Abstract
Protective immunity to Mycobacterium tuberculosis (Mtb) remains poorly understood and the role of Mtb-specific CD8+ T cells is controversial. Here we performed a broad phenotypic and functional characterization of Mtb-specific CD8+ T cells in 326 subjects with latent Mtb infection (LTBI) or active TB disease (TB). Mtb-specific CD8+ T cells were detected in most (60%) TB patients and few (15%) LTBI subjects but were of similar magnitude. Mtb-specific CD8+ T cells in LTBI subjects were mostly TEMRA cells (CD45RA+ CCR7− ), coexpressing 2B4 and CD160, and in TB patients were mostly TEM cells (CD45RA− CCR7− ), expressing 2B4 but lacking PD-1 and CD160. The cytokine profile was not significantly different in both groups. Furthermore, Mtb-specific CD8+ T cells expressed low levels of perforin and granulysin but contained granzymes A and B. However, in vitro-expanded Mtb-specific CD8+ T cells expressed perforin and granulysin. Finally, Mtb-specific CD8+ T-cell responses were less frequently detected in extrapulmonary TB compared with pulmonary TB patients. Mtb-specific CD8+ T-cell proliferation was also greater in patients with extrapulmonary compared with pulmonary TB. Thus, the activity of Mtb infection and clinical presentation are associated with distinct profiles of Mtb-specific CD8+ T-cell responses. These results provide new insights in the interaction between Mtb and the host immune response.
Keywords: Active TB disease, Cytotoxicity, Functional profile, Latent Mtb infection, Mtb-specific CD8+ T cells
Introduction
One-third of the world’s population is believed to be latently infected with Mycobacterium tuberculosis (Mtb) and two million people die of tuberculosis (TB) every year [1], thus underscoring the tremendous need for protective vaccines, new diagnostic tools, and medications.
T lymphocytes are thought to play an important role in the control of TB and Mtb may reactivate under certain conditions of immunodeficiency such as in elderly or secondary to coinfection with HIV or to immunosuppressive therapy [2, 3]. Several studies have underscored the essential role of CD4+ T cells in protection against Mtb, since CD4+ T-cell depletion is also associated with Mtb reactivation in HIV-infected individuals [4] and uncontrolled bacilli growth [5, 6]. The protective Mtb-specific CD4+ T-cell response is considered to be a typical TH1 response with CD4+ T cells producing cytokines such as IFN-γ or TNF-α that contribute to the recruitment of monocytes and granulocytes and activate the antimicrobial activity of macrophages [7, 8]. Of interest, we recently demonstrated that Mtb-specific CD4+ T-cell responses were functionally different in patients with active TB disease as compared with those in subjects with latent Mtb infection (LTBI) [9]. Several studies also suggested a role of TH17 cells in the control of TB [10, 11].
The importance and the role of Mtb-specific CD8+ T cells in the control of Mtb and their mechanism of action remain highly controversial. A number of secreted immunodominant Mtb antigens can be processed by cytosolic pathways and presented by MHC class I molecules [12–14]. Several studies performed in mice and nonhuman models have proposed a role of Mtb-specific CD8+ T cells in the control of Mtb infection [15–17]. In these models, IFN-γ and perforin production by Mtb-specific CD8+ T cells was necessary to protect mice from Mtb infection [15, 18]. Other studies performed in humans supported these conclusions [19, 20]. Although many in vitro studies indicated that perforin- and/or granulysin-containing Mtb-specific CD8+ T-cell lines were able to kill Mtb-infected macrophages or free bacteria [21–23], Mtb-specific CD8+ T cells from lung-associated tissues generally lacked expression of these effector molecules [24, 25].
In the present study, we performed broad phenotypic (T-cell differentiation and exhaustion) and functional (cytokines production, proliferation capacity, and cytotoxic potential) characterizations of Mtb-specific CD8+ T-cell responses in 326 TB and LTBI subjects and evaluated their correlation with different clinical presentations of Mtb infection. In particular, we hypothesized that the detection of Mtb-specific CD8+ T cells and their phenotype and function may vary in active TB versus latent infection.
Our results have shown differences in the prevalence, frequency, and phenotypic and functional profiles of Mtb-specific CD8+ T cells in active disease versus latent infection and between pulmonary TB (PTB) and extrapulmonary TB (ETB). These findings provide new insights in the role of CD8+ T cells in Mtb infection and disease.
Results
Identification and frequency of Mtb-specific CD8+ T cells in TB and LTBI subjects
We have studied 326 individuals with either active TB disease (TB) or LTBI.
Mtb-specific CD8+ T-cell responses were assessed using polychromatic flow cytometry following stimulation with ESAT-6 and/or CFP-10 peptide pools. The flow cytometry panel included a viability marker, CD3, CD4, and CD8 to determine T-cell lineage and IFN-γ, TNF-α, and IL-2 antibodies.
Mtb-specific CD8+ T-cell responses were detected in 52 out of the 86 (60%) TB patients, and in 37 out of the 240 (15%) LTBI subjects (p < 0.0001; Fig. 1A). Overall, 50 and 58 Mtb-specific CD8+ T-cell responses (directed against CFP-10 or ESAT-6) were observed in the 37 and 52 LTBI and TB individuals with detectable Mtb-specific CD8+ T-cell responses, respectively. These Mtb-specific CD8+ T-cell responses in both groups were directed against CFP-10, ESAT-6 or both peptide pools in 50–60%, 20–25%, and 20–30% of cases, respectively (data not shown).
Figure 1.
