Abstract
Purpose of Review:
Metformin has multiple benefits for health beyond its antihyperglycemic properties. The purpose of this manuscript is to review the mechanisms that underlie metformin’s effects on obesity.
Recent Findings:
Metformin is first line therapy for type 2 diabetes. Large cohort studies have shown weight loss benefits associated with metformin therapy. Metabolic consequences were traditionally thought to underlie this effect, including reduction in hepatic gluconeogenesis and reduction in insulin production. Emerging evidence suggests that metformin-associated weight loss is due to modulation of hypothalamic appetite-regulatory centers, alteration in the gut microbiome, and reversal of consequences of aging. Metformin is also being explored in the management of obesity’s sequelae such as hepatic steatosis, obstructive sleep apnea and osteoarthritis.
Summary:
Multiple mechanisms underlie the weight loss-inducing and health-promoting effects of metformin. Further exploration of these pathways may be important in identifying new pharmacologic targets for obesity and other aging-associated metabolic diseases.
Keywords: Metformin, Obesity, Weight Loss, Appetite Regulation, Type 2 Diabetes, Aging
Introduction
Guanidine containing extracts from Galega officinalis, the French lilac, were used for their anti-diabetic, anti-hypertensive, and anti-aging properties dating back to medieval times [1,2]. In the early 20th century, compounds related to the active agents in the French lilac, members of the biguanide family of anti-diabetic agents, were synthesized such as phenformin, buphormin, and metformin [3]. Shortly thereafter in the 1920s, and largely accidentally, the biguanides were noted to have anti-diabetic properties. These three biguanides were subsequently studied in humans and approved for use in diabetes in Europe in the 1950s [4]. Though phenformin and buphormin were removed from the market in the 1970s due to the occurrence of lactic acidosis and ensuing mortality, metformin’s use advanced, achieving approval in Canada in the 1970s and the United States in 1994. Since that time, it has become a mainstay in the treatment of type 2 diabetes (T2D). Due to its excellent tolerability, safety profile, efficacy and lack of hypoglycemia it is now considered first-line in the treatment of T2D in conjunction with lifestyle modifications [5]. Excitingly, in recent years it has become clear from epidemiological and preclinical studies that metformin has favorable effects beyond its effects on glycemia. Particularly, it has been shown to reduce body weight, reduce the incidence of and mortality from cancer, and to prolong lifespan. These effects have made metformin an attractive research opportunity for diseases associated with obesity and aging. This review explores the relationship between metformin and the treatment and management of obesity.
Metformin and Weight Loss
Initial studies examining the cardiometabolic effects of metformin at the time of its FDA approval showed modest effects on weight [6]. The Metformin Study Group showed a statistically significant decrease in 3.8 kg in the metformin group compared to no significant change in the sulfonylurea group at week 29 [4]. The United Kingdom Prospective Diabetes Study (UKPDS) showed weight neutral status of metformin compared to glibenclamide, a sulfonylurea that stimulated weight gain [7]. Kahn et al. showed that metformin treated diabetic patients experienced a 2.7 kg weight loss over a 4-year period, with rosiglitazone and glyburide both showing weight gains of 4.8 kg and 1.6 kg respectively [8]. Meta-analysis of comparator studies between different agents showed that compared to sulfonylureas metformin showed significant differences in end weight [9]. However, these meta-analyses were unable to show statistically significant differences from placebo.
Subsequent smaller studies examined metformin with weight loss as the primary outcome and in patients without diabetes. Generally, these studies did not find significant weight reductions, although they were mostly small (N < 200) and for short duration lasting from 1 month to 1 year [10]. The BIGPRO study group examined weight loss with Metformin 850 mg BID treatment of 1 year duration and found a 2 kg weight loss (P < 0.06) [11]. Paolisso et al. found a 2.8 kg greater decrease in weight over a 1-month period during a hypocaloric diet with metformin over placebo [12]. Glueck et al. examined 31 nondiabetic obese subjects on 2.55 g of metformin a day and found a statistically significant average weight loss of 13 lbs. over 28 weeks [13]. Other small studies performed in the late 1990s and early 2000s did not show statistically significant differences in weight compared to placebo [14].
The largest study to show the weight benefits of metformin is the Diabetes Prevention Study (DPP). The DPP examined the preventative impacts of metformin on metabolic parameters in individuals at high risk for T2D. It showed that the initiation of metformin reduced the incidence of diabetes by 31% in a 3-year period for these high-risk patients. Follow up studies examined effects on weight and waist circumference. Patients randomized to metformin experienced a 2.1 kg weight loss on average. The extent of weight change was strongly associated with adherence, with highly adherent patients experiencing an average of a 3.5% reduction in body mass and low adherence associated with weight neural status in 2 years of follow up [15]. Weight loss persisted in the extended follow up period over 10 years for the highly adherent group. Waist circumference was also impacted, with reduced weight circumference associated with extent of adherence [15]. The DDP showed that weight loss associated with metformin was sustained and safe, other than mild gastrointestinal (GI) side effects, but was strongly dependent on the adherence rate of the participants.
