INTRODUCTION
The medial temporal lobe (MTL) is a region canonically responsible for memory [116], but several pain neuroimaging studies have reported activation within this region. An intriguing intersection of pain and memory comes from the case of patient H.M. who had a high tolerance for heat pain, in addition to global amnesia, following bilateral MTL resection for epilepsy [51]. The MTL region consists of the hippocampus (HC) and subiculum, parahippocampal gyrus (PHG), entorhinal and perirhinal cortices, and amygdala [130]. Activation of various MTL regions is observed in response to nociceptive stimuli of various modalities [66,81,85,113,124,146], and in different tissue types—cutaneous, muscular and visceral [23,61,137]. In particular, several studies have associated MTL activation during experimental pain to emotionally-driven modulatory processes [11,114,119,121]. However, there is little specificity or congruence across studies regarding which MTL subregions are involved in pain, and no specific role has been ascribed to MTL regions in nociceptive processing. Of importance, there has yet to be a formal investigation of the spatial consistency of MTL activations in healthy participants during experimental pain.
In addition to studies in healthy participants, extant evidence indicates aberrant MTL activity in various chronic pain conditions [56,104,108]. Recent evidence in patients with back pain suggests that the transition from subacute to chronic pain may be mediated by MTL structures [134]. Specifically, reduced MTL resting state functional connectivity (rsFC) to other cortical regions [107] and smaller MTL volume [135] predict this transition. However, as with experimental pain, it is not clear which regions within the MTL are implicated in chronic pain and what their role may be.
The overall aim of this study was to determine regions of the MTL consistently involved in nociceptive processing and pain modulation in health and disease. First, we sought to determine which regions of the MTL show consistent spatial activation in response to (1) experimental pain in healthy participants compared to baseline control conditions, and (2) chronic pain patients compared to healthy participants. To that end, we performed two coordinate-based meta-analyses of functional MRI (fMRI) studies of pain that indicated MTL engagement. We expected our first meta-analysis to show consistent MTL activation in the HC and PHG across studies reporting MTL activity. We expected our second meta-analysis to show consistent MTL activation in the HC across chronic pain neuroimaging studies. These analyses are the first formalized investigation of MTL functioning in nociceptive processing.
To generate hypotheses about the mechanistic role of the HC in nociceptive processing from existing data, it is important to explore its network interactions. Thus, we aimed to determine the connectivity of those regions elucidated by our meta-analyses using data from four distinct cohorts of chronic low back pain patients (CBP). Given previous observations of abnormal MTL engagement in CBP, we predicted that CBP would show abnormal rsFC to regions involved in processing the affective dimension of pain, i.e., the anterior insula, midcingulate cortex, amygdala and medial prefrontal cortex [25,31,33,111,134].
METHODS
Our ALE meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2015 guidelines and checklist [103].
Article selection criteria
Our article selection followed the PRISMA flow diagrams [102] as shown in Fig.1. Studies were excluded based on any of the following nine criteria: (1) animal studies; (2) did not use standardized stereotactic (Montreal Neurological Institute (MNI) or Talairach) brain coordinates; (3) case reports; (4) diagnostic or surgical MRI; (5) structural imaging; (6) studies of acute medical pain conditions; (7) functional magnetic resonance imaging (fMRI) studies without a baseline control (for experimental pain studies) or control group (for chronic pain studies); (8) studies not written in the English language; and (9) studies that are not peer-reviewed. As such, we selected human neuroimaging studies with whole-brain and region-of-interest analyses, reporting stereotactic brain coordinates. Three investigators (LA, MG, MM) independently performed the database searches and followed PRISMA guidelines by screening and determining study eligibility. In addition, quality assessment for each article selected was conducted by using a modified version of Downs and Black’s quality assessment score [18,32]. We accounted for statistical significance of the results by qualitatively assigning a maximum score of 2 for articles, which applied multiple comparison corrections to analyses (the Quality Assessment Scoresheet is available in Supplementary).
Fig.1.
PRISMA flow diagram representing the process for article selection.
Database searches
Traditional databases
We conducted a systematic search through the Pubmed, Web of Science, Embase and Medline databases on November 9, 2017. A keyword search of the following terms was conducted on all databases (medial temporal lobe OR hippocamp* OR parahippocamp*) AND pain AND ((FMRI OR functional magnetic resonance imaging OR functional MRI) OR (BOLD OR blood oxygen level dependent) OR (PET OR positron emission tomography) OR (ASL OR arterial spin labelling)).
Neurosynth
We also performed a search of the Neurosynth database (http://www.neurosynth.org) [144] on November 9, 2017 as this provides the ability to cross-search keywords (‘pain’, ‘painful’ and ‘chronic pain’) with brain coordinates. This increased the sensitivity of the meta-analysis as Neurosynth extracts coordinates from the tables in a catalogued study; therefore, even if the HC was not highlighted as a finding in the title, abstract or keywords of a study, we would still be able to identify the study. The MNI coordinates used for the HC search were derived from our previous study of hippocampal parcellation [3], as follows: the right anterior HC (antHC) is (24, 13, −21); the left antHC is (−22, −12, −20); the right posterior HC is (29, −26, −9); and left posterior HC is (−27, 25, −12). We used a liberal search sphere (radius= 15 mm) to maximize the number of studies identified, and to include adjacent structures, such as the PHG and amygdala (see Supplementary Fig.1). The same article selection criteria set out for the traditional databases were applied, with the added constraint that the HC was listed as a significant finding in at least one contrast.
Coordinate-based meta-analysis
We performed a coordinate-based meta-analysis using the activation likelihood estimation (ALE) algorithm to identify consistent regions of brain activity within the MTL in healthy participants and chronic pain patients. Brain coordinates (foci) were computed using GingerALE (v3.0) to generate probabilistic maps of activation [73] (http://brainmap.org/ale).
ALE mask
We restricted the permutation space for the null-distribution of our ALE meta-analysis to the MTL region. To do so, we constructed an MTL mask on the standard MNI152 brain in FSLeyes v.0.22.6. The mask comprised of regions selected from the Jülich Histological Atlas, including the centromedial, laterobasal and superficial amygdalae, hippocampus cornu ammonis, entorhinal cortex, dentate gyrus, hippocampal-amygdaloid transition and subiculum. The final MTL mask includes the amygdala, hippocampus and parahippocampal gyrus (see Supplementary Fig.2).
Data organization
We conducted four separate meta-analyses as part of two investigations: (1) experimental pain in healthy participants and (2) chronic pain versus healthy participants. First, foci were manually extracted from selected articles, and subsequently categorized into four separate datasheets according to the following contrasts: 1) Greater MTL activation in healthy participants during experimental pain than in control conditions, such as warm, innocuous touch or baseline conditions (experimental pain > control conditions); 2) Greater MTL activation during control conditions than painful conditions in healthy participants (experimental pain < control conditions); 3) Greater MTL activation in chronic pain patients than in healthy participants (chronic pain patients > healthy participants); and 4) Greater MTL activation in healthy participants than in chronic pain patients (chronic pain patients < healthy participants). Foci derived from significant and non-significant statistical thresholds of contrasts reporting MTL activity, were extracted from all studies. Three investigators (LA, MM, MG) verified manual extraction of the foci, before running each meta-analysis.
ALE Meta-Analysis
In preparation for the ALE meta-analysis, all foci were standardized into MNI space. Foci reported using Talairach coordinates were converted with “Talairach to MNI (FSL)” [17]. For each set of foci per experiment, the number of participants were added for weighting purposes. Once finalized, each datasheet was entered and computed separately into the software using a single dataset analysis [37,38]. We used the Turkeltaub algorithm to estimate the probability of activation at each voxel in the brain by reducing both within-experiment (inter-participants) and within-group (inter-laboratory) variables [133]. The algorithm attributes an ALE score, a voxel-based value for each focus, to create a Modeled Activation (MA) map [38], calculated using the maximum value with the “random effects” selection [37]. Furthermore, each focus is blurred with a Gaussian distribution, weighted by sample size, to minimize spatial uncertainty [74]. Lastly, the cluster-level inference algorithm described by Eickhoff and colleagues [36] was used to compute the final thresholded ALE P value map with a permutation-based cluster correction p< 0.05 (cluster-forming height threshold of p< 0.005 with 1000 permutations), which uses a Monte-Carlo simulation approach. The resulting maps were visualized and labelled in MNI space using FSLeyes v.0.22.6. Slice images were visualized and labelled in MNI space using “ch2bet” on MRIcron v2016.
Functional connectivity
Given that we found consistent pain related activity in our meta-analyses in the right antHC region, and that rsFC of the antHC region has been previously shown to be a predictor for the transition from subacute to chronic back pain [107], we conducted a whole brain seed-to-voxel rsFC study of the right antHC region, to investigate whether there were connectional differences between healthy participants and CBP. Furthermore, since we have shown that the right antHC region is activated in response to experimental pain in healthy participants and has abnormal activity in chronic pain, we sought to determine whether abnormal right antHC connectivity in CBP was related to pain characteristics, including pain duration and pain intensity.
Participants
Four resting state fMRI datasets comprising a total of 156 participants (CBP (n=77) and age- matched healthy participants (n=79)) were included in the analysis. A summary of each dataset characteristics is provided in Table 1.
Table 1.
Dataset characteristics
| Dataset | N | Age (mean± SD years) |
Sex (W/M) |
Healthy participants |
CBP |
Scanner | TR (s) |
Head Coil channels |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Sex (W/M) |
Age (mean± SD years) |
N | Sex (W/M) |
Age (mean± SD years) |
Pain duration (n=69; mean ± SD years) |
Pain intensity (n=59; mean ± SD VAS (0-10)) |
|||||||
| 1 | 57 | 49.6 ± 8.4 | 26/31 | 31 | 13/18 | 49.9 ± 7.8 | 26 | 13/13 | 49.3 ± 9.1 | 15.0 ± 12.1 | 6.5 ± 1.7 | 3T Siemens Tim Trio | 2.5 | 8 |
| 2 | 27 | 44.2 ± 11.4 | 14/13 | 12 | 6/6 | 42.2 ± 11.4 | 15 | 8/7 | 45.9 ± 11.6 | 5.2 ± 5.1 | n/a | 3T Siemens Tim Trio | 2.26 | 8 |
| 3 | 48 | 42.9 ± 11.8 | 23/25 | 27 | 11/16 | 41.2 ± 11.9 | 21 | 12/9 | 45.0 ± 0.5 | 11.6 ± 9.5 | 2.6 ± 2.5 | 3T Siemens Tim Trio | 2.5 | 12 |
| 4 | 24 | 44.7 ± 11.5 | 16/8 | 9 | 5/4 | 46.7 ± 0.5 | 15 | 11/4 | 43.5 ± 11.2 | 11.7 ± 7.3 | 4.7 ± 3.0 | 3T Siemens Tim Trio | 2 | 12 |
| Total | 156 | 45.9 ± 10.8 | 79/77 | 79 | 35/44 | 45.4 ± 11 | 77 | 44/33 | 46.2 ± 10.7 | 11.5 ± 10.0 | 4.8 ± 2.8 | |||
A summary of the four datasets included in the seed-to-voxel functional connectivity.
