Significance
Death, one of the most important events for all organisms, is thought to derive from a variety of lethal assaults. The discovery of a general, reactive oxygen species (ROS)-dependent mechanism that contributes to killing by diverse stressors was surprising. The finding that ROS levels continue to surge and kill bacteria even after removal of the initiating stressor adds a new dimension to stress-mediated lethality, demonstrating that the ROS surge is self-amplifying. Once an ROS threshold is exceeded, the death process becomes self-driven (i.e., self-sustainable). In addition to providing a new way to think about the response of bacterial populations to lethal stress, our findings encourage efforts to target oxidative stress pathways as adjuncts of antimicrobial therapy that will increase lethality and help restrict the emergence of resistance.
Keywords: reactive oxygen species, poststress cellular response, antimicrobial, antioxidant, damage repair
Abstract
Antimicrobial efficacy, which is central to many aspects of medicine, is being rapidly eroded by bacterial resistance. Since new resistance can be induced by antimicrobial action, highly lethal agents that rapidly reduce bacterial burden during infection should help restrict the emergence of resistance. To improve lethal activity, recent work has focused on toxic reactive oxygen species (ROS) as part of the bactericidal activity of diverse antimicrobials. We report that when Escherichia coli was subjected to antimicrobial stress and the stressor was subsequently removed, both ROS accumulation and cell death continued to occur. Blocking ROS accumulation by exogenous mitigating agents slowed or inhibited poststressor death. Similar results were obtained with a temperature-sensitive mutational inhibition of DNA replication. Thus, bacteria exposed to lethal stressors may not die during treatment, as has long been thought; instead, death can occur after plating on drug-free agar due to poststress ROS-mediated toxicity. Examples are described in which (i) primary stress-mediated damage was insufficient to kill bacteria due to repair; (ii) ROS overcame repair (i.e., protection from anti-ROS agents was reduced by repair deficiencies); and (iii) killing was reduced by anti-oxidative stress genes acting before stress exposure. Enzymatic suppression of poststress ROS-mediated lethality by exogenous catalase supports a causal rather than a coincidental role for ROS in stress-mediated lethality, thereby countering challenges to ROS involvement in antimicrobial killing. We conclude that for a variety of stressors, lethal action derives, at least in part, from stimulation of a self-amplifying accumulation of ROS that overwhelms the repair of primary damage.
Discovering ways to manage antimicrobial resistance is among the most important medical challenges of our time (1). Since many antimicrobials can stimulate the production of resistant mutants, often via the SOS response (2–8), one way to limit the emergence of new resistance is to more rapidly and extensively reduce pathogen populations during infection. Toward that end, we and others have been studying how antimicrobials and other lethal stressors kill bacteria. Recent work has drawn attention to the contribution of stress-stimulated accumulation of toxic reactive oxygen species (ROS) (9–21). Finding ways to stimulate ROS-mediated killing could in principle enhance the efficacy of a broad range of antimicrobials. However, an ROS contribution to antimicrobial killing became controversial when the original observation (9) was challenged (22–24). Subsequent work countered many of the challenges (13–15, 25) and extended the phenomenon to thymineless death (26), phage infection (27), the type VI secretion system (27), and overexpression of a MalE-LacZ fusion (28). Moreover, nitric oxide and hydrogen sulfide interfere with antimicrobial killing by suppressing ROS generation/accumulation (17–19). Among the questions to be addressed are whether ROS accumulation is a cause or a consequence of cell death, and whether intracellular ROS levels are sufficient to kill bacteria once the original inducing stressor is removed, that is, whether ROS accumulation can be self-sustaining or even self-amplifying.
ROS, which are commonly considered to include superoxide, hydrogen peroxide, and hydroxyl radical, cause several types of intracellular damage. For example, hydroxyl radical breaks DNA, peroxidates lipids, and carbonylates proteins (14). ROS also oxidize the dGTP and dCTP pools, causing misincorporation of bases into DNA that leads to double-stranded breaks (DSBs) in DNA through disrupted repair intermediates (11, 29, 30). In principle, ROS-mediated damage, which is secondary to the primary stress-induced lesion, could stimulate additional rounds of ROS accumulation, thereby making ROS accumulation a self-amplifying, unstoppable process that could be the terminal step in a bacterial response to lethal stress. Whether endogenous ROS are sufficient to kill cells is unclear, because the lethal effect from the primary stressor and that from subsequent ROS accumulation have not been separated. Indeed, it has been suggested that endogenous ROS concentrations are unlikely to reach lethal levels (24).
In the present work, we devised a way to separate lethality due to a primary stressor from that triggered by subsequent intracellular ROS accumulation. In this assay, we treated cultured Escherichia coli with a lethal stressor, removed the stressor, and plated the bacteria on stressor-free agar containing an agent known to suppress the accumulation of toxic ROS. We found that ROS-mitigating agents, present in or on agar, interfered with killing by diverse lethal stressors, even after stressor removal. In these cases, ROS is a cause, not a consequence, of stress-induced cell death. In addition, we report a case in which primary damage to DNA was insufficient to cause cell death; accumulation of toxic ROS is required unless cells contain a deficiency in primary damage repair. The work also reveals a misconception concerning the traditional antimicrobial killing assay in which bacteria are thought to be dead at the time of posttreatment plating; they can actually still be alive, dying on drug-free agar plates during poststress recovery due to ROS accumulation. Overall, our findings provide insight into antimicrobial lethality and support efforts to find broad-spectrum ROS-related enhancers of antimicrobial action.
Results
Poststress Cell Death Associated with Antimicrobial Stress.
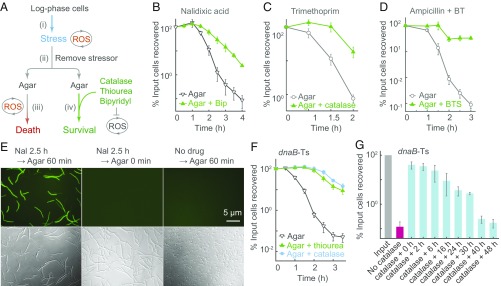
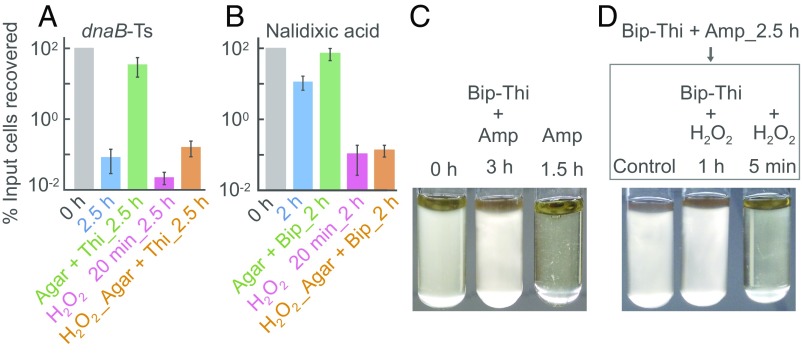
To separate lethality due to a primary stressor from that due to the accumulation of ROS, we examined ROS-mediated death occurring after removal of the primary stressor (Fig. 1A). For example, we treated E. coli cultures with nalidixic acid [∼1 minimum inhibitory concentration (MIC); SI Appendix, Table S1], washed cells to remove the drug, and then plated cells on agar containing a subinhibitory concentration (0.5 MIC) of bipyridyl to halt the production and accumulation of hydroxyl radical. The presence of bipyridyl in agar raised survival by ∼30-fold (Fig. 1B), indicating that ROS are responsible for death (SI Appendix, Fig. S1 A‒G). Residual nalidixic acid is unlikely to contribute to the ROS-mediated killing on agar, because quinolone-gyrase-DNA complexes are reversible, and washing cells to remove quinolone fully restores DNA synthesis blocked by formation of the complexes (31–33); if the complexes are rendered irreversible by crosslinking of quinolone to gyrase, DNA synthesis is not restored (32). In addition, a mass spectrometry-based assay showed that washing and diluting cells lowered intracellular quinolone concentration to 0.002 times MIC (SI Appendix, Fig. S1H). Since MIC correlates with cleaved-complex formation (34, 35), which results in the primary quinolone-mediated lesion, few complexes are likely to be present at 0.002 MIC. Moreover, no growth inhibition was observed at nalidixic acid concentrations as high as 0.5 MIC (SI Appendix, Fig. S1I). These data are consistent with biochemical studies showing that dilution reverses quinolone-topoisomerase-DNA complex formation (36). Finally, potential growth-inhibitory effects of anti-ROS agents on stressor action are irrelevant, due to stressor removal. We conclude that poststress ROS-mediated toxicity is responsible for lethality due to nalidixic acid treatment.
