The soil microbiome carries out important ecosystem functions, but interactions between soil microbial communities have been difficult to study due to the high microbial diversity and complexity of the soil habitat. In this study, we successfully obtained stable consortia with reduced complexity that contained species found in the original source soil. These consortia and the methods used to obtain them can be a valuable resource for exploration of specific mechanisms underlying soil microbial community ecology. The results of this study also provide new experimental context to better inform how soil microbial communities are shaped by new environments and how a combination of initial taxonomic structure and physical environment influences stability.
KEYWORDS: chitin, microbial consortia, microbiome, microbiome stability, model microbiome, N-acetylglucosamine, species volatility, succession, fungi, soil microbiology
ABSTRACT
Soil microorganisms play fundamental roles in cycling of soil carbon, nitrogen, and other nutrients, yet we have a poor understanding of how soil microbiomes are shaped by their nutritional and physical environment. In this study, we investigated the successional dynamics of a soil microbiome during 21 weeks of enrichment on chitin and its monomer, N-acetylglucosamine. We examined succession of the soil communities in a physically heterogeneous soil matrix as well as a homogeneous liquid medium. The guiding hypothesis was that the initial species richness would influence the tendency for the selected consortia to stabilize and maintain a relatively constant community structure over time. We also hypothesized that long-term, substrate-driven growth would result in consortia with reduced species richness compared to the parent microbiome and that this process would be deterministic with relatively little variation between replicates. We found that the initial species richness does influence the long-term community stability in both liquid media and soil and that lower initial richness results in a more rapid convergence to stability. Despite use of the same soil inoculum and access to the same major substrate, the resulting community composition differed greatly in soil from that in liquid medium. Hence, distinct selective pressures in soils relative to homogenous liquid media exist and can control community succession dynamics. This difference is likely related to the fact that soil microbiomes are more likely to thrive, with fewer compositional changes, in a soil matrix than in liquid environments.
IMPORTANCE The soil microbiome carries out important ecosystem functions, but interactions between soil microbial communities have been difficult to study due to the high microbial diversity and complexity of the soil habitat. In this study, we successfully obtained stable consortia with reduced complexity that contained species found in the original source soil. These consortia and the methods used to obtain them can be a valuable resource for exploration of specific mechanisms underlying soil microbial community ecology. The results of this study also provide new experimental context to better inform how soil microbial communities are shaped by new environments and how a combination of initial taxonomic structure and physical environment influences stability.
Author Video: An author video summary of this article is available.
INTRODUCTION
Soil microbiomes are among the most diverse microbial communities on the planet (1, 2), and the majority of soil microbes have not yet been cultivated or studied under laboratory conditions. This and other confounding properties, such as extreme spatial heterogeneity, make it difficult to study how soil microorganisms interact within natural communities (3). Despite this, a deeper understanding of the ecological properties that control the structure and function of soil microbiomes is needed, as they underpin almost every terrestrial food web (4), regulate many elements of Earth’s biogeochemical cycles (5), and are fundamental for growth of healthy crops and bioenergy feedstocks (6).
Estimates for annual CO2 emissions from soil microbial respiration are 10 times greater than the CO2 produced by fossil fuel utilization (5). Therefore, small changes in the soil carbon cycle—specifically microbiome functioning and substrate availability—can have large impacts on atmospheric CO2 concentrations. The cycling of complex biopolymers that are both produced and stored in soils largely influences the flux of CO2 to the atmosphere. Of these, chitin, an insoluble β-1,4-linked polymer of N-acetylglucosamine (NAG) (7, 8), is a major substrate for soil microbial activity (9) and represents a linkage between the carbon and nitrogen cycles in soils (10 – 12). Chitin is omnipresent in soil and is an important biopolymer synthesized by fungi (13) and many insects. However, little is known about how chitin and NAG can select for soil-specific bacterial and fungal taxa and influence the structure of microbial communities that are involved in their decomposition.
Successional dynamics of soil microbiomes are related to changes in substrate availability and are crucial to predicting ecosystem development (14 – 20). During primary succession, early-colonizing taxa shape available niche space by regulating pH and nutrient availability (16, 17, 21). However, the feedbacks and processes driving successional patterns constitute fundamental knowledge gaps in understanding trajectories of ecosystem development (16, 19). Microbial succession patterns can be influenced by available resources, including nutrient pools (19, 22), physiochemistry (23), and vegetation (24). Additionally, it is well known that soil moisture is a key determinant of microbial metabolism (25 – 27). Less is known about how the physical environment, with respect to soil or liquid-like conditions, affects microbial community succession and stability. The relative stability of microbial communities through early succession and thereafter is key to understanding and predicting microbial responses to perturbation (28 – 31). While the complexity of soil microbiomes has hindered many efforts to describe the succession dynamics to ecosystem functioning, organic matter chemistry has been identified as a key driver of primary succession (32).
In this study, we aimed to investigate processes underlying soil microbial community succession by monitoring community development in a sterile soil matrix enriched with NAG. Comparisons were made over the course of 15 weeks of succession to a liquid medium culture derived from the same inoculum. In this way, environmental successional trajectories of the soil microbiome were directly compared to community development using traditional, liquid-based culturing methods that omit the heterogenous chemical and spatial landscapes associated with the soil matrix.
We hypothesized that initial species richness would influence the succession of the consortia and their ability to stabilize with a relatively constant taxonomic structure over time. Specifically, we anticipated that consortia with lower species richness during the initial phases of succession would display higher tendencies to converge toward smaller changes in community structure between successive time points. We also hypothesized that long-term selection by NAG would result in soil microbial consortia with reduced complexity compared to the parent soil microbiome and that this process would be deterministic with relatively little variation between replicates during enrichment.
To test these hypotheses, we investigated the influence of initial richness and physical environment on the progression of chitin/NAG-enriched soil microbial consortia. We designed soil microbiome enrichment experiments with the expectation that dilution and long-term selection on chitin/NAG would dramatically reduce the initial community richness compared to the native soil. One of our aims was to use this procedure to obtain simplified, naturally adapted consortia that can serve as a valuable experimental resource that can be shared for recapitulating some soil microbiome behaviors. We also expected and found that the emergent consortia from long-term succession would show distinct differences based on the physical environment (soil versus liquid). This study has improved our understanding about the succession and stability of microbial communities in soil. Generally, these results show that the final stability of and the extent of species richness were directed by the length of succession, the initial richness, and the culturing environment.
RESULTS
Enrichment of a native soil microbiome on chitin.
Native soil was supplemented in triplicate with 3 concentrations of chitin (10, 50, and 100 ppm) for 6 weeks to select for naturally coexisting soil populations capable of using chitin as a carbon and/or nitrogen substrate. Respiration was monitored during the enrichment as a proxy for soil microbial activity during chitin decomposition. The highest respiration was observed for the highest chitin concentration, and therefore, the 100-ppm treatments were used to inoculate longer-term enrichments supplemented with NAG.