Abbreviations: BDI Beck Depression Inventory, CBP chronic low back pain patients, M men, W women, VAS visual analogue scale.
Dataset 1
The first dataset initially consisted of 68 participants, 34 CBP and 34 healthy participants from the study by Mansour and colleagues [88] and was obtained from the “cbp_resting” provided by the OpenPain Project (OPP) database (OPP; Principal investigator: A. Vania Apkarian; http://www.openpain.org). We consented to and followed OpenPain Data Use Agreement and procedures were approved by the University of Toronto’s Human Research Ethics Board prior to data analysis. As described in the original manuscript, all participants were provided with written consent and all experimental protocols were approved and conducted according to the Northwestern University’s Institutional Review Board committee. Patients’ clinical assessment included the Short-Form of the McGill Pain Questionnaire (SF-MPQ) [98], where the visual analog scale (VAS) (0= no pain, 10= worst pain imaginable) was used to evaluate pain intensity. Participants were given the questionnaire one hour before scanning.
Dataset 2
The second dataset initially consistent of 36 participants, 20 CBP and 16 healthy participants and was previously published in a study investigating rsFC in CBP and acquired from Stone, Seminowicz and colleagues [19]. The study was approved by McGill University Faculty of Medicine Institutional Review Board, the Montreal Neurological Institute (MNI) and Hospital Research Ethics Board, and the McGill University Health Centre Research Ethics Office. The participants gave written consent before starting the study. All participants completed the SF-MPQ for pain intensity prior to scanning, however VAS data were not available.
Dataset 3
The third dataset initially included a total of 63 participants from Osaka, Japan (24 CBP, 39 healthy participants). This dataset was obtained from “BrainNetworkChange_Mano” on OPP database. We consented to and followed OpenPain Data Use Agreement. The original study was approved by the Ethics Committee for Human and Animal Research of the National Institute of Information and Communications Technology, Japan (reference 20140611) [87]. All participants gave written consent before participating in the study. Pain intensity was measured on a VAS scale as part of the SF-MPQ on the day of scanning.
Dataset 4
The fourth dataset initially included 34 participants, 17 CBP and 17 healthy participants from Cambridge, UK in “BrainNetworkChange_Mano” on OPP database. We consented to and followed OpenPain Data Use Agreement. The original study was approved by the East of England NRES Committee, Norfolk, UK (reference 13/EE/0098) [87]. All participants gave written consent before participating in the study. The VAS scale as part of the SF-MPQ was to determine pain intensity on scan day.
Resting state fMRI data acquisition parameters
Dataset 1
Functional T2*-weighting brain images were acquired using a 3T Siemens Trio whole-body scanner, with an 8-channel head coil, during rest, as follows: TR= 2.5s; TE= 30 ms; flip angle= 90°; in-plane matrix resolution= 64 × 64; number of slices= 40; slice thickness= 3mm; field of view= 256 × 256 mm; and number of volumes= 244, 300, or 305 [88]. Additionally, for realignment purposes, T1-weighted brain images for each participant were acquired using the same scanner with the following parameters: isotropic resolution 1mm; TR= 2.5s; TE= 3.36 ms; flip angle= 9°; in-plane matrix resolution= 256 × 256; number of slices= 160; and field of view= 256 × 256 mm [88].
Dataset 2
Scans were acquired using a 3T Siemens Tim Trio scanner with an 8-channel head coil. Participants were instructed to “relax, keep your eyes open, and don’t think about any one thing in particular” [19]. The functional T2*-weighted imaging scans where acquired using: echo planar imaging (EPI), TR= 2.26 s; TE= 30 ms; flip angle= 90°; in-plane matrix= 64×64; number of slices= 38; slice thickness= 4 mm; field of view= 256 × 256 mm; and number of volumes= 133 [19]. The anatomical T1 scans were acquired using the following parameters: isotropic resolution 1mm; TR= 2.3 s; TE= 2.98 ms; flip angle= 9°, in-plane matrix resolution= 256 × 256; number of slices= 176; and field of view= 256 × 256 mm [19].
Dataset 3
The MRI scans were performed using a 3T Siemens MRI Scanner Tim Trio scanner with a 12-channel head coil at CiNet (Osaka, Japan). During resting state scanning, participants were given the following instructions: “please relax during the scan; do not sleep and keep looking at the fixation point (a tiny cross-hair) presented at the center of the display; do not think of anything in particular.” Functional T2* images were acquired with the following parameters: TR= 2.5s; TE= 30 ms; flip angle= 80°; in-plane matrix resolution= 64 × 64; number of slices= 41; field of view= 212 × 212 mm; and number of volumes= 234 [87]. The anatomical T1 scans were acquired using the following sequence: isotropic resolution 1mm; TR= 2.25s; TE= 3.06 ms; time of inversion= 900 ms; flip angle= 9°; in-plane matrix resolution= 256 × 256; number of slices= 208; and field of view= 256 × 256 mm [87].
Dataset 4
The MRI scans were performed with a 3T Siemens MRI Scanner Tim Trio scanner with a 12-channel head coil at Addenbrooke’s Hospital in Cambridge, UK. During resting state scanning, participants were instructed to: “please relax during the scan; do not sleep and keep looking at the fixation point (a tiny cross-hair) presented at the center of the display; do not think of anything in particular.” Functional T2* images were acquired with the following parameters: TR= 2s; TE= 30 ms; flip angle= 80°; in-plane matrix resolution= 64 × 64; number of slices= 32; field of view= 212 × 212 mm; and number of volumes= 300 [87]. The anatomical T1 scans were collected using the following sequence: isotropic resolution 1mm, TR= 2.3s; TE= 2.98ms; time of inversion= 900ms; flip angle= 9°; in-plane matrix resolution= 256 × 256; number of slices= 176; and field of view= 256 × 256 mm [87].
fMRI data preprocessing
FMRI data were preprocessed for whole brain rsFC using CONN toolbox v17f (http://www.conn-toolbox.org) implemented in Statistical Parametric Mapping software package (SPM v.12; http://www.fil.ion.ucl.ac.uk/spm/software/spm12)) and ran on MATLAB (R2016b v.9.1; Mathworks, Nantick, MA) [141]. Each participant’s anatomical T1-weighted and functional T2*-weighted scans were imported into CONN. Data was preprocessed as follows. Briefly, functional T2* scans underwent co-registration to the participant’s structural T1 scans. Functional images were spatially realigned and unwarped. Realignment is defined by default by six dimensions, three translations and three rotations along the x, y and z axes. Furthermore, each anatomical image was segmented for grey and white matter, and cerebrospinal fluid (CSF) and normalized to the T1-weighted MNI152 template. FMRI data were also aligned to the MNI template. Data was resliced after normalization using the default Tissue Probability Maps (structural target resolution= 2 mm, functional target resolution= 2 mm) and subsequently smoothed with a Gaussian kernel of 8-mm at full-width half-maximum (FWHM). Once preprocessed, we checked the histogram plots of rsFC values (voxel-to-voxel correlation coefficient between BOLD times series and 512 subset of voxels) for alignment centered at zero for quality assurance. If the data are completely without noise, then the correlation of a random subset of connections should center around zero; i.e. no correlations. Skewness in the plots indicate noise related to physiological processes or subject motion; this skewness may artificially inflate connectivity strengths. Several steps were taken to mitigate these confounds. First, physiological noise was corrected by using aCompCor, an algorithm which performs a principal components analysis on fMRI signal from the white matter and cerebrospinal fluid (CSF), areas of non-neuronal origin [10]. The white matter and CSF masks were generated by tissue segmentation performed using SPM, and then eroded to minimize partial volume effects for each subject. These masks were visually inspected. In the denoising step, we regressed the following confounds: realignment of six dimensions with their first temporal derivatives (12 components), white matter (5 components) and CSF (5 components). A band-pass filter of 0.008–0.09 Hz was additionally applied to the data. To control for excessive motion, we used a criterion of framewise displacement (FD) greater than the absolute value of 0.5mm threshold [29,87]. The mean FD (mFD) was computed using the weighted sum across six dimensions of the mean absolute scan to scan differences [117]. Participants were excluded if they met any of the following motion criteria: mFD > ∣0.5mm∣ and at least 20 % of the suprathreshold FD value > ∣0.5mm∣ [87,110]. Both mFD and maximum FD (maxFD) were also computed for comparative purposes between healthy participants and CBP for each site (see Supplementary Table 1). The maxFD was calculated using the weighted sum across six dimensions of the maximum absolute scan to scan differences [117]. The Mann-Whitney U test compared FD values between healthy participants and CBP for each site at p< 0.05. In addition, we excluded participants based on quality assurance, if they still presented a heavily skewed histogram plot of correlation coefficient following denoising. The residual fMRI signal of the included participants was used for whole brain rsFC analysis.
Participants were removed from each dataset, as follows. Further, a summary is provided in Supplementary Table 2.
Dataset 1
We excluded 11 participants (three healthy participants and eight CBP) from the initial dataset after matching for age with the other three datasets (n=1 control), after preprocessing (n=3; 1 control and 2 CBP, scans could not be aligned), after denoising (n=1 CBP, to meet quality assurance standards), and after motion correction (n=6; 1 control, 3 CBP had a mFD value > ∣0.5 mm∣ and at least 20% of the suprathreshold FD value > ∣0.5 mm∣) and 2 CBP had at least 20% of the suprathreshold FD value > ∣0.5 mm∣. We included 57 participants in our analysis, which consisted of 26 CBP and 31 age-matched healthy participants.
