Abstract
Optogenetics and chemogenetics are powerful tools, allowing the specific activation or inhibition of targeted neuronal subpopulations. Application of these techniques to sleep and circadian research has resulted in the unveiling of several neuronal populations that are involved in sleep–wake control, and allowed a comprehensive interrogation of the circuitry through which these nodes are coordinated to orchestrate the sleep–wake cycle. In this review, we discuss six recently described sleep–wake and circadian circuits that show promise as therapeutic targets for sleep medicine. The parafacial zone (PZ) and the ventral tegmental area (VTA) are potential druggable targets for the treatment of insomnia. The brainstem circuit underlying rapid eye movement sleep behavior disorder (RBD) offers new possibilities for treating RBD and neurodegenerative synucleinopathies, whereas the parabrachial nucleus, as a nexus linking arousal state control and breathing, is a promising target for developing treatments for sleep apnea. Therapies that act upon the hypothalamic circuitry underlying the circadian regulation of aggression or the photic regulation of arousal and mood pathway carry enormous potential for helping to reduce the socioeconomic burden of neuropsychiatric and neurodegenerative disorders on society. Intriguingly, the development of chemogenetics as a therapeutic strategy is now well underway and such an approach has the capacity to lead to more focused and less invasive therapies for treating sleep–wake disorders and related comorbidities.
Keywords: parafacial zone, ventral tegmental area, hypercapnia, REM behavioral disorder, aggression, photic regulation of arousal and mood
Statement of Significance
The development of molecular genetic tools such as optogenetics and chemogenetics has revolutionized neurobehavioral research in general, and sleep and circadian science in particular. In this review, we discuss six recently described sleep–wake and circadian circuits that carry great potential in leading to the development of novel pharmacological treatments and interventional strategies for reducing the burden of sleep–wake disruption and related neurological comorbidities.
Introduction: Genetically Engineered Receptors and Channels as Tools or Therapeutics?
The increasingly widespread availability of tools such as chemogenetics and optogenetics has equipped basic sleep and circadian scientists with an unprecedented ability to interrogate the neural circuitry subserving arousal state change. As our understanding of these circuits has continued to expand, so too have the opportunities for exploiting these circuits for their therapeutic potential, whether this be through the discovery of novel pharmacological targets, developing gene-based treatments or uncovering new ways in which lifestyle habits and sleep–wake hygiene can be modified to better achieve patient outcomes.
The success of genetically engineered receptors as a neuroscientist’s tool largely relies on two factors: Firstly (and arguably most importantly), these receptors are engineered such that they are no longer activated by endogenous ligands in vivo. Instead, they may be activated by injectable pharmacologically inert ligands specific to the receptor (chemogenetics) [1, 2] or the delivery of specific wavelengths of light via an implanted optical fiber (optogenetics) [3, 4], allowing an investigator temporal control over particular subsets of neurons. Secondly, these receptors can be expressed within the brain in both a neuroanatomically and neurochemically specific manner. Localization of the receptor is achieved using a highly precise brain injection of a small quantity of a viral vector (commonly, adeno-associated viral vectors [AAVs]) carrying the transcript for the receptor to the particular brain region of interest. Typically, the receptor transcript is packaged within a Flip-Excision-Switch (FLEX) cassette such that the functional receptor can be expressed only in the presence of cre-recombinase (cre). Cre-driver mouse lines are then employed whereby which cre is expressed downstream of a selected promoter, thus ensuring expression of the receptor specifically in neurons expressing the select protein. By taking advantage of these receptors as tools, investigators can effectively hijack a neural circuit and evaluate the effect that exciting or inhibiting a specific neuronal node has upon behavior [5].
In addition to their effectiveness as a neuroscientist’s tool, genetically engineered receptors show considerable potential as a therapeutic strategy in their own right. Conceptually, one can imagine a scenario whereupon the downregulation of a hyperexcitable node or the increased activation of a depressed node within a network may stabilize a neural circuit and thereby improve symptoms or normalize behavior. For instance, suppose one could express an excitatory chemogenetic receptor within a typically sleep-promoting node such as the parafacial zone (PZ) [6] in a patient with insomnia. Administration of the chemogenetic ligand would activate the PZ, trigger sleep-promoting circuitry, and bring about sleep, acting as a more selective hypnotic agent.
Attractive as such a proposal is, several hurdles must be overcome before such a scenario could become a reality. For example, before brain delivery in humans, appropriate viral vector and chemogenetic receptor combinations must be selected that display sufficient receptor expression, low toxicity, and little endogenous constitutive activity. Additionally, chemogenetic ligands are required that are both biologically inert in the absence of the chemogenetic receptor and that break down to nontoxic, biologically inert metabolites. Nevertheless, progress on all of these fronts is underway (for review see [2]), and recently, successful injections of AAVs carrying functional inhibitory chemogenetic receptors have been made in nonhuman primates [7]. There exists some uncertainty, however, upon the practicability of chemogenetically activating neuronal subpopulations in humans. Due to the potentially supraphysiological activation of neurons using this method, legitimate concerns arise regarding the risk of neurotransmitter depletion at transfected synapses, altered plasticity within neuronal circuits and the uncertainty of other long-term side-effects resulting from repetitive chemogenetic activation of a circuit. In mouse models, relatively long behavioral effects can be induced from a single low-dose CNO injection (>6 hr [6, 8, 9]), demonstrating that sustained chemogenetic activation of a neuronal population is achievable. Additionally, plasticity changes within neuronal circuits may even, in some instances, be a desirable effect of repeated chemogenetic activation. However, more thorough and more long-term studies must be carried out in animal models before the feasibility of chemogenetic activation as a therapeutic strategy can be fully assessed. Nevertheless, one chemogenetic tool that shows some therapeutic potential, and that has recently been tested in mouse models, is the inhibitory mutated human glycine receptor (hGlyR). hGlyR is an inhibitory ionotropic receptor that is gated by the drug, ivermectin (IVM) [10–12]. Since hGlyR is inhibitory, concerns about supraphysiological activation of the neurons in which it is expressed is not a concern. Moreover, because hGlyR is a human protein, and IVM has already been approved by the FDA for use in humans and animals as an antibiotic against worm infestations, this chemogenetic tool is especially well suited for clinical use.
One ongoing challenge is that the immense complexity of the circuitry governing arousal and circadian rhythms remains incompletely understood. While the elucidation of these circuits will undoubtedly lead to the development of more appropriate therapeutic targets, there have already been considerable strides in our understanding of these circuits in recent years, with several new circuits and circuit nodes revealed. This review spotlights six novel sleep–wake and circadian circuits that were presented at the recent SLEEP 2018 meeting and that demonstrate the variety of ways in which circuit-based sleep and circadian research can contribute to the development of targets for therapeutic interventions.
Parafacial zone GABAergic neurons: a new target for sleep enhancement
The past two decades of sleep research have seen a dramatic increase in the number of identified brain areas involved in slow-wave sleep (SWS, the deepest stage of nonrapid eye movement [NREM] sleep) control, including the well-known ventrolateral preoptic area (VLPO) [13, 14], nNOS-expressing cortical neurons [15], and the PZ [16]. This past year has been particularly prolific; neurons involved in SWS control have been described in the nucleus accumbens [17], zona incerta [18], the rostromedial tegmental nucleus [19], and even in the ventrolateral periaqueductal gray matter (vlPAG) [20]. Among all of these sleep-promoting neuronal populations, the PZ GABAergic neurons are arguably the most potent at inducing and maintaining deep sleep.
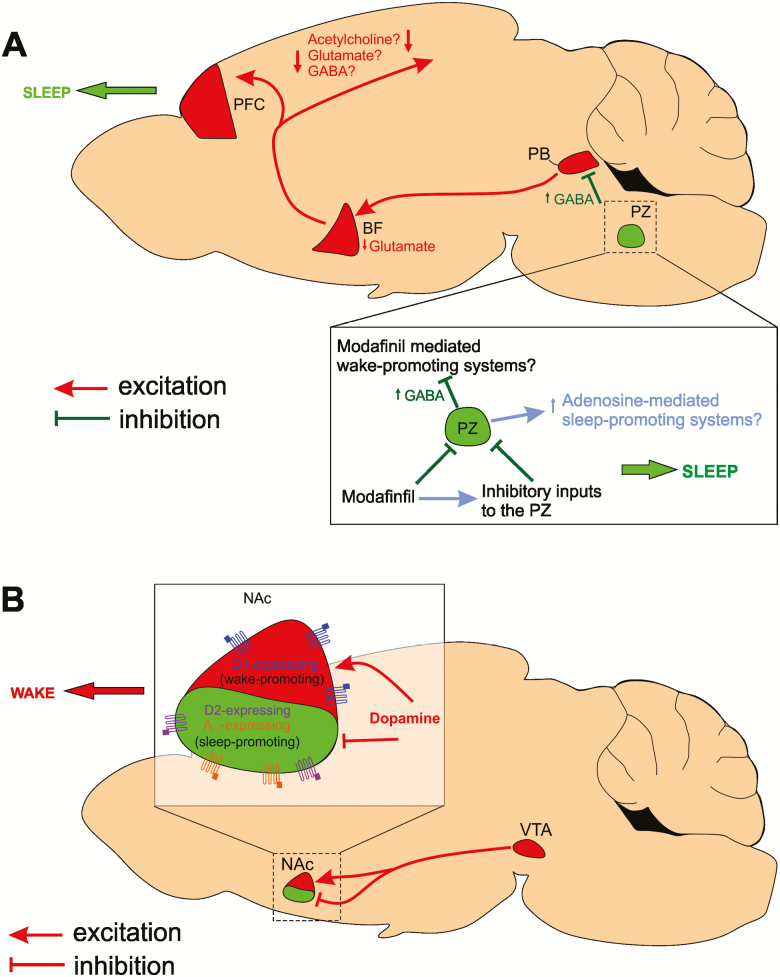
The PZ is located in the brainstem, dorsal and lateral to the facial nerve [21], and contains neurons that are specifically active during sleep [22]. PZ GABAergic neurons are not only necessary for maintaining a normal amount of sleep [21], but their activation is also sufficient to induce and maintain deep SWS [6]. PZ cell body–specific lesions and disruption of PZ GABAergic transmission result in insomnia, whereas acute and specific chemogenetic activation of PZ GABAergic neurons induces SWS with a short latency, increases SWS amount and consolidation, and enhances slow-wave activity (SWA, a marker of SWS quality) [6]. PZ GABAergic neurons likely bring about sleep through their inhibition of the parabrachial nucleus (PB) [6]. The PB in turn projects to the basal forebrain (BF), which innervates the cortex to promote electrocortical arousal [23] (Figure 1A). Impressively, the sleep-promoting drive of PZ GABAergic neurons is so powerful that it can counteract the wake-promoting action of psychostimulants such as modafinil and caffeine [13].
