking' is defined as any alcohol consumption equivalent to more than 3 AU/d or 21 AU/week (with 1 AU equating to 12 grams of ethanol) ‐ with or without symptoms of alcohol abuse or dependency. This corresponds to the amount of alcohol associated with increased postoperative complication rates in most clinical studies (Eliasen 2013; Rotevatn 2017; Tønnesen 2009).
Description of the intervention
Cochrane Reviews on treatment of alcohol dependence evaluate pharmacological and psychosocial interventions and show some efficacy for benzodiazepine for treatment of alcohol withdrawal (Amato 2010), and for acamprosate and opioid antagonists for treatment of alcohol dependence (Rösner 2010a; Rösner 2010b). Anticonvulsants have not been found efficient for treatment of alcohol withdrawal (Minozzi 2010). Disulfiram has shown some effect on short‐term abstinence and days until relapse (Jørgensen 2011).
Brief alcohol interventions include advice and a short intervention with, or without, pharmacological strategies. They are often based on motivational interviewing techniques and generally aim for reduced alcohol intake and to some degree alcohol cessation. Two Cochrane Reviews have reported that these interventions are effective in reducing alcohol intake among patients in primary care and in general hospital populations (Kaner 2007; McQueen 2009).
In the surgical setting, perioperative alcohol cessation interventions vary in intensity and timing (Egholm 2017; Shourie 2006; Tønnesen 1999a; Tønnesen 2015). Intensive interventions last from four to eight weeks and aim to achieve complete alcohol cessation before surgery. They are comparable to the gold standard intervention programmes for smoking cessation (Rasmussen 2017). Intensive alcohol cessation interventions include empowerment of the patient, information and recommendations, treatment of alcohol withdrawal, relapse prophylaxis supported by pharmacological strategies, and follow‐up provided by experienced staff. The potential effect of perioperative alcohol cessation interventions on postoperative complications is related to (1) their effect on alcohol consumption and (2) the timing and intensity of the interventions (Tønnesen 2009).
The surgical setting is characterized by a fixed operation date, a relatively short period before surgery, and a minimum length of hospital stay; therefore alcohol cessation intervention programmes in this setting must be very effective.
This challenge also applies to acute surgery, where there is no time for intervention before surgery ‐ unless it is possible to postpone surgery until the intervention has been completed.
How the intervention might work
Abstinence from alcohol may to some degree reverse the pathophysiological processes seen among risky drinkers (Tønnesen 2003). Preoperative abstinence from alcohol has been shown to significantly reduce the incidence of arrhythmia during the postoperative period (Tønnesen 1999). Two weeks of abstinence from alcohol significantly improves DHT, and after eight weeks, DHT has been shown to be normalized (Tønnesen 1992a). The prolonged bleeding time seen in the perioperative period is also reversible, and four weeks of abstinence from alcohol improves the stress response to surgery (Tønnesen 1999a).
The relatively short period of abstinence required to normalize dysfunctioning organ systems among patients with risky intake of alcohol may explain the beneficial effects of alcohol cessation interventions on postoperative complication rates.
Quitting drinking may be followed by inconvenience caused by the change in lifestyle itself or by side effects of mild or severe alcohol withdrawal symptoms. An intensive intervention programme includes pharmacological support for prevention of withdrawal symptoms along with prescribing of vitamin B. Vitamin B deficiency occurs during drinking but is seldom diagnosed until an individual has quit drinking.
Why it is important to do this review
Elective surgery often allows time for preoperative lifestyle interventions, whereas acute surgery is characterized by very short or no preoperative time for lifestyle interventions. In this review, the perioperative period is defined as preoperative, intraoperative, and postoperative times related to surgery.
This review maps out the evidence on alcohol cessation interventions in the surgical setting and describes their effects on postoperative complications. Without a rigorous review of the evidence for, and against, these interventions, the danger is that they will be adopted without providing clear benefit for patients. On the other hand, if the effectiveness of perioperative alcohol cessation interventions can be established, they may provide an effective approach for reducing postoperative complication rates and should then be routinely applied.
Perioperative screening provides not only an opportunity to identify which patients qualify for preventive perioperative interventions, but also an opportunity to screen large and diverse patient populations for risky drinking (Kip 2008). Perioperative alcohol screening, followed by effective alcohol cessation interventions, may play an important role in preventing the severe consequences of alcohol use disorder (AUD) and thereby may contribute to other health benefits (Kip 2008). Still, although a large number of patients are screened and found to be at risk, alcohol cessation interventions are seldom routinely applied (Wåhlin 2014).
Objectives
To assess the effects of perioperative alcohol cessation interventions on rates of postoperative complications and alcohol consumption.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) evaluating the effects of pharmacological and psychosocial perioperative alcohol cessation interventions on postoperative complications or postoperative alcohol consumption, or both.
Types of participants
We included studies involving participants with risky drinking who were undergoing all types of surgical procedures under general anaesthesia, regional anaesthesia, or sedation, who were given a perioperative alcohol cessation or control intervention. We included studies of participants undergoing elective or acute surgery.
(We define 'risky drinking' as alcohol consumption equivalent to more than 3 alcoholic units (AU)/d or 21 AU/week (with 1 AU equating to 12 grams of ethanol)).
Types of interventions
All interventions of interest were pharmacological and psychosocial alcohol cessation interventions, provided in relation to a surgical procedure for the purpose of stopping or reducing alcohol consumption. Among elective surgical patients, the interventions began in due time preoperatively, and among acute surgical patients, the interventions took place immediately before or after surgery and continued during the postoperative period. We considered both brief and intensive interventions, including interventions with pharmacological strategies for alcohol withdrawal and relapse prophylaxis. Control groups included surgical patients receiving treatment as usual (TAU) including an assessment of their alcohol history.
Types of outcome measures
Primary outcomes
Any type of postoperative complication (e.g. wound‐related complication, secondary surgery, cardiopulmonary complication, admission to intensive care) defined by the need for treatment
In‐hospital and 30‐day postoperative mortality
Secondary outcomes
Successful quitters (number of abstainers) at the end of the programme
Postoperative alcohol consumption (grams of alcohol/week) at the end of the programme
Length of hospital stay (LOS)
Prevalence of participants without risky drinking over the long term (three‐, six‐, nine‐, and 12‐month follow‐up)
We reported postoperative risky drinking based on the number of AUs consumed per day. Alcohol consumption was self‐reported with or without validation (by interview, breath test, or alcohol markers). We reported alcohol consumption at the end of the intervention as both number of successful abstainers and grams of alcohol consumed.
We reported LOS in number of days from admission to discharge. For patients who were readmitted because of complications during the follow‐up period, we calculated the total number of hospital days.
Search methods for identification of studies
Electronic searches
For this updated review, we identified RCTs through a literature search based on systematic and sensitive search strategies, as outlined in Chapter 6.4. of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not apply restrictions to language or publication status. We searched the following databases for relevant trials.
Cochrane Central Register of Controlled Trials (CENTRAL; September 2018, Issue 9 of 12), in the Cochrane Library (Appendix 1).
MEDLINE (Ovid SP, 1946 to 21 September 2018) (Appendix 2).
Embase (Ovid SP, 1974 to 21 September 2018) (Appendix 3).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCOhost (1982 to 21 September 2018) (Appendix 4).
We developed a subject‐specific search strategy for MEDLINE and used this as the basis for search strategies employed in searching the other listed databases. When appropriate, we expanded the search strategy by including search terms for identifying RCTs.
We scanned the following trials registries for ongoing and unpublished trials (September 2018).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP).
ClinicalTrials.gov.
We developed the search strategy in consultation with an Information Specialist.
Searching other resources
We scanned the reference lists and citations of included trials and scanned identified relevant systematic reviews for further references to identify additional trials in September 2018.
When necessary, we contacted trial authors to ask for additional information.
Data collection and analysis
Selection of studies
The PRISMA flow diagram in Figure 1 summarizes our screening and selection process. Three review authors (JE, HT, and JA) independently scanned the titles and abstracts of reports identified by the search. We retrieved and evaluated potentially relevant studies, chosen by at least one review author, using full‐text versions. If necessary, we contacted trial authors to clarify a study's eligibility. The same review authors examined the full texts of all remaining articles and made a joint decision regarding inclusion.
1.
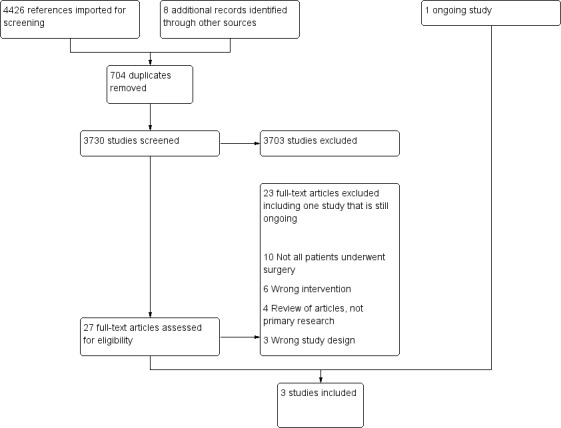
Study flow diagram.
We have listed the studies formally considered and excluded along with reasons for exclusion in the Characteristics of excluded studies table.
Data extraction and management
Two review authors (JWE and CBJ) independently performed data extraction using a tool based on guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion. When required, we obtained additional information through collaboration with the original trial author.
Assessment of risk of bias in included studies
We evaluated the quality of included trials. To avoid potential bias, we (JE, HT, and CBJ) independently evaluated the included studies and resolved disagreements by discussion until we reached consensus.
To enable us to draw conclusions about the overall risk of bias for an outcome, we evaluated domains such as random sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting, as well as recruitment, follow‐up rates, and other sources of bias. Any assessment of overall risk of bias involved consideration of the relative importance of different domains (Higgins 2011).
Some domains affect the risk of bias across outcomes in a study, for example, random sequence generation and allocation concealment; others, such as blinding and incomplete outcome data, may have different importance for different outcomes within a study. Thus, the risk of bias is not the same for all outcomes in a study (Higgins 2011). We defined trials as having low risk of bias only if they adequately fulfilled the criteria listed in the Cochrane Handbook for Systematic Reviews of Interventions. We have presented our risk of bias judgements in a 'Risk of bias summary' figure (Higgins 2011).
We performed summary assessments of the quality of evidence for each important outcome using the GRADE approach (Guyatt 2008; Guyatt 2011), which includes four levels of quality of evidence (high, moderate, low, and very low).
We assessed risk of bias in these domains according to the criteria described in Appendix 5.
Measures of treatment effect
For the primary outcomes ‐ postoperative complications, in‐hospital and 30‐day mortality ‐ we calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous data (binary outcome). We also did this for the secondary outcomes ‐ successful quitters (number of abstainers) at the end of the programme, prevalence of participants without risky drinking in the longer term (three‐, six‐, nine‐, and 12‐month follow‐up).
For continuous data, we reported the secondary outcomes ‐ length of stay (LOS), postoperative alcohol consumption (grams of alcohol/week) at the end of the programme ‐ as mean differences (MDs).
We reported postoperative alcohol consumption at the end of the intervention as the number of successful abstainers and grams of alcohol consumed, as well as the number of AUs consumed per day ‐ with or without validation (by interview, breath test, or alcohol markers) (with outcomes reported as RRs).
Unit of analysis issues
The present review presents no issues related to unit of analysis, as we have included only individually randomized trials, and the outcome of interest was the number of participants with complications. If results were given as the number of complications, we contacted study authors to request additional information. If study results included more relevant interventions, we divided the number of participants in the control group between the intervention groups.