The dominant bacterial phyla in the native soil communities were Proteobacteria, Actinobacteria, Acidobacteria, Chloroflexi, Plantomycetes, and Bacteroidetes; there were few archaea identified in high relative abundance (Fig. 1; see also Fig. S1A in the supplemental material). The dominant fungi were Ascomycota (Fig. S1B). The native soil bacterial richness had a mean of 818.5 ± 75.6 16S operational taxonomic units (OTUs) (Fig. S1C). The native soil bacterial community also exhibited high evenness, with a Simpson’s evenness score of 0.3; the most abundant OTU accounted for less than 4% of all observations. The fungal richness and evenness in the native soil were much lower than the 16S results (Fig. S1D). The mean internal transcribed spacer (ITS) OTU count was 128 ± 39, with a Simpson’s evenness score of only 0.069, and the top two OTUs together comprised 41% of the observed fungal community.
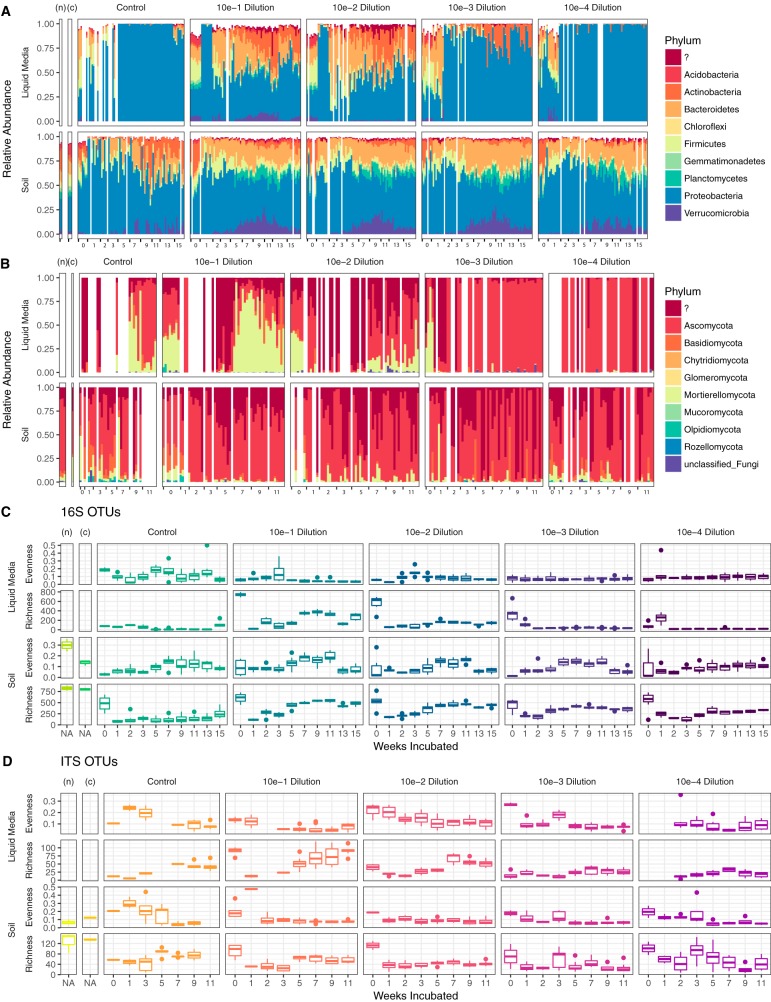
FIG 1.
The successional dynamics of microbial consortia. Differences in microbial community structure and alpha diversity are plotted with respect to native soil communities (labeled as “n”), the 6-week-chitin-enriched communities (labeled as “c”), uninoculated control (labeled as “control”), and different serial dilutions of NAG enrichment in liquid and soil treatments for 0 to 15 weeks. The results are partitioned by initial dilution and incubating conditions (NAG enrichment in liquid on the top and soil on the bottom). The most abundant bacterial (A) and fungal (B) phyla are shown over the 15-week incubation. The alpha diversities of bacteria (C) and fungi (D) were estimated using species richness and Simpson’s evenness.
The most abundant bacterial (A) and fungal (B) phyla are plotted for the native soil (labeled as “n”) and the 6-week-chitin-enriched soil (labeled as “c”). The alpha diversities of bacteria (C) and fungi (D) were estimated using species richness and Simpson’s evenness. Download FIG S1, PDF file, 0.01 MB (8.5KB, pdf) .
Copyright © 2019 Zegeye et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Following the initial 6-week chitin enrichment, the bacterial community structure shifted to a higher relative abundance of Firmicutes and Acidobacteria and fewer Actinobacteria, although Proteobacteria continued to maintain the highest relative abundance (Fig. 1A). Additionally, there were shifts in the fungal communities, with a higher relative abundance of Mortierellomycota and decreased Ascomycota abundance compared to those of the native soil (Fig. 1B). Bacterial richness remained statistically unchanged (P = 0.7624; native soil = 823.5 ± 78.5 [n = 2] and chitin-enriched soil = 801.7 ± 26.35 [n = 3]). However, bacterial evenness decreased by 55%, indicating that chitin supplementation selected for a subset of populations. The fungal species richness remained essentially unchanged by the chitin supplementation, indicating that the native fungal taxa were less responsive to chitin than were the bacteria.
The structure and taxa of soil and liquid-based consortia.
After chitin enrichment, subsequent extended enrichment was carried out over 15 weeks using 100 ppm of NAG as the major carbon and nitrogen source. The enrichments were performed in two parallel tracks using the same source inoculum (soil enriched for 6 weeks with 100 ppm of chitin) in both gamma-irradiated (sterile) soil and liquid M9 medium. The total time for the experiment using chitin enrichment followed by NAG enrichment was 21 weeks. This experimental design was used to optimize opportunities for selection of reduced complexity, naturally coexisting soil consortia and to determine the influence of the physical matrix on the enrichment process. While the physical differences between soil and liquid are paramount, it is important to note other differences, including carbon/nitrogen sources or pH, that may also have an effect on the succession of resulting consortia.
The NAG enrichments were initiated by serial 10-fold dilution of the chitin-enriched soil (dilutions ranged from 10−1 to 10−4) into the irradiated sterile soil and into liquid M9, both containing 100 ppm of NAG. The relative abundances of both 16S and ITS OTUs differed between serial dilutions and treatment conditions over the course of the experiment (Fig. 1). Proteobacteria remained the dominant bacterial phylum during the succession period in both the liquid and soil treatments (Fig. 1A). However, the NAG-enriched liquid environment showed a greater degree of change than the native source soil. In the NAG-enriched liquid medium, members of the Proteobacteria and Ascomycota phyla dominated the bacterial and fungal communities, respectively. In contrast, there was a higher diversity of phyla represented in the NAG-enriched soil environment over time. In these samples we observed increases in typical soil bacteria that are generally difficult to cultivate, namely, Planctomycetes and Verrucomicrobia. Planctomycetes were negligible in all matching liquid incubations, and Verrucomicrobia was present to a comparable degree only in the least diluted liquid sample (10−1). Simultaneously, we observed depletion of Acidobacteria and Actinobacteria in the NAG-enriched soil. We also detected a greater number of fungal phyla in communities grown on the NAG-enriched soil than in its liquid counterpart, with relatively high proportions of Mortierellomycota, Basidiomycota, and unidentified fungi at the end of the incubation period (Fig. 1B).