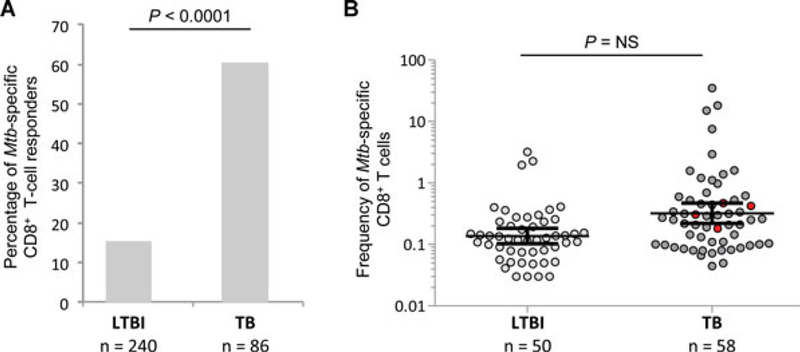
Detection and magnitude of Mtb-specific CD8+ T-cell responses in LTBI subjects and TB patients: (A) Proportion of LTBI subjects and TB patients with Mtb-specific CD8+ T-cell responses. Statistical significance was calculated using two-tailed Fisher’s exact test. CD8+ T cells were gated as shown in Supporting Information Fig. 1A. (B) Magnitude (mean with 95% CI) of Mtb-specific CD8+ T-cell responses in the 37 LTBI and 52 TB patients with Mtb-specific CD8+ T-cell responses. Mtb-specific CD8+ T-cell responses were defined by the presence of IFN-γ-producing CD8+ CD4− CD3+ T cells following stimulation with ESAT-6 and/or CFP-10 peptide pools. Red points identify Mtb-specific CD8+ T-cell responses from HIV-coinfected subjects. An unpaired two-tailed Student’s t-test was performed.
Of interest, the magnitude of Mtb-specific CD8+ T-cell responses, as determined by the frequency of IFN-γ-producing CD8+ T cells following ESAT-6 or CFP-10 stimulation, was not significantly different between LTBI and TB subjects (p > 0.05; Fig. 1B). Therefore, these results indicate that Mtb-specific CD8+ T-cell responses are a component of the host immune response during active TB disease and also potentially, to some extent, during latent infection.
T-cell differentiation of Mtb-specific CD8+ T cells in LTBI and TB subjects
We then investigated the level of T-cell differentiation of Mtb-specific CD8+ T cells in a portion of the LTBI and TB subjects. Subjects were randomly selected based on the availability of cryopre-served PBMC. For this purpose, Mtb-specific CD8+ T-cell responses were evaluated for the expression of CD45RA and CCR7. As shown in the representative subject LTBI#910, most Mtb-specific CD8+ T cells were CD45RA+ CCR7− (i.e. terminally differentiated; TEMRA), whereas in the representative patient TB#TB-3, the majority of Mtb-specific CD8+ T cells were CD45RA− CCR7− (i.e. effector memory; TEM) (Fig. 2A). Cumulative analyses confirmed that the majority of Mtb-specific CD8+ T cells were composed of TEMRA and TEM in LTBI and TB subjects, respectively (both p < 0.00001; Fig. 2B).
Figure 2.
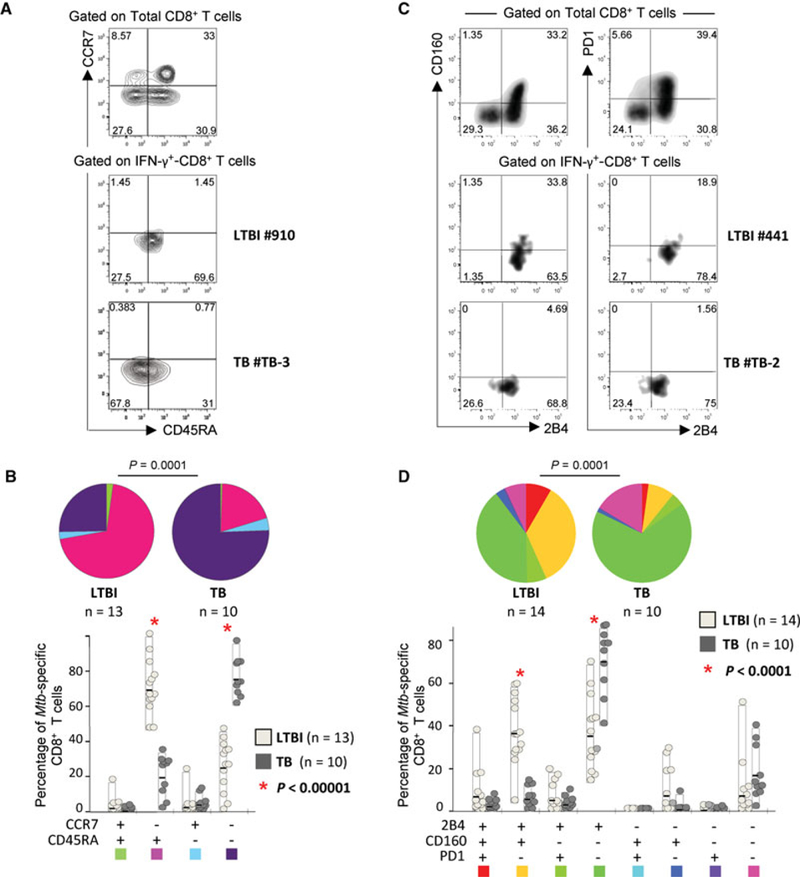
T-cell differentiation and exhaustion of Mtb-specific CD8+ T cells in LTBI subjects and TB patients. (A) Representative flow cytometry examples and (B) cumulative analyses of the expression of CCR7 and CD45RA on Mtb-specific CD8+ T cells from LTBI subjects and TB patients are shown. CD8+ T cells were gated as shown in Supporting Information Fig. 1A. As in all other flow cytometry analyses from this study, the gating is based on the distribution of the different markers on bulk CD8+ T cells (A; top) and it is then conserved in the analyses of Mtb-specific CD8+ T cells (A; bottom). (C) Representative flow cytometry examples and (D) cumulative analyses of the expression of PD-1, 2B4, and CD160 on Mtb-specific CD8+ T cells from LTBI subjects and TB patients are shown. (A, C) Flow cytometry profiles are gated on live CD3+ CD4− CD8+ T cells and Mtb-specific CD8+ T-cell responses were defined as IFN-γ-producing cells following stimulation with ESAT-6 and/or CFP-10 peptide pools. (B, D) For cumulative analyses, all the possible combinations of the different markers are shown on the x-axis whereas the percentages of the distinct T-cell subsets within Mtb-specific CD8+ T cells are shown on the y-axis. The pie charts summarize the data, and each slice corresponds to the mean proportion of Mtb-specific CD8+ T cells positive for a certain combination of markers. (B, D) Comparisons of markers distribution were performed using a Student’s t-test and a partial permutation test as described [50].