Figure 1.
PZ GABA and VTA dopamine sleep–wake circuitries. (A) Activation of PZ GABAergic neurons inhibits the parabrachial nucleus (PB), which reduces excitatory drive to the basal forebrain (BF) and subsequently the prefrontal cortex (PFC), bringing about a switch in cortical activity from wakefulness to SWS. Remarkably, PZ activation causes a wake-to-sleep switch even in the presence of psychostimulants such as modafinil and caffeine. The wake-promoting actions of modafinil may be upstream of the PZ, directly (or indirectly) inhibiting PZ GABAergic neurons. Alternatively, modafinil may have excitatory actions upon wake-promoting circuitries that are under powerful inhibition from the PZ. Additionally, the sleep-promoting actions of the PZ may utilize a common mechanism to the sleep-promoting factor adenosine since PZ-mediated SWS enhancement is partially blocked by the adenosine antagonist, caffeine. (B) Activation of VTA dopamine terminals in the nucleus accumbens (NAc) induces potent arousal. This is likely through the combined actions of dopamine inhibition of the adenosine 2A receptor (A2A)/dopamine D2 receptor (D2)-expressing sleep-promoting NAc neurons and activation of dopamine D1 receptor (D1)-expressing wake-promoting NAc neurons.
Modafinil is commonly used to treat the excessive daytime sleepiness associated with many disorders such as narcolepsy [24]. It induces long lasting wakefulness that is associated with enhanced cognitive capability but not with hyperactivity, in both human and animals [25]. However, the wake-promoting action of the modafinil R-enantiomer (armodafinil) is nullified by chemogenetic activation of PZ GABAergic neurons. Furthermore, SWS is actually enhanced, resulting in a SWS phenotype indistinguishable from that observed following chemogenetic activation of PZ GABAergic neurons in the absence of armodafinil treatment [26]. Although the mechanism of action of modafinil remains a mystery, these findings suggest some of its potential neural targets. Since armodafinil’s capacity to induce arousal is completely negated in the face of chemogenetic activation of PZ GABAergic neurons, one might conjecture that armodafinil acts upon nodes within the sleep-promoting PZ GABAergic circuit. For example, while armodafinil may act upon upstream sites to increase inhibitory tone to PZ GABAergic neurons or even directly inhibit them, chemogenetic activation of PZ GABAergic neurons supersedes this inhibitory input. Alternatively, armodafinil may act upon downstream populations that receive substantial inhibitory tone from PZ GABAergic neurons. In this case, it follows that the inhibitory tone from chemogenetically activated PZ GABAergic neurons overrules the action of armodafinil and the neural activity of downstream targets remains depressed (Figure 1A).
Similarly, chemogenetic activation of PZ GABAergic neurons significantly attenuates the wake-promoting action of caffeine, the most widely used psychostimulant [27]. However, in the presence of caffeine, the dramatic enhancement of SWS typically observed following chemogenetic activation of PZ GABAergic neurons is mitigated, and levels of arousal are comparable to the control condition (i.e. when not chemogenetically activated and in the absence of caffeine). Moreover, SWS cortical EEG is significantly more desynchronized than following chemogenetic activation alone, indicating decreased SWS quality [26]. Since activation of PZ GABAergic neurons and caffeine (a well-known adenosine antagonist) counteract each other’s effects upon arousal, it can be hypothesized that the PZ’s sleep-promoting actions could be mediated by adenosine. Adenosine is a sleep factor that has long been known to promote SWS and SWA homeostasis [28]. Because adenosine and PZ GABAergic neurons both promote sleep in a manner that is antagonized by caffeine, it is plausible that they may act upon a shared sleep-promoting circuit (Figure 1A). Understanding the relationship between the PZ and the sleep-promoting actions of adenosine may help in uncovering novel non-GABAergic pharmacological candidates to assist in the treatment of insomnia.
It is the case that new and more selective sleep-promoting drugs are in great demand in sleep medicine. Indeed, sleep-promoting drugs, and more specifically the ones enhancing GABAergic transmission, are frequently associated with next day “hangover.” It is likely that this “hangover” associated with GABAergic sleep-promoting drugs is due to the action of these drugs on ALL central GABAergic neurons (including cortical GABAergic neurons) and that a more selective activation of GABAergic neurons within the PZ alone may mitigate the “hangover” effect while still bringing about the desired sleepiness. Interestingly, a recent study suggested that activating PZ GABAergic neurons also improves learning and memory, indicating that cognitive abilities are enhanced, rather than disrupted, following PZ GABAergic activation [29]. Although such a drug may not treat the underlying cause of insomnia (which is often the consequence of other pathological changes within the brain or due to a disrupted lifestyle resulting from stress, shift work, or a noisy environment), it may well be efficacious in treating insomnia symptoms. As such, SWS induction via PZ activation is of great interest from a clinical perspective in developing therapeutics to help reduce the burden of insomnia.
In conclusion, PZ GABAergic neurons’ effectiveness in fully or partially counteracting the wake promoting action of potent psychostimulants, and to induce and enhance SWS and SWA, indicates that these neurons play a central role in sleep induction and maintenance. Critically, any drug that specifically activates PZ GABAergic neurons is likely to become a selective and specific sleep-promoting treatment.
Ventral tegmental area regulation of sleep and wakefulness
The ventral tegmental area (VTA) is a brain hub for the regulation of motivation, reinforcement learning, and reward processing [30, 31]. It contains intermingled glutamatergic, dopaminergic, and GABAergic neurons [32]. VTA dopaminergic neurons are attractive candidates for regulating sleep–wake states as pharmacological substances that modulate dopamine neurotransmission are among the strongest known stimulants [33], and their arousing effects are abolished in mice deficient in dopamine signaling [34, 35]. In humans, genetic variations in dopaminergic signaling pathways modulate the negative effects of sleep loss on behavior and performance [36]. Nonetheless, for several decades, dopamine has been considered as the only monoamine not involved in sleep–wake regulation [37, 38], mainly because early electrophysiological findings suggested that the VTA and substantia nigra pars compacta (SNc) dopaminergic neurons do not change their mean firing rates across sleep–wake states [39–42] and lesions of dopaminergic VTA and SNc neurons do not modify time spent in wakefulness [43, 44]. However, recent studies using detailed single-unit electrophysiological measurements and calcium-dependent fiber-photometry recordings have revealed robust alterations in VTA dopaminergic neuron activity across arousal states [45, 46]. Furthermore, optogenetic and chemogenetic activation of VTA dopaminergic neurons indicate that activity in these neurons can induce and maintain wakefulness [46, 47] and is potent enough to initiate arousal from isoflurane anesthesia [48]. In contrast, chemogenetic inhibition of VTA dopaminergic neurons can promote sleep [19, 46] or sleep-preparatory behaviors, despite the presence of salient stimuli such as a potential mate, predator odor, or palatable food [46]—suggesting a prominent role for VTA dopaminergic circuitry in salience-induced arousal [49].
VTA dopaminergic neurons project to many brain regions including the nucleus accumbens (NAc), central amygdala (CeA), dorsolateral striatum (DLS), and medial prefrontal cortex (mPFC). Short phasic optogenetic stimulations (5 s at 25 Hz, to mimic burst firing [45, 50]) of VTA dopaminergic projections in the NAc, CeA, and DLS, but not the mPFC, promote transitions from NREM sleep to wakefulness. Stimulating VTA dopaminergic projections within the mPFC and CeA can also initiate a transition from rapid eye movement (REM) sleep to wakefulness [46]. Importantly, stimulating VTA dopaminergic projections to the NAc can maintain wakefulness throughout the entirety of a prolonged stimulation paradigm (2 s per min at 25 Hz for 6 hr), whereas the stimulation of other projections cannot [46], highlighting the strength of the VTA-NAc connection in inducing arousal. These findings are consistent with recent studies implicating the NAc in sleep–wake regulation [17, 51–53]. Adenosine A2A/dopamine D2 receptor–expressing NAc GABAergic neurons promote NREM sleep [17], whereas dopamine D1 receptor–expressing GABAergic NAc neurons promote wakefulness [52]. Projections from VTA dopaminergic neurons to the NAc may therefore cause arousal through the inhibition of adenosine A2A/dopamine D2-expressing (sleep-promoting) NAc neurons and the excitation of dopamine D1-expressing (wake-promoting) NAc neurons (Figure 1B).
In addition to dopaminergic neurons, the VTA also contains a large GABAergic population [32]. VTA GABAergic neurons have been suggested to tightly control activity in VTA dopaminergic neurons in the context of motivation and reward [54, 55]. Interestingly, both single-unit recordings from putative VTA-GABAergic neurons [56] and recent fiber-photometry recordings from vesicular GABA transporter (VGAT) expressing VTA neurons [57] demonstrate strong activation during wakefulness and REM sleep, and low activity during NREM sleep—a similar activity pattern to VTA dopaminergic neurons [45, 46]. In apparent contradiction, however, chemogenetic activation of VTA GABAergic neurons promotes NREM sleep, whereas lesions promote wakefulness [44]. Since VTA GABAergic neurons are not physiologically active during NREM sleep, one reconciliatory explanation is that VTA GABAergic neurons have the capacity to fine-tune activity in wake-promoting populations and as such, their robust chemogenetic activation ultimately results in the inhibition of wake-promoting neurons, whereas their inhibition disinhibits these neurons, permitting wakefulness.