As the 15-week NAG enrichments were being regularly sampled for genomic DNA (gDNA) and respiration, we employed sterile controls to monitor contamination (Fig. S2). This enabled detection of cross-contamination between samples as growth in our soil and liquid medium controls. This was inferred from non-zero respiration measurements and the recovery of gDNA from liquid media (gDNA was always present in sterile soil). The cross-contamination was first observed at week 5 (Fig. S2). The most common OTU identified from the controls was of the genus Pseudomonas. This OTU was present in the native soil and chitin enrichments, indicating that it was intrinsic to the experimental system and native to the parent microbiome (Fig. S3). Although the sterile controls lacked any viable growth at the onset of the incubations (as determined by plate counting), the Pseudomonas OTU introduced during the incubations was able to grow and dominate the liquid sterile controls as well as the more dilute liquid samples (10−3 and 10−4). However, although present, the Pseudomonas OTU did not establish itself to high relative levels within the higher-richness liquid samples or any of the soil samples, likely due to the complexity and stability of the existing microbial communities already present in these sites.
Rate of respiration varies over time based on dilution and physical environment. The respiration rate was plotted for both liquid (top) and soil (bottom) for NAG enrichment cultures from weeks 2 through 7 to avoid the light spike in CO2 released immediately after inoculation. Respiration was monitored three times a week with a PP Systems (Amesbury, MA) EGM-4 environmental gas monitor. The gas analyzer was set up in static mode to measure CO2 and monitor respiration in each sample at periodic intervals. To measure CO2, a 10-ml gas aliquot was taken from the incubation container using a sterile plastic syringe with a control valve. Download FIG S2, PDF file, 0.01 MB (14.3KB, pdf) .
Copyright © 2019 Zegeye et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Bacterial OTUs observed from 16S rRNA gene amplicon products in uninoculated controls. The most abundant OTUs shown in the controls were also found in the other samples. Download FIG S3, PDF file, 0.09 MB (89.8KB, pdf) .
Copyright © 2019 Zegeye et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
We anticipated that long-term selection by NAG in a sterile soil or liquid M9 medium environment would result in soil microbial consortia with reduced complexity compared to both the native soil microbiome and the chitin-enriched soil microbiome. Overall, this was found to be true, although the initial species richness of the inoculum also played a major role. We manually reduced the complexity of the inoculum by controlling the initial species richness through dilutions. A comparison of the species richness measured on the first sampling date (week 0) across dilutions in NAG-enriched liquid media showed that the dilutions were successful in reducing the richness of the initial inoculum (Fig. 1C and Table S1). It is very likely that a corresponding initial drop in richness was also happening with the soil dilutions, although this could not be confirmed by amplicon analysis due to DNA amplification from soil microbes that were likely killed during the gamma irradiation process (33, 34). In the liquid incubations, the observed 16S and ITS OTU counts from the 10−3 and 10−4 dilutions gradually decreased over time; however, the 10−1 and 10−2 dilutions revealed sharp decreases in species richness on the first week, followed by a rebounding trend through week 15. This drop and rebounding effect after week 3 were also observed across all of the dilutions associated with the NAG-enriched soil. Fungal richness measurements followed patterns similar to those seen for bacterial richness. By the end of 15 weeks the NAG-enriched soil microbiome richness was reduced by approximately 35 to 70% (depending on dilution) compared to the original native soil (Fig. 1C and D) and the NAG-enriched liquid microbiome richness was reduced by approximately 37 to 88%. This represents a considerable decrease in species complexity from the initial native and chitin-enriched soil microbiomes and a demonstration that a combination of dilution and long-term selection on specific carbon sources can lead to consortia with reduced species complexity.
Final richness significantly varies between dilutions. Tukey’s honestly significant difference (HSD) test was performed to compare the richness between dilutions, and the P values are shown here. Download Table S1, DOCX file, 0.01 MB (13.1KB, docx) .
Copyright © 2019 Zegeye et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Physical environment and initial species richness influence stability.
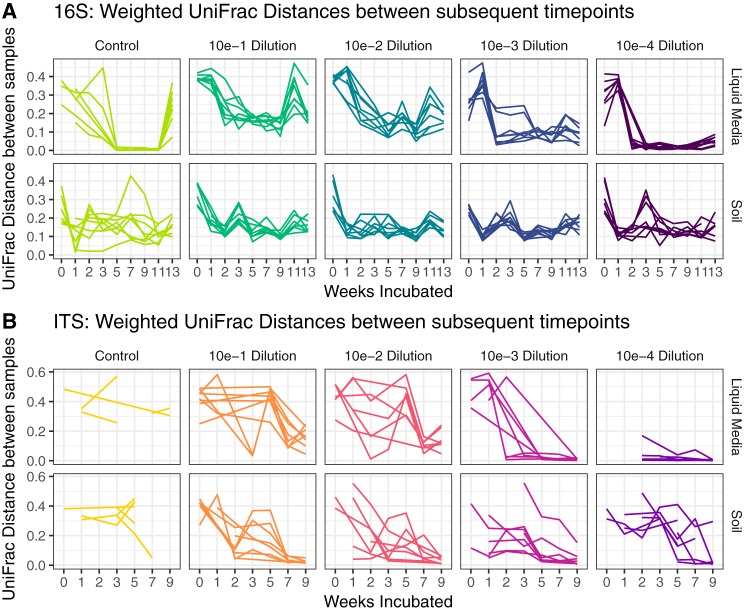
The stability of the enriched consortia was measured by comparing beta diversity over time. Specifically, we used measures of weighted UniFrac distance (35) between samples that occurred sequentially as a measure of phylogenetic volatility (Fig. 2), where consortia with lower volatility are defined as those showing a more similar community structure from one time point to the next (36). This represents a way to measure how much the community is changing from week to week, which is related to the taxonomic compositional stability over time (Fig. S4). By using this metric, it was clear that while enrichment on both NAG-containing soil and liquid media led to stable consortia, those enriched within the liquid environment became relatively stable more quickly than those in the enriched soil (Fig. 2). Consortial stability also depended on the complexity of the initial inoculum (Fig. 2 and Table S2), a factor that was controlled by dilution of the chitin-enriched input soil. Samples inoculated with the 10−4 dilution (lowest initial richness) showed the greatest tendency to stabilize.
FIG 2.
The influence of physical environment on taxonomic volatility, i.e., the tendency for the community to stabilize with respect to taxa being gained/lost over time. Each graph shows the weighted UniFrac distance for bacteria (A) and fungi (B) calculated between subsequent incubation times and plotted by weeks of incubation, dilution factor, and treatment conditions.
Patterns of variation within sample groups and within replicates. (A) Beta dispersion (within sample group variation) varies among time points as shown by the pairwise permutation test for homogeneity of multivariate dispersion at each time. Pairwise comparisons of the observed P values are tabulated for both the 16S and ITS for liquid and soil treatment conditions and indicate significant difference of within group dispersion. (B) A linear regression fit to the pairwise distance between replicates also shows changes in dispersion over time. The different dilutions are shown in separate facets and are used as strata during the permutation testing of beta dispersion. Download FIG S4, PDF file, 2.9 MB (2.9MB, pdf) .
Copyright © 2019 Zegeye et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The adonis test (permutational multivariate analysis of variance using distance matrices) was used to partition variation associated with dilution in the final time points from each treatment condition. The R2 column reports the percentage of weighted Unifrac distance that can be attributed to differing dilution. Download Table S2, DOCX file, 0.01 MB (12.9KB, docx) .