Therefore, these results indicate that Mtb-specific CD8+ T cells in TB and LTBI subjects have distinct stages of differentiation.
Expression of regulatory receptors in Mtb-specific CD8+ T cells
We then investigated the expression of regulatory receptors in Mtb-specific CD8+ T cells in a portion of LTBI and TB individuals randomly selected based on the availability of cryopreserved PBMC. For this purpose, Mtb-specific CD8+ T-cell responses were evaluated for the expression of PD-1, 2B4, and CD160, which are three relevant coinhibitory molecules whose expression is associated to functional defects [26]. As shown in the representative flow cytometric profiles and confirmed in the cumulative analyses (Fig. 2C and D), the majority of Mtb-specific CD8+ T cells in TB patients expressed 2B4 but mostly lacked PD-1 and CD160, that is, were 2B4+ PD1− CD160−. Differently, in LTBI subjects, the Mtb-specific 2B4+ PD1− CD160− CD8+ T-cell population was significantly lower (p < 0.0001); moreover a substantial proportion (about 50%) of Mtb-specific CD8+ T cells coexpressed CD160 and/or PD-1 in addition to 2B4 (Fig. 2C and D).
Therefore, Mtb-specific CD8+ T cells from LTBI and TB subjects showed significant differences regarding the expression of regulatory receptors (p < 0.0001; Fig. 2D).
Functional profile of Mtb-specific CD8+ T cells in LTBI and TB subjects
We then performed a broad characterization of the functional profile of Mtb-specific CD8+ T cells. Consistently with previous studies [27,28], we observed that in both LTBI and TB patients, the majority of Mtb-specific CD8+ T cells were composed of dual IFN-γ/TNF-α- or single IFN-γ-producing cells (Supporting Information Fig. 2). Still, Mtb-specific CD8+ T cells of LTBI subjects contained a greater (p = 0.03) proportion of triple cytokine producing CD8+ T cells (i.e. IFN-γ+ TNF-α+ IL-2+ ) whereas a higher proportion of single TNF-α-producing CD8+ T cells was found in TB patients (p = 0.03; Supporting Information Fig. 2). Considering the large number of individuals analyzed (n = 35 Mtb-specific CD8+ T-cell responses in each group), these data indicate that the cytokines profile of Mtb-specific CD8+ T cells is only slightly different between TB and LTBI subjects.
Cytotoxic potential of Mtb-specific CD8+ T cells
Previous studies suggesting a direct role of Mtb-specific CD8+ T cells in the control of Mtb infection proposed expression of perforin as a key mechanism [15, 18, 20]. We therefore analyzed perforin expression in Mtb-specific CD8+ T cells in a subset of TB and LTBI subjects randomly selected based on the availability of cryopreserved PBMCs. We found that about 10% of Mtb-specific CD8+ T cells contained perforin in TB and LTBI subjects ex vivo (Fig. 3A and B). However, a consistent proportion (20–30%) of total CD8+ T cells and of IFN-γ-producing CD8+ T cells following polyclonal stimulation (positive control) expressed perforin (Fig. 3A and B).
Figure 3.
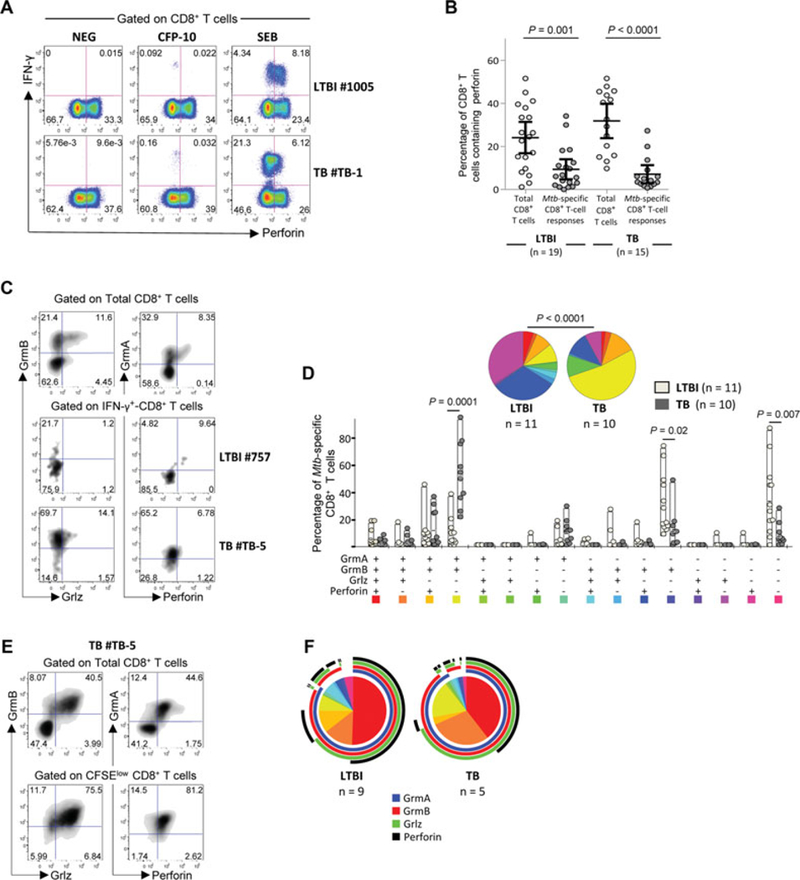
Cytotoxic potential of Mtb-specific CD8+ T-cell responses in LTBI subjects and TB patients. (A) Flow cytometric profiles showing perforin expression on Mtb-specific IFN-γ-producing CD8+ T cells in representative LTBI subjects and TB patients. CD8+ T cells were gated as shown in Supporting Information Fig. 1A. The flow cytometric profiles of unstimulated cells (negative control) and cells stimulated with a polyclonal stimulation (positive control) are also shown. Flow cytometry profiles are gated on live CD3+ CD4− CD8+ T cells and Mtb-specific CD8+ T-cell responses were defined as IFN-γ-producing cells following stimulation with ESAT-6 and/or CFP-10 peptide pools. (B) Percentages (mean with 95% CI) of perforin expression in total- and Mtb-specific CD8+ T cells from LTBI (n = 19) or TB (n = 15) patients. Unpaired two-tailed Student’s t-tests were performed. (C) Representative flow cytometry examples and (D) cumulative analyses of the expression of perforin, granzyme (Grm) B, GrmA, and granulysin (Grlz) on Mtb-specific CD8+ T cells from LTBI subjects and TB patients. All the possible combinations of the different markers are shown on the x-axis whereas the percentages of the distinct T-cell subsets within Mtb-specific CD8+ T cells are shown on the y-axis. (E) Representative flow cytometry example and (F) cumulative analyses of the expression of perforin, GrmB, GrmA, and Grlz on Mtb-specific CD8+ T cells from LTBI subjects and TB patients after 6 days of antigen-specific in vitro T-cell expansion. (D, F) The pie charts summarize the data, and each slice corresponds to the mean proportion of Mtb-specific CD8+ T cells positive for a certain combination of markers identified by the respective arcs. Regarding SPICE analyses, comparison of distributions (D) was performed using a Student’s t-test and a partial permutation test as described [50].