Fig. 1.
ROS-mediated poststress death (PSD). (A) Assay design. Cultures were stressed, the stressor was removed, and cells were plated on agar containing/lacking agents that interfere with ROS accumulation; then cfu was determined. (B) Nalidixic acid-stimulated PSD. Wild-type strain 3001, treated with drug (1 MIC), was plated on agar containing/lacking bipyridyl (Bip, 0.5 MIC; n = 5). (C) Trimethoprim-stimulated PSD. Wild-type strain (2428), treated with trimethoprim (6 MIC), was plated on agar overlaid (or not) with catalase (n = 4). (D) Ampicillin-stimulated PSD. As in B but with ampicillin (2.5 MIC) in the presence/absence of bipyridyl (0.4 MIC) plus thiourea (0.45 MIC) (BT). Cultures were plated on agar containing/lacking the bipyridyl-thiourea combination plus sucrose (BTS; n = 3). (E) ROS accumulation on agar after removal of nalidixic acid (2.5 h treatment) and pulse-labeled with carboxy-H2DCFDA (20 min) immediately after drug removal. n = 4. (F) Inhibition of dnaB-Ts–stimulated PSD. Strain 2429 was shifted to 42 °C for the indicated times and then plated at 30 °C on agar, agar plus 15 mM (0.15 MIC) thiourea (n = 5), or agar in which spotted samples were overlaid with catalase (200 U; n = 3). The percentage of cells recovered is relative to cfu at the point at which cultures were shifted to 42 °C. (G) Time for PSD completion. dnaB-Ts mutant was incubated at 42 °C for 2.5 h and then plated at 30 °C for the indicated times before catalase was added for 48 h (n = 6). n, number of independent experiments. Data are mean ± SD.
In a similar experiment, E. coli cells were treated with trimethoprim to induce a process similar to thymineless death that is also associated with ROS accumulation (SI Appendix, Fig. S2 A–D) (26, 37). As with nalidixic acid, trimethoprim (6 MIC) was removed before plating by washing and diluting cells. When applied to LB agar, the samples were overlaid with catalase to degrade peroxide and thereby remove a source of hydroxyl radical. Catalase increased survival by 5- to 25-fold (Fig. 1C and SI Appendix, Fig. S2E). Mass spectrometry measurement of intracellular drug concentration indicated that washing/dilution of trimethoprim-treated cells removed most of the drug, lowering the drug concentration to 0.06 MIC (SI Appendix, Fig. S2F). Thus, trimethoprim action is a second example of poststress ROS-mediated death. The protective effect of catalase also provides strong evidence that ROS cause death rather than resulting from it.
We next examined the lethal action of ampicillin, which inhibits cell wall synthesis and can lead to cell lysis under some conditions (38). Previous work showed that bipyridyl or thiourea, added to broth cultures during ampicillin treatment, inhibits killing (9, 10), a finding consistent with increased ROS accumulation during ampicillin treatment (SI Appendix, Fig. S2 G and H). To prevent ROS from accumulating so rapidly that cells are killed in broth culture, an event that would obscure detection of poststress death on agar (see Discussion), we added subinhibitory concentrations of bipyridyl plus thiourea to broth cultures containing ampicillin at 2.5 MIC. Subsequent plating on drug-free agar after removal of ampicillin from cells showed that thiourea plus bipyridyl in agar increased survival by 350-fold (Fig. 1D and SI Appendix, Fig. S2 I and J). Ampicillin was readily removed from cells by the washing/dilution procedure, with mass spectrometry measurements indicating 600- and 20,000-fold lower drug concentrations in cells after a single washing compared with those seen for trimethoprim and nalidixic acid, respectively. After the four 10-fold dilutions used with the poststress killing assay, the level of ampicillin was below the detection limit (a decrease of at least 100-fold relative to cells that were not diluted) (SI Appendix, Fig. S2K).
For the three antimicrobials tested above, we looked for the accumulation of ROS in cells on agar. Immediately after removal of antimicrobial, we pulse-labeled the culture for 20 min with carboxy-H2DCFDA, an ROS-sensitive fluorescent dye, plated the cells, and examined them by fluorescence microscopy. Little fluorescence was evident when cells were examined immediately after labeling with the dye, indicating that little ROS was carried over when cells were plated on agar. However, after incubation on agar for 1 h, a strong ROS signal was seen in cells that had been treated with nalidixic acid, trimethoprim, or ampicillin (Fig. 1E and SI Appendix, Fig. S3). These data indicate that damage due to the action of lethal antimicrobials stimulates a self-amplifying accumulation of ROS that continues after antimicrobial removal.
Poststress Cell Death with a dnaB-Ts Mutant.
To extend the study to a conditional lethal mutation (a nonantimicrobial stress), we examined a temperature-sensitive helicase mutant (dnaB-Ts; SI Appendix, Fig. S4A). An ROS increase was observed as elevated fluorescence of ROS-indicator dyes (carboxyl-H2DCFDA and Peroxy Orange 1) and as increased activity of the ROS-sensitive promoters of oxyS and soxS (13) (SI Appendix, Fig. S4 B–E). As expected, ROS accumulation paralleled a drop in survival (SI Appendix, Fig. S4F). Moreover, a shift to restrictive temperature for 1 h caused a twofold increase in expression of four ROS-responsive detoxifying genes (SI Appendix, Fig. S4G). The addition of a subinhibitory combination of bipyridyl plus thiourea suppressed the ROS accumulation and completely blocked killing (SI Appendix, Fig. S4 H–J). Control experiments showed that this combination of bipyridyl plus thiourea had little effect on growth rate (SI Appendix, Fig. S4K). Moreover, no ROS accumulation was detected in the dnaB-Ts mutant grown at permissive temperature or in wild-type cultures incubated at 42 °C; likewise, no autofluorescence was observed with the mutant at 42 °C (SI Appendix, Fig. S4 L–P). Thus, the temperature shift stimulated lethal events associated with toxic ROS accumulation.