Dataset 2
We excluded nine participants (four healthy participants and five CBP): two healthy participants from the initial cohort to age match with the other three datasets and two that had at least 20% of the suprathreshold FD value > ∣0.5 mm∣ . Five CBP were excluded after motion correction (n=5; one participant had a mFD value > ∣0.5 mm∣ and at least 20% of the suprathreshold FD value > ∣0.5 mm∣) and 4 CBP had at least 20% of the suprathreshold FD value > ∣0.5 mm∣ . In sum, we included 27 participants in our analysis: 15 CBP and 12 age-matched healthy participants.
Dataset 3
We excluded 15 participants (12 healthy participants and three CBP) to age-match with the other datasets (n=7 healthy participants), to exclude healthy participants with pain (n=4), to exclude one CBP after preprocessing since the skull was not properly removed (n=1), to exclude after motion correction (n=3; one healthy participant had at least 20% of the suprathreshold FD value > ∣0.5 mm∣, one CBP had a mFD value > ∣0.5 mm∣ and one CBP had a mFD value > ∣0.5 mm∣ and at least 20% of the suprathreshold FD value > ∣0.5 mm∣ . We included 48 participants in our analysis, 21 CBP and 27 age-matched healthy participants.
Dataset 4
We excluded ten participants (eight healthy participants and two CBP) to age-match with the other datasets (n=1 healthy participant), to exclude healthy participants with pain (n=6), and to exclude after motion correction (n=3; one healthy participant with at least 20% of the suprathreshold FD value > ∣0.5 mm∣, one CBP with a mFD > ∣0.5 mm∣ and one CBP with a mFD > ∣0.5mm∣ and at least 20% of the suprathreshold FD value > ∣0.5 mm∣. Finally, we included 24 participants in our analysis, 15 CBP and 9 age-matched healthy participants.
Whole brain functional connectivity
We performed seed-to-voxel rsFC of the right antHC to the rest of the brain using the CONN toolbox. The antHC mask was derived from our previous parcellation study, which delineated the region of the right antHC using a hippocampal mask based on the Harvard-Oxford subcortical atlas on FSL v4.0 [3]. This region overlaps with the result from our chronic pain meta-analysis. The center-of-gravity of the mask was (MNI=24, 13, −21) [3]. We conducted a first-level, fixed-effects analysis, which was a seed-to-voxel based correlation between the right antHC ROI timeseries and every other voxel in the brain. We then performed a second-level random effects analysis to evaluate differences between healthy participants and CBP while regressing each dataset (site) and sex as covariates of no-interest. Site was included as a covariate of no-interest to account for scanner/site specific noise. Sex was also included as a covariate as there are sex differences in resting state networks [22,45,54,60,123,140], and our samples were not sex-matched. Voxelwise correlation coefficients were z-scored using the Fisher r-to-z transformation. To test whether the functional connectivity data were normally distributed, we performed the Shapiro-Wilk’s test, with significance set at p< 0.05, in SPSS (v25, IBM corp, Armonk, NY). Group differences between CBP and healthy participants were assessed with parametric cluster-based statistics using family-wise error (FWE), and maps were thresholded using cluster-size at PFWE< 0.05 (cluster-forming height threshold of p< 0.001).
Post-hoc correlations
We performed post-hoc correlations to determine whether the aberrant antHC functional connectivity (CBP vs. healthy participants) was associated with pain characteristics (i.e., pain intensity and pain duration). Pain intensity was collected with the VAS scale on SF-MPQ on scan day in three datasets (1,3,4). Dataset 2 also collected part of the SF-MPQ, but VAS scores were not collected, as such this dataset was excluded for this analysis. In all datasets, pain duration was evaluated in number of years. We extracted the connection strength between the antHC seed and the resultant cluster (connection strength here is defined as the fisher-transformed correlation coefficient between BOLD activity in the antHC seed region and the resulting cluster) for each CBP subject, to assess whether they were correlated with clinical characteristics, including disease duration (n=69 CBP) and pain intensity (n=59 CBP) using Spearman’s rank correlation. Significance was set at p< 0.05.
RESULTS
Article selection
Our database and reference search identified a total of 49 articles that met our criteria of selection, as shown in Fig.1. Table 2 provides a summary of the 21 studies included in the first meta-analysis, which reported MTL activity in response to experimental pain, compared to a control condition. Table 3 provides a summary of the 28 studies of chronic pain patients reporting abnormal MTL activity compared to healthy participants that were included in our second meta-analysis.
Table 2.
Summary of experimental pain studies in healthy participants included in the meta-analysis
| MTL Finding |
N | Sex (W/M) |
Age (mean ±SD/range/ S.E.M. in years) |
Foci | Stimuli |
Ref. | QS (/20) |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Pain > Control |
Pain < Control |
Contrast | Modality | Body Part | ||||||
| (1) L HC | (2) L PHG | 12 | 6/6 | 28.8 (21-41) | (1) 17 (2) 9 |
(1) heat pain > warm (2) warm > heat pain |
thermal laser | R dorsum of the hand | [28]a | 17 |
| B HC | 8 | 0/8 | 22-40 | 9 | heat pain > baseline | thermal | L dorsum of the hand | [114] | 17 | |
| B HC | 14 | 1/13 | 25.8 (21-41) | 12 | thermal laser > baseline | thermal laser | B dorsum of the hand | [13] | 17 | |
| B HC | 12 | 6/6 | 25.42 ± 5.43 (20-35) | 16 | heat pain > baseline | thermal | L dorsum of the hand | [39] | 19 | |
| (1) B HC (2) L HC |
22 | (1) 9/10 (2) 9/8 |
(1) 26 ± 1/34 ± 4 (2) 26 ± 1/31± 4 |
(1) 8 (2) 6 |
(1) muscle pain (saline injections) > baseline (2) cutaneous pain (saline injections) > baseline |
chemical | (1) R muscle lower leg (2) R cutaneous lower leg |
[52] | 17 | |
| B HC | 11 | 0/11 | 28 ± 4 | 24 | pressure pain after saline injections > baseline | mechanical and chemical | R lower leg | [81] | 18 | |
| L HC, B PHG | 9 | 5/5* | 29.7 ± 8.7 | 8 | pricking pain > burning pain | thermal laser | L dorsum of the foot | [137] | 17 | |
| B PHG /HC | 61 | 33/ 28 | 26.6 ± 4.7 | 18 | low thermal pain < baseline | thermal | R volar forearm | [70] | 17 | |
| R HC | 7 | 3/4 | 18-36 | 9 | heat pain > warm | thermal | L dorsum of the hand | [91] | 17 | |
| B PHG | 11 | 11/3** | 26.9 ± 4.7 | 5 | transcutaneous stimulation > baseline | electrical | R ankle | [113] | 17 | |
| B HC | 12 | 7/5 | 20–31 | 10 | heat pain expectancy > baseline | thermal | L or R wrist | [146] | 17 | |
| R PHG | 31 | 16/ 15 | 30 (22–38) | 25 | painful esophageal stimulation > baseline | mechanical | esophagus | [23] | 17 | |
| R PHG | 34 | 18/ 16 | 23.4 ± 2.5 | 36 | heat pain > warmth baseline | thermal | L lower leg | [71] | 19 | |
| R PHG | 14 | 8/6 | 26.1 (21-37) | 13 | heat pain > baseline | thermal | L volar forearm | [64] | 19 | |
| (1) L PHG | (1) L PHG (2) B PHG |
32 | (1)16 (2)16 |
(1) 30.9 ± 7.8 (2) 27.8 ± 7.1 |
(1)57 (2)70 |
painful esophageal stimulation > baseline | mechanical | esophagus | [66] | 18 |
| R HC | 12 | 6/6 | 29.9 ± 2.5 | 22 | heat pain > baseline cerebral blood flow | thermal | L dorsum of the hand | [85]b | 18 | |
| B HC | 5 | 5/0 | 35 (26-56) | 38 | painful electrical shock > baseline | electrical | R volar forearm | [132] | 17 | |
| B PHG | 15 | 7/8 | 25.7 (22-30) | 12 | pain > baseline | chemical | R lower back muscle (4th lumbar vertebra) | [145] | 17 | |
| (1) L HC (2) R HC |
24 | 24/0 | 31.3 (24-45) | 44 | (1) laser heat > baseline (2) noxious cold > baseline |
thermal | L dorsum of hand R foot |
[15] | 18 | |
| R HC | 24 | 10/ 14 | 25 ± 5 | 13 | heat pain nocebo***< high heat pain | thermal | L volar forearm | [61] | 17 | |
| B HC/ PHG | 23 | 12/ 11 | 27.8 ± 4.2 | 15 | electrical pain > baseline | electrical | L dorsum of the hand / forehead | [124] | 18 | |
A detailed description of all 21 experimental pain fMRI studies (with the exception of one ASL and the other PET) of healthy participants included in the ALE meta-analysis.
Abbreviations: ALE activation likelihood estimation, ASL arterial spin-labeled magnetic resonance imaging, B bilateral, HC hippocampus, L left, M men, PET positron emission tomography, PHG parahipppocampal gyrus, QS quality score, R right, W women.
One participant (gender unspecified) was excluded from the study.
Three participants (gender unspecified) were excluded from the study.
Low heat pain nocebo was control to high heat pain
PET study
ASL study
Table 3.