Copyright © 2019 Zegeye et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The consortia became more stable starting at week 5, with the maximum stability reached by the end of the experiment, at week 15. However, differences were observed based on succession in liquid versus soil environments. The NAG-enriched soil microbial communities showed an initial drop in volatility (weeks 1 to 2), followed by a rise in volatility through weeks 3 to 5 (Fig. 2). After 5 weeks of enrichment in soil with NAG, the composition of the soil microbiome did not change significantly and volatility continued to drop as the experiment progressed. In contrast, NAG-enriched liquid microbiomes initially exhibited an extreme drop in volatility over the first 2 weeks and thereafter showed either a consistent volatility measurement near 0.10 (dilutions 10−3 and 10−4) or a continual gradual drop in volatility over the remaining 13 weeks down to a minimum of 0.15 (dilutions 10−1 and 10−2). Bacterial volatility showed a consistent increase around near week 11, which also corresponded with the observed decreases in the relative abundance of OTUs assigned as Verruucomicrobia and Bacteroidetes in the soil consortia (Fig. 1). More diverse microbial communities were enriched and stabilized in soil than in the liquid incubations. This demonstrates that the physical environment was a significant factor for the stability and compositional convergence of microbial consortia. These results show that the final stability of the consortia and the extent of species richness were directed by the length of succession, initial richness, and culturing environment.
Biological and physical variables underpinning observed beta diversity.
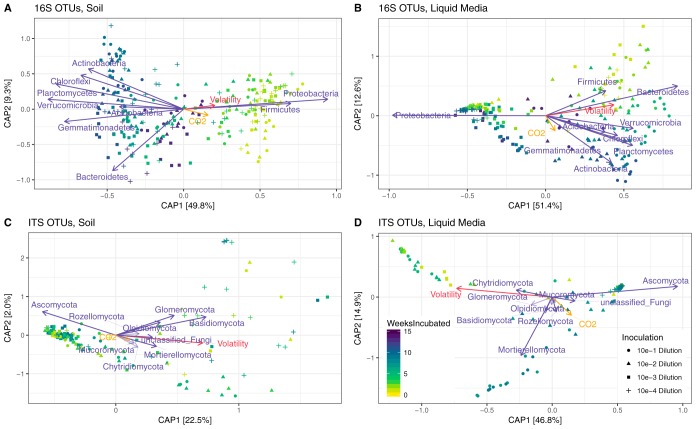
Respiration and volatility of the enriched communities were compared to phylogenetic composition over time via ordination by canonical analysis of principal coordinates using weighted Unifrac distance between rarefied samples (Fig. 3). As described earlier, changes (volatility) in community composition between time points were measured as the weighted UniFrac distance between subsequent time points (Fig. 2). In all environments, the volatility vector points were in the direction of early-stage incubation samples (Fig. 3), where large changes in the community structure occurred between time points (Fig. S4A). In liquid medium incubations, the contribution of respiration for the dissimilarity between samples becomes more prominent in the later stages of incubation time courses. The dominant phyla from both kingdoms were assessed with respect to incubation time and treatment condition. In the soil, Proteobacteria and Firmicutes covaried with volatility (Fig. 3A), as they were most abundant in the volatile initial samples and slowly decreased over time. However, in the liquid media Firmicutes and Bacteroidetes were closely aligned with volatility (Fig. 3B). Also, Proteobacteria became dominant over time in the liquid medium incubations; in particular, those that were originally inoculated with higher dilutions of the chitin-enrichment that had a lower initial species richness. For the fungi in the soil culture, volatility covaried primarily with Mortierellomycota and Chytridiomcota, motile saprotrophs with chitin-containing cells walls that are found in wet soils (Fig. 3C) (37).
FIG 3.
The biological and physical variables correlated with beta diversity. Ordinations present canonical analysis of principal coordinates (CAP) of weighted UniFrac distances between samples. The percent variation captured by the vectors is shown on each axis. Each vector has a magnitude (length) and direction of a variable’s contributions to the principal components. Vectors represent respiration (CO2 per hour) (orange), volatility (red), and the most abundant phyla (purple).
DISCUSSION
Selective enrichment of soil microbes with specific carbon substrates resulted in the formation of distinct microbial consortia that displayed reduced complexity. Those consortia that developed in NAG-enriched soil were also representative of the native soil microbiome used as the inoculum. A primary finding from this study was that the initial species richness influenced successional patterns that were enriched with a specific carbon/nitrogen source in both NAG-enriched liquid media and soil incubations. Because the experiment was well replicated (8 biological replicates per treatment), we also confirmed our hypothesis that substrate-driven soil community succession is deterministic in that all of the replicates for a given soil dilution become more similar over the course of the successional period observed (Fig. S4 and Table S3). This result was obtained in both liquid media and soil substrates, although the taxonomic structure of endpoint consortia was controlled by hydrophysical and other matrix-associated differences between soil and liquid media. The endpoint microbial community structure was well explained by the initial dilution condition, and this influence was more pronounced under the liquid than the soil treatment condition (Table S2). At the end of the enrichment period, the soil NAG enrichments showed higher species richness than equivalent liquid treatments, despite having identical inoculations, and were also more representative of microbiomes from the original native soil with respect to community composition. The persistence of members of the original soil microbiome was consistent across dilutions for the NAG-enriched soil. The 10−1 dilution had the highest species similarity to the native soil and retained a diverse community, while at the other extreme, the 10−4 dilution represented a much simpler, less rich community.
Outputs from the tests for homogeneity of multivariate dispersions performed with respect to each treatment. Download Table S3, DOCX file, 0.01 MB (15.2KB, docx) .
Copyright © 2019 Zegeye et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
This approach for developing consortia with reduced complexity is of interest as a method for obtaining simplified model microbiomes with naturally interacting members that are representative of the native soil system. This similarity to the native soil seen with the NAG-enriched soil is likely a result of the experiment being performed with soil microbes in their natural soil substrate, in contrast to a relatively foreign substrate (liquid). Another recent study also examined shorter-term succession of soil microbiomes in liquid (but not soil) and found that soil microbiomes enriched on liquid media are very different from the original-source soil microbiome (38). That study was carried out using a variety of carbon sources, resulting in microbiomes with reduced complexity, similar to what we show here. Together, these studies confirm that reduced-complexity consortia that have community membership representative of soil microbiomes are much more likely to be obtained using a soil-based enrichment than a liquid-based enrichment. In addition, our results clearly show differences in the successional dynamics and endpoint structures of each consortium with respect to their initial species richness based on the dilution of the chitin-enriched soil inoculum.
We found that the richness of the initial soil inoculum strongly impacted the alpha diversity of the resulting microbial consortia over time (Fig. 1). These results support our hypothesis that the initial species richness would influence each consortium’s tendency to converge toward smaller changes in community structure between successive time points. Results supporting this hypothesis were observed for the higher dilutions (10−3 and 10−4) for all treatments and measurements (Fig. 2). Each consortium’s tendency to converge toward smaller changes in community structure between successive time points was assessed by comparing weighted UniFrac distances between time points and was notably strongest for communities developed in the liquid media and measured by 16S rRNA sequencing rather than ITS. The generalizability of this stability convergence effect is partially supported through similar findings presented by Shade et al., who showed how rare taxa significantly influence microbial diversity (39). Dilutions are more likely to remove rare taxa, and therefore, our results provide some additional quantification of the effect presented by Shade et al. (39). However, in our current study, we could not fully decouple the effects of reduced initial richness from reduced counts of viable cells that were almost certainly created from the dilution procedure. Hence, an alternative interpretation could be formulated, as decreased viable cell numbers in early stages of succession led to decreased species richness and higher tendencies to converge toward smaller changes in community structure between successive time points.