Dealing with missing data
We contacted the authors of included studies regarding missing data. When we found that data were missing and study authors were not accessible, we calculated missing statistics (such as standard deviations (SDs)) from other quoted statistics (such as standard errors (SEs) or confidence intervals (CIs)). However, the included studies insufficiently reported some outcomes and did not include SD, SE, or CI, but only range; therefore it was not possible for review authors to include these outcomes in a meta‐analysis.
We adhered to the intention‐to‐treat (ITT) principle as far as possible. We analysed data from all participants according to the groups to which they were randomized. We reported available details in full if participants were excluded after allocation or withdrew from the trial.
Assessment of heterogeneity
We assessed clinical heterogeneity by comparing the distribution of important participant factors across trials, including age, gender, and characteristics of interventions. We assessed statistical heterogeneity by examining the I² statistic (Higgins 2002), which is a quantity that describes the proportion of variation in point estimates that is due to heterogeneity rather than to sampling error. We interpreted values of the I² statistic according to guidance provided in the Cochrane Handbook for Systematic Reviews of interventions (Higgins 2011).
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: shows considerable heterogeneity.
In cases of excessive clinical heterogeneity, we performed no statistical analyses to pool trial results. Clinical heterogeneity included types of interventions, outcome measures reported, and methodological quality.
Assessment of reporting biases
We searched for trial protocols of the included trials to assess whether outcome reporting seemed to be sufficiently complete and transparent. In the review protocol (Oppedal 2010), we stated that we would use a funnel plot analysis to examine publication bias. However, as our review included only three studies, we did not produce a funnel plot.
Data synthesis
We entered data from all trials included in the systematic review into Review Manager 5 (Review Manager 2014). We used random‐effects models because of expected large heterogeneity between studies regarding interventions, participants, and types of surgery. The outcomes of postoperative complications, 30‐day mortality, and successful quitters were dichotomous, and we pooled results as risk ratios (RRs). We calculated 95% CIs for each estimated effect size, using Mantel‐Haenszel (MH) for dichotomous outcomes. We did not pool the outcomes of postoperative alcohol consumption (grams of alcohol per week) and length of stay (days in hospital), as they were reported as median and range values.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses when data were available, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to compare:
different types of surgery (e.g. orthopaedic surgery, general surgery); and
intensive alcohol cessation interventions and brief interventions.
However, this review included too few trials to enable performance of subgroup analyses.
Sensitivity analysis
We planned to perform sensitivity analyses, when possible, to explore risk of bias. However, data were sparse, and we were not able to conduct these analyses.
'Summary of findings' table and GRADE
We developed a 'Summary of findings' table to compare results for the six main outcomes (Higgins 2011). We created this table by exporting data from Review Manager 5.3 into a web‐based version of the GRADEprofiler software (Review Manager 2014;GRADEpro). We did this according to the methods and recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We created one 'Summary of findings' table for comparison of our two primary outcomes:
any type of postoperative complication (e.g. wound‐related complication, secondary surgery, cardiopulmonary complication, admission to intensive care) defined by the need for treatment; and
in‐hospital and 30‐day postoperative mortality;
and for comparison of our four secondary outcomes:
successful quitters (number of abstainers) at the end of the programme;
postoperative alcohol consumption (grams of alcohol/week) at the end of the programme;
length of stay (LOS); and
prevalence of participants without risky drinking in the longer term (three‐, six‐, nine‐, and 12‐month follow‐up).
Methods used to assess the quality of evidence for outcomes
One review author (JE) initially evaluated the quality of evidence using the GRADE approach; remaining members of the review author group then discussed the quality of evidence ratings for each outcome until we reached consensus. We took the following factors into account: risk of bias, inconsistency of results, indirectness of evidence, imprecision of results, and publication bias. We assessed the quality of evidence for each of our six outcomes.
This review includes only RCTs, and we downgraded the evidence for each outcome from high‐quality by one level when considering a serious limitation, or by two levels when considering the issue to be a very serious limitation. In Table 1, we justified our decisions and described in the footnotes whether we had downgraded the quality of evidence, or if we had decided not to downgrade the quality of evidence.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The electronic search yielded 4426 references, along with eight additional records identified through other sources that we imported for screening: 704 were duplicates. Therefore, we screened 3730 potentially relevant studies by reviewing titles and abstracts. After completing this review, we excluded 3703 studies, leaving 27 potentially relevant studies (see Figure 1).
Included studies
We identified three eligible studies for inclusion in this review (Egholm 2017; Tønnesen 1999a; Tønnesen 2002). We included in the review data from these studies, involving 140 participants at entry. We described study characteristics in the Characteristics of included studies section.
Setting and participants
All three studies were conducted in Denmark (Egholm 2017; Tønnesen 1999a; Tønnesen 2002). The first study included 42 participants undergoing colorectal resection (Tønnesen 1999a). The second study included 28 participants undergoing elective hip arthroplasty (Tønnesen 2002), and the third study included 70 participants undergoing acute ankle fracture surgery (Egholm 2017). All three studies aimed to recruit adult women and men aged 18 years and over.
Screening
All three included studies used self‐reported alcohol consumption to identify eligible participants (Egholm 2017; Tønnesen 1999a; Tønnesen 2002).
In Egholm 2017, the eligibility criterion was weekly alcohol consumption of a minimum of 252 grams. In Tønnesen 1999a, the eligibility criterion was daily alcohol consumption exceeding 60 grams/d. In Tønnesen 2002, the eligibility criterion was alcohol consumption exceeding 60 grams/d or 420 grams/week.
Control
All three studies defined control as treatment as usual (Egholm 2017; Tønnesen 1999a; Tønnesen 2002). However, the control intervention involved a detailed assessment of the participant's alcohol history.
Interventions
The three included studies evaluated effects of intensive alcohol cessation interventions, including pharmacological strategies for alcohol withdrawal and relapse prophylaxis (Egholm 2017; Tønnesen 1999a; Tønnesen 2002).
In Egholm 2017, researchers provided the intervention for the purpose of achieving six weeks of postoperative alcohol cessation supported by disulfiram 400 mg/week, of which 200 mg was taken under supervision, and 200 mg without supervision. They offered chlordiazepoxide for withdrawal symptoms. The intervention included motivational counselling, together with a brief interview about alcohol intake (all together, about 30 minutes), every week. Project staff, who had undergone a two‐day training course, were available for participants by phone in the daytime. All participants received B vitamins and thiamine.
In Tønnesen 1999a, investigators provided the intervention to attain four weeks of preoperative support with disulfiram (800 mg), taken under supervision twice weekly until the week before surgery. They handed out prophylactic chlordiazepoxide according to the Danish guidelines for preventing withdrawal symptoms (Mundt 2003). All participants were given B vitamins. The intervention consisted of preoperative cessation from alcohol, motivational counselling, and an interview about alcohol intake.
In Tønnesen 2002, study authors provided the intervention to achieve three months of preoperative alcohol cessation (median 84 days, range 26 to 112 days) supported by disulfiram 800 mg/week ‐ 400 mg taken under supervision, and 400 mg without supervision. They offered chlordiazepoxide for withdrawal symptoms. The intervention included motivational counselling, together with a brief interview about alcohol intake (all together about 30 minutes), every week. Project staff were available for participants by phone in the daytime. All participants received B vitamins.
The intensive alcohol interventions in this review are similar to the intensive interventions developed for smokers (Fiore 2008; Rasmussen 2016). These intensive alcohol interventions comprised four or more meetings lasting at least 10 minutes each and pharmacological strategies for alcohol withdrawal and relapse prophylaxis. Brief interventions often consisted of a single session, fewer than four sessions of engagement with the participant, and provision of information and advice designed to achieve a reduction in smoking or alcohol consumption.
Outcomes
All three included studies reported postoperative complications defined as death or postoperative morbidity requiring treatment. Researchers reported complications one month postoperatively in Tønnesen 1999a and Tønnesen 2002, and at six weeks after surgery in Egholm 2017.
Researchers also reported mortality and length of stay (Egholm 2017; Tønnesen 1999a; Tønnesen 2002), as well as the number of successful quitters and postoperative alcohol consumption. The three studies also reported alcohol consumption at the time of surgery. Alcohol consumption was self‐reported as AU/d. Two studies validated self‐reported alcohol consumption by per cent carbohydrate‐deficient transferrin (CDT%) (Egholm 2017; Tønnesen 2002).
Two studies reported postoperative alcohol consumption after four weeks (Tønnesen 1999a; Tønnesen 2002). The third study reported postoperative alcohol consumption weekly over the first six weeks after surgery (Egholm 2017).
Excluded studies
We excluded 23 studies.
Four of the 23 excluded studies were reviews of articles ‐ not primary research articles (Bejou 2000; Chiang 1995; Soria 1981; Vagts 2002). One was a controlled clinical trial (Shourie 2006), one a case control study (Kaka 2017), and one a validation study (Watson 1999). We excluded 10 studies because not all participants underwent surgery (Antti‐Poika 1988; Forsberg 2000; Gentilello 1999; Heather 1996; Holloway 2007; Schermer 2006; Shepard 2016; Shetty 2011; Smith 2003; Sommers 2006), and six studies because they did not include an appropriate intervention (Avram 2009; Batioglu‐Karaaltin 2017; Schoenfeld 2007; Spies 1995; Spies 1996; Spies 2006).
We have summarized the reasons for exclusion of these possibly relevant studies in the Characteristics of excluded studies section.
Studies awaiting classification
We identified no studies awaiting classification.
Ongoing studies
We identified one ongoing study (NCT02188446). For details of this study, please see the Characteristics of ongoing studies table.
Risk of bias in included studies
We have provided details of how and why we rated study quality for included studies in the Characteristics of included studies section. Figure 2 provides a summary of overall risk of bias in the three studies judged as high, low, or unclear. Figure 3 provides details of judgements about each risk of bias domain for each study.
2.
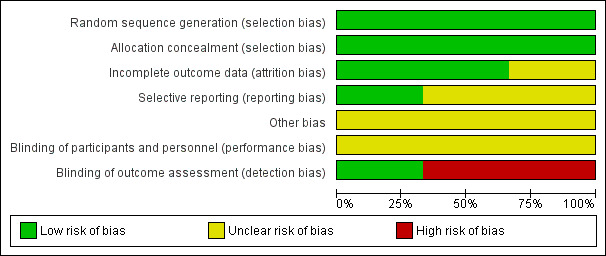
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
We deemed sequence generation for randomization to be adequate in all three included studies, as all used a computer‐generated code, off‐site data management, and opaque sealed envelopes (Egholm 2017; Tønnesen 1999a; Tønnesen 2002). All three studies used block randomization, and two applied stratification for each centre, as they were multi‐centre studies (Egholm 2017; Tønnesen 1999a). Therefore, we considered all three studies to have low risk of selection bias.
Blinding
Due to the nature of the interventions, it was not possible to blind participants or staff who provided the interventions. Egholm 2017 performed both the assessment and the statistical analysis while blinded. In Tønnesen 1999a and Tønnesen 2002, those performing the primary outcomes assessment were not blinded. Risk of detection bias based on blinding of outcome assessors in these studies was low in Egholm 2017 and unclear in Tønnesen 1999a and Tønnesen 2002.
Incomplete outcome data
All three studies assessed incomplete data (Egholm 2017; Tønnesen 1999a; Tønnesen 2002).
Egholm 2017 scheduled all participants for acute surgery before they where randomized. However, two participants did not undergo surgery and were excluded. In the remaining two studies, authors reported including participants before the final decision about surgery was made, and excluding participants after randomization if they fulfilled the exclusion criteria later in the preoperative period (Tønnesen 1999a; Tønnesen 2002).