Dysregulation of arousal is a hallmark of many psychiatric disorders [58]. Moreover, such disorders, including major depression, bipolar disorder, schizophrenia, and substance abuse, are accompanied by alterations in VTA neuronal activity [59, 60]. Despite this, very little is known about the mechanistic relationship between sleep–wake disturbances, neuropsychiatric disorders, and dysregulation of VTA neurons [58, 61]. Additionally, it is now recognized that sleep–wake disturbances are not merely a side effect of psychiatric disorders, but are in fact thought to play a causative role in the progression of these disorders [58]. The circuitry described herein highlights the importance of the VTA as an important node in sleep–wake regulation and provides a framework with which to investigate the causal role of the VTA in sleep–wake disturbances associated with psychiatric disorders. Understanding the relationship between how the VTA controls arousal and how its activity is dysregulated over the progression of neuropsychiatric disease will be fundamental for identifying and developing therapeutic interventions to improve mental health.
A dedicated brainstem circuit underlies REM sleep behavior disorder
REM sleep behavior disorder (RBD) is a neurological disorder characterized by the loss of muscle atonia during REM sleep, which can lead patients to “act out” their dreams [62–64]. Patients often display violent behavior resulting in injury to themselves or their bed partners [63–65]. More importantly, 90% of people with RBD eventually develop a neurodegenerative synucleinopathy (e.g. Parkinson’s disease [PD], Multiple System Atrophy, or dementia with Lewy bodies) 10 to 15 years after their RBD diagnosis [62, 63, 66–68]. The strong link between RBD and these neurodegenerative diseases [69, 70], and the occurrence of symptoms exclusively during REM sleep, has led to the hypothesis that neurodegeneration within the circuit controlling muscle atonia during REM sleep underlies RBD [62, 68–70].
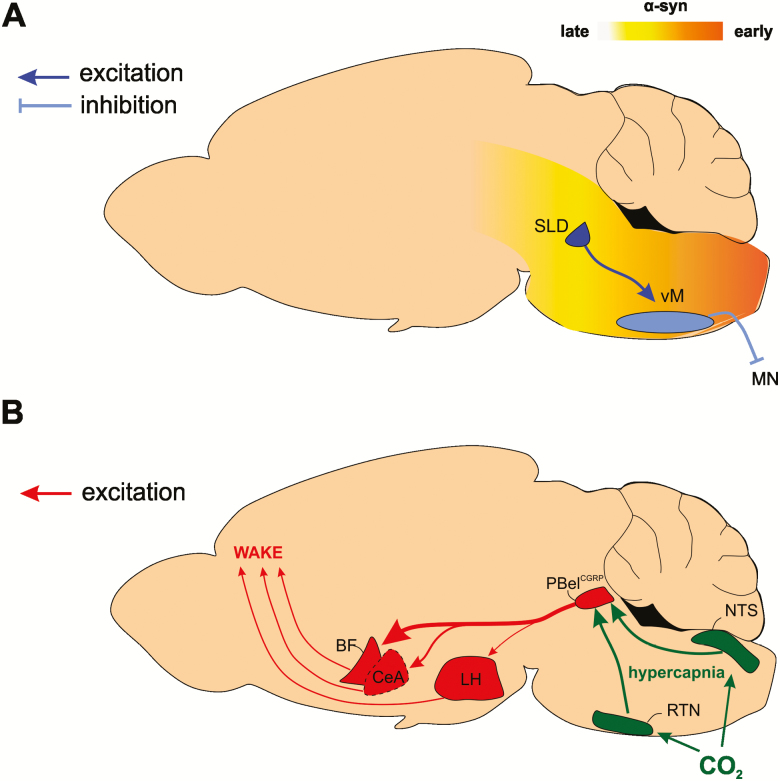
The release of GABA and glycine onto motoneurons is critical for the silencing of muscle activity during REM sleep [71]. Simultaneous antagonism of glycine, GABA-A, and GABA-B receptors causes REM sleep without atonia [72, 73], while transgenic mice lacking normal glycine and GABA transmission show motor behaviors during REM sleep similar to those observed in people with RBD [74]. A specific two-part circuit in the brainstem functions to release GABA/glycine onto motoneurons of the spinal cord and inhibit muscle activity [75, 76]. A group of cells located at the mesopontine junction called the sublateral dorsal tegmentum (SLD) has been hypothesized to not only control the entrance into REM sleep but also the muscle paralysis that characterizes the state [75–79]. Activation of cells in this region induces REM sleep muscle atonia, whereas lesions cause REM sleep without atonia [76]. The majority of REM sleep-active cells in the SLD are glutamatergic [80, 81], and long-term impairment of glutamate transmission from SLD cells using short hairpin RNA (shRNA) induces periods of REM sleep without atonia and reduces the overall amount of REM sleep [82]. Glutamate cells located in the ventral part of the SLD are thought to control the atonia of REM sleep by connecting to cells in the ventral medulla (vM) [78].
GABA/glycine cells located in the vM constitute the second part of the circuit that controls REM sleep muscle atonia by releasing these inhibitory neurotransmitters onto motoneurons. Lesions of the vM cause periods of REM sleep without atonia [83–87], and blocking GABA release from vM cells by shRNA increases motor activity during REM sleep [88]. When GABAergic transmission in vM cells is impaired, mice display periods of REM sleep with complex motor behavior such as “trying to jump or run” [88]. In addition, recent work has demonstrated that optogenetic inhibition of SLD glutamate release at the level of the vM induces intense motor activity during REM sleep [89]. Together, this evidence supports the idea that the described 2-part REM sleep circuit (i.e. glutamate SLD → GABA/glycine vM; Figure 2A) is responsible for suppression of muscle activity in REM sleep since damage to either part of this circuit leads to symptoms identical to those observed in patients suffering from RBD [68, 78].
Figure 2.
Circuitry underlying RBD and hypercapnic arousals. (A) Glutamate cells in the sublaterodorsal tegmental nucleus (SLD) cause REM sleep muscle atonia by activating GABA/glycine cells in the ventromedial medulla (vM), which in turn inhibit motor neurons (MN). RBD is caused by Lewy pathology (i.e. aggregation of α-syn) within the circuit controlling REM sleep muscle atonia (i.e. SLD → vM), which results in REM sleep without atonia and RBD. Eventually these aggregates propagate rostrally through the neural axis to cause degeneration of neurons involved in synucleinopathy (e.g. Parkinson’s disease). (B) Increased pCO2 (hypercapnia) causes activation of both central and peripheral chemoreceptors whose signals are integrated in the nucleus of the solitary tract (NTS) and retrotrapezoid nucleus (RTN). The NTS and RTN activate neurons expressing CGRP in the external lateral parabrachial (PBelCGRP) nucleus, a node that causes cortical arousal, largely through projections to the basal forebrain (BF), and to a lesser extent through the central nucleus of the amygdala (CeA) and the lateral hypothalamus (LH). The thickness of the arrows emanating from the PB represents the relative contribution of each network connection in eliciting hypercapnia-induced arousal.
Several lines of evidence support the hypothesis that targeted neurodegeneration of cells in the SLD-vM circuit leads to RBD: (1) Imaging studies show that neuronal integrity is lost in the SLD region of people with RBD [90]; (2) these patients show pathological synuclein aggregates in the brainstem including in the SLD and vM regions [91–93]; and (3) RBD is one of the strongest prodromal indicators of synucleinopathies with 90% of people with RBD eventually developing a more severe neurodegenerative disorder like PD [94–96]. Our current understanding of synucleopathic processes indicates that misfolding of the protein α-synuclein (α-syn) into fibrils leads to the formation of pathological aggregates known as Lewy bodies and neurites [63, 68, 97, 98]. These aggregates eventually interact with mitochondria to cause cell death [99] and can be transferred through cell-to-cell contact in a prion-like manner [98, 100]. Earlier signs of α-syn aggregates are present in the brainstem, including in the SLD-vM circuit [91, 92], and eventually these aggregates propagate rostrally through the neural axis to cause degeneration of neurons involved in PD (i.e. dopamine cells in the substantia nigra; Figure 2A) [68, 97]. It remains untested whether targeted degeneration of the circuit controlling REM sleep muscle atonia underlies RBD. Virally mediated overexpression of α-syn, which has been used previously to model PD [101–103], can be adapted to model RBD by targeting the SLD-vM circuit that controls REM sleep muscle atonia [68]. It is quite clear that the development of such a model, mimicking the neurodegenerative aspect of RBD and its primary symptom (i.e. loss of muscle atonia), is crucial for understanding the pathogenesis of RBD and the development of potential treatments.
Brain circuitry for regulating arousal to hypercapnia
Obstructive sleep apnea (OSA) is characterized by repeated episodes of loss of airway dilator muscle tone during sleep, resulting in airway collapse and increased circulating blood CO2 levels (hypercapnia), followed by brief arousals that restore airway patency [104–111]. These repeated arousals result in sleep disruption, which in turn causes cognitive impairment, as well as cardiovascular and metabolic morbidities [108, 112–118]. Preventing arousal from sleep, while preserving the respiratory drive that reinitiates breathing, could potentially prevent these negative consequences arising from frequent OSA-based arousals. As such, understanding the precise circuits that mediate cortical EEG and respiratory responses to apnea is critical in the development of treatments for people with OSA.
Several brainstem cell groups, such as the serotonergic dorsal raphe nuclei [119–123] and the noradrenergic (NA) locus coeruleus (LC) neurons [124–126], may play a modulatory role [127] in mediating hypercapnia-arousal circuitry. However, the parabrachial nucleus (PB) of the rostral pons likely plays a major role in triggering hypercapnia-induced arousals [128–143]. The PB serves as a hub, receiving chemosensory information from upper airway afferents (that respond to pulmonary negative pressure associated with apneas) and from the retrotrapezoid nucleus (RTN) and the nucleus of the solitary tract (NTS) in the medulla (which sense hypercapnia and hypoxia; Figure 2B), while exerting powerful control over both cortical arousal [144–148] and respiration [136, 147, 149–155]. It is thought that the ascending projections of the PB promote cortical arousal [146, 147, 156–158], whereas its descending projections regulate respiration [143, 159].