Summary of chronic pain studies included in the meta-analysis
| Pain condition |
Sex (W/M) | Age (mean ± SD/range/ S.E.M. in years) |
Foci | Stimuli | MTL Finding | Ref. | QS (/20) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | C | P | C | Contrast | Modality | Body part | P > C | P < C | ||||
| CD | 9/16 | 6/19 | 31.72 ± 8.05 | 29.24 ± 6.85 | 2 | resting state conditions | L HC/ PHG | [8] | 19 | |||
| FD | 31/18 | 25/14 | 22.55 ± 1.78 | 22.18 ± 0.85 | 4 | resting state conditions | L PHG | [80] | 19 | |||
| 20/11 | 19/11 | 22.50 ± 1.46 | 22.23 ± 0.94 | 13 | resting state conditions | L HC/ PHG | [79] | 18 | ||||
| FM | 6/0 | 8/0 | 53.0 ± 4.8 | 51.4 ± 3.9 | 14 | FDOPA uptake < control | chemical | B HC | [143] | 18 | ||
| 29/0 | 31/0 | 49.8 (25-64) | 46.3 (20-63) | 7 | incongruent > congruent stimuli | stroop color word test | L HC | [90] | 18 | |||
| 37/6 | 10/5 | 46.3 ± 11.4 | 44.1 ± 14.8 | 34 | (1) cuff stimulation > baseline (2) stimulus offset (still in pain) |
mechanical | L lower leg | (1) R HC/PHG (2)B HC/R PHG |
[126] | 17 | ||
| 15/0 | 15/0 | 52.07 ± 7.14 (42-64) | 52.67 ± 6.23 (46-69) | 1 | controllability during expectation of pain > control condition | controllability in reaction time task | R HC | [48] | 19 | |||
| IBS | 5/3 | 5/3 | 41.3 (27-64) | 39.4 (24-54) | 3 | rectal distention > baseline | mechanical/auditory | rectum | R HC | [5] | 18 | |
| 15/15 | 15/15 | 21.7 ± 3.0 | 21.4 ± 1.5 | 4 | cognitive task < baseline | stroop task | R HC | [4] | 17 | |||
| 33/0 | 18/0 | 32.5 (20-60)/40.3 (21-60) | 32.5 (21-54) | 3 | expectation of high distention > control | mechanical | rectum | R HC | [75] | 18 | ||
| 11/6 | 11/6 | 36.0 ± 10.8 | 37.4 ± 10.2 | 13 | analgesia > control | suggestion -/conditioning enhanced placebo | R PHG | [76] | 19 | |||
| 76/42 | 31/29 | 29.39 ± 9.93/35.95 ±12.97 | 30.65 ± 10.71/37.28 ± 10.75 | 3 | resting state conditions | R HC | [55] | 17 | ||||
| 15/2 | 10/11 | 47.96 ± 3.7 | 34.76 ± 2.8 | 2 | visual cue > control cue | visual task with rectal distention | rectum | B HC | [58] | 19 | ||
| 7/14 | 10/11 | 41.82 ± 11.92 | 35.91 ± 14.76 | 8 | resting state conditions | R HC | [83] | 18 | ||||
| ITN | 10/7 | 10/9 | 63.41 ± 7.25 | 62.53 ± 7.41 | 3 | resting state conditions | R PHG | [139] | 19 | |||
| LBP | 5/6 | 6/5 | 20.4 | 21.5 | 27 | task picture > no task picture | visual task picture | B PHG | [128] | 19 | ||
| 16/15 | 18/13 | 51.8 ± 9.9 | 49.3 ± 8.2 | 2 | task picture > no task picture | visual task picture | R HC | [9] | 17 | |||
| Migraine | 9/1 | 9/1 | 37.9 ± 4.7 | 37.8 ± 4.8 | 2 | pain-related words > baseline (negative words) | verbal stimuli | R HC/PHG | [35] | 19 | ||
| 55/0 | 44/0 | 34.5 ± 11.0 | 34.3 ± 14.3 | 15 | resting state conditions | B HC | [46] | 19 | ||||
| 8/3 | 8/3 | 42.5 ± 11.9 | 42.3 ± 11.9 | 2 | heat pain > baseline | thermal | painful facial side (patients)/matched (healthy participants) | R HC | [106] | 18 | ||
| 19/5 | 22/5 | 36.2 ± 11.3 | 33.7 ± 12.5 | 9 | heat pain > baseline | thermal | L volar forearm | L HC | [127] | 18 | ||
| MOH | 30/7 | 20/12 | 41.27 ± 9.27 | 41.34 ± 10.89 | 1 | resting state conditions | R PHG | [20] | 18 | |||
| MPS | 10/6 | 8/6 | 32.6 ± 7.2 | 30.6 ± 9.0 | 15 | electrical pain > baseline | electrical | L shoulder | B HC, L PHG | [108] | 16 | |
| OA | 16/0 | 0/17 | 60.87 | 64.17 | 51 | resting state conditions | L HC/PHG | [57]* | 17 | |||
| PD | 34/0 | 34/0 | 21.5 ± 1.2 | 22.2 ± 1.7 | 5 | resting state conditions | L HC | [63] | 19 | |||
| SPD | 5/7 | 4/6 | 45.83 ± 14.95 | 35.2 ± 10.78 | 10 | electrical pain > baseline | electrical | **lower leg | R PHG | [82] | 17 | |
| 11/6 | 11/6 | 44.7 ± 9.1 | 45.4 ± 9.2 | 22 | pricking > baseline | mechanical | L lower leg | B HC, L PHG | [131] | 15 | ||
| WAD | 17/4 | 9/9 | 37.0 ± 11.0 | 35.0 ± 9.0 | 15 | resting state conditions | B PHG | [78] | 17 | |||
A detailed description of 28 chronic pain studies selected for the ALE meta-analysis.
Abbreviations: ALE activation likelihood estimation, ASL arterial spin-labeled magnetic resonance imaging, B bilateral, C healthy controls, CD Crohn’s disease, FD functional dyspepsia, FDOPA 6-[18F][121] fluoro-L-dopa, FM fibromyalgia, fMRI functional magnetic resonance imaging, HC hippocampus, IBS irritable bowel syndrome, ITN idiopathic trigeminal neuralgia, L left, LBP lower back pain, M men, MOH medication overuse headache, MPS myofascial pain syndrome, OA osteoarthritis, P patients, PD primary dysmenorrhea, PHG parahippocampal gyrus, SPD somatoform pain disorder, R right, QS quality score, W women, WAD whiplash associated disorder.
ASL study, the rest of the studies used fMRI
laterality not specified
Article quality assessment
Quality scores for each article included in each of the two meta-analyses is provided in Tables 2 and 3. Average scores for all articles included in the first meta-analysis were 17.52 ± 0.75 (mean ± SD score out of 20) and 17.93 ± 1.05 for the articles in the second meta-analysis. The most notable limitation of these articles is that most did not report whether sample size calculations were conducted a priori. In addition, some articles did not account for variables of potential interest, such as sex/gender.
Contrasts results
Our experimental pain and chronic pain meta-analyses resulted in the following number of experiments (contrasts within studies were accounted), foci, and participants as follows:
From the 21 studies investigating experimental pain, and reporting HC or PHG activation:
Healthy participants during experimental pain > control conditions (baseline): 22 experiments, 398 foci, 385 participants;
-
Healthy participants during experimental pain < control conditions (baseline): 3 experiments, 100 foci, 105 participants.
From the 28 studies investigating chronic pain, and reporting HC or PHG activation:
Chronic pain patients > healthy participants: 17 experiments, 173 foci, 629 participants;
Chronic pain patients < healthy participants: 11 experiments, 81 foci, 583 participants.
ALE Meta-Analyses
Experimental pain studies
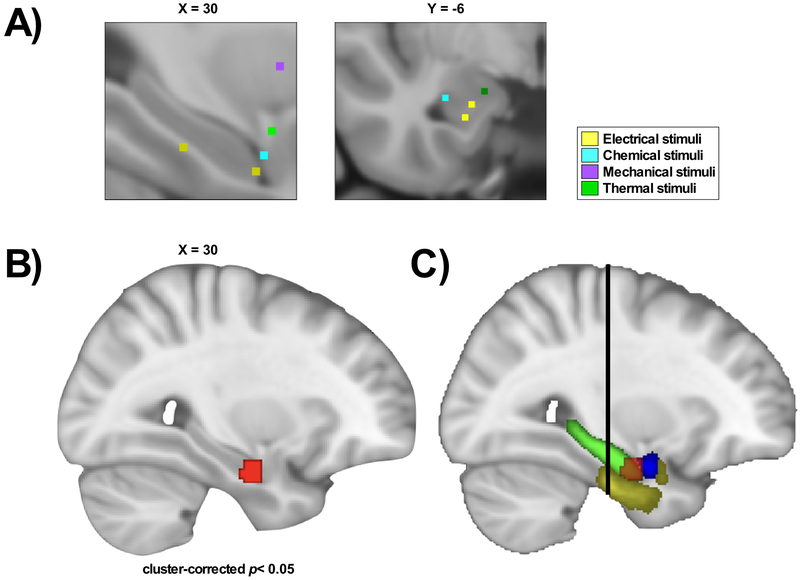
Our ALE meta-analysis of experimental pain studies in healthy participants is reported in Table 4, Fig.2 and Supplementary Fig.3. The contrast experimental pain > control conditions identified consistently greater activations in the right antHC, amygdala and parahippocampal gyrus at a cluster-corrected p< 0.05 at cluster-forming height threshold of p< 0.005 with 1000 permutations. The experimental pain < control conditions contrast did not have a sufficient number of experiments to perform the analysis.
Table 4.
ALE results of experimental pain and chronic pain studies
| Brain region | MNI |
ALE value | Cluster size (mm3) |
||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Healthy participants: Experimental pain > control conditions | |||||
| R antHC/PHG/amygdala | 30 | −10 | −24 | 0.025 | 808 |
| Chronic pain patients < healthy participants | |||||
| R antHC | 26 | −16 | −16 | 0.021 | 840 |
ALE results of all significant contrasts. One significant activation cluster resulted from the ALE meta-analysis of healthy participants during experimental pain compared to control conditions (n=21, cluster-corrected p< 0.05, threshold p< 0.005 with 1000 permutations). One significant activation cluster in chronic pain patients compared to healthy participants resulted from the ALE meta-analysis of chronic pain studies (n=28, cluster-corrected p< 0.05, threshold p< 0.005 with 1000 permutations).
Abbreviations: ALE activation likelihood estimation, antHC anterior hippocampus, BA Brodmann area, MNI Montreal Neurological Institute, PHG parahippocampal gyrus, R right.
Fig.2. Peak activation in the anterior hippocampus in healthy participants during experimental pain conditions, compared to control conditions.
The ALE meta-analysis of noxious experimental conditions and baseline conditions in healthy participants with MTL activation (n=22 experiments). (A) Close-up of foci activation in the MTL, color-labelled according to stimuli. (B) The map shows one significant cluster (red) in the right anterior hippocampus (antHC), parahippocampal gyrus and amygdala (cluster-corrected p< 0.05, cluster-forming height threshold of p< 0.005 with 1000 permutations). (C) The map shows the delineation of the significant cluster in red and the different regions of the MTL: parahippocampal gyrus (yellow), amygdala (blue) and hippocampus (green). The black line represents MNI Y=−21, which represents the border between the anterior and posterior hippocampus [116].