For all three studies, it was possible to follow each participant in the medical record system, and these studies showed no or very minor disparity between the number of participants randomized and the number analysed. Therefore, we determined that a low risk of bias judgement was appropriate for this domain for Egholm 2017 and Tønnesen 1999a. Although participants were excluded according to exclusion criteria, attrition rates were very high in Tønnesen 2002; therefore the judgement of unclear risk of bias was appropriate.
Selective reporting
Risk of reporting bias was low in one study (Egholm 2017), for which we had access to all original data and the protocol had been published. We considered the remaining two studies to have an unclear reporting bias because it was unclear if these trials reported all assessed outcomes (Tønnesen 1999a; Tønnesen 2002).
Other potential sources of bias
All three of the included studies planned to include both men and women. Egholm 2017 included 47 men and 23 women. Tønnesen 1999a included only three women, all in the control group. Tønnesen 2002 ended up including only men.
Recruitment seemed difficult in all studies, as the number needed to screen was high for identifying eligible participants consuming at least 36 grams ‐ in Egholm 2017 ‐ and 60 grams ‐ in Tønnesen 1999a and Tønnesen 2002 ‐ of alcohol, respectively.
Under‐reporting of alcohol consumption may explain this problem in part and may contribute to the low inclusion rate. General reluctance among staff to address patients' alcohol use may have added to the problem. In addition, many patients declined to participate in these studies.
There is a risk that included patients were more motivated to quit alcohol intake than patients who for one reason or another were not included. This may have led to overestimation of quit rates in these studies. Therefore, we considered this domain to be atunclear risk of bias.
Effects of interventions
See: Table 1
Egholm 2017 screened 1531 patients by medical record review; 1371 patients did not meet the eligibility criteria, leaving 160 eligible patients. Of these, 90 refused participation, leaving 70 patients for inclusion in the study. Recruitment was difficult and took over four years. Tønnesen 2002 screened about 1900 patients with a self‐administrated questionnaire: 1486 patients returned a filled questionnaire, and 1133 underwent a hip replacement. Only 48 of these patients were eligible for inclusion according to their alcohol intake; 20 were not included (11 patients were not operated, three did not want to participate, and six were missed), leaving 28 trial participants for randomization. Tønnesen 1999a appeared to have similar problems with recruitment, as it took two and a half years for researchers to recruit 42 participants.
Primary outcomes
1. Any type of postoperative complication (e.g. wound‐related complication, secondary surgery, cardiopulmonary complication, admission to intensive care) defined by the need for treatment
All three included studies reported postoperative complications requiring treatment categorized as major and minor complications (Egholm 2017; Tønnesen 1999a; Tønnesen 2002). Major complications included fascial rupture, intra‐abdominal bleeding, intra‐abdominal abscess, anastomotic leakage, ileus, cardiopulmonary insufficiency (requiring intensive care), sepsis, delirium, hepatic coma, peptic stress ulcer (bleeding), multi‐organ failure, and death. Minor complications were deep wound infections, wound haematoma, plaster cast complications, dislocated fracture, hypertension, deep vein thrombosis, pneumonia, haematemesis, subileus, dehydration, urinary infection, fistula, luxation, and withdrawal symptoms.
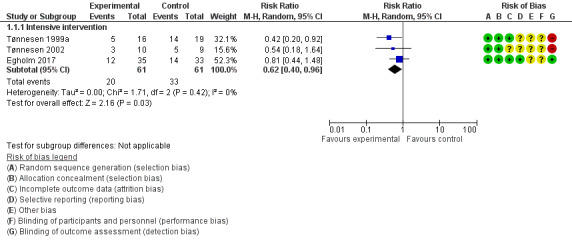
Of 61 participants, 20 in the intervention groups had complications compared with 33 of 61 in the control groups (RR 0.62, 95% CI 0.40 to 0.96; P = 0.03; Analysis 1.1; Figure 4). Review authors noted clinical differences between the three studies ‐ regarding both outcome measurements and intensity of the interventions. The alcohol cessation programmes were intensive and included pharmacological therapy. Due to the small number of trial participants, we downgraded the overall quality of the evidence for this outcome from high to moderate.
1.1. Analysis.
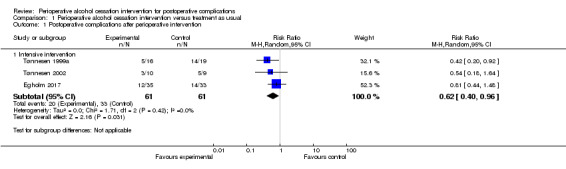
Comparison 1 Perioperative alcohol cessation intervention versus treatment as usual, Outcome 1 Postoperative complications after perioperative intervention.
4.
Forest plot of comparison: 1 Perioperative alcohol cessation intervention versus treatment as usual, outcome: 1.1 Any type of postoperative complication (e.g. wound‐related complications, secondary surgery, cardiopulmonary complications, admission to intensive care) defined by the need for treatment.
2. In‐hospital and 30‐day postoperative mortality
Overall mortality was low in the three studies (Egholm 2017; Tønnesen 1999a; Tønnesen 2002). The total number of deaths was four among 122 participants, and results show no significant differences in mortality between intervention groups (one death out of 61 participants in intervention groups, and three deaths out of 61 participants in control groups; RR 0.47, 95% CI 0.07 to 2.96; P = 0.42; Analysis 1.2;Figure 5). We downgraded the overall quality of evidence for this outcome by two levels (i.e. from high to low) because of the small numbers of participants and events reported, as well as the risk of non‐generalizability between populations of interest and those who participated in these studies; we found that data were insufficient to address effects of interventions on mortality.
1.2. Analysis.
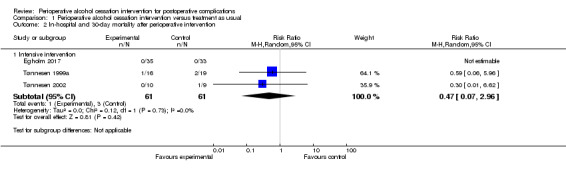
Comparison 1 Perioperative alcohol cessation intervention versus treatment as usual, Outcome 2 In‐hospital and 30‐day mortality after perioperative intervention.
5.
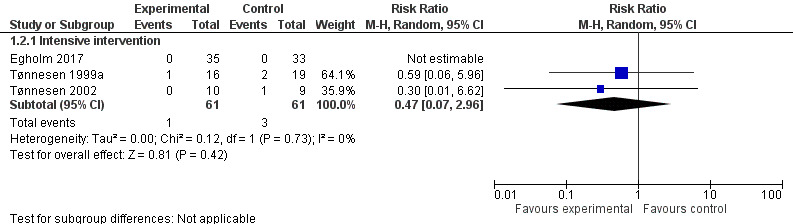
Forest plot of comparison: 1 Perioperative alcohol cessation intervention versus treatment as usual, outcome: 1.3 In‐hospital and 30‐day postoperative mortality.
Secondary outcomes
1. Successful quitters (number of abstainers) at the end of the programme
In the three included studies, intervention groups received an intensive alcohol cessation programme aimed at complete alcohol cessation in the perioperative period (Egholm 2017; Tønnesen 1999a; Tønnesen 2002). Results show a significant effect in all three studies regarding successful quitters at the end of the programme. In total, 140 participants were included in the meta‐analysis according to intention‐to‐treat analysis. Three participants were excluded and therefore were counted as non‐quitters. Forty‐one of 70 participants in the intervention groups successfully quit drinking compared with only five of 70 participants in the control groups (RR 8.22, 95% CI 1.67 to 40.44; P = 0.01). We downgraded the quality of evidence by one level due to large imprecision. We did not downgrade for indirectness. We did consider the possibility of higher motivation among participants compared to the entire sample of risky alcohol drinkers for whom this evidence should apply, but high motivation level was not an inclusion criterion, and the very low quit rates in the three control groups talked against a motivational level that could influence alcohol outcomes. We did not downgrade successful quitting for heterogeneity, as all studies provided results favouring the intervention (Analysis 1.3; Figure 6). The small numbers of participants and especially events increased the width of the 95% confidence interval.
1.3. Analysis.
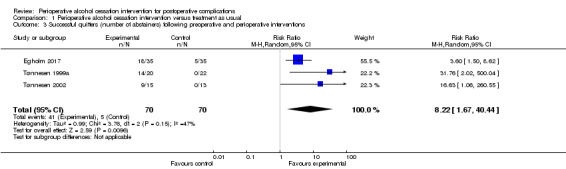
Comparison 1 Perioperative alcohol cessation intervention versus treatment as usual, Outcome 3 Successful quitters (number of abstainers) following preoperative and perioperative interventions.
6.
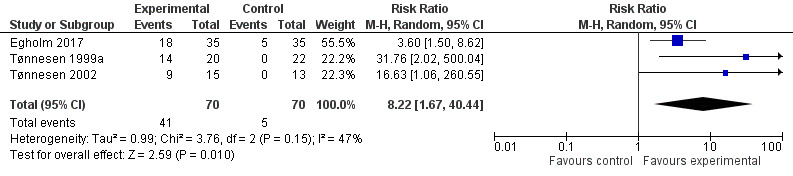
Forest plot of comparison: 1 Perioperative alcohol cessation intervention versus treatment as usual, outcome: 1.4 Successful quitters (number of abstainers) at the end of the programme.
Egholm 2017 reported a significantly larger number of abstainers at the end of the programme after six weeks in the intervention group compared with the control group (quitters: 18 of 35 participants vs five of 35 participants; RR 3.60, 95% CI 1.50 to 8.62).
Tønnesen 1999a reported that all participants in the intervention group completed the withdrawal programme with total abstinence from alcohol, including the two participants who did not undergo surgery. In the control group, participants did not reduce their alcohol consumption (quitters at four weeks: 14 of 20 participants vs none of 22; RR 31.76, 95% CI 2.02 to 500.04).
In Tønnesen 2002 at the end of the programme after three months, nine of 15 participants in the intervention group were abstainers versus none of 13 in the control group (RR 16.63, 95% CI 1.06 to 260.55).
2. Postoperative alcohol consumption (grams of alcohol/week) at the end of the programme
At the time of inclusion, median alcohol consumption in Egholm 2017 was 420 grams, ranging from 108 to 1272 grams per week in the intervention group, and 372 grams, ranging from 24 to 1368 grams in the control group. At the end of the programme, the control group had reduced alcohol consumption to 252 grams per week (0 to 864 grams) compared with 0 grams per week (0 to 512 grams) in the intervention group.
In Tønnesen 1999a, the corresponding alcohol intake was 84 grams, ranging from 60 to 480 grams per day, in the intervention group; and 72 grams, ranging from 60 to 480 grams per day, in the control group. At the end of the programme, alcohol consumption was 0 grams, ranging from 0 to 0 grams per day; and 72 grams, ranging from 60 to 480 grams per day, respectively.
In Tønnesen 2002, alcohol consumption at inclusion was 72 grams, ranging from 60 to 156 grams per day, in the intervention group; and 72 grams, ranging from 60 to 96 grams per day, in the control group. At the end of the programme, alcohol consumption was 24 grams per day and 60 grams per day, respectively.
All three studies reported alcohol consumption as median and range values; therefore it was not possible to estimate the mean and the SD, and we performed no meta‐analysis.
3. Length of hospital stay
All three included studies reported length of hospital stay. None of these studies showed a significant difference. Egholm 2017 reported five days of hospitalization, ranging from two to 28 days, in the intervention group; and five days, ranging from two to 33 days, in the control group. Tønnesen 1999a presented a hospital stay of eight days (three to 41 days) versus 10 days (four to 46 days), respectively. Tønnesen 2002 reported hospital stay of 14 days (nine to 31 days) versus 16 days (nine to 23 days), respectively. Studies reported only median and range values for length of stay; therefore it was not possible to perform a meta‐analysis.