Both bacterial and fungal populations were selected during the chitin/NAG incubation process. This suggests that the representative populations were able to either metabolize or take advantage of the added substrates through other means. Specifically, we found that members of the Acidobacteria, Actinobacteria, Bacteroidetes, Chloroflexi, Firmicutes, Gemmatimonadetes, Planctomycetes, Proteobacteria, and Verrucomicrobia were represented in the NAG incubations (Fig. 1A). In addition, the richness of Verrucomicrobia, Bacteroidetes, and Planctomycetes increased in soil incubated with NAG compared to that in native soil (Fig. 1A). Representatives of these phyla were also detected on a previous study of soil enriched with chitin (12). With respect to the fungi, we found that the Mortierellomycota phylum increased in relative abundance in the NAG-enriched consortia (Fig. 1B). Mortierellomycota are members of the Mucoromyceta, based on recent fungal taxonomy (40). They are sporangiferous, are generally saprotrophic (including being able to grow on other fungi), and are found in soil (41). The dominance of these specific bacteria and fungi suggests that their enrichment came due to their ability to use either chitin/NAG or its metabolic by-product as a substrate.
The occurrence of enriched, stable consortia with dozens to hundreds of members, found in this study and in a similar study by Goldford et al. (42), as opposed to selection of a monoculture, suggests that the compositions of the reduced microbial communities are governed by cross-feeding interactions among microbes. In our longer-term soil incubations with NAG, the microbiome converged into a less complex microbial community than that found in the native soil. This is consistent with the results of the study by Goldford et al., which also enriched a simplified microbial community, derived from soil, on single carbon sources. However, unlike the previous study, which used only liquid, we enriched on both liquid and soil and found that enrichments on soil led to a reduced-complexity community that was far more representative of the native soil microbiome than obtained with enrichment on liquid. There are several reasons why structured environments may better facilitate and stabilize social interactions, including the limited dispersal of interacting species and the physical retention of resources within the soil matrix. The close physical proximity of members of soil consortia in discrete niches would thus facilitate social activities between member populations (e.g., exchange of public goods, quorum sensing, and competition). When microbial communities have a single major carbon source, only a subset of the community will have the metabolic capability to utilize it as a substrate. For complex substrates, such as chitin, other species will be reliant on primary species to degrade the polymer to simpler compounds, thus selecting for a community that interacts by metabolic cross-feeding, interactions that positively affect both the primary degrading species and the secondary degrading species (43, 44). Positive metabolic interactions between microorganisms residing within communities have been studied in other systems as well, particularly in biofilms where species and cells are in very close proximity and must cooperate for growth (45).
Because we monitored the soil enrichments over a relatively long period, we could determine the time required for the soil microbiomes to reach stable community memberships. Stability was achieved surprisingly rapidly (3 to 5 weeks), and the resulting consortia remained stable over several months. Importantly, the development of stable, reduced complexity, naturally interacting consortia from native soil can provide representative model soil communities for future studies to study the mechanisms underlying species interactions. This valuable resource should enable deciphering of the molecular signaling mechanisms and metabolic interactions used by soil community members to decompose complex carbon substrates in soil. In addition, the information can be used to enhance in silico models of soil microbial community interactions that can be used to predict how key taxa and traits can be perturbed by environmental change.
Conclusions.
Here we demonstrate that the succession of microbial communities derived from chitin/NAG-enriched soil microbiome is strongly influenced by the initial soil microbiome richness and the hydrophysical environment. The initial species richness, which is a proxy for the complexity of a microbiome, at least partially controlled the tendency for a soil-sourced consortium to stabilize and maintain a relatively constant community structure over time. Additionally, the long-term soil enrichments resulted in a reduced-complexity representation of the initial soil microbiome diversity and richness. The results of this study show how soil microbial communities are shaped during succession and how a combination of the initial taxonomic structure and physical environment influences the tendency for a community to stabilize over time.
MATERIALS AND METHODS
Field sampling and chitin enrichment.
Soil was collected in October 2017 from a field site operated by Washington State University, located in Prosser, WA (46°15′04″N and 119°43′43″W). The soil represents a Warden silt loam that is characterized as a coarse-silty, mixed, superactive, and mesic Xeric Haplocambid. The soil represents a marginal soil with low organic matter content (3.7%) and pH of 8. All soil samples were collected in three field replicates. At each site, bulk sampling was accomplished with a shovel within a 0- to 20-cm depth from the ground, and samples were stored in plastic bags at 4°C. To exclude bigger soil aggregates and rocks, samples were sieved (4-mm mesh size). For each of the three field blocks, three homogeneous replicates of 150 g of soil were weighed out into 250-ml sterile screwcap bottles. To enrich chitin-degrading members of the microbial community, samples were incubated for 6 weeks in soil augmented with chitin [poly-(1→4)-β-N-acetyl-d-glucosamine; Sigma-Aldrich, St. Louis, MO] at different concentrations (0, 10, 50, and 100 μg of chitin/g of soil [dry weight]). Chitin was mixed and evenly distributed within the soil, and sterile water was added to reflect a 24% field water capacity. Samples were kept in the dark at 20°C. Additionally, 1 g of sample was harvested weekly from each bottle and stored in −80°C for 16S and ITS amplicon analysis.
Gamma-irradiated soil.
Prosser soil was sterilized with gamma irradiation at 85 kGy in two successive applications of 25 kGy followed by 60 kGy. Initially, 3,000 Ci of 60Co source was used in the collimated open beam irradiator. For the second irradiation, 1,300 Ci of 60Co source was used in the Gamma Bunker, which is a 1.5-ft3 closed-chamber irradiator (46, 47). Sterility of soil was confirmed by plating of several serial dilutions on LB agar plates followed by incubation at 30°C and the lack of growth.
Sterile soil incubations and liquid controls.
M9 minimal medium and sterile liquid soil extract were prepared as described by Sambrook and Russell (48) and Weaver et al. (49), respectively. N-Acetylglucosamine (NAG; Sigma-Aldrich, St. Louis, MO) was added into the M9 medium to 100 μg/ml. Ten-milliliter liquid cultures were set up in 25-ml sterile glass tubes in four successive 10-fold serial dilutions. First, 1 g of actively respiring chitinolytic enriched soil (100 μg of chitin/g of soil [dry weight]) was inoculated into the first glass tubes with 9 ml of the M9 medium (representing the 10−1 dilution) and vortexed for 30 s. This solution then was used for the subsequent serial dilutions. Uninoculated controls were also generated and incubated with the dilution samples. Each serial dilution and respective controls were performed in 8 biological replicates. Incubation was performed in the dark at 20°C, with shaking at 130 rpm. CO2 respiration was measured aseptically three times a week. Headspace was aseptically flushed with air after each sample to prevent anaerobic conditions. Additionally, 1 ml of sample was harvested weekly for the first 3 weeks, followed by biweekly sampling; samples were stored at −80°C for 16S and ITS amplicon analysis. After each sampling period, substrate and moisture levels were refreshed by adding 1 ml of M9 medium.