As such, the PB is perfectly anatomically located to simultaneously control airway dilator muscles and trigger arousals during sleep apneas. Abolishing glutamate transmission specifically in the external lateral PB (PBel), through cell body lesions or through deleting the vesicular glutamate transporter 2 (VGlut2) gene, prolongs hypercapnia-induced arousal latencies, indicating a critical role of PBel glutamate transmission in hypercapnia-induced arousal [146]. Many of the PBel neurons that display cFos activation following hypercapnia [143] also express calcitonin gene–related peptide (CGRP, PBelCGRP) [160] and project to the forebrain, suggesting that the PBelCGRP neurons may be responsible for forebrain arousal during hypercapnia. Indeed, optogenetic activation of PBelCGRP neurons triggers short latency arousals, whereas chemogenetic activation significantly increases net wakefulness [147]. Additionally, optogenetic inhibition of PBelCGRP neurons during hypercapnia significantly increases the latency to arousal or prevents arousal altogether, indicating that the PBelCGRP neurons comprise an essential node of the hypercapnia-arousal circuitry. Importantly, silencing of the PBelCGRP neurons affects neither the respiratory drive during hypercapnia, nor the arousal threshold to an acoustic, somatosensory, or vestibular stimulation [147]. Forebrain targets of PBelCGRP neurons include the substantia innominata in the basal forebrain (BF), the CeA, and the lateral hypothalamus (LH; Figure 2B). Optogenetic silencing of PBelCGRP terminals in these brain areas increases the latency to arousal during hypercapnia, demonstrating that the PBelCGRP neurons can elicit arousal responses via direct projections to multiple arousal-promoting target sites. Projections to the BF appear to be most critical for the hypercapnia-arousal response since inhibiting this pathway most potently suppresses hypercapnic arousals. In contrast, inhibiting PBelCGRP projections to the CeA results in a more modest suppression of the hypercapnia arousal response, whereas inhibition of PBelCGRP terminals in the LH is comparatively least effective in preventing hypercapnic arousals [147]. The PBel thus has the capacity to orchestrate hypercapnia-induced awakenings, preserve the respiratory drive, and thereby permit an individual to survive the apneic event.
Although pharmacotherapy is not yet available for OSA, current research focuses on identifying druggable targets that can selectively activate the circuits that regulate the upper airways [161]. Another line of investigation seeks to quantify the “arousal threshold” in people with OSA, with the goal of providing more personalized therapeutic interventions for patients with a low-arousal threshold [162]. A deeper knowledge of the neural circuitries governing hypercapnic arousal, such as the PBelCGRP circuit described here, is crucial for this endeavor since hypercapnia is not the only stimulus that activates PBelCGRP neurons, which are also responsive to various potentially dangerous or aversive stimuli [157, 163–168]. As such, it is plausible that different classes of aversive stimuli may be encoded or processed by different subpopulations of PBelCGRP neurons [169], resulting in modulation of the hypercapnia-arousal response. Understanding how these afferents modulate PBelCGRP activity may inform novel strategies for preventing the low-threshold arousals experienced during hypercapnic events. In sum, the PBelCGRP neurocircuitry has the capacity to yield valuable therapeutic targets for preventing cortical arousal while preserving respiratory drive, ultimately preventing OSA and its negative secondary health consequences.
A circuit for the circadian control of aggression
The central circadian clock, located in the suprachiasmatic nucleus (SCN) of the hypothalamus, is required for daily rhythms of physiology and behavior. SCN neurons can function as individual oscillators with rhythmic electrical activity on a period of about 24 hr [170] under the control of clock genes [171]. Although this same genetic machinery is present in cells throughout the brain and body, the SCN is necessary to synchronize such peripheral oscillators and maintain rhythmic behavior [3]. Importantly, axonal output appears to be the primary method by which the SCN establishes such synchrony in vivo [172], and the majority of SCN axons target a nearby region known as the subparaventricular zone (SPZ) [173]. Indeed, circadian rhythms of sleep–wake, locomotor activity, and feeding behavior have been shown to be regulated by a pathway from the SCN, through the SPZ [174], to the dorsomedial nucleus of the hypothalamus (DMH; Figure 3) [172, 174].
Figure 3.
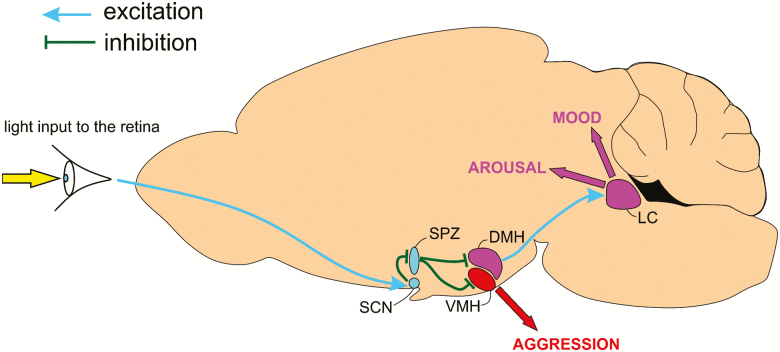
Circadian circuitry underlying behavioral aggression and the PRAM pathway. A midsaggital section of a mouse brain illustrating loci involved in circadian modulation of aggressive behavior and PRAM-associated arousal and affective behavior. Light input to the retina activates the SCN, which in turn inhibits the SPZ. Since the SPZ is primarily inhibitory, SCN activation disinhibits downstream targets of the SPZ such as the ventromedial hypothalamus (VMH), leading to aggression. Disruption of this pathway in people with AD and dementia may result in aggressive behavioral symptoms such as sundowning. Similarly, light input to the retina activates the SCN, in turn activating the DMH, possibly via disinhibition of SPZGABA neurons. Increased DMH activity stimulates the LC, increasing arousal and modulating affect. Activating this pathway carries potential as a possible therapeutic strategy for treating disorders of arousal and mood.
Neural pathways by which the circadian system directly regulates more complex behaviors, such as particular social behaviors, have remained more elusive. For example, although evidence from hamsters [175], rats [176], and humans [177] previously demonstrated temporal differences in aggressive behavior, it was unclear whether the SCN clock directly regulated a rhythm in aggression [178, 179]. Neurons within the ventromedial nucleus of the hypothalamus (VMH), specifically those that express estrogen (Esr1) and progesterone receptors in the ventrolateral VMH (VMHvl) [180, 181] regulate aggression in male mice. These neurons are not directly connected to the SCN but receive an inhibitory input from GABAergic SPZ (SPZGABA) neurons (Figure 3), which act as a critical intermediary between vasoactive intestinal polypeptide (VIP) neurons of the SCN (SCNVIP) and Esr1-expressing VMHvl neurons [10]. SPZGABA neurons that project to the VMH are predominantly active during the early light period, a time of day when nocturnal mice are usually at rest. Deletion of the VGAT gene in SPZ neurons (which eliminates the ability of these neurons to release GABA) results in increased aggression propensity in male mice during the early light period. Importantly, corticosterone levels and locomotor activity are not increased following VGAT deletion from SPZGABA neurons, indicating that the increased aggression observed during the early light period is not due to increased stress or locomotor activity in these animals. Similarly, chemogenetic inhibition of SPZGABA neurons during the early light period (but not during the early dark period) causes reversible increases in aggression. As such, it is apparent that aggression propensity in male mice follows a daily rhythm that is contingent upon proper functioning of SPZGABA neurons and that the SCNVIP → SPZGABA → VMH circuit constitutes a critical pathway through which the central circadian clock gates aggression propensity across the day.
Since circadian dysfunction and aggression are comorbid factors in numerous neuropsychiatric, neurodevelopmental, and neurodegenerative disorders, the SCNVIP → SPZGABA → VMH circuit is a potentially fruitful target upon which to develop pharmaceuticals that ameliorate circadian-related behavioral symptoms associated with these disorders. For instance, neurodegenerative diseases such as Alzheimer’s disease (AD) and related dementias are accompanied by progressive disruption in sleep–wake and other circadian rhythms. One particular feature of circadian dysfunction in people with AD and related dementias is “sundowning syndrome,” a poorly understood clinical phenomenon characterized by agitation, aggression, and delirium during the early evening [182–184]. The neurobiology of sundowning remains unknown [185]; however, the temporal periodicity of its symptoms is indicative of a possible disturbance within the SCN clock, or in the pathways by which the SCN modulates particular rhythms such as aggression. Notably, disrupting the SCNVIP → SPZGABA → VMH pathway leads to increased aggression during the early resting phase in mice [10], which is temporally analogous to the time of day that people with AD and dementia experiencing sundowning display increased agitation and aggression. Although this suggests that the SCNVIP → SPZGABA → VMH circuit may be compromised in AD and dementia, it also raises the intriguing possibility that light therapy may alleviate some of the behavioral symptoms of sundowning since the activity of the SCN is entrained by light input from the retina [186]. Indeed, studies exploiting light as a therapeutic tool in people with AD and dementia have already shown beneficial effects in reducing agitation and improving emotion [187–189]. Therefore, the SCNVIP → SPZGABA → VMH pathway as a circuit substrate is a promising therapeutic target for the treatment of circadian dysfunction and aggression in patients who display sundowning, and successful interventions acting upon this pathway have the capacity to greatly improve the quality of life for people with AD and dementia and their caretakers.
A circuit for the photic regulation of arousal and mood
The noradrenergic (NA) locus coeruleus (LC) promotes high levels of vigilance and arousal [124, 190] and tonic discharge of NA-LC cells is highest during waking, and virtually silent during REM sleep. Additionally, LC activity anticipates sleep–wake stage transitions [191], is necessary for maintaining alertness under novel conditions [192], and its activation is sufficient to alter EEG measurements of anesthetic depth and accelerate recovery of consciousness [193]. The LC is also involved in specific cognitive functions such as learning and memory [194–196], as well as attention and behavioral flexibility [197, 198].