4. Prevalence of participants without risky drinking in the longer term (three‐, six‐, nine‐, and 12‐month follow‐up)
None of the included three studies reported this outcome (Egholm 2017; Tønnesen 1999a; Tønnesen 2002).
Discussion
Summary of main results
This systematic review assessed the efficacy of preoperative and perioperative alcohol cessation interventions for postoperative complications and alcohol consumption. Evidence from the three included studies, including 140 participants contributing data to measured outcomes, shows that intensive alcohol cessation intervention given for four to eight weeks reduced the number of participants with postoperative complications, increased the number successfully quitting, and reduced postoperative alcohol consumption after the intervention. Data were insufficient to show the effect on mortality or hospital stay. No studies addressed the prevalence of risky drinking in the longer term.
Studies included participants undergoing ankle fracture surgery, hip replacement therapy, or colorectal resection and addressed only intensive interventions. In general, the World Health Organization (WHO) recommends shorter interventions but has not evaluated these interventions in randomized controlled trials (RCTs) with surgical outcomes (WHO 2010). One excluded study had investigated shorter interventions but not in a randomized setting and found no effect on complications after surgery (Shourie 2006). We have summarized the principal findings of this review in Table 1.
Overall completeness and applicability of evidence
Study participants
The three included studies used different methods to identify risky drinkers (quantity times frequency). Two of the three studies identified daily alcohol consumption (Tønnesen 1999a; Tønnesen 2002). The third study addressed weekly alcohol consumption (Egholm 2017). Only two of these studies included both sexes (Egholm 2017; Tønnesen 1999a); however the number of women included in these studies was low, which could be explained in part by the fact that the inclusion criteria for risky drinking were the same for both men and women, and men often have a higher alcohol intake than women. Another possible explanation is that risky drinking may be more taboo in women than in men.
As mentioned under Description of the condition, unhealthy alcohol intake ranges from an intake just above national alcohol limits to complicated addiction. This is also reflected in surgical studies. Although all included studies presented inclusion criteria that were based on the amount of alcohol intake, they used different criteria to diagnose addictive symptoms observed in patient characteristics. Egholm 2017 used criteria of the International Classification of Diseases and Related Health Problems, Tenth Edition (ICD‐10), and identified addiction in one‐third of the study population. Tønnesen 2002 used the CAGE test with a maximum of four points, and above two points indicating alcoholism. Participant scores ranged from 0 to 3 points, but researchers did not reveal the frequency of positive test findings. Tønnesen 1999a focused only on alcohol intake, which consisted of more than 60 grams of ethanol per day. This could be considered a study limitation; study authors have found that surgical complications are related to the amount of intake but have not yet proved that they are related to the level and symptoms of abuse.
Interventions
The three studies included in this review compared intervention versus control, which study authors defined as treatment as usual. Researchers offered intervention groups high‐intensity interventions including weekly sessions and pharmacological support for four to eight weeks. We found no RCTs that reported the effects of brief alcohol intervention on postoperative complications.
Types of outcomes
All three included studies evaluated the two primary outcomes: any type of postoperative complication, and in‐hospital and 30‐day postoperative mortality. All three studies evaluated three of the secondary outcomes in similar ways. We identified no studies that evaluated the number of participants without risky drinking in the longer term (three‐, six‐, nine‐, and 12‐month follow‐up).
The pharmaceuticals used in the intervention programme may cause severe side effects, which are however rare (e.g. disulfiram treatment may be followed by hypersensitivity, psychosis, and optic neuritis and even fulminant necrosis of liver cells, whereas diazepam/chlordiazepoxide may be followed by respiratory depression and psychosis). Study authors did not encounter these adverse events or other adverse events related to the intervention.
Length of follow‐up
The postoperative period for recording complications after surgery lasted four to six weeks. Researchers reported postoperative alcohol intake at the end of the intervention programme. No studies evaluated the long‐term effect of interventions on risky alcohol intake.
Completeness and applicability of evidence
All three included studies were conducted in Denmark, and study findings were published in English. Thus, applicability of the evidence may be limited to the Danish healthcare system. Most participants were men, and study results may not apply to women. The included participants may have been selected according to motivational readiness to change alcohol habits; therefore the effect may be overestimated. This would be the case for both intervention and control groups ‐ and thus would not influence differences between groups.
How do trial results fit into the context of current practice
In most countries, alcohol consumption is not a routine part of a medical record. Alcohol intake may be surrounded by taboo and silence. A few countries, including Denmark, record alcohol history in about 80% to 90% of medical records (Tønnesen 2008). Recently, social alcohol drinking has been included as a risk factor in the preoperative evaluation score from 1 to 5 used by the American Society of Anesthesiologists (ASA) worldwide (ASA 2014). Now, the score of 1 indicating the lowest risk at surgery requires "no or minimal alcohol use", score 2 is exemplified by "social alcohol drinker", and alcohol abuse/addiction is placed as score 3, in line with poorly controlled diabetes mellitus and chronic obstructive pulmonary disease.
In Sweden, the Swedish Society of Orthopaedics has decided to recommend preoperative alcohol cessation interventions for risky drinkers admitted for surgery (SOF 2017). The Danish National Board of Health has developed national guidelines and patient folders aimed at reducing risk by quitting risky alcohol intake before surgery (Danish Health Authority 2017). This information is handed out to all patients admitted to surgical departments as part of the clinical routine. Due to these major differences in current international practice, generalization of results could present a challenge ‐ regarding both the well‐known implementation scope and the required change in clinical culture (Nolan 2017).
Quality of the evidence
We used the GRADE system to rate the quality of outcomes included in Table 1. Ratings ranged from moderate for the outcomes of postoperative complications and successful quitting to low for the outcome of mortality.
The overall relatively small sample size presents a challenge. Conclusions based on such small numbers must be reached cautiously. Compliance with abstinence from alcohol was very high. However, the occurrence of successful quitting may be due to the low inclusion rate, introducing high risk of recruitment bias and overrepresentation of patients with high motivation for quitting, thus representing the "tip of the iceberg". The sparse data and the small numbers of events may render trial results fragile. Consequently, we downgraded the quality of evidence by one level due to risk of imprecision, and we downgraded the quality of evidence on mortality outcomes by one level due to risk of indirectness.
Inclusion of different surgical interventions led to potential risk of inconsistency, usually characterized by different levels of complications. This seems overruled by the increased complication rate across surgeries in high‐risk patients with a risky alcohol intake. Therefore we did not downgrade the quality of evidence for inconsistency in effect size.
Our search for relevant studies was thorough and included searches of relevant electronic databases and clinical trials registers and checking of reference lists in included studies. We cannot entirely exclude the possibility of publication bias, but we decided not to downgrade the quality of evidence due to publication bias.
Potential biases in the review process
One strength of the review process is that it involved a substantial search of electronic databases including clinical trials registries and manual searching of reference lists. In addition, three review authors (JE, HT, and JA) independently screened all titles and abstracts included in the search results. CBJ and JE independently collected and extracted data, assessed risk of bias, and rated the quality of evidence according to the GRADE system. We resolved disagreements by discussion and consensus. In addition, CBJ and JE entered all relevant data into Review Manager (Review Manager 2014), and BP performed double entry of the data.
This review has limitations; updates have been made regarding secondary outcomes and related analysis of data, which was not predefined in the original protocol (Oppedal 2010), but this decision was made before extraction of data and analysis for this review. As originally advised by the Cochrane Anaesthesia Review Group, we maintained exclusion of controlled clinical trials (CCTs) (Oppedal 2012). All review authors discussed the challenges encountered during the review process.
One of the co‐authors (HT) of this review has authored all three of the studies included in this review (Egholm 2017; Tønnesen 1999a; Tønnesen 2002). Four review authors (JE, HT, BP, and JA) authored one study (Egholm 2017). We have declared this information in the Declarations of interest section. To avoid potential bias, CBJ assessed the congruence of trials with inclusion criteria of the review (Appendix 6). In cases of disagreement, we would have contacted another review author (AMM) for discussion. However, we encountered no disagreements. It could be considered a limitation that two of the included studies have not been published (Egholm 2017; Tønnesen 2002); on the other hand, study data were fully accessible to review authors.
Agreements and disagreements with other studies or reviews
Several reviews on this topic have been published. Some of them are not systematic reviews (e.g. Breuer 2003), and others do not include RCTs (Tønnesen 1992c; Vagts 2003). Two other systematic reviews reported on very different interventions, such as alcohol cessation intervention and alcohol infusion in the same meta‐analysis, and included only certain types of postoperative complications: non‐surgical site infections (e.g. pneumonia, sepsis) and mortality (Shabanzadeh 2014; Shabanzadeh 2015). One of these studies identified a significant risk reduction regarding infections but not mortality, in spite of combining alcohol cessation interventions with alcohol infusion (Shabanzadeh 2015). Shabanzadeh 2014 included only two types of complications (surgical site infection and anastomotic leakage) and observed no differences in the meta‐analysis of mixed interventions.
None of the previous systematic reviews included all three of the studies included in our review. Four of these reviews ‐ Breuer 2003,Shabanzadeh 2014,Shabanzadeh 2015, and Tønnesen 1992c ‐ included only one of the RCTs included here (Tønnesen 1999a). Two systematic reviews also included Tønnesen 2002 in their reviews, which had a strong focus on the pathophysiology of alcohol‐induced organ dysfunction and recovery during abstinence from alcohol (Tønnesen 2009; Wåhlin 2014). Three reviews reached similar conclusions to those reported in our review, but they included fewer studies (Breuer 2003; Tønnesen 2009; Wåhlin 2014).
It is difficult to draw a direct comparison between some of the previous reviews and our current review because of differences in types of interventions and outcome parameters.
Authors' conclusions
Implications for practice.
Although there is wide agreement that high alcohol intake is a risk factor at surgery, only moderate‐quality evidence shows that intensive alcohol cessation interventions in the perioperative period reduce postoperative complications (e.g. wound‐related complications, secondary surgery, cardiopulmonary complications, admission to intensive care) as defined by the need for treatment.
The estimated effect could be of practical importance for patients undergoing surgery, for healthcare providers, and for society at large because complications are often followed by pain and discomfort, prolonged recovery, and increased costs. Because only a few small studies are available, no firm conclusions regarding clinical practice can be drawn. However, given that alcohol‐induced complications are severe and can be life‐threatening, side effects of the interventions are few, and the pathophysiology of surgical relevance is improved during the intensive intervention, this approach could be considered in clinical practice.
Moderate‐quality evidence shows that an intensive alcohol cessation intervention increases successful quitting (number of abstainers) at the end of the programme. Furthermore, data are insufficient to show effects on mortality.
We do not have sufficient evidence to determine if there are any differences in effect of intervention on length of stay or postoperative alcohol consumption (grams of alcohol/week) at the end of the programme because study authors did not report results as applicable for our evaluation. However, all studies noted significant reductions in alcohol consumption, which may be an implication for practice despite lack of overall evidence.
None of the included studies evaluated the prevalence of participants without risky drinking in the longer term (three‐, six‐, nine‐, and 12‐month follow‐up); therefore we are not able to determine effects on this outcome. Once published and assessed, the one 'ongoing' study identified may alter the conclusions of this review.
Implications for research.
This review has identified important areas of research concerning outcomes and study populations for new RCTs. Furthermore, researchers should evaluate different types of interventions including information technology (IT) support and should focus on evaluating clinical applicability because only intensive interventions for alcohol cessation have been investigated hitherto.