The dnaB-Ts system appeared to be suitable for assaying poststress death following a pulse of incubation at 42 °C, because downshift from 42 °C to permissive temperature (30 °C) allowed recovery of DNA synthesis in 1 h (SI Appendix, Fig. S5A). Since mutant and wild-type cells exhibited the same growth rate at 30 °C, and since the mutant showed similar cfu on agar incubated at 30 °C and 22 °C (SI Appendix, Fig. S5 B and C), the activity of mutant DnaB appears to be normal at permissive temperature, as is required for a downshift to eliminate stress.
When we exposed dnaB-Ts cultures to 42 °C for 2.5 h, followed by immediate chilling and plating at 30 °C, survival was only 0.2% relative to cell density at the time when cultures were shifted to 42 °C (Fig. 1F). However, survival was almost 100% when mutant cells incubated at 42 °C were subsequently plated at 30 °C on agar either containing thiourea or overlaid with catalase and then incubated at 30 °C (Fig. 1F). In control experiments, we found that survival of mutant cells maintained at 30 °C throughout the experiment was unaffected by either thiourea or catalase on agar; likewise, substitution of an unrelated protein (BSA) for catalase had no effect on growth/survival of the mutant incubated at restrictive temperature before plating (SI Appendix, Fig. S5 D–F). The addition of either thiourea or catalase to agar also blocked killing of a dnaB-Ts-ΔkatG hyperlethal double mutant (SI Appendix, Fig. S4H), indicating that the anti-ROS treatments were effective even when the absence of the KatG catalase allowed elevated levels of oxidative stress (20, 26). Overall, poststress ROS action accounts for DnaB-Ts–mediated killing.
Poststress death at 30 °C, stimulated by a 2.5-h treatment of the dnaB-Ts mutant at 42 °C, takes several hours to complete; even after incubation on agar at 30 °C for 2–6 h, the addition of catalase prevented the death of most cells (Fig. 1G). However, when catalase addition was delayed for longer than 40 h, little protection was observed (Fig. 1G). Consequently, by 40 h, the poststress death process was largely complete; catalase could not revive dead cells. These data show that ROS cause death, because if only bacterial growth were blocked on agar or if cells entered a viable but not culturable physiological state, then catalase-mediated rescue would have been independent of the time of catalase addition.
Genes Involved in dnaB-Ts–Triggered Poststress Cell Death.
Since poststress ROS contributes 100% to the death of the dnaB-Ts mutant (Fig. 1F and SI Appendix, Fig. S4H), we used the mutant to search for genes whose deficiency may affect poststress death. A variety of DNA repair mutations showed little effect with the dnaB-Ts mutant (SI Appendix, Fig. S6 A–C), but a deficiency in ahpC/F, which encode a peroxidase (39), almost completely suppressed the killing arising from incubation at restrictive temperature (Fig. 2A). The ahpC deficiency had no effect on culture growth of the dnaB-Ts mutant. An ahpF deficiency showed a protective behavior similar to that seen with ahpC (SI Appendix, Fig. S7 A and B). The results with ahpC and ahpF deficiencies were paradoxical, because reduced ROS detoxification should increase rather than decrease killing.
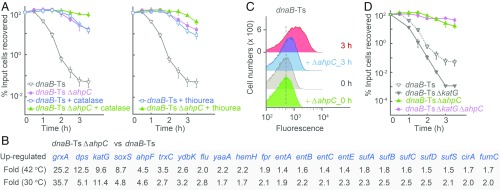
Fig. 2.
Effect of ahpC deficiency on dnaB-Ts–stimulated poststress death. (A) Inhibition of dnaB-Ts–stimulated death. Strains 2429 (dnaB-Ts) and 3780 (dnaB-Ts ΔahpC) were shifted to 42 °C for the indicated times and then plated at 30 °C on agar, agar plus catalase (Left), or agar plus 15 mM (0.15 MIC) thiourea (Right). After incubation, cfu were measured (n = 3). (B) Up-regulation of antioxidant and stress response genes associated with deficiency of ahpC in a dnaB-Ts strain (strains as in A). RNA-seq data are from two independent cultures. (C) Decrease in ROS accumulation associated with ahpC deficiency. Peroxide levels in dnaB-Ts (strain 2429) and dnaB-Ts–ΔahpC (strain 3780) during incubation at 42 °C were measured using Peroxy Orange-1 and flow cytometry. n = 5. (D) Suppression of the hyperlethal phenotype due to a ΔkatG mutation in a dnaB-Ts mutant by an ahpC deficiency (strain 4503). n = 3. n, number of independent replicates. Data are mean ± SD.
To address the ahpC/F paradox, we performed RNA-seq with the dnaB-Ts and dnaB-Ts–ΔahpC mutants (40). The combination of the two mutations elevated the expression of many genes involved in the reduction of oxidative stress; elevation occurred at both permissive and nonpermissive temperatures (Fig. 2B and SI Appendix, Table S2). Elevated expression of protective genes at permissive temperature could enable cells to better tolerate damage from subsequent stress-stimulated ROS increases. Indeed, the ahpC mutation reduced ROS accumulation at restrictive temperature when present in the dnaB-Ts mutant (Fig. 2C); it also eliminated killing in a dnaB-Ts strain that carried a deficiency in katG (Fig. 2D), thereby overcoming the loss of KatG-mediated hyperlethality. Moreover, the dnaB-Ts–ΔahpC mutant grown at permissive temperature exhibited protection from killing by β-lactams and quinolones (SI Appendix, Fig. S7 C and D). Collectively, these observations explain the protective action of the ahpC deficiency, as increased expression of protective genes and support the conclusion that ROS contribute in a general way to antimicrobial lethality.
Contribution of Primary Damage and Damage Repair to Cell Death.
To better understand the roles of ROS and primary damage in stress-mediated killing, we used RecN-YFP–based fluorescence microscopy (26, 41) to monitor the accumulation of DSBs in DNA; both quinolone and ROS are known to generate DNA breaks (11, 26, 28, 30, 42, 43). Treatment with the quinolone nalidixic acid led to rapid accumulation of DSBs; after 1 h treatment, 97% of cells scored positive (Fig. 3A). Agents that suppress ROS accumulation (thiourea plus bipyridyl) failed to block DSB accumulation but inhibited killing (SI Appendix, Fig. S1 E and F). These data indicate that (i) quinolones can generate DSBs without ROS participation and (ii) primary quinolone-mediated DSBs are insufficient to kill cells. Finding examples in which the primary damage (e.g., nalidixic acid-mediated DSBs) fails to correlate with death while ROS accumulation does correlate with death constitutes evidence for a decisive role of ROS in stress-mediated killing.