We then investigated the expression of additional cytotoxic granules such as granzyme (Grm)B, GrmA, and granulysin (Grlz) known to be also associated with the cytotoxic capacity of CD8+ T cells [22, 23]. Mtb-specific CD8+ T cells from LTBI subjects lacked expression of all cytotoxic markers in about 40% of cells and only contained GrmB in about 30% of cells. Interestingly, the majority (>55%) of Mtb-specific CD8+ T cells from TB patients were composed of cells coexpressing GrmB and GrmA (p < 0.0001; Fig. 3C and D) whereas less than 10% contained Grlz (Fig. 3A and D).
Since perforin, GrmB, and GrmA are upregulated upon T-cell stimulation and proliferation [29], CFSE-labeled mononuclear cells from LTBI subjects or TB patients were stimulated with the cognate antigens for 6 days and assessed for the expression of the cytotoxic granules after in vitro expansion. No significant difference in the proliferation capacity (i.e. the percentage of CFSElow CD8+ T cells) was observed between LTBI subjects and TB patients (data not shown) and Mtb-specific CD8+ T cells coexpressed perforin, granulysin, and granzymes in both TB and LTBI individuals (Fig. 3E and F).
Overall, these results indicate that Mtb-specific CD8+ T cells from TB and LTBI subjects express distinct patterns of cytotoxic granules ex vivo and that expression of perforin and Grlz can be induced after antigen-specific in vitro T-cell expansion.
Association between Mtb-specific CD8+ T-cell responses and clinical presentation
Since phenotypic and functional differences in Mtb-specific CD8+ T cells were observed between subjects with LTBI versus active TB disease, it was possible that the type of clinical presentation could play a role in the distinct phenotypic and functional profiles observed. Of note, among the 86 TB patients enrolled in this study, and based on WHO classification [30], 67 patients had PTB whereas 19 patients had ETB.
Mtb-specific CD8+ T cells were significantly more frequently detected in PTB as compared with ETB (67 versus 37%, respec-tively; p = 0.017; Fig. 4A). Furthermore, differently from PTB, a broader range of magnitude of Mtb-specific CD8+ T-cell responses was observed in ETB (Fig. 4B). PTB patients were also stratified according to the smear test that is commonly considered as a reflection of the bacterial burden [31]. Within PTB patients, Mtb-specific CD8+ T-cell responses were significantly greater in smear-positive as compared with those of smear-negative patients (p = 0.01; Fig. 4C). Furthermore, we also separately analyzed the two patients who were not microbiologically confirmed but were clear clinical PTB. Of interest, the magnitude of Mtb-specific CD8+ T cells from these two patients was typically in the range of that of the other smear-negative microbiologically confirmed PTB patients and thus was lower as compared with the responses from smear-positive PTB patients (Supporting Information Fig. 3). Of note, the cytokines and perforin profiles were not different between smear-positive and smear-negative, or between PTB and ETB patients, or between microbiologically confirmed and clinical TB (data not shown).
Figure 4.
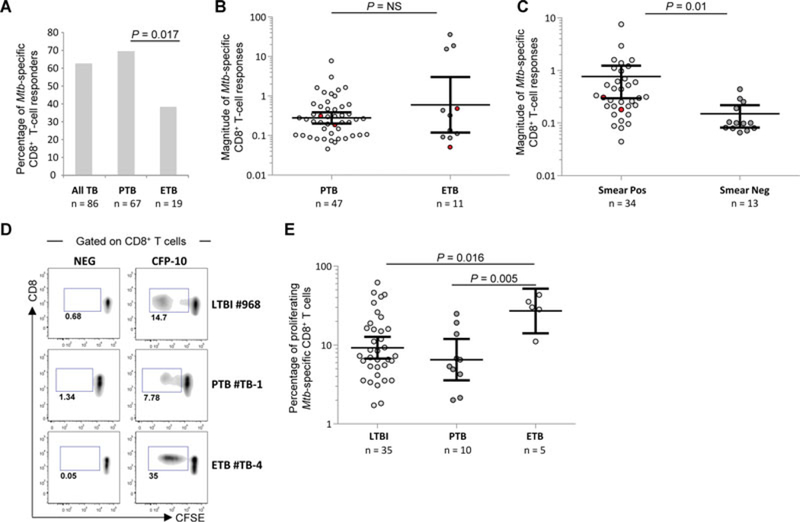
Associations between Mtb-specific CD8+ T-cell responses and clinical presentation. (A) Proportion of patients with Mtb-specific CD8+ T-cell responses in all TB patients (n = 86) and in patients with pulmonary TB (PTB; n = 67) or extrapulmonary TB (ETB; n = 19). Statistical significance was calculated using two-tailed Fisher’s exact test. (B) Magnitude (mean with 95% CI) of the frequency of Mtb-specific IFN-γ-producing CD8+ T-cell responses in PTB (n = 47) and ETB (n = 11) patients with detectable Mtb-specific CD8+ T-cell responses (two-tailed Mann–Whitney test). Red points identify Mtb-specific CD8+ T-cell responses from HIV-coinfected subjects. (C) Magnitude (mean with 95% CI) of the frequency of Mtb-specific IFN-γ-producing CD8+ T-cell responses within PTB patients subdivided into smear-positive (n = 34) and smear-negative (n = 13) patients. Red points identify Mtb-specific CD8+ T-cell responses from HIV-coinfected subjects. (D) Representative flow cytometry examples and (E) cumulative analyses (mean with 95% CI) of the frequency of Mtb-specific CD8+ T cells endowed with proliferation capacity in LTBI (n = 35), PTB (n = 10) and ETB (n = 5) patients. T-cell proliferation was determined using the CFSE dilution assay and profiles are gated on live CD3+ CD8+ CD4− T cells as shown in Supporting Information Fig. 1B. Two-tailed Mann–Whitney tests were performed.