Types of outcomes
We identified no studies that reported on long‐term outcomes. It is highly relevant to evaluate whether perioperative alcohol cessation interventions have an impact on long‐term health similar to that reported with the perioperative smoking cessation programme (Thomsen 2014).
It would be preferable if future studies would report outcomes that can be included in meta‐analyses. We were not able to evaluate the body of evidence for length of stay and alcohol consumption at the end of the programme because investigators reported the two outcomes only as median and range values.
Sizable study populations
As described under Quality of the evidence, the included studies were small and reported few events of mortality. Therefore, we need larger RCTs powered to detect effects on mortality. Strong research competencies are necessary for future studies, as recruitment to these studies seems to be difficult, reflecting mainly on recruitment of participants in all three studies. Furthermore, only one of the three included studies has been published (Tønnesen 1999a). It is interesting to note that compliance of participants was high in all three studies. It also would be relevant to investigate effects of these interventions among patient groups in other countries and research groups, such as those undergoing head and neck surgery, lung resection, or bladder cancer operations.
Interventions
More information is needed to clarify the most beneficial intervention programmes, including duration of abstinence from alcohol and possible effects of reducing alcohol consumption.
New research should target several lifestyle risk factors because many patients have other risk factors in addition to risky alcohol drinking. Costs of postoperative complications are high for patients undergoing surgery and for society; therefore it is relevant to perform regular cost‐effectiveness analyses in line with value‐based principles of priority.
What's new
| Date | Event | Description |
|---|---|---|
| 21 September 2018 | New search has been performed | We have added three new review authors (Egholm JWM, Adami J, and Juhl CB) to the team, and one review author, Oppedal K, has left the team The search was updated in August 2017, and was expanded to include preoperative, perioperative, and postoperative time periods. The search identified 10 new publications and one ongoing study (NCT02188446). We included in the review one new study with 70 participants that previously was identified as ongoing (Egholm 2017) A new secondary outcome on successful quitting at the end of the intervention programme has been added. The former two outcomes: 'Prevalence of non‐hazardous drinkers in the postoperative period (3, 6, 9, and 12 month follow up)', and 'Prevalence of non‐alcohol use disorders (non‐AUD) patients in the postoperative period (3, 6, 9, 12 month follow up)' have been replaced by the outcome 'Prevalence of participants without risky drinking in the longer term' To comply with MECIR Reporting Standards, CBJ checked data extracted and analyses of the three included studies (see Declarations of interest) We have updated the following sections in our review: Abstract, Plain language summary, Summary of main findings, Methods, Results, PRISMA flow chart, Discussion, References, Characteristics of studies, Data and analyses (1.1, 1.2, 1.3), and Additional tables. |
| 21 September 2018 | New citation required but conclusions have not changed | The conclusions of this updated review have not been changed by the inclusion of one new study (Egholm 2017) To comply with current MECIR Reporting Standards, we have updated the methods on quality assessments to incorporate the GRADE method |
History
Protocol first published: Issue 2, 2010 Review first published: Issue 7, 2012
| Date | Event | Description |
|---|---|---|
| 8 March 2013 | Amended | Contact details have been updated |
Acknowledgements
We would like to thank Bronagh Blackwood (Content Editor); Vibeke E Horstmann (Statistical Editor); Louise O'Connor, Daniel M Shabanzadeh, and Laura Amato (Peer Reviewers); Janet Wale (Consumer Editor); and Andrew Smith (Co‐ordinating Editor) for their help and editorial advice during preparation of this systematic review.
We would like to thank Kristian Oppedal, author of the previous Cochrane Review (Oppedal 2012). We also would like to thank Janne Vendt for guidance on the literature search, and Jane Cracknell (Managing Editor) for her editorial advice during the entire process. We thank Susanne Vahr for her good advice during writing of this updated systematic review.
We would like to thank Andrew Smith (Content Editor); Cathal Walsh (Statistical Editor); Laura Amato (Cochrane Drugs and Alcohol Group); Claudia Spies and Elizabeth Proude (Peer Reviewers); and Tracey Lloyd (Consumer) for their help and editorial advice during preparation of this systematic review.
We would like to thank Andrew Smith (Content Editor); Nathan Pace (Statistical Editor); Laura Amato (Cochrane Drugs and Alcohol Group); and Claudia Spies and Elizabeth Proude (Peer Reviewers) for their help and editorial advice during preparation of the protocol for this review.
Appendices
Appendix 1. Search strategy for CENTRAL (the Cochrane Library)
#1 MeSH descriptor: [Alcoholics] explode all trees #2 MeSH descriptor: [Alcoholism] explode all trees #3 MeSH descriptor: [Alcohol‐Related Disorders] explode all trees #4 MeSH descriptor: [Alcohol Drinking] explode all trees #5 (alcohol* near (drink* or use* or intake or intervention* or education or program* or misuse* or abuse* or consump*)) #6 (drink* near (behaviour or hazardous or harmful* or dependen*)) #7 #1 or #2 or #3 or #4 or #5 or #6 #8 MeSH descriptor: [Postoperative Complications] explode all trees #9 MeSH descriptor: [Perioperative Care] explode all trees #10 MeSH descriptor: [Preoperative Care] explode all trees #11 MeSH descriptor: [Perioperative Period] explode all trees #12 MeSH descriptor: [Surgical Procedures, Operative] explode all trees #13 preoperat* or perioperat* or postoperat* or pre operat* or peri operat* or post operat* #14 complication* #15 surg* or operat* #16 #14 and #15 #17 #8 or #9 or #10 or #11 or #12 or #13 or #16 #18 #7 and #17, in Trials
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1 exp Alcohol Drinking/ or Alcoholism/ or Alcohol‐Related Disorders/ or alcoholics/
2 (alcohol* adj3 (drink* or use* or intake or intervention* or education or program* or misuse* or abuse* or consump*)).mp.
3 (drink* adj3 (behaviour or hazardous or harmful* or dependen*)).mp.
4 1 or 2 or 3
5 exp Postoperative Complications/ or exp perioperative care/ or exp preoperative care/ or exp Perioperative Period/ or exp Surgical Procedures, Operative/
6 (preoperat* or perioperat* or postoperat* or pre operat* or peri operat* or post operat*).mp.
7 complication*.mp.
8 (surg* or operat).ti,ab. or surgery.fs.
9 7 and 8
10 5 or 6 or 9
11 4 and 10
12 ((randomized controlled trial or controlled clinical trial).pt. or randomi?ed.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (exp animals/ not humans.sh.)
13 11 and 12
Appendix 3. Search strategy for Embase (Ovid SP)
1 drinking behavior/ or alcoholism/ or exp alcohol abuse/ or alcohol abstinence/ or alcohol consumption/ or alcohol withdrawal/
2 (alcohol* adj3 (drink* or use* or intake or intervention* or education or program* or misuse* or abuse* or consump*)).mp.
3 (drink* adj3 (behaviour or hazardous or harmful* or dependen*)).mp.
4 1 or 2 or 3
5 (preoperat* or perioperat* or postoperat* or pre operat* or peri operat* or post operat*).ti,ab.
6 exp complication/su or exp postoperative complication/ or perioperative period/ or exp preoperative period/ or exp postoperative period/
7 (surg* or operat*).ti,ab. or surgery.fs.
8 5 or 6 or 7
9 4 and 8
10 ((CROSSOVER PROCEDURE or DOUBLE‐BLIND PROCEDURE or SINGLE‐BLIND PROCEDURE).sh. or (crossover* or cross over*).ti,ab. or placebo*.ti,ab. or (doubl* adj blind*).ti,ab. or allocat*.ti,ab. or trial.ti. or RANDOMIZED CONTROLLED TRIAL.sh. or random*.ti,ab.) not ((exp animal/ or exp invertebrate/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans or man or men or wom?n).ti.))
11 9 and 10
Appendix 4. Search strategy for CINAHL (EBSCOhost)
S1 (MH "Alcohol‐Related Disorders+") OR (MH "Alcohol Drinking+") OR (MH "Alcoholism") OR (MH "Drinking Behavior+") OR (MH "Alcohol Abuse")
S2 TX alcohol* N5 (drink* or use* or intake or intervention* or education or program* or misuse* or abuse* or consump*)
S3 TX drink* N5 (behaviour or hazardous or harmful* or dependen*)
S4 S1 OR S2 OR S3
S5 (MH "Postoperative Complications+") OR (MH "Postoperative Period") OR (MH "Postoperative Care") OR (MH "Postoperative Pain") OR (MH "Preoperative Period") OR (MH "Preoperative Care") OR (MH "Preoperative Education") OR (MH "Perioperative Care") OR TX (preoperat* or perioperat* or postoperat* or pre operat* or peri operat* or post operat*)
S6 TX complication* AND TX ( surg* or operat* )
S7 S5 OR S6
S8 S4 AND S7
S9 (MH "Clinical Trials+")
S10 PT Clinical trial
S11 TX clinic* n1 trial*
S12 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*)
S13 TX randomi* control* trial*
S14 (MH "Random Assignment")
S15 TX random* allocat*
S16 TX placebo* 36,091
S17 (MH "Placebos")
S18 (MH "Quantitative Studies")
S19 TX allocat* random*
S20 S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 982,793
S21 S8 AND S20
Appendix 5. Assessment of risk of bias in included studies
Random sequence generation
Low risk of bias: the method used generated random sequences, e.g. random number generation or toss of coin.
Unclear: no available information on random sequence generation.
High risk of bias: alternate medical record numbers or other non‐random sequence generation.
Allocation concealment
Low risk of bias: allocation method prevented investigators and participants from knowing the next allocation, e.g. central allocation; sealed opaque envelopes; serially numbered or sequentially numbered but otherwise identical vehicles, including their contents; or other descriptions of convincing concealment of allocation.
Unclear: no information on allocation method was available, or the description did not allow a clear distinction.
High risk of bias: allocation method allowed investigators or participants, or both, to know the next allocation, e.g. alternate medical record numbers; reference to case record numbers or date of birth; open allocation sequence, such as unsealed envelopes.
Incomplete outcome data
Low risk of bias: numbers and reasons for dropouts and withdrawals in the intervention groups were equal and unrelated to the intervention (e.g. emigration), or it was specified that there were no dropouts or withdrawals, or analysis was performed on an intention‐to‐treat basis.
Unclear: the report gave the impression that there were no dropouts or withdrawals, but this was not specifically stated.
High risk of bias: numbers or reasons for dropouts and withdrawals were not sufficiently accounted for ‐ or a high number of dropouts.
Selective outcome reporting
Low risk of bias: available study protocol and prespecified outcomes, or if no protocol was published, all expected outcomes had been reported.
Unclear: insufficient information provided.
High risk of bias: if no study protocol was available, and if not all prespecified outcomes had been reported on all time points.
Other bias
Could be funding of studies, baseline differences, and selective patient population.
Blinding
Low risk of bias: investigators, patients, and outcome assessors were kept unaware of intervention allocations after inclusion of participants into the study, or knowledge of allocation could not affect evaluation of the outcome (e.g. death).
Unclear: blinding was not described.
High risk of bias: no blinding of patients and outcome assessors; categorized as an open‐label study; or without use of placebo.