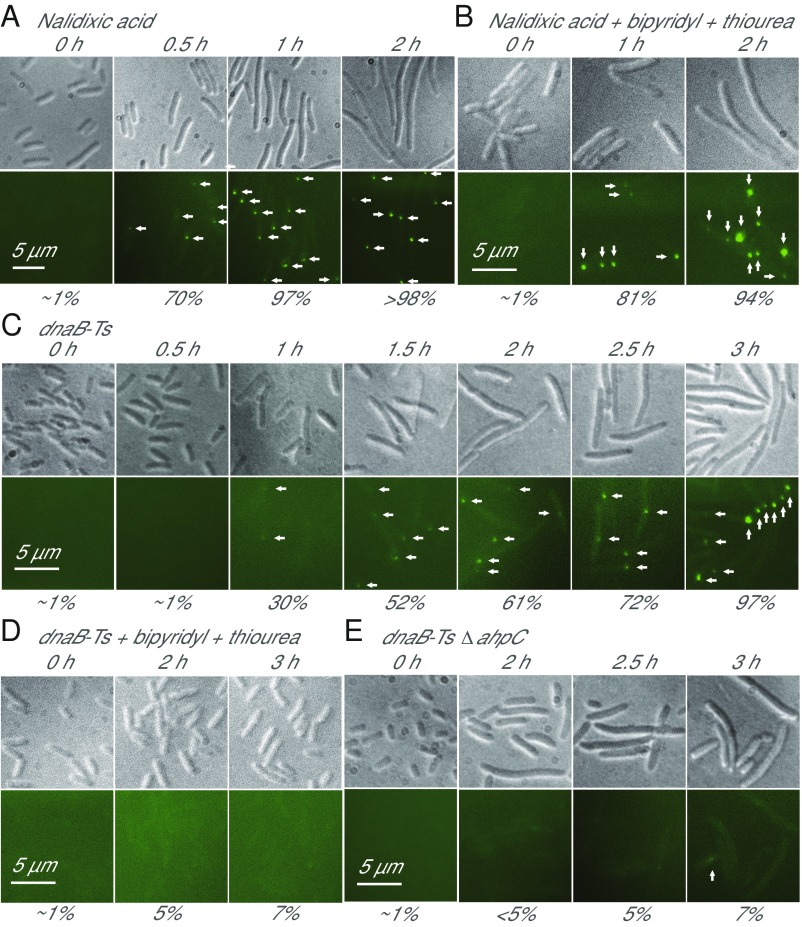
Fig. 3.
Primary and ROS-mediated DNA damage. (A) DNA DSBs due to nalidixic acid (1 MIC, 8 µg/mL). DSBs in strain 4245 were detected by fluorescence microscopy using RecN-YFP fusion. n = 4. (B) Non–ROS-mediated DSBs due to nalidixic acid treatment. As in A, thiourea (0.75 MIC), bipyridyl (0.5 MIC) were added with drug. n = 4. (C) DSB accumulation during 42 °C treatment (for indicated times) of dnaB-Ts strain 2429, detected as in A. n = 6. (D) Chemical suppression of ROS accumulation inhibits generation of DSBs during thermal stress with dnaB-Ts mutant. As in A, thiourea (0.5 MIC) and bipyridyl (0.5 MIC) were added to suppress ROS accumulation. n = 3. (E) ΔahpC inhibits accumulation of DSBs during thermal stress of dnaB-Ts strain 3780 as in A. n = 4. In all panels, values of n indicate the number of independent experiments, arrows indicate DSB fluorescent foci, and numbers below panels indicate the percentage of cells with at least one fluorescent focus. (Upper) Bright field images. (Lower) Fluorescent images.
The behavior of DNA breaks associated with the dnaB-Ts mutant differed from that described for nalidixic acid. When the dnaB-Ts mutant was shifted to 42 °C, DSBs appeared more slowly than seen with nalidixic acid, beginning at 1 h after upshifting and being seen in almost every cell by 3 h after upshifting (Fig. 3C). Moreover, dnaB-Ts–mediated DSBs were not observed when ROS accumulation was suppressed in broth cultures by bipyridyl plus thiourea or when cells contained both the ΔahpC and dnaB-Ts mutations (Figs. 2C and 3 D and E and SI Appendix, Fig. S4I). However, as with nalidixic acid, dnaB-Ts–mediated DSBs were insufficient to kill cells, since 100% of the cells in which 60% contained DSBs were viable at 2 h after the temperature upshift (Figs. 1F and 3C). These data suggest that incubation at nonpermissive temperature leads to ROS-induced DSBs, but that those initial lesions do not kill cells without additional ROS-mediated damage (Figs. 1F and 2A).
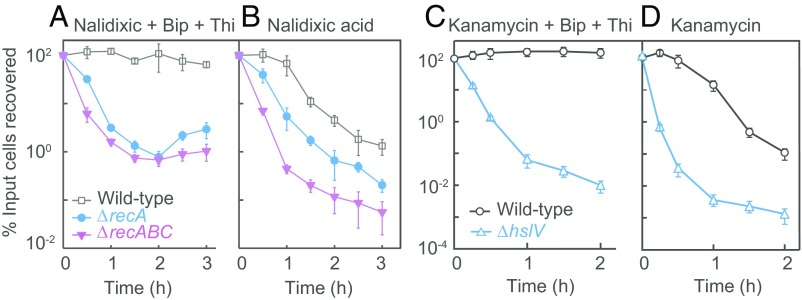
DNA repair is one explanation for the failure of the quinolone-induced DNA DSBs to kill cells when an ROS surge is blocked. With cells harboring a deficiency in recA, treatment with a bipyridyl-thiourea combination failed to fully protect cells from quinolone-mediated killing (Fig. 4 A and B and SI Appendix, Fig. S6 D and E), in contrast to the full protection seen with wild-type cells. Moreover, a combination of recA and recBC deficiencies further reduced protection by a bipyridyl-thiourea combination. These observations indicate that successive reduction in DNA repair capacity may incrementally lower the requirement for ROS to execute quinolone-mediated death. Examination of mutants deficient in repair of protein damage indicated that for kanamycin, unrepaired primary damage also reduces the contribution of ROS to death (Fig. 4 C and D). Thus, in repair-proficient strains, death may derive from ROS overwhelming repair.
Fig. 4.
Deficiency in primary damage repair reduces dependence on ROS for killing by quinolones and aminoglycosides. (A) Reduced dependence on ROS for killing by nalidixic acid in recA and recABC DNA damage repair mutants. Midlog phase cultures of wild-type strain (strain 10), recA-13 (strain 14), and recA-13 recB-21 recC-22 (strain 156) were treated with bipyridyl (Bip, 0.5 MIC) plus thiourea (Thi, 0.5 MIC) and nalidixic acid (10 MIC) for the indicated times before dilution, plating, and determination of cfu. (B) Killing by nalidixic acid increased by rec mutations. Conditions and strains were as in A, except that bipyridyl and thiourea were omitted. (C) Reduced dependence on ROS for killing by kanamycin with the hslV protein damage repair mutant. Midlog phase cultures of wild-type strain (3001) and ΔhslV (strain 3325) were treated with kanamycin (1.1 MIC) in the presence of bipyridyl (0.5 MIC) plus thiourea (0.5 MIC) for the indicated times before dilution, plating, and determination of cfu. (D) Killing by kanamycin increased by protein damage repair mutation. Strains and conditions were as in C, except that bipyridyl and thiourea were omitted. In A and C, the addition of antioxidants had no effect on antimicrobial MIC. In all panels, n = 3. Data are mean ± SD.
