Abstract
Objectives:
Pancreatic lesions in autosomal dominant polycystic kidney disease (ADPKD) are primarily cysts. They are increasingly recognized, with isolated reports of intraductal papillary mucinous neoplasia (IPMN).
Methods:
Retrospective study to determine prevalence, number, size, and location of pancreatic abnormalities using abdominal magnetic resonance imaging (MRI) of genotyped ADPKD patients (seen February 1998-October 2013) and compared with age- and sex-matched non-ADPKD controls. We evaluated presentation, investigation, and management of all IPMNs among individuals with ADPKD (January 1997-December 2016).
Results:
Abdominal MRIs were examined for 271 genotyped ADPKD patients. A pancreatic cyst lesion (PCL) was detected in 52 patients (19%; 95% confidence interval, 15%−23%). Thirty-seven (71%) had a solitary PCL; 15 (28%) had multiple. Pancreatic cyst lesion prevalence did not differ by genotype. Intraductal papillary mucinous neoplasia was detected in 1% of ADPKD cases. Among 12 IPMN patients (7 branch duct; 5 main duct or mixed type) monitored for about 140 months, 2 with main duct IPMN required Whipple resection, and 1 patient died of complications from small-bowel obstruction after declining surgical intervention.
Conclusions:
With MRI, PCLs were detected in 19% and IPMNs in 1% of 271 ADPKD patients with proven mutations, without difference across genotypes. Pancreatic cyst lesions were asymptomatic and remained stable in size.
Keywords: autosomal dominant polycystic kidney disease, intraductal papillary mucinous neoplasm, magnetic resonance imaging, pancreatic cysts
Introduction
Autosomal dominant polycystic kidney disease (ADPKD), the most common inherited renal cystic disease, is characterized by multiple renal cysts and various extrarenal cystic manifestations.1,2 Cysts have been described in the liver, pancreas, spleen, arachnoid membrane, and seminal vesicles.3,4
Pancreatic lesions, mainly pancreatic cyst lesions (PCLs), are common in the general population (prevalence, 2.5%−13.5%). The prevalence of PCLs increases with age,5–7 but these lesions increasingly are detected in younger people because of the more frequent use of cross-sectional imaging and improvements in image resolution.8
Development of invasive adenocarcinoma in PCLs is rare. An incidental cyst seen on magnetic resonance imaging (MRI) has a 10 in 100,000 chance of being a mucinous invasive malignancy and a 17 in 100,000 chance of being ductal cancer.9,10 Currently, the American Gastroenterological Association recommends MRI monitoring of PCLs <3 cm without solid components or of a dilated pancreatic duct at 1 year, then every 2 years for a total of 5 years if no change occurs in size or characteristics.9
Pancreatic cyst lesions are a recognized component of the ADPKD phenotype.2 Animal models of PKD1 and PKD2 genes also have PCLs.11,12 In a 1995 study, PCL prevalence was 5% to 9% in ADPKD cases with use of ultrasonography.13 In the Halt Progression of Polycystic Kidney Disease (HALT) trial, in which MRI was limited to the coronal plane, PCLs were identified in 1.8% of 560 patients and solitary in 8 cases (62%).14 The most comprehensive recent MRI study noted a PCL prevalence of 36.4% in ADPKD cases, compared with 22% in age- and sex-matched controls,15 with greater prevalence in individuals with PKD2 mutations. Six had PCLs of >1 cm; the PCLs of 3 patients had a connection to the main pancreatic duct (MPD) or uncinate duct, consistent with intraductal papillary mucinous neoplasm (IPMN).15 Although the malignancy potential of PCLs is miniscule, lesions connecting to the MPD may represent IPMNs and potentially have an increased cancer risk.16 Cancer risk in IPMN lesions increases when size exceeds 3 cm, with duct dilatation, or with presence of solid components.16–19 Investigation and management in patients with asymptomatic ADPKD can be hampered by altered anatomy and complex medical comorbidities.20–22
We evaluated prevalence and characteristics of PCLs in patients with genetically confirmed ADPKD who had an abdominal MRI at Mayo Clinic in Rochester, Minnesota, between February 1998 and October 2013 and compared them with matched controls without ADPKD. We determined changes in PCLs over an 8-year follow-up for individuals with repeat imaging. In addition, we evaluated clinical outcomes for patients with ADPKD who had a diagnosis of IPMN irrespective of MRI between January 1997 and December 2016.
MATERIALS AND METHODS
The Mayo Clinic Institutional Review Board (IRB) approved this study in accordance with the Declaration of Helsinki and Health Insurance Portability and Accountability Act. Mayo Clinic provided funding support. All authors had access to study data and have reviewed and approved the final manuscript.
Study Population
All patients consented to the use of their health records for research, and informed consent was waived by Mayo Clinic internal review board. All persons consented for minimal risk health record review studies. Genotyped patients with ADPKD who underwent MRI between 1997 and 2008 were eligible for inclusion and separately consented for genotyping studies. Controls matched for sex and age were selected from an archive of MRIs; they had undergone abdominal MRI between 1998 and 2013 for non–pancreas-related indications and had no cystic kidney disease. Age, sex, race/ethnicity, and estimated glomerular filtration rate (GFR) attained within 6 months of their MRI were obtained for study participants and controls. Genotypes were obtained from the Mayo Clinic Polycystic Kidney Disease (PKD) database. Estimated GFR was calculated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration equation.23 For those who underwent multiple MRIs, the first scan was analyzed for this study. For patients with PCLs noted on initial MRI with follow-up imaging available, changes in PCL characteristics were compared with their most recent abdominal MRIs.