Appendix 6. Study selection form (perioperative alcohol cessation intervention for postoperative complications)
| First author | Journal/Conference proceedings, etc | Year | Contributing author |
| Study eligibility | |||
| RCT | Relevant participant (risky alcohol consumption and surgical procedure) |
Relevant outcomes (postoperative complicationsa, in‐hospital or 30‐day mortality, successful quittersb, postoperative alcohol consumptionc, length of stayd, prevalence of participants without risky drinking in the long terme). |
|
| Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear | |
| Do not proceed if any of the above answers is 'No'. If study is to be included in the 'Excluded studies' section of the review, record below the information to be inserted into the 'Characteristics of excluded studies' table. | |||
Footnotes
aAny type of postoperative complication (e.g. wound‐related complications, secondary surgery, cardiopulmonary complications, admission to intensive care) as defined by the need for intervention.
bNumber of abstainers at the end of the programme (with or without validation by interview, breath test, or alcohol markers).
cGrams of alcohol/week at the end of the programme.
dNumber of days from admission to discharge. For patients re‐admitted due to complications during the follow‐up period, the total number of hospital days was summarized.
eFollow‐up at 3, 6, 9, and 12 months after surgery.
Data and analyses
Comparison 1. Perioperative alcohol cessation intervention versus treatment as usual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative complications after perioperative intervention | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Intensive intervention | 3 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.40, 0.96] |
| 2 In‐hospital and 30‐day mortality after perioperative intervention | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Intensive intervention | 3 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.07, 2.96] |
| 3 Successful quitters (number of abstainers) following preoperative and perioperative interventions | 3 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 8.22 [1.67, 40.44] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Egholm 2017.
| Methods | RCT | |
| Participants | Country of region: Denmark, 2 centres. Block randomization with stratification for each centre N = 70; intervention group (n = 35), control group (n = 33) Age (years); intervention group 48 (22 to 77), control group 53 (20 to 78) Sex (men); intervention group 25 (71%), control group 22 (65%) Study period: May 2010 to July 2014. Clinical setting: orthopaedic surgery Inclusion criteria
Exclusion criteria
|
|
| Interventions | Project staffs were trained at a 2‐day educational course before providing the intervention Intensive intervention
Control group
|
|
| Outcomes |
Primary outcomes: follow‐up at 6 weeks, 3, 6, 9, and 12 months
|
|
| Notes |
Funding: the study was funded by the Swedish Institute of Public Health, the Danish National Board of Health, and the Western Health Region in Norway and in the Skåne Region in Sweden The protocol is registered at ClinicalTrials.gov (ID: NCT00986791) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code (block randomization with stratification for each centre) |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes with consecutive numbers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Inclusion within 36 hours after entering the hospital. 2 participants from the control group were excluded as they did not undergo surgery Participants who withdrew: 0/33 in the control group and 4/35 in the intervention group Participants who dropped out: 1/33 in the control group and 1/35 in the intervention group Participants who cancelled the 6‐week meeting: 1/33 in the control group and 1/35 in the intervention group, but data were collected at the following meeting All participants were allowed to follow up via the medical record system. Data on all participants were available |
| Selective reporting (reporting bias) | Low risk | Selective reporting was not identified. A published protocol was available |
| Other bias | Unclear risk | Recruitment seemed to be difficult as the number needed to screen to identify eligible participants was very high. Many participants declined participation in the study. Therefore the study may not be representative of the whole population |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible. Participants were not blinded; however main outcomes were evaluated in a blinded fashion |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment of postoperative complications was performed via a double‐blinded approach |
Tønnesen 1999a.
| Methods | RCT | |
| Participants | Country of region: Denmark, 3 centres N = 42 Age (years); intervention group 58 (37 to 75), control group 61 (50 to 76) Sex (men); intervention group 16/16, control group 16/19 Clinical setting: gastrointestinal surgery Study period: November 1995 to May 1998 Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intensive intervention
Control group
|
|
| Outcomes |
Follow‐up during hospital stay
Follow‐up perioperatively
Follow‐up at 1 month
Follow‐up at 4 weeks
|
|
| Notes | In total, 42 participants were included in the study Intervention group n = 20
Control group n = 22
4 intervention and 3 control participants were therefore not included in the analyses on postoperative complications and in‐hospital and 30‐day mortality after preoperative intervention, leaving 16 participants in the intervention group and 19 participants in the control group Funding: Danish Ministry of Health fund for Alcohol Research |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code (block randomization with stratification for each centre) |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes with consecutive numbers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 2/21 in the control group and 4/20 in the intervention group. Intention‐to‐treat analysis was not performed. Study authors report including patients before the final decision regarding operation was made, and that 3 participants in the control group and 4 participants in the intervention group were excluded after randomization Data were available on most participants |
| Selective reporting (reporting bias) | Unclear risk | Selective reporting was not identified. No protocol was available, so it is unclear if all outcomes were reported. Several outcomes were reported only with mean and range values |
| Other bias | Unclear risk | Only 3 women were included; all were in the control group. Recruitment seemed to be difficult as the number needed to screen to identify eligible patients was very high. Many participants declined participation in the study. Therefore, this study may not be representative of the whole population |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible. Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | All analyses of pathophysiological parameters were performed blinded, but not the primary outcomes |
Tønnesen 2002.
| Methods | RCT | |
| Participants | Country of region: Denmark, 6 centres. Block randomization with stratification for each centre N = 28 Age (years); intervention group 57 (39 to 75), control group 63 (49 to 70) Sex: men Clinical setting: orthopaedic surgery Study period: 1997 to 1999 Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intensive intervention
Control group
|
|
| Outcomes |
Follow‐up during admission
Follow‐up at 1 month
Follow‐up at 3 months
|
|
| Notes |
Intervention group
Control group
All participants were not included in the analyses on postoperative complications and in‐hospital and 30‐day mortality after preoperative intervention Leaving 10 participants in the intervention group and 9 participants in the control group Funding: IMK Almene Fond from Denmark |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization and computer‐generated code |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes with consecutive numbers |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: 4/13 in the control group and 5/15 in the intervention group. Study authors report included participants before a final decision on the operation was made; 4 participants in the control group and 5 participants in the intervention group were excluded after randomization, as they fulfilled the exclusion criteria in the preoperative period. Study authors report both intention‐to‐treat and per‐protocol analyses based on the remaining participants. Data were available on most participants. However, attrition rates were very high |
| Selective reporting (reporting bias) | Unclear risk | Selective reporting was not identified. No protocol was available, so it is unclear if all outcomes were reported. Several outcomes were reported only as mean and range values |
| Other bias | Unclear risk | Only men were included. Recruitment seemed to be difficult, as the number needed to screen to identify eligible patients was very high. Many participants declined participation in the study. Therefore the study may not be representative of the whole population |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible. Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Biochemical analysis was performed blinded, but not the primary outcome |
ASA: American Society of Anesthesiologists (ASA); AU: alcohol unit; CTD: carbohydrate‐deficient transferrin; EKG: electrocardiogram; PEth: phosphatidyl ethanol.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Antti‐Poika 1988 | Not all participants underwent surgery |
| Avram 2009 | Wrong intervention |
| Batioglu‐Karaaltin 2017 | Wrong study design and no intervention |
| Bejou 2000 | Review of article; not primary research |
| Chiang 1995 | Review of article; not primary research |
| Forsberg 2000 | Not all participants underwent surgery |
| Gentilello 1999 | Not all participants underwent surgery. The outcome was re‐trauma, but not complications |
| Heather 1996 | Not all participants underwent surgery |
| Holloway 2007 | Not all participants underwent surgery |
| Kaka 2017 | Wrong study design |
| Schermer 2006 | Not all participants underwent surgery |
| Schoenfeld 2007 | Wrong intervention. No alcohol cessation intervention |
| Shepard 2016 | Not all participants underwent surgery |
| Shetty 2011 | Not all participants underwent surgery |
| Shourie 2006 | Wrong study design; began as a randomized trial, but was changed to a controlled clinical trial |
| Smith 2003 | Not all participants underwent surgery |
| Sommers 2006 | Not all participants underwent surgery |
| Soria 1981 | Review of articles; not primary research |
| Spies 1995 | Wrong intervention. No alcohol cessation intervention |
| Spies 1996 | Wrong intervention. No alcohol cessation intervention |
| Spies 2006 | Wrong intervention. No alcohol cessation intervention |
| Vagts 2002 | Review of articles; not primary research |
| Watson 1999 | Wrong study design |
Characteristics of ongoing studies [ordered by study ID]
NCT02188446.
| Trial name or title | STOP smoking and alcohol drinking before OPeration for bladder cancer (the STOP‐OP Study) |
| Methods | RCT |
| Participants | Country of region: Denmark, 5 centres N = 110 Age: 18 years Sex: mixed Clinical setting: urological surgery Inclusion criteria
Exclusion criteria
|
| Interventions |
Intensive intervention
Control group
|
| Outcomes |
Primary outcomes
Secondary outcome
|
| Starting date | November 2014 |
| Contact information | Susanne Vahr Lauridsen; phone: +45 35451704 Mail: susanne.vahr@regionh.dk |
| Notes | ClinicalTrials.gov identifier: NCT02188446 |
NRT: nicotine replacement therapy; RCT: randomized controlled trial.
Differences between protocol and review
We made the following changes in the update to the published review (2017).
In the review protocol (Oppedal 2010), we did not specify:
one objective, exclusively. This has been done according to the Methodological Expectations of Cochrane Intervention Reviews (MECIR), which requires one main outcome. We changed the objective from "To assess the effect of preoperative alcohol cessation interventions on the rate of postoperative complications including mortality in hazardous drinkers" and "To assess the effect of postoperative alcohol cessation interventions for hazardous drinkers on alcohol use in the postoperative period and longer term" to "To assess the effect of perioperative alcohol cessation interventions on the rate of postoperative complications";
an outcome on successful quitting (as numbers of abstainers) at the end of the alcohol cessation intervention programme, even though improvement in alcohol‐induced organ dysfunctions ‐ of importance for the surgical pathophysiology ‐ takes place only during complete abstinence from alcohol. This has now replaced the former outcome "prevalence of non‐alcohol use disorders (non‐AUD) patients in the postoperative period". AUD is often diagnosed by the AUDIT tool, which has recently been shown to not be associated with the present consumption and postoperative complications (Rubinsky 2013);
the long‐term prevalence of participants without risky drinking. However, we have included this according to the above reasons, and it replaces the previous outcomes on non‐AUD. Furthermore, the term 'risky drinking' is clearly defined by the amount of alcohol consumed per time unit; it has a close relationship to postoperative morbidity;
the intervention period to include preoperative, intraoperative, and postoperative interventions. We have included this perioperative period because the intervention does not take place only preoperatively, but also postoperatively. This is now also reflected in the title, which we have changed from "Preoperative alcohol cessation prior to elective surgery (Review)" to "Perioperative alcohol cessation intervention for postoperative complications";
the analysis of length of stay in accordance with MECIR recommendations that an estimate shall be based on the SD, which was not provided in the included studies. The previous meta‐analysis on length of stay was estimated from range and is therefore removed;
how we would apply the GRADE system to assess the quality of the body of evidence. In the review, we have now explained this in the methods of the Data collection and analysis section; or
the "postoperative alcohol consumption (grams of alcohol/week) at end of programme", which should also be measured here and not only after three, six, nine, and 12 months.
We have included:
the outcomes specified above in the search strategy (Appendix 1, Appendix 2, Appendix 3, Appendix 4), as well as in Table 1, in the Data collection and analysis section, and in Analysis 1.3;
the term 'risky drinking' instead of 'hazardous drinking', because risky drinking is used for alcohol intake above a specified limit independent of addiction symptoms and other harms related to alcohol intake. As mentioned above, this review uses a specific definition of the term related to development of complications after surgery. The definition may vary by the group of participants (e.g. risky drinking in children is defined by any alcohol intake, many countries have different thresholds for men and women). Other descriptions of alcohol consumption are weakly defined except for addiction (WHO 2014); and
new members of the review team JE, JA, and CBJ; KO has left the review team.
We made the following changes to the protocol (Oppedal 2010), in the published review (Oppedal 2012).