Chronic pain studies
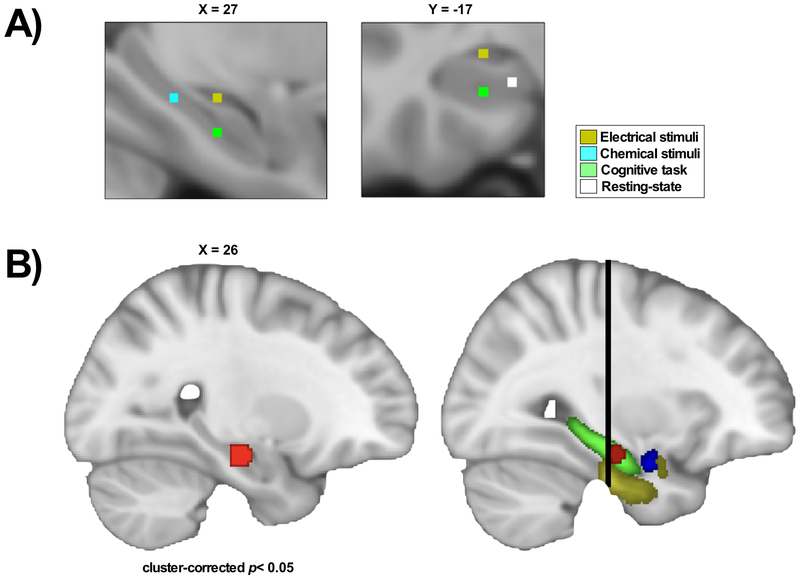
Our ALE meta-analysis of chronic pain studies versus healthy participants is shown in Table 4, Fig.3 and Supplementary Fig.4. The contrast chronic pain patients < healthy participants found consistently less activation in the right antHC at a cluster-corrected p< 0.05 at cluster-forming height threshold of p< 0.005 with 1000 permutations. There were no significant clusters for the chronic pain patients > healthy participants contrast.
Fig.3. The anterior hippocampus shows significantly less activity in chronic pain, compared to healthy participants.
The ALE meta-analysis compared chronic pain studies versus healthy participants with MTL activation (n=11 experiments). (A) Close-up of foci activation in the MTL, color-labelled according to stimuli. (B) The map represents one significant cluster in the right anterior hippocampus (antHC). Patients with chronic pain had consistently lower activity in this region compared to healthy participants (cluster-corrected p< 0.05 at cluster-forming height threshold of p< 0.005 with 1000 permutations). (C) The map shows the cluster in relation to other MTL structures: the hippocampus (green), the amygdala (blue) and the parahippocampal (yellow). The black line represents MNI Y=−21, which represents the border between the anterior and posterior hippocampus [116].
Group differences in antHC functional connectivity
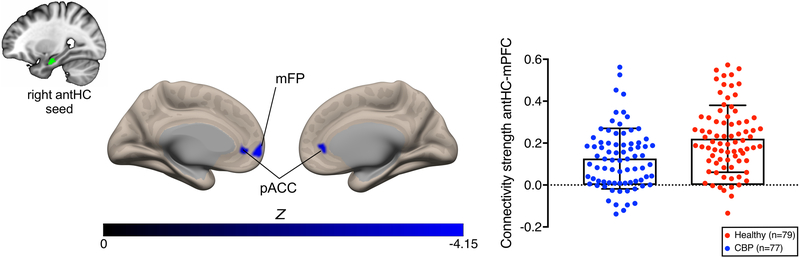
Our whole brain seed-to-voxel functional connectivity analysis of the right antHC seed yielded reduced connectivity in CBP compared to healthy participants in the medial prefrontal cortex (mPFC; peak MNI coordinates: −10, 56, −02; cluster size= 328 mm3, cluster-corrected PFWE< 0.05) including bilateral pregenual anterior cingulate cortex (pACC) and the left medial frontal pole (Fig.4). We found that the functional connectivity result was normally distributed (p>0.05). Data is shown in Supplementary Fig.5. Post-hoc analyses were performed to determine whether the aberrant connectivity was related to pain characteristics (pain intensity and pain duration). Reduced antHC-mPFC connectivity values in CBP were not significantly correlated with pain intensity (n=59 CBP, r= −0.129, p= 0.329) nor pain duration n=69 CBP, r= −0.088, p= 0.471). Post hoc analyses investigating sex-differences are reported in the Supplementary Materials.
Fig.4. CBP have weaker functional connectivity to the medial prefrontal cortex.
Whole brain seed-to-voxel resting state functional connectivity in four datasets (n=156) of the right anterior hippocampus (antHC) seed in CBP patients compared to healthy participants. Patients had weaker functional connectivity between the right antHC the medial prefrontal cortex, including the bilateral pregenual anterior cingulate cortex and the left medial frontal pole, compared to healthy participants (cluster-size PFWE < 0.05, with a cluster-forming height threshold of p< 0.001). Functional connectivity strength (mean ± SD) is represented for both patients with chronic low back pain and healthy participants. Significant clusters are shown on a semi-inflated MNI brain in CONN. Abbreviations: antHC anterior hippocampus, CBP chronic low back pain patients, mFP medial frontal pole, pACC pregenual anterior cingulate cortex.
DISCUSSION
Neuroimaging evidence suggest that MTL structures are involved in acute pain and exhibit abnormal activity in chronic pain. To determine which regions of the MTL are involved, we performed two meta-analyses. The first meta-analysis investigated MTL activation in experimental pain studies of healthy participants and found consistent activation in the right antHC. The second meta-analysis investigated MTL activation in chronic pain patients compared to controls and found that patients have consistently less activation in the right antHC. Since functional connectivity of the antHC region has been previously shown to be a predictor for the transition from subacute to chronic back pain [107], we further conducted a seed-to-voxel rsFC analysis of the right antHC region in a large sample of CBP patients pooled from four different cohorts. Our analysis showed reduced antHC-mPFC functional connectivity compared to controls. These data suggest the right antHC is involved in healthy nociception but is dysfunctional in chronic pain.
MTL activity during experimental pain
The first key ALE finding is that experimental pain leads to right antHC activation. The HC, PHG and amygdala are key structures of the Papèz circuit, which is involved in memory and emotional processing. The HC/PHG is canonically responsible for learning and retention; the HC being a key structure for consolidation of contextual and spatial memory [116]. Notably, these MTL structures show pain-related activity in animal studies [27,34,68,84,97]. In contrast, several human studies have linked HC/PHG activity to a negative affect modulation of pain [11,119,121]. For example, one study found greater activity in the entorhinal cortex (the major interface between the HC and neocortex) during painful heat stimuli under elevated anxiety, compared to a low anxiety condition [114]. Activity in entorhinal cortex was correlated with the mid-insula in anxiety-induced hyperalgesia [114]. This finding is in line with the Gray-McNaughton theory of anxiety: the hippocampal formation amplifies anxiety-related signals in threatening situations [49].
Furthermore, the amygdala plays an integral role in the affective component of pain and is an opiate rich brain region [13,41,83,86,99,119,129]. The amygdala engages autonomic and emotional responses such as unpleasantness and fear [109], suggesting an intrinsic role in pain-related negative affect processing. In the context of stress or fear, the amygdala may induce hypoalgesia to minimize pain sensation during noxious stimuli [136]. There are well-known antHC-amygdala interactions in both encoding and retrieval of affective information [31,111] which can certainly extend to situations involving experimental pain.
To our knowledge, we present the first meta-analysis of experimental pain studies with MTL activity and report that MTL structures are consistently co-activated. Thus, our findings lend some new insights into potential memory and affective processing in this context.
MTL activity in chronic pain
Our second aim was to identify which MTL region show consistent activation in chronic pain since recent studies have highlighted the MTL as a potential site for understanding the onset and development of chronic pain [6,134]. Our main finding yielded consistently less activation in the right antHC in chronic pain patients, compared to controls. This antHC region overlaps with the area activated in experimental pain in healthy participants.
The HC is longitudinally divided into the antHC and posterior HC with distinct functional properties [3,116]. The antHC is implicated in mood related functions [69,115], and stress modulation [40], likely through interactions with the amygdala. Thus, abnormal antHC activity in chronic pain may be related to dysregulation in stress modulation. Chronic pain can be considered as a stressor, eliciting a prolonged stress response [26] – i.e., chronic pain poses an allostatic load on the brain [16,96,135]. The HC is particularly sensitive to the neurotoxic effects of prolonged exposure to stress hormones [24,92], thus affecting its structure [96] and function [53]. While working memory deficits have been reported in chronic pain [12,93], there has not been a systematic investigation of types of memory processes most sensitive to hippocampal dysfunction such as associative recollection. A few neuroimaging studies addressed the interruptive function of pain in relation to memory function [14,44], where reduced right antHC activity and poor visual encoding were observed in response to pain stimuli [44]. Interestingly, HC activity is related to psychologically induced analgesia [50]. As such, our finding could reflect HC dysregulation related to prolonged stress exposure, with chronic pain as the stressor. The HC subregions could certainly be further investigated in the context of memory and pain in future mechanistic studies.
antHC functional connectivity in CBP
Our third aim was to identify whether abnormal right antHC activity was accompanied by abnormal rsFC in CBP. We found reduced right antHC-mPFC connectivity in CBP compared to controls, in line with a previous study that showed disrupted antHC-mPFC connectivity predicts the transition from subacute to chronic back pain [107]. These results suggest this aberrant connectivity is also involved in sustaining maladaptive pain, but how it is implicated in chronic pain is not well understood.
The literature indicates antHC-mPFC interaction in memory encoding and retrieval, future decision-making and autobiographical memory [95,105]. Specifically, the mPFC integrates spatial and contextual information [30,62,142], attributing behavioral, cognitive and emotional relevance to a particular stimulus [142]. As such, the antHC-mPFC connectivity facilitates the encoding and retrieval of global schemas, contextual event information and emotional cues and enables future memory-guided behaviours [105,120]. Reduced connectivity between these regions is seen in conjunction with poor autobiographical memory in patients with MTL damage [94] and is associated with deficits in emotional decision-making as observed previously in CBP [7]. Of interest, the right antHC is consistently activated to a greater extent when simulating future events compared to recalling past memories [2,122]. The antHC-mPFC functional connectivity also mediates extinction learning [65,100]. Extinction learning is the process of forming new memories that decouple conditioned responses (e.g., fear) to a stimulus (e.g., tone). This results when the stimulus is repeatedly presented without the unconditioned stimulus (e.g., electric shock) which caused the conditioned response [112,118]. Chronic stressors, including chronic pain, impair extinction learning [1,42,59,101]. In particular, chronic stress blocks long-term potentiation between the HC and mPFC [47,89] and contributes to dendritic regression in mPFC and HC [77]. In CBP, there is impaired extinction learning and slower extinction to pain and verbal responses [43], and muscular reactivity [43,125] compared to controls. Perhaps reduced antHC-mPFC connectivity in CBP reflects a memory network deficit which fails to inhibit the memory of pain or to enable non-pain memory schemas that would allow retrieval of alternate memories.