Individuals who had a diagnosis of IPMN and ADPKD reported in their health records or imaging report and had been seen at Mayo Clinic in Rochester, Minnesota, between 1997 and 2016 were identified through the clinical data repository databases using the search terms autosomal dominant polycystic kidney disease [ADPKD] and intraductal papillary mucinous neoplasm [IPMN]), the PKD database, and Mayo Clinic Medical Index (Fig. 1A). ADPKD inclusion criteria were based on having either 1) a proven pathogenic mutation associated with ADPKD, 2) modified Ravine criteria in addition to a positive family history,1 or 3) presence of >10 bilateral renal cysts and hepatic cysts in the absence of evidence for other inherited renal cystic diseases on imaging.24 Thirty-six persons were excluded from the study because they did not meet these criteria for ADPKD or had autosomal dominant liver disease or autosomal recessive polycystic kidney disease, simple PCLs not consistent with IPMN, mucinous cystic neoplasm, serous cystadenoma, or pancreatic adenocarcinoma without IPMN. Pancreatic cystic lesions deemed IPMNs were classified as 1) suspected branch duct (BD)-IPMN, PCL >5 mm in diameter that communicated with the MPD, cyst fluid analysis with carcinoembryonic antigen (CEA) >192 ng/mL, or multifocal cysts with at least 1 that clearly communicated with the MPD,25 or a combination of these; 2) suspected IPMN or segmental or diffuse dilation of the MPD >5 mm without an obvious cause of pancreatic duct obstruction25; and 3) mixed type (MT)-IPMN with cysts meeting criteria for both main duct (MD-IPMN) and BD-IPMN.25
FIGURE 1.
Flowchart of PCLs in the ADPKD Study and the Search Criteria to Identify ADPKD Patients With IPMN. A, The Advanced Cohort Explorer database was used to search for individuals presenting to Mayo Clinic with a diagnosis of PKD and IPMN. Health record review by radiology, gastroenterology, and nephrology services was used to further evaluate and exclude 60 patients as not having PCLs consistent with IPMN or having cystic kidney disease consistent with ADPKD. B, MRI scans of ADPKD patients from Mayo Clinic (n = 271) between February 1998 and October 2013 were compared with age- and sex-matched non-ADPKD individuals (n = 271). ADPKD indicates autosomal dominant polycystic kidney disease; F, female; IPMN, intraductal papillary mucinous neoplasm; M, male; MRI, magnetic resonance imaging; PCL, pancreatic cyst lesion; PKD, polycystic kidney disease.
Intraductal papillary mucinous neoplasm-related complications were assessed through evaluation of follow-up imaging (Y.K. and N.T.) and chart review (B.A.M., S.T.C., and M.C.H.). Among ADPKD patients with IPMN, follow-up imaging and health records were reviewed to assess for complications related to IPMN. Demographic and clinical variables (eg, age at PCL diagnosis, sex, race/ethnicity) were abstracted from the health records. Imaging studies were reviewed by MRI and abdominal imaging expert radiologists (Y.K. and N.T.). MRI characteristics that make IPMN more likely than PCL are its multifocal or grapelike appearance, its communication with the MPD, and absence of non-IPMN cysts (e.g., serous and mucinous cyst neoplasms, pseudocyst, other neoplasms).
Gadolinium can be used for determination of whether IPMN cysts have malignant characteristics (where GFR permits). Reports of endoscopic and pancreatic imaging were reviewed by 2 experts in the Mayo Pancreas Clinic group (A.S. and S.T.C.). The collected imaging data included, where available, the modality, cyst number, maximum cyst diameter, MPD diameter, cyst location, and observed changes in cyst characteristics. For patients who underwent endoscopic ultrasonography or endoscopic retrograde cholangiopancreatography (ERCP), the findings and CEA levels were noted, and clinical records, imaging, and pathology reports were reviewed by an expert gastroenterologist in pancreatic diseases and IPMN (S.T.C.).
PKD Gene Sequence Analysis
DNA was isolated from blood specimens with use of standard methods, and Sanger sequencing or multiplex ligation-dependent probe amplification of PKD1 and PKD2 was performed as previously described.11,26,27
Imaging Protocol
All patients underwent MRI on a 1.5- or 3.0-T magnetic resonance machine (Signa; GE Healthcare) with a body-phased-array coil for rapid image acquisition. Axial or coronal, or both, single-shot fast-spin echocardiography images were used to evaluate PCLs. These coronal and axial images were obtained over a single or multiple breath holds.
Image Analysis
Scans were reviewed by 2 radiologists with experience in genitourinary and pancreatic MRI evaluation (Y.K. and N.T.). Pancreatic cystic lesions were defined as round structures identified on MRI as sharply demarcated from the surrounding parenchyma and with homogeneous signal intensity. Location, size, and number of PCL characteristics were recorded as seen with imaging. Maximum PCL dimensions were measured from inner wall to inner wall. A minimum threshold of 2 mm was used.