To assess the nature of ROS-mediated secondary damage during stress, we treated E. coli cultures with oxolinic acid, which, like nalidixic acid, is a first-generation quinolone that generates DNA lesions (34) as primary damage. We then measured protein carbonylation and lipid peroxidation, neither of which was expected to be a direct effect of the drug (e.g., ROS-mediated secondary damage), and both were increased unless thiourea and bipyridyl were present (SI Appendix, Fig. S8 A–C). Likewise, kanamycin, which creates mistranslated/misfolded proteins (44) as primary damage, caused lipid peroxidation; polymyxin, which primarily acts on lipids (45), produced protein carbonylation, and ampicillin, which primarily blocks wall synthesis (38), created ROS-dependent DNA damage (SI Appendix, Fig. S8 D‒G). In principle, such secondary damage can stimulate additional rounds of ROS production that generate further (tertiary) damage. As expected, treatment of cells with oxolinic acid generated more lipid peroxidation in a protein repair mutant than in wild-type cells (SI Appendix, Fig. S8C). These data support the conclusion that the ROS surge can be self-amplifying; successive rounds of ROS attack occur and overwhelm cellular repair with massive secondary and tertiary damage.
Exogenous Hydrogen Peroxide Stimulates Cell Death During Stress.
To examine the converse of suppressing ROS accumulation with antioxidants, we monitored the effect of hydrogen peroxide added to cells also stressed by other agents. We first determined a sublethal dose (1.5 mM, which in a 3-h treatment did not kill otherwise unstressed cells; SI Appendix, Fig. S9 A and B). We next added sublethal peroxide for 20 min either (i) after shifting the dnaB-Ts mutant to 42 °C for 2.5 h and then to 30 °C or (ii) after a 2-h treatment with nalidixic acid (∼1 MIC). With both stressors, exogenous peroxide added as a sublethal dose only to broth cultures eliminated protection by bipyridyl subsequently added to agar following removal of the stressor (Fig. 5 A and B). Exogenous hydrogen peroxide also contributed to ampicillin-mediated death. When bipyridyl and thiourea were added to broth cultures, they inhibited cell lysis (Fig. 5C); subsequent addition of sublethal (1.5 mM) hydrogen peroxide rapidly lysed otherwise intact cells (Fig. 5D). High concentrations of peroxide alone also lysed cells (SI Appendix, Fig. S9C). These data suggest that the sublethal peroxide kills E. coli by adding to the ROS stimulated by ampicillin. As a control, we found that addition of a sublethal level of peroxide to cultures stressed with a bacteriostatic agent (chloramphenicol) showed little killing (SI Appendix, Fig. S9D).
Fig. 5.
ROS-mediated exacerbation of stress-stimulated death. (A) Exogenous peroxide-stimulated killing of dnaB-Ts mutant. Strain 2429 was shifted to 42 °C for 130 min and then treated for 20 min with 1.5 mM H2O2, a sublethal dose (SI Appendix, Fig. S9), before shifting to 30 °C and plating on agar lacking/containing thiourea (Thi) at 15 mM (0.15 MIC). n = 4. Data are mean ± SD. (B) Peroxide-stimulated killing by nalidixic acid (8 µg/mL (1 MIC) for 100 min, followed by 20 min of 1.5 mM H2O2). After drug and peroxide treatments, cells were plated on agar lacking/containing bipyridyl (Bip) at 0.5 MIC. n = 5. Data are mean ± SD. (C) Inhibition of ampicillin (2.5 MIC)-mediated lysis of strain 3001 by bipyridyl (0.4 MIC) plus thiourea (0.45 MIC). n = 4. (D) Peroxide-stimulated, ampicillin-mediated cell lysis. Following cotreatment by ampicillin (Amp), bipyridyl and thiourea (concentrations as in C) for 2.5 h, cells were resuspended in fresh medium and then treated with 1.5 mM H2O2 with/without bipyridyl and thiourea for the indicated times (n = 4). n, number of independent replicates; all replicates gave similar results.
Collectively, our peroxide data support a role for ROS in stress-mediated lethality and show that if high levels of ROS are achieved in broth, cells die before stressor removal and plating. Once cells are dead, subsequent reduction in ROS with antioxidants cannot revive them. Thus, ROS present before stressor removal may mask the detection of subsequent ROS-mediated poststress killing on agar (SI Appendix, Fig. S2 I and J).
Discussion
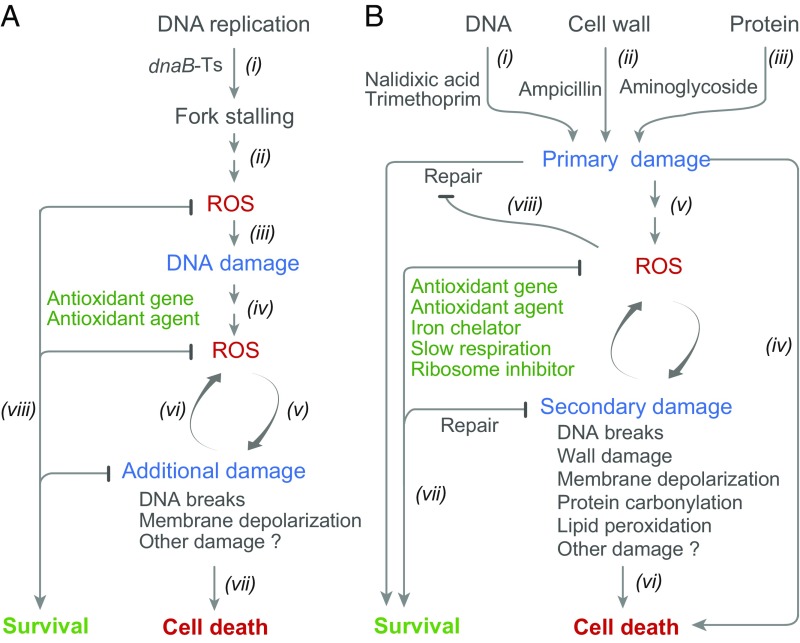
The work described above supports the idea that diverse stressors (nalidixic acid, trimethoprim, ampicillin, and thermal stress with a dnaB-Ts mutant) create potentially lethal primary lesions and also stimulate a self-amplifying accumulation of toxic ROS. The latter can be essential for cell death, as indicated by the lethal action of several diverse stressors being halted or reduced by treatment with anti-ROS agents even after removal of the primary stressor (Fig. 1 B–D, F, and G and SI Appendix, Figs. S1G, S2 E, I, and J, and S4H). As expected, ROS continued to increase inside cells after removal of stressors (Fig. 1E and SI Appendix, Fig. S3). An example was found in which a primary antimicrobial lesion was necessary but insufficient for bacterial death; when cells were treated with nalidixic acid and ROS accumulation was suppressed, death did not occur unless cells were deficient in the repair of quinolone-generated lesions (Figs. 3 A and B and 4 A and B and SI Appendix, Figs. S1 E and F and S6 D and E). A similar phenomenon was observed for kanamycin-mediated killing and hslV-mediated repair of protein damage (Fig. 4 C and D). Since ROS have many toxic effects (46), including DNA breakage (11, 26, 47), membrane depolarization (48) (SI Appendix, Fig. S10), protein carbonylation (49), and cell wall damage (Fig. 5 C and D), ROS-mediated damage likely triggers subsequent rounds of ROS production, leading to even more damage. Accumulated damage eventually overwhelms the capacity of a cell to repair potentially lethal primary lesions and ensures cell death. Our current view of stress-mediated lethality, which includes both direct lesion-based death and ROS-based death, is sketched in Fig. 6.