Due to the modest and inconsistent effects of weight loss the FDA has not approved metformin as a weight loss agent. The 2015 Endocrine Society Practice Guideline on Pharmacology for Obesity does not recommend the use of metformin as monotherapy for obese patients without metabolic complications such as diabetes [16]. The 2016 AACE/ACE guidelines on Obesity management recommend the use of metformin in obese patients with evidence of prediabetes or insulin intolerance that does not respond to lifestyle medications or other anti-obesity medications (Grade A; BEL 1) [17]. The current use of metformin as an agent exclusively for weight loss remains off-label but is frequently utilized in patients at high risk for metabolic complications and who do not tolerate other interventions.
Metformin and Weight Prevention
Metformin has been examined in multiple disease processes as a weight gain prevention tool. One area plagued by medication-induced weight gain is the treatment of psychosis and mood disorders. Atypical anti-psychotic agents are associated with reduced risk of extrapyramidal features, however frequently induce metabolic complications including weight gain and hyperglycemia. Multiple studies have examined whether the initiation of metformin prevents anti-psychotic-associated weight gain. A recent meta-analysis-examining 12 studies with a total of 743 patients treated with metformin and atypical anti-psychotics showed a significant reduction in BMI and insulin resistance but not fasting blood sugar [18].
The management of more advanced T2D involves insulin which is pro-obesogenic both from its anabolic effect on lipid accumulation and due to compensatory eating to prevent episodes of hypoglycemia [19]. In the HOME trial, metformin continued beyond the initiation of insulin therapy has been shown to prevent insulin-induced weight gain, versus when it is discontinued and insulin substituted in its place [20].
Cellular Targets of Metformin in Diabetes
Given the safety and tolerability of metformin, as well as its plural benefits on health, much attention has been devoted to deducing its mechanism of action. The most classical effects of metformin in diabetes are attributed to its ability to reduce hepatic glucose output [21, 22]. Most, but not all, literature suggests that the mechanisms by which metformin lowers hepatic glucose production relates to its modulation of mitochondrial energetics and redox potential. Metformin inhibits complex I of the electron transport chain, reducing mitochondrial adenosine triphosphate (ATP) production [23]. However, there is some controversy over whether this effect is meaningful at attainable concentrations of metformin in humans in vivo [24]. None the less, the ratio of ATP to its metabolites adenosine diphosphate and monophosphate (ADP and AMP) are thought to be one potential mechanism by which metformin lowers hepatic glucose output. One consequence of this increased AMP:ATP ratio is activation of AMP-activated protein kinase (AMPK), an inhibitor of hepatic lipogenesis and gluconeogenesis [25, 26]. However, animal models suggest that AMPK is dispensible for the antihyperglycemic effects of metformin, as animals with liver-specific genetic deletion of AMPK or its upstream kinase liver kinase B1 (Lkb1) are still able to reduce hepatic gluconeogenesis in response to metformin [27, 28]. Multiple alternative explanations have been put forward that do not include AMPK, including AMP mediated alterations in adenylate cyclase activity and cAMP concentrations, direct allosteric inhibition of gluconeogenic enzymes, and modulation of hepatic redox potential by alterations in the glycerol-phosphate shuttle [29–31]. Thus, while the role of AMPK remains controversial in hepatic metabolism, AMPK has been found to be relevant as a global energy sensor both in the liver and in the central nervous system (CNS). Below we discuss the potential ability of CNS AMPK to modulate metformin’s effect on appetite.
Metformin and Appetite
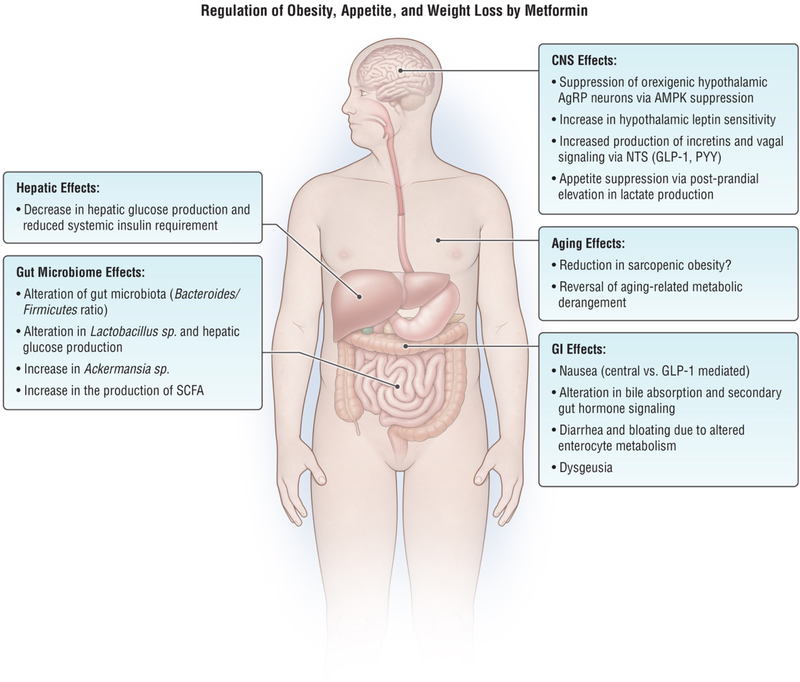
Current evidence suggests that the weight change associated with metformin is more likely to be due to decreased caloric intake versus increases in energy expenditure. Metformin appears to impact appetite regulation both directly and indirectly due to its gastrointestinal side effects. Studies supporting these conclusions are discussed below (Figure 1).
Figure 1.