Statistical Analyses
Quantitative variables are presented as mean (standard deviation [SD]) or median (interquartile range). Differences in demographic variables between patient group and control group were assessed with t test and χ2 test for quantitative and categorical data, respectively. Age- and sex-matched controls for MRI study were selected from a pool of 1,986 sequential abdomen MRI scans. The χ2 test was used to evaluate differences in cyst prevalence of persons with PKD1 or PKD2 mutations or the presence or absence of cysts of patients with ADPKD. Differences were considered statistically significant at P < 0.05.
RESULTS
Of 271 genotyped ADPKD cases with abdominal MRIs between February 1998 and October 2013, 52 (19%) had PCLs. Clinical indications for imaging (Fig. 1B) included determination of prognosis, evaluation for hypertension or kidney pain, organomegaly, and surgical renal transplant planning (n = 250). Patients with ADPKD were compared with 271 age- and sex-matched controls (without ADPKD or an underlying pancreatic condition) as their indication for MRI (Table 1). Most control MRIs were conducted with gadolinium (n = 244, 90%) compared with 38% (n =104) of gadolinium-enhanced MRI for individuals with ADPKD, reflecting reduced contrast agent use for individuals with impaired renal function due to risk of nephrogenic systemic fibrosis. Patients with ADPKD were predominantly white (n = 248, 92%); with mean (SD) estimated GFR of 69 (30) mL/min/1.73 m2. At least 1 PCL was noted in the ADPKD patients compared with 10.2% of controls (n = 28) (P = 0.03).
TABLE 1.
Comparison of Clinical and Radiologic Characteristics, Including PCL Prevalence, of Patients With ADPKD and Control Patients
| Characteristic* | ADPKD Group (n = 271) | Control Group (n = 271) | P |
|---|---|---|---|
| Age at first MRI, mean (SD), y | 42 (12) | 42 (12) | >0.99 |
| Age of PCL patients, mean (SD), y | 43 (12) | 50 (9) | <0.001 |
| Sex, male, n (%) | 109 (40) | 109 (40) | >0.99 |
| White race/ethnicity, n (%) | 247 (91) | 228 (84) | 0.02 |
| eGFR with CKD-EPI, mean (SD), mL/min/1.73 m2 | 69 (30) | 94 (28) | 0.001 |
| MRI with gadolinium, n (%) | 104 (38) | 244 (90) | 0.03 |
| Presence of ≥1 PCL, n (%) | 52 (19) | 28 (10) | 0.03 |
| No. PCL, mean (SD) | 1.6 (1.1) | 1.6 (1.2) | 0.90 |
| PCL size, mean (SD), mm | 6.4 (4.1) | 6.2 (3.1) | 0.80 |
| PCL location in pancreas (n = 52), n (%) | |||
| Head | 17 (33) | 11 (42) | |
| Body | 12 (23) | 6 (21) | |
| Tail | 12 (23) | 5 (18) | |
| Uncinate/neck | 1 (2) | 1 (4) | |
| Multiple | 10 (19) | 5 (17) | |
| No. PCL, n (%) | |||
| 0 | 219 (81) | 244 (90) | |
| 1 | 37 (14) | 16 (6) | |
| 2 | 7 (3) | 7 (3) | |
| 3–10 | 8 (3) | 5 (2) |
Values are presented as number and percentage of patients unless specified otherwise.
ADPKD indicates autosomal dominant polycystic kidney disease; CKD-EPI, chronic kidney disease–epidemiology collaboration; eGFR, estimated glomerular filtration rate; MRI, magnetic resonance imaging; PCL, pancreatic cyst lesion.
Patients with ADPKD and PCLs were younger (mean [SD] age, 43.0 [12.2] years vs 50.3 [9.1] years; P < 0.001) than controls, without differences in average number or frequency of PCLs (Table 1). Minimum PCL diameter was 2 mm (mean [SD], 6.4 [4.1] mm in ADPKD patients vs 6.2 [3.1] mm in controls; P = 0.20). In the ADPKD group, 33% (n = 17) of PCLs were in the pancreatic head; 23% (n = 12), the body; 23% (n = 12), the tail; 2% (n = 1), the uncinate; and 19% (n = 10), multiple pancreatic locations.
Among the ADPKD group, 82% (n = 221) had a PKD1 mutation and 18% (n = 50) had a PKD2 mutation (Fig. 1B and Table 2). Those with a PKD1 mutation were younger than patients with a PKD2 mutation (mean [SD] age, 41 [12] vs 49 [9] years), but the difference was not significant (P = 0.05). No difference was observed in the prevalence of PCLs between the PKD1 and PKD2 cases (18% [41/221] vs 22% [11/50], respectively; P = 0.20). A tendency toward larger PCLs was observed in the PKD2 group, but no difference was seen in the prevalence of multiple PCLs in either group (Table 2).
TABLE 2.