A distinct circadian rhythm of firing activity is unmasked when competing inputs to the LC are blocked under anesthesia, and this is under indirect control of the master circadian clock in the SCN [199, 200]. The SCN receives a major excitatory input from melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs) [201], which release glutamate and pituitary adenylate cyclase-activating polypeptide (PACAP) at the SCN, thereby entraining the circadian clock [202–204]. The SCN, in turn, mediates LC activity via a DMH relay; SCN input onto the DMH is both direct and indirect, via the SPZ [205–207] (Figure 3). Together, this retina → SCN → DMH → LC pathway can be referred to as the photic regulation of arousal and mood (PRAM) pathway, reflecting the potential impact that perturbation of this circuit has upon arousal and mood [208]. Notably, each relay along this pathway has a documented role in promoting or modulating arousal. For example, activation of RGCs induces an arousal-like phenotype in mice [209, 210] or sleep behavior, depending on the activating wavelength [210], whereas lesions of the SCN result in the disturbance of circadian rhythms in animals [211, 212] and humans [200], and DMH lesions disrupt the circadian pattern of arousal and corticosterone [174, 213]. It is currently unclear through which specific cell types arousal information is conveyed from the DMH to the LC; however, the orexin (hypocretin) neurons are an attractive candidate, as these neurons are important for sleep–wake regulation and are apparently under circadian control [206, 207]. Consistent with the proposed role of the PRAM pathway, orexin neurons are themselves critical for mood [214] and for maintaining consolidated arousal [215–217]. Furthermore, optogenetic activation of orexinergic terminals within the LC rapidly triggers arousal in mice, indicating a functional DMH-orexin → LC arousal promoting circuit, which may be the source of circadian input to the LC [124, 190, 218–222]. Additional study is required to determine whether orexin neurons and/or neurons of other phenotypes play a critical role in PRAM signaling. This knowledge will lead to a better understanding of the limits of utility of the PRAM pathway.
Since the LC is under indirect control of light stimulation at the retina, this makes the ipRGCs a prospective candidate with which one can modulate activation of the PRAM pathway to affect arousal and behavior. Importantly, ipRGCs project to and influence the activity of nonimage forming neuronal circuitries and can therefore allow for efficient control of the PRAM pathway without promoting significant effects on conscious vision [202–204]. Moreover, the retina is particularly unique as it is the only part of the central nervous system that can be accessed without the risk of invasive brain surgery, and thus, is a promising target for novel human therapeutics, such as chemogenetic therapies. As such, the eye presents an alternate route for direct administration of viral vectors that carry the genetic information required for neurons to manufacture therapeutic proteins (for example, chemogenetic receptors). Indeed, intravitreal injections (IVIs) are now commonly used in clinical ophthalmology settings as a mechanism for delivering treatments for blinding diseases. The IVI is a low-cost and rapid procedure and there is now compelling data to indicate that it is a safe and effective method of delivering treatments to the retina [223]. Appropriating this technique for the delivery of viral vectors for the treatment of neuropsychiatric disease has the capacity to offer enormous advantages over direct brain injection [224–227].
Specifically, this method of delivery may be appropriate for targeting expression of excitatory chemogenetic receptors to the retina (using specially designed promoters [e.g. PACAP] to exclusively drive expression of the chemogenetic receptor in ipRGCs), in order to acutely manipulate the PRAM pathway. Selective control of the PRAM pathway is likely to have immense clinical applications for numerous disorders, particularly for those that are characterized by dysfunctional LC activity. An obvious example of a potential future clinical application of PRAM stimulation is for the treatment of depression, as depression is often characterized by dysfunctional LC activity, as well as disruption of circadian rhythms [228] and sleep patterns [229, 230]. Additionally, the typical etiology of seasonal affective disorder (SAD), a form of depression, is decreased light availability [231]. Therefore, it is possible to envisage that patients could receive a single IVI delivery of the viral vector, which would enable the PRAM pathway to be activated by a DREADD-activating ligand, perhaps in the form of a daily pill. Previous studies investigating the effect of bright light therapy on the treatment of SAD provide encouraging evidence for retinal-based treatments for depression. Remission rates of SAD using bright light therapy range from 64% [227] to 74% [225]. Whilst attractive, bright light therapy is not without limitations. Light therapy typically involves several hours of light exposure per day which limits its feasibility. Importantly, exposure to bright blue light has been reported to cause retinal damage [224, 226], which may also limit its use as a therapy. PRAM stimulation may be superior to light therapy because it circumvents these limitations and may have fewer side effects. It is tempting to predict that activation of the PRAM pathway may be used as a novel treatment for at least some forms of depression in the future, including SAD. Although further research is required to understand the precise mechanisms underlying PRAM-mediated regulation of arousal, the PRAM pathway represents an exciting new direction for the treatment of arousal and mood disorders in humans.
Conclusion
With the advent of chemogenetic and optogenetics, much progress has been made in recent years in dissecting the neural circuitry underlying arousal state change in both health and disease. This has resulted in the characterization of a large number of previously unrecognized cell populations and circuits associated with sleep and arousal, thereby informing new and unexplored targets for therapeutic intervention. This review has highlighted some of the most promising and exciting work in circuit-based sleep and circadian research, with a particular focus on how these circuits might be exploited for their therapeutic potential. It is hoped that these advances will ultimately lead to novel treatments for sleep–wake and circadian disruption and associated comorbidities.
Funding
A.V. was supported by Sleep Research Society Career Development Award (CDA 016-JP-17) and National Institutes of Health (NS073613 and NS092652). W.D.T. was supported by Alzheimer’s Association grant (AARF-16-443613) and National Institutes of Health (NS084582-01A1 and HL00701-15). J.F. was supported by Canadian Institutes of Health Research (CIHR) and the National Science and Engineering Research Council (NSERC). H.B. was supported by National Health and Medical Research Council of Australia Early Career Fellowship: 1128089. A.E.-R. was supported by Alfred P. Sloan Foundation, the Brain and Behavior Research Foundation (NARSAD), and the Eisenberg Translational Research Award University of Michigan Depression Center. S.K. was supported by U.S. Public Health Service (USPHS) grant (2P01 HL095491). C.A. was supported by National Institutes of Health (MH103399, NS106345), Coins for Alzheimer’s Research Trust (CART), and Citizens United for Research in Epilepsy (CURE).
Conflict of interest statement. None declared.
References
- 1. Armbruster BN, et al. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104(12):5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roth BL. DREADDs for neuroscientists. Neuron. 2016;89(4):683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyden ES, et al. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(9):1263–1268. [DOI] [PubMed] [Google Scholar]
- 4. Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci. 2015;18(9):1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fuller PM, et al. How genetically engineered systems are helping to define, and in some cases redefine, the neurobiological basis of sleep and wake. Temperature (Austin). 2015;2(3):406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anaclet C, et al. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat Neurosci. 2014;17(9):1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eldridge MA, et al. Chemogenetic disconnection of monkey orbitofrontal and rhinal cortex reversibly disrupts reward value. Nat Neurosci. 2016;19(1):37–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pedersen NP, et al. Supramammillary glutamate neurons are a key node of the arousal system. Nat Commun. 2017;8(1):1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Venner A, et al. A novel population of wake-promoting GABAergic neurons in the ventral lateral hypothalamus. Curr Biol. 2016;26(16):2137–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Todd WD, et al. A hypothalamic circuit for the circadian control of aggression. Nat Neurosci. 2018;21(5):717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Machado NLS, et al. A glutamatergic hypothalamomedullary circuit mediates thermogenesis, but not heat conservation, during stress-induced hyperthermia. Curr Biol. 2018;28(14):2291–2301.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lynagh T, et al. An improved ivermectin-activated chloride channel receptor for inhibiting electrical activity in defined neuronal populations. J Biol Chem. 2010;285(20):14890–14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saito YC, et al. Monoamines inhibit GABAergic neurons in ventrolateral preoptic area that make direct synaptic connections to hypothalamic arousal neurons. J Neurosci. 2018;38(28):6366–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kroeger D, et al. Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat Commun. 2018;9(1):4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams RH, et al. Cortical nNOS/NK1 receptor neurons are regulated by cholinergic projections from the basal forebrain. Cereb Cortex. 2018;28(6):1959–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anaclet C, et al. Brainstem regulation of slow-wave-sleep. Curr Opin Neurobiol. 2017;44:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oishi Y, et al. Slow-wave sleep is controlled by a subset of nucleus accumbens core neurons in mice. Nat Commun. 2017;8(1):734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu K, et al. Lhx6-positive GABA-releasing neurons of the zona incerta promote sleep. Nature. 2017;548(7669): 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang SR, et al. The rostromedial tegmental nucleus is essential for non-rapid eye movement sleep. PLoS Biol. 2018;16(4):e2002909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weber F, et al. Regulation of REM and Non-REM sleep by periaqueductal GABAergic neurons. Nat Commun. 2018;9(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anaclet C, et al. Identification and characterization of a sleep-active cell group in the rostral medullary brainstem. J Neurosci. 2012;32(50):17970–17976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alam MA, et al. Characteristics of sleep-active neurons in the medullary parafacial zone in rats. Sleep. 2018;41(10). doi: 10.1093/sleep/zsy130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anaclet C, et al. Basal forebrain control of wakefulness and cortical rhythms. Nat Commun. 2015;6:8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Billiard M, et al. Modafinil: its discovery, the early European and North American experience in the treatment of narcolepsy and idiopathic hypersomnia, and its subsequent use in other medical conditions. Sleep Med. 2018;49:69–72. [DOI] [PubMed] [Google Scholar]