Mechanisms by which metformin impacts obesity, appetite, and weight loss. CNS: central nervous system, AgRP: Agouti-related peptide, AMPK: AMP-activated protein kinase, NTS: nucleus tractus solitarius, SCFA: short chain fatty acids, GLP-1: glucagon like peptide-1, PYY: peptide YY.
Initial studies explored the effects of metformin on food intake. Lee et al. performed appetite analysis on diabetic subjects and found that metformin had a dose dependent effect on the quantity consumed [32]. In the same study, a 24-week double-blind treatment with metformin induced up to 8 kg weight loss in subjects with diabetes. Similarly, in T2D subjects started on insulin, those continued on metformin had less weight gain, attributed to a reduction in caloric intake [19, 33]. In contrast, no change was seen in energy expenditure.
Mechanisms by which metformin suppresses appetite are still being elucidated. Metabolic acidosis in various chronic disease states including CKD is associated with relative anorexia and protein malnutrition [34]. Metformin is known to induce lactate production through suppression of complex I of the electron transport chain [35]. The ensuing decreased respiratory potential of mitochondria shunts glucose towards anaerobic respiration, driving lactate production, particularly post-prandially [6, 36]. Lactate-mediated, mild, metabolic acidosis may thus drive some metformin-mediated appetite suppression [37–39].
Metformin may also affect appetite through the gut-brain axis. Metformin has been shown to increase secretion of the weight-loss promoting incretin glucagon like peptide 1 (GLP-1) [40, 41], and the anorectic hormone peptide YY (PYY) [42]. The development of delayed release metformin has created an opportunity to study gut-specific effects of metformin. Utilizing these delayed-release formulations, Buse et al. found that the glycemic effects of metformin could be realized without any change in serum metformin concentration, suggesting that metformin exerts its effects on blood glucose through local action on the GI tract [43]. A putative additional mechanism by which metformin may suppress appetite through action in the GI tract include alteration of bile acid absorption through interaction with farnesoid X receptor [41, 44]. The alteration of bile acids is thought to have a secondary effect on secretion of appetite suppressing neuropeptides such as GLP-1 and peptide YY [45].
CNS effects of metformin, both direct and indirect have been reported. Metformin has been shown to be important in activating intestinal afferents to the Nucleus Tractus Solitarius (NTS) in a GLP-1 receptor and PKA dependent manner via AMPK [46]. Functional MRI studies have shown metformin induced cerebral metabolic changes in patients with T2D, experiencing decreased metabolism in the parahippocampal gyrus, ventromedial prefrontal cortex and fusiform gyrus [47]. These are areas associated with semantic memory and reward formation and may be altering food-reward relationships [48, 49].
Hypothalamic effects of metformin have also been reported. Intracerebroventricular (ICV) injections of metformin have been found to reduce food intake by decreasing hypothalamic expression of the orexigenic peptide neuropeptide Y (NPY) in rats [50]. ICV metformin has also shown to prevent the function of exogenous ghrelin on promoting appetite through suppression of mechanistic target of rapamycin (mTOR) [51]. There is evidence of blood-brain barrier permeability to metformin, and metformin reaches concentrations in the tens of micromolar in CSF and several brain regions, including hypothalamus, further supporting the potential for a direct CNS effect [52, 53]. STAT3 phosphorylation, which is evident after administration of the anorectic hormone leptin [54], is activated in hypothalamic neurons in obese diabetic rats with metformin administration [55]. Metformin has also been shown to impact leptin receptor expression and decrease hypothalamic leptin resistance [56, 57].
AMPK is expressed in the appetite-regulating components of the hypothalamus including the arcuate nucleus [58]. It is essential to the functioning of Agouti-related Peptide (AgRP) neurons, as deletion of AMPK in these neurons leads to a lean phenotype in mice [59]. Thus, expression of AMPK is associated with orexigenic phenotypes due to stimulation of the NPY-AgRP axis. Curiously, although metformin is a known activator of hepatic AMPK, metformin has been shown to suppress hypothalamic AMPK in vitro and in vivo, consistent with its anorexigenic effect [60]. In primary cultures of rat hypothalamic neurons, metformin inhibits AMPK and prevents increases in orexigenic NPY expression [60]. This highlights the complex, tissue specific effects of metformin on metabolism and appetite, which, in aggregate, stimulate weight loss. It should be stressed that the relevance of direct, metformin-mediated neuronal activation of AMPK to appetite regulation remains unclear, due to the reported effects of the drug on appetite regulatory signals such as incretins, leptin and peripheral metabolites.
Metformin and the GI Tract
As mentioned above, another potential mechanism by which metformin may suppress appetite is via effects in the GI tract. [61]. With oral administration, metformin reaches its highest concentrations in the GI tract in enterocytes [52]. These high metformin levels have been associated with increased enterocyte glucose uptake [62]. This glucose is utilized in an anaerobic manner driving local lactate production. Biopsy studies have shown increased lactate production with metformin infusions in the rat model [63]. The metabolic impacts of this gut lactate production may drive some of the gastrointestinal symptoms associated with metformin including diarrhea, bloating and GI discomfort. This has been examined genetically. Dujic et al. examined polymorphisms in organic cation transporter 1 (OCT1, the major transporter by which metformin is taken up into enterocytes and hepatocytes) and the likelihood of metformin intolerance [64]. They found that persons with homozygous reduced-function alleles had a 2-fold higher likelihood of intolerance symptoms to metformin, probably due to increased accumulation of metformin in the intestinal lumen [65]. Intestinal serotonin concentrations have also been found to be upregulated in metformin treatment [66]. The authors hypothesized that excess local secretion of serotonin may drive some of GI intolerance symptoms associated with metformin. The extent of weight loss and its relationship to these side effects is unclear. The DPP trial suggests that adherence correlates with weight loss, but whether GI side effects are a harbinger of metformin’s efficacy for weight loss is still to be determined.