Clinical and Radiologic Characteristics of PCLs According to PKD Gene Mutation
| Gene Mutation | ||||
|---|---|---|---|---|
| Characteristic | Total Group (n = 271) |
PKD1 (n = 221) |
PKD2 (n = 50) |
P* |
| PCL, n (%) | 52 (19) | 41 (19) | 11 (22) | 0.20 |
| Age, mean (SD), y | 43 (12) | 41 (12) | 49 (9) | 0.05 |
| Male sex with PCL, n (%) | 22 (43) | 18 (44) | 3 (27) | 0.20 |
| No. PCL, mean (SD) | 1.5 (1.2) | 1.4 (1.00) | 2.1 (1.59) | 0.06 |
| Size, mean (SD), mm | 6.4 (4.1) | 6.5 (4.8) | 6.1 (2.6) | 0.10 |
| Multiple PCL, n (%) | 10 (24) | 7 (21) | 3 (27) | 0.20 |
P values represent comparison between PKD1 and PKD2 tests (t test).
Among 52 ADPKD patients with PCLs, 75% (n =39) underwent repeat MRI over 2 to 18 years (mean [SD] follow-up, 6.1 [3.1] years). Pancreatic cystic lesions remained stable in size for 50% (n = 26), decreased for 17% (n = 9), and increased for 7% (n = 4) (Fig. 2 and Supplemental Figure 1).
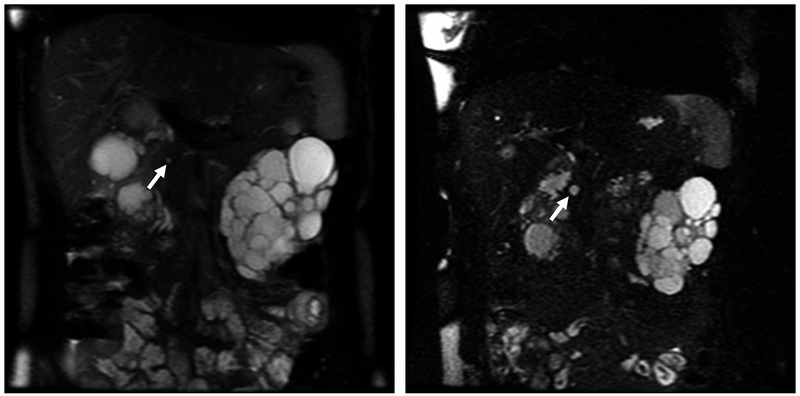
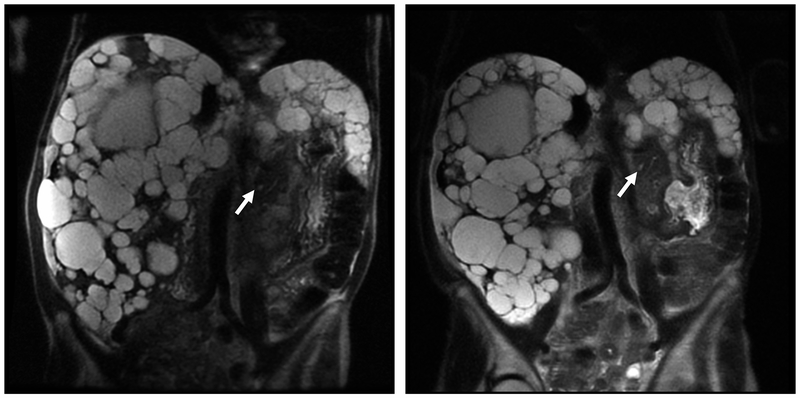
FIGURE 2.
Pancreatic Cyst Lesions (PCLs) From Magnetic Resonance Imaging (MRI) of Patient With PKD1 and PKD2 Genes. A, Coronal single-shot fast-spin echocardiography (SSFSE) MRI showing a PCL (arrow) in 28-year-old woman with PKD1 from 2006. B, Coronal SSFSE MRI showing increased PCL size (arrow) in the same woman with PKD1 in 2011. C, Coronal SSFSE and coronal half-Fourier acquired single-shot turbo-spin echocardiography (HASTE) MRI showing a PCL (arrow) in the tail of the pancreas of a 65-year-old woman with PKD2 from 2006. D, Coronal SSFSE and coronal HASTE MRI showing increase in PCL size (arrow) from 2011.
IPMN in ADPKD
Initially, PCLs that communicate with the MPD were noted in 2 of the 52 genotyped ADPKD cases, suggestive of BD-IPMN (Fig. 1B), with a prevalence of 3.8% for IPMN in ADPKD patients with PCL and 0.7% within the entire cohort of ADPKD patients. Ten additional cases were identified (Table 3) independent of the patients with ADPKD studied in the MRI cohort. Of these additional cases, 2 were diagnosed elsewhere and referred to Mayo Clinic for additional evaluation of the IPMN (case 9 and 12). Compared with the ADPKD patients who had PCLs, the IPMN patients were older (mean [SD] age, 62 [2.8] vs 43 [12] years; P < 0.001). We found BD-IPMN was present in the majority (n = 7), MD-IPMN in 3 patients, and MT-IPMN in 2 patients. Of genotyping available (n = 4), all cases were PKD1; ADPKD was confirmed with family history and imaging for 5 and radiologic criteria alone for 3 patients. Seven patients were kidney transplant recipients. Eleven of the 12 IPMN cases were observed for a mean (range) period of 61 (12–124) months, in which time 5 patients were alive without IPMN-related complications, 2 underwent the Whipple pancreatectomy procedure, 3 died of complications unrelated to IPMN, and 1 who had MPD-IPMN died of IPMN-related complications after declining surgical intervention (Fig. 3). No patient with BD-IPMN required surgical intervention or succumbed to IPMN-related disease. Figure 3 and Supplemental Figures 2–9 show computed tomography, MRI, or endoscopic evidence in each case.