We also compared the proliferation capacity of Mtb-specific CD8+ T cells between ETB and PTB patients in a subset of patients randomly selected on the basis of the availability of cryopreserved PBMC. The proliferation capacity of Mtb-specific CD8+ T cells, as determined by the frequency of CFSElow CD8+ T cells following stimulation with ESAT-6 or CFP-10, was significantly higher in ETB patients as compared with those in PTB patients (p = 0.005) and to LTBI subjects (p = 0.016; Fig. 4D and E). Of note, the proliferation capacity of Mtb-specific CD4+ T cells was not different between PTB and ETB patients (Supporting Information Fig. 4) and thus did not skew Mtb-specific CD8+ T-cell responses.
These data indicate significant associations between the clinical presentation of TB and profiles of Mtb-specific CD8+ T cells.
Discussion
Although growing evidence suggests that CD8+ T cells contribute to the control of Mtb [15–20, 32, 33], the results obtained in humans and in animal models remain controversial and the discrepancies likely result from the difficulty to compare investigations performed by in vitro studies versus in vivo observations or from the antigens utilized to discriminate between T-cell responses induced by infection versus vaccination [34, 35].
Mtb-specific CD8+ T-cell responses against ESAT-6 or CFP-10 were detected predominantly in patients with active TB disease as compared with LTBI subjects, consistently with a previous report by Day and colleagues performed on whole blood [28]. Previous studies have shown that ESAT-6 and CFP-10 antigens can identify Mtb-specific T-cell responses in 99% of LTBI and TB patients [36]. However, since Mtb expresses approximately 4000 proteins [37], we cannot exclude that additional Mtb-specific CD8+ T-cell responses may be directed particularly against latency antigens [38].
One potential mechanism to explain the greater frequency of Mtb-specific CD8+ T-cell responses in TB patients is that these responses are predominantly stimulated in the presence of higher antigen load [39]. This hypothesis is supported by a recent study performed in children showing that Mtb-specific CD8+ T cells were detected in active TB disease but not in healthy children recently exposed to Mtb, despite that similar frequencies of Mtb-specific CD4+ T-cell responses were present in both groups [40]. Along the same lines, the higher number of granulomas found in TB patients as compared with that in LTBI subjects may also be a significant determinant of the reduced proportion of Mtb-specific CD8+ T-cell responses in LTBI subjects.
Major phenotypic and functional differences were observed between TB and LTBI subjects, consistently with previous studies [27, 28]. Mtb-specific CD8+ T cells were mostly composed of TEMRA in LTBI and of TEM in TB patients. This is consistent with the observation that TNF-α blockade induced a decrease of TEMRA CD8+ T cells, underscoring the potential role of Mtb-specific TEMRA CD8+ T cells in Mtb control [19]. These results are also consistent with current models of antiviral immunity suggesting that TEMRA and TEM are associated with chronic controlled and uncontrolled virus infection, respectively [41]. Our results suggest that this may be also the case for Mtb-specific CD8+ T cells in LTBI and active disease.
It is well established that coregulatory molecules are upregulated upon activation and that coexpression of these receptors is associated with a state of T-cell exhaustion (reviewed in [26]). We have found little coexpression of PD-1, CD160, and 2B4 in Mtb-specific CD8+ T cells from TB patients. Significant differences were observed in the expression of CD160 between TB and LTBI; however PD-1 was always expressed at low levels thus questioning the hypothesis of exhaustion of Mtb-specific CD8+ T cells. The findings that Mtb-specific CD8+ T cells retained proliferation capacity and had a polyfunctional cytokines profile are also not supporting the exhaustion hypothesis.
Furthermore, there were no major differences in the cytokines profile of Mtb-specific CD8+ T-cell responses between LTBI subjects and TB patients. Still, it is interesting to note that Mtb-specific CD8+ T cells were more polyfunctional (i.e. IFN-γ+ TNF-α+ IL-2+ ) in LTBI, consistently with the current paradigm in antiviral immunity (reviewed in [41]). Also, Mtb-specific CD8+ T cells were enriched in single TNF-α-producing CD8+ T cells in TB patients, thus following the same trend than CD4+ T cells [9].
The current model for the protective role of CD8+ T cells in TB is based on the ability to lyse infected cells (in addition to cytokines release). Differently from many animal studies [42, 43], we found that Mtb-specific CD8+ T cells expressed little perforin and granulysin ex vivo. These results are consistent with in situ analyses of human tissues [25, 44]. We observed, however, that Mtb-specific CD8+ T cells expressed significant levels of GrmB and GrmA. Taken together, these observations suggest a potential perforin-independent cytotoxic mechanism of action of Mtb-specific CD8+ T cells. Furthermore, the majority of Mtb-specific CD8+ T cells expressed perforin, GrmA, GrmB, and Grlz after antigen-specific in vitro T-cell expansion, consistently with studies performed on T-cell lines [22], activated T cells [31], and animal models [15, 18].