Side effect studies have also identified that dysgeusia is a common side effect of metformin [67]. Recent studies have shown organic cation transporter 3 (OCT3) is involved in the concentration of metformin in the salivary glands [68]. A medicinal, bitter or metallic taste due to the oropharyngeal concentration of metformin may also be an appetite suppressing quality of this medication.
Metformin and the Microbiome
The accumulation of metformin in the GI tract is thought to impact not just epithelial brush border metabolism but also that of the complex bacterial community of the gut. The distribution of microbial flora has been shown to be different between obese and non-obese populations, with multiple factors thought to be playing both causative and correlative roles [69]. Multiple common enteric species have found to be negatively correlated with the obese/metabolic syndrome phenotype [70]. Studies have shown that administration of Ackermansia improves metabolic phenotypes in mice and that metformin increases the relative abundance of Ackermansia. [71, 72]. Napolitano et al. identified changes in the relative concentration of phyla Bacteroides and Firmicutes with metformin treatment [41]. One difference noted in multiple studies was the reduction in bacteria that produce short chain fatty acids. (SCFAs). SCFAs such as acetate and butyrate are being investigated as important signaling metabolites that impact hepatic gluconeogenesis and fatty acid metabolism. Increases in SCFAs are thought to contribute to decreased hepatic gluconeogenesis, reductions in FFA release from adipocytes, and suppression of appetite via the incretin system [73]. Metformin treatment in rats was found to modulate gut microbiota and increase SCFA metabolizing bacteria [74]. Additionally, metformin-mediated shifts in intestinal Lactobaclillus sp. has been shown to reduce hepatic glucose production in a rat model of T2D by restoring normal intestinal glucose sensing and sodium glucose cotransporter-1 (SGLT1) expression [75]. Human studies show favorable effects on the microbiome as well. A recent double-blind randomized control study demonstrated that the addition of metformin significantly altered relative abundance of multiple bacterial strains. Stool samples were transferred to mice which exhibited improved metabolic parameters after metformin-treated stool transplant, as well as increase expression of bacterial genes related to SCFA metabolism [76]. These studies suggest the exciting possibility that the favorable effects of metformin, including the weight effects of metformin, may be a product of a modified microbiome.
Metformin, Obesity and Aging
Obesity, while fundamentally a disorder of nutrient homeostasis, is also a disease of aging, increasing markedly worldwide as individuals age [77]. Also common in aging is the phenotype of sarcopenic obesity, where an increase in fat mass is accompanied by decreases in lean body mass [78]. This has been found to be a relatively common occurrence, with some elderly populations exhibiting prevalence of up to 15%. Anthropometric measures are unreliable in identifying this type of obesity and make elucidating eutrophic versus obese states challenging. Unfortunately, there are no agents that directly target muscle loss and sarcopenic obesity, with anabolic therapy focused on exercise as the principle method of treatment. Metformin however is being investigated as a potential tool for managing the sequelae of aging. Multiple studies in C. elegans and mouse models have shown extension of lifespan and healthspan (proportion of individual’s life where they are productive and free from disease) with metformin [79]. Epidemiologic studies have also shown decreased rate of aging-related cancer development in individuals who take metformin [80]. Multiple pathways are thought to contribute to these potential effects. Metformin is known to suppress the function of mTOR complex, increased activity of which is mechanistically linked to aging and multiple aging-related diseases [81, 82]. Wu et al. elucidated that mTOR suppression is mediated by metformin through action on transport through the nuclear pore complex (NPC) [83, 84]. Suppression of passage through the NPC via metformin’s effect on mitochondria prevents inappropriate mTOR activation caused by age-related increases in nuclear pore transport, which have been shown to be causal in aging [85]. Still other work in model systems suggests that metformin modulates aging by affecting AMPK activity or via effects on the microbiome [79, 86].
In humans, it remains unclear how metformin modulates aging. Trials are underway to investigate the effects of metformin on muscle mass and strength, and a trial has been proposed to study the effects of metformin on aging-related outcomes [87, 88]. It also remains a possibility, since elevated insulin/IGF-1 signaling has been linked to progeria, that metformin’s ability to improve insulin sensitivity and lower lifetime insulin load is a mechanistic tie to its ability to promote healthy aging.
Metformin and Sequelae of Obesity
Non-Alcoholic Fatty Liver Disease (NAFLD)
The metabolic effects of biguanides on the liver have created interest in metformin as a potential therapeutic for NAFLD, a problem intimately related to the obesity epidemic. Meta-analyses have been unable to show benefit for metformin in reducing hepatic fibrosis or clinical parameters such as reductions in aminotransferase levels. These studies however were small, heterogeneous and frequently contained diabetic patients [89]. It is also unclear whether transient impacts on these parameters are due to a primary effect of metformin on the pathogenesis of NASH or due to secondary effects from reductions in weight, appetite and insulin resistance. The American Association for the study of Liver Disease (AASLD) 2018 guidelines do not recommend the use of Metformin as an agent to treat Non-alcoholic fatty liver disease [90].