Fig. 6.
Schemes describing poststress death and relationship to direct killing by stress. (A) dnaB-Ts–mediated poststress death. Inactivation of the DnaB helicase by incubation of the dnaB-Ts mutant at nonpermissive temperature stalls replication forks (i), which stimulates ROS accumulation by unknown mechanisms (ii). ROS generate early DNA damage that is insufficient to directly cause death (iii). However, the DNA damage likely stimulates further ROS accumulation (iv). ROS attack macromolecules, causing secondary damage, such as DNA breakage, protein carbonylation, and membrane depolarization (v). Secondary damage is expected to further elevate levels of ROS (vi). Extensive damage is expected to kill cells (vii). Reduction of ROS, as seen with deficiency of ahpCF or with antioxidants (catalase, thiourea), leads to cell survival (viii). (B) Poststress death and direct killing due to chemical and physical stressors. Soon after application of stress, DNA damaging agents (i), β-lactams (ii), and aminoglycosides (iii) create primary damage. Such damage, if severe or within a primary repair deficiency background, may directly kill cells without the need for ROS (iv). Primary damage, which can be insufficient to kill cells, activates pathways leading to the accumulation of ROS (v), which can then cause secondary damage and more ROS accumulation. When damage is too great to be repaired (vi), it causes death. ROS accumulation can be blocked or reduced in several ways, such as by overexpression of antioxidant genes, the addition of antioxidants or iron chelators, and inhibition of respiration (vii). Such blockage can lead to survival, which is called tolerance. ROS may contribute to killing by overwhelming repair of primary stress damage (viii), since deficiencies in primary damage repair reduce the dependence on ROS to kill cells.
While our data do not rule out small amounts of primary, stressor-specific damage persisting after stressor removal, such damage is likely to be of little consequence once ROS become self-amplifying. In cases where anti-ROS agents blocked killing (Figs. 1, 2, and 5), any persistent primary damage must be insufficient to kill cells. We note that the poststress death assay was performed shortly after the initiation of antimicrobial treatment. If the assay is delayed, many cells die before drug removal and plating, thereby reducing the ability of anti-ROS agents to distinguish ROS-mediated poststress death from overall killing. After long exposure to stressor, survival curves with anti-ROS agents in agar approach those obtained in the absence of the agents (SI Appendix, Figs. S1G and S2E). In such situations, failure to detect ROS-mediated poststress death does not mean that ROS made no contribution to death, as ROS action likely occurred before plating on agar.
We encountered two situations in which little effect of ROS-mitigating agents was observed after washing, diluting, and plating cells. In the case of ampicillin (Fig. 1D), coadministration of bipyridyl and/or thiourea with the antimicrobial inhibits killing (9, 10), indicating that ROS contribute to ampicillin lethality. Killing via ROS apparently occurs rapidly, since observing poststress death required cotreatment of broth cultures with anti-ROS agents (Fig. 1D and SI Appendix, Fig. S2 I and J), presumably to suppress rapid ROS accumulation and death before drug removal and plating. With kanamycin treatment (Fig. 4 C and D), coadministration of bipyridyl and/or thiourea with the antimicrobial inhibits killing (9, 10), indicating that ROS contribute to cell death. Unlike the situation with ampicillin, cotreatment of kanamycin with bipyridyl plus thiourea before plating did not reveal poststress killing (SI Appendix, Fig. S10H). When we measured residual drug by mass spectrometry following washing and dilution of cells, we found that kanamycin was not effectively removed; cell-bound kanamycin decreased by only 50% (SI Appendix, Fig. S11), much less than the degree observed with other antimicrobials (SI Appendix, Figs. S1H and S2 F and K). This carryover of kanamycin precludes measurement of kanamycin-mediated poststress ROS-mediated killing using the washing/dilution method. Overall, our data emphasize that detection of poststress ROS effects requires effective residual drug removal and may require damping of ROS-mediated toxicity before plating.
How initial lesions lead to ROS accumulation is poorly understood. Since many types of stressor-specific damage occur, multiple pathways likely exist. Evidence for distinct processes is seen in a comparison of genes involved in death stimulated by quinolones and thymine starvation (genes participating in downstream events are identified by allelic deficiencies that are protective from lethal stress). With quinolones, such protective deficiencies occur in mazF, lepA, cpx, and arcA (12, 50, 51); in contrast, thymineless death, which also involves ROS accumulation, is not influenced by mutations in these genes (26). Genes involved in iron metabolism, the TCA cycle, and oxidative stress have also been implicated in ROS production and accumulation for a variety of stressors (14, 21). Learning how the effects of diverse types of stressor converge to induce an ROS cascade will require more work.
The existence of a toxic, self-amplifying process implies that protective mechanisms exist for survival in a variety of environments. Indeed, regulons that suppress the effects of oxidative stress have been studied for many years (52). In the present work, we observed a protective effect arising from a deficiency in the ahpCF peroxidase genes in the dnaB-Ts background; the deficiency elevated the expression of many other oxidative stress-mitigating genes at permissive temperature, thereby suppressing ROS-mediated death that would otherwise follow exposure to restrictive temperature or to antimicrobials (Fig. 2 and SI Appendix, Fig. S7 and Table S2). A similar phenomenon likely follows a short treatment of E. coli with hydrogen peroxide (13) or treatment with low concentrations of paraquat or plumbagin (53). The latter stimulators of superoxide production protect from killing by bleomycin (54) and quinolones (53, 55); in contrast, high concentrations are toxic (56, 57). This dichotomy suggests that superoxide is bifunctional: protective at low concentration and destructive at high levels. The protective function likely derives from low to moderate levels of superoxide and hydrogen peroxide inducing the expression of many protective oxidative stress-responsive genes (13).
The existence of a runaway (i.e., self-amplifying) death mechanism raises questions about the potential roles of ROS in developmental processes sometimes termed programmed cell death. ROS amplification could serve as the terminal stage. ROS could also be involved in the association of apoptotic biomarkers with stress-mediated bacterial death (48, 58–61). For biomarkers, a relevant question is whether the presence of a biomarker reflects death derived from a self-sustaining process or from a continuing stimulation of toxic events by the initiating stressor. To address such questions, examining the time course of events is important to establish that the observed signals reflect a death pathway rather than a breakdown product of dead cells.