Study limitations
Chronic pain conditions are heterogeneous and, accordingly, may have different patterns of brain activity. This is compounded by individual variability in chronic pain. Hence, the antHC network found in CBP is only relevant for the specific cohorts and not representative of chronic pain as a whole. For example, in burning mouth syndrome, patients had stronger antHC-mPFC connectivity than controls, but only in the presence of spontaneous pain [67]. The finding that successful recovery in patients with subacute back pain is accompanied by increased antHC-mPFC connectivity [107] suggests it is at least robust in back pain.
Furthermore, advances in MRI technology have led to variability in data quality and coordinates collected between laboratories through years of publications. To minimize this variability, we used the Turkeltaub algorithm, which accounts for such heterogeneity. In addition, foci derived from heterogeneous statistical thresholds were included in the meta-analyses—not accounting for these studies could lead to false negative findings, and thus bias the outcome of the result. Importantly, given our article selection and use of an MTL mask, our results do not speak to the consistency of MTL activation in pain in general, nor to co-activated regions outside the MTL.
In order to achieve sufficient statistical power, it has been reported that an ALE meta-analysis requires 8–15 experiments per contrast [129,138]. Based on this heuristic, our contrast of healthy participants during experimental pain < control conditions was underpowered, and this negative result should be interpreted with caution.
We hope the current findings stimulate future studies that could provide a mechanistic account of antHC in pain. The seeming contradiction of greater antHC activation in acute pain and reduced activity associated with chronic pain portrays a complex role and raises questions as to whether activation magnitude is a cause or consequence of pain experience. Experimental fMRI studies of pain and analgesia would be useful here. Furthermore, investigating functional connectivity during acute pain would be helpful to clarify how correlated activity in the target regions vary with the stimulus and task. Finally, investigating the relationship of these pain-related effects to other known functions of the antHC such as autobiographical memory retrieval or context modulation of anxiety are clearly warranted. Such studies are necessary to provide ‘meat on the bones’ for the relationship of antHC and pain that we have identified.
CONCLUSION
In conclusion, we performed the first meta-analyses of the MTL in pain and determined that in studies reporting MTL abnormalities in chronic pain, the most common subregion was the antHC. The antHC has abnormal rsFC to the mPFC in CBP, reflecting cognitive and affective abnormalities. These data shed novel and important mechanistic information on the role of the HC in chronic pain.
Supplementary Material
ACKNOWLEDGMENTS
LA was supported by the Harron G. Wilson Trust Bursary, the Harron Scholarship and the UTCSP Pain Scientist Award. MM was supported by Connaught Fund from the University of Toronto and acknowledges support from the Bertha Rosenstadt Endowment Fund. He also holds a National Science and Engineering Research Council Discovery Grant RGPIN-2018–04908. The OPP project (Principal Investigator: A. Vania Apkarian, Ph.D. at Northwestern University) is funded by the National Institute of Neurological Disorders and Stroke (NINDS) and National Institute of Drug Abuse (NIDA). DAS was funded by National Institute of Dental and Craniofacial Research R21DE023964.
Footnotes
CONFLICTS OF INTEREST
We have no conflicts to report.
REFERENCES
- [1].Abdallah CG, Geha P. Chronic Pain and Chronic Stress: Two Sides of the Same Coin? Chronic Stress (Thousand Oaks) 2017;1: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Addis DR, Cheng T, Roberts RP, Schacter DL. Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus 2011;21:1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Adnan A, Barnett A, Moayedi M, McCormick C, Cohn M, McAndrews MP. Distinct hippocampal functional networks revealed by tractography-based parcellation. Brain Struct Funct 2016;221:2999–3012. [DOI] [PubMed] [Google Scholar]
- [4].Aizawa E, Sato Y, Kochiyama T, Saito N, Izumiyama M, Morishita J, Kanazawa M, Shima K, Mushiake H, Hongo M, Fukudo S. Altered cognitive function of prefrontal cortex during error feedback in patients with irritable bowel syndrome, based on FMRI and dynamic causal modeling. Gastroenterology 2012;143:1188–1198. [DOI] [PubMed] [Google Scholar]
- [5].Andresen V, Bach DR, Poellinger A, Tsrouya C, Stroh A, Foerschler A, Georgiewa P, Zimmer C, Monnikes H. Brain activation responses to subliminal or supraliminal rectal stimuli and to auditory stimuli in irritable bowel syndrome. Neurogastroenterol Motil 2005;17:827–837. [DOI] [PubMed] [Google Scholar]
- [6].Apkarian AV, Mutso AA, Centeno MV, Kan L, Wu M, Levinstein M, Banisadr G, Gobeske KT, Miller RJ, Radulovic J, Hen R, Kessler JA. Role of adult hippocampal neurogenesis in persistent pain. Pain 2016;157:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain 2004;108:129–136. [DOI] [PubMed] [Google Scholar]
- [8].Bao CH, Liu P, Liu HR, Wu LY, Jin XM, Wang SY, Shi Y, Zhang JY, Zeng XQ, Ma LL, Qin W, Zhao JM, Calhoun VD, Tian J, Wu HG. Differences in regional homogeneity between patients with Crohn’s disease with and without abdominal pain revealed by resting-state functional magnetic resonance imaging. Pain 2016;157:1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barke A, Preis MA, Schmidt-Samoa C, Baudewig J, Kroner-Herwig B, Dechent P. Neural Correlates Differ in High and Low Fear-Avoidant Chronic Low Back Pain Patients When Imagining Back-Straining Movements. J Pain 2016;17:930–943. [DOI] [PubMed] [Google Scholar]
- [10].Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007;37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Berna C, Leknes S, Holmes EA, Edwards RR, Goodwin GM, Tracey I. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol Psychiatry 2010;67:1083–1090. [DOI] [PubMed] [Google Scholar]
- [12].Berryman C, Stanton TR, Jane Bowering K, Tabor A, McFarlane A, Lorimer Moseley G. Evidence for working memory deficits in chronic pain: a systematic review and meta-analysis. Pain 2013;154:1181–1196. [DOI] [PubMed] [Google Scholar]
- [13].Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C. Subcortical structures involved in pain processing: evidence from single-trial fMRI. Pain 2002;99:313–321. [DOI] [PubMed] [Google Scholar]
- [14].Bingel U, Rose M, Glascher J, Buchel C. fMRI reveals how pain modulates visual object processing in the ventral visual stream. Neuron 2007;55:157–167. [DOI] [PubMed] [Google Scholar]
- [15].Bogdanov VB, Vigano A, Noirhomme Q, Bogdanova OV, Guy N, Laureys S, Renshaw PF, Dallel R, Phillips C, Schoenen J. Cerebral responses and role of the prefrontal cortex in conditioned pain modulation: an fMRI study in healthy subjects. Behav Brain Res 2015;281:187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Borsook D A future without chronic pain: neuroscience and clinical research. Cerebrum 2012;2012:7. [PMC free article] [PubMed] [Google Scholar]
- [17].Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci 2002;3:243–249. [DOI] [PubMed] [Google Scholar]
- [18].Burns E, Chipchase LS, Schabrun SM. Primary sensory and motor cortex function in response to acute muscle pain: A systematic review and meta-analysis. Eur J Pain 2016;20:1203–1213. [DOI] [PubMed] [Google Scholar]
- [19].Ceko M, Shir Y, Ouellet JA, Ware MA, Stone LS, Seminowicz DA. Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment. Hum Brain Mapp 2015;36:2075–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen Z, Chen X, Liu M, Dong Z, Ma L, Yu S. Altered functional connectivity architecture of the brain in medication overuse headache using resting state fMRI. J Headache Pain 2017;18:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cho SY, Jahng GH, Park SU, Jung WS, Moon SK, Park JM. fMRI study of effect on brain activity according to stimulation method at LI11, ST36: painful pressure and acupuncture stimulation of same acupoints. J Altern Complement Med 2010;16:489–495. [DOI] [PubMed] [Google Scholar]
- [22].Clemens B, Junger J, Pauly K, Neulen J, Neuschaefer-Rube C, Frolich D, Mingoia G, Derntl B, Habel U. Male-to-female gender dysphoria: Gender-specific differences in resting-state networks. Brain Behav 2017;7:e00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Coen SJ, Kano M, Farmer AD, Kumari V, Giampietro V, Brammer M, Williams SC, Aziz Q. Neuroticism influences brain activity during the experience of visceral pain. Gastroenterology 2011;141:909–917 e901. [DOI] [PubMed] [Google Scholar]
- [24].Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev Neurosci 2008;19:395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Davis KD, Moayedi M. Central mechanisms of pain revealed through functional and structural MRI. J Neuroimmune Pharmacol 2013;8:518–534. [DOI] [PubMed] [Google Scholar]
- [26].Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage 2009;47:864–871. [DOI] [PubMed] [Google Scholar]
- [27].Delgado JM. Cerebral structures involved in transmission and elaboration of noxious stimulation. J Neurophysiol 1955;18:261–275. [DOI] [PubMed] [Google Scholar]
- [28].Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain 1997;73:431–445. [DOI] [PubMed] [Google Scholar]
- [29].Dixon ML, De La Vega A, Mills C, Andrews-Hanna J, Spreng RN, Cole MW, Christoff K. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc Natl Acad Sci U S A 2018;115:E1598–E1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dolan RJ, Fletcher PC. Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature 1997;388:582–585. [DOI] [PubMed] [Google Scholar]
- [31].Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci U S A 2005;102:2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Duerden EG, Albanese MC. Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Hum Brain Mapp 2013;34:109–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dutar P, Lamour Y, Jobert A. Activation of identified septo-hippocampal neurons by noxious peripheral stimulation. Brain Res 1985;328:15–21. [DOI] [PubMed] [Google Scholar]
- [35].Eck J, Richter M, Straube T, Miltner WH, Weiss T. Affective brain regions are activated during the processing of pain-related words in migraine patients. Pain 2011;152:1104–1113. [DOI] [PubMed] [Google Scholar]