Obstructive Sleep Apnea (OSA)
OSA is a common complication of obesity [91]. Metformin has been examined through cross sectional and retrospective studies on its impacts on OSA severity. Its use was not associated with changes in OSA prevalence [92].
Metabolic Bone Disease
Obese patients are known to have a decreased rate of fracture for a given bone mineral density, however diabetic patients are at increased risk of fractures [93, 94]. Metformin has been evaluated to see if it impacts bone turnover in vitro. The addition of metformin does not appear to increase the risk of fractures in rodents [95]. Randomized trials have shown no change in BMD in diabetic patients receiving insulin vs insulin and metformin [96]. Observational studies have not consistently shown a reduction in fractures with the use of metformin [97].
Osteoarthritis
Osteoarthritis is a common complication of obesity due to the high impact forces on joints [98]. Cohort studies have not shown that metformin use is associated with decreased prevalence of osteoarthritis. A prospective observational study from Taiwan found that patients who utilized Cox-2 inhibitors and metformin versus Cox-2 inhibitors alone were less likely to receive a joint replacement (HR 0.742) [99]. It is unclear whether this is mediated by inflammation however one study noted that metformin protected nucleus pulposus cells and prevents disc degeneration [100].
Thromboembolism
Venous thromboembolism is a serious complication of morbid obesity due to lower extremity venous stasis, prothrombotic adipokines such as leptin and resistin, and chronic inflammation [101]. Metformin has been found to decrease risk of DVT in a cohort study in Taiwan [102]. Potential etiologies for this phenomenon include inhibiting platelet activation and mtDNA release [103].
Conclusions
Despite its excellent safety and tolerability profile, metformin remains equivocal as a primary treatment for obesity and as a weight loss agent. It may serve as an adjunct therapy for those patients who are at high risk for metabolic complications or are experiencing other sequelae of obesity. Its mechanistic effects on central hypothalamic signaling, incretin secretion and alteration of the gut microbiome establish attractive areas of research in identifying new targets for obesity treatment. The long-term impacts of metformin use on aging and sarcopenia have yet to be elucidated, but they may also provide important insights into optimizing body composition with age. In the meantime, metformin will continue to serve as a mainstay treatment in the management of T2D and confer multiple metabolic effects beyond glycemic control.
Acknowledgements
We wish to acknowledge Scott Leighton for creating the figure for this review.
Funding: This work was supported by NIH R01AG058256, R01DK101522, and R01DK072041, the Weissman Family MGH Research Scholar Award, and a Glenn Award for Research in the Biological Mechanisms of Aging (to AS). AY is supported by the NIH-NIDDK funded MGH Endocrinology training grant T32DK007028.
Footnotes
Conflict of Interest:
Armen Yerevanian reports no conflict of interest.
Alexander Soukas reports no conflict of interest.
Compliance and Ethical Standards: No IRB approval was indicated for this review
Human Rights and Animal Rights Informed Consent: This article does not contain any studies with animal or human subjects performed by the author.
References
Papers of particular interest, especially those published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.Witters LA, The blooming of the French lilac. J Clin Invest, 2001. 108(8): p. 1105–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey CJ, Metformin: historical overview. Diabetologia, 2017. 60(9): p. 1566–1576. [DOI] [PubMed] [Google Scholar]
- 3.J W.E.a.B., The preparation of methylguanidine, and of ββ-dimethylguanidine by the interaction of dicyandiamide, and methylammonium and dimethylammonium chlorides respectively. J Chem Soc Trans, 1922. 121: p. 1790–1794. [Google Scholar]
- 4.DeFronzo RA and Goodman AM, Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med, 1995. 333(9): p. 541–9. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes, A., Standards of Medical Care in Diabetes-2019 Abridged for Primary Care Providers . Clin Diabetes, 2019. 37(1): p. 11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stumvoll M, et al. , Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med, 1995. 333(9): p. 550–4. [DOI] [PubMed] [Google Scholar]
- 7.Holman RR, et al. , 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med, 2008. 359(15): p. 1577–89. [DOI] [PubMed] [Google Scholar]
- 8.Kahn SE, et al. , Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med, 2006. 355(23): p. 2427–43. [DOI] [PubMed] [Google Scholar]
- 9.Saenz A, et al. , Metformin monotherapy for type 2 diabetes mellitus. Cochrane Database Syst Rev, 2005(3): p. CD002966. [DOI] [PubMed] [Google Scholar]
- 10.Golay A, Metformin and body weight. Int J Obes (Lond), 2008. 32(1): p. 61–72. [DOI] [PubMed] [Google Scholar]
- 11.Fontbonne A, et al. , The effect of metformin on the metabolic abnormalities associated with upper-body fat distribution. BIGPRO Study Group. Diabetes Care, 1996. 19(9): p. 920–6. [DOI] [PubMed] [Google Scholar]
- 12.Paolisso G, et al. , Effect of metformin on food intake in obese subjects. Eur J Clin Invest, 1998. 28(6): p. 441–6. [DOI] [PubMed] [Google Scholar]
- 13.Glueck CJ, et al. , Metformin reduces weight, centripetal obesity, insulin, leptin, and low-density lipoprotein cholesterol in nondiabetic, morbidly obese subjects with body mass index greater than 30. Metabolism, 2001. 50(7): p. 856–61. [DOI] [PubMed] [Google Scholar]
- 14.Levri KM, et al. , Metformin as treatment for overweight and obese adults: a systematic review. Ann Fam Med, 2005. 3(5): p. 457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diabetes Prevention Program Research, G., Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care, 2012. 35(4): p. 731–7.** Largest and most comprehensive study that identified persistent weight loss with metformin treatment.