The soil enrichments were set up using 5.5 g of gamma-irradiated soil in 15-ml sterile tubes in parallel with their liquid counterparts. The “sterile soil” treatments were prepared by adding 1 ml of soil extract liquid enriched with 100 ppm of NAG to each tube containing sterile soil. The soil samples were briefly mixed with a sterile spatula and preincubated in the dark at 20°C for 2 days. After preincubation, 0.5 ml of inoculum was taken from the liquid serial dilution described above and added to the counterpart sterile soil tubes. The soil enrichments were sealed with filter screw caps (nonpyrogenic and sterilized by gamma irradiation; CellTreat, China) to allow continuous airflow. Each 0.3-g sample was harvested weekly for the first 3 weeks, followed by biweekly sampling; samples were stored at −80°C for downstream molecular measurements.
Amplicon sequencing.
Total DNA was extracted using the MoBio PowerSoil DNA isolation kit (Qiagen, Carlsbad, CA) in accordance with the Earth Microbiome Project (EMP) protocols (50). Sequencing was performed on a MiSeq instrument (Illumina, San Diego, CA). Triplicate, separate 16S and ITS rRNA gene amplification reactions were performed on DNA from each extraction. The 16S primers targeted the V4 hypervariable region of the 16S small-subunit (SSU) rRNA gene using the V4 forward primer (515F) and V4 reverse primer (806R) with 0 to 3 random bases and the Illumina sequencing primer binding site (51). The ITS primers targeted the ITS1 region using the ITS1f and ITS2 primers (52).
Amplicon analysis.
The Hundo amplicon processing protocol was used to process 16S and ITS amplicons (53). In brief, sequences were trimmed and filtered of adapters and contaminants using BBDuk2 of the BBTools (“Tools”) package. VSEARCH (54) was used to merge, filter to an expected error rate of 1, dereplicate, and remove singletons before preclustering reads for de novo and reference-based chimera checking. Reads were clustered into OTUs at 97% similarity, and an OTU table in the BIOM format (55) was constructed by mapping filtered reads back to these clusters. BLAST+ (56) was used to align OTU sequences to the database curated by CREST (57) (SILVA v128 for 16S and UNITE v7 for ITS), and taxonomy was assigned based on the CREST lowest common ancestor method. Multiple sequence alignment was performed with Clustal Omega (58) and a phylogenetic tree was constructed using FastTree2 (59).
Diversity analysis.
Downstream analysis was completed in R (60), using the phyloseq (61) and vegan (62) packages. To preserve the maximum consistency within each replicate, samples were rarified to an even depth of 2,000 reads per sample. The observed counts of unique OTUs (species richness) and Simpson’s evenness were used to characterize alpha diversity (35). In order to assess microbial stability/volatility over time, we implemented the volatility analysis as previously described (36), in which the amount the community change between successive time points was measured with weighted UniFrac distances.
Data availability.
Genetic sequencing data are available on DataHub at https://doi.org/10.25584/data.2019-02.700/1506698 for both 16S and 1TS amplicons. The R markdown processing scripts used to process the data and build graphs are available on Open Science Framework at https://osf.io/6d5kz/.
ACKNOWLEDGMENTS
This research was supported by the Department of Energy Office of Biological and Environmental Research (BER) and is a contribution of the Scientific Focus Area “Phenotypic response of the soil microbiome to environmental perturbations” (70880). PNNL is operated for the DOE by Battelle Memorial Institute under contract DE-AC05-76RLO1830.
REFERENCES
- 1.Barrios E. 2007. Soil biota, ecosystem services and land productivity. Ecol Econ 64:269–285. doi: 10.1016/j.ecolecon.2007.03.004. [DOI] [Google Scholar]
- 2.Thompson LR, Sanders JG, McDonald D, Amir A, Ladau J, Locey KJ, Prill RJ, Tripathi A, Gibbons SM, Ackermann G. 2017. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 551:457–463. doi: 10.1038/nature24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramette A, Tiedje JM. 2007. Multiscale responses of microbial life to spatial distance and environmental heterogeneity in a patchy ecosystem. Proc Natl Acad Sci U S A 104:2761–2766. doi: 10.1073/pnas.0610671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender SF, Wagg C, van der Heijden MG. 2016. An underground revolution: biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol Evol 31:440–452. doi: 10.1016/j.tree.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Gougoulias C, Clark JM, Shaw LJ. 2014. The role of soil microbes in the global carbon cycle: tracking the below‐ground microbial processing of plant‐derived carbon for manipulating carbon dynamics in agricultural systems. J Sci Food Agric 94:2362–2371. doi: 10.1002/jsfa.6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang C, Jesus EC, Duncan DS, Jackson RD, Tiedje JM, Balser TC. 2012. Soil microbial communities under model biofuel cropping systems in southern Wisconsin, USA: impact of crop species and soil properties. Appl Soil Ecol 54:24–31. doi: 10.1016/j.apsoil.2011.11.015. [DOI] [Google Scholar]
- 7.Roberts WK, Selitrennikoff CP. 1988. Plant and bacterial chitinases differ in antifungal activity. J Gen Microbiol 134:169–176. doi: 10.1099/00221287-134-1-169. [DOI] [Google Scholar]
- 8.Kumar MNR. 2000. A review of chitin and chitosan applications. React Funct Polym 46:1–27. doi: 10.1016/S1381-5148(00)00038-9. [DOI] [Google Scholar]
- 9.Brzezinska MS, Jankiewicz U, Burkowska A, Walczak M. 2014. Chitinolytic microorganisms and their possible application in environmental protection. Curr Microbiol 68:71–81. doi: 10.1007/s00284-013-0440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersson M, Kjøller A, Struwe S. 2004. Microbial enzyme activities in leaf litter, humus and mineral soil layers of European forests. Soil Biol Biochem 36:1527–1537. doi: 10.1016/j.soilbio.2004.07.018. [DOI] [Google Scholar]
- 11.Beier S, Bertilsson S. 2013. Bacterial chitin degradation—mechanisms and ecophysiological strategies. Front Microbiol 4:149. doi: 10.3389/fmicb.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacquiod S, Franqueville L, Cécillon S, Vogel TM, Simonet P. 2013. Soil bacterial community shifts after chitin enrichment: an integrative metagenomic approach. PLoS One 8:e79699. doi: 10.1371/journal.pone.0079699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debode J, De Tender C, Soltaninejad S, Van Malderghem C, Haegeman A, Van der Linden I, Cottyn B, Heyndrickx M, Maes M. 2016. Chitin mixed in potting soil alters lettuce growth, the survival of zoonotic bacteria on the leaves and associated rhizosphere microbiology. Front Microbiol 7:565. doi: 10.3389/fmicb.2016.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaštovská K, Elster J, Stibal M, Šantrůčková H. 2005. Microbial assemblages in soil microbial succession after glacial retreat in Svalbard (high Arctic). Microb Ecol 50:396. doi: 10.1007/s00248-005-0246-4. [DOI] [PubMed] [Google Scholar]
- 15.Ohtonen R, Fritze H, Pennanen T, Jumpponen A, Trappe J. 1999. Ecosystem properties and microbial community changes in primary succession on a glacier forefront. Oecologia 119:239–246. doi: 10.1007/s004420050782. [DOI] [PubMed] [Google Scholar]