During the early review process, we followed the advice provided by the Cochrane Anaesthesia Review Group to not include controlled clinical trials in the review. We have also removed the additional tables from the Appendix.
Contributions of authors
Conceiving the review: JE (Julie Weber Melchior Egholm), AMM (Ann Merete Møller), BP (Bolette Pedersen), JA (Johanna Adami), CBJ (Carsten B Juhl), HT (Hanne Tønnesen), and Kristian Oppedal (KO).
Co‐ordinating the review: JE.
Undertaking manual searches: JE, HT, JA.
Screening search results: JE, HT.
Organizing retrieval of papers: JE, JA.
Screening retrieved papers against inclusion criteria: JE, JA.
Appraising the quality of papers: JE.
Abstracting data from papers: CBJ, JE.
Writing to authors of papers for additional information: JE.
Providing additional data about papers: JE, HT.
Obtaining and screening data on unpublished studies: JE, JA, BP, CBJ.
Managing data for the review: JE, BP, CBJ.
Entering data into Review Manager (Review Manager 2014): JE, BP, CBJ.
Analysing RevMan statistical data: CBJ.
Performing other statistical analysis not using RevMan: BP, CBJ.
Providing double entry of data: data entered by person one: JE and CBJ; data entered by person two: BP.
Interpreting data: JE, JA, BP, AMM, HT, CBJ.
Making statistical inferences: JE, JA, BP, AMM, HT, CBJ.
Writing the review: JE, JA, BP, AMM, HT, CBJ.
Securing funding for the review: HT.
Performing previous work that was the foundation of the present study: KO, JE, JA, BP, AMM, HT, CBJ.
Serving as guarantor for the review (one review author): JE.
Taking responsibility for reading and checking the review before submission: JE.
Sources of support
Internal sources
-
Alcohol and Drug Research, Western Norway, Norway.
Salary, Kristian Oppedal
External sources
No sources of support supplied
Declarations of interest
Hanne Tønnesen has authored all three of the studies included in this review (Egholm 2017; Tønnesen 1999a; Tønnesen 2002).
Julie Weber Melchior Egholm, Johanna Adami, and Bolette Pedersen have authored one of the included studies (Egholm 2017).
Hanne Tønnesen is also the primary investigator for the ongoing study (NCT02188446).
Therefore, to avoid any potential bias, Carsten B Juhl extracted data and checked the interpretation against study reports and any available study registration details or protocols as an independent review author.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Egholm 2017 {unpublished data only}
- Egholm JWM, Pedersen B, Oppedal K, Rasmussen M, Adami J, Lauritzen JB, et al. Scand‐Ankle: effect of patient education for alcohol cessation intervention on acute fracture surgery ‐ a randomized controlled trial. Unpublished RCT ‐ Data on file (as supplied 1 June 2017).
Tønnesen 1999a {published data only (unpublished sought but not used)}
- Tønnesen H, Rosenberg J, Nielsen HJ, Rasmussen V, Hauge C, Pedersen IK, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ 1999;318(7194):1311‐6. [PUBMED: 10323814] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tønnesen 2002 {unpublished data only}
- Tønnesen H. Intensive intervention among alcohol patients prior to elective hip replacement‐complications, life‐quality, surgical outcome. Report ‐ Unpublished data 31.07.2002.
References to studies excluded from this review
Antti‐Poika 1988 {published data only}
- Antti‐Poika I, Karaharju E, Roine R, Salaspuro M. Intervention of heavy drinking ‐ a prospective and controlled study of 438 consecutive injured male patients. Alcohol and Alcoholism 1998;23(2):115‐21. [PUBMED: 3390235] [PubMed] [Google Scholar]
Avram 2009 {published data only}
- Avram O, Grecu I, Macovei R, Marinesco O, Grintescu I. Benefits of implementations of a protocol for preoperative chronic alcohol abusers identification and alcohol withdrawal syndrome prevention. Romanian Journal of Anaesthesia and Intensive Care 2009;16:121‐7. [Google Scholar]
Batioglu‐Karaaltin 2017 {published data only}
- Batioglu‐Karaaltin A, Binbay Z, Yigit Ö, Dönmez Z, Yollu U. Can temperament and character features of patients predict alcohol use after laryngectomy?. Clinical Otolaryngolgy 2017;42:373‐80. [PUBMED: 27717234 ] [DOI] [PubMed] [Google Scholar]
Bejou 2000 {published data only}
- Bejou B, Benamouzig R. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomized controlled trial [Effect de l'abstinence sur le prognostic post‐opératorie des consommateus excessifs d'alcool]. Gastroenterérologie Clinique Et Biologique 2000;24(3):377‐8. [PUBMED: 10866517] [PubMed] [Google Scholar]
Chiang 1995 {published data only}
- Chiang PP. Perioperative management of the alcohol dependent patient. American Family Physician 1995;52(8):2267‐73. [PUBMED: 7484720] [PubMed] [Google Scholar]
Forsberg 2000 {published data only}
- Forsberg L, Ekman S, Halldin J, Ronnberg S. Brief intervention for risk consumption of alcohol at an emergency surgical ward. Addictive Behaviors 2000;25(3):471‐5. [PUBMED: 10890304] [DOI] [PubMed] [Google Scholar]
Gentilello 1999 {published data only}
- Gentilello LM, Rivara FP, Donovan DM, Jurkovich GJ, Daranciang E, Dunn CW, et al. Alcohol interventions in the trauma centre as a means of reducing the risk of injury recurrence. Annals of Surgery 1999;230(4):473‐80. [PUBMED: 10522717] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heather 1996 {published data only}
- Heather N, Rollnick S, Bell A, Richmond R. Effects of brief counselling among male heavy drinkers identified on general hospital wards. Drugs and Alcohol Review 1996;15(1):29‐38. [PUBMED: 16203349] [DOI] [PubMed] [Google Scholar]
Holloway 2007 {published data only}
- Holloway AS, Watson HE, Arthur AJ, Starr G, McFadyen AK, McIntosh J. The effect of brief interventions on alcohol consumption among heavy drinkers in a general hospital setting. Addiction 2007;102(11):1762‐70. [PUBMED: 17784901] [DOI] [PubMed] [Google Scholar]
Kaka 2017 {published data only}
- Kaka AS, Zhao S, Ozer E, Agrawal A, Kang S, Rocco J, et al. Comparison of clinical outcomes following head and neck surgery among patients who contract to abstain from alcohol vs patients who abuse alcohol. JAMA Otolaryngology‐Head & Neck Surgery 2017;143(12):1181‐6. [PUBMED: 28447103 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schermer 2006 {published data only}
- Schermer CR, Moyers TB, Miller WR, Bloomfield LA. Traumacentre brief interventions for alcohol disorders decrease subsequent driving under the influence arrests. Journal of Trauma 2006;60(1):29‐34. [PUBMED: 16456433] [DOI] [PubMed] [Google Scholar]
Schoenfeld 2007 {published data only}
- Schoenfeld H, Heymann C, Lau A, Krocker D, Neuner B, Schink T, et al. The effect of stress‐reducing, low‐dose ethanol infusion on frequency of bleeding complications in long‐term alcoholic patients undergoing major surgery. American Surgeon 2007;73(2):192‐8. [PUBMED: 17305301] [PubMed] [Google Scholar]
Shepard 2016 {published data only}
- Shepard DS, Lwin AK, Barnett NP, Mastroleo N, Colby SM, Gwaltney C, et al. Cost‐effectiveness of motivational intervention with significant others for patients with alcohol misuse. Addiction 2016;111(5):832‐9. [PUBMED: 26574195 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shetty 2011 {published data only}
- Shetty V, Murphy DA, Zigler C, Yamashita DDR, Belin TR. Randomized controlled trial of personalized motivational interventions in substance using patients with facial injuries. Journal of Oral and Maxillofacial Surgery 2011;69(9):2396‐411. [PUBMED: 21496991 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shourie 2006 {published and unpublished data}
- Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol and Alcoholism 2006;41(6):643‐9. [PUBMED: 16905552] [DOI] [PubMed] [Google Scholar]
Smith 2003 {published data only}
- Smith AJ, Hodgson J, Bridgeman K, Shepherd JP. A randomized controlled trial of brief intervention after alcohol‐related facial injury. Addiction 2003;98(1):43‐52. [PUBMED: 12492754] [DOI] [PubMed] [Google Scholar]
Sommers 2006 {published data only}
- Sommers MS, Dyehouse JM, Howe SR, Fleming M, Fargo JD, Schafer JC. Effectiveness of brief interventions after alcohol‐related vehicular injury: a randomized controlled trial. Journal of Trauma 2006;61(3):523‐31. [PUBMED: 16966982] [DOI] [PubMed] [Google Scholar]
Soria 1981 {published data only}
- Soria P, Richard P. Tiapride and alcoholism in surgical practice [Tiapride et ethylisme en milieu chirurgical]. Annales De Chirurgie 1981;35(6):425‐8. [PUBMED: 7259038] [PubMed] [Google Scholar]
Spies 1995 {published data only}
- Spies CD, Dubisz N, Funk W, Blum S, Müller C, Rommelspacher H, et al. Prophylaxis of alcohol withdrawal syndrome in alcohol‐dependent patients admitted to the intensive care unit after tumour resection. British Journal of Anaesthesia 1995;75(734):9. [PUBMED: 8672322] [DOI] [PubMed] [Google Scholar]
Spies 1996 {published data only}
- Spies CD, Nordmann, A, Brummer G, Marks C, Conrad C, Berger G, et al. Intensive care unit stay is prolonged in chronic alcoholic men following tumor resection of the upper digestive tract. Acta Anaesthesiologica Scandinavica 1996;40(6):649‐56. [PUBMED: 8836256] [DOI] [PubMed] [Google Scholar]
Spies 2006 {published data only}
- Spies C, Eggers V, Szabo G, Lau A, Dossow V, Schoenfeld H, et al. Intervention at the level of the neuroendocrine‐immune axis and postoperative pneumonia rate in long‐term alcoholics. American Journal of Respiratory and Critical Care Medicine 2006;174:408‐14. [DOI: 10.1164/rccm.200506-907OC; PUBMED: 16728716 ] [DOI] [PubMed] [Google Scholar]
Vagts 2002 {published data only}
- Vagts VDA, Noldge‐Schomburg GFE. Anesthesiological considerations in alcoholic patients. Anaesthesiologie und Reanimation 2002;27(6):160‐7. [PubMed] [Google Scholar]
Watson 1999 {published data only}
- Watson HE. Problem drinkers in acute care settings: validation of an assessment instrument. International Journal of Nursing Studies 1999;36(5):415‐23. [PUBMED: 10519686] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02188446 {published data only}
- NCT02188446. Intensive smoking and alcohol cessation intervention in bladder cancer surgery patients (STOP‐OP). clinicaltrials.gov/ct2/show/NCT02188446 (date first received 11 July 2014).
Additional references
Amato 2010
- Amato L, Minozzi S, Vecchi S, Davoli M. Benzodiazepines for alcohol withdrawal. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD005063.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
ASA 2014
- American Society of Anesthesiologists. ASA physical status classification system. https://www.asahq.org/resources/clinical‐information/asa‐physical‐status‐classification‐system 2014.