- 16.Apovian CM, et al. , Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab, 2015. 100(2): p. 342–62.* Provides the most recent guidelines on pharmacotherapy for obesity.
- 17.Garvey WT, et al. , American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr Pract, 2016. 22 Suppl 3: p. 1–203. [DOI] [PubMed] [Google Scholar]
- 18.de Silva VA, et al. , Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry, 2016. 16(1): p. 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makimattila S, Nikkila K, and Yki-Jarvinen H, Causes of weight gain during insulin therapy with and without metformin in patients with Type II diabetes mellitus. Diabetologia, 1999. 42(4): p. 406–12. [DOI] [PubMed] [Google Scholar]
- 20.Kooy A, et al. , Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med, 2009. 169(6): p. 616–25. [DOI] [PubMed] [Google Scholar]
- 21.Hundal RS, et al. , Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes, 2000. 49(12): p. 2063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inzucchi SE, et al. , Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med, 1998. 338(13): p. 867–72. [DOI] [PubMed] [Google Scholar]
- 23.El-Mir MY, et al. , Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem, 2000. 275(1): p. 223–8. [DOI] [PubMed] [Google Scholar]
- 24.He L and Wondisford FE, Metformin action: concentrations matter. Cell Metab, 2015. 21(2): p. 159–162. [DOI] [PubMed] [Google Scholar]
- 25.Zhou G, et al. , Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest, 2001. 108(8): p. 1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw RJ, et al. , The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science, 2005. 310(5754): p. 1642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foretz M, et al. , Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest, 2010. 120(7): p. 2355–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller RA and Birnbaum MJ, An energetic tale of AMPK-independent effects of metformin. J Clin Invest, 2010. 120(7): p. 2267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller RA, et al. , Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature, 2013. 494(7436): p. 256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madiraju AK, et al. , Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature, 2014. 510(7506): p. 542–6.* A novel mechanism by which metformin reduces hepatic glucose output by inhibiting the mitochondrial glycerol-phosphate shuttle, altering hepatocellular redox potential.
- 31.Madiraju AK, et al. , Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat Med, 2018. 24(9): p. 1384–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee A and Morley JE, Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res, 1998. 6(1): p. 47–53. [DOI] [PubMed] [Google Scholar]
- 33.Yki-Jarvinen H, Nikkila K, and Makimattila S, Metformin prevents weight gain by reducing dietary intake during insulin therapy in patients with type 2 diabetes mellitus. Drugs, 1999. 58 Suppl 1: p. 53–4; discussion 75–82. [DOI] [PubMed] [Google Scholar]
- 34.Kalantar-Zadeh K, et al. , Metabolic acidosis and malnutrition-inflammation complex syndrome in chronic renal failure. Semin Dial, 2004. 17(6): p. 455–65. [DOI] [PubMed] [Google Scholar]
- 35.Owen MR, Doran E, and Halestrap AP, Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J, 2000. 348 Pt 3: p. 607–14. [PMC free article] [PubMed] [Google Scholar]
- 36.Tokubuchi I, et al. , Beneficial effects of metformin on energy metabolism and visceral fat volume through a possible mechanism of fatty acid oxidation in human subjects and rats. PLoS One, 2017. 12(2): p. e0171293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Islam H, et al. , Potential involvement of lactate and interleukin-6 in the appetite-regulatory hormonal response to an acute exercise bout. J Appl Physiol (1985), 2017. 123(3): p. 614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lam CK, et al. , Central lactate metabolism regulates food intake. Am J Physiol Endocrinol Metab, 2008. 295(2): p. E491–6. [DOI] [PubMed] [Google Scholar]
- 39.Chari M, et al. , Activation of central lactate metabolism lowers glucose production in uncontrolled diabetes and diet-induced insulin resistance. Diabetes, 2008. 57(4): p. 836–40. [DOI] [PubMed] [Google Scholar]
- 40.Mulherin AJ, et al. , Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell. Endocrinology, 2011. 152(12): p. 4610–9. [DOI] [PubMed] [Google Scholar]
- 41.Napolitano A, et al. , Novel gut-based pharmacology of metformin in patients with type 2 diabetes mellitus. PLoS One, 2014. 9(7): p. e100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeFronzo RA, et al. , Once-daily delayed-release metformin lowers plasma glucose and enhances fasting and postprandial GLP-1 and PYY: results from two randomised trials. Diabetologia, 2016. 59(8): p. 1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buse JB, et al. , The Primary Glucose-Lowering Effect of Metformin Resides in the Gut, Not the Circulation: Results From Short-term Pharmacokinetic and 12-Week Dose-Ranging Studies. Diabetes Care, 2016. 39(2): p. 198–205.* Identified the importance of gut-specific effects of metformin. Metformin’s effects were present even when serum levels were minimal.