- [36].Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage 2012;59:2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Eickhoff SB, Bzdok D, Laird AR, Kurth F, Peter T. Activation Likelihood Estimation meta-analysis revisited. Neuroimage 2012;59:2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 2009;30:2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fairhurst M, Wiech K, Dunckley P, Tracey I. Anticipatory brainstem activity predicts neural processing of pain in humans. Pain 2007;128:101–110. [DOI] [PubMed] [Google Scholar]
- [40].Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010;65:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fields HL. Pain modulation: expectation, opioid analgesia and virtual pain. Prog Brain Res 2000;122:245–253. [DOI] [PubMed] [Google Scholar]
- [42].Flor H The functional organization of the brain in chronic pain. Prog Brain Res 2000;129:313–322. [DOI] [PubMed] [Google Scholar]
- [43].Flor H, Knost B, Birbaumer N. The role of operant conditioning in chronic pain: an experimental investigation. Pain 2002;95:111–118. [DOI] [PubMed] [Google Scholar]
- [44].Forkmann K, Wiech K, Ritter C, Sommer T, Rose M, Bingel U. Pain-specific modulation of hippocampal activity and functional connectivity during visual encoding. J Neurosci 2013;33:2571–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Galli G, Santarnecchi E, Feurra M, Bonifazi M, Rossi S, Paulus MP, Rossi A. Individual and sex-related differences in pain and relief responsiveness are associated with differences in resting-state functional networks in healthy volunteers. Eur J Neurosci 2016;43:486–493. [DOI] [PubMed] [Google Scholar]
- [46].Gao Q, Xu F, Jiang C, Chen Z, Chen H, Liao H, Zhao L. Decreased functional connectivity density in pain-related brain regions of female migraine patients without aura. Brain Res 2016;1632:73–81. [DOI] [PubMed] [Google Scholar]
- [47].Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol Learn Mem 2008;89:560–566. [DOI] [PubMed] [Google Scholar]
- [48].Gonzalez-Roldan AM, Bomba IC, Diesch E, Montoya P, Flor H, Kamping S. Controllability and hippocampal activation during pain expectation in fibromyalgia syndrome. Biol Psychol 2016;121:39–48. [DOI] [PubMed] [Google Scholar]
- [49].Gray JA, McNaughton N. The Neuropsychology of Anxiety. Oxford: Oxford Univ Press, 2000. [Google Scholar]
- [50].Hashmi JA, Kong J, Spaeth R, Khan S, Kaptchuk TJ, Gollub RL. Functional network architecture predicts psychologically mediated analgesia related to treatment in chronic knee pain patients. J Neurosci 2014;34:3924–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hebben N, Corkin S, Eichenbaum H, Shedlack K. Diminished ability to interpret and report internal states after bilateral medial temporal resection: case H.M. Behav Neurosci 1985;99:1031–1039. [DOI] [PubMed] [Google Scholar]
- [52].Henderson LA, Gandevia SC, Macefield VG. Gender differences in brain activity evoked by muscle and cutaneous pain: a retrospective study of single-trial fMRI data. Neuroimage 2008;39:1867–1876. [DOI] [PubMed] [Google Scholar]
- [53].Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:1201–1213. [DOI] [PubMed] [Google Scholar]
- [54].Hjelmervik H, Hausmann M, Osnes B, Westerhausen R, Specht K. Resting states are resting traits--an FMRI study of sex differences and menstrual cycle effects in resting state cognitive control networks. PLoS One 2014;9:e103492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hong JY, Kilpatrick LA, Labus J, Gupta A, Jiang Z, Ashe-McNalley C, Stains J, Heendeniya N, Ebrat B, Smith S, Tillisch K, Naliboff B, Mayer EA. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. J Neurosci 2013;33:11994–12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Howard MA, Krause K, Khawaja N, Massat N, Zelaya F, Schumann G, Huggins JP, Vennart W, Williams SC, Renton TF. Beyond patient reported pain: perfusion magnetic resonance imaging demonstrates reproducible cerebral representation of ongoing post-surgical pain. PLoS One 2011;6:e17096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Howard MA, Sanders D, Krause K, O’Muircheartaigh J, Fotopoulou A, Zelaya F, Thacker M, Massat N, Huggins JP, Vennart W, Choy E, Daniels M, Williams SC. Alterations in resting-state regional cerebral blood flow demonstrate ongoing pain in osteoarthritis: An arterial spin-labeled magnetic resonance imaging study. Arthritis Rheum 2012;64:3936–3946. [DOI] [PubMed] [Google Scholar]
- [58].Icenhour A, Langhorst J, Benson S, Schlamann M, Hampel S, Engler H, Forsting M, Elsenbruch S. Neural circuitry of abdominal pain-related fear learning and reinstatement in irritable bowel syndrome. Neurogastroenterol Motil 2015;27:114–127. [DOI] [PubMed] [Google Scholar]
- [59].Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci 2006;26:5733–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jamadar SD, Sforazzini F, Raniga P, Ferris NJ, Paton B, Bailey MJ, Brodtmann A, Yates PA, Donnan GA, Ward SA, Woods RL, Storey E, McNeil JJ, Egan GF, Group AI. Sexual Dimorphism of Resting-State Network Connectivity in Healthy Ageing. J Gerontol B Psychol Sci Soc Sci 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Jensen KB, Kaptchuk TJ, Chen X, Kirsch I, Ingvar M, Gollub RL, Kong J. A Neural Mechanism for Nonconscious Activation of Conditioned Placebo and Nocebo Responses. Cereb Cortex 2015;25:3903–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Jin J, Maren S. Prefrontal-Hippocampal Interactions in Memory and Emotion. Front Syst Neurosci 2015;9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jin L, Yang X, Liu P, Sun J, Chen F, Xu Z, Qin W, Tian J. Dynamic abnormalities of spontaneous brain activity in women with primary dysmenorrhea. J Pain Res 2017;10:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Johnstone T, Salomons TV, Backonja MM, Davidson RJ. Turning on the alarm: the neural mechanisms of the transition from innocuous to painful sensation. Neuroimage 2012;59:1594–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci 2006;26:9503–9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kano M, Farmer AD, Aziz Q, Giampietro VP, Brammer MJ, Williams SC, Fukudo S, Coen SJ. Sex differences in brain response to anticipated and experienced visceral pain in healthy subjects. Am J Physiol Gastrointest Liver Physiol 2013;304:G687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Khan SA, Keaser ML, Meiller TF, Seminowicz DA. Altered structure and function in the hippocampus and medial prefrontal cortex in patients with burning mouth syndrome. Pain 2014;155:1472–1480. [DOI] [PubMed] [Google Scholar]
- [68].Khanna S, Sinclair JG. Noxious stimuli produce prolonged changes in the CA1 region of the rat hippocampus. Pain 1989;39:337–343. [DOI] [PubMed] [Google Scholar]
- [69].Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A 2002;99:10825–10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kong J, Loggia ML, Zyloney C, Tu P, Laviolette P, Gollub RL. Exploring the brain in pain: activations, deactivations and their relation. Pain 2010;148:257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kunz M, Chen JI, Lautenbacher S, Vachon-Presseau E, Rainville P. Cerebral regulation of facial expressions of pain. J Neurosci 2011;31:8730–8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kwan CL, Diamant NE, Pope G, Mikula K, Mikulis DJ, Davis KD. Abnormal forebrain activity in functional bowel disorder patients with chronic pain. Neurology 2005;65:1268–1277. [DOI] [PubMed] [Google Scholar]
- [73].Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE Meta-Analysis Workflows Via the Brainmap Database: Progress Towards A Probabilistic Functional Brain Atlas. Front Neuroinform 2009;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 2005;25:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Larsson MB, Tillisch K, Craig AD, Engstrom M, Labus J, Naliboff B, Lundberg P, Strom M, Mayer EA, Walter SA. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology 2012;142:463–472 e463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lee HF, Hsieh JC, Lu CL, Yeh TC, Tu CH, Cheng CM, Niddam DM, Lin HC, Lee FY, Chang FY. Enhanced affect/cognition-related brain responses during visceral placebo analgesia in irritable bowel syndrome patients. Pain 2012;153:1301–1310. [DOI] [PubMed] [Google Scholar]
- [77].Leuner B, Gould E. Structural plasticity and hippocampal function. Annu Rev Psychol 2010;61:111–140, C111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Linnman C, Appel L, Soderlund A, Frans O, Engler H, Furmark T, Gordh T, Langstrom B, Fredrikson M. Chronic whiplash symptoms are related to altered regional cerebral blood flow in the resting state. Eur J Pain 2009;13:65–70. [DOI] [PubMed] [Google Scholar]
- [79].Liu P, Qin W, Wang J, Zeng F, Zhou G, Wen H, von Deneen KM, Liang F, Gong Q, Tian J. Identifying neural patterns of functional dyspepsia using multivariate pattern analysis: a resting-state FMRI study. PLoS One 2013;8:e68205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Liu P, Zeng F, Zhou G, Wang J, Wen H, von Deneen KM, Qin W, Liang F, Tian J. Alterations of the default mode network in functional dyspepsia patients: a resting-state fmri study. Neurogastroenterol Motil 2013;25:e382–388. [DOI] [PubMed] [Google Scholar]
- [81].Lorenz IH, Egger K, Schubert H, Schnurer C, Tiefenthaler W, Hohlrieder M, Schocke MF, Kremser C, Esterhammer R, Ischebeck A, Moser PL, Kolbitsch C. Lornoxicam characteristically modulates cerebral pain-processing in human volunteers: a functional magnetic resonance imaging study. Br J Anaesth 2008;100:827–833. [DOI] [PubMed] [Google Scholar]
- [82].Luo Y, Yan C, Huang T, Fan M, Liu L, Zhao Z, Ni K, Jiang H, Huang X, Lu Z, Wu W, Zhang M, Fan X. Altered Neural Correlates of Emotion Associated Pain Processing in Persistent Somatoform Pain Disorder: An fMRI Study. Pain Pract 2016; [DOI] [PubMed] [Google Scholar]
- [83].Ma X, Li S, Tian J, Jiang G, Wen H, Wang T, Fang J, Zhan W, Xu Y. Altered brain spontaneous activity and connectivity network in irritable bowel syndrome patients: A resting-state fMRI study. Clin Neurophysiol 2015;126:1190–1197. [DOI] [PubMed] [Google Scholar]
- [84].Maclean PD, Delgado JM. Electrical and chemical stimulation of frontotemporal portion of limbic system in the waking animal. Electroencephalogr Clin Neurophysiol 1953;5:91–100. [DOI] [PubMed] [Google Scholar]