TABLE 3.
Characteristics of Pancreatic IPMN Cases
| Pancreatic Cyst | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case No. |
Age at Diagnosis, y |
Type of IPMN |
Clinical Characteristics | No. | Size, mm | Location | MPD Dilatation, mm | EUS/ERCP | CEA, ng/mL |
Change in Cysts/ Progression |
Current Status | Follow-up, mo |
Image |
| 1 | 69* | BD | Incidental finding, asymptomatic | 7 | 30 | Head | 3 | No | NA | NA | No follow-up since initial imaging | NA | Figure 3A |
| 2 | 58†‡ | BD | Incidental finding, asymptomatic Transplant |
2 | 20 | Head and tail | 3 | EUS—clear communication seen with MPD | NA | Increase | Alive—no complications | 124 | Figure 3B |
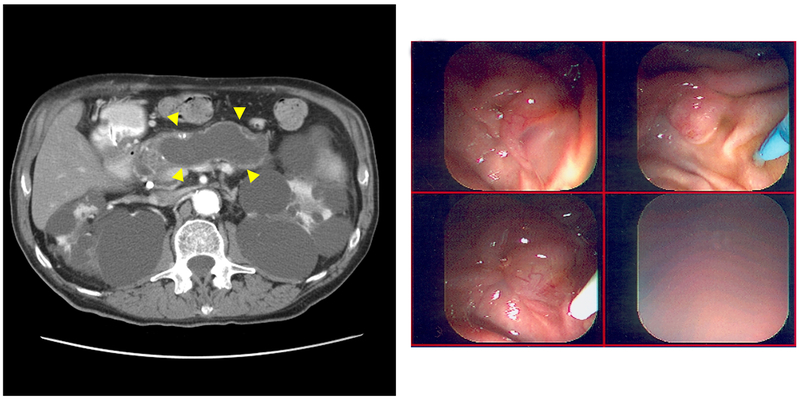
| 3 | 72§ | MD | Symptomatic, abdominal pain | NA | NA | NA | >10 | ERCP—thick mucus from widely patent MPD | NA | Increase | Deceased—IPMN-related SBO | 56 | Figure 3C and 3D |
| 4 | 60†‡ | BD | Incidental finding during biliary sepsis, asymptomatic | 8 | 5 | Head, body, and tail | 3 | No | NA | No change | Alive—no complications | 51 | Supplemental Figure 2 |
| 5 | 72† | BD | Incidental finding, asymptomatic | 2 | 11 | Body and tail | 3 | No | NA | Minimal increase | Alive—no complications | 27 | Supplemental Figure 3 |
| 6 | 47† | BD | Incidental finding, asymptomatic | 1 | 11 | Head | 2 | No | NA | No change | Alive—no complications | 12 | Supplemental Figure 4 |
| 7 | 70§ | BD | Incidental finding during biliary sepsis Transplant |
3 | 14 | Head and body | 3 | EUS—communi-cation with MPD | 315 | Decrease | Alive—no complications | 33 | Supplemental Figure 5 |
| 8 | 61* | BD | Incidental finding, asymptomatic Transplant |
1 | 21 | Head | 2 | EUS—irregular MPD with dilated branch ducts in tail | 2,400 | Increase | Deceased—unrelated to IPMN | 88 | Supplemental Figure 6 |
| 9 | 66* | MPD | Pancreatitis Transplant |
No image available pre-subtotal pancreatectomy | ERCP—outside hospital | NA | Resected | NA—total pancreatectomy | NA | NA | |||
| 10 | 75§ | MPD | Incidental finding, asymptomatic | 3 | 23 | Head and tail | 2 | EUS—clear communication with MPD | NA | NA | Deceased—unrelated to IPMN | 20 | Supplemental Figure 7 |
| 11 | 60* | MT | Abnormal LFTs Transplant |
1 | 40 | Head | 5 | ERCP—dilated side branches with mucus extruding from major and minor papilla | 1.9 | No change | Deceased—unrelated to IPMN | 112 | Supplemental Figure 8 |
| 12 | 64* | MT | Transplant | Numerous | 28 | Head and body | 8 | EUS—upstream pancreatic duct dilation, smaller cysts in pancreas tail | 40 | Increase | Alive—proceeded to Whipple procedure at outside hospital; severe dysplasia on histologic evaluation | NA | Supplemental Figure 9 |
Cysts and family history.
PKD1 mutation identified.
Patient in the ADPKD MRI cohort.
Cysts only as per Pei criteria.
BD indicates branch duct; CEA, carcinoembryonic antigen; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasonography; IPMN, intraductal papillary mucinous neoplasm; LFT, liver function test; MD, main duct; MT, mixed type; NA, not available; SBO, small-bowel obstruction.