- 44.Lien F, et al. , Metformin interferes with bile acid homeostasis through AMPK-FXR crosstalk. J Clin Invest, 2014. 124(3): p. 1037–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuhre RE, et al. , Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol Metab, 2018. 11: p. 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duca FA, et al. , Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med, 2015. 21(5): p. 506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang YC, et al. , Effects of metformin on the cerebral metabolic changes in type 2 diabetic patients. ScientificWorldJournal, 2014. 2014: p. 694326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Wit S, et al. , Differential engagement of the ventromedial prefrontal cortex by goal-directed and habitual behavior toward food pictures in humans. J Neurosci, 2009. 29(36): p. 11330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huerta CI, et al. , Neural bases of food perception: coordinate-based meta-analyses of neuroimaging studies in multiple modalities. Obesity (Silver Spring), 2014. 22(6): p. 1439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duan Y, et al. , Metformin inhibits food intake and neuropeptide Y gene expression in the hypothalamus. Neural Regen Res, 2013. 8(25): p. 2379–88.* Identified hypothalamic mechanism by which metformin could inhibit appetite.
- 51.Stevanovic D, et al. , Intracerebroventricular administration of metformin inhibits ghrelin-induced Hypothalamic AMP-kinase signalling and food intake. Neuroendocrinology, 2012. 96(1): p. 24–31. [DOI] [PubMed] [Google Scholar]
- 52.Wilcock C and Bailey CJ, Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica, 1994. 24(1): p. 49–57. [DOI] [PubMed] [Google Scholar]
- 53.Labuzek K, et al. , Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol Rep, 2010. 62(5): p. 956–65. [DOI] [PubMed] [Google Scholar]
- 54.Vaisse C, et al. , Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet, 1996. 14(1): p. 95–7. [DOI] [PubMed] [Google Scholar]
- 55.Lv WS, et al. , The effect of metformin on food intake and its potential role in hypothalamic regulation in obese diabetic rats. Brain Res, 2012. 1444: p. 11–9. [DOI] [PubMed] [Google Scholar]
- 56.Aubert G, et al. , The anorexigenic effects of metformin involve increases in hypothalamic leptin receptor expression. Metabolism, 2011. 60(3): p. 327–34. [DOI] [PubMed] [Google Scholar]
- 57.Kim YW, et al. , Metformin restores leptin sensitivity in high-fat-fed obese rats with leptin resistance. Diabetes, 2006. 55(3): p. 716–24. [DOI] [PubMed] [Google Scholar]
- 58.Stark R, Ashley SE, and Andrews ZB, AMPK and the neuroendocrine regulation of appetite and energy expenditure. Mol Cell Endocrinol, 2013. 366(2): p. 215–23. [DOI] [PubMed] [Google Scholar]
- 59.Claret M, et al. , AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest, 2007. 117(8): p. 2325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chau-Van C, et al. , Metformin inhibits adenosine 5’-monophosphate-activated kinase activation and prevents increases in neuropeptide Y expression in cultured hypothalamic neurons. Endocrinology, 2007. 148(2): p. 507–11.* Explored mechanism of metformin’s effects on hypothalamic neurons. It showed that unlike peripheral AMPK, neuronal AMPK is suppressed by metformin.
- 61.McCreight LJ, Bailey CJ, and Pearson ER, Metformin and the gastrointestinal tract. Diabetologia, 2016. 59(3): p. 426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gontier E, et al. , High and typical 18F-FDG bowel uptake in patients treated with metformin. Eur J Nucl Med Mol Imaging, 2008. 35(1): p. 95–9. [DOI] [PubMed] [Google Scholar]
- 63.Bailey CJ, Wilcock C, and Day C, Effect of metformin on glucose metabolism in the splanchnic bed. Br J Pharmacol, 1992. 105(4): p. 1009–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dujic T, et al. , Organic cation transporter 1 variants and gastrointestinal side effects of metformin in patients with Type 2 diabetes. Diabet Med, 2016. 33(4): p. 511–4.* Population study that suggested that concentrations of metformin in the gut lumen affects risk of experiencing GI side effects from metformin.
- 65.Dujic T, et al. , Association of Organic Cation Transporter 1 With Intolerance to Metformin in Type 2 Diabetes: A GoDARTS Study. Diabetes, 2015. 64(5): p. 1786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cubeddu LX, et al. , Effects of metformin on intestinal 5-hydroxytryptamine (5-HT) release and on 5-HT3 receptors. Naunyn Schmiedebergs Arch Pharmacol, 2000. 361(1): p. 85–91. [DOI] [PubMed] [Google Scholar]
- 67.Schiffman SS, Influence of medications on taste and smell. World J Otorhinolaryngol Head Neck Surg, 2018. 4(1): p. 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee N, et al. , Taste of a pill: organic cation transporter-3 (OCT3) mediates metformin accumulation and secretion in salivary glands. J Biol Chem, 2014. 289(39): p. 27055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maruvada P, et al. , The Human Microbiome and Obesity: Moving beyond Associations. Cell Host Microbe, 2017. 22(5): p. 589–599. [DOI] [PubMed] [Google Scholar]
- 70.Karlsson FH, et al. , Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature, 2013. 498(7452): p. 99–103. [DOI] [PubMed] [Google Scholar]
- 71.Shin NR, et al. , An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut, 2014. 63(5): p. 727–35. [DOI] [PubMed] [Google Scholar]
- 72.Cani PD and de Vos WM, Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front Microbiol, 2017. 8: p. 1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morrison DJ and Preston T, Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes, 2016. 7(3): p. 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang X, et al. , Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep, 2015. 5: p. 14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bauer PV, et al. , Metformin Alters Upper Small Intestinal Microbiota that Impact a Glucose-SGLT1-Sensing Glucoregulatory Pathway. Cell Metab, 2018. 27(1): p. 101–117 e5. [DOI] [PubMed] [Google Scholar]
- 76.Wu H, et al. , Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med, 2017. 23(7): p. 850–858.* Explored impacts of metformin with the microbiome. It showed through transplantation studies that metformin alters microbiome composition that is associated with improved metabolic status.