Finally, the present work addresses challenges to the idea that ROS contribute to killing from diverse antimicrobials (22–24). The ability of ROS-mitigating chemicals and especially of catalase to block death after stressor removal (Fig. 1) provides strong evidence for a causal role of ROS in antimicrobial-mediated killing. It has been argued that potential off-target effects are difficult to rule out for perturbations with chemical agents, such as thiourea and bipyridyl (23, 24); thus, statements of causality are questionable when such agents are used. Off-target effects are unlikely with enzymatic suppression of ROS, as when catalase is added to agar or when a deficiency of katG (catalase) increases killing (20, 26, 28). A causal role for ROS in stress-mediated death has also emerged from the effects of mutations in a variety of other genes, most notably genes involved in the processing of 8-oxoguanine in DNA (reviewed in ref. 30). Whether endogenous ROS levels are high enough to kill bacterial cells (24) has been addressed in part by stressors creating lesions that are hypersensitive to ROS attack (20, 26) and, in the present work, by self-amplification of ROS. We conclude that ROS contribute to the lethal action of many stressor types. A next step is to find adjunctive therapies for antimicrobials to more rapidly cause ROS to become self-amplifying.
Materials and Methods
Detailed descriptions of the construction of E. coli strains, poststress survival assay for dnaB-Ts and antibiotic-treated cells, measurement of ROS and membrane depolarization using flow cytometry, detection of DNA damage using fluorescent microscopy, assay of protein carbonylation and lipid peroxidation, determination of drug concentrations using LC/MS-MS, and RNA-seq methodology are provided in SI Appendix. The strains used in this study are listed in SI Appendix, Table S3.
Supplementary Material
Acknowledgments
We thank Marila Gennaro, Bo Shopsin, and several anonymous reviewers for critical comments; Chao Chen, Yan Pan, and Veronique Dartois for help with the LC/MS-MS analysis; and Sanjay Tyagi for help with microscopy. This work was supported by grants from the US National Institutes of Health (DP2OD007423 and R01 AI073491) and the China National Natural Science Foundation (81661138005 and 81473251).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The RNA sequencing data have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE114262).
See Commentary on page 9696.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1901730116/-/DCSupplemental.
References
- 1.Ventola CL. The antibiotic resistance crisis, part 1: Causes and threats. PT. 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 2.Malik M, Hoatam G, Chavda K, Kerns RJ, Drlica K. Novel approach for comparing the abilities of quinolones to restrict the emergence of resistant mutants during quinolone exposure. Antimicrob Agents Chemother. 2010;54:149–156. doi: 10.1128/AAC.01035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riesenfeld C, Everett M, Piddock LJ, Hall BG. Adaptive mutations produce resistance to ciprofloxacin. Antimicrob Agents Chemother. 1997;41:2059–2060. doi: 10.1128/aac.41.9.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baym M, et al. Spatiotemporal microbial evolution on antibiotic landscapes. Science. 2016;353:1147–1151. doi: 10.1126/science.aag0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, et al. Resveratrol antagonizes antimicrobial lethality and stimulates recovery of bacterial mutants. PLoS One. 2016;11:e0153023. doi: 10.1371/journal.pone.0153023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKenzie GJ, Harris RS, Lee PL, Rosenberg SM. The SOS response regulates adaptive mutation. Proc Natl Acad Sci USA. 2000;97:6646–6651. doi: 10.1073/pnas.120161797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hastings PJ, Rosenberg SM, Slack A. Antibiotic-induced lateral transfer of antibiotic resistance. Trends Microbiol. 2004;12:401–404. doi: 10.1016/j.tim.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Cirz RT, et al. Inhibition of mutation and combating the evolution of antibiotic resistance. PLoS Biol. 2005;3:e176. doi: 10.1371/journal.pbio.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Zhao X. Contribution of oxidative damage to antimicrobial lethality. Antimicrob Agents Chemother. 2009;53:1395–1402. doi: 10.1128/AAC.01087-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science. 2012;336:315–319. doi: 10.1126/science.1219192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorsey-Oresto A, et al. YihE kinase is a central regulator of programmed cell death in bacteria. Cell Rep. 2013;3:528–537. doi: 10.1016/j.celrep.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwyer DJ, et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc Natl Acad Sci USA. 2014;111:E2100–E2109. doi: 10.1073/pnas.1401876111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dwyer DJ, Collins JJ, Walker GC. Unraveling the physiological complexities of antibiotic lethality. Annu Rev Pharmacol Toxicol. 2015;55:313–332. doi: 10.1146/annurev-pharmtox-010814-124712. [DOI] [PubMed] [Google Scholar]
- 15.Zhao X, Drlica K. Reactive oxygen species and the bacterial response to lethal stress. Curr Opin Microbiol. 2014;21:1–6. doi: 10.1016/j.mib.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Acker H, Coenye T. The role of reactive oxygen species in antibiotic-mediated killing of bacteria. Trends Microbiol. 2017;25:456–466. doi: 10.1016/j.tim.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Mironov A, et al. Mechanism of H2S-mediated protection against oxidative stress in Escherichia coli. Proc Natl Acad Sci USA. 2017;114:6022–6027. doi: 10.1073/pnas.1703576114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shatalin K, Shatalina E, Mironov A, Nudler E. H2S: A universal defense against antibiotics in bacteria. Science. 2011;334:986–990. doi: 10.1126/science.1209855. [DOI] [PubMed] [Google Scholar]
- 19.Gusarov I, Shatalin K, Starodubtseva M, Nudler E. Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics. Science. 2009;325:1380–1384. doi: 10.1126/science.1175439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luan G, Hong Y, Drlica K, Zhao X. Suppression of reactive oxygen species accumulation accounts for paradoxical bacterial survival at high quinolone concentration. Antimicrob Agents Chemother. 2018;62:e01622-17. doi: 10.1128/AAC.01622-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brynildsen MP, Winkler JA, Spina CS, MacDonald IC, Collins JJ. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nat Biotechnol. 2013;31:160–165. doi: 10.1038/nbt.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keren I, Wu Y, Inocencio J, Mulcahy LR, Lewis K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science. 2013;339:1213–1216. doi: 10.1126/science.1232688. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Imlay JA. Cell death from antibiotics without the involvement of reactive oxygen species. Science. 2013;339:1210–1213. doi: 10.1126/science.1232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imlay JA. Diagnosing oxidative stress in bacteria: Not as easy as you might think. Curr Opin Microbiol. 2015;24:124–131. doi: 10.1016/j.mib.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao X, Hong Y, Drlica K. Moving forward with reactive oxygen species involvement in antimicrobial lethality. J Antimicrob Chemother. 2015;70:639–642. doi: 10.1093/jac/dku463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong Y, Li L, Luan G, Drlica K, Zhao X. Contribution of reactive oxygen species to thymineless death in Escherichia coli. Nat Microbiol. 