Next we investigated whether the bacterial load (i.e. smear-positive versus smear-negative TB) or the clinical presentation of TB disease (i.e. PTB versus ETB) correlated with distinct profiles of Mtb-specific CD8+ T-cell responses. We observed a higher prevalence of Mtb-specific CD8+ T-cell responses in PTB compared with ETB and a higher magnitude of these responses in smear-positive versus smear-negative PTB patients. We also observed limited proliferation capacity of Mtb-specific CD8+ T cells from PTB patients compared with ETB patients. This is consistent with the current paradigm associating CD8+ T-cell responses to high antigen burden [28, 39, 40]. Furthermore, as mentioned above, these functional differences may reflect different conditions in the stimulation of the immune responses in the different anatomic sites, to the tropism of responding T cells or to distinct stages of disease.
Overall, we have identified several phenotypic and functional differences in the profiles of Mtb-specific CD8+ T cells between patients with active disease and LTBI subjects.
Notwithstanding the key role of CD4+ T cells in the control of Mtb infection, we report here several associations between profiles of Mtb-specific CD8+ T cells and distinct clinical presentations. In particular, we found significant differences in the phenotypic (e.g. T-cell differentiation) and functional (e.g. GrmA expression) profiles between patients with active TB disease and subjects with latent infection. Whether these phenotypic and functional profiles reflect different levels of immune control remains to be determined.
Recent studies [45, 46] also questioned whether LTBI subjects represent a model of efficient control of Mtb. In this regard, there is growing evidence that LTBI corresponds to a broad spectrum of subjects with Mtb infection ranging from exposed uninfected subjects to subjects with subclinical TB [46]. One could speculate that the presence of CD8+ T cells (found in 15% of LTBI) may represent a marker of truly chronically infected subjects and potentially identify subjects at risk of reactivation (occurring in about 10% of subjects) that would benefit from chemoprophylaxis. Longitudinal studies are needed to confirm this hypothesis.
In conclusion, our descriptive study allowed us to identify major differences in the prevalence, function, and phenotype of Mtb-specific CD8+ T-cell responses in active TB and LTBI. Additional studies are needed to clarify the mechanisms driving these differences. Still, our results represent a step forward in the understanding of the role of CD8+ T cells in TB pathogenesis and provide new insights on the use of distinct phenotypic and functional profiles of CD8+ T cells as markers of Mtb activity and different clinical presentation of TB disease.
Materials and methods
Study groups
The majority of the 240 LTBI subjects and 86 TB patients were recruited at the Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland and few patients were also recruited at the INMI, Rome, Italy or in Cape Town and Worcester, South Africa. All TB patients had a diagnosis based on laboratory isolation of Mtb on mycobacterial culture from sputum, BALF, or biopsies and/or tuberculin skin test and/or ELISPOT and/or PCR as described [9]. The final diagnosis was given by a clinician after validation of these criteria associated with clinical symptoms. Furthermore, based on WHO classification [30], the 86 TB patients included 67 patients with PTB and 19 patients with ETB. Of note, a subset of 52 TB patients was further investigated for immunological assessments and these patients were randomly selected based on the availability of cryopreserved PBMCs (which was dependent upon the volume of blood collected). Furthermore, the selection of the type of immunological measures performed in each patient was random thus excluding any selection bias. Demographic and clinical data on the 52 TB patients are described in Supporting Information Table 1. Also, four TB patients were coinfected with HIV. Since Mtb-specific CD8+ T-cell responses from these patients were similar to those from the HIV-seronegative patients (clearly identified with red dots in all analyses), they were not excluded from the analyses.
All LTBI subjects were asymptomatic and were either healthcare workers routinely screened or were investigated for Mtb infection prior to the initiation of anti-TNF-α antibody treatment and had negative chest radiographs. All LTBI subjects were IGRA-positive, that is, had Mtb-specific T-cell responses against ESAT-6 or CFP-10 using IFN-γ ELISPOT (Supporting Information Fig. 5). Of note, Mtb-specific CD8+ T-cell responses were identified in only 37 of the 240 LTBI subjects tested. As for TB patients, a subset of the 37 LTBI subjects was further investigated for immunological measures and the selection was based on the availability of cryopreserved PBMC (which was depending upon the volume of blood collected) and the selection of immunological measures performed in each patient was random thus excluding any selection bias. None of these subjects (TB or LTBI) was under antimycobacterial treatment for more than 1 week at the time of the enrollment and analysis. These studies were approved by the Institutional Review Boards of the different Centers and informed written consent was obtained from each volunteer.
Peptides
Mtb-derived peptides covering ESAT-6 and CFP-10 proteins were pools of HPLC-purified (>80% purity) 15-mers peptides overlapping by 11 amino acids as described [9].
Intracellular cytokine staining
For intracellular cytokine staining, cryopreserved blood mononuclear cells (1 × 106) were rested for 6 h and then stimulated overnight in Brefeldin A (1 μL/mL, BD) and anti-CD28 antibodies (0.5 μg/mL, BD) containing media as described [47]. Monensin (1 μL/mL, BD) was also added in cell cultures for the assessment of cytotoxicity as described [48]. For cell stimulations, peptide pools were used at 1 μg/mL for each peptide. SEB stimulation (200 ng/mL) served as positive control. After stimulation, cells were stained for dead cells (LIVE/DEAD kit, Invitrogen), permeabilized (Cytofix/Cytoperm, BD), and stained with various combinations of antibodies depending on the analysis.
Antigen-specific in vitro T-cell expansion
Cryopreserved cells were labeled with 5,6-CFSE (Molecular Probes) as described [49]. Subsequently, cells were cultured in 6% human AB serum (Institut de Biotechnologies Jacques Boy) RPMI. For cell stimulation, peptide pools were used at 1 μg/mL for each peptide. SEB stimulation (200 ng/mL) served as positive control. After 6 days of in vitro T-cell expansion, cells were washed and stained for dead cells (LIVE/DEAD kit, Invitrogen) and with the antibody panel described below. The percentage of proliferating CD8+ T cells, that is, CFSElow cells, was determined in the live CD8+ CD4− T-cell population.