- 16.Nemergut DR, Anderson SP, Cleveland CC, Martin AP, Miller AE, Seimon A, Schmidt SK. 2007. Microbial community succession in an unvegetated, recently deglaciated soil. Microb Ecol 53:110–122. doi: 10.1007/s00248-006-9144-7. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt SK, Reed SC, Nemergut DR, Stuart Grandy A, Cleveland CC, Weintraub MN, Hill AW, Costello EK, Meyer AF, Neff JC, Martin AM. 2008. The earliest stages of ecosystem succession in high-elevation (5000 metres above sea level), recently deglaciated soils. Proc Biol Sci 275:2793–2802. doi: 10.1098/rspb.2008.0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown SP, Jumpponen A. 2014. Contrasting primary successional trajectories of fungi and bacteria in retreating glacier soils. Mol Ecol 23:481–497. doi: 10.1111/mec.12487. [DOI] [PubMed] [Google Scholar]
- 19.Knelman JE, Schmidt SK, Lynch RC, Darcy JL, Castle SC, Cleveland CC, Nemergut DR. 2014. Nutrient addition dramatically accelerates microbial community succession. PLoS One 9:e102609. doi: 10.1371/journal.pone.0102609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernstein HC, Brislawn CJ, Dana K, Flores-Wentz T, Cory AB, Fansler SJ, Fredrickson JK, Moran JJ. 2017. Primary and heterotrophic productivity relate to multikingdom diversity in a hypersaline mat. FEMS Microbiol Ecol 93:fix121. doi: 10.1093/femsec/fix121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brislawn CJ, Graham EB, Dana K, Ihardt P, Fansler SJ, Chirlser WB, Cliff JB, Stegen JC, Moran JJ, Bernstein HC. 2018. Forfeiting the founder effect: turnover defines biofilm community succession. bioRxiv 10.1101/282574. [DOI] [PMC free article] [PubMed]
- 22.Deiglmayr K, Philippot L, Tscherko D, Kandeler E. 2006. Microbial succession of nitrate‐reducing bacteria in the rhizosphere of Poa alpina across a glacier foreland in the Central Alps. Environ Microbiol 8:1600–1612. doi: 10.1111/j.1462-2920.2006.01051.x. [DOI] [PubMed] [Google Scholar]
- 23.Jangid K, Whitman WB, Condron LM, Turner BL, Williams MA. 2013. Soil bacterial community succession during long‐term ecosystem development. Mol Ecol 22:3415–3424. doi: 10.1111/mec.12325. [DOI] [PubMed] [Google Scholar]
- 24.Knelman JE, Graham EB, Prevéy JS, Robeson MS, Kelly P, Hood E, Schmidt SK. 2018. Interspecific plant interactions reflected in soil bacterial community structure and nitrogen cycling in primary succession. Front Microbiol 9:128. doi: 10.3389/fmicb.2018.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brockett BF, Prescott CE, Grayston SJ. 2012. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol Biochem 44:9–20. doi: 10.1016/j.soilbio.2011.09.003. [DOI] [Google Scholar]
- 26.Bouskill NJ, Wood TE, Baran R, Ye Z, Bowen BP, Lim H, Zhou J, Nostrand JDV, Nico P, Northen TR. 2016. Belowground response to drought in a tropical forest soil. I. Changes in microbial functional potential and metabolism. Front Microbiol 7:525. doi: 10.3389/fmicb.2016.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Šťovíček A, Kim M, Or D, Gillor O. 2017. Microbial community response to hydration-desiccation cycles in desert soil. Sci Rep 7:45735. doi: 10.1038/srep45735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Vries FT, Shade A. 2013. Controls on soil microbial community stability under climate change. Front Microbiol 4:265. doi: 10.3389/fmicb.2013.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shade A, Peter H, Allison SD, Baho DL, Berga M, Bürgmann H, Huber DH, Langenheder S, Lennon JT, Martiny JBH, Matulich KL, Schmidt TM, Handelsman J. 2012. Fundamentals of microbial community resistance and resilience. Front Microbiol 3:417. doi: 10.3389/fmicb.2012.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shade A, Caporaso JG, Handelsman J, Knight R, Fierer N. 2013. A meta-analysis of changes in bacterial and archaeal communities with time. ISME J 7:1493. doi: 10.1038/ismej.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allison SD, Martiny JB. 2008. Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci U S A 105:11512–11519. doi: 10.1073/pnas.0801925105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castle SC, Nemergut DR, Grandy AS, Leff JW, Graham EB, Hood E, Schmidt SK, Wickings K, Cleveland CC. 2016. Biogeochemical drivers of microbial community convergence across actively retreating glaciers. Soil Biol Biochem 101:74–84. doi: 10.1016/j.soilbio.2016.07.010. [DOI] [Google Scholar]
- 33.Carini P, Marsden PJ, Leff JW, Morgan EE, Strickland MS, Fierer N. 2016. Relic DNA is abundant in soil and obscures estimates of soil microbial diversity. Nat Microbiol 2:16242. doi: 10.1038/nmicrobiol.2016.242. [DOI] [PubMed] [Google Scholar]
- 34.Lennon J, Muscarella M, Placella S, Lehmkuhl B. 2018. How, when, and where relic DNA affects microbial diversity. mBio 9:e00637-18. doi: 10.1128/mBio.00637-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamady M, Lozupone C, Knight R. 2010. Fast UniFrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J 4:17. doi: 10.1038/ismej.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halfvarson J, Brislawn CJ, Lamendella R, Vázquez-Baeza Y, Walters WA, Bramer LM, D’Amato M, Bonfiglio F, McDonald D, Gonzalez A, McClure EE, Dunklebarger MF, Knight R, Jansson JK. 2017. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol 2:17004. doi: 10.1038/nmicrobiol.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeman K, Martin A, Karki D, Lynch R, Mitter M, Meyer A, Longcore J, Simmons D, Schmidt S. 2009. Evidence that chytrids dominate fungal communities in high-elevation soils. Proc Natl Acad Sci U S A 106:18315–18320. doi: 10.1073/pnas.0907303106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou J, Deng Y, Zhang P, Xue K, Liang Y, Van Nostrand JD, Yang Y, He Z, Wu L, Stahl DA. 2014. Stochasticity, succession, and environmental perturbations in a fluidic ecosystem. Proc Natl Acad Sci U S A 111:E836–E845. doi: 10.1073/pnas.1324044111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shade A, Jones SE, Caporaso JG, Handelsman J, Knight R, Fierer N, Gilbert JA. 2014. Conditionally rare taxa disproportionately contribute to temporal changes in microbial diversity. mBio 5:e01371-14. doi: 10.1128/mBio.01371-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tedersoo L, Sánchez-Ramírez S, Kõljalg U, Bahram M, Döring M, Schigel D, May T, Ryberg M, Abarenkov K. 2018. High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Divers 90:135–159. doi: 10.1007/s13225-018-0401-0. [DOI] [Google Scholar]
- 41.Hoffmann K, Voigt K, Kirk P. 2011. Mortierellomycotina subphyl. nov., based on multi-gene genealogies. Mycotaxon 115:353–363. doi: 10.5248/115.353. [DOI] [Google Scholar]
- 42.Goldford JE, Lu N, Bajić D, Estrela S, Tikhonov M, Sanchez-Gorostiaga A, Segrè D, Mehta P, Sanchez A. 2018. Emergent simplicity in microbial community assembly. Science 361:469–474. doi: 10.1126/science.aat1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esperschütz J, Pérez-de-Mora A, Schreiner K, Welzl G, Buegger F, Zeyer J, Hagedorn F, Munch J, Schloter M. 2011. Microbial food web dynamics along a soil chronosequence of a glacier forefield. Biogeosciences 8:3283–3294. doi: 10.5194/bg-8-3283-2011. [DOI] [Google Scholar]