- 77.Collaborators GBDO, et al. , Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med, 2017. 377(1): p. 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bouchard DR, Dionne IJ, and Brochu M, Sarcopenic/obesity and physical capacity in older men and women: data from the Nutrition as a Determinant of Successful Aging (NuAge)-the Quebec longitudinal Study. Obesity (Silver Spring), 2009. 17(11): p. 2082–8. [DOI] [PubMed] [Google Scholar]
- 79.Onken B and Driscoll M, Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One, 2010. 5(1): p. e8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim HJ, et al. , Metformin reduces the risk of cancer in patients with type 2 diabetes: An analysis based on the Korean National Diabetes Program Cohort. Medicine (Baltimore), 2018. 97(8): p. e0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson SC, Rabinovitch PS, and Kaeberlein M, mTOR is a key modulator of ageing and age-related disease. Nature, 2013. 493(7432): p. 338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kalender A, et al. , Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab, 2010. 11(5): p. 390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu L, et al. , An Ancient, Unified Mechanism for Metformin Growth Inhibition in C. elegans and Cancer. Cell, 2016. 167(7): p. 1705–1718 e13.* Mechanistic paper showing that metformin extends lifespan and inhibits growth of cancer cells by altering mitochondrial function, mTOR, and nucleocytoplasmic transport through the nuclear pore complex.
- 84.Castillo-Quan JI and Blackwell TK, Metformin: Restraining Nucleocytoplasmic Shuttling to Fight Cancer and Aging. Cell, 2016. 167(7): p. 1670–1671. [DOI] [PubMed] [Google Scholar]
- 85.D’Angelo MA, et al. , Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell, 2009. 136(2): p. 284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cabreiro F, et al. , Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell, 2013. 153(1): p. 228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barzilai N, et al. , Metformin as a Tool to Target Aging. Cell Metab, 2016. 23(6): p. 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Long DE, et al. , Metformin to Augment Strength Training Effective Response in Seniors (MASTERS): study protocol for a randomized controlled trial. Trials, 2017. 18(1): p. 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y, et al. , Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep, 2013. 1(1): p. 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chalasani N, et al. , The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology, 2018. 67(1): p. 328–357.* Guideline paper from the American Association for the Study of Liver Disease (AASLD) on the management of nonalcoholic fatty liver disease.
- 91.Lavrentaki A, et al. , Mechanisms of disease: The endocrinology of obstructive sleep apnoea. Eur J Endocrinol, 2018. [DOI] [PubMed] [Google Scholar]
- 92.Lin D, et al. , The Relationship between Metformin and Obstructive Sleep Apnea. J Sleep Med Disord, 2015. 2(4). [PMC free article] [PubMed] [Google Scholar]
- 93.Bonds DE, et al. , Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study. J Clin Endocrinol Metab, 2006. 91(9): p. 3404–10. [DOI] [PubMed] [Google Scholar]
- 94.de L II, et al. , Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int, 2005. 16(12): p. 1713–20. [DOI] [PubMed] [Google Scholar]
- 95.Jeyabalan J, et al. , The anti-diabetic drug metformin does not affect bone mass in vivo or fracture healing. Osteoporos Int, 2013. 24(10): p. 2659–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nordklint AK, et al. , The effect of metformin versus placebo in combination with insulin analogues on bone mineral density and trabecular bone score in patients with type 2 diabetes mellitus: a randomized placebo-controlled trial. Osteoporos Int, 2018. 29(11): p. 2517–2526. [DOI] [PubMed] [Google Scholar]
- 97.Schwartz AV, Diabetes, bone and glucose-lowering agents: clinical outcomes. Diabetologia, 2017. 60(7): p. 1170–1179. [DOI] [PubMed] [Google Scholar]
- 98.Bliddal H, Leeds AR, and Christensen R, Osteoarthritis, obesity and weight loss: evidence, hypotheses and horizons - a scoping review. Obes Rev, 2014. 15(7): p. 578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lu CH, et al. , Combination COX-2 inhibitor and metformin attenuate rate of joint replacement in osteoarthritis with diabetes: A nationwide, retrospective, matched-cohort study in Taiwan. PLoS One, 2018. 13(1): p. e0191242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen D, et al. , Metformin protects against apoptosis and senescence in nucleus pulposus cells and ameliorates disc degeneration in vivo. Cell Death Dis, 2016. 7(10): p. e2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Blokhin IO and Lentz SR, Mechanisms of thrombosis in obesity. Curr Opin Hematol, 2013. 20(5): p. 437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu DY, et al. , Metformin use in patients with type 2 diabetes mellitus is associated with reduced risk of deep vein thrombosis: a non-randomized, pair-matched cohort study. BMC Cardiovasc Disord, 2014. 14: p. 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xin G, et al. , Metformin Uniquely Prevents Thrombosis by Inhibiting Platelet Activation and mtDNA Release. Sci Rep, 2016. 6: p. 36222. [DOI] [PMC free article] [PubMed] [Google Scholar]