Flow cytometry analyses
The following antihuman monoclonal antibodies were used in various combinations: CD3, CD4, CD8, IFN-γ, TNF-α, IL-2, granzyme B, PD-1, and CD45RA were purchased from BD; granzyme A and CD160 from Lucerna Chem; CD4 and 2B4 from BioLegend; granulysin from Bender MedSystems GmbH; Perforin from Biotest AG and CCR7 from R&D System. Data were acquired on an LSRII four laser (405, 488, 532, and 633 nm) and analyzed using FlowJo version 8.8.6 (Tree Star Inc.). Analysis and presentation of distributions was performed using SPICE version 5.1, downloaded from http://exon.niaid.nih.gov/spice/ [50].
Statistical analyses
Comparisons of categorical variables were performed using Fisher’s exact test. Statistical significance (P values) of the magnitude of responses was calculated with unpaired two-tailed Student’s t-test using GraphPad Prism 5 version 5.04. Mann–Whitney test (two tailed) was used as nonparametric test. Bonferroni correction for multiples analyses was applied. Regarding SPICE analyses of the flow cytometry data, comparison of distributions was performed using a Student’s t-test and a partial permutation test as described [50].
Supplementary Material
Acknowledgements:
The research leading to these results was supported by the Swiss National Science Foundation and by the Swiss vaccine Research Institute and has received funding from the European Community’s Seventh Framework Programme ([FP7/2007–2013] [FP7/2007–2011]) under EC-GA no. [241642]. The authors thank Delphine Gani and Kim Ellefsen-Lavoie for logistic coordination. The authors also thank many additional members of the SATVI team who helped with enrollment and evaluation of participants, and finally, the participants themselves.
Abbreviations:
- ETB
extrapulmonary TB
- LTBI
latent Mtb infection
- Mtb
Mycobacterium tuberculosis
- PTB
pulmonary TB
- TB
tuberculosis
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
Additional supporting information may be found in the online version of this article at the publisher’s web-site
References
- 1.Koul A, Arnoult E, Lounis N, Guillemont J and Andries K, The challenge of new drug discovery for tuberculosis. Nature 2011. 469: 483–490. [DOI] [PubMed] [Google Scholar]
- 2.Winthrop KL, Risk and prevention of tuberculosis and other serious opportunistic infections associated with the inhibition of tumor necrosis factor. Nat. Clin. Pract. Rheumatol 2006. 2: 602–610. [DOI] [PubMed] [Google Scholar]
- 3.Bumbacea D, Arend SM, Eyuboglu F, Fishman JA, Goletti D, Ison MG, Jones CE et al. , The risk of tuberculosis in transplant candidates and recipients: a TBNET consensus statement. Eur. Respir. J 2012. 40: 990–1013. [DOI] [PubMed] [Google Scholar]
- 4.Granich R, Akolo C, Gunneberg C, Getahun H, Williams P and Williams B, Prevention of tuberculosis in people living with HIV. Clin. Infect. Dis 2010. 50(Suppl 3): S215–S222. [DOI] [PubMed] [Google Scholar]
- 5.Scanga CA, Mohan VP, Yu K, Joseph H, Tanaka K, Chan J and Flynn JL, Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J. Exp. Med 2000. 192: 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR and Flynn JL, Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J. Immunol 1999. 162: 5407–5416. [PubMed] [Google Scholar]
- 7.Walzl G, Ronacher K, Hanekom W, Scriba TJ and Zumla A, Immunological biomarkers of tuberculosis. Nat. Rev. Immunol 2011. 11: 343–354. [DOI] [PubMed] [Google Scholar]
- 8.Flynn JL and Chan J, Tuberculosis: latency and reactivation. Infect. Immun 2001. 69: 4195–4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harari A, Rozot V, Enders FB, Perreau M, Stalder JM, Nicod LP, Cavassini M et al. , Dominant TNF-alpha+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat. Med 2011. 17: 372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA and Bloom BR, An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med 1993. 178: 2249–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY et al. , Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol 2008. 180: 1962–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewinsohn DM, Grotzke JE, Heinzel AS, Zhu L, Ovendale PJ, Johnson M and Alderson MR, Secreted proteins from Mycobacterium tuberculosis gain access to the cytosolic MHC class-I antigen-processing pathway. J. Immunol 2006. 177: 437–442. [DOI] [PubMed] [Google Scholar]
- 13.Woodworth JS, Fortune SM and Behar SM, Bacterial protein secretion is required for priming of CD8+ T cells specific for the Mycobacterium tuberculosis antigen CFP10. Infect. Immun 2008. 76: 4199–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grotzke JE, Siler AC, Lewinsohn DA and Lewinsohn DM, Secreted immunodominant Mycobacterium tuberculosis antigens are processed by the cytosolic pathway. J. Immunol 2010. 185: 4336–4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brighenti S and Andersson J, Induction and regulation of CD8+ cytolytic T cells in human tuberculosis and HIV infection. Biochem. Biophys. Res. Commun 2010. 396: 50–57. [DOI] [PubMed] [Google Scholar]
- 16.Lalvani A, Brookes R, Wilkinson RJ, Malin AS, Pathan AA, Andersen P, Dockrell H et al. , Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 1998. 95: 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CY, Huang D, Wang RC, Shen L, Zeng G, Yao S, Shen Y et al. , A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog 2009. 5: e1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzaccaro RJ, Stenger S, Rock KL, Porcelli SA, Brenner MB, Modlin RL and Bloom BR, Cytotoxic T lymphocytes in resistance to tuberculosis. Adv. Exp. Med. Biol 1998. 452: 85–101. [DOI] [PubMed] [Google Scholar]
- 19.Bruns H, Meinken C, Schauenberg P, Harter G, Kern P, Modlin RL, Antoni C et al. , Anti-TNF immunotherapy reduces CD8+ Tcell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J. Clin. Invest 2009. 119: 1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper AM, Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol 2009. 27: 393–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenger S, Mazzaccaro RJ, Uyemura K, Cho S, Barnes PF, Rosat JP, Sette A et al. , Differential effects of cytolytic T cell subsets on intracellular infection. Science 1997. 276: 1684–1687. [DOI] [PubMed] [Google Scholar]
- 22.Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, Ganz T et al. , An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 1998. 282: 121–125. [DOI] [PubMed] [Google Scholar]