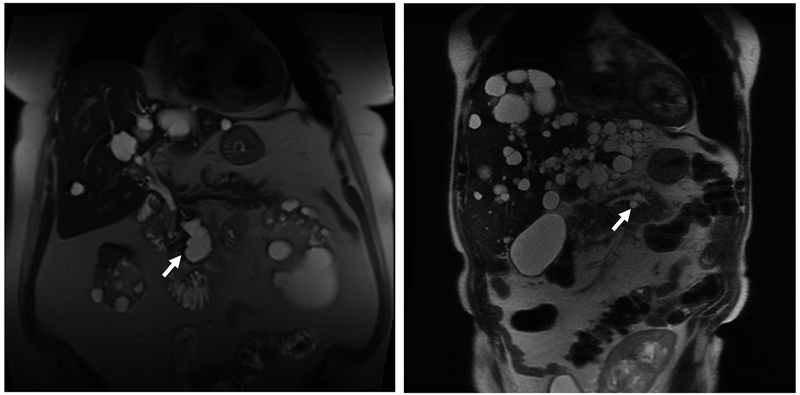
FIGURE 3.
Magnetic Resonance Imaging (MRI) Scans of Branch Duct (BD) Intraductal Papillary Mucinous Neoplasm (IPMN). A, Axial T2-weighted MRI showing a BD-IPMN (arrow) of a 69-year-old woman (case 1) with autosomal dominant polycystic kidney disease (ADPKD). Seven cysts were noted in the head of the pancreas (maximum diameter, 30 mm). The main pancreatic duct (MPD) measured 3 mm. The patient has not had follow-up imaging at this institution to evaluate any increase in size of BD-IPMN. B, Coronal single-shot fast-spin echocardiography (SSFSE) MRI of 58-year-old patient with PKD1 (case 2) with polycystic liver and bilateral nephrectomies with BD-IPMN based on endoscopic ultrasonography showing clear communication (arrow), seen with 3-mm MPD. The patient had 2 pancreatic cystic lesions (maximum diameter, 20 mm) that increased in size over 124 months but has had no indication for surgical intervention to date. C, A 72-year-old man (case 3) with ADPKD diagnosed with MPD-IPMN on presentation with abdominal pain. Abdominal computed tomography shows grossly dilated MPD (arrowheads on sagittal section) and polycystic kidneys. D, On endoscopic retrograde cholangiopancreatography, major and minor papillae were identified easily by the profuse thick mucus coming through widely patent orifices from an irregular and dilated pancreatic duct. The patient declined surgical management and succumbed to bowel obstruction secondary to the mucus at 56 months after diagnosis.
DISCUSSION
Nephrologists encounter pancreatic lesions in ADPKD patients, given widespread use of imaging, improved scan resolution, and patient survival.28 Prevalence of PCLs using MRI was 19%, higher than for non-ADPKD cohort. This is higher than previously reported using ultrasonography but lower than a recent MRI study that also found that PCLs occurred more frequently in PKD2 cases.13 Most PCLs were solitary. We found a nonsignificant tendency to greater PCL prevalence and more numerous PCLs in PKD2 patients, who comprised a smaller proportion of our cohort. Our findings reflect the population prevalence of individuals with ADPKD (~15%). Our cohort was younger and reflective of more PKD1 cases—PKD1 manifests earlier than PKD2—and with more women than men represented.
Kim et al15 observed that 3 of 40 ADPKD patients with PCLs (all asymptomatic) had MRI findings suggestive of IPMN, of which 1 underwent endoscopic ultrasonography. Several other reports describe IPMNs in ADPKD.20,21,29 Intraductal papillary mucinous neoplasias—grossly and radiographically visible epithelial tumors30—are classified into 3 types based on imaging or histology (with special sectioning), depending on the caliber of ducts involved: MD-IPMN, BD-IPMN, and a combined type involving both MPDs and BDs (MT-IPMN)31 The BD-IPMN is more likely to involve the uncinate or pancreatic tail with less likelihood of malignant transformation.16,17
In ADPKD, IPMNs may be found during assessment of kidney and liver disease, infection, or transplant. Investigation and management are not easy. We identified 12 IPMN cases, mostly BD-IPMN (noted incidentally), who underwent serial imaging without development of worrisome characteristics. Two were referred for IPMN treatment at our institution. One death was related to MD-IPMN of a patient who declined surgery; only MD- or MT-IPMN cases required intervention.
The presence of IPMN provides a management dilemma for asymptomatic persons because further investigations and therapy can be hampered by the altered anatomy, comorbidities, and the need to avoid contrast imaging because of increased risk of acute renal failure or inability to receive gadolinium.32,33 Some may be receiving immunosuppression for organ transplant (reflected in our series), adding to management complexity. In a series of 62 ADPKD patients who received donor grafts, 5 (8%) had malignancy, including 1 with pancreatic cancer, and malignancy was the main cause of death and graft failure.34 In another study, about 30% of IPMN cases had metachronous tumors.35
Nevertheless, IPMN can have a favorable prognosis (postoperative 5-year survival, approximately 100% for benign tumors and noninvasive carcinoma and approximately 60% for invasive carcinoma).35 Differences in cancer progression between MD-IPMN and BD-IPMN range from 57% to 92% and 6% to 46%, respectively. Therefore, correct sub-classification is important.31 Depending on radiologic and clinical features, surgical or conservative management may be offered.36,37 Predictive factors of malignancy include mural nodules and MPD dilatation (≥7 mm).16–18,38 Pancreatoduodenectomy and distal or total pancreatectomy performed in high-volume medical centers carries a mortality rate <5%.39 Surgical risk is greater for older patients with renal insufficiency and/or transplant recipients.