2017;2:1667–1675. doi: 10.1038/s41564-017-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong TG, et al. Generation of reactive oxygen species by lethal attacks from competing microbes. Proc Natl Acad Sci USA. 2015;112:2181–2186. doi: 10.1073/pnas.1425007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi N, et al. Lethality of MalE-LacZ hybrid protein shares mechanistic attributes with oxidative component of antibiotic lethality. Proc Natl Acad Sci USA. 2017;114:9164–9169. doi: 10.1073/pnas.1707466114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan XY, et al. Oxidation of dCTP contributes to antibiotic lethality in stationary-phase mycobacteria. Proc Natl Acad Sci USA. 2018;115:2210–2215. doi: 10.1073/pnas.1719627115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruber CC, Walker GC. Incomplete base excision repair contributes to cell death from antibiotics and other stresses. DNA Repair (Amst) 2018;71:108–117. doi: 10.1016/j.dnarep.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goss WA, Deitz WH, Cook TM. Mechanism of action of nalidixic acid on Escherichia coli, II: Inhibition of deoxyribonucleic acid synthesis. J Bacteriol. 1965;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mustaev A, et al. Fluoroquinolone-gyrase-DNA complexes: Two modes of drug binding. J Biol Chem. 2014;289:12300–12312. doi: 10.1074/jbc.M113.529164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kantor GJ, Deering RA. Effect of nalidixic acid and hydroxyurea on division ability of Escherichia coli fil+ and lon- strains. J Bacteriol. 1968;95:520–530. doi: 10.1128/jb.95.2.520-530.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snyder M, Drlica K. DNA gyrase on the bacterial chromosome: DNA cleavage induced by oxolinic acid. J Mol Biol. 1979;131:287–302. doi: 10.1016/0022-2836(79)90077-9. [DOI] [PubMed] [Google Scholar]
- 35.Chow RT, Dougherty TJ, Fraimow HS, Bellin EY, Miller MH. Association between early inhibition of DNA synthesis and the MICs and MBCs of carboxyquinolone antimicrobial agents for wild-type and mutant [gyrA nfxB(ompF) acrA] Escherichia coli K-12. Antimicrob Agents Chemother. 1988;32:1113–1118. doi: 10.1128/aac.32.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aldred KJ, et al. Role of the water-metal ion bridge in mediating interactions between quinolones and Escherichia coli topoisomerase IV. Biochemistry. 2014;53:5558–5567. doi: 10.1021/bi500682e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giroux X, Su W-L, Bredeche M-F, Matic I. Maladaptive DNA repair is the ultimate contributor to the death of trimethoprim-treated cells under aerobic and anaerobic conditions. Proc Natl Acad Sci USA. 2017;114:11512–11517. doi: 10.1073/pnas.1706236114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao Z, Kahne D, Kishony R. Distinct single-cell morphological dynamics under beta-lactam antibiotics. Mol Cell. 2012;48:705–712. doi: 10.1016/j.molcel.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storz G, et al. An alkyl hydroperoxide reductase induced by oxidative stress in Salmonella typhimurium and Escherichia coli: Genetic characterization and cloning of ahp. J Bacteriol. 1989;171:2049–2055. doi: 10.1128/jb.171.4.2049-2055.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong Y, Zhao X. 2018 RNA-seq analysis of transcriptomes of dnaB-Ts and dnaB-Ts ΔahpC at both permissive and non-permissive temperature. Gene Expression Omnibus. Available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE114262. Deposited May 9, 2018.
- 41.Kidane D, Sanchez H, Alonso JC, Graumann PL. Visualization of DNA double-strand break repair in live bacteria reveals dynamic recruitment of Bacillus subtilis RecF, RecO, and RecN proteins to distinct sites on the nucleoids. Mol Microbiol. 2004;52:1627–1639. doi: 10.1111/j.1365-2958.2004.04102.x. [DOI] [PubMed] [Google Scholar]
- 42.Chen CR, Malik M, Snyder M, Drlica K. DNA gyrase and topoisomerase IV on the bacterial chromosome: Quinolone-induced DNA cleavage. J Mol Biol. 1996;258:627–637. doi: 10.1006/jmbi.1996.0274. [DOI] [PubMed] [Google Scholar]
- 43.Malik M, Zhao X, Drlica K. Lethal fragmentation of bacterial chromosomes mediated by DNA gyrase and quinolones. Mol Microbiol. 2006;61:810–825. doi: 10.1111/j.1365-2958.2006.05275.x. [DOI] [PubMed] [Google Scholar]
- 44.Krause KM, Serio AW, Kane TR, Connolly LE. Aminoglycosides: An overview. Cold Spring Harb Perspect Med. 2016;6:1–18. doi: 10.1101/cshperspect.a027029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poirel L, Jayol A, Nordmann P. Polymyxins: Antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev. 2017;30:557–596. doi: 10.1128/CMR.00064-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi S, Ueda K, Komano T. The effects of metal ions on the DNA damage induced by hydrogen peroxide. Agric Biol Chem. 1990;54:69–76. [PubMed] [Google Scholar]
- 48.Dwyer DJ, Camacho DM, Kohanski MA, Callura JM, Collins JJ. Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Mol Cell. 2012;46:561–572. doi: 10.1016/j.molcel.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 50.Li L, et al. Ribosomal elongation factor 4 promotes cell death associated with lethal stress. MBio. 2014;5:e01708. doi: 10.1128/mBio.01708-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kohanski MA, Dwyer DJ, Wierzbowski J, Cottarel G, Collins JJ. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell. 2008;135:679–690. doi: 10.1016/j.cell.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farr SB, Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991;55:561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mosel M, Li L, Drlica K, Zhao X. Superoxide-mediated protection of Escherichia coli from antimicrobials. Antimicrob Agents Chemother. 2013;57:5755–5759. doi: 10.1128/AAC.00754-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burger RM, Drlica K. Superoxide protects Escherichia coli from bleomycin-mediated lethality. J Inorg Biochem. 2009;103:1273–1277. doi: 10.1016/j.jinorgbio.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu Y, Vulić M, Keren I, Lewis K. Role of oxidative stress in persister tolerance. Antimicrob Agents Chemother. 2012;56:4922–4926. doi: 10.1128/AAC.00921-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hassan HM, Fridovich I. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J Biol Chem. 1978;253:8143–8148. [PubMed] [Google Scholar]
- 57.Farr SB, Natvig DO, Kogoma T. Toxicity and mutagenicity of plumbagin and the induction of a possible new DNA repair pathway in Escherichia coli. J Bacteriol. 1985;164:1309–1316. doi: 10.1128/jb.164.3.1309-1316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bos J, Yakhnina AA, Gitai Z. BapE DNA endonuclease induces an apoptotic-like response to DNA damage in Caulobacter. Proc Natl Acad Sci USA. 2012;109:18096–18101. doi: 10.1073/pnas.1213332109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wadhawan S, Gautam S, Sharma A. Metabolic stress-induced programmed cell death in Xanthomonas. FEMS Microbiol Lett. 2010;312:176–183. doi: 10.1111/j.1574-6968.2010.02114.x. [DOI] [PubMed] [Google Scholar]
- 60.Gautam S, Sharma A. Rapid cell death in Xanthomonas campestris pv. glycines. J Gen Appl Microbiol. 2002;48:67–76. doi: 10.2323/jgam.48.67. [DOI] [PubMed] [Google Scholar]
- 61.Dewachter L, et al. A single amino acid substitution in Obg activates a new programmed cell death pathway in Escherichia coli. MBio. 2015;6:e01935-15. doi: 10.1128/mBio.01935-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.