- 44.Kramer S, Dibbern D, Moll J, Huenninghaus M, Koller R, Krueger D, Marhan S, Urich T, Wubet T, Bonkowski M, Buscot F, Lueders T, Kandeler E. 2016. Resource partitioning between bacteria, fungi, and protists in the detritusphere of an agricultural soil. Front Microbiol 7:1524. doi: 10.3389/fmicb.2016.01524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren D, Madsen JS, Sørensen SJ, Burmølle M. 2015. High prevalence of biofilm synergy among bacterial soil isolates in cocultures indicates bacterial interspecific cooperation. ISME J 9:81. doi: 10.1038/ismej.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ražem D, Katušin-Ražem B. 2002. Dose requirements for microbial decontamination of botanical materials by irradiation. Radiat Phys Chem 63:697–701. doi: 10.1016/S0969-806X(01)00604-1. [DOI] [Google Scholar]
- 47.Tallentire A, Miller A, Helt-Hansen J. 2010. A comparison of the microbicidal effectiveness of gamma rays and high and low energy electron radiations. Radiat Phys Chem 79:701–704. doi: 10.1016/j.radphyschem.2010.01.010. [DOI] [Google Scholar]
- 48.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 49.Weaver RW, Angle S, Bottomley P, Bezdiecek D, Smith S, Tabatabai A, Wollum A, Mickelson SH, Bigham JM (ed). 1994. Method of soil analysis. Part 2: microbiological and biochemical properties. Soil Science Society of America, Madison, WI. [Google Scholar]
- 50.Gilbert JA, Meyer F, Antonopoulos D, Balaji P, Brown CT, Brown CT, Desai N, Eisen JA, Evers D, Field D, Feng W, Huson D, Jansson J, Knight R, Knight J, Kolker E, Konstantindis K, Kostka J, Kyrpides N, Mackelprang R, McHardy A, Quince C, Raes J, Sczyrba A, Shade A, Stevens R. 2010. Meeting report: the terabase metagenomics workshop and the vision of an Earth microbiome project. Stand Genomic Sci 3:243. doi: 10.4056/sigs.1433550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walters W, Hyde ER, Berg-Lyons D, Ackermann G, Humphrey G, Parada A, Gilbert JA, Jansson JK, Caporaso JG, Fuhrman JA, Apprill A, Knight R. 2016. Improved bacterial 16S rRNA gene (V4 and V4-5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems 1:e00009-15. doi: 10.1128/mSystems.00009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown J, Zavoshy N, Brislawn CJ, McCue LA. 2018. Hundo: a Snakemake workflow for microbial community sequence data. PeerJ Preprints 6:e27272v1 https://peerj.com/preprints/27272/. [Google Scholar]
- 54.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. 2016. VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDonald D, Clemente JC, Kuczynski J, Rideout JR, Stombaugh J, Wendel D, Wilke A, Huse S, Hufnagle J, Meyer F, Knight R, Caporaso JG. 2012. The Biological Observation Matrix (BIOM) format or: how I learned to stop worrying and love the ome-ome. GigaScience 1:7. doi: 10.1186/2047-217X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lanzén A, Jørgensen SL, Huson DH, Gorfer M, Grindhaug SH, Jonassen I, Øvreås L, Urich T. 2012. CREST–classification resources for environmental sequence tags. PLoS One 7:e49334. doi: 10.1371/journal.pone.0049334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sievers F, Higgins DG. 2014. Clustal Omega, accurate alignment of very large numbers of sequences. Methods Mol Biol 1079:105–116. doi: 10.1007/978-1-62703-646-7_6. [DOI] [PubMed] [Google Scholar]
- 59.Price MN, Dehal PS, Arkin AP. 2010. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.R Core Team. 2000. R language definition. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 61.McMurdie PJ, Holmes S. 2013. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara R, Simpson GL, Solymos P, Stevens MHH, Wagner H. 2013. Package ‘vegan’. Community Ecology Package, version 2. R Development Core Team, Vienna, Austria. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The most abundant bacterial (A) and fungal (B) phyla are plotted for the native soil (labeled as “n”) and the 6-week-chitin-enriched soil (labeled as “c”). The alpha diversities of bacteria (C) and fungi (D) were estimated using species richness and Simpson’s evenness. Download FIG S1, PDF file, 0.01 MB (8.5KB, pdf) .
Copyright © 2019 Zegeye et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Rate of respiration varies over time based on dilution and physical environment. The respiration rate was plotted for both liquid (top) and soil (bottom) for NAG enrichment cultures from weeks 2 through 7 to avoid the light spike in CO2 released immediately after inoculation. Respiration was monitored three times a week with a PP Systems (Amesbury, MA) EGM-4 environmental gas monitor. The gas analyzer was set up in static mode to measure CO2 and monitor respiration in each sample at periodic intervals. To measure CO2, a 10-ml gas aliquot was taken from the incubation container using a sterile plastic syringe with a control valve. Download FIG S2, PDF file, 0.01 MB (14.3KB, pdf) .
Copyright © 2019 Zegeye et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Bacterial OTUs observed from 16S rRNA gene amplicon products in uninoculated controls. The most abundant OTUs shown in the controls were also found in the other samples. Download FIG S3, PDF file, 0.09 MB (89.8KB, pdf) .
Copyright © 2019 Zegeye et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Final richness significantly varies between dilutions. Tukey’s honestly significant difference (HSD) test was performed to compare the richness between dilutions, and the P values are shown here. Download Table S1, DOCX file, 0.01 MB (13.1KB, docx) .
Copyright © 2019 Zegeye et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Patterns of variation within sample groups and within replicates. (A) Beta dispersion (within sample group variation) varies among time points as shown by the pairwise permutation test for homogeneity of multivariate dispersion at each time. Pairwise comparisons of the observed P values are tabulated for both the 16S and ITS for liquid and soil treatment conditions and indicate significant difference of within group dispersion. (B) A linear regression fit to the pairwise distance between replicates also shows changes in dispersion over time. The different dilutions are shown in separate facets and are used as strata during the permutation testing of beta dispersion. Download FIG S4, PDF file, 2.9 MB (2.9MB, pdf) .
Copyright © 2019 Zegeye et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The adonis test (permutational multivariate analysis of variance using distance matrices) was used to partition variation associated with dilution in the final time points from each treatment condition. The R2 column reports the percentage of weighted Unifrac distance that can be attributed to differing dilution. Download Table S2, DOCX file, 0.01 MB (12.9KB, docx) .
Copyright © 2019 Zegeye et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Outputs from the tests for homogeneity of multivariate dispersions performed with respect to each treatment. Download Table S3, DOCX file, 0.01 MB (15.2KB, docx) .
Copyright © 2019 Zegeye et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
Genetic sequencing data are available on DataHub at https://doi.org/10.25584/data.2019-02.700/1506698 for both 16S and 1TS amplicons. The R markdown processing scripts used to process the data and build graphs are available on Open Science Framework at https://osf.io/6d5kz/.