This study has limitations—single center, possible selection bias for more severe cases, and controls matched for age and sex but not GFR. The role of GFR is unclear regarding PCL development. Given that this group was younger, matching for age, sex, and GFR may have resulted in a nonrepresentative control group for assessment of prevalence of PCL, which typically involves a higher prevalence due to an older age group. Not all IPMN cases underwent genotyping. Intraductal papillary mucinous neoplasias may have been underestimated in small PCLs.
We observed higher PCL prevalence for ADPKD patients than controls and report characteristics of IPMN cases with ADPKD followed approximately 10 years. In the conservatively managed cases, none had IPMN-related complications, and most did not require operative intervention. We highlight the need for multidisciplinary management involving nephrologists, gastroenterologists, surgeons, and radiologists to ensure the best outcome when PCLs are identified in ADPKD patients.
Supplementary Material
Financial Support
Grant Support: Research support for this project was from Mayo Clinic Robert M. and Billie Kelley Pirnie Translational Polycystic Kidney Disease (PKD) Center, through a grant from the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (Award P30 DK090728). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- ACE
Advanced Cohort Explorer
- ADPKD
autosomal dominant polycystic kidney disease
- BD
branch duct
- BD-IPMN
branch duct intraductal papillary mucinous neoplasm
- CEA
carcinoembryonic antigen
- ERCP
endoscopic retrograde cholangiopancreatography
- GFR
glomerular filtration rate
- HALT
Halt Progression of Polycystic Kidney Disease
- IPMN
intraductal papillary mucinous neoplasm
- IRB
institutional review board
- MD-IPMN
main duct intraductal papillary mucinous neoplasm
- MPD
main pancreatic duct
- MRI
magnetic resonance imaging
- MT-IPMN
mixed type intraductal papillary mucinous neoplasm
- PCL
pancreatic cyst lesion
- PKD
polycystic kidney disease
Footnotes
Potential Competing Interest
All authors have no financial disclosure to declare.
REFERENCES
- 1.Ravine D, Gibson RN, Walker RG, et al. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343:824–827. [DOI] [PubMed] [Google Scholar]
- 2.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. [DOI] [PubMed] [Google Scholar]
- 3.Alpern MB, Dorfman RE, Gross BH, et al. Seminal vesicle cysts: association with adult polycystic kidney disease. Radiology. 1991;180:79–80. [DOI] [PubMed] [Google Scholar]
- 4.Gabow PA, Johnson AM, Kaehny WD, et al. Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. Hepatology. 1990;11:1033–1037. [DOI] [PubMed] [Google Scholar]
- 5.Chang YR, Park JK, Jang JY, et al. Incidental pancreatic cystic neoplasms in an asymptomatic healthy population of 21,745 individuals: Large-scale, single-center cohort study. Medicine (Baltimore). 2016;95:e5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee KS, Sekhar A, Rofsky NM, et al. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079–2084. [DOI] [PubMed] [Google Scholar]
- 8.de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806–811. [DOI] [PubMed] [Google Scholar]
- 9.Scheiman JM. Pancreatic Cysts - Part 1: Using the American Gastroenterological Association Guidelines for the Management of Pancreatic Cysts-A Practical Approach. Pancreas. 2017;46:742–744. [DOI] [PubMed] [Google Scholar]
- 10.Wu BU, Sampath K, Berberian CE, et al. Prediction of malignancy in cystic neoplasms of the pancreas: a population-based cohort study. Am J Gastroenterol. 2014;109:121–129; quiz 130. [DOI] [PubMed] [Google Scholar]
- 11.Boucher C, Sandford R. Autosomal dominant polycystic kidney disease (ADPKD, MIM 173900, PKD1 and PKD2 genes, protein products known as polycystin-1 and polycystin-2). Eur J Hum Genet. 2004;12:347–354. [DOI] [PubMed] [Google Scholar]
- 12.Boulter C, Mulroy S, Webb S, et al. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc Natl Acad Sci U S A. 2001;98:12174–12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torra R, Nicolau C, Badenas C, et al. Ultrasonographic study of pancreatic cysts in autosomal dominant polycystic kidney disease. Clin Nephrol. 1997;47:19–22. [PubMed] [Google Scholar]
- 14.Hogan MC, Abebe K, Torres VE, et al. Liver involvement in early autosomal-dominant polycystic kidney disease. Clin Gastroenterol Hepatol. 2015;13:155–164 e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JA, Blumenfeld JD, Chhabra S, et al. Pancreatic Cysts in Autosomal Dominant Polycystic Kidney Disease: Prevalence and Association with PKD2 Gene Mutations. Radiology. 2016;280:762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crippa S, Bassi C, Salvia R, et al. Low progression of intraductal papillary mucinous neoplasms with worrisome features and high-risk stigmata undergoing non-operative management: a mid-term follow-up analysis. Gut. 2017;66:495–506. [DOI] [PubMed] [Google Scholar]