Babor 2001
- Babor T, Higgins‐Biddle JC, Saunders JB, Maristela MG. AUDIT. Alcohol use disorders identification test. Guidelines for use in primary care. AUDIT. Alcohol Use Disorders Identification Test. Guidelines for Use in Primary Care. Geneva, Switzerland: World Health Organization, 2001. [Google Scholar]
Breuer 2003
- Breuer JP, Neumann T, Heinz A, Kox WJ, Spies C. The alcoholic patient in the daily routine. Wiener Klinische Wochenschrift 2003;115(17‐18):618‐33. [PUBMED: 14603733] [DOI] [PubMed] [Google Scholar]
Clavien 2009
- Clavien PA, Barkun J, Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien‐Dindo classification of surgical complications: five‐year experience. Annals of Surgery 2009;250(2):187‐96. [DOI] [PubMed] [Google Scholar]
Danish Health Authority 2017
- Danish Health Authority. Alcohol and surgery ‐ prevent complications when undertaken surgery [Alkohol og operationer ‐ forebyg komplikationer når du skal opereres]. https://www.sst.dk/da/udgivelser/2012/alkohol‐og‐operationer‐forebyg‐komplikationer‐naar‐du‐skal‐opereres (accessed August 2017).
DSM‐4 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐4). https://www.psychiatry.org/psychiatrists/practice/dsm 1994 (accessed August 2018).
DSM‐5 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐5). https://www.psychiatry.org/psychiatrists/practice/dsm 2013 (accessed August 2018).
Eliasen 2013
- Eliasen M, Grønkjær M, Skov‐Ettrup LS, Mikkelsen SS, Becker U, Tolstrup JS, et al. Preoperative alcohol consumption and postoperative complications. A systematic review and meta‐analysis. Annals of Surgery 2013;258(6):930‐42. [PUBMED: 23732268 ] [DOI] [PubMed] [Google Scholar]
Ewing 1984
- Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA 1984;12(14):1905‐7. [PMID 6471323] [DOI] [PubMed] [Google Scholar]
Fiore 2008
- Fiore MC, Jaén CR, Baker WC, Bailey WC, Bennett G, Benowitz NL, et al. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US public health service report. American Journal of Preventive Medicine 2008;35(2):158‐76. [PUBMED: 18617085] [DOI] [PMC free article] [PubMed] [Google Scholar]
GBD 2015
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980‐2015: a systematic analysis for Global Burden of Disease Study 2015. Lancet 2016;388:1459‐54. [PUBMED: 27733281 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 6 August 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Knuz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Knuz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summery of finding tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI: 10.1016/j.jclinepi.2010.04.026; PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jørgensen 2011
- Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcoholism, Clinical and Experimental Research 2011;10:1749‐58. [DOI: 10.1111/j.1530-0277.2011.01523.x.; PUBMED: 21615426] [DOI] [PubMed] [Google Scholar]
Kaner 2007
- Kaner EF, Dickinson HO, Beyer FR, Campbell F, Schlesinger C, Heather N, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD004148.pub3] [DOI] [PubMed] [Google Scholar]
Kip 2008
- Kip MJ, Neumann T, Jugel C, Kleinwaechter R, Weiss‐Gerlach E, Guill MM, et al. New strategies to detect alcohol use disorders in the preoperative assessment clinic of a German university hospital. Anesthesiology 2008;109(2):169‐70. [PUBMED: 18648225] [DOI] [PubMed] [Google Scholar]
McQueen 2009
- McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD005191.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Minozzi 2010
- Minozzi S, Amato L, Vecchi S, Davoli M. Anticonvulsants for alcohol withdrawal. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD005064.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mundt 2003
- Mundt K, Jensen M, Kann A, Nielsen AS, Grønbæk M, Tønnesen H. Alkohol ‐ Forebyggelse på Sygehus, Fakta, Metoder og Anbefalinger [Alcohol ‐ Prevention in Hospitals, Facts, Methods and Recommendations]. 1st Edition. Vol. 1, Copenhagen: Klinisk Enhed for Sygdomsforebyggelse, 2003. [ISBN: 87‐91098‐02‐5] [Google Scholar]
Nolan 2017
- Nolan MB, Warner DO. Perioperative tobacco use treatments: putting them into practice. BMJ 2017;358:3340. [DOI: 10.1136/bmj.j3340; PUBMED: 28877905] [DOI] [PubMed] [Google Scholar]
Rasmussen 2016
- Rasmussen M, Tønnesen H. The smoking cessation database. Clincal Health Promotion 2016;6(2):36‐41. [Google Scholar]
Rasmussen 2017
- Rasmussen M, Fernández E, Tønnesen H. Effectiveness of the gold standard programme compared with other smoking cessation interventions in Denmark: a cohort study. BMJ Open 2017;7(2):e013553. [PUBMED: 28242770 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rotevatn 2017
- Rotevatn TA, Bøggild H, Olesen CR, Torp‐Pedersen C, Mortensen RN, Jensen PF, et al. Alcohol consumption and the risk of postoperative mortality and morbidity after primary hip or knee arthroplasty ‐ a register‐based cohort study. PLOS One 2017;12(3):e0173083. [https://doi.org/10.1371/journal.pone.0173083 Access November 2017; PUBMED: 28306737 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubinsky 2013
- Rubinsky AD, Bishop MJ, Maynard C, Henderson WG, Hawn MT, Harris AH, et al. Postoperative risks associated with alcohol screening depend on documented drinking at the time of surgery. Drug and Alcohol Dependence 2013;132(3):521‐7. [DOI: 10.1016/j.drugalcdep.2013.03.022; PUBMED: 23683792] [DOI] [PubMed] [Google Scholar]
Rösner 2010a
- Rösner S, Hackl‐Herrwerth A, Leucht S, Lehert P, Vecchi S, Soyka M. Acamprosate for alcohol dependence. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD004332.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rösner 2010b
- Rösner S, Hackl‐Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M. Opioid antagonists for alcohol dependence. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD001867.pub3] [DOI] [PubMed] [Google Scholar]
Selzer 1971
- Selzer ML. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. American Journal of Psychiatry 1971;127(12):1653‐8. [DOI] [PubMed] [Google Scholar]
Shabanzadeh 2014
- Shabanzadeh DM, Sørensen LT. Alcohol drinking does not affect postoperative surgical site infection or anastomotic leakage: a systematic review and meta‐analysis. Journal of Gastrointestinal Surgery 2014;18(2):414‐25. [DOI: 10.1007/s11605-013-2275-5; PUBMED: 23835732] [DOI] [PubMed] [Google Scholar]
Shabanzadeh 2015
- Shabanzadeh DM, Sørensen LT. Alcohol consumption increases post‐operative infection but not mortality: a systematic review and meta‐analysis. Surgical Infections 2015;16(6):657‐68. [DOI: 10.1089/sur.2015.009; PUBMED: 26244748 ] [DOI] [PubMed] [Google Scholar]
SOF 2017
- Swedish Orthopaedic Association. www.ortopedi.se (accessed August 2017).
Spies 2001
- Spies C, Tønnesen H, Andreasson S, Helander A, Conigrave K. Perioperative morbidity and mortality in chronic alcoholic patients. Alcoholism, Clinical and Experimental Research 2001;25(5):164‐70. [PUBMED: 11391067] [DOI] [PubMed] [Google Scholar]
Spies 2004
- Spies CD, Dossow V, Eggers V, Jetschmann G, El‐Hilali R, Egert J, et al. Altered cell‐mediated immunity and increased postoperative infection rate in long‐term alcoholic patients. Anesthesiology 2004;100(5):1088‐100. [PUBMED: 15114205] [DOI] [PubMed] [Google Scholar]
The ICD‐10 Classification, 1993
- The ICD‐10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva, Switzerland: World Health Organization, 1993.
Thomsen 2014
- Thomsen T, Villebro N, Møller AM. Interventions for preoperative smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD002294.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tønnesen 1992a
- Tønnesen H, Kaiser AH, Nielsen BB, Pedersen AE. Reversibility of alcohol‐induced immune depression. British Journal of Addiction 1992;87(7):1025‐8. [PUBMED: 1643394] [DOI] [PubMed] [Google Scholar]
Tønnesen 1992b
- Tønnesen H, Petersen KR, Højgaard L, Stokholm KH, Nielsen HJ, Knigge U, et al. Postoperative morbidity among symptom‐free alcohol misusers. Lancet 1992;340(8815):334‐7. [PUBMED: 1353805] [DOI] [PubMed] [Google Scholar]
Tønnesen 1992c
- Tønnesen H. Influence of alcohol on several physiological functions and its reversibility: a surgical view. Acta Psychiatrica Scandinavica Supplementum 1992;369:67‐71. [PUBMED: 1471555] [PubMed] [Google Scholar]
Tønnesen 1999
- Tonnesen H, Kehlet H. Preoperative alcoholism and postoperative morbidity. British Journal of Surgery 1999;86(7):869‐74. [PUBMED: 10417555] [DOI] [PubMed] [Google Scholar]
Tønnesen 2003
- Tønnesen H. Alcohol abuse and postoperative morbidity. Danish Medical Bulletin 2003;50(2):139‐60. [PUBMED: 12812138] [PubMed] [Google Scholar]
Tønnesen 2008
- Tønnesen H, Roswall N, Odgaard MD, Pedersen KM, Larsen KL, Mathiassen B, et al. Basic registration of risk factors in medical records. Malnutrition, overweight, physical inactivity, smoking and alcohol [Basale journaloplysninger om risikofaktorer]. Ugeskrift for Laeger 2008;170(20):1747‐52. [PUBMED: 18489891] [PubMed] [Google Scholar]
Tønnesen 2009
- Tønnesen H, Nielsen PR, Lauritzen JB, Møller AM. Smoking and alcohol intervention before surgery: evidence for best practice. British Journal of Anaesthesia 2009;102(3):297‐306. [PUBMED: 19218371 ] [DOI] [PubMed] [Google Scholar]
Tønnesen 2015
- Tønnesen H, Egholm JW, Oppedal K, Lauritzen JB, Madsen B, Pedersen B. Patient education for alcohol cessation intervention at the time of acute fracture surgery: study protocol for a randomised clinical multi‐centre trial on a gold standard programme (Scand‐Ankle). BMC Surgery 2015;15(52):1‐9. [DOI 10.1186/s12893‐015‐0035‐z; PUBMED: 25925742 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vagts 2003
- Vagts DA, Iber T, Nöldge‐Schomburg GF. Alcohol ‐ a perioperative problem of anaesthesia and intensive care medicine [Alkohol als perioperatives Problem in Anästhesiologie und Intensivmedizin]. Anästhesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie 2003;38(12):747‐61. [DOI: 10.1055/s-2003-45400; PUBMED: 14666437] [DOI] [PubMed] [Google Scholar]
WHO 2010
- World Health Organization. Global strategy to reduce the harmful use of alcohol. Geneva, Switzerland: WHO, 2010. http://www.who.int/substance_abuse/publications/global_strategy_reduce_harmful_use_alcohol/en/ (accessed August 2017).
WHO 2014
- World Health Organization. Global status report on alcohol and health. Geneva, Switzerland: WHO, 2014. http://www.who.int/substance_abuse/publications/global_alcohol_report/en/ (accessed August 2017).
Wåhlin 2014
- Wåhlin S, Tønnesen H. Time for "alcohol‐free operations". Two standard drinks a day doubles the risk of postoperative complications [Dags för »alkoholfri operation« Två standardglas per dag fördubblar risken för postoperativa komplikationer]. Läkartidningen 2014;111(44‐45):1966‐9. [PUBMED: 25349999] [PubMed] [Google Scholar]
References to other published versions of this review
Oppedal 2010
- Oppedal K, Møller A, Pedersen B, Tønnesen H. Preoperative alcohol cessation prior to elective surgery. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD008343] [DOI] [PubMed] [Google Scholar]
Oppedal 2012
- Oppedal K, Møller A, Pedersen B, Tønnesen H. Preoperative alcohol cessation prior to elective surgery. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD008343.pub2] [DOI] [PubMed] [Google Scholar]