- 25. Lin JS, et al. The unfinished journey with modafinil and discovery of a novel population of modafinil-immunoreactive neurons. Sleep Med. 2018;49: 40–52. [DOI] [PubMed] [Google Scholar]
- 26. Anaclet C, et al. Activation of the GABAergic parafacial zone maintains sleep and counteracts the wake-promoting action of the psychostimulants armodafinil and caffeine. Neuropsychopharmacology. 2018;43(2):415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mitchell DC, et al. Beverage caffeine intakes in the U.S. Food Chem Toxicol. 2014;63:136–142. [DOI] [PubMed] [Google Scholar]
- 28. Lazarus M, et al. Adenosine and Sleep. Handbook of Experimental Pharmacology. Springer, Berlin, Heidelberg. 2017. [Google Scholar]
- 29. Lu Y, et al. A critical time-window for the selective induction of hippocampal memory consolidation by a brief episode of slow-wave sleep. Neurosci Bull. 2018;34(6):1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl). 2007;191(3):391–431. [DOI] [PubMed] [Google Scholar]
- 31. Salamone JD, et al. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76(3):470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morales M, et al. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017;18(2):73–85. [DOI] [PubMed] [Google Scholar]
- 33. Boutrel B, et al. What keeps us awake: the neuropharmacology of stimulants and wakefulness-promoting medications. Sleep. 2004;27(6):1181–1194. [DOI] [PubMed] [Google Scholar]
- 34. Wisor JP, et al. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21(5):1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qu WM, et al. Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci. 2008;28(34):8462–8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holst SC, et al. Functional polymorphisms in dopaminergic genes modulate neurobehavioral and neurophysiological consequences of sleep deprivation. Sci Rep. 2017;7:45982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. España RA, et al. Sleep neurobiology from a clinical perspective. Sleep. 2011;34(7):845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saper CB, et al. Sleep state switching. Neuron. 2010;68(6):1023–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steinfels GF, et al. Behavioral correlates of dopaminergic unit activity in freely moving cats. Brain Res. 1983;258(2):217–228. [DOI] [PubMed] [Google Scholar]
- 40. Trulson ME, et al. Activity of substantia nigra units across the sleep-waking cycle in freely moving cats. Neurosci Lett. 1981;26(2):183–188. [DOI] [PubMed] [Google Scholar]
- 41. Trulson ME, et al. Dopamine-containing ventral tegmental area neurons in freely moving cats: activity during the sleep-waking cycle and effects of stress. Exp Neurol. 1984;83(2):367–377. [DOI] [PubMed] [Google Scholar]
- 42. Miller JD, et al. Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and walking in the rat. Brain Res. 1983;273(1):133–141. [DOI] [PubMed] [Google Scholar]
- 43. Jones BE, et al. The effect of lesions of catecholamine-containing neurons upon monoamine content of the brain and EEG and behavioral waking in the cat. Brain Res. 1973;58(1):157–177. [DOI] [PubMed] [Google Scholar]
- 44. Takata Y, et al. Sleep and wakefulness are controlled by ventral medial midbrain/pons GABAergic neurons in mice. J Neurosci. 2018;38(47):10080–10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dahan L, et al. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32(6):1232–1241. [DOI] [PubMed] [Google Scholar]
- 46. Eban-Rothschild A, et al. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat Neurosci. 2016;19(10):1356–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oishi Y, et al. Activation of ventral tegmental area dopamine neurons produces wakefulness through dopamine D2-like receptors in mice. Brain Struct Funct. 2017;222(6):2907–2915. [DOI] [PubMed] [Google Scholar]
- 48. Taylor NE, et al. Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc Natl Acad Sci U S A. 2016;113(45):12826–12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eban-Rothschild A, et al. Neuronal mechanisms for sleep/wake regulation and modulatory drive. Neuropsychopharmacology. 2018;43(5):937–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Adamantidis AR, et al. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci. 2011;31(30):10829–10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lazarus M, et al. Role of the basal ganglia in the control of sleep and wakefulness. Curr Opin Neurobiol. 2013;23(5):780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Luo YJ, et al. Nucleus accumbens controls wakefulness by a subpopulation of neurons expressing dopamine D1 receptors. Nat Commun. 2018;9(1):1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Qiu MH, et al. The role of nucleus accumbens core/shell in sleep-wake regulation and their involvement in modafinil-induced arousal. PLoS One. 2012;7(9):e45471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cohen JY, et al. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482(7383):85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Zessen R, et al. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73(6):1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee RS, et al. Discharge profiles of ventral tegmental area GABA neurons during movement, anesthesia, and the sleep-wake cycle. J Neurosci. 2001;21(5):1757–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Eban-Rothschild A, et al. Motivation to stay awake: VTA regulation of sleep/wake states. Presented at: Sleep, June 3–7, 2017; Boston, MA. [Google Scholar]
- 58. Wulff K, et al. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11(8):589–599. [DOI] [PubMed] [Google Scholar]
- 59. Nestler EJ, et al. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–1159. [DOI] [PubMed] [Google Scholar]
- 60. Hyman SE, et al. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. [DOI] [PubMed] [Google Scholar]
- 61. Parekh PK, et al. Circadian clock genes: effects on dopamine, reward and addiction. Alcohol. 2015;49(4):341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Peever J, et al. Breakdown in REM sleep circuitry underlies REM sleep behavior disorder. Trends Neurosci. 2014;37(5):279–288. [DOI] [PubMed] [Google Scholar]
- 63. Mahowald MW, et al. REM sleep behaviour disorder: a marker of synucleinopathy. Lancet Neurol. 2013;12(5):417–419. [DOI] [PubMed] [Google Scholar]
- 64. Schenck CH, et al. REM sleep parasomnias. Neurol Clin. 1996;14(4):697–720. [DOI] [PubMed] [Google Scholar]
- 65. Schenck CH, et al. Potentially lethal behaviors associated with rapid eye movement sleep behavior disorder: review of the literature and forensic implications. J Forensic Sci. 2009;54(6):1475–1484. [DOI] [PubMed] [Google Scholar]
- 66. Postuma RB, et al. Prodromal autonomic symptoms and signs in Parkinson’s disease and dementia with Lewy bodies. Mov Disord. 2013;28(5):597–604. [DOI] [PubMed] [Google Scholar]
- 67. Postuma RB, et al. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology. 2006;66(6):845–851. [DOI] [PubMed] [Google Scholar]
- 68. McKenna D, et al. Degeneration of rapid eye movement sleep circuitry underlies rapid eye movement sleep behavior disorder. Mov Disord. 2017;32(5):636–644. [DOI] [PubMed] [Google Scholar]
- 69. Boeve BF. Idiopathic REM sleep behaviour disorder in the development of Parkinson’s disease. Lancet Neurol. 2013;12(5):469–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. St Louis EK, et al. REM sleep behavior disorder in Parkinson’s disease and other synucleinopathies. Mov Disord. 2017;32(5):645–658. [DOI] [PubMed] [Google Scholar]
- 71. Chase MH, et al. Role of medullary reticular neurons in the inhibition of trigeminal motoneurons during active sleep. Exp Neurol. 1984;84(2):364–373. [DOI] [PubMed] [Google Scholar]
- 72. Brooks PL, et al. Glycinergic and GABA(A)-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia. J Neurosci. 2008;28(14):3535–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brooks PL, et al. Identification of the transmitter and receptor mechanisms responsible for REM sleep paralysis. J Neurosci. 2012;32(29):9785–9795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Brooks PL, et al. Impaired GABA and glycine transmission triggers cardinal features of rapid eye movement sleep behavior disorder in mice. J Neurosci. 2011;31(19): 7111–7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Peever J, et al. The biology of REM sleep. Curr Biol. 2017;27(22):R1237–R1248. [DOI] [PubMed] [Google Scholar]
- 76. Lu J, et al. A putative flip-flop switch for control of REM sleep. Nature. 2006;441(7093):589–594. [DOI] [PubMed] [Google Scholar]
- 77. Jouvet M. Neurophysiology of the states of sleep. Physiol Rev. 1967;47(2):117–177. [DOI] [PubMed] [Google Scholar]
- 78. Fraigne JJ, et al. REM sleep at its core – circuits, neurotransmitters, and pathophysiology. Front Neurol. 2015;6:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Boissard R, et al. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16(10):1959–1973. [DOI] [PubMed] [Google Scholar]
- 80. Cox J, et al. Calcium imaging of sleep-wake related neuronal activity in the dorsal pons. Nat Commun. 2016;7:10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Clément O, et al. Evidence that neurons of the sublaterodorsal tegmental nucleus triggering paradoxical (REM) sleep are glutamatergic. Sleep. 2011;34(4):419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Valencia Garcia S, et al. Genetic inactivation of glutamate neurons in the rat sublaterodorsal tegmental nucleus recapitulates REM sleep behaviour disorder. Brain. 2017;140(2):414–428. [DOI] [PubMed] [Google Scholar]
- 83. Holmes CJ, et al. Importance of cholinergic, GABAergic, serotonergic and other neurons in the medial medullary reticular formation for sleep-wake states studied by cytotoxic lesions in the cat. Neuroscience. 1994;62(4):1179–1200. [DOI] [PubMed] [Google Scholar]
- 84. Lai YY, et al. Medullary regions mediating atonia. J Neurosci. 1988;8(12):4790–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schenkel E, et al. REM sleep without atonia after lesions of the medial medulla. Neurosci Lett. 1989;98(2):159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Krenzer M, et al. Brainstem and spinal cord circuitry regulating REM sleep and muscle atonia. PLoS One. 2011;6(10):e24998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vetrivelan R, et al. Medullary circuitry regulating rapid eye movement sleep and motor atonia. J Neurosci. 2009;29(29):9361–9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Valencia Garcia S, et al. Ventromedial medulla inhibitory neuron inactivation induces REM sleep without atonia and REM sleep behavior disorder. Nat Commun. 2018;9(1): 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fraigne JJ, et al. A dedicated brainstem circuit controls REM sleep. Presented at: Sleep, June 3–7, 2017; Boston, MA. [Google Scholar]
- 90. Boucetta S, et al. Structural brain alterations associated with rapid eye movement sleep behavior disorder in Parkinson’s disease. Sci Rep. 2016;6:26782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Iranzo A, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12(5):443–453. [DOI] [PubMed] [Google Scholar]