- 17.Lawson RD, Hunt GC, Giap AQ, et al. Pancreatic cysts suspected to be branch duct intraductal papillary mucinous neoplasm without concerning features have low risk for development of pancreatic cancer. Ann Gastroenterol. 2015;28:487–494. [PMC free article] [PubMed] [Google Scholar]
- 18.Mukewar S, de Pretis N, Aryal-Khanal A, et al. Fukuoka criteria accurately predict risk for adverse outcomes during follow-up of pancreatic cysts presumed to be intraductal papillary mucinous neoplasms. Gut. 2017;66:1811–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka M International consensus on the management of intraductal papillary mucinous neoplasm of the pancreas. Ann Transl Med. 2015;3:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basar O, Ibis M, Ucar E, et al. Recurrent pancreatitis in a patient with autosomal-dominant polycystic kidney disease. Pancreatology. 2006;6:160–162. [DOI] [PubMed] [Google Scholar]
- 21.Sato Y, Mukai M, Sasaki M, et al. Intraductal papillary-mucinous neoplasm of the pancreas associated with polycystic liver and kidney disease. Pathol Int. 2009;59:201–204. [DOI] [PubMed] [Google Scholar]
- 22.Silverman JF, Prichard J, Regueiro MD. Fine needle aspiration cytology of a pancreatic cyst in a patient with autosomal dominant polycystic kidney disease. A case report. Acta Cytol. 2001;45:415–419. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pei Y, Hwang YH, Conklin J, et al. Imaging-based diagnosis of autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2015;26:746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. [DOI] [PubMed] [Google Scholar]
- 26.Porath B, Gainullin VG, Cornec-Le Gall E, et al. Mutations in GANAB, Encoding the Glucosidase IIα Subunit, Cause Autosomal-Dominant Polycystic Kidney and Liver Disease. Am J Hum Genet. 2016;98:1193–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu W, Peissel B, Babakhanlou H, et al. Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat Genet. 1997;17:179–181. [DOI] [PubMed] [Google Scholar]
- 28.Spithoven EM, Kramer A, Meijer E, et al. Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: prevalence and survival--an analysis of data from the ERA-EDTA Registry. Nephrol Dial Transplant. 2014;29 Suppl 4:iv15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naitoh H, Shoji H, Ishikawa I, et al. Intraductal papillary mucinous tumor of the pancreas associated with autosomal dominant polycystic kidney disease. J Gastrointest Surg. 2005;9:843–845. [DOI] [PubMed] [Google Scholar]
- 30.Reid-Lombardo KM, St Sauver J, Li Z, et al. Incidence, prevalence, and management of intraductal papillary mucinous neoplasm in Olmsted County, Minnesota, 1984–2005: a population study. Pancreas. 2008;37:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adsay V, Mino-Kenudson M, Furukawa T, et al. Pathologic Evaluation and Reporting of Intraductal Papillary Mucinous Neoplasms of the Pancreas and Other Tumoral Intraepithelial Neoplasms of Pancreatobiliary Tract: Recommendations of Verona Consensus Meeting. Ann Surg. 2016;263:162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonatti H, Huguet K, McLaughlin S, et al. Whipple’s procedure in a renal transplant recipient with polycystic liver disease. JOP. 2008;9:515–519. [PubMed] [Google Scholar]
- 33.Kinsella SM, Coyle JP, Long EB, et al. Maintenance hemodialysis patients have high cumulative radiation exposure. Kidney Int. 2010;78:789–793. [DOI] [PubMed] [Google Scholar]
- 34.Errasti P, Manrique J, Lavilla J, et al. Autosomal-dominant polycystic kidney disease: high prevalence of graft loss for death-related malignancies and cardiovascular risk factors. Transplant Proc. 2003;35:1717–1719. [DOI] [PubMed] [Google Scholar]
- 35.Yoon WJ, Ryu JK, Lee JK, et al. Extrapancreatic malignancies in patients with intraductal papillary mucinous neoplasm of the pancreas: prevalence, associated factors, and comparison with patients with other pancreatic cystic neoplasms. Ann Surg Oncol. 2008;15:3193–3198. [DOI] [PubMed] [Google Scholar]
- 36.Kobari M, Egawa S, Shibuya K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg. 1999;134:1131–1136. [DOI] [PubMed] [Google Scholar]
- 37.Fong ZV, Fernandez-Del Castillo C. Intraductal Papillary Mucinous Neoplasm of the Pancreas. Surg Clin North Am. 2016;96:1431–1445. [DOI] [PubMed] [Google Scholar]
- 38.Sugiyama M, Suzuki Y, Abe N, et al. Management of intraductal papillary mucinous neoplasm of the pancreas. J Gastroenterol. 2008;43:181–185. [DOI] [PubMed] [Google Scholar]
- 39.Cameron JL, Riall TS, Coleman J, et al. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.