- 23.Semple PL, Watkins M, Davids V, Krensky AM, Hanekom WA, Kaplan G and Ress S, Induction of granulysin and perforin cytolytic mediator expression in 10-week-old infants vaccinated with BCG at birth. Clin. Dev. Immunol 2011. 2011: 438463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahman S, Gudetta B, Fink J, Granath A, Ashenafi S, Aseffa A, Derbew M et al. , Compartmentalization of immune responses in human tuberculosis: few CD8+ effector T cells but elevated levels of FoxP3+ regulatory t cells in the granulomatous lesions. Am. J. Pathol 2009. 174: 2211–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson J, Samarina A, Fink J, Rahman S and Grundstrom S, Impaired expression of perforin and granulysin in CD8+ T cells at the site of infection in human chronic pulmonary tuberculosis. Infect. Immun 2007. 75: 5210–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vigano S, Perreau M, Pantaleo G and Harari A, Positive and negative regulation of cellular immune responses in physiologic conditions and diseases. Clin. Dev. Immunol 2012. 2012: 485781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caccamo N, Guggino G, Meraviglia S, Gelsomino G, Di Carlo P, Titone L, Bocchino M et al. , Analysis of Mycobacterium tuberculosis-specific CD8 T-cells in patients with active tuberculosis and in individuals with latent infection. PLoS One 2009. 4: e5528. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O’Rie T, Pienaar B et al. , Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J. Immunol 2011. 187: 2222–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harari A, Enders FB, Cellerai C, Bart PA and Pantaleo G, Distinct profiles of cytotoxic granules in memory CD8 T cells correlate with function, differentiation stage, and antigen exposure. J. Virol 2009. 83: 2862–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Global tuberculosis control: WHO (World Health Organization) Report 2011. http://www.who.int/tb/publications/global_report/archive/en/index.html.
- 31.Helke KL, Mankowski JL and Manabe YC, Animal models of cavitation in pulmonary tuberculosis. Tuberculosis (Edinb.) 2006. 86: 337–348. [DOI] [PubMed] [Google Scholar]
- 32.Behar SM, Dascher CC, Grusby MJ, Wang CR and Brenner MB, Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J. Exp. Med 1999. 189: 1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flynn JL, Goldstein MM, Triebold KJ, Koller B and Bloom BR, Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 1992. 89: 12013–12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinzel AS, Grotzke JE, Lines RA, Lewinsohn DA, McNabb AL, Streblow DN, Braud VM et al. , HLA-E-dependent presentation of Mtb-derived antigen to human CD8+ T cells. J. Exp. Med 2002. 196: 1473–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodworth JS and Behar SM, Mycobacterium tuberculosis-specific CD8+ T cells and their role in immunity. Crit. Rev. Immunol 2006. 26: 317–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lalvani A, Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest 2007. 131: 1898–1906. [DOI] [PubMed] [Google Scholar]
- 37.Ernst JD, Lewinsohn DM, Behar S, Blythe M, Schlesinger LS, Kornfeld H and Sette A, Meeting Report: NIH Workshop on the Tuberculosis Immune Epitope Database. Tuberculosis (Edinb.) 2008. 88: 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dosanjh DP, Bakir M, Millington KA, Soysal A, Aslan Y, Efee S, Deeks JJ et al. , Novel M tuberculosis antigen-specific T-cells are early markers of infection and disease progression. PLoS One 2011. 6: e28754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewinsohn DA, Heinzel AS, Gardner JM, Zhu L, Alderson MR and Lewinsohn DM, Mycobacterium tuberculosis-specific CD8+ T cells preferentially recognize heavily infected cells. Am. J. Respir. Crit. Care Med 2003. 168: 1346–1352. [DOI] [PubMed] [Google Scholar]
- 40.Lancioni C, Nyendak M, Kiguli S, Zalwango S, Mori T, Mayanja-Kizza H, Balyejusa S et al. , CD8+ T cells provide an immunologic signature of tuberculosis in young children. Am. J. Respir. Crit. Care Med 2012. 185: 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harari A, Dutoit V, Cellerai C, Bart PA, Du Pasquier RA and Pantaleo G, Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol. Rev 2006. 211: 236–254. [DOI] [PubMed] [Google Scholar]
- 42.Serbina NV, Liu CC, Scanga CA and Flynn JL, CD8 +CTL from lungs of Mycobacterium tuberculosis-infected mice express perforin in vivo and lyse infected macrophages. J. Immunol 2000. 165: 353–363. [DOI] [PubMed] [Google Scholar]
- 43.Rook GA, Lowrie DB and Hernandez-Pando R, Immunotherapeutics for tuberculosis in experimental animals: is there a common pathway activated by effective protocols? J. Infect. Dis 2007. 196: 191–198. [DOI] [PubMed] [Google Scholar]
- 44.Murray RA, Mansoor N, Harbacheuski R, Soler J, Davids V, Soares A, Hawkridge A et al. , Bacillus Calmette Guerin vaccination of human newborns induces a specific, functional CD8+ T cell response. J. Immunol 2006. 177: 5647–5651. [DOI] [PubMed] [Google Scholar]
- 45.Young DB, Gideon HP and Wilkinson RJ, Eliminating latent tuberculosis. Trends Microbiol 2009. 17: 183–188. [DOI] [PubMed] [Google Scholar]
- 46.Barry CE 3rd, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D et al. , The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat. Rev. Microbiol 2009. 7: 845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zimmerli SC, Harari A, Cellerai C, Vallelian F, Bart PA and Pantaleo G, HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. USA 2005. 102: 7239–7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hersperger AR, Martin JN, Shin LY, Sheth PM, Kovacs CM, Cosma GL, Makedonas G et al. , Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood 2011. 117: 3799–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harari A, Cellerai C, Enders FB, Kostler J, Codarri L, Tapia G, Boyman O et al. , Skewed association of polyfunctional antigen-specific CD8 T cell populations with HLA-B genotype. Proc. Natl. Acad. Sci. USA 2007. 104: 16233–16238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roederer M, Nozzi JL and Nason MC, SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 2011. 79A: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