- 92. Uchiyama M, et al. Incidental Lewy body disease in a patient with REM sleep behavior disorder. Neurology. 1995;45(4):709–712. [DOI] [PubMed] [Google Scholar]
- 93. Ehrminger M, et al. The coeruleus/subcoeruleus complex in idiopathic rapid eye movement sleep behaviour disorder. Brain. 2016;139(Pt 4):1180–1188. [DOI] [PubMed] [Google Scholar]
- 94. Al-Qassabi A, et al. Sleep disturbances in the prodromal stage of Parkinson disease. Curr Treat Options Neurol. 2017;19(6):22. [DOI] [PubMed] [Google Scholar]
- 95. Postuma RB, et al. Advances in markers of prodromal Parkinson disease. Nat Rev Neurol. 2016;12(11):622–634. [DOI] [PubMed] [Google Scholar]
- 96. Postuma RB, et al. How does Parkinsonism start? Prodromal Parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain. 2012;135(Pt 6):1860–1870. [DOI] [PubMed] [Google Scholar]
- 97. Braak H, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. [DOI] [PubMed] [Google Scholar]
- 98. Desplats P, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106(31):13010–13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ludtmann MHR, et al. α-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat Commun. 2018;9(1):2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Luk KC, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Decressac M, et al. Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of α-synuclein in midbrain dopamine neurons. Neurobiol Dis. 2012;45(3):939–953. [DOI] [PubMed] [Google Scholar]
- 102. Thakur P, et al. Modeling Parkinson’s disease pathology by combination of fibril seeds and alpha-synuclein overexpression in the rat brain. Proc Natl Acad Sci U S A. 2017;114(39):E8284–E8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dehay B, et al. Alpha-synuclein propagation: new insights from animal models. Mov Disord. 2016;31(2):161–168. [DOI] [PubMed] [Google Scholar]
- 104. Ayalon L, et al. Functional central nervous system imaging in the investigation of obstructive sleep apnea. Curr Opin Pulm Med. 2007;13(6):479–483. [DOI] [PubMed] [Google Scholar]
- 105. Berry RB, et al. Respiratory arousal from sleep: mechanisms and significance. Sleep. 1997;20(8):654–675. [DOI] [PubMed] [Google Scholar]
- 106. Blasi A, et al. Autonomic cardiovascular control following transient arousal from sleep: a time-varying closed-loop model. IEEE Trans Biomed Eng. 2006;53(1):74–82. [DOI] [PubMed] [Google Scholar]
- 107. Darquenne C, et al. Upper airway dynamic imaging during tidal breathing in awake and asleep subjects with obstructive sleep apnea and healthy controls. Physiol Rep. 2018;6(10):e13711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Malhotra A, et al. Obstructive sleep apnoea. Lancet. 2002;360(9328):237–245. [DOI] [PubMed] [Google Scholar]
- 109. Mannarino MR, et al. Obstructive sleep apnea syndrome. Eur J Intern Med. 2012;23(7):586–593. [DOI] [PubMed] [Google Scholar]
- 110. Pham LV, et al. Integrating loop gain into the understanding of obstructive sleep apnoea mechanisms. J Physiol. 2018;596(17):3819–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Schulz R. [Obstruction of the upper airways in humans and animal models]. Pneumologie. 2010;64(7):447–449. [DOI] [PubMed] [Google Scholar]
- 112. Bennett LS, et al. Sleep fragmentation indices as predictors of daytime sleepiness and nCPAP response in obstructive sleep apnea. Am J Respir Crit Care Med. 1998;158(3):778–786. [DOI] [PubMed] [Google Scholar]
- 113. Bonnet MH. Effect of sleep disruption on sleep, performance, and mood. Sleep. 1985;8(1):11–19. [DOI] [PubMed] [Google Scholar]
- 114. Bonsignore MR, et al. Metabolic syndrome, insulin resistance and sleepiness in real-life obstructive sleep apnoea. Eur Respir J. 2012;39(5):1136–1143. [DOI] [PubMed] [Google Scholar]
- 115. Drager LF, et al. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab. 2010;24(5):843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fletcher EC. Obstructive sleep apnoea and cardiovascular morbidity. Monaldi Arch Chest Dis. 1996;51(1):77–80. [PubMed] [Google Scholar]
- 117. Jun J, et al. Metabolic consequences of sleep-disordered breathing. ILAR J. 2009;50(3):289–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Malhotra A, et al. Sleep and cardiovascular disease: an overview. Prog Cardiovasc Dis. 2009;51(4):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Buchanan GF, et al. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A. 2010;107(37):16354–16359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ray RS, et al. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333(6042):637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ray RS, et al. Egr2-neurons control the adult respiratory response to hypercapnia. Brain Res. 2013;1511:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Richerson GB, et al. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol. 2005;90(3):259–266; discussion 266. [DOI] [PubMed] [Google Scholar]
- 123. Buchanan GF, et al. 5-HT2A receptor activation is necessary for CO2-induced arousal. J Neurophysiol. 2015;114(1):233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Carter ME, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13(12):1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gargaglioni LH, et al. The locus coeruleus and central chemosensitivity. Respir Physiol Neurobiol. 2010;173(3):264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yackle K, et al. Breathing control center neurons that promote arousal in mice. Science. 2017;355(6332):1411–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Smith HR, et al. Dorsal raphe serotonin neurons mediate CO2-induced arousal from sleep. J Neurosci. 2018;38(8):1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Berquin P, et al. Brainstem and hypothalamic areas involved in respiratory chemoreflexes: a Fos study in adult rats. Brain Res. 2000;857(1–2):30–40. [DOI] [PubMed] [Google Scholar]
- 129. Bochorishvili G, et al. Pre-Bötzinger complex receives glutamatergic innervation from galaninergic and other retrotrapezoid nucleus neurons. J Comp Neurol. 2012;520(5):1047–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Corcoran AE, et al. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol. 2009;168(1–2):49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Finley JC, et al. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992;572(1–2):108–116. [DOI] [PubMed] [Google Scholar]
- 132. Gonzalez C, et al. A revisit to O2 sensing and transduction in the carotid body chemoreceptors in the context of reactive oxygen species biology. Respir Physiol Neurobiol. 2010;174(3):317–330. [DOI] [PubMed] [Google Scholar]
- 133. Guyenet PG, et al. Neural control of breathing and CO2 homeostasis. Neuron. 2015;87(5):946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Guyenet PG, et al. Central respiratory chemoreception. J Comp Neurol. 2010;518(19):3883–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Izumizaki M, et al. Role of the carotid bodies in chemosensory ventilatory responses in the anesthetized mouse. J Appl Physiol (1985). 2004;97(4):1401–1407. [DOI] [PubMed] [Google Scholar]
- 136. Mizusawa A, et al. Role of the parabrachial nucleus in ventilatory responses of awake rats. J Physiol. 1995;489(Pt 3):877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Panneton WM, et al. Projections of the carotid sinus nerve to the nucleus of the solitary tract in the cat. Brain Res. 1980;191(1):239–244. [DOI] [PubMed] [Google Scholar]
- 138. Pete G, et al. CO(2)-induced c-Fos expression in brainstem preprotachykinin mRNA containing neurons. Respir Physiol Neurobiol. 2002;130(3):265–274. [DOI] [PubMed] [Google Scholar]
- 139. Roman CW, et al. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat Commun. 2016;7:11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Rosin DL, et al. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499(1):64–89. [DOI] [PubMed] [Google Scholar]
- 141. Song G, et al. Lateral parabrachial nucleus mediates shortening of expiration and increase of inspiratory drive during hypercapnia. Respir Physiol Neurobiol. 2009;165(1):9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Topchiy I, et al. Functional topography of respiratory, cardiovascular and pontine-wave responses to glutamate microstimulation of the pedunculopontine tegmentum of the rat. Respir Physiol Neurobiol. 2010;173(1):64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yokota S, et al. Respiratory-related outputs of glutamatergic, hypercapnia-responsive parabrachial neurons in mice. J Comp Neurol. 2015;523(6):907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Fuller PM, et al. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519(5):933–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Hayashi Y, et al. Cells of a common developmental origin regulate REM/non-REM sleep and wakefulness in mice. Science. 2015;350(6263):957–961. [DOI] [PubMed] [Google Scholar]
- 146. Kaur S, et al. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. J Neurosci. 2013;33(18):7627–7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kaur S, et al. A genetically defined circuit for arousal from sleep during hypercapnia. Neuron. 2017;96(5):1153–1167.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Qiu MH, et al. Stimulation of the pontine parabrachial nucleus promotes wakefulness via extra-thalamic forebrain circuit nodes. Curr Biol. 2016;26(17):2301–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Bonis JM, et al. A role for the Kolliker-Fuse nucleus in cholinergic modulation of breathing at night during wakefulness and NREM sleep. J Appl Physiol (1985). 2010;109(1):159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Chamberlin NL. Functional organization of the parabrachial complex and intertrigeminal region in the control of breathing. Respir Physiol Neurobiol. 2004;143(2-3):115–125. [DOI] [PubMed] [Google Scholar]
- 151. Chamberlin NL, et al. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J Neurosci. 1994;14(11 Pt 1):6500–6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Damasceno RS, et al. Regulation of the chemosensory control of breathing by Kölliker-Fuse neurons. Am J Physiol Regul Integr Comp Physiol. 2014;307(1):R57–R67. [DOI] [PubMed] [Google Scholar]
- 153. Díaz-Casares A, et al. Parabrachial complex glutamate receptors modulate the cardiorespiratory response evoked from hypothalamic defense area. Auton Neurosci. 2012;169(2):124–134. [DOI] [PubMed] [Google Scholar]
- 154. Miura M, et al. Circulatory and respiratory responses to glutamate stimulation of the lateral parabrachial nucleus of the cat. J Auton Nerv Syst. 1991;32(2):121–133. [DOI] [PubMed] [Google Scholar]
- 155. Yang CF, et al. Efferent projections of excitatory and inhibitory preBötzinger complex neurons. J Comp Neurol. 2018;526(8):1389–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Saper CB. Reciprocal parabrachial-cortical connections in the rat. Brain Res. 1982;242(1):33–40. [DOI] [PubMed] [Google Scholar]
- 157. Saper CB. The house alarm. Cell Metab. 2016;23(5):754–755. [DOI] [PubMed] [Google Scholar]
- 158. Saper CB, et al. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197(2):291–317. [DOI] [PubMed] [Google Scholar]
- 159. Yokota S, et al. Phrenic motoneurons receive monosynaptic inputs from the Kölliker-Fuse nucleus: a light- and electron-microscopic study in the rat. Brain Res. 2001;888(2): 330–335. [DOI] [PubMed] [Google Scholar]