- [85].Maleki N, Brawn J, Barmettler G, Borsook D, Becerra L. Pain response measured with arterial spin labeling. NMR Biomed 2013;26:664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Manning BH, Merin NM, Meng ID, Amaral DG. Reduction in opioid- and cannabinoid-induced antinociception in rhesus monkeys after bilateral lesions of the amygdaloid complex. J Neurosci 2001;21:8238–8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Mano H, Kotecha G, Leibnitz K, Matsubara T, Nakae A, Shenker N, Shibata M, Voon V, Yoshida W, Lee M, Yanagida T, Kawato M, Rosa MJ, Seymour B. Classification and characterisation of brain network changes in chronic back pain: A multicenter study. Wellcome Open Res 2018;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mansour A, Baria AT, Tetreault P, Vachon-Presseau E, Chang PC, Huang L, Apkarian AV, Baliki MN. Global disruption of degree rank order: a hallmark of chronic pain. Sci Rep 2016;6:34853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Maren S, Holmes A. Stress and Fear Extinction. Neuropsychopharmacology 2016;41:58–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Martinsen S, Flodin P, Berrebi J, Lofgren M, Bileviciute-Ljungar I, Ingvar M, Fransson P, Kosek E. Fibromyalgia patients had normal distraction related pain inhibition but cognitive impairment reflected in caudate nucleus and hippocampus during the Stroop Color Word Test. PLoS One 2014;9:e108637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Matre DA, Hernandez-Garcia L, Tran TD, Casey KL. “First pain” in humans: convergent and specific forebrain responses. Mol Pain 2010;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].May A Chronic pain may change the structure of the brain. Pain 2008;137:7–15. [DOI] [PubMed] [Google Scholar]
- [93].Mazza S, Frot M, Rey AE. A comprehensive literature review of chronic pain and memory. Prog Neuropsychopharmacol Biol Psychiatry 2017; [DOI] [PubMed] [Google Scholar]
- [94].McCormick C, Moscovitch M, Valiante TA, Cohn M, McAndrews MP. Different neural routes to autobiographical memory recall in healthy people and individuals with left medial temporal lobe epilepsy. Neuropsychologia 2018;110:26–36. [DOI] [PubMed] [Google Scholar]
- [95].McCormick C, St-Laurent M, Ty A, Valiante TA, McAndrews MP. Functional and effective hippocampal-neocortical connectivity during construction and elaboration of autobiographical memory retrieval. Cereb Cortex 2015;25:1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci 2001;933:265–277. [DOI] [PubMed] [Google Scholar]
- [97].McKenna JE, Melzack R. Analgesia produced by lidocaine microinjection into the dentate gyrus. Pain 1992;49:105–112. [DOI] [PubMed] [Google Scholar]
- [98].Melzack R The short-form McGill Pain Questionnaire. Pain 1987;30:191–197. [DOI] [PubMed] [Google Scholar]
- [99].Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature 1998;395:381–383. [DOI] [PubMed] [Google Scholar]
- [100].Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 2007;62:446–454. [DOI] [PubMed] [Google Scholar]
- [101].Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem 2006;85:213–218. [DOI] [PubMed] [Google Scholar]
- [102].Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2006;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group P-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Moisset X, Villain N, Ducreux D, Serrie A, Cunin G, Valade D, Calvino B, Bouhassira D. Functional brain imaging of trigeminal neuralgia. Eur J Pain 2011;15:124–131. [DOI] [PubMed] [Google Scholar]
- [105].Moscovitch M, Cabeza R, Winocur G, Nadel L. Episodic Memory and Beyond: The Hippocampus and Neocortex in Transformation. Annu Rev Psychol 2016;67:105–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Moulton EA, Becerra L, Maleki N, Pendse G, Tully S, Hargreaves R, Burstein R, Borsook D. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine States. Cereb Cortex 2011;21:435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Mutso AA, Petre B, Huang L, Baliki MN, Torbey S, Herrmann KM, Schnitzer TJ, Apkarian AV. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J Neurophysiol 2014;111:1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Niddam DM, Chan RC, Lee SH, Yeh TC, Hsieh JC. Central representation of hyperalgesia from myofascial trigger point. Neuroimage 2008;39:1299–1306. [DOI] [PubMed] [Google Scholar]
- [109].Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol 2004;92:1–9. [DOI] [PubMed] [Google Scholar]
- [110].Parkes L, Fulcher B, Yucel M, Fornito A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. Neuroimage 2018;171:415–436. [DOI] [PubMed] [Google Scholar]
- [111].Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol 2004;14:198–202. [DOI] [PubMed] [Google Scholar]
- [112].Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 2005;48:175–187. [DOI] [PubMed] [Google Scholar]
- [113].Piche M, Arsenault M, Rainville P. Dissection of perceptual, motor and autonomic components of brain activity evoked by noxious stimulation. Pain 2010;149:453–462. [DOI] [PubMed] [Google Scholar]
- [114].Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci 2001;21:9896–9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Pohlack ST, Nees F, Ruttorf M, Schad LR, Flor H. Activation of the ventral striatum during aversive contextual conditioning in humans. Biol Psychol 2012;91:74–80. [DOI] [PubMed] [Google Scholar]
- [116].Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci 2013;17:230–240. [DOI] [PubMed] [Google Scholar]
- [117].Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012;59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 2008;33:56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Rainville P, Bao QV, Chretien P. Pain-related emotions modulate experimental pain perception and autonomic responses. Pain 2005;118:306–318. [DOI] [PubMed] [Google Scholar]
- [120].Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci 2012;13:713–726. [DOI] [PubMed] [Google Scholar]
- [121].Roy M, Piche M, Chen JI, Peretz I, Rainville P. Cerebral and spinal modulation of pain by emotions. Proc Natl Acad Sci U S A 2009;106:20900–20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci 2007;8:657–661. [DOI] [PubMed] [Google Scholar]
- [123].Scheinost D, Finn ES, Tokoglu F, Shen X, Papademetris X, Hampson M, Constable RT. Sex differences in normal age trajectories of functional brain networks. Hum Brain Mapp 2015;36:1524–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Schmidt K, Forkmann K, Sinke C, Gratz M, Bitz A, Bingel U. The differential effect of trigeminal vs. peripheral pain stimulation on visual processing and memory encoding is influenced by pain-related fear. Neuroimage 2016;134:386–395. [DOI] [PubMed] [Google Scholar]
- [125].Schneider C, Palomba D, Flor H. Pavlovian conditioning of muscular responses in chronic pain patients: central and peripheral correlates. Pain 2004;112:239–247. [DOI] [PubMed] [Google Scholar]
- [126].Schreiber KL, Loggia ML, Kim J, Cahalan CM, Napadow V, Edwards RR. Painful After-Sensations in Fibromyalgia are Linked to Catastrophizing and Differences in Brain Response in the Medial Temporal Lobe. J Pain 2017;18:855–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Schwedt TJ, Chong CD, Chiang CC, Baxter L, Schlaggar BL, Dodick DW. Enhanced pain-induced activity of pain-processing regions in a case-control study of episodic migraine. Cephalalgia 2014;34:947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Shimo K, Ueno T, Younger J, Nishihara M, Inoue S, Ikemoto T, Taniguchi S, Ushida T. Visualization of painful experiences believed to trigger the activation of affective and emotional brain regions in subjects with low back pain. PLoS One 2011;6:e26681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D. The human amygdala and pain: evidence from neuroimaging. Hum Brain Mapp 2014;35:527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science 1991;253:1380–1386. [DOI] [PubMed] [Google Scholar]
- [131].Stoeter P, Bauermann T, Nickel R, Corluka L, Gawehn J, Vucurevic G, Vossel G, Egle UT. Cerebral activation in patients with somatoform pain disorder exposed to pain and stress: an fMRI study. Neuroimage 2007;36:418–430. [DOI] [PubMed] [Google Scholar]
- [132].Ter Minassian A, Ricalens E, Humbert S, Duc F, Aube C, Beydon L. Dissociating anticipation from perception: Acute pain activates default mode network. Hum Brain Mapp 2013;34:2228–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum Brain Mapp 2012;33:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Vachon-Presseau E, Centeno MV, Ren W, Berger SE, Tetreault P, Ghantous M, Baria A, Farmer M, Baliki MN, Schnitzer TJ, Apkarian AV. The Emotional Brain as a Predictor and Amplifier of Chronic Pain. J Dent Res 2016;95:605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Vachon-Presseau E, Roy M, Martel MO, Caron E, Marin MF, Chen J, Albouy G, Plante I, Sullivan MJ, Lupien SJ, Rainville P. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain 2013;136:815–827. [DOI] [PubMed] [Google Scholar]
- [136].Veinante P, Yalcin I, Barrot M. The amygdala between sensation and affect: a role in pain. J Mol Psychiatry 2013;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Veldhuijzen DS, Nemenov MI, Keaser M, Zhuo J, Gullapalli RP, Greenspan JD. Differential brain activation associated with laser-evoked burning and pricking pain: An event-related fMRI study. Pain 2009;141:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Wagner S, Sebastian A, Lieb K, Tuscher O, Tadic A. A coordinate-based ALE functional MRI meta-analysis of brain activation during verbal fluency tasks in healthy control subjects. BMC Neurosci 2014;15:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Wang Y, Zhang X, Guan Q, Wan L, Yi Y, Liu CF. Altered reginal homogeneity of spontaneous brain activity in idiopathic trigeminal neuralgia. Neuropsychiatric Disease and Treatment 2015;11:2659–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Weissman-Fogel I, Moayedi M, Taylor KS, Pope G, Davis KD. Cognitive and default-mode resting state networks: do male and female brains “rest” differently? Hum Brain Mapp 2010;31:1713–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012;2:125–141. [DOI] [PubMed] [Google Scholar]
- [142].Wirt RA, Hyman JM. Integrating Spatial Working Memory and Remote Memory: Interactions between the Medial Prefrontal Cortex and Hippocampus. Brain Sci 2017;7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Wood PB, Patterson JC 2nd, Sunderland JJ, Tainter KH, Glabus MF, Lilien DL. Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: a pilot study. J Pain 2007;8:51–58. [DOI] [PubMed] [Google Scholar]
- [144].Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 2011;8:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Zhang SS, Wu W, Liu ZP, Huang GZ, Guo SG, Yang JM. Altered regional homogeneity in experimentally induced low back pain: a resting-state fMRI study. J Neuroeng Rehabil 2014;11:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Ziv M, Tomer R, Defrin R, Hendler T. Individual sensitivity to pain expectancy is related to differential activation of the hippocampus and amygdala. Hum Brain Mapp 2010;31:326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.