- 160. Yasui Y, et al. Calcitonin gene-related peptide (CGRP) immunoreactive projections from the thalamus to the striatum and amygdala in the rat. J Comp Neurol. 1991;308(2):293–310. [DOI] [PubMed] [Google Scholar]
- 161. Horner RL, et al. A resource of potential drug targets and strategic decision-making for obstructive sleep apnoea pharmacotherapy. Respirology. 2017;22(5):861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Sands SA, et al. Quantifying the arousal threshold using polysomnography in obstructive sleep apnea. Sleep. 2018;41(1): zsx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Campos CA, et al. Encoding of danger by parabrachial CGRP neurons. Nature. 2018;555(7698):617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Campos CA, et al. Parabrachial CGRP neurons control meal termination. Cell Metab. 2016;23(5):811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Carter ME, et al. Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. J Neurosci. 2015;35(11):4582–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Carter ME, et al. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503(7474):111–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Han S, et al. Elucidating an affective pain circuit that creates a threat memory. Cell. 2015;162(2):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Palmiter RD. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 2018;41(5):280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Bernard JF, et al. The parabrachial area: electrophysiological evidence for an involvement in visceral nociceptive processes. J Neurophysiol. 1994;71(5):1646–1660. [DOI] [PubMed] [Google Scholar]
- 170. Welsh DK, et al. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14(4):697–706. [DOI] [PubMed] [Google Scholar]
- 171. Jin X, et al. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96(1):57–68. [DOI] [PubMed] [Google Scholar]
- 172. Saper CB. The central circadian timing system. Curr Opin Neurobiol. 2013;23(5):747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Watts AG, et al. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol. 1987;258(2):204–229. [DOI] [PubMed] [Google Scholar]
- 174. Chou TC, et al. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23(33):10691–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Landau IT. Light-dark rhythms in aggressive behavior of the male golden hamster. Physiol Behav. 1975;14(6):767–774. [DOI] [PubMed] [Google Scholar]
- 176. Haller J, et al. Ultradian corticosterone rhythm and the propensity to behave aggressively in male rats. J Neuroendocrinol. 2000;12(10):937–940. [DOI] [PubMed] [Google Scholar]
- 177. Manfredini R, et al. Day-night variation in aggressive behavior among psychiatric inpatients. Chronobiol Int. 2001;18(3):503–511. [DOI] [PubMed] [Google Scholar]
- 178. Bronsard G, et al. Rhythms, rhythmicity and aggression. J Physiol Paris. 2013;107(4):327–334. [DOI] [PubMed] [Google Scholar]
- 179. Hood S, et al. Biological clocks and rhythms of anger and aggression. Front Behav Neurosci. 2018;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Lin D, et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470(7333):221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Yang T, et al. Social control of hypothalamus-mediated male aggression. Neuron. 2017;95(4):955–970.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Bachman D, et al. “Sundowning” and other temporally associated agitation states in dementia patients. Annu Rev Med. 2006;57:499–511. [DOI] [PubMed] [Google Scholar]
- 183. Khachiyants N, et al. Sundown syndrome in persons with dementia: an update. Psychiatry Investig. 2011;8(4):275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Canevelli M, et al. Sundowning in dementia: clinical relevance, pathophysiological determinants, and therapeutic approaches. Front Med (Lausanne). 2016;3:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Bedrosian TA, et al. Sundowning syndrome in aging and dementia: research in mouse models. Exp Neurol. 2013;243:67–73. [DOI] [PubMed] [Google Scholar]
- 186. Rusak B, et al. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science. 1990;248(4960):1237–1240. [DOI] [PubMed] [Google Scholar]
- 187. Figueiro MG, et al. Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clin Interv Aging. 2014;9:1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Münch M, et al. Bright light delights: effects of daily light exposure on emotions, restactivity cycles, sleep and melatonin secretion in severely demented patients. Curr Alzheimer Res. 2017;14(10):1063–1075. [DOI] [PubMed] [Google Scholar]
- 189. Wahnschaffe A, et al. Implementation of dynamic lighting in a nursing home: impact on agitation but not on rest-activity patterns. Curr Alzheimer Res. 2017;14(10):1076–1083. [DOI] [PubMed] [Google Scholar]
- 190. Berridge CW, et al. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42(1):33–84. [DOI] [PubMed] [Google Scholar]
- 191. Aston-Jones G, et al. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1(8):876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Gompf HS, et al. Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment. J Neurosci. 2010;30(43):14543–14551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Vazey EM, et al. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(10):3859–3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Harley CW. Norepinephrine and the dentate gyrus. Prog Brain Res. 2007;163:299–318. [DOI] [PubMed] [Google Scholar]
- 195. Sara SJ. Noradrenergic modulation of selective attention: its role in memory retrieval. Ann N Y Acad Sci. 1985;444:178–193. [DOI] [PubMed] [Google Scholar]
- 196. Sara SJ. Locus Coeruleus in time with the making of memories. Curr Opin Neurobiol. 2015;35:87–94. [DOI] [PubMed] [Google Scholar]
- 197. Aston-Jones G, et al. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46(9):1309–1320. [DOI] [PubMed] [Google Scholar]
- 198. Bouret S, et al. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28(11):574–582. [DOI] [PubMed] [Google Scholar]
- 199. Aston-Jones G, et al. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4(7):732–738. [DOI] [PubMed] [Google Scholar]
- 200. Schwartz WJ, et al. A discrete lesion of ventral hypothalamus and optic chiasm that disturbed the daily temperature rhythm. J Neurol. 1986;233(1):1–4. [DOI] [PubMed] [Google Scholar]
- 201. Gooley JJ, et al. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4(12):1165. [DOI] [PubMed] [Google Scholar]
- 202. Hattar S, et al. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Provencio I, et al. A novel human opsin in the inner retina. J Neurosci. 2000;20(2):600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Sollars PJ, et al. Melanopsin and non-melanopsin expressing retinal ganglion cells innervate the hypothalamic suprachiasmatic nucleus. Vis Neurosci. 2003;20(6):601–610. [DOI] [PubMed] [Google Scholar]
- 205. Vujovic N, et al. Projections from the subparaventricular zone define four channels of output from the circadian timing system. J Comp Neurol. 2015;523(18):2714–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Yoshida K, et al. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494(5):845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Zhang S, et al. Lesions of the suprachiasmatic nucleus eliminate the daily rhythm of hypocretin-1 release. Sleep. 2004;27(4):619–627. [DOI] [PubMed] [Google Scholar]
- 208. Bowrey HE, et al. New directions for the treatment of depression: targeting the photic regulation of arousal and mood (PRAM) pathway. Depress Anxiety. 2017;34(7):588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Milosavljevic N, et al. Chemogenetic activation of melanopsin retinal ganglion cells induces signatures of arousal and/or anxiety in mice. Curr Biol. 2016;26(17):2358–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Pilorz V, et al. Melanopsin regulates both sleep-promoting and arousal-promoting responses to light. PLoS Biol. 2016;14(6):e1002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Moore RY, et al. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42(1):201–206. [DOI] [PubMed] [Google Scholar]
- 212. Stephan FK, et al. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69(6):1583–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Bernardis LL. Ventromedial and dorsomedial hypothalamic syndromes in the weanling rat: is the “center” concept really outmoded? Brain Res Bull. 1985;14(6):537–549. [DOI] [PubMed] [Google Scholar]
- 214. James MH, et al. Exercise reverses the effects of early life stress on orexin cell reactivity in male but not female rats. Front Behav Neurosci. 2014;8:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Adamantidis AR, et al. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450(7168):420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Chemelli RM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–451. [DOI] [PubMed] [Google Scholar]
- 217. Hara J, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30(2):345–354. [DOI] [PubMed] [Google Scholar]
- 218. Peyron C, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Ivanov A, et al. Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. Neuroreport. 2000;11(8):1755–1758. [DOI] [PubMed] [Google Scholar]
- 220. Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8(3):171–181. [DOI] [PubMed] [Google Scholar]
- 221. Carter ME, et al. Mechanism for Hypocretin-mediated sleep-to-wake transitions. Proc Natl Acad Sci U S A. 2012;109(39):E2635–E2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Gompf HS, et al. Role of orexin input in the diurnal rhythm of locus coeruleus impulse activity. Brain Res. 2008;1224:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Grzybowski A, et al. ; Euretina Board. 2018 Update on intravitreal injections: euretina expert consensus recommendations. Ophthalmologica. 2018;239(4):181–193. [DOI] [PubMed] [Google Scholar]
- 224. Gagné AM, et al. Atypical pattern of rod electroretinogram modulation by recent light history: a possible biomarker of seasonal affective disorder. Psychiatry Res. 2011;187(3):370–374. [DOI] [PubMed] [Google Scholar]
- 225. Meesters Y, et al. The effects of low-intensity narrow-band blue-light treatment compared to bright white-light treatment in seasonal affective disorder. J Affect Disord. 2018;232:48–51. [DOI] [PubMed] [Google Scholar]
- 226. Remé CE. The dark side of light: rhodopsin and the silent death of vision the proctor lecture. Invest Ophthalmol Vis Sci. 2005;46(8):2672–2682. [DOI] [PubMed] [Google Scholar]
- 227. Rohan KJ, et al. Randomized trial of cognitive-behavioral therapy versus light therapy for seasonal affective disorder: acute outcomes. Am J Psychiatry. 2015;172(9):862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Koenigsberg HW, et al. 24-h Monitoring of plasma norepinephrine, MHPG, cortisol, growth hormone and prolactin in depression. J Psychiatr Res. 2004;38(5):503–511. [DOI] [PubMed] [Google Scholar]
- 229. Perlis ML, et al. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord. 1997;42(2–3):209–212. [DOI] [PubMed] [Google Scholar]
- 230. Posmontier B. Sleep quality in women with and without postpartum depression. J Obstet Gynecol Neonatal Nurs. 2008;37(6):722–735; quiz 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Association AP. Diagnostic and Statistical Manual of Mental Disorders(DSM-5®). American Psychiatric Publishing, Washington, DC; 2013. [Google Scholar]